letters to nature
advertisement

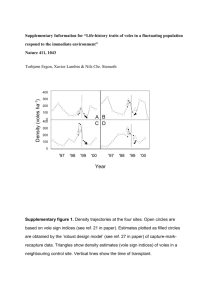
letters to nature Correspondence and requests for materials should be addressed to J.D.C. (e-mail: jcoates@micro.siu.edu). GenBank accession numbers for strains RCB and JJ are AY032610 and AY032611, respectively. ................................................................. Life-history traits of voles in a ¯uctuating population respond to the immediate environment D 21/20 20/10 16/15 C b 21 16/2 12/13 D A B 13/28 9/0 11/7 18/14 20 9/12 19 18 17 10 km B 300 200 A 100 50 10 A 300 200 100 50 A 16 A c A B C D Site A S S A S A S A S B A S S S A D 10 C 1997 Acknowledgements This work was supported in part by funding to J.D.C. by the US Of®ce of Naval Research. Funding to J.D.C. and L.A.A. was also from the US Department of Defense SERDP Program. C Body mass (g) a Density (voles ha–1) 20. Coates, J. D., Phillips, E. J. P., Lonergan, D. J., Jenter, H. & Lovley, D. R. Isolation of Geobacter species from a variety of sedimentary environments. Appl. Environ. Microbiol. 62, 1531±1536 (1996). 21. Vogel, T. M. & Grbic-Galic, D. Incorporation of oxygen from water into toluene and benzene during anaerobic fermentative transformation. Appl. Environ. Microbiol. 52, 200±202 (1986). 22. Rooney-Varga, J. N., Anderson, R. T. & Fraga, J. L. Microbial communities associated with anaerobic benzene degradation in a petroleum-contaminated aquifer. Appl. Environ. Microbiol. 65, 3056±3063 (1999). 23. Bruce, R. A., Achenbach, L. A. & Coates, J. D. Reduction of (per)chlorate by a novel organism isolated from a paper mill waste. Environ. Microbiol. 1, 319±331 (1999). 24. Coates, J. D. et al. The ubiquity and diversity of dissimilatory (per)chlorate-reducing bacteria. Appl. Environ. Microbiol. 65, 5234±5241 (1999). 25. Coates, J. D., Bruce, R. A., Patrick, J. A. & Achenbach, L. A. Hydrocarbon bioremediative potential of (per)chlorate-reducing bacteria. Bioremed. J. 3, 323±334 (1999). 26. Lovley, D. R., Fraga, J. L., Coates, J. D. & Blunt-Harris, E. L. Humics as an electron donor for anaerobic respiration. Environ. Microbiol. 1, 89±98 (1999). 27. Achenbach, L. A., Bruce, R. A., Michaelidou, U. & Coates, J. D. Dechloromonas agitata N. N. gen., sp. nov. and Dechlorosoma suillum N. N. gen., sp. nov. two novel environmentally dominant (per)chlorate-reducing bacteria and their phylogenetic position. Int. J. Syst. Evol. Microbiol. 51, 527±533 (2001). 28. Achenbach, L. A. & Coates, J. D. Disparity between bacterial phylogeny and physiology. ASM News 66, 714±716 (2000). 29. Anderson, R. T. & Lovley, D. R. Anaerobic bioremediation of benzene under sulfate-reducing conditions in a petroleum contaminated aquifer. Environ. Sci. Technol. 34, 2261±2266 (2000). 30. Coates, J. D., Bruce, R. A. & Haddock, J. D. Anoxic bioremediation of hydrocarbons. Nature 396, 730 (1998). 1998 1999 1997 1998 1999 Year Figure 1 Design of the study. a, Groups of voles were swapped between half-sides of four 1-ha unfenced trapping grids in early winter (November/December). Arrows, pairs of transplanted groups. A second transplant was done between two of the sites (B and D) in February because few voles settled in some groups. Numbers in the boxes show number of males/females that were successfully transplanted between grids and captured at least once during the spring. b, Average body mass (6s.e.) at the time of transplant in November/December among animals that had not previously bred. Open circles, females; ®lled circles, males. c, Density trajectories at the four sites plotted on a log10 scale. Density estimates denoted by S (spring) and A (autumn) are based on vole sign indices21. Estimates plotted as ®lled circles are obtained by the robust design model27 of capture± mark±recapture data. Vertical lines show the time of transplant. Density trajectories in neighbouring control areas showed similar patterns (see Supplementary Information). Torbjùrn Ergon*, Xavier Lambin² & Nils Chr. Stenseth* .............................................................................................................................................. Life-history traits relating to growth and reproduction vary greatly among species and populations1,2 and among individuals within populations3. In vole populations, body size and age at maturation may vary considerably among locations and among years within the same location4±8. Individuals in increasing populations are typically larger and start reproduction earlier in the spring than those in declining populations6±8. The cause of such life-history variation within populations has been subject of much discussion7,9,10. Much of the controversy concerns whether the memory of past conditions, leading to delayed effects on lifehistory traits, resides in the environment (for example, predators11,12, pathogens13 or food14,15) or intrinsically within populations or individuals (age distribution16,17, physiological state3, genetic18 or maternal effects19,20). Here we report from an extensive ®eld transplant experiment in which voles were moved before the breeding season between sites that differed in average overwintering body mass. Transplanted voles did not retain the characteristics of their source population, and we demonstrate an over-riding role of the immediate environment in shaping lifehistory traits of small rodents. NATURE | VOL 411 | 28 JUNE 2001 | www.nature.com Change in body mass of transplants (g) Females * Division of Zoology, Department of Biology, University of Oslo, PO Box 1050 Blindern, 0316 Oslo, Norway ² Department of Zoology, University of Aberdeen, Tillydrone Avenue, Aberdeen AB24 2TZ, UK Males 5 CtoD AtoD 4 AtoB BtoD 3 DtoB DtoC AtoD 2 CtoB CtoD CtoB DtoB 1 0 DtoC BtoC BtoD BtoA DtoA BtoA BtoC DtoA –1 –2 –1 0 1 2 3 –1 0 1 2 3 4 5 6 Site difference in body mass (g) Figure 2 Average (6s.e.) individual change in body mass of transplanted voles recaptured in January/February during the ®rst session after transplanting plotted against difference in average body mass at the sites. The site differences in body mass are calculated as average body mass in the target population during the ®rst trapping session after the transplant minus average body mass of transplants at capture during the transplantation. Voles in the transplant group are excluded from the target population when calculating the site differences. Hence, measurements are independent. Line is the unity slope through the origin, indicating perfect convergence. Plotted labels denote `source-to-target' transplant groups. See Supplementary Information. © 2001 Macmillan Magazines Ltd 1043 letters to nature Females Within A 40 source × session ** Males From A site × t NS Within A source × session NS From A site × t *** From B site × t2*** Within B source × session * From B site × t2*** Within C From C site × t * site × t2** Within C source × session NS From C site × t * Within D From D site × t *** site × t2*** Within D source × session NS From D site × t ** Body mass (g) mean ± 2s.e. 30 20 Within B 40 source × 30 session NS 20 40 source × session NS 30 20 40 source × session NS 30 20 Feb. Mar. Apr. May Feb. Mar. Apr. May Feb. Mar. Apr. May Feb. Mar. Apr. May Date This ®eld experiment was carried out in the Kielder Forest region, northern England, where ¯uctuating populations of ®eld voles (Microtus agrestis L.) inhabit distinct `clear-cuts' showing asynchronous dynamics within the region21,22. We successfully transplanted 266 voles between four clear-cuts in the forest system (Fig. 1a). At the time of transplant, average body mass at the site with the heaviest voles was 18% greater than at the site with the smallest voles (Fig. 1b). Although an estimated 98% of residents were removed before the release of the transplanted voles, 281 non-transplanted voles (mostly immigrants) were recorded in the ®rst trapping session 2±3 months after the transplant (in January and February). A further 287 overwintering voles entered the trapping grids at some time during the spring. We used capture±recapture data collected at two-week intervals throughout the spring to investigate differences in patterns of body mass development and onset of maturation between the different groups of voles. Trapping two to three months after the transplant showed that individual body mass during midwinter changed towards the mean body mass at the site to which the voles were moved (Fig. 2). Voles in the sites with the highest midwinter body mass also grew faster and reached higher body mass by the end of the study than those in the sites with low midwinter body mass (Fig. 3). Individuals originating from different sites before the transplant but living in the same site showed no clear differences in body mass trajectories through the spring. However, there was large variation in individual growth rates between sites during the spring: voles originating from the same site but transplanted to different sites showed up to 60% higher body mass in one site than at another at the same dates. Hence, individuals adjusted their midwinter body size and spring growth rate according to their immediate environment. The change in proportion of matured animals in different groups through the spring reveals a difference of around three weeks in onset of spring reproduction between sites (Fig. 4). There was no signi®cant effect of source population on timing of maturation, but 1044 Within/from D 2 effects of both time (t ) and t were included. This gave homogenous residuals that appeared normally distributed. A categorical time variable (trapping session) was used when growth patterns within sites were compared. Because there are missing combinations of `site' and `source', the population effects were examined with separate analysis for each group of `site' and `source'. Asterisks represent signi®cance levels of the interaction effect of population and time (triple asterisk; P , 0.001, double asterisk; 0.001 , P , 0.01, single asterisk, 0.01 , P , 0.05). NS, not selected by the lowest AIC. Effects of whether the voles had been transplanted or immigrated to the sites were not signi®cant at the 10% level. 1.0 Proportion perforate Figure 3 Average body mass (62s.e.) over the spring after the transplant. Each line represents a group of animals that were living in the same site and were coming from the same site before transplant. Left column, time trends are grouped according to the site the animals were living in; right column, the same time trends are grouped according to site of origin. The data were analysed with a repeated-measures analysis with individuals as random subjects (SAS Proc MIXED28). An unstructured covariance matrix (UN(3)28) was selected by the lowest AIC. When studying the `source' effect, a continuous time variable (t, number of days since the ®rst trapping session) was used because sites were not trapped on the same dates. To account for the exponential pattern in the growth curves, Within/from C D C 0.5 B A 0 1.0 Proportion lactating Within/from B 0.5 0 1.0 Proportion scrotal Within/from A 0.5 0 1 March 1 April Date 1 May Figure 4 Onset of spring reproduction measured as proportions of perforate and lactating females (top and middle panels) and proportion of males with scrotal testes (bottom panel). The Figure shows ®ts from the logistic regression models (see Methods and Table 1). Effects of whether the voles were transplanted or immigrated and effects of source population were not signi®cant (see Table 1) and hence not accounted for. the site effect was highly signi®cant (Table 1). There was a one-toone correspondence of the sites ranked according to onset of spring reproduction and ranks of average overwinter body mass and spring growth. Reproduction started earlier in the sites with higher body mass. The onset of spring reproduction may contribute profoundly to the variation in population growth rate, and is generally © 2001 Macmillan Magazines Ltd NATURE | VOL 411 | 28 JUNE 2001 | www.nature.com letters to nature Table 1 Test statistics for logistic regression of proportion of matured voles in the trapping sessions Sex (no. individuals) Response Factor F d.f.* P Deviance of model ................................................................................................................................................................................................................................................................................................................................................................... Females (n = 121) Proportion with perforate vagina Proportion lactating Males (n = 145) Proportion with scrotal testis Site Source Site ´ date Source ´ date Site Source -6 11.31 3,113 1.5 ´ 10 1.36 2.76 1.11 10.03 3,113 3,107 3,107 3,137 0.26 0.046² 0.35 5.1 ´ 10-6 0.70 3,137 0.55 107.2 84.7 111.2 ................................................................................................................................................................................................................................................................................................................................................................... All models include a `date' effect. Models with either parallel or different slopes (`group ´ date' interactions) are chosen on the basis of the models' AIC26. Because of imbalanced replication, `site' is tested assuming an effect of `source' and vice versa. Only voles that have been transplanted (that is, no immigrants) are included in the analysis. *Degrees of freedom re¯ect number of individuals, not observations (see Methods). ² Main effects in a parallel slopes model: site, F3,113 = 12.3, P = 5.0 ´ 10-7; source, F3,113 = 0.61, P = 0.6; deviance, 101.0. described as varying consistently between the phases in rodent ¯uctuations5,7. Even though the variation in life-history traits documented here is correlated with the variation in population growth rate in the spring, there is no indication that this variation re¯ects present or past densities (Fig. 1c). Our results show strong plasticity in life-history traits of voles. Individuals do not perform according to past environmental conditions. Instead, the environmental conditions during the winter and spring directly give rise to large differences in body mass and timing of maturation between sites. Thus, there is no need to invoke intrinsic mechanisms16,17,23 to explain the conspicuous variation in M these life-history traits within vole populations. Methods Transplanting voles The transplant took place in November/December, when the voles were non-reproductive. Each 1-ha trapping grid was divided into two sides, and voles were swapped between halfsides of two different grids at a time (Fig. 1). Trapping continued for 5 d until an estimated 98% of the resident animals were captured. Voles were transferred between sites in wooden boxes containing hay, sawdust, carrots and whole wheat (two individuals in each box). After the last day of trapping, the boxes were opened but left in the ®eld for at least one week. In total, 761 individually marked animals were swapped between the four locations. Of these, 266 individuals were recaptured during the following spring. Although voles that had not previously reproduced (91% of the transplanted voles) had a higher probability of being recaptured after transplant (odds ratio = 1.4, x1 = 4.84, P = 0.03), re¯ecting similar effects on natural mortality, there was no signi®cant difference in the effects of body mass and reproductive history on transplant success between the groups de®ned by `target population', `source population' and `target ´ source group' (all interactions tested in logistic regression models selected by the lowest Akaike Information Criterion26 (AIC); all P . 0.1). Thus, there is no reason to believe that differential transplant success will substantially bias our results. A detailed presentation on the analysis of transplant success is available as Supplementary Information. Population monitoring The populations at the four sites were monitored by capture±mark±recapture methods24,25. At each site, 196 traps at 7-m intervals were pre-baited with carrots 2 d before the traps were set (baited with carrots and ¯aked barley). Food was removed from the traps after each session. The ®rst trapping session after the transplant took place in January/ February. Each site was thereafter visited every two weeks (except for the ®rst interval; which varied in length between sites). In the autumn before the transplant, traps were checked every 6 h and not left set overnight. After the transplant, traps were set in the evening and checked at sunrise and sunset for the three consecutive sessions. Comparing onset of reproduction At each capture occasion, we recorded whether females had perforated vagina and whether they were lactating. Males were classi®ed as mature when they had scrotal testes with visible caput epididymis. Effects on the timing of maturation were analysed by logistic regression of the proportion of matured animals on sampling date (recapture probability was ,0.9 and not signi®cantly different between sites or source populations). As each individual will necessarily be recorded as matured on every subsequent capture after having reached maturity, the saturated model (zero deviance) is a model with individual speci®c intercepts and a common slope. Hence, we calculated the test statistic as F = ((DevH0 - DevHA)/d.f.1)/(DevHA/d.f.2) with d.f.1 being the difference in number of parameters in the two models (H0 and HA) and d.f.2 being the number of individuals minus the number of parameters in HA, and compared this with the Fisher distribution with d.f.1 and d.f.2 degrees of freedom. Received 12 December 2000; accepted 3 April 2001. 1. Stearns, S. C. The Evolution of Life Histories (Oxford Univ. Press, Oxford, 1992). 2. Roff, D. A. The Evolution of Life Histories; Theory and Analysis (Chapman & Hall, New York, 1992). NATURE | VOL 411 | 28 JUNE 2001 | www.nature.com 3. McNamara, J. M. & Houston, A. I. State-dependent life histories. Nature 380, 215±221 (1996). 4. Agrell, J., Erlinge, S., Nelson, J. & Sandell, M. Body weight and population dynamics: cyclic demography in a noncyclic population of the ®eld vole (Microtus agrestis). Can. J. Zool. 70, 494±501 (1992). 5. Taitt, M. J. & Krebs, C. J. in Biology of New World Microtus (ed. Tamarin, R. H.) 567±620 (American Soc. Mammalogists Special Publications No. 8, Shippensburg, 1985). 6. Chitty, D. Mortality among voles (Microtus agrestis) at Lake Vyrnwy, Montgomeryshire, in 1936±9. Phil. Trans. R. Soc. Lond. B 236, 505±552 (1952). 7. Krebs, C. J. & Myers, J. H. Population cycles in small mammals. Adv. Ecol. Res. 8, 267±399 (1974). 8. Tast, J. in Winter Ecology of Small Mammals (ed. Merritt, J. F.) 59±66 (Carnegie Museun of Natural History, Pittsburg, 1984). 9. Krebs, C. J. Population cycles revisited. J. Mamm. 77, 8±24 (1996). 10. Stenseth, N. C. Population cycles in voles and lemmings: density dependence and phase dependence in a stochastic world. Oikos 87, 427±461 (1999). 11. KorpimaÈki, E. & Krebs, C. J. Predation and population cycles of small mammals. BioScience 46, 754± 764 (1996). 12. Mappes, T., Koskela, E. & YloÈnen, H. Breeding suppression in voles under predation risk of small mustelids: laboratory or methodological artifact? Oikos 82, 365±369 (1998). 13. Soveri, T. et al. Disease patterns in ®eld and bank vole populations during a cyclic decline in central Finland. Comp. Immunol. Microbiol. Infect. Dis. 23, 73±89 (2000). 14. Batzli, G. O. in Wildlife 2001: Populations (eds McCullough, D. R. & Barrett, R. H.) (Elsevier Applied Sciences, London, 1992). 15. Agrell, J., Erlinge, S., Nelson, J., Nilsson, C. & Persson, I. Delayed density-dependence in a smallrodent population. Proc. R. Soc. Lond. B 262, 65±70 (1995). 16. Boonstra, R. Population cycles in microtines: the senescence hypothesis. Evol. Ecol. 8, 196±219 (1994). 17. Tkadlec, E. & Zejda, J. Small rodent population ¯uctuations: The effects of age structure and seasonality. Evol. Ecol. 12, 191±210 (1998). 18. Chitty, D. The natural selection of self-regulatory behaviour in animal populations. Proc. Ecol. Soc. Aus. 2, 51±78 (1967). 19. Boonstra, R. & Boag, P. T. A test of the Chitty hypothesis: inheritance of life-history traits in meadow voles Microtus pennsylvanicus. Evolution 41, 929±947 (1987). 20. Inchausti, P. & Ginzburg, L. R. Small mammal cycles in northern Europe: patterns and evidence for maternal effect hypothesis. J. Anim. Ecol. 67, 180±194 (1998). 21. Lambin, X., Petty, S. J. & MacKinnon, J. L. Cyclic dynamics in ®eld vole populations and generalist predation. J. Anim. Ecol. 69, 106±118 (2000). 22. Lambin, X., Elston, D., Petty, S. & MacKinnon, J. Spatial patterns and periodic travelling waves in cyclic ®eld vole, Microtus agrestis, populations. Proc. R. Soc. Lond. B 265, 1491±1496 (1998). 23. Nelson, R. J. Photoperiod-nonresponsive morphs: a possible variable in microtine population-density ¯uctuations. Am. Nat. 130, 350±369 (1987). 24. Nichols, J. D. Capture recapture models; using marked animals to study population dynamics. BioScience 42, 94±102 (1992). 25. Lebreton, J.-D., Burnham, K. P., Clobert, J. & Anderson, D. R. Modeling survival and testing biological hypotheses using marked animals: a uni®ed approach with case studies. Ecol. Monogr. 62, 67±118 (1992). 26. Burnham, K. P. & Anderson, D. R. Model Selection and Inference: a Practical Information-Theoretic Approach (Springer, New York, 1998). 27. Kendall, W. L., Pollock, K. H. & Brownie, C. A likelihood-based approach to capture-recapture estimation of demographic parameters under the robust design. Biometrics 51, 293±308 (1995). 28. Littell, R. C., Milliken, G. A., Stroup, W. W. & Wol®nger, R. D. SAS Systems for Mixed Models (SAS Institute, Cary, North Carolina, 1996). Supplementary information is available on Nature's World-Wide Web site (http://www.nature.com) or as paper copy from the London editorial of®ce of Nature. Acknowledgements The project was funded by grants from the Norwegian Research Council to T.E. and by the Natural Environment Research Council (UK) to X.L. We thank the Forestry Commission for support; C. Grif®n and J. Aars for assistance during the transplant; and R. Boonstra, L. Crespin, K. E. Hodges, R. A. Ims, R. Julliard, T. Klemola, P. Thompson, E. Tkadlec, H. Viljugrein and N. G. Yoccoz for constructive comments and advice. Correspondence and requests for materials should be addressed to N.C.S (e-mail: n.c.stenseth@bio.uio.no) and X.L. (e-mail: x.lambin@abdn.ac.uk). © 2001 Macmillan Magazines Ltd 1045