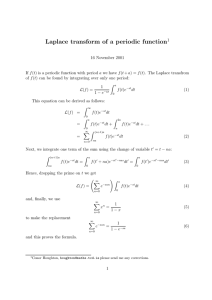
Assessing the Effects of Naphthenic Acids Using a Microbial
advertisement

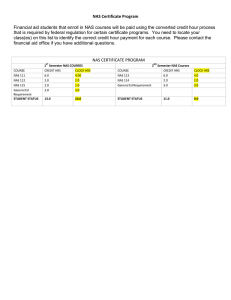
Assessing the Effects of Naphthenic Acids Using a Microbial Genome Wide Live Cell Reporter Array System Xiaowei Zhang1,2*, Steve Wiseman2, Hongxia Yu1, Honglin Liu1, John P. Giesy1,2,3,4, Markus Hecker2,5 1 State Key Laboratory of Pollution Control and Resource Reuse & School of the Environment, Nanjing University, Nanjing, China; 2Toxicology Centre, University of Saskatchewan, Saskatoon, SK, Canada; 3 Dept. Biomedical Veterinary Bioscience, University of Saskatchewan, Saskatoon, Saskatchewan, Canada; 4 Dept. Biology & Chemistry, City University of Hong Kong, Kowloon, Hong Kong, SAR China; 5 ENTRIX Inc. Saskatoon, SK, Canada Methods 100mg NAs/L 0 1 2 13 46 ssb ydcS hemC bolA sodA brnQ ybjP yjbJ dppA ycaD ybaY inaA ilvC yfcD marR araF chbA uhpT 90 120 Time (min) 180 inaA ilvC yfcD marR uhpT chbA insA_2 yajO ycaD yjeB yfbM xthA ypeA rbsD ileX rluE ppiD gadW ydcM yahD yaaW yfbE ybiH alaS insA_7 crl rph ybhK ydgH modF ribA feaB serC ppiB greA ptsG yfdU rpiA gnd araF yfeN sodA ybjP yjbJ hemC pitA gadX somA ybgI atpI yjbQ ymcC ybeB brnQ ligA cvrA yjfI trxA b2641 yjgA rob ftsK aceB ihfB htrL rpsM msrB pmrD yhiD rrnA accB trmU yejA ycfD bolA dppA ydcS ybfE ssb ybaY Figure 3. Clustering of genes modulated by NAs. A). Clustering of the concentrationand time-dependent expression of the 27 genes altered at least 2-fold change over background by NAs. B). Clustering of the time-dependent expression of the NAs altered genes selected by1.5-fold change cut-off. Gene expression in cells exposed to 1000 mg NAs/L are displayed. Classification and visualization of the gene expression were derived by use of ToxClust (Zhang et al., 2009). 100 100 150 Discrete variable Continuous variable 80 60 Discrete variable Continuous variable 60 30 Fold Change 40 0 B: 2 fold change cut-off 20 ymcC ybeB somA ybfE A: 1.5 fold change cut-off 1000 mg/L 100 mg/L 10 mg/L 0 16 4 Fold Change 1 1000mg NAs/L NAs Conc 1000mg NAs/L Response (%) 17 14 80 7 60 6 40 1 20 2 16 1 0.2 0 4 0 crl rpiA alaS insA_7 gnd Figure 1. The microbial promoter collection includes more than 1900 promoters for the E. coli K12 strain MG1655. Each of the reporter strains has a bright, fastfolding green fluorescent protein (GFP) fused to a full-length copy of an E. coli promoter (Zaslaver et al., 2006). 10mg NAs/L 1 100mg NAs/L Figure 2 A Venn diagram displaying the differentially expressed genes selected by 1.5 or 2.0 fold change cut-off at three different NAs concentrations, 10, 100, and 1000 mg/L. NAs induced a concentrationdependent response in the number of differentially expressed genes. . 0.1 10mg NAs/L Fold Change >1.5 0 Mixtures of NAs, which include cyclopentyl and cyclohexyl carboxylic acids, have been identified as major toxic components in the effluents discharged by the oil sands industry. The present study for the first time demonstrated an application of a high throughput bacterial live cell array in a genome-scale investigation of the toxic mechanisms of environmental chemicals, a commercial NAs technical mixture extracted from crude oil (Sigma). Fold Change >2.0 Response (%) Introduction Results 0.2 Real time gene profiling of time- and concentration-dependent effects of exposure to a commercial naphthenic acid (NA) mixture in live cells for three hours was conducted using a library of 1800 fluorescent transcriptional reporters for Escherichia coli growing in 384-well plates. Response patterns obtained after exposure to NAs suggested that the primary cellular responses were up-regulation of the pentose phosphate pathway, processes involved in the molecular function of NADP or NADPH binding, and down-regulation of the ATP-binding cassette (ABC) transporter complex. Transcriptional networks that were significantly modulated by NAs included those that were regulated by transcriptional factors such as CRP, RecA, and GadE. The down-regulation of the SOS response pathway suggested that DNA damage might not be the direct results of NAs within the first three hours of exposure. However, CRP-dependent genes modulated by exposure to NAs indicated that the cellular level of cyclic AMP was altered immediately upon exposure of cells to NAs. Furthermore, the linear range of the concentrationresponse curve of the selected promoter reporters encompassed a range of concentrations between 10 -1000 mg NAs /L, which covers concentrations typically observed in the environment. Thus, this assay system may represent a promising tool for the detection of environmental chemicals such as NAs. 0.1 Abstract Figure 4 Active functional modules of a transcriptional network of patters of gene responses in E coli exposed to NAs. The level of gene expression in cells exposed to 1000 mg NAs/L is indicated by the color gradient. Brown: >2 fold up regulation; gradient from Red to white: from 2 to1 fold up regulation; gradient from White to blue: from 1 to 2 fold down regulation; Gray: >2 fold down regulation. For the three TFs (crp, lexA and gadE) that displayed no significant change in response to NAs, their roles in the network modules are highlighted by in aquamarine. 10 100 1000 NAs concentration (mg/L) 10 100 NAs concentration (mg/L) 1000 Figure 5. Concentration-dependent transcriptional response to NAs. In the discrete variable approach, the number or the percentage of genes affected was used to describe the degree of chemicalinduced effects. In the continuous variable approach, the actual expression level of all the selected genes were integrated to differentiate the degree of effect induced by different concentrations of chemical. Discussion 1.Biological pathways involved in NAs effects. 1) up-regulation of the pentose phosphate pathway, 2) up-regulation of NADP or NADPH binding pathway, 3) down-regulation of the ATP-binding cassette (ABC) transporter complex. 2. Stress responsive pathway affected by NAs exposure. 1) redox-response, 2) SOS-response, 3) osmotic-response 3. Transcriptional networks involved in NAs-induced effects. Tanscriptional factors: CRP-, RecA-, and GadE 4.Potential biosensors for environmental NAs detection. Acknowledgement Jiangsu EMT grant (1012) and Canada WED grant (#6578 and 6807) Reference Zhang et al., Environ. Sci. Technol. 2008, 42 (17), 6762-6769. Zaslaver et al., Nat. Methods. 2006, 3 (8), 623-628.