Chemistry 112 First Hour Exam Name:____________ Please show all work for partial credit
advertisement
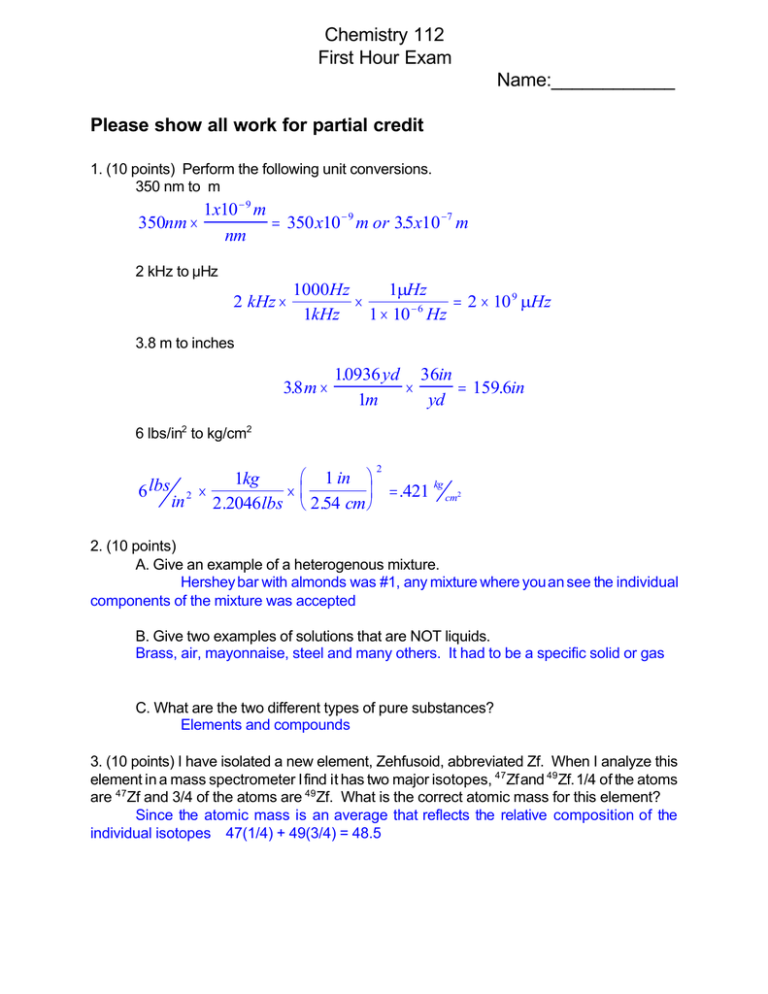
Chemistry 112 First Hour Exam Name:____________ Please show all work for partial credit 1. (10 points) Perform the following unit conversions. 350 nm to m 1x10 − 9 m 350nm × = 350 x10 − 9 m or 35 . x10 −7 m nm 2 kHz to :Hz 2 kHz × 1000Hz 1µHz × = 2 × 10 9 µHz −6 1kHz 1 × 10 Hz 3.8 m to inches 38 . m× 10936 . yd 36in × = 159.6in 1m yd 6 lbs/in2 to kg/cm2 2 6 lbs 1 in 1kg × = .421 kg cm2 2 × in 2.2046lbs 2.54 cm 2. (10 points) A. Give an example of a heterogenous mixture. Hershey bar with almonds was #1, any mixture where you an see the individual components of the mixture was accepted B. Give two examples of solutions that are NOT liquids. Brass, air, mayonnaise, steel and many others. It had to be a specific solid or gas C. What are the two different types of pure substances? Elements and compounds 3. (10 points) I have isolated a new element, Zehfusoid, abbreviated Zf. When I analyze this element in a mass spectrometer I find it has two major isotopes, 47Zf and 49Zf. 1/4 of the atoms are 47Zf and 3/4 of the atoms are 49Zf. What is the correct atomic mass for this element? Since the atomic mass is an average that reflects the relative composition of the individual isotopes 47(1/4) + 49(3/4) = 48.5 2 4. (10 points) Below are a the molecular formulas for a series of compounds, give the written names of these compounds: NaCl __Sodium chloride_______ PbO2 ___Lead(IV) oxide_______ Co2(SO4)2 __Cobalt(II) sulfate__ NO ___Nitrogen monoxide_____ P4O10 _Tetraphosphorous decaoxide 5. (10 points) Below are the names of several compounds, give their molecular formula Aluminum Chloride ____NH4Cl__________________ Mangenese(III) oxide ___Mn2O3______________________ Hydrofluoric acid ___HF(aq)________________________ Sulfur trioxide ____SO3_______________________ N2O (laughing gas) __Dinitrogen monoxide__________________________ 6. (10 points) Give the name or formula the following ions: NH4+ _Ammonium___ Nitride___N3- ______ SO3-2 _Sulfite___________ Hydride ____H-________ OH- __Hydroxide__________ 3 7. (10 points) A. What is the molar mass of the (NH4)2Cr2O7 (1.008x8) + (14.007x2) + (51.996x2) + (15.999x7) = 252.063 B. If I have 25 g of (NH4)2Cr2O7, how many moles do I have? 25g × 1mole = .0992 g 252 .063 C. If I have 25 g of (NH4)2Cr2O7, how many molecules do I have? 1 mole 6.022 x1023 molecules 25g × × = 5.973x10 23 molecules 252 .063g mole 8. ( 10 points) A 0.75 g sample of a liquid is burned in a combustion analysis. In this analysis the yields of CO2 and H2O were: 1.43 g CO2 .88g H2O What is the empirical formula for this compound? Find out how many g of C is in 1.43g of CO2 1.43g CO2 × 1mole CO2 1mole C 12 g C × × = .39 g C 44 g CO2 1 mole CO2 1 mole C Find out how many g of H is in .88g H2O .88g H2 O × 1 mole H 2 O 2 moles H 1g H × × = .098g 18 g H2 O 1 mole H 2 O 1 mole H Calculate missing oxygen .75 - .39 -.098 = .262g Find moles of C, H, and O in compound 143 . g CO2 × 1 mole CO2 1 mole C × = .0325mole C 44 g CO2 1 mole CO2 1 mole H2 O 2 moles H × = .098mole H 18 g H 2 O 1 mole H 2 O 1 mole O .262g O × = .016mole O 16 g O .88 g H 2 O × Divide by the smallest number of moles C = .0325/.016 .2; H= .098/.016.6; O= .016/.016=1 C2H6O 4 9. (10 points) Balance the following equations: N2(g) + H2(g) 6 NH3(g) N2(g) +3 H2(g) 6 2NH3(g) Fe2O3(s) + C(s) 6 Fe(s) + CO(g) Fe2O3(s) + 3C(s) 6 2 Fe(s) +3 CO(g) 10. (10 points) I am performing the following reaction in a lab 2Al(s) + 3 Br2(l) 6 2 AlBr3(s) If I start with 5 g of Al and 5 g of Br2 and have a 5 g yield of AlBr3, what was the % yield for my reaction? Yield of AlBr3 from 5g Al 5g Al × 1 mole Al 2 mole AlBr3 26.98 + 3(79.9) g AlBr3 × × = 49.4 g 26.98 g Al 2 mole Al 1 mole AlBr3 Yield AlBr3 from 5g Br2 5g Br2 × 1 mole Br2 2 mole AlBr3 29.98 + 3(79.9 ) g AlBr3 × × = 563 . g 79.9 x 2 g Br2 3 mole Br2 1 mole AlBr3 Lowest yield (5.63 g) is limiting reactant and theoretical yield % yield = Actual/theoretical X 100% = 5/5.63 x 100% = 89.9%




