ScienceDirect Aquaculture
advertisement

V U / J (v z w )
n ,? „ S ^STITUUT VOOR d e ZE£
^-ANDERS MARINE INSTITUTE
Oostencifi Belgium
A vailable o n lin e a t w w w .sc ien ce d irec t.c o m
ScienceDirect
ELSEVIER
A q u aculture 2 6 8 (2007) 156
Aquaculture
168
w w w .elsevier.com /locate/aqua-online
Deformities in larval gilthead sea bream (Sparus aurata): A qualitative
and quantitative analysis using geometric morphometries
Y ves V e r h a e g e n a, D o m in iqu e A d r i a e n s 3'*, Tania De W o l f b,
P hilippe D h c r t c, Patrick S orgelo os d
a E v o lu tio n a ry M o rp h o lo g y o f Vertebrates, G hent U niversity K .L L e d e g a n ck stra a t 35, B -9000 Gent, Belgium
b M a ric o ltu ra iii R o sig n a n a Solvay, srl, Via P. G igli (L oe LiUalro), 1-57013 R o sig n a n a S o lv a y Italy
0 ¡N V E Technologies, NV, O everstraat 7, B -9 2 0 0 B aasrode. B elgium
d L a b o ra to ry o f A q u a cu ltu re a n d A rte m ia R eference Center, G hent U niversity, R o zie r 44, B -9 0 0 0 Gent, Belgium
A bstract
D eform ities in com m ercially raised fish are a com m on source o f dow ngrading o f product value. D uring the intensive rearing o f
gilthead sea bream (Sparus a urata ), opercular deform ities are the m ost com m only observed type o f deform ation (affecting up to
80% o f the fisheries stock), som etim es show ing a severe inward folding o f the operculum . T h ey are non-lcthal m alform ations that
appear during the larval stage but affect grow th rate and m orphology, w ith a significant econom ic loss as a consequence. In o rder to
exploratory q uantity and qualify these deform ities, geom etric m orphom ctric analyses w ere perform ed on the external m orphology
from larvae w ith an age ranging from 50 to 69 days post-hatching (D PH ), and on the cranial skeleton o f 110 D PH old juveniles.
T he results show ed several osteologieal cranial shifts and a striking left right independency associated w ith deoperculation. Even
though a significant size difference w as observed at 65 DPH betw een norm al and deoperculated specim ens, allom etrics during the
exam ined grow th stages still appear to be very sim ilar in norm al and deoperculated specim ens. A t 69 D P H deoperculated
specim ens differed significantly from the norm al specim ens in th eir external m orphology based on its shape variables, but the
results suggest that discrim ination is possible from earlier stages. Further analyses are needed, but the usefulness o f this approach
tow ards developing an early detection tool could be dem onstrated.
C'12007 E lsevier B.V. All rights reserved.
K eyw ords: S p a ru s a u ra ta ; O p e rc u lar c o m p lex ; A b n o rm ality; G eom etric m o rp h o m etries; O ntogeny
1. In trod u ctio n
Gilthead sea bream (Sparus aurata), being one o f the
first intensively cultivated species in the Mediterranean
(Fishery Information. D ata and Statistics Unit, Food and
Agriculture O rganization o f the United N ations (FAQ),
2005), has becom e an important research topic over the
* C o rresp o n d in g author. T el.: +32 9 2 6 4 52 19; fax: +32 9 264 53 44.
E -m a il add ress: dom inique.ntlrinen.su! u g en l.b e (D. A driaens).
0 0 4 4 -8 4 8 6 /$ - sec fro n t m a tte r 'O' 2 007 E lse v ie r B.V. All rights reserved,
cio i : 10 . 10 16/j. aq u ac u ltu re.2 0 0 7 .0 4 .0 3 7
years (Koum oundouros cl al., 1997a). The focus has
m ainly been on aquacultural aspects, and the implemen­
tation o f techniques to improve reproductive success,
survival and growth (Tandler and Helps, 1985; Polo et al.,
1991; M ourentc ct al., 1993; Parra and Yufcra, 2000;
Papandroulakis ct al., 2002; Sadek ct al., 2004). As a
m axim al yield in growth and reproductive success may
com e into reach, problems arise w ith respect to the overall
quality (especially deformations) o f the fry and subse­
quent juvenile fish. A lthough som e studies on larval
quality have been performed in the past (e.g. Paperna,
Y. Verhaegen et al. /'A q u a c u ltu re 2 6 8 (2007) 156 168
1978; K oum oundouros ct al., 1995; Furuita ct al., 2000;
Boglione et al., 2001; Cahu et al., 2003), future research
efforts may need to concentrate even m ore on a qualitative
rather than quantitative im provem ent o f the produced fish,
especially in order to expose the nature behind devel­
opm ental and growth problem s frequently experienced
in cultivating facilities. Som e exam ples o f com mon
abnormalities in cultivated fish are axial deformities,
whether o r not related to sw im bladder abnormalities (such
as saddle-back syndrome and axial lordosis) (e.g. Alfonso
et al., 2000, Boglione et al., 2001), abnormal caudal fin
developm ent (e.g. K oum oundouros et al., 1997b) and
opercular deformations. Especially the distorted develop­
m ent o f the operculum in S. aurata, as well as in some
other cultivated species, have been a source o f product
dow ngrading and substantial econom ic losses over many
years (affecting up to 80% o f farm ed gilthead sea bream)
(Paperna, 1978; Francescoi! c t al., 1988; Chatain, 1994;
A ndrades et a l , 1996).
In order to im prove cost-efficiency for fanned
m arket-sized gilthead sea bream , a fast and early re­
cognition o f developing abnorm alities is o f utm ost
im portance for fish fanners, as w ell as to unravel the
effect o f rearing param eters in order to m inim ize
the prevalence o f these deform ities a priori. As a re­
sult, research efforts have been focusing on biometric
screening o f larvae for such defoim ities (K oum oun­
douros et ah, 1997a). H ow ever, studying the pattern
behind deform ities and related growth disturbances is
frequently based on biom etric analysis, relying on pointto-point m easurem ents o f several structures and parts o f
the body plan (K oum oundouros et al., 1997a; Lindesjöo
et al., 1994). Such an approach is useful for studying size
changes, thus growth, but is insufficient in describing
aspects o f shape adequately (especially size-independent
shape, even w hen using ratios) (see R ichtsm eier et al.,
2002; D odson, 1978), thus possibly problem atic when
studying deform ities. F or that reason, geom etric m or­
phom etries allow a m ore com plete description o f shape
and thus a m ore robust and detailed analysis o f shape
variation in growing and transform ing fishes (R o h lf and
M arcus, 1993; A dam s et al., 2004). T his approach has
proven to be useful for studying shape changes o f
com m ercially im portant fishes in the past (e.g. C aval­
canti et ah, 1999; K oum oundouros et ah, 2005; Loy
et ah, 1998; 2000; 2001 ; Valentin et ah, 2002), including
S. aurata (Loy et ah, 1999). All these shape analyses
focused on populations in the w ild, or the effect o f
rearing conditions on the overall body shape in adult
specim ens.
We perform ed a landm ark-based geom etric m orphom etric analysis on both external and internal (skeletal)
157
m orphology o f differen t age groups o f S. aurata. The
specific goals o f this exploratory study are; ( 1) to analyze
quantitatively and qualitatively the nature o f osteological
aberrations in the skull associated w ith deoperculation in
110 day old juveniles, and (2) to verify to w hat degree
shape changes in the external m orphology (total body
shape) co-occur with opercular deform ities betw een 50
and 69 days post-hatching. Such an approach will assist
in future efforts to allo w a fast recognition protocol for
detecting early opercular deform ation in cultivated
gilthead sea bream.
2. M a te ria l an d m eth o d s
2.1. R earing conditions
All specim ens originate from the commercial sea
bream hatchery M aricoltura di Rosignano Solvay (Italy).
Eggs w ere obtained from a mixed broodstock o f reared
and locally wild caught adults. The broodstock was fed a
mixed diet o f Lansy Breed*’ (IN V E A quaculture NV,
Belgium ) pellets and fresh seafood (shrimps, mussels,
crabs and fish). Spaw ning occurred under regulated light
and temperature conditions in 2800 1 tanks. The collected
eggs hatched in a 140 1 incubation tank approximately
48 h after spaw ning and w ere transferred indoors to 60001
larval rearing tanks after 24 h. Initial rearing density was
approxim ately 150 larvae/1. Larvae were raised under a
sem i-closed circuit o f filtered natural seawater (39%
salinity) originating from the nearby coast. Temperatures
and dissolved oxygen concentrations w ere continuously
kept closely to 19 °C and between 6 - 1 2 ppm , respec­
tively. The following light intensity regim e w ere applied:
0 -1 D PH at 80 lx. 2 -3 D PH at 250, 4 - 1 3 DPH at 400,
1 4 -3 9 D PH at 250 lx and subsequent w eaning at natural
shaded sunlight (4000 lx). High aeration during 0 -1 DPH
kept passively moving larvae suspended, with aeration at
low level during green w ater phase (sufficient to distribute
algae over w ater column). After this phase passive
aeration induced by w ater flow was sufficient. The
creation o f w ater currents was minimized through the
vertical position o f the w ater inlet ju st above the water
surface to allow a natural swim m ing behaviour o f the
developing fish. A s from 48 DPH, the post-metam orphic
phenom enon o f schooling was prevented by placing a
vertically submerged net in the circular tanks.
Surface skimmers w ere used to inhibit lipid film
formation on the water surface (Chatain and OunaisGuschemann, 1990). The ‘green w ater’ technique was
applied d u rin g the p eriod 3 - 2 8 D PH , u sing two
phytoplankton strains (Nannochloropsis sp. and Isochry­
sis sp.). The larvae were fed live feed starting on day three
158
Verhaegen e t al. / A qua cu ltu re 268 (2007) 156 168
(successively w ith Brachionus sp. B io type Caym an,
INV E Artemia AF strain and l’N VE Artem ia EG strain)
followed by INVE Proton 1, Proton 2/3 and Proton 2/4
artificial diets. After 48 DPH the batch was separated in
two fractions, using a grader with 1.5 mm mesh-size, and
then transferred separately to 10,000 1 w eaning tanks.
2.2. Sam ples
For the osteological analysis, eleven norm al and ten
deoperculated specimens were sampled from a batch o f
110 D PH old juveniles on the 11th o f M arch 2004. We will
apply the tenu “deoperculation” for any kind o f deforma­
tion where the operculum shows microscopically visible
signs o f opercular reduction or shape distortions. For the
external morphology, a total o f 433 specim ens were
sampled at seven time intervals (41, 45, 50, 55, 61, 65 and
69 DPH) during the period December 6th 2004 till January
3rd 2005, each sample consisting o f 3 1 -1 2 0 specimens and
representing a total standard length (Ls ) range o f 7 .2 22.0 mm. Furthenuore, five wild caught larvae (1 7 .3 18.0 m m Ls ) were obtained from a local fisherman.
All sampled specimens were anaesthetized using an
overdose o f M S-222 (>100 ppm) and w ere fixed and
stocked separately in a 4% N aH 2P 0 4 - N a 2HP 0 4 buffered
formalin.
Aw hole-m ount clearing and staining o f the 21 juveniles,
sampled for the osteological analysis o f the skull and
pectoral girdle, was carried out using the m ethods o f
Hanken and Wasscrsug (1981) and Taylor and Van Dyke
(1985). Both lateral views o f all specimens w ere photo­
graphed using a Colorview 8 digital camera m ounted on an
Olympus SXZ-9 stereoscopic microscope, with analysis
Docu 3.00 (Soft Imaging System GmbH) software. Before
being photographed, specim ens w ere positioned as
perfectly lateral as possible using the overlap o f paired
anatomical structures as an important guidance, in order to
m inimize possible shape variation due to suboptimal
positioning. 50 o f the 433 specimens for the external
morphology analysis couldn’t be positioned adequately
because o f an abnormal flexion o f the body (natural and/or
through fixation), and were thus omitted from further
analysis. A s no reliable markers for the body midline could
be discerned on the early larval stages, the unbending
module in tpsUtil was not performed (Rohlf, 2004a). All
photographs o f the right body sides where then mirrored in
order to allow the comparison o f left and right sides in a
single analysis. Standard lengths (Ls) were measured on the
digital images using analysis Docu 3.00 (Soft Imaging
System G m bH ) software. The nomenclature used to
describe the skeletal structures is based on Faustino and
Power (2000).
2.3. Landm ark data
Landmark data w ere gathered using tpsUtil (R o h lf
2004a) to construct th e tps-files and tpsD ig for digitising
landmark positions on the digital images (Rohlf, 2004b)
(freeware at http://life.sunysb.edu/m orph). F or the two
datasets, a set o f hom ologous landmarks was digitised in
two dimensions. L andm arks chosen were so-called type 1
and type 2 landmarks, thus allow ing a reliable represen­
tation o f the actual sh ap e variation (instead o f measuring
error noise) (for a definition o f these two types, see
Bookstein, 1990). A n estim ation o f digitising and
orientation error, how ever, w as perform ed (see below).
The osteology dataset included a total o f 21 homologous
landmarks that w ere digitised bilaterally on the 21
specimens, thus yielding 42 landm ark configurations to
be analysed (Fig. 1). Landm arks were chosen in order to
have a good representation o f the overall skull shape with
respect to the vertebral column, but especially w ith a
detailed representation o f the opercular region. Following
landmarks w ere used: (1) the anteroventral tip o f the
premaxillary bone (type 2), (2) the caudal tip o f the
premaxillary bone (type 2), (3) the anterior tip o f the
dcntary bone (type 2), (4) the m ediodorsal suture between
nasal and frontal bone (type 1), (5) the dorsal point o f
contact between infraorbital bones three and four (type 1),
(6) the anterodorsal tip o f the sphenotic (type 1), (7) the
postcrodorsal tip o f the supraoccipital bone (type 2), (8)
the posterior tip o f the post-tem poral bone (type 2), (9) the
postcrodorsal tip o f the preopcrcular bone (type 2), (10)
the posterodorsal tip o f the opercular bone (type 2), (11)
the caudal tip o f the opercular bone (type 2), (12) the
ventral tip o f the opercular bone (type 2), (13) the
posterior tip o f the subopercular bone (type 2), (14) the
ventral end o f the contact zone between subopercular and
interopercular bone (type 1), (15) the anterior tip o f the
interopercular bone (type 2), (16) the overlap o f the
subopercular-interopercular bone suture w ith the poste­
rior margin o f the preopcrcular bone (type 1), (17) the
anterior tip o f the preopcrcular bone (type 2), (18) the
ventral tip o f the cleithrum (type 2), (19) the caudal tip o f
the cleithrum (type 2), (20) the anterior tip o f the neural
arch o f the third vertebra (type 2), (21) the anterior tip o f
the neural arch o f the fifth vertebra (type 2).
T he external m orphology d ataset shape m odel relied
on a total o f 16 landm arks (Fig. 2). Specim ens sm aller
than 11.4 mm L s (all specim ens y o u n g er th an 50 D PH
and three specim ens aged 50 D PH ) w ere further
om itted from the geom etric m orphom etric analysis
b e c a u se o f a lack o f la n d m a rk s th a t co u ld be
hom ologised on these specim ens. O f the 310 sp eci­
m ens left and right sides w ere digitised and analysed
Y. Verhacgen e t al. / A q u a c u ltu re 2 6 8 (2007) 156 168
separately (as m ost landm arks in both view s w ere
identical as they w ere positioned in the m edial plane),
yielding tw o d atasets (left and right). A gain, landm arks
w ere o f ty p e 1 and 2, w ith the exception o f one
(landm ark 15). L andm arks w ere chosen to represent
the overall body shape, as w ell as the position o f the
p ectoral fin, the back o f the skull and the eye. As
specim ens w ere categorised as norm al or deopercu­
lated based on visib le abnorm alities in the opercular
region, the shape m odel o f the external m orphology did
not include landm arks in the opercular region, thus
allow ing an independent analysis o f shape changes co ­
occurring w ith deoperculation. F ollow ing landm arks
w ere used: (1) the anterodorsal tip o f the prem axillary
(type 1), (2) the p o ste rio r b order o f the skull (type 1),
(3) the base o f th e first spine o f the dorsal fin (type 2),
(4) the b ase o f the first so ft ray o f the dorsal fin (type 2),
(5) the b ase o f th e last soft ray o f the dorsal fin (type 2),
(6) th e dorsal onset o f the caudal fin (type 1), (7) the
m idlateral p o ste rio r ed g e o f the peduncle (type 2), (8)
the ventral onset o f th e caudal fin (type 1), (9) the base
o f the last ray o f the anal fin (type 2), (10) the b ase o f
the first ray o f the anal fin (type 2), (11) the b ase o f the
pelvic fin (type 1), (12) the posteroventral tip o f the
mandibular (type 1), ( 13) the base o f the last (upper) ray o f
the pectoral fin (type 2), (14) the base o f the first (lower)
159
ray o f the pectoral fin (ty p e 2), (15) the centre o f the eye
(type 3), (16) the posterior tip o f the skull (type 1).
2.4. M orphom etries
In order to rem ove all variation in size, rotation and
position (with respect to the im age fram e), a standardisa­
tion was done for a ll three param eters. F or that, a
G eneralised Procrustes A nalysis (GPA) was perform ed
w here all landmark configurations w ere scaled to the
sam e centroid size (CS = 1), aligned w ith centroids being
su p erim p o sed and ro ta te d until a m axim al fit o f
hom ologous landm arks (R ohlf and Slice, 1990). This
yields a dataset reflecting only differences in trae shape.
Shape differences w ere calculated between all specimens,
as w ell as between all specim ens and a consensus {i.e. an
average landmark configuration o f all specim ens in the
dataset). The fitness o f the dataset for further statistical
analysis w as tested u sing tpsSmall (Rohlf, 2003), so that
the distribution o f shape distances in the non-Euclidean
K endall shape space {i.e. Procrustes distances) between
specim ens is properly represented in the Euclidean
tangent space {i.e. tangent distances) (for a discussion
on these shape spaces, see Rohlf, 1996). All datasets
proved to be fit for analysis (correlation betw een
Procrustes distances and tangent distances w as 1.0000).
ptm
\H ?
.
F ig. 1. D raw in g o f th e cranial osteo lo g y and the landm arks applied, an, angular; art, articular; br, branchiostega! rays; cl, cleithrum ; cor, coracoid; dn,
dentary; ept, ectopterygoid; ep , epiotic; fr, frontal; lini, hyom andibular; ino 2- 6, infraorbital tw o to six; iop, interopercular; la, lacrim al; m p, m etapterygoid;
n ix , m axilla; na, nasal; op, opercular; pa, parietal; pfr, prefrontal; pi. palatine; pm x. prem axilla; pop, preopcrcular; psp , parasphenoid; pt, pterotic; ptm , post­
tem poral; q u , quadrate; sei, supracleithrum ; soc, supraoccipital; sop, subopercular; sp, sphenolic; sy, symplectic.
160
Y. Verhangen et al. / A qua cu ltu re 2 6 8 (2007) 156 -168
Fig. 2. D ra w in g o f the external m o rp h o lo g y o f a large 50 D PH old sp ec im e n (13.9 m m L s ) and th e lan d m ark s applied.
2.5. E rror analysis
Since both larval and juvenile specimens were laterally
flattened, measuring error as a result o f a reduction in
dimensions was minimal (for a discussion on measuring
errors in geometric morphometries, see A mqvist and
Martcnsson, 1998). A separate error analysis was done to
estimate the am ount o f error induced by the action o f
digitising, as well as that as a result o f variation in
positioning the specim ens for imaging. A n estimate o f
digitising error was done by digitising ten times the same
image from a single specimen o f the osteological data (all
specimens had a com parable size) and from two specimens
(the largest and smallest) from the external morphology
data. The estimate o f artificial shape variation as a result o f
variation in the positioning o f the specimens was done by
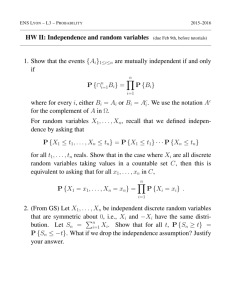
Fig. 3. External m o rphology o f the head region, show ing the on set o f deoperculation. A. Early opercular deform ation in a 40 D PH , 8.5 m m L$ post-larva;
B. severe stage o f opercular d eform ation in a 69 D PH , 19.5 m m ¿ s ju venile; C. aberrant type o f opercular deform ity in a 55 D PH , 14.7 m m Ls ju v e n ile ; D.
aberrant type o f opercular d efo rm ity in a 69 D P H 18.4 m m Ls juvenile. B lack lines indicate the caudal edge o f th e operculum and branchiostega! rays,
w h ite lines m ark the caudal e d g e o f the gili cavity. T he crest o f an abnorm ally light folding is indicated by a d a sh e d black line in Fig. 3C.
Y. Verhaegen e t al. / A qua cu ltu re 2 6 8 (2007) 156 168
■ C alculi n Bi. d e o p e rc . ■ R ig h t d e o p e rc . C i L eft d e o p e rc .
40%
35%
30%
25%
20%
15%
10%
5%
0%
41
45
50
55
61
65
69 DPH
F ig. 4. P rev alen ce o f u rinary calculi and o p e rc u la r d efo rm ities, for all
s tu d ied ag e classes.
using these three specim ens, but w hich w ere now
positioned and photographed ten times repeatedly.
The average Procrustes distances o f these datasets were
then expressed as percentage o f these distances o f the total
dataset, revealing an estimate o f the am ount o f shape
variation w hich can be attributed to digitising and
positioning errors.
2.6. Statistical analyses
Variables describing shape variation (i.e. partial
warps) in the datasets, based on the 2D -coordinates,
w ere calculated using tpsR elw (Rohlf, 2005). For the
161
statistical analysis, b o th uniform and non-uniform
partial warps w ere in cluded. The obtained m atrices o f
partial warp scores o f a ll specim ens (w eight matrix) in
both datasets w ere su bsequently subm itted to a Principal
Com ponent Analysis (also called a Relative W aip analysis
when using partial w aip scores) (Rohlf, 1993). By doing
so, the total shape variation was decomposed into a new set
o f variables (i.e. relative warps) that describe the most
important variation in th e datasets. This was also done
using tpsRelw, which w as also used to generate defor­
mation grids to visualise the explained shape variation
(with respect to the consensus configuration). Scaling was
set equal (cx=0) for local and diffuse non-uniform shape
changes (R o h lf 1993; R o h lf ct al., 1996).
For the external m o rp h o lo g y dataset, a discrim inant
function analysis (DFA ) w as perform ed on the shape
v aria b les (u sin g th e w e ig h t m a trix ) to te s t the
significance o f existing differences betw een norm al
and deoperculated specim ens fo r the different length
and age classes (S tatistica 6, StatSoft Inc.). A s m en­
tioned, a priori discrim ination betw een norm al and
deoperculated sp ecim ens w as perform ed by a m icro­
scopical analysis o f th e opercula. B oth canonical
sco res, as w ell as re le v a n t sta tistica l p aram eters
(Squared M ahalanobis d istance, F -v alu e and /7-levels)
w ere calculated usin g S tatistica 6.0 (StatSoft, Inc.). For
all statistical analyses,/7-levels sm aller than 0.05 were
considered as significant. T he shape changes asso­
ciated w ith the canonical variâtes w ere visualised using
tpsR egr (R ohlf, 2004c), including a random isation test
(with 1000 perm utations).
A s centroid size correlated highly w ith the m easured
standard lengths (R2> 0 .9 8 ,) we preferred the latter as
size param eter, because it can be m ore easily obtained
during the daily m onitoring o f stocks in fish farms.
Table 1
Percent o f variation explained b y th e first ten relative w arps o f the
osteological and bo th external m o rp h o lo g y datasets
RW
^
*
#
*•*
*-
F ig .
5. C onsensus configuration o f the osteological dataset, w ith
differences in landmark position o f aligned specim ens indicated as vectors.
% o f variation explained
O steology
L e ft external
R ig h t external
1
68.23%
5 1 .5 2 %
54.00%
2
6.66%
10.42%
3
5.56%
7.79%
9.42%
7.02%
4
3.46%
5
4 .4 5 %
3.67%
6
7
2.63%
2.06%
1.70%
8
1.54%
2.23%
2.53%
2.22%
9
10
1.39%
1.76%
1.90%
0.95%
1.69%
1.55%
3.29%
2.74%
4.42%
3.25%
2.98%
162
Y. Verhaegen e t al. / A q u a c u ltu re 2 6 8 (2007) 156 168
O Norm al
6
■ D eo p ercu lated
o Normal s i d e o f u ní-deoperculated
RW2 (6.66%)
RW1 (68.23%)
Fig. 6. S ca tte r p lo t o f relativ e w a rp I versus relative w a rp 2 scores, sh o w in g a sep a ra te c lustering o f the n o rm a l (diam onds) and abnorm al sp ecim ens
(filled sq u ares). T h e norm al sid es o f the skull o f u n id e o p e rc u late d fishes clearly fall into the norm al cluster (e m p ty squares). D otted lines interconnect
n o rm al and ab n o rm al sid es o f u n ilaterally d eo p ercu lated specim ens. S hape va ria tio n ex p lain ed by the re la tiv e w arps is v isu alise d by the deform ation
g rid s (sh o w in g the sh ap e d ifferen ces w ith respect to th e c o nsensus).
2.7. Calculation o f inflection points
proposed by Van Snik el al. (1997): the data w as logtransform ed, regression lines were calculated for .rmin
Until - T in tc rm e d ia to i and foi - ^ i n te r m e d i a te IO Tmax, w f l C l C
^ in t e r m e d ia t e was a variable iteratively ranging from.\-min+2
to xmax_ 2. Thesis were done to check w hether the group
In order to objectively calculate the m ost significant
shifts in shape changes (expressed as relative w arp 1)
versus size (log L f), inflection points were calculated as
O N o rm al
b
D e o p e r c u la te d
RW2(9.42%)
"
_
■
"
<4
%
^
o io
o
o
o o ^
m
o
-*
,o l %f o
o O
RW1 (54.00%)
-6
-4
-2
0
Fig. 7. S c a tte r plot o f relativ e w a rp I versus relativ e w a ip 2 sco res o f the analysis o f the right b o d y side, show ing the overlap o f th e norm al (diam onds)
and abnorm al sp ec im e n s (squares). S h ap e variation ex p lained b y th e relative w arps is v isualised b y the deform ation grids (sh o w in g shape difference
w ith resp ect to c o n se n su s, m irrored).
Y Verhaegen e t al. / A q u a c u ltu re 2 6 8 (2007) 1 5 6 168
O
N o rm al
h
D e o p e rc u la te d ------
163
W ild c a u g h t
a
RW1
y = 2.13x - 2.47
R2 = 0.3471
à
y = 3.76X - 4.44
R2 s 0.5847
<X>_B
Â
*
£
'
0
- o o
O.
y = 2.06X - 2.40
R2 = 0.291
y = 3.81 X - 4 .5 4
R = 0.5919
17.34 mm Ls
Fig. 8. Scatterplot o f R W l versus bod y size (log-transform ed Ls) o f the analysis o f the rig h t b o d y side, w ith the indication o f the calculated inflection point.
o f growth coefficients for x min to ^intermediate signifi­
cantly differs from the group o f growth coefficients for
■ ^ interm ediate ^ 0 •'■max- T h e ^ in t e r m e d ia t e V a l u e t h a t s h o w s t h e
largest significant f-stat is defined as the inflection point.
3 . R esults
3.1. Size and deoperculation
O percular deform ities w ere already visually detec­
table in the sm allest and youngest specim ens (8.0 mm
Ls , 40 D PH ) as a sm all notch dorsally in the free
opercular edge (Fig. 3A show s an 8.5 m m L s, 40 DPH
specim en). D uring this phase the operculum is still
largely unossified and a large posteroventral part is
resting against the pectoral girdle. As the post-larvae
grow the deform ity expands ventrally and ultimately
also affects the branchiostegal rays, with a bending o f
the operculum into the gili cavity as a result. Only four
aberrant opercular abnorm alities w ere detected after
metam orphosis: a single specim en show ed unilateral
inwardly folded branchiostegal rays w hile the operculum
did not show the expected associated deformity (Fig. 3C).
The operculum , however, showed an abnormal dorsoventral ridge, which probably is a consequence o f a
previous inward folding. In three post-m etam orphic
specim ens only the dorsocaudal tips o f the opercula
were severely folded inw ard and deformed (Fig. 3D).
The m ortality rate during the studied rearing period
w as negligible (0.03%). The prevalence o f opercular
deformities seem s to decrease during metamorphosis
(1 1 .0 -2 2 .0 T L (Total length), as mentioned by K ou­
m ound o u ro s et ah, 1997a) and to increase again
afterw ards (Fig. 4). The presence o f urinary calculi in
the gall bladder, which is a typical symptom o f stress,
show ed the sam e pattern (although not directly correlated,
w ith opercular deformities, R 2 = 0.067) (Fig. 4). O f the
383 specim ens screened 28.5% showed an opercular
deformity, o f w hich 18.3% bilateral. In both datasets there
w ere m ore left than right dcoperculations (osteological
T ab le 2
S tatistics o f th e d iscrim in a n t fu n ctio n a n aly se s o f th e five age classes
L eft
A n aly sis g roup
W ilk s’ lam bda
r
R ight
5 0 DPH
55 D PH
60 DPH
65 DPH
69 D PH
50 D PH
55 DPI!
6 0 D PH
65 DPH
0.510
2 6.270
0.123
0.660
3 4.099
0.658
0.577
0.711
0.513
3 0.520
21.418
0.073
49.606
0.124
3 9 .7 9 7
0.428
14.437
35.525
28.009
4 8.777
28
0 .0 6 9
28
28
28
28
0.984
0.198
0 .339
0.808
28
0 .007
28
0.155
28
0.464
8.005
3.439
2.570
3.690
55.972
4 2 .377
2.716
0.178
28, 4
0.998
1.235
1.100
0.666
2.665
28, 69
28. 60
0 .369
28, 26
0.853
28, 6
0.945
2 8 ,4
0.111
0.605
28
df
p -level
0.558
M ah alan o b is D 2
4 .8 4 6
F-v alu e
0.875
3 1.610
1.505
df
p-le v e i
2 8 , 26
0 .6 3 7
28, 6
0.321
0.237
6 9 DPH
28
0 .0 0 9
4.706
0.975
2.015
28, 69
0.514
28, 60
0.012
164
Y. Verhaegen et al. / A q u a c u ltu re 2 6 8 (20)07) 156 168
dataset: » = 21, o f which 33.3% left and 23.8% right
deoperculated, external m orphology dataset: » = 383, o f
w hich 20.2% left and 13.6% right deoperculated); but only
in the external morphology dataset this difference was
significant (Fisher’s exact test two-tailed ¿>-value=0.046).
O nly in the largest age class (65 D PH ) a small significant
difference in average length could be observed between
normal and deoperculated specim ens. (Ls normal= 17.2±
1.8 mm, Ls d c< > pcrc-= 16.4 ± 1.1 m m , F n 9j 7) = 2.944,
;; = 0.0074, r (43) = 2.58, ¿>= 0.013). C ontradictoiy to
these results, the deoperculated specim ens in the
osteology dataset (juveniles o f 110 D PH ) w ere bigger
(normal 24.7±1.1 m m Ls ; deoperculated 2 5 .3 ± 0 .9 mm
Ls), but this difference w as not significant (7(iç» =
-0 .7 8 7 3 8 ,/;= 0.220386).
3.2. Skeletal shape a n d deoperculation
T he analysis o f the osteological dataset yielded two
uniform and 36 non-uniform partial w arps that were
included in the analyses. The consensus configuration,
w ith aligned specim ens superim posed (thus show ing
o Normal
cv
D eoperculated
□
O O
o
o
LogLs
1.1
1.05
4
o Norm al
CV
n D eoperculated
o
3
2
1.25
1.2
1.15
o
-
o
o
1
°o
<> O o
0-
Oo
Fig. 10. D eform ation grid v isu alise d through TP SR cgr, representing the
shape difference betw een a lo w ( - 3 ,1 7 ) and high (3,35) C V -score o f the
69 D PH sam ple. T h e re g re ssio n o f the shape variables o n the CV -scores
w as subjected to a p erm u tatio n test (percent unexplained 97.90% , 0.10%
Lam bda values-< = 0 .0 0 0 0 0 0 0 3 , 9.90% G oodall F ’- v a lu c s > - 1.864).
variation in landm ark positions) show ed a differential
variation in landm ark position, with the largest variation
in the two landm arks at the posterior border o f the
opercular bone (Fig. 5). An average o f 6.78% o f the total
variation in the d ataset could be attributed to both
digitisation and orientation error. The largest digitisation
and orientation error w ere situated at landmark 12
(11.82% o f total digitisation error) and landm ark 18
(12.33% o f total orientation error) respectively. The
percentages o f variation explained by the relative w aips
(38 in total) are given in Table 1. T he first relative warp,
w hich explained 68.23% o f the total shape variation in
the dataset, clearly separated the deoperculated and
normal configurations, w hile the other relative warps
did not (Fig. 6). R elative w aip 1 thus largely represents
the shape changes in th e head region associated with
deoperculation. B esides the expected forward shift o f the
caudal m argin o f the opercular and subopercular bone (as
observed externally), oth er m ore subtle shape differ­
ences can be observed. T hese include an upw ard shift o f
the interopercle, angular, quadrate, the infraorbitals and
the preopercular. The preorbital region, thus the snout,
also shortens w ith respect to the other landm arks. M ost
striking o f this analysis, how ever, is the observation that
in unilaterally deoperculated specim ens, the normal
sides show a shape that corresponds perfectly with
bilaterally norm al specim ens, w hereas the deoperculated
sides show ed all the typical shifts in the landm ark
configuration as in bilaterally deoperculated specimens.
A pparently, the prevalence o f the deform ation associated
w ith deoperculation is com pletely decoupled bilaterally,
thus allow ing an astonishing rig h t-left asymmetry.
-1
-2
3.3. B o d y sh a p e and deoperculation
-
-3
Log Ls
-4
1.1
1.15
1.2
1.25
1.3
1.35
1.4
Fig. 9. S catterp lo ts o f th e sig n ific a n t c an o n ical variâtes versus the
logarithm o f the S tan d ard length (right b o d y side): A. 55 D P H ; B.
69 D PII.
The shape decom position o f the external m orphology
data set generated two uniform and 26 non-uniform
partial w atps. T he consensus configuration, w ith aligned
specim ens, show ed that shape difference is spread all
over the body b ut is situated m ainly in the anterior part
(head and abdom en). In this analysis, an average o f
Y. Verhaegen e t al. / A q u a c u ltu re 2 6 8 (2007) 156 168
165
T a b le 3
S tatistics o f th e d iscrim in an t function an aly ses o f the su b sets d e riv e d from the total left and right external m o rp h o lo g y d atasets ba se d o n th e inflection
p o in ts
Left
A n aly sis
G ro u p
W ilk s’ lam bda
X~
df
p -level
M ah a la n o b is D 2
F -v alue
df
/?-level
<18.01 m m ¿ s
R ight
> 18.01 m m Ls
0.510
2 6.270
28
< 1 7 .3 4 m m Ls
> 17.34 m m Ls
0.123
39.797
0 .4 2 8
1 4 .4 3 7
0.073
4 9 .606
28
28
0 .9 8 4
0.007
28
0.558
4 .8 4 6
0.069
3 1 .610
8 .0 0 5
55.972
0.875
1.505
28, 38
0 .1 7 8
2 8 , 199
28, 54
0.321
0 .9 9 8
0.111
2 8, 215
0.637
28.18% and 17.62% o f shape variation co u ld be
attributed to digitisation error for the sm allest and
largest specim en respectively, w hereas an average o f
9.06% and 16.52% o f shape variation co u ld be
attributed to orientation errors. The largest digitisation
errors are situated at landm ark 6 (18.34% o f digitisation
error) in the sm allest and at landm ark 9 (9.74% ) in the
largest specim en. The largest orientation errors w ere
situated in landm ark 2 (21.57% o f total orientation
error) and landmark 1 (19.75% ) o f the sm allest and
largest specim en respectively.
N one o f the relative warps (28 in total) explained
shape variation that allow ed to distinguish two or m ore
groups. R elative w arp 1 (Fig. 7) accounted for 54.00%
o f the total shape variation in the right side dataset
(51.52% in the analysis o f the left body sides) and
reflects an overall heightening o f the body, m ost
extensive in the posterior head region and abdom en,
as w ell as a lengthening o f those regions. Because o f the
higher percentage o f variation explained by RW1 for the
right side data, the latter will be discussed further w here
the results o f left and right body side analyses are
identical. T he largest dorsovcntral expansion seem s to
be situated in the posterior margin o f the skull and the
cleithral region, expanding the nape (the dorsal region
betw een the end o f the skull and the beginning o f the
dorsal fin) and shifting the pectoral and pelvic fins
dow nw ards. B ecause o f the expansion o f skull and
abdom en, the tail region dim inishes relatively in size.
R elative w arp 2 (Fig. 7) explains 9.42% (10.42% in the
left analysis) o f total shape variation and is characterized
by an overall dorsoventral curving o f the body, probably
as an artefact due to the form alin-fixation.
3.4. Growth allom etries and deoperculation
T he regression analysis and ¿-testing for inflection
points show s th at scores o f RW1 o f the external
2.665
m orphology analysis fit nicely onto body size (logvalues o f Ls) with an allom etric shift at 17.34 m m Ls
(18.01 mm Ls in the left dataset) (Fig. 8). N o apparent
differences in the relation between shape and size in
norm al versus d eo p ercu lated sp ecim en s could be
observed (the inflection points, the intercepts and slopes
o f the regression lines are very similar, b ut the L 2-values
for the normal specim ens, how ever, were lower).
Five specim ens, caught locally at the coast near the
hatchery, were included in the analysis for com parison
and show ed a totally different relationship betw een
shape (RW 1) and size (L og Ls ) (Fig. 8): the wild caught
specim ens obtained the sam e RW 1-scores (thus had a
com parable shape) as reared specim ens o f a m uch
sm aller size (17.3-18.1 m m Ls versus 13.3 m m L s ),
possibly indicating an accelerated grow th-developm ent
relationship under intensive rearing conditions.
It is only in the 69 D PH age class that a significant
difference between the norm al and deoperculated cluster
was observed, after a discrim inant function analysis
based on the shape variables o f the right side (%2-test,
/z<0.01 ) (Table 2, Fig. 9B). Still, a clear separation or at
least a strong polarization o f the canonical varíate scores
o f the two clusters was observed in all age classes. As
exam ple, in the 55 DPH sam ple (Fig. 9A), the difference
w as clear in a plot o f the scores b ut not significant
(F {28,6 )= 4 .1 4 7 ,/;= 0 .0 8 7 2 , D 2 = 139.97).
The shifts in body shape associated w ith deopercula­
tion at 69 DPH are minor w ith the largest differences
involving a reduced abdom inal height (m ore specifically
at the nape) and a posterior shortening o f the low er jaw
(Fig. 10).
Finally, we separated the total datasets (« = 310) o f
left and right external m orphology in two subsets each,
based on their inflection points, b ut the discrim inant
fu n ctio n an aly ses o f th e se su b sets d id n ’t reveal
significant discriminations betw een norm al and deoper­
culated specim ens (Tabic 3).
166
Y Verliaegen e t al. .'A q u a c u ltu re 2 6 8 (2007) 1 5 6 -1 6 8
4 . D iscu ssion
Both datasets show ed the occurrence o f m ore left than
right deoperculations (osteological dataset: n = 21, o f
which 33.3% left and 23.8% right deoperculated; external
m orphology dataset: « = 383, o f w hich 20.2% left and
13.6% right deoperculated). O nly for the external
m orphology dataset this difference w as significant (Fish­
er’s exact test two-tailed value= 0.046). Earlier studies
on opercular deform ities in sea bream all concluded that
unilateral deformation w as side-independent and thus was
the result o f a fluctuating asym m etry m odel (Koum oun­
douros et al., 1997a; G aleotti et al., 2000; Beraldo et al.,
2003). O ur data on the other hand suggest a directional
asym m etry model. Fluctuating asym m etry is believed to
be a consequence o f environm ental factors that have an
effect on developm ental instability during the early life
stages (A dam s and N isw ander 1967; Barahona-Fernandes, 1982), while directional asym m etry is believed
to be an inherited factor. Existing literature on the genetic
influence are contradictory: according to Tave and
H andw erker (1994) opercular deform ities arc noninheritable in Oreochromis niloticus, while inbreeding
in Cichlasoma nigrofasciatum resulted in more opercular
deformities (W inem iller and Taylor, 1982). A basic
assum ption o f asym m etry research is that left and right
side experience identical environm ental factors. This may
not be the case under rearing conditions: intensive
schooling in circular tanks for exam ple m ay cause a
different developm ent o f the sides o f the body.
The prevalence o f higher left deoperculation numbers in
the samples studied, remains to be confirmed by additional
analysis focusing on the pattern behind the asymmetry.
The transform ations co-occurring with opercular
deform ities are not only affecting the operculum itself,
but also the surrounding cranial structures. M ajor shape
ch an g es related w ith d eo p ercu latio n seem to be
concentrated in the postcrovcntral region o f the skull,
with an anterodorsally directed shift. A dditionally, such
a shift is observed in the postorbital region and the
m andibular arch. W hether both transform ations share
the sam e causal factor (e.g. gene expression pattern), are
spatially interrelated, or even have independent causes,
rem ains to be studied.
The m ost striking observations o f the cranial deforma­
tions are that in unideoperculated specim ens the deoper­
culated side shows identical transformations, with the
sam e intensity, as both sides in bideoperculated specimens,
while the normal side has an identical morphology to that
o f normal (bioperculated) specimens. Consequently, it can
be suggested that both in unilaterally and bilaterally
deoperculated larvae, the morphogenetic pattern genera­
ting the normal and/or deform ed side on the contralateral
faces in the skull is com pletely independent (at least with
respect to deoperculation).
We could not fu lly confirm the results o f Chatain
(1 9 9 4 ) and K o u m o u n d o u ro s et al. (1 9 9 7 a), that
opercular deform ities affect larval size (or are at least
related to it). A m in o r but significant size difference
could only be observ ed in the 65 D PH sam ple, where
norm al specim ens w e re slightly larger.
T he m o st im portant ontogenetic allom etries (repre­
sen ted b y relative w arp one) o f the norm al and
deoperculated groups shared a com parable slope and
intercept, as w ell as they both experienced a clear
inflection point at 1 7 .1 -1 8 .0 m m L s . w hich is situated
at the end o f the m etam orphosis period (1 1 .0 -2 2 .0 TL)
as m entioned by K o u m o u n d o u ro s et al. (1997a).
G eom etric m orphom etries can be an im portant asset in
defining the exact dem arcations o f m etam orphic phases
(as in several invertebrates) in organism s with a less
pronounced m etam orphosis such as gilthead sea bream.
Frequently, single m orphological characters are used to
dem arcate the onset o f a specific early life history phase,
such as the form ation o f fin rays or the com pletion o f the
fin ray form ation (see for exam ple Balon, 1975). O ther
studies do involve changes in allometric variables (e.g.
K oum oundouros et al., 1995). W hether or not S. aurata
undergoes a true phase o f m etam orphosis can be argued
about. In any case, this study indicates the existence o f
m arked shifts in the pattern o f their ontogenetic shape
changes at about 17 m m L$.
K oum oundouros ct al. (1997a) described a tem porary
stasis in the presence o f opercular deform ities during
m etam orphosis. O ur results, on the contrary, even
indicate a decrease in the rate o f deoperculation during
m etam orphosis, while m ortality w as negligible during
the studied stages (0.03% ). D eoperculated specim ens
can regenerate their dam aged opercula to a great extent
during later developm ent, as described by De W o lfe i al.
(2004). Therefore it is likely that regeneration m ay also
occur during m etam orphosis. T he four aberrant opercu­
lar abnorm alities, which were only encountered after
m etam orphosis, could be the result o f an incom plete
recoveiy. T his remains to be verified, however.
C hatain (1994) m icroscopically observed the earliest
opercular deform ities at 1 2 -1 5 m m TL. We could detect
opercular deformations m icroscopically from 8.0 mm
L s and 40 DPH on, as a local shortening (or possible
inward folding) o f the dorsal opercular edge. However,
earlier m icroscopical discrim ination is very likely to be
possible, as these specim ens w ere the youngest and
sm allest stages examined. T he use o f bone-staining and
h isto lo g ical m ethods allow ed the identification o f
168
Y. Verhaegen e t al. / A quaculture 2 6 8 (2007) 156 168
F au stin o , M .. Pow er, D .M ., 2000. O steo lo g ie d ev elo p m en t o f the
v iscero cran ial sk eleto n in sea b ream : a ltern ativ e o ssification
strateg ies in teleo st fish. J. Fish B iol. 5 8 , 5 3 7 -5 7 2 .
Francescoi!, A ., Freddi. A ., B arb ara. A ., G iav en n i. R ., 1988. D aurade
S p a ru s aurata L. reproduite a rtificiellem en t e t d au ra d e sauvage.
E x p érien ces paralleles en d iv erses co n d itio n s d ’élevage. A q u acu l­
ture 7 2 , 2 7 3 -2 8 5 .
Furaita, H ., Tanaka, H ., Y am am oto, T., Shiraishi, M ., Takeuclii, T., 2000.
Effects o f n-3 H U FA levels in broodstock diet on the reproductive
p erfo n n an ce and egg and la n 'a l quality o f the Japanese flounder.
P a ralichthys olivaceus. A quaculture 187, 387 -398.
Fishery Inform ation, Data and Statistics U nit, Food and Agriculture
larviculture in th e p re s e n c e o f phytoplankton w ith long photophase.
A q u a cu ltu re 204, 4 5 - 6 3 .
P aperna, I., 1978. S w im b la d d e r and skeletal d eform ations in hatchery
bred S p a ru s aurata. J. F is h Biol. 12, 1 0 9 -1 1 4 .
Parra, G ., Yufera, M ., 2 000. F e e d in g , p h y sio lo g y and grow th responses
in first-feeding g ilth e a d s e a bream (Sp a ru s aurata L.) la n a e in
relation to p re y density. J . E xp. M ar. B iol. Ecol. 243, 1 -1 5 .
Polo, A ., Yufera, M ., Pascual, E ., 1991. Effects o f tem perature o n e g g and
larval developm ent o f S p a r u s aurata L. A quaculture 92, 3 6 7 -3 7 5 .
R ichtsm eier, J.T., D eL eon, V .B ., L ele, S .R ., 2002. T h e p ro m ise o f
geom etric m o rp h o m etries. Y earb. P hys. A nthropol. 4 5 , 6 3 91.
R ohlf. F.J., 1993. R elative w a r p analysis and an exam ple o f its appli­
O rganization o f the U nited N ations (FAO), 2005. FISHSTAT Plus:
U niversal software for fishery statistical tim e series, version 2.3. (avail­
able on-line at http:/Avww.fao.orgdI/statist/FISOFT/FISHPLUS.asp).
cation to m osquito w in g s. In: M arcus, L.F., B ello, E., G arcia-
Galeotti, M .. Beraldo, P., de D om inis, S., D ’A ngelo, L ., Ballestrazzi, R.,
R ohlf. F.J., 1996. M o rp h o m e trie space, sh ap e c om ponents and the
M usetti, R., Pizzolito, S., Pinosa, M ., 2000. A prelim inary histological
effects o f lin e a r tra n sfo rm a tio n s. In: M arcu s. L.F., C orti, M ., Loy.
and ultrastructural study o f opercular anom alies in gilthead sea bream
A ., N aylor, G .J.P., Slice, D.F.. (E ds.), A dvances in M orphom etries.
P len u m P ress, N e w Y ork, pp. 117 129.
{Sparus aurata). Fish Phys. Biochem. 22, 151
157.
H anken, J., W assersug, R „ 1981. T he visible skeleton. A new double-stain
technique reveals the native o f the ‘hard" tissues. F u n d . Photogr. 16,
2 2 -2 6 .
K o u m o u n d o u ro s. G ., K iriakos, Z., D iv a n ac h , P., K cntouri, M ., 1995.
M o rp h o m etrie relationships as criteria fo r th e ev alu atio n o f la n 'a l
q u a lity o f gilthead sea bream . A q u acu lt. Int. 3, 1 4 3 -1 4 9 .
Valdecasas, A . (E ds.), C o n trib u tio n s to M orphom etries. C.S.I.C .,
M adrid, pp. 1 31-159.
R ohlf, F.J., 2 003. T psSinall: T h in P late S p lin e Sm all V ariation (V ersion
1.20). S tate U n iversity o f N e w York at S to n y B rook, S to n y B rook,
New' Y ork.
R ohlf, F.J., 2004a. T psU til: T h in Plate S pline U tility (Version 1.33). State
U niversity o f New' York a t S to n y B rook, S tony Brook, N e w York.
K o u m o u n d o u ro s, G ., O ran, G ., D iv a n ac h , P., S tefanakis, S., K entouri,
Rohlf, F.J., 2004b. TpsD ig: T hin Plate Spline Digitise (Version 1.40). State
U niversity o f New' York at S tony Brook, Stony Brook, N ew York.
M „ 1997a. T he o p ercu lar c o m p lex d e fo rm ity in in ten siv e gilthead
R ohlf, F.J., 2004c. T psR egr: T hin Plate Spline Shape R egression
soa b ream (Sp a ru s a u ra ta L.) larv icu ltu re. M o m en t o f apparition
and description. A q u acu ltu re 156, 1 6 5 -1 7 7 .
(Version 1.29). S tate U niversity o f N ew York at S tony B rook, Stony
B rook, N ew York.
K oum oundouros, G., G agliardi, F., D ivanach, P.. B oglione, C., Cataudella,
5 ., K en to u ri, M ., 1997b. N o rm a l an d abnorm al osteological
R ohlf, F.J., 2005. T psR elw : T h in Plate S p lin e R elative W arp A nalysis
developm ent o f caudal fin in S p a n is aurata L. fiy. A quaculture 149,
(V ersion 1.41). S tate U n iv e rsity o f N e w York a t S to n y B rook,
S to n y B rook. N e w York.
2 1 5 -2 2 6 .
K o u m o u n d o u ro s, G ., K o u tto u k i, S ., G eo rg ak o p o u lo u , E ., P apadakis,
R ohlf, F.J., Slice, D .E ., 1990. E x ten sio n s o f the Procrustes m ethod for
1., M ain g o t, E., K aspiris, P., K iriak o u , Y., G eo rg io u . G ., D ivanach,
P., K en to u ri, M ., M y lo n as, C .C ., 2 0 0 5 . O n to g e n y o f the shi drum
R ohlf, F.J.. M arcus, L.F., 1993. A revolution in m orphom etries. Trends
Ecol. E vol. 8, 1 2 9 -1 3 2 .
U m brina cirrosa (L innaeus 1758), a c an d id ate new' species for
R ohlf, F.J., Loy, A ., C orti, M ., 1996. M o rp h o m etrie analysis o f old
aquaculture. A quacult. R es. 3 6 , 1 2 6 5 -1 2 7 2 .
Lindesjöö, E., T hulin, J., B engtsson, B .E., T jaernlund, U ., 1994.
Abnormalities o f a gili co v er bone, the operculum , in perch Perca
fluviatilis from a pulp mill effluent area. A quat. Toxicol. 28, 189-207.
Loy, A ., M arian i, L.., B ertelletti, M ., T u n esi, L., 1998. V isualizing
allom etry: g eom etric m o rp h o m etries in th e stu d y o f sh ap e changes
in the early stag es o f the tw o -b an d ed sea bream , D ip lo dus vulgaris
(P erciform es, Sparidae). J. M o rp h o l. 2 3 7 , 1 3 7 -1 4 6 .
Loy, A ., B oglione, C ., C atau d ella, S., 1999. G eo m etric m o rphom etries
and m o rp ho-anatom y: a c o m b in e d tool in the stu d y o f sea bream
(S p a m s a u rata. S p arid ae) shape. J. A p p l, Ichthyol. 15, 104 -110.
the optim al superim p o sitio n o f landm arks. Syst. Z ool. 39, 4 0 -5 9 .
w orld T alpidae (M am m alia, Insectívora) u s in g partial-w arp scores.
Syst. B iol. 45, 3 4 4 362.
Sadek, S., O sm an, M.F.. M ansour, M .A ., 2004. Grow th, survival and feed
conversion rates o f sea bream (Sparus aurata) cultured in earthen
brackish w ater ponds fed different feed types. A quacult. Int. 12,
4 0 9 -4 2 1 .
Tandler, A ., H elps, S ., 1985. T h e effects o f pholoperiod and w ater
exchange-rate on grow th and survival o f g ilthead sea bream
(Sp a ru s au ra ta . L innaeus, Sparidae) from hatching to m e ta m o r­
pho sis in m a ss re a rin g system s. A qu acu ltu re 4 8 , 7 1 -8 2 .
T a \e , D ., H andw erker, T.S., 1994. S em i-operculum : A non-hcritable
Loy, A., B oglione, C., Gagliardi, F.. F en u cci, L., Cataudella, S.. 2000.
birth defect in Tilapia nilotica. J. W orld A quae. Soc. 25, 3 3 3 -3 3 6 .
G eom etric m orphom etries and internal anatom y in sea bass shape
Taylor, W .R., Van D yke, G .C ., 1985. R evised procedures fo r staining and
analysis (Dicentrarchus labrax L., M oronidae). A quaculture 186,
clearing sm all fishes and o th er vertebrates for bone and cartilage
study. C ybium 9, 107 -119.
33 44.
L oy, A ., B ertelletti, M .. C o sta , C ., F erlin , L.., C atau d ella, S ., 2001.
V alentin, A ., S évigny, J.M ., C h an u t, J.P., 2002. G eom etric m o rp h o ­
Shape ch an g es and gro w th tra je c to rie s in th e early stages o f three
m etries reveals b o d y shape differen c e s betw een sym patric redfish
sp ecies o f the g en u s D ip lo d u s (P ercifo rm es, S p aridae). J. M orphol.
S e b a ste s m en ta d a . Sebastes fa s c ia tu s an d th eir hybrids in the G u lf
o f S t L aw rence. J. Fish Biol. 60, 857 -875.
250, 2 4 -3 3 .
M om ente, G ., Rodriguez, A ., Tocher, D .R., Sargent, J.R., 1993. Effects o f
dietary' docosahexaenoic acid (DMA 22/6N -3) on lipid and fatty-acid
com positions and grow th in gilthead sea bream (Spam s aurata L.)
larvae during first feeding. A quaculture 112, 79 98.
P a p an d ro u lak is, N ., D iv an ach , P.. K en to u ri, M ., 2002. Enhanced
biological perfo rm an ce o f in ten siv e sea b re a m (Sparus aurata)
Van Snik, G .M .K ., Van den B oogaart. J.G .M ., O ssc, J.W .M ., 1997.
Larval grow th p atterns in C yprinus ca rp io and C larias g ariepinus
w ith attention to th e finfold. J. Fish B iol. 5 0 , 1 3 3 9 -1 3 5 2 .
W inem iller, K .O ., T aylor, D.H., 1982. Inbreeding depression in the
c o n v ic t cich lid , C ichlasom a n ig ro fa sc ia tu m (B aird an d G irard).
J. Fish Biol. 2 1 , 3 9 9 -4 0 2 .
Y Verhaegen e t al. iA q u a c u ltu r e 2 6 8 (2007) 156 168
opercular deform ities at 6.1 mm TL (K oum oundouros
et al., 1997a) and 17 DPH (Galeotti et al.. 2000) in
earlier studies, but these methods are unpractical in daily
quality assessm ent in commercial hatcheries. K oum oun­
douros ct al. (1997a) proposed a metrical m ethod to
quantify and distinguish opercular deform ities during
and after m etam orphosis for quality assessm ent and
experim entation on the causative factors. This m ethod is
based on the ratio o f (1) the length from the posterior
m argin o f the eye to the posterior m argin o f the oper­
culum (pstO r) and the sum o f (2) the eye diam eter (ED)
and (3) the length from the tip o f the snout to the anterior
m argin o f the eye (prOr) (thus ratio= pstO r/(prO r + ED)).
K oum oundouros ct al. (1995) also proposed several
classic m orphom etric ratios (for exam ple head length on
TL) for the daily assessm ent o f larval quality.
O ur m ethod, a discrim inant function analysis per­
form ed on th e shape v ariables obtain ed th ro u g h
landm ark-based geometric morphometries, could signi­
ficantly separate deoperculated from norm al specim ens at
69 D PH , based on small shape differences o f the external
morphology. O ur preliminary analysis, however, suggests
that deoperculated specimens can be detected much
earlier using geom etric morphometries (m ore specim ens
need to be analyzed, however, to support this statistically).
A n autom ated geom etric morphometric method o f the
external m orphology (e.g. based on Fourier analysis)
could thus be a reliable, time and cost efficient m ethod for
quality assessm ent o f reared fish stocks.
167
A c k n o w led g em en ts
We wish to express o u r gratitude to general m anager
A. Moretii and the co -w o rk ers at the S. aurata hatchery
M aricoltura di R o sig n an o Solvay and the associates at
INV E technologies for th e kind supply o f specim ens and
arranging o f the m eetin g s. We also w ish to thank our lab
technician M. Brunain f o r the clearing and staining o f the
specim ens w hich w ere u se d for the osteological analysis.
R eferen ces
A dam s, M .S., N isw ander, J .D ., 1967. D evelopm ental ‘n o ise ’ and
congenital malformation. G e n . Res. Com p. 10. 3 13-317.
A dam s, D.C., Rohlf, F.J., Slice, D .E.. 2004. G eom etric morphom etries: ten
years o f progress follow ing th e ‘revolution’. Ital. J. Zool. 7 1 ,5 -1 6 .
Alfonso, J.M .. M ontero, D ., R obaina, L., Astorga, N ., Izquierdo, M.S.,
G ines. R., 2000. A ssociation o f lordosis scoliosis-kyphosis deformity
in gilthead sea bream (S p a n is aurata) with fam ily structure. Fish
Physiol. Biochem. 22, 1 5 9 -1 6 3 .
A n drades. J.A ., B ecerra, J., F e m án d ez-L leb rez, P., 1996. Skeletal
deform ities in larval, ju v e n ile and a d u lt stages o f cultured gilthead
sea b ream (S p a n is a u ra ta L .). A quaculture 141, 1 -1 1 .
A m q v ist, G ., M ârtensson, T., 1998. M easurem ent error in geom etric
m orphom etries: em pirical s tra te g ies to assess and re d u c e its im pact
on m easures o f shape. A c ta Z ool. H ung. 44, 73 96.
B aló n , E.K.., 1975. T e rm in o lo g y o f intervals in fish developm ent.
J. Fish. Res. B oard C an. 3 2 , 1 6 6 3 -1 6 7 0 .
B arahona-F em andes, M .H .. 1982. Body deform ation in hatchery reared
European sea bass D icentrarchus labrax (L.). Types, prevalence and
effect on fish survival. J. F ish Biol. 21, 2 3 9 249.
B eraldo. P., Pinosa, M ., Tibaldi, E ., C anavese, B., 2003. A bnorm alities o f
the operculum in gilthead sea bream (Sparus aurata)', m orphological
description. A quaculture 2 2 0 , 8 9 -9 9 .
5 . C o n clu sio n
Boglione, C., Cagliardi, F.. Scardi, M., Cataudella, S., 2001. Skeletal
descriptors and quality assessm ent in larvae and post-larvae o f wild-
This prelim inary study, using geometric morphometries
to detect and analyse opercular deformations in S. aurata,
has yielded som e new insights in the problem. First, the
m ethodology proves to be very useful for understanding
the nature o f crania] deformations that occur in association
with opercular deformations, as well as for screening for
shape changes related to it (both in the external and the
skeletal m orphology). Second, it has been show n that there
exists a striking left-right independency tow ards deoper­
culation. Third, even though a subtle size difference was
observed at 65 D PH in norm al and deoperculated
specim ens, allometries during growth appear to be very
sim ilar in normal and deoperculated specimens.
Further sampling, however, is needed to test some o f the
observations, as well as to expand the size range in order to
evaluate the earliest possible detection o f opercular
deformities in S. aurata, from which an easy detection
protocol could b e derived. Further testing o f these
observations with experimental setups will also be needed
in order to understand causal factors behind deoperculation.
caught and hatchery-reared gilthead sea bream (Sparus aurata L.
1758). A quaculture 192, 1 -2 2 .
B ookstein, F.L., 1990. Introduction to m e th o d s fo r landm ark data. In:
R o h lf F.J., B ookstein, F.L. (E ds.), P roceedings o f th e M ichigan
M orphom etries W orkshop, pp. 2 1 5 -2 2 5 .
Cahu, C., Infante, J.Z., Takeuchi, T., 2003. N utritional com ponents affecting
skeletal developm ent in fish la n a e . A quaculture 227, 24 5 - 258.
C avalcanti, M .J., M ontciro, L .R ., Lopes, P.R .D ., 1999. L andm arkba se d m orphom etric analysis in selected species o f serranid fishes
(P erciform es: Teleostei). Z o o l. Stud. 38, 2 8 7 -2 9 4 .
C hatain, B ., 1994. E stim ation e t a m élioration des perform ances
zootechniques d e l’élevage larv aire de D icen tra rch u s la b ra x et de
S p a ru s aurata. T h è se D octo rale en S ciences, U niv ersité d ’A ixM arseille II. 199 pp.
C hatain, B., O u nais-G uschem ann, N ., 1990. Im proved ra te o f initial
sw im b la d d e r inflation in in te n s iv e ly re a re d S p a r u s aurata.
A quaculture 84, 3 4 5 -3 5 3 .
D e W olf, T., C ourtens, V., C apiferri, U ., Pirone, A ., L enzi, C ., L enzi, F.,
2004. T he influence o f light conditions on th e operculum recovery o f
sea bream (Sparus aurata L.) fry. B ook o f abstracts. A quaculture
E urope 2004, B iotechnologies for quality, B arcelona. Spain 2 0 -2 3
O ctober, 2004, p. 292.
D odson, P.. 1978. O n the use o f ra tio s in grow th studies. Syst. Z ool,
27, 6 2 -6 7 .