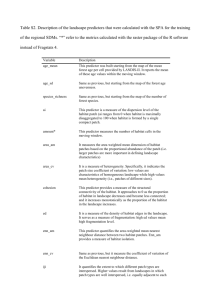
Dag Øystein Hjermann Decticus verrucivorus
advertisement

Dag Øystein Hjermann Spatial ecology of the bush-cricket Decticus verrucivorus: Movement, metapopulation dynamics and genetic variation in patchy and linear habitats Dr. scient. thesis Division of Zoology, Department of Biology, Faculty of Mathematics and Natural Sciences University of Oslo, 2000 List of papers Paper I: Hjermann, D. Ø. Do bush-crickets avoid the habitat edge? Microhabitat selection in the wart-biter (Decticus verrucivorus). Manuscript. Paper II: Hjermann, D. Ø. 2000. Analyzing habitat selection in animals without welldefined home ranges. Ecology 81: 1462-1468. Paper III: Hjermann, D. Ø. The condition of individual bush-crickets influence their spatial distribution. Manuscript. Paper IV: Hjermann, D. Ø. Why does emigration increase with decreasing patch size? An experimental test of the boundary encounter rate hypothesis. Manuscript. Paper V: Hjermann, D. Ø. and Ims, R. A. 1996. Landscape ecology of the wart-biter Decticus verrucivorus in a patchy landscape. Journal of Animal Ecology 65: 768780. Paper VI: Hjermann, D. Ø., Ims R. A. and Bondrup-Nielsen, S. Patterns and processes underlying population genetic structure in anthropogenic linear habitats: Four hypothetical scenarios and one case study. Manuscript. Paper V is reprinted by courtesy of Blackwell Scientific Publications Contents Overview and synthesis Preface 1 Scope of the thesis 3 Scaling of processes and patterns in ecology and metapopulation ecology 5 Study species and study areas 6 The wart-biter 6 Meadows - the wart-biter's natural habitat in Norway 8 The study areas 9 Movement behaviour - a "missing link" in metapopulation ecology 11 Use of area depending on habitat size 12 Use of area depending on habitat shape 15 Deciding to emigrate 16 Statistical problems in the analysis of movement behaviour 18 Interpreting occupancy patterns 19 The wart-biters in the Frogn study area 19 Metapopulation models - the good, the bad and the ugly 21 Estimating dispersal parameters from patch occupancy patterns 24 Interpreting genetic variation: invasion of new landscape elements 25 References 27 Individual papers Overview and synthesis Preface When it was time to plan my Master thesis in 1991, I found out that this Ims guy seemed quite all right to me. I mustered enough courage to knock on his office door to ask if he was willing to supervise me. He took the time to talk with me there and then (I found out later that he always does). I only knew he worked with small mammals, so I was a bit surprised when he asked if I wanted to work with wart-biters. - Wart-biters? I replied. He explained that it was a quite large bush-cricket. – Well, I knew that, I replied. (I didn't.) I went out of his office with a supervisor and a theme for my Master thesis. I had no idea (fortunately!) that I would be defending a thesis on the wart-biter nine years later. First, Rolf deserves a big THANK YOU. Rolf is the supervisor everybody should have. On the professional level, he has original research ideas, plenty of knowledge of natural history as well as ecological theory, and a lot of statistical expertise. On the personal level, he has been a pleasure to have as a supervisor. He has never had too little time to talk when I've knocked on his door; he has always given enthusiastic response on my own ideas; he even did quite much of the "farming" work for the Evenstad papers (III and IV). Rolf is simply a very nice and unselfish person that is almost unable to tell people "no". (He spends at least half of the year in the field instead.) I would also like to thank Søren. The reader should know that he and Rolf did far more field work than myself for Paper VI, and crawling along the road verges of highway with heavy traffic isn't that pleasant. Søren and Halvor Knutsen also spent much time in the allozyme lab for this paper, and I thank them for being my patient lab teachers. Nigel Yoccoz has learnt me a lot about statistics - and wart-biters - and he collected the French sample in paper VI. Then there is a bunch of other people to thank. First, my mother and father learned me to appreciate nature, both the mountains around Lærdal and the sea in Vestfold. Being the youngest in a big family, my brothers and sisters are also greatly responsible for my interest in nature and nature’s mysteries - especially Rudi, who dragged me up in the mountains from the time I was old enough to walk. (He even was in the local bird-watcher’s society, although I have a vague idea that this society also had a strong interest for “birds” with two legs and mammae). The time as an undergraduate student of biology was a fantastic time with discussions, private and organized field excursions, and countless parties. For me, this time 1 Overview and synthesis was proof that common introductory biology courses for both ecologists and molecular biologists (such as “Bio101”) is a necessary part of the biology education, both socially and scientifically. I can’t avoid mentioning my “mates” Jostein and John, but I don’t have enough space to mention the rest – you know who you are! During field work, I have enjoyed working and living together with a lot of nice people including Live, Inger, Anne, Wendy, Naomi, Harry and Gry. During the last years, when most old student friends had dispersed into computer and teacher jobs, fellow entomologists Steve and Guldborg formed much of of my social life at work. I also spent many enjoyable hours together with Jonathan working (not always so intensely) with his reindeer data; thanks for the cooperation! A considerable amount of time was spent in e-mail discussions with Yngve, Trygve, Trond-Inge, Thomas and the rest of the members of the intellectual forum "Søplegjengen", an activity which undoubtedly has enhanced my linguistic and rhetoric capabilities. Finally, I whole-heartedly thank Nils Christian Stenseth for supporting me during all these years, including giving me small jobs during one year of unemployment (after my Master) and, not least, offering me to work on his marine ecology group as a post doc. I also thank Jogeir Stokland who gave me the opportunity to work at NIJOS, which has been an interesting and rewarding experience and a valuable insight into “the world outside Blindern”. I also thank the Norwegian Research Council for supplying a million kroner or so for my doctorate and the Nansen Foundation for financial support for Paper VI. Finally, I am deeply thankful to my wife Irene. When we started on our dr. theses just a few months apart, I was a bit worried about how we would handle the stress of writing dissertations simultanously. Now, we have produced not only two (almost) finished theses, but also our beautiful daughter Inga. Because of you and your unique way of keeping our relationship strong, it has actually been four very nice years. I thank you for sharing your life with me. Oslo, November 2000 Dag Ø. Hjermann 2 Overview and synthesis Overview and synthesis Scope of the thesis This thesis deals with spatial ecology, using a bush-cricket, the wart-biter Decticus verrucivorus, as a study species. The papers do not tell a comprehensive "story" but are somewhat loosely knitted together around some key words. First, spatial variation is the lowest common denominator of all the papers. This thesis covers spatial variation on scales varying by five orders of magnitude, from movement behavior on a 5-10 meter scale to genetic variation on 50 km-scale (the papers are arranged according to spatial scale; Tab. 1). Movement behaviour is the focus of four papers (I- IV) and a fundamental part in the underlying processes of the last two. Finally, since the wart-biter is patchily distributed, metapopulation theory (Hanski & Gilpin 1997) is the ground foundation for much of my thinking and the conceptual framework for papers IV and V. The core of this theory is a drastic but useful simplification of the real world: the multitude of processes that governs the spatial distribution of a patchily distributed species can be reduced to merely two: local extinctions and recolonizations. First, in Paper I and III, I analyse movements within small habitat patches1. Within- Table 1. Short overview of the papers in this thesis. Scale of process Paper Process / pattern Spatial Temporal I Movement within natural habitat patches < 15 m 1-2 h - 3 weeks II Movement within patches III Movement within experimental patches < 15 m 1-2 h - 3 weeks IV Movement between experimental patches 15 - 300 m 1-2 h - 3 weeks V Occupancy, extinction and immigration of patches 50 - 6000 m 1-3 years VI 1 In thisand thesis, I mean exactly the habitats same by "patch" "habitat island": an area of Invasion genetic variation of linear 0.2 -and 55 km 5-20 years Statistical methodology suitable habitat (for a given species) surrounded by unsuitable habitat, usually large enough to contain one population and so far from other patches that movements between patches does not affect local population dynamics substantially. In other words, I always mean coarsegrained patchiness when I use the word "patch". 3 Overview and synthesis patch movement may ultimately influence both extinction and colonization rates. Paper II is a methodological paper where I describe the statistical method I have used in the analysis of Paper I. In Paper IV, I study emigration and movement between patches and the mechanisms that leads to emigration. In Paper V, the pattern of occupancy in an agricultural area is analysed from a metapopulation perspective. The final paper is about the effect of colonization history on population genetic patterns. It differs somewhat from the other papers, both in its focus on larger spatial and temporal scales and in its focus on genetics. Although not often explicitely mentioned, conservation biology is at the root of this thesis. The destruction, degradation and fragmentation of natural and semi-natural habitats, including the meadow habitats studied in this thesis, makes metapopulation and landscape ecology more than just academic disciplines. Many species are forced to try to survive in habitat patches that are very small and isolated from each other compared to the landscape in which they evolved. Theory often suggests that when habitat destruction goes beyond a critical threshold, the landscape becomes unsuitable for an accelerating number of species (e.g., Andrén 1994, 1999; Bascompte and Sole 1996). As there may be a considerable time lag from a species is doomed to extinction (because of habitat destruction) and the extinction itself (the "extinction debt"; Tilman et al. 1994; Pimm et al. 1995; Loehle and Li 1996; Cowlishaw 1999), we do not know how many species that "are waiting for their turn" at present. Metapopulation and landscape ecology may help discovering and understanding the threats to biodiversity before it is too late. 4 Overview and synthesis Scaling of processes and patterns in ecology and metapopulation ecology In metapopulation biology, processes are typically divided in two types based on scale: processes within each population, and processes between populations (Tab. 2). However, one should not forget that this is a simplification. First, the definition of population is, to some extent, arbitrary, and must be defined on basis of the spatial scale of some process (Addicott et al. 1987). E.g., we can define a population as the spatial area within which 95 % of matings occur between animals that both were born inside that area. However, a definition of the population that is suitable for population genetics is not necessarily suitable for population dynamics and vice versa. Moreover, the scale of each process vary between years and between different landscapes (e.g., depending on the degree of fragmentation). Thus, the framework of Tab. 2 can be extremely difficult to apply in practice. Table 2. Processes and patterns in a metapopulation perspective. Hierarchal level Processes Birth/death of individuals (depends on Populations (most individuals interact intra- and interspecific competiton, with other individuals in the predation etc.) same population) Immigration Patterns Local population size Distribution of individuals within patch Local movement (including emigration) Metapopulations (most individuals mate within populations; interactions between populations not large enough to influence local dymanics) Local extinctions (stochastic or deterministic) Collections of metapopulations (practically all individuals mate within metapopulations; interactions between metapopulations not large enough to influence metapopulation dynamics) Invasions due to spread Recolonization of empty patches Invasions/exclusions due to altered largescale conditions (e.g. climate change) Exclusions due to stochastic global extinction of metapopulations Distribution of absence/presence on habitat islands (depends on area, isolation and patch quality) Distribution pattern, differences in density among regions Geographic patterns of genotype frequencies 5 Overview and synthesis In some cases, however, populations are quite easy to identify and it is easy to classify processes as within-patch and between-patch. A typical example is animals that live in discrete, well-defined patches with room for one population and sufficiently remote from each other (compared to the species' dispersal capacities) that we can assume the populations to behave independently of each other. When metapopulation theory and other theories including space emerged, ecologists naturally looked for such systems to test the predictions of the theory. Wiens (1995) has pointed out that before space was built into ecological theory, ecologists tended to seek homogenous study areas to test theories of population dynamics, predator-prey systems, competition and so on. To a large degree, the same thing has happened in the flourishing growth of empirical metapopulation studies in the 1990s, only that the "ideal" of the completely homogenous study area was replaced by another "ideal", collections of quite small, sharply defined, homogenous areas. The wart-biter systems I have studied in Papers I-V are good examples of such systems, either naturally occuring (as in Paper I and V) or artificial (as in Paper III and IV). There is nothing wrong with seeking relatively simple systems where it is practically possible to test certain theories. However, it should be kept in mind that many, or most, species live in patches that vary continuously in quality, i.e., sourcesink systems (Pulliam 1988). Thomas & Kunin (1999) have proposed a framework that is able to incorporate source-sink-dynamics in metapopulation structures. To complicated matters even further, patches need not be stable in time (e.g., Stelter 1997), patches need not have well-defined edges, and patch quality can vary a lot in time, so that former sources can become sinks and vice versa (Boughton 1999). Study species and study areas The wart-biter The wart-biter (Decticus verrucivorus (Linneaeus)) is one of the largest large bushcrickets (Ortoptera: Tettigoniidae) of Northern Europe (body length: 24-38 mm for females, 26-44 mm for males; Harz 1960). It is a continental species that prefer hot summers; it is widespread in large parts of Eurasia, including most of Central and Southern Europe and parts of Iran, Pakistan and China (Samways and Harz 1982). In England, there are only a few populations in the extreme south. In Norway, it is found along the coast from the Swedish border to Rogaland and in some parts of the lowlands of eastern Norway (such as southern Telemark and southern Østerdal). 6 Overview and synthesis The wart-biter is winged, but unprovoked flight is extremely uncommon; Ander (1947) reported to have seen wart-biters flying longer distances (at least 20-30 m) twice during a period of 17 years. It sometimes alights when disturbed, but then usually flies only 23 meters, infrequently up to 20 m in windy weather (personal observation). Some individuals also use the wings to enhance jumping when it travels through non-habitat with little or no vegetation (personal observation). It lives on the ground in open habitats with quite low vegetation; I will discuss its habitat requirements in depth below. It is possible to rear wart-biters on purely plant food (Ragge 1965), but in nature they are partly carnivorous; adults eat 25-70 % animal food such as acridid grasshoppers and other insects (Nagy 1950, Harz 1960, Bellman 1985, Cherrill 1989, Thorens and Nadig 1997). Because adults in some habitats need to hide in tussocks to avoid predators, this may decrease their opportunities to hunt (Cherrill 1989). The wart-biter hatches in April/May and goes through seven instars (Ingrisch 1978a; Holst 1986). In Norway, the first adults appear in late June to late July, depending on weather conditions in spring and early summer. Probably because of the competition for virgin females, males become adult a couple of weeks before the females (Wedell 1992). Adult males stridulate (sing) to attract females, often climbing up on high vegetation to make the sound reach further. It needs a body temperature of 23-25 C to sing (Nielsen 1938), i.e., almost only in sunshine in Norway, and they sing mainly in the morning (9-12 a.m.) and to a lesser degree in the afternoon (around 3 p.m.). Females can approach singing males and vice versa, and each sex may turn down sexual invitations from individuals of the other sex, at least for the moment. However, it is more common thaT females tun down males than vice versa (personal observation). During mating, the male transfer a spermatophore which is attached externally to the female's genitalia. The spermatophore is quite large (9-10 % of the males' weight), it consists of an large sperm-free spermatophylax that the female eats after mating, and an ampulla with sperm that she eats after the spermatophylax (Wedell & Arak 1989). It has been suggested that the spermatophylax is a parental investment in bushcrickets, but in the wart-biter, it has low protein concentration (4.2 %), and does not increase female fecundity (Wedell & Arak 1988). Rather, it appears to be a mating investment evolved by sperm competion with other males. If the spermatophylax is large, more sperm is released from the sperm ampulla because it takes longer time before it is eaten (Wedell 1991). 7 Overview and synthesis The abundance of wart-biters has declined in Northern Europe the last decades (Ingrisch 1979; Holst 1986; Bengt Ehnström, personal communication). The reason for this is changes in landscapes and agricultural practices that makes unfertilized meadows and grasslands less common (see next chapter). The wart-biter is regarded as one of England's most acutely threatened insect species, and there is considerable effort to save it from extinction, including a captive breeding and reintroduction program (Cunningham et al. 1997). Meadows - the wartbiter's natural habitat in Norway The wart-biter lives on the ground in open habitats with quite low vegetation. The vegetation must not be too tall (at most 30-40 cm in my own experience). In Norway, various types of unfertilized meadows and grassland are suitable habitats (personal observation). The nitrophilous vegetation in fertilized meadows is too dense and tall for the species. However, it is also absent from meadows that are cut very short (Weidemann et al. 1990) or intensively grazed by, e.g., horses (personal observation). South-facing or flat habitats are preferred (Oschmann 1973; Ingrisch 1979; Cherril and Brown 1990a; Paper V). Probably, the reason for these habitat requirements is the wart-biter's temperature requirements. The species have extremely high optimum temperatures: instar development and survival is optimal at around 35 C, and not possible below 20 C, while the temperature optimum for adults is 36-40 C (Ingrisch 1978a,b). They lay their eggs in the ground in places with little or no vegetation where the soil heats up quickly in spring (dry, fine-grained substrate is preferred). Instars and adults spend much time basking in the sun to increase body temperature, positioned so that the side of the body is perpendicular to the sun's direction (personal observation). In Norway, the wart-biter appears to be a follower of agriculture, which has a 5000 Table 3. For some groups, the proportion of species on the Norwegian Red List that have the cultural landscape and meadows, respectively, as their main habitat. “Meadows” include "natural" unfertilized meadows for grazing or harvesting, and dry meadows. The cultural landscape is a wider term that in addition to meadows includes trees in the agricultural landscape, fields, stone walls, farmyards etc. The data are assembled from DN (1999a, 1999b). Red-listed species Taxon No. of species Cultural landscape % (no. of species) Total in Norway Meadows Approx. % No. of species Fungi 763 25 (189 ) 14 6000 Mosses and liverworts 216 25 (54) 20 1064 Vascular plants 255 37 (94) 32 1775 True bugs 82 38 (31) 38 445 Beetles 778 18 (141) 18 3430 Butterflies and moths 540 58 (312) 33 2111 8 Overview and synthesis year history in our country. Its primary habitat type in Scandinavia, unfertilized meadows, has been in decline for many years. The meadows are fertilised and cultivated to increase grass yields, encroached by bushes because of lack of grazing/cutting, and turned into forest, corn fields and housing estates. In Sweden, the area of unfertilized meadows for grazing was reduced by 85 % from 1850 to 1966, while the area of traditionally managed meadows for cutting has been reduced by 99.9 % (Edelstam 1995). There have been a similar trend in Norway. With the decline in unfertilized meadows, a significant part of the biological diversity is also in decline. In Norway, 874 red-listed species (28.5 % of the Norwegian Red List) are associated with the cultural landscape (DN 1999b). Many of those have meadows as their primary habitat (Tab. 3). Also, around 70 species of birds are in decline because of changes in the cultural landscape (DN 1994). The study areas We studied the natural distribution of wart-biters in two systems: in scattered meadows in an agricultural area, i.e., the "traditional" wart-biter habitat, and in roadsides along a major highway, which may be the most imortant habitat for this speciesin the future. In addition, we used an experimental area where wart-biters originally were absent. The first location for our work with the wart-biter was an agricultural landscape in Frogn, ca. 40 km from Oslo in the direction of Gothenburg (Paper I, V; see map in Paper V). This area (ca. 6 km2) is dominated by agricultural fields (with wheat, barley, rye, oats, vegetables and strawberries) with quite large patches of spruce and oak forests. The area is mostly confined by spruce forest. Most wart-biter habitats are small meadow patches in the fields where the soil is too shallow to be plowed because of rocky outcrops. Many of these meadows were grazed before. Grazing and harvesting fodder is non-existent in the area now (except for some horsegrazing, which usually renders the vegetation extremely short). It is not known whether the vegetation is stable, and if not, how rapidly bushes and trees encroaches that patches; some of the farmers cut bushes and trees to avoid shadow on the fields. Some roadsides as well as one patch of humid meadow are also suitable wart-biter habitats. This area can be said to examplify a landscape where the original wart-biter habitat exists because of the geological properties of the area, i.e., the combination of fertile soil (suitable for agriculture) and rocky outcrops. Rocky outcrops in forests are too isolated too be suitable wart-biter habitat, and in lowland agricultural landscapes without rocky outcrops, there are usually few potential wart-biter habitats because of modern agricultural practices. In 9 Overview and synthesis south-eastern Norway, unfertilized meadows that are managed by traditional farming are most common at higher altitudes, where sheep and dairy farming is common. However, the climate tend to be too cold for the wart-biter in these areas. Thus, as a combination of geological and economical factors, there are few wart-biter habitats left in meadows. However, roadsides that are managed properly, i.e., cut a couple of times each summer, have a type of vegetation that appears to be suitable wart-biter habitat. The wartbiter also seems to require that the roadside width are above of a certain limit; I have never observed populations in roadsides less than 1.5-2 m wide, and the probability of occupancy seems to increase up to 3-4 m width. Such broad roadsides are common along major, modern highways and sometimes along train lines. In Paper VI, we studied the wart-biter populations that have established along a stretch of a major Norwegian highway, E18 between Holmestrand and Larvik. This is an area with intensive agriculture and rapid urban development. There are very few meadows suitable for wart-biters left in this area, and the number is steadily decreasing. Large parts of the road has been built 5-20 years ago and cuts through forests and large fields. The roadsides along this road currently seem to be the most important wart-biter habitat in the area. The climatic conditions are similar as in the Frogn area. At last, we used an area at Evenstad field station in Østerdalen, around 150 km north of Oslo (Paper III, IV). The vegetation was suitable meadow vegetation. Compared to the two other locations, the mean temperature through the year is substantially colder, but the climate is also more continental, and summers can be quite hot. Wart-biters were collected from other places and released in the area at the start of the experiment. Movement behaviour - a "missing link" in metapopulation ecology El lobo es una cosa incognoscible. Lo que se tiene en la trampa no es mas que dientes y forro. El lobo propio no se puede conocer. Lobo o lo que sabe el lobo. Tan como preguntar lo que saben las piedras. Los arboles. El mundo. 10 Overview and synthesis Cormac McCarthy: The Crossing 2 Behaviour is the key to dispersal rate between patches in two ways. (I here assume we talk about species that have enough physical and "mental" control over their movements to stay within the natal patch if they want to. This excludes for instance many aquatic species that follow the water movements as plankton in part of their life cycle.) First, some animals decide to leave their "home" patch, i.e., they decide to emigrate. Which factors influences how many animals that emigrate, which animals that decide to do it, and when they do it? This is the subject of Paper IV. Second, after the animals have left the natal patch, they try to locate another patch while moving through the matrix (which often is drawn as a big white area in metapopulations maps, but seldom is so completely devoid of structure as such drawings seem to indicate). Which factors influence the path they follow, and more specifically: how much of the path trajectory results from innate movement patters (i.e. how often and how much they change direction), and how much depends on sensory information that they gather while moving? In this thesis, I have ignored this subject as much as possible, although I could not avoid making some assumptions about this in Paper V. It is, however, a question that is both extremely important, and in many cases extremely difficult to resolve (more on this in the chapter about Metapopulation models.) In much more indirect manner, movement behaviour also influences the probability of local extinction, the other basic process of metapopulations. First, movement behaviour may influence intraspecific competition, which in turn influences population size and thereby the risk of extinction. Second, the emigration rate can be so high (relative to immigration rate) that the risk of extinction is considerably increased. The first topic is related to the themes of Paper I and III, the second in Paper IV. Animals have presumably evolved movement behaviours that are optimal (within the constraints set by movement speed, the amount of body resources not devoted to reproduction, etc.). Animals can also be expected to make the best use of sensory information, often through indirect cues. In this connection, "optimal" means "optimal for the individual's fitness", or more precisely, the evolutionary stable strategy (ESS). In the case of dispersal rate, the ESS strategy may often be very close to the dispersal rate that maximizes 2 The wolf is an unknowable thing. What one has in the trap is nothing but teeth and fur. The wolf itself cannot be known. Wolf or what the wolf knows. It's like asking what the stones know. The trees. The world. 11 Overview and synthesis metapopulation occupancy (Hamilton and May 1977; but also see Lemire and Lessard 1997 for a different conclusion). I am not aware of similar theoretical studies of within-patch movement of animals that seek to maximise fitness (which in males often means to maximise the number of matings). However, the wart-biter's landscape has changed a lot only through the last decades, so it evolved in a landscape that was quite different from the current landscape. Movement behaviour (and other types of behaviour) can turn from adaptive to maladaptive when the landscape changes. This is relevant for within-patch movement, the decision of emigration, and the search strategy during movement between patches. E.g., if the species evolved in a fragmented landscape that is less fragmented than the current landscape, it probably has a "too high" dispersal rate, because its dispersal strategy is adjusted to the "old" level of dispersal mortality, which probably was lower than the current one. In addition, the link between an individual's sensory "input" (e.g., how often an individual encounters the habitat edge) and its behavioural "output" (e.g., the choice between staying and emigrating) may be “tuned” to be optimal in habitat patches looking quite differently than the patches in which the animals live today. E.g., suppose that the mechanism for "setting" the migration rate at a optimum level is that animals decides to disperse after encountering the edge a certain number of times. When patch size decreases, the dispersal rate will increase markedly. Thus, when the landscape is fragmented, the optimal dispersal rate decreases, while the actual dispersal rate may increase, leading to a large gap between optimal and actual dispersal strategies. Natural selection will reduce this gap, but the metapopulation may go extinct in the process. Use of area depending on habitat size In classical metapopulation theory, the patch is usually treated as a point with zero dimensions in space; i.e., all local processes are assumed to be non-spatial, and can be essentially fully understood by "classical" population dynamics of closed populations with some stochasticity thrown in (i.e., refs.). However, movement also plays a role within patches. For a species that (in part) is regulated by intraspecific competition and lives in a patch where resources are evenly distributed, any movement behaviour that leads to spatial variation in density will result in "inefficient" space use, in the sense that the population size at equilibrium is lower than it could be if the density did not vary in space. (For patches where resources are not evenly distributed, the "optimal" space use is where the pattern of species density corresponds to the pattern of in resource density.) E.g., if animals tend to aggregate in clumps although there is no variation in resources, animals experience a higher 12 Overview and synthesis density than the real overall density, which leads to higher intraspecific competition thereby lower population size. This may in turn influence the probability of local (and global) extinction. In the Frogn study area, the wart-biter occupies patches of a wide range of sizes, from large meadows of 1000 m2 to two-meter-wide road verges. In paper I, I investigated how wart-biters select habitat within the patch in two patches of different size (as well as in one road verge; see the next chapter). The positions of individually marked insects were mapped with intervals of 30 minutes to two hours during a three-week period. The data were analysed using two different methods that shed light on microhabitat selection on two different scales: which microhabitats the adult animals choose for each "move" (i.e., between two observations), and which microhabitats the adults use by large. The first question relates to the adult animal's decisions during the three-week study period and must be analysed using special methods (Paper II), while the latter question relates to the animals' decisions during all life stages, including where the females decide to lay their eggs and where the instars decide to move. On the latter scale, we found that the edge was avoided in both patches. The pattern indicated a negative edge effect for distances up two meters, and was strikingly similar between patches (Paper I, Fig. 3a). By analysing habitat selection for each move, the same pattern prevailed in the large patch, while in the small patch it seemed that both the edge and the interior were avoided (Paper I, Fig. 3b). The results showed a considerable degree of aggregation in both patches, and that large parts of the patches were effectively empty because of movement behaviour. However, without replicates of patches of the same size, it is difficult to conclude that differences in behaviour result from patch size alone. In addition, vegetation differences between edge and interior are confounding factors. In the large patch, the pattern of edge avoidance was quite consistent between habitat types, but this was not the case for the small patch. As a complementary study to Paper I, I therefore set up an experiment with patches created experimentally on Evenstad field station (Paper III and IV). Here, eight patches in three different sizes were "carved" out by mowing a larger area, and animals caught elsewhere were released in equal densities and tracked much the same way as in Paper I. The animals were of two different "qualities": animals reared in the laboratory, and animals caught as adults. Unexpectedly, animals of the latter group appeared to be in better condition: they moved more, both within and between islands, males sang more often and more loudly, and 13 Overview and synthesis females' ranges overlapped with the most active males. In this experiment, the overall use of the edge zone (here defined to be 1 m wide) corresponded to the area of the edge, with one exception: Lab-reared (low-quality) animals (of both sexes) in medium-sized patches (8 x 4 m) stayed more in the edges than expected. Why did paper I and III yield so different results with regard to edge use? Since the results of Paper I indicate that animals avoid the edge per se and not the type of vegetation found in the edge, I suggest that positive phonotaxis (that singing males attract each other) is a likely explanation that also agree well with other studies of wart-biter behaviour (Keuper et al. 1986, Weidemann et al. 1990). So why did not wart-biters avoid the edges in Paper III as well? The answer is likely to be the difference in the range of patch sizes and shapes: the smallest patch in Paper I was 390 m2, while the largest patch in Paper II was 80 m2. In addition, the patches of Paper III were "semi-linear" (the large ones measured 16 x 5 m). Weidemann et al. (1990) found that wart-biter males aggregate in clumps (a kind of "leks"). Because of the limited patch width and the relatively high density of wart-biters, the patches in Paper III were probably just large (and wide) enough to make room for a wart-biter clump, there simply was no room left outside the clumps. Weidemann et al. (1990) also found that the distribution of males within clumps was significantly more regular than a random distribution, indicating male-male interference, which also was reported by Keuper et al. (1986) for this species. In Paper III, the inferior males probably avoided the interior because they were deterred by males singing loud and frequently. The similar distribution of females may similarly have been caused by female-female interferential competition for "sexy" males. This hypothesis is more uncertain, since other authors have not reported this for the female wart-biters; however, it is (weakly) supported by my own observations of outright kicking fights between females. In the large patches, however, the inferior animals used the interior as much as the edge, perhaps because there were more room between superior animals. Papers I and III both have weaknesses in different ways. For Paper I, the lack of replication (imposed by the time constraints of a single field worker) is a severe drawback. The main drawback of Paper III is the small size (and semi-linear shape) of the patches; the "large" patches of this study were smaller than the smallest non-linear occupied patches observed in the Frogn landscape (around 100 m2; Paper V). Animal ranges did not increase from the medium to the large patches in the experimental patches (Paper III, Fig. 6), indicating that the movement of each individual, at least, was not constrained by patch size. However, 14 Overview and synthesis the addition of larger and less linear patches would have been preferable3. Nevertheless, I find that the unexpected difference between inferior and superior animals to be interesting, and to my knowledge the first Orthopteran example of spatial segregation according to social status in what appears to be a lek-like system. Another striking feature of both Paper I and III is the unexpected similarity between the sexes in many aspects of movement behaviour and microhabitat selection. One possible explanation for this could be that because the males' contribute a spermatophore (see Study species) and mate relatively infrequently, and we can expect more symmetrical mating behaviour than the common pattern of "reluctant females, ardent males" (Krebs and Davies 1987:169). However, Nina Wedell (e.g., Wedell & Arak 1989; Wedell 1991) has concluded that the spermatophore has a mating function and no effect on fecundity. A simpler and perhaps more likely hypothesis is that males simply adjust their movements to the spatial pattern of females. Use of area depending on habitat shape Linear habitats are a striking feature of landscapes modified by man, and for many species, linear habitats become increasingly important for the preservation of species in the landscape (Paper VI and references therein). Quite few studies, however, have compared linear and non-linear habitats (in contrast with the attention devoted to linear landscape elements as movement corridors). For the wart-biter in Norway, roadsides and similar habitats (e.g., verges along train tracks, grassy areas around highway intersections) seem to be making up an increasing part of the wart-biter habitat. The strong increase in this type of areas does not outweigh the loss of "traditional" farmland habitats, but it certainly decreases the negative impact landscape change and the probability of regional extinction of the species. A wide-held belief in landscape ecology is that long narrow habitats are inferior relative to non-linear patches of same size, amongst other things because of edge effects; this has also been confirmed by some studies (e.g., Major et al. 1999). However, the situation seemed to be turned around in the Frogn area, where the smallest occupied roadsides were smaller than the smallest occupied non-linear patches, and the number of singing males 3 Indeed, my original experiment included patch sizes up to 20x 20 m. I tried to implement this experiment twice: first in a meadow owned by Oslo City, which ended up being cut by a farmer, and then in a grassland at The Agricultural University at Ås, which unfortunately had too dense vegetation for the wart-biters' taste. 15 Overview and synthesis indicated a higher density of animals in the road verges (Paper I). The pattern of wart-biter movements in this habitat (Paper I; also see previous chapter) indicated that in the roadside edges were not avoided; actually, they were preferred. The preference for edges may be caused by differences in microclimate (the roadside sloped towards the east, but was flatter near the edges). Anyway, these results indicates an edge response that differs form the nonlinear patches in the same area, and may indicate a more "effective" use of the habitat area, i.e., that a smaller fraction of the habitat is effectively empty. Deciding to emigrate Which factors influences the emigration rate, and more specifically: How does habitat area and shape influence emigration rate? These questions are obviously of great importance in metapopulation biology, and essential for predictive models. In a much-cited paper, Stamps et al. (1987) described a model where animals move around the patch on random. Each time an animal encounters the patch edge, it is hypothesised to leave the patch with some probability x, and to bounce off the edge with probability 1-x. Given this model, emigration rate decreases with increasing patch area because animals encounter the edge more frequently. The predictions from this hypothesized mechanism (I will call it "the boundary encounter rate hypothesis") have often been found to be quite adequate in a metapopulation context. E.g., in a study of a metapopulation of the bush-cricket Metrioptera bicolor, Kindvall (1999) developed a simulation model based on observed daily movement distances in the habitat, daily movement rates in non-habitat, and probabilities of emigration at the boundary towards several types of non-habitat. Non-habitat movement rates and the emigration probabilities were estimated by experimentally releasing animals in the forest and on borders, respectively. By comparing with data from long-term observations, he found that this model predicted long-term net displacement distances and emigration rates quite accurately. Haddad (1999) did a similar kind of analysis to predict how movement rates of butterflies through corridors were affected by the corridor's width and length, using a simulation model which departed from correlated random walks only at habitat boundaries. In the Evenstad experiment (Paper IV; also see Use of area depending on habitat size), I clearly found that emigration rate increased with patch area. The ratios of the dispersal rates of the small, medium and large patches were 3.1 : 2.2 : 1, which fit quite well to the prediction that emigration rate is proportional to the edge/area ratio, as the boundary encounter rate hypothesis predicts (Stamps et al. 1987). 16 Overview and synthesis However, since the wart-biters adopted fairly stable ranges, we would also expect that wart-biters that stayed in the edge zone would be more inclined to emigrate compared to those who stayed more in the patch interior. However, I found no indication that this was true. Therefore, although my observations agree with the boundary encounter rate hypothesis, they do not agree with the underlying behavioural mechanism that this hypothesis assumes. I propose social cohesion as an alternative hypothesis: the social interactions between the animals function as glue that keeps them as a group. If there are few animals in a patch, there are fewer interactions and the force that keeps them staying is weaker, and they are more inclined to move away from the patch. In the case of the wart-biter, the song of the males is presumably the most important social interaction. An important difference between this hypothesis and the boundary encounter rate hypothesis is that the social cohesion hypothesis predicts that the emigration rate depends on the population size, not on the patch area. As a kind of support for this hypothesis, I found that there seemed to be a connection between the net emigration rate the first three days of the experiment (when emigration rates where high due to the unfamiliar habitat) and the emigration rate the following two weeks. Those patches that happened to be left by many individuals in the start of the experiment tended to be left by even more individuals later. Of course, this is not conclusive evidence for this hypothesis. Except for patch size, I found that a much larger proportion of migrants (individuals that emigrated at least once) among males than among females (61 versus 21 %). This was the only behavioural parameter for which we found a clear difference between the sexes. Also, I found that animals in good condition and with high movement activity migrated far more than other animals. E.g., 56% of the animals with movement activity above the median migrated, while only 30% of the others. Since condition and movement activity was closely correlated, it is impossimle to separate the effects of those factors. Thus, it did not seem that the inferior animals were forced to leave the patch; on the contrary, the typical migrant was a sexually attractive male that probably sought to increase his fitness even further. It is worth noting that after the initial bout of migration, which lasted for around three days, the migration activity was quite low for an extended period, but then increased again towards the end of the season. It seems likely that this was an adaptive strategy for males: after having secured some reproduction "at home", they emigrated, hoping to find other patches with females that had not been mated (and they did!). The difference between the sexes may indicate that males' 17 Overview and synthesis fitness is more limited by the number of matings than the females' fitness are, in spite of the male's investment spermatophore. This view is supported by Wedell (1992). Statistical problems in the analysis of movement behaviour I encountered a statistical problem when I were about to analyse the movement data: the observed use of habitat with regard to, say, vegetation type, must be compared to the availability of the different vegetation types. Of course, we could assume that the entire habitat island was available. On a large time-scale, this is not wrong: in the course of for instance the entire season, the entire habitat patch may be more or less equally available to the wart-biters. However, it was clear that many wart-biters only transversed a small portion of the habitat island during the two-three weeks of the study, and it would be entirely wrong to assume that they could select microhabitat from the entire habitat in the 1-2 hours between recordings. The answer to this problem was Arthur et al's (1996) paper on how to estimate habitat selection when availability changes. In this paper, they used the 99th percentile of observed movement distances as the radius within which all parts of the habitat were equally available. In my case, this would lead to an overestimation on the amount of habitat close to the habitat edge because of the limited size of the habitat patches. Moreover, my observation intervals were not constant, in part because not all animals were found during each searching bout, and movement lengths also depended on temperature. I therefore modified their method by letting availability vary as a continuous function of distance from last observation, time, and other variables. This modification is reported as a separate paper (Paper II), including analysis of part of the data from paper I as a worked example. Interpreting occupancy patterns The wart-biters in the Frogn study area If it looks like a duck, and quacks like a duck, we have at least to consider the possibility that we have a small aquatic bird of the family Anatidae on our hands. Douglas Adams: Dirk Gently's Holistic Detective Agency In Paper V in this thesis, Rolf A. Ims and I studied the distribution of wart-biters in the Frogn study area. During the two years of the study, we found wart-biter populations in only 27 and 16 patches of the area, respectively, although 70 patches seemed to contain suitable habitat for the species. We basically asked two questions about this pattern. First, is it a metapopulation? Second, assuming the answer to be "yes", can we calculate the average 18 Overview and synthesis dispersal distance based on the pattern of occupancy alone? The latter question will be discussed in a later chapter. Regarding the first question, we found that many aspects of the wart-biter distribution agreed well with the metapopulation concept. First, we found that the probability that a patch was occupied increased with patch area and decreased with the degree of isolation relative to occupied patches. Those two factors, when log-transformed, appeared to have additive effects on occupancy, i.e., a patch of a given area became more likely to be occupied when isolation decreased. The latter is an important point when dealing with shortterm data where little or no turnover is observed, because we might also observe significant effects of area and isolation if a species is in the process of invading an area, assuming that patches that are smaller then a certain size cannot sustain a population. We would not expect the effects of area and isolation to be additive, however, so the fact that they are additive in our study indicates turnover. Second, we observed some actual turnover. Third, we found that small, but little isolated patches tended to harbour single animals that presumably had dispersed to these patches earlier the same year. These facts together strongly suggest a metapopulation-like structure. However, not everything conformed to a classical metapopulation. First, it is not clear whether the largest patches were susceptible to extinction. Thus, the system may have some patches that practically can be looked upon as mainlands. However, a metapopulation model is not "harmed" by the inclusion of patches with extinction rates practically equal to zero; its predictive power is not diminished. Thus, this is largely a point of semantics. Second, a patch occupied in the first year was so small and isolated that it "should not" have been occupied, i.e., according to the metapopulation model we used, it was extremely unlikely to be occupied. Just how "outlying" this patch is depends partly on which model that is used, however (more on metapopulation models in the next chapter). Even if it is a single patch, a single outlier that we are unable to explain is enough to declare, with a high degree of certainty, that the model we have used is wrong. Although all models in principle are wrong, this makes the predictive power of the model somewhat questionable. A possible explanation for this patch is the time lag inherent in metapopulations; the probability that a patch is occupied does not depends on the isolation relative to occupied patches for a period of time up to this moment, not the isolation in this moment. (The outlier patch is close to a large patch that was unoccupied in 1992 and 1994, but might have been occupied shortly before our study. Later, the large patch has become occupied, while the outlier became extinct in 1994.) 19 Overview and synthesis Finally, we do not know whether the metapopulation is in dynamic equilibrium, i.e., whether the proportion of occupied patches is without a temporal trend. However, observations from the years following this study (unpublished data) do not suggest any such trend, and the landscape is fairly stable. As a conclusion, I reformulate the first question of this chapter: Is there a model (verbal or mathematical) that is simpler than the metapopulation model, and can explain the observed patterns? I cannot answer anything but "no". However, we have little direct evidence of turnover, and we know little about recolonization and extinction rates. Also, we have no clue about what causes extinctions, i.e., the relative importance of demographic stochasticity, temporal environmental variation, deterministic extinctions (e.g., because of vegetation succession) and high emigration rates. Such knowledge would of course be very important in a conservation context. 20 Overview and synthesis Metapopulation models - the good, the bad and the ugly I'm astounded by people who want to "know" the universe when it's hard enough to find your way around Chinatown. Woody Allen Metapopulation models have evolved and radiated into countless forms from Levins' original model. As far as single-species models are concerned, the main trend has been towards increasing complexity and increasing realism. Part of the reason is that metapopulation ecology has developed from a purely theoretical concept to a highly applied discipline of conservation biology. Increasingly, politicians and landscape managers demand specific advice regarding reserve and landscape design, which cannot be answered without a realistic model. Unfortunately, both of the main processes in metapopulations are infamously difficult to model realistically, and it is easy to end up with models with a lot of parameters. In addition, many of the parameters are notoriously difficult to estimate. In structured metapopulation models, for instance, local population dynamics is explicitely modelled (e.g., using the logistic growth equation). Thus, one does not only need estimates of maximum growth rate (R) and carrying capacity (K), but one also needs to know (or assume) the relationship between population size and the probability of extinction (i.e., demographic stochasisity). Unless very precies estimates are available from a species that have been studied in detail through a number of years, such models are often of limited value and. Also, small differences in models structure can have a large influence on predictions. E.g., Mills et al. (1996) used four different programs to analyse population viability based on the same data set, and found that there were large deviations between predictions from each program because of such apparent details as idiosyncracies of the input format and how density independence is incorporated. Part of the problem is that the available data commonly is snapshot or semi-snapshot patterns of occupancy, i.e., data on which patches that are occupied in one or a few years, respectively. If turnover is low, extinctions and recolonizations can be so infrequent that it is impossible to fit independent models of extinction and recolonization (as done by Sjögren Gulve and Ray 1996). Thus, one has to rely on the snapshot/semi-snapshot occupancy patterns. Thus, the question is how to squeeze maximum information out of the following data (in the most extreme case): the area of each patch, the distances between patches, and which patches that are occupied in a single year. We obviously need a model. 21 Overview and synthesis Metapopulation models for interpretation of snapshot or semi-snapshot patterns can be arranged in a continuum from mechanistic to phenomenological models. Basically, in a mechanistic model, the processes (in metapopulation studies: immigration and emigration) that link predictor variables (patch areas and distances between patches) to observed patterns (occupancy of habitat islands) are modelled, based on ecological theory. Thus, the parameters in the model have some direct interpretation in relation to the processes that are assumed to case the pattern. In contrast, in phenomenological models the link between predictor variables and the observed pattern is a statistical model that is chosen simply because it appears to fit the data well. Thus, the estimated parameters have no direct ecological interpretation. The most successful metapopulation model may be Ilkka Hanski's incidence function model (IFM; Hanski 1994). Here, occupancy is mechanistically described as a function of extinction and colonization probabilities, which in turn are more or less phenomenologically described as functions of population size (or patch area) and the number of immigrants, respectively. Both the last functions can be said to be partly mechanistic (since they use functions that ecologically makes sense) and partly phenomenological (since the parameters have no direct ecological meaning). At last, the number of immigrants is described as a function of the distance to other patches and their areas. The entire model has five parameters. Hanski recommends that one, the average dispersal distance, is estimated using dispersal data, while the other four are estimated by fitting the model to the pattern of occupancy. Thus, the number of immigrants and the probabilities of colonization and extinction can be predicted for any habitat patch on basis of the estimated parameters. The IFM has been applied in a number of studies with good results (Hanski and Thomas 1994; Hanski et al. 1996; Wahlberg et al. 1996). Finally, a purely phenomenological approach to wart-biter occupancy is to use logistic regression to model the probability of occupancy as a function of patch area and isolation. One example of this kind is Thomas et al. (1992), who studied the distribution of occupied and vacant butterfly habitats. Paper V in this thesis is another example of a purely phenomenological approach applied to the wart-biters of the Frogn study area. The approach is actually idendical to the study of Thomas et al. (1992), except that we used a theoretical dispersal function (the negative exponential, the same as in IFM) as a basis to calculate isolation indices for each patch. This study is based on patch occupancy data for two successive generations of wartbiters, i.e., typical semi-snapshot data. We assumed that the probability of occupancy in a single year was a logistic function of log(isolation index), log(area) and variables of habitat 22 Overview and synthesis quality (describing vegetation and slope). Thus, the number of parameters that must be estimated is [number of habitat quality variables + 3], with an additional parameter to estimate (average dispersal distance) if isolation indices are estimated using the theoretical dispersal function. The parameters were estimated by fitting the model to the occupancy data. Purely phenomenological models like the logistic regression and more mechanistically based models like IFM appear very different. First, the mathematical formulations of the models seem totally unrelated to each other. However, it is easy to show that in much of parameter space they may be quite similar. Second, mechanistic models based on ecological theory have parameters that have some meaning with regard to the ecological processes of extinction and recolonization, while the meaning of the parameters of the logistic models are not readily interpreted in terms of those processes (they are easily interpreted in terms of occupancy; see Discussion in Paper V). Therefore, mechanistic models can be used to simulate the metapopulation during time and how patch destruction affects metapopulation viability. It is also easy to analyse the effect of destroying patches using statistical models (i.e., a phenomenological approach); just keep the estimated parameters and calculate new isoclines after patch removal. With a statistical model and some additional information, it is also possible to deduce curves of per-year colonization and extinction rates. One advantage of statistical models is that their properties are well-known. Another advandage may be increased robustness and precision of estimates due to a lower number of parameters in the model (e.g., three in a logistic model vs. four in the IFM if there are no habitat quality variables). Generally, whichever type of model that is used, estimating parameters from snapshot or semi-snapshot patterns of occupancy involve several caveats. First, these models assume that there is no trend in the proportion of occupied patches, i.e., that the metapopulation is in a state of dynamic quasi-stability. Obviously, predicting the risk of global extinction assuming dynamic stability becomes a meaningless effort if this assumption does not hold. If the metapopulation is on its way to deterministic extinction (because patches have become too small and/or too isolated from each other) such a flawed analysis will only obscure the fact that some action must be taken. Breaking this assumption can also lead to very biased estimates also if the metapopulation is not extremely far from stability. E.g., Kindvall (1995) found that Hanski's model yielded turnover rates 2-6 higher than observed, probably because the assumption of stability did not hold because new patches had been 23 Overview and synthesis created recently before the study. Another caveat is that without knowledge of local dynamics, there may be an unknown source-sink structure in the population. Predictions about the effects of destroying patches may be far too optimistic regarding the removal of patches that in reality are source patches (and too pessimistic regarding the removal of sink patches). A third and related caveat is the assumption that migration rate is equal and not affected by for instance patch area. Small populations can be sinks simply because of high emigration rates (Kuusaari et al. 1998; Paper IV). Finally, even though there is no trend in the proportion of occupied patches, temporal heterogeneity in patterns and processes may lead to erroneous conclusions in studies based on a few years data. E.g., extinctions (or recolonizations, for that matter) may occur largely during infrequent years of unfavourable weather, and it can be difficult to foresee the extinction pattern if we have not observed such a year (e.g., Weiss et al. 1988; Solbreck 1991). One example that shows the susceptibility of data from one or a few years (how data from one or a few years can lead astray) is the Euphydryas editha metapopulation studied by Boughton (1999). Here, former sources became extinct due to an unusual frost, and were recolonized from the former pseudosinks. Estimating dispersal parameters from patch occupancy patterns Spatial ecology with predictive power depends on at least some data on dispersal between populations. However, such data may be very difficult to collect. Therefore, it would be incredibly useful to be able to estimate dispersal indirectly, using the pattern of occupied and unoccupied patches as the only raw data. Theoretically, this is possible if we make three assumptions. The first one is that the number of dispersers from an island is proportional to the area of the patch. The second is that dispersal does only depend on distance, i.e. that it does not depend on features in the landscape outside the patches. The third assumption is the form of the dispersal function, i.e., the probability that an individual that leaves a patch arrives at another patch as a function of the distance between the patches. In our case (Paper V), we assumed the negative-exponential function c = exp(-D/D'). Biologically, the assumptions behind this function is that animals spread by simple diffusion, and dispersers are "lost" at a constant rate during dispersal (Turchin 1998:188). Alternative functions could be the power function or the Gaussian function, which assumes that the ratio between long and short-range dispersers is higher and lower, respectively (Turchin 1998:192). E.g., Hill (1996) found that dispersal lengths of butterflies in a patchy landscape was well explained by the power function (see also Kot et al. 1996 and references therein). As shown in Paper V, we found a good agreement between the observed average dispersal distance (37 24 Overview and synthesis m, calculated from male dispersal only) and the average dispersal distance estimated using the pattern of occupancy (36-42 m, depending on the number of habitat variables included in the model). However, one case of good agreement does not exactly guarantee that average dispersal distance can be estimated in this way in general. Moreover, even if the average dispersal distance is can be estimated adequately, this number may not be of much use as long as the form of the dispersal function is uncertain, since the tails of this function is very important when predicting the future of the metapopulation. Thus, it is very uncertain to which degree it is practically useful to estimate dispersal parameters from the occupancy patterns. Nevertheless, for many species that disperse as spores, seeds, etc., it can be practically impossible to estimate dispersal rates. In addition it can be very difficult to estimate the probability that a new population can be established in an empty patch as a function of the number of dispersers that arrive. Thus, it would be interesting to find out, e.g., using simulation, how accurately a dispersal curve can be derived given the number of patches and the size of the geographic area that has been sampled. Interpreting genetic variation: invasion of new landscape elements As mentioned before in this synthesis, roadsides are a habitat type that is becoming increasingly important for the wart-biter. The wart-biter seems to require roadsides of a certain width; I have never observed populations in roadsides less than 2 m wide, and the probability of occupancy seems to increase up to 3-4 m width. Such broad roadsides are most common along major highways built the last 20 years. Thus, the invasion and use of such roadsides is of great interest for the conservation of the wart-biter. Roadsides, if properly managed, are potentially important habitat for many other insect species as well as plants. For instance, it is believed that 80% of the butterfly species of the Netherlands can use roadside habitat (Anonymous 1995, cited in Forman and Alexander 1998). In addition, the creation of these new habitats can be exploited as experiments on a larger scale than any ecological research project can afford. First, they provide case studies of invasion of new habitat, a field of ecology that has received quite much attention recently (e.g., Kareiva 1996). Secondly, they can be used to study gene flow and genetic differentiation in linear habitats, an area of many theoretical, but few empirical studies (Paper VI). In Paper VI, Søren Bondrup-Nielsen, Rolf Anker Ims and I discuss different ways that anthropogenic linear habitats can be created and be invaded by a species. We include 25 Overview and synthesis both linear habitats that are engineered by human beings (e.g., roadsides) and linear habitats that have been created by destroying most of a once non-linear habitat (e.g., clearcutting a forest except for river margins). To investigate how these different scenarios affect spatial genetic patterns, we do a simulation study where we follow the gene frequencies of two loci in a row of populations, starting out with different initial patterns of spatial genetic patterns, as well as varying population sizes and migration rates. Using standard analyses of genetic structuring, such as overall Fst and correlation analyses (spatial autocorrelation as well as correlation and crosscorrelation between loci), such data can help estimating migration rates, effective population sizes, and/or the age since populations were established. In some cases, genetic data can also be used as a rear-view mirror, since some types of initial conditions (specifically, concurrent genetic gradients in two loci) can be detected by the presence of correlation between loci for around fifty generations after establishment of populations. As a case study, we studied the distribution and genetic variation of wart-biters along a stretch of a major Norwegian highway (E18 Holmestrand - Larvik). Large parts of this road has been built 5-20 years ago, and currently it seems to be the most important wart-biter habitat in the area along the road, which is intensively developed for farming and housing. As genetic markers we used one allozyme loci (ME) as well as the amount of black pigmentation on the wings (wing melanism, WM). We found quite strong correlation between loci as well as spatial autocorrelation within loci. In light of the simulation results, the most likely explanation appears to be that ME and WM varied geographically in similar ways before the corridor populations established. Based on simulation results, it also seems likely that the average effective population size is in the order of 60 individuals and that migration rate (between neighbouring sample points) is above 0.05. Although these values does not seem entirely improbable, it must be emphasized that this way of using the simulation results is somewhat speculative. 26 Overview and synthesis References Addicott, J.F., Aho, J.M., Antolin, M.F., Padilla, D.K., Richardson, J.S., and Soluk, D.A. (1987). Ecological neighborhoods: scaling environmental patterns. Oikos 49: 340346. Ander, K. (1947). Flygförmågan hos våra hopprätvinger [Flight ability of our Orthoptera]. Fauna Och Flora 42: 210-221. Andren, H. (1994). Effects of habitat fragmentation on birds and mammals in landscapes with different proportions of suitable habitat: A review. Oikos 71: 355-366. Andren, H. (1999). Habitat fragmentation, the random sample hypothesis and critical thresholds. Oikos 84: 306-308. Anonymous (1995). Natuur Over Wegen. Dienst Weg- en Waterbouwkunde, Delft, Netherlands. Arthur, S.M., Manly, B.F., McDonald, L.L., and Garner, G.W. (1996). Assessing habitat selection when availability changes. Ecology 77: 215-227. Bascompte, J. and Sole, R.V. (1996). Habitat fragmentation and extinction thresholds in spatially explicit models. Journal of Animal Ecology 65: 465-473. Bellman, H. (1985). A Field Guide to the Grasshoppers and Crickets of Britain and Northern Europe. Collins, London. Boughton, D.A. (1999). Empirical evidence for complex source-sink dynamics with alternative states in a butterfly metapopulation. Ecology 80: 2727-2739. Cherrill, A. J. (1989). The diet of the Wart-biter Decticus verrucivorus (L.). Bulletin of the British Entomological Society 20: 115-118. Cherrill, A.J. and Brown, V.K. (1990a). The habitat requirements of adults of the wart-biter Decticus verrucivorus (L.) (Orthoptera: Tettigoniidae) in southern England (UK). Biological Conservation 53: 145-158. Cherrill, A.J. and Brown, V.K. (1990b). The life cycle and distribution of the water-biter Decticus verrucivorus (L.) (Orthoptera: Tettigoniidae) in a chalk grassland in southern England (UK). Biological Conservation 53: 125-144. Cunningham, A.A., Frank, J.M., Croft, P., Clarke, D., and PearceKelly, P. (1997). Mortality of captive British wartbiter crickets: Implications for reintroduction programs. Journal of Wildlife Diseases 33: 673-676. 27 Overview and synthesis DN (1994). Verdifulle kulturlandskap i Norge. Mer enn bare landskap! Direktoratet for Naturforvaltning, Trondheim. DN (1999a). Kartlegging av naturtyper. Verdisetting av biologisk mangfold. Direktoratet for naturforvaltning - håndbok 13. DN (1999b). Nasjonal rødliste for truede arter i Norge 1998. Norwegian red list 1998. Direktoratet for naturforvaltning - rapport 1999-3. Edelstam, C. (1995). Ängar. Jordbruksverket, Jönköping, Sweden. Forman, R. T. T. and Alexander, L. E. (1998). Roads and their major ecological effects. Annual Review of Ecology and Systematics 29: 207-231. Haddad, N. M. (1999). Corridor use predicted from behaviors at habitat boundaries. American Naturalist 153: 215-227. Hamilton, W.D. and May, R.M. (1977). Dispersal in stable habitats. Nature 269: 578-581. Hanski, I. (1994). A practical model of metapopulation dynamics. Journal of Animal Ecology 63: 151-162. Hanski, I. and Gilpin, M. (1997). Metapopulation Biology. Ecology, Genetics, and Evolution. Academic Press, London. Hanski, I., Moilanen, A., Pakkala, T., and Kuussaari, M. (1996). The quantitative incidence function model and persistence of an endangered butterfly metapopulation. Conservation Biology 10: 578-590. Hanski, I. and Thomas, C.D. (1994). Metapopulation dynamics and conservation: A spatially explicit model applied to butterflies. Biological Conservation 68: 167-180. Harz, K. (1960). Geradflügler oder Orthopteren (Die Tierwelt Deutschlands 46). VEB Gustav Fischer Verlag, Jena. Hill, J.K., Thomas, C.D., and Lewis, O.T. (1996). Effects of habitat patch size and isolation on dispersal by Hesperia comma butterflies: implications for metapopulation structure. Journal of Animal Ecology 65: 725-735. Holst, K. T. (1986). The Saltatoria (bush-crickets, crickets and grasshoppers) of Northern Europe. Fauna Entomologica Scandinavica 16. Ingrisch, S. (1978a). Labor- und Freilanduntersuchungen zur Dauer der postembryonalen Entwicklung einiger mitteleuropäischer Laubhauschrecken (Orthoptera: Tettigoniidae) und ihre Beeinflussung durch Temperatur und Feuchte. Zoologischer Anzeiger 200: 309-320. 28 Overview and synthesis Ingrisch, S. (1978b). Zum verhalten mitteleuropäischer Leubheuschrecken (Orthoptera: Tettigoniidae) und ihre Beeinflussung durch Temperatur und Feuchte. Deutsche Entomologische Zeitschrift 25: 349-360. Ingrisch, S. (1979). Experimentell-ökologische Freilanduntersuchungen zur Monotopbindung der Laubheuschrecken (Orthoptera: Tettigoniidae) und ihre Beeinflussung durch Temperatur und Feuchte. Beitraege zur Naturkunde der Osthessen 15: 33-95. Kareiva, P. (1996). Developing a predictive ecology for non-indigenous species and ecological invasions. Ecology 77: 1651-1652. Keuper, A., Kalmring, K., Schatral, A., Latimer, W., and Kaiser, W. (1986). Behavioural adaptations of ground living bushcrickets to the properties of sound propagation in low grassland. Oecologia 70: 414-422. Kindvall, O. (1995). Ecology of the bush cricket Metrioptera bicolor with implications for metapopulation theory and conservation (doctor dissertation). Sveriges Lantbruksuniversitet Rapport 29. Kindvall, O. (1999). Dispersal in a metapopulation of the bush cricket, Metrioptera bicolor (Orthoptera: Tettigoniidae). Journal of Animal Ecology 68: 172-185. Kot, M., Lewis, M.A., and van den Driessche, P. (1996). Dispersal data and the spread of invading organisms. Ecology 77: 2027-2042. Krebs, J. R. and Davies, N. B. (1987). An Introduction to Behavioural Ecology. Blackwell, Oxford. Kuussaari, M., Saccheri, I., Camara, M., and Hanski, I. (1998). Allee effect and population dynamics in the Glanville fritillary butterfly. Oikos 82: 384-392. Lemire, M. and Lessard, S. (1997). On the non-existence of an optimal migration rate. Journal Of Mathematical Biology 35: 657-682. Major, R.E., Christie, F.J., Gowing, G., and Ivison, T.J. (1999). Age structure and density of red-capped robin populations vary with habitat size and shape. Journal of Applied Ecology 36: 901-908. Mills, L.S. and Allendorf, F.W. (1996). The one-migrant-per-generation rule in conservation and management. Conservation Biology 10: 1509-1518. Nagy, B. (1950). Beiträge zur Kenntnis des Nahrungsbedarfs von Decticus verrucivorus L. (Orthopt.:Tettigon.). Annales Biologicae Universitatis Debreceniensis 1: 222-227. 29 Overview and synthesis Pulliam, H. R. (1996). Sources and sinks: empirical evidence and population consequences. In: O. E. Rhodes, R. K. Chesser, and M. H. Smith (eds.): Population dynamics in ecological space and time. The University of Chicago Press, Chicago. Samways, M. J. and Harz, K. (1982). Biogeography of intraspecific morphological variation in the bush crickets Decticus verrucivorus (L.) and D. albifrons (F.) (Orthoptera: Tettigoniidae). Journal of Biogeography 9: 243-254. Solbreck, C. (1991). Unusual weather and insect population-dynamics - Lygaeus equestris during an extinction and recovery period. Oikos 60: 343-350. Stamps, J.A., Buechner, M., and Krishnan, V.V. (1987). The effects of edge permeability and habitat geometry on emigration from patches of habitat. American Naturalist 129: 533-552. Stelter, C., Reich, M., Grimm, V., and Wissel, C. (1997). Modelling persistence in dynamic landscapes: Lessons from a metapopulation of the grasshopper Bryodema tuberculata. Journal of Animal Ecology 66: 508-518. Thomas, C.D. and Kunin, W.E. (1999). The spatial structure of populations. Journal of Animal Ecology 68, 647-657. Thomas, C.D., Thomas, J.A., and Warren, M.S. (1992). Distributions of occupied and vacant butterfly habitats in fragmented landscapes. Oecologia 92: 563-567. Thorens, P. and Nadig, A. (1997). Atlas de Distribution des Orthopteres de Suisse. Centre Suisse de Cartographie de la Faune, Pro Natura, Tilman, D., May, R.M., Lehman, C.L., and Nowak, M.A. (1994). Habitat destruction and the extinction debt. Nature 371: 65-66. Turchin, P. (1998). Quantitative Analysis of Movement. Sinauer, Sunderland, Massachusetts. Wahlberg, N., Moilanen, A., and Hanski, I. (1996). Predicting the occurrence of endangered species in fragmented landscapes. Science 273: 1536-1538. Wedell, N. (1991). Sperm competition selects for nuptial feeding in a bush-cricket. Evolution 45: 1975-1978. Wedell, N. (1992). Protandry and mate assessment in the wartbiter Decticus verrucivorus (Orthoptera, Tettigoniidae). Behavioral Ecology and Sociobiology 31: 301-308. Wedell, N. and Arak, A. (1989). The wartbiter spermatophore and its effect on female reproductive output (Orthoptera: Tettigoniidae, Decticus verrucivorus). Behavioral Ecology and Sociobiology 24: 117-125. 30 Overview and synthesis Weidemann, S., Stiedl, O., and Kalmring, K. (1990). Distribution and population density of the bushcricket Decticus verrucivorus in a damp-meadow biotope. Oecologia 82: 369-373. Weiss, S.B., Murphy, D.D., and White, R.R. (1988). Sun, slope, and butterflies: topographic determinants of habitat quality for Euphydryas editha. Ecology 69: 1486-1496. Wiens, J. A. (1995). Landscape mosaics and ecological theory. In: L. Hansson, L. Fahrig, and G. Merriam (eds.): Mosaic Landscapes and Ecological Processes. Chapman & Hall, London. 31 Overview and synthesis List of papers Paper I: Hjermann, D. Ø. Do bush-crickets avoid the habitat edge? Microhabitat selection in the wart-biter (Decticus verrucivorus). Manuscript. Paper II: Hjermann, D. Ø. 2000. Analyzing habitat selection in animals without welldefined home ranges. Ecology 81: 1462-1468. Paper III: Hjermann, D. Ø. The condition of individual bush-crickets influence their spatial distribution. Manuscript. Paper IV: Hjermann, D. Ø. Why does emigration increase with decreasing patch size? An experimental test of the boundary encounter rate hypothesis. Manuscript. Paper V: Hjermann, D. Ø. and Ims, R. A. 1996. Landscape ecology of the wart-biter Decticus verrucivorus in a patchy landscape. Journal of Animal Ecology 65: 768780. Paper VI: Hjermann, D. Ø., Ims R. A. and Bondrup-Nielsen, S. Patterns and processes underlying population genetic structure in anthropogenic linear habitats: Four hypothetical scenarios and one case study. Manuscript. Paper V is reprinted by courtesy of Blackwell Scientific Publication 32 Overview and synthesis Paper 1 33 Overview and synthesis Paper 2 34 Overview and synthesis Paper 3 35 Overview and synthesis Paper 4 36 Overview and synthesis Paper 5 37 Overview and synthesis Paper 6 38 Overview and synthesis Box 1: Guesstimating migration rates from observations of patches with single males In Paper V, we each year categorized patches into three types: patches with no observations or wart-biters at all, patches with observations of single males, and patches with observations of at least two animals. We can use the data in the middle group to give us a rough idea about migration rates per area in occupied patches. I here demonstrate this for the 1992 data from Frogn (Paper V). I use only data for the patches that are smaller than the smalles patch with populations any of the years, i.e., patches that appeared to be to small for establishment of a population (<120 m2). I sorted these patches by connectivity value (sensu Paper V), then calculated the running mean of connectivity value and the observed probability of observing a male on the patch. The result is the "observed" line in the figure below. Now, the connectivity value of patch i was calculated using two basic assumptions: the density of wart-biters and the migration rate is the same on all occupied patches (i.e., the migration rate does not depend on area, contrary to what I showed in Paper II). Then, the number of emigrant males from a patch equals [patch area]*[density of wart-biters]*[migration rate], or [no. of emigrant males] = area*C, where C is the number of emigrants per area. What we want is to estimate C. To find how many of the emigrants from patch j that reaches another patch i, we multiply with a function ci,j that depends only on the distance between i and j. The rest of the emigrants ends up somewhere else or dies. The expected number of emigrants that reaches i from j is therefore areaj*C*ci,j. To find the expected number of emigrants that reaches i from anywhere, we sum up this product for all patches j, i.e. [the number of immigrants] = areaj*C*ci = C*areaj*ci (since C is a constant). However, I have defined connectivity as areaj*ci (Paper V). Thus, by assuming different values of C we can plot the expected number of immigrants in the same graph as the observed numbers of males (see below). Probability of immigration of a male to an empty patch. The "observed numbers" are running means of eight islands sorted by connectivity value. We here see that for the right-hand part of the graph, C values around 0.0025 - 0.005 appears to fit quite well. For lower connectivity values, the observed values is higher, though. This may have two reasons. Low connectivity means that the surrounding patches are small and/or far away. However, Paper II clearly indicates that emigration rate is higher on small patches. In addition, long-distance migration may be more common that the negative exponential function (which we used for ci,j) indicates, and moreover, we assumed that no migrants moved longer than 400 m. I therefore think that C = 0.0025 - 0.005 might be a plausible estimate, i.e., one male emigrant per 2-400 m2 of habitat. The estimate is probably closest to the truth for medium and large patches. 39 Overview and synthesis Box 2: Guesstimating extinction and colonization probabilities from a logistic regression model Based on the rough estimate of male emigration rate described in Box 1, we can calculate both extinction and colonization rates, given a couple of assumptions more. As in Box 1, we assume that dispersers are independent and that the probability of dispersing from one place to another follow the negative exponential distribution. Let us also assume that the emigration rate of males is 2.9 times the emigration rate females is equal that of males (which is what we found in Paper II). Then, the probability of immigration by one individual, two individuals and one individual of each sex, respectively, is as depicted in the figure below. Next, we assume that the colonization probability equals the extinction probability on the 50% isocline of probability of occupancy (which implies that the metapopulation is in equilibrium). It is then easy to calculate the probability of extinction as a function of area (see figure of H. comma). 40