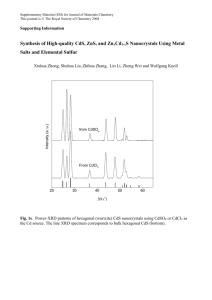
AN ABSTRACT OF THE DISSERTATION OF
advertisement

AN ABSTRACT OF THE DISSERTATION OF Yu-Wei Su for the degree of Doctor of Philosophy in Chemical Engineering presented on June 8, 2011. Title: CdS Nanocrystalline Thin Films Deposited by the Continuous Microreactor-Assisted Solution Deposition (MASD) Process: Growth Mechanisms and Film Characterizations Abstract approved: __________________________________________________________________ Chih-Hung Chang The continuous microreactor-assisted solution deposition (MASD) process was used for the deposition of CdS thin films on fluorine-doped tin oxide (FTO) glass. The MASD system, including a T-junction micromixer and a microchannel heat exchanger is capable of isolating the homogeneous particle precipitation from the heterogeneous surface reaction. The results show a dense nanocrystallite CdS thin films with a preferred orientation at (111) plane. Focused-ion-beam was used for TEM specimen preparation to characterize the interfacial microstructure of CdS and FTO layers. The band gap of the microreactor-assisted deposited CdS film was determined at 2.44 eV. X-ray Photon Spectroscopy show the bindings energies of Cd 3d3/2, Cd 3d5/2, S 2p3/2 and S 2p1/2 at 411.7 eV, 404.8 eV, 162.1 eV, and 163.4 eV, respectively. The film growth kinetics was studied by measuring the film thickness deposited from 1 minute to 15 minutes in physical (FIB-TEM) and optical (reflectance spectroscopy) approaches. A growth model that accounts for the residence time in the microchannel using empirical factor (η) obtained from previous reported experimental data. Applying this factor in the proposed modified growth model gives a surface reaction rate of 1.61*106 cm4mole-1s-1, which is considerable higher than the surface reaction rates obtained from the batch CBD process. With the feature of separating homogeneous and heterogeneous surface reaction, the MASD process provides the capability to tailor the surface film growth rate and avoid the saturation growth regime in the batch process. An in-situ spectroscopy technique was used to measure the UV-Vis absorption spectra of CdS nanoparticles formed within the continuous flow microreactor. The spectra were analyzed by fitting with the sum of three Gaussian functions and one exponential function in order to calculate the nanoparticle size. This deconvolution analysis shows the formation of CdS nanoparticles range from 1.13 nm to 1.26 nm using a residence time from 0.26 s to 3.96 s. Barrier-controlled coalescence mechanism seems to be a reasonable model to explain the experimental UV-Vis data obtained from the continuous flow microreactor, with a rate constant k’ value of 2.872 s-1. Using CFD, low skewness value of the RTD curve at high flow rate (short τ) suggests good radial mixing at high flow rate is responsible for the formation of smaller CdS nanoparticles with a narrower size distribution. The combination of CdS nanoparticle solution with MASD process resulted in the hindrance of CdS thin film deposition. It is hypothesized that the pre-existing sulfide (S2-) ions and CdS nanoparticles changes the chemical species equilibrium of thiourea hydrolysis reaction. Consequently, the lack of thiourea slows down the heterogeneous surface reaction. To test the scalability of the MASD process, a flow cell and reel-to-reel (R2R)-MASD system were setup and demonstrated for the deposition of CdS films on the FTO glass (6ʺ x 6ʺ) substrate. The film deposition kinetics was found to be sensitive to the flow conditions within the heat exchanger and the substrate flow cell. The growth kinetics of the CdS films deposited by R2R-MASD process was investigated by with a deposition time of 2.5 min, 6.3 min, and 9 min. In comparison with the continuous MASD process, the growth rate in R2R-MASD is higher, however more difficult to obtain a linear relationship with the deposition time. © Copyright by Yu-Wei Su June 8, 2011 All Rights Reserved CdS Nanocrystalline Thin Films Deposited by the Continuous Microreactor-Assisted Solution Deposition (MASD) Process: Growth Mechanisms and Film Characterizations by Yu-Wei Su A DISSERTATION submitted to Oregon State University in partial fulfillment of the requirements for the degree of Doctor of Philosophy Presented June 8, 2011 Commencement June 2012 Doctor of Philosophy dissertation of Yu-Wei Su presented on June 8, 2011. APPROVED: __________________________________________________________________ Major Professor, representing Chemical Engineering __________________________________________________________________ Head of the School of Chemical, Biological and Environmental Engineering __________________________________________________________________ Dean of the Graduate School I understand that my dissertation will become part of the permanent collection of Oregon State University libraries. My signature below authorizes release of my dissertation to any reader upon request. __________________________________________________________________ Yu-Wei Su, Author ACKNOWLEDGEMENTS I would like to sincerely express my truthful gratitude and appreciation to my advisor Prof. Chih-Hung Chang for his enormous support and guidance during the past seven years. I greatly appreciate that he gave me the opportunities to work on the GAP project of the Nanobits Inc., the NSC funded project with National Taiwan University, and the ITP project funded by the US Department of Energy. I would also like to extend my appreciation to my committee members: Dr. Daniel Palo, Prof. Goran Jovanovic, Prof. Brian K. Paul, Prof. Vinod Narayanan, and Prof. Michael Penner. Special thanks are given to the ITP team co-workers, Dr. Sudhir Ramprasad, Mr. Clayton Hires, Mrs. Supriya Pawar, Mr. Don Higgins, and Dr. Jair Lizarazo Adarme for their assistance in the flow cell design, experimental operation and technical support. Without them, I could not have produced good samples for characterization and the kinetics study could not have been accomplished. I would also like to thank Dr. Yi Liu for his assistance on focused-ion-beam and TEM operations. Thanks to every group member in Chang’s group for their help and discussion on my research. Especially, I appreciate Mrs. Kathy Han for her writing correction on this dissertation. Thanks my lovely little buddy, Ryan Chang (1.5 year-old) and his parents (Ean Chang, and Nancy Kao) leading me into a photography world. We almost stepped and took pictures on every corner in Oregon. These pictures brought me a sweet and unforgettable memory in my PhD life in USA. The last, my deepest gratitude goes to my family for their spiritual support, and to my girlfriend, Winnie Lai, for her encouragement from Taiwan every day. I am so touchable that she spends her 7-year colorful life on long-distance love with me. Finally, I want to thank all my brothers and sisters in CCCC (Corvallis Chinese Christian Church). We did fellowship on bible study and worshiped God every Sunday. In the past 7-year life, knowing God and then becoming a follower of Jesus Crist are much worth than getting the PhD degree. TABLE OF CONTENTS Page Chapter 1 Introduction .......................................................................................... 1 Chapter 2 Dense CdS Thin Films on Florine-doped Tin Oxide Surface by High-rate Microreactor-Assisted Solution Deposition ........................ 6 2.1 Introduction .......................................................................................... 7 2.2 Experimental ........................................................................................ 8 2.3 Film Characterization ........................................................................ 10 2.3.1 XRD ............................................................................................ 10 2.3.2 TEM ............................................................................................ 12 2.3.3 Surface Property ......................................................................... 12 2.3.4 Optical Property .......................................................................... 15 2.4 Conclusion ......................................................................................... 15 Chapter 3 Study of Growth Kinetics for the CdS Thin Films by Continuous Microreactor-Assisted Solution Deposition (MASD) on Fluorine-doped Tin Oxide Surface .................................................... 24 3.1 Introduction ........................................................................................ 25 3.2 Experimental ...................................................................................... 32 3.3 Characterization ................................................................................. 32 3.3.1 Thickness .................................................................................... 32 3.3.2 Micro Structure ........................................................................... 35 3.4 Growth Kinetics ................................................................................. 37 3.5 Conclusion ......................................................................................... 40 TABLE OF CONTENTS (Continued) Page Chapter 4 Investigation of CdS Nanoparticle Formation and Deposition using Continuous Microreactors.................................................................. 52 4.1 Introduction ........................................................................................ 52 4.2 Experimental ...................................................................................... 55 4.3 Results and Discussion ...................................................................... 56 4.3.1 Part 1: Nanoparticle Formation .................................................. 56 4.3.2 Part 2: Thin Film Deposition ...................................................... 60 4.4 Simulation of Residence Time Distribution (RTD) ........................... 61 4.5 Conclusion ......................................................................................... 64 Chapter 5 Influence of Flow Conditions on CdS Thin Film Growth Kinetics by Continuous Microreactor-Assisted Solution Deposition (MASD) .... 74 5.1 Introduction ........................................................................................ 74 5.2 Experimental ...................................................................................... 75 5.3 Computational Fluid Dynamics (CFD).............................................. 76 5.3.1 Stacked Heat Exchanger ............................................................. 76 5.3.2 Flow Cell .................................................................................... 77 5.4 Chapter 6 Result and Discussion ........................................................................ 79 Analysis of CdS Thin Film by Reel-to-Reel Microreactor-Assisted Solution Deposition (R2R-MASD) ................................................... 88 6.1 Introduction ........................................................................................ 88 6.2 Experimental ...................................................................................... 89 6.3 Film Characterization ........................................................................ 90 TABLE OF CONTENTS (Continued) Page 6.3.1 XRD ............................................................................................ 90 6.3.2 TEM ............................................................................................ 90 6.3.3 Surface Property ......................................................................... 91 6.3.4 Optical Property .......................................................................... 92 6.4 Result and Discussion ........................................................................ 92 Chapter 7 Conclusion and Future Work ........................................................... 104 Chapter 8 Bibliography .................................................................................... 106 Appendix A: Harmonic Oscillator Approximation ............................................. 118 Appendix B: Optical and Physical Measurement Results ................................... 119 Appendix C: De-convolution Fitting Results ...................................................... 121 Appendix D: Thickness Data Points of Large CdS Films (6ʺ x 6ʺ)..................... 122 Appendix E: Time-dependent Outlet Tracer Concentration ............................... 125 LIST OF FIGURES Figure Page 2.1 Schematic diagram of microreactor-assisted solution deposition (MASD) process……………………………………………………... 17 2.2 2.3 XRD spectrum of (a) bare FTO glass substrate, (b) CdS layer on FTO after one-pass deposition and (c) two-pass deposition, (d) SnO2 (#411445, Tetragonal), (e) CdS (#750581, Cubic), and (f) CdS (#653414, Hexagonal)……………………………………………….. 18 (a) Low magnification image of CdS/FTO structure (magnification: 75,000X) (b) HRTEM images of the CdS/FTO boundary (magnification: 620,000X). (c) Fast Fourier transformed diffraction pattern of CdS layer (region 1). (d) Fast fourier transformed diffraction pattern of FTO layer (region 2)………………………….. 19 2.4 Raman spectra of CdS layer deposited on FTO substrate………….... 20 2.5 AFM images of (a) bare FTO substrate (RMS = 8.15 nm), (b) 2.6 2.7 3.1 one-pass deposited CdS film (RMS = 11.32 nm), and (c) two-pass deposited CdS film (RMS = 8.11 nm) (insert: 3D morphological images)….…………………………………………………………..... 21 XPS spectra (dashed line: as received, solid line: after etching, red dotted line: Gaussian fitting) of the CdS films deposited by continuous MASD (a) O 1s, (b) Cd 3d, (c) C 1s, (d) S 2p……………………………………………………………………... 22 Plot of (αhν)2 versus hν showing the band gap energy of 251.72 nm CdS films by continuous MASD process……………………………. 23 Schematic diagram of continuous MASD process for CdS films growth kinetic study………………………………………………….. 42 LIST OF FIGURES (Continued) Figure Page 3.2 Measured reflectance (solid) and calculated reflectance (dotted) of the continous MASD deposited CdS films with a deposition time of (a) 1 min, (b) 2 min, (c) 3 min, (d) 4 min, (e) 5 min, (f) 8 min, (g) 10 min, and (h) 15 min…………………………………………………………. 43 3.3 Schematic diagrams (a) ~ (e) of TEM specimen prepartion by FIB process……………………………………………………………......... 3.4 3.5 3.6 3.7 3.8 3.9 4.1 44 TEM cross sectional images of the continuous MASD deposited CdS films with a deposition time of (a) 1 min, (b) 2 min, (c) 3 min, (d) 4 min, (e) 5 min, (f) 8 min, (g) 10 min, and (h) 15min………………….. 45 GIXRD of the continuous MASD deposited CdS films with a deposition time of 0 to 15 min………………………………………… 46 Instrumental calibration curve of CdS films by MASD process during various deposition times (Correlation factor is 0.9104)……………….. 47 Growth kinetics of CdS thin film deposited by batch process at 60 °C, 70 °C, and continuous MASD process. The series data of batch process are from Dona et al. [31]. Solid fitted lines are based on Kostoglue’s model (3-27) with non-fixed kH (a), and fixed kH (b)…... 48 The CdS film growth rate on SiO2/Si substrate at various surface temperature versus residence time, and the fitting curve (solid line) based on equation (3-29)………………………………………………. 50 Growth kinetics of CdS thin film deposited by continuous MASD process. The fitting line is based on the modified model (3-30). The kH is fixed at 0.0263 cm3mole-1s-1…………………………………............ 51 Schematic diagrams of in-situ spectroscopic flow cell measurement and thin film deposition by combining MASD process……………….. 65 LIST OF FIGURES (Continued) Figure Page 4.2 Absorbance spectra of CdS nanoparticles formed at various flow rates……………………………………………………………………. 66 4.3 Absorbance spectra (black solid line) of CdS nanoparticles formed at various flow rates are fitted through 1st stage calculation by using (4-9) (red dotted line), including Gaussian and exponential functions (black dashed line)…………………………………………………….. 4.4 67 Absorbance spectra (black solid line) of CdS nanoparticles formed at various flow rates are fitted through 2nd stage calculation by using (4-9) (red dotted line), including Gaussian and exponential functions (black dashed line)…………………………………………………….. 68 4.5 Energies difference of the fitted three absorption bands plotted as a function of CdS (Eg = 2.48 eV) particle size………………………….. 69 4.6 Dependence of the optical size of CdS nanopartices at various residence times after the 1st (red squares) and 2nd (black dots) stage analysis. The second analysis result was fitted by the barrier-controlled coalescence model (solid line)……………………. 69 4.7 4.8 4.9 Photograph of CdS films deposited by MASD process with and without nanoparticle precipitation…………………………………….. 70 Schematic diagram of a T-junction micromixer with extended tubes used for simulating RTD by COMSOL 4.2………………………….. 71 The plot of average time-dependent concentration profile (a) and the normalized RTD curve (b) in different inflow velocities……………... 72 4.10 The plot of coefficient of variation (σθ) and skewness (s) as a function of residence time (τ)……………………………………….................... 73 LIST OF FIGURES (Continued) Figure Page 5.1 Schematic diagram of continuous MASD system…………………… 80 5.2 5.3 Photograph (top) and cross-section diagram (bottom) of parallel flow cell and deflected flow cell…………………………………….. 80 2-D velocity and temperature profiles in the silicon heat exchanger with various inlet velocities………………………………………...... 81 5.4 Velocity contour maps of the parallel and deflected flow cell [97]…. 84 5.5 The map of 36 measurement points on a 6-inch substrate using MASD process with a flow cell……………………………………... 85 2-D CdS film thickness line profiles (row A to row F) along the flow direction of reactants in the parallel (a) and deflected (b) flow cell.…………………………………………………………………... 86 3-D thickness surface profiles of CdS films deposited by continous MASD with (a) parallel flow cell and (b) deflected flow cell………. 87 6.1 Working principle of the reel-to-reel coating machine [99]…………. 94 6.2 Schematic diagram of the reel-to-reel continuous MASD…………... 94 6.3 GIXRD of the reel-to-reel continuous MASD deposited CdS films on FTO substrate…………………………………………………….. 95 TEM cross-sectional images of the reel-to-reel continuous MASD deposited CdS films on FTO substrate. (Magnification: 125,000kX)…………………………………………………………... 96 5.6 5.7 6.4 LIST OF FIGURES (Continued) Figure Page 6.5 AFM images of (a) bare FTO substrate (RMS = 8.15 nm), (b) reel-to-reel continuous MASD deposited CdS film (RMS = 11.35 nm) at 6.5 min deposition time. (Insert: 3D morphological images)... 97 6.6 XPS spectra (dashed line: as received, solid line: after etching, red dotted line: Gaussian fitting) of the CdS films deposited by R2R-MASD (a) O 1s, (b) Cd 3d, (c) C 1s, (d) S 2p…………………. 6.7 98 Plot of (αhν)2 versus hν showing the band gap energy of the reel-to-reel continuous MASD deposited CdS films………………… 99 6.8 The location map of 36 measurement points on a 6-inch substrate using R2R-MASD process…………………………………………... 100 6.9 2-D thickness line profiles of CdS films deposited by continuous R2R-MASD during (a) 2.5 min, (b) 6.3 min, and (c) 9 min………… 101 6.10 3-D thickness surface proflies of CdS films deposited by continuous R2R-MASD during (a) 2.5 min, (b) 6.3 min, and (c) 9 min….……... 102 . 6.11 Growth kinetics of CdS thin film deposited by batch process at (a) 70°C, (b) 60°C, (c) continuous MASD process, and (d) R2R-MASD process. The series data of (a) and (b) are from Dona et al. [31]. Solid lines are fitting curves of Kostoglue’s model (3-27) and the modified model (3-30)………………………………………………. 103 LIST OF TABLES Table Page 3.1 Comparison of film thickness measurement techniques…………….. 42 4.1 Fit parameters in the de-convolution analysis procedure……………. 66 4.2 Kinetic constants of early stage CdS nanoparticles fitted by three different growth models……………………………………………………….. 70 Comparison of the residence time, coefficient of variation, and skewness at different flow rates……………………………………... 73 4.3 B.1 Four optimized parameters of harmonic oscillator approximated equation for bare FTO layer…………………………………………. 119 B.2 Optical CdS thickness at different locations measured by spectroscopic reflectance…………………………………………….. 119 B.3 Physical CdS thickness at different locations measured by TEM…… 120 C.1 First and second stage de-convolution fitting results of CdS nanoparticles by continuous MASD………………………………… 121 D.1 CdS films deposited by continuous MASD in the parallel flow cell... D.2 CdS films deposited by continuous MASD in the deflected flow cell. 122 D.3 CdS films deposited by continuous R2R-MASD at 2.5min…………. 123 D.4 CdS films deposited by continuous R2R-MASD at 6.3min…………. 123 D.5 CdS films deposited by continuous R2R-MASD at 9min…………… 124 122 CdS Nanocrystalline Thin Films Deposited by the Continuous Microreactor-Assisted Solution Deposition (MASD) Process: Growth Mechanisms and Film Characterizations Chapter 1 Introduction In the past 40 years, Cadmium sulfide (CdS) is regarded as one of the most popular semiconductor materials due to its potential application in photo-detectors [1], thin-film transistors (TFTs) [2-4], CdTe and CIGS thin film solar cells [5]. CdS is usually fabricated as a thin or thick film for various applications. For photo-detectors, the thickness should be at least 1µm to absorb enough light and have electrons excite to higher energy level. For TFT and thin film solar cells, the thickness is from 50nm to 150nm in order to show p-n diode function instead of conductive phenomena. CdS thin films can be deposited by gas and liquid process. Physical vapor deposition (PVD) and chemical vapor deposition (CVD) are belong to gas processes, which usually need expensive vacuum equipment to obtain the longer mean free path of the molecular. For a PVD process, H.S. Kowk et al. [6] reported using the ArF laser fluence (1~5 J/cm2) in laser-evaporation deposition (LEDE) in 1988. The target was made by high purity CdS powders (99.999%). The CdS film was deposited on a quartz substrate, which was heated to 350°C and located at 3cm above the target. In addition to ArF laser, Nd:YAG laser [7] at 355nm (UV) and 1064nm (IR) with 10Hz pulses are also performed in thin film deposition. Meanwhile, thermal co-evaporation [8, 9] was applied to make CdS thin films doped with various impurities, such as In, Mn, Se, and Sb. High purity CdS powders and trace amounts of dopants were co-evaporated at high temperature to the substrate. An Rf-sputtering process was also applied for CdS 2 thin film deposition in the early 1980’s. Substrate temperature (60 ~ 300 °C), power density (0.24 ~ 2 W/cm2), and gas pressure (2~10 mTorr) are experimental parameters explored in the process to study the growth rate [10] and electrical properties [11] of polycrystalline CdS thin films. The film thickness was increased with the deposition time, however the bandgap showed decreasing. This result was expected due to a thicker film caused by larger grain size. Recently, Tomakin et al. [12] reported how low substrate temperature (-173 ~ 27 °C) and post-deposition annealing (200 ~ 400 °C) affect the grain size and dark resistivity. Also, the substrate rotation was combined to reduce surface roughness and obtain the fast vapor deposition process [13]. For a CVD process, organic metal precursors are carried by nitrogen or hydrogen gasses to a low pressure chamber for reaction. Boone et al. [14] and Berry et al. [15] used dimethylcadmium (DMC) and hydrogen sulfide (H2S) diluted in helium as reagent sources. The CdS film was grown on (111) silicon substrate at 270 ~ 550 °C in the pressure of 565 ~ 2660 Pa (5 ~ 20 Torr). Uda et al. [16] used diethyl sulfide (DES) instead of H2S for the sulfide source. Ajayi et al. [17] and Fainer et al. [18] respectively used bis-(morpholinodithioato-S,S’) cadmium and cadmium dithiocarbamates as the single-source precursor instead of the conventional two precursors process. The single-source precursor was carried by nitrogen to the chamber, vaporized at 200 ~ 300 °C and then thermally decomposed to CdS on the substrate (quartz glass). The reported advantages are the simpler reactor design than conventional MOCVD and the potential to deposit large-area devices. Until recently, only more structurally complicated single-source precursors were used in CVD processes [19]. 3 The only liquid process, chemical bath deposition (CBD) has advantages of high quality film production and low cost fabrication. During 1960’s to 1970’s, Russian scientists Kitaev et al. and Betenekov et al. [20-22] started the CBD research from the quantum mechanical calculations and radiochemical experiments of the possible dissociation mechanism in thiourea and the reaction with Cd(NH)3)42+ ions. In 1980’s, Mexico scientists P. K. Nair and his colleague studied the photoconductive property [1, 23-25] of CdS films, and also the growth kinetics and material utilization yield of CBD process [26, 27]. Until the 1990’s, Dona et al [28] reported applying an electrochemical open-circuit potential change (EOCPC) method for in-situ measurement to understand the growth kinetics of CdS films by CBD process. Meanwhile, Lincot et al [29] also reported a novel measurement technique of in-situ quartz crystal microbalance (QCM) combined with electrochemical impedance spectroscopy (EIS) to study the CdS thin film layers during CBD. Ortega-Borges et al. [30] initially established a heterogeneous surface reaction mechanism by controlling different variables in the CBD reaction, such as concentrations of cadmium salt, thiourea, aqueous ammonia, ammonium salt, and reaction temperature. In 1997, Dona et al. [31] modified Ortega-Borges’s mechanism by replacing the original adsorbed complex intermediates (Cd(OH)2) to the complex (Cd(OH)2(NH3)2). Oladeji et al. [32] found the heterogeneous surface reaction competes with the homogeneous reaction, which limited the CdS film growth rate. Optimization of CBD was studied to obtain the high thin film growth rate. Kostoglou et al. [33-37] developed a detail comprehensive mathematical model to study the temporal evolution of reagent concentrations in bulk fluid. The complete numerical solutions are obtained by solving the mass balance equations 4 of each species. So far, all CBD researches encounter the co-existing of homogeneous reaction and heterogeneous reaction in the same space. A modified CBD process, continuous microreator-assisted solution deposition (MASD), was developed to spatially separate the homogeneous particle formation and heterogeneous surface nucleation. Microreactor technology has advantages of accelerating heat and mass transfer by high surface area-to-volume ratios within the microscale space. Our MASD process provides the benefit of impinging a constant flux of solution to the substrate, and efficiently controls the quantity of reagents. This continuous MASD process has been applied successfully for depositing various thin films, such as CdS [3, 38, 39], CuInS2 [40], CuInSe2 [41], CuSe [42], ZnO [43-45] and ZnS [46]. In addition to thin film deposition, the microreactor-assisted system was also applied in synthesizing polyamide dendrimers [47, 48], gold nanoparticles [49], SnTe nanorods [50] and ZnO nanowires [51, 52] for various applications. In this research, CdS films was deposited on the fluorine-doped tin oxide (FTO) coated glass by continue MASD process. Various characterization techniques (chapter 2) were performed to understand the thin film microstructure; also the growth kinetics (chapter 3) was studied by applying previous mathematical model. The early nucleation growth of CdS nanoparticles (chapter 4) in a microreactor was investigated by using UV-vis spectroscopy to realize the temporal particle size. For the industrial application, MASD process was incorporated with a closed space flow cell for large area (6 by 6 inches) thin film deposition. The influence of flow condition for thin film uniformity was elucidated in chapter 5. The further modified reel-to-reel (R2R) MASD process (chapter 6) is 5 similar as the industrial automatic production line with advantages of fast loading and unloading of the substrate. 6 Chapter 2 Dense CdS Thin Films on Florine-doped Tin Oxide Surface by High-rate Microreactor-Assisted Solution Deposition Yu-Wei Su1, Sudhir Ramprasad2, Seung-Yeol Han1, Wei Wang1, S. O. Ryu3, Daniel R. Palo2, Brian K. Paul4,5 and Chih-hung Chang1,5* 1 School of Chemical, Biological & Environmental Engineering, Oregon State University, Corvallis, OR 97330, USA 2 Energy Processes and Materials Division, Pacific Northwest National Laboratory, Corvallis, OR 9730, USA 3 School of Display and Chemical Engineering, Yeungnam University, 214-1 Dae-dong, Gyeonsan, Gyeongbuk 712-749, Republic of Korea 4 School of Mechanical, Industrial & Manufacturing Engineering, Oregon State University, Corvallis, OR 97330, USA 5 Oregon Process Innovation Center for Sustainable Solar Cell Manufacturing, Corvallis, OR 9730, USA [*] E-mail: chih-hung.chang@oregonstate.edu Abstract Continuous microreactor-assisted solution deposition (MASD) is demonstrated for depositing CdS thin film on fluorine-doped tin oxide (FTO) glass. The continuous flow system consists of a microscale T-junction micromixer with the co-axial water circulation heat exchanger to isolate the homogeneous particle precipitation from the heterogeneous surface reaction. The result shows dense nanocrystallite CdS thin films with a preferred orientation at (111) plane and a cubic structure. Focused-ion-beam was used for TEM specimen preparation to characterize the interfacial microstructure of CdS and FTO layers. Surface structural property was characterized by AFM. The band gap was confirmed at 2.44 eV by UV-vis absorption spectroscopy. 7 2.1 Introduction Cadmium sulfide (CdS) is a one of the most popular semiconductor materials due to its potential application in photo-detectors [1], thin-film transistors (TFTs) [2, 3], and most importantly CdS/Cu(In,Ga)Se2, and CdS/CdTe hetero-junction solar cells [5]. In the early 1990’s, the CdTe/CdS solar cell efficiency reached 15% [53] and the module product was commercialized by First Solar Inc. within 10 years. Many studies [53-59] have reported that chemical bath deposition (CBD) is a low-cost and stable method for the deposition of CdS thin films on transparent conducting (TCO) glass. However, the conventional CBD process has nanoparticle growth and film growth simultaneously happened in the same reactor confinement. This phenomenon causes the CdS films have high roughness and low area coverage. The proposed microreactor-assisted solution deposition (MASD) process can perform better surface coverage and uniformity of CdS films on SiO2/Si substrate in comparison with the conventional CBD process [38]. In this paper, we reported high quality CdS thin films on fluorine-doped tin oxide (FTO) coated glass substrate deposited by MASD process. Also, focus-ion-beam milling process with lift-off TEM sample preparation technique was used for observing the interface structure between CdS and FTO layers. 8 2.2 Experimental Our microreactor setup make use of a micromixer for efficient mixing of two reactant streams, and confine the homogeneous reaction in the heat exchanger before impinging on the substrate. The MASD process (Figure 2.1) in this study consists of a microprocessor-controlled peristaltic pump (Ismatec REGLO Digital) for pumping each reactant stream through a 1.22 mm ID Tygon ST tube (Upchurch Scientific). The T-junction micromixer (Upchurch Scientific) was used for mixing these two streams. Stream A was prepared by dissolving 0.073 g CdCl2 (Mw = 183.31 g/mole), 0.214 g NH4Cl (Mw = 53.49 g/mole), and 4.16 ml of 14.82 M (28 wt%) NH4OH in DI water. The total volumn was added up to 50 mL. Stream B was prepared by dissolving 0.305 g thiourea (Mw = 76.12 g/mole) in 50 mL DI water. With this recipei, the stream A stock solution contains 0.008 M CdCl2, 0.08 M NH4Cl, and 1.23 M NH4OH; the stream B stock solution contains 0.08M thiourea. After mixing by equal volumn ratio, the concentration of each components in the PEEK tube was reduced to half. The polycaryl-ether etherketone (PEEK) tube was enclosed co-axially in a tygon-tube, which serves as a shell and tube heat exchanger with hot water circulation maintained at 80-85 °C by a constant temperature bath. The flow rate of each stream was controlled at 0.434mL/min to impinge the solution onto the FTO glass substrate (Pilkington TEC 8), which was taped on a hotplate to keep the surface temperature at 80-90 °C. The residence time of the solution was controlled at 35 seconds. Once the process was completed, the substrate was rinsed by DI water and dried under nitrogen gas. To better understand the CdS crystal growth structure, a second CdS deposition process 9 was performed on the as-deposited CdS/FTO/glass. The first and second depostion process were both performed using a deposition time of 5 minutes. XRD (Bruker D8 Discover, CuKα=1.54056Å) was applied to characteize the crystal structure of the CdS films after one-pass and two-pass depositions. Focused ion beam (FEI Helios Dual Beam Microscopy) was used to prepare cross-sectional samples for TEM analysis. Platinum was deposited on a rectangular area of 10 μm (length) x 2 μm (width) with a 0.5 μm thick by electron beam (1.4 nA), which was cleaned by an ion-beam with a current of 0.2 nA. Two areas with 11 μm x 6 μm, close to the platinum deposited area, were milled down in a 3 μm depth by ion-beam (2.8 nA). The cross-section of the vertical surface was cleaned by ion-beam with a current of 0.93 nA. Finally, small pieces of specimen were mounted on an omniprobe and then transferred to a supported grid. The thickness of the specimen was again milled down to less than 0.1 μm. Surface analysis of morphology, roughness, and chemical bonding were performed by AFM (Bruker, Innova Scanning Probe Microscope), Raman (Witec, alpha 500), and XPS (ESCALAB 250). The optical property was characterized by UV-vis absorption spectroscopy using an Ocean Optics USB2000, spectrometer with a halogen lamp as light source. 10 2.3 Film Characterization 2.3.1 XRD Figure 2.2 shows the XRD diffractograms of bare FTO glass substrate (a) and CdS films by one-pass (b) and two-pass deposition (c). Compared to the tetraganol SnO2 (JCPDS #411445, d), the three major peaks at a 2θ value of 26.37°, 37.66°, and 51.42° of FTO substrate (a) shifted 0.2°~0.4° degree lower. The reason could be attributed to the larger d-spacing of fluorine doped SnO2 than the pure SnO2. All peaks showed in the FTO glass substrate are much close to SnO2, and the orientation planes can be indexed to tetraganol structure. The XRD diffractograms still resembled the diffractogram of bare FTO substrate after one-pass and two-pass deposition except the peaks around 26°. It can be observed that one-pass and two-pass deposition caused the peak at 26.39° shift to 26.63° and 26.71°, respectively (inset figure). The inset figure magnified the 2θ range from 25.5° to 27.5° and shows a small transition peak at 26.41° and a following main peak at 26.63° for one-pass deposited CdS films (b). For thicker CdS films made by two-pass deposition (c), the main peak shifted to 26.71° also the intensity increased in comparison with the previous thinner films (b). Mazon-Montijo et al. [60] reported that the underneath substrate peaks can still be seen when the top film is not sufficiently thick. Therefore, the small transition peak (26.41°) of one-pass deposited CdS films (b) matches with the 26.39° of FTO substrate, which has orientation of T(110) plan. The peak at 26.63° and 26.71° from the one-pass and two-pass deposited films could be assigned to either C(111) plane of cubic CdS (JCPDS #760581, e) or H(002) plane of hexaganol CdS (JCPDS #653414, f). Similar finding has also been reported by Chu et al [61]. Additional other peaks at 11 43.8° and 52° that could be assigned as C(220)/H(110) and C(311)/H(112). However, the peak at 43.8° was not observed in these samples. The other peak at 52° of FTO substrate is index to T(211) plan of tetraganol SnO2 (JCPDS #411445, d). According to our previous grazing incidence X-ray diffraction pattern, the C(111) plane in CdS films can be differentiated from the FTO-coated glass substrate [62]. Therefore, grazing incidence X-ray source could be used to identify any possible overlap peaks. Other peaks at 33.60°, 37.68°, 51.38°, 61.42°, and 65.40° from one-pass and two-pass deposited CdS layers could be assigned to the underneath FTO glass substrate with respective orientations of T(101), T(200), T(211), T(310), and T(301) planes. Ikhmayies et al. [63] ever reported the shifting T(200) plane of CdS:In/FTO at 480°C annealing was due to the formation of solid solution CdS1-xSnx. The non-shift T(200) plan of the as-deposited CdS films can explain no solid solution CdS1-xSnx was formed without thermal annealing. 12 2.3.2 TEM Figure 2.3(a) and (b) show TEM images of the interfacial micro structure between the CdS and FTO layers at low magnification (75,000X) and high magnification (620,000X) respectively. The thickness of the two-pass deposited CdS layer is about 251.72nm on the FTO layer with 344.83nm. The average growth rate could be calculated as 25.2 nm/min. This rate is significantly higher than batch CBD process. For example, Kokotov et al. [64] reported highly-textured, columnar CdS films with an average deposition rate of 2.5 nm/min from a CdSO4 and EDA bath and 1.67 nm/min from CdCl2 and ammonia, respectively. In figure 2.3(b), the FTO layer exhibits large single crystalline structure. At the interface, the deposited CdS layer consists of several nano-size grains and shows columnar structure [64]. A few grains are attached closely to FTO boundary. Kim et al. [65] reported that CdS nanocrystalline structure is cubic due to strong correlations with the FTO structure. It implies that lattice mismatch is reduced a lot between cubic-CdS and tetragonal FTO. The figure 2.3(c) shows the selected area diffraction of CdS (region 1) with a fractured ring pattern, which shows the nanocrystalline structure of the CdS film. The FTO substrate (figure 2.3(d): region 2) presents a highly oriented dot pattern, which presents highly single crystalline material. 2.3.3 Surface Property The Raman scattering measurement was performed at room temperature by using an Argon ion laser with excitation wavelength at 514.5nm. Figure 2.4 shows the observed Raman spectrum of the deposited CdS films on FTO coated glass substrate. Two phonon peaks at 298.5 cm-1, and 592.5 cm-1 represent 1LO 13 (longitudinal optical) mode and 2LO mode. The full with at half maximum (FWHM) of the 1LO is 24.1 cm-1. The phonon peaks position agreed well with the reported result in earlier studies [66, 67]. Compared to bulk CdS (1LO: 305 cm-1), it shows the down-frequency shift of the 1LO Raman peak. The reason may come from the grain-size effect. However, none TO (transverse optical) vibration mode was found in CdS/FTO sample. Tong et al. [68] explained that lattice mismatch and thermal expansion coefficient mismatch between CdS film and different substrates could cause the deformation potential. Figure 2.5 (a) ~ (c) show AFM scanned 2D and 3D (insert) images of the bare FTO glass (RMS = 8.15 nm), one-pass deposited films (RMS = 11.32 nm) and two-pass deposited films (RMS = 8.11 nm). The RMS roughness of the one-pass deposited CdS films is 11.32 nm, which is higher than 7nm RMS for the CdS film deposited on ITO glass. This difference comes from the FTO glass substrate with higher roughness than the ITO glass surface. Kim et al. [69] also reported the RMS roughness between 7 and 15 nm depending on the thiourea concentration in chemical solution deposition. The results show that a lower RMS value is obtained by a higher thiourea mole concentration. The other finding in this research is that the RMS value of the two-pass deposited film is lower than the one-pass deposited film because of the voids being filled out after two-pass deposition. XPS was performed to obtain the chemical binding information of the CdS films. Figure 2.6 shows the presence of oxygen (O), cadmium (Cd), carbon (C), and sulfur (S) from CdS layer. Figure 2.6 (a) shows the oxygen on the surface at 532.2 eV due to the formation of hydroxide (Cd(OH)2) layer. The data obtained were corrected by taking the specimen charging and referring to C 1s at 284.9 eV 14 (figure 2.6 (c)), which was originated in the atmospheric contamination. For the as-received condition, the binding energies of Cd 3d3/2 and Cd 3d5/2 shows at 411.7 eV and 404.8 eV respectively. These spectrums of Cd and S after etching can be fitted by Gaussian function (red dot lines). For Cd 3d energy level, the binding energy of 3d3/2 and 3d5/2 orbital are 412.7 eV and 405.9 eV respectively, with the splitting energy of 6.8eV. For S 2p energy level, the binding energies of S 2p3/2 and S 2p1/2 orbitals are 162.2 eV and 163.4eV respectively, with the splitting energy of 1.2eV. The obtained result shows exactly agreement with previous researches [70, 71]. 15 2.3.4 Optical Property The optical band gap (Eg) of CdS thin film was characterized by absorption spectroscopy and determined from the formula 𝛼ℎ𝜐 = 𝐴(ℎ𝜈 − 𝐸𝑔 )𝑛 (2-1) where hυ is the incident photon energy, A is a constant, α(cm-1) is the absorption coefficient, and the exponent n depends on the type of transition, n = 1/2 and 2 for direct and indirect transition, respectively. By using equation (2-2), the absorption coefficient α can be obtained by the measured wavelength-dependent transmittance (T), and the film thickness (t) was determined by TEM observation. 𝑇 = exp(−𝛼𝑡) (2-2) Figure 2.7 shows the Tauc plot of the 10 minutes deposited CdS thin film (251.72 nm). The optical band gap is determined by extrapolating the curve at around 2.44eV. 2.4 Conclusion The continuous MASD process was successfully demonstrated to deposit dense CdS thin films on FTO substrate. The average growth rate at 25.2 nm/min is significantly higher than batch CBD process. The CdS film shows a preferred orientation in cubic-(111), which become more significant than the tetragonal-(110) from FTO substrate with increasing the film thickness. The nanocrystalline structure was observed by HRTEM through FIB specimen preparation. Two-pass deposition can fill out the voids created by the one-pass deposition. The roughness of two-pass deposited film (RMS = 8.11nm) shows equivalent as the bare FTO substrate (RMS = 8.15nm). 16 Acknowledgement The work was funded by the US Department of Energy, Industrial Technologies Program through award #NT08847, under contract DE-AC-05-RL01830 to PNNL. We are thankful to Dr. Yi Liu in Oregon State University Microscope Facility for his assistance on FIB sample preparation and TEM operation. 17 Figure 2.1 Schematic diagram of microreactor-assisted solution deposition (MASD) process. 18 Figure 2.2 XRD spectrum of (a) bare FTO glass substrate, (b) CdS layer on FTO after one-pass deposition and (c) two-pass deposition, (d) SnO2 (#411445, Tetragonal), (e) CdS (#750581, Cubic), and (f) CdS (#653414, Hexagonal). 19 Figure 2.3 (a) Low magnification image of CdS/FTO structure (magnification: 75,000X) (b) HRTEM images of the CdS/FTO boundary (magnification: 620,000X). (c) Fast Fourier transformed diffraction pattern of CdS layer (region 1). (d) Fast fourier transformed diffraction pattern of FTO layer (region 2). 20 Figure 2.4 Raman spectra of CdS layer deposited on FTO substrate 21 Figure 2.5 AFM images of (a) bare FTO substrate (RMS = 8.15 nm), (b) one-pass deposited CdS film (RMS = 11.32 nm), and (c) two-pass deposited CdS film (RMS = 8.11 nm) (insert: 3D morphological images). 22 Figure 2.6 XPS spectra (dashed line: as received, solid line: after etching, red dot line: Gaussian fitting) of the CdS films deposited by continuous MASD (a) O 1s, (b) Cd 3d, (c) C 1s, (d) S 2p 23 Figure 2.7 Plot of (αhν)2 versus hν showing the band gap energy of 251.72 nm CdS films by continuous MASD process. 24 Chapter 3 Study of Growth Kinetics for the CdS Thin Films by Continuous Microreactor-Assisted Solution Deposition (MASD) on Fluorine-doped Tin Oxide Surface Yu-Wei Su1, Sudhir Ramprasad2, Daniel R. Palo2, Brian K. Paul3,4 and Chih-hung Chang1,4* 1 Oregon State University, School of Chemical, Biological & Environmental Engineering, Corvallis, OR 97330, USA 2 Energy Processes & Material Division, Pacific Northwest National Laboratory, Corvallis, OR 97330, USA 3 School of Mechanical, Industrial & Manufacturing Engineering, Oregon State University, Corvallis, OR 97330, USA 4 Oregon Process Innovation Center for Sustainable Solar Cell Manufacturing, Corvallis, OR 9730, USA [*] E-mail: chih-hung.chang@oregonstate.edu Abstract Microreactor-assisted solution deposition (MASD) process was used to deposit CdS thin films on fluorine doped tin oxide (FTO) glass. The film growth kinetics was studied by measuring the film thickness deposited from 1 minute to 15 minutes in physical (FIB-TEM) and optical (reflectance spectroscopy) approaches. The crystalline structure was identified by grazing-incidence XRD (GIXRD) and presented a preferred orientation at cubic (111) plan. A new proposed modified model incorporated the emperical residence time factor (η) obtaines the surface reaction rate (k0) of 1.61*106 cm4 mole-1 s-1 and R-square value of 0.9695. Comparing to the saturation growth region in the batch process, MASD process can prevent the free particle formation by separating homogeneous reaction from heterogeneous surface reaction. This unique property of MASD process alters the growth kinetics from within saturation growth to only a linear growth. 25 3.1 Introduction CdS is regarded as an excellent heterojunction partner for p-type CdTe or as a buffer layer in p-CuInSe2 solar cells. In these applications, only a thin layer around 50 to 1000 nm is required. In addition to solar cells, other applications including photochemical cells, light meters, and image intensifiers require a working thickness of CdS above 1μm which is much more challenging to achieve by a batch CBD process. For example, Oladeji [32] reported that thicker CdS film (400 ~ 500 nm) can be obtained by a batch CBD process. The entire deposition time took between 2 hours to 4 hours. Microreactor-assisted solution deposition (MASD) offers a solution to overcome this challenge by continuously supply optimum reacting chemical solution to the surface [38]. High quality, high-rate deposition of thick CdS thin films have been demonstrated by MASD approach [39]. In this paper, we use a laboratory scale MASD system to investigate the growth kinetics of CdS thin films. During the mid-1960’s to 1970’s, Kitaev et al. and Betenekov et al. [20-22] applied thermodynamic equilibrium foundation to study the solubility of cadmium hydroxide (Cd(OH)2) and cadmium-ammonia complex ions (Cd(NH3)42+) in alkaline solution. They suggested a solid phase precipitate Cd(OH)2 in the solution provides the catalytic surface for decomposing thiourea and releasing the sulfur ion (S2-) for CdS films formation. Aside from the thermodynamic study, quantum mechanical calculations and radiochemical experiments were also performed to explore the possible dissociation mechanism of thiourea and the reaction with Cd(NH3)42+ ions. Kitaev and Betenekov’s efforts have made significant contribution to better understand the solution chemistry of chemical bath 26 deposition. In the 1990’s, the growth mechanisms of CdS thin film were studied more thoroughly using in-situ measurement techniques. J. M. Dona et al [28] applied an in-situ electrochemical open-circuit potential change (EOCPC) method to study the CBD CdS growth kinetics in combination with scanning electron microscopy (SEM) observation of thin film morphology. Lincot et al [29] reported the first in-situ quartz crystal microbalance (QCM) and electrochemical impedance spectroscopy (EIS) study of CBD CdS thin film deposition. The principle of QCM for measuring film thickness is to detect the frequency changes of a quartz sensor based on different amount of material adsorbed on its surface. The frequency change data is then converted to film thickness. The other method, EIS, uses two parallel capacitors and a resistor. Equivalent film thickness data can be converted from the capacitance values. An important conclusion from these studies is to identify three major growth regimes in batch CBD CdS deposition. The first regime, induction/coalescence, and the second regime, compact layer growth, are composed of an inner compact layer. The third regime is the outer porous layer growth, which occurs at a longer reaction time. Later, Ortega-Borges et al. [30] reported the use of in-situ QCM to monitor the CdS film growth kinetics. The kinetic growth mechanism was established by investigating different variables in the CBD reaction, including concentrations of cadmium salt, thiourea, aqueous ammonia, ammonium salt, and reaction temperature. According to their mechanism, free Cd2+ ions forms due to the dissociation of cadmium salt precursor CdSO4 → Cd2+ + SO2− 4 (3-1) In the presence of ammonium hydroxide, the equilibrium of ammonia and 27 ammonium ions (as buffer reagent) follows the relationship. OH − + NH4+ ⇄ NH3 + H2 O (3-2) Then the free cadmium ion binds with ammonia molecular to form a complex with a coordination number of four. Cd2+ + 4NH3 ⇄ Cd(NH3 )2+ 4 (3-3) Thiourea (CS(NH2)2) dissociates in a basic environment to slowly release HS- ions and then undergoes a second reaction to form S2- ions. CS(NH2 )2 + OH − → HS − + CH2 N2 + H2 O (3-4) HS − + OH − ⇄ S 2− + H2 O (3-5) When the ionic product exceeds the solubility limit of CdS (~10-25), the homogeneous nucleation and particle formation of CdS begins by the reaction of free cadmium ions and sulfide ions. Cd2+ + S 2− → CdS(s) (3-6) Based on Ortega-Borges’s reported experimental data, the growth mechanism is no longer considered atom-by-atom growth but growth by complex adsorption and decomposition mechanism. The CdS formation model is proposed as following. (I). Reversible adsorption of cadmium mechanism hydroxide species k 1 Cd(OH) − Cd(NH3 )2+ 2,ads + 4NH3 4 + 2OH + site k (3-7) -1 (II). Formation of a surface complex with thiourea k 2 [CdSC(NH2 )2 (OH)2,ads ] Cd(OH)2,ads + SC(NH2 )2 ∗ (3-8) (III). Formation of CdS with site regeneration ∗ k 3 CdS + CH2 N2 + 2H2 O + site [CdSC(NH2 )2 (OH)2,ads ] (3-9) 28 The growth rate is derived from above reactions 𝑟= − 2 𝑘1 𝑘2 𝐶𝑠 [𝐶𝑑(𝑁𝐻3 )2+ 4 ][𝑆𝐶(𝑁𝐻2 )2 ][𝑂𝐻 ] 𝑘1 𝑘2 [𝑂𝐻 − ]2 [𝐶𝑑(𝑁𝐻3 )2+ 𝑘−1 [𝑁𝐻3 ]4 +𝑘1 [𝑂𝐻 − ]2 [𝐶𝑑(𝑁𝐻3 )2+ 4 ]+𝑘2 [𝑆𝐶(𝑁𝐻2 )2 ]+ 4 ][𝑆𝐶(𝑁𝐻2 )2 ] 𝑘3 (3-10) Dona et al. [31] proposed a modified heterogeneous surface reaction mechanism supported by SEM images and XPS data. The adsorbed complex intermediates Cd(OH)2 proposed by Kitav and Ortega-Borges with hydroxy ions are modified to become a dihydroxy-diammino-cadimiumn complex Cd(OH)2(NH3)2. Previous reactions proposed by Ortega-Borges were re-written in (3-11) to (3-13). The reasons behind this proposing change is that the transition metal easily exists in the form of aqueous-ammonia complexes with coordination number of four and the hydroxy (OH-) ions have a high tendency to be adsorbed on the glass surface. Reaction (3-7) is modified to k 1 [Cd(NH ) (OH) − Cd(NH3 )2+ 3 2 2,ads ] + 2NH3 4 + 2OH + site k -1 (3-11) Reaction (3-8) is modified to k 2 [Cd(NH3 )2 SC(NH2 )2 (OH)2,ads ] [Cd(NH3 )2 (OH)2,ads ] + SC(NH2 )2 ∗ (3-12) Reaction (3-9) is modified to ∗ k 3 CdS + CH5 N3 + NH3 + 2H2 O + site [Cd(NH3 )2 SC(NH2 )2 (OH)2,ads ] (3-13) The growth rate is derived from above reactions 𝑟= − 2 𝑘1 𝑘2 𝐶𝑠 [𝐶𝑑(𝑁𝐻3 )2+ 4 ][𝑆𝐶(𝑁𝐻2 )2 ][𝑂𝐻 ] 𝑘1 𝑘2 [𝑂𝐻 − ]2 [𝐶𝑑(𝑁𝐻3 )2+ 𝑘−1 [𝑁𝐻3 ]2 +𝑘1 [𝑂𝐻 − ]2 [𝐶𝑑(𝑁𝐻3 )2+ 4 ]+𝑘2 [𝑆𝐶(𝑁𝐻2 )2 ]+ 4 ][𝑆𝐶(𝑁𝐻2 )2 ] 𝑘3 (3-14) 29 So far, the study of the CdS thin films growth model was focused on heterogeneous surface reaction by introducing the assumed cadmium complex-thiourea intermediate. Considering the role of homogeneous reaction in the bulk solution phase, Kostoglou et al. [33-36, 72] developed a complete comprehensive mathematical model to study temporal evolution of reagent concentrations and the rate of nucleation, particle growth, coagulation and particular deposition. In CBD process, the mass balance equations of Cd are given as 𝑑[𝐶𝑑] 𝑑𝑡 =− 𝐴𝑟 𝑉 𝜌 ∞ 𝜌𝛼 − 𝛿 𝑚 ∫0 𝐺(𝑥)𝑓(𝑥, 𝑡)𝑑𝑥 − 𝑚 𝐻(𝑆) 𝑤 (3-15) 𝑤 [𝐶𝑑] = [𝐶𝑑 2+ ] + [𝐶𝑑(𝑁𝐻3 )2+ 4 ] (3-16) The nomenclature of all symbols is listed in table 3.1. The term on the left-hand side of equation (3-15) represents the temporal evolution of the total cadmium ions concentration, including the cadmium-ammonia complex ion. The terms on the right-hand side (from left to right) represent the Cd ions consumed for film growth, particle growth in the bulk, and generation of new nuclei. The mass balance equation of thiourea is given as 𝑑[𝑆𝐶(𝑁𝐻2 )2 ] 𝑑𝑡 =− 𝐴𝑟 𝑉 𝜌 ∞ − 𝑘𝐻 [𝑆𝐶(𝑁𝐻2 )2 ][𝑂𝐻 − ] − (1 − 𝛿) 𝑚 ∫0 𝐺(𝑥)𝑓(𝑥, 𝑡)𝑑𝑥 (3-17) 𝑤 The terms on the right-hand side of equation (3-17) represent (from left to right) consumption of thiourea for film growth, hydrolysis, and particle growth in the bulk. The mass balance equation of sulfide ions are given as 𝑑[𝑆] 𝑑𝑡 𝜌 ∞ 𝜌𝛼 = 𝑘𝐻 [𝑆𝐶(𝑁𝐻2 )2 ][𝑂𝐻 − ] − 𝛿 𝑚 ∫0 𝐺(𝑥)𝑓(𝑥, 𝑡)𝑑𝑥 − 𝑚 𝐻(𝑆) [𝑆] = [𝑆 2+ ] + [𝐻𝑆 − ] 𝑤 𝑤 (3-18) (3-19) The term on the left-hand side of equation (3-18) represents the time evolution of 30 total sulfide ions concentration, including hydrosulfide ions. The terms in the right-hand side of equation (3-18) represent (from left to right) sulfide ions produced by thiourea hydrolysis, sulfide ions consumed for particle growth in the bulk, and sulfide ions consumed for generation of new nuclei. The particle population balance is given as 𝜕𝑓(𝑥,𝑡) 𝜕𝑡 1 𝜕𝐺(𝑥)𝑓(𝑥,𝑡) 𝜕𝑥 ∞ ∞ = 2 ∫0 𝐵(𝑦, 𝑥 − 𝑦)𝑓(𝑦, 𝑡)𝑓(𝑥 − 𝑦, 𝑡)𝑑𝑦 − 𝑓(𝑥, 𝑡) ∫0 𝐵(𝑥, 𝑦)𝑓(𝑦, 𝑡)𝑑𝑦 − − 𝐷(𝑥)𝑓(𝑥, 𝑡) + 𝐻(𝑆)𝛿(𝑥 − 𝛼) (3-20) The rate of nucleation is expressed as 𝐻= 𝐷𝑛 5/3 𝑉𝑚 𝑒𝑥𝑝 ( −∆𝐺𝑐𝑟𝑖𝑡 𝑘𝐵 𝑇 ) (3-21) where Dn is the diffusivity of the nucleus, Vm is the molecular volume of CdS, kB is the Boltzmann constant, and ∆Gcrit is the maximize free energy change at critical nucleus size, which can be expressed as ∆𝐺𝑐𝑟𝑖𝑡 = 16𝜋 2 𝜎3 𝑉𝑚 (3-22) 3 (𝑘𝐵 𝑇 𝑙𝑛 𝑆)2 where σ the value of is interfacial surface energy. From equation (3-6), S is given as the supersaturation ratio. 𝑆=( [𝐶𝑑2+ ][𝑆 2− ] 𝐾𝑠𝑝 1/2 ) (3-23) Assuming all particles are spherical shape, the particle growth rate is expressed as 𝐺(𝑥) = (36𝜋)1/3 𝑥 2/3 𝑚𝑤 𝑟 𝜌 (3-24) where G(x) is a function of particle volume “x”. Mw is molecular weight of CdS, ρ is the density, and r is the surface reaction rate. The coagulation rate is used for describing particle coagulation in colloidal solution based on Brownian motion theory. The Brownian motion rate is given as 31 𝑥 1/3 2𝑘 𝑇 𝐵 𝐵(𝑥) = 3𝜇𝑊 [2 + (𝑦) 𝑐 𝑦 1/3 + (𝑥 ) ] (3-25) where (x,y) is a pair of particles with volume “x” and “y”, μis the viscosity of the liquid. Wc is a constant. The last part is the particulate deposition rate, which is given as 2/3 1/2 0.3𝐷𝑝 𝑢∞ 𝐷(𝑥) = 𝑊 𝑑𝑣 1/6 𝐿 1/2 (3-26) where Dp is the diffusivity of particles of volume x, ν is the kinetic viscosity of the solution, 𝑢∞ is a characteristic velocity of the fluid, L the characteristic length of the submerged surface, and Wd the stability ratio for particulate deposition. The computational solved results provide abundant information on temporal evolution of reagent (Cd, thiourea, and sulfide) concentrations, film thickness evolution for various ammonia concentration, and final film thickness versus ammonia concentration for various pH values. A simplified model (3-27) was proposed to predict CdS film thickness and demonstrate the capabilities of solving the complete model. ℎ= 𝑚𝑤 𝑘0 [𝐶𝑑]0 [𝑆𝐶(𝑁𝐻2 )2 ]0 [𝑂𝐻 − ] (1 𝑘𝐻 𝜌[𝑁𝐻3 ]2 − 𝑒𝑥𝑝(−𝑘𝐻 [𝑂𝐻 − ]𝑡)) (3-27) where kH is the hydrolysis constant from equation (3-4), and k0 is the overall reaction rate constant. Two years later, Kostoglou et al. [37] reported using SEM to observe CdS film surface morphology on tin-oxide-coated (TOC) glass. SEM pictures showed that the discrete CdS particles on the surface tend to coalesce with neighboring ones to form a continuous surface with time. A model was developed to examine the temporal film thickness evolution of instantaneous surface nucleation and constant surface nucleation. 32 3.2 Experimental A commercial fluorine doped tin oxide (FTO) glass (Pilkington TEC-15) was employed as the substrate for CdS thin film deposition. Positive displacement pumps (Acuflow Series III) were used to pump reagents at a constant flow rate of 31 ml/min. Cadmium source reagent consisted of cadmium chloride (0.008 M), ammonium chloride (0.08 M), and ammonium hydroxide (0.08 M) in water. Sulfur source reagent consisted of thiourea (0.08 M) in water. The reagents from the two streams were mixed in a T-junction micromixer before entering the heat exchanger. The mixed reagent was heated to 85 °C in the heat exchanger and impinged to the FTO glass substrate, which was held at a surface temperature at 80 °C. The experimental set-up is illustrated in Figure 3.1. The deposition time was varied from 1 minute to 15 minutes for kinetics study. After deposition, the film was rinsed with DI water to remove any particulates and by-products from the substrate. The thin film characterization was performed on the as-deposited films. 3.3 Characterization 3.3.1 Thickness The film thickness measurement attracts great interests due to the effects of thickness on optical and electrical properties. Many techniques have been developed so far to accurately measure the thickness of different materials. Choosing a proper technique is very important in thin film metrology. All measurement techniques can be classified as either mechanical or optical approaches. Mechanical approaches, such as AFM and surface profiler, use a tiny 33 stylus dragged on the surface, and the result is presented as a 3D surface plot or 2D line plot. Stylus surface profiler could not provide reliable measurement on these samples due to the significant roughness of FTO surface. The other approach is using microscopy, which relies on FIB (focus-ion-beam) to etch a cross-section surface for observation. This process needs to been performed in the chamber of scanning electron microscopy (SEM). The optical approach, spectroscopic ellipsometry, measures the phase shift and intensity of electromagnetic wave propagating in different media. Reflectance spectroscopy measures the intensity of reflected light from the surface. Both of these two optical approaches do not need surface contact and specimen preparation in vacuum, but refractive index (n) and extinction coefficient (k) values for thickness fitting is required. The comparison of all above approaches is showed in table 3.1. Therefore, a proper measurement approach can obtain the accurate film thickness. In this research, reflectance spectroscopy was applied to measure the film thickness. Direct observation of film thickness was done by TEM to calibrate the reflectance spectroscopy measurement. A film stack model, FTO/Soda lime glass, was initially built to fit the reflectance spectrum of a bare FTO glass substrate according to the harmonic oscillator model, which was expressed as following. m 𝜀(𝐸) = 1 + j 0 1 𝐴𝑗 𝑒 −𝑖𝜃𝑗 (𝐸+𝐸 𝑗 +𝑖Γ𝑗 1 − 𝐸−𝐸 𝑗 +𝑖Γ𝑗 ) (3-28) The (E) represent the complex dielectric constant as a function of electron energy (E). Four parameters, Aj, Ej, j, and j for j = 0, 1, 2 were obtained by getting a minimize MSE (mean square error) value. The reflectance (R) can be expressed in 34 terms of n and k values (Appendix A). The calculated thickness of FTO layer was 370 nm, which match well with TEM observation. A combination layer of CdS/void/FTO was added on top of the FTO layer with fixed parameters to improve the spectrum fitting [73] and obtain the desired CdS film thickness. Figure 3.2 (a) ~ (h) show the measured and calculated reflectance spectrum of various CdS film thickness deposited from 1 to 15 minutes. It can be observed that the fitting improves with the increased film thickness. 35 3.3.2 Micro Structure The FIB lift-out process was commonly used for TEM specimen preparation. Figure 3.3 (a) ~ (e) illustrate the FIB process for the TEM specimen preparation. First, Platinum was deposited in a rectangular area of 10 μm by 2 μm with a 0.5 μm thick by electron beam on CdS surface (Figure 3.3 (a)). The platinum coating is to protect the specimen from damage during ion milling. Then, two areas near the Platinum deposited area were milled down to a 3 μm depth by gallium ion bombardment (figure 3.3 (b)). The cross-section of the vertical surface was formed and cleaned by the ion-beam. After that, figure 3.3 (c) shows the omniprobe with a sharp tip that was inserted to approach the vertical surface and then welded with the surface by depositing Platinum ions. Then ion milling was used to cut the edges connecting to the substrate. Once the specimen was separated from the substrate, the omniprobe with specimen attached was carefully lifted (Figure 3.3 (d)) and placed onto a specimen support. The last step was to weld the specimen on a support and then detach the omniprobe (Figure 3.3 (e)). Finally, the specimen requires ion-bombardment to mill the width from 2 μm to 100 nm in order to get high quality TEM images. Figure 3.4 (a) ~ (h) show the TEM images of the CdS film deposited from 1 min to 15 min. The thickness of each specimen is averaged by 5 different locations (red double arrows). Many of the large grains beneath the CdS layer are crystalline structured FTO layer. Figure 3.5 shows the grazing-incidence (0.5⁰) X-ray diffractograms of various CdS film thicknesses. Three peaks of bare FTO layer are index to T(110), T(101), and T(200) of tetragonal SnO2 (JCPDS-411445). A comparison between 36 the CdS/FTO and the bare FTO layer reveals that the first peak appears obviously in the thicker CdS films. The first peak (26.45⁰ ~ 27.0⁰) agrees well with C(111) of cubic CdS (JCPDS-750581) and H(002) of hexagonal CdS (JCPDS-653414) [74]. Due to the absence of H(100) and H(101) plans, the as-deposited CdS films is considered to have cubic structure. 37 3.4 Growth Kinetics The growth kinetics was studied by fitting the thickness data points to a theoretical model. All spectroscopic reflectance measurement results are shown in Appendix B. Before studying the growth kinetics, calibration work was performed first to obtain the correlation of optical thickness and physical thickness. Figure 3.6 shows the correlation factor of 0.9104 (ideal value is 1) and R-square value of 0.9803, which indicate the optical measurement is quite reliable. For the conventional batch process, Kostoglue et al. [34] reported a model (3-27) to fit the experimetal results given by Dona et al. [31]. ℎ= 𝑚𝑤 𝑘0 [𝐶𝑑]0 [𝑆𝐶(𝑁𝐻2 )2 ]0 [𝑂𝐻 − ] (1 𝑘𝐻 𝜌[𝑁𝐻3 ]2 − 𝑒𝑥𝑝(−𝑘𝐻 [𝑂𝐻 − ]𝑡)) (3-27) The h is film thickness, and each chemical species is substituted by [Cd]0 = 0.025*10-3 mole/cm3, [SC(NH2)2]0 = 0.035*10-3 mole/cm3, [OH-] = 0.005*10-3 mole/cm3, [NH3] = 1.68*10-3 mole/cm3, and k0 is the surface reaction rate obtained by curve fitting. The molecular weight (MW) of CdS is 144.6 g/mole, and density (ρ) is 4.82 g/cm3. Marcotrigiano et al. [75] in 1972 reported the thiourea hydrolysis rate (kH) is about 0.0263 cm3 mole-1 s-1 at 80 °C. The kH at 60 °C, 70 °C is 0.0058 cm3 mole-1 s-1 and 0.0127 cm3 mole-1 s-1 respectively. These two values can be calculated by Arrhenius equation with the activation energy of 17867 cal/mole. Figure 3.7 shows the fitting results along with the experimental growth data from the batch procss at 60 °C, 70 °C and MASD based on equation (3-27). In this fitting analysis, k0 and kH were set as target fitting parameters in figure 3.7 (a), and k0 as only target fitting parameter in figure 3.7 (b). The adjusted R-square of these three series data all show good fitting results ( > 0.95). The fitting results were 38 listed in Table 3.2. The obtained hydrolysis rate is 306.8 cm3 mol-1 s-1, 716.8 cm3 mol-1 s-1 and 421.2 cm3 mol-1 s-1, respectively for 60oC batch, 70oC batch and MASD data. These values are significantly higher than the hydrolysis rate reported by Marcotrigiano et al. One could possibly attributed this higher thiourea hydrolysis rate to the catalytic surface provided by the Cd(OH)2 precipitaes. This explanation would also result in significantly higher homogeneous particle formation. The feature of the continous MASD process is to separate the residence time (τ) of homogenious reaction in a microreactor and the deposition time (t) of heterogenous reaction on substrate surface. From the previous result in figure 3.8 [39, 76], the growth rate of CdS films deposited on SiO2/Si substrate at various surface temperature (60 ~ 80 °C) shows an increasing trend with the residence time, and decreasing at 70 s. This observation arise a hypothesis that much longer residence time can not enhance the film growth rate because the forming of CdS nanoparticles consume the thiourea concentration. Considering the residence time effect, Yu-Jen Chang’s result [76] was used to obtain the emperical residence time factor for the continous MASD process instead of Kostoglou’s model. The modified model originated from Dona’s derived equation (3-14). 𝑟= − 2 𝑘1 𝑘2 𝐶𝑠 [𝐶𝑑(𝑁𝐻3 )2+ 4 ][𝑆𝐶(𝑁𝐻2 )2 ][𝑂𝐻 ] 𝑘1 𝑘2 [𝑂𝐻 − ]2 [𝐶𝑑(𝑁𝐻3 )2+ 𝑘−1 [𝑁𝐻3 ]2 +𝑘1 [𝑂𝐻 − ]2 [𝐶𝑑(𝑁𝐻3 )2+ 4 ]+𝑘2 [𝑆𝐶(𝑁𝐻2 )2 ]+ 4 ][𝑆𝐶(𝑁𝐻2 )2 ] 𝑘3 (3-14) Compared to k-1[NH3]2, the other terms in denominator can be neglected due to the small values [30]. The equation (3-14) is rewritten in (3-28). 𝑟=𝑘 [𝐶𝑑]0 [𝑆𝐶(𝑁𝐻2 )2 ]0 [𝑂𝐻 − ]2 [𝑁𝐻3 ]2 (3-28) The left term r (reaction rate of CdS) is rearranged and expressed in the thickness 39 growth rate. Also, the initial thiourea concentration ([SC(NH2)2] 0) should be substitute to the effective thiourea concentration by multiplying an empirical factor (η), which is a function of residence time (τ). Then, (3-28) is modified to (3-29). dh dt = mw k0 [Cd]0 [OH− ]2 [SC(NH2 )2 ]0 (exp(Aτ) ρ[NH3 ]2 − exp(Bτ)) (3-29) Equation (3-29) is used for fitting the CdS film growth rate on SiO2/Si substrate at the surface temperature from 60°C to 80 °C. The average constant of A and B are -0.003682 and -0.3106, respectively. Each chemical specie is substituted by [Cd]0 = 0.004*10-3 mole/cm3, [SC(NH2)2]0 = 0.04*10-3 mole/cm3, [OH-] = 0.001*10-3, and [NH3] = 0.655*10-3 mole/cm3. Then, a derived equation (3-30) is used for fitting the data from continous MASD process in Figure 3.9. ℎ = 1.11 × 10−7 𝑘0 𝑡(𝑒𝑥𝑝(−0.003682𝜏) − 𝑒𝑥𝑝(−0.3106𝜏)) (3-30) The thickness value of MASD process in figure 3.9 is obtained by multiplying the correlation factor (Figure 3.6) from optical thickness. The final fitting result shows R-square value and k0 are 0.9695 and 1.61*106 cm4mole-1s-1, respectively. The Kostoglue’s model (3-27) presents ideal fitting only in the initial linear region of batch process, when kH is set 0.0263 cm3mole-1s-1. Comparing to the batch process, the growth kinetics of the CdS films by MASD process presents a linear growth rate and without saturation region. It is concluded that the MASD process can improve free homogenous particle formation by continuously impinging fresh reagent to the surface. 40 3.5 Conclusion The CdS films by continuous MASD process have been characterized and calibrated by FIB-TEM and reflectance spectroscopy. GIXRD was successfully applied to identify the CdS films with a preferred orientation on the C(111) plan. The thiourea hydrolysis rate (kH) is already considered in the empirical factor (η) based on previous experimental result. Applying this factor in the proposed modified model gives a surface reaction rate of 1.61*106 cm4mole-1s-1. With the feature of separating homogeneous and heterogeneous reaction, the MASD process can alter the behavior of the saturation growth region in the bath process to linear growth. Acknowledgement The work was funded by the US Department of Energy, Industrial Technologies Program through award #NT08847, under contract DE-AC-05-RL01830 to PNNL. Additional matching funds were received from the Oregon Nanoscience and Microtechnologies Institute (ONAMI) under a matching grant to Oregon State University. We are grateful to Mr. Don Higgins for his assistance in the experimental setup and operation and to Dr. Jair Lizarazo-Adarme for his help with data acquisition and control. Special thanks to Dr. Leo Asinovski from Semiconsoft, Inc. for his help on model fitting of reflectance spectrum. We are also thankful to the staffs in Oregon State University Microscope Facility and the Center for Advanced Materials Characterization in Oregon (CAMCOR) their assistance on FIB sample preparation and TEM operation. 41 List of Symbols A B(x) D(x) f(x,t) G(x) H(x) h kH Substrate area Coagulation rate Particulate deposition rate Particle number density population Particle growth rate Nucleation rate Film thickness Thiourea hydrolysis rate constant k1 k-1 Forward rate constant of the adsorption reaction for complex cadmium and hydroxide Reverse rate constant of the dissociation reaction for complex cadmium k2 k3 Mw r S t hydroxyl ions Rate constant of the formation of a surface complex with thiourea Rate constant of the formation of CdS with site regeneration Molecular weight (g/mole) Surface reaction rate Supersaturated ratio time V x y α ρ δ Reactor volume Particle volume Particle volume Volume of nucles Density of solid CdS =0 (completely film growth) =1 (completely particle growth) 42 Figure 3.1 Schematic diagram of continuous MASD process for CdS films growth kinetic study. Vacuum Advantage Disadvantage Requirement Surface No profilometry Output Information Easy and quick Mask required to make a scan Thickness v.s horizontal scan length AFM No Precise surface Mask required morphology SEM Yes (FIB lift-out) 3D or 2D images Physical and Difficult Real surface accurate specimen morphology thickness is preparation achieved Spectroscopic No Non-destructive Collecting data and v.s Ellipsometry measurement and model wavelength fitting both take time. Reflectance spectroscopy No Non-destructive Requires film Reflectance v.s and quick stack model for wavelength measurement curve fitting. Table 3.1 Comparison of various film thickness measurement techniques. 43 Figure 3.2 Measured reflectance (solid) and calculated reflectance (dotted) of the continous MASD deposited CdS films with a deposition time of (a) 1 min, (b) 2 min, (c) 3 min, (d) 4 min, (e) 5 min, (f) 8 min, (g) 10 min, and (h) 15min. 44 Figure 3.3 Schematic diagrams (a) ~ (e) of TEM specimen prepartion by FIB process. 45 Figure 3.4 TEM cross sectional images of the continous MASD deposited CdS films with a deposition time of (a) 1 min, (b) 2 min, (c) 3 min, (d) 4 min, (e) 5 min, (f) 8 min, (g) 10 min, and (h) 15min. 46 Figure 3.5 GIXRD of the continuous MASD deposited CdS films with a deposition time of 0 to 15 min. 47 Figure 3.6 Instrumental calibration curve of CdS films by MASD process during various deposition times (Correlation factor is 0.9104). 48 Figure 3.7 Growth kinetics of CdS thin film deposited by batch process at 60 °C, 70 °C, and continuous MASD process. The series data of batch process are from Dona et al. [31]. Solid fitted lines are based on Kostoglue’s model (3-27) with non-fixed kH (a), and fixed kH (b). 49 Batch Batch MASD at 70°C at 60°C at 80°C (Dona et al.) (Dona et al.) Two fit parameters k0 = 2.9*105 kH = 716.8 k0 = 1.2*105 kH = 306.8 k0 = 7.8*105 kH = 421.2 Fit k0 (cm4 mole-1 s-1) k0 = 1.49*105 k0 = 6.55*104 k0 = 6.8*105 Fixed kH (cm3 mole-1 s-1) kH = 0.0127* kH = 0.0058* kH = 0.0263 Fit k0 (cm4 mole-1 s-1) Fit kH (cm3 mole-1 s-1) One fit parameter Table 3.2 Fitting results of Kostoglue’s redudecd model for batch and microreator-assisted processes. −𝐸 Calculated by 𝑘𝐻 = 𝐴𝑒𝑥𝑝 ( 𝑅𝑇𝑎 ), * Ea = 17867 cal/mole, 50 Figure 3.8 The CdS film growth rate on SiO2/Si substrate at various surface temperature versus residence time, and the fitting curve (solid line) based on equation (3-29). 51 Figure 3.9 Growth kinetics of CdS thin film deposited by continuous MASD process. The fitting line is based on the modified model (3-30). The kH is fixed at 0.0263 cm3mole-1s-1. 52 Chapter 4 Investigation of CdS Nanoparticle Formation and Deposition using Continuous Microreactors 4.1 Introduction In 1996, Alivisatos published an article [77], “Semiconductor clusters, nanocrystals and quantum dots” in the journal “Science”. A semiconductor cluster is defined as a fragment of semiconductor consisting of hundred to many thousands of atoms. Generally speaking, any material with a dimension of less than 100 nm should be referred to as a nanoparticle. The term “nanocrystal” or “nanocrystalline” can be used to describe nanoparticles with a single crystalline structure. A quantum dot combines all of these concepts as a semiconductor matter whose excitons are confined in all three spatial dimensions. The physical characteristic of the spatial confinement effects can cause quantum dots to have a higher band gap than the bulk material [78, 79]. So far, most science and engineering researchers regarded the nanocrystals with strongly size-dependent optical and electrical property as the quantum dots. Many researchers have reported that II-VI semiconductor nanocrystals, such as CdSe [80], CdS [81, 82], and ZnS [83, 84] can be synthesized by a continuous flow microreactor. It is well-known that the size-dependent optical property occurs when the size is smaller than the bulk Bohr radius. Edel et al. [81] first reported using a continuous flow microreactor to produce CdS nanoparticles. Sodium polyphosphate ((NaPO3)n) was added to the cadmium nitrate solution as a surfactant to stabilize the nanoparticles, and then the solution was mixed with sodium sulfide in the microchannel. Each inlet stream was split into two substreams and each substream was split again into the other two substreams. The entire micromixer part was composed of totally 16 micro-scale substreams for each inlet. The flow rate was 53 controlled at 0.001 ~ 0.3 mL/min per each inlet and total mixing volume in the micromixer was less than 0.6 μL. Comparing to the bulk CdS band gap of 2.48 ~ 2.58 eV, the produced band gaps of the nanoparticles at room temperature were estimated at 3.6 eV (3.2 nm particle) and 2.4 eV (12 nm particle). After several years, Hung et al. [85] reported an alternative droplet generation device that can make CdS nanocrystals with the sizes from 4.2 nm (Eg = 2.9 eV) to 8.2 nm (Eg = 2.6 eV). This device has advantages of rapid and highly efficient mixing in the micro-channel. Tiemann et al. [83, 84] reported a kinetics growth study of ZnS nanoparticles by in-situ stopped-flow UV/Vis absorption spectroscopy. The temporal evolution of the particle size was estimated by deconvoluting the Uv-vis absorption spectrums. Recently, Shayeganfar et al. [82] reported effective mixing by turbulence dispersion to control CdS nanoparticles. Absorbance spectroscopy and TEM were applied to characterize the optical size and image size respectively. Mullaugh et al. [86, 87] also set CdS as a model to study the size-dependent spectroscopic properties of long-tern stable nanoparticles. In this study, the spectroscopy associated with a flow cell is utilized to take an in-situ measurement of the CdS nanoparticles precipitated in a commercial T-junction micromixer. The feature of this method is optical in-situ analysis instead of ex-situ TEM analysis. Nosaka’s finite potential model [79] and Tiemann’s [83] spectrum analysis approaches were introduced to study the effect of residence time on sulfide nanoparticle size. The growth kinetics can be obtained by fitting experimental data to three previous reported models. The first model is derived from the Ostwald ripening mechanism, which describes how small crystals re-dissolve while larger crystals 54 grow by consumption of the solute species. This mechanism can be described by Lifshitz-Slyozov-Wagner (LSW) model. 𝑑 − 𝑑0 = 𝑡1/𝑛 (4-1) where d is the particle diameter, d0 is the mean particle diameter at t = 0, a is the material constant, and n is expected to be between 2 and 4. The value of n represents the ripening controlled by surface diffusion at solid/liquid interface (n = 2), volume diffusion in the liquid medium (n = 3), and dissolution kinetics of initial species (n = 4). The second mechanism is called the orientated attachment mechanism. Ribeiro et al. [88] developed a simple kinetic model to describe the crystal growth by orientated attachment. This model is referred to as the barrierless coalescence model due to assuming no activation energy needed for coalescence. The particle size is described in (4-2) 2𝑘[𝐴] 𝑡+1 1/3 𝑑 = 𝑑0 ( 𝑘[𝐴] 0𝑡+1 ) 0 (4-2) where [A]0 and d0 are the concentration of particle and the mean diameter when t = 0; k is the rate constant and approximated by 𝑅𝑇 𝑘= (4-3) where R is the universal gas constant, T is the temperature, and η is the liquid viscosity. Huang et al. [89] proposed the third model (4-4), which is referred as barrier-controlled reorientation. 3 𝑑 = 𝑑0 ( √2𝑘 𝑡+1) (𝑘 𝑡+1) The rate constant k’ is related to activation energy. (4-4) 55 4.2 Experimental This experimental section includes the CdS nanoparticles formation in a T-junction micromixer (part I) and the integration with thin film deposition by MASD process (part II). Figure 4.1 (part I) shows the nanoparticle in-situ measurement system, composed of a peristaltic pump, a flow cell and a UV/Vis spectrophotometer. CdS nanoparticles were precipitated by mixing metal salt solution (0.0004M Cd(NO3)2*4H2O, 0.0004M sodium polyphosphate) and sodium sulfide solution (0.0004M Na2S*9H2O) through a T-junction micromixer. The spectrum was captured when the flow approaches the steady state conditions. To study the effect of residence time on particle size, each spectrum was captured by varying the flow rate of each stream from 0.5 mL/min to 9 mL/min. The estimated residence time could be calculated by dividing the constant internal tube volume by the real total flow rate. The purpose of the part II experiment is to investigate the effect of CdS nanoparticles in thin film deposition. The precipitation of nanoparticles was formed by pre-mixing metal salt and sodium sulfide solution, which are the same as part I (with sodium polyphosphate). Then, the nanoparticles are mixed with the reagents, which is composed of cadmium chloride solution (0.008M CdCl2, 0.08M NH4Cl, and 1.23M NH4OH) and thiourea solution (0.08M). The flow rates are set at 0.1 mL/min and 0.434 mL/min for pump 1 and pump 2, respectively. 56 4.3 Results and Discussion 4.3.1 Part 1: Nanoparticle Formation In order to calculate the optical particle size, the de-convolution method was derived from a finite potential model. The band gap difference of a nanoparticle and bulk status is described as in (4-5). 𝐸𝑙,𝑛 − 𝐸𝑔 = 𝐸𝑙,𝑛 + 𝐸𝑙,𝑛 − 2 1. (4-5) 𝑟 The ( = r0 = 5.04*10-11) is the actual permittivity, and the subscript notation (l, n) represent (0,0), (0,0)ˊ, (1,0) for the first excitonic transition, the first splittling transition and the second excitonic transition. The kinetic energies of an electron and a hole can be expressed as (4-6). 𝑖 𝐸0,0 = 0 0,0 [ + 0,0 2 𝑚∗𝑖 (𝑟 √𝑉0 +𝑐0,0 ) 𝑚 = 𝑒, ℎ (4-6) ] Numerical values of V0 (potential depth), me* (effective electron mass), mh* (effective hole mass), and me (electron mass) are available from previous studies. According to Nosaka et al [79], this finite potential model can be applied for CdS nanoparticles. These parameters of CdS potential model are V0 = 3.6 eV, me* = 0.19 me, mh* = 0.8 me and a0,0 = 0.0025, b0,0 = 0.325, c0,0 = 0.4 for an electron; a0,0 = 0, b0,0 = 0.4, c0,0 = 0.28 for a hole. Substituting all above parameters into (4-6), then the finite potential model of CdS can be derived and approximated as 1.711 𝐸0,0 − 𝐸𝑔 = 0.00 + (𝑟+0.4 4)2 0.5 + (𝑟+0.165)2 1.554 (𝑟+0.2514)2 (4-7) And the nanoparticle size (r: nm) is 1.554 𝑟 = √𝐸 0,0 −𝐸𝑔 − 0.2514 (4-8) 57 The absorbance spectrum data was approximated by the sum of three Gaussian functions and one exponential function. 3 𝐴= i 0 𝑎𝑖 𝑒𝑥𝑝 [ 𝜋 𝑤𝑖 √ −2(𝐸−𝐸𝑖 )2 𝑤𝑖2 2 𝐸 ] + 𝑐1 𝑒𝑥𝑝 (𝑐 ) 2 (4-9) where the subscript notation “1”, ”2” and “3” represent the first excitonic (E0,0), splitting (E0,0’), and second transition (E1,0) respectively. Figure 4.2 shows how the absorption spectra of CdS nanoparticles changes steeper with the increased volumetric flow rate. This result matches well with the previous reported results [81, 83]. As can be seen clearly, the higher volumetric flow rates result smaller sizes with a shorter residence time. Also, the spectra of CdS nanoparticles have a specific wavelength at which the same absorbance for different flow rates. This point is called the isosbestic point, which is used as reference points in the study of reaction rates. The isosbestic point of CdS nanoparticles located between 387 nm ~ 393 nm (3.16 ~ 3.20 eV), which is very closed to 3.12 eV [81]. For the least-square fitting analysis, the original 11 parameters in table 4.1 were reduced to 9 unconstrained parameters for the first stage analysis by constraining a2 = 0.4a1, and w2 = w1. The fitting process was performed by the GUI function (curve fitting tool) in MATLAB. After the first stage analysis, the preliminary particle size was calculated based on the energy difference of the first excitonic transition (E1). Then the obtained first splitting excitonic transition (E2) and the second excitonic transition (E3) were put into the (4-9) for the next calculation. The final results needed to be constrained by (4-10) 𝐸2 𝑟3 𝐴 − 𝐸𝑔 = (𝑟+ )2 (4-10) 58 Also the parameters of a3 and w3 should be constrained by the linear relationship with a1 and w1 in the second stage analysis. If E2 and E3 could not be constrained by (4-10), then the calculation process need to be repeated from the first stage analysis by guessing new 9 values of parameters for fitting until obtaining the optimized results. Finally, the values of E2 and E3 calculated from (4-10), and a3 = 1.848a1, w3 = 1.56w1 were substituted into (4-9) for the second stage analysis. Then the fitting accuracy was increased by reducing the unconstrained parameters from 9 to 5. Figure 4.3 and 4.4 show the de-convolution results of each spectrum at the first and second stage analysis, respectively. The peak position (Ei), intensity (ai), and full width at half maximum (wi) values and the constants (c1, c2) of the exponential function are showed in Appendix C. Figure 4.5 shows the plot of the energy difference at three excitonic transitions versus the particle size, which is calculated based on (4-8). The real lines represent equation (4-10) with different values of A and B for E2 and E3. Figure 4.6 shows the final fitting results of CdS namoparticle size growing with the residence time. The first (red square) and second (black circle) analysis shows similar particle sizes from the 1st and 2nd stage calculation. This indicates that the fitting process is stable and reliable. In order to understand the growth mechanism, the size data of the second analysis are fitted by three proposed models, Ostwald ripening (4-1), barrierless coalescence (4-2), and barrier-controlled reorientation (4-4). All the fitted parameters of these three models are listed in table 4.2. The obtained value from the Ostwald ripening model shows n ≥ 20, beyond the reasonable range (4 ≥ n ≥ 2). Hence, the Ostwald ripening model seems highly unlikely to explain the mechanism of precipitated 59 CdS nanoparticles in the flowing status. The barrier less coalescence model shows the value of [A]0 is 10-9 mole/L, which is much lower than the initial concentration (5*10-4 mole/L) of reagent solution. The low value would mean that the most precursor ions are still in solution before ripening [90]. Therefore this mechanism is still unable to describe the real situation. The solid line in figure 4.6 shows the fitting result by the barrier-controlled coalescence model proposed by Hung et al.[89]. The modified rate constant k’ is 2.872 s-1 much higher than 0.002 s-1 [87]. The possible reason for the variance could be the reported time scale of this long-term observation was lasting for several thousand seconds. 60 4.3.2 Part 2: Thin Film Deposition Figure 4.7 shows the photograph of the CdS films deposited by MASD process with and without combining precipitated CdS nanoparticles. It can be visually observed that the combination of CdS nanoparticles does not produce a visible yellow CdS films on the substrate. That indicates the combination of CdS nanoparticles hinder the CdS thin film growth rate of the original MASD process (Figure 2.1). A possible mechanism is that the CdS nanoparticles from precipitation (3-6) in stream E (figure 4.1: part II) acts as seeds after mixing with stream F. The already existing sulfide (S2-) ions from sodium sulfide solution will also precipitate with cadmium ions (3-6) from stream F. The consuming of sulfide ions changes the chemical species equilibrium and forwards the reaction (3-5) and (3-4) going to right hand side. CS(NH2 )2 + OH − → HS − + CH2 N2 + H2 O (3-4) HS − + OH − ⇄ S 2− + H2 O (3-5) Cd2+ + S 2− → CdS(s) (3-6) Therefore, the amount of thiourea adsorbed on the substrate surface to form thiourea-hydroxyl complex was decreased, and reaction (3-8) and (3-9) were reduced significantly. k 2 [CdSC(NH2 )2 (OH)2,ads ] Cd(OH)2,ads + SC(NH2 )2 ∗ k ∗ 3 CdS + CH2 N2 + 2H2 O + site [CdSC(NH2 )2 (OH)2,ads ] (3-8) (3-9) 61 4.4 Simulation of Residence Time Distribution (RTD) Residence time distribution (RTD) was calculated in this study to better understand the impacts of flow rate on the CdS nanoparticle synthesis within the continuous flow system. Adeosum and Lawal [91-94] compared the RTD function by computational fluidic dynamic (CFD) with experimental data of a multi-laminated micromixer and a T-junction micromixer. COMSOL 4.2 is used to simulate the flow distribution in the microchannel of the in-situ spectroscopic flow cell measurement system. Figure 4.8 shows the geometry of the flow system including a T-junction micromixer, an extended PEEK tube and a connection tube. The module of laminar flow and transport of diluted species are added in a time-dependent setting and solved by incompressible Navier-Stokes equations. The boundary condition “Normal inflow velocity” was used for two inlets, “zero pressure” for outlet, and “No slip” for other boundaries set as wall. Different inflow velocities were set at 0.042, 0.085, 0.161, 0.242, 0.323, 0.402, and 0.721 m/s to observe the velocity effect on the RTD functions. The velocity is calculated by dividing the inflow volume rate (1.0, 2.0, 3.8, 5.7, 7.6, 9.5 and 17.0 mL/min) by the cross-section area of inlets. Finally, this computational velocity field is used as the initial condition for the convection term in the module of transport of diluted species. According to the experimental precursor concentration in section 4.2, the dilute tracer specie concentration in inlets was set at a value of 0.4 mole/m3 with an injection time of 0.2 seconds, and “outflow” was set in the outlet as the boundary condition. Also, the diffusion coefficient was set at a value of 1*10-9 m2/s in this system. 62 The computational time-dependent outlet tracer concentration profiles given in Appendix E show the outlet concentration decreased with the decreasing inflow velocity, since the enhanced convection in axial direction is caused by longer residence time. Therefore, the same amount tracer is diluted and lower concentration is detected in the outlet. The RTD function E(t) is mathematically expressed as 𝐸(𝑡) = 𝐶(𝑡) ∞ ∫0 𝐶(𝑡)𝑑𝑡 𝐶(𝑡𝑖 ) ∞ ∑𝑖=0 𝐶(𝑡𝑖 )∆𝑡𝑖 (4-11) where ∆ti is the time step for the measurement. Figure 4.9 (a) shows the C(t) is the outlet time-dependent tracer concentration. Each data point in a C(t) curve represent the mean concentration at a certain time step. A normalized E(θ) function in figure 4.9 (b) is used instead of E(t) when two different flow conditions need comparison. The relationship of E(t) and E(θ) RTD functions are expressed by (4-12) and (4-13). 𝐸(𝜃) = 𝜏𝐸(𝑡) (4-12) 𝑡 𝜃=𝜏 (4-13) ∞ The residence time (τ) is defined in (4-14), since ∫0 𝐸(𝑡)𝑑𝑡 = 1. ∞ 𝜏= ∫0 𝑡𝐸(𝑡)𝑑𝑡 ∞ ∫0 𝐸(𝑡)𝑑𝑡 ∞ = ∫0 𝑡𝐸(𝑡)𝑑𝑡 (4-14) The variation (σ2), and coefficient of variation (σθ) are dimensionless characteristic parameters for quantifying the mixing performance of the flow system. They can be derived from (4-15) and (4-16). ∞ 𝜎 2 = ∫0 (𝑡 − 𝜏)2 𝐸(𝑡)𝑑𝑡 𝜎𝜃 = 𝜎 𝜏 i 0 (𝑡𝑖 − 𝜏)2 𝐸𝑖 (𝑡)∆𝑡𝑖 (4-15) (4-16) 63 The variation is a parameter to measure the width of a RTD curve. Lower value means the RTD curve a narrower width. There is another useful parameter, s, which is used for identifying the skewness (4-17) of a RTD curve. ∞ 𝑠= ∫0 (𝑡−𝑡𝑚 )2 𝐸(𝑡)𝑑𝑡 𝜎 1.5 𝜏 ( ) (4-17) Figure 4.10 shows the CFD results of variation (σθ) and skewness (s) as a function of residence time (τ). The corresponded flow rate and inflow velocity are also listed in table 4.3 for comparison. The skewness measures the degree of symmetric distribution. When good radial mixing is achieved at high flow rate, the RTD curve is highly symmetric with low skewness [95]. Comparing to the particle size growth in figure 4.6, a large number of small CdS nanoparticles are formed by good radial mixing at high flow rate. On the contrary, poor mixing at slow flow rate causes few amounts of large particles within the longer residence time. However, the coefficient of variation does not present absolutely decreasing with the residence time in our T-junction micromixer system. This finding was ever reported by Adeosum and Lawal [94] but no further discussion on the relationship of coefficient of variation and residence time was addressed. Table 4.3 also shows the comparison of the residence time value obtained by estimation (τe) and RTD cure (τ). The τe is given by dividing the microreactor internal volume (0.00393 cm3) to the flow rate. This estimation method shows a good approach for a reactor with simple geometry. Experimental RTD approach is still essential and more accurate for the complicated geometric system. 64 4.5 Conclusion In this study a novel in-situ spectroscopy technique was developed to capture the UV absorption spectra of CdS nanoparticles precipitated through a T-junction micromixer. The spectra were successfully fitted by the sum of three Gaussian functions and one exponential function in order to obtain the nanoparticle size. This deconvolution analysis shows the size between 1.13 nm to 1.26 nm under the residence time from 0.26 s to 3.96 s. Barrier-controlled coalescence is a reasonable model for the precipitated CdS nanoparticles growth, and the modified rate constant k’ is 2.872 s-1. By using CFD, low skewness value of the RTD curve at high flow rate (short τ) can explain good radial mixing at high flow rate, also a large number of small CdS nanoparticles are formed in this condition. The precipitated reaction combined with MASD process for thin film deposition was found a very low surface reaction rate. The pre-existing sulfide (S2-) ions and CdS nanoparticles changes the chemical species equilibrium of thiourea hydrolysis reaction. Consequently, the lack of thiourea adsorbed to the surface for cadmium-ammonia-thiourea complex interrupts the heterogeneous surface reaction. 65 Figure 4.1 Schematic diagrams of in-situ spectroscopic flow cell measurement and thin film deposition by combining MASD process. 66 Figure 4.2 Absorbance spectra of CdS nanoparticles formed at various flow rates. Fit parameters in equation (4-9) Peak 1 1st stage analysis 2nd stage Analysis E1 Unconstrained Unconstrained a1 Unconstrained Unconstrained w1 Unconstrained Unconstrained E2 Unconstrained a2 a2 = 0.4a1 a2 = 0.4a1 w2 w2 = w1 w2 = w1 𝐸2 − 𝐸𝑔 = 3.626 (𝑟 + 0.64 1)2 Peak 2 Unconstrained E3 𝐸3 − 𝐸𝑔 = 34.06 (𝑟 + 3.6360)2 Peak 3 exponential a3 Unconstrained a3 = 1.848a1 w3 Unconstrained w3 = 1.56w1 c1 Unconstrained Unconstrained c2 Unconstrained Unconstrained Table 4.1 Fit parameters in the de-convolution analysis procedure. 67 Figure 4.3 Absorbance spectra (black solid line) of CdS nanoparticles formed at various flow rates are fitted through 1st stage calculation by using (4-9) (red dotted line), including Gaussian and exponential functions (black dashed line). 68 Figure 4.4 Absorbance spectra (black solid line) of CdS nanoparticles formed at various flow rates are fitted through 2nd stage calculation by using (4-9) (red dotted line), including Gaussian and exponential functions (black dashed line). 69 Figure 4.5 Energies difference of the fitted three absorption bands plotted as a function of CdS (Eg = 2.48 eV) particle size. Figure 4.6 Dependence of the optical size of CdS nanopartices at various residence times after the 1st (red squares) and 2nd (black dots) stage analysis. The second analysis result was fitted by the barrier-controlled coalescence model (solid line). 70 Ostwald Ripening Barrier less Barrier-controlled (4-1) Coalescence Coalescence (4-2) (4-4) d0 = 0.1997 nm d0 = 1.014 nm d0 = 1.014 nm -1 a = 0.9995 k[A]0 = 2.268 s n = 21.59 (not reasonable) k = 2.47*109 L mole-1 s-1 adjusted R2 = 0.9887 [A]0 = 0.92*10-9 mole L-1 kˊ = 2.872 s-1 adjusted R2 = 0.9580 adjusted R2 = 0.9598 Table 4.2 Kinetic constants of early stage CdS nanoparticles fitted by three different growth models. Figure 4.7 Photograph of CdS films deposited by MASD process with and without nanoparticle precipitation. 71 Figure 4.8 Schematic diagram of a T-junction micromixer with extended tubes used for simulating RTD by COMSOL 4.2 72 (a) (b) Figure 4.9 The plot of average time-dependent concentration profile (a) and the normalized RTD curve (b) in different inflow velocities. 73 Figure 4.10 The plot of coefficient of variation (σθ) and skewness (s) as a function of residence time (τ). Flow rate Velocity Residence Time (mL/min) (m/s) (seconds) σ2 Coefficient of Skewness, s variation, σθ τe τ 1 0.042 3.960 2.995 2.687 0.547 6.635 2 0.085 1.980 1.392 0.499 0.508 1.381 3.8 0.161 1.042 1.028 0.441 0.646 0.850 5.7 0.242 0.695 0.729 0.255 0.693 0.442 7.6 0.322 0.521 0.621 0.179 0.682 0.318 9.5 0.402 0.417 0.505 0.102 0.632 0.203 17 0.721 0.233 0.332 0.049 0.664 0.090 Table 4.3 Comparison of the residence time, coefficient of variation, and skewness at different flow rates. 74 Chapter 5 Influence of Flow Conditions on CdS Thin Film Growth Kinetics by Continuous Microreactor-Assisted Solution Deposition (MASD) 5.1 Introduction The conventional chemical solution reaction in a batch reactor has low thin-film production yield, which is determined by the mass ratio of final film thickness and the original reagents quantity. The reason is that the formation of homogeneous CdS nanoparticles competes with heterogenous surface reaction by consuming cadmium salt and thiourea. In order to increase the yield in a batch reactor, a small spacing distance between two substrates was performed to trap reagents via the surface tension [26, 27]. Lee et al. [96] prepared ZnO seed layer in poly-dimethylsiloxane (PDMS) microfluidic channels, and then grew ZnO nanowires by chemical vapor deposition (CVD). We presented the continuous MASD process composed of microfluidic channels and a deposition chamber to separate the homogeneous particles formation and heterogeneous surface nucleation. This MASD process has been applied in growing ZnO [43, 45] and CdS [3, 38, 39] thin films. McPeak et al. [51] designed the continuous flow reactor to make reagents contact the substrate surface in a closed space for growing ZnO nanowires. Compared to the bath reactor, this continuous flow reactor gives higher deposition rate and yield (30% ~ 50%). The result showed that higher flow rate can accelerate the nanowire growth rate, and the growth rate decreased with the downstream position from the inlet [52]. However, previous researches were all focused on the small dimensional size (less than 3 cm). In this research, the industrial pilot-scale production was firstly performed on depositing CdS films on the 6-inch squared FTO glass substrate by our 75 continuous MASD process. In reality, the original design, parallel flow cell, has parabolic flow distribution in the channel, and caused the final CdS films have large variation in thickness. A modified version, deflected flow cell, was designed to compensate the lateral flow variation across a high aspect ratio channel. Both parallel and deflected flow cells were investigated to understand how the different flow conditions affected the final CdS film thickness. 5.2 Experimental Figure 5.1 illustrates the continuous MASD system. One reactant stream was composed of 0.008 M CdCl2, 0.08 M NH4Cl, and 1.27 M NH4Cl aqueous solution. The other reactant stream was composed of 0.08 M thiourea aqueous solution. Both streams were pumped through a Tee mixer and into a stacked heat exchanger with a total flow rate of 24 mL/min. After that, the solution was guided into the flow cell section to allow the surface reaction of CdS film growth on the FTO substrate (Pilkington TEC-15). The residence time in the heat exchanger can be determined by stacking different numbers of copper plates. Figure 5.2 shows the designs of parallel and deflected flow cells. Two main features were designed for deflected flow cell. The first feature was the silicon gasket changed from the polygon shape to the curvature shape. The second feature was one more polycarbonate sheet added between the substrate and the top cover plate. Several poles with screws were installed into the top cover plate, which can push the beneath polycarbonate sheet to manipulate a deflected flow cell. This modification altered the parabolic flow profile to an even flow profile, solving the non-uniform thin film issue caused by flow pattern. 76 5.3 Computational Fluid Dynamics (CFD) 5.3.1 Stacked Heat Exchanger The momentum and heat transport of reactant flow in stacked heat exchanger are solved numerically by using finite elemental analysis CFD software (COMSOL 4.2). Navier-Stokes equation (5-1) can be expressed as velocity at xand y- coordination (5-2a, b) for the velocity profiles of incompressible flow. 𝐷 2 =− 𝑝+ 𝐷𝑡 𝜕𝑣 𝜕𝑣 ( 𝜕𝑡 + 𝜕𝑥 𝜕𝑣 𝜕𝑣 ( 𝜕𝑡 + 𝜕𝑥 + + + (5-1) 𝜕𝑣 𝜕2 𝑣 𝜕 ) = − 𝜕𝑥 + ( 𝜕𝑥 2 + 𝜕𝑦 𝜕𝑣 𝜕2 𝑣 𝜕 ) = − 𝜕𝑦 + ( 𝜕𝑥 2 + 𝜕𝑦 𝜕2 𝑣 𝜕𝑦 2 𝜕2 𝑣 𝜕𝑦 2 )+ (5-2a) )+ (5-2b) For heat transfer, the analogue form of the Navier-Stokes equation can be expressed as (5-3). Substituting in velocity vectors, it can be extended as the complete form (5-4). The left and right hand side of the equal sign represent respectively the convection and conduction in fluids. 𝑐 𝜕𝑇 𝜕𝑡 𝐷𝑇 𝐷𝑡 + =𝑘 𝜕𝑇 𝑥 𝜕𝑥 + 2 𝑇 𝜕𝑇 𝑦 𝜕𝑦 (5-3) 𝑘 𝜕2 𝑇 𝜕2 𝑇 = 𝜌𝑐 (𝜕𝑥 2 + 𝜕𝑦 2 ) 𝑝 (5-4) After drawing the geometry, the boundary conditions of incompressible Newtonian fluid module were added to simulate the velocity profile in the heat-exchanger. Figure 5.3 (a), (c), and (e) show the velocity profiles for the setting inlet velocities at 0.04 m/s, 0.008 m/s, and 0.001 m/s. The color mapping shows the dead volume in the entrance square region that is formed at high inlet velocities. Then the boundary conditions of heat conduction and convection were added and incorporated with the simulated velocity profiles. The temperature profiles corresponding to the various inlet velocities were solved and are shown in figure 77 5.3 (b), (d), and (f). With the increased fluid velocity, heat convection in the liquid becomes more significant than the heat conduction from the walls. Therefore, an obvious temperature gradient can be seen in the heat-exchanger channels. It implies that longer residence time is needed to let the high velocity fluid approach to the desire temperature (85 °C). 5.3.2 Flow Cell Figure 5.4 shows the velocity contour map of the parallel and deflected flow cell. The CFD analysis was performed by FLUENT 6.3 with an inlet velocity of 0.089 m/s. Hires [97] proposed the Hagen-Poiseuille equation (5-5) on designing the deflected flow cell. The original parallel flow cell (Figure 5.4 (a)) has three stream paths (L1 > L2 > L3) from the inlet to outlet and equivalent pressure drop (ΔP1 = ΔP2 = ΔP3). The hydraulic diameter, DH (5-6), can be thought as an imaginary rectangular long channel with a distance of width (W) and height (H). The other constant, µ, is dynamic viscosity of fluid. The relationship of hydraulic diameter and stream path is expressed in (5-7) due to the constant DH. Therefore, the velocity (v) of these three stream paths shows v1 < v2 < v3 to fulfill the equivalent pressure drop. Figure 5.4 (a) shows the parabolic velocity distribution in parallel flow with the highest velocity (v3) in middle stream path than the other paths (v1 and v2). 32𝜇𝐿 2 𝐻) ∆ = (𝐷 (5-5) 4𝑊𝐻 𝐷𝐻 = 2(𝑊+𝐻) 𝐿1 (𝐷𝐻1 ) 2 𝐿2 (𝐷𝐻1 ) (5-6) 2 𝐿3 (𝐷𝐻1 ) 2 (5-7) 78 To produce the uniform velocity profile (v1 = v2 = v3), the (5-7) is modified to (5-8), and also the DH in three paths should follow the relationship of DH1 > DH2 > DH3 to fulfill the equivalent pressure drop. 𝐿1 (𝐷𝐻1 ) 2 = 𝐿2 (𝐷𝐻1 ) 2 = 𝐿3 (𝐷𝐻1 ) 2 (5-8) According to (5-6), the height (H) is the only manipulated parameter in this flow cell system. Hires [97] provided detail data of gap distance between the bottom of cover plate and the substrate surface on different locations. Figure 5.4 (b) shows the velocity profile in the deflected flow cell is improved to more uniform from the original parabolic shape. This theoretical background gives a well support to compare the film uniformity from the parallel and deflected flow cell. 79 5.4 Result and Discussion There are totally 36 points (Figure 5.5) are measured by reflectance spectroscopy to determine the thickness profiles of the large scale CdS films deposited by using the parallel and deflected flow cell (Appendix D: Table D.1 and D.2). Figure 5.6 shows the 2-D thickness line profiles (row A to row F) of the CdS films made by the parallel (a) and deflected flow (b) cell system. The line profile is plotted parallel to the flow direction. It is observed that the deflected flow cell did improve the film uniformity and has a much lower variance than the film made by the parallel flow cell. Figure 5.7 (a, b) show the 3-D thickness surface profiles based on the line profiles in figure 5.6. The overall average thickness and standard deviation are 39.85 nm ± 22.54 nm and 33.36 nm ± 4.51 nm for parallel flow cell and deflected flow cell, respectively. For the parallel flow cell, the center part (row D) has thicker film, and also apparent thickness gradient along the flow direction. The thickness gradient is caused by the non-uniform flow patterned in the flow cell. Blasius’s solution for the laminar boundary layer on a flat plate is expressed as equation (5-9) 𝛿 = 5√𝑣 𝑥 ∞ (5-9) The boundary thickness ( δ) is inversely proportional to the square root of external flow velocity (v∞). The high velocity flow in the centre part leads the thinner boundary layer, and then shortens the diffusion length in perpendicular to the flow direction. Therefore, the reactant in solution is easily transported to the surface and forms the thicker films in the centre part than edge parts. Based on this result, it is concluded that the modified deflected flow cell can obtain uniform large area CdS films by controlling the same velocity of the 80 internal flow in each psition. Figure 5.1 Schematic diagram of continuous MASD system. Figure 5.2 Photograph (top) and cross-section diagram (bottom) of parallel flow cell and deflected flow cell. 81 (a) Velocity profile of V = 0.04 m/s (b) Temperature profile of V = 0.04 m/s 82 (c) Velocity profile of V = 0.008 m/s (d) Temperature profile of V = 0.008 m/s 83 (e) Velocity profile of V = 0.001 m/s (f) Temperature profile of V = 0.001 m/s Figure 5.3 Velocity and temperature profiles in the silicon heat exchanger with various inlet velocities. 84 Figure 5.4 Velocity contour maps of the parallel and deflected flow cell [97]. 85 Figure 5.5 The map of 36 measurement points on a 6-inch substrate using MASD process with a flow cell. 86 Figure 5.6 2-D CdS film thickness line profiles (row A to row F) along the flow direction of reactants in the parallel (a) and deflected (b) flow cell. 87 Figure 5.7 3-D CdS film thickness surface profiles by continous MASD process with the parallel (a) and deflected (b) flow cell. 88 Chapter 6 Analysis of CdS Thin Film by Reel-to-Reel Microreactor-Assisted Solution Deposition (R2R-MASD) 6.1 Introduction Solar cell is the most promising renewable energy technology in current solar energy technology. The best research-cell efficiency was reported to be 43.5% in III-V group three-junction tendon cell, 20.3% in Cu(In,Ga)Se2 cell, 17.3% in CdTe/CdS cell, and 8.3% in organic cell. The development of solar cell production technology plays an important role in PV product market. The first generation crystalline silicon solar cell has achieved mature production technology, and the commercial product still dominated the market share. Due to the high manufacturing cost in crystalline silicon, new generation low-cost CdTe and Cu(In,Ga)Se2 thin film solar cells have attracted more attention and have the biggest market share for the non-silicon solar cells. Continuous reel-to-reel process has a potential to further lower down the cost. Winkler et al. [98] reported a reel-to-reel (R2R) process for fabricating CuInS2 solar cells on the flexible Cu-tape in a non-vacuum ambient. The average efficiency of the small module reached 7.1%. Blankenburg et al. [99] fabricated P3HT/PCBM organic solar cells by using a R2R coating machine shown in figure 6.1. Low viscous polymer solution was dispensed on the moving foil substrate, and then the solvent was dried out through hot air convection. Romeo et al. [100] reported the fabrication of CdTe/CdS thin film cells by an in-line process. The whole process is composed of three deposition sections, including the ITO layer, p-n junction layers, and the back contact layer [100]. In this chapter, analysis of CdS thin films by R2R-MASD process was reported. 89 6.2 Experimental Figure 6.2 illustrates the schematic diagram of the R2R-MASD, which includes the solution reaction region and the film deposition region. In solution reaction region, the reagent of stream A was composed of 0.008 M CdCl2, 0.08 M NH4Cl, and 1.27 M NH4OH aqueous solution. The stream B was composed of 0.08 M thiourea aqueous solution. Both streams were pumped through a Tee mixer and into a stacked heat exchanger at a total flow rate of 45 mL/min. After that, the solution was guided into the nozzle to be dispensed on the substrate. In the film deposition region, the substrate was installed on a stage carried by a conveyor. In step 1, the substrate stayed at the original point and waited for pre-heating. In step 2, the substrate moved to the IR lamp pre-heating zone for maintaining the surface temperature to 80 ~ 90 °C. Then the pre-heated substrate moved to the spray zone in step 3. The reagent running through a heat exchanger was dispensed on a rotating rod, which enhance the coverage of solution on the substrate surface. After dispensing the proper amounts of reagents, the substrate moved into the heating zone for growing the CdS film in step 4. To improve the film quality, the substrate moved back and forward between the spraying and heating zones several times. Finally, the substrate moved to the vacuum zone to clean the excess residual solution on surface in step 5. All steps were controlled automatically and tuned to the optimum conditions. 90 6.3 Film Characterization 6.3.1 XRD Smaller-pieces of the sample (1ʺ x 1ʺ) were taken from the as-deposited larger size CdS/FTO (6ʺ x 6ʺ) sample for various characterizations. The CdS films were deposited by the R2R-MASD process with a deposition time of 6.5 minutes. The GIXRD (Bruker D8 Discover, CuKα=1.54056Å) scan was collected with a grazing incidence angle of 0.5°. The detector was moving to receive the signals. Figure 6.3 shows the CdS films with four reflections at 26.26°, 30.47°, 43.84°, and 52.12°, which can be indexed as C(111), C(200), C(220), and C(311) of cubic CdS (JCPDS-750581). The bare FTO substrate shows the reflection at 26.61°, 33.39°, 37.60°, and 51.55°, which can be indexed as T(110), T(101), T(200), T(211), and T(220) of tetragonal SnO2 (JCPDS-411445). A comparison between the FTO substrate with and without CdS films reveals that T(101), T(200), T(211) and T(220) can’t be observed in CdS/FTO. The other finding is that the CdS films contribute to an intense C(111) peak that block the T(110) peak from bare FTO substrate. The GIXRD result clearly identifies the formation of CdS films. 6.3.2 TEM Figure 6.4 shows the TEM (Philip CM-12) cross-sectional image of CdS/FTO structure at low magnification (125,000X). A platinum layer was deposited for the protection of samples during ion-milling, and the underneath carbon layer function as a conducting material during FIB operation. The average physical thickness of the CdS layers was determined to be around 80.66 nm and the optical thickness was determined to be 102 nm obtained by the reflectance spectroscopy. 91 6.3.3 Surface Property Surface morphology and the surface roughness were measured by AFM (Innova Scanning Probe Microscope, Bruker). The sample was cut to a small size (1ʺ x 0.5ʺ) in order to be loaded onto the sample stage. Figure 6.5 (a), (b) show AFM tapping mode images of the bare FTO substrate and the CdS films deposited at 6.5 min respectively. The RMS roughness of bare FTO substrate is 8.15 nm, which is higher than the reported value of 6.7 nm from an ITO substrate [101]. The RMS roughness of the CdS films on FTO is 11.35 nm, which is much higher than the reported value of 7 nm for CdS film on ITO with equivalent deposition time [101]. FTO surface used for PV application normally has higher roughness than typical ITO surface. Therefore, it is not surprise to see a higher surface roughness from our CdS/FTO samples. Kim et al. [69] also reported the RMS roughness around 7 ~ 15 nm depending on the thiourea molar concentration from 0.1 M to 0.4 M. The low RMS value was obtained from a batch with higher thiourea concentration. Our RMS value (11.35 nm) is reasonable in light of Kim’s reported range (7 ~ 15 nm). Mazon-Montijo et al. [60] reported the RMS value of 3.6 nm in CdS films on ITO and 2.7 nm for the bare ITO substrate. The deposited film layer has higher roughness compared to the bare substrate because of particle formation during the solution deposition process. XPS was performed to obtain the chemical binding information of the CdS film. Figure 6.6 shows the presence of oxygen (O), cadmium (Cd), carbon (C), and sulfur (S) from CdS layer. The data obtained were corrected by taking the specimen charging and referring to C 1s at 284.9 eV (figure 6.6 (c)), which was originated in the atmospheric contamination. The oxygen (figure 6.6 (a)) on the 92 surface at 532.4 eV appears to be due to hydroxide (Cd(OH)2) relative surface chemisorbed oxygen. For the as-received condition, the binding energies of Cd 3d3/2 and Cd 3d5/2 shows at 412 eV and 405.3 eV respectively. These spectrums of Cd and S after etching can be fitted by Gaussian function (red dotted lines). For Cd 3d energy level, the binding energy of 3d3/2 and 3d5/2 orbital are 411.7eV and 405 eV respectively, with the splitting energy of 6.8 eV. For S 2p energy level, the binding energies of S 2p3/2 and S 2p1/2 orbitals are 161.1 and 162.3 eV respectively, with the splitting energy of 1.2 eV. The obtained result shows excellent agreement with previous researches [70, 71]. 6.3.4 Optical Property The optical property was characterized by UV-vis absorption spectroscopy (Ocean Optics USB2000, Halogen lamp as light source). Figure 6.7 shows the plot of (αhν)2 versus hν (band gap energy) of the CdS film with a thickness of 80.66 nm. The optical band gap is determined by extrapolating the curve across the x-axis at 2.38 eV, which is quite consistent with previous reported results. 6.4 Result and Discussion The growth kinetics was studied by analyzing the film thickness. Figure 6.8 shows a 6-inch sample with 36 measurement points, which were labeled by column 1 to column 6 and row A to row F. The film thickness was determined by the spectroscopic reflectance, which was elucidated in Chapter 3. Figure 6.9 shows the 2-D thickness line profiles from row A to row F at the deposition time of 2.5 min, 6.3 min, and 9 min. Data points are showed in table D.3 ~ D.5 in Appendix D. The flat line profiles show the lower standard deviations (σ) at 2.5 min deposition 93 time. Longer deposition time change the line profiles from flat to a curvature shape, which represents uneven film obtained. Figure 6.10 (a) ~ (c) show the 3-D thickness surface profiles based on the above line profiles. The standard deviation of all 36 points is 3.94 nm, 5.18 nm, and 31.34 nm for 2.5 min, 6.3 min, and 9 min respectively. It is observed that the center region has significantly thicker films than the films around the edge for samples deposited at longer deposition time. The non-uniform film growth rates most likely come from the non-uniform distribution of the dispensed reagents on the substrate. The average thickness of all 36 points is 72.58 nm, 94.78 nm, and 232.28 nm for 2.5 min, 6.3 min, and 9 min respectively. These three points are plotted in figure 6.11 (d) and compared with the results obtained from the lab-scale continuous MASD (c), batch processes at 60 °C (a) and 70 °C (b). The equation (3-30) derived from the revised model (3-29) was applied to analyze the experimetal results. 𝑑 𝑑𝑡 = 𝑚𝑤 𝑘0 [𝐶𝑑]0 [𝑂𝐻 − ]2 [𝑆𝐶(𝑁𝐻2 )2 ]0 (𝑒𝑥𝑝(𝐴𝜏) 𝜌[𝑁𝐻3 ]2 − 𝑒𝑥𝑝(𝐵𝜏)) ℎ = 1.11 × 10−7 𝑘0 𝑡(𝑒𝑥𝑝(−0.003682𝜏) − 𝑒𝑥𝑝(−0.3106𝜏)) (3-29) (3-30) For continuous R2R-MASD process, the concentrations of each species are the same as ones previously used in Chapter 3. They are [Cd]0 =0.004*10-3 mole/cm3, [SC(NH2)2] = 0.04*10-3 mole/cm3, [OH-] = 0.001*10-3 mole/cm3, and [NH3] = 0.655*10-3 mole/cm3. The molecular weight (MW) of CdS is 144.6 g/mole, and density (ρ) is 4.82 g/cm3. The fitting result shows the k0 = 3.567*106 cm4mole-1s-1, and the R-square is 0.777. This growth rate is higher than the lab-scale continous flow MASD process. 94 Figure 6.1 Working principle of the reel-to-reel coating machine [99]. Figure 6.2 Schematic diagram of the reel-to-reel continuous MASD. 95 Figure 6.3 GIXRD of the reel-to-reel continuous MASD deposited CdS films on FTO substrate. 96 Figure 6.4 TEM cross-sectional images of the reel-to-reel continuous MASD deposited CdS films on FTO substrate. (Magnification: 125,000X) 97 Figure 6.5 AFM images of (a) bare FTO substrate (RMS = 8.15 nm), (b) reel-to-reel continuous MASD deposited CdS film (RMS = 11.35 nm) at 6.5 min deposition time. (Insert: 3D morphological images). 98 Figure 6.6 XPS spectra (dashed line: as received, solid line: after etching, red dotted line: Gaussian fitting) of the CdS films deposited by R2R-MASD (a) O 1s, (b) Cd 3d, (c) C 1s, (d) S 2p 99 Figure 6.7 Plot of (αhν)2 versus hν showing the band gap energy of the reel-to-reel continuous MASD deposited CdS films. 100 Figure 6.8 The location map of 36 measurement points on a 6-inch substrate using R2R-MASD process. 101 Figure 6.9 2-D thickness line profiles of CdS films deposited by continuous R2R-MASD during (a) 2.5 min, (b) 6.3 min, and (c) 9 min. 102 Figure 6.10 3-D thickness surface proflies of CdS films deposited by continuous R2R-MASD during (a) 2.5 min, (b) 6.3 min, and (c) 9 min. 103 Figure 6.11 Growth kinetics of CdS thin film deposited by batch process at (a) 70°C, (b) 60°C, (c) continuous MASD process, and (d) R2R-MASD process. The series data of (a) and (b) are from Dona et al. [31]. Solid lines are fitting curves of Kostoglue’s model (3-27) and the modified model (3-30). 104 Chapter 7 Conclusion and Future Work The continuous microreactor-assisted solution deposition (MASD) process was used for the deposition of CdS thin films on fluorine-doped tin oxide (FTO) glass. The MASD system, including a T-junction micromixer and a microchannel heat exchanger, is capable of isolating the homogeneous particle precipitation from the heterogeneous surface reaction. The results show a dense nanocrystallite CdS thin films with a preferred orientation at (111) plane. The film growth kinetics was studied and a growth model that accounts for the residence time in the microchannel using empirical factor (η) obtained from previous reported experimental data. Applying this factor in the proposed modified growth model gives a surface reaction rate of 1.61*106 cm4mole-1s-1, which is considerable higher than the surface reaction rates obtained from the batch CBD process. With the feature of separating homogeneous and heterogeneous surface reaction, the MASD process provides the capability to tailor the surface film growth rate and avoid the saturation growth regime in the batch process An in-situ spectroscopy technique was used to measure the UV-Vis absorption spectra of CdS nanoparticles formed within the continuous flow microreactor. The data shows the formation of CdS nanoparticles range from 1.13 nm to 1.26 nm using a residence time from 0.26 s to 3.96 s. Barrier-controlled coalescence mechanism seem to be a reasonable model to explain the experimental Uv-Vis data obtained from the continuous flow microreactor, with a rate constant k’ value of 2.872 s-1. Using CFD, low skewness value of the RTD curve at high flow rate (short τ) suggests good radical mixing at high flow rate is responsible for the formation of smaller CdS nanoparticles with a narrower size distribution. The 105 combination of CdS nanoparticle solution with MASD process resulted in the hindrance of CdS thin film deposition. It is hypothesized that the pre-existing sulfide (S2-) ions and CdS nanoparticles changes the chemical species equilibrium of thiourea hydrolysis reaction. Consequently, the lack of thiourea slows down the heterogeneous surface reaction. A flow cell and a reel-to-reel (R2R)-MASD systems were setup to test the scalability via the deposition of CdS films on the FTO glass (6ʺ x 6ʺ) substrate. The film deposition kinetics was found to be sensitive to the flow conditions within the heat exchanger and the substrate flow cell. The growth kinetics of the CdS films deposited by R2R-MASD process was investigated. In comparison with the continuous MASD process, the growth rate in R2R-MASD is higher, however more difficult to obtain a linear relationship with the deposition time. Two major areas for future work are listed here: Investigate the homogeneous nanoparticle formation, particle sticking, and surface reaction on the particle surface and substrate. Fabricate CdTe and CIGS thin film solar cells using MASD process and optimize the process for higher material utilization and cell performance. 106 Chapter 8 Bibliography [1] P.K. Nair, J. Campos, M.T.S. Nair, Opto-Electronic Characteristics of Chemically Deposited Cadmium-Sulfide Thin-Films, Semicond Sci Tech, 3 (1988) 134-145. [2] G. Arreola-Jardón, L.A. González, L.A. García-Cerda, B. Gnade, M.A. Quevedo-López, R. Ramírez-Bon, Ammonia-free chemically deposited CdS films as active layers in thin film transistors, Thin Solid Films, 519 (2010) 517-520. [3] Y.J. Chang, P.H. Mugdur, S.Y. Han, A.A. Morrone, S.O. Ryu, T.J. Lee, C.H. Chang, Nanocrystalline CdS MISFETs fabricated by a novel continuous flow microreactor, Electrochem Solid St, 9 (2006) G174-G177. [4] F.Y. Gan, I. Shih, Preparation of thin-film transistors with chemical bath deposited CdSe and US thin films, Ieee T Electron Dev, 49 (2002) 15-18. [5] R.W. Birkmire, B.E. McCandless, CdTe thin film technology: Leading thin film PV into the future, Curr Opin Solid St M, 14 (2010) 139-142. [6] H.S. Kwok, J.P. Zheng, S. Witanachchi, P. Mattocks, L. Shi, Q.Y. Ying, X.W. Wang, D.T. Shaw, Growth of highly oriented CdS thin films by laser-evaporation deposition, Appl Phys Lett, 52 (1988) 1095. [7] B. Ullrich, H. Sakai, Y. Segawa, Optoelectronic properties of thin film CdS formed by ultraviolet and infrared pulsed-laser deposition, Thin Solid Films, 385 (2001) 220-224. [8] T. Hayashi, T. Nishikura, T. Suzuki, Y. Ema, Formation and Properties of in-Doped High-Conductivity Cds Film, J Appl Phys, 64 (1988) 3542-3550. [9] F. Iacomi, M. Purica, E. Budianu, P. Prepelita, D. Macovei, Structural studies on some doped CdS thin films deposited by thermal evaporation, Thin Solid Films, 515 (2007) 6080-6084. 107 [10] I. Martil, G. González-Díaz, F. Sanchez-Quesada, M. Rodriguez-Vidal, Deposition dependence of r.f.-sputtered CdS films, Thin Solid Films, 90 (1982) 253-257. [11] I. Martil, G. Gonzalezdiaz, F. Sanchezquesada, Electrical Transport-Properties of Polycrystalline Rf Sputtered Cds Thin-Films, Journal of Vacuum Science & Technology a-Vacuum Surfaces and Films, 2 (1984) 1491-1494. [12] M. Tomakin, M. Altunbas, E. Bacaksiz, I. Polat, Preparation and characterization of new window material CdS thin films at low substrate temperature (< 300 K) with vacuum deposition, Mat Sci Semicon Proc, 14 (2011) 120-127. [13] R. Castro-Rodriguez, J. Mendez-Gamboa, I. Perez-Quintana, R. Medina-Ezquivel, CdS thin films growth by fast evaporation with substrate rotation, Appl Surf Sci, 257 (2011) 9480-9484. [14] J.L. Boone, S.A. Howard, D.D. Martin, G. Cantwell, Mocvd of Cds onto (111)Silicon Substrates, Thin Solid Films, 176 (1989) 143-150. [15] A.K. Berry, P.M. Amirtharaj, J.T. Du, J.L. Boone, D.D. Martin, Photoluminescence and Raman Studies of Cds Films Grown by Metal Organic-Chemical Vapor-Deposition on Si(111) Substrates, Thin Solid Films, 219 (1992) 153-156. [16] H. Uda, H. Yonezawa, Y. Ohtsubo, M. Kosaka, H. Sonomura, Thin CdS films prepared by metalorganic chemical vapor deposition, Sol Energ Mat Sol C, 75 (2003) 219-226. [17] O.B. Ajayi, O.K. Osuntola, I.A. Ojo, C. Jeynes, Preparation and Characterization of Mocvd Thin-Films of Cadmium-Sulfide, Thin Solid Films, 248 (1994) 57-62. [18] N.I. Fainer, M.L. Kosinova, Y.M. Rumyantsev, E.G. Salman, F.A. Kuznetsov, Growth of PbS and CdS thin films by low-pressure chemical vapour deposition using dithiocarbamates, Thin Solid Films, 280 (1996) 16-19. 108 [19] D. Barreca, A. Gasparotto, C. Maragno, E. Tondello, CVD of nanosized ZnS and CdS thin films from single-source precursors, J Electrochem Soc, 151 (2004) G428-G435. [20] G.A. Kitaev, Uritskarya, A. A.and Morkrushin, S. G. , Conditions for the chemical deposition of thin films of cadmiumn sulfide on a solid surface, Russ. J. Phys. Chem., 39 (1965) 1101. [21] G.A. Kitaev, Makurin, Y. N. and Dvoinin, V. I. , Quantum-chemical calculation of the electronicstructures of thiourea and its derivatives and comparison of the reactivity indices, Russ. J. Phys. Chem., 50 (1976). [22] N.D. Betenekov, Medvedev, V. P., Zhukovskaya, A. S.and Kitaev, G. A., Radiochemical investigation of chalcogenide films. II. Kinetics of the growth of cadmium sulfide films on the surface of glass, Sov Radiochem+, 20 (1979). [23] P.K. Nair, M.T.S. Nair, J. Campos, L.E. Sansores, A Critical Discussion of the Very High Photoconductivity in Chemically Deposited Cadmium-Sulfide Thin-Films - Implications for Solar-Cell Technology, Sol Cells, 22 (1987) 211-227. [24] M.T.S. Nair, P.K. Nair, J. Campos, Effect of Bath Temperature on the Optoelectronic Characteristics of Chemically Deposited Cds Thin-Films, Thin Solid Films, 161 (1988) 21-34. [25] M.T.S. Nair, P.K. Nair, R.A. Zingaro, E.A. Meyers, Conversion of Chemically Deposited Photosensitive Cds Thin-Films to N-Type by Air Annealing and Ion-Exchange Reaction, J Appl Phys, 75 (1994) 1557-1564. [26] A.A.C. Readigos, V.M. Garcia, O. Gomezdaza, J. Campos, M.T.S. Nair, P.K. Nair, Substrate spacing and thin-film yield in chemical bath deposition of semiconductor thin films, Semicond Sci Tech, 15 (2000) 1022-1029. [27] P.K. Nair, V.M. Garcia, O. Gomez-Daza, M.T.S. Nair, High thin-film yield achieved at small substrate separation in chemical bath deposition of semiconductor thin films, Semicond Sci Tech, 16 (2001) 855-863. 109 [28] J.M. Dona, J. Herrero, Chemical Bath Deposition of Cds Thin-Films Electrochemical Insitu Kinetic-Studies, J Electrochem Soc, 139 (1992) 2810-2814. [29] D. Lincot, R.O. Borges, Chemical Bath Deposition of Cadmium-Sulfide Thin-Films - Insitu Growth and Structural Studies by Combined Quartz Crystal Microbalance and Electrochemical Impedance Techniques, J Electrochem Soc, 139 (1992) 1880-1889. [30] R. Ortegaborges, D. Lincot, Mechanism of Chemical Bath Deposition of Cadmium-Sulfide Thin-Films in the Ammonia-Thiourea System - in-Situ Kinetic-Study and Modelization, J Electrochem Soc, 140 (1993) 3464-3473. [31] J.M. Dona, J. Herrero, Chemical bath deposition of CdS thin films: An approach to the chemical mechanism through Study of the film microstructure, J Electrochem Soc, 144 (1997) 4081-4091. [32] I.O. Oladeji, L. Chow, Optimization of chemical bath deposited cadmium sulfide thin films, J Electrochem Soc, 144 (1997) 2342-2346. [33] M. Kostoglou, N. Andritsos, A.J. Karabelas, Flow of supersaturated solutions in pipes. Modeling bulk precipitation and scale formation, Chem Eng Commun, 133 (1995) 107-131. [34] M. Kostoglou, A.J. Karabelas, Comprehensive modeling of precipitation and fouling in turbulent pipe flow, Ind Eng Chem Res, 37 (1998) 1536-1550. [35] M. Kostoglou, N. Andritsos, A.J. Karabelas, Modeling thin film CdS development in a chemical bath deposition process, Ind Eng Chem Res, 39 (2000) 3272-3283. [36] M. Kostoglou, N. Andritsos, A.J. Karabelas, Progress towards modelling the CdS chemical bath deposition process, Thin Solid Films, 387 (2001) 115-117. [37] M. Kostoglou, N. Andritsos, A.J. Karabelas, Incipient CdS thin film formation, J Colloid Interf Sci, 263 (2003) 177-189. 110 [38] P.H. Mugdur, Y.J. Chang, S.Y. Han, Y.W. Su, A.A. Morrone, S.O. Ryu, T.J. Lee, C.H. Chang, A comparison of chemical bath deposition of CdS from a batch reactor and a continuous-flow microreactor, J Electrochem Soc, 154 (2007) D482-D488. [39] Y.J. Chang, Y.W. Su, D.H. Lee, S.O. Ryu, C.H. Chang, Investigate the Reacting Flux of Chemical Bath Deposition by a Continuous Flow Microreactor, Electrochem Solid St, 12 (2009) H244-H247. [40] M.S. Park, S.Y. Han, E.J. Bae, T.J. Lee, C.H. Chang, S.O. Ryu, Synthesis and characterization of polycrystalline CuInS(2) thin films for solar cell devices at low temperature processing conditions, Curr Appl Phys, 10 (2010) S379-S382. [41] C.R. Kim, S.Y. Han, C.H. Chang, T.J. Lee, S.O. Ryu, Synthesis and characterization of CuInSe2 thin films for photovoltaic cells by a solution-based deposition method, Curr Appl Phys, 10 (2010) S383-S386. [42] C.R. Kim, S.Y. Han, C.H. Chang, T.J. Lee, S.O. Ryu, A Study on Copper Selenide Thin Films for Photovoltaics by a Continuous Flow Microreactor, Mol Cryst Liq Cryst, 532 (2010) 455-463. [43] S.Y. Han, Y.J. Chang, D.H. Lee, S.O. Ryu, T.J. Lee, C.H. Chang, Chemical nanoparticle deposition of transparent ZnO thin films, Electrochem Solid St, 10 (2007) K1-K5. [44] T.J. Lee, Y.J. Lee, N.K. Park, G.B. Han, S.O. Ryu, C.H. Chang, The preparation and desulfurization of nano-size ZnO by a matrix-assisted method for the removal of low concentration of sulfur compounds, Curr Appl Phys, 8 (2008) 746-751. [45] J.Y. Jung, N.K. Park, S.Y. Han, G.B. Han, T.J. Lee, S.O. Ryu, C.H. Chang, The growth of the flower-like ZnO structure using a continuous flow microreactor, Curr Appl Phys, 8 (2008) 720-724. 111 [46] D.H. Lee, J.Y. Jung, E.J. Bae, T.J. Lee, S.O. Ryu, C.H. Chang, Highly uniform ZnS thin film through the continuous flow reaction process, J Korean Phys Soc, 53 (2008) 102-105. [47] S. Liu, C.H. Chang, High Rate Convergent Synthesis and Deposition of Polyamide Dendrimers using a Continuous-Flow Microreactor, Chem Eng Technol, 30 (2007) 334-340. [48] S. Liu, C.-h. Chang, B.K. Paul, V.T. Remcho, Convergent synthesis of polyamide dendrimer using a continuous flow microreactor, Chem Eng J, 135 (2008) S333-S337. [49] H.D. Jin, A. Garrison, T. Tseng, B.K. Paul, C.H. Chang, High-rate synthesis of phosphine-stabilized undecagold nanoclusters using a multilayered micromixer, Nanotechnology, 21 (2010) -. [50] H.D. Jin, C.-H. Chang, Continuous synthesis of SnTe nanorods, J Mater Chem, 21 (2011) 12218-12220. [51] K.M. McPeak, J.B. Baxter, Microreactor for High-Yield Chemical Bath Deposition of Semiconductor Nanowires: ZnO Nanowire Case Study, Ind Eng Chem Res, 48 (2009) 5954-5961. [52] K.M. McPeak, J.B. Baxter, ZnO Nanowires Grown by Chemical Bath Deposition in a Continuous Flow Microreactor, Cryst Growth Des, 9 (2009) 4538-4545. [53] C. Ferekides, J. Britt, Cdte Solar-Cells with Efficiencies over 15-Percent, Sol Energ Mat Sol C, 35 (1994) 255-262. [54] T.L. Chu, S.S. Chu, C. Ferekides, C.Q. Wu, J. Britt, C. Wang, 13.4-Percent Efficient Thin-Film Cds/Cdte Solar-Cells, J Appl Phys, 70 (1991) 7608-7612. [55] J. Britt, C. Ferekides, Thin-Film Cds/Cdte Solar-Cell with 15.8-Percent Efficiency, Appl Phys Lett, 62 (1993) 2851-2852. 112 [56] M.E. Calixto, M. Tufino-Velazquez, G. Contreras-Puente, O. Vigil-Galan, M. Jimenez-Escamilla, R. Mendoza-Perez, J. Sastre-Hernandez, A. Morales-Acevedo, Study of chemical bath deposited CdS bi-layers and their performance in CdS/CdTe solar cell applications, Thin Solid Films, 516 (2008) 7004-7007. [57] S. Kohli, V. Manivannan, J.N. Hilfiker, P.R. McCurdy, R.A. Enzenroth, K.L. Barth, W.P. Smith, R. Luebs, W.S. Sampath, Effect of Chemical Treatment on the Optical Properties of a Cadmium Telluride Photovoltaic Device Investigated by Spectroscopic Ellipsometry, J Sol Energ-T Asme, 131 (2009). [58] J.F. Han, C. Liao, T. Jiang, G.H. Fu, V. Krishnakumar, C. Spanheimer, G. Haindl, K. Zhao, A. Klein, W. Jaegermann, Annealing effects on the chemical deposited CdS films and the electrical properties of CdS/CdTe solar cells, Mater Res Bull, 46 (2011) 194-198. [59] J.F. Han, C. Spanheimer, G. Haindl, G.H. Fu, V. Krishnakumar, J. Schaffner, C.J. Fan, K. Zhao, A. Klein, W. Jaegermann, Optimized chemical bath deposited CdS layers for the improvement of CdTe solar cells, Sol Energ Mat Sol C, 95 (2011) 816-820. [60] D.A. Mazon-Montijo, M. Sotelo-Lerma, L. Rodriguez-Fernandez, L. Huerta, AFM, XPS and RBS studies of the growth process of CdS thin films on ITO/glass substrates deposited using an ammonia-free chemical process, Appl Surf Sci, 256 (2010) 4280-4287. [61] T.L. Chu, S.S. Chu, N. Schultz, C. Wang, C.Q. Wu, Solution-Grown Cadmium-Sulfide Films for Photovoltaic Devices, J Electrochem Soc, 139 (1992) 2443-2446. [62] S. Ramprasad, Y.-W. Su, C.-H. Chang, B.K. Paul, D.R. Palo, Cadmium sulfide thin film deposition: A parametric study using microreactor-assisted chemical solution deposition, Sol Energ Mat Sol C, 96 (2012) 77-85. [63] S.J. Ikhmayies, R.N. Ahmad-Bitar, Characterization of the SnO2:F/CdS:In structures prepared by the spray pyrolysis technique, Sol Energ Mat Sol C, 94 (2010) 878-883. 113 [64] M. Kokotov, Y. Feldman, A. Avishai, M. DeGuire, G. Hodes, Chemical bath deposition of CdS highly-textured, columnar films, Thin Solid Films, 519 (2011) 6388-6393. [65] M. Kim, B.K. Min, C.D. Kim, S. Lee, H.T. Kim, S.K. Jung, S. Sohn, Study of the physical property of the cadmium sulfide thin film depending on the process condition, Curr Appl Phys, 10 (2010) S455-S458. [66] C. Li, X.G. Yang, B.J. Yang, Y. Yan, Y.T. Qian, Growth of microtubular complexes as precursors to synthesize nanocrystalline ZnS and CdS, J Cryst Growth, 291 (2006) 45-51. [67] Z.R. Khan, M. Zulfequar, M.S. Khan, Optical and structural properties of thermally evaporated cadmium sulphide thin films on silicon (1 0 0) wafers, Materials Science and Engineering: B, 174 (2010) 145-149. [68] X.L. Tong, D.S. Jiang, W.B. Hu, Z.M. Liu, M.Z. Luo, The comparison between CdS thin films grown on Si(111) substrate and quartz substrate by femtosecond pulsed laser deposition, Appl Phys a-Mater, 84 (2006) 143-148. [69] M.J. Kim, H.T. Kim, J.K. Kang, D.H. Kim, D.H. Lee, S.H. Lee, S.H. Sohn, Effects of the Surface Roughness on Optical Properties of CdS Thin Films, Mol Cryst Liq Cryst, 532 (2010) 437-444. [70] H. Khallaf, G. Chai, O. Lupan, L. Chow, S. Park, A. Schulte, Characterization of gallium-doped CdS thin films grown by chemical bath deposition, Appl Surf Sci, 255 (2009) 4129-4134. [71] L. Wan, Z.Z. Bai, Z.R. Hou, D.L. Wang, H. Sun, L.M. Xiong, Effect of CdCl2 annealing treatment on thin CdS films prepared by chemical bath deposition, Thin Solid Films, 518 (2010) 6858-6865. [72] M.a.K. Kostoglou, A. J., Evaluation of numerical methods for simulating an evolving particle size distribution in growth processes Chem Eng Commun, 136 (1995). 114 [73] S. Kohli, V. Manivannan, J.N. Hilfiker, P.R. McCurdy, R.A. Enzenroth, K.L. Barth, W.P. Smith, R. Luebs, W.S. Sampath, Effect of Chemical Treatment on the Optical Properties of a Cadmium Telluride Photovoltaic Device Investigated by Spectroscopic Ellipsometry, J Sol Energ-T Asme, 131 (2009) -. [74] R. Castro-Rodriguez, A.I. Oliva, V. Sosa, F. Caballero-Briones, J.L. Pena, Effect of indium tin oxide substrate roughness on the morphology, structural and optical properties of CdS thin films, Appl Surf Sci, 161 (2000) 340-346. [75] G. Marcotrigiano, G. Peyronel, R. Battistuzzi, Kinetics of the desulphuration of 35S-labelled thiourea in sodium hydroxide studied by chromatographic methods, Journal of the Chemical Society, Perkin Transactions 2, (1972) 1539-1541. [76] Y.J. Chang, Investigation of Low Temperature Solution-based Deposition Process for Flexible Electronics, in: Chem Eng-New York, Oregon State University, Corvallis, 2007. [77] A.P. Alivisatos, Semiconductor clusters, nanocrystals, and quantum dots, Science, 271 (1996) 933-937. [78] T. Trindade, P. O'Brien, N.L. Pickett, Nanocrystalline semiconductors: Synthesis, properties, and perspectives, Chem Mater, 13 (2001) 3843-3858. [79] Y. Nosaka, Finite Depth Spherical Well Model for Excited-States of Ultrasmall Semiconductor Particles - an Application, J Phys Chem-Us, 95 (1991) 5054-5058. [80] S. Krishnadasan, J. Tovilla, R. Vilar, A.J. deMello, J.C. deMello, On-line analysis of CdSe nanoparticle formation in a continuous flow chip-based microreactor, J Mater Chem, 14 (2004) 2655-2660. [81] J.B. Edel, R. Fortt, J.C. deMello, A.J. deMello, Microfluidic routes to the controlled production of nanoparticles, Chem Commun, (2002) 1136-1137. 115 [82] F. Shayeganfar, L. Javidpour, N. Taghavinia, M.R.R. Tabar, M. Sahimi, F. Bagheri-Tar, Controlled nucleation and growth of CdS nanoparticles by turbulent dispersion, Phys Rev E, 81 (2010) 026304. [83] M. Tiemann, Ö. Weiß, J. Hartikainen, F. Marlow, M. Lindén, Early Stages of ZnS Nanoparticle Growth Studied by In-Situ Stopped-Flow UV Absorption Spectroscopy, Chemphyschem, 6 (2005) 2113-2119. [84] M. Tiemann, F. Marlow, F. Brieler, M. Linden, Early stages of ZnS growth studied by stopped-flow UV absorption spectroscopy: Effects of educt concentrations on the nanoparticle formation, J Phys Chem B, 110 (2006) 23142-23147. [85] L.H. Hung, K.M. Choi, W.Y. Tseng, Y.C. Tan, K.J. Shea, A.P. Lee, Alternating droplet generation and controlled dynamic droplet fusion in microfluidic device for CdS nanoparticle synthesis, Lab Chip, 6 (2006) 174-178. [86] K.M. Mullaugh, G.W. Luther, Spectroscopic determination of the size of cadmium sulfide nanoparticles formed under environmentally relevant conditions, J Environ Monitor, 12 (2010) 890-897. [87] K.M. Mullaugh, G.W. Luther, Growth kinetics and long-term stability of CdS nanoparticles in aqueous solution under ambient conditions, J Nanopart Res, 13 (2011) 393-404. [88] C. Ribeiro, E.J.H. Lee, E. Longo, E.R. Leite, A kinetic model to describe nanocrystal growth by the oriented attachment mechanism, Chemphyschem, 6 (2005) 690-696. [89] F. Huang, H.Z. Zhang, J.F. Banfield, Two-stage crystal-growth kinetics observed during hydrothermal coarsening of nanocrystalline ZnS, Nano Lett, 3 (2003) 373-378. [90] M. Tiemann, F. Marlow, J. Hartikainen, O. Weiss, M. Linden, Ripening Effects in ZnS Nanoparticle Growth, The Journal of Physical Chemistry C, 112 (2008) 1463-1467. 116 [91] J.T. Adeosun, A. Lawal, Mass transfer enhancement in microchannel reactors by reorientation of fluid interfaces and stretching, Sensor Actuat B-Chem, 110 (2005) 101-111. [92] J.T. Adeosun, A. Lawal, Numerical and experimental mixing studies in a MEMS-based multilaminated/elongational flow micromixer, Sensor Actuat B-Chem, 139 (2009) 637-647. [93] J.T. Adeosun, A. Lawal, Numerical and experimental studies of mixing characteristics in a T-junction microchannel using residence-time distribution, Chem Eng Sci, 64 (2009) 2422-2432. [94] J.T. Adeosun, A. Lawal, Residence-time distribution as a measure of mixing in T-junction and multilaminated/elongational flow micromixers, Chem Eng Sci, 65 (2010) 1865-1874. [95] D. Boskovic, S. Loebbecke, Modelling of the residence time distribution in micromixers, Chem Eng J, 135 (2008) S138-S146. [96] S.H. Lee, H.J. Lee, D. Oh, S.W. Lee, H. Goto, R. Buckmaster, T. Yasukawa, T. Matsue, S.K. Hong, H. Ko, M.W. Cho, T.F. Yao, Control of the ZnO nanowires nucleation site using microfluictic channels, J Phys Chem B, 110 (2006) 3856-3859. [97] C.L. Hires, Uniform Residence Time in Microreactor-Assisted Solution Deposition of CdS Thin-Films for CIGS Photovoltaic Cells, in: Industrial Engineering, Oregon State University, Corvallis, 2011. [98] M. Winkler, J. Griesche, I. Konovalov, J. Penndorf, J. Wienke, O. Tober, CISCuT - solar cells and modules on the basis of CuInS2 on Cu-tape, Sol Energy, 77 (2004) 705-716. [99] L. Blankenburg, K. Schultheis, H. Schache, S. Sensfuss, M. Schrodner, Reel-to-reel wet coating as an efficient up-scaling technique for the production of bulk-heterojunction polymer solar cells, Sol Energ Mat Sol C, 93 (2009) 476-483. 117 [100] N. Romeo, A. Bosio, A. Romeo, An innovative process suitable to produce high-efficiency CdTe/CdS thin-film modules, Sol Energ Mat Sol C, 94 (2010) 2-7. [101] A.I. Oliva, R. Castro-Rodriguez, O. Ceh, P. Bartolo-Perez, F. Caballero-Briones, V. Sosa, First stages of growth of CdS films on different substrates, Appl Surf Sci, 148 (1999) 42-49. 118 Appendix A: Harmonic Oscillator Approximation According to the relationship (A-1) of dielectric constants and refractive index, the real (ε1) and imaginary part (ε2) of dielectric constant are converted to n and k, expressed in (A-2a) and (A-2b). Due to the dielectric constant being wavelength dependent, the harmonic oscillator approximated equation (2-3) can be used to fit the dielectric constant by adjusting all parameters. ε(E) = ε1 + iε2 = (n + ik)2 m 𝜀(𝐸) = 1 + 2 j 0 1 𝐸+𝐸𝑗 +𝑖Γ𝑗 − 1 𝐸−𝐸𝑗 +𝑖Γ𝑗 ) (2-3) 2 √ε1 +ε2 +ε1 n=√ 2 √ε21 +ε22 −ε1 k=√ 𝐴𝑗 𝑒 −𝑖𝜃𝑗 ( (A-1) 2 (A-2a) (A-2b) Here, the real part of the refractive index (n) indicates the phase speed, while the imaginary part (k) indicates the amount of absorption loss when the electromagnetic wave propagates through the material. When the incident angle and refracted angle are zero, the reflection coefficient also called reflectance is defined in n, k and also expressed in ε1, ε2 by substituting (A-2a) and (A-2b) into the Fresnel equation. The final calculated reflectance equation (A-3) is used for fitting the measured reflectance spectrum. ( −1)2 +k2 =( +1)2 +k2 √ε21 +ε22 +1−√2(√ε21 +ε22 +ε1 ) = (A-3) √ε21 +ε22 +1+√2(√ε21 +ε22 +ε1 ) 119 Appendix B: Optical and Physical Measurement Results Parameters Aj Ej j j 0 9.323 -0.056 -4.431 -2.203 1 -21.762 1.874 11.198 -0.258 2 0.484 3.693 0.567 0.785 j Table B.1 Four optimized parameters of harmonic oscillator approximated equation for bare FTO layer. Position Average Deposition Time (min) 1 2 3 4 5 thickness 6 (nm) Standard Deviation 15 144.27 145.46 150.92 177.25 160.6 158.00 156.08 12.25 10 108.87 105.89 110.5 106.54 112.71 112.64 109.53 2.94 8 98.67 104.15 100.82 102.59 106.29 102.34 102.48 2.63 5 68.42 67.43 71.35 66.37 72.98 76.29 70.47 3.77 4 37.83 38.34 36.95 35.40 36.38 32.74 36.27 2.02 3 29.49 32.27 29.08 30.34 30.18 29.49 30.141 1.15 2 25.36 25.20 25.29 16.59 23.99 24.48 23.49 3.42 1 ~0 ~0 ~0 ~0 ~0 Table B.2 Optical CdS spectroscopic reflectance. ~0 thickness ~0 ~0 at different locations measured by 120 Location Deposition Time (min) 1 2 3 4 5 Average (nm) Standard Deviation 15 153.06 170.68 147.65 136.82 131.39 147.92 15.34 10 88.05 107.7 87.55 117.62 88.34 97.852 13.97 8 80.46 81.42 93.03 83.34 82.93 84.236 5.05 5 69.35 59.62 62.97 52.27 54.22 59.686 6.87 4 44.84 40.45 39.38 47.38 46.63 43.736 3.63 3 26.68 36.47 38.34 50.39 33.46 37.07 8.67 2 11.19 15.1 16.98 17.18 16.3 15.35 2.46 1 N/A N/A N/A N/A N/A ~0 Table B.3 N/A Physical CdS thickness at different locations measured by TEM. 121 Appendix C: De-convolution Fitting Results 3.172 0.09 0.09 0.4625 0.4566 0.5195 3.516 3.582 3.488 3.450 0.0397 0.036 0.0406 0.036 0.036 0.4154 0.4263 0.4414 0.4625 0.4566 0.5195 3.892 3.940 3.965 3.923 3.965 3.908 3.883 a3 0.1752 0.1748 0.1730 0.1663 0.1882 0.1663 0.1736 =1.848*a1 a3 0.1817 0.6669 0.6257 0.6074 0.6480 0.6558 0.6886 0.6844 0.7123 0.6778 =1.56*w1 w3 w3 1.0E-9 2.06E-9 1.0E-9 1.0E-9 1.01E-9 2.2E-9 2.18E-7 2.43E-9 3.89E-8 1E-9 0.2226 0.2187 0.2271 0.2181 0.2159 0.2175 0.2273 0.2988 0.2286 0.2677 0.2165 0.9953 0.9972 0.9977 0.9980 0.9981 0.9974 0.9988 0.9989 0.9992 0.9992 0.9950 0.9976 Adjust 3.16 0.1016 0.4414 3.590 0.0378 0.4032 3.942 0.1781 0.6363 1.45E-9 0.2198 0.9962 a2 = 3.196 0.09 0.4263 3.548 0.036 0.4011 3.969 0.1776 0.6436 1.0E-9 0.2208 0.9927 Flow rate 3.16 0.09935 0.4154 3.463 0.0393 0.3971 3.955 0.1735 0.6243 1.09E-9 0.2217 1st 2nd 1st 2nd 1st 2nd 1st ed R2 3.224 0.09458 0.4032 3.551 0.0386 0.4079 3.959 0.1827 0.6502 1.0E-9 C2 3.18 0.09 0.4011 3.597 0.0384 0.375 3.961 0.1700 0.6148 C1 3.227 0.09832 0.3971 3.577 0.0395 0.4002 3.968 0.1723 E3 3.215 0.09659 0.4079 3.600 0.0395 0.3569 3.973 w2 = w1 1st 3.25 0.09612 0.375 3.589 0.036 0.3941 E2 2nd 3.249 0.09877 0.4002 3.600 0.0373 w1 1st 3.261 0.09885 0.3569 3.613 a1 2nd 3.263 0.09 0.3941 E1 1st 3.283 0.09324 2nd 0.4*a1 2nd 3.289 ((mL/min) 1.0 2.0 3.8 5.7 7.6 9.5 17.0 Table C.1 First and second stage de-convolution fitting results of CdS nanoparticles by continuous MASD. 122 Appendix D: Thickness Data Points of Large CdS Films (6ʺ x 6ʺ) All the thickness data are expressed as the unit of nm. σ Avg. 1 2 3 4 5 6 29.23 21.01 6.87 0.00 0.21 2.01 54.77 62.20 A 11.58 36.47 33.66 30.37 29.72 32.57 32.60 59.91 B 14.36 49.46 25.40 43.02 47.99 64.23 62.70 53.42 C 5.31 67.83 68.83 60.27 63.55 67.64 72.01 74.68 D 24.20 32.79 39.00 46.48 49.00 57.31 1.92 3.04 E 10.75 31.51 28.99 29.74 39.35 40.67 38.26 12.08 F Table D.1 CdS films deposited by continuous MASD in the parallel flow cell. (Flow direction: from right to left) std avg 1 2 3 4 5 6 5.79 29.94 30.32 34.28 32.44 35.90 26.55 20.17 A 6.51 30.57 34.91 26.70 38.75 35.26 24.09 23.69 B 4.06 35.07 36.06 40.17 37.98 34.42 28.46 33.35 C 1.78 33.75 31.10 35.16 33.22 35.17 32.42 35.44 D 2.17 35.39 33.58 38.13 34.12 35.28 37.95 33.26 E 2.17 35.45 35.94 36.62 37.73 36.51 31.71 34.20 F Table D.2 CdS films deposited by continuous MASD in the deflected flow cell. (Flow direction: from right to left) 123 σ Avg. 6 5 4 3 2 1 2.04 71.99 68.80 73.38 73.80 73.33 72.50 70.12 A 2.09 75.34 71.94 78.33 75.96 75.73 74.46 75.63 B 1.11 74.28 73.24 74.85 72.95 74.54 74.09 76.00 C 1.76 74.14 74.43 76.80 73.41 74.34 74.46 71.38 D 4.46 71.77 72.28 76.93 73.37 73.76 70.54 63.74 E 5.79 67.94 70.53 72.30 71.56 65.80 70.41 57.07 F Table D.3 CdS films deposited by continuous R2R-MASD at 2.5min. (Moving direction: from left to right) σ Avg. 6 5 4 3 2 1 5.68 91.02 93.80 99.46 93.88 88.25 86.44 84.29 A 2.29 96.93 97.58 99.12 99.99 94.96 95.02 94.90 B 5.08 97.87 99.59 102.51 99.26 101.42 95.86 88.60 C 2.71 94.81 97.91 98.50 93.67 92.53 93.85 92.38 D 6.81 96.33 97.67 104.25 99.81 100.30 88.52 87.45 E 4.76 91.71 94.46 97.74 94.54 90.96 85.40 87.15 F Table D.4 CdS films deposited by continuous R2R-MASD at 6.3min. (Moving direction: from left to right) 124 σ Avg. 6 5 4 3 2 1 24.81 188.46 173.94 198.39 215.68 208.64 185.84 148.28 A 26.91 237.46 211.33 253.17 264.58 259.17 237.59 198.93 B 18.92 252.16 236.25 266.69 271.84 263.63 250.79 223.78 C 16.71 259.22 239.76 279.90 272.90 266.77 254.86 241.12 D 20.07 239.58 223.04 274.24 247.46 235.85 238.77 218.09 E 21.50 216.78 239.77 247.40 209.90 200.63 207.35 195.59 F Table D.5 CdS films deposited by continuous R2R-MASD at 9min. (Moving direction: from left to right) 125 Appendix E: Time-dependent Outlet Tracer Concentration V = 0.042 m/s Q = 1.0 mL/min V = 0.085 m/s Q = 2.0 mL/min 126 V = 0.161 m/s Q = 3.8 mL/min V = 0.242 m/s Q = 5.7 mL/min 127 V = 0.322 m/s Q = 7.6 mL/min V = 0.402 m/s Q = 9.5 mL/min 128 V = 0.721 m/s Q = 17.0 mL/min