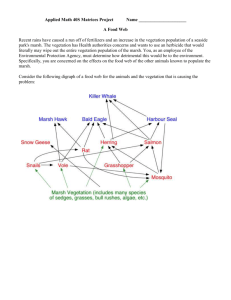
AN ABSTRACT OF THE THESIS OF
advertisement

AN ABSTRACT OF THE THESIS OF Kristin N. Jones for the degree of Master of Science in Forest Ecosystems and Society presented on November 20, 2015 Title: Combined Effects of Intensive Forest Management and Microclimate on Reproduction in a Cavity-Nesting Songbird Abstract approved: ________________________________________________________ James W. Rivers Matthew G. Betts ABSTRACT Future scenarios of global climate change rely on large-scale climate envelope models that do not account for local climatic conditions to which organisms most closely respond. Shifts in species distributions and phenology driven by climate change are welldocumented, yet we lack a strong understanding of how climate change will influence the demographic rates of animal populations, which directly determine the likelihood of species persistence. Land cover change, an agent of global change that is increasing in extent and intensity to meet the needs of a growing human population, can combine with climate change to stress animal populations by altering microclimatic conditions, as well as changing the availability of resources such as food and cover from predators. Thus, identifying the individual and combined effects of climate and land management practices is essential to accurately predict the responses of animal populations to global change. Intensive forest management results in land cover change by suppressing the growth of competing plant species in favor of commercial species and altering the abundance and composition of forest vegetation. In turn, changes in the abundance and composition of forest vegetation can modify local air temperatures. I hypothesized that herbicide application would alter the thermal environment for early-seral forest organisms (Chapter 2). If this were the case, then air temperature could be expected to increase in magnitude and variability along a gradient of herbicide treatment intensity. To test this hypothesis, I used iButton dataloggers to monitor air temperature at 160 nest boxes on 20 intensively managed early-seral forest stands (10.4 - 18.9) in the northern Oregon Coast Range, USA representing a gradient in intensive forest management (i.e., no-spray control, light, moderate and intensive herbicide application). I also measured the amount and composition of vegetation cover to test for herbicide effects on vegetation among application intensities. Using linear mixed models, I compared three measures of air temperature (mean daily minimum, mean, and maximum) and their associated coefficients of variation (CVs). Additionally, I used linear mixed models to confirm differences in total vegetation and broadleaved vegetation cover. Although mean total vegetation cover generally decreased, it did not significantly differ among herbicide treatments; in contrast, mean broadleaved vegetation cover was significantly reduced in the moderate and intensive treatments. Herbicide treatment was a significant predictor of maximum and mean temperatures, but minimum temperatures did not differ with herbicide treatment. Although there was an effect of herbicide treatment on air temperature, corrected pairwise comparisons indicated no significant differences among treatments. I note that though my power to detect statistical differences among treatments was limited, these differences were quite small (< 0.5°C) and confidence intervals were generally relatively narrow (< 1.5°C), suggesting that temperature did not differ among herbicide treatments in biologically meaningful ways. Furthermore, I did not detect an effect of herbicide treatment on temperature variability. Estimated differences in temperature variability among treatments were small (< 0.5%) and confidence intervals covered a relatively broad range of values, indicating that I did not have enough statistical power to detect effects. I found no uniform pattern in the direction (positive or negative) of the effect of herbicide treatment on temperature or CVs among treatment intensities. Daily air temperatures can strongly influence the reproductive output of earlyseral songbirds if temperatures exceed physiological tolerances of offspring, decreasing physiological performance (e.g., excessively high and low temperatures can alter metabolic rates) and survival. Moreover, the abundance and composition of early-seral forest vegetation can influence songbird reproductive output through changes in nest predator communities, food resources, or both. Thus, I further hypothesized that intensive forest management practices could combine with intraseasonal air temperatures to impact reproductive output in an insectivorous cavity-nesting songbird, the House Wren (Trogolodytes aedon) (Chapter 3). If this were the case, then House Wren nest survival, the number of offspring produced, and the quality of those offspring would decline with greater management intensity and increasing air temperatures. To test these predictions, I monitored 283 nests on 24 intensively managed early-seral forest stands (8.6 - 18.9 ha) in the northern Oregon Coast Range, USA representing a gradient in intensive forest management (i.e., no-spray control, light, moderate and intensive herbicide application). I used data from Chapter 2 to test for combined effects of air temperature and herbicidedriven vegetation changes on House Wren reproductive output. Using linear mixed models within a model selection framework, I did not find support for combined effects of temperature and herbicide treatment on nest survival. After accounting for the effects of herbicide-driven vegetation changes on reproductive output, air temperature effects were negligible. My results suggest that post-harvest vegetation management likely does not influence the number of young produced nor their quality (as indicated by body condition) in intensively managed early-seral forests, but may influence nest survival. However, nest survival did not decline along a gradient of herbicide intensity as I expected. Instead, mean nest survival was greatest in the control and most intensively managed stands (failure was greatest in the light treatment); however, these effects were so variable as to not be statistically significant. My results suggest that post-harvest vegetation management in intensively managed forests may be linked to minor changes in microclimatic air temperatures, but that there is high variation in these effects and effects are likely small. Therefore, there appears to be limited potential for vegetation control strategies through herbicides to buffer expected climate change effects on organisms in early-seral forest. My results also suggest that the potential for combined effects of herbicide application and air temperature on early-seral cavity-nesting songbirds is limited. Under current local climate patterns, air temperature appears to exert negligible effects on House Wren reproductive output after accounting for changes in vegetation cover. The effects of herbicide-driven vegetation changes on early-seral songbirds may be large, though highly variable, and not an increasing function of herbicide intensity. These effects may be primarily predator-mediated, as indicated by the large effects of herbicide treatment on House Wren nest survival but not the number of offspring produced nor their quality. My finding of limited temperature effects on House Wren reproductive output compared to the effects of forest management intensity supports predictions that, despite increasing concerns over the impacts of advancing climate change on animal populations, land cover change driven by anthropogenic land use will continue to be the primary global change driver impacting animal populations in the near future. ©Copyright by Kristin N. Jones November 20, 2015 All Rights Reserved Combined Effects of Intensive Forest Management and Microclimate on Reproduction in a Cavity-Nesting Songbird by Kristin N. Jones A THESIS Submitted to Oregon State University in partial fulfillment of the requirements for the degree of Master of Science Presented November 20, 2015 Commencement June 2016 Master of Science thesis of Kristin N. Jones presented on November 20, 2015 APPROVED: Co-Major Professor, representing Forest Ecosystems and Society Co-Major Professor, representing Forest Ecosystems and Society Head of the Department of Forest Ecosystems and Society Dean of the Graduate School I understand that my thesis will become part of the permanent collection of Oregon State University libraries. My signature below authorizes the release of my thesis to any reader upon request. Kristin N. Jones, Author ACKNOWLEDGEMENTS This project would not have been possible without the use of land provided by Weyerhauser Company, Hancock Natural Resource Group, Plum Creek Timber Company, and the Oregon Department of Forestry; collaboration with Jake Verschuyl and the National Council of Air and Stream Improvement, Inc.; and funding provided by grants from the United States Department of Agriculture, Agriculture Food and Research Initiative grant (AFRI-2009-04457), the National Council for Air and Stream Improvement, the Nobel Fund, Giustina Land and Timber, the Oregon Forest Industry Council, and the Oregon State University College of Forestry Fish and Wildlife Habitat in Managed Forests Research Program. Thanks to Daniel Ardia of Franklin & Marshall College for lending equipment and protocols and providing guidance on temperature-related measures; and to T. Manning, J. Bailey, K. Zummo, T. Barron, C. Adlam, H. Beyl, B. Cooney, G. Cummins, S. Doorly, E. Eve, D. Ferraro, B. Hardt, E. Keyes, D. Millican, L. Natola, E. White, D. Backlund, T. Stokely, C. Fitzmorris, N. Volpe, K. Wilson, S. Jordan, C. Loucks, E. Pokrivka, S. Campbell, R. Hepner, N. Garlick, A. Muniz, and J. Powell, who were absolutely instrumental to data collection. Thanks also to Forest Industry Biologists A.J. Kroll and Jack Giovanni for providing supporting data, and to cooperating forest industry biologists and managers J. Johnson, S. Keniston, M. Rochelle, T. Tompkins, J. Bakke, J. DeRoss, R. Frazzini, A. Heimgartner, T. McBride, J. Thiemens, A. Weathers, E. Finnell, T. Mortensen, M. Taylor, J. Travers, D. Irons, J. Light for providing further project support. I am deeply grateful to my advisors, James Rivers and Matthew Betts, for their boundless and creative insight into the research process, and for constantly pushing me to be a better scientist. I am also sincerely thankful to my committee members, Kerry Grimm and Joan Hagar for their support and research input. Thanks also to the Betts lab group, especially Thomas Stokely, for providing guidance and insight into research and analysis. I would like to give an especially massive thank you to Ariel Muldoon for her instrumental assistance with programming and analysis and seemingly endless patience. Thanks also to Lisa Ganio for analysis assistance and always being happily willing to discuss research, statistics, and professional development. I would also like to thank John Bliss for his wisdom and guidance, as well as Lisa Shipley and Keith Blatner for their instrumental roles in my placement in the master’s program in the College of Forestry at Oregon State University. Finally, thanks especially to my parents, Korina Layne-Jones and Christopher Jones, for keeping me “sane” throughout my master’s program and for unfailingly providing support and encouragement throughout all of life’s adventures. TABLE OF CONTENTS Page CHAPTER 1: GENERAL INTRODUCTION ................................................................... 1 CHAPTER 2: INTENSIVE FOREST MANAGEMENT EXERTS WEAK AND VARIABLE INFLUENCES ON MICROCLIMATE IN EARLY-SERAL FOREST ....... 9 2.1 Abstract ..................................................................................................................... 9 2.2 Introduction ............................................................................................................. 10 2.3 Materials and methods ............................................................................................ 15 2.3.1 Study area......................................................................................................... 15 2.3.2 Experimental design and treatments ................................................................ 16 2.3.3 Sampling design ............................................................................................... 17 2.3.3.1 Air temperature ......................................................................................... 18 2.3.3.2 Vegetation abundance and composition ................................................... 18 2.3.4 Data filtering and summary ............................................................................. 20 2.3.5 Statistical analysis ............................................................................................ 20 2.4 Results ..................................................................................................................... 22 2.4.1 Vegetation ........................................................................................................ 22 2.4.2 Mean air temperature ....................................................................................... 23 2.4.3 Variability in mean air temperature ................................................................. 24 2.5 Discussion ............................................................................................................... 25 2.6 Management implications ....................................................................................... 31 CHAPTER 3: INTENSIVE FOREST MANAGEMENT HAS A GREATER IMPACT THAN CLIMATE ON REPRODUCTIVE OUTPUT IN A CAVITY-NESTING SONGBIRD ...................................................................................................................... 41 3.1 Abstract ................................................................................................................... 41 3.2 Introduction ............................................................................................................. 42 3.3 Materials and methods ............................................................................................ 48 3.3.1 Study area and species ..................................................................................... 48 3.3.2 Experimental design......................................................................................... 50 3.3.3 Sampling design ............................................................................................... 51 TABLE OF CONTENTS (Continued) 3.3.3.1 Environmental measurements ................................................................... 51 3.3.3.2 Nest survival and productivity .................................................................. 54 3.3.4 Statistical analysis ............................................................................................ 55 3.3.4.1 Vegetation ................................................................................................. 56 3.3.4.2 Reproductive output .................................................................................. 56 3.4 Results ..................................................................................................................... 59 3.4.1 Effects of herbicide treatments on temperature and vegetation ....................... 59 3.4.2 Influence of herbicide treatment and temperature on nest survival ................. 60 3.4.3 Influence of herbicide treatment and temperature on the number of young produced .................................................................................................................... 62 3.4.4 Influence of herbicide treatment and temperature on nestling body condition ................................................................................................................... 63 3.5 Discussion ............................................................................................................... 64 CHAPTER 4: CONCLUSIONS ....................................................................................... 86 LITERATURE CITED ..................................................................................................... 88 APPENDICES ................................................................................................................ 100 Appendix A: Herbicide Prescriptions ......................................................................... 101 LIST OF FIGURES Figure Page Figure 2.1. Location of the eight study blocks used to examine the influence of intensive forest management on early-seral forest biodiversity in the Oregon Coast Range. Blocks used in this study to assess the influence if intensive forest management on microclimatic air temperature, May-August 2014, are indicated by orange squares............................... 35 Figure 2.2. Representative examples of stands spanning the gradient in herbicide intensity for (A) Control, (B) Light, (C) Moderate, and (D) Intensive treatments in the Oregon Coast Range, US, 2014 ..................................................................................................... 36 Figure 2.3. Ratio of mean percent cover among control, light, moderate, and intensive herbicide treatments, with Bonferroni-adjusted 95% confidence intervals. The dashed line at 1 represents no statistical difference. The left panel (A) depicts differences in broadleaved cover. The center panel (B) depicts differences in conifer cover. The right panel (C) depicts differences in total vegetation cover ..................................................... 37 Figure 2.4. Average daily air temperature fluctuations among herbicide treatments at each experimental block in the Oregon Coast Range, US. Lines represent the means of all sample points (8) for May-August, 2014 .......................................................................... 38 Figure 2.5. Mean air temperature differences among control, light, moderate, and intensive herbicide treatments in the Oregon Coast Range, May-August 2014, with Bonferroni-adjusted 95% confidence intervals. The left panel (A) depicts differences in mean air temperature. The right panel (B) depicts differences in air temperature variability (CVs). The dashed line at zero represents no statistical difference. Estimates on the right side of the dashed line correspond to decreases in air temperature with increasing herbicide treatment intensity, while estimate on the left side correspond to increases in air temperature .............................................................................................. 39 Figure 3.1. (A) A House Wren (Troglodytes aedon) nestling approximately 8 days after hatching being prepared for measurement of body condition, and (B) a completed clutch of House Wren eggs during incubation inside a nest box ................................................. 76 LIST OF FIGURES (Continued) Figure 3.2. General location map of the eight study blocks used to examine the influence of intensive forest management on early-seral forest biodiversity in the Oregon Coast Range. Blocks used in this study to assess the influence if intensive forest management on House Wren reproductive output May-August 2014, are indicated by orange squares ........................................................................................................................................... 77 Figure 3.3. Mean daily maximum temperature (Tmax) across all study sites in the Oregon Coast Range during the House Wren breeding season, May-August 2014. Measurements were taken every 15 min, averaged across a 24-hour period, and then averaged over all nest boxes (n=192) on 24 study sites in the Oregon Coast Range. The gray band shows the 95% confidence interval.............................................................................................. 78 Figure 3.4. Representative examples of stands spanning the gradient in herbicide intensity, including ranging from (A) Control (no-spray), (B) Light, (C) Moderate, and (D) Intensive treatments in the Oregon Coast Range, US, 2014 ...................................... 79 Figure 3.5. Plots depicting the effect of herbicide treatment and Tmax and on House Wren nest survival during the breeding season in the Oregon Coast Range, 2014. (A) Differences in odds ratio estimates of nest survival between the effect of herbicide treatments relative to control stands; odds ratios were averaged over all models in the candidate set. The dashed horizontal line represents odds ratios of the control for comparison with herbicide treatments; 95% CIs that overlap one indicate lack of significant differences relative to the control treatment. Numbers above confidence intervals indicate the number of nests monitored per treatment; there were 73 nests in the control treatment. (B) Boxplots depicting Tmax values from nests that either failed (pink) or fledged offspring (blue) across all four treatments. Vertical bars within boxes represent medians, boxes are interquartile ranges, whiskers are 1.5xinterquartile range and dots are outlying data ................................................................................................. 81 LIST OF FIGURES (Continued) Figure 3.6. Number of House Wren young produced per successful summarized by (A) herbicide treatment and (B) Tmax during the breeding season in the Oregon Coast Range, 2014. (A) Boxplots depict the number of young produced by treatment. Bars are medians, yellow diamonds are means, boxes are interquartile ranges, and whiskers are 1.5xinterquartile range. Numbers above whiskers indicate the number of successful nests per treatment. (B) Relationship between Tmax and the number of fledglings produced per successful nest. The blue line is a fitted linear regression line with 95% confidence intervals ............................................................................................................................. 83 Figure 3.7. House Wren day 8/9 body condition summarized by (A) herbicide treatment and (B) Tmax during the breeding season in the Oregon Coast Range, 2014. (A) Boxplots depict nestling body mass by treatment. Bars are medians, yellow diamonds are means, boxes are interquartile ranges, and whiskers are 1.5xinterquartile range. Numbers above whiskers indicate the number nests in which nestlings were measured per treatment. (B) Relationship between Tmax and the number of fledglings produced per successful nest. The blue line is a fitted linear regression line with 95% confidence intervals ................. 85 LIST OF TABLES Table Page Table 2.1 Means (± 1 SD) for vegetation and stand-location attributes for herbicide treatments in the Oregon Coast Range, 2014. Vegetation was measured June-early August 2014 during the height of the growing season. Vegetation values are percent (%) cover and were averaged over three sample points, then averaged over treatment units. Elevation and Aspect measured at each temperature sampling point and then averaged over treatment units. All values are stand-level means..................................................... 34 Table 3.1. A priori candidate models describing herbicide treatment and air temperature effects on House Wren nest survival, the number of young produced, per nest, and nestling body condition in the Oregon Coast Range, US, 2014 ....................................... 70 Table 3.2. Means (± 1 SD) for vegetation and stand location attributes for stands subjected to different herbicide treatments in the Oregon Coast Range, 2014. Vegetation (percent cover) was measured June-August 2014 during the height of the growing season, and was averaged over three sample points at each nest box. Measurements from each box were then averaged then averaged over all herbicide treatments units. Temperature (Tmax), elevation and aspect were measured at each nest box and then an averaged average was calculated for all boxes within stands subjected to each herbicide treatment over treatment units. All values are stand-level means..................................................... 72 Table 3.3. Model selection results from a priori candidate models describing the effects of herbicide treatment and Tmax on House Wren nest survival, the number of young produced per nest, and nestling condition in the Oregon Coast Range, 2014. Models are ranked in ascending order by Akaike’s Information Criterion adjusted for small sample sizes (AICc). The number of parameters (k), difference between the best model and all other models (ΔAICc), the relative likelihood of a model, AICc weights (wi), and evidence ratio (ER) are given for each model .................................................................. 73 Table 3.4. Results of models testing for effects of herbicide treatment and Tmax on House Wren nest survival, the number of young produced per nest, and nestling body condition in the Oregon Coast Range, 2014. Model coefficients (β) and 95% confidence intervals are given for each model. For nest survival models, odds ratios are also given. All model coefficients are model-averaged estimates ....................................................................... 74 Combined Effects of Intensive Forest Management and Microclimate on Reproduction in a Cavity-Nesting Songbird 1 CHAPTER 1: GENERAL INTRODUCTION Plant and animal populations are being markedly altered by climate change effects (Root et al., 2003; Parmesan, 2006; Cahill et al., 2012). Increasing temperatures and weather variability associated with climate change and can have direct and indirect effects on organisms. Indirect effects include earlier timing of spring events and poleward shifts in species distributions (Parmesan, 2006). Direct effects of climate change on organisms can result from precipitation and temperatures that exceed physiological tolerances (e.g., hyperthermia and water stress), potentially altering metabolic rates, decreasing physiological performance, and resulting in increased mortality rates (Helmuth et al., 2005; Buckley et al., 2012; Cahill et al., 2012). By the end of the 21st century, climate change is expected to increase global surface temperatures by more than 1.5-2 °C (IPCC, 2013, see IPCC, 2013 for full range of emissions scenarios); therefore, predicting how wildlife populations will respond to ongoing climatic shifts is critical to guiding successful conservation decisions (Dawson et al., 2011). Predictions of animal population responses to future climate change are often based on incomplete information (Sears et al., 2011; Potter et al., 2013). Some of the most widely used measures of climate change effects on populations are shifts in species ranges and phenology; however, these factors do not address the physiological and demographic mechanisms that directly determine the likelihood of species’ persistence on the landscape (Pearson and Dawson, 2003; Cooke et al., 2013). Evaluating a species’ conservation status requires information not only on species’ response to environmental pressures along spatial and temporal continuums, but also, and more importantly, on the 2 actual and potential capacity of individuals to respond to environmental pressures and how those responses impact population demographic rates (e.g., birth rates) (Vié et al., 2009; Bellard et al., 2012). Moreover, predictions of climate change impacts on animal populations rely largely on large-scale climate envelope models that do not account for the local climatic conditions to which organisms most closely respond—microclimates (Potter et al., 2013)—which can profoundly differ from surrounding macroclimates (Geiger et al. 2003). Failure to consider microclimates can lead to misleading predictions of how animal populations will respond to climate change (Gillingham et al., 2012; Varner and Dearing, 2014) by over- or under-estimating the availability of thermally suitable habitat across landscapes (Ashcroft, 2010; Suggitt et al., 2011). Microclimates are driven by several local terrain elements, including elevation, aspect, and vegetation cover (Dobrowski, 2011). Differences in the abundance and structure of vegetation cover produce different biophysical effects (e.g., albedo and surface roughness) on climate, changing the relative amount of incident solar radiation that is used by the land surface to heat the immediate environment (sensible heat) and dissipated to broader atmospheric scales (latent heat) (Bonan 2008a). Microclimate can also be influenced by vegetative composition (von Arx et al., 2012; Zhao and Jackson, 2014), which can alter biophysical characteristics such as canopy conductance (the ease with which plants can transpire water) and evaporative fractions (the fraction of available radiation used to evaporate water) to enhance or diminish heat dissipation. Therefore, it is likely that climate change effects on animal populations will be mediated by the microclimatic effects of local habitat characteristics. 3 Land cover change—the conversion of natural vegetation to alternate states— resulting from anthropogenic land use can significantly alter the microclimates to which organisms are exposed (Saunders et al., 1991; Pielke et al., 2011). It is also currently the primary driver of global declines in animal populations through habitat loss and degradation (Pereira et al., 2012; Monastersky, 2014). By decreasing habitat amount and quality (Fischer and Lindenmayer, 2007), land cover change can indirectly affect animal populations through many pathways, including altered food availability, predation, and temperature extremes (Saunders et al., 1991; Bennett and Saunders, 2010). Consequently, it is broadly expected that changes in land cover and climate change will combine in their effects on animal populations (Brook et al., 2008). However, like climate change, studies of land cover change effects on animal populations have largely focused on broad-scale measures of species change such as richness and abundance (McGarigal and Cushman, 2002), that do not directly influence the demographic rates governing species’ survival. As anthropogenic land use increases in extent (Lambin and Meyfroidt, 2011) and intensity (Tilman et al., 2011; Tscharntke et al., 2012) and the effects of climate change continue to intensify (IPCC, 2013), understanding the combined effects of land cover and climate change on demographic rates will be essential to conserving wildlife populations under global change. Forests are particularly subject to anthropogenic land cover change (Lambin and Meyfroidt, 2011; Hooke et al., 2012). Although forest cover loss has slowed globally and forest cover is even increasing in some regions (Hansen et al. 2013, Sloan et al. 2015), much of the world’s remaining forests have been degraded (Foley et al., 2005; Potapov et 4 al., 2008). Forest degradation is typically thought of in the context of old-growth forest types; however, early-seral forest degradation is receiving increasing attention (Swanson et al., 2010, DellaSala et al., 2014; King and Schlossberg, 2014). Compositionally and structurally diverse early-seral forest is declining in some locations in the northern hemisphere (Angelstam, 1998; Thomas et al., 2006), often below its historic range of variability (Spies and Johnson, 2007). This trend is of conservation concern because early-seral forest is associated with high species diversity and food-web complexity (Hagar, 2007; Swanson et al., 2010). Furthermore, the population trends of several vertebrate species have been linked with the availability of structurally and compositionally diverse early-seral forest (Hunt, 1998; Litvaitis, 1993). Forest vegetation management alters the abundance, structure, and composition of early-seral forest vegetation to favor commercially valuable trees (Balandier et al., 2006; Fortier and Messier, 2006; Wagner et al., 2006). Intensive forest management is a widely applied forest management system (Fox, 2000; Adams et al., 2005) that often uses herbicides to suppress the growth of early-seral herbaceous and broadleaf vegetation that would otherwise compete with trees for resources (e.g., soil nutrients, water, and sunlight) (Shepard et al., 2004; Wagner et al., 2006). Herbicides can significantly alter abundance, structure, and composition of early-seral forest stands (Balandier et al., 2006), potentially resulting in ecological degradation and wildlife population declines (Flueck and Smith-Flueck, 2006). Rapid declines in several Pacific Northwest songbird species are attributed to the increasing scarcity of structurally and compositionally diverse early-seral forest. Early- 5 seral vegetation shifts driven by herbicide use have been demonstrated to decrease abundance (Betts et al., 2013) and reproductive rates of several early-seral songbirds (Easton and Martin, 1998; Easton et al., 2002). Herbicides used in intensive forest management are generally assumed to have no direct effects on songbirds (Tatum, 2004; Flueck and Smith-Flueck, 2006). However, indirect effects include vegetation-mediated decreases in food availability quality (Taylor et al., 2006; Hagar 2007) and increases in predation rates (Easton and Martin, 1998). Furthermore, as land cover can function to decouple local climatic conditions from large-scale climatic shifts (Dobrowski, 2011; Varner and Dearing, 2014), herbicide-driven shifts in early-seral vegetation may provide a complex feedback mechanism that influences how early-seral organisms are impacted by climate change. The goal of my research was to examine the effects of forest cover change on abiotic and biotic processes along a gradient of management intensity. First, I evaluated the effects of herbicide treatment on microclimatic air temperature along an experimental gradient in treatment intensity in intensively managed forests in the northern Coast Range of Oregon, USA (Chapter 2). Second, I also examined the combined effects of herbicide intensity and air temperature on songbird reproductive output (Chapter 3). I asked the following research questions: 1. How does air temperature vary along a gradient in herbicide application intensity in intensively managed forests? (Chapter 2) I collected microclimatic air temperature data from 20 intensively managed forest stands throughout the northern Oregon Coast Range. Intensive forest management is a 6 commonly used forest management system in the Pacific Northwest (Adams et al., 2005; Murphy et al., 2005) and throughout the world (Fox, 2000; Paquette and Messier, 2009), thus my results may be widely applicable to timber operations globally. My results begin to fill important knowledge gaps concerning ecological impacts of forest management practices on a declining habitat type: early-seral forest. This study is a part of one of the largest experimental studies on intensive forest management practices globally. Moreover, this is the first study to examine the effects of post-harvest forest vegetation management on microclimatic air temperature within a gradient of herbicide treatment intensity. My results suggest that intensive management of early-seral forests exerts limited influence on the microclimatic air temperatures. 2. How does intensity of herbicide application interact with air temperature to affect reproductive success in a cavity-nesting songbird? (Chapter 3) I tested how the reproductive output of a locally abundant cavity-nesting songbird, the House Wren (Troglodytes aedon), was influenced by the gradient of herbicide treatment and microclimatic air temperature examined in Chapter 2. I collected data on nest survival, the number of fledglings produced per nest, and nestling body condition from 24 intensively managed forest stands throughout the northern Oregon Coast Range. My results provide insights into current patterns of the relative influence of global change drivers on animal population demographic rates. Moreover, this is the first study to examine the combined effects of a gradient in land cover change intensity and microclimatic air temperature on avian reproduction. My results suggest that microclimatic air temperature may influence reproductive success for early-seral 7 songbirds, but land cover change will likely continue to be the primary driver of changes in demographic rates in the near future. Finally, Chapter 4 summarizes the outcomes of the work described above. 8 INTENSIVE FOREST MANAGEMENT EXERTS WEAK AND VARIABLE INFLUENCES ON MICROCLIMATE IN EARLY-SERAL FOREST Kristin N. Jones, James W. Rivers, Matthew G. Betts 9 CHAPTER 2: INTENSIVE FOREST MANAGEMENT EXERTS WEAK AND VARIABLE INFLUENCES ON MICROCLIMATE IN EARLY-SERAL FOREST 2.1 ABSTRACT Climate change is expected to have far reaching impacts on global biodiversity. Negative effects of rising temperatures at large spatial scales have been widely predicted in plants and animals, but the spatial scale of such predictions is often broad and poorly matched to the fine scales at which organisms experience the environment. Such microclimate thermal regimes are often moderated by forest cover; thus, intensive forest management practices have the potential to either ameliorate or exacerbate climate change effects on biota. In this study, we examined how air temperature varied across a gradient of post-harvest vegetation management (via herbicide application) in intensively managed early-seral forests in the northern Oregon Coast Range. We evaluated standlevel air temperatures in regenerating forest stands subject to no-spray (control), light, moderate, and intensive herbicide treatments. We examined whether mean daily temperatures (minimum, mean, and maximum) and their associated coefficients of variation varied across herbicide treatments. We found that herbicide treatments significantly influenced mean and maximum air temperatures, but we did not detect an effect of herbicide treatment on minimum temperature. However, the direction (positive or negative) and magnitude of these effects were variable and small (< 0.5°C). Furthermore, we did not detect any differences in mean air temperatures in corrected pair-wise comparisons across individual herbicide treatment intensities. Additionally, 10 herbicide treatment did not exert a significant effect on within-treatment temperature variability. Our results suggest that post-harvest vegetation management has limited impacts on fine-scale air temperatures and is unlikely to either amplify or buffer the projected effects of climate change in early-seral forests. 2.2 INTRODUCTION Land cover change resulting from anthropogenic land use is currently the primary driver of global biodiversity declines (Pereira et al., 2012; Monastersky, 2014). Humans are estimated to have modified up to 58% of all ice-free land area through land uses such as urbanization and agriculture (Hooke et al., 2012), and have profoundly altered critical ecosystem functions such as climate regulation and wildlife habitat (Foley et al., 2005). At the same time, climate change is modifying ecosystems (Dawson et al., 2011). Climate change can affect organisms indirectly through shifts in species distributions and phenology (Parmesan, 2006), and directly through temperatures that exceed physiological tolerances and decrease physiological performance and fitness (Bellard et al., 2012; Buckley et al., 2012). Consequently, it is expected that changes in land cover and climate will combine in their effects on global biodiversity (Brook et al., 2008). Forests, some of the most diverse ecosystems in the world (Aerts and Honnay, 2011), have been particularly subject to anthropogenic land cover change (Rudel et al., 2005; Hooke et al., 2012). Through a combination of increasing afforestation and decreasing deforestation, global forest area is currently increasing (Sloan and Sayer, 11 2015). However, planted forest area is increasing while natural forest area is decreasing (FAO 2015). Planted forests may represent degraded forest conditions compared to naturally regenerated forests through an altered ability to support diverse ecological communities and recover from disturbance (Carnus et al., 2006; Potapov et al., 2008). Forest degradation is often considered in the context of old-growth forest (Foster et al., 1996; Spies, 2004; Thomas et al., 2006). However, increasing attention is being paid to the degradation of early-seral forests (Swanson et al., 2010; DellaSala et al., 2014; King and Schlossberg, 2014). Compositionally and structurally diverse early-seral forest is declining in some locations in the northern hemisphere (Angelstam, 1998; Thomas et al., 2006), often below its historic range of variability (Spies and Johnson, 2007). This trend is concerning because early-seral forest is associated with high food web complexity and species diversity (Hagar, 2007; Swanson et al., 2010). Furthermore, the population trends of several vertebrates have been linked to decreases in the availability of structurally and compositionally diverse early-seral forest (Litvaitis, 1993; Hunt, 1998). Forest vegetation management practices can decrease the quality of early-seral forests as habitat for wildlife (Swanson et al., 2010; Betts et al., 2013). Intensive forest management, a widely used forest management system (Fox, 2000), often uses herbicides to suppress the growth of early-seral herbaceous and broadleaved vegetation that compete with trees for resources (Fortier and Messier, 2006), leading to changes in abundance, diversity, and composition of early-seral forest (Balandier et al., 2006). The main impact of herbicides is altering the relative dominance of plant species within the community (i.e., relative increases in conifer cover and decreases in broadleaved cover) (Balandier et 12 al., 2006), which can result in negative consequences for early-seral organisms (Easton and Martin 1998; Betts et al., 2013). Microclimatic shifts are one mechanism by which herbicide-driven changes in the relative abundance of plant species in early-seral forests may influence habitat quality. One microclimatic variable important to habitat quality is air temperature (Huey, 1991). Surface air temperatures and their variability drive many ecological processes, including nutrient and water cycles and species distributions (Bonan 2008a). Forest cover primarily influences local air temperature through altering biophysical characteristics such as surface roughness (vertical irregularities in the canopy) and albedo (the fraction of solar radiation reflected by a land surface), which strongly influence the relative amount of incident solar radiation that is used by the land surface to heat the immediate environment (sensible heat) and dissipated to broader atmospheric scales through evapotranspiration (latent heat) (Bonan, 2008b; Jackson et al., 2008; Anderson et al., 2010). Previous work indicates that the relative abundance of plant species can also exert significant effects on forest microclimatic conditions (von Arx et al., 2012; Zhao and Jackson, 2014). For example, deciduous tree species have a summer albedo up to 0.1 higher than coniferous forests and canopy conductance (the ease with which plants can transpire water) and evaporative fractions (the fraction of available solar radiation used to evaporate water) approximately twice that of coniferous forests (Anderson et al., 2010). This additional transpiration and reflectivity of broadleaved vegetation tends to cool air temperatures to a greater degree than forests dominated by coniferous species. For example, von Arx et al. (2012) showed that broadleaved and non-conifer pine forests reduced daily maximum 13 temperatures twice as much as coniferous forests. This greater cooling effect of broadleaved vegetation suggests that forests with greater amounts of broadleaved vegetation cover may provide superior “micro-refugia” for species under advancing global climate change (Ashcroft, 2010; Dobrowski, 2011). To date, relatively few studies (Proe et al., 2001; Devine and Harrington, 2007; Parker et al., 2012) have examined the effects of herbicides on air temperature. Additionally, all of these studies have emphasized the average conditions of herbicide treatments, not the variation within them. Air temperature variation within treatments may allow temperature-sensitive species to persist in areas of the stand with suitable microclimates (Sears et al., 2011); conversely, temperature variation may impact earlyseral species that prefer more stable microclimatic conditions (e.g., Checa et al., 2014). Moreover, no studies have examined air temperature along a gradient of herbicide treatment intensity to evaluate the extent to which air temperature is moderated by variation in the relative abundance of broadleaved and coniferous vegetation in earlyseral forest. Furthermore, no studies have examined the effects of forest vegetation management on air temperature in the framework of intensive forest management, a management system that is projected to be applied to an increasing proportion of forested landscapes in coming years to meet the growing demand for wood products (Fox, 2000). By evaluating the effects of herbicide treatment intensity on air temperature, we can identify how forest vegetation management practices can be varied to produce thermal refuges from climate change for species associated with this forest seral stage. 14 In this study, we examined patterns of microclimatic air temperature variation across a gradient of post-harvest vegetation control in early-seral coniferous forests in the central Oregon Coast Range. By examining gradients in herbicide treatment intensity, we allowed for the potential detection of degrees of forest vegetation management that might minimize trade-offs between increases in the growth of commercially valuable conifers (via broadleaved vegetation suppression) and climate regulation in intensively managed forests. The herbicide treatments are part of a broader study that experimentally evaluates the effects of a gradient of herbicide treatment on early-seral forest biodiversity (Betts et al., 2013). We evaluated stand-level variation in microclimatic air temperature among and within herbicide treatments by addressing the following questions: (1) how do standscale patterns of surface air temperature vary with herbicide intensity? and (2) how does herbicide intensity affect the variability of surface air temperatures in managed forests? We predicted that herbicide treatments would increase surface air temperature extremes by shifting stand composition towards predominantly coniferous species, and that these increases would intensify with increasing herbicide treatment intensity. We also predicted that herbicide-treated stands would exhibit greater air temperature variability relative to untreated stands (Baker et al., 2014; Hardwick et al., 2015) by increasing the variability of day-time high and night-time low temperatures (i.e., the moderating effect of forest vegetation cover would decrease with less total cover in herbicide-treated stands). We further predicted that intermediate levels of herbicide treatment would exhibit increased spatial variability compared to intensive and control treatments because of (1) irregular shading by residual broadleaved vegetation, especially deciduous hardwood trees that 15 were not subject to herbicides in the light treatment and/or (2) patchy distribution of herbaceous species (as opposed to bare ground) in the light and moderate treatments, which can also exert moderating influences on air temperature (Geiger et al. 2003). 2.3 MATERIALS AND METHODS 2.3.1 Study area We conducted this study at five of the eight experimental blocks that comprise the broader study (see Figure 2.1) in the northern Oregon Coast Range—Black Rock, Grande Ronde, Luckiamute, Trask, and Willamina. The maritime climate of the Oregon Coast Range is characterized by cool, wet winters and mild, dry summers (Franklin and Dyrness 1988). Mean annual precipitation ranges from 165 to 330 cm with most precipitation occurring October through March. Mean annual air temperature ranges from 5-19°C (Franklin and Dyrness 1988, Taylor and Hannan 1999) Soils are dominantly moderately deep to deep well-drained silt loam soils, and are derived from basalts, sandstone, and siltstones. Topography is characterized by somewhat low, highly dissected mountains with slopes ranging from 0 to 90 percent (Knezevich 1982, Taylor and Hannan 1999). All sampled stands are in the western hemlock (Tsuga heterophylla) zone (Franklin and Dyrness 1973), ranging in elevation from 165 to 765 m. Douglas fir (Pseudotsuga menziesii) dominates early-seral forest plantations in this region, with grand fir (Abies grandis), western hemlock (Tsuga heterophylla), and western redcedar (Thuja plicata) forming minor components. Dominant shrub/woody species include big- 16 leaf maple (Acer macrophyllum), California hazelnut (Corylus cornuta californica), cascara (Rhamnus purshiana), common snowberry (Symphoricarpos albus), oceanspray (Holodiscus discolor), red alder (Alnus rubra), and vine maple (Acer circinatum). Smaller understory broadleaf species include Oregon grape (Mahonia nervosa) salal (Gaultheria shallon), and Vaccinium spp. The herbaceous community is comprised of many native and non-native herbaceous plants, with swordfern (Polystichum munitum) and brackenfern (Pteridium aquilinum) often dominating. 2.3.2 Experimental design and treatments Our study makes use of a subset of sites that occur within a larger study area that uses a randomized complete block design in which 32 stands occur in 8 separate blocks, and each of 4 levels of herbicide treatment were randomly applied to one stand within each block (Figure 2.1). All blocks are located across a 100 km (N-S) section of the northern Oregon Coast Range region (Betts et al., 2013). Stands within each block were sited > 1 km of each other to ensure spatial independence of treatments and no more than 5 km of each other to reduce within-block variation (i.e., slope, elevation, and pre-cut vegetation composition). From this larger study framework, we constrained our study on 20 forest stands located within 5 of the blocks, with each stand 10.4 - 18.9 ha because of logistical considerations that prevented us from working on all stands concurrently. All stands were clear-cut in fall 2009 and replanted in spring 2011 with Douglasfir, the most commonly commercially cultivated species throughout the region, at approximately 980 trees per hectare. A full suite of herbicides and surfactants typically 17 used in commercial timber harvesting operations was applied to stands between 2009 and 2014 in a manner that created a gradient in management intensity; see Betts et al. (2013) and Appendix A for a full description of the herbicides used, the timing with which they were applied, and their concentration. All herbicide spraying occurred in a timeframe that mimicked the typical timeframe in which vegetation control takes place in commercial operations Temperatures were measured around bird nestboxes to assess the effects of temperature on songbird reproduction in another study (Jones et al., in prep). Following harvest treatments, 8 cedar nest boxes were mounted on steel garden stakes 1 - 1.5 m from the ground on each stand (n = 160). Nest boxes were sited with several considerations in mind; (1) equal distances between boxes (> 50 m separation), (2) even stand coverage, and (3) sampling logistics (i.e. walking distance between boxes < 30 min). As distance to stand edge was not a factor in nest box siting, but has been shown to influence air temperature (Baker et al., 2014), we included it as an analysis covariate (see Statistical Analysis below). Vegetation was not a factor in box siting. 2.3.3 Sampling design We recorded air temperature during the local songbird breeding season (May August 2014) as part of a related study (Jones et al., in prep), 3 years after trees were planted. We measured air temperature at nestboxes on each stand to link temperature effects to the reproductive output of cavity-nesting songbirds because a goal of that study 18 was to evaluate the effects of intensive forest management on air temperatures experienced directly by organisms (microclimatic air temperatures). 2.3.3.1 Air temperature To measure ambient temperature, we placed iButton temperature loggers (n = 160) on the underside of nestboxes erected on study sites at a density of 8 boxes/stand. Each iButton was programmed to record temperature every 15 min and was affixed to the underside surface of each nestbox with a zip tie so that it hung freely 5 cm below the box. We covered iButtons with a section of white 10 cm diameter PVC tube with ventilation holes so as to prevent direct exposure of the iButton to solar radiation and moisture, allow for airflow, and minimize heat accumulation. Due to logistical constraints, we used two iButton models that varied slightly in their accuracy (± 1.0°C accuracy, n = 71 boxes: ± 0.5°C accuracy, n = 89 boxes). The two models were distributed irregularly among stands; thus, we note that estimated differences in air temperature between stands are within approximately 1.0°C of their true value. All iButtons were validated against an independent digital thermometer (Omega HH609R, Omega Engineering, Stamford, Conneticut, USA) prior to placement on stands; any iButtons that deviated by ≥ 0.5°C during his validation procedure were not used. 2.3.3.2 Vegetation abundance and composition We measured vegetation cover in each treatment to quantify changes in vegetation abundance and composition among the different herbicide treatments. At each 19 temperature sampling point, we measured vegetation at three distinct sampling points (3 m radius each): one was centered directly on the temperature sampling point, and the other two were located 25 m distant from the temperature sampling point. The azimuth of the initial off-nestbox point was chosen randomly, with the second point located 180° away. At each vegetation sampling point, we visually estimated percent cover to genus (in the case of Rubus, Ribes, and forbs) or species in each of three distinct vegetative strata; herbaceous (0 - 0.5 m), shrub (0.5 - 2.0 m), and canopy (> 2.0 m) layers. We aggregated some closely-related species that are functionally similar (i.e., Rubus and Ribes), as well as all herbaceous forbs, grasses, and ferns. For analysis, we summed the amount of cover for all plant species and genera in each of the three vegetation sampling points, then calculated an cover value, grouping plant types together to estimate the total amount of (1) broadleaved cover (as defined by Ellis and Betts, 2011), (2) conifer cover, and (3) vegetative cover. At each sampling point, we also measured aspect, which we transformed to “southwestness” (i.e., cos(aspect degrees - 225); Franklin et al., 2000), elevation, and distance to edge, as these variables have been shown to strongly influence microclimate (Dobrowski, 2011). Aspect was transformed because it is not an incremental metric (e.g., 0° is more similar to 350° [both north-facing] than 90° [eastfacing]). Conversely, southwestness is an index ranging from 1 (signifying southwest) to -1 (signifying northeast) that can indicate the environmental aridity with more arid environments producing greater values (Huang et al., 2012). 20 2.3.4 Data filtering and summary Temperature data were initially inspected and assessed for errors, indicated by extreme atypical values (> 50°C or < -10°C) caused by instrument malfunction or when logging stations were found to be disturbed (i.e., nestbox damaged by wildlife). For example, data were considered erroneous when temperatures logged by the same iButton within an hour were greater or less than 10°C of the record in question or temperatures recorded on the same stand at other nestboxes were 20°C greater or less than the recorded temperature in question. This led us to remove ≤ 5% of temperature values. We used iButtons to identify the minimum (Tmin), mean (Tmean), and maximum (Tmax) daily temperature at each nest box for each day during a 24-hour period starting at midnight. For each of these three measures, we then computed means at each nestbox during each 24-hour period. We used these data to calculate a stand-level mean and coefficient of variation (CV; Tmincv, Tmeancv, Tmaxcv) for all 6 air temperature response variables. 2.3.5 Statistical analysis All models were fit using the lme function of the nlme package (Pinheiro et al. 2015) in ‘R’ v3.2.0 (R Development CoreTeam 2015). First, we used mixed model analysis to test whether forest vegetation abundance and composition differed significantly among herbicide treatments. We constructed models for three response variables of forest vegetation (broadleaved, conifer, and total vegetation cover) that included fixed effects for treatment (4 levels: control, light, moderate, intensive) and one 21 random effect (block) to reflect our randomized complete block design. All vegetation responses were log transformed prior to analysis to correct for heterogeneity of variance among herbicide treatments. We back-transformed values so that values can be interpreted as the mean multiplicative increase or decrease in cover between treatments. Therefore, a treatment contrast of 1 indicates that cover is equal between treatments. Second, we used a randomized block ANCOVA with one random effect (block) to test whether mean daily air temperature and within-treatment variability differed significantly among herbicide treatments. We constructed models for 6 air temperature response variables (Tmin, Tmean, Tmax, and their associated CVs) that included fixed effects for treatment. Covariates included were stand-level averages for elevation, southwestness, and the distance to stand edge, taken over all nestboxes in a stand. We included elevation as a covariate because stands within a block could vary > 100 m due to the highly heterogeneous terrain of the Oregon Coast Range. Southwestness and distance to stand edge were included as covariates to account for any systematic variation in temperatures due to nestbox siting as, along with elevation, both factors can influence surface air temperatures (Geiger et al. 2003). No covariates were correlated (r < 0.25 in all cases). Diagnostic tests revealed minor violations of assumptions of normality and homogeneous variance among treatments, but these were not enough to warrant data transformation (Zurr et al. 2009, Ramsey and Schafer 2013). We also conducted 5 pairwise comparisons using Bonferroni-corrected 95% confidence intervals and p-values in the ‘estimable’ function of the gmodels package in R (Warnes 2013). We compared each of the 3 treatments where herbicide was applied to 22 the control (3 comparisons) to evaluate how increasing management intensity influenced air temperature. In addition, we compared the moderate herbicide treatment to the light and intensive treatments (2 comparisons) because the moderate treatment this treatment that is closest to current operational practices (Betts et al. 2013). Thus, these comparisons (5 total) allowed us to compare how alternative herbicide application may lead to changes in air temperature. We report means and associated confidence intervals; effects were considered significant at P < 0.05 for all tests. 2.4 RESULTS 2.4.1 Vegetation Herbicide treatments had a strong influence on vegetation composition (Figure 2.2) with broadleaved vegetation cover generally decreasing with increasing herbicide treatment intensity (F3, 11 = 18.68, P < 0.001; Figure 2.3a) while conifer cover increased (F 3, 11 = 9.19, P = 0.003; Figure 2.3b). However, after correcting for multiple comparisons, we only detected significant differences in broadleaved cover between the control and the moderate (t 11 = 4.88, P < 0.001, 𝛽̂ = 1.88 [0.68, 3.08]) and control and intensive treatments (t 11 = 5.04, P < 0.001 , 𝛽̂ = 1.93 [0.74, 3.12]) and the light and the moderate treatment (t 11 = 4.89, P < 0.001, 𝛽̂ = 1.87 [0.68, 3.05]) (Figure 2.3a). Furthermore, we only detected a difference in conifer cover between the control and intensive treatments (t 11 = -4.69, P < 0.001, 𝛽̂ = -0.95 [-1.58, -0.32]). We did not detect any statistically significant differences in total vegetation cover among all herbicide 23 treatments (F3, 11 = 2.070 P = 0.162; Figure 2.3c). Lastly, vegetation cover within vegetative strata (e.g., canopy cover) was similar among herbicide treatments (Table 2.1). 2.4.2 Mean air temperature Air temperature varied little among blocks (Figure 2.4), and was statistically indistinguishable among treatments for Tmin, Tmean, and Tmax (Figure 2.5a). Herbicide treatments exerted a significant effect on most temperature variables, but the direction of this effect was inconsistent. We did not detect an effect of treatment on Tmin (F3, 9 = 1.039, P = 0.421) but we did find that treatment influenced on Tmean (F3, 9 = 7.655, P = 0.008). However, this effect was driven by the significance of the intercept (control treatment) in Tmean models (𝛽̂ = 18.6, SE = 1.0, t9 = 18.6, P < 0.001). Pairwise comparisons revealed no statistically significant differences in Tmean among herbicide treatments (Figure 2.5a). All estimated differences in mean Tmean were ≤ 1.1°C, with the majority < 0.5°C (Figure 2.5a), and the direction of these differences (positive or negative) was inconsistent (Figure 2.5a). We also detected an effect of treatment on Tmax (F3, 9 = 5.34, P = 0.022). However, this effect was driven by statistically significant Tmax differences between the control and the light treatment (𝛽̂ = 1.1, SE = 0.4, t 9 = 2.67, P = 0.026) and the control and the moderate treatment (𝛽̂ = 1.0, SE = 0.4, t 9 = 2.34, P = 0.044). Mean Tmax monotonically increased with increasing treatment intensity (Figure 2.5a). Elevation was a significant predictor of Tmean (F1, 9 = 30.97, P < 0.001); Tmean decreased with increasing elevation (𝛽̂ = -0.3°C/100 m, SE = 0.06, t 9 = -4.819 , P < 24 0.001). Elevation was also a significant predictor of Tmax (F1, 9 = 114.68, P < 0.001); Tmax decreased with increasing elevation (𝛽̂ = -0.7°C/100 m, SE = 0.08 , t 9 = -8.523 , P = 0.003). In addition, distance to stand edge was a significant predictor of Tmax (F1, 9 = 6.95, P = 0.027); for every 10 m from the stand edge, Tmax increased by 0.4 (± 0.15 SE) °C. 2.4.3 Variability in mean air temperature Temperature variability did not statistically differ among herbicide treatments (Figure 2.5b). We did not detect a significant difference in Tmincv among treatments (all CIs overlapped zero). Nevertheless, compared to the control, mean Tmincv was lower in the moderate and intensive treatments, but more variable in the light treatment. Among treatments, mean Tmincv was greater in the light and moderate treatments compared to the intensive treatment (Figure 2.5b). We also did not detect a significant difference in Tmeancv among treatments (all CIs overlapped zero; Figure 2.5b). Nevertheless, mean Tmeancv was lower in all herbicide-treated stands compared to the control treatment (Figure 2.5b). Among herbicide treatments, mean Tmeancv was greater in the light compared to the moderate treatment (𝛽̂ = 0.06% [-1.87, 2.0]); conversely, mean Tmeancv was greater in the intensive treatment compared to the moderate treatment (𝛽̂ = -0.09% [2.09, 1.91]). Finally, we did not detect a significant difference in Tmaxcv among treatments (all CIs overlapped zero; Figure 2.5b). Nevertheless, mean Tmaxcv was lower in all herbicide-treated stands compared to the control (Figure 2.5b). Among herbicide treatments, mean Tmaxcv was greater in the light treatment compared to the moderate 25 treatment and in the moderate treatment compared to the intensive treatment (Figure 2.5b). Estimated differences among treatments for mean Tmincv, Tmeancv, and Tmaxcv were ≤ 1.5%, with the majority < 0.5% (Figure 2.5b). 2.5 DISCUSSION Our results suggest that herbicide treatment has little to no influence on microclimatic air temperatures in early-seral forests. We found that herbicide treatment exerted a statistically significant, though minor, effect on Tmean and Tmax, but not on Tmin. However, corrected pairwise comparisons indicated no significant differences in mean air temperatures among herbicide treatment intensities. Furthermore, air temperature variability (Tmincv, Tmeancv, and Tmaxcv) was not significantly influenced by herbicide treatment. Given the significant differences in broadleaved cover among treatments, that we detected no more than limited differences among treatments is surprising and runs counter to our initial predictions. Previous studies have found broadleaved vegetation to significantly cool air temperature relative to coniferous vegetation (von Arx et al., 2012; Zhao and Jackson, 2014) via increased evaporative fractions and higher albedo. Thus, we expected decreases in broadleaved vegetation from herbicides to result in increased air temperatures. Indeed, our results suggest that Tmax and Tmean were influenced to a degree by herbicide treatment; however, temperature differences among treatments were small, highly variable, and exhibited no uniform direction (positive or negative). For example, Tmax was greater in all herbicide 26 treatments compared to the control, but also was greater in the light and lower in the intensive treatment compared to the moderate treatment. Therefore, herbicide treatment seems to exert minimal influence on stand-level microclimatic air temperatures in earlyseral intensively managed forests in the Oregon Coast Range. One possible explanation for decreased broadleaved cover not influencing air temperature is that temperature in early-seral stands may depend more on total vegetation cover than relative composition. Indeed, the largest moderating influences on air temperature of forest vegetation are generally associated with changes in measures of total vegetation abundance such as leaf area index (Aussenac, 2000). The lack of difference in total cover among herbicide treatments was likely driven by increases in exotic ruderals, such as species in the genera Cirsium, Epilobium, and Aster (Jones et al., unpublished data). This group, which comprised the majority of non-woody vegetation in the understory of our herbicide-treated stands (Figure 2.2), substantially increased with increasing herbicide treatment (Table 2.1), though this trend was highly variable. Furthermore, total vegetation cover in early-seral forest stands is dependent on sitespecific factors, including site topography, hydrology, and seed bank composition (Lautenschlager and Sullivan, 2002); sites with more favorable growing conditions will regenerate vegetation more quickly. Thus, total vegetation cover may begin to recover fairly quickly on highly productive sites such as those in the Oregon Coast Range (Waring and Franklin, 1979). Indeed, as herbicides only alter the relative abundance of target vegetation and do not necessarily kill competitive and weedy species (Miller and Miller, 2004), such plants can rebound within 2-5 years following treatment 27 (Lautenschlager and Sullivan, 2002; Miller and Miller, 2004). Thus, increased growth of rapidly establishing exotic ruderals on our stands may have played a compensatory role in thermal regulation on sites with decreased broadleaved cover. An additional explanation for our inability to detect an effect of decreased broadleaved cover on air temperature could be that the differences in vegetation structure and composition we observed among treatments were not large enough to produce a detectable cooling effect. We only detected significant decreases in broadleaved cover in 3 of 5 comparisons among treatments, and these differences were highly variable such that the range of possible values included estimates not much different from a 1:1 ratio. (i.e., a treatment contrast of one). Previous work (von Arx et al., 2012; Zhao and Jackson, 2014) demonstrating variation in local (< 4 ha) air temperature as a function of broadleaved cover gives little indication of the exact amount of broadleaved cover required to produce detectable cooling effects. However, both of these studies compared differences in air temperature between land areas (Zhao and Jackson, 2014) and stands (von Arx et al., 2012) principally dominated by broadleaved vegetation or land cover principally dominated by other vegetation types (e.g., coniferous). From this we may be able to conclude that larger differences in broadleaved cover are required to produce detectable air temperature differences in early-seral forests. We did not detect significant effects of herbicide treatment on air temperature variability (Tmincv, Tmeancv, and Tmaxcv). This may not be surprising as temperature variability is most strongly associated with vegetation structure rather than composition (Baker et al., 2014; Hardwick et al., 2015), and we did not detect any differences in the 28 total cover among treatments (Figure 2.3c). However, there were some broad patterns in air temperature variability among treatments. Contrary to expectations, air temperature variability generally decreased in herbicide-treated stands compared to control stands. The greater air temperature variability in control treatments is likely caused by a greater spatial variability in regenerating vegetation compared to the herbicide treatments (Heithecker and Halpern, 2006; Ma et al., 2010; Xu et al., 1997). Specifically, a greater number of broadleaved hardwood trees (e.g., Acer macrophyllum and Alnus Rubra) left after harvest have grown into the canopy (Figure 2.2) in the control treatments, providing patchy shading that may increase the spatial variability of air temperatures. However, consistent with our predictions, air temperatures in the light treatment were generally more variable than the moderate treatment and air temperatures in the moderate treatment were generally more variable than the intensive treatment. This pattern is also likely driven by relatively higher spatial variability of regenerating vegetation in the less intensive herbicide treatments. Not only does the light treatment have a greater amount of residual broadleaved hardwood trees to provide patchy shading (Table 2.1), but a more variable distribution of herbaceous species and bare ground in the understory (Jones et al., unpublished data). The distribution and amount of understory cover also influences the surface energy fluxes, where air temperatures are generally more tightly coupled to atmospheric conditions (and thus more variable) over bare ground compared to ground covered with vegetation (Geiger et al. 2003). These results suggest that less intensive herbicide treatments may have a greater capacity to provide microhabitats for 29 temperature-sensitive species to persist in areas of the early-seral forest stands with suitable microclimates (Sears et al., 2011). Despite the overall patterns in air temperature variability we observed, none of these effects were significant. The small magnitude of the estimated differences in temperature variability among treatments, as well as their wide confidence intervals, indicates that, although statistical power to detect an effect may have been low (Steidl et al., 1997), herbicide treatments likely minimally influenced temperature variability. This may be because of the rapid mixing of air masses that occurs within forest clearings (Geiger et al. 2003; Bonan 2008a) and the relatively small differences in canopy cover among herbicide treatments, which is a principal driver of air temperature variability in forests (Chen et al., 1999). It is also possible that the spatial scale of our sampling influenced our inability to detect treatment effects on air temperature. The distance between sample points (> 50 m) may have been too large to capture differences in air temperature variability driven by canopy cover. It is possible that smaller scales of sampling (e.g., 1 - 5 m) may allow detection of air temperature variability associated with finer-scale differences in canopy cover. Lastly, potential differences in mean air temperatures and their variability among treatments may also be diminished by the matrix in which they occur (Dobrowski, 2011). Many factors influence air temperature within forest clearings, such as amount of solar radiation received, vegetation height, and cold air pooling (a climatic process that can occur in topographic depressions, resulting in a topographically-confined, stagnant air layer that is cooler than the air aloft; Geiger et al. 2003, Whiteman et al. 2001). This 30 matrix can reduce the effect of microclimatic differences from changes in surface roughness and albedo driven by decreased vegetation cover. One critical factor is the size of the clearing relative to the height of the surrounding trees (Geiger et al. 2003). As clearing size increases, incident solar radiation (the amount of solar radiation energy received by a land surface during a given time) increases and temperatures increase. Simultaneously, however, the clearing becomes less protected from the wind and the mixing of air masses increases, which carries away excess heat (Geiger et al. 2003, Bonan 2008a). The increase in solar radiation dominates until the clearing size reaches a threshold (i.e., a distance: height ratio of 1.8; Geiger et al. 2003); mixing dominates after this point and temperature excesses of clearings compared to forests decrease. As all treated stands were generally the same size, there was some size variation (10.4 - 18.9 ha), and the age of the forest adjacent to our stands varied in age. Therefore, it is possible that the variation in the size of the clearing relative to the height of the surrounding stands made it impossible for us to detect differences in mean air temperature or air temperature variability or that the distance:height ratio across all/most stands exceeded the threshold at which the processes influencing air temperature in forest clearings dominate. As this variable was beyond the scope of this study, we did not measure it. However, for the reasons stated above, it may be an important consideration for future studies of air temperature in intensively managed forests. Our finding of generally weak and inconsistent effects of herbicide treatment on air temperature in early-seral forests compliments the few studies to examine the effects of post-harvest vegetation management on microclimatic air temperatures. In an 31 experimental study examining the effects of vegetation control practices on microclimate in post-clearcut Sitka spruce (Picea sitchensis) stands in the United Kingdom, Proe et al. (2001) found that herbicide-driven vegetation changes only influenced near surface air temperatures when combined with other post-harvest treatments (fertilizer and whole-tree harvesting). Similarly, in a study that independently varied woody and herbaceous vegetation control in pine-dominated shelterwood stands in central Ontario, Parker et al. (2012) found minimal vegetation-mediated influences of herbicide on air temperature. Woody vegetation control produced the only difference in air temperature among forest stands but this effect only occurred in 1 out of 4 growing seasons and was relatively small (0.5°C). Taken together with the results of this study, it appears that post-harvest vegetation control in intensively managed temperate conifer forests produces negligible differences in the thermal environment during early-seral stages. 2.6 MANAGEMENT IMPLICATIONS Intensive forest management practices such as the use of herbicides to control competing vegetation during stand establishment are widely used to increase forest production (Wagner et al., 2006). Globally, the areal extent of intensively managed forests is estimated to be 135 million ha (Fox, 2000), and is projected to increase in the coming decades to meet growing demands for wood products (Fox, 2000). Although herbicide application has become a widely adopted post-harvest vegetation management strategy in silvicultural operations, the ecological costs of herbicide use in forests are not 32 well-understood (Shepard et al., 2004) and can result in decreased habitat quality for forest organisms (Swanson et al., 2010). One of the ways in which forest cover change can impact habitat quality is through altered climatic regimes (Lehtinen et al., 2003). Our results indicate that changes in the relative composition of broadleaved and coniferous vegetation cover driven by herbicide application do not cause detectable changes in air temperature in intensively managed early-seral forests. Comparisons among herbicide treatments indicated no significant differences in Tmin, Tmean, Tmax, nor their variability. This suggests that management practices in early-seral forest habitats may not alter air temperatures in a way that strongly impacts early-seral organisms. However, no data exist on this topic so future studies should focus on testing the link between intensive management practices, temperature, and measures of organism performance (e.g., fitness). Conversely, our findings also indicate there is little potential for postharvest vegetation management to ameliorate expected climate change impacts (IPCC, 2013). However, the general pattern for less intensive herbicide treatments (i.e., our light and moderate herbicide treatments) to exhibit greater air temperature variability than more intensive herbicide treatments, may indicate that less intensive herbicide applications have a greater capacity to provide microhabitats that support the persistence of temperature-sensitive species in early-seral forest stands (Sears et al., 2011). However, as our study only spans one growing season and early-seral forests are highly dynamic ecosystems (Swanson et al., 2010), studies spanning multiple years are required to build upon our results. Nevertheless, our study provides strong evidence that post-harvest vegetation management has limited impacts on fine-scale air temperatures and is unlikely 33 to either amplify or buffer the projected effects of climate change on biodiversity in early-seral forests. 34 Table 2.1 Means (± 1 SD) for vegetation and stand-location attributes for herbicide treatments in the Oregon Coast Range, 2014. Vegetation was measured June-early August 2014 during the height of the growing season. Vegetation values are percent (%) cover and were averaged over three sample points, then averaged over treatment units. Elevation and Aspect measured at each temperature sampling point and then averaged over treatment units. All values are stand-level means. Treatment Control Light Moderate Intensive Broadleaved (%) 59.4 (32.6) 59 (32.4) 13.5 (16.4) 9 (7.1) Conifer (%) 5.6 (4.5) 7.8 (6.7) 10.2 (5.1) 15 (9.4) Non-woody vegetation (%) 49 (8) 45.8 (46) 81.8 (50) 85.6 (45.5) Canopy (%) 3.6 (6.4) 4.2 (7.6) 0.4 (0.8) 0.9 (2.2) Understory 141.1 (28.4) 136 (50.4) 113.2 (48) 114.9 (37) Elevation 480.7 (181.4) 452.8 (138.8) 417.1 (134.8) 526.4 (159) Aspect 198.9 (92.7) 188.5 (101.1) 155.7 (116.6) 138.9 (81.4) 35 Figure 2.1. Location of the eight study blocks used to examine the influence of intensive forest management on early-seral forest biodiversity in the Oregon Coast Range. Blocks used in this study to assess the influence if intensive forest management on microclimatic air temperature, May-August 2014, are indicated by orange squares. 36 Figure 2.2. Representative examples of stands spanning the gradient in herbicide intensity for (A) Control, (B) Light, (C) Moderate, and (D) Intensive treatments in the Oregon Coast Range, US, 2014. 37 Figure 2.3. Ratio of mean percent cover among control, light, moderate, and intensive herbicide treatments, with Bonferroniadjusted 95% confidence intervals. The dashed line at 1 represents no statistical difference. The left panel (A) depicts differences in broadleaved cover. The center panel (B) depicts differences in conifer cover. The right panel (C) depicts differences in total vegetation cover. 38 Figure 2.4. Average daily air temperature fluctuations among herbicide treatments at each experimental block in the Oregon Coast Range, US. Lines represent the means of all sample points (8) for May-August, 2014. 39 Figure 2.5. Mean air temperature differences among control, light, moderate, and intensive herbicide treatments in the Oregon Coast Range, May-August 2014, with Bonferroniadjusted 95% confidence intervals. The left panel (A) depicts differences in mean air temperature. The right panel (B) depicts differences in air temperature variability (CVs). The dashed line at zero represents no statistical difference. Estimates on the right side of the dashed line correspond to decreases in air temperature with increasing herbicide treatment intensity, while estimate on the left side correspond to increases in air temperature. 40 INTENSIVE FOREST MANAGEMENT HAS A GREATER IMPACT THAN CLIMATE ON REPRODUCTIVE OUTPUT IN A CAVITY-NESTING SONGBIRD Kristin N. Jones, Matthew G. Betts, James W. Rivers 41 CHAPTER 3: INTENSIVE FOREST MANAGEMENT HAS A GREATER IMPACT THAN CLIMATE ON REPRODUCTIVE OUTPUT IN A CAVITYNESTING SONGBIRD 3.1 ABSTRACT Shifts in avian species distributions and phenology that are driven by global climate change are well-documented, yet we lack a strong understanding of the mechanisms behind these shifts. Increasing air temperatures, the strongest evidence of ongoing global climate change, can combine with anthropogenic land cover change to influence the demographic rates of animal populations. Therefore, identifying the individual and combined effects of climate and land management practices is essential to understand how populations will respond to global change. In a large-scale study that experimentally manipulated intensive forest management practices, we examined effects of air temperature and degree of vegetation management (via herbicides) on reproductive output in a cavity-nesting songbird, the House Wren (Trogolodytes aedon). We tested the relative effects of maximum air temperature and herbicide intensity on nest survival and the variation in the quantity and quality of offspring produced along a gradient of management intensity. We did not detect statistical differences in any measure of reproductive output across herbicide treatments, though our power to detect herbicide effects was low. Nest survival decreased from the control in the light treatment, but counter to our predictions, appeared to rebound with further management intensity (i.e., in the moderate and intensively managed treatments). Our results also suggest that the 42 effects of maximum air temperature on reproductive output in early-seral forests are negligible; we detected no link between nest temperature and measures of nest survival, number of young produced, and offspring quality temperature at the nest, nor did we find evidence for combined effects of temperature and herbicides on reproductive output. Overall, neither climate nor land cover change exerted statistically significant effects on reproductive output in house wrens. Herbicide intensity appeared to have a greater effect on reproductive output compared to temperature. We hypothesize that land use change will continue to be the primary global change driver affecting this species in the near future. 3.2 INTRODUCTION Habitat loss and degradation resulting from anthropogenic land use are the principal causes of global declines in animal populations (Vié et al., 2009; Pereira et al., 2012; Monastersky, 2014). Land cover change driven by anthropogenic land use can negatively impact demographic rates in some species (Selwood et al., 2014) as a result of altered habitat availability and quality (Pereira et al., 2012; Selwood et al., 2014). Although the effects of climate change on demographic rates are less clear (Cahill et al., 2012), an increasing number of studies have shown that climate significantly influences temporal variation in demographic rates and population size for many species (McCarty, 2001; Gallinat et al., 2015; Williams et al., 2015). Climate change can influence animal populations differentially, creating both “winners” (i.e., species that benefit from changes 43 in climate) and “losers” (i.e., species whose response to climate change is negative) (Williams et al., 2015). Thus, it is expected that changes in climate and land cover will combine (Brook et al., 2008) to produce novel and potentially deleterious environmental conditions for many species (Hobbs et al., 2006; Mantyka-pringle et al., 2012; Jantz et al., 2015). Understanding how these pressures combine to affect demographic rates is essential to conservation planning as both land cover and climate change are expected to increase in extent and intensity (Lambin and Meyfroidt, 2011; Seto et al., 2011; Tscharntke et al., 2012; IPCC, 2013). Increases in air temperature, one of the most prominent components of ongoing climate change (IPCC, 2013), can influence demographic rates through both direct (e.g., physiological performance) and indirect pathways (e.g., changes in critical resources; Chase et al., 2005; Hansen, 2009; Adamo et al., 2012; Selwood et al., 2014). For example, elevated temperatures can alter metabolic rates of organisms (Knut SchmidtNielsen 1997), resulting in decreased survival and fitness (Selwood et al., 2014). In plants, increased drought frequency may decrease fruit set and seedling survival (Selwood et al., 2014; Williams et al., 2015). In breeding animals, elevated temperatures can cause heat stress in parents (e.g., inadequate water availability for lactating females) and drive declines in neonatal survival and fecundity (Selwood et al., 2014). Birds in particular have reproductive rates that are closely tied to air temperature because of high metabolic costs incurred by flight and because their offspring originate in eggs (Chase et al., 2005; Cox et al., 2013a; Becker and Weisberg, 2014). For instance, ambient temperatures > 36 - 40.5°C can reduce egg viability (DuRant et al., 2013; Wada et al., 44 2015) and may induce heat stress in developing nestlings (Murphy, 1985; Pipoly et al., 2013). Heat stress can shift energy allocation to thermoregulation, ultimately reducing offspring growth (Murphy, 1985; Pipoly et al., 2013; Salaberria et al., 2013) which may ultimately reduce post-fledgling survival (Schwagmeyer and Mock, 2008). Furthermore, heat stress may indirectly impact offspring growth via changes in adult foraging (e.g., adults may deliver fewer and lower quality food items for the same amount of effort) (du Plessis et al., 2012; Edwards et al., 2015). The foraging activity of some nest predators has been shown to increase with increasing temperatures (Cox et al., 2013b; DeGregorio et al., 2015), resulting in another indirect impact on the survival of nests and developing young. Alternatively, slight temperature increases during the nesting period could enhance avian reproductive rates. Increased temperature may reduce the exposure of eggs to temperatures below physiological optima (~ 26°C; Conway and Martin, 2000). Eggs exposed to suboptimal low temperatures may not hatch or produce offspring with decreased immune function and/or growth rate (Ardia et al., 2010; DuRant et al., 2012), and thus decreased survival. Songbirds, especially species with female-only incubation, may be constrained in maintaining their eggs at optimal temperatures (Webb, 1987) because of tradeoffs between investing resources in energy-intensive incubation or selfmaintenance (i.e., feeding; Reid et al., 2000). Consequently, increases in ambient temperatures during incubation can increase offspring growth and survival (Pérez et al., 2008). Furthermore, increased ambient temperatures during the nestling period may benefit nestling growth and survival by reducing exposure to cold temperatures (~ 11- 45 20°C; Becker and Weisberg, 2014). Warmer temperatures may also increase food availability for insectivorous birds whose foraging efficiency can be decreased by cooler temperatures (via reduced food availability and higher thermoregulatory costs), a result that has been observed in aerial insectivores (Avery and Krebs, 1984; Winkler et al., 2002). Avian reproductive rates can also be influenced by habitat loss and degradation (Lampila et al., 2005; Becker and Weisberg, 2014; DeGregorio et al., 2014). Forested ecosystems have been particularly subject to loss and degradation resulting from anthropogenic land cover change (Hooke et al., 2012), with negative consequences for some populations (Lampila et al., 2005). Although forest cover loss has slowed globally (Sloan and Sayer, 2015), much of the world’s forests have been and continue to be degraded (Potapov et al., 2008). In particular, structurally and compositionally diverse early-seral forest is declining in the northern hemisphere (Angelstam, 1998; Thomas et al., 2006), a trend that may be linked to the rapid population declines of several bird species in the Pacific Northwest that require this habitat type during the critical breeding season (Betts et al., 2010, 2013). Intensive forest management, a widely used forest management system (Fox, 2000), may reduce the quality of early-seral forest habitat for breeding songbirds by using herbicides to suppress the growth of early-seral herbaceous and broadleaved vegetation that compete with merchantable trees for resources (Shepard et al., 2004; Wagner et al., 2006). Herbicides can significantly change the abundance, structure, and composition of early-seral forest vegetation (Balandier et al., 2006), in turn altering the relative strength 46 of factors that limit reproductive output (e.g., food, nest predation). Loss of broadleaved vegetation through intensive management may have especially strong impacts because this habitat component is positively correlated with higher densities of invertebrates in leaf litter and in foliage (Willson and Comet, 1996a; Hagar et al., 2007). In particular, the abundance of lepidopteran larvae, an important food source for developing nestlings (Arnold et al., 2010), is positively associated with broadleaved vegetation (Hammond and Miller, 1998). Moreover, nest predation rates may be higher in more homogenous forests that contain less deciduous vegetation (Easton and Martin, 1998) because of differences in the distribution of predator communities (Sieving and Willson, 1998) and because more diverse foliage allows different species of birds to partition nests among specialized microhabitats, thereby reducing predation risk (Martin, 1993a). Therefore, herbicide use in early-seral forests may negatively impact the reproductive output of birds through a reduction in nest survival, as well as offspring growth. Forest vegetation composition can influence rates of surface cooling through changes in biophysical factors such as albedo and canopy conductance (von Arx et al., 2012; Zhao and Jackson, 2014). Thus, the very changes in vegetative composition that arise from the use of herbicides may also lead to changes in temperature, and both of these factors have the potential to negatively impact songbird reproductive output (Sieving and Willson, 1998; Chase et al., 2005). However, a small number of studies (Easton and Martin, 1998; Easton et al., 2002) have examined the influence of herbicide use on avian demographic rates, and few have examined the potential for combined effects of vegetation changes and air temperature due to herbicide use in forested 47 ecosystems (Cox et al., 2013a; Becker and Weisberg, 2014; Flesch et al., 2015). Nevertheless, effective conservation approaches require more information on how land management and climate can combine to affect the demographic rates that underlie species persistence given ongoing changes in the quality of early-seral habitat (Thomas et al. 2006, Swanson et al. 2010) and rising global temperatures (IPCC, 2013). In this study, we examined the effects of herbicides and microclimatic air temperatures in intensively managed coniferous forests on the reproductive output of a cavity-nesting songbird, the House Wren (Troglodytes aedon). We selected the House Wren for this study because (1) this species has experienced a strong, significant longterm decline (3% per year; Sauer et al. 2015) in the Pacific Northwest and (2) House Wren abundance declines with herbicide treatment intensity (Betts et al. 2013), together suggesting that decreases in the quality of early-seral forest may be linked to long-term population declines. We tested the hypotheses that reproductive output was influenced by air temperature, herbicide treatment, or both acting together. We predicted that House Wren reproductive output, as measured by nest survival and the number and quality (i.e., body condition) of young produced, would decrease with increasing herbicide treatment intensity as a result of reductions in vegetation cover. We also predicted that these measures would be positively affected by increased maximum daily air temperatures throughout the breeding season up to a threshold (nestlings: > 30°C; eggs > 38 - 40.5°C) (Pipoly et al., 2013; Wada et al., 2015), beyond which reproductive output would decrease. We used mean daily maximum temperature (hereafter Tmax) because: (1) we were interested in temperature effects on reproductive output at the scale of days 48 throughout the breeding season, (2) maximum temperatures are likely to be correlated with a range of temperature summaries relevant to birds (i.e., minimum and mean temperatures) and can be used as an index of such (Cunningham et al., 2013), and (3) warm daytime temperatures are expected to have the strongest effect on birds through reductions in activity (e.g. feeding, nestling provisioning) (du Plessis et al., 2012). Taken in its entirety, our study is the first to evaluate the combined effects of herbicide intensity and microclimatic air temperature on songbird reproductive output and therefore has the potential to provide information critical to songbird conservation planning in early-seral forests. 3.3 MATERIALS AND METHODS 3.3.1 Study area and species We conducted this investigation within the framework of a larger study that is examining the tradeoffs between intensive forest management, biodiversity, and ecosystem function in the northern Oregon Coast Range (see Betts et al. 2013). Mean annual precipitation ranges from 165 to 330 cm with most precipitation occurring October through March. Mean annual air temperature ranges from 5 - 19°C (Franklin and Dyrness 1988, Taylor and Hannan 1999) Soils are dominantly moderately deep to deep well-drained silt loam soils, and are derived from basalts, sandstone, and siltstones. Topography is characterized by somewhat low, highly dissected mountains with slopes ranging from 0 to 90 percent (Knezevich 1982, Taylor and Hannan 1999). All sampled 49 stands are in the western hemlock (Tsuga heterophylla) zone (Franklin and Dyrness 1988), ranging in elevation from 165 to 765 m. Douglas fir (Pseudotsuga menziesii) dominates early-seral forest plantations in this region, with grand fir (Abies grandis), western hemlock (Tsuga heterophylla), and western redcedar (Thuja plicata) forming minor components. Dominant shrub/woody species include big-leaf maple (Acer macrophyllum), California hazelnut (Corylus cornuta californica), cascara (Rhamnus purshiana), common snowberry (Symphoricarpos albus), oceanspray (Holodiscus discolor), red alder (Alnus rubra), and vine maple (Acer circinatum). Smaller understory broadleaf species include Oregon grape (Mahonia nervosa) salal (Gaultheria shallon), and Vaccinium spp. The herbaceous community is comprised of many native and nonnative herbaceous plants, with swordfern (Polystichum munitum) and brackenfern (Pteridium aquilinum) often dominating. The House Wren is a small (10 - 12 g), insectivorous, long-distance migrant passerine that is common throughout much of North America (Johnson 2014). It nests in a wide variety of open wooded habitats in Oregon from mid-April to mid-August, preferring nest sites < 30 m from woody vegetation. House Wrens are cavity-nesting songbirds that primarily nest in pre-formed tree cavities and readily use nest boxes (Johnson 2014). Female House Wrens can be double-brooded and lay clutches of 4 - 8 eggs, of which more than ~ 90% hatch and ~ 70 - 90% successfully fledge from the nest (Johnson 2014). Females alone incubate the eggs and brood the young (Figure 3.1a; Figure 3.1b) until some point in the latter third of the nestling stage (Johnson et al., 1993); however, both adults provision nestlings (Johnson 2014). 50 3.3.2 Experimental design Our study makes use of a subset of sites that occur within a larger study area that uses a randomized complete block design in which 32 stands occurred in 8 separate blocks, and each of 4 levels of herbicide treatments were randomly applied to one stand within each block (Figure 3.2). All blocks are located across a 100 km (N-S) section of the northern Oregon Coast Range region (Betts et al., 2013; Figure 3.2). Stands within each block were sited > 1 km of each other to ensure spatial independence of treatments and no more than 5 km of each other to reduce within-block variation (i.e., slope, elevation, and pre-cut vegetation composition). From this larger study framework, we constrained our study on 24 forest stands located within 6 of the blocks, with each stand 8.6 - 18.9 ha because of logistical considerations that prevented us from working on all stands concurrently. All stands were clear-cut in fall 2009 and replanted in spring 2011 with Douglasfir, the most commonly commercially cultivated species throughout the region, at approximately 980 trees per hectare. Application of the 4 levels of herbicide treatment occurred between 2009 and 2014. A full suite of herbicides and surfactants typically used in commercial timber harvesting operations was applied to stands between 2009 and 2014 in a manner that created a gradient in management intensity; see Betts et al. (2013) and Appendix A for a full description of the herbicides used, the timing with which they were applied, and their concentration. All herbicide spraying occurred in a timeframe that 51 mimicked the typical timeframe in which vegetation control takes place in commercial operations 3.3.3 Sampling design 3.3.3.1 Environmental measurements 3.3.3.1.1 Air temperature We placed iButtons temperature loggers (n = 192) on the underside of nestboxes (see below) erected on study sites at a density of 8 boxes/stand (see below). Each iButton was programmed to record temperature every 15 min and was affixed to the underside surface of each nestbox with a zip tie so that it hung freely 5 cm below the box. We covered iButtons with a section of white 10 cm diameter PVC tube with ventilation holes so as to prevent direct exposure of the iButton to solar radiation and moisture, allow for airflow, and minimize heat accumulation. Due to logistical constraints, we used two iButton models that varied slightly in their accuracy (± 1.0°C accuracy, n = 71 boxes: ± 0.5°C accuracy, n = 89 boxes). The two models were distributed irregularly among stands; thus, we note that estimated differences in air temperature between stands are within approximately 1.0°C of their true value. All iButtons were validated against an independent digital thermometer prior to placement on stands (Omega HH609R, Omega Engineering, Stamford, Conneticut, USA); any iButtons that deviated by ≥ 0.5°C during his validation procedure were not used. 52 We recorded air temperature on the outside of nest boxes because: (1) ambient temperatures recorded by iButtons were simultaneously used as part of another study to assess the influence of herbicide treatment on microclimatic air temperatures (Jones et al. in prep), and (2) recording temperatures outside the nest box allowed us to standardize measurements in a way that was impossible inside nest boxes because of marked variation in the architecture of individual nests (see McCabe, 1965). Our pilot data indicated that temperatures inside nest boxes were 4.4°C warmer (SE = 0.8, t 3 = 5.54, P = 0.012) throughout the breeding season, so our results provide a conservative measure of the temperatures experienced by eggs and nestlings (e.g., McComb and Noble, 1981). We used temperature data from iButtons to calculate the mean daily maximum temperature (hereafter Tmax) during each observation interval (see below) and nestling cycle starting at midnight; thus, this value represents the average for 96 temperatures taken during every 24-hour period across the breeding season. Following standard approaches with small-scale temperature data (e.g., Baker et al., 2014), we removed erroneous temperature that were caused by instrument malfunction or when logging stations were found to be disturbed (i.e., nestboxes damaged by wildlife) prior to calculating Tmax from our calculations. We considered temperature data to be erroneous when values were > 50°C or < -10°C with no temporal or spatial precedence. For example, temperatures logged by the same iButton within an hour were greater or less than 10°C of the record in question or temperatures recorded on the same stand at other nestboxes were 20°C greater or less than the recorded temperature in question. This led us to remove ≤ 5% of temperature values. 53 3.3.3.1.2 Vegetation We measured vegetation cover in each treatment to quantify changes in vegetation abundance and composition among the different herbicide treatments, as vegetation cover can influence temperature (see above). At each nest box, we measured vegetation at three distinct sampling points: one was centered directly on the nest box, and the other two were located 25 m distant from the nest box; these latter two were considered to represent vegetation in the core of a wren’s territory. The azimuth of the initial off-nest box point was chosen randomly, with the second point located 180° away. At each vegetation sampling point, we visually estimated the amount of cover provided by each plant species in a 3 m radius in each of three distinct vegetative strata; herbaceous (0 - 0.5 m), shrub (0.5 - 2.0 m), and canopy (> 2.0 m) layers. We aggregated some closely-related species that are functionally similar (i.e., Rubus and Ribes), as well as all herbaceous forbs, grasses, and ferns. Vegetation measured in this way predicts avian abundance in early-seral conifer forests in our study area (e.g., Ellis and Betts, 2011; Ellis et al., 2012). For analysis, we summed the amount of cover for all plant species and genera over all three strata in each 3 m radius sampling point, and then calculated an average cover value, grouping species and genera together to estimate (1) the total amount of broadleaf cover (as defined by Ellis and Betts 2011) and (2) the total amount of vegetative cover of all species and genera. We note that summed cover values >100% can result from this analysis approach. 54 3.3.3.2 Nest survival and productivity Following harvest treatments, 8 cedar nest boxes were mounted 1 - 1.5 m above the ground on each stand using t-posts so that the box (n = 192 total boxes). Nest boxes were sited with several considerations in mind: (1) equal distances between boxes (> 50 m separation), (2) even stand coverage, and (3) sampling logistics (i.e., walking distance between boxes < 30 min). Existing vegetation was not a factor in box siting. We monitored boxes every 3 - 4 days throughout the 2014 breeding season starting from late April to early August. We checked nest boxes to record nest contents (i.e., the number of eggs and nestlings present during each visit) and to determine each nest’s fate (success or failure). A nest was considered successful if, upon termination of a nesting attempt, at least one offspring was thought to have fledged from the nest. We considered a nest successful if (1) we observed nestlings fledging from the nest, (2) we observed fledglings close to boxes, or (3) if, upon checking the nest box, no live nestlings were observed in the nest and the nest cup rim was flattened and/or excrement covered the walls (Sutherland et al. 2004). We considered a nest to have failed if (1) ≥ 1 eggs were missing or broken, (2) all nestlings were missing or dead before the expected earliest date of fledging (Martin and Geupel, 1993), or (3) the nest was abandoned by parents. A nest was considered to be abandoned if eggs did not hatch in the generally established incubation period for the species (12 - 13 days) and/or once no female nesting activity was observed around the nest for at the nest for at least three consecutive nest observation intervals. In these instances, the nest was recorded as having failed on the earliest possible end date for the incubation period (Converse et al., 2013). House Wren 55 nestlings may fledge earlier than the general nestling period (17 - 18 days) for this species (Johnson 2014), so if a nest was found empty and lacking diagnostic signs of a fledged nest (e.g., presence of pin feather sheaths), the nest was considered to have failed (Sutherland et al. 2004). Nests with uncertain fates and those who were suspected to have failed due to human activities were removed from analysis. To quantify the reproductive output of successful nests, we recorded the number of offspring produced from each nest and the body condition of those offspring as a measure of offspring quality. We recorded the number of offspring fledged from a nest as the number of nestlings present on nestling day 8/9 (where nestling day 0 is the day of hatch) minus the number of nestlings found dead in the nest after fledging. On nestling day 8/9, we measured nestling tarsus length and wing chord (± 0.5 mm, dial caliper and wing chord ruler, respectively), and body mass (± 0.1 g, digital scale) (Sutherland et al. 2004). House Wren offspring do not reach the peak of their growth until later in the nestling stage (Day 10 - 13) (Zach, 1982), but we were unable to measure nestlings on our study area at later developmental stages because of the risk of premature fledging (Jones, personal observation). We averaged across all nestlings in a brood to calculate mean age, body mass, tarsus length, and wing chord estimates for day 8/9 nestlings for each nesting attempt. Due to logistical constraints, we measured nestling body condition on 4 of the 6 study blocks (16 stands). 3.3.4 Statistical analysis 56 3.3.4.1 Vegetation All models were fit in in ‘R’ v3.2.0 (R Core Development Team 2015). First, we used mixed model analysis to test whether forest vegetation abundance and composition differed significantly among herbicide treatments using the lme function of the nlme package (Pinheiro et al. 2015). We constructed models for three response variables of forest vegetation (broadleaved, conifer, and total vegetation cover) that included fixed effects for treatment (4 levels: control, light, moderate, intensive) and one random effect (block) to reflect our randomized complete block design. To correct for heterogeneity of variance among herbicide treatments, both broadleaved and total cover were log transformed prior to analysis. We back-transformed values after analysis; therefore, values should be interpreted as the mean multiplicative increase or decrease in cover between treatments. Thus, a treatment contrast that overlaps with 1 indicates there is no evidence of a statistical difference between two treatments. 3.3.4.2 Reproductive output All models were fit in in ‘R’ v 3.2.0 (R Core Development Team 2015). Models for the number of young produced and nestling body condition were fit using the lme function of the nlme package (Pinheiro et al. 2015); in contrast, nest survival models were fit using the glmer function of the lme4 package (Bates et al. 2015). We constructed three sets of linear mixed models representing a priori hypotheses to separately model the relationship between (1) herbicide treatment treatments and reproductive output 57 measures (i.e., nest survival, number of young produced, and nestling body condition) and (2) Tmax and reproductive output measures (see Table 3.1). We included models that did and did not contain a quadratic term for Tmax because the relationship between reproductive measures and Tmax may not be linear. For example, moderate increases in Tmax may benefit nestlings, but excessive increases could cause negative consequences. We included three random effects in all models: (1) study block, (2) treatment stand, and (3) nestbox. The random effects for block and stand account for potential correlation of nest fates within blocks and stands, whereas the random effect for each nestbox accounts for potential correlation of fates between nests occurring in the same nestbox. Elevation was included as a covariate in all models to control for differences in elevation between stands and between boxes within stands. Although avian reproductive output often declines with seasonal advancement (Perrins, 1970; Martin, 1987), the date of nest initiation was highly correlated (r > 0.8) with Tmax in our study. Recent work has indicated that model averaged-coefficients based on AIC weights are invalid where there is multicollinearity among predictor variables (Cade, 2015), so we did not include a covariate for nest initiation date in our analyses. We used a second-order Akaike’s Information Criterion, AICc, to quantify the relative strength of all competing linear regression models for each response variable (Burnham and Anderson 2002; Anderson 2008). Model parameter estimates were averaged across the set of candidate models for each response variable (Anderson 2008). We used the MuMIn package (Barton 2015) to conduct the model selection and averaging process. Before performing model selection, the global model (i.e., candidate 58 model with the most parameters) for the number of young produced and nestling body condition response variables was evaluated for compliance with model assumptions (see below regarding nest survival model checking). Treatment and temperature effects were considered significant at P ≤ 0.05. We used the logistic exposure method to estimate daily nest survival rate (Shaffer, 2004), which allows for the modeling of time-dependent covariates (Grant et al., 2005; Converse et al., 2013). Logistic exposure takes into account the fate of a nest during observation intervals (between successive nest checks) by using a logistic-exposure link function that explicitly considers exposure time, as measured by the length of observation interval (Shaffer 2004). In all logistic exposure models we included a term for mean nest age (i.e., nest age at the mid-point of each observation interval; see Grant et al. 2005) because this measure can influence nest survival rates (Thompson, 2007). All continuous variables were standardized prior to analysis, and subsequently un-standardized to allow for interpretation of estimates (Hosmer et al. 2013). Currently, no effective methods exist for testing fit of logistic exposure models (Shaffer and Thompson, 2007; Shaffer, personal communication); therefore, we used binned residual plots from package ‘arm’ (Gelman et al. 2015) as a qualitative test and found little evidence of model misfit with regards to normality among model residuals (Gelman 2000). To model the number of young produced, we fit linear mixed models. For this measure, we only considered successful nests for assessing the number of young produced to remove any variation caused by differences nest predation among treatments. The resulting data subset was a left-censored Poisson distribution of the response, and 59 thus severely underdispersed (dispersion parameter < 0.4, P < 0.001). As the censored data approximately followed a normal distribution, we treated the data distribution as an approximation of the normal distribution for analysis (Greene 2005). We evaluated 6 models that tested the relative importance of herbicide treatment intensity and air temperature effects on the number of young produced (Table 3.1). All models contained a term for clutch size (i.e., the maximum number of eggs laid during a nest attempt) to control for any differences in potential brood size. To model nestling body condition, we fit linear mixed models. Although body condition indices such as body mass regression residuals are used widely in the literature (Labocha and Hayes, 2012), recent work suggests that simple measures of body mass often outperform body condition indices as a measure of energy stores (Schamber et al., 2009; Labocha and Hayes, 2012). Therefore, we included tarsus length as a size covariate in all body condition analyses to control for size (Bowers et al., 2014; Paquette et al., 2014). In addition, we included a covariate for mean nestling age when measured to control for differences when nestlings were measured for growth. 3.4 RESULTS 3.4.1 Effects of herbicide treatments on temperature and vegetation During May-August 2014, Tmax on our study sites averaged 24.8 (± 4.8) °C (range: 17.1 - 32.2°C; Figure 3.3) and varied little among treatments (Table 3.2), indicating no direct effect of herbicide treatment on air temperature. As expected, 60 herbicide treatments had a strong influence on vegetation composition (Figure 3.4); broadleaved vegetation cover generally decreased with increasing herbicide treatment intensity (F3, 11 = 18.68, P < 0.001). After correcting for multiple comparisons, we detected significant differences in broadleaved cover between the control and the moderate treatments (t 9 = 4.88, P < 0.001, 𝛽̂ = 1.88 [0.68, 3.08]), the control and intensive treatments (t 9 = 5.04, P < 0.001, 𝛽̂ = 1.93 [0.74, 3.12]), and the light and the moderate treatments (t 9 = 4.89, P < 0.001, 𝛽̂ = 1.87 [0.68, 3.05]). We also found that conifer cover increased with increases in herbicide intensity (F 3, 11 = 9.19, P = 0.003), yet we only detected a significant difference in conifer cover between the control and intensive treatments (t 9= -4.69, P < 0.001, 𝛽̂ = -0.95 [-1.58, -0.32]). We also found that conifer cover increased with increases in herbicide intensity we only detected a significant difference in conifer cover between the control and intensive treatments ( t 9 = -4.69, P < 0.001, 𝛽̂ = -0.95 [-1.58, -0.32]). We did not detect any statistically significant differences in total vegetation cover among treatments (F3, 11 = 2.070 P = 0.162). We found that vegetation cover within vegetative strata (e.g., canopy cover) was similar among herbicide treatments, and that non-woody vegetation cover increased with increasing herbicide treatment primarily because of an increase of exotic ruderals (Table 3.2). 3.4.2 Influence of herbicide treatment and temperature on nest survival 61 The total number of nesting attempts by House Wrens was similar among treatments (Figure 3.5a). The average number of nests initiated per stand was 11.8 (± 4.4), reflecting a combination of high occupancy of nest boxes and multiple nesting attempts in individual boxes. Of 282 active nests, 78 (28%) failed, with 95% of these nest failures classified as predation. We found some support for an effect of herbicide treatment on nest survival; all models containing treatment were better supported than the null model (ΔAICc = 2.69, Evidence Ratio (ER) = 3.83; Table 3.3). However, we did not detect a statistically significant effect of herbicide treatment on House Wren nest survival (Table 3.4; Figure 3.5a). Nevertheless, compared to the control treatment, herbicide treatment negatively influenced mean daily survival rate. Treatment most negatively influenced daily survival rate in the light treatment (Odds Ratio (OR) = 0.35, 95% CI: 0.09, 1.48; Table 3.4), while its effect was greatly reduced in the intensive treatment (OR = 0.78, 95% CI: 0.29, 2.10), and intermediate in the moderate treatment (OR = 0.51, 95% CI: 0.16, 1.59; Table 3.4; Figure 3.5a). Confidence intervals around parameter estimates for herbicide treatment effects on nest survival were large (Table 3.4), indicating low statistical power to detect effects. We also did not detect a significant effect of temperature on nest survival (Table 3.4; Figure 3.5b). Furthermore, we did not find any evidence of quadratic relationship between temperature and mean daily nest survival rate (𝛽̂ = -0.14, 95% CI: 0.73, 1.04; Table 3.4). We found some support for combined effects of treatment and temperature on mean daily survival rate, as the best supported model contained treatment, temperature, 62 and its quadratic term (wi = 0.35; Table 3.3). However, the best supported model containing treatment and a quadratic effect of temperature was only 1.3x more likely than the next best model, which contained treatment only (ΔAICc = 0.53, ER = 1.30; Table 3.3). We note that the comparable ΔAICc values of all models included in the model set (≤ 4.08) in addition to comparable evidence ratios (Table 3.3) strongly indicated that all hypotheses were equally plausible (Table 3.4). 3.4.3 Influence of herbicide treatment and temperature on the number of young produced On average, 4.9 (± 1.6) nestlings were produced per successful nest across all treatments combined (n = 204 successful nests; Figure 3.6). We did not find support for an effect of treatment on the mean number of young produced per nest. The bestsupported model was the null model containing elevation and maximum brood size (AICc wi = 0.62; Table 3.3). All models containing herbicide treatment had AICc weights < 0.04 and evidence ratios > 16 (Table 3.3). We did not detect a significant effect of treatment on the mean number of young produced (Table 3.4; Figure 3.6a). We also did not detect an effect of temperature on the number of young produced per nest (Table 3.4). Nevertheless, the mean number of young produced generally decreased with increasing temperature, however, this estimated effect was extremely small and non-significant (-0.01 nestlings/1°C, 95% CI: -0.07, 0.05; Figure 3.6b). We also did not detect significant evidence of a quadratic effect of temperature on the number of young produced (95% CI: -0.01, 0.01). However, comparable ΔAICc values and evidence ratios for temperature 63 (ΔAICc = 2.01, ER = 2.73) and its quadratic term (ΔAICc = 3.81, ER = 6.70) indicated that temperature is as equally as plausible an explanation as the null model (Table 3.3). Finally, we did not find support for combined effects of treatment and temperature on the mean number of young produced (Table 3.4); both models containing treatment and temperature had ΔAICc values > 7 and evidence ratios > 47 (Table 3.3). 3.4.4 Influence of herbicide treatment and temperature on nestling body condition On average, nestlings on day 8/9 averaged 8.86 (± 0.76) grams across all treatments (Figure 3.7a). The null model containing elevation, mean tarsus length, and mean nestling age was the best-supported model (AICc wi = 0.36; Table 3.3). However, that the model containing treatment was the second best supported model (ΔAICc = 0.80, ER = 1.49) suggests some support for an effect of treatment on mean nestling body condition. We did not detect a significant effect of treatment on nestling body condition (Table 3.4) We also did not detect a significant effect of temperature on nestling body condition (Table 3.4; Figure 3.7b), nor did we did not detect significant evidence of a quadratic effect of temperature on mean nestling body condition (Table 3.3). Lastly, we did not find support for combined effects of treatment and temperature on the mean number of young produced (Table 3.2). However, models containing treatment and temperature and treatment and a quadratic effect of temperature were only 4.7x and 7.5x more likely than the null to explain variation in nestling body condition, respectively (Table 3.3). All hypotheses were equally good at explaining variation in nestling body 64 condition, evidenced by comparable ΔAICc values and ERs of all models in the model set (Table 3.3). 3.5 DISCUSSION Our study suggests that in intensively managed early-seral conifer forests, the use of post-harvest herbicides may influence House Wren nest survival but not the number of young produced nor their quality. Our results also indicate that the effects of air temperature on reproductive output in early-seral conifer forests are negligible compared to herbicide-driven shifts in vegetation. Lastly, our study provides very little empirical support for a combined effect of herbicide treatment and air temperature on nest survival, the number of young produced, or offspring quality. Our finding of a lack of combined effects of air temperature and herbicide-driven changes in early-seral forest vegetation change differs from a growing number of other studies (Cox et al., 2013a; Becker and Weisberg, 2014; Flesch et al., 2015). However, those studies examined the relationship between reproductive output and temperature on a coarser scale between years whereas our study was focused on changes within the breeding season. Other investigations have found within-season effects of temperature on songbird reproduction (Salaberria et al., 2013), but our data indicate that temperatures in our study area did not reach those that could be deleterious to avian offspring (Pipoly et al., 2013; Wada et al., 2015). Indeed, Tmax taken across our study sites during the breeding season never exceed 33°C, which is well within the optimal range for eggs 65 (DuRant et al., 2013). Temperatures above > 30°C have been shown to negatively affect nestling growth (Murphy, 1985; Pipoly et al., 2013) but not survival. However, that we did not detect an effect of temperature on House Wren nesting growth may be because such temperatures were relatively infrequent on our study sites (e.g., 18% [340/1869] of all observation periods). Thus, even though we had high spatial resolution, full-season temperature measurements across a managed forest landscape, we were still unable to detect an effect of Tmax on nest survival. Coupled with the small effects of temperature on nest survival and body condition and the relatively low variability surrounding these estimates, our results suggest that any combined effects are limited if they are indeed present. In contrast, the limited empirical support for combined effects of herbicide treatment and air temperature for the number of young produced indicates that these two factors do not combine to influence reproductive output. Of the factors we assessed, herbicide treatment had the greatest impact on House Wren reproductive output through reductions in nest survival. This was indicated by our inability to detect an effect of air temperature on nest survival after considering herbicide treatment and the relatively large (though variable) effects of herbicide treatment on nest survival. The difference in House Wren nest survival between control and herbicide treatments in our study is consistent with the one other study of the effects of herbicidedriven changes in vegetation composition on songbird nest survival. Easton and Martin (1998) found the nest survival of several species of forest songbirds such as the American Robin (Turdus migratorius), Dark-eyed Junco (Junco hyema) and Dusky Flycatcher (Empidonax oberholseri) to decline with herbicide-driven decreases in broadleaved 66 vegetation abundance in managed conifer forests in British Columbia. They found daily survival probability to be as much as 18% lower between control and herbicide-treated forest stands. The commercial forest stands we studied were similar to those in Easton and Martin (1998) in their age, their dominance by conifers, and their location in the Pacific Northwest, yet our study found nest survival can decline by more than triple that amount (64% between the control and light treatments) for House Wrens. This strongly implicates local factors as being important in driving variation in avian nest predation, a topic that should be the focus of future studies. House Wren nest survival, although impacted by herbicide treatment, did not respond to management intensity as we expected. Among herbicide treatments, nest survival increased substantially; when compared to control stands, daily survival rate was 2.9x lower in the light treatment (64%) than in the intensive treatment (22%). Several studies have shown a decrease in House Wren nest survival with increasing vegetation density at the nest (Belles-Isles and Picman, 1986; Finch, 1989; Li and Martin, 1991), which may provide hiding cover for nest predators (e.g., snakes, small mammals) that are themselves preyed upon by higher-level predators (e.g., raptors). The idea that greater vegetation cover leads to reduced nest survival is not supported by our finding that predation rates were lowest in the control stands. The mechanism behind this result is unclear, but it may be that a vegetation threshold exists whereby the numbers and/or activity of nest predators changes (e.g., prey-switching; (Schmidt, 1999). Nest boxes in our study were used by three other species (Jones et al., unpublished data) and box occupancy was high on all stands, so perhaps the vegetation on light herbicide stands was 67 enough to provide hiding cover to nest predators, but not so much that it impeded their ability to search for and locate nests. Regardless of the mechanism responsible for the variation in nest survival in this study, the relationship between vegetation cover and nest survival is less clear for cavity-nesting birds than it is for open-cup nesting species (Martin, 1993b; Thompson, 2007). For example, Li and Martin (1991) examined nest survival for 14 species of cavity-nesting birds in conifer forest in central Arizona and found the height of the cavity above the ground and entrance diameter to be the strongest predictors of nest survival, which was negatively correlated with increasing vegetation cover. Cockle et al. (2015) also found entrance diameters and nest height to be significantly more important than nest-site vegetation in predicting the nest survival of 35 species of cavity-nesting birds in northeastern Argentina. Similarly, the nest survival of Western Bluebirds (Sialia Mexicana) (Kozma and Kroll, 2010) and Lewis’ woodpeckers (Melanerpes lewis) (Newlon and Saab, 2011) were found to be most strongly related to factors such as nest-initiation date and clutch size rather than nest-site vegetation characteristics. The relationship between House Wren nest survival and herbicide treatment is likely multifaceted; however, the large effects of herbicide treatment on nest survival we observed indicate that the relationship between post-harvest vegetation management and nest survival for cavity-nesting songbirds warrants further exploration. Decreased broadleaved cover may also influence the reproductive output of breeding songbirds by affecting the amount and quality of food available, as invertebrate density in leaf litter and on foliage are positively correlated with this vegetation type (Willson and Comet, 1996b; Hagar, 2007). For example, lepidopteran larvae, an 68 important source of energy and nutrients for songbird nestlings (Arnold et al., 2010), are associated with increased abundance of herbaceous and deciduous vegetation (Hammond and Miller, 1998; Miller et al., 2003). Thus, we would have expected food availability to decrease with increasing herbicide treatment intensity, and that should translate into decreased nestling body mass and/or the number of young produced (Schwagmeyer and Mock, 2008). However, that herbicide treatment was as equally likely as all other hypotheses to explain the number of young produced and their body condition in combination with its small effects suggests that herbicide treatments likely did not alter food availability for House Wrens in our study. This may be due to their general foraging habits and broad nestling diet (Pezzolesi 2000), which may not be as sensitive to decreased broadleaf vegetation as songbirds that are more strongly associated with broadleaf vegetation (Betts et al., 2010; Ellis and Betts, 2011). Our results support the notion that, despite increased concern about climate change impacts, anthropogenic land use will continue to be the primary global change driver influencing animal populations in the near future (Pereira et al., 2012). Our study suggests that current air temperature patterns do not combine with land cover change to impact the reproductive rates of animal populations in temperate, early-seral coniferous forests. However, it is plausible that projected increases in climate (IPCC, 2013) may lead to combined effects of air temperature and forest cover change that reduce reproductive rates in ways that are apparently not present at the current time. Herbicide use in early-seral forests may alter the structure and function of early-seral communities (Flueck and Smith-Flueck, 2006) and predators and prey may respond differently to 69 ongoing climatic shifts. Thus, as anthropogenic land cover change expands and intensifies (Lambin and Meyfroidt, 2011; Seto et al., 2011; Tscharntke et al., 2012) and the climate continues to change (IPCC, 2013), recognition is growing that these pressures must be considered in tandem to more accurately predict how animal populations will respond to global change. Furthermore, patterns of land use change can influence populations far more than climate (Pereira et al., 2012), so understanding the relative influence of each factor is essential to formulating successful conservation strategies (Brook et al., 2008). 70 Table 3.1. A priori candidate models describing herbicide treatment and air temperature effects on House Wren nest survival, the number of young produced, per nest, and nestling body condition in the Oregon Coast Range, US, 2014. Model name Parameters Description Nest survival Null* Elevation + mean nest age Temperature Tmax Documented variation in nest survival with elevation and nest age Daily maximum temperature influences nest survival directly via inducing nestling metabolic stress and decreased nest survival Tmax² Daily maximum temperature influences nest survival directly via inducing nestling metabolic stress and decreased nest survival and may exhibit a quadratic relationship Management intensity Herbicide treatment† Herbicide treatment influences nest survival via altered vegetative abundance, structure, and composition and thus decreased food availability Temperature + Management intensity Tmax + herbicide treatment† Herbicide treatment influences nest survival but this effect is compounded daily maximum temperatures Tmax² + herbicide treatment† Herbicide treatment influences nest survival but this effect is compounded daily maximum temperatures and may exhibit a quadratic relationship Elevation + Maximum brood size Null model accounts for documented variation in fledgling brood size with elevation and the number of nestlings present in each nest able to survive to fledging Tmax Daily maximum temperature influences fledgling brood size directly via inducing nestling metabolic stress and decreased nestling survival Tmax² Daily maximum temperature influences fledgling brood size directly via inducing nestling metabolic stress and decreased nestling survival and may exhibit a quadratic relationship Temperature² Temperature² + Management intensity Number of young produced Null* Temperature Temperature² 71 Management intensity Herbicide treatment† Herbicide treatment influences fledgling brood size via decreased food availability and thus fewer nestlings survive to fledging Temperature + Management intensity Tmax + herbicide treatment† Herbicide treatment influences fledging brood size but this effect is compounded by daily maximum temperatures Tmax² + herbicide treatment† Herbicide treatment influences nest survival but this effect is compounded daily maximum temperatures and may exhibit a quadratic relationship Elevation + Mean tarsus length + Mean nestling age Null model accounts for documented variation in songbird nestling mass with elevation with a factor to correct for nestling size Tmax Daily maximum temperature influences nestling mass directly via inducing nestling metabolic stress and thus influencing growth Temperature² Tmax² Daily maximum temperature influences nestling mass directly via inducing nestling metabolic stress and thus influencing growth and may exhibit a quadratic relationship Management intensity Herbicide treatment† Herbicide treatment influences nestling mass via decreased food availability and thus slower nestling growth and lower mass Temperature + Management intensity Tmax + herbicide treatment Herbicide treatment influences nestling mass but this effect is compounded by daily maximum temperatures Tmax² + herbicide treatment† Herbicide treatment influences nest survival but this effect is compounded daily maximum temperatures and may exhibit a quadratic relationship Temperature² + Management intensity (Global model) Nestling body condition Null* Temperature Temperature² + Management intensity (Global model) *All terms in the null model were included in the candidate set †Herbicide treatment includes four levels of treatment intensity (Control, Light, Moderate, Intense) 72 Table 3.2. Means (± 1 SD) for vegetation and stand location attributes for stands subjected to different herbicide treatments in the Oregon Coast Range, 2014. Vegetation (percent cover) was measured June-August 2014 during the height of the growing season, and was averaged over three sample points at each nest box. Measurements from each box were then averaged then averaged over all herbicide treatments units. Temperature (Tmax), elevation and aspect were measured at each nest box and then an averaged average was calculated for all boxes within stands subjected to each herbicide treatment over treatment units. All values are stand-level means. Treatment Control Light Moderate Intensive Broadleaved (%) Conifer (%) 59.4 (± 32.6) 5.6 (± 4.5) 59 (± 32.4) 7.8 (± 6.7) 13.5 (± 16.4) 10.2 (± 5.1) 9 (±7.1) 15 (± 9.4) Non-woody (%) 49 (± 38) 45.8 (± 46) 81.8 (± 50) 85.6 (± 45.5) Canopy (%) 3.6 (± 6.4) 4.2 (± 7.6) 0.4 (± 0.8) 0.9 (± 2.2) Understory (%) 141.1 (± 28.4) 136 (± 50.4) 113.2 (± 48) 114.9 (± 37) Temperature (°C) Elevation (m) 24.7 (±1.7) 480.7 (± 181.4) 25.1(±1.5) 452.8 (± 138.8) 25.1(±1.8) 417.1 (± 134.8) 24.3 (±1.4) 526.4 (± 159) Aspect (degrees) 198.9 (± 92.7) 188.5 (± 101.1) 155.7 (± 116.6) 138.9 (± 81.4) 73 Table 3.3. Model selection results from a priori candidate models describing the effects of herbicide treatment and Tmax on House Wren nest survival, the number of young produced per nest, and nestling condition in the Oregon Coast Range, 2014. Models are ranked in ascending order by Akaike’s Information Criterion adjusted for small sample sizes (AICc). The number of parameters (k), difference between the best model and all other models (ΔAICc), the relative likelihood of a model, AICc weights (wi), and evidence ratio (ER) are given for each model. Model set Nest survival Tmax² + Herbicide treatment Herbicide treatment Tmax + Herbicide treatment Tmax² Elevation + Mean nest age Tmax Number of young produced Elevation + Brood size Tmax Tmax² Herbicide treatment Tmax + Herbicide treatment Tmax² + Herbicide treatment Nestling body condition Elevation + Mean tarsus length + Mean nestling age Herbicide treatment Tmax Tmax² Tmax + Herbicide treatment Tmax² + Herbicide treatment k AICc ΔAICc wi ER 11 9 10 8 6 7 636.39 636.91 638.20 638.56 639.07 640.47 0.00 0.53 1.82 2.17 2.69 4.08 0.35 0.27 0.14 0.12 0.09 0.05 1.00 1.30 2.48 2.97 3.83 7.67 7 8 9 10 11 12 481.31 483.32 485.12 486.93 489.00 490.95 0.00 2.01 3.81 5.62 7.69 9.64 0.62 0.23 0.09 0.04 0.01 0.01 1.00 2.73 6.70 16.84 47.92 124.60 8 11 9 10 12 209.33 210.13 211.74 211.99 212.44 0.00 0.80 2.41 2.66 3.11 0.39 0.26 0.12 0.10 0.08 1.00 1.49 3.34 3.77 4.73 13 213.36 4.03 0.05 7.46 74 Table 3.4. Results of models testing for effects of herbicide treatment and Tmax on House Wren nest survival, the number of young produced per nest, and nestling body condition in the Oregon Coast Range, 2014. Model coefficients (β) and 95% confidence intervals are given for each model. For nest survival models, odds ratios are also given. All model coefficients are model-averaged estimates. Model set β Odds ratio L (95% CI) Intercept 5.40 220.52 87.78 554.02 Temperature 0.00 1.00 0.82 1.21 Temperature (squared) -0.14 0.87 0.73 1.04 Light treatment -1.03 0.36 0.09 1.48 Moderate treatment -0.68 0.51 0.16 1.59 Intensive treatment -0.25 0.78 0.29 2.10 Elevation 0.00 1.00 0.70 1.43 Mean nest age 0.03 1.03 0.81 1.31 Effect U (95% CI) Nest survival Number of young produced Intercept 0.48 -0.12 1.08 Temperature -0.01 -0.07 0.05 Temperature (squared) 0.00 -0.01 0.01 Light treatment -0.13 -0.56 0.30 Moderate treatment -0.13 -0.55 0.29 Intensive treatment 0.00 -0.45 0.44 Elevation 0.00 0.00 0.00 Maximum brood size 0.93 0.85 1.00 75 Nestling body condition Intercept 2.26 -0.16 4.69 Temperature 0.00 -0.02 0.02 Temperature (squared) 0.00 0.00 0.00 Light treatment 0.02 -0.16 0.20 Moderate treatment 0.01 -0.14 0.17 Intensive treatment 0.02 -0.17 0.21 Elevation 0.00 0.00 0.00 Mean tarsus length 2.76 2.63 2.88 Mean nestling age 0.31 0.07 0.55 76 Figure 3.1. (A) A House Wren (Troglodytes aedon) nestling approximately 8 days after hatching being prepared for measurement of body condition, and (B) a completed clutch of House Wren eggs during incubation inside a nest box. 77 Figure 3.2. General location map of the eight study blocks used to examine the influence of intensive forest management on early-seral forest biodiversity in the Oregon Coast Range. Blocks used in this study to assess the influence if intensive forest management on House Wren reproductive output May-August 2014, are indicated by orange squares. 78 Figure 3.3. Mean daily maximum temperature (Tmax) across all study sites in the Oregon Coast Range during the House Wren breeding season, May-August 2014. Measurements were taken every 15 min, averaged across a 24-hour period, and then averaged over all nest boxes (n = 192) on 24 study sites in the Oregon Coast Range. The gray band shows the 95% confidence interval. 79 Figure 3.4. Representative examples of stands spanning the gradient in herbicide intensity, including ranging from (A) Control (no-spray), (B) Light, (C) Moderate, and (D) Intensive treatments in the Oregon Coast Range, US, 2014. 80 Figure 3.5. Plots depicting the effect of herbicide treatment and Tmax and on House Wren nest survival during the 81 breeding season in the Oregon Coast Range, 2014. (A) Differences in odds ratio estimates of nest survival between the effect of herbicide treatments relative to control stands; odds ratios were averaged over all models in the candidate set. The dashed horizontal line represents odds ratios of the control for comparison with herbicide treatments; 95% CIs that overlap one indicate lack of significant differences relative to the control treatment. Numbers above confidence intervals indicate the number of nests monitored per treatment; there were 73 nests in the control treatment. (B) Boxplots depicting Tmax values from nests that either failed (pink) or fledged offspring (blue) across all four treatments. Vertical bars within boxes represent medians, boxes are interquartile ranges, whiskers are 1.5xinterquartile range and dots are outlying data. 82 83 Figure 3.6. Number of House Wren young produced per successful summarized by (A) herbicide treatment and (B) Tmax during the breeding season in the Oregon Coast Range, 2014. (A) Boxplots depict the number of young produced by treatment. Bars are medians, yellow diamonds are means, boxes are interquartile ranges, and whiskers are 1.5xinterquartile range. Numbers above whiskers indicate the number of successful nests per treatment. (B) Relationship between Tmax and the number of fledglings produced per successful nest. The blue line is a fitted linear regression line with 95% confidence intervals. 84 85 Figure 3.7. House Wren day 8/9 body condition summarized by (A) herbicide treatment and (B) Tmax during the breeding season in the Oregon Coast Range, 2014. (A) Boxplots depict nestling body mass by treatment. Bars are medians, yellow diamonds are means, boxes are interquartile ranges, and whiskers are 1.5xinterquartile range. Numbers above whiskers indicate the number nests in which nestlings were measured per treatment. (B) Relationship between Tmax and nestling mass. The blue line is a fitted linear regression line with 95% confidence intervals. 86 CHAPTER 4: CONCLUSIONS My thesis explored the effects of post-harvest vegetation control intensity on microclimate and avian population demography in intensively managed early-seral forests. I examined the microclimatic air temperature and its variability along a gradient of herbicide treatment intensity to test for combined effects of herbicide treatment intensity and air temperature on reproductive output in the House Wren, an insectivorous cavity-nesting songbird. Animal population responses to future climate change are often predicted using large-scale envelope models that do not account for the local climatic conditions to which organisms most closely respond (Potter et al., 2013). Predictions from such models can be incomplete and misleading (Gillingham et al., 2012; Varner and Dearing, 2014); resulting in misguided wildlife management decisions. Further, they do not give any information as to how land management decisions may modify the impacts of expected climatic shifts on organisms. I found that herbicide treatment may influence forest microclimatic air temperatures and their variability (Chapter 2); however, these effects, if present, are small and variable. Instead, microtopograhic variables such as elevation and distance to stand edge were the principal determinants of microclimatic air temperatures. My results support recent findings that, in addition to differences in vegetative abundance and structure, differences in forest vegetation composition may influence microclimatic air temperatures (von Arx et al., 2012; Zhao and Jackson, 2014). However, the generally small and the variable effects of herbicide-driven vegetation 87 changes on air temperature among treatments in addition to a lack of herbicide treatment effects on temperature variability indicates little potential for post-harvest vegetation management to exacerbate or ameliorate ongoing climate change effects (IPCC, 2013) on early-seral organisms in coniferous forests. My results suggest that microtopographic elements such as elevation may be the most significant modifiers of future climate change impacts on early-seral organisms in topographically heterogeneous and forested environments such as the Oregon Coast Range. In Chapter 3, I explored the potential for combined effects of air temperature and herbicides on reproductive output in a House Wren nestbox population in intensively managed early-seral coniferous forests. My results suggest that post-harvest vegetation control appears to influence songbird nest survival, but not the number of young produced or their quality. However, nest survival did not decline along a gradient of herbicide intensity as expected. Instead, nest survival rate was lowest in the light treatment and highest the intensive herbicide treatment; however, these effects, though large, were not statistically significant. Furthermore, my results suggest that the effects of air temperature on songbird reproductive output in early-seral forests is negligible compared to herbicide-driven shifts in vegetation. Lastly, my results suggest that there is limited evidence for temperature and herbicide-driven vegetation changes to combine in their effects on songbird reproductive output. My results support the prediction that anthropogenic land cover change will continue to be the most important global change driver impacting animal populations in the near-future. 88 As global climate change alters the distribution and abundance of plant species (Kelly and Goulden, 2008; Pauli et al., 2012) and anthropogenic land cover change increases in extent and intensity (Hooke et al., 2012; Tscharntke et al., 2012), it is imperative that future studies model the combined and interacting (De Chazal and Rounsevell, 2009) effects of climate change and vegetation on population dynamics to more completely understand how species will respond to global change (Brook et al., 2008; Oliver and Morecroft, 2014). Thus, mine is the first study to examine the potential for combined effects of herbicide treatment and microclimatic air temperature on avian demographic rates in intensively managed early-seral forests, and my study advances our understanding of the effects of intensive forest management practices on early-seral forest ecology. LITERATURE CITED Adamo, S.A., Baker, J.L., Lovett, M.M.E., Wilson, G., 2012. Climate change and temperate zone insects: the tyranny of thermodynamics meets the world of limited resources. Environmental Entomology 41, 1644–52. Adams, W., Hobbs, S., Johnson, N., 2005. Intensively managed forest plantations in the Pacific Northwest: Introduction. Journal of Forestry 103, 59–60. Aerts, R., Honnay, O., 2011. Forest restoration, biodiversity and ecosystem functioning. BioMed Central Ecology 11, 29. Anderson, D. R. 2008. Model based inference in the life sciences: a primer on evidence. Springer Science+Business Media, LLC, New York, New York, USA. Anderson, R.G., Canadell, J.G., Randerson, J.T., Jackson, R.B., Hungate, B.A., Baldocchi, D.D., Ban-Weiss, G.A., Bonan, G.B., Caldeira, K., Cao, L., others, 2010. Biophysical considerations in forestry for climate protection. Frontiers in Ecology and the Environment 9, 174–182. 89 Angelstam, P.K., 1998. Maintaining and restoring biodiversity in European boreal forests by developing natural disturbance regimes. Journal of Vegetation Science 9, 593–602. Ardia, D.R., Pérez, J.H., Clotfelter, E.D., 2010. Experimental cooling during incubation leads to reduced innate immunity and body condition in nestling tree swallows. Proceedings of the Royal Society B: Biological Sciences 277, 1881–1888. Arnold, K.E., Ramsay, S.L., Henderson, L., Larcombe, S.D., 2010. Seasonal variation in diet quality: antioxidants, invertebrates and blue tits Cyanistes caeruleus. Biological Journal of the Linnean Society 99, 708–717. Ashcroft, M.B., 2010. Identifying refugia from climate change. Journal of Biogeography 37, 1407–1413. Aussenac, G., 2000. Interactions between forest stands and microclimate: ecophysiological aspects and consequences for silviculture. Annals of Forest Science 57, 287–301. Avery, M.I., Krebs, J.R., 1984. Temperature and foraging success of great tits Parus major hunting for spiders. Ibis 126, 33–38. Baker, T.P., Jordan, G.J., Steel, E.A., Fountain-Jones, N.M., Wardlaw, T.J., Baker, S.C., 2014. Microclimate through space and time: Microclimatic variation at the edge of regeneration forests over daily, yearly and decadal time scales. Forest Ecology and Management 334, 174–184. Balandier, P., Collet, C., Miller, J.H., Reynolds, P., Zedaker, S., 2006. Designing forest vegetation management strategies based on the mechanisms and dynamics of crop tree competition by neighbouring vegetation. Forestry 79, 3–27. Barton, K.. 2015. MuMIn: Multi-Model Inference. R package version 1.14.0. http://CRAN.R-project.org/package=MuMIn. Bates, D., Maechler, M., Bolker, B.,Walker, S., 2015. Fitting Linear Mixed-Effects Models Using lme4. Journal of Statistical Software 67, 1-48. Becker, M.E., Weisberg, P.J., 2014. Synergistic effects of spring temperatures and land cover on nest survival of urban birds. The Condor 117, 18–30. Bellard, C., Bertelsmeier, C., Leadley, P., Thuiller, W., Courchamp, F., 2012. Impacts of climate change on the future of biodiversity. Ecology Letters 15, 365–377. Belles-Isles, J.-C., Picman, J., 1986. Nesting losses and nest site preferences in House Wrens. Condor 88, 483–486. Bennett, A.F., Saunders, D.A., 2010. Habitat fragmentation and landscape change. Conservation biology for all 93, 1544–1550. Betts, M.G., Hagar, J.C., Rivers, J.W., Alexander, J.D., McGarigal, K., McComb, B.C., 2010. Thresholds in forest bird occurrence as a function of the amount of early-seral broadleaf forest at landscape scales. Ecological Applications 20, 2116–30. Betts, M.G., Verschuyl, J., Giovanini, J., Stokely, T., Kroll, A.J., 2013. Initial experimental effects of intensive forest management on avian abundance. Forest Ecology and Management 310, 1036–1044. Bonan, G., 2008a. Ecological climatology: concepts and applications. Cambridge University Press, Cambridge, UK. 90 Bonan, G.B., 2008b. Forests and climate change: forcings, feedbacks, and the climate benefits of forests. Science 320, 1444–1449. Bowers, E.K., Hodges, C.J., Forsman, A.M., Vogel, L.A., Masters, B.S., Johnson, B.G., Johnson, L.S., Thompson, C.F., Sakaluk, S.K., 2014. Neonatal body condition, immune responsiveness, and hematocrit predict longevity in a wild bird population. Ecology 95, 3027–3034. Brook, B.W., Sodhi, N.S., Bradshaw, C.J.A., 2008. Synergies among extinction drivers under global change. Trends in Ecology and Evolution 23, 453–60. Buckley, L.B., Hurlbert, A.H., Jetz, W., 2012. Broad-scale ecological implications of ectothermy and endothermy in changing environments. Global Ecology and Biogeography 21, 873–885. Burnham, K. P, Anderson, D. R., 2002. Model selection and multimodel inference: a practical information-theoretic approach. Second edition. Springer-Verlag New York, Inc. New York, New York, USA. Cade, B.S., 2015. Model averaging and muddled multimodel inference. Ecology 96, 2370-2382. Cahill, A.E., Aiello-Lammens, M.E., Fisher-Reid, M.C., Hua, X., Karanewsky, C.J., Ryu, H.Y., Sbeglia, G.C., Spagnolo, F., Waldron, J.B., Warsi, O., others, 2012. How does climate change cause extinction? Proceedings of the Royal Society of London B: Biological Sciences Carnus, J.-M., Parrotta, J., Brockerhoff, E., Arbez, M., Jactel, H., Kremer, A., Lamb, D., OHara, K., Walters, B., 2006. Planted forests and biodiversity. Journal of Forestry 104, 65–77. Chapin III, F.S., Matson, P.A., Vitousek, P., 2011. Principles of terrestrial ecosystem ecology. Springer Science and Business Media, LLC. New York, New York, USA. Chase, M.K., Nur, N., Geupel, G.R., Stouffer, P., 2005. Effects of weather and population density on reproductive success and population dynamics in a song sparrow (Melospiza melodia) population: a long-term study. The Auk 122, 571–592. Checa, M.F., Rodriguez, J., Willmott, K.R., Liger, B., 2014. Microclimate variability significantly affects the composition, abundance and phenology of butterfly communities in a highly threatened neotropical dry forest. Florida Entomologist 97, 1– 13. Chen, J., Saunders, S.C., Crow, T.R., Naiman, R.J., Brosofske, K.D., Mroz, G.D., Brookshire, B.L., Franklin, J.F., 1999. Microclimate in forest ecosystem and landscape ecology. BioScience 49, 288–297. Cockle, K.L., Bodrati, A., Lammertink, M., Martin, K., 2015. Cavity characteristics, but not habitat, influence nest survival of cavity-nesting birds along a gradient of human impact in the subtropical Atlantic Forest. Biological Conservation 184, 193–200. Converse, S.J., Royle, J.A., Adler, P.H., Urbanek, R.P., Barzen, J.A., 2013. A hierarchical nest survival model integrating incomplete temporally varying covariates. Ecology and Evolution 3, 4439–4447. Conway, C.J., Martin, T.E., 2000. Effects of ambient temperature on avian incubation behavior. Behavioral Ecology 11, 178–188. 91 Cooke, S.J., Sack, L., Franklin, C.E., Farrell, A.P., Beardall, J., Wikelski, M., Chown, S.L., 2013. What is conservation physiology? Perspectives on an increasingly integrated and essential science. Conservation Physiology 1. Cox, W.A., Thompson, F.R., Reidy, J.L., Faaborg, J., 2013a. Temperature can interact with landscape factors to affect songbird productivity. Global change biology 19, 1064-1074. Cox, W.A., Thompson III, F., Reidy, J., 2013b. The effects of temperature on nest predation by mammals, birds, and snakes. The Auk 130, 784–790. Cunningham, S.J., Martin, R.O., Hojem, C.L., Hockey, P.A., 2013. Temperatures in excess of critical thresholds threaten nestling growth and survival in a rapidly-warming arid savanna: a study of common fiscals. PloS One 8. Dawson, T.P., Jackson, S.T., House, J.I., Prentice, I.C., Mace, G.M., 2011. Beyond predictions: biodiversity conservation in a changing climate. Science 332, 53–8. De Chazal, J., Rounsevell, M.D., 2009. Land-use and climate change within assessments of biodiversity change: A review. Global Environmental Change 19, 306–315. DeGregorio, B.A., Weatherhead, P.J., Sperry, J.H., 2014. Power lines, roads, and avian nest survival: effects on predator identity and predation intensity. Ecology and Evolution 4, 1589–600. DeGregorio, B.A., Westervelt, J.D., Weatherhead, P.J., Sperry, J.H., 2015. Indirect effect of climate change: Shifts in ratsnake behavior alter intensity and timing of avian nest predation. Ecological Modelling 312, 239–246. DellaSala, D.A., Bond, M.L., Hanson, C.T., Hutto, R.L., Odion, D.C., 2014. Complex Early Seral Forests of the Sierra Nevada: What are They and How Can They Be Managed for Ecological Integrity? Natural Areas Journal 34, 310–324. Devine, W.D., Harrington, C.A., 2007. Influence of harvest residues and vegetation on microsite soil and air temperatures in a young conifer plantation. Agricultural and Forest Meteorology 145, 125–138. Dobrowski, S.Z., 2011. A climatic basis for microrefugia: the influence of terrain on climate. Global Change Biology 17, 1022–1035. Du Plessis, K.L., Martin, R.O., Hockey, P.A., Cunningham, S.J., Ridley, A.R., 2012. The costs of keeping cool in a warming world: implications of high temperatures for foraging, thermoregulation and body condition of an arid-zone bird. Global Change Biology 18, 3063–3070. DuRant, S.E., Hopkins, W.A., Hawley, D.M., Hepp, G.R., 2012. Incubation temperature affects multiple measures of immunocompetence in young wood ducks (Aix Sponsa). Biology letters 8, 108–111. DuRant, S.E., Hopkins, W.A., Hepp, G.R., Walters, J., 2013. Ecological, evolutionary, and conservation implications of incubation temperature-dependent phenotypes in birds. Biological Reviews. Easton, W.E., Martin, K., 1998. The effect of vegetation management on breeding bird communities in British Columbia. Ecological Applications 8, 1092–1103. Easton, W.E., Martin, K., Powell, A., 2002. Effects of thinning and herbicide treatments on nest-site selection by songbirds in young managed forests. The Auk 119, 685–694. 92 Edwards, E.K., Mitchell, N.J., Ridley, A.R., 2015. The impact of high temperatures on foraging behaviour and body condition in the Western Australian Magpie Cracticus tibicen dorsalis. Ostrich 86, 137–144. Ellis, T.M., Betts, M.G., 2011. Bird abundance and diversity across a hardwood gradient within early seral plantation forest. Forest Ecology and Management 261, 1372–1381. Ellis, T.M., Kroll, A.J., Betts, M.G., 2012. Early seral hardwood vegetation increases adult and fledgling bird abundance in Douglas-fir plantations of the Oregon Coast Range, USA. Canadian Journal of Forest Research 42, 918–933. Finch, D.M., 1989. Relationships of surrounding riparian habitat to nest-box use and reproductive outcome in House Wrens. Condor 91, 848–859. Fischer, J., Lindenmayer, D.B., 2007. Landscape modification and habitat fragmentation: a synthesis. Global Ecology and Biogeography 16, 265–280. Flesch, A.D., Hutto, R.L., van Leeuwen, W.J., Hartfield, K., Jacobs, S., 2015. Spatial, Temporal, and Density-Dependent Components of Habitat Quality for a Desert Owl. PloS one 10, e0119986. Flueck, W.T., Smith-Flueck, J.A.M., 2006. Herbicides and forest biodiversity: an alternative perspective. Wildlife Society Bulletin 34, 1472–1478. Foley, J.A., DeFries, R., Asner, G.P., Barford, C., Bonan, G., Carpenter, S.R., Chapin, F.S., Coe, M.T., Daily, G.C., Gibbs, H.K., others, 2005. Global consequences of land use. Science 309, 570–574. Fortier, J., Messier, C., 2006. Are chemical or mechanical treatments more sustainable for forest vegetation management in the context of the TRIAD? The Forestry Chronicle 82, 806–818. Foster, D.R., Orwlg, D., McLachlan, J., 1996. Ecological and conservation insights from reconstructive studies of temperate old-growth forests. Trends in Ecology and Evolution 11, 419–424. Fox, T.R., 2000. Sustained productivity in intensively managed forest plantations. Forest Ecology and Management 138, 187–202. Franklin, J., McCullough, P., Gray, C., 2000. Terrain variables used for predictive mapping of vegetation communities in Southern California, in: Wilson, John P. and Gallant, John C. (Ed.), Terrain Analysis: Principles and Applications. John Wiley & Sons, Inc., pp. 331–353. Franklin, J. F., Dyrness, C. T., 1988. Natural vegetation of Oregon and Washington. Gen. Tech. Rep. PNW-8. USDA, USFS, Pacific Northwest Forest and Range Experiment Station. Friedl, M.A., McIver, D.K., Hodges, J.C., Zhang, X., Muchoney, D., Strahler, A.H., Woodcock, C.E., Gopal, S., Schneider, A., Cooper, A., others, 2002. Global land cover mapping from MODIS: algorithms and early results. Remote Sensing of Environment 83, 287–302. Gallinat, A.S., Primack, R.B., Wagner, D.L., 2015. Autumn, the neglected season in climate change research. Trends in Ecology and Evolution 30, 169–176. Geiger, R., Aron, R.H., Todhunter, P., 2003. The climate near the ground. Sixth edition. Rowman & Littlefield Publishers, Oxford, UK. 93 Gelman, A, Goegebeur, Y., Tuerlinckx, F., Van Mechelen, I., 2000. Diagnostic checks for discrete data regression models using posterior predictive simulations. Applied Statistics 49: 247-268. Gelman, A., Su, Y., 2015. Arm: data analysis using regression and multilevel/hierarchicalmodels. R package version 1.8-5. http://CRAN.Rproject.org/package=arm. Gillingham, P., Huntley, B., Kunin, W., Thomas, C., 2012. The effect of spatial resolution on projected responses to climate warming. Diversity and Distributions 18, 990–1000. Grant, T.A., Shaffer, T.L., Madden, E.M., Pietz, P.J., Johnson, D., 2005. Time-specific variation in passerine nest survival: new insights into old questions. The Auk 122, 661–672. Greene, W.H., 2005. Censored Data and Truncated Distributions. NYU Working Paper No. EC-05-08. Hagar, J.C., 2007. Wildlife species associated with non-coniferous vegetation in Pacific Northwest conifer forests: A review. Forest Ecology and Management 246, 108–122. Hagar, J.C., Dugger, K.M., Starkey, E.E., 2007. Arthropod prey of Wilson’s Warblers in the understory of Douglas-fir forests. The Wilson Journal of Ornithology 119, 533– 546. Hammond, P.C., Miller, J.C., 1998. Comparison of the biodiversity of Lepidoptera within three forested ecosystems. Annals of the Entomological Society of America 91, 323– 328. Hansen, J., Sato, M., Ruedy, R., Lo, K., Lea, D.W., Medina-Elizade, M., 2006. Global temperature change. Proceedings of the National Academy of Sciences 103, 14288– 14293. Hansen, M.C., Potapov, P.V., Moore, R., Hancher, M., Turubanova, S., Tyukavina, A., Thau, D., Stehman, S., Goetz, S., Loveland, T., others, 2013. High-resolution global maps of 21st-century forest cover change. Science 342, 850–853. Hansen, P.J., 2009. Effects of heat stress on mammalian reproduction. Philosophical Transactions of the Royal Society B: Biological Sciences 364, 3341–3350. Hardwick, S.R., Toumi, R., Pfeifer, M., Turner, E.C., Nilus, R., Ewers, R.M., 2015. The relationship between leaf area index and microclimate in tropical forest and oil palm plantation: Forest disturbance drives changes in microclimate. Agricultural and Forest Meteorology 201, 187–195. Heithecker, T.D., Halpern, C.B., 2006. Variation in microclimate associated with dispersed-retention harvests in coniferous forests of western Washington. Forest Ecology and Management 226, 60–71. Helmuth, B., Kingsolver, J.G., Carrington, E., 2005. Biophysics, physiological ecology, and climate change: does mechanism matter? Annual Review of Physiol. 67, 177–201. Hobbs, R.J., Arico, S., Aronson, J., Baron, J.S., Bridgewater, P., Cramer, V.A., Epstein, P.R., Ewel, J.J., Klink, C.A., Lugo, A.E., others, 2006. Novel ecosystems: theoretical and management aspects of the new ecological world order. Global Ecology and Biogeography 15, 1–7. 94 Hooke, R.L., Martin-Duque, J.F., Pedraza, J., 2012. Land transformation by humans: a review. GSA today 22, 4–10. Hosmer, D. W., Lemeshow, S., 2013. Applied logistic regression. Third edition. Wiley & Sons Inc., Hoboken, New Jersey, USA. Huang, C., Asner, G.P., Barger, N.N., 2012. Modeling regional variation in net primary production of pinyon-juniper ecosystems. Ecological Modelling 227, 82–92. Huey, R.B., 1991. Physiological consequences of habitat selection. American Naturalist 137, 91–115. Hunt, P., 1998. Evidence from a landscape population model of the importance of early successional habitat to the American Redstart. Conservation Biology 12, 1377–1389. IPCC 2013. The physical science basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA. Jackson, R.B., Randerson, J.T., Canadell, J.G., Anderson, R.G., Avissar, R., Baldocchi, D.D., Bonan, G.B., Caldeira, K., Diffenbaugh, N.S., Field, C.B., others, 2008. Protecting climate with forests. Environmental Research Letters 3. Jantz, S.M., Barker, B., Brooks, T.M., Chini, L.P., Huang, Q., Moore, R.M., Noel, J., Hurtt, G.C., 2015. Future habitat loss and extinctions driven by land-use change in biodiversity hotspots under four scenarios of climate-change mitigation. Conservation Biology 29, 1122–1131. Johnson, S.L., 2014. House Wren (Troglodytes aedon), The Birds of North America Online (A. Poole, Ed.). Ithaca: Cornell Lab of Ornithology; Retrieved from the Birds of North America Online: http://bna.birds.cornell.edu.bnaproxy.birds.cornell.edu/bna/species/380doi:10.2173/bn a.380 Johnson, L.S., Kermott, L.H., Lein, M.R., 1993. The cost of polygyny in the house wren Troglodytes aedon. Journal of Animal Ecology 669–682. Kelly, A.E., Goulden, M.L., 2008. Rapid shifts in plant distribution with recent climate change. Proceedings of the National Acadademy of Sciences 105, 11823–6. Knezezich, C. A., 1982. Soil survey of Polk County, Oregon. United States Department of Agriculture, Soil Conservation Service, Washington DC, USA. King, D.I., Schlossberg, S., 2014. Synthesis of the conservation value of the earlysuccessional stage in forests of eastern North America. Forest Ecology and Management 324, 186–195. Kozma, J.M., Kroll, A.J., 2010. Nest survival of Western Bluebirds using tree cavities in managed ponderosa pine forests of central Washington. The Condor 112, 87–95. Labocha, M.K., Hayes, J.P., 2012. Morphometric indices of body condition in birds: a review. Journal of Ornithology 153, 1–22. Lambin, E.F., Meyfroidt, P., 2011. Global land use change, economic globalization, and the looming land scarcity. Proceedings of the National Academy of Sciences 108, 3465–3472. Lampila, P., Mӧnkkӧnen, M., Desrochers, A., 2005. Demographic responses by birds to forest fragmentation. Conservation Biology 19, 1537–1546. 95 Lautenschlager, R., Sullivan, T.P., 2002. Effects of herbicide treatments on biotic components in regenerating northern forests. The Forestry Chronicle 78, 695–731. Lehtinen, R.M., Ramanamanjato, J.B., Raveloarison, J.G., 2003. Edge effects and extinction proneness in a herpetofauna from Madagascar. Biodiversity and Conservation 12, 1357–1370. Li, P., Martin, T.E., 1991. Nest-site selection and nesting success of cavity-nesting birds in high elevation forest drainages. The Auk 405–418. Litvaitis, J.A., 1993. Response of early successional vertebrates to historic changes in land use. Conservation Biology 7, 866–873. Ma, S., Concilio, A., Oakley, B., North, M., Chen, J., 2010. Spatial variability in microclimate in a mixed-conifer forest before and after thinning and burning treatments. Forest ecology and management 259, 904–915. Mantyka-pringle, C.S., Martin, T.G., Rhodes, J.R., 2012. Interactions between climate and habitat loss effects on biodiversity: a systematic review and meta-analysis. Global Change Biology 18, 1239–1252. Martin, T.E., 1993a. Nest predation among vegetation layers and habitat types: revising the dogmas. American Naturalist 897–913. Martin, T.E., 1993b. Nest predation and nest sites. BioScience 43, 523–532. Martin, T.E., 1987. Food as a limit on breeding birds: a life-history perspective. Annual review of ecology and systematics 18, 453–487. Martin, T.E., Geupel, G.R., 1993. Nest-Monitoring Plots: Methods for Locating Nests and Monitoring Success Journal of Field Ornithology 507–519. McCabe, R.A., 1965. Nest construction by house wrens. The Condor 67, 229–234. McCarty, J.P., 2001. Ecological consequences of recent climate change. Conservation biology 15, 320–331. McComb, W.C., Noble, R.E., 1981. Microclimates of nest boxes and natural cavities in bottomland hardwoods. The Journal of Wildlife management 45, 284–289. McGarigal, K., Cushman, S.A., 2002. Comparative evaluation of experimental approaches to the study of habitat fragmentation effects. Ecological applications 12, 335–345. Miller, J.C., Hammond, P.C., Ross, D.N., 2003. Distribution and functional roles of rare and uncommon moths (Lepidoptera: Noctuidae: Plusiinae) across a coniferous forest landscape. Annals of the Entomological Society of America 96, 847–855. Miller, K.V., Miller, J.H., 2004. Forestry herbicide influences on biodiversity and wildlife habitat in southern forests. Wildlife Society Bulletin 32, 1049–1060. Monastersky, R., 2014. Biodiversity: Life-a status report. Nature 516, 159–161. Murphy, G.E., Sutton, W.R., Hill, D., Chambers, C., Creel, D., Binkley, C., New, D., 2005. Economics of intensively managed forest plantations in the Pacific Northwest. Journal of forestry 103, 78–82. Murphy, M.T., 1985. Nestling Eastern Kingbird growth: effects of initial size and ambient temperature. Ecology 66, 162–170. Newlon, K.R., Saab, V.A., 2011. Nest-site selection and nest survival of Lewis’s Woodpecker in aspen riparian woodlands. The Condor 113, 183–193. 96 O’Connor, R.J. 1984. The growth and development of birds. John Wiley and Sons, New York, New York, USA. Oliver, T.H., Morecroft, M.D., 2014. Interactions between climate change and land use change on biodiversity: attribution problems, risks, and opportunities. Wiley Interdisciplinary Reviews: Climate Change 5, 317–335. Paquette, A., Messier, C., 2009. The role of plantations in managing the world’s forests in the Anthropocene. Frontiers in Ecology and the Environment 8, 27–34. Paquette, S.R., Pelletier, F., Garant, D., Bélisle, M., 2014. Severe recent decrease of adult body mass in a declining insectivorous bird population. Proceedings of the Royal Society of London B: Biological Sciences 281. Parker, W.C., Pitt, D.G., Morneault, A.E., 2012. Influence of herbaceous and woody vegetation control on seedling microclimate, leaf gas exchange, water status, and nutrient relations of Pinus strobus L. seedlings planted in a shelterwood. Forest Ecology and Management 271, 104–114. Parmesan, C., 2006. Ecological and evolutionary responses to recent climate change. Annual Review of Ecology, Evolution, and Systematics 637–669. Pauli, H., Gottfried, M., Dullinger, S., Abdaladze, O., Akhalkatsi, M., Benito Alonso, J.L., Coldea, G., Dick, J., Erschbamer, B., Fernández Calzado, R., Ghosn, D., Holten, J.I., Kanka, R., Kazakis, G., Kollár, J., Larsson, P., Moiseev, P., Moiseev, D., Molau, U., Molero Mesa, J., Nagy, L., Pelino, G., Puşcaş, M., Rossi, G., Stanisci, A., Syverhuset, A.O., Theurillat, J.-P., Tomaselli, M., Unterluggauer, P., Villar, L., Vittoz, P., Grabherr, G., 2012. Recent plant diversity changes on Europe’s mountain summits. Science 336, 353–355. Pearson, R.G., Dawson, T.P., 2003. Predicting the impacts of climate change on the distribution of species: are bioclimate envelope models useful? Global ecology and biogeography 12, 361–371. Pereira, H.M., Navarro, L.M., Martins, I.S., 2012. Global biodiversity change: the bad, the good, and the unknown. Pérez, J.H., Ardia, D.R., Chad, E.K., Clotfelter, E.D., 2008. Experimental heating reveals nest temperature affects nestling condition in tree swallows (Tachycineta bicolor). Biology Letters 4, 468–471. Perrins, C., 1970. The timing of birds ‘breeding seasons. Ibis 112, 242–255. Perry, D.A., Oren, R., Hart, S.C., 2008. Forest ecosystems. Johns Hopkins University Press, Baltimore, Maryland, USA. Pielke, R.A., Pitman, A., Niyogi, D., Mahmood, R., McAlpine, C., Hossain, F. and G.K.K., Nair, U., Betts, R., Fall, S., et al., 2011. Land use/land cover changes and climate: modeling analysis and observational evidence. Wiley Interdisciplinary Reviews: Climate Change 2, 828–850. Pinheiro J., Bates, D., DebRoy S., Sarkar, D., and R Core Team, 2015. nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1-122. Pipoly, I., Bókony, V., Seress, G., Szabó, K., Liker, A., 2013. Effects of Extreme Weather on Reproductive Success in a Temperate-Breeding Songbird. PloS one 8. 97 Potapov, P., Yaroshenko, A., Turubanova, S., Dubinin, M., Laestadius, L., Thies, C., Aksenov, D., Egorov, A., Yesipova, Y., Glushkov, I., Karpachevskiy, M., Kostikova, A., Manisha, A., Tsybikova, E., Zhuravleva, I., 2008. Mapping the World’s Intact Forest Landscapes by Remote Sensing. Ecology and Society 13. Potter, K.A., Woods, H.A., Pincebourde, S., 2013. Microclimatic challenges in global change biology. Global change biology 19, 2932–2939. Proe, M., Griffiths, J., McKay, H., 2001. Effect of whole-tree harvesting on microclimate during establishment of second rotation forestry. Agricultural and Forest Meteorology 110, 141–154. Ramsey, F. L., Schafer, D. W., 2013. The statistical sleuth: a course in methods of data analysis. Third edition. Brooks/Cole, Cengage Learning, Boston, MA, USA. Reid, J.M., Monaghan, P., Ruxton, G.D., 2000. Resource allocation between reproductive phases: the importance of thermal conditions in determining the cost of incubation. Proceedings of the Royal Society of London 267, 37–41. Root, T.L., Price, J.T., Hall, K.R., Schneider, S.H., Rosenzweig, C., Pounds, J.A., 2003. Fingerprints of global warming on wild animals and plants. Nature 421, 57–60. Rudel, T.K., Coomes, O.T., Moran, E., Achard, F., Angelsen, A., Xu, J., Lambin, E., 2005. Forest transitions: towards a global understanding of land use change. Global environmental change 15, 23–31. Salaberria, C., Celis, P., López-Rull, I., Gil, D., 2013. Effects of temperature and nest heat exposure on nestling growth, dehydration and survival in a Mediterranean holenesting passerine. Ibis 156, 265-275. Sauer, J. R., Hines, J. E., Fallon, J. E., Pardieck, K. L., Ziolkowski, D. J., Jr., Link, W. A., 2015. The North American Breeding Bird Survey, Results and Analysis 1966 - 2013. Version 01.30.2015 USGS Patuxent Wildlife Research Center, Laurel, MD Saunders, D.A., Hobbs, R.J., Margules, C.R., 1991. Biological consequences of ecosystem fragmentation: a review. Conservation biology 5, 18–32. Schamber, J.L., Esler, D., Flint, P.L., 2009. Evaluating the validity of using unverified indices of body condition. Journal of Avian Biology 40, 49–56. Schmidt, K.A., 1999. Foraging theory as a conceptual framework for studying nest predation. Oikos 85, 151–160. Schmidt-Nielsen, K. 1997. Animal physiology: adaptation and environment. Cambridge University Press, New York, New York, USA. Schwagmeyer, P., Mock, D.W., 2008. Parental provisioning and offspring fitness: size matters. Animal Behaviour 75, 291–298. Sears, M.W., Raskin, E., Angilletta, M.J., 2011. The world is not flat: defining relevant thermal landscapes in the context of climate change. Integrative and Comparative Biology, 1-10. Selwood, K.E., McGeoch, M.A., Mac Nally, R., 2014. The effects of climate change and land-use change on demographic rates and population viability. Biological Reviews. Seto, K.C., Fragkias, M., Güneralp, B., Reilly, M.K., 2011. A meta-analysis of global urban land expansion. PLoS One 6. 98 Shaffer, T.L., 2004. A unified approach to analyzing nest success. The Auk 121, 526– 540. Shaffer, T.L., Thompson, F.R., 2007. Making meaningful estimates of nest survival with model-based methods. Studies in Avian Biology 34, 84–95. Shepard, J.P., Creighton, J., Duzan, H., 2004. Forestry herbicides in the United States: an overview. Wildlife Society Bulletin 32, 1020–1027. Sieving, K.E., Willson, M.F., 1998. Nest predation and avian species diversity in northwestern forest understory. Ecology 79, 2391–2402. Sloan, S., Sayer, J.A., 2015. Forest Resources Assessment of 2015 shows positive global trends but forest loss and degradation persist in poor tropical countries. Forest Ecology and Management 352, 134–145. Spies, T.A., 2004. Ecological concepts and diversity of old-growth forests. Journal of Forestry 102, 14–20. Spies, T.A., Johnson, K.N., 2007. Projecting Forest Policy and Management Effects across Ownerships in Coastal Oregon. Ecological Applications 17, 3–4. Steidl, R.J., Hayes, J.P., Schauber, E., 1997. Statistical power analysis in wildlife research. The Journal of Wildlife Management 61, 270–279. Suggitt, A.J., Gillingham, P.K., Hill, J.K., Huntley, B., Kunin, W.E., Roy, D.B., Thomas, C.D., 2011. Habitat microclimates drive fine-scale variation in extreme temperatures. Oikos 120, 1–8. Swanson, M.E., Franklin, J.F., Beschta, R.L., Crisafulli, C.M., DellaSala, D.A., Hutto, R.L., Lindenmayer, D.B., Swanson, F.J., 2010. The forgotten stage of forest succession: early-successional ecosystems on forest sites. Frontiers in Ecology and the Environment 9, 117–125. Sutherland, W.J., Newton, I., Green, R.E., 2004. Bird ecology and conservation: a handbook of techniques. Oxford University Press Inc., New York, New York, USA. Tatum, V.L., 2004. Toxicity, transport, and fate of forest herbicides. Wildlife Society Bulletin 32, 1042–1048. Taylor, G.H., Hannan, C., 1999. The climate of Oregon: from rain forest to desert. Oregon State University Press, Corvallis, USA. Taylor, R.L., Maxwell, B.D., Boik, R.J., 2006. Indirect effects of herbicides on bird food resources and beneficial arthropods. Agriculture, ecosystems, and environment 116, 157–164. Thomas, J.W., Franklin, J.F., Gordon, J., Johnson, K.N., 2006. The Northwest Forest Plan: origins, components, implementation experience, and suggestions for change. Conservation Biology 20, 277–287. Thompson, F.R., 2007. Factors affecting nest predation on forest songbirds in North America. Ibis 149, 98–109. Tilman, D., Balzer, C., Hill, J., Befort, B.L., 2011. Global food demand and the sustainable intensification of agriculture. Proceedings of the National Academy of Sciences 108, 20260–20264. 99 Tscharntke, T., Clough, Y., Wanger, T.C., Jackson, L., Motzke, I., Perfecto, I., Vandermeer, J., Whitbread, A., 2012. Global food security, biodiversity conservation and the future of agricultural intensification. Biological Conservation 151, 53–59. Varner, J., Dearing, M.D., 2014. The Importance of Biologically Relevant Microclimates in Habitat Suitability Assessments. PloS one 9. Vié, J.-C., Hilton-Taylor, C., Stuart, S.N., 2009. Wildlife in a changing world: an analysis of the 2008 IUCN Red List of threatened species. IUCN. Von Arx, G., Dobbertin, M., Rebetez, M., 2012. Spatio-temporal effects of forest canopy on understory microclimate in a long-term experiment in Switzerland. Agricultural and Forest Meteorology 166, 144–155. Wada, H., Kriengwatana, B., Allen, N., Schmidt, K.L., Soma, K.K., MacDougallShackleton, S.A., 2015. Transient and permanent effects of suboptimal incubation temperatures on growth, metabolic rate, immune function, and adrenocortical responses in zebra finches. The Journal of Experimental Biology 218, 2847-2855. Wagner, R.G., Little, K.M., Richardson, B., Mcnabb, K., 2006. The role of vegetation management for enhancing productivity of the world’s forests. Forestry 79, 57–79. Waring, R.H., Franklin, J.F., 1979. Evergreen coniferous forests of the Pacific Northwest. Science 204, 1380–1386. Webb, D., 1987. Thermal tolerance of avian embryos: a review. Condor 89, 874–898. Whiteman, C.D., Zhong, S., Shaw, W.J., Hubbe, J.M., Bian, X., Mittelstadt, J., 2001. Cold pools in the Columbia Basin. Weather and forecasting 16, 432–447. Williams, C.M., Henry, H.A., Sinclair, B.J., 2015. Cold truths: how winter drives responses of terrestrial organisms to climate change. Biological Reviews 90, 214–235. Willson, M.F., Comet, T.A., 1996a. Bird communities of northern forests: patterns of diversity and abundance. Condor 98, 337–349. Willson, M.F., Comet, T.A., 1996b. Bird communities of northern forests: ecological correlates of diversity and abundance in the understory. Condor 98, 350–362. Winkler, D.W., Dunn, P.O., McCulloch, C.E., 2002. Predicting the effects of climate change on avian life-history traits. Proceedings of the National Academy of Sciences 99, 13595–13599. Xu, M., Chen, J., Brookshire, B.L., 1997. Temperature and its variability in oak forests in the southeastern Missouri Ozarks. Climate Research 8, 209–223. Zach, R., 1982. Nestling house wrens: weight and feather growth. Canadian Journal of Zoology 60, 1417–1425. Zhao, K., Jackson, R.B., 2014. Biophysical forcings of land-use changes from potential forestry activities in North America. Ecological Monographs 84, 329–353. Zurr, A. F., Ieno, E. N., Walker, N.J., Saveliev A. A., and G. M. Smith. 2007. Mixed effects models and extensions with R. Springer Science+Business Media LLC, New York, New York, USA. 100 APPENDICES 101 APPENDIX A: HERBICIDE PRESCRIPTIONS Study Treatment Year post-harvest & Season Prescription Chemical and Quantity/Acre Control Light 1-10 2 (spring) Planting (year 2) Herbaceous None 2.66 lbs. Velpar (hexazinone) 32 oz 2-4-D (2,4-dichlorophenoxy acetic acid) 3 (late summer) Woody vegetation control 1.125 qt Accord (glyphosate) 20 oz Garlon (triclopyr) Moderate 1 (late summer) 2 (spring) Site preparation Herbaceous control 1.5 oz Escort (metsulfuron methyl) 3 qts Accord (glyphosate) 24 oz Chopper (imazapyr) 3 oz Oust (sulfometuron methyl & metsulfuron methyl) 24 oz MSO (methylated seed oil) 2.66 lbs. Velpar (hexazinone) 32 oz 2-4-D (2,4-dichlorophenoxy acetic acid) 3 (late summer) Woody vegetation control 1.5 qt Accord (glyphosate) 20 oz Garlon (triclopyr) 4 (late summer) Intensive 1 (late summer) 2-10 (spring) Acer macrophyllum sprout control (as necessary) Imazapyr Acer macrophyllum sprout control follow-up (if necessary) Imazapyr Site preparation Herbaceous control 1.5 oz Escort (metsulfuron methyl) 3 qts Accord (glyphosate) 24 oz Chopper (imazapyr) 3 oz Oust (sulfometuron methyl & metsulfuron methyl) 24 oz MSO (methylated seed oil) 2.66 lbs. Velpar (hexazinone) 32 oz 2-4-D (2,4-dichlorophenoxy acetic acid) 102 Woody vegetation control (Annual review with backpack treatments as necessary). 1.125 qt Accord (glyphosate) 3-10 (late summer) 20 oz Garlon (triclopyr) 3-10 (late summer) Acer macrophyllum sprout control and follow-up (as necessary) Imazapyr