Document 11217206
advertisement

T
ION GENERATION, ELECTRON ENERGY DISTRIBUTIONS, AND
PROBE MEASUREMENTS IN A LOW PRESSURE ARC
by
SOL AISENBERG
B. S., Brooklyn College
(1951)
SUBMITTED IN PARTIAL FULFILLMENT
OF THE
REQUIREMENTS FOR THE DEGREE OF
DOCTOR OF PHILOSOPHY
at the
MASSACHUSETTS INSTITUTE OF TECHNOLOGY
May 1957
Signature of Author .
Departmnefof
Certified
by
. . . .
./
.........
Accepted
by
Physics,
'
13, 1957
afy
sisSu *..
)hesis Supe sor
.......
Chairman, Dertmental
..,,
DfrmnalCCormittee
mt ok Graduate Students
I
)
/
3.i ,I
"'.
:..I
';
, .; I
IvO
;i'
N-;
, IT. T
OCT 17
1957
:
ION GENERATION, ELECTRON ENERGY DISTRIBUTIONS, AND
PROBE
MEASUREMENTS
IN A LOW PRESSURE ARC
by
Sol Aisenberg
Submitted to the Department of Physics on May 13, 1957 in partial ful-
fillment of the requirements for the degree of Doctor of Philosophy.
ABSTRACT
A series of Langmuir probe measurements have been made on the
plasma of a low pressure mercury arc in order to obtain information about
some of the fundamental processes
in the plasma.
It is shown that the
actual ionziation is many times larger than the direct ionization, and
that the effect of the electron drift velocity on the direct ionization is
negligible. The results of this research plus the limited information
available in the literature indicate that the metastable density is independent of the electron density, and that the cumulative ionization is
a linear function of the electron density and not a quadratic one. The
effective cross-section for ionization of the 63 P states is calculated to
be at least 9. 0 times larger than that for the ground state. The mobility
of the electrons
in the plasma was measured
and it is found that the effective
cross-section for slow electrons in, the plasma is essentially constant with
an average value of 42 x 10-1
cm
.
The ambipolar
diffusion coefficient
and the ambipolar mobility coefficient are determined as a function of
E/po for an active plasma. With the aid of a specially developed theory
for ion mobility and diffusion in a strong nonuniform electric field, the
mobility and longitudinal diffusion coefficients are calculated for Hg ions
The effective cross-section for Hg ions is found to
in the plasma.
31
to 104 x 10-1 cm2 as the ion energy is increased from
from
increase
0.44 to 1.37 electron volts. There is reasonable agreement with the
limited data of others. The theories and methods developed can be used
for the measurement of ion cross-section in other gases. A study is
made of the collection of positive ions by a negative probe for comparison
with theory. Several new experimental techniques are introduced. The
partial pressures
mm
of the residual gases in the arc were
in the 10-8 to 10
-7
Hg range and were measured while the arc was in operation in the
was supplied
water bath. The probe potential
by a specially
designed
low impedance voltage source featuring a high degree of negative feedback.
A new method is developed to permit the change of probe work-function to
be directly recorded as a function of time, and may prove useful in
future experiments.
Thesis Supervisor:
W. B. Nottingham
Title:
Professor of Physics
Table of Contents
Chapter I
Theory o Probe Measurements
A.
Introduction
I
B.
C.
Current Density to a Retarding Planar Probe
Probe Current for the Case of a Maxwell-Boltzmann
Distribution and for a Druyvesteyn Distribution
2
Distribution Function from Probe Measurements
Utilization of the Probe Measurements
6
7
D.
E.
Cha ter II
A.
E.
Method for the Direct Determination
Discharge Tube and Water Bath
Auxiliary Equipment Used to Monitor and Measure
Low Residual Gas Pressure
Probes
D.
Vacuum Processing
G.
H.
Chapter III
4
Apparatus and Measuring Technique
C.
E.
F.
of the
of Tube
Voltage Source for Retarding Potential Measurements
Measuring Procedure and Electron Bombardment of
the Probes
Change of Probe Work-Function
Stability of Arc and Reproducibility of Measurements
9
10
11
14
15
16
17
22
Basic Experimental Data
A.
Retarding Potential Plots and Ion Current Extrapolation 25
C.
D.
Density, and Plasma Potential
Mercury Vapor Pressure and Density
Correction of the Plasma Radius for Wall Sheath
B.
Determination of Electron Temperature, Electron
Thickness
Chapter IV
A.
B.
C.
33
Ionization Process in the Plasma
Production of Ions in the Plasma
Measurement of the Total Ionization and Determination
of the Electron Density Distribution from Ambipolar Diffusion Theory
Calculation of the Direct Ionization Component
Including the Effect of the Electron Drift
Velocity
D.
26
29
35
36
48
Determination of the Important Ionization Processes
in the Plasma
50
rT
Chapter V
A.
B.
C.
D.
E.
Chapter VI
Electron Mobility
Ambipolar Diffusion Coefficient and Ambipolar
Mobility Coefficient
Ion Velocity as a Function of E /p
Simple Theory of Ion Mobility and Diffusion Coefficient
for Ions in a Strong Uniform Electric Field
First Order Theory for Ion Mobility and Diffusion in a
Nonuniform Strong Electric Field
B.
Theory of Harmonic Analysis of Retarding Potential
Plots, and the Effect of Superimposed ac
Experimental Ratio of Saturation Electron and
Saturation Ion Current and Comparison with
Random Current Theory
Simplified Form of Schulz's Theory for the Saturation
Current Ratio and Comparison with Experiment
Basic Theory for the Prediction of the Variation of the
Saturation Current Ratio With the Arc Parameters
E;
62
65
67
74
Measurements
Depletion of High Energy Electrons and Comparison
D.
57
Electron Energy Distribution Function and Probe
A.
C.
i
Electron Mobility, Ambipolar Diffusion Coefficient,
Ion Mobility Coefficient, Ion Diffusion Coefficient,
and Cross-Section for Hg ions in Their Parent Gas
With the Druyvesteyn Distribution
78
81
85
89
94
Chapter VII Summary and Conclusions
101
Appendix I
106
Glossary
110
References
115
Acknowledgement
118
Biographical Note
119
II
Table of Figures
II-
I1
II-la
II-2
II-3
II-4
III- 1
III -2
III-3
III-4
III-5
Probes
Measuring circuit
Work-function change
Plane center probe curve (25. 10 C, 5.0 amp)
Expanded plane center probe curve
Wire probe curve (25. 1°C, 5.0 amp)
Disk probe curve (25. 10 C, 5.0 amp)
Plane wall probe curve (25. 10C, 5. 0 amp)
9a
9b
1la.
16a
17a
25a
25b
25c
26a
26b
Plane center probe curve (30. 0°C, 5. 0 amp)
27a
Plane center probe curve (30. OOC,6. 0 amp)
28a
29a
III- 13
III- 14
Plane center probe. curve (35. 0°C, 5. 0 amp)
Wire probe curve (35. 0°C, 5..0 amp)
Plane center probe curve (45. 10 C, 4. 0 amp)
Wire probe curve (45. 10 C, 4.0 amp)
Plane center probe curve (45. 10 C, 5. 0 amp)
III- 16
Plane center probe curve (45. 10 C, 6.0 amp)
III- 6
III-7
III-8
III-9
III-10
III-11
III-12
III-15
III-17
II- 18
III- 19
III-20
III -21
IV-1
V-1
V-2
V-3
V-4
V-5
V-6
V-7
V-8
VI-1
VI-2
VI-3
I
Tube and water bath
Detailed drawing of discharge tube
Wire probe curve (30. 0°C, 5. 0 amp)
Wire probe curve (30. OOC, 6. 0 amp)
Wire probe curve (45. 10 C, 5. 0 amp)
Wire probe curve (45. 10C, 6. 0 amp)
Plane center probe curve (54. 60C, 5. 0 amp)
Wire probe curve (54. 60 C, 5. 0 amp)
Plane center probe curve (62. 6°C, 5. 0 amp)
Wire probe curve (62.6 0 C, 5.0 amp)
Ion generation as a function of q/kT_ and the production
of 63 Po, 63 P 1 and 63 P2 states
Electron drift velocity as a function of Ez/p o
Effective cross-section for slow electrons in Hg
Ambipolar mobility coefficient vs Er/p
Ion radial drift velocity as a function ofEr/Po
Er/Po
Ion mobility coefficient as a function of
Longitudinal diffusion coefficient vs Er/p o
Cross-section for Hg ions in Hg vs Er/P o
Cross-section
for Hg ions in Hg vs E
Retarding potential plot for a Druyvesteyn distribution
Ratio of electron temperature to gas temperature for Hg
Ratio of saturation electron current to saturation ion
current
27b
28b
29b
30a
30b
31a
31b
32a
32b
33a
33b
34a
34b
47a
57a
61a
64a
65a
73a
74a
75a
76a
79a
87a
88a
Appendix
Table I
Table II
Basic Probe Data
Basic Discharge Parameters
106
107
Table III
Ion Generation in the Plasma
108
Table IV
Electron Mobility and Electron Drift Energy
109
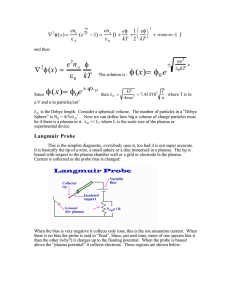
I. Theory of Probe Measurements
A. Introduction
The theory of probe measurements of arc plasmas was first
developed by Langmuir and Mott-Smith (1 ' 2 3) and made possible the
determination of many of the fundamental properties of the plasma
(4, 5)
The Langmuir probe method is very powerful because it yields the'
plasma potential Vp, the electron temperature T_, (for the range of
electron energies where the electron velocity distribution is MaxwellBoltzmann), and the electron density n.
Because of the low potential
gradients existing in the plasma (of the order of 1 volt per centimeter)
the ion and electron densities are very closely equal in the plasma (to
within about 0. 001 per cent) and therefore the Langmuir probe measurements will also yield the plasma ion densities n+.
The basic theory of the Langmuir probe method is as follows.
When the probe potential is slightly positive with respect to the plasma
potential, the current to the probe ip, is predominantly electron current
and is relatively independent of the probe potential.
For a spherically
symmetric electron velocity distribution, the saturation electron current
density J_s, is equal to the random electron current density and is thus
proportional to the product of the electron density n_, and the average
electron velocity <v_>. As the probe potential is made negative with
respect to the plasma potential, the electron current to the probe is
reduced because of the retarding field acting on the electrons, and
the ion current is increased until the probe current is predominantly
ion current and is again relatively independent of the probe potential.
The electron current i_, is found from the probe current ip, and the
extrapolatedion current
and is used to determine the eectron tem-2
extrapolated ion current i, and is used to determine the electron temperature and the electron density.
For the case where the electron
velocity distribution is Maxwell-Boltzmann (M-B), a plot of log1 0 i vs
the collector potential Vc , will be linear with a slope dlog1 0 i_/dV c
equal to 2.303 q/kT
where q/k is the ratio of the electron charge to
Boltzmann's constant and is equal to 11, 606 degrees absolute per volt.
The plasma potential Vp, is usually taken as the intersection (on the
semilogarithmic plot) of the saturation electron current extrapolation
and the extrapolation of the linear part of the retarding potential plot.
The average electron velocity (v_> found from the electron temperature
is used to calculate the electron density.
J
'
1~ R~.lll~lr'(
Crr
cant
rhi
7 t'rm nlanm'
rearding
~mn.r
nrtihp
A useful expression will be given for the electron current
density J_,to a retarding planar probe for a -common class of distriI
bution functions.
In general:
/ E7
2
,of
(I)
where J_ is the electron current density in the+x direction (charge per
unit area per unit time), vx is the electron velocity in the x direction,
and f() is the density of electrons in phase space.
defined by: m (v )2/2
and charge,
qV where m
The velocity v is
and q are the electron mass
and V(V > 0) is the retarding
potential of the probe with
respect to the plasma. This expression states that only electrons with
velocities in the +x direction that are larger than v will be able to
overcome the retarding potential and be collected.
For the case of a
spherically symmetric velocity distribution function (or for the leading
3.
spherically symmetric term of the spherical harmonic expansion in
velocity space), f(1) =, fo (v) where v 2
v2 + v 2 + v2
2
V2, and
the multiple integral may be reduced by change of variable and inte-
gration by parts to the relatively simple expression:
rin
i-2)
When there is no retarding field, then vo
X
0, and the saturation
or random current density for an arbitrary spherically symmetric distribution function is thus shown to be:
la
>
1
=
where-ra/Iv
i
t
vocCiV4
1Y Ll.
.-
L·
CI-
,
-
1_
a7cr-rd-
(I-3)
ravr
thc, Ai+Y-i1iivrhnlfvnct+irn
vvis
.- rs
Th
/ A (Ia-
-
_F '_u
-
-r r
_
~us·
-
L
.. -
distribution function averaging operator is defined by:
(A>
--" /
If the distribution function is of the general form f(v) x A exp(-B vS),
the integral may be expressed in closed form in terms of incomplete
gamma functions.
functions,
There are, however, two important distribution
the Maxwell-Boltzmann
Druyvesteyn distribution
(6'
distribution
(s
2), and the
7 8) (s , 4), which yield values of J_ that
can be expressed in terms of exponential functions and error functions.
K
T.f
~~~~~~~~~~~~~~~~~~~~~~~~~4.
C. Probe current for the case of a Maxwell-Boltzmann distribution
and for a Druy:vesteyn distribution
A M-B distribution function:
M
-; irP
(I-5)
s·~
kr,-,e
gives the following result for the current density to the probe:
x
where
the constant (8q/rm_)
(electron volt) 1
2
1/
(I-6)
=
is equal to 6.693 x 105 (meters/second)/
Thus a measurement of the current to the probe and
the saturation electron current density, plus the value of the electron
temperature obtained from the slope of the retarding potential plot will
permitthe
determination of n.
The M-B distribution is the one which
results when the electron-electron interactions are predominant and
when the effect of the field, gas atoms, ions,
and boundaries can be
neglected.
On the other hand, the Druyvesteyn distribution
(6 '
7, 8) is the
leading term of the solution (in spherical harmonic form in velocity
space) of the Boltzmann transport equation, and corresponds physically
to the following assumptions: 1. electrons move under the influence
of the field in the gas; 2.
electron-electron
and electron-ion
inter-
actions are negligible in comparison with the electron-gas interactions;
3. electron-gas collisions are elastic; 4. gas temperature is low compared to the electron temperature;
5. electron mean free path is
independent of electron velocity (hard sphere model for gas atoms).
Some of these assumptions are of course subject to question: inelastic
collisions occur;for high energy electrons, and the electron crosssection in Hg is quite velocity dependent for the faster electrons.
In
addition, since the electron-electron interactions for slow electrons
are much stronger than for fast electrons ((9) , it is expected that the
slow electrons will tend toward a M-B distribution.
The theoretical
normalized retarding potential plot for the D-D (Fig. VI-1) will show
the extreme case when the processes leading to a D-D are predominant.
The D-D is given by:
-I
(4'k )
f (1-)-
-
(-7
(I-7)
i
where '(k) is the complete gamma function, n_ is the electron density
in configuration space, and vrms is the root-mean-square
velocity
corresponding to the average energy of the distribution function. It
should be noted that the number of high energy electrons drop off much
faster for the D-D than for the M-B distribution.
The probe current density corresponding to the D-D is given in
normalized form by:
- = exy (-7 )
where y
I[(5/4)/
r'(3/4)]
.(i)
yit e
(1-8)
;
f
V/V-X 0.7397 V/V, V is the average
energy of the D-D in electron volts, V is the retarding potential, and
et {
-ch
r.r
= -* X()¢
e
(-9)
V....
6.
I
i
I
rrilho ali Ic roff^, atl+u+1r
r+iif
C;
VCIIUT:
II
The ravert
L··LL-
W.I·U
aIlul
IlLll
rr.nnt rA1Ini+'T7
fr th n nTT
ia rrivT7-nh-N7
=
r;l
fo
I.
Ir;llu
v
LL;
I
I
L
·L
V1
L·V
h
The ratio of saturation current for the D-D to the saturation current
!d
1
~for
the M-B distribution of the same electron density and same average
energy is equal to 1. 030. For the purpose of comparison with each
other and with experiment, normalized probe curves for both distributions are given in Fig. VI-1.
D. Method for the direct determination of the distribution function
from probe measurements
It was shown by Druyvesteyn (
(3 )
)
( and earlier by Mott-Smith
2
and Langmuir
.q
relationship between the distribution function f(v), and the second
derivative
q
for a spherical collector) that there is a simple
(with respect to the retarding
potential V) of the electron
current to a retarding planar, cylindrical or spherical probe. This
relationship can be obtained from Eq. I-2 by double differentiation
with respect to the potential V which is implicitly contained in the
q
definition of vo . The result is:
Numerical
graphical
or different
the J vs V data will yield (I-ll)of
Numerical or graphical differentiation of the J vs V data will yield
very 'inaccurate results because the process of differentiation will
magnify the relative effects of any small errors or fluctuations, and
a second differentiation will further increase the uncertainty.
The
2
use of more than three points in the determination of d J /dV
reduce the error but not appreciably.
Medicus
2
will
has recently developed
a convenient graphical method for determining the distribution function
from very accurate probe data but his method does not prevent an amplified
error in the second derivative.
Direct measurement of the second deri-
vative is possible through the use of harmonic analysis but it is necessary
the
to separate electrically the electron current from the ion current it'
high energy electrons are of interest.
The theory of the determination of
the distribution function through harmonic analysis will be developed in
Chapter VI along with a discussion of the limitations of the various
4i.
methods plus suggestions for an improved method. In the present
Ii
research. the distribution function was not measured.
.s
E. Utilization of the probe measurements
The basic data obtained from the probe measurements consist,
in part, of a set of electron densities, electron temperatures, plasma
potentials, and probe currents for each probe at a givernvalue of bath
temperature
Tb, and arc current Iz . This data will yield the average
electron density n_, (averaged over a cross-section of the cylindrical
plasma) provided that a reasonable representation can be assumed for
the radial electron density distribution.
the axial potential gradient E,
The basic data will also give
and the total ionization per unit volume.
This total ionization can be compared with the computed direct ionization
to demonstrate that the total ionization is much larger than the direct
ionization component. Information can be obtained about the dependence
of the total ionization upon the electron temperature and the electron
dens ity.
I
i
I
i
8.
The electron and ion mobilities will also be calculated in
Chapter V. Knowledge of the axial current density J,
and the axial
potential gradient Ez, plus the average electron density will yield the
values of the electron mobility p,,as a function of the discharge parameters and will also give the equivalent cross-section for scattering
of slow electrons in mercury.
If the requirements for ambipolar dif-
fusion are assumed to be approximately satisfied, the ambipolar diffusion coefficient Da, can also be calculated.
If the radial electron density distribution canlb.eassumed
accurately enough, the radial density gradient at the plasma edge can
be calculated from the measured electron density at the center n_01
and from the measured electron density at the plasma edge nw
The radial potential gradient E r , can then be calculated from the
electron temperature and the Boltzmann density distribution law
relating the potential and the density.
This value: of Er in conjunction
with the ion density and the ion current to the wall at floating potential
will give information about the motion of ions in the plasma.
A simple
model for ion flow in strong electric fields will permit the calculation
of the ion mobility and the diffusion coefficient.
From the measured retarding potential plots, information can
be obtained about a dimensionless form of the retarding potential
plots.
The mechanism of ion collection by a negative probe will also
be investigated.
Chapter II will describe the experimental conditions and the precautions used in making the measurements.
Chapter III will present the
basic results and will show some of the corrections necessary before the
arc parameters are specified.
II. Apparatus and Measuring Technique
A. Discharge tube and water bath
The probe measurements were made in the plasma of a low pressure
mercury arc in a pyrex envelope. An auxiliary arc (with a current Iv of about
5 aips) was produced between a mercury pool cathode and a vertical anode.
The main arc in the horizontal side arm (which carried a current I z of about
5 amps) supplied the plasma to be measured.
The use of the side arm helped
to isolate the plasma from the effects of the jets of mercury vapor given off
by the cathode spot and from the high energy electrons produced by the potential
drop across the cathode sheath.
rnlnvi-fian rn A(3wniltirjnc-afn
r
The anodes are hollow nickel cylindrical cans
i i+h thy ann
onfa frinrn t'wirmvela thy nlayma
Hollow anodes were used Instead of disk anodes to minimize the effects of fluctuating anode spots.
The horizontal arm consisted of a pyrex cylinder of 5.5 cm
inside diameter with a wall thickness of 0. 24 cm. The horizontal plasma was
about 38 cm long. Figure
-la shows the discharge tube in more detail.
The mercury vapor pressure was controlled through immersion of the
complete discharge tube in a constant temperature water bath.
The mercury
pressure in the arc is thus the vapor pressure of clean mercury at this bath
temperature with a small correction of 1 to 2 degrees C because of the thermal
drop in the pyrex wall. The bath temperature was varied from 25. 10 C to 62.60C
to within 0. 1C which corresponds to a vapor pressure uncertainty of about
0.8 per cent.
This control was obtained through the use of copper cooling coils
with circulating cold water, plus a mercury thremoregulator controlling
immersion heaters totaling 2 kilowatts.
Two stirrers
were used to
COOLING COIL
DISK PROBE
STIRRER
HEATER
I I'U
HEATER
^~.
\n
1
WATER
-
LEVEL
WALL PROBE
CONTROL
LIQUID
AIR
TRAP
VERFLOW
ORIZONTAL
NODE
Fix. 11±T,Abuead
'watsebauh
rl
V
1AL
A
SEAL
off
3.
cm
3,
c
CC
r.
.-L.
Hm~rz--rc
5C
I *
Det iled
L
V~~-l
~ iAla~
~ i/K ~
~ ~ ~
m
I
drawin-r,of disclacge¢ tube
r
n i
10.
keep the water in strong motion so as to reduce thermal gradients and
to keep the bath temperature uniform.
More information about the tube
and bath is available in Fig. II-1.
B. Auxiliary equipment used to monitor and measure low residual
gas pressures
A novel feature which was added to the usual discharge tube was
a re-entrant liquid air trap in series with a Bayard-Alpert
( 12 )
type
ionization gauge, and an "evaporated metal" getter tube. The liquid
air trap permitted the mercury vapor component to be frozen out so
that the ionization gauge could measure the partial pressure of the
non-condensable residual gases while the discharge tube was on the
vacuum system and also while the discharge tube was in operation in
the water bath. In addition, the liquid air trap served to "pump" any
condensable gases in the arc and thus helped to improve the vacuum.
The tubulation connecting the trap and the tube was built with two slight
constrictions, one before and one after a well designed to contain some
mercury.
This supply of mercury between the tube and the trap served
to reduce the mercury pumping action of the trap.
Unfortunately, the
liquid air trap needed refilling about every four hours and even more
frequently if a virtual leak was to be avoided. A re-entrant liquid air
trap was necessary in order to prevent the trap from filling with
mercury.
The bottom of the trap was connected to the discharge tube
so that the consensed mercury could return by gravity flow to the main
tube whenever the trap was allowed to warm up. The liquid air was
introduced at least 6 hours. before measurements were made.
ii.
A specially designed ionization gauge control circuit (M.I. T.
Research Laboratory of Electronics drawings number D-1607-1,
A-1608-1 and A-1609-1) was used with the ionization gauge to measure
the residual gas pressureand to display it on a 1 milliampere
chart
This procedure made the history of the background pressure
recorder.
available to aid in the vacuum processing
of the tube and also per-
mitted the anticipation of a virtual leak due to the lowering of the
liquid air level.
An overpressure protector was used to prevent the
burnout of the ionization gauge filaments as a result of the loss of
liquid air.
The getter tube contained 10 mil filaments of Mo, Ta, and W
which were used to evaporate clean metal surfaces on the pyrex walls
for the absorption of contaminating gases.
The evaporation filaments
were operated with current pulses of about 1 second duration every
6 seconds in order to reduce the heating of the filament supports and
the consequent evolution of unwanted gas.
The ionization gauge
cleanup helped to remove residual gases in addition to the cleanup
of the discharge tube itself.
As a result of all these inovations and precautions, it was possible
to make measurements in a mercury plasma with a known residual gas
pressure
ranging from about 4 x 10- 8 mm Hg to 4 x 10 -7 mm Hg
(nitrogen equivalent). If better vacuum is desired in future experiments,
it is suggested that a vacuum system be connected to the discharge tube
while it is in the water bath.
C. Probes
Five probes were built into the discharge tube: two tungsten
wire probes and three tantalum planar probes. The wire probes
L;
TUNGSTEN
GLASS SHIELD
WIRE
PROBES
TANTALUM DISK
ma:
DISK PROBE
,rr-rr7Y7777T77
.-- n I T
rumt
,
,
fff9
.
iL
Fi
li[isif
,
v
fff
INSIDE PYREX
WALL OF TUBE
77
S[St77717S7S.7.
l
WALL
PROBE
.
z
L
F.
_
.
.
consisted of 29 mil diameter W wire with glass tubing shielding all but
0.98 cm. They were mounted in the center of the horizontal cylinder
with a 10 cm separation
in the axial direction and with the probe axis
coincident with a diameter of the cylinder.
Since the radial potential
and charge density gradients are small at the center of the discharge
tube,. the wire probes were in a uniform region.
An alternate way of
positioning the wire probes would be to mount them with their axis
coincident with the cylinder axis.
In this way, the effect of the axial
drift electron current would be reduced, but the effect of the axial
potential gradient (which is about 0.3 volts/cm) would result in
rounding of the retarding potential plot near plasma potential and
would cause considerable uncertainty in the measured axial potential
gradient.
The disk probe consisted of a disk of 3 mil tantalum sheet about
4 mm diameter, mounted on 30 mil tungsten wire with a glass shield
around the wire to limit the collection area to both sides of the disk
only. In order to eliminate the effects of axial and radial drift currents,
the plane of the disk is located in a plane defined by the cylinder axis
and a diameter.
The center of the disk is located about 6 mm in from
the inner wall of the tube.
The center probe is somewhat more complicated and is illustrated
in Fig. II-2. It was mounted in the center of the cylinder with its plane
located in the plane determined by the cylinder axis and a diameter.
This probe was originally designed and constructed to permit the ion
and electron components of the probe current to be separately measured,
and consists of three concentric cylinders of 3 mil Ta sheet, plus a
wire mounted axially.
30 mil vW
The outermost cylinder acts as a
13.
shield while the next innermost cylinder supports a disk of about 4 mm
diameter with a 23 mil hole punched in it. The next smallest cylinder
and the W wire are situated within these two cylinders and are designed
to permit a transverse electric field to separate the ions and electrons
passing through the hole in the disk face.
The alignment of the con-
centric cylinders was somewhat of a problem because of the small
radial separations
(about 25 mils) and because the clearance
two innermost cylinders could not be checked visually.
of the
This problem
was solved by using aluminum foil spacers to maintain the required
clearance during assembly and then disolving the aluminum out with
hydrochloric acid.
Maintenance of this separation was more difficult because the
large length of the cylinders made it possible for a small angular
displacement of about 1 degree at the press to cause a short circuit.
Unfortunately a short circuit did occur between the wire and the next
cylinder after the tube had been processed and was in operation in
the water bath. Such a possibility had been anticipated, however, and
therefore the probe was designed to permit its use as a regular planar
probe in spite of such a short circuit..
The wall probe is the same as the center probe except that the
two innermost elements were not included, and the probe is mounted
with its face flush with the tube wall.
The cylinders were made on
the same mandrils and the face disks were punched with the same
jewelers punch.
The Ta probe elements were cleaned and degassed in the
usual ways and then were preoutgassed by electron bombardment with
il
14.
1
.
1,000 volt electrons until the pressure was 10- 9 mm Hg. They were
't
then sealed off under vacuum to keep them gas free until just before
assembly. The areas of the probes are listed below for reference.
Probe
Area
wire probes
0.227 cm2
disk probe (both faces)
0.280 cm 2
center face probe
0. 137 cm
wall face probe
0. 137 cm2
D. Vacuum Processing
4
i
After the pyrex envelope was cleaned and the probes were
sealed in, the discharge tube was sealed on the vacuum system along
i
with two mercury stills.
I
The tube and the smaller still were baked
at 4500°Cwhile the larger still outside the oven and its charge of
triple distilled clean mercury were torched out. Mercury from the
outer still was then distilled over into the smaller still and the outer
still was then sealed off. Some mercury from the inner still was then
distilled over into the discharge
tube and the arc was started with a
high voltage sparker.
The anodes were operated at overload currents until the anodes
were red hot. The probe elements were heated white hot by electron
ULVI
ALIJIJ
resistors
LUAIJIllL
LJy
I
ICiL
tUillI..
L.;.JI1
lJ.
iC1
to a high voltage, high current
AUL
W.L
dc line.
U
II-L
I.LI.
.JL.I.LbLLI
The ionization gauge
elements were again outgassed by electron bombardment, and the
evaporation filaments were again outgassed and slightly evaporated.
This outgassing procedure was repeated several times until the
L
i.
pressure with the arc in operation was in the 10
mm Hg range as
measured with the ionization gauge. The glass sealoff constriction
was preoutgassed and softened in preparation for sealoff of the
discharge tube. After sealoff and after some pulsed operation of
the evaporation getter filaments, the'residual pressure was about
10-7 mm Hg with the arc in normal operation.
of the arc and the ionization gauge resulted
Continued operation
in slow cleanup of the
residual gases and reduced the pressure to as low as 4 x 108
mm,
although subsequent electron bombardment of the tantalum probes
resulted in temporary increases of pressure to about 4 x 107 mm.
This evolution of gas was somewhat unavoidable since the press leads
and part of the probe cylinders could only be slightly outgassed by
heat conduction from parts of the probe that are subject to electron
bombardment.
E. Voltage source for retarding potential measurements
A novel and convenient source of retarding potential was
designed and used. The principle of operation is illustrated in
Fig. II-3. The desired retarding potential (with respect to the horizontal anode) was selected by a Leeds and Northrup voltage divider
(accurate to 0. 1 per cent) from a stabilized set of batteries.
This
selected retarding potential (plus a feedback voltage derived from
the Drobe otential) was fd into the rid circuit of a speciallv
designed high gain battery operated dc amplifier with a drift of less
than 0. 1 millivolt per hour.
The output of the amplifier drives a
cathode follower (composed of four 6AS7's in parallel) which supplies
the probe potential at a current of up to one ampere.
As a result of
1-.
:1
the
ner~tixrp
fPdhak
sai~ri
thb
hirLh6
n
th
L±
rtl
±
Lrr
k'nnntlL
1
Wrr LI
be within 0. 01 per cent + 3 millivolts of the retarding potential
selected on the voltage divider, and the output impedance of the cathode
follower will be less than approximately 5 milliohms.
A small
correction, however, must be made for the voltage drop across the
probe current meter.
F. Measuring technique and electron bombardment of the probe
In order to avoid the contact potential changes associated with
a contaminated probe surface(
14,
the probe was cleaned
before each current reading (or at least made reproducibly dirty)
by heating it by electron bombardment before each reading.
An
electronic timer was used to make the bombardment operation semiautomatic and reproducible in time, and a series resistor and a high
voltage source provided in effect a constant current source for the
bombardment current.
The time for each probe current measurement was about 15
seconds.
This included bombarding the probe for 5 seconds (mean-
while changing the retarding potential to the next value), reading the
probe current meter two seconds after the probe current was automatically switched into the measuring circuit, recording the data
and comparing the probe current with check points to insure that the
arc had not drifted.
The bombardment current drawn by the probe was about 10
per cent (or less) of the horizontal arc current I
.
If in the future
it is desired to avoid the possible disturbance of the plasma by the
I
4)
-A
)
1>
0t)
oa
>
4
r'
?
I
iX;
are
''
;-d
"iI.
vi
bombardment current drain, the probe can be constructed in wire or
hairpin form to permit probe cleaning by the passage of external
heating current pulses.
There is no doubt about the need for cleaning
the probe surface reproducibly before each current reading if accurate
results are desired.
G. Change of probe work-function
An interesting method was developed to permit the change of
probe work-function to be directly recorded to within a few millivolts.
A value of electron probe current was selected (usually slightly more
than the ion current at floating potential), and this current was supplied
by a constant current source (usually the ion current).
A specially
designed low drift high impedance recording dc millivoltmeter was
connected between the probe and a very stable low impedance voltage
supply which was set to the probe potential.
In this way, the recording
millivoltmeter acted as an expanded scale voltmeter.
Since the
dynamic impedance of the electron current source was quite low
(
- I'-/~,
(/./,.
the millivoltmeter,
o
are,
) compared to the input impedance of
the change of work-function
appeared across the
millivoltmeter to within a few millivolts.
The probe surface was cleaned by electron bombardment and
the change of contact potential was recorded as a function of time.
A typical plot is given in Fig. II-4 for both 'W and Ta probes.
The
probes of the same material have the same behavior indicating that
the change of contact potential is largely determined by the probe
material.
The non-monotonic behavior of the change of work-
function can be ascribed
to the presence of the residual
gases in
I
Tantalum probe
H
0
-,
Change Z
4
of work function vs.
bo
Time
after probe begins to cool
o0
Tungsten probe
2.12 x 10 - 3 mm Hg pressure
0
1
2
3
4
Time (minutes)
5
6
addition to the mercury on the probe surface.
Since mercury is electropositive,
it should reduce the work-
function of a clean metal surface although it is questionable how it
would effect the;work-function of a dirty surface.
influence different surfaces in different ways.
Various gases will
Typical values of
reported work-function changes are given below.
Surface
W
Gas
oxygen
Change (volts)
Source
+ 1.70
(17)
+ 1.20
(18)
- 0.35
- 0.85
(18)
(18)
oxygen
increase
(19)
hydrogen
decrease
(19)
Ag
oxygen
nitrogen
hydrogen
+ 1.0, + 1.4
- 0.1, - 0.2
- 0.3
(18)
(18)
(18)
Pt
oxygen
nitrogen
hydrogen
Ta
i
hydrogen
i
i
(20)
1.19
(20)
- 1.15
For the case of the tungsten probes in this experiment, the
initial increase is probably related to the absorption of oxygen (or
other electronegative gas), while the later increase can be explained
as the formation of mercury on the dirty tungsten surface.
Apparently
it takes longer for the mercury to deposit in spite of the much larger
concentration because of the thermal time lag of the cooling probe
and the strong dependence of the mercury condensation upon the
probe temperature.
Heating the same tungsten probe hotter or for
a longer time makes the decreasing part occur slightly later,
indicating that it is related to the cooling of the probe structure.
i
2
19.
In addition, the decrease in the work-function can be drastically hastened
by operating the probe an additional 10 volts negative with respect to the
plasma (for about 10 seconds), thus increasing the energy of the bom-
barding mercury ions and thereby increasing the number of mercury
atoms sticking to the probe. In addition, if the probe was not heated very
hot, only the later, decreasing part of the work-function change was
observed. These factors demonstrate that the decrease in work-function
is related to the presence of mercury on the probe.
The tungsten probe temperature was over 2400 K, as calculated
from equilibrium between electron bombardment energy input, thermal
conduction loss, and radiated energy loss, and also as calculated from
the observed electron emission from the hot probe. As a result of this
high temperature,
the W probe surface can be assumed to be quite clean
just after bombardment.
Since the probe current determines the probe temperature and
therefore the amount of mercury on the probe surface, it can be seen
that the decreasing part of the change of work-function will not be
observed at the higher probe currents.
On the other hand, if the residual
gas pressure is high enough (in other experiments), the recontamination
by the residual gases will not be observable particularly if it follows
closely the cooling of the probe.
For this experiment, the W probes were
heated hot enough to insure clean surfaces.
In addition, direct measure-
ment showed that the residual gas pressure was about 10- 7 mm Hg, and
the time calculated for the formation of a monolayer of oxygen at this
pressure was about 40 seconds.
residual gas pressure,
As a result of the clean probe and low
it was possible to observe changes of work-function
20.
that are norL-monotonic.
Two other experimenters who report the use of electron bom(15)
barded probes for plasma measurements were Howe(15) and Easley
(14)
but unfortunately they do not agree on the sign of the change of work-
function (by mercury) and they do not specify the experimental conditions
well enough to resolve the difference without some assumptions about
their experiment. Howeused probes of unspecified material (either
W or Ta) in a mercury arc and observed a monotonic decrease in workfunction, while Easley used W probes in mercury-argon
and mercury-
krypton mixtures and observed an increase in work-function (a decrease
in probe current) of several tenths of a volt. If it is assumed that the
data given by Howe for the change of work-function
is for a W probe,
then it can be seen that Howe's data is just a limiting case of the results
of this experiment and corresponds to either high residual gas pressure,
or to a probe that was not heated enough. For the case of Easley, since
her value of the difference of work-function is taken from the shift of the
retarding potential plots for measurements taken with clean and dirty
probes, and since the "body" of the "dirty probe" curve corresponds
physically to large electron currents and to a hot probe, there will be
little mercury on the surface and the uncompensated electronegative
gases on the probe surface will give a larger work-function for the
dirty probe than for the bombarded probe. It should be noted, however,
that the dirt on the probe is not mercury.
With this interpretation,
Easley's results are in agreement with the present results and with
Howe's results.
The behavior of the Ta work-function
is somewhat different but
21.
can be understood if it is realized that it is quite difficult to clean Ta
surfaces. It seems that hot Ta dissolves or absorbs the surface layer
4.
which reappears upon cooling (
. Apparently the electropositive com-
ponents appear on the surface first and reduce the work-function until
the oxygen overcomes this effect and produces a net increase in workfunction. When the dirty Ta probe is operated about 10 volts negative
with respect to the floating potential for about 10 seconds, it is observed
that the work-function decreases considerably.
Apparently mercury
atoms on the surface of a dirty probe will not reduce the work-function
appreciably but when mercury ions are driven into the probe to penetrate
the surface layers they will reduce the work-function.
It is suggested that this combination of a high impedance,
expanded scale recording millivoltmeter and a constant current source
be used in future experiments to investigate changes of work-function.
In any event, the measurements
of the change of work-function
of W and Ta that were made with the aid of the recording millivoltmeter,
and with a triggered sweep oscilloscope (Dumont 304-H) and a recording
camera (for time in the millisecond range), plus the direct knowledge of
the low residual pressure,
make it evident that whatever the detailed
mechanism of the change of work-function, accurate measurements may
be made (under the conditions of this research) if the probe currents are.
read within a few seconds of the time that the probe is cleaned by electron
bombardment. Clean probes are not really necessary, but probes with
reproducible work-functions are required.
characteristic
If the electrons have a
temperature of about 1 electron volt, and if one per cent
accuracy in electron current is required, it can be seen that a change
22.
of 10 millivolts in probe work-function can be tolerated.
Rapid reading
of the probe current reduced the drift, and in addition, the readings were
always made after a constant short time interval so that the error due to
drift of probe potential is a second order effect.
This technique was
developed because such exotic equipment as a high speed electronic
recorder was not available.
Wehner and Medicus (16 ) used an x-y plotter
to quickly take the retarding potential plot within 2 or 3 seconds after the
probe was cleaned by bombardment.
This high speed technique also
improves the apparent stability of the arc because it has less chance to
change in such a short time interval.
H. Stability of the arc and the reproducibility of the measurements
The state of the arc is primarily determined by two externally
controlled parameters.
The water bath temperature determines the
vapor pressure of the mercury (with allowance for the small thermal
drop in the tube wall), and the arc current determines the power input.
For a given tube of a given geometry and gas composition, the internal
quantities are uniquely determined by these two parameters.
Regulation
of the vapor pressure was satisfactory since the bath temperature was
stable to + 0. 10 C which corresponds to an uncertainty of 0.8 per cent
in the vapor pressure for bath temperatures above 200 C. The situation
was somewhat more difficult for the arc current since it required a
constant current source of up to 10 amperes for both anodes, and this
made it necessary to use a 220 volt dc line which was not as stable as
desired because of the varying load placed upon it by other users.
Since the majority of the arc current fluctuations was about
I
1 per cent with occasional ones of up to 5 per cent, a special technique
was developed to eliminate the effects of these large changes. Measurements were made in the late evening and early morning (between 12 a.m.
and 6 a.m. ) when the laboratory was deserted.
Precautions were taken
so that if a large change of arc conditions occurred it would be detected
without the loss of more than 5 measurements.
taking (for each probe characteristic)
This was done by first
a sequence of probe measurements
at one volt intervals, progressing toward a more negative probe.
This
sequence of measurements was then repeated with an interval of 0.2 volts
(less dense in the saturation region) and was compared with the previous
sequence to check the stability at every fifth measurement.
In this way,
a total of 5, 159 points were obtained of which 25 per cent were check
points with an average magnitude of deviation of 0.7 per cent for these
points.
An additional factor which contributed to the arc instability was
the erratic motion of the cathode spot which raced about the surface of
the mercury pool. This spot could have been made stationary by a
short section of tungsten rod extending above the surface of the cathode
pool. There was a deplorable tendency for the arc to go out probably
because of short transients in the dc line.
Since the arc had to be
reignited with a high voltage sparker, it was necessary to drain the
water tank, start the arc, fill the water tank and re-establish
temperature and the arc stability.
the bath
This process involved about 4 hours
and was a great inconvenience. It is suggested that in future experiments
of this kind, an auxiliary filament be built into the discharge tube near the
I1
24..
mercury cathode to provide a short burst of electron emission for the
re-ignition of the arc without the need to drain the tank. In addition,
the use of electronic regulators for the dc line is indicated.
III. Basic Experimenetal Data
A. Retaring
potential plots
The fundamental data obtained from the experiments takes the
forr:- of probe current ip, a a function of probe potential Vc. For
probe potentials above the plasma potential, an electron sheath forms
about the probe, andi the probe current saturates at a value equal to
the randor, electron current.
As the probe potential is reduced below
the plasma the electron sheath is replaced by an ion sheath,
and the
electron current decreases while the ion current increases.
At the
floating potential (about 4.
to 9.7 volts negative with respect to the
plasma potential) the electron and ion currents are equal and result
in a zero probe current.
Below the floating potential the probe current
is predominantly ion current and is almost independent of the probe
potential.
In order to determine the electron current component of the
pro-e current, it is necessary to determine the ion current cormponent
and use it to correct the total probe current.
In the ion current saturation
range the probe current does not vary strongly with probe voltage, (about
i per cent per volt) ancawhen plotted on a semi-logarithmic
(logi0 ip vs V prbe)
correction.
can be linearly extrapolated
scale
to give the ion current
Since the saturation ion current is about 0.2 to 0.3 per cent
of the saturation electron current, this correction is not large relatively
except near the floating potential where the extrapolation itself is more
valid.
A more satisfactory way of etermining the electron current
i
100
i
4
10
Plane
pro'
Tbath
25
Vplasma
z = 5.0 ai
i
o
0
0
1
--
/0
401
/e.
/
0o
I' Vfloating
0.1
_e
0.01
.001
I
I
-14
I
I
-12
I
.I
I
-10
I
-8
Probe Voltage (volts)
i .
Plnno
:t·
-
-
11a 0
- -. -I
-
,;_ _,I
isu"~l-
I
1
-6
-4
I
-2
r
100
0
o
:
o
10
rlWAC
-12
-10
-8
Probe Voltage
L
-6
(voltas)
-4
-2
0
F
l
r
1000
Al
Wire probe
(cathode)
Tbathe 25.1
C
Iz= 5.0 amp.
100
10
I
1:4
C.
O~
00
O
(D
/
C
0
1
0
-
/
/
lm
O
I,
C
rH
/
Vfloatin
0.1
0.01
I
-16
I
I
-14
I
I
l
I
I
-10
-12
-8
Probe Voltage (volts)
.*jbu
O
- iL-:..._.
IFg.
iu1
-
1-
F-i
to}
--In - 1:r -- I
,V-re "3
orI
I
I
I
I
-6
-4
I
26.
component is to use special probes designed to separate directly the
electron and ion components.
This has been done by others
(2 2 '
23) for
other gases and would have been possible in this experiment except for
In
the shorting of the internal cylinders of the multiple center probe..
any event, the determination of the electron temperature,
electron density,
and plasma potential is dependent upon the body of the electron energy
distribution and is relatively independent of the details of the high energy
tail.
B. Determination of the electron temperature,
electron density and
plasma potential
After the electron current is plotted in the form log 1 0 i vs
the probe potential Vc, the resulting retarding potential characteristic
is used to determine the electron temperature,
and the plasma potential.
III-21.
the electron density,
Typical plots are given in Figs. III-1 through
The relation between the electron current density J and the
probe potential Vc for a M-B distribution is given by:
.T
j
S
*)
For Vp > Vc the electron temperaturekT_/qin
from the slope of the semi-logarithmic
j.,
J 5~
(--1)
Cur (fz;
electron volts is obtained
plot and from the relation:
(111,
_r--2)
This value of kT_/q for each probe characteristic
was obtained from an
average of several determinations on a 5 cycle semi-logarithmic
plot
and from several determinations on a 2 cycle semi-log plot, in order to
reduce the error introduced by plotting and slope measurements.
L
In this
1Ga
4- CA
cp
100
Disk probe
Tbath 25.1°
10
C
I z = 5.0 amp.
1
h
,0
or
0o
0
$4
0
/
/
C)
0
/
0.1
/
/
I
*1
6
/
Vfloating
(4
C
e
0.01
0.001
I
I
I
-14
-12
I
I
-10
Probe Voltage
I
I
-8
(voltsa)
I
-6
I
I
-4
-2
h
100
Plans probe
Tbath= 25.1
10
Iz = 5.0
amp.
1
0
-o
0
/
r=
/
.
/ .
/ ·
..
_
0
S:4
0.1
Vfloati
floating
rH
O
0.01
0.001
I
I
-14
I
I
I
-12
-10
I
!I
-8
Probe Voltage (volts)
Fig
:LI
i~~~~~~~~~~~~~~~~~~~~~~~~~.
1;
ii~~~~L~~~~~~~~~~~~~~~r
I
I
-6
I
I
-4
I
I
-2
F
27.
way, the areement
between successive determinations of electron tem-
Derature from the same data was about 1 Per cent. In the calculation of
the electron density, any uncertainty in the electron temperature is
reduced by a factor of two since only the square root of the electron temperature is involved. To reduce the accumulated error
ue to rounding
off figures, four significant figures were retained through all calculations.
The latest values( 2 4) of the basic physical constants were used in the
calculations.
The value of the saturation electron current Is corresponding
to the random electron current is obtained from the intersection of the
extrapolated straight line characteristics of the retarded electron current
and the saturated electron current.
This intersection noint is also used
to determine the plasma potential. Again, repeated graphical determinations
.
are used to reduce plotting error.
from:
/k -
-
The electron density s calulated
- __&-)
(III-3)
where the average electron velocity ( v_ > for a M-B distribution is given
by: (v_)
6.693 x 105 (kT /q)1 / 2 meters/second.
the high energy electron
is not significant
The depletion
in the determination
of
of the
electron density since the various retarding potential plots have a
depletion potential + (Vp - Vd) (with respect to the plasma potential) that
is approximately related to the electron temperature by: + (Vp- Vd) = 3.39kT_/q
which corresponds to an electron current of 3.4 per cent of the saturation
current at the depletion potential thus indicating that the correction can
be at the most this value of 3.4 per cent since this corresponds to a
___
100
Plane
T
10
bath
Iz-
5.
1
Pa
0
/
4
0
///,
///1
0.1
C
V
/
floating
9
o
0
-
4
r.
0
g
0.01
0.001
XI
I_
-14
II
-12
I
-10
I
I
-8
Probe Voltage (volts)
;"I- 7-~~~~~~-
I
I
I B
-6
II
I
f
-4
I
J
I
-2
1000
Wire probe (cathode)
T
bath
100
0
30.0 °C
3
I z = 5.0
amp.
10
0P
0
C-
0
4'
/, /.
To
1,1
e
r
wo
0
/
1
V
/
J/
.
Vfloatin
$
0.1
_
0.01
I
-16
I
I
-14
I
I
I
F
;Yz
r
I
I
-12
-10
-8
(volts)
Probe Voltage
I*.
,
I
I
-6
Il
I
-4
depletion of high energy electrons and not a complete absence of them.
The values of the electron temperature thus obtained are listed
in Table I along with the electron density and plasma potentials.
There
are no significant systematic variations of the electron temperature
with probe position, probe geometry or probe material, and therefore
the average of the various electron temperatures for a given set (constant
bath temperature and arc current) is used to give a characteristic
temperature for the plasma.
electron
(See.Table II. ) The uncertainty of the
mean electron temperature averaged over the 9 sets is 1. 8 per cent and
probably corresponds to the instability of the arc, bath temperature,
arc currents.
and
The uniformity of the electron temperature for the low
pressure arc is in agreement with the findings of Killian ( 5 ) . The average
electron density at the axis of the plasma cylinder no,
is obtained from
the average of the density measurements by the wire probes and the
center plane probe. The small average uncertainty in n_ 0 (1. 5 per cent
on the average for the 9 sets) demonstrates the small axial charge density
gradient and indicates that the charge density measurements are quite
reliable.
Plasma potentials are not necessarily as accurate because of
the uncertainty of the probe work-function
due to the presence
of surface
gases. The tungsten probe measurements are more reliable, however,
since the W probes were heated quite hot and made relatively clean.
In addition, the axial potential gradient is obtained from differences of
plasma potentials for the similar tungsten probes and therefore any
systematic error in the tungsten work-function tends to cancel leaving
L
____
1000
Plane probe
30
Tbath=
100
Iz=
(center)
.0
C
6.0 amp
10
I0
v
4)
0
P
_P
)
0AO
1
0
/
/
H
!
Pq
/
/
0.1
/e
Vf loating
/
-?/
I
I
I
I
I
I
I
I
I
I
I
I
I
I
I
4
- nIL
VU.
I
-16
I
-14
I
I
I
i
-10
-12
-8
Probe Voltage (volts)
FLa.ne
661,
nt ar
:iVoi.
nu
'; s?
-6
I
-4
I
1000
Wire probe (cathode)
T
=_0
d
Tbath
bath -30.0
mp*
100
z2 6.0 amp.
r
10
0a,
0
0
C)
/0
1
0
4
/
0
/
/
/
/
.
/.
V
f1 01
Vflo
I,
I
/
/
0.1 _
I
I
$
_
0.01
l
-I
-16
I
I
I
I
I
-12
-14
I
-10
Probe Voltage (volts)
"
Fi . I 1i
4iŽ3
prGS
cuWv
I
-8
I
il
-6
Il
-4
r
only second order errors.
In several cases where wire probe measure-
ments were repeated, the calculated values of the axial gradient are in
agreement
to an average of 1 per cent.
The values of E z are given in
Table II. The potentials listed in Table I show that if the floating potentials
Vf are used to calculate E z rather than the plasma potentials Vp, there
may be an error of up to a factor of 2 at the lower pressures.
This
difference occurs because the slight differences in the retarding potential
plots for the wire probes become more important at the lower pressures
where kT /q is larger.
C. Mercury vapor pressure and density
The density of mercury atoms in the plasma is an important
Mercury vapor
parameter in the determination of the plasma processes.
pressure may be calculated as a function of the temperature of the inner
glass wall provided that the discharge tube is submerged so that some
mercury condenses on the wall to provide local equilibrium between the
mercury and the mercury vapor.
The need to submerge the whole dis-
charge tube and not only the cathode pool area may be demonstrated by
slowly raising the water level in the bath. When the water level reaches
the horizontal arm, a drastic change in probe characteristic
indicates
a strong dependence of pressure upon the submersion of the tube.
The mercury vapor pressure is predominantly controlled by
the water bath temperature Tb, but there is a correction of about 10 to
20 per cent in pressure
corresponding
to the temperature
drop AT, in
the pyrex wall, due to the conduction of heat outward to the water bath.
The electrical energy input to the plasma can be calculated from the
arc current Iz and the axial potential gradient E z . It is assumed that
100
Plane
Tbatl
Iz=
10
1
0
,-
p4
/-
00
?/
/
0.1
Vfloating
0
0o
0
e
W
e
0.01
0.001
-14
-12
-10
-8
Probe Voltage (volts)
~?.
I,,
ne c e n
l
I I
Il I I I Ill
::r
-:o
ill 10T~t. r·1
',1-11I
". '
-6
-4
-2
-2,
1000
Wire probe (cathode)
m _ an - O.
'bath =or.
100
Iz= 5.0 am]
10
C
,
0
O
0
O
C,
0C4
a,
1
/,
O
e
/0
Q
0l
O
0
rx
F4
,.4
/
V
floating
t
0.1
0.01
I
I
-16
I
I
-14
I
I
Il
I
-12
-10
Probe Voltage (volts)
.
,laai -.pz,
T J T _ l1I
I
-8
a
I
a
I
-6
I
I
-4
sof
30.
with the exception of a small fraction of this input which passes through
the pyrex wall as electromagnetic radiation, this input energy must be
fA
removed by thermal conduction. From the tube geometry, the temperature drop-AT, in the wall is given by:
,6o-
7 -
/r,)
(111-4)
where IzE z is the electrical power input per unit length, r 2 /rl
is the
ratio of outer tube radius to inner radius, and the thermal conductivity
for Pyrex (chemical glass No. 7740) is given by: K = 0. 0027 calories/
sec x cmrrx C. For the experimental tube used where the inner radius
is 2.75 cm and the wall thickness is 0.24 cm, the temperature correction
is:
where AT is in degrees C, E z is in volts/cm,
and I z is in amperes.
The significant values are given in Table II.
Very accurate data for the vapor pressure of mercury as a
function of temperature are available in the International Critical
Tables (25) , and in slightly abbreviated form in recent editions of the
Handbook of Chemistry and Physics (26) . The following empirical
expression was calculated (using least squares) to describe the dependence
of the vapor pressure
p, on the temperature T°K for the range between
00 C and 1000 C:
j,
7
(III-6)
This simple expression has an average magnitude of error of only 0.4
per cent with a maximum error of 1.2 per cent occurring at 0°C, but in
order not to introduce an avoidable error,
this expression was used to
r
.
~
Iuuu
Plane probe
Tbath
l
(center)
45.1 C
Iz= 4.0 amp.
100
10
a
I,
0
/
0
/o
/
/
00
Czr
/4
'I
//
,
oo
·
;;
;i i
·;
1
··
;·:
· i
Qp
0-
.! i
rH
e
Vfloating
/
e
0.1
m
e1
0.01
.-
I
I
-14
l
l
-12
I
I
- I
I
-10
-8
Probe Voltage (volts)
,.M L"t
t~.*
i 1
-,t,:,!
" "e; -1,--e I- -PI
' -.
I-
-, t"?
,;'
-6
i
III
-4
-2
1000
100
10
0
o
00
O1
0.1
r.0
n~ni
-16
-14
-12
-10
Probe Voltage (volts)
Pi . ii-13
-8
-6
-4
-2
31.
interpolate betweein the tabulated values rather than for direct calcuation.
From this expression
it can be seen that an uncertainty
of 0. 1°0 C in the
temperature corresponds to an error of less than 1 per cent in pressure
for temperatures above 10iC.
What is really significant in the plasma is the mercury density
The perfect gas law (P = nkT), can be
rather than the vapor pressure.
used to calculate the gas density ng, from the vapor temperature Tg,
and the vapor pressure p. In particular,
one can calculate the reduced
pressure po, which corresponds to the pressure for this same density
at OOC:
P7.3. ~
(III-7)
The reduced pressure is really only a unit of density with 3.536 x 1016
3
molecules/cm3 equal to 1 mm Hg of reduced pressure.
of pressure,
reduced pressure,
Calculated values
and gas density are listed in Table II.
The mercury vapor temperature
- Tg, is assumed to be the
same as the temperature (Tb + AT), of the inside of the pyrex envelope
but actually the vapor temperature is slightly higher because of the
"temperature jump" ( 27 ) associated with the conduction of heat from
the gas to the wall.
is small.
It will be shown that this temperature
T,
jump
Chapman and Cowling(2 8 ) give the following expression for
the temperature difference ST between a gas and a wall:
(I-8)
ar
where the accommodation coefficient 9, is a function of the gas and
the wall material.
The factor p' is a constant of order unity,
),is
the
1000
Plane probe (center)
Tbath=
100
45
.1 *C
Iz= 5.0 amp.
10
0
/'
/,
00
4)
0
0
/'
/.
/
0
C,
1
/
/
Vfloating
0.1
0.01
_
I
-18
I
'I
-16
I
I
I
I
-14
-12
-10
Probe Voltage (volts)
~1~8·1~;Y~1,5e
v ,7
,i;j
l' it'
I
-8
I
-6
r
1000
l
Wire probe (cathode)
Tbath
45
.1
C
Iz= 5.0 amp.
100
10
.
00
A.
0o
k
o-
/·
-P
/.
0a)
/
1
/
/0
H
h
'.o
P
C)
/e
a)
ri
W
/'
/,
Vfloating
/6
/
/
0.1
/
I
0.01
-18
-16
I
I
I
-14
-12
-10
Probe Voltage (volts)
I
I
I
-8
-6
I
32.
mean free path of the gas atoms and d.T/dr is the temperature gradient
in the gas. The accommodation coefficient can be interpreted
(2 7)
as the
ratio of the actual energy transfer from gas to wall compared to the
energy transfer that would occur if the gas atoms were emitted from the
wall with the characteristic
temperature of the wall.
The International
Critical Tables (29 ) give the accommodation coefficient for mercury
atoms and liquid mercury as 1. 00 + 0. 01. 'The mean free path for
mercury atoms in mercury vapor can be calculated from data given in
the Smithsonian Tables (3 0 ), and the value of po ?Yobtained is
2.4 x 10-3 cm x mm Hg. For a mercury pressure of about 10 microns,
the mean free path is about 0.24 cm which is small compared to the tube
radius.
It is somewhat difficult to determine the temperature gradient
dT/dr in the gas but an estimate can be made of its value.
The tem-
perature drop in the pyrex wall has been calculated to be about
degree C
for a thickness of 0.24 cm thus giving a temperature gradient of about
4 degrees C per crn for the pyrex wall.
The thermal conductivity of
pyrex is 2.7 x 10- 3 calories/cm
0
x sec x C, while the thermal con-
ducitivity of mercury vapor as calculated from data given by Elenbaas
is about 6 x 10 5 calories/cm
x sc
The results given by Klarfela (3
pressures
below 100 microns,
is converted into gas heat loss.
)
x C for mercury
for a mercury
(3 1 )
vapor at 300 0 K.
are indicate that for
less than 1 per centof the inputpower
With these figures it is calculated that
the temperature gradient in the gas near the pyrex wall is about 2 degrees
C per cm.
When the value of the accommodation
coefficient and the mean
free path are introduced, the temperature jump 'T, is found to be about
0.5 0 C. Since this approximate correction is only 0.2 per cent of the
1000
Plane probe (center)
Tbath= 45.1 "C
100
Iz= 6,0 amp.
10
0
(D
0
o4
·
0
a
4~
A.
0
ls,
0
1
0
0~
Vfloating
0.1
0,01
' I
-16
I
I
-14
l
1>1n3-5tH
I
I
I
I
I
I
-10
-12
I
-8
Probe Voltage (volts)
;'.
S_~~3
L2
3I.
~~.
k,.I.
~He. t Tlr
;I,
L 2 2J
,
-
C.1
I
-
I
-6
-~~~~.
I
I
-4
Wire probe (cathode)
!
i
o
a,
0
0
4"
0
I
!
o
4-
,i:
':
i,
0.1
i
!
jj
I
0
Probe Voltage
(volt
z
I~~~~~~~~~~~~~rBF
-8
-6
, *i-.
-
?:.l7X~~~~~~~~~~~~~~~~~~~~~~e
,-tD~~~~~~~~~~~~~~~~~le
ci~~~~~~~~~~~~~~tre
CU+"
:~~~~~~~~~~l
33.
approximate vapor temperature of 3000 K, the temperature jump will
be nerlected.
D. Wall sheath thickness
The diameter of the plasma does not correspond
exactly to
tube because of the formation of
the inner diameter of the discharge
an ion sheath on the insulating walls.
In order to calculate the processes
the plasma radius must be known and therefore
in the plasma,
the sheath
thickness must be calculated. This sheath thickness Ar is calculated
for the wall probe at floating potential and it is assumed that the floating
potential of the tantalum probe is the same as the floating potential of
the pyrex wall.
The difference between the probe floating potential and
the wall floating potential cannot be large because the electron current
is a strong function of the collector potential.
Since the electron density in the ion sheath is small, the ion
<
current flow to the wall is governed (in this region) by the LangmuirChilds space charge law:
',.
--
i
(III)
where J+ is the ion current density, AV is the potential difference between
Ar is the sheath thickness, and E0 in
-12
This equation
farad/m.
MKS units is equal to 8. 854 x 10
the plasma and the floating probe,
rationalized
assumes that the plasma-sheath interface has a definite location, that
the ions enter the sheath with zero velocity, and that the potential
gradient is zero at the interface.
Actually the ions enter with a directed
energy of about kT_/2q, and the potential gradient is approximately
volt/cm.
1000
Plane probe (center)
Tbath
=
54
.6
C
I z = 5.0 ampe
100
iC)
v
0
0
4
1
_D
0
0
4C)
/·
/·
3.
Q
r-
Q
Vfloating
I
0.2.
0.02.
I
I
-16
II
I
I
-14
-12
-10
-8
Probe Voltage (volts)
.i't ,* I _at i
Pln
ae. n ^ t .1_. _rb
1,
j
-.
rar,
:,,
I
I
I
I
-6
1000
Wire probe (
54 , 6
Tbath
100
I z =5.0
'
amp.
10
a)
0
p4
//.
4
f
0
0
/.
/
1
Voati
f loating
4V
0
0
Hl
I
0.1
0.01
_/
I
-16
I
I
I
-~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~
I
II
I
I
-12
-10
-8
Probe Voltage (volts)
A!.tEr I . - ,
(i?_?
rc bD
CaX27e
.........
I
I
-6
I
1
-4
I1
34.
A first order correction has been calculated for the LangmuirChilds space-charge equation, and results in a change in the calculated
sheath thickness by the factor:
o-- (I
4r2
E (
t
d
-
7
..
(III-10)
where E is the initial directed energy of the ions in electron volts as
they enter the sheath (assumed monoenergetic), AVis the potential difference between the plasma and the probe, dV/dx is the potential gradient
at the interface, and Ar is the sheath thickness as calculated from the
zeroth order Langmuir-Childs equation. When typical values are substituted in this expression, it is seen that the change in
r caused by
the interface potential gradient is quite small while the predominant
correction is the 1.5 (Eo/AV) 1/ term which is about 38 per cent.
Since the ion sheath results in a correction of less than 0.4 per cent of
the radius which is comparable to the 0.4 per cent uncertainty in the
measured tube diameter, any detailed correction is of small value.
The calculated values of the ratio of plasma radius to inner wall radius
rp/rW are listed in Table II.
F"~~~~~~
.
':!',A
1000
L
I
Plane probe
Tbath
(cer
62.6
C
Iz= 5.0 amp.
100
10
0
o
4,o
o
i
0
1
I
0
i
i
r0
i
V
W
Vfloating
0.1
0.01
_
.
-16
I'
.
*
I
-14
I
I
I
I
-10
-12
X,
I
-8
Probe Voltage (volts)
'P'--l'
.,a n e
L
- rY'
n't
~.Dv a :,,ur,; e·
I
-6
I
i
.I
-4
I
1000
Wire prol
Tbath
62
I =5.0
100
10
,0
I-
0o
a~
o
C)
v
1
To
V
floating
/'
0.1
-
0.01
I
-16
I
-14
I
I
I
-10
-8
Probe Voltage (volts)
-12
c*
.r-o
rob
CurrVe
I
I
I
-6
.I
-4
I
35.
IV. IonizationProcesses in the Plasma
A. Production of ions in the plasma
One of the important processes in the mercury arc plasma is
the production of ions through the inelastic collisions of high energy
electrons with mercury atoms. It is sometimes assumed that ions are
generated predominantly by direct ionization of atoms in the ground
state (6 SO). An alternative method of ionization involves the produnction of excited atoms (63P0o 1 2) and then the subsequent ionization
of these excited atoms to form ions. Meissner and Miller(33) find that
external irradiation of a positive column (He, Ne, A, Kr, Xe) results
in a potential increase for a constant current.
They show that practically
the entire irradiation effect for the He plasma is due to the absorption of
the 20, 581 A line which raises the metastable
21S0 state to the 21P1
(34 ) reports that for a discharge in a Hg and rare
radiating state. Kenty
gas mixture, strong illumination by Hg radiation nearly doubles V and
T (arc voltage and electron temperature).
He concludes that the P
metastable state furnishes most of the ions. These experiments indicate
that the ionization in a plasma is determined largely, by the metastable
density. In his microwave afterglow studies, Biondi (3 5 ) finds that
collisions between pairs of metastable atoms also produce significant
ionization in the decaying plasma.
These ionization processes that
take more than one step are called cumulative -ionization.
It will be shown in this chapter that the ionization in the plasma
under investigation proceeds predominantly by cumulative processes.
36.
B. Measurement of the total ionization and the determination of the
electron density distribution from ambipolar diffusion theory
It is necessary to measure the total ionization G, (ions generated
per second per unit volume) as a function of the electron density n_, the
gas density ng, and the electron temperature kT_/q, in order to obtain
the desired information about the fundamental processes involved in the
production of ions. From consideration of charge conservation, when
there is no significant volume recombination of ions and electrons, the
ions and electrons produced in the steady state plasma will all travel to
the wall where they will recombine.
The ion current density to the
wall probe when it is at floating potential J (Vf), is therefore a
measure of the total ionization averaged over a cross-section
of the
plasma G. This assumes that the effects of the secondary electron
emission from the pyrex wall and from the tantalum wall probe are small.
There is very little information available for the secondary
emission coefficients for Hg metastables,
but a value for the secondary
emission coefficient for Hg metastables on mercury has been reported
by von Engle ( 3 6 ) to be about 0. 01. This implies that the metastable
induced electron emission from the pyrex wall (with condensed mercury
on the surface) can be considered small.
In addition, measurements on
the ion current to a negative probe show that the difference between the
ion current for a clean and dirty probe (Ta and W) is less than 2 per cent,
for this mercury arc.
Since Hagstrum ( 37 ) has shown that the secondary
emission coefficients are very dependent upon the surface contamination,
it is expected that if the secondary electron emission were contributing
appreciably to the ion current measured by the wall probe, it should
37.
be possible to influence this ion current by cleaning the probe by electron
bombardment.
The absence of such an effect implies that the secondary
emission due to nr.etastables is notsignificant.
In addition, since the
electrons ejected by metastable bombardment must again be collected by
the wall, a net error is introduced only if the secondary emission
coefficient for the pyrex-mercury wall is different from the secondary
emission coefficient for the Ta wall probe. It can thus be seen that the
secondary emission due to metastable atoms is only a second order effect
of the ambipolar
in the measurement
ion current.
Since the secondary
emission coefficients appear to be only about 1 per cent for Hg, the second
order effect due to metastable induced secondary emission will be neglected.
From the continuity equation div (J+/q) X G, it can be shown
that the average total ionization G (averaged over the cross-section
of
the long cylindrical plasma), is related to the wall ion particle current
s (IV
rt+*~LY
density [7+w' the plasma radius rp, and the wall radius r w , by the
following expression:
rw )
-=~~L
(
The values of [ calculated from the experimental data are listed in
Table III.
In order to investigate the dependence of U /p n
upon kT_/q,
.it is necessary to determine the value of n_ (averaged over a crosssection of the plasma) in terms of the central density n_o, and the
wall density nw.
This makes it necessary to calculate the electron
density distribution and requires a discussion of ambipolar diffusion
theory.
If only electrons and singly charged ions are produced, the conthe
servation of. charge andlcontinuity equation for the steady state require
that:
(IV-2)
,Xv ftz-
(IV-3)
In the range where mobility and diffusion processes determine the electron
and ion flow,
(IV-4)
= D
where D+,- -,
,m/#4
D arid
-4'
-7
7 'd
(IV - 5)
_ respectively are the ion diffusion coefficient,
the ion mobility coefficient, the electron diffusion coefficient, and the
electron mobility coefficient.
The potential V, is related to the ion and
electron density by Poisson's equation:
011Iv
Yd,/
.V6('I(IV-6)
~
where e is the permittivity in rationalized MKS units.
The diffusion coefficients D+, and D, correspond to a net flow
due to a density gradient.
There is also a "temperature diffusion coef-
ficient" which: corresponds to a net flow resulting from a "temperature"
39.
gradient, but it is assumed that the "temperature gradient" is small compared to the density gradient so that the thermal diffusion can be neglected.
There are now 5 independent equations relating the 5 variables
r,-',
nn,
n,
and V (two vector and 3 scalar).
Since the differential
equations contain nonlinear terms it is necessary to resort to some
simplification following Schottky's ambipolar diffusion theory 3(
with a little more rigor in the derivation).
)8
(but
The potential gradients in the
plasma are quire small (about 1 volt per cm) and therefore according to
Poisson's equation the ion density is very closely equalS-tothe electron
density and permits the elimination of one of the equations and one of the
variables by allowing the approximation n = n+. The ion and electron
density gradients are also approximately equal although probably to a
lesser extent.
When the divergence of f+ and 7
are taken and the
term div (n_ grad V) is eliminated, it is possible, without requiring
any assumption about the equality of r'+and r, to obtain the following
expression which is valid for any geometry:
F o(IV-7)
Do j,~A_) tr^S
;,v (PI
where the ambipolar diffusion coefficient Da , is defined by:
Vhen
-, independent
teate
is assumed of position,
t(IV-8)
When Da is assumed to be independent of position, the following results:-
i)E
ELITlv ,7r^S o
fbSy
e(IV-9)
This differential equation when solved with the appropriate boundary
conditions will specify the density distribution of the electrons and ions
40.
and will permit the average electron density n_, to be calculated.
Considerable information about the properties of the plasma can
be obtained from the relationship between the currents and the potential
and density gradients. Because div Ps , div
F
and 7,
r',
the vector currents
differ only by a constant vector term B and by a curl A
term since in general div curl A X O.0.For a long cylindrical plasma the
radial component of the curlA term
coI
-.-
)
is zero because of the angular and axial symmetry.
Since the radial
}+
components of'the vectors
and
are equal at the wall (which is at
floating potential), the radial component of the constant vector B is
zero at the wall and is therefore zero throughout the plasma.
demonstrates that the radial components
/r
and fr
This
are equal for all
values of r in the plasma so that:
(IV-l0)
It should be noted that this is not necessarily true for all components of
F+and
,
as is sometimes stated or implicitly assumed. Elimination
of r between the radial components of the vector equations results in
- = o(IVI)
the relationship between the radial density gradient and the radial potential
gradient:
9 D
-Ds
-
which has as a solution:
,'
(V,
---- ~(-,,
/4g
p
>*
].
(IV-12)
41.
It will be shown that-this leads to the Einstein relation between the diffusion
coefficient and the mobility coefficient.
An insulating'surface placed in contact with the plasma will
assume a potential with respect to the plasma such that the total current
to the surface is zero.
Since the electron and ion densities are equal,
and the electron mass is much less than the ion mass, the random current
to the probe will be predominantly electron current unless the ions are
much more energetic than the electrons which is very unlikely.
Thus
the potential of the insulating surface will go negative with respect to the
plasma .until it repels all but a small fraction of the incident electrons.
At the same time, this negative probe influences the plasma (in spite of
the fact that the bulk of this potential difference appears across the small
ion sheath) and draws additional ions to the surface
in excess of the
random flow.' As a result of the negative wall, the radial flow of electrons
due to the density gradient is very nearly compensated by the opposite flow
Since the negative wall considerably reduces
due to the potential gradient.
the electron drain, the electrons are in approximate equilibrium and therefore their density may be described by the Boltzmann density distribution:
,M
ex
[
(IV- 13)
r
Since the mobility and diffusion coefficients for electrons are much larger
than for ions, these equations lead to the Einstein relation:
P-
yTr-
.,,,~->~~~~~
~
For the case where the distribution functions are not isotropic, the
Einstein relation may b expressed in tensor form(39 ' 40)
L
~(IV14).
Since the radial components of the flow vectors are equal, it is
possible to eliminate dV/dr from the vector equations to obtain the
following relation between the radial current flow and the radial density
gradient without requiring any assumptions about the constancy of Da:
(IV-15)
This equation will be used in the next chapter to calculate the ambipolar
diffusion coefficient from the measured value pf +(Vf) and from the
value of dn_/dr calculated from the assumed density distribution.
In
addition, the average electron density n_ , will be used in the calculation
of the electron mobility.
Thus it can be seen that many interesting quantities can be
calculated provided that the electron density distribution can be specified
with reasonable accuracy. The partial differential equation for the electron
density can be reduced to a differential equation in r if the plasma is long
enough so that end effects are negligible and if advantage is taken of the
cylindrical symmetry of the plasma to eliminate the angular dependence
so that:
i
Dr
'
wet- /
(IV-16)
For the case where the ion generation is assumed constant throughout
the plasma volume (only for the 'purpose of simplicity of solution), the
electron density can be expressed in the following simple form:
~'
-L
e
(Il'V-17)
% e"'
-
I
43.
where the part of the solution that is singular at r
0, is eliminated by
the physical requirement that the electron density is finite and non-zero
This solution, although easily obtained is not
at all points in the plasma.
an accurate description of the density distribution.
A much more accurate solution corresponds to the assumption
that the ion generation is proportional to the electron density and can be
written as
(IV-18)
The ionizatioh frequency
i (ions per second per electron) is a function of
the electron energy distribution function and.it is necessary to assume
that rwi contains no radial dependence in order to solve the differential
equation. The resulting linear differential equation has solutions in the
form of Bessel functions with the singular solutions excluded because
of the finiteness requirement so that
(IV- 19)
"-
where as a result of the change of variable
V
(
.v-z0)
Because negative values of n are not possible, the first zero of the
Bessel function cannot be within the plasma.
It is possible to determine
Ro by measuring the electron density at the center and at a particular
radius (for convenience at the plasma edge). In this way, the quantity v /D a
iL~~~~~~~~~~~~i
a
44.
can be calculated from the measured quantities.
The case where the ionization is assumed to be completely
quadratic ionization (G(n) = b(n )2 ), has been considered by Spenke(4 1)
-
who solved the resulting nonlinear equation to give the density as a
function of the radius:
'
)
(IV-21)
where 2. 92 is the first zero of the Spenke function,
are tabulated by Spenke for the variable
2. 92 r/R
o
and the values of S
in steps of 0.2 up to
the first zero.
The difference between the density distribution given by the
Bessel function and by the Spenke function is quite small (see Howe(l 5 )
in view of the uncertainties of electron density measurements so that it
is not easy to determine experimentally if the density distribution.fits
the Bessel function or the Spenke function better.
Howe (15) has made
measurements of.the electron density distribution in the plasma of a
low pressure mercury arc, and he obtained values of electron.. density
(for 5 different radial positions) that could be fit somewhat better by
the Spenke function than the Bessel function at the 3 highest pressures.
On the basis of this and also in view of the fact that the direct ionization
cannot account for the observed ionization, Howe concluded that the
ionization in the plasma is quadratic cumulative ionization.
Unfortunately
the accuracy of Howe's experimental results are somewhat subject to
question.
Analysis of the experimental data given by Howe in his article
shows that the electron density as measured by his wall probe is about
48 per cent (on the average) larger than the electron density as measured
45.
by the element of his multiple probe nearest the wall. Since this is
obviously impossible, the value of Howe's density measurements is
reduced.
In any event, since the average of the electron density distribution is needed for the determination is the total ionization frequency,
and for the electron mobility, the process of integration itself reduces
any small difference between the.assumed distribution and the actual
distribution. To estimate the small differences resulting from the
various assumed distributions, the average density (normalized to
unity at the center) has been calculated for the parabolic distribution,
the Bessel distribution, and the Spenke distribution, with the integration
for each taken out to the zero since the averages differ the most at this
value.
The calculated values of WI/n
o are: n/no(parabolic)
n/no (Bessel)
=
0.432, and in/no(Spenke)
of the Spenke distribution
solution given by Spenke.
0.374.
0. 500,
The average value
was calculated by integration of the series
From the above results,
it. can be seen that
the Bessel function can be used to evaluate the average electron density
quite accurately with the uncertainty not more than 13.5 per cent if the
actual density distribution falls between the Bessel distribution and the
Spenke distribution-
The properties of the Bessel function permit the
average electron density in, to be given by:
(IV-22)
where the value of x is determined from the density at the center n,.
and at the wall nw'
by
46.
'"''
x
(IV-23)
The values of n_- /no- calculated from n-ww/n -O have been listed in Table III.
It is now possible to calculate the total effective ionization frequency
reduced to 1 mm Hg and 0°C: G/ponn
In order to analyze the dependence of the total ionization G, upon
the electron density and the electron temperature, it is necessary to
consider in some detail the general problem of the production of particles
of type "B" as a result of collisions
of electrons
with particles
of type
"A". In general, the generation rate G (number per second per unit
volume) is given by:
c
00
/r(Xo=
-dZ(J
age ofg)vsvC7~
Mr(IV-24)
where the velocity of the electron is assumed to be much larger than the
velocity of particles "A" so that the relative velocity of the electrons and
particles"A" can be assumed to be equal to that of the electrons v.
When the differential cross-section Q(A-*B), which is a function of the
electron energy V, has a threshold energy V9 , and when the velocity
distribution function of the electrons is M-B with a characteristic
temperature
the form
VT
X
kT_/q,
the generation rate G, can be expressed
in
G(A)
s(/B)
"(A)
>/e M
$J)(IVWr
5)
(Iv-a5)
47.
The effective cross-section
value of
Q(A->B), can be defined as that
. constant cross-section which when used to replace the
differential cross-section
in the integral will give the same value for
G(A-*B). Thus
sso-p
PI e
> ( B)
(IV-26)
and it is seen that the major dependence of G upon VT is in the exp(- V/VT)
term since the effective cross-section
Qo is not a strong function of VT.
This expression for the ion generation suggests that the quantity
G/po _ when plotte.d (on a semi-log plot) as a function of q/kT_ will have
a slope characteristic
of the effective threshold energy V 9, for the
production of ions. It is assumed that the number of atoms of type "A"
supplying the ions (atoms in the ground and excited states) is proportional
to the reduced pressure
p
The quantity log G/pon
is plotted in
Fig. IV-1 as a function of q/kT_ and it is seen that the points fall on a
straight line as is implied by the previous equation.
The effective threshold voltage V0 , is found to be 6.26 volts,
and the average effective cross-section
QOfor ionization in the mercury
plasma is calculated to be 0.41 x 10-16 cm 2 with a rms deviation of
10 per cent.
Thus the actual ionization in the plasma can be described
quite accurately by
(> .
X-
-1
(IV-27)
V~~~~~evri
r,
-7 a
108
6 3 P2
0
co
0
02
0
o
a,
bO
4,
106
0
C)
Direct
Ionization
Q
C,
Experimental
Ionization
I:J
4 amp
®
5 amp
+
6
SC
04
104
103
amp
Mercury
I
I
Vapor
I
0.4
0.2
I
I
0.6
q/kT_
Fj ], 8,,
7" " -
I
I
0.8
(volts)-1
I
I
1.0
I
t
1.2
I
48.
If it can be assumed that the ions are produced through the ionization of the 6 P 0, 1, 2 states,
and f an estimate
can be made of the
density of these states, an estimate can then be made of the crosssection for ionization of these states.
This problem will be discussed
in section D of chapter IV. First, however, it is necessary to demonstrate
that.the actual ionization is much larger than can be accounted for by direct
ionization of atoms in the ground state.
This will be done in the next
section.
C. Calculation of the direct ionization component including the effect of
the electron drift velocity
In order to compare the direct ionization with the total ionization,
the value ofVip
data for the
of'~.[/p.. was calculated from Nottingham's(42)
Nottingham's
L
U
A
probability of direct ionization of mercury atoms by fast electrons.
M-B energy distribution for the electrons was assumed.
The ionization
frequency Vi, (neglecting the atom velocity compared to the electron
velocity) is given by
(L'v e
y=<H(V
V4 .,;
(IV-28)
< 11r)~~ ~
The term VT is the voltage equivalent of the electron temperature,
is the energy of the electron in electron volts,
V
Vi is the ionization
potential of 10.43 volts for mercury, and v_> is the magnitude of
electron velocity averaged over the distribution function and is given
by
v_) = (8q/wm_) 1 / 2 (kT_/q) 1 /2 z
6.693 x 105 (kT /q)
/2
meters/
second. The term Pi(V) is Nottingham's probability of ionization (for
electrons of energy V) measured in ions per electron per meter path
i
j
49.
at 00 C and 1 mm Hg pressure
(for po equal to 1 mm Hg).
The probability
of ionization is related to the cross-section for ionization Qi' by:
ngQi(V), where the ratio ng/Po corresponds to a density of
PoPi (V)
3.536 x 1016 molecules per cm3 for a reduced pressure
po, of 1 mm Hg.
Numerical integration yields values of the integral Vi/po, which are
tabulated in Table III and are plotted in Fig. IV-1 as a function of q/kT
for comparison with the actual ionization frequency G/pon .
Comparison
of the experimental ionization frequency and the calculated ionization
frequency for direct ionization shows that the actual ionization frequency
is much larger than the direct ionization component.
It is logically necessary to demonstrate that the electron drift
velocity superimposed upon the M-B velocity distribution does not significantly increase the direct ionization component. The distribution
function for the electrons is taken to be a displaced M-B distribution of
the form:
Fr
-)'- ~(where v
is the drift velocity).
r-
(IV-29)
The direct ionization can be calculated
by partial integration to be
V,~
-
_
>
vI-,,3-
-I-
5 tr
'f;
2,- ' Z"
A
7
V(V)e vdv
(IV-30)
50.
The drift energy
VO of the electrons
is defined by: qV
-
m v 2/2.
This integral was evaluated by numerical integration (again using
Nottingha's
data) for the lowest pressure data where the drift energy
V, is the largest fraction (4 per cent) of kT_/q and where the effect
of the drift energy on the direct ionization is the most important.
It
was found that even in this case the drift velocity has a small effect
The value
since it increases the direct ionization by only 16 per cent.
of the drift velocity used in this calculation was obtained from the results
of chapter V.
It is also necessary to point out that the actual probe curves
indicate a depletion of high energy electrons above about 3.39 kT_/q
and therefore
the assumption of a M-B distribution
in the calculation
of the direct ionization component is notJ quite accurate.
This depletion
strengthens the argument that the direct ionization is much smaller than
the observed ionization since the calculated ionization for the M-B distribution case is an upper bound for the depleted distribution.
D. Determination of the important ionization processes in the plasma
It can be seen from Fig IV-1 that the actual ion generation in
this experiment is much larger (up to a factor of 45.6 at the highest
pressure) than the component that can be ascribed to direct ionization.
This leads to the conclusion that the ions are produced predominantly
through the ionization of excited and metastable atoms.
It is first
necessary to demonstrate that the rate of production of 63 P0 1,2 states
is large enough to supply the amount required by the observedionization.
With the aid of the cross-sections
for the production of 63P 0 1, 2
states in mercury as given by Kenty
, it is possible to calculate the
generation of such states by electrons with a M-B distribution:
e PrSVt
V
~6:(1;/>.+~
=_(y>r)g
{e)
(IV-31)
~~~..
where the threshold energies V,
for the 6 P0, 12 States are 4.67, 4. 89,
and 5.47 volts respectively. The results of these numerical integrations
are given in Fig. IV-1 which shows that the production of these states is
larger than the production of ions by about a factor of 10 and therefore can
supply enough excited atoms to be ionized.
The cross-section
for ionization
of these states has been given by Klarfeld (44 ) as about an order of magnitude (actually a factor of 30 was given by Klarfeld(44) in his original
article) larger than the cross-section for ionization of the ground state.
Waymouth and Bitter
(4 5 )
report a factor of 3.3 for the ratio of the ionization
cross-section for excited states to the cross-section for ionization of
the ground state of mercury.
It is possible to calculate from the data
obtained in this experiment (with the aid of a few reasonable assumptions),
a lower bound for the effective cross-section
for ionization of the 63 P states.
This will be done later in this section.
It will be demonstrated that although the predominant ionization in
the plasma is through the ionization of excited states (cumulative ionization),
this cumulative ionization is a linear function of the electron density and
not a quadratic function.
The loss of metastable states by diffusion is not the important
factor in the determination of the density of these states.
L
This can be
shown with the aid of the diffusion coefficient for 63P2 metastable states
in mercury which is given by Biondi (4 6 ) as: D ng
cent) cm / sec x cm3
X
1.5 x 1018 (+ 10 per
The maximum density of 63P 2 states is given by
BoltzmannS theorem:
(,,.
6C)
__
(3
_
)
_-
_7
'So)
(r
(
,^
f 'So)
/
(IV-32)
where the statistical weight ratio g6 3 P 2 )/g(61 S0 ), is equal to 5. This
maximum value for the 63 P2 metastable density corresponds to the
situation where the electrons and excited states are in equilibrium.
According to some measurements made by Kenty (4 3 ) on a lower current
mercury arc, the actual population of 6 P 2 in his experiment is about
21 per cent of the maximum density given by the Boltzmann theorem.
If the maximum value of the 6P
2
density is used together with the
metastable diffusion coefficient given by Biondi, the resulting maximum
loss by diffusion (assuming a Bessel function for the metastable radial
density distribution) is given by
Aj7me
D,'PVr&Si*A
L.&s
ir
P
--
(IV-33)
If the actual metastable density distribution is more uniform than the
Bessel function distribution, the diffusion loss will be even smaller.
When the maximum diffusion loss of 63 P2 is compared to the generation
of 63P2 for set "D" of the data (corresponding
to kT /q = 0. 920 volts),
it is found that the diffusion rate is less than the generation rate by a
53.
factor of 3.4.
Since the actual metastable density'is lower than the maximum
density calculated from Boltzmann's theorem, the loss of metastables by
diffusion becomes even less significant.
The loss of metastables (at the higher pressures at least) is thus determined by collisions with electrons.
Since the generation of excited states is
proportional to the electron density, and the electron quenching rate is also
proportional to n,
the metastable density (for low Dm ) should be independent
of the electron density. An experiment by Kenty (4 3 ) has verified this statement.
He measured
the population of 6 3 P 2 and 63Po metastable
states by the
absorption of.4047A and 5461A radiation as a function of arc current (and
therefore as a function of n) and found that the absorption was essentially
constant for arc currents ranging from about 0. 1 to 0.45 amps. Thus for
large electron densities and small Dm , the populations of metastables will
be independent of n_. If the ionization is predominantly produced through
the ionization of metastables,
the ion generation
function of the electron density n,
G should be a linear
provided that the electron energy dis-
tribution function is relatively independent of n_. As the pressure decreases
the diffusion coefficient Dm for metastables (which is inversely proportional
to po) increases and the diffusion loss of metastables becomes more important.
The results of this experiment show that
electron density.
/pon_
is independent of the
For three of the nine sets of data, the arc current was
changed by about 20 per cent with respect to the remaining 6 sets.
If the
cumulative ionization were quadratic as it is sometimes assumed, the
value of /pn
_
would be a linear function of
cent for these three points.
the expectations.
_ff.
and would vary by 20 per
Such a variation is not observed and confirms
54.
As a result of the previous considerations,
it is concluded that
the ionization is proportional to the electron density, and therefore the
cumulative ionization is linear ionization and not quadratic ionization.
Any further investigation of the dependence of the ion generation upon
electron density should also consider in some detail the electron energy
distribution function, particularly for the high energy electrons.
Unfortunately,
at the present time, there is very little information available for the distribution functions.
It is possible to obtain limits for the effective cross-section for
ionization of the 63P states if it is assumed that most of the ions are
produced through ionization oi the 63P states, and if it is assumed that
the 6 P0 1,2 states have the same effective cross-section Qo(63P
ion).
The method of calculation will be outlined below. Let 63 Pj (where j
represent any one of the 63 P 0 1,2 states.
O0,1,2)
The density of the 63pj state
is given by:
/&pIV
P)
/7-
(IV-34)
where the "saturation factor" sj, is the ratio of the 63 Pj density to the
maximum density given by the Boltzmann theorem.
threshold energy for the j state.
The term Vj is the
The multiplicity term g(61S0 ) is equal
to 1, while the g(63pj) term is given by (2j + 1). The generation of ions
from the 6 Pj state is now given by
G6Ct A
= ARC tJ). fig/-)
a,Cal,#)f
tp.
J)e
(IV-3/5
55.
The threshold energy for ionization of the 6 Pj state is (10.43 - Vj
For the higher pressures
)
volts.
where VT is low, n6 1 SO) X ng, so that
do r6 °) ·-7 A_ -> aC-t
°)ie
Vsjo
(-i-
r-
/,V
-1/1
V
V7-~~~~~~·
(IV-36)
At the higher pressures where the direct ionization is a small part of the
total ionization, it may be assumed that
'4t-Z4
j XI ,L
,r-("O'
·-
(IV-37)
This observed ionization G, is thus related to the effective cross-section
by
i v 7 /11.61-r_>a. (/ t.,
e
-
,.'.V3
&~rdI
J(~.&[
7 4-
(IV-38)
If the values of sj were known, then the value of Qo (63 P-*ion) could be
calculated from the known values of V j, and from the experimental values
of G, ng, n_,v
his arc, s,
>, and VT. Kentvtys investigation (43 )indicates that for
sl, s 2 , are equal to 0.21, 0. 047, 0. 21 respectively.
Since the
56.
currents and electron densities in the present arc are much larger than
in Kenty's arc, it is expected that the values of s in this experiment
are closer to unity and that Kenty's values are a lower limit.
When the
maximum values of s (unity) are assumed, and the experimental
values of G, ng, n, cv),
and VT are introduced,
a lower bound
(minimum effective cross-section) is calculated for Q (63P-ion).
The experimental values for the highest pressure (set E) give the greatest
lower bound, and the corresponding minimum effective cross-section
for
3
-16
2
ionization of the 63P states is found to be 8.1 x 10- 16 cm . For the
same electron temperature, the effective cross-section for ionization
of the ground state is calculated from Nottingham's (42) data to be
0.90 x 10-16 cm 2 . Thus the effective cross-section
for ionization
of a 63 P. state is a minimum of 9.0 times larger than the effective crosssection for ionization of the ground state.
In addition, if the values of
sj given by Kenty for his arc are assumed to be approximately valid
for this arc, the value of Qo (63P-ion) calculated for the same set of
date (highest pressure) is 53 x 10- 16 cm or about 58 times as large as
the effective ionization cross-section for the ground state.
Since the values of s will probably be between unity and the
J
values given by Kenty, the effective ionization cross-section for the 63P
state will be between 9.0 and 58 times larger than the effective crosssection for ionization of the ground state, provided that the assumptions
made in calculating these values are valid. When more definite values
for sj (or for the densities of the 63 Pj states) are made available for
a similar Hg arc, it will be possible to specify the cross-section
Qo (63p" ion) more closely.
L~~~
V.
lectron Mobility, Anbipolar Diffusion Coefficient, Ion
Mobility Coefficient. Ion Diffusion Coefficient. and CrossSection for Hg Ions in Their-Parent Gas
A. Electron mobility
The mobility of electrons in the plasma can be obtained (from
i
the results of the previous chapters) in terms of the axial current Iz,
the plasma radius rp, the axial potential gradient Ez, and the electron
density averaged over a cross-section
drift current density J,
of the plasma n . The axial
is given by
rr, ="'fn
(V-l)
where it is assumed that the axial potential gradient and the axial drift
velocity (predominantly that of the electrons) are independent of the
radial position in the plasma.
Table I
contains values of
W9z
calculated
froIr the axial current and the plasma radius. The average electron
density n_, was calculated in chapter IM in conjunction with the deterrnination of the ionization frequency, and is available from Table III.
The resulting values of the axial drift velocity vz are listed in Table IV
and are plotted in Fig. V-1 as a function of Ez/p
is the sum of the drift velocity of the electrons
almost equal to the electron drift velocity.
small compared to the electron mobility.
of only about 0.2 per cent.
PO
/
'K
-.
This axial velocity
and of the ions and is
The ion mobility is very
This results in a correction
The values of Po
F
o.
_, defined by
(V-2)
:f 'r?
,a
II
·I
:I
:i
i
'i
·i
Ii
:i
i
;I
Ii
:i
i i Ii' ;r
ii
;i ii
i i·
o
0o
:'I
i:
II II
i I i:
iQiii:
·
;·
I :!iI;
i
ij
E
1 ·r:·, i
o
i i/ j
iI
;i
· i!
q.4
Ii
c0
-o
i
' '
0
H
106
04
'
i
10
iI
I
I
I
I
I
I
I
I
I
I
I
100
10
'
-
-
"
.rt
i_I
'
I'
'
19r.
- -, A
_
';
."
z,
{a
e~
".
"
,
-.
I
I
I
i
III
1000
x mmnHg)
Ez/po (volts/cm
n-,,i~
""I
I
1 lit",>
tc.
v
w4-',
i{ar
58.
are calculated from v and E z and are listed in Table IV.
At the same time it is possible to obtain the ratio of the electron
drift velocity to the average random velocity vz_/v_>,
along with the
ratio qV /kT _, where kT_/q is a measure of the random energy, and
V
sf
_
z
2
Vz is the axial drift energy defined by: 2qVz = m vZ_ . In this experi-
ment, the drift energy is at most 4 per cent of kT_/q, and can be used
to show that the effect of the drift energy on the direct ionization is not
significant.
It is possible to calculate the effective mean free path for slow
electrons in the mercury plasma from the relation between the electron
imobility and the mean free path. Allis and Brown (47) give an accurate
expression for the electron mobility in terms of a mean free path for
electrons which is a general function of. the electron velocity.
Their
expression was obtained by expanding the electron velocity distribution
function into spherical harmonics in velocity space and then substituting
it in the Boltzmann transport equation. If the effect of the drift velocity
on the distribution function is small enough so that only the first two
terms in the expansion need be retained, the electron mobility can be
expressed as
2
where 'm
Aa_
(
< i)(V-3)
-21_
is the momentum transfer mean free path for electrons.
When it is assumed that the electrons have a M-B velocity distribution,
this equation can be reduced by partial integration to give
Jc.. ,<
_
(V-4)
59.
The momentum transfer mean free path )m, is related to the
Qm' by: ,mngQm z 1, where ng is
momentum transfer cross-section
the gas density.
to the differential
The momentum transfer cross-section
scattering
cross-section
(a
1
t?ffen
=( l
_ ceS e)
Qm' is related
q(G), by:
(V-5)
sac /0
Electron scattering data is sometimes given in terms of the collision
cross-section Qc' which is related to the differential scattering crosssection by:
-' >~~rr/i~~
S(V-6)
a~C
g~ s~
t~
The probability of collision Pc is measured in collisions per centimeter
at 1mm Hg pressure and 0°C, and is related to the collision crosssection and the collision mean free path ic' by: PoPc
ngQc = (>c)
In order to permit the conversion of Qc to Qm the values of Qm/Qc
have been obtained by numerical integration of the angular scattering data
given by Arnot (4 8 ) for electrons in mercury, and are tabulated for the
following values of electron energy:
Electron energy
Qm/Q
2 volts
0.783
4
0.817
6
0.823
7
0.802
8
0.834
average
0.812 + 1.6 per cent rms
Unfortunately there is no angular scattering data for electrons below
2 volts.
According to Massey and Burhop
(49)
, however, the angular
distribution for elastically scattered slow electrons is uniform thus
implying that Qm/Qc approaches unity as the electron energy is reduced.
The effective mean free path can be defined as that constant
mean free path which when used to replace the velocity dependent mean
free path will give the same mobility.
The effective cross-section
can
be defined in the same way. In this experiment, it was found that the
effective mean free path m (eff.), is relatively independent of kT /q.
(where Am now corresponds
For the case of a constant-m,
to the
effective mean free path for momentum transfer) Eq. V-3 reduces to
(V-7)
It can be shown that for a Maxwell-Boltzmann velocity distribution:
p(1
r 2<,.=03fh(V-8)
(S)$
where r(k) is the complete gamma function. Thus:
' i.
(V-9)
so that
-/C"
fi- 3
3
.-.
4-
-
>(V-o0)
The Langevin (5 0 ) expression for
for the constant mean free path case.
>
the electron mobility
C-
c/
"'
(V-
1 )
is lower by a factor of 1.132.
Equation V-10 implies that if the effective mean free path is
independent of kT_/q, the quantity po lae (kT /q)
/
should be constant.
This was found to be true since the average value of poP_ (kT /q)l/2 is
1.49 x 105 (cm2 / volt x sec) (mm Hg) (volt)1/2 with a small rms deviation
from the mean of 5.6 per cent.
The effective momentum transfer cross-section for slow electrons
in the mercury plasma (about 0. 1 per cent ionized) was calculated and is
plotted in Fig. V-2 as a function of kT /q, where it is seen that the crosssection is approximately constant.
The average value of Qm (eff.) is
42 x 10 16 cm for electron temperatures ranging from 0.829 to 1.90
The values of Pc given by Brode (5 1) for slow electrons
electron volts.
in mercury indicate that Qc decreases as the electron energy increases,
and thus one would expect that Qm (eff. ) should decrease as kT /q increases.
From Brode's
(5 1 '
52) values of Pc, as a function of electron energy, it
was calculated by numerical integration that the effective momentum
transfer cross-section
Qm (eff.) is 58 x 106
a M-B distribution with a characteristic
volts in mercury.
cm2 for electrons having
temperature of 1.00 electron
Elenbaas ( 53 ) has obtained cross-sections
41 x 10-16 cm2 for a high pressure
of 43 and
mercury arc by using the Langevin
equation and the energy transfer from electrons to atoms required to
02
0
.
r4
+
@
Em
0
w
40
0
c
X
00tOCxI
C
30 _
4)
0
20
_
10
_
B
*
4 amp
5 amp
+
6 amp
Slow electrons in
Mercury plasma
c)
a,
44
i
0
0
I
0.4
I
I
I
I
I
I
I
I
2.0
1.6
1.2
0.8
kT/q (volts)
c"C9
5- r-·- I-- ;I P" .
-i.
Cs__,
,'L
__
_,
,.
.
or
4I
. e)7p
I-
.A
"
l:
_
-
iidI.1
'-
i1,ea'
62.
1
supply the heat and conduction loss.
The electron temperature for his
Adler and Margenau (5 4
arc was about 0.43 electron volts.
(from microwave Measurements),
equal to 9.5 x 10
croSs-sectLon
cm at
o01 u x iu
from 0.69 ev to 1.2 ev.
that
cm
2
ior electron
The agreement
have found
A?(effective) is constant and is
mm Hg pressure.
-16
)
This corresponds
temperatures
to a
in the range
of these cross-sections
is good,
particularly in view of the different values of kT /q and the different
methods.
A survpv
of the 1iterftnrii
wnc mncrip for informtninn
hnl]fthp
cross-section for monoenergetic slow electrons in merucry. It was
found that Brode (52 ) published results (prior to his review article) that
indicated an increase in Qc of only about 4 per cent as the electron energy
was reducer from 1 volt to about 0.5 volts, thus indicating that the crosssection for very slow electrons in mercury is approximately constant
and may even decrease for very slow electrons.
B. Ambipolar diffusion coefficient and ambipolar mobility coefficient
The abipolar
Ldiffusion coefficient Da can be calculated from
the available experimental data subject, of course, to the assumption
that the situation is describable in ambipolar diffusion terms.
It was
shown in chapter IV that the ambipolar diffusion coefficient is given by
hre
where
Pr
ct
seti
d
(V-12)
is the ion particle current density to the wall probe when
it is at floating potential.
The radial electron (and ion) density gradient
dn /dr can be calculated from the assumed electron density distribution;
but, since a derivative is involved, any uncertainty in the form of the
density distribution function results in a larger uncertainty in the gradient.
A good representation for the density distribution (n_/n_o) is the Bessel
function Jo (2.405 r/Ro) which was derived and discussed in chapter IV.
With this representation,
the density gradient
-~>)~2
(< /"J; /0
*-/ ~(V-1
was calculated at the plasma edge (r = rp) in terms of the central electron
density n_,0 and the wall density nw.
The identical values for Da may
be obtained from the relation
D), = 1,~, (' .
~r
(V-14)
which was derived in the section on ambipolar diffusion. It is not surprising
that these two expressions give the same result since they were both obtained
from the same ambipolar diffusion assumptions, involve the same Bessel
function solution and the same experimental data.
Since the electron mobility is much larger than the ion mobility,
the ambipolar diffusion coefficient may be written as
Da
Aea
(V-15)
where the Einstein relation connecting the electron diffusion coefficient
and the electron mobility coefficient has been used. An amb ipolar mobility
coefficient Pa will be defined here by:
·___
_____
64.
(V-16)
Only for the case where the mobility flow of ions to the wall is much larger
than the diffusion flow of ions to the wall (so that D+/ +
kT_/q) will
the expression for the arnbipolar diffusion coefficient reduce to the
approximation
DP..b Sfr-C s
(V-17)
The values of Pa have been calculated and are shown in Fig. V-3 as a
function of Er/Po, where Er is the radial potential gradient at the plasma
edge.
The method for the determination of Er will be described in the
next section.
With the help of the definition in Eq. V-16, values of Da
can be obtained from kTiq
and Pa'
There are very little data available for the purpose of comparison
with the results of this experiment; this makes the present results even
more important.
Biondi( 5 5) gives a value of D ng
3.6 x 1017 (cm2/sec)
(atoms/cm3) for Hg ions and thermal electrons in mercury vapor at 350 K,
which was obtained through afterglow measurements on a decaying plasma.
This value is equivalent to qDap/kT
= 3.4 x 102 (cm /volt x sec) (mm Hg).
In the present experiment, the corresponding value of qDapo/kT- varied
from 1.58 x 10
be ascribed
to 7.52 x 102 for an active plasma.
The variation can
to the change of ion mobility and diffusion as a result of
different arc conditions.
In the low pressure arc under consideration,
the mobility and
diffusion coefficients are functions of the electric field because the energy
.
?
8
H
I
0
0O
ob
o~~
cq
INI
H
o
r4.3
.<
,i
bO
o
r
O
a_4
0
0
H
P
O
,r
Ut
O
'
4
r
i.
s?
p.*
$o
*
I
oO
o
I
~
L...
~~
~
I
I
I
I
(re
I
I
x
1°&I/
I
0
+
0
I
H Ul) s6/Od
0O
65.
gained by an ion in a mean free path is large compared to the random
energy thus making the mean free time of flight of the ion dependent
upon the field.
At the edge of the plasma,
the radial electric field is
much larger than the axial electric field so that the resultant electric
field may be taken in the radial direction.
At the low pressures
in this
arc, the diffusion flow of ions is not small enough to be neglected.
in this chapter,
Later
the radial flow of ions will be decomposed into the
mobility and diffusion components with the help of a special theory for
ion mobility and diffusion in a strong electric field.
C. Ion velocity as a function of Er/Po
It is possible to calculate the radial potential gradient Er from
the radial density distribution of electrons and from the Boltzmann
relation between the electron density and the plasma potential as follows:
(V-18)
The directed ion velocity in the radial direction v (at the plasma edge)
can be obtained from the ion density (n w x n+w) at the plasma-sheath
interface and from the ion particle current density to the wall probe when
it is at floating potential.
Figure V-4 shows the ion velocity v, as a
function of Er/Po, evaluated at the plasma edge.
There is very little other data available for the velocity of
mercury ions in mercury.
Kingdon and Lawton
(5 6 )
in an abstract
give
the results of oscilloscopic transit time measurements for E/p ranging
from 20 to 6000 (volts/cm x mm Hg),
They state that the ion velocity
I
I
I
o
u0
Ip
9-_4
.!.
H
r
w:
:
0
Art
k
O
I··
0
r
-4~
!··-
O~~~~~~~~~~.-
I
0
I-,
I
I
I
I
-
I
I
-
(PUOoSQ/Mo) A4OOTA
0l
I
II
0
UoI
'-4
'I]
66.
was proportional
to (E/p) / 1
at an E/p of 1000 volts/cm
and they report an ion velocity of 10 cm/sec
x mm Hg. This corresponds
3.2 x 103 (cm/sec)/(volt/cm
/2
x mm Hg)1/2
to v./(E/p)l
1
22
The accuracy involved was
not specified nor was it demonstrated that the diffusion flow of ions across
the drift space was negligible compared to the mobility flow. What is
surprising about this result is the implication that the cross-section of
mercury ions in mercury is independent of the ion energy, thus resulting
in a constant mean free path.
For this experiment the relation between the ion velocity v+, and
Er/Po can be given by several representations
v+/
(Er/po)
) /3
v+/ Er/Po
v / E/p
of varying accuracy:
9.71 x 103 (cm/sec)(volt/cm x mm Hg)1/3 + 2.7 per cent rms
)l/2 x 4.17 x 103 (cm/sec)(volt/cm
x mm Hg)1
The difference between a variation of (Er/po) /
is a factor of (Er/Po)/
6
/2
+ 11 per cent rms
and one of (Er/po)/3
which is difficult to detect.
In order to separate the mobility and diffusion components of the
ion flow so as to experimentally determine these coefficients, it is necessary
to know the theoretical
dependence of the ion mobility and .diffusion coef-
ficients upon the various arc parameters.
Since in this experiment the
energy gained by the ions in a mean free path is large compared to the
random energy of the gas atoms, the mobility and diffusion coefficients
will be a function of the electric field.
There are no simple and satis-
factory relations available for the diffusion and mobility of ions in a
strong electric field. In the next section a simple theory will be
developed for the ion mobility coefficient and the longitudinal diffusion
coefficient for the case of a strong electric field.
67.
D. Simple theory of ion mobility and diffusion coefficient for ions in a
strong uniform electric field
In order to calculate the diffusion and mobility coefficients from
the experimental data, it is necessary to make several assumptions to
obtain a mathematically tractable model. It is assumed that the energy
obtained by an ion in a free path is large, compared to the random energy
of the ions, so that the motion of the ions between collisions is essentially
determined by the field. If the scattering of the ions is by charge transfer processes,
the new ion will have the kinetic energy of the gas atoms
and therefore may be considered as starting at rest.
of the ion-gas interaction
The cross-section
is assumed to be independent of the ion velocity.
Near the plasma edge the radial electric field Er is much larger than
the axial electric field Ez and, therefore, the resulting electric field
is taken as equal to the radial electric field. Since only the radial
diffusion flow contributes to the radial ion current, it is only necessary
to calculate the longitudinal diffusion coefficient for diffusion flow
parallel. to the strong electric field.
With the help of Professor W. P. Allis, it was possible to
derive a simple yet reasonably accurate representation for the mobility
and diffusion coefficients in a strong electric field.
Consider first the case of ions moving in a strong uniform
electric field, and assume for the present that there is no density
gradient of ions so that the diffusion flow may be neglected.
It is
desired to find the time average of the ion velocity for a single representative free flight. The time average velocity v, is given by
(V-19)
where
is the time of flight. Assume the E field is very large so that
the motion of the ion can be assumed to be parallel to the E field, and
let "s" be the distance in the x direction (paralled to E) traversed in the
Then:
time of flight.
__
Ad = r>0vxiwX srtrve
(V-Z0)
It is desired to obtain v as a function of s and E. Assume that the ion
has a terminal velocity (in the x direction) of vf at the end of time 1",
and that after the collision there is a persistence of velocity a, so that
it starts the next free flight with velocity a vf. Assume also that it
started the free flight under consideration with the same velocity a vf.
It is seen that the final velocity is related to the time of flight by:
[
..- ''(V-21)
Ofc,-~
The acceleration
,
)
"a" of the ion is given by
(V-22)
CL =
where q and m + are respectively
the charge and mass of the ion.
The
time of flight 1' is related to the distance of flight s by
t
fS ,("
da
- -x t~)
-r'.
7"
~-.
COe
(V-23
69.
Substitute vf from Eq. V-21 in Eq. V-23 and solve for 't to get
'li-= T~A
(V-24)
When Tis substituted in V-20, the time average velocity is found to be
(=4</}
(+'S
(V-25)
It isnecessary to average over the mean free path distribution.
In
general,
~
-
'
(SA-/>fi~6-/r
I/
Xe
W :
A A,IC
-
(V-26)
so that
(V-Z7)
As a result, the average velocity is given by
,; t (g)1A te/%
(V-28 )
for ion motion in a strong uniform electric field.
It is assumed that the motion of the Hg ions in the plasma is
limited by charge transfer collisions where a fast ion exchanges identity
with a gas atom. Since the velocity of the gas atom is very small compared
70.
to that of the ion (and is also randomly. directed), there is no persistence
of velocity so that a
=
0, and
Sena(57) assumed charge transfer limitedmobiity and constant A, and
obtained the same result as Eq. V-29.
There are several other theories for the motion of ions in a
strong electric field and they will be given for the purpose of comparison
with the result (Eq. V-28) derived here. An early expression calculated
by Tonks and Langmuir was
hat , s(
They assumed a constant
a)
(V-30)
and a persistence
of velocity of a
X
1/2 for
the fast ions. Wannier(58 in a more recent and much more detailed calculation which includes a constant
A
(hard sphere),nearly isotropic
scattering and persistence of velocity, obtains
/Crsh
(V-3
1)
It will.be shown that the simple theory developed here gives a value for
v which is in good agreement with that of Wannier. According to Jeans (59)
for the case of elastic scattering of a fast particle by a stationary hard
sphere of equal mass, the persistence of velocity
is 1/2, When a
1/2
is substituted into Eq. V-28, there results
4
1(ic)'
(a?)' =/.o r (a A)
(/ " ~~ )=.o
(v-32)
71.
which is very close to the value obtained by Wannier through a much more
detailed calculation.
It is assumed that the Hg ions are scattered by charge transfer
collisions so that in this case there is no persistence of velocity.
Equation
V-29 will therefore be used for the average ion velocity in a uniform
electric field. In the plasma, ions will also flow to the wall because of
the radial ion density gradient so that it is necessary to find a simple
expression for the diffusion coefficient in a strong uniform field.
Consider the particle current density for ions passing through a mathematical plane normal to the potential gradient E. The ion particle current
f"7~.(,~e
x (V
density (ions per unit second per unit area) passing through the plane. can
be given by
?,.j.
Ado
The term n
.~.L.
-
(V-33)
is the density of ions at the plane, dn/dx is the density
gradient of ions and exp (-x/ ) is the fraction of ions that will travel the
distance "x" to the plane without a collision.
expression for
This results in the following
+
(V-34)
The ion mobility coefficient
/S-
.k
.l+
is
=
1)
4
(V-35)
72.
while the longitudinal diffusion coefficient (for diffusion of ions in a
direction parallel
to the E field) is given by
= g a(V-36)
-(V,
From these two expressions,
one can obtain a form similar to the
Einstein relation
-F~(V-37)
'--"
=>
With the aid of the expressions
for the ion mobility and diffusion
coefficients, it is possible to obtain values of the effective
in the plasma.
Equations V-34 and V-29 yield
/
17tp/
where dn /dr
for the ions
/;
dn+/dr in the plasma was replaced by - nqE/kT,
the equation is evaluated at the wall.
(V-38)
and
The only unknown, qEjL/kT_, can
be solved for by convergent approximation.
This quantity, qE/kT,
represents the relative importance of the diffusion flow of ions comrnpared to the mobility flow. It ranged from 0. 42 at the highest pressure
to 0.65 at the lowest pressure.
If the pressure were higher, the
diffusion flow would be smaller.
The values of Er (calculated in the previous sectinn)plus the
values of kT /q can be used to determine the values of > from the
calculated values of qE-A/kT_. The ion mobility coefficient was
r73.
calculated and is plotted in Fig. V-5 as a function of Er/po without a
correction (at the present time) for the non-uniformity of the electric
field.
This correction will be calculated in the next section.
The longi-
tudinal diffusion coefficient is plotted in Fig. V-6. From the values of X
and the gas density, the cross-sections
Q are calculated (fromlngQ
=
and are shown in Fig. V-7 as a function of Er/P
0
1)
and in Fig. V-8 as a
function of E ) .
Although the values of
for the ions ranged from 0.26 cm to
1.3 cm and were not very small compared to the radius (2.75 cm) of
This is so
the plasma, the concept of ion mobility can still be applied.
because the charge transfer limited mobility requires only one collision
rather than the many collisions needed for regular mobility flow.
In the range of ion energy about 1 electron volt, the crosssection increases with ion energy and is many times the cross-section
-16
of 8.29 x 1016
cm 2 for Hg-Hg interactions which was obtained from
viscosity
(60)
and x-ray
(61 )
measurements.
Very little data are
available for scattering of low energy Hg ions in the parent gas. However,
data available for other ions in the parent gas indicate that as the ion
energy decreases,
the cross-section
increases up to a point and then
decreases as the ion energy continues to decrease
ment the cross-section
(62)
. In this experi-
varied from 39 to 116 x 10-16 cm
2
for the case
where the correction for the nonuniform electric field was not yet made.
Massey and Burhop (63) give an approximate theoretical maximum value
of 5 x 10 14 cm
for the charge transfer
cross-section.
Sena(6 4 57)
calculated a value of Q X 5.3 x 10- 1 5 cm 2 for Hg ions of 1.6 ev energy
(56)
in mercury vapor from the data given by Kingdon and Lawton
.
Palyukh
1000
0
e
i
0
o
Hin0
*
+
o
A
....
uncorrected for non-uniform rield
Corrected for uniform field
-
Mobility of Mercury ions in Mercury plasma
.U
t
I
I
A
I
I
I
I
I
I
(volts/cm x mm Hg)
Er/Po
r
5r ·
' ` ;:5·t i)a-7
c~~2.~
I
I
II I
1000
100
10
I
.:B,
U.L,
74.
and Sena(65) report values of the charge transfer cross-section for high
energy Hg ions in mercury vapor.
Their results give a charge transfer
(at the extremes) of 1. I x 10-14 cm 2 for 37 volt ions and 0.71 x 10 -14 cm 2
for ions with an energy of about 1, 000 volts.
Varney (66) gives values of
Q from drift velocity measurements of ions in the parent gas and reports
values of 54, 65, 134, 157 and 192 x 106
cm2 for He, Ne, A, Kr and
Xe respectively.
In view of the agreement of the present results
with the limited
data given by others for the cross-section of mercury ions in mercury,
it can be seen that the results of this experiment are of value, partio·li~clnfEl:arr
ctnon4tw
L,;ULLJ.
DLLLU::
IC;y
h
.L:IC
LJU.-t
A-4r
zi;_l.4
-
6UL: LL;CU.
T- riS;+;rr
LI
dLUULbLUlI,
ChoIfrWt;rsr
LJ.
LLUI.
LIJ.
easily obtained from probe measurements in a dc discharge.
dLLUIl
Wa;.
This
method may be applied to other gases to obtain information about the ion
mobility and diffusion coefficients and the cross-section for low energy
ions.
The ions are moving in a slightly nonuniform electric field so
that it is necessary to refine the theory of ion mobility and diffusion to
include this case.
The next section will consider this correction.
E. First order theory for ion mobility and diffusion in a nonuniform
strong electrid field
If the ions are moving in a strong nonuniform electric field
which has an appreciable change in a mean free path, it is n'ecessary
to take this into account in the calculation of the cross-section
mobility and diffusion coefficients.
in the first order form as
and the
The electric field will be expressed
>11
00
,-4
LO
O
:4
0
0
I
4
02
o.
o
C'.
Zgi
0
.1-I
m
P
frb
0
C
r
0
C::
C"~
0
o
-
bO
la
O
100
0:4
0
Ctl
I0··
t~b
k C
C)
a
0
0 r-i
0
0
o
oO
4P
r·
0i
4+
af
-ri
,x
0U)
it
':o
CrC
I
I
I
I
I
I
(oes/mo
I
I
a
x SgH m)
I
od
I
00
-i
0
-
'75.
I
is the value of E at the end of the free path. The time average
where E
ion velocity v is equal to s/1r.
of s, E
(V-39)
-(-,)*
_: E
It is desired to express
W'as a function
Since the ions are assumed to start from rest, the
and dE/dx.
1V.
-_
time of flight r is equal to
Jo
6-
OlX
(ksy ,
(V-40)
'
where the ion has passed through the potential difference (V - Vx).
is given by
From the first order expansion for the E field,
---
-----
.
I
',- Ya,
1=
Y4)iiece
For4r
i
;Y)'
(V-41)
When this integral is evaluated in series form including terms up to
the second order, it is found that the time average velocity is given by
e zo
L
/CTT~~~~L;/~~Si
1.2 Aq C/
//IV,
Pe
., Ve
(V-42)
After the terms containing "s " are averaged over the distribution in "s"
(according to Eq. V-26) the following expression results for the average
ion velocity in a nonuniform strong electric field:
.LViA
lUUL
I
Uncorrected for non-uniform field
+
0
Corrected for non-uniform field
0
H
0
02
04
0 10
C)
I
V2
".
N
Mercury ions in Mercury plasma
I
.
I
m
I
I
I
I
I
·
I
I I I
I
I
I
,
I
I
I
I
I
I
I
Er/Po (volts/cm x mm Hg)
.' 0
41;
,.
,s,,,<
i
i;
<b
*r
A
-
z;;~~~~~'
.u
e
2'ir4,
I
I
I I
....
1000
100
10
I
>_w eF,,
, _
v
.
-
$v..1_SL
I _ ,,4e"+'j
.
.s.v
F-~~~~~~~~~~~~~ C~~~~~~~~~76.
j/zT' h;>)-i-/-*g
±
zo
hi
ivx](V-43)
This is seen to reduce to the results of the previous section when dE/dx = 0.
The values of (dE/dr)/E,
can be calculated from the assumed
Bessel function electron density distribution and the Boltzmann theorem
relating the potential and density distribution.
The following expression
is obtained:
.
-
'
(Pd
](V-44)
/*(AP)
--
the quantity Xp is defined by
'
-
ohno(
_
V-45)f)
(V-4 5 )
Equation V-38 in conjunction with Eq. V-43 gives
(V-46)
which can be solved for ~ through self-consistant
mations.
convergent approxi-
From these new values of -A (which have been corrected for
the nonuniform field), the cross-sections
are calculated and are plotted
1000
Mercury ions in Mercury plasma
0
--
0rI
14
0
*1
4-)
0
100
C
02
w)
*
+
I
10
I
I
I
I
I
I
I
I
1
I
!
I
I
I
I
I
I
Uncorrected for
non-uniform field
Corrected for
non-uniform field
I
·i
I
I
I
I
i
I
I
I I
-2I.,.
II -.-n
EN (volts)
- ,i4 *_. .
-
I
10
1.0
0.1
I
-
I-i
_ , _ .
f
(,:
,
O t
_. -'
1
r
77.
in Fig. V-7 as a function of E /po, and in Fig. V-8 as a function of E
The effective corrected cross-section
.
-16
cm 2
increased from 31 to 104 x 1016
as the ion energy increased from 0.44 to 1.37 electron volts.
The ion
mobility and diffusion coefficients are calculated from the corrected
and are plotted in Fig. V-5 and Fig. V-6 as a function of Er/Po,
It was
found that when the corrections for the diffusion flow and the nonuniform
electric
field were made, the ion velocity due to the field only can be
described by
',4
=i.
which is in good agreement with the value of Yl/2
3. 2 x 103implied by
the reported results of Kingdon and Lawton( 56 ) .
The accuracy of the results of this chapter is contingent on the
validity of the assumed Bessel density distribution for the electrons and
ions. Since the mobility and diffusion coefficients are dependent on the
field, the next order of refinement involves the determination of the
density distribution for the case of a variable ambipolar diffusion '
This involves the numerical solution of a set of second
coefficient.
order nonlinear differential equations, which cannot be made dimensionless
because of the axial field parameter E z . This problem will be left for
S..
~ ~~~ ~
A....
U. LUUI'- jJCUi-.
~
~
1
r
78.
VI. Electron Energy Distribution Function and Probe Measurements
A. Depletion of the high energy electrons
and comparison
with the
Druyvesteyn distribution
It has been established in this experiment and in others(1 5' 32)
that there is a definite depletion from a M-B distribution for the high
energy electrons in the low pressure mercury arc.
This depletion can
be seen in the typical experimental retarding potential plots given in
chapter III. The depletion cannot be completely ascribed to incorrect
extrapolation of the ion current component. The discrepancy between
the probe current expected for a M-B distribution and the measured
electron current is about equal to the ion current itself and would
require that the actual ion current be larger than the measured ion
On the other
current by about a factor of 2 to remove the depletion.
hand a depletion of high energy electrons is expected because of the
various electron energy loss mechanisms.
Loss of electron energy through elastic collisions will lead
the electron energy distribution towards a Druyvesteyn type distribution and will thus result in a depletion of high energy electrons.
In addition, the inelastic collsions (which have an energy threshold)
will result in a loss of high energy electrons alone.
There is also a
preferential loss of high energy electrons which have enough velocity
to overcome the retarding potential of the negative wall.
The Druyvesteyn distribution gives a reasonable description
of the probe curve expected for the elastic collisions although inclusion
of the variation of the electron-atom
cross-section with electron energy
79.
would give a better description.
In accordance
with the expression
for
the retarding potential function obtained in chapter I, the normalized probe
curve for the D-D has been plotted in dimensionless form in Fig. VI-1
along with a dimensionless curve for a M-B distribution of the same average
energy and the same saturation current.
These curves are plotted to
show a variation of over 4 orders of magnitude and therefore do not
show details for very low values of V/V. For small values of V/V,
the quantity J/J
sat. is slightly above that for the M-B distribution.
This difference has a maximum value of about 0. 02 which occurs at
V/V approximately equal to 0. 2, and is so small that it is about equal
to the plotting' accuracy and is not apparent in the figure.
As V/V
the probe current for the D-D falls below that of the M-B
increases,
distribution.
When V/VT is about 4 the probe curve for the D-D is
about 2 orders of magnitude below that for the M-B distribution.
A typical experimental curve for the central plane probe
(Tbath
54. 6°C, Iz
2
5.0 amp) has been plotted in dimensionless
form in the same figure for comparison, and it is seen that the low
energy electrons have a distribution which is M-B while the higher
energy electrons deviate towards the D-D in agreement with expectations.
It can be seen, from the values of the plasma potential Vp and
the floating potential Vf listed in Table I for each probe, that the floating
potential measured with respect to the plasma potential is proportional
to kT /q.
The quantity Vf - Vp is equal to 5.32 kT_/q with a rms
deviation of 3 per cent.
The depletion potential Vd measured with
respect to the plasma potential is also found to be proportional to
kT /q although not as closely.
The quantity Vd - Vp is equal to 3.39
.1~~~~~~~~~~~~~~
r
Plane probe(center)
bath =
bath
54
6
°C
I z = 5.0 amp.
Maxwell-Boltmann
Di tri.butio
I
Normalized
Probe Curve for Druyveateyn Distribution
-5
-4
-3
-2
-1
0
v/7
i
- " I;
i
'
lA
t%
)
i .
I !'-IL
;,
t
<
,
t-
Be
,X' ag
-
Uo
AXS
-
_
'_ I.,
kT /q with an average deviation of 18 per cent.
This depletion potential
Vd is defined here as the probe potential at which the retarding potential
plot for the high energy electrons begins to be depleted below the linear
part characterized by the M-B distribution. These last observations
may or may not be significant but they are included here for completeness.
It can be calculated, from the experimental values of ion
generation, that at the lower pressure about 9 per cent of the input energy
goes into the production of ions through inelastic collisions, while at the
highest pressure about 18 per cent of the input energy goes into the
Thus a considerable fraction of the electron energy
production of ions.
is lost through inelastic collisions.
On the other hand, according to
Klarfeld( 3 ), the elastic losses in a low pressure mercury arc are
less than 1 per cent of the input energy.
This implies that the inelastic
processes are more important than the elastic processes in the depletion
of the high energy electrons.
It would be of considerable interest if the distribution function
for the electrons were calculated taking into account the inelastic
collisions
L-
~
iClLLV
un~zrLUrvrl13
and the electron-electron
tLIC)LLt,
vL
V.
-L fJr
tD
.L
%_1U.L.rtj
interactions.
l
Since the degree of
--
a,.tA'U
v-r.
be considered in the calculation of the distribution function. Professor
Allis has made available (6 7 ) most of the analysis necessary for this
solution.
The distribution function will not be determined in this
research.
-~
_
_
81.
B. Theory of harmonic analysis of retarding potential plots and the
effect of superimposed ac
It was shown in Chapter I that the velocity distribution for
the electrons is related to the second derivative, (with respect to the
probe potential), of the electron current to a plane probe.
Numerical
or graphical differentiation is inaccurate and tedious, so that various
methods have been developed by others to increase the accuracy and
convenience of the measurements.
If a small monofrequency ac signal
"A sin at" is added to the retarding potential V, the resulting probe
current density, J, may be written as a Fourier series and may also
be expanded as a power series in "A sin
t". When the Fourier series
is equated to the power series and multiplied by sin nwt (or cos nut),
integration plus the orthogonality properties of the sine and cosine
functions will yield the Fourier coefficients.
The complete result is
< tffl
given here for the dc term, the first harmonic, and the second harmonic:
)(KS'
) )
bas!
r~'
OI
[et
=' U~j
lW
P(A
·I·I-~c
t+1
82.
where J(k) (V) is the k' th derivative of J(V). Note that the cos wt and
the sin Zwt terms do not appear.
Inspection of this expression indicates the various methods that
In the increase in the
may be used to determine the second derivative.
dc current due to the ac signal is measured and if the amplitude "A"'
is small enough to permit the higher order terms to be neglected, this
increase is proportional to the second derivative.
In the experiments of
(2 3),
and of Pringle and Farvis
Sloane' and MacGregor(68)
this increase
is measured by balancing out the basic probe current with a constant
current
source and then measuring
directly the change in the dc current
The disadvantage of this method is
when the ac signal is introduced.
that the drift of the arc itself results in small fluctuations in the probe
current that may be comparable to the measured increase.
The method of van Gorcum
(6 4 )
eliminates
the effect of the drift
by measuring only the ac component corresponding to the sind term to
obtain the first derivative. Subsequent differentiation of this result
numerically or graphically gives the second derivative.
The disadvantage
of this method is, of course, the need for additional differentiation.
Another interesting method involves the measurement of the
amplitude of the cos 2t component since the leading term contains the
second derivative directly.
It should be noted that this term is incorrectly
written as cos wt by Sloane and MacGregor (6 8
instead of the correct
form cos 2wt given here and originally by Landale (70
)
This error is
propagated in Loeb( 71 ) . The advantage of the second harmonic method
is that the second derivative may be measured directly with a tuned
amplifier without the extreme sensitivity to drift that is implicit in the
_
I
__
--
__
_
I·
_
--I
Il---L·
·- --Z --·-
--· _
CC-·-·---
- - -
- -
83.
incremental dc method. In addition, stray linear effects 'such as
capacitance do not affect the measurement.
On the other hand, the
disadvantage of this method is that the source of the original ac voltage
"A sin wt" must have very small harmonic components.
In view of the disadvantages and limitations of these methods,
a new method is proposed for future research.
A small ac signal of
frequency w2 is added to the probe voltage and the amplitude "A" of
this signal is amplitude modulated at a much lower frequency w1 by a
mechanical chopper switch.
The resulting ac probe current is passed
through a low pass filter to remove the high frequency components.
It is then measured
to give the amplitude of the increment
in the dc
current resulting from the small ac signal without the difficulties
introduced by the drift of the arc.
A synchronous mechanical or electronic
rectifier operating at a frequency wl plus an integrating circuit may be
used to reduce the effect of stray signals.
The above statements may be
applied to the measurement of other nonlinear elements.
In conjunction with the discussion
of the determination
of the
distribution function from the probe characteristics, there are several
points worth mentioning.
The determination of the second derivative of the electron
current from the second derivative of the probe current is valid for the
range where the contribution to the second derivative by the ion current is
negligible.
If the distribution function is required for the high energy
electrons, it is then necessary to use a special screened probe to separate
the electron and ion components (2 3 ). Harmonic analysis can then be
used upon the electron component to determine the distribution function.
84.
If the ac frequency is high enough so that the ions do not respond,
then
an ordinary probe may be used without the need for separating the ion
current.
It can be shown that an upward "kink" in the retarding potential
plot does not necessarily indicate that there are more high energy
electrons
than would be expected from the M-B body of the distribution
function. For the range where the electron energy distribution is M-B,
the electron current to the probe is an exponential function of qV/kT .
The dc term in the Fourier series expansion of the probe current
(Eq. VI-1) shows that when the electron current is an exponential
function of the probe potential, the dc term separates into the same
exponential term multiplied by a function of qA/kT_. Thus, the slope
as determined from a retarding potential plot is not changed as a result
of a small monofrequency ac voltage, but the probe current is increased
except above the plasma potential.
It is possible, however, for oscillations in the plasma to produce an upward "kink" in the retarding potential plot which may increase
the apparent electron temperature and may increase the apparent
number of high energy electrons.
When the probe is slightly below
plasma potential the dynamic impedance across the plasma-probe region
is quite small (about 1 ohm) and only a small fraction of the ac voltage
is superimposed upon the retarding potential.
As the retarding potential
is increased, the probe-plasma impedance becomes larger and comparable
with the plasma impedance and the impedance of the external circuit.
A larger fraction of the ac voltage is thus added to the regarding potential
and results
in an increase
in the probe current
and an upward "kink."
85.
This upward "kink" was observed by Bailey ( 7 2 ) in a low pressure mercury
arc where the oscillations were introduced inadvertently by the sheath
around a "filtering" grid placed in the positive column.
C. Experimental ratio of saturation electron and saturation ion current
and comparison with random current theory
There is a scarcity of published data for the ratio of saturation
electron current J-s to saturation ion current J+s
The purpose of this
and the following sections is to give accurate experimental values for
this ratio and to compare them with the various theoretical values. A
new theory will be advanced later to explain the results.
In this research the saturation ion current J+s is defined in the
following way. As the probe is made more negative with respect to the
plasma potential, the electron current becomes smaller until the probe
current is equal to the ion current.
The value of probe current where
the electron current first becomes small compared to the probe current
(about 1 per cent or less) is defined as the saturation ion current.
The
probe current to a very negative probe is not a strong function of the
probe potential (about 1 per cent per volt) while the electron current is
a strong function (about iO0per cent per 0.1 volt for a kT /q of 1 electron
volt).
The ratio J s/J +s for each probe, calculated from the basic
probe data of Table I, was broken into two groups: the planar probes
and the wire probes.
For a given set of arc parameters
(Tbath and Iz),
the ratios for the plane probes are in good agreement in spite of the
different locations and orientations.
When averaged, there is an average
86.
uncertainty of 1.7 per cent in the ratios for the plane probes.
Since the
three plane probes have quite different edge surroundings and yet give
almost the same saturation current ratios, it is concluded that the edge
effects are probably not very significant for the plane probes used in this
experiment.
In particular,
the wall probe, when it is measuring the ion
current, is almost at the same potential as the floating potential of the
nedative wall which surrounds the wall probe.
As a result, the tube
wall at floating potential acts as a very large "guard ring" for the wall
probe (when it is measuring ion current) and therefore no edge effects
are expected for the wall probe measurements of the saturation ion
current.
Since the disk probe has no guard ring at all and yet gives
about the same saturation current ratio as the wall probe, it is concluded that the edge effects are not very large.
The same is true for
the center plane probe. The average saturation current ratio for the
plane probes is plotted in Fig. VI-3 as a function of p0 .
The situation for the wire probes is more complicated because
of the different geometry.
The ion collection area for the wire probe is
larger than the geometric area because of the ion sheath around the
negative probe.
The values of Js/J+s
for the wire probes when
averaged for each set have an average uncertainty of 0. 9 per cent
and are an average of 19 per cent below the ratios for the planar probes
at the same pressure.
The theories developed in this chapter will not
be applied to the results for the wire probes.
A simple theory for the saturation electron and saturation ion
current ratio assumes that the ratio of saturation currents is equal to
the ratio of the random currents.
Since the ion and electron
densities
87.
are equal, this simple theory predicts
rg(VI-2)
j
-Jv~s
(7-At
~
t
where the implicit assumption is made that the ions can be described by
a M-B energy distribution with a characteristic
/ j1
mercury ions the factor (M+/m_)
temperature T.. For
is equal to 604.6.
In conjunction
with the experimental ratios of J s/J+ s ranging from 284 to 395, this
requires that the ion temperature be about 4 times the electron temperature which is quite improbable from consideration of the basic processes in the plasma.
In fact, the ion temperature should be very close
to the gas temperature Tg because of the much lower ion mobility and the
larger energy transfer for ion-atom collisions compared to that for
electron-gas collisions. Carrying this one step further, if it is assumed
that the ions have a characteristic
temperature corresponding to random
motion, and if this ion temperature is equal to the gas temperature Tg,
the saturation current ratio J s/J+s should be given by
s
r(
(VI-3)
7-
This quantity (M+/m ) 1 /2 (T /Tg)12
is plotted in Fig. VI-3 as a function
of po. It is seen that the random current model gives the wrong sense ot
variatLon of J_s/J+s with p0 and gives a value of 10 larger than experimental values.
At the same time, the quantity T_/Tg was calculated
and is given in Fig. VI-2 for reference.
There are two types of explanation for the discrepancy between
~
WTI-"~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~'
-1
i o
o'g
t
~~~;-*
o
n
i:
0
,b
14
M
0
0
b4 4
F
8
Vl
o
P
'4
to
o
0
)
S:d
p4
N
0s
ar
o~~
C
I
II
II
II
I
I
I
I
I
0
0
I
E4ba9
I
I
I
I
r
1
'0H
-4
88.
the experimental data and the simple random current model. One
explanation is that the experimental value of Js does not correspond to
the collection of ions but is related to the secondary
from the negative probe.
emission of electrons
The other explanation is that the random current
picture for the ions is not accurate.
It can be shown that for this experi-
ment the secondary processes are not able to explain the discrepancy
and thus requires the abandonment of the random current model.
The electron emission from the negative probe can result from
three fundamental processes:
ion bombardment of the probe, meta-
stable atoras striking the probe and radiation striking the probe. Induced
electron emission resulting from the ion bombardment is not an acceptable
explanation because the discrepancy between the experimental data and
the simple theory would require that the ratio between the secondary
electrons
and the incident ions be larger than 10 which is not reasonable
for such low energy ions.
There are no experimental data available for
the secondary yield coefficient
for Hg ions on tantalum or tungsten, but
for mercury ions on mercury the secondary yield coefficient is about 0. 01
for 4 kV ions(21)
Experimental
data for Hg metastables
on Ta or W
are not available but for Hg metastables on mercury the secondary yield
is about 0. 01(36). Comparable values may be expected for the Ta and
W surfaces.
If the product of the secondary yield coefficient for meta-
stable atoms and the metastable atom current to the probe is about 10
times the random ion current to the probe, the discrepancy may be
explained in this way. The same argument holds for the radiation
induced emission from the probes. 'Although it is possible that the
electron emission effects are not completely negligible, they are not
C 10 4
0
0
40
c
Random ion current model
0
'~e.r pJI/
s,
4)
4
0C)
O
0
4Jr
Mercury plasma
0o
Ca
Schulz theory
00o
o
10
+
Experimental points for plane
probes
·
Combination of Schulz theor~
and "ion drainage theory
10
I
10
I
.
I
.
I
1
.
.
11
.
.
I1 .
.
.
.
1
.
I
.
10 '2
'
i
____
- .
_
. .
_
.
. .
-to-
,
tr
t'
'1
t (: 17,3 t ur'n,
tio,'e
tf.o n
.
.
I I
.
.
I
..
I
.
.
I
.
10-1
P (mm Hg)
24. , i "
I
ur,
r ,turr en
89.
1
capable of completely explaining the difference of a factor of 10 between
experiment and simple theory.
In addition, since Hagstrum(37) has shown that the secondary
yield coefficients are strongly related to the surface conditions, it is
expected that if the probe ion current contains a significant component
of secondary processes the probe ion current should depend upon the
time after the cleaning of the probe surface.
I
i
Measurements of the probe
ion current were made using the expanded scale meter and the recorder.
Itwas foundthatthe ion currents to the W and Ta probes remain constant
to better than 2 per cent after the probes had been cleaned by electron
bombardment.
I
I
nrrinminonflv
This indicates that the ion current is probably not
Pl1ftrnn
cbmiQinn
criirrnt n nd it lpdsrto thP intrndlilrtinn
of a more accurate theory and a better understanding of the process of
ion collection by a negative probe.
D. Simplified form of Schulz's theory for the saturation current ratio
and comparison with experiment
The assumption that the saturation ion current to the negative
probe is equal to the random ion current has been observed here and
elsewhere to be in considerable disagreement with experiment.
If it
is assumed that the ions in the plasma travel with a directed velocity
towards the probe, much better agreement with experimental data can
be obtained. This directed velocity in the plasma occurs because there
is a small potential gradient in the plasma in spite of the fact that the
major part of the potential difference between the probe and the plasma
appears across the probe sheath. As early as 1929, Langmuir ( 73 ) stated
that the ions enter the sheath with a directed velocity because the influence
9O.
of the negative probe extends into the plasma.
The collection of positive ions by a negative probe can be accurately
described if several reasonable assumptions are made. The case of an
infinite planar probe is considered in order to obtain an easily solvable
model. The plasma is defined as the region where the ion and electron
densities are approximately equal, while the ion sheath is defined as the
region where the ion density is much larger than the electron density.
Assume that the ions in the plasma enter the sheath with an arbitrary
energy E o (electron volts) in the direction normal to the plane probe
and are accelerated toward the probe without appreciable collisions.
The total energy is conserved so that
Mf.
"'=
+
(;
V)
(VI-4)
where Vb - V is the potential in the sheath with respect to the potential
Vb of the plasma-sheath interface.
In the sheath the electron density is
small so that there is no ionization,Athe ion current is constant and
(VI-5)
In the plasma the electron density is related to the plasma potential by
the Boltzmann density law
Elf
icV -If
(VI-6)
The potential gradient dV/dx is assumed to be continuous at the interface
and the electron and ion densities are assumed to be equal at the interface.
The last assumption that is needed may take either of two equivalent
forms. It may be assumed that at the interface
A_
I
./X
.4+
_
_
/IAr
-
u
_
J
(VI-7)
or one may assume that at the interface
~ik#
d~X
/sIvv)n
= -_,
(VI- 8 )
and
I
..h*
EP4'
d-f
inJO /,,f,S
/_ .,ti,,_
JTIJA
(VI-9)
These statements can be made from knowledge of the processes in the
plasma and the sheath and from the assumption that there is a gradual
transition from the plasma to .the sheath. In fact, it is difficult to define
exactly where the plasma ends and the sheath begins. The validity of
these assumptions will be tested by the agreement of the conclusions with
experiment.
The previous equations can be combined in the following way to
obtain the desired result. From Eq. VI-5 it is apparent that in the
sheath
,,l,+
From Eq. VI-6.
.z
5f
W;-Y
) i .f5
(VI-lO)
· ,,l _
,= --W-X
A~~~~X
AX(VI-11)
It~~~~
Equation VI-7 shows that at the interface
t 2 )5
*-
12)
(VI-
When the logarithmic derivative of Eq. VI-4 is taken there results
(VI- 13)
When evaluated at the interface (V z Vb) and substituted in Eq. VI-12,
the previous equation gives
(VI- 14)
Since the potential gradient at the interface dV/dx is not zero, the
following result is obtained for E
/.
(VI- 1 5)
=z,
This implies that the plasma and the sheath adjust themselves so that the
ions enter the sheath with.a directed energy E o which is comparable to the
random energy of the electrons, and is independent of the probe potential
thus giving a saturation effect.
The ion current to the probe is then
obtained from the ion density at the interface and from the directed
velocity where the directed velocity is related to E o by
Mt
- = LIZ
~(VI-16)
Fso
- ---
--
93.
Since a potential gradient in the plasma is necessary to provide the ions
with the initial energy Eo , the density of ions at the interface is less
than the density in the plasma by a factor of about exp (qE0 /kT )-(e)l/2
from the Boltzmann density law. When combined with the random current
density for the electrons (see chapter I, these considerations lead to
the following ratio of saturation electron current to saturation ion
current:
(VI-17)
For the case of mercury ions the ratio is equal to 398. This result was
given recently by Schulz and Brown (74) through similar reasoning.
It
was given even earlier in essentially the same form by Bohm, Burhop
and Massey (75)
This constant value of 398 for the saturation current ratio is
plotted in Fig. VI-3 for comparison with experiment, and it is seen
that the agreement is good particularly for the higher pressures.
the lowest pressure,
At
however, there is still a discrepancy since the
value given by this simple theory is larger than the experimental value
by a factor of 1.40. In addition, there is still a definite variation of
the saturation current ratio with p
that should be explained.
94.
I
E. Basic theory for the prediction of the variation of the saturation
current ratio with the arc parameters
It has been shown in the preceeding section that the ions enter
the probe sheath with a directed energy E0 of from 0.5 to 1 electron
volt. This result was obtained through certain assumptions about the
matching of density gradients at the plasma-sheath interface and does
not predict any variation of saturation current ratio with respect to
the arc parameters.
In this section a model will be developed which
will also consider the rate of generation of ions in the plasma and the
ion mobility limited flow of ions through the plasma towards the probe.
The validity of the assumptions
can be shown by the agreement
of the
results with the experimental values.
In order to obtain a solvable model, consider the one dimensional
problem of a large planar probe in a large uniform plasma.
When the
probe is biassed negative with respect to the plasma potential, an ion
sheath is formed near the surface of the probe. In this ion sheath the
ion density is much larger than the electron density.
Adjacent to this
ion sheath is a region of the plasma where the ion and electron densities
are about equal; but there is a potential and density gradient in the
direction.of the probe. Ions generated in this region of the plasma are
formed with very small kinetic energy and, since they are in a potential
well, they must eventually flow to the probe at a rate governed by the
rate of generation of the ions.
drainage plasma.
This region of plasma is called the ion
Adjacent to the ion drainage plasma is the relatively
undisturbed plasma where the ion and electron densities are equal and
where the density and potential gradients are small.
95.
I
A number of statements can be made which apply to all parts
of the plasma and sheath.
From the conservation of charge in the steady
state, the ion particle current is related to the generation of ions by
(VI-18)
where it is assumed that the ionization is proportional to the electron
density.
+
I.t
Poisson's equation relates the potential distribution to the
electron and ion densities as follows:
(VI-19)
-)
E = -
Since the negative probe draws very little electron current, the electron
density in the plasma and the sheath can be related to the potential by
the Boltzmann law
,,.
_
_,., / v-_ )
exD
-
(VI-20)
-
From the above equations it is possible to obtain a differential
equation relating r+, n and n+. Consider the one dimensional form of
the divergence operator and use the following change of variable:
le
a{>x
=
~**~.
allv
(VI-21)
From Eqs. VI-18, VI-20, and VI-21, it follows that:
_(6.
(VI- 22)
Equations VI-19, VI-20, and VI-21 give
-E
L
(----)
(VI-2
96.
When E is eliminated between Eqs. VI-22 and VI-23 the following
differential equation results:
2
t
=
(9;
(^)
go
h
e 0(VI-24)
This relates /+ n_ and n+. In order to obtain a differential equation
in '+, and n_ alone, it is necessary to eliminate n+. This can be done
if the ion velocity can be specified as a function of the potential distribution.
For the plasma in this experiment, the motion of the ions can
be described
in terms of the ion mobility.
Kingdon and Lawton(56) have
shown that for large values of E/p the velocity of Hg ions in mercury
is given by
Bylt , 3.2 szo
I(E~Ij
In addition, the results
(VI-25)
c^/ffC
of chapter V show that the ion velocity is given by'
At/('81,'.)-
°x
.3-
,4t°r//)
aeh/f
/9p. r, s
(E -26)
where the correction has been made for the diffusion flow and the nonuniform
field.
This formulation of mobility-limited ion flow to the probe is not
necessarily accurate since the concept of charge transfer-limited
mobility requires that the mean free path be as small as the distance
travelled.
The validity of this formulation and the relative importance
of the inaccuracy will be tested by the agreement
of the final results
with the experimental values of the saturation current ratio.
97.
+ ,+, it is possible with the help of Eq. VI-26 to
Since P+ n+
solve for nr+as a function of P and E to get the following:
(VI-27)
Aae-
When the value of E from Eq. VI-22 is substituted, there results
A(d,7/p)'yr/ 4 ) Y
,
/
(VI-28)
When n+ from En. VI-28 is substituted into Eq. VI-24 it leads to a nonlinear differential equation in T. and n_ which is difficult to solve.
An accurate simplification, however, can be made which makes
a solution of the problem easy. Because the ion current in the sheath
is constant(due to the much lower production of ions in the sheath), one
can solve the problem by considering the equations that apply to the flow
O.fions in the plasma regions.
Setting the ion density equal to the electron
density permits considerable simplification of the problem.
When E is
eliminated from Eq. VI-22 and Eq. VI-27, and the ion density is set
equal to the electron density, the following results directly:
'L Ef_
·
(VI-29)
L
This is a very simple differential equation which can be evaluated in the
·Fnl1axrin
n
a
-E W x7
C/
-f
/17.
If
·Iv~
(=J_
0
(1)
I
.,_
(VI-30)
98.
The quantity '+ is the ion current from the plasma to the sheath and it
SN)7iz
-J
becomes equal to the saturation ion current
trs as the electron density
becomes smaller. It is seen that
(?,)7 c,
* ro
'
(VI-31)
so that when n /n p is small, r+ can be set equal to
n p is the electron density in the plasma.
· =c,
C
s.
The quantity
This gives
. i-
(VI-32)
where the constant of integration has yet to be evaluated.
From the value of
r-sobtained from chapter I, it can be seen
that
I3 ?rvs)
Inspection of the factor (q/kT_)12
(()t
=V c-
' /
(V
(Vi/po) shows that as the pressure
increases the quantity J+s/J s should decrease thus implying that the
saturation current ratio J s/J+s should increase as the pressure increases.
This is in agreement with the experimental variation as can be seen from
Fig. VI-3. The factor (q/kT)
1 /Z
(Vi/po) decreases as the pressure
increases because the electron temperature decreases with increasing
pressure and the quantity
Vy/Pois a strong exponential function of the
electron temperature. Physically this means that at the higher pressures
99.
there is less ionization in the ion drainage region of the plasma and
therefore the ion drainage component contributes less to the saturation
ion current at the higher pressures.
In order to obtain numerical values of Js/J+s
for comparison
with the experimental values, it is necessary to evaluate the constant C 2 .
When the contribution of the ion drainage plasma is small enough so that
it can be neglected, the saturation ion current should be given by Schulz's
theory so that, from Eq. VI-17, it is expected that
¢,
4=~U
-A,
(VI-34)
Thus the results of this analysis predict the general relation
(P-,J
T.
~J~/r
7j/(JJ
rj
ix;/!'/
(VI-35)
which should be applicable to any discharge that satisfies the assumptions
made.
The validity of the assumptions made will be verified by the
agreement of the predictions and the experimental values.
When the experimentally determined values of V i/Po, kT_/q,
and y1/2 (which are available from the tables of basic data and from
chapters IV and V) are substituted in Eq. VI-35, and the values of J s/J+s
are calculated, they are found to be in remarkable agreement with the
experimental values. The calculated values are plotted in Fig. VI-3
for comparison with the experimental values.
The difference between
the theoretical values and the actual values is 3.4 per cent rms which
is comparable with the experimental uncertainties.
It can be concluded
100.
either that the model and the assumptions are verified, or there is a
I
I
strong coincidence.
In any event, if the assumptions and model are not acceptable,
I
i
Eq. VI-35 can be proposed as an empirical expression relating the
saturation current ratio, to the ion and electron mass, the electron temperature,
the ionization frequency, the reduced pressure,
mobility coefficient for large E/Po.
and the
It would be of interest to test the
applicability of Eq. VI-35 to probe measurements in other gases.
I
101.
VII. Summary and Conclusions
Careful measurements have been made of the electron tem-
perature, electron density, plasma potential, and probe currents for
a set of probe measurements
arc.
in the plasma of a low pressure
As a result of the precautions
mercury
used in the measurements,
the
results are accurate and reliable. Detailed analysis of the basic
data yields considerable new information about the basic processes in
the plasma.
It was shown that the actual ionization in the plasma is much'
larger (up to 45.6 times) than the direct ionization thus proving that
the ionization in the plasma is predominantly cumulative ionization.
The direct ionization was calculated for a Maxwell-Boltzmann electron
energy distribution with the aid of Nottingham's data for the probability
of direct ionization of mercury.
A calculation of the effect on the direct
ionization by the electron drift energy shows that this effect is small.
The rate of production of the 63Po 1 Z excited states was calculated
from the cross-sections
given by Kenty, and it was shown that the
rate of production of these excited states is larger than the ionization rate
thus demonstrating that cumulative ionization through these excited states
is possible.
It was shown, with the aid of the diffusion coefficient for
Hg metastables in mercury given by Biondi, that the maximum rate of
diffusion loss (corresponding to the maximum metastable density given
by the Boltzmann law) is lower than the rate of production of the meta-
stables thus indicating that the predominant loss of metastables is the
quenching by electron collision.
This implies that the density of
metastables should be relatively independent of electron density.
102.
I
This indicates that the cumulative production of ions should be a linear
function of electron density and not a quadratic one. The results of this
experiment confirm this since G/n _p
was found to be independent of n
for a variation of n of 20 per cent.
Information was found about the electron mobility in the plasma.
The average electron density,, the axial arc current, and the axial
potential gradient gave the electron mobility.
With the aid of the assumed
Bessel function density distribution, the average electron density was found
from the electron density at the center of the plasma and the electron
density at the plasma edge. It was found that the mobility could be well
represented by a velocity independent average cross-section
electron body of the distribution.
by ,L!po(kT_/q)l
Z/
an rs
The electron mobility can be described
, 1.49 x 105 (cm2 /v sec) (mmHg) (volt)1/2 with
deviation from the mean of 6 per cent.
cross-section
for the slow
The corresponding average
is 42 x 10-16 cm 2 for electrons with kT_/q ranging from
0.829 to 1.90 electron volts.
The ambipolar diffusion coefficient for an active plasma was
obtained from the ion particle current density to the wall probe, and
from the assumed Bessel function density distribution.
The values
obtained give more detailed information about Da for a mercury plasma
than has been reported before.
The Boltzmann theorem relating the density and potential dis-
tribution permits the radial electric field to be calculated from the
assumed Bessel function density distribution.
Values of the radial ion
velocity were obtained from the radial ion current to the wall probe
and from the ion density measures at the plasma edge. Thus the ion
velocity v was obtained as a function of E /Po and could be expressed
in the form
1 03.
A/r
Q7/ /0( fV/f)
*1
7'r-'
cm/3fv
or
,,At - M17
#I/%
i/,-
toftl/L
This indicates that the energy gained by the ion in one mean free path
(EA) is large compared. to the random energy.
With the aid of a specially developed theory for the mobility
and diffusion flow of ions in a strong field it was possible to calculate
the mobility and longitudinal diffusion coefficients as well as the crosssection for slow Hg ions in mercury vapor. Since the ions are moving
in a slightly nonuniform
.
electric field, it was necessary to
consider this nonuniformity. A first order theory was therefore
developed for the mobility and diffusion coefficients in a strong
nonuniform electric field. The mobility and diffusion coefficients and
cross-sections
were recalculated with this correction.
The effect
was small and resulted in a reduction for the cross-section
19 per cent on the average.
mercury
of only
The corrected cross-section for the
ions was found to increase
from 31 to 104 x 10
E x increased from 0.44 to 1.37 electron volts.
-16
cm
2
as
The methods and
theories developed in this research may be used for the measurement
of cross-sections
for low energy ions in other gases.
The ion velocity corrected for the diffusion flow and the nonIt was found that this ion velocity could
1/2
with the average constant
be described as proportional to (Er/Po)
uniform field was calculated.
of proportionality
equal to 3.05 x 103 cm/sec
per (v/cm mm Hg) 1/
104.
with a rms deviation from the average of 19 per cent.
The retarding potential plots for the electrons were found to be
intermediate between that for a Maxwell-Boltzmann and for a Druyvesteyn
distribution.
For the arc under consideration it was found that the de-
pletion potential (the potential at which the depletion of high energy
electrons started), measured with respect to the plasma potential, is
approximately equal to 3.4 kT_/q on the average.
The same is true
for the floating.potential except that the numerical factor is 5.32.
The ratio of saturation electron current to saturation ion
current was investigated both experimentally and theoretically.
It
was established that the ratios for the plane probes increase with po
In order to predict the observed variation of the saturation ration with
the arc parameters,
a model for ion collection by a negative planar
probe has been proposed.
This model includes the drainage of ions
generated in the plasma near the probe.
When combined with the
Schulz theory for the saturation current ratio, this ion drainage theory
gives values for the saturation current ratio which are in very good
agreement with the experimental values.
Various new experimental techniques were developed to increase
the accuracy and convenience of the measurements.
The retarding
potential was accurately supplied by a specially designed low impedance
voltage source which features a high degree of negative feedback. The
partial pressure
of residual
gases was kept in the 10
to 10- 7 mm Hg
range and was measured while the arc was in operation in the water
bath. The probe surface was automatically cleaned by electron
105.
bombardment before each probe current reading in order to keep the
probe work-function constant. A new method was developed to permit
the change of probe work-function to be directly recorded, and may
prove useful in future experiments.
i
i'I
Table
Tb
C
25.1
I
z
wire(a)
wire(a)
center
wire(c)
wall
disk
30.0
30.0
5.0
6.0
45.1
45.1
45.1
54.6
4.0
5.0
6.0
5.0
62.6 5.0
I
I+4
kT_/q
Vp
Vf
ma
ma
volts
volts
volts
86.5
92.8
56.0
86.4
0.375
0.400
1.91
1.92
1.91
1.93
1.96
2.58
2.68
4.37
5.30
5.10
4.87
13.13
13.28
13.98
14.46
14.35
14.10
3.35
5.38
12.87
13.98
30.3
55.4
115
73.9
117
wire(a)
center
146
95.3
146
wall
disk
5.0
-S
wire(a)
center
wire(c)
wall
disk
wire(c)
35.0
Basic Probe Data
I
Probe
amp.
5.0
I
35.3
70.2
44.2
89.0
0.202
0.370
0.108
0.189
5.87
14.42
13.97
0.570
0.317
0.550
0.149
0.288
1.63
1.63
1.63
1.56
1.41
3.46
5.34
6*33
6.25
5.97
12.85
13.98
14.82
14.38
13.91
1.88
2.03
0.490
0.530
1.42
4.04
4.10
6.21
1.88
wire(a)
center
wire(c)
wall
disk
187
121
197
41.3
0.652
0.355
0.660
0.125
108
0.303
wire(a)
center
center
wire(c)
wall
disk
231
wire(a)
center
wire(c)
wall
disk
297
202
308
65.0
wire(a)
center
wire(c)
wall
disk
403
wire(a)
584
center
wire(c)
wall
disk
351
614
173
256
411
72.4
217
88.7
300
0.590
0.560
6.25
6.17
39.5
81.9
250
51.4
135
1.02
1.67
1.60
1.46
0.512
0.119
0.250
154
158
1.10
1.10
1.65
136
142
143
1003
0.466
0.255
0.465
0.120
0.238
wire(a)
wire(a)
center
wire(c)
wall
disk
89.3
1.74
1
per m3xlO
1.59
14.77
1.47
1.60
1.49
0.761
0.774
1.88
0.963
1.00
1.44
1.36
1.40
1.40
1.23
7.22
12.27
12.30
13.86
14.84
6.99
6.74
14.52
13.88
1.14
1.29
1.14
1.05
1.18
5.05
7.00
8.41
8.20
7.48
11002
13.20
14.30
13.87
13.14
2.88
2.90
3.04
0.790
0.430
0.445
0.815
0.149
0.365
1.07
1.17
1.23
1.11
1.04
5.20
7.30
7.09
8.57
8.36
7.65
11.06
13.15
3.67
3.88
3.87
3.89
0.970
0.568
1.09
5.17
7011
8.49
8.36
7.51
11.08
13.18
5.61
7.64
8.88
10.55
12.77
13.63
13.43
12.71
0.271
1.04
0.193
0.445
1.09
1.20
1.06
0.994
1.07
1.28
0.698
1.31
0.192
0.560
0.929
1.01
0.906
0.855
0.906
1.73
0.908
1.83
0.223
0.818
0.891
0.825
0.785
0.748 0.828
8.68
7.97
5.74
7.71
8.84
8.79
7.97
13.22
14.39
14.03
13.24
14.22
13.86
13.10
10.27
12.47
13.37
13.06
12.41
1.95
2.09
1.99
0.909
0.984
1.10
1.37
1.72
4.67
5.03
4.91
1.78
2.22
6.87
6.96
7.10
2.13
3.04
10.6
10.1
11.1
2.73
4.39
7
ii
to
to
0
C\l
U
03
C
J
tl
( a) CL)o
co
a
*
0
04
0
043
o0
o0 0 00C' O)00 00 00 00 00 0n
bo
CZ
F-
I
Lo
¢
C' COC
L) L
-4
V a
00I, H
a
0
co
,
0tr(
P
P
0)
0
0
*
)
0)
L-
'4
0
to
to
(D C')C
t
H
0)
tO
0)
0
0
CD
L-
c0
)
0
O)
H
0
C
0o)
0)
-
0*
0
Cd
0
L
0 0
00 0 O
C)
m
t.o
H
0
0 0C'
C
0)
C
a)
0
)
0)
0
0)
0
0,
dI
H
0
bO )
Sz
P
to
0
0
)O 0r
H
-d4
*
0
0
H
r
0
C')
0
tlO C)
bc
0
EO o
C'
H
C
CO d)
H
*
0
0H1 0O
- H
CH
ao
'qN
a)
0
-
o
a-
a
H-
H-J
C*)
CO
C
C
COO)
to
CO
(00
000
4
H
H
H
,
E4
E-4
N
H
0 00
0L0
0
0 0
0
g
.000
U)
U)
a)
4O00
J,
o o
0
H
H
C'
CQ CQ
H
u)
(D
C-
C
0
*
0
0
0
LO
O
H
to
t
to
t
t
O
to
t
.
N
to
0
CO
O
d4
U)
H4
t
0
0
n
mr
-0
PQ
a)
iii
iI
Table
Set
nw./no_
Ion Generation
III
.n/nO_
Jw(Vf) Gtotal
x 10
15
amp./m2 (#/ec./cm3
in the Plasma
/PO
1
(direct)
v
(total)
i(direct)
(#/ec.)/mm
A
0.554
0O767
7.52
3.43
1027 x10
7
1.71
G
0.502
0.738
8.54
3.89
4.41 x10 6
2.76
H
0.499
0.737
4.00 x106
2.86
B
0.460
0.715
8.25
4.79
3.76
1.50 x10 6
4.21
I
0.374
0.666
8.83
3.55 xlO 5
6.80
C
0.358
0.657
10.6
4.02
4.82
2.60 xl0 5
8.42
F
0.364
0.660
1397
6.21
1.87 xlO5
D
0.306
0.626
13.7
6.21
3.27
E
0.257
0.597
15.8
7.20
9.08 x103
10.5
x104
11.4
26.2
45.6
I
Electron Mobility and Electron Drift Energy
Table IV
Set
Jz
vz
-3
x10
amp./m
-5
x10
Po/,.
<v.>
xlO15
V
vzZ
m/s
(cm/4T/cm)mm
Vz
kT_/q
A
2.12
1.62
1.17
5.68
0.0395
G
2.12
1.18
1.16
7.16
0.0248
H
2.54
1.12
1.14
7.52
0.0225
B
2.12
0.936
1.26
8.38
0.0182
I
1.69
0.541
1.37
13.3
0.00719
C
2.12
0.525
1.37
13.5
0.00700
F
2.54
0.492
1.34
14.2
0.00636
D
2.11
0.302
1.53
21.3
0.00282
E
2.11
0.208
1.84
29.3
0.00149
110.
Glossary
of ion, p. 68
a
acceleration
A
amplitude of small ac signal, p. 81
C1
constant of integration,
p. 97
C2
constant of integration,
p. 98
Da
ambipolar diffusion coefficient,
Dm
diffusion coefficient for metastables,
D+
ion diffusion coefficient, p. 38
D
electron diffusion coefficient, p. 38
Dl1
longitudinal ion diffusion coefficient, p. 72
eo
permittivity in rationalized MKS units, p. 33
Eo
directed energy of ion in electron volts, p. 34
Er
radial electric field (evaluated at plasma edge), p. 65
Ez
axial electric field, p. 29
E9
electric field at end of free path for ion, p. 75
f(v)
density of electrons
p. 39
p. 52
in phase space, p. 2
fo(v) spherically symmetric part of the distribution function, p. 3
.
g
statistical weight of a state, p. 52
G
total ionization in ions generated per second per unit volume, p. 36
G'
G
total ionization averaged over the cross-section
ip
P
current to the probe, p. 1
i
electron current to probe, p. 2
ie
ion current to probe, p. 25
of the plasma,
p. 37
111.
Iv
current in vertical arm of discharge, p. 9
Iz
axial arc current in horizontal arm of discharge, p. 9
Is
saturation electron current, p. 27
Jz
axial current density in horizontal plasma averaged over the plasma
averaged.over the plasma cross-section,
J
electron current density, p. 26
J-s
saturation electron current density, p. 3
Jts
saturation ion current density, p. 85
(same as z ) p. 57;
J+w(Vf) ion current density to floating wall probe, F. 36
OWBessel function of the first kind, p. 43
J1
Bessel function of the first kind, p. 45
k
Boltzmann's constant, p. 2
K
thermal conductivity of pyrex, p. 30
mn
mass of electron,
M+
mass of ion, p. 68
ng
density of mercury atoms, p. 49
nm
density of metastable atoms, p. 52
n_
electron density in number per unit volume, p. 1
n+
ion density in number per unit volume, p. 1
n_
electron density averaged over a cross-section of the cylindrical plasma
p. 2
p. 45
no
electron density at the center of the plasma, p. 45
n w
electron density at the edge of the plasma,
p
vapor pressure of mercury, p. 30
Po
reduced pressure,
p. 31
p. 45
L
112.
Pc
probability of collision in number per unit length, p. 59
Pi
probability of ionization in number per unit length, p. 48
q
magnitude of electron charge, p. 2
q(O)
differential scattering cross-section, p. 59
QC
collision coss-section,
p. 59
cross-section for ionization, p. 49
Qm
Qo
cross-section for momentum transfer, p. 59
effective cross-section,
Q(A ->B)
p. 47
cross-section for the production of species B from species A, p. 46
rp
radius of the plasma, p. 33
rW
radius of the wall, p. 30
rl
inner radius of pyrex wall (same as rw), p. 30
r2
outer radius of pyrex wall, p. 30
Ar
thickness of ion sheath on Wall, p. 33
Ro
radial location of zero of electron distribution, p. 43
distance of flight of ion, p. 68
S
s
saturation factor for the j'th state:-- ratio of the density of the state to the
maximum density given by the Boltzmann theorem, p. 54
S
Spenke function, p. 44
Tb
temperature of water bath, p. 29
Tg
temperature of mercury vapor, p. 31
T_
electron temperature in degrees K, p. 1
T+
"temperature of ions", p. 87
AT
temperature drop in the pyrex wall, p. 30
ST
temperature jump from the mercury vapor to wall, p. 31
I
r-
113.
v
Vf
vf
magnitude of velocity vector, p. 3
terminal velocity of ion at end of flight, p. 68
minimum velocity required to overcome retarding potential, p. 2
Vx
Vz.
velocity in the "'x" direction, p. 2
axial drift velocity of electrons, p. 57
vrms root mean square velocity of electrons, p. 6
v
ion velocity in the radial direction
(+w n+
v+), p. 65
time average velocity, p. 67
'i
drift velocity of electrons, p. 49
electron velocity averaged over the distribution function, p. 3
Vc
retarding potential of the probe with respect to the plasma potential, p. 2
potential of the collector (probe), p. 26
Vd
Vf
potential at which the depletion of high energy electrons starts, p. 80
floating potential of probe, p. 79
.·E
V.
ionization potential, p. 48
VP
plasma potential, p. 27
VT
voltage equivalent of T (equal to kT_/q), p. 46
Vz
axial drift energy of electrons, p. 58
V
average energy in electron volts, p. 79
drift energy of electrons in electron volts, p. 49
V.
threshold energy in electron volts, p. 46
vej
threshold energy for the j'th state, p. 54
x
2.405 r/R (evaluated at the plasma edge where xp = 2.405 rp/Rp) p. 45
YO
Y/2
0. 7397 V/V, p. 5
ratio of average ion velocity to (E/po)l/
p. 77
i14.
ac
persistence
r(k)
complete gamma function, p. 5
of velocity, p. 68
1r'w ion particle current density to the wall, p. 42
7i+
particle current density of ions, p. 38
particle current density of electrons, p. 38
-g
mean free path of the mercury atoms, p. 31
' r mean free path for the mercury ions, p. 69
cmean free path for collisions, p. 59
mean free path for momentum transfer, p. 58
I
Pa
ambipolar mobility coefficient, p. 64
p
mobility coefficient for electrons, p. 38
)+
mobility coefficient for ions, p. 38
9
accommodation coefficient for mercury atoms on pyrex, p. 31
f"
time of flight, p. 68
vi
ionization frequency in number per electron per second, p. 43
hA
change of work-function,
p. 17
115.
References
1.
2.
I. Langmuir, Gen. Elec. Rev. 26, 731 (1923)
I. Langmuir and H. M. Mott-Smith, Gen. Elec. Rev. 27, 449,
538, 616, 762, 810 (1924)
3.
H. M. Mott-Smith
4.
5.
L. Tonks and I. Langmuir, Phys. Rev. 34, 876 (1929)
T. J. Killian, Phys. Rev. 35, 1238 (1930)
6.
M. J. Druyvesteyn and F. M. Penning, Rev. Mod. Phys. 12,
and I. Langmuir,
Phys.
Rev. 28, 727 (1926)
87, (1940)
7.
P. M. Morse, W. P. Allis,
and E. S. Lamar,
Phys. Rev. 48,
412 (1935)
8.
S. Chapman and T. G. Cowling, The Mathematical
Theory of Non-
Uniform Gases, second edition, The University Press,
(Cambridge),
1953, p. 346
9.
R. F. Post,
28, 338 (1956)
Rev. Mod. Phys.
M. J. Druyvesteyn,
11.
G. Medicus,
12.
13.
R. T. Bayard andD. Alpert, Rev. Sci. Instr.
T. A. Anderson, Phil. Mag. 38, 179 (1947)
14.
15.
16.
M. A. Easley, J. Appl. Phys. 22, 590 (1951)
R. M. Howe, J. Appl. Phys. 24, 881 (1953)
G. 'WVehnerand G. Medicus, J. Appl. Phys. 23, 1035 (1952)
17.
A. L. Reimann,
18..
G. L. Weissler
19.
20.
G. L. Weissler and R. W. Kotter, Phys. Rev. 73, 538 (A) (1948)
C. W. Oatley, Phys. Soc. Proc. 51, 318 (1939)
21.
P. F. Little,
22.
R. L. F. Boyd, Proc. Roy. Soc. (London)A201, 329 (1950)
23.
D. H. Pringle and W. E. J. Farvis, Phys. Soc. Proc. B68, 836 (1955)
24.
J. Appl.
Phil.
Phys.
27, 1242 (1956)
21, 57i (1950)
Mag. 20, 594 (1935)
and T. N Wilson, J. Appl. Phys. 24, 472 (1953)
Handbuch der Physik,
Vol. XXI, 1956, p. 639
E. R. Cohen, J. W. M. DuMond, T. W. Layton, and J. S. Rollett,
Rev.
25.
Z. Physik,
64, 791 (1930)
10.
Mod. Phys.
27, 363 (1955)
International Critical Tables, Vol. III, McGraw-Hill,
New York),
1928, p. Z06
26.
27.
Handbook of Chemistry and Physics, 38 ed. Chemical Rubber
Publishing Co., (Cleveland), 1956-57, p. 2150
E. H. Kennard, Kinetic Theory of Gases, McGraw-Hill (New York),
1938, p. 311
L
F.
/
28. Chapman and Cowling, p. 104
29. International Critical Tables, Vol. V, 1929, p. 53
30. Smithsonian Physical Tables, 9th ed. Smithsonian Institute,
(Washington),
1954, p. 638
31. W. Elenbaas, The High Pressure Mercury Vapor Discharge, Interscience Publishers, (New York), 1951 p. 42
32. E. Klarfeld, Jour. Phys. U.S.S.R. 5, 173 (1941)
33. K. W. Missner and W. F. Miller, Phys. Rev. 92, 896 (1953)
34. C. Kenty, Phys. Rev. 80, 95 (L) (1950)
35. M. A. Biondi, Phys. Rev. 88, 660 (1952)
36. A. von Engel, Ionized Gases, Clarendon Press, (Oxford), 1955, p. 84
37. H. D. Hagstrum,
Phys. Rev. 104, 1516 (1956)
38. W. Schottky, Physik. Z. 25, 342 635 (1924)
39. W. P. Allis, Handbuch der Physik, Vol. XXI, 1956
40. J.O. Hirschfelder,
C. F. Curtis
andB. R. Bird, Molecular
Theory of Gases and Liquids, 1954, p. 441
41. E. Spenke, Z. Physik, 127, 221 (1950)
42. W. B. Nottingham, Phys. Rev. 55, 203 (1939)43. C. Kenty, J. Appl. Phys. 21, 1309 (1950)
44. B. Klarfeld, Tech. Phys. U.S.S.R. 5, 913 (1938)
45. J. F. Waymouth and F. Bitter, J. Appl. Phys. 27, 122 (1956)
46. M. A. Biondi, Phys. Rev. 90, 730 (1953)
47. W. P. Allis and S. C. Brown,
Phys. Rev. 87, 419 (1952)
48. F. L. Arnot, Proc. Roy . Soc. (London),A130, 655 (1930)
49. H. S. W. Massey
Phenomena,
and E. H. S. Burhop, Electronicand IonicImpact
The Clarendon Press, (Oxford), 1956, p. 121
50. K. K. Darrow, ElectricalPhenomena in Gases, The Williams and
William Co., (Baltimore) 1932, p.
00
51. R. B. Brode, Rev. Mod. Phys. 5, 257 (1932)
52. R. B. Brode, Proc. Roy. Soc. (London)A125, 134 (1929)
53. W. Elenbaas,Phillips Res. Reports, 2, 20 (1947)
54. F. P. Adler and H. Margenau, Phys. Rev. 79, 970 (1950)
55.
M. A. Biondi,
Phys.
Rev. 90, 730 (1953)
56. K. H Kingdon and E. J Lawton, Phys. Rev. 56, 215 (A) (1939)
57. L. Sena, Jour. Phys. U.S.S.R. 10, 179 (1946)
117.
58.
G. H. Wannier, The Bell System Tech. Jour. 32, 170 (1953)
59. J. H. Jeans, The Dynamical Theory of Gases, Dover Publications,
(New York) 1954, p. 263
(0. L. F Epstein and M. D. Powers, Jour. Phys. Chem. 57, 336 (1953)
61. J. S. Lukesh, W. H. Howland, L. F. Epstein and M. D. Powers,
Jour. Chem. Phys. 23, 1923 (1955)
62. Massey and Burhop, p. 526
63. Massey and Burhop, p. 444
64. Massey and Burhop, p. 413
65. B. M. Palyukh and L. A. Sena, J. Exp. Theor. Phys. U.S.S.R. 20,
481 (1950)
S. C Brown and W. P. Allis, Technical Report 283, Research
Laboratory
of Electronics,
M.I.T.
1954, p. 21
American Institute of Physics Handbook, McGraw-Hill, (New York)
(1957), p. 7-174
66.
R. N. Varney,
Phys.
Rev. 88, 362 (1952)
67. W. P. Allis, Handbuch der Physik, Vol. XXI, 1956, p. 90
68. R. H Sloane and E. I. R. MacGregor, Phil. Mag. 18, 193 (1934)
69. A. H. van Gorcum, Physica, (Hauge), 207, 1936
70. S. E. A
Landale, Proc. Camb.
Phil. Soc. 25, 355 (1929)
71. L. B. Loeb, Basic Processes of Gaseous Electronics, University
of CaliforniaPress (Berkeley) 1955, p. 345
72. J. M. Bailey,
Unpublished M.S. Thesis, Department of Physics,
M.I.T. May 1951
73. I. Langmuir, Phys. Rev. 33, 954 (1929)
74.
G. J. Schulz and S. C Brown,
Phys.
Rev.
98, 1642 (1955)
G. J. Schulz, PhD Thesis, Department of Physics, M.I. T. 1954
75.
D. Bohm,
E
H. S Burhop,
and H. S. W. Massey,
"Uses of Probes
for Plasma Explorations", in The Characteristics of Electrical
Discharges in Magnetic Fields, ed. by A. Guthrie and R. K. Wakerling,
McGraw-Hill, (New York) 1949, p. 13
/
118.
Acknowledgement
I would like to thank Professor Wayne B. Nottingham for all the
help and advice he has given me. I am grateful to Professor W. P. Allis
for valuable aid with the theory.
The interest shown by Professor
S. C. Brown and Professor F. Bitter was very encouraging.
I would also like to thank the members,
past and present,
of the
Physical Electronics group and the Microwave Gaseous Discharge group
for many enlightening discussions.
Thanks are due to Mr. L. W. Ryan and Mr. A. J. Velluto for
their expert glass-blowing, Mr. A. Berg and his wiring shop for the
construction of the electronic circuits,
Mr. L. E. Sprague for drawing
some of the figures, and Miss Anne F. Crimmings for typing the thesis.
The encouragement of my wife, Dr. Ruth Aisenberg, is gratefully
acknowledged.
119.
Biographical Note
Sol Aisenberg was born in New York City on August 26, 1928.
He was educated in the New York public schools and graduated from
Brooklyn Technical High School. He entered Brooklyn College in 1947
where he majored in physics.
In 1951 he received his B.S. cum laude
with honors in physics, and was admitted to the graduate school of the
Massachusetts Institute of Technology. From July 1951 to September
1956, he held a research assistantship in the Research Laboratory of
Electronics and the Physics Department of the Massachusetts Institute
of Technology.
He is a member of the American Physical Society, Sigma Xi,
Pi Mu Epsilon, and Phi Beta Kappa.
He was married
on February
12, 1956.
Lkaslc etarding Potential Data
Tihe following
4
original measurements
ot
a necessary
request
pages of data which represent the
for 43 retarding
poteantial
part of this thesis but are inTi-ud
eed at the
of Professors
W. P. Allis,
S
from the data for use in this thesis
rown, and '.
.
:;ttinighazm.The significant quantities
I and II.
lots are
B.
hich were calculated
are
listed
in tables
These qualtities include the electron temperature,
the electron density, the plasma potential, and the saturation
electron and ion currents for each probe.
The
current to the probe was -measured as a function
probe potential
V, where this potential is measured with
respectto the anodeIn the horizontal
tube.
of the
arm of the dischargo
Since there is a small voltage drop in the probe crrent
wneter,the probe potential
V, must be increased
according to
the following:
50 rmilivoltsfull scale for the 100 to 1000 miliampere range,
50 -ralivolts full scale for the 10 to 100 miliaapere range,
O0millvol ts full
AN
for
th.e 1 to
range,
O ailiampere
sale for the 0.1 to 1 miliamoere range,
milivolts
fullscale for the 0 to 0.1 ulliacere rane.
50 mil volts f'll
and
scale
.
uLwK
p°B
f l
(
ro)
I
=
7I,
-, 5
: 5·
Z,
-
57,
cr
/
I./
e-
a~t
r
9S
t
/ 7j g7_
V//
v
(tI
_,?
,,,
7-r)
V
( 'I,#
1't
2.o
.2
3. o7
/0
/ .
/e
.z
.;
107
/o
/o~
I°
.3
I eC3
Jle
I1
7 ~,.,
q.
o
',,_
1/.7
a- .3/
o. 7ff'~.- o. ¢
2.3/
o . q/,-
o. · ¢a
F3
/./ zy~
o. 6,r
e. 3'1
2. 02
1.7~
.?t
I~ °
I/.3
f :7 S
'I :. t
-'
,/
f. /
'f7.
9/5G
.(6 5' e3
,3'f7
J3
e92
Y73
O.
o ,~
1 o. 2 5
M
T
a.
I./Zs
o. 2/S
7/
o. 4zC
I F',o
'W3.9
~.o.PO
13el
3 7b
..33- 5(
. 3.f
e,/
o 33.-
'77.3
72.
¢,~.It
'
o
$,¢,.
7r.o
0.
O'.3 9?
- F
tS~
o.¢% .:
Cm.
o3G7
a. fl{
o LG°
o.
o3
o. °5"5
o.o/
1`7. Y
3.
,.,/
3~".$
3 .C
2.
2 q
i/7.~'
I7' .3
O
,?
O. 3
O. 0,{f
3.
2./
. f1
'ZJ.
3?
2.2.( Ss
I3ap
l/ .Cc
1·7 5
13.1
170.e
I'3
o0ry
o )XP%
o e. 0
0D.5
3
0. 33fc
2o oo8sw9>
0~ )/e
POY.
owu
19L414C"C CsoLO
7.
/.'/$
lzf.3
el.w
7. 5-/
7' /
33
·.
-,z ~'
c.~'
/t~p
~. 't ~
'~.c/
,z 3
3.
g:
2
3. 7
3 32
2 *%
1
7
o.Y/
2 . '6
w. C
:C 2. rp
DlsA
pseir
V
./
2
C,
2;7
5.
v
I
(verS) 9
A/
.
O-
/|C
0. lT
I,
_
M
I. e
G3.5
a. `L
63.3
69.
.s
GC5:
63.f
,o
G2. 7
.~.1.3
f. '
;¢.G
CL.2
t.0
/£. o
I 9Q
. /2.
Oi.q) o
e.
77
I·l~..
. (
0oG77
2.°
6/
G: C
3
0.~
0.
0.
57'60
'77
0. 5'
o.~ o7
o. /g3
o. /7/
0.1
07
0.F32'
9 . 3W/
3 .7/ 4
00.o.(G
0.233 7,
3
0. f
O¢
. o5f
/. e .
5
0(6
,'...oIe/.
05 S
ot3
.0
""-.3
.
-.'
o.~.f15..('g
o.320
5
O.· 1b76
6'
o.15
I .w O. z A
s.
g
(i/
O.
o. Lf
79
o.
5'77
Go g
/.
/6
/ 4(7
0 /S
0.1~
0.
o../2/
I~,
o. ff'
6.
0./
32 .-
Q..'
o. /33
T537
-.
25,7
I9'$
O./
IBo
/3. 0
/7,o
¢' ?3
7.13
5.70
.q~
39.'9
,.
5, /
'/5'
. /2.
SS
3. 3-
2. '/2
e °,7
2. e
2.3 6
,./ 5
I,o
19..f'
(/.
.1"
C7
°. o.,~6
o.
£.0
o,/&~
2 S. q
7
d. ofp
°g7
6.
/ire
°z /4b
(B
o9.
OI~'~
/ (1
'2.
2..
r5'."'
~'5
, .o
£. /I tG
(
O·
D. /%G
o,
'
I.C.?
° /f6
fC
0.
(
0. /
-
o.(78.
o.t/
:i
o. 03
0. {J
e. o/,
/
O./87 o.
0'/
5.F
0.
e
:'/f P / I 7_r
/ 5- CH ,O f0/7'f
A V"Oe6
c6
Ad
=
£9.
c
,9PIOPe -iRe
-7-
, ,/
.,'v
z
PPeseI
!
C7
3
t
',5,0 .#
'.
7//S/, -9
V
( ~o
I.
A_ S
O.
/0F
.
(
)
-,'f,
(, VD
(MAh)
)
I"5
o/.7I.7.
Ioq.3
97,/
-
g
/.
5-.
,,o
C
~o.G
, 2 . y3
..
3 .2-
o.3/97
F'6
/
0.
/. 55
I.¥
/, o
o.
7o.
,
y~?.2
o3-
'3. ,
77.
~/7
o.
4 .j 5
3;R.
iC7
,'3*.
r
o- ,, 2.
2rL
o . 4Ry
t>.
v/r
0.
.3
o.32
'
-3',7"
/9.0" 172
0
2£,5/' .
.o
o.
/c/
0. /
B
L
4 2
. 1Ž. o
0
. T
0,B
'- 9-./
7
Ca2. f
2 / . S'
/7. 5
//5.1
./
/ -. 5v
1''. S
35-
. 3..~
,o.3'7
'..3 3
. 3'
/.
o. 13
/3.9'
3. 1
Ž0?
b
3.
2.
Iq.
'1.. 3i
7
'7Is-
.
j
9.
.2.S
: .z
03
/. 3
/'"sz
/·
/. /3/
('"
0o.
'7V. /
7/.
2.,
e. 3
/3/6
'77;f
'7/..
3., '
e.
0.3/a7//
1. c2
/o3.y
/I2 .?
3
0,1,0) )A
2.23
/o.y
o.z~
.3/
)
v
o.
°0/f
/. 5o 3
e. 2
0.7
// /
. 2. /f
1,1'/
°.,32
o.Yr
. C7
o. r3
o. 2c ~
7 9r/
2. q3
7.te
o.L
O.
30
7. 21
/- 6"o
61/2
S. s-9
/ .°
'
J.j
3iY ° .
_
O.
O.oo3
¢
/Ars'
F'8/
~-,/
/F
:'. 6
. 35-
'. ET
3. ?
3. 5'9
.3/
Z.
o
2 /6
0.
0-
3/
S/
3.27
2 . Vr7/
2.
./7
P,, lrTf
,4-01E46
~
j,-
0//
=
T
-.
W#LL- LAmCfRif
tb x-w,
X-V
zr 5%
q
'c
- )
Zi,1,7
#'
LI
.A 4
'Vf
m
353
3 5.3
o.q
4-
3-, 7
3-,r
3,-
/6.
II
/
0
1{. e
t rj
3.7
3. 2
3J.
o.
3 . a
3.e
3 3.,
32. 3
/
I. '
o-
( 0 o2 .
.
o. .s'
./
o. ,9,,
o. 7/
.
'.
."'/3
o. a/
o, /o(
o.lq72-2'
0
a.
o.
,,(
o,
o.^S5Z
o.o 2..9'
S'
,-.a
03Y
o.
31.3
o,
o.fe o3
ooa. y9
°./2,
.° 7
a.
c0ro
/ °2_~
-
/t
e
o7
O.O ib
C. oe15-,
2-.?
$.
/7.2r
'2.
. o7.
,'to
L,/
° _
". 0. .o.o3
0.o
.oro
/
0.o,0
, 1
171
a
oo.
7.-,-
i'7.
o =, o9
/17'.
' o.-.r
o.o 9."
0.o7
0./rrf
_,'oy
(o3
O- - /
o./5
"o.° / .
o.
.o/_
0./ '5'"
3. ¢
3..9'
o...it
/o. o 7'
I.
o, /,, C
2I. /~l
o /o3
LG.
T/
c,
o.(o 7
o. oogy
o,
o
" . ,o 8.
-'
*./,/
a.,
oQ2
0. /"'S
..l1g '
o./
27o
/I
3.2.~ z
-9
o..
c /o
o.I
o. /*.
3. -7:z
I]~~~~~~~~/
-
. / Cf.
9
o.o
32.
£,o
01.
7
o. ~'°
o./(o
.C5
q
o./5'
0.,
I./
,3. _
30.0
0.
2
/. 77f
i.- 7ff
.. c 0'
33./
33.9,
3F,
/ 7./.'7
33
¥,
6'.f"
o. /o
33.
9
33.C
33.5
.
2.
72' 2
33.
33. 5
,.,,
9'?
.19.
/.3/
3Yq9
°
5'
2./ .
5,
3/'
.
2.
3 5.
.
-
,
-A.
.o
o
o./o
o,
,.Ob'
/09
3.3'
- 77
-
27- '
2 e
cKc,-
/'e..rs
fArf
A/V,46b 'C#%Vf£
/.o
r
-
t
I
I
f'-
ZI?
ct'rr,e_
/,
ffoj3
--Po,,
1z
5r-;ro5A
-
v
(v,,- TV
rg
-//r/-'
.,4 1f
h)
b. °
e3. 2
63,
G3. 9
2. 7
C3/.s
2.9
C2..
36/.
o,/ 7
2.7w
3,/.
6.6
/o
l e',
0
2. qX"
c.5
/7
/ 73
'7
,
. /
I. 9,'
C9
I.5-D
/3•
/
7
G/. 6
6/.
1.3.0
/.2 °
/,
/
oo7
o '79
C-/. 9
,9-
3.0
3 9
/5
2.
2
/5. 5-
.0
2.1•
o."7
2.22.
G.I
--
,.-
(-4
.3
333
o .,f 7
/.
2,
/7.
/
67
o .
Ao
7
.75'3
~.~./
.0
57
>
o.
c.3 2/
q-. 7
3/1
~5-.
(
Y. e
5-7.
7
5-. '7
55-0
o.
Il.
o
.,
5 .2
f.°
.
f
/
. / F'
'
7.o.G °/o
oe.
1°Ff;t'ffF ,,°/,o //, 5'7
, . o°.
ol
f.
38.
~~.0
938
p.o
3a .o 7S~
39-7
.
9/.,
.o
2. ./
23 .
7
,
/,
t
2
/-,
..
.- 2
o
2
fr
fo/M7
:
#1,,,
/. C
/3.o
/z.
C
0.
0.1t2
ii.
7./
7. 32
o.
0, /G
D
-7. $'r
6. 77
'. //
/7
0, /7
.1-/7
3. /-'
0.
,7
23.
e
/57
/2
3. 6-/
z.3
A3~r461
o2
9K
.002
cl.
c. y fv
2/.(-/
/7.3
022
,. /'Y'
o. 3 .
# 6./
+
o
e
(h-A/-e
o.
f
oo
,
AN-pe
rv"fo pp.80 C - (-)
rr/ 1 5-1 AP
v
(V. ~,v
.
O~0
L/
'
(,v
e.
Ilv
,IS
loq
t'3
/.q/
eo.'f
/eo
I.e
/f
h
o. 31
I,5"" ,.
1/
/e 75j
e3 7
. 37
/.
/o5
/ef
/o .'
,5-
I .'l
qUz
2,o
F
f3. 6
f
/.
/o.
q.
O.
ql.G
/er,
q4V-o
!9.f y
e. /
-/.
2
3 7
G
/2.°
C
.$,
l,b·^637
f>
71
7/
7
Gf 7·
e.~
3.+-736-
3
5-7.
3.7z
q/.z
a·
/C. C~
/ SX.°
/3
o.
tcz
c.e7f
37
{f3
o.
/. o
37.Sz
3o. ?C
3-
~'o.o
a
o.
2/
.2·
ao3/
2 ^. 2
e·C, f
2.
6. C
>-q.~
e6.
0~J~ 33
o~.3
. 2.~'5
7S
o. 3-
oo .,3gg72
g~
0.3
°
80f
o.3
3735'~
So
·.
o,~
O,..z
. '3
cP3/
17.
/
/ C.
ffc
o~..~
· v.V/
'"/~
° 78> o. 15-/
C°
ff
? d~ -~.'
_',c ~..?
2 2. 2
o
o. 371
F
o.7
q 37~r'~
,~, 92'7 /3 ..yW
0.3,
67
6. 2/S2 a... 37e
37.
° /5 ¢
;
5^. C
.5'C
;.1-~..
.~
.c337Cg
7 gf.1
dY.~
.8(//
7'f7
/,¢>o5~?
713
3- o
/./7/37F
7y
. G7
-2
C.
.,L a. /
.
2.$2.
.g
/. 7
jS
°.
co/
o.
33etfX
o 30tf
o
3l/
°
o.
e/2// 9
o.
-y
o.o oog-,73
o.-,<EC
1 .
ti
/.qt
/ /..~
q./ef.ag
7, o
/ 3.
9·r
3g5
o .35"
P. f26
.3GY
g
o.j7f
SS
.
3 r
S. S'
o. $y5 Zf
3. dz
C. >3
°.~
3;
5;
o.C
3. 9ry
c.36t
°. 3ff
2. 3
=
/V/
e/llr.;
3.-"
o .~3
2. 7j>
q
.3oZ
.3 F7
1P . 3 ft
/
o.3~'
o .- 3 S
o
0
.j237;
C. 't'
7./~r'
3fir
2L
b·/
aXS 9
.3SS
n
o
<~o2
/
CAr-Vof
7-
= 3
z-
-
Xv
PIf-e
LA'Ig,'
7
O 'e
3 S e Me
= s,A
II7
dqMP
,'p
V
A
V
4- e
-
"P f
t
-
(V e J)
/ el
Y,
a. 5-
1635
/Gz.
. c4
/6/.¢'
I/"'.5"
G
/ G-t.
1C'
t G-f
I/ y'~r
G;e
A-0.0/•
~,.w
:~ / ¢'
, .sr-/
/16'.
SLf 5~
6,o
/}-. f5
Ir9/I 5-3
f
0
p'
. 513
0. /v7
o. 9/:
&-e
. 4'
I '.t
I ¥1
7.0
/I'
sq.
•
//33
9'. .y'
r
/ 13..¢
f
o..GS
qq9t
- ./ o
.Y"I'
0.
3/
O.
a. 23C
a.
.
o
o. 0/3
c
5. 7
47.3
, . .3
Z~o
o4
l/C.
I q'. 1-
17. it
/7*1
Iq.7
'~
IO.P~/ .(0.7 73
7*
7.77
30
. TI'
?9
-,
'. . o
3. '(.
N/
) ,
3.'r/
3. q/
0X
2-'(4
13.0 /. -2
O.'a.7
o.L;7
Pn,7&
f f/,mrf-
/C
5o. 2
0. ,
t
o.
G. 3
3¥,~
fq
5-a-
O.
70.
3q.7t
I. 7z'
1.
a.
'/e
. o°/.Y3'
el
rel
a. - oe
-
&..ft 0
9. 8
S2.2 ~
3'?7
3o
9,
2-'?
/,f0
~,.
'
0. e5
'71.)
6eq
I.
.
Er
,7q. C'
q.,,
o. / V7
.q./o
e
/'5/5
.5S
I ,
71
)
. ' q
o.
// 5'3.5'
5'.¢'
2 19
l.'-3- f7
l71
/, 1
/.1~ G.
'Z
I'(
-rS
,4v6eR,40
clA'o-"
,A
=
/.
6
p-
I
DPs,5ff e Jo
T6 - 30,
I
'c
A?
s
7/2 d/1 -a
1/
.. ,,,.r)
3. o
/1,,
M
A-t
V
iF,
~.~.r
13.o
r.1/9.
98,2
979/
7 2
C.-7
F5'-.
r
S .F
C3
97 f
. '~3
o. 2 .
.
O
35
~7
f7-91
O.
. 3,5,
f~
°->
o.>L.eo
o.>Xo
o./75 O
"c/
L13C
~TL
0.),oe
'rG.G
/.o
737.7
'13.,7
77
¥37.7
'.t
3z.
37.
3:.(
o7 *X or
o.o.,.
2 9P
o.-v.2
2./,/r
I~,o
2 o. o
O,.,.'
O<
'.f2
I.o z.¥~.
L
((.0
O.
O.
I./,g7/.
q'I a
. /
73
.o.
a.
//1/,I.o-./.sc "f
w7
/Z
go~
o.f73
"· 3S<Y
..z3
.a.
-
7./3
I.
o o~/S
0. 0 7/
c.
o
7
·
.o.
I
.o. es
9
o. 0oe"
o0.
0.o0o]
o
7
'
/ 7,3
3.35'
~.
o.t
0.0c~
o~z
g
;
.
o, /w
a. / oo
ca,o , /
Ct zqq
us;D
17. o
'L
C
. 'a
a.
./ 7
o ./grP
L/
.o °9 a.
I/.o
q&
6.
q.
3e,
, .o
.'
YI,)
'
,,. s' o ?
c.
0.?39
ete<
o. (qo
a. 159
77. g3
/;r . O
o, IL
v. ~O. Gb
o. /'r
o.eqd-r
?
Io. o
o,~.35"
o. 9'7
'.F
/
?
, '
b.'~?
a.6 y
7-L, P/to,/r
rx'66 e
89c~cc
fw/Ar5
CKfo/c#*t
=
/, 2
t
vv/AL.
-
9a86
'L.9{A
71 /;
.'f
V
L/'
^3,
e. t
-1-o
/7V
c
o.'-"
3. 3.1
93.6
13,
. /75,
. ('5
Lz . 5
0./
°Oa.
' /T7i
. '.(-/oo
93. /
'-. 3
.o
0
5 f"
6
e .f
o, -3¥%
0.
L/.
c.2<3
o. /6/
o. ((/
o./70
O. o'7"7
q .a-
el3~~.
t o.
e. L
e'.o c7
tr'
o, o
If
'?, C
o,
o
~,ol
a.oc IV
.oot
3. q
I70
6'.
. 7
O
o. / l(
0.19/
0'e (
6e.
o.
6 e
oo
C
o. f2.l5
-2./
/ °. /
7.3
o,
wL/
.
lz
O
°Si1
-
35-.
39- -?,
23.71
/ ',7
/
0. /.
gj.
2 e, o
o. 1Z '
/2. C
f
/7
I-oIr5
C/-fHcf
Av- jeA6
C'.3-Sq
2
a.
3
3. e(
.I
o 26I
.q'
, f O:1
1.1~
'
o 772
o ." C 7
I,1
oc~
=
p/IA(
f
_
Q1*u,6-f
f z,
.
O
?
ANNPL at pe
fe Tb
c
3,
/
-._rv e's- -. ?.
1i7V
/5e6
I,O
2,
v
,'?
4r9
(
A/l
/
If
I r?
/. o
I. . y
/ &
0,. 371/
!r2.f
0. >7we
2.
/5
/ I¥
/93
I)~
/1
/35
132
II~
J
6.2
/a.q
/9). 9
3.
'7.
3/.o
o
/9.~
9.7
i. .?..
(.L
/1.1
o.~-6
l/.'7.Gt
ab.7
/g
3.f~
29-,
07
3. .§t.o?p.
3<
9.y'
5,~(
'-'Cf
5 3/
9..222.
2. 72
2. 32
r,~?
2.~'
1.70
o. 9
,~.
t7~~
f
. 7.f
¢
I./ ) v,
/1
r
,t'
'.o'('
O.°'/0.0,7
6
2.2 .0
/9.2
I./4
6 , /J
0,
o,
o.q C
. C7/ o.o2 9
.ot
7¥.0
o. '7c
7
0.53
S
0,0
o,5 ,/.
12.
2. ~. .
/~.3
'f32
j./, '(2.oq
e
o.
0.3 97(~5"'
o.a°7
5-.
5-a./
.
_o o.
,o
. q ~3_
~'
a.4
o.
7
7/ 2
8
.
o.3Yt
e z
6.
o(.
/3
0o
0.-32
/(/9. .' V
/32
2.
g
3 o/c5~
(79
I".-
5-o
952° G/f
/ /. /
I 9'.
/2.
o./e'
6'.
O.FZ
0.
( 75.
zf °
74,
o.4f
15-2
0
/4 -
/./
57
0.52/
f f/Crs = 77
/F ccz 27^{v
Avwo6 t
Har
=/
Cro
' - P;
Tj
1R,
r
v
e PP
'P
- 57,- r
:
=
II
/P
5,
4 -?
-.
7/ o/5-'
--
-
f
II.o
97
d
777
' 7.3
97. 5"
.
77./
.f
9
> 37
q5/./
77.
?G. .
0./
95.
¢6./
'73,
'
.y. 9,
'7 .
3
I- 7.
t /
9
e. 772
o.6V c.q
13. o
O 2L'
o.25/
0.
e'-. !
g3.
'./
9Y3.
/.97
/r
. '
I.°2
q.w4f
o·f7P5'
&.G%
o." e1o6
OK'Lo
.5
o.2 9
0. ~. ,'
.~ /3
o
- _
.
. f 7
f
o. 3
.t~ o.z5J ,13
o,
7t
o
°. /r.5
o. r
05"C
-.S
o.24
'2
o,ef
O. i/p
0.
' . '
I V~e' ,.,'.7
-735,
32.//
a5.
.
_
./3y, 7
a-/
0.
o
0
I.o
o.Z-5
o. ' f
1'1. o
o.~
.
I ?.
o.
0.
~.°/3' 7
o,
.0/5
$
.77
.r/2
$'
°.o
/
>7
5?.t
q3.9
/7.8
"5-, 5'
0'.
72.,
7/ . /
fJ vess>-< <sz,9,
,7.*
57. 7
. 3
3 .~
.37
/3.5
-.
/,7
o.2s"
f
. *7
-
c>7Z-
f)-
35
' c
L
4_T
Ivr
5 . Arpp
_ i O Amt
V
4)
v/
_-
_,,f
(L/O
,P
0")
IC
14'3/ev'1
2I. 33
· B
IG7
/GS'
/, O
r'
32
fc
I ~'5"
I~z
V-ro
.
/ I.~-
iat.5'
3/i~-~.s'
7.6SK
&-S
o. ~' q7
I .%5
e. 5"~
o.G'e
o· S/
o. ~o
e~ 67
,~ .4Y,~
?
,.
d~. 5"'
e.
-/
o. C/3
e'.3'~
o.
j.o-
lIst
G°. S
1/
1la
/&/
Iq~'
1qo.'
l/;S
/G
5·
I ¥,'.S5
1Sf2
o/ oeS3?
O,,
O.¢~S'
°.
o.
53
.
1
b. ooY
lq7
/.o
i3,0
I1, °°
3:z. S
'2, .T'.
o. qW
/YIr
I/Y
o. 3 e!<~
/37 </
'f.
9' S
I ,r2.
IL -
.
o.3oG;
0.5-o,~
o. o7
.5
o.L/ JX
s
°·
177
°0, 32
T.
3z.~'
IS/5H
73.
6 f. f
o. 2..e
I,/7
1-333
0o9
/7
e..5or
y-i°
° /5.
2- F:L
ro, · t %/o
o./R
10 r.
~,' .'
S
~
.o 5
. i//
l1<
'
.
0./ S
?
,. d
.;z7 ,.ec
. o3
O5
oo.5/
ff-c
1, o
-.
. f5/r
/7
5t.
(o 39s
3fz
6, o
37 2-Z
7.°
32.
~S'
2-MS
S,
/.
b ..
f. 55"
r
¥y. o
2%.7
o 'A
B.
6ff/
Z 5 crtC
z.
Z- S. °
L7.;-/
o. 5 30
1
o
l0t
o.5-'
o,
S;
, . o/ ,
C
r~ro.5"z7
. 5r S
.52 =
6. qP'
q 7L
=
o. o2
o o, r/L
vo
Oz
Y
o. s/,,
0
~Su/j
ob
rs.o
J°/arsI
.",!2
0.5"
5' ~"
~.
7 y3
2. 5S
o.
~.o32
j
~52
5
3
~ 'K
2./S
r7
o.
53
0.7
R
/,3
+
C'.F2S/
3e
0.7r
O
e . 5r P8w
o. 5 2
o, °°
,
A qppE
IQ
ppuefi
T-i :r q r,) ,
T-?
L/:'c
,
13
9 MP
-7// 7/5-C
.f
(V-rv
lul,
2o4
2L
2 6rL
t
J3o
Lf. ¥'-
2L/.
I
?-q. 7
23 1.7
2
/3/
I*'f.
C.?
7
6,/
/3/. 7
B/
S'tI.
~
72.3
7/.q
607.
ro
7
*2
..
L.~S"
/3.?
2> &
I o'.
/77
0.7
//.
7. 3
o 3r/
1.30
3.
2.g'4
o j
y
0.
.
7?
q.
3 .3 Y
o.73
1 0
O. 037
0. o 3 7
0 3/2f
o0.372
o I79'
o.7'
o.(5'.
6.3q-7
750
O.
75
L,
/7,a°
/ g, O
IZi e
b /3 r
.
O/1D
a6~
°
0.
77
0.
0e
767
0.
770_
0.
o2.
O.
3
0.
.
77
.
'
0.dl
-
0 77'
o0
. 772
, 011
6.,~
o
O.
,
0O3
. 7'7
0. 7C,
/a.O
. os
v.s
r = 7
o
O//
77-
0.. 777o
'7,
-o/.
=
f
. -5o
.
0.7C6/
7f
73
°G0
o 03/
t.
o,
o, //
O. 7.~
/6,
¢7<
.
737
7
0.
O.
i
5~,~
[I. q. , -
8.
e
~72..
153
1, ?
132.
15
/Igw
5,3. 7
Z7
1S3 r
24.'(
!
.
7
'.
2 21.
°
S-
2·3C~7
II~,
o, 7
/
°" -5
~/.
e. 76
0./
I/Soe
O.
o./ f
7
62/ O·e.7gi
o.6GY"
° G 70 0. 7 {
a. 70"
2.C8.
l~: I7
z=,40.
2 q4_
I',
0.
o . Lr~
21^5
-'0 77
2~
5~
o. 75F/
°.. 757f 3
0'.<
T5-r
3.o
o
0.
.1G. 7
2. G
-r
rt
"tf
)
ol,
-
I
Z,e
5; ,
A
/-
LI
_
-.
/I19
'p
0.3?
i
JIY,
I,' f2L
035-3
o,
o0. 33
o.
.Ž.-7
/ 7
o. Z C7
jLr,
La~
/
//'2-
° 3 y4
o. 2.7
o.35S
c0.3>/
7
..3'
O. 02
(i sL/3
i
/9/3.
0'333
i// f93.5
?.
3
3.3
0
o6
0.
357
3
°
?RS
C-
o.
/ f5-
o.
a
3
. 3r
3 5-
1e
°.3s3
(39
3
.
00
3
. o0
13'7
0,.
132.3
/-2L2
//,
-77.
8'-
7
395~-
33.2.
32.
9
2. 7.2-w
27
2-7.5-
22.5j
/572
/ . #L
O~
o/,72.72
.*'q~ .
o./
o.3
7,.yq
3. 7/
2.72.
~
.o .¾q
L,z5 y
/2.t/239•
e.3/S'
o.341
d.33.'
a. BfS2
b.3 Y7
?0.~'a,
l
. O. y3-7
3r
~-/
0.~I290
3a
0. /17
/
<>.
/6
i
5-
2•.
7. 5-'
2.. Y'
I,OF
/. /7q
,'. 3 7
o.35¼
f
0.
0,/2
,7.
b
0.30
s
s
o .. r.
z,o°
-7
7. /
ys
c9
7.~
£1-72
(#0a
3
5-, g-
5- '. 9
lu
7
o. oo 5-
0.30y
'
O -3,.
r
a.
~
3.j
j,<L
TqO
a, 2
1"-
o./'3
2I..
'.777
PoTvrf
- 73
S
(7
rC.4' ec
/ur
o
o2'_
WULq
-
PLe,qAe
f
- 57 e
~.CS)
e
L/
5'~
rl
f M +
¼
.,'_
/5-r0
o. I -
o.I~~
9
- '.
.(t'.°
d.. /R/
0. /31-
Sq.
(
0.
C-,?
/3C
5-3.
5z.
6."/
r
5-..7
5L
5-f.
5/ /
q.
L*7. 3
32. s
2 .C
2,
:2/-. 2
t'-7, ?
?. ,
/r /
537 7l
'e.
·~.1~""
/. G/
,,,
.o .- 7
I.o/3r
o. 2
°' /G Z
f
oe~
O. °
.
005
3o.
o. oe3o
. o
. o
L
5rt
,/yC
c1,0/'77
7
/.0t
I.
b·
O*/; /.°r
0 ^ {S
.*/+</
/.
t+2
',
o·7/F
,,t a. at3
o /fcf
°· 3 %3
o./Ys
o,/99b o./f3
6. 299'
6./95
a.og
r30i
o. 7
32.
(7v
3.
2 ·GC
2.°3
.
S.P
2/24'.
8s'?7
/2.-, z7.135
I.((
. 3.2
I,. o
13.o
6./
3.f'~
/2.7
6- -77
q.
12.e
7
-r~
/e. e
o. 0/ 0
~.~.007-. " o- r
,- . - - C
.
.
c.,3
. (--9
t
q' r.
3~. ?
?.
O. (V3
5b3. 2
5/.
5.0p
67 G
q, 0
(
o.1q
5"3.'7
r3. 5
-. 7
5~.
fi"
02 j
a.
7
o./V7
o. /f
7, o
_
t5.
/tj/L
//5
t>-Ofj
o<7/+7
/l
0,o6
df
o°'f 3
=-G. . r
:i o
.V E-e
F
O¢t-t
-
a
e-
_
& R-
0fe-ga opfE
7rj 55r/
1-v
2;.
_
5,
e4
eA
0;
v
/,
6 ?
-
UI
-P
15-;,
q z.
o,->.:S
,_
'"/ )
I
o.
q /G2
/ CK
v
IG/.
&
r'
9z 3
0.
o/
.
r-
.
. 0r3/
o. o-3
L/y'
1rs
I
o. o,/
/ I-f
o.
o. q Z
'/?
0
L$2
0.1
q,30
c'.
L,3o
r
/
15'
/7
/<g~
G.e
I
o
o.
/7FT. q
/I7
d19.'
7'
/.3-, 4'
/ 0. 4
z
L/~./
I,7?//
3,~.
'z.
'-FI
17. q
iVt. ~
/.3Y'
9*.3
/0.0
I/
o53o
.2.-
o.
`7. .C
.ry.
"
:'-
o. qf
i. 07
. ?
t3.
t5;i6
/
0.C?/
a.zpo
2..
I?. 0
.
~a
33ySH
2
°e.. e7eO
2_3
c7
0.
-
e' 12 P
ILf.0
.e 2
,c
.
4"-
a.q/o
.
. m/ ,
e. //
oo 5
,. C, C
" 2/
o. 3 c/u
O. R M
Cz3G2
L/ J2
/
d . L/ 1;2
C.
.
G4
r L/4
o~s
~39
/7. °
o
/o
o4'c3
o
2d o-
b·
7
/I
5-;7'
e.
(/30
,. f32
773'.~
.7. 7s"
/
f'
. Io"
0.O
.
/ .
I/ O
o.
:C
7~,
.
r
9
S/~y
/ ,rs
C/1ce
AV R
=
G
4f
pRe /Ar
e--a, c, i
-
0 ag
_
I'
q
Cr/1'L
w 1/k
1b =
/
6e
ppR
,
/7
x
: 5-- 7o A
-r -3-
, 7//I,
V
V
(voLSr
'-_
fj
{i/,,,',,~]
0.7
2
/
2 1.7
2
I/ o
q.7
c, 7r'"
27G.7
2 7'2
z.7 . 7
0.
2 G5r.
6'Ž
/7,
'f
/3
6
0
C7
-7 93
0.
,
5-/ .
C.76
5
77
.7•
7 9'/
"'
22 5. -77
23
f2 "
/6
77o
7
C7. 7
2-G°
0
.7./
0.
2L/
o.
z?
0.7.5
0
Ž22 7
2 6."
0.
69,
a G f.
27
bo
3
,,/
OK 7 3'2
O'' ·f/-76
6(/
2 -7. 7
o
0. 777
0·8
'2. '.o
zoq
2
o.3 75"
I.
TO
'.
5'.,'
0.
/
9. o
,
o.,,
"·
o/2
o2/
o/
7
3
.5-15'
29.
2 z3 7
Ilq
2-?.
°
0'.
ff"5?
a>, 7
// q
1/9. 7'
por'75
7,7
7 7
/A
93.5'
qz .
7, e
'
~.
39.5'
32.. 3
73 0
27.5
2//
22.
/g r9
I¥./
2..0
I~.o
~,~
/,aT
. /7.2-
Is, 60
3. ,Sf
3
/5;, 6
O .
0. 75"'
/. 55<
,e '
O . 6o
0.2-
o9. 7,5"
. 7C
o. 76
e
7C
a.
76
0
770
076
o,. 77/
a , o5
o. / -
o. 75S
/2.7'
0 76
2.,7•
2. -
G
_."77/
3. 5
2. 7•
&.G(
yvy3
¥,yqG
y. ~--
2.3/
/. va'
1. /c-C
. 76G7
g.,2.
.077f
'75-7
-.
z
. 5 e'o
-=
V/6C
7/
j
..
'A/"
-
_
_
I
I
#A1oPxosE
fL?
A
J5 r-, o
LEI7
V
/l
14
LI
_-
3, 0
°.4.
I-7
17o
/7/v
17'.
-
.,
M,)
. i'.3
°. 3r-
/3'
oi0 (.5,
e·7
o. 32r
/674'
i
"I'
/6 7-L
IqO
.9??
0364' o'. 3
/6V
i
.35o.~¥
3, ' o
o. s3'
o. o/s-
/6. V
~,.ol
3
.. 03/
o
0'.
O.
/53
I 6(/.
/6-7
/G
. o,/
O.ot
/6'. F
0.Q/
1, 9.
/f
. o
/5.
/,o
o.o'$','
/7. 4C// 05'
/l1¢
G.z
2w?" o97.4'
,
/r
6r.
73.9
"''
S9
S'
3o./
53
z .3
/3-7
, 3
/
.
.
q.
7.?
a. '3
2.9/
- 4'
o. 3
o. 4/3
,2.3/
.7
05-'7
0
oP.
q'
0.F'
/3, '15'
a.
0.
o .8
0. 'v'?4
4/33
/1. 7
t. t"/
3 3C
2..
'
/. 7S/
...~r i
A'5AA -, 7 CA,'sry
Dt
=
f
_
._..
=
Wlgf frebg (ct80vl
9
I - 5'
. ,
T-7
¢-t
-o
V
LI
o
L/
x..
,.
1,.'
5""?
,, _
ro
,,,,
,. o
4
,<v
-7.2
Jra
,4
,~ qV'
qf5
~,,~
e
#f
: 3.
4173
I-
¥qF
2 F,3
13-3
~
I 19
3/ -A
. 3.
,q
;,o
3'z. >
1. '
7.
3
s. q3
/,o
o
qt q ;~
.3 1
0 . ,~'2
i.0.
33
q~'~.
f'vfq
f9
3
e.G3
CZFS
q34
q7z
. ,,;
4/¥,
34eq2
o%
.tj o
/
.410
li.~
/.93' -
q44'
3>
q5
3G5r(33
Lafe
~74
/79
C72
I.~- f
i. ~,
I. /Y'
,
o C
.
f3,
1 t.-~
o. rE
I. s~,
/.~ X
0. eC~
o.~
rl.
,q
,
O.
° ' ..
.*/
/'
~3
3
dClC
°' -/#'
I
/.ze
/. 2 q
G1
/'1~?
Ysr
L/,-f
,. ,¢¢
.~-
'',
.co.q~7af
,q
¥,tz
INY
5^0
f
/.~5
I~'.e
If.~
z'
.*
o
/t3
.$~
.". . ~
I..
1.3'
I.I.33l3
I. ¥.
'ql.
YII
2, _ .. /.7t
a1.
3
f
3vX
/.r
3
/~P
I re
/1Ct
¢t e,,-~
o'.f
= 5/
7-
= z
6 C
,
O
9
i7
l'
V
( M'r)
tf,
(M)
o
23Y1
2j
33 5
232/
o
o,
e"+ l
O. -C
o. 3/
372.
2.3/.
L3z . r<
2.32.
'2/
232
,'o.'-{7o
r
O *SS
_3
2.3/f
o.
~.
2-2.
'.5
7J
2.2.
~
3
0.553
,,.S 4e
22.f
22- 7. 7
2/
.Oa
e. S'-
. 5- S
.
a. ,Y
a0-5
5'2
8'
e.oo
~'
or6
a.~
O'. 5'-'7
~/
2.12.f .~
o
'.
12, 1
'7
17, e'
II/"qr
15".2
22.2
5'
2/7..5"
5
2.
/f
2/?e
27,o2
2/0
/ 7(•r
12.
q.r
,I0g
z-3.3
I.43
tq.¢
IS f
9
/3. 9
I/.Y
7.63
6. 4?
I/0r
. '3
O.5
a- . 7
e.
32.o
.
.5
7. .
3.7
t
1.1•
I. / r
/.
0
L
q2
4'Y/
86
2.
4.
3
~
. o.- f
z ,.
o. 5-1r
3°, °.
o. G'5"
=
C/I
Of
7
,'-
h lf'e' ,-e
3 C.
0
-
-2 /
o, . c"
/7.
0.5~S ?
'2.2.8
f o/A.rr
C",o
1-7,;.
IT 0
2 6O
/a 5 <
o. y'"G
/go
°. 6F 7
0 5- 1
5V
0.
o.
C2
6?
.qf6
f
fL'y
o. (#
e, I ty
O
rf
3'
o
53-5.
e
5
2.3/.
o
.o
fO l
,.
eRlt
.
(AAu
VAe- fD
,d= 57C·CI
T}
1
S; 6 f; e
-
-T
:
s,
-'-~
A
0/3/51
V
As
(f))
(C'a
'ry
1,
L/ '5
r
_
V
i-
-" I1
It. °)
I
1-4
o. 5'f'C
. T/
,4¥z-7
I, Z G
O.
. 7vo
"e ,
I~ . ,
/2
1.2-7
/,.~ 7
1w/
I. /
' V3
1.17
P 7/
.-
o.
o.
/. 'L7
I.I
I./1
2.77
/ .w
I.~,,3
I.
7
I. 7
I.
~r
II27
},
.
V
0./ ,
I./?
0. 7 o
&.
q1-7
qf r
Firi
ll
. -7
I.?.-~"'
13.7
3 3
77:.
v7 If/te
('4.?
L/6
5 .'/
.2
I~',"
/7. O
i/q'.c,
,o.o
0· s%
7
la
,f1c(,
Of
,r.S
9
.<
~-
-,
1q
1:7.¢
'
e.· fU
0/
13,
+
°!/f.7.0
.6.1
o.
.
I/:3 / /
/3?
/3yw
, ,e°
Iq/
l2t
13
Iro
30w
23
/
I.~,'
12-9
2e
2L.e-
.o
a. o3
o. 0/
= F/
'o sr
C/A-oos
(L"-rs)
c 7rtR-
-
PL,g
ffR -i
2 2.
, ,
V
v
A'f
(M)
t,
2.70
0. 7
·27/.7
7'
O, o /5
2.7'
O.e/r
,'.6¢3'
i.
6/
O. G R3
o./a'
zGY
2.63
2.
R
0.o
. G~p
lP
253.7
0.LBo
a.
a, G3
a.T;T
o.
0.Gbq
6.66G9I'. 6F
3,.
o.6
7q/C
o.£. G.t
G
0.
6
C 67
/7'
7
6.q(
6';
Gr
.7?
0.
O.o
f
6 97
7.
. 7'
°
o.7/y
0.e°/9
z.y~O
2 .f
2r.o
O.
7.
3#e o. 7>
3 q.
2 f,
.3. /
.1f*f
lr,7
2,
/ C/fecf
/,. .
7./3
3. 7/
/.)
o.
6.C7
0 7o.
0.e3 2
o
. 03. .
.Gi
3.3
2 .7
2. 70
ff
*
7.dG'
/. ?/
q
0.32 6
e,.
9-r/
g
e 5d.
G
L
C7
. /F
rs
, 6V0O040,0C,',.'Oveg =
O.7
/1.
g
.' ,or = 7
i/ Vt.
I/.,
j3
O.tr
,°,0
2 ,o
2'L
qq..
a.
o.
f/
C. G4/
.
(/i.
o .oo
o 6G
/ C. 7
113
oo b
oo
W',
,
2/3.7
I35-
1:1
-4q I
6.6b~z
237
.7
.~. 15-o77
Z-
o.6F
2I f
5-"
6. /
6 .6 72
2. /63.-,.77
225. 7
2/. 5
o0'
c9, 03c
. 6 °t-
23
Z~7
V
_'P
( 32
e.°o 7
P7qa.
7.
i
5'r
pe.
7.9
~o. 3
,-
-4
-.
-
,. 3~,'
/r
0.
o./c3
C. /,
7o.3
2i
7.
7q.r
71.q
71,.
7,
,·
775
13 9
7',7
a.
77 7
0
/77
O. (79
o,i
0.fV~ 9z'
o./
'r
77,0
7?/
v6. '
.a
5e . oOa"
e.
3C
o0.o27
l/
G
o.. /(67
-
7G. 9
e./ 7
IK7
o.
-77.
7
,. o '
.(qff
-75-, -
7q1
°o./gZ
7y, 7'
77,°
5. L
7. 2
·e
ro
0..If
73•
3. 7
3.7
7'. bE Gp
'',a
6
F·Co
P 2.
f~f
o,((
0.
o /
o/, 6
2. 39 I
'
/ .
-
a7
~'.(Pz..
,
. oo.
oo/
0. ~..t/'
.
0/.
. eo
12.er
O./
oq'
e..213
o.
q/q
s2 °
.
D
3e.
0
0e.>t
,rs
1-3
C.e'
0. / j2
oc>
.o {l'Y
1
23.
.) .27
1,~I
c.
>7
7/ 5-
'/37'?
/ 1/
./PS'
~.17
.17/
o./ t
75"2-
7.
-.
( gq
/ ?o
O.
. / 'c
75. 7
0.007
0,
=
c fICl
y' ]RQ,
3
fPI1/Tr
¢/.AV
CC0
=
. ",T-"
I
i;
W/,e
pR.of
r7b
9/
(C,#rmTp)
6 2.
'
- I
9
'/
V
(v,,.,-)
v
..f
(Ms)
I
7o4/
7-,
"
--
3.o
,. 77
o.o 5b- ' 78"
I
a/-77e
' a7"
777
6qS
/TI 0
/ 5P-
I-72
1 77
/6.o
- 537
1-7'
.3/
o. f5
I. V-/
. 75
,T'
IF. 70
I. IFa
/.dO
i
'.3'
4,
.
a 33
2- 2/
/.
7/
/, -1
1- 7
/ 2.
6 3
1-7
/ 77
I.-'"
i
(-75'
I. 7
/.-2
I
i
1. ?I/
/
O. -c
Ff
1. 7
I. 717
'7'
t
I.
/ 77
/2.
7"r
, f73
7.
/7.
.'
I% °
Z,
G.$r
G7.
3
· I
23w
G3
°
33•
.
.(
//7 3
3.Zr
2- .30
/. 77
1 7'
Me
CfR
.
2 f, o / . f
j
-G
94'
0. I'
I. IT
/.if
o
3-o,
36.7'
7
7/
/. 32
/. 0i3
=eer
-, 7
>3 Cffc Pfs
,9
#
4/q
r
irPi pp B(i. El )
r;d = 62. '<
2t~
0
p
xI- _ ~,
( vu e )
,
-
f
v
_-
-7-
/.o
2.0
G36
C 3C
G3 Y
C3
6e5
G 30
63 o'
G/
-I-
/3
/ ~'8
.1-7
/7f
I )G
/<.
7, 3
/. 7
I/7
1 72
. 'f
1.
eo 2
cl'.OW
= /. /Ip
f
. ~?,,,
1- / '
620o
1. 3
/.'
6 7
/
o.
,,4L
eo
1.75
/
5-q
56'6 7
6/z.
6/ >
6 Oi
v·
}-
7/
2C00
/,?
/7-7
. o
2..("'
c/.
.*
II. ot
lq
1.
S70
.
4{5·
L
3S.3
,o·
11.7
1.7l
17.3
/
/3. 8
5F Z'
*1-/.3.·
73
I,
07
~
/1 7
I.
7
I.
/ 7
2 ..
I. fe.ra
.
rz G5
1.
$-2
I./ ¥i'G°
I. ¢ -
2g..
,-
/- 77
.
,o,~t
-
=
/v*'eRAf
-7 7
I
2,
2/',. ''g
I.7°
' 3Z
Ce. q~n
.· 7/
I. /
'
./
7r
T1,= 662. c
~r, :
6,
e
/9
V
('
1f
.f
-r5)
fJ65'
-
14 /
Af
/4' -
'(ml)
1/
3/8
3/7
0'7
.
3/'
lf o
3/ ?. 7
3 /3. 7
3/2.
31z-
3/2.7
-31~.
/
/I5',
°
33/./1. 771
/G, o
3/?
3.',
3"e
30
3 7. 7
17
o. 73
732
,- 7CK1
aYL
0.o/
.o/7
0.
0
.a9,
o. 7tf
0.7r2
309.7
3") .7
33eo
2
233.7
73i.7
7
2- G
-e/
a
/3 . 7
q2. ~(
13. 5
t,~
/.3/
-3
S-
33./
.
I.. oq.
'
IL·
1o3
7
4
o.1
.'7
/1.7
/. 75
3 .59
2 .C
I. 'iS
o. a, .
,5
/2.
32./
.
. f-
.
a
o
3!
073
.73
0.73
0.o 73
r
6.
e 732
0. 73e
o./q
o. /O'
0.o 'Y
r
4 v O's
f7
°
O.
o. 7Y(
. 7'.¢
7
a.
a. 79'o
a. 74/v
O. 79'Y
. 72
@
73r
0.
..72
'7I39'7
0.
737
0.
6. 7//
3/2.7
3/I
o. e C/
O- 73
o. 702.
3/3. 7
'3/3
3/o
G.'
3/ 7
Ccj4c '
ot#r
cO
ve
Al
_
,
/
TjLL
-
fL. a
'fpod
-7- _: 5o
fS,
If 0 C
Thy
c e2..A
-g~
V
(s..rs
.a
/'.
LI
_4
,_
(mi)
q. g
q C.f
)2 .
757;2
' .7
o.
0/2.
,. .
/ 5
O
9.
0.
/
g;-
o
e·
,
/
9
2/ ?
)/
. 22.
e /q
ohI0,
0. 22.0
'.o
/
c.
e..2/9
2.lr
q3.S
F'. 3
°.
.o3 /
072/
. / eZ
~,')°2
95-. 7
f3 r
.o~1
/ 73
/ 7
o./~r7
0.
e.
D/7
O
e.
//
/
1. °°3
/
e. . 22/.. /
>
5.Z
o. / 7
0,222.
.oo
o,. oo
° 2
92. 7
OZ.
0/
9 .2
2.
q, .
q0.
9W.,
7. ?'
o.
.
I7. o
o. '< 3
/7.e
0.
2..
3.o
2p.·f.2 2
I0·
?,o~
.22 .~
g
0.o.><f
.222
2..o
0.2.39
3.0
1
.559/.,
P 3 I5
3 9e
23. /
a
/ ~S5
,3!_
S03
I3.
I'. 7
i.
°.2/
S'.,f
e 2./
q.0.
0-2/
2. 3'?
e
o. 21
0I
A
e
.5-'
..o
I
o.
,2.
Iz
2.I..°3
/79
3 ','
d
6.'4
d
1.3-,
0. 135
D /-7
e'w/G
0 .,2 g
C
'41
6.
. e
50I
2-3
3
. 2-4K
rP/I7
2 /
3
0.
c
e
_
.
2..
-
C 1 cpct
7
9'
p/AI,
A YC JM cf1/6
= . 7 ,'
Cjds7W _ rL Ul
-T
= C . 6 C
-t7
-7-
PR-B
=
= -
,
S
v'
'
,e
37 r
0 9
375
-373
7
13, o
rt-
w.7'3
55
O. 35. 7/21-
37o
37/
3.375
73
37/
37e
3G
o. /.R-
O. 7Z
4. 8'~
°, /32
o.t' )
O. l 'q2
. ,,/
37
3ar
3aG
oo..~'7..
.72
3G;
36
r'-
b. ~'~,~
°. 7~'[T
36s
57,,
3 8°
3 7/
R N3
4. o
r/
37
. SS3
¢c .
°*
° 97r
0.
O. oa_ ~
.
3G
. g~re
o. o3
O
, oOf
<goe
. ezs_
3 C qr
35"'3
3
7.0
3°
3 G 7,
33 C'2
c o
1,
O,, g
5-
3~S
3C5
3LL
fi
~,7./
3
F
2./.~
I/1f
,e
/7
6.57F
° /Sr'.5q7~
. e
.4
2 f.°
o0q4f
3
°-SC
oe
L~
X7rff"
/ - 3
e. ';2
2I /.4
6.2
/~, 9I
?-'?
°.. l,7
;7.
. g/f
cf.
3.¢/
Ci'.
3/3,f+¢./C~
O
.16.
9,
5; 77
,i. ~'~
3.b
. 9t/
I. 7e
I ,o
o.
e r~,
/ r
°-3 'r
e. 9
. 8qo
I·
Sr
o. 70 %
. 4q/ v· M13
0°
O·Cb 3
0
/3
7
p e/rrs=
22
c~/e
A 6bA
17_,e
oS
o,
o= 5o,,o
9{
oo.8. /5
o
3 o.~
s'.
9;
Fo%
o, qoo
2°8 o
2
1'. 21
o
o
fr7.
I,o~
33
/G.e
O.
3rsv
333rf7
f3
° z)7
e~ qe,
a. ¢oa
/!,
G.o
o'
° os
c,4/
.,,
=
e.,
"-
Pood$ (Se
W/,r
T1 =
5, /
1, :
, $
Iv
.57
v
7
4
J?
(V--75
-4,
ill
OP.e
/.93
2. ?3
/, 00
13V
34'
33 9
/2 .F $'
I. g'Ls
3f o
33
339
33
331
337
I\· O
ii.oo
.
,,o?,
I.CI
I. '9-
/. 'f/
Ipr
° t33
o. 3 "f
O.,~'(
O· 4f
I)..
33/1. -
o. 5'~f
o.Gq3
l.o/
d $75'
a.3r7
/·
30,
3310e
/7
3325
.o7~X
0.> C7
332
3-33
1
3"
3r e
.
)
-3 V -23 Y/
l·
zq
a. 7/
13 , . .~,/~
f3e
317
3/7
3/3
0
"I
I.Pz
o. / ~1t
o ./
. 15"*
/,02
3 //
Lf~
o
y
IoL
O. ~'~
o. o/
a. q 3
o9. J
I ¥o
7
302
3//
3tt
24t
3 "3
.oZe
1.02
I.
o.o7
O`L j
o.
/. "2
O. ?f.
ot'
a . ta
/.td
o.qgf?
1, a
zy
1.03
/6 z
tr f
/3)
/37
lC3
o. ~ 9 f
/·3
o.
a. qg~
Qfg
O . q7 5
Ily
r g
1/7
-q. 1
. ,
2,4,r
7,
O. /a t
1,oz
z7f
&,,
. -/
a a3Q
/.
!o
77
/·
>.O.a;r
03
.c
3
,,
VC~
oYk
"3
/·03
/.03
2
.-.qt.It7
2SC.
7
7$
/ .3
.99
2,
.. cz.
/b
73,
'
~. ,e-~
,.
f./ f3
/2.3
,
q.7:'9
,
. /.· °e4
7~
,E S.'or
wL
e
P,I. , e s~
/. o
I'
3°
o
<,
PPeXr
5-
/,.q
evI
I
/ .0
=
A,/Oxho3. -
r
5/
Cf#cf
,
=
clol~w~l
°
: - e. S
Puy fo
3
,sA
;--
v
(£.-or5
q7
(t,)
2.00
tq.
t qq
/09.
.!
V
,, 7-
,
-e_
¢V'
e
8.
/3.7
/. 2
/3.3
/e '
9'
3.3
7 q~ .
,
1.37
9z
/ 7
/"7
(97.9'
,9 7.9
17I
I '7q
I
I q.
7
-.
1.-Y'7 '.·
3. as/
Ot.S q
,.~e
a/. .2Y 1-7 O.
1.
3.9
o/ .37.(U
'o
Iqq.
93C.V
l/ Is7f
I 92
I qZ
3.0
I
i/go
.bG
c
/.
9L3
e.3 /g
.
/'
1-3 2o
e7.
q
/
I.q
/ 1'7
o.
e 3,3q
o.q
II/7¢,
9" .'
T7'9'
/ 70. £7V
Ir
I T
/ ¢ *"
.
3
' 2^ 230
tiR .
_0
° /23
ac2Y3
3
° l 3o
o/ .33
,o a.ooY/
/L$
Oo.3q,7
'
/77
170.
172.
I/I 7'°
7.1
90
2
.0.
y
a.
2,
.
'
-7
/1/77'.
7 9'
6/
2 °-
q7
3/
.3L/5
7'
75
Ibe,.qy
o.
o-zq
.qq4o
o.L
°/7
/
0. ¢
S
.
O.
,v. 4q~
f/ ?
'.
O
0.qq
o
ff¥5
o. g5q5
wv7 a. q -7G
o.. 9 'o ~ L/j
~ o.L7L.
e eo
'° 3
oo/
63C
20.¢'
5"z.
r
179
3ef.
4
o° 3o
°
/
/l - °·o.°oo
.q7 . a.qyz
"
0.713
173
o
03
.o. e.oo
qe
c, .
° q3
a
8
7o.
-/2t/
.
qy.
fL
'
3G.7
S
3o.
2, 3
9.4/
.
2.
e.
3 0.e
0.c/77
o.
q t7s
0.~
o/rs
v rA,
r
c/,,#Al
- / 7
C
rR -f
r
=
V
= 57
V
(~' lr3
2.0
f'4Ce
sl
fe
t-fe
"e
3/
v
-If
/<.°
,.I C
,-t
IMAf
/ mh)
2J
./,
I/.
/. 32 7
2/-.5
2. /5"
2/3
z/3
-I13.
3. 5-
2 b3
2. /
I
2. (,
0 .. f-
2/'
9.o
*
0. r,/
0./7
0. 'y'
/,o
0 . 5 57
d.~2
,. G/7~..qz
*.52
o,,f~.
o.,.y3-Fc/
o.~/
P.33 9
00.-2
. r3
5.1.
eI
. 1 79
0.0
O
532
0 fZ
3°f/
1'
0..
o. oe
'
o.
o.2oq'~
3
2/0
z Tr
2.0eq
Ž10
2
o.
. ---o
/0.
O. -f-'
7
0. 0 S.
° 5-'
6. SS3
0.5r'
207T
q.5'
2. or
0.33 SŽ
05-3
2
2.04'
7.0
t'
/ 97
/g3
16C
o. 5~- C
I
/-,.
76. 3
94. li
I7.
s
5
6. 3'
c,.
5G.>,
9. o
,. 7.
>..4..?
2.
13.
7
Iq.1
11.7
7. C
6.0 7
'. qs3
,.6'
2.22
II
°t
0.
e
5
.ao
S
^ ocP
.03
-&L
O
a.
S; ·/
o .5-f2.
22.0
LC"
b
0.-71
O.
r
eo .yvrs
.57-,
0.07
0o.5
.'~
2..
o.~'"
o.-"' '1
0.5-6
~o
.0
3o.. ? 0·
..5-72.
.5rF oTr
O.·
.,
,.;
.2
2
Z. ¢
6'
o
o
0
O.00?
. c o
I , O/oO7. f- .
I/X
, f
o' .
0.60
.Z . 5
/2"
O.oc 0(0
- Y'"
2.03
2
0.
0.
O..s
.-4,
'
-,.?
3. · 2'
2 77
2.3 cpo
4v "OAO
f''-tf r
CVAA
ctg
O-
O'
o.
;
Wig
fAe
(c4rfof)
3z
I
r
='A
r,.
,°
I
v
(vj-rS
3. ,
.
f
VI
A -
,
P
t
f
'-
(mo)
35
-
. .°
3 -f7
:~.0
3r5
2~ 7 'I
1,, ,
2 ,o/
1.o0
o Id
o.LZ
d.el*. .
/,.f
/0/oo
/. (3
a. ¶25
I, l
d. ("
O. 9
2
,~G2
~.~.
/I 0
Ir"
.
, .8"'~.
I. o).F
3. '
9/
3.
3P'
q.o
35
-3 5-3
3r3
33
3
3-~3r2iq
3
3(o
35/
33-
3q/
33?
33 3'
Iq,,
&/,o3
S
.
-
33
7F
0.3/
. o'7
3/2.
3-33.- 1
3 3
2
,
33.3
3 q
30
3/a
f/
o
9
.,°) /,2f ,. 03
I'iO
I
.l
. t,~, fI.5
03 o.0°f
° f
bG20,^
c1 I /,Y
.°
Io,
0CC
2
72
,7e'.'-
o. e
La .y
2 g~¥
'(13 3
(. '13
22 ,3.
q.
.7.
°(o
f. o.. ~t
qa
2't(. /I/°, $~..
."
. 0 0/<
o
I 4o"
o
to>.S
o.
//,
.'
0.
(Jo
1 1·.
u. (~Y
3 7:3
Y
3 /
33,qV
/.
w'3
/I0
.~'
I.qo
.-65
?a
I of· fw
i/.
2.~.o
4.e'
,o~
Sr
/
I/t'4
CncYPlur
I,, o
o.e.
//,e
Ct.
e
,°
S
.
,Oo
.Io
i
I
I
WTL
- -
f~e
fL/AA'
i
I
33
T
,
,O
I
a/r 15 6
I
V
(o,,LrS)
3.o
.,'f
I
A~J,-
II,"),
73. /
73 .2
73./
7 .w
71.
7a.
72-C
0
72.-.
/
af
72/
e
7/
/3.
332
r
.
. /9'SF
o. t7,l
a
I%
"/ '
7'.3
7/
-7.,. .3
7>
>
79.
a .5/
7/
O.3c3f'
0.2 f e
. fbt87
°
o.
"(
O./ 7
7/'t>
7. 9
,'6
0. )/l
o. °/ /
7
, 06.
0.o/7
0. 03 C
0
7'~.
O. [/t~
.o.t,/1~"c
x
o.ox
W.
,,,8
oo./'S
./ ,l,,~
O.
( S
./C° 6.(g.
6o 15
6. 73 ,
8
0.
o
o.,
o/7
~0
'7-"
oo.
/(.
C 7.'
. 0Z
6.7
SC..
5.
LC
C31
Go. 8'
0
/ 7 0°
a.o03f
. oo.
o.o · !
o./2
o./q
/(2
6. .(q3
.G
7
3.3
2. ..
3. 57
2.G~
I . 9[
1. 7f
0./ 7
0, (5'
o. (r
0. 11r-5
i,2..
0.
/-p
0.,
o~
o-
t2.
°
z>
22. °
I,5~'5'-g
o./
0.
tq-,.
0.1 2
/,
B, os-i
Gc'.
e
0./92
/ Ie
7. 8
7;5s
e. /o
°"./?/¶/
0. /
2.o
2-1. o
C. 5/
o.
o, /3
o0.1 re'
6
°'./
0.
/.ci. F
7
O. 6''
otrb
d.f.)
4.
/9e
,.19b
-a., . ooe
eI .d,
d.
0o7 ,
. oo 3
3 .
e.
'0/-
G9 9
3'd...o
oa.Fr.
./. °o,°(q3
5"
o/7o
0
o. /o3
,
30.
e. { E?
0
o,
0 2
e
O.
o .
2
7
2 o9
et
,
A 6
15 ees 4 >- c-,,
C
c/x-a
"-.
vet
&
= 0. e,,;:
W/t,* ep ^
iAf
3q
= 3e.e °C
Iv~
;3o 0
7s~y
T}"
~
-.,
-,,
7/7/5
/'f)
v
(uo,l
/
Yt-
'. P
c".)
137
o7,e
13-7
135"
13
133
o.(
1·.7
7zsBg
r 7
1 3t e
I z . 'Y
-3 .Y
13
/~.
v.qz,
Jv.
~,"f "'
5e.q.3 2.
.
e.
-
oa ef'".7.
Y
/. Iz
1.5-~3
I. 3//
1. /'75' ~'
.
o,/e
13.6
/. ~5'-,
a.Ct
,.oz.oC>78e
"fo '. fz y
12
7. cf
/12S,
113~
I.~. '
e .q3'y~
e. 7 'f >
1.3. y
/11(.,Iq
a. /S' 2
22.
o. - ¢'
'+/
o. 5/ 3
c.c-/
II.
/32
/Itq
3 1
/e
/
12.5"
3 Of
Io 7 .
019. Y
-7 7·5-
o.F6.
3
17.
3,
o
2 '. ,
2.. ° /2
35I.
.~7'"
25.
·
',o3
77. 5
I/f.
qz.I
13. 5
Ia
/3
7,¥ 97
7y
V-3/2
o3 3/
37. '
°
I~,e
3 3.
/96.
Iw S
o c/.
o
3. /
/;
f,6a
16..
/1, e
o, it.7C
/..V
63
. z7r o.¥/
q/~.7
o. '41e,
G. -F"
R
ff7
ZZO
2_°.e
f
e
3.Ctr
3.
qfo ,/
,/~
o.V.6
qr z
o. q/v3
o. o70
0.0¢o
s, e cy~
0. o3 6
e. o3
D,?
., 35
/7i.7.
a 3d.
"3
a·L
3 ,.t o.~
. ~/
eS4'
. 4't
/
ac/zo. 6f 56-"
O6·.3
°I C
, <8 +
'7t~
> ;;
.t. f /-F
'. ¢31/
23.?
'.
5"
So
.
° /2
gY
o.,/W
S7 r
2. 3
7 o
o.lq63
. /72
o. /WC
o. //
'47.
'z.
G .~ 9
'3.
e','z ~-iY
,~"-'
e. ow
.'a. q~3/
$37
.o~
7e a·ct3G
°.
O.c/73
' O
o. q 3 'o
a~q,
0.q35"o
o.
',
o2.
Z
o . ow
. 'f3
o . qf3 Y
O. y
o. qS-'y
o. q/
o. q~-3
~~~z.
"z.
c.
O
z/ ,
O o/ '.
o, oo' 7
¥5-'
O Q2
2 c', o_ a.
2..0.
2.p,
o.
oa,
'ts-'y"
/
2. Ed
2.t/
.
?5
v. 3
`~ 1,/"(r
=
-2-Y
4- Y 0 RA
r
P/NS
-A.
- /
-,,q
= ,
aL
-97/
I
iOPO F
c g'ra - PCt/vg
I
I
I
.v
= ;,
E~~~~~7
-,P
/.1~
(Vo,-r)
'-t
~
-
-
(m,)
/.l·J7
7/ o
/·.
*·
a.x63
2
. 737r
70q.
s
~77.
7G.~.
7567
7;
77,
c7 /7
5o
.
0.205
-79; 9~
7fr Y
c(6./
'41,/
I7. 3
.
7 2.
.
.
23.
7. o
/.
. 9 '!{
..°.ot
'
fof
o.
.. .2.r
~'of5
.~,y 7
o.o/ '
okjck p/o/A
G o . z.-
e. o o
-
2-/.a
I.q.$
(.J
- 2 .e
.
<
.
..
0.3/
-5
¢
oG
239
36.
e,.L -?
o/lr. .g; ./3
0
o.~.
3 7/
7<2.3
/
-f,.
37zZ
9. 3,.
/32
1.
o.·.6
~..~.,.
/,I G
°.°-°"
o.
2qr' .o,.o
o.
//
.'.o°
o.l/o
°
2
4'c·.l7 ,.><f
9-7.5-
9.3-'
,-/5I$/
/
0.2',
V.
,3
7Z.7
72//
do
/.S
/ 72
'
o1
a ,,.3/
-,
77 r
7.. q
o.*
90
.7 .~q
7 4K?
3.0
/
/,"F
7d.4
/
:2.2
/71
;r0 g
7 '
05
I. q 7
//. e
I,o"/
2.0
.,
/3.
p
0.2.
G,-
0..1
7.
I "o
7 o
l.y
~1
c5. 9-
2 .l
, 2C
7.23
6.
3~.35-r
232
iY
C/. : S5
.q
3.Q
f
2 .S9
2
O.L
.Sf
2..1/
g.o
o
o..° S
OOJ
D15
?l1204
T~ = 3c.
e
'C
36
/S 7
v/
f
, _,
/.>
o
td 1,
f7
7-rrf
F7. .#
C
o. L
/
7.3
.
/3-°
75, 3
75-, C
75/,7
570.6a1
3-3 g
7.
7 -2
7
7 £
F.C
7
t-,
.
.
o. ,o!
o..'. / ~'
oe /SLf
c~. /7
6
. oe~
,,.
7
S. _ cK
o.
z P/
).
.°S~
a2. f
27
L c4./
17.0o
ZL
Q
o.~
. o15-el
.Z~
.
f~7
~,
. 2/a/3/
¢.
c.>.
. 2- 7
~.
o 7..'
Z2"f
~o23/
. ~ 3°/
o.-/~
z/. ?
/SZe
S.
o,2~ ~
. 7k
I, e
. a;
7. fF
5,3/
L',Fl
v
a.
o /8 ,
0.60 o5e`7
?
( . ?;
.,r 3
°. 23
w
o. 227
e
;
/ c/,
2 .°
0 2,30
d wjB
y*
.2 f2
z
sO5
o.o,°'&,~ tf
e oo
O.e/
o r3
D2
IS 6
3.~
Ses.
Y?. 3v
75
~./ 6/
,a
O2-77
/6.o
/
3 /.
2 . 7T
rK
e. 2'
f7
f
C2-
o q
CG/
e.
C.
3/, o
l.
5
O'
~. ,
,¥, o
3.
35.g7
.7. -~
o- 22'4
0./ 5
e'. / -e
7e./
7
57
3
.z>
C 2.1.
of
a.
se.s
O.
a. 2' 3
o~ oe 6
7.5
$/.
-7e·...;
o
a
_ o.
//f e
765
o.
-w
3fW
o. e 83
I~ .
73 '/
73.
72./
¢'
'
o7-7) e lo3
76 .5"
1>-.5'
73. c
73 <
,
1.3~f
e, -~. ~
e./3Z,
73-,
7~/5. o"
7~,~
7. °
o.1 2 a
e .Yf 3
I7. 1' F
7G
77
/.
1g 4
. ~z,7./
o.,.t'?
7, e
..
Ob~
.
. 7
7~,
7G.
.
e
/,
/
~7q. y
7
t/,
,'
I,7¢"
5/t ~'4L
S
%/,
~/.z
-7o.
o./
'27.
7g .2
7-,7
71.
-- f
, -;^
g.aoo
0,
e
5"
Y
w
° 33
L/.
3. 3/
3I,~
2. 84
3 .-c
./7
.re
o w,
3Y
X
9,
o
S
o,>
ao
PO/rs
'2- q
=
C
s
C
fl,,vrjS
yiFl-Sk
6rep
/t7"1,/9 F
=:
°
3 7-
3ak,PP-5 (
T-b
J.
3
"Io~
37
'C
5--
r tTO
'7/715-W
V
/o
1'
..
'f
(1;
ea.a
I s/
1/5-
I
I.
/5-/. V
~q
.7
. 7,
/ ' f .,
iL17
lq
/I /
IlI. o
. J
/q3. 9
/ q3
/ ,.4/
137.9
1//5
5/.a 3.0I3
3.a
/
3'
o.V/
7.7o
~q+
o. 1/
6. qo
!.,5
(. 3
,.d. q-
0/
e.'7
w
I.3.G3
9.
31
L.
/.5'"5(
'7
..o
a.92
2.1
0,-
3V''.
0 9'9
5-
e.9."
yo
13(Y 1-3 .
(1
I~'
// 339
4e
/ 3F
/3/
0. 9
1-7,3S
.
132
7`7
a..
-o I. 7et
op
. ' 7 a. l-7
°O.
/3/.
/3'
I1,
I-
.
/a.
G.7
-.3.
I Ia.
i.
o I7r~
c q. ' a.
e. c
*7~
o.
tq a.. q/3
4:7
o.3~1
e.
o.t(-~
o. c~ 7..O
___
7 o9
/2. ¢
I/. r'
/
%1. 7
o
9;o. /
13./
56 .t
/2
.
, /t ~"
6 a,.° ry
-7.
3.
O.I3
y-a. ;~
5
Cf 0. / F, ,, .
2 .o
. fse
o
. 4 7<
r
1 7. 5
,
~,/
G
/s. 9
f o/sr =
4
as'
CHtc
AJPAL2hCK~~~=
L
,-
/ .3
17./
7.
/ -,
'.3.3
0.
o.
0. qyge
ev..y 'r d.
y¢'3 o a.
'
a. qq
q/
0. C15°."
/ffq
f3c
a.
/'
o. Y7 /O.' 43 7
4O.f
Or
Pe/=r
_
.6z<G
VV,9,
p'4e0
-"C0
I-
=
: ,
/4
T =,
v
91135;
A.f
(V -
)-
w4.'
.
tf 0)
/. °
qjo, 2.
h, a
ff,
o
/
,a.)...
qo~
/. 2
O- /1
/.¥/
-
o./It
a, /I
o. 97e
3q.-'7
2.° ~.
3q.~
y34. ~,
5
/Z,, 0
2*
o. //3
/It
o.. /t3
a. t/(
3 0-3
3, 3
7.o
339=
-7.
o. 3L
ol os
13.0
. t3
a.
5, o
Y 37f
37.
3.
3 6. 3T,
. o ~r .
,
,o~, ot
.77
0O
'2 c/f 4
/q.q
I/.5'
/.7 8/S9
1,.T
o.
o. i/7
7. tl~
({
O.
H1t
O.
o.
0X(/
O. 0/ r~
y2.-A,o
1. G,
'~.f o
p.
00
OX°^G
,,. (iS't
O ,S
2.
.
I/!
.O.
/z.
o .//.9
O.
O ,Oo/
O.O// O/
O. ~/y
7.II
9 6e
{ . Gs
,. It~
o.e fl
Io.C
5;, 49
qq
5./f
0.."·/2I.
O..
0..
s/,
O
e
0.
(F·
o~28
oL .
/E
o
4·lj
OB;
3s. Gg
3, o
2. f i
'3./
-1. 77
-,. r',
Z /
/. ,'-
o, o Lf
o. (~
G. kO
G, 35
/o, o
o, o 4/?
O. (r
a. //
b.tty
. /12~
/3s1o
qo
0.
o, °~
o7(
o. /t
/ r,a
20.3
//1.~
ff.~
I¢,¢y
I~..q/
7,c
fl.
. 0,/
~ e. ~
'7,
lao7
I ', o.
o...oey'
70o
o(t~
a
3/
O. 2/
O. tS
S
S
3 r
fe
6.>
G. e
t/¢
iq ao,oae.ofef
¢-f a. N1
I~
O t{;
3G.3
3
dfr
O. ~fl
3~
172f
7t
o.Gql
O. It
3 7.
7.
3833
.a. qr8~
~r
a.if'
i. aqf
3 T4
I. Iq
O.ft'
.·
39 3
3jq.q
7.9
I. 73
li
e. /
!.
_'
I 1qf
AJRc
)9
_ CRgAf
=
g
a
=
-7
3q
( '9 vol
PA-10
1v I 9
'-
37
Ar1
/*
"I
t
r4
( t@)
t r)
1-7/
/-f
_1
.
r
* 7G
X f' o.o~5-3?/ odt5
I O.II(5
e.5
.S'
/66 s.
Is
/
/1'
/G
I'~
..x
-" /67.S
16.5
/ G'7
I 3
I&/
t Ge1
5>017
·i
o S//I5M6
55'
13Z
/lq
/ qf0
o'
f.7,
36 5
.,3.~.
'.3
S
I, 2
°° r-'q S. ,. -TI,
. "
9/
q.qo
>.-'/
7.q fY
7. '1
Lf7.C
p.-/
o,. of,te.CCk
L-,1'~
e.
3. /3
2. 7
,6
o,o_
o ,o q' c
d.. . .
o, ./
v·-SS
0._/S
' ..
.
°-·s'
P I {.W /
o. ~
0.< 0.<Y 7/t°
>,oo.53 G
~'K5~r'~
d-r6 0.fif .. ° 7
o.<ft
/2. T
0.
·(1'
. (o2
~,o,.F4-/7."°,
I.
3 /'
Iq,o
'739'
,
o.f7
186@
,
. -hf
S
°. 4f<(6.
..
Jr,
II~.~
'7. '
°5-
. 4-f
q3. g
21
.
°
c ,99/f
ITr,o
L'q.
24.y
9!
,o c..
7o.
6.
c.
o. StO
.- ..fL4. 6
e9.q
*5
,-ty
i/2
.'e. '
7 Q. G
I , II
S
o°Qo
-3
.Y~.
6. . 3c
I'.,
b.-, 7
o
G. 5g°
O ,'
,/'."
7. /
,oe
Iq
I1.
'
I ~r~.~"
/ Cs'/ 6
L~~~,
3 I.0-/2t1 G'
I.q3,
"o7
./
, o0.3
5'
/sl
i;e.S'
/ 5',
.
/
0,
/°°d.52/
o. G/3 o .
/ G61.5
2.0
2 'o
.s
.
o. 9
0
q
507
e' A.
tF
7,~,o 4'fB
,*, A...-~(/t /
"
-~ ". ' ''
=
l / pe
P
o ( c#
/t
= 3
T
-Z
o0
'G
-= ;, e
,-A t
)e
.o
Im)
/ S0
I va
I~
17f
2.o
/77
/77
I 7x'
/7317
3. e
.'f
,
/7/
/7 1
.f
l/
,
. 6"le,,.s
/ %"o.
/ 7
e.7y
S
b
/' ',~
1
,O-I
¥
1e.
7 .5e
/73.5
i
17o.
17/. 5'
/r/
,3c . 5'
lS, o
w.5-
0.o7.13
o3
°
,/~'
,· "7
..a.I1'~
7S 7..3
fW
6
. -~
o.
o'i2 '
o.3>v?
o. T 2 c
. 93 5'"
0-5L
0.~ /
T
. o~
o2sy
e.Z
=
° {7
0.
17.~.
c C -3/
3(
o.
.'30e
p.,j/r
o*S32
o. '4/
a, Y 33
,,o
.'/. .' "
5>ro7 6 @ 3 7
7-.3
I ?."
G, 37
qa.
o. :/3
. 53
~ ~l , r
~.
a
~,,/
C/
o.
o . ool~
86
.o~eo we
36
o,
o,~-e
(; f,.
o/
v
<f, ot
*
1./
/7. Y
XG
t/
29,
a .5
;
e
/ s
0. 5;Gu
. S ff
0O6ff
13.1/
/7z.
1-,~
3.q G
t
. °/ 3
O. o/
e.
LE , °
/oI.d
/. s
s.~
o.B+z/
I "y'
L
I· /.
2 .23
FZI
6f/3
.
37-f
I q.
O.
o ./'
. 5
/734.
I
,-
3rY,l
¥,r.
3.77
F.l/
.
e.,
't',. z
5"7. T
. F
G. 2
2. 3
11.5-/
o.2
o.
.
I/a
7"
7. q~
70
-~. q3.
5-
0/5)~
I ,"
1M9
/33
'°'
o
:. 7
.01
V qI~z °,
e 55/"
.
o. C
17
/7e
IZ
I~
l6°
/I/ lf3
rs
1
o. -T
3. -/ (/
I Pw .
3C
eI
. 50-
'~
.
~~.
0.
-3/L
I
I
i
e
,. 5 e
3.
1.7. ,~
/7s
, . 5r.
41
/r
= C7,-,
-Or7-J
3
C
Ff f '
/S s/OeS A
/
Sr
:r=
-,
y
7"
-
JT
= 3
C
#Ec f'
p.
I=16. e-
1v
ovrs)
( C, e-rs
s;
4/
e
i57
A
1/
,A y.
,.
_
14/
0`_
( tw,)
°. 3
1/°t3
9
L/,<j
/8.O
1/
/to
IoFb
I.o
d
/iSS
3j5- o 73,
Y··
3. 2o
11. 3
14. srI
.3 71
3
/I/o
/e.~-.
&
2..
/. /i/Z51Y5'
<
Iob.~
I.
lOb.3
I's
idOy..
7·3
/°
IO3.
/o(/
e6. r
/ 5.
C,.
o~. /,/"5-7U
Irq.$
(o.3
7'
· o~d
12
G/
3 -2e-~e. 70'e
o. 3o
o . C 7¢ C;
. 3e
o/-3'-/
Lf 5 6
0.37j
Iato
o.
,'eo
O. go>..
0. 17trir
o .o o6
0.o
."
3O
0/.j
4.
eaz
/6.
93
/e
0,7
P{
. , 3~*
{0
y·
4·, e
.88Ie
/ . /7
9.S
SS.T
7(
I<te
I1r,
P. 9o
o*.3e6
. /p"
O. 3 °7
e
egc.1gg
O.
o5
e. 3
~. 3 "o '
o
o. oilf
~.1 e~
o.
) f
O. 3/.
o e.ff
e.°..
°3'Y6
° 3'7
o.3/ e
/
b 32
0. /o
o. /1
o. 3/3
o oo'
o. o-°
a/S
O.
ooo
0. -°
3t
b.;5-
o. /
~,
oo.
.
F.5/z
s.~o7
o. 3 e/
oY
o.~l~
<,of o. 3/ 8
s,o
a 3fBf
2
33
2~",°
r
/~c.
II./
6. 5
,I~
~7~
~r..b
7. <.3
o. 3eS
e. 3ol
°3
163
{o3.
8 Y7
'zD.
G. SK
.o.
6
l.°5'
33
o. 5-0
,o.x. P/
/ o
3.. ~'
/°3
37.0'
. 5-Rb
o.3o
O f 'r.
gp.2
q.
2 G frF
e4,.
. 30o
O.3 o ¢
6e.-,~3r
5,e o.
~/.7
¥q
I-, ~'
4
O.~/y·3~
POov~
A ·
=
c2s
e
_II_
7
f HK,,
·_
=
O. If
e
· __I 1_ _111--1
wAl
-
-ry =
i,9wo
eg<Pg
e-,g
I=' s,'
(vi'
,p
1/
_'t
(M4 )
V q.
tq. ~
q q. .
I. 2'
C/.
9
L 6,
I.
1
6/ff
5"3
o5-ff
-.
LI
i'
-7. 7
. I
_qy
<
I ,t 5"
o9y' a
. 4' -
1.3-
o.lq
c tr
o. /(ft
o. d,
13. 0
e3
o.(o).
o.(fq
o. 2
°. f/'-l'
o, f
T'o
0..-22
-7. L/
c.
o
1/3
..o302
I'?
4/6.
C
t-,57,
./fLf' o.Or
57, 9,
°. f/s
L/ .
3cI./
o,
/o7
0.
(/5"
o.oZ
o. (234
i9,?
7.0
3-7
3
1
. a ~,.,. {(q.3
79
o./IL
G2.
/ G.·Ž6
/
C;.r
G
",
",
c.¢
-,
,/a
'2. f
/
3 .57
3.
2.5-?
2.
I
-'7
O./
Z/
2.7_.7/,
73
a.. (5/3/9q
,
L.9,o
/
P·o
e°
o./59
I
fPO/
2
I
=
6
4Weck °0 /A/7
/f3--HO
CAy /-f7-
.
=
.
6
D5p
7-'
3
a
_ a, e ,9
Ji,
LZ~7
5
1//a
V
v r)
(VP&
f
r)
A
/r 4 9-
-
( Nf)
0
.3
/.
/7.3
3 3'
/0
t/0.3
/a"
1.0
7
3
/e
/,#
-3 . qg
/. o
2 -ol
o..
I. S'9
/502-3
1. 7
/o .3
o.-7
/03
2.o
I oy
/03
o.75-
o.-71
I, C5
z.C/
/ .'
/.l, ~'-t'
/ff~.
1o.
. dG5
/0 .3
/
3...2.q
c4.t)..
2. IS
/0'
la 5'
I
2
LII.
93
o' .775
o o/,. "
0/
1C.-7
2?, .Sr
2.
70
0./
QG.
S5
t. -
96.5-
o.
0.2
o°.. °g°
C ./ ff ao.
tf
.S/3e/3
o,. 2-/3
. 0.71
'
0.
7-
o.
'',_9.*7
qF,o
q, ~-
o. 0oe
7
a.
q.
5
Ie." o
q.
L,0
~.
zrl ,'7a.
O.
73 .
/ C. o
. g
o.74
. wS
o.-~
G.o
s/.
I q.o o
G
/
q.
-. o
,
6
e B?
0oy
0. 3 2
o0> o.z
03.
35-R3
>
'/
o.3
i
°/2
I2. /
c>,
7.""29
6.3
1~r~
2/¶
00~/3
I ,o
.
>
R
5,
0.
"'7.
"'
, .2 7
/
.3
. e5
r,%'~~
5 5-Pc
to-
oo
c
w/Ig e pe
(sJ
i=
ff,o9
,.
1/
7ii7
A9
'6
1/
(,.)
(-7'-ry
.'C
22LI.
,
2
0~1e
o. o
0. 759
O. 52.
.
o-3
2 {?
2.1 9.
2
2 o1-..
/. .
o.o/3
I .o
'2 °7'
0.
e6 6(1
G/L
/6
o."
o. CKf
o.&o~
0.
'.3 79" 0s~
O,. 7-G
O
as/
/
q-, o
1 O.
2.
2'on. ¢
I 'f/
/ ~I. 6
(7/.
ff'.o
7%. "
6.
1
5-6. 2..
q.7.
S,~
o. 3g.'
0.6) 7
0.6
q7 . C
2
a.o
0.~l
o. o Z
o.,o 7
7'
o.
a.c~l
/ S7.0
o.
o.0
-S
.
c~o
{/
0. C5
/2.. '?
/.
Y
tt ,,
o.60
°.
/ ·{
7.67
5.4 . 3 '
O6/
f
2.C
2-.6
l1.
'.07
0. 65?
6. Scf g
13. C
5.
o
. C~fj
32.
1/ f'
0,
0YZ.
0. . 03
oh /0 8
.,. G3'3 i
2o./
0.0~
0.
0.~
o. 6 3 f'
67.
23.
'7.o
o. 67
0.~
0. o "
6.o{~
'. o3
63o
o..' ¢, O0.. 6'35
o. 6,C
,o. G'7 O. 637'
'.*6/Y
-. c a
27. 6
(.
a. G2q
63
o.
o o.. GG30/C.O a
632~
a. 635 0. C3
79./
0
7.
o. 670
.63
/32.C
1/
C.,,
C
o
o.
af r:
c
I'f
I S
I ~'Z
1/32.
o.S-.
.~'
oo .G
a 2-,~
0 (69
0.6 Cw
"½-0
0.
0.62
G2.7'
'o'F.,G
203.
~.of
. 2 S*
o.Gff
203
3.0
i
o.G'~
0. qfV
. /2.0 .3 95
('~
o.
35
o.77/
O.(o
I.,,0.
/3)
3.
o.
o.[oo
I.o
°
/. 3./
/
t, '~ "r
2. ((
/. 75"
.G/Z
'12.. -7
-3-(
'2. II
,o
/-.-
o$
I..y
1,C
2.../
'1.
t '7
22.1/
,1+
o 6V
6.6,'
o.Gf
c. 6 /
/t.e
0.6C5-S
2° -* o.56fC
q.
. °
252.6.0
2.°
o.6'rF
°. . 70C~
G
O,
2.'"0
b.7'0
0
3.26
2. 67
2 /7
A
.
,4 10,4,f
p
~T=
.
i
.X7
T-b
q, /-c
-
. -
V
(V"e.T5)
Y''" A
/r4
0A
I'
-'1-
V
.i-
-4
-1./p
P
O.G 2359.6
4
'237
'2.
' 23, 35
"I,·
2.3/
2-/ . .
23/.
23/
I 3.o
0. q24
2.3 C
0.
I ~.o
23.L
tqq'.£
2(
f ,6
'136
6.'
f:ko
".l
220
12.
tI 3
6
0¢.--
9.~
0.
2.67
0.
/
.
2/-.-
,
0.
5,
2.0 6.6C
2"3
22-"~
. . /.5"
23.
2_3. 6
/ '. c
/I
1.
0
ej'q
71. '
67.9
5- 7.2.
:3 '(·5'
2 ',/'2.
/'.6/
1,
C
2.
zS.oo
4'0.3
lP.
6
2. f
7..3.¢C
16.k
o6k
'. 635
/3. '
/(73
637
p,6 '1"
,a.
6'/3
0. 6,
0 .
3/
367
o.
o. 3~
./¢c
a.
/55
'9.233~7
o. /-31
0./0
6'
'9
o.
/3/
o.
5
o5~
06~~
o5-5
,:. o 1?
5 3 .
,. .0~.LG-S
-5
(I~',o
I 8/ c.6
" ~~.G~
1g,'
ll7. C
/751.6
3.
05 -~
9. &~
0.~o~
5
C
3
3.20
o . ..
e. 6 ,'
~. 5-51
o.~'~7
2 /., 6
2-/.
-,q9'3
3.
'7..
2., <',
6. /f$5
6
/6.o
Iji
0. 6/
8'.
2. /',#, C
/ 9q
6'
6.-
c/
o.,6. ~
63Cs'
637
22.
2.
Z2./
a,.
7.7
,.
0.
22,6.
21~,¢
22. L
~
72-
q75-7
II.,0','
.'672.
2 30. .r
9.
633
5G
0.
77
'. 6 :5-~7
/2.
23?. 6
233
9.,
'. '2
22~.,6
, 6
235;?7. 6
23?
3.0
,
C.0/
. 0.,' (3
I
2.2
. o.o
-7
L
. ~5- og
.o~~
6.'
.69'?
o.6 1'z0
1/"
,'-.
-z,-3 A l
P c107
o,4c eR -e
I-YIA
Ale-- :
-
-
LC
-
Tb = /,t c
LrJU
Zur _- e,-,o fA
7el 5
*
V
\
..14
i.X
0 /
V
-
I" -
13
/7,
. 1/
.
_-w
-
~. / o - 333
'.2..
g
4/7.1/
L/.
3 ,6.
.°L-,
'O o(
p
y
<o
o.
f >>
2.
//
O,./ C
I ,
o
/CZ
0.0q
?
5
4/f. 5
. 1o-
o,/1 /
.o
O.
0.
(/.
0
5-
0, o33
,0
ooq;
O
.64,
?'
. .>
6·
q.
', -(
7 o
0.
/ 2..
o.
/Z.
o.5.
L
el
(
r
6. (Z3
o. (t'f
0. oo
0.
00
0.00
3
/20
6.(~
Lz. 9
2. C'
'.1(. 9'
o
0 d/
2... o. /2.3
(23
0,(~1
/'/
00.
o ,-
0.
3c9.2
~.5
2~
I ~',o
33f .'Z
7
.
(z,
0.
vy(L.~
/3.
-,
0.
.1t7
I. 0.
qt%.
L4 .V
0
(/'..
o.
q3 . ,
'H.f
~;,
. 45
3Y.
Ir
6. (07
Lq , ,
q/3
, ./
0.f.5
. /'2 3
e./
z.5
°
/Dr~
o.
/(-s
° /26
0.1'
/5
0. /q
o.(2,7
.
I
9, I~,7
,/
7. II.1
7
7'30
7,.o
3I.if .37'
1{l,
5;f(
~l, '21.
/,/. 2-7
c;
1.
h-7
0,2-7
~~,0v
e .-t?_
I f1
o,(l~
2. (
2.~
,3'/
<(3,§
2e
2.y~0
2a °
6.3/
7.L~
/. ''
o.130
6.13/
O.
l 3°
0.
°
2. 47
. o 7 ,'
/. 1/
,
pc/
Arr
.L-
=
C14'60,
A "OAA
1-2 Do
~.c/.~.~/,
0. os/ 4
O.y 4q
. arv
O . w f s·
i
I
0. (z
0.
O.
7'3
((7
. (//
O. (f(
.
(o
7
5
)O/ ,
:5
CH5 AJ6 S-, =
3 C,
C EMrER
7.-
,/
.']-Z
r
Pb
/Ad
Je
, /
4o0 o
Z
Lil
-r . i-
'P
(
Itl)
Uo,)
0.3
1.0
.I,
.o
135,3
2,7 F
I 3 r,3
1'35-
-
3.
3./2
2.-.5-.5
2.03
2 .o6
13.3
1 ,o~..
/O.G73
. o,.,'
{~,
t o.
- /r. 0o.
2.
035w
/.72
0ol.o
.
O3'
/-39
3T
/3/
131..3
13/
131
13.o u. . ,'.
/
1'3e
'f2"
o.aO.;
0.0
/3o
b
. .3
33
1'3
..
a.3V
I,/
133
/3'z
3.0
4-
3
00
o. /
3 539
0.3
9 3r
O. 3'-/9
/3.3
I .1.3
/31.3
zIq
2.63
0.
. (73
o.(i/o
2o7
o.
o.07-
0.0s
/ 'q33
-Z.7
/23
12-3
1'-,o
a.~/
o. o.
0S.
,0 °03<
.. o(f
/ .3
Iz3
¢2o,.o
.o35"
o. 33¥q7'
O
qr
a.35-7
"
7 0
3/ 5
O',39./ v. 3'0.3'.f/ o.33 7
/o
0.3
/ 3
13.3
12. o
a.df0
o.
"3; 3
O. 3qo-3
112
°
-7
0,
O.
o
0.
. 3
2.3
.
0r
7.o/
o.O(
O
I I
0,0
.
'.
~O
d2
o-
0. 35,
*73.3
70.
/.y
a ''375
3.o
)P/ Atr-5-r
I~",
/0.0
lo.
3
23
o33
i
: -G..
z-2
h /C/1.EcA Jp0,~AT73
C
=
S
A "0Aekd-~ C//kAlL-% 6.·S-C
7-
= /a./
-½'-
-,:
--qr.
6,
Ac
e/>, A
=~·A
U,
vvcs
1/~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~
-/
'-c',-
M'*)
1-.
2 3
,/- .3
o.
t'z /
/z o
10
o //7
o. 0
2-0.3
/
/Z o
~'-
13'a _
72-5
0. (53
/I 2-.3
Z 0".~
/ .3
0.s' -' - -33 P
c;,
-
-r,
-P--2-0
- 2,
,i
'1
0.2-a 0(0/7
e2-/
o 73
/ t3
It,
3
I/ ff
3
/1-v.
1?
'/3
I1~
t3. 3
')
I/..3
I //
I/s 3.,
IlI
II"
I / °. 3
Ito. 3
/ 1(0
1
(07 3
o o.
.3.
7. 30
G.
'Td C'
f.
G.
5. Lt
:
. 5"
ct,./
CO
:3 Y.
a?Y
13.7
II./
·
0.3
6,3
.
q .y
3. 7/
2 .6 f
2 2S
, (4
I L ,o
o.
00.
o-,
1.
0. Zq'
I- '?'-
f
o
2'.
I, o
c'-3
o.·3o/
2.Mt°
67 St
pe / 1.7-'-1._7
LI. 33
3.M'(
I. 51
I. /
o. rcj.
/ ',o
o. ,,
:, ~/ o. of
' . ,Z .
0.L
(I9.'7
6 7&
(1z.
4..3/o
30os3
0.3
,. 3.c
q'
off ,~'
/0.o
. .,,
/02-3
(7
/2.
o
-2'r ° .7 00 F9°
o. 2.9 o_ "
I 2 .3
lIL
o0z 3. o . 3
o. 2.,'O..
0-7-
13.3
113
6.o
o . , p-
II
0.2-.'
p
zt
'f
0o. "
A
£'
= O
er/z 0-0
0
Al-fA-Af
'-v' =
.a,