Chemistry 114 Third Hour Exam Name:____________ Please show all work for partial credit
advertisement
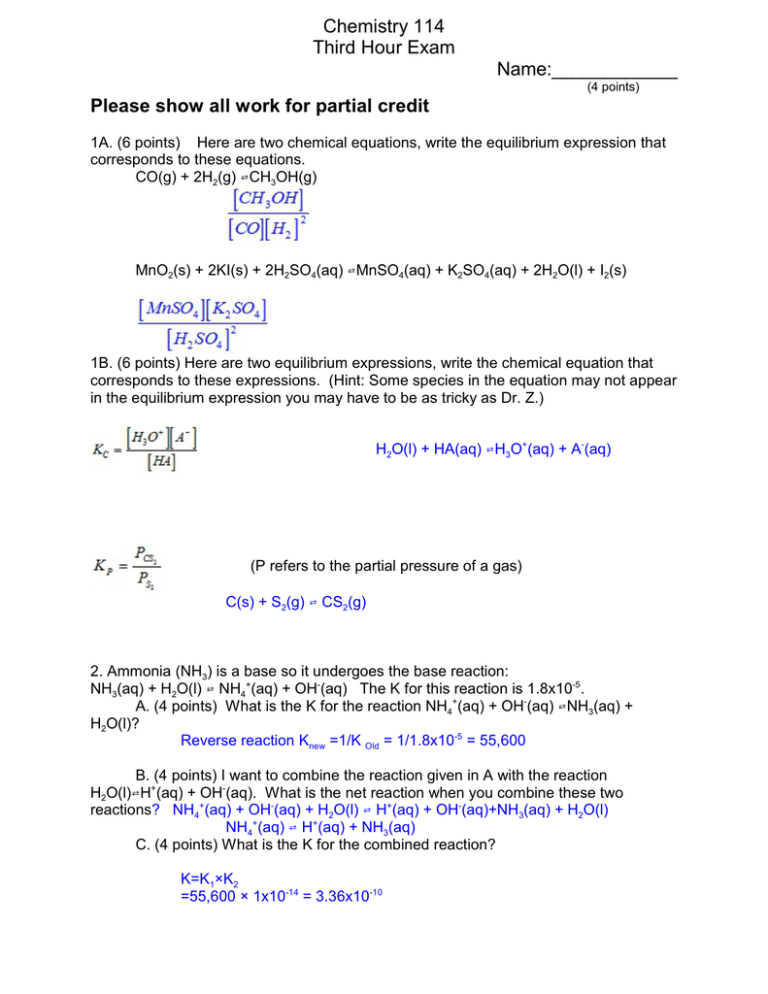
Chemistry 114 Third Hour Exam Name:____________ (4 points) Please show all work for partial credit 1A. (6 points) Here are two chemical equations, write the equilibrium expression that corresponds to these equations. CO(g) + 2H2(g) WCH3OH(g) MnO2(s) + 2KI(s) + 2H2SO4(aq) WMnSO4(aq) + K2SO4(aq) + 2H2O(l) + I2(s) 1B. (6 points) Here are two equilibrium expressions, write the chemical equation that corresponds to these expressions. (Hint: Some species in the equation may not appear in the equilibrium expression you may have to be as tricky as Dr. Z.) H2O(l) + HA(aq) WH3O+(aq) + A-(aq) (P refers to the partial pressure of a gas) C(s) + S2(g) W CS2(g) 2. Ammonia (NH3) is a base so it undergoes the base reaction: NH3(aq) + H2O(l) W NH4+(aq) + OH-(aq) The K for this reaction is 1.8x10-5. A. (4 points) What is the K for the reaction NH4+(aq) + OH-(aq) WNH3(aq) + H2O(l)? Reverse reaction Knew =1/K Old = 1/1.8x10-5 = 55,600 B. (4 points) I want to combine the reaction given in A with the reaction H2O(l)WH+(aq) + OH-(aq). What is the net reaction when you combine these two reactions? NH4+(aq) + OH-(aq) + H2O(l) W H+(aq) + OH-(aq)+NH3(aq) + H2O(l) NH4+(aq) W H+(aq) + NH3(aq) C. (4 points) What is the K for the combined reaction? K=K1×K2 =55,600 × 1x10-14 = 3.36x10-10 2 3. The reaction Al(OH)3(s)WAl3+(aq) + 3 OH-(aq) has a K of 1.3x10-33 If I mix 2 mLs of 1x10-5 M Al3+ with 8 mLs of 2x10-6 M OH- will a precipitate form? Step 1. (3 points) What is the concentration of Al3+ when you mix the two solutions? M1V1 = M2V2 V2 = 2 + 8 = 10 mL 1x10-5 M x 2mL = X M x 10 mL X = 1x10-5 x (2/10) =2x10-6 M Step 2. (3 points) What is the concentration of OH- when you mix the two solutions? M1V1 = M2V2 V2 = 2 + 8 = 10 mL 2x10-6 M x 8mL = X M x 10 mL X = 2x10-6 x (8/10) =1.6x10-6 Step 3. (3 points) What is the Q for the reaction? Q = [Al3+][OH-]3 =2x10-6 M (1.6x10-6)3 =8.2x10-24 Step 4. (3 points) Use the answer from step 3 to tell be why a precipitate does (or does not) form. Q>K Too many products Reaction will go to left Precipitate will form 4. ( 12 points) Given that the following system is at equilibrium: 2NOCl(g) 62NO(g) + Cl2(g), predict what will happen with the following changes to the system: Change [NOCl] increases The system will: Circle one Shift to Right, Shift to Left, or remain the same [Cl2] increases Shift to Right, Shift to Left, or remain the same [NO] decreases Shift to Right, Shift to Left, or remain the same He is added to system Shift to Right, Shift to Left, or remain the same Overall volume decreases Shift to Right, Shift to Left, or remain the same T increases Shift to Right, (Assume rxn is Exothermic) Shift to Left, or remain the same 3 5A. (4 points) What is the Arrhenius definition for acids and bases? Acids produce H+, Bases produce OH- B. (4 points) What is the Brønstead-Lowry definition for acids and bases? Acids are proton donors, bases are proton acceptors C. (4 points) What is the Lewis definition for acids and bases? Acids are electron pair acceptors, bases are electron pair donors 6. (12 points) Fill in the following table: [H+] .01M Hcl .01 1.58x10-9 Ca(OH)2 3.16x10-6 pH 1x10-12 2 3.16x10-6 3.16x10-6 M HClO4 Area below left blank for calculations [OH-] pOH 12 5.5 3.16x10-9 8.5 5.5 3.16x10-9 8.5 I was hoping somebody would point out my mistake in the second row. I added a base (Ca(OH)2) yet I had an acidic pH!. I would have given you BONUS points if you had said something! 4 7. An acid is 1.7% ionized when is has an concentration of .0035M. A. (6 points) What is the pH of the solution? % ionized = [A-]/[Total Acid] x 100% 1.7% = [A-]/.0035 x 100% 1.7%/100% = [A-]/.0035 .017= [A-]/.0035 [A-] = .017 x .0035 = 5.95x10-5 [H+]=[A-] so [H+] = 5.95x10-5 pH = -log(5.95x10-5) =4.26 B. (6 points) What is the KA of the acid? KA = [H+][A-]/[HA] [HA] + [A-] = Total Acid = .0035M [HA] = .0035 - [A-] [HA] = .0035 - 5.95x10-5 [HA] = .00344 KA = (5.95x10-5)2/ .00344 =1.03x10-6 8. (12 points) What is the pH of a 3.5x10-4 M solution of propanoic acid (CH3CH2COOH) with a KA of 1.4x10-5? KA = [H+][A-]/[HA] 1.4x10-5 = X2/(3.5x10-4-X) Because KA is < 10-4 I will assume 3.5x10-4-X . 3.5x10-4 1.4x10-5 = X2/3.5x10-4 1.4x10-5 x 3.5x10-4 = X2 4.9x10-9 = X2 X = SQRT(4.9x10-10 ) X = 7x10-5 = [H+] pH = -log(7x10-5) =4.15