Document 12142557
advertisement

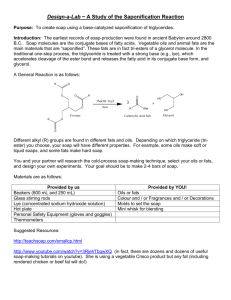
Cleaning Up the Classroom- a Traditional Way! Chemistry 20- Miss Dmytryshyn Objective: Using the soap making process you will be able to… 1. Identify changes which indicate a chemical reaction has take place 2. Recognize how chemistry has been involved in product and process development in the last 30 years. Chemical Reaction: Background Information: Although the process of making soap was not documented due to oral transmission of knowledge, Saskatchewan Metis peoples have indicated that their peoples used traditional soap making techniques for many years (Campbell, 2011). In their process, they would use woodash lye and animal fats to produce soaps for basic cleaning. Other reports indicate the use of buffaloberries or soap berries to produce a soap for cleaning or a combination of chalk and oatmeal (Coleclough, 2013). In western Chemistry, scientists believe that the production of soap is due to a chemical reaction between the lye and fats. Fatty acids are carboxylic acids (carbon atoms bonded to two oxygen atoms-­‐one single bond, one double bond) with a long carbon chain attached to the end. Three fatty acids connected by an ester produce a “tri”glyceride. Triglycerides get their name from the three fatty acid groups bonded together. More commonly, triglycerides are refered to as fats or oils. At room temperature, if trigylcerides are solid they are called fats and if they are liquid are called oils. Triglycerides are not soluble in water, however, when reacted with sodium hydroxide (sodium ion and hydroxide ion), the single bonded oxygen bond is broken to produce glycerol and crude soap (sodium stearate) in a process called saponification. In this process, the oxygen atoms joins with the sodium ion in solution to form sodium stearate (soap). In addition, glycerol is produced by the hydroxide ion bonding to the remaining chain. When the reaction is completed, the water will evaporate with excess ethanol. As a result, soap will be formed and solidified. Because of the hydrophilic (water soluble or water “liking”) end and the hydrophobic (water resistant or water “fearing”) end, the soap can create emulsions around grease molecules. The hydrophobic end will attach to the greasy, exposing the hydrophilic end to absorb into the water and move freely down the drain. In new chemical processes, industries like to use detergents in this process to increase the amount of grease collected. Definitions: Fatty Acid: ______________________________________________________________ Fats: ___________________________________________________________________ Lye: ____________________________________________________________________ Chemical Reaction: ________________________________________________________ Saponification: ___________________________________________________________ Soap: ___________________________________________________________________ Concentration: ___________________________________________________________ Saponification Number: ____________________________________________________ Questions to Ponder: 1. How might the immergence of Chemistry have influenced a change in the method of soap making? 2. How might the immergence of Chemistry have influenced the method of sharing knowledge? 3. What societal needs may have resulted in the changes in the development of soap? a. Population increase b. Resources c. Cost d. Safety e. Chemical factors Basic Soap Recipe-­‐ Courtesy of Jennifer Billinsky Ingredients: • • • • • • 340 g Olive Oil 225 g Veg Shortening 187 g Coconut Oil 105 g lye 250 g distilled water 21 g fragrance oil Instructions: Dissolve lye in water (in an ice bath to cool the reaction) and leave stirring. Measure all solid fats in a second contrainer, melt and microwave. Add liquid oils to the melted fats. When both lye and oils are at ~40ºC, pour lye slowly into the oil (be careful since fumes are given off when the two are mixed). Mix with immersion blender until just blended, then add fragrance oil or exfoliants and pour into molds. Let the soap sit for 24 hours then cut into preferred size. Then store the soap for one month before use. Makes 1L of soaps Observations: _________________________________________________________________________________________________ _________________________________________________________________________________________________ _________________________________________________________________________________________________ _________________________________________________________________________________________________ _________________________________________________________________________________________________ _________________________________________________________________________________________________ _________________________________________________________________________________________________ _________________________________________________________________________________________________ _________________________________________________________________________________________________ _________________________________________________________________________________________________ _________________________________________________________________________________________________ _________________________________________________________________________________________________ _________________________________________________________________________________________________ Questions: For each question, provide a minimum of two sentences to answer the question. 1. What is the chemical reaction for soap making? 2. What components of soap making would Western Scientists be concerned with and why? State some of your observations from the Western Science method. 3. Would the Metis have the same concerns? Give examples & explain why/why not. 4. Does the Western Science method produce better soap? 5. Why might people still use the traditional method for soap making? 6. Using your answers to the previous questions, how do you think Western Science has altered due to societal demands in regards to soap making techniques? 7. List other products that may have been changed because of societies needs. Give examples from agriculture, industry, household products, or basic needs.