Calibration and Optimization of PTR-MS for Measurement of Methyl Hydroperoxide (CH OOH)
advertisement
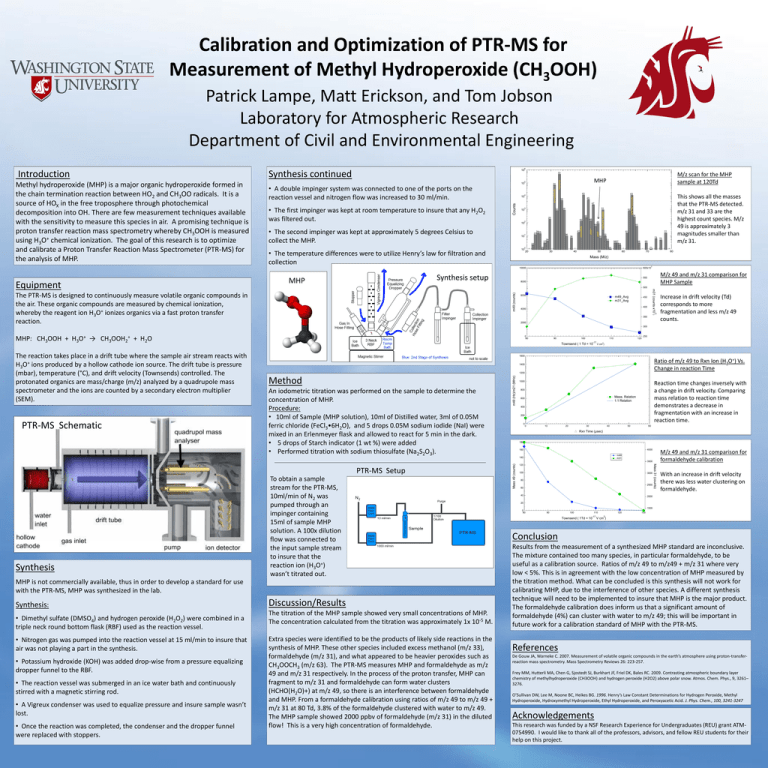
Calibration and Optimization of PTR-MS for Measurement of Methyl Hydroperoxide (CH3OOH) Patrick Lampe, Matt Erickson, and Tom Jobson Laboratory for Atmospheric Research Department of Civil and Environmental Engineering Synthesis continued 32 63 45 34 47 51 35 44 29 46 42 2 49 48 10 36 10 38 64 65 61 MHP 1 41 2425 23 NO2+ • The second impinger was kept at approximately 5 degrees Celsius to collect the MHP. 62 50 73 75 53 5657 58 22 0 • The temperature differences were to utilize Henry’s law for filtration and collection 77 52 28 27 10 26 66 69 54 20 30 40 71 50 60 70 Equipment 80 Mass (M/z) 3 10000 Synthesis setup MHP This shows all the masses that the PTR-MS detected. m/z 31 and 33 are the highest count species. M/z 49 is approximately 3 magnitudes smaller than m/z 31. potential peroxide 3 10 43 acetonitrile • The first impinger was kept at room temperature to insure that any H2O2 was filtered out. 4 10 MHP methanol hydrate • A double impinger system was connected to one of the ports on the reaction vessel and nitrogen flow was increased to 30 ml/min. 31 methanol 5 10 formaldehyde Methyl hydroperoxide (MHP) is a major organic hydroperoxide formed in the chain termination reaction between HO2 and CH3OO radicals. It is a source of HOX in the free troposphere through photochemical decomposition into OH. There are few measurement techniques available with the sensitivity to measure this species in air. A promising technique is proton transfer reaction mass spectrometry whereby CH3OOH is measured using H3O+ chemical ionization. The goal of this research is to optimize and calibrate a Proton Transfer Reaction Mass Spectrometer (PTR-MS) for the analysis of MHP. M/z scan for the MHP sample at 120Td 33 Counts Introduction 6 10 600x10 M/z 49 and m/z 31 comparison for MHP Sample 550 8000 500 m49 (counts) 6000 m49_Avg m31_Avg m31 (counts x10 The PTR-MS is designed to continuously measure volatile organic compounds in the air. These organic compounds are measured by chemical ionization, whereby the reagent ion H3O+ ionizes organics via a fast proton transfer reaction. 450 400 4000 3 ) 350 2000 Increase in drift velocity (Td) corresponds to more fragmentation and less m/z 49 counts. 300 → CH3OOH2+ 0 80 + H2O The reaction takes place in a drift tube where the sample air stream reacts with H3O+ ions produced by a hollow cathode ion source. The drift tube is pressure (mbar), temperature (°C), and drift velocity (Townsends) controlled. The protonated organics are mass/charge (m/z) analyzed by a quadrupole mass spectrometer and the ions are counted by a secondary electron multiplier (SEM). PTR-MS Schematic 90 100 110 Townsend ( 1 Td = 10 -17 120 250 2 V cm ) 1600 Ratio of m/z 49 to Rxn Ion (H3O+) Vs. Change in reaction Time 1400 1200 Method An iodometric titration was performed on the sample to determine the concentration of MHP. Procedure: • 10ml of Sample (MHP solution), 10ml of Distilled water, 3ml of 0.05M ferric chloride (FeCl3•6H2O), and 5 drops 0.05M sodium iodide (NaI) were mixed in an Erlenmeyer flask and allowed to react for 5 min in the dark. • 5 drops of Starch indicator (1 wt %) were added • Performed titration with sodium thiosulfate (Na2S2O3). m49 (Hz)/m21 (MHz) MHP: CH3OOH + H3O+ 1000 Reaction time changes inversely with a change in drift velocity. Comparing mass relation to reaction time demonstrates a decrease in fragmentation with an increase in reaction time. 800 Meas. Relation 1:1 Relation 600 400 200 0 0 10 20 30 40 50 60 Rxn Time (µsec) 180 4000 160 M/z 49 and m/z 31 comparison for formaldehyde calibration m49 m31 140 Synthesis To obtain a sample stream for the PTR-MS, 10ml/min of N2 was pumped through an impinger containing 15ml of sample MHP solution. A 100x dilution flow was connected to the input sample stream to insure that the reaction ion (H3O+) wasn’t titrated out. MHP is not commercially available, thus in order to develop a standard for use with the PTR-MS, MHP was synthesized in the lab. Synthesis: • Dimethyl sulfate (DMSO4) and hydrogen peroxide (H2O2) were combined in a triple neck round bottom flask (RBF) used as the reaction vessel. • Nitrogen gas was pumped into the reaction vessel at 15 ml/min to insure that air was not playing a part in the synthesis. • Potassium hydroxide (KOH) was added drop-wise from a pressure equalizing dropper funnel to the RBF. • The reaction vessel was submerged in an ice water bath and continuously stirred with a magnetic stirring rod. • A Vigreux condenser was used to equalize pressure and insure sample wasn’t lost. • Once the reaction was completed, the condenser and the dropper funnel were replaced with stoppers. Discussion/Results The titration of the MHP sample showed very small concentrations of MHP. The concentration calculated from the titration was approximately 1x 10-5 M. Extra species were identified to be the products of likely side reactions in the synthesis of MHP. These other species included excess methanol (m/z 33), formaldehyde (m/z 31), and what appeared to be heavier peroxides such as CH3OOCH3 (m/z 63). The PTR-MS measures MHP and formaldehyde as m/z 49 and m/z 31 respectively. In the process of the proton transfer, MHP can fragment to m/z 31 and formaldehyde can form water clusters (HCHO(H2O)+) at m/z 49, so there is an interference between formaldehyde and MHP. From a formaldehyde calibration using ratios of m/z 49 to m/z 49 + m/z 31 at 80 Td, 3.8% of the formaldehyde clustered with water to m/z 49. The MHP sample showed 2000 ppbv of formaldehyde (m/z 31) in the diluted flow! This is a very high concentration of formaldehyde. 120 100 3000 80 2500 60 40 Mass 31 (counts) PTR-MS Setup Mass 49 (counts) 3500 With an increase in drift velocity there was less water clustering on formaldehyde. 2000 20 1500 0 80 90 100 Townsend ( 1Td = 10 110 -17 120 130 2 V cm ) Conclusion Results from the measurement of a synthesized MHP standard are inconclusive. The mixture contained too many species, in particular formaldehyde, to be useful as a calibration source. Ratios of m/z 49 to m/z49 + m/z 31 where very low < 5%. This is in agreement with the low concentration of MHP measured by the titration method. What can be concluded is this synthesis will not work for calibrating MHP, due to the interference of other species. A different synthesis technique will need to be implemented to insure that MHP is the major product. The formaldehyde calibration does inform us that a significant amount of formaldehyde (4%) can cluster with water to m/z 49; this will be important in future work for a calibration standard of MHP with the PTR-MS. References De Gouw JA, Warneke C. 2007. Measurement of volatile organic compounds in the earth’s atmosphere using proton-transferreaction mass spectrometry. Mass Spectrometry Reviews 26: 223-257. Frey MM, Hutterli MA, Chen G, Sjostedt SJ, Burkhart JF, Friel DK, Bales RC. 2009. Contrasting atmospheric boundary layer chemistry of methylhydroperoxide (CH3OOH) and hydrogen peroxide (H2O2) above polar snow. Atmos. Chem. Phys., 9, 3261– 3276. O’Sullivan DW, Lee M, Noone BC, Heikes BG. 1996. Henry’s Law Constant Determinations for Hydrogen Peroxide, Methyl Hydroperoxide, Hydroxymethyl Hydroperoxide, Ethyl Hydroperoxide, and Peroxyacetic Acid. J. Phys. Chem., 100, 3241-3247 Acknowledgements This research was funded by a NSF Research Experience for Undergraduates (REU) grant ATM0754990. I would like to thank all of the professors, advisors, and fellow REU students for their help on this project.