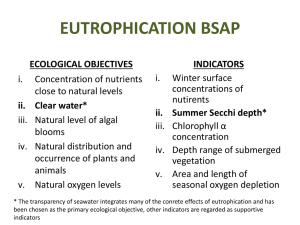
GE HAB HABs in Eutrophic Systems Harmful Algal Blooms
advertisement

GE HAB Global Ecology and Oceanography of Harmful Algal Blooms HABs in Eutrophic Systems This report may be cited as: GEOH AB, 2006. Global Ecology and Oceanography o f Harmful Algal Blooms, Harmful Algal Blooms in Eutrophic Systems. P. Glibert (ed.). IOC and SCOR, Paris and Baltimore, 74 pp. This document is GEOHAB Report # 4 . Copies may be obtained from: Edward R. Urban, Jr. Henrik Enevoldsen Executive Director, SCOR Project Co-ordinator D epartm ent of Earth and Planetary Sciences IOC Science and Communication Centre on Harm ful Algae The Johns Hopkins University University of Copenhagen Baltim ore, M D 21218 USA 0 ster Farimagsgade 2D Tel: +1-410-516-4070 DK-1353 Copenhagen K, Denm ark Fax: +1-410-516-4019 T el:+45 33 13 44 46 E-mail: scor@jhu.edu Fax: +45 33 13 44 47 E-mail: h.enevoldsen@unesco.org This report is also available on the web at: http://www.geohab.info http://www.jhu.edu/scor Primary support for this report and the workshop on which it was based was provided by the US National Oceanic and Atmospheric Administration, National Ocean Service, National Center for Coastal Ocean Science, Center for Sponsored Ocean Research. http://ioc.unesco.org/hab Cover photos Dinophysis acuminata cell. Photo: www.dnr.state.md.us Karenia m ikim otoi bloom, East China Sea, summer 2005. Photo: J. Li. Green algae bloom. Photo: www.dnr.state.md.us Ceratium furca cell. Photo: www.dnr.state.md.us Gymnodinium cell. Photo: www.dnr.state.md.us Mahoghany tide in Chesapeake Bay, USA. Photo: P. Glibert Title page photo Karenia and Gymnodinium bloom, Leigh Harbour, New Zealand, Oct 2002. Photo: Miriam Godfrey, New Zealand National Centre for Aquatic Biodiversity & Biosecurity Back cover photos Alexandrium taylori bloom, Catalan coast of Spain. Photo: M. Estrada Clockwise, starting upper left: Dinophysis acuta, Prorocentrum minimum, Pseudo-nitzschia, colony of Microcystis. Photos: www.dnr. state.md.us Red tide in Kuwait Bay, Kuwait. Photo: P. Glibert Copyright© 2006 IOC and SCOR. Published by IOC and SCOR, Paris and Baltimore, 2006. GEOHAB G lobal E cology & O c ea n o g r a ph y of H a r m fu l A lgal B loom s HABs in Eutrophic Systems (SCOR) (IOC) UNESCO S p o n s o r e d b y t h e S c ie n t if ic C o m m i t t e e o n O c e a n ic R e s e a r c h a n d the I n t e r g o v e r n m e n t a l O c e a n o g r a p h ic C o m m i s s i o n of Edited by: Patricia M. Glibert W ritten by: J. Icarus Allen, Donald Anderson, Michele Burford, Sonya Dyhrman, Kevin Flynn, Patricia M. Glibert, Edna Granéli, Cynthia Heil, Kevin Sellner, Theodore Smayda, and Mingziang Zhou Based on contributions by participants o f the 2005 GEOHAB Open Science M eeting on HABs and Eutrophication, and the GEOHAB Scientific Steering Committee Design and layout by: Jane Hawkey M ay 2006 Table of Contents Executive Summary Chapter 1. Introduction Chapter 2. Development o f the GEOHAB Programme and Its Study o f HABs in Eutrophic Systems.............................................................................................. Chapter 3. General Understanding o f HABs and Eutrophication: A Synopsis................12 Eutrophication and Its Global Effects......................................................................... 13 Sources o f N utrient Inputs............................................................................................16 The Importance o f Nutrient Quality.............................................................................18 Complexity o f Responses o f HABs to Nutrients..........................................................20 Advancing the Study o f HABs and Eutrophication Through New Techniques 22 M odelling HABs and Eutrophication...........................................................................24 Toward a Consensus on Eutrophication and HAB Proliferation...............................28 Chapter 4. Priorities and Challenges for Understanding the Relationship Between HABs and Eutrophication............................................................................................................... 32 Key Questions................................................................................................................ 34 Specific Challenges for Further Advancem ent...........................................................46 Chapter 5. Moving Forward in the Study o f HABs in Eutrophic Systems........................ 48 Applying the GEOHAB A pproach................................................................................ 48 Co-ordination o f Activities............................................................................................50 Data Management and Data Sharing..........................................................................50 Protocols and Quality C ontrol...................................................................................... 51 Capacity Building...........................................................................................................51 M odelling Activities.......................................................................................................51 Interaction w ith Other International Programmes and Projects..............................51 References........................................................................................ Appendix I. GEOHAB 2005 Open Science M eeting Programme.. Appendix II. GEOHAB 2005 Open Science M eeting Participants Appendix III. GEOHAB Scientific Steering C om m ittee................. Figure Credits................................................................................... Executive Summary utrient enrichment of both land and water is a result of increased human population growth and many associated activities for food and energy production, and discharge of associated sewage and waste. The end result of nutrient loading to inland and coastal waters is often an increase in algal biomass, frequently dominated by one or more species or species groups; this process is eutrophication. An important consequence of eutrophication is the increased prevalence of harmful algal blooms (HABs) that develop high biomass, cause fish kills, intoxicate seafood, result in oxygen depletion, and alter trophic interactions. Nutrient enrichment can stimulate HABs not only directly by stimulation of growth and biomass, but indirectly in subtle, but H a r m f u l a l g a l b l o o m s w e r e r e s p o n s ib l e f o r l a r g e f is h k il l s in AQUACULTURE FACILITIES IN THE E A S T C H IN A S E A IN SUM M ER 2 0 0 5 . P h o t o : J . L i. nevertheless significant, ways through alterations in food web and ecosystem dynamics. The interactions of these alterations on HAB proliferation are only beginning to be understood. W orld-w ide, strong relationships have been observed betw een increases in nutrient loading and proliferations o f specific types o f H A B s. In some locales, H A B s have increased in response to alterations in the type o f nutrient, not only major nutrient forms such as nitrogen and phosphorus, but changes in the chemical form o f these nutrients. O rganic, not ju st inorganic, nutrient loading is increasing world-wide and has been correlated w ith many blooms o f both dinoflagellates and cyanobacteria. A dvancements in our understanding o f the physiology o f these organisms has yielded im portant insights as to why these algal classes respond so favourably to these nutrients. 4 Increased nutrient loading from human activities is considered to be one of the reasons that HABs have been expanding in frequency, duration, and harmful properties world-wide. A lthough we have good quantitative estim ates o f many sources and forms o f nutrient loads, the transform ation processes o f these nutrients and how they are affected by landscape changes, food web alterations, and clim atic variations are not well understood. It is imperative to learn how present trends in nutrient loading relate to algal blooms in general, as well as how they prom ote the developm ent o f particular species. The key to th is knowledge is an understanding o f the ecology and oceanography o f H A B s at regional and global scales. The Global Ecology and O ceanography o f H arm fu l A lgal Blooms (G E O H A B ) Program m e is an international netw ork o f scientists and projects, under the auspices o f the Scientific C om m ittee on Oceanic Research (SC O R) and the Intergovernm ental O ceanographic C om m ission (IO C ) o f U N E S C O . It has as its fundam ental goal an improved understanding o f H A B population dynamics through the integration o f biological, chem ical, and physical studies and application o f advanced observational tools and m odelling. This report focuses on one o f the overarching questions o f G E O H A B : T o w h at e x ten t d o e s in creased S h e l l f is h a n d f in f is h m o r t a l i t y d u e t o This docum ent outlines the justification, th e priorities for study, and some o f the new approaches th a t may be brought to bear in studying the relationships betw een H A B s and eutrophication. The key questions identified here include: r e s u l t s in l a r g e P h o t o s l e f t t o r i g h t : N R C o f C a n a d a , a n d P. G l ib e r t . • A re there clusters or specific types o f H A B species th a t are indicative o f global nutrient increases? • To w hat extent do residence tim e and other physical processes im pact the relationship betw een nutrient loading and H A B proliferation? • H o w do feedbacks and interactions betw een nutrients and th e planktonic, microbial food webs im pact H A B s and their detrim ental effects? • D o anthropogenic alterations o f the food web, including overfishing and aquaculture activities, synergistically interact w ith nutrients to favour H A B s? • H o w do anthropogenic changes in land use, agricultural use o f fertiliser, N O x emissions from vehicles, and global changes in land cover affect the delivery o f nutrients to coastal waters and th e resulting incidence o f H A B s? H o w do the stoichiom etry and quality o f these nutrient sources regulate th e biological responses favouring H A B s? • D o climate change and climate variability have im pacts on ecosystems th a t augm ent th e im pacts o f eutrophication in the form ation o f H A B s? e u tr o p h ic a tio n in flu e n c e th e occu rren ce o f H A B s and th eir h a r m fu l effects? This docum ent also serves as an initial road map for the developm ent o f a Core Research Project w ith in the G E O H A B fram ew ork on H A B s in E u trop h ic System s. The overall strategy o f G E O H A B is to apply the comparative approach, to undertake research th a t is interdisciplinary and international in scope to encompass the global issues o f H A B events, and to benefit from the skill and experience o f investigators world-wide. The research questions and priorities identified herein were developed through com m unity interaction at an O pen Science M eeting held in B altim ore M aryland, U SA , in M arch 2005. HABs ECONOM IC LOSSES AS W ELL AS LOSS OF PRODUCT FOR CO N S U M PTIO N . Research on these major priority areas in eutrophication and its associated effects on HABs is welcome within the GEO HA B framework. The understanding of this important issue, and ultimately the management of nutrient loads and improved prediction and management of HABs, will require attention from the global community of scientists. 5 1. Introduction T ■ EOHAB, the Global Ecology and Oceanography of Harmful Algal Blooms Programme, sponsored by the Scientific M Committee on Oceanic Research (SCOR) and the Intergovernmental Oceanographic Commission (IOC) of UNESCO, is an international programme to foster and promote co-operative research directed toward improving the prediction of harmful algal bloom (HAB) events. GEO HA B has recognized the impacts of HABs throughout all waters of the world, but has emphasized events in marine and brackish waters because of the global significance N o c t il u c a b l o o m . H o o d C a n a l , W a s h i n g t o n , U S A , s u m m e r 2 0 0 5 . P h o t o : W . P a ls s o n , W D F W . G E dH A B T-W Jforrit 2*tï JÍWAvimóiy1. IQM B ro c h u re f o r G E O H A B O pen S c ie n c e M e e tin g o n H A B s a n d E u tr o p h ic a tio n , 7 -1 0 M a r c h 2005. 6 of these problems and the need for collaborative, international studies to address them. H A B s have been associated w ith fish and shellfish kills, hum an health im pacts, and ecosystem damage thro u g h o u t the world. C oncurrent w ith escalating influences o f hum an activities on coastal ecosystems, the environm ental and economic im pacts of H A B s and consequent challenges for coastal zone m anagem ent have increased in recent years. The relationship betw een H A B s and the increasing nutrient enrichm ent o f m any o f the w orld’s coastal and estuarine environm ents is o f particular concern. Increasing nutrient loading to coastal and enclosed or estuarine environm ents is a result o f agricultural, aquacultural, anim al operations, and industrial and sewage effluents. The relationship betw een nutrient (both inorganic and organic) loading and alteration in nutrient supply ratios and many H A B s is now recognized, but much rem ains to be understood. GEOHAB is an international programme directed toward improving the prediction of harmful algal bloom events. This report is based on th e G EO H A B Open Science M eeting on H A B s and Eutrophication and held from 7-10 M arch 2005 in B altim ore, M aryland, U SA. The program m e and participants lists are found in A ppendices I and II. This m eeting, th e th ird in a series o f O pen Science M eetings, brought together international experts to review the state o f knowledge w ith respect to our understanding o f the role o f eutrophication in th e proliferation o f H A B s world-wide and take th e initial steps tow ard designing research on comparative systems and species to address this critical global issue. H A B s in E u trop h ic S ystem s is designed as one o f the Core Research Projects in the G E O H A B Science and Im plem entation Plans (www.geohab.info). The research described herein provides a guide for this C ore Research Project. It w ill serve as a nucleus for continued planning and w ill be augm ented as research is funded, results are obtained, and new questions are form ulated. This docum ent also serves as an invitation to individuals who could not participate in the G EO H A B Open Science Meeting on H A B s and Eutrophication. H a r m f u l a l g a l b l o o m s o c c u r in a l l c o l o r s , r e f l e c t in g t h e M ULTITU DE OF SPECIES RESPONSIBLE FOR THESE EVENTS W O R LD -W ID E (LEFT t o r ig h t : L a J o l l a , C A U S A ; C a m b r id g e , M D U S A ; S o u t h A f r ic a ) P h o t o s ( l e f t t o r ig h t ) : P. F r a n k s , P. G l ib e r t , a n d G . P it c h e r . Hawkey, D arlene W indsor and Ji L i, U niversity o f M aryland C enter for Environm ental Science; and Kevin Sellner and D an G ustafson, Chesapeake Research C onsortium , for assistance w ith m eeting preparation. The G E O H A B SSC and the Conference O rganising C om m ittees are grateful for the financial support for th e conference provided by th e N ational Oceanic and A tm ospheric A dm inistration-N ational O cean Service, the US N ational Science Foundation-D ivision o f O cean Sciences, University o f M aryland C enter for Environm ental Science, US N ational Office for M arine Biotoxins and H arm fu l A lgal Blooms, M aryland D epartm ent o f N atural Resources, Chesapeake Research C onsortium , YSI E nvironm ental, and G allaudet University. The members o f the O rganising C om m ittee for the G EO H AB Open Science M eeting on H A B s and Eutrophication wish to th a n k the individuals who participated in this m eeting, as it is those people w ho contributed so substantially to the ideas presented herein. Special thanks are also given to John C ullen for his contributions to G E O H A B during its early stages, and to members o f the past and present G E O H A B Scientific Steering C om m ittee (SSC, A ppendix III). The contributors to this report wish to th a n k all those who contributed illustrations and who carefully reviewed drafts o f this report, especially R aphael Kudela, D ennis M cG illicuddy, Bob H o w arth , G reg D oucette, A lan L ew itus, Sue B anahan, and Rob M agnien. The O rganising C om m ittee also th an k s E d U rban, E lizabeth Gross and Phyllis Steiner, SC O R ; Judy K leindinst, W oods H ole O ceanographic Institution; Jane 7 2. Development of the G EO H A B Programme and Its Study of HABs in Eutrophic Systems f G E0HAB G E#H A B G I«U l i f c l «Ul O i J U U |H ^ | m*fm*rnl¿l40ttMrm, l4.u « rU l i j . ■ — ál I # I È he Global Ecology and Oceanography of Harmful Algal Blooms (GEOHAB) Programme was initiated under the auspices of the Scientific Committee on Oceanic Research (SCOR) of the International Council for Science (ICSU) and the Intergovernmental Oceanographic Commission (IOC) of UNESCO. Im p le m e n ta tio n Science FIjii -¿k Plan A) , - ^ v . B G E O H A B S c ie n c e P l a n , G E O H A B I m p l e m e n t a t io n P l a n , p u b lis h e d 2 0 0 1 p u b lis h e d 2 0 0 3 . The Mission and Scientific Goal of GEOHAB The mission of GEOHAB is to foster international co­ operative research on HABs in ecosystem types sharing common features, comparing the key species involved, and the oceanographic processes that influence their population dynamics. The scientific goal of GEOHAB is to improve prediction of HABs by determining the ecological and oceanographic mechanisms underlying their population dynamics, integrating biological, chemical, and physical studies supported by enhanced observational and modelling systems. The first G E O H A B planning m eeting was held in H avreholm , D enm ark in 1998, and a Scientific Steering C om m ittee (SSC) was formed in 1999. In 2001, the G EO H AB Science Plan (G E O H A B 2001) was published, outlining the mission, goals, and scientific objectives o f the program m e. In 2003, the G EO H AB Implementation Plan (G E O H A B 2003) was published, representing an invitation for the com m unity to collaborate on the objectives of GEOHA B. G E O H A B is an international program m e to co-ordinate and build upon related national, regional, and international efforts in H A B research. The G E O H A B Program m e assists in bringing together investigators from different disciplines and countries to exchange technologies, concepts and findings. This takes the form o f workshops and m eetings, as in the case o f the Baltim ore m eeting on H A B s and E utrophication, or more specific team s o f collaborating scientists. G E O H A B is not a funding program m e per se, but instead w ill facilitate those activities th a t require cooperation am ong nations and am ong scientists w orking in comparative ecosystems. The central feature o f th e G E O H A B Program m e im plem entation is Core Research, o f w hich H A B s in E u tro p h ic S ystem s is one priority area. Core Research is comparative, interdisciplinary, international and directly addresses the overall goals of G E O H A B as outlined in th e Science Plan. As stated in the The overall strategy of GEOHAB is to apply the comparative approach. Implementation Plan, Core Research comprises oceanographic field studies conducted in, and application o f models to, comparable ecosystems, supported by identification o f relevant organism s, and m easurem ents o f physical, chem ical, and biological processes th a t control their population dynamics. The overall strategy o f G E O H A B is to apply the comparative approach. The comparative m ethod assembles the separate realisations needed for scientific inference by recognising naturallyoccurring patterns, and tem poral and spatial variations in existing conditions and phenom ena (A nderson et al. 2005a, G E O H A B 2005). U nderstanding th e responses o f harm ful algae to the increasing nutrient load o f comparative ecosystems o f the w orld’s coastal zones w ill assist in prediction o f future patterns as well. D evelopm ent o f a C ore Research Project in G E O H A B is based upon the prem ise th a t a comprehensive understanding o f the population dynamics o f H A B s requires the integration o f oceanographic studies w ith the application o f models of comparable ecosystem types. Such comparisons w ill assist in a: • • • • definition o f com m on characteristics, including the groupings o f harm ful species from similar habitat types and identification o f functional groups; determ ination o f the prim ary physiological, genetic or behavioural processes th a t regulate cellular grow th and toxicity; identification o f th e im portant physical and chemical influences over appropriate tem poral and spatial scales; and developm ent and validation o f technologies for detailed and extensive m onitoring; establishm ent o f real-tim e observation platform s. H a r m fu l a lg a l b lo o m s affect bo th n earsh o r e a n d offsho re w aters ( l e f t t o r ig h t : S w e d e n ; H o n s h u , J a p a n ; B a l t ic S e a ) . P h o t o s : G . A neer, Y . F u k u y o , a n d K . K o n o n e n . B io diversity A d a p tiv e and S t r a te g ie s Biity yog rap hy N u trien ts Observation. Modelling, and Prediction and E u tro p h ic a tio n C o m p a ra tiv e Ecosystem s M a n a g e m e n t and M itigation The GEOHAB Science Plan The GEOHAB Science P/anoutlines five Programme Elements that serve as a guide to establish the research priorities. These elements and their overarching questions include: Biodiversity and Biogeography. What are the factors that determine the changing distributions of HAB species, their genetic variability, and the biodiversity of associated communities? Nutrients and Eutrophication. To what extent does increased eutrophication influence the occurrence of HABs and their harmful effects? Adaptive Strategies. What are the unique adaptations of HAB species and how do they help to explain their proliferations or harmful effects? Comparative Ecosystems. To what extent do HAB species, their population dynamics, and community interactions respond similarly under comparable ecosystems? Observation, Modelling, and Prediction. How can we improve the detection and prediction of HABs by developing capabilities in observation and modelling? 9 The GEOHAB Programme Multidisciplinary and Comparative Studies • B-lMJiv#rEl1.y «fld B lü g o ü g rn p tiy \ N u lritirttt 0H(lï411#n, C o m p jrtllv * and M o -d a liln g , E c o iy iltm ti E u lr o p b ic a tio n In te g r a t io n iru J P r i d le I l a n A d * p tlv * S ln lf p l« » / T h e in t e g r a t io n o f f in d in g s f r o m t h e in d iv id u a l P r o g r a m m e E l e m e n t s d e s c r ib e d in t h e G E O H A B S c i e n c e P l a n is r e q u i r e d TO ACHIEVE N O T ONLY A N UNDERSTANDING OF HAB ORGANISMS AN D ASSOCIATED EVENTS, BUT ALSO TO DEVELOP PREDICTIVE CAPABILITIES. S ource: GEOHAB 2001. Core research on H A B s in E u trop h ic S ystem s directly addresses Program m e E lem ent 2, N utrients and E utrophication, o f the Science Plan. As outlined therein, th e overall objective o f the N utrients and E utrophication Program m e E lem ent is to determ ine the significance o f eutrophication and nutrient transform ation pathways to H A B population dynamics. The identified specific objectives o f the N utrients and E utrophication Program m e E lem ent o f G E O H A B include: • determ ine the com position and relative im portance to H A B s o f different nutrient inputs associated w ith hum an activities and natural processes; • determ ine the physiological responses o f H A B and non-H A B species to specific nutrient inputs; • determ ine the effects o f varying nutrient inputs on the harm ful properties o f H A B s; and determ ine the role o f nutrient cycling processes in H A B development. 10 Smaller in scope, but nonetheless equally im portant to the success o f G E O H A B , are Targeted Research Studies. Targeted Research Studies address individual objectives outlined in the Science Plan (G E O H A B 2001). D evelopm ent o f specific models, or comparative laboratory investigations o f particular H A B species or species groups, may be investigations th a t fall under th e realm o f Targeted Research Studies. This docum ent outlines the justification, the priorities for study, and the approaches to be taken, in studying the relationship betw een H A B s and eutrophication. Research on H A B s in E u trop h ic S ystem s w ill require th e m ulti-dim ensional approaches o f a Core Research Project, com bined w ith focused or targeted research, w hich may address specific needs related to individual species, systems, or m odel development. In the following sections, a general synopsis o f the current state o f understanding w ith respect to th e role o f eutrophication in H A B s is provided, followed by an identification o f the major research priorities, and finally a roadm ap for addressing these research needs. Research on these major priority areas in eutrophication and its associated effects on H A B s is welcome w ith in the G E O H A B fram ew ork (G E O H A B 2003). 3. General Understanding of HABs and Eutrophication: A Synopsis f IM ƒ I g y Jm uch has been written in recent years È concerning the relationship between m HABs and eutrophication (e.g., Smayda 1990, Hallegraeff 1993, Riegman 1995, Richardson and j 0 rgensen 1996, Anderson et al. 2002, Glibert et al. 2005), but much uncertainty remains with respect to the process of eutrophication and how it impacts the propensity for specific blooms. Although it may seem reasonable to assume a causal relationship between human activities and an expansion of HAB events, the underlying mechanisms are not well known. It is imperative to M a s s iv e b l o o m s o f t h e d i n o f l a g e l l a t e P r o r o c e n t r u m m i n i m u m DEVELOP AN N U ALLY IN THE CHESAPEAKE B A Y , USA. PH OTO: P. G LIB ER T. learn how present trends in pollution and nutrient loading relate to algal blooms in general, as well as N u trien t C h .in g e s A lg a l R e s p o n s e s P o le n ile I Im p a c ts how they may promote the development of particular species. The key to this knowledge is an understanding of the ecology and oceanography of HABs at both regional and global scales. Highlights of current general EtotlAf m understanding are provided herein. This is not intended B arri h e IC itre a I to be a comprehensive review, but rather a synopsis of Hum an where progress has been made and where significant C hanna» m F.JwWi Malura Ptm-1 a m i Dh*llli«Ji Kill* ■nrl Fi+riHi*i N u t r ie n t c h a n g e s , a l g a l r e s p o n s e s , a n d e c o s y s t e m im p a c t s a r e LINKED AT M A N Y LEVELS. SO URCE: 12 GEOHAB 2001. questions still remain. By highlighting the critical issues, the justification for the Core Research Project on HABs in Eutrophic Systems will become apparent. "Eutrophication is the process o f increased organic enrichment o f an ecosystem, generally through increased nutrient in p u ts" Nixon 1995 i 11-10 110-30 120 U M 5 0 -1 0 0 □ ■ 100-300 m soo B l >500 Eutrophication and Its Global Effects M o d e l - p r e d i c t e d e x p o r t o f d is s o l v e d i n o r g a n i c n it r o g e n f r o m WATERSHEDS TO COASTAL SYSTEMS (KG “E utrophication” has been defined in various ways (e.g., N ixon 1995, R ichardson and Jorgensen 1996). C entral to all definitions is the concept th a t the enrichm ent o f water by nutrients causes an accelerated grow th o f algae and higher forms o f p lant life, which leads to an undesirable disturbance in the balance o f organisms present in the water and to the quality o f the water concerned. The result o f eutrophication is often an increase in to tal algal biomass, frequently dom inated by one or more species or groups. Such blooms may have deleterious effects including overgrowth and shading o f seaweeds and sea grasses, oxygen depletion o f th e water from algal respiration or decay o f algal biomass, suffocation o f fish, direct toxic effects on fish and shellfish, suffocation o f fish from stim ulation o f gili mucus production, and m echanical interference w ith filter feeding by fish and bivalve mollusks. Deleterious effects on the benthos may also be considerable. O f additional concern w ith the developm ent o f high-biom ass algal blooms is the poor transfer o f energy to higher trophic levels, as many bloom species are not efficiently grazed, resulting in decreased transfer o f carbon and other nutrients to fish stocks when they replace desirable algal species. For example, some H A B species secrete allelopathic substances th a t inh ib it co-occurring species (P ratt 1966, G entien and A rzul 1990), and suppression o f grazing occurs above a threshold concentration o f the H A B species (Tracey 1988). Recently, it has also been shown th a t an increase in the production o f allelochemicals occurs when some H A B species are subjected to unbalanced nitrogen or phosphorus conditions, w hich in tu rn are directly caused by eutrophication (G ranéli and Johansson 2003, Fistarol et al. 2005a). E utrophication o f both inland and coastal waters is a result o f hum an population grow th and the production o f food (agriculture, anim al operations, and aquaculture) and energy, and is considered one o f the largest pollution problems globally (H ow arth et al. 2002a, 2005). Population grow th and increased food production result in major changes to the landscape, in tu rn increasing sewage discharges and runoff from farm ed and populated lands. In addition to population grow th, eutrophication arises from the large increases in the use o f chemical fertilisers th a t began in the 1950s N K M '2 W ATERSHED'1 Y E AR '1. S o u r c e : S e it z in g e r a n d K r o z e 1 9 9 8 . and w hich are projected to continue to escalate in the com ing decades (Smil 2001, G alloway et al. 2004, G lib ert et al. 2006). B oth nitrogen and phosphorus are o f concern in eutrophication (N R C 2000, H o w arth and M arino 2006), but nitrogen has received far more attention because it often lim its prim ary production in estuaries and coastal waters, and because the global application o f nitrogen from synthetic fertilisers is far greater th a n th a t o f phosphorus (W assm an and O lli 2005). F urtherm ore, for many regions where phosphorus lim itation has developed, it may be the result o f excess nitrogen loading. Phosphorus loading, however, is often cited as th e major cause o f H A B s in freshwaters (Oliver and G a n f2 0 0 0 ) where nitrogen-fixers often dom inate. N utrients can stim ulate or enhance the im pact o f toxic or harm ful species in several ways (A nderson et al. 2002). A t the simplest level, harm ful phytoplankton may increase in abundance due to nutrient enrichm ent, but rem ain in the same relative fraction o f th e total phytoplankton biomass. Even though no n -H A B species are stim ulated proportionately, a m odest increase in the abundance o f a H A B species can cause it to become noticeable because o f its toxic or harm ful effects. A more frequent response to nutrient enrichm ent occurs w hen a species or group o f species begins to dom inate under the altered nutrient regime. In deeper freshwater, estuarine, and coastal marine systems, phytoplankton dom inate the algal flora. In contrast, macroalgae and benthic microalgae often dom inate many shallow lakes and poorly flushed estuaries, lagoons, and upper em baym ents, as well as coral reefs and rocky in tertid al/ subtidal habitats (H arlin 1993). In surface waters across the entire salinity gradient, there are many examples o f overgrowth and highbiomass blooms by phytoplankton, benthic microalgae (especially epiphytes), and macroalgae. In many cases, th e responding dom inant species are not toxic and, in fact, are beneficial to coastal productivity until they exceed the assimilative capacity o f the system, after w hich hypoxia and other adverse effects occur. W h en th a t threshold is reached, seemingly harm less species can have negative impacts. 13 A S ynopsis A B O V E : L a r g e b l o o m s o f K a r e n i a b r e v i s o c c u r r e d in F l o r i d a , U S A d u r i n g 2 0 0 5 , r e s u l t i n g in l a r g e f i s h k i l l s . P h o t o s : C . H e i l . BELOW: L a r g e P h o t o s : J. Li. b l o o m s o f K a r e n i a m i k i m o t o i o c c u r r e d in E a s t C h i n a S e a in s u m m e r 2 0 0 5 , r e s u l t i n g in k i l l s o f b o t h w i l d a n d f a r m e d f i s h . Increases in high-biom ass phytoplankton blooms have been reported from th e South C hina Sea (Q i et al. 1993), the Black Sea (B odeanu and R uta 1998), H o n g Kong (L am and H o 1989), and many other locations, typically in parallel w ith the nutrient enrichm ent o f coastal waters. In Chesapeake Bay, U SA , high phytoplankton biomass is typically observed in the spring, associated w ith high riverine nutrient inputs (G libert et al. 1995, M alone et al. 1996). These large spring blooms eventually settle to the bottom , where heterotrophic bacteria process a major fraction o f the organic m aterial. This can result in depletion o f oxygen as tem peratures w arm (M alone et al. 1986, Shiah and D ucklow 1994), leading to hypoxia and m ortalities o f benthic organisms (e.g., Boynton et al. 1982, M alone et al. 1983, Fisher et al. 1988, 1992, G libert et al. 1995). As another example, spring eutrophication from the nitrogen loading o f the M ississippi and A tchafalaya rivers to the G u lf o f M exico has resulted in enhanced phytoplankton production and the developm ent o f hypoxia in the 14 G u lf o f M exico, a so-called “dead zone” th a t has altered benthic food web dynamics and fish habitat substantially (Turner and Rabalais 1994, C raig and Crowder 2005). A lthough eutrophication is occurring globally, nutrient export is not evenly distributed (Seitzinger et al. 2002a, 2005a, H ow arth et al. 2005, G libert et al. 2006). Inorganic nitrogen export to coastal waters is estim ated to be highest from E uropean and A sian lands, although significant discharge also occurs from th e U nited States and other parts o f the world (Seitzinger and Kroeze 1998). F urtherm ore, th e rate o f nutrient export to coastal waters has increased dram atically in recent years in some parts o f the world. For example, C hina, w hich used less th a n 5 m illion metric tonnes o f nitrogen fertiliser annually in the 1970s, now uses more th an 20 m illion metric tonnes per year, representing 25% o f global nitrogen fertiliser consum ption (G libert et al. 2005), leading to significantly increased nitrogen pollution o f its coastal waters. A S y n o p sis others W h e r e p e o p l e l iv e o n t h e P l a n e t E a r t h , 2 0 0 3 . S o u r c e : N a t i o n a l N. America R e s o u r c e s C o n s e r v a t i o n S e r v ic e , U S D A . L a tin A m e ric a C o n t r i b u t i o n b y g l o b a l r e g io n t o t h e c o n s u m p t i o n o f t h e w o r l d ' s The w orld’s hum an population is expected to continue to increase by 1-2% per year (C ohen 2003), and eutrophication and its effects, including H A B s, hypoxia, fish kills, and loss o f habitat are expected to increase in many parts o f th e world. M o st o f these increases are expected to occur in areas th a t are already affected by H A B s. A N N U A L CURRENT USE OF 8 5 M ILLIO N TONNES OF NITROGEN. D A T A FROM the I n t e r n a t i o n a l F e r t il iz e r I n d u s t r y . S o u r c e : G l ib e r t e t a l . 2 0 0 5 . Projections of percent change in nitrogen fertiliser use for the three decades from 1990 to 2020 for some countries. Plotted from data provided in Cofala et al. 2001. 15 A S y n o p s is F r o m ( a b o v e ) s e w a g e d is c h a r g e ( p h o t o : A . K a n a ) , t o im p a c t s o f Sources of Nutrient Inputs ATMOSPHERIC EMISSIONS, TO INTENSIVE AQUACULTURE DEVELOPMENT ( p h o t o : P. G l ib e r t ) a n d a n i m a l p r o d u c t i o n f a c il it ie s , t o ( b e l o w ) RUNOFF FROM APPLIED FERTILISER A N D M ANURES (PH O TO : W W W . k u h n k n ig h t .c o m ) , t h e a n t h r o p o g e n ic s o u r c e s o f n u t r ie n t s a r e M AN Y . Global Use of Fertilisers The change in global consumption of nitrogen and phosphorus synthetic fertilisers over the past several decades (from International Fertilizer Industry 2005). Projections through 2010 are based on an annual increase of 3% (Glibert and Burkholder 2006). 16 A broad range o f anthropogenic activities result in significant alteration o f nutrient cycling o f coastal environm ents. Fertiliser application on land rem ains a major contributor to nonpoint nutrient pollution, and this source is still increasing at an alarm ing rate in many geographic regions (Vitousek et al. 1997). G roundw ater has also been identified as an im portant source o f nutrients to receiving surface waters. H u m an population grow th and agricultural practices have increased nutrient loadings to groundw ater, and th is has the potential to affect algal grow th in adjacent rivers, lakes, estuaries, and coastal zones. O n local to global scales, one o f the m ost rapidly increasing sources o f nutrients to both fresh waters and the coastal zone is th e atmosphere. A tm ospheric inputs are im portant not only because o f their m agnitude, but because th e mix o f atm ospheric nutrients - like other nutrient sources - can stim ulate some phytoplankton species disproportionately over others. A tm ospheric deposition o f nitrogen comes from both fossil fuel com bustion and from volatilization from agriculture and its m agnitude is likely to be underestim ated in many cases, since gaseous dry nitrogen deposition is seldom measured (Galloway e t al. 2004, H o w arth et al. 2002a, 2005) Blooms in the Yellow Sea o f C hina, w hich have escalated in frequency over the past several decades, have been related to atm ospheric deposition in addition to direct nutrient ru n off (Z hang 1994). It is estim ated th a t a typical rain event over the Yellow Sea may supply sufficient nitrogen, phosphorus, and silicon to account for 50-100% o f the prim ary production o f a H A B event (Z hang 1994). A quaculture ponds and cage culture systems represent another source o f nutrients, provided as feed or fertiliser and by the biological transform ations occurring in these high-biom ass systems. It has been suggested th a t these enriched systems may prom ote the grow th o f harm ful species not previously detected in the source water (A nderson 1989, H allegraeff 1993). A S y n o p sis M a n u r e N i t r o g e n L e e c h i n g V u l ne r a b i l i t y I ndex S e w a g e p l u m e m a p a s in d i c a t e d b y a15N s ig n a t u r e s o f m a c r o a l g a e DEPLOYED FOR 4 DAYS IN M A R C H , 1 9 9 8 . D IS T IN C T SEWAGE PLUME (HIG H a 15N v a l u e s ) w a s e v id e n t a t t h e m o u t h o f t h e B r is b a n e R iv e r , A u s t r a l i a . S o u r c e : D e n n is o n a n d A b a l 1 9 9 9 . A n i m a l m a n u r e s a r e a c o m m o n l y a p p l ie d f e r t il is e r . T h is m a p SHOWS THOSE WATERSHEDS M OST SUSCEPTIBLE TO LEACHING OF M ANURE NITROGEN TO COASTAL A N D ESTUARINE WATERS. M A N U R E S ARE ONE SOURCE OF NUTRIENTS TH AT HAVE INCREASED SUBSTANTIALLY IN RECENT YEARS. S o u r c e : U S D e p a r t m e n t o f A g r ic u l t u r e . Strong positive relationships have been reported betw een increasing hum an population and the intensity o f H A B outbreaks (L am and H o 1989, T rainer et al. 2003). For example, in Tolo H arbour, H ong Kong, hum an population w ithin the w atershed grew 6-fold betw een 1976 and 1986, during w hich the number o f red tide events increased 8-fold (Lam and H o 1989). In Puget Sound o f W ashington State, U SA , where consistent shellfish m onitoring has been carried out for decades, a strong positive relationship betw een the frequency o f one algal toxin, paralytic shellfish toxin (PST ), and the grow th in hum an population has recently been reported (Trainer et al. 2003). However, the im pacts o f differing anthropogenic activities are not necessarily the same. For example, nutrient delivery associated w ith sewage may bear little sim ilarity in quantity or com position to th at associated w ith inputs from agriculture, aquaculture, or dredging operations. In tu rn , nutrients from these sources may also differ in quantity and com position from those associated w ith natural delivery m echanism s such as groundw ater flow and atmospheric deposition, recognising th a t these sources may also be affected by hum an activities. The challenge th a t rem ains is understanding the conditions under w hich specific nutrient forms, or specific nutrient sources, may im part an advantage to certain H A B species and thus lead to th eir proliferation. M oreover, im pacts o f upwelling, storms, m onsoons, and/or tsunam is may also be sources o f nutrient delivery th a t have to be distinguished from those above. Therefore, it is o f critical im portance to distinguish the im pacts o f various activities on H A B developm ent, and how these im pacts may differ from natural m echanism s based on w hich nutrients are delivered to the coastal zone. Increasing HAB Occurrences Increasing HAB occurrences in the Tolo Harbour, Hong Kong, concomitant w ith the increase in human population in the region. Redrawn by Granéli from Lam and Ho 1989. S The relationship between population growth (in millions) and the average decadal maximum paralytic shellfish toxin in Puget Sound, Washington State, USA. Replotted from Trainer et al. 2003. Average D ecadal Maxim um P ST 1 2 Population (Millions) 17 A S ynopsis H i g h - b io m a s s b l o o m in T o l o H a r b o u r , H o n g K o n g , r e s u l t in g The Importance of Nutrient Quality IN DISCOLORED W ATER, H Y PO XIA, A N D TOXICITY OF FISH A N D SHELLFISH. P h o t o : M . D ic k m a n . Foams of Phaeocystis may be caused by the exudation of protein-rich foams by the algae. Photo by V. Rousseau, courtesy of the EUROFIAB Science Initiative, 1999. The change in N:P ratio is related to Phaeocystis blooms in Dutch coastal waters. From Anderson et al. 2002, based on Riegman 1995. I! 18 ■ -I- F M any factors afFect phytoplankton species com position and bloom developm ent, and am ong these is th e com position o f the nutrient pool - the forms o f th e nutrients supplied, as well as the relative abundance o f the major nutrient elements. Efforts to understand the relationships betw een nutrient loading and algal blooms have largely focused on to tal nutrient loads and altered nutrient ratios th a t result from selected nutrient addition or removal. A lterations in the com position o f nutrient loads have been correlated w ith shifts from diatom -dom inated to flagellate-dom inated algal assemblages (Smayda 1990). For example, the num ber o f red tide events in C hinese coastal waters increased sharply from 1970 to 1993, due in large p a rt to altered nitrogen-to-phosphorus ratios from increasing use o f synthetic fertilisers and increasing atm ospheric emissions and deposition o f pollutants. Perhaps the clearest dem onstration o f the effect o f altered nutrient supply ratios involves the stim ulation o f non-diatom species following increases in the availability o f nitrogen or phosphorus relative to silicate. A prom inent example o f the im portance of nutrient supply ratios in determ ining phytoplankton species com position is seen w ith the foam -producing prym nesiophyte Phaeocystis pouchetii. A 23-year tim e series off the G erm an coast o f the Baltic Sea docum ents the general enrichm ent of these coastal waters w ith nitrogen and phosphate, and a 4-fold increase in the N :Si and P:Si ratios (Radach et al. 1990). This was accom panied by a decrease in the diatom com m unity and an increase in the occurrence o f Phaeocystis blooms. A sim ilar tim e series o f alterations in the N:Si ratio has also been docum ented for N arragansett Bay, U SA , in w hich a decreased ratio o f diatoms to flagellates, and increased abundance o f H A B species such as Heterosigma akashiwo, corresponded w ith an increase in the N:Si ratio due to excess nitrogen loading from the early to late 1980s (Smayda and B orkm an, in press). A S y n o p sis M any freshwater systems, and freshwater end members o f estuarine systems as well, have seen a shift in phytoplankton species com position tow ards bloom -form ing cyanobacteria, including toxic species such as Microcystis aeruginosa, as phosphorus loads have increased in these systems (Oliver and G a n f 2000). In tributaries of C hesapeake Bay, U SA , blooms o f M . aeruginosa in 2003 and 2004 were up to 4 tim es higher th a n a decade earlier (P. T ango, unpub. data). A lthough phytoplankton have long been know n to use organic nutrients in addition to inorganic nutrients (Flynn and B utler 1986, A n tia et al. 1991), increasingly it has become recognised th a t they do so under natural conditions. Some species use organic nutrients for th eir sole nutritional requirem ents, others to supplement their use o f inorganic nutrients, while still others may use organic com pounds for their carbon dem ands as well (e.g., G ranéli et al. 1997, 1999, Stoecker 1999). A n increased understanding o f the bioavailability o f anthropogenically derived organic compounds has also developed in recent years. The chemical com position of dissolved organic m atter exported from agricultural watersheds is not know n, but up to 50% o f this m aterial can be taken up directly or indirectly by estuarine plankton com m unities (Seitzinger et al. 2002b). The chemical com position, bioavailability, and effects o f dissolved organic m atter on coastal plankton com m unities vary depending on its source, the plankton com m unity composition, and th e season (B ronk2002). O rganic nutrients have been shown to be im portant in the developm ent o f blooms o f various H A B species, in particular cyanobacteria and dinoflagellates (e.g., Paerl 1988, G libert et al. 2001) and th e im portance o f this phenom enon is beginning to be docum ented around the world (e.g., G ranéli et al. 1985,1999, Berm an 1997, 2001, Berg et al. 2003). A recent study found th a t in Florida Bay and on the southwest Florida continental shelf, the cyanobacterial fraction o f the algal com m unity was positively correlated w ith the fraction o f nitrogen taken up as urea, and negatively correlated w ith the fraction o f nitrogen taken up from the inorganic source, nitrate (G libert et al. 2004). O th er studies have shown th a t some cyanobacteria M ic r o c y s t is b l o o m o n th e lo w e r St. J o h n s R iv e r , F lo r i d a , 2005. P h o t o : B . Y a te s , w w w . c y p ix . n e t . The relationship between the percent contribution of urea to total N uptake by the algal community and the fraction o fth a t community that was cyanobacteria for Florida Bay (panel A) and for the southwest Florida continental shelf (panel B), and the relationships between percent contribution of NOyto total uptake (panels C and D) for the same sites. From Glibert and Heil (2005). C O _cro Q. o o io m io 0 40 10 20 00 40 SO 00 b C o n tr ib u tio n o f u re a to N u p ta k e □.s 0.4 0.3 0.2 0.1 40 80 O*. 100 fto b C o n tr ib u tio n o f N 0 3" to N u p ta k e grow faster on urea th a n on other nitrogen sources (Berm an and Chava 1999, M ulholland et al. 1999). W orld-w ide use o f urea has increased in recent decades, such th a t urea now represents >50% of all global nitrogen fertiliser use (G libert et al. 2006). 19 A S ynopsis “I t may be that as eutrophication progresses through its various stages, changes in life-form conditions occur which determine which life-form type ofphytoplankter w ill predominated T ed Smayda (2005) p. 96 Complexity of Responses of HABs to Nutrients Prorocentrum minimum blooms in the tributaries of Chesapeake Bay, USA, have been related to nutrient input. The extent to which this bloom depends on different forms of nitrogen is highly dependent on temperature (Fan et al. 2003). Photo: P. Glibert. Nitrate A m m o n iu m T e m p e ra tu 20 The complexity o f the relationship betw een H A B s and eutrophication is beginning to be appreciated (Smayda 1989, A nderson et al. 2002, G libert and B urkholder 2006). The ecosystem response to nutrient enrichm ent, or eutrophication, is a continual process rather th a n a static condition or a trophic state (Cloern 2001, Smayda 2005). N utrient availability m ust be m atched w ith the preferences o f the cells and th eir physiological conditions, and w ith the physical and trophic structure o f the w ater colum n at the tim e o f nutrient delivery. A recent conceptual fram ew ork o f H A B dinoflagellates has been developed. This is based on distinctive morphological and habitat preferences, ranging from invasive species th a t dom inate in habitats w ith enriched nutrient loading to those th a t thrive in more oligotrophic, stratified systems (Smayda and Reynolds 2001). Large blooms o f Prorocentrum m inim um , found in many regions affected by anthropogenic nutrient inputs, are an example o f a species th a t thrives in habitats w ith enriched nutrient loading (H eil et al. 2005). In contrast, Karenia brevis and K. mikimotoi bloom in open coastal waters, aggregate in fronts, and are transported by coastal currents (D ah l and T angen 1993, W alsh et al. 2001). N utritional mechanism s are complex. In addition to direct uptake by autotrophs, other pathways o f nutrient acquisition must be recognized. Indeed, some species, such as the ichthyotoxic dinoflagellate Pfiesteria piscicida are not autotrophs at all, and instead, rely virtually exclusively on grazing for th eir nutrition. In the case o f Pfiesteria, nutrition is provided through a com bination o f consum ption o f bacteria and other small algae, as well as bits o f epiderm al tissue o f fish (Burkholder and G lasgow 1997, L ew itus et al. 1999). Such modes o f nutrition complicate our ability to model and predict biomass developm ent based solely on nutrient loadings. Several cyanobacterial species (e.g., Trichodesmium, Anabaena, Nodularia, Aphanizomenon) are also capable o f fixing nitrogen and may therefore form massive blooms in circum stances where the grow th o f other phytoplankton is lim ited by nitrogen. A S y n o p sis Thus, while there have been many advances in our understanding o f nutrient uptake pathways by various species, these pathways and preferences are complex. It is increasingly recognized th a t certain species or groups o f species have nutritional requirem ents and preferences, and therefore may be favoured when the environm ent is altered in such a way as to increase the relative availability o f the preferred source. In addition to species-specific responses and nutrient uptake requirem ents, the geographic range and biomass o f algae are affected by physical controls and the behaviour o f the harm ful algal species. L ong-distance tran sp o rt o f organisms (e.g., Franks and A nderson 1992), accum ulation o f biomass in response to water flows, buoyancy regulation, and sw im m ing behaviour (Kamykowski 1995), and m aintenance o f suitable environm ental conditions (including tem perature, salinity, stratification, irradiance, and nutrient supply; W hitledge 1993) are critical to understanding the ecosystem response to nutrient loads. O f additional consideration are the trophic interactions controlling the grow th and accum ulation o f algal biomass. C hanges in food web structure attributable to over-fishing, habitat degradation, invasive species, and other factors also interact w ith nutrient loading to increase susceptibility o f a system to H A B s. W h en algae are released from predation due to alterations in th e grazing com m unity, they may proliferate in eutrophic environm ents due to the abundance o f nutrients (M errell and Stoecker 1998, Stibor et al. 2004, Vadstein et al. 2004). C hanges in food web structure affect grazing pressure thro u g h trophic cascading (G ism ervik et al. 1996, P ark and M arshall 2000, T urner et al. 2001); thus, changes at any level o f the cascade may ultim ately im pact the grazing on the algae. O ne area th a t is especially poorly understood is the extent to w hich, and the mechanism s whereby, nutrient enrichm ent may lead to the developm ent o f H A B species th a t are toxin producers. A lthough eutrophication may be associated w ith increasing numbers o f high-biom ass blooms or other H A B events, the specific M a n y b l o o m - f o r m i n g f l a g e l l a t e s h a v e p h a g o t r o p h ic c a p a b i l it ie s - THE AB ILITY TO ENGULF A N D INGEST PARTICULATE M ATERIAL FOR N U TR ITIO N AL PURPOSES, SUCH AS THIS PFIESTERIA CELL FEEDING O N A C R YPTO M O NAD CELL. P H O T O : M . PaR R O W . relationships leading to increased frequency o f toxin producers, and th e increased production o f toxin w ith in these organisms are often poorly characterized. A s many toxins are considered to be secondary metabolites, th eir production w ill depend on th e physiological condition o f the cells (Flynn and Flynn 1995, G ranéli et al. 1998, G ranéli and F lynn 2006). Differences in toxin production may therefore be related to changes in grow th rate or th e grow th conditions o f an organism. For Pseudo-nitzschia, for example, more domoic acid is produced under silica or phosphorus lim itation th a n under nitrogen lim itation, likely due to the fact th at domoic acid is a nitrogen-rich molecule (Bates et al. 1989, 1991). Furtherm ore, when adapted to its nitrogen source, Pseudo-nitzschia appears to produce more domoic acid when grow n on urea th a n on other nitrogen sources (A rm strong and Kudela 2003). G row th on urea is also know n to change the toxin content and composition in the genus Alexandrium (D yhrm an and A nderson 2003). These studies underscore th e im portance o f nutrient source as well as physiological condition in toxin production by H A B species. A nother area th a t is starting to gain attention is how some H A B species increase production not only o f toxins but also o f allelochemicals, particularly under conditions o f nitrogen or phosphorus deficiency. These allelochemicals enable such species to utilize the “scarse” nutrient resources by inhibiting and/or killing th eir com petitors (other algae) and even th eir grazers (G ranéli and Johansson 2003a, T illm an n 2003, Fistarol et al. 2005). In such situations, the effects o f these allelochemicals are enhanced even further, as the other algae are debilitated by nutrient depletion (Fistarol et al. 2005). M onospecific blooms are often found for such species w ith the capability o f producing potent allelochemicals, as in th e case o f Prymnesium parvum (G ranéli and Johansson 2003b, G ranéli and H ansen 2006). 21 A S ynopsis A s a further, more fundam ental complication, different strains w ithin the same species often respond to nutrients differently. A com m on m isunderstanding is th a t the characteristics o f one strain, m aintained for years under highly artificial laboratory conditions, are representative o f all strains o f th a t species in the natural environm ent. This assum ption overlooks the fact th a t for nearly all algal species studied, different strains w ithin the same species generally have shown m arked differences in fundam ental traits such as grow th characteristics, toxicity, bloom -form ing behaviour and responses to nutrients and other environm ental conditions (Cem bella et al. 1987, W ood and L eatham 1992, A nderson et al. 1994, B urkholder et al. 2001, 2005, Burkholder and G libert 2006). In the case o f the cyanobacterium Microcystis aeruginosa, high levels o f nitrogen and phosphorus were found to favour the grow th o f toxic strains over non-toxic strains (Vezie et al. 2002). There is much yet to be understood w ith respect to the response o f individual species to nutrient loading, and how changes in both quantity and quality o f nutrient loading, in conjunction w ith the physical and trophodynam ic state o f the receiving waters, may lead to H A B s. It is therefore im portant to study the grow th and behaviour characteristics o f strains o f a particular species from a num ber o f globally-distributed locations and to do so under natural nutrient concentrations and to collect data adequate for the support o f m odelling (Flynn 2005b). A u t o n o m o u s i n s t r u m e n t a t i o n is p e r m i t t i n g a c q u i s i t i o n o f a r a n g e OF DATA, IN C LUDING NUTRIENT CONCENTRATIONS, BEFORE , DU RIN G , A N D AFTER BLO O M S, AD VA NCING OUR UNDERSTANDING OF THE DYN A M IC RELATIONSHIPS BETWEEN NUTRIENTS A N D H A B OUTBREAKS. PH O TO S LEFT t o r ig h t : W. B o ic o u r t a n d M. T r ic e . Advancing the Study of HABs and Eutrophication Through New Techniques The potential for advances in understanding o f the im pacts o f nutrient pollution on, and relating eutrophication to, H A B s and th eir proliferation is large. N ew and b etter tools are providing m ethodologies for addressing questions related to the complexities o f the nutrient pool, th e tim e and space scales on w hich nutrients are delivered, and algal nutrition. O nly a few tools are illustrated here. A classic dilem m a in assessing w hether, and, to w hat extent, nutrients are stim ulatory to a given H A B is th a t the reporting and sam pling o f bloom events typically occurs after the H A B has developed. By th a t tim e, many nutrients are consum ed, leading to the potentially erroneous conclusion th a t nutrients are unavailable and therefore u nim portant. T raditional approaches for collecting inform ation on nutrient distributions, such as weekly or bim onthly sam pling, are also inadequate to detect the short-lived nutrient pulses th a t often follow meteorological events. D evelopm ent o f in situ nutrient m onitors has provided a means to begin resolving the all-im portant antecedent conditions and to provide sufficient resolution o f the tem poral scale o f bloom events. Use o f these types o f in situ instrum ents in conjunction w ith other rem ote m ethods for resolving additional environm ental characteristics (Babin et al. 2005) may yield the resolution necessary to define the ephemeral changes in nutrients th a t can be significant to phytoplankton cells. The H A B research field has led the oceanographic com m unity in species detection. Advances in molecular approaches are also allowing, for some species, the resolution o f the response o f an individual population w ithin a mixed species assemblage. For 22 A S y n o p sis Enzyme labeled fluorescence (ELF) example, major advances in m arine microbial genomics, such as the sequencing o f Thalassiosira pseudonunni! (A rm brust et al. 2004, D oney et al. 2004) are providing insight into the genetic capacity o f selected algal models. In recognition o f its significance, the Joint G enom ic Institute (http://w w w .jgi.doe.gov) is now sequencing the Aureococcus anophagefferens genome (~32Mb). The availability o f this first full H A B genome w ill allow researchers an unprecedented ability to assess physiological potential in this species, and to examine the genome for clues as to how th is model organism uses nutrients and w hat makes it so successful in certain coastal systems. These genomic advances are driving technology developm ent, and as the cost o f sequencing becomes affordable, there are an increasing num ber o f studies th a t are exam ining transcriptional processes in key organisms (Lidie et al. 2005). Tools such as quantitative polymerase chain reaction (PC R ) and m icroarray chip technology are becom ing available, and these approaches w ill increasingly allow scientists to assay (at the level of gene transcription) how different H A B species respond to changes in their exogenous nutrient environm ent. A dditional advances in protein detection and characterisation w ill help to further define how H A B species respond to eutrophication. For instance, we can now track the phosphorus physiology o f Prorocentrum minim um using antibody probes for a phosphorus-regulated protein (D yhrm an and Palenik 2001). The H A B science com m unity is now poised to bring the full potential o f these exciting advances to the study o f H A B s and eutrophication. Furtherm ore, flow cytom etry, a m ethodology for rapidly m easuring intrinsic or applied (stained) optical characteristics of individual cells, has been used to study the occurrence, abundance, physiology, and diversity o f aquatic m icroorganism s for the past tw o decades. This technology has been increasingly applied to the study o f H A B species, for example, to generally enumerate and specifically identify harm ful algal cells in cultures (Shumway and Cucci 1987, Vrieling et al. 1997) and natural samples (Costas and L opez-R odas 1996, Becker et al. 2002). This tool also has recently advanced our understanding o f H A B s and th eir relation to nutrients and for tracking changes in the complex structure o f aquatic microbial com m unities (including viruses, pathogenic L E F T : P e r c e n t a g e A n t ib o d y L a b e l e d . R I G H T : C e l l - s p e c if ic DETECTION OF ENZYME AC TIV IT Y : E N Z Y M E LABELED FLUORESCENCE ( E L F ) DETECTS A LKALIN E PHOSPHATASE AC TIVITY IN A FIELD SAMPLE FROM THE O regon C o ast. S ource a n d pho to s: S. D y h r m a n . bacteria, and harm ful algae) as related to seasonal cycles, nutrients, or bloom age (e.g., L i and Dickie 2001, Jacquet et al. 2002). Such studies, including recent in situ flow cytom etric investigations o f phytoplankton assemblages, are providing valuable insights about biological factors (e.g., infection, com petition, grazing) th a t influence the occurrence and distribution o f H A B s. Recently, the cell-sorting capacity o f some flow cytom etres has been applied, as well, to assess modes o f nutrition by H A B species (Parrow and B urkholder 2003). Knowledge is also advancing about th e chemical composition o f the nutrient environm ent in w hich H A B s may thrive. The chemical composition o f th e dissolved organic nitrogen pool has long been recognised to be complex, consisting o f a m ixture of multiple com pounds (B ronk 2002). Electrospray ionisation mass spectrom etry is a tool now allowing greater characterisation than has been possible in the past. In contrast to past methodologies for studying the organic m atter pool - w hich were mostly lim ited to bulk analysis o f total carbon or nitrogen, analyses o f a few individual com pounds (e.g. urea, am ino acids, specific proteins), or com pound classes th a t account for a small percent o f total dissolved organic m atter (e.g., total carbohydrates, total proteins; B ronk 2002) - electrospray ionisation mass spectrom etry allows for the exam ination o f a large suite o f individual com pounds in natural mixes o f dissolved organic m atter. This technique also provides molecular w eight inform ation o f ionisable com pounds (in the form o f a m ass-to-charge ratio, m /z, w hich equals molecular w eight for singly charged compounds), and a measure o f concentration as ion abundance (Seitzinger et al. 2005b). As more researchers use this type o f instrum ent, com pound libraries w ill be developed, allowing characterisation o f individual com pounds, and com parison am ong p atterns in use o f specific com pounds in comparable ecosystems and by comparable species in different environments. 23 A S ynopsis “ A subtle, but pervasive, achievement o f biological oceanography is that modelling has become a mainstream activity; it permeates so much o f our work that graduate students in the discipline assume it is integral to biological oceanography. Modelling was at one time an esoteric craft practiced by a gifted few ; now it is the norm. Today’s biological oceanography graduate student is more likely to have a model than a microscope.” Richard T. Barber and Anna K. Hilting (2000), p. 19 Modelling HABs and Eutrophication Fundam ental research on H A B s m ust be guided by and validated w ith observations o f phytoplankton dynamics in nature. To determ ine why H A B s occur, it is necessary to describe the tem poral and spatial developments o f the targ et species in specific ecosystems. This requires observation and m odelling o f the physical-chemical-biological interactions th a t support the specific life strategies o f H A B species in relation to other members o f the plankton comm unity. Consequently, measurem ents o f physical and chemical properties and processes, along w ith quantitative detection and characterisation o f the plankton, m ust be made w ithin a spatial and tem poral fram ew ork th a t is appropriate for characterising bloom dynamics. It is also critically im portant to param etrise the environm ental conditions leading up to a bloom event, and not ju st those associated w ith the term inal phases o f a bloom and its subsequent decline. Effective interaction betw een observationalists and modellers is essential (Flynn 2005b). It is typical th a t w ith in models, the more complex th e description is o f the physical environm ent, the less complex the description is o f the biology. Similarly, the more complex the biology, the less complex is the model o f the physics. A n example o f model hierarchy illustrates this. Z ero-dim ensional models are useful for describing complex biological interactions, but w ith reduced physics. These models can provide test-beds to develop and check param etrisations o f chemical-biological processes such as nutrient uptake, grow th, grazing or production o f toxins. They are appropriate for detailed descriptions o f processes w ith in the cell (e.g., interaction o f the internal nutrient pools and regulation o f toxin production; Flynn 1998). For advanced H A B models, processes such as these can be incorporated into dynamic models o f nutrient assimilation, photosynthesis and pigm ent synthesis as they influence grow th kinetics or toxin production (e.g., John and Flynn 2002, Flynn 2005b). Such form ulations are necessary to lin k nutrient assim ilation and grow th processes w ith variable nutrient quotas, 24 cell pigm ents, and bio-optical signatures o f phytoplankton (Schofield et al. 1999). F u rth er complexity can be provided through one-dim ensional w ater colum n models th a t allow the inclusion o f additional processes, such as m ixing, sinking and buoyancy, nutrient uptake, and benthic-pelagic coupling in a vertically structured environm ent (light, salinity, tem perature, nutrients). Sim ulations can be related directly to experim ents in mesocosms and to observations in the field, if advection is not too im portant. M oreover, water colum n models can be ru n w ith very high vertical resolution to study processes in th in layers (D onaghay and O sborn 1997). Extension to tw o-dim ensional models is useful for exam ining the physicalchemical-biological interactions th a t influence th e distributions and population dynamics o f phytoplankton, for example, in coastal fronts (Franks 1997). W ith the recognition th a t lig h t and nutrient history play a major role in cell grow th and metabolism (e.g., Flynn 2002), the use o f L agrangian ensemble approaches (W oods and B arkm ann 1993, A nderson et al. 2005) may be necessary to unravel the population dynamics o f H A B s in nature. T hree-dim ensional, physical-chemical-biological coupled models are required to simulate the population dynamics o f H A B s comprehensively in an oceanographic context. G iven the scales o f biological and physical variability in response to th e local forcing and far-field effects, three-dim ensional models m ust have the flexibility to allow different resolutions. O w ing to th eir modular model structure, three-dim ensional circulation models can be used for problems w ith different complexity o f the corresponding biological processes. Examples o f a simplified biological approach include the study o f driftin g cells th a t spread in response to w ind-driven circulation or populations th a t accumulate at fronts. M ore complex models w ith physiological com ponents, including dynamics o f grow th, chemical com position, and nutrient assim ilation allow, in principle, comprehensive explorations o f the physical, chem ical, and biological interactions th a t determ ine population dynamics. These models can study th e roles o f sinking, buoyancy, advection, shear flows, and small-scale turbulence. So, in A S y n o p sis METABOLISM INFLUENCED DINOFLAGELLATE DIEL VERTICAL MIGRATION external NlTR.QSEJti ÜIVIs a n < DËSCËNT A5CENÎ r r \ h nui or Listii INTEHBfTY a x is P M O T Ö S t M TM ATË IC A R B O H Y D H A T E O R U H D | addition to accounting for th e physiology o f algal grow th and toxin production, we need to determ ine th e rules th a t govern behaviour (e.g., vertical m igration and variable sinking rates) and the links betw een behaviour and physiology (Cullen and M acIntyre 1998, Kamykowski et al. 1999). T h e c o m p l e x i t ie s o f m o d e l l i n g p o p u l a t i o n d y n a m i c s a r e il l u s t r a t e d HERE FOR A SPECIES TH AT UNDERGOES VERTICAL M IG R A TIO N . T H E FUNCTIO NS (CIRCLED LETTERS) TH AT M UST BE PARAMETRISED IN THIS M O D EL INCLUDE EN VIRO NM ENTAL FORCING, PHYSIO LOG ICAL RATE PROCESSES, BIO C HEM ICAL POOL CONCENTRATIONS, CELL D IV IS IO N , A N D BEHAVIOUR. S o u r c e : G E O H A B 2 0 0 1 , m o d if ie d f r o m K a m y k o w s k i e t a l . 1 9 9 9 . Indeed, many models could be argued as having serious flaws in th eir biological structure (Flynn 2005a, b). W hile experim ental research tends to highlight differences am ong systems, modelers tend to try to identify unifying them es. W hile simplicity is the goal for all m odelling efforts, the extent and means by w hich this is achieved is controversial. M any physical and biological oceanographers claim th a t model com ponents are oversimplified. The m ain problem is th a t while physics is an exact science, biology is not; we simply know too little about the functioning o f H A B ecosystems. As a consequence, biological models often rely on outdated biological knowledge. Some im portant biological processes are often not considered at all, for example, use o f dissolved organic m atter (D O M ), allelopathy, mixotrophy, and prey selectivity related to nutrient status. Effective interactions betw een observationalists and modellers w ill depend on easy interactive access for the non-specialist to m odelling tools, including the com ponent models. In tu rn , the interaction o f observations and m odelling also requires effective means for visualisation and developm ent o f interactive capabilities. Predictions o f H A B -specific coupled models w ill be im proved by well designed m onitoring and use o f data assim ilation techniques to integrate real-tim e and near-real tim e data into a ru n n in g model. D ata assim ilation can thus provide better now -cast predictions of conditions during process studies. These predictions are useful for adaptive sam pling, and they address a principal goal o f G E O H A B , the early w arning and prediction o f H A B s. W h ile much progress has been made, there are a num ber o f critical issues w ith respect to collection o f data th a t impede our progress to m odel H A B occurrence. These include (1) the range o f data types, (2) th e frequency o f m easurem ent, and (3) the units o f m easurem ent. W h ile the im portance o f the first tw o are widely recognized in experim ental studies, the th ird is not. The consequence o f failings in any o f these three areas is th a t most experim ental studies (laboratory and field) are o f little or no use in the developm ent o f models (Flynn 2005b). In the same way th a t experim ents should be designed at th e outset to yield data amenable to statistical analysis, so they should also generate data suitable for the support o f m odelling efforts. To highlight one particular problem in th is regard, the routine m easurem ent of H A B abundance using chlorophyll in vivo fluorescence gives data o f poor value to modellers, as the chlorophylkcarbon (C hl:C ) ratio changes w ith nutrient status and irradiance (including self shading as the bloom develops), and th e ratio betw een the fluorescence signal and chlorophyll also varies w ith nutrient status (K ruskopf and Flynn 2006). F urther, the use o f chlorophyll as a biomass indicator for organisms w ith a high m ixotrophic ability is arguably illogical. For example, the dynamics o f Pfiesteria piscicida could not be m odelled as a function o f chlorophyll as it does not contain chlorophyll, other th a n th a t w hich it has eaten. Indeed, for many physiological processes, expressing th e relationship as a function of chlorophyll versus carbon yields vastly different relationships. The problem o f param etrising H A B biomass is also not resolved by identification o f individual species. To describe and model population dynam ics, distributions o f the targ et organisms m ust be determ ined at the species level; other members o f the 25 A S ynopsis jr • • • J 'ís 'h y to p la n k to n DON U p ta k e' C h em ical L eeching & Bacterial P ro ce sse s E xcretion & ^ M o r t a li t y A SCHEMATIC D IA G R A M OF A NITR OG EN -BASED M O D EL OF M IX ED -LA YER PLANK TO N A N D NITROGEN CYCLING SH OW ING THE FLOW OF NITROGEN A M O N G CO M PARTM ENTS. SO URCE: D . KELLER , R . H O O D , A N D THE I n t e g r a t io n a n d A p p l ic a t io n N e t w o r k , U n iv e r s it y o f M a r y l a n d C e n t e r f o r E n v i r o n m e n t a l S c ie n c e , w w w . i a n . u m c e s . e d u . com m unity should be identified and quantified as taxonom ic or functional groups. This analysis is traditionally done thro u g h visual microscopic exam ination, a slow and tedious process th a t is poorly suited for research on the dynamics o f H A B s. Simply, resources are often inadequate to obtain and analyse enough samples to describe adequately the distributions o f species in space and tim e. A lso, some species are extremely difficult (e.g., w ithin the genus Alexandrium) or impossible (e.g., Pseudo-nitzschia) to distinguish w ith light microscopy alone. These constraints also ham per efforts for routine m onitoring. A utom ated m ethods are needed and several approaches are being pursued, including antibody probes (Shapiro et al. 1989, A nderson et al. 2005b), nucleotide probes (Scholin et al. 1999, A nderson et al. 2005b), and other assay m echanism s such as quantitative P C R (e.g., Coyne et al. 2006) th a t can be adapted for use in sem i-autom ated bulk-sample analysis or single­ cell analysis using flow cytom etry, depending on the approach. In the m eantim e, bio-optical oceanographers continue efforts to extract inform ation on species com position o f phytoplankton from measurem ents o f ocean colour and other optical properties o f surface waters (Stuart et al. 1998, C io tti et al. 1999, Schofield et al. 1999, K irkpatrick et al. 2000). Even where it is possible to m onitor specific algal groups, however, it m ust be recognised th at presence alone does not confer the potential for a toxic or noxious bloom event. To describe and understand ecological controls on the activities and effects o f H A B s in nature, physiological status and toxicity o f the algae m ust be determ ined along w ith identification and quantification. Fortunately, labelling and detection m ethods sim ilar to those for identifying species can be used to assess biochem ical or physiological properties such as the presence o f a toxin (Lawrence and Cem bella 1999), enzyme activity (G onzálezG il et al. 1998), and photo synthetic capability (O lson et al. 1996). 26 Inherently, all models involve some form o f simplification because it is not feasible to include every organism or every species - and the interactions am ong them - explicitly. M any models aggregate the biological com ponents and abstract them into functional groups or com partm ents. Such functional groups may represent, for example, the m ain roles o f production (e.g., phytoplankton), consum ption (e.g., Zooplankton, fish) and decom position (e.g., bacteria). Functional group models are com m only used to simulate phytoplankton and nutrient cycling, but are frequently extended to include Zooplankton, detritus, and the microbial loop. There is an optim al balance betw een model detail and perform ance (Fulton et al. 2003). Too much complexity leads to uncertainty (particularly in param étré definition) and problems in interpretation o f the models’ dynamics and predictions; too little complexity and the models cannot reproduce realistic behaviour. However, a m eta­ analysis o f data for process-oriented planktonic ecosystem models (based on 153 studies published betw een 1990 and 2002) indicates th a t increasing complexity does not improve th e fit w ith data (A rhonditsis and B rett 2004, A nderson 2005). Ultimately, the selection o f model complexity should be driven by questions being asked o f th e system under study, but lim itations on the complexity may arise from the data available. M uch o f the research supporting our phenom enological understanding o f H A B species physiology is inadequate for the param etrisation o f models. Research is required to seek a more rational determ ination o f model complexity to address the problems under consideration (M oll and Radach 2003). This m ust also be considered w hen submodels are lifted from one m odelling system and applied to a new m odelling system - the original caveats in th e design and use may well be ignored. In the context o f H A B s, th e developm ent o f planktonic models w ith a stronger physiological basis are required if we are to obtain more accurate simulations. The key stages in defining the structure o f an ecological model are to concentrate the degree o f resolution or representation to the m ain targ et species and to make increasing simplifications or decrease the resolution both up and dow n from the target level (de Young et al. 2004). A S y n o p sis The Rhomboidal Modelling Approach 0.1 Predators H A B /Z ooplankton focus at & o 0,06 (5 >-■ £ 0,04 d ia to m s Life h isto ry W ith o u t life \ h isto ry ' £ Greatest fu n ctio n a l c o m p le x ity at th e area o f focus N u trie n t/B a cte ria / Protozoan focus C hem istry 1 10 100 1000 Ir ra d ia n c e ( p m d p h o to n s n r 2 s e c 1) Functional co m p le xity G e n e r a l t r e n d in t h e v a r i a t i o n s in t h e c h l o r o p h y l l a : C r a t io f o r S c h e m a t ic i l l u s t r a t i n g t h e r e l a t io n s h i p b e t w e e n t r o p h ic l e v e l PROKARYOTES A N D FOR DIATOM S AS A FUNC TIO N OF THE IRRADIANCE AT A N D FU N C TIO N A L COM PLEXITY. T H E R H O M BO ID ILLUSTRATES THE REGION W H IC H THEY WERE G RO W N . REVISED A N D REDRAWN FROM M a c I n TYRE A N D OF PRIMARY FOCUS FOR H A B M O D ELS. REVISED FROM DE Y O U N G ET AL. C u lle n 2 0 0 5 . 200 4. To model H A B s, we need to consider representing the target species explicitly, possibly including th e key stages o f its life history and its links to the environm ent. For the H A B s th a t are m ixotrophs, such an approach may be more difficult, as the H A B may serve as both prim ary producer and consumer. M odelling m ixotrophy is probably one o f the m ost challenging activities in plankton modelling. A n im portant aspect o f m odelling data is th a t it allows an expert user to infer from it aspects o f the system th a t the model is not able to simulate directly. In the same way th a t a w eather forecaster interprets inform ation about pressure and moisture fields from a num erical w eather prediction model to infer w hat the w eather w ill be, a marine environm ental forecaster may be able to take inform ation from a numerical ecosystem prediction m odel to predict aspects o f the ecosystem not directly simulated. For example, we know th a t H A B events often coincide w ith distorted nitrate:phosphate ratios and low turbulence, and th a t toxin production often occurs when the phytoplankton are nutrient stressed. C urrently, we cannot make species-specific simulations o f H A B s but we can simulate these indicators. In principle, we can combine inform ation from hydrodynam ic and ecosystem models to provide probability-based risk maps o f H A B occurrence. Modelling is intricately linked with observation and experimentation, and both models and predictive systems will be improved as new analytical approaches aid experimental research, and new monitoring systems yield data on time and space scales appropriate for the processes of interest. A generic m odelling system may facilitate comparative studies, by providing a consistent m odelling fram ew ork th a t can be applied in all regions. This fram ew ork needs to be m odular to allow simulations o f differing complexity according to the task at hand. C rucial to this is a consistent model evaluation procedure on a num ber o f levels (temporal, spatial, and functional) and the generation o f error statistics. O u r current lack o f physiologically robust species-specific H A B models implies th a t forecasting o f a species-specific H A B event is probably not consistently achievable in the short term . A lthough m odelling has evolved enormously as a discipline and as an integrated activity th roughout all o f oceanography and ecology (e.g., Barber and H iltin g 2000), if our understanding o f events surrounding the developm ent o f H A B s is to be judged by our ability to simulate th eir occurrence, then there is much w ork to be done. The developm ent o f a new generation o f physiologically robust H A B models w ill require better synergy am ong modellers, observational approaches, and experim entalists. P ro cess S tud ies E a rly W arm n g and Prediction S y stem s 27 A S ynopsis o> Tbticbatiiiii Longitude (deg.) Toward a Consensus on Eutrophication and HAB Proliferation In developing the recom m endations and priorities for study in the area o f H A B s and eutrophication, it is recognized th a t there are fundam ental processes, trends, principles, and relationships th a t are broadly accepted. G E O H A B w ill build on these, and w ill use these in guiding the developm ent o f Core Research. Several recent docum ents are highlighted here to illustrate the com m unity consensus on the role o f eutrophication in H A B proliferation. Each o f these efforts highlighted here was developed w ith com m unity involvement. W h ile it is impossible to encapsulate all o f the debate and discussion th a t occurred in developing these consensus docum ents, nor to fully represent all such docum ents em erging around the world, these examples illustrate the breadth o f issues on w hich there is broad agreement. These docum ents also consistently underscore the fact th a t an ecosystem approach w ill be required to fully understand the relationships betw een H A B s and eutrophication. W ith the role o f eutrophication in the developm ent o f many H A B s now well recognised and affirmed by various scientific and policy groups, the specific questions for fu rth er advancem ent can now be posed, and Core Research can be developed. I■ M' *■ ■¡.--.iirvT«) --------------- 28 In 1999, the US N ational C enter for C oastal O cean Science completed an assessment o f estuarine eutrophication (Bricker et al. 1999). This assessment, w hich w ill be updated in 2006, was based on results o f a survey conducted over th e years 1992-1997 on nutrient inputs, population projections, and land uses. It included more th a n 138 estuaries, representing more th a n 90% o f the estuarine surface area o f the U nited States. A m ong th e key findings T h e " D e a d Z o n e " ( r e d a r e a ) o f t h e G u l f o f M e x ic o . S o u r c e : N . R a b a l a is , L U M C O N . Gulf of Mexico, USA Hypoxia in the Gulf of Mexico, USA, is the result of nitrogen loading from the Mississippi River which drains significant agricultural lands. This nitrogen leads to large algal blooms, which, in turn, sink to the bottom and decay. The hypoxic zone in this region in the late 1990s was twice the extent that it was in the late 1980s. One HAB species, Pseudo-nitzschia, has been found to be increasing in this region in direct proportion to the increasing nitrogen loads in the Gulf of Mississippi. N itrate le a d in g (pM ) The average abundance (%) of the diatom Pseudo-nitzschia in the sedimentary record as a function of the nitrate loading in the northern Gulf of Mexico. Redrawn from Turner and Rabalais 1991 and Parsons et al. 2002. symptoms o f eutrophication are prevalent in US estuaries; hum an influence on the expression o f eutrophic conditions is substantial; and im pairm ent to estuarine resources, and fisheries in particular, is high. A S y n o p sis S e d im e n t s a n d p h y t o p l a n k t o n b l o o m , m o u t h o f t h e Y a n g t z e , E a s t L a r g e r e d t id e s w e r e v is ib l e in t h e e a s t C h i n a S e a , 2 0 0 5 . P h o t o : C h i n a S e a . P h o t o : N A S A V is ib l e E a r t h , v is ib l e e a r t h . n a s a . g o v . J . L i. China The average number of harmful blooms (red line), in addition to their areal extent and duration along the east coast of China have escalated in the past tw o decades coincident with the increasing use of synthetic fertiliser (blue bars). ie 1970 1475 1950 1455 1990 1495 B In M arch 2002, the International C ouncil for th e Exploration o f the Sea (IC E S) convened a workshop in The H ague, The N etherlands, on the linkage betw een eutrophication and its effects on phytoplankton. Consensus was reached on the following points: • There is evidence th a t anthropogenic nutrient supply to coastal waters is leading to altered phytoplankton dynamics. • W h ile the underlying factors (mechanisms) and ecological principles (e.g., dose-yield responses) apply universally, the outcome o f th e collective phytoplankton response to nutrient loading is often site-specific; functional group responses are more predictable th a n species-level responses. • Useful models (for m anagem ent purposes) o f the effects o f nutrification on phytoplankton mass balances and functional group responses are attainable. • M an y knowledge and technical gaps exists, am ong w hich are the need for field observations to be carried out on tim e and space scales th a t m atch processes, ecophysiological data in support o f models, and better understanding o f the relationships betw een food web alterations and nutrient enrichm ent. 2000 Redrawn from Anderson et al. 2002 and Zhou 2005. 29 A S ynopsis In January 2003, the US Environm ental Protection Agency sponsored a scientific “roundtable” w ith the objective o f developing a scientific consensus on the role o f eutrophication and H A B s. A pproxim ately 25 scientists from th e US were in attendance. Based on keynote presentations and discussion, the following statem ents were unanim ously adopted: • D egraded water quality from increased nutrient pollution prom otes the developm ent and persistence o f many H A B s and is one reason for their expansion in the US and the world. • The com position - not ju st the total quantity - o f the nutrient pool im pacts H A B s. • H igh-biom ass blooms m ust have exogenous nutrients to be sustained. • B oth chronic and episodic nutrient delivery prom ote H A B development. • Recently developed tools and techniques are already im proving the detection o f some H A B s, and em erging technologies are rapidly advancing tow ard operational status for the prediction o f H A B s and th eir toxins. • E xperim ental studies are critical to fu rth er the understanding o f the role o f nutrients on H A B expression, and will strengthen prediction and m itigation o f H A B s. M anagem ent o f nutrient inputs to the w atershed can lead to significant reduction in H A B s. 30 P h y t o p l a n k t o n b l o o m in G u l f o f C a l i f o r n i a . P h o t o : NASA V is ib l e E a r t h , v is ib l e e a r t h . n a s a . g o v . Gulf of California, USA Runoff from agricultural regions has recently been associated w ith massive blooms in the Gulf of California. These blooms were found to occur w ithin days of fertiliser applications (Beman et al. 2005). Total nitrogen loads from these applications are sufficient to support these massive blooms. For example, urea concentrations can exceed 40 pMol N L-1 following fertilisation (Glibert et al. 2006). 300 Nitrogen applied to Agriculture in Yaqui Valley 250 I 200 |¡ 150 I o à 100 50 19SO 1900 1970 19S0 1990 2000 Source: International Maize and Wheat Improvement Center (CIMMYT database). In 2004, the Study G roup to Review Ecological Q uality Objectives for E utrophication (S G E U T ) convened by IC E S evaluated the linkage betw een eutrophication and H A B s in E uropean coastal w aters (Aertebjerg and Sm ayda2004). The S G E U T report emphasised the complexity o f the relationship betw een H A B s and eutrophication by affirm ing th a t H A B s are an ecosystem -regulated response and not the result o f simple, linear responses to nutrients, being also influenced by microbial and grazer com m unities and physical oceanographic conditions. A S y n o p sis P h y t o p l a n k t o n b l o o m in t h e B l a c k S e a . P h o t o : N A S A V is ib l e E a r t h , v is ib l e e a r t h . n a s a . g o v . Black Sea Phytoplankton blooms in the Black Sea in the mid-1990s increased to levels more than 20-fold higher than were observed in the 1960s. These increases were especially apparent during the rapid escalation of nitrogen and phosphorus loading during the 1980s. Dinoflagellate abundance increased 4-fold over this period, while diatom abundance decreased. 16 7G U ED u 10 SV e ah £ 4 20 2 10 0 0 Modified and redrawn from Oguz 2005. 1 ■U S G S G £ i ï 1 S In O ctober 2004, the 3rd International N itrogen Conference was held in N anjing, C hina w ith an aim tow ard understanding the “im pacts o f population grow th and economic developm ent on the nitrogen cycle: consequences and m itigation at local, regional, and global scales”. The participants o f the conference affirmed, among other statem ents, that: “...in many parts o f the world, significant am ounts o f reactive nitrogen are lost to the environm ents in agricultural and industrial production and fossil fuel com bustion. This has lead to disturbances in the nitrogen cycle, and has increased the probability o f nitrogen-induced problems, such as pollution o f freshwater, terrestrial and coastal ecosystems, decreasing biodiversity, and changing clim ate, and poses a th reat to hum an health.” In 2005, the European E nvironm ental A gency reported th at “E u tro p h icatio n ...is still one o f the major environm ental problems across E urope” (www .eea. eu.int, R eport No. 7/2005). E utrophication affects waters from inland w ater bodies such as groundw ater, rivers and lakes, to transitional and coastal waters and ecosystems in open seas. They fu rth er affirmed that: ru n o ff from agricultural activities is the m ain source of nitrogen pollution and nitrogen loads parallel the surplus o f nitrogen applied to large agricultural catchments; households and industry are th e major sources o f phosphorus pollution; and discharges from nitrogen and phosphorus are showing declines based on measured to reduce these inputs, but have been larger for phosphorus w ith reduced point sources. 31 4. Priorities and Challenges for Understanding the Relationship Between HABs and Eutrophication t ¡ ~ È - , or many years, research on HABs was undertaken in response to specific events, and conducted in localised areas where specific problems occurred. Over the past decade, enormous strides have been made internationally in linking the global community of scientists to better understand the factors that lead to specific blooms, their population dynamics, and their impacts, and to do so in a comparative framework where research questions are B a l t i c S e a c y a n o b a c t e r ia b l o o m . P h o t o : K . K o n o n e n . similar. In some cases, comparative studies have shown that similar processes are controlling blooms in similar ways. In other studies, apparently similar species occur in various parts of the world, but they differ in growth dynamics or expression of harmful attributes. O f relevance to th e focus on the role o f eutrophication in H A B proliferation, a comparative approach is highly beneficial in the ability to compare gradients o f responses. For example, discharge from land in Europe and A sia appears to be significantly higher th a n in much o f the rest o f the world. These higher loads may potentially hold the key to understanding some o f th e dynamics o f differential H A B occurrences. A specific example is th a t o f the colonial prym nesiophyte Phaeocystis, w hich is th o u g h t to have proliferated in th e N o rth Sea as a result o f changing nutrient loads and ratios from the major rivers o f the region have been modified (Lancelot et al. 1987, 2005). In contrast, however, blooms of Phaeocystis have only a lim ited range in the U nited States, localised prim arily in th e G u lf o f M aine and Cape C od Bay, and are o f significantly smaller scale and duration (A nderson et al. 2005a). D oes th is difference reflect lower nutrient loads or different nutrient ratios, differences in species or strains o f Phaeocystis, or other ecological factors? 32 Key Features fa rm in g d eve lo pm en t pho sp ho ru s load w a te r tre a tm e n t plan ts n itro g e n load in d u stry a tm o sp h e ric n itro g e n tra n s p o rta tio n Prim ary Symptom Expressions R high ch lo ro p h yll X a qu a tic biota to x ic /h a rm fu l bloom SAV high dissolved oxygen m a cro alga e bloom high w a te r q u a lity Secondary Symptom Expressions « * s h e llfis h c o n ta m in a tio n o r die o ff algae die o ff fis h leave or die o ff lo w w a te r q u a lity A lo w dissolved oxygen i loss o f lig h t p e n e tra tio n a eroso lize d to x in s SAV loss H ighlighted in this chapter are th e key questions th a t can and should be studied on an international comparative basis. Examples are provided o f detailed research questions in comparative systems. In m ost cases, suitable sites and suitable comparative studies are numerous; thus, the identified questions and systems are representative but not a comprehensive review. This section concludes by outlining several specific, but tractable, challenges th a t should also be addressed w ith high priority. C o n c e p t u a l d ia g r a m o f t h e p r o c e s s o f e u t r o p h ic a t io n . S o u r c e : t h e I n t e g r a t i o n a n d A p p l i c a t i o n N e t w o r k , U n i v e r s it y o f M a r y l a n d C e n t e r f o r E n v i r o n m e n t a l S c ie n c e , w w w . i a n . u m c e s . e d u . Major HAB impacts in the coastal United States 9 C j« o o b # c t* rtí 0 N cnjrctoocic S fn flfisii ! ' * P*.*lylK A A m o « « S h e i t i i i i f e o * » n lo g ■ C i g i u t a * f n ii f o f e w m g 0 P*1*iompJe» G Brawn T«tfe □ A WUtr-.ultytr i H d if r r jC ú r i rr>h. l.w rl. rrv im iru d f t > jil.H im ijr:l jMtuAtHvMjpUnrtfl Uh V i r t u a l l y e v e r y c o a s t in t h e U n i t e d S t a t e s is n o w i m p a c t e d b y HABs, AND THE SAME IS TRUE THROUGHOUT MUCH OF THE WORLD. S ource: D. A n d e r s o n , W H O I N a t i o n a l O f f ic e f o r M a r i n e B io t o x in s a n d H a r m f u l A l g a l B l o o m s . 33 Key Q u e stio n s n < i □ 1'-io ■ fttO EZÜ20-SO ■ »100 □ io « » □ hkfwo E l >500 Key questions to be addressed in the understanding the ecology and oceanography of HABs in eutrophic systems QUESTION 1 G l o b a l d i s t r i b u t i o n o f P r o r o c e n t r u m m i n i m u m is s h o w n ( r e d d i a m o n d s ) s u p e r im p o s e d o n t h e g l o b a l e x p o r t m a p o f N f r o m r iv e r s ( f r o m S e it z in g e r a n d K r o e z e 1 9 9 8 ) . T h e h ig h e s t d e n s it y o f OBSERVATIONS OF THIS SPECIES OCCURS IN REGIONS WHERE G LOBAL D I N EXPORT IS PREDICTED TO BE H IG H . REPLO TTED FROM H E IL ET A L . 2 0 0 5 and G l ib e r t a n d B u r k h o l d e r 2 0 0 6 . Diminishing Î*, Increasing hm increasingly eutrophic coastal temperate frontal temperate shallow s h e lf O ne o f the im portant objectives o f comparative studies on H A B s is to understand com m onalities th a t drive plankton com m unity structure. The concept o f “functional groups” provides a basis for simplification in order to improve our predictive ability relative to the dynamics o f the system. The term “functional group” (or “life form ”) is operationally defined to mean “a non-phylogenetic classification leading to a grouping o f organisms th a t respond in a similar way to a syndrome o f environm ental factors” (G itay and N oble 1997). The organisms w ithin a functional group share one or more adaptations to those environm ental conditions (M argalef 1978). (sp rin g ) highly post opwelling stra tifie d tro p ic a! M a r i n e p é l a g i a l h a b it a t s a s a f u n c t i o n o f l i g h t a n d n u t r i e n t f ie l d s ( S m a y d a a n d R e y n o l d s 2 0 0 1 ) . T h e s e h a b it a t s s e l e c t f o r H A B SPECIES, FUNCTIONAL GROUPS, OR LIFE FORMS AND REFLECT THE ABILITIES Smayda and Reynolds (2001) have provided a conceptual model for classifying H A B dinoflagellates by functional group. They identified nine distinct and diverse habitat types in w hich dinoflagellates may bloom. Associated w ith each habitat type are dinoflagellates life forms th a t ordinate on a habitat tem plate along onshore-offshore gradients o f decreasing nutrients, reduced m ixing, and deepening o f the euphotic zone. Several o f these functional groups are associated w ith nutrient-rich or eutrophic waters. For example, dinoflagellate genera, such as Prorocentrum, Phaeocystis, Heterocapsa, Scrippsiella, Cochlodinium, Heterosigma and others, have adaptations m aking them suitable for thriving in nutrient-rich waters. Increasing evidence suggests th a t some o f these species also may be increasing in their global extent. 34 OF THE SPECIES TO GROW AS PHYSICS OR BEHAVIOUR ALTER VERTICAL DISTRIBUTIONS AND GROWTH POTENTIAL IN ALL HYDROGRAPHIC REGIMES. A sim ilar classification based on functionality has been developed for freshwater phytoplankton (Reynolds et al. 2002). This conceptual model is based on tolerances and sensitivities to a range o f physical and nutrient conditions. Some nitrogenfixing cyanobacteria species capable o f form ing blooms, such as Cylindrospermopsis raciborskii, are able to tolerate low nitrogen conditions, while others, such as Microcystis, appear to favour eutrophic conditions. Such a fram ew ork provides a context for comparative studies o f species. Sim ilar matrices w ill also need to be developed for other H A B species. Key Q u e stio n s A ltered nutrient loads have been associated w ith increasing abundance o f Pseudo-nitzschia in the northern G u lf o f M exico since the 1950s (Parsons et al. 2002). D u rin g the same period o f tim e, nitrogen loads have doubled, while silica availability has decreased substantially (Turner and Rabalais 1991). This relationship has also been observed in E uropean waters, where long-term increases in nitrate loading parallel the increase in abundance o f th is genus in the sedim entary record. Relatedly, in the w estern and northern A driatic Sea, a shift from predom inantly red tide blooms to more frequent m ucilaginous blooms occurred in the 1980s coincident w ith phosphorus removal from detergents and expansion o f sewage treatm ent plants (Sellner and F onda-U m ani 1999); however, cause and effect rem ain to be determ ined as m ucilaginous events were also recorded in the region more th an 200 years ago. O ne o f the high-biom ass H A B species th a t has been shown to increase in abundance following events th a t lead to runoff and increased nutrient delivery is Prorocentrum minim um (H eil et al. 2005). This species can develop to high standing stocks and result in harm ful im pacts on benthic habitat, including hypoxia/anoxia, as well as on shellfish, depending on th eir life stage. Blooms o f this species have been observed in tropical, subtropical, and tem perate regions (H eil et al. 2005). P. minimum is one o f many m ixotrophic species and w ill grow well when organic nutrients are supplied (L i et al. 1996, C arlsson et al. 1998, G libert et al. 2001). H um ic and fulvic acids from Swedish rivers increased P. minimum intracellular nitrogen pools, cell numbers, and grow th rates relative to cultures free o f natural organics (G ranéli et al. 1985). In tributaries o f Chesapeake Bay, U SA , P. minim um also appears to grow and develop into massive blooms th a t have been associated w ith declines in submerged grasses and benthic habitat (Gallegos and Jordan 2002), and have proven to be toxic under some grow th conditions (W ikfors 2005). These blooms have increased in m agnitude w ith the eutrophication o f the Chesapeake Bay, and cell counts are now comm only more th a n an order o f m agnitude higher th a n recorded ju st tw o decades ago. These blooms, like those in Sweden and many other regions o f the world, appear to intensify and expand in response to delivery o f nutrients from rainfall events, and in particular, from organic nitrogen delivery (G libert et A BLOO M OF THE DINOFLAGELLATE LINGULODINIUM POLYEDRA RESULTED IN A VISIBLE RED TIDE OFF SOUTHERN C A L IF O R N IA , U S A , SUMM ER 2 0 0 5 . P hoto: S. O h . al. 2001, H eil et al. 2005). Through comparative studies it should be possible to ascertain w hether these sim ilar factors are indeed stim ulating these blooms, and w hether these blooms are increasing in frequency globally. Com parisons o f the physical dynamics o f the regions, quantification o f nutrient delivery and the trophic dynamic com position o f th e plankton at the tim e o f nutrient inputs, and the detrim ental im pacts o f these blooms w ill be extremely valuable in developing predictive capabilities. In addition, com parison o f strains w ill yield insight into comparative physiology, including the ability o f different strains to produce toxins in response to certain environm ental factors. Suitable models are needed to explore the interactions betw een nutrient status, physiological ability, and other factors leading to changes in biomass. 1. Can functional groups of HABs be defined that are 2. Are there specific adaptive strategies used by HABs in 3. Can the nutritional strategies of different HAB species be used to predict their responses to eutrophication? 4. How does intraspecific variability (strain differences) in response to eutrophication control HAB development and 35 Key Q u e stio n s A CROSS-ISOBATH TRANSPORT MECHANISM FOR INITIATION OF At EXANDRtUM BLOOMS UPWELUNG lALTlkalr DOWNWELUNG I/AUtf; MxjtJtinfj, P h y s ic a l e n v ir o n m e n t s in t e r a c t w i t h o r g a n i s m b e h a v i o u r in BLO O M IN IT IA T IO N , AS SHOW N HERE FOR ALEXANDRIUM BLO O M S . D U R IN G UPW ELLING -FAVO UR ABLE W IN D S , THE PLUME TH IN S VERTICALLY A N D EXTENDS OFFSHORE, OVERLYING THE CYSTS SEEDBEDS. ALEXANDRIUM CELLS ARISING FROM THE CYSTS SW IM TO THE SURFACE A N D ARE TRANSPORTED ALON G THE COAST W IT H D O W N W E LLIN G -FA V O U RABLE W IN D S . SOURCE: population’s range and biomass are affected by physical controls such as long-distance tran sp o rt (e.g., Franks and A nderson 1992), accum ulation o f biomass in response to water flows and sw im m ing behaviour (Kamykowski 1974), and the m aintenance o f suitable environm ental conditions (including tem perature and salinity, stratification, irradiance, and nutrient supply; W hitledge 1993). M c G il l ic u d d y e t a l . 2 0 0 3 . QUESTION 2 To what extent do residence time and other physical processes impact the relationship between nutrient loading and HAB proliferation? O ne o f the difficulties in lin k in g nutrient loading to H A B s is the m ultiplicity o f factors contributing to H A B species responses to nutrient loading. A n understanding o f th e physical dynamics o f blooms in relation to nutrient dynamics w ill help lead to an understanding o f how bloom dynamics may differ w hen nutrient loads are sim ilar but physical controls are different. Physical processes are im portant at every stage o f bloom dynamics. The initiation o f a bloom requires successful recruitm ent o f a population into a water mass. This may result from excystment o f resting cells during a restricted set o f suitable conditions (e.g., Alexandrium in the G u lf o f M aine; A nderson 1998), tran sp o rt of cells from a source region where blooms are already established, or response to unusual clim atic or hydrographic conditions (e.g., Pyrodinium bahamense in the Indo-W est Pacific; M aclean 1989). Once a bloom is initiated, physical processes controlling bloom tran sp o rt are o f param ount im portance. C oastal currents driven by w ind, buoyancy, or other factors can tran sp o rt blooms hundreds or even thousands o f kilom eters along the coast, often from one m anagem ent area to another. U nderstanding the physical dynamics underlying these tran sp o rt pathways is essential to effective m anagem ent and m itigation o f H A B effects. A 36 Physical processes th a t are likely to influence the population dynamics o f H A B species operate over a broad range o f spatial and tem poral scales. Large-scale circulation affects the distribution o f w ater masses and biogeographical boundaries. M any examples may be found o f the influence o f mesoscale circulation on H A B population dynamics. Eddies from th e deep ocean can, for example, im pinge on slope and shelf regions, affecting the transfer o f algae and nutrients across the shelf break. This type o f tran sp o rt may be involved in th e delivery o f the Florida red tide organism Karenia brevis to nearshore waters from an offshore zone o f initiation (Steidinger et al. 1998). A lthough eddies are difficult to resolve thro u g h sam pling at sea, they can usually be detected thro u g h rem ote sensing o f tem perature, sea-surface height, or ocean colour. U nderstanding o f the m ain features o f circulation is often sufficient to devise models th a t may be used to guide decision-m akers on the movement and developm ent o f H A B s, although such predictions are still mostly rudim entary. Processes at interm ediate scales result in the form ation o f convergence zones, fronts, and upwelling. The retentive nature of some sem i-enclosed coastal systems, such as estuaries and fjords, can produce long residence tim es leading to prolonged suitable periods for cells to thrive (Cem bella et al. 2005). The im portance of fronts in bloom developm ent, including H A B s, is now recognised and included in some m odelling efforts (Franks 1992). A linkage has been dem onstrated, for example, betw een tidally generated fronts and the sites o f massive blooms o f the toxic dinoflagellate Gyrodinium aureolum (=Karenia mikimotoi) in the N o rth Sea (H olligan 1979). The typical p attern is th a t o f a high surface concentration o f cells at th e frontal convergence, contiguous w ith a subsurface chlorophyll m axim um th a t follows the sloping interface Key Q u e stio n s betw een the tw o water masses beneath th e stratified side o f the front. The signature o f the chlorophyll m axim um , sometimes visible as a “red tide”, may be 1-30 km wide. Chlorophyll concentrations are generally lower and much more uniform on the w ell-m ixed side o f the front. The significance o f th is differential biomass accum ulation is best understood when movement o f the front and its associated cells brings toxic dinoflagellate populations into contact w ith fish and other susceptible resources, resulting in massive m ortalities. This is an example where physical-biological coupling results in biomass accum ulation, and larger-scale advective m echanism s interact synergistically in a way th a t results in harm ful effects. The im portance o f small-scale physical processes in H A B developm ent is observed in the layering o f the physical, chemical, and biological environm ent in stratified coastal systems. O ff the French coast, for example, a th in layer o f dinoflagellates, including the H A B species Dinophysis cf. acuminata, has been observed in the region o f the therm ocline (G entien et al. 1995, 2005). The same pattern is found for Dinophysis norvegica in the Baltic Sea, where a 1-2 m th ick layer w ith up to 80 000 cells-1 is usually situated betw een 20-25 m depth, where light is less th an 1% (Gisselsson et al. 2002). Several H A B species are well know n for th eir ability to form these th in , subsurface layers o f uncertain cause and unknow n persistence, at scales as small as 10 cm in the vertical and 10-1000 km in the horizontal (Gisselsson et al. 2002). O ne explanation is th a t these layers result from the stretching o f horizontal inhom ogeneities by the vertical shear o f horizontal currents. This produces an environm ent potentially favouring motile organisms th a t can m aintain th eir position in th is layer or organisms able to regulate th eir buoyancy. A ny comprehensive study o f H A B s, including studies focussing on nutrient controls, should incorporate an understanding o f the physical conditions th a t can lead to cell aggregation and dispersion. L E F T : D in o p h y s is a c u m in a t a . P h o t o : w w w . s d n - w e b . d e . R I G H T : D i n o p h y s i s b l o o m in c o a s t a l N o r w a y . P h o t o : H a v f o r s k n in g s in s titu t te t, I n s t it u t e o f M a r in e R e s e a rc h , w w w . a lg e in fo . im r .n o . The need to understand physical dynamics has further im portance in understanding the relationship w ith nutrients. For example, the responses o f phytoplankton to fluctuating lig h t (due to sw im m ing behaviour and turbulence) are not the same as responses to the average exposure. There is cause to suspect th a t synergistic events may prom ote toxicity above th a t w hich would be expected from knowledge o f th e im pact o f individual processes (Flynn 2002). Similarly, for nutrient uptake, the kinetics o f uptake measured under steady-state conditions may not apply w hen cells are exposed to highly variable nutrient environm ents, w hether from changes in vertical nutrient structure or from exposure to ephemeral patches o f nutrients (e.g., G oldm an and G lib ert 1983). Thus, there are many needs for understanding the relationships between physical environm ents and the physiological responses o f the cells. Com parisons am ong sim ilar species in different hydrographic regim es, as well as comparisons across species in similar hydrographic regim es, w ill be useful in th is regard. Examples of Relevant Detailed Questions 1. Do residence time and physical processes impact the likelihood for different species to bloom under eutrophic conditions in different ways or at different stages in the life cycle? 2. What are the physical factors that contribute to the persistence of high-biomass blooms in eutrophic systems? 3. To what extent do systems w ith the same indigenous species and nutrient load develop similar blooms when physical processes differ? 4. How do physical processes control the physiological responses of different species to light and nutrients? 37 Key Q u e stio n s + W ater MPB clarity Biom ass Nutrient av ailab ility Brown tide biomoss MPB Productivity MPB Biomass ■=> % Browntide productivity Water N u trient Brawn tide clarity availability productivity n MPB s i ^ Productivity G row n tid e bio m ass C o n c e p t u a l r e l a t io n s h i p s b e t w e e n m i c r o p h y t o b e n t h o s (MPB) and QUESTION 3 blo o m s of How do feedbacks and interactions between nutrients and the planktonic, microbial food webs impact HABs and their detrimental effects? UPPER PANEL, A BENTHIC DO M IN ATE D STATE W IL L LEAD TO LO W BROW N TIDE A u r e o c o c c u s a n o p h a g e f f e r e n s ( b r o w n t id e ) . N o t e in t h e BIO M ASS, W HILE IN THE LOWER PANEL, PELAGIC NUTR IEN T- ENRICHMENT CAN LEAD TO HIGH BROWN TIDE BIO M AS S. M O D IF IE D A N D REDRAWN FROM M a c I ntyre et a l . 2 0 0 5 . N utrient enrichm ent can stim ulate H A B s not only directly by th eir support o f increased biomass, but indirectly, in more subtle but significant ways over the long term - for example, by increasing hypoxia th at, in tu rn , elim inates filter-feeding shellfish th a t w ould have consum ed H A B s but th a t can no longer survive in the habitat (Burkholder 2001, G libert et al. 2005, Smayda 2005). Similarly, im pacts o f nutrients may be rem oved in tim e and space from the nutrient source as nutrients are consum ed, recycled, and transform ed, leading to complex - and often indirect - interactions betw een nutrients and H A B s (G libert and Burkholder 2006). In addition, bacterial interactions are often o f critical im portance in the developm ent o f toxins w ithin some H A B species, and these interactions may occur differently under varying nutrient regimes. H arm fu l algae do not exist in isolation in natural ecosystems, and are influenced by interactions betw een individual cells o f the same and other members o f th e planktonic comm unity. Food-web interactions have a profound effect on the population dynamics o f H A B species. Processes such as com petition and grazing can affect the net grow th rates o f algae in nature. O n the other hand, grazing may also increase nutrient regeneration, thus altering the availability o f some nutrient forms for the algae to consume (G libert 1998, M itra and Flynn 2006). Viruses are now know n to have significant im pacts on the dynamics o f m arine com m unities and some have been found to infect algae and have been im plicated in the demise o f red or brown tide blooms (F uhrm an and Suttle 1993). Likew ise, bacteria play an im portant role in controlling many H A B s and regulating th eir im pacts, including their toxicity. Bacteria may also interact w ith H A B s in a positive m anner by stim ulating th eir grow th (Fistarol 38 et al. 2004a). Unique microbial com m unities are often associated w ith nutrient-rich systems, affecting internal nutrient cycling. C yanobacteria, in particular, establish m utually beneficial consortia, by chem otactically attracting and supporting m icro­ organisms involved in nutrient cycling and the production of grow th factors (Paerl and M illie 1996). A different type o f bacterial interaction w ith H A B species was described by Bates et al. (1995), who showed th a t th e toxicity o f the diatom Pseudo-nitzschia was dram atically enhanced by the presence o f bacteria in laboratory cultures. The extent to w hich any o f th e above interactions occur in natural waters, and affect H A B dynamics and/or toxicity, is not know n, representing an im p o rtan t line o f inquiry. The effects o f nutrient inputs on H A B s are not always direct; there are indirect pathways by w hich nutrients can influence the developm ent o f H A B s. For example, nutrients may be consum ed and/or transform ed from one form to another, thereby increasing th eir bioavailability for H A B species. O n a seasonal scale, in estuarine systems such as th e Chesapeake Bay, U SA , nutrient input in the spring is delivered largely in the form o f nitrate and is rapidly assim ilated by diatom s w hich, in tu rn , sink and decompose. Subsequently, during the w arm er sum m er m onths, nitrogen is released via sedim ent processes in the form o f am m onium , w hich th en supports an assemblage dom inated by flagellates, including dinoflagellates (M alone 1992, G libert et al. 1995). The shallow, brackish Baltic Sea is characterised by efficient cycling o f phosphorus, coupled w ith effective removal o f nitrogen, leading to a prevalence o f a low N :P ratio. These conditions are especially favourable for nitrogen-fixing cyanobacteria. Furtherm ore, cyanobacteria are favoured in conditions where anoxia in the bottom layer leads to a release o f chemically bound phosphorus Key Q u e stio n s P lan ktivorous fish C o p ep o d s M icro zoo plan kto n P hytoplankton from sediments; release o f phosphorus from th is reservoir may prolong the harm ful consequences o f anthropogenic loading for years or decades after reductions in phosphorus input into the system (C arm en and W u lff 1989). C o n c e p t u a l d i a g r a m o f t o p - d o w n c o n t r o l a n d e f f e c t o f t r o p h ic CASCADES ON SM ALL A N D LARGE CELL-SIZE P H Y TO PLA N K TO N . O n THE LEFT, PROLIFERATION OF BAITFISH RESULTING FROM O VER-FISHING OF TOP PREDATORS LEADS TO BLOO MS OF LARGE DINOFLAGELLATES (CIRCLED). A l t e r n a t iv e l y , o n t h e r ig h t , r e m o v a l o f s m a l l f is h c a n l e a d t o O n shorter tim e scales, nutrients may be taken up by a particular species in one form and released in another form by th e same organism or by its grazer. The cyanobacterium Trichodesmium, w hich forms massive blooms in tropical and subtropical waters, derives its nitrogen via the fixation o f N r However, much o f this nitrogen is rapidly released as am m onium and dissolved organic nitrogen (Karl et al. 1992, G libert and B ronk 1994, G libert and O ’N eil 1999). This released nitrogen is therefore available to support the grow th o f other microbial populations th a t cannot otherwise derive their required nitrogen in nutrient-im poverished waters. N um erous consortial associations involving other nitrogenfixing cyanobacterial bloom taxa (Anabaena, Aphanizomenon, Nodularia) and bacterial epiphytes have been shown to lead to enhanced grow th o f cyanobacterial “hosts” (Paerl 1988, Paerl and Pinckney 1996). Relationships betw een nutrient inputs, and the C o m p a ris o n o f th e a m b ie n t n u tr ie n t c o n c e n tr a tio n in s id e a n d o u ts id e a BLOO MS OF S M ALL PH YTO PLANKTO N (CIRCLED) SUCH AS BROW N TIDE. S o u r c e : D . S t o e c k e r a n d t h e I n t e g r a t io n a n d A p p l ic a t io n N e t w o r k , U n i v e r s it y o f M a r y l a n d C e n t e r f o r E n v i r o n m e n t a l S c ie n c e , w w w . i a n . u m c e s . e d u . various processes by w hich nutrients are recycled and transform ed are im portant for understanding H A B grow th strategies. It is highly likely th a t a com bination o f increases in nutrient resources and relaxation in grazing together contribute to both th e frequency and intensity o f H A B events. E utrophication alters top-dow n control as well as providing bottom -up nutrient support for blooms. T op-dow n control o f m icrozooplankton is particularly im p o rtan t as predation on the m icrozooplankton com m unity may release H A B species from grazing control. Trophic cascades, alterations in benthic-pelagic coupling, and interactions betw een all com ponents o f th e microbial com m unities need to be better understood on comparative bases. d e n s e s u rfa c e b lo o m o f T ric h o d e sm iu m (see p h o to ). D in o fla g e lla te s w e r e s ig n ific a n tly m o r e a b u n d a n t in th e E x a m p le s o f R e le v a n t D e t a ile d Q u e s tio n s p la n k to n c o m m u n ity in s id e th a n o u ts id e , w h ile d ia to m s w e r e m o r e a b u n d a n t o u ts id e th a n in s id e th e Tr i c h o d e s m i u m b lo o m . F ro m G lib e r t a n d O 'N e il 1 9 9 9 . b lo o m . P h o to : P. G l i b e r t . Inside Bloom Outside Bloom NH4 (pM) 7.0 1.4 P04(pM) 1.2 0.2 DON (pM) 10.3 6.9 Dinoflagellates 74% 54% Diatoms 26% 46% 1. What are the roles of bacteria and viruses in growth dynamics and impacts of HAB species in eutrophic environments? 2. What is the impact of eutrophication on the quality of Zooplankton food and how does that affect grazing activity? 3. Do HABs uniquely alter trophic interactions through their toxin production? 4. To what extent do bacterial interactions w ith HABs lead to toxin production that is greater in eutrophic waters? 5. How does eutrophication impact the production of allelopathic chemicals? 39 Key Q u e stio n s T h is f is h f a r m in t h e E a s t C h i n a S e a w a s s e v e r e l y i m p a c t e d b y In t h e HABs ACCIDENTALLY INTRODUCED V IA THE BALLAST WATER OF SHIPS TO THE B L A C K in 200 5. P hoto: J. Li. e a r l y 1 9 8 0 s , t h e R a in b o w J e l l y , M n e m i o p s i s l e id y i , w a s S e a w h e r e it h a d a c a t a s t r o p h i c e f f e c t o n t h e e n t ir e e c o s y s t e m . P hoto: L. K l is s u r o v , B l a c k S e a P h o t o G a l l e r y . QUESTION 4 Do anthropogenic alterations of the food web, including overfishing and aquaculture activities, synergistically interact w ith nutrients to favour HABs? Global changes in trophic structure o f nearshore habitats are occurring coincident w ith global changes in nutrient loading. A ltered grazing structure, for example, has occurred from both overfishing, as well as from the intensification o f aquaculture activities throughout th e world. The dram atic decrease o f im portant comm ercial fisheries in many parts o f the world may interact synergistically to prom ote H A B s. Studies in both lake and estuarine waters have shown th a t reductions in large piscivorous fish leads to a trophic cascade, whereby an increase in the smaller piscivorous fish is subsequently noted, w hich then exerts strong predation control on Zooplankton, thereby reducing their biomass and thus increasing th e biomass o f phytoplankton (A ndersson et al. 1978, C arpenter et al. 1985). In th e Black Sea, for example, over-fishing has led to a virtual disappearance o f the large comm ercial fish, and as a consequence there has been a dram atic increase in the predatory ctenophore Mnemiopsis leidyi, a disruption o f the pelagic food web, and increases in H A B s (D askalov 2002, L ancelot et al. 2002, Kideys et al. 2005) Similarly, in N arragansett Bay, U SA , recent evidence suggests th a t H A B s have increased as M . leidyi has become more abundant in th a t system, also decreasing th e Zooplankton consumers o f these H A B s (Sullivan et al. 2001). The synergistic im pact o f trophic cascades and nutrient loading has recently been shown by G ranéli and T urner (2002). They observed, in mesocosms experim ents, th a t by m anipulating th e food web through th e introduction o f jellyfish, phytoplankton biomass increased w ithout supplemental nutrients. O n the other hand, when nutrients were also added, the biomass o f phytoplankton, and in particular, the biomass o f H A B species, increased significantly. 40 W h ile control o f phytoplankton thro u g h Zooplankton grazing has been studied for a long tim e, there are still im p o rtan t gaps in our knowledge th a t seriously hinder our ability to model the interaction correctly (M itra and Flynn 2005) In particular, there are insufficient data for the grazing o f phytoplankton o f different nutritional status; not only does this affect our understanding o f the grazing o f no n -H A B species (Jones and Flynn 2005), but th is issue is likely to be o f even greater im portance when considering the palatability o f H A B species whose toxicity varies w ith nutrient availability. H ence, it is now becom ing increasingly recognized th a t many H A B species are not efficiently grazed. M orphological features, poor nutritional quality, as well as intracellular or extracellular toxins or other bioactive com pounds, may act as feeding deterrents (Verity and Smetacek 1996, T urner and Tester 1997). A variable susceptibility o f toxic algae to grazers is considered to reflect varying toxin levels, depending on algal grow th condition (Turner and Tester 1997, T urner et al. 1998). F urtherm ore, increasing as well as decreasing inhibition o f Zooplankton feeding by H A B species has been observed under both nitrogen and phosphorus lim itation (Nielsen et al. 1990, G ranéli and Johansson 2003a). Thus, the nutrient state o f the algae may be o f critical im portance in controlling th eir susceptibility to grazing. C orroborating these findings is the fact th a t for many H A B species the toxicity increases when they are grow n under nutrient-unbalanced conditions (G ranéli et al. 1998). The idea th at changes in higher levels o f the food web facilitate the success of H A B species by decreasing th eir grazers, and, thro u g h alterations o f the nutrient pool, fu rth er decrease th eir susceptibility to grazing by altering their toxin content, requires much more study. G razing control o f H A B s can also depend on the population density o f the harm ful algae, as dem onstrated for th e brow n tides in N arragansett Bay, U SA , where suppression o f grazing occurs above a threshold concentration (Tracey 1988). A threshold effect may also occur if the daily production o f new harm ful cells Key Q u e stio n s becomes large enough to saturate the ingestion response o f the grazers and the ability o f grazers to increase th eir populations. In th a t case, population grow th can accelerate dram atically - if sufficient nutrients are available to support grow th (D onaghay 1988). There is, however, little quantitative inform ation on how the nature o f the grazer response influences the tim in g , m agnitude, and duration o f H A B s, and for those studies where such inform ation is available, alm ost n othing is know n about w hether these responses would change under altered nutrient regimes. M odel param etrisation o f these effects is thus in very early stages o f development. However, initial m odelling studies suggest th a t the links betw een nutrient status, toxicity, poor grazing, removal o f no n-H A B species, and establishm ent o f many H A B s may be driven by a positive feedback loop (M itra and Flynn 2006). The structure o f grazing com m unities also has been altered significantly through th e developm ent o f large aquaculture facilities. A n example o f the association betw een fish farm ing operations and H A B s comes from Norway. Up to the 1970s, harm ful algal blooms were not considered a major th reat to the m arine or coastal environm ent o f Norway. Single episodic events o f mussel toxicity and m ortality o f w ild biota were recorded along the N orw egian coast, but these episodes did not attract any wide public attention or lead to any m anagem ent actions. Through the 1970s, several fish farm s were established along the coast, and since 1980, the production o f the N orw egian aquaculture industry grew significantly from a few thousand tonnes to ~500,000 tonnes o f salmon and tro u t in 2001, and N orw ay’s emergence as the w orld’s largest exporter o f A tlantic salmon. In parallel w ith this developm ent, H A B s emerged as a significant problem. In 1981, a large bloom o f th e dinoflagellate Gyrodinium aureolum caused significant m ortality o f farm ed salmon and economic losses to fish farmers. N ew blooms o f Gyrodinium followed, and during the 1980s, other phytoplankton species bloomed to cause additional problems, including Dinophysis spp. in 1984, Chrysochromulina polylepis in 1988, recurrent blooms o f Prymnesium since 1989, Chrysochromulina leadbeateri In 1991, and Chattonella m 1998, 2000, and 2001. It is, o f course, incorrect to attribute these outbreaks solely to nutrient inputs from fish farm s, but some linkage seems probable. T h e s e a q u a c u l t u r e f a c il it ie s i n K u w a i t h a v e o f t e n b e e n i m p a c t e d b y HABs. P h o t o : P. G l ib e r t . E nrichm ent o f nutrients from aquaculture activities has also been associated w ith outbreaks o f pathogens th a t synergistically interact w ith H A B s to alter fish health. For example, in Kuwait, a recent massive fish m ortality event, attributed to an outbreak o f the pathogenic bacterium Streptococcus agalactiae'was also functionally related to an outbreak o f PSP-producing dinoflagellates (Evans et al. 2002, G libert et al. 2002). It was suggested th a t as massive numbers o f fish died, nutrient release led to conditions favourable for the dinoflagellate outbreak. There is much th a t can be learned from comparative systems where major alterations o f food webs have occurred through over-fishing or over-stocking. A s new markets and regions are developing intensive aquaculture operations, there are also opportunities for gaining knowledge regarding the potential for increased H A B proliferation from comparisons w ith long-established regions. Examples of Relevant Detailed Questions 1. Under what conditions does overfishing lead to trophic cascades that may synergistically interact w ith nutrient availability to promote HABs? 2. Does nutrient loading uniquely alter HAB toxin production leading to grazing alterations? 3. Is nutrient enrichment associated with both fish farming and shellfish farming, from feed additives and nutrient regeneration, correlated w ith HAB proliferation, and are there unique species that develop under specific aquaculture nutrient regimes? 41 Key Q u e stio n s &0 7 ,0 LO 0.0 c ’f O' M o r e a n d m o r e v e h ic l e s , a s w e l l a s v e h ic l e s w i t h p o o r g a s ECONOMY, ARE CONTRIBUTING TO NOX EMISSIONS. P H O T O : P . G LIB ER T. •r. o- O'. O' C h a n g e in U S a t m o s p h e r i c N O x e m is s io n s f r o m 1 9 4 0 t o 2 0 0 0 . S o u r c e: H o w a r t h et a l . 2 0 0 2 b . QUESTION 5 How do anthropogenic changes in land use, agricultural use of fertiliser, NOx emissions from vehicles, and global changes inland cover affect the delivery of nutrients to coastal waters and the resulting incidence of HABs? A s described earlier in this report, hum an activities have altered the nutrient regimes o f coastal waters tremendously, prim arily as a result o f increased applications o f synthetic fertilisers. Population grow th and developm ent, and th e production o f food (crop and anim al production systems) result in dram atic alteration o f the landscape as well as large sewage inputs, and increased runoff from land. Increased nutrient inputs to enclosed and nearshore ecosystems have resulted in w idespread coastal eutrophication throughout Europe, Asia, and th e U nited States. The production and consum ption o f energy also results in increased atm ospheric inputs from N O x emissions, w hich can th en lead to increased nitrogen deposition. In freshwater reservoirs, increased prim ary production has been correlated w ith the proportion o f increased agricultural land in the w atershed (Knoll et al. 2003). C om parisons o f nutrients delivered from pristine, urban or agricultural land uses are sorely needed. Similarly, little is know n about comparative nutrient loading from systems undergoing rapid deforestation and/or rapid urbanisation. O f the nitrogen th a t hum ans p u t into the landscape, much is also denitrified. A better understanding o f the fluxes from land to the atm osphere from denitrification, how they change w ith climatic variations, and how they may be altered as landscape is altered, is fundam entally im p o rtan t to our understanding o f nutrient delivery to coastal waters (H o w arth et al. 2006). Y I 13» B I -t» I * HÚ BOM N ii Af r t m e w n f c N In p u t, per A n . [ Da “*j T h e r e l a t io n s h i p b e t w e e n h u m a n in p u t s o f N a n d r iv e r in e N e x p o r t FOR M AJO R RIVERINE A N D COASTAL REGIONS OF THE N O R T H A T L A N T IC O c e a n . S o u r ce: H o w ar th et a l . 1 9 9 6 . M a n - m a d e c a n a l s r e s t r ic t f l o w , r e d u c e c ir c u l a t i o n , a n d in c r e a s e SUSCEPTIBILITY OF THESE SYSTEMS TO BLOO MS FROM ELEVATED NUTRIENT loads. 42 'V P h o t o : w w w .w e n e e d a v a c a t i o n . c o m . Key Q u e stio n s In F l o r id a , USA, s o m e w a t e r f l o w is u n d e r g a t e d c o n t r o l . T here ARE O NG O IN G RESTORATION EFFORTS TO RETURN TO SURFACE FLOW, POTENTIALLY ALTERING THE FORMS A N D AM O U N T S OF NUTRIENTS TO BE DELIVERED TO THE COAST. P H O T O : E v E R G L A D E S /F l O R ID A Bay W ATE R S H ED I n C h in a , t h e T h r e e G o r g e s D a m o n the Y a n g t z e R iv e r , s c h e d u l e d FOR CO M PLETIO N BY 2 0 0 9 , W IL L BE THE LARGEST HYDROELECTRIC D A M IN THE W O R LD . It W IL L SIGNIFICANTLY ALTER THE DO W NSTREAM FLOW OF NUTRIENTS. P H O T O : W W W .A F TE R D R E A M S .C O M . M a n a g e m e n t , w w w .s f w m d .g o v . Large-scale hum an alteration o f coastlines or inland waters also need to be assessed for th eir effect on nutrient enrichm ent and supply. For example, sem i-enclosed harbours and extensive housing developm ents along artificial canals are now commonplace world-wide, from the C atalan coast o f Spain to the east coasts o f the U nited States and A ustralia. Reduced circulation w ithin these harbours has been associated w ith recurrent blooms. In South C arolina, U SA , numerous types o f H A B s have been frequently observed in coastal detention ponds used for storm w ater control in housing developm ents and go lf courses (Lew itus et al. 2003). These brackish, shallow, low-flow environm ents are depositional sites for nonpoint source pollutants, prim arily fertiliser nutrients from intensive landscape m aintenance and tu r f managem ent. Because these ponds exchange w ith tid al creeks, they are sources for H A B dispersion into adjacent estuaries. M ajor engineering projects are also projected to have im pacts on nutrient delivery and booms. E stim ates, for example, o f the potential eutrophication im pact o f the Three Gorges D am Project o f the C hangjiang (Yangtze) River, C hina, suggest th a t the nitrogen to phosphorus ratio may be significantly altered so as to yield a shift in ecosystem structure and the developm ent o f anoxia (Z hang et al. 1999). The im pending restoration o f surface water flow through the Everglades in Florida, U SA , a project o f decadal tim e scale, is expected to have major im pacts on nutrient delivery to Florida Bay. U nderstanding the im pact o f varying nutrient quality as well as quantity is im portant in understanding the type o f phytoplankton bloom th a t may develop (e.g., cyanobacteria vs. diatom ; G libert et al. 2004). A lternatively or additionally, some fundam ental change in seagrass processes and the entire benthic com m unity may be diverting recycled nutrients from sedim ent pools to the overlying water colum n, fueling phytoplankton grow th. Spatially explicit, em pirical models have previously been developed to quantify global nutrient export to the coastal zone as a function o f w atershed characteristics (D u m o n t et al. 2005, H arrison et al. 2005, Seitzinger et al. 2005a). Such models take into account w atershed characteristics including freshwater runoff, land use, atm ospheric deposition, hum an population, fertiliser use, and anim al production am ong other factors. These types o f models need to be coupled w ith long-term records o f H A B occurrences. Finally, the relationships betw een nutrient delivery via the range o f mechanism s described above and H A B developm ent also must be understood in term s o f the physics o f th e water body receiving th e nutrients. Equivalent nutrient inputs to different systems may have differential effects on H A B developm ent due to differences in ecosystem structure and function. Examples of Relevant Detailed Questions 1. To what extent do similar land-use patterns lead to similar nutrient discharges (in quantity and quality) and similar HAB outbreaks? 2. Can long-term trends in nutrient loadings be correlated w ith changes in HAB bloom patterns and dynamics? 3. What is the likelihood for long-term ecosystem restoration projects to alter nutrient delivery in such a way as to increase or decrease the frequency of HAB outbreaks? 4. How do nutrient transformation processes such as denitrification vary w ith changes in nutrient loading to the land and other landscape changes, and how do these processes affect nutrient availability for HABs? 43 Key Q u e stio n s Pro-lsabfll PoBL-lsaÍMl DMeranea between SfipWfflbe* 11. 2003 Seplcm bM 24. 20Ö3 pro- and poil-lsab cl Chi j (mg m *1- M ia I io Mí * 6 4 2 0 *65 mo longiLutíe ("WJ *6 5 *6 0 Longitude ("WJ 76 5 *60 Longrtude i ' W ) S h o r t - t e r m n u t r i e n t l o a d i n g , s u c h a s o c c u r r e d f o l l o w in g QUESTION 6 H u r r ic a n e I s a b e l w h i c h s t r u c k t h e C h e s a p e a k e B a y r e g io n , U S A , in Do climate change and climate variability have impacts on ecosystems that augment the impacts of eutrophication in the formation of HABs? 2 0 0 3 , LED TO LARGE A LG AL BLOO MS W IT H IN DAYS. SO URCE: D . M lL L E R In order to assess the extent to w hich nutrients and/or eutrophication may be an im portant causative factor o f specific blooms, the role o f interacting or confounding factors m ust also be considered. C lim ate ultim ately controls th e fundam ental param étrés relating to algal grow th, from water tem perature to nutrients, and from precipitation and ru n o ff to light. Clim ate change and climate variability can cause changes in species composition, trophic structure, and function o f marine ecosystems. C lim ate change is the long-term alteration in global and/or regional w eather due to increasing concentrations o f greenhouse gases and aerosols th a t have altered the radiation balance o f the E arth. C lim ate variability, on the other hand, is the natural variation in the im pact o f solar variation on clim ate, including periodic E l N iño-S outhern O scillation (E N SO ) phenom enon, the N o rth A tlantic O scillation (N A O ), and th e Pacific Decadal O scillation (PD O ). N utrient supply is linked to freshwater input (e.g., Caraco 1995, Vitousik et al. 1997) th a t, in tu rn , is driven by regional climate variability (Najjar 1999, M iller et al. 2005, Burkholder et al. 2006, H ow arth et al. 2006). Freshwater input determ ines, to a large extent, the spring chlorophyll a m axim um in systems such as Chesapeake Bay, U SA , by delivering nutrients (H ard in g 1994, M alone et al. 1996, Kemp et al. 2005). Freshwater input also plays a role in the physical tran sp o rt o f phytoplankton species, such as the H A B species Prorocentrum minimum (Tyler and Seliger 1978), blooms o f w hich have been shown world-wide to be associated w ith freshwater inputs and associated nutrients (Silva 1985, G ranéli and M oreira 1990, Stonik 1995, G rzebyk and Berland 1996, 44 ET A L . 2 0 0 6 . G libert et al. 2001, Springer et al. 2005). Inter-annual variability is also im portant, however, affecting the tim in g o f freshwater flow, residence tim es, and the m agnitude and tim in g o f blooms, including H A B s. In the case o f brow n tide in th e Peconic Estuary, U SA , for example, a paucity o f inorganic nitrogen addition via groundw ater during dry years has been shown to stim ulate the grow th o f the brow n tide organism , Aureococcus anophagefferens (LaR oche et al. 1997, N uzzi and W aters 2004) w hich can utilize D O N resulting from th e partial m ineralisation o f the previous year’s algal grow th or from the same year’s spring bloom (Gobler and Sañudo-W ilhelm y 2001). M ajor climatological events, such as strong storms or hurricanes, also affect estuarine conditions during short tim e periods. A s examples, H urricane Isabel in 2003 caused a large phytoplankton bloom to develop in Chesapeake Bay, U SA, w ithin days (M iller et al. 2005), as did H urricanes Charley and W ilm a off the Florida coast (H eil et al., unpub. data). A s climate w arms, one predicted scenario is for alteration in rainfall, leading to increased riverine discharge. Such an increase may lead to lowered salinity, increased abundance o f taxa th a t proliferate in lower salinity waters, including freshwater bloomform ing cyanobacteria. W ith increased discharge, such species may also be carried fu rth er seaward, leading to potential exposure o f new regions to toxin-producing cyanobacterial blooms. A dditionally, buoyant plumes could extend m uch fu rth er off the coast, selecting for m igrating taxa such as dinoflagellates, w hich recur in these coastal features (Sellner and Fonda-U m ani 1999). A lternatively, for systems w ith declining riverine discharge, more stenohaline taxa could potentially move fu rth er up estuaries, thereby exposing larger portions o f upper basins to more saline H A B taxa such as Dinophysis, Karenia, and Alexandrium. Key Q u e stio n s SH2¿ 1 -y e a r running m e a n o f S [ - Q J %\NAO\ HAB® year (20th Century) The current ecological paradigm suggests th a t an ecosystem does not exist in a unique, stable equilibrium state, but rather can easily be shifted to a new state by non-linear responses to physical forcings, such as those associated w ith climate change or variability. Biological responses are non-linear, complex m ixtures o f physiology, behaviour, life history, and physical redistribution. A major question is w hether changes in climate synergistically interact w ith changes in nutrient loads to lead to increased frequency o f H A B s. P o s s ib l e l o n g - t e r m r e l a t i o n s h i p b e t w e e n c l i m a t e v a r i a b i l i t y a n d P a r a l y t i c S h e l l f i s h P o is o n i n g e v e n t s c a u s e d b y G y m n o d i n i u m CATENATUM IN THE G A L IC IA N R iA B a IX A S ( N W IBERIAN P E N IN S U L A ). S [ Q X] A N D S [ N A O ] ARE DE-SEASONALISED CU M ULATIVE SUMS OF OFFSHORE E k m a n t r a n s p o r t ( - Q x) a n d t h e N o r t h A t l a n t i c O s c i l l a t io n in d e x ( N A O ) o v e r t h e p e r io d 1 9 8 1 - 1 9 9 8 . T h e s t r a i g h t r e d l in e in d ic a t e s YEARS OF DETECTION OF G. CATENATUM ASSOCIATION W ITH (THICK LINE) OR ABSENCE FROM (TH IN LINE) THE DETECTION OF P S P TOXINS IN SHELLFISH. A POSITIVE TREND IN S [ N A O ] INDICATES TRANSITIO N TO U PW ELLING FAVOURABLE PERIODS. A NEGATIVE TREND IN S [ N A O ] INDICATES C lim ate changes are also resulting in global variations in tem perature. W hile average global changes may be relatively small, effects o f climate w arm ing vary widely by region. Tem perature is a fundam ental regulator o f physiological processes, affecting the activity o f specific enzymes and th e cues for various life cycle events, such as excystment. Tem perature also regulates abundance and activity o f predators. The extent to w hich these physiological controls and synergistic relationships w ith other com ponents of the food web are regulated by tem perature, is far from understood. O ne example o f such interactions is th a t o f Heterocapsa triquetra, w hich blooms during w inter in N o rth C arolina, U SA (Litaker and Tester 2005). O n a physiological level, H. triquetra is favoured under conditions o f w arm tem peratures and high light. Yet, due to tem perature control on its grazer, it tends to bloom at cooler tem peratures when grazers are kept in check. I f w inters become warm er, the tim ing o f such blooms may be altered. TRANSITIO N TO DO W NW ELLIN G -FA VO U RA BLE PERIODS. P S P OUTBREAKS CAUSED BY G. CATENATUM WERE MORE FREQUENT A N D INTENSE DURING THE TRANSITIO N PERIOD ( 1 9 8 0 s TO 1 9 9 0 s ) FROM D O W N W E LLIN G - TO U P W ELLIN G -FAVO U RABLE CO N D ITIO N S . SO URCE: A lV A R E Z -S a L G A D O ET AL. 2003. Examples of Relevant Detailed Questions 1. Whatare the chemical paramétrés that are likely to change due to climate and how might they impact HAB proliferation? 2. How do either small or large changes in temperature impact cellular physiology favouring HABs? 3. Do ENSO and other large-scale physical forcings have impacts on HABs? 4. How do climate variability and changes impact river flow and the associated transport of nutrients leading to HABs? 45 Key Q u e stio n s A B a l t i c S e a b l o o m , p o s s i b ly N o d u l a r i a , J u l y 2 0 0 5 . P h o t o : J . D e s c lo itr e s , M O D I S L R R T , N A S A G S F C . Specific Challenges for Further Advancement M uch progress has been made over the past several years in many aspects o f H A B bloom ecology and dynamics. In some cases, the linkages betw een cell physiology and toxicity have become clear. Progress has also been made in understanding the influences o f physical process and other environm ental param étrés integral to harm ful algal expression. Yet, m any fundam ental physiological processes rem ain unm easured for m ost species. N ew m ethods of cell detection are becom ing more readily available, and progress has been made in the identification o f some genes involved in toxin production. F urther understanding has been gained o f the roles o f nutrients, both inorganic and organic, in bloom development. Similarly, the roles o f viruses and bacteria are better understood for some H A B s. Yet, the understanding o f the interactions o f H A B s w ith grazers, the sublethal effects o f H A B s on com m unity dynamics, and the application o f these data in developing predictive models is only ju st beginning. A ccurate and tim ely identification o f harm ful species is of fundam ental im portance to all aspects o f H A B research and m anagem ent. H istorically, species have been delineated on the basis o f m orphological, u ltrastructure, and life-stage features visible in the light and electron microscopes, as well as by pigm ent content. Increasingly, organisms are being com pared and identified on the basis o f molecular markers such as cell surface proteins, lipids, D N A sequences, and toxins. These same markers are serving as a basis for rapid assays designed to detect and quantify species specifically. W ith this progress has come an increasing dem and for identifying organisms using traditional techniques so th a t comparative studies and new findings regarding species diversity, biogeography, and toxicology can be placed in an appropriate historical context. 46 Significant advances in cellular physiology o f harm ful microalgae have been made in the past decade w ith regard to nutrient regulation o f toxin synthesis and assim ilation o f dissolved organic nutrients. However, critical inform ation required for model developm ent - such as photosynthesis-irradiance relationships, rates o f nutrient uptake, and excretion o f organic m atter - is not available for m ost species. Studies o f grow th rates as a function o f tem perature, irradiance, nutrients, and th eir ratios, are rare. M o st harm ful species employ sw im m ing or buoyancy-regulation strategies influenced by irradiance and nutrients, in addition to am bient tem perature and salinity, and these factors influence population grow th and com m unity structure in ways th a t we do not yet fully understand. The grow th rate o f many H A B s in nature largely rem ains unknow n. Collectively, our lack o f fundam ental knowledge concerning the basic physiology and behaviour o f most H A B species im pedes our ability to develop predictive models th at w ill ultim ately aid many aspects o f basic research and m anagement. N atural resource managers and public health officials need better tools to forecast im m inent H A B events so th a t m anagem ent strategies can be im plem ented effectively. Forecasting capabilities can be enhanced by im proving and expanding rem ote-sensing technologies, such as offshore in strum ent m oorings and satellite sensing, and th is inform ation w ill perm it m onitoring o f the environm ental conditions th a t could lead to H A B form ation and decline and for the tracking o f H A B s as they transit thro u g h a region. Tim e-series analyses o f existing databases for phytoplankton com m unities and environm ental variables such as m acro- or m icro-nutrients, where available, are required. Retrospective analyses o f historical data and inform ation may provide im portant insights. In some cases, the sediments may hold im portant historical inform ation. L and-use data, such as may be available from maps and geographic inform ation systems may yield trends in nutrient loadings to the landscape. W here such data are lacking, long-term consistent m onitoring program m es m ust be initiated in key regions where hum an influences are either know n or are anticipated to increase. M oored instrum entation packages - including nutrient, plankton, and optical sensors - are needed Key Q u e stio n s to resolve the tem poral relationships betw een nutrient inputs and shifts in plankton composition. Shipboard observations and field program m es would be helpful in resolving spatial differences and in the collection o f samples for w hich sensors are not currently available. The vast complexity o f th e interactions betw een H A B s and their environm ent dictates th a t we th in k in term s o f integrative models to address the problem in a way th a t our m ental com puting capability cannot (Noble 2002). M echanistic ecosystem models are widely used in marine research. M odels should be constructed at an appropriate level o f complexity to address the hypothesis being tested and th e data available to support it. However, in reality, we often lack the appropriate data to support such developments. A dditionally, we should bear in m ind th a t a w ell-constructed m odel th a t fails can be more informative th a n one th a t succeeds. A good model should be descriptive (represent the available data), integrative (dem onstrate how elements interact), and explanatory (provide biological insight). C onceptual models o f biological systems are comm on. They are com m only used as m anagem ent tools for predicting eutrophication (e.g., M oll and Radach 2003, T ett et al. 2003), to understand ecosystem processes (e.g., ecosystem dynam ics, A llen et al. 2004; response to climate change, Taylor et al. 2002) and to predict biotic responses (e.g., Besiktepe et al. 2003). C onceptual models can be viewed as having tw o com plim entary roles. The first is a heuristic role, whereby they are used to corroborate a hypothesis, illum inate areas o f fu rther study, and identify where further empirical data are required. The second role is as predictive tools, whereby the models are used to aid m arine resource m anagem ent and to assess the im pact o f hum ans on the m arine ecosystem. T h e d a r k a r e a s n e a r t h e s h o r e l in e s h o w a r e d t id e b lo o m . K a r e n ia BREVIS, ALONG THE S W COASTLINE OF FLO R ID A , M A R C H 2 0 0 2 . P H O T O : J . D e s c lo itr e s , M O D I S L R R T , N A S A G S F C . The establishm ent o f a robust m odelling approach for sim ulating H A B dynamics in eutrophic environm ents requires the following future work: • th e definition o f functional groups to describe H A B s and fu rth er developm ent and refinem ent o f conceptual process models; crucial to th is process is the definition o f life histories and hence, stage-structured models; equally critical is knowledge o f th e interaction betw een H A B s and non-H A B s (with w hich they compete for com m on nutrients) and their predators; • bioinform atics to acquire, archive, and interpret data; • th e construction, param etrisation, validation, and evaluation o f appropriate m athem atical and num erical models; • integration o f H A B models into generic coupled hydrodynam ic ecosystem models for comparative studies; and • data assimilation to improve model param étrés and forecasts, and to quantify model errors. W h ile the ultim ate goal is to develop whole ecosystem models, m uch is to be gained by construction and testing o f models as an aid to our understanding o f the biological processes underpinning H A B form ation and demise. The basis for many biological models has failed to keep pace w ith our understanding o f plankton biology (Flynn 2005). The u n dertaking o f experim ents to investigate these interactions should not be trivialised; neither should funding bodies dismiss such needs because they “have been done before”. Integrated studies o f experim ental research and m odelling are few and far betw een, and few data sets from previously conducted experim ental studies are adequate for the task. 47 5. Moving Forward in the Study of HABs in Eutrophic Systems / n accordance with the GEOHAB strategy, the approach of the Core Research Project on HABs in Eutrophic Systems will be comparative from the cellular to the ecosystem level. Research that is interdisciplinary - focussing on biological, chemical, and physical processes - will be fostered. Research will be multifaceted as the problems relating to eutrophication are complex, and interactions and processes occur on a broad range of scales. Finally, research will be international in scope to encompass the global issues of HAB events and to benefit from the skill and expertise gained by HAB investigators world­ A CYANOBACTERIAL (BLUE-GREEN ALGAE) BLOOM ON THE SHALLOW LAKE, L a k e T a i h u , J ia n g s u P r o v in c e , C h i n a . P h o t o : W . W w w w .a slo .org/ ph o to po st/ wide. ur tsba u g h , . A lthough G E O H A B is prim arily built upon a coalescence of projects at many levels, th e Core Research Project w ill also seek, thro u g h national funding mechanism s, to conduct a series o f experim ents and observations th a t directly address the key questions outlined in C hapter 4. W here possible, these efforts will capitalize on existing platform s, program m es, or ongoing studies. Research on th e major priority areas identified herein is welcome w ithin the G E O H A B framework. The understanding o f this im portant issue, and ultim ately th e m anagem ent o f nutrient loads and im proved prediction and m anagem ent o f H A B s w ill require the global com m unity o f scientists. Applying the GEOHAB Approach W h o l e lak e a n d e x p e r im e n t a l MESOCOSM STUDIES HAVE BEEN USEFUL IN UNDERSTANDING THE IMPACTS OF NUTRIENTS ON BOTH PHYSIOLOGICAL AND ECOSYSTEM levels. 48 P h o t o : D . S c h in d l e r . G lobal population is rapidly increasing and concurrent changes in land use and coastal nutrient loading have been well established for many regions o f the world. Critical to our long­ term understanding is th e need for continuous m onitoring and observation, to docum ent changes as they are occurring, and to understand the processes o f change. There is a need for a global The GEOHAB Core Research Project on HABs in Eutrophic Systems will seek to implement a range of comparative and interdisciplinary approaches. Summary of Key Research Considerations Long-term monitoring, beyond a decade, may be required to observe trends in population, climate, and nutrient loading. Research must be integrated w ith new understanding in agricultural practices, aquacultural practices, atmospheric emissions and deposition, and human demography. Ecosystem characteristics such as hydrography, residence time, stratification, and trophic interactions w ill impact the expression of eutrophication and HABs. Multiple observational, experimental, and modelling approaches w ill be required to advance the study of HABs in relation to eutrophication. M e s o c o s m s t u d ie s h a v e p r o v e n t o be a n e f fec tiv e t o o l in UNDERSTANDING POPULATION AND COMMUNITY RESPONSES TO NUTRIENT p e r t u r b a t io n s . R ig h t : M Left: M e s o c o s m s t u d ie s in e s o c o s m s t u d ie s in C h in a . P hoto: J . Ll. L o n g Is l a n d , N Y , U S A . P h o t o : T . Kana. netw ork o f sites th a t are deem ed sensitive to nutrient alteration and eutrophication. In the U nited States, numerous sites now have more th an a decade o f data on w hich to build an understanding of the relationships am ong population change, climate change, and trends in nutrient quantity and composition. In E urope, numerous sites have been designated for continuous m onitoring for benthic biodiversity and long-term phytoplankton records are know n for the Scandinavian, N orw egian, British, Irish, French, Iberian, and A driatic coasts. Japan has a long history o f harm ful algal records while C hina is rapidly expanding its coastal m onitoring and H A B specific activities. The value o f long-term records is fundam ental in understanding relationships betw een anthropogenic change, climate change, and H A B s. In a m anner analogous to the establishm ent o f oceanic long-term sites such as the JG O F S H O T (H aw aii O cean Tim e-series) and B A TS (Berm uda A tlantic T im e Series Study) sites, long-term sites for m easuring nutrient loading and H A B s m ust be m aintained. R ecognising the value of these long-term records is the first step in bringing comparative observational and m odelling approaches together. N atural experim ents are being undertaken on a broad scale th a t w ill im pact the expression o f eutrophication in several parts of the world and may be deserving o f special research attention. For example, Florida Bay, U SA , receives freshwater flow from the Everglades, but currently this flow results from m anaged discharge rather th a n from natural hydrological conditions (R udnick et al. 1991). Fong-term m anagem ent plans for the southern Florida area call for restoration o f the natural flow conditions w ith in the Everglades. Thus, key questions o f concern to both scientists and managers are to w hat extent do nutrients originating from the Everglades contribute to eutrophication and H A B s in Florida Bay and how w ill they change w ith altered flow conditions? Similarly, major engineering projects are being undertaken to alter the flow o f the Yangtze River in C hina, where diversion o f water will cause major changes in nutrient loading to receiving waters. These regions are o f key interest because the existing conditions are well characterised and thus, the im pacts o f hydrological diversion can be quantified. The value o f integrated experim ental studies also m ust be underscored. Such approaches have led to enorm ous advances in our understanding o f nutrient lim itation. For example, whole-lake m anipulations yielded trem endous insights into the differential regulation o f N and P lim itation in freshwater systems. M uch was learned regarding the regulation o f prim ary production by iron in th e IronE x and SoFex experim ents (de Baar et al. 2005), in w hich large patches o f th e ocean were fertilised w ith iron and the responses o f the plankton com m unity observed over days to weeks. Such approaches are beneficial in understanding H A B responses, both on a physiological level and also on a whole ecosystem level. Farge-system perturbations, include nutrient additions in organic form , may provide th e key to understanding how systems may respond as land use and nutrient loadings are altered. To elevate research on H A B s and eutrophication to a level consistent w ith th e G E O H A B approach o f comparative, international, m ultifaceted, and interdisciplinary studies, the Core Research Project on H A B s in E u trop h ic S ystem s will aim to undertake one or more large-scale experim ental studies th a t w ill provide opportunities for researchers from around th e world to participate. Such studies w ill be developed w ith considerable discussion w ith in the broader comm unity. These experim ental studies w ill augm ent smaller-scale studies th a t will be linked thro u g h data sharing and smaller-scale comparative studies. Thus, the G E O H A B Core Research Project on H A B s in E u tro p h ic S ystem s w ill seek to im plem ent a range o f approaches. The key questions identified in C hapter 4 w ill require different experim ental, observational, and m odelling methodologies. 49 M oving F o rw ard T he p a s t s e v e r a l d e c a d e s h a v e w it n e s s e d l a r g e c h a n g e s in TERRESTRIAL AND AQUATIC NITROGEN AND PHOSPHORUS, PRIMARILY DUE TO HUMAN ACTIVITIES SUCH AS INTENSIVE AGRICULTURE. PHOTO: J . H a WKEY. Co-ordination of Activities A n im portant aspect o f international activities like G E O H A B is the sharing o f scarce resources am ong participating nations. Such sharing makes possible research activities o f a scale and breadth th a t are not otherwise feasible, and therefore enable the comparison o f ecosystems o f a sim ilar (or contrasting) type in different parts o f the world. The C R P for H A B s in E u trop h ic S ystem s w ill prom ote the application o f additional resources - in term s o f scientific expertise, sam pling platform s, and equipm ent - to the key research questions identified in this docum ent. G E O H A B has already initiated the sharing o f expertise and developm ent o f an international research com m unity on th e topic o f H A B s and E u trop h ic S ystem s by supporting the Open Science M eeting in 2005. G E O H A B Core Research Projects (CRPs) w ill be co-ordinated by the G E O H A B Scientific Steering C om m ittee (SSC) through the establishm ent o f separate sub-com m ittees for each C R P composed o f SSC members and leaders o f the C R P activities. The sub-com m ittees w ill prim arily work by correspondence, but may meet on an opportunistic basis and when identified resources allow for m eetings to address major planning and co-ordination issues. The sub-com m ittees w ill w ork w ith the G E O H A B SSC and International Program m e Office to encourage scientific networking. G E O H A B w ill identify, and draw the attention o f responsible bodies to, opportunities for co-ordination o f resources th a t w ill add value to ongoing and planned research. Individuals involved in studying the key questions outlined herein w ill be responsible for developing plans relating to the sharing o f expertise and equipm ent and how they w ill contribute to th e continued co-ordination o f the Core Research. 50 G E O H A B and the C R P sub-com m ittee w ill encourage the publication o f results from Core Research in relevant peer-reviewed scientific journals, w ith appropriate reference to the relationship o f the research to m anagem ent issues as well as th e G E O H A B goals. G E O H A B w ill also seek to aid researchers in the dissem ination o f research results and program m e status more broadly to the world-wide com m unity o f managers and scientists interested in H A B s through publication o f articles in H arm ful Algae News, the G E O H A B W eb site, and other opportunities as become available. Each C R P sub-com m ittee w ill also be responsible for dissem inating announcem ents o f m eetings, symposia, or other special events, and is encouraged to form appropriate linkages and com m unications w ith the research and m anagem ent com m unities o f the im pacted/studied areas. Data Management and Data Sharing The collective value o f data is greater th a n its dispersed value, and comparative research requires effective data sharing among scientists w orking in different regions; therefore, data m anagem ent and data exchange are im portant com ponents o f G E O H A B C RPs. The developm ent o f an appropriate data m anagem ent plan is a fundam ental and critical activity upon w hich the ultim ate success o f G E O H A B w ill depend, and the G E O H A B SSC is working w ith other related international marine research projects to develop basic guidelines for data m anagem ent and sharing (see w ww .jhu. edu/scor/D ataM gm t.htm ). Each C R P w ill need to develop its own specific plans, conform ing w ith th e principles adopted by GEOHA B. G E O H A B w ill use a decentralized data m anagem ent and distribution system w ith a centralized m etadata index, as the program m e develops. Each C R P w ill create an inventory o f data and data products. Each G E O H A B C R P w ill address th e long­ term archival o f observational data and data products to ensure a lasting contribution to marine science. M oving F orw ard A lm o tp lw o l í 1* 1* Mrtffi QrtmrwiiM fW « li ■ — ------CmMtpy fKOdMClWn Ag4w«ihVklm «Heeia tr<K K J. f . t w r j H um an A ctivities A q u a tic E c o s y s t e m s T7?e A tffro g en C ascade • iA 0 < 4 t* t d * rw m r4 M iäe pdbrtCi^l Protocols and Quality Control T h e I n t e r n a t i o n a l N it r o g e n I n it ia t iv e is i n t e r e s t e d in The C R P on H A B s in E u trop h ic S ystem s w ill require m easurem ent o f a broad suite o f param étrés th a t may differ depending on the key question being addressed. W h ere possible, w ell-defined, internationally agreed m ethods w ill be adopted. Regardless, to ensure quality control, the protocols used for data collections w ill be fully docum ented in inform ation files (metadata) accom panying data sets. W h ere necessary, the G E O H A B SSC w ill initiate Fram ew ork A ctivities th a t lead to developm ent o f protocols or m ethods, or th eir intercalibration, to ensure th at comparisons across systems can be accomplished. THE USES OF ANTHROPOG ENIC NITROGEN (W W W .IN IT R 0G E N .O R G ) . SOURCE: UNDERSTANDING A LL ASPECTS OF THE IMPACTS OF G LOBAL INCREASES IN Capacity Building G E O H A B encourages a “train in g through research” approach th a t offers opportunities for student participation and instruction in marine research relevant to H A B s. Exchange o f post-doctoral fellows and senior scientists are equally im p o rtan t for the C RPs. T raining activities th a t w ould benefit G E O H A B research will be organised by the G E O H A B SSC and proposals for specific train in g activities can be subm itted for endorsem ent as G E O H A B activities. G allo w ay et al 2 0 0 3 . Interaction with Other International Programmes and Projects G E O H A B exists in the context o f several large international program m es and projects th a t study aspects o f global change and eutrophication th a t could be relevant to th e C R P on H A B s in E u tro p h ic System s. Some examples include: • The L and-O cean Interactions in the C oastal Z one (L O IC Z ) project has compiled inform ation about the supply o f m acro­ nutrients from the land to the ocean, inform ation th a t could be useful in studying the relationships betw een nutrient supply, type, and ratios and H A B s. • The International N itrogen Initiative (IN I), under the auspices o f the Scientific C om m ittee on Problems o f the E nvironm ent (S C O P E ) and the International G eosphereBiosphere Program m e (IG B P), is a global effort to optim ize nitrogen’s beneficial role in sustainable food production and to m inim ize nitrogen’s negative effects on hum an health and th e environm ent. A s p a rt o f its objectives, knowledge o f the flows o f nitrogen and the related problems in several targeted regions o f the globe are being developed w ith a goal o f m inim izing the negative effects o f hum an health and the environm ent. This inform ation is relevant in characterising and defining nitrogen-based eutrophication. • The Integrated M arine B iogeochem istry and Ecosystem Research (IM B E R ) project is being developed w ith a goal o f providing a comprehensive understanding of, and accurate predictive capacity for, ocean biological and chemical responses to accelerating global change and the consequent effects on th e E a rth System and hum an society. P lanned observation and process studies related to the effects o f nutrient inputs to coastal areas are relevant to H A B s. Modelling Activities As described more fully in C hapter 3 o f th is report, the G E O H A B C R P on H A B s in E u trop h ic S ystem s w ill require application o f a range o f m odelling approaches. The C R P on H A B s in E u tro p h ic S ystem s should interact w ith the G E O H A B M odelling Subcom m ittee to compare models, to participate in model inter­ com parison exercises, and share approaches. M odelling w ithin H A B s in E u trop h ic S ystem s m ust also recognise the im portance o f global nutrient, clim ate, and population models in additional to models o f population dynamics o f H A B s per se. 51 Moving F o rw ard Hunan Sysiwn Eütt-i S y a e n t i L i i Fijterai F aen g s Fwdiwcfcs 1T in x w ts Arthfopogcrtc Forciigs Ecceyslan MARINE B f o g e o c ^ ie a ' C ydes T h e I M B E R p r o j e c t is d e s ig n e d t o s t u d y t h e im p a c t s o f ANTHROPOG ENIC A N D CLIM ATIC CHANGE EFFECTS O N BIOGEOCHEM ICAL PROCESSES IN THE OCEAN ( I M B E R 2 0 0 5 ) . The C lim ate Variability and Prediction (C LIV A R ) project is studying how climate oscillations, such as E N S O and N A O , operate and are expressed in the ocean and atmosphere. Interactions betw een climate change and eutrophication are identified as one o f the key questions o f interest to H A B s in E u trop h ic System s. In summary, with the aid of co-ordination facilitated by the CRP committee and the GEOHAB SSC as a whole, the activities of core research of HABs in Eutrophic Systems will strive to undertake studies that directly address the key questions identified and The G lobal O cean O bserving System (G O O S ) is a program m e for sustained, co-ordinated international observations o f the ocean and a platform for th e generation o f oceanographic products and services. H A B s in E u trop h ic S ystem s recognises th a t traditional approaches for detecting nutrient concentrations are often inadequate to resolve short-term pulses o f nutrient loading th a t may precede H A B outbreaks. As new technologies in nutrient m onitoring and cell detection move into operational status, the interactions betw een G O O S and H A B s in E u tro p h ic S ystem s will become strengthened. The G lobal O cean Ecosystem D ynam ics (G L O B E C ) project is focussed on understanding the relationship betw een physical processes and Zooplankton in the support o f marine fisheries. A ctivities o f this project w ill be o f direct relevance to H A B s in E u trop h ic S ystem s in determ ining the interactions “top dow n” and “bottom up” factors in controlling H A B s. 52 to become integrated with many projects at many levels, in order to advance and communicate scientific understanding of the role of eutrophication in the global proliferation of HABs. GEOHAB invites all relevant contributions to this effort. WRÈOF U ^UJI mm ¿LsU G o t í f im i íf i l of C a n a d a Fiühirf,es nnd O c w n i SHELLFISH ftREA G m iv sm titiS iii d u C a n a d a Pachos *t O cénn* ZONE FEU MEN A LA MCOLTE UES M O LL U SQ U ES ICIQSEU CC BU I I LAUIA « U L r u i m la m ia i t r t H eo m B W U I A M T C h i TA n n t l i t iin m iiitiH M H iu iiH . ■E L à I H L A lK lU N H f Fn Lina TAAIHQ SMIL.LHÏH f T R I ! M I I . I» SUBJECT m c i t C u T i a h uw( t i r * i F I Í H Í M I S QUI A Í C H I D i t ï a u c t m im c o u t u i E* a TH SA r a m u t u i t EST P l U l l l i Dl c m u u l a m i TO ACT MINUTIA 0 * U S H U M ii AND M E A N S F M C A U D A n H n U lH H T 1 LIS r K R W t «■JUSTA E R E t PLUMEA DES OCEANS DU C U M A IF f ir n ITiiïl W O R L D -W ID E , FISHING, SHELLFISHING, A N D S W IM M IN G AREAS ARE FREQUENTLY CLOSED DUE TO HA R M FU L ALGAE. P H O T O S (CLOCKWISE FROM TOP LEFT): U S F is h a n d W il d l if e , N e w J e r s e y F ie l d O f f ic e ; P. G l ib e r t ; C a n a d a D e p t , o f F is h e r ie s a n d O c e a n ; a n d K . W h i t i n g - G r a n t , M a i n e S e a G r a n t . References A ertebjerg, G . and T.J. Smayda. 2004. Report o f the Study Group to R eview Ecological Quality Objectives for Eutrophication. IC E S Advisory C om m ittee on Ecosystems. IC E S C M 2 004/A C E :04 Ref. A C M E , C .E ., 47 pp. A llen, J.I., J.R . Siddorn, J.C . Blackford, and F.J. G ilbert. 2004. Turbulence as a control on the microbial loop in a tem perate seasonally stratified m arine system m o d el./. Sea Res. 52:1-20. A lvarez-Salgado, X .A ., E.G. Figueiras, F.F. Pérez, S. G room , E. N ogueira, A . Borges, L. C hou, C .G . C astro, G . M oncoiffe, A.F. Rios, A .E.J. M iller, M . Frankignoulle, G . Savidge, and R. W ollast. 2003. The P ortugal C oastal C ounter C u rren t off N W Spain: N ew insights on its biogeochemical variability. Progr. Oceanogr. 56:281-321. A nderson, D .M . 1989. Toxic algal blooms and red tides: A global perspective. In T. O kaichi, D .M . A nderson, and T. N em oto (eds.), R ed Tides: Biology, Environm ental Science and Toxicology. Elsevier Science, pp. 11-16. A nderson, D .M . 1998. Physiology and bloom dynamics o f toxic Alexandrium species, w ith emphasis on life cycle transitions. In D .M . A nderson, A .D . C em bella, and G .M . H allegraeff (eds.), Physiological Ecology o f H arm ful Algal Blooms. Springer-Verlag, Berlin, H eidelberg, N A T O A S I Series Vol. G 41:29-48. A nderson, D .M ., P.M . G libert, and J.M . Burkholder. 2002. H arm fu l algal blooms and eutrophication: N utrient sources, composition, and consequences. Estuaries 25:704-726. A nderson, D .M ., D .M . Kulis, G.J. D oucette, J.C . G allagher, and E. Balech. 1994. Biogeography o f toxic dinoflagellates in the genus Alexandrium from the northeastern U nited States and Canada. Mar. Biol. 120:467-478. A nderson, D .M ., D .M . Kulis, B.A. Keafer, K .E. G ribble, R. M arin , and C .A . Scholin. 2005b. Identification and enum eration o f Alexandrium spp. from the G u lf o f M aine using molecular probes. Deep-Sea Res I I 52:2467-2490. A nderson, D .M ., G .C. Pitcher, and M . Estrada. 2005a. The comparative “systems” approach to H A B research. Oceanography 18(2):148157. A nderson, T.R . 2005. P lankton functional type m odelling: R unning before we can walk? J. Plank. Res. 27:1073-1081. A ndersson, G ., H . Berggren, G. G ronberg, and C. G elin. 1978. Effects o f planktivorous and benthivorous fish on organisms and water chem istry in eutrophic lakes. Hydrobiologia 59:9-15. A ntia, N.J., P.J. H arrison, and L. Oliveria. 1991. The role o f dissolved organic nitrogen in phytoplankton nutrition, cell biology and ecology. Phycologia 30:1-89. A rhonditsis, G .B. and M .T. B rett. 2004. Evaluation o f th e current state o f mechanistic aquatic biogeochemical modeling. Mar. Ecol. Progr. Ser. 271:13-26 A rm brust, E.V., J.A . Berges, C. Bowler, B.R. G reen, D. M artin ez , N .H . P u tnam , S. Z hou, A .E . A llen, K .E. A pt, M . Bechner, M .A . Brzezinski, B.K. C haal, A . C hiovitti, A .K . Davis, M .S . D em arest, J.C . D etter, T. G lavina, D. G oodstein, M .Z . H ad i, U. H ellsten, M . H ildebrand, B.D . Jenkins, J. Jurka, V.V. Kapitonov, N ils Kroger, W.W.Y. Lau, T.W. L ane, F.W. L arim er, J.C . Lippm eier, S. Lucas, M . M edina, A . M ontsant, M . O bornik, M .S . Parker, B. Palenik, G.J. Pazour, P.M . Richardson, T .A . Rynearson, M .A . Saito, D .C . Schw artz, K. Tham atrakoln, K. Valentin, A . Vardi, P.P. W ilkerson, and D .S. Rokhsar. 2004. The genome o f the diatom Thalassiosira pseudonana'. Ecology, evolution and metabolism. Science 306:79-86. A rm strong, M .D . and R. Kudela. 2003. Nitrogenous Preference o f Toxic P seudo-nitzschia spp. from Enrichment Experiments Conducted in Iron Replete andiron Deplete Regions in Central California. Second Symposium on H arm fu l A lgae in the US, W oods H ole, M A , Dec. 2003 (abstract only). Babin, M ., J.J. Cullen, C .S. Roesler, P.L. D onaghay, G.J. D oucette, M . K ahru, M .R . Lewis, C .A . Scholin, M .E . Sieracki, and H .M . Sosik. 2005. N ew approaches and technologies for observing harm ful algal blooms. Oceanography 18(2):210-227. 54 Barber, R.T. and A .K . H ilting. 2000. Achievem ents in biological oceanography. In O cean Studies Board, N ational Research C ouncil (ed.), 50 Years o f Ocean Discovery: N ational Science Foundation 1950-2000. N ational Academ y Press, W ashington, D .C ., pp. 11-21. Bates, S.S., C.J. Bird, A .S.W. de Freitas, R. Foxall, M . G ilgan, L .A . H an ic, G .R . Johnson, A.W . M cC ulloch, P. O desne, R. Pocklington, M .A . Q uilliam , P.G. Sim, J.C . Sm ith, D.V. Subba Rao, E .C .D. T odd, J.A . W alker, a n d J.L .C . W right. 1989. Pennate diatom Nitzschia pungens as the prim ary source o f domoic acid, a toxin in shellfish from eastern Prince E dw ard Island, Canada. Can. J. Fish. Aquat. Sei. 46:1203-1215. Bates, S.S., A .S.W. de Freitas, J.E . M illey, R. Pocklington, M .A . Q uillam , J.C . Sm ith, a n d j. W orm s. 1991. C ontrols on domoic acid production by the diatom Nitzschia pungens f. multiseries in culture: N utrients and irradiance. Can. J. Fish. Aquat. Sei. 48:1136-1144. Bates, S.S., D.J. D ouglas, G.J. D oucette, and C. Leger. 1995. E nhancem ent o f domoic acid production by reintroducing bacteria to axenic cultures o f the diatom Pseudo-nitzschia multiseries. Nat. Toxins 3:428-435. Becker, A ., A . M eister, and C. W ilhelm . 2002. F low cytom etric discrim ination o f various phycobilin-containing phytoplankton groups in a hypereutrophic reservoir. Cytometry 48:45-57. Bem an, J.M ., K.R. A rrigo, and P A . M atson. 2005. A gricultural ru n o ff fuels large phytoplankton blooms in the vulnerable areas o f the ocean. Nature 4343:211-214. Berg G .M ., M . Balode, I. P u rin a, S. Bekere, C. B echem in, and S.Y. M aestrini. 2003. P lankton com m unity com position in relation to availability and uptake o f oxidized and reduced nitrogen. Aq. Microb. Ecol. 30:263-274. B erm an, T. 1997. Dissolved organic nitrogen utilization by wo. Aphanizomenon bloom in Lake K inneret. ƒ. Plank. Res. 19:577-586. B erm an, T. 2001. The role o f D O N and the effect o f N :P ratios on occurrence o f cyanobacterial blooms: Im plications from the outgrow th o f Aphanizomenon in Lake K inneret. Limnol. Oceanogr. 46:443-447. B erm an, T. and S. Chava. 1999. A lgal grow th on organic com pounds as nitrogen sources./. Plank. Res. 21:1423-1437. Besiktepe, S T ., P.F.J. Lerm usiuax, and A .R . Robinson. 2003. Coupled physical and biogeochemical data driven simulations o f M assachusetts Bay in late summer: Real-tim e and post-cruise data assim ilation./. Mar. Syst. 40-41:171-212. B odeanu, N . and G . Ruta. 1998. D evelopm ent o f th e planktonic algae in the R om anian Black Sea sector in 1981-1996. In B. Reguera, J. Blanco, M .L . Fernandez, and T. W y att (eds.), H arm ful Algae. X unta de G alicia and Intergovernm ental O ceanographic Com m ission o f U N E S C O , Paris, pp. 188-191. Boynton, W .R ., W .M . Kemp, and C.W . Keefe. 1982. A comparative analysis o f nutrients and other factors influencing estuarine phytoplankton production. In V. S. Kennedy (ed.), Estuarine Comparisons. Academic Press, NY, pp. 69-90. Bricker, S.B., C .G . C lem ent, D .E . Pirhalla, S.P. O rlando, and D .T.G . Farrow. 1999. N ational Estuarine Eutrophication Assessment: Effects o f N utrient Enrichment in the N ations Estuaries. N O A A , N ational O cean Service, Special Projects Office and the N ational Centers for C oastal O cean Service, Silver Spring, M D , 71 pp. Bronk, D .A . 2002. D ynam ics o f dissolved organic nitrogen. In D .A . H ansell and C .A . C arlson (eds.), Biogeochemistry o f M arine Dissolved Organic Matter. Elsevier, N ew York, NY, pp. 153-247. B urkholder, J.M . 2001. Beyond algal blooms, oxygen deficits and fish kills: C hronic, long-term im pacts o f nutrient pollution on aquatic ecosystems. In L. Bendell-Young and P. G allagher (eds.), Waters in Peril. Kluwer A cademic Publishers, N orw ell, M A , pp. 103-126. B urkholder, J.M ., Dickey, D .A ., K inder, C ., Reed, R .E ., M allin, M .A ., M elia, G ., M clver, M .R ., C ahoon, L .B ., Brownie, C ., Deamer, N ., Springer, J., Glasgow, H ., Toms, D ., Sm ith, J., 2006. Comprehensive tren d analysis o f nutrients and related variables in a large eutrophic estuary: A decadal study o f anthropogenic and clim atic influences. Limnol. Oceanogr. 51:463-487. 55 R eferen ces Burkholder, J.M . and H .B . Glasgow, Jr. 1997. Pfiesteria piscicida and other Pfiesteria-like dinoflagellates: Behavior, im pacts, and environm ental controls. Limnol. Oceanogr. 42:1052-1075. Burkholder, J.M ., H .B . Glasgow, and N.J. D eam er-M elia. 2001. O verview and present status o f the toxic Pfiesteria complex. Phycologia 40:186-214. Burkholder, J.M . and P.M . G libert. 2006. Intraspecific variability: A n im portant consideration in form ing generalisations about toxigenic algal species. S. Africa J. Mar. Sei. In press. Burkholder, J.M ., A .S. G ordon, P.D. M oeller, J.M . Law, K.J. Coyne, A.J. L ew itus, J.S. Ram sdell, H .G . M arshall, N.J. D eam er, S.C. Cary, J.W. K em pton, S.L. M o rto n , and P A . Rublee. 2005. D em onstration o f toxicity to fish and m am m alian cells to Pfiesteria species: C om parison o f assay m ethods and multiple strains. Proc. Nat. Acad. Sei. (U .S.A.) 102:3471-3476. Caraco, N.F. 1995. Influence o f hum an populations on P transfers to aquatic systems: A regional scale study using large rivers. In H . Tiessen (ed.), Phosphorus in the Global Environm ent. S C O P E 54. John W iley & Sons L td., N ew York, NY, pp. 235-247. Carlsson, P., H . E dling, and C. Bechem in. 1998. Interactions betw een a marine dinoflagellate (Alexandrium catenella) and a bacterial com m unity utilizing riverine hum ic substances. Aquat. Microb. Ecol. 16:65-80. C arm an, R. and F. W ulffi 1989. A dsorption capacity o f phosphorus in Baltic Sea sediments. Estilar. Coast. Shelf Sei. 29:447-456. C arpenter, S .R .,J.F. K itchell, an d J.R . H odgson. 1985. C ascading trophic interactions and lake productivity. BioScience 35:634-639. C em bella, A .D ., D .A . Ibarra, J. D iogene, and E. D ahl. 2005. H arm fu l algal blooms and th eir assessment in fjords and coastal embayments. Oceanography 18(2):158-171. C em bella, A .D ., J.J. Sullivan, G .L . Boyer, F.J.R. Taylor, and R.J. A nderson. 1987. Variation in paralytic shellfish toxin com position w ithin the Protogonyaulax tamarensis/catenella species complex, red tide dinoflagellates. Biochem. Syst. Ecol. 15:171-186. C iotti, A .M ., J.J. C ullen, and M .R . Lewis. 1999. A sem i-analytical model o f the influence o f phytoplankton com m unity structure on the relationship betw een lig h t attenuation and ocean color./. Geophys. Res. 104:1559-1578. C loern, J.E . 2001. O u r evolving conceptual m odel o f th e coastal eutrophication problem. Mar. Ecol. Prog. Ser. 210:223-253. Cofala, J., C. Heyes, Z . K lim ont, M . A m ann, D.W . Pearce, and A . H ow arth. 2001. Technical Report on Acidification, Eutrophication and Tropospheric Ozone in Europe: A n Integrated Economic and Environm ental Assessment. R IV M report 481505014 under contract w ith the Environm ental D irectorate-general o f the E uropean C om m ission. 93 pp. C ohen, J.E . 2003. H um an population: The next h a lf century. Science 302:1172-1175. Costas, E. and V. L opez-Rodas. 1996. Enum eration and separation o f th e toxic dinoflagellates Alexandrium m inutum from natural simples using im m unological procedures w ith blocking antib o d ies./. Exp. Mar. Biol. Ecol. 198:81-87. C oyne, K.J., S.M . H andy, E. D em ir, E .B . W hereat, D .A . H utchins, K.J. P ortune, M .A . D oblin, and S.C. Cary. 2006. Im proved quantitative real-tim e P C R assays for enum eration o f harm ful algal species in field samples using an exogenous D N A reference standard. Limnol. Oceanogr.Methods 3:381-391. Craig, J.K . and L.B. Crowder. 2005. H ypoxia-induced habitat shifts and energetic consequences in A tlantic croaker and brown shrim p on the G u lf o f M exico shelf. Mar. Ecol. Prog. Ser. 294:79-94. Cullen, J.J and J.G . M acIntyre. 1998. Behavior, physiology and the niche o f depth regulating phytoplankton. In D .M . A nderson, A .D . C em bella, and G .M . H allegraeff (eds.), Physiological Ecology o f H arm ful Algal Blooms. Springer-Verlag B erlin H eidelberg, N A T O A SI Series Vol. G 41:559-579. D ahl, E. and K. Tangen. 1993. 25 years experience w ith Gyrodinium aureolum in N orw egian waters. In T. J. Smayda and Y. Shim izu (eds.), Toxic Phytoplankton Blooms in the Sea. Elsevier, NY, pp. 15-21. Daskalov, G .M . 2002. O verfishing drives atro p h ic cascade in the Black Sea. Mar. Ecol. Prog. Ser. 225: 53-63. De Baar, H .J.W ., P W . Boyd, K .H . Coale, M .R . Landry, A . Tsuda, P. Assmy, D .C .E . Bakker, Y. Bozec, R.T. Barber, M .A . B rzezinski, K.O. Buessler, M . Boye, P L . C root, F. Gervais, M.Y. Gorbunov, P.J. H arrison, W.T. H iscock, P. L aan, C. Lancelot, C.S. Law, M . Lavasseur, A . M arch etti, F.J. M illero, J. N ishioka, Y. N ojiri, T. van O ijen, U. Riebesell, M .J.A . Rijkenberg, H . Saito, S. Takeda, K.R. T im m erm ans, M .J.W . Veldhuis, A .M . W aite, and C. S. W ong. 2005. Synthesis o f iron fertilization experiments: From the iron age in the age o f enlightenm en t./. Geophys. Res.-Oceans 110 (C9): A rt No. C09S16. 56 R e fe ren ce s D ennison, W .C . and E. A bal. 1999. Moreton Bay Study: A Scientific Basis for the Healthy Waterways Campaign. South E ast Q ueensland R egional W ater Q uality M anagem ent Strategy, Brisbane, A ustralia. de Young, B., M . H eath , F. W erner, F. C hai, B. M egrey, and R M onfray. 2004. C hallenges o f m odeling ocean basin ecosystems. Science 304:1463-1466. Donaghay, R L . 1988. The role o f tem poral scales o f acclim ation, food quality and trophic dom inance in controlling the evolution of copepod feeding behaviour. Bull. Mar. Sei. 43:469-485. D onaghay, R and T .R . O sborn. 1997. Toward a theory o f biological-physical control o f harm ful algal bloom dynamics and im pacts. Limnol. Oceanogr. 42(5, p a rt 2):1283-1296. Doney, S.C., M .R . A bbott, J.J. C ullen, D .M . Karl, and L. R othstein. 2004. From genes to ecosystems: the ocean’s new frontier. Front. Ecol. Environ 2(9):457-466. D um ont, E ., J.H . H arrison, C. K roeze, E.J. Bakker, and S.P. Seitzinger. 2005. G lobal distribution and sources o f dissolved inorganic nitrogen export to the coastal zone: Results from a spatially explicit, global model. GlobalBiogeochem. Cycles 19(4):GB4S02. D yhrm an, S. and D .M . A nderson. 2003. Urease activity in cultures and field populations o f the toxic dinoflagellate Alexandrium. Limnol. Oceanogr. 48:647-655. D yhrm an, S.T. and B. Palenik. 2001. A single-cell im munoassay for phosphate stress in the dinoflagellate Prorocentrum minimum (D inophyceae)./. Phycol. 37:400-410. E U R O H A B . 1999. E uropean Initiative on H arm fu l A lgal Blooms (E U R O H A B ): H arm fu l A lgal Blooms in European M arine and Brackish W aters, E. G ranéli, G .A . C odd, B. D ale, E. Lipiatou, S.Y. M aestrini, and H . R osenthal (eds.). E uropean C om m ission, D irectorate G eneral Science Research and Developm ent, 93 pp. Evans, J.J., P.H . Klesius, P.M . G libert, C .A . Shoemaker, M .A . A l-Saraw i, J.H . Landsberg, R .D . D urem dez, A . A l-M arzo u k , and S. A lZ enki. 2002. C haracterization o f beta-hem olytic G roup B Streptococcus agalactiae in cultured gilthead seabream, Sparus auratus (L.) and w ild m ullet, L iza klunzingeri (Day), in Kuwait. J. Fish Diseases 25:505-513. Fan, C ., P.M . G libert, J. Alexander, and M .W . Lom as. 2003. C haracterization o f urease activity in three marine phytoplankton species. Mar. Biol. 142:949-958. Fisher, T .R ., L. H ardin g , D.W. Stanley, and L .G . W ard. 1988. Phytoplankton, nutrients, and tu rb id ity in the Chesapeake, D elaware, and H udson estuaries. Estilar. Coastal Shelf Sei. 27:61-93. Fisher, T .R ., E .R . Peele, J.W . A m m erm an, and L.W . H arding. 1992. N utrient lim itation o f phytoplankton in Chesapeake Bay. Mar. Ecol. Prog. Ser. 82:51-63. Fistarol, G .O ., C. L egrand, and E. G ranéli. 2005. A llelopathic effect on a nutrient-lim ited phytoplankton species. Aquat. Microb. Ecol. 41:153-161. Fistarol, G .O ., C. L egrand, C. Rengefors, and E. G ranéli. 2004a. Tem porary cyst form ation in phytoplankton: A response to allelopathic competitors? Environ. Microbiol. 6(8):791-798. Fistarol, G .O ., C. L egrand, E. Selander, C. H u m m ert, W . Stolte, and E. G ranéli, E. 2004b. A llelopathy in Alexandrium spp.: Effect on a natural plankton com m unity and on algal m onocultures. Aquat. Microbial Ecol. 35:45-56. Flynn, K.J. 1998. Physiology o f toxic microalgae w ith special emphasis on toxin production; construction o f dynamic models. In B. Reguera,, J. Blanco, L. Fernandez, and T. W y att (eds.), H arm ful Algal Blooms. Intergovernm ental O ceanographic Com m ission o f U N E S C O , pp. 315-320. Flynn, K.J. 2002. Toxin production in m igrating dinoflagellates: A m odelling study o f PSsB-pro&vecmg Alexandrium. H arm ful Algae 1:147155. Flynn, K.J. 2005a. M odelling m arine phytoplankton grow th under eutrophic co n d itio n s./. Sea Res. 54:92-103. Flynn, K.J. 2005b. Castles built on sand: D ysfunctional plankton models and the failure o f th e biology-m odelling interface./. Plank. Res. In press. Flynn, K.J. and I. Butler. 1986. N itrogen sources for the grow th o f marine microalgae: Role o f dissolved free am ino acids. Mar. Ecol. Prog. Ser. 34:281-304. 57 R eferen ces Flynn, K.J. and K. Flynn. 1995. D inoflagellate physiology: N u trien t stress and toxicity. In P. Lassus, G. A rzul, E. E rard, P. G entien, and C. M arcaillou (eds.), H arm ful M arine Algal Blooms. Lavoisier Science Publishers, Paris, pp. 541-550. Franks, P.J.S. 1992. Sink or swim: A ccum ulation o f biomass at fronts. Mar. Ecol. Prog. Ser. 82:1-12. Franks, P.J.S. 1997. Spatial patterns in dense algal blooms. Limnol. Oceanogr. 42:1297-1305. Franks, P.J.S. and D .M . A nderson. 1992. A longshore tran sp o rt o f a toxic phytoplankton bloom in a buoyancy current: Alexandrium tamarense in the G u lf o f M aine. Mar. Biol. 116:153-164. Fulton, E .A ., J.S. Paslow, A . D .M . Sm ith, and C .R . Johnson. 2003. Effect o f complexity on marine ecosystem models. Mar. Ecol. Prog. Ser. 253:1-16. Fuhrm an, J.A . and C .A . Sutile. 1993. Viruses in marine planktonic systems. Oceanography 6:50-62. Gallegos, C .L . and T .E . Jordan. 2002. Im pact o f the spring 2000 phytoplankton bloom in Chesapeake Bay on optical properties and light penetration in the Rhode River, M aryland. Estuaries 25:508-518. Galloway, J.N ., F N . D entener, D .G . C apone, E.W . Boyer, R.W. H o w arth , S.P. Seitzinger, G.P. Asner, C. Cleveland, P A . G reen, E. H olland, D .M . Karl, A . M ichaels, J.H . Porter, A . Townsend, and C. Vorosmarty. 2004. N itrogen cycles: Past, present, and future. B iogeochemistry 70:153-226. G entien, P. and G . A rzul. 1990. Exotoxin production by Gyrodinium cf. aureolum (D inophyceae)./. Mar. Biol. Assoc. 70(3):571-581. G entien, P., P. D onaghay, H . Yamazaki, R. Raine, B. Reguera, and T. O sborn. 2005. H arm fu l algal blooms in stratified environments. Oceanography 18(2):172-183. G entien, P., M . Lunven, M . L ehaitre, and J.L . D uvent. 1995. In situ d epth profiling o f particles sizes. Deep-Sea Res. 42:1297-1312. G E O H A B . 2001. Global Ecology and Oceanography o f H arm ful Algal Blooms, Science Plan, P. G libert and G . Pitcher, (eds.). S C O R and IO C , Baltim ore and Paris, 86 pp. G E O H A B . 2003. Global Ecology and Oceanography o f H arm ful Algal Blooms, Implementation Plan, P. G entien, G. Pitcher, A . C em bella, and P. G libert, (eds.). S C O R and IO C , Baltim ore and Paris, 36 pp. G E O H A B . 2005. Global Ecology and Oceanography o f H arm ful Algal Blooms, G EO H AB Core Research Project: H AB s in Upwelling Systems, G. Pitcher, T. M oita, V. Trainer, R. Kudela, F. Figueiras, and T. Probyn, (eds.). IO C and S C O R , Paris and B altim ore, 88 pp. G ism ervik, I., T. A nderson, and O. Vadstein. 1996. Pelagic food webs and eutrophication o f coastal waters: Im pact o f grazers on algal com m unities. Mar. Poll. Bull. 33:22-35. Gisselson, L .-Ä ., P. Carlsson, E. G ranéli, and J. Pallon. 2002. Dinophysis blooms in the deep euphotic zone o f the Baltic Sea: D o they grow in the dark? H arm ful Algae 1:401-418. Gitay, H . and I.R . Noble. 1997. W h a t are functional types and how should we seek them ? In T .M . Sm ith, H .H . Shugart, and F.I. W oodw ard (eds.), Plant Functional Types: T/jeir Relevance to Functional Properties and Global Change. C am bridge U niversity Press, Cam bridge, U nited K ingdom , pp. 3-19. G libert, P.M . 1998. Interactions o f top-dow n and bottom -up control in planktonic nitrogen cycling. Hydrobiologia 363:1-12. G libert, P.M . and D .A . Bronk. 1994. Release o f dissolved organic nitrogen by m arine diazotrophic cyanobacteria, Trichodesmium spp. Appl. Environ. Microbiol. 60:3996-4000. G libert, P.M ., and J.M . Burkholder. 2006. The complex relationships betw een increasing fertilization o f the earth, coastal eutrophication and proliferation o f harm ful algal blooms. In E. G ranéli and J. T urner (eds.), Ecology o f H arm ful Algae. Springer-Verlag, B erlin/ H eidelberg. In press. G libert, P.M ., D.J. Conley, T .R . Fisher, L.W . H ard in g , Jr., and T .C . M alone. 1995. D ynam ics o f the 1990 w inter/spring bloom in Chesapeake Bay. Mar. Ecol. Prog. Ser. 122:27-43. G libert, P.M ., J. H arrison, C. H eil, and S. Seitzinger. 2006. E scalating worldwide use o f urea - a global change contributing to coastal eutrophication. Biogeochemistry 77:441-463. G libert, P.M . and C .H eil. 2005. Use o f urea fertilisers and the im plications for aquatic harm ful algal blooms. In Z . Z hu, K. M in am i, and G . X ing (eds.), 3rd International Nitrogen Conference Contributed Papers, Science Press U SA , Inc, Beijing, pp. 539-544. 58 R e fe ren ce s G libert, P.M ., C .A . H eil, D. H ollander, M . Revilla, A . H oare, J. A lexander, and S. M urasko. 2004. Evidence for dissolved organic nitrogen and phosphorus uptake during a cyanobacterial bloom in Florida Bay. Mar. Ecol. Progr. Ser. 280:73-83. G libert, P.M ., J. Landsberg, J. Evans, M .A . A l-Saraw i, M . Faraj, M .A . A l-Jarallah, A . H ayw ood, S. Ibrahem , P. Klesius, C. Powell, and C . Shoemaker. 2002. A fish kill o f massive proportion in Kuwait Bay, A rabian G ulf, 2001: The roles o f infectious bacteria, harm ful algae, and eutrophication. H arm ful Algae 12:1-17. G libert, P.M ., R. M agnien, M .W . Lom as, J. Alexander, C. Fan, E. H aram oto, M . Trice, and T .M . Kana. 2001. H arm fu l algal blooms in the Chesapeake and C oastal Bays o f M aryland, USA: C om parison o f 1997, 1998, and 1999 events. Estuaries 24:875-883. G libert, P.M . and J.M . O ’Neil. 1999. Dissolved organic nitrogen release and amino acid oxidase activity by Trichodesmium spp. In L. C harpy and A .W .D . L arkum (eds.), M arine Cyanobacteria. M onaco M usée O céanographique, B ulletin de l ’In stitu t O céanographique, M onaco, pp. 265-271. G libert, P.M ,. S. Seitzinger, C .A . H eil, J.M . Burkholder, M .W . Parrow, L .A . C odispoti, and V. Kelly. 2005. The role o f eutrophication in the global proliferation o f harm ful algal blooms. Oceanography 18(2):198-209. G obler, C.J. and S.A. Sañudo-W ilhelm y. 2001. Tem poral variability o f groundw ater seepage and brow n tide blooms in a L ong Island em baym ent. Mar. Ecol. Prog. Ser. 217:299-309. G oldm an, J.C . and P.M . G libert. 1983. Kinetics o f inorganic nitrogen uptake. In C arpenter, E.J. and C apone, D .G . (eds.), Nitrogen in the M arine Environm ent. Academic Press, NY, pp. 233-274. G onzález-G il, S., B.A. Keafer, R.V.M. Jovine, and D .M . A nderson. 1998. D etection and quantification o f alkaline phosphatase in single cells o f phosphorus-lim ited marine phytoplankton. Mar. Ecol. Prog. Ser. 164:31-35. G ranéli, E. 2005. E utrophication and harm ful algal blooms. In P. W assm ann and K. O lli (eds.), Drainage basin nutrient inputs and eutrophication: A n integrated approach. U niversity o f Trom so, Norway, pp. 99-112. G ranéli, E ., D .M . A nderson, P. Carlsson, and S.Y. M aestrini. 1997. L ight and dark carbon uptake by Dinophysis species in comparison to other photo synthetic and heterotrophic dinoflagellates. Aquat. Microb. Ecol. 13:177-186. G ranéli, E ., P. Carlsson, P. Tester, J.T. Turner, C. Bechem in, R. D awson, and F. A zam . 1999. Effects o f N :P:Si ratios and Zooplankton grazing on phytoplankton com m unities in the northern A driatic Sea. I. N utrients, phytoplankton, biomass, and polysaccharide production. Aq. Microb. Ecol. 18:37-54. G ranéli, E ., L. E dler, D. G edziorow ska, and U. N ym an. 1985. Influence o f humic and fulvic acids on Prorocentrum minim um (Pav.) J. Schiller. In D .M . A nderson, A.W . W h ite, and D .G . Baden (eds.), Toxic Dinoflagellates. Elsevier Science P ublishing C o., N orthH olland, pp. 201-206. G ranéli, E. and K.J. Flynn. 2006. Chem ical and physical factors influencing toxin content in harm ful algae. In E. G ranéli and J.T. T urner (eds.), Ecology o f H arm ful Algae. Springer-Verlag, B erlin/H eidelberg. In press. G ranéli, E. and P.J. H ansen. 2006. A llelopathy in harm ful algae: A m echanism to compete for resources? In E. G ranéli and J.T. T urner (eds.), Ecology o f H arm ful Algae. Springer-Verlag, B erlin/H eidelberg. In press. G ranéli E. and N . Johansson N. 2003a. Increase in th e production o f allelopathic substances by Prymnesium parvum cells grow n under N or P-deficient conditions. H arm ful Algae 2:135-145. G ranéli, E. and N . Johansson. 2003b. Effects o f th e toxic haptophyte Prymnesium parvum on th e survival and feeding o f a ciliate: The influence o f different nutrient conditions. Mar. Ecol. Prog. Ser. 254:49-56. G ranéli, E ., N . Johansson, and R. Panosso. 1998. C ellular toxin contents in relation to nutrient conditions for different groups of phycotoxins. In B. Reguera, J. Blanco, M .L . Fernández, and T. W yatt, (eds.), H arm ful Algae. X unta de G alicia and Intergovernm ental O ceanographic C om m ission o f U N E S C O , pp. 321-324. G ranéli, E. and M .O . M oreira. 1990. Effects o f river w ater o f different origin on the grow th o f marine dinoflagellates and diatom s in laboratory cu ltu res./. Exp. Mar. Ecol. 136:89-106. G ranéli, E. and J.T. Turner. 2002. “T op-dow n” regulation in ctenophore - copepod - ciliate - diatom - phytoflagellate com m unities in coastal waters: A mesocosm study. Mar. Ecol. Prog. Ser. 239:57-68. G rzebyk, D. and B. Berland. 1996. Influences o f tem perature, salinity and irradiance on grow th o f Prorocentrum minimum (Dinophyceae) from the M editerranean S ea./. Plankt. Res. 18:1837-1849. 59 R eferen ces Hagy, J.D ., W .R . Boynton, C.W . Keefe, and K.V. W ood. 2002. H ypoxia in Chesapeake Bay, 1950-2001: L ong-term change in relation to nutrient loading and river flow. Estuaries 27:634-658. HallegraefF, G .M . 1993. A review o f harm ful algal blooms and th eir apparent global increase. Phycologia 32:79-99. H arding, L.W ., Jr. 1994. L ong-term trends in the distribution o f phytoplankton in Chesapeake Bay: Roles o f light, nutrients and streamflow. Mar. Ecol. Prog. Ser. 104:267-291. H arlin, M .M . 1993. C hanges in major p lant groups following nutrient enrichm ent. In A.J. M cC om b (ed.), Eutrophic Shallow Estuaries and Lagoons. C R C Press, Inc., Boca Raton, F L , pp. 173-187. H arrison, J.H ., N .R Caraco, and S.P. Seitzinger. 2005. G lobal patterns and sources o f dissolved organic m atter export to the coastal zone: Results from a spatially explicit, global model. GlobalBiogeochem. Cycles 19(4):GBS406. H eil, C. A ., P.M . G libert, and C. Fan. 2005. Prorocentrum minim um (Pavillard) Schiller - A review o f a harm ful algal bloom species of grow ing worldwide im portance. H arm ful Algae 4:449-470. H olligan, P.M . 1979. D inoflagellate blooms associated w ith tidal fronts around the British Isles. In D .L . Taylor and H .H . Seliger (eds.), Toxic Dinoflagellate Blooms. Elsevier N o rth H olland, NY, pp. 249-256. H ow arth, R.W ., G . Billen, D. Swaney, A . Townsend, N . Jaworski, K. L ajtha, J.A . D ow ning, R. E lm gren, N . Caraco, T. Jordan, F. Beredse, J. Freney, V. Kueyarov, P. M urdoch, and Z. Z hao-L iang. 1996. Riverine inputs o f nitrogen to the N o rth A tlantic Ocean: Fluxes and hum an influences. Biogeochemistry 35:75-139. H ow arth, R.W ., E.W . Boyer, R. M arino, D. Swaney, N . Jaworski, and C. G oodale. 2006. The influence o f climate on average nitrogen export from large w atersheds in the northeastern U nited States. Biogeochemistry. In press. H ow arth, R.W ., E.W . Boyer, W .J Pabich, and J.N . Galloway. 2002b. N itrogen use in the U nited States from 1961-2000 and potential future trends. Ambio 31:88-96. H ow arth, R.W. and R. M arino. 2006. N itrogen as the lim iting nutrient for eutrophication in coastal marine ecosystems: Evolving views over 3 decades. Limnol. Oceanogr. 51:364-376. H ow arth, R.W ., K. R am akrishna, E. C hoi, R. E lm gren, L. M artinelli, A . M endoza, W . M oom aw, C. Palm , R. Boy, M . Scholes, and Z. Z hao-L iang. 2005. N u trien t m anagem ent, responses assessment. In: Ecosystems and H uman Well-Being, Volume 3, Policy Responses, the M illenium Ecosystem Assessment. Island Press, W ashington D C , pp. 295-311. H ow arth, R.W ., A . Sharpley, and D. W alker. 2002a. Sources o f nutrient pollution to coastal waters in the U nited States: Im plications for achieving coastal water quality goals. Estuaries 25:656-676. H um borg, C., D.J. Conley, L. R ahm , F. W ulff, A . Cociasu, and V. Ittekot. 2000. Silica retention in river basins: far reaching effects on biogeochem istry and aquatic food webs. Ambio 29:45-50. Integrated M arine B iogeochem istry and Ecosystem Research (IM B E R ). 2005. Science Plan and Implementation Strategy. IG B P R eport No. 52, IG B P Secretariat, Stockholm , Sweden. 76 pp. International Fertilizer Industry 2005. w w w .fertilizer.org/ifa/statistics. Jacquet, S., L. Prieur, C. Avois-Jacquet, J.-F. L ennon, and D. Vaulot. 2002. Short-tim escale variability o f picophytoplankton abundance and cellular param étrés in surface waters o f the A lboran Sea (western M ed iterran ean )./. Plank. Res. 24:633-651. Jang, M .H ., K. H a, G.-J. Joo, and N . T akam ura. 2003. Toxin production o f cyanobacteria is increased by exposure to Zooplankton. Freshwater Biol. 48:1540 -1550. John, E .H . and K.J. Flynn. 2002. M odelling changes in paralytic shellfish toxin content o f dinoflagellates in response to nitrogen and phosphorus supply. Mar. Ecol. Prog. Ser. 225:147-160. Jones, R .H . and K.J. Flynn. 2005. N utritional status and diet com position affect the value o f diatom s as copepod prey. Science 307:14571459. Kamykowski, D. 1974. Possible interactions betw een phytoplankton and sem idiurnal internal tid e s./. Mar. Res. 32:67-89. Kamykowski, D. 1995. Trajectories o f autotrophic m arine dinoflagellates./. Phycol. 31:200-208. 60 R e fe ren ce s K amykowski, D ., J.E . M illigan, R .E . Reed, and W . Liu. 1999. G eotaxis/phototaxis and biochem ical patterns in Heterocapsa illdefina (Dinophyceae) during vertical m igrations./. Phycol. 35:1397-1403. Karl, D., R. Letelier, D.V. H ebel, D.E. Bird, and C .D . W inn. 1992. Trichodesmium blooms and new nitrogen in th e N o rth Pacific Gyre. In E.J. C arpenter, D .G . C apone, and J.G . Reuter (eds.), M arine Pelagic Cyanobacteria: Trichodesm ium and Other Diazotrophs. Kluwer Academic Publishers, N etherlands, pp. 219-238. Kemp, W .M ., W .R . Boynton, J.E . A dolf, D.E. Boesch, W .C . B oicourt, G . B rush, J.C . C ornw ell, T .R . Fisher, P.M . G libert, J.D . H agy, L.W . H arding, E .D . H oude, D .G . K im m el, W .D . M iller, R .I.E . N ewell, M . R. R om an, E .M . Sm ith, and J.C . Stevenson. 2005. Eutrophication in Chesapeake Bay: H istorical trends and ecological interactions. Mar. Ecol. Prog. Ser. 303:1-29. Kideys, A .E ., A . Roohi, S. Bagheri, G. Finenko, and L. K amburska. 2005. Im pacts o f invasive ctenophores on the fisheries o f the Black Sea and C aspian Sea. Oceanography 18(2):76-85. K irkpatrick, G ., D.E. M illie, M .A . M oline, and O. Schofield. 2000. O ptical discrim ination o f a phytoplankton species in natural mixed populations. Limnol. Oceanogr. 45:467-471. Knoll, L .B., M .J. Vanni, and W .H . Renwick. 2003. Phytoplankton prim ary production and photosynthetic param eters in reservoirs along a gradient o f w atershed land use. Limnol. Oceanogr. 48:608-617. Kruskopf, M . and K.J. Flynn. 2006. C hi content and fluorescence response cannot be used to gauge reliably phytoplankton biomass, nutrient status or grow th rate. N ew Phytologist 169:525-536. L am , C.W.Y. and K.C. H o. 1989. Red tides in Tolo H arbour, H o n g Kong. In T. O kaichi, D .M . A nderson, and T. N em oto (eds.), Red Tides: Biology, Environm ental Science and Toxicology. Elsevier Science, pp. 49-52. Lancelot, C ., G. Billen, A . Sournia, T. W eisse, F. Colijn, M.J.W . Veldhuis, A . Davies, and P. W assm ann. 1987. Phaeocystis blooms and nutrient enrichm ent in the continental coastal zones o f the N o rth Sea. Ambio 16:38-46. Lancelot, C ., J.M . M a rtin , N . P anin, and Y. Zaitsev. 2002. The N orth-w estern Black Sea: A pilot site to understand th e complex interaction betw een hum an activities and the coastal environm ent. Estilar. Coastal Shelf Sei. 54:279-283. Lancelot, C ., Y. Spitz, N . G ypens, K. R uddick, S. Becquevort, V. Rousseau, G . Lacroix, and G . Billin. 2005. M odelling diatom and Phaeocystis blooms and nutrient cycles in th e Southern B ight o f the N o rth Sea: The M IR O model. Mar. Ecol. Prog. Ser. 289:63-78. L aR oche, J., R. N uzzi, R. W aters, K. W ym an, P.G. Falkowski, and D.W .R. W allace. 1997. Brown tide blooms in L ong Island’s coastal waters linked to variability in groundw ater flow. Global Change Biol. 3:397-410. Lawrence, J.E . and A .D . Cem bella. 1999. A n im m unolabeling technique for th e localisation o f diarrhetic shellfish toxins in individual microalgae. Phycologia 38:60-65. L ew itus, A.J., L .B . Schm idt, L.J. M ason, J.W. K em pton, S.B. W ilde, J.L . W olny, K.C. H ayes, S.N. H ym el, C.J. Keppler, and A .H . R ingw ood. 2003. H arm fu l algal blooms in South C arolina residential and go lf course ponds. Pop. Environ. 24:387-413. L ew itus, A.J., B .M . W illis, K .C. Hayes, J.M . Burkholder, H .B . Glasgow, Jr., P.M . G libert, and M .K . Burke. 1999. M ixotrophy and nitrogen uptake by Pfiesteria piscicida (D inophyceae)./. Phycol. 35:1430-1437. Li, A ., D .K. Stoecker, D.W . C oats, and E.J. A dam . 1996. Ingestion o f fluorescently labeled and phycoerythrin-containing prey by m ixotrophic dinoflagellates. Aquat. Microb. Ecol. 10:139-147. Li, W .K.W . and P.M . D ickie. 2001. M onitoring phytoplankton, bacterioplankton and virioplankton in a coastal inlet (Bedford Basin) by flow cytometry. Cytometry 44:236-246. L idie, K .L., J.C . Ryan, M . Barbier, and F M . Van D olah. 2005. G ene expression in the Florida red tide dinoflagellate Karenia brevis: analysis o f an expressed sequence tag (E S T ) library and developm ent o f a D N A microarray. Mar. Biotech. In press. L itaker, R.W. and P A . Tester. 2005. A biotic and biotic factors controlling a nutrient driven dinoflagellate bloom and likely responses to increased eutrophication. G EO H A B Open Science M eeting on H AB s and Eutrophication, B altim ore, M D (abstract only). M acIntyre, H . and J.J. C ullen. 2005. U sing cultures to investigate the physiological ecology o f microalgae. In R .A . A nderson (edI), Algal Culturing Techniques. Elsevier A cademic Press. H o n g Kong, pp. 287-326. M acIntyre, H .L , M .W . Lom as, J. Cornw ell, D.J. Suggett, C.J. G obler, E.W . Koch, and T .M . Kana. 2005. M ediation o f benthic-pelagic coupling by m icrophytobenthos: and energy- and m aterial-based model for initiation o f blooms o f Aureococcus anophagefferens. H arm ful Algae 3: 403-438. 61 R eferen ces M aclean, J.L . 1989. Indo-Pacific red tides, 1985-1988. Mar. Pollution Bull. 20:304-310. M alone, T.C . 1992. Effects o f water colum n processes on dissolved oxygen, nutrients, phytoplankton and Zooplankton. In D .E . Sm ith, M . Leffler, and G . M ackiernan (eds.), Oxygen Dynamics in the Chesapeake Bay: A Synthesis o f Research. M aryland Sea G ran t College Publication No. U M -S G -T S -9 2 -0 1 , College Park, M D , pp. 61-112. M alone, T .C ., D.J. Conley, T .R . Fisher, P.M . G libert, L.W . H ard in g , and K.G. Sellner. 1996. Scales o f nutrient-lim ited phytoplankton productivity in Chesapeake Bay. Estuaries 19:371-385. M alone, T .C ., P.G. Falkowski, T.S. H opkins, G.T. Rowe, and T .E . W hitledge. 1983. M esoscale response o f diatom populations to w ind events in the plum e o f the H udson River. Deep-Sea Res. 30:149-170. M alone, T .C ., W .M . Kemp, H .W . Ducklow, W .R . Boynton, J.H . T uttle, and R.B. Jonas. 1986. Lateral variability in the production and fate o f phytoplankton in a partially stratified estuary. Mar. Ecol. Prog. Ser. 32:149-160. M argalef, R. 1978. Life-form s o f phytoplankton as survival alternatives in an unstable environm ent. Oceanol. Acta 1:493-509. M cG illicuddy, D.J., R.P. Signell, C .A . Stock, B.A . Keafer, D .M . Keller, R .D . H etlan d , and D .M . A nderson. 2003. A m echanism for offshore initiation o f harm ful algal blooms in the coastal G u lf o f M a in e ./. Plank. Res. 25:1131-1138. M errell, J.R . and D .K . Stoecker. 1998. D ifferential grazing on protozoan m icroplankton by developm ental stages o f the calenoid copepod Eurytemona affinis Poppe. ƒ Plank. Res. 20:289-304. M iller, W .D ., L.W . H ard in g , and J.E . Adolf. 2005. The influence o f H urricane Isabel on Chesapeake Bay phytoplankton dynamics. In K.G. Sellner (ed.), Hurricane Isabel in Perspective. Chesapeake Research C onsortium Publication 05-160, Edgewater, M D . M iller, W .D ., L.W . H ard in g , Jr., and J.E Adolf. 2006. H urricane Isabel generated an unusual fall bloom in Chesapeake Bay. Geophys. Res. Lett. 33, L 06612, doi:10.1029/2005G L025658. M itra, A . and K.J. Flynn. 2005. Predator-prey interactions: Is “ecological stoichiom etry” sufficient when good food goes bad? J. Plank. Res. 27: 393-399. M itra, A . and K.J. Flynn. 2006. P rom otion o f harm ful algal blooms by Zooplankton predatory activity. Biol. Lett. D oi:10.1098/ rsbl.2006.0447. M oll, A . and G. Radach. 2003. Review o f three-dim ensional ecological m odelling related to the N o rth Sea shelf system - P a rt 1: M odels and their results. Progr. Oceanogr. 57(2):175-217. M ulholland M .R ., K. O hki, and D .G . Capone. 1999. N itrogen utilization and m etabolism relative to patterns o f N 2 fixation in cultures o f Trichodesmium NIBB1967. J. Phycol. 35:977-988. Najjar, R .G . 1999. The w ater balance o f the Susquehanna River basin and its response to climate ch an g e./. Hydrology 219:7-19. N ational Research C ouncil (N R C ). 2000. Clean Coastal Waters: Understanding and Reducing the Effects o f N u trien t Pollution. N ational Academies Press, W ashington, D C . Nielsen, T .G ., T. Kiorbo, and P.K. Bjornsen. 1990. Effects o f a Chrysochromulina polylepis subsurface bloom on the planktonic community. Mar. Ecol. Prog. Ser. 62:21-35. Nixon, S.W. 1995. C oastal marine eutrophication: A definition, social causes, and future concerns. Ophelia 41:199-219. Noble, D. 2002. The rise o f com putational biology. Nature Rev. Mol. CellBiol. 3:460-463. N uzzi, R. and R .M . W aters. 2004. L ong-term perspective on the dynamics o f brow n tide blooms in L ong Island coastal waters. H arm ful Algae 3:279-294. O guz, T. 2005. L ong-term im pacts o f anthropogenic forcing on the Black Sea ecosystem. Oceanography 18(2):112-121. Oliver, R .L. and G .C . G anf. 2000. Freshwater blooms. In B. A . W h itto n and M . Potts (ed.), The Ecology o f Cyanobacteria. Kluwer Academic Publishers, pp. 149-194. O lson, R.J., A .M . C hekalyuk, and H .M . Sosik. 1996. Phytoplankton photosynthetic characteristics from fluorescence induction assays o f individual cells. Limnol. Oceanogr 41:1253-1263. Paerl, H .W . 1988. Nuisance phytoplankton in coastal, estuarine and inland waters. Limnol. Oceanogr 33:823-847. 62 R e fe ren ce s Paerl, H .W . and D.F. M illie. 1996. Physiological ecology o f toxic cyanobacteria. Phycologia 35:160-167. Paerl, H .W . and J.L . Pinckney. 1996. M icrobial consortia: Their role in aquatic production and biogeochemical cycling. Microbiol. Ecol. 31:225-247. Park, G .S. and H .G . M arshall. 2000. E stuarine relationships betw een Zooplankton com m unity structure and trophic g rad ien ts./. Plankt. Res. 22:121-135. Parrow, M .W . and J.M . Burkholder. 2004. The sexual life cycles o f Pfiesteria piscicida and cryptoperidiniopsoids (D inophyceae)./. Phycol. 40:664-673. Parsons, M .L ., Ch D ortch, and R .E . Turner. 2002. Sedim entological evidence o f an increase in Pseudo-nitzschia (Bacillariophyceae) abundance in response to coastal eutrophication. Limnol. Oceanogr. 47:551-558. P ratt, D .M . 1966. C om petition betw een Skeletonema costatum and Olisthodiscus luteus in N arragansett Bay and in culture. Limnol. Oceanogr. 11:447-445. Q i, Y.Z., Z . Z hang, Y. H ong, S. L u, C. Z hu, and Y. Li. 1993. O ccurrence o f red tides on the coasts o f C hina. In T. Smayda and Y. Shim izu (eds.), Toxic Phytoplankton Blooms in the Sea. Elsevier Science Publishers, B.V. A m sterdam , pp. 43-46. R adach, G ., J. Berg, and E. H agm eier. 1990. L ong-term changes o f the annual cycles o f meteorological, hydrographic nutrient and phytoplankton tim e series at H elgoland and at LV Elbe 1 in the G erm an Bight. Cont. Shelf Res. 10:305-328. Reynolds, C .S., V .L.M . H uszar, C. K ruk, L. N aseill-Flores, and S. M elo. 2002. Toward a functional classification o f the freshwater phy to p lan k to n ./. Plank. Res. 24:417-428. Richardson, K. and B.B. Jorgensen. 1996. Eutrophication: D efinition, history and effects. In B.B. Jorgensen and K. R ichardson (eds.), Eutrophication in Coastal M arine Ecosystems. Coastal and Estuarine Studies, Volume 52. A m erican G eophysical U nion, W ashington, D C , p p . 1 -1 9 . Riegm an, R. 1995. N utrient-related selection mechanism s in m arine phytoplankton com m unities and the im pact o f eutrophication on the planktonic food web. Water Sei. Technol. 32(4):63-75. Schofield, O ., J. G rzym ski, W .P. Bissett, G.J. K irkpatrick, D.F. M illie, M . M oline, and C .S. Roesler. 1999. O ptical m onitoring and forecasting systems for harm ful algal blooms: Possibility or pipe dream ?J. Phycol. 35:1476-1496. Scholin, C. A ., R. M arin III, P E . M iller, G.J. D oucette, C .L . Powell, P. H aydock, J. H ow ard, and J. Ray. 1999. D N A probes and a receptorbinding assay for detection o f Pseudo-nitzschia (Bacillariophyceae) species and domoic acid activity in cultured natural sam ples./. Phycol. 35:1356-1367. Seitzinger, S.P., J.A . H arrison, E. D um ont, A .H .W . Beusen, and A.F. B ouwm an. 2005a. Sources and delivery o f carbon, nitrogen and phosphorous to the coastal zone: A n overview o f global nutrient export from watersheds (N E W S ) models and th eir application. Global Biogeochem. Cycles 19:GB4S09. Seitzinger, S.P., H . H a rtn e tt, R. Lauck, M . M azurek, T. M inegishi, G . Spyres, and R. Styles. 2005b. M olecular level chemical characterization and bioavailability o f dissolved organic m atter in stream w ater using E SI mass spectrometry. Limnol. Oceanogr. 50(1):112 . Seitzinger, S.P. and C. Kroeze. 1998. Global distribution o f nitrous oxide production and N inputs in freshwater and coastal marine ecosystems. Global Biogeochem. Cycl. 12: 93-113. Seitzinger, S.P., C. Kroeze, A.F. B ouw m an, N . Caraco, F. D entener, and R.V. Styles. 2002a. Global patterns o f dissolved inorganic and particulate nitrogen inputs to coastal systems: Recent conditions and future projections. Estuaries 25:640-655. Seitzinger, S.P., R.W. Sanders, and R.V. Styles. 2002b. Bioavailability o f D O N from natural and anthropogenic sources to estuarine plankton. Limnol. Oceanogr. 47:353-366. Sellner K .G. and S. Fonda-U m ani. 1999. D inoflagellate blooms and mucilage production. In T.C . M alone, A . M alej, L.W . H ard in g Jr, N . Sm odlaka, and R .E . T urner (eds.), Ecosystems a t the Land-Sea Margin: Drainage Basin to Coastal Sea. C oastal E stuarine Studies 55, A m erican G eophysical U nion, W ashington, D C , pp. 173-206. Shapiro, L.P., L. C am pbell, and E .M . H augen. 1989. Im m unochem ical recognition o f phytoplankton species. Mar. Ecol. Prog. Ser. 57:219224. 63 R eferen ces Shiah, F-K . and H .W . Ducklow. 1994. Tem perature regulation o f heterotrophic bacterioplankton abundance, production, and specific grow th rate in Chesapeake Bay. Limnol. Oceanogr. 39:1243-1258. Shumway, S.E and T .L.C ucci. 1987. The effects o f th e toxic dinoflagellate Protogonyaulax tamarensis on the feeding and behaviour of bivalve molluscs. Aq. Toxicology. 10:19-27. Silva, E.S. 1985. Ecological factors related to Prorocentrum minim um blooms in O bidos Lagoon (Portugal). In D .M . A nderson, A . W hite, and D. Baden, (eds.), Toxic Dinoflagellates. Elsevier, NY, pp. 251-256. Smayda, T.J., 1989. Prim ary production and the global epidemic o f phytoplankton blooms in the sea: A linkage? In E .M . Cosper, V.M. Bricelj, and E.J. C arpenter, (eds.), N ovel Phytoplankton Blooms. C oastal and E stuarine Studies No. 35, Springer-Verlag, NY, pp. 449484. Smayda, T.J. 1990. Novel and nuisance phytoplankton blooms in th e sea: Evidence for a global epidemic. In E. G ranéli, B. Sundström , L. Edler, and D .M . A nderson (eds.), Toxic M arine Phytoplankton. Elsevier Science Publishing, N ew York, pp. 29-40. Smayda, T.J. 2005. E utrophication and phytoplankton. In P. W assm ann and K. O lli (eds.), Drainage Basin N utrient Inputs and. Eutrophication: A n Integrated Approach. U niversity o f Trom so, Norway, w ww .ut.ee/~olli/eutr/, pp. 89-98. Smayda, T.J. and D .G . Borkm an. 2006. N u trien t and plankton gradients in N arragansett Bay. In B. C osta-Pierce, A . C olt, and A. D esbonnet (eds.), Ecosystem-based Management o f a Southern N ew England Estuary: A Case Study o f Narragansett Bay. Springer, N ew Y o rk . In press. Smayda, T.J. and C .S. Reynolds. 2001. C om m unity assembly in m arine phytoplankton: A pplication o f recent models to harm ful dinoflagellate bloom s./. Plankton Res. 23:447-461. Smil, V. 2001. Enriching the Earth: F ritz Haber, Cari Bosch, and the Transformation o f World Food. The M IT Press, Cam bridge, M A . Springer, J.J., J.M . Burkholder, H .B . Glasgow, P.M . G libert, and R .E . Reed. 2005. Use o f a real-tim e m onitoring netw ork (R T R M ) and shipboard sam pling to characterize a dinoflagellate bloom in the Neuse Estuary, N o rth C arolina, U .S.A . H arm ful Algae 4:553-574. Steidinger, K .A ., G .A . Vargo, P A . Tester, and C .R . Tomas. 1998. Bloom dynamics and physiology o f Gymnodinium breve w ith emphasis on the G u lf o f M exico. In D .M . A nderson, A .D . C em bella, and G .M . H allegraeff (eds.), Physiological Ecology o f H arm ful Algal Blooms. Springer-Verlag, Berlin, H eidelberg, N A T O A SI Series Vol. G 41:133-153. Stibor H ., O. Vadstein, S. D iehl, A . Glezleichter, T. H ansen, F. H antzsche, A . Katechalis, K. L oseth, C. Peters, W . Roederer, M . Sandow, L. Sundt-H ansen, and Y Olsen. 2004. C opepods act as a switch betw een alternative trophic cascades in marine pelagic food webs. Ecol. Lett. 7:321-328. Stoecker, D .K . 1999. M ixotrophy am ong dinoflagellates./. Eukaryot. Microbiol. 46:397-401. Stonik, I.V., 1995. A potentially toxic dinoflagellate, Prorocentrum minimum, in A m urskii Bay o f the Sea o f Japan. Russ. J. Mar. Biol. 20:314-320. S tuart, V., S. Sathyendranath, T. P latt, H . M aass, and B.D . Irw in. 1998. Pigm ents and species com position o f natural phytoplankton populations: Effect on the absorption sp ectra./. Plankton Res. 20:187-217. Sullivan, B .K., D. Van Keuren, and M . Clancy. 2001. T im in g and size o f blooms o f the ctenophore Mnemiopsis leidyi in relation to tem perature in N arragansett Bay, R I. Hydrobiologia 451:113-120. Taylor, A .H ., J.I. A llen, and P.A. Clark. 2002. E xtraction o f a w eak climatic signal by an ecosystem. Nature 416:629-632. T ett, P., G . G ilpin, H . Svendsen, C.P. Erlnadsson, U. Larsson, S. Kratzer, E. Fouilland, C. Janzen, J.Y. Lee, C. G renz, A . N ew ton, J.G . Ferrier, T. Fernandez, and S. Scory. 2003. E utrophication and some E uropean waters o f restricted exchange. Cont. Shelf Res. 23:16351671. T illm ann, U. 2003. Kill and eat your predator: A w inning strategy o f th e planktonic flagellate Prymnesium parvum . Aq. Microb. Ecol. 32'd?>84. Tracey, G .A . 1988. Feeding reduction, reproductive failure, and m ortality in M ytilus edulis during the 1985 “brow n tid e” in N arragansett Bay, Rhode Island. Mar. Ecol. Prog. Ser. 50:73-81. T rainer, V., B -T.L. E berhart, J.C . W ekell, N .G . A dam s, L. H anson, F. Cox, and J. Dowell. 2003. Paralytic shellfish toxins in Puget Sound, W ash in g to n ./. Shellfish Res. 22:213-223. 64 R e fe ren ce s Turner, J.T., A . Ianora, A . M iralto, M . Laabir, and F. Esposito. 2001. D ecoupling o f copepod grazing rates, fecundity and egg-hatching success on mixed and alternating diatom and dinoflagellate diets. Mar. Ecol. Prog. Ser. 220:187-199. Turner, J.T. and P.A. Tester. 1997. Toxic marine phytoplankton, Zooplankton grazers, and pelagic food webs. Limnol. Oceanogr. 42:12031214. Turner, J.T., P.A. Tester, and P.J. H ansen. 1998. Interactions betw een toxic marine phytoplankton and m etazoan and protistan grazers. In D .M . A nderson, A .D . C em bella and G .M . H allegraeff (eds.), Physiological Ecology o f H arm ful Algal Blooms. N A T O A SI Series G: Ecological Sciences, 41:453-474. Turner, R .E . and N .N . Rabalais. 1991. Changes in M ississippi River water quality this century and im plications for coastal food webs. BioScience 41(3):140-147. Turner, R .E . and N . Rabalais. 1994. C oastal eutrophication near the M ississippi river delta. Nature 368:619-621. Turner, J.T., P.A. Tester, and P.J. H ansen. 1998. Interactions betw een toxic marine phytoplankton and m etazoan and protistan grazers. In D .M . A nderson, A .D . Cem bella, and G .M . H allegraeff (eds.), Physiological Ecology o f H arm ful Algal Blooms. N A T O A SI Series G: Ecological Sciences, Vol. 41. Springer-Verlag, Berlin, pp. 453-474. Tyler, M .A . and H .H . Seliger. 1978. A nnual subsurface tran sp o rt o f a red tide dinoflagellate to its bloom area: W ater circulation patterns and organism distributions in the Chesapeake Bay. Limnol. Oceanogr. 23:227-246. Vadstein O ., H . Stibor, B. L ippert, K. L oseth, W Roederer, L. Sundt-H ansen, and Y. Olsen. 2004. M oderate increase in the biomass o f omnivorous copepods may ease grazing control o f planktonic algae. Mar. Ecol Prog. Ser. 270:199-207. Verity, P.G. and V. Smetacek. 1996. O rganism life cycles, predation, and the structure o f m arine pelagic ecosystems. Mar. Ecol. Prog. Ser. 130:277-293. Vezie, C ., J. Rapala, J. Vaitoma, J. Seitsonen, and K. Sivonen. 2002. Effect o f nitrogen and phosphorus on grow th o f toxic and nontoxic Microcystis strains and on intracellular m icrocystin concentrations. Microbial Ecol. 43:443-454. Vitousek, P.M ., J. A ber, R.W. H o w arth , G .E . Likens, P A . M atson, D.W. Schindler, W .H . Schlesinger, and G .D . T ilm an. 1997. H um an alteration o f the global nitrogen cycle: Causes and consequences. Ecol. Appl. 7:737-750. Vrieling E .G ., W .H . van de Poll, G . Vriezekolk, and W .W .C. Gieskes. 1997. Im m uno-flow cytom etric detection o f the ichthyotoxic dinoflagellates Gyrodinium aureolum and Gymnodinium nagasakiense'. Independence o f physiological sta te ./. Sea Res. 37:91-100. W alsh, J.J., B. Penta, D .A . D ieterle, and W .P. Bissett. 2001. Predictive ecological m odeling o f harm ful algal blooms. H uman Ecol. Risk Assess. 7:1369-1383. W assm ann, P. and K. O lli (eds.). 2006. Drainage Basin N utrient Inputs and Eutrophication: A n Integrated Approach. U niversity o f Tromso, Norway, w w w .ut.ee/~olli/eutr/. 325 pp. W hitledge, T .E . 1993. The nutrient and hydrographic conditions prevailing in L aguna M adre, Texas before and during a brown tide event. In T.J. Smayda and Y. Shim izu (eds.), Toxic Phytoplankton Blooms in the Sea. Elsevier Science Publishers, B.V. A m sterdam , pp. 711-716. W ikfors, G .H . 2005. A review and new analysis o f trophic interactions betw een Prorocentrum minim um and clams, scallops, and oysters. H arm ful Algae 4:585-592. W ood, A .M . and T. L eatham . 1992. The species concept in phytoplankton ecology./. Phycol. 28:723-729. W oods, J. and W. B arkm ann. 1993. The plankton m ultiplier - positive feedback in the greenhouse./. Plank. Res. 15:1053-1074. Z hang, J. 1994. A tm ospheric w et depositions o f nutrient elements: C orrelations w ith harm ful biological blooms in th e N orthw est Pacific coastal zones. Ambio 23:464-468. Z hang, J., Z.F. Z hang, S.M . Liu, Y. W u, H . X iong, and H .T. C hen. 1999. H u m an im pacts on the large world rivers: W ould the C hangjiang (Yangtze) River be an illustration? Global Biogeochem. Cycles 13:1099-1105. Z hou, M . 2005. W h ich is the trigger factor to the outbreak o f large scale Prorocentrum bloom in the E ast C h in a Sea? G EO H AB Open Science Meeting on H A B s and Eutrophication, Baltim ore, M D (abstract only). 65 A p p e n d i x I: GEOHAB O p e n S c ie n c e M e e t in g o n H AB s a n d E u tr o p h ic a tio n , M a r c h 2 0 0 5 Programme M on d ay, M arch 7, 200S SE S SIO N 1 T R E N D S IN E U T R O P H IC A T IO N A N D H A B S 12:00-13:30 (Session C hair: Patricia M . G libert) O pening Remarks - Patricia M . G libert (USA) N itrogen pollution: sources, trends, and effects globally and regionally - R obert H o w arth (USA) C O FFE E BREAK N ational and global trends in H A B s - D onald A nderson (USA) M ultidecadal changes in the diatom: Flagellate ratio and Si:N and Si:P ratios in N arragansett Bay, and influence o f SI N supply ratios on diatom species com petition - Ted Smayda (USA) and D. B orkm an LUNCH SE S SIO N 2 P H Y S IO L O G Y A N D E C O L O G Y O F H A B S W I T H R E S P E C T T O N U T R I E N T S 9:15- 9:45 9:45 - 10:25 10:25-11:00 11:00-11:30 11:30-12:00 13:30-14:10 14:10-14:30 14:30-14:50 14:50-15:15 15:15- 15:35 15:35- 15:55 15:55- 16:15 16:15 -16:45 16:45- 17:05 17:05-17:30 17:30- 19:30 (Session Chairs: E dn a G ranéli and C indy H eil) The role o f nutrient conditions on toxicity, allelopathy, and m ixotrophy in H A B s - E d n a G ranéli (Sweden) N itrogen uptake by the toxigenic diatom Pseudo-nitzschia australis - W illiam C ochlan (USA), J. H erndon, N .C . Ladizinsky and R .M . Kudela. U rea-am m onium -nitrate interactions in Thau lagoon (Southern France): Relationships w ith Alexandrium catenella blooms - Yves Collos (France), A . Vaquer, M . Laabir, E. A badie, T. Laugier, and A . Pastoureaud BREAK A role for anthropogenically derived nitrogen in the form ation o f harm ful algal blooms along th e US w est coast - Raphael Kudela (USA), M . A rm strong, W. C ochlan, and J. H erndon N itrogen preference o f the fish-killing flagellate Chattonella cf. verruculosa - Carm elo Tomas (USA) Intraspecific variability in the nutritional ecology o f harm ful algae - Jo A n n Burkholder (USA) The effects o f macro- and m icro-nutrient lim itation on karlotoxin production by Karlodinium micrum strains - Jason A d o lf (USA), T. Bachvaroff, G.F. Reidel, and A . R. Place Effect o f N :P supply ratio on biochem ical com position and toxicity in dinoflagellate Alexandrium tamarense - A i M urata (Japan), S. C. Y. Leong, Y. N agashim a, and S. T aguchi O pen discussion Poster Session O ne (see page 68) (H osted by th e U niversity o f M aryland C enter for E nvironm ental Science, the Chesapeake Research C onsortium , and the M aryland D ep artm en t o f N atural Resources) T uesday, M a rch 8 , 2005 SE S SIO N 3 T H E G E O H A B P R O G R A M M E A N D O T H E R IN IT IA T IV E S 8:30-8:45 8:45-9:05 9:05-9:35 9:35-9:55 9:55-10:20 SE S SIO N 4 1 0 : 2 0 - 11:00 11:00-11:40 11:40-12:20 12:20-14:00 14:00-14:40 14:40-15:00 66 (Session C hair: D onald A nderson) Introduction to the G E O H A B Program m e - G ran t Pitcher (S. Africa) Introduction to IO C and S C O R - H en rik Enevoldsen (IO C ) and E d U rban (SC O R) N O A A extram ural H A B research: Present and future - Q uay D ortch (USA), S. B anahan, and M . Suddleson Perspectives o f the US O cean Com m ission and the Pew O ceans Com m ission - D onald Boesch (USA) C O FFE E BREAK C O M P A R A T IV E S T U D I E S A N D IN T E R N A T IO N A L P R O G R A M M E S O N H A B S IN E U T R O P H IC AREAS (Session Chairs: M ingjiang Z hou and Lars Edler) The C hinese H A B Program m e: C E O H A B - M ingjiang Z hou (China) D o the coastal eutrophication and w arm ing cause w idespread and persistent Cochlodiniumpolykroides blooms in Korean waters? - H akG yoon Kim (Korea), C -K . Lee, W -A . Lim , S-Y. K im , and H -G . Jin Influence o f monsoons and oceanographic processes on red tides in H o n g Kong waters - Kedong Yin (H ong Kong China) LUNCH H arm fu l algae and eutrophication in the Baltic Sea A rea - L ars E dler (Sweden) O ceanographic and environm ental assessment o f K uwait’s waters in relevance to algal blooms - Faiza Al-Yamani (Kuwait), W .A . Ism ail, K.S. A l-R ifaie, and A . Lennox 15:00-15:20 15:20-15:45 15:45-16:05 16:05-16:25 16:25-16:45 16:45-17:30 H A B s in W estern A ustralia: Expressions o f eutrophication in a southern climate - M alcolm Robb (A ustralia), T. Reitsem a, W . Hosja, and A . Begum BREAK A n integrated approach to predicting harm ful algal blooms: Phytoplankton physiology, nutrient dynamics and their application in an ecosystem model - Paul A rm strong (A ustralia), P A . Thom pson, C.J.S. Bolch, S.I. B lackburn, J.P. Parslow, M . H erzfeld and K. W ild-A lien C om parative analysis o f the relationships betw een nutrient cycling and phytoplankton com m unity com position in tw o eutrophied subtropical estuaries: Florida Bay, U SA , and M oreton Bay, A ustralia - C ynthia H eil (USA), P.M . G libert, J. O ’N eil, W .C . D ennison, D. H ollander, J. G reenw ood, M . O ’D onohue, S. C ostanzo, M . Revilla, J. Alexander, A. H oare, and S. M urasko Linkages betw een land-based nutrient discharges and harm ful m acroalgal blooms: C om parative studies on coral reefs o f southeast Florida and Jam aica - Brian Lapointe (USA), B. B edford, P.J. Barile, C. H anson, and L. G etten O pen discussion W ed n esd ay, M a rch 9 , 2005 S E S SIO N 5 M A C R O N U T R IE N T IN T E R A C T IO N S W IT H O T H E R F A C T O R S C O N T R O L L IN G H A B S 8:30-8:50 8:50-9:10 9:10-9:30 9:30- 9:50 9:50- 10:20 10:20-10:40 10:40-11:00 11:00- 11:20 S E S SIO N 6 (Session C hair: Ted Smayda) H o w does eutrophication affect th e role o f grazers in harm ful algal bloom dynamics? - E dw ard Buskey (USA) Freshwater flow and nutrients: effects on top-dow n control o f bloom -form ing dinoflagellates? - D iane Stoecker (USA), M .L . Reaugh, A .E . Thessen, D .E . G ustafson, M .R . R om an, and W .C . Boicourt A conceptual model for ecosystem disruptive algal blooms: The interactive roles o f eutrophication, algal toxicity, and lim itation by nutrients and lig h t - W illiam Sunda (USA) and R. H ardison Abiotic and biotic factors controlling a nutrient driven dinoflagellate bloom and likely responses to increased eutrophication - R. W ayne L itaker (USA) and P A . Tester C O FFE E BREAK Brackish storm w ater detention ponds as prom oters o f H A B s and eutrophication along the South C arolina coast - A lan L ew itus (USA), M .K . Burke, L. J. M ason, K. N . Bunker, S.R. D rescher, and W .H .J. Strosnider Iron induced developm ent pathw ay o f H A B s com m unity and its consequence on m itigation o f eutrophication - Jun Sun (China), Y. Feng, and P. Sun. The synergy o f iron, copper and the toxicity o f diatom s - M a rk W ells (USA), C. G . T rick, W .P. C ochlan, P. H ughes, and N . C. Ladizinsky N E W C H A L L E N G E S A N D M E T H O D O L O G IE S 11:50-12:30 12:30-14:00 (Session C hair: M are Suddleson) Im plem enting the coastal module o f the Global O cean O bserving System (G O O S ): Toward rapid detection and tim ely predictions o f harm ful algal blooms - Thomas M alone (USA) N ew approaches and technologies for observing harm ful algal blooms - M arcel Babin (France) LUNCH SE S S IO N 6 (con tin u ed ) 11:20-11:50 14:00-14:20 14:20-14:40 14:40-15:00 15:00-15:30 15:30-15:50 15:50-16:10 16:10-16:40 16:45-18:45 (Session C hair: M arcel Babin) A pplication o f the environm ental sample processor (ESP) for rem ote detection o f harm ful algae and toxins they produce - C hris Scholin and G reg D oucette (USA) A utonom ous nutrient m onitoring and water sam pling as tools for studying H A B s: Progress and prospects- L ou C odispoti (USA), V. Kelly, P. G libert, and J. A lexander N ew technologies for m onitoring and assessing harm ful algal blooms and water quality in Chesapeake Bay, M aryland - C hristopher H eyer (USA), T. M .Trice, P. J. T ango, B .M ichael, L .C odispoti, V. Kelly BREAK D iagnostic indicators o f H A B nutritional physiology - Sonia D yhrm an (USA) N ew approaches to understanding the role o f dissolved organic m atter in H A B dynamics - Sybil Seitzinger (USA), P. M . G libert, J.P. Simjouw, and R. Sipler O pen discussion Poster Session Two (see page 69) 67 Program m e Thursday, M a rch 10, 2 0 0 5 SE S SIO N 7 M O D E L L IN G O F N U T R IE N T S A N D H A B S 8:30- 9:10 9:10- 9:50 9:50-10:10 10:10- 10:40 10:40- 11:00 11:00-11:30 (Session Chairs: J. Icarus A llen and Kevin Flynn) E utrophication and H A B models for the N W E uropean continental s h e lf - J . Icarus A llen (UK), F. G ilbert, J. H olt, M . H o lt, R. Proctor, a n d j. Siddorn G arbage in, G arbage out? Problems in experim ental design and m odelling o f H A B ecology - Kevin Flynn (Wales) C O FFE E BREAK Assessing the validation o f a prelim inary Karlodinium micrum nowcast model system in Chesapeake Bay and its tributaries: a fram ew ork for H A B nowcasts and forecasts - Peter Tango (USA), C.W . Brown, T.F. Gross, D .L. Ram ers, R .R . H ood, and B.D. M ichael M odelling Pfiesteria life cycle attributes and population dynamics - Raleigh H o o d (USA), X . Z hang and J.T. A nderson O pen discussion o f m odelling SE S SIO N 8 G E O H A B IM P L E M E N T A T IO N 11:30-11:45 11:45-12:45 12:45-14:15 14:15-14:45 14:45-16:00 16:00-16:15 16:15-16:45 16:45 17:00 (Session C hair: G ran t Pitcher) C harge to w orking groups - Patricia G libert and G ran t Pitcher F irst break-out groups m eet LUNCH Reports o f first break-out groups Second break-out groups meet BREAK Reports o f second break-out groups Final w rap-up A djourn M on d ay, M arch 7, 2 0 0 5 P O S T E R SE S SIO N O N E 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. 19. 20. 68 Phytoplankton indices o f eutrophication in U K coastal waters - R.J. G owen, D .K. M ills, M . Best, M .J. D evlin, M . Edw ards, J. Foden, S.J. Painting, R. Park, C. Reid, and P. T ett E utrophication and harm ful algal blooms in the Swan River estuary, W estern A ustralia - J. John The research o f the eutrophication status o f E ast C hina Sea - X .- L . W ang, X.-Y. Shi, and C .-S. Z hang C oastal nutrification following th e passage o f H urricane Charley and its relation to a subsequent Karenia brevis bloom on the W est Florida S h e lf -M .B . Neely, C .A . H eil, and G .A . Vargo The effect o f nutrient concentration at different grow th stages on hemolytic ability o f three clones o f the ichthyotoxic flagellate Prymensium parvum from blooms in the U nited States - M . Clouse and C. Tomas Strain Variation in Karlodinium micrum toxin production - T. Bachvaroff, J.E . A dolf, and A .R . Place Fatty acids and grow th in the heterotrophic dinoflagellates Pfiesteria Spp. and P L O s - L.W . H aas, V. Foster, L. O tt, W .K . Vogelbein, K.S. Reece, J.D . Shields, and P. M ason The influence o f dissolved copper on the production o f domoic acid by toxigenic species o f Pseudo-nitzschia in M onterey Bay, C alifornia - N .C . Ladizinsky, G.J. Sm ith, K .H . Coale, and W .P. C ochlan Toxin levels in the benthic cyanobacterium Lyngbya majuscula in relation to tissue nutrient content and bloom intensity - J.M . O ’Neil, S. A lbert, N . O sborne, and G . Shaw The potential role o f increased nutrient inputs to higher incidences o f ciguatera in H aw aii - M .L . Parsons N utrient regulation o f toxin production: C om parison o f hemolytic activity o f Amphidinium carterae and Amphidinium klebsii - L .A . Z im m erm ann and C .R . Tomas N itrate uptake kinetics o f th e toxic dinoflagellate Alexandrium tamarense in response to nitrate supply mode - S.C.Y. L eong, M . M aekaw a, and S. T aguchi Bioavailability o f dissolved organic phosphorus compounds to typical harm ful dinoflagellate Prorocentrum donghaiense L u - B. H uang, L. O u, H . H ong, H . Luo, and D. W ang Dissolved organic m atter concentration and characteristics during Aureococcus anophagefferens blooms in 2002 and 2003: A comparison - J.-P.Simjouw, E .C . M inor, and M .R . M ulholland The role o f natural D O M sources in Prorocentrum minim um grow th dynamics - R. Sipler, S.P. Seitzinger, and P.M . G libert Urea utilisation by harm ful algal species in the Chesapeake Bay, M aryland, U SA - C .M . Solomon and P.M . G libert A com parison o f nutrient effects on the grow th o f Chattonella subsalsa and Heterosigma akashiwo (Raphodophyceae) isolated from the Inland Bays, Delaware (U .S.A) - Y. Z h an g and D .A . H utchins The assessment o f brow n tide blooms caused by the alga, Aureococcus anophagefferens and related environm ental factors in coastal waters o f N ew Jersey (2000-2002) - M . Downes G astrich, R. L athrop, S. H aag, M .P. W einstein, M . D anko, D .A . C aron, and R. Schaffner H arm fu l algae in Suffolk C ounty (N.Y., USA) estuaries: A 30 year history - R. N uzzi O rganic nutrients and brow n tide in M aryland C oastal Bays - C. W azniak Program m e W ed n esd ay, M a rch 9 , 2005 P O S T E R SE S SIO N T W O 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. Top down control and demise o f a nutrient driven dinoflagellate bloom - P.A. Tester and R.W. Litaker M odelling the contribution o f prey deselection in th e form ation o f harm ful algal blooms - A. M itra and K.J. Flynn Raphidophyte systematics and rapid identification: Sequence analyses and real tim e P C R Assays - H .A . Bowers, C. Tomas, J.W. K em pton, S. G oto, A.J. L ew itus, and D.W. O ldach. G eographic distribution o f Pfiesteria spp. and environm ental factors - H . Z hang and S. L in Im proved accuracy o f quantitative real-tim e P C R o f H A B species in environm ental water samples using an exogenous D N A internal standard - K.J. C oyne, S.M . H andy, E. D em ir, K.J. P ortune, Y. Z hang, M .A . D oblin, D .A . H utchins, and S.C. C ary H arm fu l phytoplankton indicator species applied to eutrophication assessments o f Scottish coastal waters supporting aquaculture - M .J. G ubbins, P.J. Sammes, and I.M . Davies. M onitoring toxic phytoplankton and shellfish in support o f eutrophication assessments for Scottish coastal waters - M .J. G ubbins, E .A. Sm ith, M . G rieve, and E. Bresnan H istory o f H A B m onitoring in M aryland tidewaters: M onitoring, response, nowcasting and forecasting - P.J. Tango, B. M ichael, D. G oshorn, R. M agnien, C. Heyer, T .M . Trice, W . Butler, C. W azniak, R. K arrh, S. Bowen, R. Lacouture, H . Bowers, D. O ldach, C. L uckett, C. Poukish, D. M atuzsak, J. Ryan, H . L ynch, C. Brown, R. H ood, T. Gross, and D. Ramers A n autonom ous urea m onitor for studying H A B s - V. Kelly, L .A . C odispoti, P. G libert, and J. A lexander A pplications o f an in situ water quality m onitoring platform (M A R V IN ) for H A B research: A com parison o f data collected in the St. Johns and C aloosahatchee River systems in Florida - J. Rueter, M .B . Neely, B. Bendis, R. Pigg, K. Steidinger, and C. H eil R elationships betw een nitrogen loading and concentrations o f nitrogen and chlorophyll in coastal embayments - E .H . D ettm ann, L .B . M ason, A . Erhunse, and K .M . H enry M odeled Karenia brevis bloom initiation and subsequent accum ulation in the vicinity o f a coastal nutrient front - G .S. Janow itz and D. Kamykowski Ecosystem m odelling o f th e N W E uropean shelf seas tow ards th e forecasting o f harm ful algal blooms - J.R . Siddorn, J.I. A llen, and M . H olt E nvironm ental and behavioural influences on Karenia brevis’ nitrate uptake: A bloom initiation scenario - G . Sinclair, D. Kamykowski, E. M illig an , and B.Schaeffer A behaving drifter for sim ulating tran sp o rt o f mobile H A B organisms in nature - T .G . W olcott, D. Kamykowski, and G . Janow itz Potential roles o f Prorocentrum minimum to Chesapeake Bay dissolved oxygen and oyster dynamics - E. Brownlee, S. Sellner, and K .G. Sellner Volunteer H A B m onitoring provides a “F irst W atc h ” for resource managers and researchers in th e Delaware In lan d Bays, U SA - E. W hereat and M . Farestad Problems w ith ballast water exchange as a means o f controlling m ovem ent o f harm ful algal species th roughout th e world - C .E . O rano-D aw son, R. D awson, and D .A . W right 69 A p p e n d i x II: GEOHAB O p e n S c ie n c e M e e t in g o n HABs a n d E u tr o p h ic a tio n , M a r c h 2 0 0 5 Participants Jason Adolf Patrick Conway Robert Gross B a l t im o r e , M D U S A W a s h in g t o n , D C U S A W il m in g t o n , D E U S A J. Icarus Allen Kathryn Coyne M att Gubbins P ly m ou th U N I T E D K IN G D O M L ew es, D E U S A A berdeen U N I T E D K IN G D O M Khlood Al-Rifaie Ifeyinwa Davis Leonard W. Haas K u w a it K U W A IT W a s h in g t o n , D C U S A G l o u c e s t e r P o in t , VA U S A Donald M. Anderson Edward Dettmann Callie Haii W N arrag a nsett, R I U S A S t e n n is S pace C e n t e r , M S U S A o ods H ole, M A USA Paul Armstrong Felisa Diaz-Mendez Matthew R. Haii H B a l t im o r e , M D U S A A n n a p o l is , M D U S A obart, T a s m a n ia A U S T R A L IA Marcel Babin L. Kellie Dixon Paul E. Hargraves Sa r a s o t a , E L U S A N a rrag a nsett, R I U S A Tsvetan Bachvaroff Quay Dortch Cynthia Heil B a l t im o r e , M D U S A S ilv er S p r in g , M D U S A St . P e t e r s b u r g , E L U S A Susan Banahan Gregory Doucette Julian Herndon S ilv er S p r in g , M D U S A C h a r l e s t o n , SC U S A T ib u r o n , C A U S A V il l e f r a n c h e - su r - M er FRA N CE Jennifer Berrigan Sonya Dyhrman Christopher Heyer W a s h in g t o n , D C U S A W A n n a p o l is , M D U S A oods H o le, M A USA Donald Boesch Lars Edler Raleigh R. Hood C a m b r id g e , M D U S A A n gelholm S W E D E N C a m b r id g e , M D U S A Holly Bowers Henrik Oksfeldt Enevoldsen Robert W. Howarth B a l t im o r e , M D U S A C o pen h a g en D E N M A R K It h a c a , N Y U S A Larry Brand Chunlei Fan Bangqin Huang M B a l t im o r e , M D U S A X ia m e n P.R . C H I N A JoAnn Burkholder Muns Farestad Edythe M. Humphries R a l e ig h , N C U S A L ew es, D E U S A D over, D E U S A ia m i, EL USA Edward Buskey Betty June Farkas Muna Hussein P o r t A ra nsa s, T X U S A G a it h e r s b u r g , M D U S A A l S u r r a h K U W A IT Melissa Clouse Kevin John Flynn Dave Hutchins W il m in g t o n , N C U S A Sw a n se a U N I T E D K I N G D O M L ew es, D E U SA William Cochlan Patricia Glibert Gerald Janowitz T ib u r o n , C A U S A C a m b r id g e , M D U S A R a l e ig h , N C U S A Lou Codispoti David Goshorn Andrew Juhl C a m b r id g e , M D U S A A n n a p o l is , M D U S A P a l is a d e s , N Y U S A Yves Collos Edna Granéli Vince Kelly K alm ar S W E D E N C a m b r id g e , M D U S A M o n t p e l l ie r 70 FRA N CE Darla Konkel W a sh in g to n , D C U SA Robert Nuzzi R i v e r h e a d , N Y USA Dana Sipek W a sh in g to n , D C U SA HakGyoon Kim R E P U B L IC O F K O R E A Garvan O'Donnell B usan P a rk m o re , G a lw a y Judy Kleindinst W o o d s H o l e , M A U SA Judith M. O'Neil C a m b r i d g e , M D U SA Ted Smayda Raphael Kudela S a n t a C r u z , C A U SA A n n a p o lis, Celia Erlinda F. Orano-Dawson M D U SA Caroline M. Solomon C a m b r i d g e , M D U SA V ero B each, Brian E. Lapointe EL U SA Troy C. Osborne W a s h i n g t o n , D C U SA Diane Stoecker C a m b r i d g e , M D U SA B u ie s C r e e k , L. Michael Larsen N C U SA W a sh in g to n , Pia Marie Paulone D C U SA W ilm in g to n , Sandric Chee Yew Leong JA P A N Grant Pitcher C a p e T o w n S O U T H A F R IC A Mare Suddleson S i l v e r S p r i n g , M D U SA Alan J. Lewitus Allen Place Jun Sun Tokyo Rachel Sipler IR E L A N D N a rra g a n se tt, R I U SA Peter Strilko D E U SA USA B a ltim o re , M D U SA A n n a p o lis, Rebecca Raves M D U SA William Sunda B e a u f o r t , N C U SA Senjie Lin G r o t o n , C T U SA Tarren Reitsema E a s t P e r t h A U S T R A L IA A n n a p o lis, R. Wayne Litaker N C U SA Malcolm Robb E a s t P e r t h A U S T R A L IA Robert Magnien S i l v e r S p r i n g , M D U SA Jason Rueter C h a rle s to n , SC Ji Li C a m b rid g e, B eau fo rt, S t. M D U SA N J U SA N ew B ru n sw ick , EL U SA P e te rsb u rg , Thomas C. Malone A r l i n g t o n , VA U SA W ilm in g to n , W a sh in g to n , Anne S. Marsh D C U SA N e w B ru n sw ick , Bruce Michael M D U SA Kevin Sellner E d g e w a t e r , M D U SA Aditee Mitra S w a n s e a U N IT E D K IN G D O M John Siddorn Ai Murata JA P A N Jean-Paul Simjouw A n n a p o lis, Tokyo P e te rsb u rg , EL D E U SA Sybil Seitzinger N J U SA U N IT E D K IN G D O M Geoff Sinclair USA R a le ig h , N C P R C H IN A Peter Tango M D U SA Avery O. Tatters C a ro lin a B each, N C U SA Patricia A. Tester N C U SA B eau fo rt, Helge Abildhauge Thomsen C h a r l o t t e n l u n d DENM ARK Carmelo R. Tomas N C U SA W ilm in g to n , Charles Trick L o n d o n CANADA EricTurevon E x e te r, D evon N e w B ru n sw ick , N J Merrie Beth Neely S t. Paul Sample Q in g d a o W a sh in g to n , D C U SA Ed Urban U SA B a ltim o re , M D U SA Cathy Wazniak USA A n n a p o lis, M D U SA Participants E d w a rd W h e re a t D E USA Lew es, R o b e r t W h it m a n N ew ark, D E USA A m a n d a W illa r d W a sh in g to n , D C USA T h o m a s G . W o lc o tt R a le ig h , N C USA K e d o n g Y ln G uangzhou R R . C H IN A YaohongZhang Lew es, D E U SA M in g jia n g Z h o u Q in g d a o R R . C H IN A L e ig h Z im m e r m a n n W ilm in g to n , 72 N C U SA A p p e n d ix III: G lobal E cology a n d O c e a n o g r a ph y of H a r m f u l A lgal B loom s S c ie n tific S te e r in g C o m m itte e fo r G EO H A B 2006 Raphael M. Kudela USA Members Robin Raine C h a ir , I K U D E L A @ U C S C .E D U reland R O B IN .R A IN E @ N U IG A L W A Y .IE Alicia Lavin S pa in Marcei Babin V ic e -c , F h a ir m arcel @ a l i c i a .l a v i n o b s - v l f r .f r Dennis McGillicuddy USA Allan Cembella (ex- o ffic io C R P ), G , C @ s t .i e o .e s rance o D M C G IL L IC U D D Y @ W H O I.E D U -C h a ir of F jo rd s and C oastal E mbayments erm any Grant Pitcher a c e m b e lla @ a w i-b re m e rh a v e n .d e (ex -o ffic io , C h a i r o f U p w e llin g S y stem s C R P ), S o u th A fric a Einar Dahl N ORWAY g p itc h e r@ sfri2 .w c a p e .g o v .z a E in a r.D a h l@ im r.n o Beatriz Reguera (ex -o ffic io , C h a ir , Wolfgang Fennel (ex- o ffic io , C h a ir of w o l f g a n g .f e n n e l IP H A B ), S p a in b e a triz .re g u e ra @ v i.ie o .e s M @ io -w o d el l in g Su b c o m m it t e e ), G erm any a r n e m u e n d e .d e Mingjiang Zhou C Ken Furuya h in a -B m jzh o u e ijin g @ m s .q d i o .a c .c n Japan FU R U Y A @ F S. A .U -T O K Y O .A C .JP Sponsor Representatives Patrick Gentien (ex- o ffic io pg e n t ie n @ , C h a ir of St r a t if ie d Sy s t e m s C R P ), F rance i f r e m e r .f r H A B @ B O T .K U .D K Patricia Glibert (ex -o ffic io , C h a i r o f E u tr o p h ic S y stem s G L IB E R T @ H P L .U M C E S .E D U Henrik Enevoldsen IO C C R P), USA Ed Urban SC O R E d .U r b a n @ j h u .e d u Leonardo Guzmán C h il e L G U Z M A N @ IF O P .C L 73 Figure Credits p. 13. Model-predicted export of dissolved inorganic nitrogen. Reprinted from Seitzinger and Kroeze (1998), Global Distribution o f Nitrous Oxide Production and N Inputs in Freshwater and Coastal Marine Systems. Global Biogeochemical Cycles 12:100, with permission from Springer Science and Business Media. p. 15. Contribution by global region. Reprinted from Glibert et al. (2005), The Role o f Eutrophication in the Global Proliferation o f Harmful Algal Blooms. Oceanography 18(2):200, w ith permission from The Oceanography Society. p. 17. Sewage plume map. Reprinted from Dennison and Abal (1999), Moreton Bay Study. Published by the Southeast Regional Water Quality Management Strategy, p. 131 w ith permission from the authors. p. 17. Increasing HAB occurrence. Reprinted from Granéli (2005), Eutrophication and harmful algal blooms, Chapter 7 in P. Wassmann, and K. Olli (eds.), Drainage Basin Inputs and Eutrophication, w w w .ut.ee/~olli/eutr/, w ith permission from the author. p. 17. The relationship between population growth. Redrawn from Trainer et al. (2003), Paralytic Shellfish Toxins in Puget Sound, Washington. Journal o f Shellfish Research 22:221, w ith permission from the journal. p. 18. The change in N:P ratio. From Anderson et al. (2002), based on Riegman (1995), Harmful Algal Blooms and Eutrophication: Nutrient Sources, Composition and Consequences. EstuarieslS (4b):714, w ith permission from the Estuarine Research Federation. p. 19. The relationship between the percent contribution of urea to total N uptake. Reprinted from Glibert and Heil (2005). Use o f Urea Fertilizers and the Implications for Increasing Harmful Algal Blooms in the Coastal Zone. Contributed papers, the 3rd International Nitrogen Conference, Science Press USA Inc., 2005, p. 542, w ith permission from the authors. p. 20. Prorocentrum minimum blooms in the tributaries of Chesapeake Bay. Reprinted from Fan et al. (2003), Characterization o f the A ffin ity for Nitrogen, Uptake Kinetics, and Environmental Relationships for Prorocentrum minimum in Natural Blooms and Laboratory Cultures. Harmful Algae 2:293, w ith permission from Elsevier. p. 34. Marine pelagial habitats. From Smayda and Reynolds (2002), Community Assembly in Marine Phytoplankton: Application o f Recent Models to Harmful Dinoflagellate Blooms. Journal o f Plankton Research 23:456, with permission from Oxford University Press. p. 36. Physical environments interact w ith organism behaviour. From McGillicuddy et al. (2003), A Mechanism for Offshore Initiation o f Harmful Algal Blooms in the Coastal Gulf o f Maine. Journal o f Plankton Research 25(9):1131 -1138, w ith permission from Oxford University Press. p. 38. Conceptual relationships. Redrawn from MacIntyre et al. (2005), Mediation o f Benthic-Pelagic Coupling by Microphytobenthos: and Energy- and Material-Based Model for the Initiation o f Blooms o f Aureococcus anophagefferens. Harmful Algae 3:421, with permission from Elsevier. p. 42. Change in US atmospheric NOx emissions. Reprinted from Howarth et al. (2002b), Nitrogen Use in the United States from 19612000 and Potential Future Trends. Ambio 31 (2):89, w ith permission from The Royal Swedish Academy of Sciences. p. 42. The relationship between human inputs of N and riverine N export. Reprinted from Howarth et al. (1996), Regional nitrogen budgets and riverine N and P fluxes. Biogeochemistry35 (1):75-139, w ith permission from Springer Science and Business Media. p. 44. Short-term nutrient loading, such as occurred following Hurricane Isabel. Reprinted from Miller et al. (2006), Hurricane Isabel Generated an Unusual Fall Bloom in Chesapeake Bay. Geophysical Research Letters 33, L06612, w ith permission from the American Geophysical Union. p. 45. Possible long-term relationship. Reprinted from Alvarez-Salgado et al. (2003), The Portugal Coastal Counter Current O ff NW Spain: New Insights on Its Biochemical Variability. Progress in Oceanography 56:281-321, with permission from Elsevier. p. 51. The International Nitrogen Initiative. Reprinted from Galloway et al. (2003), Emerging Challenges- New Findings. The Nitrogen Cascade: Impacts o f Food and Energy Production, Overfishing: Harvesting Fish Faster Than They Can Reproduce. GEO Year Book 2003, p. 57, w ith permission from the author. 74 www.geohab.info