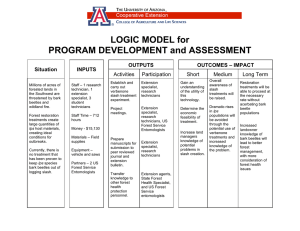
Proceedings of a Workshop on Bark Beetle Genetics: Current Status of Research
advertisement

United States Department of Agriculture Forest Service Pacific Southwest Research Station General Technical Report PSW-GTR-138 Proceedings of a Workshop on Bark Beetle Genetics: Current Status of Research May 17-18, 1992, Berkeley, California Hayes, Jane L.; Robertson, Jacqueline L, tech. coords. 1992. Proceedings of a workshop on bark beetle genetics: current status of research. May 17-18, 1992, Berkeley, California. Gen. Tech. Rep. PSW-GTR-138. Albany, CA: Pacific Southwest Research Station, Forest Service, U.S. Department of Agriculture; 31 p. The Proceedings reports the results of a workshop focusing on the topic of bark beetle genetics. The workshop evolved because of the growing interest in this relatively unexplored area of bark beetle research. Workshop participants submitted brief descriptions of their views of the current status of bark beetle genetic research and needs for the future. Contributions vary from broad overviews to detailed descriptions of specific projects. A common theme is the potential for rapid advances in this area of research given recent technological advances in the field of genetics. Also included is an extensive reference list. Retrieval Terms: Dendroctonus species, Ips species, scolytid genetics, taxonomy Dedication We dedicate this report to the memory of Gerald Lanier, a valued colleague to all who share an interest in bark beetle research, and a dear friend of many of the workshop participants. It is particularly fitting that we pay tribute to Gerry for his pioneering efforts in bark beetle genetics and in recognition of his lasting contributions in the area of scolytid systematics. Technical Coordinators: Jane L. Hayes is a research entomologist and Project Leader of the Ecology, Behavior, and Management of Southern Pine Beetle Unit of the Southern Forest Experiment Station, USDA Forest Service, 2500 Shreveport Highway, Pineville, LA 71360. Jacqueline L. Robertson is a research entomologist and Project Leader of the Insect Biochemistry, Genetics, and Biometry Unit of the Pacific Southwest Research Station, 800 Buchanan Street, Albany, CA 94710. Acknowledgments: We thank Sue Moore for handling the innumerable workshop arrangements and mailings, and for assembling the draft report. We are also grateful to David Wood and the School of Forestry, University of California, Berkeley, for accommodating the Workshop on Day 1 and to Garland Mason and the Pacific Southwest Research Station for accommodations on Day 2. We extend special thanks to Maureen Stanton for providing the "outsider's" point of view and invaluable insight during the course of the Workshop. Publisher: Pacific Southwest Research Station Albany, California (Mailing address: P.O. Box 245, Berkeley, California 94701-0245 Telephone: 510-559-6300) November 1992 Proceedings of a Workshop on Bark Beetle Genetics: Current Status of Research May 17-18, 1992, Berkeley, California Jane L. Hayes and Jacqueline L. Robertson, Technical Coordinators CONTENTS Preface....................................................................................................................................................iii An (Ecologically Biased) View of the Current Status of Bark Beetle Genetics and Future Research Needs ............................................................................................................... 1 Jane L. Hayes and Jacqueline L. Robertson Qualitative Genetics of Mountain Pine Beetle in Central Oregon .......................................................... 3 Lula E. Greene Biochemical Indicators of Population Status of Pine Bark Beetles ........................................................ 4 M. Carol Alosi and Jacqueline L Robertson Spatial Analysis in Bark Beetle Research ............................................................................................... 5 Haiganoush K. Preisler Bark Beetle Genetics .............................................................................................................................. 6 Wyatt W. Anderson and C. Wayne Berisford Isozyme Studies of Bark Beetle Population Genetics and Systematics .................................................. 7 Molly W. Stock, Gene D. Amman, and Barbara J. Bentz Biosystematics of Ips mexicanus and Ips plastographus (Coleoptera: Scolytidae) and Their Fungal Symbionts .................................................................................................................. 10 David L Wood, Andrew J. Storer, Thomas R. Gordon, Steven J. Seybold, and Marion Page The Status of Scolytid Genetics―A Reductionist's View .................................................................... 12 Steven J. Seybold Genetic Characters for Bark Beetle Phylogenies .................................................................................. 14 James H. Cane Cuticular Hydrocarbon Research .......................................................................................................... 16 Marion Page CONTENTS Bark Beetle Genetics―An Overview ................................................................................................... 17 Stephen Teale and Barbara Hager Current Status and Critical Research Needs in Scolytid Genetics ........................................................ 18 Kenneth F. Raffa Genetic Basis of Semiochemically Mediated Bark Beetle-Predator Coevolution: Implications for Developing Mass-Trapping Technologies ............................................................ 20 Robert A. Haack, Daniel A. Herms, Bruce D. Ayres, Matthew P. Ayres, and M.L Doozie Snider Population Genetics of Spruce Bark Beetle Ips typographus (Col., Scolytidae) and Related Ips Species.................................................................................................................... 22 Christian Stauffer, Renate Leitinger, and Erwin Fuehrer Novel Bark Beetle Research Possible with New Genetic Techniques .................................................. 23 Ken Hobson and Owain Edwards Systematics Research ............................................................................................................................ 25 Donald E. Bright References ............................................................................................................................................. 26 Workshop Participants .......................................................................................................................... 31 ii USDA Forest Service Gen. Tech. Rep. PSW-138. 1992. Preface This report is the result of a workshop focusing on the topic of bark beetle genetics held May 17-18, 1992, in Berkeley, Calif. Bark beetles play a significant role in pine forest ecosystems throughout the world; this group of insects often causes severe economic damage and loss of wildlife habitat. In general, bark beetles threaten forest health in the short term. In the long term, however, they have served as natural agents to thin overcrowded stands in pine forests. Although bark beetles have been the focus of intensive research efforts, relatively little effort has been devoted to understanding bark beetle genetics. Our Workshop evolved because of (and reflects) the growing interest in this relatively unexplored area of bark beetle research. The objectives of this workshop were fourfold: 1. To promote interaction among researchers and exchange of ideas; 2. To foster collaboration; 3. To summarize the state of knowledge; and 4. To identify research needs for the future. To accomplish the last two goals and to set the stage for discussions, participants were asked before the workshop to submit brief descriptions of their views of the current status of bark beetle genetics research and the needs for the future. How this assignment was fulfilled was as diverse as the participants' approaches to the topic. Contributions varied from broad overviews to detailed descriptions of specific projects. As a whole, these statements provide an insightful description of the state of knowledge and research in the area of bark beetle genetics. Students of bark beetle biology will find this review and the combined reference list a valuable source of current information on this topic. Significant advances have been made in the available technology in the decade since the topic of bark beetle genetics was first reviewed (Mitton and Sturgeon 1982). Our knowledge of bark beetle biology has advanced slowly; however, as many contributors indicate, the potential for more rapid advances is imminent. During the two-day workshop, participants gave brief presentations on the status of their ongoing research efforts. A general group discussion of research needs and goals followed. A topic of considerable concern to the group was the lack of recognition of the need and support for a continuous effort in the area of systematics. Declining support for systematics work has resulted in lack of training and career opportunities for the next generation of skilled systematicists. Without a commitment by academic and funding institutions to support this fundamental research, we face a future without advances in this essential specialty area. USDA Forest Service Gen. Tech. Rep. PSW-138. 1992. To structure our general discussions, priority research needs were identified by participants. The group recommended that research be initiated or continued on these 15 topics: • understanding ancestry of scolytid species • biosystematics of important species, including symbionts • monitoring endemic vs. epidemic conditions, and understanding processes that trigger changes • spatial statistical techniques for mapping geographic patterns of gene frequency (and changes in relation to population dynamics of bark beetles) • interaction between phylogeography and population growth status • genetics of variation in pheromone systems • understanding individual variation in pheromone and other qualitative characteristics of individual beetles • genetics of variation in interaction with natural enemies genetics of cold-hardiness • understanding selection pressure (i.e., stage and mechanism) • artificial rearing techniques for major species (especially Dendroctonus spp.) • molecular population analyses that focus on phylogeography, gene flow, paternity • gene expression (description of genome, protein and enzyme level, transcription) for key traits • survey of polymorphisms identified from different analytical techniques • applying information from other well-researched systems (e.g., Drosophila, Tribolium) to bark beetle genetics Because items on this list were intentionally expressed in relatively broad terms and covered multiple levels of analysis (i.e., molecular to population to phylogenic levels), we did not attempt to refine or prioritize this general list any further. Instead, we sought to identify more specific questions and technological advances necessary to address these questions. This discussion is summarized in table 1. Through this report we seek to continue the productive efforts described at this workshop: to share information and ideas, and to encourage the pursuits of others with interests in the challenges of research into genetics of bark beetles. All participants welcome further inquiries―addresses and telephone numbers are provided. We hope that this publication will aid those seeking to learn more about the topic, and we encourage efforts in studying new topics that are not mentioned here. Jane L. Hayes Jacqueline L. Robertson Technical Coordinators iii Table 1―Specific questions and technological advances necessary to address priority research needs. Levels of Analysis Specific Questions Techniques 1. Molecular genetics a. Study patterns of expression for genes involved in semiochemical pathways, e.g., detoxification, pheromones. Determine more biochemical pathways. b. Study genetics of development, e.g., stage-specific gene (adult vs. juvenile, overwintered vs. pre-winter, etc.) Develop DNA probes associated with key developmental events. c. Screen for specific patterns of expression associated with dispersal vs. feeding adults; cold hardiness; different seasonal cohorts; host-tree species. Develop systems to describe transformation of trees or beetles. d. Development of molecular markers for ecological genetics. RAPDS, VNTR loci, mtDNA e. Studies of sex ratio anomalies (e.g., in Ips spp. etc.) (see Level 2) 2. Quantitative genetics of ecologically important traits. Once appropriate rearing techniques are established for controlling environmental and genetic background, focus on traits, establish role of genes and environment as influencing variations among individuals. Need rearing techniques for studying similarity within "constant" environment and for manipulation of environment. a. Semiochemicals, correlations between them (production and response profiles) Artificial media vs. outdoor trees, logs. b. Cuticular hydrocarbons, e.g., changes during development and relationship with host compounds Explore Kermit Ritland's technique for h2 estimation based on electrophoretic data. c. Cold-hardiness: examine patterns of expression in larvae d. Microbial interactions: involvement with overcoming host defenses, increased attack success, pheromone synthesis e. Morphological/physiological traits (e.g., elytra loadings, lipid contents) Fluctuating asymmetry analysis. f. Genetics of host selection g. Trees: genetic and environmental determinants of susceptibility 3. Selection and population dynamics a. Stages and sources of selection in mortality during the life cycle due to host stress and predation and parasitism at various stages. b. Dynamics of beetle interactions (e.g., competition) as a function of population phase in the context of tree interactions. c. Do patterns of selection differ between population phase? (e.g., rvs. k-type) Rearing techniques. d. Potential for selection experiments for key characterization (e.g., thick vs. thin phloem) (See level 4c and d). dispersal: density and "phase"-dependency Techniques for following cohorts of beetles below the bark (acoustics, radiography, infrared detection) population structure: “phase”-dependency continued iv USDA Forest Service Gen. Tech. Rep. PSW-138. 1992. Table 1, continued Levels of Analysis Specific Questions Techniques 4. Population structure and phylogeography a. Calculate hierarchical F-statistics: between geographic regions, between populations, within populations (between host plants, pheromone "groups", emergence groups, families) (See Level 5a) Also relevant to Biosystematics b. Exploit existing data sets: Dendroctonus spp., I. pini (requires systematic sampling and coordination between groups). Spatial statistics development. c. Spatial analysis, interaction with GIS data now available. d. Dependence of population structure on: 1. local density 2. stage of population growth (See Level 3d) e. Direct analysis of dispersal, gene flow in relation to elemental labelling rare or unique alleles, mutants (beetles or symbionts) (See Level 3d) 5. Phylogenetic reconstruction and biosystematics. a. Species taxonomy of bark beetles is relatively complete, but need phylogenetic reconstruction. b. Focus on Ips, Dendroctonus, especially 1) species relationships within these groups, and 2) ancestral relationships between them. Elemental labelling (Mark-Release-Recapture). Develop discrete characters for: 1. morphometrics 2. mtDNA, nuclear markers 3. sequence data on rib. DNA (larger scale) 1. develop discrete characters for cladistic analysis, 2. continue applying isozymes. c. Based on phylogeny, consider evolution of following traits: Apply new methods of phylogeny reconstruction and analysis. 1. pheromone production and composition (semiochemistry), 2. behavior, including isolation mechanisms, 3. aggregation response, and 4. cuticular hydrocarbons USDA Forest Service Gen. Tech. Rep. PSW-138. 1992. v An (Ecologically Biased) View of the Current Status of Bark Beetle Genetics and Future Research Needs1 Jane L. Hayes and Jacqueline L. Robertson2 Bark beetles are commonly considered the most destructive insect pests in pine forests in North America. Despite the relatively intense interest in this group because of their pest status and uniquely complex ecological and evolutionary relationships, many aspects of their biology, including genetics, are poorly understood. Most genetic research on bark beetles has concerned inferences about the evolution of species, especially the phylogeny of important genera (e.g., Cane and others 1990b, Lanier 1967, 1981, Lewis and Cane 1990b, Mitton and Sturgeon 1982). Relatively little emphasis has been placed on population genetics, particularly on genetic variation within and among populations over time and space (reviewed in Milton and Sturgeon 1982, and ref. below). The natural history of this group is probably the single most significant deterrent to studies of population genetics, because sampling and laboratory rearing is either difficult or impossible. Improved sampling of adults has paralleled advances in semiochemical research, which in turn have prompted and permitted recent investigations into variation in the production and response to semiochemicals at the population level (e.g., Birgersson and others 1984, Herms and others 1991, Miller and others 1989, Teale and Lanier 1991). However, most of the life cycle is spent under bark, limiting observations and necessitating destructive sampling of brood in order to obtain requisite estimates of fitness (e.g., fecundity, age to first reproduction). Most species are relatively intractable in the laboratory, thus precluding classical genetic studies, estimations of heritability, and development of molecular genetics techniques based on phenotypic studies. It is not surprising that recent advances in our understanding of both molecular and phenotypic variation are concentrated in the more easily reared species such as Ips spp. (e.g., Cane and others 1990b, Gast 1987, Lanier and others 1972, 1980, Miller and others 1989, Piston and Lanier 1974, Stauffer and others 1992a; but see Langor and Spence 1991, Teale 1990, Teale and Lanier 1991). The newest molecular techniques (e.g., Restriction Fragment Length Polymorphism and Randomly Amplified Polymorphic DNA) may mitigate sampling deficits, are likely to lead to gene mapping, and vastly accelerate the molecular exploration of genetic variation. Even with these advanced techniques, however, dramatic advances depend on novel application and well1 An abbreviated version of this paper was presented at the Bark Beetle Genetics Workshop, May 17-18, 1992, Berkeley, California. 2 Supervisory Research. Entomologist, Southern Forest Experiment Station, USDA Forest Service, 2500 Shreveport Highway, Pineville, LA 71360, and Supervisory Research Entomologist, Pacific Southwest Research Station, USDA Forest Service, Albany, Calif. USDA Forest Service Gen. Tech. Rep. PSW-138. 1992. designed experimentation. Ultimately, these results must be tied to ecologically relevant characteristics (phenotypic expression). Emphasizing an ecological genetics approach, our (other team members include Alosi, Greene, and Preisler, see contributions this proceedings) investigations have involved estimates of both genotypic and phenotypic variation within and among bark beetle populations and communities, as well as across ecologically similar species (mountain, western, and southern pine beetles). Our research includes aspects of the technology such as starch gel electrophoresis that were used in the earlier studies of differences between populations (e.g., Roberds and others 1987, Stock and others 1979, 1984). Besides genetic inferences made by electrophoresis, we are examining differences in the levels of the key enzymes that help to adapt beetles to the presence of toxic secondary chemicals in their host trees (see Alosi this proceedings). In contrast with previous gross examinations of terpene levels in host trees (Coyne and Lott 1976, Smith 1975)―their primary defense― we are examining differences in terpene composition by means of more sensitive analyses to detect and monitor changes consistent with changes in population structure. To date, information acquired from five years (1985-90, see Greene this proceedings) of monitoring mountain pine beetle populations in Central Oregon suggests that patterns of esterase enzymes detected by electrophoresis have shifted concomitantly with a shift in population status from endemic to epidemic and back to endemic. In addition, quantities of esterases measured biochemically have changed, even for mountain pine beetles in the same host species. Glutathion S-transferase levels also seem to be involved in the transition. We have initiated (1991-present) comparable studies with western and southern pine beetles and begun investigations of phenotypic variation between and within populations including analyses of behavioral (pheromone response), physiological (lipid content) and morphological (fluctuating asymmetry) variation. These studies represent modifications to previous efforts to quantify and relate phenotypic variations to genotypic variation among beetle populations (e.g., Langor and Spence 1991, Sturgeon and Mitton 1986). For example, morphological variability within individuals may provide a valuable early guide to changes in beetle quality. Fluctuating asymmetry (nondirectional deviations from bilateral symmetry) has been shown to be an effective and inexpensive tool for detecting stress in natural populations (Leary and Allendorf 1989) and in cultures (Clarke and McKenzie 1992), because it provides a reasonably direct measure of developmental stability (Palmer and Strobeck 1986). Environmental and genetic perturbations could affect fluctuat- 1 ing asymmetry analogously, i.e., as the level of stress of any kind increases, fluctuating asymmetry and its variability should increase provided that the stress is sufficiently intense to produce an effect (Parsons 1990a, b). In addition, in most genetic studies done to date, standard statistical methods involving chi-square comparisons of observed gene frequencies versus those predicted by HardyWeinberg equilibrium have been used for data analyses. Our investigations emphasize the use of exploratory statistics to examine actual numbers rather than frequencies (see Preisler this proceedings). Uses of these new procedures will permit us to model patterns in biotic communities of which bark beetles are a part, to estimate realistic models of species overlap, and to formulate causal hypotheses about changes in bark beetle populations in transition from endemic to epidemic states. Priority Research Needs 1. Long-term (multi-generation, multi-season) qualitative and quantitative genetics studies are needed to detect genetic changes that result in changes on the population structure. 2. Likewise, changes in the component(s) that comprise the gene-environment interaction (e.g., host-insect interactions) 2 must be assessed to understand the sources of pest population variation. 3. Continuous rearing technology (e.g., artificial bark beetle diet) must be developed for Dendroctonus spp. Needs in Quantitative Studies 1. Estimate heritabilities of key traits over space and time 2. Identify genetic markers and develop DNA/RNA probes, estimate frequencies, and study changes in population structure over space and time 3. Having identified one or more markers that differ constantly with space, time (or both), quantitative investigations are needed to establish sources of variation (selection pressures), including a. Biochemical traits: levels of key enzymes such as esterases, mixed function oxidases, or any other enzyme that can be directly linked to survival (fitness) b. Phenotypic or quantitative traits: the expression of life history traits (e.g., fecundity, longevity, age to first reproduction, phenology), or behavioral, physiological or morphological traits. USDA Forest Service Gen. Tech. Rep. PSW-138. 1992. Qualitative Genetics of Mountain Pine Beetle in Central Oregon1 Lula E. Greene2 The mountain pine beetle (MPB), Dendroctonus ponderosae, is a predator of all native and many introduced pines in western North America (Furniss and Schenk 1969, McCambridge 1975, Smith and others 1981, Wood 1982). The most important hosts of MPB are ponderosa, lodgepole, sugar, and western white pine. Although these pine species occur sympatrically throughout portions of their ranges, MPB are rarely found in more than one host species in any given locality. Along the Front Range in the Colorado Rockies, this bark beetle is found primarily in ponderosa pine, but throughout the northern Rockies, its preferred host is lodgepole pine. In Oregon, the major hosts are both ponderosa and lodgepole pines, but in California, the major host is sugar pine. Nevertheless, small areas can be found in which this beetle has colonized two or more hosts at the same time. No factor in the insect/host interaction influences the life of an endoparasitic phytophagous insect more than its host (Diel and Bush 1984). Because plant species differ chemically, physically, and biologically, they can present markedly different selective environments to insects feeding on them (Bush 1969, Wood 1980). Differential selection pressures provided by different host species can cause genetic differentiation of an insect species along host lines. Significant genotype variation in forest trees (the hosts) have been identified for bark beetles (Berryman 1972, Smith 1963, Smith 1972). Host species affect emergence, egg gallery characteristics, fecundity, development, and mortality of MPB (Amman 1982, Amman and Cole 1983, Amman and Pasek 1986, Schmid 1972). Sturgeon and Robertson (1985) measured microsomal polysubstrate monooxygenase (PSMO) activity in western pine beetle from ponderosa pine and in MPB from both lodgepole and ponderosa pines. Although they detected no significant difference in the PSMO activity between the two beetle species, PSMO activity in MPB from ponderosa pine was significantly lower than in MPB from lodgepole pine. These results suggest that MPB in the two different host species might have qualities that vary with respect to their ecological genetics. Isozyme electrophoresis is another means that can be used to track changes in beetle quality; in such studies, the genetic structure of insect populations is used to measure genetic variation. Data from electrophoretic surveys have provided direct measures of genetic variation within and genetic similarity among populations (Stock and others 1978). Stock and associates (1984) have surveyed much of the northern range of the MPB for electrophoretically detectable genetic variation. Beetles collected from populations in Oregon, Washington, Idaho, and Montana could be differentiated at two enzyme loci, although 1 An abbreviated version of this paper was presented at the Bark Beetle Genetics Workshop, May 17-18, 1992, Berkeley, California. 2 Research Entomologist, Pacific Southwest Research Station, USDA Forest Service, 800 Buchanan Street, Albany, CA 94710 USDA Forest Service Gen. Tech. Rep. PSW-138. 1992. similarity coefficients suggested that they were closely related (Stock and Guenther 1979). Coastal and inland populations of MPB collected from varieties of lodgepole pine showed significant differentiation (Stock and others 1978). In Utah, geographically separated populations of MPB from the same host were more similar than adjacent populations of MPB in diffeent hosts (Stock and Amman 1980). These studies showed that there is great genetic variation within and among the populations of MPB in the same host in addition to variation due to geography. Host tree species is implicated as a factor in maintaining differences among the MPB populations (Stock and Amman 1985). However, the different host trees were not sampled at the same site, so the significance of host variation as a factor cannot be separated from natural variation and geographic variation. Studies by Sturgeon and Mitton (1986) and Langor (1989) indicate that significant genetic divergence does exist in cooccurring populations of MPB in different hosts at the same locality. These differences, they conclude, can be attributed to either selection, substructuring populations of MPB according to host, or nonrandom mating in the hosts' population. Either of these events could lead to host-race formation, and ultimately, to speciation. To gather more information to clarify insect/host interactions, we initiated a 10-year project on the Deschutes National Forest in central Oregon in 1985. One of our objectives is to determine the effects of secondary plant chemicals on MPB infesting five host trees over time and space. This should aid in developing a basic understanding of host-induced genetic changes in bark beetles. Our long-term study will enable us to monitor changes in allelic frequencies and genotype structuring of populations for trends that indicate changes in the population status (endemic to epidemic). At present, we are analyzing data from the first five years of our study. Esterase enzymes are involved in the insect's ability to withstand toxins in its environment and its ability to survive. The esterase enzymes reveal four loci and show the most genetic variation. At the beta-esterase 1 locus, there is decided preference for heterozygotes, with one heterozygote genotype having a frequency that ranges 0.720 to 1.00 in 3 out of 39 samplings. This locus seems promising as a diagnostic indicator of host preference and perhaps, population status. Although our present focus is on MPB, information gained can provide insights for research into other polyphagous insects and other insects that persist at low levels for long periods of time and suddenly explode to devastate entire forests. No one biotechnology investigatory tool presently available can provide all of the information required to satisfy the needs of entomologists, population geneticists, and systematicists, but several used in concert can validate or bring a greater degree of credibility to the information obtained through their use. 3 Biochemical Indicators of Population Status of Pine Bark Beetles1 M. Carol Alosi and Jacqueline L. Robertson2 Background The processes by which insects cope with toxic chemicals in their environment are commonly classified as class 1 and 2 reactions (Dauterman and Hodgson 1978). Class 1 reactions include oxidations, reductions, and hydrolyses. By these processes, a hydrophilic group is added onto the foreign molecule and the molecule is then eliminated. Class 2 reactions consist of conjugations, i.e., glutathione conjugations, glucoside formation, and amino acid conjugation. Sturgeon and Robertson (1985) examined the comparative activity of polysubstrate monooxygenases (PSMO = mixed function oxidases) of mountain pine beetle (MPB) in lodgepole pine (the preferred host) and ponderosa pine (alternate host). PSMO activity of western pine beetle (WPB) in ponderosa pine was compared to that of MPB in lodgepole and ponderosa pine. Results of this investigation, done with MPB in an endemic population in the Deschutes National Forest in 1982, suggested that host preference is reflected in levels of this detoxification enzyme. By 1985, the Deschutes population reached epidemic levels; MPB infested five pine species (lodgepole, ponderosa, western white, sugar, and whitebark). For the past seven years, we have collected emerging and attacking MPB from a number of locations in the vicinity of La Pine, Oregon (Deschutes and Winema National Forests) for biochemical and qualitative genetic studies. These studies have been done to test the hypothesis that population status of this bark beetle species is associated with biochemical diversity (i.e., differences in class 1 and 2 reactions are associated with host tree species) that varies over time. We have quantified levels of PSMO's and esterases in class 1, and glutathione Stransferase (GST) in class 2. We have detected changes in all three enzyme systems. During 1991, we expanded our attention to western and southern pine beetles, and to various Ips species. GST was chosen for study at the molecular level because methods have been developed for various Dipteran species and are being developed for a tortricid leafroller, Epiphyas postvittana, in New Zealand. GST are enzymes that enable cells to detoxify harmful compounds. Various forms of GSTs, representing allelic variation and sometimes complex gene families, are found in all eucaryotic organisms. There is some evidence that insects utilize the GST-enzyme systems to detoxify plant allelochemicals that they encounter during herbivory (Wadleigh and Yu 1987). In bark beetles, levels of GST may vary with host and with population status (endemic versus epidemic) (Robertson and others in progress). These observations have led us to propose that a study of the structure and expression of GST genes in beetles will provide us with molecular indicators of population status. In addition, studies of GST genes in beetles will provide insight into the mechanism of metabolic resistance in polyphagous insects. Method of Study The immediate goal of our investigation is to isolate cDNA clones of expressed GST genes from mountain, western, and southern bark beetles. In order to do this we will construct cDNA libraries of all three beetles and screen the libraries with heterologous probes derived from Drosophila and lepidopteran cDNAs; alternatively, GST clones may be identified using lepidopteran-derived GST antibodies. Positive GST isolates from the libraries will provide us with homologous probes. Homologous probes are necessary to attain the sensitivity necessary to detect allelic variations of genes and to identify (for example) evidence of gene amplification in relationship to insect population dynamics. Presently, our work on this project includes developing methodology for purification of RNA and DNA from bark beetles. In addition, we are conducting hybridization studies between a Drosophila GST cDNA probe and bark beetle RNA and DNA. These preliminary studies provide us with information useful for establishing experimental conditions for screening beetle cDNA libraries. 1 An abbreviated version of this paper was presented at the Bark Beetle Genetics Workshop, May 11-18, 1992, Berkeley, California. 2 Research Molecular Geneticist and Supervisory Research Entomologist, respectively, Pacific Southwest Research Station, USDA Forest Service, 800 Buchanan Street, Albany, CA 94710. 4 USDA Forest Service Gen. Tech. Rep. PSW-138. 1992. Spatial Analysis in Bark Beetle Research1 Haiganoush K. Preisler2 Genetic and ecological information about bark beetle populations most often consists of data collected over space and time. However, when these data are analyzed, little attention is usually given to relationships operating in the spatial dimension. One of the reasons for the small number of studies that have examined the spatial aspects of bark beetle populations is the lack of statistical tools for the analysis of spatial-temporal data. One statistical technique that can be adapted for the analysis of spatial data is the methodology of generalized linear regression models. This method was used in a series of articles by Mitchell and Preisler (1991, Preisler [in press], Preisler and Mitchell [unpublished]). By fitting an auto-logistic model, we were able to study the factors affecting the probabilities of trees being attacked by mountain pine beetle (MPB). Those factors included characteristics of individual trees (such as tree size and vigor), in addition to the locations of each tree within a stand. We have found that the framework of generalized linear models is quite flexible and very helpful as an aid for understanding spatial patterns of discrete data points and for relating those patterns to specific environmental factors or tree characteristics. The second technique that might be useful for the analysis of spatial data is the methodology of Geographic Information Systems (GIS). GIS can be used to store spatial and temporal information on insect populations. Genetic information can then be added to the system and used to analyze changes over space and time; interactions among and within various factors can be investigated. Although GIS allows entomologists to compile spatial data, deriving inferences from these data is still extremely difficult because appropriate statistical techniques are lacking. A few references that discuss statistical techniques for analyzing geographic information and gene-frequency maps are Brillinger (1990), Kemp and others (1989), Liebhold and Elkinton (1989), Piazza and others (1981). Further statistical research is needed in this area. 1 An abbreviated version of this paper was presented at the Bark Beetle Genetics Workshop, May 12-18, 1992, Berkeley, California 2 Research Statistician, Pacific Southwest Research Station, USDA Forest Service, 800 Buchanan Street, Albany, CA 94710. USDA Forest Service Gen. Tech. Rep. PSW-138. 1992. 5 Bark Beetle Genetics1 Wyatt W. Anderson and C. Wayne Berisford2 1. Previous work A. Protein electrophoresis the basic technique for most work. B. Genetic variability has been characterized. C. Population differences demonstrated and analyzed. D. Genetic population structure assessed. E. Basic molecular systematics explored; phylogenetic trees developed. F. Attempts to link protein loci to phenotypic characters such as behavior. 2. Our work at University of Georgia A. Starch gel electrophoresis of Dendroctonus and Ips populations, in terms of genetic variability, population structure, and population differences. B. mtDNA studies, using restriction site analysis and DNA sequencing. (1) Cloned part of mitochondrial DNA (2) Completed restriction site analysis of SPB populations (3) Begun sequencing of several mtDNA segments 3. Work to be done A. Long-term study to follow genetic variability and population structure in relation to population cycles of SPB. Electrophoresis and perhaps restriction mapping techniques of choice. B. Studies using mtDNA as molecular markers of population history in relation to geographic location―phylogeography. Restriction mapping and sequencing of PCR-amplified sequences techniques here. C. Molecular systematics of bark beetle taxa such as Dendroctonus species, using mtDNA and nuclear loci. Restriction mapping and sequencing of PCR-amplified sequences techniques here. 1 In lieu of a full narrative paper, the authors submitted this outline for publication. Any questions should be directed to the authors. 2 Professor of Genetics, Dept. of Genetics, 101B Life Sciences Bldg., University of Georgia, Athens, GA 30602 and Professor of Forest Protection, University of Georgia, Athens, GA 30602 6 USDA Forest Service Gen. Tech. Rep. PSW-138. 1992. Isozyme Studies of Bark Beetle Population Genetics and Systematics1 Molly W. Stock, Gene D. Amman, and Barbara J. Bentz2 Electrophoretic analysis was used extensively in the 1970's to estimate genetic relationships among populations, species, and closely related genera of many different plants and animals, including many insect groups. Some of the first electrophoretic work on bark beetles was done by Anderson and others (1979), Anderson and others (1983), Florence and Kulhavy (1981), Florence and others (1982), and Namkoong and others (1979). These studies demonstrated that bark beetles are highly polymorphic and amenable to population studies using this technique. The first isozyme study of the mountain pine beetle (MPB) Dendroctonus ponderosae (Stock and Guenther 1979) showed differentiation among geographically separated populations. A subsequent study of MPB from four locations (Stock and Amman 1980) suggested that there might also be genetic differences at individual gene loci between MPB from lodgepole and ponderosa pine, and greater overall heterozygosity in beetles from ponderosa pine. Some preliminary data also showed differences between beetles emerging from trees with thin and thick phloem and between early- and late-emerging beetles from the same tree. Inferences that could be made from these observations of differences related to host species were, however, confounded by geographic distance among sites. Sturgeon (1980) and Sturgeon and Mitton (1986) studied sympatric groups of MPB attacking different hosts in mixed pine stands and found significant differences related to host tree species. Further evidence of differentiation of MPB by host was found in a comparison of isozyme frequencies of MPB collected from lodgepole and ponderosa pine at a single site in Utah (Stock and Amman 1985). Deviations in genotype frequencies from Hardy-Weinberg (random mating) expectations occurred when isozyme data from all beetles were pooled (Wahlund effect); frequencies were much closer to random mating expectations when separate analyses were conducted for beetles from each of the host species. Once again, greater heterozygosity was observed in beetles from ponderosa pine. Overall, beetles from thin-phloem lodgepole were more heterozygous than those from thick phloem, though these differences were not detected in beetles from ponderosa pine. 1 An abbreviated version of this paper was presented at the Bark Beetle Genetics Workshop, May 17-18, 1992, Berkeley, California. 2 Professor of Forest Resources, Dept. of Forest Resources, College of Forestry, University of Idaho, Moscow, ID 83843; and Supervisory Research Entomologist and Research Entomologist, respectively, Intermountain Research Station, USDA Forest Service, Ogden, Utah. USDA Forest Service Gen. Tech. Rep. PSW-138. 1992. Heterozygosity and Environmental Stress Males were more differentiated than females among sites and numbers of emerging males more variable in thin-phloem trees (Stock and Amman 1980). In a later study (Stock and Amman 1985), males emerging from thin-phloem lodgepole pine were found to be more heterozygous and more variable from tree to tree, and fewer and more variable males (relative to females) emerged from thin phloem. We suggested that genetic diversity of MPB (as measured by average heterozygosity) might increase in response to increased severity of environmental conditions (i.e., stress), a response observed in numerous other organisms by other workers (e.g., Samollow and Soule 1983). Male MPB are generally thought to be more sensitive to microenvironmental conditions than females, and therefore would be expected to have greater genetic diversity if a generalized population-level response to increased selective pressures was an increased level of heterozygosity. Further evidence to support this hypothesis was provided by a study of isozyme variation observed over six consecutive generations of MPB laboratory-reared in thick and thin phloem; heterozygosity increased in both groups over the generations, but more rapidly in the beetles in thin phloem (Amman and Stock unpublished). In Ips pini (Gast and Stock unpublished), genetic diversity was significantly higher in overwintered than in non-overwintered beetles. However, Langor and Spence (1991) observed changes in gene frequencies but no consistent increase in heterozygosity in MPB with overwintering. These authors attribute the genetic differentiation they observed to differential survival in the hosts, rather than to differential host preference of beetle genotypes. Thus, one might see, as did Gast and Stock (unpublished), greater differences between beetles that have lived for a longer time in different hosts or in the same host species under more severe environmental conditions. Other evidence supports this hypothesis. For example, Gast (1987) reported that overwintered I. pini responding to pheromone were significantly more heterozygous than overwintered beetles responding to host volatiles. Heterozygosity levels in hostresponding, pheromone-responding, and nonresponding beetles that had not overwintered were very similar across response categories. Time of collection can therefore have an important influence on the type of genetic variation observed and interpretations that can be made from it. Langor and Spence (1991) and the work of Gast (1987) suggest that laboratory rearing of beetles collected from logs cut before winter reduces the differential selective influence of host tree species. This possibility could help explain the fact that Sturgeon (1980) and Sturgeon and Mitton (1986) found genetic differences between MPB 7 from lodgepole and limber pine, whereas Langor and Spence (1991) did not. Sturgeon's (1980) beetles were collected in summer, just before emergence from their host trees; they represented survivors of within-tree mortality estimated to average 85 to 99 percent of the brood which had been established the previous fall. Langor and Spence (1991) used beetles reared in the laboratory and collected in the fall and suggested that data collected from laboratory-reared beetles better reflect possible effects of host selection behavior and the genetic makeup of colonizing beetles. We compared heterozygosity of earlyand late-emerging MPB reared from fall-collected lodgepole logs from six sites (Stock and Amman unpublished). In beetles from four of the six sites, heterozygosity went up over the emergence period. It is possible that such trends would be even more apparent in samples of beetles collected as they emerged in the field the following year. Heterozygosity and Species Distribution Langor and Spence (1991) attributed the low levels of heterozygosity (10.0-11.2 percent) they observed in MPB from Alberta and British Columbia (compared to heterozygosity in MPB from U.S. studies) to the location of their populations near range margins. In contrast, our studies suggest that populations near the margins of species distribution (presumably living under suboptimal conditions) could be expected to be more heterozygous. The highest level of polymorphism that we observed in a MPB population (more than 17 percent) occurred in a population from California, another area that might be considered near the range margin for this species (Higby and Stock 1982). Heterozygosity levels of Langor and Spence's (1991) Canadian populations were lower than in this California population, but approximately equal to heterozygosities in almost all other populations we studied―including a population from the Black Hills (South Dakota), a third area that could be considered marginal for the species and which had an average heterozygosity of 11.1 percent (Stock and others 1984). Genetic Indicator of Population Phase Some very early electrophoretic work with the Douglas-fir tussock moth (Stock and Robertson 1977) revealed an esterase allozyme that appeared only in low-density populations. Populations sampled two or more times during 1976 and 1977 showed changing frequencies of this allozyme (relative to the other two allozymes at that locus) that seemed to parallel changes in population density. At that time, we hypothesized that relative frequencies of these three allozymes might be used as a genetic indicator of future population trends in the Douglas-fir tussock moth. Hayes and Robertson (these proceedings) describe allozyme variation at an esterase locus showing similar correlation with population status in the MPB. Average heterozygosity also appears to be related to population phase, reaching highest levels as epidemic populations reach peak numbers and begin to decline (Stock and others 8 unpublished). Thus, there may be a correlation between factors of increased environmental stress that act to end an outbreak and the higher levels of heterozygosity observed at this time. One might infer a relationship between heterozygosity and population size, but we need to keep in mind that the processes underlying observed genetic variation are very complex and that factors that influence genetic variation under one set of conditions (e.g., during outbreaks or epidemics) can be very different under another set of conditions (e.g., during the lowdensity or endemic population phase). For example, inbreeding in low-density populations might act to reduce heterozygosity and counterbalance any tendencies toward increased heterozygosity related to environmental stressors that might be keeping the population at a low level. In the field, heterozygosity appears to be lower in endemic than in epidemic populations (Stock and others unpublished), and laboratory studies (Amman and Stock unpublished) showed that small numbers of parent beetles per log resulted in an F1 population with a much lower level of heterozygosity than the parental population. On the basis of these ideas, Stock and others (unpublished) hypothesized that heterozygosity increasing over time might indicate that a population was approaching the end of an epidemic; conversely, a stable or decreasing heterozygosity in a high-density population could indicate that the population was unstressed and that population numbers would increase or stay high. Predictions of population trend based on these hypotheses for six MPB populations were good in four of six cases. Systematics of Dendroctonus Species Using electrophoretic techniques, Higby and Stock (1982) identified diagnostic isozymes for sympatric populations of the sibling species D. ponderosae and D. jeffreyi in California. An earlier study (Stock and others 1979) showed very similar levels of differentiation between D. pseudotsugae populations from Idaho and Oregon, but the wide geographic separation between these two populations precluded any definitive statements about species status. Wood (1963) used anatomical and biological characters to rank Dendroctonus species in order of increasing evolutionary specialization relative to other Scolytidae. Lanier (1981) also suggested an order of specialization based on cytogenetic characteristics. Although Wood and Lanier placed the species into nearly identical species groups, their postulated order of specialization of these groups were nearly opposite. Bentz and Stock (1986) used electrophoretic data from 22 populations, representing 10 Dendroctonus species, in an attempt to unravel the discrepancies in phylogenetic interpretations. Although the species groups identified using electrophoretic data were similar to those previously identified by both Wood (1963) and Lanier (1981), the implied phylogeny was independent of the species groupings. Some members of a species group were more specialized than other members in the same group. On the basis of these data, it appears that no single species group can be said to be more advanced or more primitive than another. USDA Forest Service Gen. Tech. Rep. PSW-138. 1992. The phylogenetic tree produced using electrophoretic data suggests that D. rufipennis, D. adjunctus, and D. approximatus were the most primitive species, followed by D. ponderosae and D. brevicomis; D. valens, D. terebrans, D. simplex, D. pseudotsugae, and D. frontalis are among the more evolutionarily advanced species in the genus. There were no discernible trends in level of heterozygosity (by species) based on evolutionary position in the hypothesized phylogenetic tree. These interpretations of the phylogenetic relationships among Dendroctonus species could be useful in analysis of behavioral strategies used in scolytid genera, such as production and response to semiochemicals, mating strategies, and host tree associations. Stock and others (1987) compared the great European spruce bark beetle, D. micans, to the 10 North American Dendroctonus species studied by Bentz and Stock (1986). Average heterozygosity was 5.3 percent in D. micans; North American species ranged from 11.4 to 22.6 percent. Cluster analysis suggests that D. micans is most closely related to D. terebrans and D. valens, species with which it shares the characteristic of gregarious larval feeding in the phloem of living hosts. More recently, Gast and Furniss (unpublished) compared isozyme characteristics of D. micans with its morphologically similar, spruce-infesting, North American counterpart, D. punctatus. USDA Forest Service Gen. Tech. Rep. PSW-138. 1992. Research Needs (1) Test the heterozygosity model of Stock, Schmitz, and Amman for predicting phase (endemic, building, epidemic, postepidemic) of MPB populations. (2) Investigate further the change from endemic to building phase: What are the influencing factors associated with it (population size, mixing of demes, synchrony of emergence and attack, host characteristics, etc.)? (3) Continue to investigate differences in MPB response to verbenone (and possibly other pheromones): Are they genetically based and, if so, what factors are causing selection? (4) Dispersal: Are there physiological and/or genetic differences among individuals with different flight behavior (long vs. short fliers) and do these relate to population phase? (5) The effect of temperature on MPB is very important in Rocky Mountain populations. Is cold tolerance genetically based, possibly showing up as geographic differences associated with elevation and latitude? (6) Continue study of bark beetle systematics using electrophoresis and other, newer, molecular genetic techniques. 9 Biosystematics of Ips mexicanus and Ips plastographus (Coleoptera: Scolytidae) and Their Fungal Symbionts1 David L. Wood, Andrew J. Storer, Thomas R. Gordon, Steven J. Seybold, and Marion Page2 Ips mexicanus (Hopk.) (concinnus group of S.L. Wood 1982) and I. plastographus (plastographus group) share common coniferous hosts throughout western North America (S.L. Wood 1982). In California their distribution in the coastal pines (Pinus radiata, P. muricata, P, contorta contorta, and P. contorta bolanderi) and in the high elevation P. contorta murrayana of the Sierra Nevada Mountains (and Cascade Mountains) is especially interesting because of their wide separation by the Sacramento and San Joaquin Valleys. This separation is 2-400 km from Redding in the north to Bakersfield in the south, a latitudinal distance of about 1200 km. Thus these populations have been noninterbreeding populations for thousands of years. Furthermore, these coastal and montane populations probably come together where P. contorta and P. contorta murrayana or P. c. contorta hybridize. Native Monterey pine (Pinus radiata) is now represented by only three small separated coastal populations. Other hosts of I. mexicanus and I. plastographus occur on small islands off the southern California coast and into the mountains of Mexico (Bright and Stark 1973, S.L. Wood 1982, Seybold and others 1992a). On the basis of morphological criteria and reduced hatching in the F1, Lanier (1970b) defined subspecies of I. plastographus: I. plastographus maritimus Lanier for the coastal form and I. plastographus plastographus (Leconte) for the montane form. In limited cross-mating studies, he showed that both populations were interfertiles at the F1 and at the parental backcrosses. Similar limited studies were conducted with the cohabiting I. mexicanus (Lanier 1967). However, in recent studies of the distribution of ipsenol (2-methyl-6methylene-7-octen-4-ol) and ipsdienol (2-methyl-6-methylene2.7-octadien-4-ol), pheromone components in Ips spp., Seybold and others (1992b) found both components in montane I. mexicanus but only ipsdienol in coastal I. mexicanus. The enantiomeric com-position of ipsdienol is ~90 percent-(-) for both populations. Although the status of these compounds as pheromone compo-nents for these species has not been established, they have been shown to be pheromone components in all species studied to date (D.L. Wood 1982). Nevertheless, the presence of ipsenol in only the montane population offers considerable opportunity to investigate the genetics of these two populations. 1 An abbreviated version of this paper was presented at the Bark Beetle Genetics Workshop, May 17-18, 1992, Berkeley, California. 2 Professor of Entomology and Post-Doctoral Researcher, respectively, Dept. of Entomological Sciences, University of California, 201 Wellman Hall, Berkeley, CA 94720; Assistant Professor, University of California, Berkeley, CA; Post Doctoral Scholar and Research Entomologist, Pacific Southwest Research Station, USDA Forest Service, 800 Buchanan Street, Albany, CA 94710. 10 Very little is known about the fungal symbionts of these species. Recently Parmeter and others (unpublished data) have isolated unidentified species in the genera Ophistoma, Leptographium, and Graphium from I.p.. maritimus and coastal populations of I. mexicanus. These fungi were inoculated into living trees. Through dye studies (Parmeter and others 1989) some isolates were shown to interrupt water transport. The role of symbiotic fungi in the biology of bark beetles has been studied extensively, especially in the Ophistoma spp./ Dendroctonus spp. system (reviewed exhaustively in Schowalter and Filip 1992; see for example, Owen and others 1987, Parmeter and others 1989, 1992). In Ips spp./fungi associations, Ophistoma spp. have been shown to inhibit oviposition but not development (Yearian and others 1972) and to interrupt water transport (Parmeter unpublished data, Homvelt and oth-ers 1983). Although no specialized morphological structures are apparent in Ips spp. for carrying these fungi [such as the pronotal mycangium of Dendroctonus brevicomis and D. frontalis and maxillary mycangia of D. jeffreyi and D. ponderosae (Whitney 1982)], there is a consistent and ubiquitous association between bluestain fungal invasion of the sapwood and phloem and the gallery systems of these bark beetles. Therefore, studies of relatedness and divergence in I. mexicanus and I. plastographus would be enhanced by similar analyses of these phoretic symbionts. Evidence of divergence with either or both taxa would be of great value in understanding the evolution of these species. Studies of relatedness in Ips spp. have focused on I. pini (eastern/western populations) and on the grandicollis group sibling species (paraconfusus, confusus, and hoppingi). In I. pini, no post-mating barrier has evolved between eastern populations (Ontario, Canada) crossed with western populations (California and Arizona) (Lanier 1972). However, a premating barrier has evolved between these two populations. There pheromone component, ipsdienol, is produced by both populations. However, in the California population (as well as in most western North American populations―see Seybold and others 1992d), ipsdienol is produced as a mixture of 98 percent (-)and 2 percent (+-)-ipsdienol. The (+)- isomer is an interruptant, whereas the (-)- isomer is a powerful aggregation attractant (Birch and others 1980; Seybold and others 1992d). In the eastern population a mixture of about 40 percent (-) and 60 percent (+)-ipsdienol is produced. Here both isomers function as an attractant. We have found only a few beetles responding to the racemate in California (Seybold and others 1992d). Eastern populations respond at low levels to 98 percent (-)ipsdienol (Teale 1990). In addition to ipsdienol, lanierone (2hydroxy-4,4,6-trimethyl-2.5-cyclohexadien-l-ol) has recently USDA Forest Service Gen. Tech. Rep. PSW-138. 1992. been isolated and shown to be an important component of the pheromone of the eastern population (Teale and others 1991). Although this compound caused an increased response to ipsdienol in California, it has not been found in California populations (Seybold and others 1992c). As with ipsenol in the montane population of I. mexicanus, the presence or absence of lanierone and the differences in enantiomeric composition of ipsdienol offer opportunities to investigate the genetics of these two populations of I. pini. In the work with the grandicollis group, Lanier (1970a and b) and Merrill (1991) established the presence of postmating barriers among I. paraconfusus, I. hoppingi, and I. confusus. Premating barriers are weakly developed or not developed at all (Cane and others 1990c, Fox and others 1991 a). Although there is pheromonal cross-attraction among these species (Lanier and Wood 1975; Cane and others 1990a, 1990c), we have found nonreciprocal divergence in these pheromone-elicited behaviors, i.e., I. paraconfusus females do not discriminate between conspecific and I. confusus males, whereas I. confusus females prefer conspecific males in the host pine species (Cane and others 1990c). Furthermore, females join heterospecific males in host and nonhost pines (Fox and others 1991 a). Morphometric, electrophoretic (Cane and others 1990b), and cuticular hydrocarbon (Page and others 1992) analyses support the species status of these sibling 5-spined Ips described by Lanier (1970b). Thus we see the evolution of postmating and weak-tononexistent premating barriers in the sibling grandicollis group and the reciprocal scenario in I. pini. The studies we propose will add to the knowledge base developed over the past 20 years on the biosystematics of these two Ips spp. complexes. At the same time, expanding our investigation to include the symbiotic fungi should give us greater insights into the evolution of this group of organisms. Also of importance to the proposed study is the recent discovery of the pitch canker fungus, Fusarium subglutinans, in Monterey pine (P. radiata) plantings in the vicinity of Santa Cruz, Calif. Our studies have shown that I. paraconfusus and I. mexicanus adults carry propagules of this fungus (Fox and others 1990, 1991b). Furthermore we have demonstrated that attraction of I. mexicanus to Monterey pines results in pitch USDA Forest Service Gen. Tech. Rep. PSW-138. 1992. canker infections associated with attacking adults. This fungus, which is native to pines of the southern U.S. (Correll and others 1992), causes extensive tip dieback followed by numerous resinous bole-cankers. We believe that this fungus further weakens trees so that they are more easily killed by these Ips species. Our studies suggest that progress of this disease from urban plantings of Monterey pine in the Santa Cruz area into our native forests will likely be a consequence of propagule transmission via I. plastographus maritimus and I. mexicanus. We predict transmission from I. mexicanus to I. plastographus in the northernmost native stands of Monterey pine (Ano Nuevo State Park) to urban plantings of Monterey and bishop pine (P. muricata), to native stands of bishop and shore pine (P. contorta contorta) on the coast and ultimately inland to the Cascade and Sierra Nevada Mountains, perhaps via P. ponderosa. Ips mexicanus and I. plastographus cohabit these coastal pine species as well as P. contorta murrayana (I.p. plastographus) in the above mountain ranges. Thus our studies on the coastal and montane populations of these Ips spp. will be important in predicting the ultimate distribution of this newly introduced fungal pathogen. At the same time we are in a unique position to investigate the coevolved system of these bark beetle species with their symbiotic fungi. Such studies will provide baseline information which can be used to understand the new symbiotic relationship that we expect will develop when F. subglutinans becomes a part of this community. Research Objectives 1. Determine the extent of postmating barriers to gene flow between coastal and montane populations of I. mexicanus. 2. Determine the extent of premating barriers to gene flow between the above populations. 3. Determine the presence or absence of ipsenol in the above crosses of I. mexicanus and in the backcrosses. 4. Compare the genetic constitution of the above populations using mitochondrial DNA and cuticularhydrocarbon analyses. 5. Compare the genetic constitution of the fungal symbionts commonly associated with the above populations using mitochondrial DNA analyses. 11 The Status of Scolytid Genetics―A Reductionist's View1 Steven J. Seybold2 As biologists considering studies of scolytid genetics we are fortunate that an insect model exists that has been the target of genetic analyses on both the individual and population levels for most of the twentieth century. The knowledge base associated with Drosophila melanogaster3 and congenerics is staggering, and it is a tribute to scientific inquiry that so much is known about organisms of such little economic importance. The goal of the classical genetic crosses of D. melanogaster begun in the lab of T.H. Morgan in 1909 was to infer the genetic basis of the phenotype (phenotype →a gene). The development of P-element-mediated transformation (Rubin and Spradling 1982) allows "reverse genetic" studies where the gene of choice is placed into the organism and the resulting individual is assayed to infer the phenotypic product of the gene (gene → phenotype). During the period between the advent of each of these approaches with Drosophila, serendipity and intensive screening procedures revealed a wide array of morphological, developmental, and behavioral mutants; biochemical studies characterized nucleic acids and proteins; genetic studies led to the development of physical, cytological maps of polytene chromosomes; and, soon, the entire genome will be sequenced (Merriam and others 1991). The phenomenon of transposition ("jumping genes") has been actively investigated in D. melanogaster (Green 1980, Engels 1983), and the transposons themselves have been the targets of studies of population genetics (Charlesworth and Langley 1989). In addition to the biochemical and molecular work, the drosophilids have been the subject of studies of population biology, phylogeny, and pheromone production (Bartlet and others 1986, Kaneshiro 1988, Singh 1989). In the Scolytidae, no mutants have been isolated, prospects for long-term culture have not been developed, most crosses have been performed to determine species status of wild populations rather than to assay for intraspecific phenotypic variants, and few biochemical reactions have been characterized. When evaluated against the background of Drosophila genetics, the status of scolytid genetics can be described as primitive at best. Thus, it is in an area of enormous opportunity, where, 1 An abbreviated version of this paper was presented at the Bark Beetle Genetics Workshop, May 17-18, 1992, Berkeley, California. 2 Post Doctoral Scholar, Pacific Southwest Research Station, USDA Forest Service, 800 Buchanan Street, Albany, CA 94710. 3 An organization called the Drosophila Information Service catalogs and communicates biological (mostly, genetic) data about D. melanogaster and related species. In addition, there is an encyclopedia of Drosophila mutations authored by Lindsley and Grell, and various stock cultures of wild type and mutant strains are maintained around the world. 12 because of the stature of the insects in forest management, minor scientific gains may have large economic impacts. However, rather than repeat the past 80 years of Drosophila genetics with the Scolytidae, we should apply the Drosophila knowledge base to scolytids as often as possible. No scolytid is likely to become a model for eukaryotic biology, but by applying the tools used with Drosophila, we may discover some interesting and perhaps unique biological phenomena in the Scolytidae. For the purposes of organization, it is useful to divide scolytid genetics into the reductional and holistic approaches. By reductional I mean the analysis of biochemical, morphological, or behavioral traits of individuals and the interpretation of the results from a molecular or mechanistic perspective. By holistic I mean the analysis of the same traits from pooled samples of individuals and the interpretation of the results from an ecological or population perspective. The following are several reductional areas of study that seem relevant to scolytid genetics. 1) Genetic analysis of morpho- and chemosystematic characters: A variety of useful morphological characters have been identified for distinguishing scolytid taxa (Bright 1976, Bright 1992, Bright and Stock 1982, S.L. Wood 1982). However, mutations in these characters have not been studied in a systematic fashion [e.g., red vs. white eye color, vestigial vs. wild-type (normal) wing morphology in Drosophila]. Recently there has been considerable interest in establishing chemical phenotypes for some species in the Scolytidae, and using the newly discovered chemosystematic characters in constructing phylogenetic relationships (cuticular hydrocarbons―Page and others 1990a, b, 1992; Bright 1992; chirality of pheromone components―Seybold and others 1992b; and migration of metabolic enzymes in starch gels―Cane and others 1990b, and other studies). However, while typical chemical phenotypes have been characterized, nothing is known about the genetic regulation leading to these phenotypes and possible mutations that might lead to altered chemical phenotypes. Furthermore, because of the insensitivity of the chemical assays, the chemical phenotypes have often been determined on pooled samples of individuals, whereas we ought to evaluate the phenotypes of individuals. It will be important to standardize laboratory and statistical procedures used to evaluate a range of individual normal chemotypes so that mutants can be recognized when they are encountered. This is not an easy problem. For example, the cuticular hydrocarbon profile from a species typically contains 60 to 80 hydrocarbon components which vary in presence, USDA Forest Service Gen. Tech. Rep. PSW-138. 1992. absence, and quantity. Here are two interesting problems that seem appropriate to this topic. (a) Chirality of ipsdienol as a heritable trait of individual male Ips pini. Ips pini occurs in two basic pheromone types (Birch and others 1980, Lanier and others 1972, Lanier and others 1980, Miller 1990, Teale 1990, Miller and others 1989). The eastern form produces and responds maximally to ipsdienol with an enantiomeric composition of 30 percent to 60 percent-(4R)-(-), whereas the western form produces and responds maximally to ipsdienol with an enantiomeric composition of 93 percent to 100 percent-(4R)-(-). The eastern form produces and responds strongly to lanierone (Teale and others 1991); the response of the western form is enhanced by lanierone, but males from this population do not appear to produce lanierone (Seybold and others 1992c). Populations from North America have been surveyed (Miller 1990, Seybold and others 1992b), and a zone of sympatry between the forms appears to occur in British Columbia. Teale (1990) has crossed eastern (New York) and western (Arizona) forms and demonstrated heritable patterns to the chirality of male-produced ipsdienol. He has also found a correlation between the response to enantiomeric blends by males and production in the eastern form of I. pini. We are pursuing similar studies with the western population. (b) Ipsenol as chemotypic marker for two populations of Ips mexicanus. Ips mexicanus occurs as three distinct populations in western North America: coastal in Pinus radiata, P. muricata, and P. contorta; montane in P. contorta murrayana and P. contorta latifolia; and interior in other Pinus spp. The I. plastographus and I.integer complex can be broken down into three similar entities. In a survey of the enantiomeric composition of ipsenol and ipsdienol produced by male Ips, we have found that the coastal and montane populations of I. mexicanus differ in their ipsenol-ipsdienol phenotype. Coastal I. mexicanus produce ~90 percent-(4R)-(-)-ipsdienol, whereas montane I. mexicanus produce the same ipsdienol and >99 percent-(4S)-(-)ipsenol. We have not studied an interior population of I. mexicanus. Thus, interbreeding studies of coastal and montane populations of I. mexicanus could be followed by traditional traits such as larval: egg niche ratios (Lanier 1967), as well as by ipsenol production. Coastal I. mexicanus could be treated as a USDA Forest Service Gen. Tech. Rep. PSW-138. 1992. strain of I. mexicanus that is deficient in the ability to produce ipsenol. 2) Characterization of biochemical pathways: To accompany classical genetic analyses, we need to expand our knowledge of bark beetle biochemistry. Because of a series of pheromone biosynthesis studies with I. pini and I. paraconfusus using labelled precursors (Fish and others 1979, 1984, Hendry and others 1980, Vanderwel and Oelschlager 1987, Vanderwel 1991), these two species make particularly good candidates for initial studies on the enzymes involved in pheromone biosynthesis. 3) Sex ratio condition: One intriguing genetic problem that bears further study is the sex ratio condition discovered in Ips latidens and other species (Lanier and Oliver 1966). In this phenomenon individuals from certain populations carried a maternally transmitted factor that resulted in selective mortality of male embryos. A similar sex ratio condition also occurs in Drosophila spp. (Sakaguchi and others 1965, Ikeda 1965, Novitski and others 1965), and in recent studies the causative microorganisms have been isolated and cultured (Hackett and others 1986). Other sex ratio distortions in Drosophila spp. are caused by chromosomal and extrachromosomal factors. Molecular studies of a distortion in the latter group (hybrid dysgenesis) led to the discovery of the P-element transposon (Rubin and Spradling 1982). Application of modern biochemical and molecular biological techniques to the sex ratio condition in Ips may lead to similar discoveries. Lanier never published the fact that the condition also occurs in another species of Ips [confusus (LeConte)] and in the scolytid genera Pityophthorus and Dendroctonus. 4) Construct gene libraries: With the cooperation of Carol Alosi (Pacific Southwest Research Station, USDA Forest Service), we have begun to construct a c-DNA library of genes for I. pini. Large numbers of male and female western I. pini have been frozen for this purpose. Genomic libraries for the major scolytid species should be constructed and made centrally available to all researchers (the beginnings of a Scolytid Information Service?). 13 Genetic Characters for Bark Beetle Phylogenies1 James H. Cane2 The current taxonomic classification of scolytid bark beetles is based largely upon external morphological characters. This will remain the status quo into the foreseeable future for rea­ sons of cost, time, convenience, and reproducibility. For genera of particular economic interest, notably Dendroctonus and Ips, additional taxonomic characters have been sought more or less successfully through karyological and electrophoretic studies and applied to teasing apart sibling species complexes and circumscribing species groups (e.g., Lanier 1967, 1970a, 1970b, 1972, 1981; Cane and others 1990b). As noted by Bright (this workshop), this task of describing species of the North Ameri­ can scolytid fauna is virtually complete, thanks to the works of S. L. Wood, D. E. Bright, the late G. N. Lanier and G. R. Hopping and many others (Bright 1992). Before us lies the task of developing supportable phylogenetic hypotheses for key bark beetle taxa, essentially converting lists of species and higher taxonomic groups into ancestor-descendant hierarchies or genealogical lineages. How can we benefit from having rigorous phylogenetic hypotheses for bark beetles? Certain bark beetle taxa offer us unusual opportunities for insights into the evolution of po­ lygyny, mate choice, pheromonal and stridulatory courtship communication, host association, and predator-prey interac­ tions. For instance, does pheromonal specificity result from ecological interaction of co-occurring species, or does it reflect the intervening time since common ancestry? For species of the grandicollis group of Ips, at least, only the latter hypothesis is supported (Cane and others 1990a, 1990c, Lewis and Cane 1990b). Furthermore, it appears that divergence in specificity of stridulatory communication parallels that of pheromone speci­ ficity (Lewis and Cane 1992) despite its role in defense (Lewis and Cane 1990a). Evolutionary biologists and ecologists are coming to appreciate the value of a proper phylogeny for the testable evolutionary scenarios that it can offer to comparative or evolutionary questions in chemical ecology, plant-herbivore interactions, parasitology, and studies of symbioses (Cane 1983, Gittleman and Kot 1990, Mitter and others 1991, Wanntorp and others 1990). Rigorous, testable evolutionary hypotheses for bark beetle behavior and ecology require equally rigorous phylogenetic statements that are defendable on their scientific merits. Unfor­ tunately, mere character similarity is insufficient evidence for quantifying such phylogenetic relationships, as it confounds the possible sources of similarity. Similarity may be the result 1 An abbreviated version of this paper was presented at the Bark Beetle Genetics Workshop, May 17-18, 1992, Berkeley, California. 2 Associate Professor Entomology, Dept. Entomology, 301 Funchess Hall, Auburn Univ., Auburn, AL 36849-5413. 14 of evolutionary convergence, as in the stridulatory capabilities of taxonomically disparate bark beetle taxa. Even if characters shared by two taxa are indeed homologous, such as some of the saturated straight chain cuticular hydrocarbons of Dendroctonus spp. (Page and others 1990b), they are shared with many other insect genera and so are uninformative in describing phyloge­ netic relationships within any one lesser taxonomic unit. This dilemma is best resolved using cladistics, an approach which has emerged from the fires of heated debate over the past 20 years as the most appropriate method for reconstructing phylo­ genetic relationships (Wiley 1981). Cladistic algorithms hierar­ chically arrange species into ancestor-descendant lineages (a genealogical tree) supported by shared, derived characters, thus formalizing what good taxonomists have done all along. Recent experimental work with a virus has shown that a known ancestral genealogy can be fully recovered using a cladistic analysis of nucleotide sequences of the terminal viral families (Hillis and others 1992). Bark beetles had been too wee for molecular methods using DNA until the advent of the polymerase chain reaction, or PCR. Now, isolated mitochondrial, ribosomal or nuclear DNA can, with skill, determination and some luck, be itera­ tively amplified to provide enough reliably replicated DNA for RFLP's (restriction fragment length polymorphisms) or actual nucleotide sequencing. These data can have advantages over electrophoretic and morphometric data in that they provide discrete characters suitable for binary, presence-absence cod­ ing. Because the cladistic approach works with patterns of shared, derived characters and not clustering based upon overall similarity, character states must be discrete rather than continuous so that they can be polarized as either ancestral or derived at every juncture or branch in the phylogenetic tree. It is comparatively easy to imagine an ancestral Ips either possess­ ing or lacking a third, hooked spine on its elytra. However, for a continuous measure such as size, is a length of 4.6 mm primi­ tive or derived? The same problem can be encountered with karyological, electrophoretic or biochemical data. Nucleotide sequences may provide us with the supplemental discrete, heri­ table characters needed to establish trees of phylogenetic rela­ tionships for our evolutionary questions (Gittleman and Kot 1990, Cracraft and Helm-Bychowski 1991). I have emphasized the application of genetic information to the solution of phylogenetic and evolutionary questions for bark beetles because of the topic's familiarity to me and rel­ evance to this workshop. Obviously, there are a number of other prominent and perhaps more pressing genetic questions regarding bark beetles that demand our attention as well. 1. What is the genetic structuring of select scolytid popula­ tions? In particular, what is the size of a deme for a given species during eruptive and quiescent periods of population USDA Forest Service Gen. Tech. Rep. PSW-138. 1992. cycles? What are the respective rates of gene flow during these periods? Do allele frequencies shift as a result of natural or sexual selection during either part of these population cycles? If there is an oscillation in selective regimes, is its effect roughly reciprocal, such that selection in one phase cancels that in the next? Or do populations gradually change genetically as a result of such cyclical selection? Or is it not selection at all but drift during the phase of population crash that fixes genetic change in populations, the so-called "flush-crash" hypothesis of Hampton Carson? 2. Over what distances do some individuals of a species migrate? Are outbreaks intensified by an influx of beetles from neighboring populations, or do they result from expansion of the local population? Do outbreaks spawn greater proportions of dispersing individuals? USDA Forest Service Gen. Tech. Rep. PSW-138. 1992. 3. It is crucial that we gain an understanding of the relative contributions of environment and inheritance to the variance we see in bark beetle phenotypes, particularly in their produc­ tion, perception and response to semiochemicals. Teale and Hager's work (this workshop) with heritability and covariance in pheromone production and response, and its responsiveness to imposed selection, is some of the first work in this exciting and much-needed line of investigation. 4. We must not allow ourselves to become complacent or flip about the methodological hurdles that lie in wait of bark beetle biologists wishing to make use of the tools of molecular biology and genetics. As noted by Seybold, by Alosi, and by Anderson (this workshop), we can benefit from the genetic and molecular knowledge already accrued for other insect taxa in our hunt for methods to answer our own genetic questions about scolytids. 15 Cuticular Hydrocarbon Research1 Marion Page2 We have been studying existing taxonomies of forest insects that are based on morphological, genetic and/or behav­ ioral characteristics to evaluate the utility of cuticular (surface) hydrocarbons as taxonomic characters (Haverty and others 1988, 1989, Page and others 1990a, 1990b). Cuticular hydrocarbons are relatively stable metabolic end products that appear to be genetically fixed. Because the insects studied so far synthesize all or most of their hydrocarbon components, hydrocarbon composition and taxonomic grouping should be related. Ideally, we would use these chemical characters much as clas­ sical taxonomists use morphology, behavior or genetics, i.e., to sort the groups of insects on the basis of surface chemical characters first, rather than after groups have already been sorted on the basis of existing (nonchemical) character criteria. However, by comparing our taxonomic separations on the basis of cuticular hydrocarbons with existing taxonomic divisions based on other characteristics, we are broadening the data base of cuticular hydrocarbons as potential taxonomic characters for forest insects. We initially started our studies on the dampwood termites, Zootermopsis, while trying to understand a synonymy of two species of scolytid cone beetles, Conophthorus ponderosae and C. lambertianae. Since these beetles have few useful diagnostic morphological characters, we decided to examine cuticular hydrocarbons as taxonomic characters. To test our understand­ ing of the current methodology, we repeated the results of Blomquist and others (1979) on Z. angusticollis. Our initial investigation produced hydrocarbon profiles that differed from those they had published. Had we made an error in methodol­ ogy? Was their hypothesis about species and caste-specific hydrocarbons correct? Had we observed population or colony variation unreported by them? On the basis of morphological characters, our termite speci­ mens were identified as Z. nevadensis, not Z. angusticollis. Blomquist (personal communication) suggested that both our laboratories reevaluate our termite collections using identical methods and gas chromatography parameters. Surprisingly, Blomquist's and our laboratory data were identical to those in our first trial but different than his published data. Had we discovered a sibling species? Additional collections and analy­ ses led us to identify an "extra" hydrocarbon phenotype of Zootermopsis and to find a morphological character for un­ equivocal identification of the three described species of Zootermopsis (Thome and Haverty 1989). We have separated all species of the dampwood termite by hydrocarbon pheno- types. Two phenotypes within a species were so different that we used these data as a basis for proposing two subspecies, Z. nevadensis nevadensis and Z. nevadensis nuttingi. Confident that we were capable of proceeding with our original purpose involving cone beetles, we embarked on stud­ ies of Conophthorus spp. and [testing hypothesis on] select species of forest insects. We conducted a study to determine the degree of similarity or diversity or both among eight of the 15 described species of Conophthorus (Page and others 1990a). From these species we identified 140 hydrocarbons occurring as individual and isomeric mixtures. Many hydrocarbons were species specific. We discovered that the relatedness of hydrocarbon profiles for each species parallels existing morphologi­ cal keys. These data support the synonymy of C. monticolae with C. ponderosae. Conophthorus from sugar pine could com­ prise a sibling species. Hydrocarbon mixtures of two eastern species, C. resinosae and C. banksianae, are identical, support­ ing the suspicion that C. banksianae may not be a valid species. Closely related pinyon cone beetles, C. cembroides and C. edulis, have similar combinations of hydrocarbons except for a unique and abundant alkene (C27:1) in C. edulis and two dimethylalkanes in C. cembroides. To date we have published the only studies on the cuticular hydrocarbons of scolytid beetles (Page and others 1990a, 1990b). We examined four species of Dendroctonus that comprise two sibling species and one pair of morphologically similar species (Page and others 1990b). Each of these four species has an abundance of information on behavioral classifications (i.e., location and patterns of larval and adult galleries), host associa­ tions, and taxonomic classification based on host-finding and mating behaviors, pheromone chemistry, and classical mor­ phological traits. The mountain pine beetle and Jeffrey pine beetle are sibling species and are difficult to separate on the basis of morphological characters. The western pine beetle can be distinguished from its close relative, the southern pine beetle, by a few morphological characters, such as larger body size, and on the basis of geographical distribution. We were able to ascertain that the cuticular hydrocarbon mixtures in these four species are species specific. The hydrocarbon patterns of the sibling species corroborate their similarity, yet a few hydrocar­ bon components are unique enough to allow separation. Western pine beetle and southern pine beetle have hydrocarbon mixtures that are not qualitatively identical. However, they are similar enough to each other to confirm that these species are closely related, as suggested by electrophoresis analyses. 1 An abbreviated version of this japer was presented at the Bark Beetle Genetics Workshop, May 17-18, 1992, Berkeley, California. 2 Research Entomologist, Pacific Southwest Research Station, USDA Forest Service, 800 Buchanan Street, Albany, CA 94710. 16 USDA Forest Service Gen. Tech. Rep. PSW-138. 1992. Bark Beetle Genetics―an Overview1 Stephen Teale and Barbara Hager2 Our understanding of bark beetle genetics is fairly rudi­ mentary. A few studies have used enzyme variation to docu­ ment population structure of Ips and Dendroctonus species in relation to geographic separation (Anderson and others 1979, 1983, Namkoong and others 1979), host trees (Langor and Spence 1991, Sturgeon and Milton 1986), dispersal (Florence and others 1982), and phenology (Gast 1987). Electrophoretic data have also been used to look at distance relationships of Ips species (Cane and others 1990b). Preliminary studies have investigated genetics of morphological (Linton and others 1984) and pheromonal (Piston and Lanier 1974) traits. The three main priority areas that we see in need of par­ ticular attention (not necessarily in this order) are areas that, until recently, have not received attention: 1. Heritable variation in traits affecting bark beetle-natural enemy interactions, in particular, the use of bark beetle phero­ mones by predators and parasitoids in prey location and how it affects heritable qualities of bark beetle populations as well as population size. The basis for this research need is reports by Raffa and Klepzig (1989) and Herms and others (1991) show- ing that the pheromone systems of Ips pini is significantly altered by predator eavesdropping. An understanding of the genetic control of pheromone production and response is essen­ tial to understanding these reciprocal predator-prey adapta­ tions. 2. Heritable variation in host selection traits of bark beetles and how it can influence population dynamics through interac­ tive selection between host trees and beetles under varying population densities. The groundwork in this area was laid by Raffa and Berryman (1987). The genetic component involved in interactions both among and within trophic levels has gener­ ally not been considered in bark beetle population biology. 3. The use of DNA techniques, particularly sequencing, in phylogenetic reconstruction. With an unparalleled array of mating systems, the family Scolytidae begs to become a model for the study of the evolution of mating systems as well as the evolu­ tion of host selection (i.e., which beetle taxa feed on which tree taxa). This can be done only in a phylogenetic context, and DNA sequences yield the most robust phylogenies. 1 An abbreviated version of this paper was presented at the Bark Beetle Genetics Workshop, May 17-18, 1992, Berkeley, California. 2 Assistant Professor and Post Doctoral Associate, respectively, Dept. of Environmental and Forest Biology, Syracuse University of New York, College of Environmental Science and Forestry, Syracuse, NY 13210. USDA Forest Service Gen. Tech. Rep. PSW-138. 1992. 17 Current Status and Critical Research Needs in Scolytid Genetics1 Kenneth F. Raffa2 During the past two decades, our knowledge of bark beetle behavior and ecology has greatly expanded. In particular, stud­ ies of pheromone communication, host plant relations, and population dynamics have increased our basic knowledge of bark beetle biology, improved our ability to manage pest spe­ cies, and provided strong models for research on other insect groups. However, bark beetles remain our most damaging forest pests nationwide. In addition, the various components of scolytid biology are usually studied in isolation, and some key gaps in our knowledge preclude effective synthesis. In my opinion, a major reason for these difficulties is that insufficient attention has been given to bark beetle genetics. Increased knowledge in this area could serve as a powerful unifying theme among the various subdisciplines, and improve our man­ agement capabilities. The lack of attention to scolytid genetics is well evidenced by the major books and review articles on this family. Most texts and chapters make no, or relatively brief, mention of genetics or the role genetics could play in population behavior. Some areas of scolytid genetics have received well-focused attention, in particular isozyme variation within and between species (Langor and Spence 1991, Stock and Guenther 1979, Stock and others 1984). These studies established polymor­ phism, heterozygosity, and population structuring across geo­ graphic regions and sometimes host species. Some basic work has been done on cytology (e.g., Lanier 1981), before the development of modern methods. However, a number of criti­ cal areas, ranging from the molecular to population levels, remain unexplored. A number of new tools are now available for studying insect genetics. However, I feel the most critical need is to generate testable hypotheses regarding how heritable aspects of scolytid biology relate to their behavior and population dynam­ ics. Milton and Schneider (1987) summarized two basic ap­ proaches to the role of genetics in insect outbreaks: (1) start with a heritable marker, and conduct a broad search for correla­ tions between gene frequencies and population fluctuation, and (2) identify life history traits whose variation would greatly influence population behavior, and determine heritable varia­ tion. Both approaches are valid, and both must be pursued to make relevant gains. As a population ecologist, I will approach the issue from the latter perspective. Hopefully, this perspec­ tive will complement equivalent suggestions arising from the marker-up, and more molecular approaches. From an ecologi­ cal/population management view, I think that three critical genetic issues must be addressed. 1 An abbreviated version of this paper was presented at the Bark Beetle Genetics Workshop, May 17-18, 1992, Berkeley, California. 2 Forest Entomologist, Dept. of Entomology, University of Wisconsin, Madison, WI 53706 18 Host Selection and the Genetics of Outbreak Behavior Genetic traits associated with host selection may be useful in predicting the onset of bark beetle outbreaks. Low density populations are typically concentrated within severely stressed trees. Yet during outbreaks, almost all trees are attacked and killed, regardless of their vigor. The mechanisms by which high beetle numbers kill trees is fairly well understood: aggre­ gation pheromones coordinate rapid mass attacks on entered trees, and the tree's resistance capabilities are exhausted. More beetles are required to kill vigorous trees than stressed trees. However, this tells us nothing about how beetles' decisions to enter trees could be influenced by their population densities. Each beetle undergoes a strict behavioral repertoire, which culminates in either host entry or resumed flight (Raffa 1988). This suggests that different selective pressures may operate on individual beetles at different beetle densities. At low popula­ tions, the likelihood of an entered beetle being joined by enough recruits to successfully colonize healthy trees is low, and so relatively discriminating behavior may be favored. Conversely, at high densities, beetles that enter healthy trees are more likely to be joined by sufficient recruits to kill the host, and so less discriminating behavior is adaptive (also, discriminating types may never find acceptable hosts, as the weakest trees are colonized by generations leading to the outbreak). Therefore, changes in gene frequencies associated with host acceptance could both result from, and partially cause, bark beetle popula­ tion increases (Raffa and Berryman 1983, Raffa 1988). The relatively high levels of within-tree, compared to spatial and temporal, genetic (electrophoretic) variation in Dendroctonus frontalis (Florence and Kulhavy 1981), and the high levels of genetic diversity that can be maintained in dispersed popula­ tions (Florence and others 1982) could lead to such directional selections. Observed behavioral differences between outbreak and nonoutbreak Ips typographus japonicus (Futura 1989) are consistent with such a pattern. There are alternative explanations of how host selection could change through time, such as individuals becoming less discriminating as their stores deplete, or pheromones eliciting host entry in addition to attraction [although Hynum (1978) observed the proportion of beetles resuming flight after landing does not decrease during mass attack]. However, the genetics of host selection should be considered a high-priority area. This can be approached by: (1) quantifying for heritable variation in beetle selectivity, (2) quantifying genetic diversity throughout the endemic and epidemic phases within an area, and between endemic and epidemic populations at one time, using polymor­ phic DNA markers such as RFLP's or RAPD's, and (3) testing alternative hypotheses. USDA Forest Service Gen. Tech. Rep. PSW-138. 1992. Role of Natural Enemies Pheromone Synthesis and Attraction Biological control of bark beetles has received only scant attention, largely because the major pests are native species. However, geographic and local variation in predator behavior may provide some avenues for better exploiting natural en­ emies. These include introducing predators from distant popu­ lations, and biasing against natural enemy removal during semiochemically based population control measures. The major insect predators locate prey by responding to bark beetle aggregation pheromones. Prey mortality is often extremely high. Thus, selective pressures exerted by predators may favor alterations in prey semiochemistry that reduce their attractiveness to predators, yet retain intraspecific functional­ ity. Several lines of evidence support this view: (1) predacious beetles, although attracted to scolytid pheromones, may show greater affinity for stereoisomers that are relatively less attrac­ tive to (and produced by) the prey (Herms and others 1991, Payne and others 1984, Raffa and Klepzig 1989); (2) minor pheromone components can have markedly different effects on predator and prey species (Seybold and others 1992c, Miller and others personal communication); (3) local predator guilds may be more attracted to distant than local populations of the same prey species (Lanier and others 1972, Raffa and Dahlsten personal communication). The underlying genetics of predator-prey interactions are completely unknown. No studies on the heritability of predator responses to bark beetle pheromones have been conducted, and only recently has individual variation in bark beetle semiochemistry been explored (see #3 below). Studies should be aimed at: (1) conducting classical hybridization studies of divergent perception phenotypes within predator species, (2) identifying DNA markers associated with pheromone percep­ tion among both predators and prey, and (3) quantifying selec­ tive pressures imposed by predators on beetle reproductive success (at both the colonizing cohort and individual beetle levels) and on predators to locate a particular prey guild. Pioneering work by Lanier (1970c) demonstrated Mende­ lian control over Ips pheromone production and response. Such inheritance patterns can contribute to demic structuring (Cane and others 1990c). Recent studies of scolytid pheromone com­ munication have shown strong inter- and intrapopulation dif­ ferences within species (Berisford and others 1990, Birgersson and others 1988, Miller and others 1989, Seybold and others 1992c). These results could have strong implications for bark beetle biology and management, particularly in guiding semiochemically based management strategies and biological control (see above). Several factors have been proposed as possible contribu­ tors to heritable genetic variation in scolytid semiochemical synthesis and response. For example, it has been proposed that some beetles do not actively engage in host searching, but only react to pheromone plumes indicative of others' success. The frequency of such types may change with beetle density (Birgersson and others 1988), in a fashion similar to the host selection model proposed in #1. If so, accompanying genetic or phenotypic markers may be useful in predicting outbreak be­ havior. Genetically based variation in pheromone chemistry may also be important in maintaining the structure of scolytid mating populations (Teale personal communication). Studies should (1) conduct classical hybridization tests among beetles, putatively divergent types (based on arrival time in aggregation sequence and relative responsiveness to host cues in laboratory assays), (2) identify DNA markers associated with putatively different behavioral types, and (3) determine the level of gene flow and demic integrity among putatively different types. USDA Forest Service Gen. Tech. Rep. PSW-138. 1992. 19 Genetic Basis of Semiochemically Mediated Bark Beetle-Predator Coevolution: Implications for Developing Mass-Trapping Technologies1 Robert A. Haack. Daniel A. Herms, Bruce D. Ayres, Matthew P. Ayres, and M.L. Doozie Snider2 Background The pheromone chemistry of Ips pini (Coleoptera: Scolytidae) has been extensively studied in recent years. In eastern North America, I. pini populations respond most strongly to ipsdienol with an enantiomeric composition of about 40 percent to 75 percent-(S)-(+) (Herms and others 1991, Lanier and others 1980, Raffa and Klepzig 1989, Teale 1990). In western populations, I. pini typically responds maximally to ipsdienol with an enantiomeric composition of 93 percent to 100 percent-(R)-(-) (Lanier and others 1980, Miller and others 1989, Seybold and others 1992d). In eastern populations of I. pini, both isomers of ipsdienol function as attractants (Teale 1990), whereas in most western populations the (-)-isomer serves as an attractant and the (+)isomer functions as an interruptant (Birch and others 1980, Seybold and others 1992d). Recently, lanierone was demon­ strated to be an important component of the aggregation phero­ mone of eastern populations of I. pini (Teale and others 1991), but it was not found in western populations (Seybold and others 1992c). Teale (1990) demonstrated in I. pini that the chirality of male-produced ipsdienol was heritable. In addition, Teale and others (personal communication) have shown a strong, positive correlation between male response to and production of enan­ tiomeric blends of ipsdienol. If positive assortive mating were common among individuals with similar ipsdienol preference/ production, then genetic substructuring of I. pini populations could easily occur. Moreover, if movement by Ips adults were limited between habitat patches (e.g., isolated pine woodlots), then further genetic divergence could result on a spatial scale. Thanasimus dubius (Coleoptera: Cleridae) is a specialist predator of Ips and other bark beetles, and can cause significant bark-beetle mortality (Riley and Goyer 1986, and references therein). Thanasimus dubius uses the pheromone of I. pini as a kairomone in prey location, and like I. pini, demonstrates prefer­ ences among different blends of ipsdienol (Herms and others 1 An abbreviated version of this paper was presented at the Bark Beetle Genetics Workshop, May 17-18, 1992, Berkeley, California. 2 Supervisory Research Entomologist, North Central Forest Experiment Station, USDA Forest Service, Stephen S. Nisbet Bldg., 1407 S. Harrison Road, East Lansing, MI 48823; Staff Entomologist, The Dow Gardens, Midland, Mich.; President, Woods Run Forest Products, Colfax, Wis.; Research Ento­ mologist, Southern Forest Experiment Station, USDA Forest Service, Pineville, La.; Research Technician, Michigan State University, East Lansing, Mich. 20 1991, Raffa and Klepzig 1989). Such variation in the kairomone response of Thanasimus could lead to differential mortality of Ips adults that respond/produce different enantiomeric blends of ipsdienol. Ips that respond/produce low-risk ipsdienol blends theoretically could escape intense predation and increase in numbers. This change in Ips pheromone production would, eventually exert a reciprocal selective force on its predators, resulting in a coevolutionary feedback system. Similar to the selective pressures described above for Thanasimus, mass-trapping with a single enantiomeric blend of ipsdienol may also cause differential mortality of Ips popula­ tions and lead to an increase in Ips that respond/produce blends of ipsdienol that differ from the pheromone bait being used. In other words, there is potential for Ips populations to evolve resistance to mass-trapping programs that utilize a single blend of ipsdienol. Current Research Objectives We are interested in developing mass-trapping technology for managing I. pini populations in midwestern pine woodlots. This will require an understanding of the relative genetic and environmental components to variation in the pheromone re­ sponses of I. pini and its specialist predators (Herms and others 1991). Heritable variation in beetle responses to different enan­ tiomeric blends of the aggregation pheromone ipsdienol im­ plies the potential for Ips populations to evolve resistance to mass-trapping programs that use individual blends of ipsdienol. This risk is especially great if Ips populations are genetically substructured on the basis of pheromone preferences or if gene flow between pine woodlots is rare. The risks are complicated by secondary impacts on predators that exploit ipsdienol as a kairomone for prey location. Mass-trapping has potential as an economically viable, ecologically sound control measure, but its sustained effectiveness may be compromised by disregard­ ing the above risks. We will develop models to predict evolu­ tionary responses of Ips and their predators to different masstrapping scenarios. The expected product is an economical resistance management program, probably based on sequential or simultaneous use of different ipsdienol blends, and including considerations of predator-prey dynamics. Current Research Question To what extent are populations of I. pini and T. dubius genetically substructured within and between habitat patches (pine woodlots)? USDA Forest Service Gen. Tech. Rep. PSW-138. 1992. Experiments in Progress Future Research Plans 1. Electrophoretic studies to test for genetic differentiation within and among populations of I. pini (and its clerid predator, T. dubius) based on ipsdienol-blend preferences. 2. Mark-recapture studies to test pheromone-response specificity of I. pini. 3. Comparison of pheromone-response profiles of I. pini populations from several isolated red-pine stands in Michigan. Assuming a genetic component exists to the observed intrapopulation variation, then genetic drift or differential selection pressures could result in stand-to-stand differences on a local scale. We plan to initiate replicated mass-trapping studies in several isolated red pine woodlots in Michigan in 1992. If there is a genetic component to the observed variation in pheromone response, we should be able to manipulate pheromone response profiles by exerting selection through mass-trapping. Directional selection (e.g., using one pheromone blend) should change the response profile, whereas stabilizing selection (e.g., using several blends) should maintain it. If the genetic component to pheromone response is weak relative to environmental influences, or if there is substantial gene flow within and among woodlots, then mass-trapping should have little effect on response profiles of local I. pini populations. USDA Forest Service Gen. Tech. Rep. PSW-138. 1992. 21 Population Genetics of Spruce Bark Beetle Ips typographus (Col., Scolytidae) and Related Ips Species1 Christian Stauffer, Renate Leitinger, Erwin Fuehrer2 Enzyme electrophoresis proved to be a useful method to study population genetic aspects in Ips typographus (L.) because of three highly polymorphic loci. Aspartate aminotransferase locus-2 (Aat-2), physiologically involved in the transfer of aminogroups, has six alleles per population. Amylase locus1 (Amy-1), physiologically involved in the hydrolysis of starch, has 10 alleles per population, and esterase locus-2 (Est-2), physiologically involved in processes of detoxification and the metabolism of juvenile hormone, has 16 alleles per population. The allozymes, pattern, and the mode of inheritance of nearly all alleles were genetically proved. The different amount of alleles among allozymes could reflect the physiological role of the enzymes (Johnson 1974); therefore most studies were calculated individually (Stauffer and others 1992b). A related but distinctly different species is the spruce bark beetle Ips amitinus. This relationship could be also demonstrated by enzyme electrophoresis. The existence of I. amitinus and I. amitinus var. montana (Fuchs 1913), which appeared to be doubtful, was investigated by behavioral, morphological and biochemical (cuticular analysis and enzyme electrophoresis) techniques. Because no obvious differences between the two races could be found, the distinction of a var. montana becomes very unprobable (Zuber and others 1992). Efficiency of a commercial pheromone product, which is commonly used in central Europe for trapping I. typographus, proved to be regionally different. A genetic basis of these differences was assumed. Seventeen European populations of this species, collected from infested logs, were studied. The dendrograms and an Alscal analysis of Aat-1 and Est-2 clustered three groups―the northern (Scandinavian) populations, the southern (alpine) populations, and the middle European (prealpine/Czechoslovakian) populations. Distribution of these three genetic groups corresponds to the three parts of natural distribution of the host tree, Picea abies, in Europe, each of them originating from different glacial refuge areas (Central Russia, Carpathian Mountains, Dinaric Alps, respectively). Thus it seems likely that I. typographus populations had been subject to genetic differentiation by long-term isolation during the glacial period and postglacial remigration to middle and north Europe, parallel to its host tree (Leitinger and others 1992). Preliminary results indicate, moreover, that within I. typographus populations the genetic variance of samples collected from pheromone traps differs more or less from those which had been collected from infested logs. As to the genetic divergence between log-trapped and pheromone-trapped samples, a slight trend from middle European lowland populations toward upland and Scandinavian populations, respectively, was ascertained (Leitinger 1991). Because subpopulations of I. typographus, which are colonizing different spruce logs, also differ genetically, a genetically determined variance of orientation patterns within this bark beetle species is assumed. This phenomenon may concern the pioneer problem too. Voltinism is another property with epidemiological significance, which seems to be genetically determined within populations of I. typographus. Depending on different altitudes, the proportion of obligately and facultatively diapausing beetles is different. First attempts to biochemically identify both diapausing types revealed promising results. From the epidemiological point of view, work will be focused on functional and ecological population genetics. Using the allozyme method for identification of different "types," the functionality of enzymes as well as enzyme activity must be studied more in detail. We proposed to begin with the juvenile hormone esterase (Stauffer and others 1992a), the monooxygenase (Sturgeon and Robertson 1985), and the general esterases. 1 An abbreviated version of this paper was presented at the Bark Beetle Genetic Workshop, May 17-18, 1992, Berkeley, CA. 2 Institute of Forest Entomology, Forest Pathology and Forest Protection, Universität für Bodenkultur, A-1190 Vienna, Austria. 22 USDA Forest Service Gen. Tech. Rep. PSW-138. 1992. Novel Bark Beetle Research Possible with New Genetic Techniques1 Ken Hobson and Owain Edwards2 Prospects The new molecular techniques in genetics offer solutions to questions about bark beetle biology in two principal areas. The first of these is dominated by questions of phylogenetic interest. For example, the unity of Dendroctonus valens as a species (e.g., Pajares and Lanier 1990) could be explored with precision. Geographic substructuring of other scolytid taxa, including regional differences in pheromone production and response, partial mating barriers in sibling Ips and so on, share this phylogenetic approach. Much of this work is well underway. The second area, which is wide open for new ideas, uses the ability of the new techniques to discriminate individuals and populations to address questions from population dynamics and behavior (Slatkin 1987). It is possible now with mammalian and bird DNA to characterize individuals and verify genetic contributions of parents to their offspring. Paternity analyses of this sort have recently provided some surprising insights in ornithological literature where extra-pair copulations have been found to be much more common than was previously recognized. Little is known about bark beetle behavior under the bark―e.g., whether supposedly monogamous Dendroctonus females sometimes mate with more than one male before ovipositing their first clutch of eggs, whether there is selection by females for males with particular characteristics, and so on. The ability to identify individuals and their descendants would allow paternity analyses of eggs from scolytid galleries. Male-male competition may be occurring in D. valens galleries where there are three adults present (Hobson 1992). Paternity analysis of egg clutches of even single pairs of beetles could provide insights into the frequency of pre-emergence mating. The ability to identify beetles from subpopulations and track their descendants may allow us to answer questions such as whether patch kills of trees by D. brevicomis are caused by beetles which are the descendants of an earlier nearby infestation or instead are beetles which are drawn from the general population of all dispersing adults. Similarly, typical bark beetle dispersal distances can be assessed by looking at variability along transects through beetle populations. The number of migrants per generation between populations can be estimated from FST statistics as estimated using the allele frequencies of several different genes. Studies employing electrophoresis have had difficulty in the past find1 An abbreviated version of this paper was presented at the Bark Beetle Genetics Workshop, May 17-18, 1992, Berkeley, California. 2 Graduate Students, Dept. of Entomology, Univ. of California, 201 Wellman Hall, Berkeley, CA 94720. USDA Forest Service Gen. Tech. Rep. PSW-138. 1992. ing enough polymorphism to conduct very fine-grained analyses of this sort. Now several of the new molecular techniques permit rapid analysis of DNA with enough resulting information to carry out gene flow and dispersal analyses with dozens of markers resulting in more precise estimates. Contrasts that exist between beetles in an endemic or early infestation population and an epidemic or late infestation population may involve genetic drift or perhaps repeating cycles of shifting gene frequencies. Methods Sequencing of both mitochondrial and genomic DNA has provided information previously unattainable to systematists. These data, in conjunction with other chemical phenotype data, such as cuticular hydrocarbon analysis (e.g., Page and others 1990b), will provide answers to many bark beetle phylogenetic questions. However, discrimination of bark beetle populations requires molecular techniques that detect higher levels of polymorphism. DNA sequencing of highly polymorphic regions of DNA can be useful, but these regions must first be located. Sequencing is costly and labor-intensive, so its application in population genetics can only be limited. Restriction Fragment Length Polymorphism (RFLP) has also been used for the detection of DNA polymorphism. However, RFLP analysis requires that knowledge of a polymorphic region to target exists, and its sequence must be known so that a DNA probe can be constructed. RFLP analysis is much less labor-intensive than sequencing, but it is still quite costly. Radioactivity is also necessary for both sequencing and RFLP. Because of the drawbacks of these DNA techniques, protein allozyme analysis has been the most popular method for performing genetic polymorphism studies. Allozyme analysis is much less expensive, but it is often difficult to find polymorphic loci. The polymerase chain reaction (PCR) has made it much simpler and more cost-effective to perform molecular analyses at the level of the DNA (Arnheim and others 1990). Because PCR dramatically increases the copy number of specific DNA sequences, analyses using PCR can be performed with very little starting DNA. DNA sequencing of PCR-amplified DNA eliminates the need for cloning, which dramatically reduces the cost and effort. RFLP analysis using PCR-amplified DNA eliminates the need for a probe, so less sequence information is required, and the need for radioactivity is eliminated. However, the level of polymorphism detected using these techniques may still limit their usefulness in answering some population genetics questions. 23 In the last two years, a PCR-based technique called Ran­ domly Amplified Polymorphic DNA (RAPD) has proven very successful for performing genetic analyses in plants (Williams and others 1990). Its advantages are that it detects higher levels of polymorphism than either isozyme or RFLP analyses, and that no prior DNA sequence knowledge is necessary. A disad­ vantage to this technique is that the electrophoretic bands it produces are inherited as completely dominant traits as op­ posed to semi-dominant traits as in RFLP or isozyme analysis, which can make genetic analyses somewhat more complicated. It has also been reported that some RAPD bands are not inher­ ited in a Mendelian fashion (Riedy and others 1992). Success with this technique has been reported with some insects (Edwards, unpublished results), including haplodiploid species where the dominance problem can be avoided by performing the analysis on males. The characterization of individuals, as needed for paternity analysis, requires an analysis that targets highly variable re­ 24 gions of DNA. In humans, this is accomplished by analyzing Variable Number Tandem Repeats (VNTR's), which are highly mutable (10-3-10-4) regions of repetitive DNA (Jeffreys and others 1985). The major disadvantage of this technique is that few VNTR's have been found in insects (Bigot and others 1990, Blanchetot 1991, Moritz and others 1991), so it is likely that the application of this analysis to the study of bark beetles would first require significant time and effort to locate the repetitive DNA. Once this was accomplished a very high de­ gree of polymorphism would be available for free grain dis­ persal studies and behavioral investigations requiring charac­ terization of an individual and its progeny. Given the diversity of techniques for assessing polymor­ phism and discriminating groups under study, one of our first goals might be to survey the level of polymorphism that is found in the groups of interest. Then the appropriate technique for a particular problem can be chosen. USDA Forest Service Gen. Tech. Rep. PSW-138. 1992. Systematics Research1 Donald E. Bright2 The following comments about bark beetle genetics reflect my current thoughts on the status of bark beetle systematics and present some of my ideas concerning future needs. The basic alpha taxonomy of North American bark beetles is essentially finished. All of the species occurring in North America are, more or less, well characterized at the alpha level and their taxonomy is well established. An authoritative name can now be consistently given to almost any specimen or series of specimens. Keys and descriptions to all species in all genera of North American scolytids are now available. A catalog of the world fauna will soon be available. It is extremely unlikely that very many morphologically distinct, unnamed species of North American Scolytidae remain to be discovered. Any newly dis­ covered species will probably be found in either host plants of very restricted distributions (e.g., Santa Lucia fir), in the vari­ ous desert shrubs or deciduous trees, or as currently unrecog­ nized sibling species. I would estimate that fewer than 10 morphologically distinct, unnamed species remain undiscov­ ered in North America. This, however, does not mean that systematic investiga­ tions are no longer needed. Indeed, we are now able to embark on the most exciting aspect of systematic research. With all of this basic knowledge, we are now left with the task of resolving a number of species groups, subspecies, host/geographic races, sibling species, and so on. Unresolved species groups are known to exist in Dendroctonus, Ips, Pityophthorus, probably Scolytus, and undoubtedly in a number of less studied genera. Also, on a more fundamental level, there is a need to develop information that will improve understanding of phylogeny, evolution, ge­ neric and tribal relationships, and other such basic systematic questions. Morphological studies have been the backbone of taxo­ nomic research for decades and will probably remain of pri­ mary importance for decades to come. However, we now have an exciting array of other methods that may prove to be of immense importance. Isozyme analysis, cuticular hydrocarbon analysis and PCR techniques will be increasingly used in the future to help resolve some of the questions mentioned above. A molecular systematics laboratory is being established at the Biological Resources Division, Canada Department of Ag­ riculture, in Ottawa, Ontario. I hope it will be able to address some of the questions raised above. One concern for the future of scolytid systematics is that in less than 10 years the currently working North American systemists will have all retired. I am not aware that any current graduate student is working on scolytid systematics, nor are any young, currently employed researchers working on this group. I urge university faculty to seek out qualified individuals and guide them into the systematic study of the Scolytidae. Forest entomology has been fortunate during the past three decades in having several active individuals interested in sys­ tematic studies of bark beetles, and this interest needs to be continued. 1 An abbreviated version of this paper was presented at the Bark Beetle Genetics Workshop, May 17-18, 1992, Berkeley, California. 2 Research Scientist, Canada Dept. of Agriculture, Systematics Research, Centre for Land and Biological Resources Research, Ottawa, Ontario K1A 0C6 USDA Forest Service Gen. Tech. Rep. PSW-138. 1992. 25 References Alosi, M. Carol; Robertson, Jacqueline L. 1992. Biochemical indicators of population status of bark beetles [this proceedings]. Amman, G.D. 1982. Characteristics of mountain pine beetles reared in four pine hosts. Environmental Entomology 11: 590-593. Amman, G.D.; Cole, W.E. 1983. Mountain pine beetle dynamics in lodgepole pine forest. Part II: population dynamics. Gen. Tech. Rep. WT-145. Ogden, UT: Intermountain Forest and Range Experiment Station, Forest Service, U.S. Department of Agriculture; 59 p. Amman, G.D.; Pasek, J.E. 1986. Mountain pine beetle in ponderosa: effects of phloem thickness and egg gallery density. Res. Paper INT-376. Ogden, UT: Intermountain Forest and Range Experiment Station, Forest Service, U.S. Department of Agriculture; 7 p. Amman, G.D.; Stock, M.W. Effect of phloem thickness on heterozygosity of laboratory-reared mountain pine beetles (Dendroctonus ponderosae Hopkins, Coleoptera: Scolytidae). Unpublished draft supplied by author. Anderson, W.W.; Berisford, C.W. 1992. Bark beetle genetics. [this Proceed­ ings] Anderson, W.W.; Berisford, C.W.; Kimmich, R.H. 1979. Genetic differences among five populations of the southern pine beetle. Annals of Entomo­ logical Society of America 72: 323-327. Anderson, W.W.; Berisford, C.W.; Tumbow, R.H.; Brown, C.J. 1983. Genetic differences among populations of the black turpentine beetle, Dendroctonus terebrans, and an engraver beetle, Ips calligraphus (Coleoptera: Scolytidae). Annals of Entomological Society of America 76: 896-902. Arnheim, N.; White, T.; Rainey, W.E. 1990. Application of PCR: organismal and population biology. BioScience 40: 174-182. Bartlet, R.J.; Schaner, A.M.; Jackson, L.L. 1986. Aggregation pheromones in five taxa of the Drosophila virilis species group. Physiological Entomol­ ogy 11: 367-376. Bentz, B.J.; Stock, M.W. 1986. Phenetic and phylogenetic relationships among ten species of Dendroctonus bark beetles (Coleoptera: Scolytidae). Annals of Entomological Society of America 79: 527-534. Berisford, C.W.; Payne, T.L.; Berisford, Y.C. 1990. Geographical variation in response of southern pine beetle (Coleoptera: Scolytidae) to aggregat­ ing pheromones in laboratory assays. Environmental Entomology 19: 1671-1674. Berryman, A.A. 1972. Resistance of conifers to invasion by bark beetlesfungus associations. BioScience 22: 598-602. Bigot, Y.; Hamelin, M: H.; Periquet, G. 1990. Heterochromatin condensation and evolution of unique satellite-DNA families in two parasitic wasp species: Diadromus pulchellus and Eupelmus vuilleti (Hymenoptera). Molecular Biology and Evolution 7: 351-364. Birch, M.C.; Light, D.M.; Wood, D.L.; Browne, J.E.; Silverstein, R.M.; Bergot, B.J.; Ohloff, G.; West, J.R.; Young, J.C. 1980. Pheromonal attraction and allomonal interruption of Ips pini in California by the two enantiomers of ipsdienol. Journal of Chemical Ecology 6(3): 703-717. Birgersson, G.; Schlyter, F.; Bergstrom, G.; Lofquist, J. 1988. Individual variation in the aggregation pheromone of the spruce bark beetle, Ips typographus. Journal of Chemical Ecology 14: 1737-1761. Birgersson, G.; Schlyter, F.; Lofqvist, J.; Bergstrom, G. 1984. Quantitative variation of pheromone components in the spruce bark beetle Ips typographus from different attack phases. Journal of Chemical Ecology 10: 1029-1055. 26 Blanchetot, A. 1991. A Musca domestica satellite sequence detects indi­ vidual polymorphic regions in insect genome. Nucleic Acid Research 19: 929-932. Blomquist, G.J.; Howard, R.W.; McDaniel, C.A. 1979. Structure of the cuticular hydrocarbons of the termite Zootermopsis angusticollis (Hagen). Insect Biochemistry 9:371-374. Bright, D.E. 1976. The insects and arachnids of Canada, Part 2. The bark beetles of Canada and Alaska. Publication 1576. Ottawa, Ontario, Canada: Canadian Department of Agriculture; 241 p. Bright, D.E. Systematics of bark beetles. In: Schowalter, T.D.; Filip, G.M., eds. Beetle-pathogen interactions in conifer forests. New York: Aca­ demic Press. [In press.] Bright, Donald E. 1992. Systematics research [this Proceedings] Bright, D.E., Jr.; Stark, R.W. 1973. The bark and ambrosia beetles of Califor­ nia: Scolytidae and Platypodidae. Bulletin California Insect Survey 16. Berkeley: University of California Press; 169 p. Bright, D.E.; Stock, M.W. 1982. Taxonomy and geographic variation. In: Mitton, J.B., Sturgeon, K.B., eds. Bark beetles in North American coni­ fers. Austin: University of Texas Press; 46-73. Brillinger, D.R. 1990. Spatial-temporal modelling of spatially aggregate birth data. Survey Methodology 16: 255-269. Bush, G.L. 1969. Sympatric host race formation and speciation in frugivo­ rous flies in genus Rhagoletis (Diptera, Tephritidae). Evolution 23: 237251. Cane, J.H. 1983. Chemical evolution and chemosystematics of the Dufour's gland secretions of the lactone-producing bees (Hymenoptera: Colletidae, Halictidae, Oxaeidae). Evolution 37: 657-674. Cane, James H. 1992. Genetic characters for bark beetle phylogenies [this Proceedings]. Cane, J.H.; Merrill, L.D.; Wood, D.L. 1990a. Attraction of pinyon pine bark beetle, Ips hoppingi, to conspecific and I. confusus pheromones (Co­ leoptera: Scolytidae). Journal of Chemical Ecology 16(10): 2791-2798. Cane, J.H.; Stock, M.W.; Wood, D.L.; Gast, S.J. 1990b. Phylogenetic rela­ tionships of Ips bark beetles (Coleoptera: Scolytidae): Electrophoretic and morphometric analyses of the grandicollis group. Biochemical Sys­ tematics and Ecology 18: 359-368. Cane, J.H.; Wood, D.L.; Fox, J.W. 1990c. Ancestral semiochemical attrac­ tion persists for adjoining populations of sibling Ips bark beetles (Co­ leoptera: Scolytidae). Journal of Chemical Ecology 16: 993-1013. Charlesworth, B.; Langley, C.H. 1989. The population genetics of Drosophila transposable elements. Annual Review of Genetics 23: 251-287. Clarke, Geoffrey M.; McKenzie, Leslie J. 1992. Fluctuating asymmetry as a quality control indicator for insect mass rearing processes. Journal of Economic Entomology 85: 2045-2050. Correll, J.C.; Gordon, T.R.; McCain, A.H. 1992. Genetic diversity in Califor­ nia and Florida populations of the pitch canker fungus Fusarium subglutinans f. sp. pini. Phytopathology 82: 415-420. Coyne, J.F.; Lott, L.H. 1976. Toxicity of substances in pine oleoresin to southern pine beetles. Journal of Georgia Entomological Society 11: 301305. Cracraft, J.; Helm-Bychowski, K. 1991. Parsimony and phylogenetic infer­ ence using DNA sequences: some methodological strategies. In: Miyamoto, M.M.; Cracraft, J., eds. Phylogenetic analysis of DNA sequences. New York: Oxford University Press; 184-220. USDA Forest Service Gen. Tech. Rep. PSW-138. 1992. Dauterman, W.C.; Hodgson, E. 1978. Detoxification mechanisms in insects. In: Rockstein, M., ed. Biochemistry of insects. New York: Academic Press: 541-577. Diel, S.R.; Bush, G.L. 1984. An evolutionary and applied perspective of insect biotypes. Annual Review of Entomology 29: 471-504. Engels, W.R. 1983. The P family of transposable elements in Drosophila. Annual Review of Genetics 17: 315-344. Fish, R.H.; Browne, L.E.; Bergot, B.J. 1984. Pheromone biosynthetic pathways: conversion of ipsdienone to (-)-ipsdienol, a mechanism for enantioselective reduction in the male bark beetle, Ips paraconfusus. Journal of Chemical Ecology 10: 1057-1064. Fish, R.H.; Browne, L.E.; Wood, D.L.; Hendry, L.B. 1979. Pheromone biosynthetic pathways: Conversions of deuterium labelled ipsdienol with sexual and enantioselectivity in Ips paraconfusus Lanier. Tetrahedron Letters 17: 1465-1468. Florence, L.Z.; Johnson, P.C.; Coster, J.E. 1982. Behavioral and genetic diversity during dispersal: analysis of a polymorphic esterase locus in southern pine beetle, Dendroctonus frontalis. Environmental Entomology 11: 1014-1018. Florence, L.Z.; Kulhavy, D.L. 1981. Genetic variation and population structure of the southern pine beetle, Dendroctonus frontalis. In: Stock, M.W., ed. Application of genetics and cytology in insect systematics and evolution: proceedings of symposium, National ESA Meeting; December 1-2, 1980; Atlanta, GA. Moscow, ID: Forest, Wildlife, and Range Experiment Station, Univ. of Idaho; 141-152. Fox, J.W.; Wood, D.L.; Cane, J.H. 1991 a. Interspecific pairing between two sibling Ips species (Coleoptera: Scolytidae). Journal of Chemical Ecology 17: 1421-1435. Fox, J.W.; Wood, D.L.; Koehler, C.S. 1990. Distribution and abundance of engraver beetles (Scolytidae: Ips Species) on Monterey pines infected with pitch canker. Canadian Entomologist 122: 1157-1166. Fox, J.W.; Wood, D.L.; Koehler, C.S.; O'Keefe, S.T. 1991b. Engraver beetles (Scolytidae: Ips species) as vectors of the pitch canker fungus, Fusarium subglutinans. Canadian Entomologist 123: 1355-1367. Fuchs, A.G. 1913. Forstzoologiche Ergebnisse einer Sommerreise ins Engadin. III. Die Arven, Larchenund Fichtenborkenkafer des Engadin. Naturwissenschaftliche Zeitschrift fur Forst-und Land-wirtschaft 11(2); 65-86. Furniss, M.M.; Schenk, J.A. 1969. Sustained natural infestations by the mountain pine beetle in seven new Pinus and Picea hosts. Journal of Economic Entomology 62: 518-519. Futura, K. 1989. A comparison of endemic and epidemic populations of the spruce beetle (Ips typographus japonicus Niijima) in Hokkaido. Journal of Applied Entomology 107: 289-295. Gast, S.J. 1987. Factors affecting host and pheromone attraction and a comparison of genetic diversity in consecutive generations of Ips pini (Say)(Coleoptera: Scolytidae) in Idaho. Moscow: Univ. of Idaho. M.S. Thesis. Gast, S.J.; Furniss, M.M. Electrophoretic comparison of Dendroctonus punctatus LeConte and D. micans (Kugelann) (Coleoptera: Scolytidae). Unpublished draft supplied by author. Gast, S.J.; Stock, M.W. Genetic diversity in consecutive generations of Ips pini (Say) (Coleoptera: Scolytidae) in Idaho. Unpublished draft supplied by author. Gittleman, J.L.; Kot, M. 1$90. Adaptation: statistics and a null model for estimating phylogenetic effects. Systematic Zoology 39: 227-241. Green, M.M. 1980. Transposable elements in Drosophila and other Diptera. Annual Review of Genetics 14: 109-120. USDA Forest Service Gen. Tech. Rep. PSW-138. 1992. Greene, Lula E. 1992. Qualitative genetics of mountain pine beetle in central Oregon [this Proceedings]. Haack, Robert A.; Herms, Daniel A.; Ayres, Bruce D.; Ayres, Matthew P.; Snider, M.L. Doozie. 1992. Genetic basis of semiochemically mediated bark beetle-predator coevolution: implications for developing mass-trapping technologies [this Proceedings]. Hackett, K.J.; Lynn, D.E.; Williamson, D.L.; Ginsberg, A.S.; Whitcomb, R.H. 1986. Cultivation of the Drosophila sex-ratio spiroplasma. Science 232: 1253-1255. Haverty, M.I.; Page, M.; Blomquist, G.J. 1989. Value of cuticular hydrocarbons for identifying morphologically similar species of pine cone beetles. Proc. 3rd Cone and Seed Insect Working Party, IUFRO, Victoria, B.C., Canada. 50-62. Haverty, M.I.; Page, M.; Nelson, L.J.; Blomquist, G.J. 1988. Cuticular hydrocarbons of the dampwood termites, Zootermopsis: Intra- and intercolony variation and potential as taxonomic characters. Journal of Chemical Ecology 14: 1035-1058. Hayes, J.L.; Robertson, J.L. 1992. An (ecologically biased) view of the current status of bark beetle genetics and future research needs. [this Proceedings] Hendry, L.B.; Piatek, B.; Browne, L.E.; Wood, D.L.; Byers, J.A.; Fish, R.H.; Hicks, R.A. 1980. In vivo conversion of a labelled host plant chemical to pheromones of the bark beetle Ips paraconfusus. Nature 284: 485. Herms, D.A.; Haack, R.A.; Ayres, B.D. 1991. Variation in a semiochemicalmediated prey-predator interaction: Ips pini (Scolytidae) and Thanasimus dubius (Cleridae). Journal of Chemical Ecology 17: 1705-1714. Higby, P.K.; Stock, M.W. 1982. Genetic relationships between two sibling bark beetle species, the Jeffrey pine beetle and the mountain pine beetle, in northern California. Annals of Entomological Society of America 75: 668-674. Hillis, D.M.; Bull, J.J.; White, M.E.; Badgett, M.R.; Molineux, I.J. 1992. Experimental phylogenetics: generation of a known phylogeny. Science 255: 589-592. Hobson, K.R. 1992. Studies on host selection and biology of Dendroctonus valens and fungal host interactions of Dendroctonus brevicomis. Berkeley: University of California. Dissertation. Hobson, Ken; Edwards, Owain. 1992. Novel bark beetle research possible with new genetic techniques. [this Proceedings] Homvelt, R.; Christiansen, E.; Solheim, H.; Wang, S. 1983. Artificial inoculation with Ips typographus-associated fungi can kill Norway spruce trees. Meddelelser fra Norsk Instituut for Skogforskning 38(4): 1-20. Hynum, B.G. 1978. Migration phase interactions between the mountain pine beetle and lodgepole pine. Pullman: Washington State University. Dissertation. Ikeda, H. 1965. Interspecific transfer of the "sex-ratio" agent of Drosophila willistoni in Drosophila bifasciata and Drosophilia melanogaster. Science 147: 1147-1148. Jeffreys, A.J.; Wilson, V.; Thein, S.L. 1985. Hypervariable "minisatellite" regions in human DNA. Nature 314: 67-73. Johnson G.B. 1974. Enzyme polymorphism and metabolism. Science 184: 283-292. Kaneshiro, K.Y. 1988. Speciation in the Hawaiian Drosophila. BioScience 38: 258-263. Kemp, W.P.; Kalaris, T.M.; Quimby, W.F. 1989. Rangeland grasshopper (Orthoptera: Acrididae) spatial variability: Macroscale population assessment. Journal of Economic Entomology 82: 1270-1276. 27 Langor, D.W. 1989. Host effects on the phenology, development, and mortality of field populations of the mountain pine beetle, Dendroctonus ponderosae Hopkins (Coleoptera: Scolytidae). Canadian Entomologist 121: 149-157. Langor, D.W.; Spence, J.R. 1991. Host effects on allozyme and morphological variation of the mountain pine beetle, Dendroctonus ponderosae Hopkins (Coleoptera: Scolytidae). Canadian Entomologist 123: 395-410. Langor, D.W.; Spence, J.R.; Pohl, G.R. 1990. Host effects on fertility and reproductive success of Dendroctonus ponderosae Hopkins (Coleoptera: Scolytidae). Canadian Entomologist 121: 149-157. Lewis, E.E.; Cane, J.H. 1992. Inefficacy of courtship stridulation as a premating ethological barrier for Ips bark beetles (Coleoptera: Scolytidae). Annals of Entomological Society of America 85(4): 517-524. Liebhold, A.M.; Elkinton, J.S. 1989. Characterizing spatial patterns of gypsy moth regional defoliation. Forest Science 35: 557-568. Linton, D.A.; Safranyik, L.; Whitney, H.S.; Spanier, O.J. 1984. Possible genetic control of color morphs of spruce beetles. Research Notes 4: 53. Victoria, B.C., Canadian Forest Service. McCambridge, W.F. 1975. Scotch pine and mountain pine beetles. Green Thumb 32: 87. Lanier, G.N. 1967. Biosystematic, cytological, and sex ratio studies of closely related bark beetles (Coleoptera: Scolytidae). Berkeley: University of California; 110 pp. Dissertation. Merriam, J.; Ashbumer, M.; Hard, D.L.; Kafatos, F.C. 1991. Toward cloning and mapping the genome of Drosophila. Science 254: 221-225. Lanier, G.N. 1970a. Biosystematics of the genus Ips (Coleoptera: Scolytidae) in North America. Hopping's group III. Canadian Entomologist 102: 1404-1423. Merrill, L.A. 1991. Biological barriers to hybridization in closely related species of Ips (Coleoptera: Scolytidae). Berkeley: University of California; 121 p. Dissertation. Lanier, G.N. 1970b. Biosystematics of North American Ips (Coleoptera: Scolytidae): Hopping's group IX. Canadian Entomologist 102: 11391163. Lanier, G.N. 1970c. Sex pheromones: abolition of specificity in hybrid bark beetles. Science 169: 71-72. Lanier, G.N. 1972. Biosystematics of the genus Ips (Coleoptera: Scolytidae) in North America. Hopping's groups IV and X. Canadian Entomologist 104: 361-388. Lanier, G.N. 1981. Cytotaxonomy of Dendroctonus. In: Stock, M.W., ed. Application of genetics and cytology in insect systematics and evolution. proceedings of symposium, National ESA Meeting; December 1-2, 1980; Atlanta, GA. Moscow, ID: Forest, Wildlife, and Range Experiment Station, University of Idaho; 33-66. Lanier, G.N.; Birch, M.C.; Schmitz, R.F.; Furniss, M.M. 1972. Pheromones of Ips pini (Coleoptera: Scolytidae): variation in response among three populations. Canadian Entomologist 104: 1917-1923. Lanier, G.N.; Claesson, A.; Stewart, T.; Piston, J.; Silverstein, R.M. 1980. Ips pini: the basis for interpopulational differences in pheromone biology. Journal of Chemical Ecology 6: 677-687. Lanier, G.N.; Oliver, J.H. 1966. Sex-ratio condition: unusual mechanisms in bark beetles. Science 153: 208-209. Lanier, G.N.; Wood, D.L. 1975. Specificity of response to pheromones in the genus Ips (Coleoptera: Scolytidae). Journal of Chemical Ecology 1(1): 913. Miller, D.R. 1990. Reproductive and ecological isolation: community structure in the use of semiochemicals by pine bark beetles (Coleoptera: Scolytidae). Burnaby, B.C., Canada: Simon Fraser University; 166 p. Dissertation. Miller, D.R.; Borden, J.H.; Slessor, K.N. 1989. Inter- and intrapopulation variation of the pheromone ipsdienol produced by male pine engravers, Ips pini (Say) (Coleoptera: Scolytidae). Journal of Chemical Ecology 15(1): 233-247. Mitchell, R.G.; Preisler, H.K. 1991. Analysis of spatial patterns of lodgepole pine attacked by outbreak populations of the mountain pine beetle. Forest Science 37: 1390-1408. Mitter, C.; Farrell, B.; Futuyma, D.J. 1991. Phylogenetic studies of insectplant interactions: insights into the genesis of diversity. Tree 6: 290-293. Mitton, C.; Schneider, J.C. 1987. Genetic change and insect outbreaks. In: Barbosa, P.; Schultz, J.C., eds. Insect outbreaks. New York: Academic Press; 505-532. Mitton, J.B.; Sturgeon, K.B. 1982. Bark beetles in North American conifers: a system for the study of evolutionary biology. Austin: University of Texas Press; 527 p. Moritz, R.F.A.; Meusel, M.S.; Haberl, M. 1991. Oligonucleotide DNA fingerprinting discriminates super- and half-sisters in honeybee colonies (Apis melifera L.). Naturwissenschaften 78: 422-424. Namkoong, G.; Roberds, J.H.; Nunnally, L.B.; Thomas, H.A. 1979. Isozyme variations in populations of southern pine beetles. Forest Science 25: 197-203. Leary, R.F.; Allendorf, F.W. 1989. Fluctuating asymmetry as an indicator of stress: implications for conservation biology. Trends in Ecology & Evolution 4: 214-217. Novitski, E.; Peacock, W.J.; Engel, J. 1965. Cytological basis of "sex ratio" in Drosophila pseudoobscura. Science 148: 516-517. Leitinger, R. 1991. Populationsgenetische Untersuchungen an Ips typographus (Col., Scolytidae) outer geographischem and verhaltens-biologischem Aspekt. Dipl. Thesis BOKU Vienna. Owen, D.R.; Lindahl, K.Q., Jr.; Wood, D.L.; Parmeter, J.R., Jr. 1987. Pathogenicity of fungi isolated from Dendroctonus valens, D. brevicomis, D. ponderosae to ponderosa pine seedlings. Phytopathology 77: 631-636. Leitinger R.; Stauffer, Ch.; Fuehrer, E. 1992. Postglacial remigration of the European Ips typographus (Col.: Scolytidae) populations from three refugial areas. Entomologia generalis. Unpublished draft supplied by author. Lewis, E.E.; Cane, J.H. 1990a. Stridulation as a primary antipredator defence of a beetle. Animal Behavior 40: 1003-1004. Lewis, E.E.; Cane, J.H. 1990b. Pheromonal specificity of Southeastern Ips pine bark beetles reflects phylogenetic divergence (Coleoptera: Scolytidae). Canadian Entomologist 122: 1235-1238. 28 Page, Marion. 1992. Cuticular hydrocarbon research [this Proceedings]. Page, M.; Nelson, L.J.; Blomquist; G.J.; Seybold, S.J. 1992. Cuticular hydrocarbons as chemosystematic characters of pine engraver beetles (Ips sp.) (Coleoptera: Scolytidae) in the Grandicollis subgeneric group. Unpublished draft supplied by author. Page, M.; Nelson, L.J.; Haverty, M.I.; Blomquist, G.J. 1990a. Cuticular hydrocarbons of eight species of North American cone beetles, Conophthorus Hopkins. Journal of Chemical Ecology 16: 1178-1193. Page, M.; Nelson, L.J.; Haverty, M.I.; Blomquist, G.J. 1990b. Cuticular hydrocarbons as chemotaxonomic characters for bark beetles: Dendroctonus USDA Forest Service Gen. Tech. Rep. PSW-138. 1992. ponderosae Hopkins, D. jeffreyi Hopkins, D. brevicomis LeConte and D. Frontalis Zimmerman (Coleoptera: Scolytidae). Annals of Entomologi­ cal Society of America 83: 892-901. Pajares, J.A.; Lanier, G.N. 1990. Biosystematics of the turpentine beetles D. terebrans and D. valens (Coleoptera: Scolytidae). Annals of Entomologi­ cal Society of America 83: 171-188. Palmer, A.R.; Strobeck, C. 1986. Fluctuating asymmetry: measurement, analysis, patterns. Annual Review of Ecology and Systematics 17: 391421. Parmeter, J.R., Jr.; Slaughter, G.W.; Chen, M.-M.; Wood, D.L. 1992. Rate and depth of sapwood occlusion following inoculation of pines with bluestain fungi. Forestry Science 38: 34-44. Parmeter, J.R., Jr.; Slaughter, G.W.; Chen, M.-M.; Wood, D.L.; Stubbs, H.A. 1989. Single and mixed inoculations of ponderosa pine by fungal associ­ ates of Dendroctonus sp. Phytopathology 79: 768-772. Parsons, P.A. 1990a. Fluctuating asymmetry: an empigenetic measure of stress. Biological Reviews 65: 131-145. Parsons, P.A. 1990b. Fluctuating asymmetry and stress intensity. Trends in Ecology & Evolution 5: 97-98. Payne, T.L.; Dickens, J.C.; Richerson, J.V. 1984. Insect predator-prey coevo­ lution via enantiomeric specificity in a kairomone-pheromone system. Journal of Chemical Ecology 10: 487-491. Piazza, A.; Menozzi, P.; Cavalli-Sforza, L. 1981. The making and testing of geographic gene-frequency maps. Biometrics 37: 635-659. Piston, J.J.; Lanier, G.N. 1974. Pheromones of Ips pini (Coleoptera: Scolytidae). Response to interpopulational hybrids and relative attractiveness of males boring in two host species. Canadian Entomologist 106: 247-251. Preisler, Haiganoush K. 1992. Spatial analysis in bark beetle research [this Proceedings]. Preisler, H.K. Modeling spatial patterns of trees killed in clusters by an epidemic population of bark beetle. Applied Statistics [In Press]. Preisler, H.K.; Mitchell, R.G. 1992. Colonization patterns of the mountain pine beetle in thinned and unthinned lodgepole pine stands. Unpublished draft supplied by author. Raffa, K.F. 1988. Host orientation behavior of Dendroctonus ponderosae: integration of token stimuli and host defenses. In: Mattson, W.J.; Levieux, J.; Bernard-Dagan, C., eds. Mechanisms of woody plant resistance to insects and pathogens. New York: Springer-Verlag; 369-390. Raffa, Kenneth F. 1992. Current status and critical research needs in scolytid genetics [this Proceedings]. Raffa, K.F.; Berryman, A.A. 1983. The role of host plant resistance in the colonization behavior and ecology of bark beetles. Ecological Monographs 53: 27-49. Raffa, K.F.; Berryman, A.A. 1987. Interacting selective pressures in coniferbark beetle systems: a basis for reciprocal adaptations. American Natural­ ist 129: 234-262. Raffa, K.F.; Klepzig, K.D. 1989. Chiral escape of bark beetles from predators responding to a bark beetle pheromone. Oecologia 80: 556-569. Riedy, M.F.; Hamilton, W.J.; Aquadro, C.F. 1992. Excess of non-parental bands in offspring from known primate pedigrees assayed using RAPD PCR. Nucleic Acid Research 20: 918. Riley, M.A.; Goyer, R.A. 1986. Impact of beneficial insects on Ips sp. (Coleoptera: Scolytidae)bark beetles in felled loblolly and slash pines in Louisiana. Environmental Entomology 15: 1220-1224. USDA Forest Service Gen. Tech. Rep. PSW-138. 1992. Roberds, J.H.; Hain, F.P.; Nunnally, L.B. 1987. Genetic structure of southern pine beetle populations. Forest Science 33: 52-69. Rubin, G.M.; Spradling, A.C. 1982. Genetic transformation of Drosophila with transposable element vectors. Science 218: 348-353. Sakaguchi, B.; Oishi, K.; Kobayashi, S. 1965. Interference between "sexratio" agents of Drosophila willistoni and Drosophila nebulosa. Science 147: 160-162. Samollow, P.B.; Soule, M.E. 1983. A case of stress-related heterozygote superiority in nature. Evolution 37(3): 646-649. Schmid, J.M. 1972. Emergence, attack densities and seasonally trends of mountain pine beetle (Dendroctonus ponderosae) in the Black Hills. Res. Note RM-21. Fort Collins, CO: Rocky Mountain Forest and Range Experiment Station, Forest Service, U.S. Department of Agriculture; 4 p. Schowalter, T.D.; Filip, G.M. 1992. Beetle-pathogen interactions in conifer forests. New York: Academic Press. [In press]. Seybold, Steven J. 1992. The status of scolytid genetics―a reductionist's view. [this Proceedings] Seybold, S.J.; Milstead, J.E.; Wood, D.L. 1992a. Notes on the co-coloniza­ tion of Monterey pine, Pinus radiata by Ips sp. (Coleoptera: Scolytidae) and new host associations for Ips mexicanus, I. paraconfusus, I. plastographus maritimus and Dendroctonus valens. Unpublished draft supplied by author. Seybold, S.J.; Ohtsuka, T.; Wood, D.L.; Kubo, J. 1992b. The enantiomeric composition of ipsenol and ipsdienol from species in the subgeneric groups of Ips (Coleoptera: Scolytidae). Unpublished draft supplied by author. Seybold, S.J.; Teale, S.A.; Wood, D.L.; Zhang, A.; Webster, F.X.; Lindahl, K.Q., Jr.; Kubo, I. 1992c. The role of lanierone in the chemical ecology of Ips pini (Say)(Coleoptera: Scolytidae) in California. Unpublished draft supplied by author. Seybold, S.J.; Wood, D.L.; Ohtsuka, T.; Nomura, M.; Kubo, I.; Schultz, M.E. 1992d. The response of the pine engraver, Ips pini (Say) (Coleoptera: Scolytidae) and its insect associates of the pure enantiomers of ipsdienol in California. Unpublished draft supplied by author. Singh, R.S. 1989. Population genetics and evolution of species related to Drosophila melanogaster. Annual Review of Genetics 23: 425-453. Slatkin, M. 1987. Gene flow and the geographic structure of natural popula­ tions. Science 236: 787-792. Smith, R.H. 1963. Toxicity of pine resin vapors to three species of Dendroctonus bark beetles. Journal of Economic Entomology 56: 827-831. Smith, R.H. 1972. Xylem resin in the resistance of the Pinaceae to bark beetles. Gen. Tech. Rep. PSW-1. Berkeley, CA: Pacific Southwest Forest and Range Experiment Station, Forest Service, U.S. Department of Agri­ culture; 7 p. Smith, R.H. 1975. Formula for describing effect of insect and host tree factors on resistance to western pine beetle attack. Journal of Economic Entomology 68: 841-844. Smith, R.H.; Cramer, J.P.; Carpender, E.J. 1981. New record of introduced hosts for the mountain pine beetle in California. Res. Note PSW-354. Berkeley, CA: Pacific Southwest Forest and Range Experiment Station, Forest Service, U.S. Department of Agriculture; 3 p. Stauffer Ch.; Huang, W.; Harshman, L.; Hammock, B.D. 1992b. Character­ ization of the juvenile hormone metabolites of Ips typographus (Col., Scolytidae) and Tenebrio molitor (Col., Tenebriodiae). Unpublished draft supplied by author. 29 Stauffer, Christian; Leitinger, Renate-, Fuehrer, Erwin. 1992a. Population genetics of Ips typographus (Col., Scolytidae) and related Ips species [this Proceedings]. Stauffer, Ch.; Leitinger, R.; Simsek, Z.; Schreiber, J.; Fuehrer, E. 1992. Allozyme variation among nine Austrian Ips typographus populations. Journal of Applied Entomology 114: 17-25. Stock, M.W.; Amman, G.D. 1980. Genetic differentiation among mountain pine beetle populations from lodgepole pine and ponderosa pine in northeast Utah. Annals of Entomological Society of America 73: 472-478. Stock, M.W.; Amman, G.D. 1985. Host effects on the genetic structure of mountain pine beetle, Dendroctonus ponderosae, populations. In: Safranyik, L., ed. The role of the host in the population dynamics of forest insects. Victoria, B.C., Canada: Pacific Forest Research Centre; 83-95. Stock, M.W.; Amman, G.D. Genetic diversity in early and late-emerging mountain pine beetle. Unpublished draft supplied by author. Stock, Molly W.; Amman, Gene D.; Bentz, Barbara J. 1992. Isozyme studies of bark beetle population genetics and systematics [this Proceedings]. Stock, M.W.; Amman, G.D.; Higby, P.K. 1984. Genetic variation among mountain pine beetle (Dendroctonus ponderosae) (Coleoptera: Scolytidae) populations from seven western states. Annals of Entomological Society of America 77: 760-764. Teale, Stephen A.; Hager, Barbara. 1992. Bark beetle genetics―an overview [in Proceedings]. Teale, S.A.; Lanier, G.N. 1991. Seasonal variability in response of Ips pini (Coleoptera: Scolytidae) to ipsdienol in New York. Journal of Chemical Ecology 17: 1145-1158. Teale, S.A.; Webster, F.X.; Zhang, A.; Lanier, G.N. 1991. Lanierone: a new pheromone component from Ips pini (Coleoptera: Scolytidae) in New York. Journal of Chemical Ecology 17: 1159-1176. Thorne, B.L.; Haverty, M.I. 1989. Accurate identification of Zootermopsis species (Isoptera: Termopsidae) based on a mandibular character of nonsoldier castes. Annals of Entomological Society of America 82: 262266. Vanderwel, D. 1991. Pheromone biosynthesis by selected species of grain and bark beetles. Simon Fraser University; 230 p. Dissertation. Vanderwel, D.; Oehlschlager, A.C. 1987. Biosynthesis of pheromones and endocrine regulation of pheromone production in Coleoptera. In: Prestwich, G.D.; Blomquist, G.J., eds. Pheromone biochemistry. Orlando, FL: Aca­ demic Press; 175-215. Wadleigh, R.W.; Yu, S.J. 1987. Glutathione transferase activity of fall armyworm larvae toward alpha, beta-unsaturated carbonyl allelochemicals and its induction by allelochemicals. Insect Biochemistry 17: 759-764. Stock, M.W.; Gregoire, J.C.; Furniss, M.M. 1987. Electrophoretic compari­ son of European Dendroctonus micans and ten North American Dendroctonus species. Pan-Pacific Entomologist 63(4): 353-357. Wanntorp, H.-E.; Brooks, D.R.; Nilsson, T.; Nylin, S.; Ronquist, F.; Steams, S.C.; Wedell, N. 1990. Phylogenetic approaches in ecology. Oikos 57: 119-132. Stock, M.W.; Guenther, J.D. 1979. Isozyme variation among mountain pine beetle (Dendroctonus ponderosae) populations in the Pacific Northwest. Environmental Entomology 8: 889-893. Whitney, H.S. 1982. Relationships between bark beetles and symbiotic or­ ganisms. In: Mitton, J.B.; Sturgeon, K.B., eds. Bark beetles in North American conifers: a system for the study of evolutionary biology. Aus­ tin: University of Texas Press; 183-211. Stock, M.W.; Guenther, J.D.; Pitman, G.B. 1978. Implications of genetic differences between mountain pine beetle populations to integrated pest management. In: Berryman, A.A.; Amman, G.D.; Stark, R.W.; Kibbee, D.L., eds. Theory and practice of mountain pine beetle management in lodgepole pine forests―a symposium; April.25-27, 1978. Moscow, ID: University of Idaho, College of Forest Resources; 197-204. Stock, M.W.; Robertson, J.L. 1977. Genetic polymorphism in the Douglas-fir tussock moth. USDA Forest Service Expanded Douglas-Fir Tussock Moth Program, Final Report, December 31, 1977. Unpublished draft supplied by author. Stock, M.W.; Pitman, G.B.; Guenther, J.D. 1979. Genetic differences between Douglas-fir beetles (Dendroctonus pseudotsugae) from Idaho and coastal Oregon. Annals of Entomological Society of America 72: 394397. Wiley, E.O. 1981. Phylogenetics. New York: John Wiley and Sons; 439 p. Williams, J.K.G.; Kubelik, A.R.; Livak, K.J.; Rafalski, J.A.; Tingey, S.V. 1990. DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acid Research 18: 6531-6535. Wood, D.L. 1982. The role of pheromones, kairomones, and allomones in the host selection and colonications behavior of bark beetles. Annual Review of Entomology 27: 411-446. Wood, David L.; Storer, Andrew J.; Gordon, Thomas R.; Seybold, Steven J.; Page, Marion. 1992. Biosystematics of Ips mexicanus and Ips plastographus (Coleoptera: Scolytidae) and their fungal symbionts [this Proceedings]. Wood, S.L. 1963. A revision of the bark beetle genus Dendroctonus Erichson (Coleoptera: Scolytidae). Great Basin Naturalist 23: 1-117. Stock, M.W.; Schmitz; R.F.; Amman, G.D. Heterozygosity and density change in mountain pine beetle (Dendroctonus ponderosae) (Coleoptera: Scolytidae) populations. Unpublished draft supplied by author. Wood, S.L. 1982. The bark and ambrosia beetles of North and Central America (Coleoptera: Scolytidae), a taxonomic monograph. Great Basin Naturalist Memoirs No. 6. 1359 p. Sturgeon, K.B. 1980. Evolutionary interactions between the mountain pine beetle, Dendroctonus ponderosae Hopkins, and its host trees in the Colorado Rocky Mountains. Boulder: University of Colorado, Disserta­ tion. Wood, T.K. 1980. Divergence in the Enchenop binotata Say complex (Homoptera: Membracidae) effected by host plant adaptation. Evolution 34: 147-160. Sturgeon, K.B.; Mitton, J.B. 1986. Allozyme and morphological differentia­ tion of mountain pine beetles Dendroctonus ponderosae Hopkins (Co­ leoptera: Scolytidae) associated with host tree. Evolution 40: 290-302. Sturgeon, K.B.; Robertson, J.L. 1985. Microsomal polysubstrate monooxygenase activity in western and mountain pine beetles. Annals of Entomological Society of America 78: 1-4. Yearian, W.C.; Gouger, R.J.; Wilkinson, R.C. 1972. Effects of the bluestain fungus, Ceratocystis ips, on development of Ips bark beetles in pine bolts. Annals of Entomological Society of America 65: 481-487. Zuber, M.; Stauffer, Ch.; Leitinger, R.; Nelson, L.; Page, M.; Bright, D. 1992. Biochemical, morphological and behavioural analysis of the relationship between Ips amitinus and Ips amitinus var. montana (Col., Scolytidae). Unpublished draft supplied by author. Teale, S.A. 1990. Ecology and evolution of pheromone communication in Ips pini (Coleoptera: Scolytidae). New York: State University of New York; 109 p. Dissertation. 30 USDA Forest Service Gen. Tech. Rep. PSW-138. 1992. Workshop Participants Alosi, M. Carol, Pacific Southwest Research Station, USDA Forest Service, 1027 Trenton Ave., Bend, OR 97701; (503) 388-5422, FAX: (503) 3835733. Amman, Gene D., Intermountain Research Station, USDA Forest Service, 507 25th Street, Ogden, UT 84401; (801) 625-5393, FAX: (801) 6255723. (retired) Anderson, Wyatt W., Department of Genetics, 101B Life Sciences Bldg., University of Georgia, Athens, GA 30602; (706) 542-3326, FAX: (706) 542-3910. Bentz, Barbara J., Intermountain Research Station, USDA Forest Service, Forestry Sciences Lab, 860 No. 1200 East, Logan, UT 84321; (801) 7521311, FAX: (801) 753-8625. Bright, Donald E., Canada Dept. of Agriculture, Systematics Research, Cen­ tre for Land and Biological Resources Research, Ottawa, Ontario, Canada K1A OC6; (613) 996-1665, FAX: (613) 995-1823 or 7283. Cane, James H., Dept. of Entomology, 301 Funchess Hall, Auburn Univ., Auburn, AL 36849-5413; (205) 844-5006, FAX: (205) 844-3005. Gordon, Thomas R., Dept. of Entomological Sciences, University of Califor­ nia, Berkeley, CA 94720; (510) 642-3874, FAX: (510) 642-3845. Mitchell, Russ, 20454 Steamboat, Bend, OR 97702, (503) 388-2869. (retired) Page, Marion, Pacific Southwest Research Station, USDA Forest Service, 800 Buchanan Street, Albany, CA 94710; (510) 559-6300, FAX: (510) 559-6440. Preisler, Haiganoush K., Pacific Southwest Research Station, USDA Forest Service, 800 Buchanan Street, Albany, CA 94710; (510) 559-6300, FAX: (510) 559-6440. Raffa, Kenneth F., Dept. of Entomology, University of Wisconsin, Madison, WI 53706; (608) 262-1125, FAX: (608) 262-3322. Robertson, Jacqueline L., Pacific Southwest Research Station, USDA Forest Service, 800 Buchanan Street, Albany, CA 94710; (510) 559-6300, FAX: (510) 559-6440. Seybold, Steven J., Pacific Southwest Research Station USDA Forest Ser­ vice, 800 Buchanan Street, Albany, CA 94710; (510) 559-6477, FAX: (510) 559-6440. Stanton, Maureen, Department of Botany, Univ. of California, Davis, CA 95616; (916) 752-2405, FAX: (916) 752-5410. Greene, Lula E., Pacific Southwest Research Station, USDA Forest Service, 800 Buchanan Street, Albany, CA 94710; (510) 559-6300, FAX: (510) 559-6440. Stauffer, Christian, Institute of Forest Entomology, Forest Pathology and Forest Protection, Universität für Bodenkultur, A-1190 Vienna, Austria. Present address: Dept. of Entomology, Univ. of California, Davis, CA 95616; (916) 752-0492. Haack, Robert, North Central Research Station, 1407 S. Harrison Road, East Lansing, MI 48823; (517) 355-7740, FAX: (517) 335-5121. Stock, Molly W., Dept. of Forest Resources, College of Forestry, University of Idaho, Moscow, ID 83843; (208) 885-7033, FAX: (208) 885-6226. Hager, Barbara, Dept. of Environmental and Forest Biology, State Univ. of NY, College of Environmental Science and Forestry, Syracuse, NY 13210; (315) 470-6758, FAX: (315) 470-6934. Storer, Andrew J., Dept. of Entomological Sciences, University of Califor­ nia, 201 Wellman Hall, Berkeley, CA 94720; (510) 643-5806, FAX: (510) 642-7428. Hayes, Jane L., Southern Forest Experiment Station, USDA Forest Service, 2500 Shreveport Highway, Pineville, LA 71360; (318) 473-7232, FAX: (318) 473-7222. Teale, Stephen, Dept. of Environmental and Forest Biology, State Univ. of NY, College of Environmental Science and Forestry, Syracuse, NY 13210; (315) 470-6758, FAX: (315) 470-6934. Hobson, Ken, Intermountain Research Station, USDA Forest Service, For­ estry Sciences Lab, 860 No. 1200 East, Logan, UT 84321; (801) 7521311, FAX: (801) 753-8625. Wood, David L., Dept. of Entomological Sciences, University of California, 201 Wellman Hall, Berkeley, CA 94720; (510) 642-5538, FAX: (510) 642-7428. USDA Forest Service Gen. Tech. Rep. PSW-138. 1992. 31 The Forest Service, U.S. Department of Agriculture, is responsible for Federal leadership in forestry. It carries out this role through four main activities: • Protection and management of resources on 191 million acres of National Forest System lands • Cooperation with State and local governments, forest industries, and private landowners to help protect and manage non-Federal forest and associated range and watershed lands • Participation with other agencies in human resource and community assistance programs to improve living conditions in rural areas • Research on all aspects of forestry, rangeland management, and forest resources utilization. The Pacific Southwest Research Station • Represents the research branch of the Forest Service in California, Hawaii, American Samoa and the western Pacific. Persons of any race, color, national origin, sex, age, religion, or with any handicapping conditions are welcome to use and enjoy all facilities, programs, and services of the U.S. Department of Agriculture. Discrimination in any form is strictly against agency policy, and should be reported to the Secretary of Agriculture, Washington, DC 20250. United States Department of Agriculture Forest Service Pacific Southwest Research Station General Technical Report PSW-GTR-138 Proceedings of a Workshop on Bark Beetle Genetics: Current Status of Research May 17-18,1992, Berkeley, California