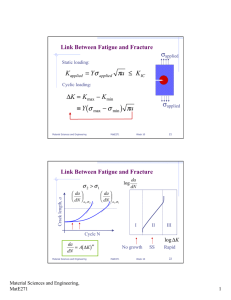
EFFECT OF OXYGEN ON CREEP CRACK by
advertisement

EFFECT OF OXYGEN ON CREEP CRACK GROWTH IN NICKEL-BASE SUPERALLOYS by Kenneth Rees Bain B.M.E., General Motors Institute (1980) S.M., Massachusetts Institute of Technology (1982) SUBMITTED TO THE DEPARTMENT OF MATERIALS SCIENCE AND ENGINEERING IN PARTIAL FULFILLMENT OF THE REQUIREMENTS OF THE DEGREE OF DOCTORATE IN PHILOSOPHY at the MASSACHUSETTS INSTITUTE OF TECHNOLOGY September 1983 @ Massachusetts Institute of Technology 1983 Signature of Author: Department odfMaterials Science and Engineering August 5,1983 Certified by: Crii -Regis by: M. Pelloux Thesis Supervisor Accepted by: pm .)_Bernhardt J. Wuensch Chairman, Depatrntal Graduate Committee ArchiveS MASSACHUSETTS INSTITUTE OFTECHNOLOGY OCT 14 1983 LIBRARIES EFFECT OF OXYGEN ON CREEP CRACK GROWTH IN NICKEL-BASE SUPERALLOYS by KENNETH REES BAIN of Materials Science and to the Department Submitted Engineering on August 5, 1983 in partial fulfillment of the requirements for the Degree of Doctorate in Philosophy. ABSTRACT (CCGR) of four PM/HIP The creep crack growth rates nickel-base superalloys are measured in the range of CCGR from 10 -9 m/s to 10-5 m/s and a range of stress intensity, K, from 10 to 120 MPa/F at 7040 C in air and in 99.999% pure The alloys tested are Low Carbon Astroloy, Merl-76, argon. (60 and 120 mesh size Low Carbon IN-100, and Rene-95 Crack length was measured on single edge notched powders). specimens using the D.C. potential drop technique. Crack growth rates were observed to accelerate up to 1000 times in an air environment over the CCGR measured in 99.999% pure argon. The fracture path was totally intergranular in all the The fracture path in argon follows grain CCG tests. boundaries which coincide with prior powder boundaries for PPB grain boundary There was no preference (PPB). cracking in CCGR tests in air. The CCGR behavior of the Ni-base alloys tested was shown to correlate with the stress intensity factor, K. The CCGR versus K curves exhibit three stages of behavior which respectively with an initial transient in are associated damage accumulation ahead of the crack, power law dependent CCGR on K and fast fracture at KIC. The measured CCGR for an alloy is shown to be significantly affected by changes in test procedure, specimen design, and initial K. A comparison of the notched stress rupture results gives a qualitative measure of the CCGR behavior observed for the nickel-base alloys. Tensile, creep, and creep rupture tests at 704C for 4 alloys were performed to obtain the constitutive equations. These relationships along with the microstructure were used to model the CCGR behavior of the alloys based on the accumulation of damage ahead of the crack tip. The computer model predicts the effect of microstructure, mechanical properties, and load history on creep crack growth rates. Thesis Supervisor: Title: Dr. Regis M. Pelloux Professor of Materials Engineering 3 TABLE OF CONTENTS CHAPTER TITLE PAGE PAG 1 ABSTRACT 2 TABLE OF CONTENTS LIST OF FIGURES LIST OF TABLES 3 6 11 ACKNOWLEDGEMENTS 12 1. INTRODUCTION 13 2. LITERATURE 16 REVIEW 2.1. Metallurgy of Ni-Base Alloys 2.1.1. Chemistry of Ni-Base Alloys 2.1.2. Strengthening Mechanisms 2.1.3. Grain Boundary Morphology 2.1.4. Heat Treatment 16 16 22 25 28 2.2. Environmental Embrittlement of Ni-Base Alloys 2.2.1. Determination of Embrittling Elements 2.2.2. Theories for Environmental Embrittlement 2.2.3. Effect of Grain Boundary Chemistry on Environment Embrittlement 29 Creep Crack Growth 2.3.1. Analysis of CCGR Testing Methods 2.3.2. CCGR in Various Alloys 2.3.3. Air Embrittlement of Ni-Base Alloys 2.3.4. Effect of Microstructure on CCGR in Ni-Base Alloys 42 43 48 52 Theories of Creep Crack Growth 2.4.1. Crack Tip Stress Distribution 2.4.2. CCGR Models 2.4.2.1. Diffusional Creep Models 2.4.2.2. Creep Constrained Cavity 57 57 63 63 65 2.3. 2.4. 33 38 54 Deformation Controlled 2.4.3. CCGR Models Iterative CCGR Models 66 4 TABLE OF CONTENTS (cont'd) CHAPTER3. EXPERIMENTAL PROCEDURES 3.1. 69 Materials 69 3.3. 3.1.1. Chemistry and Processing 3.1.2. Microstructural Characterization Mechanical Testing 3.2.1. Tensile Testing 3.2.2. Smooth Bar Creep Testing 3.2.3. Notched Stress Rupture Testing 3.2.4. Creep Crack Growth Rate Testing 3.2.4.1. D.C. Potential Drop Technique 3.2.4.2. Data Analysis Pre-exposure Oxygen Penetration Tests 70 72 77 77 79 79 81 83 91 93 3.4. Fractography 93 3.2. 4. PAGE EXPERIMENTAL 4.1. 4.2. 4.3. 4.4. RESULTS 95 Tensile Properties at 7040C Smooth Bar Creep Results 4.2.1. Minimum Creep Rate Results 4.2.2. Creep-Rupture Results Notched-Rupture Results in Air 4.3.1. Constant Load Results 4.3.2. Air Pre-exposure Results Creep Crack Growth Rate Results 4.4.1. CCGR for Five Ni-Base Alloys 4.4.1.1. CCGR of PM/HIP Low C Astroloy 4.4.1.2. CCGR of PM/HIP Merl-76 4.4.1.3. CCGR of PM/HIP Low C IN-100 4.4.1.4. CCGR of PM/HIP Rene-95 95 96 96 98 100 100 100 102 102 102 104 106 106 (60 mesh) 4.4.1.5. CCGR of PM/HIP Rene-95 (120 mesh) 4.4.2. Effect of Initial Stress Intensity Factor 4.4.3. Comparison of CCGR in Air 4.4.4. Comparison of CCGR in Argon 4.4.5. Validity of K Correlation of CCGR 4.4.6. Fractography of CCG Tests 4.4.6.1. Argon Tests 4.4.6.2. Air Tests 4.5. G. B. Penetration of Oxygen Results 109 109 112 112 115 116 117 118 118 5 TABLE OF CONTENTS (cont'd) CHAPTER 5. AN ITERATIVE MODEL FOR CREEP CRACK GROWTH 130 5.1. 'Introduction 130 5.2. Numerical Procedures 5.2.1. Calculation of Stress and Strain 5.2.2. Accumulation of Strain 131 131 136 5.3. Effect 140 5.4. 5.5. 143 Predictions of Creep Crack Growth Rates 148 Effect of Critical Parameters 5.5.1. Effect of Critical Strain to Fracturel 48 5.5.2. Effect of Grain Size 149 149 Constant K Calculation of CCGR 153 Effect of dK/da on CCGR 155 Effect of Temperature and Yield Strength Predictions of Creep Crack Initiation Times 158 5.6. 5.7. 5.8. 5.9. 6, PAGE of Oxygen 159 DISCUSSION 6.1. CCGR Modelling 6.1.1. Effect of Triaxiality 6.1.2. Effect of Oxygen Concentration 6.1.3. Limitations of the CCGR Model 62. CCGR of PH/HIP 6,2.1. Effect 6.2.2. Effect Notched-Stress 6.3. Ni-Base Alloys of Test Procedures of Oxygen Rupture versus CCGR Tests 159 164 165 167 169 169 170 173 7. CONCLUSIONS 177 8. RECOMMENDATIONS FOR FUTURE WORK 179 8.1. 8.2. Grain Boundary Chemistr,y Variations Effect of Test Procedures and Specimen Design 179 180 APPENDIX I Table of CCGR Test Results 181 APPENDIX II Computer Program for CCGR Prediction 183 APPENDIX III Calculation of Ductile-Brittle Creep 186 Transition Times References 190 6 LIST OF FIGURES FIGURE 2.1. PAGE Alloying elements in Nickel-Base alloys. 19 (ref. 6) 2.2. Gamma prime volume fraction versus weight percent Al + Ti in Ni-Base Superalloys. (ref. 2.3. Comparison 21 8) of reduction in area for tensile tests on Ni-270 following 10000 C, 200 hour exposure to various gaseous environments. 31 (ref. 34) 2.4. Fracture ductility versus temperature for both inert and air exposure at 100 0 °C for 200 hours. 2.5. (ref. 34) Effective diffusivity of oxygen in pure Nickel versus 1/T. 32 34 (ref. 30) 2.6. Effect of alloying additions on the rupture 40 life of IN-738. Results given as stress versus the Larson-Miller Parameter. (ref. 22) 2.7. Creep strain versus time in PE16 at 6500 C with and without Boron and Zirconium additions. 2.8. 2.9. (ref. 32) Typcial CCGR behavior found in Nickel-Base alloys. 41 46 (ref. 64) Effective of initial K on CCGR results on .5% Cr-.5%Mo-.25%V steel at 565 0C. (ref. 41,66) 2.10. CCGR versus K for several Ni-base alloys at 47 50 7040 C. 2.11. The effect of temperature on CCGR in PM/HIP low carbon Astroloy. (ref. 63) 51 2.12. Comparison of CCGR results in an air environment and in an inert environment for Ni-Base alloys at6500 C. 53 2.13. Initial K versus time to failure for CCGR tests on IN-792 in air at 7040 C. (ref. 35) 55 2.14. Effect of heat treatment on CCGR results on 56 IN-100 in air at 700 0C. (ref. 57) 7 2.15. Calculated stress field ahead of a crack tip for PM/HIP low carbon Astroloy. (ref. 63) 62 2.16. Stress and Creep Strain ahead of a crack tip versus time, used in modelling CCGR. 68 3.1. Microstructure of PM/HIP Low Carbon Astroloy. 73 3.2. Microstructure of PM/HIP Merl-76. 74 3.3. Microstructure of PM/HIP Low Carbon IN-100. 75 3.4. Microstructure of PM/HIP Rene-95. 76 3.5. Specimen geometry used for both tensile and creep-rupture testing. 78 3.6. NSR specimen geometry 80 3.7. Single Edge Notched specimen geometry used for CCGR testing. 82 3.8. Theoretical and experimental d.c. potential drop calibration of SEN specimen geometry. 85 3.9. Schematic test system. 87 of CCGR 3.10. Variation in the calculated a/w from the d.c. potential drop technique with varying load spacing, Y. 90 4.1. Stress versus minimum creep rate results at 70°0 C for 4 Ni-Base Alloys. 97 4.2. Stress versus time to rupture for NSR tests on 4 alloys in air at 704C. 99 4.3. CCGR results for PM/HIP Low Carbon Astroloy 103 at 704C in both air and 99.999% pure argon. 4.4. CCGR results for PM/HIP Merl-77 at 704 0 C in both air and 99.999% pure argon. 4.5. CCGR results for PM/HIP Low Carbon IN-100 107 at 7040 C in both air and 99.999% pure argon. 4.6. CCGR results for PM4HIP Rene-95 (60 mesh powder) at 704 C in both air and 99.999% pure argon. 108 4.7. CCGR results for PM/HIP Rene-95 (120 mesh powder) at 7040 C in both ari and 99.999% pure argon. 110 105 8 4.8. CCGR results for PM/HIP Merl-76 in air at 7040°C with varying initial K. 111 4.9. CCGR results for five PM/HIP Ni-Base 113 in air at 7040 C. alloys 4.10. CCGR results for five PM/HIP Ni-Base alloys in argon at 7040 C. 114 4.11. Fractograph of the Fatigue precrack creep crack growth transition in Rene-95 (120 mesh) and in IN-100. 120 4.12. Fractograph of the creep crack - Fast 121 fracture transition in In-100 at 704 C in air. 4.13. Fractograph of the CCG - Fast fracture transition at 7040 C. in IN-100 in pure 122 argon 4.14. S.E.M. Fractographs of CCG fracture surface for PM/HIP Astroloy in air and in 99.999% pure argon. Tests performed at 704°C. 123 4.15. S.E.M.Fractograph of a typical creep crack fracture in arson for PM/HIP Low Carbon In-100 at 704 C. 124 4.16. S.E.M. Fractograph of a typical CCG fracture surface for Rene-95 (60 mesh) tested in argon at 7040 C. 125 4.17 S.E.M.Fractographs of the typical creep crack fracture surface in argon at 704°C for the four Ni-Base alloys tested. 126 4.18. Cavity like features observed on Rene-95 (60 mesh) and Merl-76 fracture surfaces at 127 10,000x. Tests run at 704 C in argon. 4.19. S.E.M. Fractographs of typical creep crack fracture surfaces in air at 7040C for the four Ni-Base alloys tested. 128 4.20. S.E.M. Fractograph of a creep crack fracture surface tested in air for PM/HIP Low Carbon Astroloy. 129 5.1. 135 Graphic illustration of the stress field ahead 5.2. Graphic of a creep illustration advance. crack at t=0. of the Dt for crack 139 9 5.3. Ratio of critical creep ductility in air 142 and argon versus the ratio of grain boundary concentration of Boron to that of Astroloy. 5.4. Predicted and Actual CCGR results for PM/HIP Low Carbon Astroloy. 144 5.5. Predicted and Actual CCGR results for PM/HIP Merl-76. 145 5.6. Predicted and Actual CCGR results for PM/HIP Low Carbon IN-100. 146 5.7. Predicted and Actual CCGR results for PM/HIP Rene-95 (60 mesh). 147 5.8. Plot of predicted constant K CCGR versus critical strain for Astroloy at 7040 C in air. 150 5.9. Plot of predicted constant K CCGR versus grain size for Astroloy at 7040 C in air. 150 5.10. Effect of grain size on predicted CCGR curves for PM/HIP Low Carbon Astroloy at 7040C, and actual CCGR results. 151 5.11. Predicted CCGR versus the number of crack advances for constant K tests. Results based on constitutive relationships for Astroloy in air at 7040 C. 152 5.12. Effect of dK/da on the predicted CCGR for PM/HIP Low Carbon Astroloy in air at 7040 C. 154 5.13. Comparison of Predicted and Actual CCGR versus K data obtained by Huang (63) for PM/HIP Low Carbon Astroloy in air. 156 5.14. Predicted constant K CCGR versus yield strength for Astroloy in air at 7040 C. 157 5.15. Plot of initial K versus predicted time to first crack advance. Prediction based on data for Astroloy in air at 7040 C. 157 6.1. Graphic illustration of the effect of 162 dK/da on measured CCGR. 6.2. Predicted effect of plane stress on the CCGR for Astroloy in air at 704 0C. 166 6.3. CCGR measured 172 in air at K = 30 MPa/im 704 C for 4 Ni-base alloys versus the 10 product of the effective diffusivity of oxygen along the grain boundaries and the grain size. 6.4. CCGR in air at K = 30 MPa/ii at 704 C for 4 Ni-Base alloys versus the NSR time to failure. 175 11 LIST OF TABLES TABLE 2.1. PAGE Chemical Composition of selected Nickel-Base superalloys. 18 (ref. 9) 2.2. Ratio of rupture strength for various additions of solid solution strengtheners to pure nickel at 6150 C and 815 C. 23 2.3. Creep Rupture properties of U-500 at 8700 C with additions of Boron and Zirconium. (ref. 15) 27 2.4. CCGR Results for Ni-Base alloys in published literature. 49 3.1. Powder size before HPing alloys studied. for the Ni-Base 69 3.2. Thermal Processing used on Ni-Base alloys studied. 70 3.3. Alloy chemistries and microstructure for 4 Ni-Base alloys studied. 71 4.1. Tensile 95 4.2. Minimum creep rate results at 7040 C. 96 4.3. Creep-rupture results for 4 Ni-Base alloys in air at 7040 C and 801 MPa. 98 4.4. Notched stress rupture results on Rene-95 (60 mesh) in air at 7040 C, following pre-exposure to air at 7040 c with and without a load. 101 4.5. Grain boundary embrittlement study results. 100 hour air pre-exposure in air at 704C. 119 5.1. Comparison of Critical strain ratio to average 141 concentration of Boron on the grain boundary in Ni-Base alloys. test results at 704 G. 12 ACKNOWLEDGEMENTS The author gratefully acknowledges the support of the following people and institutions. Their encouragement and assistance greatly contributed to the completion of this thesis. Professor Regis Pelloux, the author's thesis advisor whose guidance, encouragement, and assistance throughout the author's graduate studies were invaluable. Professor Andre Pineau whose many discussions and critiques on modelling of creep crack growth were very helpful. Professor F.A. McClintock and Professor R. Ballinger for their reviews and comments of the final thesis. Mr. Lenny Sudenfield for his help in operating and later teaching the operation of the scanning electron microscope at M.I.T. The author would like to thank Philippe Bensussan and Bill Moshier, graduate students at M.I.T., for their help and discussions on occasions too numerous to count. The author would also like to acknowledge all the members of the creep and fatique research group for their interesting discussions and assistance throughout the author's graduate career. My wife, Amy, who typed this document and without whose love and constant support this research would have been impossible. The author would like to acknowledge the financial support given by the Air Force Office of Scientific Research and the Cabot Corporation in the form of a Cabot Fellowship. The author would also like to acknowledge the Center for Material Science and Engineering for support from a NSF-MRL grant, and the AT-1, High Temperature Fracture, group for their many interesting discussions. 13 1. INTRODUCTION Creep crack growth is a process in which a single crack advances intergranularly tensile stress at is inert environment growth possible consist a material under a constant temperatures where at least local creep deformation test in of (T>.4 Tm; melting point). In an the micromechanisms of creep crack nucleation, growth, and coalescence of grain boundary cavities. The forms the recent of interests high temperature mechanisms for crack advance are result concepts of the desire to incorporate fracture mechanics into advanced retirement-for-cause life of in creep crack growth and other as design a criteria. The concept of method for extending the useful of many high temperature components is but one example these area of gas advanced concern turbines criteria (1). Creep crack growth is an in nickel-base superalloys used in modern hot section components and stainless steel in nuclear and conventional power plants. Current based on design are statistical chance inch for gas turbine engines are bulk creep deformation and crack initiation. components 1/32 criteria crack. removed that from one service when there The is a part in 1000 has developed a This conservative life limit results in the retirement of 999 out of 1000 components, many of which may have replacing up to 10 times more useful life. The cost of these components may reach $100 million a year by 14 The 1985. life of these components critical could be by periodically inspecting the part for cracks and extended replacing spacing those parts in which cracks are found. only the of inspection of understanding the periods requires both limits of detection reasonable The an of a and of the rate at which this flaw or crack propagates flaw The accuracy required in the prediction to final fracture. of a creep crack propagation time is critical since failure cf a component such as a turbine disk could be costly. Creep is time-dependent deformation of a material under stress. The mechanisms of creep and creep fracture have The notched stress rupture extensively studied (2-4). been test is a simple test designed to measure the time to (NSR) rupture to creep processes in the vicinity of a stress due concentration site. Extensive NSR and smooth bar test have 'indicated that the time to rupture is reduced results in the presence of the high stresses at the root of a notch. The creep severe crack NSR growth test test can be thought of as a more in which the time to form the macroscopic crack is eliminated. The test environment has been shown to significantly affect the results of smooth bar and NSR tests (5), environment will also have an effect on the the and creep crack growth rate (CCGR). crack Creep severe tip form acts produces as of a locally growth testing can be thought of as a notched stress rupture testing. severe stress concentration The crack site which large stresses, which may relax with time 15 due to creep advances as deformation a time-dependent and result crack and fracture. The crack front of creep damage which is due to the tip stresses. Crack tip plasticity, environmental embrittlement ahead of the crack tip play an important role in controlling CCGR. This current Then thesis includes a brief review of the literature on the metallurgy of nickel-base alloys. the Ni-base literature alloys and literature review mechanical test Finally, presented, given. first a and on environmental creep crack growth is reviewed. of The is followed by a detailed description of procedures, computer a embrittlement materials, model of creep discussion of the and test results. crack growth is critical results is 16 2. LITERATURE REVIEW 2.1. Metallurgy of Ni-Base Alloys Nickel-base superalloys are the material used in almost every modern gas turbine disk and blade. outstanding low-cycle these tensile fatigue alloys > 6000 C) Typical modern 100,000 hr. stability. strength, at which properties, the are high common turbine lives capability weight and creep-rupture and corrosion operating strength, resistance of temperatures (i.e. in modern gas turbine engines. engines are designed for 5000 to which therefore require long time alloy Turbine maximum This is due to the operating conditions are pushed to the of the alloy in the interest of minimum maximum operating efficiency. All these requirements are generally fulfilled by Ni-base superalloys. The following sections detail the chemistry, microstructure, strengthening mechanisms and heat treatment of nickel-base superalloys. 2.1.1. Chemistry of Ni-Base Alloys The chemistry of nickel-base superalloys is complex with at least 12 carefully controlled alloying elements. In general these alloys contain 10-20 percent chromium, up to 8 percent aluminum zirconium, specific and titanium, and small amounts of boron, and carbon. properties, other properties. Other elements are added to enhance but usually at the expense of some 17 The alloying elements can be divided into three classes which are: Elements which prefer to form the face-centered 1. cubic (FCC) austenite (gamma,y ) matrix (i.e. nickel, iron, chromium, cobalt, molybdenum, tungsten, and vanadium) 2. Elements which form the gamma prime phase (y ') (i.e., aluminum, titanium, columbium, and tantalum) 3. Elements which segregate to the grain boundary (i.e., magnesium, boron, carbon, zirconium, and undesirable impurities). 2.1. Table chemical gives superalloys nickel-base compositions for several currently being used in modern gas turbine applications (9). The most common alloying additions in nickel-base alloys and the area of their greatest influence in the alloy is shown in Figure The (gamma, Y MC ). boundaries of a consists mainly of microstructure large and 2.1. (6). solid embedded carbides in ' precipitates the austenite matrix y' precipitates are also found at the grain along with M 2 solution of molybdenum, and tungsten. 6 carbides. nickel The matrix consists with cobalt, chromium, This combination of phases allows the utilization of the alloys at up to .8 (melting point) 18 C- 0 \O \O O 0 I0 0 0 \O 0 0 I I 0 o C .9-I a) 0O H OH \0 0 0 I 0 0 0 00 \0 0(~ I kH ,0 O m 0 O O O 00O 0OH-0\C\ H 00 'f0 u00 00 oN O ' 0 m 0 03: --- i I zrq .H1 E4 r-4 C 0a) O la *H H. C O. n 0 ' O O) U :z ,0 alE0 Pq C k I ! I I· o0O C- 00 01 rl . I . n. c . . c'-s O' O Ez :0 0 0 (: O k ·O 0rZ 0~ . \o 0 i* o0 0 0 CO0 H H- H H M, H Err H U) O 0 o 0 0 H oo 0 0 I 0 -6 0 O 0- '0 0 0 0N n0 O' \ ' O C) . CZ 0 V\ H : C) H1Iw C'- 0r CZ CN2 O O P. H _ E- C.- 19 Nv8 InA rlA -21 +6 +- 429 27 - -.AF- 7.66 IV A 49 _; VA - r. - -666 +28 __ V A VI A V ¥ - -VI A Co 4.66 4.66 No 1.71 066 N, n Atomic Difference o. DiameterfromNickel 5.66 1,.66 +13 Ta - O +12 Nb +18 -. +1 Fe Cr 5 66 +18 +3 3 . W 5.66 4.66 .. . . . !, AtomicDiameter of Carbon.Soron.Zrcni Magnesum - Goldschmidt forCN12 AtomicDianeterof OtherElements from tticeParameterEffectn NickelBinaryAlys " El~ement Partitions to FIGURE 2.1) D Element Partitions to ' 1 Partitions to GrainBoundary 3 1 Element Periodic table of alloying elements in Nickel-Base Superalloys. (ref. 6) 20 for times as long as 100,000 hrs (6). attack down slow or inhibit which Al203 oxides at the surface and Cr203 form aluminum and Additions of chromium by oxygen, nitrogen, sulfur, and other aggressive atmospheric elements (7). main precipitate phase in the gamma matrix (y ) is The ( y'). gamma-prime "B" and a phase with the general FCC with "A" being either nickel, iron, or cobalt A 3 B, formula is It aluminum, either being titanium, or columbium. precipitates were initially spherical in early alloys These treatments heat advanced but result in shape cube a precipitate which enhances strengthening. The tantalum. aluminum, of percent atomic y' is a function of the combined percent weight The result of y' columbium, ti tanium, analyses of 15I superalloys are summarized in Figure 2.2(8). It and nickel-base can be seen that additions of aluminum, titanium, columbium and tantalum will increase Y' formation. The presence of Cobalt and Vanadium will also enhances the formation of y' from the y matrix. are Carbides (usually boundary boundary phases in Ni-base alloys. microstructure grain major sliding. M23C6) of the grain boundary Carbides, located at the markedly inhibit grain These grain boundary carbides, however, can and will serve as nucleation sites for creep cavities. One the major problems with Ni-base superalloys is of the formation TCP phases of topologically closed-packed phases (TCP). usually have body centered tetragonal crystal The Libraries Massachusetts Institute of Technology Cambridge, Massachusetts 02139 Institute Archives and Special Collections Room 14N-118 (617) 253-5688 This is the most complete text of the thesis available. The following page(s) were not included in the copy of the thesis deposited in the Institute Archives by the author: H.] i 22 A Laves phase has the chemical formula structure. and cobalt "B" being either Mo or Ta. (Ni,Co)y where phase is generally of the form (Cr,Mo Sigma x being usually "A" and can vary from 1 to 7, but usually are both around y as hard flat plates or needles form phases These unity. B with which will reduce alloy ductility (10). The Fe to the matrix enables the of y" , eta, and delta phases which strengthen precipitation Columbium When alloy. the of addition IN-718, the primary centered tetragonal is present, such as in alloy strengthening phase is ", phase with the composition Ni 3Cb. body y" can degenerate into the orthorhombic delta phase. The third phase enabled by iron is called eta, which is Ni 3Ti and has a hexagonal close packed (HCP) structure. is a IN-901, strengthening phase in alloys such as A-286, primary and Pyromet 860. However, the frmation IN-706, brittle large Eta particles of of either eta or delta phase will reduce rupture life and ductility. 2.1.2. Strengthening Mechanisms Several Ni-Base strengthening alloys give to mechanisms are usually used in high short and long time strength over a wide range of operating temperatures. Several alloys as harden the between the elements are added to the solid solution alloys solute strengtheners. matrix of Ni-base These elements as a result of the atomic size mismatch atoms and the nickel matrix. The most 23 potent solid elements, Rene-95 solution strengtheners are the slow diffusing such has as both molybdenum and tungsten. Mo and W additions. The effect of solid solution strengtheners is shown in Table 2.2. 10% additions Ni is reported The most The effect of of Mo, Cr, and W on rupture strength of pure (12). most temperatures For instance, up common (Ni,Co)3 (Al,Ti). important to mechanism for 7600C is precipitation hardening. The and This strengthening effective precipitate phase a has face is ¥ ', centered cubic 24 structure which is compatible with the FCC a 0 1% to latice mismatch. homogeneous nucleation of the stability. Other also on The mismatch allows y' precipitate and long time such as y", eta, and delta can effect of the precipitates on strength depends The the size, shape, and volume fraction of the precipitate. strength optimum third The of formation act to The formation from strengthening a mixture of coarse and (7, 13). mechanism involves the beneficial carbides at grain boundaries which inhibit grain of boundary migration and hence creep. blocky MC and M23C 6 carbides along grain lock the grain boundaries and decrease the creep boundaries at comes y' precipitates shaped fine, cubic rate low from the matrix and act to strengthen the precipitate alloy. phases The matrix and has high temperature. The carbides can also form as M6C, Cr7C3, and as a film of M23C 6 along the grain boundary, all of which will embrittle the grain boundaries and result in premature fracture. The above effects (and others) can be summarized in the following improve guidelines for alloy design and heat treatment to the balance struck tensile and creep strength: between creep, ductility, and 25 1. Solid solution strengthen y 2. Increase volume percent 3. Increase coherence strains for less than 0.6 Tm. 4. Decrease ripening rate for greater than 0.6 Tm. 5. Solid solution strengthen 6. Minimize formation of TCP embrittling phases such as Ni 3Nb, Laves, and a phases. 7. Control carbides and grain boundary rupture strength. 8. Careful control of heat treatment to develope y'. '. y' to enhance microstructure. 2.1.3. Grain Boundary Morphology Creep fracture crack and growth therefore is a process of intergranular the control of the composition and microstructure of the grain boundaries is of great interest. Several alloying magnesium,and elements carbon such segregate as boron, zirconium, to the grain boundaries in Ni-base alloys. Addition zirconium have properties.(7) times, of less been These elongation than .1 weight percent of boron and shown additions to increase increased creep-rupture lifetime by 13 by 7 times, rupture stress by 1.9 times, and they law creep. (7, 12) Magnesium also is thought to improve the creep also increased the stress exponent, N, properties, element. for power but it is not widely used as an alloying 26 While it beneficial not effect, the mechanisms by which B and Zr act are clearly grain is known that these B and Zr elements have a understood. boundaries B because and Zr will segregate to the of their atomic sizes 21 percent undersize and 29 percent oversize, respectively. zirconium have been shown to retard grain boundary cracking Boron and in U-500, and boron alone has been shown to resist oxidation damage along grain boundaries in IN-738 (14). Boron boundary was crack indicates that observed initiation upon the loading onset in of grain U-500, which When the concentration of boron is 120 ppm, it will form M 3 B 2 borides with the "M" being primarily as reduce it may be a critical element for inhibiting grain boundary fracture. above to molybdenum and chromium. These g.b. borides act a source of boron as well as molybdenum and chromium for the grain boundary (7). It zirconium Reduced has also and diffusion formation creep-rupture (15). retard rates grain-boundary diffusion. along grain boundaries reduce the y' nodules around M 2 3 C 6 carbides in U-500. of region of fine strength. suggested that additions of boron, magnesium tendency to form The been y' nodules depletes the grain boundary y' which results in a loss of grain boundary The effect life of of boron Udimet 500 and zirconium on the is shown in Table 2.3. 27 TABLE 2.3. Creep Rupture of Udimet 500 at 870 C Alloy a =17.6MPa, hrs. Life at Base Alloy +.19% Zr +.009% B +.009% B + .01% Zr Creep Exponent, N 50 2.4 140 400 647 4.0 7.0 9.0 The boron and zirconium additions reduced the number of y' nodules observed and the number of microcracks. The alloys MC, M 23 C6, Cr 7C 3 of carbon at grain boundaries in Ni-Base is generally better understood. marked MC effect Cr 7 C3 or tendency for carbides carbides boundary M 6 C carbides. grain Carbon forms either M 23 C6 carbides have a boundaries, as well as M 6 C and which are observed as grain boundary films. are y' (15) important and M 2 3 C6 for the carbides formation through the of grain following reaction: 6MC + Y M23C6 + Y' This reaction occurs between 7600°C and 9800 C. Carbides and grain creep have crack growth. boundaries reducing cavitation creep positive and negative effects on creep Blocky M inhibit rates. They grain also 23 C 6 and MC carbides at boundary sliding thus act as sites for creep and tend to increase the CCGR as observed by Law 28 (16), Blackburn and in (18) al. of Cr7C 3 or M6C both have a strong negative grain the embrittling of effect Grain alloys. Ni-base wrought different films boundary Pelloux and Huang (17) and Saegusa et boundaries and reducing stress rupture strength. 2.1.4. Heat Treatment Proper for critical development the Wrought alloys, mainly Y following temperature varies temperature this and matrix from prepares nickel-base superalloys is of treatment heat of strength and ductility. MC y', consist of of solutioning Y' The carbides. solvus 10400 C to 12300 C, and aging above the matrix for precipitation of y' on subsequent cooling and/or aging. Following precipitate to given Rupture phases. precipitating 24-hour solutioning a series of aging treatments are y' period. and creep and in develop the major strengthening the This resistance range is is obtained of 8400 C to 1100 0 C for a followed by an aging approximately 7600 C to complete the development of fine Good by at '. tensile strength at lower temperatures is developed by precipitating fine y' by aging slowly at 7600 C. This also minimizes the formation of carbides at grain boundaries (7). Several variations of the general heat treatments are used to develop various microstructures. Alloys which depend on y" for strength, such as IN-718, usually require a longer time for precipitation at a 29 temperature. lower is to used avoid The lower temperature (650 C-760 C) age the of eta phase which will formation reduce creep-rupture life. (11) Environmental Embrittlement of Ni-Base Alloys 2.2. degradation in properties of metallic materials in The environments various long been observed. Nickel-base operating at temperatures above 500 C in air exhibit alloys a has marked decrease in their creep-rupture life (24), and a decrease in notched stress rupture life (16), lower fracture (34), ductility at increase in fatigue crack growth rates in creep crack growth rates (17). and (19), an temperatures high in air has been Embrittlement observed for iron-nickel alloys (21), cobalt alloys (20), and nickel-base alloys (22-24, 26, 27, 30, 33, 34). in strongest Embrittlement in air is the nickel-base alloy systems. Only a slight of air is observed on cobalt systems and iron-nickel effect Air systems. embrittlement is not observed in copper base alloys, and in aluminum alloys (36). 2.2.1. Determination of Embrittling Elements Chaka and McMahon (33) have demonstrated that air will creep-rupture life of Udimet 700 at 9250 C by a reduce the factor of 2 over the creep-rupture life in vacuum. results Similar have been observed by Shahiniam (24) and Prager and Sines (23) Hosoi and on several wrought and cast nickel-base alloys. Abe (26) indicated that small amounts of oxygen 30 can rupture All of time studi'es were performed on Ni-base alloys at 7500 C. above Most Ni-base alloys will have It has of their strength at these temperatures. lost most been shown pre-exposure IN-738 9000 C. causing the initiation of surface cracks. by these temperatures and the surface of IN-617 and reduce the creep decarburize that a to air when (30, loss in at 1000°C is observed for pure nickel at temperatures between 6000 C and tested 34) ductility following tensile Exposure to air results in brittle intergranular fracture. Ni-base gaseous elements. Nimonic 115, presence of are alloys embrittled by a variety of other Fatigue crack growth rates in IN-718 and Ni-base both gas hydrogen alloys, at are increased in the 6500 C (35). The presence of carbon dioxide was found to embrittle alloy PE 16 and reduce creep-rupture lives the environments 7000 C at presence (25). Along with the above of sulfur containing gases will ductility and rupture times for nickel-base alloys decrease (37). Recent is oxygen the temperatures were tensile measured. species in air at high Round specimens .10 inches in diameter to a variety of atmospheres at 10000C for These environments included vacuum, N2 , H , CO2, H20, 02, and air. time Ni-270 (99.98% Ni) has shown that embrittling (34). pre-exposed 200 hours. on research test The specimens were then failed in a short at 8000C and the reduction in area was The results (See Figure 2.3) indicate that oxygen 31 NI 270, 1000 C EXPOSURE FOR 200 HRS. IN VARIOUS ENVIRONMENTS - VAC 10c 90 C02 I-- 80 tm -- 7 I H2 CO .- N2 r _ 70 I-Cu .- 60 C: 0 '-H Ii W O o ad C0 50 40 .m 30 V20 m AIR 10 02 I LI k./ "I w FIGURE 2.3) __ _ __ · I w m I m m Percent reduction in area for NI 270 following 200 hr. exposure to various gaseous environments at 1000 C. (ref. 34) 32 100 90 r 80 : z-70 E450 z r 40 "a 30 20 10 0 0 200 400 600 800 TEMPERATURE ( C) FIGURE 2.4) Reduction in area results for NI 270 versus test temperature following a 10000 C/200 hr. exposure to either air or a vacuum. (ref. 34) 1000 33 is the embrittling embrittling species. effect of oxygen Figure is 2.4 only shows that the detectible over a narrow range of temperatures from 5000 C to 9000 C (34). Oxygen penetration along grain boundaries in pure nickel and nickel-base alloys produces severe grain boundary embrittlement. The depth of grain boundary embrittlement of pure Ni in air from 9000 C to 11000 C was measured on fracture surfaces after tensile tests (30). generate a plot of the The results were used to effective diffusivity of oxygen versus temperature 1l' (K) of oxygen exposure. diffusivity along of calculated as fracture, and plot D of 64 Kcal/gm -10 D=X 2 /t. t atom is grain the depth of intergranular 1/T gives an boundaries (Figure 2.5). activation energy was The of and values of diffusivity which range from -9 cm /s to 10 7040 C(977K) (X the is the exposure time) versus 2 10 oxygen The effective 2 cm /s. indicate Extrapolation of these results to an oxygen diffusivity along the grain boundaries of approximately 10-1 4 cm2 /s. The results gaseous oxygen reported is a by many have demonstrated that severely embrittling element in both pure nickel and nickel-base alloys. most severe along oxygen crack embrittlement grain This embrittlement is boundaries, which indicates that will be an important factor in creep growth at the temperatures of interest in this study. 2.2.2. Theories of Environmental Embrittlement While the embrittlement of nickel-base alloys by oxygen has been observed, the mechanism by which oxygen causes 34 0.1 CN '4 Cn U 4- 4 0 -ri Ai U 0 1/T (K-1)x 104 FIGURE 2.5) Depth of penetration of oxygen, X, in NI 270 + 0.005% S as a function of exposure temperature. (ref. 30) 35 forth put unknown. remains embrittlement Several theories have been to explain oxygen embrittlement. These theories are: 1. Gamma prime-oxygen reaction 2. Reduction of surface energy at 3. Complex oxide formation along grain boundaries 4. Carbon dioxide bubble formation 5. Sulfur y-y ' interfaces due to oxidation of grain boundary release sulfides. The first mechanism is the It proposed been has grain boundaries fine y' y'-oxygen reaction process. oxygen will diffuse along the that ahead of the crack tip and react with the precipitates oxide particles and . along This reaction is shown below: Y' + (0)-- + Mxy 0 removal The of fine (Equ. 2.1) y' particles along grain reduce the strength of the boundaries and would boundaries to form the grain boundaries promote brittle intergranular fracture. Oxygen boundary grain heat has been observed to increase the rate of grain cracks at boundary treated y' nodules in Udimet 500 (7). instability superalloys A severe can exist in poorly alloyed or when aided by an applied tensile 36 Here large M 2 3 C 6 carbides nucleate large nodules of stress. Y ' that hypothesized and matrix the the cohesive nodules (23). ' Y' fine oxygen atomic reduces boundaries its of boundary grain which deplete the surrounding perimeter their around particles. It is diffusing down the grain strength between the y The resulting cracks and the weakened grained boundaries result in a brittle fracture along the grain boundaries. A mechanism third proposed by Woodford and Bricknell 33) involves the formation of complex oxides along the (30, grain by boundaries the mechanism given in equation 2.1. These complex grain boundary oxides then serve as additional The formation nucleation sites for grain boundary cavities. of numerous creep will greatly accelerate grain cavities boundary cracking and promote brittle fracture. Another alloys involves the formation of CO 2 bubbles at nickel-base grain proposed mechanism for oxygen embrittlement of boundary carbides. (22) This mechanism is similar to the well known methane bubble formation in Cr-Mo-V steels in the diffuses of of presence the reacts hydrogen at high temperatures. Oxygen into the material along the high diffusivity paths grain with MC boundaries. Once in the alloy, the oxygen and M 2 3 C6 carbides to form CO 2 as shown in equation 2.2: 12(0) + M 2 3C6 - Y + 6C02 (Equ. 2. 2) 37 thermodynamic feasibility of carbon dioxide bubble The has formation pressure will in result growth of cavities. these These link and cause a reduction in creep strength boundary grain premature through nucleation of bubbles at carbides and the early the will cavities The partial CO 2 gas is large (approximately 2000 MPa), and of accelerate determined by Dyson (31). been fracture. This theory that a reduction in carbon content will reduce CO2 suggests bubble and formation fracture intergranular reduction since creep crack growth is an it will reduce the CCGR. A process in carbon content will detrimentally affect other creep properties and therefore may not be feasible. mechanism Another into the sulfur grain release oxidation involves the release of free sulfur by a reaction with oxygen. boundary to the Free boundaries results from the grain MnS particles in the grain boundaries through of the following reaction: (0) + MnS - The presence concentrations severly of as embrittle has MnO + S free low as sulfur 10 (Equ. 2.3) in nickel alloys in to 20 ppm has been shown to grain boundaries (38). The oxidation of been observed in IN-738 and Ni-270 with MnS particles the use of Auger microscopy (28, 29). While the release of 38 free sulfur affected was observed, it could not be determined if it the ductility of these alloys since the formation of CO 2 bubbles was also observed. All of the above embrittlement mechanisms depend on the diffusion rates of oxygen along grain boundaries. Elements which segregate to grain boundaries and reduce the number of vacancies oxygen can along be expected grain to reduce boundaries. increase the cohesive interface will inhibit Alloying additions which strength the the diffusivity of of nucleation the carbide-g.b. of grain boundary cavities and reduce the amount of embrittlement in the alloy by oxygen. 2.2.3. Effect of Grain Boundary Chemistry on Environmental Embrittlement Several' researchers alloying nickel have investigated the effect of additions on the extent of oxygen embrittlement in base alloys (22, 28, 30, 32). Woodford (22) varied the composition of IN-738 in order to study the susceptibility effect of boron, hafnium, and yttrium on the of IN-738 to oxygen embrittlement. The time to rupture was measured in air and in vacuum for four heats of IN-738. reduced after 200 hours. for bars 1000°C Rupture lives of smooth bars were severely pre-exposures to oxygen or air at 10000 C for A slight decrease in rupture life was observed exposed to N 2 gas, while exposure to a vacuum at for 200 hours resulted in the longest rupture lives. 39 The lives rupture were severely reduced after oxygen pre-exposure at 10000 C when tested in a range of temperature from 7000 C to 8000 C. Oxygen pre-exposure had no detrimental effects on rupture life at 10000 C. results are Larson-Miller Figure The parameter. 2.6 as stress versus the addition of 1.5% hafnium to The addition the longest rupture time in air. gave IN-738 in shown The above creep rupture of only 0.1% boron also increased the rupture time in air, but the addition of 0.5% yttrium reduced the rupture times for IN-738. to time of the heats gave approximately the same All tested in a vacuum. when rupture This indicated that the addition of these elements affected the interaction between the alloy and oxygen. 0.005% Zr and .05% zirconium reduced the minimum creep did additions sliding, boundary both cracks in strain versus for and boron (32) found that the addition of and increased the time to rupture for alloy PE16. rate B Davidson and Floreen PE16 with not reduce The the amount of grain but did inhibit the formation of surface and air helium environments. The creep time at 6500C measured in air and in helium and without B and Zr additions is shown in Figure 2.7. The observed addition of boron to Ni200 (99.54% pure nickel) is to eliminate grain boundary embrittiement in air (30). The fact that Ni200 and Ni270 (99.99% pure nickel) do show severe containing grain environments boundary embrittlement by oxygen indicate that oxygen embrittlement 40 600 500 400 X 300 rn O 200 100 n v16 18 20 22 24 P=T(°K)[20+log (tR(hrs.))]x10 FIGURE 2.6) 26 28 3 Comparison of creep-rupture life of IN-738 with four alloy variations with a pre-exposure in air and without a pre-exposure in air. (ref. 22) 41 6 Creep Elong. 4 2 t 0 200 400 600 800 1000 1200 Time (h) FIGURE 2.7) Comparison of smooth specimen creep tests for the alloy PE16 with both boron and zirconium or with neither boron nor zirconium. Tests performed in either air or helium at 650 C and a stress of 380 MPa. All tests interupted at approximately 4% Elongation. (ref. 32) 1400 42 is a phenomenon is inherent in the nickel alloy Since boron additions eliminate embrittlement in models based on system. Ni200, which ' - reaction, oxygen possible, are not required for embrittlement. to while Boron appears be very effective in eliminating the effect of oxygen on a fundamental level. While additions exact the role segregate which of to boron and other alloying the grain boundaries is not The effect of boron clear, some conclusions can be derived. and zirconium additions on embrittlement of grain boundaries in nickel alloying these are they the alloys pronounced. The mechanisms by which work are very fundamental, since additions effective in very pure nickel. of diffusion occupying is along oxygen the Boron may inhibit grain boundaries by vacancies and slowing grain boundary diffusivity. (30) 2.3 Creep Crack Growth Creep crack growth rates (CCGR) measured in several alloy systems: steels wide (43-48), variety aluminum alloys (39-42), and nickel base alloys (29, 35, 48-63). A of alloys, test specimen geometries, and test been reported. Several review papers have been written (63, 64, 66). The following section will conditions also have recently been have review the published work to date on creep crack growth rate results and test procedures. 43 2.3.1. Analysis of CCGR Testing Methods CCGR tests geometries tension including specimens (DCB), (64). performed center (CT), (SEN), Several using many specimen cracked panels (CCP), compact double specimen and wedge researchers specimens (39, been cantilever beam specimens double edge notch specimens (DEN), single edge notch specimens test have to surfaces, opening load specimens (WOL) have added side grooves to the promote a plane strain condition at the and to eliminate crack tip tunnelling 47, 51, 53, 55, 57, 58, 62, 63). Crack tip tunnelling results from the creep crack growth rate being faster in the specimen center which specimen sides. Side the triaxiality has greater triaxiality than the grooves are often added to increase at the surfaces of the specimen. All of the above specimens have certain advantages, but the CT specimen and the SEN specimen geometries are the most widely used. stress intensity with crack advance and the test procedures and The K-calibration CT specimen are well affords a low rise in the documented. The SEN specimen allows a complete CCGR versus K plot to be generated from a single test because of a steep dK/da, and the K-calibration is been equally observed measurement CCGR well known. of results questionable to Since the lack of side grooves has result in crack tip tunnelling, accurate crack length obtained (39, 59). from is impossible, and therefore ungrooved specimens are 44 Crack length potential is usually determined via the electrical difference technique (58, 59). compliance techniques for crack length measurement are also employed (47). accurate for of grooves side Optical (47) and therefore Optical crack length measurement is only a polished smooth specimen surface. all will result results The lack in crack tip tunnelling and obtained by optical techniques will under-estimate the crack length and the CCGR. Creep crack growth rates have been correlated using the stress intensity (COD), the for time stress J-Integral dependent the (41, correlated by determine crack from a C* have the net section been published The CCGR is best the C* parameter in creep-ductile materials. however, that the method used to yields a rate. (64,66) rate growth rate. studies and 46, 52, 53, 59, 64, 66). possibility, growth the Several (C), (J), the C*-integral practical applicability of these correlating parameters is for plasticity plasticity (anet ) comparing There factor (K), the crack opening displacment of COD. value of C* which is a function of The C* parameter is measured This depends strongly on the crack Correlations of CCGR data with C* when C* is a function of the CCGR results in the situation of correlating CCGR versus appear CCGR (67). While the correlations with C* good, the results cannot be used to predict the CCGR behavior when different initial conditions of load and crack length are used. and IN-100 CCGR tests on IN-718 (52), Udimet 700 (53) (59), all nickel-base alloys, indicate that the 45 factor, intensity stress K, gives the best correlation of The definition of creep-brittle and creep-ductile are CCGR. given in section 2.4. The creep quickly K . The function unique values initial behavior does not appear to be a stage I of and tests which are started at high K of K may only exhibit Stage I and Stage III Figure 2.9 shows results for .5% Cr-.5% Mo-.25% V behavior. at 565 0C by Nikbin steel CCGR with K. Stage II is similar to Stage III corresponds to a rapidly increasing CCGR growth. near increasing known Paris Law regime observed for fatigue crack well the rate versus K curves usually three stages (Figure 2.8) (64). Stage I is a region exhibit of growth crack et. al. (41) and Neute and Siverns (66), and the effect of initial K on the CCGR behavior of an are alloy obvious. versus CCGR curve. The K and A higher initial K resulted in a lower a steeper slope for the CCGR versus K higher initial K tests did not have a Stage II region of CCG. In length reported complex general the test procedures, specimen design, crack measurement for CCGR array of technique, testing in and correlating parameters the literature result in a often conflicting and inconsistant data. These differences reflect the lack of a proper understanding of the creep crack growth fracture processes. 46 iE z'U U U TIME _ Il I- 0 U 0 ICa J I-- I -- LOG STRESS INTENSITY FiCuRE28) Schematic illustration of crack growth data. (ref. 64) 47 .- 6 iU 10- 7 da dt (m/s) 10- 8 10 10 20 40 60 80 100 STRESS INTENSITY FACTOR,K (PaM) FIGURE 2.9) Comparison of CCGR results for 0.5Cr-0.5Mo-0.25V steel in air at 565 C with different initial K. (ref. 41,66) 48 CCGR in Various Nickel Base Alloys 2.3.2. have CCGRs both been determined for many Ni-base alloys in and inert environments from 530 C to 850 C. air CCGR in the literature for Ni-base alloys are listed in Table 2.4. The results in Table 2.4. are for a variety of test conditions, results techniques. measurement designs, specimen In general and crack length the stress intensity factor was observed to give the best correlation of the test results. Figure the CCGRs for these alloys at 704 0C are shown in The 2.10. Ni-base A wide range of results has been obtained for alloys. originates from conditions which appear Some inaccurate of the scatter in the results tests procedures and initial result in CCGR versus K data which do not to be a unique function of K. 49 TABLE 2.4. CCGR Results for Nickel-Base Alloys from Literature ALLOY Astroloy Astroloy Astroloy TEMPERATURE (C) ENVIRONMENT 655, 704, 725, 760 655, 704, 760 Vacuum 704 650, 750, 850 850 850 Udimet 700 Udimet 700 Udimet 700 IN-100 IN-100 X-750 X-750 X-750 X-750 IN-718 IN-718 IN-718 IN-718 IN-718 IN-718 IN-718 NIMONIC 115 AF 115 NIMONIC 105 IN-738 LC B6 704 540, 650 540, 650 650 538, 650, 704, 760 704 650 650 650 540, 650 540, 650 704 The 48 59 57 61 58, 62 58 50 48 48 55 55 52 50 49 56 56 48 48 50 Air Argon Air Air Air Vacuum Air Vacuum Air Air Air Air Air Air Air 704 704 650 650 effect 48 Vacuum 704 750 850 PE16 PE16 53 Vacuum 650 Waspaloy 50 Air Air Air Air Argon Air Air 650, 704, 760 650, 760 Rene-95 Rene-95 51, 63 51, 63 Vacuum 650, 700 704 Rene-95 Air Air Air Air 732 MERL-76 REFERENCE 61 54 54 61 50 32 32 Helium of temperature on CCGR behavior in Ni-Base alloys has been extensivly studied. (48, 51, 52, 58, 62, 63) The results for PM/HIP low Carbon Astroloy by Huang Figure 2.11 temperature are on given CCGR. as an example of (63) in the effect of The results of all reported research 50 - 1r -3 Iv L- -I I I I Creep Crack Growth Rate, Air, 704°C 1 2 3 10 ASTROLOY (ref. 63) IN-100 (ref. 57) MERL-76 (ref. 61) 4 .- .- , e ,I e \ / 5 6 7 10-5 da dt 8 _ 9 6 10- 6 1 (m/s) 10- 7 -8 10 io8 10 M o-9 - -- 10 - L- I 20 I _ I 40 I 60 I I 80 100 STRESS INTENSITY FACTOR, K (MPa-i) FIGURE 2.10) Typical CCGR data for several Nickel -base alloys in an air environment at 7040 C. 51 1 --" . I 10 II II U i I[' it L/C ASTROLOY - / i. Air Ha o I /LD -d5 700 C I I -6 10 10'E u I0 E E oIw -7 10 =o1 -8 0 -g FIGURE 2.11) I I I-I 20 30 LJ~ II 40 50 .K, MPa j .... I. I 70 I I I 100 Comparison of CCGR results for PM/HIP low Carbon Astroloy tested in air at three different test temperatures. (ref. 63) 52 that in the range of temperatures tested the CCGR indicates increases significantly with increasing temperature. The surfaces for all the Ni-Base alloy tests fracture was totally intergranular, and the tests in air displayed an fracture brittle extremely mode with very little visible ductility or cavitation. Air Embrittlement of Ni-base Alloys 2.3.3. The oxygen on the CCGR has been studied by of effect (63) on Astroloy, Sadananda and Shahinian (49, 51) on Huang In-718, X-750, and Udimet 700, Pineau (56) on IN-718, and by The effect (32) on PE16, and Bain (58) on Rene-95. Floreen of oxygen on CCGR ranges from only a slight increase in CCGR for Astroloy to a 1000 times increase in the CCGR for IN-718 and Rene-95. 2.12 - shows Figure results environment The CCGR range of surprising creep results for in well CCGR since range the several of and air inert Ni-base alloys at 650C. an inert environment are in a narrow below all the the air results. This is not alloys have essentially similar and tensile properties at this temperature. The CCGR in air were much faster than in an inert environment but the amount alloy of to strongly increase alloy. on embrittlement. The each The in the CCGR varies significantly from effect alloy's of on oxygen ability to CCGR depends resist oxygen ability of an alloy to resist CCGR in air are probably linked to the same alloying additions which 53 0 da dt (m/s) STRESS INTENSITY FACTOR, K (MPa1m) FIGURE 2.12) CCGR results for several Nickel-Base alloys for both air and an inert environment 54 have been shown to eliminate oxygen embrittlement in Ni-base alloys described in section 2.2. 2.3.4. Effect of Microstructure Floreen on the (35, CCGR increasing decrease in The the in Figure factor of 68) has studied the effect of grain size IN-792 in air grain size the CCGR. 2.13 versus on CCGR in Ni-Base Alloys as at 7040 C. It was found that from 8um to 250um results in a The results for IN-792 are reported a plot of the initial stress intensity time to failure for precracked CT specimens. finer grain size produced a sharp reduction in the time to failure. IN-718 Similar results were obtained by Pineau (67) on 6500°C and by Law and Blackburn (61) on AF 115 at at 7040 C. Wu and Pelloux (57) varied the heat treatment of IN-100 and studied its effect on CCGR at 7000 C in air. are shown in eliminated showed a Figure grain 2.14. boundary reduction in The carbides heat The results treatment (treatment C which and D) stage II.creep crack growth rates. Heat treatment C produced a smaller grain size (3.5 um grain size), resulted than in heat an treatment D (40 um grain size) which increase in the CCGR for treatment C. The results indicate that the CCGR of an alloy are significantly affected by thermal treatment and processing. 55 Un cc 0 o % 0 oo e CU &l; CM C; _ at X X :D (Z/.'NW) 4!suolul ss8J4s IO!l!ul X I I - - - Ir I I 1 r 1 I I I Iv' I 1 z - id E ( 4 -A I0 lot 700'C A B 10 - C ! r- zU I 40 I . ,,~~~~~~~~~~ 60 I 89 ! ! 100 I 150 K MPa/m FIGURE2.14) Comgarison of CCGRresults for IN-100 in air at 700 C with four different heat treatments (A-D). (r-f. 57) 57 2.4. Theories of Creep Crack Growth Creep crack growth is generally thought to be a process in which a single macro-crack advances through a material at growth the and existance of grain boundary cavities ahead of coalesence crack. Tm) as a result of the nucleation, (T>.5 temperature high and growth result from the nucleation Cavity high stresses and strains in the region ahead of of the crack tip. Cavity are theories growth based on either the diffusional growth of cavities by vacancy transport or creep is essentially exception Creep constrained cavity growth cavity growth. constrained that as diffusional growth with the the same the cavity growth rates are limited by the accommodation of the matrix via bulk creep plasticity. one of models the above based are been developed on the assumption of have models CCGR cavity on mechanisms. growth the time dependance of These CCGR crack tip stresses. 2.4.1. A Crack Tip Stress Distribution study of creep crack growth theories must begin with a description of the state of stress ahead of the crack tip. Several reviews of crack tip stress calculations have good been written by McClintock and Bassani (65), Huang (63), and Bensussan complicated et. by al. (39). The stresses ahead of a crack are the accumulation of creep deformation which relieves the stresses with time. -58 the In are dominant, elastic region where the elastic strains ( e the ) stresses, for small scale yeilding, are given by the usual singular field (69): KI fi (0) (Equ. 2.4) aij /2Jrr Where KI is the mode I stress intensity factor, "r" is the distance ahead of the crack tip, and f(8)is a function which varies with the angle from the plane of the crack. In tip, plastically deformed region ahead of the crack the the ( P ) by material is assumed to harden with plastic strain ower law hardening: sP=Bp (a)NP where eP dependant where is the plastic parameters. plastic strain (Equ. 2.5) strain, Bp and Np are material The stresses at time=0 in the region dominates are represented by the Hutchinson, Rice, and Rosengren (HRR) singularities (70-72): J aij= (l/(Np+l)) ' Bp r (Equ. 2.6) fij (Np,®) Np where N p = the parameter loading is the J-integral (73), and X As time increases creep strain will accumulate ahead of the accumulating The crack. creep strain will relax the crack tip stresses. The minimum creep rate with applied stress is given by the following power law expression: (Equ. 2.7) 6C=BC (a)NC where iC is the creep dependent parameters. the stresses dominent in factor the are rate, Be and N are material If the plastic strains are neglected, region where the creep strain is the given by the Riedel and Rice (RR) singularity (74) which is analogous to the HRR singularity: 60 ai. C(t) )(1/(NC+) ) ij (B I r fij (Nc,0) (Equ. 2.8) where C(t) is given below: where the given J t<t tr C(t) = t>t tr C(t) -- . (Equ. 2.9a) C* (Equ. 2.9b) (Nc+l) t C* is the time dependent C* integral (75), and ttr is creep transition time. The creep transition time is by: J ttr Another expression (N¢+l) for (Equ. 2.10) C* the transition time has been suggested by McClintock and discussed by Huang (63): - ,e ttr where c anet E nc £ (Equ. 2.11) is the creep rate in the far field, E is Young's modulas, and a net is the net section stress. transition time approximates the time to relax the The stresses the tip of the crack. at If t<< transition time, the stresses have not relaxed significantly and the alloy is called If creep-brittle. the time/= transition time, the stresses will have significantly relaxed. The for small scale yielding condition can J-integral be approximated as for plane strain (73): J= (1-v2 ) KT 2 (Equ. 2.12) E Therefore, when the conditions of small scale yielding apply in a creep brittle material the stresses and strains ahead of the conditions modern gas crack tip can now be described by KI . generally turbines, These apply for Ni-base alloys operating in and, therefore, K I is the parameter which is being used to correlate CCGR data. 61 - 62 CreeDi n C_(t ) fia, 1 :=0 ~0 () V) 1 E I - in alostic field /* .4 . -- - Log r --- - (a) -- _L nc > np O<t t 2 t3 FIGURE 2.15) Schematic crack tip stress distribution versus time in a creeping solid. (ref. 63) 63 above relationships are used to calculate The of ahead (63). (Figure 2.15) The stresses are highest upon loading at t=0 and are calculated by given crack tip in Astroloy a stresses the HRR singularity. As time progresses the stresses given by the RR singularity slowly relax. 2.4.2. CCGR Models There have been several attempts to predict creep crack The models are based on growth rates in metallic materials. either law by diffusional of cavities (76-79) or by power growth creep deformation controlled cavity growth as suggested (80). Hancock law Power has creep been diffusional cavity growth by Argon (81) and Chen 2.4.2.1. coupled to (82). Diffusional Creep Models Diffusion have models been proposed by Vitek (83), Pilkington (84), and Raj. et al. (85): da Vitek: .516 6 DB 4 4 (Equ. 2.13) ) 413 (2 2 D da Pilkington: . d K s 1 2( 2 1)d)/ Raj et al.: da 7 x K 3 ) kT 1s (1-TB/S) n 6 D K (Equ. 2.14) )(2 (x-Ro) 32 2 (Equ. 2.15) 1 05 E k T x 3 64 where: - Young's modulus E - Grain boundary thickness multiplied by grain 6DB boundary diffusion coefficient - Atomic volume of controlling diffusion species k - Boltzman constant T - Temperature (absolute) s - Crack width K - Stress intensity factor Ds - Surface diffusion coefficient for controlling species Ts - Surface energy of matrix 8B - Surface energy of grain boundary Ro - Radius of cavity nuclei x .One-half cavity spacing. . The diffusional models predict a CCZR dependance on K with a slope between 2 and 4. CCGR tests performed environments, however., have given higher in (63) strain is not slope results which in inert have a the Stage IIregion of creep crack growth. These models do not account for the effect of plastic on the crack tip stress field, environmental damage considered, predicted. and the Stage I CCGR behavior is not 65 2.4.2.2. Deformation Controlled CCGR Models (84) Barnby, (86) Nix, (87) and Pilkington. N da dt Barnby: Nc B K da Nix: dt C net o r dt L = been proposed by have models controlled Deformation (Equ. 2.16) oo (d) (-Nc/2 + 1) (Equ. 2.17) N /2 ln() c (N-2) (rr) N da Pilkington: dt BcK c (2A) - (RL)°i ln(R) (-Nc /2 + 2) (Equ. 2.18) N /2 ) (Nc-2) (2r) 0 "d" where the is grain size, and a net is the net section stress ( C=B CNc ). The deformation models predict on K is the approximately behavior, predicted. models do by given but the that the CCGR dependence N , the creep exponent. slope of the observed This.slope Stage I is CCGR observed stage two CCGR behavior is not Again, as in the diffusional CCGR models, these not predict any effect of environment. of CCGR models was given by Huang (63) A review 66 2.4.3. Iterative CCGR Models An iterative Huang (63). computer This model calculated damage ahead of the crack tip in the grain boundaries terms of average grain boundary cavity radius along advances The crack tip when the average cavity size the cavity spacing in the grain immediately ahead of crack tip. HRR, ahead of the crack tip. by one grain-diameter equals the model of CCGR was attempted by RR, and Huang calculated the stress field using the elastic singularities already described, and the stresses were allowed to relax with time. The idea of damage accumulation and creep relaxation of stress represent a realistic view of the actual conditions which are believed to exist during creep crack advance. slope of crack advance and K. decrease model CCGR as damage proposed on decreases the the predicted K in CCGR versus K curves will vary with The slope will be initially large and accumulates by Huang the The ahead of the crack. The (63) predicts a high dependance of region of Stage I growth. The slope with. increasing K as a result of the increase in number of grains in the plastic zone ahead of the crack tip. The the only major problem in the Huang model results from calculation loading rather direction than the of stress versus time. was used equivalent The stress in the to predict cavity growth rate stress. The stress in the 67 is much larger than the equivalent stress. loading direction An adjustable parameter prediction of stress. over relaxation no a11 (0) was used to correct for this The model also assumes there is of stresses ahead of the crack tip until the the RR-singularity stresses predicted by stresses calculated from the HRR-singularity. the ahead of after loading crack plastic the creep creep with tip should begin to relax immediately The RR singularity is only valid at t=O strains are small when compared to the strains. However, This is not the situation ahead of tip at t=O in creep crack growth. The stress and crack the The stresses due to-the high stresses in the plastic zone ahead of the crack. when drop below the strain accumulation at a point ahead of the crack tip time is shown schematically in Figure 2.15. The consequence of assuming the stress relaxation beginning with t = 0 is that creep strain accumulates too fast and the creep crack growth rate is over-predicted. 68 HRR ""I 3) TIME ,-,r .c HUANG CREEP STRAIN TIME FIGURE 2.16) Stress and strain ahead of the crack tip versus time from the computer model given by Huang(63) and the predicted values in the present model. 69 EXPERIMENTAL PROCEDURES 3.1 Materials Four / y' nickel base superalloys were chosen for this study. They IN-100, and varying susceptibility oxygen. alloys each are Rene-95. The alloys into alloy Low Carbon Astroloy, Merl-76, Low Carbon These alloys were chosen for their to grain boundary embrittlement in were produced by HIP processing of PM 9/16" diameter rods. is shown particle diameter. in The powder mesh size for Table 3.1 along with the maximum Rene-95 was obtained in two mesh sizes. Table 3.1 - Powder Mesh S.ize Particle diameter, pm Size Astroloy 100 149 Merl-76 325 45 IN-100 60 250 Rene-95 60 250 Rene-95 120 125 70 Chemistry and Processing 3.1.1. The thermal and HIP processing alloys are given in Table 3.2. chosen to yield parameters for the The heat treatment used was similar mechanical properties for all the alloys. TABLE 3.2. -- I Thermal Processing -- 1. HIP Cycle a. Astroloy - 12320C/4 hours/Furnace cool/15 Ksi b. IN-100, Merl-76, Rene-95 - 11770 C/4 hours/ Furnace Cool/15 Ksi 2. Heat Treatment Solution: Age: 1177 C/4 hours/air cool 871°C/8 hours/air cool 982 C/'4 hours/air cool 650 0 C/24 hours/air cool 760C/8 The alloy hours/air cool chemistries were determined adsorption and wet chemistry by Luvac, Inc. using atomic The chemistries 71 and microstructures The calculated given Table in elements for the alloys are given in Table 3.3. volume fraction for each alloy is also y' 3.3. (3.1) The trace which segregate to the grain boundaries such as B, Zr, C, O, P and S were determined. powder of concentration particle size were The grain size and prior determined via linear the intercept method. (98) TABLE 3.3. Alloy Chemistries and Microstructure Sample 1 Molybdenum Columbium Aluminum Titanium Hafnium Vanadium Nitrogen 3.97 3.39 14.0 7.71 3.33 3.36 3.31 2.41 12.2 17.8 3.20 1.36 4.71 4.19 .01 .01 .10 12.2 18.3 3.39 <.01 4.88 4.17 <.01 <.001 .,007 .009 .97 .044 .082 .034 .025 .037 .0129 <.001 .014 .0008 .007 .020 .050 .0238 .082 .021 .037 Silicon Iron Tungsten y' Volume Fraction (Calculated) Grain Size (um) Prior Powder Size (um) Remainder 0.50 28 I 95 , .064 .0137 .001 <.001 .0020 I 4 .0111 <.001 <.001 <.001 <.001 .07 .18 .02 .24 Nickel Sample IN-100 .004 Carbon Boron Zirconium Oxygen Sulfur Phosphorus 3 Sample Merl-76 14.8 16.3 4.82 Cobalt 2 Rene-95 Astroloy Chromium Sample .0029 .0016 .10 .04 .077 .082 3.42 Remainder Remainder Remainder 0.52 25 and 24 11 70 and _ 34 _ I, 22 0.58 0.63 23 I - 35 - , - -72 3.1.2. Microstructural Characterization in of heat treated material were mounted samples Several Buehler plastimet, ground on 240, 320, 400, and 600 grit paper, polished with 3 um diamond paste on carbide silicon with polished finally and cloth, nylon silica ..solution) on nylon cloth. (colloidal Nalcoag 1060 The specimens etched using No. 2 stainless reagent (100 ml methanol, were 50 1 HC1, and 5 gm FeC1 3 ). specimens were observed under both a Zeiss The/ etched Astroloy have a coarse a finer grain size with large ' particles along the boundaries. Prior boundaries powder all in magnification usually have Rene-95 and and carbides decorating the grain boundaries. with size primary result were easily observed at low the alloys tested. These boundaries grain boundaries. The prior powder with coincide boundaries the IN-100 respectively. Merl-76 AMR-1000 A scanning an microstructures of Astroloy, Merl-76, IN-100, and treatment Rene-95 and Figures 3.1 - 3.4 show the after heat microscope. electron grain microscope optical Universal from the existence of large carbides on surface of the powder particles. These carbides do not go into solution on subsequent heat treatment. In the case of Rene-95 are known to be and Columbium carbides, probably are segregate to while Titanium during precipitate these Merl-76 the in carbides Astroloy carbides. solidification powder surfaces. (3.2) of The and IN-100 These the carbides powder prior they and particle 73 (a) (b) FIGURE 3.1) Photomicrographs of PM/HIP low carbon Astroloy. (a) optical, (b) SEM (etchant: 100ml Methanol, 50ml HC1, and 5g FeC13 ) 74 (a) (b) FIGURE 3.2) Photomicrographs of PM/HIP MERL-76. (a) optical (b) SEM (etchant: 100ml Methanol, 50ml HCl, and 5g FeC13 ) 75 (a) (b) FIGURE 3.3) Photomicrographs of PM/HIP low carbon IN-100. (a) optical, (b) SEM. (etchant: 100 ml Methanol, 50 ml HC1, and 5 gm FeC1 3 ) 76 (b) (a) (c) FIGURE 3.4) Photomicrographsof PM/HIP RENE-95. (a) optical (60 Mesh), (b) optical (120 mesh), (c) SEM. (etchant:100ml Methanol, 50ml HC1, and 5g FeC13) 7-7 boundaries grain have a boundaries higher which concentration are not along of carbides than the prior powder particle boundaries. 3.2. Mechanical Testing Different the mechanical tests were performed to determine high temperature tensile, creep, and creep crack growth behavior of the alloys. The test procedures are described in the following sections. 3.2.1. Tensile Testing Tensile tests were performed using a screw driven floor mounted Instron Tensile Tester. and An The tests were run at 7040 C a crosshead displacement rate of .02 inches per minute. A.T.S. three-zone resistance heater with a Leeds and Northrup Electromax III temperature controller were used for specimen a The heating. Load vs. Displacement was recorded using strip chart recorder incorporated in the Instron machine. .2% yield stress measured graphically. measured and ultimate tensile strength were Elongation and reduction of area were directly off the failed test specimen. illustrates the Figure 3.5 specimen geometry used in both tensile and creep-rupture tests. -78 2 - - NF-3A 2 PLACES FIGURE 3.5) Smooth bar creep-rupture and tensile specimen geometry. (Dimensions in inches) Smooth Bar Creep Testing 3.2.2. were tests Creep conducted obtain the power law to constitutive equations for the purpose of theoretical creep accurate was temperature test The determine the Creep tests were within stress range of 600 to 1200 MPa. 704C at conducted the alloys. of properties creep-rupture to and growth, crack creep of modeling to within 4°C. The elongation was measured using an extensometer connected to a tester. arm level data per points mV, and a stress versus minimum creep rate Smooth specimen. bars were tested at a and the time to rupture was recorded as well load constant 100 The stress was increased in steps in several obtain to per The minimum creep rate was recorded for several stress levels. order range inch Tests were conducted on an A.T.S. 1 micron. of resolution .25 a with LVDT dc-dc as the minimum creep rate. 3.2.3. Notched Stress Rupture Testing root of radius air in .33 mm, The notch has 60° flank angles, a and factor of 3.2. concentration in 3.6.) (Figure testing. an heater. recorded. The The tests a calculated elastic stress Tests were conducted at 7040 C level arm test system with a 3-zone A.T.S. resistance specimens were used for NSR notched Circumferentially time to rupture for each test was were performed in a range of stress from 400 MPa to 800 MPa. Several specimens of PM/HIP Rene-95 80 2ZO-UF-3A 2 PLACES I1 FIGURE 3.6) Notched Stress-Rupture (NSR) specimen geometry. (Dimensions in inches) 81 pre-exposed to air at 7040 C to determine the effect of were oxygen on the notched stress rupture (NSR) behavior. Creep Crack Growth Rate Testing 3.2.4. a constant applied load in a level arm tester supplied with by growth rate tests were conducted at 7040C crack Creep was controlled using specimen System Company (ATS). Testing Applied a in 4°C within resistance 3-zone retort argon. A tests. Argon ATS by supplied gauge section of the heater. Tests were air and 99.999 percent pure in two environments: conducted the The temperature was used in the argon tests were conducted at a pressure of 5 psig in order to insure no back streaming of air. A crack creep has side grooves Tunnelling growth rate tests. growth added specimen was used in the test edge-notched single to (Figure 3.7) The specimen prevent crack tip tunnelling. of the crack results from the slower creep crack rate in the plane stress condition which would exist on the specimen surface without side grooves. A notch is cut using a 150 um thick diamond Specimens were fatigue precracked at room temperature. saw. The starter maximum always stress K, used in precracking was intensity, less than the initial stress intensity in subsequent 0 creep crack growth testing at 704 C. 82 i 2 PLACES I I .I ~. %A . . I CHAM.1.5-2.0 x 45APPROX. 2PLACES 25.4 -59 - - - - 76.2 REF. - P I J-- ' "'IA " I - " 111.7 j 4.76R 4 PLACES . I i l) ' H 127-20-UNF-3A 2 PLACES 4 PLACES 1 r -,4.76R 476~ Y U-- FIGURE 3.7) \ 0114.4b\ rT7sz2T I A r I E Single Edge Notched (SEN) specimen geometry used in CCGR tests. (Dimensions in mm) 83 3.2.4.1. D.C. Potential Drop Technique electrical potential drop method is based on d.c. The the fact such as in a current carrying body, a discontinuity that a crack, results in a disturbance in the potential field. order to monitor the crack length, a current is In passed through across the length causes either side the mouth is measured. crack monitoring potential the of this and the electrical potential specimen the crack (V), to two fixed points on increase. increase potential reference potential between An increase in crack By continuously and comparing it to a the crack length to specimen width ratio (a/w) can be inferred. Several potential solution theoretical have by solutions for crack length versus been made by solving Laplace's equation. Johnson (88) developed A for a center-cracked plate specimen with a razor slit starter is as follows: cosh-l V V Johnson 1 cosh(7Y/W) cos(lwa/W) - cosh 1 cshYW(Equ. 3.1) cos (raY/W) later worked out a similar solution for a SEN specimen with a razor sharp slit starter notch (89): 84 cosh 1 V 0 = c° h 1 V0 In both cosh_ cosh(wY/2W) (Equ. 3.2) cos(Tra/2W) cosh(nY/2W) 1 cos(ao/2W) equations V is the measured potential, V is 0 the measured potential for a crack of length a , Y is one 0 half the leads, distance 'a' specimen and 2a is between the crack length the two potential measurement (for the center-cracked the crack length and reference crack length are 2a respectively), and W is the specimen width. Other Che-Yu theoretical calibrations have been performed by Li and Wei (93) who modified Johnson's relations for an elliptical starter notch. Recent work has been performed on modeling the potential distributions using finite element techniques (94). The finite element solutions verify equation 3.2 by Johnson. The shown to theoretical calibration by Johnson (89) has been be valid for the SEN specimen geometry in several experimental calibrations (63, 90-92, 94). Figure 3.8 shows the results of a theoretical and an experimental calibration for was the SEN specimen shown in Figure 3.7. accomplished by growing temperature while monitoring was measured a fatigue The calibration crack at room the potential. The crack length by periodically altering the R-ratio to beach 85 L -J I D.C. POTENTIAL DROP: EXPERIMENTAL CALIBRATION Linear Approximation - - -Theoretical - Calculation (ref. 89) (W=11.7mm; a=1.05mm; Y=1.30mm) 5 4 V -IJ, v ao 3 _ /~~~~P 2 ] 2 1 3 4 5 a/a O FIGURE 3.8) - Experimental calibration of the d.c. potential drop technique for crack length measurement for the SEN specimen shown in figure 3.7. 6 86 fracture the mark surface. Agreement between the actual data and the theoretical calculation were obtained. 3.9. shows a schematic for the test system used Figure in creep was growth testing. crack off-the-sheif and did not All of the equipment used have to be specifically manufactured. There exists several sources of potential errors in this method of crack length measurement from both electrical and configurational source A from of sources. electrical error which is possible comes the DC power supply used as a constant current source. A Hewlett- Packard Regulated DC Power Supply Model 6295B was used in this study. Possible errors are: 1.) Current Drift -The current output can drift by .05% in an 8 hour period at constant temperature. 2.) Temperature Variation -Ambient temperature variation can cause a variation of .012%/°C in a range from 20 to 400 C. 3.) AC Line Variation -Variation in AC line current can cause .012% variation in DC output current. 40) Ripple -Background current fluctuations of .05% peak to peak are possible with system. 5.) Insufficient Warm Up Time -Significant variation in output current and voltages can occur if the power source is not allowed a 30-minute warmup. 6.) Plotter Accuracy -The Omega plotter has an accuracy of .5% of full scale. With a typical full scale of 1OmV the error becomes 50 uV. -87 DC POWER SUPPLY HP - G2 59B O.-- 50AMP O-10V SOL __ rEC DB-2 RECORDER 10 MV± 0.05 MV 'FIGURE _____ 3.9) Schematic of the d.c. potential drop system for crack length measurement. 88 In a time 24-hour .33% maximum variation in output current is a temperature, is small and not detectable with variation This possible. a 100 C variation in with period Using the calibration curve the recorder used in the study. in Figure 3.8 and assuming an initial voltage of 2 mV yields a minimum resolution of 60 microns change in crack length. of Analysis by performed of variation, electrical temperature Electrical variations. geometry and variation have been calculated to cause a limit temperature of been The sources and variations, has several researchers (90-92, 95). include error error of sources potential in crack length resolution for a compact tension 100 um specimen (95). position can Configurational cause variations errors extreme such as lead and should be tightly controlled. The potential difference method requires careful d.c. positioning measurement of both leads. leads current-input The and potential positioning will affect the lead sensitivity and precision. The current leads should be positioned at a distance of at least of the Closer positioning 2w from the crack plane (90). leads reproducibility the increases and increased sensitivity insulated from the therefore is sensitivity, demanded. loading only should frame but be reduces used when The specimen must be in order to force the current to pass only through the specimen. The current lead be large enough to conduct up to 50 amps d.c. should wires current. close as leads measurement potential The should be placed as possible and across the mouth of the crack. This has been shown to give the best reproducibility positioning sensitivity (90). while maintaining The contact area of the potential lead wires should be small, and wires on the order of .2 mm in diameter are usually used. high creep in leads leads measurement discharge welding to is the These welds should be strong enough to endure the environment oxidizing and temperature during potential electrical by accomplished specimen. the of Attachment crack growth rate testing. studies several have experienced The current input been attached outside the heating furnace and passing the current through the specimen grips thus eliminating some problems of attachment. The calculated value for crack length given by equation will vary significantly with the potential lead spacing 3.2 The variation in the theoretical calibration is shown ''V. in for 3.10. Figure three values of Y which represent a lower limit, upper limit, and nominal spacing. errors can be eliminated by stopping the Configuration test before in temperature final crack surface. termination and failure fatigue. length can breaking An be the accurate obtained specimen at room measurement of the from the fracture This along with the potential measurement at test will give a point with which to calibrate the 90 rb 7 6 V V0 i 4 3 2 .1 a/W FIGURE 3.10) - Comparison of the effect of potential measurement lead spacing, Y, on the potential versus crack length curve. SEN specimen geometry. (equation 3.2) 91 The test. used are and initial crack lengths and potentials final to the eliminates the lead spacing Y. calculate major of source This procedure configurational error in crack length measurement. effect The crack tip bowing on the d.c. potential of drop technique has been analyzed by Druce and Booth a significant under-prediction indicate short if cracks addition side of (39, researchers a length for of crack crack front is assumed. straight on grooves (92) who SEN 63) eliminates specimens by The several the plane stress condition at the specimen surface and reduces crack tip bowing. Data Analysis 3.2.4.2. The the crack potential potential, V, length versus to versus time data is calculated from The conversion from time crack results. length, 'a', is given by solving equation 3.2. for crack length: aljn) gg i,( -1 2 ccosh(crrY/2W) cV - l Jo lrs C IVo ~ The slope points of 1=osh(Y/2W) (Equ. 3.3) cos(0rao/2W) crack growth rate is calculated by determining a must least span squares fit to 3 data points. .2 mV in measured potential times the limit of resolution). the The data (which is 4 92 The stress intensity factor, K, for the SEN specimen is given by Brown and Strawly (96): K = ar (1.12-.23(a/w)+10.6(a/w) +30.4 (a/w) 4 ) K where is the 2 -21.7(a/w) intensity stress 3 factor, a (Equ. 3.4) is the gross section stress in MPa, 'a' is the crack length, and w is the of width notched the specimen. specimen (SEN) This equation is for a single edge which is free to bend. The gross section stress for a notched specimen is given as: P a= (Equ. 3.5) W V"B*BN .I, where P is the applied load, B is the gross thickness, and BN is the net section thickness N (97). 93 3.3 Pre-exposure Oxygen Penetration Tests in of each material was air at 7040°C for 100 hours with no applied load to exposed 3.7) (figure specimen SEN One The order to allow oxygen to diffuse into the material. were bars using notched fatigue-precracked room at temperature exposure. a 150 um thick diamond saw, and before temperature the high The bars were pulled to failure in an 0 with extension screw driven tensile tester at 704 °C Instron rate of .2 inches/minute. The depth of inter-granular fracture is measured. fracture intergranular of (30). alloys the The is a result of oxygen embrittlement An apparent diffusivity of oxygen is calculated using the following equation: D= X2 /t x where exposure is time the (Equ. 3.6) depth of intergranular fracture, t is the (3.6 x 10 seconds), and D is the apparent diffusivity of oxygen along the grain boundary (30). 3.4. Fractography The scanning fractography electron was performed microscrope. using an AMR-1000 The specimens were cleaned -\~~ with acetone and oxidized surfaces. coated with gold to avoid charging of ~~9 95 4. EXPERIMENTAL RESULTS 4.1. at 7040 C Tensile Properties The 4.1. tensile The results at 7040 C are shown in Table test 0.2% yield strength, Elastic modulas, and U.T.S. measured for all the alloys are approximately total elongation Rene-95 to ductility exhibited cracks. The (P= The varies by a factor of three from 5.0% for 15.4% for Astroloy. The specimens with low failure via the propagation plastic proportionality the same. strain constant B hardening are of surface exponent Np and also given in Table 4.1. (/ B )P ) TABLE 4.1. 704 0C Tensile Test Results U.T.S. .2% Y.S. (MPa) (MPa) Rene-95 1199 947 IN-100 1167 MERL-76 Astroloy . , % El. E Bp Np (GPa) (MPa) 5.0 167 1785 1012 8.4 162 1454 16.39 1164 1012 13.1 160 1448 16.67- 1200 950 15.4 170 1662 8.93 9.71 i .. 96 4.2. Smooth Bar Creep Results Minimum Creep Rate Results 4.2.1. The air 7040 C at in a range applied stress from 600 to of The results are given in Figure 4.1 as a plot of 1200 MPa. stress creep rate for each alloy was measured in minimum The creep exponents, Nc, versus minimum creep rate. constants, Bc, for the-power law equation for secondary and creep rate are given in Table 4.2. range from 10 -8 sec all -4 to 10 sec -1 The creep . alloys measured is similar. four exponent creep -1 The minimum creep rates behavior of Rene-95 has a larger (Nc) and proportionality constant (Bc) than the other alloys. TABLE 4.2. Minimum Creep Rate Results at 7040 C . I gc(sec I -1) I I = Bc(a(MPa)) B I Ill I I Nc 6.29 x 10 61 18.5 Merl-76 1.66 x 10- 65 20.0 IN-100 7.81 x 10-61 18.5 (60 meesh) 4.03 x II c Astroloy Rene-95 II 10 - 3 7 10.3 --- 97 __ _ _ _ C I I .7 _ I .X C I - o c Ca In u>. C C 0 _ z! - .. Z 0000 1 M'0I e l0aCI . M I = I O Z _ oC .. , = C i-C *E- C 11 -4 _ C. C Z . oT. I I C C I CC X o , U _ 'S I. a . C. O c: =: r 2 _ C S r. v, 1I O O - o, _ 1 ~41 C O O N o _ 1I O O 0 1I I I 00 0% 00 c 00 I ' - 0 0 'tl I oC. I __ __ .: C_ C CD Tr U. 98 4.2.2. Creep Rupture Results Four creep-rupture performed at 800 MPa tests in air (one from each alloy) were at 704 C. The results for the four alloys were similar with the exception of Merl-76 which gave a longer time to rupture. The individual results are shown below: - - Table 4.3. Creep-Rupture Results, Air, 7040 C, 800 MPa t (hrs) ecritical Rene-95 7.2 .0083 IN-100 4.7 .0068 Merl-76 28.7 .0202 Astroloy 5.8 .0068 - - Table failure. minimum constant 4.3. The creep also shows the secondary creep strain at secondary creep strain is the product of the rate and the time to rupture. This value is for an alloy at a given stress, which is the basis for the well known Larson-Miller parameter used to correlate creep-rupture results. - ___ _ _~~~~~~~~~~~~~~~~~~~ I I I IU I I I O I I _z 0di 0 z Z. - -o 0 0 0 -e *4 el~4 _- -w z = I-r£ n V) Of w oI w 0D -LDt: _g -L LL 0 Ho LU -U -I o0 . L. o C; ou o 0r 0 -7 es 'i co -14 0 C 14 z :0 0 0 el .: -I .tQ q -7< 00 0cu a. I 00 0 1-1 II 00 m I I 0 I I 00 O P, I ! I Y G 0(~ 10C Notched Stress Rupture Results 4.3. 4.3.1. Constant Load Results Notched all rupture (NSR) tests were performed on alloys in air at 704°C. four Figure 4.2. rupture stress The results are given in The results indicate that Rene-95 has a shorter time than the other three alloys tested. At high Merl-76 gave the longest time to rupture, but at the stress lower stress a crossover occurs and Astroloy has the longest time to rupture. NSR results can be interpreted as giving a measure The of the relative CCGR behavior of the four alloys when tested in Astroloy air. had the longest time to rupture at the lower stress, but at the higher stress Astroloy'fails sooner than IN-100 and Merl-76. 4.3.2. Air Pre-Exposure Results Three for 100 hours specimens were exposed to air at 7040 C prior to NSR testing. Some specimens had a applied load during the pre-exposure. small run Rene-95 to failure at a higher stress i, air results are summarized in Table 4.4. They were then t 7040 C. The 101 TABLE 4.4. Notched Stress-Rupture of Rene-95, 704 C Pre-exposure Test Results 408 MPa/403.4 hrs. 675 MPa/<1 min. No Pre-exposure 591 MPa/137.2 hrs. 0 MPa/73.4 hrs. 591 MPa/237.8 hrs. 338 MPa/69.0 hrs. 571 MPa/153.8 hrs. The tests were inconclusive but they did present a few interesting tensile with no results: 1) pre-exposure in air with a small stress was more damaging than a pre-exposure to air stress; and 2) a long pre-exposure with a small stress exhausted the residual life at the higher load. 1C2 Creep Crack Growth Rate Results 4.4. CCGR for Five Ni-Base Alloys 4.4.1. air The creep crack growth rates were measured at 7040 C in and in low They were low carbon IN-100, Rene-95 Merl-76, Astroloy, carbon The on all five alloys. argon were all in the PM/HIP condition. tested alloys pure 99.999% (60 mesh), and Rene-95 (120 mesh). could be not made to grow if the K used in pre-cracking the specimen was higher than maximum K initial the cracks creep The CCGR probably is growth for to imposed stresses compressive due The lack ofcreep crack testing. the by presence the of residual larger plastic zone remaining from the fatigue precracking. 4.4.1.1. CCGR of PM/HIP low C Astroloy The results of 6 CCGR tests on PM/HIP low C Astroloy in Figure 4.3. are shown and exhibited an Four tests were performed in air increasing da/dt versus K, but the CCGR not linear over the entire range of K tested. curve was three stage creep crack growth curve was obtained. A The lowest initial K in air was 22 MPavi, and the lowest initial K in argon was 33.2 MPa i. The by a tests had an initially large rise in CCGR followed region of decreasing slope. The slope in the second region was approximately 3.5 in air and 7.0 in argon. 103 c o\ oC.5 C'# -o 0 s.o 0 I , w4 U,# oc r4 of CO cv, I 0 It1 iInco 1 1 Ia CZ 1 i -4 (S/W) ?P/DP 104 at argon CCGR for but K = 70 MPa i the air and are the same at 3.5 x 1-6 m/s. curves The two at high crack growth rate indicating that the merge curves 100 times faster in air than in is air K = 40 MPa'm-, argon was advancing faster by creep fracture with no front crack in CCGR The effect of oxygen embrittlement. CCGR of PM/HIP Merl-76 4.4.1.2. Four CCGR curves in air and two CCGR curves in argon at 7040 C were obtained. The results in air were (Figure 4.4) and an for gross section stress from 145.6 MPa to 655.0 Ma, length 1.01 mm from to 2.62 mm. The CCGR initial crack results in air increased 20 times over the CCGR in argon at K = 40 MPa-m. The behavior in air and in argon are similar Astroloy. All the tests have an initially fast increase to in the CCGR followed by a region of linearly increasing CCGR on a log-log plot. slope The of the air CCGR versus K curves are approximately 3, and the CCGR tests in argon had a slope of A observed 4. rise definite in the CCGR measured failure at K = 80 MPa/, before in air was while in an argon atmosphere the final K was approximately 110 MPaii. measured CCGR The in air away from the initial transient was slightly higher in specimens which had a lower gross- section change in stress. dK/da with This effect is probably due to the gross stress in the specimen. result will be discussed later. This 105 c a, 0 0 C) ,-, 4I c 04 u1 .To ¥4 CA o I C)0o 3 r-I 1-4 i I 1- (S/W) 'O1 0 0 ?P/OP 1O 0 1O I C)~~~~~~v 1C6 4.4.1.3. The CCGR of PM/HIP Low C IN-100 Both air and high purity argon CCGR results are Figure 4.5. in CCGR The given. for PM/HIP Low C IN-100 at 7040 C is shown in CCGR air increased to 4 times the CCGR in The apparent at K = 40 MPaAH. argon for IN-100 KIC in both air and argon was 100 MPF/m. The effect of dK/da on the CCGR in an argon atmosphere is easily observed. The higher the gross section stress the lower the CCGR for the same stress intensity factor, K. 4.4.1.4. CCGR of PM/HIP Rene-95 (60 Mesh) (60 Rene-95 mesh) showed the largest environment on the creep crack growth behavior. The effect of (Figure 4.6) CCGR in air was 300 times higher than the CCGR measured in argon. CCGR KIC at 704 0 C was observed to occur at 80 MPai. tests in air were performed over a range of gross 91 MPa from to 291 MPa, and a range of section stresses initial crack lengths from 1.40 mm to 3.35 mm. of air the tests were similar, The results but the effect of gross stress on the Stage II CCGR was observed in this alloy as in all the previous alloys tested. The behavior. and in CCGR CCGR The increased CCGR was results crack indicate similar CCGR growth rates were initially very low rapidly with crack advance. followed initial This sharp rise by a region of gradually increasing over a broad range of K. The slope of the CCGR versus 107 c o' 0" 00c C. 00 I- ,-I 0 4J ,-4 C, CD rT OI V-4 IoCc I c.fV4 (S/W) l l Wv4 0) IaC i 4 - V- 108 C , . i i ' I i j , I I .C -c 4.1 0DO ,- -Q E 4.. -. p' ,I I 0 I to -II _: L | C) I i CI ;a' h o3 __ J_-_ , I « : r./3 04 rn _ _ __ 1-j~~~~~~~~~~~~~ _ _ _ _ _ _ " _ - _ _ _. . __· %D CD I0 0II- Co Ia C~4 C '.4 (S/ UW) 7P/'aP 0 0I ~J 1C9 K II in air was 2, and for purified argon the Stage curve slope was 4. CCGR of PM/HIP Rene-95 (120 mesh) 4.4.1.5. change in the CCG behavior at 704 0C between an air The and an argon atmosphere was not as pronounced as atmosphere was for observed larger mesh size. (Figure 4.7) The the CCGR in air over the CCGR in argon is only in increase the 10 times. The CCGR in air for the finer 120 mesh size is the same as the larger 60 mesh size Rene-95. a faster CCGR in an argon atmosphere. result The difference is the size and powder particle size variations. grain of The finer mesh size has the prior particle boundaries in argon, follows The crack but it follows the grain boundaries in air. The finer mesh size has a faster CCGR in argon because of the smaller prior particle size, but the grain size for both mesh sizes is the same (grain size = 25um.) Effect of Initial Stress Intensity Factor 4.4.2. The from K 704 C initial stress = 14.2 MPam in quickly 43.2 MPaii air atmosphere. an to to intensity factor, Ki, was varied for PM/HIP Merl-76 at (Figure 4.8) The CCGR rises a region of gradually increasing CCGR versus K. The initially low CCGR results from the lack of damage ahead of the occurred, crack. the Once the first jump in the crack front has crack tip encounters material which has been 110 a) C. oN 0a, I Z -o ai 0rZ a,4 0 Co C o C; In ': (s/W) co rC "o " -4o co o c) o 4 111 - u) u4 0 u.4 co -n I u - -Y co cn I0 us tn l I 'o4 1 (87W) co i O l'C i 1o 7p/oP i 0 I a) X 0 (/) 112 creep and oxidation damaged ahead of the crack tip. This damaged material requires less time (and creep ductility) to fracture and increases ahead the until of the crack growth increases. The CCGR the dynamic process of damage accumulation crack tip and equilibrium. The *.CCGR depends history, stress the rate the on intensity crack advance changes factor, and in reach the load dK/da in the specimen. 4.4.3. Comparison of CCGR in Air A comparison of CCGR versus K curves for all the alloys in air indicate to in is given in Figure 4.9. The results all the alloys exhibit CCGR between 10 - 7 m/s that 10-5 m/s slopes 4. 7040 C at in a range of K from 20 to 100 MPa/i. The the stage II region of the curves vary from 2 to Astroloy and Merl-76 have the lowest CCGR in air while Rene-95 (60 and 120 mesh) and IN-100 have the fastest CCGR. In all the intergranular. intergranular alloys Fracture the fracture surfaces in path is totally Astroloy have prior particle boundary cracking at K greater than 60 MPai-. 4.4.4. A alloys in The Comparison of CCGR in Argon comparison of the CCGR versus K curves for all the in argon at 7040C is shown in Figure 4.10. argon are compared The CCGR to the air results in Figure 4.11. CCGR for all the alloys is higher in air than in argon. 113 0 CD IT r, 0 -H 0co a 0 cn a IZc C-q H4 <: t; t CT I W.4 0 m' I4 C~4 034 W-0 (S/UW) co C- 0 V-4 ?P/OP 0) C I -4 C) 0r V-C 114 - C; 0 o0 0s- C; o .a 0 0rU, U) QI 1- C.) .) C-m 9c- 0 -0 O - (S/W) C: D I-9-4 -4 P/OP O 9i 115 The slopes of the stage II region of crack growth in argon is higher than in air and it ranges from 3.5 to 6. has Astroloy the lowest CCGR at low K, but Merl-76 becomes slower at higher K values. Rene-95 (120 mesh) and IN-100 have the fastest CCGR in argon at all values of K. fracture ..path The grain boundaries which in argon are also is intergranular, along prior powder particle boundaries. 4.4.5. Validity of K Correlation of CCGR CCGR curves for Merl-76 in air are shown in Figure The These results are for a range of gross section stress 4.8. from 145.6 MPa Stage II to and 655 MPa. The CCGR for Merl-76 in the Stage III regions of creep crack growth rate versus K curves indicate a K-dependancy on crack growth with a scatter of approximately of magnitude in There is higher crack growth rate for a lower initial K and a lower gross section value of stress. dK/da 5. A trend an order CCGR. chapter a half in the in the results which indicate a This behavior is attributed to the specimen; it will be explained in good correlation exists between da/dt and K for all the alloys tested in both air and argon with scatter being result within should creep-brittle one half an order of magnitude in CCGR. not and characterized by K. be the surprising crack This since the materials are tip stresses are well 116 initial transient behavior, Stage I cracking, does The crack initial is this not cracking, growth rate increases with increasing K, but but Tests which are performed at a true. always initial large In general it is observed that the K. with correlate not intensity stress directly proceed never exhibit stage II from Stage I to Stage III crack growth. I Fractography of CCG Tests 4.4.6. fracture path for all the creep crack growth tests The was intergranular. The tests in argon fractured along grain boundaries which coincided with prior powder boundaries. In air the cracks were not limited to prior powder boundaries. precracking was performed at room temperature. Fatigue fracture path of the fatigue precrack The crystallographic can be seen in Rene-95 (120 mesh) and IN-100. (Figure 4.11) The transition crack growth to creep crack as a sharp fractographic transition for all appears growth fatigue from the alloy tested. The transition from creep crack growth to fast fracture is also transition very the which ductile, is transition more mixed between difficult surface observed for CCG in air and fracture observed to The sharp from the difference between the brittle results intergranular the air tests in air. for sharp intergranular-transgranular for fast fracture fracture. (Figure 4.12) The creep crack growth and fast fracture is detect in tests performed in argon. 117 Figure 4.13 for IN-100 4.4.6.1. in air. general the boundaries.. boundary Figure from CCG to fast fracture Argon Tests In powder shows the transition The cavitation 4.14 fracture for in crack argon Astroloy. for argon in argon follows prior fracture surface shows more grain in surfaces respectively creep than in air as is shown in Figures 4.15 and 4.16 show the IN-100 7040 C. at and Rene-95 (60 mesh) The round prior powder particles can be easily detected on the fracture surface. Figure 4.17 shows the typical fracture appearance for argon CCG tests for the 4 alloys. are easily particles seen is in all determined the by The round prior particles fractures. the mesh The size of the size used for each alloy. High magnification argon revealed These cavity Rene-95 surfaces (60 fracture like mesh) photomicrographs features features and are which of the alloys in may be cavities. shown in Figure 4.18 for Merl-76 at 10,000 x. The fracture in Figure 4.18 were from CCGR tests in argon which were not oxidized. 118 Air Tests 4.4.6.2. Figure 4.19 shows the typical fracture surfaces for the path fracture The the when alloys four featureless CCG tests were run in air at 704C. totally was boundaries intergranular flat with characteristic of brittle fracture. The surfaces obtained in air tests are heavily oxidized as a of exposure to oxygen at 7040 C . result High magnification failed to reveal any cavity-like features. photomicrographs (Figure 4.20) CCGR The versus the intersect CCGR results K results for obtained Astroloy in in Air argon at K = 60 MPamiii. The fracture surfaces of the CCG tests in air have a transition at K= 60 MPa-/m from intergranular, brittle fracture to a predominantly prior particle boundary fracture which path fracture has been surfaces is transition shown obtained physical to be characteristic of the in an argon environment. This evidence supporting the conclusion at high creep crack growth rates in Astroloy the crack that grows faster than the embrittling effect of oxygen. The tests fracture surfaces for the notched stress rupture in air are the same as that observed for the air CCGR tests. 4.5. Penetration of Oxygqen Results The exposure depth at of 7040°C intergranular fracture following oxygen varied from alloy to alloy. Astroloy exhibited the smallest depth of damage and Rene-95 (60 mesh) 119 the showed largest depth of intergranular damage. The results are summarized in Table 4.5. TABLE 4.5 Depth of Intergranular Embrittlement after a 100 hour Exposure at 7040 C in Air I Average Depth of Embrittlement (um) Alloy Effective Calculated Diffusivity (m2/s) D = x2/t 84 1.96 x 10-14 Merl-76 310 2.67 x 10-13 Low C IN-100 720 1.44 x 10-12 Rene-95 (60 mesh) 840 1.96 x 10-12 Low C Astroloy The results indicate that Astroloy and Merl-76 are only slightly crack embrittled growth rates and in therefore should have lower creep air than either IN-100 or Rene-95. This result has been verified in section 4.4.3. 120 I FIGURE4,11) FRACTOGRAPHOF THE FATIGUEPRECRACK-CCG TRANSITION 0 IN PM/HIPRENE-95 (120MESH POWDER)TESTEDAT 704C IN AIR, 121 FIGURE 4,12) FRACTOGRAPH OF THE CCG-FASTFRACTURE TRANSITION IN PM/HIP Low C IN-100 TESTED AT 7040 C IN AIR. 122 (a) (b) FIGURE 4.13) Fractographs of the CCGR-Fast Fracture transition in PM/HIP Low C IN-100 tested at 704-C. (a) in 99.97O pure argon; (b) in air. 123 (a) (b) FIGURE 4.14) Fractogrphs of PM/HIP Low C ASTROLOY at 704 C. (a) CCGR test in air; (b) CCGR test in 99.999% pure argon. 124 0 FIGURE4,15) FRACTOGRAPHS OF CREEDCRACKFRACTURES IN 99.999%PURE ARGON,(A)PM/HIPLow C IN-100,(B)PM/HIPRENE-95 (120 MESH POWDER) 125 FIGURE 4.16) Fractograph of a CCGR specimen tested in air at 7040 C. (PM/HIP RENE-95 (120 mesh powder)) 126 (a) (b) (c) (d) FIGURE 4.17) Typical fractographsof four PM/HIP Nickel-Basealloys 0 tested in argon at 704 C. (a) Low C ASTROLOY; (b) MERL-76; (c) Low C IN-100; (d) RENE-95 (60 mesh). 127 (a) (b) FIGURE 4.18) Fractographs of cavity like featurew on creep crack fracture surfaces from CCGR tests in argon at 704 0 C. (a) PM/HIP MERL-76. PM/HIP RENE-95 (60 mesh); (b) 128 (a) (c) (b) (d) FIGURE 4.19) Typical creep crack fracture surfaces in air at 704 C in four PM/HIP Nickel-Basealloys. (a) Low C ASTROLOY; (b) MERL-76; (c) Low C IN-100; (d) RENE-95 (60 mesh). 129 FAST FRACTURE PRIORPOWDER BOUNDARY FRACTURE INTERGRANULAR FRACTURE FIGURE4.20) TYPICAL FRACTOGRAPH OF A CCGRTEST ON PM/HIPLowC 0 ASTROLOY TESTED IN AIR AT 704C. 130 5. AN ITERATIVE MODEL FOR CREEP CRACK GROWTH 5.1. Introduction A a specimen conditions creep model for creep crack growth is developed the accumulation of damage ahead of the crack tip on based for computer which is creep brittle and satisfies the of small scale yielding. The damage is based on accumulation in elements which are assumed to strain have the dimension of the critical microstructural parameter (i.e. and grain size or prior powder size). primary contribution by advances of strain are assumed to make a negligible creep to The plastic strain the total amount of damage. The crack one element when the element immediately ahead the crack tip achieves a critical value of creep strain. After a crack advance, the stresses in all the elements are reset to calculated initial HRR stress. the stresses The assumption that reset to the HRR stress after a crack advance was done in the interest of simplicity. However, the actual stress will be slightly lower as a result of the accumulated creep accumulation and Rice crack. (63) which strain in gone before. Creep strain is calculated using the stress given by Riedel (74) This has in model creeping material ahead of a stationary differs from an earlier model by Huang placing an upper bound on stress at the crack tip, and by using the equivalent stress rather than stress in the y-direction. relaxing Crack tip stresses are assumed to begin from their HRR values immediately after loading as 131 a result of the accumulating creep strain. (See Figure 2.16). The effect of oxygen on crack growth is modelled by a change in element size from prior powder size to grain size and reduction a in the value of the critical strain necessary for crack advance. The function model of strongly predicts K-history dependent the and upon K. changes strength, and critical strain. the predicted CCGR is creep crack growth rate as a CCGR in is also shown to be creep rate, yield The effect of temperature on accounted for in secondary creep rate and yield strength. changes in the The critical creep strain is assumed not to change with temperature. 5.2. Numerical Procedures 5.2.1. Calculation of Stress and Strain Stresses ahead of the crack tip at time=O must first be determined. with This increasing initial stress distribution then relaxes time, t>O. The stress in the far field is co' Closer to the crack tip, at radius "r" the stresses are described by the elastic stress intensity factor, KI (99). aE aE j, T fE(a) fE() (Eq.-. 5.1) 132 ( f ) is a factor for each stress component that depends only on the angle (O ) from the plane of the crack. The equivalent stress, aE , in the plastic region ahead of the crack at t=O is given by the HRR singularity (70, 71, 72): KJ2(1/(Npi)) E (Equ. 5.2) Np is the stress exponent from equation 5.3: eP = B (a) P p where cp e (Equ. 5.3) is the plastic strain. The size expression of the plastic zone, Rp, is estimated by the for plane strain loading and non-hardening plasticity (99): R where Y is = P. .1 the P 2 I (-EC. Yj yield strength. 5. ) The calculation for the plastic zone size can be combined with the HRR stress field: 133 ~~p~ i(1/ At r-Rp the (Eq. Y .RJ ( (l/(Np+1)) (Eq. 5.6) equivalent stress calculated by the model ahead of crack tip at t=0 is illustrated in Figure approximation of equation 5.3 and stresses which are slightly above the actual by 5.5) aEIY, so equation 5.5 gives: aE The (NP+)) the HRR corrected the stress-strain substituted singularity. into The effect 5.1. The relationship given by equation 5.6 yields stresses given of a lower stress is by assuming a 10-20% smaller critical strain than is obtained from smooth bar creep rupture results. For t>O relax as a the stresses in the plastic and elastic zones result of creep strain ahead of the crack tip. The relaxation is described by Riedel and .29 K2 = ,E r E (Nc+l) B Rice (74): (1/(Nc+l)) t LY ~~\b](1/(NcCl)) fE (O,NN,) (Eq. 5.7)' 134 are the material constants for Norton's law: N where Bc an ic Bc(a) c (Eq. 5.8) is the minimum creep rate as determined via smooth bar iC The term f ( O,N ) is taken as 1. creep rate measurements. RR stress calculation indicates that at time 0 the The ( aE ) stress impossible predicted and for upper an bound HRR. by t=0 infinity. at starts A on This stress transition is obviously is the stress time can be calculated at which the creep-relaxed stress given by the RR calculation (5.7) the same as the stress is t=0 given by HRR (5.2). at=O at r and (See Figure 2.16) · 29 i r (::c+l)r L-ransition BcE (:cO) )(:"c. This calculated transition time does not relate to the real test time, but it is only a starting time for subsequent creep strain calculated according to the RR equation (5.7). 135 PM/HIP low C ASTROLOY, On= 200 MPa, O,= 950 MPa, a=lmm -1 (1 = -. 093 (Np+l) 0' r I I I I I 1 I 2 I I I I I I I I I I I Iv 10- 2 100 102 r/a FIGURE 5.1) Equivalent stress ahead of a creep crack at time=0 in a PM/HIP nickel-base superalloy at 704°C. 136 5.2.2. The Accumulation of Strain The of time creep strain ahead of the crack tip as a function is found by integrating the Norton Law expresssion with time using the stress given from the RR analysis. ec(t)= tf tf f6c dt= fBc( RR)NC dt 0 0 (Eq. 5.10) where Ec(t) is the creep strain as a function of time, tf is the time interval time-dependent to stress advance given the by crack, and aRR is the the RR singularity. The result of integrating equation 5.10 is: =c EC~) =[ ~ .29 K 2 E r Equation crack, very but 5.11 for c ( +1) Bc (Nc+l) predicts short t( (Eq ( ) the creep strain ahead of the times the accumulation of strain is fast resulting from the infinite stresses predicted at t=0 by the RR singularity. The ahead of model calculates the creep strain for each element the crack tip within the plastic zone. When the 137 strain in an element reaches the critical creep fracture strain ( Crit ) the crack advances by one element. For each calculated strain. t=O element a transition time for aRR =HRR is and this time is used to calculated a transition This transition time given by equation 5.9 becomes and is combined with the calculation of creep strain with time in equation 5.11 to calculate a transition strain: 29 K' r E at= transition) (E It is interesting to note that this strain is independent of Bc, Nc, and time. from the This transition strain must be subtracted calculated over-prediction in creep stress strain as to compensate for the short times by the RR singularity. The time increment to advance one element is calculated as: (Nc+l) At = -t transition (Eq., 5.13) where the crit is the critical strain for fracture and accumulated strain in that element from ei is previous 138 =0 c (note: iterations. for the first jump) This is graphically illustrated in figure 5.2. The is tip creep change K with advances in crack length, and in have will history load The plastic zone will accumulate. and calculated the size, or each element ahead of the crack f, strain a strong effect on the predicted creep crack growth rate. is calculated [r 2K Ec(At) strain in an element ahead of the crack tip creep The as: 2 ) - (Eqr(t N Bc (Nc+l)(t+ ttra trans. (Eq. Creep strain plastic zone. to accumulate values K of significant. stresses crack at length. for elements 5.14) within the Elements outside the plastic zone are assumed creep negligible stresses smaller only accumulates 5.14trans. in in creep The stresses given Once the However, at low elastic region. the the t=O strain as a result of the the elastic region may become are reset to the calculated by the HRR singularity for the new stresses are reset the entire process begins again for the next jump in crack length. 139 1n c (E cri;- Ei ) I 5 I I I 10 _ .At ~ ~ ~ ~ ~ ~~~~~I _ I I~_ III - I I I I I I I 0 6 tran 8 TIME (sec) FIGURE 5.2) Schematic representation of the time for a crack advance in CCG, &t. I 140 5.3. The Effect of Oxygen Oxygen diffuses grain boundaries. ahead of the crack and embrittles the This results in a loss of creep ductility and an increase in creep crack growth rate. fracture alloy. The decrease in ductility in air is observed to vary from alloy to In fracture addition path to a change in ductility, a change in occurs from prior powder boundaries to grain boundaries. This decreases the element size and results in an in the creep crack growth rates. increase strain The critical for an inert environment is obtained from short time creep-rupture tests by and the time to failure. by the Monkman-Grant multiplying the minimum creep rate This is the critical strain given relationship (100). In air the ductility is determined by fitting the predicted CCGR to the actual CCGR. The allpo.yis given required in table change in ductility for each 5.1. -- TABLE 5.1 crit/air Alloy w/o B in G.B. crit/argon Astroloy 1.0 MERL-76 IN-100 0.2 0.71 RENE-95 1.0 .30 .70 0.1 . I II w/o B in G.B. Astroiov .26 .II 1. 141 ratio of the grain boundary concentration of Boron The to the grain boundary concentration of Boron in Astroloy is also of This expression shows the relative amount presented. per boron unit of grain boundary volume. The weight B per unit grain boundary volume is proportional to percent the w/o by the B in the bulk times the average grain size divided grain boundary thickness (6). Table 5.1 is graphically illustrated in Figure 5.3. Woodford and (22, 30, 101) have shown that Bricknell additions of as little as .1 w/o B added to Ni-200 or IN-738 will totally The than on with alloys grain a the embrittling effect of oxygen. large amount of boron per unit area of have less of a reduction in creep ductility boundary alloy with less boron. an The concentration of boron grain boundaries was found to be 1000 times greater the than eliminate the bulk for PM/HIP Rene-95 as determined by Auger in Spectral analysis. segregates to the segregate to grain zirconium should This indicates grain boundary. boundaries that most of Boron Other elements which such as carbon, sulfur and also have an influence on the creep crack growth rates which is a process of grain boundary fracture. Predictions of Creep Crack Growth Rates 5.4. The alloys for model studied. both was used o predict the CCGR for the four The predicted and actual results are shown the air and the argon CCGR results in figures 5.4 142 1. 0. 0.1 crit. air crit. argon 0. 0.2 o.( 0.0 0.2 0.4 0.6 I[W/oB*GS/6/6 ]/[w/oB*GS//6 FIGURE 5.3) 0.8 1.0 ASTROLOY Ratio of the critical strain in air to the critical strain in argon versus the ratio of the grain boundary concentration of boron in the alloy to that in ASTROLOY. (6=Grain Boundary Thickness,assumend to be a constant for all the alloys; GS=Grain Diameter). Assuming the boron in the bulk segregated to the grain boundaries the amount of boron will form a zone 17 mono-layers thick in PM/HIP Low C ASTROLOY. 143 to 5.7 for Astroloy, MERL-76, IN-100, and RENE-95 (60 mesh) respectively. The factor an model predicts the experimental results within a of 2 for all the alloys. The predicted results show initial transient until damage is established throughout the plastic versus K plot elements size is The variation in the slope of the da/dt a accumulating increases. because the zone. result of strain (damage) as the plastic zone This effect the increasing varies number of from alloy to alloy the variation in grain size leads to a variation in number Damage is of elements assumed to within the plastic zone changes. accumulate only in the region of plastic deformation. 5.5. Effect of Critical Parameters 5.5.1. Effect of Critical Strain to Fracture Increasing in the critical strain results in an increase the time to accumulate this ( Ecrit) strain and therefore decreases the predicted CCGR. The model predicts the relationship between critical strain and da/dt is non-linear and for larger values of the critical strain the CCGR becomes exponentially smaller. Figure strain K=50 MPa K test on 5.8 the . shows CCGR the effect of varying the critical predicted for Astroloy in air, at The CCGR is determined for a simulated constant after a sufficient number of crack advances for the 144 0 0 o Ca .,I Ca .,- 14 p. 0 C; .3o -W O 0 d-i 04 p, 4 0 10 W-. W I 'C I 'oI I I I-I (S/W) p/op lp - 145 ¥ 0 0-T I,4-J cto -r_ .. C: - .-a) I I I I p4 H u3 .14 10 I0 _ w.. 1C (: In i CD co co i C ) U I o!o CD u (7S/W) :p/op r 146 0Co 0 c ro c0 o0 ,-i fl oo H '0 u W0 )> s-I we 40A ~ C_ IV0 l 1 0 C: (S/W) I 0o -9 10 -4 0 Y-4 o Y-4 C: ?P/OP 44 147 Q 0o rlC 0o b4 .f-4 so Cu 9: la co $54 '-H ddN0 as,4 ow f% C7% C- 1r w zw0) C) "0 0) II z 04 pi 0 la u -ri A4 m ·ct CDo aa a 9-* t-4 in 0o / (S/WU) co o - 7p/CP Cr 0 p ,-4 xp 148 CCGR to become constant. The number of crack advances is on the order of the plastic zone divided by the grain size. The slope of the steady (da/dt)ss versus steeper increasing with fracture -1 strain results rates state critical creep strain strain. crack growth rate plot For a becomes small the slope of figure 5.8 is -1. from ever critical A slope of the fact that for fast creep crack growth the stresses at the tip of the crack have little time to relax. 5.5.2. Effect of Grain Size The into effect account size. of grain size on CCGR predictions is taken by changing the element size or crack step This will cause changes in both the slope of the CCGR versus K curve and in the magnitude of the CCGR predicted as a result the of stress at each element node. effect Figure 5.9 shows of grain size on the predicted constant K CCGR. The curve is similar to figure 5.8 with the constant K creep crack growth rate exponentially decreasing with increasing grain size. The predicted effect of changing grain size on the CCGR versus K curve for PM/HIP Astroloy tested in air is shown in figure slope 5.10. and The a larger grain size resulted in a higher lower creep crack growth rate. The change in slope resulted from the number of elements which accumulated damage. within The CCGR will increase with the number of elements the plastic zone. However, the CCGR is lower as a 149 result of a decrease in the rate of creep strain accumulation with increasing r as given by equation 5.11. 5.6. Constant K Calculations The been creep predicted Astroloy of crack in air. strain growth transient occurred of The until to a bring accumulation is in figure 5.11. for PM/HIP the crack tip affects the creep crack crack the should growth curves have an initial sufficient number of crack advances has process equilibrium shown The plot illustrates how the accumulation ahead rate. creep and growth rate for constant K tests has crack to a growth dynamic correspond to an rate and the damage equilibrium. upper limit for the crack growth rate in an increasing K test. constant, longest an time element ahead in region the of of This When K is the crack experiences the plastic deformation and therefore accumulates the most creep strain. 5.7. Effect of dK/da on CCGR The of creep crack growth has been modelled as a process damage accumulation ahead of a crack tip. Therefore, the load history an element experiences before advances through it will become important. or decreasing intensity important. crack K factor A advance CCGR with situation test the crack in the crack In an increasing rate of change of stress advance, dK/da, becomes which the rise in K with each becomes so large as to not allow the dynamic 15i )4°C, air =50 MPaS 10 -6 (m/s) 1-7 10 ,^-8 - --- .0 01 I I .01 0.1 Ecritical FIGURE 5.8) 10 M Predicted effect of Ecritical on the CCGR of a PM/HIP Superalloy at 7040 C. Z_ m PM/HIP low C ASTROLOY, 704°C, air, K=50 MPaYm --- ass (m/s) -6 10 - 10 1 I 10 I 100 GRAIN SIZE (um) FIGURE 5.9) Predicted effect of grain size variations on the CCGR of a PM/HIP nickel-base superalloy at 7040 C. 151- 0o o .0 0 C-, 3 0 v-I P-I U U ~ N o U) -0v'p0r 1-I n c:I 9-4 t loI -0 lo Ic~ o tn co Io tl- (S/W) 7p/lop DCDDi- oI CC: 152 d1o _ I __ I I I I ._ I I I 1 =70 MPa-F K:=50 MPa-m -6 10 d aim da dt (m/s) Pa-i 1-7 10 0 I 1 I 2 II . 3 I 4 5 I 6 I1 7 8 I 9 NUMBER OF CRACK ADVANCES (X) FIGURE 5.11) Predicted CCGR versus the number of crack advances for a constant K test. (based on data for PM/HIP low carbon ASTROLOY at 704°C in air.) 10 153 process will of strain accumulation to reach equilibrium. result in the prediction of a different creep crack growth rate than is actually possible at a given K. illustrated Astroloy in specimens dK/da in and or figure air at This is 5.12 for CCGR predictions on PM/HIP 704 C. procedures a This large A careful is examination of test recommended to avoid a large initial K which will give a non-conservative creep crack growth rate. The load effect history prediction of not reaching equilibrium as a result of will of temperature. become extremely creep-fatigue This process, important interactions though noted, in at will the high not be discussed here. 5.8. Effect of Temperature and Yield Strength The K curve obtained effect of temperature on the predicted CCGR versus is illustrated in figure 5.13 with the results by Huang (63) for PM/HIP Astroloy at 650 0C and 760 C. Temperature changes result in changes in the creep strain rate and a change will maintain a high yield strength at high temperature and will increasethe This will result in the yield strength. secondary in creep rate Ni-base alloys with temperature. a large increase in CCGR. rate for Astroloy is given below (Huang(63)): The creep 154- 0 o -T I0 -J 0 It -o 0A 00) · 0) P4 Ec 44 'I< I ct ~~ Ila I c: -(S/W) 1 a l I O I p I w H /p//p X 155 12.31 =0-(MPa) (sec-l) exp -122.2 (Kcal-mole -1 (Eq. The changes in s 5.15) for Astroloy account for the change in CCGR between 650 C and 760 C. As yield decreases ahead strength decreases the predicted CCGR also as a result of a lower stress in the plastic zone of the illustrated crack. The effect of varying Y.S. is in figure 5.14 for the predicted CCGR of PM/HIP Astroloy at a constant K=50 MPa/m. strongly on stress, and a small decrease in yield strength results been in a observed, groups. creep The creep rate depends large decrease in the CCGR. but not reported, This result has by industrial research The process of reducing yield strength to decrease crack susceptibility is refered to as "de-tuning" an alloy. The with yield strength from hot tensile tests will vary strain rate at temperatures where creep deformation is possible. Faster yield strength. strain rate tests have a larger value of Therefore, the value of yield strength from tensile tests used in the model may not be totally accurate. However, all the tensile results were obtained at the same strain rate and should be self-consistant. 156 16 ~_ ---- ---------- - - - -- rI I I 1 I Js r 1 L/C ASTROLOY Air C 760 --- p o65 0 / 700 OC _ . 10 0 10 650 C i IL - 3 U E 1/I, E -7 10 Cy +j ! 'IA -8 -9 0 l !I 20 FIGURE 5.13) _ I _I X 30 I. I' 40 I -C 50 ' -LI 7 /U II ! --- ~ t l -- r IUU K, MPaclm Predicted and actual CCGR results for PM/HIP low carbon Astroloy (ref. 63) for different temperatures. 157 PM/HIP low C ASTROLOY; .704°C; air; K=50 MPa~Y 10- 5 asS (m/s 10- 6 855 FIGURE 5.14) . 950 YIELD STRENGTH (MPa) Predicted effect of yield strength on the steady state CCGR of a nickel-base superalloy. An IUU mm PM/HIP low C ASTROLOY; 7040C; air 60 Kinit. (MPaVffi) 20 a 0 I 100 10 _ ,I I 104 f ___ ,,, 106 - 108 I 1010 INITIATION TIME (sec) FIGURE 5.15) Predicted time to begin CCG in a nickel-base superalloy. 158 5.9. Predicted Creep Crack Initiation Times The effect initiate creep specimen has of initial stress intensity on the time to crack been Charpigny (103). the to time growth reported It initiate from by a fatigue precracked Floreen (50), Bain (102), is found that as initial K decreases a creep crack becomes exponentially large. The crack the model can be used to predict the time to initiate growth from a sharp notch by determining the time for first results versus crack obtained time advance. for Figure 5.15 shows the predicted PM/HIP to initiation. Astroloy in air as initial K At low values of K the time to initiation becomes extremely long as a result of the rapidly reducing stresses at the crack tip. is similar to what is observed. The behavior predicted 159 6. DISCUSSION 6.1. CCGR Model The computer model presented in chapter 5 predicts some surprising results for creep-brittle materials in a small scale yielding condition. 1) The apparent These predictions are: lower limit for creep crack growth occurs when the plastic zone ahead of the crack becomes less than half an average grain diameter; 2) The CCGR in the stage II region depends on the rise in K with crack advance; 3) The stage I CCGR observed is an initial transient and is not a unique function of K; 4) The CCGR of an alloy is a strong function of the yield strength and creep strain rate of a material; 5) Load history can affect creep crack growth rate; and 6) function The function The time to initiate creep crack growth is a unique of K. lower limit in K of the grain size. for CCG is predicted to be a This lower limit, K threshold, for CCG is given below: KTH Y GS (Equ. 6.1) where KTH is the threshold stress intensity for creep crack growth, Y is the yield strength, and GS is the average grain 16 diameter. Floreen for intensity function (104) has shown that the initial stress in 100 hours at 704°C failure GS A in IN-792 is a true threshold may not exist for creep crack growth since there will always be some yielding at the tip a crack and for creep crack of limit therefore some damage. growth (K ) should The apparent be given by equation 6.1. The region of Paris Law type growth is affected by the dK/da for a specimen geometry. A Paris type relationship is given below: da/dt = AKd (Equ. 6.2) where A and variation are CCGR material dependent constants. an in increase dK/da a crack element drawn characterized ahead for constant The CCGR is determined by the stresses ahead of the material as At a given results in less time for damage accumulation in an element ahead of the crack tip. for The with dK/da results from a change in the history for an element ahead of the crack. load K, in N by K, and the load history for the of the crack tip. the K test. CCGR A series of curves can be versus number of crack advances for a The CCGR increases with each jump until a dynamic equilibrium occurs between the rate of crack advance and the rate of damage accumulation ahead of the crack. The 161 equilibrium value represents the CCGR for a given constant K without any history effects. In actual CCGR tests the value for K increases with each crack advance. The CCGR at a value of K depends cin the K-history which results from the dK/da This process is graphically illustrated in in the specimen. Ais the dK/da increases within a specimen, the 6.1. Figure CCGR K; curve versus grained large in pronounced decreases. This effect materials and will be should be considered when measuring the CCGR for a material. The I stage transient CCGR behavior jumps. results an initial undamaged material for the first Thi s results in a lower CCGR upon loading which quickly will is whichI results from the fact that the crack tip is advancing throug;h initially few measured rise reported to in the stage II region. Many of the the literature reflect primarily this initial transient, and any conclusions based on Stage I CCGR data are questionable. The be CCGR measured for all materials has been shown to a strong function of the stress at the crack tip and the of creep strain accumulation (Chapter 5). rate Any changes in heat treatment or processing which alters either of these material properties will result in a marked affect on the observed CCGR behavior in a material. The the measured amount of CCGR at a value of K depends strongly on creep deformation accumulated just ahead of the crack tip. of a Variations in the grains in the load history specimen or component which will affect the amount of 162 K KK X a _ 1 I I I I I I I 1 Ki FIGURE6.1) ! _ _ I _ I K2 Schematic showing the effect of dK/da on CCGR results. 163 crack creep strain ahead of a crack will affect the growth rate. The crack growth rate depends on creep accumulated the amount of damage a grain receives from previous loadings which gives study on the the a "memory" of past loadings. material effect of load history is vital A if the between fatigue and creep crack growth are the .interactions be understood. shown in chapter 5, the time to initiate creep was As from growth crack initial a crack depends strongly on the sharp This result is important when one considers the K. between interaction creep crack growth and fatigue. If a fatigue crack advances through the damaged zone ahead of the in crack tip crack growth exhibit time less time than is required to initiate creep for a sharp crack, then the crack should not significant time dependent behavior. becomes important when The initation considering whether or not a fatigue crack will advance during a tensile hold period at a high temperature. Tensile hold periods are common in modern aircraft gas turbine components during flight. 164 Effect of Triaxiality 6.1.1. It much often been observed that creep crack growth is has slower in the plane stress condition than in the plane strain condition. any show growth shown stress rate critical that can be to change the creep crack shown a material via changes in the value of the of strain function The affect of a triaxial effect of stress state. of state in section 5 does not The model presented for fracture. Rice and Tracey (105) have the critical strain to fracture a material is a of the state of stress. The expression for the critical strain is given by: e where "C" is hydrostatic plane have plane A material, cH is the aE is the equivalent stress. The The ratio of the strain for plane stress to the critical strain for strain as given by equation 6.3. is approximately 18. in smooth significant 75 0 C. the worked out by Hutchinson (72). been reduction versus and for (Equ.6.3) and plane strain stress fields at a crack tip stress critical constant stress, [(. C expI 23Ja rtcal by the creep Dyson creep ductility for notched specimens rupture specimens was shown to be and Loveday (106) for Nimonic 80A at 165 The effect increasing of critical of 18 for PM/HIP plane stress CCGR is shown in Figure 6.2. is much behavior The plane stress than the CCGR in plane strain. slower accounts strain by a at 704 0 C in air to simulate factor CCGR Astroloy the This the crack tip tunnelling phenomenon for observed in CCGR tests without side grooves. 6.1.2. Effect of OxygenConcentration model The the strain and a change in the element size. critical in reduction critical mechanism element size change in in air assumes tests. mystery. A reduction in the path from predominantly prior particle argon CCGR in air tests to CCGR tests. predominantly The change in crack crack runs This to The model growth rate which is not observed in all in air on PM/HIP Astroloy indicate that the Tests out growth rate for Astroloy. constant reduction in the critical strain in air creep da/dt. CCGR a CCGR tests results from the observed fracture creep a alloys. crack will result from any of the size alone is sufficient to explain the increase in element with air in intergranular the remains fracture boundaries strain The mechanisms suggested in section 2.2., but the embrittlement exact the CCGR in air by a reduction in predicts the embrittling effect of oxygen at high change is reflected in the transition at fast a prior particle boundary fracture path in the air 166 to cn co 0 co - co 0 .-4 t0 uC P4- UI .;4 .h 0 00 cV 0oI I 0- C0 - I! ( (IS/ W) I '0 ?P/OP II i 0C -4 I - 0 I - P 167 6.1.3. Limitations of the CCGR Model There are several points in the model which may give rise to errors in calculating the CCGR of a material. include: crack These 1) The procedure used to update the stresses upon advance; 2) The effect of strain rate on plasticity; 3) The estimation of the plastic zone size; 4) The effect of time independent plastic strain on CCGR; and 5) The effect of strain rate on yield strength. The stresses ahead of the crack tip are updated to the calculated values given at t=O which are by an approximation of the HRR singularity. result of creep iterations. stresses of creep strain accumulation from previous This may result in an slight over-estimation of at CCGR. The stresses are not reduced as a the crack tip and therefore an over-estimation This strains over-estimation are generally should be small since the small when compared with the plastic strain. At high temperatures the strain hardening behavior of a material The becomes a function of both strain and strain rate. measured increasing yield strain strength rate. of a material increases with A change in yield strength would result in a considerable effect on the predicted CCGR for an alloy. at All the tensile tests in this study were performed the same strain rate, and therefore should be comparible and self consistant. The scaling plastic zone is calculated as .1 (K2 /y 2). parameter for the plastic zone is 0.1. The Estimations 168 of plastic zone size vary with angle ahead of the crack tip, For mode I loading in state of stress, and mode of loading. plane strain estimates for the scaling factor vary from .018 to .32. scaling 0.1 is a good estimate While (69) factor, it may not be the best. of the average Changing the of the plastic zone will affect the slope of the estimation CCGR versus K curve, but such changes will be minor. model does not calculate any damage as a result of The the time independent plastic strain ahead of the crack tip. Plastic strain has been shown strain by Nix (107). fracture reduce to the critical The effect of plastic strain on the critical strain may be a source of error in predicted CCGR results. At this time there exists no consistant way of predicting this damage. The yield 0.2% strength for temperature varies with strain rate. a strength will affect tested were at high Any change in yield The yield strength of the the CCGR. obtained material The yield strength has affect on the predicted CCGR. large alloys a at the same strain rate (2%/minute) and should be self-consistant. The various sources of error in CCGR prediction can be significant, but hopefully these errors are systematic and therefore act only as scaling factors which will adjust the predicted CCGR in a self-consistant manner. The fact that model predicts the CCGR for the alloys tested based on measurable input parameters indicates that these errors are the systematic. 169 CCGR of PM/HIP Ni-Base Alloys 6.2. for a PM/HIP Ni-base alloy has been shown to CCGR The considerably. from alloy to alloy in both air and inert vary measured The environments. CCGR will vary as a result of test procedure, and environment. and creep-brittle, tip crack well are conditions of small scale yielding the III) Therefore, the stresses ahead of the (Appendix apply. The alloys tested were all characterized by K, and K becomes a natural parameter against which CCGR behavior is correlated. Effect of test procedures 6.2.1. CCGR The and load initial the in air for an alloy varied with the applied K. The stage II CCGR decreased with The effect of applied load is a result of load. increasing changes in dK/da associated with changing load. The dK/da for the SEN specimens tested is given by: dK/da /7;P OF N (.56-.345/w+26.5(a/W2 )-76.0(a2/W 3 ) /W3 4 )) +136.8(a (Equ 6 4) The dK/da varies only with load, "P", for a fixed a/w. The tests exhibit a region of sharply increasing CCGR CCGR upon initial loading. This stage I region of growth is not unique and occurs for any initial K. high initial CCGR versus K K. resulted Tests performed at in the lack of a stage II region This indicates that the condition required 17C at equilibrium reach- dynamic to crack tip were not the acheived before the value of KIC was reached. fact that the applied load and initial K will have The a profound effect on the CCGR results coupled with the such effect of triaxiality on the CCGR indicates that most of the CCGR on results published for Ni-base alloys high Systematic tests may have significant errors. temperatures at with low dK/da, low initial K, and specimens which have been to avoid crack tip tunnelling are required to grooved side achieve accurate CCGR results. 6.2.2. Effect of Oxygen presence of oxygen acted to significantly increase The the In addition to measured CCGR in all the alloys tested. increasing the path fracture CCGR, from oxygen resulted to boundaries particle prior in a change in the grain boundary cracking. hour 100 that indicated the grain results air exposure at been to a considerable of also on 4 alloys depth. Similar obtained by Woodford and Bricknell (30) and Pineau (56) using Ni-base alloys. that 7040 C the oxygen diffuses into the material along boundaries have tests The results indicated the alloys which had the highest effective diffusivity oxygen had were IN-100 and Rene-95 (60 mesh). the embrittlement fastest in the CCGR time in for The depth of oxygen air. one These alloys crack advance can be calculated from the following expression: 171 (Equ. 6.5) is X where the Assuming a minimum depth of oxygen grain diameter. average a" is the CCGR, and "GS" is the in section 4.5, determined embrittlement ahead is the of oxygen along the grain boundaries diffusivity effective of oxygen embrittlement, D depth the of crack tip is required for accelerated fracture in air, equation 6.5 indicates that the CCGR maximum at air Figure 6.3 7040 C and K = 30 MPami versus A good correlation of the CCGR data is observed, but DO GS. the air is proportional to Do-GS. in CCGR the shows in slope of the line is -.5 indicating the depth of oxygen embrittlement is not the only criteria for fracture. CCGR rate the CCGR the Stage observed Merl-76. limited by the diffusion of oxygen and embrittlement, then when the diffusion of oxygen subsequent becomes is oxygen in If the the slope of the stage II region of limiting versus K curves will be reached. A reduction of II CCGR slope from the predicted slope in air is in Rene-95 to a lesser degree in IN-1OQ and and While the change in slope may be partially due to the change in fracture path, these results indicate that the of rate limiting similar diffusion oxygen rate to and embrittlement does become a step for CCGR in air. the well known stress This behavior is very corrosion phenomenon observed in aquious environments. cracking 172 . -5 _ 'U _ _ _ I I da dt I (m/s) -6 10 1 I v -7 - - - _ l _ 10-18 _ _ _ _._ _ I 7- _ 10-17 10 -j 10 10 D *GRAIN SIZE (m3/s) 0 FIGURE 6.3) Comparison of the CCGR in air at 7040 C, K=30 MPa-f versus the effective diffusivity of oxygen * grain size for four PM/HIP Nickel-Base Superalloys. 10 173 Alloying magnesium additions which such as boron, zirconium, and inhibit the embrittlement of oxygen can be expected to reduce the CCGR measured in an oxygen containing environment. The predicted over-predicted preditions by for over-prediction reduced CCGR the upon model. Rene-95 comes initial (60 from before boundaries neglected oxygen in air is This is most obvious in the mesh) the critical strain in air. required loading can in air at 7040C. The assumption of a constant, Actually some time will be diffuse and embrittle the crack tip. along the grain Since this time is in the model, an over-prediction of the CCGR will occur. Some indicate crack CCGR results in air for IN-718 by Pineau (56) that the diffusion of oxygen down the crack to the tip may limit the availability of oxygen. These CCGR results indicate that as the initial crack length increases, the would creep crack occur growth rate decreases for a given K. This if diffusion of oxygen to the crack tip became the rate limiting step in oxygen embrittlement. In general, these effectswere not observed in the alloys tested. 6.3. time Notched Stress Rupture Tests versus CCGR The notched stress to failure for an alloy from creep mechanisms in the rupture presence of a stress concentration. involves the (NSR) test indicates the The fracture mechanism initiation of cracks in the specimen followed 174 by propagation of a single dominant crack until KIC is the and reached At high stresses the occurs. fracture fast net section of the notched specimen goes plastic and entire failure usually occurs from subsurface cracks which initiate The failure. stress lower fractures. intergranular to These to from one dominant crack which propagates to link cracks surface cracks. from results failure the stress, shown At lower net section center of the specimen. (106) the in fractures are usually brittle The presence reduce significantly the of oxygen has been time to failure in nickel-base alloys. (5) of a as crack. test at low net section stress can be thought NSR The which measures the time to initiate a creep test If crack initiation in the NSR test results from the nucleation, growth and coelescence of cavities in the stress field at tests can root the the notch, then the results of NSR of the relative CCG behavior of different indicate alloys and/or different heat treatments. 6.4 Figure for air from have while highest performed at 675 MPa in air at 704°C. tests results CCGR be of the alloys tested versus the time to rupture 4 results NSR to NSR compares the CCGR at 704 0 C, K=30 MPa/-m, in the Rene-95 CCGR. for The the alloys follow the same ranking as the for the same 4 alloys. longest gave time the Astroloy was observed to rupture and the lowest CCGR shortest time to rupture and the These results indicate that the NSR test may a quick, simple and inexpensive test to compare the CCGR 175 S 10 _ _ da dt (m/s) -6 10 l A-7 LU 10 100 1000 RUPTURE TIME (HRS.) FIGURE 6.4) Comparison of the CCGR in air at 7040 C, K=30 MPa-i versus the time to rupture a NSR specimen. 176 behavior changes. of various alloys, heat treatments and processing 177 7. CONCLUSIONS 1. Creep crack growth rates were measured for 4 Ni-base, y -'; strengthened Ni-base superalloys at 704 C in both air and in a 99.999% pure argon environment. The four alloys tested were all PM/HIP alloys and they included low Carbon Astroloy, Merl-76, Low Carbon IN-100, Rene-95 (60 mesh powder), ad Rene-95 4(120 mesh powder). The CCGR ranged from 10- m/ s to 10 m/s and the stress intensity ranged from 10 MPa-m- to 120 MPaii: The presence of oxygen during CCG resulted in an increase in the measured CCGR for a given K in all the alloys tested. The increase in CCGR varied for the alloys tested, but Rene-95 (60 mesh) had the largest increase in CCGR and Low Carbon IN-100 had the smallest increase in CCGR. CCGR increases for the alloys ranged from 10 times to 1000 times in air over the CCGR measured in pure argon. 2. The increase in the measured CCGR in air resulted from a change in fracture path and a decrease in creep ductility. The CCG fracture path was intergranular for both the air and the pure argon environments, but the creep crack followed grain boundaries which were coincident with prior particle boundaries in argon tests. The predominantly PPB cracking in argon can be attributed to the increased number of carbides which segregate to the PPB in PM/HIP alloys. In air the fractures followed the nearest grain boundary, regardless of whether it was a PPB or not. 3. The CCGR behavior of Ni-base alloys exhibited three stages. Stage I is an initial transient, which is not a unique function of K, and results from the crack propagation through initially undamaged material. Stage II is a region of gradually increasing CCGR with K, and stage III is associated with KIC and fast fracture. 178 4. A computer model was developed to predict the CCG The model is based on the of the alloys. behavior accumulation of damage in the form of creep strain ahead of the crack tip. The results of the model were in excellent agreement with actual CCGR results, and the model provided some insights into the CCG process. The model predicts that grain size, critical strain, and creep rate will all significantly affect the CCGR. The model also predicts that load history effects will significantly alter the CCGR measured. 5. CCGR behavior depends on both the stress intensity factor and the load history applied to the specimen. The intensity factor, K I, describes the crack tip stress stresses and therefore the CCGR for creep-brittle materials when the conditions-of small scale yielding are satisfied. The CCGR measured depends strongly on the load history. The effect of load history on CCGR was observed with the effect of dK/da on CCGR. The initial transient in CCGR upon loading also indicates the importance of load history. The CCGR test procedures and the specimen geometry 6. used in CCGR testing will have a strong effect on the measured CCGR behavior of an alloy. In all cases the errors in CCGR measurements resulting from improper test procedure lead to a non-conservative estimate of CCGR, and in a large over-prediction of actual component life. Notch stress rupture test can be used to evaluate 7. the CCG resistance of a material when the net section stress is low. 8. The grain boundary chemistry of an alloy is in determining it's susceptibility to oxygen critical Alloys with high concentrations of boron embrittlement. tend to have a smaller reduction increep ductility and lower increase in CCGR in air than alloys with a low B content. 179 8. RECOMMENDATIONS FOR FUTURE WORK following The are for research that recommendations extend the understanding of creep crack growth and the will effects of alloy chemistry on CCG behavior. Effect of Test Procedures and Specimen Design 8.1. It by has been shown that the CCGR results are influenced rise the a with crack advance and thevalue of the K The development of general testing criteria and K. initial in standard geometry specimen used in creep crack be to rate measurement are required to insure accurate and growth test repeatable These results. results can then be incorporated in a design criteria for operating components. tests CCGR such geometries edge single varying on several specimen performed cantilever beam specimen, double double specimen, specimen notched from obtained the as tension compact be should notched specimen, edge and others. The results specimens with a specific alloy while these the specimen dimensions should be analyzed to only determine if such parameters as grain size to specimen width ratio and dK/da Since CCGR is a boundaries ahead surprising if measured dK/da CCGR. are size ratio are important. process of damage accumulation on grain of crack the affected boundaries grain versus Once analyzed, the number by the of tip, it randomly would oriented not be grain crack tip would influence the the effects of specimen geometry and a coherent test procedure could be 180 conceived repeatable insure would which comparible and results. Grain Boundary Chemistry 8.2. The of chemistry boundary grain alloys is Ni-base strongly affected by minor alloying additions. Elements like C, B, and Zr segregate to the grain boundaries, and have the potential of strongly the creep crack growth influencing behavior of an alloy. A of study the effect of elements which segregate to grain boundary should be attempted. the commercial Ni-base systematic alloying elements. The oxygen such be can procured ofgrain additions which have specific boundary selected for the study should have a alloy eff ect of environment on CCGR, since the interaction strong of alloy Several heats of a as acceptable and Rene-95, IN-718, in regard. additions of yttrium, those elements is also of interest. which this INCONEL Different X-750 are all heats should have like boron, zirconium, hafnium, and elements are and Alloys known to affect sensitivity to oxygen embrittlement. The alloy is changes. development of a new creep crack growth resistant conceivable The by making only minor alloy chemistry development commercial applications. of such an alloy has obvious 181 APPENDIX I CCGR TEST RESULTS 182 TABLE I.1 LIST OF CCGR TESTS GROSS STRESS TES ALLOY tP Kinitial (PROPAGATION TIME) (hours) Ao (mm) (MPa m) (MPa) _ . . tf (FAILURE TIME) (hours) . 236.( 2.40 29.0 1.5 1.5 135 145.6 2.68 19.4 68.6 117.3 137 191.1 2.28 22.1 20.9 20.9 149 191.1 3.52 34.6 1.9 1.9 150 191.1 2.36 23.0 2.1 2.1 153 236. 1.78 22.6 28.0 31.6 140' 141.1 3.48 33.2 63.6 63.6 145' 291.2 2.45 35.6 70.0 70.0 655.C 1.01 43.2 2.1 71.6 132 155.C 1.81 15.1 1.5 7.4 138 200.2 2.62 26.2 2.3 2.3 139 236.6 1.87 23.3 2.1 2.1 152 145.6 1.84 14.2 12.1 12.1 141* 291.2 2.48 36.0 41.0 84.3 144* 241.2 2.32 33.8 31.5 51.2 528.0 1.03 35.4 5.5 5.5 131 164.0 2.08 17.6 7.6 30.2 148 182.0 2.13 20.0 4.6 22.8 142* 291.2 2.13 31.7 7.5 7.5 147* 145.6 2.54 18.3 23.7 23.7 241.0 1.40 23.6 6.5 124.9 145.6 1.42 12.0 2.1 4.6 151 91.0 3.35 15.3 1.2 1.2 154 164.0 1.91 16.5 1.0 26.2 143* 236.6 2.03 24.9 36.2 36.2 441.0 1.01 32.4 2.8 71.3 145.6 1.57 13.2 6.8 58.2 236 . 6 1.37 18.6 43.7 66.0 130 128 127 Low C Astrolo: MERL-76 Low C IN-100 Rene-95 (60 mesh) 133 136 129 Rene-95 (120 mesh 134 146* * 99-999%pure Argon;** time to failure includes time spent at lower loads 183 APPENDIX Computer Program: II CCGR Prediction Model (Written for the IBM-PC in -BASICA) Portions of the text on the following page(s) are not legible in the original. 184 I 2 . 4 5 6 REM t*S**t** REM * REM * F:EM * REM * REM * 7 REM* X*t*** * ************* CCGRMOD9 WRITTEN :)4-14-EWRITTEN BY: ENJETH R. EAIrJ MASSACHUSETTS INSTITUTE OF TECHNOLOGY THIS PROGRAM CLCULATES da/idt vs FOR SPECIFIC INPUT VALUES RAND MATE:IALS FARAMENTERS t** *******s **** *******YIB$t$ttt ***trW **** **** i':,DIM EG (2':~,:) DADTS 2') W=.0117 21 0=') 22 FOR Z=1 TO ZOO)C:) O. EG(Z) =' 24 C02(Z)=': 25 NEXT Z 26 CLS S (50) ,C02 5 $ * * (2'C:,::) 29 RESTORE .0 GOSUB 2(:,":)' 40 Tl=O 50 A=AO 60 I=1 65 PI=5.14159 7') K=SI*SOR(PI*A)*(1.12-.2*A/W+10).6*(A/W) "2-21.7*(A/W)' 7-+.4*(A/W) 72 RP=(K/SY)2/10 76 IF I=1 THEN A2=A 77 IF I=1 THEN T2=O '4) IF K'=K:IC THEN GOTO 250 90 IF A(.6*W) THEN GOTO 20 91 NC1=(NC/(1+NC)):ECCON=(.29*K'2/BC/E*(l+NC)'(l/NC))'NC1 95 EP1=ECCON/GSNC1 96 R=GS 99 GOSU 1000 106 T=((EPC-EG(I)+ECTR)/EP1)"(NC+I)-TTRAN 1JO> N%=RP/GS 140 FOR J=2 TO N% 150 R=GS*J 152 GOSUS 1000 155i L-I+J-1 159 TT=T+TTRAN 160I EG(L)=EG(L)+ECCON*(TT'(1 /NC)/R)"NC1-ECTR 170 I NEXT J 180 I A=A+GS 190 I TI-TI+T 191 IF I-1 THEN GOTO 201 195 IF K (S1.5*K1) THEN GOTO 210 201 Q=Q+ 202 DADTS () = (A-A2)/(T1-T2) 20.: KS(Q)=(K1+K)/ 2 204 KI=K 205 IF I=1 THEN KS(Q)-K1 206 A2-A 207 208 210 220 2.0 T2:=T1 PRINT D ADTS(Q):KS(Q);A;T1 I=I+1 GOTO 70 GOSUBP 000 INPUT "DO YOU WANT ANOTHER RUN (Y/N)"; Q09 9 2J3 IF 09$-"Y" OR Q0 $"y" THEN GOTO 20 240 END 1000)TTRAN=NC.LOG(BC)+LOG(.29*K-2/E/R/(NC+1))-(NC+1)*LOG(SY(RP/R)(1/(NP+I))I 1010i TTRAN=EXP(TTRAN) 1020 ECTR=ECCON*(TTRAN (1/NC)/R)"NCI 1050 F PETURN 200: i PRINT "WELCOME TO CCGRMOD9: 2010 i PRINT "'INPUT ALLOY: ASTROLOY PRTNT_" _- _ R_-7_ WRITTEN BY KENNETH BAIN":PRINT 1" 185 :040 2050 2060 2070 PRINT " IN-t100 PRINT " RENE-95 (60 mesh) 4" PRINT " RENE-95 (120 mesh) 5" INPUT " OTHER 6":02 IF 02, 1 OR 02;5 THEN GOTO 2100 2075 FOR Z=l TO 02 READ C,NC.BP.NP.GS,PPS,E,fIC.EPCK:ICAR.SY.D02 2085 NEXT Z 2090 GOTO 2210 INPUT "ENTER BC (MPa); (creep rate=(stress/BC)'NC)";BC 2110 INPUT "ENTER NC" :NC 2120 INPUT "ENTER NP";NP 2140 INPUT "ENTER GRAIN SIZE (um)";GS 2145 GS=GS / 1 0004: :C) ' 2160 INPUT "ENTER MODULUS (MPa)";E 2170 INPUT "ENTER KIC (MPa*sqrt(m))":KIC 2180 INPUT "ENTER CRITICAL STRAIN";EPC 2190 INPUT "ENTER YIELD STRENGTH (MPa)":SY 2200 INPUT "ENTER DUCTILITY REDUCTION IN OXYGEN";D02 221 0 INPUT "ENTER INITIAL STRESS (MPa)":SI :21':0 2220 INPUT "ENTER INITIAL CRACK LENGTH (mm)";AO AO=AO/1000 2225 INPUT "IS OXYGEN PRESENT (Y/N)";Q8$ 2240 IF Q8$="Y" OR 085="y" THEN GOTO 2270 2247 IF 02=6 THEN GOTO 2270 2250 GS=PPS 2260 KIC=I ICAR 2270 INPUT "ENTER TITLE";TITL$ 2272 DATA 1795,18.4,1662,9.70,28E-6 95E-6,17E+4,88..008,88,.95(:0.1.0) 2275 DATA 1734,19.9,1448,16.6,11E-6,22E-6,16E+4,84,.05.120,1012,.2 2274 DATA 1774.18.5,1454, 16.3,23E-63,. 5E-6, 16E+4,72. 007,105.1012,.714 2275 DATA 416,10.3.1785,8.90,25E-6,7E-6,167E+3.,71..00.90.950j,. _-...5 2276 DATA 416.10..-.1785,8.90:,22E-6, :-.:)50,. 00.9,95 34E-6,167E-43 71 .. 2278 IF Q8$="Y" OR oes="y" THEN EPC=EPCtDO2 2280 RETURN 5000 LPRINT TITLS:LPRINT " 1c000 5005 AOOAO LPRINT "INITIAL CRACK LENGTH (mm)=";AOO 3015 o15 LPRINT "INITIAL STRESS (MPa)=";SI 3020 LPRINT "OXYGEN (Y/N)=":$SS LPRINT 30.5 "BC=" ; C;" LFRINT " ":LPRINT " " LPRINT "da/dt (m/s) 3.040 FOR 0Q=1 " YS="; SY;" GS = ": GS " K (MPatsqrt(m))":LPRINT TO 3050 LPRINT USING " ##.##. 3060 NEXT Q1 5070 RETURN = NC ":NC "':DADTS(O),.KS(01) 186 APPENDIX III CALCULATION OF DUCTILE-BRITTLE TRANSITION TIMES 187 The tested alloys to time assumption the can creep-brittle condition for the be verified by comparing the total test calculated transition time. creep-brittle the a of time transition Calculations for have been suggested by Riedel and Rice (74) and by McClintock (108). The C*-integral must be calculated for the SEN geometry calculate the transition time from Riedel and Rice (74). to The C*-integral can inferred be calculation of Shih (63). from the J-integral The expression for C* is: (1+N) C= N )c (r~Ea a hh(a/wN) (a/w l a a n 1 +wa2] (w-a) m= 1.455 for plane strain m= 1.072 for plane stress N ) (Equ. A.3.1) 188 e=( C/Bc) Cr is the gross section stress, where c, and h1 (a/w,N ) is a geometric constant which is equal to .96 for a/w = .25 and N When = 10; and .23 for a/w = .15 and Nc = 20. CX= specimen is a/w = .25, 300 MPa, in plane strain, w = 11.7 mm, the and calculated the value for transition time is given in Table A.3.1. The calculation in Table tested A.3.1. are for the constituative relationships alloy using the at 704°C. TABLE A.3.1. Transition Times for Several Ni-base Alloys at 7040 C ttrans (sec) C*(MPa'm-s ) ttrans (RR)(sec) McClintock -14 ASTROLOY 8.2 x 10 14 MERL-76 1.6 x 101 IN-100 8.8 x 10 Rene-95 1.3 x 10 1.1 x _ 15 105 2.3 9 x 1010 5.1 x 1015 1.2 x 109 1.0 x 2.3 x 106 9.4 x 10 10 1.2 x 10 (60 mesh) K = 43.5 MPa-m which corresponds results indicate for the conditions used in Table A.3.1. to an average, K in CCGR testing. The that the transition time calculation from McClintock is more conservative, time. CCGR tests lasted giving a shorter transition only 4 x 10 5 seconds for Astroloy, and less time for alloys which have a faster CCGR. The total a longer transition times are much larger than the CCGR test time which indicates that the assupmtion of creep-brittle condition is a valid assumption. When the 189 total test time is on the order of the transition time then no conclusions are possible. 190 REFERENCES 1.) C.C. Annis, Jr., M.C. VanWanderham, J.A. Harris, Jr., and D.C. An Application Sims, "Gas-Turbine Disk Retirement-for-Cause: of Fracture Mechanics and NDE", A.S.M.E., March 10, 1980. 2.) D. McLean, B.F. Dyson, and M.R. Taplin, Fracture 1977, vol. 1, ICF4, Waterloo, Canada, p. 325. 3.) A.S. Argon, Scripta Met., vol. 17,p. 5, 1983. 4.) A.S. Argon, "Recent Advances in Creep & Fracture of Engineering Materials and Structures", edited by B. Wilkshire and D.R.J. Owen, Pineridge Press, Swansea, U.K., 1982. 5.) C.C. Law and M.J. Blackburn, "Plastic Flow and Fracture Processes in Powder Metallurgical Nickel-Base Superalloys", Pratt and Whitney Aircraft, AFOSR report No. EII 80-200-7053-FR, p. 75. 6.) R.F. Decker and C.T. Sims, The Superalloys, Chapter 2, edited by C.T. Sims and W.C. Hagel, John Wiley and Sons, New York, p. 2. 7.) R.F. Decker, Strenghening Mechanisms in Nickel-Base Superalloys, published by International Nickel, New York, 1970, p.2 . 8.) O.H. Kriege and J.M. Baris, Transactions of the ASM, vol. 62, 1969, p. 195. 9.) Metals Progress Databook 1980, p. 90. 10.) C.T. Sims, The Superalloys, Chapter 9, edited by C.T. Sims and W.C. Hagel, John Wiley and Sons, New York, 1972, p.25 4 . 11.) D.R. Muzyka, ibid., p. 113. 12.) R.M. Pelloux and N.J. Grant, Trans. AIME, 218, 1960, p.23 2 . 13.) N.S. Stoloff, The Superalloys,ibid., p.79. 14.) D.A. Woodford, Met. Trans. A, 12A, 1981, p. 229. 15.) R.F. Decker and J.W. Freeman, Trans. AIME, vol. 218, 1960, p. 277. 16.) C.C. Law and M.J. Blackburn, Met. Trans. A, 11A, 1980, p. 495. 191 17.) R.M. Pelloux and J.S. Huang,Creep-Fatigue-Environment Interactions, edited by R.M. Pelloux and N.S. Stoloff, published by A.I.M.E., 1980, p. 151. 18.) T. Saegusa, M. Uemura, and J.R. Weertman, Met. Trans. A, 11A, 1980, p. 1453. 19.) J. Reuchet and L. Remy, Met. Trans. A, 14A, 1983, p. 141. 20.) D.A. Woodford and R.H. Bricknell, Met. Trans. A, 12A, 1981, p. 1945. 21.) R.H. Bricknell and D.A. Woodford, Met. Trans. A, 12A, 1981, P. 1673. 22.) D.A. Woodford, Met. Trans. A, 12A, 1981, p. 299. 23.) M. Prager and G. Sines, Journal of Basic Eng., 1971, p. 225. 24.) P. Shahinian, Transactions of the ASME, 1965, p. 344. 25.) R.C. Lobb, Mat. Sci. and Eng., vol. 38, 1979, p. 249. 26.) Y. Hosoi and S. Abe, Met. Trans. A, 6A, 1975, p. 1171. 27.) M.L. Sessions, C.J. McMahon, Jr., and J.L. Walker, Mat. Sci. and Eng., vol. 27, 1977, p. 17. 28.) R.H. Bricknell, R.A. Mulford, and D.A. Woodford, Met. Trans. A, 13A, 1982, p. 1223. 29.) R.A. Mulford, Met. Trans. A, 14A, 1983, p. 865. 30.) D.A. Woodford and R.H. Bricknell, Met. Trans. A, 12A, 1981, p. 1467. 31.) B.F. Dyson, Acta. Metall.,.Vol. 30, 1982, p. 1639. 32.) S. Floreen and J.M. Davidson, Met. Trans. A., 14A..1983 p. 895. 33.) P.N. Chaka and C.J. McMahon, Jr, Met. Trans A., vol 5., 1974, p. 441. 34.) R.H. Bricknell and D.A. Woodford, Met. Trans. A., 12A, 1981, p. 425. 35.) S. Floreen and C.J. White, Met. Trans. A., 12A, 1981, p. 1973 36.) P.L. Bensussan, private communication, M.I.T. 192 37.) S. Floreen and R.H. Kane, Nickel Topics, Vol. 34, No. 2, 1981 p. 6. 38.) D.A. Kraui and S. Floreen, Trans. TRS-AIME, vol. 230, 1964, p.8 3 3 . 39.) P.L. Bensussan, D.A. Jablonski and R.M. Pelloux, "A Study of Creep Crack Growth in 2219-T851 Aluminum Alloy Using a Computerized Testing System", research at M.I.T. and Instron Inc., submitted to Met. Trans. 1983. 40.) J.G. Kaufman, K.O. Bogardus, D.A. Mauney and R.C. Malcom, ASTM STP 540, 1976, 41.) p. 144. K.M.Nikbin and B.A. Webster, Micro and Macro Mechanisms of Crack Growth, edited by K. Sadananda, BB. Ruth and D.J. Mickel, AIME, 1982, p. 137. 42.) V.M.Radhakrishnan and A.J.McEvily, Joun. of Eng. Mat. and Tech., Trans. of ASME, 102, 1980, p. 200. 43.) D.J. Michel and H.H. Smith, Creep-Fatigue-Environment Interactions, edithed by R.M. Pelloux and N.S. Stoloff, The Metallurgical Society of AIME, 1980, p. 129. 44.) L. Coffin, ibid, p.l. 45.) C.L. Jones and R. Pilkington, Met. Trans. A., 9A, 1978, p. 865. 46.) D.J. Smith and G.A. Webster, "Influence of Cyclic Loading on Crack Growth of .5% Cr, .5% Mo, .25% V steel", to be presented at the Fourth International Conf. on Behavior of Materials, Stockholm, August, 1983. 47.) K. Sadananda and P. Shahinian, Met. Trans. A., 11A, 1980, p. 267. 48.) K. Sadananda and P. Shshinian, Mat. Sci. and Eng., vol. 43, 1980, p. 159. 49.) K. Sadananda and P. Shahinian, Creep-Fatigue-Environment Interactions, p. 86. ibid., p. 112 50.) S. Floreen, 51.) R.M. Pelloux and J.S. Huang, ibid., p. 151. 52.) K. Sadananda and P. Shahinian, Met. Trans. A., 8A, 1977, p. 439. 53.) K. Sanananda and P. Shahinian, Met. Trans. A., 9A, 1978, p. 79. 193 54.) 55.) R.B. Scarlin, Mat. Sci. and Eng., vol. 30, 1977, p. 55. F. Gabrielli and R.M. Pelloux, Met. Trans. A., 13A, 1982, p. 1083. 56.) A. Pineau, "Subcritical Crack Grwoth Due to Fatigue, Stress Corrosion and Creep", ISPRA, 1981. 57.) Bogun Wu and R.M. Pelloux, "High Temperature Creep Crack Growth in In-100 Alloy", unpublished research at M.I.T. 58.) K.R. Bain and R.M. Pelloux, "Effect of Environemnt on Creep Crack Grwoth in Rene-95", submitted to Met. Trans., 1982. 59.) R.C. Donath, J. Nicholas and C.S. Fu, Fracture Mechanics: Thirteenth Conference, ASTM STP 743, edited by R. Roberts, p. 186. 60.) J.M. Larson and S. Floreen, Met. Trans. A., 8A, 1977, p. 51. 61.) C.C. Law and M.J. Blackburn, Met. Trans. A.,1lA, 1980, p.495. 62.) R. McCabe, "Creep Crack Growth in Rene-95 at 6500 C and 7600 C", S.M. Tehsis, M;I.T., February, 1981. 63.) J.S. Huang, "Fatigue Crack Growth and Creep CRack Growth of PM/HIP Low Carbon Astroloy at High Temperature", Sc. D. thesis, M.I.T., February, 1981. 64.) L.S. Fu, Eng. Fract. Mech., vol 13, 1980, p. 307. 65.) F.A. McClintock and J.L. Bassani, "Problems in Environmentally Affected Creep Crack Growth", Proceedings of the IUTAM Symposium, Dourdan, 1980. 66.) H.P. Van Leeuwen, Eng. Fract. Mechanics, vol. 9, 1977, p. 951. 67.) A. Pineau, private communication, May 1983. 68.) S. Floreen, Mat. Trans. A., 6A, 1975, p. 1741. 69.) David Broek, Elementary Engineering Fracture Mechanics, Sijthoff and Noordhoff Publishers, Netherlands, 1980, p. 10. 70.) J.R. Rice and G.F. Rosengren, J. Mech. Phys. Solids, 16, 1968, 71.) p. 2. J.W. Hutchinson, J. Mech. Phys. Solids, 16, 1968, p. 13. 194 72.) J.W. Hutchinson, J. Mech. Phys. Solids, 16, 1968, p. 337. 73.) J.R. Rice, J. of App. Mech., Trans. of ASME, 1968, p. 379. 74.) H. Riedel and J.R. Rice, ASTM STP 700, 1980, p. 112. 75.) J,D. Landes and J.A. Begley, ASTM STP 590, 1976, p. 128. 76.) D. Hull and D. Rimmer, Phil. Mag., vol. 4, 1959, p. 673. 77.) M.U. Speight and W. Beere, Metal Sci., vol. 9, 1975. 78.) R. Raj and M.F. Ashby, Acta, Met., vol. 23, 1975, p. 653. 79.) M.U. Speight and Harris, Met. Sci. J., vol. 1, 1967, p. 83. 80.) J.W. Hancock, Mat. Sci., 1976, p. 319. 81.) A.S. Argon, Scripta Met., vol. 17, 1983, p.5. 82.) I-Wei Chen, Scripta Met., vol. 17, 1983, p. 17. 83.) V. Vitek, Acta. Met., 26, 1978, p. 1345. 84.) R. Pilkington and D. Miller, Met. Trans. A., 11A, 1980, p. 177. 85.) R. Raj and S. Baik, Metal Science, vol. 14, 1980, p. 385. 86.) J.T. Barnby, Eng. Fracture Mech., vol. 7, 1975, p. 299. 87.) W.D. Nix, D. Matlock, and R. Dimelfi, Acta. Metal., vol. 25, 1977, p. 495. 88.) H.H. Johnson and A.M. Willner, App. Mats, Reserach, vol. 4, 1965, p. 34. 89.) H.H. Johhson, Materials Research and Standards, vol. 5, 1965, p. 442. 90.) M.D. Halliday and C.J. Beevers, The Measurement of Crack Length and Shape During Fracture and Fatigue, editor C.J. Beevers, Chameleon Press Ltd., London, p. 85, 1980. 91.) J.F. 92.) S.G. Druce and G.S. Booth, ibid, p. 136. 93.) Che-Yu Li and R.P. Wei, Materials Research and Standards, 1966, p. 392. Knott, ibid, p. 113 195 94.) R.D. Ritchie and K.H. Bathe, International Journal of Fracture, vol. 15, 1979, p. 47. 95.) R.D. Ritchie, "Crack Growth Monitoring: Some Considerations on the Electrical Potential Method", University of Cambridge, January, 1972. 96.) W.F. Brown and J.E. Strawly, Plane Strain Crack Toughness Testing of High Strength Metallic Materials, ASTM STO 410, Philadelphia, 97.) 196.6. C.F. Shih, H.F. deLorenzi, Int. Journ. of Materials, 1, 1966, p. 770. 98.) "Estimating the Average Grain Size of Metals", ANSI/ASTM E112-77, 1977, p. 205. 99.) D. Broek, Elementary Engineering Fracture Mechanics, Sijthoff and Noordoff ed., 1978. 100.) F.C. Monkman and N.J. Grant, Proc. ASTM, vol. 56, 1956, p. 593. 101.) D.A. Woodford and R.H. Bricknell, Proc. Fourth Int. Conf. on Superalloys, Seven Springs, Sept. 1980, ASM. 102.) K.R. Bain, S.M. Thesis, M.I.T., February 1982. 103.) Charpigny, Doctoral Thesis, Ecole de Mine, Paris 104.) S. Floreen, Met. Trans. A., 6A, 1975, p. 1741. 105.) J.R. Rice and D.M. Tracey, J. Mech. Phys. Solids, vol. 17, 1969, p. 201. 106.) B.F. Dyson and M.S. Loveday, Creep in Structures, p. 406, ed. A.R.S. Ponter and D.R. Hayhurst, Springer-Verlag, New York, 1981. 107.) G.M. Pharr and W.D. Nix, Acta. Met., 27, p. 1615 (1979). 108.) F.A. McClintock and J.C. Bassani, Three Dimensional Constitutive Relationships and Ductile Fracture, Eds. J. Zurka and S. Nemat-Nasser, Dourdan, (1981), p. 119.