Chemistry 221 - Test 2 Review Sheet
advertisement

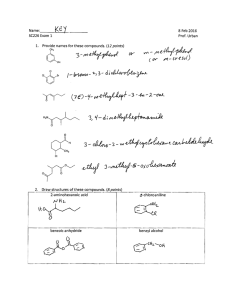
Chemistry 221 - Test 2 Review Sheet (Chapters 6-9) 1. Fill in the missing starting material(s), reagent(s), or product(s). Show all reasonable answers. Name the reactions. HBr Br 1. O3 2. Zn, H3O+ KMnO4 (acidic) OH OH 1) OsO4 , pyridine 2) NaHSO 3, H 2O consider product stereochemistry HIO4 O O 1) O3 KMnO4 (acidic) 2) H3O+ Cl Cl 2. Draw the cyclic osmate for the oxidation of . 3. Write all possible products and the mechanism for the reaction: HBr (consider Markovnikov’s rule and rearrangement) What evidence do we have to support this mechanism? Draw a potential energy diagram for the overall reaction labeling the pertinent sites. 4. Name: Br Cl Cl Br Br Br 5. Rank the following molecules by the stability of the pi bonds. (#1 most stable) 6. Why are trans-alkenes more stable than cis-alkenes? 7. Draw the rearrangement product (if any) for: HBr HBr HBr 8. Why do propagation steps occur as many as 10,000 times before termination? How does a termination step stop the chain reaction? 9. Assign the R and S configuration to each of the following stereogenic centers. Which two represent the same molecule. CH 3 H Br Cl H CH 3 CH 3 CH 3 H CH 3 CH 3 CH 3 Br Cl H H Cl Br H H CH 3 H Cl Br Draw all the isomers of C3 H5 ClBr2 and identify any stereogenic centers. Draw the stereoisomers of those molecules in Fischer projection. H O-H H H Rank the relative acidities of: H2 O N H Using 1-butyne and 1-iodopropane (and any inorganic reagents) prepare 3-heptyne.