Hydroxylation and decarboxylation of hydroxybenzoic acids by Fe -chelates
advertisement

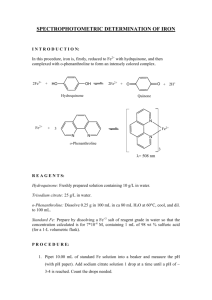
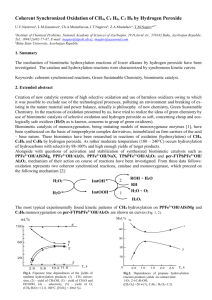
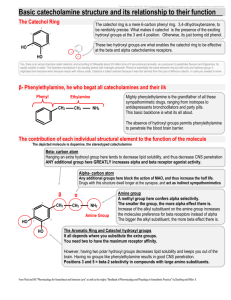
Hydroxylation and decarboxylation of hydroxybenzoic acids by Fe2+-chelates Halvor Aarnes, Department of Biology, University of Oslo, POB 1066, Blindern, N-0316 Oslo, NORWAY; E-mail: halvor.aarnes[at]bio.uio.no Unpublished research paper based on experiments done in 1998-1999. Abstract Hydroxylation and decarboxylation of hydroxybenzoic acids occurs rapidly at pH 3 to 6.5 in a system containing FeSO4 and Na2EDTA. EDTA could be replaced by citric acid. In this in vitro system 4hydroxybenzoic acid is hydroxylated to 3,4-dihydroxybenzoic acid (protocatechuic acid) and decarboxylated to hydroquinone. In an analogous reaction 2-hydroxybenzoic acid (salicylic acid) is hydroxylated to 2,3-dihydroxybenzoic acid and 2,5-dihydroxybenzoic acid (gentisic acid) and decarboxylated to catechol. Surprisingly the reactions showed Micahelis-Menten saturation kinetics for the products, and this paper is the first description of these reactions. From these data it is also cautioned to use hydroxylation of hydroxybenzoic acids as an indicator of oxidative stress and hydroxyl radicals in biological systems without proper controls. Keywords- Catechol, decarboxylation, Fe 2+-chelates, hydroxybenzoic acids, hydroquinone, hydroxylation . (Coudray et al. 1995; Ste-Marie et al. 1996; Myhre et al. 2000; Liu et al. 2002,). Stringent tests for the experimental system in order to avoid OH• -production from non-biological sources is necessary (Montgomery et al. 1995; Myhre et al. 2000, ). During our work trying to detect OH• I observed the possibility of artifactual production of hydroxylated hydroxybenzoic acids. The results presented here show that iron-chelates without ascorbate can hydroxylate and decarboxylate the hydroxybenzoic acids 2hydroxybenzoic acid and 4-hydroxybenzoid acid. Decarboxylation of 4-hydroxybenzoic acid produced hydroquinone. INTRODUCTION Since Udenfriend et al. (1954) and Brodie et al. (1954) reported that ascorbic acid, Fe2+ , EDTA and oxygen at pH 7 hydroxylated aromatic compounds, a large number of model systems for aromatic hydroxylation have been presented A large number of model systems for aromatic hydroxylation have been presented (Halliwell 1978; Grootveld.& Halliwell1986; Tamagaki 1989, Liu et al. 2002). In most cases the hydroxylation is performed in a Fenton-like reaction by hydrogenperoxide and ferrous ion (Fe2+). In biological systems, the presence of hydroxyl radicals (OH•) is regarded to be an indicator of oxidative stress. Trapping by hydroxybenzoic acid is a widely used sensitive technique to detect OH• down to picomol concentrations (Coudray et al. 1995; Ste-Marie et al. 1996) using HPLC with electrochemical detection. The salicylic acid method depends on the formation of the two stable compounds 2,3dihydroxybenzoic acid and 2,5-dihydroxybenzoic acid from 2-hydroxybenzoic acid (Coudray et al. 1995). Catechol is a also a product in the salicylate assay (Grootveld.& Halliwell1986) . The 4-hydroxybenzoic acid method is based upon the formation of 3,4-dihydroxybenzoic acid MATERIALS AND METHODS Chemicals All chemicals were obtained from SigmaAldrich Norway, except ethylacetate, FeSO4 •7H2O, and Titriplex III (Na2EDTA) which were from Merck (Germany), and HPLC-grade methanol from Rathburn (Scotland). Hydroxylation and decarboxylation assay Detection of 2,3-dihydroxybenzoic acid, 2,5dihydroxybenzoic acid, catechol, 3,41 dihydroxybenzoic acid and hydroquinone were based upon the following assays: The assay reaction mixture in glass tubes contained 2 mM 2-hydroxybenzoic acid (or 4-hydroxybenzoic acid), 2 mM Titriplex III (Na2-EDTA), 2 mM FeSO4@7H2O (freshly made), 20 mM Na-acetate pH 4.0 (or pH5.0) and water in a final volume of 1 ml. Reactions were started by addition of FeSO4 and whirlmixed for 5 s. The reaction mixture was diluted 200 x with the mobile phase (20 mM citrate, 30 mM Na-acetate, 0.1 mM EDTA and 7 % (v/v) methanol (pH 4.0)) and injected into the chromatograph. In some of the experiments 2 ml ethylacetate was added to each reaction mixture and immediately whirlmixing for 20 s. When the layers were separated, 0.5 ml of the supernatant was taken out, dried to dryness overnight with circulating air in drams glass at room temperature and dissolved in 1 ml of the mobile phase. The dihydroxybenzoic acids were measured by reversed-phase HPLC (Schimadzu SCL-6A) equipped with an electrochemical detector ( Schimadzu L-ECD-6A) set at +0.7V versus a Ag/AgCl reference electrode. A Brownlee Spheri-5 RP-18, 5: (220 x 4.6 mm) with a RP-18 precolumn was used with the mobile phase at a flowrate 0.2 - 0.8 ml min-1 at ambient temperature. The output of the the detector was registred and the peak areas were calculated as volt per second and quantified using authentic standards of dihydroxybenzoic acids, Catechol and Hydroquinone and corrected for the volumes applied. The production of 3,4-DHBA and Hydroquinone were 76 % and 24 %, respectively, of the total hydroxylated products formed in the reaction. Figure 1. Production of 3,4-dihydroxybenzoic acid (•) and hydroquinone (#) at different concentrations of FeSO4 and at constant concentration of 4-hydroxybenzoic acid (2 mM) and Na2EDTA (2 mM). The time course of the reaction was very fast and finished in less than seconds. Iron in the form of ferritin or K4Fe(CN)6 , was ineffective in the reaction. Citric acid could replace EDTA in the reaction, as could malic acid and oxalic acid could to some extent, but higher concentrations were necessary (data not shown). EDTA could not be replaced by 1,10-phenanthronline or the calcium chelating agent EGTA. Standard solutions of 2,4-dihydroxybenzoic acid, 2,6dihydroxybenzoic acid, resorcinol, and 2,4,6trihydroxybenzoic acid could not be measured when the detector response was set at 0.7 V. However, standard solutions of 3,4,5trihydroxybenzoic acid (gallic acid) and 2,3,4trihydroxybenzoic acid could be detected. RESULTS The results showed that FeSO4 and Na2-EDTA form stable adducts of 2,3-dihydroxybenzoic acid and 2,5-dihydroxybenzoic acid via an aromatic hydroxylation of 2-hydroxybenzoic acid. In addition decarboxylation of 2-hydroxybenzoic acid gave catechol (Figure 1, 2 & 3). The production of 2,3-dihydroxybenzoic acid, 2,5dihydroxybenzoic acid and catechol were 50 %, 37 % and 13 %, respectively, of the total hydroxylated products formed in the reaction. Hydroxylation of 4-hydroxybenzoic acid gave 3,4-dihydroxybenzoic acid and decarboxylation of 4-hydroxybenzoic acid gave hydroquinone. 2 The pH optimum of the hydroxylation and decarboxylation reactions were between pH 3 to pH 5, but with lower activity above pH 7. Increasing the concentrations of the reactants 4hydroxybenzoic acid, and FeSO4 and citric acid resulted in increasing amounts of products showing saturation kinetics (Figure 1 & 2). The same result was obtained varying the concentrations EDTA, Fe2+-EDTA and citrate (Figure 3). The results were confirmed with separate experiments ex tracting the hydroxybenzoic acids into etylacetate at pH 3. Production of the products 2,3-dihydroxybenzoic acid, 2,5-dihydroxybenzoic acid and catechol from the reactants 2-hydroxybenzoic acid , FeSO4 and Na2-EDTA or citric acid followed the same pattern. Also in this case saturation kinetics were obtained with increasing concentrations of the substrates in the reaction (see appendix). Figure 3 Production of 3,4-dihydroxybenzoic acid (•) and hydroquinone (#) at different concentrations of Na2EDTA and at constant concentration of FeSO4 (2 mM) and 4hydroxybenzoic acid (2 mM). See appendix for additional data. Figure 2. Production of 3,4-dihydroxybenzoic acid (•) and hydroquinone (#) at different concentrations of 4-hydroxybenzoic acid and at constant concentration of FeSO4 (2 mM) and Na2EDTA (2 mM). 3 Figure 4. Summary of the Fe2+-EDTA induced hydroxylation and decarboxylation of 2-hydroxybenzoic acid giving 2,5-dihydroxybenzoid acid , 2,3-dihydroxybenzoic acid and catachol. The products from Fe2+-EDTA induced hydroxylation and decarboxylation of 4-hydroxybenzoic acid are 3,4dihydroxybenzoic acid and hydroquinone. DISCUSSION This work show that Fe2+-EDTA or Fe2+-citrate at pH 3-6.5, without hydrogenperoxide or ascorbate, can induce hydroxylation and decarboxylation of 2-hydroxybenzoic acid and 4-hydroxybenzoic acid. This work also suggests that 4-hydroxybenzoic acid can be hydroxylated and decarboxylated to give 3,4-DHBA and HQ, respectively, in an analogous reaction. Formation hydroquinone from 4hydroxybenzoic acid by Fe2+-chelates has not been reported earlier. Not only EDTA, but also citric acid can be used as a iron-chelate in the reaction. It is well known that Fe2+ can react with hydroxybenzoic acid in siderophore-complex (Montgomery et al. 1995). Thus, it is likely that no free intermediates occur in the reaction. The time course of the reaction indicate a fast reaction kinetics as reported earlier for the ascorbate-dependent hydroxylation (Ste-Marie et al. 1999 ). Ascorbate, in addition to be an antioxidant, can work as an enzyme cofactor of hydroxylase enzymes. In biological systems enzymes are known to catalyze the same type of reactions presented in this paper e.g. salicylate 1-monooxygenase (EC 1.14.13.1) catalyzing oxidative decarboxylation of 2hydroxybenzoic acid and 4-hydroxybenzoate 3-monooxygenase (EC 1.14.13.2) catalyzing hydroxylation of 4-hydroxybenzoic acid, but in these cases NAD(P)H are used as an electron donor 4 instead of Fe2+. In addition, the cytochrome P450 system can hydroxylate hydroxybenzoic acids (14). Because only a limited number of hydroxybenzoic acids can give response when the detector was set to the potential +0.7 V, there is a possibility that also other products can be produced in the reactions investigated here. The biological relevance of addition of Fe2+-chelate to a biological system in order to produce OH• can be questioned. It is cautioned to use hydroxylation of hydroxybenzoic acids as an indicator of oxidative stress in biological systems without proper controls. Data from such measurements can be at least partially artifactual and should be interpreted with caution. It is suggested that the oxidative hydroxylation and decarboxylation of hydroxybenzoic acids can happen without involvement of hydroxyl radicals. REFERENCES Brodie, B.B.; Axelrod, J.; Shore, P.A.; Udenfriend, S. Ascorbic acid in aromatic hydroxylation. II. Products formed by reaction and substrates with ascorbic acid, ferrous ion, and oxygen. J. Biol. Chem. 208: 741-750; 1954. Coudray, C.; Talla; M.; Martin, S.; Fatôme, M. & Favier, A. 1995. High-performance liquid chromatography-electrochemical determination of salicylate hydroxylation products as an in vivo marker of oxidative stress. Anal. Biochem. 227:101-111. Grootveld, M.& Halliwell.1986. Aromatic hydroxylation as a potential measure of hydroxyl-radical formation in vivo. Identification of hydroxylated derivatives of salicylate in human body fluids. Biochem. J. 237:499-504. Halliwell, B. 1978. Superoxide-dependent formation of hydroxyl radicals in the presence of iron chelates. FEBS Letters 92:321-326. Liu, M., Liu, S., Peterson, S.L., Miyake, M. & Liu, K.J.: On the application of 4-hydroxybenzoic acid as a trapping agent to study hydroxyl radical generation during cerebral ischemia and reperfusion. 2002. Mol.Cell.Biochem. 234/235:379-385. Montgomery, J.; Ste-Marie, L.; Boismenu, D.& Vachon, L. 1995. Hydroxylation of aromatic compounds as indices of hydroxyl radical production: a cautionary note revisited. Free Radic Biol. Med. 19:927-933. Myhre, O.; Vestad, T.A., Sagstuen, E.; Aarnes, H.& Fonnum; F. 2000. The Effects of Aliphatic (n-nonane), Naphtenic (1,2,4-trimethylcyclohexane) and Aromatic (1,2,4trimethylbenzene) Hydrocarbons on Respiratory Burst in Human Neutrophil Granulocytes. A Fluorescence and Electron Paramagnetic Resonance (EPR) Spectroscopy Study. Toxicol. Appl. Pharmacol. 167: 222-230. Ste-Marie, L.; Boismenu, D.; Vachon, L.& Montgomery,J. 1996. Evaluation of sodium 4hydroxybenzoate as an hydroxyl radical trap using gas chromatography-mass spectrometry and high-performance liquid chromatography with electrochemical detection. Anal. Biochem. 241: 6774. 5 Ste-Marie, L.; Vachon, L.; Bémeur, C.; Lambert, J.; Montgomery, J. 1999. Local striatal infusion of MPP+ does not result in increased hydroxylation after systemic administration of 4-hydroxybenzoate Free Radic. Biol. & Med. 27: 997-1007. Tamagaki, S.; Suzuki, K.& Tagaki, W. 1989. Aromatic hydroxylation with an iron(III)-catecholH2O2 system. Mechanistic implication of the role of catechol. Bull. Chem. Soc. Jpn. 62:148-152. Udenfriend, S.; Clark, C.T.; Axelrod, J.; Brodie, B.B. Ascorbic acid in aromatic hydroxylation.I. A model system for aromatic hydroxylation. J.Biol. Chem. 208: 731-739; 1954. Appendix Appendix 1. Production of 3,4-dihydroxybenzoic acid (•,34DHB) and hydroquinone (#,HQ) at different concentrations of citrate (0-5 mM) and at constant concentration of FeSO4 (2 mM) and 4hydroxybenzoic acid (2 mM). 6 Appendix 2: Production of 2,3-dihydroxybenzoic acid (•, 2,3-DHBA) and 2,5 dihydroxybenzoic acid (B, 2,5-DHBA) at different concentrations of Na2EDTA (0-4 mM) and at constant concentration of FeSO4 (2 mM) and 2-hydroxybenzoic acid (2 mM). Appendix 3: Production of 2,3-dihydroxybenzoic acid (•, 2,3-DHBA) and 2,5 dihydroxybenzoic acid (B, 2,5-DHBA) at different concentrations of 2-hydroxybenzoic acid (2-HBA) and at constant concentration of FeSO4 (2 mM) and Na2EDTA (2 mM). 7 Appendix 4: Standard curve for 2,5-dihydroxybenzoic acid (2,5-DHBA) measured by HPLC equipped with electrochemical detector set at 0.7V. The concentration of 2,5-DHBA was measured as volt@second (Vs). The same kind of standard curve was made for all the compounds used in this paper.. Appendix 5: Standard curve for 3,4-dihydroxybenzoic acid (3,4-DHBA) measured by HPLC equipped with electrochemical detector set at 0.7V. The concentration of 3,4-DHBA was measured as volt@second (Vs). 8 Appendix 6: Production of 2,3-dihydroxybenzoic acid (•, 2,3-DHBA) and 2,5 dihydroxybenzoic acid (B, 2,5-DHBA) at different concentrations of FeSO4- Na2EDTA 0-4 (mM) and at constant concentration of 2-hydroxybenzoic acid ( mM). 9 Appendix 7: A. Detection of 2,3-dihydroxybenzoic acid (23DHB); 2,5-dihydroxybenzoic acid (25DHB) and catechol (CC) from 2-hydroxybenzoic acid diluted 100x with the mobile phase. Detection with HPLC equipped with electrochemical detector (ECD) at 0.7V and the amount of products is measured as millivolt (mV). B. Elution of a standard mixture containing 1 :M 2,5dihydroxybenzoic acid (25DHB); 2,3-dihydroxybenzoic acid (23DHB); 3,4-dihydroxybenzoic acid (34DHB) and catechol (CC). C. Chromatogram of reaction mixture with 4-hydroxybenzoic acid and Fe2+-EDTA diluted 100x with the mobile phase. D. Elution of a standard mixture containing 1:M of 2,3,4-trihydroxybenzoic acid (234THB); 3,4,5-trihydroxybenzoic acid (345THB), hydroquinone (HQ); 2,3-dihydroxybenzoic acid (23DHB); 3,4-dihydroxybenzoic acid (34DHB) and catechol (CC). 10