AP Chemistry - Notes - Chapter 12 - Kinetics
advertisement

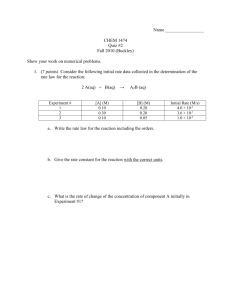
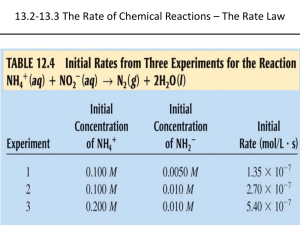
AP Chemistry - Notes - Chapter 12 - Kinetics Chapter 12 outline : Chemical kinetics Page 1 of 7 A. Chemical Kinetics - chemistry of reaction rates 1. Reaction Rates a. Reaction rate- the change in concentration of reactant or product per unit of time - always positive (absolute change per unit of time) - units are mol/L • s (or Ms-1) - the instantaneous rate (rate at a particular time) can be determined by calculating the slope of a line tangent to that point in time For the reaction A Æ B, a reaction progress curve might look like : [A] a. b. t1 Time (s) c. t2 The rate of the reaction is -∆[A] / ∆t (negative ∆[A] because A is being used up as it changes to B) - final value will be positive (absolute value) - the slope of the line tangent to point a. is the initial rate t ≈ 0 - the slope of the line tangent to point b. is the rate at time = t1 - the slope of the line from a. to c. is the average rate from t = 0 to t = t2 - the stoichiometry of the equation determines the relative rates of consumption of reactants or production of products (e.g. in the reaction 2H2O Æ 2 H2 + O2, water is used up at the same rate hydrogen is produced, per mole, and oxygen is produced at half the rate of hydrogen) Sample problems : 1. The reaction for the Haber process used for the production of ammonia is N2(g) + 3 H2(g) Æ 2 NH3(g). a. Compare the rate at which the ammonia will be produced to the rate at which nitrogen and hydrogen will be consumed. Answer : Ammonia will be produced at twice the rate at which nitrogen is consumed due to the mole ratio of N2 to NH3 in the balanced equation which equals 1:2. Ammonia will be produced at two-thirds the rate at which hydrogen is consumed due to the mole ratio of H2 to NH3 in the balanced equation which equals 3:2 b. If hydrogen gas is used up at a rate of 24.0 L/s, what is the rate at which ammonia is produced? Answer : 16.0 L/s Solution : Ammonia is produced at a 2/3 rate of hydrogen so : 2/3 x 24.0 L/s = 16.0 L/s AP Chemistry - Notes - Chapter 12 - Kinetics Page 2 of 7 2. Rate Laws : An Introduction a. Reactions are reversible, therefore the rate of the forward reaction equals the difference in the forward and reverse rates. If we examine a reaction in which only the reactants are present, the reverse reaction can be ignored. For the following reaction : 2NO2 Æ 2NO + O2, then becomes : Rate = k[NO2]n , where is the rate constant and n is the order of the reactant (n must be determined experimentally and may be an integer (including zero) or a fraction). - Care must be taken on how the rate is defined. In the reaction above is the rate determined by how much nitrogen dioxide is used up?, How much oxygen is produced? These will be different due to the mole ratio in the balanced equation. - rate is dependent on concentration - k is independent of concentration (k is temperature dependent) b. Types of rate laws : - Differential rate law - expresses how the rate depends on concentration - Integrated rate law - expresses how the concentrations of species depend on time - which rate law is used is based on the types of information obtained from a reaction or what types of information are needed - since the two types of rate laws are related in a defined way, only one of the rate laws for a reaction need to be determined -The importance of the rate law is that it reveals information about the steps in which a reaction occurs 3. Determining the Form of the Rate Law a. Determining the order of a reaction : - The order of a reactant is the power to which the concentration of the reactant must be raised to produce the amount of product indicated by the reaction. observe the following data for the reaction : aA + bB ------> cC + dD Initial [A] .02 .04 .04 Initial [B] .01 .01 .02 Initial rate(mol/L-s) .003 .012 .024 - Comparing the first two lines of data we see that [A] is doubled (.02 to .04) and the reaction rate is increased by a factor of four(.003 to .012). line 1 = line 2 .003 k(.04)x(.01)y = .04x = .012 k(.02)x(.01)y .02x , 4 = 2x , x = 2 The order of reactant A is therefore 2, or second order (22=4). If [B] is doubled the reaction rate is doubled and it is therefore a first order reactant (21=2). the overall reaction order is the sum of each of the orders of the reactants and is therefore a third order reaction. The rate law equation for this reaction can then be written as rate = k[A]2[B] - Here is another example: Determining the orders of each reactant and the overall reaction rate for the following reaction. aA + bB + cC -----> dD + eE Data : Initial [A] Initial [B] Initial [C] Initial rate(mol/L-s) .10 .06 .20 6.400 .05 .06 .20 3.200 .05 .03 .20 .800 AP Chemistry - Notes - Chapter 12 - Kinetics Page 3 of 7 .05 .03 .10 .200 Order of A is 1 : [A] is cut in half (lines 1-2) and the reaction rate is cut in half (½ )1=½ Order of B is 2 : [B] is cut in half (lines 2-3) and the reaction rate is cut to one-fourth (½)2=¼ Order of C is 2 : [C] is cut in half (lines 3-4) and the reaction rate is cut to one-fourth (½)2=¼ Overall order is 5 : equals the sum of the orders of each reactant - Writing the equation for the rate law for this reaction we get : rate = k[A][B]2[C]2 b. Calculation of k : k can be determined by rearranging the rate law to solve for k using each line of data and then averaging the values - units for k : Units of the rate constant depend on the overall order of the reaction. These are the units necessary to cancel properly and give the rate in units of Ms–1 ( mol/L • s). Overall Order of Reaction 0 1 2 3 Units of k Ms –1 (or mol/L • s) s–1 ( or 1/s) M –1s–1 (or L/mol • s) M –2s–1 ( or L2/mol2 • s) 4. The Integrated Rate Law - expresses the concentration of the reactant as a function of time a. first order reactions : for the reaction : aA Æ products, the integrated first-order rate law is : ln[A] = -kt +ln[A]0 where [A] is the concentration of A at time t, [A]0 is the initial concentration of A - this allows us to calculate [A] at any time if t and k are known - it is an equation for a straight line (y= mx + b) where the slope = -k To determine which - a plot of ln[A] vs t gives a straight line order a reactant is the - a useful derivation is ln ([A]0/[A]) = kt data has to be plotted to - half-life of a first-order reaction (t½) (half life depends on k alone) see which type of data - equation : t½ = 0.693/k gives a straight line. b. Second-order rate laws -second-order rate law : 1/[A] = kt + 1/[A]0 - a plot of 1/[A] will produce a straight line with the slope equaling k - t½=1/k[A]0 (half life depends on k and[A]0 and the length of each half life is twice the half-life before it) Sample problem : The following data were obtained for the reaction of the decomposition of nitrogen dioxide in the gas phase at 300 ºC, NO2(g) Æ NO(g) + ½O2(g) Time (s) [NO2] (M) 0.0 0.01000 50.0 0.00787 100.0 0.00649 200.0 0.00481 300.0 0.00380 Determine the rate law for this reaction and the value of k. Solution : To determine the order of NO2, we need to plot ln[NO2] and 1/[NO2] vs t to determine if it is first or second order. Calculating the values we get : AP Chemistry - Notes - Chapter 12 - Kinetics Sample problem cont'd) Time 0.0 50.0 100.0 200.0 300.0 [NO2] 0.01000 0.00787 0.00649 0.00481 0.00380 Page 4 of 7 ln[NO2] -4.610 -4.845 -5.038 -5.337 -5.573 1/[NO2] 100 127 154 208 263 Constructing the plots we would get : ln[NO2] 1/[NO2] Time (s) Time (s) Since the plot of 1/[NO2] is linear the reaction is second order with regards to [NO2] giving the rate law of Rate = k[NO2]2. Calculating the slope would give a value of 0.543. For a second order reaction k = slope and therefore k = 0.543 L/mol • s Sample problems : Half-life 1. A certain first-order reaction is 35.0% complete in 45.0 s. What are the values of the rate constant and half-life for this reaction? Solution : Use the derivation ln ([A]0/[A]) = kt to solve for k . Assume 100.0 for [A]0 , and then at 45.0 s when the reaction is 35.0% complete [A] = 65.0. ln (100.0/65.0) = k(45.0s), k = 9.57 x 10-3L/mol • s The equation for the half-life of a first-order reaction is t½ = 0.693/k Solving t½ = 0.693/9.57 x 10-3L/mol • s = 72.4 s 2. In a second-order reaction it took 112 s for 50.0% of a substance to decompose. If the initial concentration was .20 M, what is the rate constant for this reaction? Solution : For a second-order reaction t½ = 1/k[A] 0 or k = 1/t½[A]0 Solving : k = 1/(112s • .20 M) = 4.5 x 10-2 L/mol • s c. Zero order rate laws : - rate is constant and does not change with changes in concentration -zero-order rate law [A] = -kt + [A]0 - zero-order reactions usually involve a metal surface as a catalyst or an enzyme (these factors control reaction rate rather than concentration) - a plot of [A] vs t would give a straight line with the slope = -k - t½ = [A] 0 /2k AP Chemistry - Notes - Chapter 12 - Kinetics Page 5 of 7 d. Integrated Rate Laws for Reactions with More than One Reactant - can be studied one reactant at a time by keeping the reactant concentration of the reactant in question small compared to other reactants much larger concentrations can be considered constant in comparison to the small concentration of the reactant in question) 5. Summary of rate laws Summary of Kinetics for a zero, first or second order reaction of [A] in which A Æ Products Order : 0 Rate Law Integrated Rate Law Plot needed for a straight line Relationship of rate constant to the slope of straight line Half-life 1 2 Rate = k [A] = -kt + [A]0 [A] vs t Rate = k[A] ln[A] = -kt +ln[A] 0 ln[A] vs t Rate = k[A]2 1/[A] = kt + 1/[A] 0 1/[A] vs t Slope = -k Slope = -k Slope = k t½ = [A] 0 /(2k) t½ = 0.693/k t½ = 1/(k[A] 0) 6. Reaction Mechanisms - the series of steps by which a reaction occurs - the balanced equation tells us what we start and end with, the reaction mechanism tells us what's in between - the reaction mechanism must agree with the experimentally determined rate law - the sum of the elementary steps must give the overall balanced equation for the reaction a. intermediate - a species that is formed and then changed in a reaction mechanism - it is neither a reactant nor a product b. elementary step - a reaction whose rate law can be written from its molecularity (the number of species that must collide to produce the reaction indicated by that step) - unimolecular step - involves only one molecule - bimolecular step - involves two species - always second order (e.g. k[A]2 for a single reactant or k[A][B] for two reactants) - termolecular step - involves three species (rare) Elementary step A Æ products 2A Æproducts A + B Æ products 2A + B Æ products A + B + C Æ products Molecularity Unimolecular Bimolecular Bimolecular Termolecular Termolecular Rate Law Rate = k[A] Rate = k[A]2 Rate = k[A][B] Rate = k[A]2[B] Rate =k [A][B][C] Sample Problem : Write the rate laws for the following elementary reactions : a. A(g) Æ B(g) b. A(g) + B(g) Æ C(g) c. 2A + B Æ 2C(g) Solution : In elementary reactions the rate law can be determined using the coefficients in the balanced equation as the order of each reactant. a. Rate = k[A] b. Rate = k[A][B] c. rate = k[A]2[B] AP Chemistry - Notes - Chapter 12 - Kinetics Page 6 of 7 - the rate determining step - the slowest step of the reaction mechanism which determines the overall speed of the reaction - reaction mechanisms can never be absolutely proven Sample problem : From the equations below which describe the two elementary steps of a process by which a certain chemical reaction occurs, write the rate law and the balanced chemical equation for the overall reaction. Step 1 : Step 2 : A + B Æ C + D (slow step) C Æ E + F (fast step) Solution The rate law can be written from the rate determining step (slow step) : Rate = k[A][B] The balanced chemical equation can be determined by adding the two reaction and canceling out like terms on the reactant and product sides. A + B Æ C + D + C Æ E + F____ = A + B Æ D + E + F 7. A Model for Chemical Kinetics a. Collision Model - molecules must collide in order to react - Factors affecting the effectiveness of collisions : - temperature - concentrations of reactants - nature of reaction (e.g. states of reactants) - presence of catalysts b. activation energy - energy needed to overcome the energy barrier - the energy needed to break the bonds of the reactants - kinetic energy becomes the potential energy - energy stored in bonds c. activated complex (transition state) - the temporary combination of reactant species at the top of the energy barrier - number of collisions with activation energy = (total number of collisions)e-Ea/RT Ea = activation energy R- universal gas constant e-Ea/RT - fraction of collisions with energy Ea or greater at temperature T d. molecular orientation - molecules must collide with proper orientation to each in order for the collision to be effective (assuming enough energy in collision) d. In order for a collision to be effective it must meet both of the above criteria (enough energy and proper orientation) - Arrhenius equation : k = Ae-Ea/RT where : A is the frequency factor - taking the natural log of the Arrhenius equation : ln(k) = (- Ea/R)(1/T) + ln(A) - to determine the activation energy : ln(k2/k1)=(Ea/R)(1/T1-1/T2) 8. Catalysis a. enzymes -organic catalysts (substances which speed up a reaction without being changed or used up) - catalysts work by lowering activation energy (provide alternate pathway (mechanism)) - homogeneous catalysts - same phase as reacting molecules - heterogeneous catalysts - different phase than reacting molecules (usually a solid) AP Chemistry - Notes - Chapter 12 - Kinetics Page 7 of 7 b. Heterogeneous catalysts - catalysts is in a different phase (state) as reactants - adsorption - the collecting of one substance on the surface of another substance - common heterogeneous catalysts -platinum, palladium, and transition metal oxides - typical heterogeneous catalysis involves four steps : - adsorption and activation of reactants - migration of adsorbed reactants on the surface - reaction of adsorbed substances - escape or desorption of products - examples of heterogeneous catalysis -hydrogenation of oils, conversion of sulfur dioxide to sulfur trioxide, catalytic converters c. Homogeneous catalysis - catalyst is in the same phase as reactants -example- NO and freons (chlorofluorocarbons) in the upper atmosphere causes the destruction of ozone