THE EFFECT OF -GLUCAN STRUCTURE ON THE RHEOLOGICAL by SPIROS JAMAS
advertisement

THE EFFECT OF
-GLUCAN STRUCTURE ON THE RHEOLOGICAL
PROPERTIES OF YEAST CELL WALLS
by
SPIROS JAMAS
S.B., Chemical Engineering
University of Manchester Institute of Science and Technology
(1981)
Submitted
to the Department
of
Nutrition and Food Science
in Partial Fulfillment
of the
Requirements of the Degree of
MASTER OF SCIENCE
at the
MASSACHUSETTS INSTITUTE OF TECHNOLOGY
May, 1983
®
Massachusetts Institute of Technology 1983
Signature of Author:
-
Department of Nutrition and Food Science, May 18, 1983
Certified
by:
J 'Thesis Supervisor
Accepted by:
Chairman, Committee on Graduate Students,
Department of Nutrition and Food Science
/
Archives
MASSACHUSETTS
INSTITUTE
OFTECHNOLOGY
JUN
2
Wj83
LIRPARIES
-1-
THE EFFECT OF -GLUCAN STRUCTURE ON THE RHEOLOGICAL
PROPERTIES OF YEAST CELL WALLS
by
SPIROS JAMAS
Submitted to the Department of Nutrition and Food Science on
May 18, 1983 in partial fulfillment of the requirements for
the degree of Master of Science in Food Science
ABSTRACT
The structure-function relationships of yeast glucans
were studied. Rheological measurements were performed on
glucan and on glucan subjected to chemical and
whole
enzymatic modification. A linear model has been developed to
hydrodynamic
Thus,
the
these
measurements.
analyse
properties of the yeast glucan samples have been determined
quantitatively. These results enable us to gain valuable
insight to the 3-dimensional (tertiary) structure of the
yeast glucan matrix.
from Saccharomyces
isolated
samples
were
Glucan
cerevisiae A364A (wild type) and its cell division cycle
mutants, 374 (cdc8) and 377 (cdcll). Whole glucan from the
two mutants exhibit higher thickening properties than the
wild type glucan. The thickening properties of the mutants'
be
enhanced by chemically extracting the
can
glucan
interchain crosslinks. Furthermore, glucans from strains 374
and 377 consist of a denser matrix than the wild type glucan
with a high degree of crosslinking. This crosslinking plays
an important structural role.
Treatment by a lytic enzyme (laminarinase) generally
causes a decrease in the thickening properties, however,
this effect
is less pronounced
in 374 and 377 glucan.
required application (eg. food
on
the
Depending
thickening) the structure-function relationships of yeast
and enzymatic
by
chemical
adjusted
be
can
glucan
modification. The linear model will provide the qualitative
standards in the process.
Thesis Supervisor: Dr. Anthony J, Sinskey.
Title: Professor of Applied Microbiology
-2-
ACKNOWLEDGMENTS
I
would
father.
like
to dedicate this thesis to my mother and
Their continuous support and encouragement has been
unsurpassed by anyone in my student years.
I
J.
wish
Sinskey
years
and
at
her
to express my appreciation to Professor Anthony
for
his guidance and patience throughout my two
M.I.T.
group
I would also like to thank Dr. Chokyun Rha
for their valuable cooperation and criticism
of this work.
Finally,
I
would
laboratories
16-222
constructive
criticism
would
like
to
like to thank all the inhabitants of
and
16-210 for their friendship, their
and
their destructive criticism.
I
thank Cheryl O'Brien for maintaining a level
of human insanity in the lab.
My
trainee
deserves
special
laboratory
technician
Marianne
Wenckheim
mention for her valuable technique of test
tube mixing.
This project was funded by the Center for Biotechnology
Research.
I acknowledge the Center's support and interest in
this work.
-3-
TABLE OF CONTENTS
Page
..............
................................
2
LIST OF TABLES .........................................
5
LIST OF FIGURES ........................................
6
ABSTRACT
1. INTRODUCTION
2. LITERATURE
.......................
SURVEY
.................
....................................
10
19
3. DEVELOPMENT AND APPLICATION OF
THEORY TO VISCOMETRY STUDIES ........................
40
4. MATERIALS AND METHODS ...............................
50
4.1 Strains .......................................
50
4.2 Growth Media ..................................
50
4.3 Yeast Fermentation ............................
51
4.4 Glucan Extraction
.............................
4.5 Infra-red Spectroscopy ......................
52
53
53
..........................
4.6 Capillary Viscometry
4.7 Laminarinase Digest ...........................
54
4.8 Acetic Acid Extraction ........................
55
4.9 Total Carbohydrate Assay
......................56
5. RESULTS AND DISCUSSION ..............................
57
6. SUMMARY AND CONCLUSIONS ............................. 126
7. SUGGESTIONS FOR FUTURE RESEARCH ..................... 128
REFERENCES..
............................. 130
-4-
LIST OF TABLES
Table No.
Title
Page
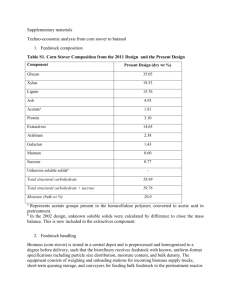
1. Chemical Composition of Yeast Cells ............. 12
2. Chemical Composition of Yeast Cell Walls ........ 12
3. Composition of Media ............................
51
4. Yeast Fermentation .............................
57
5. Glucan Extraction
............................. 77
6. Hydrodynamic Properties of Whole Yeast Glucan...
98
7. Hydrodynamic Properties of Glucan after
Acetic Acid Extraction .......................... 109
8. Extraction
of
(1-6) Glucan
.....................
111
9. Hydrodynamic Properties of Glucan after
Laminarinase Digest .............................119
10. Effect of Laminarinase digestion time on the
Hydrodynamic Properties of A364A Glucan ......... 121
-5-
LIST OF FIGURES
Figure No.
Title
Page
1. Viscosity Profiles of Three Different
Morphologies
....................................
2. Summary of Viscosity Profiles of Yeast Cells
and Cell Wall Suspensions .......................
13
14
3. Viscosity Profile of Cell Wall Suspensions of
S. cerevisiae A364A.............................. 15
4. Viscosity Profile of Cell Wall Suspensions of
S. cerevisiae JD7 Grown in SDC
..................
16
5. Viscosity Profile of Cell Wall Suspensions of
Mycelium-like S. cerevisiae JD7.................
17
6. Yield Stress-Concentration Relationships of
Mycelium-like Cell Wall Suspensions and CMC .....
18
7. Arrangement of Mannoprotein in the Yeast
Cell Wall ....................................... 21
8. Pathways in Mannan Biosynthesis .................
23
9. Arrangement of Glucan and Mannan in the
Yeast Cell Wall ................................. 26
10. General Structure of the Alkali Soluble
Glucan Fraction ................................ 28
11. General Structure of the Alkali Insoluble
Glucan Fraction ................................ 30
12. The Proposed Mechanism for Glucan Synthetase
Regulation .
.....................................36
13. Growth Curve for Saccharomyces cerevisiae A364A.
58
14. Growth Curve for Saccharomyces cerevisiae 374...
59
15. Growth Curve for Saccharomyces cerevisiae 377...
60
16. Morphology of Saccharomyces cerevisiae A364A....
61
-6-
.
Morphology of Saccharomyces cerevisiae 374
Grown at 280 C ...........................
63
17b . Morphology of Saccharomyces cerevisiae 374
after 4 hours at 37uC...................
65
17a
........
a.
Morphology of Saccharomyces cerevisiae 377
Grown at 280 C ...........................
67
18b . Morphology of Saccharomyces cerevisiae 377
after 4 hours at 37UC..................
69
18
........
19. A364A Glucan
.................................. 71
20. 374 Glucan ......................................
73
21.
75
377
Glucan...
....
..
...................
22. I.R. Spectrum of Laminarin
(2% w/w sample concentration)...................
23. I.R. Spectrum of -Gentiobiose
(2% w/w sample concentration)
79
...................
80
24. I.R. Spectrum of A364A Glucan
(2% w/w sample concentration)...................
81
25. I.R. Spectrum of 374 Glucan
(2% sample concentration) .......................
82
26. I.R. Spectrum of 377 Glucan
(2% w/w sample concentration)...................
83
27. I.R. Spectrum of A364A Glucan
(8% w/w sample concentration).................
84
28. I.R. Spectrum of 374 Glucan
(8% w/w sample concentration)
................
85
29. I.R. Spectrum of 377 Glucan
(8% w/w sample concentration)
................
86
30. I.R. Spectrum of A364A Glucan after
Acetic Acid Extraction (8% w/w) .................
87
31. I.R. Spectrum of 374 Glucan after
Acetic Acid Extraction (8% w/w).................
88
32. I.R. Spectrum of 377 Glucan after
Acetic Acid Extraction (8% w/w).................
89
-7-
33. Cooperative Hydrogen Bonding in Adjacent
Cellulose
Mo
lecules ...
........................
90
34. Inter-Molecular Hydrogen Bonding within a
O(1-3) Glucan Molecule ...........................
35. Viscosity Profiles of Yeast Glucan Comparing
Different Cell Morphologies .....................
90
94
36. Plot of the Modified Mooney Equation for
A364A
Glucan ..............
...
...................
95
37. Plot of the Modified Mooney Equation for
374 Glucan
......................................
96
38. Plot of the Modified Mooney Equation for
377 Glucan
......................................
97
39. Viscosity Profile of A364A Glucan Showing the
Effect of 3h. Extraction in Acetic Acid ......... 100
40. Viscosity Profile of 374 Glucan Showing the
Effect of 3h. Extraction in Acetic Acid ......... 101
41. Viscosity Profile of 377 Glucan Showing the
Effect of 3h. Extraction in Acetic Acid ......... 102
42. Plot of the Modified Mooney Equation for
A364A Glucan
....................................
103
43. Plot of the Modified Mooney Equation for
A364A Glucan after 3h. Extaction in Acetic Acid. 104
44. Plot of the Modified Mooney Equation for
374 Glucan ......................................
105
45. Plot of the Modified Mooney Equation for
374 Glucan after 3h. Extraction in Acetic Acid.. 106
46. Plot of the Modified Mooney Equation for
377 Glucan
after 3h. Extraction
in Acetic
Acid..
107
47. Viscosity Profile of A364A Glucan Showing the
Effect of 4h. Laminarinase Digest ............... 113
48. Viscosity Profile of 374 Glucan Showing the
Effect of 4h. Lamminarinase Digest .............. 114
49. Viscosity Profile of 377 Glucan Showing the
Effect of 4h. Laminarinase Digest ...............115
-8-
50. Plot of the Modified Mooney Equation for
A364A Glucan after 4h. Laminarinase Digest ...... 116
51. Plot of the Modified Mooney Equation for
374 Glucan after Laminarinase Digest ............ 117
52. Plot of the Modified Mooney Equation for
377 Glucan after
4h. Laminarinase
Digest ........
118
53. Viscosity Profile of A364A Glucan Showing the
Effect of Incubation Time with Laminarinase..... 122
54. Plot of the Modified Mooney Equation for
A364A Glucan - the Effect of Incubation
Time with Laminarinase .......................... 123
55. Linear Correlation of a Hydrodynamic Parameter
of A364A Glucan to Incubation Time with
Laminarinase ....................................
-9-
124
1.
INTRODUCTION
The
study
biopolymers
With
of
of genetic engineering, biopolymers can be
advent
the
modified
of
a field of increasing importance.
becoming
is
relationships
structure-function
vivo to produce molecules with altered physical
in
properties.
which
Polysaccharides
the
have
world
microbial
already
noted
been
their
for
responsible for maintaining
are
and
importance
structural
the bulk of biopolymers in
form
the integrity of bacteria and fungi.
An
of
Food
Science,
of
properties
branched
and
viscosity
in
have
yield
stress
compared
the rheological
A364A
and
its
cervisiae
JD7.
results
indicate that the
of
impart
solution
lower
The
cells
than
the
critical
JD7
a
higher
spherical A364A cells and
concentration
(see
Figs.
Furthermore, the cell wall components exhibit
3-4
viscosities
1981)
Saccharomyces
elongated
a
1,2,3,4,5,6).
performed by Liu (S.M. Thesis, Dept.
M.I.T.,
mutant
morphological
also
study
initial
of
times
higher
than the whole cells and the
the cell wall components was comparable to
other hydrocolloidal polysaccharides.
These
cell
was
wall components consisted mainly of glucan,
also observed that the morphological mutant JD7
and
it
had
a higher glucan content than the yeast-like A364A (Table
1,2).
This
study
is
therefore
directed
-10-
to investigate the
phase
in
of yeast glucan as a first
relationships
structure-function
the development of a scheme to produce glucan with
desirable physical properties through directed biosynthesis.
For
their
the
this
study,
division cycle were chosen in order to elucidate
cell
changes
in
understood
have
is
induced.
that
occur
when
a
This is important since
biosynthesis can be achieved only once
directed
genetically
structure
glucan
change
morphological
we
morphological mutants with a block in
the
machinery and information that the
cell requires to synthesize new glucan.
Various
processing procedures can be used to remove the
mannan and glycogen components of yeast cell walls and
outer
obtain
either
glucan.
For
initial phases of this study, the alkali-
the
insoluble
glucan
generally
contains
branched
B(1-3)
(3) and a minor
Studies
on
or insoluble (3,4) fractions of
(2)
soluble
fraction
a
has
major
glucan
component
been
component
which
which
rheological
used.
3%
is a
-11-
(about
85%)
of
a
8(1-6) interchain links
(1-6) glucan.
properties
obtained for these glucan samples.
This fraction
and
structure was
Table
1
Chemical Composition of Yeast Cells
(S)
JD7-YPD*
JD7-SDC
Trehalose
4.5.+0.4
3. 2+0.8
Alkali-soluble 5.4+0.7
Glycogen
Total
Glycogen
Mannan
Glucan
Total Carbo-
hydrate
6.4+1.1
A364A-YPD
(1)
A364A-SDC
10.1+0.6
5.7+0.2
5
9
17.3
18.2
13.8
20.3
12.8+0.1
11.6+0.4
13.8+o.3
16.30.4
12.1±0.5
12.1
14.6+0.9
50
54
Protein
11.5
43
49
41
45
52
43
* Strain-Medium
Table
2
Chemical Composition of
Composition(%)
Trehalose
Alkali-soluble
Glycogen
Acid-soluble
JD7-Y PD'
Yeast Cell Wall(l)
JD7-SDC
A364A-YPD
0.7
1.4
1.6
39.8
31.2
37.9
40.5
35.6
ND
35.9
38.3
ND
38.3
75
80
Glycogen
Total Glycogen
Mannan
Glucan
Total carbohydrate
Protein
ND
39.5
78
ND
ND - not detected
*strain-medium
-12-
ND
ND
20
(n
0.
15
I
t0
0q)
10
z
03
a.
a.
5
0
0
10
20
30
40
DRY WEIGHT
Figure 1: Viscosity Profiles of Three
Morphologies
-13-
50
(gm
60
/I
Different
70
-o- J D 7-Y PD-WALL
-
18
-7D n-ft. I I
16
r0
14
%.0
12
>-
.--
U)
0
0
>
cr 6
04
0..
0
10
20
30
40
50
60
70
CONCENTRATION(G/L)
Figure 2: Summary of Viscosity Profiles of Yeast
Cell Walls Suspensions
-14-
Cells
and
-0-
APPARENT VISCOSITY
V6 DRY WEIGHT OF
RESInUE
-0-
60
APPARENT VISCOSITY
VS DRY WT. BEFORE
(4
HOT ALKALINE
TREATMENT
50
--.a- - VISCOSITY PROFILE
OF WHOLE CELL
SUSPENSION
)
40
I
rI
(.n
0I- 30
m
z
/II
,/
G:
0-
20
,/
I
//,
10
0
0
20
6~
40
60
DRY WEIGHT
Figure 3:Viscosity
_
80
100
(ginmI).
Profile of Cell Wall Suspensions of
Saccharomyces cerevisiae
-15-
A364A
-0- APPARENT V ICOSITY
VS DRY WEIGHT OF
30
.
RESIDUE
-I-APPARENT
VI SCOSITY
VS DRY WT. BEFORE
HOT ALKALINE
TREATMENT
25
-'-VISCOSITY
PROFILE
OFWHOLE CELL
0o
I--
20
SUSPENSION
/
Is
z
L
Ct:
20
40
60
80
100 120 140 160 180 200
DRY WEIGHT
Figure
( m/
4: Viscosity Profile of Cell Wall Suspensions of
Saccharomyces cerevisaie JD7 Grown in SDC
-16-
20 -
I
I
I
-
I
I
I
18
16
APPARENT VISCOSITY
VS DRY WEIGHT
OF
RESIDUE
-*-APPARENT VISCOSITY
I
VS DRY WT. BEFORE
I
HOT ALKALINE
I
TREATMENT
16
14
-6-V ICOSITY PROFILE
OF WHOLE CELL
SUSPENSION
12
I-
0
10
C-
8
z
I
*
/''/~~
U
6
or
<
A
Ii
I
4
/
2
oL
10O
DRY
m
20
30
WEIGHT
I50
40
50
I
I60
60
-
70
(gm/I )
Figure 5:Viscosity Profile of Cell Wall SusDenslons of
Mycelium-like Saccharomyces cerevisiae JD7
v-
-17-
14
o JD7-YPD-WALL
CMC
o JD7-YPD-CELL
E
0
>4
V)
U)
Cx
(n
0I
w
(
CONCENTRATION
/. ) -
*
Relationships of
Figure 6: Yield Stress-ConcentrationSuspensions
and CMC
Mycelium-like Cell Wall
-18-
2. LITERATURE SURVEY
2.1 The Yeast Cell Wall
cells are encapsulated in a rigid wall consisting
Yeast
entirely
almost
chemical
but
of
structure
unique
each.
to
the
structure
functions
able
this careful tuning of
with
Hence,
is
cell
The
components is relatively simple
the
of
components.
polysaccharide
three
assign
to
specific physical
to
properties)
(structure-function
these
polysaccharides.
The
components
three
mannose
glucose,
independent
short
peptide
for
evidence
in
moiety.
cell
wall.
Only
as
mannan
However, recent studies(6,7) provide
or
between
linkage
possible
directly
glucan-either
the
occur
of
known(5) to be covalently linked to a
is
a
and
N-acetylglucosamine
and
structures
(mannose polymer)
homopolysaccharides
are
chitin
and
short peptide sequences
through
containing mainly lysine and citrulline.
Glucan
(the
of
rigidity
morphology
of
glucose
yeast
cell
A
yeasts.
polymer)
walls
provides the structural
and
hence
maintains
the
detailed review on the structure
and biosynthesis of yeast glucan will be included below.
The
cells
by
alkali.
mannoprotein
heating
This
in
component can be extracted from whole
citrate
treatment
buffer (pH 7.0) or in dilute
solubilizes
-19-
.^.
the
mannoprotein and
depending
degrees
have
on
the
heterogenous
assigned
two
the
component.
ranging
of
groups
cell
on
wall.
the
location
cerevisiae
been
have
a
chain
cell
outer
wall
distinguished(8).
chain of 15-17
first
residues
mannose
in
unit
to
the
is
of
the
be
structural
The second
expansion enzymes (exoenzymes)
core
the
short
In Saccharomyces
and an inner mannan core
The inner mannan core consists
(1-6) linked mannose units.
varying
inner
asparagine
acetylglucosamine
consists
can
Mannan provides a matrix for
highly branched through
is
The
the basis of their functionaal
The
mannan
oligomannose
bonds
obtained
surface(8) acting as a cement.
of
an
x106 .
from 25,000 to
as invertase and acid phosphatase(5).
such
with various
The mannan fragments
mannoprotein
the enzyme bound component.
the
fragments
is interspersed throughout the cell wall and
It
covers
is
of
weights
fractions
to
in
also
procedure
of disruption are obtained(5).
molecular
role
exact
(1-2) and
in
core
length.
This main
(1-3) bonds to
The
terminal
is linked through
(1-4)
unit(5) of the peptide through an
bridge.
In
addition
the
inner
core
oligomannoside chains linked directly to
the serine and threonine of the proteins(5).
The
core mannan chain consists of a much longer
outer
a(1-6) linked
a(1-2) and
backbone
a(1-3)
with heterogenous branching through
linkages.
Phosphodiester bonds are also
found linking the side-chains(8).
-20-
I
4GLNAc
GLcNAc
[M61M
AS N
M
I
I
I
I
I
INNER
OUTER CORE
CORE
'R
1 2
IR)
Mi
Ml
M _1
Figure 7.
The
pathways
chitin
are
biosynthesis.
Also,
nature
in
_2
the
of mannan follows rather more complex
and
the
yeast
complicated
the
In
glucan.
to
known(8)
intermediates
component
2M 1
Arrangement of Mannoprotein
in the Yeast Cell Wall
biosynthesis
than
2M1 2
fact
cell
be
that
wall
fact, lipid-linked
involved
mannan
to
elucidation
in
is
mannan
the
only
be of glycoprotein
of
these
pathways
further.
The
isolation and characterization of mannan mutants of
-21-
Saccharomyces
antibiotic
yeast
cerevisiae
along
tunicamyacin(9)
with
inhibits
the
finding
that
mannan biosynthesis in
protoplasts played an important role in clarifying the
structure and biosynthetic pathways involved.
Initial
formation
from
of
lysed
(8,9)
were
residues
enzyme
the
protoplasts
shown
a
no
The
catalyse
the
carlsrbergensis
transfer
of
mannose
precursor to form mannan.
The
is
required.
acetolysis
The presence of Mn
fragments
of
the
is
synthesized
revealed a structure resembling that found in vivo
on
biosynthesis.
the
Mn
2+
effects of cations(9) provided evidence for
of
It
or
intermediate
was
produced(9).
In
residues
Saccharomyces
nucleotide
primer
involvement
either
to
of
Enzyme preperations
preparation is specific for the precursor GDP-mannose
essential.
Studies
the carbohydrate portion.
from
however,
mannan
studies on mannan biosynthesis investigated the
lipid
was
Mg
2+
observed
as
formed
the
bound
the
intermediates
that
in
the
in
mannan
presence of
only cation a mannosyl-lipid
from GDP-mannose but no mannan was
presence
of
both
cations
mannosyl
were transfered from the nucleotide-sugar precursor
to the lipid bound intermediate and then to mannan.
-22-
Mn 2 +
GDP-mannose
GDP-mannose
GDP-mannose
8.
Figure
The
mannan
-
-
g
> lipid(mannan)-
Mn 2+
2
Mg
lipid(mannan)
intermediate
lipid
of
and
In
addition
mannose
Recently the
(DMP-mannose).
mannan
of
the
to
occurs
However,
in
protein,
the
intermediate.
units
the
linked
sequences
linked
lipid
intermediate
to
only the
protein
is
The subsequent
directly
from
the
the complete role of
synthesis
of
the
high
mannan of the inner and outer core has not
understood.
The current working hypothesis is that an
oligosaccharide
core
be
weight
molecular
been
to
identified(9)
was
residues
precursor.
nucleotide-sugar
lipid
the
from
of
short
directly
residue
incorporated
the
the
threonine
mannosyl
mannan
the formation of mannoproteins was
in
DMP-mannose
clarified(9).
serine
-
Pathways in mannan biosynthesis.
dolichyl-monophosphate-mannose
role
>mannan
is
first
moiety
which
represents
N-glucosidically
linked
part of the inner
to
the
protein's
in a lipid intermediate involving step.
asparagine
residue
Once
step is completed further mannosylation occurs by
this
direct
transfer
from
GDP-mannose.
-23-
The
formation
of the
specific
( a(1-2), a(1-3), a(1-6) ) in the outer core
bonds
branches
and
controlled
is
specific
by
mannosyl
transferases(9).
Most
is
walls
the chitin found in yeast (Saccharomyces) cell
of
located
it
walls
fungal
the
bud
Although
septum.
primary
in
only
approximately 1% of the
constitutes
It is a linear polymer of
a(1-4) linked N-acetylglucosamine residues.
chitin
constitutes the
a major component of
is
chitin
cell wall polysaccharides.
yeast
and
scars
The structure of
In yeasts the a
been chracterized in detail(8).
has
form
crystalline
each
to
antiparallel
intramolecular
form chitin chains run
this
In
occurs.
Hence, chitin is
bonding to occur.
hydrogen
extensive
allowing
thus
other
resistant to chemical extraction and insoluble in water.
As
mentioned
is
yeast
budding,
between
above
located
chitin
90%
the chitin found in
of
in the bud scar region(10).
appears
and
mother
the
over
as
ring
a
daughter
During early
surrounding
cells.
the neck
Before each cell
division
chitin grows over the annulus between the two cells
forming
a
et
Molano
al
anthrone
an
material
could
is
disc-shaped
distributed
by
(10) on purified septa showed the presence of
reacting
constituted
possibly
studies
However,
cross-wall.
agent
approximately
be glucan.
as
in
the
septal
15%
of
disc.
the
This
septa and
The remaining fraction of chitin
lozenge-shaped particles over the entire
-24-
cell wall.
Chitin
budding
region
with
block
a
is
synthesis
of
a
In fact, studies made on yeast
cell.
in the cdc24 gene(8) revealed that chitin was
randomly
synthesized
necessarily localised at the
not
over
entire surface of unbudding
the
cells.
The
biosynthesis
which
synthetase
enzyme
of
an
occurs
unknown
inactive
activating
in the native state(9).
The
to
The existing
destruction of an inhibitor which is
proteolytic
bound
factor or trypsin.
is that activation of chitin synthetase
mechanism
by
tightly
is
be activated by mild proteolysis in the presence
can
tentative
chitin involves the enzyme chitin
of
the
enzyme.
The
pathway
for
chitin
synthesis
resembles
the mannan pathway in that a nucleotide
precursor
transfers
the
sugar
residues
to
an endogenous
In yeasts Mg2 + and Mn 2 + cations are essential for
receptor.
this pathway(8).
2n UDP-N-a-D-GlcNac
endogenous acceptor is chitin itself however, it is
The
not
known
chitin
an acceptor
if
synthesis.
stimulated
their
2n UDP + (GlcNac a(l-4)GlcNac)
------
exact
the
for the initiation
of
Oligomers of N-acetylglucosamine clearly
enzyme
role
is required
as
preparation
primers
clear.
-25-
of
from
fungi(9) however,
chitin
synthesis is not
CYTOPLASM
Figure 9.
Arrangement of Glucan and Mannan in the
Yeast Cell Wall
2.2. The Structure of Yeast Glucan
Glucan,
walls
or
cell
and
and
is
(1-6)
walls
a
the
a
major
structural
polymer
in
yeast cell
polymer of glucose linked through either
glucosidic linkages.
was
soluble
enzymatic
understood
(1-3)
The role of glucan in yeast
after removal of mannan, chitin
glucan component from cell walls by chemical
treatment
had
morphology(9).
-26-
no
effect
on
the
cell
As
shown
(11)
B(1-3)
the
cell
branches
in Figure 3., and determined by Kopecka et al
linked
wall
glucan
adjacent
forms a fine fibrillar
to
the
cytoplasm.
layer
The
in
B(1-6)
and other polysaccharides are located on this inner
fibrillar
layer.
considered
layers.
However,
the yeast cell wall must not be
as a structure containing seperate polysaccharide
The
glucan provides a gross structural matrix onto
which the other polysaccharide components are located.
Treatment
a(1-3)
and
of
8(1-6)
digestion
began
proceeded
in
that
of
yeast
glucanase
at
all
Molano
(Saccharomyces)
the
outer
directions.
et
al(9)
that
cell
enzymes(ll)
bud
scar
walls with
revealed
that
regions and then
This observation agrees with
glucan is present in yeast
septa.
Glucans
alkali.
are
grouped on the basis of their solublity in
Three
fractions
identified(12,13,14).
component,
(1-3)
mainly
an
linkages
of
These
insoluble
and
of
glucan
are
an
component
have
alkali
consisting
been
soluble
mainly
of
a highly branched component, consisting
(1-6) linkages, which is tightly associated with
the former.
-27-
G1
Figure 10.
20%
dilute
of
this
the
glucan
a
consisted
of
80-85%
of
(1-3)
linkages, 8-12%
This glucan
insoluble
Thus
, thus, it is very similar to
250,000
Furthermore, both glucans
fraction.
3-4% branching through
the
only
characteristics
of
solubility
the
soluble fraction contains 8-12%
Consequently,
determining
(1-3) and
(1-6)
structural difference explaining
the
results
little
When extracted with
and 3-4% heterogenous branches.
approximately
linkages.
in
of
Structural analyses showed that
wall(2).
cell
weight
alkali
show
is
component
degree of polymerization of approximately 1,500 and a
molecular
the
soluble
sodium hydroxide this component comprises about
6(1-6) linkages
has
alkali
importance to the cell wall.
structural
cold
General Structure of the Alkali Soluble
Glucan Fraction
minor
The
G-
3
1
the two glucans is that
(1-6) linked residues.
the arrangement of these linkages is important
the
physical
properties
of
glucans.
The
of Smith-degradation(2) on this glucan suggested the
-28-
general structure shown in Figure 10.
The
the
major insoluble glucan fraction accounts for 85% of
total
linked
yeast
glucan.
of
backbone
8(1-6) interchain
8(1-3) linked
This glucan consists of a
high
molecular
linkages(3).
backbone
after
240,000.
However
comparison
Bacon
al
and
et
(3)
S(1-6) linkages
have
of
evaluation
the
extraction
of
in acetic acid is
obtained by
results
the
that the
et al (3) indicate
insignificant
effect on the final
weight,
molecular
containing 3%
weight
The molecular weight of the
Manners
an
(1-3)
that
showing
these
linkages are present in minor quantities.
ability
The
of
envelope
insoluble
laminarin
example,
of
polymerization
in
soluble
containing
10(3)
a
and
glucose
residues
by
applying
Manners
al
(3)
an
backbone
this
its
has
degree
a
For
of
low degree of branching is
are
laminarin
insoluble
knowledge
to
molecules
in water.
yeast
glucan
assumed that the high molecular weight
were
essentially linear and by the
8(1-3) glucan
molecules
appropriate
inter-chain
and
crosslinking
an
matrix
information
tuning
average
linear
But,
Therefore,
et
B(1-3)
the
has
which
water.
20
fine
on
the solubility of the molecule.
on
effect
significant
of
cell with an
the
provide
depends
clearly
length
The
structure.
to
glucan
insoluble
available
intra-molecular
is
formed.
8(1-6)
With
the
to date on glucan structure it can be
-29-
postulated
that
dimensional
the
insoluble
(1-3) component forms a two
tree-type
structure
random
proposed
for
existing
wholly
amylopectin.
or
Evidence
partially
been found.
was
found to be about 1500 (3).
each
chains.
yeast
glucan
The degree of polymerization of this glucan
glucan
The
for
that
in a triple-helical form has
not
that
resembling
molecule
Hence, it can be calculated
contains
approximately 50 side
glucan structure proposed by Manners et al (3)
is illustrated below.
---
1
3 1
3 1
6-----
G
-G
1G 3G
!
1
3G1
6)
g-1
- --
Figure 11.
The
degree
of
molecule
smaller
This
has
component
3G
high
(endo-
in
proportion
(19%) of
can
component
isolated
from
is a highly branched glucan with a
polymerization
proportion
enzymolysis
sample
insoluble
cerevisiae
a
36
e
31e
General Structure of the Alkali Insoluble
Glucan Fraction
minor
Saccharomyces
31
be
-(1-3)
the
of
range
130-140.
This
(1-6) linkages with a
(1-3) glucosidic linkages(4).
isolated
glucanase)
either
of
or by extraction in hot acetic acid.
-30-
a
by
selective
whole
glucan
Both procedures
yield
very
this
glucan
glucanase
glucan
similar
fraction
to
degradation
the
Therefore,
by
endo-
-(1-3)
(1-3) glucosidic linkages are
shows that adjacent
absent.
The resistance of
structures.
(1-3) linkages present act as
19%
inter-chain or intra-molecular linkages(4).
Extraction
that
due
it
is
soluble
the
to
in aqueous solution.
This is possibly
degree of branching in the molecule which
high
the
prevents
of this fraction in hot acetic acid revealed
of adjacent molecules to
allignment
suitable
form an insoluble matrix.
In
the
B-glucan
above
on
The
important
role
shows
evidence
a
after
and
made
structure
on
the structure of insoluble
to
explain
these
covalent
first
solubility
the
-glucans definitely plays an
of
properties.
linkage
proposed
the
However,
recent
-glucans is affected
that the solubility of
possible
Wessels(6)
of
basis of inter-chain and intra-molecular
the
branching.
by
were
attempts
properties
review
with chitin.
existance
of
Sietsma and
this linkage
studying the effects of selective enzymatic hydrolysis
chemical
fungus
in
a
the
purified
cell
preparations of the
wall
Degradation
of
the chitin
alkali insoluble fraction of the cell walls
significant
B-glucan. Chitin
on
commune.
Schizophyllum
present
caused
treatment
was
chitinase
increase
removed
in
the
either
by
solubility of the
digestion with a
or by deacetylation with alkali followed
-31-
by
depolymerization
two
-glucan
single
in nitrous acid.
Both methods released
components, an alkali insoluble component with
glucose
unit
branches,
and a highly branched water
soluble component.
Treatment
glucanase
of
the
dissolved
residue
could
glucan/chitin
90%
be
the
of
hydrolysed
with
(1-3)
-glucan and the remaining
with
chitinase
to
yield
N-acetylglucosamine
and
citrulline
The glucan/chitin complex was found to
contain
complex.
50%
lysine,
an
complex
N-acetylglucosamine-lysine-
20% citrulline and approximately 12.5%
glutamic acid(6).
Recently,
evidence
Sietsma
for
Saccharomyces
the
and
Wessels(7)
covalent
cerevisiae.
insoluble
after
could
be
solubilized
Since
nitrous
obtained
glucan-chitin
The
glucan
similar
linkage
fraction
in
remaining
extraction in 40% sodium hydroxide at 100°C
acid
after
treatment
with nitrous acid.
depolymerises the acetylated chitin the
possibility of a glucan/chitin linkage is supported.
2.3 Glucan Biosynthesis
The
glucans
of three distinct, and chemically related
presence
in
the
understanding
of
cell
walls
their
of
yeast hinders the complete
respective
functions
and modes of
biosynthesis.
In
contrast to the structural importance of
this
in
yeast
compound
cell
-32-
walls,
little
information
concerning
glucan
incorporation
is
biosynthesis
available.
using whole cells were used to obtain
studies
on the overall biosynthetic pathway.
information
Initially,
Sentandreu
et
al (13) used Saccharomyces cerevisiae cells permeabilized
by
treatment with toluene and ethanol.
1 4 C]glucose
UDP-[U-
labelled
with
cells
,
synthesis of a
No labelled lipids were
(1-3)glucan was observed.
labelled
On incubation of the
detected.
The
a
step in understanding glucan biosynthesis was
next
study
by
performed
glucan
investigated
transcriptional
RNA
ts(-136).
non-permissive
(37
temperature
the
a
mRNA's
of
By
rate.
observed
the
5
blocking
by incubation at the
Cycloheximide was used
After shifting log phase cells
This
decrease
in
mannan
observation indicated that
have a relatively slow decay
peptides
protein
a
glucan synthesis continued
however
hours.
wall
0 C).
temperature
non-permissive
further
blocked
is
synthesis
to
for
workers
level by employing Saccharomyces cerevisiae
block protein synthesis.
was
The
(14).
the translational and
at
biosynthesis
to
formation
al
et
Elorza
synthesis
mannan
formation
was
blocked
after
glucan
formation
was
unaffected.
with cycloheximide
a few minutes whereas
This result suggests a
high degree of stability for the glucan synthetases.
Lopez-Romero
free
glucan
and Ruiz-Herera(15) demonstrated that cell
extracts from Saccharomyces cerevisiae could synthesize
containing
both
(1-3)
-33-
and
B(1-6)
linkages.
The
of
incorporation
glycosyl
residues from UDP-(U-1 4 C)glucose
Only trace amounts
GDP-(U-14C)glucose was investigated.
and
of
glucose
glucan
were
both
containing
synthesized
from
the
and
B(1-3)
UDP-sugar
the
fractions
cell-wall
from
incorporated
latter, however a
0(1-6)
linkages
was
Membrane and
precursor.
used, however activity was higher
were
in the cell wall fraction.
An
that
a
interesting
from this study was
arose
2.5%
wall
resembles
vivo
was formed using the cell
synthesized
from
the
latter
alkali insoluble glucan fraction found
However,
the
in
vitro
synthesized glucan is
in alkali possibly due to a lower molecular weight.
soluble
these
From
linkages
glucan
major
the
.
(1-6)
The
fraction.
(1-6) linkages whereas a glucan
0.6%
containing
containing
results
walls.
it
can
be
assumed
transferase
is
mostly
Biosynthesis
of
B-glucan
B(1-6)glucosyl
cell
that
a mixed membrane preparation catalysed the formation of
glucan
in
point
that
associated
was
the
with the
specific
for
UDP-glucose.
The
glucan
a
regulation
(1-3)-glucosidic bond formation in
of
was studied in detail by Shematek and Cabib(16) using
membrane
UDP-glucose
from
preparation
was
used
as
the
Saccharomyces
cerevisiae
.
sugar donor and the reaction
addition
of glycerol, bovine serum albumin and ATP or GTP to
activate
the membrane preparation.
the
workers
obtained
Under optimal conditions
20-50% incorporation of the substrate
-34-
in
20
minutes.
alkali
of
soluble, and was characterized to be a
degree
of
requirement
polymerization 60 to 80.
of
intermediate
Equivalent
unit
The glucan produced was water insoluble and
a
was
primer
was
involved
amounts
transfered
of
found
(1-3) glucan
No evidence for the
and
no
lipid linked
in the synthesis of the glucan.
UDP
hence,
were liberated for each glucose
the
following
equation
can
be
written:
>UDP + ((1- 3 )glucose)n+l
UDP-glucose+ ((1-3)glucose)n
In
contrast
transfer
of
to
the
mannan
sugar
and
chitin
biosynthesis, the
from the nucleotide-sugar precursor
does not require the presence of divalent cations.
During
the
material
is
example,
during
the
yeast
the
synthesis
of wall
at
whereas new cell wall material is actively
the
growing bud.
activation/inactivation
implied.
(1-3)
For
budding cell wall synthesis is quiescent in
cell
synthesized
bound
cycle
not evenly distributed over the cell wall.
mother
therefore
cell
scheme
The need for a reversible
for
glucan
synthesis
is
A regulatory mechanism for the membrane
synthetase is essential to localize the enzyme
activity where required.
Shematek
synthetase
inhibited
is
by
and Cabib(17) observed that the membrane bound
stimulated
the
by ATP or GTP.
presence
of
-35-
ATP activation is
EDTA whereas GTP activation
requires
EDTA.
transfer
the
enzyme
not
Several
GTP
analogs
y-phosphate also
which
acted
are unable to
as stimulants of the
preparation indicating that the y-phosphate of GTP is
involved
in
enzyme
activation.
The
simple
working
hypothesis derived from these observations is:
GTP
stimulation
while
ATP
into
of
occurs by binding to a site on the membrane
converts a loosely bound phosphorylated substrate
a product tightly bound to the enzyme.
Mg
product
an
endogenous
hydrolytic
into
ATP
back
the
In the presence
enzyme converts the ATP
substrate
hence
allowing
reversible activation/inactivation of the enzyme.
Now,
the
to
simplify this mechanism it can be assumed that
phosphorylated
substrate
and
the
product are GDP (or
GMP) and GTP, respectively.
~ .
+Px
J1tE
--
2+
~Ma
,
endogenous phosphatase
1
[E]
~
N[
>[E] -P,X + ATP I
ADP + [E] -P.XP-
- -1
L
2
-P1x
GTP
I1
[E]i-GTP
(
[E]
i + X +P
[E]i= inactive enzyme
[E] = active enzyme
Figure 12. The Proposed Mechanism for Glucan
Synthetase Regulation(17).
-36-
2.4 Cell Division Cycle Mutants
or
mitosis
by
size
bud
a
reveals
division cycle.
cell
through a series of
progresses
which include DNA replication at the beginning of the
nuclear
the
quarters
the mother
mechanisms
Thus,
cell, and cell separation
that
Now,
since the size of the bud indicates
cell
in
are
us
temperature
defective
during
the
must be
size
the position
of the
cell division cycle, it has been made possible
the
screen
cell
control
to those that control cell wall synthesis.
related
closely
of
size
the bud is about three
when
division
bud and the mother cell are approximately the same
the
size.
to
The size of the
of the cell in the cell division
cycle
mitotic
cycle,
when
its surface and this bud grows in
on
position
the
The
events
cell
bud
the
throughout
cycle.
A single cell begins the mitotic cycle
budding.
initiating
is a yeast that reproduces by
cerevisiae
Saccharomyces
genes
in
cell
sensitive (ts) mutants of yeast that
that are induced at specific times
This technique allows
division cycle(18).
to determine the time in the cell division cycle that the
product
particular
gene
relevantly
the
involved.
About
completes
biosynthetic
step
its
in
function
cell
and more
wall synthesis
150 such mutants wre isolated that defined
32 genes in the cell division cycle(19).
In
dependent
the
cerevisiae
Saccharomyces
sequence
of
events
have
-37-
been
cell
cycle
two
identified(20).
These
are
DNA
sequence,
replication
and
cytokinesis
bud
in
and
nuclear
emergence,
nuclear
the second sequence.
dependent
sequence,
completion
of
each
division
one
migration
and
In other words, in the
can
event
in
the preceeding event.
proceed
only
upon
The cell cycle is made
up of a large number of these ordered events(20).
must
It
be
controlling
for
illustrated
by
mutants
several
the
that
cell
implied
above
cycle
most
are
not
This
progress.
of
can
the
rate
be
various temperature sensitive, cell division
Saccharomyces
cycles
synthesis
of
cerevisiae
before total arrest.
the
gene
product
is
which
undergo
In these mutants
the
temperature
event hence, material synthesized before the shift
dependent
in
the
of
cell
however,
functions
gene-controlled
cycle
noted
temperature
explains
the
remains
observation
functional(20).
This
supposition
of continuous cell wall synthesis
in these ts mutants most appropriately.
In
cycle
words,
mass
yeasts,
the major rate controlling step in the cell
the actual rate of increase of cell mass. In other
is
the
is
cell division cycle is initiated once a critical
obtained.
Saccharomyces
cerevisiae
cells
for
example, form buds only when a certain size is reached.
Blocks
yeasts
of
the
at
affects
various stages in the cell division cycle of
the
structural
employment
cell morphology and hence the synthesis
polymers
in the cell walls.
Hence, the
of ts cell division cycle mutants may prove to be
-38-
a
useful
tool
in elucidating
the structural
properties of these polymers.
-39-
and functional
3. DEVELOPMENT AND APPLICATION OF THEORY
TO GLUCAN VISCOMETRY STUDIES
3.1 Rheology of Suspensions
The
cell
extraction process used in this work yields
glucan
composed of a rigid matrix containing mainly
ghosts
-glucans.
These
original
cell
glucan
In
the
glucans,
and
morphology
size range (2.0-5.0
have
maintained
the
a very narrow discrete
have
m) as determined by coulter counter.
studies
dispersed
particles
on
rheological
system
properties
of
these
models have been used to analyze
the results.
The
experimental
capillary
viscometer
distilled,
deionized
modelled
larger
than
this
element
water
75).
were
Glucan
used.
dispersions
in
This system can be
solvent
of
the
nature
molecules
but
smaller
experimental apparatus.
the
microscopic
viscosity
than
the
In a dispersion
(consider
an
of solvent not occupied by glucan particles) remains
unchanged,
condition
phase
(size
used was the Cannon-Fenske
as one in which the solid glucan particles are much
dimensions
of
apparatus
is
Therefore,
viscometer
i.e.,
is
based
not
the
solvent viscosity is governing.
the
on
the
soluble
results
to
assumption
any
degree
obtained
that the suspended
in
from
the
solvent.
the
capillary
are a measure of the macroscopic viscosity.
-40-
This
This
viscosity
is
the
result of distorted flow lines due to the
large suspended particle.
The
used
on
three
to
dimensional
calculate
the
equation
of
flow(21) has been
the effect of a large suspended particle
flow of a fluid.
Using Stoke's equation to simplify
the equation of flow and the following boundary conditions:
1.
Flow velocity u, at all points far removed from the
suspended particles have an assigned value
determined by the rate of flow.
2.
Flow velocity u, at the surface of the particle is
equal to the particle velocity in magnitude and
direction.
Einstein
macroscopic
(22)
obtained
viscosity
the following equation for the
of
a
dilute
suspension of rigid
spheres:
n = no( 1 + 2.50 )
where, n = macroscopic viscosity of the
suspension
no= viscosity of the solvent
0 = volume fraction of suspended
paricles
This
treatment
particles.
with
respect
In
this
to
was
case
extended
to
apply
to asymmetric
the orientation of the particles
the flow lines is an important parameter.
-41-
At
are
low
velocity gradients one can assume that the particles
randomly
oriented.
For
this
case the Einstein-Simha
equation describes the macroscopic viscosity,
n = n(
1 + V
)
where, V = shape factor
V = 2.5 for spheres
V > 2.5 for ellipsoids
This
dilute
equation,
systems
Mooney
however,
in which
(23)
can
only
be
used
for very
0 < 0.02.
extended Einstein's equation to apply to a
suspension of finite concentration ( 0
n
nr
= exp[
o
< 0.2)
V0
1- 0/0
where, nr = relative viscosity
0m
The
= maximum packing fraction
term, 0 , is experimentally difficult to determine,
hence we can substitute
0 = cv into Mooney's equation,
where,
c = concentration
v = hydrodynamic volume
-42-
Hence, Mooney's equation becomes:
n
Vcv
= expt
]
1- cv/0m
In
order
values
for
following
employ this equation to obtain meaningful
to
the
parameters
rheological
algebraic
of
glucans,
the
manipulations must be made to the above
equation.
Take log:
Vcv
ln(nr)
1- cv/
Now,
for
constant
can
be
in
a
the
expressed
constants,
particular
m
glucan
sample assume that v =
operating range of c.
solely
in
terms
Hence, the equation
of
c
kl, k 2.
klc
ln(n ) =
r
1 - k2c
where, k
k2
= Vv
_v
9m
-43-
by defining two
Expanding the above equation yields:
ln(nr) - k2cln(nr) = klc
ln(nr ) = k 1 c + k2 cln(nr)
ln(nr) = [ kl
+ k21ln(nr) ]c
ln(nr)
ln(n
k
k1 ++ kk21n
(nr )
Inverting:
1
kl + k2 1n(nr)
C
ln(n )
r
Therefore:
1
c
1
k
=
1
+
ln(nr)
Hence,
linear
Mooney's
equation
relating
model
has
reciprocal
k
2
been
used
to obtain a
concentration
to
the
for
the
reciprocal loge(relative viscosity).
The
parameters
the
correlations
kl,
samples
dimension/width
k 2.
mean
was
on
studies
theological
resulting
equation
above
the
enabled
used
as
glucan
the
a
model
dispersions.
determination
of
The
the
Upon microscopic observation of
values
dimension)
of
of
-44-
the
each
aspect
ratio
(length
sample were determined
hence using the equation (24):
- 1 )5
V = 0.4075(
+ 2.5
L
where, D = aspect ratio of
the suspended particle
of the shape factor, V
values
exact
Hence,
the
values
1/c vs
/ln(nr).
v,
were calculated.
0m were determined from the plots of
3.2 Capillary Viscometry
As
discussed
above, the time taken for a suspension of
to flow through a capillary is proportional to the
particles
macroscopic viscosity of this suspension.
Ideal
the
theory
conditions
for
are
capillary
considered in the development of
viscometers(25).
Therefore, the
following assumptions must be made:
1.
Steady flow
2.
No radial or tangential components of fluid
velocity, i.e., laminar flow
3.
Axial velocity is only a function of the radial
coordinate
4.
Boundary layer at capillary walls, i.e., fluid
velocity is zero at wall
5.
End effects are negligible
6.
Incompressible fluid
-45-
7.
No external forces
8.
Isothermal conditions
9.
Viscosity is not a function of the changing
pressure down the capillary
To
derive
viscosity
the
relationship
between
efflux
time and
for the above ideal conditions, a force balance is
made on an element of the capillary.
Viscous restraining
"forces,
redial
(2nrL)s
coordinate
I-~~
Forces on
nd
I .I
Forces on end
of column of flul
i2A
_
ITI GAp
-Direction
of flow
Direction of flow
nr2Ap = (2nrL)s
where,
s = shearing stress
L = capillary length
r = radial
coordinate
p = pressure difference over the
capillary
-46-
For a Newtonian fluid,
n = s/(-du/dr)
s = rp/L
Now,
-du/dr = Apr/2nL
thus,
Integrating:
u(r) = (ApR2/4nL)(1 -(r/R)2)
The
volumetric flow rate, Q, can be expressed as the surface
integral
of
the
the
over
velocity
cross-section
of the
capillary:
QIRi
2 nru (r)dr
Hence,
substituting
for
u(r)
into
integrating, with respect to r:
Q =
R4Ap/8nL
-47-
the above equation and
Now
let a total volume, VT flow through the capillary in
time t.
VT
VT
-
=
R
4
p/8nL
t
For a fluid flowing under gravity,
where,
p = hpg.
h = hydrostatic head
p = fluid density
g = acceleration due to gravity
T
hence,
t
hgt
7R4
8nL
Rearranging the equation gives,
_ =_R
p
4
8 LVT
where, the term
Since
the term
Since
4the
term
7TRh
hgt
P
= kinematic viscosity
is constant for a given viscometer
8 LVT
we can write:
n
-- = Ct
Hence,
by
determining the density and efflux time of a
-48-
suspension
glucan
the
macroscopic
viscosity,
n
can
be
calculated.
However,
viscosity
since
we
are
( n/n o ) where n
interested
in
the
relative
is the viscosity of the
solvent,
for
(n/p)
Ct
(no/P)
Ct
the range of glucan concentration used it was determined
that P = Po
hence,
The
n
r
experimental
n _t
no
results
to
could
therefore
be
directly
converted to relative viscosity using the above equation.
-49-
4. MATERIALS AND METHODS
4.1 Strains
Saccharomyces
(a,
adel,
these
A364A
which has the genotype
his7, tyrl, gall) was used as a control in
studies.
strains
cell
ade2,
cerevisiae
S.
cerevisiae
374
and
377 are ts mutant
which have the same genotype as A364A but harbor the
division cycle mutations cdc8 and cdcll, respectively.
These
mutants
grow
temperature
280 C
but
temperature
37 0 C,
a
leads
physiologically
when
block
at
the
permissive
grown at the non-permissive
in
the cell division
cycle
to one or more elongated buds which remain attached to
the parent cell.
4.2 Growth Media
Complex
media
yeast strains.
The
yeast
YPG and YEPD were used for growth of the
All media are kept at pH = 5.5
strains
have been maintained on YPAD slants
at 40 C.
The composition
of the media is as follows:
-50-
Table
3
Component
% v/v
YPG
Dextrose
2
Peptone
1
Yeast extract
0.3
YEPD
Dextrose
2
Peptone
2
Yeast extract
1
YPAD
Dextrose
2
Peptone
2
Yeast extract
1
Agar
2
4.3 Yeast Fermentation
The
fermentations
operation.
The
fermenter
itself.
overnight culture.
101
were
culture
The
all
carried
medium
inoculum
out
was sterilized in the
size
was
250
The operating conditions were:
Stirrer speed, N = 400 RPM
-51-
under batch
ml
of an
Aeration, Q = 1 vvm
pH = 5.5 (maintained with 10 M NaOH)
Temperature = 280 C (or 370 C after
shift for 374, 377)
of the cells was followed by removing 10 ml
Growth
samples
and
turbidity
measuring
in
a
Klett-Summerson
colorimeter.
were
Cells
harvested
by
centrifugation at 12,000 RPM
for 20 minutes.
4.4 Glucan Extraction
In
glucan
order
as
to
in
found
formulated
obtain
a glucan sample representative of
vivo,
glucan extraction scheme was
a
by combining extraction methods for beta(1-3) and
beta(1-6) glucans used by other workers (1,2,3).
The
yeast
growth
exponential
glycogen
the
with
ratio
cool
were
phase
phase
rigorous
1000 C for 1 hour.
and
material
min.
allowed
buffer
was
recovered
by
to
of the
material
750 C
cool
was
for
to
at
pH
5.5.
The first step
The suspension was then allowed to
water
at
end
extraction of the cells in 11, 1N NaOH
distilled
extracted
the
order to maximize the glucan:
in
11
This
at
(13,14). The cells were washed twice
citrate-phosphate
a
harvested
since glycogen synthesis is turned on during
stationary
involves
at
cells
was
room
The
insoluble
centrifuging at 2000 RPM for 15
then
3
added.
suspended in 11 3% NaOH and
hours.
The
temperature
-52-
suspension
and
was
the extraction
continued
with
for
11
16
hours.
distilled
The
suspension was then diluted
water.
The
insoluble
residue
was
recovered by centrifugation at 2000 RPM for 15 min.
The
brought
material
to
insoluble
3
4.5
then
with
finally
HC1
extracted
75 0 C
at
for
in 3% NaOH
1 h.
The
residue was recovered by centrifugation and washed
times
with
pH
was
with
ethyl
distilled
ether.
water, once with ethanol and twice
The resulting glucan was then air dried
at 370 C.
4.5 Infra-red Spectroscopy
I.R.
Spectra
fractions.
50
w/w.
The
were
sample
obtained
for
all
the
glucan
was prepared in solid KBr discs.
A
mg sample size was used with a glucan concentration of 2%
To
obtain
higher resolution of the characteristic
~(1-6) peak, a glucan concentration of 8% w/w was used in the
KBr disc.
Standard
(isolated
glucan
from
BSgentiobiose
spectra
Laminaria)
as
were obtained using Laminarin
as
a
S(1-3)
standard
and
(1-6) standard.
4.6 Capillary Viscometry
The
the
principle
flow-rate
viscosity
driving
of
of
that
force
is
of the capillary viscometer is to relate
fluid
fluid
through
(25).
a narrow capillary to the
In
this
viscometer,
the
the hydrostatic head of the sample fluid,
-53-
hence kinematic viscosity is measured directly.
A
for
Cannon-Fenske
all
the
immersed
glucan
viscometer
in
a
samples
and
water,
latter
times
efflux
The
measurements
per
bath
was
used
The
as
a
control.
The
the range 2-5 minutes approximately.
in
were
repeated
to
concentration.
obtain
4
consistent
running
After
each
viscometer was cleaned with chromic acid
the
concentration,
at 25°C.
for each glucan was determined so as to
range
give
water
The viscometer was
suspended in distilled, deionized
all
were
viscometer (size 75) was used
measurements.
well-stirred
the
concentration
results
Routine
and dried with acetone.
4.7 Laminarinase Digest of Glucan
A
mg/ml
400
ml
solution
Laminarinase
buffer
370 C
4
hours.
was
held
solution
the
order
The solution was incubated at
At
end of the incubation
the
5000
removed
an
solution
is
diluted
to
obtain
20
min.
380
ml
order
to
bring
the
glucan
into
recovered
by
for
original
to
to deactivate
was
rpm
in
the
residue
remaining
back
residues
This
were
pH 7.0.
at 700 C for 15 minutes
at
centrifugation
supernatant
at
The
enzyme.
(1-3) glucanase) was prepared in
(endo
phosphate
for
containing 1 mg/ml glucan and 0.25
of
concentration of 20 mg/ml.
range of concentrations in
viscosity measurements of the Laminarinase
-54-
degraded
glucan
effectively
performed
glucan.
samples.
removed
as
Since
the
enzyme
could not be
from solution, a control experiment was
above
where the incubated enzyme contained no
Such readings were then used to correct the solvent
viscosity
accounting
for
the contribution
of the enzyme
to
the macroscopic solution viscosity.
4.8 Acetic Acid Extraction
Repeated
extraction
preparations
in
acetic
of
alkali
acid
removes
insoluble
glucan
the highly branched
a(1-3)glucan component(4).
500mg
0.5M
acetic
suspension
At
of
the
removed
glucan
a
whole
acid
to
glucan preparation was suspended in
a final concentration of 2mg/ml.
The
was continuously stirred at 900 C for three hours.
end
by
of
the
extraction
centrifugation
residue
was
ethanol
and
ether,
initial
suspension
at
washed
and
and
was
the
the
insoluble
glucan was
5000rpm for 20 minutes.
The
in distilled, de-ionized water,
then
air dried at 37°C.
supernatant
total carbohydrate.
-55-
were
The
assayed for
Total Carbohydrate Assay
Total
method.
carbohydrate
In
carbohydrate
such
constituents
measured
or
yeast
using
cell
the
walls
phenol
certain
(glucose, mannose) predominate to
an extent that the effects of other constituents on the
assay
become
quantitate
The
strong
furfurals.
negligible.
Hence,
this assay was chosen to
-glucan preparations.
protocol
Thilly(26).
in
bacterial
was
The
used
is
described
by
Langer
and
carbohydrates in the sample are dehydrated
(98%) sulphuric acid to give derivatives known as
These
derivatives
form
which absorb ligt at 488nm.
-56-
complexes
with phenol
5. RESULTS AND DISCUSSION
The
Figs.
377
30
growth
13,14,15.
was
strains
The
A364A,
374,
377 are shown in
temperature shift for strains 374 and
made at the early exponential growth phase at about
Klett.
was
of
In
observed
this
but
case an increased specific growth rate
the cells moved into the stationary phase
shortly after.
Table
4
Yeast Fermentation
Strain
Temperature (C)
Specific Grywth
Rate (h )
Doubling
Time
(min)
A364A
374
30
28
0.45
0.16
92
246
374
37
0.27
154
377
28
0.25
162
377
37
0.32
129
The
Figs.
morphologies of the strains used are illustrated on
16,17,18.
illustrate
Figures
1 7b
and
18 b
clearly
the incomplete budding process which has resulted
in elongated buds remaining attached to the parent cell.
-57-
10 liter
BATCH FERMENTATION
S. cerevislae A364A
ure
200
o
0
@
0
100
0
la
A
03
50*
#'
I-
z 30I-
20-
(1/h)
n
0
w
mu10-
5-q
a
0
o
o
-
I
I
I
I
I
1
2
3
4
T
I
-
6
i
8
TIME hours)
Figure 13.
-58-
I
I
I
I
1'0 il
1
a
---
1'3 1
200.
2 80 C * 370 C
100.
,I ·
I- 50.
I-
I
W1
30
20.
I
10
5
4
3.
2
I
* .
41
0
2
4
6
S
*
10
*
12 14
*
a*
16 18
a
20
a
22 24
26
Time(hours)
Figure
14: Growth curve with temperature shift
Saccharomyces cerevlslae 374
-59-
200.
I.
.0
/I. /·
100.
I·
280C
50
'-
40'
ID
30'
20'
b,
. dI
I v
/
5
4'
3
I
2
1
Figure
.
*
J
U
U_
0
2
4
U
U
*
^
6
8
10
*
12
15: Growth curve with temperature shift
Saccharomyces cerevislae 377
-60-
14
16
Time(hours)
Figure 16.
Morphology of Saccharomyces cerevisiae A364A
-61-
-62-
Figure
1 7 a.
Morphology of Saccharomyces cerevisiae 374
Grown at 28°C
-63-
-64-
Figure
17
b.
Morphology of Saccharomyces cerevisiae 374
Grown at 37C
-65-
-66-
Figure
18
a.
Morphology of Saccharomyces cerevisiae 377
Grown at 28°C
-67-
-68-
Figure
1 8b
Morphology of Saccharomyces cerevisiae 377
Grown at 37C
-69-
-70-
Figure 19.
A364A Glucan
-71-
-72-
Figure
20.
374 Glucan
-73-
-74-
Figure
21.
377 Glucan
-75-
-76-
Table 5
GLUCAN EXTRACTION
(Alkali Insoluble)
DRY CELL WEIGHT (g)
14.03
15.25
9.78
GLUCAN FRACTION (g)
1.86
1.64
1.53
%DCW EXTRACTED
13
11
15
TOTAL CELL GLUCAN
1.68
1.83
1.17
7.33
8.07
8.77
(12% dcw)
AA CONTENT
Lysineequiv. (mg/ggucan)
-77-
The
yields
accurately
lengthy
from
the
glucan
determined
due
to
extraction
overall
view
extractions
(Fig.
cell
of
of
the
that
of
However,
yields
the
confirming
biopolymer
loss
A364A glucan.
19,20,21)
shape
process.
extraction
could not be
sample
Table
obtained
in
during the
5
gives
an
3
different
It was interesting to observe
glucan preparations retained the
glucans role as the major structural
in the yeast cell wall.
Ninhydrin protein assays
on the glucan samples clearly indicated the presence of
made
amino acids as observed by other workers (6,7).
The
whole
glucan
characteristic
spectra
22,23,24,25,26).
glucosidic
obtained
whole
All
bonds(1)
for
glucan
samples
both
of
the
at
from all three strains gave
glucans
peaks
7.95,
laminarin
preparations
11.0 1amwhich is characteristic of
illustrated
in
the
owever,
377
glucan
the
shoulder
at
this
characteristic
8.35,
and
also
wavelength
(1-6) glucan.
Figures
of X(1-3)
and 11.3pm
8.7
the glucan samples.
were
The
exhibited a slight peak at
(1-6) glucosidic bonds as
B-gentiobiose
sample
(see
only
spectra
gave
indicating
a
(Figure
23).
very
small
a
possible lower
To magnify the peak intensity, the
degree
of
glucan
concentration in the sample was increased from 2% w/w
to
w/w.
8%
27,28,29.
slight
The
spectra
obtained
are
shown
in Figures
Even at this concentration 377 glucan only gave a
(1-6) shoulder.
-78-
· · 1--
?~
·-r
C
i
J
111
..
. .
I I i !: :i .,I...
4 - +i- +i' -- i t f-i
++t-ti ,t
I+1:4
tS
44-J44 ti _t tttt-1-t
I +I44I±+-T4+ i-+Hi
.
.ii.'iII
!,
-1A
.
0_
---
T Tii
I
CCL-L 44
-_-H
i
Il
T
I
' ' ''''''
I -- q41-~tI L......'.
I
III
II
T-1
,IIIII
1 .
I-T 1111
I
'4ar
--+ f
I-I
1-1- -1-+~
X *t
tfrI
rr
1<:·
-J-
·~1-~
1 1 1
111I1
II''lIII'llIITI
!.-l-4-4.-
-
. .
.
t+.
1
T
II
-
1
I
.
;
.! illl7f.Ii,
-- 1-- + I -t 1
I. .
""-N.- i I]
'1r4T
!
'4ltdI
LJ_
I
I I
- TT TT
:14i.I--i4--I4 I
t-..I
II-
il11
i-H,
1Hi-t
*1
t
H
i .
H4t-
I
---
r:-~
i
tII
oi
i
.
I
<
44t
TT,
s
l::
T
i 14t,tj.---Y-tsfitt+,j
tft--
I
I.l.ii
I
w1'-
t-i+ L11A-
: ]
i . I .+ +
r1I
s
4' 1 I,,.
~f~-m#ltl
.
.
H-
_
. III·l, .
::
......
t#
ft
I+4
Lt
.4-4-+t1f-f1 H4
fi;ittij~
TT1
14-ti
4Th4l
.,.....
J
?
~~~~~~~
~
l-1
71+ ±t
. , I
til4
I1
I-tt
- t4-4+H444 tI-H
ti 4±'r
·
-t
f t- H-- -t-i-H- -,4
i44-L
4 _.i
.S
iI
I
-II 44
I1I
l,,.,, ·
l
I I
t--i -4-
-
il'!l:;!;
-:9
~.-rl
_
H-At4-tF1
l~r
-Hf
I I I I I 1:
. Iitf++++H.,§ fH
I,f-Ic+F
I I 1 1 1 irrrsT7r
IlIllI~TT
I !,
r
'U
o0
Xs
I Ht+'-+ft+H1
tcr-·HHH+iH+~H
-s-rc -I ft4-H-I-H
i i d-·--+I'i. 1+1F
r
i-H fH+44-444+1
H +4-H,-:
+,4-4
t- tH- -cL
r·.+±f
- -~i-f k!
i--- ii--· ~ c~, ~:·
c ... c~iur~
.i H.l4l
ii iih t4 -. .tt(
V4'1 !t
. ..
I
ith-H--tt
I
I
] I
I
1J
lllll
4
Ii;
-t
_IiI . .
..,..
I
,,
-I-
j
I :i
-317-444]" 4m1
t
j4 -_4
ti44 4 4T444tt4-4Hi
I
141-
:-7:~
j J'
£,1
A-;.,tq
I -it
I
•444444
'+j1!,
fHfH
t
-
1-
z
,-4i-4---
- ' 1 -' 4W4
t
tt -
1+ it f1+-Hi
---,
i. I
t.
i
; .. . i
h t.t 144
Th
WW4t
.
III
1
. .....
I
Z
Z
..
[i#SH
-+
I
4--L
ru
.
O
o
3n
.. . .. .
i--
.o.
1lyil-;SESX21
'Tj
C
~,
'
l1
#!
44
!4
.
t
A
O
0inr
0~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~
' JE
-i-i-LtWf-Ti
ii
:CttiXtift
SttXt~i~ft~
xI
M
Zu
4C
3:
Figure 22. I.R. Spectrum of Laminarin
(2% w/w sample concentraion)
-79-
r4q.
--
r;
I I
-
I I
l l
i ,
I I I
I
_,/l
I I l1
I1
I I
I 1111111111111
RIH
t44,4 14-4'44444
-4-t
t+t1-+4
+I !
H
I
!,1~~~~~
1, 1 i
. I
O_
H
,
1
I
I I ;
I
!
1
I
. Ii !'I
1
I I[
i .
LIf
J.r
-
UI-
it
9:
-4/4
[ttH-Ht -Ht+H
ti
H
T-
H+4
-+t-H4
4z
_
,
-t-
-.
t L
t
HT,4
1
MM~r;tT
44lof
1i4-4ttq
i:- t
FLai
.,-4
4-
Li44
. .
l
-1
4+1H
IH4 XfH
±-L
4
-t-4t
4
1
1-4
4i ; t14t41,I44444-11
I+ !t-i4_.l-Is [.t!t- -I+---
.
| ':
j
F-z-6-- . . 1 ! 1
- ;:
_
-
.
+1-4
-i..,
,4'1T.
_
,
1!
rr
I,4-,
t
,
~
I
'
!
7, i4L-!+.+t-tI-ifii:47+
'
!
l
OF
44--
I-
L
4:-
4Thtt+H.i34
O
a
i;
H t+iq t
HI ti
- -- ~-
4
.1
1
]--.-
,
-L4I
i
15C--ttH1 H--
T'~~~~~~~~
. .
L r
+44-44
- ! - --:~--~, ~ i
.i i11
±H-ltStHflifIH-; Ahft
t4
-rc~~~rl~~~-
I
*r
+--,,i
-'+ I
HIAt - 55~
-ttd
'-
V--I
-
I
.
Li#
,
4--·I--
Z.
l; '
'
II'
'
'
~~~~t~~~~~~mf~~~~~~~~i
~
~
It"14a,~f
-~
4
I
1
i-
·
4--Ht4tH+FH
Ic fi-
itI-IHf
1-t444+4-t-4-+
'
'
II .
I.
I
I
-. ,
l .
ift
.
1-I-
-
___
_
'
1
'
Aw4
99'Mit
-"
I H44
VT
iL-4-44444444-~
i4-7; Thf
I-I
Kii
i
Cr
:.-
IlZ -1 i,
i
'u
Wf
tW- thitHA44
4
+H4+-
:, '-
__
I
,i;C
i 4-4+1
'-14 -
t.
Irw
4t;
F
4--
t-
m
4
1,,
:P
4-
ta
LL1444
44
I I I
I I
I I i
1
. .ll~i
. .l . . .1 .'
L+43
t±1
-
1
I
,
I
111111
4
11-
0-
J'
II
L
-~~~i~~illL
i
;,
I
rI I
1- -
-444
11
I t44
I
..
J
0
II
:_i;
I
-
4-
0
U
i-
t
-T,
-
--
9-
Trt±L~-
I
71-1
iSI
3-
t
~l-I-~III1~'ii~~TTh.
L
I IIll
I I'I--
ILIALIL
i~T~TTTTT7I ·
I:,r
ii
1
:4'
t4414-TF4F
; .t
I Lf~'! . '
-
.,,
!'Ci
I
·
!
i
I-
I
z
I_
C-
-+tf if
: iM,111!
I
,
I-' -
,-w+:-+-
-'71m:Th tti
1cC
-
+r
1
7=
-T:ITRTMT~~~~~~L
~L~~~?~ilft-"i-~tf
t tt, -4111ff
I ITIII~~~~~~~~~f
t-i
a
LI
!A
1-c f-H
_ -'.-LIAI
l
0
0
An
04
-
,
. T .
I
I .
.
I I
I .
I
,
?-~-c~t
-!: 1
.,1 4
r4rid4
t+-4-44444-.'
4+'t-t
lc~H
1! 44-'
144+- i4- -- i414-414L:-f~t
' -L4.4~ti
4
-
±tti7
X
I S
m-
tttdtt
lil
Hiitht:
.Ct
'~ -H~ H-4-4-+4-~
F
lb
4I &IL
. 4- 1I-t-f
+-IIIa
rl 14+ 11%P 1i!
II~
i ', '-
-
!
M-'-
--
-t
+-LI-H....
i -~,~~~~~~~~~~~~~~~~~~~~~~~~~~~~~
C1 '
I
t:;
(3 P
I--n:
9
.
T'T
E i-rT
~~~~~~~~~~~~~~~~~~~,4
1
~~~~~~~~~~~~~~~
L (-~~~~~~~~~~
~~~~~~~~~~~~~~~~,,,,
tl~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~i
·
kl~~~~~~~lf
"4
1
I~~~~~~~~~~~~~~~~I
?
I~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~
44#
7
'
nt
--
,
uJ
~tt
11
Li
;44+
_W-t
4-
_
-
_
__
_
.
.
_
.
.
.
.
,
..
....
Figure 23. I.R. Spectrum of -Gentiobiose
(2% w/w sample concentration)
-80-
....
i
__
:;m
I
-7-
I
-TITI
'1-
ZZ T
MY
1I
:
i[
I
4;
SO I
!' ' ' '
:,ii
i·
,
L.--:
-}
I,,_
_
..
I
IE
_r.
?1
- +
0a
I
*, -1f
-- I
4L"
_l
:. - .
~
-t, -- P-4
,Ii~~--?
4- fl
I I--:-!-L
4t±L
144+4
H.I +444411t .
. .
i:
[Lmj
; i vI ~
0r.
-
ii:
1_L
'1
t II
_ZL__
. . . .
+t
-L
.,
.:
-ii:---
I I
-~
`-
MI
-H
i
-.~~~
I
I--I
. . .
m
mttt
H~~~~~~~~'''
-±H:U
........
,,
: J
t
rt-
,- .. .
H-ti!i-H r
i I : ' I
l
.11I
' li
111
la
T_
-''
-
T
r
--L;
-
.
4+
NI
--. 'r-T
LITI
-L.
44I
-:
"!
n4
I I' I
I
i
1.-t
T
rI
.
t-'t--t
' '!t1'
I. I,
I :, . I.a .,, .
f .
1 it
.tt .-
Ii
mtf
t-f -ft
if I ii
-H4]A4±
t-
_.
-.-! fi - i
.i rt" +i -tf
tHti-
U4-
it i
9-
Ht+t
hI I
Ht·
-t11§
F
-. +H
4
--- -++-,+4
0
'
-444
o
"i
I
-
l'
__.
,_
4
m
C
r 1' 1
.
J,.I
I
,4
01
-4-ft 4i
.i
.v'
I I
:
'T'T C
rT-14
: i
1T
-
l
+
ia
UI
""!"'1 Ittt
t-f-,Ff
Iti11 .i -_
___1
.
. . .i---1. .II .
tt
set t~ftttf
I
1.1.I
L-
''
-:H,I t1-.
-LL
4+tffH
.- -
tm t'tm
`L'·]I
f
',U
I
2.
W4
A
,
I4i,q
ttiI I I I
.
....
J
I JI
i' ' I
-i - - - -I -Ik--. -.I...-I...
- -I -t -I- .. - .
cC)
C
e+~fCt+lf++-e-- - - -tm
+t-4-i41
I
ii
4-L-
It1
-.--111
-Lt-1
'4
-4
. ..
,--i-
I . . .
-H'Ht
---
r+
4I1
+rhd
I-~44~
___i
LY
_'-
l
42_
' I I I , . ,
I
,S,
.
.
_
H
m-l-
~
;*
L
,L
1
I,
I'
I
-~~~~~~~~~~~
4
1;
w
I
I
;T
4----4'it*--T~lllllll
-
e~tl
'
t
I.4
't
:i
,1fi
It
0
oan
o,
--
~~~~~~~4i
i~~~~~~~~~i
1_i.
I I.jjw ,T.
4
~~~T
/I~
T
4t
:z
4 P_
.1
E-1
I.
I
ir
T<
MiwX
ij-z
g
J
|
mM
3..t~.
m
_'1
-
1' '
. I.--
fiizltt-:-t4-ttt
. . |·
l I II- ! E
-4_--_
to
-I- klJ. i
i _i; t i-- l
.I
IIE._;]l .-ltt-Rtfi-'+lt'-r-l- I il.I. Ill
I.I - - II]-I
=#ffft1#HV1=Jfi
%_
,
1- Il
-,
i
A
V.IV,__
t
1;V-,-1
-.
. i I 1
I . rt- t+Tti4ttt-
fl I 4#ff#-TtA'14~#
1
,
I-
-.
Ii
VTi
.l tft1
· ' rI I. .I
4-4t
.
] -
.
I
ei!.
4144#1t
X--'F
|
.e
'
-
-t
- r'i
.ttt- i .I.
.LL
- - 4- 1411I13LU11~~
'K-rn
Figure 24. I.R. Spectrum of A364A Glucan
(2% w/w sample concentration)
-81-
; 9I I &ii . :Mrf
.Y--tH
--
0
Q
ITiJ_!'
·
_
L|
,_
:
.
7f
-
·
· ..l
4 III4u.1.
I
.
:i i
. ,!~:
~lI
I4- I
:I!
, 1 :
II
I
I
-
I
i
i
',~ X
T
H-
g'1·1·
Tr
_-
''-
---
I:
I
I
H.L++K
4-4t
ITR
444-1I
z
911
;:,
- .- -
fT::
C
+ +-.il
I .H
I*1 -
I
*';'
.il
4
-r
II
II
I
I
III
I
I
I
:
III
r
1
I
I
I .
1
( ,
i-H
---1
I
r
I
I
I
I
· ·'
I
I
I
I
'
I
I
C
--
I
p
'
;
- -;4,--
-
.
-l -
'
AT
. .
hi.
.
.
. . . ..
.
. .
-
I 0i
. .
. . . .
T
1
0
-I
"H
·
J --
l"Hi i
-
e
T
+
_4
'I
__
_
i-
L'
_
Mo.
6&U
+
i-
I-4-t-.- i 444-
Jt~JJ
- .
! ! !
J
-
um
£
C
.
q*
L'?
.
4
. 7 -
.
.. ..
-CD4-i-i_tIJ
4.
- ..
IiL__
~
:IIL:LI
L
I
· '·
I'1
4
__'El2
. . R
Iiff
'I
-
_
I
"~~'·
t- 17h-I
I-iLA
·
4t'4.t!
--
-1
- b
-
i
[
L.
_L
;,
y
'I
-
_!J11
l
T-
j
I
L
l
i
i
?
tm
I]I
1rr
I
; 11-; s :1-i+'1 i 111.......
Ic _r-C L...
~~~~~~~~~~~~~~~~~~~~~~~~~~~~.
i ez444!
H
F
'
-- iI i
.
-4
9
- 1 .
9in
--
·
'1iI
__
liI
1
IIl1 :
!LLL
.
]]
I.i,
_
I!
S4-g1-.me-{.
. -4z.X
~
'
i
-
d_L.___
l
1'_'L!
-JL.
'1 _La
,
I
1i'-
I
I
I
I
I
._LJLL
iL
:E
Mi
__z...
~:
4--' .~'-
:~' ~:T~
_'.~:
~-~~
,
~i~.
:~1:~=
---
...-
~~
_
.......
.;.
~J-t-
_
,
. ..,~
......
~.
....
~......_
-'T'-- -~e~i-- :T ~.~ :.T._
,'T.~.
.~_
.4---~
~-_-_:::---4'-i-:_~
I
I
4:;
:: .
'#
.
r-4r.
..
.,
.,,
U...-_
L;4-L +J'4-'
,,
_
:
i . ! :' . .
...~ .. .~I..~
.
.
-,~__
'~-''~
'
~-":-7-~':~.T
-- ~'~---:;Ti',
!',-
%~.'''
''
' '-:
i~~. .._'L~..~ , -~4';:.-~-~
,,,,
.
it
IM
3:
·,.~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~
..
'''''
I.l.,
I...
i
.
...
.1,
,-i-zA
*_
Figure 25. I.R. Spectrum of 374 Glucan
(2% w/w sample concentration)
-82-
~'
+ -z
. . ..........
.-
~
4-,!,
t- -,ti
Tt
|.
- 711241
:m
Lit---T
t
,.
- I-t- --
j--4
1-
-
_,
7L
_1=F=:
|~~~~~~~~~:
L-.-
___
--j H
4-
t-
4
;
,
F
I
-
t- :1,
.___
,z=~
-
t;--
'-'
_i.,~-
. -.
C
i
-H-
tL
L --
z.!T
ur-i.t-et-t- ¼'
'
Ilt]
~~
___ ______ _-t
=_
_
___
___Th___J
_X_'_'_
_ m.q
i
I '
~
J4_
'-
q'.
,
-
*-,
_ _-e
,
-fr-
ti
P.
_
T5,
t-
5>
>
1
,
T
tT
!o
|
-+___
l
, .
1
-
' 'S-''-i__
.-r-'-2F
-- >__
j,
'
.
_
--t-r- r--
1>--=tt -
It 1-t
g-i=
m- -- 1--- V-E-
t
2112
D,,
,
4
1-
1
+
I
'
1-
,
l__
A==
-t t-ti-- '----L- -__
t=--7Cr
t--t
t --
t
-:TTT,'I :;l:,'T-
I
_
l
_1:_
1 _
C4
=iTT,1 _'_-4'
,
1
_4. r-1 '
_
1'
.
1
t
~-ci,"
=
--
-+ -- 44-t
*__I
t
4
I-A
.
1 1 , I=- 1_
|
|
t--tH--
=r=m
E
-H-
__=
i
H.
i
_,
s
I
l
t-
j
I
_
1:_-- _G-:
I
__W_
F__t
-4
---4---
_
|
_
t--<4
__ I
t-
..-- -+ _--L-:
01
--
m----
t | - *1 1 * t
C
im
_
_.
T
_S
_t s4 i _ t+
u-=_- I':¥ 'I1--H-,4
a
i
aT4__
:,~~~?_ ~_l
TPr_Z
tC
:
W_+-,
44
4,
PC
I
---
m
'
-
_
I
, .,
1 '_L
I'
4a-4__
4
k
a
_
_
4__
r;H-
!'
'F-
,
1:
I
.:
.
'
-
-4
CD
:)
_
.
W
t:-4, '
4
+.
I,
J ' ', ,,_~
.,- 4-
,
I _
. m-_
l._.
.
I
I
-4
-tr
__4
I
>,n,,< t _
_
4
t-~. -4
4
._1.
-n
t
.
.
.
Xe'. -
_ 1 .__.Ir_;_,_
_4-__
_-
___
d-_
.
.
.
__ _ _._
t__
t
21
l --1
i-e''
i,,lMl.v.
_
.t
_
1
_
_
g
___
I
.,
,
t1
t-t|
_.T
>-+-tll,,
T' l
LH__
I
t---5-----t
|
='li#'
I
t31==z-
-jz
I_
' I+-
W-t-
1-t-
',, t
--
t--+-
-
2f ,;
I
--
@
f
t-__e'd_ t_
'
'
f
_ _ _-.
|
>
4
t-
_.
_
t - ---
|_
tI_
_
_._
F___f__.
2
_
__r
,K,-:1-_=
--f-t-t
4, ---t---t=
9
._
:-4---t --'---t--
ill
[
7
_
_
1- -:
J I-__
-.: -T
__
r-_~-- --- I--- ---
--- j
r----- _.
-__
-- -
Figure
T.-
f
_- -T
_-
;:=
-:
l~
- _
.i
-- _ -i -. __ ----
7.
,
F,
- - '
-.
I
_-'
S~--s?
L_
_ --. I
l
-
-
_
&
l
I-
_-£-t= _-_ -_
---1------_...
I -t
f, A
---
26. I.R. Spectrum
-
-,
--f - __f-___
- - ---: -- :~-=t _ 9+-I1 1--f ±7
4 -_-i
--
_
,
i
.
i
_
-_H
-t
.
.
I
SM
-t ----
.
Z
IM
I.-
:
7
_
....
-4 -:.
.
--
-
_
,
_ -- _I _
271141
--
I - --I - 4 -- --- -4-_
_
.
of 377 Glucan
(2% w/w sample concentration)
-83-
X
,-------.I..
,
-u-
i
-- --u ----
--
+ :-f:j4
:: .=__
_
_
F--.---8
T
I
I
|
,
,M
§7tllf I- j221
t_
=t=
.X.
J
&
, .
_
_
.
_
-27±+
-7
12171
>7j
:
fF _
t_ =
-:---"
---': '-. -- -.
I
-, - r-__ - 1
I'--- ·------ - _-_ --:------:
--.--- --- L- :-_ - :T-L-Z~-~l.ZE
~XjL'i_~___I~.~L.
_
_..
.......
........-.......
.....
.........
'
_---_
--........
__
.
_._____--._ __
I.,
-~-;':-::- -::4-::'.~:::--_
~__::---t
..:..::---:--'- -"1 ....--- .:---7.'------:--:-:-'--.
~-_-----
-
_.-
r~~'--'~~:::::
---- _ - :_S-
z
_-_
.
-
....
,---
.
----
-----· '-
--------------
T--- '
----
-_
- . . F--
............
_- --t----:.
_
_
.
.'
-=
-
-
- ---_
- -
-t--.
_-
r--
_.
,. ___Z._7
_ -_ _4__
_ a----
r
-
·
-- : -- ·-·---
---
,__
I._ -..
t --
r
-
------
f
-
-_
-
--
-----
-r
-
-_
P.:
__. _.______..__I
_
-
_.
_1.___
CA
Cc
w
__
_
I
-- ^--` -I
__-_ - - I - --
._..__^
- ---
I:
--
__·
-·
2
C
I
Cc
---
---
------
wt.--
r--------
-.
S
q
-- tt~t--
... ---..---- ·- '-----·-- c--···-e-----
--
I
r
I
.-.-
---
_
-- ?--·-
_
----__
__
.
I_
_
. -------::
_
_
_
_
_
-·-----
T_--l-
-----------t-------.I.------ -- -- I·----c--i----i
___iZ---;--L_1--tir_
_
1
_
_
_
_
--
----
-- '::---:. _r.._. ,rrr-i__f_
,_
-
"-
_
::- __
1
--- :---
f--_i.
·)- !
Figure 2.
--
E-EF-HE
-
t--
I .
--
_
T
-.
.....-: I
--.--- - -(----C
_
_.
_ ._
.
+---
+
-
o
0
!--
-i1
8
(Z
r-w
_-_
.--
_·
_..-_,_
I
_-_.__
i
·------
-.-
---
0
*---r--·---.
---1.
··----. ,-.:-.----I---
_
t
_
_
.
_
_
_
_
j
_
.
o
co
-1
-----
I
··
· ------ ---'
1c
--
-
--..-
--
-j--J__-_
-t --?-
.
---- -
|
-7-+-----
-_:-
-r
_
:
_.
--- · · t
_
_
_
__
--- 1-----1----
_~~~~
I.R. Spectrum of A364A Glucan
(8% w/w sample concentration)
I'C1
_
2
---- +----C·---i----r
-----
.-.
-- r--
Q
t
------ ;--·---- -, -··-- -
r..
t------ ·
i
-··1-··--- *-·----r.-I -- -- ·---------------
-·--- ·
.-
I
I
-- ILI-
.___r:--· i -·-
-84-
_
_
_If
--
I.--
I_;t
I
r
_j
___
.-. cu..--- I·----------
-···
--
__
_
_
1 . R
_...
t'--
.
___.
__.4
-- ·----I-- - -· i
t -----
1:
--- C-I-C-------
c
.i
CY
---- r
-
--
---------,----------i---·--i-
-t
-I,
--
------------
t-l
-----
i- -·-----·--r-·---
.I-LIT11___
--;---L-
·-
---
L·---+
:
I
I------L
------r-·-.----
'---
-.
_
-+-
--
--
.__- !
.
I
-.- L --L--L-f,-:
-------
-C--.-·--
1i
I
.
_
---
r
---
'---
rI__
_:LL-::
-- · ·
-L---
i'
=I--::
-.i-·
----
-- i-
·-
:lj
. .
-- ------ -,-.
--
--r --
J_
-i--
i-
.
f-
-.-.
.- c-.-.
.- :--.-r..
----- C· ·-- rr -- i·--·
-·-- ------1-· - --------·
----.
·
'- I-...-1--...-----;---i-----L
-- ----I
i--- ·--I-- -L--- --- ·-i ---- --------- · - · ---- ·--- -- · r - - -- · · --. ----·
1...
.-.-. L. . --.--t-·---- ·+.
·
----- · r-I - -- · ·---- r- ·--..-.
--L-. -C. -.
··
! ------ · - -- (----_.__I-.,
_._.
I
t.
C!
._
-_
I__
I .__...
---
_
----
_____.
-·r---i---
3--
-!----J-
,.._.
__
_
I_
a.
___i--
___
__
_
i __
-____
---
-c----:
-- ·---- '--I--I
--
-- 4
-.---
L----.-.L
------
- -_
_
t- _ ..--t-------- ----- f----
:_-:-
,
-
.
- t---c----
-1-·----
____I _ _
__
....--i -· -t-
_
--.
t--
f- -·-i----$--7
---t---'-------
...........
_ _
.
;-----
tf---;
-(----
-t--L----t----C---C-
--- t--
---
......
--. __
-+-- ----c
-
:---
{
__._4
-
----
LF---
-- ;------- -----
--
I
--
-
-- +
t-----1--- $
.
·--
-I
----c...-l-
C
-- f---l
------t--_._1__,=
f
I~-- ----
_i ---
I
t----1---1--
-- t---·-----C
-- - --_i--:---
.
__
_I
.-T---i----:
.
4 .-- .__
·_
1----)
-- -'
L-C--II1-i-=
__
.
I_
~
- .-I -- __
--
- ---- -! --L--
.
___ _ _. _
.hi.-
,- -- ------------ ----I
--'-.-'-`'.
---·--------'
---------------.----.- ----- -- - -·------ --- -- f ---11....-..
- -- `- .___
_.-_.-)---c
---- ;-·-
4
--7:±=
_. I---
-- j----·-
--I-..--.__------
C
-
-
-- r.----_I_-!
I
LLI
·
.
:i6
. =c
d-tn
~7
.........
:
_
·
t
_ _._
,.
_
--- ·- -- e
--
-- c,-- - t __:-.-=- ___T__----
_- __L_
.
I
X.
i
-
.
_....._.
j Ij ----.
__,2
i
-1
·-----t
__
-
iI---- -- -
1
__:_
-
·
_.--
-.
-
j Tf
__-..
_ _ _ _.. -I- -,,__
__
______
_
i
, ..
-
_......
-
_·-+--
r
-c--,----------I
·
-- ---------·--·-- i--_.i---·- -- '--------=---.-i -.,--.
__
r_._
--- · · ------$-----.-·
ri-- --I-·
--------C---- -- -- t ----- ; --- -·t- - -·- -- - ---- r
____ -- ---- ·--- ·-----i----- +----------· ---- ·- -----------,-i-- - - - .-- i----+
.---- .-------II
.
,
I
,
r---_
-----=--I
_
----_
! _-
- -_ _ __.
-,
.-r,--"
.---------
.
_
.-
J__ .= ,
- ---
_g$-
r--
I t._--
-t--:
4
t
I-
---
I-_.
_4 __
_ _.
+
c
_.
Z.
I---
_u_-
Cj·----L---
=__ i-,-------,r
~~---- .
-f--·--:--·
'---1-
---
t= _1
-- - -
ZLt--7Lj--
--c-_-_
o.
r
---
_
__I--1--11
I
i------·-l
--
o0
L_
f;-l_f_
--- ·-- r·----
-- t--·--- t··-
-------
·--i--·---
i
---
..
-
__.-1 -_
I
..
'
___71
.
I
2W-_:._:----
:r~~~~~~r~_
.-.::_:.._
p:;~
. -~~, - --'=~
-t--. _T;_
: --.-- - r--,-_, --~~~' --- -I .-..-__
.-Z
_
_ _
t.
1--I-
r-- 1
- -=-'.-
---
.
i--_=1--=
--1
t
-4- - ---
--
t;------+---L- · -·t_
_j
O0
_. i-
_ IL
--'--t - ·-
··
·-
_.-
t::_.
-. -
_·---- --- --
-
I
-:
_.ffi_·$--i-----
-------- -- t---·---+---·----·--- ,----·
_
----~------ --- --- 4-+-
_ _
I---t-- .
4___ . - --- ;; --__----- __
__. - -,.-- ;- -- -- 4-- _ _=- _
=
i
t:, :,-::
t------- f---+---
i...
I-.--.--:
-L.-.--.-
LL!
Ltl-`-il-I
..--__
__..
---- r·· --= _
m =
_,
j
_
-
t
--
------------i-----------
_
-7
----------- '-- ·--·- ----
C ---
i-- -------!-.---."
'-f--____lz
· --r_.
----
-----
i:' _
- _::)-_= T
___..
-----
i
o
_
--__-_..... f....-;.....:
..---.
--. -__-- I-_---_+
--.- . .. .....
............................
--,- 4-_.._._.
- -+.
_' 4-._ _ ---. _
~-____._
....
~..................'
.......... ___'___
_
-----r- _
*-- --__
-------- _ - +1------t-~-*~---=~---~-_--=--:---
C%
q.
....~::-- :,:.-.--!
_tii
.c-
,,=,
t-
co
N
-3 Z
LU
]
w
I
I
r
-
4
_I
l
l
T
=
E
^
. L
777
I!£'
,,
.
N
7-r
-
F--:7
'
I
__
i.. .
_
S
_-r
___
, _I
_
I
,
F,
.
_
_-·-~-
~-
r--
~I
r
d-,
- -
;e--
_-__i
___
C--* ·) II
:
-
I---
I
·T--i
r
_ -i-L1
___
1---
_C
4
-
-'
:-
--
I
I
.
,
I.
f
i_ Hf
L__-_
.
-,
,
5I
_-_
I, i
C+----:
it---i-
7J
:_
T~
I
", .~
4H4_~-i
F+=-
--
I--
H4Ei4t5I
+3-t-c-·+-
c
I
0.
ii:
---
,1
-t
---
---
-
-- r·
o
,------$$
--r:
-"
---
----
-- --_ _ I
o
.---
---
I
, . --,
-- t-----+
e
+-
-
t---iIt
t
-+-
a. -1III
t----
I-
-II
:
.
._
I
-=
,
-- ·----
rr-- ---f
-
_+
=1__,
---
|
CC
J-L
-
-`-
-
---
-T
--- t
--
_
_
,
,
7"N-''--v
V---T"tt--1
.
=
'I
1
-
-11
1-
,-
1
-
1
-
'I
I= 477-
- :_
---
, I
;
..
I
0-
7--_
-
B-. | -t
o
--
--
.-. .
__
A-
·
-·-t-
i
0
o
I4§=4±-±fi±5Fli-3r
0
I..-
:ft-
0
v0
C_
I
.
.
--
t
F-
H--_
.
I
I
.
}
.
.
.
_
__
_+.b
.-x
I
|
-I
'.
"
:1~
.
I. I
i i Le'
-cI
'!
§
.,
.
,,
,.
I
{
I
I
§,
i
-4
. . '
1i
1 -- I-4
-
-4
00
_
__
7 44--t
_
,
___1
,i
-I
~ .
I_
i
l
+_--f
---
I
-4 - - I-
I
. ' zt-
-4 .- 4:-
CY
__
--
=4
HI-
f-
- -I
-;
I
i
-
r -
7T:T
=JT=
7
r
- --
7._
+
t
-
_
_
_
_s
_
i:
_
_
0
C'
-4IFE7T ZiZZE7A ZZ
--C
9I~iL
_
I
-
l'
i
I-~
' |
!
-1--4---
-=.
_---
;T
'
_ .
*_
- -,i
I
--t7I------
'
+-3
_
-i
I
_
-t
-__'
1
'
I
.
-
-
,
:
'
,'
;''
I
,
I
t
i
,
I
'~
= --
I
";
I
_ _
II
I
I
---i=.
I=
,
I
i
'
:
I .,
-- _A;----.-4--A__
._-
I
I_
'LI
I --=
_
I
E
-C-
0
b z:::- ,1
Ui
m
I
Z
--
I_
i·
I
t_
' : ' --!I
_|L'|'I_]_
-w
-
!..
-
:
T----t-
-
_
1
!
Figure 28. I.R. Spectrum of 374 Glucan
(8% w/w sample concentration)
-85-
1
--
f
r:._ _-+-
--4 .-
W
N.
0o
0
0
0
o
o
0-
0
-
_
.I--
_
4__
: -
-
-
--
-
-
1
-1-
--
I
----
=i-
B
O.
---
_I=-7----
_--
t----
.
---
1---
I
+.-.
-
ii
-u
r
-'
!!
_
_
_
.
:=
_
!-
I
I=
_.
]i
_
.
_--
I-
=
1-
_
1
I
-l-f:;-- -:l-
-
-
·-
c
-
ran
t--I
--..
--
C
,
-
I
-
-
-kt-i ---
-- -
^^^^^^^---
^
--- ,
_
_
1-IE
_ _
:
--
1+1
oD
:L Y9
IC
i
-
i-
i.
t- 1
-
-. I
t--
--4-- . I--
--2-
i
-- ---
- '-T-I
_
__-
=
_
0
0
WD
1
._
'|.
:Z--i 7-
--
-- -
,-t=
=t---I
- --i----
Ir=
-----------
ThsC$-2~
--
,l
T--
-
22L
Z
-L ·
77----47 -
t---
0
0
Go
----
==t
I
--
_
---r---
w
I-
-
I
,
t
i
-i
=%-
--
-==
--
-
w
LU
m
,
=---W_
-==-- --- H-t --- ---
iF
4+
-
He·
-- --
-"
-I-
-
=_--4-----t
5-·27+:
.,
1-
-
]
__
1 ....
---
-
'
r--
--1.
--
_
.
-
1
--
------ I--_
._-------C---- ·---
I
-
--
--
-
II
.- 7
·-·e
_
.
-+---
_-
4
----
1
_<_~
_-
_
__
.
1
--
---
--
---
_
---
--- -- L
---
-- -r---Ylf--
.
Ft-seas
_1-
r_._
,
1
_
i--
-
--
I.
l
..-iH=
31
n
i
H
.
,
_
- -1
i
- --
- --
.
I-
t--
.
I_...
---- CE-
F
III_
A
-
=i
_
I
I
,
i,:L,
- --
-t---.--
;
=-
:
--
:=
| --
- =-|-
i
(0
ttt
_
o
t
-- ,-
t
C
~r
r
t
___
--
_
Ii
I-
i-e -- t--r
4__
4+:1
I1
1
_L_
---
--
=
r-F-
+4__
---
I
--
1-- -----
-
---
!
J---- -
--
-- ·C-
ft-
,
.I
---t
LI-
W
I.T-----
I
:Zt--
->
---
J
LO
0
c'J
I
22i~-~
T---
'
-- '-
t-'
L
0
:
t-__
----
I
1F---t
-t=7
.~
-7't-
'-C
- '--
---
·--
--- *
'Tf
----··
---
-iL- --
'`-
----
7=4=---
!-
t-m---t---
0
-t
---
--'
_
-T--_= t
I--.
-P
--
--+i
-
`--
-i
--
-L_'
I.R. Spectrum
-I
---
I
I
TV
+tT
-C-
+--_
-
|
=
|
|-i
1,
:- ---t
i
of 377 Glucan
(8% w/w sample concentration)
-86-
---
---
C
---- t- --- r
!
Figure 29.
----
-'
t-- ---
-----
.
T
;
I0
0
C.)
-
C
ir
·
I
---
_
----
!--
------
1
w
w
2
z
w
5
I
g
I
_^
-
-r-
.... lb- Z. . 4 _+L_'--
[---.
---
--
i
-
---
,.
...
_.
.
C -
I
!
w
.
---...
i----
--
F
-!
_
__
___
A_
__
l-C--
I-I
.
__
:
--
1 _
._
z,-
=
_._
._
=
[, =:
+ L
+-_y-`--Z- ---
r
..
~t
.
_
-
__~
O.
....
-
-- I
-
.._
i
I-
Z=IL-,
---3-.-
T.=-:
--
i
.
-
.
,
^
. ...
r
-
.
.
.
:
r-- 00
_
l
0
Ov
0r
i
1'll
l.
_
O
---
:
.
:' . _
--
lJ
t .
:-_-F-.-t--.----_: _
-·
-_
=-_=
L+---
----4--- 4T---
-....
e .
--t
o
._
i
.
__
,
_.--
--
---
------
-------
.-
_
-
-
=L:-,
r--- -i----
__-,
'--·- --- -
:L- ---I---._--
T·---
-4---
t _
-;----=-
-7
I -
-
-
---
t
_
t
-
_
--
'----·-
F---t-
:--t'--
4
-
w,-
--
_
F--.-
t--ft _t
.----
4 ----
..-
-
_
--
t
__
g_
.
I-
0Ir
-L-=' .: , -- %
;- _= ,-_--1:'Fz
-_-._':':_._-__
-s__H_
+.:-I
_,
§
-- __= --.t.
t.=. _
_
|--
---
--
-
t-'
-
+
..-:--)-......
t
-
1-
4-
1
·
,
I
.
-
t--
, ----
-
--.
-_
._
-
t$-_
t- - .
o
0
r--+,_._
F----H
_
,,
---
I_.__ _,a_-- a.F:-_
- ------
-+
----
---
--
--
M--
~~~~~~~~_ I-
-i'---
t
-4
~~~-
'
--
__
UO
CV
F
1
-
--
_vE
_4
-
0
-·
,
-.--
-_-
--
-----
- t1
t-
X-'fT-
--
--#-+-
~-
j---
4--
;-
t
---
t--
---
r---~---+_
--- 4---
i
----
-4
, H e t~~~~~~~~~~--t-1
--- s-
II.
^e--t-----1--'
o-- -t--
-~
---- |~
e--r--__
-
1--
4--=.
=
_
|
t
,
_
--------
T
t
-
o
oD
-- ___
-
i
.
--
I
-1-4--
-
---
-r
L---
----
C.
-f-
r)
I~~~~
t---
I
4
-L
-
--- &4
I
-'I
--_~_-_-.
---------------I-~ '_
5A7?
-.~ |
| -§~~----I .- - I .__
l I
II.
- ..tX---
C
o
D
c1~
·---
-r-L'----:
-__l_l
'I
'C
----
~~-
t
---
t
-
.
-__F__:
:~- :-~:-~_
'- -
C
Ca
- .7-
-
-'
. . _-_-T::
.
L-'TI ._.-...
w
t----1--
EJ IE--
__
1
-7
t=iS
t
-i--1F:---.
-
___
4_ ___--- r-
2
,
I1I
_r-_
-
-_i
-
.
--_t
.1-' . 1- - -
---
---- t---.--
.
-
------4EEI-(a
----
_ __
- --
_ -4_
---
_
__
1----
---
---
_.-
'
---
--
1 -- ·
----
-i
_
_
__.=_
-·-
1
L-+-_
-T-
---
2-
_-
·
|
--
; -I
_ --· tC·--·
--C-·
*--
H
_
·.
---.-
F-
--
--
_
'
-- -
_-
---
=--=LL-
-
--
....
T --- ._^Ei.--
L..--- L
sI4-
__
--
= - -- __--.
Z:TZ--
t-t-
2
l__
=.-o
omD
w
U1
==_
___~
__
-·--0-+
i-I--z
,..
+
+.
-
|
_
I
_ -
F-~~
_t
t--+
t---I
|
t--
--
4
0
0
m
-
M
_
_+
_
-+
-
_
--------
,
~~ ~ ~ ~ ~
--
t
_
-
-+-
-
I__
, |+-| ,_+
,-_9 -t
~
-+-
t
C
--
I
--
-
-
§
-
---
t
-
-
-t
.
l
--
-l
-1
i
-
l
1
1
|
|
I-
::)
-
C.
w
z)
Z
E
=:-:--
___f7-4--- ___j
=-___:F
_ __
-.-4' - .
,
-
,
_
I - - -
,__
"
-t--. -
I
.
:. --
-
-.
!__
--
7'_ a
--Ii
- _
------7--1
-----T-
3C
;
Figure 30. I.R. Spectrum of A364A Glucan after Acetic Acid
Extraction (8%w/w sample concentration)
-87-
- -
,-ri-- r
.--
I
:1I - -
'
_
, ,
f--
. l
.
.
....=--:::::T
-
.---
--.
1.- ...
-i----
-
:r-
- 2--
ok
-
0c. 1
6-
--
|
2
=
7-_
_
---- -t ---
0"
0.
-
.
_
._-
-
.
--
_c-.
--: =
: A_
-J''
--__+-
0
I_
___
__
.
_ _ :_X_
.<
_
.
.
_
_
|
-
_
------
=Z4=--
__
_
....---
t --
4
t
-
-
-
I8.
uJ0-
_
t.
. -
-
_
_
--
_
_
---
=..E
----I_-$1-11-7-'=1
-- 1
·----- ·- ·---..
-----
-r
·
--..
,
~'l
,
_
I--
.-r-
-.
=
-
-
-
*-
--
--
-
-
___
-
=
-
-- r--C-
--
_
__
-r-.
·-4]
-~~ ~~ ~~
- --
-
'
-?----
4--
--
t--.
'
._-__
j
to 0
I
cc -
----
TTLif
__
I
;----
,
~-
;1I
':
I
I,
....
}
T
1"
:
I
_R-:
~. --
__' . .
--
-
t·
-·-
-"I
.
1 '7-
------
---
---1
--
-·C·T----
--
--
7-r
+-+
---- ·
rit
--
-
_
----· 1__
---·r·r-
r--
ID
!|~
F_
t~F--4·
---
1
-Z
-·-
--
-ii
--
---
---------
_:
!:
.x
__
I
_
I
--
:
-
*
I
Q
i
1
1
i 1
Im
,|
I.
_
t-
--
-Ir
---
·-
·---
-4._-
.
--_- z==_---<
-, +r
I
-+---e -- ·- -
--
t--
b
iiri.:. -4
-·
[---4
-C-·
4-- -A--t-- +::I
. I
I.
' '
---
., ,_
I
,1 1
i-:
C
-T-
I
_-I I
,;----
Ij
----
r-t
·
_
'!|
-
-.--
*·
-C
--
_
. ·,
--
--
_..____x.
, -- 4--'
--L
--
7-- . .
i
-
--
·- I ·-
-- ·-
-- · e
-- T
------
t-
__
_
--
I
I
_--
f*
c
c
c
IO'e
-
*: II[-'|-II I : 1 Il
:-T
' i
----
t ., _
-
I
-*
.. I'.
---
F-
I-_ i
t---
I
-- i
----
q-
O
1-
-z--_ I
- ---- +
-.
-- tt~~~~t,F
-_-1
=tt--
U
-
.__L-'
+rT' J
*I
;zz.
'liii1,
-1-
----
;r------- i
C3
.-
_
--
-t-1
t-t
_ I _:,i
--
_
t=t---
-- ,,-- 4.;
~
]-
_1
-
-r
-f-
., - w
. - . -K
__
A_
Z-- H
1-lo--+_
-
.___
--
-- r
0.
0
0
t. r = I
;i-. 4
-a--If_-6-0
s-1w:
.;.I
I
-
:-L
-H_--
__H
_
--
.-
--
-r
'·I
------·
-
--
:: .-
I
..~.
-t i -i ,tr----- +-.
.F
-
- --
-----
--
,-------
-
_
r~--
---1- --- -- r---1
---e -----------1----i
_
f -.
---.
;:i
-- H
LEE-i-3--
-t
1-- :_
:1- I
,
-j
-1--
-=
L
-f
_
'
- --- i --
--
=
wI
..
1
1:
Y
. -
-T; !--on
__i
__
77-2
-
__
-T ---
__
;----:
-- -- -
--_
·----
._4_.
__
S
-
:I
.
t---
' -·
-
-
-
-
- -t
-
*
.-
e
A:7,.
l
H___
__
S
-
I.'
..
--eL
---- t----l
,__
--- t-w
- --
-
_
._
H-:--
_
s
.,
,
-J- -j~
:_
_
. 4+I
'i--J
.
-i
-t--. --·-Y--t-'--1
f
._
|
ii
-:-~-R_?
1. .--
|
--
- |-
----
-_l.
.
1,C_-:-
._
8
-
_ -
_
.
.
_?-
_
=
---
.
.
_T=.-
.
-- 1-i
i-TVL
;
_-
-·-
-
_
-l--
--
I
~
---
|-
-i-.
,-
.
-3:
__
F--A- .-_
' ,4I_
T-
T--I ..
-
1
It
. ',
.
----- _
U=
---
-
|
-
*
-
-
-
-
----
-
I
.
)--1
'--^'S
'--
--
-
F
- =---
I--- -3--
-
·-.
+
--
_
9
;
r--- .-
t-
E t
tt~-~
__1-
t
re
-
-
-C-
I-
. .~-y
§ e
_
_-
_
't- --.1
-
I
.
I
-
:
I .:
I '_
I
I
t- it ==tntt=,I
"
1
4t
r
H
·
1
Li
U
.;it
;E
3:
.
Figure 31. I.R. Spectrum of 374 Glucan after Acetic Acid
Extraction (8% w/w sample concentration)
-88-
~_
·
!
.
.
.
.
.
.-
i_-_--F-
-I
C,
E
L
o0i.
-1-~~~~~~~~~~~~~~~~
0
-4----
0
Ut
- ~:
i-~L:
-·--i
..-
I ...
4_
-
----'
-
----
: .--
----:_- .--_-___-"---
0~
nc
UJ
co
~-_,.
88
IV.
_-_-_LZ
-'.....--
~.---_ -
---~ - _
0'
(0
.... .
.
I
I.
.
0-
- ~
-- I-----~~~~'
~
C.
---------4-
...
o..9
CV
-----
q_
M
--
----------
-
---,--
co
0
0
00
-
--
1
----
W
Co
,---
I
-
0
0
U,
--
--c----
7:=
--
--------
: --t--
-----,ccc--·
-----
---------
--------
I
.--,----.
I
'I
--
-"'
.---
-----··-- -r·--T--·
i-· ·- -------i:----
4--- I__ s- !__
--
C.)
-4·
---
·-----
:0
4)t
L-- ---------
-
.---
~IT-
-------+----·
--- --- ---1 -------f
-- r--------·--- --
-
-4-----
·---?--
--
----.--..
1
:_ i:!
--
EE3
---- I
--...-,--·_:___
----'--_I___
___
U: .
I
FE#±r
+9-f
o
----
-1-------
I-
00
r -1
i - -- -- I -
I ~ ~~ ~ ~
W
L _
'--
.
C-I
I
-4--
-- 1S--
-- 4---
' ~ ......... I [
w
---
:
:-----~:=~- -~----
-
-4--
1--
e'~ .
~- :-- _-+-
----- --.~~--_ -
(/).
3:
z
J
1::'.,
---.-
-----
.
-i
_
-7--"
·-------
r.- __._.---- ,
q_
w
m
---_:..T-,F-._:_?::- i -
-- :
i .:'i -~--
·.......--
;----
2
·
h-
-4
:=L]._
_7·
__
*--i
f
C.
w
:---.---
.I--
-
e
-:::I
-l--i:--
;- ---·
t.-
7.
zw
,.I
HE
_--
- -T
I
;----
I--
I
I .....
----- ·-
---
--
LZ
_7T_
·
Figure 32. I.R. Spectrum of 377 Glucan after Acetic Acid
Extraction (8% w/w sample concentration)
-89-
C
v
HO
V -H
11%
Figure 33. Cooperative Hydrogen Bonding in adjacent
Cellulose Molecules
0
__OH
HO
Figure 34. Inter-Molecular Hydrogen Bonding
within a (1-3) Glucan Molecule
-90-
An
important observation from these spectra is that all
gave
a
peak at 2.7pm adjacent to the strong hydroxide (-OH)
peak
(3.0 pm),
hydroxyl
bonded
dry
or
inter-
occurs
along
H-bonds
the
reluctance
to
cellulose
which
molecular
orientation
can
of
a
For
H-bonding
makes
this
the
800,
its
explain
consider
linked
between the closely
compound insoluble in
(1-3)-D-glucan of
is readily soluble in
explained by the fact that the
bonds
of
formation of
example,
However, laminarin, a
be
molecules
will
chain
Given the
B(1-4)-D-glucosidically
approximately
weight
This
water.
a
large
cooperative
solution.
chains
adjacent
very
the
of
Cooperative
(Figure 33).
water
ring
into
is
polysaccharide.
stacked
length
go
are
,
250,000
weight
molecular
this H-bonding to occur.
chains
glucan
that
chains arrange themselves
glucan
that
It is
in the glucans.
intra-molecularly
allow
to
geometrically
the H-bonding observed probably
discs
therefore,
possible
fact
KBr
of hydrogen
presence
the
Since the spectra obtained were of
groups.
in
samples
indicates
which
(1-3)
brings the oxygen in the glucose
close to the hydroxide on the 4thcarbon of the adjacent
glucose
thus promoting inter-molecular H-bonding.
unit
electronegative
H-bonded
to
(Figure
34).
therefore
O -atoms
hydroxyl
not
in the glucose rings are therefore
hydrogens of the same glucan molecule
Cooperative
strong
The
enough
H-bonding
to
between
is
resist H-bonding to water
molecules, hence, laminarin goes into, solution.
-91-
chains
The
explain
since
above
the
criteria
physical
however,
are
not
sufficient
properties of the yeast glucan matrix
this is composed of more complex branched chains.
theological
studies
to
have
these
elucidated
The
properties and
will be discussed later.
As
shown
in
Figures
30,31,32 extraction of the whole
samples in acetic acid resulted in partial removal of
glucan
the
(1-6)
glucan
H-bonding.
in addition to destruction of
(1-6) glucan component to
of the
in the whole glucan matrix has not been studied to
The
component
glucan
relation
The
H-bonding
date.
component
only information available(4) is that this minor
is
closely
matrix.
It
associated
is
not
if
insoluble
results
provided above infer that the highly branched
glucan
extracted
supported
high
by
the
electrostatic attractions.
the
H-bonding
fact
3(1-3)
this association is
covalent
by
or
known
the
through
relatively
bonds
to
acetic
acid
potential.
treatment
The
(1-6)
has
a
This supposition is
that this component becomes soluble
after the extraction.
Only
one
evidence into consideration.
experimental
the
major
the
aid
model can be proposed by taking all the above
alkali
of
(1-3) glucan component - with
insoluble
B(1-6)
inter-molecular
cooperative
H-bonding
into
the highly branched
bound
which
through
-
provides
H-bonding.
The model is that
crosslinking
and
a rigid insoluble matrix
(1-6) component is strongly
In
-92-
the
presence
of
this
macroscopic
solution.
matrix,
However,
cooperative
H-bonding
the
(1-6)
upon
rigorous
is
component does not go into
chemical
treatment the
disrupted and the minor component
becomes soluble in water.
An
insoluble
additional
comment
nature
the
of
that
8(1-3)
can
be
glucan
made is that the
(in
contrast
to
laminarin) is due to two factors:
1. the tight geometric arrangement of the
chain due to (1-6) crosslinks(28)
2. a high density of cooperative H-bonding
(a function of chain length/molecular weight)
-93-
_
4.0
_
4A
>
3.0
0U
)i,
a
I-
0
,.
2.0
1.0
0
concentration (g/dl)
Figure
35 : Viscosity profiles of yeast glucan comparing different
cell morphologies
-94-
2.0
I /c
1.5
1.0
0.5
1.0
2.0
3.0
4.0
5.0
6.0
7.0
8.( Do
1/lnl(r)
Figure 36 : Plot of the modified Mooney equation for A364A glucan
-95-
2.0
1/c
1.5
1.0
0.5
C
1.0
2.0
3.0
4.0
5.0
6.0
1/nlr(r)
Figure
37. Plot of the modified Mooney equationfor 374 glucan
-96-
2.0
1/c
1.5
1.0
0.5
1/Inl(r)
Figure
38 : Plot of the modified Mooney equation for 377 glucan
-97-
Figure
glucan
35
compared
division
cell
and
377
viscosity
can
behaviour
of
the
these
can
be
that
and
at
of
(critical
In fact, the
A364A glucan does not appear to reach
in the concentration range used.
A
particles.
with
respect
This
more
concise explanation of
to macrosopic glucan structure
using the plots of the linear model
by
at
in
developed
36,37,38).
inflection
be grossly explained by the shape differences
arrived
was
an
reach
a much lower concentration.
point
glucan
results
It can be clearly seen that 374
impart higher viscosities in suspension to
profile
inflection
377 glucan after inducing the
and
374
cycle block.
glucan
concentration)
an
to
glucans
A364A
the viscosity profiles of A364A
illustrates
theory
the
section
(see
Figs.
Table 6 summarizes the obtained information.
Table
6
Hydrodynamic Properties of Whole Yeast Glucan
Glucan
Sample
Regression
Coefficient
r
kl
k2
Shape Hydrodynamic
Factor
Volume
V
v (dl/g)
A364A
0.9986
0.23
0.15
2.5
0.092
0.63
374
0.9987
0.36
0.24
4.1
0.088
0.36
377
0.9974
0.37
0.20
4.1
0.091
0.45
-98-
We
can
A364A
cells
from
the
that
wild
type glucan from spherical
has a higher hydrodynamic volume, v than glucan
elongated
374
and
377
cells.
From
Mooney's
be
seen that relative viscosity increases
v.
Therefore,
an adverse behavior is observed, since
would
expect
equation
with
we
observe
can
it
hydrodynamic
overpowered
since
nr
coulter
that
374
volume
by
is
the
and
than
377
A364A
glucan.
considerably
proportional
to
glucan
higher
exp
(V)).
to
have
(This
shape
higher
effect is
factor, V,
Furthermore,
counter studies on the various glucan samples showed
377
and
374
glucan
have
a
higher
mean
effective
diameter than A374A glucan.
The
and
377
glucan,
glucan
possibly
crosslinks
points
above observations therefore indicate that the 374
particles
are denser structures than A364A
containing
between
the
a higher proportion of
8(1-3) backbone
chains.
(1-6)
These
will be elucidated and have been confirmed with other
experiments discussed below.
-99-
.I1LI,.A
-I..-J
Il
4.0
;xtraction
3.0
0
m
to
2.0
2.0
1.0
0.5
1.0
1.5
2.0
2.5
concentration (g/dl)
Figure
39 : Viscosity profile of A364A glucan showing the effect
of 3h. extraction In acetic acid
-100-
after
eYtracttinn
whole alucan
4.0
),
-6
3.0
0
o
a
2.0
1.0
0.5
1.0
1.5
2.0
2.5
concentration (g/dl)
Figure
40: Viscosity profile of 374 glucan showing the effect
of 3h. extraction
in acetic acid
-101-
Ill
-84 -
b 14
4.0
--
raction
an
3.0
0.
0
a
N.
2.0
1.0
concentration (g/dl)
Figure
41 : Viscosity profile of 377 glucan showing the effect
of 3h. extraction In acetic acid
-102-
2.0
1/c
1.5
1.0
0.5
I
1/in rq(r)
Figure
42: Plot of the modified Mooney equation for A364A glucan
-103-
2.0
1/c
1.5
1.0
0.5
C
8.0
1/In l(r)
Figure
43: Plot of the modified Mooney equation for A364A glucan
after 3h. extraction In acetic acid
-104-
2.0
1.5
1/c
1.0
0.5
I
V
1.0
2.0
3.0
4.0
5.0
6.0
1/In r(r)
Figure
44 :
Plot of the modified Mooney equation for 374 glucan
-105-
2.0
1.5
1/c
1.0
0.5
i
1/in l(r)
Figure
45 : Plot of the modified Mooney equation for 374 glucan
after 3h. extraction In acetic acid
-106-
2.0
I /c
1.5
1.0
0.5
1.0
2.0
3.0
4.0
5.0
1/In 1(r)
Figure
46: Plot of the modified Mooney equation for 377 glucan
after 3h. extraction In acetic acid
-107-
The
8(1-6)
performed
to
structural
40,
and
and
A364A
this
clarify
the
41 illustrate
glucan.
role
of
this
fraction
Figures 39,
the effects of this extraction
Before
in the
on 377
proceeding on the discussion of
particular experiment it must be noted that microscopic
extraction
of
the
glucan
revealed
allowed
throughout
of
hydrodynamic
the
plots
samples
before
and
after the
no visual (shape related) differences.
us to assume that the shape factor, V remained
constant
(nr)
extraction in 0.5M acetic acid was
integrity of yeast cell wall glucan.
observation
This
glucan
(see
the experiment, and enabled calculation
parameters
Figs.
from
the
42,43,44,45,46).
summarizes these results.
-108-
1/c
vs.
Table
1/ln
7
Table
7
Hydrodynamic Properties of Glucan after Acetic Acid
Extraction
Sample
r
kl
k2
Shape
Factor
V
Hydrodynamic
Volume
v (dl/g)
0
m
A364A
Whole
glucan
0.9999
0.27
0.23
2.5
0.106
0.46
A364A
After
extn.
0.9974
0.26
0.22
2.5
0.103
0.47
374
whole
glucan
0.9981
0.36
0.15
4.1
0.088
0.60
374
after
extn.
0.9995
0.42
0.23
4.1
0.103
0.44
377
Whole
glucan
0.9997
0.37
0.20
4.1
0.091
0.45
377
After
extn.
0.9995
0.42
0.23
4.1
0.103
0.44
An
very
and
of
initial observation is that
small
in
the
effect
fact
glucan
(1-6) extraction has a
on the viscosity profile of A364A glucan
a slight decrease in the thickening properties
occurs.
The
values
-109-
of v,
m also remain
essentially
constant.
glucan
a
has
A364A
These
results indicate that
B(1-6)
minor structural role in the glucan matrix of
cell walls and is possibly present in relatively small
quantities with respect to
On
the
a significant and converse effect was
on 374 and 377 glucan after the
observed
The
contrary
(1-3) glucan.
8(1-6)
considerably
extracted
higher
374
and
377
(1-6) extraction.
glucans
properties
thickening
exhibited
with a critical
concentration
10% and 25% respectively, lower than for whole
glucan.
extraction
The
in
reflected
volume
of
fact
the
of
words,
with
the
the
of
the
value for hydrodynamic
matrix in suspension.
maximum
unchanged
the
8(1-6) glucan can also be
the
This increase indicates a drop in the
glucan
that
essentially
size
increase
(see Table 7).
density
the
the
of
packing fraction,
glucan
indicates
particle
difference
in
Furthermore,
m , remains
that the overall shape and
remains unchanged.
rheological
In other
properties observed
B(1-6) extraction is not due to shape and size effects
but due to more subtle structural alterations.
Hence
important
we
can
structural
conclude
role
that
in
374
B(1-6)
and
glucan plays an
377
glucan and is
present in relatively high proportions.
must
be
samples
were
used to follow the extraction in acetic acid.
Before
mentioned
that I.R. spectra of the glucan
It
the extraction, the spectra showed the characteristic
8(1-6) peak at 11.0
m.
I.R. spectra of glucan samples after
-110-
the
extraction
clearly
showed
only
a very low background
at 11.0 pm, thus confirming that the extraction was
response
effective.
The
peaks
characteristic
of
B(1-3)
glucan
remained unchanged.
Table 8
Extraction of
Total
Carbohydrate
before extn.
(mg/ml)
Sample
%
8(1-3)
4.31
0.18
4.2
374
3.23
0.13
4.0
377
4.75
0.15
3.3
be
to
laminarinase
the
established.
cases
obtain
a
pronounced
information.
Since
sites
8(1-3) glucosidic
can
be
47,48,49 illustrate the effect of the
on the glucan samples.
digest
In all three
decrease in viscosity imparting ability is observed
inflection
However,
of
Figures
enzyme
structural
(1-3) lytic activity, information on
has only
availability
hour
digest of the glucan samples can also
laminarinase
used
and
0(1-6)
Total
Soluble
Carbohydrate
(mg/ml)
A364A
The
4
(1-6) Glucan
it
points
occur
at
higher
concentrations.
is clearly shown that this treatment has a more
effect
on
A364A
glucan
glucan.
-111-
than
on
374
and 377
example,
For
of
A364A glucan suspension drops by 38% whereas for 374
the
the
glucan
observed.
that
2.0 g/dl concentration, the viscosity
at
377
drop
These
is
19%
results
and for 377 glucan
agree with the earlier conclusion
and 374 glucan particles have a denser matrix than
thus
having
a lower availability of
A364A
glucan,
sites
for cleavage by the enzyme.
proportion
decrease
an 11% drop is
of
the
S(1-6)
Furthermore, an increased
crosslinks
availability
of
(1-3)
in the mutants will also
B(1-3)
more resistant matrix.
-112-
sites and provide
a
whole glucan
4.0
3.0
0
o
0
0
0
enzyme digest
-0
-4.'
U
0
h.
2.0
1.0
w
0.5
1.0
1.5
2.0
2.5
concentration (g/dl)
Figure
47:Viscosity
profile of A364A glucan showing the effect
of 4h. laminarinase digest
-113-
l
4.0
zyme digest
3.0
4'
10
a
a
i0
U.
ab
O
tJ
m
.m
e
2.0
1.0
Iv
0.5
1.0
1.5
2.0
2.5
concentration (g/dl)
Figure
48: Viscosity profile of 374 glucan showing the effect
of 4h. laminarinase
digest
-114-
can
4.0
nzyme digest
3.0
a
I
a
2.0
1.0
concentration (g/dl)
Figure
49: Viscosity profile of 377 glucan showing the effect
of 4h. laminarlnase digest
-115-
2.0
1/c
1.5
1.0
0.5
1/In q(r)
Figure
50: Plot of the modified Mooney equation for A364A glucan
after 4h. laminarinase digest
-116-
2.0
1/c
1.5
1.0
0.5
([
1.0
2.0
3.0
4.0
5.0
6.0
7.0
1/inr(r)
Figure
51: Plot of the modified Mooney equation for 374 glucan
after 4h. laminarinase digest
-117-
2.0
1/c
1.5
1.0
0.5
{)
I
1/In(r)
Figure
52 :Plot of the modified Mooney equation for 377 glucan after
4h. laminarinase digest
-118-
The
plots
of
the
linear
model
(see Figs. 50,51.52)
provide the following information.
Table
9
Hydrodynamic Properties of Glucan after Laminarinase Digest
Sample
r
k1
k2
Shape
Factor
v(dl/g)
0m
V
A364A
Whole
glucan
0.9999
0.27
0.23
2.5
0.106
0.46
A364A
After
digest
0.9998
0.14
0.21
2.5
0.057
0.27
374
Whole
glucan
0.9987
0.36
0.24
4.1
0.088
0.36
374
After
digest
0.9985
0.23
0.23
4.1
0.070
377
Whole
glucan
0.9974
0.37
0.20
4.1
0.091
0.45
377
After
digest
0.9989
0.27
0.24
4.1
0.065
0.27
The
effect
significant
of
decrease
laminarinase
in
both
the
-119-
is
0.30
reflected
in
a
hydrodynamic volume and
in
decrease
that
on
important
modified
reflected
glucan
observation of the results in Table 9, is
Mooney plot), remains essentially constant
The effect of the enzymatic
in
the
modified
To
plot.
establish
this
fact
profile
continuous
activity
since
hour
no
Figure
action
of
of
the
piece
of
53.
the
The results
This plot only illustrates the
enzyme
is
enzyme
The
on
the
essentially
substrate.
The
lost after 1 hour
further degradation of the glucan occured in the 4
incubation.
equation
of each sample was obtained.
on
plotted
are
an
was performed in which A364A glucan was subjected
laminarinase digest for a range of incubation times.
viscosity
is
of the parameter k 1 - the slope of
value
Mooney
of the glucan
properties
the hydrodynamic
on
experiment
to
the
particles with a higher proportion
laminarinase digest.
the
degradation
the
of
surface
each glucan sample, the value of k 2 (the intercept
for
after
the
due to the lysis of
m is a result of this effect.
The decrease in
the
is
The
in a densely packed matrix resistant to the
crosslinking
An
on
bonds
yields
This
particles.
enzyme.
volume
hydrodynamic
glucosidic
8(1-3)
of
fraction for all three glucan samples.
packing
maximum
(Figure
for
54)
essentially
constant
is
reflected
these results gives an important
The
information.
clearly
the plot of the modified Mooney
However,
intercept
(k2 ) has remained
and
the effect of the incubation time
in
the slope which is a hydrodynamic
-120-
parameter
hydrodynamic
of
consisting
volume,
v.
shape
the
These
factor,
V,
and
the
results are shown in Table
10.
Table
10
Effect of Laminarinase Digestion Time on the Hydrodynamic
Properties of A364A Glucan
Incubation
k
=
;V
Time
(min)
0
0.27
15
0.23
30
0.18
60
0.14
240
0.14
-121-
*
whole glucan
o 15 minutes
o
30 minutes
a
60 minutes
4.0
X
3.0
0
Io
o
I.
2.0
2.0
u
0.5
1.0
1.5
2.0
2.5
co nc entration (g/dl)
Figure
5 3 :Viscosity
profile of A364A glucan showing the effect
of incubation time with laminarinase
-122-
*
whole glucan
o
15 minutes
0
30 minutes
A
2.0
S.
.
1.5
1/c
1.0
0.5
I
1.0
2.0
3.0
4.0
1/in
Figure
5.0
6.0
7.0
l(r)
54: Plot of the modified Mooney equation for A364A glucan
- the effect of incubation time with laminarinase
-123-
8A.0
0.
II
.; 0.'
L.
O
Q
E
C
'O
0
co
.
O
0.'
0
,m
0.
10
20
30
40
s0
60
Incubation time (minutes)
Figure
55 :Linear correlation of a hydrodynamic parameter of A364A glucan
to Incubation time with laminarinase
-124-
4h
A
of the data in Table 10 is shown in Figure 54.
plot
linear
Using
incubation time can be correlated
regression,
for times up to 60 minutes.
to k
The correlation obtained is:
k1 =
-0.0021( time )
+
0.26
regression coefficient = -0.98
glucan
sample
properties
the
that for a given
this
result
this
case
A364A glucan) the thickening
can be adjusted to desired levels.
previous
properties
(in
is
of
implication
The
knowledge
(shape
factor,
of
the
initial
This requires
hydrodynamic
maximum packing fraction) of the
glucan which were obtained using capillary viscometry.
-125-
6. SUMMARY AND CONCLUSIONS
The
mutants
alkali-insoluble
374,
377
glucan
fractions
of
the
cdc
exhibit higher thickening properties than
the wild-type A364A glucan.
Glucan
isolated
from
Saccharomyces cerevisiae 374 and
377 have denser matrices than wild type A364A glucan.
S(1-6)
structural
was
also
glucan
for
also
glucosidic
plays
an
important
role in the glucan isolated from the mutants.
found
samples.
the
crosslinking
to
in
related
to
H-bonding in the whole
This H-bonding is, however, not responsible
heological
present
be
It
properties of the glucans since it was
A364A glucan which was not affected by its
absence.
Chemical
an
extraction
insignificant
effect
of the
(1-6) glucan fraction has
on
rheological properties of
the
A364A glucan.
Chemical
377
glucan
extraction of the
enhanced
its
(1-6) glucan fraction from
thickening
properties
without
altering its gross size and shape.
Laminarinase
digestion
of the glucan samples decreased
-126-
the
viscosity imparting characteristics in all 3 cases.
The
effect was more pronounced for the wild type A364A glucan.
The
effect
can
be correlated
the
glucan.
of
This
laminarinase
directly
enables
treatment on yeast glucan
to the hydrodynamic
accurate
prespecified thickening properties.
-127-
parameters
of
design of glucan with
7. SUGGESTIONS FOR FUTURE RESEARCH
The
ultimate
objectives of this line of research is to
develop:
1. genetic techniques to control/direct glucan
biosynthesis
2. a system for the production
of yeast
glucan
using spent yeast or a continuous regeneration
model
It
program
to
and
is
therefore
firstly
cell
to
formulate
a
research
in the areas of yeast genetics with respect
division
secondly
necessary
cycle
events and cell wall biosynthesis,
for the optimal extraction of glucans from the
yeast cell wall.
The
yet
work
unique
presented in this thesis establishes a simple
method
Therefore,
by
cerevisiae
cell
differences
of
can
be
to
characterize
utilizing
division
the
cycle
glucan
existing
mutants,
structure.
Saccharomyces
the
structural
glucan at different stages of the cell cycle
determined.
This
will
provide
two
pieces
of
important information:
1. mode of glucan biosynthesis during the cell
division cycle in yeast
2. extensive understanding of the structurefunction relationships of yeast glucan
The
research
approach
for
-128-
the
second objective will
utilize
with
a
similar
glucan
located
mannan
in
strategy.
extraction
the
inner
layer.
It
enzymes
or
mutants
blocked
chemical
in
mannan
to
study
mutants
temperature
the bulk of the glucan is
cell wall and is covered by an outer
extraction.
characterized(5).
glucan
that
is therefore unavailable for cleavage by
and
mannan
is
The major problem associated
Saccharomyces cerevisiae
biosynthesis have been isolated
These
mutants can therefore be used
extraction/regeneration.
can
be
sensitivity,
mutagenized
cell
division
mutants
with altered glucan structure.
with
a
block
will
contain
in
a
the
described,
more
a
a
higher proportion of
two
cycle
screened
mutants
for
or
For example, mutants
(1-6) glucosyl transferase activity
more susceptible to cleavage by
Although
and
Furthermore,
part
(1-3) glucan which is
(1-3) glucanases.
research
approach
has
been
combination of the two approaches will yield a
conclusive
understanding
of
structure-function relationships.
-129-
glucan formation and its
References
1.
Pei-Syan, L. (1981), Rheological Properties of Yeast
Wall Glucan. S.M. Thesis, Dept. of Nutrition and Food
Science, M.I.T.
2.
Fleet, G.H., and Manners, D.J. (1976), Isolation and
composition of an alkali soluble glucan from the cell
walls of Saccharomyces cerevisiae .J.Gen.Micro. 94,
180-192.
3.
Manners,
Masson,
D.J.,
and
A.J.,
J.C.
Patterson,
(1973a), The structure of a (1-3)-D-glucan from yeast
cell walls. Biochem. J. 135, 19-30.
4.
D.J.,
Masson,
H., and Lindberg.
Manners,
Bjorndahl,
(1-6)-D-glucan
J.C.,
A.J.,
Patterson,
(1973b), The structure of
from yeast cell walls. Biochem. J.
135,
31-36.
5.
Ballou,
of the
and biosynthesis
(1976), Structure
C.E.
cell
Mannan component of the yeast
Microb. Physiol. 14, 93-158.
envelope.
Adv.
6.
Wessels, J.G.H. (1979), Evidence
Sietsma, J.H., and
-glucan in a
for covalent linkages between chitin and
fungal wall. J. Gen. Micro. 114, 99-108.
7.
Sietsma, J.H., and Wessels, J.G.H. (1981), Solubility
-D-glucan
in fungal walls:
-D/(1-6)of
(1-3)of presumed linkage between glucan and
Importance
chitin. J. Gen. Micro.
8.
E.,
Cabib,
yeast
125, 209-212.
Robert,
and
(1982), Synthesis
R.
of the
wall and its regulation. Ann. Rev. Biochem.
cell
51, 763-793.
9.
Rogers,
Perkins,
H.J.,
and Wand,
H.R.,
J.B.
(1980),
Microbial Cell Walls and Membranes. Chapman and Hall
(editors), London, New York.
10.
Molano,
J.,
Distribution
Bowers,
of
B.,
and
Cabib,
E.
(1980),
chitin in the yeast cell wall. J. Cell
Biol. 85, 199-212.
11.
Kopecka,
M.,
Phaff,
H.J.,
and
Fleet,
G.H.
(1974),
Demonstration of a fibrillar component in the cell wall
of the yeast Saccharomyces cerevisiae and its chemical
nature. J. Cell Biol. 62, 66-76.
-130-
12.
Phaff, H.J. (1971), Structure and Biosynthesis of the
Yeast Cell Envelope. The Yeasts. A.H. Rose and J.S.
Harrison (editors), New York: Academic Press, 2, 135.
13.
Sentandreu, R.,Victoria Elorza, M., and Villanueva,
J.R. (1975), Synthesis of yeast wall glucan. J. Gen.
Micro., 90, 13-20.
14.
Victoria
Elorza,
M.,
Lostau,
C.M., Villanueva,
J.R.,
and
Sentandreu,
R.
(1976),
Cell wall synthesis
regulation in Saccharomyces cerevisiae. Effect of RNA
and protein inhibition.
Biochimica et Biophys. Acta,
454, 263-272.
15.
Lopez-Romero,
E.,
and
Ruiz-Herrera,
J.
(1977),
Biosynthesis of
~-glucan by cell-free extracts from
Saccharomyces cerevisiae. Biochem. Biophys. Acta, 500,
372-784.
16.
Shematek,
F.M.,
Braatz,
J.a.,
and Cabib,
E. (1980),
Biosynthesis of the yeast cell wall. 1. Preparation and
properties of
(1-3) glucan synthetase. J. Biol. Chem.
255, 888-894.
17.
Shematek,
F.M.,
and Cabib,
E. (1980), Biosynthesis
of
the yeast cell wall. 2. Regulation of
(1-3) glucan
synthetase by ATP and GTP. J. Biol. Chem. 255, 895-902.
18.
19.
Hartwell, L.H. (1967), Macromolecule synthesis in
temperature-sensitive mutants of yeast. J. Bact. 93,
1662-1670.
Hartwell,
L.H.,
Mortimer,
R.K.,
Culotti,
J.,
and
Cullotti,
M. (1973), Genetic control of the cell
division cycle in yeast. V. Genetic analysis of CDC
mutants. Gen. 74, 267-286.
20.
Nurse,
In:
P.
(1981), Genetic Analysis of the Cell Cycle.
Genetics
as a Tool in Microbiology.
31st Symposium
of Soc. for Gen. Microb., Cambridge.
21.
Bird, R.B., Stewart,
W.E.,
and Lightfoot,
E.N.,
(1960),
Transport Phenomena. John Wiley and Sons, ed.
22.
23.
Einstein,
A.
(1906),
molekuldimmensionen.
Ann.
289-306.
Mooney,
M.
suspension
(1951),
of
The
spherical
Eine neue bestimmung
Phys., Paris
IV ,
viscosity
der
19,
of a concentrated
particles. J. Colloid. Sci.,
6, 162-170.
-131-
24.
Biomaterials Science and Engineering Laboratory, Food
Rheology: Principles and Practice, 1982.
25.
Van
Wazer
et
al.
(1963),
measurement, Ed., Interscience.
26.
Langer, R.S., and Thilly, W.G. (1981), Analytical
Practices
in
Biochemistry.
M.I.T.
Department of
Nutrition and Food Science.
-132-
Viscosity
and
flow