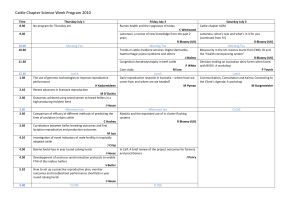
Recent Developments in Reproductive Technologies
advertisement

Recent Developments in Reproductive Technologies Presentation 8- DR Fulvio Gandolfi , Laboratory of Biomedical Embryology, Department of Stem Cell Research, Faculty of Veterinary Medicine, Milan, Italy, *Kings College, London “Advantages and limitations of reproductive technologies applied to conservation programs” (P. 58) Presentation 9- DR GREGG P. ADAMS,Veterinary Biomedical Sciences, University of Saskatchewan, CANADA “Reproductive biotechnology as a solution to endemic disease in Canada’s threatened Wood Bison herd” (P. 62) Presentation 10 - DR GYÖRGY GÁBOR , Research Institute for Animal Breeding and Nutrition, Herceghalom, Hungary “Factors influencing pregnancy rate in dairy cattle” (P. 69) Presentation 11- DR GARETH EVANS, Faculty of Veterinary Science, The University of Sydney, Sydney,, Australia “Application of sexed sperm in animal breeding and conservation programs” (P. 74) Presentation 12- DR PETER J. HANSEN, Dept. of Animal Sciences and D.H. Barron Reproductive and Perinatal Biology Research Program, University of Florida, Gainesville, FL, USA “Prospects for Genetic Modification to Improve Dairy Cow Reproduction During Heat Stress” (P. 82) Presentation 13- DR WILLIAM W. THATCHER, Department of Animal Sciences, University of Florida, Gainesville, FL 32611-0910 “Has The Loss of Genetic Diversity Adversely Affected The Holstein?” (P. 87) “International Strategic Program for Conservation of Animal Genetic Resources for Food and Agriculture” sponsored by OECD Co-operative Research Programme 57 © F Gandolfi, 2010, All rights reserved Presentation-8 Advantages and limitations of reproductive technologies applied to conservation programs Fulvio Gandolfi and Cecilia E. Gandolfi* Laboratory of Biomedical Embryology, Department of Stem Cell Research, Faculty of Veterinary Medicine, Milan, Italy, *Kings College, London Correspondence: fulvio.gandolfi@unimi.it Introduction The application of assisted reproduction methods can be an effective tool for managing conservation programmes of endangered breeds. However the efficiency of each method varies between individual species. Furthermore it is important to understand the advantages and limitations of each method in order to select the one more suitable for any specific goal. A brief explanation will be provided for the most common reproductive techniques together with a critical evaluation of their applicability in the different species. In vivo production of embryos Superovulation and embryo transfer are well established techniques widely applied to all domestic and wild species. They represent the golden standard since this is the method that produce the embryos of highest quality allowing a fertility almost identical to natural mating or artificial insemination. Most importantly these embryos can be efficiently cryopreserved thus enabling an easy storage and a convenient way of transport and distribution. In vitro production of embryos (IVP) In farm animals IVP starts with the in vitro maturation of the oocyte. This consists of the resumption of the meiotic division that was arrested at prophase of the first meiotic division during the foetal life. This is the same process that takes place in vivo at the time of ovulation. However the important difference is that in vivo this can occur only to fully developed oocytes whereas, in vitro, any oocyte that has reached a minimum size can mature. The further away the oocyte is from its full development, the lower are its chances to be fertilized and to sustain embryonic development to term. As a consequence, in species where this technique is more developed, as in ruminants, on average, no more than 40% of the oocytes matured in vitro can develop to the blastocyst stage. At this point they can be transferred into a recipient animal where only 40 to 60% of the embryos goes to term. These values refer to cattle IVP embryos and to experienced laboratories. Other species, like pig or horse, typically show lower results. “International Strategic Program for Conservation of Animal Genetic Resources for Food and Agriculture” sponsored by OECD Co-operative Research Programme 58 © F Gandolfi, 2010, All rights reserved In order to use IVP for preserving genetic diversity it is important to collect the oocytes from living animals as opposed to using slaughter house material traditionally utilized to produce embryos for research. This can be accomplished by the ultrasound-guided transvaginal aspiration of oocytes from small and preovulatory follicles. Its efficiency is comparable with that of traditional embryo transfer with the added possibility to use pregnant and clinically infertile animals. However individual variation strongly affects the final results in a similar way as superovulation, and the use non surgical procedures can be limited by the size of the species. Figure 1. The scheme describes the different phases required for the in vitro production of embryos. The time frame refers to bovine species. Cryopreservation of embryos and gametes The possibility of cryopreserving embryos adds a lot of potential to embryo transfer or IVP, allowing the storage of large numbers of individuals in a limited space. This enables the creation of repositories and facilitates the logistic and sanitary management of long range transport. In cattle, the same pregnancy rates are obtained with fresh and cryopreserved embryos if produced in vivo, whereas IVP embryos are more sensitive to temperature stress. It has been estimated that freezing and banking 300 non-sexed embryos collected from 90 unrelated donors would be sufficient to maximize genetic diversity and save a rare bovine breed. The same is even more true in case of sperm cryopreservation. Semen cryopreservation is a widespread and extensively applied technique with high or acceptable rates of success in virtually any species. Oocyte cryopreservation is less efficient, but constant progress is obtained in laboratory species as well as in non-human primates, horses and ruminants. These studies have made substantial progress in using ultrarapid freezing protocols for retaining the stability of oocyte cytoskeleton and, therefore, could be applied for oocyte cryopreservation of rare breeds in case embryos cannot be obtained at a specific location. “International Strategic Program for Conservation of Animal Genetic Resources for Food and Agriculture” sponsored by OECD Co-operative Research Programme 59 © F Gandolfi, 2010, All rights reserved Reproductive cloning The possibility to obtain a new individual from any somatic cell through nuclear transplantation, in theory, makes the task of preserving a rare breed or even resurrecting an extinct one very easy. The principle is now well known and is based on the transplantation of a somatic cell into an enucleated egg. The cytoplasmic components present in the egg cytoplasm are able to reprogram the somatic nucleus back to its embryonic stage transforming a fully differentiated cell into a totipotent zygote. The potential of this technique is only equal to its limitations. First of all the reprogramming efficiency is very low therefore it is necessary to perform anything between 100 and 500 nuclear transfers for obtaining one live offspring. This implies the need for a large number of oocytes that becomes increasingly difficult to obtain with the decrease of the prolificacy of the species of interest. In any case, if the goal is the conservation of a breed within a numerous species, the limit can be overcome with enough financial and technical support. If, on the contrary, the goal is to save a species with a limited number of individuals whose oocytes are, therefore, very rare and valuable, SCNT is unlikely to be a viable method as it must rely on the use of oocytes from a different species. This makes the whole procedure even more inefficient and in most cases simply impossible. Figure 2. A muflon born after interspecific somatic cell nuclear transfer with a sheep oocyte and transfer into a sheep recipient. (From Loi et al. 2001. Genetic rescue of an endangered mammal by cross-species nuclear transfer using post-mortem somatic cells. Nat Biotechnol 19:962-964.) The reasons for this dramatic drop in efficiency have not been fully elucidated but it is thought that the inability of oocyte mitochondria to communicate with the donor nucleus is a likely cause. In addition the asynchrony of genome activation between donor and recipient seems to play a crucial role. “International Strategic Program for Conservation of Animal Genetic Resources for Food and Agriculture” sponsored by OECD Co-operative Research Programme 60 © F Gandolfi, 2010, All rights reserved The role of the oocyte At the end of this brief overview it is important to realize that the possibility to apply all the techniques described above, both in endangered or widely available breeds, depends on the availability and quality of a large number of oocytes. Increasing the number of fertilisable oocytes by removing them from the ovary before ovulation has a price. Large field trials performed under commercial conditions, in fact, showed that pregnancy rates after transfer of fresh or frozen-thawed in vitro-produced embryos are significantly reduced compared to the rates achieved with their in vivo produced counterparts. At present, successful development in vitro is limited to the use of fully grown oocytes from antral follicles; although, the vast majority of oocytes present in an ovary at any given time are enclosed in primordial and primary follicles. Current studies are preparing the way for the exploitation of this vast source of genetic material; however; considerable biological and technical hurdles still need to be overcome. Suggested readings Andrabi SM, Maxwell WM. 2007. A review on reproductive biotechnologies for conservation of endangered mammalian species. Anim Reprod Sci 99(3-4):223-243. Brevini Gandolfi TAL, Gandolfi F. 2001. The maternal legacy to the embryo: cytoplasmic components and their effects on early development. Theriogenology 55(6):1255-1276. Brevini TA, Cillo F, Antonini S, Tosetti V, Gandolfi F. 2007. Temporal and spatial control of gene expression in early embryos of farm animals. Reprod Fertil Dev 19(1):35-42. Galli C, Lazzari G. 2008. The manipulation of gametes and embryos in farm animals. Reprod Domest Anim 43 Suppl 2:1-7. Gandolfi F, Brevini TA. 2010. RFD Award Lecture 2009. In vitro maturation of farm animal oocytes: a useful tool for investigating the mechanisms leading to full-term development. Reprod Fertil Dev 22(3):495-507. Gandolfi F, Brevini TA, Cillo F, Antonini S. 2005. Cellular and molecular mechanisms regulating oocyte quality and the relevance for farm animal reproductive efficiency. Rev Sci Tech 24(1):413-423. “International Strategic Program for Conservation of Animal Genetic Resources for Food and Agriculture” sponsored by OECD Co-operative Research Programme 61 © GP Adams, 2010, All rights reserved Presentation-9 Reproductive biotechnology as a solution to endemic disease in Canada’s threatened Wood Bison herd Gregg P. Adams1, Robert B. McCorkell2, Carl Lessard1 1Veterinary 2Faculty Biomedical Sciences, University of Saskatchewan, CANADA of Veterinary Medicine, University of Calgary, CANADA Background The Committee on the Status of Endangered Wildlife in Canada (COSEWIC) has defined the wood bison (Bison bison athabascae) as a threatened species. The largest herd of free-ranging wood bison are in Wood Buffalo National Park on the border between northern Alberta and the Northwest Territories. However, wood bison herds in and around Wood Buffalo National Park remain endemically infected with tuberculosis and brucellosis (Gates et al 1992, Gates et al 2001). The eradication of bovine tuberculosis and brucellosis from domestic animals was first initiated in Canada in 1907 and then again in 1928. Canada was finally declared free of brucellosis in domestic animals in 1985. Endemic disease in wood bison threatens to infect neighboring healthy wood bison herds and domestic cattle and bison ranches (Gates et al 2001, Mitchell & Gates 2002). In addition, the presence of diseased herds prevents the reintroduction of healthy wood bison into much of their original range (Gates et al 2001). In 1990 the Federal Environmental Assessment Review Organization (FEARO) concluded that eradication of diseased wood bison herds is the only method that will eliminate the risk of transmission of brucellosis and tuberculosis to domestic cattle and otherwise uninfected wood bison (FEARO 1990). The panel further recommended that the area should be repopulated with healthy wood bison obtained through genetic salvage operations (FEARO 1990). One method of genetic salvage is the cryopreservation of gametes (sperm and ova) and embryos. Animals with the same genetic heritage can then be produced at a future date using the technologies of in vitro fertilization and embryo transfer (Solti et al 2000). The application of these reproductive technologies to bison has been attempted but the results have been disappointing (Dorn 1995). The primary reason for difficulty in applying advanced reproductive technologies is the lack of knowledge about the reproductive physiology of the target species (Pukazhenthi & Wildt. 2004). Recent studies in the our laboratory, using daily transrectal ultrasonography and blood hormone analysis, have led to a more complete understanding of ovarian function in cattle, llamas, sheep, muskoxen, wapiti, and humans. (Adams et al 1990, Adams 1999; Baerwald et al 2003, Hoare et al 1997, McCorkell et al 2004). This knowledge has resulted in improved methods of estrus synchronization and superovulation in cattle. Ultrasonography has been used to study bison (Matsuda et al1996, Othen et al 1999), however, it has not been done in a serial fashion necessary for characterization of ovarian function during the annual reproductive cycle. Recently, we have successfully adapted the technique of daily transrectal ultrasonography to nondomestic species including wapiti, white tailed deer, and muskoxen (Hoare et al 1997, McCorkell et al 2001, 2004, 2007; Kemp “International Strategic Program for Conservation of Animal Genetic Resources for Food and Agriculture” sponsored by OECD Co-operative Research Programme 62 © GP Adams, 2010, All rights reserved et al 2003). We have initiated a similar study in our research herd of wood bison, and have documented the feasibility of the ultrasound technique for the purposes of preservation of genetic material (McCorkell et al., 2008). Objectives and Outcomes Experiment 1. The objective was to determine the feasibility of daily examination in wild-caught wood bison for the purpose of data collection on reproductive physiology. Three year old female wood bison (n=15) from Elk Island National Park were placed in our Native Hoofstock Centre at the University of Saskatchewan. Following acclimation to the handling procedure, transrectal ultrasonography was done on a daily basis. Ovarian follicular dynamics were succesfully followed for a period of 40 days. We concluded that habituated bison will tolerate daily transrectal ultrasonography and that this approach will allow the characterization of ovarian follicular and luteal events (Fig. 1). Figure 1. Wave pattern of follicle development in wood bison during the non-breeding season. Experiment 2. The objective was to characterize ovarian events, endocrine profiles and mating behavior that occur during the annual reproductive cycle of wood bison. The previously habituated Wood bison were examined daily by transrectal ultrasonography for 6-weeks during January and February (a time when the ovulatory season of bison was thought to end). No spontaneous ovulations were observed and the follicular dynamics of the early anovulatory period were characterized (Fig. 1). A second period of examinations started in July and extended into October. This period encompassed the late anovulatory period, the transistion into the ovulatory season, and the early part of the normal breeding season. Blood sampling and ultrasound examinations were done simultaneously to relate ovarian and endocrine events during the estrous cycle. Ovarian dynamics, and circulating concentrations of sex steroids and gonadotropins “International Strategic Program for Conservation of Animal Genetic Resources for Food and Agriculture” sponsored by OECD Co-operative Research Programme 63 © GP Adams, 2010, All rights reserved were compared with behavioural changes monitored by the HeatWatch system. The HeatWatch® system is based on a radio transmitter affixed to the female which signals a remote receiver when triggered by mounting of a male. This non-intrusive system allows us to gather continuous detailed information about behavioural estrus (time, frequency and duration) in a wild species. The use of the HeatWatch® system has not been previously reported in bison. In addition all bison were fitted with proximity collars that recorded the identity and timing of all interactions between collared individuals. This information will provide evidence about when the bull detects a cow in estrus and how long he tends her before and after breeding. Endocrine and ovarian dynamics are summarzed in Fig. 2. Figure 2. Diameter profiles (mean ±SEM) of successive dominant ovarian follicles and corpora lutea, and circulating concentration of progesterone during the transition from the nonbreeding to the breeding season in wood bison (n=19). Table 1. Synchronization of ovarian follicle wave emergence (mean±SEM) in bison pretreated with or without a long-acting neurolept tranquilizer (Piportil; Experiment 3). Control Control + Piportil Combined Wood bison Plains bison Wood bison Plains bison Day of follicle wave 4.3±0.8 4.5±0.3 4.5±1.2 2.8±0.9 4.0±0.4 emergence Ablation Ablation + Piportil Day of follicle wave 1.0±0.0 1.0±0.0 1.1±0.1 1.0±0.0 1.0±0.2 emergence Estradiol Estradiol + Piportil Day of follicle wave 3.3±0.5 2.8±0.4 2.6±0.2 4.2±0.8 3.3±0.3 emergence “International Strategic Program for Conservation of Animal Genetic Resources for Food and Agriculture” sponsored by OECD Co-operative Research Programme 64 © GP Adams, 2010, All rights reserved Experiment 3. The objective was to develop treatment protocols to control the reproductive cycle of female wood bison. The protocols will provide control of ovarian follicle development that is required for superstimulation and superovulation. These protocols will make it possible to conduct embryo transfer and timed artificial insemination. Information gathered in Experiment 2 was critical in the design of an experiment to test ovarian synchronization and superstimulation protocols similar to those we have developed for use in cattle. In addition to addressing the stated objective, an attempt was made to evaluate the effect that long acting neurolept tranquilizers (e.g., Piportil) might have on reproductive function. It is anticipated that the use of drugs in this class may be necessary to ensure success when working with wild, unconditioned animals (Ebedes, 1992). The experiment was successfully completed and the preliminary results are summarized in Table 1. Transvaginal ultrasound-guided follicle ablation (Bergfelt et al., 1994) shortened (P<0.05) and synchronized (P<0.05) the interval to new wave emergence compared to controls; estradiol was intermediate in effect. The neurolept tranquilizer appeared to have the desirable result of no effect on reproductive function. Experiment 4: Results of Experiment 3 were used to conduct a preliminary experiment on fixed-time artificial insemination. As part of a separate study on methods for cryopreservation of bison semen, we collected semen from our bison during the summer and fall and successfully implemented a freeze-thaw process that preserved sperm viability (Bogle et al., 2010). Frozen-thawed wood bison semen was used for fixed-time artificial insemination in our bison cows previously synchronized as described above. Of 12 synchronized cows, 8 (67%) ovulated within the scheduled 48 hr window for fixed-time AI, and 3 of the 8 (38%) become pregnant. All 3 bison cows gave birth to healthy calves in mid-June of the following year. Although the pregnancy rate was modest, due perhaps to issues with semen quality, timing of AI or quality of the ovulated oocyte, this represents the first report of pregnancy in bison from fixed-time insemination. On-going studies: Our next objective is to establish effective ovarian superstimulatory protocols for bison as a necessary prelude to oocyte collection for in vitro fertilization, or in vivo fertilization and embryo transfer. We will compare the ovarian response and oocyte quality in bison given a superstimulatory dose of equine chorionic gonadotropin (eCG) or follicle stimulating hormone (FSH) with or without a follow-up dose of luteinizing hormone (LH) during the anovulatory and ovulatory seasons. Follicular wave emergence will be synchronized among bison by transvaginal ultrasound-quided follicular ablation (Experiments 3 and 4). After preliminary analysis of initial superstimulation trials, we conclude that induction of a superstimulatory response is possible in wood bison during both the anovulatory and ovulatory seasons. Treatment with FSH was more effective than eCG to induce ovarian superstimulation and good quality of COC in bison, but follow-up treatment with LH had no apparent effect on oocyte quality. “International Strategic Program for Conservation of Animal Genetic Resources for Food and Agriculture” sponsored by OECD Co-operative Research Programme 65 © GP Adams, 2010, All rights reserved Conclusion Considering the iconic importance of wood bison as a uniquely Canadian species, and the threat posed by federally reportable diseases (endemic tuberculosis and brucellosis) to wood bison and to the national domestic livestock herd, it is astounding that funding to support this type of research is so scarce. It is equally astounding that prior to this study, so little was known of the normal reproductive physiology of bison. By taking a deliberate and systematic approach, we anticipate efficient production of disease-free germ plasm and embryos derived from captive and free-ranging bison, based on a solid foundation of normal reproductive patterns and protocols designed to influence these patterns. It is only a matter of time before a substantial germ bank for wood bison is established. The next challenge will be to effectively extirpate the endemically diseased population before repopulating with healthy animals. Acknowledgments This research was supported by grants from the Advancing Canadian Agriculture and Agri-Food Fund, the Agri-Food Innovation Fund, Parks Canada, the World Wildlife Fund, and the Northwest Territories. We thank Parks Canada for providing wood bison from Elk Island National Park, the University of Saskatchewan for bison maintenance and handling facilities, and the Provincial and National Bison Associations for Bison handling and consultation. References Adams GP, Sumar J, Ginther OJ. 1990. Effects of lactational and reproductive status on ovarian follicular waves in llamas (Lama glama). Journal of Reproduction Fertility 90(2): 535-545. Adams GP (1999) Comparative patterns of follicle development and selection in ruminants. Journal of Reproduction and Fertility, supplement 54, Reproduction in Domestic Ruminants IV, pp 17-32. Adams GP, MCCorkell RB, Jurgielewicz VC, Ambati D, Woodbury MR (2009) Estrous synchronization and fixed-time ai in wood bison (Bison bison athabascae). Reprod Fert Dev. (Jan. abstr). Baerwald, A. R., G.P. Adams, & R.A. Pierson. 2003. A new model for folliculogenesis during the menstrual cycle in women. Fertility and Sterility 80: 116 – 122. Bergfelt DR, Lightfoot KC, Adams GP. Ovarian synchronization following ultrasoundguided transvaginal follicle ablation in heifers. Theriogenology 1994;42:895-907. Bogle OA, Lessard C, McCorkell RB, Grafton T, Adams GP (2010) The effect of Bovipure gradient on bison sperm cryopreservation. Reprod Fert Dev. (Jan. abstr). Dorn, C.G. 1995. Application of reproductive technologies in North American bison (Bison bison). Theriogenology 43: 13 – 20. Ebedes H. Long-acting neuroleptics in wildlife. The use of tranquilizers in wildlife. Proceedings of the Wildlife Tranquilizer Symposium (ed. H. Ebedes). National Zoological Gardens, Pretoria, Republic of South Africa. 1992; 31–37. “International Strategic Program for Conservation of Animal Genetic Resources for Food and Agriculture” sponsored by OECD Co-operative Research Programme 66 © GP Adams, 2010, All rights reserved Federal Environmental Assessment Review Organization (FEARO). 1990. Northern diseased bison. Report No.35 of the Environmental Assessment Panel. Hull, Quebec, Federal Environmental Assessment Review Office. Gates C.C., T. Chowns & H. Reynolds. 1992. Wood buffalo at the crossroads. In J.E. Foster, D. Harrison & I.S. MacLaren (Eds) Buffalo. Edmonton, Alberta, The University of Alberta Press. P. 139 – 165. Gates C.C., R.O. Stephenson, H.W. Reynolds, C.G. van Zyll de jong, H. Schwantje, M. Hoefs, J. Nishi, N. Cool, J. Chilsholm, A. James, & B. Koonz. 2001.National recovery plan for the wood bison (Bison bison athabascae), National recovery plan No. 21. Recovery of nationally endangered wildlife. Ottawa, Ontario Hoare E.K., S.E. Parker, P.F. Flood & G.P. Adams. 1997. Ultrasonic imaging of reproductive events in muskoxen. Rangifer 17: 119 - 123. Kemp K, McCorkell RB, Woodbury M, Adams GP (2003) Ovarian follicle development in white-tailed deer (Odocoileus virginianus) in the anestrous season. Theriogenology 59: 395. Matsuda D.M., A.C. Bellem, C.J. Gartley, V. Madison, W.A. King, R.M. Liptrap & K.L. Goodrowel. 1996. Endocrine and behavioral events of estrous cyclicity and synchronization in wood bison (Bison bison athabascae). Theriogenology 45: 1429 – 1441. McCorkell R.B., L. MacDougall & G.P. Adams. 2001. Transrectal ultrasonography in female wapiti: a feasibility study. in: L.A. Renecker & T.A. Renecker (Eds) Biodiversity, Management, Ecotourism, Traditional Medicine and Health. Toronto, Canada, Renecker and Assoc. Inc. p. 282 – 290. McCorkell R.B., L. MacDougall & G.P. Adams. 2004. Ovarian follicle development in wapiti (Cervus elaphus) during the anovulatory season. Theriogenology 61(2-3): 473-483. McCorkell RB, Woodbury MR, Adams GP (2007) Evaluation of an ovarian synchronization scheme for fixed-time artificial insemination in wapiti. Theriogenology 67:1217-1223. McCorkell RB, Woodbury MR, Adams GP (2008) Serial ovarian ultrasonography in wildcaught wood bison (Bos bison athabascae). Reproduction in Domestic Animals 43 Supplement 3: 91. McCorkell RB, Paziuk W, Smart L, Woodbury M, Adams GP. 2010. Exogenous control of follicular wave emergence in wood bison (Bison bison athabascae). Reprod Fert Dev. (Jan. abstr). Mitchell, J.A. & C.C. Gates. 2002. Status of the wood bison (Bison bison athabascae) in Alberta. Wildlife status report No. 38. Edmontan, Alberta, Alberta Sustainable Resource Development, Fish and Wildlife Division, and Alberta Conservation Association. Othen L.S., A.C. Bellem, C.J. Gartley, K. Auckland, W.A. King, R.M. Liptraps & K.L. Goodrowel. 1999. Hormonal control of estrous cyclicity and attempted superovulation in wood bison (Bison bison athabascae). Theriogenolgy 52: 313-323. Palomino JM, McCorkell RB, Balog B, Ambati D, Woodbury M, Adams GP. 2010. Induction of follicular wave emergence in wood bison by follicular ablation or treatment with estradiol and progesterone. Reprod Fert Dev. (Jan. abstr). Pukazhenthi B.S. & D.E. Wildt. 2004. Which reproductive technologies are most reel“International Strategic Program for Conservation of Animal Genetic Resources for Food and Agriculture” sponsored by OECD Co-operative Research Programme 67 © GP Adams, 2010, All rights reserved vant to studying, managing and conserving wildlife? Reproduction, Fertility and Development 16: 33-46. Solti L., E.G. Crichton, N.M. Loskutoff, & S. Cseh. 2000. Economical and ecological importance of indigenous livestock and the application of assisted reproduction to their preservation. Theriogenology 53: 149 - 16 “International Strategic Program for Conservation of Animal Genetic Resources for Food and Agriculture” sponsored by OECD Co-operative Research Programme 68 © G Gábor, 2010, All rights reserved Presentation-10 Factors influencing pregnancy rate in dairy cattle György Gábor and Orsolya Balogh Research Institute for Animal Breeding and Nutrition, Herceghalom, Hungary Introduction Efficient reproduction in dairy cattle herds is of great economic importance. Fertility, one of the most complex measures of reproduction, is undisputedly influenced by genes and the environment. Although these two components act in concert, they synergistically mask the contribution of the other, thereby confounding selection strategies for fertility and, ultimately, affecting reproductive performance [1]. Increased milk production together with suboptimal farm management can dramatically reduce fertility. However, other factors, such as reproductive diseases or season of calving, are relatively more important than milk yield in influencing reproductive performance. To address decreased fertility in many countries, cow longevity, health, and fertility have been used as selection criteria in many breeding programs. In addition, the contribution of environmental factors and management errors (e.g. ambient temperature, housing, bedding, assistance and hygiene at calving etc.), should also be considered. Trends in genetic selection Extreme selection pressure for milk production in dairy cows has been highly successful. Unfortunately, there are clear negative genetic relationships (several times stronger than phenotypic ones) between milk yield and fertility, presence of mastitis, and other health traits. Surprisingly, large genetic variation is found in these fitness- and welfarerelated traits, despite their low heritability. Reasons for the decline in fertility are multifactorial and not exclusively associated with increased milk production. In many studies, higher producing cows are often the healthiest cows due to better feeding and reproductive management. However, unfavorable correlations were mostly ~0.2–0.4, indicating that selection for milk yield alone would reduce fertility and animal health, thus seriously affecting cow welfare. However, since these correlations are far from unity, there are individual cows (e.g. in Sweden) with high milk yield and concurrently good health and fertility, provided the cow has inherited good genes for both milk production and fertility and/or that high-quality management can overcome the negative genetic effect [1]. Most modern dairy AI-bulls have been primarily selected for milk yield, with dramatic increases in genetic gain, but concurrent decreased reproductive performance (presumably due to the insufficient correlation between these two traits). The industry has used genetic markers to track recessive genetic disorders (qualitative traits), but the impact of tracking quantitative traits is yet limited. Although the heritability of fertility is low (<5%), Höglund et al. [2] identified 26 QTL for fertility traits on 17 chromosomes (best evidence was found on chromosomes BTA1, BTA7, BTA10, BTA26). Although the currently used genomic selection seems to be an appropriate method for selection, in “International Strategic Program for Conservation of Animal Genetic Resources for Food and Agriculture” sponsored by OECD Co-operative Research Programme 69 © G Gábor, 2010, All rights reserved case of enexpected outcomes, the role of genetic (semen) banks may invaluable in the future. Environmental factors In order for dairy cows to reach their genetic potential, and appropriate production environment is essential; in other words, production and reproduction are highly influenced by the environment. 1. Nutrition Neuro-endocrine regulation of ovarian activity is highly affected by nutrition. In high producing dairy cows, negative energy balance (NEBAL) frequently occurs, causing metabolic and reproductive failures. In that regard, NEBAL affects the resumption of cyclicity, the time of first insemination, and conception rate. In general, NEBAL begins a few days before calving, and reaches its most negative level approximately 2 weeks post partum. Furthermore, the NEBAL nadir has been implicated in the timing of first ovulation, which occurs on average at ~30 days postpartum (range, 17–42 days). Variation in the severity and duration of NEBAL is primarily related to differences in dry matter intake and its rate of increase during early lactation [3]. Cows in NEBAL mobilize energy from body fat, with subsequent increases in plasma concentrations of nonesterified fatty acid (NEFA) and beta-hydroxybutyrate (BHB). High plasma NEFA concentrations can also cause this compound to accumulate, with deleterious effects on granulosa and theca cells and oocytes in vitro and probably also in vivo [4,5]. Uterine involution and environment are also affected by β-carotene and antioxidant content of the forage; in many cases, RFM and puerperial metritis occur when these compounds were deficient. In addition, low plasma concentrations of β-carotene and antioxidants can also affect bull fertility. 2. Calving assistance Calving disorders have a strong influence on the post partum period. Retained fetal membranes (RFM) and puerperial metritis are more frequent when calving is assisted, as shown (experimental video-recordings of 58 calvings, unpublished data, Table 1). No. cases of No. cases No. cases metritis % of RFM % Regular calving (no assistance) 33 9 27.3 2 6.1 Assisted calving 25 12 48.0 3 12.0 Table 1: Effect of kind of calving on puerperial disorders During the postpartum period, rapid and uneventful involution of the uterus, early resumption of normal ovarian activity, and accurately detected estrus are required [6]. Most cows develop a mild non-pathological endometritis during the early puerperal phase [7]. This physiological condition, together with the reduced immuncompetence (due to high milk production and NEBAL, as well as poor calving hygiene), can cause “International Strategic Program for Conservation of Animal Genetic Resources for Food and Agriculture” sponsored by OECD Co-operative Research Programme 70 © G Gábor, 2010, All rights reserved puerperial endometritis. Metritis causes endometrial damage, which can delay conception. In postpartum dairy cows, irregular luteal forms, including a corpus luteum with cavity, and cystic ovarian disease, frequently occur. Some of these phenomena occur due to metritis, but there is a possible effect of NEBAL (due to increased plasma concentrations of NEFA). Oocytes can accumulate fatty acids, which may change their lipid content and composition. The accumulation of lipid in oocytes and embryos can reduce their quality and cryotolerance. Perhaps irregular luteal forms are caused by the effects of NEFA on theca and granulosa cells; in that regard, formation of irregular luteal forms seems to be associated with higher plasma NEFA concentrations [7]. 3. Ambient temperature In heat-stressed dairy cows, the reduction of dry matter intake causes an energy deficit, thereby accentuating NEBAL. Furthermore, high ambient temperature are associated with deficits in β-carotene and antioxidant capacity. The effects of heat stress on may also be due to a direct impact of high ovarian temperatures on oocyte quality. The intrauterine environment is also compromised in heat-stressed cows, with reduced blood flow to the uterus and increased uterine temperature; this may inhibit embryonic development, increase early embryonic loss, and reduce the proportion of successful inseminations [8]. During the warm summer period, poor expression of estrus, the rapid decrease in pregnancy rate, and a pronounced increase of late embryonic losses (LEL) are common. Based on assessment of ~68 000 samples for serum PSPB concentration and re-checking the pregnant cows by transrectal palpation (Figure 1), rate of pregnant samples (RPS) decreased while LEL increased in association with daily average temperature. 60% 45% 30% 15% 0% -12 -9 -7 -5 -3 -1 1 3 5 7 9 11 13 15 17 19 21 23 25 27 Average ambient temperature Pregnant samples% LEL% Figure 1: Relationship between rate of pregnant samples (RPS) and late embryonic losses (LEL) with ambient temperature “International Strategic Program for Conservation of Animal Genetic Resources for Food and Agriculture” sponsored by OECD Co-operative Research Programme 71 29 © G Gábor, 2010, All rights reserved 4. Time of first AI The time of the first successful insemination is influenced by the severity and duration of NEBAL and involution. Gábor et al. [10] reported that the efficiency of the AI (Figure 1) was significantly lower within 60 days post-partum (P < 0.001); consequently, the voluntary waiting period is usually at least 60 days. Figure 2: Effect of interval from calving to insemination on pregnancy rate (Gábor et al., 2007) In conclusion, improved genetic selection and nutrition should increase fertility, even in high-producing dairy cows. Proper housing and calving management (appropriate hygiene) should also enhance pregnancy rate and decrease late embryonic losses. References [1] Rodriguez-Martinez H., Hultgren J., Båge R., Bergqvist A.S., Svensson C., Bergsten C., Lidfors L., Gunnarsson S., Algers B., Emanuelson U., Berglund B., Andersson G., Håård M., Lindhé B., Stålhammar H., Gustafsson H.: ] Reproductive performance in high-producing dairy cows: can we sustain it under current practice? In: IVIS Reviews in Veterinary Medicine, I.V.I.S. (Ed.). International Veterinary Information Service, Ithaca NY (www.ivis.org), Last updated: 11-Dec-2008; R0108.1208 [2] Höglund J.K., Guldbrandsten B., Su G., Thomsen B., Lund M.S.: Genome scan detects quantitative trait loci affecting female fertility traits in Danish and Swedish Holstein cattle. J. Dairy Sci. 2009;92:2136-2143. “International Strategic Program for Conservation of Animal Genetic Resources for Food and Agriculture” sponsored by OECD Co-operative Research Programme 72 © G Gábor, 2010, All rights reserved [3] Butler W.R.: Energy balance relationships with follicular development, ovulation and fertility in postpartum dairy cows. Livestock Prod. Sci. 2003;83:211-218. [4] Vanholder T., Leroy J.L.M.R., Van Soom A., Maes D., Coryn M., Fiers T., de Kruif A., Opsomer G.: Effect of non-esterified fatty acids on bovine theca cell steroidogenesis and proliferation in vitro. Anim Reprod Sci 2006;92:51-63. [5] Vanholder T., Leroy J.L.M.R., Van Soom A., Opsomer G., Maes D., Coryn M., de Kruif A.: Effect of non-esterified fatty acids on bovine granulosa cell steroidogenesis and proliferation in vitro. Anim Reprod Sci 2005;87:33-44. [6] Compendium of animal reproduction, 9th revised edition 2006. [7] Balogh O.G., Fébel H., Huszenicza G., Kulcsár M., Abonyi-Tóth Z., Endrődi T., Gábor G.: Seasonal fertility differences in synchronized dairy cows: ultrasonic, metabolic and endocrine changes. J. Reprod. Dev. 2010. (submitted) [8] Thatcher W.W., Bilby T.R., Bartolome J.A., Silvestre F., Staples C.R., Santos J.E.P.: Strategies for improving fertility in the modern dairy cow. Theriogenology 2006;65:30-44. [9] De Rensis F., Scaramuzzi RJ.: Heat stress and seasonal effects on reproduction in the dairy cow – a review. Theriogenology 2003;60:1139-1151. [10] Gábor G., Tóth F., Ózsvári L., Abonyi-Tóth Z., Sasser R.G.: Early detection of pregnancy and embryonic loss in dairy cattle by ELISA tests. Reprod. Domest. Anim. 2007;42:633-636. “International Strategic Program for Conservation of Animal Genetic Resources for Food and Agriculture” sponsored by OECD Co-operative Research Programme 73 © G Evans, 2010, All rights reserved Presentation 11: Application of sexed sperm in animal breeding and conservation programs Gareth Evans and Simon de Graaf Faculty of Veterinary Science The University of Sydney Sydney, NSW 2006, Australia Introduction Since the first report of birth of live offspring in mammals after insemination with sexsorted sperm (Johnson et al. 1989), there have been rapid developments in the technology of sperm sorting and its application in a range of species (Maxwell et al. 2004; O'Brien et al. 2009). Today, bull sexed semen is available worldwide, predominantly from dairy breeds where there is a strong commercial imperative and farmers are prepared to pay a substantial premium. There have been many reports of live offspring born from sexed semen in other species, but the technology has not yet become sufficiently advanced and/or economically viable to reach the open market, except perhaps in small scale fledgling markets in horses and deer. In this review the development of sperm sexing technology in a variety of species and its application to breeding and conservation programs will be discussed. Sperm sorting technology Despite numerous postulated methods of pre-determining the sex of offspring throughout history, the only validated methods are of embryo-biopsy and genetic analysis, which is wasteful of unwanted embryos, and the more efficient sperm sorting via flow-cytometry. The method of flow-cytometry involves incubation and staining of sperm DNA with a fluorescent dye, passing the sperm in single file through an orientating nozzle at high pressure, laser activation of the dye, and detection of the intensity of fluorescence according to the overall amount of DNA in the sperm nuclei (Sharpe and Evans 2009). The overall difference in DNA content between X and Y chromosomebearing sperm is in the range of 3-4% for common mammalian species, but varies up to 7.5% for the chinchilla (Garner 2006). The sperm, contained in a small droplet of carrier fluid, are each segregated into a droplet, which is charged according to the fluorescence, and deflected into collection tubes accordingly. Sperm which cannot be orientated, are defective, or which cannot be resolved are passed through to waste (5560%; Sharpe and Evans 2009). Using a modern high-speed flow cytometer (MoFlo XDP, Beckman Coulter) sperm of most species hitherto tested can be sorted into both X- and Y-enriched sperm populations of greater than 90% purity, depending on sorting rates chosen, and high percentage motility. Sperm of sheep and cattle are usually sorted at a rate of up to 8,000 sperm per second (25-30 million/hr) to obtain >90% purity, but sperm of other species can only be sorted at lower speeds for similar purity (O’Brien et al., 2009). However, “International Strategic Program for Conservation of Animal Genetic Resources for Food and Agriculture” sponsored by OECD Co-operative Research Programme 74 © G Evans, 2010, All rights reserved the whole process involves a number of stressors which reduce individual sperm viability (Seidel and Garner 2002), though the overall population of sorted sperm may appear more viable due to elimination of defective sperm (de Graaf et al. 2006). Further, sorted sperm need to be re-concentrated by centrifugation, and then frozen for maximum flexibility of use, including transport and long-term storage, and this further reduces motility and/or viability (de Graaf et al. 2006). Live offspring have been born from sex-sorted sperm of humans (Levinson et al. 1995), cattle (Cran et al. 1993b), sheep (Catt et al. 1996), horses (Buchanan et al. 2000), pigs (Johnson 1991), rabbits (Johnson et al. 1989), elk (Schenk and DeGrofft 2003), buffaloes (Presicce et al., 2005), cat (Pope et al. 2009), dog (Meyers et al. 2008), goat (RA Bathgate et al., unpublished data), bottle-nosed dolphin (O'Brien and Robeck 2006) and deer (Gao et al. 2010), which demonstrates that the technology is widely applicable across species, and in the case of cattle, sheep and horses at least, across different breeds within a species. In addition, sperm of a host of other species, including endangered wildlife species, have been sorted using flow cytometry (O’Brien et al, 2009), leading to the prospect of using sex-sorted sperm in wildlife and rare breed conservation via cryobanking. In both cases, X-sperm-enriched semen is the chosen type as female offspring are preferred for rapid proliferation of a limited gene pool. In the case of captive wildlife breeding programs, females are strongly preferred in alpha male society structures, where surplus males are expensive to maintain in individual or bachelor group housing (O'Brien et al. 2009). Notwithstanding the wide-ranging possibilities for sorting sperm of many species, some species provide more challenges than others. In general, species with lesser difference in DNA between X and Y sperm and/or with more irregular sperm morphology, e.g. human and western lowland gorilla, are more difficult to sort and take much longer sorting times to provide a sample suitable for AI (Garner 2006; O'Brien et al. 2009). Indeed, experience in sheep, cattle and horses has also demonstrated different `sortability’ between individual sires and even between ejaculates, related in part to an inability to uniformly stain the sperm DNA (Clulow et al. 2009; de Graaf et al. 2009). In addition, the well-known inherent differences in individual male fertility and `freezability’ of semen add another layer of uncertainty to the whole process of cryobanking for conservation (Frijters et al. 2009). Conservation of sex-sorted sperm It appears that conventionally-frozen sperm of sheep and cattle can be frozen indefinitely without loss of viability (Leibo et al. 1994; Salamon et al. 2004), and hence there is the prospect of conserving gene pools indefinitely via sexed-sorted sperm. Regardless, sorted sperm require freezing to facilitate maximum flexibility of use. Indeed, sorting machines are not transportable and are usually located in bull semen centres or in research laboratories, far from the intended site of use for AI. Fortunately, sorted sperm can generally be frozen successfully by conventional freezing methods, with minor modification (Seidel and Johnson 1999; Hollinshead et al. 2002). One major sup“International Strategic Program for Conservation of Animal Genetic Resources for Food and Agriculture” sponsored by OECD Co-operative Research Programme 75 © G Evans, 2010, All rights reserved plier of sorted bull semen has adopted gradient freezing technology (Arav et al. 2002) to good effect but, equally, in sheep, standard straw or pellet-freezing methods are effective (Hollinshead et al. 2002; de Graaf et al. 2007d). The sorting process removes seminal plasma, which often adds natural protection to sperm, but there are conflicting reports of the benefits of replacing seminal plasma to sorted sperm before freezing (de Graaf et al. 2007b; Leahy et al. 2009), and otherwise sorted sperm do not require particular modification of cryoprotective extenders. Notwithstanding, there has been little success with frozen sorted semen of stallions (Clulow et al. 2008) and boars (Bathgate et al. 2008), even after deep uterine AI, so more work is required to improve viability of sperm through the sorting and/or freezing processes. An interesting technique which may find application to wildlife conservation in particular is that of sorting of already-frozen sperm. This allows opportunistic collection of semen from valuable individual males of rare breeds or species remotely from a sorting facility, freezing the semen, transporting it to the sorter, and then removing the cryoprotectant before sorting the sperm and re-freezing. This technique, first described by O'Brien et al. (2003) has proved to be remarkably successful for ram semen, where double-frozen and sorted semen has been used successfully with conventional intrauterine AI techniques (de Graaf et al. 2007c). The same level of success has, unfortunately, not been obtained with in vivo insemination of double-frozen sorted bull semen (Underwood et al. 2010a; Underwood et al. 2010b), though it has been successfully used in conjunction with in vitro insemination of in vivo or in vitro matured oocytes (Underwood et al. 2010c). Insemination of sex-sorted sperm Ideally, sex-sorted frozen sperm could be used with standard AI techniques. This has been achieved in sheep with equal fertility to unsorted frozen semen (de Graaf et al. 2007d; Beilby et al. 2009), and in cattle with acceptably high, albeit slightly reduced, fertility (DeJarnette et al. 2009; Schenk et al. 2009). Reduced fertility in cattle may be associated with the relatively low numbers used (Frijters et al. 2009), and with reduced fertility in lactating dairy cows (DeJarnette et al. 2008), but in sheep it appears that the sorting process selects a highly fertile population of sperm which has high fertility even with low AI doses (de Graaf et al. 2007d; Beilby et al. 2009). In sheep, close synchronisation of oestrus and ovulation certainly facilitates successful AI. In order to increase efficiency of use, particularly in the early days of slow and inefficient sorters, a variety of other approaches to use of sorted sperm has been used in sheep, cattle, pigs and horses, including synchronisation of ovulation (sheep: Hollinshead et al. 2003), deep uterine or endoscopically-guided insemination (pigs: Grossfeld et al. 2005; horses: Lindsey et al. 2002), superovulation and embryo transfer (sheep: de Graaf et al. 2007a; cattle: Hayakawa et al. 2009), in vitro fertilisation of in vitro or in vivo matured oocytes (cattle: Cran et al. 1993a), including juvenile in vitro embryo production and transfer (sheep: Morton et al. 2004), and even intracytoplasmic sperm injection (sheep: Catt et al. 1996). Unfortunately, in vitro fertilisation (IVF) technology itself is not well-advanced in many species, particularly in many endangered wildlife species (O’Brien et al. 2009), “International Strategic Program for Conservation of Animal Genetic Resources for Food and Agriculture” sponsored by OECD Co-operative Research Programme 76 © G Evans, 2010, All rights reserved but even in some common species such as the horse IVF is not a currently viable option for use of sex-sorted sperm. Hence, sex-sorted sperm is currently an effective option for aiding conservation of domestic species such as cattle, sheep, pigs, and possibly goats and deer, but further work is required on many other species before one could use it with assurance. Sexed sperm in breeding and conservation programs In cattle, sexed-semen consumers purchase X-semen almost exclusively as it is predominantly used to breed replacement dairy heifers and heifers for sale to developing economies, though Y-semen is used to breed steers for meat or bulls as sires. The limited demand in other species is also for females, often because they offer the opportunity to more rapidly change genotype, eg in newly imported or fashionable breeds, with limited reference to improving genetic background of flocks or herds. Equally, `female’ sperm would be preferred in conservation programs for endangered breeds and wildlife. Hence, the demand is ideally for 100% pure X-sperm, but this is not feasible in practice and a limited number of Y-sperm are always found in the X-fraction. There is a compromise between sorting rates and sorting purity, and sorting gates are usually set for 90% purity of either X or Y sperm. Hence, `normal’ sorting of X-sperm would still provide a minority bank of Y-sperm within. In a breeding program aimed at genetic improvement of a breed, when a reasonable population of sires is required to provide appropriate selection intensity, the optimum purity for X-sperm could be less than 90%. This would allow for greater sorting speeds and fewer sperm rejected as being unresolvable. Conclusion Sex-sorting technology using high-speed flow cytometry is well-advanced in common agricultural species, and could certainly be utilised for gene banking or conservation of rare breeds in select (not every individual) males of cattle and sheep. In some other species, sex-sorted frozen semen could potentially be used with modified AI techniques or IVF of matured oocytes. However, in most species, there is much research needed on preservation of viability of sperm through the sorting and cryopreservation process, as well as on control of oestrus and ovulation in females, AI and IVM-IVF techniques. References Arav A., Zeron Y., Shturman H. and Gacitua H. (2002) Successful pregnancies in cows following double freezing of a large volume of semen. Reproduction, Nutrition, Development 42, 583-6. Bathgate R., Grossfeld R., Susetio D., Ruckholdt M., Heasman K., Rath D., Evans G. and Maxwell W. M. C. (2008) Early pregnancy loss in sows after low dose, deep uterine “International Strategic Program for Conservation of Animal Genetic Resources for Food and Agriculture” sponsored by OECD Co-operative Research Programme 77 © G Evans, 2010, All rights reserved artificial insemination with sex-sorted, frozen-thawed sperm. Animal Reproduction Science 104, 440-444. Beilby K. H., Grupen C. G., Thomson P. C., Maxwell W. M. C. and Evans G. (2009) The effect of insemination time and sperm dose on pregnancy rate using sex-sorted ram sperm. Theriogenology 71, 829-835. Buchanan B. R., Seidel G. E., Jr., McCue P. M., Schenk J. L., Herickhoff L. A. and Squires E. L. (2000) Insemination of mares with low numbers of either unsexed or sexed spermatozoa. Theriogenology. 53, 1333-44. Catt S. L., Catt J. W., Gomez M. C., Maxwell W. M. C. and Evans G. (1996) Birth of a male lamb derived from an in vitro matured oocyte fertilised by intracytoplasmic injection of a single presumptive male sperm. Veterinary Record 139, 494-5. Clulow J. R., Buss H., Sieme H., Rodger J. A., Cawdell-Smith A. J., Evans G., Rath D., Morris L. H. A. and Maxwell W. M. C. (2008) Field fertility of sex-sorted and non-sorted frozen-thawed stallion spermatozoa. Animal Reproduction Science 108, 287-297. Clulow J. R., Evans G., Morris L. H. A. and Maxwell W. M. C. (2009) Factors influencing the "sortability" of stallion spermatozoa into X- and Y-chromosome bearing populations. Animal Reproduction Science 113, 220-228. Cran D. G., Cochrane D., Polge C., Johnson L. A. and Miller N. G. A. (1993a) Production of bovine calves following separation of X- and Y-chromosome bearing sperm and in vitro fertilization. Bovine Practitioner 0, 143-144. Cran D. G., Johnson L. A., Miller N. G. A., Cochrane D. and Polge C. (1993b) Production of Bovine Calves Following Separation of X-Chromosome and Y-Chromosome Bearing Sperm and In Vitro Fertilization. Veterinary Record 132, 40-41. de Graaf S. P., Beilby K. H., O'Brien J. K., Osborn D., Downing J. A., Maxwell W. M. C. and Evans G. (2007a) Embryo production from superovulated sheep inseminated with sex-sorted ram spermatozoa. Theriogenology 67, 550-555. de Graaf S. P., Beilby K. H., Underwood S. L., Evans G. and Maxwell W. M. C. (2009) Sperm sexing in sheep and cattle: the exception and the rule. Theriogenology 71, 8997. de Graaf S. P., Evans G., Gillan L., Guerra M. M. P., Maxwell W. M. C. and O'Brien J. K. (2007b) The influence of antioxidant, cholesterol and seminal plasma on the in vitro quality of sorted and non-sorted ram spermatozoa. Theriogenology 67, 217-227. “International Strategic Program for Conservation of Animal Genetic Resources for Food and Agriculture” sponsored by OECD Co-operative Research Programme 78 © G Evans, 2010, All rights reserved de Graaf S. P., Evans G., Maxwell W. M. C., Cran D. G. and O'Brien J. K. (2007c) Birth of offspring of pre-determined sex after artificial insemination of frozen-thawed, sexsorted and re-frozen-thawed ram spermatozoa. Theriogenology 67, 391-398. de Graaf S. P., Evans G., Maxwell W. M. C., Downing J. A. and O'Brien J. K. (2007d) Successful low dose insemination of flow cytometrically sorted ram spermatozoa in sheep. Reproduction in Domestic Animals 42, 648-653. de Graaf S. P., Evans G., Maxwell W. M. C. and O'Brien J. K. (2006) In vitro function of fresh and frozen-thawed ram spermatozoa after sex-sorting and re-freezing. Reproduction, Fertility, & Development 18, 867-874. DeJarnette J. M., Nebel R. L. and Marshall C. E. (2009) Evaluating the success of sexsorted semen in US dairy herds from on farm records. Theriogenology 71, 49-58. DeJarnette J. M., Nebel R. L., Marshall C. E., Moreno J. F., McCleary C. R. and Lenz R. W. (2008) Effect of Sex-Sorted Sperm Dosage on Conception Rates in Holstein Heifers and Lactating Cows. Journal of Dairy Science 91, 1778-1785. Frijters A. C. J., Mullaart E., Roelofs R. M. G., van Hoorne R. P., Moreno J. F., Moreno O. and Merton J. S. (2009) What affects fertility of sexed bull semen more, low sperm dosage or the sorting process? Theriogenology 71, 64-67. Gao Q. H., Wei H. J., Han C. M., Du H. Z., Zhang Z. G., Zhao W. G., Zhang Y. and Li S. (2010) Successful low dose insemination of flow cytometrically sorted Sika (Cervus nippon) sperm in Wapiti (Cervus elaphus). Animal Reproduction Science 118, 89-93. Garner D. L. (2006) Flow cytometric sexing of mammalian sperm. Theriogenology 65, 943-957. Grossfeld R., Klinc P., Sieg B. and Rath D. (2005) Production of piglets with sexed semen employing a non-surgical insemination technique. Theriogenology 63, 2269-2277. Hayakawa H., Hirai T., Takimoto A., Ideta A. and Aoyagi Y. (2009) Superovulation and embryo transfer in Holstein cattle using sexed sperm. Theriogenology 71, 68-73. Hollinshead F. K., Gillan L., O'Brien J. K., Evans G. and Maxwell W. M. C. (2003) In vitro and in vivo assessment of functional capacity of flow cytometrically sorted ram spermatozoa after freezing and thawing. Reproduction, Fertility and Development 15, 351359. Hollinshead F. K., O'Brien J. K., Maxwell W. M. C. and Evans G. (2002) Production of lambs of predetermined sex after the insemination of ewes with low numbers of frozenthawed sorted X- or Y-chromosome-bearing spermatozoa. Reproduction, Fertility, & Development 14, 503-8. “International Strategic Program for Conservation of Animal Genetic Resources for Food and Agriculture” sponsored by OECD Co-operative Research Programme 79 © G Evans, 2010, All rights reserved Johnson L. A. (1991) Sex preselection in swine: altered sex ratios in offspring following surgical insemination of flow sorted X- and Y-bearing sperm. Reproduction in Domestic Animals 26, 309-314. Johnson L. A., Flook J. P. and Hawk H. W. (1989) Sex preselection in rabbits: live births from X and Y sperm separated by DNA and cell sorting. Biology of Reproduction. 41, 199-203. Leahy T., Marti J. I., Evans G. and Maxwell W. M. C. (2009) Seminal plasma proteins protect flow-sorted ram spermatozoa from freeze-thaw damage. Reproduction Fertility and Development 21, 571-578. Leibo, S.P., Semple, M.E. and Kroetsch, T.G. (1994) In vitro fertilization of oocytes by 37-year-old cryopreserved bovine spermatozoa. Theriogenology 42, 1257-1262 Levinson G., Keyvanfar K., Wu J. C., Fugger E. F., Fields R. A., Harton G. L., Palmer F. T., Sisson M. E., Starr K. M. and Dennison-Lagos L. (1995) DNA-based X-enriched sperm separation as an adjunct to preimplantation genetic testing for the prevention of X-linked disease. Human Reproduction. 10, 979-982. Lindsey A. C., Schenk J. L., Graham J. K., Bruemmer J. E. and Squires E. L. (2002) Hysteroscopic insemination of low numbers of flow sorted fresh and frozen/thawed stallion spermatozoa. Equine Veterinary Journal 34, 121-7. Maxwell W. M. C., Evans G., Hollinshead F. K., Bathgate R., de Graaf S. P., Eriksson B. M., Gillan L., Morton K. M. and O'Brien J. K. (2004) Integration of sperm sexing technology into the ART toolbox. Animal Reproduction Science 82-83, 79-95. Meyers M. A., Burns G., Arn D. and Schenk J. L. (2008) Birth of canine offspring following insemination of a bitch with flow-sorted spermatozoa. Reproduction Fertility and Development 20, 213-213. Morton K. M., Catt S. L., Hollinshead F. K., Maxwell W. M. C. and Evans G. (2004) Production of lambs after the transfer of fresh and cryopreserved in vitro produced embryos from prepubertal lamb oocytes and unsorted and sex-sorted frozen-thawed spermatozoa. Reproduction in Domestic Animals 39, 451-461. O'Brien J. K., Hollinshead F. K., Evans K. M., Evans G. and Maxwell W. M. C. (2003) Flow cytometric sorting of frozen-thawed spermatozoa in sheep and non-human primates. Reproduction, Fertility, & Development 15, 367-75. O'Brien J. K. and Robeck T. R. (2006) Development of sperm sexing and associated assisted reproductive technology for sex preselection of captive bottlenose dolphins (Tursiops truncatus). Reproduction Fertility and Development 18, 319-329. “International Strategic Program for Conservation of Animal Genetic Resources for Food and Agriculture” sponsored by OECD Co-operative Research Programme 80 © G Evans, 2010, All rights reserved O'Brien J. K., Steinman K. J. and Robeck T. R. (2009) Application of sperm sorting and associated reproductive technology for wildlife management and conservation. Theriogenology 71, 98-107. Pope C. E., Crichton E. G., Gomez M. C., Dumas C. and Dresser B. L. (2009) Birth of domestic cat kittens of predetermined sex after transfer of embryos produced by in vitro fertilization of oocytes with flow-sorted sperm. Theriogenology 71, 864-871. Salamon S., Gillan L., Evans G. and Maxwell W. M. C. (2004) Fertility of ram semen after 35 years of frozen-storage. In '15th International Congress on Animal Reproduction, Porto Seguro, Brazil' p. 476 Schenk J. L., Cran D. G., Everett R. W. and Seidel Jr G. E. (2009) Pregnancy rates in heifers and cows with cryopreserved sexed sperm: effects of sperm numbers per inseminate, sorting pressure and sperm storage before sorting. Theriogenology 71, 717728. Schenk J. L. and DeGrofft D. L. (2003) Insemination of cow elk with sexed frozen semen. Theriogenology 59, 514 (Abstr.). Seidel G. E., Jr. and Garner D. L. (2002) Current status of sexing mammalian spermatozoa. Reproduction 124, 733-43. Seidel G. E., Jr. and Johnson L. A. (1999) Sexing mammalian sperm--overview. Theriogenology 52, 1267-72. Sharpe J. C. and Evans K. M. (2009) Advances in flow cytometry for sperm sexing. Theriogenology 71, 4-10. Underwood S. L., Bathgate R., Ebsworth M., Maxwell W. M. C. and Evans G. (2010a) Pregnancy loss in heifers after artificial insemination with frozen-thawed, sex-sorted, re-frozen-thawed dairy bull sperm. Animal Reproduction Science 118, 7-12. Underwood S. L., Bathgate R., Maxwell W. M. C. and Evans G. (2010b) Birth of offspring after artificial insemination of heifers with frozen-thawed, sex-sorted, re-frozenthawed bull sperm. Animal Reproduction Science 118, 171-175. Underwood S. L., Bathgate R., Pereira D. C., Castro A., Thomson P. C., Maxwell W. M. C. and Evans G. (2010c) Embryo production after in vitro fertilization with frozenthawed, sex-sorted, re-frozen-thawed bull sperm. Theriogenology 73, 97-102. “International Strategic Program for Conservation of Animal Genetic Resources for Food and Agriculture” sponsored by OECD Co-operative Research Programme 81 © PJ Hansen, 2010, All rights reserved Presentation-12 Prospects for Genetic Modification to Improve Dairy Cow Reproduction During Heat Stress Peter J. Hansen Dept. of Animal Sciences and D.H. Barron Reproductive and Perinatal Biology Research Program, University of Florida, Gainesville, FL 32611-0910 USA The growing impact of heat stress on dairy cow reproduction There are three major effects of heat stress on reproductive function of lactating dairy cows – reduced expression of estrus, decreased fertilization rate, and increased embryonic mortality (1). These effects are a consequence of the physiological changes made by the female to regulate its body temperature as well as the damage to gametes and embryos caused by maternal hyperthermia. The advent of ovulation synchronization programs such as Ovsynch means that effects of heat stress on estrus detection can be overcome but the problem of infertility has proven difficult to solve. Heat stress is a widespread and growing problem. It is widespread because hyperthermia can occur in intensively-managed lactating cows at air temperatures as low as 25-28oC (2, 3). In fact, reduced fertility during summer is seen in Wisconsin and Alberta (3,4). Increased milk yield makes regulation of body temperature during heat stress more difficult (4,5) and exacerbates effects of heat stress on fertility (6). The impact of heat stress is growing, even without considering global climate change, because the continual improvement in average milk yield per cow can result in increasingly larger effects of heat stress on reproduction. Such a historical trend has been documented in northeastern Spain (see Figure 1; 7). The impact of heat stress will grow even more severe if predictions of global climate change prove accurate because heat stress will be greater in magnitude and of longer duration in much of the world than is presently the case. Figure 1. Changes in pregnancy rate per insemination for lactating Holsteins in northeastern Spain from 1991-2000 in the cool and warm seasons of the year. From reference 7. Given its growing impact on reproduction as well as on milk yield and other traits, multiple approaches to reducing the impact of heat stress is should be pursued. One potential strategy for doing so is to change the cow genetically to improve thermal resistance. Such an approach, which has been the cornerstone for developing beef cattle in hot climates, has not been the focus of concerted effort for dairy cattle. Nonetheless, there are genes that confer thermotolerance in cattle, both with respect to regulation of body tempera- “International Strategic Program for Conservation of Animal Genetic Resources for Food and Agriculture” sponsored by OECD Co-operative Research Programme 82 © PJ Hansen, 2010, All rights reserved ture and cellular responses to elevated temperature. Increasing the frequency of these genes in dairy cattle populations in hot climates could represent a sustainable approach to improve reproduction and milk yield. This paper will focus on various approaches for doing so. Quantitative genetic selection Progress in selecting animals for resistance to heat stress should be possible. There are breed differences in thermoregulation (8,9), heritability estimate for rectal temperature (in beef cattle) range from 0.25-0.65 (10) and there is a genetic component to seasonal variation in days open (11). One problem with any selection scheme to improve thermotolerance is that sires are typically used globally and location x genotype interactions are typically not estimated and indeed often deemed unimportant (12). A potential limitation with selecting for thermotolerance is a possible genetic correlation between resistance to heat stress and lower feed intake or milk yield. An alternative approach is to select for increased production among animals raised in hot environments. In Australia, beef cattle selected for growth rate also became more thermotolerant (13). Selection using molecular markers The advent of molecular genetics offers the possibility to select genes conferring thermotolerance without also selecting genes that reduce production. One approach, called the candidate gene approach, is to identify single nucleotide polymorphisms (SNPs) in the promoter or coding region of specific genes that confer some beneficial change in phenotype. Several SNPs in HSPA1A (previously called HSP70) have been related to fertility in Brahman crossbred cattle (14) and a SNP in the calpastatin gene has been related to daughter pregnancy rate in dairy cattle (15). A SNP in ATP1A1 (previously called Na+/K+ ATPase) has been identified in Holsteins that is related to body temperature regulation (16). Given the array of molecules involved in regulation of body temperature and cellular responses to hyperthermia, it is likely that many other genes contain allelic variants that affect thermotolerance. A second genomic approach is to conduct genome-wide association studies with SNPs located at random positions throughout the chromosomes to identify loci near where genes controlling a specific trait are located. For quantitative traits, these loci are referred to as quantitative trait loci (QTL). It is not necessary to identify the specific gene or genes controlling a trait to achieve selection. The advent of the BovineSNP50 Bead Chip in cattle and the planned production of more dense chips is making marker assisted selection a practical tool in dairy cattle selection. To date, few QTL for thermotolerance in dairy cattle have been identified. In a novel approach, Hayes et al. (17) estimated genetic merit of bulls for thermotolerance by determining the change in milk yield of their daughters with changes in temperature-humidity index. A SNP on BTA29 located close to FGF4 was identified in Holsteins and Jerseys that was related to thermotolerance. “International Strategic Program for Conservation of Animal Genetic Resources for Food and Agriculture” sponsored by OECD Co-operative Research Programme 83 © PJ Hansen, 2010, All rights reserved Introgression of genes from thermotolerant breeds to Northern European dairy breeds The origin of most dairy breeds in Northern Europe has probably resulted in the number of genes conferring thermotolerance being smaller than for cattle that were developed in hot climates. Genes from thermotolerant beef or dairy breeds can, however, be introduced into breeds like the Holstein and Jersey. The simplest way to do so is through crossbreeding. When nutrient and other resources are limited, Bos indicus x Holstein F1 crosses can outperform cows that have a higher percentage of Holstein genes (18). When resources are more abundant, production increases with increasing percentage of Hosltein genetics (18). Another strategy is to introduce specific genes controlling thermotolerance via introgression using crossbreeding followed by backcrossing while selecting for the desired genotype. Olson et al. (19) and colleagues identified a gene in the Senepol and Carora breeds called the slick hair gene that controls hair length. Animals with the dominant allele have a very short, sleek, and sometimes glossy coat. Milk yield for Carora × Holstein cows in Venezuela was higher for animals with slick hair than for their contemporaries with wild-type hair (19). The slick hair gene has been introduced into Holsteins by introgression. Holsteins with the slick hair phenotype have superior ability to regulate body temperature during heat stress (20). Though not identified, there are also genes that confer thermoprotection at the cellular level. Exposure to elevated temperature reduced development of bovine preimplantation embryos but the magnitude of the reduction was less for Brahman, Nelore, and Romosinuano embryos than for Holstein or Angus embryos (21-23). In addition, Holstein cows bred to Gyr bulls during heat stress had slightly higher fertility than Holsteins bred to Holstein bulls (24). The advantage for the crossbred embryos could reflect expression in the embryo of genes controlling cellular thermoprotection or be a result of heterosis. Identification of genes controlling cellular resistance to elevated temperature may result in their transfer to Holsteins to produce a cow more resistant to disruption of cellular function by heat shock. REFERENCES 1. Hansen PJ. Exploitation of genetic and physiological determinants of embryonic resistance to elevated temperature to improve embryonic survival in dairy cattle during heat stress. Theriogenology 2007; 68S, S242-S249. 2. Berman A, Folman Y, Kaim M, Mamen M, Herz Z, Wolfenson D, Arieli A, Graber Y. Upper critical temperatures and forced ventilation effects for high-yielding dairy cows in a subtropical climate. J Dairy Sci 1985;68:1488-95. 3. Sartori R, Sartor-Bergfelt R, Mertens SA, Guenther JN, Parrish JJ, Wiltbank MC. Fertilization and early embryonic development in heifers and lactating “International Strategic Program for Conservation of Animal Genetic Resources for Food and Agriculture” sponsored by OECD Co-operative Research Programme 84 © PJ Hansen, 2010, All rights reserved cows in summer and lactating and dry cows in winter. 2002;85:2803-12. J Dairy Sci 4. Ambrose DJ, Govindarajan T, Goonewardene LA. Conception rate and pregnancy loss rate in lactating Holstein cows of a single herd following timed insemination or insemination at detected estrus. J Dairy Sci. 2006;89 (Suppl 1):213-4 (abstr). 5. Umphrey JE, Moss BR, Wilcox CJ, Van Horn HH. Interrelationships in lactating Holsteins of rectal and skin temperatures, milk yield and composition, dry matter intake, body weight, and feed efficiency in summer in Alabama. J Dairy Sci 2001;84:2680-5. 6. Al-Katanani YM, Webb DW, Hansen PJ. Factors affecting seasonal variation in 90 day non-return rate to first service in lactating Holstein cows in a hot climate. J Dairy Sci 1999;82:2611-5. 7. López-Gatius F. Is fertility declining in dairy cattle? A retrospective study in northeastern Spain. Theriogenology 2003;60:89-99. 8. Correa-Calderón A, Armstrong D, Ray D, DeNise S, Enns M, Howison, C (2004) Thermoregulatory responses of Holstein and Brown Swiss heat stressed dairy cows to two different cooling systems. Int J Biometeorol 48:142–8. 9. Dikmen S, Martins L, Pontes E, Hansen PJ. Genotype effects on body temperature in dairy cows under grazing conditions in a hot climate including evidence for heterosis. Int J Biometeorol 2009; 53: 327-31. 10. Finch VA. Body temperature in beef cattle: its control and relevance to production in the tropics. J Anim Sci 1986; 62: 531–42. 11. Ravagnolo O, Misztal I (2002) Effect of heat stress on nonreturn rate in Holstein cows: genetic analyses. J Dairy Sci 85:3092-100. 12. Lillehammer M, Hayes BJ, Meuwissen TH, Goddard ME. Gene by environment interactions for production traits in Australian dairy cattle. J Dairy Sci 2009; 92:4008-17. 13. Frisch JE. Changes occurring in cattle as a consequence of selection for growth rate in a stressful environment. J Agric Sci 1981; 96: 23-38. 14. Rosenkrans C Jr, Banks A, Reiter S, Looper M. Calving traits of crossbred Brahman cows are associated with heat shock protein 70 genetic polymorphisms. Anim Reprod Sci 2010;119:178-82. “International Strategic Program for Conservation of Animal Genetic Resources for Food and Agriculture” sponsored by OECD Co-operative Research Programme 85 © PJ Hansen, 2010, All rights reserved 15. Garcia MD, Michal JJ, Gaskins CT, Reeves JJ, Ott TL, Liu Y, Jiang Z. Significant association of the calpastatin gene with fertility and longevity in dairy cattle. Anim Genet 2006;37:304-5. 16. Liu Y, Li D, Li H, Zhou X, Wang G. A novel SNP of the ATP1A1 gene is associated with heat tolerance traits in dairy cows. Mol Biol Rep 2010; in press. 17. Hayes BJ, Bowman PJ, Chamberlain AJ, Savin K, van Tassell CP, Sonstegard TS, Goddard ME. A validated genome wide association study to breed cattle adapted to an environment altered by climate change. PLoS One. 2009;4:e6676. 18. Madalena FE, Lemos AM, Teodoro RL, Barbosa RT, Monteiro JBN. Dairy production and reproduction in Holstein-Friesian and Guzera crosses. J Dairy Sci 1990; 73: 1872–86. 19. Olson TA, Lucena C, Chase CC Jr, Hammond AC Evidence of a major gene influencing hair length and heat tolerance in Bos taurus cattle. J Anim Sci 2003; 81:80-90. 20. Dikmen S, Alava E, Pontes E, Fear JM, Dikmen YK, Olson TA, Hansen PJ. Differences in thermoregulatory ability between slick-haired and wild-type lactating Holstein cows in response to acute heat stress. J Dairy Sci 2008; 91:3395-402. 21. Paula-Lopes FF, Chase CC Jr, Al-Katanani YM, Krininger CE 3rd, Rivera RM, Tekin S, Majewski AC, Ocon OM, Olson TA, Hansen PJ. Genetic divergence in cellular resistance to heat shock in cattle: differences between breeds developed in temperate versus hot climates in responses of preimplantation embryos, reproductive tract tissues and lymphocytes to increased culture temperatures. Reproduction 2003;125:285-94. 22. Hernández-Cerón J, Chase CC Jr, Hansen PJ. Differences in heat tolerance between preimplantation embryos from Brahman, Romosinuano, and Angus breeds. J Dairy Sci 2004; 87:53-8. 23. Eberhardt BG, Satrapa RA, Capinzaiki CR, Trinca LA, Barros CM. Influence of the breed of bull (Bos taurus indicus vs. Bos taurus taurus) and the breed of cow (Bos taurus indicus, Bos taurus taurus and crossbred) on the resistance of bovine embryos to heat. Anim Reprod Sci 2009;114:54-61. 24. Pegorer MF, Vasconcelos JL, Trinca LA, Hansen PJ, Barros CM. Influence of sire and sire breed (Gyr versus Holstein) on establishment of pregnancy and embryonic loss in lactating Holstein cows during summer heat stress. Theriogenology 2007; 67:692-7. “International Strategic Program for Conservation of Animal Genetic Resources for Food and Agriculture” sponsored by OECD Co-operative Research Programme 86 © WW Thatcher, 2010, All rights reserved Presentation-13 Has The Loss of Genetic Diversity Adversely Affected The Holstein? William W. Thatcher Department of Animal Sciences, University of Florida, Gainesville, FL 32611-0910 Abstract The purpose of this overview is to review some of the major advances in reproductive technologies and genomics, and how they may be applied in forecasting the advances to meet the challenge of enhancing reproductive efficiency in the high producing dairy cow. There is a perception of a loss in genetic diversity that has compromised reproductive efficiency. The current population of high producing dairy cows is considered to be sub-fertile, as characterized by low pregnancy rates and high rates of embryonic mortality. Coordinated systems of reproductive management have been developed based upon a thorough understanding of the endocrine, cellular and molecular factors controlling ovarian and uterine function. These systems have partially restored herd reproductive performance and will contribute to an improved management environment to permit further advances for genetic selection of production traits, as well as less heritable health and reproductive traits. Advances in other reproductive technologies offer possibilities for wider use of superior germplasm. Technologies genomic selection and preimplantation genetic diagnosis offer the potential to enhance the impact of superior animals on production of food for human consumption. Genomic selection for production, health and reproductive traits will be the wave of the future as genomic and bioinformatic tools continue to be expanded and refined. Indeed increased production and efficiency of production is contributing to reductions in use of resources and mitigating adverse environmental impacts. Additional research is needed to counter the higher rates of embryonic and fetal mortality associated with some of these technologies and high production responses. Utilization of genomics, proteomics, metabolomics and bioinformatics in the study of reproduction will undoubtedly provide investigators with a greater understanding of the limitations to efficient reproductive processes in the sub-fertile lactating dairy cow in a changing world environment. Introduction Modern dairy practices require considerably fewer resources than dairying in 1944 with 21% of animals, 23% of feedstuffs, 35% of the water, and only 10% of the land required to produce the same 1 billion kg of milk in the USA. Waste outputs in modern dairy systems (i.e., manure, methane and nitrous oxide) have been reduced per billion kg of milk compared with equivalent milk from historical dairying in the 1940s (Capper et al., 2009). To fulfill the increasing requirements of the world population for dairy products, it is essential to adopt management practices and technologies that improve production efficiency, while reducing use of resources and lighten the environmental impact. The current high producing dairy cow has evolved through continued genetic progress for milk production coupled with nutritional management to obtain production potential. “International Strategic Program for Conservation of Animal Genetic Resources for Food and Agriculture” sponsored by OECD Co-operative Research Programme 87 © WW Thatcher, 2010, All rights reserved Recombinant bovine somatotropin (rbST) has been developed for the improvement in both milk production and efficiency of production. Somatotropin is a key homeorhetic control of nutrient partitioning. Administration of rbST to dairy cows increases milk production, improves the efficiency of milk synthesis (Bauman, 1999), and its timely use is associated with an increase in reproductive efficiency to first service after parturition (Moreira etal., 2001). When introducing new biotechnology to the dairy industry, it is essential to balance their biological benefits and efficiency to potential environmental impacts. Furthermore it is of paramount importance to properly and accurately extend to consumers the pros and cons of such technology, as related to health and well being to both cattle and consumers. For example, producers that implement rbST supplementation can glean an improvement in individual cow production, with reductions in nutrient input and waste output per unit of milk produced. From an industry and consumer perspective, supplementing one million cows with rbST reduced feedstuff and water use, cropland area, N and P excretion, greenhouse gas emissions, and fossil fuel use compared with an equivalent milk production from unsupplemented cows (Capper et al., 2008). Overall, rbST appears to represent a valuable management tool for use in dairy production to improve efficiency of production and to have less negative effects on the environment than conventional dairying. Acceptance and utilization of such breakthrough technologies will be essential over the next 20 years to meet the world’s food production needs at a time when consumer demands have also evolved. Continued genetic progress for milk production coupled with nutritional management of high producing dairy cows, without attention to reproductive performance, has contributed to an inverse relationship between milk production and reproduction (Lucy, 2001). A recent review documented the perceived multiplicity of factors contributing to low reproductive performance of dairy cows (Rodriguez-Martinez et al., 2008). Various factors identified with low fertility were: insufficient weight within genetic selection programs for longevity, health and fertility; interrelated factors such as negative energy balance, level of milk production, dystocia, retained placenta, twinning, stillbirths, and endometritis that reduce the risk for pregnancy; as well as inadequate attention to body confirmation, nutrition and reproductive management, infectious diseases, animal comfort and housing. Various short term and long term strategies were described to improve fertility that may or may not sustain high levels of milk production. The Situation Intensive genetic selection for milk production without attention to reproductive performance has contributed to the low herd pregnancy rates in current production systems. Inclusion of Productive Life, Daughter Pregnancy Rate, utilization of timed artificial insemination (TAI) programs and more recently the availability of Sire Conception Rate, as a measurement of phenotypic service-sire fertility, appear to have reduced the rate of decline in fertility in the USA (Norman et al., 2007). Pregnancy rate over a 21 day period for the national herd of dairy cows in the United States is approximately 16.2%. Reproductive management of the lactating dairy cow has been a challenge because of poor expression of estrus and low fertility to insemination at a detected estrus. The duration of estrus is reduced as milk production increases, and the frequency of double “International Strategic Program for Conservation of Animal Genetic Resources for Food and Agriculture” sponsored by OECD Co-operative Research Programme 88 © WW Thatcher, 2010, All rights reserved ovulations and subsequent occurrence of twins also is increased in cows with high levels of milk production at the time of the breeding period (Lopez et al., 2005) The high producing dairy cow of today expresses estrus for approximately 7 hours during which time an average of 6.5 standing events takes place with an accumulative period of standing of 20 seconds (i.e., 3 seconds per standing event; Lopez et al., 2004). Thus the ability to successfully develop a timed insemination program that controls the times of insemination and ovulation that result in good fertility is important to the dairy industry. Presently in 2010, systems have been developed to optimize the beginning of a TAI program, period of follicle dominance has been optimized to improve fertility, the need to sustain progesterone exposure throughout the period of follicular synchronization is realized, complete regression of the CL is essential in lactating dairy cows, and timing of AI relative to induction of ovulation with GnRH needs to be optimized and is somewhat dependent upon the TAI system utilized. These advancements allow on farm pregnancy rates of 40 to 50% for first and second service. However, they require maximal compliance in protocol implementation, integration with on farm computer monitoring for lists of cows to be handled, treated, and efficiency of the system to be monitored. It is essential that dairy producers, farm staff, nutritionists, and veterinarians understand the physiological underlying reasons why certain components of the reproductive management program are able to improve reproductive performance or conversely why a misunderstanding of the program can lead to catastrophic pregnancy results. Further future developments will entail early diagnosis of pregnancy, online monitoring of cycling and health status in the milking parlor with the use of nanotechnology. Such technology combined with optimized housing to achieve animal comfort, health and well-being will further allow high producing lactating dairy cows to successfully reproduce and yet produce high levels of production. Genomics Evolutionary Genomics: Since completion of the human genome sequencing project in 2001, the genome for the bovine has been sequenced in 2004 and further refined in 2009 to allow for evolutionary comparisons with other species and more detailed identification of expressed genes and their proteins. Sequencing of the bovine genome was a world-wide endeavor (for more information on the project and selected references see www.hgsc.bcm.tmc.edu/projects/bovine). It has been estimated that the cattle genome, comprised of 30 pairs of chromosomes, contains approximately 3 billion nucleotides with roughly 1% coding for functional genes. The high degree of conservation of genetic sequences across different species is providing valuable comparisons of genomic sequences to help in the discovery of genes and to map their location to bovine chromosomes. Latest estimates have identified at least 22,000 protein coding genes and 496 miRNA genes that are capable of differentially regulating gene expression. What applications and inferences does technology associated with elucidation of the bovine genome offer to dairy producers? First there are the evolutionary implications associated with the ruminant and importance of lactation (Elsik et al., 2009). Seventysix percent (778 out of 1020) of sequential duplications corresponded to complete or “International Strategic Program for Conservation of Animal Genetic Resources for Food and Agriculture” sponsored by OECD Co-operative Research Programme 89 © WW Thatcher, 2010, All rights reserved partial gene duplications with high sequence identity (median 98.7%). This suggests that many of these gene duplications are specific to either the Bos lineage (i.e., wild and domestic cattle) and tend to encode proteins that often interface with the external environment, particularly immune proteins and sensory and/or olfactory receptors and include defensins, and pregnancy-associated glycoproteins. Duplications that are present exclusively in cattle may have functional implications for their unique physiology, environment that they subside in, and diet of cattle. An overrepresentation of genes involved in reproduction in cattle is associated with several gene families expressed in the ruminant placenta. These gene families encode the intercellular signaling proteins pregnancy-associated glycoproteins, interferon tau (IFNT) and prolactin-related proteins These genes regulate ruminant-specific aspects of early pregnancy recognition, fetal growth, maternal adaptations to pregnancy, and the coordination of parturition. Examples of genes varying in cattle relative to mouse include a cluster of b-defensin genes, which encode antimicrobial peptides. Compared to the human and mouse genome, the cattle genome has increased changes in the numbers of interferon genes and the number and organization of genes involved in adaptive immune responses. This extensive duplication and divergence of genes involved in innate immunity may be because of the substantial load of microorganisms present in the rumen of cattle, which increases the risk of opportunistic infections at mucosal surfaces and positive selection for the traits that enabled stronger and more diversified innate immune responses at these locations. Another possibility is that immunity may have been under selection due to the herd structure, which can promote rapid disease transmission. Also, immune function–related duplicated genes have gained nonimmune functions, e.g., IFNT that in addition to regulating antiviral activity is involved in maintenance of the corpus luteum in early pregnancy by its actions on the uterus to ultimately suppress secretion of prostaglandin F2α; the C-class lysozyme genes, which are involved in microbial degradation in the abomasum. A summary of these evolutionary comparisons among species indicate that the biological systems most affected by changes in the number and organization of genes in the cattle lineage include reproduction, immunity, lactation, and digestion. These changes in the cattle lineage probably reflect metabolic, physiologic, and immune adaptations due to microbial fermentation in the rumen, the herd environment and its influence on disease transmission, and the reproductive strategy of cattle. Mapping of the cattle genome and associated resources will facilitate the identification of novel functions and regulatory systems as well as the tools for genetic improvement within the dairy industry. Furthermore, there is a greater homology of the bovine genome with the human genome than the genomes of the traditional mouse or rodent models used for humans. Functional genomics: Gene discovery says nothing of gene function. However, searching databases from other species and now the bovine genome is helping to predict gene function, particularly for single gene traits. Other methods are also helping us to identify functional roles of genes and include gene chips or microarrays, gene knockouts and gene knockdowns. Affymetrix Inc. now markets a bovine genotyping chip “International Strategic Program for Conservation of Animal Genetic Resources for Food and Agriculture” sponsored by OECD Co-operative Research Programme 90 © WW Thatcher, 2010, All rights reserved based on this work, allowing broader translation of the genome project into applications. Microarrays are nylon or glass slides or “chips”, as they are commonly called, that are spotted with partial gene coding sequences. Chips are incubated with fluorescently tagged complementary DNA (cDNA) from tissues of interest to determine what genes are being expressed. For example, we have compared uterine-endometrial tissue gene expression between lactating and non-lactating dairy cows of cyclic versus pregnant cows at 17 days after a synchronized LH surge that is comparable to onset of an estrus. A number of up-regulated expression of genes were detected due to lactation that were related to to immune function, particularly of B cells and γδ T cells (Cerri and Thatcher unpublished observations, 2010). Conversely developmental genes related to limb and neural development and glucose homeostasis were down-regulated by lactation. The genes associated with immune function and developmental genes expressed in the endometrium, that are impacted by lactational state, are possible candidate genes for interventions aiming to improve fertility of lactating dairy cows and warrant further investigation relative to alteration of immune function during lactation. Genomic selection: Mapping of the bovine genome has facilitated the ability to complement direct genetics with traditional quantitative genetics that will benefit the dairy industry. The genetic contribution of many multi-gene traits in cattle (e.g., milk production) is well documented, and this knowledge has provided the basis for the identification and mapping of a growing number of quantitative trait loci (QTL). The only limitation to performing direct genetic experiments and identifying genes underlying these traits is the lack of a complete genome sequence, which is now available for the bovine. Selection experiments, heterosis studies and breed comparisons have all been used in bovine genetic studies. Many populations have been used to map genes to large chromosomal regions but positional mapping the gene has been difficult. Sequencing the bovine genome and identifying “Single Nucleotide Polymorphisms” (SNPs) will provide additional polymorphic markers and positional candidate genes derived from the human and bovine genomic maps. Indeed due to the higher homology between the bovine with the human genome compared to the genome of the mouse, the functional genomics of the bovine is probably more applicable than using the mouse as an experimental model. The populations with designed mating generated by natural reproduction, artificial insemination or assisted reproductive technologies provides unique opportunities for selection and propagation of efficient dairy cattle in the future that can perhaps both produce milk and reproduce efficiently. Clones can also be generated from fibroblasts or stem cells and cryopreserved. Instead of thinking that genetic diversity has decreased in Holstein cattle one should envision a greater opportunity to identify genes that are impacting reproductive processes that can be incorporated into our selection programs. The imprints of domestication and breed development on the genomes of livestock likely differ from those of companion animals. A deep draft sequence assembly of shotgun reads from a single Hereford female and comparative sequences sampled from six additional breeds were used to develop probes to interrogate 37,470 singlenucleotide polymorphisms (SNPs) in 497 cattle from 19 geographically and biologically “International Strategic Program for Conservation of Animal Genetic Resources for Food and Agriculture” sponsored by OECD Co-operative Research Programme 91 © WW Thatcher, 2010, All rights reserved diverse breeds (Gibbs et al., 2009). These data show that cattle have undergone a rapid recent decrease in effective population size from a very large ancestral population, possibly due to domestication, selection, and breed formation. Domestication and artificial selection appear to have left detectable signatures of selection within the cattle genome, yet the current levels of diversity within breeds are at least as great as exists within humans. The availability of high-throughput assays for genotyping single nucleotide polymorphisms (SNP) has led to the genotyping of thousands of dairy cattle using the BovineSNP50 BeadChip (Illumina, Inc., San Diego, CA) or similar platforms. The SNP markers represent single base changes (A, T, C, or G) within the DNA sequence of a bull or cow. This technology provides the ability to carry out 54,000 DNA SNP marker tests simultaneously; SNPs are throughout the bovine genome of approximately 3 billion base pairs. Consequently, the SNPs become genetic markers for individual animals such as progeny tested bulls in artificial insemination (AI) programs or young bulls that are candidates for such programs. A study at the USDA-ARS Beltsville Agricultural Research Center established the SNP genotypes for 5,369 Holstein bulls and cows (VanRaden et al., 2009; Weigel et al., 2010). The genotype data of the bulls were used to estimate the effects of 38,416 SNP markers on production, type, longevity, udder health and calving ability. Based on the estimated SNP associations on these phenotypic traits from this parent population, a genomic predicted transmiting ability (PTA) was determined for each of 2,035 young Holstein bulls born from 2000 to 2003 that had no progeny. In 2009 the PTA of each young bull was determined from its progeny and compared with the traditional PA (Parental Average) and the genomic PTA computed from the 2004 data. The same process was performed in the Jersey breed (1361 older animals and 388 young bulls) and the Brown Swiss breed (512 older animals and 150 young bulls). Results in Table 1 show the increase in reliability (REL) due to genomic information, as compared with the REL from parent average information only. Gains in REL from genomic information were positive for almost all responses. Gains in REL for Jerseys and Brown Swiss were not as large as for Holsteins and this is largely due to a fewer number of progeny tested bulls that were genotyped. For each trait, a young animal’s PA can be combined with information from the BovinSNP50 bead Chip to obtain a genomic PTA of much greater accuracy. For a bull calf, REL of the genomic PTA is equivalent to what could be obtained by measuring performance on 25 or 30 test daughters. “International Strategic Program for Conservation of Animal Genetic Resources for Food and Agriculture” sponsored by OECD Co-operative Research Programme 92 © WW Thatcher, 2010, All rights reserved Table 1. Changes in reliability due to the inclusion of genomic data in national genetic evaluations in the United States (VanRaden et al., 2009). Trait Net Merit Milk Yield Fat Yield Protein Yield Fat Percentage Protein Percentage Productive Life Somatic Cell Score Daughter Pregnancy Rate Holstein +24% +26% +32% +24% +50% +38% +32% +23% +28% Jersey Brown Swiss +8% +9% +6% +17% +11% +10% +2% +14% +36% +8% +29% +10% +7% +12% +3% +17% +7% +18% Weigel et al., (2010) compared how well genomic evaluations were performing for the young bulls of 2000-2003 that in 2009 had both genomic data and at least 50 milking daughters. Parent averages (PA), Genomic Predicted Transmitting Abilities (GPTA); and Daughter Yield Deviations (DYD; contains no genomic information) of the bulls. A total of 238 Holstein bulls had official genomic PTAs for milk, fat, protein, somatic cell score (SCS) in January 2009 that were based solely on genomic information and the bulls had at least 50 milking daughters in August 2009. Only 60 bulls had at least 50 daughters in their genetic evaluations for daughter pregnancy rate (DPR). Comparisons of reliability (REL) for PA (Jan. 2009), GPTA (Jan. 2009) and DYD (Aug. 2009) are very insightful and are presented in Table 2. The average January 2009 RELs for PA was 42% for yield traits, 39% for SCC, and 26% for DPR; whereas RELs of the genomic PTA, which include both pedigree and genomic information, averaged a higher 72%, 67% and 62%, respectively. When examining actual production responses of the daughters (DVD in August, 2009), the average REL of 84% for yield traits, 67% for SCS and 62% for DPR. The correlations between August 2009 DYD from progeny testing and January 2009 PA and GPTA for each trait were much higher with the inclusion of genomic information (Table 2). PTA of young bulls via genotyping of SNPs without progeny test estimates allows for the use of young bulls with some degree of confidence. This would allow dairy producers to use a larger number of young bulls that would lower the risk associated with the use of lower REL bulls. Producers who supplement their traditional sire selections with a group of superior genome-tested bulls (i.e., each used in moderation) will achieve the greatest genetic progress. “International Strategic Program for Conservation of Animal Genetic Resources for Food and Agriculture” sponsored by OECD Co-operative Research Programme 93 © WW Thatcher, 2010, All rights reserved Table 2. Comparison of January 2009 parent averages (PA) and genome-enhanced predicted transmitting abilities (GPTA) for milk, fat, protein, somatic cell score (SCS), and daughter pregnancy rate (DPR) with August 2009 daughter yield deviations (DYD) for US Holstein bulls whose first-crop daughters calved between January and August. Milk Fat Prot SCS DPR No. Bulls 238 238 238 237 60 Reliability (Jan ‘09 PA) 42% 42% 42% 39% 36% Reliability (Jan ‘09 GPTA) 72% 72% 72% 67% 62% No. Daughters (Aug ’09 DYD) 71 71 71 71 62 Reliability (Aug ‘09 DYD) 84% 84% 84% 67% 52% Correlation (Jan ‘09 PA, Aug ‘09 0.444 0.540 0.476 0.376 0.213 DYD) Correlation (Jan ‘09 GPTA, Aug ‘09 0.624 0.695 0.632 0.531 0.341 DYD) The lower REL for DPR and SCS illustrates the greater difficulty in improving lower heirtable fertility and health traits through genetic selection although progress can be made. However, with good reproductive management, as described earlier, the opportunities to improve reproductive performance through selection will be enhanced. The ability to estimate genomic Use of genomic technology has identified a potential gene or associated locus that is related to bull fertility (Feugang et al., 2009). A Phase I comprehensive genome wide analysis of SNPs for bull fertility identified a total of 97 SNPs that were significantly associated with fertility (P < 0.01). In Phase II, the four most significant SNPs of Phase I were tested in 101 low fertility and 100 high-fertility bulls. One of the SNPs, rs41257187 (C → T) is in the coding region of the integrin beta 5 gene on chromosome 1. The SNP rs41257187 induces a synonymous (Proline → Proline) suggesting disequilibrium with the true causative locus. However, incubation of bull spermatozoa with integrin beta 5 antibodies significantly decreased the ability to fertilize oocytes. These insightful findings indicate that the bovine sperm integrin beta 5 protein plays a role during fertilization and could serve as a positional or functional marker of bull fertility. This genomic approach enters into the tool box for strategies to improve dairy cattle fertility. Sequencing the bovine genome and further advances in functional genomics promises great benefits to the dairy industry. As genes for production traits are identified, genetic selection strategies can be improved. One can envision making improvements in milk yields and milk fat and protein composition, as well as herd health and reproductive performance. As genes for production traits are identified, gene selection will be reduced to simply running a genetic test for the particular gene(s) of interest. “International Strategic Program for Conservation of Animal Genetic Resources for Food and Agriculture” sponsored by OECD Co-operative Research Programme 94 © WW Thatcher, 2010, All rights reserved Inbreeding and Heritability Inbreeding: As chromosomes are paired, there are two genes one inherited from each parent. Inbreeding is an outcome of any genetic selection program. In order to increase the proportion of animals displaying a favorable trait or phenotype, relatives are mated because they utilize the common genetic material allowing expression of favored phenotypes. In the mating of relatives, the chance of the offspring receiving identical genes is enhanced. An inbreeding coefficient is an estimate of the percentage of identical genes pairs inherited by an animal. The average level of inbreeding in the US Holstein population is approximately 5 % having increased from approximately 0 % since 1960 (AIPL, 2010; Figure 1). The DPR is estimated to decrease by 0.08 when inbreeding increases by 1 % (AIPL, 2007). Expected inbreeding values through 2010 appear to be in a plateau period likely indicative of efforts to attenuate the increase in inbreeding within existing breeding programs. One beneficial aspect of genomic selection will be the potential differential identification of full sibs with different alleles from those with similar alleles (e.g., SNP analysis). Simulation studies indicated that importation of genetic material from Nordic Holsteins may slow down the deterioration of animal health and reproduction in US Holsteins (Buch et al., 2009). There are extreme examples of the negative effects of inbreeding. Markers for several recessive diseases have been developed through the use of Marker Assisted Selection. Examples of diseases that severely impact reproductive performance, but that have been reduced to minor concerns because of the use of genetic markers, are BLAD “International Strategic Program for Conservation of Animal Genetic Resources for Food and Agriculture” sponsored by OECD Co-operative Research Programme 95 © WW Thatcher, 2010, All rights reserved (Bovine Leukocyte Adhesion Deficiency), DUMPS (Deficiency of Uridine - 5Monophosphate Synthase) and CVM (Complex Vertebral Malformation). Heritability: Heritability is the proportion (percentage) of the observed variation in a trait that is due to genetics. It is difficult to make genetic improvement in reproductive traits because the heritability of reproductive traits is relatively low (i.e., 0.05 or less) compared to production traits like milk yield (i.e., 0.30). For example less than 5% of the variation between cows in their reproductive function is caused by differences in genes. There are several factors contributing to the low heritability of reproductive traits. These include the large number of genes regulating tissues and biological functions that contribute to reproductive processes, the large number of non-genetic factors affecting reproductive herd responses such as herd nutrition, climatic conditions contributing to heat stress, on farm reproductive management skills (abilities of the inseminator, etc.). However, one should not conclude that low heritability means that there are no genes controlling reproduction. With mapping of the genome and use of SNPs, more genes affecting reproduction function are being detected or associations with reproductive performance identified. Furthermore, as reproductive and dairy management skills are improved the proportion of observed variation in reproductive traits due to genetics will be increased. Recent estimates of very specific reproductive tissue responses have been quantified to monitor anovulation by progesterone and embryo loss by ultrasound/rectal palpation. Bamber et al. (2009) estimated heritabilities for anovulation at ~50 d in milk and pregnancy loss occurring between first and second pregnancy diagnoses after artificial insemination. Anovulation data consisted of 5,818 records from 13 studies in 8 herds with an overall prevalence of 23.3%. The mean heritability estimate for anovulation was 0.171. Bivariate analysis of BCS with anovulation revealed a genetic correlation of −0.301. Pregnancy-loss data consisted of 3,775 records from 14 studies in 8 herds with an overall prevalence of 14.4%. A sire–maternal grandsire model yielded heritability for pregnancy loss of 0.489. Differential analyses indicated that the embryo’s ability to survive has a greater affect on pregnancy loss than does the cow’s ability to maintain the pregnancy. These results suggest that genetic improvement of reproductive performance could be enhanced by selection for fundamental measures such as abnormally long periods of postpartum anovulation and pregnancy loss. In 2003, the USDA began estimating the genetic merit of bulls for reproduction. The trait used is Daughter Pregnancy Rate (DPR). This term is calculated from days open and is directly related to the proportion of females eligible to become pregnant in a 21day period that actually become pregnant (i.e. the 21-day pregnancy rate). The heritability of DPR is only 0.04. Weigel (2006) reported that the top 10% of Holstein bulls had a DPR 4.9% higher than bulls in the lowest 10%. This difference corresponds to a difference in days open of 20 days (a 1% difference in DPR is equal to a 4 day difference in days open). “International Strategic Program for Conservation of Animal Genetic Resources for Food and Agriculture” sponsored by OECD Co-operative Research Programme 96 © WW Thatcher, 2010, All rights reserved Conclusion Tremendous advances have been made for improving milk production, but have in turn, resulted in an overall decline in reproductive efficiency for the dairy industry. To combat this problem, research must focus on improvements in three main areas: the cow, the gametes and the environment. Problems associated with the cow include inability to properly detect estrus, and altered hormone profiles resulting in low conception rates and increased early embryonic death. Coordinated systems of reproductive and nutritional management offer the vehicles to improve herd reproductive performance. Advances in reproductive technologies offer wider use of germplasm. However, at this time additional research is needed to counter the higher rates of embryonic and fetal mortality. Detailed studies of tissue and cell biology, utilizing the techniques of genomics, proteomics and bioinformatics, will undoubtedly allow investigators to understand the limitations to efficient reproductive processes of the sub-fertile lactating dairy cow. Genomic selection for production, health and reproductive traits will be the wave of the future as genomic and bioinformatic tools continue to be expanded and refined. Such technology combined with optimal reproductive management and the repertoire of assisted reproductive technologies will go hand in hand to improve reproductive performance coupled with increased milk production, as immune, metabolic and nutritional components are co=regulated to optimize cow performance and well being. References AIPL (Animal Improvement Programs Laboratory). 2010. Trend in inbreeding. http://aipl.arsusda.gov/eval/summary/inbrd.cfm Accessed July 10, 2010. AIPL (Animal Improvement Programs Laboratory). 2007b. Changes to evaluation System: August 2007. http://aipl.arsusda.gov/reference/changes/eval0708.html Accessed July 10, 2010. Bauman DE. (1999). Bovine somatotropin and lactation: From basic science to commercial application. Domes Anim Endocrinol 17:101–116. Bamber RL, Shook GE, Wiltbank MC, Santos JEP, Fricke PM. (2009). Genetic parameters for anovulation and pregnancy loss in dairy cattle. J. Dairy Sci. 92:5739–5753. Buch LH, Sørensen AC, Lassen J, Berg P, Christensen LG, Sørensen MK. (2009). Factors affecting the exchange of genetic material between Nordic and US Holstein populations. J. Dairy Sci. 92: 4023–4034. Capper JL, Cady RA, Bauman DE. (2009).The environmental impact of dairy production: 1944 compared with 2007. J Anim Sci. 87:2160-2167. “International Strategic Program for Conservation of Animal Genetic Resources for Food and Agriculture” sponsored by OECD Co-operative Research Programme 97 © WW Thatcher, 2010, All rights reserved Capper JL, Castañeda-Gutiérrez E, Cady RA, Bauman DE.(2008). The environmental impact of recombinant bovine somatotropin (rbST) use in dairy production. Proc. Natl. Acad. Sci. USA 105:9668–9673. Elsik, CG, Tellam, RL, Worley, KC, Gibbs, RA, Muzny, DM, Weinstock, GM, Adelson, DL, Eichler, EE et al. Bovine Genome Sequencing and Analysis Consortium; The genome sequence of taurine cattle: a window to ruminant biology and evolution. Science 2009 Apr 24; 324(5926):522-8. Feugang JM, Kaya A, Page GP, Chen L, Mehta T,Hirani K, Nazareth L, Topper E, Gibbs R, Memili E. (2009). Two-stage genome-wide association study identifies integrin beta 5 as having potential role in bull fertility. BMC Genomics, 10:176-186. Gibbs, RA, Taylor, JF, Van Tassell, CP, Barendse, W, Eversole, KA, Gill, CA, Green, RD, Hamernik, DL et al. Bovine HapMap Consortium; Genome-wide survey of SNP variation uncovers the genetic structure of cattle breeds. Science 2009 Apr 24; 324(5926):528-32. Lopez H, Caraviello DZ, Satter LD, Fricke PM, Wiltbank MC. (2005). Relationship between level of milk production and multiple ovulations in lactating dairy cows. J. Dairy Sci. 88, 2783-2793. Lopez H, Satter LD, Wiltbank MC. (2004). Relationship between level of milk production and estrous behavior of lactating dairy cows. Anim. Reprod. Sci. 81, 209-223. Lucy MC. (2001). Reproductive loss in high-producing dairy cattle: where will it end? J. Dairy Sci. 84, 1277-1293. Moreira F, Orlandi C, Risco CA, Mattos R, Lopes F, Thatcher WW. (2001). Effects of presynchronization and bovine somatotropin on pregnancy rates to a timed artificial insemination protocol in lactating dairy cows. J. Dairy Sci. 84: 1646-1659. Norman HD, Wright JR, Hubbard SM, Kuhn MT, Miller RH. (2007). Genetic Selection for Reproduction: Current Reproductive Status of the National Herd; Application of Selection Indexes for Dairy Producers. In: Proc. Dairy Cattle Reproductive Conference, Eds. W.W. Thatcher and E.R. Jordan, Dairy Cattle Reproductive Council, Hartland, WI, pp 69-78 Rodriguez-Martinez H, Hultgren J, Båge R, Bergqvist A-S, Svensson C, Bergsten C, Lidfors L, Gunnarsson S, Algers B, Emanuelson U, Berglund B, Andersson G, Håård M, Lindhé B, Stålhammar H, Gustafsson H. (2008). Reproductive performance in highproducing dairy cows: can we sustain it under current practice? In: IVIS Reviews in Veterinary Medicine, I.V.I.S. (Ed.). International Veterinary Information Service, Ithaca NY (www.ivis.org), Last updated: 12-Dec-2008; R0108.1208 (Open Journal). VanRaden PM, Van Tassell CP, Wiggans GR, Sonstegard TS, Schnabel RD, Taylor J F, Schenkel FS. 2009. Invited review: Reliability of genomic predictions for North American Holstein bulls. J Dairy Sci. 92:16–24. “International Strategic Program for Conservation of Animal Genetic Resources for Food and Agriculture” sponsored by OECD Co-operative Research Programme 98 © WW Thatcher, 2010, All rights reserved Weigel KA, de los Campos G, Vazquez AI, Van Tassell CP, Rosa GJM, Gianola D, O’Connell JR, VanRaden PM, Wiggans GR. Genomic Selection and its Effects on Dairy Cattle Breeding Programs. Available at: http://www.aipl.arsusda.gov/publish/other/2010/submit_9wcgalp_vanraden_kw.pdf Accessed July 5, 2010. “International Strategic Program for Conservation of Animal Genetic Resources for Food and Agriculture” sponsored by OECD Co-operative Research Programme 99