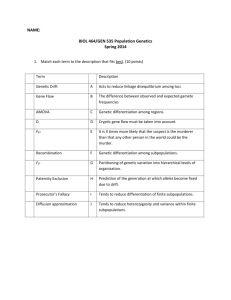
Document 11867132
advertisement

POTENTIAL IMPACT OF FOREST DISTURBANCE ON GENETIC DIVERSITY OF AMPHIBIAN POPULATIONS by RASHIDAH HALIMAH FARID A THESIS Submitted in partial fulfillment of the requirements for the degree of Masters of Science in the Department of Biological and Environmental Sciences in the School of Graduate Studies Alabama A&M University Normal, Alabama 35762 May 2014 Submitted by RASHIDAH FARID in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE specializing in PLANT AND SOIL SCIENCE. Accepted on behalf of the Faculty of the Graduate School by the Thesis Committee: _____________________________ _____________________________ _____________________________ Major Advisor _____________________________ _____________________________ Dean of the Graduate School _____________________________ Date ii Copyright by RASHIDAH HALIMAH FARID 2014 iii The thesis is dedicated to my sisters Samaiyah, A’ishah, Sabaah, and Janet and to my Grandmothers Rose and Effie who gave me breath of life when I had none. For without their strength and courage I am nothing. iv POTENTIAL IMPACT OF FOREST DISTURBANCE ON GENETIC DIVERSITY OF AMPHIBIAN POPULATIONS Farid, Rashidah, M.S., Alabama A&M University, 2014. 84 pp. Thesis Advisor: Dr. Khairy Soliman, Ph.D Populations of many amphibian species have declined because of habitat destruction, fragmentation, and alteration. In a forest community that has experienced dynamic changes in habitat structure and composition, it is expected that amphibian populations’ genetic variations could be affected due to declined success of breeding and survivorship, which might lead to the bottleneck effect over multiple generations. A study was initiated at Bankhead National Forest in Alabama to examine how past forest management practices have affected genetic structures of pool-breeding amphibian species. Molecular markers simple sequence repeats (SSR) were used to assess the level of heterozygosity among and within individual populations and species in the area of study. Five vernal pools of different size and disturbance history were selected and tissue samples were collected from two targeted species: Ambystoma maculatum and Notophthalmus viridescens viridescens. Heterozygosity of Ambystoma maculatum populations ranges from 35-55%, with inbreed coefficient not exceeding 55%. However, homozygosity was highly prevalent in all Notophthalmus viridescens viridescens populations. All three populations of Eastern Red Newt exhibited bottleneck events; allele frequency (0-0.22) was lowest in the first distribution class. Two populations of Spotted Salamander v exhibited bottleneck events; allele frequencies were 0 and 0.21. The results provided a glimpse into the landscape’s genetic connectivity and created a baseline for future genetic monitoring studies of these species. KEY WORDS: salamanders, gene drift, habitat fragmentation, bottleneck vi TABLE OF CONTENTS LIST OF TABLES ............................................................................................................. ix LIST OF FIGURES ........................................................................................................... xi LIST OF ABBREVIATIONS ........................................................................................... xii BNK - William Bankhead National Forest ....................................................................... xii ACKNOWLEDGMENTS ............................................................................................... xiii CHAPTER 1 ....................................................................................................................... 1 INTRODUCTION .............................................................................................................. 1 1.2 Statement of the Problem ................................................................................... 3 1.3 Objectives of the Study ...................................................................................... 4 CHAPTER 2 ....................................................................................................................... 5 LITERATURE REVIEW ................................................................................................... 5 2.1 Amphibian Decline ............................................................................................ 5 2.2 Bottleneck Theory.............................................................................................. 6 2.3 Mutation-Drift Equilibrium ............................................................................... 6 2.4 Application of Microsatellites............................................................................ 7 2.5 Impacts of Timber Harvest ................................................................................ 8 2.6 Spotted Salamander (Ambystoma maculatum) Ecology .................................... 9 2.7 Red-Spotted Newt (Notophthalmus viridescens viridescens) Ecology ........... 10 CHAPTER 3 ..................................................................................................................... 12 MATERIALS AND METHODOLOGY .......................................................................... 12 3.1. Sampling ......................................................................................................... 12 3.2 Genomic Deoxyribonucleic Acid Purification................................................. 15 3.3 Random Amplified Polymorphic DNA: SSRs Identification & PCR ............ 17 3.4. Bacteria Transformation and Cloning ............................................................. 19 3.5. Sequencing of Plasmid DNA .......................................................................... 26 3.6. Population Genetic Analysis with Microsatellites .......................................... 29 CHAPTER 4 ..................................................................................................................... 34 RESULTS ......................................................................................................................... 34 4.1. Genomic Deoxyribonucleic Acid Quantification ........................................... 34 4.2. Random Amplified Polymorphic DNA .......................................................... 38 vii 4.3. Bacteria Transformation and Cloning ............................................................. 42 4.3.2. Plasmid DNA Extracted from Positive Clones ............................................ 44 4.4. Sequenced Plasmid DNA ................................................................................ 45 4.5. Fragment Analysis .......................................................................................... 46 4.6. Bottleneck Analysis ........................................................................................ 52 CHAPTER 5 ..................................................................................................................... 56 DISCUSSION ................................................................................................................... 56 REFERENCES ................................................................................................................. 64 VITA ..................................................................................................................................... viii LIST OF TABLES Table 1. SSR Spotted Salamander Primer Set. ................................................................. 31 Table 2. SSR Eastern Red Newt Primer Set. .................................................................... 31 Table 3. One hundred twenty-two DNA samples with 260/280 ratios between 1.7 and 1.8...................................................................................................................................... 34 Table 4. Eastern Red Newt DNA samples used for RAPD analysis are highlighted. ...... 39 Table 5. : Spotted Salamander DNA samples used for RAPD analysis are highlighted. . 39 Table 6. Random Amplified Polymorphic DNA Kit, US Biological .............................. 40 Table 7. Amplified Spotted Salamanders Polymorphic Regions...................................... 42 Table 8. Amplified Eastern Red Newt Polymorphic Regions .......................................... 42 Table 9. Selected Clones for Sequencing from Bacteria PCR. ......................................... 45 Table 10. Eastern Red Newt heterozygosity and inbreeding coefficient per locus within BaPo 2 population. ............................................................................................................ 46 Table 11. Eastern Red Newt heterozygosity and inbreeding coefficient per locus within BaPo 3 population. ............................................................................................................ 46 Table 12. Eastern Red Newt heterozygosity and inbreeding coefficient per locus within BaPo 8 population. ............................................................................................................ 47 Table 13. Observed Heterozygosity of Eastern Red Newt Populations by Locus. .......... 47 Table 14. ANOVA of Loci Heterozygosity (Eastern Red Newt) Across and Within Populations........................................................................................................................ 48 Table 15. Spotted Salamander heterozygosity and inbreeding coefficient per locus within BaPo 6 population. ............................................................................................................ 48 Table 16. Spotted Salamander heterozygosity and inbreeding coefficient per locus within BaPo 4 population. ............................................................................................................ 49 Table 17. Spotted Salamander heterozygosity and inbreeding coefficient per locus within BaPo 3 population. ............................................................................................................ 49 ix Table 18. Spotted Salamander heterozygosity and inbreeding coefficient per locus within BaPo 2 population. 50 Table 19. Observed Heterozygosity of Spotted Salamander Populations by Locus......... 51 Table 20. ANOVA of Loci Heterozygosity (Spotted Salamander) Across and Within Populations........................................................................................................................ 51 Table 21. Wilcoxon Test for Heterozygosity for Eastern Red Newt Populations. ........... 53 Table 23. Wilcoxon Test for Heterozygosity for Spotted Salamander Populations. ........ 54 x LIST OF FIGURES Figure 1. Map of pCR 4-TOPO. The sequence and restrictions sites are labeled to indicate actual cleavage sites (Life Technologies, Inc.) ................................................................. 21 Figure 2. Flow chart of experimental steps of cloning (Life Technologies, USA). ......... 24 Figure 3. Life Technology visual depiction of the incorporation of terminating fluorescent nucleotides. ....................................................................................................................... 26 Figure 4. Chromatogram of successfully sequencing of short PCR products using BigDye Terminator chemistry; sample run of ABI 3100 Genetic Analyzer using POP-6 Polymer (Life Technologies USA). ................................................................................................. 27 Figure 5. Gel electrophoresis of seventy one genomic DNA samples on a ethidium bromide stained 1% agarose 1 x TBE gel; 80 voltages for 2.5 hours. Samples represented are from study species; in numerical order. ...................................................................... 38 Figure 6. Transformed Bacteria Plates.............................................................................. 43 Figure 7. Allele Frequency Distribution for Eastern Red Newt Populations. .................. 53 Figure 8. Allele Frequency Distribution for Spotted Salamander Populations. ............... 55 Figure 9. BaPo 8 Stand First Year. ................................................................................... 57 Figure 10. Location of Sites BaPo 2 and BaPo 6. ............................................................. 59 Figure 11. Bankhead National Forest Study Sites Locations. .......................................... 60 xi LIST OF ABBREVIATIONS BNK - William Bankhead National Forest DNA - Deoxyribonucleic Acid dNTP - 2’-deoxyribonucleotide triphosphate NSF - National Science Foundation PCR - Polymerase Chain Reaction RNA - Ribonucleic Acid SSR - Simple Sequence Repeats USDA - United States Department of Agriculture CDC - Charge-capture Digital Camera HWE - Hardy-Weinberg Expectations S.O.C. Medium - Super Optimal with Catabolism repression TAE Buffer - Tris-Acetate-EDTA Buffer TBE Buffer - Tris-Borate-EDTA Buffer EDTA Buffer - Ethylenediaminetetraacetic Acid TE Buffer - Tris-EDTA Buffer xii ACKNOWLEDGMENTS I would like to thank my advisors, Dr. Khairy Soliman and Dr. Yong Wang, for their guidance. I am sincerely grateful to my advisory committee members, Dr. William Stone and Dr. Luben Dimov, for their expertise and time. My research would not have been possible without the financial support of the CREST Program and the National Science Foundation (NSF) and tissue collection permission from the US Forest Service and the Alabama Department of Conservation and Natural Resources. I am thankful to my colleagues for their moral support, vast knowledge, and expertise in the molecular field: Dr. Govind Sharma, Dr. Ramesh Kantety (late), Safira Sutton, Abreeotta Williams, Fetun Desta, Angelica Durrah, Dr. Venkateswara Sripathi, Dr. Seloame Nyaku, Timley Watkins, and Sarah Cseke. Thank you Mrs. M. Saintjones for all of your help. For their time and dedication, I would also like to thank my students: Rogericus Denish, Meseret Sima, and Calvin Means. Finally, I would like to thank the support staff of the Agricultural Research Center and CREST Program: Penny Stone, Mila Sangalang, and Lisa Gardner. xiii CHAPTER 1 INTRODUCTION Although many amphibian adults are faithful to their breeding pools across breeding seasons, they are increasingly being forced to move to more suitable pools for breeding to increase their probability of breeding success (Gibbs, 1998; Chan-Mcleod, 2003). The combination of fewer breeding adults and reduced pool suitability could result in fewer breeding events and egg masses at these breeding pools. These demographic changes may lead to increasingly smaller population sizes of some pool breeding amphibians in the areas being impacted by forest management practices (Renken et al., 2004; Patrick et al., 2006). With smaller populations or more isolation, gene frequencies can drift dramatically by chance effect. A reduction in population that is maintained at a smaller size for several generations will have different genetic characteristics than it had prior to the reduction in size. This reduction will change the populations genetically owing to the loss of connectivity between subpopulations and genetic drift and increases in the possibilities of inbreeding (Andersen et al., 2004). Genetic drift and inbreeding could result in predicted low levels of genetic variation as detected by significant deviations from Hardy-Weinberg expectations (HWEs) (Frankham et al., 2002). 1 Reductions in population size can also be changes in forest composition. Tree composition of the canopy directly determines the type and abundance of organic matter and therefore indirectly regulates microbial community and subsequently the macro invertebrate community. Dietary requirements vary considerably between species and life stages. For those species dependent of macroinvertebrates, species presence is directly correlated to sediment load, chemical concentrations, and humus. Therefore in a forest community that has experienced dynamic changes in tree composition, amphibian populations’ genetic variations would reflect periods of resources limitation or abundance throughout multiple generations. An understanding of genetic variation in amphibian populations and how these variations limit species abundance could have significant impacts on the way forest and mitigate wetlands are managed. For this study, three species were initially sampled to assess population effective size number and species comparison feasibility to include: the Spotted Salamander (Ambystoma maculatum), Red-Spotted Newt (Notophthalmus viridescens viridescens), and Southern Leopard Frog (Lithobates sphenocephalus utricularius). Additionally, tissue from the following species was included when time permitted: Marble Salamander (Ambystoma opacum), Green Frog (Lithobates clamitans melanota), and Eastern Spadefoot (Scaphiopus holbrookii). To study amphibian genetic variation in response to changes in forest management practices, The William Bankhead National Forest (BNF) was chosen because of its management history. Originally a mixed hardwood forest, the BNF was cut and converted into agricultural fields and pastures prior to 1914. As small agricultural use gradually declined (USDAFSBNF 2003), the USDA Forest Service (FS) purchased the land and reforested it 2 with loblolly pine, a fast-growing species that is excellent for erosion control and quality timber. With the older pine stands dilapidated, due to severe inter-specific competition and southern pine beetle infestations, sites quickly began regenerating with native hardwood species such as oaks, red maple, black gum, and yellow poplar. Presently, a thriving amphibian population is well established within its forest boundaries (USDAFS BNF 2003). The management practices and changes in forest community provide a unique opportunity to study forest restoration effects (Wang et al., 2010) on long term amphibian population vitality. 1.2 Statement of the Problem The effect of forest disturbances specifically prescribed as thinning and burning, on animal communities with a focus on herpetofauna was conducted. In recent years, several research studies examined the effect of these treatments and their interactions on species richness and abundance and how the mechanisms (microclimate, vegetation structure, food availability, density of competitors, vernal pool hydrology, and metapopulations dynamics) are responsible for the changes in population demographics. In this study, the main focus was to examine such effects by experimentally determining the specific habitat features, landscape level changes, and metapopulation genetic dynamics and structures (Wang et al., 2010). This research will address the specific research hypotheses that silvicultural practices have had an evident effect on the genetic diversity of pool breeding amphibians. 3 1.3 Objectives of the Study The overall objectives of this research project are to (1) evaluate if genetic bottleneck events are present and (2) determine the current level of genetic variation per species. The specific objectives of this study were to: (1) calculate species effective population size per species, (2) determine expected allele/ heterozygosity ratios, (3) analyze data for environmental correlations between species abundance and environmental factors, (4) conduct DNA extraction and amplification, and (5) per species, evaluate presence of bottle neck in each population. 4 CHAPTER 2 LITERATURE REVIEW 2.1 Amphibian Decline Loss and degradation of habitat are considered the foremost causes of amphibian populations decline worldwide (Collins and Storfer, 2003). Amphibians have relatively low dispersal abilities and are often philopatric, leading to distinct populations that can represent unique genetic entities despite geographic proximity (Kimberling et al., 1996; Waldmann & Tocher, 1997; Driscoll, 1999; Scribner et al., 2001). With life cycles based both in water and on land, amphibians are dramatically affected by changes in canopy cover and demographics, hydrology, climate change and tonicity levels. Amphibians are well suited to address questions at the level of metapopulations (Hanski, 1997), and have in recent years become a focus for studies on the effect of landscapes and landscape alterations to wildlife (Halley et al., 1996; Vos et al., 2001). The restoration of habitat has shown to have a direct effect on the recovery of a species population numbers, as the correlation between environmental variation and fitness components are widely accepted (Bruce and Stiven, 1988). It has been debated that the success of the population is dependent on the extent and prolonged degradation of the habitat as well as the associated recovery time laps following the habitat degradation. It is difficult to distinguish real 5 long-term downward trends from natural population oscillations (Beebee and Rowe, 2001). In many cases, statistical analysis of demographic information is often the only way of determining population declines (Reed and Blaustein, 1995). 2.2 Bottleneck Theory Bottlenecks, long standing reductions in effective population size, were effectively determined by measuring genotype frequencies at multiple polymorphic loci at a single point in time (Beebee and Rowe, 2001). Cornuet and Luikart (1996) developed an approach to identify recent population bottlenecks by genetic analysis. The test requires the determination of genotype frequencies at multiple polymorphic loci at a single point in time, based on the expectation that a bottlenecked population will demonstrate an excess of heterozygosity over the expected, under mutation-drift equilibrium. The test also assumes that such loci evolved according to the Infinite Allele Model (IAM) and Stepwise Mutation Model (SMM); and the relationship between excess heterozygosity and observed number of alleles is a function of time elapsed since the beginning of the bottleneck (Cornuet and Luikart, 1996). Testing for bottleneck events has become increasingly more accessible by the application of routine sequencing and Polymerase Chain Reactions; particularly, with the application of DNA-based markers such as microsatellite loci (Jehle and Arntzen, 2002). 2.3 Mutation-Drift Equilibrium Bottleneck calculations assume mutation-drift equilibrium to compute the distribution of gene diversity expected from the observed number of alleles. 6 Microsatellite genetic diversity can be used to calculate estimates of evolutionarily effective population size (Ne) given particular assumptions about mutation patterns and rates. The expected average heterozygosity is calculated through simulations of Infinite Allele and Stepwise Mutations Models (Knaepkens et al., 2004). Under Infinite Allele Model (IAM) each mutation that arises is unique. Mismatch at a given allele is observed as an occurrence of a single mutation. At equilibrium, the heterozygosity under the infinite allele model (IAM) is expressed as: , which leads to (Crow and Kimura, 1970), which µ is the mutation rate (Knaepkens et al., 2004). In Stepwise Mutation Model (SMM), actual mutations are observed and scored on a frequency spectrum at a given loci; no mismatch (0), (1) one mismatch, (2) two mismatch, etc. The simplest form of SMM is a one-step symmetric model where it is assumes that each mutation is a single step with equal probability of increasing (+1) or decreasing (-1) (Walsh, 2001). Under SMM at equilibrium, the heterozygosity is equal to: ), which yields: (Ohta and Kimura, 1973). Populations without a recent change in size will be in mutation-drift equilibrium where the expected heterozygosity (HEQ) will equal the Hardy–Weinberg equilibrium heterozygosity (HE) (Knaepkens et al., 2004). 2.4 Application of Microsatellites Microsatellites (SSRs) consist of tandem repetitive units of DNA, typically less than five base pairs in length. The number of alleles segregating over a panel of microsatellite loci enables the genetic recognition of individuals without physical marking (Luikart, 1999). On average, microsatellites exhibit mutation rates between 10-3 7 and 10-5 (Goldstein and Schlötterer, 1999) and mostly mutate through the addition or deletion of one repeat unit, following a stepwise mutation model or alternatively, by a certain number of repeats simultaneously (Goldstein and Schlötterer, 1999). Beebee and Rowe (2001) found genetic analysis using microsatellite loci was effective in distinguishing between short-term fluctuation and long-term trend in accurately assessing amphibian declines. Seven microsatellite loci were used in examine the relationship between the current six of bullhead fish and genetic diversity in the 2004 study by Knaepkens. Though the loci number was below the recommended 10-15 (Cornuet and Luikart, 1996), Knaepkens et al. (2004) were able to observe a positive correlation between genetic variability and the size of the population implying that concerns about loss of genetic diversity in small populations are indeed warranted. 2.5 Impacts of Timber Harvest Prior to the 1960s, selective timber harvest, leaving a few remaining large trees was the preferred method of cutting. Since the 1960’s, clear cutting has almost completely replaced selective cutting as the preferred method of timber harvesting by the U.S. Forest Service in the southern Appalachians (Petranka et al., 1993). However since FEMAT (1993) percent shelter wood, or partly removal of canopy, harvest has dominated as the preferred method on USFS lands. Amphibians’ skin must be kept moist to facilitate gas exchange. Consequently, adults generally are restrict their activity to moist forestfloor microhabitats in the day and are active on open surface ground only at night when humidity is high, to reduce the chance of dehydration. Although limited studies have been conducted on clear cutting effects on amphibians, studies collectivity suggest that 8 timber harvesting is detrimental to amphibians, especially salamanders, in eastern forests (Bury and Corn, 1988; Raphael, 1988; Welsh, 1990). Clear cutting degrades forest-floor microhabitats for salamanders by eliminating shading, reducing leaf litter, increasing soil surface temperature and reducing soil-surface moisture (Bury, 1983; Ash, 1988; Raphael, 1988; Welsch, 1990). Petranka et al. (1993) concluded that most animals died, estimated loss of 75-80%, from physiological stress following the removal of trees from sites. Increased sedimentation and general deterioration of stream quality may also have contributed to the decline of species with aquatic larval stages (Bury and Corn, 1988). Despite their ecological importance, amphibians (especially salamanders) are often neglected in forest management studies (Petranka et al., 1993). 2.6 Spotted Salamander (Ambystoma maculatum) Ecology Spotted salamanders are large, metamorphs 50 mm and adults 228 mm, dark body salamanders with yellow and/or orange spots on the tail, body, and head. However, spots do not appear until several months after metamorphosis. Occurring throughout the southeast and along the east coast into Canada, spotted salamanders prefer primarily hardwood and mixed deciduous forests. Breeding is restricted to vernal or ephemeral wetlands with mature upland terrestrial habitats (Mitchell and Gibbons, 2010). Adults breed in the winter and early spring; often following rainy or foggy nights. Breeding in Alabama has been documented in late December and early February (Mount, 1975). Males normally arrive earlier than females and are 1.5 times more numerous (Flageole and Leclair, 1992). Adults are fateful to specific breeding ranges, often by pass other closer ponds and navigated to their original home range (Downs, 1989). 9 Olfaction has been suggested to guide migration routes and facilitate entry and exit near the same point yearly (Petranka, 1998). During mating, males deposits spermatophores on substrate near a courted female. Females absorbs multiple, 15-20, spermatophores with her cloacal lips before the close of mating season (Petranka, 1998). Females can deposit between one-four clutches of eggs, each up to 250 eggs, within a single season. However, embryonic mortality can be as high as 75%, depending on depth of water, time of season, and temperature at time of deposit. Clutch incubation period normal lasts between 4-7 weeks. After hatching, larvae feed on a variety of zooplankton and macroinvertebrates. Transformation into metamorphs occurs within 2-4 weeks. Metamorphs disperse into nearby forest within weeks of their transformation and will not return to breeding pond until sexually mature 2-5 years. Adults feed on forest floor invertebrates and live an average of 6-8 years and a maximum of approximately 20 years (Petranka, 1998). 2.7 Red-Spotted Newt (Notophthalmus viridescens viridescens) Ecology N. virdescens, with exception populations, has four complex and distinct life history stages: the egg, aquatic larva, terrestrial red eft (juvenile stage), and aquatic adult. Two stages, red eft and aquatic adult are distinctively identifiable. The juvenile stage, red eft, migrates to land and remains for several years, approximately seven, until sexual maturity. The in the red eft stage, individuals are gill less with lungs; the larvae losses gill function prior to existing the aquatic environment as an eft. Red efts bright red coloration is visible within two weeks after metamorphosis (Chadwick, 1950). A second transformation occurs at maturity were terrestrial red efts metamorph into a gill-less, aquatic adult and remain aquatic for extent of their lives. The morphological changes 10 include a dorsal tail fin and development of non granular skin. Aquatic adults measure approximately 90- 165 mm of total length. Eastern Newts (N. virdescens) are the second widest distributed salamander in North American; their range extending as west as Texas, south to Florida and north into southern Canada (Petranka, 1998). 11 CHAPTER 3 MATERIALS AND METHODOLOGY 3.1. Sampling Sampling was carried out in multiple steps due to the nature of amphibian breeding and migratory patterns. Amphibian populations are highest during the breeding seasons, and can vary depending on specific species. Breeding season may begin in early spring and extend throughout the fall. Sampling was conducted monthly during the breeding months of each species and sampled randomly during periods of high rainfall. Sampling occurred in the fall and spring seasons or when populations were most abundant; throughout the study period of approximately two years. Minnow net/traps were used for surveying tadpoles, hatchlings and larva. A minimum quantity of tissue was collected, to minimize stress and reduce morality, from each of the 5 research breeding pools; BaPo 2, BaPo 3, BaPo 4, BaPo 6, and BaPo 8. BaPos 2, 3, 4, 6 and 8 were sampled at higher priority level due to their uniformity of two are more species presences: Spotted Salamander (Ambystoma maculatum), Red-Spotted Newt (Notophthalmus viridescens viridescens), and/or Southern Leopard Frog (Lithobates sphenocephalus). Tissue samples from the tail and/or toe were taken of no greater than 10% of total body mass of any specimen to minimize stress. Toe clippings were taken 12 from adults. Tail clippings were collected from metamorphs with a total body mass equal or greater than 2 grams. For metamorphs and larva less than 2 grams or eggs, the whole specimen was sacrificed; not exceeding more than ten individuals or 1% of a given species per site within a sampling season. Whole specimens were euthanized in a benzocaine hydrochlorid bath (CCAC, 2006). Samples were kept in separate, labeled test tubes and stored with dry ice in the field. Samples were stored long term at -80oC. 3.1.2 Sample Size Recent BNF amphibian population estimates are currently undetermined. However, a sample size between 20-30 individuals per species/site is considered adequate in assessing several estimates of genetic diversity (number of alleles per locus, average observed heterozygosity, and average expected heterozygosity) for both within and between, species specific, pool populations (Pruett and Winker, 2007). Therefore, ponds of high estimated population densities, a minimum of 20 samples were collected for analysis. Additionally, more tissue samples were collected, to account for potential loss due to degradation during handling. Sampling varied with species and site resulting in N values form 5-20. Estimates of alleles per locus at small sample sizes can be greatly biased, especially when compared to populations from which a larger sample size is obtained (Pruett & Winker, 2007; Petit et al., 1998). Therefore to account for allele diversity, we used the rarefaction method (smallest N value 5) of to normalize standard error across sample sizes. Rarefaction is commonly used to compare allelic diversity across unequal sample sizes (Petit et al., 1998, Leberg, 2002). 13 3.1.3 Live Capture Precautions Minnow nets were positioned to ensure the air-water interface is maintained to avoid drowning or asphyxiation. Due to amphibian sensitivity to temperature and dehydration, care was taken to handle specimens quickly. Contact was limited by examining individuals on plexiglass, to avoid the transfer of body heat. Handling time was minimized in compliance with CCAC protocol. Trap deaths from exposure to unfavorable temperatures, drowning, shock, predation and desiccation was minimized by checking traps daily and not sampling during unfavorable weather conditions (CCAC, 2006). Sampled individuals were released at individual prospective capture sites. 3.1.4 Protocol for Pathogens (Chytrid Fungus) Due to the risk the potential persistence of amphibian pathogens in aquatic environments and the risk of transporting them, the Southeast Partner’s in Amphibian and Reptile Conservation protocol for disinfecting equipment was followed. This protocol is sufficient in eliminating the transport of chytrid fungus between wetlands or aquatic sites. Once sampling at an aquatic site was completed and before moving to a new site or returning from the field, all field equipment (e.g., nets, buckets, and water quality meters) and personal gear (e.g., boots and waders) were rinsed with water, and all debris and mud removed. If the tires of a vehicle contacted water with amphibians, they will be cleaned. A 10% bleach disinfectant solution or equivalent was used to kill pathogens. The disinfectant remained in contact with equipment or personal gear for at least 2-5 minutes to ensure complete inactivation pathogens. Equipment and footwear were rinsed with municipal water after the minimum disinfecting time to remove residual chemical, which 14 can be toxic to aquatic life. Disinfectant solutions were discarded and replaced after 5 days (Miller and Gray, 2009). 3.2 Genomic Deoxyribonucleic Acid Purification 3.2.1 Genomic DNA Extraction Deoxyribonucleic acid (DNA) was purified from a 5-20 mg sample of frozen toe, tail, skin, or other tissue using Qiagen, Gentra Puregene Tissue Kit. Tissue was prepared using liquid nitrogen and either mortar and pestle or the TissueLyser and 8 mm steal beads. The TissueLyser and steal beads were used for skin and tail samples. However it was not efficient in homogenizing hard tissues from toe cuttings; therefore, mortar and pestle was used. Following homogenization, chemical treatment was the same. Generally, cells were lysed with an anionic detergent in the presence of a DNA stabilizer. Tissue homogenates were digested at 55°C with proteinase K (0.2mg/mL) in the presence 0.5– 1% SDS before extraction with phenol, phenol–chloroform (1:1 v/v), and chloroform – isoamyl alcohol (25:1 v/v). Ribonucleic acid (RNA) was removed by treatment with RNase at 37°C; followed by salt precipitation of proteins and contaminants. Finally, purified DNA was recovered by supernatant with isopropanol; then washed the 70% ethanol and dissolved in hydration solution (1 mM EDTA, 10 mM Tris-Cl pH 7.5) for 1 hour at 65°C. Genomic DNA was stored at -20ºC long term use. 3.2.2 Spectrophotometric Analysis A spectrophotometer (NanoDrop 1000, Thermo Fisher) was used to determine the concentration of nucleic acid (ng/µl) and purity of the each purified sample. The 15 spectrophotometer is design to distinguish between protein, RNA and DNA by the molecular weight at a given absorbency range (at wavelength of 260/280 nm). The absorbance analysis is conducted at a baseline established from the sample suspension buffer. A liquid column (1 mm) is formed from the sample between a upper and lower optical base. Light is pass through the sample, quantifying a molecule concentration within a know path length (l mm). A second measurement is taken at an adjust height of 0.2 mm to provide a reliable concentration range and estimate of sample purity (NanoDrop Technologies, USA). 3.2.3 NanoDrop Procedure The instrument was calibrated and initialized using 2 µl of RNase and DNase free water; following by blanking with 2 µl DNA hydration solution (1 mM EDTA, 10 mM Tris-Cl pH 7.5). DNA samples were loaded (2 µl) individual on the optical base, labeled and measured. Each sample was assigned a unique name in which the first letter and number represent site name, middle letters indicate species, and latter numbers indicated sequential sample extracted per site/species. 3.2.2 Gel Electrophoresis Gel electrophoresis was used to visualize the physical quality and condition of the nucleic acid. The electrophoresis apparatus uses a DNA stabilizing and conductive buffer to create an electrical current. Moving from negative and positive electrodes, electricity passes through a porous gel of polyacrimanide or agarose at a set voltage and prescribed time. Ethidium bromine was used to stain the DNA for florescence. Because, DNA is 16 negative, larger fragments move relatively short distances versus small fragments, when compared to a commercially produced DNA ladder of predetermined fragment sizes. Therefore, sample fragment length, containments presences and shearing are visualized under UV light. 3.3 Random Amplified Polymorphic DNA: SSRs Identification & PCR Random Amplified Polymorphic DNA (RAPD) is fragments of high GC content (Allison, 2007) between 50-65% designed to amplify genomic region of high polymorphism. SSRs are found within regions of high polymorphic variation. A preliminary screening was performed with DNA from several individuals from the studied populations to allow for the identification of RAPD primers that yielded distinct, well-separated and reproducible bands. These bands were subsequently chosen for final analyses. Band repeatability for each primer was confirmed by duplicate PCRs with DNA from a subset of each population sampled. Forty RAPDs were used to target regions that may contain SSRs. Repeating PCR product size was desired from multiple samples before presiding to cloning and sequencing. 3.3.1 Introduction: Polymerase Chain Reaction Polymerase Chain Reaction (PCR) (Allison, 2007) is a technique to directly amplify target regions of a genome. A primer anneals and flanks the desired region followed by repeated polymerase enzymatic cycles. These repetitive cycles produce (amplification) multiply copies of the desired gene. The PCR product of desire gene can 17 be concentrated and quantified using gel electrophoresis for downstream application such as cloning. 3.3.2 RAPDs: PCR Protocol PCR was carried out conducted using a MJ Research PTC 200 or a Bio-Rad Tetrad 2 peltier thermal cycle. Each 12.5µl reaction consisted of 100-200 ng of DNA, 6.5 µl of Qiagen Taq PCR Master Mix (2.5 units of Taq DNA Polymerase, 1 X Qiagen PCR buffer, 200 µM of each dNTP, 1.5 µM of mM MgCl2), 2 µl of each primer (0.2 µM) and 1-3 µl of DNase free water. The following PCR program was conducted: initialized at 95°C for 2-5 minute, 30-35 cycles of denatured for 30 seconds at 95°C, annealing at either 39.5°C or 43.6°C, extension at 72°C for 1 minute and final extension for 10 minutes at 72°C and storage at 4°C. The follow samples were tested. 3.3.3 RAPDs: Electrophoresis and Band Extraction Identified products were subjected to electrophoresis on a 2% agarose, 1x TAE (Tris-Acetate Electrophoresis) buffer gel for 90 minutes at 80 volts. DNA bands were stained with ethidium bromide and visualized under UV light. One-hundred base-pair marker was use to determine base size. Desired products were cut from the gel using a dark light eliminator and DNA was extracted use a PureLink Quick DNA Gel Extraction Kit (Life Technologies, USA). The gel bands were dissolved in solvent release DNA fragments. The solution was added to a silica spin column. DNA was adhered to the spin column, washed to remove containments, and eluted in a TE buffer. Selected products were cloned via chemically competent E.coli transformation. 18 DNA Gel Extraction Procedure Gel bands were excised from gel using a scapula, minimizing excess agarose. Band was when weighed to ensure a 3:1 ratio of Gel Solubilization Buffer to DNA band in a 1.6 ml polypropylene microcentrifuge tube. Gel was incubated at 50°C for 10 minutes and remained in a dry block for additional 5 minutes. After dissolving, content was transferred to extraction column/tube apparatus and centrifuged for 1 minute at >12000 x g. Filter column was washed with 500 µl of wash buffer, containing ethanol, and centrifuged again. Residual wash buffer was removed by centrifuging at >1200 x g for an addition 1-2 minutes. DNA was eluted through incubation at room temperature for 1 min with 50 µl of Elution Buffer; followed by recovery centrifugation at > 12000 x g for 1 min. Expected DNA recovery was between 85% - 95% of original concentration. 3.4. Bacteria Transformation and Cloning 3.4.1 Cloning Introduction The TOPO TA Cloning Kit (Life Technologies, USA) was used for direct insert of PCR products into pCR 4-TOPO plasmid vector. Plasmid used has a single 3’ deoxthymidine (T) residues allowing for efficient bind to the 5’ poly- A tails added with use of Taq polymerase. TOPO cloning kit exploits the enzymatic reaction of topoisomerase I from Vaccinia virus. The DNA phosphodiester backbone is cleaved after 5’ –CCCTT by topoisomerase I in one strand; energy release is conserved through the formation of covalent bond between 3’ phosphate and tyrosyl residual of Topisomerase I. Five prime hydroxyl of the original stand ultimately attaches to the phopho-tyrosyl bond reversing the reaction and releasing the topoisomerase (Life Technologies, USA). Vector 19 pCR 4-TOPO also contains the lethal ccdB gene allowing for direct positive screening. The ccdB gene fuses directly to the c-terminus of the LacZα fragment. Only the ligation of a PCR fragment disrupts the expression of the lacZα -ccdB gene permitting only positive recombinants to be transformed into chemically competent E. coli cells (Life Technologies, USA). Figure 1 depicts plasmid structure and recognition sites. 20 Figure 1. Map of pCR 4-TOPO. The sequence and restrictions sites are labeled to indicate actual cleavage sites (Life Technologies, Inc.) ________________________________________________________________________ __________________________________________________________________ 21 3.4.2 LB + Ampicillin Media Plates Nutrient rich LB+ ampicillin plates were prepared before 2 days before transformation to allow for any contaminated plates to be eliminated. Standard LB broth was prepared with 10 grams of bactro- tryptone, 5 grams of bactro- yeast extract and 10 grams of NaCl. Contents were heat dissolved in 750 ml of ddH2O, pH adjusted to 7.5 with sufficient 1 M NaOH. Final solution volume was brought to 1 liter. Lastly, 15 grams of bacro- agar was added. LB broth was autoclaved at 121°C for sterilization for minimum of 35 minutes. After sterilization, broth was allowed to cool to 50°C before added 100 mg of ampicillin dissolved in 2 ml of ddH2O; stock ampicillin concentration of 50 mg/ml. Plates were poured immediately in a sterilized hood; after solidification, plates were sealed, bagged and stored at 4°C for 48 hours or until the night before use. Plates were heated at 37°C overnight before use to ensure (1) no contaminate growth occurs and (2) pre-warm the plates to encourage colony growth prior to spreading transformed E. coli. 3.4.3 TOPO Cloning Reaction Procedure Fresh PCR product (4 µl) was gently mix with 1 µl of salt solution (1.2 m NaCl; 0.06 M MgCl2) and 1 µl of TOPO vector; incubated for 5 minutes at room temperature. The reaction was placed on ice for 5-30 minutes until ready of transformation into competent cells. 22 3.4.4 Chemical Transformation Procedure In each vial of One Shot chemically competent E coli, 2 µl of cloning reaction was added, mix gently, and incubate for 5 – 30 minutes on ice. Cells were heat shocked for 30 seconds at 42°C using a dry heat block; then immediate transfer back onto ice. While remaining on ice, 250 µl of room temperature S.O.C. medium was added to each vial. Vials were shaken (200 rpm) horizontally at 37°C for one hour. Two volumes, 20 µl and 50 µl, of the each transformation were plated on the pre-warm ampicillin treated LB plates and allowed to incubate for 48 hours. Positive clones were selected for bacteria PCR analyzes. Figure 2 describes a complete layout of cloning procedure. 23 Figure 2. Flow chart of experimental steps of cloning (Life Technologies, USA). _______________________________________________________________________ 3.4.5. Analysis of Transformants by Bacteria PCR Positive single colonies were selected for bacteria PCR analyzer. Sterile toothpicks were gently dabbed into a colony and mixed into 0.2 µl tube with 6.25 µl of Taq master mix, 1 µl of appropriate RAPD primer, and 5.25 µl DNA grade water. The reactions were incubate at 94°C for 10 minutes to lysed the cells; followed by amplification for 20-30 cycles and final extension for 10 minutes at 72°C. PCR products were quantified using the Tape Station. Positive clones with the target gene were selected for plasmid DNA extraction and sequencing. 24 3.4.6. Plasmid DNA Extraction form E. coli Each selected clone was grew overnight in Innova 44 Incubator Shaker. Five milliliters of ampicillin treated of sterile LB broth was placed in a 50 mL conical tube along with the selected colony; colonies were incubated at 37°C and shaken at 200 rpm to encourage growth. Cells were harvested by centrifuging 1 mL of the overnight LB culture in a 1.6 µl micro-centrifugation tube. Gathered pellet was processed using the PureLink Quick Plasmid Miniprep Kit (Life Technologies, USA). Harvested pellet was resuspended with 250 µl of buffer R3 ( 50mM Tris- HCL, pH 8.0; 10 mM EDTA) with RNase A (20 mg/mL in R3 buffer); mixed by pipeting until homogeneous. Lysis buffer (250 µl; 200mMNaOH, 1% w/v SDS) was added add gently mix by inverting; incubated at room temperature for 5 minutes. Proteins were precipitated with 250 µl of Precipitation Buffer, added to each tube; mix vigorously by inversion and centrifuge at > 12,000 x g for 10 minutes. Supernatant was then loaded onto a spin column / wash tube apparatus and centrifuged at 12,000 x g for l minute. Plasmid DNA bound to the spin column was then washed with 700 µl of 70% ethanol washing buffer and centrifuge at 12,000 x g for 1 minute; washing was repeated and followed by a dry centrifugation of 12,000 x g for l minute to remove residual ethanol. Plasmid DNA was finally eluted from the spin column into a clean 1.6 mL tube with 50-75 µl of preheated TE buffer. TE buffer was incubated at the center of the spin column at room temperature for 1 minute and centrifuge at 12,000 x g for 2 minutes. Plasmid DNA recovered was stored at -20°C to 4°C until sequencing. 25 3.5. Sequencing of Plasmid DNA 3.5.1. Sequencing Platform and Chemistry Introduction Sequence was conducted on the ABI 3100 Genetic Analyzer. Samples were prepared with the Big Dye Terminator v3.1 Standard kit. Big Dye chemistry works by incorporating signature fluorescent (A, T, C, G) nucleotides into the amplification of the target gene’s PCR products. As a fluorescent nucleotide is incorporated, the extension of the primer stops; allowing for a single terminal nucleotide per fragment. This incorporated continues until the target gene sequence is represented by a multiple of single fluorescent nucleotide fragments. ________________________________________________________________________ ________________________________________________________________________ Figure 3. Life Technology visual depiction of the incorporation of terminating fluorescent nucleotides. Nucleotides fluoresce during capillary (16 capillary/ 50 cm) electrophoresis at a distinct wavelength. This flexible chemistry allows for reads up to 850 bps. The result is visualization of the DNA sequencing in a chromatogram. See (Figure 4). 26 _______________________________________________________________________ Figure 4. Chromatogram of successfully sequencing of short PCR products using BigDye Terminator chemistry; sample run of ABI 3100 Genetic Analyzer using POP-6 Polymer (Life Technologies USA). 3.5.2. Sequencing PCR Reaction Reaction volumes of 20 µl consisted of 8 µl of master mix, 3 ng of plasmid DNA, 3.2 pmol of M13 primer, and de-ionized water. Cycle sequencing occurred using a thermal cycler. After mixing, reactions were denatured at 95°C for 5 minutes; followed by 50-70 cycles of 95°C for 30 sec, annealing at 50-55 °C for 10 seconds, and extension for 4 minutes at 60°C. PCR products were held at 4°C until purification (Life Technologies USA). 3.5.3. PCR Purification The ethanol-EDTA purification method was used to precipitate PCR products within a 96 well reaction plate. EDTA (125 µl, 125mM) and 60 µl of 100% were added to the bottom of each well. The reaction plate was sealed with aluminum tape and mixed thoroughly by inverting approximately four times. After incubated at room temperature for 15 minutes, the plate was centrifuged at 3000 x g for 30 minutes. The reaction plate was immediately inverted and centrifuged at 185 x g for 1 minute to remove all buffer. 27 Reactions were washed in 60 µl of 70% ethanol and spun at 4°C and 1600 x g for 15 minutes. Plate was immediately inverted again and spun at 185 x g for 1 min. Purified reactions were re-suspended in 10 µl of formamide, cover with aluminum foil to protect from light and stored at 4°C (Life Technologies USA). 3.5.4. Sequencing Parameters The ABI 3100 Analyzer was standardized with BigDye Terminator v3.1 Matrix Standard kit. A multi-component matrix was required to normalize the data collection software to the four different colored fluorescent dyes labeling DNA fragments in a single capillary (Life Technologies USA). Matrix Standard (5 µl) was mixed with 195 µl formamide; 1:40 dilution. Reaction denature at 95 °C for 2 minutes. Reaction mixture was used to run a spectral calibration for the dye set Z; A-green, C-blue, G-yellow and Tred. A spatial analysis was also conducted for each capillary array. Spatial test checks for even light penetration and absorbency through each of the 16 capillaries (Life Technologies USA). The Run 3100 Data Collection v2.0 software allows for the labeling and sequence run parameters to be set using the Plate Manger application. Samples identification was paralleled with well position, dye set, sequence protocol, and data storage location. After designing plate parameters, reaction plate was linked to run manger until sequencing was completed. Data was formatted, viewed and analyzed using Sequencing Analysis 5.1.1 software. Sequences results were BLAST to determine whether or not they were from know origins or presence unknown and potentially new microsatellites. 28 3.6. Population Genetic Analysis with Microsatellites 3.6.1. Fragment Analysis Platform and Chemistry Introduction Fragment Analysis chemistry works by comparing the known signature standard dye marker (ROX) fragments peaks size to the amplified target gene’s PCR products. This comparison allows for the determination of fragment size and genetic variation of alleles. Target loci are distinguished by fluorescent dyes colors: 6-FAM is blue, HEX is green, and NED is yellow. Labels fluoresce during capillary (16 capillary/ 50 cm) electrophoresis, as they pass in front of a laser. The fluorescence is captured by the charge-capture digital camera (CDC). The result is visualized on an electropherogram. For each fragment a single peak represents the relative dye concentration, used as a label, against time of exposure. Resolution, Rs, of two peaks in an electropherogram is defined as: Rs = (Life Technologies, 2010). Fragment analysis was conducted on the ABI 3100 Genetic Analyzer. PCR products were prepared with the ROX 400 or 500 Dye Standard; 50 µl of Standard with 1000 µl of formamide. ROX Dye Standards contain florescent fragments of sizes ranging from 40- 400 or 500 bps. Unlike sequencing, fluorescent primers are used to tag desired loci. The diluted PCR product (1 µl) was then added to the dye standard; heated at 95°C for 5 minutes, then immediately returned to ice for at least 5 minutes. The dye standard serves as a genetic marker within each well, determining the base pair size for each fragment. Each analysis reaction contained 1 µl of dilution PCR product and 6-8 µl of dye standard solution. 29 3.6.2. Fluorescent Primers and PCR A total of ten microsatellite primers were using for Spotted Salamanders (Ambystoma maculatum) isolated by Wieczorek et al. (2002). The seven polymorphic microsatellites isolated by Croshaw and Glenn 2003 for Red Newts (Notophthalmus viridescens) were also used. Primers were synthesized by Operon, Inc. with either a HEX or 6-FAM labeled forward primer 5’ end. Sample size ranged from five to eight depending on pool site and species tissue availability. Each reaction contained 20-100 ng of DNA, 6.25 µl of Taq Qiagen Master Mix, 2 µl of 100 mM primer and DNA grade water. Thermal cycles were conducted according to the authors’ recommendations using Bio-Rad Tetrad 2 Cycler. Wieczorek et al. (2002) loci were amplified under the following conditions: 5 minutes of denaturation at 95°C; 35 cycles of 30 seconds at 95°C, 30 seconds at specific annealing temperature, 1 min at 72°C; and final extension for 10 minutes at 72°C (Table 1). Croshaw & Glenn (2003) loci for red newts were amplified under a touchdown program with two annealing temperature groups (either 60-50°C or 55-45°C); additional modifications of a denaturation and final extension period were added, program as follows: 3 minutes of denaturation at 95°C; 5 cycles of 96°C for 20 seconds, 30 seconds at the highest annealing temperature (either 60° or 55°C), and 72 °C for 1 minute; 21 cycles of 96 °C for 30 seconds, highest annealing temperature (either 60° or 55°C), minus 0.5 °C each cycle for 30 seconds, and 72 °C for 1 minute; lastly 10 cycles of 96°C for 30 seconds, the lower annealing temperature (either 50° or 45°C), for 30 seconds, and 72°C for 1 minutes; and final extension of 72°C for 3 minutes. PCR product purification is not required for fragment analysis. However, products must be diluted in DNA grade water to minimize background under analysis. PCR products were 30 diluted to a 1:20 ratio; 1 µl of product mix with 20 µl of DNA grade water for each reaction. Table 1. SSR Spotted Salamander Primer Set. Wieczorek et al. (2002). Table 2. SSR Eastern Red Newt Primer Set. Primer Primer Sequence 5' - 3' Dye GenBank Number Touchdown Temp. Nvi2F AGC CAC TTG TAA GAA TTG T CCA TCA CAC ACG TTA TTT TGC CTT GCT GTG ATT C GGA CAT TCA AGC TCA CAT ACT GGG AGA GAG GAA TAG AC ATG GTA TTG TGA TTA CTC TAT HEX AY29145 2 60 Nvi2R Nvi7 F Nvi7 R Nvi11 F Nvi11 R 6-FAM HEX AY29145 4 AY29145 5 31 60 60 Size of Range (bps) 176194 127157 178214 No. of Alleles HO HE 6 0.38 0.33 0.88 0.77 0.5 0.62 0.63 0.75 0.88 0.85 0.75 0.81 8 8 (Continued). Table 2. SSR Eastern Red Newt Primer Set. Primer Primer Sequence 5' - 3' Nvi14 F Nvi14 R Nvi18 F Nvi18 R Nvi19 F Nvi19 R Nvi24 F Nvi24 R AAG GTC ATC TAA CAA AAG AGT ACA GCA TGG CAC AGT AT TAT GGA GTC CTT TGT ATT TTT TTC AGG CTT CAT C TGT CAC CCA CTT CAG TA GTG GCG ACT TGT ATG T CCT CCA TGT TCT CTC ATA CTC ATT CCA ACA CTT AAC TAT Dye 6FAM HEX 6FAM HEX GenBank Number Touchdown Temp. Size of Range (bps) No. of Alleles HO HE AY291456 60 284-308 6 0.63 0.78 0.38 0.76 0.5 0.87 0.38 0.88 0.75 0.83 0.88 0.86 0.38 0.81 0.25 0.9 AY291457 AY291458 AY291459 60 60 60 146-190 163-201 100-163 10 8 10 Croshaw & Glenn (2003). 3.6.2. Fragment Analysis Parameters The ABI 3100 Analyzer was standardized with Multi-Capillary DS-30 Matrix Standard Kit. A multi-component matrix was required to normalize the data collection software to the four different colored fluorescent dyes (6-FAM, Hex, NED and ROX dyes) labeling DNA fragments in a single capillary. Matrix Standard (5 µl) was mixed with 195 µl formamide; 1:40 dilution. Reaction denature at 95 °C for 5 minutes. Reaction mixture was used to run a spectral calibration for the DS-30 dye set D; green, blue, red (ROX standard), and yellow. A spatial analysis was also conducted for each capillary 32 array. Spatial test checks for even light penetration and absorbency through each of the 16 capillaries. The Run 3100 Data Collection v2.0 Software allows for the labeling and fragment analysis run parameters to be set using the Plate Manger application. Samples’ identification was paralleled with well position, dye set, sequence protocol, and data storage location. After designing plate parameters, reaction plate was linked to run manger until sequencing was completed. 3.6.3. Fragment Analysis Parameters Data were formatted and analyzed using the GeneMapper Software v3.5. GeneMapper Software is designed to analyze the data generated using several fragment analysis chemistry kits on the ABI PRISM® 3100 Genetic Analyzer. Run results were exported in a .csv format and later viewed with Excel for data cleaning and formatting. CONVERT software (Glaubitz, 2004) was used to convert the cleaned data into a useable format for heterozygosity analysis using GENEPOP (Raymond & Rousset, 1995). CONVERT program is designed to transfer codominant, diploid genotypic data outputs form common genetic software packages. Excel files of clean codominant marker data were used as an input file for conversion into GENEPOP format (Glaubitz, 2004). GENEPOP software package computes exact tests: for Hardy-Weinberg equilibrium, population differentiation, classical Fis, allele frequencies and allele size-based statistics (Raymond & Rousset, 1995). To determine bottleneck presence in each population, BOTTLENECK program was used. Bottleneck is a program for detecting recent effective population size reductions from allele data frequencies (Piry et al., 1999). 33 CHAPTER 4 RESULTS 4.1. Genomic Deoxyribonucleic Acid Quantification Approximately 230 tissue samples were collected; of which, total genomic DNA was extracted from 107 samples. DNA samples were allocated towards SSR identification and isolation. Following isolation, DNA samples were quantified and qualified for downstream application using the NanoDrop Spectrophotometer and assigned a distinctive code. The following table displaces all genomic DNA samples with a 260/280 value of between 1.7- 2.0, indicating purity of sample. Table 3. One hundred twenty-two DNA samples with 260/280 ratios between 1.7 and 1.8. Sample ID B10SS1 B10SS2 B1ES3 B1ES4 B1ES5 B1ES7 B1ES8 B1ES9 B1SS2 B2GF1 Date 8/28/2012 8/28/2012 8/28/2012 8/28/2012 8/28/2012 8/28/2012 8/28/2012 8/28/2012 8/28/2012 8/28/2012 Time 6:59 PM 6:37 PM 6:52 PM 6:53 PM 6:54 PM 6:56 PM 6:58 PM 6:36 PM 6:46 PM 10:22 AM ng/ul 284.04 108.32 47.78 48.1 61.26 38.46 32.05 25.42 43.68 68.29 34 A260 5.681 2.166 0.956 0.962 1.225 0.769 0.641 0.508 0.874 1.366 A280 3.027 1.159 0.522 0.562 0.684 0.403 0.34 0.27 0.474 0.703 260/280 1.88 1.87 1.83 1.71 1.79 1.91 1.89 1.88 1.84 1.94 (Continued) Table 3. One hundred between 1.7 and 1.8. Sample ID Date Time B2GF2 8/28/2012 10:24 AM B2LF15 8/28/2012 4:50 PM B2LF15 8/28/2012 4:51 PM B2LF22 8/28/2012 5:00 PM B2LF24 8/28/2012 5:51 PM B2LF29 8/28/2012 5:57 PM B2LF30 8/28/2012 5:58 PM B2LF31 8/28/2012 6:10 PM B2RN10 8/28/2012 10:39 AM B2RN11 8/28/2012 10:40 AM B2RN20 8/28/2012 4:57 PM B2RN21 8/28/2012 4:58 PM B2RN32 8/28/2012 6:11 PM B2RN4 8/28/2012 10:27 AM B2RN6 8/28/2012 10:33 AM B2RN7 8/28/2012 10:34 AM B2RN8 8/28/2012 10:35 AM B2SS14 8/28/2012 4:48 PM B2SS16 8/28/2012 4:52 PM B2SS17 8/28/2012 4:53 PM B2SS18 8/28/2012 4:54 PM B2SS28 8/28/2012 5:56 PM B2SS34 8/28/2012 6:39 PM B3MB10 8/28/2012 6:28 PM B3MB5 8/28/2012 6:19 PM B3MB5 8/28/2012 6:21 PM B3MB8 8/28/2012 6:25 PM B3MB9 8/28/2012 6:26 PM B3RN1 8/28/2012 6:15 PM B3RN1 8/28/2012 6:14 PM B3RN2 8/28/2012 6:16 PM B3RN3 8/28/2012 6:17 PM B3RN7 8/28/2012 6:23 PM B4SS11 8/28/2012 11:04 AM B4SS12 8/28/2012 4:41 PM twenty-two DNA samples with 260/280 ratios ng/ul 65.51 21.51 20.95 12.71 22.41 65.81 25.6 301.51 43.47 53.22 50.22 109.33 110.66 95.52 114.18 90.13 251.06 344.57 6.01 430.21 201.27 46.77 114.22 29.05 1556.63 688.66 49.13 21.06 157.63 146.4 208.66 56.94 214.72 53.53 35.5 35 A260 1.31 0.43 0.419 0.254 0.448 1.316 0.512 6.03 0.869 1.064 1.004 2.187 2.213 1.91 2.284 1.803 5.021 6.891 0.12 8.604 4.025 0.935 2.284 0.581 31.133 13.773 0.983 0.421 3.153 2.928 4.173 1.139 4.294 1.071 0.71 A280 0.679 0.222 0.224 0.13 0.251 0.688 0.278 3.227 0.446 0.559 0.53 1.131 1.255 0.965 1.178 0.927 2.615 3.754 0.062 4.775 2.207 0.514 1.186 0.332 16.872 7.438 0.54 0.246 1.809 1.722 2.284 0.646 2.293 0.568 0.367 260/280 1.93 1.93 1.87 1.95 1.78 1.91 1.84 1.87 1.95 1.9 1.89 1.93 1.76 1.98 1.94 1.95 1.92 1.84 1.93 1.8 1.82 1.82 1.93 1.75 1.85 1.85 1.82 1.72 1.74 1.7 1.83 1.76 1.87 1.88 1.93 (Continued) Table 3. One hundred between 1.7 and 1.8. Sample ID Date Time B4SS15 8/28/2012 4:46 PM B4SS16 8/28/2012 5:02 PM B4SS18 8/28/2012 5:04 PM B4SS30 8/28/2012 6:00 PM B4SS31 8/28/2012 6:02 PM B4SS33 8/28/2012 6:05 PM B4SS35 8/28/2012 6:07 PM B4SS5 8/28/2012 10:55 AM B6MB2 8/28/2012 5:05 PM B6SS1 8/28/2012 4:42 PM B6SS10 8/28/2012 5:15 PM B6SS11 8/28/2012 5:17 PM B6SS12 8/28/2012 5:18 PM B6SS13 8/28/2012 5:21 PM B6SS14 8/28/2012 5:22 PM B6SS15 8/28/2012 5:25 PM B6SS16 8/28/2012 5:29 PM B6SS17 8/28/2012 5:31 PM B6SS18 8/28/2012 5:32 PM B6SS19 8/28/2012 5:33 PM B6SS20 8/28/2012 5:35 PM B6SS21 8/28/2012 5:36 PM B6SS22 8/28/2012 5:38 PM B6SS23 8/28/2012 5:39 PM B6SS24 8/28/2012 5:41 PM B6SS25 8/28/2012 5:43 PM B6SS26 8/28/2012 5:44 PM B6SS27 8/28/2012 5:45 PM B6SS28 8/28/2012 5:47 PM B6SS29 8/28/2012 5:48 PM B6SS30 8/28/2012 7:00 PM twenty-two DNA samples with 260/280 ratios ng/ul 11.25 12.41 22.52 13.55 22.83 27.78 27.26 9.23 38.97 15.79 13.63 37.81 123.12 95.97 266.05 55.7 195.79 50.37 79.74 339.59 315.3 171.44 242.4 262.87 347.5 146.98 289.22 349.33 276.28 244.12 360.63 36 A260 0.225 0.248 0.45 0.271 0.457 0.556 0.545 0.185 0.779 0.316 0.273 0.756 2.462 1.919 5.321 1.114 3.916 1.007 1.595 6.792 6.306 3.429 4.848 5.257 6.95 2.94 5.784 6.987 5.526 4.882 7.213 A280 0.12 0.142 0.235 0.142 0.238 0.29 0.289 0.094 0.439 0.177 0.146 0.43 1.419 0.999 3.035 0.632 2.145 0.539 0.891 3.648 3.655 1.826 2.609 2.857 3.763 1.554 3.157 3.851 2.937 2.614 3.878 260/280 1.87 1.75 1.91 1.91 1.92 1.92 1.89 1.96 1.78 1.79 1.87 1.76 1.73 1.92 1.75 1.76 1.83 1.87 1.79 1.86 1.73 1.88 1.86 1.84 1.85 1.89 1.83 1.81 1.88 1.87 1.86 (Continued) Table 3. One hundred twenty-two DNA samples with 260/280 ratios between 1.7 and 1.8. Sample ID Date Time ng/ul A260 A280 260/280 B6SS4 8/28/2012 5:08 PM 24.38 0.488 0.279 1.75 B6SS5 8/28/2012 5:09 PM 133.96 2.679 1.441 1.86 B6SS6 8/28/2012 5:10 PM 38 0.76 0.411 1.85 B6SS7 8/28/2012 5:11 PM 162.72 3.254 1.873 1.74 B6SS8 8/28/2012 5:13 PM 137.4 2.748 1.486 1.85 B6SS9 8/28/2012 5:14 PM 303.09 6.062 3.225 1.88 B8SS1 8/28/2012 6:41 PM 153.39 3.068 1.669 1.84 B8SS2 8/28/2012 6:42 PM 183.22 3.664 1.925 1.9 B9SS1 8/28/2012 6:38 PM 29.93 0.599 0.315 1.9 ___________________________________________________________________ In each label: first two spaces represent site name, middle two letters represent common name species, last digits are order of quantification. Note the tables include all samples and species extracted; however three species were targeted for SSR primer development. Purity and quality of genomic DNA were verified by gel electrophoresis. Samples were run on a 1 x TBE 1% agarose gel. Below in figure 5, 60 genomic samples were visualized. A one kb genetic marker was used to visualize the percentage of genomic DNA above 2,000 bps. Shearing of DNA was also visible in some samples as a sign of contamination and degradation. Samples with contamination or significant shearing were removed from the study. 37 ________________________________________________________________________ Figure 5. Gel electrophoresis of seventy one genomic DNA samples on a ethidium bromide stained 1% agarose 1 x TBE gel; 80 voltages for 2.5 hours. Samples represented are from study species; in numerical order. 4.2. Random Amplified Polymorphic DNA Random Amplified Polymorphic DNA (RAPDs) primers are being used to isolate highly polymorphic fragments of the conservative regions of the genome, pre species. Initial PCR was conducted on twelve DNA samples for each of the 40 RAPD primers. PCR trials produced adjusted annealing temperatures and primer concentrations that will be use for the remaining DNA samples. In Table (7 & 8) below, highlighted samples were amplified with RAPD primers. After gel electrophoresis, products proceeded to cloning. 38 Table 4. Eastern Red Newt DNA samples used for RAPD analysis are highlighted. Sample ID B2RN13 B2RN12 B2RN4 B2RN10 B2RN7 B2RN6 B2RN21 B2RN8 ng/ul 46.87 40.4 95.52 43.47 90.13 114.18 109.33 251.06 260/280 2.23 2.12 1.98 1.95 1.95 1.94 1.93 1.92 Sample ID B2RN11 B2RN20 B3RN7 B3RN2 B2RN32 B3RN3 B3RN1 B3RN1 ng/ul 53.22 50.22 214.72 208.66 110.66 56.94 157.63 146.4 Table 5. : Spotted Salamander DNA samples used for RAPD analysis are highlighted. Sample ID B10SS1 B10SS2 B2SS14 B2SS17 B2SS18 B2SS34 B4SS14 B6SS12 B6SS13 B6SS13 B6SS14 B6SS16 B6SS19 B6SS20 B6SS21 ng/ul 284.04 108.32 344.57 430.21 201.27 114.22 95.54 123.12 91.22 95.97 266.05 195.79 339.59 315.3 171.44 260/280 1.88 1.87 1.84 1.8 1.82 1.93 2.01 1.73 2.03 1.92 1.75 1.83 1.86 1.73 1.88 Sample ID B6SS22 B6SS23 B6SS24 B6SS25 B6SS26 B6SS27 B6SS28 B6SS29 B6SS30 B6SS5 B6SS7 B6SS8 B6SS9 B8SS1 B8SS2 ng/ul 242.4 262.87 347.5 146.98 289.22 349.33 276.28 244.12 360.63 133.96 162.72 137.4 303.09 153.39 183.22 260/280 1.86 1.84 1.85 1.89 1.83 1.81 1.88 1.87 1.86 1.86 1.74 1.85 1.88 1.84 1.9 A number of primers produced distinctive polymorphic regions, visualize on a 2% agarose 1X TBE buffer gel under UV light. Six primers yielded tight reproducible bands for Red-Spotted Newt, 9 primers for Spotted Salamander. The results for Spotted Salamanders and Red Newts are listed in Table 7 and 8 prospectively. In these tables 39 RAPD primers are listed by their working identification name. The specific sequence and catalog numbers corresponding to each working name are listed in Table 9 below. Table 6. Random Amplified Polymorphic DNA Kit, US Biological Random Amplified Polymorphic DNA Kit, BioAssay: Lot No. L10041901 & L12102655 GC Content TA: 39.5°C Primers Catalog no. Sequence % TM Primer Working Name 1 R1125-01-3 AGT CAG CCA C 60% 39.5 C 2 R1125-01-4 AAT CGG GCT G 60% 39.5 C 3 R1125-01-5 AGG GCT CTT G 60% 39.5 C 4 R1125-01-7 GAA ACG GGT G 60% 39.5 C 5 R1125-01-8 GTG ACG TAG G 60% 39.5 C 6 R1125-01-10 GTG ATC GCA G 60% 39.5 C 7 R1125-01-11 CAA TCG CCG T 60% 39.5 C 8 R1125-01-12 TCG GCG ATA G 60% 39.5 C 9 R1125-01-14 TCT GTG CTG G 60% 39.5 C 10 R1125-01-15 TTC CGA ACC C 60% 39.5 C 11 R1125-01-16 AGC CAG CGA A 60% 39.5 C 12 R1125-01-17 GAC CGC TTG T 60% 39.5 C 13 R1125-01-18 AGG TGA CCG T 60% 39.5 C 14 R1125-01-19 CAA ACG TCG G 60% 39.5 C 15 R1125-01-20 GTT GCG ATC C 60% 39.5 C 16 R1125-02-01 GTT TCG CTC C 60% 39.5 C 17 R1125-02-02 TGA TCC CTG G 60% 39.5 C 18 R1125-02-04 GGA CTG GAG T 60% 39.5 C 40 (continued) Table 6. Random Amplified Polymorphic DNA Kit, US Biological Random Amplified Polymorphic DNA Kit, BioAssay: Lot No. L10041901 & L12102655 GC Content TA: 39.5°C Primers Catalog no. Sequence % TM Primer Working Name 19 R1125-02-11 GTA GAC CCG T 60% 39.5 C 20 R1125-02-12 CCT TGA CGC A 60% 39.5 C 21 R1125-02-15 GGA GGG TGT T 60% 39.5 C 22 R1125-02-16 TTT GCC CGG A 60% 39.5 C 23 R1125-02-17 AGG GAA CGA G 60% 39.5 C 24 R1125-02-18 CCA CAG CAG T 60% 39.5 C 25 R1125-02-20 GGA CCC TTA C 60% 39.5 C GC Content TA: 42°C Primers Cat. # Sequence % TM 26 R1125-01-01 CAG GCC CTT C 70% 43.6 C 27 R1125-01-02 TGC CGA GCT G 70% 43.6 C 28 R1125-01-06 GGT CCC TGA C 70% 43.6 C 29 R1125-01-09 GGG TAA CGC C 70% 43.6 C 30 R1125-01-13 CAG CAC CCA C 70% 43.6 C 31 R1125-02-03 CAT CCC CCT G 70% 43.6 C 32 R1125-02-05 TGC GCC CTT C 70% 43.6 C 33 R1125-02-06 TGC TCT GCC C 70% 43.6 C 34 R1125-02-07 GGT GAC GCA G 70% 43.6 C 35 R1125-02-08 GTC CAC ACG G 70% 43.6 C 36 R1125-02-09 TGG GGG ACT C 70% 43.6 C 37 R1125-02-10 CTG CTG GGA C 70% 43.6 C 38 R1125-02-13 TTC CCC CGC T 70% 43.6 C 39 R1125-02-14 TCC GCT CTG G 70% 43.6 C 40 R1125-02-19 ACC CCC GAA G 70% 43.6 C 41 Table 7. Amplified Spotted Salamanders Polymorphic Regions Primer # bands Band Size I Size Size Size Comments 1 3 490 194 110 3 2 955 534 5 3 927 737 269 2 CLEAR BANDS 27 3 793 556 281 31 4 1116 796 320 95 32 33 35 38 2 7 4 3 660 739 705 1003 1080 525 565 673 307 391 304 106 366 Table 8. Amplified Eastern Red Newt Polymorphic Regions Primer # bands Band Size Size 1 3 350 600 6 2 700 800 7 2 495 600 8 2 512 800 10 1 750 19 2 350 675 21 2 21 75 26 1 166 27 1 175 118 39 1 213 VERY CLEAR 660 VERY CLEAR 5 CLEAR BANDS VERY CLEAR Size 820 4.3. Bacteria Transformation and Cloning RAPD PCR products bands were exorcized from agarose gels; then dissolved and DNA was extracted. Fragments were inserted into vectors and transformed into e. coli; in the presence of ampicillin for selection. The growth of white colonies indicates successful transformation of plasmid into bacteria. A number of positive clones are visible in Figure 6. Of the white colonies, single isolates were selected for bacteria PCR 42 to determine which fragments were inserted. Clones within fifty base pair range of the original isolated product from RAPD PCR were selected for sequencing. Figure 6. Transformed Bacteria Plates. 43 (continued) Figure 6. Transformed Bacteria Plates. 4.3.2. Plasmid DNA Extracted from Positive Clones For the study species, ten individual transformation plates were selected for sequencing. However, twenty other plates of positive colonies were sequences what were not from the study species. Colonies were replicated overnight in 5 mL of LB broth in the presence of ampicillin. Selected colonies and fragment insert sizes are listed below in Table 10. 44 Table 9. Selected Clones for Sequencing from Bacteria PCR. Final Selected Clones for Sequencing by Fragment Size Clone ID Colony 1 Colony 2 Colony 3 Colony 4 1SS33 2SS33 198/364 86/852 137/461 3SS5 710 57 172 787 4SS27 163/626 84 630 61/539/632 5SS5 375 605 6SS27 101 X X 7SS27 8SS33 45 X X X 9SS31 10SS31 11SS3 12SS27 13SS35 14SS33 15SS33 16SS5 535 247/450 20RN27 425 427 21RN27 418 425 427 22RN6 310 563 23RN6 24RN39 4.4. Sequenced Plasmid DNA From thirty sequenced plasmids, only 3 produced unknown sequence. However, these sequences for another species not included in the remainder of study. All positive clones were replicated overnight in LB broth in the presence of ampicillin; shaken at 200 rpm for aeration, over night at 37°C. A total of 2 mL of LB broth with bacteria was suspended in sterile glycerol and stored at -80°C for later sequencing. 45 4.5. Fragment Analysis Eastern Red Newt Table 10. Eastern Red Newt heterozygosity and inbreeding coefficient per locus within BaPo 2 population. Bapo 2 Eastern Red Newt Locus Ho (1-Qintra) He (1-Qinter) Fis (per locus) Nvi11 0.25 0.9167 0.7273 Nvi14 0 1 1 Nvi18 0 0 Nvi19 0 0.6667 1 Nvi24 0.3333 0.8333 0.6 Nvi7 0 0.9048 1 Nvi2 0 0 POP(wt.) 0.087 0.7174 0.8788 The eastern red newt populations at site BaPo 2 had an average observed heterozygosity of 8.7 %; the expected heterozygosity for this population was 71.7 %. This population was found to be more than 90% homozygosis and 87.8 % (Fis) inbred. Eight individuals were analyzed and 5 loci amplified. Table 11. Eastern Red Newt heterozygosity and inbreeding coefficient per locus within BaPo 3 population. Bapo 3 Eastern Red Newt Locus Ho (1-Qintra) He (1-Qinter) Fis (per locus) Nvi11 0.6667 0.9833 0.322 Nvi14 0.6 0.95 0.3684 Nvi18 0 0.6667 1 Nvi19 0.3333 1 0.6667 Nvi24 0 0.8333 1 Nvi7 0 0.6667 1 Nvi2 POP(wt.) 0.32 0.866 0.6305 46 The eastern red newt populations at site BaPo 3 had an average observed heterozygosity of 32 %; the expected heterozygosity for this population was 86.6 %. This population was found to be more than 65% homozygosis and 63 % (Fis) inbred. Seven individuals were analyzed and 6 loci amplified. Table 12. Eastern Red Newt heterozygosity and inbreeding coefficient per locus within BaPo 8 population. BaPo 8 Eastern Red Newt Locus Ho (1-Qintra) He (1-Qinter) Fis (per locus) Nvi11 0.6 0.65 0.0769 Nvi14 Nvi18 0 1 1 Nvi19 0.2 0.5 0.6 Nvi24 0.3333 0.8333 0.6 Nvi7 Nvi2 POP(wt.) 0.3333 0.6833 0.5122 The eastern red newt populations at site BaPo 8 had an average observed heterozygosity of 33.3 %; the expected heterozygosity for this population was 68.3 %. This population was found to be more than 67% homozygosis and 51 % (Fis) inbred. Five individuals were analyzed and 4 loci amplified. Table 13. Observed Heterozygosity of Eastern Red Newt Populations by Locus. Eastern Red Newts Observed Heterozygosity per Population by Locus Locus Nvi11 Nvi14 Nvi19 Nvi24 Nvi18 Nvi7 Nvi2 B2 0.25 0 0 0.3333 0 0 0 B3 0.6667 0.6 0.3333 0 0 0 B8 0.6 0.2 0.3333 0 47 MEAN 0.50557 0.3 0.17777 0.2222 0 0 0 SD 0.22383 0.42426 0.16776 0.19243 0 0 0 Table 13 summarizes observed heterozygosity across all populations of Eastern Red Newt by locus. Of the seven loci, only one (Nvi11) was heterozygous in all three populations. Three loci, (Nvi18, Nvi7, Nvi2), were homozygous in each population. All loci without a value failed to amplify within a given population. Table 14. ANOVA of Loci Heterozygosity (Eastern Red Newt) Across and Within Populations. ANOVA Source of Variation SS Between Groups 0.189729 Within Population 0.410541 Total df MS F P-value F crit 3 0.063243 1.078338 0.418128 4.346831 7 0.058649 0.600271 10 No significant difference (p = 0.41) was observed of heterozygosity between populations or within populations when grouped by loci; p < 0.05. The variation from loci is not contributing significantly to the heterozygosity variations between populations. Spotted Salamanders Table 15. Spotted Salamander heterozygosity and inbreeding coefficient per locus within BaPo 6 population. BaPo 6 Spotted Salamander Locus Ho (1-Qintra) He (1-Qinter) Fis (per locus) AMA07 0.5 0.875 0.4286 AMA112B 0.1429 0.8333 0.8286 AMA127 0 0 AMA2C2 0.25 1 0.75 AMA33 0.875 0.9643 0.0926 AMA34 0.3333 1 0.6667 AMA410 AMA51 0 0 AMA61 0.2857 0.7024 0.5932 AMA94 0.4 0.9 0.5556 POP(wt) 0.4286 0.8801 0.513 48 The spotted salamander population at site BaPo 6 had an average observed heterozygosity of 42.9 %; the expected heterozygosity for this population was 88 %. This population was found to be more than 57.1% homozygosis and 51.3 % (Fis) inbred. Eight individuals were analyzed and 7 loci amplified. Table 16. Spotted Salamander heterozygosity and inbreeding coefficient per locus within BaPo 4 population. BaPo 4 Spotted Salamander Locus Ho (1-Qintra) He (1-Qinter) Fis (per locus) AMA07 0.1429 0.1429 0 AMA112B 0.5 0.6964 0.2821 AMA127 0.4286 0.7143 0.4 AMA2C2 0.7143 0.8571 0.1667 AMA33 0.7143 0.8571 0.1667 AMA34 0.875 0.8214 -0.0652 AMA410 0.4286 0.7857 0.4545 AMA51 0 0.4 1 AMA61 0.75 0.7589 0.0118 AMA94 0.75 0.9464 0.2075 POP(wt) 0.5556 0.7123 0.2201 The spotted salamander population at site BaPo 4 had an average observed heterozygosity of 55.6 %; the expected heterozygosity for this population was 71.2 %. This population was found to be more than 44.4% homozygosis and 22.0 % (Fis) inbred. Eight individuals were analyzed and 9 loci amplified. Table 17. Spotted Salamander heterozygosity and inbreeding coefficient per locus within BaPo 3 population. BaPo 3 Spotted Salamander Locus Ho (1-Qintra) He (1-Qinter) Fis (per locus) AMA07 0.25 0.5179 0.5172 AMA112B 1 0.5 -1 AMA127 0 0.25 1 AMA2C2 0.375 0.4464 0.16 49 (Continued). Table 15. Spotted Salamander heterozygosity and inbreeding coefficient per locus within BaPo 3 population. Locus Ho (1-Qintra) He (1-Qinter) Fis (per locus) AMA33 0.625 0.875 0.2857 AMA34 0.2857 0.5476 0.4783 AMA410 0 0.5714 1 AMA61 0.75 0.7589 0.0118 AMA94 0.75 0.9464 0.2075 POP(wt) 0.5556 0.7123 0.2201 The spotted salamander population at site BaPo 3 had an average observed heterozygosity of 35.5 %; the expected heterozygosity for this population was 57.8 %. This population was found to be 64.5% homozygosis and 38.7 % (Fis) inbred. Eight individuals were analyzed and 9 loci amplified. Table 18. Spotted Salamander heterozygosity and inbreeding coefficient per locus within BaPo 2 population. BaPo 2 Spotted Salamander Locus Ho (1-Qintra) He (1-Qinter) Fis (per locus) AMA07 0.3333 0.6667 0.5 AMA112B 0.5 1 0.5 AMA127 AMA2C2 0.5 0.9583 0.4783 AMA33 0.4 1 0.6 AMA34 AMA410 0 0 AMA51 0 0 AMA61 0.25 1 0.75 AMA94 0.5 0.5 0 POP(wt) 0.36 0.7933 0.5462 The spotted salamander population at site BaPo 2 had an average observed heterozygosity of 36 %; the expected heterozygosity for this population was 79.3 %. 50 This population was found to be 64 % homozygosis and 54.6 % (Fis) inbred. Five individuals were analyzed and 8 loci amplified. Table 19. Observed Heterozygosity of Spotted Salamander Populations by Locus. Locus AMA07 AMA112B AMA2C2 AMA33 AMA34 AMA61 AMA94 AMA51* AMA127 AMA410 Mean Pop. Spotted Salamanders Observed Heterozygosity per Population by Locus BaPo 6 BaPo 4 BaPo 3 BaPo 2 0.5 0.1429 0.25 0.3333 0.1429 0.5 1 0.5 0.25 0.7143 0.375 0.5 0.875 0.7143 0.625 0.4 0.3333 0.875 0.2857 0.2857 0.75 0.875 0.25 0.4 0.75 0.4286 0.5 0 0 0 0 0.4286 0 0.4286 0 0 0.3097 0.5304 0.4266 0.3104 Mean 0.30655 0.53573 0.45983 0.65358 0.498 0.54018 0.51965 0 0.14287 0.14287 SD 0.15068 0.35233 0.19798 0.19819 0.32736 0.3189 0.15922 0 0.24745 0.24745 Table 19 summarizes observed heterozygosity across all populations of Spotted Salamanders by locus. Of the ten loci, one locus (AMA51) was homozygous in all populations of Spotted Salamander. All other loci, except AMA127 and AMA 410, were heterozygous in at least three populations. All loci without a value failed to amplify within a given population. Table 20. ANOVA of Loci Heterozygosity (Spotted Salamander) Across and Within Populations. ANOVA Source of Variation Between Groups Within Groups SS 0.89691 1.516364 Total 2.413274 df MS F P-value F crit 8 0.112114 1.774463 0.1322638 2.355081495 24 0.063182 32 51 No significant difference (p = 0.13) was observed of heterozygosity between populations or within populations when grouped by loci; p < 0.05. The variation from different loci is not contributing significantly to the heterozygosity variations between populations. 4.6. Bottleneck Analysis Summary of Heterozygosity and Fis Correlation Heterozygosity is the individual of population level parameter measuring gene diversity. Heterozygosity ranges from 0-1.0 and reflects the proportion of loci expected to be heterozyotes. Observed heterozygosity is the calculated as the number of heterozygotes of the total loci. Expected heterozygosity is calculated as 1 minus the sum of squared gene frequencies. Fis, inbreeding coefficient, is the proportion of the variance in the sub population contained in an individual. Bottleneck calculations assume mutation-drift equilibrium to compute the distribution of gene diversity expected from the observed number of alleles; i.e. the various molecular weights of allele. Expected heterozygosity is computed from Hardy- Weinberg equilibrium (HWE) (He= 1) (Knaepkens et al., 2004). Populations without a recent change in size will be in mutation-drift equilibrium where the expected heterozygosity (HEQ) will equal the Hardy–Weinberg equilibrium heterozygosity (HE) (Knaepkens et al., 2004). A normal Lshaped distribution of allele frequency across distribution classes is expected in a normal population. The loss of heterozygosity in the first distribution class (0-0.1) represents gene drift and a bottleneck event (Piry et al., 1999). 52 Eastern Red Newt Table 21. Wilcoxon Test for Heterozygosity for Eastern Red Newt Populations. Wilcoxon Test (Loci Heterozygosity) BaPo 2 BaPo 3 One Tail Excess 0.01563 0.01563 Two Tail Ex or Def. 0.03125 0.03125 One Tail Deficiency 1 0.99219 BaPo 8 0.03125 0.0625 1 Excess heterozygosity was significant, where p < 0.5, in all populations of Eastern Red Newt p- values ranging from (0.01- 0.03). Figure 7. Allele Frequency Distribution for Eastern Red Newt Populations. In all populations of Eastern Red Newt, allele frequency (0-0.22) was lowest in the first generation class (0-0.1) and highest in the second distribution class (0.101-0.2) in 53 BaPo 2 and BaPo 3. In the BaPo 3 population, allele frequency was the highest in the first class and resembles an L-shape distribution. In BaPo 8, allele frequency was highest in distribution class 0.5-0.6 and least resembles an L-shape distribution. Table 22. Wilcoxon Test for Heterozygosity for Spotted Salamander Populations. Wilcoxon Test One tail He Deficiency One Tail He Excess Two tail Ex. or Def. BaPo 2 0.98438 0.02344 0.04688 BaPo 4 0.34766 0.6875 0.69531 BaPo 6 0.53125 0.53125 1 BaPo 3 * Raw data error Excess heterozygosity was significant, where p < 0.5, in only the BaPo 2 population of Spotted Salamanders; p- value 0.02. BaPo 4 and Bapo 6 p-values were not significantly different from a normal population variation. Raw data formatting error prevented Wilcoxon analysis for the BaPo 3 population. 54 Figure 8. Allele Frequency Distribution for Spotted Salamander Populations. Normal L- shape distribtion of allele frequency was observed in populations BaPo 4 and BaPo 6; allele frequency was highest in the first distruction class (0-0.1). Bapo 3 allele frequency (0.207) was lower in the first class than the second distrubtion class (0.241). BaPo 2 allele frequency (0) was lowest in the first distrubution class. 55 CHAPTER 5 DISCUSSION In all populations of eastern red newts, observed heterozygosity was less than expected heterozygosity. Further the results were supported by Fis values indicating inbreeding was highly present. Excess heterozygosity was significant, where p < 0.5, in all populations of Eastern Red Newt p-values ranging from (0.01- 0.03). These results were consistent with the allele frequency distribution results. In all populations of Eastern Red Newt, allele frequency (0-0.22) was lowest in the first generation class. All three populations exhibited bottleneck events with gene drift occurring most recently in BaPo 3. In the BaPo 3 population, allele frequency was the higher (0.222) in the first class and resembled an L-shape distribution. It is possible BaPo 3 population is recovering from a recent bottleneck which would be further substantiated with additional study of the migration rate of the population. Site locations are mapped below in Figure 8. In BaPo 8, allele frequency was highest in distribution class 0.5-0.6 and least resembles an L-shape distribution; indicating a bottleneck event occurred with a recovery time much slower than other populations. Bapo 8 is located within meters of a dirt road and within a FS stand with forest disturbance dating, by first year, to approximately 1960 (USFS-CISC database). See Figure 7. What portion of the canopy cover removed and by what means 56 the disturbance was caused requires further research. Little to no migration is expected for this site without of nearby pond sites. The semi-aquatic adults’ movement is limited; only the eft juveniles’ dispersal movement is a migration factor in the metapopulation genetic variation (Williams, 2009). Figure 9. BaPo 8 Stand First Year. The observed heterozygosity levels differ from the results of Croshaw & Glenn (2003). However, considering sample size, these results may not be representative of the whole populations for two reasons. The markers used were developed from another population of the same species but not the same sub species. Additionally, the markers 57 used were designed to amplify consistently in populations of average to high expected heterozygosity. High homozygosity was present in these populations which could account for the over estimation of inbreeding. Spotted Salamander Heterozygosity of spotted salamander populations ranges from 35-55%, with inbreed coefficient not exceeding 55%. Collectively these populations exhibited normal range of heterozygosity. Population BaPo 4 had the highest level of heterozygosity at 55.6% and the lowest lever of inbreeding (22%) presences. However, populations BaPo 3 (0.3548) and BaPo 2 (0.36) heterozygosity levels did not exceed 40%; both populations Fis also exceeded 50 percent. Populations BaPo 6 (0.4286) and BaPo 4 (0.5556) heterozygosity levels were highest; allele frequency distributions were also normal Lshape. Bapo 3 allele frequency (0.207) was lower in the first class than the second distrubtion class (0.241); representative of a bottleneck. BaPo 2 allele frequency (0) was lowest in the first distrubution class; representatiove of the most recent bottleneck of all populations of Spotted Salamanders. The occurrence of a bottlenck in Spotted Salamander population is BaPo 2 is unexpected. BaPo 2 and BaPo 6 are within migrational range of for the species. However, BaPo 2 site is a permanent pond, located near a clearing cultivated as a wildlife food plot. Spotted Salamanders are known to prefer vernal pools and avoid clearings during migration. The FS stand in which BaPo 2 is located is dated, by first year, to approximately 1993 (USFU- CISC database) compared to BaPo 6, by first year, Stand date of approximately 1935 (USFU- CISC database). See Figures 8 and 9. Again, what portion of the canopy cover removed and by what means the disturbance was caused 58 requires further research. It is also worth noting after AL State Highway 33 is location between these sites. Figure 10. Location of Sites BaPo 2 and BaPo 6. 59 Figure 11. Bankhead National Forest Study Sites Locations. 60 CHAPTER 6 CONCLUSION This research has contributed to establishing a long-term genetic study of amphibian populations at Bankhead NF (1) by establishing a base line of heterozygosity to study gene drift in established populations and (2) by determined expected allele/ heterozygosity ratios. The project sample size was greatly limited because of (1) DNA and tissue samples lost, and (2) the availability of chemicals for fragment analysis. A considerable about of samples were lost due to power outages cause by weather. Additional samples were lost when the sequencing platform was changed from Ion Torrent Touch to ABI 3100; samples prepared for the Ion Torrent could not be used for the ABI 3100 fragment analysis process. Final since ABI 3100, was not the intended platform for analysis, this project was preformed with remaining reagents from another project. However, both objectives were accomplished for this project. This research has provided better insight into distinguishing short and long term population declines; as well as contributed to understanding why genetic diversity is important for longer population fitness- and ultimately the survival of a species. Without specific data on the types of forest disturbance that have occurred surrounding these study sites, no management recommendations can be made. 61 The study concluded: 1. Establishing a base line of heterozygosity to study gene drift in established populations. 2. In all populations of eastern red newts, observed heterozygosity was less than expected heterozygosity. 3. Excess heterozygosity was significant, where p < 0.5, in all populations of Eastern Red Newt p-values ranging from (0.01- 0.03). 4. Fis values indicating inbreeding was highly present in all populations of Eastern Red Newts (RN). 5. High homozygosity was presence in RN populations could account for the over estimation of inbreeding. 6. In all populations of Eastern Red Newt, allele frequency (0-0.22) was lowest in the first distribution class. 7. All three populations of RN exhibited bottleneck events with gene drift occurring most recently in BaPo 3. 8. In RN BaPo 3 population, allele frequency was the higher (0.222) in the first class and resembled an L-shape distribution. 9. In RN population BaPo 8, allele frequency was highest in distribution class 0.5-0.6 and least resembles an L-shape distribution. 10. Heterozygosity of spotted salamander populations ranges from 35-55%, with inbreed coefficient not exceeding 55%. 11. Collectively Spotted Salamander (SS) populations were with normal range of heterozygosity. 62 12. SS population BaPo 4 had the highest level of heterozygosity at 55.6% and the lowest lever of inbreeding (22%) presences. 13. SS Populations’ BaPo 3 (0.3548) and BaPo 2 (0.36) heterozygosity levels did not exceed 40%; both populations Fis also exceeded 50 percent. 14. In Spotted Salamanders populations’ BaPo 6 (0.4286) and BaPo 4 (0.5556) heterozygosity levels were highest; allele frequency distributions were also normal L- shape. 15. Spotted Salamander population Bapo 3 allele frequency (0.207) was lower in the first class than the second distrubtion class (0.241); representative of a bottleneck. 16. Spotted Salamander population BaPo 2 allele frequency (0) was lowest in the first distrubution class; representative of the most recent bottleneck of all populations of Spotted Salamanders. 63 REFERENCES Allison, L.A. (2007). Fundamental molecular biology. Oxford: Blackwell. Andersen LW, Fog K, Damgaard C. (2004). Habitat fragmentation causes bottlenecks and inbreeding in the European tree frog (Hyla arborea). Proc Roy Soc London B 271: 1293–1302. Ash, A. (1988). Disappearance of salamanders from clearcut plots. Journal of the Elisha Mitchell Scientific Society 104:116–122. Beebee, T. & Rowe, G. (2001). Application of Genetic Bottleneck Testing to the Investigation of Amphibian Declines: A Case Study with Natterjack Toads. Conservation Biology, Vol. 15, No. 1: 266-270. Bury, R.B. (1983). Differences in amphibian populations in logged and old growth redwood forest. Northwest Science 57:167–178. Bury, R.B. and P.S. Corn. (1988). Responses of aquatic and streamside amphibians to timber harvest: a review. Pp. 165–181. In Raedeke, K.J. (Ed.), Streamside Management: Riparian and Forestry Interactions. Institute of Forest Resources, Contribution 59, University of Washington, Seattle, Washington. Canadian Council on Animal Care. (2006). CCAC species-specific recommendations on amphibians and reptiles. Chadwick, C.S. (1950). Observations on the behavior of the larvae of the common American newt during metamorphosis. American Midland Naturalist 43:392–398. Chan-McLeod, A.C.A. (2003). Factors affecting the permeability of clearcuts to redlegged frogs. Journal of Management 67: 663-671. Close, Bryony, K. Banister, V. Baumans, E. Bernoth, N. Bromage, J. Bunyan, W. Erhardt, P. Flecknell, N. Gregory, H. Hackbarth, D. Morton and C. Warwick. (1996). Recommendations for euthanasia of experimental animals: Part 2. Laboratory Animals. 30: 293-316. Cushman, S.A. (2006). Effects of habitat loss and fragmentation on amphibians: A review and prospectus. Biological Conservation, 128(2), 231–240. doi:10.1016/j.biocon.2005.09.031. Collins, J.P. & Storfer, A. (2003). Amphibian declines: sorting the hypotheses. Diversity and Distributions 9, this issue. 64 Corn, P.S. and R.B. Bury. (1989). Logging in western Oregon: responses of headwater habitats and stream amphibians. Forest Ecology and Management 29:39–57. Cornuet, J.M. & Luikart, G. (1996). Descriptions and power analysis of two tests for detecting recent population bottlenecks from allele frequency data. Genetics 144, 2001–2014. Croshaw, D. a., & Glenn, T. C. (2003). Seven polymorphic microsatellite DNA loci from the red-spotted newt (Notophthalmus viridescens). Molecular Ecology Notes, 3(4), 514–516. doi:10.1046/j.1471-8286.2003.00496.x Crow, J.F., Kimura, M., (1970). An Introduction to Population Genetics Theory. Harper and Row Publishers, New York, Evanston and London. Downs, F.L. (1989). Ambystoma maculatum (Shaw), spotted salamander. Pp. 108– 125. In Pfingsten, R.A. and F.L. Downs (Eds.), Salamanders of Ohio. Ohio Biological Survey Bulletin, New Series, Volume 7, Number 2, Columbus, Ohio. Driscoll, D.A. (1999). Genetic neighbourhood and effective population size for two endangered frogs. Biological Conservation 88, 221–229. FEMAT. (1993). Forest ecosystem management: An ecological, economic, and social assessment – report of the Forest Ecosystem Management Assessment Team. USDA-Forest Service. Portland, Oregon. Online at: http://pnwin.nbii.gov/nwfp/FEMAT/ Flageole, S. and R. Leclair Jr. (1992). Etude demographique d'une population de salamanders (Ambystoma maculatum) a l'aide de la methode squelettochronologique. Canadian Journal of Zoology 70:740–749. Frankham, R., Ballou, J.D. & Briscoe, D.A. (2002). Introduction to conservation genetics. Cambridge.University Press, Cambridge, U.K. Gibbs, J.P. (1998). Distribution of woodland amphibians along a forest fragmentation gradient. Landscape Ecology 13: 253-264. Glaubitz, J.C. (2004). CONVERT: a user friendly program to reformat diploid genotypic data for commonly used population genetic software packages. Molecular Ecology Notes 4: 309-310. Glenn, T. C., & Schable, N. A. (2007). Isolating Microsatellite DNA Loci *, (2005), 202– 222. Goldstein, D.B. & Schlötterer, C., eds. (1999). Microsatellites: evolution and applications. Oxford University Press, Oxford, U.K. 65 Halley, J. M., R.S. Oldham, and J.W. Arntzen. (1996). Predicting the persistence of amphibian populations with the help of a spatial model. The Journal of Applied Ecology 33(3): 455-470. Hanski, I.A. and M.E. Gilpin. eds. (1997). Metapopulation Biology: Ecology, Genetics, and Evolution. Academic Press, San Diego, California. Hanski, I.A. (1999). Metapopulation ecology. Oxford University Press, New York. Hoffman, R.L., G.L. Larson and B.J. Brokes. (2003). Habitat segregation of Ambystoma gracile andAmbystoma macrodactylum in mountain ponds and lakes, Mount Ranier National Park, Washington, USA. Journal of Herpetology 37:24–34. Jehle, R., Arntzen, J.W. (2002). Review: microsatellite markers in amphibian conservation genetics. Herpetological Journal 12, 1–9. Kimberling, D.N., A.R. Ferreira, S.M. Shuster and P. Keim. (1996). RAPD marker estimation of genetic structure among isolated northern leopard frog populations in the southwestern USA. Molecular Ecology 5:521–529. Knaepkens G., Bervoets L., Verheyen E., Eens M. (2004). Relationship between population size and genetic diversity in endangered populations of the European bullhead (Cottus gobio): implica-tions for conservation. Biol Cons 115:403–410. Leberg, P. L. (2002). Estimating allelic diversity: Effects of sample size and bottlenecks. Molecular Ecology 11:2445-2449. Lemmon, E. M., Murphy, M., & Juenger, T. E. (2011). Identification and characterization of nuclear microsatellite loci for multiple species of chorus frogs (Pseudacris) for population genetic analyses. Conservation Genetics Resources, 3(2), 233–237. doi:10.1007/s12686-010-9330-2 Life Technologies. (2002). Cycle Sequencing Kit Protocol. ABI 3100 Genetic Analyzer. Life Technologies. (2010). ABI User’s Manual. ABI PRISM 3100 Genetic Analyzer. Life Technologies. (2011). PureLink ® Quick Gel Extraction Kit Guide, (25). Luikart, G., Allendorf, F.W., Cornuet, J.M. & William, B.S. (1998). Disproportion of allele frequency distributions provides a test for recent population bottlenecks. Journal of Heredity 89, 238–247. 66 Miller, D. L. & M. J. Gray. (2009). Disinfection of field equipment and personal gear. Southeastern Partners in Amphibian and Reptile Conservation, Disease, Pathogens and Parasites Task Team, Information Sheet #10. Mitchell, J., & Gibbons, W. (2010). Salamanders of the Southeast. The University of Georgia Press, Athens. Mount, R.H. (1975). The Reptiles and Amphibians of Alabama. Agricultural Experimental Station, Auburn University Press, Auburn, Alabama. Patrick, D.A., M.L. Hunter Jr., & A.J.K. Calhoun. (2006). Effects of experimental forestry treatments on a Maine amphibian community. Forest Ecology 234: 323332. Petit, R.J., A. El Mousadik & O. Pons. (1998). Identifying populations for conservation on the basis of genetic markers. Conservation Biology 12:844–855. Petranka, J. W., M. E. Eldridge, and K. E. Haley. (1993). Effects of timber harvesting on southern Appalachian salamanders. Conservation Biology 7: 363–370. Petranka, J.W. (1994). Response to impact of timber harvesting on salamanders. Conservation Biology 8:302–304. Pentranka, J.W. (1998). Salamanders of the United States and Canada. Smithsonian Institution Press. Washington, DC. Pidancier, N., C. Miquel & C. Miaud. (2003). Buccal swabs as a non-destructive tissue sampling method for DNA analysis in amphibians. Herpetological Journal, Vol. 13: 175-178. Piry S, Luikart G, Cornuet JM. (1999). BOTTLENECK: a computer program for detecting recent reductions in the effective population size using allele frequency data. Journal of Heredity, 90, 502–503. Pruett CL, Winker K. (2008). The effects of sample size on population genetic diversity estimates in song sparrows Melospiza melodia. J. Avian Biol. 39: 252–256. Ohta, T., Kimura, M. (1973). A model of mutation appropriate to estimate the number of electrophoretically detectable alleles in a finite population. Genetical Research, 22, 201-204. Raphael, M.G. (1988). Long-term trends in abundance of amphibians, reptiles, and mammals in Douglas-fir forests of northwestern California. Pp. 23–31. In Szaro, R.C., K.E. Severson and D. Patton (Tech. Coords.), Management of Amphibians, 67 Reptiles, and Small Mammals in North America. U.S.D.A. Forest Service, Technical Report, RM-166, Rocky Mountain Forest and Range Experiment Station, Fort Collins, Colorado. Raymond M., Rousset F. (1995). GENEPOP (Version 1.2): population genetics software for exact tests and ecumenicism. J Hered 86: 248-249. Reed, J. M., and A. R. Blaustein. (1995). Assessment of “nondeclining” amphibian populations using power analysis. Conservation Biology 9:1299–1300. Renken, R. B., W. K. Gram, D. K. Fantz, S. C. Richter, T. J. Miller, K. B. Ricke, B. Russell, and X. Wang. (2004). Effects of forest management on amphibians and reptiles in Missouri Ozark Forests. Conservation Biology 18: 174–188. Scribner, K.T., Arntzen, J.W., Burke, T., Cruddace, N. & Oldham, R.S. (2001). Environmental correlates of toad abundance and genetic diversity. Biological Conservation 98, 201–210. Stiven, A. E., & R. C. Bruce. (1988). Ecological genetics of the salamander Desmognathus quadramaculatus from disturbed watersheds in the southern Appalachian biosphere reserve cluster. Conserv. Biol. 2: 194-205. United States Department of Agriculture, Forest Service, Southern Region, Bankhead National Forest. (2003). Final Environmental Impact Statement: Forest Health and Restoration Project. Management Bulletin R8-MB 110B: p. 351. United States Department of Agriculture, Forest Service, Continuous Inventory Stand Condition (CISC) Database. Vos, C.C., Antonisse-de Jong, A.G., Goedhart, P.W. & Smulders, M.J.M. (2001). Genetic similarity as a measure for connectivity between fragmented populations of the moor frog (Rana arvalis). Heredity 86, 598–608. Waldman B., Tocher M. (1997). Behavioural ecology, genetic diversity, and declining amphibian populations. In: Behavioural Ecology and Conservation Biology (ed. Caro T), 394–448. Oxford University Press, Oxford. Walsh, Bruce. (2001). Estimating the time to the MRCA for the Y chromosome or mtDNA for a pair of individuals, Genetics 158: 897—912. Wang, Yang, Dimov, L., Schweitzer, C., Tadesse, W. (2010). Center of Forest Ecosystem Assessment (CFEA): Subproject 1. Forest Community Responses and Dynamics. Center for Research Excellence in Science and Technology. National Science Foundation Proposal, 10-519. 68 Welsh, H.H., Jr. (1990). Relictual amphibians and old-growth forests. Conservation Biology 4:309–319. Wieczorek, A.M, Zamudio K.R, King T.L, Gjetvaj B. (2002). Isolation of microsatellite loci in spotted salamanders (Ambystoma maculatum). Molecular Ecology Notes, 2, 313-315. Williams, S. (2009). A Review of the Life History and Ecology of the Eastern Newt (Notopthalmus viridescens), 1–16. 69 VITA Rashidah Halimah Farid, the daughter of Wali Farid and Dr. Ashanty Farid, was born on April 10, 1985 in Abbeville, Alabama. She received her Bachelor of Science degree in Animal Science from Tuskegee University in May 2008. She later returned to graduate school at Alabama Agricultural and Mechanical University in May of 2011 to begin the Master of Science degree program in conservation genetics. Miss. Farid received her Master of Science degree in May 2014 from the department of Biological and Environmental Sciences with a concentration in Plant and Molecular Biology.