Name:_____________ Chemistry 114 First Hour Exam
advertisement
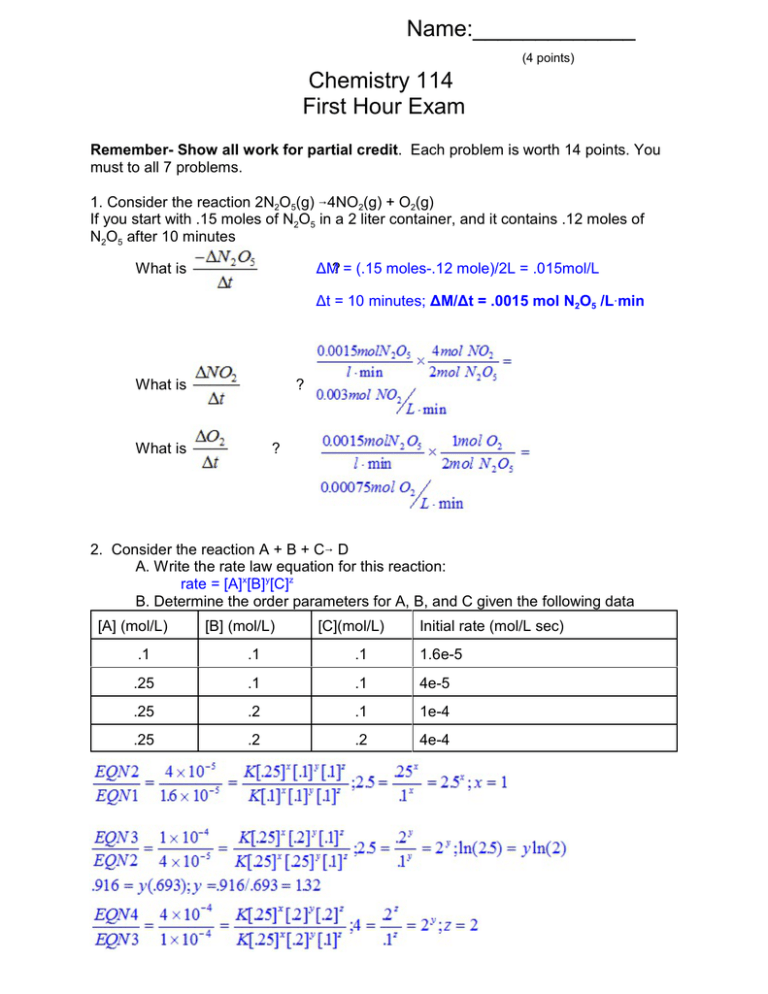
Name:_____________ (4 points) Chemistry 114 First Hour Exam Remember- Show all work for partial credit. Each problem is worth 14 points. You must to all 7 problems. 1. Consider the reaction 2N2O5(g) 64NO2(g) + O2(g) If you start with .15 moles of N2O5 in a 2 liter container, and it contains .12 moles of N2O5 after 10 minutes What is ÄM? = (.15 moles-.12 mole)/2L = .015mol/L Ät = 10 minutes; ÄM/Ät = .0015 mol N2O5 /LAmin What is ? What is ? 2. Consider the reaction A + B + C6 D A. Write the rate law equation for this reaction: rate = [A]x[B]y[C]z B. Determine the order parameters for A, B, and C given the following data [A] (mol/L) [B] (mol/L) [C](mol/L) Initial rate (mol/L sec) .1 .1 .1 1.6e-5 .25 .1 .1 4e-5 .25 .2 .1 1e-4 .25 .2 .2 4e-4 3. You are studying the reaction A + B 6 C. If the reaction is 1st order with respect to A and 2nd order with respect to B, and [A]=.001 M while [B] is 1M, predict the appearance of the following plots Plot Slope (+, 0, or -) Linearity (straight or curved) Y = [A], X=t negative Curved Y = ln[A], X=t negative Straight Y = 1/[A], X =t Positive Curved Y=[B], X=t zero Straight Now I change the conditions and [A]=1M and [B]= .001 M Plot Slope (+, 0, or -) Linearity (straight or curved) Y = [B], X=t negative Curved Y = ln[B], X=t negative Curved Y = 1/[B], X =t Positive Straight Y=[A], X=t zero Straight 4. Define the following terms: Intermediate A species that is both generated and consumed in a reaction mechanism Elementary step A step in a reaction mechanism whose molecularity can be written directly from the reaction. Bimolecular step A step in which only two molecules must collide. Rate determining step The slow step in a reaction pathway. Steric Factor The factor ñ in the equation rate = Zñe-Ea/RT that refers to the orientation of a molecule. Inhibitor A substance that slow a reaction down. Equilibrium Constant When the reaction aA + bBWcC + dD reaches equilibrium; K = [C]c[D]d/[A]a[B]b 5. Give the Arrhenius plot below, determine the activation energy for this reaction. Ea = ? Slope = -Ea/R; -1804=-Ea/8.314J/KAmol Ea=1804×8.314=15000J/mol = 15kJ/mol Given that one point on this line is the point k=.02 T=25oC, what is the k of this reaction when T = 200oC? 6. Write the reaction quotient for the following chemicals reactions: H2(g) + F2(g) W2HF(g) Q=[HF]2 / [H2][F2] N2(g) + 3H2(g) W2NH3(g) Q=[NH3] / [N2][H2]3 Cu(NH3)62+ (aq) + 6Cl-(aq) WCuCl62+(aq) + 6NH3(aq) Q=[CuCl62+][NH3]6 / [Cu(NH3)62+][Cl-]6 2C4H10(g) + 13O2(g) W8CO2(g) + 10H2O(g) [CO2]8[H2O]10 / [C4H10]2[O2]13 7. The reaction 2SO2(g) + O2(g)W2SO3(g) has an equilibrium constant (in terms of concentration) of 3.57 What is the equilibrium constant (in terms of concentration) for the reaction 2SO3(g)W2SO2(g) + O2(g) This is reverse of original equation so Knew = 1/Kold =1/3.57 = .280 What is the equilibrium constant (in terms of concentration) for the reaction 4SO3(g)W4SO2(g) + 2O2(g) This is the previous equation multiplied by 2 so Knew = (Kold)2 =.2802 = .0785 If T = 25oC,what is the equilibrium constant (in terms of pressure) for the reaction 2SO2(g) + O2(g) W 2SO3(g) This is the original KC now being expressed in terms of pressure KP = KC (RT)Än; Än=2-(2+1) = -1 3.57(RT)-1 =3.57 / (.08206)(25+273) =.146 What is the equilibrium constant (in terms of pressure) for the reaction 2SO3(g)W2SO2(g) + O2(g) This is the reverse of the above equation so Knew = 1/Kold =1/.146 = 6.85