Chemistry 112 First Hour Exam Name:____________ Please show all work for partial credit
advertisement
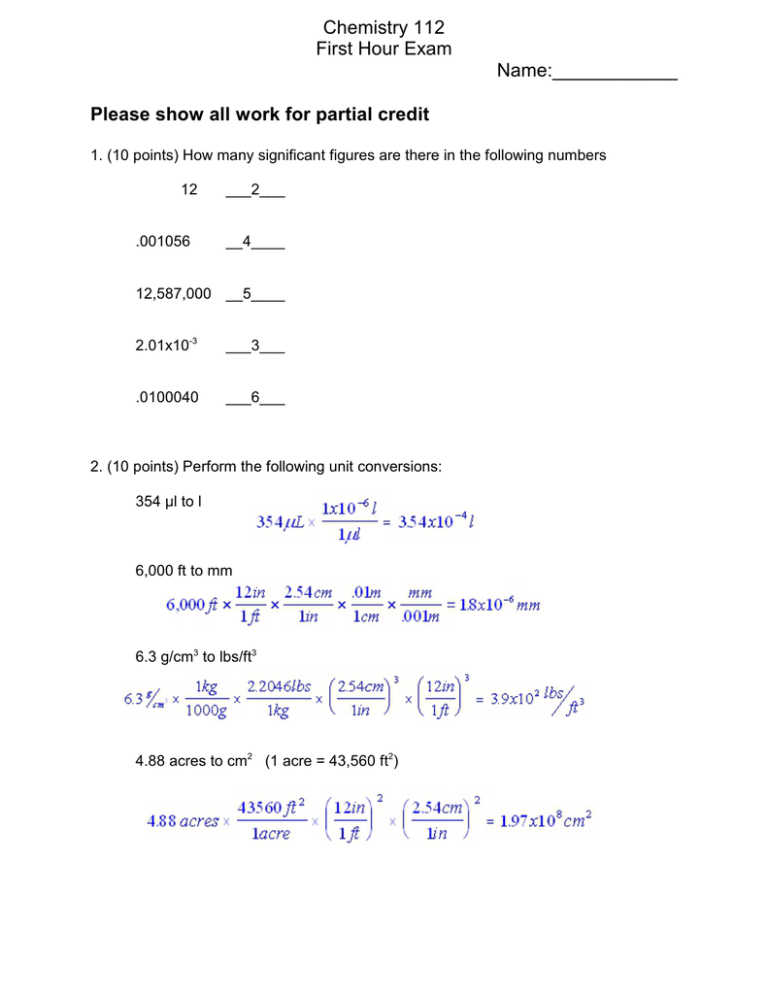
Chemistry 112 First Hour Exam Name:____________ Please show all work for partial credit 1. (10 points) How many significant figures are there in the following numbers 12 .001056 ___2___ __4____ 12,587,000 __5____ 2.01x10-3 ___3___ .0100040 ___6___ 2. (10 points) Perform the following unit conversions: 354 :l to l 6,000 ft to mm 6.3 g/cm3 to lbs/ft3 4.88 acres to cm2 (1 acre = 43,560 ft2) 2 3. (10 points) Perform the following mathematical operations and express the result with the correct number of significant figures: .05 + .000458 + 100. + 24.5 = 125 (3 sig fig) .05 x .000458 x 100. X 24.5 = .06 (1 sig fig) (.05 + .000458) x (100. + 24.5) = 6 (1 sig fig) 4. (10 points) Name the following elements The alkali metal in the third period ___Na___________________ Any 2 noble gases ____He, Ne, Ar, Kr, Xe, or Rn___________ A halide in the second period ____F_______________ Any transition metal __Anything from the middle of the table__ 5. (10 points) How many protons, neutrons and electrons are there in each of the following atoms or ions # protons # neutrons # electrons 5 6 5 20 20 20 53 74 54 3 6. (10 points) Give the names of the following compounds NaCl Sodium chloride SF6 Sulfur hexafluoride Ca(NO2)2 Calcium nitrite H2SO4(aq) Sulfuric acid FeCl3 Iron(III) chloride 7. (10 points) Give the molecular formula of the following compounds Sodium oxide ___Na2O__________ Dinitrogen tetrahydride __N2H4____________ Lithium sulfate __Li2SO4______________ Hydrobromic acid __HBr(aq)_________________ Lead(IV) sulfide __PbS2________________ 8. (10 points) Natural copper has an atomic mass of 63.55 amu. The two most common isotopes of Cu are 63Cu and 65Cu. What are the relative abundances of these two isotopes in natural copper. 4 9A (5 points) I have 10 grams of water. How many moles of water is this? Molar mass of water = 16.00 + 2(1.008) = 18.016g 9B (5 points) I have 10 grams of water. How molecules of water is this? 0.56 moles water x 6.022×1023 molecules/mole = 3.3×1023 molecules 10. (10 points) What is the percent composition of every element in the compound aluminum sulfate? Aluminum sulfate = Al2(SO4)3 Molar mass Al 2x26.98 = 53.96 S 3x32.07= 96.21 O 12x16 = 192 Total = 342.17 g % compositions Al: 53.96/342.17 x 100% = 15.77% Al S: 96.21/342.17 x 100% = 28.12% S O: 192/342.14 x 100% = 56.11% O Sum of %’s = 100.00%