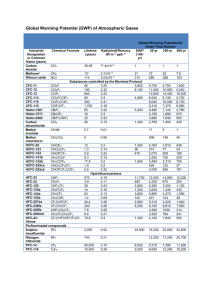
This report contains the collective views of an international group... necessarily represent the decisions or the stated policy of the...
advertisement

This report contains the collective views of an international group of experts and does not necessarily represent the decisions or the stated policy of the United Nations Environment Programme, the International Labour Organization, or the World Health Organization. Concise International Chemical Assessment Document 28 METHYL CHLORIDE Note that the layout and pagination of this pdf file are not identical to those of the printed CICAD First draft prepared by Agneta Löf, National Institute of Working Life, Solna, Sweden Maria Wallén, National Chemicals Inspectorate (KEMI), Solna, Sweden, and Jonny Bard, Åseda, Sweden Published under the joint sponsorship of the United Nations Environment Programme, the International Labour Organization, and the World Health Organization, and produced within the framework of the Inter-Organization Programme for the Sound Management of Chemicals. World Health Organization Geneva, 2000 The International Programme on Chemical Safety (IPCS), established in 1980, is a joint venture of the United Nations Environment Programme (UNEP), the International Labour Organization (ILO), and the World Health Organization (WHO). The overall objectives of the IPCS are to establish the scientific basis for assessment of the risk to human health and the environment from exposure to chemicals, through international peer review processes, as a prerequisite for the promotion of chemical safety, and to provide technical assistance in strengthening national capacities for the sound management of chemicals. The Inter-Organization Programme for the Sound Management of Chemicals (IOMC) was established in 1995 by UNEP, ILO, the Food and Agriculture Organization of the United Nations, WHO, the United Nations Industrial Development Organization, the United Nations Institute for Training and Research, and the Organisation for Economic Co-operation and Development (Participating Organizations), following recommendations made by the 1992 UN Conference on Environment and Development to strengthen cooperation and increase coordination in the field of chemical safety. The purpose of the IOMC is to promote coordination of the policies and activities pursued by the Participating Organizations, jointly or separately, to achieve the sound management of chemicals in relation to human health and the environment. WHO Library Cataloguing-in-Publication Data Methyl chloride. (Concise international chemical assessment document ; 28) 1.Methyl chloride - toxicity 2.Risk assessment 3.Environmental exposure I.International Programme on Chemical Safety II.Series ISBN 92 4 153028 6 ISSN 1020-6167 (NLM Classification: QV 633) The World Health Organization welcomes requests for permission to reproduce or translate its publications, in part or in full. Applications and enquiries should be addressed to the Office of Publications, World Health Organization, Geneva, Switzerland, which will be glad to provide the latest information on any changes made to the text, plans for new editions, and reprints and translations already available. ©World Health Organization 2000 Publications of the World Health Organization enjoy copyright protection in accordance with the provisions of Protocol 2 of the Universal Copyright Convention. All rights reserved. The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the World Health Organization concerning the legal status of any country, territory, city, or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters. The Federal Ministry for the Environment, Nature Conservation and Nuclear Safety, Germany, provided financial support for the printing of this publication. Printed by Wissenschaftliche Verlagsgesellschaft mbH, D-70009 Stuttgart 10 TABLE OF CONTENTS FOREWORD . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1 1. EXECUTIVE SUMMARY . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4 2. IDENTITY AND PHYSICAL/CHEMICAL PROPERTIES . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5 3. ANALYTICAL METHODS . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5 4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6 5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION . . . . . . . . . . . . . . . . . . . . . . 7 6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9 6.1 6.2 7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY MAMMALS AND HUMANS . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10 7.1 7.2 7.3 7.4 8. Absorption . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Distribution . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Metabolism and elimination . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Genetic polymorphism and sex, strain, organ, and species differences . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11 11 11 11 EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS . . . . . . . . . . . . . . . . . . . . . . . . . . . 13 8.1 8.2 8.3 8.4 8.5 8.6 8.7 8.8 9. Environmental levels . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9 Human exposure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10 Single exposure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Irritation and sensitization . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Short-term exposure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Medium-term exposure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Long-term exposure and carcinogenicity . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Genotoxicity and related end-points . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8.6.1 Studies in vitro . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8.6.2 Studies in vivo . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Reproductive and developmental toxicity . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8.7.1 Effects on fertility . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8.7.2 Developmental toxicity . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Immunological and neurological effects . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13 13 13 15 16 18 18 18 20 20 22 22 EFFECTS ON HUMANS . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 23 9.1 9.2 9.3 Studies in volunteers . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 23 Case reports . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 23 Epidemiological studies . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 23 10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 23 10.1 10.2 Aquatic environment . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 23 Terrestrial environment . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 24 iii Concise International Chemical Assessment Document 28 11. EFFECTS EVALUATION . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 24 11.1 11.2 Evaluation of health effects . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11.1.1 Hazard identification and dose–response assessment . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11.1.2 Criteria for setting tolerable intakes or guidance values for methyl chloride . . . . . . . . . . . . . . . . . . . . . . 11.1.3 Sample risk characterization . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Evaluation of environmental effects . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 24 24 26 27 27 12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 28 REFERENCES . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 29 APPENDIX 1 — SOURCE DOCUMENTS . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 36 APPENDIX 2 — CICAD PEER REVIEW . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 37 APPENDIX 3 — CICAD FINAL REVIEW BOARD . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 38 INTERNATIONAL CHEMICAL SAFETY CARD . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 39 RÉSUMÉ D’ORIENTATION . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 41 RESUMEN DE ORIENTACIÓN . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 43 iv Methyl chloride While every effort is made to ensure that CICADs represent the current status of knowledge, new information is being developed constantly. Unless otherwise stated, CICADs are based on a search of the scientific literature to the date shown in the executive summary. In the event that a reader becomes aware of new information that would change the conclusions drawn in a CICAD, the reader is requested to contact IPCS to inform it of the new information. FOREWORD Concise International Chemical Assessment Documents (CICADs) are the latest in a family of publications from the International Programme on Chemical Safety (IPCS) — a cooperative programme of the World Health Organization (WHO), the International Labour Organization (ILO), and the United Nations Environment Programme (UNEP). CICADs join the Environmental Health Criteria documents (EHCs) as authoritative documents on the risk assessment of chemicals. Procedures The flow chart shows the procedures followed to produce a CICAD. These procedures are designed to take advantage of the expertise that exists around the world — expertise that is required to produce the highquality evaluations of toxicological, exposure, and other data that are necessary for assessing risks to human health and/or the environment. CICADs are concise documents that provide summaries of the relevant scientific information concerning the potential effects of chemicals upon human health and/or the environment. They are based on selected national or regional evaluation documents or on existing EHCs. Before acceptance for publication as CICADs by IPCS, these documents undergo extensive peer review by internationally selected experts to ensure their completeness, accuracy in the way in which the original data are represented, and the validity of the conclusions drawn. The first draft is based on an existing national, regional, or international review. Authors of the first draft are usually, but not necessarily, from the institution that developed the original review. A standard outline has been developed to encourage consistency in form. The first draft undergoes primary review by IPCS to ensure that it meets the specified criteria for CICADs. The primary objective of CICADs is characterization of hazard and dose–response from exposure to a chemical. CICADs are not a summary of all available data on a particular chemical; rather, they include only that information considered critical for characterization of the risk posed by the chemical. The critical studies are, however, presented in sufficient detail to support the conclusions drawn. For additional information, the reader should consult the identified source documents upon which the CICAD has been based. The second stage involves international peer review by scientists known for their particular expertise and by scientists selected from an international roster compiled by IPCS through recommendations from IPCS national Contact Points and from IPCS Participating Institutions. Adequate time is allowed for the selected experts to undertake a thorough review. Authors are required to take reviewers’ comments into account and revise their draft, if necessary. The resulting second draft is submitted to a Final Review Board together with the reviewers’ comments. Risks to human health and the environment will vary considerably depending upon the type and extent of exposure. Responsible authorities are strongly encouraged to characterize risk on the basis of locally measured or predicted exposure scenarios. To assist the reader, examples of exposure estimation and risk characterization are provided in CICADs, whenever possible. These examples cannot be considered as representing all possible exposure situations, but are provided as guidance only. The reader is referred to EHC 1701 for advice on the derivation of health-based tolerable intakes and guidance values. The CICAD Final Review Board has several important functions: – – – International Programme on Chemical Safety (1994) Assessing human health risks of chemicals: derivation of guidance values for health-based exposure limits. Geneva, World Health Organization (Environmental Health Criteria 170). 1 – to ensure that each CICAD has been subjected to an appropriate and thorough peer review; to verify that the peer reviewers’ comments have been addressed appropriately; to provide guidance to those responsible for the preparation of CICADs on how to resolve any remaining issues if, in the opinion of the Board, the author has not adequately addressed all comments of the reviewers; and to approve CICADs as international assessments. Board members serve in their personal capacity, not as representatives of any organization, government, or 1 Concise International Chemical Assessment Document 28 CICAD PREPARATION FLOW CHART SELECTION OF PRIORITY CHEMICAL SELECTION OF HIGH QUALITY NATIONAL/REGIONAL ASSESSMENT DOCUMENT(S) FIRST DRAFT PREPARED PRIMARY REVIEW BY IPCS ( REVISIONS AS NECESSARY) REVIEW BY IPCS CONTACT POINTS/ SPECIALIZED EXPERTS R E V I E W O F C O M M E N T S ( PRODUCER/RESPONSIBLE OFFICER), PREPARATION OF SECOND DRAFT 1 FINAL REVIEW BOARD FINAL DRAFT 2 3 EDITING APPROVAL BY DIRECTOR, IPCS PUBLICATION 1 Taking into account the comments from reviewers. 2 The second draft of documents is submitted to the Final Review Board together with the reviewers’ comments. 3 Includes any revisions requested by the Final Review Board. 2 Methyl chloride industry. They are selected because of their expertise in human and environmental toxicology or because of their experience in the regulation of chemicals. Boards are chosen according to the range of expertise required for a meeting and the need for balanced geographic representation. Board members, authors, reviewers, consultants, and advisers who participate in the preparation of a CICAD are required to declare any real or potential conflict of interest in relation to the subjects under discussion at any stage of the process. Representatives of nongovernmental organizations may be invited to observe the proceedings of the Final Review Board. Observers may participate in Board discussions only at the invitation of the Chairperson, and they may not participate in the final decision-making process. 3 Concise International Chemical Assessment Document 28 1. EXECUTIVE SUMMARY CFC-11, which has an ODP of 1. Methyl chloride is not thought to contribute significantly to either global warming or photochemical air pollution. The assessment of human health aspects in this CICAD on methyl chloride was based primarily on a review prepared by the Nordic Expert Group in collaboration with the Dutch Expert Committee for Occupational Standards (Lundberg, 1992). Relevant databases covering the years 1992–1999 were searched to identify additional data. For the environmental and ecotoxicological aspects of methyl chloride, BUA (1986), ATSDR (1990), WMO (1994), and HSDB (1996) were used as primary sources. ATSDR (1990) was updated in 1998; where ATSDR (1998) provided new information, this has been taken into account. Additional data on environmental issues were identified in relevant databases covering the years 1989–1997. Information concerning the nature and availability of the source documents is presented in Appendix 1. Information on the peer review of this CICAD is presented in Appendix 2. This CICAD was approved as an international assessment at a meeting of the Final Review Board, held in Stockholm, Sweden, on 25–28 May 1999. Participants at the Final Review Board meeting are listed in Appendix 3. The International Chemical Safety Card (ICSC 0419) for methyl chloride, produced by the International Programme on Chemical Safety (IPCS, 1999), has been reproduced in this document. The dominant loss mechanism for methyl chloride in water and soil is volatilization. Slow hydrolysis and possibly biotic degradation may contribute to the loss in deeper soil layers and in groundwater. However, little information is available concerning biodegradation. The most important route of exposure of methyl chloride in humans is via the respiratory pathway. In humans as well as in experimental animals, methyl chloride is readily absorbed through the lungs following inhalation. Following exposure to 14C-radiolabelled methyl chloride, the radioactivity is found throughout the body. Although a large portion of the radiolabelled substance is incorporated into protein through the onecarbon pool, methyl chloride may also bind to protein by direct alkylation. However, if methyl chloride is an alkylating agent, it is so to a very small extent. Methyl chloride is metabolized in mammals either by conjugation with glutathione or, to a lesser extent, through oxidation by cytochrome P-450; the glutathione pathway yields methanethiol, and both pathways yield formaldehyde and formate. Metabolites from methyl chloride are excreted via the urine and by exhalation. Methyl chloride is also exhaled unmetabolized. In humans, there are large interindividual differences in the uptake and metabolism of methyl chloride. These differences depend on the presence or absence of the enzyme glutathione transferase T1 (GSTT1), which displays genetic polymorphism. Humans can be phenotyped as high conjugators, low conjugators, or nonconjugators of GSTT1. However, as it is not evident if high conjugators or non-conjugators incur the highest risk, one must consider all phenotypes as sensitive to methyl chloride exposure. Methyl chloride (CAS No. 74-87-3) is released mainly to air during its production and use and by incineration of municipal and industrial wastes. However, natural sources, primarily oceans and biomass burning, clearly dominate over anthropogenic sources. The total global release of methyl chloride from all sources is estimated to be about 5 × 106 tonnes per year. The contribution from natural sources has been estimated to be well over 90%, and perhaps as much as 99%, of the total release. Methyl chloride is present in the troposphere at a concentration of approximately 1.2 µg/m3 (0.6 ppb). The acute inhalation toxicity of methyl chloride in rats and mice seems to be fairly low, with an LC50 value above 4128 mg/m3 (2000 ppm). No data on irritation or sensitizing properties were located in the literature. The principal sink for methyl chloride in the troposphere is chemical reaction with hydroxyl radicals, and the atmospheric lifetime is estimated to be 1–3 years. A certain amount of methyl chloride reaches the stratosphere; there, photodissociation generates chlorine radicals, which contribute to ozone depletion. Estimates of the amount of methyl chloride reaching the stratosphere, and thus depleting ozone, vary widely. As estimated from figures presented by the World Meteorological Organization (WMO), methyl chloride contributes approximately 15% of the total equivalent effective stratospheric chlorine. The stratospheric ozone depletion potential (ODP) of methyl chloride has been determined to be 0.02 relative to the reference compound The main target organs after short-term inhalation exposure to methyl chloride seem to be the nervous system, with functional disturbances and cerebellar degeneration in both rats and mice, as well as testicles, epididymis, and kidneys in rats and kidneys and liver in mice. In a 2-year inhalation study in mice, axonal swelling and degeneration of lumbar spinal nerves were observed at 103 mg/m3 (50 ppm) in exposed animals compared with controls, but without an apparent 4 Methyl chloride 2. IDENTITY AND PHYSICAL/CHEMICAL PROPERTIES dose–response relationship. At the end of the study, cerebellar degeneration in mice of both sexes and renal adenocarcinomas in male mice were observed at 2064 mg/m3 (1000 ppm). These effects were not observed in the rat at 2064 mg/m3 (1000 ppm). Methyl chloride (CAS No. 74-87-3; CH3Cl; chloromethane) is a colourless gas at ambient temperatures. It can be compressed to a liquid, which has a weak ethereal smell. The odour threshold has been estimated to be 21 mg/m3 (10 ppm) (ASTM, 1973). Methyl chloride decomposes in water with a half-time of 4.66 h at 100 °C (IARC, 1986). Methyl chloride is clearly genotoxic in in vitro systems in both bacteria and mammalian cells. Although the positive effects seen in a dominant lethal test most likely were cytotoxic rather than genotoxic, methyl chloride might be considered a very weak mutagen in vivo based on some evidence of DNA–protein crosslinking at higher doses. Methyl chloride is marketed as a liquefied gas under pressure. The purity of a representative technical grade of methyl chloride is close to 100%. Impurities include water, hydrochloric acid, methyl ether, methanol, and acetone (Holbrook, 1992). Testicular lesions and epididymal granulomas followed by reduced sperm quality lead to reduced fertility in rats at 980 mg/m3 (475 ppm) and to complete infertility at higher doses. Methyl chloride has a very high vapour pressure and a high solubility in water. The value of its Henry’s law constant is high, which suggests that volatilization of methyl chloride will be significant in surface waters. The calculated octanol/water partition coefficient (log Kow) is low, indicating a low potential for bioaccumulation and low tendency of adsorption to soil and sediment. Methyl chloride induced heart defects in mouse fetuses when dams were exposed to 1032 mg/m3 (500 ppm) during the gestation period. Effects on humans, especially on the central nervous system, can be clearly seen after accidental inhalation exposure. In short-term exposure of volunteers to methyl chloride, no significant effects were seen that could be attributed to the exposure. There are insufficient epidemiological data available to assess the risk for humans to develop cancer as a result of methyl chloride exposure. Some relevant physical and chemical properties of methyl chloride are listed in Table 1. Additional physical/chemical properties are presented in the International Chemical Safety Card, reproduced in this document. In conclusion, the critical end-point for methyl chloride inhalation toxicity in humans seems to be neurotoxicity. Guidance values of 0.018 mg/m3 (0.009 ppm) for indirect exposure via the environment and 1.0 mg/m3 (0.5 ppm) for the working environment were derived. Although the nerve lesions were seen at lower exposure levels than those at which infertility in rats (980 mg/m3 [475 ppm]) and renal tumours in male mice (2064 mg/m3 [1000 ppm]) occurred, emphasis should also be laid on these very serious effects in a qualitative risk characterization of methyl chloride. 3. ANALYTICAL METHODS In air, methyl chloride can be analysed by Method 1001 of the US National Institute for Occupational Safety and Health (NIOSH, 1994). Analysis is performed by gas chromatography (GC), and the sample detection limit is 3.1 µg/m3 (1.5 ppb). Using the method of Oliver et al. (1996), the detection limit is 1.1 µg/m3 (0.53 ppb). Few data were found on the short-term toxicity of methyl chloride to both aquatic and terrestrial organisms. No data were found on long-term toxicity. The existing data indicate that methyl chloride has a low acute toxicity to aquatic organisms. The lowest LC50 value for fish is 270 mg/litre. As measured concentrations of methyl chloride in surface waters are generally several orders of magnitude less than those demonstrated to cause effects, it is likely that methyl chloride poses a low risk of acute effects on aquatic organisms. Only very limited data are available on the effects of methyl chloride on terrestrial organisms. The use of carbon disulfide at dry ice temperature for desorbing the analyte has also been described, as well as a thermal desorption technique as an alternative (Severs & Skory, 1975). A thermally desorbable diffusional dosimeter for monitoring methyl chloride in the workplace has also been described (Hahne, 1990). Very low concentrations (0.006–0.1 µg/m3 [0.003–0.05 ppb]) of methyl chloride (in ambient air) can be analysed by the use of photoionization, flame ionization, and electron capture detectors in series (Rudolph & Jebsen, 1983). 5 Concise International Chemical Assessment Document 28 !160 °C, thermal desorption, separation on a GC capillary column, and detection with ion trap MS. The detection limit is about 0.06 µg/m3 (0.03 ppb) for methyl chloride. Table 1: Identity and physical/chemical properties of methyl chloride. Property Value Relative molecular mass 50.49 Melting point !97.7 °C !97.1 °C Holbrook, 1992 Weast, 1988 Boiling point !23.73 °C !24.2 °C Holbrook, 1992 Weast, 1988 0.920 g/ml 2.3045 g/litre Holbrook, 1992 Holbrook, 1992 Density liquid at 20/4 °C gas at 0 °C, 101.3 kPa Specific gravity 1.74 (air = 1) Holbrook, 1992 Solubility in water at 25 °C 5.325 g/litre 4.800 g/litre Horvath, 1982 Holbrook, 1992 3.04 × 10 5 Pa 5.75 × 10 5 Pa BUA, 1986 BUA, 1986 0.1977 ± 1.4% Moore et al., 1995 BUA, 1986 Vapour pressure at 5.5 °C 25 °C Henry’s law constant at 3 °C (seawater, salinity 30.4‰)a 25 °C a Reference 4.15–6.05 kPa@m 3/mol Log K ow 0.91 Hansch & Leo, 1985 Conversion factor ppm (v/v) to mg/m 3 in air at 25 °C 1 ppm = 2.064 mg/m 3 1 mg/m 3 = 0.4845 ppm ATSDR, 1990 In water, methyl chloride can be analysed by EPA Method 502.2 at a detection limit of 0.1 µg/litre (US EPA, 1986b). Other methods for the detection of volatile organic substances in water are EPA Method 502.1, which has a detection limit of 0.01 µg/litre (US EPA, 1986b), and EPA Method 524.2, with a detection limit of 0.05 µg/litre (US EPA, 1986b). Another method involving a solid-phase micro-extraction technique has a detection limit of <25 µg/litre (Shirey, 1995). EPA Method 601 (purgeable halocarbons) is suitable for measuring methyl chloride in wastewater. The detection limit is 0.06 µg/litre (US EPA, 1982; CFR, 1990). A similar method is EPA Method 624 (purgeables), with a detection limit of 2.8 µg/litre (US EPA, 1982; CFR, 1991). A third method used for analysis of wastewater is EPA Method 1624, with a minimum detection level of 50 µg/litre (CFR, 1991). In soil and solid waste, EPA Method 5030 (US EPA, 1986a) may be used to analyse methyl chloride. Analysis is performed by different EPA methods. In Method 8010B, the limit of detection is 12.5 µg/kg for high-concentration soils and sludges (US EPA, 1986a). Gomes et al. (1994) describes another method to collect and analyse methyl chloride, which can also be used to analyse contaminants in groundwater. 4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE Henry’s law constant is defined as the concentration in air divided by the concentration in water at equilibrium; unit of Henry’s law constant: dimensionless (Moore et al., 1995). Exposure to methyl chloride can also be monitored in air by a direct-reading infrared analyser, at minimum detectable concentrations of 800–3100 µg/m3 (390– 1500 ppb) (IARC, 1985). Natural sources of methyl chloride dominate over anthropogenic sources. The major source appears to be the marine/aquatic environment, likely associated with algal growth. Other sources are biomass burning (forest fires), degradation of wood by fungi, and direct and indirect anthropogenic sources. Stratospheric air samples are often concentrated by a cryogenic procedure, at liquid nitrogen or argon temperature, followed by GC analysis employing electron capture detection (Rasmussen et al., 1980; Singh et al., 1983, 1992; Rudolph et al., 1992, 1995; Khalil & Rasmussen, 1993; Fabian et al., 1996) or with GC/mass spectrometry (MS) (Schauffler et al., 1993). The GC may be equipped with a flame ionization detector (Evans et al., 1992) or a mass selective detector (Atlas et al., 1993). Almasi et al. (1993) described a modified version of a method commonly used by the US Environmental Protection Agency (EPA) to analyse low levels of volatile organic compounds in air (EPA Method TO-14). It includes sample concentrations on glass beads at Methyl chloride is produced industrially by reaction of methanol and hydrogen chloride or by chlorination of methane (Key et al., 1980; Edwards et al., 1982a; Holbrook, 1992). In almost all of the commercial uses, methyl chloride is reacted to form another product (ATSDR, 1998). The current principal uses are in the production of silicones and also as a general methylating agent. The use of methyl chloride in the manufacture of synthetic rubber, its refrigerant and extractant applications, and its use as a tetramethyllead intermediate now have secondary importance (Holbrook, 1992). 6 Methyl chloride Indirect sources of methyl chloride are tobacco smoke, turbine exhaust (Wynder & Hoffmann, 1967; Graedel, 1978; Häsänen et al., 1990), incineration of municipal and industrial waste (Graedel & Keene, 1995), chlorination of drinking-water, and sewage effluent (Abrams et al., 1975). (Isidorov, 1990). Estimates of the global yearly release of methyl chloride from marine sources are in the range 1–8 × 106 tonnes (Watson et al., 1980; Singh et al., 1983; Isidorov, 1990). Terrestrial species also produce methyl chloride. The activity of methyltransferases, believed to be responsible for methyl chloride production, has been observed in several herbaceous species (Saini, 1995). According to Harper et al. (1988), 34 species of fungi are also known to biosynthesize methyl chloride. The current production capacity of methyl chloride in the USA has been estimated to be about 0.417 × 106 tonnes per year (CMR, 1995). The production in Japan in 1996 was 0.13 × 106 tonnes (Chemical Daily Co. Ltd., 1998). Estimates of the global annual release of methyl chloride from biomass burning are in the range 0.4–1.8 × 106 tonnes (Watson et al., 1980; Andreae, 1991, 1993; Lobert et al., 1991; Rudolph et al., 1994, 1995). The major part of the methyl chloride released from biomass burning originates from forest fires in the tropics (Andreae et al., 1994). The estimated global release of methyl chloride from temperate and boreal biomass fires has been calculated to be 0.012 × 106 tonnes per year (Laursen et al., 1992). Low-intensity, inefficient combustion and high chlorine content of the biomass promote methyl chloride formation (Reinhardt & Ward, 1995). It has been concluded that well over 90%, and perhaps as much as 99%, of ambient air concentrations on a global scale appear to originate from natural sources rather than from anthropogenic sources (ATSDR, 1998). Edwards et al. (1982b) estimated the emissions of methyl chloride during production, transport, storage, and use to be approximately 0.02 × 106 tonnes per year, corresponding to nearly 6% of the amount produced. According to this estimate, anthropogenic sources would account for 1–2% of the total release, including natural sources. Other estimates of the global yearly release from anthropogenic sources are in the range of 0.024–0.6 × 106 tonnes (Watson et al., 1980; Gribble, 1992; Dowdell et al., 1994), the higher estimate including indirect anthropogenic sources and possibly also biomass burning. 5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION Methyl chloride, which is the most prevalent halogenated methane in the atmosphere, is present in the troposphere at a concentration of about 1.2 µg/m3 (0.6 ppb) (WMO, 1994). It has been calculated that at a production rate of about 3.5 × 106 tonnes per year, the steady-state mixing ratio of 1.2 µg/m3 (0.6 ppb) is maintained given an atmospheric lifetime in the order of 2 years (WMO, 1994). Estimates of the total global annual release of methyl chloride from all sources are around 5 × 106 tonnes (Rasmussen et al., 1980; Logan et al., 1981; Edwards et al., 1982b; Dowdell et al., 1994; WMO, 1994; Fabian et al., 1996). According to ATSDR (1998), the total release from all sources amounts to approximately 3.2–8.2 × 106 tonnes per year. Most methyl chloride discharged to the environment will be released to air. The principal sink for methyl chloride in the troposphere is chemical reaction with hydroxyl radicals (ASTDR, 1990; Graedel & Keene, 1995; Fabian et al., 1996). The rate constant for this reaction is approximately 4.3 × 10–14 cm3/s per molecule at 25 °C (NASA, 1981; Atkinson, 1985). Atmospheric lifetime estimates range from 1 to 3 years (Atkinson, 1985; BUA, 1986; Warneck, 1988; ATSDR, 1990; WMO, 1990, 1994; Fabian et al., 1996; Houghton et al., 1996). Surface deposition, rainout, and washout are unimportant sinks for methyl chloride (Graedel & Keene, 1995). Estimates of the amount of methyl chloride reaching the stratosphere vary considerably. Borchers et al. (1994) claim that the contribution of methyl chloride to the stratospheric chlorine budget is significant. Crutzen & Gidel (1983) estimated the flux of methyl chloride to the stratosphere to be about 2 × 106 tonnes per year or 20–25% of the total annual stratospheric chlorine input. According to Fabian et al. (1996), only a fraction (less than 10%) of the amount of methyl chloride emitted reaches the stratosphere. Edwards et al. (1982b) claim that about 6% of the methyl chloride released reaches the stratosphere (corresponding to 0.3 × 106 tonnes per year). According to Graedel & Crutzen Over the Pacific Ocean, the concentration of methyl chloride is higher in the lower troposphere than in the higher layers. However, over the continents, the concentration is independent of the altitude. Thus, the ocean seems to be a source of methyl chloride (Geckeler & Eberhardt, 1995). In the oceans, algae, especially planktonic algae, are considered to be responsible for most of the methyl chloride production. However, this has not been fully proven. Phytoplankton have been shown to produce methyl chloride in laboratory studies (Tait & Moore, 1995). An alternative model is that methyl chloride is formed as a result of exchange processes between methyl iodide and chlorine ions in seawater 7 Concise International Chemical Assessment Document 28 HO-reaction 4 ± 1 x 106 tonnes Marine algae 4 ± 2 x 106 tonnes Biomass combustion 0.5 ± 0.2 x 106 tonnes CH3Cl β= 3.7x106 tonnes ∆≈ 0 Industrial 0.3 ± 0.1 x 106 tonnes Ocean hydrolysis 0.3 ± 0.6 x 106 tonnes Transport to the stratosphere 0.03 ± 0.01 x 106 tonnes Rainout/washout ≈ 0.0012 x 106 tonnes β= Tropospheric burden ∆= Tropospheric growth rate Figure 1. Global budget for tropospheric methyl chloride (as tonnes chlorine per year). The budget is based on an average concentration of methyl chloride in the troposphere of 1.3 µg/m3 (0.62 ppb), a lifetime of 1.5 years, and a tropospheric burden of about 3.7 × 106 tonnes chlorine per year (adapted from Graedel & Keene, 1995). (1993) and Graedel & Keene (1995), only 0.8% (corresponding to 0.03 × 106 tonnes chlorine per year) is expected to reach the stratosphere. A global budget for tropospheric methyl chloride is shown in Figure 1. dissociation rate of each compound involved in ozone depletion. A radiative forcing value of 0.0053 W/m2 per part per billion has been determined for methyl chloride. This value is about 2% of the forcing of CFC-11 and about 300 times the forcing of carbon dioxide, on a per molecule basis (Grossman et al., 1994). Houghton et al. (1996) gave the radiative forcing value as 0 W/m2 for methyl chloride. The global warming potential (GWP) has been calculated to be about 25, relative to carbon dioxide (GWP = 1), at a time scale of 20 years (Grossman et al., 1994). The ability of the ozone layer to absorb ultraviolet radiation shorter than 290 nm should exclude direct photolysis in the troposphere, because methyl chloride does not absorb any radiation above 290 nm (BUA, 1986). In the stratosphere, photodissociation will occur at a rate approximately equal to its reaction with hydroxyl radicals (Robbins, 1976). The chlorine radicals that are generated contribute to ozone depletion. Methyl chloride has been shown to photochemically decompose at 185 nm. Photooxidation products in the gas phase were carbon dioxide, carbon monoxide, formic acid, formyl chloride, water, and hydrogen chloride (Gürtler & Kleinermanns, 1994). As the current concentration of methyl chloride in the atmosphere is relatively low, approximately 1.2 µg/m3 (0.6 ppb), the contribution of this substance to the greenhouse effect will not become a problem unless large releases of this gas occur (Grossman et al., 1994). WMO (1994) also considers that the contribution of methyl chloride to climate forcing is minimal. The stratospheric steady-state ODP of methyl chloride has been determined to be 0.02 relative to CFC11 (ODP = 1) (Solomon et al., 1992; WMO, 1994; Fabian et al., 1996). Estimates of the amount of methyl chloride reaching the stratosphere, and thereby also its contribution to ozone depletion, vary considerably. However, as estimated from figures presented by WMO (1994), methyl chloride contributes approximately 15% (0.5 ppb) of the total (3.3 ppb) equivalent effective stratospheric chlorine. The term “equivalent effective stratospheric chlorine” includes both stratospheric chlorine and bromine ("1 = 40) and also considers the The contribution of methyl chloride to the creation of photochemical air pollution is not significant because of its relatively low reactivity and low amounts emitted. The photochemical ozone creation potential (POCP) of methyl chloride has been determined to be 3.5 (integrated ozone formation over 5 days) relative to that of ethylene (POCP = 100) (Derwent et al., 1996). Reactive chlorine in the lower atmosphere (as distinguished from chlorofluorocarbon-derived chlorine 1 In the stratosphere, each bromine atom is assumed to be 40 times more damaging to ozone than each chlorine atom (WMO, 1994). 8 Methyl chloride in the stratosphere) is supposed to be important in considerations of precipitation acidity, corrosion of metals and alloys, foliar damage, and chemistry of the marine boundary layer. The tropospheric reactive chlorine burden of approximately 8.3 × 106 tonnes chlorine is dominated by methyl chloride (~45%) and trichloroethane (~25%) (Graedel & Keene, 1995). layers may to some extent leach into the groundwater, as well as diffuse to the surface and volatilize (ATSDR, 1990; HSDB, 1996). In groundwater, methyl chloride is expected to biodegrade or hydrolyse very slowly (ATSDR, 1990; HSDB, 1996). The cumulative volatilization loss of methyl chloride, from a depth of 1 m beneath ground, has been calculated to be at least 70% and 22% in 1 year for a sandy soil and a clay soil, respectively (Jury et al., 1990). If methyl chloride is released into water, it will be lost primarily by volatilization. The volatilization half-life has been calculated to be 2.1 h in a model river (Lyman et al., 1982). The volatilization half-lives of methyl chloride in a pond and in a lake have been estimated to be 25 h and 18 days, respectively, using the model EXAMS (ATSDR, 1990). The low log Kow (0.91) of methyl chloride indicates that the substance does not tend to concentrate in sediments. There are no experimental studies on bioaccumulation. However, only a minor accumulation in biota would be expected on the basis of the low log Kow. A bioconcentration factor of 2.9 has been calculated based on the log Kow (ATSDR, 1990). 6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE The transformation of methyl chloride by hydrolysis is probably negligible under acid and neutral conditions. Under basic conditions, slow hydrolysis takes place, yielding methanol as a transformation product (Simon, 1989). Hydrolytic half-lives range from 31 days (pH 11) to 2.5 years (pH not given) at 20–25 °C (Zafiriou, 1975; Mabey & Mill, 1976, 1978; Simon, 1989). The hydrolytic half-life of methyl chloride in seawater varies with temperature (0–30 °C) from 0.5 to 77 years (Elliott & Rowland, 1995). Laboratory data indicate that the photochemical transformation of methyl chloride in water is negligible (Mabey & Mill, 1976). 6.1 Environmental levels Background concentrations of methyl chloride in the troposphere are around 1.2 µg/m3 (0.6 ppb), ranging from about 1.0 to 1.4 µg/m3 (from 0.5 to 0.7 ppb) (Cox et al., 1976; Cronn et al., 1976, 1977; Pierotti & Rasmussen, 1976; Singh et al., 1977, 1979, 1983; Graedel, 1978; Khalil & Rasmussen, 1981, 1993; Guicherit & Schulting, 1985; Gregory et al., 1986; Warneck, 1988; Rudolph et al., 1992; Singh et al., 1992; Atlas et al., 1993; WMO, 1994; Graedel & Keene, 1995; Fabian et al., 1996). In the stratosphere, the concentration of methyl chloride decreases with altitude. Concentrations in the Arctic stratosphere in March 1992 ranged from 0.60 to 0.082 µg/m3 (from 0.29 to 0.04 ppb) at altitudes of 11–22 km (von Clarmann et al., 1995). In May 1985, at a latitude of 26–30 °N, Zander et al. (1992) found methyl chloride concentrations ranging from 0.12 to 0.050 µg/m3 (from 0.058 to 0.024 ppb) at altitudes of 12–22 km. Near the tropical tropopause (23.8–25.3 °N, 15–17 km altitude), the mean methyl chloride concentration was measured to be 1.1 µg/m3 (0.531 ppb) during January–March 1992 (Schauffler et al., 1993). Methyl chloride was not readily biodegraded in a standardized “closed bottle test” (MITI, 1992). However, several isolated bacterial strains have been shown to degrade methyl chloride under both aerobic (Stirling & Dalton, 1979; Hartmans et al., 1986; Bartnicki & Castro, 1994; Chang & Alvarez-Cohen, 1996) and anaerobic conditions (Traunecker et al., 1991; Braus-Stromeyer et al., 1993; Dolfing et al., 1993; Leisinger & BrausStromeyer, 1995). A half-life of less than 11 days was estimated for the anaerobic biodegradation of methyl chloride in groundwater, based on laboratory data obtained under conditions favourable for anaerobic biodegradation (Wood et al., 1985). The very low log Kow (0.91) of methyl chloride indicates that it will not tend to adsorb to soil (Lyman et al., 1982). The adsorption coefficient, Koc, has been calculated to be 5, based on physical/chemical data (ATSDR, 1990). The very high vapour pressure and low adsorption to soil suggest that methyl chloride present near the soil surface will rapidly be lost by volatilization (ATSDR, 1990; HSDB, 1996). As it is not expected to adsorb to soil, methyl chloride present in deeper soil Numerous measurements of methyl chloride levels in air have been performed, especially in the USA. The mean or median concentrations of methyl chloride measured in the air of rural/remote sites in the USA were about 1.0–2.7 µg/m3 (0.5–1.3 ppb), with the majority of the values below 2.1 µg/m3 (1.0 ppb); the maximum concentration measured was 4.3 µg/m3 (2.1 ppb) (Grimsrud & Rasmussen, 1975; Robinson et al., 1977; 9 Concise International Chemical Assessment Document 28 Singh et al., 1977, 1981b; Brodzinsky & Singh, 1983; Rasmussen & Khalil, 1983; Shah & Singh, 1988). In samples from urban/suburban areas in the USA, the mean/median concentrations were in the range of 0.27– 6.2 µg/m3 (0.13–3.0 ppb), with the majority of the values in the range 1.0–2.3 µg/m3 (0.5–1.1 ppb); the highest concentration found was 25.0 µg/m3 (12.1 ppb) (Singh et al., 1977, 1979, 1981a, 1982, 1992; Brodzinsky & Singh, 1983; Edgerton et al., 1984; Shah & Singh, 1988; Rice et al., 1990; US EPA, 1991a, 1991b; Evans et al., 1992; Kelly et al., 1994; Spicer et al., 1996). Methyl chloride concentrations in three Japanese cities ranged from 4.5 to 35 µg/m3 (from 2.2 to 17 ppb) (Furutani, 1979). In Delft, the Netherlands, and Lisbon, Portugal, concentrations of 6.2 µg/m3 (3.0 ppb) (Guicherit & Schulting, 1985) and 4.5 µg/m3 (2.2 ppb) (Singh et al., 1979), respectively, were found. methyl chloride was mostly found at 0.01–0.05 µg/litre (Lovelock, 1975; Pearson & McConnell, 1975; NAS, 1978; Singh et al., 1979, 1983; Edwards et al, 1982b); however, a higher concentration of 1.2 µg/litre was reported from a measurement near the shore of California, USA (Singh et al., 1979). Methyl chloride was detected in soils at 34 waste sites and in sediments at 13 waste sites in the USA (HazDat, 1998) and in 1 of 345 sampling stations of the US EPA STORET database, at a concentration of <5 µg/kg (Staples et al., 1985). Methyl chloride was also detected in soil at an electronic industrial site in São Paulo, Brazil (Gomes et al., 1994). No data were found on methyl chloride levels in sediment. According to the US EPA STORET database, methyl chloride was detected in 1% of analysed samples of fish and seafood (Staples et al., 1985). From these data, it appears that the concentrations of methyl chloride are slightly higher in the air of urban/ suburban sites than at rural/remote sites. However, a direct comparison is difficult, because samples in urban/ suburban areas were probably often taken at ground level, while several measurements of rural/remote areas were made at higher altitudes. 6.2 Human exposure Data given in section 6.1 suggest that humans are exposed to methyl chloride in ambient air. Background concentrations are around 1.2 µg/m3 (0.6 ppb). In urban areas, mean and median concentrations generally seem to be slightly higher, 1.0–2.3 µg/m3 (0.5–1.1 ppb). However, individual measurements may be much higher. The highest value found in the literature was 35 µg/m3 (17 ppb). Methyl chloride has also been occasionally detected in water, soil, and biota. A few studies on measurements of methyl chloride in drinking-water were identified, most of them performed in the USA and Canada (Abrams et al., 1975; Coleman et al., 1976; Burmaster, 1982; Mariich et al., 1982; Otson et al., 1982; Otson, 1987). A maximum concentration of 44 µg/litre was measured in a drinking-water well (Burmaster, 1982). Workplace concentrations have been measured in four US chemical plants (NIOSH, 1980). Three of the plants produced methyl chloride. The personal 8-h timeweighted average concentrations in the three plants ranged from 18.4 to 25.6 mg/m3 (from 8.9 to 12.4 ppm), from <0.4 to 15.5 mg/m3 (from <0.2 to 7.5 ppm), and from <0.2 to 26.2 mg/m3 (from <0.1 to 12.7 ppm), respectively. In the fourth plant, where methyl chloride was used as a blowing agent in the production of polystyrene foam, the personal exposures ranged from 6.2 to 44.2 mg/m3 (from 3.0 to 21.4 ppm). In a Dutch methyl chloride production plant, individual 8-h time-weighted averages of methyl chloride exposure in the air ranged from 62 to 186 mg/m3 (from 30 to 90 ppm) (van Doorn et al., 1980). In measurements of groundwaters in the USA, concentrations of methyl chloride ranged from not detectable up to 100 µg/litre, found at a former waste site of a chemical factory (Page, 1981; Burmaster, 1982; Sabele & Clark, 1984; Lesage et al., 1990; Plumb, 1991; Rosenfeld & Plumb, 1991). The substance was detected in groundwaters at 20 of 479 waste disposal sites in 1991 (Plumb, 1991). In surface water samples in North America, the concentrations ranged from not detectable up to 224 µg/litre, the highest value reported from New Jersey, USA, in the 1970s (Page, 1981; Otson et al., 1982; Great Lakes Water Quality Board, 1983; Granstrom et al., 1984; Staples et al., 1985; Otson, 1987). In the only European study found (Hendriks & Stouten, 1993), a maximum concentration of 12 µg/litre in the river Rhine was reported. In seawater samples collected near the surface, 7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY MAMMALS AND HUMANS The most important route of exposure of methyl chloride in humans is via the respiratory pathway. Data 10 Methyl chloride Metabolites from methyl chloride are excreted in the urine and in the expired air. S-Methylcysteine has been identified in the urine of occupationally exposed humans and rats (van Doorn et al., 1980; Landry et al., 1983), and formic acid has been found in rat urine (Kornbrust & Bus, 1983). Further, carbon dioxide has been shown to be the major final metabolite of methyl chloride, accounting for nearly 50% of the radiolabelled material recovered after a 6-h exposure of rats to methyl chloride (Kornbrust & Bus, 1983). Methyl chloride is also excreted unmetabolized via the lungs, as seen in studies in volunteers (Stewart et al., 1980; Nolan et al., 1985; Löf et al., 2000). on methyl chloride toxicokinetics cover only inhalation; no relevant information on other routes of administration was located in the literature. 7.1 Absorption In humans as well as in experimental animals, methyl chloride is readily absorbed through the lungs following inhalation (Andersen et al., 1980; Stewart et al., 1980; Landry et al., 1983; Nolan et al., 1985; Löf et al., 2000). In human volunteers exposed to 21 or 103 mg methyl chloride/m3 (10 or 50 ppm) for 6 h or to 21 mg/m3 (10 ppm) for 2 h, steady state was reached during the first exposure hour (Nolan et al., 1985; Löf et al., 2000). In rats, equilibrium between uptake and elimination was also obtained within 1 h (Landry et al., 1983). 7.2 The plausible metabolic pathways of methyl chloride in mammals are shown in Figure 2. Distribution 7.4 After rats were exposed to 14C-labelled methyl chloride by inhalation, radioactivity was found to the largest extent in the liver, kidneys, and testes and to a smaller extent in the brain and lungs (Redford-Ellis & Gowenlock, 1971; Kornbrust et al., 1982; Landry et al., 1983). The presence of residues was, however, attributed to the metabolism of methyl chloride to formaldehyde and formate and subsequent incorporation of the radiolabelled carbon atom into tissue macromolecules through single-carbon anabolic pathways (Kornbrust & Bus, 1982; Kornbrust et al., 1982). Methyl chloride may also bind to macromolecules, especially protein, and to a minimal extent probably also to DNA (Kornbrust et al., 1982; Vaughan et al., 1993). 7.3 Genetic polymorphism and sex, strain, organ, and species differences In several studies in volunteers, large interindividual differences in concentrations of methyl chloride in breath and blood and amounts of excreted urinary metabolites have been observed (Stewart et al., 1980; van Doorn et al., 1980; Putz-Anderson et al., 1981a; Nolan et al., 1985; Löf et al., 2000). One explanation for the large interindividual differences in uptake and elimination of methyl chloride in humans is the presence or absence of the enzyme GSTT1 (Coles & Ketterer, 1990). The presence of the GSTT1 gene leads to conjugation between glutathione and methyl chloride (GSTT1+), and the absence of the gene leads to no conjugation (GSTT1!) (Pemble et al., 1994). Metabolism and elimination In humans as well as in animals, methyl chloride is mainly metabolized by conjugation with glutathione. S-Methylglutathione can then be further metabolized to S-methylcysteine and methanethiol (Redford-Ellis & Gowenlock, 1971; Bus, 1982; Landry et al., 1983). To a lesser extent, methyl chloride is also metabolized microsomally via cytochrome P-450 in rat liver, resulting in the formation of formaldehyde and formate (Kornbrust & Bus, 1983). Formaldehyde and formate may also be formed via the glutathione pathway (Kornbrust & Bus, 1983). About 60% of blood samples from a German population showed a significant metabolic elimination of methyl chloride, whereas 40% did not (Peter et al., 1989). In a Swedish population, Warholm et al. (1994) found a large interindividual variation in the glutathione transferase activity in erythrocytes treated with methyl chloride, as 43% had a high activity, 46% had a medium activity, and 11% lacked activity. Nelson and co-workers (1995) mapped the ethnic differences in the prevalence of the null genotype (GSTT1!) and found the highest prevalence among Chinese (64%), followed by Koreans (60%), African-Americans (22%), and Caucasians (20%), and the lowest among Mexican-Americans (10%). Warholm et al. (1994) concluded that the GSTT1 polymorphism leads to three different phenotypes in humans — namely, non-conjugators (NC), low conjugators (LC), and high conjugators (HC). In a comparison involving the three human phenotypes and experimental animals, Thier et al. (1998) established that GSTT1 activity towards methyl chloride in human erythrocytes Inhalation of methyl chloride by male B6C3F1 mice resulted in a concentration-dependent depletion of glutathione in liver, kidney, and brain. The depletion was most pronounced in the liver, where a 6-h inhalation exposure to 206 mg/m3 (100 ppm) decreased the glutathione level by 45%, and exposure to 5160 mg/m3 (2500 ppm) reduced the glutathione content to 2% of control levels (Kornbrust & Bus, 1984). 11 Concise International Chemical Assessment Document 28 excretion of CH3 Cl in exhaled air CH3 Cl Methyl chloride + glutathione +glutathione transferase +Cytochrome P450 GS CH3 S-Methylglutathione NH2 CH3SCH2CHCOOH S-Methylcysteine CH3 SH Methanethiol alkylation of macromolecules, especially proteins HCHO Formaldehyde HCOOH Formic acid one carbon pool CO2 H2S Hydrogen sulphide incorporation in the tissues via metabolism 2- SO4 Figure 2: Metabolic pathways for methyl chloride (slightly modified after Bus, 1982). 12 excretion via urine exhalation Methyl chloride (HC, LC, or NC) and in liver and kidney cytosol in experimental animals decreased in the following order: female mouse (B6C3F1) > male mouse (B6C3F1) > HC > rat (Fischer 344) > LC > hamster (Syrian golden) > NC. In animals, GSTT1 activity towards methyl chloride was 2–7 times higher in liver cytosol than in kidney cytosol (Thier et al., 1998). details. In another experimental series, where no clinical acute toxicity symptoms except for lethality were reported, five male B6C3F1 mice were exposed (whole body) to methyl chloride at concentrations of 1032– 5160 mg/m3 (500–2500 ppm) in increments of 1032 mg/m3 (500 ppm) (Chellman et al., 1986a). The 6-h LC50 value was determined to be 4540 mg/m3 (2200 ppm). In this study, lethality as well as hepatotoxicity, renal toxicity, and cerebellar degeneration were prevented in mice exposed to 5160 mg/m3 (2500 ppm) for 6 h by pretreatment with the glutathione synthesis inhibitor L-buthionine-S,R-sulfoximine, indicating that metabolism of methyl chloride by glutathione conjugation increases the toxicity. The human GSTT1 polymorphism was illustrated in a study on the toxicokinetics of methyl chloride in volunteers phenotyped for GSTT1 activity (HC, LC, and NC) (Löf et al., 2000). It was seen that conjugators with the fast GSTT1 activity (HC) had the highest respiratory net uptake (respiratory net uptake equals the difference between the amount of methyl chloride in inhaled and exhaled air during exposure) of methyl chloride, whereas subjects with no GSTT1 activity (NC) had a smaller respiratory net uptake. At the end of the exposure, the concentration of methyl chloride in blood declined more rapidly among volunteers with high (HC) and intermediate (LC) GSTT1 activity than in those with no activity (NC). The area under the curve for NC was higher than those for HC and LC, and the area under the curve for LC was higher than that for HC. Further, the clearance of methyl chloride by metabolism was high in fast conjugators (HC) and close to zero in subjects with no GSTT1 activity (NC). Although other single-exposure inhalation toxicity studies in small rodents exist, they are very old (published before 1950) and do not meet current standards, and they are therefore not included in the present evaluation on methyl chloride. In any case, the results are similar to those reported here. No single-exposure studies on methyl chloride toxicity following other routes of administration were located in the literature. In conclusion, based on scarce data, the acute inhalation toxicity in male mice seems to be fairly low, with an LC50 value above 4128 mg/m3 (2000 ppm). In mice, a sex difference in susceptibility to methyl chloride was indicated. In an investigation by Dekant et al. (1995), sex-, strain-, and species-specific bioactivation of methyl chloride by cytochrome P-450 2E1 (CYP2E1) was seen in the liver and kidneys of rats and mice. In kidney microsomes, the rate of oxidation of methyl chloride was significantly higher in male mice than in female mice and in rats of both sexes than in mice. It was also observed that the rate of oxidation in kidney microsomes was faster in CD-1 mice and NMRI mice than in C3H/He and C57BL/6J mice. In erythrocytes from other species — rats, mice, cows, pigs, sheep, and rhesus monkeys — no conversion of methyl chloride was seen in erythrocyte cytoplasm (Peter et al., 1989). 8.2 No data on irritation or sensitization were available. 8.3 Short-term exposure The toxic response to methyl chloride was studied in Fischer 344 rats (10 animals per sex per group) exposed by inhalation to 0, 4128, 7224, or 10 320 mg methyl chloride/m3 (0, 2000, 3500, or 5000 ppm) for 6 h/day for 9 days (5 days of exposure followed by a 2day break in exposure, then a further 4 days’ exposure) and in C3H, C57BL/6, or B6C3F1 mice (5 animals per strain per sex per group) exposed by inhalation to 0, 1032, 2064, or 4128 mg methyl chloride/m3 (0, 500, 1000, or 2000 ppm) for 6 h/day for 12 days (Morgan et al., 1982). The animals were sacrificed 18 h after their last exposure or immediately after the day’s exposure if they were found to be moribund. Clinical observations and histopathological investigations of the brain, liver, kidneys, and adrenal glands in both species and of testes and epididymis of rats were reported. As a result of high intoxication, some rats from the two highest dose groups were sacrificed in extremis (6 males, 5 females: 8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS 8.1 Irritation and sensitization Single exposure In B6C3F1 mice, the LC50 of methyl chloride via inhalation for 6 h was reported to be 4644 mg/m3 (2250 ppm) in males and 17 544 mg/m3 (8500 ppm) in females (White et al., 1982). The data on lethal doses were obtained from an abstract without any further 13 Concise International Chemical Assessment Document 28 10 320 mg/m3 [5000 ppm]; 2 females: 7224 mg/m3 [3500 ppm]). No information was given on whether effects were seen in animals with a fulfilled exposure scheme or with an interrupted scheme. cellular effects. A no-effect level could not be obtained for either species. The ultrastructure of the methyl chloride-induced cerebellar lesions observed in mice and rats by Morgan and co-workers (1982) and in guinea-pigs (reported under section 8.4) by von Kolkmann & Volk (1975) was further studied by Jiang et al. (1985) in female C57BL/6 mice. The mice were exposed for 6 h/day, 5 days/week, for 2 weeks to 0 or 3096 mg methyl chloride/m3 (0 or 1500 ppm). In all treated mice, degenerative changes of varying severity were observed in the granular cell layer of the cerebellum. The lesions in the granular cells were characterized by nuclear and cytoplasmic condensation of scattered granule cells and also by watery swelling and disruption of granule cell perikarya. From poorly reported clinical observations, neurological deficiency in motor coordination was seen. Few kidney abnormalities were detected, indicating that the cerebellar degenerations were not secondary to kidney lesions. Clinically, especially in the higher dose groups, the rats were seriously affected by the exposure, and symptoms such as lack of coordination of the forelimbs, paralysis of the hindlimbs, convulsive seizures, perineal urine staining, and diarrhoea were recorded. In the kidneys, concentration-related degeneration and necrosis of the proximal convoluted tubules could be seen (lowestobserved-adverse-effect level or LOAEL [males] = 4128 mg/m3 [2000 ppm]; LOAEL [females] = 7224 mg/m3 [3500 ppm]). Testicular degeneration in the seminiferous tubules (LOAEL = 4128 mg/m3 [2000 ppm]) and adrenal fatty degeneration (LOAEL [males and females] = 7224 mg/m3 [3500 ppm]) were also concentration related. Most animals showed minimal hepatocellular response, including loss of normal areas of cytoplasmic basophilia and variable degeneration. Rats in the 10 320 mg/m3 (5000 ppm) group showed degeneration of the cerebellar granular layer. In a study primarily designed to investigate the correlation between neurotoxicity and continuous versus intermittent exposure to methyl chloride, Landry et al. (1985) exposed female C57BL/6 mice for 11 days either continuously (22.5 h/day) to 31, 103, 206, 310, or 413 mg/m3 (15, 50, 100, 150, or 200 ppm) or intermittently (5.5 h/day) to 310, 826, 1651, 3302, or 4954 mg/m3 (150, 400, 800, 1600, or 2400 ppm). A quantitative relationship between neurotoxicity and continuous and intermittent exposure was not observed. The lowest effect level for clinical observations, similar to those reported earlier by Dunn & Smith (1947) and later by von Kolkmann & Volk (1975), Morgan et al. (1982), and Jiang et al. (1985), was 206 mg/m3 (100 ppm) for continuous exposure and 3302 mg/m3 (1600 ppm) for intermittent exposure. Cerebellar lesions were recorded at 206 and 826 mg/m3 (100 and 400 ppm) for continuous and intermittent exposure, respectively. A statistically significant decrease was observed in relative and absolute thymus weights at the 31, 103, and 310 mg/m3 (15, 50, and 150 ppm) exposure levels (continuous exposure) and also at the 3302 and 4954 mg/m3 (1600 and 2400 ppm) levels (intermittent exposure). Although the decrease in thymus weight at 31 mg/m3 (15 ppm) suggests that this level might be a LOAEL, the absence of a methyl chloride-induced effect on the thymus or its function in long-term studies indicates that these weight decreases are of uncertain significance. From the results, a LOAEL of 826 mg/m3 (400 ppm) for intermittent exposure (cerebellar lesions) and of 206 mg/m3 (100 ppm) for continuous exposure can be concluded. All mice in the highest dose group died before or were moribund at exposure day 5. No apparent strain differences could be seen from available mortality data. Prior to death, some of the animals developed moderate to severe ataxia, and all females developed haematurea. In the 2064 mg/m3 (1000 ppm) group, females developed haematurea to a much larger extent than males. Cerebellar degeneration of the same type as in rats was seen at the 2064 and 4128 mg/m3 (1000 and 2000 ppm) concentration levels in female C57BL/6 mice only. The other two mouse strains did not develop brain lesions. On the contrary, in all three mouse strains, degeneration in the kidneys was found at 4128 mg/m3 (2000 ppm), and basophilic renal tubules were observed at 2064 mg/m3 (1000 ppm). Hepatocellular necrosis was confined to the 4128 mg/m3 (2000 ppm) group in male C57BL/6 and B6C3F1 mice. Hepatocellular degeneration was seen in lower dose groups, mainly in the 1032 and 2064 mg/m3 (500 and 1000 ppm) groups of male and female C57BL/6 mice. Liver damage in the low dose groups was considered mild and consisted of, for example, variable degrees of glycogen depletion and cytoplasmic vacuolization. From these studies, which highlight species and sex differences in methyl chloride-induced toxicity, a rat LOAEL of 4128 mg/m3 (2000 ppm) could be derived from the testicular, epididymal, renal, and to some extent hepatocellular findings, and a mouse LOAEL of 1032 mg/m3 (500 ppm) could be derived from the hepato To assess the role of inflammation in the toxicity of methyl chloride, especially to sperm, Chellman et al. 14 Methyl chloride No toxicity data for short-term exposures other than those obtained from administration via the respiratory pathway were located in the literature. (1986b) exposed male Fischer 344 rats for 5 days, 6 h/day, to 0 or 10 320 mg methyl chloride/m3 (0 or 5000 ppm) with and without the presence of the anti-inflammatory agent 3-amino-1-(m-[trifluoromethyl]phenyl)-2-pyrazoline (BW755C), an inhibitor of leucotriene and prostaglandin synthesis. Lesions that were induced by exposure to methyl chloride alone were epididymal sperm granulomas, degeneration of cerebellar granule cells, necrosis of renal proximal tubules, cloudy swelling of hepatocytes, and vacuolization of cell cytoplasm in the outer region of zona fasciculata in the adrenal glands. Virtually none of these effects was seen when BW755C was given in parallel with methyl chloride, strongly suggesting an inflammatory response. 8.4 Medium-term exposure To investigate methyl chloride-induced neurotoxicity, von Kolkmann & Volk (1975) exposed 19 guineapigs to 41 280 mg methyl chloride/m3 (20 000 ppm; 2 vol% in a pressurized vessel) by inhalation for 61– 70 days (10 min/day, 6 times/week). Clinically, in approximately half of the animals in the treated group, ataxia, paresis of the hind legs, staggering, atactic moving of the head, and retardation in spontaneous reaction and mobility were observed. No animal died during the exposure period. Histopathologically, necroses were seen in the cerebellar cortex in the granular cell layer. Further, Purkinje’s cell necrosis occurred. Considering the extremely high exposure concentrations, the study can be used for descriptive purposes only. The question as to whether methyl chlorideinduced renal tumours in male mice are evoked by the metabolic intermediate formaldehyde was studied by Jäger et al. (1988). Fischer 344 rats and B6C3F1 mice of both sexes in groups of five were exposed to 0 or 2064 mg methyl chloride/m3 (0 or 1000 ppm) for 6 days, and DNA lesions (cross-links and single-strand breaks), glutathione transferase (GST) activity, and formaldehyde dehydrogenase (FDH) activity were measured. It was shown that the tumour formation in male mice is not based on any obvious biochemical sex differences in enzymatic transformation with respect to FDH. Neither is the metabolically formed formaldehyde likely to be the effective carcinogen, as the characteristic formaldehydeinduced genetic damage is absent. However, the significant species difference between mice and rats — in that mice, due to higher GST activity, especially in the kidneys, seem to be more susceptible to methyl chloride treatment — could not be ruled out. It was, for example, not shown if toxicity caused by the glutathione conjugation pathway was due to a metabolite formed or to glutathione depletion, as suggested by Jäger et al. (1988). In a subchronic toxicity study, 80 Fischer 344 rats (40 per sex) and 80 B6C3F1 mice (40 per sex) were exposed to methyl chloride by inhalation at concentrations of 0, 774, 1548, or 3096 mg/m3 (0, 375, 750, or 1500 ppm) for 90 days (CIIT, 1979). Clinical observations and data on food consumption, body and organ weights, haematology, clinical chemistry, urinalysis, ophthalmoscopic examination, gross pathology, and histopathology were recorded. Female mice in the 3096 mg/m3 (1500 ppm) dose group had significantly depressed total body weight at the end of the exposure period. Absolute and/or relative organ weights were increased for heart, brain, spleen, liver, kidneys, and lungs in female mice (mainly in the highest dose group) and in pancreas in male mice. Cytoplasmic vacuolization of hepatocytes occurred in the two highest dose groups and was considered compound related. In the 1548 mg/m3 (750 ppm) dose group, vacuolization was seen 5 times as frequently in females as in males. Exposure-related fluctuations in haematology and in clinical chemistry were observed but not considered significant, as they were within the control range. Further, the methyl chloride-exposed mice had a high incidence of a mucopurulent conjunctivitis. However, this effect was probably not related to methyl chloride exposure, as it was seen mainly in the 774 mg/m3 (375 ppm) dose group. The available results indicate that female mice are more sensitive than male mice to methyl chloride exposure. In conclusion, after short-term exposure, the target organ in both rats and mice is the nervous system, with functional disturbances and cerebellar degeneration. The LOAELs in mice are 206 and 826 mg/m3 (100 and 400 ppm) upon continuous and intermittent exposure, respectively. Higher levels of exposure caused toxicity in the kidney and liver in mice and in the testes, epididymis, and kidney in rats. A mouse LOAEL of 1032 mg/m3 (500 ppm) could be derived from liver toxicity data. The decrease in thymus weight, unaccompanied by histopathological changes, in mice exposed to 31 mg/m3 (15 ppm) was not corroborated by either a 90-day (CIIT, 1979) or a 2-year study (CIIT, 1981) (reported in sections 8.4 and 8.5, respectively). Because of this lack of corroboration, the decrease in thymus weight will not be forwarded to the sample risk characterization. Male rats in all dose groups and females in the two highest dose groups showed significantly decreased absolute body weight. In rats (males and/or females; mainly in the highest dose group), absolute and/or rela- 15 Concise International Chemical Assessment Document 28 Total body weight gain was significantly reduced throughout the exposure period for male and female rats in the 2064 mg/m3 (1000 ppm) exposure group. Although female rats in the 464 mg/m3 (225 ppm) group and female mice in the 2064 mg/m3 (1000 ppm) group also had significantly decreased growth rates, these occurred periodically and were not observed at the end of exposure. The relative heart weight was increased in female mice and male and female rats at 2064 mg/m3 (1000 ppm). Otherwise, changes in relative or absolute organ weights were seen at the 2064 mg/m3 (1000 ppm) exposure level for kidney, liver, heart, and brain in both species and in testes in rats. For comparison with the short-term exposure study by Landry et al. (1985), thymus weight was not affected by methyl chloride exposure in the 2-year study. tive organ weights were increased for heart, brain, testes, ovaries, spleen, liver, kidney, pancreas, and adrenals. In the 1979 CIIT study (which served as a pilot for the 2-year chronic toxicity/carcinogenicity study by CIIT [1981]), no compound-related lesions were reported from gross pathology or histopathology on kidneys, heart, or testes. The absence of recorded organ lesions in mice could be due to a fairly high mortality, especially in the highest dose group, in combination with the histological examination applied. In the histopathological examination, as a first step, the highest dose group was compared with the control group. In the case of positive findings, the control animals were thereafter compared with the 1548 mg/m3 (750 ppm) dose group and then the 375 ppm (774 mg/m3) dose group. This procedure might suffer from a high mortality in the 3096 mg/m3 (1500 ppm) dose group and give rise to false negatives. No similar explanation could be given for rats, as the mortality during the exposure period was low. 8.5 Clinical observations on toxicity to the central nervous system (hunched posture, tremor, and paralysis) were seen in mice in the highest dose group but not in rats. Long-term exposure and carcinogenicity In mice, statistically significant hepatocellular changes (vacuolization, karyomegaly, cytomegaly, and degeneration) were seen in male and female mice from the 2064 mg/m3 (1000 ppm) group. In male mice exposed to 2064 mg methyl chloride/m3 (1000 ppm), significantly elevated serum glutamic–pyruvic transaminase (SGPT) values were seen, coupled with histopathological findings in the liver. Elevated SGPT values were also observed in the lower dose groups but were not correlated with any histopathological findings. In a 2-year inhalation study, Fischer 344 rats and B6C3F1 mice (120 animals per sex per group) were exposed to 0, 103, 464, or 2064 mg methyl chloride/m3 (0, 50, 225, or 1000 ppm) for 6 h/day, 5 days/week, with the objective of determining the potential toxicological and oncogenic effects (CIIT, 1981). Planned interim necropsies of the experimental animals were completed at 6, 12, 18, and 24 months following initiation of exposure. As a result of high mortality in the mouse high-dose group, the scheduled 24-month sacrifice was carried out after 21 or 22 months of exposure. After 6 or 12 months, 10 rats per sex per dose group were scheduled to be sacrificed, and after 18 or 24 months, 20 and 80 rats per sex per dose group, respectively. Mice were scheduled for sacrifice in groups of 10 per sex per dose group after 6, 12, or 18 months and in groups of 90 per sex per dose group after 24 (or 21, 22 months). Data on body weights, clinical signs of toxic effects, clinical chemical analyses, gross pathology, and histopathology were recorded. In the 2064 mg/m3 (1000 ppm) dose group in male mice, a large, statistically significant increase (P > 0.05) in the development of renal tubuloepithelial hyperplasia, hypertrophy, and/or karyomegaly, with onset at 12 months, was observed. Further, in the same group, significant exposure-related increases (P < 0.05) in numbers of observed renal cortical adenomas as well as renal adenocarcinomas (including those designed as renal cortical adenocarcinomas and renal cortical papillary cystadenocarcinomas) were noted in animals sacrificed or dying between 12 and 21 months (incidences of cortical renal lesions are found in Table 3). Cortical adenomas were also seen in two male mice in the 464 mg/m3 (225 ppm) group. Although this increase was not of statistical significance, the adenomas were similar to those that occurred in the 2064 mg/m3 (1000 ppm) group and were therefore judged to be associated with the methyl chloride exposure. In the 103 mg/m3 (50 ppm) group, there was a slightly increased incidence of renal cortical microcysts in male mice sacrificed at 24 months compared with the control males (6/32 vs. 1/20). Renal microcysts were also observed in the 464 mg/m3 (225 ppm) group; the During the exposure period, rat survival was not affected by methyl chloride exposure. However, mouse survival was low in the 2064 mg/m3 (1000 ppm) dose group compared with the control animals. The high mortality occurred predominantly during the first 6 months and was attributed by CIIT (1981) to fighting for dominance. The number of rats and mice that died during the 2-year study for reasons other than planned sacrifice is given in Table 2. 16 Methyl chloride Table 2: Number of rodents that died during the exposure period for reasons other than scheduled sacrifice. Number of rodents that died 0 ppm Species 50 ppm 225 ppm 1000 ppm male femal e male female male female male female Rats 15 23 12 19 12 23 14 19 Mice 75 33 62 34 62 25 93 73 Table 3: Total number of significant cortical renal lesions (malign and benign) observed in male B6C3F1 mice exposed to methyl chloride for 2 years. Number of renal lesions/number of animals necropsied 0 ppm Renal lesions 50 ppm 225 ppm 1000 ppm m f m f m f m f Cortex, adenocarcinoma 0/120 0/120 0/118 0/100 0/117 0/123 5/120 0/109 Cortex, papillary cysts, adenocarcinoma 0/120 0/120 0/118 0/100 0/117 0/123 1/120 0/109 Cortex, adenoma 0/120 0/120 0/118 0/100 2/117 0/123 12/120 0/109 Cortex, papillary cysts, adenoma 0/120 0/120 0/118 0/100 0/117 0/123 2/120 0/109 Cortex tubuloepithelium, hypertrophy, hyperplasia, and/or karyomegaly 0/120 0/120 0/118 0/100 0/117 0/123 44/120 0/109 2064 mg/m3 [1000 ppm]: 3/7 males and no data on females). The effects at each exposure level were significantly increased in each dose group as compared with control animals. However, no concentration– response relationship could be established. In the 2064 mg/m3 (1000 ppm) dose group sacrificed at 22 months, minimal to moderate swelling and degeneration of the lumbar spinal nerves were recorded in 13 of 18 female mice. Twelve of 18 females had similar lesions in the thoracic spinal cord, and 6/18 females in the cervical spinal cord. Histopathological examinations of mice in the 2064 mg/m3 (1000 ppm) dose group with unscheduled death showed high incidences of cerebellar lesions and axonal degeneration of lumbar spinal nerves, lesions similar to those found at the 18-month sacrifice. increase, as compared with the control group, was not, however, significant in either males or females. The incidence of renal cortical microcysts was not reported in animals from the highest dose group. Since the microcysts appear to be variations of the same lesion observed at higher exposure levels, they should be considered to be related to methyl chloride, although no concentration dependence could be established. Further, at 2064 mg/m3 (1000 ppm), degeneration and atrophy of the seminiferous tubules were seen, as well as lymphoid depletion and splenic atrophy. At the 18-month sacrifice, cerebellar lesions (degeneration and atrophy of the cerebellar granular layer) were noted in male and female mice at the 2064 mg/m3 (1000 ppm) level. Mice from the control, low, and intermediate exposure groups did not have lesions in the granular cell layer of the cerebellum. At the 22month sacrifice, similar but more extensive observations in 17/18 females were reported from the 2064 mg/m3 (1000 ppm) group (the only group examined at this time period). In rats, exposure to methyl chloride at 2064 mg/m3 (1000 ppm) caused testicular lesions (bilateral, P > 0.05, and unilateral, P > 0.05, diffuse degenerations and atrophies of the seminiferous tubules). These lesions were statistically significant as compared with the control group and were first observed at the 6-month sacrifice. Sperm granulomas were noted in three male rats at 2064 mg/m3 (1000 ppm). No statistically significant changes other than the effects on body weight gain were seen in female rats. This indicates that the maximum tolerated dose used in the CIIT (1981) study might have been too low to induce toxicity in female rats. At the 18-month sacrifice, axonal swelling and degeneration of minor severity were observed in the spinal nerves and cauda equina associated with the lumbar spinal cord. The effects occurred in most treated animals in all dose groups (controls: 1/5 males and 2/10 females; 103 mg/m3 [50 ppm]: 4/5 males and 10/10 females; 464 mg/m3 [225 ppm]: 5/5 males and 5/5 females; Significant findings from the long-term studies in mice are disturbances of the nervous system and 17 Concise International Chemical Assessment Document 28 In vitro, 1–10% methyl chloride caused induction of unscheduled DNA synthesis (UDS) in rat spermatocytes and hepatocytes (Working et al., 1986). induction of tumours and microcysts in male mice. Axonal swelling and degeneration of spinal nerves in all exposure groups suggest a LOAEL of 103 mg/m3 (50 ppm). This LOAEL is forwarded to the sample risk characterization. The observation of renal microcysts in the 103 mg/m3 (50 ppm) dose group (although not concentration related) supports the LOAEL of 103 mg/m3 (50 ppm). Further significant findings are the testicular lesions in rats. In male rats, testicular lesions occurred at 2064 mg/m3 (1000 ppm). No toxic effects in females were reported. Methyl chloride was shown to directly bind to bovine serum albumin. No further details were available (Kornbrust et al., 1982). In TK/6 human lymphoblasts, methyl chloride induced a statistically significant concentration-related induction of sister chromatid exchange (SCE) frequency as well as significantly declined mitotic index and a significant concentration-related increase in seconddivision metaphases (Fostel et al., 1985). No toxicity data from long-term exposure to methyl chloride other than those obtained from administration via the respiratory pathway were located in the literature. 8.6.2 8.6 In vivo, methyl chloride did not cause induction of UDS in rat spermatocytes, hepatocytes, or tracheal epithelial cells at exposure concentrations of 6192– 7224 mg/m3 (3000–3500 ppm) for 6 h/day for 5 days. However, exposure to 30 960 mg/m3 (15 000 ppm) for 3 h did cause a marginal increase in UDS in hepatocytes (Working et al., 1986). The doses used were considered below, but close to, the maximum tolerated dose. For an overview of, and details on, the genotoxicity studies, see Table 4. In all studies referred to below, methyl chloride was administered by inhalation, unless otherwise noted. 8.6.1 Studies in vivo Genotoxicity and related end-points Studies in vitro Using the Ames assay, methyl chloride was shown to induce gene mutations in Salmonella typhimurium TA100 (Simmon et al., 1977) and in S. typhimurium TA1535 (Andrews et al., 1976) in both the presence and absence of metabolic activation. Further, a concentration-related increase in the 8-azaguanine-resistant fraction in S. typhimurium was observed (Fostel et al., 1985). In a macromolecular binding study, male rats were exposed to 14C-labelled methyl chloride (specific activity 25–70 dpm/nmol = 11.2–31.4 × 10–3 mCi/mmol), and accumulation of 14C was measured in lipid, RNA, DNA, and protein from isolated liver, kidneys, lungs, and testes (Kornbrust et al., 1982). Radiolabelled carbon was found in all tissues and fractions studied; however, methylation was not found. Pretreatment with the protein synthesis inhibitor cycloheximide or the folic acid antagonist methotrexate, which interferes with singlecarbon metabolism, to a large extent inhibited most of the 14 C-incorporation in proteins and macromolecules, respectively. Further, the extent of incorporation of methyl chloride into proteins and lipids was consistent with the rates of turnovers in these macromolecules. Therefore, the most likely mechanism for uptake of methyl chloride into macromolecules is via the onecarbon pool. However, this does not exclude the possibility that methyl chloride might bind directly to macromolecules to a lesser extent. Methyl chloride induced the adaptive response to alkylation damage in Escherichia coli regulated by the ada protein, which suggests that methyl chloride is a direct DNA-alkylating agent (Vaughan et al., 1993). Methyl chloride caused gene mutations in vitro in TK6 human lymphoblasts, as shown by a dose-related increase in the mutant fraction (Fostel et al., 1985). In the study by Fostel and co-workers (1985), no increase in DNA strand breaks as measured by alkaline elution was observed. However, the outcome from the positive control (methyl methane sulfonate [MMS]) was questionable, as unexpectedly high doses were needed for a positive result. Thus, the mutagenic lesions produced by methyl chloride might be either different from those produced by MMS or formed at a level below the threshold of detection of alkaline solution. In a DNA binding assay, in which Peter et al. (1985) exposed rats and mice to 14C-labelled methyl chloride, no methylation of guanine in DNA at N7 and/or O6 in liver and kidneys by methyl chloride was found. The specific activity in this study (13 mCi/mmol = 2.9 × 104 dpm/nmol) was about 3 orders of magnitude higher than that used by Kornbrust et al. (1982). DNA damage following methyl chloride exposure was shown as a statistically significant enhanced transformation of Syrian hamster embryo cells by SA7 adenovirus (Hatch et al., 1983). 18 Methyl chloride Table 4: Genotoxicity of methyl chloride and related end-points. Table 4 (contd) Species Protocol Result Reference Gene mutation in vitro; bacteria S. typhimurium TA100 Ames test 2.5–20%; 8 h; ± S9 mix positive Simmon et al., 1977 S. typhimurium TA1535 Ames test 0.5–20.7%; ± S9 mix positive Andrews et al., 1976 S. typhimurium TM677 (8-azaguanine resistance) bacterial forward mutation assay 0, 5, 10, 20, or 30%; 3 h; strain deficient in metabolizing xenobiotics; no metabolizing system was added positive adaptive response to alkylation damage (ada gene) positive Vaughan et al., 1993 positive Fostel et al., 1985 Fostel et al., 1985 DNA damage in vitro; bacteria E. coli B F26 Gene mutation in vitro; mammalian cells human lymphoblasts TK6 (trifluorothymidine resistance) gene mutation 0, 1, 2, 3, 4, or 5%; 3 h positive controls: ethyl methane sulfonate (EMS) or methyl methane sulfonate (MMS) DNA damage in vitro; mammalian cells human lymphoblasts TK6 alkaline elution 0, 1, 3, or 5% positive controls: EMS or MMS negative Fostel et al., 1985 Syrian hamster embryo cells (primary SHE cells) DNA damage and repair assay (DNA adenovirus SA7 transformation) 0, 6000, 12 000, 27 000, 52 000, or 103 000 mg/m 3 (0, 3000, 6000, 13 000, 25 000, or 50 000 ppm); 2–20 h positive Hatch et al., 1983 rat, F-344 hepatocytes spermatocytes unscheduled DNA synthesis 1–10% positive Working et al., 1986 bovine serum albumin protein binding assay positive Kornbrust et al., 1982 positive Fostel et al., 1985 Working et al., 1986 Chromosomal effects in vitro; mammalian cells human lymphoblasts TK6 sister chromatid exchange assay 0, 0.3, 1.0, or 3.0% positive control: EMS DNA damage in vivo; mammals rat, F-344 hepatocytes spermatocytes tracheal epithelial cells unscheduled DNA synthesis 6192–7224 mg/m 3 (3000–3500 ppm), 5 days, 6 h/day negative unscheduled DNA synthesis 30 960 mg/m 3 (15 000 ppm); 3 h weakly positive rat, F-344, males 3 or 6 animals per group (no further information on groups was available) DNA binding study 1032, 3096 mg/m 3 (500, 1500 ppm); 6 h + 24 h postexposure specific radioactivity: 25–70 dpm/nmol = 11.2–31.4 × 10 –3 mCi/mmol liver, kidney, lung, testes negative Kornbrust et al., 1982 mouse, B6F3C1, males and females 6 animals per group DNA–protein cross-links 0 or 2064 mg/m 3 (0 or 1000 ppm); 8 h indications (male mice) Ristau et al., 1989 19 Concise International Chemical Assessment Document 28 Table 4 (contd) Species Protocol Result Reference rat, F-344, males and females 5 animals per group DNA binding study exposure for 4 h of rats in a closed chamber; initial concentration approximately 2064 mg/m 3 (1000 ppm) (reading off from graphs) specific radioactivity: 13 mCi/mmol = 2.9 × 10 4 dpm/nmol negative Peter et al., 1985 mouse, B6C3F1, males and females 25 animals per group DNA binding study exposure for 4 h of rats in a closed chamber; initial concentration approximately 2064 mg/m 3 (1000 ppm) (reading off from graphs) specific radioactivity: 13 mCi/mmol = 2.9 x 10 4 dpm/nmol ngative Peter et al., 1985 rat, F-344, males and females 5 animals per group DNA–protein cross-links 2064 mg/m 3 (1000 ppm) approximately 6 h/day for 6 days indication Jäger et al., 1988 mouse, B6F3C1, males and females 5 animals per group 2064 mg/m 3 (1000 ppm) approximately 6 h/day for 6 days animals sacrificed 6 h postexposure Chromosomal effects in vivo; mammals rat, Fischer 344, males 80 animals per group dominant lethal test 6 h/day for 5 days + 17 exposure-free weeks 0, 2064, 6192 mg/m 3 (0, 1000, 3000 ppm) + positive control triethylenemelamine (TEM) Using the alkaline elution technique, no cross-links in male mouse kidneys could be detected after exposure to 2064 mg methyl chloride/m3 (1000 ppm) for 6 days, but some indications of DNA single-strand breaks were obtained (Jäger et al., 1988). However, when mice were exposed to 2064 mg/m3 (1000 ppm) for 8 h only, DNA cross-links were seen in renal tissue of male mice but not in female mice or in hepatic tissues (Ristau et al., 1989). In an attempt to investigate the time-course of the DNA lesions, Ristau et al. (1990) again exposed male mice to 2064 mg methyl chloride/m3 (1000 ppm) for 8 h. In renal tissue, it was observed that DNA–protein cross-links were removed at a fast rate, whereas single-strand breaks appeared to accumulate. At 48 h postexposure, all lesions had disappeared. negative (probably a cytotoxic effect) Working et al., 1985a origin. However, a genotoxic effect should not be totally excluded. The role of epididymal inflammation in the induction of lethal mutations was studied by Chellman et al. (1986c) in an assay with a test protocol similar to the OECD test guideline for dominant lethal mutations. Rats were exposed to methyl chloride in the presence or absence of the anti-inflammatory agent BW755C. BW755C was effective against the postimplantation losses induced by methyl chloride, but not against preimplantation losses. The authors’ conclusions, based on unpublished data mentioned in Chellman et al. (1986c), are that the increase in preimplantation losses might be a consequence of testicular lesions caused by methyl chloride and that BW755C is effective against epididymal injuries only, thus indicating that epididymal inflammation has a role in the induced infertility. In a dominant lethal assay, performed according to Organisation for Economic Co-operation and Development (OECD) test guidelines, male rats were exposed to methyl chloride (Working et al., 1985a). The numbers of live and total implants were decreased, there was an increase in the percentage of preimplantation loss at weeks 2, 4, 6, and 8 postexposure, and there was an increase in the percentage of postimplantation loss at week 1 postexposure. The changes observed were not concentration related. A true dominant lethal effect of genetic origin could be questioned, as the time-courses of the pre- and postimplantation losses after methyl chloride exposure were not the same as those obtained after administration of the positive control, triethylenemelamine (TEM). The development of sperm granulomas in the epididymis, the effects seen in the dominant lethal assay, seems to be cytotoxic rather than genotoxic in In conclusion, methyl chloride is clearly genotoxic in in vitro systems, in both bacteria and mammalian cells. Methyl chloride binds to protein. Methyl chloride is possibly an alkylating agent; however, the available studies do not allow any quantification. Although the positive effects seen in a dominant lethal test were most likely cytotoxic rather than genotoxic, methyl chloride might be considered a very weak mutagen in vivo based on some evidence of DNA–protein cross-linking at higher doses. 20 Methyl chloride 8.7 Reproductive and developmental toxicity 8.7.1 Effects on fertility Chapin et al. (1984) investigated the development of lesions induced in testes and epididymis and effects on reproductive hormones in F-344 rats after exposure to 0 or 6192 mg methyl chloride/m3 (0 or 3000 ppm) for a total of 9 days (6 h/day; approximately 60 exposed animals and 16 control animals). Testicular lesions in the form of delay in spermiation, germinal epithelial vacuolization, and cellular exfoliation as well as bilateral epididymal granulomas were seen. The effects were observed in most animals, with the onset at day 9 or 11 after the beginning of the exposure. In general, lesions seen in In the 1981 CIIT study, referred to in section 8.5, exposure to methyl chloride at 2064 mg/m3 (1000 ppm) caused testicular lesions in rats. The lesions seen were bilateral and consisted of diffuse degeneration and atrophy of the seminiferous tubules. Table 5: Breeding results in rats in the F0 and F2 generations after methyl chloride exposure. Breeding results 0 ppm 150 ppm 475 ppm 1500 ppm F0 generation: number of exposed males proven fertile when mated to exposed females 18/40 (45%) 20/39 (51%) 12/40 (30%) 0/40 (0%) F0 generation: number of exposed males proven fertile when mated to unexposed females 23/28 (82%) 21/28 (75%) 12/28 (43%) 0/26 (0%) F1 generation: number of exposed males proven fertile when mated to exposed females 31/40 (78%) 26/40 (65%) 14/23 (61%) – animals at day 19 were of higher severity than those seen earlier. In rats killed 70 days or more after the onset of the exposure, 70–90% of the seminiferous tubules lacked any germinal cells; in 10–30% of the tubules, varying degrees of recovery of spermiation were observed. The LOAEL in this study must be set at 6192 mg/m3 (3000 ppm). size, sex ratio, pup viability, or pup growth were found among the 980 mg/m3 (475 ppm) and 310 mg/m3 (150 ppm) groups compared with the control F0 group. A trend towards decreased fertility was also found in the 980 mg/m3 (475 ppm) dose group in the F1 generation. A LOAEL of 980 mg/m3 (475 ppm) (infertility) was derived from the two-generation study. Breeding results in rats in the F0 and F2 generations after methyl chloride exposure are shown in Table 5. In a dominant lethal assay in rats exposed to methyl chloride for 5 days, described in section 8.6, visible sperm granulomas in the epididymis were present in the 6192 mg/m3 (3000 ppm) group 17 weeks postexposure but not in the 2064 mg/m3 (1000 ppm) group or in the control group. After exposure to 6192 mg/m3 (3000 ppm), the number of live and total implants was decreased, and there was an increase in postimplantation loss. In both treated groups, there was an increase in preimplantation losses (Working et al., 1985a). The LOAEL for preimplantation loss was 2064 mg/m3 (1000 ppm). A two-generation inhalation study in Fischer 344 rats was carried out at methyl chloride concentrations of 0, 310, 980, or 3096 mg/m3 (0, 150, 475, or 1500 ppm) (Hamm et al., 1985). The F0 generation (40 males and 80 females per exposure group) was exposed for 10 weeks and during a 2-week mating period (6 h/day, 5 days/week, and 6 h/day, 7 days/week, respectively). A similar exposure schedule was used for the F1 generation, with the exclusion of the 3096 mg/m3 (1500 ppm) exposure level. In the high-dose F0-generation males sacrificed immediately after 12 weeks of exposure, treatment-related lesions were found, consisting of minimal to severe atrophy of the seminiferous tubules (10/10 males examined) and granulomas in the epididymis (3/10). Severely affected tubules were lined by Sertoli’s cells and by occasional stem cell spermatogonia. In the less affected tubules, decreased numbers of spermatogonia, primary spermatocytes, and/or secondary spermatocytes were found. In a subsequent study, Working et al. (1985b) characterized the effect of methyl chloride exposure on sperm quality and histopathology in rats in more detail. Male Fischer 344 rats (80 animals per group) were exposed to 0, 2064, or 6192 mg methyl chloride/m3 (0, 1000, or 3000 ppm) for 5 days, 6 h/day. Besides significantly decreased testis weights in the high-dose group 3–8 weeks postexposure and the findings that more than 50% of the treated animals showed sperm granulomas in the epididymis in the same dose group, observations indicating cytotoxic effects on sperm quality were made. Further, in the F0 generation, no litters were born when high-dose males were mated to exposed or unexposed females, and significantly fewer litters were born to unexposed females mated to the males in the 980 mg/m3 (475 ppm) dose group. No differences in litter 21 Concise International Chemical Assessment Document 28 Observations made at the 6192 mg/m3 (3000 ppm) level included significant decreases in testicular spermatid head counts, delay in spermiation, epithelial vacuolization, luminal exfoliation of spermatogenic cells, and multinucleated giant cells. Further, sperm isolated from the vasa deferentia had significantly depressed numbers and an elevated frequency of abnormal sperm head morphology by week 1 postexposure and significantly depressed sperm motility and increased frequency of headless tails by week 3 postexposure. These changes were all within or close to the normal range by week 16 postexposure. A LOAEL of 6192 mg/m3 (3000 ppm) could be derived based on the histopathological findings. In parallel with the rat study, Wolkowski-Tyl et al. (1983a) also evaluated teratogenicity in pregnant C57BL/6 mice (33 mice per dose group) carrying B6C3F1 fetuses exposed through gestation days 6–17 following the same exposure schedule as the rats. Dams in the 3096 mg/m3 (1500 ppm) group died or were killed in extremis due to very high toxicity (tremor, hunched appearance, difficulty in righting, vaginal bleeding, bloody urine, cerebellar granular cell necrosis and degeneration, etc.). No other maternal toxicity was observed in the other exposure groups. In the 1032 mg/m3 (500 ppm) group, the fetuses (male and female) had a small but significant increase in heart defects (reduction or absence of the atrioventricular valves, chordae tendineae, and papillary muscles). In both the 1032 and 206 mg/m3 (500 and 100 ppm) groups, a significant increase in degree of ossification in the hindlimbs was seen as compared with control animals. A LOAEL for heart defects in fetuses of 1032 mg/m3 (500 ppm) was obtained. The cause of preimplantation loss induced by methyl chloride was further investigated in rats by Working & Bus (1986). Fischer 344 rats (10–30 animals per group) inhaled 0, 2064, or 6192 mg methyl chloride/m3 (0, 1000, or 3000 ppm) 6 h/day for 5 days or received a single injection of TEM as a positive control for genotoxicity. At weeks 1–3 postexposure in the 6192 mg/m3 (3000 ppm) group, preimplantation losses did not exceed unfertilized ova, which was the case for the positive control. From these data, the authors suggested that preimplantation losses are due to a failure in fertilization rather than to an increase in embryonal deaths. In a subsequent study, Wolkowski-Tyl et al. (1983b) again exposed pregnant C57BL/6 mice carrying B6C3F1 fetuses with the aim of confirming the earlier findings, elucidating the nature of the heart defects more clearly, and establishing a concentration–effect relationship. Approximately 75 mice per dose group were exposed to 0, 516, 1032, or 1548 mg methyl chloride/m3 (0, 250, 500, or 750 ppm) for 6 h/day during gestation days 6–18. In the 1032 and 1548 mg/m3 (500 and 750 ppm) groups, an exposure-related increase in heart defects (involving effects on the atrioventricular valves, chordae tendineae, and papillary muscles) was observed. Dams were affected at the 1548 mg/m3 (750 ppm) exposure level (decrease in body weight and body weight gain). No maternal toxicity, embryotoxicity, fetotoxicity, or teratogenicity was associated with exposure to methyl chloride at 516 mg/m3 (250 ppm). In this study, the LOAEL for heart defects was 1032 mg/m3 (500 ppm), the NOAEL was 516 mg/m3 (250 ppm), and the maternal LOAEL was 1548 mg/m3 (750 ppm). In conclusion, testicular lesions and epididymal granulomas followed by reduced sperm quality lead to reduced fertility as well as complete infertility in rats. A LOAEL of 980 mg/m3 (475 ppm) and a no-observedadverse-effect level (NOAEL) of 310 mg/m3 (150 ppm) were identified from the two-generation study of Hamm et al. (1985). 8.7.2 Developmental toxicity In a study designed to study structural teratogenicity, pregnant Fischer 344 rats (25 rats per dose group) were exposed to 0, 206, 1032, or 3096 mg methyl chloride/ m3 (0, 100, 500, or 1500 ppm) for 6 h/day through gestation days 7–19 (Wolkowski-Tyl et al., 1983a). In the highest dose group, significant reductions in fetal body weight and female crown–rump length were observed. Further, skeletal immaturities such as reduced ossification (metatarsals and phalanges of the anterior limbs, thoracic vertebral centra, pubis of pelvic girdle, and metatarsals of the hindlimbs) were seen. Although these findings were seen in the presence of significantly decreased maternal food consumption, body weight, and weight gain in the same dose group, they should be considered as serious and exposure related. A fetal LOAEL for skeletal immaturities as well as a maternal LOAEL for effects on body weight and food consumption of 3096 mg/m3 (1500 ppm) were obtained. No other effects, including heart defects, were reported from the 206 and 1032 mg/m3 (100 and 500 ppm) dose groups. In a number of different experiments on small numbers of animals, pregnant C57BL/6 mice carrying B6C3F1 fetuses were exposed to methyl chloride at concentrations of 516, 619, or 2064 mg/m3 (250, 300, or 1000 ppm) for 12–24 h during gestation day 11.5–12.5 (John-Greene et al., 1985). The exposure time was chosen as a critical period in development of cardiac defects. The authors found heart defects when the test was nonblind but not when the technician was unaware of which fetuses were exposed. Further, John-Greene et al. (1985) had concerns regarding the technique used by Wolkowski-Tyl et al. (1983a). However, the investigation by John-Greene et al. (1985) is difficult to evaluate, as a small number of animals were used and as the exposure period was not similar to those in the Wolkowski-Tyl et al. (1983a, 1983b) studies. No LOAEL could be established. 22 Methyl chloride disturbances, mental confusion, and paraesthesis. Neurotic and depressive symptoms are also described. Further, gastrointestinal symptoms (nausea, vomiting, abdominal pain, etc.) have been observed, as well as jaundice. In general, the symptoms seem to develop soon after the exposure. However, recovery periods vary to a large extent; for example, in seamen highly exposed to methyl chloride, effects on the nervous system were observed 13 years after the accident (Gudmundsson, 1977). In conclusion, from the studies by Wolkowski-Tyl and co-workers (1983a, 1983b), it seems that methyl chloride could induce heart defects in mice exposed to 1032 mg/m3 (500 ppm) when dams were exposed through gestation days 6–18. A NOAEL of 206 mg/m3 (100 ppm) was established from the developmental toxicity studies. 8.8 Immunological and neurological effects 9.3 No specific reports on immunological or neurological effects caused by methyl chloride were found in the literature. Performance and cognitive functions were adversely affected in workers manufacturing foam products. There was also an increase in the magnitude of finger tremors. The workers were exposed for 2 years to approximately 72 mg methyl chloride/m3 (35 ppm), as well as other chemicals (NIOSH, 1976). However, insufficient information was available on exposure to the other chemicals and lifestyle factors, and no relationship could be established between methyl chloride exposure and the various psychological and personality tests employed. 9. EFFECTS ON HUMANS 9.1 Studies in volunteers In order to monitor the physiological response to methyl chloride in healthy volunteers (eight men and nine women) with no previous methyl chloride exposure, Stewart et al. (1980) exposed the volunteers to methyl chloride at concentrations of 0, 41, 206, or (men only) 310 mg/m3 (0, 20, 100, or [men only] 150 ppm), 1, 3, and 7.5 h/day, 5 days/week, for 6 weeks in an exposure chamber. Using a wide battery of behavioural, neurological, electromyographic, and clinical tests, no significant decrements were found in the exposed volunteers as compared with controls. For interindividual differences in methyl chloride concentrations in blood and expired air, see section 7. A mortality follow-up study was conducted of 852 male workers employed for at least 1 month between the years 1943 and 1978 in a butyl rubber manufacturing plant using methyl chloride (Holmes et al., 1986). For each cohort member, complete work history and death information were obtained. No information on lifestyle factors was reported. The exposure to methyl chloride and other compounds used in the butyl rubber manufacturing plant was estimated in three categories (high, medium, and low). No detectable excess mortality from any specific cause of death including all cancers was found in the study population after analysis by level and duration of exposure. In volunteers, Putyz-Anderson and co-workers (1981a, 1981b) found minimal or no effects on performance after exposure to methyl chloride at 206 or 413 mg/m3 (100 or 200 ppm) for 3h (n=56) and at 413 mg/m3 (200 ppm) for 3.5 h (n=84), respectively 9.2 Epidemiological studies In a 32-year follow-up study by Rafnsson & Gudmundsson (1997), indications of elevated mortality from cardiovascular disease after high accidental methyl chloride exposure were seen in Icelandic seamen (deckhands: relative risk [RR] = 3.9, 95% confidence interval [CI] = 1.0–14.4; officers: RR = 1.7, 95% CI = 0.3–6.4). The small number of observed cancers (all cancers and lung cancers) in the exposed group provides an insufficient basis for assessing the cancer risk in humans. The reference group used was controlled for age, occupation, social class, and lifestyle factors. Case reports Available information related to the toxic effects on humans exposed to high concentrations of methyl chloride is mainly derived from accidental exposures in connection with the use of methyl chloride in the production of polystyrene foams and also from refrigerator leakages. Among symptoms described in case reports (see, for example, MacDonald, 1946; McNally, 1946; Hansen et al., 1953; Thordarson et al., 1964; Scharnweber et al., 1974; Spevak et al., 1976; Gudmundsson, 1977; Lanham, 1982) are effects on the nervous system, such as dizziness, weakness, blurred vision, muscular incoordination, drowsiness, sleep In conclusion, effects on humans, especially on the central nervous system, can clearly be seen after accidental (mostly high) exposure or after normal work exposure levels. A rough estimation of the degree of exposure from case reports might be in the order of approximately 200–2000 mg/m3 (100–1000 ppm). In shortterm exposure of volunteers, no significant effects were 23 Concise International Chemical Assessment Document 28 Table 6: Short-term toxicity to aquatic organisms. Organism End-point Toxicity (mg/litre) Reference Cyanobacteria Microcystis aeruginosa Toxicity threshold, EC3 (cell multiplication inhibition test) 550 Bringmann & Kühn, 1976 Toxicity threshold, EC3 (cell multiplication inhibition test) 1450 Bringmann & Kühn, 1980 Toxicity threshold, EC5 (cell multiplication inhibition test) >8000 Bringmann & Kühn, 1980 Green algae Scenedesmus quadricauda Protozoa Entosiphon sulcatum Fish Bluegill sunfish (Lepomis macrochirus) 96-h LC50 550 Dawson et al., 1977 Tidewater silverside (Menidia beryllina) 96-h LC50 270 Dawson et al., 1977 Table 7: Short-term toxicity to terrestrial organisms. Organism End-point Toxicity Reference Bacteria Methanogenic bacteria (35 °C, pH 7, anaerobic conditions) 48-h IC50 (inhibition of gas production) 50 mg/litre Blum & Speece, 1991 Nitrobacter (25 °C, pH 9.1) 24-h IC50 (inhibition of NO 2-N production) 2010 mg/litre Tang et al., 1992 Pseudomonas putida Toxicity threshold, EC3 (cell multiplication inhibition test) 500 mg/litre Bringmann & Kühn, 1976 Higher plants Several speciesa (3-h exposure in gas phase) a 5000–10 000 mg/m 3 (2400–4800 ppm) >5000 mg/m 3 (>2400 ppm) >5000 mg/m 3 (>2400 ppm) Visible symptoms Photosynthesis Transpiration Christ, 1996 Tested plant species were tomatoes (Lycopersicum esculentum Miller), sunflower (Helianthus annuus L.), bush bean (Phaseolus vulgaris L.), nasturtium (Tropaeolum majus L.), sugar-beet (Beta vulgaris L.), soya bean (Glycine maxima (L.) Merill), and wheat (Triticum aestivum L.). seen. There are insufficient data available to assess the risk for humans to develop cancer as a result of methyl chloride exposure. (see Table 6). The acute toxicity to the two fish species was determined under static conditions, and the concentration of the test substance was not measured. This means that the toxicity may have been underestimated by the test, if significant amounts of the test substance volatilized during the test. The 96-h LC50 values for the freshwater species, bluegill sunfish (Lepomis macrochirus), and the saltwater species, tidewater silverside (Menidia beryllina), were determined to be 550 and 270 mg/litre, respectively (Dawson et al., 1977). 10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD 10.1 Aquatic environment 10.2 Few data were found on the short-term toxicity of methyl chloride to aquatic organisms, and no data were found on long-term toxicity. The existing data for a cyanobacterium, a green alga, a protozoan, and two fish species indicate a low acute toxicity to aquatic species Terrestrial environment Data on the short-term toxicity of methyl chloride were found only for three species of bacteria and some higher plants (see Table 7). No chronic toxicity data were found for terrestrial organisms. 24 Methyl chloride Table 8: Summary of animal inhalation toxicity studies with relevance for risk characterization. Study duration Species End-point LOAEL, mg/m3 (ppm) NOAEL, mg/m3 (ppm) Reference Short-term exposure mouse, C3H, females; C57BL/6, males and females 12 days hepatocellular necrosis and degeneration 1032 (500) – Morgan et al., 1982 mouse, C57BL/6, females 11 days, continuous exposure cerebellar lesions 206 (100) – Landry et al., 1985 826 (400) – intermittent exposure Long-term exposure rat, F-344 2 years testicular lesions 2064 (1000) 464 (225) CIIT, 1981 mouse, B6C3F1 2 years renal tumours in males; significant increase at 2064 mg/m 3 (1000 ppm) 2064 (1000) 464 (225) CIIT, 1981 development of renal microcysts in males; also seen at 464 mg/m3 (225 ppm); no dose–response 103 (50) nerve axonal swelling and degeneration; seen in all treated groups; effects significant as compared with control 103 (50) Reproductive toxicity — fertility rat, F-344 9 days testicular lesions and epididymal granulomas 6192 (3000) – Chapin et al., 1984 rat, F-344 twogeneration infertility; dose dependence or trend 980 (475) 310 (150) Hamm et al., 1985 rat, F-344 5 days preimplantation loss 2064 (1000) – Working et al., 1985a rat, F-344 5 days effects on sperm quality 6192 (3000) 2064 (1000) Working et al., 1985b Reproductive toxicity — development rat, F-344 gestation days 7–19 skeletal immaturities in the presence of effects of maternal body weight and food consumption 3096 (1500) 1032 (500) Wolkowski-Tyl et al., 1983a mouse, B6C3F1 gestation days 6–17 heart defects in fetuses; significant increase as compared with control animals 1032 (500) 206 (100) Wolkowski-Tyl et al., 1983a mouse, B6C3F1 gestation days 6–18 heart defects in fetuses; dose dependence 1032 (500) 516 (250) Wolkowski-Tyl et al., 1983b administration. However, as the major route of human exposure to methyl chloride seems to be by the respiratory pathway, the lack of data from other routes of administration is of minor concern. It should be pointed out that there are surprisingly few recent investigations of methyl chloride toxicity for all end-points except genotoxicity. In Table 8, data from inhalation toxicity studies on methyl chloride in experimental animals are summarized. 11. EFFECTS EVALUATION 11.1 Evaluation of health effects 11.1.1 Hazard identification and dose–response assessment The database for methyl chloride risk assessment is in general acceptable in terms of toxicity after inhalation exposure. Few data could be located in the literature on methyl chloride toxicity after dermal or oral Considering the human GSTT1 polymorphism and the suggested metabolic pathways of methyl chloride (Figure 2), it is not possible to conclude whether a rapid metabolic clearance of methyl chloride as in high 25 Concise International Chemical Assessment Document 28 seen at 464 mg/m3 (225 ppm). Development of renal cortical microcysts in mice was seen in the 103 mg/m3 (50 ppm) dose group and to some extent in the 464 mg/m3 (225 ppm) group (CIIT, 1981); however, no concentration–response relationship could be established Although there are indications of low CYP2E1 activity in male human kidney microsomes as compared with male CD-1 mice, as shown by Speerschneider & Dekant (1995), the presence of human renal CYP2E1 cannot be excluded. Further, the presence of CYP2E1 in human tissues other than kidneys might lead to the induction of tumours in other organs. Therefore, the findings of renal tumours in male mice should be considered as relevant for humans. conjugators (HC) leads to a higher or lower risk than a longer retention of methyl chloride in the body as in low conjugators (LC) or non-conjugators (NC). Further, as the GSTT1 activity decreases in the order mouse liver and kidney cytosol > HC > rat > LC > hamster > NC, it is impossible to select one species of experimental animal as preferable in the risk assessment of methyl chloride when extrapolating animal effects data to humans. Because of these uncertainties, no species of experimental animal can be ruled out in favour of another, and human high conjugators, low conjugators, and non-conjugators must be considered as sensitive to methyl chloride. Consequently, the lowest appropriate LOAEL or NOAEL from any species could be chosen for further risk characterization. Methyl chloride is clearly genotoxic in in vitro systems in both bacteria and mammalian cells. Methyl chloride can bind to protein. However, if methyl chloride is an alkylating agent, it is so to a very small extent. Further, methyl chloride might be considered only a very weak mutagen in vivo. Data on single exposures are poor for methyl chloride, and no firm conclusions can be drawn. However, the acute inhalation toxicity in rats and male mice after single exposure seems to be fairly low, with an LC50 value above 4128 mg/m3 (2000 ppm). In mice, there might be a sex difference in susceptibility to methyl chloride, as the LC50 value obtained for female mice was 17 544 mg/m3 (8500 ppm). Testicular lesions and epididymal granulomas followed by reduced sperm quality lead to reduced fertility as well as complete infertility in rats. A LOAEL of 980 mg/m3 (475 ppm) could be derived from the reproductive toxicity data where a concentration–response could be established (NOAEL = 310 mg/m3 [150 ppm]). No data on irritation and sensitization were available. However, from short- and long-term exposure data, no indications of respiratory irritation caused by methyl chloride exposure have been reported. Thus, methyl chloride is probably not a strong respiratory irritant. It seems from the Wolkowski-Tyl et al. (1983a, 1983b) studies that methyl chloride could induce heart defects in mice exposed to 1032 mg/m3 (500 ppm) when dams are exposed through gestation days 6–18. The development of heart defects was concentration dependent. A NOAEL of 206 mg/m3 (100 ppm) could be estimated from the developmental toxicity studies. The principal target in both rats and mice upon short-term exposure is the nervous system, with animals exhibiting functional disturbances and cerebellar degeneration. The LOAEL (based on cerebellar degeneration) in mice is 206 mg/m3 (100 ppm) upon continuous exposure. Higher levels of exposure caused toxicity in the kidney and liver in mice and in the testes, epididymis, and kidney in rats. The decrease in thymus weight, unaccompanied by histopathological changes, in mice exposed to 31 mg/m3 (15 ppm) was not corroborated by either a 90-day or a 2-year study. Effects on humans, especially on the central nervous system, can clearly be seen after accidental or normal work exposure levels. A rough estimation of the degree of exposure from case reports might be in the order of approximately 200–2000 mg/m3 (100–1000 ppm). In short-term exposures of volunteers, no significant effects on the nervous system were seen. There are insufficient data available to assess the risk for humans to develop cancer as a result of methyl chloride exposure. In the long-term studies, exposure to 103 mg/m3 (50 ppm) caused nerve lesions, such as axonal swelling and degeneration. The effects at each exposure level were significantly increased in each dose group as compared with control animals. However, no concentration–response relationship could be established. Significant degenerative effects on nerve fibres were also seen at higher doses, although the observations were not concentration related. Further, testicular lesions in rats and renal lesions in male mice were important findings obtained from the 2-year toxicity study. Renal tumours as well as testicular lesions were seen at the 2064 mg/m3 (1000 ppm) exposure level, and, although not of statistical significance, cortical adenoma was also 11.1.2 Criteria for setting tolerable intakes or guidance values for methyl chloride Human data supplemented by data from short-, medium-, and long-term studies in laboratory animals, principally the mouse, clearly indicate that the nervous system is a particularly vulnerable target of methyl chloride exposure. Histopathological effects in spinal cord nerves were observed in a 2-year exposure of mice (CIIT, 1981) at a LOAEL of 103 mg/m3 (50 ppm), in the 26 Methyl chloride absence of renal and hepatic effects. At 2064 mg/m3 (1000 ppm), cerebellar degeneration was noted. This latter lesion, which appears to be of a progressive and persistent nature, has also been observed in mice exposed in other studies (e.g., Landry et al., 1985) for much shorter periods of time. In addition, evidence of functional impairment noted in short-term studies is consistent with the spinal cord and the brain being potential sites of injury under chronic exposure conditions. 11.1.3 Sample risk characterization Based on the sample estimate of exposure, indirect exposure via the ambient air, 0.0012 mg/m3 (0.6 ppb), is 15 times below the guidance value of 0.018 mg/m3 (0.009 ppm). Also, the sample median exposure estimate for urban air, 0.0010–0.0023 mg/m3 (0.5–1.1 ppb), is 18 to 8 times below the guidance value. Finally, the maximum value obtained from individual measurements in urban air, 0.035 mg/m3 (17 ppb), is 2 times above the guidance value. From the available exposure data, a risk was identified from exposure in urban air but not ambient air. Consequently, the LOAEL of 103 mg/m3 (50 ppm), derived from the 2-year study by CIIT (1981) for effects on the nervous system, was chosen to be used in the risk characterization. As no estimation from the available exposure database can be made on the allocation of the tolerable intake from environmental air and from air in the working environment, two separate guidance values were derived. The quality of human exposure data in the present report is fairly poor, which will contribute to uncertainties in the outcome of the sample risk characterization for occupational exposure. However, when more applicable exposure data can be used, the quality of the risk assessment will improve. The range of the sample estimate of workplace exposure, 0.2–186 mg/m3 (0.1–90 ppm), is 5 times below and 180 times above the guidance value of 1.0 mg/m3 (0.5 ppm), respectively. Thus, a comparison of the available methyl chloride exposure concentrations in the working environment and the guidance value derived from effects on the nervous system leads to the identification of a risk. Although the nerve lesions were seen at lower exposure levels than those at which infertility in rats (980 mg/m3 [475 ppm]) and renal tumours in male mice (2064 mg/m3 [1000 ppm]) occurred, emphasis should also be laid on these very serious effects in a qualitative risk characterization of methyl chloride. A guidance value for indirect inhalation exposure to methyl chloride via the environment for the general population was estimated to be: (103 mg/m3 × 1.0 × 6/24 × 5/7) / 1000 = 0.018 mg/m3 (0.009 ppm) where: • 103 mg/m3 (50 ppm) is the LOAEL, • 1.0 refers to 100% allocation of the tolerable intake to intake via the environmental air, • 6/24 and 5/7 are the conversion of 6 h/day and 5 days/week to continuous exposure, and • 1000 is the uncertainty factor (×10 for intraspecies variation, ×10 for interspecies variation, and ×10 for the poor database and the use of a LOAEL instead of a NOAEL). 11.2 Evaluation of environmental effects The troposphere, where methyl chloride reacts with hydroxyl radicals, is the main environmental sink for the chemical. A certain amount of the tropospheric methyl chloride reaches the stratosphere. In the stratosphere, photolysis produces chlorine radicals, which in turn will react with ozone. Estimates of the amount of methyl chloride reaching the stratosphere, and thereby also its contribution to ozone depletion, vary considerably. However, as estimated from figures presented by the WMO, methyl chloride contributes approximately 15% of the total equivalent effective stratospheric chlorine.1 The relative contribution from methyl chloride to the depletion of the ozone layer will probably increase A guidance value for occupational inhalation exposure was estimated to be: (103 mg/m3 × 1.0) / 100 = 1.0 mg/m3 (0.5 ppm) where: • 103 mg/m3 (50 ppm) is the LOAEL, • 1.0 refers to 100% allocation of the tolerable intake to intake via workplace air, and • 100 is the uncertainty factor (×10 for interspecies variation and ×10 for the poor database and the use of a LOAEL instead of a NOAEL). 1 The term “equivalent effective stratospheric chlorine” includes both stratospheric chlorine and bromine and also considers the dissociation rate of each compound involved in ozone depletion (e.g., chlorofluorocarbons). In the stratosphere, each bromine atom is assumed to be 40 times more damaging to ozone than each chlorine atom. No correction was made for continuous exposure in the working environment. 27 Concise International Chemical Assessment Document 28 in the future, as the use of chlorofluorocarbons and hydrochlorofluorocarbons is expected to decrease. The stratospheric ODP of methyl chloride has been determined to be 0.02 relative to that of CFC-11 (ODP = 1). Methyl chloride is not thought to contribute significantly to global warming or to the creation of photochemical air pollution. The dominant loss mechanism for methyl chloride in water and soil is volatilization. Slow hydrolysis and possibly biotic degradation may contribute at deeper soil depths and in groundwater. However, little information is available concerning biodegradation. Data on short-term toxicity to both aquatic and terrestrial organisms are sparse. No information was found on long-term toxicity. The available data show that methyl chloride has a low acute toxicity to tested aquatic organisms (e.g., protozoa, green algae, and fish). The lowest LC50 value for fish is 270 mg/litre. As concentrations of methyl chloride in surface waters (maximum 0.22 mg/litre) are generally several orders of magnitude less than those demonstrated to cause effects, it is likely that methyl chloride poses a low risk of acute effects on aquatic organisms. Data on terrestrial organisms are even more sparse than data on aquatic organisms. The only information found shows that methyl chloride causes acute effects (i.e., visible symptoms and effects on photosynthesis and transpiration) on higher plants at concentrations above 5000 mg/m3 (2400 ppm), which is about 105 times higher than the highest concentration found in urban air (i.e., 0.035 mg/m3 [0.017 ppm]). 12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES In its series of monographs, the International Agency for Research on Cancer (IARC, 1986) has evaluated methyl chloride. Based on the data available, the Task Group concluded that there is inadequate evidence for the carcinogenicity of methyl chloride to experimental animals and to humans. In the overall assessment of data from short-term tests, the Task Group concluded that there is sufficient evidence for genotoxic activity. In the overall evaluation (IARC, 1987), methyl chloride was placed in group 3 as being not classifiable as to its carcinogenicity to humans. 28 Methyl chloride Bartnicki EW, Castro CE (1994) Biohalogenation: rapid oxidative metabolism of mono- and polyhalomethanes by Methylosinus trichosporium OB-3b. Environmental toxicology and chemistry, 13(2):241–245. REFERENCES Abrams EF, Derkics D, Fong CV, Guinan DK, Slimak KM (1975) Identification of organic compounds in effluents from industrial sources. Washington, DC, US Environmental Protection Agency (NTIS Accession No. PB-241 641/0; EPA-560/3-75-002) [cited in ATSDR, 1990; HSDB, 1996]. Blum JW, Speece RE (1991) A database of chemical toxicity to environmental bacteria and its use in interspecies comparisons and correlations. Research journal of the Water Pollution Control Federation, 63(3):198–207. Almasi E, Kirshen N, Kern H (1993) The determination of sub part-per-billion levels of volatile organic compounds in air by pre-concentration from small sample volumes. International journal of analytical chemistry, 52:39–48. Borchers R, Gunawardena R, Rasmussen RA (1994) Long term trend of selected halogenated hydrocarbons. Pasadena, CA, National Aeronautics and Space Administration, pp. 259–262 (NASA Conference Publication 3266; NTIS/N95-10652/2). Andersen ME, Gargas ML, Jones RA, Jenkins LJ Jr (1980) Determination of the kinetic constants for metabolism of inhaled toxicants in vivo using gas uptake measurements. Toxicology and applied pharmacology, 54:100–116. Braus-Stromeyer SA, Cook AM, Leisinger T (1993) Biotransformation of methyl chloride to methanethiol. Environmental science and technology, 27:1577–1579. Bringmann G, Kühn R (1976) Vergleichende Befunde der Schadwirkung wassergefährender Stoffe gegen Bakterien (Pseudomonas putida) und Blaualgen (Microcystis aeruginosa). Gas- und Wasserfach; Wasser/Abwasser, 117(9):410–413 [cited in BUA, 1986; HSDB, 1996]. Andreae MO (1991) Biomass burning: Its history, use and distribution and its impact on environmental quality and global climate. In: Levine JS, ed. Global biomass burning — atmospheric, climatic and biospheric implications. Cambridge, MA, MIT Press, pp. 3–21. Bringmann G, Kühn R (1980) Comparison of the toxicity thresholds of water pollutants to bacteria, algae and protozoa in cell multiplication inhibition test. Water research, 14:231–241 [cited in BUA, 1986; HSDB, 1996]. Andreae MO (1993) The influence of tropical biomass burning on climate and atmospheric chemistry. In: Orleand RS, ed. Biogeochemistry of global change — radiatively active trace gases. New York, NY, Chapman & Hall, pp. 113–150 [cited in WMO, 1994]. Brodzinsky R, Singh HB (1983) Volatile organic chemicals in the atmosphere — an assessment of available data. Research Triangle Park, NC, US Environmental Protection Agency, Environmental Sciences Research Laboratory, 207 pp. (NTIS/PB83-195503) [cited in HSDB, 1996]. Andreae MO, Fishmann J, Garstang M, Goldammer JG, Justice CO, Levine JS, Scholes RJ, Stocks BJ, Thomson AM, van Wilgen B, STARE/TRACE-A/SAFARI-92 Science Team (1994) Biomass burning in the global environment: first results from the IGAC/BIBEX field campaign STARE/TRACE-A/SAFARI-92. In: Prinn RG, ed. Global atmospheric-biospheric chemistry. Proceedings of the 1st Scientific Conference on the International Global Atmospheric Chemistry (IGAC) Project, April 18–22, 1993, Eilat, Israel. New York, NY, Plenum Press, pp. 83–101 (Series on Environmental Science Research 48). BUA (1986) Chloromethane. GDCh-Advisory Committee on Existing Chemicals of Environmental Relevance (BUA). Weinheim, VCH Verlagsgesellschaft mbH; and New York, NY, VCH Publishers, Inc. (BUA Report 7). Burmaster DE (1982) The new pollution — groundwater contamination. Environment, 24:6–13, 33–36 [cited in ATSDR, 1990; HSDB, 1996]. Andrews AW, Zawistowski ES, Valentine CR (1976) A comparison of the mutagenic properties of vinyl chloride and methyl chloride. Mutation research, 40:273–276. Bus JS (1982) Integrated studies of methyl chloride toxicity. Chemical Industry Institute of Toxicology (CIIT) activities, 2(1):3–4. ASTM (1973) Compilation of the odor and taste threshold values data. Philadelphia, PA, American Society for Testing and Materials (ASTM Data Series DS 48). CFR (1990) Code of Federal Regulations, Title 40 Protection of Environment, Volume A, Part 136 Guidelines establishing test procedures for the analysis of pollutants. US Code of Federal Register (7/1/90) [cited in HSDB, 1996]. Atkinson R (1985) Kinetics and mechanisms of the gas-phase reactions of the hydroxyl radical with organic compounds under atmospheric conditions. Chemical reviews, 85:69–1 [cited in ATSDR, 1990; HSDB, 1996]. Atlas E, Pollock W, Greenberg J, Heidt L, Thompson AM (1993) Alkyl nitrates, nonmethane hydrocarbons and halocarbon gases over the equatorial Pacific Ocean during Saga 3. Journal of geophysical research, 98(D9):16 933–16 947. CFR (1991) Code of Federal Regulations, Title 40 Protection of Environment, Volume A, Part 136 Guidelines establishing test procedures for the analysis of pollutants. Appendix A Methods for organic chemical analysis of municipal and industrial wastewater. US Code of Federal Register (7/1/91) [cited in HSDB, 1996]. ATSDR (1990) Toxicological profile for chloromethane. Atlanta, GA, US Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry (Report No. TP-90-07). Chang H-L, Alvarez-Cohen L (1996) Biodegradation of individual and multiple chlorinated aliphatic hydrocarbons by methane-oxidising cultures. Applied and environmental microbiology, 62(9):3371–3377. ATSDR (1998) Toxicological profile for chloromethane (update). Atlanta, GA, US Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry (Report No. 205-93-0606). Chapin RE, Russell DW, Morgan KT, Bus JS (1984) Studies of lesions induced in the testis and epididymis of F-344 rats by inhaled methyl chloride. Toxicology and applied pharmacology, 76:328–343. 29 Concise International Chemical Assessment Document 28 Chellman GJ, White RD, Norton RN, Bus JS (1986a) Inhibition of the acute toxicity of methyl chloride in male B6C3F1 mice by glutathione depletion. Toxicology and applied pharmacology, 86:93–104. Dawson GW, Jennings AL, Drozdowski D, Rider E (1977) The acute toxicity of 47 industrial chemicals to fresh- and saltwater fishes. Journal of hazardous materials, 1:303–318. Dekant W, Frischmann C, Speerschneider P (1995) Sex, organ and species specific bioactivation of chloromethane by cytochrome P4502E1. Xenobiotica, 25(11):1259–1265. Chellman GJ, Morgan KT, Bus JS, Working PK (1986b) Inhibition of methyl chloride toxicity in male F-344 rats by the anti-inflammatory agent BW755C. Toxicology and applied pharmacology, 85:367–379. Derwent RG, Jenkin ME, Saunders SM (1996) Photochemical ozone creation potentials for a large number of reactive hydrocarbons under European conditions. Atmospheric environment, 30(2):181–199. Chellman GJ, Bus JS, Working PK (1986c) Role of epididymal inflammation in the induction of dominant lethal mutations in Fischer 344 rat sperm by methyl chloride. Proceedings of the National Academy of Sciences of the United States of America, 83:8087–8091. Dolfing J, van den Wijngaard AJ, Janssen DB (1993) Microbiological aspects of the removal of chlorinated hydrocarbons. Biodegradation, 4:261–282. Chemical Daily Co. Ltd. (1998) 13398 chemical products. Tokyo, The Chemical Daily Co. Ltd., p. 796. Dowdell DC, Matthews GP, Wells I (1994) An investigation into the sensitivity of the atmospheric chlorine and bromine loading using a globally averaged mass balance model. Atmospheric environment, 28(12):1989–1999. Christ RA (1996) Die Wirkung von Organischen Substanzen (Lösemitteln) in der Gasphase auf höhere Planzen. Gefharstoffe -Reinhaltung der Luft, 56:345–350. Dunn RC, Smith WW (1947) Acute and chronic toxicity of methyl chloride. Archives of pathology, 43:296–300. CIIT (1979) Final report on a 90-day inhalation toxicology study in rats and mice exposed to methyl chloride. Prepared for Chemical Industry Institute of Toxicology. Columbus, OH, Battelle Columbus Laboratories. Edgerton SA, Khalil MAK, Rasmussen RA (1984) Estimates of air pollution from backyard burning. Journal of the Air Pollution Control Association, 34:661–664 [cited in HSDB, 1996]. CIIT (1981) Final report on a chronic inhalation toxicology study in rats and mice exposed to methyl chloride. Prepared for Chemical Industry Institute of Toxicology. Columbus, OH, Battelle Columbus Laboratories [published as an abstract in Pavkov KL, Kerns WD, Chrisp CE, Thake DC, Persing RL, Harroff HH (1982) Major findings in a twenty-four month inhalation toxicity study of methyl chloride in mice and rats. Toxicologist, 2:161]. Edwards PR, Campbell I, Milne GS (1982a) The impact of chloromethanes on the environment. Part 1. The atmospheric chlorine cycle. Chemistry and industry, 16:574–578 [cited in ATSDR, 1998]. Edwards PR, Campbell I, Milne GS (1982b) The impact of chloromethanes on the environment. Part 2. Methyl chloride and methylene chloride. Chemistry and industry, 17:619–622 [cited in ATSDR, 1998]. CMR (1995) Chemical profile: Methyl chloride. Chemical marketing reporter, March 6:44–45 [cited in ATSDR, 1998]. Elliott S, Rowland FS (1995) Methyl halide hydrolysis rates in natural waters. Journal of atmospheric chemistry, 20:229–236. Coleman WE, Lingg RD, Melton RG, Kopfler RC (1976) The occurrence of volatile organics in five drinking water supplies using gas chromatography/mass spectrometry. In: Keith L, ed. Analysis and identification of organic substances in water. Ann Arbor, MI, Ann Arbor Science [cited in ATSDR, 1990]. Evans GF, Lumpkin TA, Smith DL, Sommerville MC (1992) Measurements of VOCs from the TAMS network. Journal of the Air and Waste Management Association, 42:1319–1323. Coles B, Ketterer B (1990) The role of glutathione and glutathione transferases in chemical carcinogenesis. CRC critical reviews in biochemistry and molecular biology, 25:47–70. Fabian P, Borchers R, Leifer R, Subbaraya BH, Lal S, Boy M (1996) Global stratospheric distribution of halocarbons. Atmospheric environment, 30(10/11):1787–1796. Cox RA, Derwent RG, Eggleton AEJ, Lovelock JE (1976) Photochemical oxidation of halocarbons in the troposphere. Atmospheric environment, 10:305–308 [cited in BUA, 1986]. Fostel J, Allen PF, Bermundez E, Kligerman AD, Wilmer JL, Skopek TR (1985) Assessment of the genotoxic effects of methyl chloride in human lymphoblasts. Mutation research, 155:75–81. Cronn DR, Rasmussen RA, Robinson E (1976) Measurements of tropospheric halocarbon by gas chromatography – mass spectrometry. Research Triangle Park, NC, National Environmental Research Centre (report for phase I of EPA Grant No. R 0804033-01) [cited in BUA, 1986]. Furutani C, Sadayoshi K, Tanabe H, Hayata K, Sueda A, Kitakawa Y (1979) Air pollution by halogenated hydrocarbons, II: Chlorinated hydrocarbons. Yamaguchi-Ken, Kogai Senta Nenpo, 5:13–17 [cited in BUA, 1986]. Geckeler KE, Eberhardt W (1995) Biogene Organochlorverbindungen - Vorkommen, Funktion, Umweltrelevanz. Naturwissenschaft, 82:2–11. Cronn DR, Rasmussen RA, Robinson E (1977) Halogenated compound identification and measurement in the troposphere and lower stratosphere. Journal of geophysical research, 82:5935–5944 [cited in ATSDR, 1990]. Gomes DC, Alarsa M, Salvador MC, Kupferschmid C (1994) Environmental soil and groundwater assessment using high resolution passive soil-gas samplers — PETREX method: Methodology and results of a case study performed in Brazil. Water science and technology, 29(8):161–172. Crutzen PJ, Gidel LT (1983) A two-dimensional photochemical model of the atmosphere. 2. The tropospheric budgets of the anthropogenic chlorocarbons CO, CH4, CH3Cl and the effect of various NOx sources on tropospheric ozone. Journal of geophysical research, 88:6641–6661. Graedel TE (1978) Chemical compounds in the atmosphere . New York, NY, Academic Press [cited in BUA, 1986; HSDB, 1996]. 30 Methyl chloride Graedel TE, Crutzen PJ (1993) Atmospheric change: An earth system perspective. New York, NY, WH Freeman, pp. 298–299. Häsänen E, Manninen PKG, Himberg K, Väätäinen V (1990) Chlorine and bromine contents in tobacco and tobacco smoke [letter]. Journal of radioanalytical and nuclear chemistry, 114(5):367–374. Graedel TE, Keene WC (1995) Tropospheric budget of reactive chlorine. Global biochemical cycles, 9(1):47–77. Hatch GG, Mamay PD, Ayer ML, Casto BC, Nesnow S (1983) Chemical enhancement of viral transformation in Syrian hamster embryo cells by gaseous and volatile chlorinated methanes and ethanes. Cancer research, 43:1945–1950. Granstrom ML, Ahlert RC, Wiesenfeld J (1984) The relationship between the pollutants in the sediment and the water of the Delaware and Raritan canal. Water science and technology, 16:375–380 [cited in ATSDR, 1990]. HazDat (1998) Hazardous Substance Release and Health Effects Database. Atlanta, GA, Agency for Toxic Substances and Disease Registry [cited in ATSDR, 1998]. Great Lakes Water Quality Board (1983) An inventory of chemical substances identified in the Great Lakes ecosystem. Vol. I. Summary. Report to Great Lakes Water Quality Board. Windsor, Ontario, Great Lakes Water Quality Board, pp. 1–8, 11, 59, 90–91 [cited in ATSDR, 1990]. Hendriks J, Stouten MDA (1993) Monitoring the response of micro-contaminants by dynamic Daphnia magna and Leuciscus idus assays in the river Rhine delta: biological early warning as a useful supplement. Ecotoxicology and environmental safety, 26:265–279. Gregory GL, Harriss RC, Talbot RW, Rasmussen RA, Garstang M, Andreae MO, Hinton RR, Browell EV, Beck SM (1986) Air chemistry over the tropical forest of Guyana. Journal of geophysical research, 91:8603–8612 [cited in HSDB, 1996]. Holbrook MT (1992) Chlorocarbons, hydrocarbons (CHCI3). In: Kroschwitz JI, Howe-Grant M, eds. Kirk-Othmer’s encyclopedia of chemical technology, 4th ed. New York, NY, John Wiley & Sons [cited in ATSDR, 1998]. Gribble GW (1992) Naturally occurring organohalogen compounds — a survey. Journal of natural products, 55(10):1353–1395. Holmes TM, Buffler PA, Holguin AH, Hsi BP (1986) A mortality study of employees at a synthetic rubber manufacturing plant. American journal of industrial medicine, 9:355–362. Grimsrud EP, Rasmussen RA (1975) Survey and analysis of halocarbons in the atmosphere by gas chromatography – mass spectrometry. Atmospheric environment, 9:1014–1017 [cited in BUA, 1986; ATSDR, 1990; HSDB, 1996]. Horvath AL (1982) Halogenated hydrocarbons. Solubilitymiscibility with water. New York, NY, Marcel Dekker, Inc. [cited in ATSDR, 1998]. Grossman AS, Grant KE, Wuebbles DJ (1994) Radiative forcing calculations for CH3Cl. Livermore, CA, Lawrence Livermore National Laboratory (UCLR-ID-118065). Houghton JT, Meira Filho LG, Callander BA, Harris N, Kattenberg A, Maskell K, eds. (1996) Climate change 1995 — the science of climate change. Contribution of working group I to the second assessment report of the Intergovernmental Panel on Climate Change. Cambridge, Cambridge University Press. Gudmundsson G (1977) Methyl chloride poisoning 13 years later. Archives of environmental health, 32:236–237. Guicherit R, Schulting FL (1985) The occurrence of organic chemicals in the atmosphere of The Netherlands. Science of the total environment, 43(3):193–219 [cited in HSDB, 1996]. HSDB (1996) Hazardous substances data bank. Bethesda, MD, National Library of Medicine. Gürtler KR, Kleinermanns K (1994) Photooxidation of exhaust pollutants — II. Photooxidation of chloromethanes: Degradation efficiencies, quantum yields and products. Chemosphere , 28(7):1289–1298. IARC (1985) Environmental carcinogens; selected methods of analysis. Vol. 7. Some volatile halogenated hydrocarbons. Lyon, International Agency for Research on Cancer, pp. 107–140 (IARC Scientific Publications No. 68). Hahne RMA (1990) Evaluation of the GM Systems Inc., thermally-desorbable diffusional dosimeter for monitoring methyl chloride. American Industrial Hygiene Association journal, 51:96–101. IARC (1986) Some halogenated hydrocarbons and pesticide exposures. Lyon, International Agency for Research on Cancer (IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Humans, Vol. 41). Hamm TE, Raynor TH, Phelps MC, Auman CD, Adams WT, Proctor JE, Wolkowski-Tyl R (1985) Reproduction in Fischer-344 rats exposed to methyl chloride by inhalation for two generations. Fundamental and applied toxicology, 5:568–577. IARC (1987) Overall evaluations of carcinogenicity: an updating of IARC Monographs volumes 1 to 42. Lyon, International Agency for Research on Cancer (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Suppl. 7). Hansch C, Leo AJ (1985) Medchem project. Claremont, CA, Pomona College (Issue No. 26) [cited in ATSDR, 1998]. IPCS (1999) International Chemical Safety Card — Methyl chloride. Geneva, World Health Organization, International Programme on Chemical Safety (IPCS 0419). Hansen H, Weaver NK, Venable FS (1953) Methyl chloride intoxication. Report from fifteen cases. Archives of industrial hygiene and occupational medicine, 8:328–344. Isidorov VA (1990) Organic chemistry of the Earth’s atmosphere . Berlin, Springler-Verlag, pp. 89–92. Harper DB, Kennedy JT, Hamilton JTG (1988) Chloromethane biosynthesis in poroid fungi. Phytochemistry, 27:3147–3153 [cited in ATSDR, 1990]. Jäger R, Peter H, Sterzel W, Bolt HM (1988) Biochemical effects of methyl chloride in relation to its tumorigenicity. Journal of cancer research and clinical oncology, 114:64–70. Hartmans S, Schmuckle A, Cook AM, Leisinger T (1986) Methyl chloride: naturally occurring toxicant and C-1 growth substrate. Journal of general microbiology, 132:1139–1142. Jiang XZ, White R, Morgan KT (1985) An ultrastructural study of lesions induced in the cerebellum of mice by inhalation exposure to methyl chloride. NeuroToxicology, 6(1):93–104. 31 Concise International Chemical Assessment Document 28 John-Greene JA, Welsch F, Bus JS (1985) Comments on heart malformations in B6C3F1 mouse fetuses induced by methyl chloride — Continuing efforts to understand the etiology and interpretation of an unusual lesion. Teratology, 32:483–487. evaluation of biomass burning emissions — nitrogen and carbon containing compounds. In: Levine JS, ed. Global biomass burning — atmospheric, climatic and biospheric implications. Cambridge, MA, MIT Press, pp. 289–304 [cited in WMO, 1994]. Jury WA, Russo D, Streile G, El Abd H (1990) Evaluation of volatilization by organic chemicals residing below the soil surface. Water resources, 26(1):13–20 [cited in HSDB, 1996]. Löf A, Johanson G, Rannug A, Warholm M (2000) Glutathione transferase T1 phenotype affects the toxicokinetics of inhaled methyl chloride in human volunteers. Pharmacogenetics, 10:645–653. Kelly TJ, Mukund R, Spicer CW, Pollack AJ (1994) Concentrations and transformations of hazardous air pollutants. Environmental science and technology, 28(8):378–387. Logan JA, Prather MJ, Wofsy SC, McElroy MB (1981) Tropospheric chemistry: A global perspective. Journal of geophysical research, 86(C8):7210–7254. Key JA, Stuewe CW, Standifer RL, Hobbs FD, Pitts DM (1980) Organic chemical manufacturing. Vol. 8. Selected processes. Research Triangle Park, NC, US Environmental Protection Agency (NTIS/PB81-22076; EPA-450/3-80-028a) [cited in ATSDR, 1998]. Lovelock JE (1975) Atmospheric halocarbons and stratospheric ozone. Nature, 252:292–294 [cited in BUA, 1986]. Khalil MAK, Rasmussen RA (1981) Atmospheric methyl chloride (CH3Cl). Chemosphere , 10(9):1019–1023 [cited in HSDB, 1996]. Lundberg P (1992) Methyl chloride. NEG and DECOS basis for an occupational standard . Solna, National Institute of Occupational Health, Nordic Council of Ministers (Arbete och Hälsa 27). Khalil MAK, Rasmussen RA (1993) Arctic haze: patterns and relationships to regional signatures of trace gases. Global biogeochemical cycles, 7(1):27–36. Lyman WJ, Reehl WF, Rosenblatt DH (1982) Handbook of chemical property estimation methods. New York, NY, McGrawHill, pp. 15.1–15.34 [cited in HSDB, 1996]. Kornbrust DJ, Bus JS (1982) Metabolism of methyl chloride to formate in rats. Toxicology and applied pharmacology, 65:135–143. Mabey WR, Mill T (1976) Kinetics of hydrolysis and oxidation of organic pollutants in the aquatic environment. In: Extended Abstracts from the Symposium on Nonbiological Transport and Transportation of Pollutants on Land and Water, May 11–13. Gaithersburg, MD, Bureau of Standards [cited in BUA, 1986; ATSDR, 1990]. Kornbrust DJ, Bus JS (1983) The role of glutathione and cytochrome P-450 in the metabolism of methyl chloride. Toxicology and applied pharmacology, 67:246–256. Mabey WR, Mill T (1978) Critical review of hydrolysis of organic compounds in water under environmental conditions. Journal of physical and chemical reference data, 7(2):383–415 [cited in BUA, 1986; ATSDR, 1990; HSDB, 1996]. Kornbrust DJ, Bus JS (1984) Glutathione depletion by methyl chloride and association with lipid peroxidation in mice and rats. Toxicology and applied pharmacology, 72:388–399. Kornbrust DJ, Bus JS, Doerjer G, Swenberg JA (1982) Association of inhaled [ 14C]methyl chloride with macromolecules from various rat tissues. Toxicology and applied pharmacology, 65:122–134. MacDonald JDC (1946) Methyl chloride intoxication. Report of 8 cases. Journal of occupational medicine, 6:81–84. Mariich LI, Kasimova LZ, Krasnozhenenko DA (1982) Gas chromatographic determination of chlorinated impurities of the methane series in water. Khimiia Tekhnologiia Vody, 4(6):542–545 [cited in BUA, 1986]. Landry TD, Gushow TS, Langvardt PW, Wall JM, McKenna MJ (1983) Pharmacokinetics and metabolism of inhaled methyl chloride in the rat and dog. Toxicology and applied pharmacology, 68:473–486. McNally WD (1946) Eight cases of methyl chloride poisoning with three deaths. Journal of industrial hygiene and toxicology, 28:94–97. Landry TD, Quast JF, Gushow TS, Mattsson JL (1985) Neurotoxicity of methyl chloride in continuously versus intermittently exposed female C57BL/6 mice. Fundamental and applied toxicology, 5:87–98. MITI (1992) Biodegradation and bioaccumulation data on existing chemicals based on the CSCL, Japan. Tokyo, Ministry of International Trade and Industry, Chemicals Inspection and Testing Institute, Supervision of Chemical Products Safety Division, Basic Industries Bureau. Lanham JM (1982) Methyl chloride: an unusual incident of intoxication. Canadian Medical Association journal, 126:593. Laursen KK, Hobbs PV, Radke LF (1992) Some trace gas emissions from North American biomass fires with an assessment of regional and global fluxes from biomass burning. Journal of geophysical research, 97(D18):20 687–20 701. Moore RM, Geen CE, Tait VK (1995) Determination of Henry’s law constants for a suite of naturally occurring halogenated methanes in seawater. Chemosphere , 30(6):1183–1191. Leisinger T, Braus-Stromeyer SA (1995) Bacterial growth with chlorinated methanes. Environmental health perspectives, 103(5):33–36. Morgan KT, Swenberg JA, Hamm TE Jr, Wolkowski-Tyl R, Phelps M (1982) Histopathology of acute toxic response in rats and mice exposed to methyl chloride by inhalation. Fundamental and applied toxicology, 2:293–299. Lesage S, Ritch JK, Treciokas EJ (1990) Characterisation of groundwater contaminants at Elmira, Ontario, by thermal desorption solvent extraction GC-MS and HPLC. Water pollution research journal of Canada, 25(3):275–292. NAS (1978) Chloroform, carbon tetrachloride and other halomethanes — an environmental assessment. Washington, DC, National Academy of Sciences, National Research Council [cited in BUA, 1986]. Lobert JM, Scharffe DH, Hao WM, Kuhlbusch TA, Seuwen R, Warneck P, Crutzen PJ (1991) Biomass burning — experimental NASA (1981) Chemical kinetic and photochemical data for use in stratospheric modelling evaluation number 4. Pasadena, CA, 32 Methyl chloride National Aeronautics and Space Administration, NASA Panel for Data Evaluation (Report No. NASA-CR-163973) [cited in ATSDR, 1990]. Pierotti D, Rasmussen RA (1976) Interim report on the atmospheric measurement of nitrous oxide and the halocarbons. Washington, DC, National Aeronautics and Space Administration (NASA Grant NSG 7214) [cited in BUA, 1986]. Nelson HH, Wiencke JK, Christiani DC, Cheng TJ, Zuo Z-F, Schwartz BS, Lee B-K, Spitz MR, Wang M, Xu X, Kelsey KT (1995) Ethnic differences in the prevalence of the homozygous deleted genotype of glutathione S-transferase theta. Carcinogenesis, 16(5):1243–1245. Plumb RH Jr (1991) The occurrence of Appendix IX organic constituents in disposal site ground water. Ground water monitoring reviews, 11(2):157–164. Putz-Anderson V, Setzer JV, Croxton JS, Phipps FC (1981a) Methyl chloride and diazepam effects on performance. Scandinavian journal of work, environment, and health, 7:8–13. NIOSH (1976) NIOSH technical information: Behavioral and neurological effects of methyl chloride. Cincinnati, OH, US Department of Health, Public Health Service, Centers for Disease Control, National Institute for Occupational Safety and Health (Publication No. 77-125). Putz-Anderson V, Setzer JV, Croxton JS (1981b) Effects of alcohol, caffeine and methyl chloride in man. Psychological reports, 48:715–724. NIOSH (1980) Extent-of-exposure survey of methyl chloride. Washington, DC, US Department of Health and Human Services, National Institute for Occupational Safety and Health (Technical Report: Publication No. 80-134). Rafnsson V, Gudmundsson G (1997) Long-term follow-up after methyl chloride intoxication. Archives of environmental health, 52(5):355–359. NIOSH (1994) Method 1001: Methyl chloride. In: NIOSH manual of analytical methods, 4th ed. Cincinnati, OH, US Department of Health and Human Services, Public Health Service, Centers for Disease Control, National Institute for Occupational Safety and Health [cited in ATSDR, 1998]. Rasmussen RA, Khalil MAK (1983) Natural and anthropogenic trace gases in the lower troposphere of the arctic. Chemosphere , 12:371–375 [cited in ATSDR, 1990]. Rasmussen RA, Rasmussen LE, Khalil MAK, Dalluge RW (1980) Concentration distribution of methyl chloride in the atmosphere. Journal of geophysical research, 85(C12):7350–7356. Nolan RJ, Rick DL, Landry TD, McCarty LP, Agin GL, Saunders JH (1985) Pharmacokinetics of inhaled methyl chloride (CH3Cl) in male volunteers. Fundamental and applied toxicology, 5:361–369. Redford-Ellis M, Gowenlock AH (1971) Studies of the reaction of chloromethane with preparations of liver, brain and kidney. Acta Pharmacologica et Toxicologica, 30:49–58. Oliver KD, Adams JR, Daughtrey EH Jr (1996) Technique for monitoring toxic VOCs in air: Sorbent preconcentration, closedcycle cooler cryofocusing, and GC/MS analysis. Environmental science and technology, 30(6):1939–1945 [cited in ATSDR, 1998]. Reinhardt TE, Ward DE (1995) Factors affecting methyl chloride emissions from forest biomass combustion. Environmental science and technology, 29:825–832. Otson R (1987) Purgeable organics in Great Lakes raw and treated water. International journal of environmental analytical chemistry, 31:41–53 [cited in ATSDR, 1990; HSDB, 1996]. Rice J, Moore WH, McAllister RA, Bowles EG (1990) 1989 National urban air toxics monitoring program. In: Proceedings for the 83rd Annual Meeting of the Air and Waste Management Association, June 24–29, 1990, Pittsburgh, PA. Pittsburgh, PA, Air and Waste Management Association, Vol. 9, pp. 1–15. Otson R, Williams DT, Bothwell PD (1982) Volatile organic compounds in water at thirty Canadian potable water treatment facilities. Journal of the Association of Official Analytical Chemists, 65(6):1370–1374 [cited in HSDB, 1996]. Ristau C, Bolt HM, Vangala RR (1989) Detection of DNA–protein crosslinks in the kidney of male B6C3F1 mice after exposure to methyl chloride. Archives of toxicology, Suppl. 13:243–245. Page GW (1981) Comparison of groundwater and surface water for patterns and levels of contamination by toxic substances. Environmental science and technology, 15:1475–1481 [cited in BUA, 1986; ATSDR, 1990]. Ristau C, Bolt HM, Vangala RR (1990) Formation and repair of DNA lesions in kidneys of male mice after acute exposure to methyl chloride. Archives of toxicology, 64:254–256. Robbins DE (1976) Photodissociation of methyl chloride and methyl bromide in the atmosphere. Geophysical research letters, 3:213–216 [cited in HSDB, 1996]. Pearson CR, McConnell G (1975) Chlorinated C1 and C2 hydrocarbons in the marine environment. Proceedings of the Royal Society of London, Series B: Biological Sciences, 189:305–332 [cited in BUA, 1986]. Robinson E, Rasmussen RA, Krasnec J (1977) Halocarbon measurements in the Alaskan troposphere and lower stratosphere. Atmospheric environment, 11:215–223 [cited in ATSDR, 1990]. Pemble S, Schroeder KR, Spencer SR, Meyer DJ, Hallier E, Bolt HM, Ketterer B, Taylor JB (1994) Human glutathione Stransferase theta (GSTT1): cDNA cloning and the characterisation of a genetic polymorphism. Biochemical journal, 300:271–276. Rosenfeld JK, Plumb RH Jr (1991) Ground water contamination at wood treatment facilities. Ground water monitoring reviews, 11(1):133–140. Peter H, Laib RJ, Ottenwälder H, Topp H, Bolt HM (1985) DNAbinding assay of methyl chloride. Archives of toxicology, 57:84–87. Rudolph J, Jebsen C (1983) The use of photoionization, flame ionization and electron capture detectors in series for the determination of low molecular weight trace compounds in the non urban atmosphere. International journal of environmental analytical chemistry, 13:129–138. Peter H, Deutschmann S, Reichel C, Hallier E (1989) Metabolism of methyl chloride by human erythrocytes. Archives of toxicology, 63:351–355. 33 Concise International Chemical Assessment Document 28 Rudolph J, Khedim A, Clarkson T, Wagenbach D (1992) Longterm measurements of light alkanes and acetylene in the Antarctic troposphere. Tellus, 44(4):252–261. Environmental Protection Agency, Environmental Sciences Research Laboratory (EPA 600/3-81-055; NTIS PB82-24902) [cited in ATSDR, 1990]. Rudolph J, Khedim A, Koppmann R, Helas G, Bonsang A (1994) Field study of the halocarbon emissions from biomass burning in Africa. Physico-chemical behaviour of atmospheric pollutants, 1:424–430. Singh HB, Salas LJ, Stiles RE (1982) Distribution of selected gaseous organic mutagens and suspect carcinogens in ambient air. Environmental science and technology, 16:872–880 [cited in BUA, 1986; ATSDR, 1990]. Rudolph J, Khedim A, Koppmann R, Bonsang A (1995) Field study on the emissions of methyl chloride and other halocarbons from biomass burning in western Africa. Journal of atmospheric chemistry, 22:67–80. Singh HB, Salas LJ, Stiles RE (1983) Methyl halides in and over eastern Pacific (40°N–32°S). Journal of geophysical research, 88:3684–3690. Singh HB, Salas LJ, Viezee W, Sitton B, Ferek R (1992) Measurement of volatile organic chemicals at selected sites in California. Atmospheric environment, 26A(16):2929–2946. Sabel GV, Clark TP (1984) Volatile organic compounds as indicators of municipal solid waste leachate contamination. Waste management research, 2:119–130 [cited in ATSDR, 1990; HSDB, 1996]. Solomon S, Mills M, Heidt LE, Pollock WH, Tuck AF (1992) On the evaluation of ozone depletion potentials. Journal of geophysical research, 97(D1):825–842. Saini HS, Attieh JM, Hanson AD (1995) Biosynthesis of halomethanes and methanethiol by higher plants via a novel methyltransferase reaction. Plant, cell and environment, 18:1027–1033. Speerschneider P, Dekant W (1995) Renal tumorigenicity of 1,1dichloroethane in mice: The role of male-specific expression of cytochrome P450 2E1 in the renal bioactivation of 1,1-dichloroethene. Toxicology and applied pharmacology, 130:48–56. Scharnweber HC, Spears GN, Cowles SR (1974) Case reports. Chronic methyl chloride intoxication in six industrial workers. Journal of occupational medicine, 16(2):112–113. Spevak L, Nadj V, Fellé D (1976) Methyl chloride poisoning in four members of a family. British journal of industrial medicine, 33:272–278. Schauffler SM, Heidt LE, Pollock WH, Gilpin TM, Vedder JF, Solomon S, Lueb RA, Atlas EL (1993) Measurements of halogenated organic compounds near the tropical tropopause. Geophysical research letters, 20(22):2567–2570. Spicer CW, Buxton BE, Holdren MW, Smith DL, Kelly TJ, Rust SW, Pate AD, Sverdrup GM, Chuang JC (1996) Variability of hazardous air pollutants in an urban area. Atmospheric environment, 30(20):3443–3456. Severs LW, Skory LK (1975) Monitoring personnel exposure to vinyl chloride, vinylidene chloride and methyl chloride in an industrial work environment. American Industrial Hygiene Association journal, 36:669–676. Staples CA, Werner FA, Hoogheem TJ (1985) Assessment of priority pollutant concentrations in the United States using STORET database. Environmental toxicology and chemistry, 4(2):131–142 [cited in HSDB, 1996]. Shah JJ, Singh HB (1988) Distribution of volatile organic chemicals in outdoor and indoor air. Environmental science and technology, 22:1381–1388 [cited in ATSDR, 1990]. Stewart RD, Hake CL, Graff CA, Forster HV, Keeler WH, Lebrun AJ, Newton PE, Soto RJ (1980) Methyl chloride: Development of a biologic standard for the industrial worker by breath analyses. Cincinnati, OH, National Institute for Occupational Safety and Health (NTIS PB81-167686). Shirey RE (1995) Rapid analysis of environmental samples using solid-phase microextraction SPME and narrow bore capillary columns. HRC: Journal of high resolution chromatography, 18(8):495–499 [cited in ATSDR, 1998]. Stirling DI, Dalton H (1979) The fortuitous oxidation and cometabolism of various carbon compounds by whole-cell suspensions of Methylococcus capsulatus (Bath). FEMS microbiology letters, 5:315–318 [cited in ATSDR, 1990]. Simmon VF, Kauhanen K, Tardiff RG (1977) Mutagenic activity of chemicals identified in drinking water. In: Scott D, Bridges BA, Sobels FH, eds. Progress in genetic toxicology. Amsterdam, Elsevier, pp. 249–258. Tait VK, Moore RM (1995) Methyl chloride (CH 3Cl) production in phytoplankton cultures. Limnology and oceanography, 40(1):189–195. Simon PB (1989) Hydrolysis of methyl chloride as a function of pH following TSCA test standard 796.35000 as amended with cover letter. Ann Arbor, MI, Ann Arbor Technical Service Inc. Tang NH, Blum DJW, Nirmalakhandan N, Speece RE (1992) QSAR parameters for toxicity of organic chemicals to Nitrobacter. Journal of environmental engineering, 118(1):17–37. Singh HB, Salas LJ, Shigeishi H, Crawford A (1977) Urban– nonurban relationships of halocarbons, SF6, N2O and other atmospheric trace constituents. Atmospheric environment, 11(9):819–828 [cited in BUA, 1986; ATSDR, 1990]. Thier R, Wiebel FA, Hinkel A, Burger A, Brüning T, Morgenroth K, Senge T, Wilhelm M, Schulz TG (1998) Species differences in the glutathione transferase GSTT1-1 activity towards the model substrates methyl chloride and dichloromethane in liver and kidney. Archives of toxicology, 72:622–629. Singh HB, Salas LJ, Shigeishi H, Scribner E (1979) Atmospheric halocarbons, hydrocarbons and sulfur hexafluoride — global distribution, sources and sinks. Science, 203:899–903 [cited in BUA, 1986; HSDB, 1996]. Singh HB, Salas LJ, Smith AJ (1981a) Measurements of some potentially hazardous organic chemicals in urban environments. Atmospheric environment, 15:601–602 [cited in ATSDR, 1990]. Thordarson O, Gudmundsson G, Bjarnason O, Jóhannesson T (1964) Metylkloridforgiftning. Nordisk Medicin, 18:150–154. Traunecker J, Preuss A, Diekert G (1991) Isolation and characterisation of a methyl chloride utilising, strictly anaerobic bacterium. Archives of microbiology, 156:416–421. Singh HB, Salas LJ, Stiles R (1981b) Trace chemical in the “clean” troposphere . Research Triangle Park, NC, US 34 Methyl chloride US EPA (1982) Methods for organic chemical analysis of municipal and industrial waste water. Methods 601 and 624. Cincinnati, OH, US Environmental Protection Agency, Environmental Monitoring and Support Laboratory (Report No. EPA-600/4-82-057) [cited in ATSDR, 1990]. WMO (1994) Montreal protocol on substances that deplete the ozone layer — Scientific assessment of ozone depletion: 1994. Geneva, World Meteorological Organization (Global Ozone Research and Monitoring Project Report No. 37). Wolkowski-Tyl R, Phelps M, Davis JK (1983a) Structural teratogenicity evaluation of methyl chloride in rats and mice after inhalation exposure. Teratology, 27:181–195. USEPA (1986a) Test methods for evaluating solid waste. Methods 5030, 8000, 8010, 8015, 3rd ed. Cincinnati, OH, US Environmental Protection Agency, Office of Solid Waste and Emergency Response (Report No. SW-846) [cited in HSDB, 1996; ATSDR, 1998]. Wolkowski-Tyl R, Lawton AD, Phelps M, Hamm TE Jr (1983b) Evaluation of heart malformations in B6C3F1 mouse fetuses induced by in utero exposure to methyl chloride. Teratology, 27:197–206. US EPA (1986b) Methods for determination of organic compounds in finished drinking water and raw source water. Washington, DC, US Environmental Protection Agency [cited in HSDB, 1996]. Wood PR, Lang RF, Payan IL (1985) Anaerobic transformation, transport and removal of volatile chlorinated organics in ground water. In: Ward CH, Giger W, McCarty Pl, eds. Groundwater quality. New York, NY, John Wiley & Sons, Inc., pp. 493–511 [cited in ATSDR, 1990]. US EPA (1991a) Nonmethane organic compound and three-hour air toxics monitoring program, 1990. Research Triangle Park, NC, US Environmental Protection Agency (Report No. EPA-450/4-91008). Working PK, Bus JS (1986) Failure of fertilisation as a cause of preimplantation loss induced by methyl chloride in Fischer 344 rats. Toxicology and applied pharmacology, 86:124–130. US EPA (1991b) Urban air toxics monitoring program, 1990. Research Triangle Park, NC, US Environmental Protection Agency (Report No. EPA-450/4-91-024). Working PK, Bus JS, Hamm TE (1985a) Reproductive effects of inhaled methyl chloride in the male Fischer 344 rat. I. Mating performance and dominant lethal assay. Toxicology and applied pharmacology, 77:133–143. van Doorn R, Borm PJA, Leijdekkers C-M, Henderson PT, Reuvers J, van Bergen TJ (1980) Detection and identification of S-methylcysteine in urine of workers exposed to methyl chloride. International archives of occupational and environmental health, 46:99–109. Working PK, Bus JS, Hamm TE (1985b) Reproductive effects of inhaled methyl chloride in the male Fischer 344 rat. II. Spermatogonial toxicity and sperm quality. Toxicology and applied pharmacology, 77:144–157. Vaughan P, Lindahl T, Sedgwick B (1993) Induction of the adaptive response of Escherichia coli to alkylation damage by the environmental mutagen, methyl chloride. Mutation research, 293:249–257. Working PK, Doolittle DJ, Smith-Oliver T, White RD, Butterworth BE (1986) Unscheduled DNA synthesis in rat tracheal epithelial cells, hepatocytes and spermatocytes following exposure to methyl chloride in vitro and in vivo. Mutation research, 162:219. von Clarmann A, Linden A, Oelhaf H, Fisher H, Friedl-Vallon F, Piesch C, Seefeldner M (1995) Determination of the stratospheric organic chlorine budget in the spring arctic vortex from MIPAS B limb emission spectra and air sampling experiments. Journal of geophysical research, 100(D7):13 979–13 997. Wynder EL, Hoffmann D, eds. (1967) Tobacco and tobacco smoke: Studies in environmental carcinogenesis. New York, NY, Academic Press, p. 454. Zafiriou OC (1975) Reaction of methyl halides with seawater and marine aerosols. Journal of marine research, 33(1):75–81 [cited in BUA, 1986; HSDB, 1996]. von Kolkmann FW, Volk B (1975) Über Körnerzellnekrosen bei der experimentellen Methylchloridvergiftung des Meerschweinchens. Experimentelle Pathologie, 10:298–308. Zander R, Gunson MR, Farmer CB, Rinsland CP, Irion FW, Mahieu E (1992) The 1985 chlorine and fluorine inventories in the stratosphere based on ATMOS observations at 30° north latitude. Journal of atmospheric chemistry, 15:171–186. Warholm M, Alexandrie A-K, Högberg J, Sigvardsson K, Rannug A (1994) Polymorphic distribution of glutathione transferase activity with methyl chloride in human blood. Pharmacogenetics, 4:307–311. Warneck P (1988) Chemistry of the natural atmosphere . London, Academic Press, pp. 267–272. Watson AJ, Lovelock JE, Stedman DH (1980) The problem of atmospheric methyl chloride. In: Nicolet M, Aikin AC, eds. NATO Advance Study Institute on Atmospheric Ozone: Its variation and human influence. Washington, DC, Department of Transportation, pp. 365–373 (Report No. FAA-EE-80-20). Weast RC (1988) CRC handbook of chemistry and physics, 69th ed. Boca Raton, FL, CRC Press [cited in ATSDR, 1998]. White RD, Norton R, Bus JS (1982) Evidence for S-methyl glutathione metabolism in mediating the acute toxicity of methyl chloride (MeCl). Pharmacologist, 24:172. WMO (1990) Scientific assessment of stratospheric ozone: 1989. Geneva, World Meteorological Organization, p. 158 (Global Ozone Research and Monitoring Project Report No. 20). 35 Concise International Chemical Assessment Document 28 from the ATSDR reviewed the peer reviewers’ comments and determined which ones to include in the profile. APPENDIX 1 — SOURCE DOCUMENTS The responsibility for the content of the profiles lies with the ATSDR. Lundberg P (1992) Methyl chloride. NEG and DECOS basis for an occupational standard. Solna, National Institute of Occupational Health, Nordic Council of Ministers (Arbete och Hälsa 27) BUA (1986) Chloromethane. GDCh-Advisory Committee on Existing Chemicals of Environmental Relevance (BUA). Weinheim, VCH Verlagsgesellschaft mbH; and New York, NY, VCH Publishers, Inc. (BUA Report 7) Copies of the Arbete och Hälsa document on methyl chloride (ISSN 0346-7821; ISBN 91-7045-179-6) may be obtained from: National Institute for Working Life Publications Department S-171 84 Solna Sweden Copies of the BUA report on chloromethane (ISBN 3-52728558-X [Weinheim] and 1-56081-734-8 [New York]) may be obtained from VCH in Weinheim, Basel, Cambridge, and New York. The document, which is focused on health effects only, was prepared in the series of criteria documents from the Nordic Expert Group for Documentation of Occupational Exposure Limits (NEG) in collaboration with the Dutch Expert Committee for Occupational Standards (DECOS) of the Dutch DirectorateGeneral of Labor. The draft was reviewed by the Dutch Expert Committee as well as by the Nordic Expert Group. The reviewers were industrial and academic specialists who were chosen either because they have an extended knowledge of methyl chloride itself or because they are specialists in the critical effect area of the chemical. BUA reports are written by the largest German producer of the chemical. The draft is examined by BUA (GDCh-Advisory Committee for Existing Chemicals of Environmental Relevance), which consists of representatives from government agencies, industry, and the scientific community. Resulting questions are clarified by the authors as well as by further research (resulting in updated reports). After an average of two readings in the work group and discussions with experts, the BUA plenum debates the report before it is published. More detailed information on the BUA reports is found in Assessment of existing chemicals. A contribution toward improving the environment, a 1993 report by BUA. ATSDR (1990) Toxicological profile for chloromethane. Atlanta, GA, US Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry (Report No. TP-90-07) HSDB (1996) Hazardous substances data bank. Bethesda, MD, US National Library of Medicine The version of HSDB used for this CICAD is included in the CD-ROM CHEM-BANK (July 1996), published by: ATSDR (1998) Toxicological profile for chloromethane (update). Atlanta, GA, US Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry (Report No. 205-93-0606) Silver Platter Information Inc. 100 River Ridge Drive Norwood, MA 02062-5043 USA HSDB is also available on CD-ROM from the Canadian Centre for Occupational Health and Safety (CCINFOdisc 2) and on-line by Data-Star, DIMDI, STN International, Toxicology Data Network (TOXNET). HSDB is built, reviewed, and maintained on the National Library of Medicine’s TOXNET. HSDB is a factual data bank, referenced and peer reviewed by a committee of experts (the Scientific Review Panel). All data extracted from HSDB for use in this CICAD were preceded by the symbol denoting the highest level of peer review. Copies of the ATSDR Toxicological profile for chloromethane may be obtained from: Agency for Toxic Substances and Disease Registry Division of Toxicology 1600 Clifton Road NE, E-29 Atlanta, Georgia 30333 USA The date for the last revision or modification of the record on methyl chloride was June 1996. A peer review panel was assembled for the Toxicological profile on chloromethane (1990), including Dr Anthony DeCaprio and Dr Nancy Reiches (private consultants), Dr Theodore Mill (SRI International), and Dr Nancy Tooney (Department of Biochemistry, Polytechnic University). A joint panel of scientists from ATSDR and EPA reviewed the peer reviewers’ comments and determined which ones to include in the profile. For the updated profile (1998), the panel consisted of Dr Herbert Cornish (private consultant), Dr Anthony DeCaprio (Associate Professor, State University of New York at Albany), Dr Theodore Mill (Senior Scientist, SRI International), and Dr Nancy Tooney (Associate Professor, Brooklyn, NY). Scientists 36 Methyl chloride APPENDIX 2 — CICAD PEER REVIEW WMO (1994) Montreal protocol on substances that deplete the ozone layer — Scientific assessment of ozone depletion: 1994. Geneva, World Meteorological Organization (Global Ozone Research and Monitoring Project Report No. 37) The draft CICAD on methyl chloride was sent for review to institutions and organizations identified by IPCS after contact with IPCS national Contact Points and Participating Institutions, as well as to identified experts. Comments were received from: Copies of this report (ISBN 92-807-1449-X) for scientific users may be obtained from: M. Baril, International Programme on Chemical Safety/ Institut de Recherche en Santé et en Sécurité du Travail du Québec, Montreal, Quebec, Canada World Meteorological Organization attn. Dr Rumen Bojkov P.O. Box 2300 1211-Geneva Switzerland R. Benson, US Environmental Protection Agency, Denver, CO, USA R. Cary, Health and Safety Executive, Bootle, United Kingdom The WMO (1994) report was the latest in a series of scientific assessments of ozone depletion, prepared under the auspices of WMO and UNEP, that was available during the preparation of the CICAD on methyl chloride. The genesis of the WMO (1994) report occurred at the 4th meeting of the Conference of the Parties to the Montreal Protocol held in Copenhagen, Denmark, in 1992, at which the scope of the scientific needs was defined. In 1993, an international steering group outlined the report and suggested scientists to serve as authors. The first draft was examined by the authors and a small group of experts, and the second draft was sent to a large number of scientists worldwide for review. At a Panel Review Meeting in July 1994, final changes were discussed and decided upon. The scientists who prepared the report (230) and participated in the peer review process (147) are listed in the report. R. Chhabra, Department of Health and Human Services, National Institute of Environmental Health Sciences, Research Triangle Park, NC, USA P. Edwards, Department of Health, Protection of Health Division, London, United Kingdom M. Greenberg, US Environmental Protection Agency, Research Triangle Park, NC, USA Martin Matisons Environmental Health Service, Health Department of Western Australia H. Nagy, National Institute for Occupational Safety and Health, Cincinnati, OH, USA W. Rawson and L. Neuwirth, Methyl Chloride Industry Association, Washington, DC, USA M. Warholm, Institute of Environmental Medicine, Karolinska Institute, Stockholm, Sweden P. Yao, Ministry of Health, Institute of Occupational Medicine, Chinese Academy of Preventive Medicine, Ministry of Health, Beijing, People’s Republic of China K. Ziegler-Skylakakis, GSF-Forschungszentrum für Umwelt und Gesundheit, Neuherberg, Oberschleissheim, Germany 37 Concise International Chemical Assessment Document 28 Dr A. Poole (representing CEFIC), Dow Europe S.A., Horgen, Switzerland APPENDIX 3 — CICAD FINAL REVIEW BOARD Dr K. Ziegler-Skylakakis, GSF-Forschungszentrum für Umwelt und Gesundheit, Institut für Toxikologie, Neuherberg, Oberschleissheim, Germany Stockholm, Sweden, 25–28 May 1999 Members Secretariat Mr H. Abadin, Agency for Toxic Substances and Disease Registry, Centers for Disease Control and Prevention, Atlanta, GA, USA Dr A. Aitio, Programme for the Promotion of Chemical Safety, World Health Organization, Geneva, Switzerland Dr B. Åkesson, Department of Occupational and Environmental Health, University Hospital, Lund, Sweden Ms M. Godden, Health and Safety Executive, Bootle, United Kingdom Dr T. Berzins (Chairperson), National Chemicals Inspectorate (KEMI), Solna, Sweden Ms L. Regis, Programme for the Promotion of Chemical Safety, World Health Organization, Geneva, Switzerland Mr R. Cary, Health and Safety Executive, Bootle, Merseyside, United Kingdom Dr P. Toft, Division of Health and Environment, World Health Organization, Regional Office for the Americas/Pan American Sanitary Bureau, Washington, DC, USA Dr R.S. Chhabra, General Toxicology Group, National Institute of Environmental Health Sciences, Research Triangle Park, NC, USA Dr M. Younes, Programme for the Promotion of Chemical Safety, World Health Organization, Geneva, Switzerland Dr S. Dobson (Rapporteur), Institute of Terrestrial Ecology, Monks Wood, Abbots Ripton, Huntingdon, Cambridgeshire, United Kingdom Dr H. Gibb, National Center for Environmental Assessment, US Environmental Protection Agency, Washington, DC, USA Dr R.F. Hertel, Federal Institute for Health Protection of Consumers and Veterinary Medicine, Berlin, Germany Dr G. Koennecker, Chemical Risk Assessment, Fraunhofer Institute for Toxicology and Aerosol Research, Hannover, Germany Dr A. Nishikawa, National Institute of Health Sciences, Division of Pathology, Tokyo, Japan Professor K. Savolainen, Finnish Institute of Occupational Health, Helsinki, Finland Dr J. Sekizawa, Division of Chem-Bio Informatics, National Institute of Health Sciences, Tokyo, Japan Ms D. Willcocks (Vice-Chairperson), Chemical Assessment Division, National Occupational Health and Safety Commission (Worksafe Australia), Sydney, Australia Professor P. Yao, Institute of Occupational Medicine, Chinese Academy of Preventive Medicine, Ministry of Health, Beijing, People’s Republic of China Observers Dr N. Drouot (representing ECETOC), Elf Atochem, DSE-P Industrial Toxicology Department, Paris, France Ms S. Karlsson, National Chemicals Inspectorate (KEMI), Solna, Sweden Dr A. Löf, National Institute of Working Life, Solna, Sweden 38 METHYL CHLORIDE 0419 March 1999 CAS No: 74-87-3 RTECS No: PA6300000 UN No: 1063 EC No: 602-001-00-7 TYPES OF HAZARD/ EXPOSURE Chloromethane Monochloromethane CH3Cl Molecular mass: 50.5 ACUTE HAZARDS/SYMPTOMS PREVENTION FIRST AID/FIRE FIGHTING FIRE Highly flammable. Heating will cause rise in pressure with risk of bursting. NO open flames, NO sparks, and NO smoking. Shut off supply; if not possible and no risk to surroundings, let the fire burn itself out; in other cases extinguish with water spray. EXPLOSION Gas/air mixtures are explosive. Closed system, ventilation, explosion-proof electrical equipment and lighting. Use non-sparking handtools. In case of fire: keep cylinder cool by spraying with water. Combat fire from a sheltered position. EXPOSURE STRICT HYGIENE! Inhalation Staggering gait. Dizziness. Headache. Nausea. Vomiting. Convulsions. Unconsciousness. See Notes. Ventilation, local exhaust, or breathing protection. Fresh air, rest. Artificial respiration if indicated. Refer for medical attention. Skin MAY BE ABSORBED! ON CONTACT WITH LIQUID: FROSTBITE. Cold-insulating gloves. Protective clothing. ON FROSTBITE: rinse with plenty of water, do NOT remove clothes. Eyes (See Skin). Safety goggles, face shield, or eye protection in combination with breathing protection. Ingestion SPILLAGE DISPOSAL PACKAGING & LABELLING Evacuate danger area! Consult an expert! Ventilation. NEVER direct water jet on liquid. (Extra personal protection: complete protective clothing including self-contained breathing apparatus). F+ Symbol Xn Symbol R: 12-40-48/20 S: (2-)9-16-33 UN Hazard Class: 2.1 EMERGENCY RESPONSE STORAGE Transport Emergency Card: TEC (R)-41/20G41 NFPA Code: H2; F4; R0 Fireproof. Ventilation along the floor. IPCS International Programme on Chemical Safety Prepared in the context of cooperation between the International Programme on Chemical Safety and the European Commission © IPCS 2000 SEE IMPORTANT INFORMATION ON THE BACK. 0419 METHYL CHLORIDE IMPORTANT DATA Routes of exposure The substance can be absorbed into the body by inhalation and through the skin. Physical State; Appearance COLOURLESS LIQUEFIED GAS. Physical dangers The gas is heavier than air and may travel along the ground; distant ignition possible, and may accumulate in low ceiling spaces causing deficiency of oxygen. See Notes. Inhalation risk A harmful concentration of this gas in the air will be reached very quickly on loss of containment. Chemical dangers The substance decomposes on burning producing toxic and corrosive fumes including hydrogen chloride and phosgene. Reacts violently with powdered aluminium, powdered zinc, aluminium trichloride and ethylene causing fire and explosion hazard. Attacks many metals in the presence of moisture. Effects of short-term exposure The liquid may cause frostbite. The substance may cause effects on the central nervous system. Exposure may result in unconsciousness. Exposure far above OEL may result in liver, cardiovascular system and kidney damage. Medical observation is indicated. Occupational exposure limits TLV: 50 ppm; (skin) (ACGIH 1998). TLV (as (STEL) ): 100 ppm; (skin) (ACGIH 1998). Effects of long-term or repeated exposure The substance may have effects on the central nervous system, resulting in effects measured using behavioural tests. Animal tests show that this substance possibly causes toxic effects upon human reproduction. PHYSICAL PROPERTIES Boiling point: -24.2°C Melting point: -97.6°C Relative density (water = 1): 0.92 Solubility in water, g/100 ml at 25°C: 0.5 Vapour pressure, kPa at 21°C: 506 Relative vapour density (air = 1): 1.8 Flash point: Flammable Gas Auto-ignition temperature: 632°C Explosive limits, vol% in air: 8.1-17.4 Octanol/water partition coefficient as log Pow: 0.91 ENVIRONMENTAL DATA NOTES Following intoxication patient should be observed carefully for 48 hours. Check oxygen content before entering area. ADDITIONAL INFORMATION LEGAL NOTICE Neither the EC nor the IPCS nor any person acting on behalf of the EC or the IPCS is responsible for the use which might be made of this information ©IPCS 2000 Methyl chloride RÉSUMÉ D’ORIENTATION relatives à la proportion de chlorure de méthyle qui atteint la stratosphère et contribue par là à la destruction de la couche d’ozone, sont très variables. Selon les chiffres donnés par l’Organisation météorologique mondiale (OMM), le chlorure de méthyle contribue à hauteur de 15 % à la teneur totale de la stratosphère en chlore actif. Le chlorure de méthyle a un potentiel de destruction de l’ozone stratosphérique de 0,02 par rapport au composé de référence, le CFC-11 dont le potentiel est égal à 1 par définition. On ne pense pas que le chlorure de méthyle ait une influence sensible sur le réchauffement climatique ou sur la pollution de l’air d’origine photochimique. L’évaluation des effets du chlorure de méthyle sur la santé humaine qui figure dans le présent CICAD repose principalement sur une mise au point rédigée par le Groupe nordique d’experts en collaboration avec le Comité néerlandais pour les normes d’hygiène sur les lieux de travail (Lundberg, 1992). Un dépouillement des banques de données couvrant la période 1992-1999 a été effectué afin de compléter les données disponibles. En ce qui concerne les effets environnementaux et écotoxicologiques du chlorure de méthyle, les principales sources d’information utilisées sont les suivantes : BUA (1986), ATDSR (1990) et WMO/OMM (1994). La banque de données ATDSR a été mise à jour en 1998; chaque fois que cette banque de données contenait de nouvelles informations, elles ont été prises en compte. La consultation des banques de données pertinentes couvrant la période 1989-1997 a permis d’obtenir des données supplémentaires sur les questions d’ordre écologique. On trouvera à l’appendice 1 des renseignements sur la nature des sources documentaires existantes. Des informations sur l’examen par des pairs du présent CICAD sont données à l’appendice 2. Ce CICAD a été approuvé en tant qu’évaluation internationale lors d’une réunion du Comité d’évaluation finale qui s’est tenue à Stockholm (Suède) du 25 au 28 mai 1999. La liste des participants au Comité d’évaluation finale figure à l’appendice 3. La fiche internationale sur la sécurité chimique (ICSC 0419) du chlorure de méthyle, établie par le Programme international sur la sécurité chimique (IPCS, 1999), est également reproduite dans le présent document. Le chlorure de méthyle disparaît principalement de l’eau et du sol par évaporation. Dans les couches profondes du sol et dans les eaux souterraines, sa disparition pourrait s’expliquer notamment par une lente hydrolyse et éventuellement par une biodégradation. On sait cependant peu de choses sur ce dernier point. La voie la plus importante d’exposition humaine au chlorure de méthyle est la voie respiratoire. Chez l’Homme comme chez l’animal de laboratoire, il est rapidement absorbé au niveau des poumons après inhalation. Après exposition à du chlorure de méthyle marqué au 14C, la radioactivité se répartit dans l’ensemble de l’organisme. Le produit radiomarqué est incorporé en grande partie aux protéines par le canal du pool des substances à un atome de carbone, mais le chlorure de méthyle peut également se fixer aux protéines par alkylation directe. Toutefois, si le composé se comporte comme un agent alkylant, c’est en très faible proportion. Chez les mammifères, il est métabolisé par conjugaison avec le glutathion et, dans une moindre mesure, par oxydation au niveau du cytochrome P-450. La conjugaison avec le glutathion conduit à la formation de méthanethiol et les deux voies métaboliques ont pour point d’aboutissement le formaldéhyde et le formiate. Les métabolites du chlorure de méthyle sont excrétés dans les urines ainsi que dans l’air expiré, qui contient également une certaine proportion du composé initial. Lorsque du chlorure de méthyle (No CAS 74-87-3) est libéré dans l’atmosphère, c’est surtout au cours de sa production, de son utilisation ou encore lors de l’incinération de déchets municipaux ou industriels. Quoi qu’il en soit, les sources naturelles de chlorure de méthyle (en premier lieu les océans et la combustion de la biomasse) l’emportent largement en importance sur les sources d’origine humaine. On estime que l’ensemble de ces sources libère chaque année quelque 5 × 106 tonnes de chlorure de méthyle. La part des sources naturelles dans ce bilan dépasse largement 90 % selon les estimations et pourrait même atteindre 99 %. Le composé est présent dans la troposphère à une concentration approximativement égale à 1,2 µg/m3 (0,6 parties par milliard). Chez l’Homme, on observe d’importantes différences individuelles concernant l’absorption et la métabolisation du chlorure de méthyle. Ces différences sont dues à la présence ou à l’absence d’une enzyme, la glutathion-transférase T1 (GSTT1), qui présente un polymorphisme génétique. On distingue en effet 3 phénotypes chez l’Homme qui correspondent à une conjugaison forte, faible ou nulle. De toute manière, comme on voit pas très bien si le risque le plus élevé correspond à une conjugaison forte ou à une conjugaison nulle, il faut considérer que tous les phénotypes ont la même sensibilité au chlorure de méthyle. Dans la troposphère, le piégeage du chlorure de méthyle s’effectue principalement par sa réaction sur les radicaux hydroxyle et l’on estime que sa demi-vie atmosphérique est de 1 à 3 ans. Une fraction s’échappe dans la stratosphère où le composé subit une photodissociation donnant naissance à des radicaux chlore qui attaquent la couche d’ozone. Les estimations 41 Concise International Chemical Assessment Document 28 La toxicité aiguë du chlorure de méthyle après inhalation semble assez faible pour le rat et la souris, puisque la CL50 est supérieure à 4128 mg/m3 (2000 ppm). On n’a pas trouvé dans la littérature de données qui indiquent que le composé soit irritant ou sensibilisant. En conclusion, on peut dire que chez l’Homme, le point d’aboutissement de l’action toxique du chlorure de méthyle est vraisemblablement le système nerveux central. On a pu en tirer des valeurs-guides de 0,018 mg/m3 (0,009 ppm) pour une exposition indirecte dans l’environnement et de 1,0 mg/m3 (0,5 ppm) pour une exposition sur le lieu de travail. Bien que les lésions nerveuses aient été constatées chez le rat à des doses plus faibles que celles qui provoquaient la stérilité des mâles (980 mg/m3, soit 475 ppm) ou des lésions rénales chez des souris mâles (à la concentration de 2064 mg/m3, soit 1000 ppm), c’est ces très graves effets qui sont à prendre en compte pour toute caractérisation qualitative du risque que comporte une exposition au chlorure de méthyle. Il semble qu’après inhalation, les principaux organes cibles du chlorure de méthyle soient le système nerveux (avec des troubles fonctionnels ainsi qu’une dégénérescence du cervelet chez le rat et la souris) ainsi que les testicules, l’épididyme et le rein chez le rat ou encore le rein et le foie chez la souris. Lors d’une étude de 2 ans au cours de laquelle on a fait inhaler du chlorure de méthyle à des souris, on a constaté qu’à la concentration de 103 mg/m3 (50 ppm), les nerfs rachidiens lombaires présentaient un gonflement de l’axone et des signes de dégénérescence par comparaison aux animaux témoins, sans qu’on puisse toutefois mettre en évidence une relation dose-réponse. Au terme de cette étude, on a observé chez les souris des deux sexes une dégénérescence du cervelet et chez les mâles, des adénocarcinomes rénaux à la concentration de 2064 mg/m3 (1000 ppm). Ces effets n’ont pas été observés chez le rat à cette concentration. On n’a guère trouvé de données sur la toxicité à court terme du chlorure de méthyle pour les organismes aquatiques ou terrestres. Dans le cas de la toxicité à long terme, c’est même une absence totale. Les données existantes indiquent que le composé n’a qu’une faible toxicité aiguë pour les organismes aquatiques. Chez les poissons, la plus faible valeur de la CL50qui ait été obtenue est de 270 mg/litre. Comme les dosages donnent pour les eaux de surface des concentrations de chlorure de méthyle généralement inférieures de plusieurs ordre de grandeur à celles qui produisent effectivement des effets, il est vraisemblable que ce composé ne présente pas de risque important d’intoxication aiguë pour les organismes aquatiques. On ne possède que des données très limitées concernant les effets du chlorure de méthyle sur les organismes terrestres. Le chlorure de méthyle se révèle nettement génotoxique in vitro, tant vis-à-vis des bactéries que des cellules mammaliennes. Même si les effets observés lors d’un test de létalité dominante étaient très vraisemblablement plutôt cytotoxiques que génotoxiques, on pourrait considérer le chlorure de méthyle comme très faiblement mutagène in vivo, eu égard à certains signes témoignant de la présence de pontages ADN–protéines aux doses élevées. Chez des rats soumis à une concentration de 980 mg/m3 (475 ppm), on a observé la présence de lésions testiculaires et notamment de granulomes épididymaires, puis une réduction de la vitalité des spermatozoïdes qui a conduit à une baisse de la fertilité aboutissant à une stérilité totale à plus forte dose. Le chlorure de méthyle a produit des anomalies cardiaques chez des foetus de souris dont la mère avait été exposée à une concentration de 1032 mg/m3 (500 ppm) pendant la gestation. Après inhalation accidentelle de chlorure de méthyle, les effets sont nets chez l’Homme, notamment au niveau du système nerveux central. Chez des volontaires brièvement exposés à du chlorure de méthyle, on n’a pas constaté d’effets sensibles qui puissent être attribués à ce composé. On n’a pas suffisamment de données épidémiologiques pour évaluer le risque de cancer chez l’Homme par suite d’une exposition au chlorure de méthyle. 42 Methyl chloride RESUMEN DE ORIENTACIÓN equivalente total de cloro estratosférico efectivo. Se ha determinado que el cloruro de metilo tiene un potencial de destrucción del ozono estratosférico de 0,02 en relación con el compuesto de referencia, el CFC-11, cuyo potencial es igual a 1. No parece que el cloruro de metilo contribuya de manera significativa al calentamiento mundial o a la contaminación fotoquímica del aire. La evaluación de los aspectos relativos a la salud humana de este CICAD sobre el cloruro de metilo se basó fundamentalmente en un estudio preparado por el Grupo de Expertos Nórdicos en colaboración con el Comité de Expertos Neerlandeses en Normas del Trabajo (Lundberg, 1992). Se realizó una búsqueda en las bases de datos pertinentes para el período de 1992-1999 con objeto de obtener datos adicionales. Se utilizaron como fuentes principales para los aspectos ambientales y ecotoxicológicos del cloruro de metilo BUA (1986), ATSDR (1990), WMO/OMM (1994) y HSDB (1996). La publicación ATSDR (1990) se actualizó en 1998; cuando esta versión actualizada proporcionaba información, se ha tenido en cuenta. Se obtuvieron datos adicionales sobre cuestiones ambientales en bases de datos pertinentes para el período 1989-1997. La información relativa al carácter y a la disponibilidad de los documentos originales se presenta en el apéndice 1. La información sobre el examen colegiado de este CICAD figura en el apéndice 2. Este CICAD se aprobó como evaluación internacional en una reunión de la Junta de Evaluación Final, celebrada en Estocolmo (Suecia) del 25 al 28 de mayo de 1999. La lista de participantes en esta reunión figura en el apéndice 3. La Ficha internacional de seguridad química (ICSC 0419) para el cloruro de metilo, preparada por el Programa Internacional de Seguridad de las Sustancias Químicas (IPCS, 1999), se reproduce en el presente documento. El mecanismo predominante de eliminación del cloruro de metilo en el agua y el suelo es la volatilización. La hidrólisis lenta y posiblemente la degradación biótica pueden contribuir a su desaparición en las capas más profundas del suelo y en el agua freática. Sin embargo, hay poca información sobre su biodegradación. La ruta de exposición más importante del ser humano al cloruro de metilo son las vías respiratorias. En las personas, así como en los animales de experimentación, el cloruro de metilo se absorbe con rapidez a través de los pulmones después de la inhalación. Tras la exposición a cloruro de metilo marcado con 14C, se detecta radiactividad en todo el organismo. Aunque una gran parte de la sustancia marcada se incorpora a las proteínas a través del “pool” de moléculas de un átomo de carbono, el cloruro de metilo también se puede unir a proteínas por alquilación directa. Sin embargo, si bien es un agente alquilante, lo es en un grado muy pequeño. El cloruro de metilo se metaboliza en los mamíferos por conjugación con el glutatión, y en menor proporción mediante oxidación por el citocromo P-450; la vía del glutatión produce metanotiol y ambas vías dan lugar a formaldehído y formato. Los metabolitos del cloruro de metilo se eliminan con la orina y por exhalación. También se exhala cloruro de metilo sin metabolizar. El cloruro de metilo (CAS Nº 74-87-3) se libera fundamentalmente en el aire durante su producción y uso y por la incineración de residuos municipales e industriales. Sin embargo, las fuentes naturales, en particular los océanos y la combustión de biomasa, predominan claramente sobre las fuentes antropogénicas. La emisión mundial total de cloruro de metilo de todas las fuentes se estima en alrededor de 5 × 106 toneladas al año. Se ha calculado que la contribución de las fuentes naturales es muy superior al 90%, y tal vez hasta del 99%, de la emisión total. El cloruro de metilo está presente en la troposfera en una concentración aproximada de 1,2 µg/m3 (0,6 ppmm). En las personas, hay grandes diferencias individuales en la absorción y el metabolismo del cloruro de metilo. Estas diferencias dependen de la presencia o ausencia de la enzima glutatión transferasa T1 (GSTT1), que presenta polimorfismo genético. Se distinguen en el ser humano tres fenotipos, correspondientes a una conjugación alta, baja o nula de la GSTT1. Sin embargo, como no está claro si el mayor riesgo corresponde a una conjugación alta o a una conjugación nula, hay que considerar que todos los fenotipos son sensibles a la exposición al cloruro de metilo. El principal medio de absorción del cloruro de metilo en la troposfera es la reacción química con los radicales hidroxilo y su vida en la atmósfera se estima que es de uno a tres años. Cierta cantidad de cloruro de metilo llega a la estratosfera; allí, la fotodisociación genera radicales de cloro, que contribuyen a la destrucción de la capa de ozono. Las estimaciones de la cantidad de cloruro de metilo que llega a la estratosfera y destruye pues la capa de ozono varían ampliamente. A partir de las cifras presentadas por la Organización Meteorológica Mundial (OMM), se estima que el cloruro de metilo contribuye con alrededor del 15% al La toxicidad aguda por inhalación de cloruro de metilo en ratas y ratones parece ser bastante baja, con un valor de la CL50 superior a 4128 mg/m3 (2000 ppm). No se han encontrado en la bibliografía datos indicativos de que el compuesto sea irritante o sensibilizante. Los principales órganos destinatarios tras una exposición breve por inhalación al cloruro de metilo parecen ser el sistema nervioso, con trastornos funcionales y degeneración cerebelar tanto en las ratas como en los ratones, así como los testículos, el 43 Concise International Chemical Assessment Document 28 Se encontraron pocos datos sobre la toxicidad a corto plazo del cloruro de metilo para los organismos acuáticos o terrestres. No se obtuvo ningún dato sobre la toxicidad a largo plazo. Los datos disponibles indican que el cloruro de metilo tiene una toxicidad aguda baja para los organismos acuáticos. El valor más bajo de la CL50 para los peces es de 270 mg/litro. Teniendo en cuenta que las concentraciones de cloruro de metilo que se han determinado en las aguas superficiales son generalmente varios órdenes de magnitud inferiores a las que se ha demostrado que son causantes de esos efectos, es probable que el cloruro de metilo represente un riesgo bajo de efectos agudos para los organismos acuáticos. Solamente se dispone de datos muy limitados sobre los efectos del cloruro de metilo en los organismos terrestres. epidídimo y los riñones en las ratas y los riñones y el hígado en los ratones. En un estudio por inhalación de dos años con ratones se observaron inflamación y degeneración axonal de los nervios de la médula espinal lumbar con 103 mg/m3 (50 ppm) en los animales expuestos en comparación con los testigos, pero sin una relación dosis-respuesta aparente. Al final del estudio se detectaron degeneración cerebelar en los ratones de ambos sexos y adenocarcinomas renales en los ratones machos con 2064 mg/m3 (1000 ppm). Estos efectos no se observaron en las ratas con 2064 mg/m3 (1000 ppm). El cloruro de metilo es claramente genotóxico en sistemas in vitro, tanto en bacterias como en células de mamífero. Aunque los efectos positivos observados en una prueba de dominancia letal fueron con toda probabilidad citotóxicos más que genotóxicos, el cloruro de metilo podría considerarse un mutágeno muy débil in vivo sobre la base de algunos signos de entrecruzamiento proteína-ADN a dosis más altas. Las lesiones testiculares y los granulomas epididimales seguidos de una disminución de la calidad del esperma dieron lugar a una reducción de la fecundidad en las ratas con 980 mg/m3 (475 ppm) y a su pérdida completa a dosis superiores. El cloruro de metilo indujo afecciones cardíacas en fetos de ratones cuyas madres estuvieron expuestas a 1032 mg/m3 (500 ppm) durante el período de gestación. Se pueden observar claramente efectos en las personas, especialmente en el sistema nervioso central, tras la exposición accidental por inhalación. En la exposición breve de voluntarios al cloruro de metilo no se observaron efectos significativos que pudieran atribuirse a ella. Hay pocos datos epidemiológicos que permitan evaluar el riesgo de aparición de cáncer para las personas como resultado de la exposición al cloruro de metilo. En conclusión, el efecto final crítico para la toxicidad por inhalación de cloruro de metilo en las personas parece ser la neurotoxicidad. Se obtuvieron valores guía de 0,018 mg/m3 (0,009 ppm) para la exposición indirecta a través del medio ambiente y de 1,0 mg/m3 (0,5 ppm) para el entorno de trabajo. Aunque las lesiones nerviosas se observaron a niveles de exposición inferiores a los que provocaron la pérdida de fecundidad en las ratas (980 mg/m3 [475 ppm]) y los tumores renales en los ratones macho (2064 mg/m3 [1000 ppm]), en la caracterización del riesgo cualitativo del cloruro de metilo se debe hacer hincapié también en esos efectos muy graves. 44 THE CONCISE INTERNATIONAL CHEMICAL ASSESSMENT DOCUMENT SERIES Azodicarbonamide (No. 16, 1999) Benzoic acid and sodium benzoate (No. 26, 2000) Benzyl butyl phthalate (No. 17, 1999) Biphenyl (No. 6, 1999) 2-Butoxyethanol (No. 10, 1998) Chloral hydrate (No. 25, 2000) Crystalline silica, Quartz (No. 24, 2000) Cumene (No. 18, 1999) 1,2-Diaminoethane (No. 15, 1999) 3,3'-Dichlorobenzidine (No. 2, 1998) 1,2-Dichloroethane (No. 1, 1998) 2,2-Dichloro-1,1,1-trifluoroethane (HCFC-123) (No. 23, 2000) Diphenylmethane diisocyanate (MDI) (No. 27, 2000) Ethylenediamine (No. 15, 1999) Ethylene glycol: environmental aspects (No. 22, 2000) 2-Furaldehyde (No. 21, 2000) HCFC-123 (No. 23, 2000) Limonene (No. 5, 1998) Manganese and its compounds (No. 12, 1999) Methyl methacrylate (No. 4, 1998) Mononitrophenols (No. 20, 2000) Phenylhydrazine (No. 19, 2000) N-Phenyl-1-naphthylamine (No. 9, 1998) 1,1,2,2-Tetrachloroethane (No. 3, 1998) 1,1,1,2-Tetrafluoroethane (No. 11, 1998) o-Toluidine (No. 7, 1998) Tributyltin oxide (No. 14, 1999) Triglycidyl isocyanurate (No. 8, 1998) Triphenyltin compounds (No. 13, 1999) To order further copies of monographs in this series, please contact Marketing and Dissemination, World Health Organization, 1211 Geneva 27, Switzerland (Fax No.: 41-22-7914857; E-mail: bookorders@who.int)