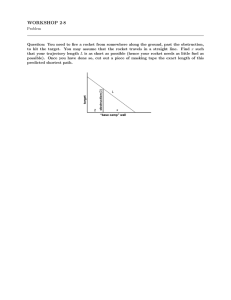
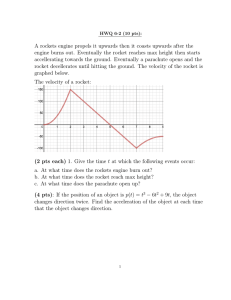
ChemRocket NOTES Integrated Vista Overview
advertisement

Integrated Vista ChemRocket NOTES Overview Students demonstrate Newton’s Laws of Motion, describe factors affecting the rate that a solid dissolves, balance a chemical equation, and describe the reactants and products. General Time Frame Pre-laboratory experience (55 minutes) ChemRocket Learning Experience (Generally two to three 55-minute periods for phases 1 and 2. Phases 3 and 4 may take more time.) Background Information for the Teacher The IPC Texas Essential Knowledge and Skills (TEKS) require the use of inquiry-based science, which allows students to collect and process data as they work to solve a problem. Many cookbook-type science activities can be modified to inquiry/research-based laboratory investigations with the use of “phase” strategies. In “cookbook” science, the teacher directs decision-making from topic to conclusion. Students follow the teacher’s directions in the same way a cook follows a recipe where deviations are not acceptable. Although it is an improvement over the 45- to 90-minute lecture and not necessarily bad science, it is not inquiry science. By using phase strategies, teachers can shift students’ reliance on “cookbook” activities to student-directed investigations that challenge students to use higher-order thinking skills. In addition, different phase strategies can be used with the same lesson to meet the varied needs of students for more or less teacher direction. Phase strategies coincide with other approaches that may be more familiar to some teachers, such as the traditional laboratory approach, structured inquiry approach, student-directed inquiry approach, and student research approach. The following list shows the relationship between these approaches and the different phase strategies: The Charles A. Dana Center at UT Austin 1 NOTES Phasing Phase 1—Traditional Laboratory Approach Phase 1 provides students with laboratory activities written in the scientific method, which allows them to become familiar with scientific processes. These activities provide students with the opportunity to complete appropriate charts and graphs, and answer challenging discussion questions. Phase 2—Structured Inquiry Approach The structured inquiry approach requires students to make their own conclusions based on supportive evidence. In the learning experience, parts of the scientific method are excluded. Charts, data tables, and graphs are usually removed, requiring students to design a method of collecting and communicating data. Phase 3—Student-Directed Inquiry Approach At this level, although the teacher selects the topic, it is the student who identifies the question, selects the materials, designs the investigation, analyzes the results, and reaches supportable conclusions. The investigation involves a real-world scenario and an outline of the scientific method. The outline requires students to identify the problem, formulate a hypothesis, and communicate data effectively to draw conclusions about the results. Initially, the teacher may need to assist students with question development, but this type of input should diminish with time. Phase 4—Student Research Approach The student research approach is scientific inquiry, and the most challenging for many students. A real-world problem is presented to students who must solve this problem using their own design, creativity, and testing procedures. Methods for communicating results vary in this phase because students must give scenario-specific conclusions to the appropriate parties. Examples of methods that could be used include data reports to supervisors, letters to officials, journal entries, and working models. Students may require some support and guidance from the teacher. Not all students will reach this phase, but the teacher should be ready to meet the needs of students who are ready to be challenged by this level. 2 Integrated Physics and Chemistry Institute – Fall 2004 Choosing a Phase Strategy Inquiry learning is a process that can be accomplished in differing degrees. The following learning experience, Chemrocket, demonstrates the four phases. It is not the intent of this model to suggest that all teachers should be doing exactly the same thing. It is up to each teacher to determine the needs of his or her students and to guide them to reach their highest levels. NOTES Phase Strategies Rubric Phase 1 Cookbook/ Traditional Phase 2 Structured Inquiry Phase 3 StudentDirected Inquiry Phase 4 Student Research Topic Teacher Teacher Teacher Teacher/ Student Problem Teacher Teacher Student Student Materials Teacher Teacher Teacher/ Student Student Procedures Teacher Teacher Teacher/ Student Student Results/ Analysis Teacher Student Student Student Conclusion Teacher/ Student Student Student Student Permission to use the modified Phasing Strategies Rubric was granted by: Lisa Duvall and Cindy Bagwill © RonJon Publishing, Inc. An integrated learning experience ChemRocket is a good example of a learning experience that integrates physics and chemistry concepts. Students will use their knowledge of force and motion as well as that of chemical reactions, rate of solution and scientific processes. Even though this learning experience may be view as time intensive, in 3-4 days students have actually discussed and investigated many of the TEKS for IPC. Following is a list of the TEKS that are covered in ChemRocket. Teachers can modified the types of questions and/or conclusions that students complete based on where students are in their understanding of these concepts. This experience could be conducted at the beginning of the year, in order to determine the level of understanding students have of these concepts from middle school science. The learning experience may be conducted as a study in motion, but then reviewed with students when chemical reactions or solutions are being studied. It is important for teachers to look for inquiry-based activities that illustrate the integrated nature of science. The Charles A. Dana Center at UT Austin 3 NOTES Materials Printed Materials Included in this Vista: ChemRocket Pre-Laboratory Experience investigation pages ChemRocket—Phase 1 investigation pages ChemRocket—Phase 2 investigation pages ChemRocket—Phase 3 investigation pages ChemRocket—Phase 4 investigation pages Materials for the Teacher Each learning experience has a list of all necessary equipment and materials. However, it is not the intention of TEXTEAMS to dictate the types and quantities of materials/equipment to use for the learning experiences. All the materials/equipment that are listed in the learning experiences are suggestions. Teacher’s notes give specific instructions for areas where the author has experienced the need for a specific item. Substitutions for materials/ equipment should be based on local budgets, availability, and facilities. Correlation to National Science Education Standards (NSES) 4 9–12 Abilities necessary to do scientific inquiry 9–12 Understandings about scientific inquiry 9–12 Structure and properties of matter 9–12 Chemical reactions 9–12 Motions and forces 9–12 Abilities of technological design 9–12 Systems, order, and organization 9–12 Evidence, models, and explanation 9–12 Constancy, change, and measurement Integrated Physics and Chemistry Institute – Fall 2004 The Charles A. Dana Center at UT Austin 5 IPC (A) plan and implement investigative procedures including asking questions, formulating testable hypotheses, and selecting equipment and technology; (B) collect data and make measurements with precision; (C) organize, analyze, evaluate, make inferences, and predict trends from data; and (D) communicate valid conclusions. The student is expected to: (4) Science concepts. The student The student is expected to: knows concepts of force and (B) investigate and describe applications motion evident in everyday of Newtonʼs laws such as vehicle life. restraints, sports activities, geological processes, and satellite orbits; (2) Scientific processes. The student uses scientific methods during field and laboratory investigations. (B) make wise choices in the use and conservation of resources and the disposal or recycling of materials. (A) demonstrate safe practices during field and laboratory investigations; and The student is expected to: Student Expectations FOR Knowledge and Skills S KILLS AND T EXAS E SSENTIAL K NOWLEDGE (1) Scientific processes. The student, for at least 40% of instructional time, conducts field and laboratory investigations using safe, environmentally appropriate, and ethical practices. (A)e Correlation of the TEKS to the ChemRocket Vista √ √ √ √ √ √ √ √ √ √ √ √ ChemRocket—Phase I ChemRocket—Phase II ChemRocket—Phase III ChemRocket—Phase IV ChemRocket—Phase I ChemRocket—Phase II ChemRocket—Phase III ChemRocket—Phase IV ChemRocket—Phase I ChemRocket—Phase II ChemRocket—Phase III ChemRocket—Phase IV Learning Experience Objective 5 4(B) Objective 1 2(A)(B)(C)(D) Objective 1 1(A) Grade 10 TAKS C HEM R OCKET V ISTA Objective 5 4(B) Objective 1 2(A)(B)(C)(D) Objective 1 1(A) Grade 11 TAKS 6 Integrated Physics and Chemistry Institute – Fall 2004 (9) Science concepts. The student knows how solution chemistry is part of everyday life. (8) Science concepts. The student knows that changes in matter affect everyday life. IPC (E) demonstrate how factors such as particle size, influence the rate of dissolving. (D) demonstrate how various factors influence solubility including temperature, pressure, and nature of the solute and solvent; and The student is expected to: (A) distinguish between physical and chemical changes in matter such as oxidation, digestion, changes in states, and stages in the rock cycle. The student is expected to: Student Expectations FOR Knowledge and Skills S KILLS AND T EXAS E SSENTIAL K NOWLEDGE √ √ √ √ √ √ √ √ ChemRocket—Phase I ChemRocket—Phase II ChemRocket—Phase III ChemRocket—Phase IV ChemRocket—Phase I ChemRocket—Phase II ChemRocket—Phase III ChemRocket—Phase IV Learning Experience Objective 4 9(D) Objective 4 8(A) Objective 4 9(D) Objective 4 8(A) Grade 10 TAKS Grade 11 TAKS C HEM R OCKET V ISTA ChemRocket Laboratory Investigation NOTES Background Information Background Information for the Teacher for all Phases As noted in the chart below, students have prior knowledge of Newton’s Laws of Motion, acceleration, rate of dissolving and simple chemical reactions from grades 6, 7, and 8. Grade 6 Grade 7 Grade 8 6.6(A)(B) 7.6(A)(B) 8.7(A) 6.7(A) 7.7(A) 8.9(A)(C) Physics Content Newton’s First Law states that an object at rest tends to remain at rest, while an object in motion tends to remain in motion unless acted upon by some outside force. This law is sometimes called the law of inertia. Newton’s Second Law states that the acceleration (a) of an object is inversely proportional to its mass (m), provided the force (F) acting on the object stays the same. F=ma Mathematically, acceleration can be determined from the following formula: a = F/m Newton’s Third Law states that for every action, there is an opposite and equal reaction. The rocket in this investigation lifts off because an unbalanced force (Newton’s First Law) acts upon the film canister. The rocket moves only after the addition of this force. This force is produced when the film canister’s lid blows off from the force of the gas formed in the canister from the reaction of the effervescent tablet and water. The rocket is then pushed upward by a force equal to and opposite of the force of the water pushing downward (Newton’s Third Law). The Charles A. Dana Center at UT Austin 7 NOTES F1 = F2 F1 F2 This upward force is directly proportional to the mass of water and gas expelled from the rocket and its acceleration rate (Newton’s Second Law) and can be expressed as the relationship F = ma. Although we are using the term “rocket” to describe the canister, the motion that is observed is more like that of a projectile––an object that has one short initial explosion that propels it. A rocket actually has a longer impulse that propels it into space––a force that is sustained over a greater period of time. Chemistry Content Some effervescent antacid tablets contain sodium hydrogen carbonate (commonly called sodium bicarbonate) and citric acid. When such a tablet is placed in water, aqueous citric acid [H3C6H5O7(aq)] is formed, which then reacts with the sodium bicarbonate. H3C6H5O7(aq) + 3NaHCO3(s) Na3C6H5O7(aq) + 3H2O(l) + 3CO2(g) The resulting chemical reaction forms aqueous sodium citrate (Na3C6H5O7(aq)), water, and carbon dioxide gas. The rate of dissolving is determined by the temperature of the water placed in the canister and the size of the tablets placed in the canister. The warmer the water, the faster the tablet will dissolve and the more quickly the rocket will launch. Instructional note: Student proficiency of the written balance reaction will rely upon instruction prior to this activity. If students have past experience in writing and balancing reactions, then supplying the formulas for sodium citrate and citric acid may be the only prompt necessary. If students have only middle school experience with reactions, then an acceptable answer should reflect the understanding of products and reactants but not balancing. Other IPC TEKS that could be addressed with modifications to the activity are: 4(D), 6(A), 8(B)(C)(E), 9(D). 8 Integrated Physics and Chemistry Institute – Fall 2004 Mathematics/Technology Content Students are asked to determine the height of their rockets’ paths, which can be done in several ways, including NOTES 1. judging against a background of known height, such as a brick wall, and 2. using a method of triangulation with a protractor and a small mass (see Figure 1). Figure 1 Device to measure height of rocket A student measures the angle ø from his/her eye level to the highest point of the rocket’s path.The altitude (h) is equal to the tangent of the measured angle multiplied by the baseline (distance between the launch point and observer) plus the observer’s height at eye level [ h = tan ø (baseline + observer’s height at eye level)]. This method assumes the flight path is perpendicular to the ground. 3. Assuming that the only acceleration on the canister after the explosion is due to gravity, we can use a constant acceleration relationship by measuring the time of the flight of the rocket (or better, the time it takes to fall from its maximum height). d = distance vi = initial velocity a = acceleration d = vit + 1/2at2 t = time of flight In this case, the initial velocity (vi) is zero, so by neglecting air resistance we can reduce the equation to d = 1/2at2. Using the Data Table provided in the ChemRocket Phase 1 investigation pages, students should begin to make a relationship between the time of the flight and the height the rocket achieves. In Phases 2 through 4, students will have to determine on their own what relationships are necessary to complete the task. The Charles A. Dana Center at UT Austin 9 NOTES 4. To analyze the motion and collect data from the rocket, students can use a video camera and a VCR with frame-by-frame capability, or a video camera and public-domain digitizing software (http://cipe.com/). 5. To record the motion of the rocket directly to a graphing calculator, students can use a calculator-based ranger (CBR) or other motion detector probes. 10 Integrated Physics and Chemistry Institute – Fall 2004 ChemRocket Pre-Laboratory Experience NOTES ABORATORY EXPERIENCE Teacher note: Before starting any learning experience with students, it is important to begin with an activity that focuses the students’ interest, rather than merely giving instructions, distributing materials, and telling the students to “get to work.” The following pre-laboratory experience gives the teacher an opportunity to focus the students’ thinking in the right direction. Therefore, no matter which phase strategy you have chosen to use for the ChemRocket learning experience, have all students first complete the ChemRocket Laboratory Investigation. Description: This pre-laboratory experience is designed to help students understand the TEKS concepts of Newton’s Laws of Motion. Time Frame: 55 minutes Materials: Balloon (1 per student group) Snack-size, zipper-type plastic bag (1 per student group) Water Effervescent tablet (1 per student group) Safety goggles (1 pair per student) Laboratory apron (1 per student) Chemrocket Pre-Laboratory Experience pages (included in the Blackline Master section at the end of this vista) Advance Preparation: Make copies of the ChemRocket Pre-Laboratory experience pages for each student group. Procedures: SAFETY: Caution students about the danger of chemical splashes. Students must wear safety goggles and chemical-resistant aprons during this activity. The Charles A. Dana Center at UT Austin 11 NOTES 1. Distribute copies of the ChemRocket Pre-Laboratory Experience pages. Have students complete the pages based on their understanding of forces. 2. Have students place an effervescent tablet in the snack-size, zipper-type plastic bag with approximately 10 mL of water. Students should make sure the bag is tightly closed as they observe the reaction. 3. Introduce students to an example of Newton’s Laws of Motion by having them release air-filled balloons. At this point, do not mention the laws of motion, but discuss the ideas of “pushes” or “pulls” as related to forces. 4. As a class, have students discuss their responses to the Pre-Laboratory investigation pages. Pre-Laboratory Questions (possible student responses): Teacher note: At this point, do not correct any misconceptions, as this is only an opportunity for the teacher to identify them 1. Use chemical formulas and/or equations to describe what happens when an effervescent tablet and water are sealed in a container together. [Reaction of citric acid and sodium hydrogen carbonate (commonly called sodium bicarbonate) to produce aqueous sodium citrate, water, and carbon dioxide.We observed bubbles, dissolving, disappearing, bag expanding, and it feels cold.] 2. Predict what will happen when an effervescent tablet is placed in an open container of water. [the same but the open container does not expand] 3. Based on your observations, illustrate and identify the types of forces that were involved when the balloon was released. [air pushing in on the outside walls of the balloon, gas pushing outward on the inside walls of the balloon, gas pushing out through the valve, air pushing inward on the valve] 4. Determine whether the forces involved in the release of the balloon would apply to the launch of a rocket. 5. Describe some factors that may affect how high a rocket can go. [fuel, payload, weather, weight of rocket, shape of rocket] 6. Make a sketch of a simple rocket leaving a launch pad. Using arrows, indicate the direction that the rocket is traveling and the direction of the burned fuel as it flows from the rocket. 7. Describe the motion of rockets A and B represented in the graphs shown below. [A: rocket traveled to a height of 5m in 2s (speed of 2.5 m/s) at a relatively constant speed, then changed direction ( reduced height) at a relatively constant rate of 5m in 6.5s] [B: rocket traveled to a height of 5m in 3s (slower AVERAGE speed of 1.7 m/s), then changed direction (reduced height) at an average rate of 5m in 5s (“something occurred” that caused the accelerated ascent and descent)] 12 Integrated Physics and Chemistry Institute – Fall 2004 ChemRocket Pre-Laboratory Experience The study of rockets began in the early eleventh century. Since then, rockets have been used to launch many things, including fireworks, arrows, missiles, and satellites. Pre-Laboratory Questions: 1. Use chemical formulas and/or equations to describe what happens when an effervescent tablet and water are sealed in a container together. 2. Predict what will happen when an effervescent tablet is placed in an open container of water. 3. Based on your observations, illustrate and identify the types of forces that were involved when the balloon was released. 4. Determine whether the forces involved in the release of the balloon would apply to the launch of a rocket. 5. Describe some factors that may affect how high a rocket can go. 6. Make a sketch of a simple rocket leaving a launch pad. Using arrows, indicate the direction that the rocket is traveling and the direction of the burned fuel as it flows from the rocket. The Charles A. Dana Center at UT Austin 13 7. Describe the motion of rockets A and B represented in the graphs shown below. Flight of Rocket A Flight of Rocket B 14 Integrated Physics and Chemistry Institute – Fall 2004 ChemRocket—Phase 1 NOTES Learning Experience 1 Description: This learning experience is designed to help students understand the TEKS concept of force and motion. Time Frame: 55 minutes Materials: 3L plastic bottle (1 per student group) Black, opaque, or transparent film canisters with tight-fitting lids (3 per student group) Nail (1 per student group) Modeling clay (1 package per student group) 24-30cm-long, 3/16-inch-diameter dowel rod (1 per student group) Hot glue gun or masking tape (1 per student group) 4cm-long section of plastic drinking straw (1 per student group) Index card or sheet of construction paper (1 per student group) Scissors (1 per student group) Effervescent tablets (3 per student group) Graduated cylinder (1 per student group) Graphing calculator (1 per student group) Calculator-based ranger (optional, 1 per student group) Water Student laboratory notebook (1 per student) Safety goggles (1 pair per student) Stopwatch (1 per student group) Laboratory apron (1 per student) ChemRocket—Phase 1 investigation pages (included in the Blackline Masters section at the end of this vista) Background Information for the Teacher: This learning experience is an example of a traditional Phase 1 activity. The size of the effervescent tablet used in the film canister will influence the height at which the “rocket” rises. Students may design their rocket cone or fins in a variety of ways. The design of the rocket parts is not as important as the development of the concepts involving Newton’s Laws of Motion. The Charles A. Dana Center at UT Austin 15 NOTES Conduct this learning experience with student teams consisting of no more than four students in any one group. Teacher note: Remember: Have students begin the ChemRocket-Phase1 learning experience after successfully completing and discussing the prelaboratory experience. Figure 1 Rocket launcher assembly Advance Preparation: Make copies of the ChemRocket—Phase 1 investigation pages for each student group. Procedures: SAFETY: Caution students about splashes. Students must wear safety goggles and a chemical-resistant apron during this activity. Also, remind them that hot glue may cause severe burns if it comes in contact with the skin. 1. Distribute copies of the ChemRocket—Phase 1 investigation pages to the students. Make sure students are aware of the safety precautions involved in the investigation. Teacher note: Since this is a Phase 1 investigation, do not let students stray from directions. 2. Make sure that student groups are working far enough apart so that rockets cannot come in contact with students. If weather permits, students should launch the rockets outdoors. 16 Integrated Physics and Chemistry Institute – Fall 2004 Formative Assessment: Formative assessments can be made while the teacher observes the students during the two demonstrations. Since the pre-laboratory experience is given before any instruction concerning Newton’s Laws, the teacher should be able to establish a baseline of student understanding of these concepts. In addition, student conversations and actions can indicate the level at which the students understand the concepts. NOTES Summative Assessment: After the students complete the questions at the end of the investigation, ask them to justify their designs and explain how the designs influence the forces involved in launching the rocket. Ask the students to include Newton’s Laws of Motion in their justifications and explanations. The Charles A. Dana Center at UT Austin 17 ChemRocket—Phase 1 In this investigation, you will demonstrate Newton’s Laws of Motion, describe factors affecting the rate at which a solid dissolves, balance a chemical equation, and describe the reactants and products from a chemical reaction. Materials: 3-L plastic bottle, plastic film canisters with lids, nail, modeling clay, dowel rod, hot glue gun or masking tape, plastic drinking straw, index card or sheet of construction paper, scissors, effervescent tablets, graduated cylinder, water, student laboratory notebook, graphing calculator, calculator-based ranger (optional), safety goggles, stopwatch, laboratory aprons Procedures: SAFETY: There is a danger of chemical splashes to your eyes.You must wear safety goggles and chemical-resistant apron during this activity. Hot glue may cause severe burns if it comes in contact with the skin. Rocket Launcher Assembly 1. Cut the top off the 3-L plastic bottle. 2. Use a nail to make a hole in the center of the lid of one of the film canisters. Put a small amount of clay in the film canister. Push a dowel rod through the hole into the clay and to the bottom of the film canister. Make sure the lid is securely on film canister (In Figure 1, this film canister is identified as film canister A.). 3. Put a 1/2-inch cube of clay in the bottom of the 3-L bottle, and use it to attach the film canister A/dowel rod assembly to the inside bottom of the 3-L bottle (see Figure 1). Building the Rocket 4. To make the rocket, attach the 4–cm section of the plastic drinking straw to the side of the other film canister. (In Figure 1, this film canister is identified as film canister B.) Use as little glue/tape as possible throughout the construction of the rocket. 18 Integrated Physics and Chemistry Institute – Fall 2004 5. Make a nose cone for the top of the rocket using an index card or construction paper. Carefully attach the nose cone to the base (outside bottom) of film canister B (see Figure 1.) You may also glue small “fins” on the rocket. Figure 1 Rocket Launcher Assembly The Launch 6. Using the hot glue gun, carefully attach an effervescent tablet to the inside of the film canister lid that is part of the rocket. The tablets are very easy to break, and it is okay if only a piece of the tablet is securely glued. 7. Use the graduated cylinder to measure 15 mL of water. Pour the water into film canister B. Replace the lid (be careful to keep the canister upright at this time). YOU MUST WEAR SAFETY GOGGLES. 8. Have one member of your team use the stopwatch to time the rocket’s flight. 9. When the timer is ready, quickly invert the rocket and slide the straw over the dowel rod. Step back—the rocket may launch quickly or it may take several seconds. 10. Start timing as the rocket reaches its highest point and stop when it hits the ground. 11. Repeat the launching process at least two additional times, and record all data in your journal. The Charles A. Dana Center at UT Austin 19 Data Table Record data in the table. Find the average height your rocket attained using the formula d = 1/2at2. TRIAL Time(s) t2 Acceleration 9.8 m/s2 Distance (height in m) 1 t1= d1 = 2 t2= d2 = 3 t3 = d3 = davg = Average Height Questions: 1. How are Newton’s Laws of Motion illustrated by the behavior of your rocket when it was launched? 2. Would these same laws apply to the launching of a space shuttle rocket? Explain your answer. 3. Explain what happened when the effervescent tablet came in contact with the water. How did this reaction provide the “fuel” for the rocket? Write the balanced chemical equation for the reaction and identify the reactants and the products. 4. What factors determined how high the rocket traveled? 5. What factors influenced the rate at which the effervescent tablet dissolved? Explain your response. 6. Would any of these factors apply to the launching of a full-scale rocket? Explain your answer. 7. Explain the impact of your design on the successful launch and flight of the rocket. Conclusions: Discuss the launch of your rocket and explain how it compares to the launch of a full-scale rocket. 20 Integrated Physics and Chemistry Institute – Fall 2004 ChemRocket—Phase 2 NOTES Learning Experience 2 Description: This learning experience is designed to help students understand the TEKS concept of force and motion. Time Frame: 55 minutes Materials: 3-L plastic bottle (1 per student group) Black, opaque, or transparent film canisters with tight-fitting lids (3 per student group) Nail (1 per student group) Modeling clay (1 package per student group) 24-30 cm-long, 3/16-inch-diameter dowel rod (1 per student group) Hot glue gun or masking tape (1 per student group) 4-cm-long section of plastic drinking straw (1 per student group) Index card or sheet of construction paper (1 per student group) Scissors (1 per student group) Effervescent tablets (3 tablet per student group) Graduated cylinder (1 per student group) Water Graphing calculator (1 per student group) Calculator-based ranger (optional, 1 per student group) Student laboratory notebook (1 per student) Safety goggles (1 pair per student) Stopwatch (1 per student group) Laboratory apron (1 per student) ChemRocket—Phase 2 investigation pages (included in the Blackline Masters at the end of this vista) Background Information for the Teacher: The difference between a Phase 1 and Phase 2 version of the ChemRocket learning experience is that Phase 2 requires the students to design their own methods of collecting data and communicating the data. The Charles A. Dana Center at UT Austin 21 NOTES Students may design their rocket cone or fins in a variety of ways. The design of the rocket parts is not as important as the development of the concepts involving Newton’s Laws of Motion. Conduct this learning experience with teams of students consisting of no more than four students to a group. Teacher note: Remember: Have students begin the ChemRocket-Phase 2 learning experience after successfully completing and discussing the prelaboratory experience. Building the Rocket Launcher Figure 1 shows the students how to build a rocket launcher with a predesigned rocket. Figure 1 Rocket Launcher Assembly 22 Integrated Physics and Chemistry Institute – Fall 2004 Advance Preparation: Prepare copies of the ChemRocket—Phase 2 investigation pages for each student group. NOTES Procedures: SAFETY: Caution students about the danger of chemical splashes. Students must wear safety goggles and chemical-resistant apron during this activity. Also, remind them that hot glue may cause severe burns if it comes in contact with the skin. 1. Distribute copies of the ChemRocket—Phase 2 investigation pages to the students. Make sure students are aware of the safety precautions involved in the investigation. 2. Make sure that student groups are working far enough apart so that rockets cannot come in contact with students. If weather permits, students should launch the rockets outdoors. Formative Assessment: Formative assessments can be made while the teacher observes the students during the two demonstrations. Since the pre-laboratory experience is given before any instruction concerning Newton’s Laws, the teacher should be able to establish a baseline of student understanding of these concepts. In addition, student conversations and actions can indicate the level at which the students understand the concepts. Summative Assessment: After the students complete the questions at the end of the investigation, ask each student group to justify its design and explain how the design influences the forces involved in launching the rocket. Ask the students to include Newton’s Laws of Motion in their justifications and explanations. The Charles A. Dana Center at UT Austin 23 ChemRocket—Phase 2 In this investigation, you will demonstrate the application of Newton’s Laws of Motion to the launch of a model rocket, describe factors affecting the rate at which a solid dissolves, balance a chemical equation, and describe the reactants and products from a chemical reaction. Materials: 3-L plastic bottle, plastic film canisters with lids, nail, modeling clay, dowel rod, hot glue gun or masking tape, plastic drinking straw, index card or sheet of construction paper, scissors, effervescent tablets, water, graduated cylinder, graphing calculator, calculator-based ranger (optional), student laboratory notebook, safety goggles, stopwatch, laboratory aprons Procedures: SAFETY: There is a danger of chemical splashes to your eyes.You must wear safety goggles and chemical-resistant apron during this activity. Hot glue may cause severe burns if it comes in contact with the skin. Building the Rocket Launcher Figure 1 Rocket Launcher Assembly 24 Integrated Physics and Chemistry Institute – Fall 2004 Rocket Launcher Assembly 1. Cut the top off the 3-L plastic bottle. 2. Use a nail to make a hole in the center of the lid of one of the film canisters. Put a small amount of modeling clay in the film canister. Push the dowel rod through the hole into the clay and to the bottom of the film canister. Make sure the lid is securely on the film canister. (In Figure 1, this film canister is identified as film canister A.) 3. Put a 1/2-inch cube of modeling clay in the bottom of the 3-L bottle, and use it to attach the film canister A/ dowel rod assembly to the inside bottom of the 3-L bottle (see Figure 1). Building the Rocket 4. To make the rocket, attach the 4–cm section of the plastic drinking straw to the side of the other film canister (In Figure 1, this film canister is identified as film canister B.). Use as little glue/tape as possible throughout the construction of the rocket. 5. Make a nose cone for the top of the rocket using an index card or construction paper. Carefully attach the nose cone to the base (outside bottom) of film canister B (see Figure 1).You may also glue small “fins” on the rocket. The Launch 6. Using the hot glue gun, carefully attach an effervescent tablet to the inside of the film canister lid that is part of the rocket. The tablets are very easy to break, and it is okay if only a piece of the tablet is securely glued. 7. Use the graduated cylinder to measure 15 mL of water. Pour the water into film canister B. Replace the lid (be careful to keep the canister upright at this time). YOU MUST WEAR SAFETY GOGGLES. 8. Have one member of your team use the stopwatch to time the rocket’s flight. 9. When the timer is ready, quickly invert the rocket and slide the straw over the dowel rod. Step back—the rocket may launch quickly or it may take several seconds. 10. Start timing as the rocket reaches its highest point and stop when it hits the ground. 11. Repeat the launching process at least two additional times, and record all data in your journal. Data Record the data and find the average height the rocket attained. Show all calculations in the space below. Questions: 1. How do Newton’s Laws of Motion illustrate the behavior of the rocket when it was launched? 2. Would these same laws apply to the launching of a space shuttle rocket? Explain your answer. 3. Explain what happened to the effervescent tablet when it came in contact with the water. How did this reaction provide the “fuel” for the rocket? Write the balanced chemical equation for the reaction, and describe the physical and chemical changes that occurred with the reaction. 4. What factors determined how high the rocket traveled? 5. What factors influenced the rate at which the effervescent tablet dissolved? Explain your response. 6. Would any of these factors apply to the launching of a full-scale rocket? Explain your answer. 7. What features of the rocket launcher assembly and the design of the rocket led to the successful launch and flight of the rocket? Conclusions: Compare the launch of your model rocket to the launch of a full-scale rocket. The Charles A. Dana Center at UT Austin 25 NOTES ChemRocket—Phase 3 Learning Experience 3 NG EXPERIENCE 3 Description: This learning experience is designed to help students understand the TEKS concept of force and motion. Time Frame: 55 minutes Materials: 3-L plastic bottle (1 per student group) Black, opaque, transparent plastic film canisters with tight-fitting lids (3 per student group) 24-30 cm-long, 3/16-inch-diameter dowel rod (1 per student group) Effervescent tablets (3 per student group) Graphing calculator (1 per student group) Calculator-based ranger (optional, 1 per student group) Student laboratory notebook (1 per student) Safety goggles (1 pair per student) Laboratory apron (1 per student) ChemRocket—Phase 3 investigation pages (included in the Blackline Masters at the end of this vista) Teacher note: In Phase 3, additional materials (e.g., hot glue guns, masking tape, modeling clay, nails, plastic drinking straws, index cards, construction paper, scissors, graduated cylinders, water) should be available throughout the room for students to use if they choose. Background Information for the Teacher: The difference between the Phase 2 and Phase 3 versions of the ChemRocket learning experience is that Phase 3 requires students to analyze their data and draw conclusions from their data. The teacher selects the topic and assists the students in identifying the problem to be solved. Some groups may require more teacher guidance than others, but assistance should be minimal. 26 Integrated Physics and Chemistry Institute – Fall 2004 Students are asked to build a launch pad that might be based on the example in Figure 1. Students may design their rockets in a variety of ways. The design of the rocket is not as important as the development of the concepts involving Newton’s Laws of Motion. NOTES Conduct this learning experience with student teams consisting of no more than four students in any one group. Teacher note: Remember: Have students begin the ChemRocket-Phase 3 learning experience after successfully completing and discussing the pre-laboratory experience. Figure 1 Rocket launcher assembly Advance Preparation: Prepare copies of the ChemRocket—Phase 3 investigation pages for each student group. Procedures: SAFETY: Caution students about the danger of chemical splashes. Students must wear safety goggles and chemical-resistant apron during this activity. Remind students that use the hot glue gun that hot glue may cause severe burns if it comes in contact with the skin. The Charles A. Dana Center at UT Austin 27 NOTES 1. Distribute copies of the ChemRocket–Phase 3 investigation pages to the students. Make sure students are aware of the safety precautions involved in the activity. 2. Set the stage by telling students that they have been hired by the LIFT-OFF Company to design and build a rocket. Upon completion of the rocket, the students will be required to conduct test flights and record data from each flight. They will make a presentation to the company’s executives and communicate their findings for review. 3. Make sure that student groups are working far enough apart so that rockets cannot come in contact with students. If weather permits, students should launch the rockets outdoors. Teacher note: The teacher should inspect and approve the rocket launcher to ensure that it is a safe working design. Formative Assessment: Formative assessments can be made while the teacher observes the students during the two demonstrations. Since the pre-laboratory experience is given before any instruction concerning Newton’s Laws, the teacher should be able to establish a baseline of student understanding of these concepts. In addition, student conversations and actions can indicate the level at which the students understand the concepts. Summative Assessment: In the student summary, students should justify their designs and explain how the designs influence the forces involved in launching the rocket. Students should include Newton’s Laws of Motion in their justifications and explanations. 28 Integrated Physics and Chemistry Institute – Fall 2004 ChemRocket—Phase 3 In this investigation, you will demonstrate Newton’s Laws of Motion, describe factors affecting the rate at which a solid dissolves, balance a chemical equation, and describe the reactants and products from a chemical reaction. An aerodynamics company named LIFT-OFF has asked teams of scientists to develop a rocket that can deliver a specified payload into space.You will be competing against other teams for the award of a contract to develop this rocket for the LIFT-OFF Company.Your team is to design, construct, and successfully launch a model rocket that will soar higher than any other team’s rocket. Materials: 3-L plastic bottle, plastic film canisters with lids, dowel rod, effervescent tablets, graphing calculator, calculator-based ranger (optional), student laboratory notebook, safety goggles, laboratory aprons List any additional teacher-approved materials: ______________________________________________________________________________________________ ______________________________________________________________________________________________ Procedures: SAFETY: There is a danger of chemical splashes to your eyes.You must wear safety goggles and may choose to wear a chemical-resistant apron during this activity. Hot glue may cause severe burns if it comes in contact with the skin. Directions: 1. Design and construct a launch pad and a model rocket, using the materials listed above. If there are additional materials you want to use, you must get your teacher’s approval before you begin. 2. You are limited to three test launches of your model rocket. 3. All observations, data records, analysis of data, and conclusions must be recorded in your journal. Conclusions: Write a summary of concepts that were involved in the successful launch of your model rocket. Include physical laws and chemical reactions in the summary statements. Compare these concepts and reactions to those used in the launching of a full-scale rocket. Make a presentation of your findings to the LIFT-OFF Company. The Charles A. Dana Center at UT Austin 29 NOTES ChemRocket—Phase 4 Learning Experience 4 Description: This learning experience is designed to help students understand the TEKS concept of force and motion. Time Frame: 2 lessons (55 minutes each) Materials: Graphing calculator (1 per student group) Calculator-based ranger (optional, 1 per student group) Student laboratory notebook (1 per student) Safety goggles (1 pair per student) Laboratory apron (1 per student) ChemRocket—Phase 4 investigation pages (included in the Blackline Masters at the end of this vista) Teacher note: In Phase 4, all the materials used to construct the rocket and launch pad from Phase 1 should be available throughout the room for students to choose from. Background Information for the Teacher: The difference between a Phase 3 and a Phase 4 version of the ChemRocket learning experience is that Phase 4 requires students to identify the problem, design and test the procedures, and communicate the results in a presentation or report. This phase should require some support and little guidance from the teacher. There may be some students who may not reach the phase 4 level of inquiry. Students may design their rockets in a variety of ways. The design of the rocket is not as important as the development of the concepts involving Newton’s Laws of Motion. Conducted this learning experience with student teams consisting of no more than four students in any one group. 30 Integrated Physics and Chemistry Institute – Fall 2004 Teacher note: Remember: Have students begin the ChemRocket-Phase 4 learning experience after successfully completing and discussing the pre-laboratory experience. NOTES Advance Preparation: Prepare copies of the ChemRocket—Phase 4 investigation pages for each student group. Procedures: SAFETY: Caution students about the danger of chemical splashes. Students must wear safety goggles and chemical-resistant apron during this activity. Remind students who use the hot glue gun that hot glue may cause severe burns if it comes in contact with the skin. 1. Begin the investigation by telling students that they have been hired to design and build a rocket by the LIFT-OFF Company. Upon completion of the rocket, the students will be required to conduct test flights and record data from each flight. When confident in their design and the rocket’s operation, students will make a presentation about their findings to the company’s executives. 2. Students should build the rockets and rocket launchers, keeping accurate records of the process and procedures used in their journals. 3. Distribute copies of the ChemRocket—Phase 4 investigation pages to the students. Make sure students are aware of the safety precautions that must be followed while completing the investigation. 4. Make sure that student groups are working far enough apart so that rockets cannot come in contact with students. If weather permits, students may launch the rockets outdoors. Teacher note: The teacher should inspect and approve the rocket launcher to ensure that it is a safe working design. Formative Assessment: Ask students questions about their design and results as you circulate around the room observing the progress on the learning experience. Make sure that students are correctly identifying the forces that are necessary for the rocket to launch and that students understand Newton’s Laws of Motion. Summative Assessment: During the student presentations to the class, students should justify their designs and explain how the designs influence the forces involved in launching the rocket. Students should include Newton’s Laws of Motion in their justifications and explanations. The Charles A. Dana Center at UT Austin 31 ChemRocket—Phase 4 An aerodynamics company named LIFT-OFF has asked teams of scientists to develop a rocket that can deliver a specified payload into space. You will be competing against other teams for the award of a contract to develop this rocket for the LIFT-OFF Company. Your team is to design, construct, and successfully launch a model rocket that will soar higher than any other team’s rockets. Due to budget restraints, each team is limited to six launches. LIFT-OFF requires your team to make a presentation of your findings, including a summary of the physical laws and chemical reactions that were involved in the successful launch of your model rocket. A record of all observations, data records, and analyses of data and conclusions that were recorded in your journal must be available to verify the results of your launch. Materials: Graphing calculator, calculator-based ranger (optional), student laboratory notebook, safety goggles, laboratory aprons There are a number of items placed around the room that you may use for constructing your launch pad and rocket. If you need any other materials please see the project manager (teacher). List all materials selected and give their purpose: ______________________________________________________________________________________________ ______________________________________________________________________________________________ Project Manager (teacher) sign off of the rocket design: __________________________________________ SAFETY: There is a danger of chemical splashes to your eyes.You must wear safety goggles and may choose to wear a chemical-resistant apron during this activity. 32 Integrated Physics and Chemistry Institute – Fall 2004 ChemRocket Student Blackline Masters The Charles A. Dana Center at UT Austin 33 ChemRocket Pre-Laboratory Experience The study of rockets began in the early eleventh century. Since then, rockets have been used to launch many things, including fireworks, arrows, missiles, and satellites. Pre-Laboratory Questions: 1. Use chemical formulas and/or equations to describe what happens when an effervescent tablet and water are sealed in a container together. 2. Predict what will happen when an effervescent tablet is placed in an open container of water. 3. Based on your observations, illustrate and identify the types of forces that were involved when the balloon was released. 4. Determine whether the forces involved in the release of the balloon would apply to the launch of a rocket. 34 Integrated Physics and Chemistry Institute – Fall 2004 5. Describe some factors that may affect how high a rocket can go. 6. Make a sketch of a simple rocket leaving a launch pad. Using arrows, indicate the direction that the rocket is traveling and the direction of the burned fuel as it flows from the rocket. 7. Describe the motion of rockets A and B represented in the graphs shown below. Flight of Rocket A The Charles A. Dana Center at UT Austin 35 Flight of Rocket B 36 Integrated Physics and Chemistry Institute – Fall 2004 ChemRocket—Phase 1 In this investigation, you will demonstrate Newton’s Laws of Motion, describe factors affecting the rate at which a solid dissolves, balance a chemical equation, and identify the reactants and products from a chemical reaction. Materials: 3 L plastic bottle 3 plastic film canister with lid nail modeling clay dowel rod hot glue gun or masking tape plastic drinking straw index card or sheet of construction paper scissors effervescent tablets graduated cylinder water student laboratory notebook graphing calculator calculator based ranger (optional) safety goggles stopwatch laboratory aprons Procedures: SAFETY: There is a danger of chemical splashes to your eyes.You must wear safety goggles and a chemical-resistant apron. Hot glue may cause severe burns if it comes in contact with the skin. Building the Rocket Launcher Figure 1 Rocket launcher assembly The Charles A. Dana Center at UT Austin 37 Rocket Launcher Assembly 1. Cut the top off the 3 L plastic bottle. 2. Use a nail to make a hole in the center of the lid of one of the film canister. Put a small amount of clay in the film canister. Push a dowel rod through the hole as far as it will go into the clay and to the bottom of the film canister. Make sure the lid is securely on the film canister. (In Figure 1, this film canister is identified as film canister A) 3. Put a 1/2-inch cube of clay in the bottom of the 3-L bottle, and use it to attach the film canister/dowel rod assembly to the inside bottom of the 3-L bottle (see Figure 1). Building the Rocket 4. To make the rocket, attach the 4–cm section of the plastic drinking straw to the side of the other film canister (In Figure 1, this film canister is identified as film canister B). Use as little glue/tape as possible throughout the construction of the rocket. 5. Make a nose cone for the top of the rocket using an index card or construction paper. Carefully attach the nose cone to the base (outside bottom) of film canister B (see Figure 1). You may also glue small “fins” on the rocket. The Launch 6. Using the hot glue gun, carefully attach an effervescent tablet to the inside of the film canister lid that is part of the rocket. The tablets are very easy to break, and it is okay if only a piece of the tablet is securely glued. 7. Use the graduated cylinder to measure 15 mL of water. Pour the water into film canister B. Replace the lid (be careful to keep the canister upright at this time). YOU MUST WEAR SAFETY GOGGLES. 8. Have one member of your team use the stopwatch to time the rocket’s flight. 9. When the timer is ready, quickly invert the rocket and slide the straw over the dowel rod. Step back— the rocket may launch quickly or it may take several seconds. 10. Start timing as the rocket reaches its highest point, and stop when it hits the ground. 11. Repeat the launching process at least two additional times, and record all data in your journal. 38 Integrated Physics and Chemistry Institute – Fall 2004 Data Table Record data in the table. Find the average height your rocket attained using the formula d = 1/2at2. TRIAL Time(s) t2 acceleration 9.8 m/s2 Distance (height in m) 1 t1= d1 = 2 t2= d2 = 3 t3= d3 = Average Height davg = Questions: 1. How are Newton’s Laws of Motion illustrated by the behavior of your rocket when it was launched? 2. Would these same laws apply to the launching of a space shuttle rocket? Explain your answer. 3. Explain what happened when the effervescent tablet came in contact with the water. How did this reaction provide the “fuel” for the rocket? Write the balanced chemical equation for the reaction, and identify the reactants and the products. 4. What factors determined how high the rocket traveled? The Charles A. Dana Center at UT Austin 39 5. What factors influenced the rate at which the effervescent tablet dissolved? Explain your response. 6. Would any of these factors apply to the launching of a full-scale rocket? Explain your answer. 7. Explain the impact of your design on the successful launch and flight of the rocket. Conclusions: Discuss the launch of your rocket, and explain how it compares to the launch of a full-scale rocket. 40 Integrated Physics and Chemistry Institute – Fall 2004 ChemRocket—Phase 2 In this investigation, you will demonstrate the application of Newton’s Laws of Motion to the launch of a model rocket, describe factors affecting the rate at which a solid dissolves, and describe chemical and physical changes involved in a chemical reaction. Materials: 3 L plastic bottle, plastic film canister with lid, nail, modeling clay, dowel rod, hot glue gun or masking tape, plastic drinking straw, index card or sheet of construction paper, scissors, effervescent tablets, water, graduated cylinder, graphing calculator, calculator-based ranger (optional), student laboratory notebook safety goggles, stopwatch, laboratory aprons Procedures: SAFETY: There is a danger of chemical splashes to your eyes.You must wear safety goggles and a chemical-resistant apron. Hot glue may cause severe burns if it comes in contact with the skin. Building the Rocket Launcher Figure 1 Rocket launcher assembly The Charles A. Dana Center at UT Austin 41 Rocket Launcher Assembly 1. Cut the top off the 3-L plastic bottle. 2. Use a nail to make a hole in the center of the lid of one of the film canister. Put a small amount of modeling clay in the film canister. Push a dowel rod through the hole as far as it will go into the clay and to the bottom of the film canister. Make sure the lid is securely on the film canister. (In Figure 1,this film canister is identified as film canister A.) 3. Put a 1/2-inch cube of modeling clay in the bottom of the 3-L bottle, and use it to attach the film canister/dowel rod assembly to the inside bottom of the 3-L bottle (see Figure 1). Building the Rocket 4. To make the rocket, attach the 4–cm section of the plastic drinking straw to the side of the other film canister (In Figure 1, this film canister is identified as film canister B). Use as little glue/tape as possible throughout the construction of the rocket. 5. Make a nose cone for the top of the rocket using an index card or construction paper. Carefully attach the nose cone to the base (outside bottom) of t film canister B (see Figure 1).You may also glue small “fins” on the rocket. The Launch 6. Using the hot glue gun, carefully attach an effervescent tablet to the inside of the film canister lid that is part of the rocket. The tablets are very easy to break, and it is okay if only a piece of the tablet is securely glued. 7. Use the graduated cylinder to measure 15 mL of water. Pour the water into the film canister B. Replace the lid (be careful to keep the canister upright at this time). YOU MUST WEAR SAFETY GOGGLES. 8. Have one member of your team use the stopwatch to time the rocket’s flight. 9. When the timer is ready, quickly invert the rocket and slide the straw over the dowel rod. Step back— the rocket may launch quickly or it may take several seconds. 10. Start timing as the rocket reaches its highest point, and stop when it hits the ground. 11. Repeat the launching process at least two additional times, and record all data in your journal. Data Record the data and find the average height the rocket attained. Show all calculations in the space below. 42 Integrated Physics and Chemistry Institute – Fall 2004 Questions: 1. How do Newton’s Laws of Motion illustrate the behavior of the rocket when it was launched? 2. Would these same laws apply to the launching of a space shuttle rocket? Explain your answer. 3. Explain what happened to the effervescent tablet when it came in contact with the water. How did this reaction provide the “fuel” for the rocket? Write the balanced chemical equation for the reaction, and describe the physical and chemical changes that occurred with the reaction. 4. What factors determined how high the rocket traveled? 5. What factors influenced the rate at which the effervescent tablet dissolved? Explain your response. 6. Would any of these factors apply to the launching of a full-scale rocket? Explain your answer. 7. What features of the rocket launcher assembly and the design of the rocket led to the successful launch and flight of the rocket? The Charles A. Dana Center at UT Austin 43 Conclusions: Compare the launch of your model rocket to the launch of a full-scale rocket. 44 Integrated Physics and Chemistry Institute – Fall 2004 ChemRocket—Phase 3 In this investigation, you will demonstrate Newton’s Laws of Motion, describe factors affecting the rate at which a solid dissolves, balance a chemical equation, and describe the reactants and products from a chemical reaction. An aerodynamics company named LIFT-OFF has asked teams of scientists to develop a rocket that can deliver a specified payload into space.You will be competing against other teams for the award of a contract to develop this rocket for the LIFT-OFF Company. Your team is to design, construct, and successfully launch a model rocket that will soar higher than any other team’s rocket. Materials: 3-L plastic bottle, plastic film canister with lid, dowel rod, effervescent tablets, graphing calculator, calculator-based ranger (optional), student laboratory notebook, safety goggles, laboratory aprons List any additional teacher approved materials: ____________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ __________________________________________________________________ Procedures: SAFETY: There is a danger of chemical splashes to your eyes.You must wear safety goggles and a chemical-resistant apron. Hot glue may cause severe burns if it comes in contact with the skin. Directions: 1. Design and construct a launch pad and a model rocket, using the materials listed above. If there are additional materials you want to use, you must get your teachers approval before you begin. Your instructor must approve additional materials before you construct the launch pad. 2. You are limited to three test launches of your model rocket in this competition. 3. All observations, data records, analysis of data, and conclusions must be recorded in your journal. The Charles A. Dana Center at UT Austin 45 Conclusions: Write a summary of the physics and chemistry concepts that were involved in the successful launch of your model rocket. Include physical laws and chemical reactions in the summary statements. Compare these concepts and reactions to those used in the launching of a full-scale rocket. Make a presentation of your findings to the LIFT-OFF Company. Be sure to include a cost analysis and efficiency analysis of your model rocket 46 Integrated Physics and Chemistry Institute – Fall 2004 ChemRocket—Phase 4 An aerodynamics company named LIFT-OFF has asked teams of scientists to develop a rocket that can deliver a specified payload into space.You will be competing against other teams for the award of a contract to develop this rocket for the LIFT-OFF Company. Your team is to design, construct, and successfully launch a model rocket that will soar higher than any other team’s rocket. Due to budget restraints, each team is limited to six launches. LIFT-OFF requires your team to make a presentation of your findings including a summary of the physical laws and chemical reactions that were involved in the successful launch of your model rocket. A record of all observations, data records, and analyses of data and conclusions that were recorded in your journal must be available to verify the results of your launch Materials: Graphing calculator, calculator-based ranger (optional), student laboratory notebook, safety goggles, laboratory aprons There are a number of items placed around the room that you may use for constructing your launch pad and rocket. If you need any other materials, please see the project manager (teacher). List all materials selected and give their purpose: ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ________________________________________________ Project Manager (teacher) sign off of the rocket design: _____________________________________________________________ SAFETY: There is a danger of chemical splashes to your eyes.You must wear safety goggles and a chemical-resistant apron during this activity. The Charles A. Dana Center at UT Austin 47