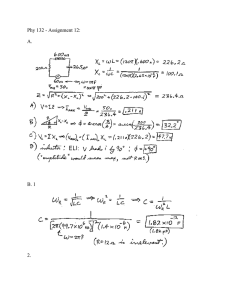
Document 12079265
advertisement

AN ABSTRACT OF THE THESIS OF Lucas J. Longway for the degree of Master of Science in Forest Ecosystems and Society presented on November 13, 2015 Title: Comparing Ectomycorrhizal Communities of Understory Giant Chinquapin (Chrysolepis chrysophylla) and Overstory Pinaceae Trees in a Mixed Conifer Forest in Central Oregon Abstract approved: _________________________________________________________________________ Jane E. Smith Giant chinquapin (Chrysolepis chrysophylla) is an evergreen hardwood often found as a shrubby understory component of coniferous forests in the Pacific Northwest United States. Due to its ability to sprout quickly after disturbances such as fire and logging it is often viewed as a pest by forest managers. Like its associated overstory conifers, giant chinquapin forms ectomycorrhizae. However, the ectomycorrhizal fungus communities associated with giant chinquapin found in the Pacific Northwest have not been investigated. To further explore giant chinquapin’s ecological roles in central Oregon’s forests we compare ectomycorrhizal communities associated with giant chinquapin and cooccurring overstory Pinaceae trees in the Pringle Falls Experimental Forest, central Oregon. Ectomycorrhizal communities of Pinaceae trees had a greater taxa richness than those found associated with giant chinquapin. However, 57% (8 of 14) of the taxa found in 31% (5/16) of study areas on Pinaceae trees were found associated with giant chinquapin. Four taxa (Cenococcum geophilum 1 & 2, Piloderma 2, Byssoccorticium 1), likely important for host water and nutrient access, were found in 31% of study areas associated with both chinquapin and Pinaceae hosts. Sixty-four percent (23 of 36) of the ectomycorrhizal taxa found on giant chinquapin associated with Pinaceae trees and every genus associated with giant chinquapin in our study has been reported to form ectomycorrhizae with Pinaceae trees in this or other studies. Based on these results, it is likely that giant chinquapin is supporting a subset of the ectomycorrhizal community associated with Pinaceae hosts. Giant chinquapin, with its ability to quickly sprout after disturbance, could be beneficial to local conifer seedlings as a source of ectomycorrhizal innoculum should overstory conifers decrease as a a result of a stand replacing disturbance. ©Copyright by Lucas J. Longway November 13, 2015 All Rights Reserved Comparing Ectomycorrhizal Communities of Understory Giant Chinquapin (Chrysolepis chrysophylla) and Overstory Pinaceae Trees in a Mixed Conifer Forest in Central Oregon by Lucas J. Longway A THESIS submitted to Oregon State University in partial fulfillment of the requirements for the degree of Master of Science Presented November 13, 2015 Commencement June 2016 Master of Science thesis of Lucas J. Longway presented on November 13, 2015 APPROVED: _________________________________________________________________________ Major Professor, representing Forest Ecosystems and Society _________________________________________________________________________ Head of the Department of Forest Ecosystems and Society _________________________________________________________________________ Dean of the Graduate School I understand that my thesis will become part of the permanent collection of Oregon State University libraries. My signature below authorizes release of my thesis to any reader upon request. _________________________________________________________________________ Lucas J. Longway, Author ACKNOWLEDGEMENTS I would like to express my sincere appreciation to my major professor, Jane E. Smith for guiding me through the ups and downs of executing a scientific study and obtaining a master’s degree. My heartfelt thanks also goes to my committee members, Paul Anderson Jeff Hatten, and Daniel Luoma, who have offered invaluable support throughout this process. I would also like to thank everyone who helped with data collection and sample processing, Donaraye McKay, Elizabeth Bowman, Joseph Cagle. I would also like to express my gratitude to my lab mates Ariel Cowan, Maria Osuna Garcia and Benjamin Hart, my partners in PCR, Richard Cronn, Joyce Eberhart, Tara Jennings, Thomas Mullins and my map expert Lucy Romeo, for helping, listening and offering feedback every step of the way. For teaching me things I really needed to know, I would like to thank Lisa Ganio, Bruce McCune, Shawn O’Neil, and Matthew Powers. For thesis formatting help I would like to thank Jerry Mohr. For meeting with me every week for precisely 3.2 bajillon weeks and talking about numbers, I would like to thank Ariel Muldoon. Finally, I would like to thank my friends and my family for supporting me though this process. My wife, Kelly Longway, deserves a medal of honor and perhaps a Master's in ‘keeping Lucas alive’ and for her I am eternally grateful. Last, but most certainly not least, I would like to thank Sam and Napoleon. TABLE OF CONTENTS Page STUDY OVERVIEW AND RELATED TOPICS ................................................................1 USDA Forest Service Pringle Falls Experimental Forest ..............................................................1 History & Research ....................................................................................................................1 PFEF Geologic History ..............................................................................................................1 Soil .............................................................................................................................................2 Climate & Vegetation ................................................................................................................2 Fire .................................................................................................................................................3 Fire Adapted Environments in the Pacific Northwest................................................................3 Fire & Climate Change ..............................................................................................................4 Ecosystem Changes with Fire & Ectomycorrhizal Refuge Plants .............................................5 Overarching Study .....................................................................................................................6 Mycorrhiza .....................................................................................................................................7 History........................................................................................................................................7 Mycorrhizal Classification .........................................................................................................8 Ecological Roles ......................................................................................................................10 EMF, Nitrogen & Phosphorus..................................................................................................10 Growth, Reproduction, Molecular Advances...........................................................................14 Common Mycorrhizal Networks..............................................................................................15 Generalists & Specialists..........................................................................................................16 Chinquapin ...................................................................................................................................16 Biology & Ecology ..................................................................................................................16 Economic Significance.............................................................................................................17 Cultural Significance................................................................................................................17 Ecosystem Services..................................................................................................................18 Chinquapin & Fire ..................................................................................................................18 Competition..............................................................................................................................19 Chinquapin Ectomycorrhiza.....................................................................................................20 Pinaceae........................................................................................................................................21 Pacific Northwest Genera and Species ....................................................................................21 Economic Significance.............................................................................................................21 Pinaceae Range & Biology ......................................................................................................22 Summary ......................................................................................................................................26 STUDY MANUSCRIPT......................................................................................................32 Introduction ..................................................................................................................................32 Materials & Methods....................................................................................................................35 Study Area................................................................................................................................35 Climate .....................................................................................................................................35 Vegetation ................................................................................................................................35 Soils..........................................................................................................................................36 Study Design ............................................................................................................................36 Vegetation & Soil Moisture .....................................................................................................37 Soil Sampling & Nutrients .......................................................................................................38 Ectomycorrhiza Root Collection & Host Identification...........................................................38 Statistical Analyses ..................................................................................................................41 Results ..........................................................................................................................................42 Vegetation Community ............................................................................................................42 EMF Communities ...................................................................................................................43 EMF Community Comparisons ...............................................................................................43 EMF Communities & Environmental Variables......................................................................44 Discussion ....................................................................................................................................45 EMF Community & Soil Nutrients ..........................................................................................46 EMF Community Comparisons ...............................................................................................47 EMF & Fire..............................................................................................................................49 Limitations & Future Directions ..............................................................................................50 Conclusions & Implications.....................................................................................................51 BIBLIOGRAPHY................................................................................................................67 LIST OF FIGURES Figure Page 1. Study area overview and design....................................................................... 52 2. Lookout Mountain study area .......................................................................... 53 3. Box and whisker plot of basal area (m2/ha) of overstory Pinaceae trees. ........ 54 4. Basal area (m2/ha) of overstory Pinaceae trees per plot by elevation (m). ...... 55 5. Pinaceae basal area (m2/ha) per plot ................................................................ 56 6. Taxa found on both Pinaceae hosts and giant chinquapin by frequency of occurrence on transect pairs. ............................................................................ 60 7. Species rarefaction curves and 95% confidence intervals ............................... 61 8. Number of ascomycetes and basidiomycetes on transect pairs ....................... 62 9. Dominant EMF taxa or EMF taxa.................................................................... 63 10. Frequencey of all EMF taxa found by transect pair......................................... 64 11. NMDS showing EMF taxa in transect space. .................................................. 66 LIST OF TABLES Table Page 1. Comparisons of EMF genera found associated with Pinaceae and chinquapin to genera found on hosts phylogenetically related to chinquapin. 27 2. Environmental variable measurements ............................................................ 57 3. Spearman ranked correlation coefficient (ρ) of pH correlated with chinquapin percent cover ................................................................................. 57 4. Spearman ranked correlation coefficients (ρ) of environmental variables correlated to Pinaceae basal area (m2/ha)......................................................... 57 5. Spearman ranked correlation coefficients (ρ) for environmental variables correlated with each other ................................................................................ 58 6. Root tip success summary. ............................................................................... 58 7. Count and percent of unique and shared fungal taxa found by host. ............... 58 8. Count and percent of unique and shared fungal taxa. ...................................... 58 9. Count and percent of total fungal taxa found by host. ..................................... 59 10. Spearman ranked correlation coefficients (ρ) for Pinaceae EMF descriptors that were correlated with environmental variables .......................................... 65 11. Spearman ranked correlation coefficients (ρ) for Chinquapin EMF descriptors that were correlated with environmental variables........................ 65 LIST OF APPENDICES Page APPENDICES ....................................................................................................................88 Appendix A ................................................................................................................................. 89 Appendix B ...............................................................................................................................103 Selected Scatterplots Involving Environmental Variables ....................................................103 Selected Scatterplots Involving EMF Variables and Root tip Counts ..................................112 Selected Scatterplots Involving Overstory Pinaceae Basal Area ..........................................121 LIST OF APPENDIX FIGURES Figure Page 1. Scatter plot of chinquapin % cover vs. pH per transect pair. ......................... 103 2. Scatter plot of bray phosphorus (mg/kg) vs. pH per transect pair. ................ 104 3. Scatter plot of bray phosphorus (mg/kg) vs. elevation (m) per transect pair . 105 4. Scatter plot of total elevation (m) vs. phosphorus (mg/kg) per transect pair . 106 5. Scatter plot of total nitrogen (%) vs. mineralizable nitrogen (mg/kg) per transect pair .................................................................................................... 107 6. Scatter plot of average volumetric soil moisture (m3/m3) vs. mineralizable nitrogen (mg/kg)............................................................................................. 108 7. Scatter plot of total phosphorus (mg/kg) vs. Bray phosphorus (mg/kg) per transect pair .................................................................................................... 109 8. Scatter plot of total carbon (%) vs. mineralizable nitrogen (mg/kg) per transect pair. ................................................................................................... 110 9. Scatter plot of total nitrogen (%) vs. total carbon (%) per transect pair. ....... 111 10. Scatter plot of Pinaceae root tips vs. Pinaceae EMF Shannon’s Diversity per transect pair. ................................................................................................... 112 11. Scatter plot of Pinaceae root tips vs. chinquapin root tips per transect pair. . 113 12. Scatter plot of Pinaceae root tips vs. Pinaceae EMF richness per transect pair.................................................................................................................. 114 13. Scatter plot of Pinaceae root tips vs. chinquapin EMF Shannon’s Diversity per transect pair. ............................................................................................. 115 14. Scatter plot of Pinaceae root tips vs. chinquapin EMF Richness per transect pair.................................................................................................................. 116 15. Scatter plot of chinquapin root tips vs. chinquapin EMF richness per transect pair. ................................................................................................... 117 16. Scatter plot of chinquapin root tips vs. Pinaceae EMF richness per transect pair.................................................................................................................. 118 17. Scatter plot of chinquapin root tips vs. chinquapin EMF Shannon’s diversity per transect pair. ............................................................................................. 119 18. Scatter plot of chinquapin root tips vs. Pinaceae EMF Shannon’s diversity per transect pair. ............................................................................................. 120 19. Scatter plot of elevation (m) vs. grand fir basal area (m2/ha) per transect pair.................................................................................................................. 121 20. Scatter plot of Bray phosphorus (mg/kg) vs. lodgepole pine basal area (m2/ha) per transect pair. ................................................................................ 122 21. Scatter plot of Pinaceae EMF Shannon’s diversity vs. lodgepole pine basal area (m2/ha) per transect pair. ........................................................................ 123 22. Scatter plot of Pinaceae EMF richness vs. lodgepole pine basal area (m2/ha) per transect pair .............................................................................................. 124 LIST OF APPENDIX TABLES Table Page 1. EMF taxa found on Lookout Mountain. .......................................................... 89 2. Full Spearman ranked correlation (ρ) tables of environmental variables correlated with each other ................................................................................ 98 3. Spearman ranked correlation coefficients (ρ) for comparing environmental variables to chinquapin EMF variables.......................................................... 100 4. Spearman ranked correlation coefficients (ρ) for comparing environmental variables to Pinaceae EMF variables ............................................................. 101 5. Spearman ranked correlation coefficients (ρ) for most frequently occurring Pinaceae overstory species compared to environmental and EMF variables (n = 12)........................................................................................................... 102 1 STUDY OVERVIEW AND RELATED TOPICS USDA Forest Service Pringle Falls Experimental Forest History & Research Thornton T. Munger founded the Pringle Falls Experimental Forest (PFEF), the first experimental forest in the Pacific Northwest, in 1931 (Camp and Youngblood 2006). It was comprised of the Pringle Butte unit (3,043 ha) and was established to provide a large scale study area in which to investigate the best silvicultural practices for spacing, thinning, and fertilization of ponderosa pine in order to achieve the greatest growth and yield (Camp and Youngblood 2006). In 1936 the Lookout Mountain unit (1,413 ha) was added to the forest (Adams et al. 2004, Youngblood 2009). From inception through 1993, 119 scientific publications have come out of the PFEF area (Youngblood 1995), including studies focusing on competition effects to ponderosa pine from shrubs and studies investigating the negative effects of insect pathogens to forest health (Camp and Youngblood 2006). Current studies taking place at Pringle Falls tend to focus on investigating long-term processes that influence forest structure and composition (Adams et al. 2004). PFEF Geologic History The Pringle Falls Experimental Forest, located within the Deschutes National Forest, is part of the Sierra Nevada – Cascade Mountain physiographic province; specifically the Middle Cascades section (Simpson 2007). The province is volcanically influenced, containing several large volcanoes and many smaller cinder cones (Simpson 2007). Lookout Mountain, a 300,000-year-old shield volcano (Adams et al. 2004), has bedrock of mixed basalts, andesites, and rhyolites, underlying approximately 50-125 cm of andisolic soils layers (Larsen 1976). 2 Soil The upper layers of soil on the mountain are comprised mainly of ash and tephra from the Mt. Mazama eruption 6,600 years ago. (Camp and Youngblood 2006). The ash layer is approximately 0.5 to 2 m (2 to 3 ft) thick on the study site (Adams et al. 2004; Simpson 2007). According to a report on the first official soil survey at Pringle Falls (Tarrant 1947), the soils are classified under the Lapine soil series, which is an Ashy­ pumiceous, glassy Xeric Vitricryand, meaning that the soils are composed of volcanic ash and pumice, while also being glassy, excessively well drained, dry, cold and andisolic in nature (Soil Survey Staff 2014). These soils tend to be limited in plant available phosphorus and nitrogen and relatively low in organic matter content as well (Adams et al. 2004; Soil Survey Staff 2014). Additionally, the permanent plant wilting point of these soils is at 15 to 16% soil moisture (NCSS 2015). Despite these harsh soil conditions the majority of the area is classified as highly productive forestland by the USDA Forest Service (Larsen 1976). Climate & Vegetation The Lookout Mountain unit experiences an average of 1020 mm of precipitation a year, which is greater than the immediately surrounding areas such as Pringle Butte, which receives only 610mm (24 in) on average annually (Adams et al. 2004). Most of this precipitation falls as snow (Adams et al. 2004). Precipitation is seasonal in this area and the soils tend to be moister during the spring snowmelt than during the dry and warm summer months. On average summer high temperatures range from 21° to 35° C (Adams et al. 2004). Forest composition present on Lookout Mountain varies with elevation and aspect; ponderosa pine dominant stands occupy the lower elevations, gradually shifting to mixed conifer stands higher on the mountain (Adams et al. 2004). In 2011 the mixed conifer stands on Lookout Mountain were found to contain, grand fir (Abies grandis (Dougl. ex D. Don) Lindl.), subalpine fir (Abies lasiocarpa (Hook.) Nuttall), California red fir (Abies 3 magnifica A. Muray), lodgepole pine (Pinus contorta Dougl. ex Loudon), sugar pine (Pinus lambertiana Dougl.), western white pine (Pinus monticola Douglas ex D. Don), ponderosa pine (Pinus ponderosa Dougl. ex Laws.), Douglas-fir (Pseudotsuga menziesii (Mirb.) Franco), and mountain hemlock (Tsuga mertensiana (Bong.) Carr.). Understory shrubs and shrubby trees found on Lookout Mountain were: antelope bitterbrush (Purshia tridentata (Pursh.) DC.), wax currant (Ribes cereum Dougl.), bitter cherry (Prunus emarginata (Dougl. ex Hook) D. Dietr.), pinemat manzanita (Arctostaphylos nevadensis A. Gray), greenleaf manzanita (Arctostaphylos patula Greene), snowbrush ceanothus (Ceanothus velutinus Dougl. ex Hook.), and giant chinquapin (Chrysolepis chrysophylla (Dougl. ex Hook.) Hjelmq.) (henceforth referred to as ‘chinquapin’). The forests on the mountain within the study site fall into three classifications: Pinus ponderosa/Purshia tridentata-Ceanothus velutinus (CPS-312) at the lowest elevations does not support chinquapin; Mixed conifer/Ceanothus velutinus-Arctostaphylos patula (CWS-112) at mid-elevation does support chinquapin; and Mixed Conifer/Ceanothus velutinus-Carex stoliferus (CWS-115) at the highest elevation in the study site also supports chinquapin (Volland 1985; Youngblood 2009). Fire Fire Adapted Environments in the Pacific Northwest Fire is a common ecological disturbance in the Pacific Northwest (Agee 1993). Many of the drier ecosystems in the Pacific Northwest are fire adapted, meaning the vegetation communities have ways to survive fires or recolonize quickly after fire (Agee 1993). Fire in an ecosystem is typically described via particular fire regimes, which are complex interactions among fire frequency, size, intensity, severity, type, and seasonality (Flannigan et al. 2000); fire frequency is the average time between fires, size is the area burned, intensity is the amount of energy released from fuels, severity is the amount and type of fuel consumed, type is where the fire is located in the forest (crown, surface, 4 ground), and seasonality is when in the year the fire takes place (Flannigan et al. 2000). All of these factors are linked and influenced by weather and topography (Flannigan et al. 2000). Specific ecosystems tend to develop with specific fire regimes (Agee 1993). For instance, the ponderosa pine dominated forests and the mixed conifer stands found on the Lookout Mountain unit were historically relatively well adapted to fire with a return interval of 7 to 20 years (Adams et al. 2004). In areas like Pringle Falls, frequent burns were usually low-intensity and served to maintain a low fuel level by thinning understory trees and seedlings. However, fire regimes changed drastically in 1910 after large forest fires in the Northwestern United States caused policy makers in Washington to change the primary goal of the nascent U.S. Forest Service to fire suppression in order to preserve forest resources and timber for industry and human use (Fitzgerald 2005; Silcox 1911; Southard 2011). In fact, the last stand replacing fire to burn through the Lookout Mountain area of Pringle Falls was in 1845, however part of the mountain burned in 1914 as well (Youngblood 2009, Final 2010). These fires gave rise to the 166 and 97-year-old stands that were present on the mountain until 2011 (Youngblood 2009, Final 2010). No standreplacing or large fires have burned through the area since 1914 and without the routine maintenance of fire, the forest on Lookout Mountain, like many of the forests in the western United States, has become dense, brushy, and a risk for large burns (Fitzgerald 2005; Youngblood 2009, Final 2010). Fire & Climate Change In addition to the problem of dense forests, global climate change also impacts fire regimes (Running 2006; Krawchuck et al. 2009; Adams 2013). In the Pacific Northwest, summers have been warming, winter precipitation has been decreasing, and spring snowmelt has been happening earlier in the year (Running 2006; Westerling et al. 2006). Fire seasons from 1987 to 2003 have become longer than those in 1970 to 1986 by an average of 78 days (Westerling et al. 2006). Increase in fire season length from the mid 5 1980s to 2003, due to longer and warmer summers, has increased the land area burned in the United States by more than six times the average from 1970 to 1986 (Westerling et al. 2006). Correspondingly, there has been increasing frequency of large, highly destructive fires in the western United States and in other areas worldwide (Westerling et al. 2006; Running 2006; Adams 2013). Future climate models have predicted increased summer warming with increased winter precipitation for the Pacific Northwest United States (Mote & Salathé 2010). Despite increasing winter precipitation, the increase in summer warming combined with earlier spring snowmelt will likely exacerbate current fire risks, leading to greater frequency of fire (Westerling et al. 2006). Ecosystem Changes with Fire & Ectomycorrhizal Refuge Plants Small, frequently occurring, low intensity fires are necessary for optimal ecosystem function for ponderosa pine dominated forests of the Pacific Northwest (Fitzgerald 2005). However, fuel loading from extreme fire suppression has led to conditions where fires have the capacity to become large and severe (Fitzgerald 2005). These large fires can remove or kill the majority of above-ground vegetation and significantly change vegetation structure and soil properties (Adams et al. 2013). However, even less severe fires can cause large ecosystem changes, such as leaving trees weakened and susceptible to beetle attack (McHugh et al. 2003; Fitzgerald 2005) and fungal attack (Gara et al. 1985; Parker et al. 2006). In the case of stand replacing fires, years may elapse before the historically dominant forest species can re-establish dominance in a stand (Savage and Mast 2005). Part of ensuring that overstory dominant species can recolonize a burned site is maintaining appropriate underground fungal partners, or mycorrhiza, for them. Most tree and plant species are dependant on mycorrhizal fungi for growth (Smith and Read 2008). All trees in the Pinaceae form a particular type of mycorrhizal partnership called ectomycorrhiza (ECM) (Brundrett 2009). When much of the vegetation in a forest is removed, such as in a large burn, the initial diversity of local ectomycorrhizal fungal 6 populations may decrease (Visser 1995; Bruns et al. 2002b). There are, however, several ways in which ectomycorrhizal fungi (EMF) may quickly re-enter a system. Some EMF have moderately heat resistant spores which can survive some fires and quickly recolonize roots of germinating seedlings (Baar et al. 1999; Peay et al. 2009). Vegetative mycelium and spores from nearby undisturbed areas can act as innoculum (Horton et al. 1998; Hagerman et al. 1999; Krannabetter et al. 1999; Nara and Hogetsu 2004; Dickie and Reich 2005; Hewitt et al. 2013). Additionally, certain fire adapted plant species may be able to act as a refuge for EMF species (Molina et al. 1992; Hewitt et al. 2013). This ability is due to their root systems and underground burls remaining relatively intact after fire, allowing them to resprout (Kauffman and Martin 1990). Chinquapin, a shrubby evergreen member of the beech family (Fagaceae) that grows beneath Pinacaeae trees on much of Lookout Mountain, may serve as an EMF refuge after fires in central Oregon. Overarching Study The dense state – increasing the likelihood of mountain pine beetle (Dendroctonus ponderosae Hopkins) attack or stand replacing fire, of this outdoor laboratory combined with its rich history of scientific study, inspired the U.S. Forest Service to begin a large study testing fuel reduction methods to restore the forest to an open state (Youngblood 2009). This ongoing study began in 2011 on the Lookout Mountain unit of the PFEF. This study split the Lookout Mountain unit into four blocks, which were then each subdivided into five treatment units (Youngblood 2009). The five treatments consisted of a control treatment and four thinning treatments combined with understory mastication and prescribed burning. Prior to these treatments a nine-hectare (ha) sampling unit, comprised of twenty-five points arranged on a 50 × 50 m grid, was established on each treatment unit for gathering pre-treatment data (Youngblood 2009). At each point, a central circular plot (.04 ha) was delineated for vegetation surveys, and two parallel transects (17 m long and 22.5 m apart), one on either side of the circular vegetation plot, were established in a randomly selected cardinal direction for the placement of soil moisture tubes (Youngblood 7 2009). Treatments were applied between 2011 and 2015. Post treatment data on the sampling units is ongoing. The large fuel reduction study incorporates many simultaneously occurring smaller studies. One of these studies was conducted to observe the response of the understory shrub, chinquapin, to forest thinning and fuel reduction techniques (Youngblood 2009, Anderson and Smith 2011). Chinquapin, with its propensity to sprout and spread after disturbance, competes with overstory conifers for nutrients and water (Barrett et al. 1983; McKee 1990; Kauffman and Martin 1990; Donato et al. 2009; Meyer 2012). Additionally, chinquapin can contribute to fuel loads and is considered moderately flammable (Weatherspoon and Skinner 1987). Chinquapin is an ectomycorrhizal-forming shrub, but little is known with regards to its mycorrhizal symbionts. A key aspect of this study was to investigate the differences in EMF community composition and/or diversity between the overstory Pinaceae and the understory chinquapin hosts to see if, after fires or other disturbance, chinquapin could serve as an EMF refuge plant for future germinating Pinaceae trees. This study also explored the response of EMF communities to environmental variables with the hypothesis that EMF communities would be influenced by soil nutrients, specifically nitrogen and phosphorus, regardless of host. Mycorrhiza History The mycorrhizal lifestyle for fungi is ancient and found globally (Smith and Read 2008; Kohler et al. 2015). The earliest evidence of mycorrhizal-like fungi comes from 460 million year old fossils from the mid-Ordovician Guttenberg Formation (Redecker et al. 2000). The fossilized spores and hyphae found resemble those from the extant group Glomales (Redecker et al. 2000). Approximately 60 million years later in the Lower Devonian an arbuscule, concrete evidence of a mycorrhizal association, was fossilized in the Rhynie Chert in Scotland (Remy et al. 1994; Strullu-Derrien et al. 2014). This finding indicates that arbuscular mycorrhizae (AM) were likely the first mycorrhizal type to arise 8 (Remy et al. 1994; van der Heijden et al. 2015). However, there is evidence that fungi from the Mucoromycotina may have been forming mutualisms at nearly the same time as AM fungi since fossils of Mucoromycotina associating with early land plants were recently found in the 400 million-year-old Rhynie Chert (Strullu-Derrien et al. 2014). Many mycorrhizal fungi were originally saprophytic or parasitic in nature and gradually lost some to most of their capacity to degrade complex molecules and therefore began to form symbiotic relationships with plant roots as an alternative (Tedersoo et al. 2010; Kohler et al. 2015). The ECM condition has arisen from the loss of saprotrophy independently in at least 78 to 82 individual lineages of fungi according to Tedersoo and Smith (2013). The earliest fossil record of an ECM root tip was found in the middle Eocene Princeton Chert in British Colombia and dates back to 50 million years ago (LePage et al. 1997). However, Berbee and Taylor (2001) found that early EMF lineages could have arisen as far back as 200 million years ago. Regardless, ECM development was much later than that of AM (van der Heijden et al. 2015). Mycorrhizal Classification The classification of mycorrhizal types has evolved as understanding of the mycorrhizal associations has increased. In 1885 Frank coined the term ‘mycorrhiza’ or ‘fungus-root’ (Frank 2005). Others have subdivided mycorrhiza into different groups, but Peyronel et al. (1969) grouped mycorrhiza into three main categories (endomycorrhiza, ectomycorrhiza, and ectendomycorrhiza), based on physiological characters of the root and fungus interaction. With increased understanding of mycorrhizal associations, the classification scheme has expanded to include seven types (arbuscular (AM), ectomycorrhiza (ECM), ectendomycorrhiza, arbutoid, monotropoid, ericoid and orchid) (Smith and Read 2008). However, an alternative classification lists only five types (vesicular-arbuscular (VAM), ectomycorrhizal (ECM), orchid, ericoid, and subepidermal), which then contain subcatagories (Brundrett 2004). In this classification scheme, the 9 arbutoid and monotropoid types are placed as sub-categories of ectomycorrhiza and the ectendomycorrhiza are deemed a morphotype and not a true category (Brundret 2004). Regardless of categorical hierarchy, there tend to be typical physiological characters associated with types, or sub-categories, of mycorrhiza (Brundrett 2004; Smith and Read 2008). VAM (AM) are formed by aseptate fungi and found within the root cortical cells of plants and can be either linear or coiling types (Smith and Read 2008). They form arbuscules (nutrient exchange sites) within plant cells and vesicles (Brundrett 2004; Smith and Read 2008). They are most common when considering all vascular land plants (Brundrett 2009). In contrast, ECM form a fungal sheath encompassing the roots and a Hartig net for nutrient transfer is formed by hyphae penetrating between, but not within, hosts plant root cells. However, in Brundrett’s (2004) classification the transfer cell, monotropoid, and arbutoid sub-catagories of ECM there is hyphal penetration of plant cells. ECM associations are highly important due to the widespread and dominant nature of key hosts such as conifers and many hardwoods (Brundrett 2009). Despite differing interpretations of their hierarchical classification, arbutoid and monotropoid associations form with plants in the Ericales and Monotropoideae respectively (Smith and Read 2008). Smith and Read (2008) classify ectendomycorrhizae as mycorrhizae that have a developed Hartig net and root cell penetration, but with a reduced or absent sheath. Both Smith and Read (2008) and Brundrett (2004) agree on the classification of ericoid and orchid mycorrhizae, the former usually associating with plants in the Ericales and the latter forming with Orchidales. However, Smith and Read (2008) point out that ericoid mycorrhizae also form with bryophytes and Brundrett (2004) further sub-divides the orchid mycorrhizae into categories based on hyphal location and commensalist vs. exploitative nutritional status. Many mycorrhizal fungi can form different kinds of associations depending on the identity of the host plant (Smith and Read 2008). For instance, the same fungus can form ecto-, arbutoid-, monotropoid, or orchid mycorrhizae (Smith and Read 2008). This variability would suggest that the mycorrhizal form is determined by both the host and the fungus and is highly versatile. 10 Ecological Roles Mycorrhizal fungi are generally essential for plant growth, with more than 80% of all flowering plant species being mycorrhizal (Smith and Read 2008; Brundrett 2009; Simard and Austin 2010). When considering AM and ECM associations, seedlings of various trees are typically not viable without appropriate fungal partners (Smith and Read 2008). In general, AM and ECM fungi assist in the uptake of plant essential nutrients such as phosphorus and nitrogen (Read and Perez-Moreno 2003; Smith and Read 2008), and allow for expedited uptake of water during dry periods due to improved nutrition and to smaller diameter hyphae which have greater surface area for absorption than larger roots (Lehto 1992; Auge 2001 (AM); Smith and Read 2008 (ECM)). Mycorrhizae also confer benefits to the host plant such as protection from some fungal pathogens and some protection from heavy metal contamination (Newsham et al. 1995; Leyval et al. 1997; Regvar 2010). Additionally, mycorrhizae as beneficiaries of plant fixed atmospheric carbon, are essential in terrestrial ecosystems as they facilitate the connection between above ground and below ground nutrient cycles (Simard and Austin 2010). EMF, Nitrogen & Phosphorus Plant uptake of nitrogen, a limiting soil nutrient in many temperate forest ecosystems, can be enhanced via EMF colonization (Finlay et al. 1992; Smith and Read 2008). Rygiewitz et al. (1984) found that Douglas-fir, Sitka spruce (Picea sitchensis (Bong.) Carr.) and western hemlock (Tsuga heterophylla (Raf.) Sarg.) had increased ammonium uptake when colonized by Hebeloma crustuliniforme (Bull. ex St. Amans.) Quél. in comparison to non-mycorrhizal trees. Most EMF can utilize inorganic (nitrate and ammonium), organic, or both sources of nitrogen, however, different taxa have varying abilities in regards to the forms of nitrogen they best utilize (Finlay et al. 1988; Finlay et al. 1992). Finlay et al. (1992) investigated the abilities of ten EMF taxa to utilize various forms of inorganic nitrogen in pure culture and found that they all grew well on ammonium and less well on nitrate, despite variability in strain response. However, 11 Hebeloma crustuliniforme, Laccaria proxima (Boud.) Pat., and Paxillus involutus (Batsch) Fr. grew only slightly less well on nitrate in comparison to ammonium (Finlay et al. 1992). Finlay et al. (1988) investigated the uptake of ammonium specifically, and allowed mycelium of Paxillus involutus, Pisolithus arhizus (Scop.) Rauschert (prev. tinctorius), Rhizopogon roseolus (Corda) Th. Fr. and Suillus bovinus (L.) Roussel, growing in association with Pinus sylvestris L., to access peat sources labeled with 15N ammonium. They showed that labeled glutamate/glutamine and aspartate/asparagine were present in significant amounts in every fungus species except for P. involutus (Finlay et al. 1988). Bending and Read (1995) investigating organic N uptake, performed a similar experiment, and found that when mycelium from Suillus bovinus and Thelephora terrestris Ehrh., in association with Pinus sylvestris, colonized organic soil layers from a pine forest, the organic nitrogen content of the soil decreased significantly for both fungi in comparison to controls. However, it seemed that S. bovinus was able to utilize more organic nitrogen than T. terrestris (Bending and Read 1995). Piloderma species have been found growing in dense and extensive mycelial mats in organic soil horizons where they alter local nutrient uptake (Kluber et al. 2010; Kluber et al. 2011; Zeglin et al. 2012; Phillips et al. 2012). Kluber et al. (2011) found that Piloderma mats maintained greater concentrations of a chitin degrading enzyme, providing greater access to organic N, than non-mat soils throughout the year. In a similar study, Kluber et al. (2010) found that mycorrhizal mats, some formed by Piloderma sp., in organic soil horizons and in mineral soils, maintained greater levels of chitinase, pheneloxidase and phosphatase in comparison to non-mat soils. Interestingly, the mats found in organic layers were comprised of different fungal species and had different enzyme expression than mats found in mineral layers (Kluber et al. 2010). Additionally, Phillips et al. (2012) investigated respiration rates of Piloderma mycorrhizal mats and found that respiration was increased by an average of 16% in comparison to non-mat soils and that enzyme levels were positively correlated with respiration rates in mat soils. EMF have the capacity to alter nitrogen nutrition, but soil nitrogen levels can also impact EMF community richness, structure and composition (Lilleskov et al. 2002; Avolio 12 et al. 2009; Kranabetter et al. 2009a; b; LeDuc et al. 2013). For instance, increases in inorganic soil nitrogen caused a decrease in fungal diversity in a white spruce (Picea glauca (Moench) Voss) forest in Alaska (Lilleskov et al. 2002). The decrease in fungal diversity was likely because the spruce trees had a surplus of nitrogen and began to invest in mycorrhizal fungi that were less specialized at nitrogen uptake (Lilleskov et al. 2002). However, nitrogen deposition (gaseous ammonia) was exaggerated in the study area due to anthropogenic factors (Lilleskov et al. 2002). In a forest with a natural nitrogen gradient, Kranabetter et al. (2009b) found that EMF richness of fruiting bodies first increased and then decreased as combined soil inorganic and organic nitrogen increased from 10 to 60 kg ha-1 and that different EMF species had different tolerances for amounts and types of soil nitrogen. Additionally, at the same study site, Kranabetter et al. (2009a) found that EMF detected belowground increased in richness along a foliar nitrogen gradient of 9 to 15 g kg-1 while individual species differed in percent colonization of root tips. Similarly, LeDuc et al. (2013) investigated EMF community shifts along a fifty-one year chronosequence after stand-replacing fire in jack pine (Pinus banksiana Lamb.) dominated stands in Michigan. They found that the community shifted from one dominated by Suillus brevipes (Peck) Kuntze and Thelephora terrestris towards one of greater complexity (increases of Clavulina J. Schröt., Cortinarius (Pers.) Gray, and Russula Pers. taxa) over time and as organic nitrogen and amino acid nitrogen increased; however the mechanism behind this relationship was unclear (LeDuc et al. 2013). It seems that the type of nitrogen added to a system and host tree species may also play key roles in EMF response as Avolio et al. (2009) found that additions of organic nitrogen to oak seedlings actually increased total root colonization in comparison to controls whereas mixed results were seen on pine seedlings receiving organic nitrogen inputs. Phosphorus (P) is also a key nutrient for plant growth and like nitrogen, can be a limiting soil nutrient (Attiwill and Adams 1993; Plassard and Dell 2010; Binkley and Fisher 2013). Generally plants and mycorrhiza solubilize and take up inorganic forms of phosphorus (Pi) such as orthophosphates (Smith and Read 2008; Plassard and Dell 2010) but many ectomycorrhizal fungi have the capacity to take up organic forms of phosphorus 13 (Po) as well (Dighton 1991; Cairney 2011). EMF fungi can take up Pi in solution via specific P transporters (Tatry et al. 2009) or by exuding low weight molecular acids that weather soil minerals and solubilize soil Pi (Barker et al. 1998; Landeweert et al. 2001; Hagerberg et al. 2003; Plassard and Dell 2010). They utilize P from organic phosphorus (Po) sources by secreting phosphatases into the soil that act to free Po from organic complexes (Perez-Moreno and Read 2000; Courty et al. 2006; Plassard and Dell 2010). This capacity for mycorrhizal fungi to improve plant P nutrition (Chalot et al. 2002; Plassard and Dell 2010) can be essential in soils influenced by volcanic ash, since phosphorus sources are often bound to ash surfaces (Appelt et al. 1975; Andregg 1988; Page-Dumroese 2007). Different EMF taxa have different capacities for both organic and inorganic P uptake (Wallander 2000; Courty et al. 2005; Cairney 2011). Taxa in several genera (Cortinarius, Hebeloma, Lactarius, Paxillus, Piloderma, Pisolithus and Suillus) release significant amounts of low weight molecular acids (Courty 2010, Plassard and Dell 2010), central in mineral weathering processes (Drever and Stillings 1997) that free Pi. Specifically, Wallander (2000) found in a pot experiment that a strain of Suillus variegatus (Sw.) Kuntze and an unidentified fungus positively influenced P nutrition in Pinus sylvestris seedlings when apatite was the only source of P. Mahmood et al. (2002) found that a Piloderma species may have been important for Pi acquisition from granulated wood ash based on the frequency of granule colonization in a fertilization study in a Norway spruce (Picea abies (L.) Karst.) stand in Sweden. As for utilization of Po sources, Courty et al. (2006) found that three dominant EMF (Lactarius quietus (Fr.) Fr., Cortinarius anomalus (Fr.) Fr., Xerocomus chrysenteron (Bull.) Quél.) in an oak (Quercus spp. L.) forest in France followed similar trends in phosphatase production throughout the year. In a similar study, Courty et al. (2005) investigated enzymatic activity of ten EMF taxa at different soil layers in an oak forest and found that many taxa displayed differential enzymatic activity depending on soil layer. Interestingly, Lactarius quietus (Fr.) Fr. showed increased phosphatase moving from the A1 to the A2 layer, while Cortinarius olivaceofuscus Kühner showed a decrease in phosphatase activity from A1 to A2 (Courty 14 et al. 2005). From a community stand point, Baxter and Dighton (2005) found that increased EMF diversity positively improved Po uptake by Pinus rigida P. Mill. seedlings, but that the EMF community composition did not impact P uptake. Growth, Reproduction, Molecular Advances Ectomycorrhizal host plants generally dominate the forests of boreal and temperate areas like the Pacific Northwest (Smith and Read 2008). In central Oregon the forests are mainly composed of coniferous ECM forming hosts and often maintain components of understory trees, which associate with EMF (Simpson 2007; van der Heijden et al. 2015). The majority of EMF that reproduce sexually do so by forming epigeous or hypogeous fruiting bodies that release spores. New ECM colonization can come about via spore germination as well as by colonization by extra-radical mycelium from other plant roots (Baar et al. 1999; Horton et al. 1999; Bruns et al. 2002a; Peay et al. 2009). Some EMF are not known to produce a fruiting body and tend to multiply via asexual means, like Cenococcum geophilum Fr. which tends to spread via sclerotia (Massicotte et al. 1992). Molecular methods for identifying fungal species from ECM plant root tips became prevalent in the mid-1990s and revolutionized the understanding of belowground mycorrhizal communities (Smith and Read 2008). Prior to this time, estimates of EMF diversity from sporocarp studies were useful for understanding food web functions and for comparison of current with historic studies to identify trends in fungal communities, but they incompletely documented diversity (Smith et al. 2002). Gardes and Bruns (1996) sampled EMF sporocarps found above-ground and ECM root tips below-ground in a bishop pine (Pinus muricata D. Don) stand for four years and found that sporocarps were not adequately representative of the belowground EMF community. However, while we now know that many trees host a great diversity of EMF we are still only scratching the surface of how these fungal communities function and the role of each fungus within an ecosystem. In a pioneering continent-wide study, Talbot et al. (2014) found that many 15 EMF perform similar roles across ecosystems and that functional convergence was common in North America. Common Mycorrhizal Networks An EMF individual can colonize more than one host, and because many plants in PNW forests are ECM forming, high probability exists for extensive EMF connections among plants (Amaranthus and Perry 1994; Simard 1997; Smith and Read 2008). Common mycorrhizal networks are important infrastructures that contribute to below and aboveground community success. Simard et al. (1997a) found that labeled carbon could travel bi-directionally between the hosts Betula papyrifera Marsh. and Douglas-fir via an established mycorrhizal network. Another function of CMNs is possibly connecting larger trees with seedlings or germinants that would provide them with mycorrhizal innoculum and may be important for resource sharing (Teste et al. 2009). Teste et al. (2009) investigated this possibility in a dry Douglas-fir forest in Canada and found that CMNs were important for nutrient transfer and survival of germinated seedlings. In addition to positive effects from nutrient transfers, Bingham and Simard (2011) found that laboratory seedlings placed under drought stress had a greater survival rate when connected via a CMN to another seedling that had access to adequate water, than those not connected to CMNs. Beiler et al. (2010) did not investigate water or nutrient movement via CMN, but in a mapping study found that mycorrhizal networks formed by Rhizopogon spp. in a Douglas-fir forest were extensive and served to promote new Douglas-fir seedling establishment and development. Aside from nutrient and water transfer and facilitation of regeneration, CMNs may play a role in plant responses to pathogens as it has been shown that CMNs comprised of AM fungi can facilitate transport of signals from infected plants to non-infected plants (Song et al. 2010; Babikova 2013). Song et al. (2010) facilitated formation of a CMN between pairs of tomato plants (Lycopersicon esculentum Mill.) with Glomus mosseae (T.H. Nicolson & Gerd.) Gerd. & Trappe and when a pathogen was introduced onto one tomato plant, genes for defensive enzymes were upreglated in the 16 other. Similar results were reported with bean plants (Vicia faba L.) connected by a CMN and attacked by aphids (Acyrthosiphon pisum Harris). The function, frequency, and extent of these networks continues to be explored, however there is evidence that they are essential to forest ecosystems. Generalists & Specialists Two categories of EMF as far as host associations are concerned are generalists and specialists. Generalist fungi can associate with many different plant species, and specialists associate with a single species or genus (Molina et al. 1992). One exception to specialization is in cases of epiparisitsm where fungi that would be host-specialists, are also associated with achlorophylous heterotrophic (Monotropoideae, Epidendroideae, Orchidoideae, Vanilloideae) plants that utilize carbon transferred from associated autotrophic plants for all or part of their lives (Luoma 1987; Smith and Read 2008). An example of this is Rhizopogon subcaerulescens A.H. Sm., which is specific to Pine hosts, but is the only fungus known to associate with Pterospora andromedea Nutt., a myco­ heterotropic plant in the Monotropoideae (Leake 1994). However, in general, CMNs between dissimilar host species would be comprised of generalist fungi. One of the ecological advantages of having generalist fungi in an EMF community is that if there are disturbances that damage one forest patch or particular host tree then sufficient fungal innoculum may be present on the roots of nearby trees for the recolonization of newly germinating seedlings (Hagerman et al. 1999; Kranabetter et al. 1999; Nara and Hogetsu 2004). Chinquapin Biology & Ecology Chinquapin ranges from west-central Washington south to northern California, however it is most prevalent in Oregon and California (McKee 1990; Niemiec et al. 1995; 17 Meyer 2012). There are two growth forms of chinquapin, a tree form and a shrub form (McKee 1990; Niemiec et al. 1995). Generally, neither growth form occurs in pure stands, but is instead often found mixed with conifers (McKee 1990; Niemiec et al. 1995). The tree form tends to grow on sites with more precipitation than sites where the shrub form is found and it is generally found from Lane County, OR south to Marin County, CA (McKee 1990). The shrub form of chinquapin can grow in harsh conditions and is often found at high elevations and dry sites with rocky soils, such as parts of the Cascade Range and just east of the Cascade Range in central Oregon (McKee 1990; Niemiec et al. 1995). Chinquapin flowers from June to midwinter (McDonald 2008) and produces mature seed in the fall two years later (Neimiec et al. 1995; Baldwin et al. 2012). It is evergreen, sclerophyllous and relatively shade tolerant (Keeler-Wolf 1988; Neimiec et al. 1995; Hunter 1997). Chinquapin rooting habits have not been studied in detail, however it has been reported that they initially develop a deep taproot and then, as they age, further develop a spreading lateral root system (Neimeic et al. 1995). Economic Significance The tree form of chinquapin can be used for furniture making or paneling, however it is not often cultivated for this purpose as low volumes from mixed stands are generally not worth milling (McKee 1990; Niemiec et al. 1995). Whereas the wood machines and sands easily, it can be difficult to work with if not air-dried prior to kiln drying because of its propensity to check (Niemiec et al. 1995). Cultural Significance Chinquapin nuts and sometimes leaves have been eaten or used as tea by at least seven indigenous tribes in the PNW (Coville 1897; Chesnut 1902; Schenck and Gifford 1952; Mahar 1953; Gifford 1967; Baker 1981). The Southwestern Pomo people of the Sonoma County area of California purportedly ate chinquapin nuts when they were available, however the nuts were recorded as being from the tall coastal tree growth form 18 of chinquapin (Gifford 1967). Chesnut (1902) recorded that chinquapin nuts were also occasionally eaten by the Native Americans in Mendocino County, California, but were reportedly eaten more frequently by Native Americans further north. An example is the more northerly Klamath, Yurok and Tolowa tribes eating the nuts (Coville 1897; Baker 1981). Additionally, it seems the Paiute people of the Warm Springs Indian Reservation used the leaves for tea (Mahar 1953). The Karok tribe in Humboldt and Siskiyou Counties, California, also ate the nuts, but nuts from tanoak (Notholithocarpus densiflorus (Hook. & Arn.) Manos, Cannon & S.H. Oh) were preferred (Schenck and Gifford 1952). Ecosystem Services Chinquapin in the central Oregon forests is important for a variety of ecosystem services. It recovers quickly from damage and is useful for ameliorating effects of erosion in watersheds after fire disturbance (McKee 1990; Meyer 2012). Additionally, it is one of the only mid-level trees on Lookout Mountain to provide mast for small mammals, birds and insects (McDonald et al. 1983; McKee 1990; Neimeic et al. 1995). It is also one of the few hosts of the golden hairstreak butterfly (Habrodais grunus Boisduval) (Shoal 2009). Chinquapin provides cover for birds and small mammals. On the west side of the Oregon Cascades sightings of Red-breasted nuthatch (Sitta canadensis Linnaeus), Empidonax flycatchers (dusky (E. oberholseri Phillips or Hammond’s (E. hammondii Xantus de Vesey)), and Pine siskin (Carduelis pinus A. Wilson) were positively associated with small and medium sized chinquapin trees (Gilbert and Allwine 1991b). Presence of red tree voles (Arborimus longicaudus True) and Pacific shrews (Sorex pacificusi Coues) was positively associated with chinquapin as well (Gilbert and Allwine 1991a). Chinquapin & Fire Chinquapin, with its ability to regenerate from root crowns, burls and root sprouts (Kauffman 1986), can generally withstand fires (McKee 1990, Kauffman and Martin 1990; Niemiec et al. 1995; McDonald 2008). Chinquapin’s aboveground vegetation tends to die in fire, causing basal diameter, crown volume, cover and aboveground biomass to decrease 19 after fire, but vigorous spouting, especially of larger individuals occurs within 2 seasons (Kauffman and Martin 1990; Donato et al. 2009). Kauffman and Martin (1990) found that on average 27% to 78% of chinquapin individuals exposed to prescribed burns survived and resprouted, depending on the season of the burn (early fall vs. early spring). Donato et al. (2009) showed that larger trees were more likely to survive and resprout after burning. Kauffman (1986) found that chinquapin possesses root meristematic tissue in the mineral soil that would partially account for its regenerating capabilities. Despite the evidence that chinquapin can survive and flourish after forest fire, there are conflicting results about how well chinquapin recovers after severe fire. Donato et al. (2009) report that chinquapin survived and showed no change in resprouting ability after two severe forest fires (occurring within 15 years of each other) compared to its respouting ability after one severe forest fire in the Klamath-Siskiyou Mountains in Oregon. In contrast, Halpern and Spies (1995) report that chinquapin in plots in the H. J. Andrews Experimental Forest in Oregon that were logged and heavily burned in 1963, did not resprout or recolonize the site even up to 27 years later. These contrasting results may be because different environmental factors affecting chinquapin’s tolerance to fire. For example, Kauffman and Martin (1990) found that fuel consumption, season, shrub size, and growth stage all impacted shrub survival after prescribed fires in the Sierra Nevada. Competition Chinquapin is controlled in plantations to prevent water, nutrient, or shade induced stress that negatively affects conifer seedling growth (McKee 1990; Nambiar and Sands 1993; Zhang et al. 2006). However, Keys and Maguire (2005) synthesized results from three studies and concluded that initial shrub cover in ponderosa pine stands facilitates pine germinant survival for the first one or two summers of growth. Thus, although reasons to control chinquapin in silvicultural situations are valid, reasons to retain it include providing valuable mast and cover for small mammals and birds (McKee 1990), enhancing survival 20 of some Pinaceae germinants (Keyes and Maguire 2005), and potentially supporting mycorrhizal communities on which Pinaceae species depend (Kauffman and Martin 1990). Chinquapin Ectomycorrhiza Investigation of chinquapin EMF communities in the Pacific Northwest is rudimentary, although it has been reported to be a host for matsutake (Tricholoma magnilevare (Peck) Redhead) (Lefevre 2002). However, because chinquapin is phylogenetically related to old-world Lithocarpus Blume, old and new world Quercus L., Castanea Mill. in the northern hemisphere, Notholithocarpus Manos, Cannon & S.H. Oh, and Asian Castanopsis (D. Don) Spatch (Manos et al. 2008, Oh & Manos 2008), we hypothesized that the EMF communities on chinquapin might resemble those on species found in these genera. Unfortunately, detailed studies of the mycorrhizal associations of southeast Asian Lithocarpus pachylepis A. Camus and Lithocarpus xylocarpus (Kurz) Markgraf, chinquapin’s nearest phylogenetic neighbors (Manos et al. 2008), have not yet been undertaken. However, there have been studies investigating ECM communities on Castanopsis fargesii Franchet in southwest China (Wang et al. 2011), a Castanopsis forest in central Nepal (Christensen 2009), Quercus liaotungensis Koidz. in northern China (Wang et al. 2012), Quercus garryana Dougl. ex Hook. in southern Oregon (Valentine et al. 2004), Notholithocarpus densiflorus in northern California (Bergemann and Garbelotto 2006), and on Castanea dentata (Marshall) Borkh. in western Wisconsin and Ohio (Palmer et al. 2008; Bauman 2010). Lactarius Pers., Russula Pers., Tomentalla G.H. Cunn., Boletus L., and Scleroderma Pers. were found with oaks in the compared literature almost ubiquitously (Table 1). On average, 14 genera were found associated with these oaks, with a minimum of six being found on Castanopsis fargessii and a maximum of 35 being found on Notholithocarpus densiflorus (Table 1). The oaks investigated had an average of 6 unique genera, with a minimum of zero and a maximum of 22. Differences in methods for EMF identification or study location were not taken into account in this summary. 21 Pinaceae Pacific Northwest Genera and Species Coniferous trees in the Pinaceae dominate the forests of the PNW, including those of central Oregon (Simpson 2007). This family encompasses many economically, ecologically, and socially valuable trees (Campbell et al. 2002; Campbell et al. 2003). Six Pinaceae genera are represented in Oregon’s forests (fir (Abies Mill.), larch (Larix Mill.), spruce (Picea A. Dietrich), pine (Pinus L.), Douglas-fir (Pseudotsuga Carr.), and hemlock (Tsuga (Endlicher) Carr.)) (Campbell et al. 2002; Campbell et al. 2003). Douglas-fir dominates most of the forests in western Oregon (Campbell et al. 2003). However, significant percentages of hemlocks, firs, and pines inhabit western forests as well (Campbell et al. 2002). Central and eastern Oregon forests are often dominated by ponderosa pine but also maintain other Pinaceae genera (Campbell et al. 2003). In Oregon some of the most economically and ecologically valuable trees in the Pinaceae are Douglas-fir and ponderosa pine (Lowerey 1984; Gale et al. 2012). Economic Significance The logging industry in Oregon was built around fast growing conifer trees, particularly Douglas-fir, hemlocks, and pines such as ponderosa pine (Lowerey 1984; Gale et al. 2012). These giants provided for generations of individuals and a thriving logging industry (Graham and Jain 2005; Andrews and Kutara 2005). Oregon has been, and continues to be a top producer of U.S. timber (Andrews and Kutara 2005; Gale et al. 2012). The logging industry took several economic hits in the past two to three decades, due to changes in Federal land regulation and the economic downturn in the mid 2000’s (Gale et al. 2012). In 2007 Oregon’s timber production started to decline, reaching a low point in 2009 and reports showed that by 2010 the industry had lost approximately 14,400 jobs and $527 million since the downturn (Gale et al. 2012; Oregon 2015). However, timber production rebounded and reached pre-recession levels by 2013 (Oregon 2015). Despite variability in timber harvests, the standing timber volume of Oregon’s forests in 22 2010 remained unchanged from the volume available in 1953 (Gale et al. 2012). The industry has undergone major operational changes, but continues to play a large economic role in the state providing for 76,000 jobs statewide and generating $5.2 billion in revenue per year (Gale et al. 2012). This figure represents 11% of the statewide economic base (Gale et al. 2012). Harvests have declined in federally owned forest lands throughout Oregon in the past 20 to 30 years due to changes in federal land management and listing of threatened species such as the northern spotted owl (Strix occidentalis caurina (Xantus de Vesey) and the marbled murrelet (Brachyramphus marmoratus (Gmelin)) (Gale et al. 2012). Specifically, harvests of pine from federally owned forests in Oregon have declined from 18% of the harvest in the 1980’s to only 4% in 2008, likely due to reduced harvest of pondersoa pine in central and eastern Oregon (Gale et al. 2012). This transition is likely because federal agencies have shifted towards multiple-objective management of forests including: timber production, wildlife habitat, ecosystem services, and recreation (Hessel 1988). It is recognized that central Oregon’s pine dominated forests are an important region of the PNW forests aside from timber harvests as they are responsible for storing at least 33kg m-2 of carbon (Birdsey 1992; Turner et al. 1995; Smithwick et al. 2002), generating state recreational revenue (Loomis 2005) as well as being important habitat for wildlife (Gilbert and Allwine 1991a; b; Woodward 2011). Pinaceae Range & Biology The most abundant Pinaceae species found in the study site on Lookout Mountain are ponderosa pine, lodgepole pine, western white pine, and grand fir. Despite all being in the same family, each of these species is unique and occupies a different niche within forest ecosystems (Burns et al. 1990). Below are summaries of each species range and biology. 23 Ponderosa pine (Pinus ponderosa Dougl. ex Laws.) Ponderosa pine is a three needle pine, (sometimes having only 2 needle fascicles depending on race), that ranges from Canada south to Mexico and east to Nebraska from the Pacific Ocean (Oliver and Ryker 1990). Ponderosa pine is known to be competitive on relatively dry sites (Oliver and Ryker 1990). Ponderosa pine’s drought tolerance partially stems from the ability to increase transpiration rates when heat stressed due to its deep root system (Kolb and Robberecht 1996). Ponderosa pine seedlings invest early in growing a deep taproot (Berndt and Gibbons 1958; Larson 1963). Roots of mature trees can grow to depths of more than two meters in loose soils or even down to 12 m if there are fissures in the bedrock (Oliver and Ryker 1990). Additionally, they produce a large lateral root system that can extend out past the crown in open stands, but is less extensive in dense stands (Oliver and Ryker 1990). Ponderosa pine also tolerates lower soil nutrient levels and requires lower levels of foliar N and P than some other conifers (Oliver and Ryker 1990). Ponderosa pine is also highly resistant to fire due to its thick bark and ability to survive even after fifty percent of its crown has been singed (Oliver and Ryker 1990). This resistance has helped it retain dominance, despite shade intolerance, in some areas due to the lesser ability of some other conifers to withstand fires (Oliver and Ryker 1990). Historically one of the major timber producing species of the western United States, Ponderosa pine has been used to make doors, paneling, moldings, poles, posts, plywood, pulp and for a myriad of other uses. (Lowerey 1984). Lodgepole pine (Pinus contorta Dougl. ex Loud.) Lodgepole pine is a two needle pine found from the Yukon Territory of Canada south to Baja California and east to the Black Hills of South Dakota from the Pacific Ocean (Lotan and Critchfield 1990). On Lookout Mountain, lodgepole pine is found mainly at the lower and mid elevations (Results Section). In general lodgepole pine grows better on moister soils than ponderosa pine, however it thrives in a variety of different 24 environmental conditions and is capable of growing in relatively infertile soils (Horton 1956; Lotan and Critchfield 1990). Root systems develop more slowly and are generally shallower than those of ponderosa pine (Lotan and Critchfield 1990). A first year seedling’s root system is generally shallow and lacks a deep taproot (Noble 1979). Root systems also tend to be fairly shallow in rocky areas (Lotan and Critchfield 1990). Lodgepole pine is often found on andisolic soils, such as those on Lookout Mountain, throughout their range in central Oregon (Lotan and Critchfield 1990). Similar to ponderosa pine, lodgepole pine is shade intolerant (Lotan and Critchfield 1990). However, lodgepole pine is not as fire tolerant as ponderosa pine due to thinner bark (Lotan and Critchfield 1990). The species adapts to fire via quick regeneration post fire from serrotinous cones (cones that open with heat) (Lotan and Critchfield 1990). However, the degree of serotiny in lodgepole stands can vary based on previous fire history and genetics (Lotan 1967; 1976; Parchman et al. 2012). Lodgepole pine wood is used for framing, paneling, posts, poles, railroad ties and wood pulp (Lotan and Critchfield 1990). Western white pine (Pinus monticola Dougl. ex D. Don) Western white pine is a five needle pine that ranges from southern British Columbia south along the Cascade Range into California (Graham 1990). Additionally, a separate population occurs in northern Idaho and extends into southern Canada (Graham 1990). On Lookout Mountain western white pine is found only at high elevations (Results Section). Throughout its range it is found mainly in relatively moist areas like streambeds and northern slopes (Graham 1990). Western white pine develops a relatively shallow root system with the bulk of absorptive mass located in the top 65cm of soil (Graham 1990). Additionally, development of roots and shoots is slow in seedlings, and despite being relatively shade tolerant, development is slower when they are shaded (Graham 1990). This species tends to be present in mixed stands and is often seral and non-dominant (Graham 1990). 25 Western white pine is less fire resistant than ponderosa pine, due to thinner bark and relatively flammable foliage (Starker 1934; Graham 1990). However, as a seral species, it depends on fire to reduce competition for establishment (Graham 1990). Western white pine timber is used for specialty work like trims, moldings and cabinets due to its non-resinous nature and more generally for plywood (Graham 1990). Grand fir (Abies grandis (Dougl. ex D. Don) Lindl.) Grand fir is found from southern British Colombia, Canada south to the Pacific coast in northern California (Foiles et al. 1990). It is prevalent west of and throughout the Cascades in Washington and Oregon (Foiles et al. 1990). Additionally, a large population of the subvariety, A. grandis var. idahoensis Silba, is present in northern Idaho extending north into southern Canada (Silba 1990; Foiles et al. 1990). Grand fir is generally found with other conifers, and on moist sites, it can be a competitive component of the overstory (Foiles et al. 1990). In the Oregon pumice zone this species can grow well on shallow and exposed mountain ridges as long as there is enough moisture (Foiles et al. 1990). When growing in drier areas, grand fir compensates by growing a deep root system and can be fairly resistant to drought and heat injury (Foiles et al. 1990). In seedlings, deep taproots tend to develop much faster if grown in full sun (Foiles et al. 1990). While grand fir can be shade tolerant, seedlings are initially more susceptible to drought when grown in shade due to their shallower root systems (Foiles et al. 1990). When grand fir is found on dry sites, it tends to be more fire resistant than individuals grown in mesic areas (Foiles et al. 1990). This fire resistance is due to the development of thicker bark and deeper roots in dry areas (Foiles et al. 1990). Generally, since grand fir is a soft wood, its industrial uses are limited to wood pulp production, however it is also cultivated for use as Christmas trees (Foiles et al. 1990). 26 Summary The Lookout Mountain unit of the PFEF is an ideal location for the study of Pinaceae and chinquapin EMF communities. The area has been extensively studied as a result of its incorporation into the PFEF (Youngblood 1995) and its disturbance history is well known (Youngblood 2009). The PFEF is representative of other volcanically influenced Pinaceae dominated forests in central Oregon. Because PFEF is an area prone to frequent fire (Adams et al. 2004), and possibly stand replacing fire due to climate change (Westerling et al. 2006) and fuel accumulation (Youngblood 2009), makes the investigation of chinquapin as a possible EMF refuge plant viable and relevant for this location. 27 Table 1. Comparisons of EMF genera found associated with Pinaceae and chinquapin to genera found on hosts phylogenetically related to chinquapin. Host species abbreviations and reviewed literature citations below table. Fungal Genus Amanita Amphinema Byssocorticium Cenococcum Cortinarius Elaphomyces Gautieria Hygrophorus Hysterangium Inocybe Lactarius Leucogaster Leucophleps Lyophyllum Melanogaster Phellodon Phialocephala Piloderma Pseudotomentella Ramaria Rhizopogon Russula Pinaceae Chinq. CADA a & b CAFAc CAfor.d NODEe QUGA f POg QULI h QUsp.a QUsp.i RT RT RT RT FB RT RT FB & RT RT RT RT * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * 28 Table 1 (Continued) Sebacina Sistotrema Suillus Thelephora Tomentella Tomentellopsis Tricholoma Wilcoxina Alpova Antrodiella Astraeus Aureoboletus Austroboletus Balsamia Bankera Barssia Boletellus Boletinus Boletopsis Boletus Brauniellula Cadophora Camarophyllus Cantharellus Capronia Ceratobasidium Pinaceae * * * * * * * * Chinq. * CADA a & b * CAFAc CAfor.d * * * * * NODEe * QUGA f POg * QULI h * QUsp.a QUsp.i *^ * * * * * * *^ * * * * * * * * * * * * * * * * * * * * * * * * 29 Table 1 (Continued) Pinaceae Chalara Chalciporus Choiromyces Chromelosporium Chroogomphus Clavulina Coltricia Cortinomyces Craterellus Cryptococcus Dentinum Destuntzia Divide Endogone Entoloma Galiella Genabea Genea Geoglossum Geopora Gomphidius Gomphus Gymnomyces Gyroporus Hebeloma Helvella Humeria Chinq. CADA a & b CAFAc CAfor.d NODEe * QUGA f POg QULI h Qusp.a Qusp.i * * * * * * * * * * * * * * * * * * * * * 30 Table 1 (Continued) Pinaceae Hydnellum Hydnoplicata Hydnotrya Hydnotryopsis Hydnum Hymenogaster Laccaria Lachnum Leccinum Lyophyllum Macowanites Marasmius Neonectria Pachyphloeus Peziza Phialocephela Phialophora Phylloporus Pisolithus Scleroderma Tarzetta Tricholoma Tuber Chinq. CADA a & b CAFAc CAfor.d NODEe * QUGA f POg QULI h QUsp.a QUsp.i * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * 31 Table 1 (Continued) Pinaceae Tylopilus Xerocomus Chinq. CADA a & b CAFAc CAfor.d * NODEe QUGA f * POg * QULI h QUsp .a QUsp.i * ^Sebacinales & Thelephoraceae found CADA = Castanea dentate, CAFA = Castanopsis fargesii, CAfor. = Castanopsis forest, NODE =Notholithocarpus densiflorus, QUGA = Quercus garryanna, PO = Pine-Oak, QULI = Quercus liaotungensis, QUsp. = Quercus spp. a Palmer et al. 2008, bBauman 2010, cWang et al. 2011, dChristensen 2009, eBergemann and Garbelotto 2006, fValentine et al. 2004, gDokmai et al. 2015, hWang et al. 2012, iMorris et al. 2009 32 STUDY MANUSCRIPT Introduction The forests of the western United States support recreational pursuits (Loomis 2005), provide vital wildlife habitat (Woodward et al. 2011), sequester carbon (Smithwick et al. 2002) and sustain a timber industry (Gale et al. 2012). With climate change occurring, these forests need to be adaptable and resilient if they are to thrive. Resilience is the capacity for an ecosystem to accommodate change while still retaining its core characteristics and function (Walker and Salt 2006). Several climate models predict warmer and drier summers and wetter winters in the Pacific Northwest (Mote and Salathé 2010). These changes will likely alter regional fire regimes, defined as the interactions among fire frequency, size, intensity, severity, type, and seasonality (Flannigan et al. 2000; Dale et al. 2001; Hardy et al. 2001; Rogers et al. 2011). One potential way to achieve resiliency in these forests is to ensure that mycorrhizal fungus communities, belowground partners to trees, are maintained in the probable increases in disturbance from fire. Mycorrhizal fungi form a mutually beneficial, obligatory symbiosis with most tree species. The fungus obtains soil nutrients and water for the tree and the tree provides simple sugars for the fungus (Smith and Read 2008). In the Pacific Northwest, trees in the Pinaceae dominate the forests and form a type of mycorrhiza called ectomycorrhiza. The fungi that participate in these relationships are called ectomycorrhizal fungi (EMF). More than 6,000 fungal species form ectomycorrhizal associations (Brundrett et al. 2002). It is well known that many trees, including the trees in the Pinaceae, would be untenable without their belowground EMF partners (Smith and Read 2008). Therefore, the health of these forests, and the industries that rely on them, rests partially upon the functionality of the EMF belowground community. Many Oregon forests are managed for timber production (Gale 2012) as well as recreation (Loomis 2005). Historically, in order to preserve forest resources, fire has been suppressed causing a build-up of fuels (Review 2001; Sommers et al. 2011; Rogers et al. 33 2011). The increased fuel load combined with the hotter and drier summers predicted by climate change models will likely cause an increase in large and severe fires (Rogers et al. 2011). These changes may already be occurring; Kasischke and Stocks (2000) report that the land area being burned annually in the United States has increased by three times from 1970 to 2000. For Pacific Northwest forests specifically, Rogers et al. (2011) estimate that area burned by fire in the next 100 years will increase from 76% to 310% with an increase in both burn intensity and severity. Severe fires can have a negative impact on the diversity of EMF communities and the recovery of complex EMF communities can be a long-term process. Stand replacing fires remove or kill the majority of above ground vegetation, significantly changing the vegetation structure (Sommers et al. 2011). As the ectomycorrhizal relationship is generally obligatory for both the fungal partner and the plant host, damage or death of the host from fire or other disturbances can also lead to decreased diversity in the EMF communities necessary for future tree growth and health (Barker et al. 2013). EMF communities can recolonize a site from heat-resistant soil spores (Baar et al. 1999; Peay et al. 2009), windborne spores (Bruns et al. 2002b), or from mycelium adjacent to EMF hosts (Horton et al. 1999). Depending on environmental factors, it can take at least a decade for a community to fully recover (Treseder et al. 2004). For instance, Treseder et al. (2004) found that it took up to 15 years for EMF communities to return to pre-burn levels of colonization in an Alaskan forest after severe fire. In contrast, Barker et al. (2013) reported that Douglas-fir seedlings planted in a low severity and high severity burn site were fully colonized by EMF after just one year. Although the seedlings were fully colonized after a year, the number of frequently occurring EMF taxa decreased at the high severity site, with only Wilcoxina and Rhizopogon species dominating the community (Barker et al. 2013). Typically, EMF form mycorrhizae with more than one host species within an ecosystem (Molina and Trappe 1982a; Molina et al. 1992; Simard et al. 1997b; Kennedy et al. 2003) and plants of the same or different species can be linked by their EMF hyphae (Molina and Trappe 1982b, Amaranthus and Perry 1994; Simard 2009). Therefore, an EMF community may be maintained through a disturbance event by refuge plants, species 34 of ectomycorrhizal plants that persist after disturbance, typically by resprouting (Baar et al. 1999; Hagerman et al. 2001). Hagerman et al. (2001) found that manzanita (Arctostaphylos uva-ursi (L.) Spreng.) maintained a similar mycorrhizal community to the overstory Douglas-fir (Pseudotsuga menziesii (Mirb.) Franco) even three years after the Douglas-fir had been harvested. In a similar study, Nara and Hogetsu (2004) found that a shrubby willow (Salix) pioneer species, which forms ectomycorrhizal associations, acted to facilitate fungal inoculation of birch (Betula) and larch (Larix) species in a primary succession area of a volcanic desert in Japan. Giant chinquapin (Chrysolepis chrysophylla (Douglas ex Hook.) Hjelmq.) is one possible refuge plant found in the mixed conifer forests of central Oregon. Giant chinquapin (henceforth ‘chinquapin’) is an evergreen, sclerophyllous understory shrub that infrequently achieves small tree stature in the forests of central Oregon. It is typically viewed as competition for soil nutrients and water (Nambiar and Sands 1993) to the timber producing trees in the Pinaceae (Meyer 2012). Chinquapin contributes to fuel loads and is considered moderately flammable (Weatherspoon and Skinner 1987). It can also shade seedlings of cultivated coniferous species, reducing initial conifer growth (Meyer, 2012). Since chinquapin sprouts from stumps and roots it generally recovers quickly after cutting or fire damage (McKee 1990; Kauffman and Martin 1990; Niemiec et al. 1995; Donato et al. 2009). Due to these characteristics chinquapin is often controlled in managed forests (Meyer 2012). As an ectomycorrhizal host spieces, chinquapin may help to support the diversity of essential fungal root symbionts in an ecosystem. If Pinaceae trees and chinquapin support similar EMF communities, the quick sprouting nature of chinquapin may allow it to maintain the EMF community after disturbances that damage overstory trees such as a stand replacing fire. Resprouting chinquapin could act as a refuge plant and provide fungal innoculum and access to mycorrhizal networks for germinating or out-planted Pinaceae seedlings, making it an essential part of early ecosystem recovery. Our objective was to determine whether or not chinquapin could serve as an EMF refuge plant. We investigated the similarity of EMF communities of overstory Pinaceae 35 trees and understory chinquapin in the fire prone, mid to high elevation forests on Lookout Mountain in central Oregon. Specifically, we wanted to know if EMF communities on Pinaceae hosts and chinquapin were similarly rich (number of taxa) and diverse (Shannon’s Diversity) and if the EMF community composition was similar between hosts. We also explored the response of EMF communities to environmental variables with the hypothesis that EMF communities would be influenced by soil nutrients, specifically nitrogen and phosphorus, regardless of host. Materials & Methods Study Area We conducted this study on the Lookout Mountain unit in the Pringle Falls Experimental Forest (PFEF) within the Deschutes National Forest in central Oregon. The study site elevations range from about 1400 m to 1700 m (Deschutes National Forest 2001). The majority of the sampling locations face in an easterly direction, from 68 ° to 187 ° (Deschutes National Forest 2001). Maps of the study area can be seen in Figures 1 and 2. Climate The PFEF, located just east of the Oregon Cascade Range, generally experiences relatively dry weather with most precipitation falling as snow (Adams et al. 2004). Standing at 1900 m, Lookout Mountain receives 1020 mm of precipitation a year on average (Adams et al. 2004). High temperatures in the summer range from 21 to 32 °C and nights are cool with frost possible during the growing season (Adams et al. 2004). Vegetation The Lookout Mountain Unit of the PFEF is dominated by ponderosa pine (Pinus ponderosa Dougl. ex Laws.) at mid and lower elevations of the study site. As elevation 36 increases the forest shifts to a mixed-conifer stand maintaining: grand fir (Abies grandis (Dougl. ex D. Don) Lindl.), rocky mountain fir (A. lasiocarpa (Hook.) Nuttall), red fir (A. magnifica A. Muray), lodgepole pine (Pinus contorta Dougl. ex Loudon), sugar pine (P. lambertiana Dougl.), western white pine (P. monticola Douglas ex D. Don), ponderosa pine (P. ponderosa), Douglas-fir (Pseudotsuga menziesii), and mountain hemlock (Tsuga mertensiana (Bong.) Carr.). The forests on the mountain fall into three classifications: Pinus ponderosa/Purshia tridentata-Ceanothus velutinus (CPS-312) which is at the lowest elevations and does not support chinquapin; Mixed conifer/Ceanothus velutinus-Arctostaphylos patula (CWS-112) which is at mid-elevation and does support chinquapin; and Mixed Conifer/Ceanothus velutinus-Carex stoliferus (CWS-115) which is at the highest elevation in the study site and also supports chinquapin (Youngblood 2009, Volland 1985). Soils The soils in the area are defined as Ashy-pumiceous, glassy Xeric Vitricryands as described in the Lapine Soil Series (Soil Survey Staff 2014). The area was heavily influenced by the Mt. Mazama eruption approximately 6,600 years ago (Youngblood 1995). Mazama ash fallout accumulates up to half a meter in some areas of the PFEF (Adams et al. 2004). This ash layer adds to the andisolic nature of the soils and contributes to its excessively well-drained and nutrient poor properties (Busse and Riegel 2004; Soil Survey Staff 2014). Study Design The current study was devised as a complementary investigation to an experiment focusing on increasing resilience of the forest via thinning the overstory to different densities then masticating and underburning the groundcover (Youngblood 2009). This larger study was set up as a randomized complete block design that split Lookout Mountain into four blocks which were then each subdivided into five treatment units 37 (Youngblood 2009). On each treatment unit, a nine-hectare (ha) sampling unit, comprised of twenty-five points arranged on a 50 × 50 m grid was established (Youngblood 2009). Finally, around each point, a central circular plot (400m2) was delineated for vegetation surveys, and two parallel transects (17 m long and 22.5 m apart), one on either side of the circular vegetation plot, were established in a randomly selected cardinal direction (Youngblood 2009) (Fig. 1). As a part of this larger study, soil moisture measurements were conducted on one transect for a subset of the vegetation plots containing chinquapin. To decide which transects would be used for soil moisture measurement, central vegetation plots from a pool of all vegetation plots containing chinquapin, were chosen randomly until two vegetation plots per chinquapin containing sampling unit were chosen; then one transect of the transect pair was randomly selected. Since treatment units and sampling units were arranged on Lookout Mountain based on elevation and overstory basal area (BA) class, the selected vegetation plots and the associated pairs of transects covered the range of elevations in the study site and all but the extreme ends of the range of overstory BA. For our study of EMF we sampled the 16 pairs of transects where one transect was used for volumetric soil moisture measurement (Fig. 2). Vegetation & Soil Moisture On each transect selected for volumetric soil moisture measurment, two soil moisture tubes (PVC pipe, 5 cm diameter) were installed to a maximum depth of 75 cm and then capped. Volumetric soil moisture data used in this study were collected every two weeks during July and August 2012 with frequency domain reflectometry using a Troxler Century 200 moisture meter (Troxler Electronic Laboratories, Inc. Research Triangle Park, NC). Vegetation surveys were conducted on the central circular vegetation plots (400m2) during July and August of 2011 or during July, August, and September of 2012. Overstory and understory vascular plants were all counted and identified to species (Youngblood 2009). Percent cover for understory plants was ocularly estimated (1% resolution from 1­ 38 10% cover, 5% resolution from 11-100% cover) and the diameter at breast height (1.37m) of overstory trees was measured to the nearest 0.1 cm with a diameter tape (Youngblood 2009). Soil Sampling & Nutrients In July and August of 2012, we obtained soil and root tips for ectomycorrhizal community analysis by collecting four soil cores (10 cm depth, 5 cm diameter) (AMS, Inc., American Falls, ID) at least two meters apart on each transect. We preferentially targeted chinquapin roots and so ensured a ≥ 75% chinquapin canopy cover over the square meter of grount surrounding each soil core location. We collected mineral soil to a 10 cm depth at each of these sampling locations and combined soil from each sampling location in a transect transect for chemical analyses. All samples were transported on ice to the laboratory after which they were stored at -20 °C for a maximum time of nine months. For processing, the soil samples were thawed and sieved to 2 mm, and the fine fraction was air-dried for at least five days and then finely ground (200-150 mesh) in a BICO Model UA Pulverizer (Preiser Scientific, St. Albans, WV, Louisville, KY & Beijing, China). Soil pH was measured in the 1:2 soil to water method (Horneck et al. 1989) via an accuFlowTM pH Combination Electrode with accupHastTM (Fisher ScientificTM, Pittsburgh, PA). Measurements of Bray phosphorus (Bray P), total phosphorus (Total P), ammonium (NH4-N), incubation nitrogen (Inc. N), mineralizable nitrogen (Min. N), total carbon (%C) and total nitrogen (%N) were performed by the Central Analytical Laboratory at Oregon State University utilizing the methods found in Horneck et al. (1989). Ectomycorrhiza Root Collection & Host Identification Ectomycorrhizal root tips were selected from soil samples that were washed through a series of 2 mm and 1 mm soil sieves (U.S.A. Standard Testing Sieves) to remove silt and sand particles. The remaining roots were placed in petri dishes, viewed with a Stemi SV6 stereomicroscope (Carl Zeiss Microscopy, LLC, Thornwood, NY) and hand­ 39 sorted to separate live ectomycorrhizal root tips from dead organic material and remaining soil particles. The live root tips from each sample were spread evenly on a 39 ×18 cm tray overlaid with a 4 × 4 cm numbered grid (36 total grid squares). The root tip closest to the center of 12 randomly chosen grid squares was selected for further processing. Additional root tips were selected from each sample if they appeared to be unique morphotypes that had not been selected in the random pick. This method resulted in approximately 12 random root tips with an average of 4.5 extra root tips per sample, for a total of 1,724 root tips. Once root tips were selected, they were cleaned of all clinging organic material, described, and then ground in 0.5 ml microcentrifuge tubes. The remaining fine roots and root tips were air dried, then oven dried at 37 °C for a maximum of three days and weighed to the nearest 0.0001 g. The DNA from the ground root tips was extracted with an altered method of Extract-N-AmpTM (Sigma Aldrich, St. Louis, Missouri) procedure created by L. Kluber (Unpublished 2010). This method called for the addition of 10 µl of Extract-NAmpTM extraction solution to the ground root tip. Next, the samples were vortexed, spun and incubated at 90 °C for 10 minutes. Then 20 µl of Extract-N-AmpTM dilution solution was added and the samples were vortexed and frozen or taken through the next steps of a Polymerase Chain Reaction (PCR). The extracted DNA was taken through the process of PCR twice in order to identify both the tree host and the fungus. Fungal specific primers ITS1F (Gardes and Bruns 1993) and ITS4 (White et al. 1990) and plant specific chloroplast primers trnH and psbA were used (Sang et al. 1997; Tate and Simpson 2003). The PCR program for the fungal primers was as follows: 33 cycles of amplification (94 °C 30 s, 50 °C 1 min, 72 °C 1:30 min) with a total volume of 40 µl per reaction (23.95 µl deionized H2O, 10 µl 5× PCR buffer, 4 µl 10× dNTPs, 0.4 µl of each primer, 0.85 µl MgCl, 0.4 µl BSA, 0.32 µl GoTaq® (Promega, Madison, Wisconsin)). The PCR program for the plant specific primers was as follows: 31 cycles of amplification (94 °C 30 s, 62 °C 1 min, 72 °C 1:30 min) with a total volume of 20 µl per reaction (12.9 µl deionized H2O, 4 µl 5× PCR buffer, 2 µl 10× dNTPs, 0.2 µl each primer, 0.5 µl MgCl, 0.2 µl BSA, 0.23 GoTaq®). After the PCR, the resulting products were electrophoresed through 2.5% agar gels at 100 V for approximately two hours to check that the DNA had amplified. Once 40 imaged under ultraviolet light (AlphaImager EC, Alpha Innotech Corp. (acquired by Cell Biosciences, Inc.), Santa Clara, CA) the DNA bands for the plant primers could be assigned to either overstory Pinaceae trees or to chinquapin trees simply based on height of the bands in relation to control DNA from host leaves and needles. The fungal PCR products were imaged and those that showed amplified fungal DNA in a bright single band on the gel, indicating the presence of one fungus in the sample, were cleaned with ExoSapIT® ((Affymetrix, Santa Clara, California) 1 µl PCR product and 6 µl ExoSap-IT®) and 2.5 µl of the resulting solution was run through a 2.5% agarose gel at 100 V for approximately 1.5 hours. Based on the images of the resulting gel, a 12 µl solution of DNA template, deionized water, and 1.2 µl of 10 µM primer was created. To prepare the samples for sequencing, 5 µl of the DNA template and primer solution for each sample were placed into plates and each was mixed with 5 µl of a solution composed of: 2 µl deionized water, 1 µl ABI BigDye® Terminator 3.1 (Applied Biosystems, Foster City, California), 1.5 µl 5X buffer, 0.5 µl dimethyl sulfoxide (DMSO). The plates were then placed in a Peltier Thermal cycler (BioRad DNAEngine PTC-100, MJ Research, Inc., St. Bruno, Quebec, CAN) and the following program was executed: initial step of 96 °C for 5 minutes followed by 25 cycles of amplification (96 °C 30 s, 50 °C 15 s, 60 °C 4 min) with a total volume of 10 µl per reaction. Fungal samples were sent to either the Center for Genome Research and Biocomputing (CGRB) at Oregon State University or the Advanced Genetic Technologies Center (AGTC) at the University of Kentucky for Sanger sequencing. All samples were sequenced on an Applied BiosystemsTM ABI 3730 DNA analyzer (ThermoFisher Scientific, Pittsburgh, PA). The resulting sequence files were uploaded into the program GeneiousTM (version 7.1.7, Biomatters Ltd.). The sequences were trimmed to remove basepairs below a 0.05 error probability limit. A total of 762 sequences were then assembled with the Geneious Assembler via DeNovo assembly and sequences were aligned according to 97% similarity. Consensus sequences were formed from the 99 resulting consensus sequence groups and these were compared to sequences in the GenBank nucleotide database using the National Center for Biotechnology Information's (NCBI) Basic Local Alignment Search Tool (BLAST) program. BLAST results were 41 filtered to remove target sequences labeled as ‘clonal’ or ‘metagenomic’. Species names were assigned to the consensus sequences based on a 97% or above percent similarity to the target sequences. Higher taxonomic names were assigned to consensus sequences which did not as closely match the best target sequences or which matched several species/genera equally well. Statistical Analyses The tree host and fungal community data were initially explored using summary statistics to create graphs and identify possible trends. Spearman’s rank correlation coefficient was used to assess possible relationships among the measured variables (A. Muldoon pers comm. 2015). The observational nature of the study prevented the extensive use of typical test based statistics. However, the number of ascomycete taxa compared to basidiomycete taxa found on both Pinaceae hosts and chinquapin was compared using a two-sided t-test in RStudio® (RStudio Team 2013, version 0.98.1056). Despite efforts to sample preferentially under chinquapin shrubs, the majority of root tips collected came from overstory conifer trees. In order to adequately compare the taxa richness between the two hosts despite the unbalanced sample size we selected 6 transect pairs from the Pinaceae and 5 from chinquapin where identification of the EMF fungi was greater than 55% and 60%, respectively. Using these data we generated species rarefaction curves to estimate the likely number of taxa we would have found if we had greater fungal identification success for the 16 total transect pairs (Colwell 2013, EstimateS version 9.1.0). In order to determine whether or not the EMF communities found on Pinaceae and chinquapin hosts differed in composition and structure, we performed a Blocked MultiResponse Permutation Procedure (MRBP) in PC-ORDTM (version 6, MJM Software Design) (McCune and Mefford 2011) with the fungal community matrix blocked by transect pair and grouped by host tree. Then to visualize the communities and assess impacts of environmental variables, we performed a Non-metric Multidimensional Scaling (NMDS) on the presence-absence taxa data for both host groups considered separately 42 (McCune and Mefford 2011). The Pinaceae matrix used for this test was comprised of fungi found in three or more transects and the chinquapin matrix included fungi found in two or more transects. The distance matrices used for the NMDS analysis were calculated with a Sørensen distance measure from a random starting configuration where ties were penalized according to Kruskal’s secondary approach. Final solutions were tested against a Monte Carlo randomization test (500 runs). When NMDS was successful, bi-plot overlays were added to the plots to assess the effect of measured environmental variables on the distribution of the taxa. Environmental variables that were correlated with an r2 value of 20% or more for the Pinaceae community and 15% or more for the chinquapin community were considered significantly explanatory of the variation in the data. Results Vegetation Community Of the Pinaceae community, ponderosa pine had the greatest basal area with an average of 37.6 m2/ha per vegetation plot (Fig. 3). It was also dominant (greatest basal area) or co-dominant in all plots except for the second to highest elevation plot where western white pine and grand fir shared dominance (Fig.4). Grand fir basal area increased with elevation and it shared dominance with ponderosa pine at the highest elevation plot (Fig. 4). Lodgepole pine was present with a relatively low basal area in nearly 75% of the vegetation plots, but had the greatest basal area at the lowest elevation site that also had the highest level of Bray phosphorus. Red fir was entirely absent from the lower elevations and only a minor component at the highest elevations. Chinquapin was found on all transect pairs in the study and ranged in percent cover from 1.5% to 85% per transect pair. The most diverse Pinaceae communities in the study were found in unit 12, at the highest elevations, whereas the least diverse Pinaceae community was in vegetation plots 31_22 and 33_12, which contained only ponderosa pine (Fig. 5). It should be noted that data on overstory Pinaceae was not available for vegetation plots 11_18 and 11_20. 43 Of the 11 environmental variables used in this analysis (Table 2), chinquapin percent cover was positively correlated with pH (Table 3). In the Pinaceae, basal area of grand fir was positively correlated with elevation (Table 4) and basal area of lodgepole pine was positively correlated with plant available P (Table 4). Additionally, elevation and total and plant available P were negatively correlated (Table 5). EMF Communities In total, we collected 1724 root tips, 1186 from Pinaceae trees, 384 from chinquapin and 154 from unidentified trees and shrubs (Table 6). Of the 787 mycorrhizal root tips assigned to a taxon, 77% (603/787) were Pineaceae roots and 23% (184/787) were chinquapin roots (Table 6). In all but one pair of transects, a greater number of Pineaceae root tips were found than chinquapin root tips. Ninety-nine consensus sequences were assembled from 762 sequence reads. An additional 59 taxa were found only once throughout the study and could not be assigned to a consensus sequence but were of identifiable quality. Pinaceae hosts and chinquapin shared 23 EMF taxa (Table 7 and Fig. 4); a greater number of taxa asssociated only with Pinaceae hosts than only with chinquapin (Table 8). Overall, 92% of the detected EMF taxa were associated with Pinaceae hosts and 23% were associated with chinquapin (Table 9) (See Appendix A, Table S1 for taxa list). EMF Community Comparisons The rarefaction analysis showed that there would be a significant difference in the EMF community richness between Pinaceae and chinquapin if sample size had been equal and if fungal identification rate had been ≥ 55% for Pinaceae root tips and ≥ 60% for chinquapin root tips (Fig. 7). When compared with a Blocked Multi-Response Permutation Procedure (PC-ORD v.6) the EMF communities found on Pinaceae roots and chinquapin roots were slightly more different than would be expected by chance (A= 0.07, p = 44 0.0001). However, the effect value (A statistic) is so low as to make this test relatively meaningless (McCune and Grace 2002). Basidiomycetes comprised the majority of the EMF community on both Pinaceae and chinquapin hosts (Fig. 8). At the transect pair level, on average, significantly more basidiomycetes than ascomycetes associated with both host groups (Pinaceae: paired t-test, t = -9.3, df = 15, p < 0.001, chinquapin: paired t-test, t = -2.8, df = 15, p = 0.04). However, as Figure 5 and the t-tests show, the difference in occurence between ascomycetes and basidiomycetes is much greater in Pinaceae than in chinquapin. The Pinaceae and chinquapin EMF communities were dominated by Cenococcum geophilum 1, which occurred on Pinaceae in all pairs of transects (Fig. 9). Piloderma 2, the second most dominant EMF on chinquapin, occurred on Pinaceae hosts in a similar number of transect pairs (Fig. 9). The dominant taxa, those that occur on either host group in 31% (5/16) or more transect pairs, are shown in Figure 9. The EMF communities on chinquapin and Pinaceae hosts were both relatively diverse with an average Shannon’s Diversity of 1.7 (±0.5 SD) for chinquapin and 2.9 (±0.3 SD) for Pinaceae per transect pair. However, the majority of taxa found on Pinaceae and chinquapin were rare, with more than half of each respective community being found in fewer than five transect pairs (Fig. 10). EMF Communities & Environmental Variables Pinaceae EMF species richness and Shannon’s diversity were not well correlated with any of the measured environmental variables (Appendix A, correlation table S4). However, Pinaceae EMF richness and Shannon’s diversity were positively correlated with the number of Pinaceae root tips collected and negatively correlated with the number of chinquapin root tips collected (Table 10). Similarly, species richness and Shannon’s diversity for chinquapin EMF were positively correlated with the number of chinquapin root tips collected and negatively correlated with the number of Pinaceae root tips collected (Table 11). Additionally, the number of taxa shared by Pinaceae hosts and 45 chinquapin per transect pair ranged from 1 to 5 and did not correlate well with any of the measured environmental variables (Appendix A, correlation table S2). Non-metric Multidimensional Scaling (NMDS) of the Pinaceae EMF community indicated that a three-dimensional solution best represented the data, with a minimum stress of 18.8 after 127 iterations. The final solution passed the Monte Carlo randomization test (Monte Carlo, 200 random runs, p = 0.02). Axes 1, 2, and 3, accounted for 29.9%, 19.2%, and 18.6% of the variation respectively (Fig. 11a). The bi-plot overlays show variables with a significance of r2 ≥ 18% that are affecting the distribution of the EMF taxa. A three-dimensional solution, with a minimum stress of 16.1 after 88 iterations, also best represented the chinquapin EMF community. The final solution passed the Monte Carlo randomization test (Monte Carlo, 200 random runs, p = 0.02). Axes 1, 2, and 3, accounted for 23.5%, 18.4%, and 32.7% of the variation respectively (Fig. 11b). The biplot overlays show variables with a significance of r2 ≥ 15% that are affecting the distribution of the EMF taxa. In the case of Pinacaee EMF total phosphorus had the greatest effect, whereas for the chinquapin EMF community Bray (plant available) phosphorus was most important. However, in both ordinations, the majority of the shared dominant taxa were present in areas of lower relative phosphorus (Fig. 11ab). Discussion Despite preferentially sampling beneath chinquapin shrubs we found a greater abundance of Pinaceae root tips than chinquapin root tips overall (Table 6). Sampling to a depth of 10cm in forests east of the Cascade Mountain Range in Oregon has typically yielded abundant Pinaceae root tips (Smith et al. 2004; 2005; Garcia et al. in press). However, deeper sampling of the soil profile may have resulted in greater numbers of chinquapin roots. Lopez et al. (2001) found that in a Mediterranean Quercus ilex L. forest with hot, dry summers, fine root numbers measured for each 10cm interval to a depth of 60 46 cm tended to decrease with increasing depth but were greater in the 10-20 cm stratum than between 0 and 10cm. Unexpectedly, there appeared to be a negative relationship between pH and Bray P, however, the trend is likely influenced by one data point of very low pH (see Appendix B Fig. S2) and thus the possibility of this relationship should be further investigated. In general, P decreased with increasing elevation and this relathionship is likely linked with the vegetative communities and carbon cycling. Volcanically influenced soils tend to easily bind P sources, however soil organic matter (SOM) also binds to soil particles and can act as a buffer, freeing more P (Anderegg and Naylor 1988). Certain volcanic soils can preferentially bind P over SOM (Appelt et al. 1975), however the slight decrease in soil C with increasing elevation and increase with increasing plant available P on Lookout Mountain would suggest that it is acting as a buffer and making phosphorus more available. EMF Community & Soil Nutrients Total P and plant available P influenced the fungal communities of Pinaceae hosts and chinquapin, respectively (Fig. 11). The most frequently found shared fungi, Byssocorticium 1, Cenoccocum geophilum 1 and 2, and Piloderma 2 were all found more frequently in mid to low P areas except for C. geophilum 1 (Fig. 11). Piloderma species have been linked with improved host P nutrition (Jongmans and van Breeman 1997; Tuason and Arocena 2009). Jongmans and van Breeman (1997) found evidence that a Piloderma species was directly impacting plant inorganic P nutrition via dissolution of soil rocks and rock fragments. Further, Tuason and Arocena (2009) found that Piloderma fallax (Lib.) Stalpers produced increased amounts of oxalate when grown in a P limited culture. In addition to improving access to P, mat-forming Piloderma species have been found to improve nitrogen nutrition in Douglas-fir forests (Kluber et al. 2010; 2011; Phillips et al. 2012; Zeglin et al. 2012). It is likely therefore, that their prevalence in low P 47 locations for Pinaceae and chinquapin hosts is due to preferential carbon allocation from the host in exchange for improved P and possibly, N uptake. The dominant strains Cenococcum geophilum 1 and 2 are likely important for soil water uptake specifically as it has been well documented that C. geophilum is prevalent in dry environments (Pigott 1982; Buée et al. 2005) where it improves host survival by increasing host plant water potential (Hasselquist et al. 2005). Byssocorticium 1 may also be assisting in plant water uptake. Shi et al. (2002) found that Byssocorticium atrovirens was abundant on Fagus sylvatica L. across five ecotypes in Italy, independent of drought status. Whereas this finding does not confirm that Byssocorticium was helpful to its hosts in more extreme drought areas, it does suggest that Byssocorticium taxa show environmental plasticity and a certain tolerance for water stress. We did not see a response in the EMF community to the local N gradient. However, Lilleskov et al. (2002) found that excessively high levels of soil nitrogen, which can be caused by deposition from industry, can decrease EMF diversity (Lilleskov et al. 2002). Our local N gradient ranged from 3 to 29 mg/kg, whereas Lilleskov et al. (2002) investigated a mineral N gradient of 13 to 243 mg/kg. The comparatively small N gradient on Lookout Mountain was likely the reason we did not see an EMF response. EMF Community Comparisons Nine taxa (Byssocorticium 1, Cenococccum geophilum 1, 2, and 3, Cortinarius 13, Elaphomyces 1, Hygrophorus 1, Inocybe 1, and Piloderma 2) associated with giant chinquapin in five or more transect pairs were considered dominant. With the exception of Elaphomyces 1 and Inocybe 1, these taxa also associated with Pinaceae hosts in our study. However, Elaphomyces and Inocybe have been found in association with ponderosa pine and Douglas-fir (Barroetaveña et al. 2007) and are likely not unique to chinquapin in our study area. Eleven infrequently detected taxa (representing: Cortinarius, Hygrophorus, Hysterangium, Inocybe, Melanogaster, and Tomentella) associated with giant chinquapin 48 were not found with Pinaceae hosts. However, it is unlikely they are unique to chinquapin as they all have been found associated with ponderosa pine and Douglas-fir (Barroetaveña et al. 2007). Generally EMF distribution is patchy (Horton and Bruns 2001; Korkama et al. 2006), suggesting that the presence of chinquapin contributes to maintaining EMF diversity in our central Oregon site. Cenococcum geophilum 3, was found more often on chinquapin roots than on Pinaceae roots (5 transect pairs vs. 1 transect pair) despite the discrepancy in sample size. This apparent difference in association may be because fewer fungi associated with chinquapin, which led to greater opportunity for a less competitive strain of C. geophilum to gain a foothold, or because of a slight preference of this strain for chinquapin roots over Pinaceae roots. Given the small sample size and observational nature of this study, further research would be needed to test these hypotheses. Eighty-four percent (122/145) of taxa found on Pinaceae hosts, but not on chinquapin (See Appendix A, table S1 for taxa lists), comprised 13 genera: Gautieria, Leucogaster, Leucophleps, Lyophyllum, Phellodon, Phialocephala, Pseudotomentella, Rhizopogon, Sistotrema, Suillus, Tomentellopsis, Tricholoma, and Wilcoxina that are known to associate with Pinaceae hosts (Table 1) (Jumpponen and Trappe 1998). Rhizopogon and Suillus are generally restricted to hosts in the Pinaceae so their absence from the chinquapin community was expected (Molina and Trappe 1982a; Molina et al. 1992; Molina and Trappe 1994, Bruns et al. 2002a). The remaining fungal genera have been found associated with roots of tree genera with close phylogenetic relationships to chinquapin (Manos et al. 2008; Oh and Manos 2008) and are displayed in Table 1 (Fogel 1979; Walker 2003; Dickie et al. 2009; Jumponnen et al. 2010); or in the case of Leucogaster, found fruiting in a mixed Pinus-Quercus forest in Mexico (Cázares et al. 1992). With greater sampling effort these genera may have been detected on chinquapin. The EMF community associated with Pinaceae hosts was 75% more species rich (Fig. 7) and more diverse (Shannon’s Diversity) than the chinquapin EMF community. This difference in richness and diversity is likely due to finding more Pinaceae root tips than chinquapin root tips, but may partially be because more host-specialist fungi associate 49 with species in the Pinaceae than with chinquapin and because we sampled from multiple host species in the Pinaceae. A total of 23 shared taxa associated with chinquapin and Pinaceae hosts (Fig. 6). These taxa represent 64% of the chinquapin EMF community, but only 16% of the Pinaceae EMF community. The majority of the taxa associated with chinquapin also associated with Pinaceae hosts suggesting that further sampling of chinquapin roots would likely show an increase in the number of shared taxa. However, an increase in shared taxa may not occur if further sampling included Pinaceae roots, as the Pinaceae EMF species rarefaction curve indicated that the community was not entirely sampled. The most frequently found taxa occurred on both Pinaceae and chinquapin hosts, but these common taxa were often found on fewer transect pairs when associated with chinquapin. These results show that chinquapin maintains a sub-set of the EMF community associated with Pinaceae trees, including several fungi important for improved water uptake and P nutrition. EMF & Fire EMF are an important component of the forest recovery process after fire (Baar et al. 1999; Bruns et al. 2002b). Different methods of EMF recolonization after burns likely exert influence on post burn EMF community structure and complexity. Baar et al. (1999) found that fungal taxa, such as Rhizopogon species, with resistant spores or propagules in the soil profile were the most abundant after stand replacing wildfire in a Pinus muricata D. Don forest on the California coast. Similarly, early stage colonizers like Hebeloma (Deacon and Flemming 1992) were found in a post-burn area and likely recolonized by airborne spores (Baar et al. 1999). Additionally, fungi already present on mature hosts can recolonize a site via mycelial spread or spores from fruiting bodies formed next to the mature host, which can be important for the retention and spread of complex EMF communities (Krannabetter et al. 1999). Vegetative spread of fungi from mature hosts to regenerating seedlings presents the possibility for the formation of common mycorrhizal 50 networks, which can improve plant survival due to water (Bingham and Simard 2011) and nutrient transfer (Simard et al. 1997a; Teste and Simard 2009). Retaining diverse EMF communities throughout a potential burn site via fire resistant ectomycorrhizal forming hosts could be beneficial to the long-term maintenance of plant communities. A shrubby, sprouting, fire-resistant ectomycorrhizal forming birch (Betula nana L.), was able to retain a viable EMF community after fire in Arctic tundra (Hewitt et al. 2013). Hewitt et al. (2013) found similar EMF communities on sites with un-burned shrubs and sites with shrubs that had resprouted after burning. They concluded, based on the typically late-stage nature of the dominant EMF species detected (Russula, Lactarius, Inocybe), that the EMF had been maintained in a mycelial state through the fire (Hewitt et al. 2013). Similarly, Horton et al. (1999) showed that fungi forming arbutoid mycorrhizae with the shrub species manzanita (Arctostaphlos uva-ursi) assisted in establishment in Douglas-fir seedlings in Chaparral communities in central California. Further, as found by Hagerman et al. (2001), the maintenance of diverse mycorrhizal community by manzanita three years after overstory removal is strong evidence supporting the idea that an understory species could serve as an EMF refuge plant after disturbance to the overstory. As a stump and root sprouter, chinquapin is highly resilient to fire (McKee 1990; Kauffman and Martin 1990), and can re-sprout even after repeated severe burns (Donato et al. 2009). This ability to retain live roots may mean that it is capable of maintaining EMF partners after fire despite the temporary halting of photosynthetic C flowing to the roots. Bauhus (1994, cited in Bauhus and Bartsch 1996) found that two years after cutting, Fagus sylvatica L. stumps maintained live fine roots. Limitations & Future Directions This observational study provides important insight into the EMF of a poorly investigated ectomycorrhizal host on one mountain in central Oregon. The PFEF is representative of many forests in central Oregon and these results can be cautiously applied to different situations and conditions. We now have a glimpse of the EMF community 51 associated with chinquapin and its relation to Pinaceae EMF communities, but there is likely much left to learn and further studies investigating this topic in different locations and environments would be beneficial. Additionally, investigations of the chinquapin EMF after fire or clearing could further elucidate if chinquapin can serve as an EMF refuge plant and innoculum source for Pinaceae seedlings after disturbance. Conclusions & Implications Pinaceae hosts and chinquapin maintain a similar EMF community that is dominated by fungi known to improve plant nutrition (Jongmans 1997; Tuason and Arocena 2009; Kluber et al. 2011) and survival under moisture stress (Hasselquist et al. 2005). This information is important for management decisions regarding forests comprised of these trees in central Oregon, as chinquapin is often controlled to reduce fire risks and competition for the more economically valuable pine species (Barrett et al. 1983). Although competition by understory shrubs is a valid concern (Busse et al. 1996; Zhang et al. 2006), chinquapin could factor into maintaining the health of Pinaceae-dominated forests in several ways. Keys and Maguire (2005) synthesized results from three studies and concluded that initial shrub cover in ponderosa pine stands facilitates pine germinant survival for the first one or two summers. Additionally, because chinquapin is a root and stump sprouting species (Barrett et al. 1983; McKee 1990) and associates with many of the dominant EMF taxa found with Pinaceae hosts, it could play a vital role as a refuge plant in keeping a remnant EMF community in place after disturbances such as fire, harvest damage, or removal of overstory Pinaceae. Considering the similarities found between the EMF communities on chinquapin and the associated Pinaceae overstory, an investigation to identify if chinquapin could maintain a diverse EMF community (including key players such as C. geophilum and Piloderma sp.) and be able to function as a source of fungal innoculum for future Pinaceae seedlings after disturbance, would be beneficial. 52 http://0.tqn.com/d/gonw/1/0/U/w/- / - /oregonstatetopo.gif Figure 1. Study area overview and design. a) Oregon Map, Blue square = Lookout Mountain area; b) Lookout Mountain study area, Yellow squares = EMF sampling areas; c) Study transect pair diagram, Blue/shaded circles = soil moisture tube approximate locations. Central circle = overstory vegetation survey area. 53 Figure 2. Lookout Mountain study area. Yellow squares = Location of vegetation plots and transect pairs where soil moisture and EMF were sampled. 54 Pinus ponderosa Tree Species Pinus monticola Pinus contorta Abies magnifica Abies grandis 0 20 40 60 Basal Area (m / ha) Figure 3. Box and whisker plot of basal area (m2/ha) of overstory Pinaceae trees, where the upper and lower hinges of box = 1st and 3rd quartiles of the data, the mid-box line = 2nd quartile and the whiskers = 1.5* IQR (inter-quartile distance, the distance between the 1st and 3rd quartile). Outliers are beyond the 1.5*IQR cutoff. When a box is defined by only two data points the ends of the whiskers are the data points and the box is located mid-way between them. Dot-plot overlay shows basal area per species per vegetation plot. 55 60 Basal Area (m2/ha) per Plot Name Abies grandis Abies magnifica Pinus contorta 40 Pinus monticola Pinus ponderosa Total BA 20 0 1400 1500 1600 1700 Elevation (m) Figure 4. Basal area (m2/ha) of overstory Pinaceae trees per vegetation plot by elevation (m). (n = 14) 56 12_20 12_24 14_16 14_6 15_2 15_24 25_2 25_20 31_12 31_22 32_14 32_2 33_12 33_16 Pinus ponderosa Species Pinus monticola Pinus contorta Abies magnifica Abies grandis 0 204060 0 204060 0 204060 0 204060 0 204060 0 204060 0 204060 0 204060 0 204060 0 204060 0 204060 0 204060 0 204060 0 204060 Basal Area (m /ha) Figure 5. Pinaceae basal area (m2/ha) per vegetation plot (excepting those in plots 11_18 and 11_20) 57 Table 2. Environmental variable measurements averaged across 16 study transect pairs. Means are listed with standard deviations in parentheses. Elevation (m) Aspect (º) pH Vol. Water (m3/m3) Bray P (mg/kg) Total P (mg/kg) Mineralizable N (mg/kg) Total C (%) Total N (%) Root Biomass (g) Chinquapin Cover (%) Average 1553 (94.6) 102.9 (31.4) 5.76 (0.21) 0.042 (0.018) 19.82 (2.05) 922.94 (149.81) 10.98 (7.12) 3.47 (1.21) 0.08 (0.03) 0.20 (0.05) Minimum 1401 68.43 5.16 0.001 15.38 712.41 3.1 1.74 0.03 0.15 Maximum 1703 186.1 6.04 0.073 40.88 1333.35 28.64 5.38 0.14 0.3 44.2 (24.0) 1.5 85.2 Dates Collected 7-8/2012 7-8/2012 7-8/2012 7-8/2012 7-8/2012 7-8/2012 7-8/2012 7-8/2012 7-8/2011 or 7-9/2012 Table 3. Spearman ranked correlation coefficient (ρ) of pH correlated with chinquapin percent cover (Plots in Appendix B). Bold = relationship strong enough to merit attention. pH Chinquapin % Cover 0.51 Table 4. Spearman ranked correlation coefficients (ρ) of environmental variables correlated to Pinaceae basal area (m2/ha). (Plots in Appendix B). Bold = relationship strong enough to merit attention. Elevation (m) Bray P (mg/kg) Pinaceae EMF Richness Pinaceae EMF Shannon’s Div. Abies grandis 0.76 -0.26 -0.27 -0.27 Pinus contorta -0.35 0.83 -0.52 -0.50 Pinus ponderosa -0.01 -0.24 0.00 -0.03 58 Table 5. Spearman ranked correlation coefficients (ρ) for environmental variables correlated with each other (Plots in Appendix B). Bold = relationship strong enough to merit attention. See Appendix A table S2 for full table. pH Elevation (m) Bray P (mg/kg) Total P (mg/kg) Mineralizable N (mg/kg) Total C (%) Total N (%) pH 1.00 0.32 -0.50 0.06 -0.46 -0.32 -0.47 Avg. Vol. Water (m3/m3) Pinaceae root tips Chinquapin root tips -0.08 0.26 0.11 Elevation (m) Bray P (mg/kg) Mineralizable N (mg/kg) Total C (%) 1.00 -0.72 -0.61 0.12 -0.46 -0.44 1.00 0.58 0.03 0.49 0.47 1.00 0.59 0.64 1.00 0.93 0.43 -0.33 0.26 -0.41 0.12 -0.21 0.66 -0.05 -0.28 0.24 0.13 -0.19 Table 6. Root tip success summary. Pinaceae Count 1186 603 Initial Sample Final Output Success Rate % 69 77 51% Chinquapin Other Total Tips Count 384 184 Count 154 - % Count 9 1724 787 46% % 22 23 48% Table 7. Count and percent of unique and shared fungal taxa found by host. Pinaceae Unique Shared Total Count 122 23 145 Chinquapin % of Total 84 16 100 Count 13 23 36 % of Total 36 64 100 Table 8. Count and percent of unique and shared fungal taxa. Unique to Host Shared Total Count 135 23 158 % of Total 85 15 100 59 Table 9. Count and percent of total fungal taxa found by host. Pinaceae Giant Chinquapin Total Count 145 36 158 % of Total 92 23 Taxa 60 Cenococcum geophilum 1 Byssocorticium 1 Cortinarius 1 Cenococcum geophilum 2 Piloderma 2 Piloderma 1 Russula 1 Cortinarius 4 Byssocorticium 2 Lactarius resimus Cortinarius 5 Cortinarius laetissimus Piloderma 5 Cortinarius 6 Piloderma olivaceum 2 Helotiales 1 Piloderma olivaceum 3 Helotiales 2 Cortinarius 12 Russula 3 Hygrophorus 1 Cenococcum geophilum 3 Cortinarius 13 Host Pineaceae Chinquapin 0 5 10 15 Frequency of taxa by transect pair Figure 6. Taxa found on both Pinaceae hosts and giant chinquapin by frequency of occurrence on transect pairs. 200 200 150 150 Estimated Number of Taxa Estimated Number of Taxa 61 100 100 50 50 0 0 4 8 Transect Pairs 12 16 4 8 Transect Pairs 12 16 Figure 7. Species rarefaction curves and 95% confidence intervals for a) Pinaceae (estimated from six transect pairs with a ≥ 55% fungal identification rate), and b) chinquapin (estimated from five transect pairs with a ≥ 60% fungal identification rate) 0 5 Number of Taxa 10 15 20 25 62 Ascomycete Basidiomycete Ascomycete Basidiomycete Figure 8. Number of ascomycetes and basidiomycete taxa on transect pairs for a) Pinaceae (paired t-test, t=-9.3, df = 15, p < 0.001, and b) chinquapin (paired t-test, t = -2.8, df = 15, p = 0.04). Boxplots represent the distribution of fungal taxa where the upper and lower hinges of box = 1st and 3rd quartiles of the data, the mid-box line = 2nd quartile and the whiskers = 1.5* IQR (inter-quartile distance, the distance between the 1st and 3rd quartile). Outliers are beyond the 1.5*IQR cutoff. 63 a. b. Cenococcum geophilum 1 Cenococcum geophilum 1 Byssocorticium 1 Piloderma 2 Cortinarius 1 Cenococcum geophilum 2 Inocybe 1 Wilcoxina rehmii Hygrophorus 1 Taxa Taxa Piloderma 2 Sistotrema alboluteum Russula 1 Rhizopogon salebrosus 1 Elaphomyces 1 Cortinarius 13 Rhizopogon ochraceorubens Byssocorticium 1 Piloderma 1 Suillus tomentosus Cenococcum geophilum 3 Cortinarius 4 Cenococcum geophilum 2 Cortinarius 3 0 5 10 Frequency of taxa by transect pair 15 0 5 10 15 Frequency of taxa by transect pair Figure 9. Dominant EMF taxa or EMF taxa associated with a) Pinaceae and b) Chinquapin hosts that occurred on five or more transect pairs. Filled arrows indicate shared dominant taxa. Clear arrows indicate shared taxa. 64 b. Taxa Taxa a. 4 8 12 Frequency of taxa by transect pair 16 3 6 9 Frequency of taxa by transect pair Figure 10. Frequencey of all EMF taxa found by transect pair for a) Pinaceae hosts and b) chinquapin 65 Table 10. Spearman ranked correlation coefficients (ρ) for Pinaceae EMF descriptors that were correlated with environmental variables (Plots in Appendix B). Bold = relationship strong enough to merit attention. C tips P tips Richness Shannon’s Diversity -0.5 0.88 -0.52 0.88 Table 11. Spearman ranked correlation coefficients (ρ) for Chinquapin EMF descriptors that were correlated with environmental variables (Plots in Appendix B). Bold = relationship strong enough to merit attention. C tips P tips Richness Shannon’s Diversity 0.89 -0.68 0.89 -0.65 66 a. b. Cenococcum geophilum 2 Cenococcum geophilum 2 Total P Cenococcum geophilum 1 Byssocorticium 1 Axis 3 (r = 32.7%) Axis 3 (r = 18.6%) Piloderma 2 Piloderma 2 Byssocorticium 1 Bray P Cenococcum geophilum 1 Axis 2 (r =19.2%) Axis 1 (r =23.5%) Figure 11. NMDS showing EMF taxa in transect space. Triangles = dominant taxa shared by Pinaceae and chinquapin. Circles = EMF taxa. a) Pinaceae EMF. Environmental variable shown as bi-plot overlay has r2 ≥ 20%. b) Chinquapin EMF. Environmental variable shown as bi-plot overlay has r2 ≥ 15%. 67 BIBLIOGRAPHY Adams M (2013) Mega-fires, tipping points and ecosystem services: Managing forests and woodlands in an uncertain future. Forest Ecology and Management 294:250–261. doi: 10.1016/j.foreco.2012.11.039 Adams MB, Loughry LH, Plaugher LL (eds) (2004) Experimental Forests and Ranges of the USDA Forest Service. General Technical Report GTR-NE-321 Revised, USDA Forest Service Northeastern Research Station, Newtown Square, PA, pp178 Agee J.K. (1993) Fire ecology of Pacific Northwest forests. Island Press, Washington D.C., pp 505 Amaranthus MP, Perry DA (1994) The functioning of ectomycorrhizal fungi in the field: linkages in space and time. Plant and Soil 159:133–140. Anderegg J, Naylor DV (1988) Phosphorus and pH relationships in an andic soil with surface and incorporated organic amendments. Plant and Soil 107:273–278 Anderson P, Smith JE (2011) Autecology of giant chinquapin (Chrysolepis chrysophylla). In: Proposals for research at Lookout Mountain, Pringle Falls Experimental Forest. USDA Forest Service Pacific Northwest Research Station, Corvallis, OR, pp 17-25 Andrews A, Kutara K (2005) Oregon’s timber harvests: 1849–2004. Oregon Department of Forestry, Salem, OR 154 p. Appelt H, Coleman NT, Pratt PF (1975) Interactions Between Organic Compounds, Minerals, and Ions in Volcanic-Ash-Derived Soils: II. Effects of Organic Compounds on the Adsorption of Phosphate. Soil Science Society of America Journal 39:628–630 Attiwill PM, Adams MA (1993) Tansley Review No . 50 Nutrient cycling in forests. New Phytologist 124:561–582 Auge RM (2001) Water relations, drought and vesicular–arbuscular mycorrhizal symbiosis. Mycorrhiza 11:3–42 Avolio ML, Tuininga AR, Lewis JD, Marchese M (2009) Ectomycorrhizal responses to organic and inorganic nitrogen sources when associating with two host species. Mycological research 113:897–907. doi: 10.1016/j.mycres.2009.05.001 Baar J, Horton TR, Kretzer AM, Bruns TD (1999) Mycorrhizal colonization of Pinus muricata from resistant propagules after a stand-replacing wildfire. New Phytologist 143:409–418. 68 Babikova Z, Gilbert L, Bruce TJ, Birkett M, Caulfield JC, Woodcock C, Pickett JA, Johnson D (2013) Underground signals carried through common mycelial networks warn neighbouring plants of aphid attack. Ecology Letters 16:835–43. doi: 10.1111/ele.12115 Baker MA 1981 The Ethnobotany of the Yurok, Tolowa and Karok Indians of Northwest California. M.A. Thesis, Humboldt State University Baldwin BG, Goldman DH, Keil DJ, Patterson R, Rosatti TJ, Wilken DH (eds) (2012) The Jepson Manual: Vascular Plants of California, 2nd edn. University of California Press, Berkeley Barker JS, Simard SW, Jones MD, Durall DM (2013) Ectomycorrhizal fungal community assembly on regenerating Douglas-fir after wildfire and clearcut harvesting. Oecologia 172:1179–1189 Barker WW, Welch SA, Chu S, Banfield JF (1998) Experimental observations of the effects of bacteria on aluminosilicate weathering. 83:1551–1563. Barrett JW, Marting RE, Wood DC (1983) Northwestern Ponderosa Pine and Associate Species. In: Silvicultural Systems for the Major Forest Types of the United States. Burns RM (tech compil.) Agriculture Handbook 445, USDA Forest Service, Washington, DC, pp 16-18 Barroetaveña C, Cázares E, Rajchenberg M (2007) Ectomycorrhizal fungi associated with ponderosa pine and Douglas-fir: a comparison of species richness in native western North American forests and Patagonian plantations from Argentina. Mycorrhiza 17:355–73. doi: 10.1007/s00572-007-0121-x Bauhus J, Bartsch N (1996) Fine-root growth in beech (Fagus sylvatica) forest gaps. Canadian Journal of Botany 26:2153–2159 Bauman JM (2010). Ectomycorrhizal communities associtated with restoration plantings of american chestnut (Castanea dentata) seedlings on Ohio mine lands: planting methodologies to promote root colonization. Dissertation, Miami University Baxter JW, Dighton J (2005) Phosphorus source alters host plant response to ectomycorrhizal diversity. Mycorrhiza 15:513–23. doi: 10.1007/s00572-005­ 0359-0 Beiler KJ, Durall DM, Simard SW, Maxwell SA, Kretzer AM (2010) Architecture of the wood-wide web: Rhizopogon spp. genets link multiple Douglas-fir cohorts. New Phytologist 185:543–53. doi: 10.1111/j.1469-8137.2009.03069.x 69 Bending GD, Read DJ (1995) The structure and function of the vegetative mycelium of ectomycorrhizal plants V. Foraging behaviour and translocation of nutrients from exploited litter. New Phytologist 130:401–409 Berbee ML, Taylor JW (2001) Fungal Molecular Evolution: Gene Trees and Geologic Time. The Mycota VII Part B. Systematics and Evolution 229–245 Bergemann SE, Garbelotto M (2006) High diversity of fungi recovered from the roots of mature tanoak (Lithocarpus densiflorus) in northern California. Canadian Journal of Botany 84:1380–1394. doi: 10.1139/B06-097 Berndt HW, Gibbons RD (1958) Root distribution of some native trees and understory plants growing on three sites within ponderosa pine watersheds in Colorado. Station Paper 37. USDA Forest Service Rocky Mountain Forest and Range Experimental Station, Fort Collins, Colorado, 14 pp Bingham MA, Simard SW (2011) Do mycorrhizal network benefits to survival and growth of interior Douglas-fir seedlings increase with soil moisture stress? Ecology and Evolution 1:306–16. doi: 10.1002/ece3.24 Binkley D, Fisher RF (2013) Ecology and Management of Forest Soils 4th ed. Wiley Blackwell, West Sussex Birdsey RA (1992) Carbon Storage and Accumulation in United States Forest Ecosystems. General Technical Report WO-59:51, USDA Forest Service Northeastern Forest Experiment Station, Radnor, PA, 51 pp Brundrett MC (2002) Tansley Review No. 134. Coevolution of roots and mycorrhizas of land plants. New Phytologist 154:275–304 Brundrett M (2004) Diversity and classification of mycorrhizal associations. Biological reviews of the Cambridge Philosophical Society 79(3):473–495 Brundrett MC (2009) Mycorrhizal associations and other means of nutrition of vascular plants: understanding the global diversity of host plants by resolving conflicting information and developing reliable means of diagnosis. Plant and Soil 320:37–77. doi: 10.1007/s11104-008-9877-9 Bruns T, Tan J, Bidartondo M, Szaro T, Redecker D (2002a) Survival of Suillus pungens and Amanita francheti Ectomycorrhizal Genets Was Rare or Absent StandReplacing wildfire. New Phytologist 155:517–523 70 Bruns TD, Bidartondo MI, Taylor DL (2002b) Host specificity in ectomycorrhizal communities: what do the exceptions tell us? Integrative and Comparative Biology 42:352–9. doi: 10.1093/icb/42.2.352 Buée M, Vairelles D, Garbaye J (2005) Year-round monitoring of diversity and potential metabolic activity of the ectomycorrhizal community in a beech (Fagus silvatica) forest subjected to two thinning regimes. Mycorrhiza 15:235–45. doi: 10.1007/s00572-004-0313-6 Burns RM, Honkala BH, (tech coords) Silvics of North America: 1. Conifers, 2. Hardwoods. Agriculture Handbook 654. USDA Forest Service, Washington, D.C. pp 877 Busse MD, Cochran PH, Barrett JW (1996) Changes in Ponderosa Pine Site Productivity following Removal of Understory Vegetation. Soil Science Society of America Journal 60:1614. doi: 10.2136/sssaj1996.03615995006000060004x Busse MD, Riegel GM (2005) Managing Ponderosa Pine Forests in Central Oregon: Who Will Speak for the Soil? In: Ritchie MW, Maguire DA, Youngblood A (tech coords) Proceedings of the Symposium on Ponderosa Pine: Issues, Trends, and Management. General Technical Report PSW-GTR-198, USDA Forest Service Pacific Southwest Research Station, Klamath Falls, OR, pp 1614 -1621 Cairney JWG (2011) Ectomycorrhizal fungi: the symbiotic route to the root for phosphorus in forest soils. Plant and Soil 344:51–71. doi: 10.1007/s11104-011-0731-0 Camp A, Youngblood A (2006) Silvicultural and Other Long-term Research at the Pringle Falls Experimental Forest. In: Irland LC, Campe AE, Brissette JC, Donohew ZR. Long-Term Silvicultural and Ecological Studies Results for Science and Management. Research Paper 005, Global Institute of Sustainable Forestry, New Haven, CT, pp 182–186 Campbell S, Azuma D, Weyermann D (2004a) Forests of Western Oregon : An Overview. General Technical Report PNW-GTR-525 Revised. USDA Forest Service Northwest Research Station, Portland, OR, pp 27 Campbell S, Azuma D, Weyermann D (2004b) Forests of Eastern Oregon : An Overview. General Technical Report PNW-GTR-578 Revised, USDA Forest Service Pacific Northwest Research Station, Portland, OR, pp 31 Cázares E, García J, Castillo J, Trappe JM (1992) Hypogeous fungi from Northern Mexico. Mycologia 84:341–359. 71 Chalot M, Javelle A, Blaudez D, Lambilliote R, Cooke R, Sentanac H, Wipf D, Botton B (2002) An update on nutrient transport processes in ectomycorrhizas. Plant and Soil 165–175. Chesnut VK (1902) Plants used by the Indians of Mendocino County, California. Contributions from the U.S. National Herbarium, USDA Division of Botany, Washington, DC. 7(3): 295-408 Christensen M (2009) Phenology of ectomycorrizal fungi in subtropical evergreen Castanopsis forest. Botanica Orientalis 6:8–11 Colwell RK (2013) EstimateS: Statistical estimation of species richness and shared species from samples, Version 9.1.0. http://purl.oclc.org/estimates. Accessed 2014 Courty P-E, Buée M, Diedhiou AG, Frey-Klett P, Le Tacon F, Rineau F, Turpault M-P, Uroz S, Garbaye J (2010) The role of ectomycorrhizal communities in forest ecosystem processes: New perspectives and emerging concepts. Soil Biology and Biochemistry 42:679–698. doi: 10.1016/j.soilbio.2009.12.006 Courty P-E, Pouysegur R, Buée M, Garbaye J (2006) Laccase and phosphatase activities of the dominant ectomycorrhizal types in a lowland oak forest. Soil Biology and Biochemistry 38:1219–1222. doi: 10.1016/j.soilbio.2005.10.005 Courty P-E, Pritsch K, Schloter M, Pritsch K, Schloter M, Hartmann A, Garbaye J (2005) Activity profiling of ectomycorrhiza communities in two forest soils using multiple enzymatic tests. New Phytologist 167:309–319. doi: 10.1111/j.1469­ 8137.2005.01401.x Coville FV (1897) Notes on the plants used by the Klamath Indians of Oregon. Contributions from the United States National Herbarium 5:87–108 Dale VH, Joyce LA, Mcnulty S, Neilson RP, Ayres MP, Flannigan MD, Hanson PJ, Irland LC, Lugo AE, Peterson CJ, Simberloff D, Swanson FJ, Stocks BJ, Wotton BM (2001) Climate Change and Forest Disturbances. BioScience 51:723–734 Deacon JW, Flemming LV (1992). Interactions of ectomycorhizal fungi. In Allen MJ (ed.) Mycorrhizal Functioning an Integrative Plant-Fungal Process. Chapman and Hall, New York Deschutes National Forest (2001) 40ftContour.shp. USDA Forest Service, Deschutes National Forest Data Library. http://www.fs.fed.us/r6/data-library/gis/deschutes/. Accessed April 2014 72 Dickie IA, Dentinger BTM, Avis PG, McLaughlin DJ, Reich PB (2009) Ectomycorrhizal fungal communities of oak savanna are distinct from forest communities. Mycologia 101:473–483. doi: 10.3852/08-178 Dickie IA, Reich PB (2005) Ectomycorrhizal fungal communities at forest edges. Journal of Ecology 93:244–255. doi: 10.1111/j.1365-2745.2005.00977.x Dighton J (1991) Acquisition of nutrients from organic resources by mycorrhizal autotrophic plants. Experientia 47:362–369 Dokmai P, Phosri C, Khangrang R, Suwannasai N (2015) Above- and Below-Ground Ectomycorrhizal Diversity in a Pine-Oak Forest in Northeastern Thailand. Chaing Mai Journal of Science 42:79–87 Donato DC, Fontaine JB, Robinson WD, Kauffman JB, Law BE (2009) Vegetation response to a short interval between high-severity wildfires in a mixed-evergreen forest. Journal of Ecology 97:142–154. doi: 10.1111/j.1365-2745.2008.01456.x Drever JI, Stillings LL (1997) The role of organic acids in mineral weathering. Colloids and Surfaces A: Physicochemical and Engineering Aspects 120:167–181. doi: 10.1016/S0927-7757(96)03720-X Final (2010) Final Environmental Impact Statement EXF Thinning, Fuels Reduction and Research Project. Pringle Falls Experimental Forest. Bend/Ft. Rock Ranger District, Deschutest National Forest, Deschutes County, Oregon. USDA Forest Service Pacific Northwest Research Station, Portland, OR, pp 283, 5 appendices Finlay RD, Ek H, Odham G, Soderstrom B (1988) Mycelial uptake, translocation and assimilation of nitrogen from 15 N-labelled ammonium by Pinus sylvestris plants infected with four different ectomycorrhizal fungi. New Phytologist 110:59–66 Finlay RD, Frostegård Å, Sonnerfeldt A-M (1992) Utilization of organic and inorganic nitrogen sources by ectomycorrhizal fungi in pure culture and in symbiosis with Pinus contorta Dougl . ex Loud . New Phytologist 120:105–115 Fitzgerald SA (2005) Fire Ecology of Ponderosa Pine and the Rebuilding of Fire-Resilient Ponderosa Pine. In: Ritchie MW, Maguire DA, Youngblood A (tech coords) Proceedings of the Symposium on Ponderosa Pine: Issues, Trends, and Management. General Technical Report PSW-GTR-198, USDA Forest Service Pacific Southwest Research Station, Klamath Falls, OR, pp 197-225 Flannigan MD, Stocks BJ, Wotton BM (2000) Climate change and forest fires. The Science of the Total Environment 262:221–229 73 Foiles MW, Graham RT, Olson DF Jr (1990) Grand Fir. In: Burns RM, Honkala BH, (tech coords) Silvics of North America: 1. Conifers, 2. Hardwoods. Agriculture Handbook 654. USDA Forest Service, Washington, DC. http://www.na.fs.fed.us/pubs/silvics_manual/Volume_1/abies/grandis.htm Accessed 2015 Fogel R (1979) The genus Leucophleps (Basidiomycotina, Leucogastrales). Canadian Journal of Botany 57:1718–1728 Frank B (2005) On the nutritional dependence of certain trees on root symbiosis with belowground fungi (an English translation of A.B. Frank’s classic paper of 1885). Mycorrhiza 15:267–275. doi: 10.1007/s00572-004-0329-y Gale CB, Keegan III CE, Berg EC, Daniels J, Christensen GA, Sorenson CB, Morgan TA, Polzin P (2012) Oregon’s Forest Products Industry and Timber Harvest, 2008: Industry Trends and Impacts of the Great Recession Through 2010. General Technical Report PNW-GTR-868. USDA Forest Service, Pacific Northwest Research Station, Portland, OR, 55 p Gara RI, Littke WR, Agee JK, Geiszler D, Stuart J, Driver C (1985) Influence of fires, fungi and mountain pine beetles on development of a lodgepole pine forest in south-central Oregon, In: DM Baumgartner, Krebill RG, Arnott JT, Weetman GF (eds) Lodgepole Pine: The Species and Its Management Symposium Proceedings. Washington State University, pp 153-162 Garcia MO, Smith JE, Luoma DL, Jones MD (In press) Ectomycorrhizal communities of ponderosa pine and lodgepole pine in the south-central Oregon pumice zone. Mycorrhiza Gardes M, Bruns TD (1996) Community structure of ectomycorrhizal fungi in a Pinus muricata forest: above- and below-ground views. Canadian Journal of Botany 74:1572–1583 Gardes M, Bruns TD (1993) ITS primers with enhanced specificity for basidiomycetes ­ application to the identification of mycorrhizae and rusts. Molecular Ecology 2:113–118 Gifford EW (1967) Ehtnographic notes on the Southwestern Pomo. Anthropological Records 25. University of California Press, Berkeley and Los Angeles Gilbert FF, Allwine R (1991a) Small mammal communities in the Oregon Cascade Range. In: Ruggiero LF, Aubry KB, Carey AB, Huff MH, (tech coords) Wildlife and vegetation of unmanaged Douglas-fir forests. General Technical Report PNW­ 74 GTR-285, USDA Forest Service Pacific Northwest Research Station, Portland, OR, pp 257-268 Gilbert FF, Allwine R (1991b) Spring bird communities in the Oregon Cascade Range. Pages 145-160 In: Ruggiero LF, Aubry KB, Carey AB, Huff MH, (tech coords) Wildlife and vegetation of unmanaged Douglas-fir forests. General Technical Report PNW-GTR-285, U.S. Department of Agriculture Forest Service Pacific Northwest Research Station, Portland, OR, pp 145-160 Graham RT (1990) Western White Pine In: Burns RM, Honkala BH, (tech coords) Silvics of North America: 1. Conifers, 2. Hardwoods. Agriculture Handbook 654. USDA Forest Service, Washington, DC. http://www.na.fs.fed.us/pubs/silvics_manual/Volume_1/pinus/monticola.htm. Accessed 2015 Graham RT, Jain F (2005) Ponderosa Pine Ecosystems. In: Ritchie MW, Maguire DA, Youngblood A (tech coords) Proceedings of the Symposium on Ponderosa Pine: Issues, Trends, and Management. General Technical Report PSW-GTR-198, USDA Forest Service Pacific Southwest Research Station, Klamath Falls, OR, pp 1-32 Hagerberg D, Thelin G, Wallander H (2003) The production of ectomycorrhizal mycelium in forests : Relation between forest nutrient status and local mineral sources. Plant and Soil 252:279–290. Hagerman SM, Jones MD, Bradfield GE, Gillespie M, Durall DM (1999) Effects of clear­ cut logging on the diversity and persistence of ectomycorrhizae at a subalpine forest. Canadian Journal of Forest Research 29:124–134 Hagerman SM, Sakakibara SM, Durall DM (2001) The potential for woody understory plants to provide refuge for ectomycorrhizal inoculum at an interior Douglas-fir forest after clear-cut logging. Canadian Journal of Forest Research 31:711–721. doi: 10.1139/cjfr-31-4-711 Halpern CB, Spies TA (1995) Plant species diversity in natural and managed forests of the Pacific Northwest. Ecological Applications 5:913–934 Hardy CC, Schmidt KM, Menakis JP, Sampson RN (2001) Spatial data for national fire planning and fuel management. International Journal of Wildland Fire 10:353–372 Hasselquist N, Germino MJ, McGonigle T, Smith WK (2005) Variability of Cenococcum colonization and its ecophysiological significance for young conifers at alpine­ treeline. New Phytologist 165:867–873. doi: 10.1111/j.1469-8137.2004.01275.x 75 Hessel DL (1988) The future of ponderosa pine forestry—A public manager’s perspective. In: Baumgartner DM, Lotan JE. Proceedings of the Symposium on Ponderosa Pine the species and its management. Cooperative Extension, Washington State University, Pullman, WA Hewitt RE, Bent E, Hollingsworth TN, Chapin III SF, Taylor DL (2013) Resilience of Arctic mycorrhizal fungal communities after wildfire facilitated by resprouting shrubs. Ecoscience 20:296–310 Horneck DA, Hart JM, Topper K, Koepsell B (1989) Methods of Soil Analysis Used in the Soil Testing Laboratory at Oregon State University. SM 89:4, Agriculture Experiment Station, Oregon State University, Corvallis, OR Horton K (1956) The ecology of lodgepole pine in Alberta and its role in forest succession. Forest Research Division Technical Note. 45, Department of Northern Affairs and National Resources Forestry Branch, Edmonton, Alberta, Canada, pp 29 Horton TR, Bruns TD (2001) The molecular revolution in ectomycorrhizal ecology: peeking into the black-box. Molecular Ecology 10:1855–1871 Horton TR, Bruns TD, Parker VT (1999) Ectomycorrhizal fungi associated with Arctostaphylos contribute to Pseudotsuga menziesii establishment. Canadian Journal of Botany 77:93–102. doi: 10.1139/cjb-77-1-93 Horton TR, Cázares E, Bruns TD (1998) Ectomycorrhizal, vesicular-arbuscular and dark septate fungal colonization of bishop pine (Pinus muricata) seedlings in the first 5 months of growth after wildfire. Mycorrhiza 8:11–18 Hunter JC (1997) Correspondence of environmental tolerances with leaf and branch attributes for six co-occuring species of broadleaf evergreen trees in northern California. Trees 11:169–175 Jongmans AG, van Breeman N (1997) Rock-eating fungi. Nature 389:682–683 Jumpponen A, Jones KL, Mattox JD, Yaege C (2010) Massively parallel 454-sequencing of fungal communities in Quercus spp. ectomycorrhizas indicates seasonal dynamics in urban and rural sites. Molecular ecology 19:41–53. doi: 10.1111/j.1365-294X.2009.04483.x Jumpponen A, Trappe JM (1998) Performance of Pinus contorta inoculated with two strains of root endophytic fungus, Phialocephala fortinii: effects of synthesis system and glucose concentration. Canadian Journal of Botany 76:1205–1213 76 Kasischke ES, Stocks BJ (2000) Fire, climate change, and carbon cycling in the boreal forest. Springer-Verlag, New York Kauffman JB (1986) The Ecological Response of the Shrub Component to Prescribed Burning in Mixed Conifer Ecosystems. Dissertation, University of California, Berkeley Kauffman JB, Martin RE (1990) Sprouting Shrub Response to Different Seasons and Fuel Consumption Levels of Prescribed Fire in Sierra Nevada Mixed Conifer Ecosystems. Forest Science 36:748–764 Keeler-Wolf T (1988) The role of Chrysolepis chrysophylla (Fagaceae) in the Pseudotsuga-hardwood forest of the Klamath Mountains of California. Madroño 35:285–308 Kennedy PG, Smith DP, Horton TR, Molina RJ (2012) Arbutus menziesii (Ericaceae) facilitates regeneration dynamics in mixed evergreen forests by promoting mycorrhizal fungal diversity and host connectivity. American journal of botany 99:1691–701. doi: 10.3732/ajb.1200277 Keyes CR, Maguire DA (2005) Positive Seedling-Shrub Relationships in Natural Regeneration of Ponderosa Pine. In: Ritchie MW, Maguire DA, Youngblood A (tech coords) Proceedings of the Symposium on Ponderosa Pine: Issues, Trends, and Management. General Technical Report PSW-GTR-198, USDA Forest Service Pacific Southwest Research Station, Klamath Falls, OR, pp 95-107 Kluber LA, Smith JE, Myrold DD (2011) Distinctive fungal and bacterial communities are associated with mats formed by ectomycorrhizal fungi. Soil Biology and Biochemistry 43:1042–1050. doi: 10.1016/j.soilbio.2011.01.022 Kluber LA, Tinnesand KM, Caldwell BA, Dunham SM, Yarwood RR, Bottomley PJ, Myrold DD (2010) Ectomycorrhizal mats alter forest soil biogeochemistry. Soil Biology and Biochemistry 42:1607–1613. doi: 10.1016/j.soilbio.2010.06.001 Kohler A, Kuo A, Nagy LG, Morin E, Barry KW, Buscot F, Canbäck B, Choi C, Cichocki N, Clum A, Colpaert J, Copeland A, Costa MD, Doré J, Floudas D, Gay G, Girlanda M, Henrissat B, Herrmann S, Hess J, Högberg N, Johansson T, Khouja H­ R, LaButti K, Lahrmann U, Levasseur A, Lindquist EA, Lipzen A, Marmeisse R, Martino E, Murat C, Ngan CY, Nehls U, Plett JM, Pringle A, Ohm RA, Perotto S, Peter M, Riley R, Rineau F, Ruytinx J, Salamov A, Shah F, Sun H, Tarkka M, Tritt A, Veneault-Fourrey C, Zuccaro A, Mycorrhizal Genomics Initiative Consortium, Tunlid A, Grigoriev IV, Hibbett DS, Martin F (2015) Convergent losses of decay mechanisms and rapid turnover of symbiosis genes in mycorrhizal mutualists. Nature Genetics. doi: 10.1038/ng.3223 77 Kolb PF, Robberecht R (1996) High temperature and drought stress effects on survival of Pinus ponderosa seedlings. Tree Physiology 16:665–672 Korkama T, Pakkanen A, Pennanen T (2006) Ectomycorrhizal community structure varies among Norway spruce (Picea abies) clones. New Phytologist 171:815–24. doi: 10.1111/j.1469-8137.2006.01786.x Kranabetter JM (1999) The effect of refuge trees on a paper birch ectomycorrhiza community. Canadian Journal of Botany 77:1523-1528 Kranabetter JM, Durall DM, MacKenzie WH (2009a) Diversity and species distribution of ectomycorrhizal fungi along productivity gradients of a southern boreal forest. Mycorrhiza 19:99–111. doi: 10.1007/s00572-008-0208-z Kranabetter JM, Friesen J, Gamiet S, Kroeger P (2009b) Epigeous fruiting bodies of ectomycorrhizal fungi as indicators of soil fertility and associated nitrogen status of boreal forests. Mycorrhiza 19:535–48. doi: 10.1007/s00572-009-0255-0 Krawchuk MA, Moritz MA, Parisien M, Van Dorn J, Haeyhoe K (2009) Global Pyrogeography: the Current and Future Distribution of Wildfire. PLoS ONE 4:1­ 12. doi: 10.1371/journal.pone.0005102 Landeweert R, Hoffland E, Finlay RD, Kuyper TW, van Breeman N (2001) Linking plants to rocks: ectomycorrhizal fungi mobilize nutrients from minerals. Trends in Ecology & Evolution 16:248–254 Larsen DM (1976) Soil Resource Inventory: Deschutes National Forest. USDA Forest Service Pacific Northwest Region, Portland, OR, pp 381 Larson MM (1963) Initial root development of ponderosa pine seedlings as related to germination date and size of seed. Forest Science 9(4):456-460 Leake JR (1994) Tansley Review No. 69 The biology of myco-heterotrophic (’ saprophytic ') plants. New Phytologist 127:171–216. LeDuc SD, Lilleskov EA, Horton TR, Rothstein DE (2013) Ectomycorrhizal fungal succession coincides with shifts in organic nitrogen availability and canopy closure in post-wildfire jack pine forests. Oecologia 172:257–69. doi: 10.1007/s00442-012­ 2471-0 Lefevre CK (2002) Host associations of Tricholoma magnivelare, the American Matsutake. Dissertation, Oregon State University 78 Lehto T (1992) Mycorrhizas and Drought Resistance of Picea sitchensis (Bong.) Carr. I. In Conditions of Nutrient Deficiency. New Phytologist 122:661–668 LePage B, Currah R, Stockey R, Rothwell G (1997) Fossil ectomycorrhizae from the Middle Eocene. American Journal of Botany 84:410-412 Leyval C, Turnau K, Haselwandter K (1997) Effect of heavy metal pollution on mycorrhizal colonization and function: physiological, ecological and applied aspects. Mycorrhiza 7:139-153 Lilleskov EA, Fahey TJ, Horton TR, Lovett GM (2002) Belowground ectomycorrhizal fungal community change over a nitrogen deposition gradient in Alaska. Ecology 83:104–115 Loomis J (2005) Updated Outdoor Recreation Use Values on National Forests and Other Public Lands. General Technical Report PNW-GTR-65, USDA Forest Service Pacific Northwest Research Station, Portland, OR, pp 26 López B, Sabaté S, Gracia CA (2001) Vertical distribution of fine root density, length density, area index and mean diameter in a Quercus ilex forest. Tree physiology 21:555–60. Lotan JE (1967) Cone Serotiny of Lodgepole Pine Near West Yellowstone, Montana. Forest Science 13:55–59 Lotan JE (1976) Cone Serotiny - Fire Relationships in Lodgepole Pine Fire Relationships. In: Tall Timbers Fire Ecology Conference Proceedings 14, Tall Timbers Research Center, Tallahassee, FL, pp 267–278 Lotan JE, Critchfield WB (1990) Lodgepole Pine. In: Burns RM, Honkala BH, (tech coords) Silvics of North America: 1. Conifers, 2. Hardwoods. Agriculture Handbook 654. USDA Forest Service, Washington, DC. vol.2, 877 p Lowerey DP (1984) Ponderosa Pine An American Wood. FS-254 Revised, USDA Forest Service Intermountain Forest and Range Experiment Station, Ogden, UT, pp 7 Luoma D (1987) Synecology of the Monotropoideae within Limpy Rock Research Natural Area, Umpqua National Forest, Oregon. Master’s Thesis, Oregon State University Mahar MJ (1953) Ethnobotany of the Oregon Paiutes of the Warm Springs Indian Reservation. B.A. Thesis, Reed College. 79 Mahmood S, Finlay RD, Wallander H, Erland S (2002) Ectomycorrhizal colonisation of roots and ash granules in a spruce forest treated with granulated wood ash. Forest Ecology and Management 160:65–74. Manos PS, Cannon CH, Oh S (2008) Phylogenetic Relationships and Taxonomic Status Of the Paleoendemic Fagaceae Of Western North America: Recognition Of A New Genus, Notholithocarpus. Madroño 55:181–190. doi: 10.3120/0024-9637-55.3.181 Massicotte HB, Trappe JM, Peterson RL, Melville LH (1992) Studies on Ceonococcum geophilum. II. Sclerotium morphology, germination, and formation in pure culture and growth pouches. Canadian Journal of Botany 70:125–132 McCune B, Grace JB (2002) Analysis of Ecological Communities. MjM Software, Gleneden Beach McCune B, Mefford MJ (2011) PC-ORD. Multivariate Analysis of Ecological Data, Version 6. MjM Software, Gleneden Beach, OR, USA McDonald PM (2008) Chrysolepis Hjelmqvist: chinquapin. In: Bonner FT, Karrfalt RP (eds) Woody plant seed manual. Agricultural Handbook No. 727, USDA Forest Service, Washington, DC, pp 404-406 McDonald PM, Minore D, Atzet T (1983) Southwestern Oregon-northern California hardwoods. In: Burns RM (comp) Silvicultural systems for the major forest types of the United States. Agricultural Handbook 445, USDA Forest Service, Washington, DC, pp 29-32 McHugh CW, Kolb TE, Wilson JL (2003) Bark Beetle Attacks on Ponderosa Pine Following Fire in Northern Arizona. Environmental Entomology 32:510–522 McKee A (1990) Giant chinkapin. In: Burns RM, Honkala BH (eds) Silvics of North America: Volume 2, hardwoods, Agricultur. Forest Service, U.S. Deparatment of Agriculture, Washington, DC, pp 234–239 Meyer R (2012) Chrysolepis chrysophylla. In: Fire Effects Information System. USDA Forest Service Rocky Mountain Research Station, Fire Sciences Laboratory. http://www.fs.fed.us/database/feis/plants/tree/chrchr/all.html Accessed 2015 Molina R, Trappe JM (1982a) Patterns of Ectomycorrhizal Host Specificity and Potential Among Pacific Northwest Conifers and Fungi. Forest Science 28:423–458 Molina R, Trappe JM (1982b) Lack of mycorrhizal specificity by the ericaceous hosts Arbutus menziesii and Arctostaphylos uva-ursi. New Phytologist. 90:495-509 80 Molina R, Trappe JM (1994) Biology of the ectomycorrhizal genus, Rhizopogon. I. Host associations, host specificity and pure culture syntheses. New Phytologist 126:653­ 675 Molina RJ, Massicotte HB, Trappe JM (1992) Specificity phenomena in mycorrhizal symbiosis: community ecological consequences and practical implications. In: Allen MJ (ed.) Mycorrhizal Functioning an Integrative Plant-Fungal Process. Chapman and Hall, New York Morris MH, Pérez-Pérez MA, Smith ME, Bledsoe CS (2009) Influence of host species on ectomycorrhizal communities associated with two co-occurring oaks (Quercus spp.) in a tropical cloud forest. FEMS Microbiology Ecology 69:274–87. doi: 10.1111/j.1574-6941.2009.00704.x Mote PW, Salathé Jr. EP (2010) Future Climate in the Pacific Northwest. Climatic Change 102:29–50 Nambiar EKS, Sands R (1993) Competition for water and nutrients in forests. Canadian Journal of Forest Research 23:1955-1968 Nara K, Hogetsu T (2004) Ectomycorrhizal Fungi on Established Shrubs Facilitate Subsequent Seedling Establishment of Successional Plant Species. Ecology 85:1700–1707 NCSS (2015) National Cooperative Soil Survey. National Cooperative Soil Survey Characterization Database. http://ncsslabdatamart.sc.egov.usda.gov/ Accessed December 07, 2015 Newsham KK, Fitter AH, Watkinson AR (1995) Arbuscular mycorrhiza protect an annual grass from root pathogenic fungi in the field. Journal of Ecology 83:991–1000 Niemiec SS, Ahrens GR, Willits S, Hibbs DE (1995) Hardwoods of the Pacific Northwest. Research Contribution 8. Oregon State University, Forest Research Laboratory, Corvallis, OR pp 115 Noble DL (1979) Roots of lodgepole pine seedlings reach depth of only 3 to 4 inches their first season. Research Note RM-36, USDA Forest Service Rocky Mountain Forest and Range Experiment Station, Fort Collins, CO, pp 3 Oh S, Manos PS (2008) Molecular phylogenetics and cupule evolution in Fagaceae as inferred from nuclear CRABS CLAW sequences. TAXON 57:434–451. Oliver W, Ryker R (1990) Ponderosa Pine In: Burns RM, Honkala BH, (tech coords) Silvics of North America: 1. Conifers, 2. Hardwoods. Agriculture Handbook 654. 81 USDA Forest Service, Washington, DC. http://www.na.fs.fed.us/pubs/silvics_manual/Volume_1/pinus/ponderosa.htm. Accessed 2015 Oregon (2015) Oregon forest facts & figures 2015-16. Oregon Forest Resources Institute. Portland, OR, pp 32. http://oregonforests.org/sites/default/files/publications/pdf/OFRI_FactsFigures_201 5-16.pdf Page-Dumroese D, Ferguson D, McDaniel P, Johnson-Maynard J (2007) Chemical Changes Induced by pH Manipulations of Volcanic Ash-Influenced Soils. In: PageDumroese D, Miller R, Mital J, McDaniel P, Miller D, (tech eds) Volcanic-AshDerived Forest Soils of the Inland Northwest: Properties and Implications for Management and Restoration. Proceedings RMRS-P-44, USDA Forest Service Rocky Mountain Research Station, Fort Collins, CO, pp 185-202 Palmer JM, Lindner DL, Volk TJ (2008) Ectomycorrhizal characterization of an American chestnut (Castanea dentata)-dominated community in Western Wisconsin. Mycorrhiza 19:27–36. doi: 10.1007/s00572-008-0200-7 Parchman TL, Gompert Z, Mudge J, Schilkey FD, Benkman CW, Buerkle A (2012) Genome-wide association genetics of an adaptive trait in lodgepole pine. Molecular Ecology 21:2991–3005. doi: 10.1111/j.1365-294X.2012.05513.x Parker TJ, Clancy KM, Mathiasen RL (2006) Interactions among fire, insects and pathogens in coniferous forests of the interior western United States and Canada. Agricultural and Forest Entomology 8:167–189 Peay KG, Garbelotto M, Bruns TD (2009) Spore heat resistance plays an important role in disturbance-mediated assemblage shift of ectomycorrhizal fungi colonizing Pinus muricata seedlings. Journal of Ecology 97:537–547. doi: 10.1111/j.1365­ 2745.2009.01489.x Perez-Moreno J, Read DJ (2000) Mobilization and transfer of nutrients from litter to tree seedlings via the vegetative mycelium of ectomycorrhizal plants. New Phytologist 145:301–309. Peyronel B, Fassi B, Fontana A, Trappe JM (1969) Terminology of Mycorrhizae. Mycologia 61:410–411 Phillips CL, Kluber LA, Martin JP, Caldwell BA, Bond BJ (2012) Contributions of ectomycorrhizal fungal mats to forest soil respiration. Biogeosciences 9:2099– 2110. doi: 10.5194/bg-9-2099-2012 82 Pigott CD (1982) Survival of Mycorrhiza Formed by Cenoccocum geophilum Fr. in Dry Soils. New Phytologist 92:513–517 Plassard C, Dell B (2010) Phosphorus nutrition of mycorrhizal trees. Tree physiology 30:1129–39. doi: 10.1093/treephys/tpq063 Read DJ, Perez-Moreno J (2003) Mycorrhizas and nutrient cycling in ecosystems – a journey towards relevance? New Phytologist 157:475–492 Redecker D, Kodner R, Graham LE (2000) Glomalean Fungi from the Ordovician. Science 289:1920–1921 Regvar M, Likar M, Piltaver A, Kugonič N, Smith JE (2010) Fungal community structure under goat willows (Salix caprea L.) growing at metal polluted site: the potential of screening in a model phytostabilisation study. Plant and Soil 330:345–356. doi:10.1007/s11104-009-0207-7 Remy W, Taylor TN, Hass H, Kerp H (1994) Four Hundred-Million-Year-Old Vesicular Arbuscular Mycorrhizae. Proceedings of the National Academy of Sciences of the USA 91:11841–11843 Review (2001) Evolution of Federal Wildland Fire Management Policy. In: Review and Update of the 1995 Federal Wildland Fire Management Policy, BLM Office of Fire and Aviation, National Interagency Fire Center, pp 1-8 Rogers BM, Neilson RP, Drapek R, Lenihan JM, Wells JR, Bachelet D, Law BE (2011) Impacts of climate change on fire regimes and carbon stocks of the U.S. Pacific Northwest. Journal of Geophysical Research 116:G03037. doi: 10.1029/2011JG001695 RStudio Team (2013) RStudio: Integrated Development for R. RStudio, Inc., Boston, MA. http://www.rstudio.com/. Accessed 2013 Running SW (2006) Is global warming causing more, larger wildfires? Science 313:927– 928. Rygiewicz PT, Bledsoe CS, Zasoski RJ (1984) Effects of ectomycorrhize and solutin pH on [15N]ammonium uptake by coniferous seedlings. Canadian Journal of Forest Research 14:885–892 Sang T, Crawford DJ, Stuessy TF (1997) Chloroplast DNA phylogeny, reticulate evolution and biogeography of Paeonia (Paeoniaceae). American Journal of Botany 84: 1120–1136 83 Savage M, Mast JN (2005) How resilient are southwestern ponderosa pine forests after crown fires? Canadian Journal of Forest Research 35:967–977. doi: 10.1139/X05­ 028 Schenck SM, Gifford EW (1952) Karok Ethnobotany. University of California Anthropological Records 13:377–392 Shi L, Guttenberger M, Kottke I, Hampp R (2002) The effect of drought on mycorrhizas of beech (Fagus sylvatica L.): changes in community structure, and the content of carbohydrates and nitrogen storage bodies of the fungi. Mycorrhiza 12:303–311. doi: 10.1007/s00572-002-0197-2 Shoal R (2009) Occurrence and habitat status evaluation for Golden Chinquapin (Chrysolepis chrysophylla (Dougl. ex Hook.) Hjelmqvist ) on the Olympic National Forest. Interagency Special Status Species Program Report, USDA Forest Service Olympic National Forest, pp 10 Silba J. (1990) A supplement to the international census of the Coniferae II. Phytologia 68(1):7-78 Silcox FA (1911) Fire prevention and control on the national forests. In: Arnold JA (ed) Yearbook of the United States Department of Agriculture for 1910. Government Printing Office, Washington D.C. pp 413-424 Simard SW (1997) Net transfer of carbon between ectomycorrhizal tree species in the field. Nature 388:579-582 Simard SW (2009) The foundational role of mycorrhizal networks in self-organization of interior Douglas-fir forests. Forest Ecology and Management 258:S95–S107. doi: 10.1016/j.foreco.2009.05.001 Simard SW, Austin ME (2010) The role of mycorrhizas in forest soil stability with climate change. In: Simard S (ed.) Climate Change and Variability. InTech. doi: 10.5772/9813 Simard SW, Jones MD, Durall DM, Perry DA, Myrold DD, Molina R (1997a) Reciprocal Transfer of Carbon Isotopes between Ectomycorrhizal Betula papyrifera and Pseudotsuga menziesii. New Phytologist 137:529-542 Simard SW, Molina R, Smith JE, Perry DA, Jones MD (1997b) Shared compatibility of ectomycorrhizae on Pseudotsuga menziesii and Betula papyrifera seedlings grown in mixture in soils from southern British Columbia. Canadian Journal of Forest Research 27:331-342 84 Simpson M (2007) Forested Plant Associations of the Oregon East Cascades. Technical Paper R6-NR-ECOL-TP-03-2007, USDA Forest Service Pacific Northwest Region, pp 602 Smith JE, Mckay D, Brenner G, McIver J, Spatafora JW (2005) Early impacts of forest restoration treatments on the ectomycorrhizal fungal community and fine root biomass in a mixed conifer forest. Journal of Applied Ecology 42:526–535. doi: 10.1111/j.1365-2664.2005.01047.x Smith JE, Mckay D, Niwa CG, Thies WG, Brenner G, Spatafora JW (2004) Short-term effects of seasonal prescribed burning on the ectomycorrhizal fungal community and fine root biomass in ponderosa pine stands in the Blue Mountains of Oregon. Canadian Journal of Forest Research 2491:2477–2491. doi: 10.1139/X04-124 Smith SE, Read DJ (2008) Mycorrhizal Symbiosis. 3rd edition. Elsevier, New York, NY Smithwick EAH, Harmon ME, Remillard SM, Acker SA, Franklin JF (2002) Potential upper bounds of carbon stores in forests of the Pacific Northwest. Ecological Applications 12:1303–1317 Soil Survey Staff (2014) Lapine Series. Official Soil Series Descriptions, USDA Natural Resources Conservation Service. http://soils.usda.gov/technical/classification/osd/index.html Accessed 2014 Sommers WT, Coloff SG, Conard SG (2011) Synthesis of Knowledge: Fire History and Climate Change. JFSP Synthesis Reports, Paper 19, pp 341 Song YY, Zeng RS, Xu JF, Li J, Shen X, Yihdego WG (2010) Interplant communication of tomato plants through underground common mycorrhizal networks. PloS ONE 5(10):e13324. doi: 10.1371/journal.pone.0013324 Southard LF (2011) The History of Cooperative Forest Fire Control and the Weeks Act. Forest History Today Spring/Fall 2011:17-20 Starker TJ (1934) Fire resistance in the forest. Journal of Forestry 32(4):462–467 Strullu-Derrien C, Kenrick P, Pressel S, Duckett JG, Rioult J-P, Strullu D-G (2014) Fungal associations in Horneophyton ligneri from the Rhynie Chert (c. 407 million year old) closely resemble those in extant lower land plants: novel insights into ancestral plant-fungus symbioses. New Phytologist 203:964–79. doi: 10.1111/nph.12805 Talbot JM, Bruns TD, Taylor JW, Smith DP, Branco S, Glassman SI, Erlandson S, Vilgalys R, Liao H-L, Smith ME, Peay KG (2014) Endemism and functional convergence across the North American soil mycobiome. Proceedings of the 85 National Academy of Sciences of the USA 111:6341–6. doi: 10.1073/pnas.1402584111 Tarrant RF (1947) First Forest Soil Survey Gives Significant Results. Forest Research Notes 36. USDA Forest Service Pacific Northwest Forest Experiment Station, Portland, OR, pp 4 Tate JA, Simpson BB (2003) Paraphyly of Tarasa (Malvaceae) and diverse origins of the polyploid species. Systematic Botany 28: 723 –737 Tatry M-V, El Kassis E, Lambilliotte R, Corratge C, van Aarle I, Amenc LK, Alary R, Zimmermann S, Sentenac H, Plassard C (2009) Two differentially regulated phosphate transporters from the symbiotic fungus Hebeloma cylindrosporum and phosphorus acquisition by ectomycorrhizal Pinus pinaster. The Plant Journal 57:1092–102. doi: 10.1111/j.1365-313X.2008.03749.x Tedersoo L, May TW, Smith ME (2010) Ectomycorrhizal lifestyle in fungi: global diversity, distribution, and evolution of phylogenetic lineages. Mycorrhiza 20:217–63. doi: 10.1007/s00572-009-0274-x Tedersoo L, Smith ME (2013) Lineages of ectomycorrhizal fungi revisited: Foraging strategies and novel lineages revealed by sequences from belowground. Fungal Biology Reviews 27:83–99. doi: 10.1016/j.fbr.2013.09.001 Teste FP, Simard SW, Durall DM, Guy RD, Jones MD, Schoonmaker AL(2009) Access to Mycorrhizal Networks and Roots of Trees : Importance for Seedling Survival and Resource Transfer. Ecology 90:2808–2822. Treseder K, Mack MC, Cross A (2004) Relationships among fires, fungi, and soil dynamics in Alaskan boreal forests. Ecological Applications 14:1826–1838 Tuason MMS, Arocena JM (2009) Calcium oxalate biomineralization by Piloderma fallax in response to various levels of calcium and phosphorus. Applied and Environmental Microbiology 75:7079–85. doi: 10.1128/AEM.00325-09 Turner DP, Koerper GJ, Harmon ME, Lee JJ (1995) A Carbon Budget for Forests of the Coterminous United States. Ecological Applications 5:421–436 Valentine LL, Fiedler TL, Hart AN, Petersen CA, Berninghausen HK, Southworth D (2004) Diversity of ectomycorrhizas associated with Quercus garryana in southern Oregon. Canadian Journal of Botany 82:123–135. doi: 10.1139/B03-117 86 van der Heijden MGA, Martin FM, Selosse M-A, Sanders IR (2015) Mycorrhizal ecology and evolution: the past, the present, and the future. New Phytologist 205:1406– 1423. doi: 10.1111/nph.13288 Visser S (1995) Ectomycorrhizal fungal succession in jack pine stands following wildfire. New Phytologist 129:389–401 Volland LA (1985) Plant associations of the central Oregon pumice zone. R6-ECOL-104­ 1985, USDA Forest Service Pacific Northwest Region, Portland, OR pp 138 Walker JF, Horton JL (2003) Diversity and ecology of mycorrhizal fungi associated with oak seedlings in the Appalachian Mountains. Dissertation, Virginia Polytechnic Institute and State University. Walker B, Salt D (2006) Resilience thinking: sustaining ecosystems and people in a changing world. Island Press, Washington D.C. Wallander H (2000) Uptake of P from apatite by Pinus sylvestris seedlings colonised by different ectomycorrhizal fungi. Plant and Soil 218:249–256. Wang Q, Gao C, Guo L (2011) Ectomycorrhizae associated with Castanopsis fargesii (Fagaceae) in a subtropical forest, China. Mycological Progress 10:323–332. doi: 10.1007/s11557-010-0705-2 Wang Q, He XH, Guo L (2012) Ectomycorrhizal fungus communities of Quercus liaotungensis Koidz of different ages in a northern China temperate forest. Mycorrhiza 22:461–70. doi: 10.1007/s00572-011-0423-x Weatherspoon PC, Skinner CN (1987) An Assessment of Factors Associated with Damage to Tree Crowns from the 1987 Wildfires in Northern California. Forest Science 41:430–451 Westerling AL, Hidalgo HG, Cayan DR, Swetnam TW (2006) Warming and earlier spring increase western U.S. forest wildfire activity. Science 313:940–943 White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds.) PCR Protocols: a guide to methods and applications. Academic Press, New York, pp 315–322 Woodward J, Strong N, Coe FC, Cloughesy M (2011) Wildlife in Managed Forests-­ Oregon Forests as Habitat. Oregon Forest Resources Institute, Portland, OR, pp 40 87 Youngblood A (2009) Study Plan Forest Dynamics After Thinning and Fuel Reduction in Dry Forests. SP-PNW-4577- USDA Forest Service Pacific Northwest Research Station, Portland, OR, pp 38 Youngblood A (comp) (1995) Research Publications of the Pringle Falls Experimental Forest, Central Oregon Cascade Range, 1930 to 1993. General Technical Report PNW-GTR-347, USDA Forest Service Pacific Northwest Research Station, Portland, OR, pp 45 Zeglin LH, Kluber LA, Myrold DD (2012) The importance of amino sugar turnover to C and N cycling in organic horizons of old-growth Douglas-fir forest soils colonized by ectomycorrhizal mats. Biogeochemistry 112:679–693. doi: 10.1007/s10533­ 012-9746-8 Zhang J, Oliver WW, Busse MD (2006) Growth and development of ponderosa pine on sites of contrasting productivities: relative importance of stand density and shrub competition effects. Canadian Journal of Forest Research 36:2426–2438. doi: 10.1139/X06-078 88 APPENDICES 89 Appendix A Table S1. EMF taxa found on Lookout Mountain. Bold = only on chinquapin, Shaded = on chinquapin & Pinaceae Consensus # Taxa GenBank Target Name #ACN Max ID # of P transect pairs # of C transect pairs 94 Agaricomycetes 1 Amphinema sp. JN943925.1 94% 1 0 124 Agaricomycetes 2 Amphinema sp. JN943925.1 90% 1 0 125 Agaricomycetes 3 Hydnum sp. FN669208.1 94% 1 0 133 Agaricomycetes 4 Sistotrema sp. KF218963.1 97% 1 0 47 Amanita aprica Amanita aprica KF561972.1 99% 1 0 153 Amanita pantherina Amanita pantherina EU525997.1 100% 1 0 121 Amphinema 1 Amphinema sp. JN943898.1 99% 1 0 23 Amphinema byssoides Amphinema byssoides JQ711816.1 99% 3 0 135 Ascomycota 1 Ascomycota sp. JQ711841.1 87% 0 1 98 Basidiomycete 1 Basidiomycete sp. DQ365644.1 93% 1 0 6 Byssocorticium 1 Byssocorticium atrovirens AJ889936.1 92% 10 6 21 Byssocorticium 2 Byssocorticium atrovirens AJ889936.1 89% 4 4 1 Cenococcum geophilum 1 Cenococcum geophilum JX145390.1 97% 16 11 5 Cenococcum geophilum 2 Cenococcum geophilum JQ711896.1 99% 9 5 9 Cenococcum geophilum 3 Cenococcum geophilum AY394919.1 99% 1 5 4 Cortinarius 1 Cortinarius hemitrichus DQ097870.1 99% 9 2 90 Consensus # Taxa GenBank Target Name #ACN Max ID # of P transect pairs # of C transect pairs 81 Cortinarius 10 Cortinarius sp. FN669181.1 95% 0 1 92 Cortinarius 11 Cortinarius cf biformis HQ604700.1 99% 2 0 29 Cortinarius 12 Cortinarius fulvscens GQ159914.1 99% 2 4 11 Cortinarius 13 Cortinarius acutus GQ159881.1 100% 1 6 88 Cortinarius 14 Cortinarius fulvscens HQ604731.1 96% 1 0 103 Cortinarius 15 Cortinarius sp. GQ159878.1 89% 1 0 130 Cortinarius 16 Cortinarius caput-medusae HQ845170.1 93% 0 1 142 Cortinarius 17 Cortinarius casimiri HQ604719.1 98% 1 0 151 Cortinarius 18 Cortinarius sp. FJ717589.1 99% 1 0 152 Cortinarius 19 Cortinarius obtusus GQ159764.1 87% 1 0 59 Cortinarius 2 Cortinarius malachius KJ705143.1 100% 2 0 154 Cortinarius 20 Cortinarius ferrugineovelatus NR_131875.1 99% 1 0 158 Cortinarius 21 Cortinarius caput-medusae HQ845170.1 95% 0 1 132 Cortinarius 22 Cortinarius sp. FJ717527.1 97% 1 0 134 Cortinarius 23 Cortinarius casimiri GQ159814.1 98% 1 0 22 Cortinarius 3 Cortinarius candelaris GQ159883.1 99% 5 0 18 Cortinarius 4 Cortinarius acutovelatus AY669655.1 99% 5 1 42 Cortinarius 5 Cortinarius sp. GQ159878.1 99% 4 1 44 Cortinarius 6 Cortinarius casimiri HQ604719.1 99% 3 2 91 Table S1. (Continued) Consensus # Taxa GenBank Target Name #ACN Max ID # of P transect pairs # of C transect pairs 43 Cortinarius 7 Cortinarius colymbadinus NR_131819.1 98% 2 0 86 Cortinarius 8 Cortinarius volvatus NR_130287.1 99% 0 1 83 Cortinarius 9 Cortinarius sp. EU821651.1 99% 1 0 99 Cortinarius acutus Cortinarius acutus FJ157002.1 98% 1 0 48 Cortinarius alboviolaceus Cortinarius alboviolaceus JF899552.1 99% 0 2 137 Cortinarius boulderensis Cortinarius boulderensis NR_121207.1 99% 1 0 77 Cortinarius clandestinus Cortinarius clandestinus GQ159862.1 99% 1 0 150 Cortinarius firmus Cortinarius firmus AF389163.1 99% 1 0 31 Cortinarius laetissimus Cortinarius laetissimus GQ159898.1 99% 3 1 93 Cortinarius limonius Cortinarius limonius GQ159869.1 99% 1 0 148 Cortinarius obtusus Cortinarius obtusus FJ717550.1 100% 1 0 24 Cortinarius pingue Cortinarius pingue GQ159874.1 99% 3 0 15 Elaphomyces 1 Elaphomyces sp. JQ272414.1 97% 0 6 112 Gautieria 1 Gautieria sp. AF377089.1 94% 1 0 45 Gautieria monticola 1 Gautieria monticola AF377094.1 99% 2 0 34 Gautieria monticola 2 Gautieria monticola AF377075.1 99% 2 0 73 Gautieria monticola 3 Gautieria monticola AF377101.1 98% 1 0 19 Helotiales 1 Melinomyces sp. KC007335.1 97% 2 3 92 Table S1. (Continued) Consensus # Taxa GenBank Target Name #ACN Max ID # of P transect pairs # of C transect pairs 26 Helotiales 2 Melinomyces bicolor AY394885.1 98% 2 2 101 Helotiales 3 Helotiales sp. EU880594.1 99% 1 0 7 Hygrophorus 1 Hygrophorus persoonii JF908067.1 91% 1 6 96 Hygrophorus 2 Hygrophorus persoonii JF908067.1 87% 0 2 126 Hygrophorus 3 Hygrophorus unicolor AY242857.1 87% 1 0 127 Hygrophorus aureus Hygrophorus aureus JF908076.1 97% 1 0 72 Hygrophorus cf subalpinus Hygrophorus cf subalpinus JN021041.1 99% 1 0 89 Hygrophorus purpurascens Hygrophorus purpurascens HQ650731.1 99% 1 0 140 Hysterangium separabile Hysterangium separabile EU563921.1 98% 0 1 20 Inocybe 1 Inocybe flocculosa var. flocculosa HQ604185.1 99% 0 6 49 Inocybe 2 Inocybe albietis HQ604165.1 99% 1 0 97 Inocybe 3 Inocybe nitidiuscula HQ604085.1 82% 0 1 100 Inocybe 4 Inocybe flocculosa var. flocculosa HQ604084.1 97% 1 0 116 Inocybe 5 Inocybe nitidiuscula HQ604260.1 90% 1 0 149 Inocybe 6 Inocybe pupureobadia JN580875.1 94% 1 0 107 Inocybe silvae-herbaceae Inocybe silvae-herbaceae NR_119991.1 99% 1 0 108 Lactarius 1 Lactarius deliciosus JQ711835.1 94% 1 0 157 Lactarius 2 Lactarius deliciosus JQ711835.1 92% 1 0 93 Table S1. (Continued) Consensus # Taxa GenBank Target Name #ACN Max ID # of P transect pairs # of C transect pairs 50 Lactarius deliciosus Lactarius deliciosus var. deterrimus EF685051.1 99% 3 0 8 Lactarius resimus Lactarius resimus JF899563.1 99% 4 1 37 Lactarius rufus Lactarius rufus JQ712001.1 99% 1 0 51 Lactarius xanthogalactus Lactarius xanthogalactus EU726293.1 99% 1 0 114 Leucogaster 1 Leucogaster microsporus EU846312.1 91% 1 0 67 Leucophleps 1 Leucophleps spinispora AY621788.1 79% 2 0 63 Leucophleps 2 Leucophleps spinispora AY621775.1 79% 2 0 80 Leucophleps 3 Leucophleps spinispora AY621784.1 76% 1 0 129 Leucophleps 4 Leucophleps spinispora AY621796.1 80% 1 0 64 Leucophleps spinispora AY621764.1 99% 3 0 102 Lyophyllum 1 Leucophleps spinispora Lyophyllum semitale var. intermedium KP192604.1 99% 1 0 74 Melanogaster 1 Melanogaster sp. KC152159.1 96% 2 0 106 Melanogaster 2 Melanogaster intermedius EU784372.1 82% 0 1 91 Pezizaceae 1 Sarcosphaera coronaria DQ200843.1 89% 2 0 87 Phellodon melaleucus Phellodon melaleucus AY228355.1 99% 1 0 110 Phialocephala 1 Phialocephala cf. fortinii KM460828.1 99% 1 0 111 Phialocephala 2 Phialocephala sp. JX243870.1 94% 2 0 105 Phialocephala 3 Phialocephala sp. KJ542273.1 94% 1 0 94 Table S1. (Continued) Consensus # Taxa GenBank Target Name #ACN Max ID # of P transect pairs # of C transect pairs 60 Phialocephala fortinii 1 Phialocephala fortinii KJ817278.1 98% 4 0 131 Phialocephala fortinii 2 Phialocephala fortinii KF850367.1 99% 1 0 10 Piloderma 1 Piloderma olivaceum JQ11915.1 93% 6 2 155 Piloderma 10 Piloderma olivaceum JQ711915.1 87% 1 0 2 Piloderma 2 Piloderma sp. JQ711935.1 99% 7 8 69 Piloderma 3 Piloderma sp. JQ711984.1 88% 1 0 85 Piloderma 4 Piloderma sp. FN669236.1 90% 2 0 40 Piloderma 5 Piloderma olivaceum JQ711802.1 88% 3 1 58 Piloderma 6 Piloderma olivaceum JQ711915.1 93% 3 0 68 Piloderma 7 Piloderma sp. JQ711984.1 96% 2 0 136 Piloderma 8 Piloderma sp. JQ711951.1 90% 1 0 138 Piloderma 9 Piloderma fallax DQ365665.1 93% 1 0 70 Piloderma byssinum 1 Piloderma byssinum KF359605.1 99% 2 0 95 Piloderma byssinum 2 Piloderma byssinum DQ365683.1 100% 1 0 25 Piloderma lanatum Piloderma lanatum JQ711873.1 99% 4 0 41 Piloderma olivaceum 1 Piloderma olivaceum JQ11915.1 97% 3 0 57 Piloderma olivaceum 2 Piloderma olivaceum JQ711901.1 99% 3 1 30 Piloderma olivaceum 3 Piloderma olivaceum 3 JQ11924.1 99% 2 2 95 Table S1. (Continued) Consensus # Taxa GenBank Target Name #ACN Max ID # of P transect pairs # of C transect pairs 118 Pseudotomentella 1 Pseudotomentella sp. AB848560.1 88% 1 0 141 Pseudotomentella nigra Pseudotomentella nigra AF274770.1 98% 1 0 76 Ramaria 1 Ramaria sp. EU444537.1 94% 2 0 90 Ramaria 2 Ramaria sp. DQ365606.1 99% 1 0 147 Ramaria 3 Ramaria sp. DQ365629.1 99% 1 0 62 Ramaria largentii Ramaria largentii EU652343.1 99% 1 0 27 Rhizopogon 1 Rhizopogon evadens KJ595006.1 99% 4 0 28 Rhizopogon 2 Rhizopogon luteorubescens GQ267482.1 99% 3 0 113 Rhizopogon 3 Rhizopogon bacillisporus EU837230.1 84% 1 0 120 Rhizopogon 4 Rhizopogon luteorubescens GQ267482.1 87% 1 0 122 Rhizopogon 5 Russula sp. EF458016.1 95% 1 0 128 Rhizopogon 6 Rhizopogon sp. DQ680181.1 96% 1 0 146 Rhizopogon 7 Rhizopogon roseolus AJ810045.1 87% 1 0 36 Rhizopogon arctostaphyli Rhizopogon arctostaphyli NR_121275.1 99% 4 0 14 Rhizopogon ochraceorubens Rhizopogon ochraceorubens AF062928.1 99% 6 0 17 Rhizopogon salebrosus 1 Rhizopogon salebrosus KC170128.1 98% 6 0 38 Rhizopogon salebrosus 2 Rhizopogon salebrosus HQ914265.1 99% 2 0 3 Russula 1 Russula aff. subsect. Nigricantinae JX030254.1 99% 6 1 96 Table S1. (Continued) Consensus # Taxa GenBank Target Name #ACN Max ID # of P transect pairs # of C transect pairs 39 Russula 2 Russula nigricans DQ367915.1 99% 2 0 71 Russula 3 Russula ochraceorivulosa JQ902087.1 85% 2 1 66 Russula cascadensis Russula cascadensis EU526006.1 99% 1 0 16 Russula tenuiceps Russula tenuiceps DQ974756.1 99% 2 0 12 Russula turci Russula turci JQ11969.1 98% 3 0 145 Sebacina 1 Sebacina vermifera JQ711842.1 96% 1 0 79 Sebacina vermifera Sebacina vermifera JQ711843.1 98% 2 0 46 Sistotrema 1 Sistotrema pistilliferum KF218964.1 96% 3 0 32 Sistotrema 2 Sistotrema sp. FN669255.1 95% 1 0 13 Sistotrema alboluteum Sistotrema alboluteum AJ606043.2 98% 6 0 78 Suillus 1 Suillus sp. JQ711888.1 98% 1 0 139 Suillus 2 Suillus variegatus JX907819.1 95% 1 0 75 Suillus placidus Suillus placidus KM882921.1 98% 2 0 35 Suillus tomentosus Suillus tomentosus FJ845441.1 99% 5 0 56 Thelephora terrestris Thelephora terrestris HM189958.1 99% 1 0 115 Thelephoraceaea 1 Thelephoraceae sp. JX243818.1 73% 2 0 54 Tomentella 1 Tomentella sp. JQ711794.1 99% 2 0 53 Tomentella 2 Tomentella bryophila JQ711917.1 95% 2 0 97 Table S1. (Continued) Consensus # Taxa GenBank Target Name #ACN Max ID # of P transect pairs # of C transect pairs 55 Tomentella 3 Tomentella badia JQ711987.1 98% 3 0 52 Tomentella 4 Tomentella sp. U92537.1 99% 2 0 65 Tomentella 5 Tomentella ramosissima JX129141.1 97% 1 0 104 Tomentella 6 Tomentella sp. AJ534914.1 95% 1 0 119 Tomentella 7 Tomentella bryophila JQ711917.1 95% 1 0 143 Tomentella atramentaria Tomentella atramentaria DQ974772.1 98% 0 1 123 Tomentella bryophila Tomentella bryophila JQ711917.1 99% 1 0 109 Tomentellopsis 1 Tomentellopsis zygodesmoides AJ410761.1 96% 1 0 144 Tomentellopsis submollis Tomentellopsis submollis JQ711898.1 98% 1 0 61 Tricholoma 1 Tricholoma myomyces JN389299.1 99% 1 0 84 Tricholoma 2 Tricholoma equestre HM590873.1 93% 1 0 117 Tricholoma 3 AF458435.1 99% 1 0 156 Tricholoma 4 Tricholoma ustale Tricholoma saponaceum var. saponaceum DQ370440.1 86% 1 0 82 Tricholoma focale Tricholoma focale DQ367920.1 99% 2 0 33 Wilcoxina rehmii Wilcoxina rehmii AF266708.1 99% 8 0 98 Table S2. Full Spearman ranked correlation (ρ) tables of environmental variables correlated with each other (n=16). Bold = relationships strong enough to merit attention, values presented in Results section. pH pH Elevation (m) Aspect (º) Bray P (mg/kg) Total P (mg/kg) Mineralizable N (mg/kg) Incubation N (mg/kg) %C %N Root Biomass (g) Chinq. % Cover Avg. Vol. Water (m3/m3) Shared EMF (count) Chinq. Root tips Pina. Root tips Basal Area/plot -0.5 -0.72 -0.28 1 0.58 Total P (mg/kg) 0.06 -0.61 -0.13 0.58 1 Mineralizable N (mg/kg) -0.46 0.12 0.46 0.03 -0.21 Incubation N (mg/kg) -0.42 0.16 0.48 -0.01 -0.26 0.46 0.48 0.19 0.11 0.38 -0.43 0.03 -0.01 0.49 0.47 -0.11 -0.34 -0.21 -0.26 0.32 0.22 0.21 -0.17 1 0.98 0.59 0.64 -0.22 -0.37 0.98 1 0.62 0.65 -0.28 -0.31 0.38 0.11 -0.08 0.17 0.24 -0.41 0.09 -0.21 0.12 -0.36 -0.29 0.08 0.09 0.2 -0.25 0.66 -0.09 -0.28 -0.05 0.18 0.66 0.03 -0.19 -0.1 0.17 1 0.32 -0.19 -0.5 0.06 Elevation (m) 0.32 1 0.24 -0.72 -0.61 Aspect (º) -0.19 0.24 1 -0.28 -0.13 -0.46 -0.42 -0.32 -0.47 0.2 0.51 0.12 0.16 -0.46 -0.44 0.01 0.3 -0.08 0.06 0.11 0.26 0.09 0.43 0.07 0.26 -0.33 0.38 Bray P (mg/kg) 99 Table S2. (Continued) Avg. Vol. Water (m3/m3) -0.08 0.43 0.38 -0.41 -0.29 Shared EMF (count) -0.32 -0.46 0.19 0.49 0.32 -0.47 -0.44 0.11 0.47 0.22 0.2 0.01 0.38 -0.11 0.21 Chinq. % Cover 0.51 0.3 -0.43 -0.34 -0.17 0.59 0.62 1 0.93 -0.23 -0.25 0.64 0.65 0.93 1 -0.42 -0.28 -0.22 -0.28 -0.23 -0.42 1 0.12 -0.37 -0.31 -0.25 -0.28 0.12 1 0.66 0.66 0.24 0.25 0.08 0.05 -0.09 0.03 0.04 0.09 -0.22 0.09 -0.28 -0.19 -0.19 -0.13 -0.29 0.38 -0.05 -0.1 0.13 -0.01 0.28 -0.38 0.18 0.17 0.12 0.09 0.02 0.16 0.24 0.04 -0.19 0.13 0.12 0.25 0.09 -0.13 -0.01 0.09 0.08 -0.22 -0.29 0.28 0.02 0.05 0.09 0.38 -0.38 0.16 1 -0.05 -0.08 -0.18 0.17 -0.05 1 0.47 -0.14 -0.22 -0.08 0.47 1 -0.7 0.05 -0.18 -0.14 -0.7 1 0.04 0.17 -0.22 0.05 0.04 1 %C pH Elevation (m) Aspect (º) Bray P (mg/kg) Total P (mg/kg) Mineralizable N (mg/kg) Incubation N (mg/kg) %C %N Root Biomass (g) Chinq. % Cover Avg. Vol. Water (m3/m3) Shared EMF (count) Chinq. Root tips Pina. Root tips Basal Area/plot Root Biomass (g) %N 0.06 0.07 0.11 0.09 0.08 Chinq. root tips 0.11 0.26 -0.08 -0.21 0.09 Pine root tips 0.26 -0.33 0.17 0.12 0.2 Basal Area/plot 0.09 0.38 0.24 -0.36 -0.25 100 Table S3. Spearman ranked correlation coefficients (ρ) for comparing environmental variables to chinquapin EMF variables (n = 16). Bold = relationships strong enough to merit attention, values presented in Results section. Richness Shannons Div. Simpsons Div. Chinq. Root tips Pine Root tips Basal Area/plot pH 0.16 0.15 0.09 0.11 0.26 0.09 Elevation (m) 0.36 0.3 0.2 0.26 -0.33 0.38 Aspect (º) -0.18 -0.2 -0.21 -0.08 0.17 0.24 Bray P (mg/kg) -0.12 -0.07 0.01 -0.21 0.12 -0.36 Total P (mg/kg) 0.07 0.12 0.15 0.09 0.2 -0.25 Mineralizable N (mg/kg) -0.28 -0.18 -0.04 -0.28 -0.05 0.18 Incubation N (mg/kg) -0.2 -0.1 0.02 -0.19 -0.1 0.17 %C -0.27 -0.12 0.05 -0.19 0.13 0.12 Table S3. (Continued) Richness Shannon’s Div. Simpson’s Div. Chinq. Root tips Pine Root tips Basal Area/plot %N -0.16 Root Biomass (g) -0.34 Chinq. % Cover 0.34 Avg. Vol. Water (m3/m3) -0.09 -0.03 -0.44 0.28 0.13 -0.55 -0.13 -0.01 0.09 1 Shannon’s Div. 0.95 Simpson’s Div. 0.82 Chinq. Root tips 0.89 Pine Root tips -0.68 Basal Area/plot -0.03 -0.02 0.95 1 0.95 0.89 -0.65 -0.05 0.13 0.04 0.82 0.95 1 0.78 -0.58 -0.08 -0.29 0.28 0.38 -0.38 -0.08 -0.18 0.89 -0.68 0.89 -0.65 0.78 -0.58 1 -0.7 -0.7 1 0.05 0.04 0.02 0.16 0.17 -0.03 -0.05 -0.08 0.05 0.04 1 Richness 101 Table S4. Spearman ranked correlation coefficients (ρ) for comparing environmental variables to Pinaceae EMF variables (n = 16). Bold = relationships strong enough to merit attention, values presented in Results section. Richness Shannon’s Div. Simpson’s Div. Chinq. Root tips Pine Root tips Basal Area/plot pH 0.28 0.24 0.19 0.11 0.26 0.09 Elevation (m) -0.33 -0.34 -0.36 0.26 -0.33 0.38 Aspect (º) 0.25 0.22 0.2 -0.08 0.17 0.24 Bray P (mg/kg) -0.08 -0.03 0.02 -0.21 0.12 -0.36 Total P (mg/kg) 0.16 0.14 0.12 0.09 0.2 -0.25 Mineralizable N (mg/kg) -0.06 -0.02 0.01 -0.28 -0.05 0.18 Incubation N (mg/kg) -0.08 -0.03 0.01 -0.19 -0.1 0.17 %C 0.07 0.12 0.16 -0.19 0.13 0.12 Table S4. (Continued) Richness Shannon’s Div. Simpson’s Div. Chinq. Root tips Pine Root tips Basal Area/plot %N -0.01 Root Biomass (g) 0.19 Chinq. % Cover -0.35 Avg. Vol. Water (m3/m3) -0.1 0.04 0.14 -0.35 0.08 0.09 -0.13 -0.01 0.09 Shannon’s Diversity 1 1 Simpson’s Diversity 0.98 -0.1 1 1 1 -0.52 0.88 -0.16 -0.36 -0.09 0.98 1 1 -0.55 0.88 -0.17 -0.29 0.28 0.38 -0.38 -0.08 -0.18 -0.5 0.88 -0.52 0.88 -0.55 0.88 1 -0.7 -0.7 1 0.05 0.04 0.02 0.16 0.17 -0.14 -0.16 -0.17 0.05 0.04 1 Richness Chinq. Root tips -0.5 Pine Root tips 0.88 Basal Area/plot -0.14 102 Table S5. Spearman ranked correlation coefficients (ρ) for most frequently occurring Pinaceae overstory species compared to environmental and EMF variables (n = 12). Bold = relationships strong enough to merit attention, values presented in Results section. Tree Count Basal Area pH Elevation (m) Aspect (º) Bray P (mg/kg) Total P (mg/kg) Mineralizable N (mg/kg) Incubation N (mg/kg) %C %N Root Biomass (g) Chinq. % Cover Avg. Vol. Water (m3/m3) Shared EMF (count) Chinq. Richness Chinq. Shannon's Div. Chinq. Simpson's Div. Pina. Richness Pina. Shannon's Div. Pina. Simonson's Div. Chinq. Root tips Pine Root tips Abies grandis Basal Area 0.93 1.00 0.49 0.76 -0.13 -0.26 -0.22 0.09 0.01 -0.28 -0.38 0.29 0.14 0.46 -0.48 -0.24 -0.18 -0.12 -0.27 -0.27 -0.25 -0.44 0.11 Pinus contorta Basal Area 0.42 1.00 -0.47 -0.35 -0.33 0.83 0.26 -0.19 -0.18 0.41 0.33 0.01 0.15 -0.47 -0.07 -0.20 -0.17 -0.14 -0.52 -0.50 -0.48 -0.28 0.00 Pinus ponderosa Basal Area -0.05 1.00 -0.12 -0.01 0.40 -0.24 0.01 0.19 0.20 0.28 0.26 0.08 0.05 0.12 0.02 0.04 -0.02 -0.09 0.00 -0.03 -0.07 0.30 -0.01 103 Appendix B Selected Scatterplots Involving Environmental Variables ● 6.0 ● ● ● ● ● ● ●● ● 5.8 ● ● pH ● 5.6 ● ● 5.4 5.2 ● 0 20 40 Chinquapin % Cover 60 80 Fig. S1 Scatter plot of chinquapin % cover vs. pH per transect pair. 104 ● 40 ● ● B ra y Phospho r u s (m g /k g ) 35 30 ● 25 ● ● 20 ● ● ● ● ● ● ● ● 15 5.2 5.4 5.6 5.8 ● ● 6.0 pH Fig S2 Scatter plot of pH vs. bray phosphorus (mg/kg) per transect pair. 105 ● 40 ● ● B r a y P h o s p h o ru s (m g /k g ) 35 30 25 ● ● ● ● ● 20 ● ● ● 1400 1500 ● ● ● 15 ● ● 1600 1700 Elevation Fig. S3 Scatter plot of bray phosphorus (mg/kg) vs. elevation (m) per transect pair. 106 ● T o ta l P h o s p h o ru s (m g /k g ) 1200 ● ● 1000 ● ● ● ●● ● ● ● ● 800 ● ● ● ● 1400 1500 1600 1700 Elevation (m) Fig. S4 Scatter plot of elevation (m) vs. total phosphorus (mg/kg) per transect pair. 107 ● ● Mineralizable Nitrogen (mg/kg) 20 ● ● ● ● ● 10 ● ● 0.025 ● ● ● ● ● ● 0.050 0.075 Total Nitrogen (%) 0.100 0.125 Fig. S5 Scatter plot of total nitrogen (%) vs. mineralizable nitrogen (mg/kg) per transect pair. 108 Mine ralizable Nitrogen (mg/kg) ● ● ● ● 20 ● ● ● ● ● ● ● ● 10 ● ● ● ● ● ● ● ● 0.00 0.02 ● ● ● ● ● ● ● ● ● ● ● ● 0.04 Avg. Vol. Soil Moisture (m /m ) 0.06 Fig. S6 Scatter plot of average volumetric soil moisture (m3/m3) vs. mineralizable nitrogen (mg/kg). 109 ● 40 ● ● Bray Phosphorus (mg/kg) 35 30 ● 25 ● ● ● ● 20 ● ●● ● ● ● 15 800 ● ● 1000 Total Phosphorus (mg/kg) 1200 Fig. S7 Scatter plot of total phosphorus (mg/kg) vs. Bray phosphorus (mg/kg) per transect pair. 110 ● ● Mineralizable Nitrogen (mg/kg) 20 ● ● ● ● ● 10 ● ● ● ● ● ● ● ● ● 2 3 4 5 Total Carbon (%) Fig. S8 Scatter plot of total carbon (%) vs. mineralizable nitrogen (mg/kg) per transect pair. 111 ● ● ● 5 ● ● Total Carbon (%) 4 ● ● ● ● ● ● 3 ● 2 ● ●● ● 0.025 0.050 0.075 Total Nitrogen (%) 0.100 0.125 Fig. S9 Scatter plot of total nitrogen (%) vs. total carbon (%) per transect pair. 112 Selected Scatterplots Involving EMF Variables and Root tip Counts ● ●● 3.2 ● ● ● ● ● ● Pina. EMF Shannon’s Div. 3.0 ● ● 2.8 ● 2.6 ● ● ● ● 2.4 10 20 30 40 Pinaceae root tips/transect pair 50 60 Fig. S10 Scatter plot of Pinaceae root tips vs. Pinaceae EMF Shannon’s Diversity per transect pair. 113 ● 30 Chinquapin root tips/transect pair ● ● 20 ● ● ● ● ● 10 ● ● ● ● ● ● 10 20 30 40 Pinaceae root tips/transect pair ● 50 ● 60 Fig. S11 Scatter plot of Pinaceae root tips vs. chinquapin root tips per transect pair. 114 ● ●● 25 ● ● ● ● ● Pinaceae EMF Richness ● 20 ● ● 15 ● ● ● ● ● 10 20 30 40 Pinaceae root tips/transect pair 50 60 Fig. S12 Scatter plot of Pinaceae root tips vs. Pinaceae EMF richness per transect pair. 115 ● ● 2.5 ● ● Chinq. EMF Shannon’s Div 2.0 ● ●● ●● ● 1.5 ● ● ● ● ● 1.0 ● 10 20 30 40 Pinaceae root tips/transect pair 50 60 Fig. S13 Scatter plot of Pinaceae root tips vs. chinquapin EMF Shannon’s Diversity per transect pair. 116 ● ● Chinquapin EMF Richness 12 ● 8 ● ● ●● ●● ● ● 4 ● ● ● ● ● 10 20 30 40 Pinaceae root tips/transect pair 50 60 Fig. S14 Scatter plot of Pinaceae root tips vs. chinquapin EMF Richness per transect pair. 117 ● ● Chinquqpin EMF Richness 12 ● 8 ● ● ●● ● 4 ●● ● ● ● ● 10 20 Chinquapin root tips/transect pair 30 Fig. S15 Scatter plot of chinquapin root tips vs. chinquapin EMF richness per transect pair. 118 ● 25 ● ● ● ● ●● Pinaceae EMF Richness ● ● 20 ● ● 15 ● ● ● ● ● 10 20 Chinquapin root tips/transect pair 30 Fig. S16 Scatter plot of chinquapin root tips vs. Pinaceae EMF richness per transect pair. 119 ● ● 2.5 ● ● Chinq. EMF Shannon’s Div. 2.0 ● ●● ● ●● 1.5 ● ● ● 1.0 ● 10 20 Chinquapin root tips/plot 30 Fig. S17 Scatter plot of chinquapin root tips vs. chinquapin EMF Shannon’s diversity per transect pair. 120 ● ● 2.5 ● Chinq. EMF Shannon’s Div. 2.0 ● ● ●● ●● ● 1.5 ● ● ● 1.0 ● 10 20 Chinquapin root tips/plot 30 Fig. S18 Scatter plot of chinquapin root tips vs. Pinaceae EMF Shannon’s diversity per transect pair. 121 Selected Scatterplots Involving Overstory Pinaceae Basal Area 30 ● Grand Fir Basal Area (m2/ha) 20 ● 10 ● ● ● 0 ● 1400 ●● ● 1500 1600 1700 El evation (m) Fig. S19 Scatter plot of elevation (m) vs. grand fir basal area (m2/ha) per transect pair. 122 ● Lodgepole Pine Basal Area (m2/ha) 15 10 ● 5 ● ● ● 0 ● ●● 15 ● ● 20 30 25 Bray Phosphorus (mg/kg) 35 40 Fig. S20 Scatter plot of Bray phosphorus (mg/kg) vs. lodgepole pine basal area (m2/ha) per transect pair. 123 ● Lodgepole Pine Basal Area (m /ha) 15 10 ● ● 5 ● ● ● ●●● 0 2.6 2.8 3.0 Pinaceae EMF Shannon's Div. ● 3.2 Fig. S21 Scatter plot of Pinaceae EMF Shannon’s diversity vs. lodgepole pine basal area (m2/ha) per transect pair. 124 ● Lodgepole Pine Basal Area (m /ha) 15 10 ● ● 5 ● ● ● ● ● ● 0 16 20 Pinaceae EMF Richness ● 24 Fig. S22 Scatter plot of Pinaceae EMF richness vs. lodgepole pine basal area (m2/ha) per transect pair. 125