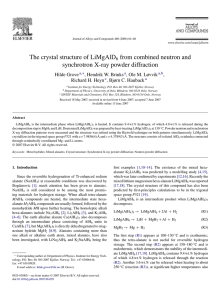
Structure and stability of possible new alanates
advertisement

EUROPHYSICS LETTERS 15 August 2004 Europhys. Lett., 67 (4), pp. 607–613 (2004) DOI: 10.1209/epl/i2004-10105-x Structure and stability of possible new alanates O. M. Løvvik 1 and O. Swang 2 1 Center for materials science and nanotechnology, University of Oslo Gaustadalléen 21, N-0349 Oslo, Norway 2 Department of Hydrocarbon Process Chemistry, SINTEF Materials and Chemistry P.O. Box 124 Blindern, N-0314 Oslo, Norway (received 9 February 2004; accepted in final form 11 June 2004) PACS. 61.18.-j – Other methods of structure determination. PACS. 71.20.-b – Electron density of states and band structure of crystalline solids. Abstract. – Three new stable bialkalimetallic alanates are predicted by accurate density functional calculations: K2 LiAlH6 , K2 NaAlH6 , and KNa2 AlH6 . Their detailed crystal structure has been determined by a systematic search through a large part of the probable space of crystal structures. They are thermodynamically stable at 0 K compared to their monoalkali constituents by 9 to 49 kJ/mol formula units. The crystal structure of the already known alanates Li3 AlH6 , Na3 AlH6 , K3 AlH6 , and LiNa2 AlH6 were also determined, and found to be in excellent agreement with experimental data where available. The two last bialkali alanates studied, Li2 NaAlH6 and KLi2 AlH6 , were found to be unstable. Introduction. – The search for light-weight hydrogen storage materials has led to the discovery of reversible hydrogen storage in the alanates NaAlH4 [1], Na3 AlH6 [1], LiNa2 AlH6 [1], and KAlH4 [2], and possibly also in Li3 AlH6 [3]. Reversible hydrogenation is only obtained (except in the case of KAlH4 ) after the addition of small amounts of a transition metal, e.g. Ti, which also leads to a dramatic increase of reaction rate. The most studied hydrogen storage material among the alanates is so far NaAlH4 , which decomposes at moderate temperatures and atmospheric pressure, and where reversible storage of more than 5 wt% hydrogen has been achieved [4]. This is, however, still not satisfactory, since the international goal of the gravimetric hydrogen density in a hydrogen storage unit is at least 5 wt% hydrogen, including the tank. Additionally, the kinetics leaves something to be desired. The large difference in thermodynamic stability of the two Na alanate phases is also disadvantageous, since this may make it necessary to operate at two different temperatures or pressures, and thus increases the energy necessary for carrying out the hydrogenation/dehydrogenation cycle. Hence, there is a need to find other materials with higher gravimetric hydrogen concentration, and with improved hydrogen sorption properties. Using theoretical band structure calculations, we have in the present study investigated existing and hypothetical compounds of the form A3−x Bx AlH6 , where A and B are Li, Na, or K, while x is 0, 1, 2, or 3. The three monoalkalimetallic compounds are already well known [5,6] (but the crystal structure of K3 AlH6 has not yet been experimentally determined), c EDP Sciences 608 EUROPHYSICS LETTERS Table I – The model structures used as input for the structural optimizations. Listed are the space group names and numbers, the crystal lattice type, the number of formula units in the unit cell Z, and the compounds on which the model structures were based. Since we have investigated two different structures in space group 14, the second one is marked by a prime. For references to the model structures, see ref. [8]. Space group P 1̄ P 21 /c P 21 /c P na21 Immm R3̄ F m3̄m No. 2 14 14 33 71 148 225 Type Triclinic Monoclinic Monoclinic Orthorhombic Tetragonal Rhombohedral Cubic Z 2 2 2 4 2 2 4 Based on Ti3 NiS6 K3 Fe(CN)6 Na3 AlD6 Li3 AlF6 Na3 AlF6 Ti3 NiS6 K3 MoF6 while members of the Li3−x Nax AlH6 system are, to our knowledge, the only bialkalimetallic alanates that have been described in the literature [7]. For each of the compounds, we have performed a full relaxation of both atomic positions, unit cell size, and shape, using a number of different structures as starting points. This puts us in a position to predict not only whether a mixed phase would be stable compared to its monoalkali constituents, but also to a certain extent the structure of the stable compound. A recent study on Li3 AlH6 gave excellent results compared to low-temperature data using the same technique [8], while a similar study on NaAlH4 checking several structures without allowing the symmetry to change also gave very good agreement with experimental data [9]. There has also recently been a theoretical study on the mixed Li3−x Nax AlH6 system [10], but the authors did only regard two possible structures for the mixed alanates, and did not allow the structure to relax from the starting symmetry. They predicted a stable mixed phase for LiNa2 AlH6 and Li1.5 Na1.5 AlH6 , but Li2 NaAlH6 was predicted to be unstable. They also compared different distributions of alkali metal atoms in the lattice, and found that the most stable arrangement of alkali atoms was when the distance between similar alkali atoms was maximized. Computational details. – All calculations were carried out using the Vienna Ab-initio Simulation Packace (VASP) [11, 12], which calculates band structures based on density functional theory and plane-wave basis sets. The projector augmented wave (PAW) scheme [13,14] was used for the wave functions, with energy cut-offs of 780 eV. A 7 × 7 × 7 Monkhorst-Pack grid was employed for the k-point integration. The PW91 gradient-corrected density functional [15] was used for all calculations. The convergence criterion for the self-consistent electronic calculations was an energy difference between iterations of less than 10−5 eV. By comparing representative calculations to calculations using higher accuracy (more k-points, etc.), we have estimated that the overall error in the total energy due to all numerical approximations (apart from the functional and the PAW method) was around 1 meV. For each of the nine compounds in our study we have used the seven start geometries listed in table I. Starting from each of these geometries, full geometry optimization has been carried out without any assumptions of symmetry, and without any constraints on the atomic positions or cell parametres. This procedure should give a good probability of finding the global energy minima of the compounds under study [8]. The relaxation was performed in several steps, with increasing precision. The first steps used the conjugate gradient method for the ionic movement, while the final steps with high precision used the RMM-DIIS implementation of the quasi-Newton method. O. M. Løvvik et al.: Structure and stability of possible new alanates 609 Table II – Resulting space group symmetry for the different input structures and compounds Am Bn AlH6 together with the total free energy in kJ/mol for all the final structures. The most stable structure is marked in boldface for each compound. The first row lists the input structures. Am Bn Li3 Na3 K3 K2 Li K2 Na KLi2 KNa2 Li2 Na LiNa2 P 1̄ R3̄ −3166 R3̄ −2904 P 1̄ −2814 P 1̄ −2928 P 1̄ −2855 P 1̄ −3023 P 1̄ −2869 P 1̄ −3066 P 1̄ −2978 P 21 /c P 21 /c −3148 P 21 /c −2844 P 21 /c −2817 P 21 −2869 P 21 −2800 P 21 /c −2976 P 21 −2842 P 21 −3036 P 21 −2928 P 21 /c P 21 /c −3156 P 21 /c −2907 P 21 /c −2845 F m 3̄m −2995 P 4/mnc −2915 P 21 −3040 P 21 −2895 P 21 −3071 P 21 /c −3005 P na21 P na21 −3157 P na21 −2891 P na21 −2843 P na21 −2956 P na21 −2856 P na21 −3036 P na21 −2874 P na21 −3068 P na21 −2966 Immm F m3̄m −3082 F m3̄m −2873 F m3̄m −2841 P 42 /mmc −2810 P 42 /mmc −2807 F m3̄m −2777 F m3̄m −2765 F m3̄m −2915 P 42 /mmc −2892 R3̄ R3̄ −3169 R3̄ −2904 R3̄ −2814 P1 −2914 P1 −2845 P1 −3008 P1 −2868 P1 −3066 P1 −2978 F m3̄m F m3̄m −3082 F m3̄m −2873 F m3̄m −2841 F m 3̄m −2995 F m 3̄m −2915 F 4̄3 m −3018 F 4̄3 m −2890 F 4̄3 m −3038 F m3̄m −3002 When bialkali alanates were constructed from monoalkali alanate structures, the alkali metal atoms were substituted so that the distance between the least abundant alkali metal (e.g., between Li in LiNa2 AlH6 ) was maximized, corresponding to the most stable arrangement of the alkali atoms found in ref. [10]. We also checked, however, whether other arrangements could be more stable, and found that the energy difference was very small for most of the cases. We attribute this small discrepance with ref. [10] to our relaxation of the structure. The energy of the most stable arrangement is presented throughout. In the following, all molar energies refer to one mol formula units. Results. – Since the relaxation procedure allows both cell size and shape to relax, it is interesting to see how often the symmetry is changed during the relaxation. Table II lists the final symmetry of all the structures of all the compounds, and we see that of the 63 structures, 13 moved to a higher symmetry, while 17 lost symmetry after finishing the relaxation. In particular, we note that the tetragonal Immm structure is metastable for all the compounds, and relaxes to the cubic F m3̄m or the tetragonal P 42 /mmc (space group 131) space groups. On the opposite side, the orthorhombic P na21 structure is stable for all the structures. The P 1̄ structure, which was made by deliberately breaking the symmetry of R3̄, is able to gain back the lost symmetry for Li3 AlH6 and Na3 AlH6 , but for no other compounds. Parallel to this, the R3̄ structure remains as R3̄ for all the monoalkali alanates, while all its symmetry is lost for all the bialkali alanates. The two monoclinic structures in the P 21 /c space group are quite different (the monoclinic angles are, for instance, 107.3 and 89.9◦ , respectively, and the coordination numbers differ) and the one based on K3 Fe(CN)6 usually ends up in the lowest symmetry, except for KLi2 AlH6 . The one based on Na3 AlD6 is both gaining and losing symmetry upon relaxation, and is the starting structure that ends up as the thermodynamically most stable one for all the compounds except Li3 AlH6 . For most of the compounds, there are pairs of final structures in the same space group, 610 EUROPHYSICS LETTERS some of them even have approximately the same total free energy. Are these pairs degenerate structures, or do they have different arrangements of the atoms? As previously shown, both these possibilities were found in the Li3 AlH6 system [8]. The Cartesian coordinates of the F m3̄m structures resulting from Immm and F m3̄m were the same within a few picometers, and the same was the case with the R3̄ structures resulting from P 1̄ and R3̄. The two P 21 /c structures, however, only differing by about 8 kJ/mol, were not the same —both the unit cell and atomic arrangement of the two structures differed significantly. Exactly the same trend is seen here for the other two monoalkali alanates; the P 1̄ and R3̄ starting structures lead to the same R3̄ structure, while the Immm and F m3̄m structures lead to the same F m3̄m structure. The exception is the P 1̄ structure in the K3 AlH6 system; it has moved to a hexagonal unit cell, but remains low in symmetry. The P 1̄ structure is, however, not far from the R3̄ structure; the lattice constants of the two final structures originating from P 1̄ and R3̄ differ by up to 6 pm, while the angles differ by up to 0.8◦ . The Cartesian coordinates are also equivalent within about 10 pm. By inspection, all the input structures P 21 /c , P na21 , Immm and F m3̄m actually end as almost the same structure for K3 AlH6 , with close to or perfect F m3̄m symmetry. Moving to the bialkali alanates, the picture changes. The P 1̄ structure was made by deliberately breaking symmetry of the R3̄ structures, so it was not surprising that these two starting points gave the same result for the monoalkali alanates. For the bialkali alanates, however, they end as different structures for all the compounds except for LiNa2 AlH6 , where the angles differ by up to 0.6◦ , the lattice constants by up to 4 pm, and the Cartesian coordinates by up to 12 pm. However, the P 1̄ and R3̄ structures still have some features in common for the bialkali alanates: All of them end as triclinic, with at most two symmetry operators. The only exception is the KLi2 AlH6 structure that started as R3̄, where the final structure kept the rhombohedral form of the unit cell, but the symmetry was broken. The Immm and F m3̄m structures also end as different structures for all the bialkali alanates, with the highest final symmetry yielded in some cases by the Immm initial structures (the KLi2 , KNa2 , and Li2 Na alanates), and in the other cases by the F m3̄m structure. The only additional structures that end as degenerate for the bialkali alanates are P 21 /c and F m3̄m, which are very similar for all the compounds except KLi2 AlH6 and Li2 NaAlH6 . The total free energy differs by 0.01 to 5 kJ/mol, the angles by 0.03 to 0.56◦ , the lattice constants by 1 to 7 pm, and the Cartesian coordinates by 3 to 51 pm. The differences are large enough to place the structures in different space groups, but closer inspection shows that the main difference usually is the distortion of the AlH6 octahedra in the P 21 /c structure. The most stable structures of each compound from table II are compared to available experimental structures in table III. Apart from Li3 AlH6 , all the final structures are quite similar, with the largest differences being the unit cell size, the angle β, and the distortion of the AlH6 octahedra. The final structure of LiNa2 AlH6 is typical, and is shown in fig. 1. The calculated structures are very similar to the already known experimental structures. The Li3 AlH6 system has already been treated in detail by the same methods as those used in the present work, and the cell angle of the calculated structure differed by 0.05◦ , the lattice constants by less than 2 pm, and the Cartesian coordinates by up to 9 pm [8]. The calculated Na3 AlH6 structure also shows excellent agreement with experimental data at 7 K. The cell angles differ by less than 0.4◦ , the lattice constants by less than 0.6 pm, and the Cartesian coordinates by less than 3 pm between the calculated and experimental structures. The K3 AlH6 structure has not yet been experimentally determined, but we predict that it has a crystal structure quite similar to Na3 AlH6 , either in the P 21 /c or the F m3̄m space groups. As mentioned above, the P 21 /c structure is very similar to the F m3̄m structure for LiNa2 AlH6 ; the cell shape differs by 0.03◦ , the lattice constants by up to 7 pm, and the atomic positions by O. M. Løvvik et al.: Structure and stability of possible new alanates 611 30 Mixing enthalpy (kJ/mol) 20 ← Li3-xKx 10 ← Li3-xNax 0 -10 -20 Na3-xK x → -30 -40 -50 0 1 2 3 x Fig. 1 Fig. 2 Fig. 1 – The predicted crystal structure of LiNa2 AlH6 . The space group is P 21 /c, the lattice constants are 516, 525, and 733 pm, and the cell angles are 90.00, 90.03, and 90.00◦ . The AlH6 complexes are drawn as octahedra, the Li ions as light grey balls, and the Na ions as dark balls. Fig. 2 – The mixing enthalpy of the six bialkali alanates presently investigated in kJ/mol. A negative mixing enthalpy means that the bialkali alanate is stable compared to its two constituting monoalkali alanates. The compounds are in the form A3−x Bx AlH6 , where A = Li and B = Na (solid line), A = Na and B = K (dashed line) and A = Li and B = K (dotted line). up to 51 pm. The experimentally reported space group for LiNa2 AlH6 is F m3̄m [16], but the details of that structure has not yet to our knowledge been reported. Since our calculations are done at 0 K, temperature effects may easily change the relative stability of these phases —we are thus not able to distinguish between P 21 /c and F m3̄m for LiNa2 AlH6 . In conclusion, all the calculated structures show very good agreement with the available experimental data. We predict that four of the six studied bialkali alanates are thermodynamically stable with respect to their monoalkali precursors, but only one of them (LiNa2 AlH6 , see above) has been described in some detail before [1, 7, 16]. The most stable structure of both K2 LiAlH6 and K2 NaAlH6 is the cubic fcc structure similar to the experimental LiNa2 AlH6 structure. They are significantly more stable than the second most stable structure in both cases, so the F m3̄m structure is the most probable to observe experimentally. KLi2 AlH6 and KNa2 AlH6 , on the other hand, are most stable in the monoclinic P 21 space group (No. 4), i.e. a rather low symmetry. As discussed above, these structures are, however, not far from the cubic structure, neither from a structural point of view nor energetically. We are thus not able to state clearly that these compounds would synthesize in the P 21 space group; it may easily be that the F 4̄3 m structures are more stable at room temperature. 612 EUROPHYSICS LETTERS Table III – The optimized structures for the nine compounds in study, which are in the form A3−x Bx AlH6 . The space group, mass density ρm , the hydrogen volume density ρH V , and the hydrogen mass density ρH m of the most stable structure for each compound are listed. Also listed is the calculated mixing enthalpy Hm for the bialkali alanates. We have compared to experimental data where available. The P 21 /c structure of LiNa2 AlH6 is very close to F m3̄m (see the text for details). A3−x Bx Li3 Li3 expt.(a ) Na3 Na3 expt.(b ) K3 K2 Li K2 Na KLi2 KNa2 Li2 Na LiNa2 LiNa2 expt.(c ) Space group R3̄ R3̄ P 21 /c P 21 /c P 21 /c F m3̄m F m3̄m P 21 P 21 P 21 P 21 /c F m3̄m ρm (kg/m3 ) 1019 1012 1479 1475 1582 1636 1680 1338 1566 1270 1434 1405.99 ρH V (kg/m3 ) 57.2 56.8 43.9 43.7 31.8 41.9 37.9 47.0 40.1 54.9 50.5 49.46 ρH m (%) 5.61 5.61 2.96 2.96 2.01 2.56 2.25 3.52 2.56 4.33 3.52 3.52 Hm (kJ/mol) N/A N/A N/A N/A N/A −41.8 −49.3 20.8 −9.2 10.9 −10.9 (a ) Ref. [5]. (b ) Ref. [6]. (c ) Ref. [16]. To judge whether a bialkalimetallic alanate phase is thermodynamically stable compared to the two constituting monoalkali alanates, we have calculated the mixing enthalpy Hm , defined as Hm (A3−x Bx AlH6 ) = E(A3−x Bx AlH6 ) − 3−x x E(AAlH6 ) − E(BAlH6 ), 3 3 (1) where E(BAlH6 ) is the total energy of the compound BAlH6 , etc. If Hm is negative, the mixed alanate is stable compared to its constituents. This is valid at 0 K only, and temperature effects may influence our conclusions. Kinetic lattice effects may both enhance and hinder the formation of mixed phases, while entropy effects may further aid the thermodynamics of synthesis. The results are presented in table III and fig. 2, and we see that all the compounds but Li2 NaAlH6 and KLi2 AlH6 are stable. It is evident from fig. 2 that the mixed compounds with two heavy alkali atoms in the formula unit are more stable than those with only one. This is most pronounced for the Li-K system, and least for the Na-K system. It may be understood rather simplistically by regarding the ions as spheres: The large difference in ionic radius between the Li ion and the other alkali ions makes it difficult to create a stable stacking when most of the alkali atoms are Li. The bialkali alanates, apart from LiNa2 AlH6 that we predict to be stable, have, to our knowledge, not been described at all in the literature so far. We hope that our results can encourage new synthesis efforts on these mixed alanate phases. Conclusions. – Gradient-corrected density functional theory has been used to predict new stable crystal phases of binary alkali alanates in the form A3−x Bx AlH6 , where A and B are Li, Na, or K and x is 0, 1, 2, or 3. Seven different structures were used as starting points for the relaxation, and both cell shape and size were allowed to relax along with the atomic positions. This made it possible both to break and gain symmetry, and a large portion of the probable space of crystal structures has hence been searched through. This led to some of the compounds relaxing to almost the same structure from different starting points, which yields a measure of the uncertainty of the method. The cell parameters of the resulting degenerate O. M. Løvvik et al.: Structure and stability of possible new alanates 613 structures differed by up to 0.5◦ and 7 pm. The Cartesian coordinates differed by up to 51 pm, due to different tilting of the AlH6 octahedra. The results were compared to experimental results where available, and the agreement is excellent with most of the known data. The difference between the calculated results and the experimental data is negligible for most of the cases, and in all cases within the same order of magnitude as the methodological uncertainty quoted above. The calculated mixing enthalpy at 0 K of the bialkali alanates compared to the monoalkali alanates showed that four of the six studied bialkali phases are stable compared to the monoalkali phases: K2 LiAlH6 , K2 NaAlH6 , KNa2 AlH6 , and LiNa2 AlH6 . We propose that a systematic experimental search for these and related compounds is started, in order to clarify their real life stability at normal conditions. ∗∗∗ Stimulating discussions and valuable input from S. Opalka are gratefully acknowledged by OML. Furthermore, thanks are due to the Norwegian Research Council for economic support and generous grants of computational resources via the NOTUR project. REFERENCES [1] Bogdanovic B. and Schwickardi M., J. Alloys Comp., 253 (1997) 1. [2] Morioka H., Kakizaki K., Chung C. S. and Yamada A., J. Alloys Comp., 353 (2003) 310. [3] Chen J., Kuriyama N., Xu Q., Takeshita H. T. and Sakai T., J. Phys. Chem. B, 105 (2001) 11214. [4] Bogdanovic B., Felderhoff M., Germann M., Härtel M., Pommerin A., Schüth F., Weidenthaler C. and Zibrowius B., J. Alloys Comp., 350 (2003) 246. [5] Brinks H. W. and Hauback B. C., J. Alloys Comp., 354 (2003) 143. [6] Rönnebro E., Noreus D., Kadir K., Reiser A. and Bogdanovic B., J. Alloys Comp., 299 (2000) 101. [7] Zaluski L., Zaluska A. and Ström-Olsen J. O., J. Alloys Comp., 290 (1999) 71. [8] Løvvik O. M. and Opalka S. M., Phys. Rev. B, 69 (2004) 134117. [9] Vajeeston P., Ravindran P., Vidya R., Fjellvaag H. and Kjekshus A., Appl. Phys. Lett., 82 (2003) 2257. [10] Arroyo y de Dompablo M. E. and Ceder G., J. Alloys Comp., 364 (2004) 6. [11] Kresse G. and Hafner J., Phys. Rev. B, 47 (1993) 558. [12] Kresse G. and Furthmüller J., Phys. Rev. B, 54 (1996) 11169. [13] Kresse G. and Joubert D., Phys. Rev. B, 59 (1999) 1758. [14] Blöchl P., Phys. Rev. B, 50 (1994) 17953. [15] Perdew J. P., Chevary J. A., Vosko S. H., Jackson K. A., Pederson M. R., Singh D. J. and Fiolhais C., Phys. Rev. B, 46 (1992) 6671. [16] Claudy P., Bonnetot B., Bastide J.-P. and Létoffé J.-M., Mat. Res. Bull., 17 (1982) 1499.