Toxicology Modulation of steroidogenic gene expression and hormone synthesis in H295R
advertisement

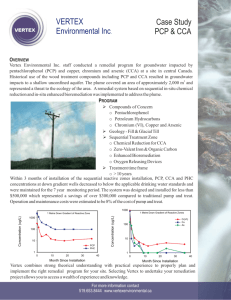
Toxicology 282 (2011) 146–153 Contents lists available at ScienceDirect Toxicology journal homepage: www.elsevier.com/locate/toxicol Modulation of steroidogenic gene expression and hormone synthesis in H295R cells exposed to PCP and TCP Yanbo Ma a,b , Chunsheng Liu a , Paul K.S. Lam c , Rudolf S.S. Wu d , John P. Giesy c,d,e,f,g,h , Markus Hecker f , Xiaowei Zhang f , Bingsheng Zhou a,∗ a State Key Laboratory of Freshwater Ecology and Biotechnology, Institute of Hydrobiology, Chinese Academy of Sciences, Wuhan 430072, China Graduate School of the Chinese Academy of Sciences, Beijing 100039, China Department of Biology and Chemistry, City University of Hong Kong, Kowloon, Hong Kong, China d School of Biological Sciences, The University of Hong Kong, Hong Kong, China e Department of Veterinary, Biomedical Sciences, University of Saskatchewan, Saskatoon, Canada f Toxicology Centre, University of Saskatchewan, Saskatoon, Canada g Zoology Department, College of Science, King Saud University, P.O. Box 2455, Riyadh 11451, Saudi Arabia h Department of Zoology, and Center for Integrative Toxicology, Michigan State University, East Lansing, MI, USA b c a r t i c l e i n f o Article history: Received 23 September 2010 Received in revised form 13 January 2011 Accepted 31 January 2011 Available online 4 February 2011 Keywords: Chlorophenol Endocrine-disruption Gene expression Steroid hormone cAMP H295R a b s t r a c t Chlorophenols (CPs) have been suspected to disrupt the endocrine system and thus affect human and wildlife reproduction but less is known about the underlying mechanism. In this study, we investigated the effects of pentachlorophenol (PCP) and 2,4,6-trichlorophenol (TCP) on human adrenocortical carcinoma cell line (H295R). The H295R cells were exposed to environmentally relevant concentration (0.0, 0.4, 1.1, 3.4 M) of PCP and TCP for 48 h, and expression of specific genes involved in steroidogenesis, including cytochrome P450 (CYP11A, CYP17, CYP19), 3ˇHSD2, 17ˇHSD4 and StAR was quantitatively measured using real-time polymerase chain reaction. The selected gene expressions were significantly down-regulated compared with those in the control group. Exposure to PCP and TCP significantly decreased production of both testosterone (T) and 17-estradiol (E2). Furthermore, a dose-dependent decrease of cellular cAMP was observed in H295R cells exposed to both PCP and TCP. A time-course study revealed that the observed selected steroidogenic gene expressions and protein abundance (StAR) are consistent with reduced cellular cAMP concentrations. The results showed that PCP and TCP may inhibit steroidogenesis by disrupting cAMP signaling. The research indicates that H295R cells can be used as an in vitro model for endocrine disruption assay for chlorophenols and the mechanism involvement of disturbing cAMP signaling. © 2011 Elsevier Ireland Ltd. All rights reserved. 1. Introduction Pentachlorophenol (PCP) has been extensively used worldwide as a pesticide and wood preservative. As a consequence, the global environment is contaminated with PCP. Because of its relatively high hydrophobicity and environmental persistence, PCP is readily bioaccumulated (Reigner et al., 1993; ATSDR, 2001). Partial dechlorination of PCP can generate more toxic intermediate compounds such as 2,4,6-trichlorophenol (TCP) (Eker and Kargi, 2007). Due to the toxicity of PCP and the fact that it is a probable human carcinogen, some countries have banned or control the use of PCP (Baynes et al., 2002), but other countries still use PCP to prevent fungal attacks on wood (Jensen, 1996). Hence PCP and its intermediate compounds are still detected in the aquatic environment ∗ Corresponding author at: Institute of Hydrobiology, Chinese Academy of Sciences, Wuhan 430072, China. Tel.: +86 27 68780042; fax: +86 27 68780123. E-mail address: bszhou@ihb.ac.cn (B. Zhou). 0300-483X/$ – see front matter © 2011 Elsevier Ireland Ltd. All rights reserved. doi:10.1016/j.tox.2011.01.024 (Bhattacharya et al., 1996; Chen and Parker, 2004; Hanna et al., 2004; Fernández Freire et al., 2005; Farhadi et al., 2009). PCP was used in China during the 1970s to control schistosomiasis (Wang et al., 2008). For this reason greater concentrations of PCP (up to 103.7 g/L) were detected in Dongting Lake (Zheng et al., 2000). PCP was banned in China as a pesticide in 1997 (Zha et al., 2006). However, PCP is still used as a wood preservative (Zheng et al., 2000). Concentrations of PCP as great as 0.59 g/L, 2,4-dichlorophenol as great as 20.0 g/L and 2,4,6-trichlorophenol as great as 29.0 g/L were observed in surface water of seven major watersheds and three drainage areas of China (Gao et al., 2008). Due to their toxicity and adverse effects on humans and wildlife, the US EPA classified PCP, 2,4,6-trichlorophenol, 2,4-dichlorophenol as priority pollutants (Ramamoorthy and Ramamoorthy, 1997). The results of previous studies have indicated that the toxic effects of PCP are related to uncoupling of oxidative phosphorylation in mitochondria and generation of reactive oxygen species (ROS) (Proudfoot, 2003; Dong and Jiang, 2009). Exposures to PCP affect the endocrine system of vertebrates and may lead to Y. Ma et al. / Toxicology 282 (2011) 146–153 147 and various model chemicals (Zhang et al., 2005) on steroidogenic pathways. Although several studies of the endocrine-modulating effects of PCP have been conducted, the underlying mechanisms of these effects have remained largely unknown. Therefore, the purpose of this study was to assess the non-receptor mediated effects of pentachlorophenol (PCP) and 2,4,6-trichlorophenol (TCP) on H295R cells. Expression of key genes involved in steroidogenesis, including StAR (steroidogenic acute regulatory protein), CYP11A (cholesterol side-chain cleavage), 3ˇHSD2 (3-hydroxysteroid dehydrogenase), CYP17 (steroid 17␣-hydroxylase/17,20-lyase), CYP19 (aromatase) and 17ˇHSD4 (17-hydroxysteroid dehydrogenase) were examined. The production of two steroid hormones: T and E2 were measured. Since cAMP is an important secondary messenger to modulate steroidogenic genes and steroid hormone biosynthesis in the human adrenal cortex (Sewer and Waterman, 2001; Stocco et al., 2005), the role of cellular cAMP in regulation of steroidogenic pathway in H295R cells upon exposure to PCP and TCP was also investigated. 2. Materials and methods Fig. 1. Schematic representation of the steps involved in steroid hormone synthesis. dysfunction of the immune system and disruption of normal sexual, cognitive, physical and emotional development (O’Donoghue, 1985; Daniel et al., 1997; Yin et al., 2006; Zhang et al., 2008). The mechanisms of endocrine disruption caused by PCP have been studied in vitro and in vivo. For example, PCP was shown to be a partial agonist for the estrogen receptor (ER) in the cellular proliferation of MCF-7 cells (Suzuki et al., 2001) and other estrogenic activity, such as induction of vitellogenin (VTG) in the cultured hepatocytes of male channel catfish (Dorsey and Tchounwou, 2004). Alternatively, the results of other studies have indicated that PCP did not exhibit estrogenicity, but rather was shown to be anti-estrogenic in the yeast two-hybrid assay (Jung et al., 2004) and in cultured goldfish hepatocytes (Zhao et al., 2006). In fish, estrogenic activities (induction of VTG), and reproductive impairment have been reported in male Japanese medaka (Zha et al., 2006), while significantly more testosterone (T) was observed in the serum crucian carp exposed to PCP (Zhang et al., 2008). Using a recombinant yeast screen assay, a recent study showed antiestrogenic/antiandrogenic activity of PCP in cultured Xenopus oocytes and inhibition of ovarian steroidogenesis, accompanied by decreased production of both progesterone and T (Orton et al., 2009). Chemicals can cause endocrine disruption by either direct interaction with receptors or alter enzymes involved in steroid hormone synthesis and metabolism. In the latter case, chemicals can alter steroidogenic gene expression or enzyme activities and have the potential to alter concentrations of hormones in blood and tissues (Hilscherova et al., 2004). In this regard, the utility of in vitro assay systems, the human adrenocortical carcinoma cells (H295R), has been developed for rapid screening of endocrine disrupting potencies of chemicals or toxicants and identification of novel mechanisms of endocrine disruption (Sanderson et al., 2000; Gracia et al., 2006). H295R cells maintain physiological characteristics of zonally undifferentiated fetal adrenal cells and express all genes involved in steroidogenesis (Fig. 1). Using this system, numerous studies have been conducted on the assessment of endocrine disruption via affects of environmental contaminants, such as pesticides (Sanderson et al., 2002), polychlorinated biphenyls (PCBs) (Li and Wang, 2005), polybrominated diphenyl ethers (PBDEs) (Cantón et al., 2006; He et al., 2008; Song et al., 2008), 1H,1H,2H,2H-perfluoro-decan-1-ol (8:2 FTOH) (Liu et al., 2010), fungicide (Ohlsson et al., 2009), bisphenol A (Letcher et al., 2005), 2.1. Chemicals Pentachlorophenol (PCP) (>99%, CAS No. 87-86-5) was purchased from Sigma (St. Louis, MO, USA). 2,4,6-Trichlorophenol (TCP) (100%, CAS No. 88-06-2) was purchased from AccuStandard Inc. (New Haven, CT, USA). They were dissolved in dimethyl sulfoxide (DMSO), and were stored at 4 ◦ C. LDH-Viability Assay Kit was purchased from GenMed Scientifics Inc. (Washington, DC, USA). The SYBR Green PCR kit was purchased from Toyobo (Osaka, Japan). Enzyme-linked immunosorbent assay (ELISA) kits for T, E2 and cAMP were obtained from Cayman Chemical Company (Ann Arbor, MI, USA). All other chemicals used were of analytical grade. 2.2. Cell culture The H295R cells were cultured in DMEM/F12 medium supplemented with of 1% insulin-transferring sodium selenite plus Premix (ITS) (BD Bioscience, Bedford, USA), 2.5% Nu-Serum (BD Bioscience, Bedford, USA), 2.5% 100 U/mL of penicillin, and 100 g/mL of streptomycin. The cells were maintained at 37 ◦ C in an atmosphere of 5% CO2 . The culture medium was changed every 2–3 days. 2.3. Experimental design PCP and TCP were dissolved in DMSO as a stock solution, and the exposure and control groups were received 0.1% DMSO. For the experiment of gene expression and hormone measurement, the cells were grown in 12-well plates, and 2 mL of cell suspension was added to each well. Quantification of cAMP was conducted in 6-well cell culture plates with 2.5 mL of a cell suspension to each well. Experiments were conducted with a density of 4 × 105 cells/mL. After 24 h, the cells were exposed to 0.0, 0.4, 1.1, 3.4 M for 48 h. The selected exposure concentration was based on the measured concentration in the surface water (Zheng et al., 2000). Three wells were used for each treatment and control as triplicates. 2.4. Cell viability assay Cell viability was determined by measuring LDH activity by use of previously described methods (Arechabala et al., 1999). Briefly, H295R cells were seeded into 24-well plates (Corning Life Sciences, Corning, NY, USA) at a density of 3 × 105 cells/mL. After culture for 24 h, cells were exposed to 0.0, 0.4, 1.1, 3.4 M PCP or TCP for 48 h, the culture medium was removed. The LDH activity was assayed utilizing a commercial kit (GMS 10073, GenMed Scientifics Inc). The reduction of NADH was recorded with a microplate reader (Molecular Device, M2) at 490 nm and room temperature. The LDH release was expressed as a percentage of the LDH release of the control. Three wells were used for each treatment and each treatment was tested in triplicate. 2.5. RNA isolation and quantitative real-time polymerase chain reaction The procedures for RNA extraction and mRNA expression pattern analysis were performed as described previously by Ding et al. (2007). Total RNA was isolated with the SV Total RNA Isolation system® (Promega, WI, USA) following the manufacturer’s instructions. Total RNA concentration was assayed at 260 and 280 nm by using a spectrophotometer (M2, Molecular Devices, CA, USA). The purity of the RNA in each sample was verified by determining the A260/A280 ratio and by confirming 1.0 g RNA on 1% agarose-formaldehyde gel electrophoresis with ethidium bromide 148 Y. Ma et al. / Toxicology 282 (2011) 146–153 Table 1 Primer sequences for the quantitative reverse transcription-polymerase chain reaction. Gene name Sense primer (5 –3 ) Antisense primer (5 –3 ) Product length (bp) -Actin CYP11A StAR 3ˇHSD2 CYP17 CYP19 17ˇHSD4 CACCTTCCAGCCTTCCTTCC GAGATGGCACGCAACCTGAAG GTCCCACCCTGCCTCTGAAG TGCCAGTCTTCATCTACACCAG AGCCGCACACCAACTATCAG AGGTGCTATTGGTRCATCTTGCTC TGCGGGATCACGGATGACTC AGGTCTTTGCGGATGTCCAC CTTAGTGTCTCCTTGATGCTGGC CATACTCTAAACACGAACCCCACC TTCCCAGAGGCTCTTCTTCGTG TCACCGATGCTGGAGTCAAC TGGTGGAATCGGGTCTTTATGG GCCACCATTCTCCTCACAACTC 100 137 168 95 134 128 121 staining. Purified RNA was used immediately for reverse transcription (RT) or stored at −80 ◦ C until analysis. Synthesis of cDNA was performed by use of the Superscript first-strand synthesis system® (Invitrogen, CA, USA). Briefly, total RNA (2 g) was combined with 0.5 g of biotinylated oligo (dT)12–18 and 0.5 mM deoxynucleotide triphosphate nucleotides, then diethylpyrocarbamate (DEPC)-treated water was added to a final volume of 10 L. Samples were denatured at 65 ◦ C for 5 min and then incubated on ice for 5 min. Reverse transcription was performed using 9 L of a master mix containing: 2 L of 10× RT buffer, 4 L of 25 mM MgCl2 , 1 L of RNase OUT (40 U/L; Invitrogen), and 2 L of RNase-free H2 O. The mixtures were incubated at 42 ◦ C for 2 min, then 50 U of SuperScript II RT (Invitrogen) were added. The reaction was incubated at 42 ◦ C for 50 min and then inactivated by heating at 70 ◦ C for 15 min. Finally, 1 L of RNase H (2 U/L) was added to each tube and incubated at 37 ◦ C for 20 min to digest the RNA. Quantitative real-time polymerase chain reaction (q-RT-PCR) was performed by using the SYBR Green PCR kit (Toyobo, Tokyo, Japan) and an ABI 7300 System (PerkinElmer Applied Biosystems, CA, USA). The primer sequences of the selected genes were previously published (Ding et al., 2007) and are given (Table 1). The thermal cycle for the q-RT-PCR procedure was as follows: samples were denatured at 95 ◦ C for 10 min, followed by 40 cycles of denaturation at 95 ◦ C for 15 s, annealing with extension for 1 min at 60 ◦ C, and a final cycle of 95 ◦ C for 15 s, 60 ◦ C for 1 min, and 95 ◦ C for 15 s. Melting curve analyses were performed after the 60 ◦ C stage of the final cycle to differentiate between desired PCR products and primer–dimmer or DNA contaminants. Q-RT-PCR reactions were performed in triplicate and also repeated three times. For quantification of PCR results, the Ct (the cycle at which the fluorescence signal is first significantly different from background) was determined for each reaction. The expression profile of the target gene was normalized to the corresponding -actin mRNA content. Fold change in mRNA expression of the relevant genes was analyzed by the 2−CT method (Livak and Schmittgen, 2001). and protein abundance were further investigated. The intracellular concentrations of cAMP and the StAR gene expression were measured at 6, 12, 24, and 48 h and Western blotting analysis was performed at 12, 24 and 48 h exposure. The hormone (T and E2) levels were quantified at 12, 24, and 48 h exposure. Western blotting analysis was performed as previously described (Liu et al., 2010). Briefly, the H295R cells were seeded in 6-well plates (Corning Life Sciences). After exposure to TCP (0, 3.4 M), the cells were lysed and the protein content was determined. In total, 50 g cytoplasmic protein were denatured, electrophoresed and transferred onto a polyvinylidene difluoride (PVDF) membrane. The transferring efficiency was evaluated for equal protein in each lane using a reversible dye (PIERCE, IL, USA). The membrane was blocked and blots were probed with an antihuman StAR antibody (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA). Following primary antibody incubation the membrane was washed and incubated with a horseradish peroxidase-conjugated anti-mouse antibody (Santa Cruz Biotechnology Inc.). The secondary antibody was diluted (1:2000) in skim milk blocking solution. The immunoblot analysis was performed using the AmershanmTM ECL Plus Western 2.6. Hormone measurements Hormone extraction method was based on previously described (Hecker et al., 2006). After 48 h exposure, culture medium was transferred to an Eppendorf tube and stored at −80 ◦ C until quantification of hormones. Frozen medium was thawed on ice, the 500 L culture medium was extracted twice with 2.5 mL diethyl ether in glass tubes, and phase separation was achieved by centrifugation at 2000 × g for 10 min. Solvent was evaporated under a stream of nitrogen, and the residue was dissolved in 250 L. ELISA buffer from Cayman Chemical Company and was either immediately measured or frozen at −80 ◦ C for later analysis. Hormones in culture medium were measured by competitive ELISA using the manufacturer’s recommendations (Cayman Chemical Company, Ann Arbor, MI; testosterone [Cat # 582701], 17-estradiol [Cat # 582251]). Extracts of culture medium were diluted 1:2 for estradiol, and 1:75 for testosterone prior to use in the ELISA assay. 2.7. Cyclic AMP measurements Intracellular concentrations of cAMP were determined using a commercial ELISA (Cat # 581001, Cayman Chemical Company, MI, USA) according to the protocol provided by the manufacturer. Briefly, after H295R cells were exposed to chemicals for 48 h, the culture medium was removed and the cells were washed with 0.9% NaCl (PBS was not used because phosphate interferes with the immunoassay). Cells were lysised for 20 min in 300 L of 0.1 M HCl at room temperature; cells were scraped off the surface with a cell scraper and the mixture was dissociated by pipetting up and down until the suspension was homogenous. Then the lysate was transferred to a 1.5 mL plastic vial, vortexed, and centrifuged at 1000 × g for 10 min. The supernatant was diluted 1:2 with the assay buffer provided by the kit and underwent all other steps, including an acetylation step according to the instructions of the supplier. cAMP was quantified by comparing to an external standard curve. 2.8. Time-course response of cAMP, StAR gene expression, protein abundance and hormone levels The steroidogenic acute regulatory (StAR) protein is a central regulator in steroidogenesis (Sewer and Waterman, 2001). To evaluate the involvement of cAMP signaling in the steroidogenic pathway, TCP (3.4 M) was exposed to the H295R cells and the time-course response of cAMP concentrations, StAR gene expression Fig. 2. Expression of mRNA steroidogenic genes in H295R cells exposed to 0.0, 0.4, 1.1 or 3.4 M of pentachlorophenol (PCP) (A) or 2,4,6-trichlorophenol (TCP) (B) for 48 h. Mean ± SEM of three replicates. Significance of the difference between the control and exposure groups is indicated by *p < 0.05, **p < 0.01. Y. Ma et al. / Toxicology 282 (2011) 146–153 149 Fig. 3. Concentrations of testosterone (T) in media of H295R cells exposed to 0.0, 0.4, 1.1 or 3.4 M (A) pentachlorophenol (PCP) or (B) 2,4,6-trichlorophenol (TCP) for 48 h. Concentrations of 17-estradiol (E2) in media of H295R cells exposed to 0.0, 0.4, 1.1 or 3.4 M (C) pentachlorophenol (PCP) or (D) 2,4,6-trichlorophenol (TCP) for 48 h. Mean ± SEM of three replicate samples.*p < 0.05 indicates significant difference between exposure groups and the corresponding control. Blotting Detection System (GE Healthcare, Baie-d’Urfe, QC, Canada). The quantification of the relative expression of StAR enzyme was performed by using BandScan 5.0 software. Three replicates were used in each experiment. 2.9. Statistical analysis The normality of the data was checked using the Kolmogorov–Smirnov test, and if necessary, data was log-transformed to approximate normality. The homogeneity of variances was analyzed by Levene’s test. The differences in the data were evaluated by use of a one-way analysis of variance (ANOVA) test followed by a Tukey’s multiple range tests using SPSS 13.0 (SPSS, Chicago, IL, USA). The criterion for statistical difference was set at p < 0.05. All values were expressed as the mean ± standard error (SEM). 3. Results 3.1. Cell viability None of the concentrations of neither PCP nor TCP caused any statistically significant leakage of LDH from cells (data not shown). This result is consistent with no change in viability of the cells. 3.2. Gene-expression profile PCP caused statistically significant down-regulation of all the steroidogenic genes tested (Fig. 2A). Expression of the StAR gene was significantly down-regulated 1.3-, 1.3- and 1.7-fold by 0.4, 1.1 and 3.4 M PCP, respectively (Fig. 2A). Expression of CYP11A was significantly inhibited in a concentration-dependent manner of 1.5-, 1.7-, and 2.2-fold (Fig. 2A). Expression of 3ˇHSD2 was down- regulated 2.9- and 3.0-fold and expression of CYP17 mRNA was down-regulated 1.6- and 2.0-fold by 1.1 and 3.4 M PCP. Downregulation of CYP19 (1.4-fold) and 17ˇHSD4 (1.7-fold) was observed in cells exposed to the greater concentration of 3.4 M PCP (Fig. 2A). Expression of StAR was significantly down-regulated 1.9- and 2.5-fold by 1.1 and 3.4 M TCP (Fig. 2B). CYP11A and 3ˇHSD2 were down-regulated 1.4- and 2.4-fold in cells exposed to the greater concentration of TCP. Expression of CYP19 was down-regulated 6.1- and 7.0-fold and expression of 17ˇHSD4 mRNA was downregulated 3.2- and 4.0-fold by 1.1 and, 3.4 M TCP, respectively (Fig. 2B). Expression of CYP17 mRNA was not significantly altered by either concentration of TCP (Fig. 2B). 3.3. Hormone production Concentrations of both T and E2 were affected by exposure to PCP or TCP. Concentrations of T were 18% and 31% less in media of cells exposed to 1.1 or 3.4 M PCP, respectively (Fig. 3A). Concentrations of T were 18%, 21% and 31% less in the media of cells exposed to 0.4, 1.1, or 3.4 M TCP, respectively (Fig. 3B). Concentrations of E2 were 12% less in the medium of cells exposed to 3.4 M PCP, while 0.4 and 1.1 M PCP exposure caused no statistically significant effects on E2 production (Fig. 3C). E2 concentration was reduced 15% when cells were exposed to 3.4 M TCP (Fig. 3D). 3.4. Cellular cAMP levels Both PCP and TCP caused a reduction in concentration of cAMP relative to that in control cells. PCP caused a concentration- 150 Y. Ma et al. / Toxicology 282 (2011) 146–153 4. Discussion Fig. 4. Concentrations of cAMP in H295R cells exposed to 0.0, 0.4, 1.1 or 3.4 M pentachlorophenol (PCP) (A) or 2,4,6-trichlorophenol (TCP) (B) for 48 h. Mean ± SEM from three replicate samples. *p < 0.05 and **p < 0.01, significant differences between treatments and control. dependent reduction in concentration of cAMP. The reductions in cAMP relative to that of the controls were 16%, 23%, and 37% for 0.4, 1.1, 3.4 M PCP, respectively, with the effect statistically significant at only the greatest concentration of 3.4 M PCP (Fig. 4A). TCP also caused a statistically significant, concentrationdependent and lesser concentration of cAMP relative to that of the controls. cAMP concentrations were 23%, 49% and 53%, less than that of controls for the three concentrations, respectively (Fig. 4B). 3.5. Time-course response of cAMP, StAR gene expression, protein abundance and hormone levels In the control group, the cellular cAMP levels remained stable during the exposure period (Fig. 5A). Exposure to 3.4 M TCP decreased cellular cAMP levels at 6, 12, 24 or 48 h by 15%, 21%, 31.8% and 40.4%, respectively, compared with the control (Fig. 5A). The StAR gene expression was down-regulated at 12, 24, and 48 h by 1.2-, 1.5- and 3.2-fold, respectively (Fig. 5B). Exposure to 3.4 M TCP also down-regulated StAR protein expression by 1.3- and 2.7-fold after 24 and 48 h, respectively, compared with the control (Fig. 5C and D). Concentrations of T were 49% and 30% less in media of the cells exposed to 3.4 M TCP at 24 and 48 h, respectively, relative to the correspondence control (Fig. 5E). There were no significant differences in the E2 concentrations upon exposure to 3.4 M TCP at 12 and 24 h (Fig. 5F), while concentrations of E2 were 20% less in media at 48 h exposure (Fig. 5F). The mechanism by which PCP decreased production of the two steroid hormones (T and E2) is consistent with down-regulation of gene expressions of enzymes involved in their production. Down-regulation of gene expression of steroidogenic enzymes was associated with decreased cellular cAMP content, which is consistent with regulation of steroidogenesis networks via cAMPdependent signaling. The statistically significant down-regulation of StAR, CYP11A, CYP17 gene expressions caused by PCP and TCP could result in changes in steroid hormones. The protein encoded by the StAR gene plays a key role in the acute regulation of steroid hormone synthesis, while CYP11A1 catalyzes the first step in steroid hormone biosynthesis which forms pregenolone through side chain cleavage of cholesterol, thus potentially affecting the levels of all adrenal steroid hormones. The CYP17 enzyme functions as two different catalysts steroid 17␣-hydroxylase and 17,20-lyase and is responsible for the production of dehydroepiandrosterone (DHEA), which is synthesized in the adrenal gland of humans (Chen et al., 2004). Inhibition of CYP17 would result in less formation of 17␣-OH-prognnolone and 17␣-OH-pregesterone, suppression of DHEA activity, and ultimately suppression of production of androstenedione. Therefore, inhibition of CYP11A and CYP17 gene expression observed in this study could lead to non-selective inhibition of other cytochrome P450 enzymes and affect steroidogenesis, which could result in less synthesis of weaker androgens, such as DHEA and consequently affect production of T and E2. Inhibition of E2 secretion has been shown to be due to inhibition of CYP17 when human luteinizing granulosa cells were treated with 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) (Moran et al., 2003). Some PBDEs and their derivatives, including hydroxyl brominated diphenylethers (OH-BDEs) and methoxylated brominated diphenylethers (MeO-BDEs) can inhibit CYP17 activity in H295R cells (Cantón et al., 2006). The 3ˇ-HSD is responsible for the oxidation and isomerization of 5-ene-3-hydroxy steroids to the corresponding 4-ene-3ketosteroids, which is a required step in biosynthesis of not only androgens and estrogens but also of mineralocorticoids and glucocorticoids (Labrie et al., 1992; Mason, 1993). In humans, two closely related types of 3ˇ-HSD (3ˇ-HSD1 and 3ˇHSD2) have been identified and 3ˇHSD2 is exclusively expressed in the adrenal cortex and gonads (Mason et al., 1997). Since the 3ˇHSD family is required for the biosynthesis of all classes of steroid hormones, down-regulation of 3ˇHSD2 is consistent with the decrease in concentration of T being partially the result of decrease production of up-stream hormones, such as 17␣-OH-progesterone, DHEA. Prochloraz significantly inhibited the expression of 3ˇHSD2, which was correlated down-regulation of steroidogenesis (Ohlsson et al., 2009). 17ˇHSD enzyme catalyzes the final step of sex steroid biosynthesis, which controls estrogen and androgen concentrations. Exposure to PCP inhibits steroidogenesis, accompanied by a decrease in production of T in cultured Xenopus oocytes (Orton et al., 2009). While there are no reports of PCP affecting steroidogenesis in H295R cells, tribromophenol (TBP) has been reported to modulate 3ˇHSD2 and CYP17 involved in steroid synthesis of H295R cells (Ding et al., 2007). Exposure of zebrafish to TBP significantly down-regulated expression of 3ˇHSD2, 17ˇHSD4, CYP17 and decreased concentration of T in plasma of females (Deng et al., 2010). The authors speculated that the decreased concentration of T was due, at least in part, to reduced expression of these genes. Taken together, these results suggest that PCP and TCP decreased mRNA expression and act at the level of gene transcription. Aromatase (CYP19) catalyzes the final and rate-limiting step in conversion of androgen to estrogen (Hilscherova et al., 2004). Var- Y. Ma et al. / Toxicology 282 (2011) 146–153 151 Fig. 5. Time-course of cellular cAMP concentration at 3, 6, 12, 24, and 48 h (A); StAR gene expressions (B); representative Western blotting of abundance of StAR enzymes from control and 3.4 M exposed cells for 12, 24 and 48 h (C); quantification of the relative expression of StAR enzyme in control and treatment group (D); concentrations of testosterone (T) (E) and 17-estradiol (E2) (F) in media of H295R cells exposed to 0.0, or 3.4 M 2,4,6-trichlorophenol (TCP). Student’s t-test was performed to indicate statistical significant differences between exposure group with corresponding control. Mean ± SEM from three replicate samples. *p < 0.05 and **p < 0.01, significant differences between treatments and control. ious fungicides are known to inhibit aromatase activity in H295R cells (Mason et al., 1987; Ayub and Levell, 1988; Vinggaard et al., 2000; Cantón et al., 2005). It has been hypothesized that the ability of various chemicals to alter the activity of CYP19 represents a potential mechanism of endocrine disruption (Sanderson et al., 2002). For example, pesticides such as imazalil and prochloraz inhibit CYP19. Since this is the enzyme that controls the rate of conversion of androgens into estrogens, in the study reported upon here, the significant decrease in expression CYP19 mRNA in H295R cells is likely the reason for decreased synthesis of E2. cAMP is an important secondary messenger that stimulates steroid hormone biosynthesis in the human adrenal cortex (Sewer and Waterman, 2001; Stocco et al., 2005). Most steroidogenic genes, including StAR, CYP11A, CYP11B, CYP17 and CYP21 are cAMPdependent (Sewer and Waterman, 2001). 3ˇHSD2 in H295R cells can be induced in H295R cells by stimulation of cAMP (Martin and Tremblay, 2005). In the study reported upon here, concentra- 152 Y. Ma et al. / Toxicology 282 (2011) 146–153 tion of cAMP in H295R cells was significantly less and expression of several key steroidogenic genes was down-regulated. This result is consistent with cellular cAMP regulating steroidogenesis. This is consistent with the observation that several chemicals, including triazines, atrazine, vinclozolin, flavonoid and methylxanthine, can modulate steroidogenesis through the cAMP pathway (Sanderson et al., 2000, 2002, 2004; Hilscherova et al., 2004; Suzawa and Ingraham, 2008). For example, treatment of H295R cells with forksolin, an inducer of cAMP resulted in greater 3ˇHSD2 expression (Hilscherova et al., 2004). In addition, co-exposure to 3-methyl-4-nitrophenol and cAMP significantly up-regulated 17ˇHSD4 expression in H295R cells (Furuta et al., 2008). This is also consistent with cAMP modulating steroidogenesis. This study further examined whether the inhibitory effects of CPs on steroidogenesis (including decreased mRNA expression, protein abundance and hormone levels) resulted from the reduction of cAMP. Among of these enzymes involvement of steroidogenesis, steroidogenic acute regulatory (StAR) protein is a central regulator in steroidogenesis (Sugawara et al., 2006). Therefore, StAR was selected for testing the time-course response of cAMP, gene expression, and the enzyme protein levels upon H295R exposure to TCP. cAMP content was significantly decreased by 31.8% and 40.4% at 24 and 48 h exposure, respectively, and StAR gene expression and protein abundance as well as hormone levels were all decreased, which indicates that the decrease in cellular cAMP may lead to inhibition of steroidogenesis. This result is consistent with those of the previous study reporting that a decrease in cellular cAMP level significantly inhibited StAR mRNA, protein and testosterone production in primary rat Leydig cells exposed to perfluorododecanoic acid (Shi et al., 2010). Taken together, we propose that PCP and TCP may alter steroidogenesis and hormone via modulating cAMP signaling in H295R cells. In summary, we have shown that PCP and TCP affect production of T and E2 in H295R cells. These effects are probably mediated by inhibition of the steroidogenic enzymes via decreased cellular concentration of cAMP. Other regulatory factors, such as steroidogenic factor 1 (SF-1) can regulate steroidogenic gene expression (Li et al., 2004; Sugawara et al., 2006) and thus future studies that investigate whether SF-1 modulates expression of these steroidogenic genes will provide new insights into the underlying mechanisms. Further in vivo investigation to elucidate the effects of the gene and hormone levels and reproduction is warranted. In addition, evaluating the effects of mixture of chlorophenols in vitro and then combining with in vivo study will provide more comprehensive information of an impact on homeostasis and organism health. Conflict of interest The authors declare no conflict of interest. Acknowledgements This work was supported by Chinese Academy of Sciences (KZCX2-YW-Q02-05), the NSFC of China (20890113), the FEBL project (2008FBZ10) and a Discovery Grant from the NSERC of Canada (326415-07), and a grant from the Western Economic Diversification Canada (6578 and 6807). Prof. Giesy was supported by the Canada Research Chair program and an at-large Chair Professorship at the Department of Biology and Chemistry and State Key Laboratory in Marine Pollution, City University of Hong Kong. References Arechabala, B., Coiffard, C., Rivalland, P., Coiffard, L.J., de Roeck-Holtzhauer, Y., 1999. Comparison of cytotoxicity of various surfactants tested on normal human fibroblast cultures using the neutral red test, MTT assay and LDH release. J. Appl. Toxicol. 19, 163–165. ATSDR, 2001. Toxicological Profile for Pentachlorophenol. Agency for Toxic Substances and Disease Registry, Public Health Service, U.S. Department of Health and Human Services, Atlanta, p. 316. Ayub, M., Levell, M.J., 1988. Structure–ctivity relationships of the inhibition of human placental aromatase by imidazole drugs including ketoconazole. J. Steroid Biochem. 31, 65–72. Baynes, R.E., Brooks, J.D., Mumtaz, M., Riviere, J.E., 2002. Effect of chemical interactions in pentachlorophenol mixtures on skin and membrane transport. Toxicol. Sci. 69, 295–305. Bhattacharya, S.K., Yuan, Q., Jin, P., 1996. Removal of pentachlorophenol from wastewater by combined anaerobic–erobic treatment. J. Hazard Mater. 49, 143–154. Cantón, R.F., Sanderson, J.T., Letcher, R.J., Bergman, A., van den Berg, M., 2005. Inhibition and induction of aromatase (CYP19) activity by brominated flame retardants in H295R human adrenocortical carcinoma cells. Toxicol. Sci. 88, 447–455. Cantón, R.F., Sanderson, T., Nijmeijer, S., Bergman, Å., Letcher, R.J., Van den Berg, M., 2006. In vitro effects of brominated flame retardants and metabolites on CYP17 catalytic activity: a novel mechanism of action? Toxicol. Appl. Pharmacol. 216, 274–281. Chen, C., Parker Jr., C.R., 2004. Adrenal androgens and the immune system. Semin. Reprod. Med. 22, 369–377. Chen, Y., Chen, H., Xu, Y., Shen, M., 2004. Irreversible sorption of pentachlorophenol to sediments: experimental observations. Environ. Int. 30, 31–37. Daniel, V., Huber, W., Bauer, K., Opelz, G., 1997. Impaired in vitro lymphocyte responses in patients with elevated pentachlorophenol (PCP) blood levels. Arch. Environ. Health 50, 148–149. Deng, J., Liu, C., Yu, L., Zhou, B., 2010. Chronic exposure to environmental levels of tribromophenol impairs zebrafish reproduction. Toxicol. Appl. Pharmacol. 243, 87–95. Ding, L., Murphy, M.B., He, Y., Xu, Y., Yeung, L.W.Y., Wang, J., Zhou, B., Lam, P.K.S., Wu, R.S.S., Giesy, J.P., 2007. Effects of brominated flame retardants and brominated dioxins on steroidogenesis in H295R human adrenocortical carcinoma cell line. Environ. Toxicol. Chem. 26, 764–772. Dong, Y., Jiang, S., 2009. Induction of oxidative stress and apoptosis by pentachlorophenol in primary cultures of Carassius carassius hepatocytes. Comp. Biochem. Physiol. 150C, 179–185. Dorsey, W.C., Tchounwou, P.B., 2004. Pentachlorophenol-induced cytotoxic mitogenic, and endocrine-disrupting activities in channel catfish, Ictalurus punctatus. Int. J. Environ. Res. Public Health 1, 90–99. Eker, S., Kargi, F., 2007. 2,4,6-Trichlorophenol containing wastewater treatment using a hybrid-loop bioreactor system. J. Environ. Eng. 133, 340–345. Farhadi, K., Farajzadeh, M.A., Matin, A.A., Hashemi, P., 2009. Dispersive liquid–liquid microextraction and liquid chromatographic determination of pentachlorophenol in water. Cent. Eur. J. Chem. 7, 369–374. Fernández Freire, P., Labrador, V., Pérez Martín, J.M., Hazen, M.J., 2005. Cytotoxic effects in mammalian Vero cells exposed to pentachlorophenol. Toxicology 210, 37–44. Furuta, C., Noda, S., Li, C., Suzuki, A.K., Taneda, S., Watanabe, G., Taya, K., 2008. Nitrophenols isolated from diesel exhaust particles regulate steroidogenic gene expression and steroid synthesis in the human H295R adrenocortical cell line. Toxicol. Appl. Pharmacol. 229, 109–120. Gao, J., Liu, L., Liu, X., Zhou, H., Huang, S., Wang, Z., 2008. Levels and spatial distribution of chlorophenols 2,4-dichlorophenol, 2,4,6-trichlorophenol, and pentachlorophenol in surface water of China. Chemosphere 71, 1181–1187. Gracia, T., Hilscherova, K., Jones, P.D., Newsted, J.L., Zhang, X., Hecker, M., Higley, E.B., Sanderson, J.T., Yu, R.M., Wu, R.S., Giesy, J.P., 2006. The H295R system for evaluation of endocrine-disrupting effects. Ecotoxicol. Environ. Saf. 65, 293–305. Hanna, K., de Brauer, C., Germain, P., Chovelon, J.M., Ferronato, C., 2004. Degradation of pentachlorophenol in cyclodextrin extraction effluent using a photocatalytic process. Sci. Total Environ. 332, 51–60. He, Y., Murphy, M.B., Yu, R.M., Lam, M.H., Hecker, M., Giesy, J.P., Wu, R.S., Lam, P.K., 2008. Effects of 20 PBDE metabolites on steroidogenesis in the H295R cell line. Toxicol. Lett. 176, 230–238. Hecker, M., Newsted, J.L., Murphy, M.B., Higley, E.B., Jones, P.D., Wu, R., Giesy, J.P., 2006. Human adrenocarcinoma (H295R) cells for rapid in vitro determination of effects on steroidogenesis: hormone production. Toxicol. Appl. Pharmacol. 217, 114–124. Hilscherova, K., Jones, P.D., Gracia, T., Newsted, J.L., Zhang, X., Sanderson, J.T., Yu, R.M.K., Wu, R.S.S., Giesy, J.P., 2004. Assessment of the effects of chemicals on the expression of ten steroidogenic genes in the H295R cell line using real-time PCR. Toxicol. Sci. 81, 78–89. Jensen, J., 1996. Chlorophenols in the terrestrial environment. Rev. Environ. Contam. Toxicol. 146, 25–51. Jung, J., Ishida, K., Nishihara, T., 2004. Anti-estrogenic activity of fifty chemicals evaluated by in vitro assays. Life Sci. 74, 3065–3074. Labrie, F., Simard, J., Luu-The, V., Belanger, A., Pelletier, G., 1992. Structure, function and tissue-specific gene expression of 3-hydroxysteroid dehydrogenase/5-ene-4-ene isomerase enzymes in classical and peripheral intracrine steroidogenic tissues. J. Steroid Biochem. Mol. Biol. 43, 805–826. Letcher, R.J., Sanderson, J.T., Bokkers, A., Giesy, J.P., van den Berg, M., 2005. Effects of bisphenol A-related diphenylalkanes on vitellogenin production in mal carp (Cyprinus carpio) hepatocytes and aromatase (CYP19) activity in human H295 adrenocortical carcinoma cells. Toxicol. Appl. Pharmacol. 209, 95–104. Li, L., Chang, Y., Wang, C., Tsai, F., Jong, S., Chung, B., 2004. Steroidogenic factor 1 differentially regulates basal and inducible steroidogenic gene expression and Y. Ma et al. / Toxicology 282 (2011) 146–153 steroid synthesis in human adrenocortical H295R cells. J. Steroid Biochem. Mol. Biol. 91, 11–20. Li, L., Wang, P., 2005. PCB126 induces differential changes in androgen, cortisol, and aldosterone biosynthesis in human adrenocortical H295R cells. Toxicol. Sci. 85, 530–540. Liu, C., Zhang, X., Chang, H., Jones, P., Wiseman, S., Naile, J., Hecker, M., Giesy, J.P., Zhou, B., 2010. Effects of fluorotelomer alcohol 8:2 FTOH on steroidogenesis in H295R cells: targeting the cAMP signaling cascade. Toxicol. Appl. Pharmacol. 247, 222–228. Livak, K.J., Schmittgen, T.D., 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−CT method. Methods 25, 402–408. Martin, L.J., Tremblay, J.J., 2005. The human HSD3B2 promoter is a novel target for the immediate early orphan nuclear receptor nur77 in steroidogenic cells. Endocrinology 146, 861–869. Mason, J.I., Carr, B.R., Murry, B.A., 1987. Imidazole antimycotics: selective inhibitors of steroid aromatization and progesterone hydroxylation. Steroids 50, 179–189. Mason, J.I., 1993. The 3-hydroxysteroid dehydrogenase gene family of enzymes. Trends Endocrinol. Metab. 4, 199–203. Mason, J.I., Keeney, D.S., Bird, I.M., Rainey, W.E., Morohashi, K., Leers Sucheta, S., Melner, M.H., 1997. The regulation of 3-hydroxysteroid dehydrogenase expression. Steroids 62, 164–168. Moran, F.M., VandeVoort, C.A., Overstreet, J.W., Lasley, B.L., Conley, A.J., 2003. Molecular target of endocrine disruption in human luteinizing granulosa cells by 2,3,7, 8-tetrachlorodibenzo-p-diox: inhibition of estradiol secretion due to decreased 17alpha-hydroxylase/17,20-lyase cytochrome P450 expression. Endocrinology 144, 467–473. O’Donoghue, J.L., 1985. In: O’Donoghue, J.L. (Ed.), Neurotoxicity of Industrial and Commercial Chemicals, vol. 2. CRC Press, Boca Raton, FL, pp. 99–120. Ohlsson, A., Ullerås, E., Oskarsson, A., 2009. A biphasic effect of the fungicide prochloraz on aldosterone, but not cortisol, secretion in human adrenal H295R cells—underlying mechanisms. Toxicol. Lett. 191, 174–180. Orton, F., Lutz, I., Kloas, W., Routledge, E.J., 2009. Endocrine disrupting effects of herbicides and pentachlorophenol: in vitro and in vivo evidence. Environ. Sci. Technol. 43, 2144–2150. Proudfoot, A.T., 2003. Pentachlorophenol poisoning. Toxicol. Rev. 22, 3–11. Ramamoorthy, S., Ramamoorthy, S., 1997. Chlorinated Organic Compounds in the Environment. Regulatory and Monitoring Assessment. Lewis Publishers, Boca Ration. Reigner, B.G., Bois, F.Y., Tozer, T.N., 1993. Pentachlorophenol carcinogenicity: extrapolation of risk from mice to humans. Hum. Exp. Toxicol. 12, 215–225. Sanderson, J.T., Seinen, W., Giesy, J.P., van den Berg, M., 2000. 2-Chloro-s-triazine herbicides induce aromatase (CYP19) activity in H295R human adrenocortical carcinoma cells: a novel mechanism for estrogenicity? Toxicol. Sci. 54, 121–127. Sanderson, J.T., Boerma, J., Lansbergen, G.W.A., Van den Berg, M., 2002. Induction and inhibition of aromatase (CYP19) activity by various classes of pesticides in H295R human adrenocortical carcinoma cells. Toxicol. Appl. Pharmacol. 182, 44–54. Sanderson, J.T., Hordijk, J., Denison, M.S., Springsteel, M.F., Nantz, M.H., Van den Berg, M., 2004. Induction and inhibition of aromatase (CYP19) activity by natural and 153 synthetic flavonoid compounds in H295R human adrenocortical carcinoma cells. Toxicol. Sci. 82, 70–79. Sewer, M.B., Waterman, M.R., 2001. Insights into the transcriptional regulation of steroidogenic enzymes and StAR. Rev. Endocr. Metab. Disord. 2, 269–274. Shi, Z., Feng, Y., Wang, J., Zhang, H., Ding, L., Dai, J., 2010. Perfluorododecanoic acid-induced steroidogenic inhibition is associated with steroidogenic acute regulatory protein and reactive oxygen species in cAMP-stimulated leydig cells. Toxicol. Sci. 114, 285–294. Song, R., He, Y., Murphy, M.B., Yeung, L.W., Yu, R.M., Lam, M.H., Lam, P.K., Hecker, M., Giesy, J.P., Wu, R.S., Zhang, W., Sheng, G., Fu, J., 2008. Effects of fifteen PBDE metabolites, DE71, DE79, and TBBPA on steroidogenesis in the H295R cell line. Chemosphere 71, 1888–1894. Stocco, D.M., Wang, X., Jo, Y., Manna, P.R., 2005. Multiple signaling pathways regulating steroidogenesis and steroidogenic acute regulatory protein expression: more complicated than we thought. Mol. Endocrinol. 19, 2647–2659. Sugawara, T., Sakuragi, N., Minakami, H., 2006. CREM confers cAMP responsiveness in human steroidogenic acute regulatory protein expression in NCI-H295R cells rather than SF-1/Ad4BP. J. Endocrinol. 191, 327–337. Suzawa, M., Ingraham, H.A., 2008. The herbicide atrazine activates endocrine gene networks via non steroidal NR5A nuclear receptors in fish and mammalian cells. PLoS one 3, e2117. Suzuki, T., Ide, K., Ishida, M., 2001. Response of MCF-7 human breast cancer cells to some binary mixtures of oestrogenic compounds in vitro. J. Pharm. Pharmacol. 53, 1549–1554. Vinggaard, A.M., Hnida, C., Breinholt, V., Larsen, J.C., 2000. Screening of selected pesticides for inhibition of CYP19 aromatase activity in vitro. Toxicol. In Vitro 14, 227–234. Wang, X., Li, Y., Dong, D., 2008. Sorption of pentachlorophenol on surficial sediments: the roles of metal oxides and organic materials with co-existed copper present. Chemosphere 73, 1–6. Yin, D., Gu, Y., Li, Y., Wang, X., Zhao, Q., 2006. Pentachlorophenol treatment in vivo elevates point mutation rate in zebrafish p53 gene. Mutat. Res. 609, 92–101. Zha, J., Wang, Z., Schlenk, D., 2006. Effects of pentachlorophenol on the reproduction of Japanese medaka (Oryzias latipes). Chem. Biol. Interact. 161, 26–36. Zhang, M., Yin, D., Kong, F., 2008. The changes of serum testosterone level and hepatic microsome enzyme activity of crucian carp (Carassius carassius) exposed to a sublethal dosage of pentachlorophenol. Ecotoxicol. Environ. Saf. 71, 384–389. Zhang, X., Yu, R.M., Jones, P.D., Lam, G.K., Newsted, J.L., Gracia, T., Hecker, M., Hilscherova, K., Sanderson, T., Wu, R.S., Giesy, J.P., 2005. Quantitative RT-PCR methods for evaluating toxicant-induced effects on steroidogenesis using the H295R cell line. Environ. Sci. Technol. 39, 2777–2785. Zhao, B., Yang, J., Liu, Z., Xu, Z., Qiu, Y., Sheng, G., 2006. Joint anti-estrogenic effects of PCP and TCDD in primary cultures of juvenile goldfish hepatocytes using vitellogenin as a biomarker. Chemosphere 65, 359–364. Zheng, M., Zhang, B., Bao, Z., Yang, H., Xu, X., 2000. Analysis of pentachlorophenol from water, sediments, and fish bile of Dongting Lake in China. Bull. Environ. Contam. Toxicol. 64, 16–19.