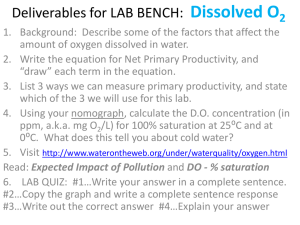
Dissolved oxygen How Does Dissolved Oxygen Affect Freshwater Ecosystems? Ecology Dissolved Oxygen.notebook
advertisement

Ecology Dissolved Oxygen.notebook September 30, 2014 Dissolved oxygen Information from "Water's The Matter" module How Does Dissolved Oxygen Affect Freshwater Ecosystems? Mar 19­7:45 AM 1 Ecology Dissolved Oxygen.notebook September 30, 2014 Dissolved oxygen MS LS2 I can construct an argument with empirical evidence to show that changes to physical or biological components of an ecosystem can affect populations. Learning Target ­ 2 Pre Big Post Assess Ideas Assess I can define dissolved oxygen and can explain a variety of circumstances that cause D.O. levels to change. Mar 19­7:45 AM 2 Ecology Dissolved Oxygen.notebook September 30, 2014 Dissolved Oxygen in Freshwater Ecosytems. I can define dissolved oxygen and can explain a variety of circumstances that cause D.O. levels to change. Organisms must have a minimum level of dissolved oxygen. The level varies for organisms, but in general a level of 4 mg/L is required. An increase in water temperature can stress or kill aquatic organisms, but there is not an upper limit or maximum amount for dissolved oxygen. In other words, there is no such thing as "too much" dissolved oxygen. 0­2 mg/L: not enough oxygen to support life. 2­4 mg/L: only a few fish and aquatic insects can survive. 4­7 mg/L: good for many aquatic animals, low for cold water fish 7­11 mg/L: very good for most stream fish Mar 19­7:45 AM 3 Ecology Dissolved Oxygen.notebook September 30, 2014 How does oxygen get in the water? I can define dissolved oxygen and can explain a variety of circumstances that cause D.O. levels to change. Basically, oxygen gets into water by three different ways. • Diffusion from surrounding air. The air in the atmosphere has a higher concentration of oxygen than water so oxygen diffuses or is "pushed" from the air into the water. • Aeration of water. Churning waters causes air to hit the water at a high pressure, allowing more oxygen to become dissolved (rapids and waterfalls). • Waste products of plants. Plants use carbon dioxide to make food and generate oxygen as a waste product. This oxygen is immediately dissolved into the water. Mar 19­7:45 AM 4 Ecology Dissolved Oxygen.notebook September 30, 2014 Aquatic Plants I can define dissolved oxygen and can explain a variety of circumstances that cause D.O. levels to change. Although plants produce oxygen during the day as they produce their own food, the situation is reversed at night. aquatic vegetation In darkness, plants will consume oxygen as fuel (cellular respiration). Thus, a body of water with a high plant density will have high dissolved oxygen levels during the day and low levels of dissolved oxygen at night. Mar 19­7:45 AM 5 Ecology Dissolved Oxygen.notebook September 30, 2014 Source: http://www.hws.edu/fli/lessons_teach_oxygen.aspx Dissolved oxygen I can define dissolved oxygen and can explain a variety of circumstances that cause D.O. levels to change. Mar 19­7:45 AM 6 Ecology Dissolved Oxygen.notebook September 30, 2014 Dissolved oxygen I can define dissolved oxygen and can explain a variety of circumstances that cause D.O. levels to change. What factors affect the amount of dissolved oxygen? • Temperature. The amount of DO is highly dependent on the water temperature. As temperature increases dissolved oxygen decreases. (This is called an inverse relationship). • Altitude. As altitude increases, the atmospheric (barometric) pressure decreases. Thus, the amount of oxygen diffused into the water decreases. • Organic material. Organic material comes from parts of trees and plants that fall into a body of water. Organic material also includes decaying algae, dead aquatic plants, dead fish or other organisms, and human and animal wastes. Organic material does not directly remove the DO, but it creates conditions where large amounts of bacteria accumulate. These bacteria consume large amounts of DO, driving the overall oxygen level down. Mar 19­7:45 AM 7 Ecology Dissolved Oxygen.notebook September 30, 2014 Dissolved oxygen I can define dissolved oxygen and can explain a variety of circumstances that cause D.O. levels to change. What factors affect the amount of dissolved oxygen? • Nitrates and Phosphates. Nitrates and phosphates are the main component in fertilizer. Fertilizers are used to promote plant growth. When these chemicals are in high concentrations in water, they do the same thing. They fertilize. This causes algae and aquatic plants to thrive. As a result, two things happen: 1. A rate of plant growth occurs that cannot be sustained. Plants grow so dense that eventually they choke each other off. Large amounts of plant material accumulates, creating organic matter and large amounts of oxygen­demanding bacteria. 2. Algae grow and create an unstable DO level. A large algae population will create oxygen during the day (photosynthesis), but only consumes it during the night (no light for photosynthesis). This results in a very low DO level just before sunrise, creating stressful conditions for some aquatic organisms. Mar 19­7:45 AM 8 Ecology Dissolved Oxygen.notebook September 30, 2014 Dissolved oxygen I can define dissolved oxygen and can explain a variety of circumstances that cause D.O. levels to change. How does human intervention affect the amount of dissolved oxygen that is present in water? • Dams Dams slow the flow of water, reducing the amount of aeration and increasing the temperature. • Human Waste Besides creating a health hazard, human waste carries with it a large amount of oxygen consuming bacteria. Sometimes this is a problem during floods when sewage treatment plants overflow and are not able to treat all the water. Raw sewage can spill into the waterway and introduce bacteria into the water that will use up a large amount of the available dissolved oxygen. • Fertilizers Poor farming practices result in large amounts of fertilizer being washed into rivers and lakes. This creates algae blooms and large growths of other plants, which ultimately result in too much organic matter and oxygen consuming bacteria. Farms and golf courses are places where fertilizer use is high. Mar 19­7:45 AM 9 Ecology Dissolved Oxygen.notebook September 30, 2014 Dissolved Oxygen Testing Part One 1. Obtain a water sample (upstream from where you are standing ­ no air bubbles). Fill it all the way to the top! 2. Add 8 drops of Manganous Sulfate. (#1) 3. Add 8 drops of Alkaline Potassium Iodide Atide. 4. Cap the bottle and mix by inverting several times. A precipitate forms. 5. Allow precipitate to settle below the shoulder of the bottle. 6. Add one level measure of Sulfamic Acid Powder. 7. Cap and gently invert the bottle to mix the contents until the precipitate and the reagent have totally dissolved. The solution will be CLEAR YELLOW ­ no orange. Sep 19­9:50 PM 10 Ecology Dissolved Oxygen.notebook September 30, 2014 D.O. Testing Part Two 8. Fill the lined vial to the 20 mL mark with the fixed water sample. Cap the vial. 9. Fill the titrator with Sodium Thiosulfate (number 4) by inserting the titrator tip into the Sodium Thiosulfate bottle. 10. Pull the plunger of the titrator out so that it will fill with the solution. If there are air bubbles, you must expel them back into the solution bottle and try again. 11. Add one drop of solution from the titrator to the water sample. Swirl the vial as you add drops. SLOW and Careful! This step is MOST IMPORTANT. 12. Continue to add drops until the sample becomes pale yellow. Put the titrator to the side. DO NOT empty it! 13. Uncap the vial and add 8 drops of Starch Indicator. The sample will turn blue. 14. Recap the vial. Reinsert the titrator and begin adding drops and swirling after each drop. BE CAREFUL and GO SLOW. Add drops until the blue color disappears. 15. To get dissolved oxygen reading, read the titrator meets the scale. Sep 19­9:56 PM 11 Ecology Dissolved Oxygen.notebook September 30, 2014 • 1.Record your measurement on the board. • 2.Empty fixed sample down drain. • 3.Put any solution from the titrator back into the Sodium Thiosulfate bottle. • 4.Recap bottles, reassemble kit and clean area. Sep 19­10:27 PM 12 Ecology Dissolved Oxygen.notebook September 30, 2014 Sep 28­9:30 PM 13