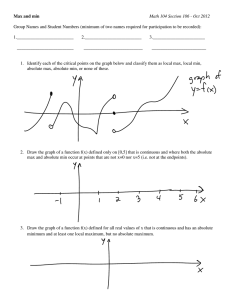
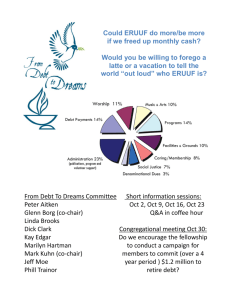
High Speed Data Acquisition System for BARKER 200
advertisement

High Speed Data Acquisition System for Optical Coherence Tomography BARKER MASSACHUSETTS INSTITUTE OF TECHNOLOGY by APR 2 4 200 Tony Hong-Tyng Ko LIBRARIES B.S., Electrical Engineering and Computer Science B.S., Bioengineering University of California at Berkeley, 1997 Submitted to the DEPARTMENT OF ELECTRICAL ENGINEERING AND COMPUTER SCIENCE in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE at the MASSACHUSETTS INSTITUTE OF TECHNOLOGY October 2000 ©Massachusetts Institute of Technology 2000 All rights reserved Signature of Author Department of Eflectrical Engineering and Computer Science October 2000 Certified by Professor James G. Fujimoto 0he i ervisor Accepted by ulnairman, Department C6mftfiittee on Graliuate Students High Speed Data Acquisition System for Optical Coherence Tomography by Tony Hong-Tyng Ko Submitted to the Department of Electrical Engineering and Computer Science on September 30, 2000 in partial fulfillment of the requirements for the degree of Master of Science Abstract Data acquisition systems currently utilized for optical coherence tomography (OCT) imaging have limited data acquisition rates that hinder high speed systems such as the clinical OCT system. Recently, advances in OCT technology have enabled the imaging of the tissue functional state in addition to the imaging of tissue microstructure. These functional imaging techniques such as Doppler OCT and Spectroscopic OCT require the digitization of the full interferometric fringe pattern instead of digitizing just the demodulated fringe pattern envelopes. Because the current data acquisition rates are not sufficient to acauire the full fringe pattern, the development of a high-speed data acquisition system for OCT signal measurement is required to study Doppler and Spectroscopic OCT effects. In this project, we propose to develop a PCbased high-speed data acquisition system that is capable of being used in a variety of OCT systems and a number of OCT delivery devices. The development of this high-speed data acquisition system will enable a number of OCT projects, in both clinical and laboratory settings, to be investigated. Thesis supervisor: Professor James G. Fujimoto Department of Electrical Engineering and Computer Science 2 Acknowledgements I wish to thank my research advisor, Professor Jim Fujimoto, for providing me the guidance, the resources, and the environment for completing this thesis work. Post docs Xingde Li, Wolfgang Drexler, Ingmar Hart and Christian Chudoba have been tremendously in helping me understand the science of OCT as well as the science of pursuing rigorous academic research. Their help was also essential in the obtaining the wonderful images from the OCT systems. I would like to thank UROP student Michael Liu who was instrumental in setting up the DirectX technology to allow real-time display of our OCT images. HST MD student Ravi Ghanta is responsible for the development of the display and analysis portion of the system presented in this thesis. He has been a friend and a great programming partner in this long process. Costas Pitris provided a lot of technical help and useful suggestions with our setup and acquisition system. Pei-lin Hsiung has also been very helpful with setting up the OCT systems for the clinical runs as well as providing technical suggestions. Finally, I would like to thank my family for their love and support. 3 Table of Contents Abstract ................................................................................................................... 2 Acknowledgements .................................................................................................. 3 Table of Contents ................................................................................................... 4 Chapter 1 Introduction 1.1 ............................................................................. 6 Scope of Thesis................................................................................................9 Chapter 2 Overview of O CT System s................................................ .10 2.1 Bench-top OCT Imaging System........................................................................ 10 2.2 Clinical OCT Imaging System........................................................................ 12 2.3 Ultrahigh Resolution OCT Imaging System......................................................... 16 2.4 Spectroscopic OCT Imaging System............................................................... 17 Chapter 3 High Speed Data Acquisition for OCT ....................................... 19 3.1 Double Buffering.......................................................................................... 19 3.2 Scan Clock Gating...................................................................................... 21 3.3 DirectX Display........................................................................................... 22 3.4 Bench-top OCT Acquisition System............................................................... 24 3.5 Clinical OCT Acquisition System..................................................................... 27 3.6 Interferometric OCT Acquisition System........................................................ 33 4 Chapter 4 Processing and Display of OCT Images................................ 35 4.1 Image Processing and Display during Acquisition............................................ 35 4.2 Post-Acquisition Image Processing and Display............................................... 42 Chapter 5 OCT Imaging Applications of the High Speed Data Acquisition System ................................... 45 5.1 Bench-top OCT System Images..................................................................... 45 5.2 Clinical OCT System Images....................................................................... 46 5.3 Herriott Cell Delay Line............................................................................. 49 5.4 Spectroscopic OCT Imaging........................................................................ 50 Chapter 6 Future Work and Conclusion...... .................. 52 6.1 Doppler OCT............................................................................................ 52 6.2 Hilbert Transform Technique....................................................................... 53 6.3 Ultrahigh Resolution Doppler OCT .............................................................. 54 6.4 Conclusions............................................................................................. 55 References..........................................**G 5 56 Chapter 1 Introduction Optical coherence tomography (OCT) is an emerging medical imaging technology that enables high-resolution cross-sectional imaging of internal microstructure of biological tissue1 . Since the technique was first presented in 1991, there has been a tremendous surge in the adaptation of OCT technology to various fields of clinical and laboratory medicine. To date, a number of studies have demonstrated the application of OCT in imaging ophthalmic, arterial, gastrointestinal, pulmonary, reproductive, and neural pathologies 2 -15 . In addition, OCT also has been investigated for use in surgical guidance 16 -19 and developmental biology 20 -23 . These wide applications of OCT technology are possible because the fundamental basis of the technology affords it many advantages over other conventional and competing medical imaging modalities. OCT is based on a technique known as optical coherence-domain reflectometry (OCDR). OCDR is a one-dimensional optical ranging technique which was developed in optoelectronic for finding faults in fiber-optic cables and network components 24 , 25. It was quickly recognized that OCDR has the ability to probe the eye and other biological tissues. Optical coherence tomography, a two-dimensional extension of OCDR, was then developed as a new kind of medical imaging modality. OCT is somewhat analogous to ultrasound B mode imaging except that imaging is performed by measuring the intensity of reflected or backscattered light rather than acoustical waves 26 -29 . Whereas ultrasound echo delay time can be measured electronically, the echo delay time of OCT backscattered light must be measured interferometrically because of the extremely high velocity of light. Figure 1 shows the schematics of a typical OCT system 6 setup. Measurements are performed using a fiber-optically implemented Michelson interferometer with a low coherence light source. One arm of the interferometer is a modular probe which directs the light onto the sample and collects the retroreflected signal. The second reference arm includes a translating retroreflecting mirror or scanning delay line. Low coherence light can be generated by a compact superluminescent semiconductor diode or other sources such as a solid-state laser. Optical interference between the light from the sample and the reference mirror occurs only when the optical distances traveled by the light in both the tissue sample and reference path match to within the coherence length of the light, 30, 31. The use of a low coherence light source with short coherence lengths permits the echo delay time and magnitude of the light reflected from internal tissue microstructures to be measured with extremely high accuracy and sensitivity. Figure 1. Schematics of a typical OCT system setup Beam Delivery Instrument Low-Coherence Light Source Specimen Fiber Optic 50/50 !Cpler Optical Pathlength Scanning Photodetector Badps FITr -- Envelope Detector-.A- -- Cmur The principle of optical coherence tomography imaging is shown in Figure 2. Tomographic OCT images are generated by scanning the optical beam across the tissue and measuring the echo delay time (or optical path lengths) and intensity of the backscattered light as a function of transverse position. By exploiting the short temporal coherence of a broadband 7 light source, OCT can image tissue microstructures at depths beyond the reach of conventional bright-field and confocal microscopes. Unlike ultrasound, optically based OCT can acquire images without direct contact with the biological tissue. The fiber optic implementation also provides a compact and low-cost system which can be easily interfaced with a wide range of clinical imaging instruments such as catheters, endoscopes, hand-held probes, colposcopes, biopsy needles, and microscopes. Therefore, OCT can function as a type of "optical biopsy" to permit the imaging of tissue microstructure with resolution approaching that of standard excisional biopsy and histopathology, except that imaging can be performed in real time and without the removal of the tissue specimen 2 , 32, 33. However, in order to be useful for surgical guidance or clinical screening, the data acquisition and OCT image display must be done in such a way as to provide the clinician with real-time imaging and feedbacks. Transverse Scanning Backscattered Inten ity Axial Position (Depth) 2D Grey Scale or False Color Image of Optical Backscattering Figure 2. OCT generates cross-sectional images of tissue internal microstructure by scanning across the tissue to measure the backscattered intensity as a function of axial and transverse position. A two-dimensional grayscale or false color image can then be displayed. 8 Recently, advances in solid-state laser technology have led to the development of broad bandwidth laser light sources capable of achieving OCT imaging 34 . The high power output and extremely broad bandwidth of these light sources have enabled the development of ultrahigh resolution OCT imaging at 1-3 jim resolution 35 . The bandwidth of these light sources also enables functional imaging techniques such as spectroscopic OCT imaging 36 and possibly ultrahigh resolution Doppler OCT imaging. To extract spectroscopic and Doppler information, it is necessary to record the full interference fringe signal instead of only the fringe envelope. Therefore, the development of a data acquisition system capable of achieving high speed analogto-digital conversions of the interferometer output is required to study Doppler and spectroscopic OCT effects. Additionally, a high speed data acquisition system capable of real-time acquisition, display, and archiving needs to be developed before meaningful OCT imaging studies can be conducted in the clinic. 1.1 Scope of the Thesis The need for a high speed data acquisition system is motivated by the demanding requirements of spectroscopic and Doppler OCT imaging and real-time clinical OCT imaging. Therefore, the goal of this thesis is to develop a complete high speed data acquisition system capable of accomplishing the numerous tasks and demands of the different OCT systems. The first part of the thesis (Chapter 2) is an overview of the different OCT systems, their different data acquisition demands and software control issues. The data-acquisition system setup, its theories of operation, and its associated waveform generation and controls are discussed in Chapter 3. Data analysis, processing, and display of the OCT images taken with the new acquisition system is described in Chapter 4. The next section (Chapter 5 & 6) explores the integration of new acquisition system to various OCT systems. Chapter 5 examines and assesses the system performance using both in vitro and in vivo OCT images as well as spectroscopic OCT images. Chapter 6 investigates the feasibility of using the high speed acquisition system to obtain ultrahigh resolution Doppler OCT images. The future scientific extension of the system developed in this thesis is also discussed in this chapter. 9 Chapter 2 Overview of OCT Systems In order for the data acquisition system to be useful, it must be compatible with all the currently available OCT systems as well as anticipate future changes and additions. This chapter reviews the current state of OCT technology and the various laboratory and clinical imaging systems that the data acquisition system must interface with. 2.1 Bench-top OCT imaging system The standard implementation of the OCT system as shown in Figure 1 (Chapter 2) is to use a fiber optic coupler and a galvanometer scanner to mechanically scan the reference arm retroreflector in a linear fashion. The motion of the reference arm mirror scans the specimen axially while precision stages translate the specimen along the two other dimensions. This way OCT imaging can be done on the specimen across any desired cross section. The linear scanning mirror will introduce a Doppler shift in the detected interferometric signal frequency. This new Doppler shifted central frequency will be: 2v fd = 2 (v = scanning velocity, X0 = light source central wavelength) The subsequent detection electronics and filters must be optimized for the Doppler frequency and bandwidth in order to ensure that the digitized signal will be the most optimum. The acquisition system must generate waveforms to drive the linear scanning galvanometer and 10 control the two translation stages. Furthermore, the acquisition must be timed such that data is only collected during the linear scanning (constant velocity) portion of the galvanometer scanner in order to ensure that all the acquired signals have the same Doppler shifted central frequency. Several light sources can provide the required low-coherence light for this system. Depending on the desired central frequency, spectral bandwidth, penetration depth, and incident power, either an AFC amplified SLD (X0 = 1.3 gm; resolution = 15 gm), a solid state Cr:Forsterite laser (X0 = 1.3 gm; resolution = 6 gm), or a solid state Ti:Sapphire laser (Xo = 0.80 gm; resolution = 1 gm) can be used. Light sources with longer central wavelengths can provide better penetration into scattering tissue while sources with broader spectral bandwidths can provide higher image resolutions. For ophthalmic imaging, 800 nm low-coherence light sources are used in order to minimize the effect of water absorption presented by the vitreous of the eye. For in vitro imaging, the beam delivery optics for this system usually consists of a microscope setup which focuses the OCT beam onto a specimen that sits atop of the translation stages. The bench-top system can also be easily integrated into an ophthalmic slit-lamp to achieve in vivo ophthalmic imaging of the different structures in the eye. Figure 3 illustrates the different delivery optics for this system. Lm ouce CA Collimating Lens Fiber Mirror.. Fiber-Optic Intebfer o Z translation rr r Detector stage Transverse S .. ...... . !!!!!!!Scanning Microscope Condersing LensObjective Bearmsplitter Slit-lamp Viewing Objective A Sample Imnage Plane B x-Y translation stages Figure 3. Two different beam delivery devices for the bench-top OCT system. A: Bench-top OCT system setup and the ophthalmic slit lamp beam delivery system. B: OCT imaging microscope beam delivery system setup. 11 Both delivery systems require a combination of collimating lens and focusing objectives in order to focus the OCT beam onto the desired imaging plane. The slit-lamp system also consists of a pair of computer-controlled orthogonal galvanometers to scan the beam in the eye. The orthogonal galvanometer scanners are required because they can scan any arbitrary pattern and can do it much faster and easier than the translating stages. The detection electronics for this system consist of a photodetector that is connected to a transimpedance amplifier. The signal then passes through a bandpass filter which removes background noise. The bandpass filter is centered around the Doppler shifted center frequency and has a broad enough bandwidth to pass through all the signal frequencies while removing much of the background noise. The signal is then demodulated using either a linear envelope detector or a logarithmic envelope detector before the envelopes are digitized by the analog-todigital converter of the computer. The electronic circuitry is optimized for each OCT system based on the acquisition speed, Doppler frequency, and light source central wavelength and bandwidth that is required. 2.2 Clinical OCT Imaging System The bench-top OCT imaging systems just described utilizes a linear scanning reference arm mirror to perform axial scanning. The reference arm retroreflector is mounted at the end of a lever arm that is rotated by a galvanometer. Mechanical inertia limits this scanning delay line setup to a maximum of -200 axial scans per second before the galvanometer response completely loses its linear scanning portion. Although this axial scanning speed is adequate for in vitro OCT and in vivo ophthalmic OCT imaging, it is not sufficient for clinical in vivo OCT imaging where motion artifacts require higher acquisition speeds to compensate. For example, in order to maintain 4 frames per second video rate at 500 axial scans per frame, an axial scanning rate of 2000 Hz is required. Since it is not possible for the linear mechanical scanner to achieve this acquisition rate, a different type of delay line setup needs to be used for the clinical OCT imaging system. 12 An optical phase delay line was developed to address the issues that real-time in vivo OCT imaging presents 37 . In this setup, the collimated reference arm light enters the delay line and hits a grating which separates its component wavelengths. After a lens collimates the diffracted wavelengths, the whole beam then hits a rotating mirror set at the focal distance of the lens. The mirror tilt introduces different travel paths for the different wavelengths in the beam which results in a wavelength-dependent phase delay. Because the scanning mirror is set in the Fourier plane of the lens, the linear phase difference in the Fourier domain translates into a time delay in the time domain. Therefore, axial scanning in this delay line setup is accomplished by scanning the angle of the mirror to produce a time dependent optical group delay. The introduction of the optical group delay in the reference arm of the OCT setup is equivalent to changing the optical path length in the reference arm, thereby achieving axial scanning of the sample. Figure 4 illustrates the basic setup of the optical phase delay line. collimator e f ce fiber f grating d Y () 0XX rotation axi k.j scanning mirror L Xo Figure 4. Schematics of the optical delay line in the reference arm of the OCT system. The beam enters the setup and hits the grating which separates the component wavelengths. The linear phase delay at the Fourier plane introduced by the scanning galvanometer mirror introduces an optical group delay in the time domain. Since the galvanometer can achieve axial scanning simply by rotating the mirror, a much higher axial scan rate than the mechanical delay line can be achieved due to the diminished 13 rotational inertia. The grating-based optical phase delay line has been demonstrated to sustain axial scanning rates of up to 2000 per second. In this setup, the data acquisition system must generate the waveform to drive the galvanometer scanner and to account for the non-linearity and hysteresis that are inherent in high-speed mechanical motion. Furthermore, the acquisition must be timed such that data is only collected during the linear portion of the galvanometer scanning. The low coherence light source currently used in this clinical OCT imaging system is an amplified Superluminescent diode (SLD) made by AFC Technologies. This source was chosen for the in vivo system because of its compact, portable and robust design. It generates low coherence light centered at 1.3 ptm with enough spectral bandwidth to support 15 gm OCT imaging resolution. By using an amplified SLD, the AFC source also can generates an order of magnitude more power than traditional semiconductor SLDs. In the future, it is anticipated that solid state laser sources such as the Cr:Forsterite and Ti:Sapphire laser will be integrated into the clinical system to provide even higher resolution for clinical in vivo OCT imaging. The delay line setup, acquisition system and control software all need to be designed to take into account this possibility. Many different beam delivery systems exist for the clinical in vivo OCT system. These delivery mechanisms can easily integrate into current clinical diagnostic instruments and have allowed the in vivo system to image in a vast number of different clinical areas. These beam delivery devices include a forward imaging OCT probe, an OCT imaging colposcope, and an OCT imaging catheter/endoscope. The forward imaging probe was developed for imaging in either an open surgical field, a laparoscopic setting, an oral cavity or on the surface of the skin. In this design, a galvanometer scans a collimated beam to a lens relay which translates the galvanometer rotational scanning to a linear displacement of the OCT beam at the imaging plane of the device. The distal optics of the forward scanning probe can be removed and sterilized, and the whole probe can be draped with a sterile plastic bag for imaging in a sterile surgical field. The OCT integrated colposcope was developed because the cervix is an ideal clinical scenario for the investigation of OCT imaging possibilities. Using this device, the in vivo OCT imaging system is directly integrated with the optics of a standard colposcope. This setup ensures better 14 registration of the images by allowing both colposcopic and OCT imaging to be done at the same time. Two orthogonal galvanometer mirrors are used in this system to scan the OCT beam, permitting fast and easy transverse scanning in any desired pattern. A fiber optic catheter that can be easily inserted into the accessory port of an endoscope was developed to investigate OCT imaging of lumenal structures. The beam is focused by a graded index (GRIN) lens and is directed perpendicularly out of the catheter by a microprism. The entire optical setup is enclosed in a transparent housing which can be sterilized. The OCT beam can be scanned either circumferentially for the small lumens of the cardiovascular system or linearly for the large lumens of the gastrointestinal or pulmonary system. Circumferential scanning is done by rotating the speedometer cable attached to the distal optics inside the transparent and stationary enclosure while linear scanning is done by translating the same cable in a push-pull fashion. Figure 5 illustrates the different beam delivery devices for the in vivo OCT system. The acquisition system must generate the drive waveforms for each of these beam delivery systems as well as ensure that the OCT beam scanning is synchronized with the data acquisition. Vertical scanning Samnple A fib scanning galvanometer SPEEDOMETER CABLE OPTICAL RBER GFN LENS RGHT ANGLE PFISM II OUTER SHEATH (STIONARY) MANSPARENT vwmOw V ) Fiber Dubet+ connector lens B Figure 5. Schematics of the different beam delivery mechanisms for the in vivo OCT system. A: The forward imaging translates rotational galvanometer scanning into linear scanning of the beam at the focal plane. B: The OCT imaging catheter can be easily inserted into an endoscope accessory port to C: The OCT integrated colposcope can achieve lumenal imaging. images of the cervical structures. colposcopic simultaneously obtain OCT and 15 * 30 cm C 2.3 Ultrahigh Resolution OCT Imaging System Since the axial resolution of OCT is determined to the spectral bandwidth of the lowcoherence light source, fundamental advancements in the light source are required to improve the resolution of OCT imaging. A state of the art Titanium Sapphire solid state laser which can support broad spectral bandwidths has been developed to improve the resolution of OCT imaging. This ultrahigh resolution OCT imaging system is similar to the bench-top imaging system except that it utilizes the Ti:Sapphire laser as the light source and all the optics have been optimized to support the broad bandwidth of this source while keeping the dispersion balanced. It has been demonstrated that this system can achieve an imaging resolution of 1.5 pm in air or 1 pm in tissue 35 . The ultrahigh resolution OCT system can be interfaced with either the OCT microscope, the slit-lamp ophthalmoscope, or the forward imaging probe. Therefore, ultrahigh resolution OCT can offer an order of magnitude better resolution for in vitro imaging, ophthalmic imaging, and in vivo surface imaging. Figure 6 demonstrates the concept behind the ultrahigh resolution OCT imaging system. Ti:Sapphire 260 nmn 1 Z\ 0.5 11.5pm - 0.5 3 1.5pm -8 -4 0 650 700 750 850 900 800 Wavelength (nm) 950 1000 -12 0 Delay (pm) -4 -8 +12 Figure 6. Ultrahigh resolution OCT imaging concepts. A: The spectrum delivered through the OCT system with the Ti:Sapphire laser (solid line) versus the standard SLD (dashed line). The full-width-half-maximum (FWHM) bandwidths are indicated. B: The OCT imaging resolution of the system with Ti:Sapphire laser (solid line) is 1.5 tm versus the 11.5 ptm of the standard SLD (dashed line). The data acquisition for the ultrahigh resolution system must be able to acquire samples fast enough in order to have the adequate pixel density to accommodate the higher resolution. 16 For example, at a galvanometer linear scanning speed of 82 mm/s, it takes the galvanometer 12.2 ms to scan a distance of 1 mm. In order to get 0.2 pm pixel density, 5000 pixels are required for every axial scan which translates into a data acquisition rate of 410 kHz. At this scan velocity and distance, a single axial scan is completed in about 20 ms, so the computer needs to transfer, process, and display the acquired 5000 pixels in less than 8 ms. 2.4 Spectroscopic OCT Imaging System The extremely broad bandwidths which are available using novel solid state laser sources will also enable spectroscopically resolved OCT imaging. By using the same Ti:Sapphire laser as in ultrahigh resolution OCT, it has been demonstrated recently that the entire spectrum of the backscattered light can be measured simultaneously on a micron scale 36 . Figure 7 shows a schematic of how spectroscopic OCT works in comparison with conventional OCT. - (Red) Envelope: OCT Reflectivity I Scattering Carrier Spectrum: Spectroscopic OCT -o Spectral Reflection /Scattering = Absorption Figure 7. Standard OCT images measure the backreflected light intensity by digitizing the demodulation envelope of the interferometric output. Spectroscopic information can be obtained by detecting and digitally processing the full interference signal. Since the different wavelengths of the OCT light are mapped into different frequencies of the interference signal, the broad spectral bandwidth (260 nm) of the Ti:Sapphire laser will allow simultaneous investigation of the scattering properties of tissue across many wavelengths. Laser light sources that can support broad spectral bandwidths have been developed for the near 17 infrared wavelength region of 700-1500 nm. This wavelength region overlaps the biologically interesting region of hemoglobin and water absorption bands. Therefore, the wavelength dependent scattering of the tissue measured by spectroscopic OCT can be used to improve image contrast between different tissue morphologies, functioning as a kind of "spectroscopic staining" in analogy to histologic staining36 . In order to extract spectroscopic information it is necessary to record the full interference fringe signal instead of only the envelope. Spectroscopic information can then be calculated at any point in the OCT interference signal by using Fourier, wavelet, or Hilbert transform analysis. Doppler flow measurements can also be detected from the full interference signal by using simple modifications of this approach 38 -40 . Recent studies have demonstrated extremely high sensitivities to Doppler flows as small as 10 Jtm/s by using OCT interferometric signals and phase retrieval techniques 41 . By utilizing the novel laser light sources of ultrahigh resolution OCT, it may be possible to accomplish ultrahigh resolution and ultrahigh sensitivity Doppler flow measurements. However, both spectroscopic OCT and Doppler OCT require a data acquisition system that can perform extremely high speed analog-to-digital (A/D) conversion of the interferometer output. Continuing the example from the last section, at a galvanometer linear scan speed of 82 mm/s, it takes the galvanometer 12.2 ms to scan a distance of 1 mm. In order to acquire the interferometric signal, we have to know that there are about 4 fringes in the fullwidth half-maximum region of 1.5 pim. Oversampling the fringes to give 15 data points per fringe means that our pixel density has to be 1.5 gm / 60 = 25 nm. This means that at least 40,000 points needs to be acquired in 12.2 ms; this translates into a data acquisition rate of 3.28 MHz. At this scan velocity and distance, a single axial scan is completed in about 20 ms, so the computer needs to transfer, demodulate, and display the acquired 40,000 pixels in less than 8 ms. The development of an acquisition system that can satisfy these requirements will be the focus of the rest of this thesis. 18 Chapter 3 High Speed Data Acquisition for OCT As pointed out in the previous chapter, a high speed data acquisition system must be able to digitized, display, and archive the data in real-time and without interruptions. This chapter will address some of fundamental issues involved in attaining accurate and complete acquisition of the streaming data. Double buffering techniques are used to ensure adequate memory handling of the streaming data while scan clock gating is used to guarantee continuous triggering for the complete acquisition of the axial scan data set. In a real-time data streaming and display situation, DirectX technology is employed to render high-speed images to the screen. Additional system-specific waveform generation and high-speed timing synchronization solutions of the acquisition system are also presented in this chapter. 3.1 Double Buffering In most common data acquisition situations, the buffering method used is called single buffering. In a single-buffered input operation, a fixed number of samples are acquired at a specified rate and transferred into the computer's system memory. After all the samples have been stored in the memory buffer, the computer can analyze, display, or save the data to the hard disk for later processing. Although single-buffering operations are relatively simple to implement, they suffer from several disadvantages that render it ineffective for high-speed data 19 acquisition. The major drawback of a single-buffered operation is that the acquired data cannot be accessed for processing or displayed until the entire acquisition task is finished. This presents a problem because, for most OCT applications, a real-time update of the most recently acquired axial scan is desired. It is possible to get around this problem by setting the single buffer to equal to the size of a single axial scan. However, this setup requires the computer to restart the single buffering operation after each axial scan, and the time involved in setting up and restarting the acquisition can cause individual axial scans to be skipped over by the acquisition. Another disadvantage of single buffering is that the amount of data that it can input or output at any one time is limited to the amount of available system memory, and the number of data points to input or output must be known ahead of time. In a clinical situation, there is no way to know a priori how many images will be collected by the OCT system and thus it is impossible to use the single buffering acquisition method. The solution to these problems is to use a double-buffered data acquisition setup. In a double-buffered operation, the acquisition data storage in the system memory is configured as a circular buffer consisting of two half-buffers. Once the acquisition card has filled up the first half-buffer with data, it can be transferred to a separate storage buffer in memory for processing and display while the acquisition card continues to fill up the second half-buffer. When the end of the second half-buffer is reached, the device returns to the beginning of the first half-buffer and fills it up with data once again. This process can continue without end unless it is interrupted by a hardware error or cleared through a function call. In this manner, a doublebuffered operation is able to input or output an infinite number of data points without requiring an infinite amount of system memory, and real-time display of the collected scans can be achieved. Although the double-buffered acquisition scheme is suitable for high-speed OCT imaging, there are a couple of potential setbacks for this acquisition method. The first is the possibility that the acquisition device has overwritten one of the half-buffers before the computer has a chance to transfer the data out. In this situation, the untransferred data is lost forever and cannot be recovered. A second possibility is that the device starts to overwrite one of the halfbuffers while the computer is in the process of copying that data to a storage buffer. In this 20 situation, the transferred data is corrupted and contains a combination of the new and old data values that are present in the half-buffer. Both of these scenarios would stop the double-buffered data acquisition process and return an error to the application program. Therefore, the limitation of a double-buffered operation is determined by the ability of the CPU to process the data within a given period of time. Specifically, the data in one half-buffer must be transferred and processed by the software application in the amount of time it takes for the acquisition card to fill up the other half-buffer with data. The effectiveness of the double-buffered operation thus depends on the speed and efficiency of the computer system and the programming language. 3.2 Scan Clock Gating In most OCT acquisition situations, a trigger signal is used to initiate the start of digitization for each axial scan. However, it is not possible to have the digitization repeatedly triggered by a series of start signals. In a fashion similar to the single-buffered operation, the triggering circuit must be turned off and restarted before the digitization can be re-triggered, and the time involved in restarting the triggering circuit can lead to the skipping over of individual axial scans in between restarts. The solution to this problem is to gate the digitization with a method called scan clock gating. Here, the internal scan (digitization) clock of the data acquisition card is gated by an external TTL signal. The acquisition can be set up such that the scan clock pauses while the gating signal is low and resumes when the gating signal goes high (or vice versa). Because this triggering scheme does not require the triggering circuit to be turned off during acquisition, all of the axial scan are guaranteed to be digitized. Figure 8A illustrates the timing diagram for this scan clock gating mechanism. It is important to note that the scan clock is based on an underlying pixel clock whose output frequency is set to the desired digitization frequency at the start of acquisition. As Figure 8B points out, it is important for the rising edge of the gating signal to be synchronized in relation to the pixel clock. If this is not the case, signal digitization also will not be synchronized among the different axial scans. The gate signal can easily be synchronized to the pixel clock by making the period of the gate signal a multiple of the period of the pixel clock. This way, the 21 gating signal will always be initiated, in relation to the pixel clock, at the same time; and the signal digitization is guaranteed to be equivalent scan after scan. Gate Signal Gate Signal Pixel Clock Scan Clock Scan Clock Each rising edge indicates Different gate initialization time results in different data digitization data digitization B A Figure 8. A: Scan Clock Gating Timing Diagram. The scan clock pauses when B: the gat signal is low and resumes when the gate signal is high. Unsynchronized initialization of the gating signal can lead to differences in the data digitization. 3.3 DirectX Display Through the use of double-buffering and scan clock gating, it is possible to digitize a streaming set of OCT signals into the system memory at extremely high speeds. However, for the digitized data in the system memory to be useful, it needs to be processed, displayed, and eventually stored to the computer hard disk. It was pointed out in Section 3.1 (Double Buffering) that the CPU only has a limited amount of time to transfer, process, and display the data before the circular buffer is overwritten. Therefore, it is highly desirable to minimize the CPU time necessary to accomplish these tasks. The amount of time required to transfer data out of the double-buffered storage and into the hard drive can be minimized through the use of Direct Memory Access (DMA) transfers. DMA transfers use hardware to accelerate the transfer of data between the system memory and storage devices. The DMA chip performs the transfers independent of the CPU, making it available to execute other instructions in the application. However, the CPU and the DMA chip 22 share control of the same bus, so some decline in computer performance can occur even when using DMA transfers. In order to display the OCT images onto the computer screen in a real-time manner, only a small amount of processing is needed. Under most situations, the acquisition software simply needs to determine where on the screen each data point needs to be displayed and what color it should be displayed as. For interferometric OCT imaging, each axial scan needs to be digitally demodulated before it can be displayed onto the computer monitor. Data processing algorithms used for real-time display need to be optimized for speed in order to minimize the CPU utilization, and these issues will be discussed in Chapter 4. However, the biggest problem with real-time display under a double-buffered acquisition scheme is the speed of the actual image display onto the screen. In the Windows operating system environment, the Graphics Device Interface (GDI) is responsible for rendering images onto the computer screen. However, the Windows GDI is much too slow for real-time multimedia applications and displays. Therefore, it is necessary to use DirectX technology for the real-time display portion for our OCT application. DirectX was created for computer game developers to address the real-time response issues of the Windows operating environment. For our application, we only need to access DirectDraw, the portion of DirectX that deals with real-time displays to the screen. It is composed of a number of fast and low-level libraries that allow the software application to directly access the video card memory and interface with the underlying hardware. DirectDraw is also able to display the image onto the screen faster than Windows GDI because it can use the hardware assistance of the computer's video card. When the hardware provides support for a particular operation such as image stretching or rotation, DirectDraw can just set up the hardware and then immediately returns control of the CPU. If the hardware does not support a particular function, DirectDraw will emulate that operation in software with the most efficient algorithm. In this manner, DirectDraw is able to minimize the CPU utilization time and achieve real-time display under a double-buffered acquisition situation. The integration of DirectDraw into our OCT application was found to be absolutely essential in order to achieve real-time imaging of several frames per second in the clinical OCT system. 23 3.4 Bench-top OCT Acquisition System Double-buffered acquisition, scan clock gating, and DirectX display are fundamental components that are used in every high speed OCT acquisition system. However, each OCT system has specific waveform generation, data synchronization, and real-time display requirements. This section will describe the specific acquisition and waveform control issues of the bench-top OCT system. The acquisition software for the bench-top OCT system needs to generate the drive waveform for the linear scanning galvanometer, control the transverse scanning stages, control the slit-lamp orthogonal galvanometers, and synchronize the data acquisition with each of these beam scanning mechanisms. 3.4.1 Linear Scanning Delay Line The sawtooth drive waveform of the linear scanning galvanometer consists of a linear scanning portion and a half-sinusoid return portion. The linear portion of the waveform must have the correct slope in order to yield the scan velocity required by the detection electronics (see Chapter 2). Since no axial scanning can be done in the return portion of the waveform, it is desirable to minimize this return time. The performance perimeter of the galvanometer response determines how much of the return time can be minimized without significantly affecting the linear scanning portion of the waveform. This minimum galvanometer return times can be experimentally determined for the different galvos used. The sluggish galvanometer response also dictates that the linear region of the waveform has to be 1.3 times longer than the actual desired axial scan depth. This adjustment is necessary to ensure that the desired axial imaging depth can be scanned by the galvanometer in a truly linear fashion. Figure 9 illustrates the drive waveform of the scanning delay line along with the acquisition timing scheme. In Figure 9, the timing signal generated by analog output channel 2 is synchronized to the start of the waveform generation at analog output channel 1. The galvanometer response to the drive waveform of channel 1 is delayed in time due to the mechanical inertia of the galvo. The gate signal which controls signal digitization through scan clock gating is triggered by the start of the timing signal and is placed in the linear scanning region of the galvanometer response. 24 Since the acquisition is done in a double-buffered manner, the duration of the gate signal must be controlled carefully so that only the exact amount of data points fall under the acquisition gate. Because of the continuous nature of double-buffered data acquisition, a single-pixel error in the gate duration time will cause the error to propagate throughout all the axial scans and lead to a skewed image. As previously described in the scan clock gating section, it is important for the gating signal to be synchronized to the pixel clock in order to ensure equivalent signal digitization conditions for the different axial scans. In addition, the pixel clock of our particular acquisition card is based upon a 20-MHz update clock; therefore, only acquisition rates that are divisible from 20 MHz are available for digitization. For example, the card can acquire data at 20 MHz and 10 MHz, but cannot acquire data at 15 MHz. In certain cases, this limitation prevents the acquired data points from completely spanning the desired axial scan depth and this deviation must be noted to the user. Analog Output Channel 1 Gate Signal Analog Output Channel 2 Time Figure 9. The drive waveform and acquisition timing scheme for the bench-top OCT system. Analog output channel 1 represents the sawtooth drive waveform of the linear scanning delay line. Analog output channel 2 represents the timing signal which is synchronized to the start of sawtooth waveform generation. The dashed line represents the delayed galvanometer response. The gate signal (shaded lines) indicates data acquisition and is triggered by the timing signal. 3.4.2 Methods of Acquisition There are two modes of operation for the bench-top OCT system, an aiming mode and an acquisition mode. In the aiming mode of operation, the acquired signal is continuously displayed onto the computer screen without being saved to the hard disk. Therefore, the double buffer is 25 set up such that each half-buffer can only receive the amount of data for one axial scan. The acquired data is then transferred into a separate storage buffer, equal in size to the half-buffer, in the system memory. The data in the storage buffer is processed through a position and color map to determine their display parameters. After the axial scan is display, the data in the storage buffer can be discarded because aiming mode does not require the data to be saved onto the hard disk. With the use of double buffering and a very small storage buffer, the aiming mode of acquisition can continue to display axial scan information until the operator can make all the adjustments necessary to place the desired OCT signal onto the screen. In the acquisition mode of operation, the OCT software needs to generate the necessary scanning commands for the translation stages or the slit-lamp galvos before the start of acquisition. Again, the double buffer is set up for each half-buffer to store the data of a single axial scan. However, the storage buffer in system memory now has to be large enough to store the entire OCT image because the data will not be saved to the hard disk until the end of image acquisition. Doing the saving at the end of acquisition prevents the possibility for the hard disk saving operation to interrupt with the double-buffered acquisition operation, but it also limits the image data size to the amount of available system memory. Except for interferometric (spectroscopic or Doppler) OCT images, this constraint does not present a problem for most imaging situations. In both modes of operation, a display window will plot the data values of the current axial scan in a manner similar to that of an oscilloscope to give the operator more realtime feedbacks. 3.4.3 Beam Delivery Devices To accomplish accurate OCT imaging, the acquisition software needs to control and synchronize the beam delivery scanning device to the data acquisition. The easiest way to accomplish this is to design the scanning pattern such that its number of update points is equal to the number of axial scans in the image. By using the gating signal of the acquisition as the update clock, the scan pattern will then only advance one point for every axial scan. To ensure that the OCT beam is not moving during signal digitization, the delivery device will only advance to the next scan point upon the falling edge of the gating signal. 26 Both slit-lamp galvanometer and translation stage movements can be synchronized using this acquisition scheme, but the translation stages can only move one winding tick (either 1 gm or 0.1 jim) for each pulse of the update clock under this step-and-repeat modality. A faster scanning method (continuous scanning) for the translation stages is usually desired. In that scenario, a set of General-Purpose Interface Bus (GPIB) commands can be used to instruct the translation stages to move at a desired linear scanning speed. However, a non-linear acceleration and deceleration phase is required at the beginning and end of the translation stage movement in order to achieve constant speed scanning. These non-linearities will introduce non-uniform transverse pixel densities to a beginning and end section of the image. Another problem presented by this scanning method is that the OCT beam is constantly moving across the tissue sample, even during periods of signal digitization. This effectively causes the OCT beam to axially scan into the tissue in a slanted fashion. Since the problems caused by the continuous scanning of the translation stages cannot be easily overcome, adequate pixel densities must be used to minimize the problems' effects on the appearance of OCT images. 3.5 Clinical OCT Acquisition System This section will describe the specific acquisition and waveform control issues of the clinical OCT system. The acquisition software for the clinical OCT system needs to generate the drive waveform for the phase delay line, control and synchronize the various beam delivery devices, display and archive the acquired images, and provide real-time adjustments to the displayed image to assist the operating clinician. 3.5.1 Optical Phase Delay Line In order to increase the axial scan acquisition rate of the clinical OCT system, the optical phase delay line is driven with a triangle waveform to eliminate the non-scanning galvo return portions that are present in the sawtooth waveform. However, at high speed scanning, the presence of galvanometer non-linearity and hysteresis makes it difficult to perform OCT imaging. Figure 10A illustrates these non-linear effects on the galvo response. 27 When the galvanometer is driven with a triangle waveform, the actual response will have an overshoot portion caused by the rapid accelerations involved at the turnaround point. This overshoot gives an asymmetry between the upward and the downward phase of the triangular waveform. Because data is acquired during both phases, this asymmetry in galvanometer motion will cause adjacent axial scans in the OCT image to mismatch, creating the so-called "zipper" effect. Driving the galvanometer with a more complicated pattern to compensate for the non-linearity is one way to solve this asymmetry problem. A delta function with an exponential decay can be used to compensate for the overshoot at the turnaround point of the triangle waveform. The delta function can quickly reverse the galvanometer motion direction while the leading edge of the exponential decay attempt to compensate for the overshoot. Figure 10B illustrates a compensated drive waveform which yields a more symmetric galvanometer response curve. The depth of the delta function and the rate of the exponential decay that produces the most symmetric galvanometer response in the region of data acquisition can be experimentally determined. Figure 10. The solid line represents the drive waveform for the optical phase delay line scanning galvanometer and the dashed line represents the actual galvo response. A: The triangle drive waveform at high speed results in an asymmetric galvanometer response. B: The triangle drive waveform with compensatory delta function and exponential decay results in a more symmetric galvanometer response. Even with this compensated drive waveform, it was found that only about 55% of the galvanometer response curve is linear and symmetric enough to obtain high quality OCT images. Therefore, to obtain a 2.25 mm axial scan, a scanning drive waveform of 4.1 mm is needed. At 28 this scanning distance, an increased slope in the linear scanning region is required to maintain the axial scanning rate at 2000 Hz. This increased galvanometer scanning speed will not change the detection electronics of the clinical OCT system because the heterodyne modulation frequency of the optical phase delay line is set independent of the scanning speed. Analog Output Channel 1 Gate Signal Analog 1 1 2 2 1 2 Output Start Trigger Time - Figure 11. The drive waveform and acquisition timing scheme for the clinical OCT system. Analog output channel 1 represents the triangle drive waveform of the optical phase delay line scanner. The dashed line represents the delayed galvanometer response. Analog output channel 2 represents the timing signals which are placed around the galvo turnaround point. The gate signal (shaded lines) indicates data acquisition and is triggered by the timing signal. The start trigger is needed to ensure that the displayed OCT image is always upright. Figure 11 illustrates the acquisition timing scheme for the clinical OCT system. Analog output channel 2 generates two timing signals for every cycle of the triangle waveform. The timing signals are placed at the turnaround point of the galvanometer response curve. One of the timing signals (#1) triggers the acquisition for the downward phase of the galvanometer scanning while the other timing signal (#2) triggers the acquisition for the upward phase of the galvanometer response. Each timing signal will trigger a gate signal which allows signal digitization to commence. Similarly to the bench-top OCT system, the gate signal duration has 29 to be controlled carefully so that only the desired amount of pixels is acquired for every axial scan. A single-pixel error in one axial scan will propagate across all the OCT images and render the acquired data useless. Since data is acquired for both upward and downward phases of the galvanometer scanning, every other axial lines need to be flipped before they are displayed onto the screen so that all the axial lines would appear to be scanned from the same position. To achieve the desired match between the regular axial scans and the flipped axial scans of the OCT image, the gating signal is placed in the region of the galvanometer scanning that is the most linear and symmetric. In addition, the time difference between the first timing signal and the second timing signal can also be adjusted to minimize the "zipper" effect caused by inexact pixel matching. In the clinical OCT system, data is acquired during both the upward and downward phase of the triangular waveform. There needs to be a mechanism for the acquisition software to differentiate the difference between the two different timing signals. Otherwise, there would be a 50% chance that the acquisition would start on the wrong phase of the triangular waveform and the acquired image would appear to be upside-down. An additional start trigger signal is used to solve this problem. This signal is triggered by the first timing signal and has a pulse duration that lasts the entire cycle (2 axial scans) of the triangular waveform. In this manner, the acquired OCT images will always be upright if the onset of the acquisition operation is triggered by the start signal. 3.5.2 Method of Acquisition In the clinical OCT system, the double buffer is set up for each half-buffer to store the data of an entire OCT image. The acquired OCT image is then transferred into a storage buffer of the same size in the system memory. Once in the storage buffer, the data is processed using a position and color map to determine the screen display parameters. After the image is displayed onto the screen, the program can choose to either discard the image in the storage buffer or save it onto the hard disk. Because it takes longer to acquire an entire image than to acquire a single axial scan, this acquisition setup allows the CPU more time to process, display, and save the OCT data before it has to handle the next OCT image. Therefore, real-time display and storage 30 of OCT images can be accomplished using this acquisition scheme. In actual imaging situations, the clinical OCT system can continuously acquire data at 4 frames per second (500 axial scans per frame) and save each acquired image onto the hard disk. This process is limited by the access rate of the PCI bus which can ideally moved 100 MB per second. For an OCT image size of 1 MB, it takes 2 transfers on the PCI bus to save the image into the hard drive. One transfer is from double buffer memory to the system memory storage while the other transfer is from the system memory to the hard disk. Theoretically, this would limit the acquisition rate to 50 frames per second. However, the PCI bus never operates continuously at 100 MB/s transfer rate, and other computer devices such as the acquisition card and the CPU are also constantly transferring data through the PCI bus. Therefore, the practical frame rate of this system would be much less than 50 frames per second. The clinical OCT system is capable of changing the image quality of the displayed OCT images in a real-time manner. A histogram of the digitized values in the displayed image is available to the user for facilitate the setting of black (noise) and white (maximum) image display levels. By properly setting the black and white levels, it is possible to remove some of the speckle noise artifacts and improve the contrast of the displayed OCT image. A "zipper" adjustment is also available to remove any remaining mismatch between adjacent lines. All of these display adjustments can enhance the quality of the OCT images in real-time in order to provide the operating clinician with a more informative viewing. During the data acquisition session, the entire computer display is recorded along with the operating clinician's running commentary onto Super-VHS tape for archival and future registration purposes. In endoscopic or colposcopic runs, a simultaneous CCD video view of the imaged tissue area is available and this view is also recorded onto Super-VHS tape to enhance future registration of OCT images with tissue histology. If a simultaneous video view is not available, a photograph of the imaged site is usually taken. 3.5.3 Beam Delivery Devices One of the challenges of building an acquisition system for the clinical OCT system is that it has to interface with a wide variety of different beam delivery devices. The system needs 31 to generate the desired scanning pattern as well as synchronizing the OCT beam scanning to the data acquisition. As described in the bench-top acquisition system section, the simplest way to accomplish synchronization is to use the gating signal as the update clock of the beam scanning waveform. When the scanning waveform has the same number of points as the number of image axial scans, the beam scanning will be completed just as the entire image has been acquired. As the system continues to acquire the next image, the beam scanning also repeats from the beginning of its waveform and this process can be continued forever. This scanning waveform control is so general that it can be used in all three beam-delivery devices of the clinical OCT system. However, a unique problem exists in scanning the OCT beam at such high acquisition speeds. Most beam delivery devices for the clinical OCT system are scanned in a sawtooth fashion with the presence of a half-sinusoid return curve at the end of the waveform. Since every axial scan triggers exactly one update in the beam scanning position, it is not possible to prevent the return portion signals from being acquired by the system. If the return portion of the waveform consists of 4% of the entire scanning waveform, the last 4% of the acquired OCT image will usually be cropped off before it is displayed onto the screen. However, this cropping does not always correspond to the axial scans in the scanning galvo return portion. This is because very high data acquisition rates require the beam-scanning device to also move its scanner very quickly. The mechanical inertia of such delivery device scanners usually prevent it from responding very quickly and a time lag will develop between the drive waveform and the actual scanning. This scanning lag time means that the return portion of the beam scanning would show up in the middle of the image instead being cropped off at the end. In order to fix this problem, the drive waveform needs to be shifted by an amount equal to the lag time. In this manner, the delayed scanning of the beam delivery device will actually produce the desired synchronization with the rest of the acquisition system setup. This concept is illustrated in Figure 12. It is important to note that the computer controlled scanning scheme would not be applicable in the circumferential scanning catheter. In that system, the catheter is rotated by an external mechanical transducer independent of computer control. A synchronization signal is 32 generated by the scanner every time if finishes one revolution and repeats the circumferential scanning. Therefore, we must make sure the acquisition system is synchronized to this external signal instead of the internally generated signal. AP Image #1 Image #2 Image #3 /X Time - - Figure 12. The delivery beam scanning drive waveform (solid line) and the delayed response (dashed lines). The shifted drive waveform, with the scanning return portion in the middle of the waveform, is required to produce the desired beam scanning response necessary for image synchronization. A correct synchronization means that the scanning return portion is cropped from the image and not displayed. 3.6 Interferometric OCT Acquisition System The system setup for collecting interferometric OCT signals is similar to that of the bench-top OCT system or the ultrahigh resolution OCT system. A linear scanning delay-line is used along with a broad spectral bandwidth laser light source to generate axial scanning of the sample. The interferometric OCT system also shares the same methods of acquisition and beam delivery mechanisms as the bench-top OCT system. In this system, however, high-speed digitization of the interferometric fringes of the detector output is required for spectroscopic and 33 Doppler OCT applications. The digital processing requirements for these types of OCT imaging also dictate that the interferometric fringes needs to be vastly over-sampled, placing even more demand on the acquisition system. With the use of double buffering techniques, scan clock gating, and a fast (5 MHz) acquisition card, it is possible to collect data quickly enough to capture the interferometric fringes. However, this data needs to be digitally demodulated in software before it can be processed through a position and color map and then be displayed onto the screen. This additional data processing along with the inherent large data-block transfers of the interferometric system place tremendous pressure on the performance of the hardware and the computer's CPU. Therefore, the processing and display algorithms need to function efficiently in order for the double-buffered acquisition to operation without hardware error interruptions. These topics will be discussed in the next chapter. 34 Chapter 4 Image Processing and Display of OCT Images An integral part of the high-speed OCT system is the real-time processing and display of images during the acquisition process. In order to prevent hardware errors from interrupting the double-buffered acquisition, it is necessary to minimize the CPU utilization time of the processing and display algorithms. This chapter will discuss solutions which maximize the speed and efficiency of these processes in order to achieve real time OCT imaging. Post- acquisition image processing algorithms and image display issues are also presented. 4.1 Image Processing and Display during Acquisition During the acquisition of OCT data, speed is the most important issue facing the processing and display of the acquired image. The acquisition program must be able to display the image in the desired fashion (linear, transposed, or polar) and with the correct color scheme (grayscale or false-color). For interferometric OCT data, the fringe data must be demodulated first in software before it can be display onto the screen in a meaningful fashion. The triangle axial scanning waveform of the clinical OCT system requires that every other scans to be flipped before they can be displayed. Real-time image contrast and "zipper" adjustments are also implemented to provide the operating clinician with the best possible image displays. 35 4.1.1 Position Mapping Since different OCT systems have different beam delivery devices and acquisition modalities, it is important for the displayed image to accurately represent the underlying acquisition method and imaged structure. For most systems, the axial scans are displayed vertically, and an image is formed by placing a series of adjacent axial scans in a horizontal fashion. This image display modality accurately represents the axial and transverse scanning methods of most OCT systems. In some ophthalmic imaging situations, the axial scans are displayed horizontally and placed from the top of the screen down to better emphasize the actual path direction of the OCT beam in the eye. In circumferential imaging situations, the use of a rotational imaging catheter requires the acquired data to be displayed in a polar-transformed fashion. Therefore, the acquired OCT data set can be displayed in three different manners linear, transposed, or polar. A position look-up table provides the program with the quickest way to map the OCT data set onto the screen in the desired manner. The position look-up table would consist of a two-dimensional array of pointers each of which represents a single pixel on the screen. Before the start of double-buffered acquisition, the program calculates the desired algorithm to map the OCT data set onto the screen by using one of the three methods. The software then goes through the position look-up table and assigns each pixel pointer to a location in the data set. All the calculations and assignments are done before the start of acquisition to prevent them from interfering with the acquisition process, during which the software simply looks up the required data value to display through the assigned pixel pointers. The use of the pointer look-up table avoids the need to calculate the required display position during acquisition, thus minimizing the CPU utilization time. For the clinical OCT system with the triangle axial scanning pattern, every other axial lines that are displayed needs to be flipped in order to form a proper image. This flipping of the data set can be done with a memory swapping process, but this would take too long to keep up with real-time acquisition. Instead, the flipping of the axial lines is performed on the position map before the start of acquisition. This shifts the burden of accurate image display from the 36 CPU back to the pre-processed position map and allows the acquisition to continue without interruptions. By the same logic, a "zipper" adjustment can also be implemented in the position map to eliminate inexact pixel matching between adjacent axial scans. The "zipper" adjustment would determine how much shift to incorporate to every other axial scans such that their flipped version matches up exactly with the other axial scans. During circumferential OCT scanning, the circular pixel density is much greater near the center of the catheter than away from it. This means that circumferential image displays require some data decimation and interpolation in order to render a complete polar image. The decimation is done in the position map by skipping through certain axial data values when the pixel densities are too high. A linear interpolation of adjacent pixel values is used to calculate missing display values when the pixel densities are too low. This interpolation must be done during acquisition, but it is simple enough that it will not require lots of processing time. Figure 13 provides a flow chart which summarizes the functions of the positional lookup table. Before Acquisition Determine Linear - - Transposed]--- Display Type Flip Calculate and construct the every other - axial Make zipper adjustments? 10 position lookup table for display -- kPoarscan? During Acquisition Position Aquisitio ffre d ata set P lar? NLobokup Yes Interpolate and decimate Figure 13. Block diagram showing the process of the position lookup table construction and the flow of OCT data during acquisition. This figure only shows the processes involved in the positional lookup table, the processes involved in the color lookup table is not included. 37 Dspa 4.1.2 Color Mapping In displaying the OCT image onto the screen, a Red/Green/Blue (RGB) color scheme is utilized. In this color scheme, each pixel has a 24-bit color value which represents the screen color for that pixel. The 24-bit color value is divided into three 8-bit values each of which represents the screen intensity of a fundamental color. A high value represents high screen intensity while a low value represents low screen intensity for that color. Therefore, the white color would be represented as a value of 255 for each of the three fundamental colors while the black color would be represented as a value of zero in each of the three 8-bit values. By mixing different intensities of the red, green, and blue color, it is possible to obtain around 16 million (24 bits) different colors for screen display. A grayscale color scheme can usually be derived from the RGB color scheme by setting all the 8-bit fundamental color values to be the same. Therefore, only 256 (8 bits) possible shades of gray can be displayed. Once the program has obtained the OCT data value and its display locations on the computer screen, it must figure out how to display that data. OCT images have many different display color schemes. For most systems, images are displayed on an 8-bit grayscale which can either have black signal on white background or white signal on black background. The black on white background grayscale was found to give better image contrasts in most situations. However, the clinical OCT system utilizes a white on black background grayscale because most clinicians are already familiar with standard ultrasound display that uses this color scheme. For in vivo human ophthalmic imaging, an 8-bit false-color display scheme is used to represent the acquired OCT image. The false-color display scheme is determined from the signal-to-noise ratio (SNR) of the image and is set to only span the range of acquired reflectivities in order to provide better ophthalmic image contrasts. Care must be used in interpreting ophthalmic image features because a specific color could represent different features under different SNR conditions. Similar to the positional mapping, a display color look-up table provides the program with the quickest way to determine how to render a data value onto the screen. The color lookup table would consist of an array of RGB values, each representing a specific grayscale or false- 38 color value. The array would have one element for every possible digitization data value. Before the start of acquisition, the program calculates the RGB values, based on the particular color scheme used, for each corresponding data value. Black and white level adjustments are also figured into the RGB value calculation at this point of the calculation. The data values greater than the white level would be displayed as white while the data values smaller than the black level would be displayed as black on the screen. During acquisition, the software simply takes the acquired data value and does an array element lookup to access the RGB color that needs to be displayed on the screen. Since CPU-intensive calculations are all done before the start of acquisition, the display color map allows quick and accurate display of OCT values without affecting the acquisition. Another complication that needs to be addressed in the display color table is the differences between log demodulation and linear demodulation of the interferometric signal. Because OCT signals suffer from an exponential attenuation as it propagates through the tissue, OCT images are usually displayed on a logarithmic scale of reflectivity to offset this effect. This is accomplished by taking the log of the digitization value before mapping that result onto the RGB color element array. However, sometimes a log demodulation of the interferometric signal is required before digitization to compensate for the limited dynamic range (12-bit) of the high speed data acquisition card. Since the log demodulator performs in hardware the same task as the logarithmic color table, a linear color table should be used in these cases. The differences between linear or log demodulation are taken into account during the calculation of the RGB color look-up table before the start of acquisition. 4.1.3 Digital Demodulation Since interferometric OCT data values did not go through either a log or a linear demodulator, they must be demodulated in software before the color mapping can be applied. The quickest way to demodulate the interferometric fringes is to use data averaging methods. In this algorithm, the collected data is first rectified before an average of the data values inside an averaging window is calculated. The averaged value is then assigned to a new demodulated data set for display onto the screen. Data averaging is equivalent to low-pass filtering of the rectified 39 interference signal because it is similar to a boxcar convolution. The averaging window size is dependant on the sampling rate of the interferometric data because the window has to be large enough to incorporate at least one interference fringe. If an averaging window of 8 is used, a 40000x1000 interferometric data set would yield a 5000x1000 demodulated image which can be easily displayed through the use of positional and color mapping. Therefore, higher sampling rates (with more axial points) would yield a better resolution of the demodulated images. Since averaging requires few floating-point operations, it can be processed quickly and the real-time display of interferometric data can be achieved. Additional digital filters such as bandpass filtering can also easily implemented because it is just a weighted sum of time-shifted signals. Figure 14 provides a flowchart that summarizes the processing and display of OCT images. Before Acquisition Linear Determine Display Type -- Flip every Tassd other -+ Make zipper adjustments? axial ___ sanfor -> -+ Plar Calculate and construct the position lookup table display During Acquisition ntberbuferenc em Yes Rectify OCT interference data Before Acquisition Determine Color Type DemodlateInterpolate Oemodausin Generated a boxcar demod convolution data White background -WFasecoordiply -sAdjust -+ Lookup Table Lokup Table Yes decimateolo deOd OCT dat usin Black background _- Polar? at st ak boT software? dipa oAcquisition black and white display 4 level and decimate Determine the color -ytable type: Log or Linear Calculate and construct the lo color lookup table for display Figure 14. Block diagram showing the processing of the position lookup table construction, the color lookup table construction, and the flow of OCT data during acquisition. This flowchart does not include the real-time image display adjustments which changes the position and color mapping of the OCT data on- the-fly. 40 Display 4.1.4 Clinical Feedback In addition to real-time display of acquired OCT images, the acquisition software contains several display features that are designed to provide maximum operator feedback. In most systems, a real-time oscilloscope output of the acquired axial scan is displayed next to the OCT image that is being formed. scheme. This display requires a different position/color mapping The color of the points in the oscilloscope display is always the opposite of the background color for increased visibility while the horizontal and vertical positions of the points are determined respectively by the sequential location and value of the acquired data. Because the oscilloscope display window is small, only a fraction of the actual axial scan data set is mapped onto the screen and it can be displayed very quickly. In a clinical OCT system, the oscilloscope display would not be informative because each axial scan is coming into the image buffer too quickly. Instead, a histogram of all the acquired data values in the image is used to convey the quality of the OCT image. The histogram can also assist in improving the image contrast. Two red lines in the histogram window denote the current black and white level of the OCT image display. Careful adjustment of the black/white level can minimize background speckle noise by thresholding the speckles to black and improve image contrast by increasing the dynamic range of the color scale. Keyboard shortcuts and user interfaces are provided so that the operator can quickly and easily change the black and white level of the image in a clinical setting. In an ophthalmic system, knowledge of the signal-to-noise ratio is important in determining the quality of the acquired image and the color table that needs to be used. Because the OCT beam has to travel through non-reflecting medium (either air or vitreous) before it reaches eye structures (retina or cornea), it can be assumed that the first few points in every axial scan contains only noise signal. Using these data values as the noise floor, the signal-to-noise ratio of the image can be easily determined. This information is displayed to the operator after image acquisition and is saved along with the data. 41 4.2 Post-Acquisition Image Processing and Display Although the ability to survey and post-process previously acquired OCT images is not critical for high-speed data acquisition, their inclusion into the acquisition program will enhance its functionality and usability. In clinical situations, medical specialists often request to view the acquired images after the imaging session in order to obtain a quick evaluation of the clinical run. Different imaging-device handling techniques are often evaluated in the clinic by viewing the acquired images to provide the device operator with more timely suggestions. This section will discuss the post-acquisition image processing and display features of the program. 4.2.1 Image Processing In post-acquisition image viewing, all of the acquisition image-processing functionalities are available for enhancing the displayed image quality. Therefore, the different positional and color mappings, black/white display level adjustments, individual axial scan views, and digital demodulation are all implemented into the post-acquisition image viewing. In addition, simple image processing algorithms are also implemented to remove motion artifacts and enhance feature detection in certain images. For most OCT systems under in vivo operation, motion artifacts are usually present in the acquired images and pose a problem in image interpretation. Motion artifacts can arise from involuntary muscle activities, such as heart beat or respiration, of the patient of anesthetized animal. If the axial scans are acquired with sufficient speed and pixel densities, we can assume that there is no motion artifacts within a single axial scan and that adjacent axial scans will represent similar tissue structures. These assumptions lead us to use a cross-correlation algorithm to remove motion artifacts from in vivo images. The algorithm is a stand cross- correlation implementation in which adjacent axial scans are compared to find the pixel shift that would produce the maximum correlation in data values. The shift values are then used to match all the axial scans with their adjacent neighboring scans, thus producing an image with minimum motion artifacts. However, one must use caution in interpreting cross-correlated images because 42 gross morphologic features may be removed. For example, the curvature of the retina in an OCT image may not be present after processing through the cross-correlation algorithm. For most OCT images, the vertical pixel number is usually larger than the screen resolution while the horizontal pixel number is usually smaller than the screen resolution. Therefore, the display of the OCT image onto the screen requires data decimation in the vertical direction and data interpolation in the horizontal direction. In data decimation, pixels at a certain calculated interval are not displayed in order to fit the entire OCT line into the screen. In data interpolation, a bilinear interpolation technique is utilized. In this algorithm, the value at each interpolated point is calculated from the linear combination of the values at the four surrounding points. The use of bilinear data interpolation will produce a less pixelated OCT image on the computer screen than the use of simple pixel duplication. For ophthalmic OCT images, it is desirable to have algorithms that can automatically outline and analyze the significant retinal features in the eye. However, this feature extraction algorithm is very complicated and may need to be updated upon different changes in OCT system performance. The standard algorithm used to detect the different features usually consist of smoothing the image with a two-dimensional kernel to remove background speckle noise, edge-detect the different layer features in the image, and then correct for the errors caused by signal dropout. For now, the standard implementation of the feature extraction process will be incorporated into the software; however, the program is designed such that it will be easy to add and include new changes into the ophthalmic feature extraction process. 4.2.2 Image Display One of the problems related to high speed image acquisition is the difficulty in handling a large image data set. In a clinical OCT system operating at four frames per second, a fiveminute image session would typically produce more than a thousand saved OCT images. It is impractical for the user to open each of these images one at a time in order to screen for interesting morphologic features. An image collage view was created to solve the problems caused by a large data volume. In the collage view, every OCT image in a particular folder is 43 opened at the same time, but only a small preview image of each OCT image is displayed onto the screen. The preview images usually contain 250x250 pixels so that most features of the underlying OCT images are still displayed. The user can survey the preview images for interesting morphologic structures and obtain image acquisition parameters by single-clicking on the preview image to obtain information such as pixel numbers, scan lengths, and the image file name. Double-clicking on the desired preview image opens up the corresponding OCT image display. Once the OCT image display is opened, a small display is used to inform the user of the current pixel location that is under the cursor. Zooming is another feature of the program that is used to improve image display. The user can select a rectangular region of interest in the OCT image and zoom into that region for a closer look. Bilinear interpolation is used to render an acceptable zoomed image onto the screen. This process can be continued until the entire screen is confined to within a single image pixel. Zooming out back to the original image is also possible. Once the program has processed the image to yield the best possible display, the image on the screen can be exported out as a bitmap file to ensure easy transfer into commercial software packages for further processing and display purposes. 44 Chapter 5 OCT Imaging Applications of the High Speed Data Acquisition System The development of the high speed data acquisition system is motivated by several different OCT imaging modalities (in vitro, in vivo, ultrahigh resolution, spectroscopic). Integration of the new acquisition module into existing OCT systems will allow these different imaging studies to be realized. This chapter evaluates the performance of the high speed acquisition system by presenting a sampling of images taken from the various OCT systems. In vitro mouse prostate images as well as in vivo hamster cheek pouch images were taken on the ultrahigh resolution bench-top OCT system. Cervical, gastrointestinal, and oral cavity images were taken with the clinical OCT system. Spectroscopic OCT data was also obtained from the mouse retina on the ultrahigh resolution system to demonstrate the interferometric data acquisition capabilities of the system. 5.1 Bench-top OCT System Images The first system in which the new high speed acquisition setup was evaluated on was the bench-top OCT system. Ultrahigh resolution (3 pm) OCT images of mouse prostate gland was obtained in vitro while ultrahigh resolution OCT images of hamster cheek pouch was obtained in vivo. Figure 15 shows examples of these images. 45 rid". Figure 15. OCT images (top) and their corresponding H&E stained histology (bottom). Ultrahigh resolution OCT image of the mouse prostate gland in vitro is on left while ultrahigh resolution OCT image of the hamster cheek pouch in vivo is on the right. The black scale bars in that OCT image indicates distance in both the axial and transverse directions. OCT images (1mm x 1mm) contain 2000 axial pixels and 1000 transverse pixels representing 0.5 pm axial pixel density and 1 pm transverse pixel density. Images have been cropped for display purposes. Both of these OCT images were acquired with the linear mechanical galvanometer scanning at 82 mm/s and a data acquisition rate of 164 kHz. The transverse scanning precision stage provided constant speed scanning of the tissue in the transverse direction. Both in vitro and in vivo OCT images show features that have correspondence to structures in the histology preparation. These images and their corresponding histology demonstrate that the high speed acquisition setup is able to acquire and display the acquired OCT images in a precise and accurate fashion. 5.2 Clinical OCT System Images After the bench-top OCT system, the high speed acquisition setup was integrated into the clinical OCT system with the AFC amplified SLD light source. High resolution (15 pm) OCT 4A; images of normal esophagus and Barrett's esophagus were acquired in vivo using the linear scanning catheter while cervical tissues were imaged in vivo using the OCT integrated colposcope. In addition, high resolution OCT images were also taken in the oral cavity with a modified hand-held probe. Figures 16 through 18 demonstrate the images taken from the clinical OCT system. These images were taken with the high speed phase delay line scanning at 2000 Hz. Each image (2.25mm x 5mm) contains 693 axial pixels and 480 transverse pixels corresponding to 3.25 pm axial pixel density and -10 pm transverse pixel density. Since only 55% of the scanning waveform is used for acquisition, this scanning speed and pixel density corresponds to an acquisition rate of 2.5 MHz. The OCT images in Figures 16 through 18 have all been cropped for display purposes. Figure 16. Clinical OCT imaging of the human esophagus in vivo. A: An endoscope camera view of the OCT catheter inside the esophagus. B: Biopsy histology of normal esophageal tissue. C: OCT image of normal esophagus in vivo. Five distinct layers can be differentiated (ep: epithelial layer, lp: lamina propria, mm: muscular mucosa, sm: submucosa, mp: muscular propria.). D: An endoscope camera view of the OCT catheter imaging the Barrett's epithelium. E: Biospy histology of Barrett's epithelium. F: OCT image of Barrett's epithelium showing the loss of distinct layers and the presence of glands (g) and crypts (c). 47 Figure 17. Clinical OCT imaging of the human cervix in vivo. A: OCT image of normal cervical area, note the distinct layered structure at the top of the tissue surface. B: OCT image of per-cancerous dysplasia taken from the same patient. Note the loss of the distinct layered structure in this OCT image. Figure 18. Clinical OCT imaging of the oral cavity in vivo. A: OCT image of normal human check tissue. Note the distinct layer below the epithelium. B: OCT image of a leukoplakia legion inside the oral cavity of the same patient. Note the loss of distinct layer structure and the corrugated appearance of the leukoplakia surface. 48 At 2000 Hz axial scanning rate, the new acquisition system was able to operate the clinical OCT system at 4 frames per second and control the different delivery devices such as the linear scanning catheter (Figure 16), the colposcope (Figure 17), and the hand-held probe (Figure 18). These images also demonstrate that the acquisition system was able to acquire and display the images with improved pixel density and data storage capacities. The acquisition system was also responsible for driving the phase delay line scanning system with the modified triangular waveform. As these OCT images show, the compensatory measures taken in the drive waveform was so impressive in achieving galvanometer linearity and scanning symmetry that no "zipper" pixel adjustments needed to be made. 5.3 Image Processing Figure 19 demonstrates the utilization of the cross correlation algorithm to remove excess motion artifacts. Figure 19. OCT imaging of an African tadpole in vitro with the Herriott Cell delay line. A: OCT image of the dorsal side of the tadpole. Noise modulation in the delay-line drive waveform generates the motion artifacts in this image. B: Demonstration of the cross-correlation algorithm and its effectiveness in removing motion artifact from the image. 49 The image in Figure 19A was obtained using a new type of delay line scanning mechanism for OCT called Herriott Cell multi-pass cavity. Noise modulation in the drive waveform for this delay line creates cavity movements that manifest themselves as motion artifacts in the image. Figure 19B demonstrates how the cross-correlation algorithm is able to remove much of the motion artifacts from the raw OCT image. These images also illustrate the acquisition system's versatility and expandability by showing its effectiveness in interfacing with a new delay line scanning mechanism. 5.4 Spectroscopic OCT Imaging One of the major reasons for developing the high speed acquisition system is its ability to take interferometric fringe data to study spectroscopic and Doppler OCT effects. Figure 20 presents an example of spectroscopic OCT imaging of the mouse retina. Figure 20. Amplitude (top) and spectroscopic OCT (bottom) imaging of the mouse retina in vivo. In the spectroscopic OCT image, the longer backscattered wavelengths show up shifted toward red while the shorter backscattered wavelengths show up shifted toward green. The highlighted box shows different scattering/absorption layers in the spectroscopic OCT image that is not readily resolved in the amplitude OCT image. Spectroscopically resolved imaging can be a powerful method for enhancing the differentiation of retinal structures as well as for assessing their functional state. 50 In these OCT image, raw interference fringes of the detector were recorded by the acquisition system at extremely high speeds. For digital processing requirements of the spectroscopic OCT, the sampling rate must be such that the interferometric fringes are vastly over-sampled. The amplitude OCT image was created by demodulating the digitized interference fringes in software. The interferometric data is first rectified and then demodulated using a boxcar convolution averaging process. The spectroscopic OCT was created by detecting the varying reflectivity spectrum of scatterers in the tissue. Several methods can be used to extract the spectroscopic information from the OCT signal. These include short time Fourier transformations with a rectangular window or wavelet transformations with a Gaussian window. There is a trade off between the spectral resolution and the spatial resolution using these techniques. A large transform window would provide better spectral resolution at the expense of spatial resolution while a smaller transform window would provide better spatial resolution but sacrifices spectral resolution. The wavelet transformation is used in this case to extract the frequency content in order to prevent the windowing artifacts of the short time Fourier transform rectangular window. The spectrum of the backscattered light is calculated for each point in the image. In order to facilitate display, a hue, luminescence, and saturation color scheme is used instead of the RGB color scheme. The hue maps the shift in the center of gravity of the spectra while the saturation measures the intensity of the backscattered light and the luminescence is kept constant. Therefore, a shift in hue (green or red shift) indicates the backscattered light has a spectral shift (shorter or longer wavelengths). Through the use of a broadband light source and wavelet transformation, spectroscopic OCT is capable of obtaining the spectroscopic information of many different wavelengths in a single measurement. As Figure 20 demonstrates, the absorption/scattering properties of tissue that is detected by spectroscopic OCT can be used to increase image contrast that is not present in the amplitude OCT image, thereby achieving "spectroscopic staining." 51 Chapter 6 Future Work and Conclusions With successful development of the high speed data acquisition system and the demonstration of its use in spectroscopic and other conventional OCT systems, the next challenge is the utilize the system for the acquisition of Doppler OCT interferometric signals. Recent advances in Doppler OCT measurements have demonstrated high flow sensitivities of 10 pm/s by using phase retrieval techniques 41 . With the combination of ultrahigh resolution OCT system and the phase retrieval techniques, it may be possible to achieve ultrahigh resolution reflectivity measurements with ultrahigh sensitivity Doppler flow detection. This combination promises to yield unprecedented structural and functional tissue resolutions in the same image. 6.1 Doppler OCT Doppler OCT utilizes the Doppler principle with OCT to simultaneously produce highresolution images of moving and stationary scattering particles in biological tissue. The flow velocity of the moving particles can be determined by measuring the Doppler shift in the fringe frequency of the interference signal. A short-time Fourier transform window is usually employed to calculate this Doppler shift. Since detection of the Doppler shift requires the transform window to incorporate at least one fringe cycle, the minimum detectable Doppler shift frequency is inversely proportional to the short-time Fourier transform window size (AT). The velocity sensitivity (vmi) is given by 52 V1 = " where 2n cos(O)AT k = light-source center wavelength, n = sample refractive index The 0 in the equation represents the angle between the OCT beam and the direction of the Doppler flow. Therefore, the larger the window size (AT), the higher the velocity sensitivity. However, spatial resolution (Ax) is linearly proportional to the window size of the short-time Fourier transform. The spatial resolution is given by Ax = V(AT) where V = Linear velocity of the delay line scanning Therefore, in a short-time Fourier transform situation, the spatial resolution and velocity sensitivity are coupled. A large transform time window increases velocity sensitivity but decreases spatial resolution. This coupling of spatial resolution and velocity sensitivity prevents the acquisition of OCT images that contain both high spatial resolution and high flow sensitivity. Therefore, another OCT Doppler flow extraction technique is desired in order to detect very low Doppler flows with very high sensitivity and spatial resolution. 6.2 Hilbert Transform Technique To address this problem, a new technique was recently developed which uses the phase change between sequential Doppler OCT scans to determine the flow velocity 41 . In this technique, the sample is kept stationary while repeated axial scans are performed at the same transverse location. The Doppler OCT signal phase can be determined from the analytic continuation of the measured interference fringes calculated from a Hilbert transformation 'FOCT ( = OCT (CT +d = A(t)exp[i#(t)] where FOCT (t) is the interference fringe function, the P denotes the Cauchy principle value and A(t) and 0(t) are the amplitude and phase of the equation. Dividing the phase change in each pixel between two sequential axial scans by the axial scan time interval will then yield the Doppler frequency shift (w = A#/T ). Because the axial scan time interval is much longer then the pixel clock time period, this flow extraction method is able to decouple spatial resolution with velocity sensitivity. This Hilbert transform technique was demonstrated on an OCT system 53 with 15 jim spatial resolution scanning at 400 axial scans per second, and a flow velocity sensitivity of 10 ptmls was achieved. 6.3 Ultrahigh Resolution Doppler OCT Since the Hilbert transform technique is able to decouple the spatial resolution with velocity sensitivity in Doppler flow measurements, the acquisition of OCT images with ultrahigh structural and functional resolution seems possible. Using the ultrahigh resolution OCT system to acquire interferometric Doppler OCT signals, it is possible to employ the Hilbert transform technique to achieve flow and structural resolution on the scale of 1 jim. However, for the Hilbert transform method to be successful, the absolute phase throughout the entire axial scan must be controlled extremely carefully so that only the flow scatterers contribute to the phase difference between sequential axial scans. Any presence of noise source that contributes to the phase difference would render this technique ineffective. So far, repeated attempts on the ultrahigh resolution OCT system to obtain Doppler flow measurements in both in vivo animal and in vitro phantom experiments were unsuccessful. We have determined the problem to be the presence of a noise source somewhere in the OCT system that is adding a phase difference to the collected Doppler OCT signals. Since the noise source contributes a phase difference that is over one oscillation cycle of the interference fringe, it effectively overwhelms the smaller phase differences created by the flowing scatterers. However, the frequency spectrum of this noise source contains some very sharp and well-defined spectral peaks that suggest isolation of the noise source is feasible. Possible causes of the noise source could be galvanometer vibrations or tissue movements. Because the ultrahigh resolution OCT system uses a linear mechanical delay-line scanner, any vibrations or changes that alter the movement of galvanometer among different axial scans would manifest itself as a phase noise source between sequential scans. Proper galvanometer dampening and improved tissue isolation would be possible methods to eliminate this noise source. The problem of the phase noise source has to be resolved before ultrahigh flow sensitivity Doppler OCT measurements can be accomplished with this acquisition system. 54 6.4 Conclusions In conclusion, a high-speed data acquisition system was developed for the measurement of OCT signals. It is able to interface with multiple OCT systems including the bench-top OCT system, the clinical OCT system, the ultrahigh resolution OCT system, and the spectroscopic OCT system. OCT images in a variety of laboratory and clinical settings were obtained using this new acquisition system to demonstrate and evaluate its capabilities. However, more work needs to be done on the isolation of phase noise sources before the system can be used to acquire ultrahigh sensitivity Doppler measurements. 55 References 1. D. Huang, E. A. Swanson, C. P. Lin, J. S. Schuman, W. G. Stinson, W. Chang, M. R. Hee, T. Flotte, K. Gregory, C. A. Puliafito, and et al., "Optical coherence tomography," Science, vol. 254, pp. 1178-81, (1991). 2. M. E. Brezinski, G. J. Tearney, B. E. Bouma, J. A. Izatt, M. R. Hee, E. A. Swanson, J. F. Southern, and J. G. Fujimoto, "Optical coherence tomography for optical biopsy. Properties and demonstration of vascular pathology," Circulation, vol. 93, pp. 1206-13, (1996). 3. J. A. Izatt, M. D. Kulkarni, W. Hsing-Wen, K. Kobayashi, and M. V. Sivak, Jr., "Optical coherence tomography and microscopy in gastrointestinal tissues," IEEE Journal of Selected Topics in Quantum Electronics, vol. 2, pp. 10 17-28, (1996). 4. G. J. Tearney, M. E. Brezinski, J. F. Southern, B. E. Bouma, S. A. Boppart, and J. G. Fujimoto, "Optical biopsy in human gastrointestinal tissue using optical coherence tomography [see comments]," Am J Gastroenterol, vol. 92, pp. 1800-4, (1997). 5. K. Kobayashi, J. A. Izatt, M. D. Kulkarni, J. Willis, and M. V. Sivak, Jr., "High-resolution crosssectional imaging of the gastrointestinal tract using optical coherence tomography: preliminary results," Gastrointest Endosc, vol. 47, pp. 515-23, (1998). 6. C. Pitris, C. Jesser, S. A. Boppart, D. Stamper, M. E. Brezinski, and J. G. Fujimoto, "Feasibility of optical coherence tomography for high-resolution imaging of human gastrointestinal tract malignancies," J Gastroenterol, vol. 35, pp. 87-92, (2000). 7. M. E. Brezinski, G. J. Tearney, N. J. Weissman, S. A. Boppart, B. E. Bouma, M. R. Hee, A. E. Weyman, E. A. Swanson, J. F. Southern, and J. G. Fujimoto, "Assessing atherosclerotic plaque morphology: comparison of optical coherence tomography and high frequency intravascular ultrasound," Heart, vol. 77, pp. 397-403, (1997). 8. G. J. Tearney, M. E. Brezinski, J. F. Southern, B. E. Bouma, S. A. Boppart, and J. G. Fujimoto, "Optical biopsy in human urologic tissue using optical coherence tomography," J Urol, vol. 157, pp. 1915-9, (1997). 9. G. J. Tearney, M. E. Brezinski, J. F. Southern, B. E. Bouma, S. A. Boppart, and J. G. Fujimoto, "Optical biopsy in human pancreatobiliary tissue using optical coherence tomography," Dig Dis Sci, vol. 43, pp. 1193-9, (1998). 56 10. C. Pitris, M. E. Brezinski, B. E. Bouma, G. J. Tearney, J. F. Southern, and J. G. Fujimoto, "High resolution imaging of the upper respiratory tract with optical coherence tomography: a feasibility study," Am J Respir Crit Care Med, vol. 157, pp. 1640-4, (1998). 11. C. Pitris, A. Goodman, S. A. Boppart, J. J. Libus, J. G. Fujimoto, and M. E. Brezinski, "Highresolution imaging of gynecologic neoplasms using optical coherence tomography," Obstet Gynecol, vol. 93, pp. 135-9, (1999). 12. J. M. Herrmann, M. E. Brezinski, B. E. Bouma, S. A. Boppart, C. Pitris, J. F. Southern, and J. G. Fujimoto, "Two- and three-dimensional high-resolution imaging of the human oviduct with optical coherence tomography," Fertil Steril, vol. 70, pp. 155-8, (1998). 13. J. M. Herrmann, C. Pitris, B. E. Bouma, S. A. Boppart, C. A. Jesser, D. L. Stamper, J. G. Fujimoto, and M. E. Brezinski, "High resolution imaging of normal and osteoarthritic cartilage with optical coherence tomography," J Rheumatol, vol. 26, pp. 627-35, (1999). 14. C. A. Jesser, S. A. Boppart, C. Pitris, D. L. Stamper, G. P. Nielsen, M. E. Brezinski, and J. G. Fujimoto, "High resolution imaging of transitional cell carcinoma with optical coherence tomography: feasibility for the evaluation of bladder pathology," Br J Radiol, vol. 72, pp. 1170-6, (1999). 15. S. A. Boppart, A. Goodman, J. Libus, C. Pitris, C. A. Jesser, M. E. Brezinski, and J. G. Fujimoto, "High resolution imaging of endometriosis and ovarian carcinoma with optical coherence tomography: feasibility for laparoscopic-based imaging," Br J Obstet Gynaecol, vol. 106, pp. 1071-7, (1999). 16. M. E. Brezinski, G. J. Tearney, S. A. Boppart, E. A. Swanson, J. F. Southern, and J. G. Fujimoto, "Optical biopsy with optical coherence tomography: feasibility for surgical diagnostics," J Surg Res, vol. 71, pp. 32-40, (1997). 17. S. A. Boppart, M. E. Brezinski, C. Pitris, and J. G. Fujimoto, "Optical coherence tomography for neurosurgical imaging of human intracortical melanoma," Neurosurgery, vol. 43, pp. 834-41, (1998). 18. S. A. Boppart, B. E. Bouma, C. Pitris, G. J. Tearney, J. F. Southern, M. E. Brezinski, and J. G. Fujimoto, "Intraoperative assessment of microsurgery with three-dimensional optical coherence tomography," Radiology, vol. 208, pp. 81-6, (1998). 19. S. A. Boppart, J. Herrmann, C. Pitris, D. L. Stamper, M. E. Brezinski, and J. G. Fujimoto, "HighResolution Optical Coherence Tomography-Guided Laser Ablation of Surgical Tissue," J Surg Res, vol. 82, pp. 275-284, (1999). 20. S. A. Boppart, B. E. Bouma, C. Pitris, J. F. Southern, M. E. Brezinski, and J. G. Fujimoto, "In vivo cellular optical coherence tomography imaging," Nat Med, vol. 4, pp. 861-5, (1998). 21. S. A. Boppart, M. E. Brezinski, B. E. Bouma, G. J. Tearney, and J. G. Fujimoto, "Investigation of developing embryonic morphology using optical coherence tomography," Dev Biol, vol. 177, pp. 54-63, (1996). 57 22. S. A. Boppart, B. E. Bouma, M. E. Brezinski, G. J. Tearney, and J. G. Fujimoto, "Imaging developing neural morphology using optical coherence tomography," Journal of Neuroscience Methods, vol. 70, pp. 65-82, (1996). 23. S. A. Boppart, G. J. Tearney, B. E. Bouma, J. F. Southern, M. E. Brezinski, and J. G. Fujimoto, "Noninvasive assessment of the developing Xenopus cardiovascular system using optical coherence tomography," Proc Natl Acad Sci U S A, vol. 94, pp. 4256-61, (1997). 24. R. Youngquist, S. Carr, and D. Davies, "Optical coherence-domain reflectometry: a new optical evaluation technique," Optics Letters, vol. 12, pp. 158-60, (1987). 25. K. Takada, I. Yokohama, K. Chida, and J. Noda, "New measurement system for fault location in optical waveguide devices based on an interferometric technique," Applied optics, vol. 26, pp. 1603-8, (1987). 26. F. Kremkau, Diagnostic ultrasound: principles, instrumentation, and exercises, 2nd ed. Philadelpha: Grune and Stratton, 1984. 27. F. Kremkau, Doppler ultrasound: Principles and instruments. Philadelphia: W. B. Saunders, 1990. 28. P. Fish, Physics and instrumentation of diagnostic medical ultrasound. New York: John Wiley and Sons, 1990. 29. W. Ziebel and R. Sohaey, Introduction to ultrasound.Philadelphia: W. B. Saunders, 1998. 30. E. A. Swanson, D. Huang, M. R. Hee, J. G. Fujimoto, C. P. Lin, and C. A. Puliafito, "High-speed optical coherence domain reflectometry," Optics Letters, vol. 17, pp. 151-3, (1992). 31. E. A. Swanson, J. A. Izatt, M. R. Hee, D. Huang, C. P. Lin, J. S. Schuman, C. A. Puliafito, and J. G. Fujimoto, "In vivo retinal imaging by optical coherence tomography," Optics Letters, vol. 18, pp. 1864-6, (1993). 32. J. G. Fujimoto, M. E. Brezinski, G. J. Tearney, S. A. Boppart, B. Bouma, M. R. Hee, J. F. Southern, and E. A. Swanson, "Optical biopsy and imaging using optical coherence tomography," Nat Med, vol. 1, pp. 970-2, (1995). 33. G. J. Tearney, M. E. Brezinski, B. E. Bouma, S. A. Boppart, C. Pitris, J. F. Southern, and J. G. Fujimoto, "In vivo endoscopic optical biopsy with optical coherence tomography [see comments]," Science, vol. 276, pp. 2037-9, (1997). 34. B. E. Bouma, G. J. Tearney, I. P. Bilinsky, B. Golubovic, and J. G. Fujimoto, "Self-phasemodulated Kerr-lens mode-locked Cr:forsterite laser source for optical coherence tomography," Optics Letters, vol. 21, pp. 1839-41, (1996). 35. W. Drexler, U. Morgner, F. X. Kartner, C. Pitris, S. A. Boppart, X. D. Li, E. P. Ippen, and J. G. Fujimoto, "In vivo ultrahigh-resolution optical coherence tomography," Optics Letters, vol. 24, pp. 1221-3, (1999). 58 36. U. Morgner, W. Drexler, F. X. Kartner, X. D. Li, C. Pitris, E. P. Ippen, and J. G. Fujimoto, "Spectroscopic optical coherence tomography," Optics Letters, vol. 25, pp. 111-13, (2000). 37. G. J.-Tearney, B. E. Bouma, and J. G. Fujimoto, "High-speed phase- and group-delay scanning with a grating-based phase control delay line," Optics Letters, vol. 22, pp. 1811-13, (1997). 38. Z. Chen, T. E. Milner, D. Dave, and J. S. Nelson, "Optical Doppler tomographic imaging of fluid flow velocity in highly scattering media," Optics Letters, vol. 22, pp. 64-66, (1997). 39. J. A. Izatt, M. D. Kulkami, S. Yazdanfar, J. K. Barton, and A. J. Welch, "In vivo bidirectional color Doppler flow imaging of picoliter blood volumes using optical coherence tomography," Optics Letters, vol. 22, pp. 1439-1441, (1997). 40. M. D. Kulkarni, T. G. van Leeuwen, S. Yazdanfar, and J. A. Izatt, "Velocity estimation accuracy in color Doppler optical coherence tomography," presented at Advances in Optical Imaging and Photon Migration, Orlando, FL, 1998. 41. Z. Chen, Y. Zhao, S. M. Srinivas, J. S. Nelson, N. Prakash, and R. D. Frostig, "Optical doppler tomography," IEEE Journal of Selected Topics in Quantum Electronics, vol. 5, pp. 1134-1142, (1999). 59