Microstructured extracellular matrices in tissue engineering and development Celeste M Nelson Tien
advertisement

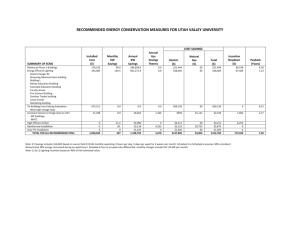
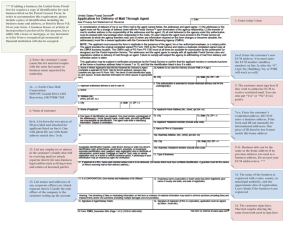
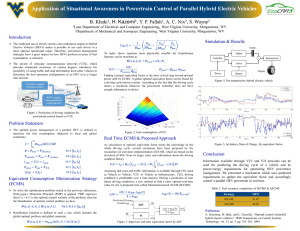
Microstructured extracellular matrices in tissue engineering and development Celeste M Nelson1 and Joe Tien2 Microscale heterogeneity in the extracellular matrix (ECM) provides spatial information that allows tissues to develop and function properly in vivo. This heterogeneity in composition (chemistry) and structure (geometry) creates distinct microenvironments for the cells that comprise a tissue. In response, populations of cells can coordinate their behaviors across micrometer-to-millimeter length scales to function as a unified whole. We believe techniques to mimic the microscale heterogeneity of the ECM in vitro will revolutionize studies that examine how large groups of cells interact. Micropatterned ECMs used for engineering perfused microvascular networks and functional epidermis and for understanding symmetrybreaking events in epithelial morphogenesis illustrate potential applications in tissue engineering and development. Addresses 1 Life Sciences Division, Lawrence Berkeley National Laboratory, Berkeley, CA 94720, USA 2 Department of Biomedical Engineering, Boston University, Boston, MA 02215, USA Corresponding authors: Nelson, Celeste M (cmnelson@lbl.gov); Tien, Joe (jtien@bu.edu) Current Opinion in Biotechnology 2006, 17:518–523 This review comes from a themed issue on Tissue and cell engineering Edited by James L Sherley Available online 12th September 2006 0958-1669/$ – see front matter # 2006 Elsevier Ltd. All rights reserved. DOI 10.1016/j.copbio.2006.08.011 Introduction The extracellular matrix (ECM) environments of most tissues and organs are inherently heterogeneous [1]. This heterogeneity, whether chemical or structural in nature, is critical for proper tissue form and function. For example, a dense basal lamina that separates epithelial and endothelial cells from the underlying interstitial ECM is required for correct cellular polarity and differentiation [2]. Likewise, periodic or fractal ECM geometries are needed for efficient transport in absorptive and secretory tissues [3]. For the past thirty years, investigations of the behavior of cultured cells and tissues have relied heavily on the use of homogeneous two-dimensional (2D) and three-dimensional (3D) ECMs. Although these systems have shed light on the basic biology of cell adhesion, the differences between normal and malignant cells, and the mechanisms underlying tissue-specific gene expression [4], they Current Opinion in Biotechnology 2006, 17:518–523 cannot replicate the complex structure of ECMs in vivo, in which bends, folds, channels and branches — at the size scale of groups of cells — are plentiful [5]. Thus, the cooperative behavior of cells across large length scales (e.g. in morphogenesis, physiology and tissue engineering) has been nearly impossible to study in vitro, other than with explanted tissues. What if researchers could create artificial ECMs that replicate the heterogeneity of native ones? For example, what if one could engineer a collagen gel so that it contained a branching network that mimicked the scale and shape of actual glands in vivo? What studies would these designer ECMs enable? This review describes recent uses of microstructured ECMs (ECMs that possess texture at the 5–1000 mm scale) in tissue engineering and studies of development, and provides evidence that cells grown in these ECMs exhibit unique behaviors not present in homogeneous cultures. Examples of microstructured ECMs designed for microvascular and epidermal tissue engineering and for recapitulation of epithelial development in vitro illustrate these ideas. Why introduce structure into ECMs? Traditional view: dominance of chemistry The ECM consists of glycoproteins (such as collagen, fibronectin and laminin), proteoglycans and glycosaminoglycans that undergo self-assembly as well as cell-directed assembly to form a complex organized meshwork [6]. Besides serving as a scaffold to which cells adhere, ECMs act as reservoirs that sequester and release growth factors and other molecules that affect cellular behavior [7]. ECM molecules and their receptors are required during development, because null mutations in either generally lead to embryonic or perinatal lethality, or to severe abnormalities shortly after birth [8,9]. Many ECMs also play central roles in homeostasis [2], wound repair [10], and diseases such as atherosclerosis [11] and cancer [12,13], partly through mechanical alterations [14]. Whereas the composition and structure of the ECM varies depending on the tissue and organ, in general ECMs are compliant viscoelastic materials with bulk properties of hydrogels [15]. Reconstitution of ECMs in vitro often proceeds by gelation of a liquid mixture of collagens and other proteins; hence, the final gels are invariably homogeneous at the micrometer scale (although not at the nanometer scale) [16,17]. Extensive studies of cellular behavior in these homogeneous gels have understandably favored the view that biochemical composition plays the dominant role in ECM functionality. www.sciencedirect.com Extracellular matrix applications Nelson and Tien 519 Emerging view: importance of form Figure 1 In contrast to the bulk hydrogels traditionally used in culture models, ECMs within native tissues contain as much architecture as their constituent cells. Organs are built of tissues in which the cells and ECMs take the form of sheets (e.g. squamous epithelia, basal lamina), tubes (e.g. vessels, bronchioles, glands), branches (e.g. blood and lymphatic vessels, lung, kidney and mammary epithelia), folds (e.g. dermal papillae, intestinal villae), and bends (e.g. vessels). Form is often viewed as the output of a biological process but it also directly affects cellular behavior, in part by controlling the magnitude and distribution of mechanical stresses within the tissue [18]. For example, angiogenesis is believed to occur preferentially at the convex walls of microvessels where mechanical forces are often greatest in vivo [19]. As we and others have shown through manipulating the shapes of cultured cells, form also exerts a strong effect on cellular behavior in vitro: the shape of a cell controls several functions, including proliferation, apoptosis, glucose metabolism, RNA processing, tissuespecific gene expression and differentiation, and stem cell commitment [20–28]. Similarly, individual cells within a contiguous aggregate display different behaviors that result from the interplay between cellular location, overall aggregate shape, and mechanical forces [29,30,31]. Thus, form can be viewed as an independent determinant of ECM functionality. Schematic of soft lithography as applied to biological materials. (a) Elastomeric poly(dimethylsiloxane) (PDMS) stamps are cured against a photolithographically created silicon master. Peeling the stamp leaves a bas-relief of the original pattern. (b) PDMS can then be coated with ECM and stamped against a solid substratum to transfer the protein (microcontact printing), sealed against a solid substratum to create channels into which a liquid solution of ECM is perfused (microfluidics), or used as a mold against which ECM hydrogels are cured (micromolding). Methods for patterning ECMs To build in vitro systems that faithfully reproduce the structure of tissues probably requires the synthesis of ECMs with microscale heterogeneity. To date, several synthetic schemes have been developed to form 2D patterns of matrix proteins on rigid substrata. Early work used photolithography — a light-based patterning technique akin to high-resolution photography — to indirectly control where proteins could adsorb on glass or silicon [32]. More recently, ‘soft’ lithographic techniques, originally developed by Whitesides and colleagues [33], that use elastomeric stamps to pattern ECM have grown in popularity, largely because of ease of use (Figure 1). Patterning usually takes place through contact printing or adsorption in microfluidic channels; with stamps that have multiple levels of features, it is possible to generate complex 2D mosaics and gradients of ECMs [34,35]. Because cells grown on these patterned substrata often behave differently from cells in homogeneous monolayer culture, 2D patterns have provided a useful tool for investigating the role of microenvironment in basic cell biology [28]. In contrast to the extensive work in 2D patterning of adsorbed or printed ECMs, only recently have investigators focused on recapitulating the 3D architecture of ECM gels. Initial attempts used light to photopolymerize small organic molecules into hydrogels and required www.sciencedirect.com specialized chemistries [36]. To synthesize 3D patterned ECMs consisting of natural proteins, we and others have recently developed several techniques to mold macromolecular gels [37,38,39]. These methods rely on molds that are treated so that their surfaces are non-adherent; liquid precursors (e.g. an acid extract of type I collagen or cold matrigel) that are gelled against these molds detach easily to yield gels with sharply defined features with <1 mm resolution (Figure 2a) [37]. In addition to introducing surface texture onto a gel, lithographic techniques can be used to form monolithic gels that contain internal surfaces, such as cavities, channels and networks (Figure 2). Formation of internal patterns requires the use of sacrificial materials, which are initially embedded in a gel and then removed to yield an open internal space. Examples of such sacrificial materials include paraffin [40], matrigel [41] and gelatin (AP Golden and J Tien, unpublished). Another strategy for patterning ECMs in 3D relies on stacking or direct ink-jet printing of microstructured gels to form multilayered laminates that localize distinct populations of cells to different planes or areas [37,42]. These methods attempt to build ECMs and tissues layer by layer, and still need to address issues of resolution, speed and alignment. Current Opinion in Biotechnology 2006, 17:518–523 520 Tissue and cell engineering Figure 2 Images of microstructured gels. (a) Arrays of posts in type I collagen. (b) Arrays of matrigel embedded in type I collagen. (c) A cylindrical channel in fibrin. (d) An open network in type I collagen, perfused by a suspension of red blood cells. Scale bars refer to 100 mm in (a–c) and 500 mm in (d). [Image in (b) adapted from [41] with permission]. Much work remains to be done to replicate the full diversity of tissues in these in vitro systems. In particular, the engineering of ECMs suitable for modeling tissues with scant stroma and high cellularity (e.g. renal medulla) remains a challenge. Nevertheless, several groups have begun to exploit the unique architectures of these microstructured ECMs for tissue engineering and development. ECMs for tissue engineering A promising use of patterned ECMs lies in tissue engineering, as shown below by recent examples in engineering functional microvessels and epidermis in vitro. Efforts in tissue engineering are currently biased towards designing synthetic biomaterials (e.g. scaffolds) and growth factors, with the expectation that specific molecules can direct cells seeded within them to achieve tissuespecific function and histology [43,44]. Although this approach can form tissues with simple laminated organization (skin, cornea and arteries), it has difficulty in forming functional complex tissues. For example, vascular endothelial cells seeded within bulk ECM gels or synthetic polymers will form random cords, but these cords do not coalesce into an open microvascular network, even in the presence of large amounts of growth factors [45]. The absence of flow in homogeneous constructs in vitro can predispose endothelial cords to apoptosis. As a result, several groups have used various strategies to enhance survival of engineered microvascular tissues, such as transfection with anti-apoptotic genes [46] and introduction of mesenchymal cells [47]. How can microstructured ECMs help in forming functional microvessels? As originally envisioned by Vacanti Current Opinion in Biotechnology 2006, 17:518–523 and colleagues [48], using an ECM with pre-formed channels that could be perfused upon cell seeding would enhance the stability of the construct. This approach provides immediate exposure to shear stress and chemical factors, and thus should avoid the regression observed with cells seeded in bulk gels. Using patterned ECMs, we have recently demonstrated that perfused endothelial tubes remained patent for weeks without any observable changes in cellular organization (Figure 3). These tubes were formed by seeding endothelial cells through ECM gels that have open channels spanning the gels [38]. Over time, these endothelial tubes developed functional behaviors typical of microvessels in vivo, such as barrier function and support of leukocyte adhesion. Thus, the use of microstructured ECMs can enhance the stability and functionality of engineered microvessels. An appropriate 3D organization appears to play a role beyond simply providing ready perfusion. In fact, perfusing embedded endothelial cells via interstitial flow does not lead to formation of stable vessels (GM Price and J Tien, unpublished). The combination of perfusion and correct polarization, both of which stem from the presence of an open channel, may be required for stable vessels to develop. Use of microstructured ECMs has also led to enhanced differentiation in engineered epidermis: Toner, Pins and colleagues [49,50] reasoned that ECMs that mimic the wavy geometry of rete ridges (the interdigitations between epidermis and dermis) should provide seeded keratinocytes with topographic cues present in native skin, and thus should lead to more functional tissue, compared with cells seeded on flat ECMs. To test their hypothesis, they created a synthetic ‘basal lamina’ by polymerizing collagen or gelatin against an undulating silicone elastomer surface. When cultured on top of these Figure 3 Images of perfused microvessels and microvascular networks comprised of microstructured type I collagen gels and human dermal microvascular endothelial cells. (a) An eight-day-old endothelial tube. (b) A solution of fluorescently labeled albumin, perfused through a five-day-old endothelial tube. The cells form a strong barrier that confines the protein to the luminal compartment. (c) A six-day-old patterned microvascular network. Scale bars refer to 100 mm. www.sciencedirect.com Extracellular matrix applications Nelson and Tien 521 ECM membranes, keratinocytes conformed to the micrometer scale ridges on the surface and showed enhanced stratification and expression of differentiation markers in deep undulations. Figure 4 ECMs for the study of development A second application of patterned ECMs is in the study of tissue development. A better understanding of the mechanisms that direct development and functional differentiation will not only suggest strategies to treat developmental defects, but will also advance efforts to engineer complex, functional tissues. A large subset of tissues and organs — kidney, lung, pancreas and prostate, salivary and mammary glands — develop by a process called branching morphogenesis [51]. This process starts with local invagination of an epithelial sheet to form a primary placode or bud [52]. This primary structure then undergoes reiterative bifurcation and/or lateral branching at non-random sites. The specific distribution of branch sites and lengths is unique for each tissue and generates tissue-specific 3D geometries. How can microstructured ECMs aid in the study of development? Culture models developed by Bissell and colleagues [53] and in vivo studies have established that morphogenesis and functional differentiation of epithelial cells are both critically dependent on the ECM, and have proven the importance of three-dimensionality in biological signaling [54]. In particular, collagen and fibronectin fibrils accumulate at sites of clefting and branching in embryonic salivary gland, lung and kidney [55–59]. Despite the recognition that branching morphogenesis takes place in a complex heterogeneous 3D ECM, the biochemical requirements for branching in the mammary gland have been defined using in vitro models of mammary epithelial cells cultured within homogeneous gels of collagen I or matrigel [60–62]. These models have helped determine the signals that are absolutely required for branching to occur. Nevertheless, cells in homogeneous gels branch randomly rather than in the characteristic arborized pattern of the gland in vivo. Use of these gels has thus provided a limited understanding of the signals that determine sites of branching in the mammary gland and other organs. We and others have recently begun to use microstructured ECMs to examine how the placement of epithelial cells can, by itself, control the development of form [18,29,63]. Numerical simulations and experimental cultures both suggest that the form of the pre-existing tissue instructs several of the symmetry-breaking events during morphogenesis [29,64], a concept introduced as early as the late 1800s by Wilhelm His [65]. For instance, by culturing cells on large (100–1000 mm) 2D islands of ECM, it was found that whether individual cells within an epithelial sheet proliferate depended on their position within the sheet and its overall geometry [29]. Slight www.sciencedirect.com Images of proliferation and invasion in structured ECMs. (a) Phase contrast image of cells cultured on 2D pattern of asymmetric (off-center) annulus pattern. (b) Colorimetric image of proliferation on 2D asymmetric pattern, generated by stacking images from 50 samples to show the frequency of cells proliferating as a fuction of space. A pixel value of 0.20 indicates that 20% of cells at that location proliferated. (c) Abrogation of shape-induced selectivity in proliferation by disruption of cell–cell adhesion using a dominant-negative cadherin construct. Proliferation becomes more uniform across the monolayer with this treatment. (d) Epithelial cells cultured within cubic cavities migrate specifically from the vertices. Scale bars refer to 100 mm in (a–c) and 50 mm in (d). [Images in (a–c) reproduced from [29] with permission, copyright 2005, National Academy of Sciences, USA.] asymmetry in the shape of the sheet of cells altered the pattern of proliferation (Figure 4). These events require integrity of the cellular sheet, because disrupting connections between the cells leads to unpatterned proliferation. We speculate that, in 3D, these spatial variations induce feed-forward events that magnify and preserve an original slight heterogeneity so that a stable, complex tissue can emerge. To test this hypothesis, we have studied the response of epithelial cells within patterned cavities in collagen gels to morphogens. Stereotyped sites of invasion developed in these 3D cultures, indicating that tissue structure not only affects proliferation, but migration as well (CM Nelson and MJ Bissell, unpublished). These results suggest that the 3D architecture of a tissue can alter further morphogenesis and support the idea that form should be viewed as an independent effector of development. Microstructured ECMs thus allow us to address how form affects its own evolution in the presence of morphogens. Conclusions The development of methods to deliberately introduce microscale variations in culture models has enabled the possibility of testing how ECM aids in the proper Current Opinion in Biotechnology 2006, 17:518–523 522 Tissue and cell engineering development of tissue form and function. ECM is not simply a glue that binds cells into tissues, nor is it just a reservoir of immobilized growth factors, proteases and matrix proteins. Heterogeneity in the ECM — whether in terms of geometry or chemical composition — appears to provide signals designed to integrate the behavior of populations of cells across large distances. Although in their infancy, the applications of microstructured ECMs to the study of tissue engineering and development seem especially promising, and could one day lead to a rational, quantitative description of morphogenetic processes. In retrospect, it is not surprising that, even in a material as well studied as type I collagen [16], there are ways to enhance functionality simply by adding structure. Acknowledgements CMN is a postdoctoral fellow in Mina Bissell’s laboratory. Our work was supported by grants from the Department of Defense (W81XWH-04-10582 to CMN and DAMD17-02-1-438 to MJB), the National Institute of Biomedical Imaging and Bioengineering (EB002228 and EB003157 to JT), the National Cancer Institute (CA64786 and CA57621 to MJB), the Whitaker Foundation (RG-02-0344 to JT), and a Boston University Provost’s Innovation Award (to JT). We would especially like to thank Mina Bissell for her encouragement. References and recommended reading Papers of particular interest, published within the annual period of review, have been highlighted as: of special interest of outstanding interest 1. Young B, Heath JW: Wheater’s Functional Histology: A Text and Colour Atlas. Churchill Livingstone; 2000. 2. Vracko R: Basal lamina scaffold–anatomy and significance for maintenance of orderly tissue structure. Am J Pathol 1974, 77:314-346. 3. 4. Murray CD: The physiological principle of minimum work applied to the angle of branching of arteries. J Gen Physiol 1926, 9:835-841. Nelson CM, Bissell M: Of extracellular matrix, scaffolds, and signaling: Tissue architecture regulates development, homeostasis, and cancer. Annu Rev Cell Dev Biol 2006, 22:287-309. 5. Kessel RG, Kardon RH: Tissues and Organs: A Text-Atlas of Scanning Electron Microscopy. WH Freeman; 1979. 6. Hay ED (Ed.): Cell Biology of Extracellular Matrix. Edited by Hay ED. Plenum Press; 1991. 7. Folkman J, Klagsbrun M, Sasse J, Wadzinski M, Ingber D, Vlodavsky I: A heparin-binding angiogenic protein–basic fibroblast growth factor is stored within basement membrane. Am J Pathol 1988, 130:393-400. 8. Aszodi A, Pfeifer A, Wendel M, Hiripi L, Fassler R: Mouse models for extracellular matrix diseases. J Mol Med 1998, 76:238-252. 9. Colognato H, Yurchenco PD: Form and function: the laminin family of heterotrimers. Dev Dyn 2000, 218:213-234. 10. Ross R: Wound healing. Sci Am 1969, 220:40-50. 11. Skalen K, Gustafsson M, Rydberg EK, Hulten LM, Wiklund O, Innerarity TL, Boren J: Subendothelial retention of atherogenic lipoproteins in early atherosclerosis. Nature 2002, 417:750-754. 12. Dvorak HF: Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med 1986, 315:1650-1659. Current Opinion in Biotechnology 2006, 17:518–523 13. Toole BP, Wight TN, Tammi MI: Hyaluronan-cell interactions in cancer and vascular disease. J Biol Chem 2002, 277:4593-4596. 14. Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D et al.: Tensional homeostasis and the malignant phenotype. Cancer Cell 2005, 8:241-254. This paper provides the first mechanistic demonstration of how ECM rigidity affects malignant progression. 15. Hsu S, Jamieson AM, Blackwell J: Viscoelastic studies of extracellular matrix interactions in a model native collagen gel system. Biorheology 1994, 31:21-36. 16. Bornstein MB: Reconstituted rat-tail collagen used as substrate for tissue cultures on coverslips in Maximow slides and roller tubes. Lab Invest 1958, 7:134-137. 17. Kleinman HK, McGarvey ML, Hassell JR, Star VL, Cannon FB, Laurie GW, Martin GR: Basement membrane complexes with biological activity. Biochemistry 1986, 25:312-318. 18. Ingber DE: Mechanical control of tissue growth: function follows form. Proc Natl Acad Sci USA 2005, 102:11571-11572. This paper briefly reviews the evidence that mechanical forces affect the directional growth of tissues. 19. Hudlicka O: What makes blood vessels grow? J Physiol 1991, 444:1-24. 20. Boudreau N, Werb Z, Bissell MJ: Suppression of apoptosis by basement membrane requires three-dimensional tissue organization and withdrawal from the cell cycle. Proc Natl Acad Sci USA 1996, 93:3509-3513. 21. Bissell MJ, Farson D, Tung AS: Cell shape and hexose transport in normal and virus-transformed cells in culture. J Supramol Struct 1977, 6:1-12. 22. Farmer SR, Ben-Ze’av A, Benecke BJ, Penman S: Altered translatability of messenger RNA from suspended anchoragedependent fibroblasts: reversal upon cell attachment to a surface. Cell 1978, 15:627-637. 23. Ben-Ze’ev A, Farmer SR, Penman S: Protein synthesis requires cell-surface contact while nuclear events respond to cell shape in anchorage-dependent fibroblasts. Cell 1980, 21:365-372. 24. Emerman JT, Burwen SJ, Pitelka DR: Substrate properties influencing ultrastructural differentiation of mammary epithelial cells in culture. Tissue Cell 1979, 11:109-119. 25. Roskelley CD, Desprez PY, Bissell MJ: Extracellular matrixdependent tissue-specific gene expression in mammary epithelial cells requires both physical and biochemical signal transduction. Proc Natl Acad Sci USA 1994, 91:12378-12382. 26. McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS: Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell 2004, 6:483-495. 27. Folkman J, Moscona A: Role of cell shape in growth control. Nature 1978, 273:345-349. 28. Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE: Geometric control of cell life and death. Science 1997, 276:1425-1428. 29. Nelson CM, Jean RP, Tan JL, Liu WF, Sniadecki NJ, Spector AA, Chen CS: Emergent patterns of growth controlled by multicellular form and mechanics. Proc Natl Acad Sci USA 2005, 102:11594-11599. This paper provides the first evidence in vitro that the shape of an aggregate of cells can influence preferential sites of cell division, in part by controlling the distribution of mechanical forces in the aggregate. 30. Farge E: Mechanical induction of Twist in the Drosophila foregut/stomodeal primordium. Curr Biol 2003, 13:1365-1377. 31. Hove JR, Koster RW, Forouhar AS, Acevedo-Bolton G, Fraser SE, Gharib M: Intracardiac fluid forces are an essential epigenetic factor for embryonic cardiogenesis. Nature 2003, 421:172-177. 32. O’Neill C, Jordan P, Ireland G: Evidence for two distinct mechanisms of anchorage stimulation in freshly explanted and 3T3 Swiss mouse fibroblasts. Cell 1986, 44:489-496. www.sciencedirect.com Extracellular matrix applications Nelson and Tien 523 33. Whitesides GM, Ostuni E, Takayama S, Jiang X, Ingber DE: Soft lithography in biology and biochemistry. Annu Rev Biomed Eng 2001, 3:335-373. 34. Tien J, Nelson CM, Chen CS: Fabrication of aligned microstructures with a single elastomeric stamp. Proc Natl Acad Sci USA 2002, 99:1758-1762. 50. Downing BR, Cornwell K, Toner M, Pins GD: The influence of microtextured basal lamina analog topography on keratinocyte function and epidermal organization. J Biomed Mater Res A 2005, 72:47-56. This study, along with [49], illustrates how the use of a patterned ECM enhances the functional differentiation of epidermal keratinocytes for skin tissue engineering. 35. Jeon NL, Dertinger SKW, Chiu DT, Choi IS, Stroock AD, Whitesides GM: Generation of solution and surface gradients using microfluidic systems. Langmuir 2000, 16:8311-8316. 51. Davies JA: Do different branching epithelia use a conserved developmental mechanism? Bioessays 2002, 24:937-948. 36. Nguyen KT, West JL: Photopolymerizable hydrogels for tissue engineering applications. Biomaterials 2002, 23:4307-4314. 52. Affolter M, Bellusci S, Itoh N, Shilo B, Thiery JP, Werb Z: Tube or not tube: remodeling epithelial tissues by branching morphogenesis. Dev Cell 2003, 4:11-18. 37. Tang MD, Golden AP, Tien J: Molding of three-dimensional microstructures of gels. J Am Chem Soc 2003, 125:12988-12989. 38. Chrobak KM, Potter DR, Tien J: Formation of perfused, functional microvascular tubes in vitro. Microvasc Res 2006, 71:185-196. This study demonstrates how the use of a patterned ECM enables tissue engineering of functional microvessels. 39. Cabodi M, Choi NW, Gleghorn JP, Lee CS, Bonassar LJ, Stroock AD: A microfluidic biomaterial. J Am Chem Soc 2005, 127:13788-13789. 40. Vernon RB, Gooden MD, Lara SL, Wight TN: Native fibrillar collagen membranes of micron-scale and submicron thicknesses for cell support and perfusion. Biomaterials 2005, 26:1109-1117. 41. Tang MD, Golden AP, Tien J: Fabrication of collagen gels that contain patterned, microscale cavities. Adv Mater 2004, 16:1345-1348. 42. Odde DJ, Renn MJ: Laser-guided direct writing for applications in biotechnology. Trends Biotechnol 1999, 17:385-389. 43. Lutolf MP, Hubbell JA: Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol 2005, 23:47-55. This paper thoroughly reviews the potential of using chemistry to design new materials for tissue engineering. 44. Levenberg S, Langer R: Advances in tissue engineering. Curr Top Dev Biol 2004, 61:113-134. 45. Montesano R, Pepper MS: Three-dimensional in vitro assay of endothelial cell invasion and capillary tube morphogenesis. In Vascular Morphogenesis: In Vivo, In Vitro, In Mente. Edited by Little CD, Mironov V, Sage EH. Birkhaüser; 1998:79-110. 53. Schmeichel KL, Bissell MJ: Modeling tissue-specific signaling and organ function in three dimensions. J Cell Sci 2003, 116:2377-2388. 54. Abbott A: Cell culture: biology’s new dimension. Nature 2003, 424:870-872. 55. Sakai T, Larsen M, Yamada KM: Fibronectin requirement in branching morphogenesis. Nature 2003, 423:876-881. 56. Fukuda Y, Masuda Y, Kishi J, Hashimoto Y, Hayakawa T, Nogawa H, Nakanishi Y: The role of interstitial collagens in cleft formation of mouse embryonic submandibular gland during initial branching. Development 1988, 103:259-267. 57. Wicha MS, Liotta LA, Vonderhaar BK, Kidwell WR: Effects of inhibition of basement membrane collagen deposition on rat mammary gland development. Dev Biol 1980, 80:253-256. 58. Silberstein GB, Daniel CW: Glycosaminoglycans in the basal lamina and extracellular matrix of the developing mouse mammary duct. Dev Biol 1982, 90:215-222. 59. Silberstein GB, Strickland P, Coleman S, Daniel CW: Epitheliumdependent extracellular matrix synthesis in transforming growth factor-b1-growth-inhibited mouse mammary gland. J Cell Biol 1990, 110:2209-2219. 60. Brinkmann V, Foroutan H, Sachs M, Weidner KM, Birchmeier W: Hepatocyte growth factor/scatter factor induces a variety of tissue-specific morphogenic programs in epithelial cells. J Cell Biol 1995, 131:1573-1586. 61. Soriano JV, Pepper MS, Nakamura T, Orci L, Montesano R: Hepatocyte growth factor stimulates extensive development of branching duct-like structures by cloned mammary gland epithelial cells. J Cell Sci 1995, 108:413-430. 46. Enis DR, Shepherd BR, Wang Y, Qasim A, Shanahan CM, Weissberg PL, Kashgarian M, Pober JS, Schechner JS: Induction, differentiation, and remodeling of blood vessels after transplantation of Bcl-2-transduced endothelial cells. Proc Natl Acad Sci USA 2005, 102:425-430. 62. Simian M, Hirai Y, Navre M, Werb Z, Lochter A, Bissell MJ: The interplay of matrix metalloproteinases, morphogens and growth factors is necessary for branching of mammary epithelial cells. Development 2001, 128:3117-3131. 47. Koike N, Fukumura D, Gralla O, Au P, Schechner JS, Jain RK: Creation of long-lasting blood vessels. Nature 2004, 428:138-139. 63. Huang S, Brangwynne CP, Parker KK, Ingber DE: Symmetrybreaking in mammalian cell cohort migration during tissue pattern formation: role of random-walk persistence. Cell Motil Cytoskeleton 2005, 61:201-213. 48. Kaihara S, Borenstein J, Koka R, Lalan S, Ochoa ER, Ravens M, Pien H, Cunningham B, Vacanti JP: Silicon micromachining to tissue engineer branched vascular channels for liver fabrication. Tissue Eng 2000, 6:105-117. 64. Shraiman BI: Mechanical feedback as a possible regulator of tissue growth. Proc Natl Acad Sci USA 2005, 102:3318-3323. 49. Pins GD, Toner M, Morgan JR: Microfabrication of an analog of the basal lamina: biocompatible membranes with complex topographies. FASEB J 2000, 14:593-602. 65. His W: Unsere Körperform und das physiologische Problem ihrer Entstehung. Leipzig: F.C.W. Vogel; 1874. [Translation: Our body form and the physiological problem of its development.] www.sciencedirect.com Current Opinion in Biotechnology 2006, 17:518–523