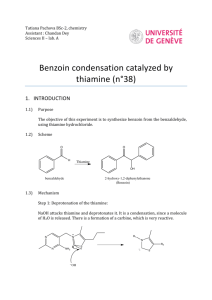
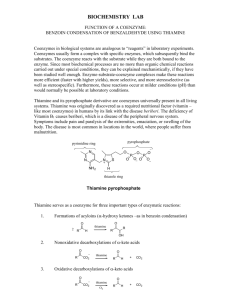
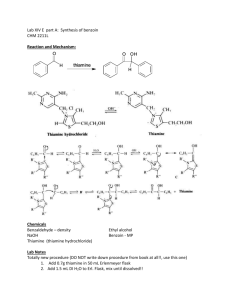
Document 11836159
advertisement

AN ANSTBACT 0' TBB TELESIS OP Elizabeth Willard. Cox for the Ph. D. in Pood.e and Nutrition te Thesis Presented: August 12, 1949 The Determination of Thiamine in the Blood of HUmP't Subjects Redacted for privacy Abstract Approved: or Professor Title: The blood thiamine concentration of 11 subjects was determined by means of the iedemann and Kmieciak (1943) method. every 5 days during a 30-day experimental period. Study I was conducted on 5 students in 1947 and Study II was conducted on 6 students in 1948. The subjects in both studies were on diets in which their intake of ascorbic acid only was controlled. A record was kept of each subjectts food intake. The daily values for total Calories, non-fat Calories and thiamine in the diet were obtained from food. tables. Non-fat Calories, the ratio of non-tat Calories to total Calories, thiamine to 1000 Calories and thiamine to non-fat Calories were calculated.. The blood, thiamine values for the subjects in Study I (all girls) ranged from 4.91 to 10.85 meg per cent. There was a general decrease throughout the study in. the mean intake of thiamine expressed in terms The greater of meg per 1000 Calories and meg per 1000 non-fat Calories. the decrease in thiamine intake in terms of meg per 1000 non-fat Calories the greater was the loss of blood thiamine. The values for thiamine in the blood of the boys in Study II ranged from 8.00 to 15.32 meg per cent The and for the one girl in. Study II from 8.35 to 13.93 meg per cent. blood thiamine values for the subjects in Study II did not indicate the same relationship to thiamine intake as did. those in Study I. There was an increase in the concentration of the thiamine in the blood even though there was a decrease in the mean thiamine intake from the beginning to the end. of the experiment. It would appear, therefore, that the boys obtained. sufficient thiamine throughout the 30-day period. No data have been obtained which indicate whether or not there are variations from day to day in. the concentration of thiamine in the blood when subjects are maintained on a. constant intake of thiamine. A metabolism study using 5 adult women as subjects was planned in order to determine the daily values for thiamine in the blood when subjects were maintained on a controlled diet for a period of 52 days. An unpublished micro-method for the determination of thiamine in the blood developed by Dr. H. Burob (1948) was to be used. The experimental diet consisted. of a basal diet providing approximately 1000 Calories and 300 meg o thiamine with additions to the basal diet planned in units providing approximately 500 Calories and 150 meg of thiamine. The values for protein, fat, carbohydrate and total Calories gi -. were obtained, from food tables. lTon-.fat Calories were calculated. and the thiamine coutent of the foode was determined by analysis. LU 5 subjects ate the same food. each day. In spite of the fact that considerable preliminary work was performed. before the study started. the blood. thiamine values obtained in the nutrition laboratory were always significantly lower than results It was decided. to freeze the blood. samples obtained. by other workers. after the protein had been removed and work on certain aspects of the method before any analyses of the blood thiamine were made. the Burch method. included. variation in the amounts of potassium acetate used, use of different trichioroacetic acid. reagents, PU.rther study of tests for enzyme activity, variations in the incubation procedure, variations in the procedure for the oxidation to tbioobrome and. in the extraction of thiochrome, reading samples sooner after transfer to optically matched tubes, variations in the irradiation procedure, use of all new reagents, use of another miero-pbotofluorometer, development of standard. curves and the determination of the response of two subjects to an oral test dose of thiamine hydrocloride. 1one of these variations resulted. in a solution of the problem, Suggestions for future work with the Buroh method are discussed, TEE iTFRMX}I&TIO! OF THIJMIEE IN TEE BLOOD OF HUMA1I SUMECTS ELIZA.BETK WILLLRD OOX A TEESIS submitted. to OREGON STATE OOLIGE in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY Augast l9i.9 APPROVD: Redacted for privacy Professor of Foods and nutrition In Cbarge of Major Redacted for privacy Head of partment of Foods and. Nu.trition Redacted for privacy hairun of School Gradugte Committee Redacted for privacy Dean of Graduate School Shirley Kosko: Typist AC1NOWLEDGME1TS Sincere gratitude is expressed. to Dr. Clara A. Storvick for her constant assistance and. encouragement thronbout the thiamine studios. The author has learned mach from her valuable criticisms and. suggestions. Appreciation is also expressed to Dr. Margaret L. Fincke for her interest in the problem, careful reading of the thesis and the helpful suggestions she has given. Grateful acknowledgment is made to Dr. Helen B. Burch for allowing us to work with. her npublished micromethod for the determination of thiamine and, for her patience in answering in detail our many questions. The cooperation, interest and enthusiasm of the subjects and the other workers in the laboratory lightened considerably the long hours of research involved. To each of them appreciation is expressed. To Mr. C. H. Wang for checking the calibration of the micro pipettes, to Dr. 3. 3. Christensen for help in the redistillation of trichloroacetic acid and to Dr. J. R. Haag for the use of some of his preparation of redistilled trichioroacotic acid the author expresses her appreciation. Last of all, my thanks to Shirley Kosko who used such painstaking care not only as a laboratory assistant bat in the typing o± this thesis. TABLE OF OONTENTS Page CHAPTER I . .. REVIEW OF LITERATURE 1 METHODS FOR ASSESSING THE STATE OF NUTRITION WITH RESPECT TO THIAMINE... Clinical ... examination...... Demonstration of Biochemical . 1 or Physio1gica1 1 . 1 Physiologicaltests ..................... .... 4 Past Dietary Intake ............ .. 4 Studies on urinary excretion Studies on blood or blood fractions ........ . Estimation of 3 METHODS FOR TEE flETERMINT ION OF INTERMEDIARY METABOLITES, THIAMINE AND COCARXYLASE IN BLOOD ORBLOODACTIONS ........... 5 . 1yruvieLacticAcid.s ....... . 5 Thiamine in Plasma and Sorwn Compared with that Q, Whole Blood.. Thiamine in. the . . . 6 ...... . ......... Formed Elements of the Blood 7 Cocarboxylase in Whole Blood .................. 8 Thiamine inWhole Blood ........................ 9 Phycomyces blakesleearnis .................... Fermentation ................... , ............ Thiochrome .......... 9 9 10 THE QUESTION OF THE SIGNIFICANCE OF BlOOD THIAMINE VALUES IN ASSESSING THE STATE OF NUTRITION WITH RESPECT TO THAT VITAMIN ....... .... CEAPTER II PROCEDURE THE EXPERIMENTAL STUDY 14 WHICH IEDEM1UThT AND KMIECIAK METHOD WAS USED FOR TUE DETERMINAT ION OF BlOOD THIAMINE ............... ..... 18 PLAN OF EXPERIMENTS ............................. . 18 TABLE OF CONTENTS (continued.) Page CHAPTER II (continued) ........ 19 ONEBALDAILYPROOEDURE.............. 20 DESCRIPTION OP SUBJECTS CHAPTER III ANAMTICAL PROCEDURE FOR TEE ThIEDEMANN ICMIECIAK METHOD ............................... EQJUIPI4ENT USED. .............. . . .................. 21 REAGENTSUSED .................................. .. 22 DETERMINATION OF THIAMINE IN TILE BLOOD ......... .. 25 Collection of Blood Sample .................... . 25 Digestion with Phosphatase and Precipitation theProtein .................................... 26 Adsorption Elution ........................ 28 Oxidation to Thioclirome ........................ 29 DETEBMINAT ION OF STANDARD CURVE ................ CHAPTER 30 WH IV RESULTS AND DISCUSSION OF THE STUDIES USED IN KMIEOIAX METHOD FRIEBEMANN DETEBMINATIONOFBLOODTHIAMINE CHAPTER 2]. ............ .. V PROCEDURE FOR THE EXPERIMENTAL STUDY IN WHICH IT WAS PLA11iED TO USE THE BUBCH METHOD FOR THE DETERMINA.TION OP BLOOD THIAMINE ..................... PLAN OF THE STUDY ............................... . 45 EXPERIMENTAL DIET ............................ .. CHAPTER VI DESCRIPTION OF THE SUBJECTS ..................... . 5]. ICAL PROCEDURE FOR THE BURCH METHOD ....... ... 55 EQ,UIPMENT USED ................................ 0ø 55 TABLE OF CONTENTS (continued.) Page CHAPTER VI CHAPTER VII (continued) REAGENTS USED 57 DETERMINATION OF THIAMINE IN THE BLOOD 59 Co1leotionofBloodSaxp1e ..................... 59 Precipitation of the Protein ................ 59 Digest ion with Phosphatase .................... 60 Oxidation to Tbiochrome ....................... 61 Calculations 62 ....................... DETERMINATION OF EEMATOCRIT ......................... 6k NEED FOR DETERMINATION OF REMATOCRIT ............ 6k EQ,UIPMENT USEI) ................................... 6k PROCEDURE FOR DETERMINATION OF H.RMATOCBIT ....... 65 SelectionofTubes ............................. 6 Collectionof Blood ............................ 65 Oentrifu.ation ................................. 65 Caleu.lation .................................. 66 RESULTS OF THE DETERMINATION OF HEMATOCRIT CHAPTER VIII FURTHER STUDY OF TEE BURGH METHOD .............. NEED FOR FURTHER STUDY OF THE BURGH METHOD 66 67 67 METHODS USED FOR FURTEER STUDY OF THE BURGH METHOD ............................................ 67 Varying the Amounts of Potassium Acetate Used.. 67 TABLE OF CONTENTS (continued) Page OKAPTER VIII (continued) The determination of the pH of the blood supernatant using varying amounts of potassiumacotate ........................ .... 67 The determination of the pH of the blood supernatant plus varying amounts of potassium acetate and acid. phosphataso....... 68 Determination of thiamine in blood when varying amounts of potassium acetate were used. ............................... . . * 68 The Use of Different Trichloroacetic AcidReagents .................................. 72 The use of red.istilled. trichloroacetio acid4. 72 Use of different brands of trichloroacetic acid ........................................ . 73 Enzyme Aetivi..... ............... .. 73 Tests Determination of blood thiamine with and. without the use of clarase ............... 73 Varying the amount of olarase used ........... 74 Use of acid phosphatase for enzyme hydrolysis 74 Testing the activity of enzymes using cocarboxylase ................................ 75 Variations in Incubation Procedure ............ . 76 Using longer time for incubation ............ . 76 Using higher incubation temperature ........ . 77 Variations in the Procedure for Oxidation Thiochrome and in the Extraction of Thiochrome. 79 Use of larger amounts of potassium ferncyanide...................................... 79 TABLE OP CO1TENTS (continued.) Page CEAPTER VIII (continued.) Omission of the sodium dihydrogen phosphateperoxide mixture ............................ 79 Variation in the amount of sodium dihydrogen phosphate-peroxide mixture used. .......... ... 80 Use of iso-butyl alcohol instead. of n-butyl alcohol .............................. 82 Readin, Samples Sooner after Transfer Optically Matched Pubes ....................... . 83 Variation in the Irradiation Procedure ........ . 83 Use of a new irradiation lamp ............... . 83 Variation in the time of irradiation ......... jReagents ............................ 85 Use of Another Parrand. Micro-Photofluorometer.. 85 The Determination 86 Stand Curves. ......... . Addition of thiamine hydrochloride to blood CHAPTER IX supernatants ................................ . 86 Addition of thiamine hydrochloride to blood.. 90 Determination of a curve using thiamine .................... . hydrochloride alone 90 Determination of a curve using cocarboxylase a lone ........................................ 91 Addition of cocarboxylase to blood ........... 92 Determination of Blood ThIamine after an Oral Test Dose of Thiamine Hydrochloride ......... .. 92 SUMMARY OF WORK WITH THE BURGH METHOD ............ .. 95 TABLE OF CONTENTS (continued) Page CHAPTER X SUGGEST IONS FUTURE WORK BIL A MIORO-.METHOD . * . . BIBLIOGRAPHY. . . . . APPENDIX I. Direction Sheet Given to 97 100 . 106 the Subjects in Study II ...... .. 107 Table 28 - Intake of Total and. Non-Fat Oalories, Ratio of Total to Non-Pat Calories, Thiamine Intake y, Meg Per 1000 Oalories, in. Terms of Meg Per Meg per 1000 Non-Fat Calories and the Values for thiamine in the Blood. During a 30-my Experimental Period ......................... .. 108 III. Directions to Subjects. .................................. 130 IV. Calibration of Micro-Pipettes.. ........................ .. 132 Hematocrit for 5 Subjects for 52 Iys ................ .. ..................... 133 II. V. Table 29 - Values for LIST OF TABLES Page 1 SUMMARY OF STUDIES IN TEE LITERATtJRE ON THE THIAMINE CONTENT OF BLOOD. . . 12 2 DESCRITIONOFTHESU:BJECTS ....... 19 3 RESULTS OF TEE DETERMINATION OF STANDARD CURVES FOR THIAMINEBYTEREEDIPEEBENTMETHODS ................... .. 31 4 TEE CONCENTRATION OF ThIAMINE IN THE BLOOD IN RELATION TO THE MEAN INTAKE OP TOTAL AND NONPAT CALORIES, RATIO OP TOTAL TO NONFAT CALORIES, THIAMINE INTAKE IN TERMS OF MOG PER DAY, MOG PER 1000 CALORIES, MCG PER 1000 NONFAT CALORIES FOR EACH GROIJP OP 5 DAYS FOR AN EXPERIMENTAL PERIODOF3O DAYS ....................................... I ........................................ . . StudyII ............ ... ........................... .. Stu.dy 5 35 35 37 TEE DECREASE OF MEAN VALUES FOR THIAMINE IN THE BLOOD, DECREASE OF THIAMINE PER 1000 CALORIES AND DECREASE IN THIAMINE PER 1000 NONFAT CLORIES DURING STUDY I........ 6 14.1 THE INCREASE OF MEAN VALUES FOR THIAMINE IN TILE BLOOD, AND DECREASE OF THIAMINE INTAKE PER 1000 CALORIES AND DECREASE OF THIAMINE INTAKE PER 1000 NONFAT CALORIES 7 DURING STUDY II ........................................ . 4.3 OOMPOSITIONOFBAS.ALDIET ................................ 4.9 CONPOSITIONOFUNITADDEDTOBASALDIET .................. 4.9 8 COMPOSITIONOFEISCUITMIX ............................... COMPOSITIONOFCOOKIEMIX ............................... . 50 50 9 THE DIET CONSUMED BY 5 SUBJECTS FROM JANUARY 31, 194.9, THROUGH MARCH 21, 191+9 52 10 .. COMPARISON OF TEE THIAMINE INTAKE WITH CALORIC INTAKE FOR 5 SUBJECTS DURING THE PERIOD JANUARY 31, 194.9, THROUGH MARCH21, 1914.9 .......................................... .. 53 11 DESCRIPTION OF THE SUBJECTS ........................... 0* 54. 12 METHOD OF DETERMINING TILE AVERAGE CORRECTED READING USED IN THE CALCULATION OF BLOOD THIAMINE ..................... 62 LIST OF TABLES (continued) Page 13 THE pH OF THE BLOOD SUPERNA!WIT WHEN VARYING AMOUNTS OF POTASSITJMACETATEWEREADDED. 69 1k THE pH OF THE BLOOD SUPERNATANT AFTER ADDING VARYING AMOUNTS OF POTASSIUM ACETATE AND ACID PHOSPHATASE ...... . 70 15 16 17 DETERMINATION OF BLOOD THIAMINE WHEN VARYING AMOUNTS OF POTASSIUM ACETATE WERE USED ............................. . 70 THE DETERMINAT ION OF BLOOD THIAMINE WHEN 2 DIFREPENT ENZYMES WEBB ADDED TO ALIQ.UOTS OF BLOOD SUPEBEATANT EBOM SUBJECT BWC.. ........................................... . 77 DETERMINATION OF THE CONCENTRATION OF THIAMINE IN THE BLOOD APER 1 18 AND 2* HOURS OF INCUBATION ............... 78 THE pH OF OXIDIZED SAMPLES AF2ER THE ADDITION OP VARYING AMOUNTS OF SODIUM DIHYDROGEN PHOSPHATE-PEROXIDE EAGENT ................................................. 19 THE CONCENTRATION OF THIAMINE IN THE BLOOD OF .5 SUBJECTS AS DETERMINED WHEN THIOOHROI4E WAS EXTRACTED USING ISO- BUTYLALOOHOL ............................................ 20 21 81 82 THE EFRECT OF IRRADIATING THE SAMPLES OR 30 AND 60 MINUTES ............................ 8k THE AVER&GE CORRECTED READING OF SAMPLES AJER BEING IRRADIATED ROR VARYING LENGThS OP TIME ................... 85 22 AVERAGE CORRECTED READINGS OF BLOOD SUPERNATANT PLUS THIAMINE HYDROCHLORIDE MINUS READINGS FOR BLOOD 23 SUE'ERNA.TAMf ........................................... . . . 88 TUE AVERAGE CORRECTED READING OF BLOOD (GAS) PLUS THIAMINE HYDROCHLORIDE MINUS TEE BEADING FOR BLOOD ..... . 90 2k AVERAGE CORRECTED READING USING THIAMINE HYDROCHLORIDE ALONE CARRIED THROUGH TUE ENTIRE PROCEDURE ............... 25 91 AVERAGE CORRECTED BEADING USING 000ARBOXYLASE ALONE CARRIED THROUGH THE ENTIRE PROCEDURE .................... . 91 LIST OF TABLES (continued) Page 26 AVERAGE CORRECTED READING OF BLOOD (GAS) PLUS COCARBOXYLASEMINUSTEEREADINGFORBLOOD ................ 27 28 THE CONCENTRATION OF THIAMINE IN THE BLOOD JJER THE ADMINISTRATION OF A 5 MG ORAL TEST DOSE OF THIAMINE EYDROCHIOB.IE ............................................ INTAKE OF TOTAL AND NON-FAT CALORIES, RATIO OF TOTAL TO NON-FAT CALORIES, THIAMINE INTAKE IN TERMS OF MCG PER DAY, MOG PER 1000 CALORIES, MOG PER 1000 NON-FAT CALORIES AND THE VALUES FOR THIAMINE IN THE BLOOD DURING A 30 DAY EPERIMENTAL PERIOD ..................................... Study I - Nornia. ................................. Study I - Barbara ................................. StudyI-Marion ................................... Study Study 9.4. 108 108 110 112 111.1. 116 - Torn. . ....... . .......................... Dave ..................................... Wesley ................................. . Don ...................................... Keith ......................... Ruth ..................................... 11.8 - 120 122 VALUES FOR EEl tATOCRIT FOR 5 SUBJECTS FOR 52 DAYS ...... ... 133 Study Study Study Study Study Study 29 I - Roberta .................... I - Bessie ...................... 92 II II II II II II 121.1. 126 128 LIST OF FIGURES Page 1 2. 3, Li.. I. * sT.A.NDAEi O1JRVS. * . . MB1A1 5fl&Y THIAMNE INT.AIcE LND BLOOD THIAMINE VALUES F0R 5 SUBJECTS IN STUDY 32 39 NEAE 5DA.Y THIAMINE INTCE AM]) BLOOD THIAMINE VALUES OR 6 SUBJECTS IN STUDY II ..................................... .. STADABDOURESUSINGTBEBU.CHNETHOD ..... ...... 89 THE IIETERMINATION OF THIAMINE IN THE BLOOD OF HUMAN SUBJECTS CHAPTER I LITERATURE REVIEW METHODS FOR ASSESSING THE STATE OF IDTRIT ION WITH RESPECT TO THIAMINE According to ]nn and 1.rby (1914.5) the methods for determining the state of thiamine nutrition may be divided into three main classes (1) clinical examination, (2) demonstration of biochemical ological lesions and (3) estimation of the past or physi dietary intake. Clinical Examination Benson, Witzberger and Slobod.y (1941) used absence of anorexia, fatigue, constipation, calf-mu.scle tenderness and abnormal cardiac or neurologic changes as indications of lack of thiamine Salcedo, Oarrasco, Jose and Valenzu.ela incidence of beriberi in the deficiency. (1948) in their studies on the Philippines made physical inspections for signs and. symptoms of thiamine deficiency occurring in various body regions and systems, i.e., in the mouth (cyanosis, numbness and tremor of the tongue); in the gastrointestinal tract of the lips (anorexia, vomiting, constipation and. waist tightness); in the cardiovascular system (fatigability, palpitation, taehycard.ia, murmurs, enlarged heart and edema); in the nervous system (tingling, and paresthesia); in the skin and muscles numbness, reflex changes (pallor, tenderness of calf muscles and. cramps). Demonstration of Biochemical or Physiological Lesions Studies on urinary excretion - The excretion of thiamine in the urine 2 of untreated subjects has been studied. in various ways: 24-hour urinary thiamine excretions (Harris and Leang, 1936, Benson, Witzberger and. Slobody, 191+1, Mason and. Williams, 1942, Melnicic and. Field, 1942, Keys, HenBchel, Mickelsen and. Brozek, 191+3, Najjsr, 191+3, Perkins, 191+3, Gifft and. Hauck, 191+6, Hathaway and Strom, 1914.6, Mickolson, Caster and. fasting bourn excretion of thiamine in Keys, 1914.6); the the urine (Najjar, 191+3, Salced.o, Carrasco, Jose and Valenzuela, 191+8) and. thiamine excretion expressed in terms of the per cent of thiamine intake (G.ifft and Hauck, 191+6). The urinary response after an or oral test dose of thiamine (t1load 'satnration'1 tests) has been used. as a measure of the state of thiamine nutrition (Melnick, Field. and Robinson, 1939, Melnick and. Field, 191+2, Keys, Henschel, Mickelsen and Brozek, 191+3, Perkins, 191+3, Gifft and. Hauck, 191+6). plete absorption of thiamine in In order to eliminate such variations as incom- of thiamine from the intestine the or poasible destraction gastrointestinal tract some investigators have pre- ferred. to measure the urinary response to a parenteral test dose of thiamine (Melnick, Field anti Robinson, 1939, Mason and. Williams, 191+2, Melnick and Field, 191+2, Alexander, Land.wehr and. Mitchell, 191+6). Pollack, Ellenberg and. Dolger (1941) suggested. that the determi- nation of free thiamine in the urine was an. index of the immediately preceding vitamin intake only, whereas the pyrimid.ine excretion more faithfully indicated a protracted insufficiency of thiamine. Because of the above possibility, the excretion of pyramine (2-methyl- 1+ amino ethoxymethyl pyrimidino), pyrimid.ine or PAYF (pyrimid.ine in the urine which accelerates yeast fermentation) has been measured (Pollack, Ilger, 11enberg and Cohen, 1940, Pollack, ].lenberg and Dolger, 1941, Alexander, Land.wehr and Mitchell, 1946, Mickolaon, Caster and lCeys, 1946). Studies on blood or blood fractions - In an attempt to determine the circulating thiamine in the blood some investigators have measured the concentration of thiamine in whole blood (Meiklejohu, 1937, Rowlands and Wilkinson, 1938, Sinclair, 1938, 1939, Kennessy and. Cereoed.o, 1939, Rit8ert, 1939, Widenbauer, 1939, Fiedemann and. Kmieciak, 1943, Knott, Kleiger and Torres-3racamonte, 19213, Oldharn, Johnston, Kleiger and Hedderich-Arismendi, 1944, Greenberg and. Rinehart, 1945, Oldbasn, Roberts and. Yonng, 1945, Oldbam, Ivis and Roberts, 1946). Other investigators have studied the in red. and white cells concentration of thiamine (Good.hart and Sinclair, 1940, Gorham, Abels and. Robbins, 191+2, Blancha.er and Cameron, 191+8, Plorijn and Smits, 1948). Since most of the thiamine in the blood is in the form of cocarborlase, the concentration of this phosphorylated. form of thiamine in blood has been determined. (Goodbart and Sinclair, 1939, Good.hart, 19/+0, Good.hart and Sinclair, 191+0). Since thiamine is essential for the complete metabolism of pyruvic acid and. there is a relationship between pyruvic and. lactic acid, these intermediary metabolites have been determined as an indirect means of detecting thiamine deficianey (Bu.eding and. Wortis, 191+0, Bueding, Stein and Wortis, 1941, hied.emann and. Barborka, 1941, Friedemann and. Haugen 1943, Horwitt, Liebert, Kreisler and Wittman, 4 1948, Horwitt and. ICreisler, 1949). Physiological tests - Perfornce tests, such as strength, work, muscular efficiency, endurance tests and. psychomotor responses have been made (Keys, Hensohel, Mickolsen and. Brozek, 1943, l'farlin, 1944). Archdeacon and. Since these physiological responses have some relation- ship to thiamine nutrition they, too, are indirect means of det8rminlug the state of nutrition with respect to thiamine. The effect of therapeutic doses of thiamine on cardiac syitoms of thiamine deficiency has been studied. electrocardiographically (Weiss, 1938). Estimation of Past Dieta Intake Studies on the dietary intake of thiamine have been used. in the interpretation of clinical and. biochemical data (Benson, Wltzberger and. Slobody, 1941, 1942, Mason and Williams, 1942, Melnick and. Field, 1942, Keys, Hensohel, Miakelsen and Brozek, 1943, liaj jar, 1943, Archdeacon and. Murlin, 1944, Old.hain, Johnston, Kielger and Hed.d.erich-Arisrnendi, 19)44, OlcIham, Roberts and Young, Gifft and. Hauck, 1945, 1946, Hathaway and Alexander and. Lsndwehr, 1946, Strom, 1946, Mickelsen, Caster and Keys, 1946, and Oldham, Ivis and Roberts, 1946). The relationship of thiamine to total Calories and. of thiamine to non-fat Calories have been used as other measures to determine the adequacy of the thiamine intake (Oowgill, 1934, Williams and. Spies, 1938). It seems to be the general opinion that no one test alone is sufficient to determine thiamine deficiency or sufficiency. The consideration of the relationship of one test to another and. of the conditions under which the tests are performed is of necessity a Mason and. Williams (191+2) stress the need of requirement. intelligent interpretation of these various indices. THODS OR TIlE ITERMINAT ION OP INTERI.tEDIARY METABOLITES, THIAMI1E A1 000ABBOXYLA.SE IN WHOLE BLOOD OR BLOOD PRACTIONS Pyru.vic and lactic Acids Thiamine pyrophosphate, or cocarboxylase, is the coenzyme necessary for the normal catabolism of pyruvic acid in the body tissues. Since there is an accumulation of pyruvic acid. in the blood in cases of thiamine deficiency, Bu.eding and. Wortis (191+0) proposed a method for the determination of blood pyrvate which is a modification of the method of Lu (1939). In order to emphasize the presence of the deficient state, some workers have added a 'metabo1ic loads such as exercise. and blood pyruvate both increase after exercise. Blood lactate iedemann and Barborlca (191+1) found that there was a definite ratio between lactic acid. and pyruvic acid in the blood. proposed. a method for Barker and Swnmereon (191+1) have the determination of lactic acid and. Friedom-nn and Haugen (191+3) have proposed a method. for the determination of keto acids in the blood. Baeding, Stein and Wortis (191+1) increased. the metabolic load by giving their subjects an oral dose of glucose. They determined. the pyruvic acid and. the glucose of the blood from subjects in the fasting state and repeated their determinations at intervals after the test dose of glucose was given. They found that in deficient subjects there was a prolonged elevation of the pyruvic acid. content of the blood and. a slightly elevated blood sugar content when compared with values for normal subjects. Ho itt and. Kreisler (19k9) and. Horwitt, Liebert, Kreisler and wittman (l9Li.8) found that in the fasting state and. in the absence of exercise there was no consistent rise in the basal levels of lactic acid. and pyruvic acid in mild. degrees of thiamine deficiency. They combined glucose ingestion and. mild exercise to form a double meta- bolic load. thus providing a still greater strain on the mechanism of carbohydrate metabolism. The authors suggested that early thiamine deficiency could. be diagnosed if the glucose level of the blood was correlated with the lactate and pyruvate levels of the blood when both were determined under the double metabolic load, of glucose ingestion and exorcise. The difficulty with these methods is that so many factors influence the concentration of pyruvic acid, in the blood. The concen- tration of pyruvic acid depends greatly on the degree of physical activity which makes difficult the interpretation of slight changes in the pyruvic acid. content of the blood (Nutrition Reviews, l98). Thiamine in asma and Serum Compared with that of Whole Blood Meiklejobn (1937) found that whole blood has greater growthpromoting activity for Phycomyces blakesleeanus than did plasma. He found that about 20 per cent of the total thiamine was present in the plasma but Good.hart and. Sinclair (1939) reported that this value may be high since any hemolysis which might have occurred. would increase greatly the value obtained for the thiamine content of plasma. irthermore, Gorham, .Abels and Bobbins (l9L2) found that the plasma of a group of normal individuals was substantially free of thiamine. Goodhart and. Sinclair (1939) reported that 90 per cent of the thiamine in the blood is in the form of cocarboxylase and. that all of the blood. cocarboxylase is contained in the blood cells. Benson, Witzberger, Slobody and. Lewis (191+2) stated. that only about 10 per cent of the total blood thiamine is present in the plasma and that this is present as free thiamine. These studies would indicate that very little thia- mine, either free or combined is present in the serum or plasma. For this reason determinations on these blood fractions would not be a satisfactory index of the amount of thiamine in the blood. Therefore, determinations for total thiamine, i.e., cocarboxylase plus free thiamine, must be carried out on whole blood rather than plasma or serum. Thiamine in the Formed Elements of the Bloo4 Goodhart and Sinclair (191+0) indicated. that a mach larger amount of cocarboxylase was found in mrnlian leucocytes than in erythrocytes. Gorbam, Abels and. Bobbins (191+2) adapted the micro-tecbnique of Atkin, Schultz and Frey (1939) and. found in the white blood cells of 30 normal adults a total thiamine concentration of 1+8 to 183 meg per 100 ml or an average of 99.8 meg per 100 ml. The total thiamine in the erythro- cytes of 21+ of the 30 normal adults was 3.7 to 38.0 meg per 100 ml or an. average of 10.3 meg per 100 ml. The average thiamine level for normal white cells was therefore about ten times the amount contained in normal erythrocytes. The authors stated that this was similar to E1 observations on the distribution of riboflavin and ascorbic acid in blood cells. They suggested that the greater concentration of thiamine, riboflavin and ascorbic acid in the white cells may be explained by the fact that of the several blood components, the white cells most closely resemble actively metabolizing tissue. Blanchaer and Cameron in 19148 using the Lactobacillus fermentim method (Sarett and Cheldelin, 19144, Cheldelin, Bennett and. ICornberg, 19146) found 10.8 to 27.6 meg of thiamine per 100 ml of blood cells in 23 patients who exhibited a wide range of nutritional status. !lorijn and. Smits (19148) determined the thiamine pyrophosphate content of red and white corpuscles. They found that the average thiamine pyrophosphate content of red cells of 8 normal men was 1.149± 0.08 meg per 1011 cells and, of 114 normal women, was 1.28 meg per 1011 cells. , 0.09 The average thiamine pyrophosphate content of white cells was 280 145 meg per 1011 cells. Thus, for human beings, Tlorijn and Smits found the ratio of the cooarbolase content of a red. to a white cell was about 1:200. The sex difference was significant for red. cells but not for white cells. Cocarboxylase in Whole iood The cocarboxylase method (GQodbart and Sinclair, 1939, Goodhart, 19140) is based on the fact that thiamine markedly stimulates the decarboxylation of pyruvic acid, by alkaline washed yeast in the presence of cocarboxylase. in Warburg manometers. The evolution of carbon dioxide is measured Since nearly 90 per cent of the thiamine in the blood is in the phosphorylated. form (Goodhart and Sinclair, 19140) this method estimates the major portion but not the total amount of thiamine I.J in the blood. Thiamine in Whole Blood Phycomyces blakesleean'as - Meiklejohn (1937) suggested the use of a mold, Phycomyces blakesleeanus, as a test ornism for the determination of total thiamine since small amounts of thiamine have a growthpromoting activity on this organism. Rowlands and Wilkinson (1938) and Sinclair (1938, 1939) have also used. this method with modifications. It is assumed that the growth of the mycelium obtained with blood. represents the true maximum growth that the thiamine present in the blood. can effect. Sinclair (1938) criticized the method. of Meiklejolrn since the method. is not specific for thiamine. Both thiamine and its breakdown products aid. in the growth of Phycomyces blakesleeanus when both of the products are supplied together. Fermentation - In 1937 (a, b), Schultz, Atkin and Frey noted that thiamine exerts a "powerful action" on the rate of alcoholic fermentation. This rate of fermentation is expressed by the amount of gas evolved in a given time interval. In 1939, Atkin, Schultz and ey modified the test for use with a Warburg apparatus in an atmosphere of nitrogen. The fermentation stimulation is not a specific test for thiamine since 2 - methyl - 5 ethoxymethy3. - 6 aminopyrimidine is also effective in stimulating fermentation (the thiazole group is inactive). The authors suggested that the results be correlated with the animal growth method to check the accuracy of the indirect method for the determination of thiamine in blood.. Knott, Kleiger and Torres-Bracamonte (l9J43) suggested the use of 10 the modified Atkin, Schultz and Prey method in the determination of blood thiamine0 This method was used by Oldham and coworkers in three studies (1) Oldham, Johnston, Kielger and Hed.dericb-Arismendi (19)44) in a study on two 5-year old. boys, (2) Oldbam, Roberts and. Young (194.5) on children 6 to 15 years of age and (3) Old.ham, 1vis and. Roberts (194.6) in a study on young women 19 to 32 years of age. Thlochrome - The thioclirorne method for the determination of thiamine is a chemical procedure and depends on the oxidation of an alline solution of thiamine by potassium ferricyanide which converts the thiamine present to thiochrome, a yellow substance having an intense blue fluorescence. The thiochrome is extracted with isobutyl alcohol which separates it from any physical interfering substances occurring in the sample or from the reagents used in the analysis. fluorescence is considerably brighter in Thiochrome isobutanol than in water so this adds to the sensitivity of the method (Hennessy, 194.7). blood Ritsert (1939) suggested a thiocbrome method for after precipitation of the protein with saturated sodium sulfate at 80 to 90 degrees Centigrade. free thiamine He concluded that in human blood there was only and. no pyrophosphate present. Wideubauor (1939) suggested that Ritsert precipitated the unsplit cocarboxylase - protein comulex with sodium sulfate and this accounted for the fact that the discarded water medium showed no blue fluorescence. Wid.enbau.er suggested the splitting of the cocarboxylase-protein complex by heating serum, water and hydrochloric acid. in a hot water bath for 10 minutes. Honnessy and Cereced.o (1939) suggested a method for the estimation 1]. of free and. phosphorylated thiamine by the thiocbrome method. They suggested enzymatic hydrolysis to obtain all of the thiamine in the free form and the use of base exchange to replace the adsorption technique that had. been used. previously to separate the thiamine from other constituents contained in the medium. (19ii-3). published a (l9L5) Kmiociak method using five ml of blood, that was a simplification over previous Rinehart Friedemaun and thiochrome methods and Greenberg and suggested a further simplification of the thiociromo method. using only one ml of blood. The phosphorylated. thiamine is not measured satisfactorily by the thiochrome method. without preliminary enzymatic hydrolysis. There are fluorescing substances other than thiochrome in biologic material which result in some lack of specificity but Youmaus and. Patton (l9Al2) stated that under ordinary conditions this is not a serious objection to the method. However, they also stated that the method. requires considerable technical care to be used satisfactorily. Ranges of values for the thiamine content 0 whole blood. as determined by the various methods are: Phycomyces blakeseeanus, 6.5 to 16.5 mcg per cent; Fermentation, 2.6 to 7.7 meg per cent; Thiocirome, 2.8 to 15 mog per cent (Table 1). In both the fermentation and. thiochrome methods the lowest values, 2.6 and 2.8 meg per cent are associated with lowered thiamine intake. 12 Table 1 SIJIvINA.RY OF STUDIES Ii - T1 LITERATURE OI THE T1II.AM1EE C0I'ITENT CF BLOOD Thiamhie Inta1 - Goodhart and Sinclair 1939 cocerboxylase Goodhart cocrboxylase Rlood Thiamine Mean Range rncJl0OOQ 26 men mc mc 7.0+2.1 4.5-10 7.0±1.53 S.D. (2o-Mo yrs) 28 boys (5-is 1940 yrs) Powlands and Wilkinson 1938 Phycomyces adults Sinclair 1939 Phycomyces 144 observations on author 6.5-16.5 over period of 3 yrs, various conditions 45 adults (both 8.0-13.5 9.5±1.5 5.5-10.5 7.l4-l.LL sexes) adults Goodhart and Nitzberg 1941 microfermentation 14.5 Knott, Kleiger, TorresBracamonte microfermentation 37 lactating women 3.1- 9.2 fasting blood bloods taken 8 or lO:3O not fasting 5.39 5.01 1 9143 6.i6 15 non-lectating women (caic) OldMm, Johnston, Kleiger and Heddrich-Arismendi microfermentation 2 boys (5 yrs old) fasting blood, 3 months study, 36 periods of 3 days each 19414- 225,250 475,500 39 children (Oct) children (May) (6-15 yrs old) 4-0 fasting blood 3.6 575,575 750,750 775,825 900,875 8.4, 7.1 7.5, 7.7 800,800 microfermentation 4.9, 6.9, 4.8 6.3, 5.3 4-800,48oO OldEam, Roberts and Young 1945 S.D. l4..5._12.O - - - 8.4, 6.5 5.c 7.3 13 Table 1 (continued) J Blood Thiamine Range Mean Authors Oldham, Davis and Roberts 1946 microfermentation 12 women (19-32 yrs) 8 months stidy, Blood tsken at end of each period preliminary (7 days) samples - 59 days 47 days - 295 424 140 200 days 742 937 4900 4914 1027 45 41 days 7 days 7 days 20 days 4.0-6.7 5.2 3.8 360 510 2.6-5.0 3.7-6.7 6.3-7.2 4.8-6.4 540 5.5-6.7 6.2 - - E!ennessy and Cerecedo 1939 thiochrome 9 -12 Ritsert 1939 thiochrome 3 -15 Benson, Witzberger, thiochrome Friedemann and Kmieciak thiochrome 1943 GreenberE and Rinehart 1945 45 children (L_12 Slobod.y, Lewis 1942 thiochrome yrs) fasting blood 1 week cal. ad. lib (1624-3051) 7 women 29 men not fasting blood not fasting blood BJ 3 weeks study high calorie diet beginning 2d & 3d week TF 3 month (3 detns) TF 2 weeks 630-1170 4.8-12.3 3.0-9.2 3.8-11.2 5.3 6.8 5.6 - - 7.81.3 5.60 5.73 1500-1900 6.1 2500 300 4.3-5.5 4.7 7.0,9.9,9. 8.9 2.8 5 -10 - '4 T CTJESTI0N OF THB SIGNIFICANCE OF BLOOD THIAMINE VALUES IN ASSESSING TNE STATE OF TJTRITION WITH BESPEOT TO THAT VITAMIN There are many differences of opinion regarding the value of the determination of thiamine in the blood as an indication of nutritional status with respect to that vitamin. Goodhart and Nitzberg (1941) found in their subjects whose dietary intake ranged. from 898 to 1493 could be found vitamin to between meg of thiamine daily that no correlation urinary values. the thiamine intake, Calorie ratio of the diet or fasting However, they found that there was a peripheral excretion1 definite the blood. levelsof thiamine. association between acute neuropatby of the alcohol addict and. low blood thiamine The incidence of peripheral neuropathy among subjects with low blood thiamine values was so high that the authors stated. that it would seem likely that further work would prove a blood thiamine value below 3.0 meg per cent, by the fermentation method, a indication of a thiamine deficiency definite state. Benson, Witzberger, Slobody and Lewis (1942) carried out a study on 45 hospitalized children who were clinically healthy and known to be in a state of thiamine saturation. Four pre-breakfast blood determi- nations were made during one week. They found that the daily variation in blood thiamine of an individual child did not follow the urinary thiamine output and that it was not proportional to the amount of thiamine excreted or to the per cent of dietary thiamine excreted. in the urine. They suggested that there may be an individual fixed blood thiamine concentration in a thiamine saturated individual which cannot 15 be raised.. later, in 1943, Benson, Witzberger and Slobody compared the above study with blood values obtained on 121 children who were hospitalized, 22 of whom showed iinquestionable evidence of tissue unsaturat ion. cally healthy They found similar blood values for both the 4,5 cliniThey children and the 12]. children in the later study. concluded that the concentration of thiamine in the blood is not indicative of the state of nutrition with respect to that vitamin. Youmans and. Patton (1942) stated that the value of blood thiamine determination in the diagnosis of thiamine deficiency seems to be limited to instances of severe clinical deficiency, e.g., peripheral neuritis. They stated that their investigations indicated that the thiamine content of the course of a deficiency. blood. does not decrease until late in the Lto to these observations they felt that the "estimation of the thiamin level in the blood does not serve as a satisfactory index of thiamin deficiency previous to the onset of outspoken clinical evidence of the disease". Oldham, Johnston, Xleiger and Heddericb-.Arismendi (1944) found in studies on two 5-year old boys that the correlation coefficients of blood. thiamine with fasting one hour excretion, with 4-hour, with 24-hour return of a test dose and with average intakes were 0.58, 0.40 and 0.45, respectively. Old.ham et a].. concluded 0.66, that blood thiamine seems less reliable than excretion tests as a measure of nutritional status. Hennessy and Cerecedo (1939) stated that they found the concentration of thiamine in the blood and in the urine to be of the same magnitude. 16 Knott, Kleiger and Torres-Bracamonte (l913) analyzed Ui samples of milk from 50 women and also determined the blood thiamine of 37 of these women. They found a definite tendency for low milk values to be associated with low blood thiamine values and higher milk thiamine with higher blood thiamine values. Friedemann and Kmieciaic (191+3) stated that the total thiamine content of blood does not fluctuate as widely as urinary thiamine excretion and that it is not as rapidly affected by of thiamine. the dietary intake However, they felt that there was a very definite correlation between the concentration of total thiaminein the blood and. the incidence of deficiency symptoms. One subject on an intake of approximately 2500 meg of thiamine per day had. values of 7.0, 9.9 and 9.9 meg per cent of thiamine in. the blood when determinations were made at monthly intervals. After two weeks during which the subject received a diet containing about 300 meg of thiamine per day the blood level fell to 2.8 meg per cent. In most of these studies determinations of blood thiamine were made on only a few subjects, and, in general, the concentration of thiamine in the blood was followed for only a short period of time. some investigations, the fasting state. In the blood samples were not drawn from subjects in Because of the differences of opinion regarding the significance of blood, thiamine values it seemed of value to carry out a controlled study on the thiamine content of the blood of human subjects: (1) when the subjects were allowed thiamine-containing foods 17 ad. 1ibitui and. the food. values calculated. for that period and (2) when subjects were maintained on a controlled diet, supplemented by two levels of thiamine for 3 weeks each, 18 OE&PTER II PRO CEDtJPE FOR THE EXPERIMENTAL STUDY IN WHICH THE IEDEMA.NN KMIECIAX METHOD WAS USED FOR TEE DETERMINATION Q ELOOD THIAMINE PLAN OF EXPERIMENTS Study I was conducted. on. 5 students from October 22 to November and Study II was conducted on 16, 6 students from January 31 to February 29, 19L8. The subjects wore on 30day studies in. which their intake of ascorbic acid only was controlled. consecutive 10day periods. During period 1 the ]iitum at meals and a record was all foods ascorbic acid each day in order to vitamin C. containing kept of each subject's significant amounts of ascorbic acid, supplement During period 3 recommended allowance 2 the of crystalline ascorbic acid so that equal to the recommended allowance Research Council. to 20 mg This of necessity limited or fruits, cabbage and potatoes. During period were given a intake was than the allowed achieve saturation with respect to of ascorbic acid in their food. such as citrus the were During periods 2 and 3 the subjects were restricted eliminated all foods subjects subjects Daring this period the subjects were also given 200 rng of food intake. or less The study was divided into three the of the of the National subjects were given 10 mg less National Research Council for ascorbic acid. Study I was conducted at Snell Hall, a girl's dormitory on the Campus near the Home Economics building. A special table was assigned 19 Before each meal the research workers for the experimental subjects. The hot foods were weighed out and weighed out all the cold foods. served when the subjects arrived. Study II was conducted at the Memorial Union Cafeteria. The same procedure of weighing and serving foods was carried out there also. SCBIPT ION OF SUBJECTS The subjects were all presumably normal, derately active students who were healthy individuals. Eight of the students were 18-year old Freshmen and. three were adults. A description of the subjects in terms of age, height, weight at the beginning and end of the study, weight range and average weight is given in Table 2. Table 2 DESCRIPTION OP THE SUBJECTS Body Weight Beg. of Study lb End of A1e yr Heibt Norma Barbara Marion Roberta Bessie 18 18 18 18 27 65 1/2 65 65 1/2 117 116 117 111.9 1114 1/LI. 6L4. 1/2 156 1/2 110 151 Tom ve Wesley Dou Keith 18 18 18 73 1/2 73 1/2 1814. 66 18 70 1/2 126 150 27 30 67 1113 624. 153 1/2 Subject Ru.th in 62 1/2 Study lb 1/11. Average Bane Wei,g lb lb 117 116 109 3/24' 115 1/4-118 1/14. 117 1/2 ll4 1149 1411. -156 1/2 151 108 3/11-lu 186 183 183 178 186 182 128 1/2 126 1/4-131 153 1116 3/24*153 3/14. 151 1115 1111 l/2_1L1.5 1/2 11421. 124.2 1/2 121.1 1/2-153 1/14. 116 1111. 1/2 178 1/2 Weight 1/14. -189 185 1115 153 110 129 20 GEIRAL DAiLY PRO OEDTJEi The subjects were given a general direction sheet so they could acquaint themselves with the procedure and what was expected of them. All subjects were very cooperative in eating all the food served and adhering to the necessary restrictions imposed upon them. A copy of the directions given in Study II is found in. Appendix I. The daily procedure was the same throughout the study. The subjects reported to the nutrition research laboratory at 6:Li5 A.M. They weighed themselves (without shoes) daily and recorded. their weights Five on a record sheet posted near the scales inside the laboratory. ml of blood were taken by vonipuncture every 5th day for the determination of the amount of thiamine in the blood. The subjects reported to Snell Hail (Study I) or to the Memorial Union (Study II) at meal times when all the foods were weighed out for them. spring balance was used. for weighing the foods. the food intake of each of the subjects. as possible. A Ohatillon A record was kept of Mixtures were avoided as much Pits or rin.&s were removed from fruit. Bone and. gristle, and some fat if necessary, were removed from the meat so that the edible portion only was weighed. Eggs were weighed in the shell and then the shells were weighed back and. subtracted to obtain the weight of the edible portion. Values were calculated for protein, fat, carbohydrate and. thiamine content of the foods. 21 CRAPTER III KMIEOIAX 1ETH0D ANALYTICAL PROCEDURE FOR THE FRIEDEMA11I( EQUIPMENT USED The method. of Fried.omann and. Kmieciak (].9M.3) was followed. for the determination of thiamine in. the blood. The eauipment used for this procedure was as follows: 1. 5 ml hypodermic 8yringee 2. 20 and 22 gauge 3. Oxalated. tubes prepared by adding by burette 0.1 ml of 2% lithium oxalate hypodermic for needles each ml of blood taken. dried aro'und. 60 degrees Centigrade. The tubes were Too high a temperature dioxide from the oxalate. will drive off carbon Non-protein-nitrogen tubes (graduated at 25 and 50 ml) 5. Footed stirring rods - long rods were made with a 0penny" shaped end. that fitted the bottom of the type of rod. the contents of the PN tube. With this tube were agitated violently by rapidly pulling the rod. up and. down in the 1N tube. 6. Boiling water bath 7. Incubator 8. Large centrifuge and cups 9. Centrifuge tubes - heavy-walled, 90 ml and. 50 ml capacity 10. Erlenmeyer flasks - 150 ml capacity 11. Stop watch 12. Set-up for suction 22 13. Oapil1ay tube for use with suction 11+. Coleman photofluorometer 15. Optically matched. cuvottes for the Coleman photofluorometer 16. Hennessy adsorption columns 17. Thiamine reaction vessels (Wilkens-Anderson Co., Chicago) 50 ml capacity 18. Syringe pipettes to hold. 10 ml and 20 ml hypodermic syringes (Northern Tool and Instrument 00., Flushing, N. L) 19. Automatic timer 20. Volumetric pipottes - 2, 21. Graduated pipettes - 2, 5, 10, 25 ml 22. 25 ml graduated 5, 10 ml cylinders, with, glass stoppers. REAGEITS USED The reagents used, in the Fried.emann and Kmieciak method. were the following: H01 1. Approximately 1.0 2. Approximately 1.0 4 NaH003 *3 10% metapbosphoric acid, prepared fresh each day. in cold water. *Lf. Disso1ed kept In refrigerator. Phosphatase - io% Clarase 300, prepared. daily. If the clarase thus prepared was not clear, it was either filtered. or centrifuged.. 5. Ion exchange adsorbent - 1 kilogram of Decalso (artificial zeolite) was covered. and. mixed with four changes of acidified. * Reagents prepared daily 23 solution of NaOl. (2 ml concentrated EC1 per liter) 25 When ordinary table salt was used in Study I the solution was filtered bofore the addition of acid. removed by decantation each time. The supernatant was The zeolite was washed five or more times by decantation with acidified distilled water (1 ml glacial acetic acid per liter). Washing removed the finer material as well as the excess salt. The Decalso was then transferred to a large Buchner funnel and washed well with distilled water and dried at room temperature in. a shallow pan. To have the Decalso in the maximum expanded state a small portion of the activated Decalso was kept motstened in a wide mouth bottle. 6. Sodium chloride solution - 250 grams of 0. P. NaCl plus 2 ml of concentrated HOl were diseolved and diluted to one liter. According to riedomann and Kxnieciak (l9L3), salt solutions which are prepared from ordinary table salt, contain materials which slightly reduce the yield. of thioohrome. When table salt was used. (in Study I) the solution was filtered before acidification with HOl. *7. Oxidizing reagent - mixture of nine parts of 10 part of 1% potassIum ferricyanide. HaOH and. one This mixture was freshly prepared each day. 8. Iso-btityl alcohol - with a fluorescence no greater than distilled. water. If the alcohol contained. much fluorescing material it was acidified with concentrated HC1, dehydrated * Reagents prepared daily 2J4 The distillate with anbydrous sodium sulfate end distilled. which came over between 106 to 108 degrees Centigrade was collected and. used. in the extraction of thiochrome. Used alcohol was poured into a bottle containing excess HOl and. sodium sulfate and this was later rediatilled. All new iso- butyl alcohol was redistilled. collecting only the 106 to 108 degree boiling fraction. 9. quinine sulfate stock standard - 100 mg quinine sulfate, U.S.P., made up to one liter with approximately 0.1 acid. bottle. This was stored in the refrigerator in an amber-colored. A solution kept in this manner should be stable for at least one year (Friedemaim and Kmieciak, 10. sulfuric l9i4.3). quinine sulfate intermediate standard. - 25 ml stock standard diluted to 100 ml with approximately 0.1 sulfuric acid. This was stored in the refrigerator in an amber-colored bottle. The intermediate standard was made up new about once a week although it probably would. have been stable over a considerably longer period of time. *11. quinine sulfate working standard - exactly 5 ml of the intermediate standard were diluted to 500 ml with approximately 0.1 N sulfuric acid. This solution was prepared each day. Until used the working standard. was protected from the light by storing in the desk in the dark. 12. Thiamine stock standard - exactly 50 mg of anhydrous thiamine hydrochloride (dried several weeks over sulfuric acid in a * Reagents prepared. daily 25 desiccator) were dissolved in 25 per cent ethyl alcohol in approximately 0.01! H01. When brought to a voimme of 500 ml with this solution each ml contained 100 meg of thiamine hydrochloride. If kept cold thia stock solution should be stable at least six months (Fried.emann. and Kmieoiak, l93). Thiamine intermediate standard - 2.5 ml of stock solution 13. were made up to a volume of 250 ml with approximately 0.01 N HOl. One ml contained 10 meg of thiamine hydrochloride Thiamine working standard. - 5 ml of the intermediate solution were diluted to 250 ml with approximately 0.01 ml of the working standard contained 0.2 meg of HOl. One thiamine hydrochloride. Indicator - brom phenol blue. 15. Five drops of a 0.L4. per cent solution were used. Os.prylic alcohol. 16. TERNIWION OF THIAMI1B IN TI BLOOD Collection of Blood Sa1e Five ml of blood were collected by venipucture in. a hypodermic The blood was delivered into an oxalated tube and. shaken syringe. vigorously until the dark red venous blood. bad. changed to a bright red color. One drop of eaprylic alcohol was added to reduce foaming. Since the thiamine content of the blood gradually changes even in the cold, it is necessary to perform the analyses without delay. For this reason, the thiamine analyses were begun as soon as the samples were measured. Peciitation Digestion with Phophatase 1. xactly t Protein 5 ml of blood were delivered into a large heavy- walled test tube (1'N tube) containing 25 ml of distilled easier water (if the blood was cooled slightly it was pipette since fewer bubbles were produced in to pipetting). The 5 ml volumetric pipette was rinsed by drawing up the waterblood mixture and blowing it out into the tube. This was repeated five times. 2. The contents Two ml of approximately 1.0 ! HO]. were added. of the tube were mixed with a footed glass rod. kept in the tube rntil motaphosphoric acid 3. the The rod was protein was precipitated with as described in paragraph 8. The tubes were heated 10 minutes in a boiling water bath and the contents stirred frequently. Lj. The tubes were cooled by placing in a pan of cold. water. drop of caprylic alcohol was added. One 1 to 2 ml of approximately 1.0 j NaBCO3 was added slowly with stirring. The volume of the NaHCO3 must be sufficient to bring the pH between 14.5 and 5.5. In this study 1.3 ml were used to produce a pH close to 5.0. 5. exactly 2 ml of the clarase solution were added. 6. The tubes were then kept 1.5 honre in a water bath at 140 to £5 degrees Centigrade. by stirring. We The contents were mixed every 15 minutes placed the tubes in a pan of water which was between kO to 145 degrees Centigrade and put the pan in an incubator to maintain the tubes at that temperature range. The temperature was checked each time the tubes were mixed to be sure the incubation temperature was being maintained. 7. The blanks were prepared by order indicated. adding the same reagents in the These were incubated and. carried through exactly the same procedure as the blood 8. samples. Ten ml of io% nietaphosphoric acid were added with a syringe pipette. The volume was adjusted to 50 ml with distilled water and the contents were mixed. This mixture was poured into a centrifuge tube and centrifuged 15 minutes. supernatant was poured off into a 150 ml The rlenmoyer flask. Since, according to Friedemann and Kmieciak (19)43), the precipitate may contain some adsorbed thiamine which may be recovered by re-extraction, 2.5 ml of metaphosphoric acid were added to the N tubes and the volume again brought to 50 ml with distilled water. The contents of the tube were transferred to the centrifuge tube containing the precipitate and mixed well. This process rinsed the NPN tube. The rod was rinsed with distilled water and the washings allowed to flow into the centrifuge tube. The tube was centrifuged again for 15 minutes and this supornatant was added to the same Erlenmeyer flask so that the final volume was about 100 ml. 9. five drops of brom phenol blue were added to the Jrlenmeyer flask containing the combined extracts. adjusted to pH 3.0 to 3.6 with 1.0 The contents were NaHCO3. To check on. the colcr and pH, the pH of one flask was checked with the Becan pH meter and then all the other samples adjusted to that color. At this point the solutions were stored in the refrigerator overnight. Adsorption and. Elutio 10. The solutions were removed from the refrigerator and the color noted to be sure that there had been no change in pH. 11. Preparation of adsorption columns - these were made up ahead of time, usually the night before, and kept in a beaker of distilled water to cover until ready for use. A plug of glass wool was pushed into the bottom of a Honnessy tube. The tube was filled with distilled water and the Decalso added, a small amount at a time, while the tube was held at a slight angle. Air bubbles were avoided. Decalso was added until an excess, forming a layer several mm deep, was present in the bell above the column. The adsorbent is more uniformly packed when floated into place this way. The excess Decalso in the bell prevents clogging of the column by solid or colloidal material. This also prevents access of air into the column provided the drained column is not jarred (Friedemann and Kmieciak, l9Ll3). Just before ready to use, the adsorption column was placed in a holder (spring typo broom holders were used) fastened on a shelf above the desk and allowed to drain. The extract was passed through the adsorption column. 12. The 150 ml Erlenmeyer flask was rinsed. 3 times with 10 ml portions of distilled water and the washings passed. through the adsorption column. The column was allowed to drain 29 completely after each washing. The bell of the column was then washed once with 5 ml of distilled water and the column again allowed to drain completely. 13. Three ml portions of the acidified NaOl solution were added until exactly 25 ml were eluted into the 25 ml graduated cylinders. Oxidation to Thiochrome Two 10 ml aliqu.ots of the eluate were transferred to thiamine reaction vessels of 50 ml capacity. 15. Five ml of the oxidation mixture were added with a syringe pipette. Thia oxidation mixture was added rapidly to produce mixing. 16. Thirteen ml of iso-butyl alcohol were added immediately with a syringe pipette. 17. Thiamine reaction vessels were stoppered and shaken vigorously for 90 seconds. 18. The reaction vessel was centrifuged 2 minutes at slow speed to separate the layers. The lower layer was drawn off by means of a capillary tube attached to suction. 3y passing the capillary tube rapidly through the upper layer the lower layer was easily removed with negligible loss of the iso-butyl alcohol layer. 19. Three to four 0.2 were added. solution gram samples of anhydrous sodium sulfate The first addition entrapped the remaining at the bottom and the addition of more sodium sulfate removed the water from the alcohol phase. The tube was shaken 30 gently and centrifuged 1 minute. If the alcohol layer was not crystal clear more sodium sulfate was added and the reaction vessel recentrifuged. 20. The clear iso-butyl alcohol layer was poured into a matched optically clean cuvette. The fluorescence was measured in the Coleman photofluorometer against the quinine sulfate working standard set at 70. 21. The thiamine content was determined from a standard curve representing the increase in fluorescence after the addition of 0.2, 0.6, 0.8 and. 1.0 meg of thiamine hydrochloride to blood samples which were analyzed by this procedure. That is, to 5 ml blood samples were added 0, 0,2, 0./+, 0.6, 0.8 or 1.0 mog of thiamine hydrochloride. These were carried through the procedure after which the value for blood thiamine only was subtracted to give the fluorescence due to the added thiamine. A new standard curve was set up for each study just before or during the course of the study. DTBMflATI01 OP STJDABD CURY During the preliminary work standard curves were determined by 3 different methods: 1. Pure thiamine solutions were carried through the oxid&tion procedure only. 2. Pure thiamine plus blood minus the thiamine of the blood alone: In this method, 25 to 30 ml. of blood were collected and known 31 amounts of standard thiamine solution were added to 5 ml samples of blood. method. These were carried through the entire The reading for blood thiamine alone was subtracted. from the reading of blood plus thiamine to give standard. (This curve was the one finally used in curve figures. calculating blood thiamine). 3. Pure thiamine treated as blood: carried through the entire The sample Figure 1. Pure thiamine solutions were procedure. readings minus blenk readings are given in Table 3 and. There was a than greater similarity between curves 2 and 3 between curves 2 and 1 since the entire procedure was not followed to obtain the results for curve 1. Table 3 RESULTS OP TH DETEBMINAT ION OF STARflARD OURVES FOP. THIAMINE BY THREE DIFFERENT METHODS Sample reading - blazk reading 2 3 Pure thiamine Pure thiamine Pure thiamine treated the same + blood - blood as blood thiamine 1 Mcg thiamine 0.2 J4..75 6.09 10.75 10.78 9.50 0.6 18.00 16.50 lLf.50 0.8 25.25 20.60 19.00 1.0 3A4..0O 2.lO 2L.13 32 STANDARD CURVES FIGURE 1. £1 (0 h F I 'I II 1 II a, 'I II 'I I 0.1 / / II 07 / / / 1 / / II Ii II -..1 '' ,/ II I, / , / / / 4j Qs CVRVE 1: / I 1,7 THIAP/INE HYDROCHLORIDE / 'I C(/RVE 2: 1,'! (THIAMiNE HYDROCHLORIDE + BL 000) -BL 000 THIA Il/N E /7 0.2 -----C(IRVE3: THIAMINE HYOROCHLOR IDE TREATED AS BLOOD 01 00 0 5 10 15 20 25 30 O8SERVED READING - BLANK READ/N& 33 In calculating results on blood alone, the reading for the sample minus the blank reading was read. from the standard curve and. the resulting figure multiplied by 20 to obtain mcg of thiamine per 100 ml of blood. For example, if one obtained. a uluorometer reading of 10.5 (which is the reading for the sample minus the reading for the blank) then the concentration of thiamine as read from the curve was 0.39 meg of thiamine in 5 ml of blood or 7.80 meg of thiamine in 100 ml of blood. In a personal communication, Dr. Friedemann stated that be calculated his values in the same manner as above. 3A1. CEAPTIJR I'V ThiEDEMA1N RESULTS AND DISCUSSION OF TUE STUDIES IN WHICH AND ICMIEO IAN }EIETHOD WAS USED FOB TEE DETERMINATION ,Q BLOOD THIAMIUE The daily values for total Calories, non-fat Calories and. thiamine See the footnote in. in the diet were calculated from food tables. Table 28 in Appendix II. Non-fat Calories were calculated by u1ti- plying the number of grams of protein and. carbohydrate in. the food. These daily values, in. addition. to the ratio of non-fat Calories by i. to total Calories, thiamine to 1000 fat Calories are given in Table 28 Calories and. thiamine to 1000 non- in Appendix II. The amount of thiamine in the blood was determined every 5 days. A summary of results for both Study I and Study Table A+ and. Figures 2 and 3. The blood thiamine value for each period II are given in refers to the determination of blood. thiamine from a fasting blood sample taken the morning following that period, for example, on February 9, 5 ml of blood were taken by venipimcture and. analyzed for thiamine content in order to determine whether or not the previous 5-day (February L through February 8) dietary experience had influenced. the concentration of thiamine in the blood. The blood thiamine values L.9l to 10.85 mog per cent. for the subjects in. Study I ranged from These subjects were all girls. From the beginning to the end of the experimental period, there was a loss in blood thiamine for all of the subjects in Study I. There was also a decrease in the mean intake of thiamine expressed in terms of mcg per 1000 Calories and meg per 1000 non-fat Calories as shown in. Table k and. Table 4 THE CONCENTRATION OF THIAMINE IN THE BLOOD IN RELATION TO THE MEAN INTAKE OF TOTAL AND NOR-FAT CALORIES, RATIO OP TOTAL TO NON..FAT CALORIES, THIAMINE INTAKE IN TERMS OF MCG PER DAY, MOG PER 1000 CALORIES, MOG PER 1000 NONPAT CAL RIES FOR EACH GROUP OP 5 DAYS FOR AN EERIMENT.AL PERIOD OP 30 DAYS Study I Thiamine Inta Ca1oio_Intake 1te Subject Total NPC Ratio of NPC to Total Cal 1947 lorma arbara 61 6 Oct. Oct. Oct. Nov. Nov. Nov. 18-21 22-26 27-31 1 - 5 6 -10 11-15 2195 2468 2201 2063 2472 2500 13324. 1324 1524 1559 64 62 Oct. Oct. Oct. Nov. Nov. Nov. 18-21 22-26 27-31 1 - 5 6 -10 11-15 2163 2487 2302 2244 2728 2571 1356 1639 1509 63 66 65 114.34 64 1697 1599 62 62 1599 1410 6/+ 63 Per 1000 Per Thay Ca]. Per 1000 NYC mog Thiamine In Blood meg og meg 1205 1228 1191 1026 1161 1039 546 518 545 499 478 417 901 788 849 9.57 9.76 77/+ 7.52 7.72 6.12 1236 1323 1282 1134 1239 1041 570 550 558 510 461 412 911 823 773 665 85/4. 79/i. 737 654 8.814. 9.40 9.93 7.12 6.92 6.12 4.91 Table 4 Study I (continued) te Subject Caloric_Intake Ratio of NPC to Total Cal Total O 1947 larion Roberta Beesie Oct. Oct. Oct. Nov. Nov. Nov. 18-21 22-26 27-31 1 - 5 6 -10 11-15 1971 2244 2179 2056 2539 2339 1216 1493 1425 1344 1600 1481 62 67 66 6 63 Oct. Oct. Oct. Nov. Nov. Nov. 18-21 22-26 27-31 1 - 5 6 -10 11-15 2110 2275 2192 2062 2542 2449 1280 1461 1437 1322 1589 1524 61 Oct. Oct. Oct. Nov. Noy. Nov. 18-21 22-26 27-31 1 - 5 6 -10 11-15 2191 2374 2137 2099 2377 2220 1306 1508 1388 1329 1531 1396 60 64 65 64 64 65 611. 63 6 64 65 61+ Thiamine Intake y Per mcg 1100 1148 1224 1015 1192 929 Per 1000 Per 1000 Gal NJ'O mog meg 531 565 495 476 904 787 861 755 750 634 10.85 9.93 9.03 5.47 6.72 6.32 9.93 10.12 8.09 9.02 8.28 5.91 9 93 9.57 6.92 7.12 7.52 6.72 1+08 1125 1225 1252 1062 1260 971 534 1+04 887 540 876 801 799 640 1136 1176 1168 1031 1231 895 519 504 872 791 550 572 514 504 51+9 81+6 493 528 776 80? 647 411+ Thiaaino In Blood meg 0' Table 4 (continued.) Stu4 II Oa]jrie_Int-ke Ratio of ]te Subiect IWO to Total IWO Total Cal 1948 Tom Per 1000 Per 1000 Cal IWO meg meg meg meg 2225 538 513 448 911 10.35 12.00 12.27 Per 1y 4103 2449 60 Feb. 14 - 8 14.54.5 4.912 59 59 2303 Feb. 9 -13 2698 2873 Feb. 14-18 Feb. 19-23 Feb. 24-28 4942 4632 4351 2858 2812 58 61 1449 776 14.51 7144 25144 59 2216 2082 1838 427 728 63 61 57 Feb. 114-18 14.999 2537 2788 2991 2889 Feb. 19-23 Feb. 24-28 5158 4572 3014.1 2578 58 59 56 2290 2280 2242 2218 2228 1738 553 Feb. 9 -13 4082 4636 5298 382 892 823 745 768 739 680 Jan. 31 Jan. 31-Feb. 3 3671 2215 61 2015 546 908 - 8 9 -13 3943 4363 2376 2504 60 57 1898 1865 797 752 Feb. 14-18 Feb. 19-23 Feb. 24-28 3909 2282 2280 2192 59 59 59 1678 1722 1429 481 431 4.30 Feb. 14. -8 Feb. 4. Feb. Thiamine In Blood 9.30 Jan. 31 Jan. 31-Feb. 3 Jan. 31 Jan. 31-Feb. 3 iesley Thamine_Intairs__________ 2194. 861 762 8.00 11.18 13.08 8.70 384.7 3709 496 4.23 444 1432 4448 388 737 767 657 8.0 8.314. 10.00 7.32 9.30 12.00 9.00 10.32 11.01 11.16 8.70 10.32 15.32 Table 4 Study II (continued) ect .te Caloric_Intake Ratio of NYC to NYC Total Cal Total Jan. 31 Jan. 31-Feb. 3 - 8 Feb. Feb. 9 -13 2638 3630 Cal meg meg 1474 1675 1949 2097 2105 1626 595 61 59 58 58 60 60 1597 63 63 62 63 979 863 696 736 723 678 15144 59 2129 2601 59 4141J4. Feb. 114-18 14.187 214.75 Feb. 19-23 Feb. 24-28 4375 3932 2631 2280 2868 2261 3158 3204 3294 3087 1750 1606 1778 1695 1588 1454 1663 1012 1109 1041 1003 930 1106 14. eith Jan. 31 Jan. 31-Feb. 3 Feb. 14. * 8 Feb. Feb. Feb. Feb. Ruth 9 -13 14-18 19-23 24-28 Jan. 31 Jan. 31-Feb. 3 Feb. 14. - 8 Feb. 9 -13 Feb. 114-18 Feb. 19-23 Feb. 24-28 123 1830 1853 1991 1858 Per 1000 Per ]y 19148 on Thiamine Intake 59 59 60 58 10214W 1227 1294 1280 1156 Thiamin In Bloo Per 1000 NFO mog mog% 1481 1025 783 755 852 800 14.16 720 10.70 10.70 10.85 9.30 9.15 11.01 14.20 56 450 388 405 388 923 767 670 699 649 626 9.30 11.01 9.00 9.68 8.00 11.32 13.93 14.63 1I45 502 378 8.70 614. 67 4114. 961 802 671 471 525 471 764 825 618 599 504 9.O 10.00 11.00 8.35 13.93 L U, FIGURE 2. IIEAN 5-DAY TH/Af1/NE INTAKE AND BLOOD TH/At1INE VALUE5 FOR 5 SUB JECT5 IN STUDY t 4- -C'-) OCT 1MEAN NOV DITES OF EXPERIPIENT -OAY TN/A1INE IWTAKE FOR PERIOD I tA) "0 F/CL/RE 3. IIEAN 5-DAY 77-/IA/I/NE INTAKE AND BLOOD TH/A11/NE VALUES FO/ 6 .SLJ5JEC TS IN STUDY 1T -.- 9 JAN FEB DATES OF EXPERItIENT 4(IEAM u-DAY THIAflINE INTAKE FOR PERIODI Figures 2 and 3. The average values for thiamine intake for Norma and Marion for the last three 5day periods, November 1 through 5, November 6 through 10 and November 11 through 15, were below the National Research Council recommended allowance (1948) of 500 meg per 1000 Calories. Barbara's intake was below the recommended. allowance for thiamine during periods November 6 through 10 and November 1]. through 15. The same was true for Bessie during the periods November 1 through 5 and November 11 through 15 and. for Roberta in the last period only, November 11 through 15. There appears to be a relationship between the loss of blood. thiamine and. the decrease in thiamine intake in terms of meg per 1000 Calories in 4 of the 5 subjects as can be seen in Table 5. table 5 THE 10RESE OF MEAN VAItIES FOR THIAMINE IN TEE BLOOD, DECREASE OF THIAMINE PER 1000 CALORIES AND DECREASE IN THIAMINE PER 1000 NONFAT CALORIES DURING STTJDY I Bessie 3.21 105 225 Norma 3.45 129 236 Roberta 4.02 130 247 Barbara 4.49 158 257 Marion 4.53 149 270 42 The relationship is even more marked. between the loss of blood thiamine and the decrease in thiamine intake in terms of mcg per 1000 non-fat Calories. That is, the greater the decrease in thiamine intake in terms of mcg per 1000 non-fat Calories the greater is the loss of blood thiamine. Williams and. Spies (1938) have suggested that the ratio of thiamine to non-fat Calories is a more accurate index of the adequacy of the intake of thiamine than the ratio of thiamine to total Calories. The ratio of thiamine to non-fat Calories appears to be the one most closely related to the values for thiamine in the blood. for the subjects in Study I. The Food and. Thitrit ion Board of the National Research Council (1948) recommends that for adolescents 30 to per cent of the total Calories should be provided in. the form of fat and for adults 20 to 25 per cant of the total Calories should be provided in the form of fat. This was met or exceeded in all cases as may be seen in Table 2 in Appendix II. As may be seen in in Study I Figure 2, blood stayed in. similar thiàmine values for each subject relative positions, e.g., Marion had the highest blood thiamine value at the beginning of the study and. next to the highest at the end of the study even though she lost the most in blood thiamine during the 30-day period. Bessie was next to highest at the beginning of the study and the highest study. at the the end of the Barbara had the lowest blood. thiamine value at both the beginning and end of the study. The boys in Study II bad higher values for thiamine in the blood. than did the girls in Study I. The values for thiamine in the blood for the boys in Study II ranged from 8.00 to 15.32 meg per cent. The one girl in Study II had blood thiamine values ranging from 8.35 to 13.93 meg per cent. The results for the subjects in Study II do not indicate the 88 relationship, as do those in Study I, between thiamine intake per 1000 Calories or thiamine intake per 1000 non-fat Calories and thiamine in the blood as can be seen in Table 6. There was an increase in the concentration of the thiamine in the blood even though there was a decrease in the mean thiamine intake from the beginning to the end of the experiment. Table 6 THE INCREASE OF MEAN VALUES FOR THIAMIHE IN THE BLOOD, AND DECREASE OF TRIAMIHE IETAXE PER 1000 CALORIES AIW DECREASE OF THIAMIHE INTAXEPER1000 NON-FAT CALORIES DURING STUDY II 3.30 171 212 Don 3.50 179 305 Tom 3.78 11]. 183 Keith Jf.63 297 178 Rith 5.23 128 343 Wesley 6.32 158 251 ve When the average values are considered, Tomts intake was wellabove the recommendEd al1oance of thiamine per 1000 Calories in all periods. The intakes of Dave, Wesley and Don wore above the recommended allowance for thiamine in. all periods except that from 28. through February bruary 2k KeIth's thiamine intake was somewhat lower than the recommended allowance in all periods except the first one. general, all the boys except Keith Ingested In much more liberal amounts of thiamine with respect to total Calories than did the girls in. Study I. Since the recommended allowance for thiamine provides a 100 per cent margin of safety and the subjects in Study II in many eases ingested higher amounts than the recommended aflowanoe, it woild appear that they obtained sufficient thiamine throughout the 30-day period. Ruth lost 11 3/Li. pounds on the 30-day study as a result of voluntarily ingesting an average of less than 1800 Calories per day throughout the study. Her mean intakes of thiamine from food were only slightly below 500 meg per 1000 Calories, the recommended allowance. In addition, some of her energy reqiirement must have been met from body stores of fat. Since Ruth was not maintaining weight the amount of body fat metabolized would need to be considered along with eaten. the food. This would. change appreciably the ratio of thiamine to Calories and. would tend. to result in a "sparing action on thiamine and may be a partial explanation for the increase of thiamine in. the blood despite a lowered thiamine intake. 4.5 CHLPTER V PROCEDURE ICR TEE EXPERIMENTAL STUDY IN WHICH IT WAS PLAN1ED TO USE TEE BURCE METHOD FOR THE DETERMINATION 01 BLOOD THIAMXRE PLAN OF TEE STUDY There are few data available concerning the concentration of thiamine in the blood and. no data have been obtained which indicate whether or not there are variations from day to day in the concentration of thiamine in the blood when subjects are maintained, on a constant intake of thiamine. The Friedernann and Kmieciak method. requires 5 ml of blood obtained by venipuncture and the method takes 2 days to complete the analyses. This of necessity limits the frequency with which determinations can be made both from the standpoint of time to carry out the analyses and the objection to frequent venipunctures. Because of the above reasons it seemed of interest to carry out analyses using an unpublished micro-method. that has been developed by Dr. Helen Burch (1948). This method has the advantages of using only 50 cmm of blood obtaifled from a finger puncture and the fact that the analysis can be completed in one day. A metabolism study1 using human subjects was planned in order to determine the daily values for thiamine in the blood when subjects were maintained. on a controlled diet over a period of 52 days. The study in which the Burch method was attempted. involved. analyses 1 For the support of this research, appreciation is expressed to the Research Corporation of New York City for a grant from the Williams-Waterman Fund for the Combat of Dietary Diseases. of both blood and urine so that the responsibility of the subjects included (1) (2) the eating the diet prepared for them in the laboratory, collection of 214-hour urine samples, (3) providing fasting blood samples by finger puncture each morning and (Lf) taking ments of crystalline thiamine and ascorbic acid. supple- A copy of the directions given to the subjects is given in Appendix III. In general, the subjects arrived at the nutrition research laboratory at 5:45 A.M. each day. The subjects weighed themselves (without shoes) and. recorded their weights in the research book provided for that purpose. The individual voidings and total volume of urine excreted. in the preceding 214-hours were recorded iu addition to menstrual period, number of bowel movements, unusual exercise or any observation that might aid in later interpretation of the data. The 24-hour period for the collection of urine began aster the first voiding on the first day and included the first voiding on the next day. Twenty-four hour urine specimens were collected each day throughout the experimental studr. Each voiding was divided so that one-half of the total volume could be kept without a preservative and. to the other half of the volume a preservative (io% by volume of metaphosphoric acid, in 2 sulfuric acid.) could. be added. All of the urine samples were kept in the refrigerator or in the cold. and were analyzed on the day following the collection. The preserved. urine was analyzed for the excretion of thiamine and ascorbic acid., and the unpreserved urine was used. for the croatinine and bomogentisic acid analyses. Fasting blood samples were taken by finger puncture for the L17 analyses. The hand. was warmed under running warm water, dried and the finger punctured deeply enough by means of a Bard-Parker blade to obtain 3.5 ml of blood to provide a sufficient volume of blood. for the analyses. These analyses included the determination of thiamine in the blood, hematoorit, total and reduced ascorbic acid in the serum and. plasma by two different methods and the determination of the ascorbic acid. content of the white-cell-platelet layer. For all of the above determinations it was essential that the punctured finger was not squeezed except, if necessary, for the collection of serum. Supplements of crystalline ascorbic acid and. thiamine hydro- chloride, dissolved in water, were taken after all of the blood samples were obtained. laboratory since Breakfast was served at tables in the nutrition research all of the subjects were busy in the laboratory in the morning. carrying out analyses Lunch and. dinner were eaten in one of the dining rooms in the Home Economics building. Even though the same foods were eaten each day over a period of 7 weeks the foods were eaten readily by all of the subjects. They maintained good general health throughout the study and no subjectl.ve symptoms of deficiency were noted. EXPERIh4ENTAL DIET The experimental diet consisted. of a basal diet with additions to it planned in units. The plan was to set up a basal diet providing approximately 1000 Oalories and 300 mcg of thiamine and. a unit to provide approximately 500 Calories and 150 meg of thiamine. The determined values for the basal diet (Table 7) provided 1021 Calories and 305 meg of thiamine and. each and. 153 meg of thiamine. unit (Table 7) provided 513 Calories Yor the sake of clarity these diets will be referred to using the approximate values. The values for protein, fat, carbohydrate and. total Oalories were Nonfat Calories (NYC) wire calculated by obtained. from food tables. multiplying the total number of grams of protein and carbohydrate by 1+. The foods were bought in case lots at the beginning of the study in amounts sufficient to last The biscuits and. cookies (Table 8) through the entire experimental period.. were prepared. using unenriched. flour. Samples of the foods were analyzed for their content of thiamine and ascorbic acid. The method of Hennessy and Cerecedo (1939) as modified. by Merck and Company (191+1) was used for the analysis of thiamine and. the method of Loeffler and Pont ing (191+2) was used for the analysis of the ascorbic acid content of the food.. Studies on ascorbic acid the thiamine study. metabolism were carried. out along with It was planned that the subjects would be main tained. on a constant intake of 25 mg of ascorbic acid. per day through out the experimental period.. ascorbic acid. The basal diet provided 9.16 mg of A daily supplement of 15.81+ mg of crystalline ascorbic acid was given to provide a total of 25 mg of ascorbic acid per day. The subjects were told. that they would be allowed 3 days to decide on the level of Calories that they would choose to eat. Three of the subjects started at a level of 2500 Calories, i.e., the basal diet plus 3 units, and 2 subjects started at a level of 2000 Calories, i.e., 49 Table 7 COQOSITI0N OF BASAL DIET C00SITI0N OF tflIT ADDED TO BASAL DIET gin gui gui gin Ascorbi Total Non-fat Ascorbi acid mg Total Non-fat tProtFatCarb0alQTAineS mcg gui gin gin meg gin mg Milk, evap.1 100 7.0 7.9 9.9 139 68 21 0.03 Biscuits4 55 3.0 7.8 21.0 166 96 12 0 Oarrots, canned3 100 1.0 0.3 7.6 37 34 14 3.1.12 Cookie4 48 2.11 9.3 215 131 11 0 &ef, round' 100 19.3 13.0 0 194 77 36 1.26 Bu.tter1 10 0.1 8.1 0 73 0 0 0 Pears, canned' 100 0.2 0.1. 18.4 7 74 7 0.16 Sugar, white1 0 0 5.0 20 20 0 0 Pruxies, dr., stewed2 100 1.7 0.5 53.3 224 220 51 3.23 Wheat 2.5 1.0 5.0 39 30 130 0 100 1.0 0.1 3.3 18 17 46 1.06 8.0 26.2 61.4 513 277 153 0 108 105 11 0 85 29 32 0 Gr. beans, canned3 Cream of wheat1 30 3.5 0.3 22.8 Eggs, E.P.3 54 6.9 6.2 0.L Cheese, cheddar1 30 7.2 9.7 0.5 118 31 9 0 6 1.5 0.6 3.0 23 18 78 0 49.3 38.7119.2 1021 673 305 Wheat germ' 1 2 erm1 5 10 9.16 Values from U.S.D.A. Misc. Pub. 572, except for thiamine and ascorbic acid. Calculated, on the basis that 75 gin of dried prunes is equivalent to 100 gin cooked prunes (values from U.S.D.A. Misc. Pub. 572, except for thiamine and ascorbic acid.). 3 4 5 6 Values from U.S.D.A. Circ. 549, except for thiamine and ascorbic acid. Values calculated from ingredients (values for ingredients obtained from U.S.D.A. Misc. Pub. 572, except for thiamine and ascorbic acid). Foods were analyzed for thiamine content by Miss ilsi Usuan Yu. Foods were analyzed for ascorbic acid content by Miss Bessie Ivey and Miss Mel-lirig Wu. Table 8 COMPOSITION OF BISCUIT MIX Total Nonf atl Fbod. Flour unenriched.2 Crisco2 Salt(1T) Baking powder, Royal Water Amount Protein Tht gin gin gin Cal 243 1620 0 0 0 0 0 0 0 0 71.3 185.9 500.9 3963 71.3 55 0 0 0 Cal gin 500.9 660 180 15 400 1310 Carbohydrate 5.9 180.0 0 0 0 2289 Baked at 4500 for 12 minutes COMPOSITION OF COOKIE MIX Total Nonfat1 Food. Flour uueririched.2 Sugar, brown2 Crisco2 Eggs (2)2 Salt(1T) Soda(t) Vanilla (iT) Ainotuit Protein Fat gin gin gin gin 4.1 341.6 429.8 450 450 220 100 48.6 0 0 0 1220 Baked at 375 1 0 0 Carbohydrate 0 220.0 11.5 0 0 0 0 0 0 0 0 0 0 61.4 235.6 772.1 12.8 0.7 Nonfat Calories were calculated from Values were Cal 1598 1719 1980 158 0 0 0 5455 3334 for 10 minutes the total grams of protein and. carbohydrate malt iplied. by 4. 2 Cal obtained from U.S.D.A. Misc Pub. 572. 51 the basal diet plus 2 units. After the second. day, however, all of the subjects decided to eat 2000 alories1. Thus, for the rest of the experimental study (1f9 days) all 5 subjects were containing approximately 2000 Calories. in grams, of the 5 subjects from maintained on a diet The dietary intake expressed. January 31, 19LI9, through March 21, l9J9, is given in Table 9. During the first 31 days (Period. I) the subjects were given a supplement of kOO meg of thiamine thiamine up to the Eational hydrochloride to bring the intake of esearch Council's recommended allowance of 500 meg of thiamine per 1000 Calories. Daring the next 21 days (Period. II) the subjects were given no additional supplement of thiamine hydrochloride so their intake was reduced to approximately 300 meg of thiamine per 1000 Calories (Table 10). DESCRIPTI0T OP THE SUBJECTS Five adult women were subjects during the 52-day study. all moderately active and presumably normal2, healthy They were individuals. each of the subjects also had. responsibility for some phase of the laboratory work so all had a more than usual interest in and understanding of the details of the study0 height, weight at A description the beginning of the subjects in terms of age, and the end. of the study, weight range and average weight is given in. Table 11. 1 2 In BWC's diet was slightly modified as can be noted. in. Table 10. addition, this subject s diet was supplemented with a commercial vitamin-mineral preparation (Stuart Formula) which contained at least 2 mg of thiamine chloride. BLD had had poliomyelitis when young, WO has nephritis. 52 Table T 9 DIET CONSUMED BY 5 SUBJECTS ThOM J.AEUABY 31, 19Lf9 THROUGH MARCH 21, 1949 BLD BWO1 MLW KHY CAS Cream of Wheat 30 30 30 30 30 Eggs2, B.?. 54 54. 54. 54. Cheese 30 30 30 30 30 Green Beans 100 100 100 100 100 Prunes 100 100 100 100 100 Biscuits 55 n°'° Cookies 48 48 48 .48 48 Meat 100 none 100 100 100 Carrots 100 100 100 100 100 Pears 100 200 100 100 100 Biscuits 55 55 55 55 .55 Cookies 48 48 48 48 48 100 100 100 100 100 Bu.tter 20 40 20 20 20 Sugar 10 15 10 10 10 Food. Breakfast Lunch !ood.s consumed. by the subjects during the day Milk 1 2 26 26 26 26 Wheat term 26 BWC had. some dietary restrictions and. took a commercial vitaminmineral supplement. Although her intake was not the same as the others it was constant throughout the stu&y. each subject. One egg was served. to B.?., was 54. grams. The average weight of the eggs, .53 Table 10 COMPARISON OF THLMflTh II'ITAXE I4ITH CALORIC INTAKE FOR 5 SUBJECTS ThIRING THE PERIOD JANUARY 31, 1949, THROUGH MARCH 21, 1949 Cioric Intake Thiamine Intake Ratio of NFC1to Prot Fat Oarb 3LD 65.3 91.1 WC 43.4' Su1c,t HEY I 2 3 1+ 5 4On-!at PeriotI McgOQ Total NEC1 242.0 2047 1227 59.94 86.6 244.4 1928 1148 59.54 65.3 91.1 242.0 2047 1227 59.94 1011 65.3 91.1 21+2.0 2047 1227 59.94 65.3 91.1 21+2.0 2047 1227 59.94 ca!OrleS al Period II Ji000 ii Mc Mc ,Ji000 Cal McOOO NEC 498 824 611 25874' 25705 494' 824 611 298 498 1011 l94 824 611 298 498 1011 494 824' 611 298 498 1011 494 298 22395 Thiamine content of the food. plus supplement of 400 mcg thiamine hydrochloride. Thiamine content of the food only. Thiamine content of the food plus a supplement of 400 mc of thiamine hydrochloride plus 2000 cg thiamine chloride from a commerca1 multiple vitaminmineral preparation (Stuart Formula). Thiamine content of the food plus 2000 mcg thiamine chloride from a commercial multiple vitamin and miera1 preparation (Stuart Formul&). 5k Table 11 DESCRIPTION OF TEJ SUBJECTS Body Weight Beg. End. of Study Weight Range lb Average Wei lb Age yr Heigjit in of Study lb ELD 28 62 3/k 107 1/2 108 1/2 106 3/11-110 3/k 108 1/2 EWO 3]. 62 116 112 111 3/11-116 1/k 113 1/2 314. 65 1/2 125 i/k 123 122 -126 123 3/k 36 6k 128 125 125 -128 126 1/2 14.2 68 1/2 114.9 114.7 1k6 -11.9 1k7 Subject jyl US 1 These subjects are Chinese. lb CH.PTER VI EQUIPMET USED The method of Burch (unpublished) was u.sed in studying a micro method for the determination of thiamine in blood. The equipment used. for this proced.ire was as follows: 1. BardParker blade, size 11 2. Small vials having a capacity of approximately 1 ml. These were made from 10 mm glass tubing. der number 2. 3. Small corks to fit the vials dsoribed Li. Test tubes, 6 x 50 mm 5. Pyrex test tubes, 10 x 75 mm, unselected 6. Pyrex test tubes, 10 x 75 mm, optically matched tubes for use in the microphotofimorometer 7. I4icropipettea - 5, 20, 23, 50, 200 and 225 emm capacity. These pipettes were made in the laboratory according to the directions given by Bessey, Lowry and Brook (l9Li6). 'or calibration of the micropipettes see Appendix IT. 8. Buzzer - A buzzer was improvised. by using the rapi&].y whirling nut on the wheel of an Amend grinder. By touching the bottom of the test tube against the whirling nut the contents of the tube were violently agitated. 9. 10. bber caps to fit the 6 x 50 mm test tubes Metal racks to hold the 6 x 50 mm test tubes 11. Wooden racks to hold the 10 x 75 mm test tubes 12. Wire basket woven with string in test tubes upright and order to hold to separate them from the 10 x 75 mm each other while in the incubator. 13. Clinical centrifuge 1)4.. Parafi].m 15. Syringe pipettos]. - to hold. 1 ml and 2 in]. hypodermic syringes 16. Hypodermic syringes - 1 in]. and 2 ml capacity 17. Hypodermic needles - 20 18. Transfer gauge pipettes - made in the laboratory by making a constriction pipette that wou.ld transfer about 1 ml of solution. 19. Incubator 20. Jrrand micro-photofluorometer - An extra gelatin filter, Jo. 2A (Eastman Kodak Co.) was used to reduce the fluorescence resulting from the filters themselves (Lowry, 19Lf8). 21. H-5 mercury vapor lamp 22. Socket and. transformer for the H..5 mercury vapor lamp 23. Irradiation rack - made in such a way that the 10 x 7.5 mm tubes were irradiated while standing upright ectly 3 inches from the filament of the H-5 mercury vapor lamp. This set-up is similar to that described by Bessey, Lowry, Brock and Lopez (19k6). 1 Syringe pipettes were obtained from Mr. H. Iff Northern Tool and Instrinent Co. l61_21 Northern Blvd. flushing, New York 57 2L1. Stop-.watch 25. Automatic timer 26. Volumetric pipettes RAGTS USED The reagents1 used. for the determinatIon of thiamine in the blood by the Burch method were as follows: 1. 5% triohioroacetic acid. 2. k 3. Phosphatase - Either one of the following was used potassium acetate a. Olarase was prepared by Dr. H. Bu.rch b. Acid. phosphatase was prepared from hux:n seminal fluid by diluting one part of seminal fluid with 5 parts of redistilled. water. acetic acid.. The pH was adjusted to 5.0 with This solution was centrifuged at high speed. for one hour and. stored in the refrigerator. Care was taken to see that the preparation was kept cold but did. not freeze. k. 30% sodium hydroxide (carbondioxide free) * A saturated solution of sodium hydroxide was prepared by adding 1100 grams of C.P. sodium hydroxide to 1000 ml of water redistilled in glass. This solution was considered to contain 75 grams of s dium hydroxide per 100 ml of solution (Hawk, Oser and S mmerson, p. 123k, 19k7). 1 Dilutions were made to 30% sodium All reaents were made up with water red.istilled. in glass. hydroxide from this reagent. 5. 2% potassium ferricyanid.e - made up once each month 6. 5.5 N sodium dihyd.rogen phosphate containing 0.015% hydrogen peroxide. We used. 189.82 gin NaH2?0L1.. H20 plus 0.125 gin hydrogen peroxide (sp.g. 1.1) made up to a volume of 250 ml with water redistilled in glass. 7. N-butyl alcohol - All n-butyl alcohol was redistilled. in an all glass still collecting the 116 to 118 degree Centigrade boiling fraction. 8. Approximately 1.0! sulfuric acid from which approximately 0.1 N sulfuric acid was prepared 9. Approximately 1.0 ! hydrochloric acid, from which approximately 0.1 10. hydrochloric acid 'was prepared. quinine sulfate stock standard - 100 mg quinine sulfate, U.S.?., was made up to a volume of 1 liter with approximately 0.1 N sulfuric acid. This solution was stored in the refrigerator in an amber-colored 'bottle. 11. quinine sulfate working standard - 1 ml of uiniue sulfate stock standard was diluted to a volume of 200 ml with approximately 0.1 R sulfuric acid. This reagent was prepared daily and protected from the light until used.. 12. Thiamine stock standard - Exactly 50 mg of anhyd.rous thiamine hydrochloride (dried several weeks over sulfuric acid) were made up to a volume of 500 ml with approximately 0.01 hydrochloric acid. hydrochloride. Thus, 1 ml contained 100 mcg of thiamine 13. Thiamine working standard - 1 ml of thiamine stock standard was diluted to a volume of 100 ml with approximately 0.01 hydrochloric acid. Thus, 1 ml contained 1 mcg of thiamine hydrochloride. PERMINAT ION OP THIAMflIE IN THE BLOOD The determination of thiamine in the blood was as follows: Collection of Blood Sample 1. A finger was lanced with a Bard-Parker blade. The first drop of blood was wiped off and the next 6 or 7 drops of blood were collected in a small vial. This amol2nt of blood would give 3 to k samples if the collection and measuring of blood was carried out without delay. 2. The vial was stoppered. with a cork and inverted several times so that the blood was well mixed. Precipitation 3. the Protein Immediately after mixing, 50 cmm samples of the blood were delivered into a 6 x 50 mm tube containing 225 cmm of 5 per cent trichioroacetic acid. 11. The tubes containing the blood plus trichioroacetic acid wore buzzed immediately. 5. After buzzing, the tubes containing the blood.-tricbloroacetic acid mixture were covered with small rubber caps and allowed to stand at room temperature 30 minutes after the last blood was measured. 6. The above tubes were buzzed again and. then centrifuged at full speed. for 10 minutes to colete1y separate the precipitated. protein from the supernatant. 7. 200 emm of the supernatant wore drawn off into 10 x 7.5 mm tubes. Diestipn with Pbosbatase potassium acetate were added.. 8. 20 cmm of L 9. 23 cmm of Buroli clarase or 10 omm of acid. phosphatase were added.. 10. The tubes were covered. with Parafilm, placed in tie wire basket and incubated 2* hours in a humidity-controlled incubator maintained at 37 to 38 degrees Centigrade. 11. Blanks were prepared by adding 200 cmm of a diluted .5 per cent trichloroacetic acid. solution (2.3 ml of 5 per cent trichioroacetic acid plus 0.5 in]. of red.lstilled water) to the 10 x 75 mm tubes. 20 cmm of potassium acetate and 23 cmm of Burch clarase or 10 cinm of acid phosphatase were added.. These were covered with Parafilm and. incubated 2* hours along with the blood samples. 12. Standards were prepared by adding 5 cmiii of the thiamine working standard solution to 200 omm of a diluted 5 per cent trichioroacetie acid (2.3 ml of 5 per cent trichioroacetic acid plus 0.5 nil, of redistilled water). 20 cmm of potassium acetate and 23 cmm of Burch clarase or 10 emni of acid. phosphatase were added. These tubes were covered with Parafilm and incubated 2* hours along with the blood samples. ri Oxidation to Thiochrome 13. ]3y The entire amount in each tube was used. for oxidation. means of a syringe pipette, 0.1 ml of a mixture of 30 per cent sodium hydroxide containing 0.12 per cent potassium ferricyanide was added to blood. samples, standards and. b1axks. Tor the oxidation mixture we used. 1 potassium ml of 2 per cent ferricyanide and made it up to a volume of 25 ml with 30 per cent sodium hydroxide. This mixture was prepared. immediately before use and was not kept more than an hour. 1)4. with The tubes were mixed at once by tapping the finger for exactly 15 seconds. 15. Immediately after the 15 dihydrogen seconds, 0.1 ml of 5.5 sodium phosphate containing 0.015 per cent hydrogen peroxide was added. with a syringe pipette. a syringe pipette. 16. 1 ml. of n-butyl alcohol was added with 17. The tubes were buzzed for 15 seconds and centrifu.ged for 2 minutes to separate the layers. The clear n-butyl alcohol layer was transferred to an optically matched 10 x 75 mrs tube by means of a transfer pipette. A clean dry transfer pipette was used to transfer each sample. 18. All tubes were read in the Jarrand. micro-photofluorometer which was set at 90 with the quinine sulfate working standard.. Diaphragm opening number 6 was used on the Farrand. microphotofluorometer. 19. After reading the optically matched tubes in the microphotofluorometer, all the tubes were irradiated for 30 minutes 62 3 Inches from the filament of the R-5 mercury vapor lamp. The H-5 lamp was allowed to warm up for 10 minutes before the tubes were irradiated. 20. Tho optically matched tubes were then reread in. the micro- photofluorometer. Calculations 21. The irradiated reading was subtracted from the reading before Irradiation and then the average blank reading subtracted from the remainder to give the final reading which was used in. the calculations, as shown in Table 12. Table 12 TH0D OF DETERMINING TEE AVERAGE CORRECTED BEADING USED IN TEE üALOULAT ION OF BlOOD TEIAMflE Tube Contents Read. Before Read. After Read. Average Before- Blank Irrad.. Irrad.. After (Col.1i-Col.5) Average Corrected Reading 8.95 Corrected Reading Irrad. Blood (BC) L4.2.25 30.00 12.25 1.75 10.50 Lf2.50 32.00 10.50 1.75 8.75 Li.6.00 35.00 11.00 1.75 9.25 Li.2.25 32.00 10.25 1.75 8.50 112.00 32.50 9.50 1.75 7.75 63 supernøt b1ood(° \ total yalume ml biood per aliquot rnl ml blood per aliquot (0.05) (2Q 275J 0.O36 ml (100 ' = meg % thiamine ( mince thiamine \ f Corrected read. sampi '4m]. blood/aliquOt I Corrected read. stan.dardi '4.1000) = 2.05 meg thiamine CEAPFER VII D.ETRMflt&TION 91 EEMTOORfl' ItEED R THE TERMII(LTIOI OF HEMATOORIT Gorham, Abels and Robbins (19k2) and Florijn and Smite (1948) foud. most of the thiamine in the blood was contained in the blood Hennessy (19k?) states that "The thiamin of blood is cells. virtually all in the cellular elements and. a mere statement of the thiamin content of whole blood is therefore almost meaningless.... An approximation can be made in the absence of leukemia if at least the hematocrit value is given in addition to the thiamin content of the whole blood." For this reason it was planned to determine the beinatocrit in addition to total thiamine in order to aid in the later interpretation of the data. The equipment used for the determination of the hematoorit included the following: 1. Small rubber vaccine bulbs 2. Spot plate 3. Capillary tubes 14 Pyseal cement 5. Alcohol lamp 6. Clinical centrifuge 7. Micropipette, approximately 1 c capacity 65 F1 PROCEDURE FOR DETERMINATION OF HEMLTOCRIT The procedure for the determination of the hematocrit was as follows: Selection of Pubes Oapillary tubes were selected that were of uniform bore (3mm) and 4 inches in length. The ends of the tube were brushed with a piece of screen if necessary so that they were smooth. These capil- lary tubes were washed and dried so that they were chemically clean. Using a micro-pipette, 1 cmm of heparin solution (Roche-Ornon, 10 mg in 1 ml) was placed in the capillary tubes one-half inch from the end and. the tubes were allowed to dry. Collection of Elood. Rubber bulbs were placed on the ends of the capillary tubes opposite the beparinized. ends. After the finger had. been lanced with a Eard-Parker blade and. the sample collected for the blood thiamine determination, the blood (about 0.1 ml) was allowed to flow up the capillary tube until it reached about one-fourth inch from the end.. The blood was mixed with the heparin by using the small rubber bulb to express the blood in and out of the tube into a spot plate. mixing was carefully done so that no bemolysis would occur. This The blood was finally drawn into the tube and. the unheparinized end sealed with Pyseal. CentrifuEation The tubes were labelled and. then centrifuged one hour at full speed in a clinical centrifuge in the cold. Our centrifuge was kept in a refrigerator throughout the experimental period. After the one hour centrifugation, the tubes were removed and length of the packed cells and the total read. by reading the length of cells plus plasma. Cal culat ion The length of the red blood. length of the cell layer divided by the total cells plus plasma and. multiplied, by 100 gives the per cent of the blood volue occupied by the red. cells. RESULTS OP THE DTERMINAT ION OP HEMATOOHIT The values for hematocrit were to be of the results of the the Barch method. used in the interpretat1on concentration of thiamine in the blood using Since the latter values have not been all of the data for hematocrit are listed in Table 29 determined, in the appendix. 67 CHAPTER VIII In spite of the fact that considerable preliminary work was performed before the study started our blood thiamine a1ues were always significantly lower than results (I+.8 to 6.5 meg per cent) obtained by Dr. Bureh in her laboratory, than other values reported in the literature or our method. results with the Friedemann. and Kmieciak It was planned to follow the concentration of thiamine in the blood during the first few days of the study when the subjects were receiving a own amount of thiamine to observe whether any change occurred in the blood thiamine value, but they continued at the same After 10 days of the experimental period it was decided to low level. freeze the blood supernatant (the 200 0mm of supernatant removed after precipitation of the protein and centrifugation) until the problem was solved. Fasting blood samples were obtained and deproteinized. each day throughout the study. Aliquots (200 cmm) of the supernatant were frozen in dry ice and are still being held frozen until the problem is solved before the thiamine analyses are attempted. METHODS USED FOR FURTHER STUDY OP THE BUBCH METHOD Varying the .&mounts of Potassium Acetate Used The determination of the pH of the blood supernatant using varying amounts of potassium acetate - One of the first questions that came to ri mind, was whether or not the pH was optimum for the enzyme hydrolysis. On Janunry 18, 1949, Dr. Buroh had suggested that a check be made on the pH after the neutralization with potassium acetate to be sure that the pH was between 4.6 and. 4.8. Sinoe we were still having difficulty, on March11, 1949, D. Bu.rch suggested that we try less potassium acetate resulting in a pH of about 4.4 for enzymatic hydrolysis in order to decrease the salt concentration. This suggestion was made since it is known that high sodium acetate inhibits the enzyme. inhibition might be overcome by dilution. This The various amounts of potassium acetate added to the blood supernatant and. the resulting pH are given in Table 1'. The determination of the pH of the blood supernatant plus varying amounts of potassium acetate and acid phosphatase - Since the ensyme itself is adjusted to a pH of about 5.0 the effect of the ad.d.itin of 10 cmm or 23 cmm of acid phosphatase (P) on the pH after addition of varying amounts of potassium acetate was observed. The following results (Table 14) indicate that little change in pH occurred with the addition of the enzyme. Determination of thiamine in fasting blood when varying amounts of potassium acetate were used - In no case did the results indicate a much higher blood thiamine value due to the possibility of a more optimum pH by the addition of varying amounts of potassium acetate. The results on March 18, 1949, when 15 omm of potassium acetate were used, suggested that there was a change in the blood value but another study on March 19 did. not confirm this (Table ii). Table 13 THE pH OF THE BLOOD SUPEPEATANT WHEN VARYING AMOUNTS OF POTASSIUM ACETATE WERE ADDED Amount of Potassiwn Acetate cmm pH I.te (1949) 15 4.33 BWC Mar. 18 17.5 4.53 BLD Mar. 17 20 14.72 14.72 MLF BLD EWO BWC 27 27 18 18 4.501 CAS Jan. Jan. Feb. Feb. Mar. 23 4.92 NLF Jan. 27 28 1+.821 GAS GAS GAS Mar. 10 Mar. 10 Mar. 10 4.68 4.71 4.621 1 Subject 10 Those d.eterrnination.s of pH were made with email micro-pR-beakers (Dietz, 19148). These micro-beakers reauired only a drop or two of solution but were a little difficult to handle to get consistent results. It is suggested that for the instrument in the nutrition laboratory the depressions into which the solution is placed be hollowed out still further. This would require a slightly larger volume but would probably give more reliable readings. 70 Table 14 TEE pH OF TEE] BLOOD SUPEBNATANT AFTER ADDING VARYING AMOUNTS OF POTASSIUM ACETATE AND ACID PHOSPHATASE Amount of Blood Sernatant Potassium Amouxit of pH Acetate Added .AP Added cmm cinm cmx 200 200 200 200 15 20 23 28 4e53 200 200 200 200 15 20 23 28 Li.53 .83 Li..95 5.12 4.86 4.90 5.12 pH 10 10 10 10 k.51 k.75 23 23 23 23 4.50 4.74 4.85 5.05 95 5.02 Table 15 DETERMINATION OP BLOOD THIAMINE WREN VARYING AMOUNTS OP POTASSIUM ACETATE WERE USED Amount of Potassium Acetate cmx Blood Thiamine BWC 15 5.27 non-fasting blood 23 cmx clarase extraction with isobutyl alcohol Mar. 18 EWO 15 5.29 Non-fasting blood Mar. 18 Su.bject Remarks mog% te (1949) 10 cmx .AP extraction with isobutyl alcohol 71 Table 15 (continued) Arnoimt of' ?otassi Acetate cmm Blood Thiamine BLD 15 2.83 BWC MLW OAS 15 15 15 15 3.93 1.96 2.03 2.93 BLD 17.5 1.28 Subject Remarks mcg% Date (19J+9) extraction with isobutyl alcohol I' Mar. 19 It 0 I' non-fasting blood Mar. 15 23 cinm elarase BLD 17.5 2.16 BLD 17.5 2.31 Mar. 17 non-fasting blood Mar. 15 10 cmm P BLD 17.5 1.96 Mar. 17 CAS 20 2.66 Mar. 10 BLD 20 1.19 Mar. 13 BWC 20 20 20 2.5A' CAS 20 3.85 Mar. 22 QAS, HB 20 20 20 2.82 3.01 Mar. 2 20 20 20 20 2.61+ BLD 23 1.73 Mar. 13 BLD 28 2.48 Mar. 13 (pooled) BWC 2.BLi. use of new reagents II Mar. 29 ft It 2.76 Ii 2.91+ 2.69 2.80 3.19 Mar. 31 0 II 'I II 72 The determinations on March 18 were carried, out on blood collected at 5 P.M. (non-fasting) and iso-'butyl alcohol was used. for the extraction instead of n-butyl alcohol. The use of iso-butyl alcohol did not explain this difference in value, however, as will be discussed later in this section. Twenty cmm of potassium acetate was finally decided upon as the volume to be used in future analyses because that was the amount suggested by Dr. Btu'ch and. because more or less than that amount was of no advantage. The Use of Different Trichioroacetic Acid Reagents The use of redistilled trichioroacetic acid. - On March 27, 1949, it was learned. that redistillod trichioroacetic acid was used in the original method since the commercial product varies widely. Coleman and. Bell1s trichioroacetic acid was used. for all of the experimental blood samples Both that are being kept frozen until the problem of method is solved. Dr. H. Burch and Dr. 0. H. Lowry have given directions for the redistillation of trichloroacetic acid. in an all glass still under reduced pressure. An attempt was made to redistill some triohioroacetic acid. with the assistance of Dr. B. B. Christensen of the Chemistry Department. Some redistilled trichioroacetie acid. was obtained but the resulting product was not as pure white as was the Coleman and Bell reagent. It was felt that further work should be done on a set-up for the distillation of this reagent since the method is more involved. than it would. appear to be. Dr. 3. H. Haag of the Agricultural Chemistry Department gave us some trichioroacetic acid that he had. redistilled in his laboratory 73 several years ago which was used. in the following experiment. The concentration of thiamine in the bloo& was determined, when the protein of 50 cmm blood sales was precipitated by 5 per cent redistilled. trichioroacetic acid. and when 50 cmm aliq,uots of the same blood. were precipitated by 5 per cent trichioroacotic acid as we had prepared it from the trichioroacetic acid. which had not been red.istilled.. difference in values was noted. No real In fact, in these two analyses a lower value was obtained for blood thiamine with the redistilled. trichioroacetic acid.. Use of different brands of trichioroacetic acid. - Coleman and Bell's, Baker's and Mallinckrod.t's trichioroacetic acid were used to determine blood. thiamine and no difference in values was observed.. Teats for Enzyme Activity Determination of blood. thiamine with and. without the use of olarase - The values obtained in the nutrition laboratory were mu.ch lower than Dr. Burch had obtained with the micro-method so an. answer to the discrepancy was sought in the q,uestion of enzyme activity. The clarase used. with the micro-method was an especially pnrified preparation from Dr. Bu,rch's laboratory. Coerôial clarase, without purification, produces too high a blank for the micro-method.. Before cocarboxylase (thiamine pyrophosphate) was obtained, the activity of the Burch clarase was tested. by noting a difference in the reading for blood. thiamine when clarase was added to some blood s'a.pornatauts and not added to others. 1 This procedure was followed in Throughout the discussion fasting blood. was used. trn].ess otherwise stated.. 74 5 different experiments at varying times during the year and. in every case the corrected. reading was zero or almost zero when no clarase was added to the blood. supernatants. When clarase was added to the blood. supernatants the blood thiamine determinations were similar to those already reported in this thesis. Varying the amount of clarase used. The Bu.rch clarase preparation had. been prepared over a year before this study began. It was thought possible that the activity of the enzyme had. decreased or that 23 cmm of clarase was not the optimum amount for hydrolysis. 1949, fasting blood. from AS was collected. On February 25, To 50 cmrn aliquots of the blood. filtrate were added 23, 30 and. 40 cmm of clarase resulting in blood thiamine values of 3.49, 3.18 and 2.82 mcg per cent, respectively. Increasing the amount of clarase used was of no value. Less than 23 cam of clarase was not sufficient. On January 28, 1949, when 20 and 23 cam of clarase were used, the blood. thiamine values were 1.46 and 3.06 mcg per cent, respectively. Use of acid. phosphatase for enzyme hydrolysis - On February 10, 1949, Dr. Bu.rch suggested the preparation of an acid. phosphatase from human seminal fluid according to the directions of Dr. Gerhard Schmidt. This enzyme had been used for a few determinations by Dr. Birch and 10 cam of the preparation was sufficient for their experiments. The seminal phosphatas? (acid phosphatase) was prepared. according to the directions of Dr. Gerhard Schmidt (1949). 1 Human seminal fluid was obtained from the laboratory of Dr. Car]. J. Heller, University of Oregon Medical School, Portland., Oregon. 75 The possible use of another enzyme was encouraging since we had. been dependent upon the limited supply given us by Dr. 3urch. The method of purification of the clarase, furthermore, was not always successful in producing an active preparation even in the hands of those who had. prepared. it. nation Comparison of the two enzymes by determi of the concentration of thiamine in the blood indicated that 10 cmm of acid. phosphatase were as effective as 23 omm of c].arase (Table 16). In the few cases in which 23 cmm of acid. phosphatase were used the values were as high or even slightly higher than those obtained with 10 cma of acid. phosphatase. the larger amount of However, the blanks were so high with enzyme that it was decided. to use the smaller amount. Testing the activity of enzymes using cocarboxylase - From the above studies, it was 1town that the enzymes were active but it was not own whether or not they were 100 per cent active. Dr. J. B. Sumner (191+9) suggested that the enzyme be tested using sodium pyrophosphate. In order to make the test more specific a small amount of thiamine pyrophosph.ate1 was obtained in March, 191+9. A cocarbolase solution was made up to be eauiyalent to the thiamine hydrochloride stock standard as follows: Thiamine chloride hydrochloride Thiamine pyrophosphate Mol. Wt. - 337.276 Mol. Wt. - 1+60.787 50 : 337.276 :: x : 1+60.787 x = 68.3 mg thiamine pyrophosphate which is equivalent to 50 mg of thiamine chloride hydrochloride 1 Appreciation is expressed to Merck and. Company for supplies thiamine pyrophosphate and thiamine hydrochloride. of z1 Dilutions of both the thiamine hydrochloride and. cocarboxylase stock solutions were d.e in exactly the same mauner, corresponding to 1 mog Equivalent amounts of cocarboxylase per ml of thiamine hydrochloride. or thiamine hydrochloride standard. solutions were athied to aliquots of blood supernatants and. these samples were carried through the procedure. using The per cent activity of the enzyme in question was determined. the average corrected readings, e.g.: corr. read. cocarboxylase corr. read. thiamine hydrochloride (100) % activity of the enzyme The results using the above procedure (March 7, 1949) and. 23 crnm clarase indicated a 102 per cent activity for the clarase. results The using the above procedure and 10 cmm of acid phosphatase indicated a 99 per cent activity for the acid. phosphatase. The experiment was repeated on March 15, 1949, when 23 omrn of clarase were found to be 109 per cent active and 10 cmm of acid phosphatase were These figures seem to indicate that found to be 109 per cent active. both enzymes are satisfactory in the quantities used. for the hydrolysis of thiamine pyrophosphate to free tiiamine. !iations in. Incubation Procedure Using longer time for incubation - The procedure as first described suggested 1 to l- hours for incubation. On January 18, 1949, Dr. Bu.rch suggested a 2 hour incubation period with the statement"tbere may be proteolytic enzymes still present which tend to promote hydrolysis to fluorescent materials on long incubation, which increase the irradiated reading. The effect of these should be avoided". 77 Table 16 THE DETMIN.TION OF BLOOD THIAMIHE WEBB 2 DIFRER1RF ENZYMES WERE ADD TO 44LIQUOTS OF BLOOD SUPER1IkTJUT BOM SUBJECT BWO ite clarase 19Ls.9 23 cimu acid phosphatase 23 osm 10 cmm 1h60 k.86 Feb. 22 3.86 3.68 Mar. 1 3.12 Mar. 29 2.5k 2.76 1.57 Feb. 20 3.87 2.58 2.84 3.19 Mar. 31 2. 6L 2.69 2.80 The determination of the concentration of blood thiamine after 1 and 2* hours of incubation are given in Table 17. In no case did the values approach those found in normal subjects by D. Burcb. 2 However, hours of incubation did. not seem to produce any increase in fluorescence so this amount of time was throughout this work. incubation nation of blood satisfactory It may be that later work will prove 1 hours sufficient incubation Using higher considered to 2 time. temperature - In macromethods for the d.etermi thiamine higher incubation temperatures,.k2 to5 degrees Centigrade have been used. (Friedemaun and Kmieoiak, I9J43, Greenberg and Rinehart, 19L.5). The incubation temperature used. in this micromethod. for the determination of blood thiamine was 37 to 38 degrees Centigrade. On March 1, 19k9, both clarase and acid phosphatase were used to test This the response of both enzymes to increased incubation temperature. was done by following the usual procedure for the determination of blood thiamine except for the addition of 10 cmm of acid phosphatase to half of the blood supernatant samples and. 23 cmm of c].arase to the rest of the blood supernatants and. incubating all of the samples at an average temperature of 144 degrees Centigrade for Z- hours. Table 17 DETEB4INAT ION OF THE OONCETBAT ION OF THIAMINE IN THE BLOOD AFTER 1* AIW 2 HOURS OF INCUBAT ION l Hours of Incubation Blood Subject 3te 19149 Apr. 12 BWC Thiamine 2 1.te Hours of Incubation Blood Subject Thiamine moo mc 19149 2.05 Feb. 22 EWO 3.68 3.86 3.87 1.5 31W 14.147 Apr. 1J4 EWO 3.75 Apr. 21 BWC 3.17 Feb. 25 OAS 3.149 Apr. 5 CS 1.92 Apr. 26 CAS 1.714. Apr. The concentration of blood. thiamine (31W) was found to be 3.12 mcg per cent when 23 cmrn of clarase were used and 2.58 mcg per cent when 79 10 cmm of acid phosphatase were used. These values are similar to those obtained with incubation at 37 to 38 degrees Centigrade. Variations in the Procedure for Oxidation to Thiochrome and in the Extraction of the hiochrome Use of larger amounts of potassium ferricyanide - In all cases the solution remained yellow for the entire 15 seconds after the oxidation mixture was added. Ohemists, This has Inc., 19i47) to be been considered (Association of Vitamin evidence that sufficient potasiuin ferricyanide was present. To confirm this obeervation for the micro-method, one experiment was performed using an oxidation mixture containing 0.16 per cent potassium ferricyanide instead of the usual 0.12 per cent of potassium ferricyanide. The usual procedure was followed for the determination of blood thiamine for half of the blood samples and to the other half was added the oxidation mixture containing the higher amount of potassium ferricyanide. No significant difference was noted in the results since a thiamine value of 2.21 meg per cent was obtained with the usual oxidation mixture and a value of 2.3LI. meg per cent was obtained for the stronger oxidation mixture. Omission of the sodium dihydrogen phosphate - peroxide mixture - Most of the macro-methods for the determination of blood thiamine the sodium dihydrogen phosphate-peroxide mixture. do not use It was decided to determine the effect of the omission of this reagent. On February 26, 19J4.9, the micro-thiamine procedure was carried out according to the usual method using only half of the samples. The other half of the samples were carried through the same procedure up To the latter samples were added 0.1 ml of the to the oxidation step. oxidation mixture, each tube was tapped for 1 seconds, the sodium dihydrogen phosphate-peroxide mixtu.re was omitted and 1.0 ml of n-butyl alcohol was added immediately. The blood thiamine value (OAS) following the usual 1.52 meg per cent. method was The blood thiamine value (OAS) when the sodium dihydrogen phosphate-peroxide mixture was omitted was L.29 meg per cent. This higher value is not a valid one because the n-butyl alcohol layer from these samples was very cloudy. nor the addition of a few crystals of anhydrous sodium sulfate remcved. this cloudy appearance. about The blank was very high, 13.95, instead of 3.80, and all of the readings were very erratic. 27, i9L.9, On March 11 and Neither repeated centrifugation Dr. Bureb wrote that the sodium phosphate-peroxide mixture was added to lower the pH and stated that if the solution was too alkaline there would be turbidity in the u-butyl alcohol layer. laboratory. The same observation had been made earlier in this The chief reasons for the use of the sodium phosphate- peroxide mixture are: (1) to lower the blaflk reading (2) to avoid turbidity in the n-butyl alcohol layer (3) to remove the yellow color Variation in the amount of sodium dihydrogen phosphate-peroxide mixture used. * In the determination of thiamine in the urine it bad been found (Mickelsen, Condiff and Keys, 19145) that lowering the pH before the extraction of the thiocbrome aided in the selective absorption of 81 thiochrorne. ven though it is possible to get only an approximate pH with the hi salt concentration present the pH (Table 18) was determined after the addition of varying amounts of the sodium The micro-pR beakers were used. dihydrogen phosphate-peroxide mixture. for these determinations. Table 18 TE pH OF OXIDIZED SALES AFTER TI] ADDITION OF VARTING AMOUNTS OF SODIUM DIHYDROGEN PRO SPHATE - PEROX IDE BEAGENT pH Amount of (average of det 'us. 2POL1. - Apr. 1 and 2, 19k9) Added 9.90 10.00 9.27 8.8k 8.56 100 120 130 ikO 150 On April 1, l9k9, the usual procedure for the determination of blood thiamine was followed except for the addition of 100 cmrn or 130 cmm of sodium dihydrogen phosphate-peroxide reagent to aliquots of blood filtrates (BWC) resulting in blood thiamine readings of 2.91 and 2.3k mcg per cent, respectively. On April 2, 19149, the experiment was repeated using 100 cmm or 1L0 omm of the sodium dihydrogen phosphate-. peroxide reagent. In this case the blood thiamine values were 2.7k and 3.17 mog per cent, respectively. A change in the pH at this step in the procedure was, therefore, of no added value. Use of iso-butyl alcohol instead of n-butyl alcohol - If the thiamine was not lost or destroyed and if the thiamine was properly oxidized to thiochrome it was thought that possibly the n-butyl alcohol was not Since, in other methods for the extracting all of the thiochromo. determination of thiamine (Hennessy Straub, 191i1, Friedemann and and. Oereoed.o, 1939, Kznieciak, 1914.3, Greenberg and Rinehart, 1914.5) iso-butyl alcohol had. been used t was decided to try iso-butyl for the extraction of thiochrome procedure as usual and the thiocbrome The values obtained. for blood was taken, carried concentration of thiamine through the extracted with iso-butyl alcohol. thiamine were 5.27 and 5.29 meg per cent. These were some of the highest values obtained not On alcohol in this micro-procedure. March 18, 1911.9, non-fasting blood (Ewo) but the results were Conner and under any circumstances confirmed on the next day, March 19, when the in the blood was determined on 5 subjects as indicated in Table 19. Table 19 TEE CONCENTRATIOI OF THIAMIEE IN TEE BLOOD OP 5 SUBJECTS AS DETEBMIEED WHEII THIOOERO WAS EXTRACTED USING ISO-BUTYL ALCOHOL !.1y. BID BWC MLW KEY GAS 2.83 3.93 1.96 2.03 2.93 83 Reading Samples Sooner after Transfer to pptically Matched Tubes Some workers (Conner and Straub, 191f1, Friedemann and Kmieciak, l9L.3) have indicated that the tubes should be read as soon as possible after the oxidation to thioehronie. The usual procedure in the laboratory was to oxidize all of the tubes, which might be as many as 50 or 60, and. then to extract the thiochrome and transfer the alcohol layer of all of these tubes before reading the samples. In macro- thiamine determitious in blood carried out in the nutrition laboratory little difference had been found in the thiochrome readings whether they were read immediately or held. at room temperature for a short while and then read.. However, on March 31, 1914.9, the time before the tubes were read. was shortened by carrying only 15 tubes through the oxidation, extraction and transferral of the n-butyl alcohol layer and tben reading them before continuing with the next group of 15 tubes. The values ranged from 2.614 to 3.19 meg per cent which were similar to those which had. not been read immediately after transfer. Variation in the Irradiation Procedure Use of a new irradiation lamp - The drop in readings after irradiation was not as great as Dr. Bu.rcb had. found. It was thought possible that the H-5 lamp was not destroying all of the thioclirome present. H-5 lamp was obtained. A new On May 17, 191+9, half of the blood samples were irradiated with the irradiation lamp we had been using and the other half were irradiated with the new H-5 lamp. irradiated 30 minutes. All of the samples were The values for blood thiamine when the o1dtt E-5 lamp was used. was 1.68 meg per cent and with the w" E-5 lamp was 1.17 meg per cent. ven though the irradiation Variation in the time of irradiation - lamp seemed adequate it was thought possible that all of the thiocliromo was not destroyed in 30 mInutes so on February 22, l9Jl9, tubes were read. after 30 minutes of irradiation, put back and irradiated an of 60 minutes, and. then read. additional 30 minutes, or a total time No consistent difference in. the readings occurred. as a result again. of longer irradiation (Table 20). Table 20 T 1TEOT OF IBRLDIATXNG ThE SA14PL1S FOR 30 AND 60 MINThS Lverae Corrected Raadins 30 Minutes of Irrad.iat ion 60 Minutes of Irradiation 2k.00 23.00 2L$.50 23.00 19.75 22.50 20.00 20.75 19.50 22.50 19.50 22.75 It was suggested that irradiation of the samples for as long as 30 minutes might increase or produce extraneous fluorescence in the tubes resulting in only a slight final decrease read after 30 minutes of before irradiation. when the tubes were irradiation when compared with the reading Therefore, on April 29, l9L9, aliquots of the same blood carried through the usual procedure were irradiated. 5, 10, 15, 20, 25 and. 30 mizmtes (Table 21). me decrease in fluorescence due to irradiation seemed to come in the first 5 minutes and irradiation for longer periods was without effect. Table 21 THE AVEBAGE CORRECTED READING OF SANPIS AFTER BEING IRPADIATED FOR VARYING LENGTHS OF TI) Average Corrected Reading Length of Irradiation Period. mm. 8.57 7.97 9.00 6.11 12.02 8.38 5 10 15 20 25 30 Use of New Reagents All new reagents were obtained and. new solutions made up from these reagents. Each new reagent was then checked individa11y by determining the concentration of blood thiamine using the new reagent and the old reagent with aliquots of the same blood. Experiments were performed using these new reagents on March 17, 21, 23, 29 and. April 2, 1949. A].]. of the blood thiamine values when the new and the old reagents were used were still between 2 and 4 meg per cent. Use of Another Farrand. iicroPhotofluorometer The Farrand. microphotofluorometer used. during the study belonged to the Western Regional Project. Diring the course of this study, the rrand micro-photofluorometer ordered. by the nutrition research laboratory arrived.. This afforded an opportunity to road the blood samples on another instrument. On March 5, 1949, both micro- photofluorometers were used. to read. the same blood samples. No essential difference in the readings was found between the two instruments. The Determination of Standard Ourves Addition of thiamine hydrochloride to blood supernatants - Experiments were performed. on April 5, 12, 14, 1, 16 and 21, 1949, in which standard thiamine solutions of varying concentrations were added to blood supernatants and these were carried through the procedure. By subtracting the value for blood thiamine from the value for blood thiamine plus added. thiamine hydrochloride the reading for the standard thiamine hydrochloride itself was obtained. Details of the above experiments performed on April 5 are described below: Twenty-five 50 cmm samples of fasting blood. were delivered. into 6 x 50 mm test tubes containing 225 cunu of 5 per cent trichioroacetic acid. These tubes were buzzed immediately, covered with rubber caps and allowed. to stand 30 minutes. After the 30 minutes the tubes were centrifuged at lull speed for 10 minutes and. then 200 cmm of the supernatant were drawn off into 10 x 75 mm tubes. The 25 blood samples were divided into five groups and treated as follows: Group 1 - Blood samples were carried through the usual procedure; I.e., 20 omm of 4 M potassium acetate, 23 cmm of clarase 87 (incubation period of 2 hours at 37 to 38 degrees Centigrade), 100 cmm of oxidation mixture and 100 omm of sodium dihydrogen phosphate containing 0.015 per cent hydrogen peroxide were added and the extraction was carried out with 1 ml of n-butyl alcohol. 55 Group 2 - 5 cmm of standard thiamine hydrochloride solution containing 1.25 mmog of thiamine hydrochloride in the 5 cmm were added to each blood supernatant. The rest of the procedure was the same as described for group one. Group 3-5 cium of a standard thiamine solution containing 2.50 mmcg of thiamine hydrochloride in the 5 cmiii were added to each blood supernatant in the same manner as group two. Group 4 - 5 cmiii of a standard thiamine solution containing 3.75 mmcg of thiamine hydrochloride were added. to each blood supernatant in the same manner as in group two. of a standard thiamine solution containing 5.00 mmcg Group 5 - 5 c of thiamine hydrochloride were added to each blood supernatant in the same manner as in group two. In order to be able to express the mmcg of thiamine hydrochloride added in terms of meg per cent, as we did with the blood values, the following calculations were made: according to the formula used, the 5 cmm sample containing 5 mmcg of thiamine would be equivalent to a blood reading of 13.75 meg per cent of thiamine. For example: fmmc thiamine hy&rochloride\(corr. read. sampleS) f 100 \ ((m). blood) vol. supernatant ) corr. read. std. ) l000J total volume 1 I ((0.05) V64..00\(o\= 13.75 mcg meg thiamine thiamine J6k.00/(loo0J 275/ So: 1.25 mmcg of thiamine hydrochloride equivalent to 3.4.4 meg % thiamine 6.88 2.50 10.31 3.75 13.75 5.00 The average corrected readl.n.gs for the sarples were: AVERAGE CORRECTED READING TUBE CONTENTS Blood only Blood + 1.25 nlmcg thiamine 8.83 18.05 Blood + 24.50 mmcg thiamine Blood + 3.7.5 mmcg thiamine Blood + 5.00 mmcg thiamine 32.25 14.3.80 When the valt 52.08 for blood thiamine was subtracted from the value for blood thiamine plus stand&rd thiamine hydrochloride, the results were: AMOUBT OF THIAMIiE READING FOR BLOOD THIAMINE + STAI1)&RD THIAMINE HYDROCHLORIDE - READING FOR BLOOD THIAMINE ALONE (18.05 - 8.83) 9.22 mmeg (32.25 * 8.83) (1+3.80 - 8.83) 34.97 5.00 mmcg (52.08 * 8.83) 1+3.25 1.25 mmcg 2.50 mmcg 23.1+2 This same procedure was followed for all of the following experiments also and the results are given in Table 22 and Figure 1+. Table 22 AVERAGE CORRECTED READINGS OF BLOOD SDPEBNATANT PLUS THIAMINE HYDROCHLORIDE MINUS READINGS FOR BLOOD SUP] RNATA'NT Thiamine Apr, 12 .te (191+9) Apr. 114 - 4.94. 3.05 4.67 4.22 Hyd.ro- oride Air. Apr. 15 br. 21 Avera mmcg 0.625 - 1.25 9.22 9.30 9.85 7.36 8.65 8.88 2.50 23.1+2 19.18 17.140 18.88 20.17 19.81 3.75 34.97 35.25 33.90 24.1+5 30.85 31.88 5.00 43.25 48.80 - 42.1+3 43.25 44.4.3 /G(JRE LI. 5 TANDARD CURVES USING TH BURCH t1ETHOD BLOOD THIAMINE (NCCr %) . -,, 70 , a a ... r 4.. r 0. U U .1 .L.J. L(...'. .J 4. CURVE 1: (THIAMINE 65 HYDROCHLORIDE -#- BLOODS.N)-8LOODS.N' -- - CURVE 2. // (THIAMUvE /1 HYDROCHLORIDE * BLOOD) -BLOOD THIAMINE / --CURVE 3: TI-I/AN/NE ss HYDROCHLORIDE TREATED AS BLOOD ---CURVE'-I:COCARBOXYL15E 50... TREATED AS BLOOD Q CURVES.(COCARBOXYLASE+ LU BLOOD)- BLOOD THIAMINE K 35_ Li Qz LU / / / / / / / / /'/ /'/ /'/ 14.J LU - // // LU 0 / / / ...2 / ..,' / / // // 30 /.' / // 25 - / // // ,' - /1 20 25 ,/ ,%;' ;, 1' I 0 I 0.625 .1.25 2.50 3.75 frIIlC& TH/Af'IINE HYDROCHLORIDE 'SUPER WA TAN T 5.00 The average corrected reading for blood (BWC) on April 2, 19k9, was 12.95 or a concentration of thiamine in the blood of 2.7k mcg per cent. If this value is read from gure if, the value of 12.95 would be equivalent to 1.75 mmcg of thiamine hydrochloride. Then: 5: 13.75 :: 1.75 : x x if.81 mog thiamine in the blood (Ewe) Addition of thiamine hydrochloride to blood - When similar dilutions of thiamine hydrochloride were added to blood itself rather than to the blood supernatant on April 26 and 28, 191f9, the values were lower than when the thiamine hydrochloride was added to the blood filtrate as may be seen in Table 23. Table 23 TRE AVERAGE CORRECTED READING OF BLOOD (GAS) PLUS THIAi'1IE HYDROCHLORIDE }4I1US THE RFDING FOR BlOOD Thiamine Hydrochloride mmcg 0.625 1.25 2.50 3.75 5.00 Average Corrected Read.ins 2.56 7.Lf6 18.91 21f.k8, 38.30 Determination of a curve using thiamine hydrochloride alone - Solutions of thiamine hydrochloride alone were carried through the entire procedure. The following values were obtained (Table 2/+ and Figure ): 91 Table 24 AVERAGE CORRECTED READING USING THIAMINE HYDROCHLORIIIEI ALONE CARRIED THROUGH THE ENTIRE PROCEDURE Average Corrected Beading Thiamine Hydrochloride mmcg 0.625 1.25 2.50 3.75 5.00 7.75 15.00 31.13 M.6.35 67.00 Determination of a curve using cocarboxylase alone - Varying concén-. trations of cocarboxylase equivalent to the above amounts of thiamine hydrochloride were carried through the same procedure as described on April 5. The results are tabulated in Table 25 and Figire k. Table 25 AVERAGE COBBE (YiED BEADING USING 000AR3OXYLA.SE ALONE CARRIED THROUGH THE ENTIRE PROCEDURE Cocarboxylase Equivalent to the Ibliowing Amounts of Thiamine Hydrochloride Average Corrected Beading mineg 0.625 8.90 1.2.5 2.50 3.75 5.00 33.09 50.96 66.50 92 Addition of cocarbor1ase to blood - Varying concentrations of cocarboxylase equivalent to the above amounts of thiamine hydrochloride were added to blood. The results are given in Table 26 and Figure 14.. Table 26 AVERAGE CORRECTED READING OF BLOOD (cAs) PLUS 000ARBOXYLASE MIIWS THE READING FOR BLOOD Cocarboxylase Equivalent to the Following Amounts of Thiamine Hydrochloride mmcg Average Corrected Reading 0.625 1.25 2.50 7.95 19.15 3.75 5.00 29.85 38.90 As can be seen from Figure £ and as was found in the Priedemaxm and Kmieciak method, the standard curve if used, must be defined. It would seem that the curve resulting from the addition of thiamine hydrochloride or cooarboxylase to the blood itself and then carrying these samples of blood plus standard through the entire procedure, subtracting the value for blood thiamine alone, would be the most valid curve to use for the determination of blood thiamine. Determination of Blood Thiamine After Oral Test Dose Thiamine H.ydro chloride The daily blood samples in the studies reported in this thesis were taken from subjects in the fasting state. The possibility that our blood thiamine values were lower than those reported in the literature 93 was considered because some investigators bad used blood which was drawn at varying times during the day and, therefore, might have been influenced by the immediately preceding thiamine intake. No reports on the effect of an oral dose of thiamine on the concentration of thiamine in the blood have been found in the litera ture, although blood thiamine concentrations after intramuscular and intravenous injections of thiamine have been determined (Bowlands and Wilkinson, 1938, Ritsert, 1939). Por this reason the effect of an oral test dose of thiamine on the concentration of that vitamin in the blood was studied on April 7, 19k9. Blood sales were collected from AS and BLD while they were In the fasting state and at definite time intervals after the ingestion of 5 mg of thiamine hydrochloride and a lowthiamine breakfast. Blood samples were collected at 8 A.L (this was considered zero time) and. then the 5 mg dose of thiamine hydrochloride was taken. blood samples were taken at , Additional 1, l, 2, 3 and 1+ hours after the test dose was given. The usi.m]. procedure for the determination of blood thiamine was followed except that the blood samles collected on Apiil 7th were carried through the transfer of the 200 cmm of blood supernatant to each 10 x 75 mm tube. At this point the tubes were covered, frozen in dry ice and kept frozen until the next day, April 8, when the analyses were completed. The results are tabulated in Table 27. 914. Table 27 T OO1OE1TBATION OF THIAMIEE HI T ELOOD AER THE A MIEISTBATIO1I OF A 5 MG ORA.L TEST DOSE OF THIAMIEE HYDROOHLORI Subject Tjrne ELD OAS 2.59 2.63 2.65 2.85 3.32 3.46 3.13 2.58 2 3.26 2.93 3 2.89 2.30 4 2.88 2.77 0 1 A peak in the blood, thiamine level was observed. j to 1 hour after the oral ingestion of 5 mg of thiamine hydrochloride. The blood thiamine value after 1 hour represented approximately a 30 increase over the fasting level. per cent CHAPTER IX SUMMARY Q WORK WITH BURGH METHOD The blood, thiamine values obtained in the nutrition laboratory were much lower than those obtained by Dr. Burch, values reported in the literature or results obtained in the nutrition laboratory with the Priedemairn and Kmieciac method. The steps in the procedure for the Burch method were studied in an attempt to find an explanation for these low values. Varying the amout of potassium acetate used. in the procedure produced no significant change in blood thiamine values. Different brands of trichioroacetic acid. or the use of redistilled trichioroacetic acid produced no significant change in the blood thiamine values. The activity of the enzyme was tested by the determi- nation of blood. thiamine (1) with and without the use of clarase and (2) by using cocarboxylase. The amount of clarase used. was varied and another enzyme, acid phosphatase, was used in the method. Both of the enzymes proved to be active so the explanation for the low blood thiamine values did not seem to lie in this step in the procedure. Longer time and higher temperature for incubation were tried without success. The oxidation procedure was studied by observing the results of the use of larger amounts of potassium forricyanide, the omission of and the use of varying amounts of the sodium dihydrogen phosphateperoxide reagent. These variations gave no clue to the problem. Iso- butyl alcohol proved to be no better as an extractant than xi-butyl alcohol. A delay of 30 to 14.5 minutes in reading the tubes after oxidation to thiochrome had no effect on the blood thiamine reading. Variation in the time of irradiation or use of another irradiation lamp or another Farrand rnicro-photofluorometer resulted in no significant change in blood thiamine values. When all new reagents were obtained and. tested indivithially in the procedure, no change in blood thiamine value was observed. Standard. curves were determined by .5 different methods C].) addition of thiamine hydrochloride to blood supernatant (2) addition of thiamine hydrochloride to blood (3) use of thiamine hydrochloride alone (J+) use of cocarboxylase alone (5) addition of cocarboxylase to blood. curve (2) or (5) seemed to be the more valid curves for Either use in the determination of the concentration of thiamine in the blood. since in these two cases the standard. was subjected to the same manipulations as blood itself. A 5 mg oral test dose of thiamine subjects while in the fasting hydrochloride was given to 2 state, Blood samples were collected at definite time intervals and analyzed for their concentration of thiamine according to the Burch method. A peak in blood. thiamine level occurred to 1 hour after the ingestion of the thiamine hydrochloride. The blood. thiamine value after 1 hour represented approximately a 30 per cent increase over the fasting level. 97 CHAPTER X 3TURE WORK ON A MICRO-THOD SUGGESTIONS YOR BLOOD THIAMflIE Modifications in the amounts of reagents, time of reading or incubation and tests of the reagents themselves have been performed without solving the problem of our low thiamine values as compared with those values obtained by Dr. Buroli. It seems as though our problem does not lie within any of these modifications that have been tested. The major portion of blood thiamine occurs in the phosphorylated. form and much of this may be combined with protein forming a complex (Good.hart and Sinclair, 1939). There is a strong possibility that some of the thiamine is lost in the step in which the protein is precipitated by trichioroacetic acid. Hennessy (19M7) stated that "before the elimination of blood protein is brought about, both thiamin and cocarbor1ase must be separated from any combination with the protein". A suggestion for a future experiment would be to delay the precipitation of the blood protein until after enzymatic hydrolysis had. been carried out. A general plan for this experiment would consist of the following steps: 1. Deliver 50 cmm samples of blood into 6 x 50 mm test tubes containing 100 cmm of redistilled water. Buzz the tubes immediately. 2. Add. 10 cmm of 1.0 N hydrochloric acid to produce a pH of about 2 to L4. 3. Oheck the pH. Buzz the hemolyzed acidified samples. Li.. Heat the tubes in a boiling water bath for 1 to 2 minutes. 5. Cool the tubes and add 20 cmm of potassium acetate to produce a pH of M..6 to Li.8. Check the pH. 6. Add 10 cmm of acid phosphatase to the test tubes. 7. Cover the tubes with Parafilm and incubate at 37 to 38 degrees Centigrade for 1* to 2* hours. 8. Add. 100 omm of 5% triobloroacetic acid. 9. Buzz the samples thoroughly. 10. Centrifuge the tubes for 10 minutes. 11. Remove 200 cmm of the supernatant to 10 x 75 mm tubes. 12. Add 0.1 ml of oxidation mixture and tap for 15 seconds. 13. Add 0.1 ml. of sodium dihydrogen phosphate-peroxide reagent. lLi.. Add. 1.0 ml of n-butyl alcohol. 15. Buzz the tubes 15 seconds. 16. Centrifuge the tubes to separate the layers. 17. Transfer the n-butyl alcohol layer to optically matched 10 x 75 mm tubes. 18. Carry the standards and blanks through the same procedure as the blood samples. 19. Read. bloods, standards and. blanks against a quinine sulfate standard as described under 20. the Burch method. Irradiate all of the tubes 30 minutes, 3 filament of the H-5 lamp. 21. Reread all of the tubes. 22. Qalculate the results. inches from the / The Bu.rch method eliminates the step in which the thiamine is adsorbed on calso. Another suggested experiment is to use this adsorbent in the method. Sodium hydroxide could be added to the above tubes after incubation to bring the pH to 3.0 to 3.5 and. a small amount of Decalso added. This mixture could. be shaken, allowed to stand for a few minutes, shaken again, the s-upernatant removed and the Decalso washed in the tube several times with redistilled water. could be removed from the Decalso by adding a acidified definite The thiamine volume of 25 per cent potassium acetate, mixing, centrifuging and using the supernatant for oxidation as described above (except for the omission of the sodium dihydrogen phosphate-peroxide mixture). These modifications have not been used. before this because they involve changes in the Burch method rather than variations in the procedure. It is to be hoped that a solution may be found soon so that a method. may be developed that can be used satisfactorily for the determination of thiamine in small amounts of blood. BIBLIOGRAPHY 1. Alexander, B. and Landwehr, G. Studies of Thiamine Metabolism in Man. I. Thiamine Balance. The Normal Requirements of Vitamin i and the Role of Focal Thiamine in Human Nutrition. J. Olin. Investigation 25: 287-293, 194.6. 2. Studies of Thiamine Alexander, B., Landwehr, G. and Mitchell, F. Metabolism in Man. II. Thiamine and Pyrimid.ine Excretion with Special Reference to the Relationship Between Injected and. Excreted Thiamine in Norma]. and Abnormal Subjects. J. Olin. Investigation 25: 294-303, 194.6. 3. Archdeacon,J. W. and. Marlin, J. R. The Effect of Thiamine Depletion and Restoration of Mu.scular Efficiency and Endurance. J. Nutrition 28: 24.1-2514., 1944. Li. Association of Vitamin Chemists, Inc. Methods of Vitamin Assay. New York, Interecience Publishers, Inc., 194.7. 5. Atkin, L., Schultz, A. S. and. Prey, C. N. Ultramiorodetermination of Thiamine by the Fermentation Method. 3. Biol. Ohem. 129: 4.71-4.76, 1939. 6. Barker, S. B. and Suinmerson, S. W. The Colorimetric Determination of Lactic Acid, in Biological Material. 3. Biol. Ohem. 138: 535-554., 194.1. 7. Benson, R. A., Witzberger, 0. M. and Slobody, L. B. Excretion of Thiamin in Normal Children. 18: 617-620, 194.1. 8. Benson, R. A., Witzberger, C. M. and. Slobody, L. B. An Evaluation of the Blood and Urinary Thiamine Determinations in Vitamin B1 Subnutrition. J. Pediat. 23: 4.37-445, 194.3. 9. Benson, H. A., Witzberger, C. L, Slobody, L. 3. and Lewis, L. The Blood Level of Vitamin i in Healthy Children and its Relation to the Urinary Thiamine. 3. Pediat. 21: 659- The Urinary 3. Pediat. 664, 194.2. 10. Bessey, 0. A., Lowry, 0. H., Brook, M. 3. and Lopez, 3. A. The Determination of Vitamin A and. Carotene in Small Quantities of Blood Serum. 3. Biol. Chem. 166: 177-188, 1946. 11. Blanchaer, H. 0. and Cameron, E. The Relation of Thiamine Clearance to Blood. Levels in Man. J. Nutrition 35: 391-397, 19148. 10]. 12. Bueding, B. and. Wortis, H. The Stabilization and. Determination of Pyruvic Acid in the Blood. J. Biol. Ohem. 133: 585591, 1940. 13. Bueding, B., Stein, M. H. and Wortis, H. Blood Pyruvate Curves Following Glucose Ingestion in Normal and. ThiamineDeficient Subjects. J. Biol. Obem. 140: 697-703, 1941. 14. Thirch, H. B, 15. Cowgill, (. B. The Vitamin B 16. Modifications Cheld.elin, V. H., Bennett, M. J. and Kornberg, H. A. in the Lactobacillus Permenti 36 Assay for Thiamine. J. Biol. Chem. 166: 779, 1946. 17. 18. 19. Personal Communication. New York City, Department of Chemistry, Columbia University, 1948, 1949. Requirement of Man. Yale University Press, 193k. J. The Determination of Thiamin by Conner, B. T. and. Straub, G. the Thiochrome Reaction. Lid. and Engin. Ohem., Anal, Ed. 13: 380-384, 1941. Dana, W. J. and Darby, W. J. The Appraisal of Nutritional Status (Nutriture) in Huma with Especial Reference to Vitamin Deficiency Diseases. Physiol. Rev. 25: 326-346, 1945. Dietz, V. H. A Simple Micro-Beaker for Use with the Beckman pH Meter (Mod1 G). 20. 21. New Haven, Corin., Science 108: 338-339, l9J48. .Lneurin-Pyrophosphate Content of Red and White Blood Corpuscles in the Rat and in Man. Nature 162: 220-221, 1948. Florijn, B. and. Smits, G. Friedemann, T. B. and Barborka, C. J.. The Significance of the Ratio of Lactic to Pyruvic Acid. in the Blood. after Excercise. J. Biol. Chem. 141: 993-994, 1941. 22. Friedemann, T. B. and. Haugen, G. B. 23. Friedeniann, T. B. and. Kmieciak, T. C. The Determination of Thiamin 3. Lab. and. Clin. Med. 28: 1262-1268, 1943. in Blood.. 24. Gifft, H. H. and Hauck, H. M. Pyruvic Acid. II. The Determination of Keto Acids in Blood and Urine. J. Biol. Chem. 147: 415-442, 1943. A Comparison of Four Methods for Studying the Urinary Excretion of Thiamine. 3. Nutrition 31: 635-646, 1946. 102 25. A Revaluation of the Methods Described by Goodhart, H. S. Goodhart and Sinclair for the Determination of Blood Cocarboxylase Values. J. Biol. Chem. 135: 778L!, 19Lo. 26. Goodhart, H. S. and Nitzberg, T. The Thiamine Content of Human Blood and Urine as Determined by the Fermentation Method. J. Olin. Investigation 20: 625-630, 1914l. 27. Goodhart, H. S. and Sinclair, H. M. The Estimation of Cocarboxylase (Vitamin 1 Diphosphate Ester) in Blood. Biochem. 3. 33: 1099-1108, 1939. 28. 29. Goodhart, H. S. and. Sinclair, H. M. Deficiency of Vitamin Man as Determined by the Blood Cocarboxylase. 3. Biol. Ohem. 132: 11-21, l9LfO. li Gorham, A. T., Abéls, 3. 0. and Robbins, A. L. The Measurement and the Metabolism of Thiamine and of a Pyrimidine Stimulating Yeast Fermentation found in Blood Cells and Urine of Normal Individuals. 3. Olin. Investigation 21: 161-176, l9J+2. 30. Methods for Determination of Greenberg, L. D. and. Rinehart, 3. F. Thiamine in Blood and Tissues with Observations on Relative Contents. Proc. Soc. Eer. Biol. and Med.. 59: 9-13, 19k5. 31. The Harris, L. 3. and Leong, P. C. Vitamins in Human Nutrition Excretion of Vitamin i in Human Urine. Leacet 230: 88689A4, 1936. 32. Hathaway, M. and. Strom, 3. B. A Comparison of Thiamine Synthesis and Excretion in Human Subjects on Synthetic and Natural Diets. J. Nutrition 32: 1-8, 19146. 33. Hawk, P. B., Oser, B. L. and Summerson, W. H. Practical Physiological Chemistry, 12th edition. Philadelphia, Pa., 314. The Blakiston co., l9L17. Hennessy, D. J. The Chemical Methods for the Determination of Biological Symposia XII: 86-120, 1914.7. Thiamin. 35. The Determination of Free and Hennessy, D. 3. and Cerecedo, L. H. Phosphorylated Thiamine by a Modified Thiochrome Assay. 3. Am. Chem. Soc. 61: 179-183, 1939. 36. The Determination of Early Horwitt, M. K. and Kreisler, 0. Thiamine-Deficient States by Estimation of Blood Lactic and. Pyruvic Acids after Glucose Administration and Exercise. 3. Nutrition 37: 14.11L28, 19149. 103 37. Horwitt, M. K., Liebert, L, Kreisler, 0. and. Wittinan, P. Investigations of Human Requirements for B-Complex Bulletin of National Research Council No. Vitamins. 116, Washington, D. C., National Research Council, National Academy of Sciences, 1948. 38. Keys, A., Henschel, A. F., Mickelsen, 0. and Brozek, J. M. The Performance of Normal Young Men on Controlled Thiamine Intakes. J. Nutrition 26: 399-415, 1943. 39. Knott, E. M., Kleiger, S. C. and Torres-Bracamonte, F. Factors Affecting the Thiamine Content of Breast milk. J. Nutrition 2k: 49-58, 1943. 40. Ascorbic Acid. Rapid DetermiLoeffler, A. J. and. Pouting, J. S. nation in Fresh, Frozen or Dehydrated Fruits and md.. Engin. Chem., Anal. Ed. 1k: 846, 1942. Vegetables. 41. Lowry, 0. H. A Micro Photofluorometez'. 682, 1948. 42. Lu, G. D. 43. Mason, H. L. and. Williams, H. D. The Urinary Excretion of Thiamine as an Index of the Nutritional Level: Assessment of the J. Olin. Investigation 21: 21+7 Value of a Test Dose. 255, 1942. 44. Meiklejohn, A. P. The Estimation of Vitamin :B,1 in Blood by a Modification of Schopfer's Test. Biochem. 3. 31: 14411k5l, 1937. 45. Melnick, D. and. Field, H. Thiamine Clearance as an Index of Nutritional Status. 3. Nutrition 24: 131-138, 1942. 46. Melnick, D., Field, H. and Robinson, W. D. A Q,uantitative Chemical Study of the Urinary Excretion of Thiamine by Normal Individuals. 3. Nutrition 18: 593-610, 1939. 47. Merck and. Company Determination of Thiamin Hydrochloride by the Thiochrome Method, with the Adaptation of the Hennessy and Cerecedo Procedure, as Carried out in the Labs. of Merck and. co. Inc., Rahway, N. 3., 1941. J. Biol. Chem. 173: 677- Studies on the Metabolism of Pyruvic Acid in Normal and. Vitamin B1 Deficient States. 2. Blood Pyruvate Level in the Rat, Pigeon, Rabbit and Man. 3. The Relation of BloodPyruvate to Cardiac Changes. Biochem. J. 33: 774-786, 1939. 104 48. Mickelsen, 0., Caster, W. 0. and Keys, A. Urinary Excretion of Thiamine as a Characteristic of the Individual. Proc. Soc. Exper. Biol, and Med.. 62: 254-258, 1946. 49. Mickelsen, 0., Condiff, H. and Keys, A. 50. Najjar, V. A. The Biosynthesis of Thiamine Assn. 123: 683-684, 1943. 51. National Research Council The Determination of Thiamine in Urine by Means of the Thiochrome Technique. J. Biol. Ohem. 160: 361-370, 1945. Revised 1948. in Man. J. Am. Med., Recommended Dietary Allowances. Washington 25, D. C., Food. and. Nutrition Board, National Research Council, 2101 Constitution Ave., 1948. 52. Nutrition Reviews Lactate-Pyruvate Relations in Blood of Man. Nitrition Reviews 6: 259-261, 1948. 53. Thiamine Excretions Qldham, H. G., 1.vis, M. V. and. Roberts, L. J. and. Blood Levels of Young Women on Diets Containing Varying Levels of the B Vitamins, with Some Observations J. Nutrition 32: 163on Niacin and. Pantothenic Acid. 180, 1921.6. 54. Oldham, H. G., Johnston, P., Kieigor, S. and Hedderich-Arismendi, H. A Study of the Riboflavin and Thiamine Requirements of Nutrition 27: 435-446, Children of Preschool Age. J. 1944. 55. Old.ham, H. CL, Roberts, L. J. and. Young, M. Results of Providing a Liberally Adequate Diet to Children in an Institution. III. Blood and. Urinary Excretion Studies Before and After Dietary Improvement. J. Pediat. 27: 24.18, 1945. 56. Perkins, J. L. The Effect of Different Levels of Thiamine Intake Corvallis, on the Urinary Excretion of Thiamine. Thesis. Oregon, Oregon State College, 1943. 57. Pollack, H., Dolger, H., Ellenberg, M. and. Cohen, S. 58. Pollack, H. L., Ellenberg, N. and. Dolger, H. Excretions of Thiamin and its Degradation Products in Man. Proc. Soc. Exper. Biol. and. Med.. 47: 414-417, 1941. 59. Ritsert, IC. A Test Proposed to Measure Vitamin B1 Saturation in Hums. Proc. Soc. Exper. Biol. and Med.. 44: 98-100, 1940. Die Aneurinbestimmung in ICleinen. Blutmengen Nach der Tbiochromverfahren. Klin. Wchschr. 18: 852-854, 1939. 105 The Clinical Significance and. Estimation of Blood Vitamin B. Brit. M. J. 2: 878-883, 1938. 60. Rowland.s, E. N. and. Wilkinson, J. F. 61. Salcedo, J., Carrasco, B. 0., Jose, F. R. and. Valensuela, R. 0. Studies on Beriberi in an Endemic Sub-Tropical Area. J. Nutrition 36: 561-578, 19148. 62. The Use of Lactobacillus Sarett, H. P. and. Cheldelin, V. H. Fermentwn 36 for Thiamine Assay. S. Biol. Obem. 155: 153-160, 19144. 63. Schmidt, G. 614. Schultz, A. 5., Personal Communication. Boston, Massachusetts, Tufts College Medical School, l9M9. Atkin, L. and Frey, 0. N. (a) A Fermentation Test for Vitamin i J. Am. Ohem. Soc. 59: 9148, 1937. (b) II. A Fermentation Test For Vitamin B. S. Am. Ohem. Soc. 59:214.57-21460, 1937. 65. The Estimation of Vitamin B1 in Blood. Sinclair, H. M. Biochem. J. 32: 2185-2199, 1938. 66. Sinclair, H. M. The Estimation of B1 in Blood. Modification of Meiklejohn's Method. II. A Further Biochem. S. 3: 2027-2036, 1939. 67. Ithaca, New York, Sumner, J. B. Personal Communication. Laboratory of Enzyme Chemistry, Cornell Uni-ersity, 19149. 68. Weiss, Soma The Application of Electrocardiography in the Detection of Avitaminosis B Nutrition: The Newer New York, Milbank Memorial Fund, Diagnostic Methods. 1938. 69. Wi&enbauer, P. Uber den Cocarboxylase-Gehalt des Menschliches Blat. Kim. Wchschr. 18: 1392-13914, 1939. 70. Williams, B. B. and Spies, T. D. Vitamin B1 (Thiamin) in Medicine. and its Use New York, Macmillan co., 1938. Diagnosis and Treatment. Nutritional Deficiences. Philadelphia, Pa., S. B. Lippincott co., 19143. 71. Younians, S. B. 72. Youinans, S. B. and Patton, B. W. The laboratory Diagnosis of Nutritional Deficiences, Clinics, Vol. I, No. 2, Philadelphia, Pa., S. B. Lippincott co., 19142. APPENDIX 107 APPENDIX I DIRECTION SHEET GIVEN TO THE ST3BJECTS IN STtJDY II January 19148 1. Finger prick blood samples will be taken each day (come to laboratory at 6:11.5 each morning; 7:45 on Sunday). 2. Venip'uncture samples will 3. The subject mast be taken every 5th day. eat all fruits, vegetables and milk that are for him. The amount of weighed out bread, butter, meat, sweet etc. may vary according to the capacity of each subject. The subject mast not eat any foods other than those weighed out rolls, Black coffee will for him at fliealtlmes. meals, that is, no sugar or be permitted. between cream or milk may be added. 14.. The subject mast not take any vitamin pills, aspirin or anymed.icine. 5. Each morning a solution of crystalline ascorbic acid. dissolved in water will be given after the blood sa1e has been taken. 6. Daring the second and. third periods (last 20 solution of ascorbic acid will be given days) an after the evening meal (7:00 to 7:30 or as soon as the foods have been 7. The study will last 8. analyzed). from January 31st through the taking of a samule on the morning of March let (30 days). subject mast additional be present at blood This means that the fl meals. Schedule: Come to laboratory - 6:4.5 .LM. on week days; 7:14.5 A.M. on Sunday 700 week days, 8:00 Sundays Breakfast n u " 1:00 12:00 Lunch " " 5:30 6:oo Dinner 108 APPENDIX II Table 28 INTAKE OF TOTAL AND NON-FAT CALORIES, RATIO OP TOTAL TO NON-FAT CALORIES, THIAMINE INTAKE IN TERMS OF MCG PER BkY, MOG PER 1000 CALORIES MCG. PER 1000 NON-FAT QALORIES AND TKE VALUES OR THIAMINE IN TKE BLOOD DURING A 30 DAT EU'ERIMENTAL PERIOD1 Study I Norma caloric Intake Ratio of FF0 to Total Oal Total FF0 ]te 19k? Oct. 18 2195 1288 1168 1464 19 1976 20 24.51 21 2158 Mean 2195 1334. Oct. 22 2958 23 2089 179? 1521 2379 25 2099 26 2817 14.32 24. 14.16 24.68 1393 1850 1599 Oct. 27 2362 1534. 28 29 30 31 2034. 1263 24.77 14.54 1464 1336 Mean 2048 2082 2201 Nov. Mean Mean 1 Thiamine Intake Per 1000 Per mcg y Cal mog Per 1000 NEC mog Thiamine In Blood mcg 59 59 60 66 61 1198 1033 1558 1029 1205 54.6 930 523 636 477 88k 106k 61 124.3 4.20 73 60 66 66 6 1621 1166 1276 835 1228 776 692 1066 4.90 814. 6o8 296 518 916 k51 788 65 62 59 71 1070 1248 1269 1300 1066 1191 453 698 988 873 888 798 849 9.76 8.84. 4.92 785 835 757 561 820 64. 14.10 64 1 2110 14.77 2 2321 3 1962 4. 1872 5 2051 2063 1325 1276 1280 1262 1324 70 57 6 68 62 64 1159 1106 966 1050 850 1026 See footnote at the end of thiE table. 54.6 614. 512 635 512 54.5 54.9 477 72? 901 4.14. 674. 499 774 9.57 Table 28 Stud.y I Norma (continue&) Thiamine Intake Caloric Intake Ratio of ThC to I.te Total NC Total Cal 191+7 Nov. 6 2578 161+2 7 2505 8 2205 9 3035 11+75 Per I.y meg 614 145]. 1281 1003 975 109k 1161 Per 1000 Per 1000 Cal )IPC Thiamine In Blood meg meg acg% 563 511 8814. 321 538 868 760 521 83k 1+78 773 628 800 697 21+72 1521+ 59 60 62 65 62 Nov. 11 2582 16714. 65 11+07 64 1051 1125 1+07 12 2187 13 3288 11+ 2505 15 1936 Mean 2500 424 337 6014. Nov. 16 2313 114.96 10 Mean 1320 1872 1312 1999 1398 1315 1559 14.55 5114. 61 1391+ 56 81i4 68 63 781 1039 1+03 59J 417 665 65 741 320 1+95 7.52 7.72 6.12 110 Table 28 (continued) Study I Barbara ]te 1947 Thiamine Intake Caloric Intake Ratio of Per 1000 Per 1000 NFC to NF0 Cal Total Cal Per ]y NFO Total meg meg - meg Oct. 18 2092 19 2164 20 2296 21 2098 Mean 2163 Oct. 22 2986 23 2190 2585 25 1961 26 75 Mean 2487 24. Oct. 27 2331 28 2141 29 2373 30 280 31 2287 Mean 2302 Nov. Mean 1565 1332 1395 1744 1.508 1509 1611 1323 1443 1434 237 Mean Nov. 1662 1543 1293 1852 1639 3 2545 5 22144 6 7 8 9 10 2856 3030 2463 3106 2187 2728 1475 1003 1236 644.2 478 570 62 76 60 66 68 66 1444. 484. 1873 1277 1238 781 1323 855 67 62 59 73 66 65 1093 1431 1177 70 58 63 68 62 64 184.6 11i46 194.9 552 606 63 1 2052 2 2335 4. 1154 1312 1321 1382 1357 1365 1356 134.6 1883 181 1528 18714. 1419 1697 64 59 65 63 66 59 62 60 65 62 14.94 631 288 550 949 1087 735 911 782 1127 828 957 422 696 107l4 1190 1163 1199 1139 980 580 823 4.98 864. 4.71 744 584 4.19 861 679 11314. 510 794. 1411 1373 1193 4.94 74.9 14.53 771 781 557 829 737 484 104.3 36 1176 1239 538 461 940 823 668 496 672 486 558 1111 1282 meg 87'4 4.68 1OO Thiamine In Blood 9.93 844 917 737 854 7.12 6 92 111 Table 28 Study I Barbara (contirn2ed.) Date 1947 Caloric Inta]e Ratio of PC to Total Cal ILFC Total Thiamine Intake Per 1000 6.12 4.91 Cal meg meg 391 549 337 436 412 599 82? 590 616 639 654 380 594 1679 1512 1758 1497 1549 1599 65 66 62 1006 1250 1037 922 990 1041 Nov. 16 2231 1425 64 847 57 56 68 Thiamine In Blood Per Iy 2575 2277 3079 2650 2 2571 Nov. 11 12 13 14 15 Mean Per 1000 N'0 meg 3148 mcg% 112 Table 28 (continued) Study I Marion Thiamine Intake Caloric Intake Ratio of I0 to i.te 1947 Total NPC Total Cal Per 1000 Ca]. meg meg 9214 1214.7 576 566 578 80k 90k 1211 1164 1281 120? 1216 62 62 59 63 62 1119 1065 90 509 1100 557 62 76 65 67 67 1167 1556 1080 1155 781 1148 414.9 22144 1618 1480 1402 1221 1745 1493 Oct. 27 20814. 1350 28 2023 29 2327 30 2214.7 31 2233 Mean 2179 13311. 65 66 995 1541 1379 1610 1453 1425 59 72 66 66 11511. Nov. 70 59 66 69 ink Oct. 18 19 20 21 Mean 1943 1882 2156 190k 1971 Oct. 22 2600 23 1957 2179 25 1884 26 2599 24. Mean 20714. 1420 1298 1353 1333 1315 2056 13144 214.73 1669 1680 1372 1 2029 2 2203 3 2036 4. 5 Mean Nov. Mean 6 7 8 9 10 1939 2771 2235 3002 2213 2539 614. 63 65 14614. 67 61 61 60 66 1600 63 18114. Per 1000 NYC mog Per ]y Thiamine In Blood aog$ 915 973 10.85 613 721 1051 770 946 30]. 4148 531 787 477 762 496 630 458 565 737 1155 837 880 697 861 9.93 1012 980 1077 893 1015 785 780 9.03 4.59 481 555 724 808 4.31 679 755 1296 1370 1065 1051 1179 1192 524 494 1416 1013 122sf 795 496 511-9 495 477 350 533 476 777 815 776 579 805 750 8.47 113 Table 28 Study I Marion (continued.) Thiamine Intake Caloric Intake Batlo of N'C to 1911.7 Nov. U 2301 14.67 64. 12 1832 13 2856 1255 1718 1593 Per 1000 Per 1000 meg meg meg 383 601 790 15 1956 Mean 2339 1374. 69 60 58 70 1481 64. 882 992 938 1018 81? 929 Nov. 16 2'496 1630 65 1080 14. 2751 54.1 328 370 Thiamine meg 6.72 54.6 4.18 639 595 4.08 634. 11.33 663 6.32 114 Table 28 (continued.) Study I Roberta te Caloric Intake Ratio of NPC to 1I'C Total Gal Total 19147 Oct. 18 2136 19 1958 20 2143 21 2202 Mean 2110 Oct. 22 2628 23 2145 1245 1200 1216 1457 1280 58 61 60 72 61 Thiamine Intake Per 1000 Per D9y meg Cal meg 1088 1103 1252 io6 509 563 584 480 1125 53k l434 614. 1141 1925 1078 1317 664 1225 57 66 61 Mean 2275 1568 1552 1356 1316 1512 1461 Oct. 27 28 29 30 31 2135 2005 2225 2339 2254 2192 1392 1246 1318 1700 1529 1437 65 62 59 73 68 65 1000 1409 1095 1545 1211 1252 468 1 2220 2 2239 1573 1268 1366 1185 1217 1322 71 57 1324 1021 1125 989 849 1062 596 456 515 553 451 1592 1606 1520 1771 1458 1589 67 58 63 60 66 63 211. 2222 25 2022 26 2356 Mean Toy. 3 2183 Mean 1787 5 1882 2062 Noy. 6 2372 14. 7 8 9 10 Mean 2762 2418 2947 2211 2542 6.5 614. 63 66 65 64 1409 111-02 1247 1005 1236 1260 897 Thiamine In Blood mog$ 87k 919 1030 725 887 728 1240 1485 79.5 651 282 550 999 439 718 1131 831 909 792 876 703 492 661 537 572 ' Per 1000 1FC meg 9.93 8140 10.12 842 805 5114. 835 698 801 594 508 516 341 885 873 820 567 559 8148 504 799 9.02 115 Table 28 Study I Roberta (continued) Thiamine Intake Caloric Intake Ratio of Per 1000 Ii'C to ]te Total 1IFO Total Cal l9Lf7 12 2052 1 2836 1k 2689 15 2005 1721 1370 1615 1530 1386 21414.9 15214. 57 57 69 63 Nov. 16 21450 1569 614 Nov. 11 266.5 Mean 65 67 Per y meg Oa.l mog 1114.7 14.30 1078 86k 525 305 352 9146 Per 1000 NYC meg 666 787 535 618 821 971 1409 592 140k 6140 9142 38k 600 Thiamine In Blood acg$ 8.28 5.91 116 Table 28 (continued.) Stu4y I Beesie Calorie Intake Ratio of NFC to Total Total Cal 1170 Date 1911.7 Oct. 18 2248 19 1997 1323 1173 1353 59 59 58 1376 1306 63 60 Oct. 22 3007 23 2068 184O 24 2311.9 1314.1 25 2062 26 2383 1291 1593 1508 61 71 57 63 67 20 2351 21 2169 Mean 2191 Mean 23711. Nov. Per 1000 Cal 1170 meg meg 1107 1083 1276 1077 1136 492 837 923 943 783 872 11449 4.82 14.221. 689 1090 1234 685 1176 4621. 63 63 62 71 6s 65 2147 2 2251 3 zo6o 4. 1897 42 5 2099 1537 1259 1235 1284 1328 1329 72 56 6o 68 62 64 1200 1010 963 1066 6 2274. 7 25214. 8 2264 9 2786 1608 1526 71 60 1i449 6li. 1659 1415 1531 60 69 65 1537 1323 1110 936 1251 1231 28 1792 29 2362 30 2351 31 1946 Mean 2137 Mean 64. Per 1000 Per Day meg 1403 1122 1473 1675 1267 1388 Oct. 27 2233 Nov. 114.73 Thiamine Intake 3. 10 2.037 Mean 2377 1096 1181 54.2 543 24.97 519 598 28? 788 967 813 956 2430 791 4.91 781 1053 768 906 721 846 9.57 781 802 780 6.92 '1131 1517 914 1168 470 549 1031 incg 504. 659 479 9114. Thiamine In Blood 64.5 559 1449 4.67 562 427 493 80 676 524 956 867 766 4.90 688 776 336 614 564. 528 807 884. 7.12 117 Table 28 Study- I Beesie (contiuu.ed.) Thiamine Intake Caloric Intake Ratio of NTC to meg 19k7 6k 67 58 851 967 8k2 1k 2327 15 198k Mean 2220 IL45 1228 1561 13k6 1399 1396 58 88k 71 64 931 895 Nov. 16 2379 1549 65 777 Nov. 11 2259 12 1820 13 2710 Per 1000 Per 1000 Thiamine meg meg mcg% 377 531 311 380 469 7.52 414 589 787 539 657 665 647 327 502 6.72 118 Table 28 (continued) Study II Tom Caloric Intake Ratio of MFC to Total Cal Total NYC ]te 191i.8 6L40 595 1911 2808 2225 652 538 1008 823 1174 911 2258 2243 1936 4.14J44 2652 2752 2149 3030 2707 2698 64 2300 2950 2938 3518 2658 2873 Feb. 14 5507 15 14586 16 1+895 17 14.466 18 5258 2962 2593 2979 2858 5 5039 6 3621 7 5144 8 4476 Mean Feb. 1+514.5 9 1+035 10 11 12 13 Mean 5293 14550 6055 14625 4912 Mean 49142 Feb. 19 1+2142 20 21 22 23 14726 Mean 14700 1+707 14786 14632 mog 1421 21449 1 meg 55 59 59 60 59 508 1445 2303 535 398 678 513 57 17140 1+31 56 1914.9 65 58 59 2506 2780 1997 2194 368 551 2973 54 21437 414.3 27814. 61 61 58 2172 2112 2044 2316 2216 4714. 2547 2957 2841 2852 2865 2812 Per 1000 NYC meg 25141 14.103 Feb. Cal 1639 3 14308 Mean Per 1000 Per Iy 66 59 59 56 60 2560 2522 2323 2392 Jan. 31 3891 Feb. 1 14.268 2 3914.5 Thiamine Intake 57 57 58 60 63 60 61 60 61 2014.6 303 1459 1432 1448 1431 1458 1440 1449 2324 514.8 20140 1432 1849 1806 2393 2082 393 381+ 500 1+51 Thiamine In Blood acg% 9.30 792 815 901 675 1120 861 10.35 757 661 853 790 751 762 12.00 820 780 713 788 777 776 12.27 912 690 651 633 835 744 8.00 119 Table 28 Studi II Tom (continnea) Date 19k8 Caloric Intake Ratio of NFC to Total Cal 1FC Total 7eb. 2k k120 25 14474 26 3490 27 4904 28 4?69 Mean 4351 Feb. 29 4989 Thiam Intake Per 1000 Per 1000 Thiamine In Blood, Per Dajy Cal meg meg meg mcg% 1911 1701 1715 1874 1988 1838 k6k 380 491 382 427 762 679 819 633 749_ 728 11.18 25444 61 6 60 60 56 59 2806 56 2052 411 731 13.08 2507 2507 2093 2959 2655 24.1? 120 Table 28 (continued) Study II Ive Caloric Intake Ratio of NFO to Total Gal Total C te 1948 Jan. Feb. 1989 11.79 675 8.70 211.140 443 506 381 673 496 784 678 1135 823 23.57 614. 19714. 3 39 63 3036 2290 6149 14082 2238 2537 4151 2911.6 71 5 5508 3113 2233 2898 6 3730 7 5151 8 14639 .27148 57 6o 56 39 14636 2788 61 48148 2692 3348 56 9 10 11 12 Feb. 14 6104 4982 6653 3905 5298 5314.3 15 4657 16 4888 17 5049 18 59 Mean 14.999 19 20 21 22 23 5297 5240 5327 5154 1888 19614 20 2280 841.5 1995 2166 2707 2907 1435 1412 714.1 55 60 56 355 647 910 783 57 22142 1423 7145 2902 2770 2919 2986 2870 2889 511. 2156 2215 1986 2385 14.04 10 00 23147 464 2218 4144 743 800 680 799 818 768 2925 55 65 58 58 2914.1 555 376 380 1005 7.32 29711W 3713 222? 2991 31416 3098 2997 59 6o 59 5? 58 77_2768 5158 acg 553 2 3692 14 Thiamine In 3lood 8.70 1501 2650 3701 Per 1000 NYC meg 633 988 838 1109 892 406 623 535 63 Mean Mean meg 64 2.3 Feb. Cal 2372 2682 Mean Feb. Per 1000 Per y meg 1 14257 3]. Mean Feb. Thiamine Intake 3041 59 1972 2023 2154 2048 2228 5L1.3 437 367 1476 1406 1472 8.314 6144 1418 577 653 719 1429 7140 1432 739 121 Table 28 Study II 1ve (continued) Caric Intake Thiamine Intake Batio of NFC to .te Total NFC Total Ca]. 1948 Feb. 24. 4444. 2529 25 4513 24.58 57 54 26 14.079 4.572 2228 3108 2562 2578 55 60 55 56 Feb. 29 5217 2800 514. 27 514.5 28 k78 Mean Per Iy Cal Per 1000 FC Thiamine In Blood mog meg meg meg% Per 1000 1773 1261 1879 1909 1867 1738 399 279 70]. 4.61 84.3 371 399 382 614 72? 680 2049 393 732 9.30 513 12.00 122 Table 28 (continued) Studs II Wesley Thiamine Intake Caloric Intake Ratio of FC to mcg Jan. 31 3670 Feb. 1 3558 2 3501 3 1956 Mean Feb. Feb. meg meg 1522 2278 14.15 65 57 16114. 2614.5 461 669 63 221+3 61+0 61 2015 51+6 2678 2398 2114 2230 2461 2376 64 2106 1883 1835 1369 2298 1898 507 56 13 331 2022 2499 2593 3311 2096 4363 2501+ 44. L4.155 6 3570 7 3716 8 Q10 3943 9 3637 10 1+J+35 11 4302 12 5608 Mean Feb. 14 3891 2222 1960 2516 16 1+134 17 3865 2185 18 4311_ 2525 2282 Mean 3909 15 3284 Feb. 19 3886 20 21 22 23 Mean Per 1000 2215 367]. 5 1+266 Mean 2296 2312 2010 Per 1000 4537 3520 3567 3726 3847 2099 2918 2042 2091 2250 2280 56 59 60 61 60 56 60 59 55 57 1690 1568 2355 2177 1536 1865 444.]. 514 368 73 1+81 1+65 354 51+7 388 Thiamine meg 663 985 803 1179 908 9.00 786 785 868 10.32 611+ 9 797 836 627 908 658 11.01 1+01 11.31 752 17011. 438 14.31 767 723 696 778 721 737 11.16 1417 1750 1700 1821 1678 51i. 2228 573 171+9 58 59 60 1392 1509 17.3k 1722 385 395 1061 599 682 722 8.70 61+ 57 60 6]. 57 58 59 59 423 41+0 416 430 1+23 465 1#1.5 767 123 Table 28 Study II Wesley (continued.) Caloric Thiamine Intake jitake Ratio of WPC to meg 1948 3781 25 3584 26 29514. 27 4162 28 o62 Mean 3709 2248 1996 1794 2585 2338 2192 59 56 61 62 58 59 1532 1196 1333 1354 b. 29 3872 2245 58 Feb. 211. Per 1000 Per 1000 ThiamiEe meg meg meg $ 14.05 68]. 10.32 334 599 1+51 71+3 325 5221. 128 1+2 111.29 388 657 1314.0 314. 597 15.32 124 Table 28 (continned) Stmiy II Don Caloric Intake Ratio of NFC to NPO Total Cal Total te 1948 Jan. 31 4089 Feb. 1 3629 2 3 924 0 Thiamine Intake Per 1000 Cal 11TC meg meg meg 429 577 587 78? 595 721 987 985 1408 1025 10.70 448 317 6k 403 585 463 718 581 947 721 947 783 10.70 420 377 550 746 643 871 709 10.85 2436 2121 550 1068 1544 60 58 6o 1756 2093 542 56 15014 59 1474 1308 Mean 2638 Feb. 4 2919 1822 2325 1983 1941 2574 2129 62 55 60 56 62 Mean 5 4255 6 3332 7 3475 8 4168 3630 59 1878 1400 2437 1675 4069 4719 4083 5513 2291 2763 2578 3297 56 59 63 60 1710 1777 2246 2356 Feb. 9 10 11 12 13 Thiamine In Blood meg Per 1000 Per]y 150 421i. Mean 755 Feb. 14 397? 15 4277 16 4103 1.7 4359 18 421? Mean 4187 2289 2597 2565 2480 2444 58 61 63 57 247,5 59 Feb. 19 4703 20 4683 21 3993 22 4607 23 3888 Mean 4375 2861 2918 2464 2627 61 62 62 2284 59 60 2631 58 .57 2549 2431 1681 1924 1898 2097 641 568 410 441 2614 2040 2122 2034 1715 2105 556 436 531 442 441 481 145o 502 1114 9.30 936 776 777 852 914 699 861 774 751 800 9.15 125 Table 28 Study II Don (continued) 1te Caloric Intake tio of NYC to Total Cal NYC Total 19kB 368 1+18 71+7 14.16 720 1487 81+6 1+96 58 58 2213 56 3932 2280 Feb. 29 1+547 2617 Per 1000 NYC meg 713 683 852 603 1698 1608 1692 1626 __2265 Mean meg 383 61 28 mcg 1+17 266.5 26 31+26 27 1+375 Ca]. 141+2 58 56 58 25 3765 Per 1000 Per ]y 1688 2367 2110 1992 Feb. 21+ 1+049 Thiamine Intake Thiamine In Blood acg% 11.01 1k.20 126 Table 28 (eontirnze) Stnd.y II Keith Caloric Intake Ratio o1 NFC to Total PC Total Gal te 1948 Thiamine In B10 9.30 14.07 63k 622 455 2868 1212 1930 1187 2057 1597 960 790 1307 923 3321 1949 1188 1908 2938 2261 1949 1149 709 1145 1662 1323 59 59 60 60 57 59 1332 811 538 809 1628 1024 401 416 453 9 2503 1378 1900 zio6 2335 1429 1830 55 993 1096 397 323 164.9 1489 1505 93 1227 374 352 388 64.5 1890 1732 1863 1811 1969 1853 58 1291 1260 398 425 683 56 60 11514. 34.9 1345 44.4 5? 14.22 409 58 1294 4.05 1828 2554 1753 2116 1705 1991 56 66 58 59 62 60 1714 1377 526 355 328 381 348 388 4. Mean 10 11 12 13 Mean 3394 3369 4024 2498 3158 Feb. 14 3247 15 16 17 18 2964. 3306 3028 3472 3204 Feb. 19 3260 20 3878 21 3014 22 3577 23 274]. Mean meg Per 1000 }WC meg 65 58 57 61 5 6 7 8 Mean Gal 64. Mean Feb. Per 1000 y Per meg 1911 2011 1502 1574 1750 Jan. 31 2978 Feb. 1 3105 2 2609 3 2781 Feb. Thiamine Intake 3294 56 63 58 57 58 58 990 1364 953 1280 74.0 556 424. 554 450 meg 683 706 759 707 980 767 11.01 721 9.00 577 783 625 670 9.68 727 619 743 722 699 938 539 565 64.5 559 64.9 8.00 12? Table 28 Study II Keith (continued) Thiamine Intake Caloric Intake Ratio of NFC to te Total I1'C Total Ca]. l9Lt8 Per 1000 Per meg .y C meg meg 619 566 719 520 61 57 62 62 59 60 1176 378 10144 32k 1166 1063 1332 1447 3087 1900 18k3 1622 2044 1882 1858 116 32k k15 378 Feb. 29 393k 2205 56 114.7k 375 Feb. 2k 25 26 27 3109 3227 2607 3285 326 Mean Per 1000 Ca]. Thiamine In Blood 11.32 626 668 13.93 128 Table 28 (continued) Stu4 II Ruth Caloric Intake Batlo of FC to NFC Total Cal Total te 1948 Jan. 31 1461 Feb. 1 1728 501 383 532 370 736 863 5014. 922 1154 1053 1273 804 1041 62 55 6 1448 63 6i 62 66? 730 678 859 545 696 1177 997 61 4. 1566 1081 5 6 7 8 1374. 9 111.89 Mean 2083 1610 2024 1271 1695 Feb. i14. 1932 15 1555 16 1832 17 1375 18 12148 Mean 1588 Feb. 19 1098 20 1817 2]. 1080 22 2011.7 23 1227 Mean 1454. meg 784 889 745 761 Feb. 10 11 12 13 mog 69 59 68 59 6o 63 1012 Feb. Cal 444 698 Mean Mean Per 1000 Per ]y 648 1206 725 1336 979 1019 1049 937 2 1481 3 1754 1606 2322 1400 2059 1543 1778 Thiamine Intake 1011.2 958 1212 922 1109 70 6]. 63 59 63 614. 1314.3 777 719 1003 57 702 1216 675 1301 758 930 64 58 63 67 63 64 62 64 490 762 599 350 421 Per 1000 NYC meg 636 1150 774 1282 Thiamine In B10 mog% 8.70 96]. 725 647 778 628 1231 802 9.30 723 633 10.00 14.29 644 675 678 4.14 671 779 403 662 673 632 869 982 764 11.00 8.35 424 67]. 14.32 811.9 463 675 706 736 4.91 951 708 866 390 4.514. 4.20 1355 582 673 85]. 4.16 6514. 652 723 531 860 825 566 14.71 525 129 Table 28 Study II Ruth (continued.) De.te Caloric Intake Batto of NFC to Total Oal NFC Total 1948 Feb. 24 25 26 27 28 Mean 981 1192 1196 1106 740 626 685 677 663 678 1462 60 860 1212 Feb. 29 2442 94.9 meg Per 1000 NPC meg 386 398 490 406 611 660 698 568 376 55)4. 471 618 352 588 Per 1000 y Per meg 63 60 70 72 68 67 1915 1573 1399 1666 1261 1663 1 Thiamine Intake Gal Thiamine In Blood 13.93 Values for total Calories and. thiamine were obtained, from the following references. The nonfat Calories (NFC) were calculated from the values for protein and carbohydrate obtained from the same tables. Bowes, A. and Church, 0. F. Food Values of Portions Commonly Used. Philadelphia, Philadelphia Ohild Health Society, 311 8. 2d. ed.. Juniper St., 1939. Bureau of Euwan Nutrition and. Home Economics, U.S.D.A., in cooperation with the National Beseareh Council. Tables of Food Composition in Terms of Eleven Nutrients. U.S.D.A. Misc. Pub. No. 572. New York, Houghton Chaney, M. S. and. Ahlborn, M. Nutrition. 3d. ed. Mifflin Co., 194.3. Cooper, 1. F., Barber, B. N. and. Mitchell, H. Nutrition in Health and. Philadelphia, J. B. Lippincott co., 194.7. 10th ed.. Disease. Federal Security Agency, U.S. Public Health Service. Food Value Table for Calculation of Diet Becord.s. Nutritional Charts. 12th ed.. Pittsburgh, Pa., Heinz, H. .7. Company. Research Dept., H. .7. Heinz Co., 1946. Lowe, Belle. Lowe Dietetic File. 2d. ed. Ames, Iowa, Belle Lowe, Iowa State College, 1929. New York, 4th ad.. Rose, M. S. A Laboratory Handbook for Dietetics. Macmillan Co., 1937. Taylor, C. K. Food. Values in Shares and Weights, New York, Macmillan Co., 194.2. 130 APPEIThIX III DIR0T IONS FOR SUBJECTS A. Saturday, A..M., January 29 The study will begin officially on between 6:15 and 6:30 A.M. the Each person will report to laboratory at that time daily. If all goes well, the experiment will end. with the completion of analyses on March 12, l9iJ9. B. The study will be divided into 2 parts of 3 weeks each. Period I. Supplements of crystalline thiamine will be given each day to bring the total thiamine intake up to the recommended allowance of the National Research Council. The basal diet will contain 300 micrograms per 1000 Calories. Period II. The basal diet will not Each person will, thiamine The total for be supplemented this therefore, receive 300 period. of micrograms every 1000 Calories consumed. daily ascorbic acid intake Supplements of ascorbic acid will will be 25 mg per day. be given daily. airing the first three days the supplements may be given at night, but beginning with the kth day the supplements will be given in the morning. 0. The basal diet contains about 1000 Oalories. No foods (except coffee without cream and sugar) will be allowed libitum. Additions to the diet are planned in units which contain about 500 Calories and. 150 mcg of thiamine. You may decide during the first three days, how many of these units you need in addition to the basal diet, but once you make your decision you are expected to 131 consume the same amount of food. each day. D. 3].00d. samples will E. Urine collections be taken daily. for 2Lhour periods will begin after the first voiding on the first day and. will include the first voiding on the following day. volume, of 2% You will preserve each collection with io%, O3 in 2 2 days. You will be given 7. Do not take 2SO4. This preservative is a fresh supply every stable for other day. aspirin, vitamin pills or any medicine during the course of the entire experiment. (Subject BWC has some dietary restrictions prescription and medication by and. although her regime will not be the same as the others, it will be constant throughout the study.) by 12 .APPENDIX IV CALIBRTI0N OF THE MICBO-PIPETTRS Micro-ipettes were made in the laboratory and were calibrated by delivering the liquid into a graduated pipette and noting the volume delivered and. consistency of delivery of that pipette. To be sure that calibration in this manner was reasonably accurate the consistency and volume of delivery of the micro-pipettes was determined by weighing the volume delivered on a micro-balance (the author delivered the samples as done in the laboratory and. Mr. C. H. Wang of the Chemistry Department carried. out the weighing on the micro-balance). The readings by weight on the micro-balance and calibration by volume as carried out in the nutrition laboratory were very close so that our method of calibration Results of the weighings in comparison could be considered acceptable. with the calibration by volume are given below: Volume of A. micro-pipettes as determined. by delivering water into a graduated pipette and reading volume delivered. 50 cmm Weight of water delivered. by the same M. micropipettes at 18.2 degrees Centigrade i9,Lj99 mg L8.736 mg Li.9.597 mg 20 emm 20.L58 mg 20.284 mg 50 cmm 19.875 ing L.9.592 mg cmm 23.350 mg 2 23.561+ mg The 50 cmm pipette for delivery of blood was also checked by delivery of blood. rather than water and found to deliver the same volume. 133 .APPDIX V Table 29 VALWS FOR HMA.T0ORIT OR 5 StJBJCTS FOR 52 fl&YS Sujects te BLD BWC MLW HY GAS 26.50 33.33 35.10 32.21 37.93 35.71 42.37 4.0.00 42.65 38.81 39.29 38.62 4.2.00 - 24.76 37.40 36.85 35.34 33.83 1914.9 Jan. 29 30 Jan. 31 Jafl. Feb. Feb. Feb. Feb. Feb. Feb. Feb. Feb. Feb. Feb. Feb. Feb. Feb. Feb. Feb. Feb. Feb. Feb. Feb. Feb. Feb. Feb. Feb. Feb. Feb. Feb. Feb. Feb. 1 2 3 Li. 5 6 7 39.31. 43.31 36.36 41.10 - * 144.29 40.59 41.96 42.25 41.48 - 4.3.20 144.14 4.0.27 140.91 4.1.46 41.35 41.12 41.67 34.88 39.43 42.31 - - 13 14. 38.54. 39.55 15 38.09 36.71 33.86 34.78 4.1.00 16 17 18 19 20 40.89 4.4.114. 41.00 42.47 4.3.33 42.16 43.94 2i 36.56 36.36 22 23 33.90 36.97 214. 35.94. 25 26 27 28 36.48 36.03 35.09 42.34 43.28 42.22 37.04. 37.19 38.27 4.1.16 34.51 38.83 30.89 33e74 34.76 34.48 8 9 10 11 12 38.14. 4.1.78 4.3.20 4.3.10 4.0.94 37.17 40.85 40.18 42.73 36.00 40.63 40.00 40.37 4.2.65 44.40 4.2.28 1i1.l8 32.614 - 39.06 38.69 36.69 36.39 37.63 30.20 39.85 - 4.2.54. - 42.97 - 38.44 39.65 39.13 39.00 38.80 33.90 42.98 39.76 4.2.86 4.1.60 46.02 40.92 44.38 144.88 4.2.93 4.2.94. 43.25 4.2.35 4.3.89 144.09 4.2.52 39.91 39.02 39.17 38.61 4.2.03 4.2.55 4.3.10 43.92 134 Table 29 (continued.) Subjects ]te BLD BWC MIJW QLS 1949 Mar. Mar. Mar. Mar. 2 3 4 34.59 36.41 37.24 37.50 43.22 42.12 43.02 43.06 43.46 42.31 41.00 43.18 Mar. 5 36.73 43.46 40.57 Mar. Mar. Mar. Mar. Mar. Mar. Mar. Mar. Mar. 6 7 - 36.36 42.52 43.90 42.64 8 35.17 41.59 9 36.51 43.59 10 11 12 13 36.63 33.95 37.25 1l4 Mar. Mar. Mar. Mar. Mar. Mar. Mar. Mar. 1 33.81 38.58 37.88 43.98 41.66 42.01 37.46 42.53 39.71 38.91 43.18 43.42 43.44 38.24 42.65 38.22 42.62 40.35 41.77 42.64 39.42 39.87 39.22 40.46 38.87 38.93 38.81 43.13 42.63 42.19 33.82 36.46 40.93 42.86 39.36 40.99 37.69 38.33 41.86 48.28 15 33.46 42.39 44.12 36.51 34.96 43.56 40.38 39.93 16 17 18 19 20 21 22 40.85 40.00 39.71 - 44.29 44.23 34.29 40.30 Jii.61 40.16 43.17 37.28 34.85 36.80 35.34 41.94 39.60 38.98 43.36 35.98 39.39 40.93 43.89 34.81 43.08 38.51 - 36.36 39.69 38.57 45.45 -