Coarse Woody Habitat Fish Community and Food Web Responses
advertisement

FEATURE
FISH
HABITAT
Fish Communityand FoodWeb
Responsesto a Whole-lake Removalof
CoarseWoodyHabitat
Greg G. Sass
JamesE Kitcheil
StephenR. Carpenter
Thomas
R. Hrabik
Anna E. Marburg
Monica
G. Turner
ABSTRACT: As lakeshoresare developed,propertyownersoften thin the $ass is a research associate at the Center for
riparianforestand removeolder logsor fallen limbsfrom the adjacentlit- Linmology, University of Wisconsin-Madison
and
can
be
reached
at
toral zone.This practicealtersfishhabitatandproducesunknownecosystem
ggsass@w•sc.edu.
Kitchell •s the Arthur D.
changes.To assess
potentialeffectson fish communitie•and foodweb inter- Hasler Professorof Zoologyand Director,
actions,we removedmore than 75% of the coarsewoodyhabitat (CWH)
Center for Limnology, University of
from the treatmentbasinof Little Rock Lake,Wisconsin,while leavingthe Wisconsin--Madison. Carpenter is the
referencebasin unaltered. Prior to CWH removal, the food webs in both StephenA. ForbesProfessor
of Zoologyat
the
Center
for
kimnology,
University
of
basinswere similar and dominatedby aquaticprey.After CWH removal,
Wisconsin--Madison.
Hrabik is an associate
largemouthbass(Micropterussalmoides)
in the treatment basin consumed
lessfish, ate moreterrestrialprey,and grewmoreslowlyrelativeto the pop- professor of biology, University of
Minnesota--Duluth.Marburgis a graduate
ulation in the reference basin. Yellow perch (Perca fiavescens)in the studentin the Departmentof Zoologyat the
treatmentbasindeclinedto extremelylow densitiesasa consequence
of pre- Universityof Wisconsin--Madison.Turner
dation and little or no recruitment.In contrast,perch in the referencebasin is the EugeneE Odum Professor
of Zoology
werereplenishedby severalsuccessful
cohortsproducedin consecutive
years. Universityof Wisconsin--Madison.
Maintenanceof CWH appearsto be crucialfor sustainingdesirable fishesand fisheriesin lakes. Changesin CWH produce
complex,long-lastingeffectsat the ecosystem
scale.
refugefor smallfishesbecause
physical structure decreases
Coarsewoodyhabitat(CWH) maybe
the foragingsuccess
of their
a criticalfeatureof freshwater
ecosystems.
predators(Savino and Stein
In lakes,unlike1oticsystems
(e.g.,Beechie
1982). Savino and Stein
and $ibley 1997; Keim et al. 2002), the
(1982} found decreases in
role of CWH rarelyhas been evaluated.
CWH supportsperiphyton,althoughthe largemouthbass(Micropterus
directcontributionof epixylicalgaeto pri- salmoides}foraging success
mary productivity in small lakes is with increasinglevelsof simrelatively minor (Vadeboncoeur and ulated aquatic vegetation,
Lodge2000). The indirect influencesof and similar constraints could
CWH, sucha• increasedorganicsediment be providedin the interstitial
retention,may be importantfor epipelic spaces created by CWH.
algae,whichcan provide50-80% of total Loss of littoral refuge may
production (Hilton
et al. 1986; result in changesin behavior
Vadebonct•urand Lodge2000). In addi- (Scheuerell and Schindler
INTRODUCTION
tion, CWH
serves as a substrate for
2004) and increasedmortal-
A largemouthbass(Micropterus
salmoides)
swimsamong
benthic invertebrateproduction,thereby ity rates of juvenile and submergedCWH in AndersonLake,VilasCounty,WI.
providingenergyto upper trophic levels smallfishes,whichultimately
(Angermeier and Karr 1984; Vander depressesgrowth rates for
Zanden and Vadeboncoeur2002).
their predators(Schindleret
CWH playsan importantrole in the al. 2000) and increases
the potentialfor tionship existed between CWH abunand
lakeshore
residential
life histories
of manyfishspecies
by offer- depensatory population dynamics dance
ing protection to nesting sites,a spawning (Walters and Kitchell 2001).
developmentin northernWisconsinand
substrate,
andan areaof greaterpreyavailPropertyownersoften reduceriparian upperMichigan lakes(Christensenet al.
ability (Hjelm et al. 2000; Hunt and tree densities and remove CWH from the 1996; Jenningset al. 2003; Marburget al.
Annett 2002). CWH may also provide littoral zonesof lakes.A negative rela- in press;Figure1). Empiricaland modelFisheries
ß VOL 31 r•o 7 ß JULY 2006
ß •/V•N•N.FISHERIES.ORG
3•)1
Representative
photographs
compareundevelopedand
developedlakeshorestypical
of northern Wisconsin lakes
Thephotographof an
undevelopedshorelinewas
taken from the treatment
basinof LittleRockLakeprior
to the CWH removal.
Developed
CWH
Removal
Damon Krueger,Greg Sass,
Brian Roth, Jeff B•ermann,
and Motomi
"Genkai"
Kato
removecoarsewoody
habitat
li
322
Fisheries
ß VOL 31 NO 7 ß JULY 2006
ß WWW.FISHERIES.ORG
ing studiesdemonstrate
extremelylonglasting negative effects of lakeshore
residentialdevelopmenton CWH pools
becauseinput rates and decayare slow
processes,
while humanremovalratesare
fast (Guyette and Cole 1999; Roth
unpublished
data).
In northern Wisconsin and upper
Michigan lakes, growth rates of largemouth bass and bluegill (Lepomis
macrochirus)
were highestin lakeswith
little or no lakeshore
residentialdevelop-
1981).
The
contradiction
between
MATERIALS
AND
METHODS
expectation and observationsuggests
that the availabilityof CWH maycreate StudySite
complex relationshipsbetween ecosysLittle Rock Lake is an 18 ha, oligtem productivity, fish growth, and otrophicseepagelake located in Vilas
exploitation.
County,Wisconsin.
The lakehasno resiTo better understand the role of CWH
in aquaticecosystems,
we removedCWH
dential development,has been closedto
publicaccess
andfishingsince1984,andis
from the littoral zone of the treatment
dividedintotwobasins
byan impermeable
curtain which createsa reference(8 ha)
developmentandno fishery.CWH levels andtreatmentbasin(10 ha) for whole-lake
basin of a lake that had no residential
studies.
The treatmentbasinwasexperimentally acidifiedthroughoutthe late
1980sandthen allowedto recoverduring
the
1990s.The aquaticcommunities
were
This result was surprising because coexistingfishpredatorand preypopulasimilar
in
both
basins
prior
to
conducting
increased nutrient loading due to tion. We specifically
examinedthe effects
our experiment(Sampson1999; Hrabik
lakeshore development generally
of CWH removalon the aquaticfood andWatras2002).
increaseslake productivity,and thereweb and the diet and growthrate of the
Fish speciesassemblages
in northern
fore, fish growth rates (Hanson and
Leggett1982). Further,lakeshoredevel- dominant predator (largemouthbass). Wisconsinlakesare generallydominated
popula- by cyprinid-Umbracommunitieswhere
opment is generally associatedwith We also testedfor depensatory
tion
growth
dynamics
in
the
dominant
winterkillis prevalentor by centrarchidincreased angler exploitation, which
should reduce density-dependentcon- prey population, yellow perch (Perca esocid-percid communities where
winterkill is uncommon and habitat availstraintson growth (Goedde and Coble fiavescens).
ability and predator-preyinteractions
determinecommunitystructure(Tonnand
Figure1. The relationship
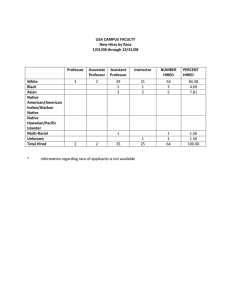
betweenCWH abundanceandthe numberof buildings
per km of
Magnuson1982).Little RockLakeis repment where CWH is most abundant, in the reference basin of the lake were
althoughtrendsfor basswerenot statisti- not manipulated.In this study,we examcally significant(Schindleret al. 2000). ined the influence of CWH on a
shoreline
for severalnorthernWisconsin
andupperMichiganlakes.DatafromChristensen
et al.
(1996;CWH>5 cmdiameter),Marburget al. (in press),
andpreviously
unpublished
data(CWH
>10 cm diameter).
lOO0
resentative of other northern Wisconsin
lakes where winterkill rarely, if ever,
occurs.The fish communityis dominated
by largemouth
bassandyellowperch.Less
abundant
fishspecies
includeblackcrappie
( Pomoxis nigromaculatus
), rock bass
(Ambloplites
rupestris),
and central mudminnow (Umbra limO. We conducted
900-
8002
pre-manipulation
monitoringof the fish
communities in both basins during
July-August
2000, May-September
2001,
700-
and May-Juneof 2002.
Priorto manipulation,the littoralzone
600
in the treatmentbasinhad475 largelogs
(>10 cm diameter)per km of shoreline.
500-
This level of CWH
30,- ?
2004
.
I
1o.o"øß ß
0
ß
0
10
20
30
40
BuildingDensity (no./km)
50
abundance was above
the median for undevelopedlakes in
northernWisdonsinand upperMichigan
(Christensen
et al. 1996;Marburget al. in
press;Figure2). CWH wasthe dominant
form of littoral structurepresent.Sparse
standsof burreeds(Sparganium
spp.)were
also presentat low stem densities(4.8
stems/m2).
The littoralzoneof the referencebasinhad 344 piecesof largeCWH
perkm of shoreline
throughout
the study.
During July--August 2002, we
removedmostof the largeCWH fromthe
littoralzoneof the treatmentbasin.Logs
and limbsencountered
to a depthof 2 m
were removedusingwinchesor by hand
afterreducing
the sizeof the logswith axes
We reducedlarge CWH abundance
mostsmallsticksand logs(<10 cm diame- from 475 logs/kinof shorelineto 128
ter) encountered including three logs/kin (73% reduction) during the
removal (Figure 2). Following CWH
abandoned
North
American
beaver
or chainsaws.In addition, we removed
removal, the treatment basin had CWH
(Castorcanadensis)
lodgesa•d their associ- abundances commensurate with lakes havated food caches. Removed CWH
this region (Christensenet al. 1996;
Marburget al. in press;Figure1). Postmanipulation monitoring of the fish
communities was conducted in both basins
duringAugust•September
2002 and the
May•September
periodsof 2003and2004.
was
ing housingdensities
of 2-8 buildings/kin FishSampling
placedonshoreabovethehighwatermark of shoreline,which is representative
of a
Methodsemployedin this studyare
of the lake.
relativelymodestlevelof development
for
detailed in Sass (2004) and briefly
recountedhere.We collectedgrowthand
Figure2.. Aerialphotograph
of LittleRockLakewithabundances
of large(>10 cm diameter)
CWH labeledand represented
by white dotsbeforeand afterthe CWH removalin the treatment
basin(north).The referencebasra(south)of LittleRockLakehad344 Iogs/kmof shoreline
throughoutthe study.
Pre-CWH
Removal
diet information from the dominant fish
species
at biweeklyintervalsduringMaySeptember2001-2004.Largemouth
bass,
blackcrappie,
androckbasswerecollected
by hook-and-lineanglingbecause
the low
conductivityof the waterprecluded
effective electroshocking.
We collecteda total
of 963 and 1,209 bassfrom the reference
and treatmentbasins,respectively,
over
the4-yearstudy.Perchwerecollected
with
mi•mow traps and beach seines.Only
seinedperchwereusedfor dietmmlysis
to
preventbiasdue to digestionof gut contents from perch capturedin mi•mow
traps.We usedperchcapturedin minnow
trapsandbyseineto determinepopulation
abundances. We collected a total of 781
and 240 perch from the referenceand
treatment basins, respectively, from
2001-2004. Eachfish capturedwasmeasured,weighed,and severalscaleswere
taken for age and growthdetermination.
Fisheslargerthan 150 mm total length
were tagged(numberedFloy• tag). We
determineddiet composition
biweeklyby
performing
gastriclavageon up to 15 fish
perspecies.
Diet itemswereseparated
into
majortaxonomiccategories,
enumerated,
344
Iogs/km
Post-CWH
Removal
and driedto determinethe dry massproportionof eachpreyitem in the diet.
Scales were analyzed to determine
mem•sizeat ageand size-specific
growth
ratesfor largemouthbassand perch.Our
methods for determining size-specific
growthratesandstatistical
analyses
canbe
foundin Schindleret al. (2000). Size-specific growthtateshave greaterstatistical
power than other indicatorsto detect
effects of habitat manipulations
(Carpenteret al. 1995}.Am•ually,wecollectedscales
frombehindthe pectoralfin
from 5 individualfish of eachspecies
for
everyavailable10mmincrement
of length
(100-109, 110-119 ram, etc.) captured.
Bassandperchscales
werepressed
between
glassslidesand photographedwith a
PolaroidDMC 2 digital camera.Scales
werereadusinga Fishomatic
opticalimaging systemdevelopedby the Center for
128
ß
: Iogs/km.,...-'...'
Iogs/km
Fisheries
• VOL 31 •0 7 o JULY2006
Limnology at
the
132,post-= 186) and93 perch(pre- = 68, nowtrappingduringJulyandwerelessthan
post-= 25) wereanalyzedfor growthfrom 75 mm in length.Eachtrapwasdeployed
individual'sgrowthrate in the previous the reference basin in 2001-2004. We ana- for fivedaysandcatcheswerecountedand
year. Growth was determined by the lyzed320 bass(pre-CWH removal= 134, emptiedat oneor twodayintervals.
Fraser-Lee
methodof back-calculating
the post-= 186)and96 perch(pre-= 57, postAnalysis
length of the previousyear. We then = 39) from the treatment basin over the
We used a Model I single factor
regressed
logcgrowthrate(mm/y;depen- sametimeperiod.
We useda Chapman-modified,
continu- Analysisof Variance (ANOVA) within
dent variable) on fish size (mm;
population basins(Zar 1996)andpairedt-testsamong
independent
variable)for eachspecies
in ous Schnabelmark-recapture
estimation
procedure
to
estimate
adultbass basinsto testfor differences
in the proporeachyearof the studyto determinemean
andtotalperchabundances
in eachyearof tion of terrestrialprey itemsand average
growthratesforfourcommonsizeclasses
of
the study(Ricker 1975). We did not esti- weightper bassdiet beforeand after the
bass(100, 200, 300, 400 mm total length)
mate young-of-year (YOY)
bass CWH removal.To testfor statistically
sigand perch (50, 100, 150, 200 mm total
abundances.
To determineyoung-of-year nificant differencesin size-specific
bass
length;Carlander1982;Schindleret al.
(YOY) and total perchcatchper unit of growth rates, we examined 95% confi2000). Only one size-specific
growthrate effort (CPUE), we deployed10 minnow
dence intervalsfrom the regression
line
was calculated from each individual fish. A
trapsin 2003and20 trapsin 2004biweekly relationships
betweengrowthrateandfish
total of 318 bass(pre-CWH removal =
in eachbasinat pre-speci- sizein eachbasin.All leastsquares
regresWisconsin--Madison
University of
to determine
an
fied near-shore locations
from
May-September.
sionsfor the relationship
betweenloge
growthrate(mm/y)versus
fishsizeforeach
Figure 3. Proportionof largemouthbass(Micropterus
typically specieswere statisticallysignificant(P
salmoides)diets(A) consistingof yellow perch(Percaflavescens) Perch YOY
became vulnerable to minand density(B)of yellowperchin the reference(R)and
<0.001)andhadr2 valuesranging
from
treatment(T) basinsof LittleRockLakepriorto (pre-)and
following(post-)the CWH removal
Figure 4. Trendsin the proportionof terrestrialpreyand the averagedry
weight per diet for largemouthbass(Micropterus
salmoides)in the reference
(R, •, solid line) and treatment (T, &, dashed line) basinsof Little RockLake
priorto (pm-) and following(post-)the CWH removal.Errorbarsrepresent
0.9
the standard error about the mean.
0.8
0.7
60
o.s
,
T
0.5
0.4
0.3
'• 30-
0.:2
,'"'
0.1
0.0
•
10-
looo
900
(B)
R
800
0.3
7OO
T
600
•,500
400
=00
2OO
100
I
Pre-
Post-
CWH Removal
Fisheries
ß VOL 31 No 7 ß JULY 2006
Pre-
CWH
i
Post-
removal
0.33 to 0.63. Overlapof 95% confidence crappieandrockbassdietswerecomprised
intervals for a particular size-specific of dipteranlarvae (Chaoborus
spp.) and
invertebrates
(Odonata,
growthrate (i.e., x = 100, 200, 300, 400 benthic
mm for largemouthbass)betweenbasins Trichoptera),
respectively,
throughout
the
representedno significantdifferencein study.
growthrates.Statisticaldifferences
in all
The terrestrial
component
of bassdiets
metrics were assessedat the P = 0.05 level
(pairedt-test;n = 10; df = 9; t = 1.3;P =
with a null hypothesis
of no difference 0.22) and total weightper diet (pairedt-
diets(ANOVA; n = 25; df = 1,23;F = 2.3;
between means.
Post-CWH Removal (Treatment Basin)
test;n = 10; df = 9; t = 0.34; P = 0.74) did
P = 0.14) or in consumptionrates
(ANOVA; n = 25; df = 1,23;F = 0.62; P =
0.44) in the referencebasinthroughout
the study(Figure4). Terrestrialpreycomprised17% to 19% of bassdiets in the
reference
basinfollowingthe manipulation
of the treatment basin.
The treatment basin food web switched
not differ betweenbasinsprior to the
byaquaticpreyto one
CWH removal(Figure4). Terrestrialver- fromonedominated
subsidized
by terrestrialprey
tebratesand invertebrates
madeup 5% to increasingly
FoodWebResponses
9% of bassdietsby dryweightin the refer- followingCWH removal.After the CWH
removal,perchaveraged
only 14% of the
ence basin and 9% to 12% in the
Pre-CWH Removal (Both Basins)
The food webs of both basins were
treatment basin prior to the CWH diet of bassin the treatmentbasin(Figure
3A). The terrestrialcomponentof bass
dominatedby aquaticprey prior to the removal.
diets increasedsignificantlywithin the
CWH removal.Priorto manipulation,
bass Post-CWH Removal (Reference Basin)
Little changewasobserved
in the food treatment basin (ANOVA; n = 26; df =
primarilyconsumed
perchandperchcon1,25; F = 8.6, P = 0.007) and between
sumedbenthicinvertebrates.
Yellowperch web of the referencebasinfollowingthe
basins
(pairedt-test;n = 15; df = 14; t =
averaged
93% and81% of the totaldiet of CWH removal. Perch consumption
4.5,
P
<0.001) following the CWH
bassin the referenceand treatmentbasins, decreased from 93% to 62% of the total
removal
(Figure 4). In addition, we
in 2004
respectively
(Figure3A). Perchdietsdid bassdietbydrymass,but increased
observed
a
significantdecreasein connot changeduring the study and were after an initial decrease(Figure3A). No
sumptionratebybasswithinthe treatment
changewasobserved
in the prodominatedby consumption
of trichopter- significant
basin (ANOVA; n = 26; df = 1,25; F =
ans,dipterans,
and odonatelarvae.Black portion of terrestrialprey found in bass
10.7;P = 0.003) and when comparedto
the reference
basin(pairedt-test;n = 15;df
RESULTS
Figure5. Ratioof treatmentbasin(T)largemouth
bass(Micropterus
salmoides)
size-specific
growthrateto referencebasin(R)size-specific
growthratefor 100, 200, 300, and400 mm size
classes
in LittleRockLakebefore(pre-)and after(post-)the CWH removal.
= 14; t = 2.5; P = 0.02; Figure 4).
Terrestrial vertebrates and invertebrates
comprised
51% to 55% of treatmentbasin
bassdietsby drymassfollowingthe CWH
removal.
1.30
GrowthResponses
Pre-
Growth rates of bass in the treatment
Post-
basin decreased relative to those observed
1.15
in the reference
basinfollowingtheCWH
removal.Priorto manipulation,
meansizeat-ageandsize-specific
growthratesat four
lengthsweresignificantly
higherforbassin
the treatmentbasin(Sass2004;Figure5).
After manipulation,meansizeat ageand
size-specificgrowth rates declined and
were most notable for youngerage and
-
,I I
1.00
smaller size classesof bassin the treatment
basin(Sass2004;Figure4). No changein
perch growthoccurredduringthe study
period.
0.85
-
FishCommunity
Responses
The perchpopulationof the treatment
basindeclinedrapidlyfollowingthe CWH
removal and remained at low abundances.
0.70
1 O0 200 300 400
1 O0 200 300 400
Length (mm)
326
Fisheries
In contrast,thedensityof perchin the referencebasinincreasedduringthe study
period(Figure3B). Averagedensityof the
populationin the reference
basinwas815
perch/haduringthestudy.Estimated
population densityin the treatmentbasinwas
141 perch/ha prior to CWH removal.
ß VOL 31 No 7 ß JULY 2006
ß Vl/Vl/Vl/.FISHERIES.ORG
Populationestimates
for perchcouldnot
be calculated in the treatment basin after
the CWH removal because no marked
perchwererecaptured.
Basedon catchrate
data,currentdensities
of perchhavebeen
as low as five perch/hain the treatment
basin. Adult bassdensitieswere higher
within andamongbasinsduringthe study
rangingfrom56 (95% confidence
interval;
41, 86) to 112 (85, 159) bass/hain the reference basin and from 60 (44, 82) to 82
(63, 113) bass/hain the treatment basin,
but the increases
werenot statistically
significant(P >0.05). Blackcrappieandrock
bass
densities
remained
low
and
unchanged
throughout
the study.
Young-of-year
perch recruitmentwas
minimalin the treatmentbasinfollowing
the CWH
removal. The
total catch of
dizedby terrestrialsourcesof food after sities.Mass-balance
food web modehng
CWH removal.Forexample,about50%of exercises
simulatingthe Little Rock Lake
treatmentbasinbassdietsby dry weight manipulationsuggestextirpationof the
were terrestrial vertebrates and inverteperchpopulation
andincreased
basspopubratesfollowingthe removalascompared lationbiomasses
followingCWH removal
to about10% prior to manipulation.The (Roth unpublished
data).Mechanistically,
diets of bass in the treatment basin
bassbiomassreplacesperch biomassin
reflectedoptimalforagingtenetsfollowing orderto returnthe systemto its carrying
the removal of CWH (Werner and Hall capacity(Roth unpublished
data).Similar
1974). Hodgson and Kitcheil (1987) ecosystem-scale
mass-balancemodeling
reporta similarresultwhere bassin two approaches,
suchasEcopathwith EcoSim,
lakesmaintainedhigh diet breadthwhen showcompensatory
biomassincreasesin
intra-specificcompetitionwas high, and predators
or preywhenone biomass
pool
then switchedto more profitableprey declinesandpoolsare linkedby foodweb
items(e.g., fishes,odonatenymphs)and interactions(Walterset al. 2000;Hinke et
reduceddiet breadthwhen competition al. 2004).
wasrelaxedfollowinga 50% reductionin
Patternsobserved
in bassdiet composibassdensity.Removalof CWH from the tion,perchpopulationestimates,
andYOY
treatment basin and the associated decline
YOY perch(n = 20 YOY perch/10ha) fol- in perch abundancecoincidedwith bass
lowing the CWH removal in the relianceupon terrestrialprey (e.g., frogs,
treatmentbasinindicateda potentialden- snakes,rodents,insects),benthic invertesity of 2 YOY/ha. The densityof YOY brates,and lessabundantandsmallerprey
perchin the reference
basinwasup to 16 fishessuchasYOY bass,YOY perch,and
timesgreater(n = 256 YOY perch/8ha) centralmudminnow.
The changein dietto
duringthe sameperiod.
lessabundantand lessenergetically
favorable
prey
foreshadowed
the
observed
DISCUSSION
declinesin largemouthbassgrowthrates
Changesin the dietsof treatmentbasin andwasconsistentwith trendsreportedby
bassfollowingthe CWH removal were Schindler et al. (2000). Cannibalism in
only from bassin
causedby rapidreductions
in perchabun- this studywasobserved
dance due to intensifiedbasspredation, the treatment basin following CWH
and perhapsfrom lossof woodysubstrate removal.Similarly,basscannibalismconof the diet
for benthic invertebrate production tributedto a majorproportion
(Angermeierand Karr 1984). The slight in upperMichiganlakeswith no lakeshore
high densitiesof
reductionin perchconsumption
by refer- residentialdevelopment,
ence basin bass following the CWH basswith poorgrowthrates,andlowalterremovallikely represents
a cyclic,stable native preyfish availability(Schindleret
predator-prey
dynamicbetweenbassand al. 1997).
Depressed
growthratesand increased
perch(Hinke2001).Perchpopulations
are
generallydominatedby a strongcohort incidenceof cannibalismmay result in
that isreducedin numberovertime bypre- stuntingof a basspopulation(Schindleret
dation
until
a
compensatory al. 1997; Postet al. 1998; Post 2003) and
stock-recruitmentresponseoccurs and also createsthe potential for the perch
another strong year class is produced. populationto recoverif cyclicpredatorof
Declinesin perchconsumption
by refer- preyinteractionsoccurasa consequence
encebasinbassimmediately
followingthe compromisedbass recruitment (Hinke
a lowprobability
CWH removallikelyrepresent
the preda- 2001). Our studysuggests
adultbassdensitor-induced perch decline and their of perchrecoverybecause
throughoutthe study(proxy
decreased
overallavailability.In contrast tiesincreased
to the treatmentbasin,perchconsumption for sufficient bassrecruitment), the inciby referencebasin basshas increased dence of cannibalism observedwas low,
towardpre-manipulation
levelscoincident and cyclic predator-preydynamicsare
with several strong, consecutive year often habitat-mediated (Hinke 2001).
classesof perch and their subsequent More likely, perch population biomass
declinesfrom basspredationand subseincreased
availability.
Althoughbothbasins
havesimilarmor- quent energy transfer to the bass
phometry and therefore, similar populationin the treatmentbasinhave
availability
of terrestrial
prey,thefoodweb resultedin improvedbassrecruitment,as
of the treatmentbasinwas largelysubsi- suggested
bythe increase
in adultbassdenFisheries
ß vol 31 •ao 7 ß suLY 2006
perchcatch ratesin the treatmentbasin
evidencea rapidand persistent
declinein
the perchpopulation
followingthe CWH
removal.Although perch abundancein
each basin was variable and both were
declining, but persistent,prior to the
CWH
removal (Swenson 2002; Sass
2004), the referencesystemdemonstrated
an oppositeresponsethrough compensatoryrecruitmentand the productionof
severalcohortsof perch.PerchuseCWH
asa spawningsubstrate,
foragingsite,and
as a refugefrom predators.
Therefore,the
removalof CWH imposedan increasein
predationmortality,a decreasein prey
availability,
anda lossof spawning
habitat.
This combinationmayhavedecreased
the
reproductivepotential of the treatment
basinpopulationto levelsat or belowthe
replacement
ratedueto the additiveeffects
of depensatorymechanismsand could
causethe populationto collapse(Postet
al. 2002;Carpenter2003). The treatment
basinpopulationmayhave an extremely
low probabilityof recoveryand couldbe
vulnerableto extirpationasa consequence
of: (1) low abundanceof spawningsubstrate,(2) few or no adult spawners,
(3)
continuedpredationpressureby basson
the fewremainingadultperch,(4) intense
predationby basson anyYOY perchproduced,and (5) extremelyslowinputrates
of natural CWH.
Much the same set of
constraintswould be imaginedfor other
preyfishspecies
and,overtime,mightproduce a less diverse fish community.
Openingthe lake to fisheryexploitation
mightreversethat trend,but the interactions of habitat changeand exploitation
effectshave not been evaluatedexperimentally.
Representative
photographsof pristine
undevelopedlakeshores
on northern
Wisconsinlakes.Bryozoansand
bluegill(Leporn•s
rnacrochirus)
use
submergedCWH.
Our studysuggests
that CWH mayplay
a similar,butalsopotentiallydifferent,role
asaquaticmacrophytes
and otherformsof
structure(e.g.,rockyshorelines)
in lakes.
High densitiesof simulatedand natural
aquaticmacrophytes
decrease
the foraging
success
of predators(Savino and Stein
1982;Gotceitasand Colgan 1989, 1990).
The interstitialspaces
and structuralcomplexity providedby high abundances
of
littoralCWH likelyplaya similarrole in
decreasing
foragingsuccess
of predators,
as
evidenced
bythe rapiddeclineof the perch
populationfollowingthe CWH removalin
thisstudy.In contrastto ourstudy,cutting
channelsthroughdensebedsof the invasive macrophyteEurasianwater milloil
(Myriophyllumspicatum)has elevated
growth rates of largemouth bass and
bluegill(Olsonet al. 1998). In this case,
cutting channels increasedhabitat by
increasingthe length of the weedline.
While low levelsof CWH (Schindleret al.
residential
development
pressures
in a sim- communitystructure
in a numberof northilar fashion as CWH
(Radomskl and
Goeman 2001; Jennings et al. 2003).
ern Wisconsin lakes.
MANAGEMENT
Establishmentof exotic rusty crayfish
IMPLICATIONS
(Orconectes
rusticus)in many northern
Wisconsin lakes and their negative
The manipulationof Little Rock Lake
impacts on aquatic macrophyte abundances also reduce available structure for
fishes(Wilson 2002). However,in contrast
to the high regenerativecapabilitiesof
aquaticmacrophytes
(Olson et al. 1998;
Wilson 2002), natural replacementand
degradationrates of CWH in northern
lakes are very slow (Guyette and Cole
changedCWH abundances
from numbers
commensuratewith other undeveloped
lakes in northern Wisconsin to those with
modestor intermediatelakeshore
housing
densities (Christensen et
al.
1996;
Marburget al. in press;Figure1). More
buildingsand peoplegenerallycorrespond
with increased
fisheryexploitation(NRC
1999).Thus,CWH losslitlayhavegreater 1992), lower fish population densities
and longer-termefi•cts on fish popula- (Swenson2002), and greaterfish growth
tions. Solely,or in concert,CWH and ratesin responseto reducedcompetition
aquaticmacrophyte
removalsmayresultin (Goedde and Coble 1981). Instead, we
fishspecies
diversitylosses
and depressedspeculatethat fisheryexploitationinterfish growth rates. Indeed, Tonn and acts with removal of CWH to create a
Magnuson(1982) found that predator- changein ecosystem
statewherefishpopprey interactionsand structuralhabitats ulationsexhibitthe paradoxof bothlower
werecriticalvariablesin determiningfish populationdensitiesand reducedindivid-
2000; Sass2004) and high densitiesof
aquaticmacrophytes
(Olson et al. 1998)
mayresukin depressed
fishgrowthrates,
intermediatelevelsof structuremay provide the highestfish growthratesbecause
predators
areableto foragesufficiently,
but
arenot capableof annihilatingpreypopulations (Crowder and Cooper 1982).
Because
CWH cannotprovidethe impenetrablecoverof certainmacrophyte
species
(e.g., Eurasianwater railfoil), CWH loss
mayhavegreaterimpactson fishpopulationsthan macrophyteloss(Schindleret
al. 2000; Sass2004).
While
some
lakes
in
northern
Wisconsinare known for high floristic
quality,aquaticmacrophyteabundances
are alsobeingcompromised
by lakeshore Bnan
328
Roth removes CWH from the treatment
Fisheries
basin of L•ttle Rock Lake
' VOL 31 NO 7 ß JULY 2006
ß WWW.FISHERIES.ORG
ual growthrates(Roth unpublished
data).
Although such patterns have been
observed
forbluegillpopulations
(Ehlinger
1997; Schindleret al. 2000), in general,
ourresultssuggest
that fishproduction
had
substantially
declined.
In small, oligotrophiclakessuch as
thosein northernWisconsin,benthicpri-
the extent of shoreline development REFERENCES
and/or the amount of trees or CWH
removedfrom the riparian and littoral
zones of the lake, respectively.
Alternatively,undeveloped
shorelinecan
bepromotedasan ecological
reserve
essen-
Angermeier,P. L., and J. R. Karr. 1984.
Relationshipbetweenwoodydebrisand
fish habitat in a small warmwater stream.
Transactions of the American Fisheries
Society113:716-726.
Beechie,T. J., madT. H. Sible¾.1997.
websthat supportimportantrecreational
Relationshipsbetweenchannel characmaryproductionandexogenous
sources
of fisheriesand/or CWH can be addedback
teristics,wooddebris,and fishhabitatin
carbon fuel aquatic food webs to create littoral habitat. An ongoing
northwestern Washington streams.
(Vadeboncoeurand Lodge2000; Pace et whole-lakestudy,whichaddedtreesto the
Transactions of the American Fisheries
al. 2004}. Our experimentalremovalof littoral zone of a lake with low amounts of
Society126:217-229.
CWH showsthat this aspectof human CWH to detennine if CWH loss was
Carlander,K. D. 1982.Standardintercepts
developmenthasrapidand strongeffects reversible,will shedlight on the latter asa
for calculatinglengthsfrom scalemeaon the food webs and fish communities of
viable managementoption (Sassunpubsurements for some centrarchid mad
lakes.Removalof CWH may result in lished data}. Clearly, there are strong
percid fishes. Transactions of the
decreasedbenthic invertebrateproduc- trade-offs
betweenlandscaped
lawnswith
AmericanFisheries
Society111:332-336.
tion, a reduction or collapse of prey clean sand beaches and the natural littoral Carpenter,S. R. 2003.Regimeshiftsin lake
populationsthat depend on CWH for zonehabitatsthat supportdesirablefishecosystems:pattern and variation.
refuge and spawning substrate, and eries. In both cases, education and
International
Ecology Institute,
depressed
fishgrowthratesandproduction. outreach are essential as we learn more
Oldendorf/Luhe,
Germany.
The inverse correlation of lakeshore develabout the ecologicalbenefitsof leaving Carpenter, S. R., P. Cunningham, S.
opmentand fish growth(Schindleret al.
Gafn¾, A. Munoz del Rio, N.
tial to the maintenance of desirable food
logsin thelakes.
•
2000)maybebestexplained
bystrongbottom-upeffectsof decreasing
prinmryand ACKNOWLEDGEMENTS
secondaryproductivity and strong topdown effectsby predatorsdepletingprey
We thank Jeff Biermann, Andrea
Fowler,
Jon Hansen, Adam Kautza,
fish resources when CWH is relnoved.
Michelle Nault, Steve Reinhardt, Scott
While winterkilleventsandpredationare
key factorsstructuring
fish communities, Van Egeren, J. J. Weis, and Michele
Nibbelink, M. Olson, T. Pellet, C.
Storlie,and A. Trebitz.1995.Responses
of bluegill to habitat manipulations:
powerto detecteffects.North American
Journalof Fisheries
Management15:519527.
Christensen,D. L., B. R. Herwig, D. E.
Woodford for field assistance. We are
adequaterefugemaydepress
predatorforSchindler,and S. R. Carpenter. 1996.
agingsuccess
andmediatethe coexistence indebtedto all (n -30) whohelpedwith
hnpactsof lakeshoredevelopmenton
of predatorand preypopulationsin lakes the CWH removal and in particular,
coarsewoodydebrisin north temperate
appreciatethe hardworkof Matt Helmus,
(Tonn and Magnuson1982).
lakes. EcologicalApplications6:1143Motomi "Genkai"Kato, DanaonKrueger,
CWH can be removed from lakes and
1149.
shorelinesin a few monthsor yearsas BradRay,andBrianRoth.We owespecial Crowder,L. B., madW. E. Cooper. 1982.
shorelinedevelopment
graduallyproceeds, thanksto the WisconsinDepartmentof
Habitat structuralcomplexityand the
for approving
thisprobut natural replacementtakes centuries NaturalResources
interactionbetweenbluegillsand their
(GuyetteandCole 1999).Asdevelopment ject and are particularlygratefulto Jeff
prey.Ecology63:1802-1813.
proceeds,CWH declinesover time and Bode,SteveGilbert, andJayneWade for Ehlinger, T. J. 1997. Male reproductive
wasfundedby
predatorpopulations
maydeclineto abun- their support.This research
competition
andsex-specific
growthpata
National
Science
Foundation
Integrated
dances that can be supportedby the
ternsin bluegill.NorthAmericanJournal
Education
and
Research
reducedprey populations.Thus, CWH Graduate
of Fisheries
Management
17:508-515.
removalmayhaveextremelylonglasting Traineeship(IGERT) awardedto G. G. Goedde, L. E., and D. W. Coble. 1981.
or even permanentconsequences
for fish Sassand an NSF biocomplexitygrant
Effectsof magling
on a previously
fished
and an unfished wamawater fish commupopulations,
fisheries,
and the food webs (DEB-0083 545) awarded to S.R.
that supportthem.
Carpenter.Equipmentwas providedby
nity in twoWisconsin
lakes.Transactions
Managementpoliciescan respondto the Anna Grant BirgeMemorialfund to
of the American FisheriesSociety
110:594-603.
thisreality.Limitationscan be placedon G. G. Sass.
Fisheries
ß VOL 31 NO 7 ß JUrY 2006
ß WWW.FISklERiE$.OR{}
329
Gotceitas,V., andP. Colgan. 1989.Predator Keim, R. F., A. E. Skaugset,and D. S.
mouthbassand bluegillsasinfluenceby
Bateman.2002.Physicalaquatichabitat
foragingsuccess
and habitatcomplexity:
simulated, submersed vegetation.
Transactions of the American Fisheries
II. Poolsand cover affectedby large
quantitativetestof the thresholdhypothwoodydebrisin three westernOregon
esis.Oecologia80:158-166.
Society111:255-266.
streams.North American Journal of Scheuerell, M.D., and D. E. Schindler.
__.
1990.The effectsof preyavailability
FisheriesManagement22:151-164.
andpredationriskon habitatselectionby
2004.Changesin the spatialdistribution
juvenile bluegill sunfish. Copeia Marburg, A. E., M. G. Turner, and T. K.
of fishesin lakes along a residential
1990:409-417.
Kratz. 2006. Natural and anthropogenic
development
gradient.Ecosystems
7:98106.
variation in coarse wood among and
Guyette,R. P., and W. G. Cole. 1999.Age
within lakes.Journalof Ecology:
in press. Schindler,D. E., J. IL Hodgson,and J. F.
characteristics
of coarsewoody debris
Research
Council.
1992.
(Pinusserobus)in a lake littoral zone. National
Kitcheil. 1997. Density-dependent
Restoration of aquatic ecosystems.
Canadian Journal of Fisheries and
changes
in individualforaging
specializaNational Academy Press,Washington,
AquaticSciences56:496-505.
tion of largemouth bass. Oecologia
Hanson, J. M., and W. C. Leggett. 1982.
Empiricalpredictionof fish biomass
and
DC.
110:592-600.
Olson, M. H., S. R. Carpenter, P. Schindler, D. E., S. I. Geib, and M. R.
Cunningham,S. Gafny, B. R. Herwig,
yield.CanadianJournalof Fisheries
and
Williams. 2000. Patternsof fish growth
N. P. Nibbelink, T. Pe!!ett, C. Storlie,
AquaticSciences39:257-263.
alonga residential
development
gradient
A. S. Trebitz, and K. A. Wilson. 1998.
Hilton, J., J.P. Lishman,and P. V. Allen.
in north temperatelakes. Ecosystems
Managing aquatic macrophytes to
1986. The dominant mechanisms of sed3:229-237.
improvefishgrowth:a multi-lakeexperi- Swenson, W. A. 2002. Demographic
iment distribution and focusingin a
ment. Fisheries23(2):6-12.
small, eutrophic, monomictic lake.
changesin a largemouthbasspopulation
Limnologyand Oceanography
31:125- Pace,M. L, J. J. Cole, S. R. Carpenter,J.
followingclosureof the fishery.Pages
F. Kitcheil,J. R. Hodgson,M. C. Van de
133.
627-637 in D. P. Philipp and M. S.
Bogert,D. L Bade,E. S. Kritzberg,and
Hinke, J. T. 2001. Trophicinteractionsof
Ridgway,
eds.Blackbass:
ecology,
conserD. Bastviken. 2004. Whole-lake carbonjuvenileyellowperch(Percaflavescens)
vation, and management. American
13 additionsrevealterrestrialsupportof
and young-of-yearlargemouth bass
Fisheries
Society,Bethesda,
Maryland.
aquaticfoodwebs.Nature427:240-243.
(Micropterus
salmoides):
the influencesof
Tonn, W. M., and J. J. Magnuson.1982.
Post, D. M. 2003. Individual variation in
density,size,and growth.Master'sthesis,
Patternsin species
compositionand richthe timingof ontogeneticnicheshiftsin
Universityof Wisconsin Madison.
ness of fish assemblages
in northern
largemouth
bass.Ecology84:1298-1310.
Hinke, J. T., I. C. Kaplan,K. Aydin, G. M.
Wisconsin
lakes.Ecology63:1149-1166.
Post, D. M., J. F. Kitcheil, and J. IL
Watters,R. J. Olson, andJ. F. Kitcheil.
Vadeboncoeur,
Y., andD. M. Lodge.2000.
Hodgson. 1998. Interactions among
2004. Visualizingthe food-webeffectsof
Periphytonproductionon woodand sedadultdemography,
spawning
date,growth
fishingfor tunas in the PacificOcean.
iment: substratum-specific
responseto
rate, predation,overwinteringmortality,
Ecology
andSociety9(1).
laboratory and whole-lake nutrient
and the recruitmentof largemouthbass
Hjelm, J., L. Petsson,and B. Christensen.
manipulations.Journal of the North
in a northernlake. CanadianJournalof
2000. Growth, morphological
variation
American
Benthological
Society19:68Fisheries
and AquaticSciences55:258881.
and ontogeneticniche shifts in perch
2600.
(Percafiuviatilis)in relation to resource Post, J. R., M. Sullivan, S. P. Cox, N. P. Vander Zanden, M.
J., and Y.
availability.
Oecologia
122:190-199.
Vadeboncoeur.
2002.
Fishes
as integraLester, C. J. Walters, F. A. Parkinson,
Hodgson,J. R., and J. F. Kitcheil. 1987.
tors
of
benthic
and
pelagic
food
websin
A. J. Paul, L. Jackson,andB. J. Shuter.
Opportunisticforagingby largemouth
lakes.Ecology83:2152-2161.
2002. Canada's recreational fisheries: the
bass(Micropterus
salmoides).
American
Walters, C. J., D. Pauly, V. Christensen,
invisiblecollapse
?Fisheries
27( 1):6-17.
Midland Naturalist 118:323-336.
and J. F. Kitcheil. 2000. Representing
Radomski, P., and T. Geoman. 2001.
Hrabik, T. R., and C. J. Watras. 2002.
densitydependentconsequences
of life
Consequences
of humanlakeshore
develRecentdeclinesin mercuryconcentrahistory
strategies
in
aquatic
ecosystems:
opment on emergentand floating-leaf
tion in a freshwater
fishery:isolatingthe
EcoSimII. Ecosystems
3:70-83.
vegetationabundance.North American
effects of de-acidification and decreased
Journalof FisheriesManagement21:46- Walters, C. J., and J. F. Kitcheil. 2001.
atmospheric
mercurydeposition
in Little
Cultivation/depensation
effectson juve61.
Rock
Lake.
Science
of
the
Total
Environment 297:229-237.
Hunt, J., andC. A. Annett. 2002. Effectsof
Ricker, W. E. 1975.Computationand interpretationof biologicalstatisticsof fish
populations.FisheriesResearchBoardof
nile
survival
and
recruitment:
implicationsfor the theory of fishing.
Canadian Journal of Fisheries and
AquaticSciences58:39-50.
habitat manipulationon reproductive
Canada Bulletin 191.
success
of individuallargemouth
bassin Sampson,C. J. 1999. Aquaticchemistryof Werner, E. E., and D. J. Hall. 1974.
an Ozark reservoir. North American
Optimalforaging
andthesizeselection
of
LittleRockLake,Wisconsin,
duringacidJournal of Fisheries Management
prey
by
the
bluegill
sunfish
(Lepomis
ification and recovery. Ph.D thesis,
22:1201-1208.
macrochirus).
Ecology55:1042-1052.
Universityof Minnesota--TwinCities.
Jennings,M. J., E. E. Emmons, G. R. Sass,G. G. 2004.Fishcommunity
andfood Wilson, K. A. 2002. Impactsof the invasive
Hatzenbeler, C. Edwards, and M. A.
rusty crayfish(Orconectes
rusticus)in
webresponses
to a whole-lake
removalof
Bozek. 2003. Is littoral habitat affected
northern Wisconsinlakes. Ph.D. thesis,
coarse woody habitat. Ph.D. thesis,
by residentialdevelopmentand landuse
Universityof Wisconsin--Madison.
Universityof Wisconsin--Madison.
in watersheds of Wisconsin lakes? Lake
Savino, J. E, and R. A. Stein. 1982. Zar,J. H. 1996.Biostatistical
analysis,
third
andReservoirManagement19:272-279.
Predator-prey
interactionbetweenlargeedition.PrenticeHall, NewJersey.
330
Fisheries
' VOL 31 NO 7 ø JULY 2006
ø WWW.FISHERIES.ORG