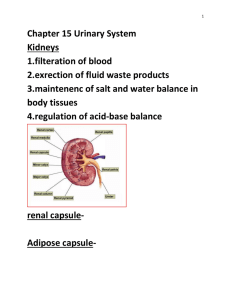
AN ABSTRACT OF THE THESIS OF
advertisement

AN ABSTRACT OF THE THESIS OF Timothy McLagan Hazzard for the degree of Doctor of Philosophy in Animal Science presented on August 26. 1997. Title: Mechanisms of Estradiol -1713- Induced Down- Regulation of Ovine Uterine Oxytocin Receptors During the Estrous Cycle. Abstract Approved: Redacted for Privacy Fredrick Stormshak To determine whether chronic treatment with estradio1-17f3 (E2) maintained the corpus luteum (CL), 10 mature ewes were injected s.c. for 20 days beginning on Day 4 of the cycle with either 500 lig E2 in corn oil or vehicle. Laparotomy of E2-treated ewes on Day 24 revealed the presence of previously marked original CL. Treatment increased the mean interestrous interval for 4 of 5 animals (p < 0.001). Therefore, to determine whether the increase in the interestrous interval was due to an effect on the function and (or) concentration of uterine oxytocin receptors (OTR), 20 ewes were injected with E2 or vehicle as above from Days 4-14 of the cycle. On Day 15, ewes were injected i.v. with oxytocin (OT) or saline, and blood samples were collected for 13,14-dihydro-15-ketoprostaglandin F2, (PGFM) analysis at frequent intervals for 60 min and progesterone (P4) analysis at time 0. Laparotomy was performed after blood collection to remove uterine endometrium from saline-treated ewes only for OTR analysis. Estradiol treatment maintained mean serum concentrations of P4 (p < 0.05), and reduced uterine OTR concentrations (p = 0.002). Treatment with E2 reduced OT-induced concentrations of PGFM (p < 0.01). These data suggest that chronic E2 treatment can prolong the interestrous interval by reducing uterine concentrations of OTR and hence OT-induced secretion of prostaglandin Flo. (PGF2.). To examine the effects of E2 and P4 on uterine OTR, OTR mRNA and P4 receptors (PR), 15 ewes were injected with either 250 lig E2 or vehicle as above or 10 mg P4 on Days 11-14 following vehicle treatment on Days 4-10. Endometrium and CL were removed on Day 15 of the cycle. Mean luteal weights were greater in E2- and P4-treated ewes (p < 0.05). Endometrial OTR and OTR mRNA were greater in control than in E2 and P4-treated ewes. In a subsequent experiment it was determined that endometrial PR did not differ between P4-treated and control ewes. To ascertain whether chronic E2 treatment of ewes during the cycle would alter PGF2.-induced secretion of luteal OT, 20 ewes were injected with 250 pg E2 or vehicle as above. On Day 15, ewes were injected i.v. with PGF2c, or saline and blood samples were collected for OT analysis at frequent intervals for 30 min. Prostaglandin Fu-induced secretion of OT was similar between treatment groups (p > 0.05), suggesting that chronic E2 treatment of ewes during the cycle does not act directly on the ovary to alter production of OT. Collectively, these data suggest that in cycling ewes exposure to increased concentration of E2 or P4 causes down- regulation of the OTR as a consequence of genomic suppression of the OTR gene. ©Copyright by Timothy McLagan Hazzard August 26, 1997 All Rights Reserved Mechanisms of Estradio1-1713 -Induced Down-Regulation of Ovine Uterine Oxytocin Receptors During the Estrous Cycle by Timothy McLagan Hazzard A THESIS submitted to Oregon State University in partial fulfillment of the requirements for the degree of Doctor of Philosophy Completed August 26, 1997 Commencement June 1998 Doctor of Philosophy thesis of Timothy McLagan Hazzard presented on August 26, 1997 APPROVED: Redacted for Privacy Major Professor, representing Animal Science Redacted for Privacy Head of Depa ment o Animal Sciences Redacted for Privacy Dean of Graduat chool I understand that my thesis will become part of the permanent collection of Oregon State University libraries. My signature below authorizes release of my thesis to any reader upon request. Redacted for Privacy Timoth McLagan Hazz , Author ACKNOWLEDGEMENTS I would like to take this time to thank a number of friends and supporters that have helped me to survive the last few years. First, I would like to thank my mentor Dr. Fredrick "Stormy" Stormshak for his invaluable guidance and fatherly advice, humorous anecdotes, and many "swift kicks" that he has freely offered (sometimes too freely!). I would like to thank by fellow graduate students for their help and support through the long days we have shared in the laboratory. First, Jennifer Bertrand who, although often quite a pessimist, was always willing to spend a little extra time helping me figure out problems both scientific and personal. Diana Whitmore-Takahashi and Joan Burke for quickly breaking me in to the "do's and don'ts" of the Stormshak lab. Shelby Filley for her philosophy on "working hard to get somewhere". Ursula Bechert for hours of entertaining e-mail, animal stories and always making me feel like I know everything, "where is the...., How do you do....". Sara "Superchick" Supancic (who got while the gettin's good) for her smiles and extremely positive attitude about everything. Kelly Pinckard (Hazzard) my student, labworker, labmate and finally fiancee who I will mention later. I must also thank many of my graduate peers. Derek "the sperm man" McLean for his quick wit and scientific inspiration, Susan "llama lady" Powell for making me take lunch breaks, Jennifer "loud mouth" Duncan for her frank yet sincere comments, and Tina "major of the week" Clark for her ability to become the sister I never had. I would like to thank those faculty members who served on my graduate committee, especially Dr. Fred Menino for his humor (I think!), and athletic prowess (Go Squirrels!) and Dr. Robert Mason for our many runs and his ever enlightening but always profane chats. I would like to express my sincere gratitude to the many student workers that helped "check the animals", particularly Clint Abbe who not only gave me a wonderful yet browbeaten view of married life (Yes dear!), but for opening up his home for me during the last month of my stay. I would like to acknowledge Dr. Dave Thomas of the Department of Statistics for his assistance in the statistical analyses, and Drs. Mark Mirando and OV Slayden for their advice in performing the radioreceptor assays. Additionally, I would like to thank Dr. Gordon Niswender for the progesterone antiserum, Dr. Dieter Schams for the oxytocin antiserum, Dr. William Thatcher for the PGFM antiserum, and Dr. Tadashi Kimura for the oxytocin receptor cDNA, for without these supplies there would be no project. Most importantly I want to express my appreciation and love to my family and future family. My parents have always been a source of never ending support for any endeavor I have chosen to undertake. They have always been proud of me and have never hesitated to express this. They should be credited for the educational status I have attained. I also want to thank my "unbenounced" cousin Laura Hulburd (Hazzard) for offering her wisdom, support and home to me over the past few years, she has quickly become family to me. I would like to thank "mom and dad" Pinckard for including me in their family times, and many home cooked meals. Finally, I want to thank my fiancee Kelly Pinckard and her "our" daughter Alexa for hours of down-time and fun. "My student" Kelly has become an important part of my life since the time I asked her in the 4°C walk-in "so..are you married?", to the time that I proposed. She has been supportive and my partner in crime both in the lab and especially when the "cat" is away. TABLE OF CONTENTS REVIEW OF THE LITERATURE CHARACTERISTICS OF THE MAMMALIAN ESTROUS CYCLE: A BRIEF OVERVIEW Page 1 1 HYPOTHALAMO-PITUITARY-OVARIAN MECHANISMS Hypothalamic and Pituitary Hormones The Ovary Feedback Mechanisms 3 FOLLICULAR PHASE Stages of the Follicle Recruitment Selection and Dominance Follicular Atresia Hormones of the Ovarian Follicle Two Cell Theory of Estradiol Production Oxytocin Ovulation Morphology and Physical Changes Inflammatory-like Reaction 9 9 Steroid ogenesis LUTEAL PHASE Luteinization The Corpus Luteum Morphology Progesterone synthesis Luteotropins Prolactin Maternal Recognition of Pregnancy Luteolysis Prostaglandin F2a Mechanisms of action for PGF Pulsatile Requirement of PGF2. Oxytocin Oxytocin-PGF2. Feedback Loop 3 7 8 11 12 14 15 15 16 18 18 20 21 23 23 25 25 27 32 36 37 45 48 49 52 53 55 TABLE OF CONTENTS (CONTINUED) Steroid Regulation Progesterone Estrogen Receptor Interaction Page 56 57 59 62 STATEMENT OF THE PROBLEM 65 DOWN-REGULATION OF OXYTOCIN RECEPTORS AND SECRETION OF PROSTAGLANDIN F2, AFTER CHRONIC TREATMENT OF EWES WITH ESTRADIOL-17p 67 INTRODUCTION 67 MATERIALS AND METHODS Animals Treatments Experiment 1 Experiment 2 Steroid Radioimmunoassays Progesterone Assay Estrogen Assay Prostaglandin Metabolite Assay Oxytocin Receptor Analysis Estrogen Receptor Exchange Assay Statistical Analysis 68 68 69 69 69 70 70 72 72 74 76 77 RESULTS Experiment 1 Experiment 2 78 78 78 DISCUSSION 82 IMPACT OF CHRONIC TREATMENT OF EWES WITH ESTRADIOL -17[3 OR PROGESTERONE ON OXYTOCIN RECEPTOR GENE EXPRESSION AND OVARIAN OXYTOCIN SECRETION INTRODUCTION 87 87 TABLE OF CONTENTS (CONTINUED) MATERIAL AND METHODS Animals Treatments Experiment 1 Experiment 2 Experiment 3 Oxytocin Receptor Analysis Progesterone Receptor Exchange Assay Steroid Radioimmunoassays Progesterone Assay Oxytocin Assay RNA Extraction and Northern Blotting Statistical Analysis Page 89 89 90 90 91 91 92 92 93 93 93 94 96 RESULTS 97 DISCUSSION 101 GENERAL CONCLUSIONS 105 BIBLIOGRAPHY 109 LIST OF FIGURES a Figure 1A. 1B. 2. 3. 4. Saturation analysis of OTR in ovine endometrium. Duplicate aliquots of membrane protein from ewes (n=5) were incubated with each concentration of [311] -0T alone (total binding) or with a 200-fold excess of unlabeled OT (nonspecific binding). Specific binding was determined as the difference between total and nonspecific binding. 79 Regression of bound OT in endometrial membranes as determined using a single saturating concentration of [3H] OT (Y = 0.52x - 4.51, R2 = 0.99). 80 Plasma concentrations of PGFM in control (CO) and E2-treated ewes on Day 15 of the cycle after i.v. injection of saline (S) or 20 IU USP OT. Because of different mean basal concentrations of PGFM among groups, each point represents the adjusted mean for the covariate (-5 and 0 time points) for each ewe. Standard errors are given for each mean in the results. 81 Northern Blot of OTR mRNA from Day 15 ovine endometrium. Each lane represents one ewe. Lane 1: ovine nonlactating mammary gland tissue (negative control). Lanes 2-4: animals received 10 mg progesterone s.c. Days 10-14. Lanes 5-7: animals received 250 lig E2 s.c. Days 4-14. Lanes 8-10: animals received 2 ml CO s.c. Days 4-14 (positive controls). The mRNA for OTR is represented by four differentially polyadenylated variants (1.65-6.20 kb). 18S rRNA was probed to determine equality of loading. 98 Plasma concentrations of oxytocin in control (CO) and E2-treated e wes on Day 15 of the cycle after iv. injection of saline (S) or 125 pg PGF2a (Estrumate ®) at time 0. The common estimate of the standard error was 15.18. Each point represents the mean concentration of OT of five animals/group. 99 MECHANISMS OF ESTRADIOL-17P-INDUCED DOWNREGULATION OF OVINE UTERINE OXYTOCIN RECEPTORS DURING THE ESTROUS CYCLE REVIEW OF THE LITERATURE CHARACTERISTICS OF THE MAMMALIAN ESTROUS CYCLE: A BRIEF OVERVIEW The mammalian estrous cycle is characterized by four distinct stages: proestrus, estrus, metestrus and diestrus. The hormonal and utero-ovarian morphological events that occur during each stage control the behavior of the animal and prepare the reproductive tract for the possibility of a pregnancy, respectively. Estrus, or the period of the estrous cycle when mating occurs, usually coincides with ovulation in domestic mammals. Primates have a menstrual cycle with ovulation occurring at the end of an extended follicular phase, which does not coincide with behavioral signs of male receptivity in most species. Estrus is characterized by high systemic concentrations of estrogen secreted by preovulatory Graafian follicles. High serum concentrations of estrogen stimulate the pulsatile release of gonadotropin-releasing hormone (GnRH) from the hypothalamus and a subsequent release of luteinizing hormone (LH) from the anterior pituitary. Preovulatory serum concentrations of LH rapidly increase in response to the estrogen-induced release of GnRH, and initiate mechanisms in the Graafian follicle that result in ovulation. Follicular rupture is then followed by the LH- 2 induced transformation of the granulosa cells into lutein cells, which become the only major source of progesterone for most mammalian species (Hafez, 1993). The transition period between female receptivity (estrus) and the rise in concentration of progesterone is termed metestrus. At this time, the corpus luteum (CL) is developing from the collapsed follicle into a transient endocrine organ that will maintain the uterus in a state capable of maintaining a pregnancy. During diestrus, the comparatively longest stage of the estrous cycle, the CL matures and continues to produce progesterone. Luteal progesterone acts to suppress further release of LH and thus inhibits ovulation. Serum concentrations of progesterone continue to increase over the subsequent 6 to 10 days before leveling off and eventually declining if the ovulated ovum was not fertilized to initiate development of a viable embryo. Proestrus is marked by the decline in progesterone concentrations and the development of a dominant follicle(s) that is destined to ovulate in response to the LH surge. Follicular growth occurs in response to increased basal concentrations of LH whose secretion from the pituitary is provoked by the increasing levels of estrogen being secreted by the developing follicle. Concomitantly, structural regression of the CL occurs with the consequent reduction in systemic concentrations of progesterone. This sequence of events promotes behavioral estrus with ovulation. The duration of each stage varies among different mammalian species, and thus, so do the lengths of the estrous cycles. Importantly, some animals (cow, sow) cycle throughout the year and only become acyclic due to pregnancy or environmental stressors 3 such as temperature. Others experience a period of anestrus, which is linked to the annual photoperiod. In species such as sheep and horses, reproduction of females commences due to the decreasing or increasing of daylight hours, and are termed "short-day breeders" and "long-day breeders", respectively (Hafez, 1993). HYPOTHALAMO-PITUITARY-OVARIAN MECHANISMS Hypothalamic and Pituitary Hormones Hormonal regulation of bodily processes such as metabolism, growth, lactation and reproduction all begin in a region of the brain, the hypothalamus, that differentiates from the ventral portion of the diencephalon during brain development. The hypothalamus consists of multiple neurosecretory nuclei producing a number of neurohormones affecting the pituitary gland. Many of these hormones are secreted into a highly vascularized region called the median eminence, and are absorbed into a portal system that supplies the anterior pituitary gland. Thus, these neurohormones are known as releasing or inhibiting hormones because they exert a direct effect on the pituitary to either release or suppress specific hormones. For example, GnRH from the hypothalamus induces a release of LH/follicle-stimulating hormone (FSH) from the anterior pituitary. Although many of the nuclei in the hypothalamus more commonly secrete one neurohormone only, a great deal of overlap in production of these hormones exists. For example, the supraoptic nuclei and the paraventricular nuclei both produce oxytocin and vasopressin (Hadley, 1992). 4 However, it is more commonly thought that the source of oxytocin is the paraventricular nuclei. The pituitary gland is located beneath the third ventricle of the brain, and is composed of the neurohypophysis (posterior pituitary) and the adenohypophysis (anterior pituitary). The neurohypophysis originates from an evagination of the diencephalon while the anterior pituitary develops from an outpocketing of the oral cavity known as Rathke's pouch. Although the fusion of these developmentally different areas forms the pituitary gland, clearly each area maintains a different function (Hadley, 1992). The adenohypophysis is a glandular structure with no continuous neural innervation from the hypothalamus. This portion of the pituitary is composed of multiple cell types that respond to the hypothalamic releasing or inhibiting hormones secreted into the median eminence and carried by the hypothalamo-hypophyseal portal system. There are three categories of tropic hormones produced and secreted by the adenohypophysis, separated by similarity of chemical structure. The glycoproteins all consist of two peptide subunits, a and p, and an assortment of carbohydrate moieties. The a subunits are the same for LH, FSH and thyroid-stimulating hormone (TSH), however the 13 subunits are different and denote the specificity of each hormone (Boothby et al, 1981). The gonadotropins, LH and FSH, provoke a response in the ovaries or testes. Luteinizing hormone causes the synthesis of testicular androgens in males and follicular androgens in preovulatory (proestrus) females as well as gamete release (ovulation in females). After ovulation, LH is also responsible for the morphological change of granulosa cells to the lutein cells that hypertrophy to form the corpus luteum. Follicle-stimulating hormone is 5 involved with gamete preparation (follicular development in the mammalian female), as well as the conversion of androgens to estrogens by follicular granulosa cells. The third glycoprotein secreted by the adenohypophysis is TSH. As the name suggests, this hormone stimulates the thyroid gland to release triiodothyronine (T3) and thyroxine (T4), which influence metabolic homeostasis (Norris, 1997). The second category of tropic pituitary hormones consists of growth hormone (GH) and prolactin (PRL). These are both large polypeptide hormones that are very similar in structure, and overlap in function. Some common effects of these two hormones involve osmoregulation, selective tissue growth and lactation. However, PRL has weaker effects on body growth and metabolism than GH. Although GH causes growth in many tissues, it does so indirectly through production of insulin-like growth factors (IGF's). Therefore, the main effect of GH is to stimulate the release of IGF's from the liver, which are carried systemically to target tissues, or induce a release of IGF's at a more local level (Norris, 1997). The third category of adenohypophyseal tropic factors consists of several hormones derived from a prohormone known as proopiomelanocortin (POMC). Hormones such as adrenocorticotropic hormone (ACTH), melanocyte-stimulating hormone (MSH), lipotropin (LPH), and [3-endorphin are all variations in posttranslational processing of POMC. Corticotropic cells in the adenohypophysis produce ACTH and pLPH as cleavage products of POMC. However, enzymes in melanotrope cells of the pars intermedia (fused area between the adenohypophysis and neurohypophysis), differentially cleave ACTH to a-MSH; and cleaves 13-LPH to I3-endorphin. Adrenocorticotropic 6 hormone stimulates release of corticosteroids from the adrenal cortex, which are involved in intermediary metabolism and other bodily functions. Alpha-MSH induces the release of melanin from skin cells, which give the skin or hair a darker color (Norris, 1997). Unlike the adenohypophysis, the neurohypophysis is an extension of the neurosecretory neurons originating in the hypothalamus. Therefore, the hormones secreted from the neurohypophysis are actually produced in the nuclei of the hypothalamus. Oxytocin (OT) and vasopressin are both nonapeptides originating in the hypothalamus and are stored, pending proper neuro-stimulus for secretion, in the neurohypophysis. Nonapeptides are synthesized as a larger propeptide, which is hydrolyzed to yield the appropriate nonapeptide and neurophysin (approximately 92 amino acid peptide). Neurophysins are thought to play some carrier function in transporting the nonapeptide to the axonal storage site in the neurohypophysis. Vasopressin acts differentially based on the concentration in the blood. In high doses, vasopressin increases blood pressure by affecting the vascular smooth muscle, which causes diuresis through an increase in the glomerular filtration rate in the kidneys. However, in low doses vasopressin acts as an antidiuretic thus preventing diuresis. Because of this effect, vasopressin is also termed antidiuretic hormone (ADH). Oxytocin stimulates smooth muscle contraction in the reproductive tract of both males (vas deferens) and females (myometrium) as well as contraction of myoepithelial cells around the alveoli and ducts of the mammary gland in females. Muscular contractions in the reproductive tract serve the purpose of both propelling sperm during ejaculation, and movement of sperm in the female to enhance fertilization. Oxytocin has also been used medically during pregnancy to successfully 7 induce labor. Release of OT involved in milk letdown is part of a neuroendocrine reflex arc. Stimulation of the teat by the newborn sends neural impulses to the brain, which reach the hypothalamus and initiate an action potential in the neurosecretory neurons storing oxytocin in the neurohypophysis, resulting in secretion of the hormone (Hadley, 1992; Norris, 1997). The Ovary The ovary is the female gonad that produces the gametes necessary for reproduction. However, the ovary is also a major endocrine organ responsible for the production of sex steroid hormones (estrogen and progesterone), prostaglandins and multiple peptide hormones (relaxin, inhibin, activin, follistatin and oocyte maturation inhibitor). These compounds are produced and secreted by two distinct cell populations in the follicles, and in some species, by the supporting stroma (interstitial cells; Hansel et al., 1973). Follicular development occurs in waves throughout the estrous cycle of domestic animals and ultimately the dominant follicle(s) of a wave ovulate to form corpora lutea. Those follicles not ovulating undergo a form of degeneration referred to as atresia (Norris, 1997). Follicular thecal cells are the primary source of androgens while granulosa cells are the primary source of estrogens in the ovary (follicular development will be discussed in further detail in subsequent sections). Importantly, many of these compounds (estrogen, inhibin and activin) are produced and secreted due to stimulation by pituitary gonadotropes, FSH and LH. Thus, regulation of follicular development and ovulation 8 depends on a cascade of hormonal release originating in the brain and continuing in the ovary, forming what is termed the hypothalamic-pituitary-ovarian axis. Feedback Mechanisms Feedback mechanisms are common throughout biochemical pathways and are usually characterized as being negative or positive. As stated above, hypothalamic hormones act in a positive manner to induce the release of pituitary hormones, which in turn stimulate ovarian secretion of steroids. However, the possibility of retrograde flow from the hypothalamo-hypophysial-portal system (Page and Bergland, 1977), and the accumulation of pituitary hormones in the circulatory system, provide a means by which these hormones can feedback to inhibit secretion of their corresponding hypothalamic releasing hormones. Synthesis, storage and release of hypothalamic hormones are regulated by three types of feedback mechanisms: "ultra-short loop", "short loop" and "long loop" feedback (Hafez, 1993). An example of "ultra-short loop" feedback would be the regulation of hypothalamic hormone production and release by the same hormone, thus it is actually a form of self-regulation. Modification of hypothalamic hormone secretion by pituitary hormones would be "short loop" feedback, while stimulated production of gonadal hormones traveling through the circulatory system to affect hypothalamic or pituitary output would be an example of "long loop" feedback. All three types of feedback mechanisms can have either a positive or negative effect on the 9 secretory activity of the stimulating hormones, and examples of both will be mentioned throughout this thesis. FOLLICULAR PHASE Stages of the Follicle As previously mentioned, the ovary is not only the site of gametogenesis, but also a major endocrine organ. However, the established connections between gametogenesis and endocrine factors cannot be explained without first detailing the physical structure and components of the ovary: the follicle. At any time during the reproductive years of a female, there are constant morphological changes occurring in the ovary, representing the development of the primordial follicles to mature follicles that will either ovulate or undergo atresia. Unlike males, who produce sperm throughout their lives, the female produces all of the primordial follicles of her reproductive life during fetal development (primates, ruminants) or early in neonatal life (rodents, rabbits; Fortune, 1994). At this point, the oocyte located within the follicle, is arrested in the diplotene stage of meiosis I of development (referred to as dictyate stage), and awaits the appropriate signals necessary to initiate resumption of meiotic division. Stages of follicular development are classified by changes in oocyte morphology and size, as well as the organization of the granulosa (cumulus oophorus) cells surrounding the oocyte (Gore-Langton and Armstrong, 1994). 10 Follicular development begins with the primordial follicles, which constitute a nongrowing follicle "reserve" in the ovarian cortex. Depletion of this "reserve" occurs throughout the reproductive life of the female, and is characterized by the growth of the primordial follicle. During the onset of follicular growth, the oocyte begins to grow and the flattened pre-granulosa cells of the primordial follicle become cuboidal and begin to proliferate (Hirshfield, 1991). The factors that initiate follicle development remain unknown (Fortune, 1994). However, both primordial and primary follicular development are independent of gonadotropins (Richards and Midgley, 1976), and recently Wandji et al. (1996), suggested that the ovarian stroma exerts inhibitory control over the initiation of primordial follicle growth. Secondary follicles are characterized by the presence of the zona pellucida, a glycoprotein matrix immediately surrounding the oocyte, as well as the proliferation of granulosa cells into two or more continuous layers. Granulosa cells are surrounded by the basement lamina, which separates the granulosa from the thecal cells. Tertiary (antrum, Graafian) follicles contain an antrum filled with follicular fluid, composed of secretions from the surrounding cells (Edwards, 1974), as well as a continually proliferating granulosa cell layer (Gore-Langton and Armstrong, 1994). The hypothesis for recruitment of the tertiary follicle for ovulation or atresia is discussed below. 11 Recruitment Although the female has a reserve of nongrowing follicles in the ovary, the patterns of development of large follicles in mammals do not appear to be random. Instead, development occurs during particular times of the reproductive cycle. Follicular development in rats, humans and pigs, occurs only during the follicular phase, and is otherwise suppressed (Fortune, 1994). Pache et al. (1990) demonstrated in humans that a cohort of follicles begin to develop during the early follicular phase, from which only one follicle continues to grow in the late follicular phase while others regress. Importantly, no ovulatory-size follicles develop during the luteal phase in humans, rats and pigs. In contrast, follicular development involving ovulatory-size follicles throughout the cycle occurs in cattle, sheep and horses. In heifers, ultrasonography revealed that two or three times during the cycle a wave of 3 to 6 follicles begin to grow larger than 4 to 5 mm in diameter (Sirois and Fortune, 1988). After several days of growth, one follicle continues to grow and the others regress. Similarly, in ewes both laparoscopy and ultrasonography revealed that peaks of follicular activity above a constant basal level occurs two or three times during the cycle (Noel et al., 1993; Schrick et al., 1993). However, these peaks of follicular activity result in multiple follicles that do not regress to leave a single dominant follicle. Thus, ewes are considered to display a continuous entry, growth and atresia of follicles that result in the presence of several ovulatory-size follicles (Schrick et al., 1993). In fact, the number of large follicles was demonstrated to be 2 to 3 times the ovulation rate in ewes (Noel et al., 1993). These data suggest a lack of follicular dominance in this 12 polyovular species. Nevertheless, two follicular wave patterns may occur during the estrous cycle of ewes while in heifers two or three waves have been detected. However, only the wave immediately preceding luteal regression (second or third wave), results in ovulation of the largest follicle(s) (Ginther et al., 1989; Noel et al., 1993). Thus, a signal is responsible for the initiation of growth or recruitment of a group of follicles in a non- random pattern. Experiments measuring plasma concentrations of FSH throughout the bovine estrous cycle have demonstrated slightly higher concentrations before the first, second and third follicular waves (Walters and Schallenberger, 1984; Adams et al., 1992). Gong et al. (1996) demonstrated in heifers, that FSH was required for antral follicles mm to continue growth up to 9 mm in diameter. In primates, plasma FSH is slightly elevated during the early follicular phase as compared with the late follicular and luteal phases (Abraham et al., 1972). Thus, slight elevations in plasma FSH concentrations are the required signal that stimulates follicular recruitment in mammals (Fortune, 1994). Selection and Dominance Finally, recruitment ends with the selection of one follicle to continue to grow at the expense of the other follicles in its cohort. These follicles are called "dominant" follicles because not only do they continue to grow, but they differentiate functionally from the subordinate follicles in ways that prepare them for ovulation. For instance, one prominent characteristic of the dominant follicle is the ability to produce tremendous 13 amounts of estradiol- 1713 (Ireland et al., 1983). Increased estradiol production appears to arise from increases in the ability of granulosa cells to aromatize androgens, which will be discussed below. More recently, researchers have examined the mRNA expression of enzymes responsible for steroid synthesis in an effort to determine molecular markers characteristic of dominant follicles (Tian et al., 1995; Xu et al., 1995, Bao et al., 1997a,b). Activity of steroidogenic enzymes such as cytochrome P450 side-chain cleavage (P450scc), 3P-hydroxysteroid dehydrogenase (313-HSD), and cytochrome P450 17ahydroxylase all increased in thecal cells within 24 hours after prostaglandin Fla (PGF2.)- induced luteolysis in heifers (Tian et al., 1995). However, the expression of 3P-HSD was higher in healthy follicles as compared with atretic follicles, and dominant follicles expressed 3P-HSD in granulosa cells (Bao et al., 1997b). Therefore, expression of 3PHSD mRNA in granulosa cells may be associated with the mechanism of dominant follicle selection during the follicular wave. Alternatively, the expression of mRNA for FSH receptor (FSHr) and LH receptor (LHr) was detected in granulosa and thecal cells, respectively (Xu et al., 1995). However, these researchers further demonstrated the expression of LHr in granulosa cells of healthy >9 mm follicles, suggesting the importance of LHr expression in selection of follicular dominance. Finally, Bao et al. (1997a) suggested that follicular recruitment in cattle was associated with granulosa expression of P450scc and cytochrome P450 aromatase (aromatase), while the process of follicular selection was associated with initiation of LHr mRNA in granulosa cells. In any case, hormonal steroidogenic expression, feedback mechanisms, and receptor expression are all 14 cues temporally spaced to bring about the final selection that establishes dominance among a cohort of follicles. Follicular Atresia Although the penultimate reason for folliculogenesis is ovulation, many follicles develop before the appropriate signals are present to cause ovulation. Follicular development is initiated, but, at a certain point these follicles undergo a form of degeneration termed atresia. Atresia is associated with several characteristics such as degeneration of the oocyte, hypertrophy of theca cells (Erickson et al., 1985), and the appearance of pyknotic granulosa cells (Byskov, 1978). When and why atretic follicles appear remains to be a contested point. Richards (1980) suggested that most follicles undergo atresia at an early stage of growth in the absence of appropriate hormonal assistance (FSH and LH). Conversely, Hirshfield (1991) implied that most follicles become atretic near the end of development. Uniquely, this latter study described follicular development in the rat as a series of follicular cell generations, with atresia most commonly occurring during the 8th or 9th granulosa cell generation. Similarly, the highest rates of atresia occur near the end of follicular development in cattle (generations 10 to 13; Lussier et al., 1987). Only those follicles that are exposed to specific signals at the time they reach this stopping point (8th or 9th granulosa cell generation) will continue to develop subsequent granulosa cell generations and ovulate. Although FSH has been previously described to be necessary in recruitment of follicles, a variety of hormonal 15 triggers in addition to FSH have been hypothesized to cause atresia. Circulating concentrations of gonadotropins, androgens and estrogens in excess (Harman et al., 1975; Clark et al., 1981) or insufficient quantities of gonadotropins and estrogens (McNatty et al., 1979; Richards, 1980), have all been associated with atresia. In fact one of the primary characteristics of the impending atresia is a high follicular fluid concentration of androgens due to decreased aromatase activity (Maxson et al., 1985). Further, metabolic stress involved with increasing cell number has also been implicated with atresia (Hirshfield, 1991). However, a definitive cause for why follicles do not continue to grow past a certain point without the proper signals remains to be clarified. Hormones of the Ovarian Follicle Two Cell Theory of Estradiol Production As mentioned previously, estradiol is produced by follicles. This steroid hormone is reproductively important because it is responsible for estrous behavior in some species, and is involved in a feedback mechanism with the hypothalamus, regulating the LH surge and ovulation. Interestingly, in many species the mechanisms by which estradiol is synthesized involves the coordinated effort of both follicular cell types; theca and granulosa cells. The two-cell theory of estrogen production states that LH acts on the theca interna to stimulate production of androgens from cholesterol derived progestins (Falck et al., 1962; Richards, 1980). While granulosa cells lack the enzyme (17ahydroxylase) necessary for androgen production, androstenedione more so than 16 testosterone (Fortune and Hansel, 1985), does diffuse across the basement membrane to the granulosa cells. These cells contain the FSH-induced enzyme aromatase (Berndtson et al., 1995), which converts thecal derived androgens to estrogens. Although both cell types produce progestins (Fortune and Hansel, 1979a), incubations of follicle wall (granulosa and theca interna cells) yielded greater production of androstenedione than theca cells alone (Fortune, 1986), suggesting that granulosa cells supply the necessary progestins for androgen biosynthesis by the theca cells. Thus, the production of steroids from theca interna and granulosa cells are dependent on the specific precursors from each cell type as well as regulation by the gonadotropins. Oxytocin Oxytocin is another important hormone produced by the preovulatory follicles of cows (Wathes et al., 1984; Schams et al., 1985) and ewes (Wathes et al., 1986). However, the production of follicular OT seems to be species specific as Stock et al. (1995), demonstrated that preovulatory follicles of mares are not a source of OT. Bovine granulosa cells from preovulatory follicles have been identified as the source of OT in vitro (Schams et al., 1985), and the location of OT mRNA synthesis (Holtorf et al., 1989). This led to a series of investigations into the role of OT in regulating preovulatory follicular steroidogenesis. Aladin-Chandrasekher and Fortune (1990), demonstrated that OT stimulated progesterone production by granulosa cells in vitro, and this occurred just prior to the LH/FSH surge (Voss and Fortune, 1991a). Thus, OT was hypothesized to have a role in the steroidogenic shift from estradiol production to progesterone 17 production. A subsequent study was conducted to determine the developmental pattern of OT secretion and the response to gonadotropins by the granulosa and thecal cells during the follicular phase (Voss and Fortune, 1991b). This study demonstrated that granulosa cells are the primary source of OT throughout the follicular phase, as OT secretion was only produced in theca cell cultures contaminated by granulosa cells. Further, more oxytocin was secreted during coculture of early and midfollicular phase theca and granulosa cells than by granulosa cells alone, and this response was induced by LH and FSH. Results of this study suggest that the LH/FSH surge stimulates the secretion of oxytocin. Increased secretion of oxytocin after the LH/FSH surge is attributable to the accumulation of oxytocin/neurophysin I mRNA in bovine granulosa cells (Voss and Fortune, 1992). Voss and Fortune (1993) examined the effect of estradiol on oxytocin secretion by bovine granulosa cells, and demonstrated that estradiol in low doses stimulated oxytocin secretion while high doses inhibited secretion. These data suggest that the elevated concentrations of estradiol in the follicular fluid of early and midfollicular phase follicles (Fortune and Hansel, 1985) may inhibit OT production, while the decline in estradiol levels after the LH surge may allow stimulation of oxytocin synthesis by gonadotropins. In addition, progesterone stimulated OT secretion by cultures of bovine granulosa cells (Voss and Fortune, 1993). Thus, both OT and progesterone may amplify the ability of gonadotropins to stimulate the production of the other hormone. However, whether progesterone regulates OT mRNA expression is unclear. In any case, a role for steroids in modulating follicular oxytocin secretion exists, suggesting that the 18 presence of OT during the periovulatory period may involve regulation of luteinization and/or ovulatory events. Ovulation Mammalian ovulation is a unique event that involves the rupture of healthy tissue at the surface of the ovary (Espey, 1994). Ovulation is initiated at the moment a surge of gonadotropins stimulates the dominant follicle(s) (Espey and Lipner, 1994). The pituitary surge of gonadotropins consists of both LH and FSH, and although an LH surge can lead to as much as a 100-fold increase in circulating concentrations of this hormone (Scaramuzzi et al., 1972), FSH has also been demonstrated to induce ovulation by itself in monkeys (Schenken et al., 1984). In fact, many researchers have suggested that a combination of LH and FSH are needed to ensure an optimal ovulatory stimulus (Nalbandov et al., 1973; Galway et al., 1990). In either case, the surge of gonadotropins that initiates ovulation bind to receptors in the follicular cells activating signal transduction pathways that result in phosphorylation processes and protein synthesis (Espey and Lipner, 1994). Morphology and Physical Changes Although the pituitary surge of gonadotropins appears to initiate ovulatory events, many morphological changes must occur in the dominant follicle to cause rupture. Rupture occurs at the apex of the follicle (where the stigma forms) and consists of 19 multiple tissue layers (Espey and Lipner, 1994). The outermost layer is a single-celled layer of cuboidal epithelial cells that surrounds the entire ovary. The second layer is the tunica albuginea, a fibroblast and collagen sheath, which surrounds the ovary and provides external structural integrity. The third layer is the theca externa cells of the follicle, and also contains fibroblasts within this collagen bound matrix of cells. The fourth layer consists of the steroid secreting theca interna cells that line the basement membrane of the follicle, and the fifth and final layer is the granulosa cell layer surrounding the antrum. Thus, the release of the oocyte from the follicle depends on the dissociation of multiple cell layers, as well as the degradation of the collagen matrix that maintains ovarian integrity (Espey, 1994). With the activation of phosphorylation events and protein synthesis within follicular cells, it is not too surprising that researchers have studied proteolysis as a means for follicular rupture. Plasminogen activator is a protease associated with the preovulatory degradation of the follicle wall (Galway et al., 1990). Plasminogen activator cleaves both serum and follicular fluid plasminogen to the active enzyme plasmin, which activates collagenase to degrade the collagen matrix supporting the follicle wall and results in ovulation (Strickland and Beers, 1976; Beers and Strickland, 1978). Galway et al. (1990) demonstrated that recombinant FSH injected into immature rats stimulated tissue plasminogen activator (tPA) enzyme activity and mRNA expression to reach peak levels 12 hours after injection, providing evidence for the role of tPA in the FSH induction of ovulation. However, FSH is not the only gonadotropin capable of stimulating PA. Reich et al. (1985) demonstrated that LH was as potent a stimulator of PA activity as FSH. More importantly, this latter study illustrated the importance of 20 follicular steroidogenesis in ovulation by demonstrating the ability of exogenous estradiol to enhance the LH induction of PA. Inflammatory-like Reaction Ovulation has been likened to an inflammatory reaction (Espey, 1994). Inflammation generally occurs as a result of a disturbance to the plasma membrane, such as occurs when epithelial cells of the skin are perturbed by abrasion. With any disturbance of the plasma membrane, prostaglandins are produced from a release of arachidonic acid from the membrane phospholipids (Espey and Lipner, 1994), initiating a cascade of events leading to the activation of prostaglandin synthetase and lipoxygenase. Importantly, part of the inflammatory process includes vasodilation, hyperemia, edema and collagenolysis, suggesting a synergistic role with PA and proteolysis. The first implication of an association of prostanoids with the ovulatory process was presented in a report by Labhsetwar (1971), who found that prostaglandin Fla (PGF2a) could induce both luteolysis and ovulation in hamsters. Evidence of a role of prostanoids at the follicular level was further documented by findings that intrafollicular concentrations of PGF2a and prostaglandin E2 (PGE2) increased in the ovulatory follicle just prior to ovulation (Ainsworth et al., 1975; Armstrong and Zamecnik, 1975; Bauminger and Linder, 1975). The action of at least PGE2 has been demonstrated to mimic the action of FSH on granulosa cells and LH on theca cells (reviewed by Armstrong, 1981). Thus, PGE2 may be able to substitute for or enhance the function of gonadotropins at certain stages of follicular development. Inhibition of prostaglandin synthesis by drugs does not interfere 21 with ovum maturation, activation of adenylyl cyclase, steroidogenesis, nor luteinization (Tsafriri and Dekel, 1994). In fact, an inhibitor of prostaglandin synthesis, indomethacin, which causes a reduction in PGE2 and PGF2 within minutes of an intravenous (i.v.) injection in rabbits (Espey et al., 1986), was found to inhibit only follicular rupture in rats, rabbits, gilts and ewes (Armstrong and Grinwich, 1972; O'Grady et al., 1972; Tsafriri et al., 1972; Ainsworth et al., 1979; Murdoch and McCormick, 1991), without the inhibition of luteinization and progesterone synthesis (Espey and Lipner, 1994). Another factor involved with the inflammatory response is tumor necrosis factor (TNF), which is present in the ovary (Brannstrom et al., 1994; Terranova, 1997). Tumor necrosis factor has been demonstrated to induce ovulation in rats, but not as successfully as LH. However, when TNF and LH were added simultaneously to ovaries in vitro, the number of ovulations increased fourfold over LH alone (Brannstrom et al., 1995). Thus, TNF is involved with ovulatory events, but the mechanism by which TNF mediates ovulation remains unknown. Steroidogenesis Changes in steroidogenesis occur in the preovulatory follicle during the late follicular phase primarily consisting of a transition from the production of androgens and estrogens to that of progestins (Fortune and Hansel, 1979b). The change in steroidogenesis has been associated with the gonadotropin surge and ovulation (Espey and Lipner, 1994). At the beginning of ovulation, estradiol synthesis is high while progesterone synthesis is very low. However, during the first half of the ovulatory process estradiol production drops to negligible quantities while progesterone production 22 increases substantially. Thus, it is logical to assume that estradiol is not needed for ovulation, and that luteinization of follicular tissue begins to occur before actual ovulation (Espey and Lipner, 1994). This brings into consideration the importance of progesterone in ovulation, as ovulation cannot occur unless a follicle begins to luteinize and produce progesterone (Espey, 1994). Snyder et al. (1984) demonstrated the necessity of progesterone in the ovulatory process by using an inhibitor of 3 P-HSD, epostane. In this study, rats were used to demonstrate that the inhibition of follicular steroidogenesis and ovulation induced by epostane, could be overcome by administration of exogenous progesterone. A further study with ewes treated with isoxazol, an inhibitor of progesterone synthesis, resulted in the inhibition of ovulation in this species (Murdoch et al., 1986). Thus, the presence of progesterone at the time of ovulation is somehow involved in regulating ovulation. One possible effect of progesterone was demonstrated by Tanaka et al. (1992) who measured ovarian PA and kallikrein (a serine protease) activity in rats. Epostane was used to block ovulation, and was also found to inhibit PA and kallikrein activities in a dose-dependent manner. The inhibitory action of epostane was reversible by the treatment of animals with progesterone, suggesting the increase of follicular progesterone at the time of ovulation regulates ovarian kallikrein and PA activities. 23 LUTEAL PHASE Luteinization Luteinization and the resulting formation of the corpus luteum are a direct result of the preovulatory gonadotropin surge. Theca interna and granulosa cells of the preovulatory follicle undergo morphological and biochemical changes that transform these follicular cells into the lutein cells of the corpus luteum. Following the ovulatory stimulus, the basement membrane breaks down, and blood vessels from the theca interna invade the ruptured antral cavity (Corner, 1919). Growth of new capillaries, angiogenesis, begins chronologically at the time of basement membrane breakdown (Tsukada et al., 1996), forming a complex network of vessels in the previously avascular granulosa cell layers (Bassett, 1943). Angiogenesis plays an important role in the development and nourishment of the corpus luteum, thus opening investigation into the role of angiogenic factors in corpus luteum development. Gospodarowicz et al. (1985) purified a diffusible substance responsible for triggering the early vascular changes that occur in the developing corpus luteum, and found it to be related to fibroblast growth factor (FGF). Previous research suggested FGF to be mitogenic for cultured granulosa cells in rats, pigs and humans (Gospodarowicz and Bialecki, 1979). However, FGF lacks a hydrophobic signal peptide thought to be necessary for extracellular transport (Redmer et al., 1985). Thus, although FGF has mitogenic activity, other growth factors with diffusible properties were thought to be responsible for neovascularization in the corpus luteum. Leung et al. 24 (1989) identified a potent mitogen for vascular endothelial cells called vascular endothelial growth factor (VEGF). Importantly, VEGF is a secreted protein with a potential to be involved in the regulation of vascular endothelial growth and angiogenesis. Vascular endothelial growth factor expression has been demonstrated in the corpus luteum of rats (Phillips et al., 1990), ewes (Redmer et al., 1996), and primates (Ravindranath et al., 1992). Further, VEGF expression is upregulated during early luteal development (Ravindranath et al., 1992; Redmer et al., 1996), when vascular growth is at its peak. Finally, Koos (1995) demonstrated that human chorionic gonadotropin (hCG) induces VEGF expression in female rats, suggesting a role of this peptide in the ovulatory process as well as in luteal vascular growth. Therefore, angiogenic activity in the early development of the corpus luteum is of fundamental importance in promoting the early development of this ephemeral endocrine gland. Further morphological and functional changes occur to the thecal and granulosa cells during luteinization. Thecal cells undergo significant hypertrophy and hyperplasia (O'Shea et al., 1980). Within 24 hours after the LH surge, these cells begin to migrate into the ruptured follicular cavity (O'Shea et al., 1979), and become dispersed among the luteinizing granulosa cells. Granulosa cells, meanwhile, have intracellular morphological changes, such as the accumulation of smooth endoplasmic reticulum, and the development of tubular cristae in the mitochondria (Niswender and Nett, 1994). These changes are characteristic of steroid-secreting cells. As previously mentioned, steroidogenesis changes from androgen/ estrogen production to progestin production at the time of ovulation. Enzymatically, the activity of cytochrome P450scc increases following the gonadotropin 25 surge, while 17a-hydroxylase activity decreases (Rodgers et al., 1986, 1987; Lauber et al., 1991), thus enhancing the conversion of cholesterol to progestins and preventing further conversion of progesterone to androgens. Morphological changes also occur at the cell surface. Following ovulation, FSH receptors in plasma membranes of granulosa cells are internalized and gene expression for these receptors is reduced (Nakamura, et al., 1991); although FSH receptors have been detected in corpora lutea of hamsters (Oxyberry and Greenwald, 1982) and cows (Matins et al., 1984). Receptors for LH in granulosa cells are also down-regulated following the LH surge (Segaloff et al., 1990; Lapolt et al., 1990; Nakamura et al., 1991), but expression of these receptors reoccurs approximately 48 hours after the gonadotropin surge (Braden et al., 1994). The importance of these surface receptor changes will be discussed below. The Corpus Luteum Morphology The direct result of luteinization in normally cycling animals, is the formation of a transient endocrine organ called the corpus luteum. Morphologically, the corpus luteum consists of at least four types of luteal cells: two types of steroid-secreting cells, vascular endothelial cells, and fibroblasts (Wiltbank, 1994). Steroidogenic luteal cells appear to be derived from the two distinct follicular steroid-secreting cells; thecal and granulosa cells. Therefore, it is of no surprise that the steroidogenic cells comprising the corpus luteum, 26 can be differentiated into two distinct cell types. Cell size, among other differences, is the most obvious characteristic that distinguishes these cells, and has led to their designation as small and large luteal cells (Koos and Hansel, 1981). Small and large luteal cells contain numerous mitochondria, and an abundance of smooth endoplasmic reticulum, which is common in steroid-secreting cells. However, unlike small luteal cells, large luteal cells also contain a rough endoplasmic reticulum and secretory granules indicative of protein secreting activity (Niswender and Nett, 1994). Secretory granules contain a number of different components including oxytocin (Rodgers et al., 1983; Wathes et al., 1983; Sawyer et al., 1986), neurophysin (Fields and Fields, 1986; Sawyer et al., 1986), and relaxin (Fields and Fields, 1980; Fields, 1984), which have been demonstrated to be released by exocytosis (Sawyer et al., 1979). Furthermore, the number of secretory granules present in the large luteal cells changes during the estrous cycle of the cow, with the highest percentage of these cells containing secretory granules during the mid-luteal phase (Fields et al., 1992). Small and large luteal cells also differ morphologically in ways that affect their response to hormones. Variations in cell response are mainly due to differences in receptor expression in the cell membrane. Fitz et al. (1982) demonstrated that small luteal cells from superovulated ewes possess substantially more receptors for LH, while large luteal cells contain the majority of receptors for prostaglandins. However, other researchers have demonstrated that large and small luteal cells from normally cycling ewes (Harrison et al., 1987), cows (Chegini et al., 1991), and rats (Nelson et al., 1992), contain similar numbers of LH receptors. Chegini et al. (1991) also suggested that small luteal 27 cells contain a significant number of prostaglandin receptors, but this is probably due to the presence of high-affinity binding sites for PGF2. on large luteal cells and low-affinity binding sites on small luteal cells (Balapure et al., 1989). Whatever the case, both steroidogenic luteal cell types have cell surface receptors that bind ligands and initiate some form of intracellular activity. The processes of ovulation and luteinization includes inflammatory-like events and neovascularization. Therefore, it is no surprise that the other luteal cell types are vascular endothelial cells and fibroblasts. Vascular endothelial cells, which line the lumen of blood vessels in the corpus luteum, make up the largest percentage of luteal cells (approximately 50%), but are only a small percentage of the total corpus luteum volume (10%; Wiltbank, 1994). However, the role of vascular formation is extremely important in establishing the luteal blood flow necessary to provide steroid precursors and to transport secreted hormones to the general circulation. Fibroblasts infiltrate the corpus luteum at the time of basement membrane breakdown, and have been demonstrated to induce luteal cell division in vitro (Gospodarowicz and Birdwell, 1977). Further, FGF infused into the ovarian artery for 12 hours suppressed the secretion of estradiol and androstenedione, suggesting a paracrine effect on luteal function (Scaramuzzi and Downing, 1995). Progesterone synthesis Progesterone is the main steroid hormone produced and secreted by the corpus luteum. This steroid is the ovarian "hormone of pregnancy" (Corner and Allen, 1929; Allen and Corner, 1930), and is responsible for preparing the reproductive tract for 28 embryo implantation, as well as the subsequent maintenance of the pregnancy. Incredibly, corpus luteum production of progesterone reaches approximately 1016 molecules of progesterone . min"' . g"' of luteal tissue during the midluteal phase of the cycle in ewes (Stormshak et al., 1963). These secretion rates are dependent on the activity and efficiency of both steroidogenic luteal cells, and the rapid transport of secreted progesterone to the general circulation. The apparent production of progesterone is reliant on the presence of precursors necessary for continuous metabolic synthesis, and the factors that regulate such a system. The synthesis of progesterone requires a source of cholesterol, which can come from either de novo synthesis or plasma lipoproteins. Luteal cells have been demonstrated to produce cholesterol from acetate as first demonstrated by Savard and Casey (1964), but appear to prefer the delivery of cholesterol by way of lipoproteins (Gwynne and Strauss, 1982). Cholesterol bound to lipoproteins is delivered either as low-density lipoproteins (LDL) or high-density lipoproteins (HDL) depending on the species. Progesterone secretion has been demonstrated to increase in cattle, after luteal cells were incubated with LDL or HDL (Pate and Condon, 1982; Carroll et al., 1992). In sheep, both LDL and HDL stimulated progesterone secretion by large luteal cells and LH-stimulated small luteal cells (Wiltbank et al., 1990); however, HDL-induced release of progesterone was greater. These researchers also demonstrated that small luteal cells utilize the endogenous lipid droplets, within the cytoplasm, to produce progesterone. These cholesterol stores were eventually depleted during long cultures or after LH stimulation. Ovine large luteal cells 29 do not contain lipid droplets (Niswender and Nett, 1994), and appear to need lipoproteins for progesterone production (Wiltbank et al., 1990). Low-density and high-density lipoproteins bind to specific receptors on the surface of the steroidogenic luteal cells, which facilitate the transport of the lipoprotein from the outside to the inside of the cell. The uptake of cholesterol carried by LDL occurs by way of receptor-mediated endocytosis (Brown and Goldstein, 1986). Once the LDL is internalized it is combined with lysosomes and degraded to liberate cholesterol. Free cholesterol is then utilized for steroid biosynthesis, or esterified and stored as lipid droplets (Flint and Armstrong, 1973). Cholesterol uptake by luteal cells is mainly related to concentrations of lipoprotein receptors. In the human, LDL receptors increased in granulosa cells treated with hCG or 8-bromo-cAMP (Golos and Strauss, 1987). Rat luteal tissue treated with hCG or LH increased binding of HDL (Strauss et al., 1982). Importantly, progesterone production is enhanced when bovine luteal cells were incubated in the presence of either LDL or HDL (Pate and Condon, 1982; 0' Shaughnessy and Wathes, 1985). However Carroll et al. (1992) demonstrated that lysine-modified LDL (which does not bind to the LDL receptor) stimulated progesterone secretion by bovine luteal cells, suggesting the delivery of LDL cholesterol may not require receptor binding. Further, a mechanism other than receptor-mediated endocytosis may be responsible for cholesterol transfer from HDL, because Paavola et al. (1985) demonstrated that delivery of cholesterol from HDL may not require internalization. Whatever the type of lipoprotein or the mechanism for cholesterol delivery, the source of cholesterol for steroid biosynthesis is lipoproteins (Wiltbank, 1994). 30 Cholesterol is converted to pregnenolone in the mitochondria and this steroid eventually gives rise to progesterone in the endoplasmic reticulum. Thus, cholesterol transport across the mitochondrial membrane in necessary for steroid synthesis to occur. Ghosh et al. (1987) suggested that cholesterol transport from the outer to the inner mitochondrial membrane is the rate-limiting step in steroid synthesis. However, until recently the transport process was an enigma. Clark et al. (1994) demonstrated that a LHinduced regulatory protein was responsible for cholesterol transport into the mitochondria, and proposed to call this protein the "steroidogenic acute regulatory protein" (StAR). Further investigations suggested that mRNA expression of StAR was closely associated with steroid production in mice (Clark et al., 1995), thus, considerably strengthening the evidence that StAR is a key mediator of steroidogenesis. Presence of mRNA for StAR protein declined in hypophysectomized ewes; however, treatment with LH rescued StAR protein expression to concentrations not different from those of control ewes (Juengel et al., 1995). Finally, the human ovary expresses mRNA for StAR, which was induced by 8bromo-cAMP, a cyclic nucleotide analogue, suggesting a signal transduction link to LH (Kiriakidou et al., 1996). Cholesterol transported by StAR reaches the inner mitochondrial membrane, the site of P4508. This enzyme converts cholesterol to pregnenolone, which is then changed in the smooth endoplasmic reticulum to progesterone by 313-HSD. The resulting final product is then secreted, because the capacity of the luteal cell to store progesterone is limited (Niswender and Nett, 1994). The basal synthesis and secretion of progesterone, regardless of species, appears to be the result of LH stimulation. In small luteal cells, progesterone secretion increases 31 greatly when stimulated by LH in sheep and rats (Fitz et al., 1982; Nelson et al., 1992), and ovine luteal LH receptor mRNA is highest during the midluteal phase of the cycle (Smith et al., 1996). Luteinizing hormone binds to a G-protein coupled receptor in the plasma membrane, and activates adenylyl cyclase. Activation of adenylyl cyclase increases the intracellular second messenger, cAMP, which stimulates protein kinase A (PKA). Marsh (1975) suggested that cAMP is the intracellular signal by which LH activates the small luteal cell. The activated catalytic subunit of PKA then uses Mg*ATP to phosphorylate various regulatory proteins (enzymes) within the cell. Luteinizing hormone may stimulate progesterone secretion through changes in PKA activation, cholesterol transport, or uptake of cholesterol from lipoproteins. Cholesterol esterase, which releases cholesterol from pools of esterified fatty acids, is activated by cAMP-dependent protein kinase (Caffrey et al., 1979). Although cholesterol esterase appears to be a target for LH-induced regulation of steroidogenesis, other steroidogenic enzymes (P450scc and 3r3-HSD) are apparently not regulated by LH (Wiltbank et al., 1993). Although LH induces progesterone secretion from small luteal cells, LH has very little effect on large luteal cell secretion of this steroid in ewes (Fitz et al., 1982; Nelson et al., 1992). Furthermore, incubation of ovine large luteal cells with LH does not increase intracellular cAMP concentrations or secretion of progesterone (Hoyer et al., 1984). However, large luteal cells secrete the majority of luteal phase progesterone in ewes (Niswender et al., 1985), and tend to secrete progesterone at a higher rate than small luteal cells under basal conditions (Fitz et al., 1982; Alila et al., 1988; Nelson et al., 1992). 32 Mechanisms responsible for the LH-independent secretion of progesterone by large luteal cells are not well understood, but possible explanations have been proposed. Lucy et al. (1993) demonstrated that growth hormone receptors (GHr) were present on bovine large luteal cells, suggesting that GH may enhance steroidogenesis in these cells. However, this appears to be species specific, as highly specific expression of GHr was not detected in porcine cells (Yuan and Lucy, 1996). As previously mentioned, large luteal cells contain prostaglandin receptors (Fitz et al., 1982; Feng and Almond, 1996), suggesting a role for prostaglandin regulation of large luteal cells. Richards et al. (1994) demonstrated that prostaglandin E2 (PGE2) stimulates progesterone production from porcine large luteal cells but not small luteal cells. Interestingly, the LH surge stimulates PGF2a receptor mRNA expression in bovine corpora lutea (Tsai et al., 1996), however, mRNA expression was not defined to luteal cell type. Differential regulation of progesterone production by small and large luteal cells may occur to ensure that systemic concentrations of progesterone are more than adequate for establishment and maintenance of pregnancy. Luteotropins The common definition of a "tropic" hormone is defined as "influencing the activity of a specified gland". Thus, in the case of the corpus luteum, the primary "luteotropic" hormone in most species is LH. As previously discussed, LH is important in ovulation and luteinization. However, the role of LH in regulating luteal function has been an active area of research for many years. In what follows an attempt will be made to describe data of studies demonstrating a pituitary origin for luteotropins in various species. 33 In the cow, LH was first suggested to be luteotropic by Simmons and Hansel (1964). In these early studies, the estrous cycle was prematurely shortened by injections of oxytocin on days 2 to 6 after estrus, so the effect of gonadotropin preparations could be observed on luteal weights and progesterone contents. The results suggested that LH or LH-containing pituitary preparations were capable of overcoming the inhibitory effects of oxytocin on the corpus luteum. A subsequent study, demonstrated that pure bovine LH injected into cows on day 16 extended the cycle from 20 days in control animals to 36 days in LH-treated animals, and this effect was lost when LH was heated with 6 M urea to denature this compound (Donaldson and Hansel, 1965). Finally, anti-bovine LH administered on days 2 to 6 of the cycle to remove endogenous plasma LH, reduced luteal weights and progesterone contents in intact heifers (Snook et al., 1969). Thus, without doubt, many studies demonstrated the necessity of LH for maintenance of the corpus luteum in the bovine. In ewes, hypophysectomy on the day of ovulation prevented the formation of the corpus luteum, while hypophysectomy on day 5 resulted in regression of the partially formed corpus luteum (Kaltenbach et al., 1968a). Similarly, hypophysectomy of pregnant ewes on days 20 to 23 of gestation resulted in corpus luteum regression and reabsorption of the embryos and membranes 7 days later. However, treatment of hypophysectomized ewes with a mixture of LH-FSH maintained pregnancy until days 27 to 29 of gestation (end of the study) in these animals (Kaltenbach et al., 1968b). Hypophysectomy on day 5 resulted in fewer small luteal cells than in controls, but no change in the number of large luteal cells was observed (Farin et al., 1990). Apparently, an LH-deprived corpus luteum 34 is incapable of a normal pattern of growth and development, but continues to function for a week in the absence of this gonadotropin. Conversely, intact ewes continuously infused with ovine LH beginning 10 to 12 days after estrus, prolonged the life span of the corpus luteum until infusion was terminated on day 30 after ovulation (Karsch et al., 1971b). Considerably less information is available about the role of LH as a luteotropin in the sow and mare. Spies et al. (1967) treated pregnant gilts on days 25 to 29 of gestation with anti-ovine LH and demonstrated the complete loss of embryos as well as corpus luteum regression due to treatment. However, these researchers were unable to cause luteal regression in non-pregnant gilts after treatment with the same antiserum on days 7 to 11 of the estrous cycle. Furthermore, corpora lutea developed after hypophysectomy (du Mesnil du Buisson and Leglise, 1963) or hypophysial stalk transection (Anderson et al., 1967) at estrus in gilts, but were smaller than those of controls at day 12 postestrus. Interestingly, exogenous estradiol -1713 has been demonstrated to maintain the corpora lutea of cyclic gilts (Gardner et al., 1963; Garbers and First, 1969). Thus, it seems likely that follicular estradiol may act locally or in combination with LH as the luteotropic hormones in the sow. In the mare, the corpus luteum regresses after treatment with antiserum against a gonadotropin fraction of equine pituitary extracts (Pineda et al., 1972), suggesting a role for LH in the maintenance of the corpus luteum for horses. In the rat and mouse it appears that luteal function during the estrous cycle is minimally dependent upon hypophyseal gonadotropins. Hypophysectomy of the rat shortly after ovulation does not alter normal life span of the corpora lutea during the cycle (Smith, 1930). However, the anterior pituitary hormone PRL is luteotropic in 35 hypophysectomized rats when administered within 56 hours after an ovulatory injection of LH (Malven, 1969). Interestingly, this study further demonstrated that PRL was luteolytic if it was not administered within 80 hours after ovulation. Therefore, the effects of PRL appear to depend on the age of the corpora lutea. In the case of the hamster, a "luteotropic complex" consisting of PRL, LH and FSH are required for normal progesterone secretion (Grady and Greenwald, 1968). The rabbit, having no cycle, is an induced-ovulator. Thus, the ovulatory surge of LH occurs as a result of cervical stimulation. Uniquely, in this species it appears that LH functions to cause ovulation only, as 50 lig of LH administered on day 9 of pseudopregnancy caused luteal regression (Stormshak and Casida, 1964). However, the presence of follicular estradiol is essential for maintenance of luteal function (Hilliard, 1973). Removal of follicular estradiol by way of x-irradiation (Keyes and Armstrong, 1968), or luteinization in response to exogenous LH (Kelly and Stormshak, 1969), results in corpus luteum regression. The rabbit corpus luteum is richly endowed with estrogen receptors suggesting that the effect of estrogen is mediated via a genomic pathway (Lee et al., 1971). Finally, pituitary luteotropins in the bitch have been investigated. Luteinizing hormone was demonstrated to be involved with ovulation and progesterone synthesis in this species (Wildt et al., 1979). Concannon (1980) examined the importance of LH as a luteotropin in the dog. In this study, bitches hypophysectomized 10, 35, 40, 45 and 50 days postestrus had declining systemic progesterone concentrations within 3 to 17 days of surgery. Importantly, plasma progesterone concentrations were increased within 3 hours 36 after subsequent treatment with LH. More recently, however, the role of PRL as a luteotropin in the bitch was investigated by Onclin and Verstegen (1997). In this study, cabergoline, a dopamine agonist inhibitory to PRL release was injected into pregnant and non-pregnant bitches 30 days after the LH surge. Mean plasma PRL, and subsequently progesterone concentrations, were decreased for 4 to 5 days following treatment, suggesting the luteotropic importance of PRL in dogs. However, exogenous PRL given twice daily on days 30 or 40 of gestation did not induce progesterone secretion in this species. Thus, the bitch has two luteotropins, LH and PRL, which appear to work in concert to maintain the corpus luteum and stimulate progesterone production. Prolactin As previously mentioned, PRL has been demonstrated to be luteotropic in rats and dogs (Wildt et al., 1979; Concannon, 1980; Niswender et al., 1985; Onclin and Verstegen, 1997). However, very little evidence exists for the role of PRL as a luteotropin in most domestic animals. In fact, plasma PRL concentrations rise slightly at the time of estrus, but no marked changes have been correlated with corpus luteum function in heifers (Swanson and Hafs, 1971). McCracken et al. (1971) infused prolactin into the ovarian artery of luteal phase ewes and were unable to stimulate progesterone secretion. Furthermore, infusion of intact ewes with PRL failed to prolong the life span of the corpus luteum (Karsch et al., 1971a). Interestingly, PRL maintained progesterone secretion by aged corpora lutea in hysterectomized gilts treated from days 110 to 120 postestrus (Felder et al., 1988). A subsequent study by Li et al. (1989) demonstrated that PRL 37 maintains progesterone secretion as well as morphology of aging corpora lutea for at least 10 days after hypophysectomy in hysterectomized gilts. Thus, PRL appears to have a luteotropic role in pigs. Maternal Recognition of Pregnancy In the event of a pregnancy, a luteotropic signal is necessary to inhibit the demise of the corpus luteum, thus ensuring a continuous production of progesterone to maintain the proper uterine environment for the developing embryo. However, unlike during the cycle, the luteotropins of pregnancy are from an embryological source. The phrase "maternal recognition of pregnancy" has been used to describe how the mother responds to the presence of the conceptus within her, and importantly how the conceptus acts to maintain corpus luteum function. There are species differences in the methods utilized for maintaining a functional corpus luteum, therefore a species by species approach is taken to define these differences. The primate and rodent have the most invasive placentas. When the blastocyst hatches from the zona pellucida, it immediately attaches to the uterine wall. Thus, implantation can occur and placental membranes develop connecting the maternal and fetal blood supply. In the primate, the corpus luteum is maintained due to the trophoblast secretion of chorionic gonadotropin (CG), which is very similar to LH and also classified in the same group of glycoprotein hormones. Secretion of CG occurs by days 7 to 9 of pregnancy, at the time of implantation (Hearn et al., 1991), and is detectable in the maternal circulation by days 8 to 11 (Lenton et al., 1982). The human placenta is capable 38 of producing enough progesterone to support the pregnancy after the first month of gestation (Auletta and Flint, 1988). Thus initially, CG appears to be essential to sustain luteal progesterone concentrations (Hodgen and Itskovitz, 1988). The rat and primate both have a hemochorial discoid placenta, however, the luteotropins necessary for pregnancy maintenance of the corpus luteum are different. Gestation length in the rat is 22 days, however, an infertile mating will result in a pseudopregnancy of about 12 to 13 days. Thus, the corpus luteum of pseudopregnancy regresses well before parturition would normally occur in a pregnant rat, suggesting the role of a luteotropin in both early pregnancy and the second half of gestation. Prolactin is the luteotropin of early pregnancy (Morishige and Rothchild, 1974), rescuing the corpus luteum of the estrous cycle from regression. However, these researchers demonstrated that the corpus luteum is dependent on PRL for only the first 7 days of early pregnancy. The second half of pregnancy in rats is dependent on a few placentally derived hormones. Placental luteotropin increased uterine weight on day 12 in pseudopregnant hypophysectomized rats (Linkie and Niswender, 1972), which coincides with the time of regression in the pseudopregnant animal. Rat placental lactogen (rPL), isolated from placental tissue and uterine decidua, displays prolactin-like activity (Kelly et al., 1975; Robertson and Friesen, 1975), and was demonstrated to be detectable in serum on days 8 thru 15 (Kelly et al., 1975; Robertson et al., 1982). The rat placenta also secretes a luteotropin with LH-like activity, rat chorionic gonadotropin (rCG), which is not secreted by the decidua (Jayatilak et al., 1984). Finally, intraluteal estrogen has been demonstrated to be important for elevated serum concentrations of progesterone during the second half 39 of pregnancy (Gibori and Keyes, 1978). However, the rat corpus luteum only produces large quantities of estrogens in the presence of androgens (Gibori and Keyes, 1978), which are elevated during days 12 to 22 of gestation (Gibori et al., 1979). Gibori and Sridaran (1981) hypophysectomized and hysterectomized pregnant rats to determine the source of late pregnancy androgens. These data demonstrated the placenta to be the source of androgens converted by the ovaries and fetuses to estrogens during the second half of pregnancy. Thus, for the rat a series of placental substances replace pituitary PRL of early pregnancy (and pseudopregnancy) at about mid-gestation, providing the necessary signals needed in maternal recognition of pregnancy (Roberts et al., 1996). Corpora lutea of the sow are the only source of progesterone for maintenance of pregnancy, and progesterone replacement in ovariectomized sows is sufficient for pregnancy to continue (Ellicott and Dzuik, 1973). Thus, to prolong the life span of the corpus luteum during pregnancy, a luteotropin is necessary. Estrogen released by the trophoblast as it elongates is probably the signal for maternal recognition of pregnancy in this species. As previously mentioned, estrogen is luteotropic during the estrous cycle of the sow. However, exogenous estrogens have been demonstrated to extend luteal function for several months in non-pregnant sows after only 5 days of treatment (days 11 to 15; Gardner et al., 1963; Frank et al., 1977). Relative to luteal maintenance, treatment with estrogens is similar to the effects of estrogen synthesized by the conceptus as it begins to elongate. In the sow, the conceptus synthesizes and secretes estrogens by day 12 of pregnancy (Gadsby et al., 1980; Heap et al., 1981). Thus, the corpus luteum of 40 early pregnancy appears to be maintained, at least initially, by a conceptus source of estrogens. Maternal recognition of pregnancy in the mare also involves the conceptus. As the conceptus grows, it remains spherical, and the trophoblast does not elongate like in the sow (Roberts et al., 1996). However, the conceptus must come in contact with the entire endometrium to prevent luteal regression (Niswender and Nett, 1994). Thus, during the critical period for recognition of pregnancy (days 12 to 14), the embryo migrates throughout the uterus to establish contact with the endometrium (Ginther, 1993). The embryo traverses the entire uterus about every 2 hours during the recognition period (Leith and Ginther, 1984). Although embryo migration involves the secretion of some factor that inhibits luteal regression, the factor(s) remains unknown (Roberts et al., 1996). Goff et al. (1993) have suggested that 17a-hydroxyprogesterone, the major steroid synthesized by the equine blastocyst, may have a role in pregnancy recognition because it is a prominent metabolite during this critical period. The horse conceptus does secrete chorionic gonadotropin (eCG), which appears in the maternal serum at about day 35 of gestation (Squires and Ginther, 1975). Equine CG induces premature ovulation of developing follicles, producing secondary corpora lutea, which aid in progestational support of the pregnancy. Thus, eCG is luteotropic but obviously not responsible for recognition of pregnancy in the mare. The conceptuses of the ewe and cow are well known to secrete an antiluteolytic trophoblast protein, responsible for pregnancy recognition. Through molecular cloning techniques, bovine trophoblast protein-1 (bTP-1) was found to have 89.7% homology 41 with ovine trophoblast protein-1 (oTP-1), and an amino acid sequence homology of 80.4% (Imakawa et al., 1989). Thus, because bTP-1 and oTP-1 are similar trophoblast interferons, Roberts et al. (1992) recommended the common nomenclature for ruminant trophoblast interferon, as interferon tau (INFT). Thus, for ease of explanation the identification and characterization of INFT for both species will be discussed together. In ewes, early investigations established a window of early pregnancy whereby the embryos presence was necessary to extend the life span of the corpus luteum (Moor and Rowson, 1966a,b). In these studies, removal of the embryo before day 12 resulted in luteal regression. However, embryos removed after day 13 did not initiate immediate luteal regression, which did ultimately occur. Thus, maternal recognition of pregnancy occurs during days 12 to 13 in the ewe. Similarly, the critical period in cows was determined to be days 16 to 17 (Betteridge et al., 1980; Northey and French, 1980). The importance of the embryo was further demonstrated when homogenates of day 14 to 16 ovine conceptuses (Ellinwood et al., 1979a; Martal et al., 1979), or 17 to 18 day-old bovine conceptuses ( Northey and French, 1980), were injected into the uterine lumen of these animals. These studies demonstrated that conceptus homogenates prolonged luteal function when injected into the uterine lumen. Martal et al. (1979) suggested that the substance secreted by the conceptus was a protein, because this component was no longer functional after heating or proteolytic digestion. In 1982, Godkin et al. described the purification of a low molecular weight protein released into culture medium by day 13 to 21 ovine conceptuses. This secretory protein had a molecular weight of about 17,000, and was soon designated ovine trophoblast 42 protein-1 (Godkin et al., 1984a). Ovine trophoblast protein-1 (oTP-1) extended the life span of the corpus luteum when infused into the uteri of non-pregnant ewes (Godkin et al., 1984b; Vallet et al., 1988). Vallet et al. (1988) also demonstrated that when this protein was removed from a pool of conceptus secretory products by immunoadsorption, infusion of the resulting products failed to increase the interestrous interval. Thus, oTP-1 and the discovery of a similar protein in cows (bTP-1; Bartol et al., 1985), were established as antiluteolysins. Unlike CG of primates, these TP's are secreted into the lumen of the uterus and do not enter the maternal circulation nor do they have a direct luteotropic effect (Godkin et al., 1984a). These authors also demonstrated that incubation of oTP-1 with endometrial explants from day 12 non-pregnant ewes, resulted in increased protein release into the incubation medium. Thus, these proteins appear to have a local effect on the uterus (Bazer et al., 1986). The luteolysin PGF2a is secreted by the uterus and enters the circulatory system through the uterine vein. Inskeep et al. (1975) suggested that a factor from the conceptus was capable of overcoming the luteolytic effect of PGF2a when mated ewes did not return to estrus after injection of this luteolysin. Progesterone concentrations in pregnant ewes treated with PGF2a between days 10 and 30 were different depending on the day of treatment (Silvia and Niswender, 1986). Specifically, progesterone concentrations in pregnant ewes on days 13 and 16 initially declined within 24 hours following PGF2a treatment, however, serum progesterone returned to pre-treatment concentrations 36 hours later. Conversely, pregnant ewes treated with PGF2c, on other days (10, 19, 22, 26 and 30) did not return to pre-treatment concentrations, suggesting 43 that the embryo provides a temporary resistance to luteal regression. Interestingly, the concentrations of PGF2a are higher in pregnant ewes than non-pregnant ewes (Peterson et al., 1976). Zarco et al. (1988a) investigated the mode of PGF2a secretion at the time of luteolysis or maintenance of pregnancy in the ewe. In this study, pulse frequencies and serum concentrations of prostaglandin F24 metabolite (PGFM) were measured in pregnant and non-pregnant ewes from days 12 to 17 post-estrus. Basal concentrations were higher in pregnant ewes on days 13 to 17; however, the pulse frequencies of PGF2c, were lower in these ewes. Thus, modification of pulsatile release of PGF2c, appeared to be an important difference that may be linked to luteal maintenance during pregnancy. Fincher et al. (1986) demonstrated that day 12 to 14 uterine infusions of conceptus secretory proteins suppressed the estradiol-induced rise in PGFM concentrations in non-pregnant ewes, and this suppression was similar to that of estradiol-treated pregnant ewes. In any case, the secretion of oTP-1 by the conceptus seems likely to be responsible for the altered pattern of PGF2c, secretion in pregnant ewes (Godkin et al., 1982; Godkin et al., 1984b; Fincher et al., 1986; Vallet et al., 1988). The ovine and bovine conceptus also secretes PGF,c, and PGE2 as well as IFM (LaCroix and Kahn, 1982; Findlay et al., 1983). LaCroix and Kann (1982) determined that PGE2 synthesis from incubated endometrium was twice as great in pregnant than non- pregnant ewes. These data established the conceptus as another source of this eicosanoid, which has been observed to be elevated during the critical period for maternal recognition of pregnancy, and suggested it to be the factor responsible for prolonging the life span of the corpus luteum (Ellinwood et al., 1979b). In support of this possibility, PGE2 has been 44 demonstrated to be antiluteolytic when infused into the uterine lumen of non-pregnant ewes (Pratt et al., 1977; Magness et al., 1981; Reynolds et al., 1981). The mechanisms by which IFNt alters the secretion of PGF2a is still under investigation. Mirando et al. (1990) demonstrated that ovine conceptus proteins blocked oxytocin stimulation of inositol triphosphate (1P3) production, the second-messenger for oxytocin-stimulated secretion of PGF2, in ovine endometrium. These data suggested a role for IFNT in the inhibition of oxytocin receptor formation, revealing a need for further investigation into the oxytocin receptor changes associated with pregnancy. Ovine conceptus secretory proteins injected twice daily from days 11 to 15 post-estrus, reduced endometrial receptor concentrations for estrogen, progesterone and oxytocin in cyclic ewes (Mirando et al., 1993b). Spencer and Bazer (1995) investigated the tissue-specific expression of these steroid hormone receptors in both cyclic and pregnant ewes. Results of this research illustrated a pattern of receptor expression that varied between pregnant and non-pregnant ewes. Specifically, endometrial estrogen receptor (ER) mRNA and progesterone receptor (PR) mRNA in cyclic ewes increased between days 11 to 15 and 13 to 15, respectively. Endometrial PR mRNA similarly increased between days 11 to 17 in pregnant ewes. However, endometrial ER mRNA expression decreased between days 11 to 15 in pregnant ewes. Therefore, endometrial expression of ER mRNA is specifically suppressed during the early stages of pregnancy, while PR mRNA displays a similar pattern in both cyclic and pregnant ewes. Spencer et al. (1995a) demonstrated that IFNt infused into ovariectomized ewes treated with steroids did not stabilize PR mRNA or protein expression in the endometrium. However, IFNt did suppress endometrial ER 45 mRNA expression and oxytocin receptor formation. Because estradiol treatment increased oxytocin receptor formation in ovariectomized control ewes (no IFNt), Spencer et al. (1995b) infused cyclic ewes with IFNt beginning on day 10 post-estrus and subsequently injected estradiol benzoate on day 12. Results of this study demonstrated that ovine IFNt inhibited the estradiol-induced increase in ER mRNA and oxytocin receptor formation, suggesting that IFNt prevents luteolysis in pregnant ewes by down- regulating these receptors. Finally, IFNt has been suggested to suppress ER transcription through a negative-acting transcriptional mechanism which prevents the estradiol-induced increases in endometrial oxytocin receptor expression (Spencer and Bazer, 1996). Thus, exposure to IFNt differentially inhibits endometrial receptor expression, apparently altering the uterine response to endocrine signals, and ultimately resulting in maternal recognition of pregnancy. Luteolysis A cycle is defined as, "an interval of time during which a sequence of a recurring succession of events or phenomena is completed." If an animal does not become pregnant, the reproductive cycle must come to an end so that a nonpregnant animal can return to a potentially fertile state. Because the corpus luteum is the transient endocrine organ that appears and disappears in each cycle, the regulation of this organ dictates the cycle length. Previously, I have discussed the luteotropins that maintain the corpus 46 luteum. Obviously in the absence of an embryo there must be luteolysins, which cause the demise of the corpus luteum, and terminate the cycle. A uterine factor has been demonstrated to be involved in luteolysis in cattle, sheep (Wiltbank and Casida, 1956), pigs (Spies et al., 1960), horses (Stabenfeldt et al., 1974) and pseudopregnant rats (Butcher et al., 1969). In these studies, animals were hysterectomized to determine the effect of the uterus on the life span of the corpora lutea. In all cases, hysterectomy prolonged the life span of the corpus luteum. However, hysterectomy does not extend the life span of corpora lutea in primates (Castracane et al., 1979) and dogs (Hadley, 1975). These data suggest that the uterus does not play an active role in luteolysis of all species. Although hysterectomy suggested that the uterus produced a luteolysin of general systemic nature, many studies have demonstrated that the effects of the uterus on luteal life span are exerted locally. Inskeep and Butcher (1966) demonstrated that unilaterally hysterectomized ewes did not display the same pattern of corpora lutea regression as observed in the completely hysterectomized ewe. Instead, these authors discovered that the presence of one uterine horn was sufficient to cause luteal regression in the adjacent ovary, while luteal regression did not occur in the ovary adjacent to the hysterectomized horn. These effects of hysterectomy were subsequently confirmed in cattle (Ginther et al., 1967). However, no effect of unilateral hysterectomy has been demonstrated in rabbits (Hunter and Casida, 1967) and horses (Ginther and First, 1971). In these latter studies, there was no significant evidence of differences in local action of the uterine horn on corpora lutea maintenance or regression. Results of these investigations suggest that in 47 some species with bipartite or bicornuate uteri a factor produced within the uterine horn must act locally to regulate luteal life span. A mechanism for transport of a uterine derived luteolysin to the ovary has been established for the ewe and cow. Although no portal system exists between the uterus and ovary, blood vessels supplying the anterior portion of the uterine horn were ligated above and beside the ovary in ewes (Inskeep and Butcher, 1966), and cows (Hixon and Hansel, 1974), resulting in maintenance of the corpus luteum. Ligation of the uterine vein draining the intact horn and reconnection to the remaining portion of the uterine vein of the hysterectomized horn resulted in luteal regression of those corpora lutea in the ovary adjacent to the removed horn in ewes (Ginther et al., 1973), and cows (Mapletoft et al., 1976). Similarly, this investigation demonstrated that the ovarian artery adjacent to the uterine intact side could be ligated and reconnected to the ovarian artery on the hysterectomized side to cause luteal regression. Thus, although no direct vascular connections exist between the ovary and uterus, the luteolytic factor appears to pass directly from the uterine vein to the ovarian artery (Ginther, 1974). Anatomically, the ovarian artery of sheep and cattle is convoluted and in close apposition to the uterine vein. Thus, a local counter-current transfer of the uterine luteolysin, (found in high concentrations in the uterine vein) can cross into the ovarian artery (area of low concentration) to affect the corpus luteum (Stabenfeldt, 1992). Interestingly, the rabbit and horse do not have extensive contact between the uterine vein and ovarian artery (Ginther, 1974). This anatomical difference provides an explanation for the previously 48 mentioned exception that in these two species there is no effect of unilateral hysterectomy on corpus luteum function (Hunter and Casida, 1967; Ginther and First, 1971). Prostaglandin F2, Phariss and Wyngarden (1969) were first to demonstrate that the abundant uterine prostaglandin, PGF2a, could be administered in high doses (1 mg kg-1 day') to rats and cause a significant shortening of pseudopregnancy by luteolysis. This breakthrough in research led to subsequent studies, the results of which demonstrated that PGF2a was luteolytic in sheep (McCracken et al., 1970; Thorburn and Nicol, 1971), cattle (Rowson et al., 1972), horses (Allen and Rowson, 1973), pseudopregnant mice (Bartke et al., 1972), hamsters (Harris and Murphy, 1981), rabbits (Scott and Rennie, 1970), and to a limited extent in swine (Gleeson, 1974). In these studies, PGF2a was demonstrated to be luteolytic when infused into the uterine vein, uterine body or ovarian artery, supporting the idea of a counter-current mechanism of transfer. However, luteolysis only occurs after prolonged treatment with high concentrations of PGF2a in primates (Auletta et al., 1984) and dogs (Concannon and Hansel, 1977). The uterus has no luteolytic effect in these species, suggesting that if PGF2a is luteolytic in these animals it must arise elsewhere in the body. Prostaglandin Fla was further confirmed to be the luteolytic factor when the prostaglandin inhibitor, indomethacin, was demonstrated to block spontaneous luteolysis in cattle and sheep (Lewis and Warren, 1977). These data document the importance of synthesis and secretion of PGF2a in luteolysis. Finally, active and passive immunization against PGF2a was demonstrated to prevent luteal regression in sheep (Scaramuzzi and 49 Baird, 1976) and cattle (Fairclough et al., 1981). Together, these studies support a role for PGF2a as the luteolysin for large domestic species and rodents. Mechanisms of action for PGF2a Progesterone concentrations decrease during luteolysis and this decline has been correlated with increasing concentrations of PGF2a (McCracken et al., 1972). Thus, the effect of PGF2a on the luteal cells has been investigated to elucidate the mechanism by which decreased steroidogenesis occurs. As previously mentioned, large luteal cells possess most of the receptors for PGF2a (Fitz et al., 1982). In these cells, PGF2a binds its receptor and activates the signal transduction pathway involving protein kinase C (PKC; Leung et al., 1986), which phosphorylates further proteins including an actin-binding protein called myristoylated alanine-rich C kinase substrate (MARCKS; Orwig and Stormshak, 1994). Protein kinase C can be activated by the phorbol ester, phorbol 12myristate 13-acetate (PMA), which has been demonstrated to inhibit the production of progesterone in ovine large and LH-stimulated small luteal cells (Wiltbank et al., 1989b). McGuire et al. (1992) examined the in vivo effect of PMA by treating ewes with the phorbol ester on day 10 postestrus and found a rapid reduction in serum concentrations of progesterone. Thus, the effects of phorbol esters in vitro and in vivo appear to be similar. Wiltbank et al. (1989a) demonstrated that in vitro treatment with PGF2a increased free calcium concentrations in ovine large but not small luteal cells. The increase in intracellular calcium concentrations was dose- and time-dependent, suggesting calcium is a second messenger mediating the luteolytic action of PGF2a. Prostaglandin Fa, has also 50 been suggested to have a direct cytotoxic effect on luteal cells (Fitz et al., 1984), which appears to be mediated by intracellular calcium concentrations (Sawyer et al., 1990). However, intracellular signaling may effect steroid biosynthesis. Pate and Condon (1989) treated bovine luteal cells with PGF2a and demonstrated a decrease in de novo cholesterol synthesis. Hawkins et al. (1993) treated ewes with PGF2a on day 10 postestrus to determine how steady state levels of mRNA encoding 3P-HSD changed in the corpus luteum. These researchers found that corpora lutea collected 1 or 8 hours after PGF2c, injection had decreased (>80% reduction) expression of 3P-HSD compared with controls. Murdoch et al. (1996) proposed a possible mechanism by which steroidogenic enzymes, ultimately responsible for progesterone synthesis and luteal function, are inactivated by PGF2a. These investigators measured quantities of mRNA for ubiquitin, an early marker protein of cell death that binds other cellular proteins targeting them for degradation, in corpora lutea collected from ewes 2 or 16 hours following treatment with PGF2.. Results of this study demonstrated that prostaglandin Fla induced ubiquitin expression in large luteal cells before the precipitous decline of progesterone characteristic of luteolysis. These data suggest that PGF2c, disrupts progesterone biosynthesis by the ubiquitination of steroidogenic regulatory enzymes. Because LH induces progesterone secretion from small luteal cells, PGF2c, regulation of LH receptor (LHr) and LHr mRNA expression has been investigated. The number of LHr binding sites present in the ovine corpora lutea decrease during luteolysis (Diekman et al., 1978a). In a subsequent study, these authors demonstrated that the reduction in LHr binding sites at luteolysis is due to PGF2e, secretion (Diekman et al., 1978b). Smith et al. (1996) demonstrated that LHr mRNA content in day 51 10 ovine corpora lutea collected after PGF2a-induced luteolysis, decreased by 6 hours after treatment and were approximately 60% of control concentrations within 24 hours. In any case, the cellular and molecular actions of PGF2a ultimately decrease the synthesis and secretion of progesterone that occurs during luteolysis. Prostaglandin Fla may stimulate factors from endothelial cells that effect steroidogenesis. As previously mentioned, angiogenesis occurs at the time of corpora lutea formation. Thus, endothelial cells, the most abundant cell type in the corpora lutea, are present throughout the life span of the corpus luteum, possibly mediating the effects of hormones on the large and small luteal cells. Endothelial cells have been demonstrated to produce a family of peptides called endothelins (Levin, 1996). Girsch et al. (1995) examined the luteolytic effect of PGF2a on bovine large luteal cells cultured alone or with endothelial cells in the presence of forskolin, an activator of adenylyl cyclase. Prostaglandin Fa, inhibited forskolin activity only when incubated in co-cultures, suggesting an endothelial cell involvement in paracrine regulation of steroidogenic function. A subsequent study demonstrated that PGF2a inhibited progesterone production from bovine luteal cells and concomitantly stimulated the production of an endothelial cell factor, endothelin-1 (ET-1; Girsh et al., 1996a). Prostaglandin F2, inhibition of progesterone only occurred in cultures with both luteal and endothelial cells. Girsh et al. (1996b) also examined the regulation of ET-1 in the bovine corpus luteum by PGF2a. Expression of ET-1 mRNA was demonstrated to be highest during luteolysis, which was further confirmed when intact cows were treated with PGF2a on day 10 postestrus. Collectively, these studies suggest a role for ET-1 in PGF2a- induced luteolysis. Although 52 the mechanism of ET-1 action remains unclear, these latter researchers suggested that PGFacinduced ET-1 production causes hypoxic conditions that lead to luteal regression by causing constriction of the arterioles supplying the corpora lutea. Further, it has been hypothesized that binding of ET-1 to luteal cells may mediate PGF2einduced changes in steroidogenesis that decrease progesterone production around luteolysis. Pulsatile Requirement of PGF2c, Interestingly, PGF2a is released from the uteri of ewes (Barcikowski et al., 1974), and cows (Nancarrow et al., 1973) as a series of discrete pulses that first appear immediately prior to the onset of luteal regression (Thorburn et al., 1972). Pulse duration and magnitude are different among these species, but they typically occur at 6 to 8 hour intervals. In sheep, pulses of uterine PGF2a release occur every 6 hours with the duration of the pulse lasting about 1 hour (McCracken, 1984). The number of episodic pulses of PGF2a in a 24 hour period appears to be critical in causing luteolysis. Schramm et al. (1983) demonstrated that four 1 hour pulses of PGF22. infused into the ovarian artery every 6 hours over a period of 19 hours on day 12 resulted in regression of corpora lutea in only one out of four ewes. However, the addition of a fifth pulse within a 25 hour period resulted in luteolysis in four out of four animals. Further, a single 1 hour pulse of PGF2a was ineffective in causing luteal regression, and resulted in a temporary decrease in serum progesterone concentrations. Thus, for physiological regression of the corpus luteum, a relatively short pulse frequency of PGF2c, (every 6 hours) over the period of a day is required. 53 Oxytocin Secretion of PGF2a from the uterus during luteolysis, is stimulated by oxytocin (Roberts and McCracken, 1976; Fairclough et al., 1980, 1984). Pulses of oxytocin or neurophysin VII have been demonstrated to coincide with pulses of PGF2a during luteolysis in sheep (Fairclough et al., 1980; Flint and Sheldrick, 1983), and cattle (Vighio and Liptrap, 1986). Wathes and Swann (1982) discovered the presence of oxytocin in the corpora lutea of ewes. This ovarian source of oxytocin contributes to the serum concentrations of oxytocin during the luteal phase of the estrous cycle (Sheldrick and Flint, 1981; Flint and Sheldrick, 1982). Specifically, Rodgers et al. (1983) concluded that the ovine large luteal cells almost exclusively store oxytocin over small luteal cells, and these cells are capable of oxytocin synthesis in vitro. Further, the use of immunohistochemistry and electron microscopy localized oxytocin-neurophysin peptide to the secretory granules of ovine and bovine large luteal cells (Fields and Fields, 1986), suggesting that this luteal peptide is secreted by exocytosis. Ivell and Richter (1984) sequenced the bovine oxytocin gene products of luteal and hypothalamic origin, and found both sources of the gene produced identical transcripts except for a difference in polyadenylation. Expression of the luteal oxytocin gene has been demonstrated to be highest early in the estrous cycle in cows and ewes (Ivell et al., 1985; Fehr et al., 1987; Jones and Flint, 1988). Northern blot analysis revealed that luteal oxytocin-neurophysin mRNA in the ewe increases during luteinization, reaches maximum concentrations by day 3 and declines to low levels by day 9 of the estrous cycle (Jones and Flint, 1988). Interestingly, this study measured maximal luteal oxytocin concentrations on 54 day 7. Thus in the ewe and cow (Fehr et al., 1987), a lag between peak mRNA and oxytocin concentrations is present, and may be attributable to the time taken for posttranslational processing of the oxytocin-neurophysin prohormone. Although oxytocin was known to induce PGF2c, secretion from the uterus, the source of oxytocin was initially thought to be the posterior pituitary. Thus, with the discovery of an alternative source of oxytocin (ovarian), experiments were conducted to examine the regulation of luteal oxytocin secretion. Flint and Sheldrick (1983) demonstrated through frequent plasma sampling that two-thirds of detected surges of uterine secretion of PGF2a were accompanied by increased serum concentrations of oxytocin. Further, ewes injected with cloprostenol (PGF2a analogue) on day 8 postestrus had decreased serum concentrations of progesterone, followed 24 hours later with a decrease in jugular oxytocin concentrations. Importantly, the corpus luteum was considered to be a significant source of oxytocin when 98% of the available oxytocin was secreted within 45 minutes of cloprostenol treatment. Regulation of oxytocin production may be through estrogen as estrogen response elements (ERE) and estrogen responsiveness have been demonstrated for the 5' flanking region of the human and rat oxytocin gene (Richard and Zingg, 1990; Adan et al., 1991). However, estrogen responsiveness appears to be species specific as the imperfect ERE in the upstream promoter sequence of the bovine oxytocin gene does not respond to estrogen (Adan et al., 1991). Recently, the oxytocin gene has been demonstrated to be expressed in the uterine epithelium of the pregnant rat (Lefebvre et al., 1992). This implies a paracrine or autocrine control of oxytocin on uterine oxytocin receptors. A 55 subsequent study investigated the role of ovarian steroids in the regulation of the oxytocin gene (Lefebvre et al., 1994). This study demonstrated that exogenous estradiol increased uterine oxytocin mRNA after 2 days of exposure in ovariectomized rats. Administration of progesterone alone does not change the mRNA concentration, however, this steroid increased oxytocin mRNA by sevenfold when given concomitantly with estradiol. These data suggest that the rat oxytocin gene is regulated by steroids in vivo, and further that progesterone and estradiol act synergistically to attain maximal expression of this gene. In any case, with multiple sources of oxytocin available, the potential roles of uterine PGF2c, and oxytocin in luteolysis appeared to involve a positive feedback loop. Oxytocin-PGF2c, Feedback Loop As with any positive feedback loop, activation must start at one site or the other. Considering the functional relationship between the uterus and ovary, the question "what comes first the pulse of PGF2a or oxytocin" was asked. Moore et al (1986) demonstrated that concentrations of PGF2,, in utero-ovarian venous plasma temporally increased before concentrations of oxytocin were detectable, suggesting that the initiation of the positive feedback loop occurs in the uterus. However, oxytocin was only suggested to be the signal that stimulates PGF2a secretion. Hooper et al. (1986) illustrated the importance of pituitary oxytocin by collecting carotid artery and jugular venous plasma for analysis of this hormone. In this study, pituitary and luteal releases of oxytocin occurred simultaneously, and were frequently associated with pulses of PGF2a. Further, more oxytocin pulses were detected in the jugular vein than the carotid artery or vena cava of 56 ewes on day 13 postestrus (Walker et al., 1997). Neurohypophyseal oxytocin is regularly released, during the follicular phase of intact ewes and in ovariectomized ewes receiving estrogen replacement, in episodic bursts lasting a few minutes and occurring at a frequency of 3 pulses/hour (Silvia et al., 1991). These observations suggest a role for estrogen in stimulating pituitary oxytocin release. Treatment of ovariectomized ewes with estradiol provoked high frequency pulses of oxytocin release that occurred every 3 hours beginning 24 hours after treatment, which led McCracken et al. (1991) to propose that alteration in pulses of pituitary oxytocin is the signal that stimulates pulsatile secretion of uterine PGF2, initiating the positive feedback loop. Steroid Regulation It is clear that the positive feedback loop is important for maintaining regular estrous cycles in ruminants. However, this utero-ovarian feedback loop is not functional for most of the estrous cycle. In fact, oxytocin stimulated release of uterine PGF2c, occurs during only a short portion of the estrous cycle (Roberts and McCracken, 1976; Roberts et al., 1976; Fairclough et al., 1984; Silvia and Taylor, 1989), and the uterine response to oxytocin develops late in the estrous cycle as well. Thus, it is of no surprise that many studies have investigated the regulation of this mechanism by steroids, peptides, embryos, as well as many other factors. 57 Progesterone Progesterone has been demonstrated to be important for the proper timing of luteolysis (Ginther, 1968; Ottobre et al., 1980). Progesterone administered 8 and 32 hours postestrus shortened the estrous cycle to 11 days in treated ewes (Ottobre et al., 1980) and this response was caused by premature secretion of PGF2,. This is supported by the data of Scaramuzzi et al. (1977) who showed that treatment of ovariectomized ewes with progesterone for 7 to 14 days restored normal serum concentrations of PGF2. from low basal levels. Additionally, ovariectomized ewes treated with progesterone twice daily to mimic the increasing concentrations of luteal phase progesterone, only responded with an oxytocin-induced release of PGF2a after 10 days of treatment (Homanics and Silvia, 1988). Apparently, the uterus has to be under a long period of progestational influence (10 to 14 days) for spontaneous secretion of PGF2. to occur. The effect of progesterone on the uterus may be to regulate the concentrations of oxytocin receptors, which have been demonstrated to be present in the endometrium of ewes during proestrus and estrus (Sheldrick and Flint, 1985). Vallet et al. (1990) increased concentrations of endometrial oxytocin receptors in ovariectomized ewes after progesterone treatment for 10 to 12 days. In ovariectomized cows treated with exogenous hormones to mimic the pattern observed during the estrous cycle, prolonged treatment with progesterone increased endometrial oxytocin receptor concentrations by day 16 (Lamming and Mann, 1995). This rise in uterine oxytocin receptors was similar to that observed during the luteal phase of intact cyclic cows. Further, Raw and Silvia (1991) found that prolonged exposure of ovine endometrial tissue to progesterone in vivo 58 increased oxytocin-induced PKC activity, which stimulates prostaglandin secretions. Thus, the presence of progesterone appears to be necessary to induce luteolysis. Leavitt et al. (1985) infused ovariectomized ewes with progesterone for 5 days during continuous infusion with estradiol -17p to examine changes in endometrial receptor concentrations. In this study, the withdrawal of progesterone after 5 days of treatment increased endometrial concentrations of estrogen, progesterone, and oxytocin receptors compared with controls (continued progesterone infusion). Similarly, oxytocin receptor concentrations increased 2 days after progesterone withdrawal in ovariectomized ewes continuously treated for days with this steroid (Zhang et al., 1992). 9 Thus, progesterone appears to be suppressing endometrial oxytocin receptor activity in ewes. Such an effect of progesterone is consistent with an early proposal by McCracken et al. (1984) that this steroid regulated estrogen and oxytocin receptors. Results of research conducted by McCracken et al. (1984) suggested that progesterone inhibits synthesis of receptors for estradiol, and that synthesis of oxytocin receptors is an estrogen-dependent process. Accordingly, as long as progesterone suppressed estrogen receptor concentrations, estradiol could not induce oxytocin receptor synthesis. In general, the effects of progesterone appear to be either stimulatory or inhibitory, but most likely progesterone acts congruently with other factors to regulate the life span of the corpus luteum. 59 Estrogen Endogenous estradiol also appears to affect the timing of luteolysis. However, the response of various species to exogenous estradiol has varied depending on dose of steroid administered, time of the estrous cycle administered and duration of treatment. Supraphysiological doses of estradiol -17(3 injected into intact ewes beginning on day 4 postestrus and continued for up to 18 days (day 22 postestrus) blocked luteolysis in intact ewes (Piper and Foote, 1968,1970). Corpora lutea in estradiol-treated ewes did not regress by the end of treatment as determined by similar luteal weights between treated and control ewes. However, estradiol was demonstrated to be necessary for luteolysis when ovarian follicles were destroyed by irradiation (Karsch et al., 1970; Zhang et al., 1991). Further, estradiol administered during the midluteal phase of the estrous cycle will induce premature luteal regression in ewes and cows (Wiltbank et al, 1961; Stormshak et al., 1969; Hawk and Bolt, 1970; Warren et al., 1973; Hixon and Flint, 1987), suggesting that estradiol has a paradoxical effect on luteal life span depending on when it is administered during the estrous cycle. The apparent paradoxical affect of estradiol on luteolysis seems likely to depend upon previous exposure of the uterus to progesterone. Ovariectomized ewes treated with progesterone for 14 days, and subsequently infused with estradiol -1713 48 hours later, had increased plasma concentrations of PGF2c, (Scaramuzzi et al., 1977). Estradiol administration for as little as 6 hours increased oxytocin-induced uterine secretion of PGF2. after 10 days of progesterone treatment in ovariectomized ewes, but had no effect on days 2 or 6 of progesterone treatment (McCracken, 1980). However, estradiol 60 injected into seasonally anestrous ewes whose uteri were not exposed to progesterone increased plasma PGF2e, concentrations, which were further increased by exogenous oxytocin (Sharma and Fitzpatrick, 1974). In contrast, chronic exposure of uteri of ovariectomized ewes to physiological concentrations of estradiol (15 days) did not result in any oxytocin-induced release of PGF2a on days 5, 10 or 15 of treatment (Homanics and Silvia, 1988). Nevertheless, this study demonstrated that exogenous estradiol enhanced the ability of progesterone to stimulate uterine responsiveness to oxytocin, which occurs in intact ewes as well (Hixon and Flint, 1987). Systemic concentrations of estradiol also appears to have an effect on uterine responsiveness to oxytocin. Quantitative effects of estradiol and progesterone concentrations on oxytocin-induced PGF2a release were investigated by Beard et al. (1994). In this study, ovariectomized ewes were treated with either low to high or high to low ratio of estradiol and progesterone, succeeded by an injection of oxytocin 12 days after treatment commenced. Results suggested that concentrations of high estradiol and low progesterone stimulated oxytocin-induced PGFM response, while high progesterone and low estradiol concentrations inhibited this response. Mann and Lamming (1995b) demonstrated a reduction in the oxytocin-induced secretion of PGF2c, in steroid-treated ovariectomized cows infused with subphysiological concentrations of estradiol. Thus, these studies suggest that systemic concentrations of estradiol appear to have an effect on the strength of the luteolytic signal. Uterine changes in oxytocin receptor concentrations are influenced by the spatiotemporal presence of estrogen and progesterone. Estradiol treatment alone 61 suppresses oxytocin receptor concentrations by day 7 in ovariectomized ewes (Val let et al., 1990). However, estradiol treatment preceded by 10 days of progesterone treatment increased concentrations of oxytocin receptors in ovariectomized (Vallet et al., 1990) and intact ewes (Hixon and Flint, 1987). Progesterone withdrawal after concomitant treatment of ovariectomized ewes with silastic implants containing estradiol increased concentrations of oxytocin receptors (Zhang et al., 1992). Onapristone, a progesterone receptor antagonist, injected on day 13 following injections of estradiol on days 11 and 12 increased uterine concentrations of oxytocin receptors (Walker et al., 1997). Thus, the ability of estradiol to increase uterine concentrations of oxytocin receptors appears to be dependent on a progesterone primed uterus. Although regulation of the oxytocin receptor by the estrogen receptor is still not clear, evidence exists for the regulation of the oxytocin receptor gene by estradiol. Estradiol has been demonstrated to up-regulate oxytocin receptor mRNA levels in the uterus, anterior pituitary and central nervous system (ventromedial hypothalamus, amygdala, and hippocampus) of the ovariectomized rat (Quinones -Jenab et al., 1997). The rat oxytocin receptor gene and 5' flanking region have been characterized (Rozen et al., 1995). Analysis of the 5' flanking region of the oxytocin receptor gene failed to reveal the presence of a classical palindromic estrogen response element (ERE), but six widely spaced half-palindromes of the ERE were detected. Theoretically, estrogen could mediate activation of the gene through these sites. More recently, Bale and Dorsa (1997) used an alternative technique to sequence the 5' flanking region of the rat oxytocin receptor gene and identified an internal segment of this area that was additional to the originally 62 published sequence. Upon examination, this segment contained the sequence representing a palindromic ERE with a difference in only one base. Importantly, estradiol was demonstrated to induce oxytocin receptor gene expression after binding to the newly characterized ERE. Thus, in the rat, estradiol does directly induce oxytocin receptor expression. Receptor Interaction The significance of uterine receptors for estrogen, progesterone, and oxytocin have been mentioned throughout this section. Development of oxytocin receptors in the luminal epithelium of the uterine endometrium is a critical step in the initiation of luteolysis in the ewe (Wathes and Lamming, 1995). As previously suggested, luteolysis, and thus the timing of oxytocin receptor development is determined by the concentration (Mann and Lamming, 1995a,b) and most importantly the secretory pattern (Beard et al., 1994) of estradiol and progesterone. To further understand the role of these receptors in luteolysis and maternal recognition of pregnancy, a temporal view of receptor development and steroid regulation will be discussed. McCracken et al. (1984) proposed a model to explain the hormone mediated events controlling the secretion of PGF2c, from the uterine endometrium. According to this hypothesis, the uterus eventually becomes refractory to luteal phase concentrations of progesterone probably due to down-regulation of the progesterone receptor. The declining action of progesterone on the uterus eventually enables serum concentrations of estradiol to up-regulate estrogen receptor concentrations. Increased concentrations of 63 estrogen receptor stimulates the expression of the oxytocin receptor gene, with a consequent increase in endometrial oxytocin receptors. At this point, systemic concentrations of oxytocin can interact with the newly synthesized oxytocin receptors to stimulate the release of uterine PGF2., initiating the utero-ovarian feedback mechanism and luteolysis. This hypothesis suggests that interrelationships between different receptor types must exist to coordinate the events surrounding luteolysis or maternal recognition of pregnancy. Wathes and Hamon (1993), demonstrated that oxytocin receptors first develop in the luminal epithelium on day 14 postestrus in the ewe. However, these authors observed that the rise in oxytocin receptor concentrations precedes estrogen and progesterone receptor development in the luminal epithelium. A subsequent study examined the timing and additive effects of estrogen, progesterone and oxytocin in ovariectomized ewes on the expression of uterine receptors for these hormones (Wathes et al., 1996). In this study, ewes were treated with hormones to mimic the natural pattern and concentrations found during the estrous cycle. As expected, progesterone treatment (overlapping low doses of estradiol), immediately decreased estrogen receptor, oxytocin receptor, and its own receptor over a period of time. Estradiol treatment alone increased estrogen and progesterone receptors, but decreased oxytocin receptors and sustained this decrease until day 14. Progesterone-induced inhibition of oxytocin receptors only appeared to be in the luminal epithelium (the required site for oxytocin receptor expression to induce luteolysis) and declined by day 14. However, no progesterone receptors were present in luminal epithelial cells between days 8 and 14. Therefore, the inhibitory effect of progesterone on 64 oxytocin receptor concentrations is unlikely to be the direct effect of this steroid. Further, the rise in estrogen receptors that occurred in progesterone-treated ewes by day 10 preceded the rise in oxytocin receptors by 2 days, suggesting that estrogen may not be essential in the initiation of oxytocin receptor synthesis. In conclusion, it appears at this time that the presence of the oxytocin receptors in the luminal epithelium is necessary for luteolysis. However, the role of estrogen and progesterone in regulating the entire process of luteolysis seems unlikely. It is likely that factors other than these steroid hormones are contributing to the timing of oxytocin receptor development. 65 STATEMENT OF THE PROBLEM Use of commercially available PGF2 analogues to induce luteolysis and synchronize estrus in domestic species is well established. However, PGF2c, is not widely used in some animal production industries because of the variability in time to estrus by individual animals (Zarco et al., 1988b), which precludes maximizing the use of artificial insemination. Thus, the study of factors regulating the estrous cycle, especially those that induce luteolysis, are of significance from the standpoint of improving the technology for animal production. The current dogma with respect to luteolysis in sheep and cattle is based upon the proper initiation of a utero-ovarian feedback mechanism involving secretion of the oxytocin-induced uterine luteolytic factor, PGF2, upon oxytocin binding to its endometrial receptor (McCracken et al., 1972; Roberts and McCracken, 1976; McCracken et al., 1984). Over the last three decades, research has been conducted to examine the various components of the luteolytic mechanism. Some of these studies have focused on measuring changes in ovarian secretion of hormones and uterine receptors for progesterone and estrogen, or measuring changes in concentrations of oxytocin receptors (OTR) during the various stages of the cycle or in hormone-treated ovariectomized ruminants. During pregnancy in sheep and cattle, the embryo interferes with the luteolytic mechanism (Bazer, 1992, Ott et al., 1993; Meyer et al., 1995). Thus, the study of pregnancy maintenance and "maternal recognition of pregnancy" has provided valuable information about the role of the embryo in suppressing luteolysis. In these species the 66 embryo secretes a protein that inhibits luteolysis by regulating uterine concentrations of endometrial estrogen and oxytocin receptors (Mirando et al., 1993b; Spencer et al., 1995c), which are integral components of the luteolytic mechanism. These studies have clearly shown the importance of suppressing estrogen and oxytocin receptors to prevent luteolysis. The need to develop improved contraceptives to control our ever-increasing world population and to have commercially available more effective agents to synchronize estrus in livestock serves as the impetus for the present research to further explore and define the utero-ovarian factors involved in regulating the life span of the corpus luteum. It has long been known that exogenous estradiol can alter the duration of the cycle. However, the role of estrogens in regulating the life span of the corpus luteum is a paradox. Administration of estradiol at midcycle in the ewe causes premature luteal regression (Stormshak et al, 1969; Warren et al., 1972) through an increase in uterine oxytocin receptors (Hixon and Flint, 1987). Conversely, Piper and Foote (1968) demonstrated that chronic treatment of ewes with estradiol at the beginning of the cycle prolonged luteal life span and increased the interestrous interval. Thus, investigating mechanisms by which estrogen treatment regulates the life span of the corpus luteum provides a useful model in which to further study the interrelationships among factors that govern luteolysis. It is anticipated that defining these factors and their interrelationships may lead to more precise and defined methods of regulating the estrous cycle and thus promote development of better products and methods of synchronizing estrus in domestic animals. 67 DOWN-REGULATION OF OXYTOCIN RECEPTORS AND SECRETION OF PROSTAGLANDIN F2, AFTER CHRONIC TREATMENT OF EWES WITH ESTRADIOL-1713 INTRODUCTION Administration of estradio1-17r3 (E2) to ewes during the mid-luteal phase of the estrous cycle causes premature luteal regression (Stormshak et al., 1969; Warren et al., 1973). However, daily injection of E2 initiated early during the cycle prolongs the life span of the corpus luteum (Piper and Foote, 1968). Thus, exogenous E2 appears to have a paradoxical effect on the life span of the corpus luteum (CL); accordingly it can either promote luteal maintenance or regression in the ewe. Prostaglandin F2, (PGF2a) secreted by the uterine endometrium is considered to be the luteolytic hormone in the ewe (McCracken et al., 1972; Roberts et al., 1976). This eicosanoid has been demonstrated to cause the secretion of luteal oxytocin (Wathes and Swann, 1982) at the end of the cycle in ewes (Flint and Sheldrick, 1983), which binds to high affinity binding sites for oxytocin in the endometrium during proestrus and estrus (Sheldrick and Flint, 1985) to stimulate the pulsatile secretion of PGF2a. Consequently, a functional interrelationship exists between the CL and the uterus through a positive feedback mechanism (McCracken et al., 1984). Uterine responsiveness to oxytocin (OT) in the ewe develops prior to luteal regression (Fairclough et al., 1980; Silvia et al., 1992) and as systemic concentrations of progesterone (P4) wane, ovarian secretion of E2 promotes increased concentration of endometrial OT receptors (Sheldrick and Flint, 68 1985). Results of research with ovariectomized ewes injected with E2 and P4, to mimic the hormonal pattern in estrous ewes, indicate that an increase in uterine oxytocin binding sites occurs in response to a treatment regimen of P4 followed by E2 (Vallet et al., 1990; Beard et al., 1994). However, Vallet et al. (1990) also demonstrated that injections of E2 alone or preceding P4 treatment reduced the number of OT receptors (OTR) in the uterus by Day 7 after treatment. The ability of exogenous E2 to either shorten or prolong luteal life span is presumably related to its capacity to alter uterine concentrations of OTR and secretion of PGF2. The present study consisted of two experiments; the objective of the first being to determine if chronic exposure of ewes to E2 initiated early in the estrous cycle would prolong the life of the CL. Because CL were maintained by this chronic treatment regimen, the objectives of the succeeding experiment were to determine whether chronic E2 treatment of ewes during the cycle would alter uterine concentrations of OT and estrogen receptors and, if so, test the ability of the uterus to secrete PGF2a in response to exogenous OT. MATERIAL AND METHODS Animals Mature ewes were checked for behavioral estrus twice daily with a vasectomized ram. After at least one estrous cycle of normal duration (15.5-18.0 days), ewes were 69 assigned randomly to treatments. All experimental procedures and surgeries were performed in accordance with the Institutional Animal Care and Use Committee. Treatments Experiment 1 Ten mature ewes were assigned randomly in equal numbers to one of two groups receiving: 1) corn oil (CO) or 2) 500 pg of E2 (Sigma Chemical Co., St. Louis, MO) dissolved in 2 ml of CO. It is recognized that this daily dosage of E2 exceeds physiological levels but was considerably less than the maximal dosage used by Piper and Foote (1968). Ewes in both groups were injected s.c. daily from Day 4 of the cycle (estrus = Day 0) until detected behavioral estrus or until Day 24 post-estrus, whichever occurred first. All ewes were subjected to midventral laparotomies on Day 7 of the cycle to mark the existing CL with India ink. Animals were anesthetized by i.v. injection of 5% sodium pentothal and anesthesia was maintained by halothane-oxygen inhalation. All surgical procedures were performed under aseptic conditions. Subsequent laparotomies of Eftreated ewes were performed on Day 24 to determine if the marked CL were maintained and whether secondary CL formation occurred as a result of treatment. Experiment 2 Twenty mature ewes were assigned randomly in equal numbers to four groups in a 2x2 factorial arrangement. Treatment consisted of two concentrations of E2 dissolved in 70 2 ml of CO (0 and 500 gg) and two concentrations of OT (0 and 20 IU USP). Injections (s.c.) of estradio1-1713 were initiated on Day 4 of the cycle and continued daily until Day 14. Jugular blood was collected by use of vacutainer tubes on Day 15, approximately 24 h after the last E2 injection, to measure serum concentrations of E2 and P4. On Day 15, ewes received either 1 ml of physiological saline or 1 ml of saline containing OT (20 IU USP) i.v. with jugular blood collected into heparinized vacutainer tubes at -5 and 0 min prior to injection and then 2, 5, 10, 20, 30, 40, 50, and 60 min post-injection. Tubes were placed on ice for transport to the laboratory where blood was immediately centrifuged at 4°C for 10 min at 2450 x g and the plasma separated and stored at -20°C until assayed for 13,14dihydro-15-keto prostaglandin P2a, the PGF2a metabolite (PGFM). Laparotomies were performed to expose the uterus only in those ewes receiving saline injection on Day 15. Approximately 3 g of caruncular and intercaruncular endometrium were collected from the horn adjacent to the ovary bearing the CL. If CL were present in both ovaries, endometrium was collected from both uterine horns. Tissue was immediately frozen in liquid N2 and stored at -80°C until assayed for OTR. Steroid Radioimmunoassays Progesterone Assay Progesterone was extracted from 100 111 of plasma in duplicate with benzene:hexane (1:2) by vortexing for 30 sec. Samples were incubated at -20°C overnight before the organic phase was decanted into new tubes, dried down and used in the assay. 71 A third tube for each sample containing 100 Ill plasma plus 4000 cpm [3H] progesterone (92 Ci/mmol, Dupont NEN, Boston, MA) was extracted to determine the extraction efficiency. Mean extraction efficiency measured for all samples was 95 f 0.6%. Radioimmunoassay was performed using P4 standards (5 pg\ tube to 800 pg\ tube) in ethanol. Ethanol standards (100 111, in triplicate) or extracted samples (100 gl in duplicate) were pipetted into 12 x 75 mm borosilicate glass tubes and evaporated under air. When tubes were dry, 100 ill of phosphate buffered saline with gelatin (GPBS; 0.01 M NaPO4 pH 7.0, 0.14 M NaCI, 1:10000 thimerosal, 0.1% gelatin) were added to the total count and non-specific binding tubes. Standards and sample tubes received 100 IA of GPBS with anti-progesterone-11-BSA (Dr. Gordon Niswender, Colorado State University, Fort Collins, CO) in a 1:2400 dilution. All tubes were vortexed and incubated at room temperature for 30 min before adding 20000 cpm [3H] progesterone in 100 ill GPBS. Tubes were vortexed and incubated overnight at 4°C. The following day, 1 ml of cold GPBS was added to the total count tubes, and 1 ml dextran-coated charcoal [2.5 g/1 washed neutral writ charcoal (Sigma), 0.25 g/lDextran T-70 (Pharmacia, Uppsala, Sweden)] in GPBS was added rapidly to all other tubes. Tubes were vortexed and incubated at 4°C for 15 min, then centrifuged at 2450 x g for 10 min. The supernatant from each tube was decanted into a 20 ml glass scintillation vial containing 6 ml Ecolume scintillation cocktail (ICN Pharmaceutical Inc., Irvine, CA). Vials were counted in a Beckman LS-6000 liquid scintillation counter. Standard curves were plotted and unknown concentrations determined using the RIA AID computer program (Robert Maciel Association, Inc, Arlington, MA). Analysis 72 of 18 replicates of a pooled plasma sample (70 ±2 pg/tube) resulted in an intraassay coefficient of variation of 6.93%. Sensitivity of the assay was 0.05 ng/tube. Estrogen Assay Estradiol was analyzed by use of RIA in a single assay (David Hess, Oregon Regional Primate Research Center) as described by Goodman (1978). Analysis resulted in an intraassay coefficient of variation of 7.4%. Sensitivity of the assay was 0.5 pg/tube. Prostaglandin Metabolite Assay Plasma samples were analyzed for PGFM using a modified procedure described by Guilbault et al. (1984). Prostaglandin free plasma was obtained from two anestrous ewes by administering two 4 ml injections of a cylcooxygenase inhibitor (flunixin meglumine; Banamine, Butler Company, Dublin, OH; 50 mg/ml) i.m. at 16 h intervals. Blood was collected into evacuated 100 ml bottles containing 1430 USP units of heparin- sodium salt 4 h after the second injection. Blood was immediately centrifuged at 4°C for 20 min at 2450 x g and the plasma was separated and stored at -20°C. Standards of PGFM were prepared by serial dilution of a stock solution (1 mg/ml in ethanol, Cayman Chemical Co., Ann Arbor, MI). Final standard concentrations were 0, 2.5, 3.75, 5, 10, 25, 50, 100, and 200 pg/100 IA in duplicate. Each tube contained 200 jAl prostaglandin free plasma and 100 pi of appropriate PGFM standard. Experimental samples (200iil plasma) were added to 100 til buffer. All tubes received 100 p1 of bovine y globulin (5 g/100 ml), were briefly vortexed and incubated for 15 min. A 100 p1 aliquot of 1:25000 73 rabbit antiserum for PGFM (gift from W.W. Thatcher, University of Florida) was added to all tubes except the non-specific binding tubes, which received 100 'al of buffer. Tubes were briefly vortexed and incubated for 30 min. Finally, 100 ill of 18000 dpm 13,14dihydro-15-keto[5,6,8,9,11,12,14(n)-311] prostaglandin Fa, (178 Ci/mmol; Amersham Corp., Arlington Heights, IL) were added to make a final volume of 600 gl/tube. All tubes were briefly vortexed and incubated for 1 h followed by overnight incubation at 4°C. A 750 IA aliquot of cold 40% solution of polyethylene glycol-8000 in water (40% PEG) was added to precipitate the bound PGFM. Tubes were vortexed for 1 min followed by centrifugation at 1620 x g for 30 min at 4°C to separate bound from free hormone. Tubes were then inverted to drain the supernatant for 10 min followed by the addition of 750 i_il of buffer and 750 i_il 40% PEG. All tubes were vortexed for 1 min after each addition followed by a final centrifugation for 30 min. Tubes were again inverted to drain the supernatant and the pellets were resuspended in 1 ml of buffer by vortexing for 2 min. Tube contents were poured into a scintillation vial containing 6 ml Ecolume cocktail (ICN Pharmaceutical Inc.). Vials were vortexed and allowed to stand overnight before counting. The sensitivity of the assay was established at 25 pg/ml, thus all values measured at less than the level of sensitivity were given this value. Six repeated measurements of a single pooled plasma sample (206 ±5 pg/ml) among five assays resulted in intra- and interassay coefficients of variation of 5.3 and 6.2%, respectively. 74 Oxytocin Receptor Analysis Endometrium was analyzed for oxytocin receptors using a modified procedure described by Mirando et al. (1993a). Approximately 1 g of tissue was rapidly minced while frozen with a single-edge razor blade and placed into a 50 ml conical tube containing 20 ml of ice-cold wash buffer A (0.9% NaCI, 1 mM EDTA). The tube was briefly vortexed and centrifuged at 2000 x g for 10 min at 4°C. Supernatant was discarded and the pellet was rinsed twice with 20 ml buffer A. Final resuspension was in 15 ml of homogenization buffer B (25 mM Tris-HC1, 0.25 M sucrose). Tissue was homogenized six times with a Tekmar (Cincinnati, OH) tissumizer at speed 60 (medium-high) for 5 sec and then allowed to cool in an ice bath for 15 sec between each homogenization. The homogenate was transferred to a Duall glass tissue grinder and further homogenized before centrifuging at 2000 x g for 10 min (4°C). The supernatant was transferred to a 25 ml tube and centrifuged at 4°C for 90 min at 80000 x g. The supernatant was discarded and the pellet gently rinsed three times with 5 ml membrane diluting buffer C (25 mM Tris -HCI, 0.01% NaN3) with final resuspension in 3 ml. Prior to storing the pellet suspension at -80°C, an aliquot was analyzed for protein concentration using the Bio-Rad Protein Assay (Bio-Rad Laboratories, Hercules, CA). Specific binding of OT to endometrial membrane preparations (110 gg protein/100 Ill) from each ewe was determined in duplicate samples by adding increasing concentrations of [tyrosy1-2,6-314]-0T (0.125 - 1 pM) alone or in combination with 200 - fold excess of unlabeled OT for a total volume of 200 IA Labeled OT (48.5 Ci/mmol, 75 Dupont NEN) was diluted to appropriate concentrations in buffer D consisting of 25 mM Tris-HC1, 0.01% NaN3, 0.2% BSA and 20 mM MnC12. Sample tubes were vortexed and incubated for 60 min at 25°C. Thereafter, incubation tubes were placed on ice and to each was added 750 IA of cold buffer D and 1 ml of membrane pelleting buffer E (40% polyethylene glycol, 25 mM Tris-HC1, 0.01% NaN3). Tubes were then vortexed and centrifuged at 2000 x g for 15 min (4°C). The supernatant was decanted and the tubes inverted for 10 min (4°C) before resuspension by addition of 1 ml each of buffers D and E, followed by centrifugation as described above. After decanting the supernatant and allowing the inverted tubes to drain for 10 min the pellet was resuspended in 500 µl of buffer C and decanted into scintillation vials containing 5 ml of Cytoscint ES+ (ICN Pharmaceutical Inc., Irvine, CA). Vials were vortexed and allowed to stand overnight at room temperature before counting. Oxytocin specifically bound to membrane receptors was determined by difference between total binding (samples incubated with labeled OT) and nonspecifically bound hormone (samples incubated with labeled OT and a 200-fold excess of unlabeled OT). Dissociation constants (Kd) for each treatment group were then determined by Scatchard analysis using LIGAND (Munson and Rodbard, 1980). Binding of [311]-0T to ovine endometrial tissue preparations was saturable (Fig. 1A). Assay validity was determined by measuring [3H] -OT bound to increasing protein concentrations prepared from endometrial tissue collected on Day 15 post-estrus (Fig. 1B). A regression of fmol OT bound/µg membrane protein assayed (Y = 0.52x - 4.51, R2 =0.99) was linear. Intra- and interassay coefficients of variation, determined from ovine 76 endometrium preparation assayed at 2.5 nM [3H] -OT in each assay, were 2.6 and 4.2%, respectively. Estrogen Receptor Exchange Assay Uterine tissue was analyzed for estrogen receptors (ER) using a modified version of the progesterone receptor exchange assay described by Slayden et al. (1994). Briefly, 400 mg frozen endometrium were homogenized in 10 vol of TEDGM [10 mM Tris -HCI, 1.5 mM EDTA, 1 mM dithiothreitol (DTT), 10% glycerol, 25 mM sodium molybdate] on ice with a Duall glass tissue grinder. The homogenate was centrifuged at 2000 x g for 10 min at 4°C to separate the cytosolic fraction (supernatant) from the nuclear fraction. The nuclear pellet was washed three times by gentle resuspension in 1 ml of TEDGM before recentrifuging as above. After washing, the pellet was resuspended in 4 ml TEDK (10 mM Tris-HC1, 1.5 mM EDTA, 1 mM DTT, 0.8 M KC1) and extracted on ice for 1 h. A 200-p1 aliquot of the extract was frozen for DNA analysis using the procedure described by Schneider (1957). The nuclear and crude cytosolic fractions were then centrifuged at 100000 x g for 1 h. The resulting supernatants were stored on ice until required. Radioligand [6,7-3H(N)] E2 (50 Ci/ mmol, Dupont NEN Boston, MA) was diluted to a concentration of 120 nM (333000 dpm/ 25 pl) in both TEDGM and TEDK. Nuclear and cytosolic fractions (100 pl) were each assayed in duplicate by addition of increasing concentrations of labeled E2 (0.94 - 60 nM) or labeled E2 plus a 200-fold excess of unlabeled E2 for a total volume of 150 pl. Nuclear fractions were incubated at 37°C for 77 30 min and then both nuclear and cytosolic fractions were incubated overnight (18 h) at 4°C. Bound receptors were separated from the free ligand using Sephadex (Pharmacia and Upjohn, Kalamazoo, MI) LH-20 mini columns at 4°C. Briefly, columns were rinsed three times with 300 ill TEDGM or TEDK before the samples were loaded onto the top of the columns. After 30 min the columns were eluted into scintillation vials with two 300- µl aliquots of respective buffer. The eluate was then diluted with 5 ml of Ecolume cocktail, vortexed, and counted. Binding of [3H] -E2 to ovine endometrial tissue preparations was saturable at a concentration of 4 nM [3H] -E2. Specifically bound hormone was determined by subtracting nonspecifically bound steroid from total bound steroid. The Kds were then determined by Scatchard analysis using LIGAND (Munson and Rodbard, 1980). Statistical Analysis Effects of E2 treatment on the duration of the interestrous interval, the serum concentrations of E2 and P4 on Day 15, and the uterine concentrations of OTR and ER protein were all tested for significance by t-test using Statgraphics (STSC, Inc., Rockville, MD). Data on PGFM concentrations were log transformed because of heterogeneity of variance and subjected to repeated measures analysis of covariance using the general linear model procedure of SAS (1993). Covariance analysis was used because of differing baseline serum concentrations of PGFM among groups prior to injection of OT or saline 78 (p<0.05). The covariate consisted of the average of the -5 and 0 h concentration of PGFM for each ewe, which was used to adjust subsequent mean concentrations of PGFM. Data presented in graphic form are of non-log transformed values (Fig. 2). RESULTS Experiment 1 Administration of E2 (500 gg/day) from Days 4 to 24 post-estrus increased the mean interestrous interval compared with that of controls ( mean ± SE; treated, 51.8 ± 1.7 vs. controls, 17.6 ± 0.4 days, p < 0.001). Marked CL were maintained in 4 of 5 treated animals by Day 24 post-estrus with no newly induced CL present. A single ewe detected in estrus prior to the end of treatment (Day 19) was excluded from the statistical analysis for mean interestrous interval. Experiment 2 Administration of E2 from Days 4 to 15 maintained the function of CL in treated ewes as determined from an increased concentration of P4 on Day 15 (treated, 2.20 ± 0.40 vs. controls, 0.97 ± 0.30 ng/ml; p < 0.05). Serum concentrations of E2 in treated ewes on Day 15 of the cycle were increased compared with concentrations in control ewes (14.3 ± 3.5 vs. 6.5 ± 0.8 pg/ ml; p < 0.05). Concentrations of OTR in the endometrium from 79 0 2 4 6 8 10 [31-1]-0XYTOCIN (nM) Figure 1A. Saturation analysis of OTR in ovine endometrium. Duplicate aliquots of membrane protein from ewes (n=5) were incubated with each concentration of [3H] -OT alone (total binding) or with a 200-fold excess of unlabeled OT (nonspecific binding). Specific binding was determined as the difference between total and nonspecific binding. 80 120 100 80 60 40 20 00 I I I I 50 100 150 200 250 MEMBRANE PROTEIN (jig) Figure 1B. Regression of bound OT in endometrial membranes as determined using a single saturating concentration of [3H] -OT (Y = 0.52x - 4.51, R2 = 0.99). 81 TIME (min) Figure 2. Plasma concentrations of PGFM in control (CO) and E2-treated ewes on Day 15 of the cycle after i.v. injection of saline (S) or 20 IU USP OT. Because of different mean basal concentrations of PGFM among groups, each point represents the adjusted mean for the covariate (-5 and 0 time points) for each ewe. Standard errors are given for each mean in the results. 82 E2-treated ewes were lower than in controls (26 ± 11 vs. 252 ± 58 fmol/mg protein, Kd =1.54 nM, p=0.002). Because of an OTR concentration of 988 frnol/mg protein, one control animal was considered an outlier and was not included in the statistical analysis for OTR concentration. Chronic E2 treatment had no effect on total nuclear or cytoplasmic endometrial ER concentration compared to controls (nuclear ER: treated, 0.61 ± 0.23 vs. controls, 0.44 ± 0.16 finol/pg DNA; Kd = 0.55 nM, p = 0.55; cytosolic ER; treated, 2.33 ± 0.48 vs. controls, 4.21 ± 1.09 fmol/pg DNA; Kd = 0.38 nM, p = 0.15). Response of ewes to injection of OT on Day 15 of the cycle, as determined by measurement of plasma concentrations of PGFM, differed markedly between control and E2-treated ewes (estradiol x oxytocin x time interaction, p < 0.01; Fig. 2). Plasma concentrations of PGFM in E2-treated and control ewes injected with saline on Day 15 did not differ. However, injection of OT into control ewes on Day 15 caused a rapid increase in plasma PGFM that attained maximal levels by 10 min after injection and remained elevated for the ensuing 50 min sampling period. In contrast, injection of OT into E2-treated ewes provoked a slight increase in plasma concentration of PGFM that returned to basal level by 20 min. DISCUSSION Results of our research indicate that chronic administration of E2 to ewes beginning on Day 4 of the cycle prolongs the life span of the CL. These data confirm those of Piper and Foote (1968) who first reported that chronic E2 treatment of ewes prolonged luteal life 83 span. Serum concentrations of P4 in E2-treated ewes on Day 15 of the cycle were significantly greater than those of control ewes, and were comparable to those of ewes in the mid-luteal phase of the cycle as previously measured in our laboratory (Slayden and Stormshak, 1990). Corpora lutea in E2-treated ewes that were marked with India ink on Day 7 of the cycle were still present on Day 24 post-estrus. It is not known how long these corpora lutea remained functional but these ewes did not exhibit behavioral estrus until approximately another 27 days had elapsed. The possibility exists that chronic E2 treatment may have stimulated secretion of luteinizing hormone in quantities that were luteotropic in the ewe (Kaltenbach et al., 1968b; Karsch et al., 1971b). However, based upon available data, it is more likely that the effect of E2 on luteal maintenance was due to altered uterine function. McCracken (1984) demonstrated that E2 acts to up-regulate OTR at the end of the normal cycle ( Day 15), and subsequently proposed (McCracken et al., 1984) that luteolysis in the ewe involves a positive feedback loop in which luteal OT binding to uterine OTR stimulates secretion of PGF2c, and vice versa. If one accepts this hypothesis, it is logical to assume that failure of uterine OTR to be upregulated at the appropriate time would result in luteal maintenance. Indeed, maintenance of CL in the pregnant ewe is due to the ability of a trophoblast protein, interferon t, to reduce the concentrations of uterine estrogen and oxytocin receptors (Spencer et al., 1995a). In our study, E2 treatment resulted in a 10-fold reduction in the concentration of uterine OTR on Day 15. These data are similar to those of Vallet et al. (1990) who reported that E2 injections into ovariectomized ewes decreased uterine OTR concentrations when quantified 7 days after 84 initiating treatment. Thus, the response of ewes to chronic treatment with pharmacological levels of E2 during the luteal phase is not comparable with endogenous E2-induced upregulation of OTR dictated by the McCracken model. It is unlikely that chronic E2 stimulated secretion of posterior pituitary OT in sufficient quantities to cause OTR down-regulation. Although i.v. infusion of E2 (2-4 µg/h) into ovariectomized ewes for 12-18 h decreased the interval between pulsatile bursts of OT secretion, the quantity of hormone released from the neurohypophysis was approximately only one-tenth the amount normally released from the CL (McCracken et al., 1995). Rat uterine epithelium is a source of OT (Lefebvre et al., 1992), suggesting that in this species uterine OT may contribute to systemic concentrations of the hormone as well as act locally to down-regulate the concentration of uterine OTR. Administration of E2 to ovariectomized rats resulted in a sevenfold increase in uterine OT mRNA (Lefebvre et al., 1994), and this response was further increased in the presence of P4 suggesting a synergistic effect of these steroids. However, preliminary data from our laboratory fail to provide evidence that chronic treatment of ewes with E2 results in any increase in systemic concentrations of OT of pituitary or uterine origin (Hazzard et al., 1996). The observed reduction in uterine concentration of OTR in E2-treated ewes cannot be attributed to a reduction in uterine estrogen receptors. Uterine concentrations of estrogen receptor in treated ewes were similar to those of control ewes in which luteal regression was underway. Similarly, Spencer et al. (1995b) reported up-regulation of uterine OTR without a change in ER in ovariectomized ewes treated with P4 and E2 85 compared with animals receiving P4 alone. In our study, E2 treatment markedly increased serum concentration of this steroid which apparently was able to stimulate uterine production of ER even though systemic concentrations of P4 were increased. Ability of a comparable dosage of estradiol to induce uterine ER in the face of high systemic concentrations of P4 in the ewe has been reported (Zelinski et al., 1980). Others have also reported that exogenous estrogen, presumably acting via its receptor, can up-regulate ovine endometrial OTR formation when systemic concentrations of P4 are increased and hence result in luteal regression (Hixon and Flint, 1987; Beard and Lamming, 1994). The possibility that E2-induced down-regulation of uterine OTR is mediated indirectly by sustained luteal production of P4 cannot be excluded. In ewes continuously infused with OT to promote down-regulation of uterine OTR and luteal maintenance, a PGF2a-induced reduction in serum concentrations of P4 resulted in uterine OTR up-regulation (Flint and Sheldrick, 1985; Sheldrick, 1992). Estradiol administered to ewes on a daily basis apparently acts directly on the uterus to suppress concentrations of OTR. Estradiol has been demonstrated to induce uterine OTR mRNA in rats (Larcher et al., 1995), but not through binding of ER to the classical estrogen response elements in the 5' flanking region of the OTR gene (Zingg et al., 1995). It is conceivable that the markedly increased concentration of E2 in treated ewes suppresses synthesis of uterine OTR via regulation of other transcriptional factors (Webb et al., 1993). Chronic E2 treatment of immature rats drastically suppresses uterine metabolism and protein synthesis (Stormshak et al., 1976). 86 Although the exact mechanism to explain the reduction in OTR concentration is unclear at this time, exogenous E2 may affect the ability of the uterus to synthesize and secrete PGF2a. In our study, E2 reduced the basal concentration of PGFM compared to that of control ewes, but did not impair OTR function as reflected by the OT-induced secretion of PGF2a on Day 15. However, the magnitude of the response to OT was significantly less in E2-treated than OT-treated control ewes. These data are similar to those of Sharma and Fitzpatrick (1974), who also demonstrated an OT-induced release of PGF2a in E2-treated anestrous ewes. Vallet et al. (1990) demonstrated that by Day 7 after initiating treatment, exogenous E2 had reduced uterine OTR concentrations in ovariectomized ewes. However, these receptors were not functional as determined by lack of OT-induced increases in serum concentrations of PGF2a metabolite (PGFM). In conclusion, the results of this study can be compared to the regulatory events that occur in the pregnant ewe. The conceptus of the pregnant ewe produces interferon ti which has been demonstrated to inhibit endometrial ER and OTR formation (Spencer et al., 1995a), leading to the suppression of the OT-induced release of PGF2c, and hence luteolysis. In cyclic ewes, the present study suggests chronic E2 treatment similarly downregulates the concentration of OTR and reduces the OT-induced release of PGF2a. However, there was no measurable change in the concentration of endometrial ER in the E2-treated ewe. Therefore, chronic treatment of ewes with E2 has the same effect in terms of luteal maintenance as the presence of a conceptus, but appears to act via an entirely different mechanism(s) to down-regulate the OTR. 87 IMPACT OF CHRONIC TREATMENT OF EWES WITH ESTRADIOL-1713 OR PROGESTERONE ON OXYTOCIN RECEPTOR GENE EXPRESSION AND OVARIAN OXYTOCIN SECRETION INTRODUCTION During the past two decades it has become central dogma that luteolysis during the estrous cycle of ruminants occurs as a consequence of a positive feedback system between luteal oxytocin (OT), which stimulates secretion of the uterine luteolysin, prostaglandin Fa, (PGF2a), and vice versa (Flint and Sheldrick, 1982; Flint and Sheldrick, 1983; McCracken et al., 1984). An integral aspect of this feedback system is the coordinated actions of estrogen and progesterone in regulating uterine concentrations of receptor to which OT binds to initiate the pulsatile release of PGF2a. We have previously demonstrated that chronic treatment of ewes with estradiol1713 (E2) beginning on Day 4 of the estrous cycle prevented an increase in endometrial concentrations of oxytocin receptors (OTR) at the expected time of luteolysis, prolonged luteal life span and maintained normal systemic concentrations of progesterone (Hazzard and Stormshak, 1997). However, the acquired data failed to provide a clue as to whether the suppression in uterine concentrations of OTR was due to a direct effect of E2 or indirectly via the sustained production of progesterone. Progesterone suppresses uterine concentrations of estrogen receptor (ER) in E2 treated ovariectomized ewes (Koligian and Stormshak, 1977) and uterine concentrations of OTR in intact ewes (Roberts et al., 1976; 88 Sheldrick and Flint, 1985) and ovariectomized ewes (Lau et al., 1992a; Zhang et al., 1992). Thus, the sustained production of progesterone in ewes subjected to chronic E2 treatment may account for the observed reduction in uterine OTR. Regardless of which steroid in chronic E2-treated ewes causes the change in uterine OTR, it is not known whether the effect is genomic or nongenomic. There is at least some circumspect basis for assuming that E2 may act directly on the genome to alter uterine production of OTR. In rats, an estrogen response element (ERE) has recently been reported to exist in the 5' flanking region of the OTR gene (Bale and Dorsa, 1997). It is conceivable that the promoter region of the ovine OTR gene also contains an ERE, thus providing for E2 regulation of gene expression. Presently, there is no evidence for the existence of a progesterone response element (PRE) present in the promoter region of the OTR gene (Rozen et al., 1995; Bale and Dorsa, 1997). On the other hand, there is some evidence that progesterone may be acting via a nongenomic mechanism to regulate uterine production of OT in the rat (Zingg et al., 1995). Whether the sustained concentrations of progesterone present in the chronic E2-treated ewe alters uterine concentration of progesterone receptor (PR) during the otherwise normal period of luteolysis is of associated relevance in ascertaining if its mode of action is genomic. Ovine large luteal cells that are the source of OT (Rodgers et al., 1983) have been reported to contain estrogen receptors (Glass et al., 1984) and the gene for OT has been shown to contain an ERE upstream of the transcription start site (Richard and Zingg, 1990; Zingg et al., 1995). Because chronic exposure of the ewe to E2 results in a pharmacological state, it is conceivable that this treatment regimen may alter synthesis and 89 (or) storage of luteal OT. Such an effect of this treatment could be ascertained by challenging the chronic E2-treated ewe with PGF2a to induce the secretion of luteal OT (Flint and Sheldricic, 1982). A reduction in luteal OT production may in part contribute to absence of a luteolytic mechanism in the chronic E2-treated ewe. The present study was conducted to: 1) examine the effects of chronic E2 and progesterone treatment on uterine concentrations of OTR and associated changes in OTR mRNA and progesterone receptors during the late luteal phase of the cycle, and 2) ascertain whether chronic E2 treatment of ewes during the cycle would alter luteal OT production. MATERIALS AND METHODS Animals Mature ewes were checked for behavioral estrus twice daily with a vasectomized ram. After at least one estrous cycle of normal duration (15.5-18.0 days), ewes were assigned randomly to treatment groups. All experimental procedures and surgeries were performed in accordance with the NIH guide for the Care and Use of Laboratory Animals at Oregon State University. 90 Treatments Experiment 1 Fifteen mature ewes were assigned randomly in equal numbers to three treatment groups to compare the effects of exogenous E2 and progesterone on uterine concentrations of OTR and OTR mRNA. Daily dosage of E2 administered to ewes in this experiment (and Exp. 3 below) was one-half of that recently used in a similiar series of experiments in our laboratory (Hazzard and Stormshak, 1997) but, nevertheless, was considered to be sufficiently pharmacological to alter uterine function as based upon the observations of Hawk and Bolt (1970). Treatments consisted of a control group and two groups receiving either 250 iig of E2 or 10 mg of progesterone in 2 ml of corn oil (CO) /day. Injections of E2 (s.c.) were initiated on Day 4 of the cycle and continued for a total of 11 days; progesterone-treated ewes received 2 ml CO s.c. on Days 4-10 and progesterone on Days 11-14. Ewes were anesthetized and laparotomized on Day 15 as previously described (Hazzard and Stormshak, 1997). All CL were counted, enucleated, and weights were recorded. An incision was made through the antimesometrial uterine tissue parallel to the long axis of the horn to expose the lumen. Approximately 3 g of caruncular and intercaruncular endometrium from the uterine horn adjacent to the ovary bearing the CL were collected, immediately frozen in liquid N2, and stored at -80°C until assayed for OTR and OTR mRNA. 91 Experiment 2 This experiment was conducted to determine whether progesterone treatment of ewes, as described for Exp. 1 above, altered uterine concentrations of PR. Ten mature ewes were assigned randomly in equal numbers to two treatment groups. Treatments consisted of two concentrations of progesterone (0 or 10 mg) dissolved in 2 ml of CO. Injections (s.c.) were initiated on Day 11 of the cycle and continued for a total of 4 days. All ewes were laparotomized on Day 15, and endometrial tissue was removed from the uterine horn adjacent to the ovary bearing the CL, maintained at 4°C and immediately assayed for PR. Experiment 3 The primary objective of this experiment was to examine the effect of chronic E2 treatment of ewes on luteal secretion of OT in response to a PGF2c, challenge. Twenty mature ewes were assigned randomly in equal numbers to four treatment groups in a 2x2 factorial arrangement. Treatment consisted of two doses of E2 (0 or 250 lig) dissolved in 2 ml of CO and two doses of PGF2a analogue (Estrumate ®, Mobay Corp., Shawnee, KS; 0 or 125 gg). Control and E2-treated ewes were injected s.c. for 11 days as described for Exp. 1. Jugular blood samples collected by use of vacutainer tubes on Day 15 were allowed to clot overnight at 4°C before centrifuging at the same temperature for 10 min at 2,450 x g. Sera were removed and stored at -20°C until assayed for progesterone (n=9 each for control and E2-treated groups). After collection of blood samples for progesterone analysis, all ewes received either 1 ml physiological saline or 1 ml of PGF2. 92 analogue (125 gg) i.v. on Day 15, with jugular blood collected in heparinized vacutainer tubes at 0 min before injection and then 2, 5, 10, 20, and 30 min after injection. Upon collection, 10 I.LI of 5 mg/ml 1,10-phenanthroline (Sigma Chemical Company, St. Louis, MO) in ethanol and 20 gl 0.5M EDTA were added to each tube to suppress oxytocinase activity. All tubes were mixed by inversion and placed on ice for transport to the laboratory where blood was immediately centrifuged as described above. Plasma was removed and stored at -20°C until assayed for OT. Oxytocin Receptor Analysis Endometrium was analyzed for OTR with a procedure described by Hazzard and Stormshak (1997) by methods adapted from Mirando et al. (1993a). Single-point saturation analysis of ovine endometrium preparation assayed at 3.0 nM [3H] -OT resulted in intraassay and interassay coefficients of variation of 4.3 and 14.0%, respectively. Progesterone Receptor Exchange Assay Endometrial PR concentrations were analyzed using a procedure described by Slayden et al. (1994), and modified as follows. Radioligand promegestone, [17a-methyl311]-R5020 (86 Ci/mmol; Dupont NEN, Boston, MA) was diluted to a concentration of 120 nM (573000 dpm/25 gl) in the appropriate buffers. Nuclear and cytosolic fractions were each assayed in duplicate (100 gl/ tube) by addition of 25 gl of labeled R5020 (3-120 93 nM) or labeled R5020 plus 25 gl of 200-fold excess unlabeled R5020 for a total volume of 150 IA To determine affinity of R5020 for glucocorticoid receptors, dexamethasone (10 nM) was added to additional samples, and assayed in duplicate at 8 nM concentration of [31-1]-R5020. There was no indication of binding of [311] -R5020 with the glucocorticoid receptor (p > 0.05, Student's t-test). Specifically bound hormone was determined by substracting nonspecifically bound steroid (3.7 ± 0.7%) from total bound steroid. Binding of [31-1]-R5020 to ovine endometrium was saturable at a concentration of 8 nM of labeled ligand. Steroid Radioimmunoassays Progesterone Assay Progesterone was extracted from 100 gl of serum with benzene:hexane (1:2) and subsequently quantified by use of RIA as described by Koligian and Stormshak (1976). Analysis of a quality-control sample of progesterone (75 pg/tube) resulted in an intraassay coefficient of variation of 7.8%. Sensitivity of the assay was 0.05 ng/tube. Oxytocin Assay Oxytocin was extracted from 1 ml of plasma and assayed as described by Bertrand and Stormshak (1996) using methods adapted from Abdelgadir et al. (1987) and Schams (1983). For plasma extraction, a Waters vacuum manifold was utilized with Sep-Pak Plus C-18 cartridges (Waters Chromatography Division, Millipore Corp., Milford, MA). Mean 94 extraction efficiency was 61 ± 0.5%. Oxytocin antibody was generously provided by Dr. Dieter Schams, Technical University of Munich, Freising-Weihenstephan, Germany. Analysis of a stock plasma sample of OT (3.5 pg/tube) resulted in intraassay and interassay coefficients of variation of 5.9 and 15.6%, respectively. Sensitivity of the assay was 0.125 pg/tube. RNA Extraction and Northern Blotting Endometrial tissues (approximately 300 mg) from each of three control, chronic E2-treated and progesterone-treated ewes and an equivalent quantity of nonlactating ovine mammary gland tissue (negative control) were pulverized with a mortar and pestle over a dry-ice/ethanol bath and then placed in 10 vol (3 ml) TRI REAGENT (Molecular Research Center, Inc., Cincinnati, OH), a single-step total RNA isolation reagent consisting of a mono-phase solution of phenol and guanidine thiocyanate (Chomczynski, 1993). Extraction of total RNA was performed according to the manufacturer's protocol, which included procedures for homogenization of tissue, precipitation of RNA with isopropanol, and centrifugation. Final resuspension of recovered RNA was in 50 ill diethylpyrocarbonate-treated (DEPC) water, which was incubated at 55-60°C for 10 min. Recovered RNA was quantitated by A260 spectrophotometric measurement and assessed for purity by A260/280 ratio. Samples were denatured at 65°C for 10 min with 4 X Sample Buffer (1 part 10 X Northern Buffer [0.2 M HEPES, 10 mM EDTA, pH 7.8], 1.6 parts 37% formaldehyde, 95 and 5 parts deionized formamide) in a ratio of 1:3. Denatured samples (30 pg total RNA) plus 2 pi of 10% Bromophenol Blue were loaded onto a 1.25% formaldehyde agarose gel and electrophoresed in 1 X Northern Buffer for 2 h at 70 V. The gel was then rinsed for 40 min in DEPC-treated water before equilibrating in 10 X SSC buffer (1 X SSC = 0.15 M NaC1, 15 mM sodium citrate, pH 7.0) for 20 min. Transfer of RNA onto MagnaGraph, Nylon membrane (Microns Separations Inc., Westboro, MA) was performed by capillary transfer. After 12-18 h, the membrane was rinsed for 10 min in 6 X SSC, allowed to air dry on filter paper (10-15 min) and the RNA UV-crosslinked to the membrane for 30 sec. The membrane was remoistened in 1 X SSPE (3 M NaC1, 0.2 M NaH2PO4H20, 20 mM EDTA, pH 7.4) before staining for 3 min (0.02% Methylene Blue, 0.3 M sodium acetate, pH 5.5), and destaining for 15 min in 1 X SSPE to visualize the 18S and 28S rRNA bands. Following visualization and destaining, the membrane was baked for 1 h at 80°C in a vacuum oven before removing the stain from the membrane with a strip buffer (0.1 X SSPE/0.1% SDS) for 15 min. Probes for OTR and 18S RNA were made as follows: The template for the OTR probe was a 0.8 kb BamBI/Pst I fragment excised from the human OTR gene plasmid insert (a gift from Dr. T. Kimura, Osaka University Medical School, Osaka, Japan). The probe was made from the cDNA template by random hexanucleotide priming with EasytideTM a-32P-dCTP (Dupont NEN) as the radioactive label (Prime-a-Gene® Labeling System, Promega, Madison, WI). Unincorporated label was removed by passage through a Sephadex® 0-50 column (Quick SpinTM columns, Boehringer Mannheim, Indianapolis, IN). The 18S probe was made from eukaryotic rRNA (1406R; Olsen et al., 1986) and 96 end-labeled with y-32P-dATP (Dupont NEN) using T4 polynucleotide kinase (Promega). Unincorporated label was removed using a Sephadex® G-25 spin column (Boehringer Mannheim). Hybridization buffer (5 X SSPE, 5 X Denhardt's solution [0.1% each BSA, Ficoll and polyvinylpyrrolidone; Sigma], 1% SDS, 100 mg/ml salmon testes DNA for hybridization [Sigma], and 50% deionized formamide) was incubated with the membrane at 42°C for at least 4 h by automated rotation in a hybridization oven (Robbins Scientific, Sunnyvale, CA). Following incubation, the OTR cDNA probe was added to the hybridization solution to obtain approximately 4 x 106 cpm/ml, and incubated overnight at 42°C. Membranes were washed at room temperature for 15 min three times in 1 X SSPE/0.1%SDS, followed by one wash for 15 min at 50°C in strip buffer. Blots were then wrapped in plastic wrap and exposed to a storage phosphor screen (Molecular Dynamics, Sunnyvale, CA) for 4-5 days. Screens were scanned by a Phosphor Imager SI and visualized with Image QuaNT software (Molecular Dynamics). Before using the 18S rRNA probe, blots were incubated at 70°C for 30 min in strip buffer to remove any OTR probe. Thereafter, the blots were prehybridized and then rehybridized overnight at room temperature and visualized as mentioned above. Statistical Analysis Effect of E2 treatment on the uterine concentrations of OTR were tested for significance by ANOVA using Statgraphics (STSC, Inc., Rockville, MD). Because 97 uterine concentrations of OTR in progesterone-treated ewes were below the detection limits of the assay, data on these animals were not included in the statistical analysis. Effects of treatments on luteal weights were tested by analysis of covariance using number of CL as the independent variable among groups. Differences among group means were further tested for significance by using orthogonal contrasts. Data on serum concentrations of progesterone and effects of progesterone treatment on the uterine concentrations of PR were tested for significance by t-test using Statgraphics (STSC, Inc.). Plasma concentrations of OT were subjected to repeated measures analysis using the general linear model procedure of SAS (1993). Data were considered significantly different at (p < 0.05). RESULTS Chronic treatment of ewes with E2 from Days 4-14 of the estrous cycle or daily injection of progesterone during Days 11-14 of the cycle reduced endometrial concentrations of OTR compared with that of controls (E2, 14 ± 11, progesterone < 1 vs. controls, 348 ± 140 frnol/mg protein; p < 0.05). These data are supported by analysis of endometrial OTR mRNA, which demonstrated that both E2 and progesterone treatments may inhibit expression of the OTR gene (Fig. 3). All four differentially polyadenylated variants of mRNA (1.65, 2.5, 3.9 and 6.2 kb) for the ovine OTR gene (Flint et al., 1995) were visible in Day 15 endometrium of control ewes (Fig. 3; lanes 8-10) but none were 98 1 2 3 4 5 6 7 8 9 10 - 0. 6.2 3.9 4. 2.5 1.65 18S Figure 3. Northern Blot of OTR mRNA from Day 15 ovine endometrium. Each lane represents one ewe. Lane 1: ovine nonlactating mammary gland tissue (negative control). Lanes 2-4: animals received 10 mg progesterone s.c. Days 10-14. Lanes 5-7: animals received 250 pig E2 s.c. Days 4-14. Lanes 8-10: animals received 2 ml CO s.c. Days 4-14 (positive controls). The mRNA for OTR is represented by four differentially polyadenylated variants (1.65-6.20 kb). 18S rRNA was probed to determine equality of loading. 99 -- 200 E 7:3) a. E 180 0 0 160 TIME (min) Figure 4. Plasma concentrations of oxytocin in control (CO) and E2-treated ewes on Day 15 of the cycle after i.v. injection of saline (S) or 125 gig PGF2a (Estrumate ®) at time 0. The common estimate of the standard error was 15.18. Each point represents the mean concentration of OT of five animals/group. 100 evident in endometria of steroid-treated ewes. Absence of OTR mRNA in endometria of treated ewes was not due to unequal loading of total RNA because no differences in quantities of 18S rRNA among samples were detected. Additionally, the absence of detectable OTR mRNA in nonlactating mammary gland tissue is consistent with the data reported by Flint et al. (1995) and indicates the specificity of the probe for the OTR mRNA. Treatment of ewes with progesterone from Days 11-14 (Exp. 2) did not alter uterine concentrations of cytoplasmic PR compared with that of control ewes (progesterone-treated, 200 ± 40 vs. controls, 180 ± 40 fmol/ mg DNA; p > 0.05). Unfortunately, uterine concentrations of nuclear PR could not be quantitated because concentrations were below the level of sensitivity of the assay. Chronic treatment of ewes with 250 pg of E2 daily during the cycle or administration of progesterone during the late luteal phase of the cycle resulted in CL on Day 15 that were heavier than those of controls (E2-treated, 394 ± 23, progesterone-treated, 492 ± 67 vs. controls, 307 ± 43 mg; p < 0.05 for each treatment compared with controls). Consistent with these weights of CL, mean serum concentrations of progesterone in chronic E2-treated ewes on Day 15 (Exp. 3) were greater than in controls (1.33 vs. 0.92 ng/ ml), but the difference was not significant statistically. However, the mean of control ewes is based upon eight animals because one ewe in this group had a serum concentration of 4.36 ng/ ml, fivefold greater than the mean serum concentration of progesterone in the other animals. Because of this unusually increased serum concentration of progesterone, this ewe was considered to be an outlier and the datum of this animal was not included in the statistical analysis. 101 Injection of PGF2a analogue on Day 15 of the cycle did not alter induced secretion of OT in chronic E2-treated and control ewes (p > 0.05; Fig. 4). However, compared with saline-injected ewes, injection of PGF2a into both chronic E2-treated and vehicle-injected ewes markedly increased plasma concentrations of OT (p < 0.05). DISCUSSION Results of these research endeavors indicate that chronic treatment of ewes with E2 from early to late in the estrous cycle suppresses endometrial OTR gene expression as reflected by the absence of measurable quantities of OTR mRNA on Day 15. In view of this observation, it is not surprising that analysis of the same endometrial tissue for OTR also revealed a significant reduction in quantities of this receptor in E2-treated ewes. This latter finding is consistent with our recent data demonstrating that chronic E2-treatment of ewes during the cycle caused down-regulation of uterine OTR without affecting uterine concentrations of ER (Hazzard and Stormshak, 1997). The dosage of E2 used in our current study, although only one-half of that used in our previous experiments, was apparently sufficient to provoke similar changes in uterine concentrations of OTR. Similarly, the effectiveness of this dosage of E2 in altering the luteolytic mechanism is supported in part by the greater weight of CL and serum concentrations of progesterone in treated compared with those of control ewes on Day 15. Thus, there is some indication that daily administration of 250 tig of E2, like the dosage of 500 lig of E2 used previously 102 (Hazzard and Stormshak, 1997), did sustain luteal function but no prediction can be advanced as to how long these CL would persist and continue to function once treatment ceased. Ewes treated with 500 lig of E2 from Days 4-24 postestrus exhibited a mean interestrous interval of over 50 days (Hazzard and Stormshak, 1997). Because our previous studies demonstrated that chronic treatment of ewes with E2 during the cycle prolonged luteal function, it was hypothesized that the sustained production of progesterone actually mediated the effect of chronic E2 in down-regulating uterine OTR. This hypothesis was tested in Exp. 1 by administering progesterone to ewes during the late luteal phase of the cycle (Days 11-14). The effect of progesterone on endometrial concentrations of OTR and OTR mRNA was similar to that evoked by chronic E2; i.e., endometria contained no OTR mRNA and corresponding concentrations of OTR were too low to measure with accuracy. On this basis one might conclude that the effect of chronic E2 treatment of ewes on OTR gene expression is indeed mediated by progesterone. The ability of this steroid to suppress OTR gene expression is consistent with the report of Stewart et al. (1993) who found an absence of endometrial OTR mRNA in the ewe during the progesterone-dominated luteal phase of the cycle. The mechanism by which progesterone acts to suppress expression of the OTR gene is not known. Regardless of the specific mechanism by which progesterone acted to inhibit OTR gene expression this steroid must have exerted its effect when bound to its receptor. However, there was no significant difference in the endometrial concentration of cytoplasmic PR between control and progesterone-treated ewes. Cytoplasmic concentrations of PR were lower than those reported to be present during the midluteal phase of the cycle of the ewe 103 (Zelinski et al., 1980) and nuclear concentrations of PR were below the detection limits of the assay. Based upon the data of Wathes et al. (1996) it is likely that the PR measured on Day 15 in progesterone-treated ewes were localized predominantly in the caruncular stroma. On Day 14 of the cycle OTR are found primarily in the uterine lumenal epithelium (Wathes and Hamon, 1993; Wathes et al., 1996). If one accepts this apparent difference in tissue distribution of OTR and PR then it is logical to propose that progesterone acts on stromal cells to stimulate the production of some factor capable of suppressing OTR gene expression in the lumenal epithelial cells. The fact that OTR apparently increase after prolonged treatment of ovariectomized ewes with progesterone (Vallet et al., 1990) may be due to eventual accumulation of E2 resulting from the peripheral conversion of the progesterone to this estrogen (Beard et al., 1994). In view of the above possible mode of action of progesterone, it is conceivable that the suppressive effects of chronic E2 and progesterone on OTR gene expression likely involve independent mechanisms. For example, in intact ewes (Sheldrick and Flint, 1985) and cows (Jenner et al., 1991) endometrial concentrations of OTR are maximal at estrus as systemic concentrations of E2 are increasing and then decline by Days 3-5 of the cycle. The reduction in uterine OTR cannot be attributed to inhibition by progesterone because luteal production of this steroid is minimal at these times. Further, a similar response to E2 has been observed to occur in ovariectomized cows after pretreatment with progesterone to mimic the natural luteal phase of the cycle (Lamming and Mann, 1995) and in ovariectomized ewes given no previous progesterone treatment (Vallet et al., 1990). These data suggest that in the intact ewe and cow, follicular secretion of E2 at estrus 104 causes down-regulation of uterine OTR, perhaps by the same mechanism as chronically administered E2 in the ewe. Flint and Sheldrick (1982) were the first to demonstrate that an exogenous analogue of PGF2a induced the secretion of luteal OT in ewes during the late stages of the estrous cycle. Because ovine large luteal cells are the source of OT and also contain ER (Glass et al., 1984) it was hypothesized that chronic treatment of ewes with E2 may alter luteal production/secretion of this nonapeptide. However, results of the present study indicate that chronic treatment of ewes with E2 did not affect PGF2a- induced secretion of OT on Day 15. These data suggest that although chronic E2 treatment interferes with luteolysis by down-regulating OTR, the PGF2c, inducible component of the positive feedback system in these animals is fully functional. In conclusion, it appears that chronic treatment of ewes with E2 during the entire cycle or treatment of ewes with progesterone only during the late luteal phase of the cycle, suppresses OTR gene expression with a concomitant reduction in endometrial concentration of the receptor. Data from this study suggest that the suppressive effects of each steroid on endometrial OTR are the consequence of each hormone acting through a distinctly different mechanism. Further, at this time, there is no evidence that chronic E2 treatment of ewes during the cycle has any impact on the ability of the CL to secrete OT. 105 GENERAL CONCLUSIONS Research was conducted to establish that daily administration of E2 could extend the interestrous interval in ewes when treatment was initiated on Day 4 postestrus. Chronic E2-treated ewes failed to exhibit behavioral estrus within the normal 17 day estrous cycle characteristic of sheep. While these data support those of Piper and Foote (1968), the mechanism by which luteolysis was inhibited remained to be clarified. In a subsequent study, examination of ovine uterine tissue on Day 15 demonstrated that chronic E2 treatment of ewes down-regulated endometrial OTR concentrations. McCracken et al. (1984) proposed that follicular phase E2 increases production of uterine ER, which are requisite for stimulating OTR gene expression. However, in our study the concentration of endometrial ER were not altered by E2 treatment. Thus, the reduction in OTR observed in the E2-treated ewe could not be attributable to a reduction in endometrial concentrations of ER. Similarly, up-regulation of uterine OTR without a change in ER has been reported in ovariectomized ewes treated with progesterone and E2 as compared with animals receiving progesterone alone (Spencer et al., 1995b). Thus, changes in uterine OTR concentrations in steroid-treated ewes do not always heed to the hypothesis of McCracken (1984). Uterine oxytocin receptors are a necessary component of the luteolytic mechanism, therefore the decreased concentration of these receptors could prevent the necessary secretion of the luteolytic hormone, PGF2a. In actuality, measurable PGF2a secretion (PGFM) in response to exogenous oxytocin was reduced in chronic E2-treated animals. 106 The attenuation of oxytocin-induced secretion of PGFM in E2-treated ewes suggests that receptor function was not impaired by treatment, but was characteristic of decreased concentrations of OTR. Similarly, research in ovariectomized ewes demonstrated that oxytocin-induced secretion of PGFM did not occur in ewes without significant uterine OTR concentrations (Vallet et al., 1990). In contrast, chronic E2 treatment of ewes for 11 days does not appear to alter Day 15 PGF2-induced secretion of oxytocin. Although chronic E2 treatment down-regulates OTR, altering the luteolytic mechanism, the PGF2a- induced component of the luteolytic mechanism remains intact in the face of supraphysiological concentrations of E2. These data suggest that the effect of chronic E2 treatment appears to specifically affect the uterine component of the luteolytic mechanism. Chronic treatment of ewes with E2 maintained the life span of corpora lutea beyond Day 15 postestrus. Serum concentrations of progesterone in the first set of experiments were also maintained at midluteal phase concentrations in treated ewes. These data suggest that the functionality of corpora lutea in E2-treated ewes is extended, and thus elevated concentrations of progesterone on Day 15 may play a role in regulating the luteolytic mechanism. Lau et al. (1992a) showed that progesterone treatment decreased concentrations of OTR in ovariectomized ewes. Further, a subsequent study demonstrated that although progesterone reduced endometrial concentrations of OTR in ovariectomized ewes, upon withdrawl of exogenous progesterone, OTR concentrations increased to pre-treatment levels (Lau et al., 1992b). Therefore, in our study the midluteal phase serum concentrations of progesterone measured on Day 15 suggest that chronic E2 treatment may reduce endometrial concentrations of OTR indirectly through the sustained 107 production of progesterone. Consequently, subsequent experiments were conducted to determine if exogenous progesterone could also induce a reduction in endometrial concentrations of OTR similar to that observed after chronic treatment with E2. Results of our research indicated that progesterone treatment from Days 11 to 14 of the estrous cycle inhibited the increase in endometrial concentrations of OTR associated with luteolysis. Analysis of mRNA for the OTR gene in Day 15 endometria demonstrated that the effect of chronic E2 or progesterone treatment was to suppress expression of the OTR gene. These data support the hypothesis that the effect of chronic E2 treatment in the ewe may be mediated by the sustained luteal phase concentrations of progesterone, and suggests that E2 and progesterone may act genomically to block the increase in endometrial OTR. However, the genomic mechanisms of action of chronic E2 and progesterone appear to be different. Although an estrogen response element (ERE) has been demonstrated in the 5' flanking region of the rat OTR gene, to date no response element for progesterone has been detected in this region (Rozen et al., 1995; Bale and Dorsa, 1997). However, the promoter region of the ovine OTR gene has not been characterized, thus the possibility of E2 and progesterone response elements in this region cannot be excluded. Interestingly, although progesterone has been demonstrated to inhibit OTR formation (Zhang et al., 1992), through endometrial progesterone receptors (PR; McCracken et al., 1984), recent data on localization of endometrial PR and OTR suggests that on Days 14 and 15 of the cycle, the two receptor types are not colocalized within the same endometrial cell. Wathes et al. (1996) demonstrated that OTR are found principally in the luminal epithelium on Day 14 in steroid-treated ovariectomized ewes. Conversely, 108 progesterone receptors are found predominantly in the caruncular stroma, and are not detectable in the luminal epithelium after Day 8 of the cycle. However, progesterone must act through a receptor mediated response. In our study progesterone treatment of ewes did not induce a change in endometrial PR concentrations, suggesting that progesterone was acting through the basal concentrations of PR present in the stroma. These data suggest that in the chronic E2- or progesterone-treated ewe, progesterone may act genomically to stimulate the production of some factor(s) from the endometrial stromal cells that suppress OTR gene expression in luminal epithelial cells with a consequent down-regulation of endometrial concentrations of OTR. It has long been known that exogenous E2 can regulate the estrous cycle. The treatment of ewes with chronic estradiol has provided us with a model to examine the mechanism by which steroids can regulate luteolysis. Future research is needed to sequence the 5' flanking region of the ovine OTR gene. The promoter sequence of this gene would prove invaluable for further contemplation of factors that regulate the OTR, as well as furnish evidence for steroid involvement. Differential tissue expression of OTR and PR could be examined using a nuclear transcription runoff assay to measure specific steroid-induced gene transcription in endometrial stromal and epithelial cells. This procedure would hopefully elucidate possible factors, stimulated by progesterone, that may regulate OTR gene expression in luminal epithelial cells. Isolation of a factor(s) from these cells could then be tested in a promoter construct system, to determine if the element could induce expression of the OTR gene. These experiments would, without doubt, provide insight into the molecular control of luteolysis. 109 BIBLIOGRAPHY Abraham, GE, WD Odell, RS Swerdloff and K Hopper. 1972. Simultaneous radioimmunoassay of plasma FSH, LH, progesterone, 17-hydroxyprogesterone, and estradiol -1713 during the menstrual cycle. J Clin Endocrinol Metab 34:312318. Abdelgadir, SE, LV Swanson, JE Oldfield and F Stormshak.1987. Prostaglandin F2.induced release of oxytocin from bovine corpora lutea in vitro. Biol Reprod 37:550-557. Adams, GP, RL Matteri, JP Kastelic, JCH Ko and OJ Ginther. 1992. Association between surges of follicle-stimulating hormone and the emergence of follicular waves in heifers. J Reprod Fertil 94:177-188. Adan, RAH, N Walther, JJ Cox, R Ivell and JPH Burbach. 1991. Comparison of the estrogen responsiveness of the rat and bovine oxytocin gene promoters. Biochem Biophys Res Comm 175:117-122. Ainsworth, L, RD Baker and DT Armstrong. 1975. Preovulatory changes in follicular fluid prostaglandin F levels in swine. Prostaglandins 9:915-925. Ainsworth, L, BK Tsang, BR Downey, RD Baker, GJ Marcus and DT Armstrong. 1979. Effects of indomethacin on ovulation and luteal function in gilts. Biol Reprod 21:401-411. Alila, HW, RA Corradino and W Hansel. 1988. A comparison of the effects of cyclooxygenase prostanoids on progesterone production by small and large bovine luteal cells. Prostaglandins 36:259-270. Allen, WM and GW Corner. 1930. Physiology of corpus luteum VII. Maintenance of pregnancy in rabbit after very early castration, by corpus luteum extracts. Proc Soc Exp Biol Med 27:403-405. Allen, WR and LEA Rowson. 1973. Control of the mare's oestrous cycle by prostaglandins. J Reprod Fertil 33:539-543. Anderson, LL, GW Dyck, H Mori, DM Henricks and RM Melampy. 1967. Ovarian function in pigs following hypophysial stalk transection or hypophysectomy. Amer J Physiol 212:1188-1194. 110 Armstrong, DT. 1981. Prostaglandins and follicular functions. J Reprod Fertil 62:283-291. Armstrong, DT and DL Grinwich. 1972. Blockade of spontaneous and LH-induced ovulation in rats by indomethacin, an inhibitor of prostaglandin biosynthesis. Prostaglandins 1:21-28. Armstrong, DT and J Zamecnik. 1975. Preovulatory elevation of rat ovarian prostaglandin F and its blockade by indomethacin. Mol Cell Endocrinol 2:125-131. Auletta, FJ and AP Flint. 1988. Mechanisms controlling corpus luteum function in sheep, cows, nonhuman primates, and women especially in relation to the time of luteolysis. Endocrine Rev 9:88-105. Auletta, FJ, DL Kamps, S Pories, J Bisset and M Gibson. 1984. An intra-corpus luteum site for the luteolytic action of prostaglandin Fla in the rhesus monkey. Prostaglandins 27:285-298. Balapure, AK, IC Caicedo, K Kawada, DS Watt, CD Rexroad Jr. and TA Fitz. 1989. Multiple classes of prostaglandin Fa, binding sites in subpopulations of ovine luteal cells. Biol Reprod 41:385-392. Bale, TL and DM Dorsa. 1997. Cloning, novel promoter sequence, and estrogen regulation of a rat oxytocin receptor gene. Endocrinology 138:1151-1158. Bao, B, HA Garverick, GW Smith, MF Smith, BE Salfen and RS Youngquist. 1997a. Changes in messenger ribonucleic acid encoding luteinizing hormone receptor, cytochrome P450-side chain cleavage, and aromatase are associated with recruitment and selection of bovine ovarian follicles. Biol Reprod 56:1158-1168. Bao, B, HA Garverick, GW Smith, MF Smith, BE Salfen and RS Youngquist. 1997b. Expression of messenger ribonucleic acid (mRNA) encoding 3P-hydroxysteroid dehydrogenase A', 6,5 isomerase (313-HSD) during recruitment and selection of bovine ovarian follicles: Identification of dominant follicles by expression of 313HSD mRNA within the granulosa cell layer. Biol Reprod 56:1466-1473. Barcikowski, B, JC Carlson, L Wilson and JA McCracken. 1974. The effect of endogenous and exogenous estradiol-1713 on the release of prostaglandin Fla from the ovine uterus. Endocrinology 95:1340-1349. Bartke, A, AP Merrill and CF Baker. 1972. Effects of prostaglandin F2, on pseudopregnancy and pregnancy in mice. Fertil Steril 23:543-547. 111 Bartol, FF, RM Roberts, FW Bazer, GS Lewis, JD Godkin and WW Thatcher. 1985. Characterization of proteins produced in vitro by periattachment bovine conceptuses. Biol Reprod 32:681-693. Basset, DL. 1943. The changes in the vascular pattern of the ovary of the albino rat during the estrous cycle. Amer J Anat 73:251-292. Bauminger, S and HR Lindner. 1975. Periovulatory changes in ovarian prostaglandin formation and their hormonal control in the rat. Prostaglandins 9:737-751. Bazer, FW. 1992. Mediators of maternal recognition of pregnancy in mammals. Proc Soc Exp Biol Med 199:373-384. Bazer, FW, JL Vallet, RM Roberts, DC Sharp and WW Thatcher. 1986. Role of conceptus secretory products in establishment of pregnancy. J Reprod Fertil 76:841-850. Beard, AP, MG Hunter and GE Lamming. 1994. Quantitative control of oxytocin-induced PGFa, release by progesterone and oestradiol in ewes. J Reprod Fertil 100:143150. Beard, AP and GE Lamming. 1994. Oestradiol concentration and development of the uterine oxytocin receptor and oxytocin-induced PGF2a release in ewes. J Reprod Fertil 100:469-475. Beers, WH and S Strickland. 1978. A cell culture assay for follicle-stimulating hormone. J Biol Chem 253:3877-3881. Berndtson, AK, SE Vincent and JE Fortune. 1995. Low and high concentrations of gonadotropins differentially regulate hormone production by theca interna and granulosa cells from bovine preovulatory follicles. Biol Reprod 52:1334-1342. Bertrand, JE and F Stormshak.1996. In vivo and in vitro responses of the bovine corpus luteum after exposure to exogenous gonadotropin-releasing hormone and prostaglandin Fla. Endocrine 4:165-173. Betteridge, KG, MD Egglesome, GCB Randall and D Mitchell. 1980. Collection, description and transfer of embryos from cattle 10-16 days after oestrus. J Reprod Fertil 59:205-216. Boothby, M, RW Ruddon, C Anderson, D McWilliams and I Boime. 1981. A single gonadotropin a-subunit gene in normal tissue and tumor derived cell lines. J Biol Chem 256:5121-5127. 112 Braden, TD, CJ Belfiore and GD Niswender. 1994. Hormonal control of luteal function. In: Molecular Biology of the Female Reproductive System. JK Findlay, ed. Academic Press, Inc, San Diego. pp 259-287. Brannstrom, M, N Bonello, LJ Wang and RJ Norman. 1995. Effects of tumour necrosis factor alpha (TNFa) on ovulation in the rat ovary. Reprod Fertil Dev 7:67-73. Brannstrom, M, RJ Norman, RF Seamark and SA Robertson. 1994. Rat ovary produces cytokines during ovulation. Biol Reprod 50:88-94. Brown, MS and Goldstein, JL. 1986. A receptor-mediated pathway for cholesterol homeostasis. Science 232:34-47. Butcher, RL, DA Barley and EK Inskeep. 1969. Local relationship between the ovary and uterus of rats and guinea pigs. Endocrinology 84:476-481. Byskov, AG. 1978. Follicular atresia. In: The Vertebrate Ovary. RE Jones, ed. Plenum Press, New York. pp 533-562. Caffrey, JL, PW Fletcher, MA Diekman, PL O'Callaghan and GD Niswender. 1979. The activity of ovine luteal cholesterol esterase during several experimental conditions. Biol Reprod 21:601-608. Carroll, DJ, RR Grummer and FL Mao. 1992. Progesterone production by cultured luteal cells in the presence of bovine low- and high-density lipoproteins purified by heparin affinity chromatography. J Anim Sci 70:2516-2526. Castracane, VD, GT Moore and AA Shaikh. 1979. Ovarian function in hysterectomized Macaca fascicularis. Biol Reprod 20:462-472. Chandrasekher, YA and JE Fortune. 1990. Effects of oxytocin on steroidogenesis by bovine theca and granulosa cells. Endocrinology 127:926-933. Chegini, N, ZM Lei, CV Rao and W Hansel. 1991. Cellular distribution and cycle phase dependency of gonadotropin and eicosanoid binding sites in bovine corpora lutea. Biol Reprod 45:506-513. Chomczynski P. 1993. A reagent for the single-step simulataneous isolation of RNA, DNA and proteins from cell and tissue samples. BioTechniques 15:532-537. 113 Clark, JR, DJ Dierschke and RC Wolf. 1981. Hormonal regulation of ovarian folliculogenesis in rhesus monkeys: III. Atresia of the preovulatory follicle induced by exogenous steroids and subsequent follicular development. Biol Reprod 25:332-341. Clark, BJ, SC Soo, KM Caron, Y Ikeda, KL Parker and DM Stocco. 1995. Hormonal and developmental regulation of the steroidogenic acute regulatory protein. Mol Endocrinol 9:1346-1355. Clark, BJ, J Wells, SR King and DM Stocco. 1994. The purification, cloning, and expression of a novel luteinizing hormone-induced mitochondrial protein in MA-10 mouse Leydig tumor cells. Characterization of the steroidogenic acute regulatory protein (StAR). J Biol Chem 269:28314-28322. Concannon, P. 1980. Effects of hypophysectomy and of LH administration on luteal phase plasma progesterone levels in the beagle bitch. J Reprod Fertil 58:407-410. Concannon, PW and W Hansel. 1977. Prostaglandin F induced luteolysis hypothermia and abortions in beagle bitches. Protaglandins 13:533-542. Corner, GW. 1919. On the origin of the corpus luteum of the sow from both granulosa and theca interns. Amer J Anat 26:117-183. Corner, GW and WM Allen. 1929. Physiology of the corpus luteum. II. Production of a special uterine reaction (progestational proliferation) by extracts of the corpus luteum. Amer J Physiol 88:326-346. Diekman, MA, PO O'Callaghan, TM Nett and GD Niswender. 1978a. Validation of methods and quantification of luteal receptors for LH throughout the estrous cycle and early pregnancy in ewes. Biol Reprod 19:999-1009. Diekman, MA, PO O'Callaghan, TM Nett and GD Niswender. 1978b. Effect of prostaglandin F on the number of LH receptors in ovine corpora lutea. Biol Reprod 19:1010-1013. Donaldson, LE and W Hansel. 1965. Prolongation of the life span of the bovine corpus luteum by single injections of bovine luteinizing hormone. J Dairy Sci 48:903-904. du Mesnil du Buisson, F and PF Leglise. 1963. Effet de l'hypophysectomie sur les corps jaunes de la truie. Resultats preliminaires. C R Acad Sci 257:261-263. Edwards, RG. 1974. Follicular fluid. J Reprod Fertil 37:189-219. 114 Ellicott, AR and PJ Dzuik. 1973. Minimum daily dose of progesterone and plasma concentration for maintenance of pregnancy in ovariectomized gilts. Biol Reprod 9:300-304. Ellinwood, WE, TM Nett and GD Niswender. 1979a. Maintenance of the corpus luteum of early pregnancy in the ewe. I. Luteotropic properties of embryonic homogenates. Biol Reprod 21:281-288. Ellinwood, WE, TM Nett and GD Niswender. 1979b. Maintenance of the corpus luteum of early pregnancy in the ewe. II. Prostaglandin secretion by the endometrium in vitro and in vivo. Biol Reprod 21:845-856. Erickson, GF, DA Magoffin, CA Dyer and C Hofeditz. 1985. The ovarian androgen producing cells: A review of structure/function relationships. Endocrine Rev 6:371-399. Espey, LL. 1994. Current status of the hypothesis that mammalian ovulation is comparable to an inflammatory reaction. Biol Reprod 50:233-238. Espey, LL and H Lipner. 1994. Ovulation. In: The Physiology of Reproduction, Second Edition. E Knobil and JD Neill, eds. Raven Press Ltd, New York. pp 725-780. Espey, LL, C Norris and D Saphire. 1986. Effect of time and dose of indomethacin on follicular prostaglandins and ovulation in the rabbit. Endocrinology 119:746-754. Fairclough, RJ, LG Moore, LT McGowan, AJ Peterson, JF Smith, HR Tervit and WB Watkins. 1980. Temporal relationship between plasma concentrations of 13,14dihydro-15-keto-prostaglandin F and neurophysin I/II around luteolysis in sheep. Prostaglandins 20:199-208. Fairclough, RJ, LG Moore, AJ Peterson and EB Watkins. 1984. Effect of oxytocin on plasma concentrations of 13,14-dihydro-15-keto prostaglandin F and the oxytocinassociated neurophysin during the estrous cycle and early pregnancy in the ewe. Biol Reprod 31:36-43. Fairclough, RJ, JF Smith and LT McGowan. 1981. Prolongation of the oestrous cycle in cows and ewes after passive immunization with PGF antibodies. J Reprod Fertil 62:213-219. Falck, B, K Menander and 0 Nordanstedt. 1962. Androgen secretion by the rat ovary. Nature 193:593-594. 115 Farin, CE, TM Nett and GD Niswender. 1990. Effects of luteinizing hormone on luteal cell populations in hypophysectomized ewes. J Reprod Fertil 88:61-70. Fehr, S, R Ivell, R Koll, D Schams, M Fields and D Richter. 1987. Expression of the oxytocin gene in the large cells of the bovine corpus luteum. FEBS Lett 210:43550. Felder, KT, J Klindt, DJ Bolt and LL Anderson. 1988. Relaxin and progesterone secretion as affected by luteinizing hormone and prolactin after hysterectomy in the pig. Endocrinology 122:1751-1760. Feng, SM and GW Almond. 1996. Identification and distribution of protaglandin E receptors on porcine luteal cells. Biol Reprod 54:1366-1374. Fields, PA. 1984. Ultrastructural localization of relaxin in corpora lutea of pregnant, pseudopregnant and cycling gilts using porcine relaxin antiserum and goat antirabbit IgG-colloidal gold. Biol Reprod 30 (Suppl 1):116A. Fields, MJ, CM Barros, WB Watkins and PA Fields. 1992. Characterization of large luteal cells and their secretory granules during the estrous cycle of the cow. Biol Reprod 46:535-545. Fields, MJ and PA Fields. 1986. Luteal neurophysin in the nonpregnant cow and ewe: Immunocytochemical localization in membrane-bound secretory granules of the large luteal cell. Endocrinology 118:1723-1725. Fields, MJ, PA Fields, A Castro-Hernandez and LH Larkin. 1980. Evidence for relaxin in corpora lutea of late pregnant cows. Endocrinology 107:869-876. Fincher, KB, FW Bazer, PJ Hansen, WW Thatcher and RM Roberts. 1986. Proteins secreted by the sheep conceptus suppress induction of uterine prostaglandin F2, release by oestradiol and oxytocin. J Reprod Fertil 76:425-433. Findlay, 1K, C Noelene, J Swaney and B Doughton. 1983. Prostaglandin F and 13,14dihydro-15-keto prostaglandin F in the endometrium and uterine flushings of sheep before implantation. J Reprod Fertil 68:343-349. Fitz, TA, MH Mayan, HR Sawyer and GD Niswender. 1982. Characterization of two steroidogenic cell types in the ovine corpus luteum. Biol Reprod 27:703-711. Fitz, TA, EJ Mock, MH Mayan and GD Niswender. 1984. Interaction of prostaglandins with subpopulations of ovine luteal cells. II. Inhibitory effects of PGF2c, and protection by PGE2. Prostaglandins 28:127-138. 116 Flint, APF and DT Armstrong. 1973. Activities of enzymes responsible for steroid biosynthesis and cholesterol ester metabolism in rabbit ovarian interstitial tissue and corpora lutea. Biochem J 132:301-311. Flint, APF, PR Riley, S Kaluz, HJ Stewart and DRE Abayasekara. 1995. The sheep endometrial oxytocin receptor. In: Oxytocin. Cellular and Molecular Approaches in Medicine and Research. R Ivell and JA Russell, eds., Plenum Press, New York. pp 281-294. Flint, APF and EL Sheldrick. 1982. Ovarian secretion of oxytocin is stimulated by prostaglandin. Nature, Lond 297:587-588. Flint, APF and EL Sheldrick. 1983. Evidence for a systemic role for ovarian oxytocin in luteal regression in sheep. J Reprod Fertil 67:215-225. Flint, APF and EL Sheldrick. 1985. Continuous infusion of oxytocin prevents induction of uterine oxytocin receptor and blocks luteal regression in cyclic ewes. J Reprod Fertil 75:623-631. Fortune, JE. 1986. Bovine theca and granulosa cells interact to promote androgen production. Biol Reprod 35:292-299. Fortune, JE. 1994. Ovarian follicular growth and development in mammals. Biol Reprod 50:225-232. Fortune, JE and W Hansel. 1979a. The effects of 1713-estradiol on progesterone secretion by bovine theca and granulosa cells. Endocrinology 104:1834-1838. Fortune, JE and W Hansel. 1979b. Effects of the LH surge on steroid secretion by theca and granulosa cells of bovine preovulatory follicles. Biol Reprod 20(Suppl 1):70. Fortune, JE and W Hansel. 1985. Concentrations of steroids and gonadotropins in follicular fluid from normal heifers and heifers primed for superovulation. Biol Reprod 32:1069-1079. Frank, M, FW Bazer, WW Thatcher and CJ Wilcox. 1977. A study of prostaglandin F2a as the luteolysin in swine. III. Effects of estradiol valerate on prostaglandin F, progestin, estrone and estradiol concentration in the utero-ovarian veins of nonpregnant gilts. Prostaglandins 14:1183-1196. Gadsby, JE, RB Heap and RD Burton. 1980. Oestrogen production by blastocyst and early embryonic tissue of various species. J Reprod Fertil 60:409-417. 117 Galway, AB, PS Lapolt, A Tsafriri, CM Dargan, I Boime and AJW Hsueh. 1990. Recombinant follicle-stimulating hormone induces ovulation and tissue plasminogen activator expression in hypophysectomized rats. Endocrinology 127:3023-3028. Garbers, DL and NL First. 1969. The effects of injected oestradio1-1713, progesterone and dietary ICI 33,828 on ovarian and pituitary functions in the sow and gilt. J Reprod Fertil 20:451-464. Gardner, ML, NL First and LE Casida. 1963. Effect of exogenous estrogens on corpus luteum maintenance in gilts. J Anim Sci 22:132-134. Gibori, G, RT Chatterton Jr. and JL Chien. 1979. Ovarian and serum concentrations of androgen throughout pregnancy in the rat. Biol Reprod 21:53-56. Gibori, G and PL Keyes. 1978. Role of intraluteal estrogen in the regulation of luteal progesterone secretion in the rat during the period after day 12 of pregnancy. Endocrinology 102:1176-1182. Gibori, G and R Sridaran. 1981. Sites of androgen and estradiol production in the second half of pregnancy in the rat. Biol Reprod 24:249-256. Ginther, OJ. 1968. Influence of exogenous progesterone and the uterus on ovarian activity in sheep. Endocrinology 83:613-615. Ginther, OJ. 1974. Internal regulation of physiological processes through venoarterial pathways: A review. J Anim Sci 39:550-564. Ginther, OJ. 1993. Mobility of the equine conceptus. Theriogenology 19:603-611. Ginther, OJ, CH Del Campo and CA Rawlings. 1973. Vascular anatomy of the uterus and ovaries and the unilateral luteolytic effect of the uterus: A local venoarterial pathway between uterus and ovaries in sheep. Amer J Vet Res 34:723-728. Ginther, OJ and NL First. 1971. Maintenance of the corpus luteum in hysterectomized mares. Amer J Vet Res 32:1687-1691. Ginther, OJ, L Knopf and JP Kastelic. 1989. Temporal associations among ovarian events in cattle during oestrous cycles with two and three follicular waves. J Reprod Fertil 87:223-230. 118 Ginther, 0J, CO Woody, S Mahajan, K Janakiraman and LE Casida. 1967. Effect of oxytocin administration on the oestrous cycle of unilaterally hysterectomized heifers. J Reprod Fertil 14:225-229. Ghosh, DK, WR Dunham, RH Sands and KMJ Menon. 1987. Regulation of cholesterol side-chain cleavage enzyme activity by gonadotropin in rat corpus luteum. Endocrinology 121:21-27. Girsh, E, Y Greber and R Meidan. 1995. Luteotrophic and luteolytic interactions between bovine small and large luteal-like cells and endothelial cells. Biol Reprod 52:954962. Girsh, E, RA Milvae, W Wang and R Meidan. 1996a. Effect of endothelin-1 on bovine luteal cell function: Role in prostaglandin Fu-induced antisteroidogenic action. Endocrinology 137:1306-1312. Girsh, E, W Wang, R Mamluk, F Arditi, A Friedman, RA Milvae and R Meidan. 1996b. Regulation of endothelin-1 expression in the bovine corpus luteum: Elevation by prostaglandin Fu. Endocrinology 137:5191-5196. Glass, JD, TA Fitz, GD Niswender. 1984. Cytosolic receptor for estradiol in the corpus luteum of the ewe: Variation throughout the estrous cycle and distribution between large and small steroidogenic cell types. Biol Reprod 31:967-974. Gleeson, AR. 1974. Luteal function of the cyclic sow after infusion of prostaglandin Fla through a uterine vein. J Reprod Fella 36:518-522. Godkin, JD, FW Bazer, J Moffatt, F Sessions and RM Roberts. 1982. Purification and properties of a major, low molecular weight protein released by the trophoblast of sheep blastocysts at day 13-21. J Reprod Fertil 65:141-150. Godkin, JD, FW Bazer and RM Roberts. 1984a. Ovine trophoblast protein 1, an early secreted blastocyst protein, binds specifically to uterine endometrium and affects protein synthesis. Endocrinology 114:120-130. Godkin, JD, FW Bazer, WW Thatcher and RM Roberts. 1984b. Proteins released by cultured day 15-16 conceptuses prolong luteal maintenance when introduced into the uterine lumen of cyclic ewes. J Reprod Fertil 71:57-64. Goff, AK, S Leduc, P Poitras and D Vaillancourt. 1993. Steroid synthesis by equine conceptuses between days 7 and 14 and endometrial steroid metabolism. Dom Anim Endocrinol 10:229-236. 119 Go los, TG and JF Strauss III. 1987. Regulation of low density lipoprotein receptor gene expression in cultured human granulosa cells: Roles of human chorionic gonadotropin, 8-bromo-3',5'-cyclic adenosine monophosphate, and protein synthesis. Mol Endocrinol 1:321-326. Gong, JG, BK Campbell, TA Bramley, CG Gutierrez, AR Peters and R Webb. 1996. Suppression in the secretion of follicle-stimulating hormone and luteinizing hormone, and ovarian follicle development in heifers continuously infused with a gonadotropin-releasing hormone agonist. Biol Reprod 55:68-74. Goodman RL. 1978. A quantitative analysis of the physiological role of estradiol and progesterone in the control of tonic and surge secretion of luteinizing hormone in the rat. Endocrinology 102:142-150. Gore-Langton, RE and DT Armstrong. 1994. Follicular steroidogenesis and its control. In: The Physiology of Reproduction, Second Edition. E Knobil and JD Neill, eds. Raven Press Ltd, New York. pp 571-627. Gospodarowicz, D and H Bialecki. 1979. Fibroblast and epidermal growth factors are mitogenic agents for cultured granulosa cells of rodent, porcine and human origin. Endocrinology 104:757-764. Gospodarowicz, D, J Cheng, GM Lui, A Baird, F Esch and P Bohlen. 1985. Corpus luteum angiogenic factor is related to fibroblast growth factor. Endocrinology 117:2283-2391. Gospodarowicz, D, CR Ill and CR Birdwell. 1977. Effects of fibroblast and epidermal growth factors on ovarian cell proliferation in vitro. II. Proliferative response of luteal cells to FGF but not EGF. Endocrinology 100:1121-1128. Grady, KL and GS Greenwald. 1968. Gonadotrophic induction of pseudopregnancy in the cyclic hamster. Endocrinology 83:1173-1180. Guilbault, LA, WW Thatcher, DB Foster and D Caton. 1984. Relationship of 15-keto13,14-dihydro-prostaglandin Fla concentrations in peripheral plasma with local uterine production of F series prostaglandins and changes in uterine blood flow during the early postpartum period of cattle. Biol Reprod 31:870-878. Gwynne, JT and JF Strauss III. 1982. The role of lipoproteins in steroidogenesis and cholesterol metabolism in steroidogenic glands. Endocrine Rev 3:299-329. 120 Hadley, JC. 1975. The effect of serial uterine biopsies and hysterectomy on peripheral blood levels of total unconjugated oestrogen and progesterone in the bitch. J Reprod Fertil 45:389-393. Hadley, ME. 1992. Endocrinology. Prentice-Hall Inc. Englewood Cliffs, NJ. Hafez, ESE. 1993. Reproduction in Farm Animals, 6th Edition. Lea & Febiger, Philadelphia. Hansel, W, PW Concannon and JH Lukawzewska. 1973. Corpora lutea of the large domestic animals. Biol Reprod 8:222-245. Harman, SM, J-P Louvet and GT Ross. 1975. Interaction of estrogen and gonadotrophins on follicular atresia. Endocrinology 96:1145-1152. Harris, KH and BD Murphy. 1981. Luteolysis in the hamster: Abrogation by gonadotropin and prolactin pretreatment. Prostaglandins 21:177-187. Harrison, LM, N Kenny and GD Niswender. 1987. Progesterone production, LHreceptors, and oxytocin secretion by ovine luteal cell types on days 6, 10 and 15 of the oestrous cycle and day 25 of pregnancy. J Reprod Fertil 79:539-548. Hawk, HW and DJ Bolt. 1970. Luteolytic effect of estradiol -17(3 when administered after midcycle in the ewe. Biol Reprod 2:275-278. Hawkins, DE, CJ Belfiore, JP Kile and GD Niswender. 1993. Regulation of messenger ribonucleic acid encoding 313-hydroxysteroid dehydrogenase/g-e4 isomerase in the ovine corpus luteum. Biol Reprod 48:1185-1190. Hazzard, TM, KL Pinckard and F Stormshak. 1996. Changes in ovine uterine oxytocin receptors and luteal oxytocin secretion after progesterone and estradiol treatment. Biol Reprod 54(Suppl. 1):120 (abstract 254). Hazzard, TM and F Stormshak. 1997. Down-regulation of oxytocin receptors and secretion of prostaglandin F chronic treatment of ewes with estradiol -1713. Biol Reprod 6:1576-1581. Heap, RB, APF Flint, PE Hartman, JE Gadsby, LD Staples, N Ackland and M Hamon. 1981. Oestrogen production in early pregnancy. J Endocrinol 89:77-94. Hearn, JP, GE Webley and AA Gidley-Baird. 1991. Chorionic gonadotrophin and embryomaternal recognition during the peri-implantation period in primates. J Reprod Fertil 92:497-509. 121 Hilliard, J. 1973. Corpus luteum function in guinea pigs, hamsters, rats, mice and rabbits. Biol Reprod 8:203-221. Hirshfield, AN. 1991. Development of follicles in the mammalian ovary. Internat Rev Cytol 124:43-101. Hixon, JE and APF Flint. 1987. Effects of a luteolytic dose of oestradiol benzoate on uterine oxytocin receptor concentrations, phophoinositide turnover and prostaglandin F2, secretion in sheep. J Reprod Fertil 79:457-467. Hixon, JE and W Hansel. 1974. Evidence for preferential transfer of prostaglandin F2, to the ovarian artery following intrauterine administration in cattle. Biol Reprod 11:543-552. Hodgen, GD and J Itskovitz. 1988. Recognition and maintenance of pregnancy. In: The Physiology of Reproduction. E Knobil and JD Neill, eds. Raven Press, New York. pp 1995-2021. Holtorf, AP, K Furuya, R Ivell and CA McArdle. 1989. Oxytocin production and oxytocin messenger ribonucleic acid levels in bovine granulosa cells are regulated by insulin and insulin-like growth factor-I: Dependence on developmental status of the ovarian follicle. Endocrinology 125:2612-2620. Homanics, GE and WJ Silvia. 1988. Effects of progesterone and estradio1-17P on uterine secretion of prostaglandin F2, in response to oxytocin in ovariectomized ewes. Biol Reprod 38:804-811. Hooper, SB, WB Watkins and GD Thorburn. 1986. Oxytocin, oxytocin-associated neurophysin, and prostaglandin F2, concentrations in the utero-ovarian vein of pregant and nonpregnant sheep. Endocrinology 119:2590-2597. Hoyer, PB, TA Fitz and GD Niswender. 1984. Hormone independent activation of adenylate cyclase in large steroidogenic ovine luteal cells does not result in increased progesterone secretion Endocrinology 114:604-608. . Hunter, GL and LE Casida. 1967. Absence of local effects of the rabbit uterus on weight of corpus luteum. J Reprod Fertil 13:179-181. Imakawa, K, TR Hansen, PV Malathy, RV Anthony, HG Polites, KR Marotti and RM Roberts. 1989. Molecular cloning and characterization of complementary deoxyribonucleic acids corresponding to bovine trophoblast protein-1: A comparison with ovine trophoblast protein-1 and bovine interferon-as. Mol Endocrinol 3:127-139. 122 Inskeep, EK and RL Butcher. 1966. Local component of utero-ovarian relationships in the ewe. J Anim Sci 25:1164-1168. Inskeep, EK, WJ Smutny, RL Butcher and JE Pexton. 1975. Effects of intrafollicular injections of prostaglandins in non-pregnant and pregnant ewes. J Anim Sci 41:1098-1104. Ireland, JJ and JF Roche. 1983. Growth and differentiation of large antral follicles after spontaneous luteolysis in heifers: Changes in concentration of hormones in follicular fluid and specific binding of gonadotropins to follicles. J Anim Sci 57:157-167. Ivell, R, KH Brackett, MJ Fields and D Richter. 1985. Ovulation triggers oxytocin gene expression in the bovine ovary. FEBS Lett 190:263-267. Ivell, R and D Richter. 1984. The gene for the hypothalamic peptide hormone oxytocin is highly expressed in the bovine corpus luteum: Biosynthesis, structure and sequence analysis. EMBO J 3:2351-2354 Jayatilak, PF, LA Glasser, ML Warshaw, Z Herz, JR Gruber and G Gibori. 1984. Relationship between luteinizing hormone and decidual luteotropin in the maintenance of luteal steroidogenesis. Biol Reprod 31:556-564. Jenner, LJ, TJ Parkinson and GE Lamming. 1991. Uterine oxytocin receptors in cyclic and pregnant cows. J Reprod Fertil 1:49-58. Jones, DSC and APF Flint. 1988. Concentrations of oxytocin-neurophysin prohormone mRNA in corpora lutea of sheep during the oestrous cycle and in early pregnancy. J Endocrinol 117:409-414. Juengel, JL, BM Meberg, AM Turzillo, TM Nett and GD Niswender. 1995. Hormonal regulation of messenger ribonucleic acid encoding steroidogenic acute regulatory protein in ovine corpora lutea. Endocrinology 136:5423-5429. Kaltenbach, CC, JW Graber, GD Niswender and AV Nalbandov. 1968a. Effect of hypophysectomy on the formation and maintenance of corpora lutea in the ewe. Endocrinology 82:753-759. Kaltenbach, CC, JW Graber, GD Niswender and AV Nalbandov. 1968b. Luteotrophic properties of some pituitary hormones in nonpregnant or pregnant hypophysectomized ewes. Endocrinology 82:818-824. 123 Karsch, FJ, B Cook, AR Ellicott, DL Foster, GL Jackson and AV Nalbandov. 1971a. Failure of infused prolactin to prolong the life span of the corpus luteum in the ewe. Endocrinology 89:272-275. Karsch, FJ, JW Noveroske, JF Roche, HW Norton and AV Nalbandov. 1970. Maintenance of ovine corpora lutea in the absence of ovarian follicles. Endocrinology 87:1228-1236. Karsch, FJ, JF Roche, JW Noveroske, DL Foster, HW Norton and AV Nalbandov. 197 lb. Prolonged maintenance of the corpus luteum of the ewe by continuous infusion of luteinizing hormone. Biol Reprod 4:129-136. Kelly, PA, RPC Shiu, MC Robertson and GH Friesen. 1975. Characterization of rat chorionic mammotropin. Endocrinology 96:1187-1195. Kelly, HE and F Stormshak. 1969. Effect of luteinizing hormone on progestin levels in rabbit corpora lutea. J Reprod Fertil 20:171-174. Keyes, PL and DT Armstrong. 1968. Endocrine role of follicles in regulation of corpus luteum function in the rabbit. Endocrinology 83:509-515. Kiriakidou, M, JM McAllister, T Sugawara and JF Strauss III. 1996. Expression of steroidogenic acute regulatory protein (StAR) in the human ovary. I Clin Endocrinol Metab 81:4122-4128. Koligian, KB and F Stormshak. 1976. Progesterone synthesis by ovine fetal cotyledons in vitro. J Anim Sci 42:439-443. Koligian, KB and F Stormshak. 1977. Progesterone inhibition of estrogen receptor replenishment in ovine endometrium. Biol Reprod 17:412-416. Koos, RD. 1995. Increased expression of vascular endothelial growth/permeability factor in the rat ovary following an ovulatory gonadotropin stimulus: Potential roles in follicle rupture. Biol Reprod 52:1426-1435. Koos, RD and W Hansel. 1981. The large and small cells of the bovine corpus luteum: Ultrastructural and functional differences. In: Dynamics of Ovarian Function. NB Schwartz and M Hunzicker-Dunn, eds. Raven Press Ltd, New York. pp 197-203. Labhsetwar, AP. 1971. Luteolysis and ovulation induced by prostaglandin F2, in the hamster. Nature 230:528-529. 124 LaCroix, MC and G Kann. 1982. Comparative studies of prostaglandins F2 and E2 in late cyclic and early pregnant sheep: In vitro synthesis by endometrium and conceptus effects of in vivo indomethacin treatment on establishment of pregnancy. Prostaglandins 23:507-526. Lamming, GE and GE Mann. 1995. Control of endometrial oxytocin receptors and prostaglandin F2 production in cows by progesterone and oestradiol. J Reprod Fertil 103:69-73. LaPolt, PS, M Oikawa, X-C Jai, C Dargan and MW Hseuh. 1990. Gonadotropin-induced up- and down-regulation of rat ovarian LH receptor message levels during follicular growth, ovulation and luteinization. Endocrinology 126:3277-3279. Larcher, A, J Neculcea, C Breton, A Arslan, F Rozen, C Russo and HH Zingg. 1995. Oxytocin receptor gene expression in the rat uterus during pregnancy and the estrous cycle and in response to gonadal steroid treatment. Endocrinology 136:5350-5355. Lau, TM, CB Gow and RJ Fairclough. 1992a. Differential effects of progesterone treatment on the oxytocin-induced prostaglandin F2 response and the levels of endometrial oxytocin receptors in ovariectomized ewes. Biol Reprod 46:17-22. Lau, TM, DJ Kerton, CB Gow and RJ Fairclough. 1992b. Increase in concentration of uterine oxytocin receptors and decrease in response to 13,14-dihydro-15-keto prostaglandin F2 in ewes after withdrawal of exogenous progesterone. J Reprod Fertil 95:885-893. Lauber, ME, MR Waterman and ER Simpson. 1991. Expression of genes encoding steroidogenic enzymes in the bovine corpus luteum. J Reprod Fertil Suppl. 43:5764. Leavitt, WW, WC Okulicz, JA McCracken, W Schranun and WF Robidoux Jr. 1985. Rapid recovery of nuclear estrogen receptor and oxytocin receptor in the ovine uterus following progesterone withdrawal. J Steroid Biochem 22:687-691. Lee, C, PL Keyes and HI Jacobson. 1971. Estrogen receptor in rabbit corpus luteum. Science 173:1032-1033. Lefebvre, DL,R Farookhi, A Larcher, J Neculcea and HH Zingg. 1994. Uterine oxytocin gene expression. I. Induction during pseudopregnancy and the estrous cycle. Endocrinology 134:2556-2561. 125 Lefebvre, DL, A Giaid, H Bennett, R Lariviere and BR Zingg. 1992. Oxytocin gene expression in rat uterus. Science 256:1553-1555. Leith, GS and OJ Ginther. 1984. Characterization of intrauterine mobility of the early equine conceptus. Theriogenology 22:401-408. Lenton, EA, LM Neal and R Sulaiman. 1982. Plasma concentrations of human chorionic gonadotropin from the time of implantation until the second week of pregnancy. Fertil Steril 37:773-778. Leung, DW, G Cachianes, W Kuang, DV Goeddel and N Ferrara. 1989. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 246:13061309. Leung, DW, T Minegishi, F Ma, F Zhou and B Ho-Yeun. 1986. Induction of polyphosphoinositide breadown in rat corpus luteum by protaglandin F2, Endocrinology 119:12-18. . Levin, ER. 1996. Editorial: Endothelin-1, prostaglandin Fla, and the corpus luteum: The crisis of lysis. Endocrinology 137:5189-5190. Lewis, PE and JE Warren Jr. 1977. Effect of indomethacin on luteal function in ewes and heifers. J Anim Sci 45:763-767. Li, Y, JR Molina, J Klindt, DJ Bolt and LL Anderson. 1989. Prolactin maintains relaxin and progesterone secretion by aging corpora lutea after hypophysial stalk transection or hypophysectomy in the pig. Endocrinology 124:1294-1304. Linkie, DM and GD Niswender. 1972. Characterization of rat placental luteotropin: Physiology and biochemical properties. Biol Reprod 8:48-57. Lucy, MC, RJ Collier, ML Kitchell, JJ Dibner, SD Hauser and GG Krivi. 1993. Immunohistochemical and nucleic acid analysis of somatotropin receptor populations in the bovine ovary. Biol Reprod 48:1219-1227. Lussier, JG, P Matton and JJ Dufour. 1987. Growth rates of follicles in the ovary of the cow. J Reprod Fertil 81:301-307. Magness, RR, JM Huie and CW Weems. 1981. Effect of chronic ipsilateral or contralateral intrauterine infusion of prostaglandin E2 (PGE2) on luteal function of unilaterally ovariectomized ewes. Prostaglandins Med 6:389-401. 126 Malven, PV. 1969. Luteotrophic and luteolytic responses of prolactin in hypophysectomized rats. Endocrinology 84:1224-1229. Mann, GE and GE Lamming. 1995a. Progesterone inhibition of the development of the luteolytic signal in cows. J Reprod Fertil 104:1-5. Mann, GE and GE Lamming. 1995b. Effect of the level of oestradiol on oxytocin-induced prostaglandin F2a release in the cow. J Endocrinol 145:175-180. Maims, JG, GD Niswender and T Braden. 1984. FSH receptors in the bovine corpus luteum. Theriogenology 22:321-328. Mapletoft, RJ, CH Del Campo and OJ Ginther. 1976. Local venoarterial pathway for uterine-induced luteolysis in cows. Proc Soc Exp Biol Med 153:289-294. Marsh, J. 1975. The role of cAMP in gonadal function. Adv Cyclic Nucleotide Res 6:137199. Martal, J, MC LaCroix, C Loudes, M Saunier and S Winterberger-Torres. 1979. Trophoblastin, an antiluteolytic protein present in early pregnancy in sheep. J Reprod Fertil 56:63-73. Maxson, WS, AF Haney and DW Schomberg. 1985. Steroidogenesis in porcine atretic follicles: Loss of aromatase activity in isolated granulosa and theca. Biol Reprod 33:495-501. McCracken, JA. 1980. Hormone receptor control of prostaglandin F2a, secretion by the ovine uterus. In: Advances in Prostaglandin and Thromboxane Research. Samuelsson, Ramwell and Paoletti, eds. Raven Press Ltd, New York 8:1329-1344. McCracken, JA. 1984. Update on luteolysis-receptor regulation of pulsatile secretion of prostaglandin F2, from the uterus. Res Reprod 16:1-2. McCracken, JA, DT Baird and JR Goding. 1971. Factors affecting the secretion of steroids from the transplanted ovary in the sheep. Recent Prog Horm Res 27:537582. McCracken, JA, JC Carlson, ME Glew, JR Goding, K Green and B Samuelsson. 1972. Prostaglandin F2, identified as a luteolytic hormone in sheep. Nature New Biol 238:129-134. 127 McCracken, JA, EE Custer, JC Lamsa and AG Robinson. 1995. The central oxytocin pulse generator: A pacemaker for luteolysis. In: Oxytocin. Cellular and Molecular Approaches in Medicine and Research. R Ivell and JA Russell, eds., Plenum Press, New York. pp 133-154. McCracken, JA, ME Glew and R Scaramuzzi. 1970. Corpus luteum regression induced by prostaglandin F. J Clin Endocrinol Metab 30:544-546. McCracken, JA, W Schramm and WC Okulicz. 1984. Hormone receptor control of pulsatile secretion of PGF2. from the ovine uterus during luteolysis and its abrogation in early pregnancy. Anim Reprod Sci 7:31-55. McCracken, JA, TT Smith, JC Lamsa and AG Robinson. 1991. The effect of estradiol1713 (E) and progesterone (P) on the oxytocin (OT) pulse generator in the ovexed sheep. Biol Reprod 44(Suppl 1):87. McGuire, WJ, DE Hawkins and GD Niswender. 1992. Activation of protein kinase (PK)C inhibits progesterone production in vivo. Biol Reprod 46(Suppl 1):84. McNatty, KP, A Makris, C DeGrazia, R Osathanondh and KJ Ryan. 1979. The production of progesterone, androgens, and estrogens by granulosa cells, thecal tissue, and stromal tissue from human ovaries in vitro. J Clin Endocrinol Metab 49:687-699. Meyer, MD, PJ Hansen, WW Thatcher, M Drost, L Badinga, RM Roberts, J Li, TL Ott and FW Bazer. 1995. Extension of corpus luteum lifespan and reduction of uterine secretion of prostaglandin F of cows in response to recombinant interferon-tau. J Dairy Sci 78:1921-1931. Mirando, MA, WC Becker and SS Whitaker. 1993a. Relationships among endometrial oxytocin receptors, oxytocin-stimulated phosphoinositide hydrolysis and prostaglandin Fes,, secretion in vitro, and plasma concentrations of ovarian steroids before and during corpus luteum regression in cyclic heifers. Biol Reprod 48:874882. Mirando, MA, JP Harney, Y Zhou, TF Ogle, TL Ott, RJ Moffat and FW Bazer. 1993b. Changes in progesterone and oestrogen receptor mRNA and protein and oxytocin receptors in endometrium of ewes after intrauterine injection of ovine trophoblast interferon. J Mol Endocrinol 10:185-192. Mirando, MA, TL Ott, JL Vallet, M Davis and FW Bazer. 1990. Oxytocin-stimulated inositol phosphate turnover in endometrium of ewes is influenced by stage of the estrous cycle, pregnancy, and intrauterine infusion of ovine conceptus secretory proteins. Biol Reprod 42:98-105. 128 Moor, RM and LEA Rowson. 1966a. The corpus luteum of the sheep: Functional relationship between the embryo and corpus luteum. J Endocrinol 34:233-239. Moor, RM and LEA Rowson. 1966b. The corpus luteum of the sheep: Effect of the removal of embryos on luteal function. J Endocrinol 34:497-502. Moore, LG, VJ Choy, RL Elliot and WB Watkins. 1986. Evidence for the pulsatile release of PGF2c, inducing the release of ovarian oxytocin during luteolysis in the ewe. J Reprod Fertil 76:159-166. Morishige, WK and I Rothchild. 1974. Temporal aspects of the regulation of corpus luteum function by luteinizing hormone, prolactin and placental luteotrophin during the first half of pregnancy in the rat. Endocrinology 95:260-274. Munson, PJ and D Rodbard. 1980. Ligand: A versatile computerized approach for characterization of ligand binding system. Anal Biochem 108:193-208. Murdoch, WJ, Austin, KA and TR Hansen. 1996. Polyubiquitin up-regulation in corpora lutea of prostaglandin-treated ewes. Endocrinology 137:4526-4529. Murdoch, WJ and RJ McCormick. 1991. Dose-dependent effects of indomethacin on ovulation in the sheep: Relationship to follicular prostaglandin production, steroidogenesis, collagenolysis, and leukocyte chemotaxis. Biol Reprod 45:907911. Murdoch, WJ, TA Peterson, EA Kirk, DL Vincent and EK Inskeep. 1986. Interactive roles of progesterone, prostaglandin, and collagenase in the ovulatory mechanism of the ewe. Biol Reprod 35:1187-1194. Nakamura, K, T Minegishi, Y Takakura, K Miyamoto, Y Hasegawa, Y Ibuki and M Igarashi. 1991. Hormonal regulation of gonadotropin receptor mRNA in rat ovary during follicular growth and luteinization. Mol Cell Endocrinol 82:259-263. Nalbandov, AV, LWL Kao and EE Jones. 1973. Effects of intrafollicular injection of hormones and drugs. J Reprod Fertil Suppl 18:15-22. Nancarrow, CD, J Buckmaster, W Chamley, RI Cox, IA Cumming, L Cummins, JP Drinan, JK Findlay, FR Goding, BJ Restall, W Schneider and GD Thorburn. 1973. Hormonal changes around oestrus in the cow. J Reprod Fertil 32:320-321. Nelson, SE, MP McLean, PG Jayatilak and G Gibori. 1992. Isolation, characterization, and culture of cell subpopulations forming the pregnant rat corpus luteum. Endocrinology 130:954-966. 129 Niswender, GD and TM Nett. 1994. Corpus luteum and its control in infraprimate species. In: The Physiology of Reproduction, Second Edition. E Knobil and JD Neil, eds. Raven Press Ltd, New York. pp 781-816. Niswender, GD, RH Schwall, TA Fitz, CE Farin and HR Sawyer. 1985. Regulation of luteal function in domestic ruminants: New concepts. Rec Prog Horm Res 41:101-151. Noel, B, JL Bister and R Paquay. 1993. Ovarian follicular dynamics in Suffolk ewes at different periods of the year. J Reprod Fertil 99:695-700. Northey, DL and LR French. 1980. Effect of embryo removal and intrauterine infusion of embryonic homogenates on the lifespan of the bovine corpus luteum. J Anim Sci 50:298-302. Norris, DO. 1997. Vertebrate Endocrinology, 3rd Edition. Academic Press, Inc. San Diego. O'Grady, JP, BV Caldwell, FJ Auletta and L Speroff. 1972. The effects of an inhibitor of prostaglandin synthesis (indomethacin) on ovulation, pregnancy, and pseudopregnancy in the rabbit. Prostaglandins 1:97-106. Olsen, GJ, DJ Lane, SJ Giovannoni, NR Pace and DA Stahl. 1986. Microbial ecology and evolution: A ribosomal RNA approach. Annu Rev Microbiol 40:337-365. Onclin, K and JP Verstegen. 1997. In vivo investigation of luteal function in dogs: Effects of cabergoline, a dopamine agonist, and prolactin on progesterone secretion during mid-pregnancy and -diestrus. Dom Anim Endocrinol 14:25-38. Orwig, KE and F Stormshak. 1994. Phosphorylation of the myristoylated alanine rich C kinase substrate (MARCKS) in the bovine corpus luteum. Biol Reprod 50(Suppl 1):143. O'Shaughnessy, PJ and DC Wathes. 1985. Role of lipoproteins and de-novo cholesterol synthesis in progesterone production by cultured bovine luteal cells. J Reprod Fertil 74:425-432. O'Shea, JD, DG Cran and MF Hay. 1979. The small luteal cell of the sheep. I Anat 128:239-251. O'Shea, JD, DG Cran and MF Hay. 1980. Fate of the theca interna following ovulation in the ewe. Cell Tissue Res 210:305-319. 130 Ott, TL, G Van Kee le, CE Hotstetler, TK Schalue, JJ Olmsted, HM Johnson and FW Bazer. 1993. Intrauterine injection of recombinant ovine interferon-tau extends the interestrous interval in sheep. Theriogenology 40:757-769. Ottobre, JS, GS Lewis, WV Thayne and EK Inskeep. 1980. Mechanism by which progesterone shortens the estrous cycle of the ewe. Biol Reprod 23:1046-1053. Oxberry, BS and GW Greenwald. 1982. An autoradiographic study of the binding of 'I-labeled follicle stimulating hormone, human chorionic gonadotropin and prolactin to the hamster ovary throughout the estrous cycle. Biol Reprod 27:505516. Paavola, LG, JF Strauss III, CO Boyd and JE Nestler. 1985. Uptake of gold- and [3H]cholesteryl linoleate-labeled human low density lipoprotein by cultured rat granulosa cells: Celluar mechanisms involved in lipoprotein metabolism and their importance to steroidogenesis. J Cell Biol 100:1235-1247. Pache, TD, JW Wladimiroff, FH de Jong, WC Hop and BCJM Fauser. 1990. Growth patterns of nondominant ovarian follicles during the normal menstrual cycle. Fertil Steril 54:638-642. Page, RB and RM Bergland. 1977. The neurohypophyseal capillary bed. I. Anatomy and arterial supply. Amer J Mat 148:345-358. Pate, JL and WA Condon. 1982. Effects of serum and lipoproteins on steroidogenesis in cultured bovine luteal cells. Mol Cell Endocrinol 28:551-562. Pate, JL and WA Condon. 1989. Regulation of steroidogenesis and cholesterol synthesis by prostaglandin F2, and lipoproteins in bovine luteal cells. J Reprod Fertil 87:439446. Peterson, AJ, HR Tervit, RJ Fairclough, PG Havik and JF Smith. 1976. Jugular levels of 13,14-dihydro-15-keto-prostaglandin F and progesterone around luteolysis and early pregnancy in the ewe. Prostaglandins 12:551-558. Phariss, BB and L Wyngarden. 1969. The effect of prostaglandin Fa, on the progestogen content of the ovaries of pseudopregnant rats. Proc Soc Exp Biol Med 130:92-94. Phillips, HS, H Jeanne, DW Leung and N Ferrara. 1990. Vascular endothelial growth factor is expressed in rat corpus luteum. Endocrinology 127: 965-967. 131 Pineda, MH, OJ Ginther and WH Mc Shan. 1972. Regression of corpus luteum in mares treated with an antiserum against an equine pitutitary fraction. Amer J Vet Res 33:1767-1773. Piper, EL and WC Foote. 1968. Ovulation and corpus luteum maintenance in ewes treated with 173 -oestradiol. J Reprod Fertil 16:253-259. Piper, EL and WC Foote. 1970. The effect of 173-estradiol on corpus luteum function in sheep. Biol Reprod 2:48-52. Pratt, BR, RL Butcher and EK Inskeep. 1977. Antiluteolytic effect of the conceptus and of PGE2 in ewes. J Anim Sci 45:784-791. Quinones - Jenab, V, S Jenab, S Ogawa, RAM Adan, JPH Burbach and DW Pfaff. 1997. Effects of estrogen on oxytocin receptor messenger ribonucleic acid expression in the uterus, pituitary, and forebrain of the female rat. Neuroendocrinology 65:9-17. Ravindranath, N, L Little-Ihrig, HS Phillips, N Ferrara and AJ Zeleznik. 1992. Vascular endothelial growth factor messenger ribonucleic acid expression in the primate ovary. Endocrinology 131:254-260. Raw, RE and WJ Silvia. 1991. Activity of phospholipase C and release of prostaglandin F2 by endometrial tissue from ovariectomized ewes receiving progesterone and estradiol. Biol Reprod 44:404-412. Redmer DA, Y Dai, J Li, DS Charnock-Jones, SK Smith, LP Reynolds and RM Moor. 1996. Characterization and expression of vascular endothelial growth factor (VEGF) in the ovine corpus luteum. J Reprod Fertil 108:157-165. Redmer, DA, JD Rone and AL Goodman. 1985. Evidence for a non-steroidal angiotropic factor from the primate corpus luteum: Stimulation of endothelial cell migration in vitro. Proc Soc Exp Biol Med 179:136-140. Reich, R, R Miskin and A Tsafriri. 1985. Follicular plasminogen activator: Involvement in ovulation. Endocrinology 116:516-521. Reynolds, LP, J Stigler, WL Hoyer, RR Magness, JM Huie, TP Huecksteadt, GL Whysong, BR Behrman and CW Weems. 1981. Effect of PGEI or PGE2 on PGF2-induced luteolysis in nonbred ewes. Prostaglandins 21:957-972. Richard, S and HH Zingg. 1990. The human oxytocin gene promoter is regulated by estrogens. J Biol Chem 265:6098-6103. 132 Richards, JS. 1980. Maturation of ovarian follicles: Actions and interactions of pituitary and ovarian hormones on follicular cell differentiation. Physiol Rev 60:51-89. Richards, RG, JE Gadsby and GW Almond. 1994. Differential effects of LH and PGE2 on progesterone secretion by small and large porcine luteal cells. J Reprod Fertil 102:27-34. Richards, JS and AR Midgley Jr. 1976. Protein hormone action: A key to understanding ovarian follicular and luteal cell development. Biol Reprod 14:82-94. Roberts, RM, JC Cross and DW Leaman. 1992. Interferons as hormones of pregnancy. Endocrine Rev 13:432-452. Roberts, JS and JA McCracken. 1976. Does PGF released from the uterus by oxytocin mediate the oxytocic action of oxytocin? Biol Reprod 15:457-463. Roberts, JS, JA McCracken, JE Gavagan and MS Soloff. 1976. Oxytocin-stimulated release of prostaglandin F2, from bovine endometrium in vitro: Correlation with estrous cycle and oxytocin-receptor binding. Endocrinology 99:1107-1114. Roberts, RM, X Sancai and N Mathialagan. 1996. Maternal recognition of pregnancy. Biol Reprod 54:294-302. Robertson, MC and HG Friesen. 1975. The purification and characterization of rat placental lactogen. Endocrinology 97:621-629. Robertson, MC, B Guillespie and HG Friesen. 1982. Characterization of the two forms of rat placental lactogen (rPL): rPL-I and rPL-II. Endocrinology 111:1862-1866. Rodgers, RJ, JD O'Shea, JK Findlay and APF Flint. 1983. Large luteal cells the source of oxytocin in sheep. Endocrinology 113:2302-2304. Rodgers, RJ, MR Waterman and ER Simpson. 1986. Cytochrome P-450,, P-45017, adrenoxin, and reduced nicotinamide adenine dinucleotide phosphate-cytochrome P-450 reductase in bovine follicles and corpora lutea. Endocrinology 118:13661374. Rodgers, RJ, MR Waterman and ER Simpson. 1987. Levels of messenger ribonucleic acid encoding cholesterol side-chain cleavage cytochrome P-450, 17a-hydroxylase cytochrome P-450, adrenoxin, and low density lipoprotein receptor in bovine follicles and corpora lutea throughout the ovarian cycle. Mol Endocrinol 1:274279. 133 Rowson, LEA, HR Tervit and A Brand. 1972. The use of prostaglandins for synchronization of oestrus in cattle. J Reprod Fertil 29:145. Rozen, F, C Russo, D Banville and HH Zingg. 1995. Structure, characterization, and expression of the rat oxytocin receptor gene. Proc Natl Acad Sci USA 92:200204. SAS (Version 6.07). 1993. SAS Institute Inc., Cary, NC. Savard, K and PJ Casey. 1964. Effects of pituitary hormones and NADPH on acetate utilization in ovarian and adrenocortical tissues. Endocrinology 74:599-610. Sawyer, HR, JH Abel Jr., MC McClellan, M Schmitz and GD Niswender. 1979. Secretory granules and progesterone secretion by ovine corpora lutea in vitro. Endocrinology 104:476-486. Sawyer, HR, CL Moeller and GP Kozlowski. 1986. Immunocytochemical localization of neurophysin and oxytocin in ovine corpora lutea. Biol Reprod 34:543-548. Sawyer, HR, KD Niswender, TD Braden and GD Niswender. 1990. Nuclear changes in ovine luteal cells in response to PGF2a. Dom Anim Reprod 7:229-238. Scaramuzzi, RJ and DT Baird. 1976. The oestrous cycle of the ewe after active immunization against prostaglandin Fu. J Reprod Fertil 46:39-47. Scaramuzzi, RJ, DT Baird, HP Boyle, RB Land and AG Wheeler. 1977. The secretion of prostaglandin F from the autotransplanted uterus of the ewe. J Reprod Fertil 49:157-160. Scaramuzzi, RJ, CA Blake, H Papkoff, J Hilliard and CH Sawyer. 1972. Radioimmunoassay of rabbit luteinizing hormone: Serum levels during various reproductive states. Endocrinology 90:1285-1291. Scaramuzzi, RJ and JA Downing. 1995. The in vivo effects of fibroblast growth factor and epidermal growth factor on the secretion of oestradiol, androstenedione and progesterone by the autotransplanted ovary in the ewe. J Endocrinol 146:301-311. Schams D. 1983. Oxytocin determination by radioimmunoassay. III. Improvement to subpicogram sensitivity and application to blood levels in cyclic cattle. Acta Endocrinol (Copenh) 103:180-183. Schams, D, TAM Kruip and R Koll. 1985. Oxytocin determination in steroid producing tissues and in vitro production in ovarian follicles. Acta Endocrinol 109:530-536. 134 Schenken, RS, RF Williams and GD Hogden. 1984. Ovulation induction using "pure" follicle-stimulating hormone in monkeys. Fertil Steril 41:629-634. Schneider WC. 1957. Determination of nucleic acids in tissues by pentose analysis. Meth Enzymol 3:680-684. Schramm, W, L Bovaird, ME Glew, G Schramm and JA McCracken. 1983. Corpus luteum regression induced by ultra-low pulses of prostaglandin F2, . Prostaglandins 26:347-364. Schrick, FN, RA Surface, JY Pritchard, RA Dailey, ED Townsend and EK Inskeep. 1993. Ovarian structures during the estrous cycle and early pregnancy in ewes. Biol Reprod 49:1133-1140. Scott, RS and PIC Rennie. 1970. Factors controlling the lifespan of the corpora lutea in the pseudopregnant rabbit. J Reprod Fertil 23:415-422. Segaloff, DL, H Wang and JS Richards. 1990. Hormonal regulation of luteinizing hormone/chorionic gonadotropin receptor mRNA in rat ovarian cells during follicular development and luteinization. Mol Endocrinol 4:1856-1865. Sharma, SC and RJ Fitzpatrick. 1974. Effect of oestradiol-1713 and oxytocin treatment on prostaglandin F alpha release in the anoestrous ewe. Prostaglandins 6:97-105. Sheldrick EL. 1992. Effect of continuous infusion of oxytocin on ovarian function and uterine oxytocin receptor concentration in the cyclic ewe. Reprod Fertil Devel 4:505-513. Sheldrick, EL and APF Flint. 1981. Circulating concentrations of oxytocin during the estrous cycle and early pregnancy in sheep. Prostaglandins 22:631-636. Sheldrick, EL and APF Flint. 1985. Endocrine control of uterine oxytocin receptors in the ewe. J Endocrinol 106:249-258. Silvia, WJ, GS Lewis, JA McCracken, WW Thatcher and L Wilson Jr. 1991. Hormonal regulation of uterine secretion of prostaglandin Fa, during luteolysis in ruminants. Biol Reprod 45:655-663. Silvia, WJ and GD Niswender. 1986. Maintenance of the corpus luteum of early pregnancy in the ewe. IV. Changes in luteal sensitivity to prostaglandin Fa, throughout early pregnancy. J Anim Sci 63:1201-1207. 135 Silvia, WJ, RE Raw, SL Aldrich and SH Hayes. 1992. Uterine secretion of prostaglandin F2, in response to oxytocin in ewes: Changes during the estrous cycle and early pregnancy. Biol Reprod 46:1007-1015. Silvia, WJ and ML Taylor. 1989. Relationship between uterine secretion of prostaglandin F2, induced by oxytocin and endogenous concentrations of estradiol and progesterone at three stages of the bovine estrous cycle. J Anim Sci 67:2347-2353. Simmons,KR and W Hansel. 1964. Nature of the lueotrophic hormone in the bovine. J Anim Sci 23:136-141. Sirois, J and JE Fortune. 1988. Follicular dynamics during the estrous cycle in heifers monitored by real-time ultrasonography. Biol Reprod 39:308-317. Slayden, 0 and F Stormshak. 1990. Suppressive action of gonadotropin-releasing hormone and luteinizing hormone on function of the developing ovine corpus luteum. J Anim Sci 68:2425-2429. Slayden, 0, MB Zelinski-Wooten, RL Stouffer and RM Brenner. 1994. Radioligand binding assay of progesterone receptors in the primate corpus luteum after in vivo treatment with the 3P-hydroxysteroid dehydrogenase inhibitor, trilostane. J Clin Endocrinol Metab 79:620-626. Smith, PE. 1930. Hypophysectomy and a replacement therapy in the rat. Amer J Anat 45:205-273. Smith, GW, PC Gentry, RM Roberts and MF Smith. 1996. Ontogeny and regulation of luteinizing hormone receptor messenger ribonucleic acid within the ovine corpus luteum. Biol Reprod 54:76-83. Snook, RB, MA Brunner, RR Saatman and W Hansel. 1969. The effect of antisera to bovine LH in hysterectomized and intact heifers. Biol Reprod 1:49-58. Snyder, BW, GD Beecham and HP Schane. 1984. Inhibition of ovulation in rats with epostane, an inhibitor of 3P-hydroxysteroid dehydrogenase (41865). Proc Soc Exp Biol Med 176:238-242. Spencer, TE and FW Bazer. 1995. Temporal and spatial alterations in uterine estrogen receptor and progesterone receptor gene expression during the estrous cycle and early pregnancy in the ewe. Biol Reprod 53:1527-1543. 136 Spencer, TE and FW Bazer. 1996. Ovine interferon tau suppresses transcription of the estrogen receptor and oxytocin receptor genes in the ovine endometrium. Endocrinology 137:1144-1147. Spencer, TE, WC Becker, P George, MA Mirando, TF Ogle and FW Bazer. 1995a. Ovine interferon-T regulates expression of endometrial receptors for estrogen and oxytocin but not progesterone. Biol Reprod 53:732-745. Spencer, TE, WC Becker, P George, MA Mirando, TF Ogle and FW Bazer. 1995b. Ovine interferon-T inhibits estrogen receptor up-regulation and estrogen-induced luteolysis in cyclic ewes. Endocrinology 136:4932-4944. Spencer, TE, NH Ing, TL Ott, JS Mayes, WC Becker, GH Watson, MA Mirando and FW Bazer. 1995c. Intrauterine injection of ovine interferon-T (INF--c) alters oestrogen receptor and oxytocin receptor expression in the endometrium of cyclic ewes. J Mol Endocrinol 15:203-220. Spies, HG, AL Slyter and SK Quadri. 1967. Regression of corpora lutea in pregnant gilts administered antiovine LH rabbit serum. J Anim Sci 26:768-771. Spies, HG, DR Zimmerman, HL Self and LE Casida. 1960. Effect of exogenous progesterone on the corpora lutea of hysterectomized gilts. J Anim Sci 19:101108. Squires EC and OJ Ginther. 1975. Follicular and luteal development in pregnant mares. J Reprod Fertil Suppl 23:429-433. Stabenfeldt, GH. 1992. Control of ovulation and the corpus luteum. In: Textbook of Veterinary Physiology. JG Cunningham, ed. WB Saunders Company, Philadelphia. pp 447-445. Stabenfeldt, GH, JP Hughes, JD Wheat, JW Evans, PC Kennedy and PT Cupps. 1974. The role of the uterus in ovarian control in the mare. J Reprod Fertil 37:343-351. Stewart, HJ, KR Stevenson and APF Flint. 1993. Isolation and structure of a partial sheep oxytocin receptor cDNA and its use as a probe for northern analysis of endometrial RNA. J Mol Endocrinol 10:359-361. Stock, AE, RT Emeny, J Sirois and JE Fortune. 1995. Oxytocin in mares: Lack of evidence for oxytocin production by or action on preovulatory follicles. Dom Anim Endocrinol 12:133-142. 137 Stormshak, F and LE Casida. 1964. Effect of gonadotropins on corpora lutea of pseudopregnant rabbits. Endocrinology 75:321-325. Stormshak, F, EK Inskeep, JE Lynn, AL Pope and LE Casida. 1963. Progesterone levels in corpora lutea and ovarian effluent blood of the ewe. J Anim Sci 22:1021-1026. Stormshak, F, HE Kelley and HW Hawk. 1969. Suppression of ovine luteal function by 17(3-estradiol. J Anim Sci 29:476-478. Stormshak, F, R Leake, N Wertz and J Gorski. 1976. Stimulatory and inhibitory effects of estrogen on uterine DNA synthesis. Endocrinology 99:1501-1511. Strauss, 1F, III, LC MacGregor and JT Gwynn. 1982. Uptake of high density lipoproteins by rat ovaries in vivo and dispersed ovarian cells in vitro: Direct correlation of high density lipoprotein uptake with steroidogenic activity. J Steroid Biochem 16:525-531. Strickland, S and WH Beers. 1976. Studies on the role of plasminogen activation in ovulation. J Biol Chem 251:5694-5702. Swanson, LV and HD Hafs. 1971. LH and prolactin in blood serum from estrus to ovulation in Hostein heifers. J Anim Sci 33:1038-1041. Tanaka, N, LL Espey, S Stacy and H Okamura. 1992. Epostane and indomethacin actions on ovarian kallikrein and plasminogen activator activities during ovulation in the gonadotropin-primed immature rat. Biol Reprod 46:665-670. Terranova, PF. 1997. Potential roles of tumor necrosis factor-a in follicular development, ovulation , and the life span of the corpus luteum. Dom Anim Endocrinol 14:115. Thorburn, GD, RI Cox, WB Currie, BJ Restall and W Schneider. 1972. Prostaglandin F concentration in the utero-ovarian venous plasma of the ewe during the oestrous cycle. J Endocrinol 53:325-326. Thorburn, GD and DH Nicol. 1971. Regression of the ovine corpus luteum after infusion of prostaglandin F2a into the ovarian artery and uterine vein. J Endocrinol 51:785786. 138 Tian, XC, AK Berndtson and JE Fortune. 1995. Differentiation of bovine preovulatory follicles during the follicular phase is associated with increases in messenger ribonucleic acid for cytochrome P450 side-chain cleavage, 3f3-hydroxysteroid dehydrogenase, and P450 17a-hydroxylase, but not P450 aromatase. Endocrinology 136:5102-5110. Tsafriri, A and N Dekel. 1994. Molecular mechanisms in ovulation. In: Molecular Biology of the Female Reproductive System. JK Findlay, ed. Academic Press, Inc, San Diego. pp 207-258. Tsafriri, A, HR Lindner, U Zor and SA Lamprecht. 1972. Physiological role of prostaglandins in the induction of ovulation. Prostaglandins 2:1-10. Tsai, S-J, MC Wiltbank and KJ Bodensteiner. 1996. Distinct mechanisms regulate induction of messenger ribonucleic acid for prostaglandin (PG) G/H synthase-2, PGE (EP3) receptor, and PGF2a receptor in bovine preovulatory follicles. Endocrinology 137:3348-3355. Tsukada, K, T Matsushima and N Yamanaka. 1996. Neovascularization of the corpus luteum of rats during the estrus cycle. Pathol Int 46:408-416. Vallet, JL, FW Bazer, MFV Fliss and WW Thatcher. 1988. Effect of ovine conceptus secretory proteins and purified ovine trophoblast protein-1 on interoestrous interval and plasma concentrations of prostaglandins F2, and E and of 13,14dihydro-15-keto prostaglandin F2, in cyclic ewes. J Reprod Fertil 84:493-504. Vallet, JL, GE Lamming and M Batten. 1990. Control of endometrial oxytocin receptor and uterine response to oxytocin by progesterone and oestradiol in the ewe. J Reprod Fertil 90:625-634. Vighio, GH and RM Liptrap. 1986. Plasma concentrations of oxytocin, prostaglandin and ovarian steroids during spontaneous luteolysis in the cow. Dom Anim Endocrinol 3:209-215. Voss, AK and JE Fortune. 1991a. Oxytocin stimulates progesterone production by bovine granulosa cells isolated before, but not after, the luteinizing hormone surge. Mol Cell Endocrinol 78:17-24. Voss, AK and JE Fortune. 199 lb. Oxytocin secretion by bovine granulosa cells: Effects of stage of follicular development, gonadotropins, and cocultrue with theca interns. Endocrinology 128:1991-1999. 139 Voss, AK and JE Fortune. 1992. Oxytocin/neurophysin-I messenger ribonucleic acid in bovine granulosa cells increases after the luteinizing hormone (LH) surge and is stimulated by LH in vitro. Endocrinology 131:2755-2762. Voss, AK and JE Fortune. 1993. Estradio1-1713 has a biphasic effect on oxytocin secretion by bovine granulosa cells. Biol Reprod 48:1404-1409. Walker, DL, PG Weston and JE Hixon. 1997. Influence of estradiol and progesterone withdrawal on the secretion of and the temporal correlation between pulses of oxytocin and prostaglandin Fla in ewes. Biol Reprod 56:1228-1238. Walters, DL and E Schallenberger. 1984. Pulsatile secretion of gonadotrophins, ovarian steroids and ovarian oxytocin during the periovulatory phase of the oestrus cycle in the cow. J Reprod Fertil 71:503-512. Wandji, SA, V Srsen, AK Voss, JJ Eppig and JE Fortune. 1996. Initiation in vitro of growth of bovine primordial follicles. Biol Reprod 55:942-948. Warren Jr., JE, HW Hawk and DJ Bolt. 1973. Evidence for progestational priming of estradiol-induced luteal regression in the ewe. Biol Reprod 8:435-440. Wathes, DC, SEF Guldennar, RW Swann, R Webb, DG Porter and BT Pickering. 1986. A combined radioimmunoassay and immunocytochemical study of ovarian oxytocin production during the periovulatory period in the ewe. J Reprod Fertil 78:167-183. Wathes, DC and M Hamon. 1993. Localization of oestradiol, progesterone and oxytocin receptors in the uterus during the oestrous cycle and early pregnancy of the ewe. J Endocrinol 138:479-491. Wathes, DC and GE Lamming. 1995. The oxytocin receptor, luteolysis and the maintenance of pregnancy. J Reprod Fertil Suppl 49:53-67. Wathes, DC, GE Mann, JH Payne, PR Riley, KR Stevenson and GE Lamming. 1996. Regulation of oxytocin, oestradiol and progesterone receptor concentrations in different uterine regions by oestradiol, progesterone and oxytocin in ovariectomized ewes. J Endocrinol 151:375-393. Wathes, DC and RW Swann. 1982. Is oxytocin an ovarian hormone? Nature 297:225-227. Wathes, DC, RW Swann, SD Birkett, DG Porter and BT Pickering. 1983. Characterization of oxytocin, vasopressin and neurophysin from the bovine corpus luteum. Endocrinology 113:693-698. 140 Wathes, DC, RW Swann and BT Pickering. 1984. Variations in oxytocin, vasopressin and neurophysin concentrations in the bovine ovary during the oestrous cycle and pregnancy. J Reprod Fertil 71:551-557. Webb, DK, BC Moulton and SA Khan. 1993. Estrogen induces expression of c-jun and jun-B protooncogenes in specific rat uterine cells. Endocrinology 133:20-28. Wildt, DE, WB Panko, PK Chakraborty and SWJ Seager. 1979. Relationship of serum estrone, estradio1-1713 and progesterone to LH, sexual behavior and time of ovulation in the bitch. Biol Reprod 20:648-658. Wiltbank, MC. 1994. Cell types and hormonal mechanisms associated with mid-cycle corpus luteum function. J Anim Sci 72:1873-1883. Wiltbank, MC, CJ Belfiore and GD Niswender. 1993. Steroidogenic enzyme activity after acute activation of protein kinase (PK) A and PKC in ovine small and large luteal cells. Mol Cell Endocrinol 97:1-7. Wiltbank, JN and LE Casida. 1956. Alteration of ovarian activity by hysterectomy. J Anim Sci 15:134-140. Wiltbank, MC, MG Diskin, JA Flores and GD Niswender. 1990. Regulation of the corpus luteum by protein kinase C. II. Inhibition of lipoprotein-stimulated steroidogenesis by prostaglandin Fla. Biol Reprod 42:239-245. Wiltbank, MC, PB Guthrie, MP Mattson, SB Kater and GD Niswender. 1989a. Hormonal regulation of free intracellular calcium concentrations in small and large ovine luteal cells. Biol Reprod 41:771-778. Wiltbank, JN, JE Ingalls and WW Rowden. 1961. Effects of various forms and levels of estrogens alone or in combinations with gonadotropins on the estrous cycle of beef heifers. J Anim Sci 20:341-346. Wiltbank, MC, JJ Knickerbocker and GD Niswender. 1989b. Regulation of the corpus luteum by protein kinase C I. Phosphorylation activity and steroidogenic action in large and small ovine luteal cells. Biol Reprod 40:1194-1200. Xu, Z, HA Garverick, GW Smith, MF Smith, SA Hamilton and RS Youngquist. 1995. Expression of follicle-stimulating hormone and luteinizing hormone receptor messenger ribonucleic acids in bovine follicles during the first follicular wave. Biol Reprod 53:951-957. 141 Yuan, W and MC Lucy. 1996. Messenger ribonucleic acid expression for growth hormone receptor, luteinizing hormone receptor, and steroidogenic enzymes during the estrous cycle and pregnancy in porcine and bovine corpora lutea. Dom Anim Endocrinol 13 :431-444. Zarco, L, GH Stabenfeldt, S Basu, GE Bradford and H Kindahl. 1988a. Modification of prostaglandin F2a synthesis and release in the ewe during the initial establishment of pregnancy. J Reprod Fertil 83:527-536. Zarco, L, GH Stabenfeldt, JF Quirke, H Kindahl and GE Bradford. 1988b. Release of prostaglandin F2c, and the timing of events associated with luteolysis in ewes with oestrous cycles of different lengths. J Reprod Fertil 83:517-526. Zelinski, MB, NA Hirota, EJ Keenan and F Stormshak. 1980. Influence of exogenous estradiol -17(3 on endometrial progesterone and estrogen receptors during the luteal phase of the ovine estrous cycle. Biol Reprod 23:743-751. Zhang, J, PG Weston and JE Hixon. 1991. Influence of estradiol on the secretion of oxytocin and prostaglandin F2a during luteolysis in the ewe. Biol Reprod 45:395403. Zhang, J, PG Weston and JE Hixon. 1992. Role of progesterone and oestradiol in the regulation of uterine oxytocin receptors in ewes. J Reprod Fertil 94:395-404. Zingg, HH, F Rozen, C Breton, A Larcher, J Neculcea, K Chu, C Russo and A Arslan. 1995. Gonadal steroid regulation of oxytocin and oxytocin receptor gene expression. In: Oxytocin. Cellular and Molecular Approaches in Medicine and Research. R Ivell and JA Russell eds., Plenum Press, New York. pp 395-404.