Co-authors: Yi Peng, Feng Wang
advertisement

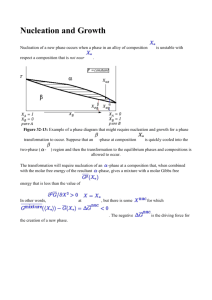
Yilong Han, yilong@ust.hk Co-authors: Yi Peng, Feng Wang Nucleation in solid-solid transitions of colloidal crystals Solid-solid phase transitions between different crystalline structures are ubiquitous in nature, but their kinetic pathways and mechanisms present formidable challenges for theory, simulation and experiment. Here we directly imaged the solid-solid transitions in colloidal thin films composed of diameter-tunable NIPAM microspheres with single-particle resolution by video microscopy. We discover a surprising two-step diffusive nucleation behavior for transitions from square- to triangular-lattices with an intermediate liquid stage. The observations and resulting theoretical analysis suggest that, provided solid-liquid interfacial energies are sufficiently small, s-s transitions in most traditional metals and alloys should follow this two-step nucleation with intermediate liquid stage, and should generally arise in 2D, 3D and thin-film single crystals and polycrystals. The nucleation precursors are particle-swapping loops rather than structural defects, which, in turn, provide a new relaxation mode that makes s-s transitions easier and faster. This new kinetic factor controlling the s-s transition rate has never been considered and should be incorporated in future s-s transition theories. Applying a small anisotropic strain can reduces the liquid nucleus size. Above a threshold of the applied strain, the intermediate liquid nuclei vanished. Instead, a few pairs of dislocations were first generated from the square lattices as nucleation precursors, which triggered tens of particles to collectively transform to a triangular-lattice nucleus and then grew diffusively. This martensitic transformation at the early stage and the diffusive nucleation at the later stage is another novel type of kinetic pathway in solid-solid transition. In addition, we observed that the coherent and incoherent facets of the evolving nuclei exhibit different energies and growth rates which can dramatically alter nucleation kinetics. The coalescence of two crystalline nuclei exhibits different behaviors for different lattice angles. Nucleation in Solid-solid Transitions of Colloidal Crystals Yilong Han 韩一龙 Department of Physics CMDS13, Salt Lake City, 2014 Introduction Two-step nucleation One-step nucleation Other kinetics Solid-solid Transitions widely exist in nature… Steel-production Man-made Diamond … Earth science Nano-materials Classification Military transformation (Martensitic): all particles move collectively, e.g. Civilian transformation (Diffusive): particles diffuse from mother phase to daughter phase. Nucleation: a free energy barrier ∆G = −V ∆µ + Aγ + Estrain − Edefect only for crystalline mother phase Difficulties in Solid-solid Transitions Theory: lack a group-subgroup relationship in symmetry. Simulation: small systems (ambiguous results) anisotropic pressure catastrophic transition at strong superheating to speed up the sluggish dynamics, but they promote martensitic transformation and suppress nucleation. Atomic experiment: X-ray & STM cannot resolve nucleation process, no single-particle dynamics. Colloid— One Class of Soft Material What are Colloids? — small particles dispersed in a solution Particle size: 10nm −10µm, kBT dominated, Brownian motion… milk, inks, paints, blood, smoke… 1.6 micron silica spheres Colloids as Model Systems Science 292, 258 (2001) Science 314, 795 (2006) Nucleation in crystallization Sublimation of colloidal crystals Science 309, 1207 (2005) Heterogeneous melting of colloidal crystals Colloidal Particle → Big Atom — watch each atom! Thermodynamic variable is volume fraction φ instead of temperature. Science 270, 1177 (1995) Science 287, 5453 (2000) Glass transition Diameter-Tunable NIPA Microgel Spheres in Water NIPA: N-isopropyl acrylamide heat water squeezed out ~96% water; ~ 4% NIPA polymers Dynamic light scattering pair potential Look into the Bulk focal plane Objective The refractive indexes of spheres and water are very close. Phase Diagram of Hard-Sphere Thin Films 1△ 2□ 2△ 3□ 3△ 4□ 4△… M. Schmidt and H. Löwen, Phys. Rev. Lett. 76, 4552 (1996). H/σ σ↓⇒ n□ → (n-1)△ Phase behavior is controlled by volume fraction φ and film thickness H/σ. φ A. Fortini and M. Dijkstra, J. Phys.: Condens. Matter 18, 371 (2006) Sample Preparation 1△ 2□ 2△ 3□ 3△ 4□ 4△… e.g. 4 layers at the center, 6 layers at the edges in a (2cm)2 sample ⇒ uniform thickness in 0.1mm region Mechanical and thermal anneal >106-particle large crystal domain How to heat ? Transitions always start from interface. … 4□ 4△ A focused beam of light heats the interior of a crystal domain. Heated region ∆T = 1.6°C Steady temperature reached in 2 s 5□ 5△ … ~80µm Introduction Two-step nucleation Y. Peng, F. Wang, Z. Wang, A. Alsayed, Z. Zhang, A. G. Yodh and Y. Han*, Nature Materials, in press One-step nucleation Other kinetics ‘Homogeneous’ Nucleation Two steps: 5□ ⇒ liquid ⇒ 4△ Nucleus precursor: Particle-swapping loops instead of defects This novel relaxation mode makes transition in solid easier. Diffusive Nucleation 50× real time Lindemann Parameter 0.02 0.2 Transition Path: metastable 5□ crystal ⇒ post-critical liquid nucleus (metastable) ⇒ 4△ nucleus ⇒ 4△ crystal (stable phase) Heterogeneous Nucleation Nucleation from dislocations Nucleation from a grain boundary Diffusive Nucleation on a Grain Boundary 100× real time Lindemann parameter 0.02 5□ crystal ⇒ liquid nucleus ⇒ 4△ nucleus ⇒ 4△ crystal θ1 θ2 lattice orientation g.b. θ1 ≠ θ2 ⇒ asymmetric nucleus 0.2 Why □ ⇒ liquid ⇒ △ ? ∆G = −V ∆µ + Aγ + Estrain = −V ( ∆µ − ∆ε ) + Aγ Dominates in large nuclei Dominates in small nuclei liquid is more favorable for small nuclei ⇔ γliquid-□ < γ△-□ γ□-liquid < γ△-□ γ□-liquid > γ△-□ θ Why □ ⇒ liquid ⇒ △ ? ∆G = −V ∆µ + Aγ + Estrain = −V ( ∆µ − ∆ε ) + Aγ Dominates in large nuclei Dominates in small nuclei liquid is more favorable for small nuclei ⇔ γliquid-□ < γ△-□ γ□-liquid < γ△-□ θ 0 liquid γ□-liquid > γ△-□ △ Why □ ⇒ liquid ⇒ △ ? ∆G = −V ∆µ + Aγ + Estrain = −V ( ∆µ − ∆ε ) + Aγ Dominates in large nuclei Dominates in small nuclei liquid is more favorable for small nuclei ⇔ γliquid-□ < γ△-□ Hold in 2D, 3D and thin films (wall-nucleus interface can be absorbed into the bulk term) Hold with or without defects (Edefect = constant) Ostwald’s step rule Wilhelm Ostwald (1853-1932) Intermediate States in Crystallization liquid with middleranged order PNAS 107, 14036 (2010) liquid dense liquid droplet Small BCC nucleus, Science 277, 1975 (1997) PRL 105, 025701 (2010) PRL 41, 702 (1978), PRL 75, 2714 (1995) classical nucleation theory FCC nucleus Intermediated States in S-S Transitions intermediate state crystalline lattices (with group-subgroup relations) martensitic nucleation liquid (highest symmetry) observed in molecular crystals observed in colloidal crystals Why not observed in simulations? Small system, strong superheating or anisotropic stress promotes martensitic transformation and suppresses two-step nucleation. Intermediate liquid was only suggested in a graphite-diamond experiment: Bull. Mater. Sci. 24, 1-21 (2001). Liquid in S-S Transitions of Metal and Alloy? γliquid-solid < γsolid-solid ⇒ liquid is more favorable for small nuclei For most metals and alloys: solid-liquid γ ~30 -250 mJ/m2 < solid-solid γ ~ 500-1000 mJ/m2 ⇒ Intermediate metastable liquid should exist Introduction Two-step nucleation Facet growth, critical size … One-step nucleation Other kinetics Three Types of S-S Interfaces small nuclei: more irregular shape medium nuclei: more circular large nuclei: faceted Coherent low interfacial energy γ Semi-coherent Incoherent high γ Facet Growth Speed 730s Incoherent b0 a0 Semi-coherent c0 d0 e0 c 796s r c0 d Coherent f0 − coherent coherent ⇔ v||incoherent < v|semi < v | || b a e f v⊥incoherent > v⊥semi −coherent > v⊥coherent ⇒ elongates along the coherent facet (lower surface energy) Coherent Facet Pinned During Shrinking Switch off the local heating Not a barrier-crossing process ⇒ No intermediate liquid Broad Angle Distribution ⇒ Not Martensitic 50 experiments A typical method to identify martensitic in molecular crystals. Critical Nucleus Size Method 1 Method 3 Method 2 Apply a Stress (small flow < 1 particle/100 sec) Liquid vanishes at flow > 0.007 µm/s ! Introduction Two-step nucleation One-step nucleation Under small flow (anisotropic stress) Other kinetics Martensitic + Diffusive Nucleation One-Step Nucleation Flow Transition Path: 5□ crystal ⇒ 4△ nucleus ⇒ 4△ crystal ‘One-step’ Nucleation in a Defect-Free Region 394s 398s 409s 400s 5μm 415s 460s 420s 45o Martensitic Diffusive One-step: n□ → (n-1)△. The nucleus precursor is dislocation pairs which glide as a “zipper” to trigger more pairs. The later growth is diffusive although with a fixed angle 45°. Near a Dislocation Similar to defect-free regions: martensitic first, then diffusive nucleation Parameter Regimes for 1-step & 2-step one-step two-step Flow in colloids (Mg, Fe)2SiO4 in Earth’s mantleα-lattice ⇒ γ- lattice Low stress: Diffusive. High stress: Martensitic P.C. Burnley & H.W. Green II, Nature 338,753 (1989) Stress in molecular crystals 1-step vs 2-step Nucleation Flow rate Diffusive ≈ 0 (<0.01 µm/s) Martensitic small (0.01-0.1 µm/s) Nucleation path two-step: civilian ‘one’-step: military + civilian No dislocation pairs 45o Intermediate state liquid nucleus Precursor swapping loops Angle between two random lattices Nucleus shape evolution circular → faceted ellipse → parallelogram Most above behaviors in defect-free regions also hold near dislocations or grain boundaries. Introduction Two-step nucleation One-step nucleation Under small flow (anisotropic stress) At some tri-junctions (can have no flow) Other kinetics 5 □ ⇒ 4△ at a Trijunction all three facets are coherent, γcoherent < γ□ -liquid ⇒ no liquid Introduction Two-step nucleation One-step nucleation Other kinetics Nuclei coalescence Nuclei Coalescence 1: liquid + liquid Can merge then transform to △, or transform to △ then merge. Liquids formed around vacancies are more mobile than those from dislocations. No attraction/repulsion between liquids and dislocations/g.b. Nuclei Coalescence 2 & 3: solid + solid (large / small angle) B-D: large angle between two △ lattices grain boundary ⇒ propagate through small nucleus E-H: small angle between two △ lattices dislocations ⇒ diffuse into large nucleus Nuclei Coalescence 4: solid + solid (// lattices) When distance is ~5 particles, □ lattice in between rotates and collectively transforms to △ Nuclei Coalescence 5: solid + solid (⊥ lattices) small △ nucleus ⇒ liquid ⇒ absorbed by big △ nucleus Why liquid? Summary 1st experiment on solid-solid transition with single-particle dynamics. Discovered a novel intermediate liquid state and understood its mechanism which should hold in 2D, 3D, thin films, most metals & alloys, with or without defects. A novel relaxation mode before s-s transition (loop-motion as nucleus precursor). A novel (martensitic + diffusive nucleation) kinetic path under flow. Acknowledgement HKUST Ph.D. Students: Yi Peng 彭毅 Feng Wang 王峰 Ahmed Alsayed, Arjun Yodh synthesized NIPA spheres