--- -, , , , -- 1. . -7
advertisement

I
- 1.- - - . IIIIIIIIIIIIIIIIIIIIIIIIIIIIIIIIE
,',-, ,11
--- -,
,1erl
.,:J.-.1;"",.,_:--.:,:.T.
, , ",..N 11
I,+
- -,--,,-7,
,I " . z:, + 11
l ii4
i71,, *,+e
I"I. '+', "!,
,
-, --1, $:
1,
,-7
'illl
,Iv.--: iIr,I,,k.
,I-,.1,
.I'.,
-,,,7,.7-, ,1+
, 1,
,":
,- - -,1,- ,, -- ,,--,-O,,,
.,
.--7
:ii:II
i7.:, .t-,+TIi- 'I I ,+
,IIII+I 1.
, -., - ,,i:,-.i,,,,,-. .:"
I,'W4JIi!,
,I, I ,."I1'+'W
".1: -- . I--1"A%
1.
,I
I
I
I .I,',,,.1I,."7 ,,,,i,, ;, , ,. 1.
-,
,,,J:.I, ,, , ,A, + ,'."-,...i",r"
,, q.+'I*',
, ,I:,t.vflI.tl,,,,tIi,, ,, I+tr",f,I
, + -"
i.
i
..
.
.
,
I .1I,t,'V, -1,
0V
I1,.1,
I
,
,
,
,
,
,
.
,,41,
;,
I..-i
.1
,&
,
I
,
+
.
',O
*11
l
,
I
.
.
,.
.,
+ ?. . FA, I II,.., I, , ,I j.1:'
:*,
Ili.q,,,, -, I 11 I , I..
I, ++
", , '
iI:, "
-+P
, 1'
- I ...
I ++, , .1
11
.II.P1
IWl
, I ...,,, I-..:.,, , , ?.-, ,
, .. !, ,, ,.-. , I . -,!
...
I..,
,, I
II
--
--
+
!-
I"
.,"
, "
..
,
..
,::
11.-
-
-
:, I
.1. .
. . ,.
- - ,
, + , +
,+
,
,
e
,
I,1
I
, . ., , : , ...
6
.
+ I1,
, ,
,
. .Ik
-- lt,,,
I - ' , ,
" ,
"
i , , - ,
,
. :,, ,
. ' l 1. ,
I
1- I
', II t -,I
, +I
"
:::
It
I
. :
. , -..-. , I .... : - , , I.. , . ,,,. , +, I .,
. I 1:
:".
.
,
,
. , , .- , :., -1, , II+ , I
,
I
+II I I
I ..
I
I w
,
,
1 -14
,
i I'. , " -1I -"...
I ",
+ t l %
.
I
,
,
,
,
I
,
,
;
,
,
i lllr
'
.1
, , , ,
,,
"
1 ,
" +
,
, , ,
I
...
"., z.: .I.- l I I
, :
., " ,I, , +, 1,I 1+
+
, , , , z
,
,.
,
+;. , .
, II
- ,.. . . .
,
, ,
.
- ,I
- II
, i I,
,
, 'K
, .
.
. ...
.
, -, , ,
, 0
1 I
, , , +,
I
I
,
I II I
I, , + IIt, 1",
, I-* 'i
.e .
i I!
,
,
. N
I-11"I.1I
. :: " I,
,
,
. I
.. ,
-- : .:. ",
. +. -I
, ; ,I , ., ,
I
, . ; 11: , . .
::i
+%, I
,
le
11
i 4 , .
j::
,
I
., .., I,
I
I
I
+
,
t
I1,
I
.
AI
,
+ '! I ,I:1 I,+ . II
:. .1
. k11
:. ,
,,, ,- , +
, ,
. , , ,
,I
,,
II
.
11% ',', I
+ +
I
I
I
11
i
I - + + I I+ ,
I
I I
, 11
"I
I. .., I
I ".
" ,, * ,
,
I"
It
.1
.
I
.. , - ; : .
,Z... .
!--' ' . I, , I,
. .1 . Ik ,
- , , .. i. .
,
., . ,:4 tj1,
, , I" JI
, , ,I, , , l ,
.e
I; t-, I- f"+l,
,. .'..,
IAl
. I
I *T, C
,
, It
, "
,
, , to,
,
'Io'.i
I
,
..
.. - ,
+ ,
'? 1,
,
..
-.. . . ....
.
I
,
: 1: , , I.
v
. , , I, , .
..
. ..
, , . , , , ', II +*- , "
"I
+ ,
. .
,. . . . ,
'.
.
11, . 4
I
, ,,- .,
.
. ,
I
..
, - . - I :. _.
,
,
..
I I
,
" " ,
, "" : +" '
+,, , * " ,
'. . , 4
I .
I .. ,
.
.
, -'l
, , I
, i .
..
I
.1
0
,
III-0
,
I#
, , ,F 1+
,,A
, -119
1,
:
, ,
..
1,
, , ,
,
.
.. .
II
1,
.
e +
1. I
- '
. I
.
..
, It"
II .
..
I ,4
2.
+1+
I
-, ,
+
,
,
:"
, , -...
, 4, C,,. . I
: I::
,
, t,
., v
I
, %
,,. :C . I . : :.:? +,I,
.. x
- ,,llcl
I + A -" :
t, .,
.
+
- -, ,
k,
,
" t
+
...
::. i ; I
11
.
,. :
I
I,
I, I I
1,
.- , . .. , - . , , , I;
L
... : .
,.
)t ,
I.. ",Fliji
.1 , :::
..., : ". ,.. ,"..
,+
. , .'.-$ i
xl'
.
I I I4T ..I tI,
.
I
I .....
I - .
" I.1.1
,
, ,,,
' +III,
I
I, " - t,'
,I ,
t
, -I + I I I
,
, I
I, I + I , , , ,,,
I
.. I
.
..
, . . . .
, . ,.:,
,
r
"
j
.
,
,
t
,
;,
.! , ,
:
!, , t . .II I +
- ,
, I
I +
I:
,
- ,,
-, -111
,
II .
, ,;i
, ,
'O'
,
+ ,
t,
+.. - .
.11I . . : I., , I
.
, I, I
+
.
:
. .1
, -.. A.
- ,
, ,I
, + l
, " 1,
t+ l
,.. - .. J ,e . i . , .
.
.
I
"I
.
.
I
I
I.,
':,
,
,
jl
'+
,
,
,
t
I
,
...I.
,
, 1,
, ,, t-Y
,
.
I. , ,
'11)I
+
,
I
. . 1 9f, J
......
,
+
I..
. .
,
,
.11I 'I
-i , , '..
, I ,
0
,
I
.
, I
I
.. .. . .1 . I
, ,
, +
I , I ,I + +
1',.
4,
I
.
"
,
I ,
, ,: ,
,
..
I II
::
.:.
,
11 .
,"
.
. , , ,+ , ,I ,
I
, I
-jF
.,. , - . 1.
I
+
, I
I, 1-1,1,
;:.. "", ,
I,
1.
,
., .
,
r
- ,
I
.. - : I
.. , -, I ,
I
I
.
I
), . ,.,
>,",'4
"I :
:i,. I
. .! "o
:
,
I +,
I .I ,
+
. z
,
"'11
,
1,
I, ,
. .. . :
- .:
, i ....., -h
- '. :
I ,,,, ,
I ,
--.: 11
4
Z.
t,
:
i
J
, P I' , . + ), , + I,, , ,
.., l I,
. " ,I I I
!
.. ... .1
.,
I
+
". k ,
1;
I+
.
x
....
,
: -2 Z,
- ,
l,
,
,1 : ,
, , l, ' ,-: v
,
I z
.
; ll.
.
, I, "I
,
, -.
... 1,
, ,
, ,
id,
,
, " 'm
,',
I I, (
: -, ;'
+ ' l" I'41't
, , , "....
,
,
1 .
, ,, . .. - -. 11,
.
,
" ,
AT "
.
Z, I - i
,
, " l 4
,
A
.1 - ,.
::
.
, I
I
1,
"
I ., IIII.I PIr,"
,
,
..
4
11,
,
I
.-,
I
,
,
,
,
. .
I
ID.. :.I.A
,
A
. :
..
. .
, , , ,
ll i,*l A,+, I ,' I, , -, 1+
I
I ,-1
. .1
I,, ,
11
,
+ ,
:I j
+
.1
I ,
', ,
,+I
,; I ,
1,
,
':
,. ,
A
.. , . 0
, ,
...
'
: - ."
,
, I II
,
,
,
,
1
"
, . .1
'' ', -]
, , ,',r i +,,
,
I: ,
,
_,
4
. -.-11.
, j ,. :?
- .1
.
, I ,, ,,,
, , , , I ,, 1,
-I k..
0 &. W I.., i I
.. + I
III+f"
I ,
:
,
, ,
I
,
I
+
, , , tI
I ++
.
,I , , , " I . .. . ..
,
, . ,'. .,
. ....
.....
..
..
.I :
,
,
++ + I ., , . I, ,
1,
, :
"
1
, +
:
..
... '
,
,41
,, I :
r ,.
. - .-11 , ,.
.:'
- -+ r
' + , I, , ,
I
, II
Z:,
I . -l + p
,
.1. 1.
.
N* b ,
., , 1
.
.
I,I
+
I :.
. , , .. ..
,I' ,
I
i r
' L + ,
, "'
h
:
I
,
, It.+ .:
,
. . ,
:. , , .,
I
+ I,
1,
+ r,' ,
'
* , ,', I , . , i,, ,
, - + . ..
,
+ II
7' 1. , - "lj",
I. , .. -.1
I I... I
I I, I , I
- I-.zw,
..
; ,4
-",I
1:,,
,
.., ,
: 'L V
f7. . . II.
1
. :.....
r.,., I I I
1.
I t
'T II
Ii,
, ..
I
5 I
,
1
.. I
. .
,+
. I ,,
I
::
.. 1
I..',1'.
1.
! ,
.
. . ., , :
.
I
I
I
1,
- I
'+
I
; .
I .., i 1.I
,r.
11
::,
0. .
, , , , I ,
.
. % 1: ,
""
;I
11
- i:I
,
.1 . ,,,
, I, 11,
I
... .. ,r,
.
., I .
,
I,
,
,
,
,
r
I
,
...
I
,
.
.
_
.
..
.
I
11
,
!
11 ...I . I
+, I
,
. e , .. I
!. ..,
.
,
,
, 1+
I ,
I
I . , , "..
JI ,
, I:
.II%..111.
- .. I
,
.
,
"
'f
,
'r
I
,
I
l..
',,
r
I
"
I
, At *
.
,
,
,
I'
, , '' . .I , l
I
I
-. . '.... , I
:
,
,
,
I, ., .
.1,
I
:11
:,
, , P. ,::,
.". ,
p
:
I II
I11 1'-,+i
11,
I
11
,
: .O",:
.
.
.1 : I
I , " -...
.
.1
.
.:%, , +
, . . I , ,
.
I
- - I
: ,
, 9.
.
..
. . ::...
.,+-,
I
,- I
: ,
,I
-, .
. f- -, ' r- '
,
.. , ,. ,
I
1: i.
,,, , ,
, r
-"
,
I
I !,
." "I
I
,
o'.? , ..
I I
,
,,
I,
.1. . . : ,I . z I " I
I
,:.
, I I1. , , r ' 4',I ,I
. 'A
I I4'
, .
.
. I
I +-,
, ,
,1+
,
, . ,4 I : . I
, "I
j ,- i,
, "' q
..iz:,4
. ._
. - , , ., I. Z
1'.-.ll. , .
v,
, , ,,
I,
, "I'll
"I'll
"
, I 'C+S
,
, , . , , .. , ,
- '
II
",ll
-......
I
I, I
:.
I
,
;; ' r
'
I
I
.. ,
+
'.11:
".,
':
...
,I
1;
I
I
.
'
IN-1.1
11
I
I
,
I,
,
,
dw!I!
!I!
III
,
_
I
I
1
!
ti
%
1.
I
I
I 'j ,
. 11 I
'I.1.
. I
,
'
I f.
.
,
R
,
jt
I
, ,
I
'
+'
,
_
't
,_
_
,
,,
I
,
4
I
1,
"!,+i
,
. , r
1
'! T
,
.
,
I
)
,
I
, I
II
I
:.,
,
,
,,-,
...
+
,
II
r
)
,
+
I,
"
, " -,
4, +
"
-. I
I . 'r
I ,
' "K 14
,
I
...
, ,
; , I I1.11 -, I
-,
+
,
I
.
l
I
I
,
I11
+
c
,
I
,
I
I
,
.I
r
I
ll
,
, i I
i
,
it, i',-'
I,
+
I I , IIII,
,,, 1: A
..
z
I
I "
I
I ,, ,,
14
"I'll
......
, 'i ,
+ ,I
I
I
1 ,
I
-J
+'I, , , , ,, ,
, :
+
,
1,
,
r
I
I,+ l,
"
. , ,I .
),
r
,
I
, r 11
, t +
- I
,
4
+ ,
,
I
:, ;::.. ., .
: , I:%
'
' 'I I,
,
1, I I + I+"
:
: ,
,I ,
I 1 1
4+
t, I
, ,, , ..
+ ,
II "
I
,
,
j I
,, '
r
I.
:- r
-t
,
, , I
1,
I 1I I
I
I
I
-,+1--' '
. , 1 , , - " , j,"
,,, ,
I -"
f t ""',
-I ,
+ ", o
I"
I
+ I I
,,,
It ., , , , I
' [
: .
!, l,II. '. ..
I1,
, I
,
,
I'l+ ,,
,
-f
4
+,, I 1
kl I r
tj , " I
+ ,
,"
I, I , II 1
r
, " ,
,
I
'
, ,
; r
:
11,
1 1
+, ,
I
. .
*, $ + , , IV-" +I , t
I r ,
I
1
',
,
:: , , q ,, z
"
,
,
,
"
I
I
,
$
,
,
"
,
"
+ "
" r,.
, ,+ r+,, , "
I
I'll,Ip I +,
,
+
" 1
, :
..
z11 . , , , . - ,
1
....
L
I.I
1
-, 1, ,
11+, I
. ', q I , , ,
,
, I- ,
I I I,
I
, ,
I*I
I, .
" : ', I, "'
,Y
,
+ I
,
, '4'* + + , ,
, ,
I , , - , -,
IA
I ,
, I
I'I, r ' , ;1.. , ,
11
-I
I,
...I - , . '..
+
I .,-,
, +
+ ,
,,, .
,
0 I ,
+ y
rrr I
I
,
I+, 1,
I
I"I'
It ,
1, , I*, , r.
+,,'
I
,
,
I1, - r I iL , , + ,+ _ r ,
I
:, :
, I.. I
- . ..I; ., l I
, I,
,;
I -I
I
, I1 11
,
I
, r
11
's 1 4 I
- ,
,
, +.
, ,
t, , t
,
,
,
++
I
, ,
I
'I
, rj
-, I I , I
,
,
, ,
3I
+,
, , +I , I ,
r, .,I- , ,
i *, , 1 q, l , , A
, I
,I
+
I I,I
,,,
, , ,
.. I ..
:
,
, , ',
, , Ir
,
+ , ,
I , , ,
,
+ f,,,,
r
+1"I"
.
,
- , , +
- , , ,
, ,
, " T"
I 1 V
1,
l
I 4II , ,
,rf
. .
I
.
I I
-, , I
- , t, , , +, . + r+I
,+
l ,, I
,, ,
-4 ,
; ++
1l
1 1 -, ,
,
,
1
I
+
"
+1
I
,
,
+
I
I
, ;, i
,
..
I
I
,
It , ,
, +4 ,
r
I I,
11 10
11
,
I
I . .v
I,
11 I
, I
I I
-' "
I '' r
4,
1
I- , ,,+,
,
1,-+*,"
,
..
,
,
, ,
,I1,
1.
. .4
I
.
-1
I
k,.,
, , :.._
- 0
r,
"'
, l I, $ %
,4 ,
'
' I,' :r,
.'
,
;
1
.. ,. P "
I I
,,o ,
,
.) . ... z, ...
, + t, +
' ',,
' 'I,
' I 14II+ ' I
,
I
_
r
,
.,
,
31
v
1,
I
+,
+
I
I
,
,
f
lr
!i
V
t,
!
,+
,
I
,+
,
1,
I
,
,
,
, , I
I .
,, ,
. .
, It
I
, I l
r, +
,
.:K,.
, :,
- IJ,+.1,,v
, + ', L
- I
-, i,-1
+ ,_
'I.
- I, li
, ,,,
k
,
,
"
.1
,
,
II
1
"
.,
",
"
.
I
I
I
,;
"
f
:,
:
,+C)11
+
,
,
7,
,
,
, ,
I,
'M 1
,+
,
4, +,
. I -,
:
-4
, "
k
,
, 4f i l .1,-.
I
N ,, ,
.
, , , ..1 ... I
,V I
-I I+'
I
? I,: I; .1
I 1, I ,
+ I, , f,,,
I
I ".
. 0 . . II
, ,+,, , , ,I, II .., ...]
111
:
tIiI-, I+ , +,,, , I
,
, I , <.:ll , .: - , 17.
I I,,+
I'It , ,
,,
v
-, , "
,
+ -,
-1
A
,
- '
:
,
I, o, I,
,
,
I
- e,
1 4 'I '4 '
, ,
' ,
" ,.Z
e. -1
: . I II.
,I - " , I 1, 1, ti ++ , "
+- ,
II "'t,
JJ
I
, I , , L,
1, ) "' ,"" ., I I
I ,
,
, r
.
.
z
,!;.
'i
I +
-.
- II I,
,
z I,
" - IIr+
,j -,
+ .+, "- 4
- , I-'r 1,
I
l. I - I T ,
t ,,- f r
I
LI
Z.
I
, vf+j
I
+ I,
,
+
IL, I I I
+
1:
1
,
I
,,
;,
V
.
,
,
,
..
,
I
,
,
++
I
,
.
.
.
..
...
.,
.%
.
,
.
,
':, , 9. , ",
I
1"
': I
_rI
,,
I
,rr ,
I
I
1lly
I
* . , 1'..",
,
,
,
'f
,
I
.
,
,
:
v
I
'+
:
:.
:.
:
:
L
-1
,
it
!i, , ,
, " i I , , -,,,
-,., ,
I
,
I , ,14
I I
+ +, It f,
I
I,
.
,
.
, V
.
e:
..
I
I
, +,'4 %. * " , 't ',, Itr$ , :, ,
I
,
1 I
.
I
"I
A ,1-tl' ) : ; .
, 4
1:, "Ol,
. i , t,
I11I
." , '+ L
l 'P:" , I,
4:
u
-4
*e , 1, ,
,-r I
IY
I. .1
,
"
,
1,
,"
+ ,
1,- - It 11
I
. .
" -' , jI+
I
I
"+ , " +
,
" III,
1,
'l
, f
tl, , I
I
l
r'
,+"
", + "
j -, , , , -' , , I, - ]*, ,-, , ,:I,
I,
4
..
P,I
I
, ,
, - + I.,,, - ,
, -" ,
,
'r
I ,
- ,
I I ' .i' '
_
-,r
I
I
.. , ':..
I
.
lk'.%..
.
'I
I'
'
t
- L I ,'I
II I
It'III, , +j rI
II
,
,
,'
)
"
..
N
,,
,
"
I
.
l
I
,
,
I
-4
"
,
,
,
,
"
,
,
",-,
,
,,,
"
-,J.
.
.11
'%'
"'
,
" , j,
,
,
.'
,
,
:
, I . .1 ...
, . ,
+ I + 1- .
'I
, , , , ,I "
, z
. '. - ,;,I),
I.
1 4 "
I
;
4rr,
, lk
, ,
i
" 'i ,
:
I, ,
I, I
I
I
Jr
-Z
m
I
t -t* It I
:.. , 4 I , .
+ l .
---, L
I
I
'
i A,
L :
, 4
, , ,
-, , ,
': I
- -+
I
.
11I , 'lK ,
, ., : : .
I , .1
. -, : ,. : .
,
,.
I
+
',
.
I ,-- ,F,'
* I
. "I 1, 1
_V'
,
, ''
r'
'; I
+
: .. , ' r , , '
I
- , I
t, .,
",
,
+1,
..
I
," 1 - 1,
T
T;' -I
'4 -- "
"+
I
+
I
"
,
,
.
..
I
I
I
r
,
,
,
,
.
::
:
.
k'%
,
:.
4
i
,
I
,
J
,
,
:
..
,
,
4
;
-1...
I-''
I
- , ,. ,
'+
.
I t, " , ",
?
I +,
1
, ,
I l
I ,,,I +
7
+
, r , , , - It'Ii' + ,- ' "- I, - ,,
I l
,I , I 'A
.. - .
.
,
.- ,Oti
I
,
.;. . I ,,
'I,
,
,
+,
,
,,"N
'1
,
,!
,
."I
:
-1
,
"
r
,f,
;
l
1:
,
,
ltv
I
..
11
.. , I . I
.. . - ...
I
0'.#"
. v,
.. I , . :
.
,
11
I 11
I,
I
, * i II
1
It+,
" . , I , ,
,
II
; , , I , 4,',t +1, 1, +
, I
' +,,
;'r ,
+
I
-I, I,
' 'I
"
'
-. ' ' l
, I
, - ' :
.11
11
i
I
!
'
y,
.
,-"
*
+
. .,4. .I
,:
I I
+
"
,
fJT
,
_1
.,
.
i
....
,
,
L
1
'
I
t
I
:
I
I
1
1+
",
.?i
k
i
'
..4
!Q
,
,
, ,
,
,
,-. , ,
vz
I " ,I II ' 'r - +
r
+ +, , " , * I" I
, ,I I ,,
I
' k
' l';" - L
t
"O'
, - , -, - ,f+,+,
, +
,_, I
1, I
4 na,
! , , +oq+:
, I* Z
'
- II
I -L
+ '
I
, .
... .
I
.
k +,
I
.
1,
I
il
.'. ., . .
' t,
II - I
I
I
I
,
I
I
I
I 1
,
II.I,
, I
.tll 'je -,
.
4
- - JA
,
, .. I.. . .
, , ,
'r
'l
A
,,,,,,%
' Ls '
_a
I
,
I 1
." - I,
,
I
..
, L , -4i&jNltl
,,. : '.
i
- ', , , ', ;,I
,r
i
[ '
.
I
I"
,
,, I,+
+ t', ,
I
,
,
i..
'L; " "
L
,
" r , ,, I
II,,
'+
I .1 1 4,
I , , ', , %', j , ++I
'" '
, ',
t ' 4r I I , , I' ,
-I*,I q- , ,+, II
II
, , -,
... . " . ._ j..I1 x..
:.:
1, t,,, tj OA
1,
, , -,111
I Lti-,II
Ii. t"L , " ,,
,
T
,o
I
III'!
','
i
,
,...
...
,
A
.,:
..
I
I
I
+
y
+
I
,
11
I
-:
,
,
I
"
I
t
vo
,
l
'"
11
1 ..
:" ,
I %
"'l,
,
I
'I,.. ,. : .. .
, ,
,
,
*" :'
I
,I
,
_+
I
I
I
+
T
I
+
,,
I
I
T-,
L
*i,
1,
L
"
'L
$
+
,
,
,
+
+4
,
j,
I
,IV
,
41
,
+
, I +
+, I
I11 - ,- , I, " , +
.
L,
I
I?
, I
l
., , I"I ,
4
+ L+ 'j' , " .r r
'
.. .. I.
,,:I"-Z:..I I .1
. ,
, I
r; I,
,
I+
1 ,' +, , - 'Y ,
: :
l L' '
j f -L
' 1, , , I',
'
, M
, '
1
,*
IV
'-*'I
,+ ,
1,
II
I
I *
1,
L
I
+ 11
I
,
j
I I
. . ,II
, ,
+
I ,,'. -,' ,
, ) - , +,
+
- +'I
Ir
,
Lj + I
L
I
r
"I .-v :.
, ,
II
I
- ..,I. I ,
,
: +," L;
,
14
,"
I I -, I
, , I I
1I
* I 1
L,"I 'A +1;'
I A, 7
+
- I -,I
+
I
. .. I "
I
I
,;
+
'
.11
,
,
'
1,
'
,
I
It
I
I
I
I
',
,
I
,
,
I
I
,
+
11
- I",
i,
+
, "I
.
I
I
"
,
' 'I
,
le ,
,
' y .
. 1,
lp
, , , ,,, r
11, I
,
:
"""I
" , Ii"
II t 't
I .
,
.
I , I I
+,
i
II
- I, I , I, I
', I , , + , , - ' ' I I
+ " ' .- .1, , . , '1 "'
I
I
,
.,
.A
..
I
4
':
'
I
'
'
,
!
'.1
,
4
I
I
"
i;
z
.
,
.,.:
,
. ,
I,
,
.
,
+
, 'I"l . + t IT- ' , - rL ,
.t+ , , r
,
,
,
: 1v1.. :
I
"
4
I
_,
.
I
"+ i r,. ,'
, I II- -, ,
4
I I " L
I
I
,
, I r
I
r
, ,;
,
.
.. 4
..
I,
, I
1
,
, I,
11,11 , 4- 4
8 , ',
.
I
,
.
V
,
,
t
T
I
,
"
Ik
,+
,
4
_
,
,
L
+
,
,
,
I
I
t
"
I
,
,'+
P
1,
,
,
I
,
I
I
I
I
I
I
..
..
.
,
,
.
:
:
:. 'I
I, I
.. ,
, +. t 1 ,
,
I
,
, ,
I I.1 Ij , N , ' , I
I
; ,+ , "
I .11
I , t I , 1:, ,
,
, , ,
L
I ,
,
1, 1,,
I
"I I
I I, I
I
, l ..,e ;, .! It, I
. , :
..I :
- . +
+,
II
I : .- , Y. ,
.. I
j + ' 1" , + ' ' r ,
, ,- , r I
, I ).
.
I ..
+
I
. %..:
I l
,
I
I II
I ' ,
I: II
. : - ,1.. I ,
1
.. ,
,, ,
I
j
1
r
,
L
L
,
'+
,
I
I
.
,
:.. ..
, ,
:1
,
I
r
'- I I
,
'r
r
! 'r"
I
I
., I'..
. .
:
, , ,-I.
.. - ,
I
.11
I" - 4' r
", :, .. . 'k
. I
iO , V , .
+
j + 1
I ':
I
,
L I
+I I
II II
,7K, "
,
I , , I
, ,,
,
I , , I
...
,,
i ,
....
L
'1 4
.. . ..
. -. .,
,
I
-, , . I
r
...
1
,
' 'LI
,
, , , ,I
,,
LI
11--.: .:.i' e,
I
,
, , ""'I : ,,11-"I
I
,
:::II .11.
, , I
11
I! , ,
I
,,
, 'I , I - I
L
, . . :
+, I+,
111
, t I, , , , , ', I
I,
t,
,,
'
I
..
.
I
I.
014;I, I
.. .
,
+,I
, , , ,J
+, I,
I, I, II,
. ,
,
'. , %: , :, ,. :...,
, .. 'ilo
, I . .. I.
.1 I
i, ,
.. . ::,
+ I
..
II
[
,
r,
,
,
+
,
,
,
r',
1
4
1
,
"i
'r
rl
"
-,+,
,
*
"
I
,
I
,
..
:
:
,
,
,
,
,
,
I
I
r,T 4
, , '!
I r I
I..,i ,
V;
I
.:. I , .
.:1:. I., . , ,
I ( IA
I
. .
.....
. ., ,
,,
, , 1,
r
1
+,
, ,
,
l
'4
_
j L
- I11, ,
I
I'
r
L,
4,
.
?" L
I, ,, , IZ.
.... .... : 11 .
.
.I
,
r I
%.
. z .
I
I
, I
, , t
+
*' , -4
I , +I ! , I , L
,
.
.i
z.. : . , :1,
. :
, . , . 4, .
I
I
.
I IV
+ ,
,
_
- li I , ,I
..
, ,
,
,
I
.
. .
. I.
1,
. 1,
, :.I . I....
I
11
r 1, "
_ L + I' ' ,I
),
I
. .. - - I
II"',
,..
.
- :I
. I: .
, - "'
I
11.. I
...
r
,
,
,
'
r
.
.
I
I
r
,
,
,
-,
L
,&
,
++
I
.
!
,,,
I I:
I
I
. ,,. .: I
+
11
I
LL
'I
, *1 , Ir
'I I
I
L
"6 1 r,
I
I
I ,
, 4 l " , , , -'
1,
, r
.
'
I I+ , +
. .. e .
I ,, I
..
I
,
,
- . : I ...
. , :.,
.1 I
+
I
+ +,
,
- +, ,
:1
I
I *
I
I
I
I
,
,
, L,,
z .. , .
I
jz ,
z
I,
, :
, I' +
'+
,
, , L
: I. .:. . I
L'I
,"
,
'T
I
I
,
- I
,
I
,
-- , ,. , ,
j I
,- ,
''
I
r
I1,
, I.. . .
I4 " , ,,, 'i-T
I.
i. ..
, I , ' I- I '
.. , ,
' *'
m, j , : : :
r
:, , i, , 1,- , ,
I l
, - I".
- :
"
, ,,, - , .I
. :a..'w
I
I f'L
.:-1 . I ,
.
.
I
I .
r
.:
eI'.::,
I
.
,-I 't
I
:
.:
II ' I
'++f
,
"
II " I
II ,
+
- 0+
'LI ,
I jl
4
-,,
Ij I + I
,
,+, , , + " "
I
:
,
.
1
,
+
,
-,
I
I
,
I
,
,
r
I
I
4
,
I
,
rl'
L
.
'1
..
.
I
,I
D
v
l
I. I 11
I:
. 1,:1
; ., ;..:
I
I+ ,
IIf I ('111"
,
, I -1 , f ,
.i
1,
,
k
, .
, +
",
;
, A;,,
I
I
'I
. . 1.
_ r, ,
',
I
I
L
I
.4
....1, 1I .V. ,
, "
t,
+ ,4
,
, ,
I
I
,+
I
-llp ,* ,+
, L,,,
"I 't., ,, , * , ,
,
I I j,
4
I
f
,+ ,; , I
I , ,, I , I I r *1
,,,I, 1*li1, .
:
t ,, , _,t, 1
,
, , 1, , iV71,+I..
I
, I
,
I
; I
r,
"
'll . ; , "
., I,
. I, , .
1t
i
II
I ,
I
: - w
.
,
"'
I
,
I I
., , , , , , ++ T,
" ,4, " I, ;, ,,,
I I I,I- ,,
. I
I
, .
,
.I I
II
,, ,
.: '. . T z :
* , ,
.. . .
t
t, lit],a , ,+ II- lp
,
1+' ' ' r, *,
,
! -f
,. . . I
, I* , , ,,l
I,
11
,
I
I
1,
1.
I
.
I I , ,,,
I
I
, 1, +
'*
I - -,
i+ , , '+'
I
..
, z9 ,
-' ' , "f ) , + I-, ' r
,- IT
,
, - I , I';
II
;IIJ
a z, ,
.1
I, I
+
- I
+
,
, ,.
.
I
,
.;.
+, , . , I
+-11t-+, +
, +- _ ' L+
_"_
,
1;
+
,
I
i
ItI
I
I
+,I
,%
,
,
,I
I
z
:..
;
1,
'..,. I.
,
" r
, 'W
.
'r - ,
L __
, ,+
P+.
LI
+ ",1 I
f,It.,I
:I,, 'l
',
+
j* I
, -JI,
, - Iij,4c, IL
- , - II-, l
,
y , -,)V
I
" 'L j
,
11
I
. : ..
. ::,
I
, " , , i, , t,
+"It
I
.,
,
I
.- . z, - -,
,
"
.- .
+
,
,4
7,
*tA
,!
.:
,
.
,
+
.
,
I
,:
V I I,I
, .I-- - - ,-,i
-,
,+ -tit
,I
I
.
. I "
1.
r
I, I
,
' + ., , l , I""
"I ; , .. i. ,
II , . , ,+,o , '
,+ , i
:
... , ,
I,
,
t ,+N +
.
'I
I.
u,! ,
,s
fIA
, , , ,
.. . . .
,
I
...
.i.A
,r
, '
,-P,
+
4
I
L,
. .
. .
,
,,
t; , - , t,;,P
,
-%
I
I - ..... , I
rrr", I
,,,,,
'W
" , L
'.
A -+ * ,+ i !
:.A
, I
, I
+,
, , ,
, ,+ - *4+ , -, 4, I
k
t
I
L
1,
J,
,
"
I?
'
'
,
,
,
,
,
I
I
, - , z
I
.
p
.1
,
. , k,,+ , ,*
I
;
"
-j
.
I
I
I
I,
N
,
1;+
V,-P
A
',
"t,
r
+ *
-, *
+-e
,,,
-+ ,*
' 'r I
W
I:
r
I,"'n
I
,
, ,
:'
+ ' '
:
,
.. ..1
'. . '-i :
, L'
I
+ I
I
.
, :.
'L r , L
'.
'
. ::
'.. ; I z
I
, 51
, ,
,
!
*1
I
dr
,,
,
+
..
1'
, 4;
I
: ,+f
, , , + I, , ,
,+
.
,l
-
''."I"
t 1+r
"I r Ir',,. 1,.,.-,,
,- +1IV--l'
IV
'41
11+
r,, ,,''4Aj j II ii
,,---;
.-,ll,
41I+T ,r",",$_,''I
-, "I'
11
1,i,I(1
-,1,, ,, II I
I--
*
,
,
,,,
11+1
.o.u
'!
... ,v
: ,
+k
.1
-,- z4- .. , I ,, [ K
,
"
,
,
I I+V" I
+
I
r
-... P',.
,
I-,,, l -t : 1'' ; - - +'r+V +l, .. .4.
11
, ',
11, I. 11 lllili.*.K ++ll
,
i,
A " ,
,, l I
, II;' ,
I I
'M , - , , I r
- - r, I7 A
I ,
, +' I
,, ,-," -, ++'
.1
-Z
"
+
+
r
I
I
1:1 ,
Ok,' +
I
I . III 1,+ , A"t+'
,,+
I
, +
I
I
r I ", I I,,,,
I
1"
,1
, *
++*
1
4 ';',4 ft '
-I , 1
-I
',,, +r' h,14. -,
rjo
,IF-,
,
11- I ", I , , , , ttr ,
tI,
+I
, 11
l ,-1
,
,
I
,
..
141111
i- -% I+
,+ '+ - ,,.,
ll +
,
- -0*-'1
I
'.
11
e;4" ;.
l
,
15
, , "-""
,
-
I
I
I
',:..
. ,. ..I'. .I.I
,
-"
'
I
. : .:..
I
I
-
'9
X. 4
"
1, ,
r
,,
" i 1 +
,
t
IA"
4# j+f. 4+
I
-r+
II I. ' "...
,
Ltt',
r
"I
+J'A, ' ,
- t?11
-L -', -1111 '',.AVI
- . J' i', -':I &", "1,-
-
-1 , -
,,, I
,A -71
i-t,;
t , , (,+
Ir , ,IT, , ,+,+
,
1,
''. t
I I, :"4t
i-;
I I ,f+ r$f"
, I ,1, ,1,,t,,,:+
q
-
: :'A.. F I
I
I
I ., , -
,"
.
- .1 )M
I
.
'lI
T
-
.
.1 7
.1 ''? ,,
I I Al ':
+
I
.1
'f
+
I
.
..
, +
..
,
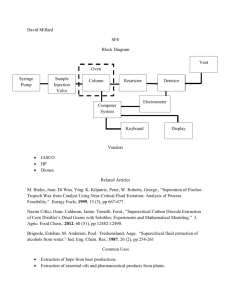
MITNE-31
Equilibrium Extraction Characteristics
of
Alkyl Amines and Nuclear Fuels Metals
in
Nitrate Systems
Progress Report for the Period
April 1 - December 31, 1962
Progress Report XI
by
Edward A. Mason
Takashi Watanabe
April 1, 1963
Work Performed Under Subcontract No. 1327
Under Contract No. W -7405 - Eng - 26
with
Union Carbide Nuclear Corporation
Oak Ridge, Tennessee
Massachusetts Institute of Technology
Cambridge 39, Massachusetts
TABLE OF CONTENTS
Page
No.
Text
I.
II.
III.
IV.
V.
Summary
1.1 Niobium
1
1
1
1.2
Ruthenium
1.3
2
Effect of High Uranium Loading on
Zirconium and Ruthenium Extraction by TLA
Niobium Studies
2.1 Objective
2.2 Procedure
2.3 Results
2.3.1 Distribution Ratio
2.3.2 Equilibrium Distribution Ratio
2.4 Program of Investigation for the
Next Period.
3
3
3
4
4
4
16
Ruthenium Studies
Effect of Uranium Loading on Extraction of
Zirconium and Ruthenium by TLA
18
20
Appendix
25
5.1 Detailed Procedures
25
5.1.1 Preparation of Niobium Stock Solution 25
5.1.2 Gravimetric Analysis of Niobium Stock
Solution
25
5.1.3
5.1.4
5.2
Preparation of the Aqueous Phase
Titration of Organic Phase for
Nitric Acid Content
References
26
26
28
LIST OF FIGURES
Page
2.1
Effect of Nitric Acid Concentration on the
Distribution Ratio of Niobium with 0.3 M
Primene JMT in Toluene
5
2.2
Effect of Nitric Acid Concentration on the
Distribution Ratio of Niobium with 0.2M
Primene JMT in Toluene
6
2.3
Effect of Nitric Acid Concentration on the
Distribution Ratio of Niobium with 0.06 M
Primene JMT in Toluene
7
2.4
Effect of Nitric Acid Concentration on the
Distribution Ratio of Niobium with 0.3 M
Primene JMT in Amsco
8
2.5
Effect of Nitric Acid Concentration on the
Distribution Ratio of Niobium with 0.2 M
Primene JMT in Amsco
9
2.6
Effect of Nitric Acid Concentration on the
Distribution Ratio of Niobium with 0.06 M
Primene JMT in Amsco
10
2.7
Effect of Amine Concentration on the
Distribution Ratio with Primene JMT in Amsco
11
2.8
12
Effect of Amine Concentration on the
Toluene
in
Distribution Ratio with Primene JMT
2.9
Effect of Contact Time on the Distribution
Ratio of Niobium
13
2.10 Effect of Aging on the Distribution Ratio
of Niobium
15
2.11 Variation in the Amount of TBP Inextractable
Niobium with the Nitric Acid Concentration
17
4.1
Extraction of Uranium with 0.3 M TLA
21
4.2
Ruthenium Distribution Ratio with 0.3 M TLA
22
4.3
Zirconium Distribution Ratio with 0.3 M TLA
23
5.1
Organic Phase Titration
27
1
1.0 Summary
1.1
Niobium
During this period, April 1 through December, 1962, the
following variables were studied in connection with the
investigation of the extraction of niobium from nitric acid
solution by alkyl amines:
(1) The distribution ratio of niobium as a fuoction of
nitric acid concentration for Primene JMT in toluene.
(2) The distribution ratio of niobium as function of
amine concentration for Primene JMT in toluene.
(3) The distribution ratio as function of contact time
for Primene JMT in toluene.
(4) The effect of diltent on niobium extraction by
Primene JMT.
It
was found that:
(1) The distribution ratio of niobium is greater than 10
for high acid and amine concentrations.
(2) There is a very strong dependence of distribution
ratio on nitric acid concentration in the range of 8-12N.
(3) There is a marked dependence of distribution ratio
on contact time.
(4) There is no significant effect of aqueous aging.
(5) The effect of different diluents is small.
1.2 Ruthenium
The previous work on the extraction of nitrato complexes
of nitrosylruthenium by the tertiary amine, TLA, (1) was
extended to include studies with the primary amine, Primene
JMT, and the quaternary amine, Aliquat Q-336, (2). At low
concentrations of HNO (2N) the order of decreasing ruthenium
extraction was found io be Q-336, TLA, and JMT. At high HNO
concentration (9N) Primene JMT had the highest ruthenium
extractability.
Rapid dilution experiments were carried out with TLA and
Q-336. The results plus those of the earlier TLA studies (1)
were analyzed using an IBM 7090 to determine the number and
aqueous phase concentrations of extractable species of
ruthenium and the amine partition coefficients for thsse
species. In accord with the earlier work (1), two extractable
species were found. The mole fractions found in the TLA and
(Q-336) experiments agreed (as predicted by the asanedmodd) and
!!!M!IMP"i
111
r)
4-
were found to increase with increasing HNO concentration.
The partition coefficients of Q-336 were found to be about
an order of magnitude greater than those of the tertiary
amine.
1.3
Effect of High U Loading on Zr and Ru Extraction by TLA
Limited studies in the effect of partial uranium
saturation of TLA on the extraction of zirconium and
nitrosylruthenium nitrato complexes were carried out at 25 C
in 7N and 9N HNO using 0-3M TLA in toluene (3). There was
consIderable scalter in the limited results obtained which
can be represented by
EA(metal) = Ae-0.02CU + 40%
Where U = organic phase uranium concentration (gm/l) and
the constant A varies with the metal specie and nitric acid
concentration.
3
2.0 Niobium Studies
2.1
Objective
The objective of the work with niobium for this period
was to establish a procedure which would yield reproducible
results for the extraction of niobium into Primene JMT,
primary amine with a highly branched chain mixture of 18-22
carbon atoms each.
During the investigation of the
extraction behavior, the distribution ratio was found to be
time dependent, and this was investigated further.
2.2
Procedure
The experimental procedure adopted is presented in
detail in Appendix 5.1. Here, only the main features of the
procedure will be discussed.
Niobium-95 was obtained in form of oxalate complex in
5% oxalic acid solution for use as a tracer. A stock
solution of natural niobium in oxalate complex form was
obtained from potassium hexaniobate (K8 Nb 0 -16H 0) by
dissolution of the hexaniobate in water, f§l1wed y the
precipitation of niobic acid and subsequent dissolution of
the precipitate by oxalic acid. Before dilution to the
desired concentration, the natural niobium stock solution
was analyzed by a gravimetric method (sEe Appendix). The
stock natural niobium solution of
10 M was used to
adjust the total concentration of niobiuiim in each run,
while the tracer niobium concentration was kept at less
than 1% of the natural niobium concentration. The mixture
of natural and tracer niobium oxalates was converted into
nitrate complexes by evaporation with hydrogen peroxide and
nitric acid to a few milliliters under an infrared lamp.
Then the solution was diluted to the required gcid
concentration and heated for
1.5 hr at
100 C. The
organic phase, prior to contact with the aqueous phase, was
contacted with nitric acid of a concentration slightly
higher than the aqueous phase to be used in actual run, to
minimize the change in acid conditions during the run. The
two phases (i.e. the aqueous phase containing niobium and
the precontacted organic phase) were shaken together in a
constant temperature bath at 25 0 for a desired contact
time. Then the mixture was centrifuged to separate the
phases. Samples of each phase were counted directly for
the y activity using a sodium iodide scintillation counter.
(Radiation Instrument Development Laboratory Model 10-2)
Each phase was titrated for acid concentration. A typical
titration curve for organic phase is presented in Appendix
Section 5.14.
4
2.3
Results
2.3.1
Distribution Ratio
Data showing the effect of HNO concentration on the
niobium distribution ratbs using Primene JMT in toluene and
Amsco are presented in Figures g.1 - 2.6. The distribution
ratios for 24 hr. contact at 25 C are plotted versus final
aqueous acid concentration on logarithmic coordinates. The
use of the 24 hour values for the distribution ratios will
be discussed later.
The main features of Fig. 1-6 are:
(a) The high value of the distribution ratio, which is
about 10 for high acid concentration (12N) and high amine
concentration (0.3M.)
(b) The very strong dependence of distribution ratio on
acid concentration.
(c) The nearly linear relationship between log of
distribution ratio and the log of acid concentration at
acid strength of 8-12N.
(d) The relatively weak dependence of distribution
ratio on the acid concentration at acid concentrations of
2-4N.
(e) Slightly lower distribution ratios were obtained
with toluene as diluent than with Amsco, at low acid
concentrations, and higher distribution ratios were obtained
with toluene at the high acid concentration.
(f) Fig. 2.7 and 2.8 are plots of logarithm of
distribution ratio versus logarithm of Primene JMT
concentration for various aqueous phase acid concentrations.
The experimental uncertainty due to the very sharp
dependence on acid concentration accounts for the large
uncertainties for 8-12N. The uncertainty at 2N was
probably due to the presence of large percentage of
inextractable polymeric species. The presence of polymeric
species will be discussed in the following section.
2.3.2_Equilibrium Distribution Ratio
Early in the investigation extensive runs were made
to determine the time required for the contact of organic
and aqueous phase to reach an equilibrium value of the
distribution ratio. Results of these runs are presented in
Fig. 2.9.
As can be seen, the distribution ratio has not
reached constant value after 460 hours for 0.06 M Primene
JMT contacted with 8N nitric acid.
pig.
1
-j
V
I
-
I
:2
-wa
t
14~
rL7
-
I
AC
7
T- T~ T
1-
I
0
4.)
ctj
~
4
I
4-4-1
-I
'/
4'
jg~~
4.)
i
rMC3
-2
U.. ~
U. 4
*
I
m
I4
10O
-
re
r- -
-T -
- -r r - -
-- -
-F
r --- i
It
-vt
I
--
t
-r-
-7
t
itt
-..
-.---..-
7
-F
i
24
Cotact
jI-
--
+4
;
4'
. -2
44
5t
*-- -
.
-2
-14
.
.
-A
-
- -p
-
j2
-
-3
2V
- 4)4)
--
-
+
-
7~
-
r--
471-4T-
f7
-
4--
- --
<Pt7
--
-1
---
-- -'-
-I
-
10
-
L
-
- -
..
-
- -
4 r
Tt
L*:1
-
-1
-4t --
1.1
* i-i
-T
1
.
* I
- -r
'IC3f
*
j
_
I.
- -L
-- T
I
-* -
r
-T 4--
p[~io~J 1
-
TI
t
Lg~
60.
V6o
.. A
~2~
I.-.
44
iT
~
4..
~'T"~
JFF"~ 7F1
~~*1.
ti'
-7 T
*-.
-.
af
Q
-.
4
~TF-
-4
-.
QN
.
44
.
1
.-.
..
tl
44.-
jj~.:
4.
.. ~...:.....j....
-1+
±~..4.
47 ±4
~1It4rl
4---
. .
)
&(
.-.
L'
9
4
-
Tilt. 1171i7d712477&
q4.i..
4 ~ . 4 . -4
G%
.
jiZtI. :~''i±u.§17
.
41T
-
........
~~
4-ivj
~Hz
At~
.
* ,.,..iiI
O
.
;41
L
-
t
-4-
ill 7
.
w-r*
.
. 4.
N*--
-
-
.
T7
L..
I.
1
-
_o_
-
....-
-
'-
.4...t...4...4....~
......
_"_
-
4
1
4.......
--
-
I,
3 1G
-. +-,- f .
3 tj 7
-4
.
.
'I
.-
I,
.±
r-..
h
..-
.K*
Ii':,
*...
I
2.1
ti
..
4
cu
0
co
r
p.-.
1
~.
im'.
j~
t
4.
t
-
1_
4.
tati
~
,4-
-
4
4. P84
--
.....
.4.
: ~
+7
ii
-4 +-
A!,
.........
.....
-
.
~*
--
.
-
b'
____
t-t
-C-
.....
U-
:tr~v~..
7 7 7.......4
4,4~-............
-
H'I~77477
pt
4*
-
.....
..
f- .
..........
T
.~'......
.....
4
.~1.1i
p...................[.........
41
......
,
-
--
......
.........:..
..
.....
-
~1
r4
4
-m
4
.4
.4
.~.
4
.4
.4
I
k-A
.
I
4
~~77~7
4
4
--
~~.
i
4
4
4
-.
ri
0
g94
o
co
.-.------.-..-.-.-.-.
*
01
I
~~~~1
4.
.
1
N
9
106
17
-T
7
--
--T
-
-
-
-
-7fMe7 --
22fq
*0.~
I xp nrm.
Onat
t
-
-
-
-
1
1
- --i
m
---
. .
f8
J
1-
'1 -**
iI
T
-
44
1--
-
1T-
--
0-*
T -
I:
1
11~
1
--T-
-7
-
-
-
-
-4
-.4
-
-t -
4
+2I
-
i
'
'-4
-
.
-
-
--
-
t-
-
1.
44
v
V#Q
1~o
4- -
III
10
IA
C.
I
A-
'
}-
-
7
T
-~I
A
i6A.I
--
-
I_
Diiuent -A460
1T
-
$1
1d
7
4.-A
~i
1;
-
t
1
iti
-4
I
:
0
-
-7
7
-
-
11
IC):
. We4t o mir oenteturi on the
M
Dist.,vi butaon a to with Pmdn-e
~in~iic
'F
~~
.
4
.3
Ff
4
-7
4
4-'
-
-T4-
--
-T
-~~-q
- -
---
1 -T
T
7
t
--
- -7 -
It
4
-
-
44 4-t- --
1
-
-
r
-17
-
71
4T
A
-4--
1
4
.--
-
-
-
-
-
--
T
T
--
-
-
"44
-
iK
1
1
44
-4-
Q-P
'7--
zizi
0 0
~~r'
ar4
* '0
V
4-
-
~r,
j7r
t-~ 4...~
~
,
~
..y.....
4.47
..
t.E4.
4.
)
V
4.
4,
'1
T
-T
-
i
8---4-t
-
-
t
3
--
- -
3:T
4-
b
r
2-
-La-x-
Tt
T
-- -- -y
-
-t-T-
~4-
--
-
T
-v
t7
l
tt
wTr
2.5-
-,4
--
-
-
~TTF
*
44-
-
-n4
o
--
-
-
-
4tt
-4
TT
r-
1.5-~~rr
TT-
-
4'4r
-4
-7t
P~
-~
-
7
7.
_t:
-
~~
-'rt
T
-
-T
.
r- - -
H1
--
7
5-4
+-
-
-.
-
t,
-t
-
-T
- -
-
-IT-
,r
t-
T
- --
-T
--
-
1
-
-
2--
-r
-44t
1.5---
+--
-
-
-
1-
-
--
t
-
rT
- t -
-
-
T
9T
I
.5
5
7
;
-
14
There are several possible explanations for the phenomena observed in Fig. 2.9. One possibility, the simplest
one, is a lack of efficient contact between aqueous and
the organic phases, but considering the long contact times
used this &jpears an unlikely explanation. Another
possibility is slow diffusion of the extractable complexes
of niobium. This again was not considered a likely
explanation in view of the long contact times and since
cerium studied previously on this project has not shown
such
behaviors (4). Another possibility is the presence
of an Jnextractabre polymeric form of niobium which is
convertible to the extractable form. There are two
possibilities under this category. One is the possibility
that the inextractable form is in unfavorable equilibrium
with respect to extractable form of niobium and the rate at
which equilibrium is achieved is slow. The other is that
the equilibrium favors the inextractable species, but again
with a slow rate of exchange between species. To separate
these two possibilities, runs were carried out using aged
aqueous solutions. The aqueous phase, after preparation by
the standard method, was stored for various lengths of time
before contact with the organic phase. The results are
presented in Fig. 2.10. Up to 77 hours of aqueous phase
aging, no significant increase in extraction was noticed
between aged and unaged solutions. Thus the increasing
distribution ratio observed with increasing contact time in
Fig. 2.9 does not appear to be due to a slow conversion from
inextractable to extractable species, with equilibriun hghly
favoring the extractable species. An explanation for Fig.
2.9 is am equilibrium in which the extractable specie is not
favored coupled with a slow rate of conversion from specie
to specie. If the inextractable species are in equilibrium
with the extractable nitrate complexes of niobium,
continuous extraction of extractable species into organic
phase could gradually cause more and more niobium to be
converted from inextractable to extractable specie.
To
determine the presence of inextractable species, several
attempts were made early in the investigation to analyze
for the percentage of inextractable species present using
the Martin technique (5).
This technique consists of a
plot of organic phase concentration versus aqueous phase
concentration of niobium for various volume ratios of
organic to aqueous. For extractable species in rapid
equilibrium a straight line passing through the origin
results. The presence of inextractable species in slow
equilibrium shifts the intercept of the straight line .
on the aqueous phase concentration axis to a point
proportional to the quantity of inextractable species
present. The early experiments made using this method to
determine the presence of the inextractable species were
failures because, at the time, the very sharp dependence of
the distribution ratio on the acid concentration was not
taken into account. These experiments are to be repeated
shortly.
~ ~
--
-4
-A-----
7~ T F~ f0T ~T
-
~
*
--
~
---
~
- ---
j
--
EHH
4~i
C'4I
;
Lt
IIt-
j.
-- ai CL)r-
,
n
Iz
(1-1
tlj
- Cn 771
I-
It
m~
.- a)A A a)r-
)
T)
'r
(n
('
16
In a study of Nb-ANO -TBP system, Hardy and Scargill (6)
at Harwell made use of th Martin technique to determine the
percentage of niobium present as inextractable species.
Their results are reproduced on Figure 2.11. Their data wre
fbr inextractable species present after about 5 minutes of
contact. Since the details of the procedure used at Harwell
and the procedure used on this project is slightly different,
no attempt was made to use the Harwell data quantitatively
for the analysis of our results. The presence of a large
quantity of inextractable, polymeric species at 2-4N
indicated by the data from Harwell is probably responsible
for large uncertainty in our data at 2-4N acid concentration.
Conversely presence of only small quantities of niobium in
inextractable polymeric form at 8-12N probably accounts for
the better reproducibility of our data at these acid
concentrations.
2.4
Program of Investigation for the Next Period
(a) Determination of the contact time required to have
the extractable species reach equilibrium.
(b) Determination of the quantity of inextractable
species present as a function of acid concentration.
(c) Studies of disttibution ratios with TLA, S-24 and
Aliquat 336, as functions of acid concentrations, amine
concentrations and types of diluent.
t
4
-
-
4
I
-
-
4
t
t
.
T
..
-
-
-
4
4
1-;~
7
-
-
-
+
--
-
-
-
H
-
,
-
-
-
-
-
I
-
:,-
..-
I,-
-
-
4
,
*-
-
-4
.
-;
-
-
t
t'..
*
-
I ,
.~~
- -
-,i
-
,
-. *
+ -
-:
4
,
--
.T.I:-
I+. I-.*++ ,
4tm~
,~
---
4,
-
-
~
I~~
-
--
t7
~~~
I
.++
t
,I+
I.-c ;
+
- ,' 1I-
-,
-
-
4-
I
t
'-+-. I-4 -+
4
-
-I
-.
-
+
-
-
+
I
, I
I
-
-
4.
-
. *
i~.I
-
,
-
-
*.
4~
I4
f
i
-
-
-
-
-
-
-
t
*
4
-++
,++
.
I.
'
,
:
+
-
-
..+I..
I
-
11~~
-
-
-
I
-
-
.
I
-
I,
1
.
-
-
*
-.
+-
,
-
-
..-
I
-
''
-
-
+
I-I
:
I-- --
-
-I
g
-
-
.
--
.
-
--
-,
.
.
.
-
I.
-
.
-.
-
.
;t
-
-
+-
-
-
.
.4
,
-
-
-
+
t
t-
.
--
4 --
-
- -
*
.I-
.+--*.
1+-.
t
-
t,,
.. +, -+.4-v4
+ +++
~~~~~~~~~~~~~~~
-
-
++
.I-
, -+
.
-,
*.. ,
. -
*
-
I
-
-
T ,
-.-
1 ;
-
-
,*
-
4
-
.
-
.-
t
-
-
-
m
;-
-
+
-
-
-
:
-
,,
+
-
-
*
*
,-
-
-
4
-
+-
-+
*
.4
".
-
--
+
+i
-I-
.
+.
,
,
++
4
++
-
-
-
. .
p
,
-+
-
-
-
.
-:
+.
-
-
--
-
,i
-
-
~
-
--
-
- -
-
++.
++
+
.
a1
-
'
-
.
.
-
-
-
+
t
++
-
+
+
-
--
-
,
-
-
-
-
.
-
..
-
-
.
-
,,
-
+
t
-
-
-
.
- -,
.+
-
+
-
--
:,
-
*.4
-
-
-
-
t
-
-M
t
-
-
I
-
,.
-
-
-
-,
-
'
*.
-..
-
+
-
'
--
ii
.+
+
-
.
:,..,
-
-
-
+
-1+
4
4
-
-
-
-
-
-
--
:.+- II
-+
..
+ + + + +
-,
+.
-.
-.
-
- .,
-.
-
-
- -
++
-
d.
- -
-I
-.
++
+ 4, -.
:
* *
-
-.
,,
-1 J ++,+
*
,
-.
'
:IIi,
''.
- -
-
:.:,4
-I
+
.-
-+
.
-
-
-.
-+
-
*
++
-.
--- -
m
-
- --
-
-
...
-e
-
'-
,
.. *
++-
-
-
-.
:
4.
-
-.
..
* i
.
-
.
-+...
+:4
+,
...
-
-4+-+
+...
+ - .
- --
- -
.
-
-
1-:
u+ .-+.I
-
-
; .
t
++
+ ,'4, t
+.
I
4 .. ....4 - ..
+
1
--
t
- :-
.
-d
mI- ":-
-
-
-.
-
t
-
.
- .
.:::I
. -
!,
-_
'
-
-+
- -
*
-t
-:
*
I.
-
*+
-
- -
+
-1,*-
...
-
-
--
-
t
-.
-
-
-
-'I
,.
-
.
-
-
.
+--
,
-
--
-
-
.-
--
it
.
+
-
--
-
-
- .t
-+
-
-
?
-
-
+ +
.
I
+,
-
-
*
-
,
-
1,I:'
.
t
:
-
--
...
ft
.-
.I-
-
+
-
:::
'
-
-
.
,,.
*
-
*
-
t
-
:
'''
,+
.
-
-
+
-
- -
-
-
-
-
-
,
,
:.
,
-
-
-
.
.
-
I
I.
-
+' -
-
-
'
-,
.
-
-
I
-
-- -
,,m ,
--
.
-,
+
-
'
--
t.
.,,,
I
-
.
-
, M 1
-
-
-"
-
;
*
--
-
,
t
-
-
r
-
-.
-
'
-
.
-
-
-
,
-I
-
-
-
-.
,
*.
-+
.
..
-
-
-+
*
--
-
,
-
1
-
-
-
-
-
.
*
'
-
-
t
-
-.
-
j
.
Dt
+
-
--
*
-
-r+
.. ::I::.
-
T
,I-
-
-
-
I.
I
-
-..
+
-
t
+
.4
.T
-
.
-{,4
..
- -
-
-
-
..-
.
.m-..
- t
.
- +
- . --- .-.
-
i
+--
-
-
-+
'
--
- _, -
-
-
- .
t
-
.I
)
-
-.
.. !.4 7*
-
-
-.
-
.
*
- .
+
-+, - : -- t7o
. , ,
...4-
- -
.
t
.
-- -,I+
-
-
.
- -
++
'+
-
-
::I-
- -
-
- +
+
.+"+t'.
- -...- +
-,
-
+++
- -
-
.
'
-
-
-- -
,.
+
-+ +
'''
.
:i
4
-
**
.'
'
,- -+
'
-.
+++
.
.I
t
':
,
-I- -,
.-
I
*
4
+.-
+- ++
4f--4I
l
-
1?
+4 +
''.
4
-.
-,
.
.+ e .t+++
'
+ -..
.
-
::-
I,,,,
-+
-
-+
-
---
+ -
+
+*4.
++
+
I
.I-*
''+
+.
: -
..
+..
I+
-
-+
-.-
-
1
t+
'I
-
.
.-
.*
++
4.
- - -
.
-
-
.
'-.
t
t
7
--
-+
+
+.
+ - - -*+
-
-
. .
,,
--
-
0
.
. +
'
t
+ ''
* -'
----.++
- -4.
.-'+
-
.'+
-
-
-
-
-
*
-
-
+
.
,
'
-
-,
.
-
-
-
--
-
.
..
-
--
-
!
-
-
-
-
t
-
-
t?
I
H
-
.
-
-
I - m. -
,
lt
-
t
-
-
,-
++
-
-
M.
--
-
-
-
- ' -*
-:.:::*,IT'
-
-
----
-+
*-+-.
f
+
-
-
-
:t-
*
-
...
..
+
t
- -
t1-
-
-
+
.--
+
-
m.
.-
-
-
:.-
-
-
-
t
I
-
-
+-- - -
-
-
-
-
-
-
18
3.0 Ruthenium Studies
The effect of amine type on the solvent extraction
behavior of the mixed nitrosyl ruthenium nitrato complexes in
nitrate systems was investigated. The amines used were a
primary amine (Primene JMT) and a quarternary amine
(Aliquat 336), and the results of this study were compared
with the extraction behavior of the tertiary amine, TLA (1).
The extraction characteristics of the amines were determined by
varying the aqueous nitric acid concentration, NaNO salting
concentration, amine concentration, phase contact time, and
phase volume ratio. The effect of these variables on the
nitric acid extraction into the organic phase was also
thoroughly investigated.
The order of increasing nitric acid extraction for the
three amines was found to be Primene JMT, TLA, and Aliquat
336. As expected, salting with sodium nitrate increased the
unbound nitric acid in the organic phase significantly in all
three cases. The nitric acid extraction data for Primene JMT
and Aliquat 336 and for both salted and unsalted aqueous phases
correlated well on the basis of the undissociated nitric acid
activity in the aqueous phase. A similar result was found for
The unbound organic acid concentration was found to
TLA (1).
be linear with amine concentration in the region 0.26M to
0.50M for Aliquat 336.
The tertiary amine, TLA, and the quarternary amine,
Aliquat 336, exhibited similar trends in ruthenium extraction
over the range of nitric acid concentration studied. The
peak extraction for both amines was found to occur about 1.5N
aqueous HNO . The primary amine, Primene JMT, exhibited an ~
increasing kuthenium extraction with increasing agueous nitric
acid concnetration and showed no maximum up
to
The order of increasing ruthenium distribution 9.9N
ratio HNO3'
is
Primene JMT, TLA, and Aliquat 336 up to about 5N aqueous HNO
but above about 8.5N HNO this order becomes TLA, Aliquat 336,
and Primene JMT. At contant aqueous HNO concentration,
salting with NaNO was found to increase Lhe ruthenium
distribution rati for all three amines at low acid concentrations but decreased the distribution ratio of TLA and
Aliquat 336 at high acid concentrations. Between 0.26M and
0.50M amine concentration the ruthenium distribution ratio for
Aliq~iat 336 was found to depend approximately otn the 0,5
power of the amine concentration. Washing prior to precontacting had little effect on the extraction behavior of
Primene JMT.
Forty-two rapid dilution extractions were alanyzed to
determine the number of extractable species of nitrosylruthenium in nitric acid systems, the mole fractions of these
species in the aqueous phase at various HNO3 concentrations,
and corresponding partition coefficients of the amines for
these extractable species. This study included 29 TLA
extractions by Skavdahl (1), 3 TLA extractions and 10 Q-33 6
19
extractions from this investigation. JMT was not suitable
for rapid dilution extraction because of low distribution
ratios. Computer analysis indicated that the extraction
data could be represented only by assuming two extractable
species. The mole fractions and partition coefficients of
these two extractable species were determined as functions of
the initial aqueous HNO concentration and the final aqueous
HNO concentration, res ectibely, by a least-squared-error
analysis.
The mole fractions of both species in the aqueous
phase were found to increase with increasing aqueous HNO
concentration, reaching a combined mole fraction for the 3 two
species of 0.35 at the highest HNO3 concentration investigated,
lO.lN. The partition coefficients of TLA for the extractable
species were found to decrease exponentially with increasing
HNO concentration. The more extractable specie had a
pa; ition coefficient 12-40 times greater than the less
extractable specie in the 1.ON-4.9N HNO concentration
region. Q-336 was found to have partition coefficients at
5N HNO concentration which were about an order of magnitude
greate than corresponding TLA partition coefficients.
Equations for the mole fractions and the TLA partition
coefficients over the range of acid concentrations
investigated have been developed. It was found that significant amounts of both extractable species are extracted
into the organic phase urder all conditions of HNO concentration and phase volume ratios used in these rapid dilution
extractions.
The details of this study are presented in a
separate report (2).
20
4.0 Effect of Uranium Loading on Extraction of Zirconium
and Ruthenium by TLA
Limited studies on the effect of partial uranium
saturation of TLA on the extraction of zirconium and
nitrosylruthenium complexes were performed by Price (3).
The objective was to determine the effect of large
amounts of uranium in the organic phase on the extraction of
zirconium and the aged nitropyl ruthenium (RuNO) nitrato
complex by trilaurylamine (TLA) in toluene. Since uranium is
extracted to a much greater degree than zirconium or
ruthenium, the effect of uranium competition is to lower the
free amine concentration and thus to lower ruthenium and
zirconium extraction.
Conditions of the extraction were 7N and 9N aqueous nitric
acid, 0.3M TLA ia toluene as the organic phase, and 24 hour
contacting at 25 C and 1:1 phase volume ratio. This
represents equilibrium contacting for uranium and zirconium,
and an intermediate condition of very slowly varying
ruthenium extraction.
Conventional chemical separation and spectrophotometric
analysis was used. The uranium spectrophotometric analysis
is based on the thiocyanate complex. Ruthenium was removed
if present in more than (1:100 = Ru:U) by volatilization as
the tetroxide, and carbonate by acidification and boiling.
Ruthenium was separated by the tetroxide distillation, and
collected and measured in basic solution by precipitation as
barium fluozirconate, carried with barium fluosilicate;
fluoride was removed, the acidity adjusted, and the Alizarine
Red S color developed and read. Other methods were used when
the separation was not required.
Uranigm distribution ratios in the presence of ruthenium
and zirconium are shown in Figure 4.1. There is little
effect of either metal. Uranium extraction at high loadings
continues to rise, causing failure of a correlation found
valid by Vaugha and Mason at lower loadings, and indicating
that there are significantly fewer than two TLA molecules
associated with each uranium atom in the organic phase at
high loadings. Further investigation of this, varying total
amine concentration, and at the conditions of salting and
acidity of previous work is recommended.
Ruthenium and zirconium distribution ratios are shown in
Figures 4.2 and 4.3 as functions of the organic phase
uranium concentration. Considerable scatter of the points
due to imprecise analyses is evident. This is due partly to
the low concentrations of zirconium and ruthenium in the
organic phase. Improvements in the precision could be made by
using tracer amounts of radioisotopes for extraction and
100
- F~.
1 Extraecion of Uanium with 0.3 M TLA
-
-..
DI ITM 'Ls24 -hour coht'4c0ting
24 September 1962
R. E. Price
'-4
LoAdinz i f r
p-4
ii
-. 1
*
P
~-A~-.
r-.
2.0
A
- ~-~*~1-~
Legend
Presenat
7
.4
iveso
HNO
9N HNO
A
A
Se Appendix for A ndun.
10
IQO
0
Uranium Concentration,
Aqueous Phase
(gm/1)
V
I
4.
--
-
.-
~
%
,~,-
..I
9
Agr
iron
C
.
ot
*
R. i
le
pik
thI
(
-4
-7-
r
t
i.
LV
44
PrI~4
p~r
4
1
t-.
8
'4
I.
I
A
'
5
_____
4-
~
2.+'F
'17
-
-7
C
4
IS
I
4
(4
i-'
IJ
4
I.
~-'~1
A,
j
4!
-
2
e
44
tbergJ
0.3 Ka' TLA Unsbalted 24 -BOr
(K~4
'1
7
r
I
.1INK
J
I.
00
IT
A
7
--
;
T,
710~
-~T
A7;7
*
I
.4
-~
1
7
'I
T--
;~4
T-
~
-~
-*
*4-1
11 A
I
*1~
V
K.
-~
LL
I
n
0*
h~sTrai4
7,*
C
I
II..'
24
radioanalysis. An added advantage would be the lower
aqueous ruthenium:uranium and zirconium:uranium ratios
required and closer conformity with expected process
conditions. The lines in Figures 4.2 and 4.3 are given by:
Ea = Ae-0.02U
where U = organic uranium concentration, gm/l
and A
Metal
= constant for each case as follows:
Aqueous HNO3 Concentration
7N
9N
Ru
0.0034
0.0059
Zr
0.0013
0.0026
Within the units of the scatter of the experimental data,
the results can be correlated by the same function.
25
oApendix
5.1
Detailed Procedures
5.1.1
Preparation of Niobium Stock Solution
1) Dissolve 2.6g K Nb
(Dis tilled, Deigni
00 16 H 0 in 20 ml of DDW
Vater)-
2) Heat to nearly boiling.
3) Add 1.5 ml conc. HNO3'
4) Continue heating and stirring for 2-3 min.
5) Centrifuge to collect the precipitate.
6)
Decant.
7) Add 5.0 ml hot 2/ NH4 NO3 solution.
8) Centrifuge.
9) Repeat steps (6), (7) and (8) three times.
10)
Add 20 ml of sat. oxalic acid.
11)
Heat with stirring until niobic acid dissolves.
12)
Cool and dilute with DDW to required concentration.
5.1.2
Gravimetric Analysis of Niobium stock solution
1)
Add 12 ml 6M HNO and 0.4 g of KC1'03 to 2 ml of the
stock solutl~on to be analyzed.
2)
Heat to boiling
3) Boil gently with occasional stirring for
5 min.
stirring
.
4) Cool the mixture, add conc. NH OxHwith
to pH of 8-10.
5) Filter on a Whatman No. 42 filter paper (Recycle
filtrate if not clear)
6) Wash with hot DDW.
7)
Ignite in a porcelain crucible at
for 15 min.
8) Weigh as Nb205
8000C.
26
5.1.3
Preparation of the Aqueous Phase
1)
1.0 pc/ml solution of Nb-95 into
Place 10 ml of
a siliconed graduated test tube.
2)
Add 2 ml of 5.6 x 10~7 M natural niobium stock
solution (in 5% oxalic acid).
3)
Add 0.75 of 30.4% H 2 0 2.
4)
Add 5 ml 15.4 N HNO3'
5)
Evaporate under infrared heater to 2 ml.
6)
Add 3 ml 15.4 M HNO
3
7)
Evaporate under infrared heater to 3 ml.
8)
Add HNO 3 to make 7.5 ml of 9.65 N solution.
9)
Place in capped, silicongd glass tube and heat in
100 C.
1 hr at
oven for
10)
Make up desired concentration of aqueous phase by
dilution or by addition of HNO3'
Heat in 8 apped, siliconed glass tube for 1.5 hr
100 C.
at
11)
12)
5.1.4
Cool to room temp.
Titration of Organic Phase for Nitric Acid Content
Figure 5.1 shows a typical titration curve obtained
of the organic phase with sodium hydroxide.
titration
by
determines the unbound nitric acid
inflection
The first
determines the total acid. The
inflection
and the second
by the difference betw~een
is
represented
bound acid
the two inflections.
1
I -
-4-
I
,
.
I
. I
27"
1
. I
1~-
*
I
.
-.
71j
:;:
-
iLl
___
* 4..
~---
-~
~
I
-
EIL 1iT___
-
-4-.
I
-4±
to
.1
.
.
. .
.
-T
-
.-
.
-
-*1
*1
-
.
.
. .t.
.
. .
-
1-
-t
I
4
14
r-
4--
t
pp
-tt
2"
I.
rzf.-
1
-
-+*
.+-
2
-.
*-
--
-
-
I +
I
1
4
.
..
.
+
T
T
V
,..i
.
l
4;
-
t
---
- -t
-
MIt
-
-
--
-
-
o
7 -e
-
. . ....
t
t --
14-
-
-
4
4--
. ...
28
5.2
References
1.
R. E. Skavdahl and E. A. Mason "The Solvent
Extraction of Nitrosylruthenium by Trilaurylamine
in Nitrate Systems". Summary Report, July 1, 1960
to March 31, 1962. Department of Nuclear
Engineering, M.I.T.,
.
June 1, 1962 (MITNE-20)
Also released as TID-16139.
2.
T. H. Timmins and E. A. Mason, "The Effect of
Alkyl Amine Type on the Extraction of Nitric Acid
and Nitrosylruthenium Nitrato Complexes", Department
of Nuclear Engineering, M.I.T., April 1, 1963
(MITNE-30).
3.
R. E. Price "Zirconium and Ruthenium Extraction by
Trilaurylamine at High Uranium Loading", S. M.
Thesis, Department of Nuclear En ineering, M.I.T.,
Cambridge, Mass. (January, 1963).
4.
V. C. A. Vabghen and E. A. Mason, "Equilibrium
Extraction Characteristics of Alkyl Amines and
Nuclear Fuels Metals in Nitrate Systems" Summary
Report, July 1, 1958 to July 1, 1960. Department
of Nuclear Engineering, M.I.T., October 1, 1960.
Also released as TID-12665.
5.
F. S. Martin, "The Chemistry of Ruthenium Part 1.
The Formation and Examination of an Extractable
Ruthenium Nitrate in Macroscopic Amounts", E. a,
AERE- C/R''816 -951)
**
6.
C. J. Hardy and D. Scargill, "The Extraction of
Niobium (V) from Nitric Acid Solution by
Tri-n-Butl Phosphate (TBP) J. Inorg. Nucl. Chem.,
1960 Vol. 13, pp. 174-180
rs/4/8/63