Spatial distribution of composition and misfit dislocations on the surface of alloys
advertisement

Semicond. Sci. Technol. 11 (1996) 717–721. Printed in the UK
Spatial distribution of composition
and misfit dislocations on the surface
of alloys
N V Fomin and D V Shantsev
Ioffe Physico-Technical Institute, 26 Polytekhnicheskaya St., St Petersburg 194021,
Russia
Received 25 January 1996, accepted for publication 26 February 1996
Abstract. The Frenkel–Kontorova theory of phase transitions into an
incommensurate phase with the formation of a superlattice of misfit dislocations on
a surface is extended to the case of alloys for which the lattice mismatch depends
on the local composition. A model is considered that takes into account the
possibility of atomic diffusion between the surface and the bulk. Peculiarities of the
conditions at the surface are accounted for by introducing a chemical potential for
one type of the atoms of the alloy which leads to a difference in composition
between the bulk and the surface layer. We calculated the average mismatch
between the surface and the bulk lattices and the critical value of chemical
potential at which the phase transition into an incommensurate phase with misfit
dislocations occurs. A phase diagram of the system in coordinates of temperature
and chemical potential is presented for the alloy Inx Ga1−x As.
1. Introduction
In previous works [1, 2] we discussed the specificity of
the appearance of misfit dislocations and incommensurate
structures in the surface layer or adsorbed film of alloys.
It is well known that the origin of misfit dislocations
is a mismatch of the crystal lattice parameters of the
adsorbed film (or surface layer) and the bulk. A classic
model describing transitions between incommensurate and
commensurate phases is the Frenkel–Kontorova model [3],
which considers a linear chain of adatoms connected to
one another by elastic springs of length a and placed in
a cosinusoidal potential with period a0 induced by the
substrate. For the case of weak interaction between adatoms
and the substrate this problem was solved by Frank and van
der Merwe [4]. The energy of the chain can then be written
as
Z 2
1
2πu(x)
V 0
FFK =
u (x) − δ
+
dx
V0 1 − cos
a0
a0
2
(1)
where u(x) represents the displacements of adatoms with
respect to their positions in the commensurate phase, i.e.
minima of the cosine potential (for the continual approach
to be valid u(x) must be a slowly varying function of
the coordinate x along the chain, i.e. u0 = ∂u/∂x 1),
V and V0 are parameters characterizing the interaction
of adatoms with the substrate and with one another, and
δ = (a − a0 )/a 1 is the misfit characterizing the lattice
c 1996 IOP Publishing Ltd
0268-1242/96/050717+05$19.50 mismatch. When the misfit exceeds its critical value
δFK =
4
√
π α
α=
V
V0
(2)
the model gives the solution in the form of a lattice of
solutions—misfit dislocations—with the period depending
on the misfit.
In alloys the lattice parameter a depends on the
composition c (we will restrict our consideration to binary
or pseudobinary alloys Ac B1−c or Ac B1−c C), and therefore
a change of the local lattice parameter a(c) is made
possible by a change of local composition [5]. This spatial
redistribution of composition proceeds owing to atomic
diffusion in the crystal lattice. In reference [1] this effect
was taken into account in terms of a continual Frenkel–
Kontorova model considering the misfit δ as a function of
the coordinate. Then the energy functional can be written
in the form
Z 2
2π u(x) α 0
V0
u (x) − δ(x)
+
1 − cos
F =
a0
a0
2
β
δ(x) − δ̄ dx
(3)
+
2
where the overbar designates averaging over the coordinate,
and the parameter
β=
fch00 (c)a03
η2 V0
(4)
where η = (1/a)(∂a/∂c), is proportional to the second
derivative of the specific chemical energy of the alloy
717
N V Fomin and D V Shantsev
fch with respect to composition c. In expression (3)
the term containing β accounts for the increase in the
alloy energy caused by a spatially nonuniform distribution
of composition (we consider only the case of positive
β, which corresponds to an alloy stable against spinodal
decomposition). Furthermore the composition modulation
in the vicinity of dislocations lowers the elastic energy by
reducing the local mismatch. The competition of these two
mechanisms leads to a value of critical misfit which is less
than that given by equation (1):
s
s
β
β
4
δc =
= δFK
< δFK .
(5)
π α(α + β)
α+β
At β → ∞, when a nonuniform distribution of composition
is energetically unfavourable, expression (5) naturally
transforms into (2), while small enough β values provide
instability of the commensurate phase for as small a misfit
δ as desired. Another interesting feature of the Frenkel–
Kontorova model for alloys is the power-law dependence
of the interaction between two misfit dislocations on the
dislocation spacing l:
Fint = 8π 2 V0
a0 α
l β
(6)
with the interaction sign corresponding to repulsion.
Therefore the phase transition can be described here as a
second-order phase transition on the basis of the Ginsburg–
Landau theory with an order parameter of 1/ l, while in
the classic Frenkel–Kontorova model the dependence of
the energy on the order parameter at the critical point
is not analytical. In generalizing the Frenkel–Kontorova
model to the case of a substrate with finite rigidity many
authors took into account the interaction between misfit
dislocations through elastic strain in the substrate (see [6]
and references therein). It should be mentioned that the
elastic interaction of misfit dislocations is proportional to
1/ l 2 at large distances, i.e. it decays more quickly than
would be expected from equation (6).
An important point for the analysis carried out in
reference [1] is the assumption that the average composition
in the chain is constant, which rules out the possibility of
atomic diffusion between the chain and the bulk. In the
present paper we give up this assumption and allow atomic
diffusion along the chain as well as beyond it (surely, if
an atom of type A leaves a site of the crystal lattice, it
is replaced by another atom of type B). As a result, and
as will be shown below, the conclusion of reference [1]
that the critical misfit δ tends to zero when the parameter
β approaches zero remains valid, while the power-law
interaction (6) between dislocations disappears.
2. Generalization of the Frenkel–Kontorova model
to alloys with regard to atomic diffusion between
the chain and the substrate
It is supposed that the atoms of the surface layer of an alloy
are placed in peculiar conditions with respect to the atoms
in the bulk since some bonds of surface atoms are dangling
or their hybridization takes place. Then it can be expected
718
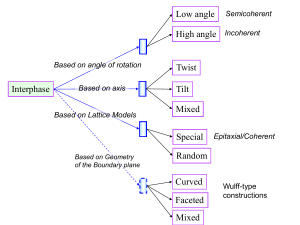
Figure 1. Black and white circles represent atoms of two
types for a binary alloy. Peculiar conditions at the surface
provide a difference in compositions and hence a mismatch
of lattice parameters at the surface and in the bulk.
(a ) A coherent structure (mismatch is sufficiently small);
(b ) presence of misfit dislocations (mismatch is large).
that it is energetically more favourable for atoms of one
type to be at the surface than for atoms of the other type.
This tendency should lead to a change of alloy composition
at the surface and hence to a mismatch between lattice
parameters at the surface and in the bulk (see figure 1).
If the composition change is small, a linear approximation
can be used for this dependence.
Let us write the total energy of the system. In the
spirit of the Frenkel–Kontorova model we will suppose that
the bulk of the alloy is rigid and has a spatially uniform
composition c. Then the linear term in the chemical free
energy expansion in terms of composition variation
fch (c + 1c) = fch (c) + fch0 (c)1c + 12 fch00 (c)(1c)2
(7)
vanishes when integrated over the whole material and the
integral of the quadratic term over the bulk is far less than
that over the surface. Thus, the total energy of the system
can be written as an integral over just the surface layer:
Z 2
V0
2π u(x) α 0
u (x) − δ(x)
F =
+
1 − cos
a0
a0
2
β
+ [δ(x)]2 − γ δ(x) dx
(8)
2
where δ is the misfit between the lattice constant at the
surface and the average lattice constant. Here we used
Vegard’s rule for the linear dependence of the lattice
parameter on composition δ = η1c. The parameter γ
is related to the effective chemical potential µ (the energy
associated with exchange of an atom of type A and an
atom of type B between the surface and the bulk) by the
following expression:
γ =
µ
ηV0
(9)
where V0 is, as before, the amplitude of the cosinusoidal
potential characterizing the interaction between the chain
of the surface atoms and the bulk.
Varying (8) with respect to δ, we obtain the Euler–
Lagrange equation:
α 0
γ
δ(x) =
u (x) +
.
(10)
α+β
α
Misfit dislocations on the surface of alloys
Substitution of δ, expressed in terms of u by (10), into (8)
allows us to write the energy as
Z V0
2πu(x) α∗ 0 2 α∗ γ 0
F =
u dx
1 − cos
+ (u ) −
a0
a0
2
β
(11)
where
αβ
α∗ =
(12)
α+β
and the energy reference point is chosen so that the energy
of the commensurate phase is zero, F [u(x) ≡ 0] = 0.
Expression (11) differs from the related one in reference
[1] by the absence of the term with (ū0 )2 responsible
for the interaction of dislocations. This is because in
reference [1] the misfit δ̄ was predetermined since the
average composition in the chain was assumed to be
constant. This time, however, the predetermined value is
the chemical potential µ, while δ̄ is defined by the condition
of minimization of the energy and, according to (10), it
increases when a lattice of misfit dislocations with period
l = a0 /ū0 appears:
α a0
γ
δ̄ =
+
.
(13)
α+β l
α
Note that expression (11) coincides with the corresponding
expansion in the Frenkel–Kontorova model with misfit γ /β
and elastic constant α∗ .
Now let us find the function u(x).
Varying
equation (11) with respect to u gives the sine-Gordon
equation:
2πu a0 2
2πu 00
sin
=
α∗
(14)
a0
2π
a0
whose first integral is
− cos
2πu
α∗ 0 2
1
(u ) − 2
=
a0
2
z
(15)
where 1/z 2 is an integration constant which must be
determined from the condition of minimization of the
energy. It is easy to see that z takes on values from 0
to 1. Expressing the function cos u through (15), we can
write the average energy density as a function of z:
V0
γ
1
0 2
¯
f (z) = 3 1 − 2 + α∗ (ū ) − α∗
ū0
(16)
z
β
a0
where the dependence of u0 on z is determined by (15). It
is convenient to express (ū0 )2 and ū0 in terms of elliptic
integrals:
Z 2π p
E(z) =
1 − z 2 cos ϕ dϕ
0
Z
K(z) =
0
2π
dϕ
p
.
1 − z 2 cos ϕ
(17)
The functions u(x), solutions of (15), describe a lattice of
misfit dislocations with period l defined as
√
Z a0
α∗ zK(z)a0
l(z) =
(u0 )−1 du =
.
(18)
√
2 2π
0
Further we have
(ū0 )2 = l −1
Z
a0
0
E(z)a0
u0 du = √
2α∗ π zl(z)
ū0 = a0 / l.
Substituting these expressions into (16), we obtain
√
α∗ E(z) α∗ γ a0
1
V0
¯
.
−
f (z) = 3 1 − 2 +
√
z
β
l
a0
2π z
(19)
(20)
(21)
The value z = 1 corresponds to the commensurate structure
(u(x) ≡ 0). An incommensurate structure emerges if
f (z) has a minimum at z = z0 lying within the interval
[0, 1]. Near the point of phase transition (z = 1) the
dislocation density 1/ l is small and the expression d =
√
V0 (4 α∗ /π − α∗ γ /β) has the meaning of the energy
necessary for one dislocation to be formed. If this energy
is negative, formation of a dislocation is favourable. The
generation of dislocations proceeds until the interaction
(repulsion) between them comes into force. Since K(z)
and hence, according to (18), l(z) diverge logarithmically
at z = 1, the interaction between dislocations decays
exponentially with their spacing which, in turn, implies a
singular dependence l −1 (d ).
The condition of the transition into the incommensurate
phase can be written in the form
γ
4
> √
β
π α∗
(22)
by analogy with the Frenkel–Kontorova model (see
equation (2)).
Now let us turn to more convenient variables. Applying
the regular solution approximation [7], we can write the
specific free energy of a binary alloy as
1
{cµA + (1 − c)µB + c(1 − c)
Wmole
+ RT [(1 − c) ln(1 − c) + c ln c]}
fch (c) =
(23)
where µA and µB are the chemical potentials of atoms of
type A and B, is the interaction parameter, R is the gas
constant, T is temperature and Wmole is the molar volume.
Applying (4) and (23), we obtain the following expression
for β:
β = tα.
(24)
Here t is linear in temperature:
t=
RT − 2c(1 − c)
c(1 − c)NA V η2
(25)
where NA is the Avogadro constant. Substituting (9) and
(24) in (22), we obtain the applicability condition for our
model
p
4η p
µ > µc =
V0 V t (1 + t).
(26)
π
The fulfilment of this condition means that the chemical
potential µ, providing for atoms of one type an excess
concentration at the surface with respect to that in the bulk,
is high enough for the average misfit between surface and
√
bulk lattices to achieve the critical value 4/(π α∗ ). The
719
N V Fomin and D V Shantsev
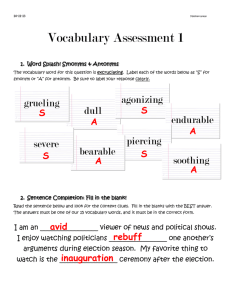
Figure 2. Phase diagram in coordinates of the effective
chemical potential and temperature for the alloy
Inx Ga1−x As. The critical line separating commensurate (C)
and incommensurate (I) phases is given by equation (26);
parameters are taken from [9]; α = 20. The (C) phase
corresponds to a coherent structure at the surface. The (I)
phase corresponds to the presence of misfit dislocations.
The full line indicates the temperature range where our
model is applicable (see (30)). Tc is the critical temperature
of spinodal decomposition.
phase diagram of the system for the alloy Inx Ga1−x As is
presented in figure 2. Since it was assumed that β > 0, our
analysis is valid only for T > Tc where Tc = 2c(1−c)/R
is the critical temperature of spinodal decomposition of the
alloy. According to experimental data (see e.g. [8]), for
III–V compounds Tc is in the range 300–1000 K, which
corresponds to typical temperatures of epitaxial growth.
Theoretically, at T close to Tc , equation (26) gives µc → 0.
It should be noted, however, that in this case we appear to
be beyond the continual approach.
Next, let us find the range of applicability of our model.
For harmonic expansion of the chemical energy to be valid
the following condition must be fulfilled:
δ
|aA − aB |
=η
a
(27)
where aA and aB are the periods of monolattices consisting
of atoms of type A and type B respectively. In addition,
for the continual approach to be applicable it was supposed
that
1
(28)
u0 ≈ √ 1.
α∗
According to (10) and (20) in the vicinity of the phase
transition, δ is given by
s
β
4
.
(29)
δ≈
π α(α + β)
Replacing strict inequalities by nonstrict ones and dropping
numerical coefficients, we obtain from (24) and (27)–(29)
the range of applicability of our model:
αη2
1
t .
α−1
1 − αη2
720
(30)
It can be seen that at η < α −1 the model is inapplicable
at any temperature. Since the parameter η is always less
than unity (for real compounds far less than unity), the
approach discussed is appropriate only for the case of
weak interaction between surface atoms and the substrate
(α 1). In principle, it is beyond reason to expect such
a large difference in interactions of surface atoms with one
another and with the substrate, though if it is assumed that
not one but n near-surface layers are placed in peculiar
conditions then α for such a system increases n times.
Nevertheless there is reason to believe that in a qualitative
sense our analysis adequately accounts for the enhanced
tendency of alloys to form dislocations owing to the
spatially nonuniform distribution of composition and that
it can also be used for describing the thermodynamically
non-equilibrium process of epitaxial growth. Finally it
should be mentioned that in the case of n near-surface
layers we should replace the quantity µ by nµ, and from
(26) it follows√that the critical value of chemical potential
µc decreases n times.
As an example, we propose a numerical estimation
using parameters for which our model is correct. According
to (9), for Inc Ga1−c As = 2.9 kcal mol−1 , η = 0.069, and
to estimate the quantity V , the elastic energy per atom,
we take the value of a pertinent combination of elastic
moduli which is ≈ 730 kcal mol−1 ; then at c = 1/2,
α = 20 and T = 770 K condition (30) takes the form
0.05 0.08 0.11, which is appropriate for a qualitative
description of the situation.
3. Conclusion
In summary, we have developed a model describing the
surface of an alloy in terms of the atomic diffusion between
the surface and the bulk. It was assumed that conditions for
the composition parameter in the surface layer differ from
those in the bulk, which results in a mismatch of surface and
bulk lattice constants. If this mismatch is large enough there
occurs a phase transition into an incommensurate phase
with the appearance of misfit dislocations. At temperatures
far from the melting point atomic diffusion is suppressed
because for an atom to hop into an interstitial it is necessary
to overcome a potential barrier of the order of the bonding
energy, and that is why the mechanism discussed in this
paper can be responsible for slow degradation of alloys.
However, our chief tendency that the appearance of misfit
dislocations is facilitated in alloys owing to the possibility
of a spatially nonuniform distribution of composition seems
to be appropriate also for the process of epitaxial growth
when the analysis in terms of equilibrium thermodynamics
used here is inapplicable. Allowing atomic diffusion into
the bulk leads to the disappearance of strong interaction
between dislocations, which takes place in the chain with a
fixed average composition. As a result, the model predicts
a phase transition similar to that considered by the model
of Frenkel–Kontorova, i.e. with a singular dependence of
the free energy on the order parameter (superlattice period).
The results obtained can be useful from the standpoint of
choosing the optimum conditions for the epitaxial growth
of non-defect structures of alloys.
Misfit dislocations on the surface of alloys
Acknowledgments
The authors are grateful to V A Shchukin and R A Suris for
fruitful discussions and interest in the work. The work was
supported by the Foundation for Fundamental Research of
Russia, grant no 93023199.
References
[1] Fomin N V 1994 Sov. Phys. Solid State 36 754
[2] Fomin N V and Shantsev D V 1995 Fiz. Tverd. Tela at press
[3] Frenkel’ F I and Kontorova A A 1939 J. Phys. (USSR)
1 137
[4] Frank F C and Van der Merwe J H 1949 Proc. R. Soc. 198
206
[5] Khachaturyan A G 1983 Theory of Structural
Transformations in Solids (New York: Wiley)
[6] Fomin N V 1991 Sov. Phys. Solid State 33 111
[7] Ilegems M and Panish M B 1974 J. Phys. Chem. Solids 35
409
[8] Ipatova I P, Malyshkin V G and Shchukin V A 1993
J. Appl. Phys. 74 7198
[9] Glas F 1987 J. Appl. Phys. 62 3201
721