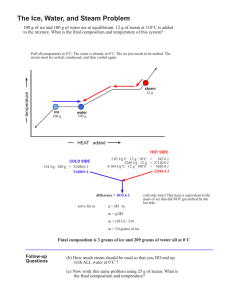
Phy 131 - Assignment 13 A. ∑Q = 0 + (mL
advertisement

Phy 131 - Assignment 13 A. ∑Q = 0 (m c ΔT)tea + (mLf + m c ΔT)ice = 0 The tea just changes temperature. The ice changes state and changes temperature. mtea: 1 liter = 1000 ml = 1000 cm3, and water has a density of 1 g/cm3. Tea is basically water, so its specific heat is cwater = Above 0°, the ice has melted. So, its specific heat is also water’s, not ice’s. (1000 g)( )(10° - 30°) + m( )+m( )(10° - 0°) = 0 - 20 000 + 79.6 m + 10 m = 0 89.6 m = 20 000 m= 223 g B. 1. a. Conduction & convection. Heat can’t flow by collisions of air molecules that aren’t there, or by the circulation of air that isn’t there. b. Radiation. A shiny surface not only absorbs radiation poorly, it also emits it poorly. (So, it doesn’t let energy in or out.) 2. C. 1. The steam gives off a large amount of heat as it condenses. For example, 1/10 gram of water cooling from 100°C to 30°C (the temperature of your hand) gives off m c ΔT = 7 calories. But 1/10 gram of 100°C steam gives off mLv + m c ΔT = 61 calories. 2. ∑Q = 0 (m c ΔT)iron + (m c ΔT)water = 0 (1.5 kg)( )(Tf - 600°) + (20 kg)( 672 Tf – 403 200 + 83 720 Tf – 2 093 000 = 0 84 393 Tf = 2 496 200 Tf = 29.6°C )( Tf - 25°) = 0 D. E. 1. The alcohol’s temperature will increase about twice as much as the water’s. 2. F.1. Glass has a much lower thermal conductivity than metal, so heat flows along that rod much slower. (A popular wrong answer is that glass has a lower specific heat. The specific heat of glass is actually similar to aluminum’s.) 2.