Kara Phillips , Jeremy Eskelsen , Ursula Mazur , K. W. Hipps
advertisement

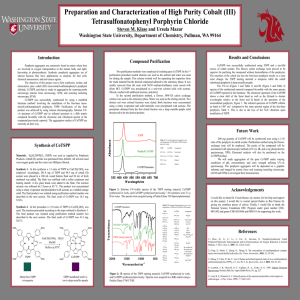
Kara Phillips1, Jeremy Eskelsen2, Ursula Mazur2, K. W. Hipps2 NSF REU Characterization of Advanced Materials at WSU 1School of Materials Science and Engineering, Clemson University 2School of Chemistry and Materials Science and Engineering, Washington State University RESULTS INTRODUCTION Porphyrins are well known for their high absorption in the visible region making them prime materials for many applications, such as: •catalysts •photoconductors •electrical conductors •sensors •light harvesting[1] FIGURE 1: UV-visible spectra of TSPP, TAPP, and TAPP+TSPP mixture showing the formation of an H-aggregate in the mixture [2]. FIGURE 2: UV vis. for differing ratios of TAPP+TSPP filtered after 20 minutes with little absorption for 1:1 suggesting a reaction ratio of around 1:1. EXPERIMENTAL FIGURE 8: TEM image of TAPP+TSPP mixed at room temperature (20 ⁰C). Structures are around 12.628 nm wide and 47.75 nm long. TAPP+TSPP Cross Section 100 ⁰C 60 TAPP+TSPP Cross Section 50 ⁰C 25 20 Height (nm) 80 Height (nm) Samples were made from stock solution then diluted to desired micromolar concentrations. Solutions were acidified with HCl to pH 3. The mixture was made by first pouring TAPP [meso-tetra (4aminophenyl) porphine] then TSPP [mesotetra (4-sulfonatophenyl) porphine] each time. UV-Visible Spectroscopy •Concentration and time dependent studies were evaluated. •Perkin Elmer 330 spectrophotometer was used. Microscopy •Atomic Force Microscopy • Samples were deposited from the bottom of solution on peeled mica then let set for one minute and spun for 30 seconds (process was repeated 5 times). • Images taken on Digital Instruments AFM in tapping mode. •Transmission Electron Microscopy • Samples were deposited on formvar coated Nickel TEM grids. • Images were taken on Phillips CM200 TEM at 200 keV. FIGURE 3: UV-vis. time study of TAPP+TSPP mixture suggesting that the reaction reaches completion after one day because there is little change in the absorption peaks after that time. 15 40 10 20 0 0 100 200 Width (nm) 300 400 FIGURE 4: AFM image of TAPP+TSPP structures formed at 100 ⁰C with cross section showing an average height of 89 nm and width of 159 nm. 5 Temperature (⁰C) Height (nm) Width (nm) Length (nm) 0 --- 7.68 28.82 20 --- 12.628 47.75 50 21 46 414.5 100 89 159 >5000 TABLE 1: Average height, width, and length of TAPP+TSPP nanorods formed at different temperatures. Measurements for 50 and 100⁰ samples were taken from AFM images. Data for 0 and 20⁰ samples was taken from TEM images. 0 0 100 200 Width (nm) 300 FIGURE 5: AFM image of TAPP+TSPP structures formed at 50 ⁰C with cross section showing an average height of 21 nm, width of 46 nm, and length of 414.5 nm. TSPP TAPP CONCLUSIONS & FUTURE WORK •At pH 3, the TAPP and TSPP compounds react to form small rod-like structures. •This reaction is completed in one day and occurs in approximately a 1:1 ratio (elemental analysis will be completed in the future to confirm). •The size of the rods changes significantly with reaction temperature. • Lower temperatures result in more nucleation sites showing large numbers of smaller rods. • Higher temperatures result in more growth showing larger rods. • Future studies will concentrate on the conductive and structural properties of the self assembled nanostructures. ACKNOWLEDGEMENTS FIGURE 6: AFM image of TAPP+TSPP structures formed at 20 ⁰C. FIGURE 7: AFM image of TAPP+TSPP structures formed at 0 ⁰C. [1] Friesen, Benjamin, Bryan Wiggins, Jeanne L. McHale, Ursula Mazur, and K. W. Hipps. "Differing HOMO and LUMO Mediated Conduction in a Porphyrin Nanorod." Journal of the American Chemical Society 132.25 (2010): 8554-556. Web. [2] Eisfeld, A., and J. S. Briggs. "The J- and H-Bands of Organic Dye Aggregates." Chemical Physics 324.2–3 (2006): 376-84. Web. The funding for this research was provided by the National Science Foundation grants CHE1152951 and REU Program DMR-1062898 as well as the Department of Chemistry and Materials Science and Engineering at WSU.