Monday, 24 February 2014
advertisement
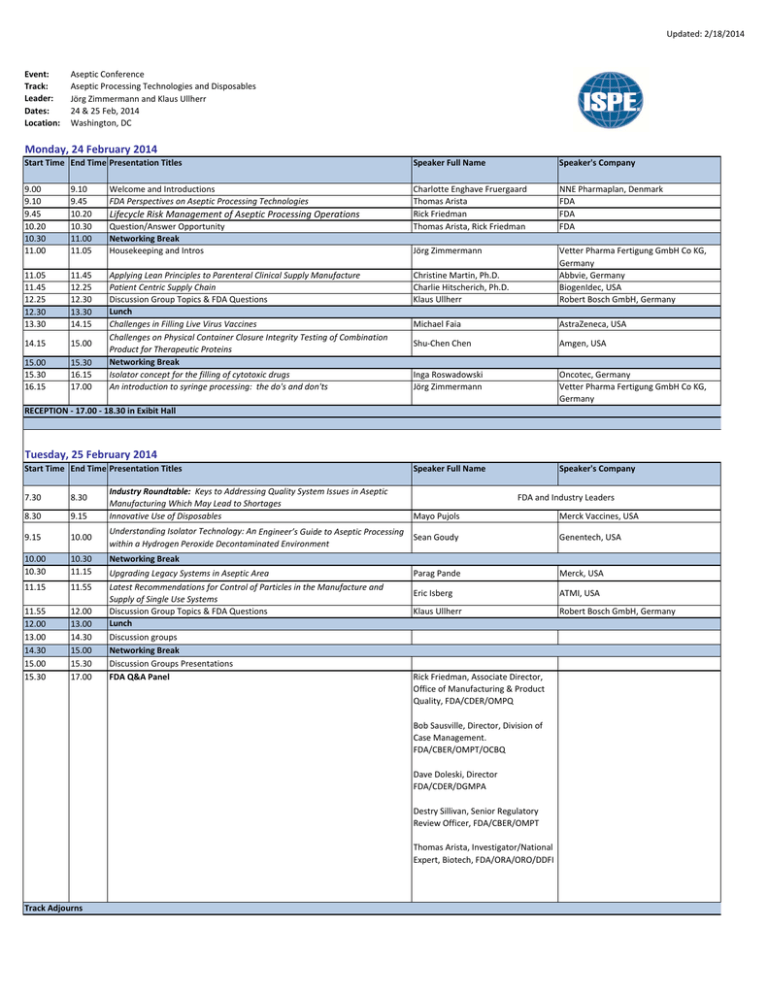
Updated: 2/18/2014 Event: Track: Leader: Dates: Location: Aseptic Conference Aseptic Processing Technologies and Disposables Jörg Zimmermann and Klaus Ullherr 24 & 25 Feb, 2014 Washington, DC Monday, 24 February 2014 Start Time End Time Presentation Titles Speaker Full Name Speaker's Company 9.00 9.10 9.45 10.20 10.30 11.00 9.10 9.45 10.20 10.30 11.00 11.05 Welcome and Introductions FDA Perspectives on Aseptic Processing Technologies Charlotte Enghave Fruergaard Thomas Arista Rick Friedman Thomas Arista, Rick Friedman NNE Pharmaplan, Denmark FDA FDA FDA Jörg Zimmermann 11.05 11.45 12.25 12.30 13.30 11.45 12.25 12.30 13.30 14.15 Christine Martin, Ph.D. Charlie Hitscherich, Ph.D. Klaus Ullherr Michael Faia AstraZeneca, USA 14.15 15.00 Shu‐Chen Chen Amgen, USA 15.00 15.30 16.15 15.30 16.15 17.00 Applying Lean Principles to Parenteral Clinical Supply Manufacture Patient Centric Supply Chain Discussion Group Topics & FDA Questions Lunch Challenges in Filling Live Virus Vaccines Challenges on Physical Container Closure Integrity Testing of Combination Product for Therapeutic Proteins Networking Break Isolator concept for the filling of cytotoxic drugs An introduction to syringe processing: the do's and don'ts Vetter Pharma Fertigung GmbH Co KG, Germany Abbvie, Germany BiogenIdec, USA Robert Bosch GmbH, Germany Inga Roswadowski Jörg Zimmermann Oncotec, Germany Vetter Pharma Fertigung GmbH Co KG, Germany Speaker Full Name Speaker's Company Lifecycle Risk Management of Aseptic Processing Operations Question/Answer Opportunity Networking Break Housekeeping and Intros RECEPTION ‐ 17.00 ‐ 18.30 in Exibit Hall Tuesday, 25 February 2014 Start Time End Time Presentation Titles 7.30 8.30 8.30 9.15 9.15 10.00 10.00 10.30 10.30 11.15 11.15 11.55 11.55 12.00 13.00 14.30 15.00 15.30 12.00 13.00 14.30 15.00 15.30 17.00 Industry Roundtable: Keys to Addressing Quality System Issues in Aseptic Manufacturing Which May Lead to Shortages Innovative Use of Disposables FDA and Industry Leaders Mayo Pujols Understanding Isolator Technology: An Engineer’s Guide to Aseptic Processing Sean Goudy within a Hydrogen Peroxide Decontaminated Environment Genentech, USA Networking Break Upgrading Legacy Systems in Aseptic Area Latest Recommendations for Control of Particles in the Manufacture and Supply of Single Use Systems Discussion Group Topics & FDA Questions Lunch Discussion groups Networking Break Discussion Groups Presentations FDA Q&A Panel Parag Pande Merck, USA Eric Isberg ATMI, USA Klaus Ullherr Robert Bosch GmbH, Germany Rick Friedman, Associate Director, Office of Manufacturing & Product Quality, FDA/CDER/OMPQ Bob Sausville, Director, Division of Case Management. FDA/CBER/OMPT/OCBQ Dave Doleski, Director FDA/CDER/DGMPA Destry Sillivan, Senior Regulatory Review Officer, FDA/CBER/OMPT Thomas Arista, Investigator/National Expert, Biotech, FDA/ORA/ORO/DDFI Track Adjourns Merck Vaccines, USA





