Does Shared Treatment Decision-Making Improve Asthma Adherence and Outcomes?
advertisement

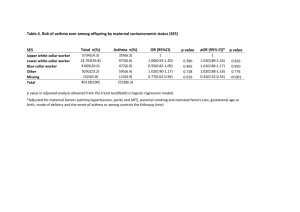
Does Shared Treatment Decision-Making Improve Asthma Adherence and Outcomes? Supported by grants from the National Heart, Lung and Blood Institute 1R01 HL69358 (PI: SWilson) and 1R18 HL67092 (PI: ASBuist) Only ~50% of patients take asthma medications at effective doses Documented problems: Under-use of controller medications Over-use of relievers & OTC medications Poor inhaled medication technique Failure to fill/refill prescriptions Failure to keep medications available when and where they are needed Known contributors to non-adherence Patient Younger age Low socioeconomic status Lack of education Memory problems Lack of understanding of the disease Regimen Longer duration of treatment Higher cost Complexity, more frequent dosing Properties (bad taste, more side effects, etc.) Physician-patient relationship Inadequate monitoring Failure to explain side effects Failure to analyze patient’s medication-taking behaviors Failure to address the patient’s individual situation and preferences Models of Clinician-Patient Interaction Traditional model: Interaction is directive; Clinician makes the treatment decision Evidence-based management usually follows a traditional model Pt MD Informed decision-making model: Clinician provides information to the patient Patient makes the decision MD Pt Shared decision-making model: Mutual exchange of information and treatment preferences between clinician & patient Both participate in treatment decisions Each brings unique knowledge to the interaction MD Pt Hypothesis: Involving patients in treatment decisions should result in: Better adherence to treatment Better asthma control Greater patient satisfaction Design of the BOAT trial Three-arm, randomized controlled trial SDM = shared decision making care management MBG = guidelines-based traditional care management UC = usual medical care Data collection Baseline and 12-mos. post-randomization Questionnaire PFT 12-mos. pre and 24 mos. post-randomization (36 mo.) Asthma medications dispensed All health care utilization BOAT study hypotheses regarding adherence and disease outcomes SDM > MBG SDM > UC Study Outcomes Primary Adherence to asthma medications Asthma-related quality of life Asthma-related health care utilization Secondary Asthma control Use of reliever medications Symptom-free days; Lung function Satisfaction with asthma care Preferences, values, & attitudes towards adherence Total asthma health care utilization Asthma-related health care costs Both the SDM & MBG Interventions: Target patients with poorly controlled, moderate-severe asthma Involve 2 in-person sessions, approximately 1 mo. apart, plus 3 follow-up calls at 3 mo. intervals Conducted by asthma care managers: Clinical pharmacists Nurse practitioners and registered nurses Physician assistants Respiratory therapists Parallel written protocols (scripts) guide both SDM and MBG clinician-patient interactions Structured to enable tailoring to the individual patient Instructional aides and worksheets are included in the interventionist manual SDM and MBG Interventions* Set the Stage Establish rapport Describe session schedule Describe shared decision making approach Gather patient information Asthma symptoms Perceptions of control Medication use Use of alternative therapies Environmental triggers Patient goals & preferences Provide information Assess understanding of asthma Review asthma and how it is treated Confirm comprehension Negotiate (SDM)/Prescribe (MBG) • Summarize patient goals and priorities Review PFTs with patient Assess symptom control using objective criteria Determine asthma severity per GINA guidelines Define medication preferences Discuss +/- of each treatment option per patient goals and preferences Negotiate a treatment decision Wrap Up Write Rx Give Asthma Action & Management Plan Teach proper inhaler use Give asthma diary Schedule follow-up appointment * White = MBG and SDM Gold = SDM only Inclusion Criteria Recent ED/hospital visit for asthma and/or evidence of over-use of rescue medication 18-70 years of age KFHP member ≥ 1 year Self-reported, doctor-diagnosed asthma Currently Rxed asthma medications Meets obstruction reversibility criterion One or more asthma control problems (ATAQ score ≥1) Exclusion Criteria Mild intermittent/seasonal asthma Regular use of oral corticosteroids Currently receiving asthma care-management Not able to speak, read, and understand English Planning to move out of area within two years Randomization* Eligible Patients SDM (N= 204) MBG (N= 205) (N=613) UC (N= 204) * Adaptive randomization algorithm (Pocock, 1983) - ensures better than chance balance and increases likelihood of better than chance balance on correlated characteristics. Demographic characteristics* N=613 Age Gender Ethnicity % 18-34 yrs. 20 35-50 yrs. 42 51-70 yrs. 38 Male 44 Female 56 Hispanic Asian Native Hawaiian/Pacific Islander % Level of education 80% Annual family income 4 10 8 Black/African American 16 White/Caucasian 62 38% < High School Diploma HS Diploma/GED 16 Technical/Some College 43 4-Year Degree/BA/BS 22 Graduate Degree 17 $20,000 8 $20,001 - $40,000 21 $40,001 - $60,000 25 $60,001 - $80,000 18 $80,001 24 DK/Refused To Answer * 2 No significant group differences. 4 Baseline asthma status* 60 Percent 50 40 SDM MBG UC 30 20 10 0 d d ly n d th ily aily ek cte icte dicte on eek ofte i we < da D d / m d w e / 1 re re re 2x t < mor < but f p of p of p u £ o b or ek % % 0% h we n t kly 80 -80 6 / o e 1 > < e m / 60 > W 2x > Symptom Frequency Nocturnal Symptoms FEV1 % predicted * No significant group differences in symptom frequency, nocturnal symptoms, or FEV1 % predicted at baseline. De facto medication regimen and asthma control* 60 Percent 50 40 SDM 30 MBG 20 UC 10 0 t t nt nt e te n en t t e t i t s s i s i i rm e rs ers ers p nte p p i ld e ld ere Mi rat Mi v e d Se Mo ed l led ro lle d l led l l o o r o r t t t ntr on on on o c c c c l l l y el rl y orl w We o o o y P tel yp r a r e V de Mo Medication regimen * No significant group differences at baseline. Asthma Control • Did the SDM patients’ medication choices differ from the MBG care managers’ guidelines-based Rx? Medication SDM N=191 MBG N=186 Beclomethasone 80 90 (50%) 108 (61%) Fluticasone 220 78 (43%) 53 (30%) Other ICS2 13 (7%) 17 (10%) 181 (95%) 178 (96%) Any ICS Leukotriene modifier Theophylline Any Controller3 pvalue1 0.03 0.67 14 ( 7%) 14 (8%) 0.94 4 ( 2%) 1 (1%) 0.37 181 (97%) 1.00 186 (97%) 1. Chi-square or Fishers exact test. 2. Includes Beclomethasone and Fluticasone at lower strengths, and Budesonide. 3. Includes ICSs, leukotriene modifiers, and theophylline; excludes LABAs and oral prednisone. Adherence measure = Continuous Measure of Medication Acquisition (CMA) CMA = Number of days’ supply of a medication dispensed/365 days Proportion of days on which medication was available for use on Rxed regimen A commonly used indicator of adherence to the intended daily regimen Data from the HMO’s pharmacy database ~95% of patients obtain all their medications from the HMO pharmacy Cumulative medication acquisition (CMA) values pre and post randomization, by experimental group CMA index – Mean (SD) Baseline Yr. Any ICS Any Controller Follow-up Yr. Any ICS Any Controller UC MBG SDM N N=203 N=203 N=204 N=610 0.32 (0.32) 0.32 (0.31) 0.33 (0.34) N=204 N=205 N=204 0.41 (0.47) 0.38 (0.37) 0.40 (0.43) N=203 N=202 N=204 0.39 (0.37) 0.54 (0.36) 0.62 (0.38) N=204 N=205 N=204 0.49 (0.52) 0.59 (0.45) 0.69 (0.45) p-value 0.8986 N=613 0.9490 N=609 SDM vs MBG p=0.0162 SDM vs UC p<0.0001 MBG vs UC p<0.0001 N=613 SDM vs MBG p=0.0095 SDM vs UC p<0.0001 MBG vs UC p=0.0014 Conclusions: For non-adherent patients with poorly controlled asthma - Involving patients in a meaningful way in treatment decisions does not result treatment regimens that conflict with standard guidelines, assuming patients have a basic understanding of: asthma their current level of disease control the medical rationale for asthma treatment. Conclusions: For non-adherent patients with poorly controlled asthma, care management that utilizes a shared clinician-patient approach to selection of the treatment regimen significantly improves adherence to asthma controllers over a one year period when compared with both: usual medical care, and traditional, prescriptive care management Intervention effects did not differ as a function of ethnic group (Caucasian, Asian and African American) Conclusions - continued Clinical approaches of asthma care managers can be shaped such that treatment decision making is shared with the patient in a meaningful way. This required use of a detailed intervention protocol, training, and ongoing feedback. Patients evaluate their own vs. the clinician’s influence on treatment decisions differently when they experience a shared decision making approach than when they experience prescriptive care management Questions being investigated by analyses in process Does shared decision-making lead to: better asthma control? better asthma-related quality of life? reduced asthma health care utilization? increased patient satisfaction? Are adherence outcomes mediated by patient perceptions of their influence on treatment decisions? Are disease outcomes mediated by medication adherence? Process outcomes • How closely did interventionists follow the protocol • Who made the treatment decisions? 5 Protocol Adherence Protocol Adherence QC rater Mean rating 4 Decision Roles Decision Roles 3 * p=0.47 Care manager 2 * p<0.001 * 1 0 QC rater SDM * Patients MBG Rating scales: Protocol Adherence 1 = Relevant elements not covered 3 = All elements covered, but some briefly, incompletely, or inadequately 5 = All topics covered completely, thoroughly, and accurately Decision Roles - Treatment decisions were made by: 1 = Care manager alone 2 = Care manager mostly 3 = Patient and care manager equally 4 = Patient mostly 5 = Patient alone Investigators Sandra Wilson, PhD, PI (PAMFRI, SUSM) Sonia Buist, MD, PI (OHSU, CHR) William Vollmer, PhD (CHR) Tom Vogt, MD (CHR) Nancy L. Brown, PhD (PAMFRI, SU) Philip Lavori, PhD (SUSM) Margaret Strub, MD (TPMG) Stephen VanDenEeden, PhD (KRFI/DOR) Clinical Site Co-investigators Faith Bocobo, MD (TPMG) Christine Fukui, MD (TPMG) Donald German, MD (TPMG) John Hoehne, MD (TPMG) Matthew Lau, MD (TPMG) Myngoc Nguyen, MD (TPMG) Consultants Amiram Gafni, PhD Elizabeth Juniper, PhD Cynthia Rand, PhD Sean Sullivan, PhD Kevin Weiss, MD (SDM only) Post-randomization CMA indices for inhaled corticosteroids, by group1 Overall p<0.00012,3 CMA FOR ICS 1.5 1.0 Mn = 0.62 N = 204 0.5 Mn = 0.54 N = 202 Mn = 0.39 N = 203 0.0 MBG 1. 2. 3. SDM GROUP UC N=504. Excludes 4 patients with mild persistent asthma for whom no ICS was prescribed. Overall test of group differences, Wilcoxon/Kruskal Wallis test. Multiple comparisons: SDM vs. MBG, p=0.02; SDM vs. UC, p<0.0001; MBG vs. UC, p<0.0001. Post-randomization CMA indices for all asthma controllers combined, by group1 3 CMA FOR CONTROLLERS Overall p<0.00012,3 2 Mn = 0.69 N = 204 1 Mn = 0.59 N = 205 Mn = 0.49 N = 204 0 MBG SDM UC GROUP 1. 2. 3. N = 504. Excludes 4 patients with mild persistent asthma, for whom no controller was prescribed. Overall test of group differences, Wilcoxon/Kruskal Wallis test. Multiple comparisons: SDM vs. MBG, p=0.02; SDM vs. UC, p<0.0001; MBG vs. UC, p=0.0023. Pre-randomization CMA for all controllers, by ethnicity, within relevant sites Northern CA & Hawaii Northern CA & Portland 3 CMA 2 for Controllers CMA 2 for Controllers 3 2 1 Mn = 0.40 N = 94 Mn = 0.41 N = 344 2 1 Mn = 0.36 N = 59 Mn = 0.47 N = 205 0 0 African American Asian White ETHNICITY White Post-randomization CMA for all controllers, by group, separately for Whites and Asians. White 2 1 0 Mn=0.66 Mn=0.74 N = 68 N = 68 MBG SDM Group Asian 3 CMA 2 for Controllers CMA 2 for Controllers 3 Mn=0.52 N = 69 UC 2 1 0 Mn=0.78 Mn=0.87 Mn=0.52 N = 18 N = 19 N = 22 MBG SDM Group Regression model Group comparison: p-value <=0.0001. Group x Ethnicity interaction: p-value = 0.4478 UC Post-randomization CMA for all controllers, by group, separately for Whites and African Americans White African American 3 CMA 2 for controllers CMA 2 for Controllers 3 2 1 0 Mn = 0.63 N = 113 MBG Mn = 0.74 Mn = 0.53 N = 116 N = 115 SDM UC 2 1 0 Mn = 0.55 N = 33 MBG Mn = 0.51 Mn = 0.34 N = 32 N = 29 SDM Group UC Group Regression model Group comparison: p-value <=0.0001; Group X Ethnicity interaction: p-value = 0.6993.