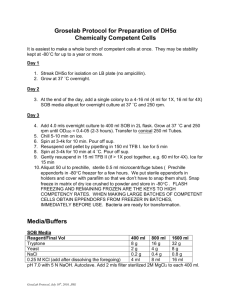
Name:_____________ Chemistry 232 Test II Take home exam
advertisement

Name:_____________ Chemistry 232 Test II Take home exam Ground Rules -You are free to use any books on campus, but you may not talk to anyone except Dr. Zehfus about the problems. You are free to use the notes and problems available for this class on the web, but you cannot use any other web resources beyond that. I understand and agree to the above rules___________________ (Please sign) The test contains a total of 120 points. You may skip one 20 point problem, or you can do all the problems and I will eliminate your worst 20 point problem. 1. (20 points) I am going to titrate a 30 mLs of.08512 M of Cu(NO3)2 with 0.1948 MNa2CrO4. What is the pCu initially, and after 10, 20 and 30 mls of titrant have been added? Where was the equivalence point, and what was the pCu at the equivalence point. (You can find the answers in any order your want, just as long as you find them) 2. (10 points) What is the ionic strength of a solution containing 1.5 M (NH4)SO4 and 1M Acetic acid buffer, pH 4.76? 3. (20 points) Using extended Debye-Hückel equation to account for activity, find the [Tl] in a saturated solution of TlSCN. (If you use the method of successive approximations, do one complete cycle of calculations and describe where you would go from there.) 4. (A - 5 points) When you make a saturated solution of KCl, the solution is 3.7 F in KCl. Ignoring activity effects, what is the Ksp of KCl. (B- 15 points) I am going to mix 50 ml of 3.7 M KCl with 50 mL of 12 M HCl. Does a precipitate form? What are the concentrations of all chemical species in the solution? 5 (20 points) CaHPO4 has a solubility product of 2.6x10-7 (ions are Ca2+ and HPO42-) Phosphoric acid is a multiprotic acid: H3PO4WH2PO4- + H+ , Ka1=7.11x10-3; H2PO4WHPO42- + H+, Ka2=6.32x10-8; HPO42-WPO43- + H+, Ka3 = 7.1x10-13. I want to calculate the concentration of all species in a solution that occurs after I mix 50 mLs of .050M CaCl with .050 F phosphoric acid, pH=8. A. List all species in the solution B. List as many equation as you can that can be applied to this system. C. Do you have enough equation to solve this system? D. What assumptions do you think you can make in this system? E. Do NOT attempt to solve the system. (Unless you really, really want to) 6. (20 points) A. What is the pH of a 5.76x10-7 M solution of LiOH? B. What is the pH of a 5.76x10-3 M solution of 1-Naphthol? C. What is the pH of a 5.76x10-1 M solution of Iodic Acid? 7. (10 points) What is the fraction dissociation of .05 F Formic acid at pH 3, 4, and 5?