Satellite D N A and speciation: of Drosophila guanchel RAAB
advertisement

2. zool. Syst. Evo1ut.-forsch. 27 (1989) 84-93
0 1989 Verlag Paul Parey, Hamburg und Berlin
ISSN 0044-3808
Received on 1. March 1989
Lehrstuhl Populationsgenetik der Eberhard-Karls- Universitat Tiibingen,FRG
Satellite D N A and speciation:
A species specific satellite D N A of Drosophila guanchel
By L. BACHMANN,M. RAABand D. SPERLICH
Abstract
The heterochromatin of the chromosomes of Drosophila uunche consists mainly of a satellite
DNA composed of multiple, tandemly arranged copies o f a 290 bp basic se uence. Five clones
containing one or two copies of the basic unit were sequenced. As expectedqfrom CsCl density
centrifugation and AT specific staining of mitotic chromosomes the sequence is AT rich. The
average nucleotid variability between the cloned sequences is 11.6 %. In situ hybridization on
the mitotic chromosomes revealed, that this satellite DNA is present in the centromeric regions
of all chromosomes but the Y. The nucleotide variability between co ies of different tandem
clusters seems to be higher than between members of the same cluster.$he copy number of the
sequence in the haploid genome was estimated to be approximately 80000. The sequence is
species specific and is not present in the enome of sibling species D . subobscwa and D . madeirenSIS. The evolutionary origin of the satebte D N A and its possible role in species formation is
discussed.
Chromosome evolution - Satellite DNA - Repetitive
Key words: Drosophila obscura-grou
DNA - Species specific &,A
Introduction
The classical method of evolutionary biology and systematics is the analysis and the comparison of organismic structures, whatever this might be, in order to see, whether or not
relations can be found between different organisms in this respect and whether these relations, if they exist, can be used for classification and/or phylogenetic studies. Very recently, however, molecular techniques have provided new and extremely precise methods
for another kind of analyses and comparisons which appear heuristically valuable and fascinating too. The first advance in this field was the introduction of electrophoretic allozyme techniques in population genetics, evolutionary studies and taxonomy Very
shortly later a great variety of molecular DNA techniques became available which appeared very interesting for further investigations. It was found that the genome of
eukaryotic organisms is not only composed of unique structural genes but contains also
DNA sequences which exist in the genome in a high number of multiple copies. According
to the grade of repetition this DNA is called middle or moderately repetitive (100-1000
copies) or highly repetitive (many thousands of copies) DNA respectively
The most curious fraction of these DNA classes is the highly repetitive DNA which
usually consists of short sequences repeated many times in tandem clusters. The base composition of this DNA is frequently distinct from the genome average, so that it can be
separated from the main DNA by density centrifugation in one or more satellite fractions.
It was further observed, that this so called satellite DNA lies often in heterochromatin,
This paper is dedicated to Prof. Dr. Dr. h. c. WOLFHERRE
on the occasion of his 80th birthday
with great affection.
U. S. Copyright Clearance Center Code Statement: 0044-3808/89/2702-0084/$02.50/0
Satellite D N A and speciation: A species specific satellite D N A of D. guanche
85
especially in the centromeric region of the chromosomes. Even closely related species can
differ very much from each other with respect to the specific amount as well as to the
specific repetition sequences of their satellite DNA.
In this paper we will present some data from our investigation of highly repetitive
D N A in the Drosophila obscura group. The phylogenetic relationship of the species of this
group has been examined already very intensively from various points of view. Cytological
and NOVITSKI
1941) and the comparistudies of metaphase chromosome sets (STURTEVANT
son of chromosome banding pattern of polytenic giant chromosomes (DOBZHANSKY
and
EPPLING1944; BOHMet al. 1987; KRIMBAS
and LOUKAS1984; MOLT^ et al. 1987) as well as
biochemical approaches (LAKOVAARA
et al. 1976; CABRERA
et al. 1983) have resulted in a
well founded phylogenetic tree of the species. From these dendrograms it can be seen that
some of the species are very closely related with each other constituting a species cluster or
a phylade in the dendrogram. One of this phylades is formed by the three species D.subobscura, D.madeirensis, and D.guanche. The latter two species are endemic to the Islands
of Madeira and to the Canary Archipelago, respectively, whereas D. subobscura is spread
all over Europe (including Madeira and Tenerife), North Africa and Asia Minor. The three
species are sibling species. They share the same karyotype of five acrocentric chromosomes what, according to MULLER(19401, corresponds with the supposed ancestral
karyotype of the genus Drosophila. Interspecific crossings between the three species are
possible to some extent. The pairing of the homologous strands in the polytenic chromosomes of the hybrid larvae reveals that the species specific chromosomes differ from each
other only by a modest number of paracentric inversions but have otherwise almost the
same banding pattern (KRIMBAS
and LOUKAS1984). More obvious differences were only
found for the sex chromosomes (= elements A) of the species MOLT^ et al. 1987;
PAPACEIT
and PREVOSTI
1989). Yet, the cytological divergence between the three species is
considerably lower than the intraspecific variation of chromosome structures in the populations of D.subobscura where more than 50 different chromosomal inversions have been
described so far (SPERLICHand PFRIEM1986) compared to 10 to 15 inversion differences
between the sibling species.
Most of cytological studies in the D.subobscura phylade have dealt hitherto with the
banding pattern of polytene chromosomes and very little is known about the highly repetitive or satellite D N A of these species. No information about this D N A fraction can be
gained from the polytene chromosomes since the sections of the chromosomes which
contain this D N A fraction are significantly underreplicated in giant chromosomes (GALL
et al. 1971). However, a first analysis with the differential staining techniques of C-, G-,
and R-banding UOHN et al. 1985) of the mitotic chromosomes of the three species revealed
that very suiking differences must exist'between the three species in the general amount of
heterochromatin as well as in their base pair compositions (RAAB et al., unpubl.). The
chromosomes of D. madezrensis and of D. guanche possess much more heterochromatin
than those of D. subobscura. A rough estimation is a 5:l ratio. Furthermore, the heterochromatin of D.guanche is, according to its strong staining with D A N (see Fig. I), composed of AT-rich DNA, whereas that of D.madeirensis and D.subobscura is definitely
GC-rich (RAAB et al., unpubl.). In this paper we will describe especially the main component of the satellite D N A of D.guanche. Also it will be discussed whether the great similarity between the three species of D.subobscura phylade with respect to organismic structures, karyograms, and aminoacid sequence of enzyme proteins is also visible in their
satellite DNA's.
86
L. Bachmann, M . Raab and D. Sperlich
Material and methods
Strains: All the strains of D. guanche, D. madeirensis, and the strain H271 of D. subobscura
were provided by A. PREVOSTI,
Barcelona.
Isolation and cloning of highly repetitive D N A of D . guanche: Total genomic D N A
of D. guanche, extracted according to PREISSet al. (1988), was digested with HindIII or
DraI, respectively, and electrophoretically fractionated on a 5 per cent Acrylamidgel. The
heavily staining bands appearing at a fragment lengths of 290 bp and 580 bp were considered to contain highly repetitive sequences. The gel regions with the two bands were cut
out and the D N A recovered from them by overnight incubation in 500mM NaAc; 1mM
EDTA. The eluted D N A fragments were then ligated in the HindIII or SmaI site of the
plasmid pUC8 according to KINGand BLAKESLEY
(1986). Cells of E. coli K12 JM 103 were
transformed with the recombinant plasmids and selected with the blue-white colour
system of the P-galactosidase gene (DAVISet al. 1986).
Sequencing: Preparation of plasmid DNA and sequencing was performed as described in
the manual of p U C sequencing kit (Boehringer, No. 30 13 106).
CsC1-density centrifugation: A CsCl solution containing 50 to 1OOpg genomic D N A
was adjusted to a refraction index of 1,393 and spun on a TI50 rotor (Beckmann) at
42000rpm for 48h. The gradient was collected in 35 fractions by an ISCO-fractionizer
and the content of D N A was simultaneously recorded photometrically at a wave length of
h = 253nm. For a better resolution of the D N A fragments with different AT-composition
a CsCl solution containing 0.85 pg Hoechst 33258/pg D N A was used in another run
(MANUELIDIS
1977). The gradient was treated otherwise in the same way as above. The
density of the fractions was determined indirectly by measuring the refraction index.
et al.
From this, the GC-content of the DNAs was calculated according to SCHILDKRAUT
(1962). Hybridization to filter bound DNA: Either 32P-dCTPor biotin-dUTP labelled
probes were used for hybridization. The 32P-signals were detected by autoradiography
and the biotin labels enzymatically by alkaline phosphatase reaction according to CHANet
al. (1985). All filter hybridization were performed under standard conditions as described
et al. (1982).
by MANIATIS
Estimation of the proportional amount of satellite D N A in the total genomic D N A :
Solutions containing a defined concentration of genomic D N A or of the specific satellite
D N A cloned in the pUC plasmid, respectively, were prepared and blotted on a Hybond
N membrane (Amersham) by means of a Schleicher and Schuell Minifold apparatus. The
filter bound DNAs were then hybridized with a probe of 32P-labelled satellite D N A from
the specific clone. After autoradiography that pair of spots from the genomic D N A solutions and the satellite D N A solutions was searched for, that exhibited the same labelling
intensity. Then, it was assumed that these spots must contain the same amount of satellite
DNA. Knowing the concentration and the volume of the test solutions for the spots the
proportion of satellite D N A in the total D N A could be estimated. The method could be
quantified, when the radioactivity of the spots is measured in a scintillation counter.
Preparation of mitotic chromosomes: Neural ganglia of third instar larvae were dissected in 0.9 YONaCl solution and then treated for 15 minutes in a hypotonic solution of 1YO
sodium citrate. After 10 min. fixation in cold a ethano1:acetic acid (3:1) mixture the ganglia
were transferred to a droD of 45 % acetic acid on a clean slide warmed UD to 42 "C. The
spreading of the chromosomes was achieved by moving the drop slowly on the slide. After
drying, the chromosomes were dehydrated stepwise in ethanol (70 Yo, 90 Yo, absol.) and
then air dried.
Satellite D N A and speciation: A species specific satellite D N A of D . guanche
87
Stainining of chromosomes: The C-banding as well as the fluorescent DAPI staining
was carried out according to SCHWEIZER
(1981).
In situ hybridization: Chromosome D N A was denatured at 60°C in 60% acetic acid
and the preparation dehydrated stepwise in ethanol. The biotinylated probe was denaturated by heating in 4xSSC with 50 YO formamide. The in situ hybridization was performed
on the slides under a 18mm square coverslip in a total volume of 1 5 ~ 1The
.
slides were
placed in a wet chamber and incubated overnight at 37°C and subsequently washed according to AMBROS
et al. (1986). Signal detection was carried out as described in the
DETEK instruction manual ( E N 2 0 Biochem. Inc., N.Y.). After 5 min. development in
diaminobezidine at room temperature the reaction was stopped by rinsing the slide with
destilled water. The slides were air-dried and analyzed microscopically with reflection
contrast (LANDEGENT
et al. 1985).
Results
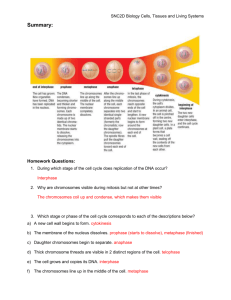
Total genomic D N A of D. guanche was centrifuged in a standard CsCl density gradient.
The distribution of the concentrations of DNA fragments along the gradient is shown in
Fig. 2a. A slight shoulder on the low density side of the main D N A distribution curve
indicates that an AT rich satellite exists in the genome of D. guanche. This observation was
confirmed through another density centrifugation where the separation of the fragments
with different bouyant densities was enhanced through the addition of Hoechst 33258 to
the gradient (Fig. 2b). A clear and destinct AT-rich satellite becomes now visible. An estimation of the G C content in the main band of D. guanche from the refraction indices gives
an value of 39 %. This is quite a low value compared to 56 Y' O in D. subobscuru and 58 % in
D. mdeirensis (BACHMANN,unpubl.). Such a result was already expected from cytological
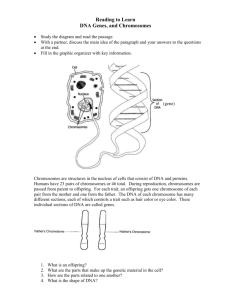
studies on the mitotic chromosomes of D. guanche. As can be seen from Fig. la, all
chromosomes of the set are heavily stained in the centromeric regions when the constitutive heterochromatin specific C-banding technique is applied. Almost the same regions
are stained with the AT specific DAPI technique (Fig. 1b).
Fig.1. Analysis of mitotic chromosomes of D.guancbe. a: The C-banding shows the high proportion of heterochromatin probably built by satellite DNA; b: the fluorescent DAPI-staining
(G-bandin ) reveals that most of the heterochromatin is formed by AT-rich sequences; c: the in
situ hybrikzation with pGH 290 DNA gives a pattern very similar to those of the C- and
G-banding. This indicates that the 290 bp satellite DNA represents the major part of the total
satellite DNA of D. guanche
88
a
L. Bachmann, M . Raab and D. Sperlich
n
b
c
c
c
a
l
4-
c
W
L
C
::
1
0
8
I
I
Q
z
0
buoyant demi ty
buoyant density
Fi . 2 . Total genomic DNA
o f D . guanche se arated in a
standard CsCl Bensity gradient (a) and in a CsCl
density gradient containing
Hoechst 33258 (b). The
slight shoulder (a) and the
se arated peak (b) on the left
si8e of the diagrams indicate
the presence of a high proportion of AT-rich satellite
DNA
A more detailed information about the composition of the satellite D N A of D. guanche
came from filter hybridization experiments. Five clones with inserts from the satellite fraction of D.guanche D N A were available:
pGH 290/1-3, each containing a 290bp HindIII fragment;
pGH 580, containing a 580bp HindIII fragment;
pGH 290, containing a 290bp DraI fragment.
Total genomic D N A of D. guanche was Hind I11 digested, electrophoretically separated on agarose gel, and the fragments transferred to a filter by Southern blotting technique. The filters were then used for hybridization experiments with the five clones described above as probes. The result is in all cases a pattern as shown in Fig. 3, lane c. The
ladderlike repetition of hybridizing fragment length classes proves that all the clones
contain a satellite fragment of the same type and that a basic repetition unit with a length
of 290bp must be present in the genome in tandemly arranged clusters. Similar hybridization experiments with total D N A from D. subobscuru or D.madeirensis with the same
clones as probes gave no hybridization at all. Thus, this satellite sequence of D.guanche is
species specific.
The next step was the sequencing of the five satellite clones of D. guanche. The consensus sequence is shown in Fig. 4. It has a length of 290bp in all cases; the 580bp clone
contains two subunits. There is a clear excess of AT versus G C in the sequence. The
Fig 3. a: Total genomic DNA of D. guanche digested
with the restriction enzymes SspI (IaneA), DraI
(IaneB), HindIII (IaneC) and AsnI (laneD) was
separated on a 0.9 % agarose gel. In the central lane
Eco RI/Hind I11 digested L D N A serves as a length
standard. The DNA was blotted to a Hybond N
membrane and h bridized with 32P-labeled pGH 290
DNA; b: the lacrderlike hybridization pattern in the
autoradiography shows that the 290 bp re eat is tandeml arranged in the genome. The Dra I &ane B) and
Hind;II (lane C) restriction sites seem 10 be characteristic for this satellite DNA. (For further explanation see text.)
Satellite DNA and speciation: A species specific satellite D N A of D . guanche
89
1
C T T T T C C G A G ACGGTAGGCC AAAACCACAC AGAGTTTGGA T C C A T T T T A T AGCAGTATCA A T C A A C T T T T CAGATAATCA
&
C
TA C
GGC A T A
TA
A A
T
T
El
>
AAGTTTTTTA CAACCAGATC TAAGATACTT AAGTTATGTG A A A A A A C A A A CCGAAACCTC TGAAAAAACC TTAAACTAAA
C
G G
C AT CT
T
T C
AC
A G
161
AATTACAAAA T T A A T A G T T C T T A A A A A A C A AGTTCTGCCA T A G A A A A T A A A A C C G A C T T T T C C C G A C T T T T T A A A G T C A A
G TTTC
GT A
A
T
C G
T
C
G
A T
G
cc
G
241
290
AATTTCGAAA AAAAAAATCT CACAACAATC AACATTTTTG CATTTCAAAG
G
CTT
A
A
CC
G
R . 4 . The consensus sequence of the 290 bp repeat of D . guanche shown as a Hind I11 fra rnent.
Tie sequence was derived from sequencing of pGH 290/1-3, PGH 580 and GD 290. ?he detected base substitutions in the different clones are noted at their positions unier the sequence
average AT content is 70.4 %. N o short internal repetition subunit could be detected. As
can be seen from Fig. 4, some variation exists between the five clones mainly due to single
base pair substitutions. Transitions as well as tranversions are found with n o significant
deviation from expectation. The average variability was calculated to be 11.6%. This all
agrees very well with the conclusions from density centrifugation and filter hybridization.
In order to confirm the assumption that the cloned satellite sequences come from the
heterochromatic part of the chromosomes and to see where and to which amount they are
present there, in situ hybridizations with the pGH 290 clones on the mitotic chromosomes of D. guanche were performed. The result is shown in Fig. 1c. All chromosomes
with exception of the Y chromosome are densely marked in the centromeric regions. A
comparison with the metaphase chromosomes in Figs. 1a and 1b, respective15 reveals that
the hybridyzing regions are almost identical with the differentially stained regions there,
which are specific for constitutive heterochromatin (Fig. 1a) or AT-rich D N A (Fig. 1b).
From that, one may conclude that most, of the satellite DNA of D. guanche is composed
of sequences homologue to the 290 bp sequence of our clones.
Since the intensity of the hybridization signals on the filter is proportional to the
number of molecules bound there, this can be used for a rough estimation of the proportional frequency of a given sequence in the genome (see also Materials and methods).
Filters with dot blot hybridizations of genomic D N A of D. guanche at increasing concentrations, and of insert D N A from one of the satellite clones at increasing concentrations,
respectively, probed with the same satellite DNA, are shown in Fig. 5. All three filters
were treated identically with exception of hybridization temperature which corresponded
to low, moderate and high stringency conditions. The hybridization signals on the autoradiogram of the filters show dots of various intensities. Yet, at low stringency conditions,
the approximately same dot intensities appear at the position, where an amount of 50ng
genomic D N A and long cloned satellite DNA, respectively, were blotted (Fig. 5c). From
this comparison, supplied by data about the radioactivity of the bound D N A measured
by in a scintillation counter, it can be concluded that approximately 16 % of the D. guanche
DNA consist of repetitive copies of the cloned 290 bp sequence. This is certainly a considerably high proportion of the total satellite D N A of this species. If we assume a haploid
genome size of Drosophikz of 150 x I06bp, the estimate of the copy number of our repetition unit in the haploid genome becomes 82 800. However, the values deduced from such
comparisons are very rough estimates. The incorrectness in the technique of pipetting
small volumes may be the main source of errors. Yet, for an informative approximation
and in the context with other observations, the above estimations might be acceptable.
L. Bachmann, M . Raab and D. Sperlich
90
68 "C
63"C
58 "C
Fig. 5. Estimation of the frequency of the 290 b repeat in the genome of D. guanche at high (a),
moderate (b and low (c) stringency. Genomic &NA was blotted in amounts of 100, 50,25, 10,5
and 2.5ng ( eft lanes) and cloned satellite DNA in amounts of 10, 5, 2, 1, 0.5 and 0.1 ng (ri ht
lanes) on a Hybond N membrane. The filters were hybridized with 32P-labeledcloned satelfite
DNA. (For further explanation see text.)
1
The similarity of the results under the three stringency conditions is an indication that the
variation between the copies is not greater than concluded from the variation in the cloned
sequences.
Another question which appeared important was, wether the variation in the base pair
composition of the various copies is evenly distributed in the repetition clusters of the
chromosomes o r whether the copies of the same cluster are more similar to each other
than to copies of different clusters. An indication that this is so, comes from filter hybridizations with genomic DNAs that were digested with different restriction enzymes. Fig. 3
gives an example. The D N A of lane A was cut with SspI, that of lane B with DraI, that of
lane A with HindIII, and that of lane D with AsnI. The probe D N A was from one of the
290bp clones. A typical ladder like pattern appears in lanes B and C . The explanation is
that a modest variation exists among the copies in the recognition sites of the two restriction enzymes. This leads to the origin of polymeric fragments increasing in size in multiples of the basic unit. The pattern of lane A, however, is different. Besides the usual ladder
a strong smear is seen in the region of long fragment sizes. This might indicate that a
fraction of D N A sequences of the genome have a great homology to the consensus sequence but have uniformly no recognition site for SspI. This might be the case if subfamilies exist which are more similar to each other and have a11 lost the recognition site by
basepair substitution. The pattern of lane D is due to the fact that the restriction site for
AsnI more or less randomly appearing in the fragments.
Discussion
From our experiments described above it is evident that a satellite D N A exists in the
genome of D. guanche, which has a 290 bp repetition unit, that is arranged in tandem clusters. It has been also shown that this satellite D N A constitutes a great part of the total
heterochromatin of the species and is present in all but the Y chromosomes. The copy
Satellite D N A and speciation: A species specific satellite D N A of D. guanche
91
number of the basic unit is high and some, though modest, variation exists between the
many copies. Members of the same cluster seem to be more similar to each other than to
members of other clusters. The satellite is specific for D.guanche and is not present in the
two sibling species D.subobscura and D.madeirensis (and in no other species of the D.
obscura group).
The main question in this context is certainly whether the evolutionary origin of this
satellite D N A was substantial for the species formation itself, or whether the repetitive
clusters have originated only by chance after speciation? Some authors believe that satellite
DNA has no function at all, but is only composed of selfish D N A which has acquired a
mechanism for a somewhat higher replication efficiency than the rest of the genomic D N A
(ORGEL
and CRICK1980; DOOLIITLEand SAPIENZA1980). Many other students of
molecular evolution, however, believe that satellite D N A has some, perhaps yet unknown
function in the organisms. Among other hypotheses, it is frequently assumed that satellite
D N A is important in the regulation of recombination frequency (see MIKLOS1985). It is
also postulated, that satellite D N A might play a role in the recognition of homologous
chromosomes in mitotic and meiotic cell cycles (WALKER1971; CORNEO1978). A high
divergence in the satellite DNAs may be the reason for hybrid sterility and might so act as
a postmating isolation factor. For the D.subobscura phylade this hypothesis would fit quite
well to the present situation. If we suppose that the continental species D.subobscura is the
parental species from which D.guanche and D.madeirensis originated through founder
effect and geographic isolation, the evolution of the satellite D N A of D.guanche can be
described in the following way First, as a consequence of founder effect, inbreeding and
isolation, the basic composition of the satellite changed as a matter of chance. There are
several hypotheses, which explain the general observation, that highly repetitive DNAs
evolve much quicker than unique DNAs. Especially the supposed mechanism that
unequal crossing over between tandemly repeated sequences can increase or decrease the
copy number of a basic unit comparatively quick and can homogenize with the same
mechanism the members of the clusters effectively (SMITH1976; OHTA1983), could be
responsible that D . guanche has developed its species specific satellite in a very short
period. As could be shown by SCHWEIZER
and coworkers (see SCHWEIZER
et al. 1987),
heterochromatin exchange can also appear between non-homologous chromosomes. This
would explain, why the satellite of D.guanche has spread to all chromosomes and has
consequently acquired in the course of its transpositions also a somewhat higher variability between the clusters. Later, after this divergence in satellite DNA had succeded, at a
second invasion of D. subobscura to the Canary Islands, the satellite could serve as an
isolating factor, so that D.guanche was equipped with a mechanism to maintain its species
status. Satellite DNA evolution, in this way, would not be assumed as the cause for
cladogenesis but the preposition for the genetically diverged pre-guanche population to
become a distinc species reproductively isolated from D.subobscura.
Though the above explanation is consistent in itself, it has to be considered as a very
preliminary working hypothesis. Satellite DNA evolution has been studied in other
Drosophik groups too. Especially in the D.meknogaster group (for summary see BERIDZE
1986) and in the D.virilis group (GALLet al. 1973) a number of different satellites have
been detected which are mainly repetitive copies of very small basic units (e. g. 7bp) and
show interspecific similarities. All the different repetition units can be easily arranged in a
dendrogramm and even the subunits of D.virilis can be included in that diagram (MULLINS
1979). It remains open whether the mechanism proposed for D.guanche
and BLUMENFELD
is a specific evolutionary peculiarity or typical for species formation through geographical
isolation.
The species specificity of our satellite of D.guanche could be certainly used as a, though
curious, discriminating trait in taxonomy. Nobody, certainly would use such a character
for practical purposes in the field. However, very recently, species specific D N A se-
L. Bachmann, M . Raab and D . Sperlich
92
quences have been discussed as a very sensitive and reliable trait under certain circumstances. Especially in parasitology very often the problem appears that species must
be determined at larval or prelarval stages. Morphological characters are usually insufficient there. In this cases dot blot in situ hybridizations of a species specific probe with the
DNA of an even tiny organism might allow an exact and reliable taxonomic determination
(see POSTand CRAMPTON
1987). The 290bp satellite DNA of D. guanche might serve as a
good example for highly repetitive DNA as a good candidate in the search for species
specific DNA sequences.
Acknowledgements
We are greatly indebted to our colleagues of the Genetic Department of our University for many
valuable technical advices. One of us (M. RAAB)had the opportunity to see and learn specific
staining techniques in the laboratory of Prof. SCHWEIZER
in Vienna. We thank also very much
KARINSTOGERER
and CHARLOTTE
REHMfor their help in the preparation of the manuscript.
Zusammenfassung
Satelliten-DNA und Artbildung: Eine artspezifische Satelliten-DNA bei Drosophila guanche
Das Heterochromatin der Chromosomen von Drosophila guanche besteht zum groaten Teil aus
einer Satelliten-DNA, die aus vielfachen Kopien einer 290 Basenpaar Ian en Grundsequenz aufgebaut ist. Funf verschiedene Klone, die ein oder zwei Kopien der Grunfeinheit besagen, konnten sequenziert werden. Wie bereits aus der Dichtegradienten-Zentrifugation und aus AT-spezifischen Farbungen des Mitosechromosomes erwartet wurde, erwies sich die Sequenz als ATreich. Die durchschnittliche Nucleotidvariabilitat zwischen den klonierten KO ien betrug
11,6 %. In situ-Hybridisierun der Sequenz an den Mitosechromosomen zeigte, dalsie im Zentromer-nahen Bereich aller Ctromosomen mit Ausnahme des Y-Chromosoms vorkommt. Die
Nukleotid-Variabilitat zwischen den Einheiten verschiedener Tandem-Gru pen scheint hoher zu
sein als zwischen den Einheiten derselben Gruppe. Die Kopienzahl der gquenz im ha loiden
Genom wurde auf ungefahr 80 000 geschatzt. Die Sequenz 1st artspezifisch und kommt {ei den
Geschwisterarten D. subobscura und D . madeirensis nicht vor. Die evolutive Entstehung der Satelliten DNA und deren mogliche Rolle bei der Artbildung wird diskutiert.
Literature
AYBROS,P. F. ; MATZKE,
M. A. ; MATZKE,
A. J. M., 1986: Detection of a 17 kb unique sequence
(T-DNA) in plant chromosomes by in situ hybridization. Chromosoma 94, 11-18.
BERIDZE,
T., 1986: Satellite DNA. Berlin: Springer-Verlag.
BOHM, I.; PINSKER,
W.; SPERLICH,D., 1987: Cytogenetic mapping of marker genes on the
chromosome elements C and E of Drosophila pseudoobscura and D . subobscura. Genetica75,
89-101.
V. M.; GONZALES,
A. M.; LARRUGA,
J. M.; GULLON,
A., 1983: Genetic distance and
CABRERA,
evolutionary relationships in the Drosophila obscura group. Evolution 37, 675-689.
CHAN,V. T. W.; FLEMING,
K. A,; MCGEE,J. 0. D., 1985: Detection of subpicogram quantities
of specific D N A sequences on blot hybridization with biotinylated probes. Nucleic Acids
Research 13, 8083-8091.
CORNEO,
G., 1978: Satellite DNAs in eukaryotes: a non-ada tive mechanism of speciation
which originated with sexual reproduction. Experientia, Basi34, 1141-1142.
DAVIS,
L. G. ; DIBNER,M. D. ; BATTEY,
J. F., 1986: Basic Methods in Molecular Biology. New
York: Elsevier.
C., 1980: Selfish genes, the phenotype paradigm and genome evoluDOOLITTLE,
G. ;SAPIENZA,
tion. Nature, London284, 601-603.
GALL,J. G.; COHEN,E. H.; POLAN,M. L., 1971: Repetitive D N A sequences in Drosophila.
Chromosoma 33, 319-344.
GALL,[. G.; COHEN,E. H.; ATHERTON,
D. D., 1973: The satellite DNAs of Drosophila virilis.
Co d Spring Harbour Symp. Quant. Biol. 38, 417-421.
JOHN,3.;KING,M.; SCHWEIZER,
D.; MENDELAK,
M., 1985: Equilocality of heterochromatin
distribution and heterochromatin heterogeneity
in acridic grasshoppers.
Chromosoma 91,
__
185-200.
KING,P. V.; BLAKESLEY,R. W., 1986: Optimizing DNA ligation for transformation. Focus 8,
1-3.
Satellite DNA and speciation:A species spebfic satellite DNA of D . guanche
93
KRIMBAS,
C. B.; LOUKAS,
M., 1984: Evolution of the obscura group DYOSO
hila species. I. Salivary chromosomes and quantitative characters in D. subobscura a n l t w o closely related
species. Heredity 53, 469-482.
LAKOVAARA,
S.; SAURA,
A. ; LANKINEN,
P.;POHJOLA,L. ; LOKKI,J., 1976: The use of isozymes
in tracing evolution and classifying Drosophilidae. 2001. Scripta 5, 173-179.
LANDEGENT,
J. E. ;JANSEN
IN DE WAL,N.; VAN OMMEN,
G. J. B. ;BAAS,E ;DE VIJLDER,J. J. M. ;
VAN DUIJN, l?; VAN DER PLOEG,M., 1985: Chromosomal localization of a unique gene by
non- autoradiographic in situ hybridization. Nature 317, 175-177.
MANIATIS,
T.; FRITSCH,
E. F.; SAMBROCK,
J., 1982: Molecular cloning. A laboratory manual.
Cold Spring Harbor Lab.
MANUELIDIS,
L., 1977: A simplified method for preparation of mouse satellite DNA. Anal.
Biochem. 78,561-568.
MOLT^, M. D. ; DE FRUTOS,R. ; MARTINEZ-SEBASTIAN,
M. J., 1987: The banding pattern of
oolvtene chromosomes of Droso~hila”wanche comuared with that of D . subobscura.
Genetica75, 55-70.
MULLER.
H. 1.. 1940: Bearines of the DrosoPhila work on svstematics. In: The New Svstematics.
J. S. Oxrord: Oxford Univ. Press and London and New York: klarendon.
Ed. by H~XLEY,
1
,
185-268.
M., 1979: Satellite 1 c: a possible link between the satellite DNA of
MULLINS,. I. ;BLUMENFELD,
D. virils and D. melanogaster. Cell 17, 615-621.
ORGEL,
L. E. ;CRICK,F. H. C., 1980: Selfish DNA: The ultimate parasite. Nature, London 284,
604-60%
POST,R. J. ;CRAMPTON,
J. M., 1987: Probing the unknown. Parasitology Today3, 380-383.
PREISS,A. ; HARTLEY,
D. A. ;ARTAVANIS-TSAKONAS,
S., 1988: Molecular enetics of Enhancer of
split, a gene required for embryonic neutral development in Drosopfila. Embo J. 12, 3917392%
SCHILDKRAUT,
C. L.; MARMUR,
J.; D o n , P., 1962: Determination of the base composition of
desoxyribonucleic acid from its buo ant density in CsCI. J. Mol. Biol. 4, 430-443.
SCHWEIZER,
D., 1981 : Counterstain-enhTanced chromosome banding. Hum. Genet. 57, 1-14.
SCHWEIZER,
D. ; LOIDL,J. ; HAMILTON,
B., 1987: Heterochromatin and the phenomenon of
chromosome bandin . In: Structure and function of eukaryotic chromosomes. Ed. by W.
HENNIG.Berlin, Heiielberg, New York, Tokyo: Springer Verlag.
SMITH,G. P., 1976: Evolution of repeated DNA sequences by unequal crossover. Science, Wash.
DC 191, 528-535.
SPERLICH, D. ; PFRIEM,P., 1986: Chromosomal polymorphism in natural and experimental
M.;
populations. In: The genetics and biology of Drosophila. Vol. 3 e . Ed. by ASHBURNER,
CARSON,
H. L.; THOMSON,
J. N. London: 257-309.
WALKER,
P. M. B., 1971: “Repetitive” DNA in hi her organism. In: Biophys. Ed. by BUTLER,
J.
A. Y ; Noble, D. Mol. Biol. Vol. 23. Oxford: Bergamon Press. 147-190.
Authors’ address: L. BACHMANN,
M. RAABand D. SPERLICH,Lehrstuhl fur Populationsgenetik,
Fakultat fur Biolo ie, Eberhard-Karls-UniversitatTiibingen, Auf der Morgenstelle 28, D-7400 gubingen, FRG