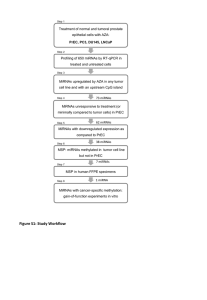
I AUG 16 by
advertisement

Role of the Interaction of proHB-EGF with Heparan Sulfate Proteoglycans MASSACHUSETTS INSTITUTE OF TECHNOLOGY by I AUG 16 2010 Robin N. Prince B.S. Mechanical Engineering University of Arkansas, 2003 1 S.M. Mechanical Engineering Massachusetts Institute of Technology, 2005 LIBRARIES ARCHNES SUBMITTED TO THE DEPARTMENT OF BIOLOGICAL ENGINEERING IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY IN BIOLOGICAL ENGINEERING AT THE MASSACHUSETTS INSTITUTE OF TECHNOLOGY AUGUST 2009 C 2009 Massachusetts Insitute of Technology, All Rights Reserved The author hereby grants to MIT permission to reproduce and to distribute publicly paper and electronic copies of this thesis document in whole or in part in any medium now known or hereafter created. Signature of Author: Department of Biological Engineering August 14, 2009 Certified By: Dou's.7 auffenburger Professor of Biological Engineering Thesis Supervisor Certified By: Richard T. Lee Professor of Medicine, Brigham and Women's Hospital, Harvard Medical School Thesis Supervisor Accepted By: tRoger D. Kamm Professor of Biological Engineering Thesis Committee Chair Thesis Committee: Douglas A. Lauffenburger, Thesis Supervisor, MIT Richard T. Lee, Thesis Supervisor, BWH & Harvard Medical School Roger D. Kamm, MIT Matthew Nugent, Boston University School of Medicine Role of the Interaction of proHB-EGF with Heparan Sulfate Proteoglycans by Robin N. Prince Submitted to the Department of Biological Engineering on August 24, 2009 in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Biological Engineering Abstract Heparin-binding epidermal growth factor-like growth factor (HB-EGF) exhibits activity as a juxtacrine, paracrine, and autocrine ligand for the epidermal growth factor receptor (EGFR), and possesses the ability to bind heparan sulfate proteoglycans (HSPGs). The interaction of HB-EGF with HSPGs has been previously studied only with the soluble (autocrine/paracrine) form of the protein (sHB-EGF), produced after proteolytic cleavage of the transmembrane form (proHB-EGF) from the cell surface. It was hypothesized that HSPGs interact with proHB-EGF in ways that could alter behavior of the transmembrane form of this ligand and consequent processes. Using an engineered form of proHB-EGF that allowed for independent tracking of the extracellular domain and the C-terminal tail, proHB-EGF was observed primarily at sites of cell-cell contact. However, a dramatic change in this localization was observed upon the addition of exogenous heparin, heparan sulfate, heparinase III or mutation of the heparin-binding domain of proHB-EGF, suggesting that an interaction with HSPGs is responsible for localizing proHB-EGF to sites of cell-cell contact. Further studies in wild-type CHO-Ki cells and heparan sulfate deficient CHOpgsD-677 cells demonstrated that a trans interaction between proHB-EGF and HSPGs on neighboring cells was responsible for this localization. Additionally, this interaction inhibited proteolytic processing of the ligand, as heparin and mutation of the heparin-binding domain increased the amount of sHB-EGF accumulated in the media. Acknowledgements This work would not have been possible without the support and guidance of my two wonderful advisors, Douglas Lauffenburger and Richard T. Lee. I would like to thank them for their excellent mentorship, guidance, support, and allowing me to pursue my own avenues of investigation and interest during my graduate studies. I would like to thank our collaborators Steve Wiley and Alice Ting, who have provided scientific insight and reagents that were crucial for this work. I am additionally grateful to my thesis committee members, Matthew Nugent and Roger Kamm for their guidance and scientific advice. I also would like to recognize Linda Griffith for her enthusiastic support and mentorship through my graduate research endeavors. I would like to thank all members of the Lee, Lauffenburger, and Griffith labs, as I have learned a great deal being part of them all and have thoroughly enjoyed my time here. I would particularly like to recognize and thank Jun Yoshioka for providing excellent training that was crucial in my transition to a biological researcher. Special thanks go to Eric Shreiter for his help in designing the acceptor peptide HB-EGF construct, Peng Zou for making mSA-AF568, and Hyung Do Kim for doing monolayer migration experiments. I would like to thank my funding sources, the NSF graduate fellowship and the Poitras Predoctoral Fellowship. To Mom, Dad, Wade, and Grandma, I thank you all for your love, support, patience and encouragement. To my boyfriend, Ohia, thank you for all your encouraging advice, your love and support, and your always open ear. Also, thank you to so many great friends and colleagues I have met in Boston, you have all made my time here so special. I would additionally like to recognize my undergraduate research mentor Ajay Malshe at the University of Arkansas, and my high school and mentors at the Arkansas School for Mathematics and Sciences, as they were crucial in preparing me for this life path. Table of Contents Abstract ............................................................................................................................... 3 Acknowledgements...................................................................................................... 4 Table of C ontents.......................................................................................................... 5 Chapter One: Introduction ............................................................................................. 9 1.1.1 Growth factor signaling .................................................................................... 9 1.1.2 The EGFR system............................................................................................. 9 1.1.3 HB-EGF autocrine signaling and EGFR transactivation..................................... 11 1.1.4 Heparin and heparan sulfate proteoglycans ...................................................... 12 1.1.5 Physiologic and pathophysiologic roles of HB-EGF........................................ 13 1.1.5.1 HB-EGF in development ........................................................................... 13 1.1.5.2 Diphtheriatoxin ......................................................................................... 14 1.1.5.3 HB-EGF in wound healing ........................................................................ 15 1.1.5.4 HB-EGF in cancer.................................................................................... 15 1.1.5.5 HB-EGF in the cardiovascularsystem ...................................................... 16 1.1.5.6 HB-EGF in pregnancy ............................................................................... 17 1.1.5.7 HB-EGF in the kidney ................................................................................ 18 Chapter Two: Experimental approach for visualization of HB-EGF ........................... 20 2 .1 Introdu ction ............................................................................................................ 20 2.2 Materials and Methods....................................................................................... 23 2 .3 Resu lts.................................................................................................................... 30 2 .4 D iscussion .............................................................................................................. 34 2 .5 F igu res..................................................................................................................... 35 Figure 2.5.1: Two methods of BirA (biotin ligase) labeling of the acceptor peptide pro tein. ...................................................................................................................... 35 Figure 2.5.2. Design of a monovalent streptavidin............................................. 36 Figure 2.5.3 Validation of AP-HBEGF constructs............................................... 37 Figure 2.5.4 Final gene map of AP-HBEGF-GFP and bioactivity....................... 38 Figure 2.5.5 AP-HBEGF-GFP is localized to cell-cell contact sites.................... 39 Chapter Three: Polarization of HB-EGF at the wound edge ........................................ 41 3 .1 Introdu ction ............................................................................................................ 41 3.2 Materials and Methods....................................................................................... 43 3 .3 Resu lts............................................................................................................... 44 3.3.1 ProHB-EGF is missing from the wound edge ................................................ 44 3.3.2 HB-EGF loss from wound edge is not due to proteolysis .............. 45 3.3.3 Newly synthesized HB-EGF localizes to cell-cell contact sites only ......... 46 3 .4 D iscu ssion .............................................................................................................. 48 3 .5 F igures.................................................................................................................... 49 Figure 3.5.1 HB-EGF is absent from the wound edge.................... 49 Figure 3.5.2 Effect of inhibitors on polarization of HB-EGF at wound edge. ........ 50 Figure 3.5.3 Turnover of cell surface HB-EGF at wound edge............................. 51 Chapter Four: The heparin-binding domain mediates localization of proHB-EGF to cellcell contact sites................................................................................................................ 53 4 .1 Introdu ction ............................................................................................................ 53 54 4.2 M aterials and M ethods...................................................................................... -- - .........- 59 4.3 Results........................................................................ 59 proHB-EGF.............. of localization the alter sulfate 4.3.1 Heparin and heparan BirA to 4.3.2 ProHB-EGF in microdomains at cell-cell contact sites is inaccessible ..... 60 and streptavidin................................................................................. site 4.3.3 Pro-HB-EGF interaction with HSPGs controls cell-cell contact - ......... 61 .. localization.......................................................................... 62 4.3.4 ProHB-EGF interacts with HSPGs in trans ............................................... 4.3.5 Heparin-binding controls amphiregulin localization, but engineered heparin64 binding is insufficient for cell-cell contact localization......................................... ... ..... 66 . ... 4.4 D iscussion....................................................................... 66 4.4 Discussion................................................................................ 69 . ---.... - ----- -----.................. 4.5 Figures.................................................................-------..... from HB-EGF of localization the changed Figure 4.5.1. Heparin and heparan sulfate cell-cell contact sites to a homogenous distribution over the cell surface............ 69 Figure 4.5.2 Localization change of HB-EGF after addition of heparin over time.. 70 Figure 4.5.3 Heparin increases accessibility of cell surface AP-HBEGF-GFP....... 71 Figure 4.5.4 HSPGs target pro-HB-EGF to cell-cell contact sites. ..................... 72 Figure 4.5.5. The heparin-binding domain targets pro-HB-EGF to cell-cell contact 73 sites. .................................................................................---------------------------.............. for required are Figure 4.5.6 HSPGs and the heparin-binding domain of HB-EGF 74 localization to cell-cell contact sites. ..................................................................... 75 Figure 4.5.7. HSPGs interact in trans with pro-HB-EGF. ................................... Figure 4.5.8 ProHB-EGF is preferentially localized to cell-cell contact sites when 76 neighbored by a non-expressing cell.................................................................... Figure 4.5.9 The heparin-binding domain of amphiregulin controls localization to ... 77 cell-cell contact sites ...................................................................................... 80 Chapter Five: Role of the heparin-binding domain ...................................................... 80 5.1 Introduction................................................................................ ... 81 5.2 M aterials and Methods.................................................................................. 83 ---------------------................... . 5.3 Results............................................................................ 5.3.1 The heparin-binding domain controls cell surface localization.................... 83 86 5.3.2 ProHB-EGF does not cluster EGFR ............................................................... 5.3.3 The heparin-binding domain controls ectodomain shedding........................ 88 89 5.3.4 Wound healing and migration....................................................................... 91 5.4 Discussion................................................................................. . ---------------------.............. 93 5.5 Figures............................................................................----. the cell surface decreases domain heparin-binding Figure 5.5.1 Mutation on the 93 fraction of HB-EGF. ........................................................................ Figure 5.5.2 The heparin-binding domain mutant is primarily localized in the ..... 94 intracellular space. ................................................................................. 95 activation.................................................... Figure 5.5.3 EGFR localization and 96 Figure 5.5.4 Heparin reduces ERK activation. ..................................................... 97 cleavage................. pro-HB-EGF reduces Figure 5.5.5. Interaction with HSPGs 98 Figure 5.5.6 Wound healing in COS-7 cells......................................................... 99 monolayer. confluent a within HMECs Figure 5.5.7 Migration speed of individual Chapter Six: Conclusions and future directions............................................................. 6.1 Future directions: Use of AP-tagged EGFR ligands............................................ 6.2 Future directions: Signaling with heparin-binding domain mutant HB-EGF...... 6.3 Future directions: HB-EGF localization change in vivo..................................... 6.4 Future directions: Structure of the HSPG-proHBEGF-CD9 complex................ 6.5 Future Directions: Computational analysis......................................................... Referen ces....................................................................................................................... 101 101 103 103 104 104 106 8 Chapter One: Introduction 1.1.1 Growth factor signaling Signaling by soluble extracellular ligands is classified by the distance in which the signal acts and the source of the ligand. Free ligands can signal in an endocrine, paracrine, or autocrine manner. Endocrine signaling molecules travel the farthest, from an endocrine organ to target cells, where the molecules typically travel through blood or extracellular fluids (Lodish, 2003). However, on a much shorter length scale, paracrine and autocrine signaling affect cells only in close proximity. In paracrine signaling, a cell produces a soluble ligand which diffuses to and binds another cell. Autocrine signaling differs in that the cell which produces the soluble ligand is also activated by that ligand. However, not all extracellular signals are diffusible. Some growth factors are active in their membrane bound form before cleavage, which defines juxtacrine signaling. 1.1.2 The EGFR system The epidermal growth factor receptor (EGFR) system is a well studied example of cell communication in regards to growth, motility, and development. The EGFR family consists of four receptor tyrosine kinases: erbB1/HER1, referred to as the epidermal growth factor receptor (EGFR), erbB2/HER2, erbB3/HER3, and erbB4/HER4. Upon ligand binding, the EGF receptors dimerize and stimulate intracellular signal transduction pathways which encourage the cell to proliferate, differentiate, or survive (Lodish 2004). Ligands for the EGFR system include HB-EGF, epidermal growth factor (EGF), amphiregulin, transforming growth factor-a (TGF-a), neuregulin, betacellulin and epiregulin. Ligands in the EGFR family are synthesized in membrane anchored proforms and are subsequently cleaved at an extracellular site via a metalloprotease to release the soluble growth factor (Asakura et al., 2002; Sahin et al., 2004). However, the ability of some EGFR ligands to activate their corresponding receptors is not dependent on cleavage; both the pro-membrane and soluble forms can serve as cellular signals (Massague and Pandiella, 1993). Binding of ligands to receptor tyrosine kinases activates various intracellular signaling pathways. In particular, EGFR activation signals through the Ras-MAP kinase pathway. Activation of the EGFR leads to the recruitment of Grb2 and Shc, which leads to activation of the intercellular membrane-bound protein, Ras. Ras then activates an intracellular kinase cascade including MAP kinase, also known as ERK, Jun N-terminal Kinase (JNK), and p38, which are active in their phosphorylated form. Activated ERK dimerizes and can translocate into the nucleus to activate various transcription factors. In addition to the Ras-MAP kinase pathway, the EGFR has also been linked to the JAK-STAT pathway. JAK is a tyrosine kinase and STAT is a transcription factor. HB-EGF is expressed primarily in the lung, skeletal muscle, brain and heart (Abraham et al., 1993) and induces proliferation and migration of smooth muscles cells, fibroblasts and keratinocytes (Raab and Klagsbrun, 1997; Yahata et al., 2006). Heparinbinding EGF (HB-EGF) was discovered in 1991 in the medium of cultured macrophagelike cells (Higashiyama et al., 1991). HB-EGF has several key features which differ from traditionally studied EGF. HB-EGF shares the same binding domain to EGFR as EGF which consists of six conserved cysteines, however, only approximately 40% of the carboxyl portion protein sequence is homologous to EGF (Higashiyama et al., 1991). Additionally, as its name suggests, HB-EGF binds strongly to heparin. EGF is selective for the EGFR, yet HB-EGF binds to EGFR, erbB4/HER4 (Elenius et al., 1997), and heparan sulfate proteoglycans (Higashiyama et al., 1993) present on the cell surface and within the extracellular matrix. HB-EGF is an 8.3 kDa protein; however, after heavy 0glycosylation it has an estimated mass of 20-22 kDa on an SDS-PAGE gel. The ability of HB-EGF to signal in a juxtacrine manner is increased by association with the tetraspanin protein CD9 (Higashiyama et al., 1995; Iwamoto et al., 1991), which also links HB-EGF to a31 integrins (Nakamura et al., 1995). In vivo staining shows co- localization of all three proteins in many tissues (Nakamura et al., 2001). The membrane-anchoring domain of HB-EGF is also crucial in juxtacrine signaling, as domain swapping with the membrane-anchoring domain of EGF, which does not participate in juxtacrine signaling, led to loss of the ability of HB-EGF to signal in a juxtacrine manner (Dong et al., 2005). Pro-HB-EGF has also been reported to interact with the tetraspanins CD63, CD81 and CD82 on the cell surface; however, no known phenotype has been reported for this interaction (Nakamura et al., 2000). Interestingly, HB-EGF juxtacrine and autocrine/paracrine signaling have been shown to elicit different phenotypes, with autocrine/paracrine activity leading to cell proliferation and juxtacrine activity leading to growth inhibition in some cell lines. DER cells, a hematopoietic cell line, and human luteinized granulosa cells undergo growth inhibition and apoptosis when stimulated with proHB-EGF (Iwamoto et al., 1999; Pan et al., 2002). However, proHBEGF juxtacrine signaling has also been reported to protect renal epithelial cells and Madin-Darby canine kidney epithelial cells from apoptosis (Singh et al., 2007b; Takemura et al., 1997). Therefore, the cell fate resulting from HB-EGF juxtacrine stimulation may be cell-type specific. Recently, it has been reported that the C-terminal fragment of HB-EGF is also a signaling molecule. This fragment translocates to the nucleus, where it reverses gene repression of the transcriptional repressors promyelocytic leukemia zinc finger protein (PLZF) and Bcl6 (Kinugasa et al., 2007; Nanba et al., 2003). The C-terminal tail of HBEGF can interact with BAG-1, which can increase proHB-EGF proteolysis, increase resistance to apoptosis, and decrease cell adhesion (Lin et al., 2001). The cytoplasmic domain of HB-EGF is phosphorylated after treatment with various stimuli, and mutation of this phosphorylation site does not affect ligand cleavage, but reduces tumorigenicity (Wang and Dey, 2006). 1.1.3 HB-EGF autocrine signaling and EGFR transactivation Upon the discovery of autocrine signaling in the EGFR system, it was originally hypothesized to be a malignant phenotype (Spom and Todaro, 1980). However, autocrine signaling has become widely accepted as a major form of cell communication regulating a host of different processes. Those specific to HB-EGF include wound healing in corneal epithelial cells (Xu et al., 2004) and keratinocytes (Piepkorn et al., 1998), and the response to mechanical stress in bronchial epithelial cells (Chu et al., 2005). In the HB-EGF/EGFR system, autocrine signaling is initiated with proteolytic cleavage of HB-EGF with a disintegrin and metalloprotease (ADAM). The specific ADAM which is responsible for HB-EGF cleavage is a topic of debate and tends to vary depending on cell type, however ADAMS 10, 12, and 17 have been identified (Higashiyama and Nanba, 2005). HB-EGF plays a prominent role in EGFR transactivation, typically through activation of G-protein coupled receptors (GPCRs). Activation of various GPCRs with GPCR agonists, such as angiotensin II (Yahata et al., 2006), endothelin-1 (Chansel et al., 2006), lysophosphatidic acid (Xu et al., 2007), ATP (Yin and Yu, 2009), histamine (Ancha et al., 2007), interleukin-8 (Itoh et al., 2005), and extracellular Ca (Yano et al., 2004) has been demonstrated to lead to HB-EGF cleavage. After proteolytic release, the mature, soluble form of HB-EGF is then free to diffuse and bind to HSPGs, EGFRs or erbB4 receptors on the cell surface. This system has the potential for positive feedback in that EGFR activation can lead to transcription of additional growth factors and activation of EGFR ligand shedding (Citri and Yarden, 2006). 1.1.4 Heparin and heparan sulfate proteoglycans HB-EGF was first identified as a growth factor purified from a heparin column which stimulated fibroblast and smooth muscle cell growth (Higashiyama et al., 1991). HB-EGF has the ability to bind heparin via a heparin-binding domain that consists of multiple basic lysine and arginine residues, which interact with negatively charged heparin (Thompson et al., 1994). Heparin, a highly sulfated glycosaminoglycan, is a widely used anticoagulant as it activates antithrombin, which inactivates blot clotting enzymes, such as thrombin and factor Xa (Chuang et al., 2001). In humans, heparin is produced by mast cells residing in vascularized serosal cavities, and upon degranulation heparin is released (Kalesnikoff and Galli, 2008). Proteoglycans are a type of glycoprotein which have glycosaminoglycan (GAG) side chains. Four major classes of proteoglycans exist, which are classified by the type of GAG chains expressed: heparan sulfate, chondroitin sulfate, dermatan sulfate, and keratan sulfate. Heparan sulfate proteoglycans (HSPGs) consist of repeating units of Dglucouronic or L-iduronic acid with N-acetyl or N-sulfo-D-glucosamine disaccharides with chains approximately 200 units long (Lodish 2004). As heparan sulfate is similar to heparin in its structure and sulfation pattern, it also has the ability to bind HB-EGF and other heparin-binding growth factors. Likely, it is the interaction of HB-EGF with HSPGs that is more physiologically relevant, as HSPGs are present in the extracellular matrix and cell surface glycocalyx of most cells. HSPGs have the ability to modulate the activity of many heparin-binding growth factors. The heparin-binding domain of HBEGF appears to be inhibitory, however, upon heparin or heparan sulfate binding, the ability of HB-EGF to activate the EGFR is increased (Higashiyama et al., 1993; Takazaki et al., 2004). Heparan sulfate side chains and heparin can be altered via enzymatic cleavage. There are three major heparin/heparan sulfate lyases purified from Flavobacterium heparinum (heparinase I, II and III), which cleave specific linkages present in heparin and/or heparan sulfate (reviewed in (Capila and Linhardt, 2002)) and are commonly used for experimental purposes. Mammalian heparanase is a pro-enzyme, that is cleaved into two subunits that associate to form active heparanase (reviewed in (Vlodavsky et al., 2007)). Additionally, there are the enzymes HSulf-1 and HSulf-2, which are secreted endosulfatases that degrade heparan sulfate (Morimoto-Tomita et al., 2002). 1.1.5 Physiologic and pathophysiologic roles of HB-EGF 1.1.5.1 HB-EGF in development HB-EGF is an important growth factor in mammalian development, particularly for the cardiovascular system. Most HB-EGF knockout mice die within the first postnatal week and have grossly enlarged ventricular chambers and cardiac valves (Iwamoto et al., 2003), while smooth muscle and endothelial cell specific knock-outs show a similar phenotype (Nanba et al., 2006). Mice expressing only a non-cleavable form of proHB-EGF also die of severe heart failure, suggesting that autocrine stimulation is required for proper heart development, while mice expressing the soluble form of HBEGF, lacking the C-terminal and transmembrane domain, develop severe hyperplasia of the skin and heart (Yamazaki et al., 2003). HB-EGF expression is observed in smooth muscle cells of the aortic wall of babies and children, however, expression is decreased in young adults (Miyagawa et al., 1995). HB-EGF also plays a role in the development of other tissues and organs. HBEGF knock-out mice show enlarged mesenchymal tissue of the lung and heart, and have immature aveoli (Jackson et al., 2003). HB-EGF knock-out mice also have delayed eyelid closure during development, where HB-EGF is expressed solely at the leading edge of the migrating epithelial sheet (Mine et al., 2005). Mice expressing an uncleavable form of HB-EGF display identical defects in wound closure, suggesting that HB-EGF autocrine signaling is crucial for driving persistent cell migration to close the wound (Mine et al., 2005). HB-EGF is expressed at higher levels in the embryonic and neonatal kidney compared to the adult, and may play a role in the development of renal collecting ducts and promote renal epithelial cell branching (Takemura et al., 2001; Takemura et al., 2002). 1.1.5.2 Diphtheriatoxin ProHB-EGF was first studied as the unidentified receptor for diphtheria toxin (Naglich et al., 1992). Diphtheria was once a serious world health threat that has largely been eliminated in developed nations after introduction of a vaccine in the early 1900s. Diphtheria is caused by the protein diphtheria toxin, which is secreted by Corynebacteriumdiphtheriae. The toxin consists of two fragments (A and B chain), of which the B chain binds to the EGF-like domain of proHB-EGF and serves as the toxin's route to enter the cell via receptor-mediated endocytosis (Naglich et al., 1992). Once internalized, the A chain inhibits protein synthesis by catalyzing ADP-ribosylation of elongation factor-2, rendering it inactive (reviewed in (Neville and Hudson, 1986)). Just as association of proHB-EGF with CD9 upregulates juxtacrine activity, it also increases binding affinity and sensitivity of diphtheria toxin binding to proHB-EGF (Cha et al., 2000; Iwamoto et al., 1991). Additionally, the association of proHB-EGF with heparin or HSPGs on the cell surface increases in the affinity of diphtheria toxin for proHB-EGF (Shishido et al., 1995). The conjugation of bacterial immunotoxins, such as diphtheria toxin, to cell specific targeting molecules is in investigation for treatment of cancer (Reviewed in (Kreitman, 2009)). Additionally, proHB-EGF's role as the diphtheria toxin receptor has been used for targeted cell ablation by expressing the human form of proHBEGF in a targeted cell type within the mouse (Saito et al., 2001). As diphtheria toxin does not interact with rat or mouse proHB-EGF, transgenic human proHB-EGF expression under a tissue or cell-specific promoter in mice can lead to conditional and tissue-specific cell ablation by administering diphtheria toxin. CRM-197 is a non-toxic mutant of diphtheria toxin, which inhibits HB-EGF by preventing it from binding to EGF receptors (Mitamura et al., 1995) and is under investigation as a treatment for ovarian cancer (reviewed in (Miyamoto et al., 2006)) 1.1.5.3 HB-EGF in wound healing HB-EGF autocrine signaling is a crucial regulator of keratinocyte migration and re-epithelialization in skin wound healing. HB-EGF leads to an increased rate of keratinocyte migration (Shirakata et al., 2005; Tokumaru et al., 2000), and topical application to bums increases wound closure (Cribbs et al., 1998). Additionally, HBEGF is also found in wound fluid (Marikovsky et al., 1993). HB-EGF expression is upregulated in keratinocytes at the margin of the wound and expression is increased by disruption of lipid rafts (Mathay et al., 2008; McCarthy et al., 1996). HB-EGF also plays a role in closing wounds of the eye, as it is an autocrine factor secreted by corneal epithelial cells in response to wounding, and inhibition of HB-EGF leads to impaired wound closure (Xu et al., 2004). Experiments suggest that sudden reduction of spatial constraints is sufficient for HB-EGF release and EGFR activation to stimulate corneal epithelial cell migration (Block et al., 2004). 1.1.5.4 HB-EGF in cancer HB-EGF gene expression is increased in many types of cancer, including tumors of the pancreas, liver, esophagus, skin, colon, stomach, ovary, bladder and brain and is associated with the acquisition of malignant phenotypes (reviewed in (Miyamoto et al., 2006)). Additionally, HB-EGF is involved in chemotherapy resistance, as chemotherapeutic agents can cause HB-EGF ectodomain shedding and protect tumor cells from apoptosis (Wang et al., 2007). Much attention has been directed to the role of HB-EGF in ovarian cancer, as HB-EGF is associated with poor clinical outcome (Tanaka et al., 2005). High mortality is predominantly caused by spread of the tumor into the peritoneal cavity (reviewed in (Miyamoto et al., 2008)). HB-EGF appears to be involved in tumor formation and survival in the peritoneal cavity, as tumor formation by injection of the human ovarian cancer cell line SKOV3 and RMG1 in nude mice is enhanced by proHB-EGF expression and blocked completely blocked by inhibition of HB-EGF gene expression (Miyamoto et al., 2004). Soluble HB-EGF levels are significantly elevated in the peritoneal cavity at levels sufficient for ovarian cancer cell survival (Miyamoto et al., 2004). 1.1.5.5 HB-EGF in the cardiovascularsystem HB-EGF is a strong mitogen and chemoattractant for smooth muscle cells, whose expression is significantly increased in smooth muscle cells and macrophages of atherosclerotic plaques (Miyagawa et al., 1995; Nakata et al., 1996). Therefore HB-EGF has been suggested to play a role in smooth muscle cell proliferation and migration in atherosclerosis. HB-EGF expression is increased in restricted carotid arteries, and is associated with increased lumen narrowing, thickening of the artery wall, and increased circumference that is not observed in HB-EGF knock-out mice (Zhang et al., 2008). EGFR transactivation via HB-EGF shedding in response to GPCR agonists additionally plays a role in vascular biology, as HB-EGF induced EGFR activation is necessary for endothelin-1 induced vasoconstriction response (Chansel et al., 2006), and HB-EGF is shed by angiotensin II stimulation in smooth muscle cells, causing proliferation and migration (Yang et al., 2005). Cardiac hypertrophy occurs when individual cardiomyocytes (the beating heart cell) expand in size under excess mechanical force to increase output and meet physiologic demands (Chien, 1999). As a normal adaptive response mechanism, cardiac hypertrophy is not dangerous until the condition of persistent stress over time evolves into dysfunction and myocardial failure (Chien, 1999). Cardiac hypertrophy is associated with an increase in cell size, protein synthesis, and re-expression of fetal genes (Sadoshima and Izumo, 1997). Increasing evidence suggests that stress on cardiomyocytes leads to production of endothelin-1, which induces metalloprotease cleavage of pro-HB-EGF (Anderson et al., 2004). The soluble growth factor is then free to diffuse and activate the EGFR and subsequent intracellular signaling pathways which lead to cardiac hypertrophy. Evidence which supports this hypothesis includes: strain on myocytes activates the EGFR and increases the concentration of HB-EGF in cell medium (Anderson et al., 2004), inhibition of pro-HBEGF cleavage prevents GPCR agonist induced hypertrophy (Asakura et al., 2002); and inhibition of NAD(P)H oxidase, which leads to endothelin-1 release, inhibits HB-EGF shedding (Anderson et al., 2004). Studies suggest that ADAM12 is the metalloprotease responsible for hypertrophic HB-EGF shedding in the heart (Asakura et al., 2002). overexpression Our own studies with adenovirus of HB-EGF the heart show that HB-EGF expression leads to cardiomyocyte hypertrophy and degradation of connexin43, a crucial gap junctional protein in the heart in a localized area around the site of transfection (Yoshioka et al., 2005). This study also demonstrates that the spatial range of HB-EGF diffusion in the heart was very restricted, only affecting the cell which produced HB-EGF and its immediately adjacent neighbor. 1.1.5.6 HB-EGF in pregnancy HB-EGF expression 6-7 hours prior to attachment of the blastocyst to the luminal epithelium is the earliest known marker for blastocyst implantation. The blastocyst exists early in embryogenesis and is covered by an outer layer of cells composing the trophectoderm. During implantation, the blastocyst adheres to the luminal epithelium of the endometrial wall, and stromal cells that surround the blastocyst decidualize to embed the embryo in the stromal bed (reviewed in (Wang and Dey, 2006)). HB-EGF is involved in early cross-talk between the luminal epithelium and the blastocyst during implantation. ErbB4 and EGFR are expressed at the apical surface of trophectoderm cells (Paria et al., 1999). Pro-HBEGF is expressed in the luminal epithelium, only surrounding the blastocyst, and juxtacrine interaction with EGFR and HSPGs on the blastocyst increases attachment during the implantation process (Das et al., 1994; Raab et al., 1996). Blastocysts synthesize the HSPG perlecan on the trophectodermal surface, which is required for attachment, and this synthesis is increased during the periimplantation period (Carson et al., 1993; Farach et al., 1988). Addition of exogenous heparin or digestion of cell surface heparan sulfate with heparinase considerably reduces the rate of attachment of embryos to monolayers of uterine epithelial cells (Farach et al., 1987). The blastocyst itself also expresses HB-EGF, and the paracrine HB-EGF produced may trigger HB-EGF gene expression in the luminal epithelium (Hamatani et al., 2004). HB-EGF null mice show deferred implantation, however, amphiregulin, but not epiregulin, can partially compensate for the loss of HB-EGF (Xie et al., 2007). 1.1.5.7 HB-EGF in the kidney In the adult kidney, HB-EGF is primarily localized to tubular epithelial cells of the S3 segment of the outer stripe in the outer medulla (Nakagawa et al., 1997). HB-EGF expression is induced in the distal tubules injured by ischemia/reperfusion (Takemura et al., 1997), in epithelial cells involved in the formation of lesions of focal and segmental glomerular sclerosis (Paizis et al., 1999), in renal epithelial cells in the obstructed kidney acting to inhibit apoptosis (Nguyen et al., 2000), in mesangial cells driving proliferation in glomerulonephritis (Takemura et al., 1999b), and in polycystic kidney disease (MacRae Dell et al., 2004). Interestingly, proHB-EGF juxtacrine signaling, but not autocrine/paracrine signaling, promotes renal epithelial cell survival which is increased by co-expression of CD9 (Takemura et al., 1999a; Takemura et al., 1997) and increases transepithelial resistance in polarized Madin-Darby canine kidney (MDCK) cells (Singh et al., 2007a). CD9 and p1 integrin expression are upregulated in the medullary thick ascending limbs (nephron segments that are normally exposed to higher and variable extracellular osmolality) after dehydration, where HB-EGF is also expressed, suggesting they may form an osmotically relevant membrane signaling complex (Sheikh-Hamad et al., 2000). Urea activation of GPCRs in renal medullary cells leads to HB-EGF cleavage and EGFR activation, which may prevent hypertonic stress induced damage (Zhao et al., 2003). 19 Chapter Two: Experimental approach for visualization of HBEGF 2.1 Introduction Traditional methods of visual protein tracking in cell culture is accomplished with either antibody detection or fusion of a fluorescent protein. Antibody detection, also known as immunocytochemistry, requires an antibody that recognizes the protein or an epitope tag which has been fused to the protein of interest. However, antibody detection for intracellular domains of the protein requires cell fixation and permeabilization, and therefore rules out any live cell tracking of the protein of interest. Fusion of a fluorescent protein is accomplished by transfecting circular DNA of the gene of interest with the DNA of the fluorescent protein, such as green fluorescent protein (GFP), to either the N or C-terminus of the molecule. This method allows for live cell tracking of the molecule; however, neither method is ideal for the extracellular domain of the protein of interest in this study, HB-EGF. Antibody detection is not ideal, as live cell imaging is desirable for this study, and our previous experience has shown that antibodies to HB-EGF are not very sensitive and produce high levels of background. Conjugation of a fluorescent protein is feasible for HB-EGF, but only for the C-terminal tail as the N-terminus is cleaved during processing to form mature proHB-EGF. As the extracellular domain can be detached from the C-terminal intracellular domain via protease cleavage, and both can serve as signaling molecules, the ability to track both independently is desirable. In order to visually track the extracellular domain of HB-EGF, we chose the method of acceptor peptide biotinylation with the enzyme biotin ligase. Biotin ligase is an E. Coli enzyme that can specifically biotinylate one lysine residue within a fifteen amino acid acceptor peptide sequence (Figure 2.5. 1a) in the presence of ATP (Beckett et al., 1999). The acceptor peptide sequence is the minimal substrate required from the biotin carbonyl carrier protein subunit of acetyl-CoA carboxylase that biotin ligase will specifically recognize. Once placed within the extracellular region of a protein, biotin ligase can be used to covalently attach one biotin molecule per protein. Then, as no extracellular proteins are endogenously biotinylated, these proteins can be specifically visualized taking advantage of the high binding affinity between streptavidin conjugated labels and biotin, which can minimize the background signal. Additionally, there is no need to fix the cells, which gives the flexibility of live-cell imaging. As the C-terminus of HB-EGF can become separated from the extracellular domain via protease cleavage and serve as a signaling molecule after translocation to the nucleus, GFP was conjugated to the C-terminus of HB-EGF to allow for independent tracking of the extracellular domain versus the C-terminal tail. The mouse HB-EGF gene was chosen for fusion of the acceptor peptide to keep the method flexible for future in vivo experiments. The acceptor peptide must be inserted into an extracellular region of HB-EGF which does not affect the protein's structure or function. Using domain analysis, four potential insertion sites for the acceptor peptide were chosen in an effort not affect protein cleavage, glycosylation, or binding to the receptor and heparan sulfate. Therefore, the following proposed insertion sites are not within the EGF-like domain or heparin-binding domain. I. After Asparagine 91: This is two amino acids before the last putative N-terminal cleavage site predicted from the human gene. The exact site of the mouse gene is unknown, therefore it was assumed to be similar to the human site. Insertion at this site would label all size isoforms of HB-EGF produced after N-terminal cleavage. II. After Aspartic Acid 106: This site lies between the heparin-binding and EGF-like domain. However, a very small portion of the heparin-binding domain overlaps the EGF-like domain, so insertion in this site could alter heparin binding. III. After Aspartic Acid 63: This site lies just before the last N-terminal cleavage site. This will label only the largest of the five size isoforms of HB-EGF, as it lies after the other four protease cleavage sites. IV. After Threonine 147: This insertion site is two amino acids away from the Cterminal cleavage site for HB-EGF at 149. This region is between the juxtamembrane stalk and the EGF-like domain, which could affect cleavage of the protein. Biotinylation and visualization of acceptor peptide tagged proteins is accomplished through two different methods depending on the objective of the experiment. The first method utilizes commercially available biotin ligase. Cells expressing acceptor peptide tagged proteins are incubated with biotin ligase, ATP, and biotin in a buffer solution, which biotinylates any acceptor peptide that is expressed extracellularly (Figure 2.5.1 B). After washing, the cells are incubated with a streptavidin-conjugated fluorophore, and washed again, then visualized with fluorescence microscopy. A second, less time consuming and less expensive method of visualization of the acceptor peptide tag is cotransfection with the DNA for BirA-ER (Figure 2.5.1 C). The BirA-ER plasmid encodes for biotin ligase fused to an endoplasmic reticulum (ER) localization sequence. Therefore when a cell expresses both the acceptor peptide tagged protein, and the BirAER protein, the acceptor peptide tag is biotinylated in the endoplasmic reticulum before presentation on the cell surface utilizing the cells own ATP and biotin supplemented into the culture media. The cell surface biotinylated acceptor peptide is then visualized after incubation with a streptavidin-conjugated fluorophore. However, the downside of this method is a higher degree of cell-cell variability in the amount of acceptor peptide biotinylated, as it alters with the amount of BirA-ER expressed, which varies depending on the degree of plasmid uptake and the co-transfection ratio. The use of streptavidin-conjugated fluorophores to visualize acceptor peptide biotinylation leads to good labeling with little background. However, wild-type streptavidin exists as a tetramer with the ability to bind four biotin molecules. Therefore, labeling cell surface biotinylated acceptor peptide tagged proteins with wild-type streptavidin likely leads to crosslinking on the cell surface (Figure 2.5.2 B), which could affect protein cleavage or endocytosis. To circumvent this problem, the Ting lab has engineered a monovalent streptavidin protein that only binds one biotin molecule (Figure 2.5.2 A). This protein consists of one active, wild-type subunit of streptavidin, with three subunits that are dead in their biotin binding ability. After purification in the Ting lab, the monovalent streptavidin is labeled with Alexa-Fluor 568 to allow for fluorescent detection of the marker. 2.2 Materials and Methods Reagents from Alice Ting's laboratory (MIT) The BirA-ER plasmid was produced in Alice Ting's laboratory at MIT. The plasmid encodes biotin ligase (BirA) fused to an endoplasmic reticulum localization sequence. Monovalent streptavidin fused to Alexa Fluor 568 (mSA-AF568) was produced by Peng Zou in Alice Ting's laboratory according to the published protocol (Howarth and Ting, 2008). Biotin ligase enzyme was purified in Alice Ting's laboratory at MIT according to the published protocol (Howarth and Ting, 2008). Cell culture COS-7 cells were cultured in DMEM (Gibco 11965) supplemented with 10% FBS and 1%penicillin-streptomycin. Cell surface acceptor peptide labeling for imaging COS-7 cells were plated onto 0.1% gelatin coated 35mm Mattek glass bottom dishes at 225,000 cells per dish. After 24 hours, the cells were cotransfected with an acceptor peptide fusion protein and BirA-ER at 1:1 molar ratios using Fugene6 (Roche) or Mirus LTI transfection reagent (Mirus) according to the manufacturer's instructions. Media was supplemented with 10 ptM biotin and incubated for 24 hours. Cells were plated on ice and washed twice with cold PBS+ (PBS supplemented with calcium and magnesium), then incubated with 10 tg/mL of monovalent streptavidin-alexa fluor 568 (mSA-AF568) and 1% pre-dialyzed bovine serum albumin (BSA) for 10 minutes on ice. Cells were washed twice with PBS+ and imaged in PBS+. Cell surface acceptor peptide labeling for western blotting COS-7 cells on tissue culture plastic were washed twice with cold PBS+ (PBS supplemented with calcium and magnesium)and placed on ice. The cell surface acceptor peptide was biotinylated with a solution of 0.3 ptM biotin ligase, 1 mM ATP, and 10 pM biotin in PBS+ for 20 minutes on ice. Cells were washed twice with cold PBS+ and immediately lysed with RIPA lysis buffer (lx PBS, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, 1 mM sodium orthovanadate, 0.57 mM PMSF, 1x protease inhibitor cocktail (Sigma P2714)) on ice for 10 minutes, then scraped to a tube. After gel electrophoresis and membrane transfer of the lysate, the membrane was blocked for 1 hour in a solution of TBS/T supplemented with 3-5% BSA, then the biotinylated acceptor peptide was probed with streptavidin-HRP (Molecular Probes S911) at 1:3000 in a solution of 3-5% BSA for at least 1 hour at room temperature. Imaging Phase contrast and fluorescent images were obtained with a digital CCD camera (CoolSNAP HQ, Roper Scientific) and an inverted microscope (Olympus IX-70). Phase contrast images were acquired at 30x with a 20x phase-contrast objective (Olympus LCPlanF NA 0.40) and an additional 1.5x magnification on the microscope. Fluorescent images were acquired using a 40x water immersion objective (Olympus UApo/340 NA 1.15). Phase contrast and fluorescent images were aligned manually using Hoescht stained nuclei (images not shown). Western blotting Western blotting was performed with the following antibodies: anti-phospho-p42/44 MAPK (Cell Signaling Technology 9101S) and goat polyclonal human HB-EGF antibody (Calbiochem PC319L). Constructing the mouse HB-EGF acceptor peptide plasmids The mouse HB-EGF gene was previously inserted into the pShuttle-IRES-hrGFP-1 plasmid (Stratagene, Catalog #240082) by Scott Perkins in Richard Lee's laboratory. The 15 amino acid acceptor peptide sequence, GLNDIFEAQKIEWHE, represented by the DNA base pair sequence, GGCCTGAACG ACATCTTCGA AGCCCAGAAA ATCGAATGGC ACGAA, was inserted into the HB-EGF mouse gene in four different spots. The four insertion sites are after amino acids N91, D106, D63, and T147 of murine HB-EGF. The insertion was done with the Qiagen QuikChange Site-Directed Mutagenesis Kit (Stratagene, Catalog # 200518), which is designed to change one amino acid by making a primer complimentary upstream and downstream of the mutation site. However, it has been reported that the kit can also be used to integrate large fragments at any site within a plasmid (Geiser et al., 2001). This was accomplished by using primers with a region of approximately 20 base pairs complimentary to the DNA sequence upstream of the insertion site, then the 45 base pair acceptor peptide sequence, then approximately 20 more base pairs complimentary to the plasmid downstream of the insertion site. The second primer in the mix is the reverse compliment of the above. The primers used for the N91 insertion site were 5'-GGC CAC CCC AAG CAA AGA AAG GAA TGG CCT GAA CGA CAT CTT CGA AGC CCA GAA AAT CGA ATG GCA CGA AGG GAA AAA GAA GAA GAA AGG AAA GGG GTT-3' and its reverse complement. The primer used for the D105 insertion site were 5'-AGG AAA GGG GTT AGG GAA GAA GAG AGA CGG CCT GAA CGA CAT CTT CGA AGC CCA GAA AAT CGA ATG GCA CGA ACC ATG CCT CAG GAA ATA CAA GGA CTA CTG-3' and its reverse complement. The primers used for the D63 insertion site were 5'-GTG CTC AGG GGG TCC AGG ACG GCC TGA ACG ACA TCT TCG AAG CCC AGA AAA TCG AAT GGC ACG AAT TGG AGG GGA CAG ATC TGA ACC TTT TCA-3' and its reverse complement. The primers used for the T147 insertion site were 5'-GAC ACA GGT GTC ATG GGC TGA CTG GCC TGA ACG ACA TCT TCG AAG CCC AGA AAA TCG AAT GGC ACG AAC TAC CAG TGG AGA ATC CCC TAT ACA CAT ATG A-3' and its reverse complement. All primers were synthesized and PAGE purified by Integrated DNA Technologies. The PCR reaction consisted of a 50 IL volume consisting of 100 ng of the pShuttle-IRES-hrGFP- 1 + HB-EGF (9.4 kb) with 125 ng each of the insertion primers plus 1 piL of PfuUltra Hotstart DNA Polymerase (Stratagene, Catalog #6003 90), 5 pL of lOx PfuUltra HF Reaction Buffer and 200 piM of each dNTP from a deoxynucleotide mix (Sigma, Catalog #D-7295). The PCR reaction consisted of a denaturation step at 95'C for 5 minutes, followed by 18 cycles of 50 seconds at 95"C, 50 seconds at 55'C, and 2 minute/kb template plasmid DNA at 68'C. The cycle ended with 7 minutes at 68"C. All thermal cycling was performed in capped PCR tubes in a MJ Research, PTC-200 Peltier Thermal Cycler using the heated lid. The restriction enzyme Dpn I (New England Biolabs, Catalog #R0176S) was used (1 ptL in 50 ptL PCR sample) to digest methylated template DNA at 374C for >3 hours. The PCR reaction was transformed into XL10-Gold Ultracompetent Cells using the protocol present in the Quikchange II XL Site-Directed Mutagenesis Kit (Stratagene, Catalog #200521), plated onto kanamycin agar dishes and incubated at 370 C overnight. The following day colonies were picked and growth in 3 mL of kanamycin supplemented LB medium with vigorous shaking at 37"C overnight. Media was collected and the plasmid DNA was purified with the Qiagen QlAprep Spin Miniprep Kit (Qiagen, Catalog #27104). In order to check for the presence of the acceptor peptide sequence, the purified DNA was subjected to a PCR reaction with the end primers HB-EGF forward (5'-ATA TAT ACT AGT ATA TGA AGC TGC TGC CGT C-3') and HB-EGF reverse (5'-ATA TAT CTC GAG TCA GTG GGA GCT AGC C-3)' with RedTaq DNA Polymerase (Sigma, Catalog #D-4309). The PCR thermal cycle consisted of 5 minutes at 94'C, followed by 30 cycles of 94"C for 30 seconds, 55*C for 30 seconds and 72'C for 1 minute, with a follow up step of 72*C for 5 minutes. The product was loaded onto a 1.2% agarose gel and the plasmids that contained HB-EGF with the acceptor peptide could be identified by the 45 base pair increase in the DNA fragment size. The positive fragments were subcloned into the pcDNA3.1N5-His TOPO (Invitrogen, Catalog #K4800-01) plasmid vector using the TOPO ligation feature according to the manufacturer's instructions. The plasmid was then transformed into One Shot TOP10 Chemically Competent E. Coli cells (Invitrogen, Catalog #C4040-10) and spread onto ampicillin agar plates and incubated at 370C overnight. The following day colonies were picked and cultured in 3 mL of LB-medium supplemented with ampicillin and subjected to vigorous shaking at 37*C overnight. The following day, the plasmid DNA was purified with the Qiagen QlAprep Spin Miniprep Kit. In order to assay for correct directionality of the insert, a third PCR reaction was performed with the T7 forward sequencing primer provided by with the pcDNA3.1 N5-His TOPO plasmid vector and the HB-EGF reverse primer. Only correct directionality of HB-EGF will result in a PCR product with this method. The PCR reaction was run on a 1.2% agarose gel to check for bands. Positive plasmids were sequenced in the forward and reverse direction with the T7 forward primer and the BGH reverse primer provided with the pcDNA3.1 /V5-His TOPO plasmid vector by the Brigham and Women's Hospital DNA Core Sequencing Facility (Boston, MA). Plasmids with the correct sequence were mass produced by culturing transformed OneShot TOP 10 E. Coli cells in 100 mL ampicillin supplemented LB medium overnight with vigorous shaking at 37C. The following day, the plasmid DNA was isolated and purified with the Qiagen Plasmid Maxi Kit (Qiagen, Catalog #12162). The concentration of the purified DNA was measured with a Smart Spec 3000 Spectrophotometer using absorbance measurements at 250, 280, and 320 nm. Constructing the mouse HB-EGF-GFP acceptor peptide plasmid The AP-HBEGF plasmid at the N91 insertion site was fused to GFP at the C-terminus by subcloning the AP-HBEGF-AP DNA sequence in pEGFP-N1 (Clontech) using the XhoI and EcoRI restriction sites. AP-HBEGF (N91) was amplified from the plasmid in pcDNA3.1 with primers containing the XhoI and EcoRI restriction sites flanking at the ends. The XhoI sense primer contains a two base pair overhang, the 6 base pair XhoI restriction site, a Kozak sequence, and 23 base pairs complementary to HB-EGF including the start codon (5'- TAC TCG AGA CCA TGA AGC TGC TGC CGT CGG TG -3'). The EcoRI reverse primer contains a two base pair overhang, the 6 base pair EcoRI restriction site, one base pair to put GFP in frame with HB-EGF, and 27 base pairs complementary to the end of HB-EGF minus the stop codon (5' - ATG AAT TCA GTG GGA GCT AGC CAC GCC CAA CTT CAC - 3'). The sense and antisense primers were used at a concentration of 25 pM to amplify the AP-HBEGF(N91) template (10 ng of template was used in a 25 pL volume). The PCR reaction was run on an agarose gel and the ~650 base pair AP-HBEGF(N91) product was cut out and purified with a QAIExII Purification Kit (Qiagen). The AP-HBEGF(N91) purified fragment and the pEGFP-N1 vector were double digested with the restriction enzymes XhoI and EcoRI overnight at 370 C. The restriction enzyme digest reaction was run on an agarose gel and the cut pEGFP-N 1 vector and AP-HBEGF(N9 1) fragment was gel purified. The cut ends the pEGFP-N1 vector were dephosphorylated with shrimp alkaline phosphatase for 2 hours are 37C to prevent self-ligation, then the DNA was purified via ethanol precipitation. The cut pEGFP-N1 vector and AP-HBEGF(N91) were fused together using the T4 ligation enzymes for 10 minutes at room temperature, then the reaction was transformed into GC10 Chemically Competent E. Coli (Genechoice) and plated onto kanamycin agar dishes for incubation overnight at 370 C. The following day colonies were picked and tested for correct insertion of the AP-HBEGF(N91) fragment into pEGFP-N1 by PCR. Positive colonies were grown overnight in LB Medium with kanamycin and DNA was purified the following day with the Qiagen Maxiprep Kit. After further analysis, an error was found in the primer design for this subcloning. The EcoRI antisense primer encodes a stop codon consisting of the overhang DNA plus the EcoRI restriction sequence. Therefore, when expressed in cells, AP-HBEGF(N91) was expressed and correctly biotinylated with biotin ligase, but GFP was not fused to the Cterminal as determined by fluorescent imaging and western blotting. To correct this problem, site directed mutagenesis was performed to change a T to an A immediately after the HB-EGF protein sequence. Two complementary primers were designed that have 27 base pairs complementary to the end of HB-EGF and 29 base pairs complementary to the EcoRI restriction site and the following multiple cloning region of pEGFP-N1 (5'- GTG AAG TTG GGC GTG GCT AGC TCC CAC CGA ATT CTG CAG TCG ACG GTA CCG CGG GCC -3' and in reverse 5'- GGC CCG CGG TAC CGT CGA CTG CAG AAT TCG GTG GGA GCT AGC CAC GCC CAA CTT CAC -3'). The site-directed mutagenesis reaction was completed in the presence of 1 tg of the APHBEGF(N91)-GFP plasmid with a stop codon, 210 ng of each primer, 1 unit of Pfu HotStart Ultra DNA Polymerase (Stratagene 600390) and 1 ptL of a 10 mM dNTP mix in a reaction volume of 50 iL. The reaction was denatured at 95'C for 3 minutes, following by 18 cycles of denaturing at 95*C for 50 seconds, annealing at 60'C for 50 seconds, and elongation at 68 0 C for 5 minutes and 21 seconds (based on 1 minute/lkb of plasmid length). The last cycle contained a long elongation step of 7 minutes. The completed 0 PCR reaction was incubated with the restriction enzyme DpnI at 37 C overnight to digest the original methylated DNA, leaving only the newly formed plasmid generated via PCR intact, which was used to transform GC10 Competent K Coli cells and plated on kanamycin agar dishes. Colonies were picked the following day and grown in 3 mL of LB medium with kanamycin overnight at 37'C with shaking at 225 rpm. Successful mutagenesis was confirmed with sequencing on DNA purified with the Qiagen Miniprep Kit. The leftovers from the 3 mL starter culture were used to start a 100 mL culture in LB medium with kanamycin overnight at 37'C with shaking at 225 rpm, and the APHBEGF(N91)-GFP plasmid was purified from this bacteria with the Qiagen Maxiprep Kit and the concentration was measured with absorbance. Purification of HB-EGF Media was collected from 10 cm dishes of COS-7 cells after 48 hours of transfection with HB-EGF plasmids. The media was concentrated via filtration and biotinylated with biotin ligase in solution. The purified media was subjected to western blotting with streptavidin-HRP as described above. Cell lysates from AP-CFP-TM transfected COS-7 cells were used as a positive control for streptavidin-HRP binding. Bands above -32 kDa represent endogenous proteins in the media which bind streptavidin. AP-HBEGF is represented by a faint band around 28 kDa, with T147 showing the highest level of HBEGF expression in the media. Note that much of the sample may be lost during concentration with the centricon filter, as HB-EGF sticks to the filter membrane. Therefore, western blotting may not accurately represent the amount of HB-EGF present in the media. However, it is assumed that the relative amounts of HB-EGF between samples are correct. Bioactivity assay COS-7 cells (3.7x106 cells) were cultured in 150 cm2 cell culture flasks in DMEM with 10% fetal bovine serum, and 1%penicillin-streptomycin. The following day, the media was replaced with DMEM without penicillin-streptomycin and 10 IM biotin, and cells were co-transfected with AP-HBEGF(N91) and BirA-ER, and BirA-ER alone for the control. After twenty-four hours of transfection, the media was supplemented with sodium chlorate (50 mM) to reduce HSPG sulfation and prevent HB-EGF from binding to HSPGs rather than being released into the conditioned medium after cleavage. Twenty four hours later, the media was removed and replaced with 15 mL of PBS with calcium and magnesium, 200 ig/mL phorbol 12-myristate 13-acetate (PMA) (Sigma P8139) and 0.01% hydrogen peroxide (Sigma 216763), which have been reported to stimulate cleavage of HB-EGF. After twenty-four hours incubation with cleavage activators, conditioned media was collected and combined with DMEM removed the previous day, then floating cells were removed via centrifugation. A positive control for heparin bead binding was made, consisting of a solution of 30 mL of PBS+ with 50 mg/mL of recombinant human HB-EGF (Sigma E4643). Heparin acrylic beads (H5263) were washed twice with PBS, and 50 iL of the bead slurry was incubated with the conditioned medium and positive control on a rotator at 4'C for >8hours. Beads were washed with PBS+ and HB-EGF was eluted with a 2 M NaCl solution at 37"C for 1 hour. The control eluant, biotinylated AP-HBEGF and positive control eluant were run on a gel and probed with streptavidin-HRP to detect the biotinylated form of the protein to confirm the presence of AP-HBEGF. To test the activity of the AP-HBEGF heparin bead eluant versus the control eluant, serum starved confluent monolayers of naYve COS-7 cells were treated with 0, 0.01, 0.1, 1 and 10 iL of the heparin bead eluant, with and without pretreatment for 30 minutes with 10 pM of the EGFR tyrosine kinase inhibitor AG1478. Cells were lysed after 15 minutes of stimulation and lysates were probed via western blotting for ERK phosphorylation. 2.3 Results The fifteen amino acid acceptor peptide sequence was successfully inserted into the mouse HB-EGF gene at four different locations: after asparagine 91 (N91), aspartic acid 106 (D106), aspartic acid 63 (D63), and threonine 147 (T147). All four AP-HBEGF constructs, including HB-EGF without the AP tag were successfully expressed in COS-7 cells and recognized by a polyclonal human HB-EGF antibody (Figure 2.5.3 A). The fifteen amino acid insertion caused a shift in mobility, with the acceptor peptide HB-EGF samples appearing slightly larger than the wild-type HBEGF. Additionally, HB-EGF was not endogenously expressed at levels high enough to be detected via western blot in COS-7 cells, as shown by the GFP transfected and wild-type, non-transfected controls (Figure 2.5.3 A). The AP-HBEGF construct existed as multiple bands, denoting the multiple N-terminal cleavage sites which results in five different HB-EGF size isoforms. The protein ran at an approximate molecular weight of 22 kDa; however, the positive control, which is recombinant human HB-EGF ran at approximately 9 kDa. This observation has been reported in the literature, and is attributed to heavy 0-glycosylation of the eukaryotic synthesized protein causing a large shift in electrophoretic mobility. Additionally, the AP-HBEGF is expressed in the pro-form (uncleaved), while the recombinant protein is only the mature, soluble extracellular domain. The AP-HBEGF constructs expressed in COS-7 cells were biotinylated on the cell surface with exogenous biotin ligase, which does not penetrate the plasma membrane. After biotinylation the cells were lysed and subjected to SDS PAGE gel electrophoresis. The membrane was then probed for biotinylated proteins with streptavidin-HRP (Figure 2.5.3 B). The upper bands represent endogenously biotinylated proteins that exist inside the cell, as the sample transfected with HB-EGF without the acceptor peptide has identical positive bands. Two of the four constructs show successful biotinylation of APHBEGF (N91 and D63) indicating that the construct was correctly incorporated into the plasma membrane and expressed on the cell surface of COS-7 cells. The D63 AP- HBEGF construct is representative of only one isoform of the five HB-EGF different size isoforms, while the N91 constructs shows all five HB-EGF bands. The positive control in this experiment is a construct engineered in Alice Ting's lab in the MIT chemistry department. This construct consists of an extracellular cyan fluorescent protein molecule and an acceptor peptide tag fused to the transmembrane domain of the PDGF receptor (AP-CFP-TM) (Chen et al., 2005). The D106 construct had no detectable cell surface biotinylation. It is speculated that the acceptor peptide inserted after D106 is sterically unavailable to biotin ligase as it lies immediately after the highly structured EGF-like domain and before the heparin-binding domain. The T147 construct has the acceptor peptide inserted after the juxtamembrane stalk of HB-EGF, therefore increasing the length of the pro-HBEGF stalk. One study reported that increasing the stalk length of HB-EGF increased the cleavage efficiency by TACE/ADAM17 (Hinkle et al., 2004). Additionally, a blot of media from COS-7 cells transfected with the various HB-EGF plasmids had a high level of biotinylated T147 in the media compared to N9 1, D106 and D63 (Figure 2.5.3 C). Therefore, we speculate that the lengthened stalk of HB-EGF in the T147 construct leads to increased cleavage so that it is present in undetectable amounts on the cell surface and accumulates in the media. Interestingly, the different size isoforms of HB-EGF identified by a HB-EGF polyclonal antibody versus cell surface biotinylation of the acceptor peptide tag differ. Biotinylated cell surface HB-EGF N91 shows the presence of a strong band at approximately 14 kDa (Figure 2.5.3 B), which is not observed with the HB-EGF antibody (Figure 2.5.3 A). This suggests that the 14 kDa band is not recognized by the HB-EGF antibody, perhaps due removal of the recognition epitope. As D106 did not get biotinylated, T147 caused altered cleavage rates, and D63 did not label all size isoforms of HB-EGF, N91 was chosen as the best construct with properties closest to wild-type HB-EGF. As the C-terminus of HB-EGF can be detached from the extracellular domain via protease cleavage, a method to independently track the C-terminus from the extracellular domain would allow for independent tracking and assessment of cleavage. The N91 APHBEGF gene was cloned in the plasmid vector pEGFP-N1 to attach GFP to the Cterminal tail of proHB-EGF, which is referred to here as AP-HBEGF-GFP (Figure 2.5.4 A). To assure that the acceptor peptide, and biotinylation of the acceptor peptide does not alter the protein's activity, the AP-HBEGF extracellular domain was tested for EGFR bioactivity. Biotinylated AP-HBEGF was precipitated from the conditioned medium of COS-7 cells with heparin acrylic beads, and a control heparin bead eluant was also prepared from conditioned medium of COS-7 cells that were not transfected with APHBEGF. Both heparin bead eluants were added to naYve COS-7 cells in increasing dosages, lysed and assayed for phospho-ERK. The biotinylated AP-HBEGF heparin bead eluant activated phospho-ERK at levels much higher than the control bead eluant (Figure 2.5.4 B). Additionally, this activation was inhibited by preincubation of the cells with the EGFR tyrosine kinase inhibitor, AG1478, suggesting that the ERK activation is indeed mediated by the EGFR. Therefore, biotinylated AP-HBEGF has the ability to activate phospho-ERK through the EGFR in a paracrine fashion. As the biotinylated APHBEGF protein was purified by heparin beads, this additionally demonstrates that insertion of the acceptor peptide after N91 does not interfere with HB-EGF's ability to bind heparin. Transfection of this construct into COS-7 cells and imaging of the extracellular acceptor peptide tag shows that the C-terminal tail (GFP, green) and the extracellular domain (AF568, red) signals overlapped, suggesting HB-EGF was primarily in the proform (Figure 2.5.5). Additionally, epifluorescence imaging shows that HB-EGF was localized primarily to sites of cell-cell contact in a confluent monolayer of COS-7 cells (Figure 2.5.5 A). This fact is highlighted when the cells were sparsely plated, as the majority of HB-EGF concentrated only at the cell-cell junction rather than at the free edges (Figure 2.5.5 B). 2.4 Discussion Biotin ligase and acceptor peptide labeling proved to be a viable technique for HB-EGF, with little background noise. The technique is powerful for the case of HBEGF to be able to distinguish the cell surface pool of HB-EGF from that in intracellular compartments. Additionally, with the dual labeling technique presented here for independent tracking of the C-terminal tail with EGFP and the extracellular domain with the acceptor peptide tag, proteolytic cleavage of HB-EGF could be visualized due to differential localization of the fluorescent signals. However, HB-EGF shows no difference in the extracellular and intracellular tags, indicating that the protein is primarily uncleaved, which is validated via western blotting. Utilization of the co- transfection method with BirA-ER makes the protocol cost sensitive, as no purified recombinant biotin ligase is necessary. This technique allows for live cell tracking of the HB-EGF ligand, as cell fixation is not required with the acceptor peptide labeling method. The utilization of an engineered monovalent streptavidin prevents streptavidin from cross-linking biotinylated proteins on the cell surface. However, the large size of monomeric streptavidin tetramers (53 kDa) may inhibit native processing of the protein, such as endocytosis or cleavage. Additionally, biotinylation of HB-EGF at the acceptor peptide tag does not affect the activity of the protein, however, a streptavidin complexed to the biotinylated acceptor peptide tag could introduce steric effects preventing HB-EGF from activating the receptor, even though the acceptor peptide tag is sufficiently far away from the EGF-like domain. 2.5 Figures AF568 Biotin G LN DIF EAQ K I EW HE Acceptor Peptide (AP) c b B B 4BirAadded AP B BirAER Figure 2.5.1: Two methods of BirA (biotin ligase) labeling of the acceptor peptide protein. (A) The enzyme BirA (biotin ligase) covalently attaches a biotin to one lysine residue within the fifteen amino acid acceptor peptide sequence. The acceptor peptide is then visualized by the addition of a streptavidin-conjugated fluorophore, such as Alexa-Fluor (AF568) (B) The acceptor peptide tagged protein is produced in the endoplasmic reticulum (ER), and then presented on the cell surface. Exogenous BirA (biotin ligase) enzyme is added to the cell media along with ATP and biotin, leading to biotinylation of only the extracellular fraction of the acceptor peptide tagged protein. The biotinylated acceptor peptide tag can then be visualized with a streptavidin-fluorophore conjugate. (C) The acceptor peptide tagged protein is coproduced along with the construct BirA-ER, which encodes for biotin ligase with an endoplasmic reticulum (ER) localization sequence. Acceptor peptide tagged proteins are biotinylated with BirA-ER in the endoplasmic reticulum (ER) using the cells own intracellular ATP and biotin supplemented in the culture media. The acceptor peptide tagged protein is presented on the cell surface already biotinylated, and the biotinylated acceptor peptide can be visualized with a streptavidin-fluorophore conjugate. (Figure2.5.1B&C adaptedfrom Howarth et al, 2008 (Howarthand Ting, 2008)) ......................................................................... . ................ . . ........ -- - ......... ........... . ....... BI jB Engineer_, Wild-type streptavidin (A4) Kd4.4 x 10-14 M Monovalent streptavidin (A1D3) Kd4.8 Divalent antibody Wild-type streptavidin x10-14 M Monovalent streptavidin Out Cell surface protein Figure 2.5.2. Design of a monovalent streptavidin. (A) Wild-type streptavidin is a tetramer with four biotin [B] binding sites [A4]. The Ting lab has engineered streptavidin that is dead in the biotin binding pocket [gray, D]. Monovalent streptavidin is engineered by combining three subunits of mutant streptavidin unable to bind biotin with one of the wild-type subunits fused to a His-Tag [AlD3], which is able to retain low Kd values. (B) Divalent antibodies and wild-type streptavidin cross-link proteins on the cell surface with their ability to bind multiple proteins. Monovalent streptavidin is designed to reduce protein cross-linking on the cell surface as it can only bind one biotinylated protein. (Figureadaptedfrom Howarth et al, 2008 (Howarth and Ting, 2008)) . . . .. .... .. . ...... . ........ 0 4/ 0k 49 - 38 - 28 - 14 - 0 0 (0 39- 4. C 2814- 28- Figure 2.5.3 Validation of AP-HBEGF constructs. (A) Expression of all four AP-HBEGF plasmids named by their insertion site (N91, D106, D63, T147), and wild-type HB-EGF in COS-7 cells was assessed by western blotting with a human HBEGF polyclonal antibody. GFP serves as a transfection control, wild-type (WT) represents non-treated COS-7 cells, and the positive control represents recombinant human HB-EGF. (B) Cell surface biotinylation of AP-HBEGF was successful only in the N91 and D63 constructs. The extracellular acceptor peptide was biotinylated in COS-7 cells expressing the AP-HBEGF plasmids, then lysed and detected with streptavidin-HRP after gel electrophoresis. N91 shows biotinylation of several size isoforms of HB-EGF between 14 and 34 kDa, and D63 shows biotinylation of the largest size isoform around 34 kDa. No detectable biotinylation is observed for D106 or T147. The positive control represents AP-CFP-TM showing biotinylation at 42 kDa. The two large molecular weight bands and the one at 36 kDa represent endogenously biotinylated proteins, as they are also present in the HB-EGF (no acceptor peptide) control. (C) The T147 protein is enriched in the cell media in the large molecular weight form at around 28 kDa. However, little is detected in the media for N9 1, D106 and D63. .............. ............. AP a N:SH PRO ....... -- .. .............. GFP D EGC soluble HB-EGF pro HB-EGF Heparin Bead Eluant Control HB-EGF-Biotin 0 10-210-1 1 10 0 1 21 11 10 b Phospho-ERK Phospho-ERK +AG1478 Figure 2.5.4 Final gene map of AP-HBEGF-GFP and bioactivity. (A) Schematic representation of the final mouse HB-EGF construct (AP-HBEGF-GFP) with the acceptor peptide inserted after N91. The arrows show sites of modification, where EGFP was inserted at the C-terminus, and the acceptor peptide was inserted after N91. Triangles represent sites of cleavage at the N and C-terminus of the human protein. The gene is broken up into the secretory region (SEC), the pro-region (PRO), the heparinbinding domain (HBD), the EGF-like domain (EGF), and a transmembrane domain. (B) Bioactivity of the soluble, mature form of biotinylated AP-HBEGF-GFP was assessed by a concentration dependent phosphorylation of ERK after 15 minutes incubation with the roughly purified protein eluted from heparin beads compared to a control eluant. The numbers above the blot represent the volume of heparin bead eluant in microliters that cells were stimulated with, as the concentration of HB-EGF here is unknown. The biotinylated HB-EGF activates ERK at lower concentrations than the control eluant. Additionally, most of the ERK activation at lower concentrations is through the EGFR, as preincubation of naive COS-7 cells for 30 minutes with 10 pM AG1478, an EGFR kinase inhibitor, blocked activation of ERK with the heparin bead eluant. b Figure 2.5.5 AP-HBEGF-GFP is localized to cell-cell contact sites. (A) After 24 hours of co-transfection of AP-HBEGF-GFP with BirA-ER in COS-7 cells, the biotinylated, extracellular acceptor peptide in AP-HBEGF-GFP was labeled with monovalent streptavidin-alexa fluor 568 (red, left) and imaged alongside the cytoplasmic tail conjugated to EGFP (green, middle), and phase contrast (right) in a confluent monolayer and (B) in sparsely plated cells. Arrows show localization of HB-EGF to sites of cell-cell contact. Each row represents the same field. Scale bars are 40 pm. 40 Chapter Three: Polarization of HB-EGF at the wound edge 3.1 Introduction HB-EGF is a chemotactic factor for many cell types, as activation of the EGFR often leads to an increase in migration. However, HB-EGF specifically has been identified as an autocrine factor released by keratinocytes (Shirakata et al., 2005; Tokumaru et al., 2000) and comeal epithelial cells (Block et al., 2004; Xu et al., 2004) upon wounding that is crucial to activate cell migration to drive wound closure. The direct stimuli that lead to HB-EGF shedding varies depending on the system, however ATP released after wounding (Yin and Yu, 2009), or simply the increase in available area for cell migration can lead to cleavage of the pro-form of HB-EGF to produce the soluble form for autocrine signaling (Block et al., 2004). It has been hypothesized that the EGF-EGFR system has potential for spatial localization of the autocrine signaling loop (Maheshwari et al., 2001). The Lauffenburger lab has computationally predicted that spatially localized autocrine signaling is possible (Maly et al., 2004) and has produced experimental data of human mammary epithelial cell migration which suggests a spatially orientated autocrine loop may exist and drive persistent cell migration (Maheshwari et al., 2001). The computational kinetic model of EGFR autocrine signaling includes ligand shedding, activation of the EGFR, recruitment of Grb2 and Sos, and phosphorylation of Raf, MEK, and ERK within one cell. In addition, the model includes positive feed back, where ERK activates additional ligand shedding as well as negative feedback where ERK phosphorylates Sos, rendering it unable to participate in the EGFR-Grb2-Sos complex. This model predicted three stables states of autocrine signaling: no signaling, symmetric EGFR signaling and polarized EGFR signaling. In the polarized state, EGF release as well as ERK activation is concentrated to one pole of the cell. The stable state which the system adopts was dependent on the density of cell surface receptors; densities of approximately 400 to 800 receptors/ptm 2 resulted in polarized signaling. Experimental evidence for a spatially polarized autocrine loop was discovered in pro-EGF expressing human mammary epithelial cells, which had increased directional persistence (Maheshwari et al., 2001). In this study, cell lines were created overexpressing the pro-membrane bound form of EGF (EGF-Ct) and a form of EGF that contains the soluble portion only without the transmembrane stalk (sEGF). EGF expression in both forms (sEGF & EGF-Ct) was shown to increase migration speed in all samples, however only pro-EGF expression led to an increase in persistence time and path length. Interestingly, the addition of exogenous HB-EGF to these cells abrogated the increased directional persistence. Therefore, it was hypothesized that the increased directional persistence was due to an asymmetrical autocrine signaling loop, leading the cell to migrate in the direction of EGFR activation. An additional quantitative experimental study of ligand capture in the EGF-EGFR system in the Lauffenburger lab outlines conditions that must be met for autocrine, rather than paracrine signaling (DeWitt et al., 2001). In this study, the rate of EGF ligand production was altered using a tet-off expression system, metalloprotease inhibitors were utilized to block the amount of ligand released, and an EGFR blocking antibody (mAb225) was used to vary the number of receptors accessible on the cell surface. This study showed that the amount of ligand captured by the cell that released it was dependent on the ratio of the ligand production rate (VLT) to the receptor production rate At VLT/VR values of 0.3 or less, the system was dominantly autocrine, and captured the majority of ligand released. However as the ratio increased, more ligand (VR)- was released into the bulk medium. EGF binds to the EGFR only; however HB-EGF binds not only to the receptors, but to HSPGs, which are plentiful on the cell surface. Therefore, as the number of potential binding sites on the cell surface for HB-EGF is much higher than that of EGF, one would predict HB-EGF signaling to be predominantly autocrine in nature. As HB-EGF serves as an autocrine chemotactic ligand during wound healing, and the EGFR system may have the ability to produce spatially polarized autocrine signaling, we sought to test the hypothesis that spatially polarized HB-EGF autocrine signaling loops exist and drive persistent cell migration to close wounds. 3.2 Materials and Methods Materials Batimastat (BB-94) was a generous gift from Steve Wiley's lab at Pacific Northwest National Laboratory where it was custom synthesized by Kimia Corporation (Santa Clara, CA). The EGFR blocking antibody (mAb225) was isolated from a hybridoma cell line obtained from the American Type Culture Collection (Gill et al., 1984). We additionally used the following: GM6001 (Calbiochem 364206), 2R-[(4-biphenylsulfonyl)amino]-Nhydroxy-3-phenylpropionamide (BiPS) (Calbiochem 444249), PD98059 (Calbiochem 513000), AG1478 (Calbiochem 658552), PP2 (Calbiochem 529576). Adenoviruses for wild-type dynamin-1, wild-type dynamin-2, dynamin-1 S45N, dynamin-2 1690K, and tTA were a generous gift from the Schmid laboratory (Soulet et al., 2006). Wound healing assay Mattek 35 mm glass bottom dishes were coated with 0.1% gelatin for > 1 hour at 370 C. COS-7 cells were plated in a confluent monolayer at 225,000 cells per dish. Wounds were induced by dragging a P200 pipette tip with constant velocity over the cell monolayer. Cell surface labeling with mSA-AF568 was performed as described in section 2.2 with gentle washing, as not to disrupt cells on the edge of the wound. In the dual-labeling experiment streptavidin-fluorescein was utilized (Vector Laboratories SA5001) Plasmids For all experiments in this chapter and on, the HB-EGF construct has the AP insertion after amino acid N91 in murine HB-EGF, which is referred to as AP-HBEGF-GFP. The construct AP-CFP-TM consists of an extracellular acceptor peptide, cyan fluorescent protein, and the transmembrane domain of the PDGFR (Chen et al., 2005). This construct was made in Alice Ting's laboratory, MIT Chemistry Department to serve as a positive control for acceptor peptide biotinylation and visualization. 3.3 Results 3.3.1 ProHB-EGF is missing from the wound edge In order to track the localization of HB-EGF during cell migration, we chose the wound healing assay. This assay is a convenient method to measure and stimulate cell migration with the advantage of a known direction of cell migration toward wound closure without time lapse imaging, as is required for single migrating cells. A wound is induced by scratching a confluent monolayer of cells with a P200 pipette tip, then the remaining cells proliferate and migrate in to close the free area of the wound, typically over timescales of 12 to 48 hours. COS-7 cells are rather stationary and migrate minimally until stimulated by creation of a wound. In order to track HB-EGF during wound healing, the AP-HBEGF-GFP construct was transfected into confluent monolayers of COS-7 cells. After twenty-four hours a wound was induced in the APHBEGF-GFP expressing cells and the wound was allowed to heal for four hours, then the extracellular acceptor peptide tag was labeled with mSA-AF568. It was observed that AP-HBEGF-GFP was absent from free or leading edge of cells on the wound edge (Figure 3.5.1 A). However, cells within the monolayer maintained a symmetrical distribution of HB-EGF at cell-cell contact sites (data not shown). Additionally, the control construct AP-CFP-TM does not change localization at the wound edge after four hours of wound healing when compared to cells in the monolayer (Figure 3.5.2 B). As AP-CFP-TM is still detectable at the leading edge, it is not likely that thinning of the cell, and therefore less fluorescence at the lamellipod is the cause of the loss of HB-EGF. We hypothesized that the loss of HB-EGF at the leading edge of the wound was due to proteolytic cleavage of the extracellular domain from the leading edge and fast internalization of the cleaved C-terminal domain. Additionally, we hypothesized that this spatially localized proteolytic cleavage led to EGFR activation at the leading edge which may lead to chemotactic cell migration in the direction of would closure. 3.3.2 HB-EGF loss from wound edge is not due to proteolytic release In order to test this hypothesis, we utilized many reagents to block the loss of HBEGF from the leading edge after wounding, internalization of the C-terminal tail, and other intracellular signaling nodes that may cause positive or negative feedback in the cascade. First, reagents aimed at blocking protease cleavage of HB-EGF at the leading edge were utilized, including batimastat (BB-94), GM6001 and BIPS. Batimastat is a broad spectrum matrix metalloproteinase (MMP) inhibitor that inhibits MMP activity by binding the zinc ion in the activate site of the MMP and has been shown to inhibit ovarian and breast cancer tumor growth (Davies et al., 1993; Low et al., 1996). Batimastat has been demonstrated to block cleavage of EGF ligands, including HB-EGF (Prenzel et al., 1999; Sahin et al., 2004). GM6001 is a broad spectrum hydroxamic acid inhibitor of matrix metalloproteases that also interferes with the zinc binding site of MMPs and has been experimentally shown to inhibit proHB-EGF cleavage (Armant et al., 2006). BiPS and TAPI-2 are also broad spectrum matrix metalloproteinase inhibitors, and BiPs has been shown to inhibit HB-EGF cleavage in COS-7 cells specifically (Mifune et al., 2005). Cells were pretreated with the metalloproteinase inhibitors for one hour, then a wound was induced and the wound was allowed to close for 4 hours in the presence of the inhibitors. No change in HB-EGF localization was observed in the presence of metalloproteinase inhibitors batimastat (10 pM), GM6001 (10 pM), BiPs (10 pM), or TAPI-2 (20 pM) (Figure 3.5.2 B-E), suggesting that ligand cleavage is not responsible for the loss of HB-EGF at the leading edge of cells at the edge of the wound. To test if loss of HB-EGF from the wound edge was due to cleavage induced by positive feedback through EGFR signaling or downstream mediators, agents aimed at blocking parts of this pathway were targeted. First, directly blocking the EGFR was tested with AG1478 and mAb225. AG1478 is a tyrosine kinase inhibitor for the EGFR that blocks downstream intracellular signaling triggered by the EGFR. mAb225 is a mouse monoclonal antibody that binds to the EGFR and blocks ligands from binding and activating the receptor, therefore inhibiting downstream signaling (Gill et al., 1984). Pretreatment of monolayers of COS-7 cells with 10 piM AG1478 or 10 ptg/mL of mAb225 for one hour prior to induction of the wound, and continuous incubation during four hours of wound healing did not reduce the loss of HB-EGF at the wound edge (Figure 3.5.2 F-G). To investigate other pathways downstream of EGFR, monolayers of COS-7 cells were pretreated for one hour prior to induction of the wound with 25 piM of the MEK1 inhibitor PD98059 that blocks activation of ERK, 20 pM of the protein kinase C (PKC) inhibitor rottlerin, 10 pM of the PKC inhibitor bisindolylmaleimide I (BIM I), 100 nM of the phosphatidylinositol-3 kinase (P13K) inhibitor wortmannin, 10 pM of the Src inhibitor PP2, and 10 ptg/mL of the P13K inhibitor LY294002. However, none of the inhibitors stopped loss of HB-EGF from the wound edge (Figure 3.5.2 H-M). BIM I treatment did lead to poor labeling of the extracellular acceptor peptide tag and spotting in all cells, even those that were non-transfected. Likely BIM I was cytotoxic and led to leaky plasma membranes, allowing streptavidin to permeate the cell and bind to endogenous biotin in the mitochondria; or BIM I caused large amounts of HB-EGF cleavage and the extracellular domain remained on the cell surface in a spotted pattern. As the loss of HB-EGF from the leading edge is not likely due to ligand cleavage or EGFR activation, it was hypothesized that extension of the lamellipodia may push proHB-EGF to the rear of the cells. In order to inhibit cytoskeletal processes during wound healing, actin polymerization was blocked with 200 nM cytochalasin D, 10 pM of the microtubule stabilizer taxol, and 300 nM of the microtubule polymerizing inhibitor nocodazole. However, none of these agents inhibited the loss of HB-EGF at the edge of the wound (Figure 3.5.2 N-P). Therefore, action of the cytoskeleton during wound healing is unlikely to control the loss of HB-EGF at the wound edge. 3.3.3 Newly synthesized HB-EGF localizes to cell-cell contact sites only As protease inhibitors did not stop the loss of HB-EGF at the wound edge, it is likely that the entire pro-form of HB-EGF is removed from the leading edge, with the extracellular domain still attached to the C-terminal domain. One possible mechanism for loss of the pro-form of the protein could be internalization from the leading edge via endocytosis. In order to block internalization, the temperature was lowered to 4"C, which blocks internalization of cell surface proteins. Preincubation of COS-7 cells at 4"C for one hour prior to induction of the wound, then continuous incubation of the cells for the entire four hours of wound healing did inhibit loss of HB-EGF at the wound edge and extension of lamellipodia into the wound area (Figure 3.5.2 R). However, lowering the cell temperature to 4'C likely inhibits many processes, including incorporation of new protein into the plasma membrane. Therefore, it could be that only newly synthesized protein localizes away from the wound edge, and the protein at the leading edge is turned over at an average rate. Recycling of all proteins was blocked with the chemical inhibitor monensin at 10 [M. Monensin did not lead to complete inhibition of the loss of HB-EGF at the wound edge, however, it was reduced (Figure 3.5.2 Q), suggesting that newly synthesized or recycled proHB-EGF is localized preferentially at sites of cell-cell contact rather than at the leading edge. The chemical inhibitor of endocytosis, phenyl arsine oxide (PAO), was utilized, however was toxic to COS-7 cells at concentrations required for endocytosis inhibition. To block the clathrin-mediated endocytosis pathway more specifically, dominant negative mutants in dynamin were employed. The S45N and 1690K mutants in dynamin1 and dynamin-2, respectively, in adenovirus forms were transfected into COS-7 cells (Soulet et al., 2006). However, inhibition of endocytosis, as assessed by EGF induced EGFR downregulation, was never achieved with these constructs in confluent monolayers of COS-7 cells. These constructs were communicated to work inefficiently in confluent monolayers of cells (unpublished data), however, confluency is required for wound healing experiments. Therefore, endocytosis inhibition was never achieved here and the role of clathrin mediated endocytosis at the wound edge could not be tested. In order to investigate the turnover of cell surface HB-EGF during the wound healing experiment, a pulse-chase experiment was performed. A confluent monolayer of COS-7 cells was co-transfected with AP-HBEGF (without the GFP tag on the cytoplasmic tail) and BirA-ER and cultured for 24 hours with biotin supplemented in the media. Cells were washed and the extracellular biotinylated acceptor peptide tag on HBEGF was labeled with mSA-AF568 (red), which does not allow protein cross-linking. Cells were placed back in full media with serum supplemented with biotin, and wounds were produced. After four hours of wound healing, the cell surface pool of HB-EGF was labeled with streptavidin-fluorescein (green), and the cells were immediately imaged (Figure 3.5.3). The cell surface fraction of AP-HBEGF labeled before production of the wound was primarily localized inside the cell (Figure 3.5.3 A). However, the cell surface pool of AP-HBEGF detected after four hours showed localization to sites of cell-cell contact and was missing from the wound edge, as expected (Figure 3.5.3 B). As the bulk of the mSA-AF568 signal was internal, this suggests that newly synthesized HB-EGF becomes preferentially localized to sites of cell-cell contact rather than at the wound edge over the experimental timecourse of four hours. 3.4 Discussion In summary, the discovery that HB-EGF was missing from the wound edge in migrating monolayers of COS-7 cells led us to hypothesize that this loss was due to preferential proteolytic cleavage from the leading edge. However, this hypothesis is likely not correct for the COS-7 cell line, as inhibition of several points along this pathway did not prevent loss of HB-EGF from the wound edge. Rather it appears that most of the HB-EGF present on the cell surface at the time of induction of the wound is internalized over the experimental time course of four hours. Rather newly synthesized or recycled HB-EGF is localized preferentially at sites of cell-cell contact and not at the edge of the wound. ProHB-EGF could be preferentially deposited at sites of cell-cell contact, or could be incorporated uniformly in the plasma membrane, then diffuse to and stay at cell-cell contact sites. However, this raises the question: what molecular interactions govern proHB-EGF localization at sites of cell-cell contact? The known protein interactions of HB-EGF include the EGFR, ErbB4, HSPGs, and CD9 on the extracellular domain, and BAG-1 for the intracellular domain. As BAG-1 does not have any discovered cytoskeletal binding properties, we hypothesize that an interaction between the extracellular domain of HB-EGF and one of its binding partners on neighboring cells holds HB-EGF at cell-cell contact sites. This ensuing hypothesis is pursued in Chapter 4. -- - - - ----- 3.5 Figures (A) Figure 3.5.1 HB-EGF is absent from the wound edge. After 24h of co- transfection of (A) AP-HBEGF-GFP or (B) AP-CFP-TM with BirA-ER in COS-7 cells, the monolayer was wounded with a P200 pipette tip. After four hours of wound healing, the biotinylated, extracellular acceptor peptide was labeled with monovalent streptavidinalexa fluor 568 (red, left) and imaged alongside (A) EGFP (green, middle) or (B) CFP (cyan, middle), and phase contrast (right). Note that the cells were pre-treated with Hoescht to label nuclei blue to assist with image alignment between the fluorescent images and the phase contrast images, as they were taken with different objectives, then compensated for scale during image processing. Therefore the presence of the light cyan nuclei in (B) is due to Hoescht blue fluorescence overlap into the cyan channel. Only the large brightly labeled cell in the middle is AP-CFP-TM transfected. Each row represents the same field. Scale bars are 40 pm. ....... .. ....................... .......... (A) Control (B) Batimastat (C) GM6001 (D) BiPs (E) TAPI-2 (F) AG1478 (G) mAb225 (H) PD98059 (1 Rottlerm (J) BIM (K) Wortmaninin (L) PP2 (M)LY294002 N) Cytochalasin D (Q) Monensin (R) 4C I O0 Nocodazolo P) Taxol Figure 3.5.2 Effect of inhibitors on polarization of HB-EGF at wound edge. After four hours of wound healing, the extracellular domain of HB-EGF is missing from the wound edge in COS-7 cells transfected with AP-HBEGF in the (A) untreated control. Pretreatment with inhibitors for 1 hour prior to wounding and continuous incubation with inhibitors after induction of the wound did not prevent the loss of HB-EGF at the leading edge of cells at the would edge (B-M). Polarization of HB-EGF at the wound edge was tested after incubation with the protease inhibitors (B) batimastat (10 iM), (C) GM6001 (10 p.M), (D) BiPs (10 piM), and (E) TAPI-2 (20 pM). EGFR signaling was inhibited with (F) AG1478 (10 pM) and (G) mAb225 (10 Ig/mL). Intracellular signaling pathways were inhibited with (H) PD98059 (25 pM), (I) rottlerin (20 pM), (J) BIM 1 (10 pM), (K) wortmannin (1 iM), (L) PP2 (10 pM), (M) LY294002 (10 ig/mL). Cytoskeletal components were inhibited with (N) cytochalasin D (200 nM), (0) nocodazole (300 nM), and (P) taxol (10 pM). Protein recycling was blocked with (Q) monensin (10 ptM) and (R) endocytosis was inhibited by lowering the incubation temperature to 4C. The dotted line represents the wound edge with empty space on the right side. Scale bar represents 20 im. AP Taa Blotinviation (A (mSA-AF568) AP Taa Biotinviation (SA-Fluoresceini (c) (B) Hoescht Figure 3.5.3 Turnover of cell surface HB-EGF at wound edge. Confluent monolayers of COS-7 cells were co-transfected with AP-HBEGF (without the GFP tag on the cytoplasmic tail) and BirA-ER and cultured for 24 hours with biotin supplemented in the media. Cells were washed and the extracellular biotinylated acceptor peptide tag on HB-EGF was labeled with monovalent-streptavidin-AF568 (mSA-AF568, red) in (A), then cells were placed back in full media and a wound was induced with a pipette tip. After four hours of wound healing, the extracellular biotinylated acceptor peptide tag on HB-EGF was labeled again, this time with streptavidin-fluorescein (SA-fluorescein, green) in (B), the nuclei were stained with Hoescht (C), and then cells were immediately imaged. After four hours of wound healing most of the cell surface HB-EGF was internalized into the cell (red), and newly synthesized HB-EGF exists at sites of cell-cell contact (green). The dotted line represents the wound edge. Scale bar represents 40 jim. 52 Chapter Four: The heparin-binding domain mediates localization of proHB-EGF to cell-cell contact sites 4.1 Introduction Heparan sulfate proteoglycans (HSPGs), present on the cell surface and in the extracellular matrix, are capable of binding many growth factors. A traditionally proposed purpose of this interaction is to restrain a soluble ligand to the cell surface and increase the local concentration to activate a receptor (Schlessinger et al., 1995). Most ligands reported to interact with HSPGs are soluble secreted factors, such as fibroblast growth factors (Gospodarowicz et al., 1984; Maciag et al., 1984; Shing et al., 1984), vascular endothelial growth factor (Ferrara and Henzel, 1989), hepatocyte growth factor (Nakamura et al., 1984; Zhou et al., 1999) and platelet-derived growth factor (Schilling et al., 1998). However, HSPGs also interact with a few growth factors that are anchored to the cell surface via a transmembrane domain, particularly those that belong to the epidermal growth factor receptor (EGFR) ligand family, including HB-EGF (Higashiyama et al., 1991), amphiregulin (Cook et al., 1991), betacellulin (Shing et al., 1993), and certain isoforms of neuregulin (Holmes et al., 1992; Loeb and Fischbach, 1995). All ligands in the EGFR family have the ability to activate their receptors in the diffusible form produced after proteolytic release from the cell surface (autocrine/paracrine signaling). However, of the EGFR family ligands, only HB-EGF (Higashiyama et al., 1995), amphiregulin (Inui et al., 1997), transforming growth factor-a (Anklesaria et al., 1990) and betacellulin (Tada et al., 1999) have been reported to activate their receptors in the pro-form, while anchored to the membrane before cleavage (juxtacrine signaling). Autocrine/paracrine signaling with these ligands has been studied much more extensively than juxtacrine signaling; however, the majority of HB-EGF remains on the cell surface in the pro-form at sites of cell-cell contact (Goishi et al., 1995). Therefore, here we focus on the role of HSPG binding to the pro-form of HBEGF capable of juxtacrine signaling. 4.2 Materials and Methods Constructing heparin-binding domain mutants of AP-HBEGF-GFP Four constructs with various mutations to the heparin-binding domain of AP-HBEGFGFP were produced. The region of the heparin-binding domain which is not included within the EGF-like domain (#93-105) was deleted (AP-delHBD-HBEGF-GFP), the first five lysine residues of the heparin-binding domain (#93-97) were mutated to alanine (AP97A-HBEGF-GFP), all lysine and arginine residues in the heparin-binding domain which lies outside of the EGF-like domain (93-105) were mutated to alanine (AP-105AHBEGF-GFP), and finally all lysine and arginine residues in the entire heparin-binding domain (93-113), including the portion that is included in the EGF-like domain, were mutated to alanine (AP-1 13A-HBEGF-GFP). The AP-delHBD-HBEGF-GFP mutant was constructed using a primer complementary to AP-HBEGF-GFP for 20 amino acids before amino acid 93 and after amino acid 105 (5' - AAA TCG AAT GGC ACG AAG GGG ACC CAT GCC TCA GGA AAT A -3') and its reverse complement. In order to make AP-97A-HBEGF-GFP, the primer HBD-A(93-97)F (5'- AAA TCG AAT GGC ACG AAG GGG CGG CAG CCG CTG CGG GAA AGG GGT TAG GGA AGA AGA 3') and its reverse complement were designed to be complementary to AP-HBEGF-GFP for 20 and 22 amino acids before and after the region of the heparin-binding domain to be mutated (93-97) with a five residue stretch of alanine to replace the five lysine residues. The AP-105A-HBEGF-GFP plasmid was made based on the AP-HBEGF-GFP plasmid and using a primer, ALA-HBD-F, (5'- AAA TCG AAT GGC ACG AAG GGG CGG CAG CCG CTG CGG GAG CAG GGT TAG GGG CGG CAG CCG ACC CAT GCC TCA GGA AAT A-3') and its reverse complement that is complementary before amino acid 93 and after 105 for twenty amino acids. The amino acid sequence AAAAAGAGLGAAA, as a replacement for KKKKKGKGLGKKR, was in between the complementary regions. The AP-1 13A-HBEGF-GFP plasmid was constructed by mutating amino acids RKYK in stretch 110-113 in the EGF-like domain of AP- 105AHBEGF-GFP to AAYA. The primer, RKA1 13 SEM Forward, (5'- CAG CCG ACC CAT GCC TCG CAG CGT ACG CAG ACT ACT GCA TCC ACG GGG A -3') has 17 base pairs complementary to AP- 105A-HBEGF-GFP before amino acid 110 and 20 base pairs after 113 with an the sequence for AAYA in between. Using the above primers and base plasmids, site-directed mutagenesis via whole plasmid PCR was completed with an annealing temperature of 50'C and an elongation time of 14 minutes. The PCR reaction was digested with DpnI restriction enzyme for >6 hours, transformed into XL- 10 Gold E. Coli, and plated onto kanamycin agar dishes. The following day, colonies were picked and placed into 3 mL of LB Media with kanamycin and cultured on a shaker overnight at 225 rpm and 37*C. The following day, 1.5 mL of the bacteria culture was used to purify the plasmid with the Qiagen Miniprep Kit. Miniprep samples were sent for sequencing to screen colonies for the correct mutation. Positive cultures were expanded into 100 mL of LB Media with kanamycin, and large amounts of the plasmid were purified with the Qiagen Maxiprep Kit. Constructing AP-Amphiregulin-GFP plasmid The human amphiregulin DNA coding sequence in pBM-IRESpuro was a generous gift from Steve Wiley. Amphiregulin DNA was removed from pBM-IRESpuro and cloned into pEGFP-N1. First, human amphiregulin was amplified by PCR from pBM-IRESpuro with the forward primer AR-XhoI-fwd (5'- TAC TCG AGA TGA GAG CCC CGC T -3'), which contains an overhang with the XhoI restriction enzyme recognition sequence, and the reverse primer AR-EcoRl-rev (5'- ATG AAT TCT TGC TAT AGC ATG TAC ATT TCC ATT CTC TTG -3'), which contains an overhang for the EcoRI restriction enzyme. The PCR fragment was gel purified with the Qiaquick gel purification kit. The PCR fragment and the pEGFP-N1 plasmid backbone were subjected to restriction enzyme digestion with XhoI and pEGFP-N1 at 37'C for >1hour. The digested samples were run on a gel, bands corresponding to the amphiregulin digested PCR product and linearized pEGFP-N1 were cut out and gel purified. The PCR fragment was ligated into linearized pEGFP-N1 with T4 DNA ligase. This was transformed via T4 ligation into E. Coli and plated onto kanamycin agar dishes, and incubated overnight at 370 C. The following day, >5 colonies were picked into 3 mL of LB media with kanamycin and incubated overnight at 37'C with shaking at 225 rpm. DNA was purified from 1-2 mL of bacterial culture with the Qiagen Miniprep Kit. Insertion of amphiregulin was confirmed via sequencing with above forward primer. Positive colonies were expanded into 10OmL of LB media with kanamycin overnight at 37"C at 225 rpm shaking. Large amounts of plasmid DNA were purified with the Qiagen maxiprep kit. The fifteen amino acid acceptor peptide sequence was then inserted into the AR-GFP plasmid after valine 107 in amphiregulin. This was achieved by whole plasmid PCR with the forward primer AR-APV107 Fwd (5'TCA GTC AGA GTT GAA CAG GTA GTT GGC CTG AAC GAC ATC TTC GAA GCC CAG AAA ATC GAA TGG CAC GAA AAG CCC CCC CAA AAC AAG -3') and its reverse complement, AR-APV107 Rev (5'- CTT GTT TTG GGG GGG CTT TTC GTG CCA TTC GAT TTT CTG GGC TTC GAA GAT GTC GTT CAG GCC AAC TAC CTG TTC AAC TCT GAC TGA -3'). The forward primer consists of the first 24 base pairs of the human amphiregulin sequence before the insertion site after V107, the fifteen amino acid acceptor peptide DNA sequence, then 18 more base pairs analogous to amphiregulin after V107. PCR was performed with 18 rounds, and an annealing temperature of 50'C, and an extension temperature of 68*C for 12 minutes per round. The reaction was digested with the DpnI restriction enzyme, then transformed into XL 10 Gold Cells (Stratagene) and plated onto kanamycin plates. The following day positive colonies were selected into 3 mL of LB media with kanamycin and incubated overnight at 37'C at 225 rpm shaking. The following day, DNA was purified with the Qiagen Miniprep kit and sent for sequencing to check for correct insertion of the acceptor peptide with the AP-XhoI-Fwd primer. Colonies positive for the acceptor peptide were grown into 100 mL of LB media with kanamycin and large amounts of plasmid DNA was purified with the Qiagen maxiprep kit. Constructing the heparin-binding domain mutant of amphiregulin (AP-143AAmphiregulin-GFP) In order to mutate all basic lysine and arginine residues to neutral alanine in the heparinbinding domain of human amphiregulin in AP-AR-GFP (corresponding to residues 123- 143 in the human amphiregulin gene sequence) (KPKRKKKGGKNGKNRRNRKKK mutated to APAAAAAGGANGANAANAAAA), AP-AR-GFP was subjected to whole plasmid PCR with the forward primer (AR-HBD-R/KtoA Fwd: 5'- AAG ACG GAA AGT GAA AAT ACT TCA GAT GCT CCC GCT GCT GCT GCT GCT GGA GGC GCT AAT GGA GCT AAT GCT GCT AAC GCT GCT GCT GCT AAT CCA TGT AAT GCA GAA TTT CAA AAT TTC T -3') and its reverse complement (AR-HBDR/KtoA Rev: 5'- AGA AAT TTT GAA ATT CTG CAT TAC ATG GAT TAG CAG CAG CAG CGT TAG CAG CAT TAG CTC CAT TAG CGC CTC CAG CAG CAG CAG CAG CGG GAG CAT CTG AAG TAT TTT CAC TTT CCG TCT T -3'). The mutagenesis primer consists of 27 base pairs of the human amphiregulin sequence before the heparin-binding domain, then the DNA sequence for the mutated heparin-binding domain, and then 31 base pairs of amphiregulin after the heparin-binding domain. PCR with these primers replaced the wild-type heparin-binding domain with the mutated sequence. PCR was performed with 18 cycles of PCR at an annealing temperature of 504C and an extension temperature of 68'C for 12 minutes per round. The reaction was digested with the DpnI restriction enzyme, then transformed into XL10 Gold Cells (Stratagene) and plated onto kanamycin plates. The following day positive colonies were selected into 3 mL of LB media with kanamycin and incubated overnight at 370 C at 225 rpm shaking. The following day, DNA was purified with the Qiagen Miniprep kit and sent for sequencing to check for mutation of the heparin-binding domain with the APXhoI-Fwd primer. Positive colonies were expanded into 100 mL of LB media with kanamycin and large amounts of plasmid DNA were purified with the Qiagen maxiprep kit. Addition of the heparin-binding domain to AP-CFP-TM The heparin-binding domain of mouse HB-EGF, consisting of the portion that lies outside the EGF-like domain (#93-105), was added to the AP-CFP-TM control protein between the acceptor peptide and the CFP molecule. The heparin-binding domain was inserted after a three amino acid linker before CFP, then another three amino acid linker was inserted after the heparin-binding domain. Therefore the sequence consists of the acceptor peptide, the linker Gly-Ala-Pro, the heparin-binding domain, the linker Ala-Gly- Gly, then CFP. This was accomplished with the primer CFPAP-HBD-F (5'- AGT GGC ACG AGG GCG CGC CGA AAA AGA AGA AGA AAG GAA AGG GGT TAG GGA AGA AGA GAG CGG GCG GCA TGG TGA GCA AGG GCG AGG A -3') and its reverse complement, whose first and last twenty amino acids are complementary to APCFP-TM. The PCR reaction was run under the same conditions as the AP-HBEGF-GFP plasmid, digested with the DpnL restriction enzyme, transformed into XL-10 Gold Cells (Stratagene) and plated on ampicillin agar dishes. Colonies were picked 24 hours after plating and grown in 3 mL of LB media with ampicillin overnight with shaking at 225 rpm at 37"C. The following day, 1 mL of the culture was harvested and DNA was purified using the Qiagen Miniprep kit. The purified DNA was sent for sequencing with the T7 forward sequencing primer to verify correct insertion of the heparin-binding domain. After identifying a positive colony, this colony was allowed to grow overnight in 100 mL of LB Media and ampicillin shaking at 225 rpm. The following day, the bacteria was harvested and purified with the Qiagen Maxiprep Kit. After further analysis of the structure of cyan fluorescent protein, I noticed that the N-terminus of the protein actually ends at the same side of the protein as the C-terminus. This means that the protein is likely oriented so that the acceptor peptide and the heparin-binding domain are pointing down toward the membrane rather than out into the media. In order to correct this, I chose to insert a flexible protease-resistant linker of 20 amino acids. The linker sequence was designed based on a study of linker sequences and protease resistance (Robinson and Sauer, 1998). The length was set at 20 residues, resulting in a linker of approximately 80 angstroms to reach around the CFP molecule (-50 angstroms). I chose to build off of the AGG sequence already present in the 3 amino acid linker and follow it with SEGGGSEGGTSGATG. The insert was made with a PCR reaction on the AP- HBD-CFP-TM plasmid with the following forward CFPAPHBD Linker Insert Forward Primer (5'- GAA GAA GAG AGC GGG CGG CTC TGA AGG CGG CGG CAG CGA AGG CGG CAC CAG CGG CGC GAC CGG AAT GGT GAG CAA GGG CGA GGA - 3') and its reverse complement, which contains 19 and 20 amino acids complementary to the AP-HBD-CFP-TM before and after the linker insertion site, respectively. The methods for inserting the linker are identical to those presented in this section above for insertion of the heparin-binding domain. 4.3 Results 4.3.1 Heparin and heparan sulfate alter the localization of proHB-EGF As proHB-EGF was observed primarily at sites of cell-cell contact, the question arose of what molecular interactions between cells may lead to proHB-EGF concentration in this area. As the extracellular domain of proHB-EGF has the ability to interact with HSPGs, which are present on the cell surface, we hypothesized that this interaction may control localization of HB-EGF to cell-cell contact sites. To test the hypothesis, we sought to compete for HSPG binding to proHB-EGF with exogenous heparin and heparan sulfate. Heparin and heparan sulfate (100 pig/mL) dramatically changed the localization of AP-HBEGF-GFP (Figure 4.5.1 A&B). The extracellular (AF568) and intracellular (GFP) domain of AP-HBEGF-GFP changed from localization primarily at cell-cell contact sites to a homogenous distribution over the entire cell surface. However, the addition of the glycosaminoglycan chondroitin sulfate (100 ig/mL) did not affect the localization of AP-HBEGF-GFP (Figure 4.5.1 C). Images shown are after four hours of treatment; however changes in proHB-EGF localization were observed as soon as five minutes after the addition of heparin (Figure 4.5.2). The timecourse depicted in figure 4.5.2 illustrates a highlighted area of cell-cell contact where proHB-EGF is localized to cell-cell contact sites. Heparin (100 ig/mL) was added after the first frame (0 seconds), and after only 20 seconds the intensity of HB-EGF was reduced at cell-cell contact sites. The intensity of HB-EGF here continued to decrease, and was observed to be primarily diffusely localized throughout the membrane of the transfected cell after 300 seconds of heparin treatment. To analyze this in more detail, the fluorescence intensity per pixel along the vertical line graphed in figure 4.5.2 B is mapped over time after the addition of heparin. The fluorescence intensity at the cell-cell contact site was at maximum before the addition of heparin. This peak intensity decreased over time, and reached its half-maximal value at approximately five minutes post heparin addition. After the removal of heparin for 24 hours, AP-HBEGF-GFP localized back to sites of cell-cell contact as observed before the addition of heparin (data not shown). 4.3.2 ProHB-EGF in microdomains at cell-cell contact sites is inaccessible to BirA and streptavidin. The amount of HB-EGF on the cell surface can be distinguished from the total pool of HB-EGF by biotinylating the cell surface fraction with exogenously added biotin ligase. After cell lysis and gel electrophoresis, the biotinylated protein can be detected with streptavidin-HRP. In this experiment, it was observed that pretreatment of the APHBEGF-GFP expressing COS-7 cells with 100 ptg/mL of heparin in the media led to a larger amount of biotinylated AP-HBEGF on the cell surface (Figure 4.5.3 A). It was therefore hypothesized that heparin increased the cell surface fraction of HB-EGF by increasing the translocation of HB-EGF from the cytoplasm to the plasma membrane, or stabilized HB-EGF on the cell surface, slowing the protein's cell surface half life. However, further study showed that the heparin-induced increase in the biotinylated cell surface fraction was not time dependent, and even at times as little as 1 minute showed no change in the amount of HB-EGF on the cell surface than at 60 minutes (data not shown). Additionally, preincubation for 30 minutes and continued treatment throughout the experiment with 10 pM monensin or at 4'C had no effect on the amount of biotinylated HB-EGF on the cell surface after the addition of heparin. Monensin is a carboxylic acid ionophore that disrupts the transport of membrane vesicles from the golgi complex to the plasma membrane (Stein et al., 1984) and lowering the temperature to 4'C inhibits endocytosis and protein trafficking. As neither of these pretreatments stopped the heparin-induced increase of HB-EGF on the cell surface, it is unlikely that heparin is affecting the translocation of HB-EGF to the cell surface. It was additionally observed in fluorescent microscope imaging of biotinylated AP-HBEGF that although the majority of the GFP signal overlapped the extracellular mSA-AF568 signal, there were some areas that were unlabeled (Figure 4.5.3 B). These areas are typically flat planar-like structures where two cells overlap. The initial hypothesis was that HB-EGF was cleaved in these areas and only the C-terminal tail was left, however, western blotting with a GFP antibody for total HB-EGF shows no evidence of a C-terminal tail fragment (data not shown). Additionally, these patch-like structures were highly mobile upon the addition of heparin (data not shown), suggesting that HB-EGF is in the pro-form and interacting with HSPGs. Therefore, it is assumed that these patch-like areas are tightly closed and sterically unavailable to biotin ligase for biotinylation or mSA-AF568 to label the prebiotinylated protein. However, upon the addition of heparin, HB-EGF is released from HSPGs and cell-cell contact sites, and accessibility to the acceptor peptide is unhindered. 4.3.3 ProHB-EGF interaction with HSPGs controls cell-cell contact site localization The change in HB-EGF localization after the addition of heparin away from cellcell contact sites suggests that interaction with HSPGs was responsible for localizing HBEGF to this area. To further test this hypothesis, we aimed to diminish HSPG-proHBEGF interactions by two alternative means: (a) removing HSPGs, and (b) altering the proHB-EGF sequence. Removal of HSPGs by two different methods changed the localization of AP-HBEGF-GFP from cell-cell contact sites to a homogenous distribution over the cell surface, as seen with the addition of heparin. Sodium chlorate is an inhibitor of protein sulfation that blocks the enzyme ATP-sulfurylase (Baeuerle & Huttner, 1986). Culture of COS-7 cells expressing AP-HBEGF-GFP with 50 mM sodium chlorate for 24 hours led to a homogeneous distribution of AP-HBEGF-GFP over the cell surface (Figure 4.5.4 A). Additionally, digestion of cell surface heparan sulfate with heparinase III (1.6 mU/mL) for four hours similarly changed the localization of AP-HBEGF-GFP to a homogenous distribution over the cell surface, although some AP-HBEGF-GFP remained at cell-cell contact sites (Figure 4.5.4 B). Thus, the reduction of all sulfation with sodium chlorate, or the removal of cell surface heparan sulfate with heparinase III reduced the amount of AP-HBEGF-GFP at cell-cell contact sites, leading to a more homogenous distribution over the cell surface. It may be that heparan sulfate chains bound to HB-EGF are relatively protected from degradation by heparinase, as this is the case with bFGF and mammalian heparanase (Tumova & Bame, 1997). For a converse experiment, we made four different mutants of AP-HBEGF-GFP to inhibit the ability of HB-EGF to interact with heparan sulfate (Table 1). The heparinbinding domain of HB-EGF consists of amino acids 93-113 of the mouse protein, which contains a combination of 12 basic lysine and arginine residues, which have been shown to be responsible for the HB-EGF interaction with heparin (Thompson et al., 1994). However, residues 108-113 lie within the EGF-like domain and contain three of the basic amino acids. Deletion of the portion of the heparin-binding domain that lies outside the EGF-like domain (93-105) (AP-delHBD-HBEGF-GFP) led to a homogeneous distribution of HB-EGF over the cell surface (Figure 4.5.5 A), similar to that seen with the addition of heparin. In contrast, mutation of the first five positive lysine residues (9397) in the heparin-binding domain to non-polar alanine (AP-97A-HBEGF-GFP) had no effect on the localization of HB-EGF (Figure 4.5.5 B). Mutation of an additional four lysine and arginine residues to alanine (93-105) (AP-105A-HBEGF-GFP) was nonetheless sufficient to change the localization of AP-HBEGF-GFP to be homogenously spread over the cell surface (Figure 4.5.5 C). The same result was observed with the mutation of all 12 basic amino acids in the heparin-binding domain to alanine, including those within the EGF-like domain (AP-1 13A-HBEGF-GFP) (Figure 4.5.5 D). These data suggest that binding of HB-EGF via the heparin-binding domain to HSPGs on the cell surface is required for HB-EGF localization to cell-cell contact sites. Name AP-HBEGF-GFP AP-delHBD-HBEGF-GFP AP-97A-HBEGF-GFP AP-105A-HBEGF-GFP AP-113A-HBEGF-GFP Heparin-Binding Domain Sequence KKKKKGKGLGKKRDPCLRKYK --------------------------- DPCLRKYK AAAAAGKGLGKKRDPCLRKYK AAAAAGAGLGAAADPCLRKYK AAAAAGAGLGAAADPCLAAYA # Basic AAs 12 3 7 3 0 Table 1. Mutations in the heparin-binding domain of HB-EGF. Mutations of basic lysine and arginine residues to alanine in the twenty-one amino acid heparin-binding domain of murine HB-EGF, with the unaltered heparin-binding domain listed in AP-HBEGF-GFP with the number of basic amino acids left after deletion. A '-' represents amino acid deletion, rather than mutation. 4.3.4 ProHB-EGF interacts with HSPGs in trans To further investigate the role of HSPGs on the localization of HB-EGF to cellcell contact sites, wild-type CHO-K1 cells and mutant CHOpgsD-677 cells, which are deficient in heparan sulfate production were utilized (Lidholt et al., 1992). Confluent monolayers of each cell type were transfected for 24 hours with AP-HBEGF-GFP and the localization of HB-EGF was evaluated with the GFP-tagged cytoplasmic tail. The wildtype CHO-K1 cells, which have functional HSPGs, show localization of HB-EGF to sites of cell-cell contact (Figure 4.5.6 A), as observed with COS-7 cells. However, transfection of AP-HBEGF-GFP into the CHOpgsD-677 cells that lack heparan sulfate showed no HB-EGF localization to cell-cell contact sites (Figure 4.5.6 C). As observed in the COS-7 cells, AP-1 13A-HBEGF-GFP was diffusely localized over the cell, and not concentrated at sites of cell-cell contact in both CHO-Ki (Figure 4.5.6 B) and CHOpgsD677 cells (Figure 4.5.6 D). Despite localization of AP-HBEGF-GFP to cell-cell contact sites in CHO-Ki cells, large flattened areas of the membrane with strong planes of proHB-EGF concentration (as shown in Figure 4.5.3 B) was not observed in this cell line. Therefore, this type of localization may be controlled by a second interaction partner in addition to HSPGs that is present in the COS-7 cells and not the CHO-Ki cells. Demonstrating that HSPGs were required for HB-EGF localization to cell-cell contact sites raised the question of the source of HSPGs for this interaction. HB-EGF could interact in cis with HSPGs on the same cell that expresses HB-EGF, holding it at cell-cell contact sites. Alternatively, HB-EGF may interact with HSPGs in trans, and therefore HSPGs would be required on the neighboring cell for localization to cell-cell contact sites. To distinguish between these two possibilities, CHO-Ki (wild-type) and CHOpgsD-677 cells were transfected with AP-HBEGF-GFP (green) or mCherry (red) and co-cultured together in different combinations. The positive control (Figure 4.5.7 A) showed AP-HBEGF-GFP at sites of cell-cell contact between mCherry and AP-HBEGFGFP transfected wild-type CHO-Ki cells. Additionally, the negative control (Figure 4.5.7 D) showed no cell-cell contact localization of AP-HBEGF-GFP between mCherry and AP-HBEGF-GFP transfected CHOpgsD-677 cells. However, CHOpgsD-677 cells transfected with AP-HBEGF-GFP showed localization of HB-EGF to the cell-cell contact sites with a wild-type CHO-Ki mCherry transfected neighbor (Figure 4.5.7 B). Wildtype CHO-Ki cells transfected with AP-HBEGF-GFP showed no HB-EGF localization to sites of cell-cell contact with mCherry transfected CHOpgsD-677 neighbors (Figure 4.5.7 C). These data demonstrate that the localization of proHB-EGF to sites of cell-cell contact is dependent on interaction with HSPGs on a neighboring cell only; therefore proHBEGF interacts with HSPGs in trans. As transient transfection is utilized to express AP-HBEGF-GFP in COS-7 cells, not all of the cells in the monolayer take in the plasmid and express the construct. Therefore, there is a heterogeneous mix of AP-HBEGF-GFP transfected and non- transfected cells on the dish in these experiments. It was observed that HB-EGF localization to cell-cell contact sites it strongest between a transfected and a nontransfected cell (Figure 4.5.8). The GFP-tagged cytoplasmic tail of HB-EGF is observed to be missing, or weakly localized to cell-cell contact sites between two transfected cells, as highlighted by the white arrows. It is hypothesized here that proHB-EGF competition with HSPGs on neighboring cells leads to less HB-EGF at cell-cell contact sites when two neighboring cells both overexpress AP-HBEGF-GFP. However, it is unclear whether this is due to overexpression of the protein, or if this could happen at physiologic expression levels. However, if this is a natural phenomenon, it may suggest that strong localization of HB-EGF to sites of cell-cell contact is reserved for non-homogenous tissues where HB-EGF expression is not equal between two different cells types. Another hypothesis here is that HB-EGF is excluded from sites of cell-cell contact during mitosis and continues to be excluded after return to GO/G1. As shown in Figure 4.5.8 C, HB-EGF is missing from the area that will become the site of cell-cell contact between the cells in the late phase of mitosis, with the nuclei almost completely separated. It should be noted here that many studies on the localization of HB-EGF are done in stably transfected cell lines, where all cells are expressing HB-EGF, and the localization of HBEGF to cell-cell contact sites in these experiments is likely diminished compared to the studies here with heterogeneous HB-EGF expression. 4.3.5 Heparin-binding controls amphiregulin localization, but engineered heparinbinding is insufficient for cell-cell contact localization HB-EGF is not the only ligand in the EGFR family capable of heparin-binding, as amphiregulin, betacellulin and some isoforms of neuregulin also interact with HSPGs. HB-EGF and amphiregulin have similar heparin-binding domains located at the Nterminus of the protein after the EGF-like domain. These domains are both twenty-one amino acids in length, with over half of the residues represented by basic lysine or arginine, which allow the domain to interact with negatively charged heparin and heparan sulfate (Thompson et al., 1994). We sought to test whether heparin changed the localization of amphiregulin to see if this mechanism was common for other heparinbinding EGFR ligands. Acceptor peptide labeling of the extracellular portion of amphiregulin, and imaging of the C-terminal GFP tag, showed that amphiregulin is similar to HB-EGF in that the extracellular and intracellular fluorophores were colocalized, suggesting that amphiregulin is primarily present in the pro-form (Figure 4.5.9 A). Additionally, amphiregulin was concentrated around the perimeter of the cell at sites of cell-cell contact. The addition of heparin (100 tg/mL) for four hours led to a homogenous distribution of amphiregulin over the cell surface, as seen with HB-EGF (Figure 4.5.9 B). Therefore, it is likely that the heparin-binding domain of amphiregulin is also responsible for its localization to sites of cell-cell contact. The heparin-binding domain of amphiregulin is very similar to HB-EGF, consisting of a 21 amino acid region of which 14 of the amino (KPKRKKKGGKNGKNRRNRKKK). acids are basic lysine or arginine In order to stop the ability of amphiregulin to interact with HSPGs, all of the lysine and arginine residues were mutated to neutral alanine (APAAAAAGGANGANAANAAAA). The heparin-binding domain mutant of amphiregulin similarly led to less amphiregulin at sites of cell-cell contact (Figure 4.5.9 C) as observed after the addition of heparin. This suggests that the same interaction of HSPGs in trans with proHB-EGF to localize the protein to sites of cell-cell contact applies for pro-amphiregulin. In order to test whether the heparin-binding domain of HB-EGF was sufficient to localize any transmembrane protein to cell-cell contact sites, an engineered construct was made consisting of an extracellular acceptor peptide sequence, followed by the portion of the heparin-binding domain of HB-EGF which lies outside the EGF-like domain (residues 93-105), cyan fluorescent protein, then the transmembrane domain of the PDGF receptor. This construct (AP-HBD-CFP-TM) (Figure 4.5.10 B) showed the same localization as the control construct (Figure 4.5.10 A) without a heparin-binding domain or flexible linker (AP-CFP-TM), with a homogenous localization over the cell surface. Therefore, the heparin-binding domain alone was insufficient to localize any protein to cell-cell contact sites. This suggests that an additional co-factor which interacts with HBEGF and amphiregulin may be required for HSPG-mediated localization to cell-cell contact sites, or removal of proteins unassociated with HSPGs, or a particular conformation of the heparin-binding domain is required that is not present in AP-HBDCFP-TM. 4.4 Discussion The key finding of Chapter 4 is that a trans interaction between HSPG and the HB-EGF heparin-binding domain is responsible for localizing proHB-EGF to sites of cell-cell contact. HSPGs change the localization of pro-amphiregulin in a similar manner, suggesting that this may be a common mechanism for transmembrane heparin-binding ligands of the EGFR family. As HB-EGF (Higashiyama et al., 1995), amphiregulin (Inui et al., 1997), and betacellulin (Tada et al., 1999) are heparin-binding and capable of signaling in juxtacrine mode, and the heparin-binding domain of HB-EGF and amphiregulin inhibits binding to the receptor until in complex with heparin or heparan sulfate (Higashiyama et al., 1993; Johnson and Wong, 1994; Piepkorn et al., 1994; Takazaki et al., 2004), the role of this interaction may be to assist in holding prospective juxtacrine ligands at sites of cell-cell contact, likely in concert with CD9, to bind a receptor on a neighboring cell. HB-EGF is localized to sites of cell-cell contact in the two cell lines explored in this chapter which express HSPGs, COS-7 and CHO-Ki cells. However, COS-7 cells, in addition to localizing HB-EGF at sites of cell-cell contact, have proHB-EGF localized in large planar areas (Figure 4.5.3 B) that are not accessible to streptavidin or BirA, suggesting that they are tightly sealed junctions, sterically inaccessible to exogenously added proteins. These structures were quickly dissolved by the addition of heparin, however, they were not observed in the CHO-Ki cell line. These structures are very similar to tetraspanin enhanced microdomains observed by Singethan and colleagues (Singethan et al., 2008) after treatment with a CD9 clustering antibody. Therefore, we hypothesize that proHB-EGF transfection may have the ability to cluster CD9 into tetraspanin microdomains at cell-cell contact sites along with proHB-EGF-HSPGs, as proHB-EGF interacts with CD9 via its heparin-binding domain to the second extracellular loop of CD9 (Sakuma et al., 1997). Additionally, heparin may have the ability to dissipate CD9 from cell-cell contact sites, as it does for proHB-EGF. Evidence to support this hypothesis is the fact that the CD9 clustering antibody (K41) binds the same region of CD9 that HB-EGF interacts with (Singethan et al., 2008), and these structures are not present in CHO-Ki cells that have been demonstrated to have very little to no CD9 expression (Jennings et al., 1994). The interaction of HSPGs with pro-HB-EGF in trans was demonstrated with wildtype and mutant CHO cells which lack heparan sulfate (Figure 4.5.7). The localization of HB-EGF to cell-cell contact sites is unlikely to depend on interaction with EGFR, as CHO-Ki cells lack endogenous EGFR, yet still show strong localization of HB-EGF to cell-cell contact sites. This experiment also suggests that HSPGs are not required as an intracellular chaperone for proHB-EGF during transport to the cell surface, as cells lacking heparan sulfate were still able to localize HB-EGF to sites of cell-cell contact when neighbored with a HSPG producing cell. HSPGs have previously been shown to interact in trans with VEGFR-2 in VEGFR-mediated angiogenesis (Jakobsson et al., 2006) and the Xenopus receptor caALK4 via the co-factor Vgl during mesoderm migration in early left-right development (Kramer and Yost, 2002). Additionally, this interaction likely plays a role in blastocyst implantation during pregnancy, as the interaction between HB-EGF on the luminal epithelium with EGFR and HSPGs on the adjacent blastocyst are required for successful attachment, which is reduced by exogenous heparin or blastocyst heparinase treatment (Farach et al., 1987; Farach et al., 1988; Raab et al., 1996). The trans interaction also likely plays a role in diphtheria toxin infection, as the presence of heparan sulfate is required for diphtheria toxin binding to proHB-EGF (Shishido et al., 1995). The localization of proHB-EGF to sites of cell-cell contact sites between two APHBEGF-GFP transfected cells is much weaker than observed between a transfected and a non-transfected cell (Figure 4.5.8). We speculate that this is due to competition between the heparin-binding domain of proHB-EGF and HSPGs at the cell-cell junction between two transfected cells. This would suggest that strong localization of HB-EGF to cell-cell contact sites may be reserved for junctions between two different cell populations, with only one expressing proHB-EGF. Given HB-EGF's role in embryonic development and that proHB-EGF juxtacrine EGFR activation can be growth inhibitory, this may serve as a signal for tissue polarization and boundary formation. In the kidney, HB-EGF is localized to the basolateral surface of collecting tubule cells, and is absent from the apical surface of collecting tubules, where cell-cell contact is missing from the basolateral surface in contact with urine (MacRae Dell et al., 2004). This localization is similar to what we observed in wounded COS-7 cells, which are also kidney cells from the African green monkey (Figure 3.5.1A). However, in mouse models of autosomal-recessive polycystic kidney disease, HB-EGF is mis-localized in the collecting tubules, with increased expression and localization to the apical surface in addition to the basolateral surface (MacRae Dell et al., 2004). Additionally, EGFRs are mis-localized to the apical surface of the related autosomal dominant autosomal-recessive kidney disease (Du and Wilson, 1995). Polycystic kidney disease is not well understood, but is a genetic disease leading to renal failure associated with over-proliferation of epithelial cells and is linked to a defect in cilia and polarization of cells in the collecting tubules. Mis-localized proHB-EGF may contribute to or be a consequence of loss of cell polarity in polycystic kidney disease, or contribute to epithelial cell proliferation. The change in localization of HB-EGF in the collecting tubules from healthy mice compared to mouse models of autosomal-recessive polycystic kidney disease is reminiscent of the change in localization observed with the addition of heparin in COS-7 cells (Figure 4.5.1 and Figure 4.5.2). 4.5 Figures Extracellular HB-EGF AP Tag Blotinylation (mSA-AF568) C-Terminal HB-EGF Tail EGFP Phase Contrast (A) Heparin (B) Heparan Sulfate (C) Chondroitin Sulfate Figure 4.5.1. Heparin and heparan sulfate changed the localization of HB-EGF from cell-cell contact sites to a homogenous distribution over the cell surface. After 24h of transfection in COS-7 cells with AP-HBEGF-GFP, the biotinylated, extracellular acceptor peptide in AP-HBEGF-GFP was labeled with monovalent streptavidin-alexa fluor 568 (red, left) and imaged alongside the cytoplasmic tail conjugated to EGFP (green, middle), and phase contrast (right). Addition of (A) heparin (100 gg/mL) or (B) heparan sulfate (100 ptg/mL) for four hours changed the localization of both the extracellular and intracellular domains of AP-HBEGF-GFP to a diffuse distribution over the cell surface, rather than at cell-cell contact sites. (C) Addition of chondroitin sulfate (100 pg/mL) for four hours had no effect on localization of AP-HBEGF-GFP. Each row represents the same field. Scale bars are 40 pim. .......... :m::::::r ............. :........... ... - ._ _ '&A - - - __ - __=W (A) AP-HBEGF-GFP Fluorescence intensity at Cell-Cell Junction After Heparin Addition -Om (B) -Im 2m -3m -4m -5m -7mSm 140 120- -aSm -- 100 - 9m 10m 80 11M 12m 14m - 60 40 60 -14m 17m U.- -18 20 00 10 20 30 40 50 60 70 Pixels Figure 4.5.2 Localization change of HB-EGF after addition of heparin over time. (A) Confluent monolayers of COS-7 cells were transfected with APHBEGF-GFP for 24 hours. Cells were placed in PBS+, and heparin (100 pg/mL) was added after time zero. Images were collected every 20 seconds and AP-HBEGF-GFP localization was monitored via the GFP-tagged cytoplasmic tail. Individual frame width is 65.5 pm. (B) The fluorescence intensity of the GFP signal is plotted versus pixel position along the yellow line drawn on the inset fluorescence image, with the top of the line representing pixel zero. Fluorescence intensity at the cell-cell contact site peaks at time zero and is reduced over time, reaching the half maximal value at approximately five minutes. 0 1 2 3 4 hours heparin 51kDa 51kDa- 51kDa- 4C s Monensin Figure 4.5.3 Heparin increases accessibility of cell surface AP-HBEGFGFP. (A) Confluent monolayers of COS-7 cells expressing AP-HBEGF-GFP had heparin (100p g/mL) added to the cell culture medium. At the indicated times, cells were washed and the cell surface acceptor peptide was biotinylated with exogenously added BirA on ice, then cells were immediately lysed. Lysate was probed after gel electrophoresis with streptavidin-HRP for biotinylated proteins. After the addition of heparin, an increase in the biotinylated cell surface form of pro-HBEGF is observed, and this pattern is not changed with preincubation for 30 minutes and incubation during the experiment length at 4"C or with monensin. (B) In confluent monolayers of COS-7 cells expressing AP-HBEGF-GFP and BirA-ER, cell surface biotinylated AP-HBEGF-GFP was labeled with mSA-AF568 (red, left) and imaged alongside the C-terminal tail with the EGFP label (green, middle). The merged image (right) combines the red extracellular domain with the green intracellular domain, where yellow represents co-localization of the two signals. Flat areas of the cell with concentrated GFP signal are not labeled with the extracellular mSA-AF568 red signal. Scale bar represents 10 im. Extracellular HB-EGF AP Taa Biotinviation (mSA-AF568) C-Terminal HB-EGF Tail EGFP Phase Contrast (A) Sodium Chlorate (B) Heparanase IlIl Figure 4.5.4 HSPGs target pro-HB-EGF to cell-cell contact sites. (A) After 24h of transfection of COS-7 cells with AP-HBEGF-GFP, cells were treated with 50 mM sodium chlorate in media without penicillin-streptomycin. After 24h of sodium chlorate treatment, the biotinylated, extracellular acceptor peptide in AP-HBEGF-GFP was labeled with monovalent streptavidin-alexa fluor 568 (red, left) and imaged alongside the cytoplasmic tail conjugated to EGFP (green, middle), and phase contrast (right). Sodium chlorate treatment led to a homogenous distribution of AP-HBEGF-GFP over the cell surface, rather than at cell-cell contact sites (B) After 24 hours of transfection with AP-HBEGF-GFP, cells were treated with 1.6 mU/mL heparinase III for 4 hours, then labeled with monovalent streptavidin-alexa fluor 568 (red, left), alongside the cytoplasmic tail (EGFP) and phase contrast (right). Heparinase III treatment also led to a homogenous distribution of AP-HBEGF-GFP over the cell surface. Each row represents the same field. Scale bars are 40 pm. Extracellular HB-EGF AP Tag Biotinylation (mSA-AF568) C-Terminal HB-EGF Tail EGFP Phase Contrast (A) AP-delHBD HBEGF-GFP (B) AP-97A HBEGF-GFP (C) AP-105A HBEGF-GFP (D) AP-113A HBEGF-GFP Figure 4.5.5. The heparin-binding domain targets pro-HB-EGF to cellcell contact sites. After 24 hours of plasmid transfection, the biotinylated, extracellular acceptor peptide in the HB-EGF mutants was labeled with monovalent streptavidin-alexa fluor 568 (red, left), and imaged along side the cytoplasmic tail of HBEGF conjugated to EGFP (green, middle), and phase contrast (right). (A) Deletion of the portion of the heparin-binding domain of AP-HBEGF-GFP which lies outside of the EGF-like domain (AP-delHBD-HBEGF-GFP), led to a homogenous distribution of HBEGF over the cell surface. (B) Mutation of the first five positively charged lysine residues in the heparin-binding domain of HB-EGF to alanine (AP-97A-HBEGF-GFP) had no effect on the localization of the protein. (C) Mutation of all nine positive lysine and arginine residues of the heparin-binding domain, which lie outside the EGF-like domain (AP- 105A-HBEGF-GFP) led to a more diffuse distribution of HB-EGF over the cell surface, however some remains localized at cell-cell contact sites. (D) Mutation of all twelve positive lysine and arginine residues in the heparin-binding domain of HBEGF to alanine (AP-113A-HBEGF-GFP), both those outside and inside the EGF-like domain, led to a homogenous distribution of HB-EGF over the cell surface. Each row represents the same field. Scale bars are 40 ptm. AP-HBEGF-GFP AP-113A-HBEGF-GFP CHO-KI (WT) CHOpgsD-677 (-HS) Figure 4.5.6 HSPGs and the heparin-binding domain of HB-EGF are required for localization to cell-cell contact sites. Wild-type CHO-K1 cells (A) & (B) and CHOpgsD-677 mutant cells (C) & (D), which do not synthesized heparan sulfate, were transfected with either AP-HBEGF-GFP (A) & (C) or AP-l 13A-HBEGFGFP (B) & (D) and HB-EGF localization was determined based on the GFP-tagged cytoplasmic tail. AP-HBEGF-GFP was localized to cell-cell contact sites only in CHOK1 cells (A), and was localized homogenously over the cell surface in CHOpgsD-677 cells (C). AP- 113A-HBEGF-GFP was not localized to cell-cell contact sites in either cell line (B) & (D). Arrows highlight HB-EGF localization to cell-cell contact sites. Scale bar represents 40 tm. CHO-K1 (WT) mCherry transfected -a A CH~pgsD-677 (-HS) mCherry transfected B ~LL 0 LL Positive control Ci s C D Tranis Negative conitrol ~0 ~U) 0 W 13L Figure 4.5.7. ..... HSPGs interact in trans with pro-HB-EGF. . Confocal imaging of the localization of HB-EGF (green) at the junction of an mCherry (red) transfected cell and an AP-HBEGF-GFP transfected cell. (A) Positive control sample with AP-HBEGF-GFP and mCherry both in CHO-Ki (wild-type) cells had HB-EGF at cell-cell contact sites (white arrows). (B) Cis binding with AP-HBEGF-GFP in a CHOKl (wild-type) cells and mCherry in CHOpgsD-677 cells (- HS) showed little HB-EGF at cell-cell contact sites. (C) Trans binding with AP-HBEGF-GFP in CHOpgsD-677 (-HS) cells and mCherry in CHO-Kl (wild-type) cells showed HB-EGF present at the cell-cell junction (white arrows). (D) Negative control sample with AP-HBEGF-GFP and mCherry in CHOpgsD-677 (-HS) cells showed no concentration of HB-EGF at the cellcell junction. Scale bars are 20 pm. ............ .. II- - - - , : - - - - - - - - - - - I (A) (B) (C) Figure 4.5.8 ProHB-EGF is preferentially localized to cell-cell contact sites when neighbored by a non-expressing cell. Monolayers of COS-7 cells transfected with AP-HBEGF-GFP for 24 hours were Hoescht stained (blue), and HBEGF localization was assessed by imaging of the GFP-tagged cytoplasmic tail. (A) & (B) HB-EGF is strongly localized to sites of cell-cell contact when neighbored with a non-transfected cell. However, HB-EGF localization to cell-cell contact sites between two AP-HBEGF-GFP cells is much weaker. This is also true for cells during division, as (C) shows two AP-HBEGF-GFP cells in the late stages of mitosis. White arrows highlight cell-cell contact sites that are missing strong AP-HBEGF-GFP localization between two transfected cells. Images are 225 pLm wide. Extracellular AP Tag Biotinylation C-terminal Amphiregulin Tail mRA.AF rAR (:|I:P Phann Contrant (A) AP-AR-GFP (B) AP-AR-GFP + heparin (C) AP-HBDmut AR-GFP Figure 4.5.9 The heparin-binding domain of amphiregulin controls localization to cell-cell contact sites. After 24 hours of plasmid co-transfection with BirA-ER, the biotinylated, extracellular acceptor peptide in amphiregulin was labeled with monovalent streptavidin-alexa fluor 568 (red, left), and imaged along side the EGFP-tagged cytoplasmic tail of amphiregulin (green, middle), and phase contrast (right). (A) The extracellular and intracellular domains of AP-AR-GFP were localized to sites of cell-cell contact. (B) Addition of heparin (100ptg/mL) for four hours changed the localization of both the extracellular and intracellular domains of AP-AR-GFP to a diffuse distribution over the cell surface, rather than at cell-cell contact sites. (C) Mutation of all basic lysine and arginine residues in the heparin-binding domain of amphiregulin to neutral alanine led to a diffuse distribution of amphiregulin over the cell surface, not concentrated to sites of cell-cell contact. Each row represents the same field. Scale bars are 40 pm. ............ Extracellular AP Tag Biotinylation mSA-AF568 *vt'raiI'*r r-PP Phan& Contrat (A) AP-CFP-TM (B) AP-HBD CFP-TM Figure 4.5.10 Addition of a heparin-binding domain to an engineered transmembrane protein was insufficient for localization to cell-cell contact sites. After 24 hours of plasmid transfection, the biotinylated, extracellular acceptor peptide was labeled with monovalent streptavidin-alexa fluor 568 (red, left), and imaged alongside the extracellular CFP-tagged control construct (cyan, middle), and phase contrast (right). (A) The control construct AP-CFP-TM had a diffuse localization of the protein over the cell surface, and (B) the addition of a heparin-binding domain to the control construct (AP-HBD-CFP-TM) did not alter this localization. Each row represents the same field. Scale bars are 40 im. 79 Chapter Five: Role of the heparin-binding domain 5.1 Introduction Heparan sulfate proteoglycans (HSPGs), present on the cell surface and in the extracellular matrix, are capable of binding many growth factors. A traditionally proposed purpose of this interaction is to restrain a soluble ligand to the cell surface and increase the local concentration to activate a receptor (Schlessinger et al., 1995). Additionally, heparan sulfate binding can modulate the activity of signaling molecules or protect them from proteolytic degradation (Conrad, 1998). Binding to heparin or HSPGs is required for both amphiregulin and HB-EGF to activate EGFR in an autocrine/paracrine manner (Higashiyama et al., 1993; Johnson and Wong, 1994; Piepkom et al., 1994; Takazaki et al., 2004). Takazaki and colleagues have suggested that the three basic residues of the heparin-binding domain of HB-EGF that lie within a cysteine di-sulfide loop of the EGF-like domain repels the rest of the basic residues on the heparin-binding domain, which alters the conformation of the EGF-like region, making it unable to activate the receptor (Takazaki et al., 2004). However, when the heparin-binding domain is bound to heparan sulfate, the basic amino acids are neutralized, changing the conformation of the EGF-like domain and increasing its affinity for the EGFR. 5.2 Materials and Methods Western blotting Western blotting was with the following antibodies: anti-phospho-p42/44 MAPK (Cell Signaling Technology 9101S), anti-pY 148 Phospho-EGFR Antibody (Cell Signaling Technology 4404S), anti-EGFR (C74B9) (Cell Signaling Technology 2646S), anti-GFP (Abcam Ab 6556), anti-Actin (Sigma A5060), anti-GAPDH (Sigma G8795) and appropriate secondaries conjugated to horse radish peroxidase. Detection of biotinylated proteins was accomplished with streptavidin-horse radish peroxidase (Molecular Probes S911) with 3-5% BSA as the blocking and incubation buffer. Confocal imaging Confocal images were acquired with an Olympus FB1000 confocal microscope with a slice height of 0.3 pm using a 60x oil objective. Constructing AlkPhos-AP-HBEGF-GFP and AlkPhos-AP-113A-AlkPhos-HBEGFGFP A plasmid for human HB-EGF conjugated to human placental alkaline phosphatase (Raab et al., 1996) was obtained from Roselyn Adam at Children's Hospital that was originally produced in Michael Klagsbrun's lab at Children's Hospital, Boston. fusion protein is in the pRc/CMV vector (Invitrogen). The Human placental alkaline phosphatase was amplified from this vector with the primers Fwd Alk PhosF2 (5'-CCT GGC CAC CCC AAG CAA AGA AAG GAA T ATC ATC CCA GTT GAG GAG GAG AAC CCG GAC TTC TGG AAC CGC-3') and reverse primer Rev Alk Phos R2 (5'TTT CTG GGC TTC GAA GAT GTC GTT CAG GCC GTC GGT GGT GCC GGC GGG G -3'). The primers are designed to PCR human placental alkaline phosphatase out of the plasmid with overhangs that are complementary to AP-HBEGF-GFP before and after the N91 insertion site. Adding 10% DMSO and 3 mM total MgCl 2 to the reaction mixture increased the yield of the appropriate PCR product. The reaction was run on an agarose gel, and the band corresponding to alkaline phosphatase was cut out and purified with the Qiaquick gel purification kit (Qiagen). This PCR product then served as the primer for a quickchange mutagenesis reaction modified for large inserts on the APHBEGF-GFP and AP-1 13A-HBEGF-GFP plasmids. This purified primer was in the PCR reaction at a concentration of 130 ng/pL with 20 ng of the base plasmid, 3 mM total MgCl 2 , Pfu Ultra DNA polymerase, Pfu Ultra buffer, and dNTPs at standard concentrations recommended by the manufacturer. Eighteen cycles of PCR were performed at 95'C for 50 seconds, 60"C for 50 seconds, then 72'C for 14 minutes and then digested with DpnI for 2 hours and transformed into GC10 E. Coli (GeneChoice). Alkaline phosphatase release experiment COS-7 cells were plated on 96-well dishes (6,500 cells per well) for one day, then transfected with AlkPhos-AP-HBEGF-GFP or AlkPhos-AP-113A-HBEGF-GFP. The following day, samples were pretreated with batimastat (10 pM) in PBS+ for 1 hour, then stimulated with a 100 ptL solution of 100 pg/mL heparin in PBS+ supplemented with 1% BSA. Supernatants were collected at various time points, then washed for one minute with 100 ptL of 1.5 M NaCl in PBS+ supplemented with 1% BSA to remove any soluble HB-EGF bound to HSPGs. The salt wash was combined with the supernatant and 40 ptL was removed to a new plate and combined with 100 jiL of p-Nitrophenyl Phosphate (Millipore ES009), incubated for 2 hours at 37'C, then the optical density was read at 410 nm. Wound healing COS-7 cells were plated in 96-well dishes at 6,500 cells/well. The following day, the cells were serum starved and transfected with either AP-HBEGF-GFP, AP-1 13AHBEGF-GFP or GFP. Inhibitors were placed in serum free media and pre-incubated with the monolayer for one hour. The inhibitor solution was removed, wounds were immediately produced with a P200 pipette tip in PBS+, the PBS+ was removed and the inhibitor solution in serum free media was replaced. Images were taken immediately at 4x. Wounds were allowed to close for 24 hours at 374C, 5% CO 2 , and wound area images were taken again at this time. Wound area was measured in Image Pro (Version 4.5.0.29, Media Cybernetics) by highlighting the open wound area. Wound area closed was calculated by subtracting the wound area at the beginning of the experiment from the wound area measured at 24 hours. Monolayer migration speed measurements Epithelial and mesenchymal human mammary epithelial cells were each incubated with 8 ptM green-fluorescent CMFDA (Invitrogen) for 20 minutes and washed twice with PBS before trypsinizing. Labeled and unlabeled cells were mixed at a 1:20 ratio and seeded at 60,000 cells/cm 2 for epithelial cells and 30,000 cells/cm 2 for mesenchymal cells in 24well tissue culture plates. This procedure creates a homogeneous monolayer with only a fraction of the cells labeled to facilitate tracking of individual cells within the monolayer. Cells were incubated overnight (16-18 hours) and serum-starved for 24 hours before growth factor stimulation. Samples were imaged with a Nikon TE2000 microscope (Nikon Instruments; Melville, NY) equipped with a Solent environmental chamber (Solent Scientific; Segensworth, United Kingdom) at 37'C and 5% CO 2 . After 1 hour of stimulation with added factors, cells were imaged with a 4x DIC objective via brightfield and 488 nm mercury excitation over 18 hours with 30 minute time intervals. (Experimentsperformed by Hyung-Do Kim, Lauffenburger lab) (Method adaptedfrom Hyung-Do Kim's Ph.D. thesis, Quantitative Analysis of 2D and 3D Models for Epidermal Growth Factor Receptor-Dependent Cell Migration in the Context of the ExtracellularMicroenvironment, December 2008, MIT, Chapter3.2) 5.3 Results 5.3.1 The heparin-binding domain controls cell surface localization In Chapter 4, it was demonstrated that proHB-EGF interacts with HSPGs on neighboring cells. We hypothesized that this interaction may stabilize proHB-EGF on the cell surface and protect it from constitutive turnover, therefore increasing the cell surface fraction of the protein. To test this hypothesis, the amount of proHB-EGF expressed on the cell surface was evaluated in the different heparin-binding domain mutants. Cell surface HB-EGF was tagged by biotinylation of the extracellular fraction with exogenously added biotin ligase after heparin pretreatment for five minutes to make all cell surface HB-EGF equally accessible to biotin ligase. The cell surface fraction of HB-EGF was detected via western blotting with streptavidin-HRP and total HB-EGF was detected with a GFP antibody to the C-terminus. Mutation of basic arginine and lysine residues to neutral alanine in the heparin binding domain led to less cell surface proHBEGF (Figure 5.5.1 A). The amount of HB-EGF on the cell surface appears to scale with the number of basic residues mutated. The mutants with fewer lysines and arginines mutated had less on the surface, which suggests that heparin-binding affects cell surface levels of HB-EGF. In Chapter 4, it was also demonstrated that the addition of the heparin-binding domain of HB-EGF to the control construct AP-CFP-TM was not sufficient to localize it to sites of cell-cell contact. However, the addition of the heparin-binding domain did increase the cell surface fraction of the protein dramatically (Figure 5.5.1 B). COS-7 cells transfected with either AP-CFP-TM or AP-HBD-CFP-TM for 24 hours were biotinylated with biotin ligase, then lysed. The cell surface fraction of the protein is shown with streptavidin-HRP blotting and the total protein with an anti-GFP antibody, which recognizes CFP. The cell surface fraction of the protein is represented by one band, and the addition of the heparin-binding domain and linker led to an appropriate increased in size. The GFP blot for total AP-CFP-TM shows multiple size isoforms of both AP-CFP-TM and AP-HBD-CFP-TM, which may be degradation products. This experiment suggests that the interaction of the heparin-binding domain with HSPGs increases the cell surface fraction of the protein. To further investigate this hypothesis, the cell surface fraction of AP-HBEGFGFP and the heparin-binding domain mutant (AP- 113A-HBEGF-GFP) was evaluated in CHO-KI versus CHOpgdD-677 cells, which lack heparan sulfate. Surprisingly, the amount of cell surface HB-EGF was equal in CHO-Ki and CHO-pgsD677 cells, and a reduced amount of AP-1 13A-HBEGF-GFP mutant was observed on the cell surface of both cells lines (Figure 5.5.1 C). This finding is not supportive of the hypothesis that the interaction of the heparin-binding domain with HSPGs increases the cell surface fraction of transmembrane proteins. This experiment suggests that the larger amount of AP- HBEGF-GFP on the cell surface compared to AP-l13A-HBEGF-GFP is independent of heparan sulfate, as both CHO-KI and CHOpgsD-677 cells without heparan sulfate have identical cell surface expression. In both COS-7, CHO-Ki and CHOpgsD-677 cells, the distribution of HB-EGF between the different size isoforms is altered when comparing the AP-HBEGF-GFP and the AP- 113A-HBEGF-GFP mutant, as demonstrated with the GFP blot for total HB-EGF (Figure 5.5.1 A,C). Wild-type AP-HBEGF-GFP is primarily expressed at the 39 kDa form in all three cells types. However, the heparin-binding domain mutant AP-1 13AHBEGF-GFP is expressed in the larger 51 kDa form. In the CHO cells, the larger 51 kDa is not presented on the cell surface. Therefore, this larger form which accumulates in the AP-1 13A-HBEGF-GFP mutant may represent altered N-terminal processing. As less of the AP-l 13A-HBEGF-GFP mutant is presented on the cell surface compared to wild-type AP-HBEGF-GFP, the localization of the remaining AP- 13AHBEGF-GFP was investigated. In order to address this, confocal imaging was employed to determine the intracellular localization of the protein by imaging one optical slice through a cell. COS-7 cells were cultured in confluent monolayers and transfected with either AP-HBEGF-GFP or AP-1 13A-HBEGF-GFP with BirA-ER for one day with biotin supplemented in the media. Cells were washed, and the extracellular biotinylated acceptor peptide was labeled with mSA-AF568. The cells were then fixed and samples were stained with Hoescht to determine localization of the nucleus. Confocal imaging (Figure 5.5.2) shows that wild-type AP-HBEGF-GFP had little intracellular signal, with the majority of the extracellular and intracellular C-terminal tail co-localized in the plasma membrane (Figure 5.5.2 A). However, the AP-1 13A-HBEGF-GFP mutant had a dramatically different intracellular distribution of the GFP C-terminal tail (Figure 5.5.2 B). The majority of the C-terminal tail was in intracellular structures that resemble the rough endoplasmic reticulum. The GFP C-terminal tail was additionally localized to the nuclear envelope. Only a fraction of the total AP-1 13A-HBEGF-GFP was in the plasma membrane, labeled by mSA-AF568 (red). However, less AF568 is also expected in the plasma membrane as it is known to be distributed over the entire cell surface, while the AP-HBEGF-GFP construct is concentrated at cell-cell contact sites. The heparin-binding domain controls the amount of HB-EGF and AP-CFP-TM on the cell surface, however it is not due to an interaction with HSPGs. Therefore, it is possible that the heparin-binding domain interacts with a second partner, or that the intracellular accumulation and/or differential N-terminal processing of AP-l 13AHBEGF-GFP alters the steady state cell surface expression. The second extracellular loop of CD9 has been shown to interact with a peptide corresponding to the heparinbinding domain of HB-EGF (Sakuma et al., 1997), which may control cell surface expression. 5.3.2 ProHB-EGF does not cluster EGFR As a trans interaction between HSPGs and proHB-EGF clusters HB-EGF to cellcell contact sites, the question arose of whether a trans reaction between proHB-EGF and EGFR could cluster EGFR at cell-cell contact sites. Two populations of COS-7 cells were co-cultured together after transfection with AP-HBEGF-GFP alone or a construct for acceptor peptide tagged EGFR (AP-EGFR) and BirA-ER. Without co-transfection of BirA-ER with AP-HBEGF-GFP, only AP-EGFR is biotinylated and visualized with mSA-AF568, and AP-HBEGF-GFP is visualized with the C-terminal GFP tag only. APEGFR was localized homogenously over the cell surface, with no preferential localization to sites of cell-cell contact. AP-EGFR cells in contact with AP-HBEGF-GFP expressing cells showed no change in localization of either construct or any significant enrichment of EGFRs at sites of cell-cell contact (Figure 5.5.3 A). This suggests that proHB-EGF does not cluster EGFRs at cell-cell contact sites. As HSPGs cluster HB-EGF at cell-cell contact sites, the role of this cluster may be to activate the EGFR in a trans manner. HB-EGF is known to signal with EGFR and ErbB4 in a juxtacrine manner. As the addition of heparin or mutation of the heparinbinding domain diminishes the clustering of HB-EGF at cell-cell contact sites, this localization change may decrease juxtacrine activation of the EGFR as less is available at cell-cell contact sites for activation of the receptor. Therefore, we hypothesized that the localization change induced by mutation of the heparin-binding domain may decrease juxtacrine signaling and lead to a drop in EGFR phosphorylation. However, no change in EGFR phosphorylation was observed after transfection of AP-HBEGF-GFP or AP-l 13A- HBEGF-GFP compared to GFP transfected COS-7 cells (Figure 5.5.3 B). To reduce noise from autocrine signaling, samples were treated with batimastat (10 [M) for five hours before lysis. Transfection with AP-HBEGF-GFP or AP-113A-HBEGF-GFP did not affect ERK or EGFR activation, nor led to downregulation of the EGFR. Reducing autocrine signaling with batimastat reduced phospho-EGFR and phospho-ERK, however there was still no change across different transfection conditions. As an endogenous EGFR mediated autocrine signaling loop is present in COS-7 cells (Kain and Klemke, 2001), we hypothesize that transfection with HB-EGF does not additionally contribute to EGFR signaling. In order to test the role of heparin on HB-EGF signaling, confluent monolayers of COS-7 cells expressing AP-HBEGF-GFP, AP-113A-HBEGF-GFP or GFP and serum starved for 24 hours were treated with heparin (100 tg/mL) for various amounts of time, then lysed. The addition of heparin led to a dramatic decrease in phospho-ERK levels after only five minutes (Figure 5.5.4). It was originally hypothesized that this drop in ERK signaling was due to an immediate decrease in juxtacrine signaling after the addition of heparin as proHB-EGF is moved away from sites of cell-cell contact. However, pre-treatment of the cells with AG1478, GM6001, or batimastat (data not shown) led to a dramatic reduction in phospho-ERK levels at all time points, suggesting that baseline ERK activation and the heparin-induced drop was due to EGFR autocrine signaling rather than juxtacrine activation. Additionally, the drop in ERK activation was observed in GFP transfected cells (data not shown), and AP-1 13A-HBEGF-GFP transfected cells; however, the heparin-induced decrease for AP- 113A-HBEGFP-GFP was time delayed compared to AP-HBEGF-GFP (Figure 5.5.4). This suggests that the drop in phosho-ERK levels was due to an endogenous ligand or signal, rather than the transfected HB-EGF construct. A similar heparin-induced drop in phospho-ERK was observed in transfected and non-transfected HeLa cells (data not shown). Total ERK levels remained constant after heparin addition in COS-7 and HeLa cells (data not shown). 5.3.3 The heparin-binding domain controls ectodomain shedding As heparin dramatically changed the localization of proHB-EGF from sites of cell-cell contact to a homogeneous distribution over the cell surface, we hypothesized that this localization change may increase access to proteases and affect ligand cleavage. To assess release of HB-EGF into the media, human placental alkaline phosphatase was inserted into the extracellular domain of AP-HBEGF-GFP and AP-1 13A-HBEGF-GFP near the N-terminus. This allows for sensitive detection of HB-EGF release by assaying for alkaline phosphatase activity in the media of transfected cells. As HB-EGF release is typically low in non-stimulated conditions, and the basic residues in the heparin-binding domain likely make the protein non-specifically sticky, ELISA measurements of HBEGF concentration in the media is challenging due to the need for sample concentration and significant sample loss. The addition of heparin (100 [ig/mL) to confluent monolayers of COS-7 cells transfected with wild-type HB-EGF (AlkPhos-AP-HBEGFGFP) increased alkaline phosphatase activity in the media (Figure 5.5.5 A), suggesting that the heparin-induced localization change of proHB-EGF away from cell-cell contact sites upregulates ligand cleavage. Treatment with the protease inhibitor batimastat (lOpM) inhibited heparin-induced cleavage of both wild-type and mutant HB-EGF. Interestingly, the heparin-binding domain mutant alkaline phosphatase fusion (AlkPhosAP- 113A-HBEGF-GFP) had higher levels of cleavage compared to wild-type HB-EGF and was unaffected further by the addition of heparin. These data suggest that the trans interaction of pro-HB-EGF with HSPGs at cell-cell contact sites prevents proteolytic release of the ligand. However, the interaction of HB-EGF with CD9, which also involves the heparin-binding domain (Sakuma et al., 1997), may serve to inhibit proteolytic ligand release, as we found a similar increase in alkaline phosphatase activity in the medium for AlkPhos-AP- 113A-HBEGF-GFP when using the HSPG-lacking CHOpgsD-677 cells (Figure 5.5.5 B). Since the CD9 interaction appears to operate in cis (Sakuma et al., 1997), and therefore ought not to depend on HB-EGF localization to cellcell contact regions, the two heparin-binding domain interactions may work in series to provide a multi-layer control on ligand release. 5.3.4 Wound healing and migration As HB-EGF is released upon the addition of heparin, we sought to explore activation of EGFR pathways and its role on migration. As heparin increases proHBEGF cleavage, we hypothesized that HB-EGF transfection and heparin stimulation may increase migration and wound healing by inducing autocrine HB-EGF signaling. Wound healing assays in COS-7 cells transiently expressing AP-HBEGF-GFP and AP-1 13AHBEGF-GFP showed no change in wound healing compared to the GFP transfected control (Figure 5.5.6 A). However, treatment of the samples with batimastat reduced wound closure, suggesting that cleavage of an endogenous cell surface ligand is driving wound closure. In order to investigate the driving forces of endogenous wound closure in COS-7 cells in more detail, an investigation of the EGFR pathway in COS-7 cells shows that preincubation with the tyrosine kinase inhibitor AG1478 for one hour, and continued inhibition during the wound healing experiment significantly reduced wound closure (Figure 5.5.6 B). Additionally, inhibition of MEKI with the inhibitor PD98059 or protease cleavage with the inhibitor GM6001 decreased wound closure rates, but not to the same degree as AG1478. It should be noted, however, that the addition of rhHBEGF (100 ng/mL) did not increase the rate of wound closure, nor did an HB-EGF blocking antibody significantly decrease wound closure rates. These data suggest that wound healing is mediated through the EGFR system in COS-7 cells, however, endogenous signaling in this system is already saturated. Therefore the addition of rhHB-EGF, or transfection with HB-EGF has no additive response. This hypothesis is supported by the fact that AP-HBEGF-GFP transfection shows no increase in phospho-EGFR levels, the heparin induced drop in phospho-ERK is not affected by transfection either, and a background signaling loop has been previously observed by others in COS-7 cells (Kain and Klemke, 2001). Heparin may increase rates of proHB-EGF cleavage, however, heparin has an inhibitory effect on wound closure in a dose dependent manner, with inhibition observed at heparin concentrations of 10-100 ptg/mL (Figure 5.5.6 C). To investigate the role of heparin on migration in further detail, studies were completed in human mammary epithelial cells (HMECs). Two different HMEC cell lines were used. The first (epithelial) were derived from a reduction mammoplasty tissue sample (Elenbaas et al., 2001). The second cell line (mesenchymal) was derived from the first, as transformation with three genes (SV40 large-T antigen, the telomerase catalytic subunit, and an H-Ras oncoprotein) caused the cells to form tumors when injected into mice (Elenbaas et al., 2001). The tumors formed were poorly differentiated carcinomas that lacked the estrogen receptor, and therefore may serve as a model for estrogen receptor-negative breast cancers (Elenbaas et al., 2001). Individual COS-7 cells are not migratory when cultured in confluent monolayers with cell-cell contact, however wound healing does stimulate COS-7 migration. However, wild-types HMECs are very dynamic and migrate even in a confluent monolayer. This model system allows for analyzing single cell migration behavior, with the role of juxtacrine signaling, as the cells can remain in contact. Individual cell migration of HMECs in a monolayer is measured by labeling a subpopulation of the cells (10%) with a cell tracker fluorophore, then the coordinates of the labeled cells are tracked over time. The migration speed of epithelial, but not mesenchymal HMECs was increased significantly by the addition of 100 ng/mL of EGF or HB-EGF, indicating EGFR signaling drives migration in the non-transformed epithelial cell line (Figure 5.5.7). HMECs have been shown to produce amphiregulin at high levels, as well as transforming growth factor-a, epiregulin, and HB-EGF (Dong et al., 1999; Rodland et al., 2008). As amphiregulin and HB-EGF are both heparin-binding proteins, whose localization is changed upon the addition of heparin, we sought to test the effect of heparin (100 ptg/mL), heparan sulfate (100 pg/mL) and heparinase III (1.6 mU/mL) on migration. Similar to COS-7 cell wound healing, heparin inhibited the migration speed of epithelial-like HMECs, but not mesenchymal-like (Figure 5.5.7). Heparinase III treatment was also inhibitory, as heparan sulfate chains produced by heparinase likely act in the same manner as endogenously added heparan sulfate, however the change was not as strong. Together, the COS-7 cell wound healing results taken with the HMEC migration speed results indicate the heparin inhibits cell migration. In summary, heparin may increase rates of cleavage, however, it does not lead to phenotypic changes associated with increased autocrine signaling, such as enhanced migration or EGFR activation. We hypothesize that heparin may disrupt extracellular localized autocrine signaling loops of heparin-binding growth factors. This disruption may lead to an overall decrease in receptor activation in various pathways which affect migration or disrupt the chemotactic gradient produced by ligand cleavage, binding and diffusion. 5.4 Discussion Here we show that mutation of the heparin-binding domain or the addition of heparin upregulates proteolysis of HB-EGF (Figure 5.5.5 A). It is conceivable that heparin and heparan sulfate also dissociate proHB-EGF from HSPGs and thus cell-cell contacts sites and cause ligand cleavage in vivo. Heparin and low-molecular weight heparin are administered via intravenous infusion for their anti-coagulant properties in the clinic for prevention and treatment of thromboembolic disorders. Intravenous injection of heparin likely does not affect HB-EGF, as it stays within the bloodstream, and HB-EGF does not appear to be expressed by endothelial cells (Nakata et al., 1996). However, heparin is cleared by the kidney's renal tubular cells (Young et al., 2004), which may interact with basolateral HB-EGF expressed in collecting tubules and change the basolateral localization of the growth factor to a homogenous distribution over the cell surface. Additionally, free heparin is secreted by mast cells upon degranulation. Mast cells and HB-EGF signaling are both implicated in the biological processes of wound healing (reviewed in (Noli and Miolo, 2001) (Tokumaru et al., 2000), angiogenesis (reviewed in (Galinsky and Nechushtan, 2008) (Ongusaha et al., 2004), and the pathogenesis of atherosclerosis (reviewed in (Kalesnikoff and Galli, 2008) (Nakata et al., 1996). Additionally, dermal mast cells themselves express HB-EGF mRNA (Artuc et al., 2002). Aside from the pro-angiogenic effects of mast cells in tumor angiogenesis, separate studies show mast cell heparin to inhibit tumor growth (reviewed in (Galinsky and Nechushtan, 2008)). Extracellular free heparan sulfate is generated by degradation of cell surface HSPGs with heparanase, whose expression is upregulated in all analyzed human cancers (Vlodavsky et al., 2007). It is possible that heparanase leads to a local concentration of free heparan sulfate high enough in the interstitial space to dissociate proHB-EGF from cell-cell contact sites and stimulate cleavage of proHB-EGF. Heparin and mutation of the heparin-binding domain of HB-EGF led to increased rates of proHB-EGF proteolysis and accumulation in the media. We hypothesize that clustering of HB-EGF at cell-cell contact sites via a trans interaction with HSPGs may prevent proteolysis of the ligand by restricting access to the transmembrane ADAMs. Therefore, upon the addition of heparin or mutation of the heparin-binding domain, HBEGF is dissociated from cell-cell contact sites and access to ADAMs is increased, resulting in increased rates of cleavage. Interestingly, it has recently been reported that heparan sulfate interacts with ADAM12, which controls ADAM12 sheddase activity by serving as a molecular switch (Sorensen et al., 2008). Additionally, removal of cell surface heparan sulfate has been reported to increase TACE activity and ErbB4 cleavage (Maatta et al., 2009). Therefore, in our experiments, stimulation with heparin may also increase ADAM activity; however, this is not expected from mutation of the heparinbinding domain. Therefore, despite the role heparan sulfate may play on ADAM activation, the heparin-binding domain of HB-EGF also controls proteolysis. To our surprise, despite the upregulation in release of HB-EGF upon the addition of heparin, heparin had an inhibitory effect on cell migration in COS-7 wound healing (Figure 5.5.6 B) and individual cell migration of confluent monolayers of epithelial HMECs (Figure 5.5.7). Heparin also dramatically reduced ERK phosphorylation immediately after its addition (Figure 5.5.4), which may play a role in inhibiting migration, however ERK levels return to baseline after a few hours and cell migration continued to be inhibited for experimental lengths of 12-24 hours. Interestingly, the mesenchymal transformed HMEC cell line, which is more tumorigenic than the epithelial HMECs, migrated at lower speeds and did not respond to heparin addition, or even stimulation with EGF or HB-EGF (Figure 5.5.7). Therefore, the addition of heparin, heparan sulfate, or degradation of HSPGs with heparinase made the epithelial HMECs act more like the tumorigenic mesenchymal HMECs. We hypothesize that HB-EGF may play a crucial role in maintaining cell polarity, as it is primarily localized at cell-cell contact sites between non-homogenous cell-cell contact sites (Figure 4.5.8) and proHBEGF juxtacrine signaling can be growth inhibitory (Iwamoto et al., 1999). The loss of cell polarity of luminal HMECs has been suggested to play a key role in the morphogenesis to a cancerous phenotype (Zhan et al., 2008). Therefore, we hypothesize that a localization change of proHB-EGF and/or pro-amphiregulin due to degradation of HSPGs by heparanase may contribute to a loss of polarization in luminal HMECs and contribute to cancer morphogenesis. 5.5 Figures 4: 4', / * Ilk PC 4', (A) $ Cell Surface HB-EGF - 51kDa - 39kDa Streptavidin-HRP Total HB-EGF Anti-GFP - 'fi Cell Surface Fraction 39kDa I CHO-KI St eptavidin-HRP 39kDa- Cell Surface HB-EGF Streptavidin-HRP - CHO-677 I - SikD* - 51kDa otal Construct Anti-GFP Total HB-EGF Anti-GFP Anti-Actin Figure 5.5.1 Mutation on the heparin-binding domain decreases the cell surface fraction of HB-EGF. (A) Cell surface expression of AP-HBEGF-GFP and three of the heparin-binding domain mutants in order of increasing number of lysine and arginine mutations show decreasing cell surface expression with increasing mutation of lysine and arginine. Confluent monolayers of COS-7 cells transfected for 24 hours were washed, heparin treated (100 ptg/mL, 5 minutes), then cell surface biotinylated with biotin ligase on ice. Cells were washed, then lysed and run on SDS PAGE. Blotting with streptavidin-HRP shows the cell surface expression of each protein, and the total protein is shown with blotting to the GFP C-terminal tail. (B) Addition of the heparin-binding domain (AP-HBD-CFP-TM) to the control construct AP-CFP-TM increased the fraction of the protein on the cell surface. GFP shows the total amount of the construct, as it also recognizes CFP. Actin served as a loading control. (C) Confluent monolayers of CHOK1 and CHOpgsD-677 (CHO-677) cells transfected for 24 hours with AP-HBEGF-GFP or the heparin-binding domain mutant AP-113A-HBEGF-GFP show decreased expression of the mutant on the cell surface of both the CHO-Ki and CHOpgsD-677 cells. Total HB-EGF is probed with an antibody to GFP. .......... .- ........................ ....................... .... .. .................... .. .... .... .... ............. ........ .......... Extracellular HB-EGF AP Tag Blotinylation (mSA-AF568) C-Terminal HB-EGF Tail EGFP Merge (A) AP HBEGF-GFP (B) AP-113A HBEGF-GFP Figure 5.5.2 The heparin-binding domain mutant is primarily localized in the intracellular space. Confluent monolayers of COS-7 cells were transfected with AP-HBEGF-GFP (A), or the heparin-binding domain mutant AP-1 13A-HBEGFGFP (B) along with BirA-ER and biotin supplemented in the media. After 24 hours, cells were washed and the extracellular biotinylated acceptor peptide was labeled with mSAAF468 (red, left). Cells were washed, then fixed with 4% paraformaldehyde and nuclei were stained with Hoescht (blue). The AP-1 13A-HBEGF-GFP has increased GFP Cterminal tail (green, middle) localization in the intracellular space compared to APHBEGF-GFP. The merged imaged shows co-localization of the extracellular biotinylated AP tag and the GFP C-terminal tail (yellow, merged, right) in the plasma membrane. Scale bars are 20 pm. C-Terminal HB-EGF Tail EGFP Extracellular EGFR AP Tag Biotinylation (mSA-AF568) Overlay Batimastat Pre-treatd HB 113A GFP HB 113A GFP Total EGFR ~m 7 pY1148 Phospho-EGFR - Phospho-ERK Figure 5.5.3 EGFR localization and activation. (A) COS-7 cells transfected with AP-EGFR/BirA-ER and AP-HBEGF-GFP were co-cultured in confluent monolayers. After 24 hours, the biotinylated AP-EGFR was visualized with mSA-AF568 and the non-biotinylated AP-HBEGF-GFP was visualized via the GFP-tagged cytoplasmic tail. Junctions between a HB-EGF expressing cell and an EGFR expressing cell show no concentration of EGFR at cell-cell contact sites, as is seen around the rest of the cell periphery. Scale bars are 20 ptm. (B) COS-7 cells transfected with AP-HBEGFGFP, AP-l 13A-HBEGF-GFP, or GFP and serum starved for 24 hours were western blotted for total EGFR, phospho-tyrosine 1148 EGFR, and phospho-ERK. Batimastat (10 pM) treatment was for five hours before lysis. 0 15 30 45 60 90 120 180 240 Time (minutes) AP-HBEGF-GFP Phospho-ERK AP-113A-HBEGF-GFP Phospho-ERK AP-HBEGF-GFP GAPDH AP-113A-HBEGF-GFP GAPDH Figure 5.5.4 Heparin reduces ERK activation. Confluent monolayers of COS7 cells were transfected with AP-HBEGF-GFP or AP- 13A-HBEGF-GFP and serum starved for 24 hours. Cells were stimulated with heparin (100 pg/mL) for various times and lysed. Heparin led to a drop in phospho-ERK activation at 30 minutes, which rose back to baseline levels by 3-4 hours in AP-HBEGF-GFP transfected cells. However, cells transfected with AP- 113A-HBEGF-GFP showed a similar, but time delayed decrease in phosho-ERK. GAPDH is a loading control. Alkaline phosphatase-AP-HBEGF-GFP release 0.5- 0.4. E c 0.3- -- WT HB-EGF -a- HBD mutant HB-EGF - e--- WT - 0 0.2- HB-EGF (+heparin) HBD mutant HB-EGF (+heparin) a WT HB-EGF (+BATI +heparin) o HBD mutant HB-EGF (+BATI +heparin) 0.1! 00 30 60 90 120 150 180 210 240 Time (minutes) Alkaline Phosphatase-AP-HBEGF-GFP Release in CHOpgsD-677 cells -.- WT HB-EGF -o- HBD Mutant HB-EGF Time (minutes) Figure 5.5.5 Interaction with HSPGs reduces proHB-EGF cleavage. COS-7 cells (A) or CHO-pgsDO677 cells (B) transfected with wild-type HB-EGF (AlkPhos-AP-HBEGF-GFP) (e) or the heparin-binding domain mutant (AlkPhos-AP113A-HBEGF-GFP) (o) were either pre-treated with 10 pM batimastat (BATI) or PBS+ alone for 1 hour. Cells were stimulated with either heparin (100 tg/mL) alone (black dashed line), or heparin and batimastat (gray dashed line), or unstimulated (black solid line) and media was collected at various time points, then cells were incubated with a 1.5 M salt solution for 60 seconds to release any soluble HB-EGF bound to HSPGs. AlkPhos-AP-HBEGF-GFP release is increased by treatment with heparin, and cleavage is further increased by mutating the heparin-binding domain (AlkPhos-AP-l 13A-HBEGFGFP). Pretreatment with batimastat before the addition of heparin blocks both AlkPhosAP-HBEGF-GFP and AlkPhos-AP- 13A-HBEGF-GFP cleavage. Data shown is average and standard deviation of three biological replicates from one of three independent experiments. (A) 1000000 ~ MAP-HBEGF-GFP 900000 - 60000 MAP-113A-HBEGF-GFP GFP t B 800000 - 0i41 Wound Closure After Twenty-Four Hours Wound Closure After Twenty-Four Hours E2 700000 40000 3 600000 MGFP 20000 0 - 500000 400000 S20000(0 - 300000- C 10000 0 - 200000 100000 0- Control Batimastat Wound Area Closed After Twenty-Four Hours 700000600000 500000- * AP-HBEGF-GFP 400000- * AP-1 13A-HBEGF-GFP 300000- * GFP 2000001000000- ,, 100 I 10 I 1 0.1 0.01 1 0.001 j 0 Heparin (pg/mL) Figure 5.5.6 Wound healing in COS-7 cells. Confluent monolayers of COS-7 cells were transfected with the plasmids indicated for 24 hours and serum starved. Wounds were made with pipette tip in 96 well dishes (n=8), wound area was measured, then the wounds were allowed to close for 24 hours, and wound area was measured again. Wound area closed was calculated by subtracting the wound area produced at the beginning of the experiment, and subtracting the wound area measured at 24 hours. (A) AP-HBEGF-GFP transfection does not change the wound closure rate compared to the GFP transfected control. Batimastat (10 pM) pre-treatment for one hour prior to induction of the wound and treatment throughout the experiment decreased wound closure rates independent of the transfection condition. (B) GFP transfected COS-7 cells were pre-treated with AG1478 (10 pM), PD98059 (25 pM), HB-EGF blocking antibody (10 pg/mL), GM6001 (10 pM), and recombinant human HB-EGF (rhHBEGF) (100 ng/mL) for one hour prior to induction of the wound and continuous treatment throughout the experiment. AG1478 drastically reduced wound closure rates; however, stimulation of wound healing with rhHBEGF had no effect. (C) Treatment of transfected COS-7 cells with heparin throughout the wound healing experiment reduced wound closure at high heparin concentrations (100 pg/mL) independent of the transfection condition. Data shown is average and standard deviation of eight biological replicates. Human Mammary Epithelial Cell Migration Speed 30 0 Control (Serum Free) U EGF N HB-EGF 25 20 Heparinase III 0 Heparin _ E D:Heparan Sulfate 15 a, U) 10 0~ 0 Epithelial Mesenchymal Figure 5.5.7 Migration speed of individual HMECs within a confluent monolayer. Confluent monolayers of epithelial and mesenchymal human mammary epithelial cells were seeded with 5% of the cells labeled with green fluorescent CMFDA. The coordinates of fluorescently labeled cells were tracked with timelapse microscopy over 18 hours with data points acquired every 30 minutes and migration speed was calculated based on the distance each individual cell traveled per time. Stimulation with recombinant EGF (100 ng/mL) and HB-EGF (100 ng/mL) led to a large increase in migration speed in epithelial, but not mesenchymal HMECs. However, the addition of heparin (100 [tg/mL), heparan sulfate (100 [tg/mL), or heparinase III digestion (1.6 mU/mL) of cell surface HSPGs led to a reduction in migration speed in epithelial, but not mesenchymal HMECs. This experiment was executed and analyzed by Hyung-Do Kim (LauffenburgerLab, MIT) 100 Chapter Six: Conclusions and future directions In summary, we have developed a novel strategy for two-color labeling and tracking of murine heparin-binding epidermal growth factor in living cells, with the ability to distinguish the C-terminal tail from the extracellular EGF-like domain. Using this construct, we discovered that a trans interaction between proHB-EGF and HSPGs is responsible for localization of proHB-EGF to sites of cell-cell contact. Additionally, the heparin-binding domain increases the cell surface fraction of transmembrane proteins and prevents proteolytic processing of proHB-EGF. As proHB-EGF signaling in juxtacrine mode can cause growth inhibition, and autocrine signaling leads to cell proliferation and migration, the role of HSPGs in regulating this balance likely plays a crucial role in the resulting cell fate. This balance may be upset in tumors, as enzymes that alter the number of extracellular heparan sulfate chains on proteoglycans have been associated with cancer, such as heparanase and HSulfl. 6.1 Future directions: Use of AP-tagged EGFR ligands Acceptor peptide tagging and biotinylation with biotin ligase proved to be very specific labeling method for ligands in the EGFR family. Additionally, this technique allowed us to distinguish the cell surface fraction from the total pool of HB-EGF. One powerful use for the AP-HBEGF-GFP construct that was not adequately explored in this thesis would be for ratiometric studies of fluorescence of the extracellular domain compared to the C-terminal tail of the ligand in individual cells, allowing one to study 101 ligand cleavage. The cell surface levels of proHB-EGF could be detected with the acceptor peptide tag, and compared to the fluorescence intensity of the GFP C-terminal tail via flow cytometry to allow for individual cell measurements of proteolysis. In addition to HB-EGF, we have also produced identical constructs for amphiregulin and transforming growth factor-a. Another possible avenue of investigation with AP-tagged EGFR ligand is measurements of internalization and recycling. This avenue has been explored in depth with the EGF receptor, as receptor downregulation and recycling has important consequences on EGFR signaling, however very little information exists on this dynamic for the ligand. The steady state internalization and recycling of the pro-form of EGFR ligands likely plays a role in juxtacrine EGFR activation and the conversion to the soluble form for autocrine signaling. Endocytosis of wild-type versus the heparin-binding domain mutant would be interesting to compare. The acceptor peptide tag inserted into the extracellular domain of EGFR ligands could be utilized for these measurements. To accomplish this, the cell surface pool of the protein is biotinylated, then at different times monovalent streptavidin is added to quench the cell surface pool and the cells are immediately lysed. As the biotin-streptavidin bond is extremely strong, under the right lysis and gel electrophoresis conditions, the bond can remain intact. Therefore, after gel electrophoresis, the biotinylated protein that was internalized can be detected by western blotting and probing the gel with streptavidin-HRP. A similar strategy could be used to measure recycling of the protein back to the cell surface. The cell surface pool of the AP-tagged EGFR ligand would be biotinylated with exogenously added biotin ligase and allowed to internalize for a sufficient amount of time. Then all samples in the timecourse would have the cell surface pool of biotinylated protein quenched with monovalent streptavidin (un-labeled). After this, at various timepoints, the internalized biotinylated non-quenched ligand that returned to the cell surface would be detected with monovalent-streptavidin-AF568 (or perhaps a fusion of monovalent streptavidin with alkaline phosphatase or horseradish peroxidase) over time. These methods could allow for quantification and comparison of endocytosis and recycling of EGFR ligands. 102 6.2 Future directions: Signaling with heparin-binding domain mutant HB-EGF COS-7 cells were an excellent experimental system for evaluating the localization of HB-EGF to cell-cell contact sites as they are easily transfected, large cells that localize HB-EGF to large planes of cell-cell contact. However, unfortunately, COS-7 cells were not properly suited for the study of HB-EGF signaling via the EGFR or subsequent downstream effects, such as cell migration, proliferation, or growth inhibition. Therefore, studies on the effect of mutation of the heparin-binding domain of proHB-EGF would be an interesting avenue of pursuit in a proper model system that responds to HB-EGF autocrine signaling through growth and migration, and responds to HB-EGF juxtacrine signaling via growth inhibition or apoptosis. Using this new model system, one could determine whether the localization change induced by heparin, heparan sulfate, or heparinase digestion of HSPGs could lead to less juxtacrine signaling, and higher levels of autocrine signaling. Additionally, we hypothesize that proHB-EGF juxtacrine signaling may serve as a contact inhibition signal between two different tissue types and provide a barrier for cell growth of one tissue into another. As the heparin-binding domain mutant leads to loss of localization at cell-cell contact sites, and therefore would likely lead to loss of tissue polarization, it would be interesting to evaluate the outcome of expression of the heparin-binding domain mutant in vivo. This could be achieved by making a knock-in of the heparin-binding domain mutant HB-EGF in mice. Additionally, injection of tumor cells expressing wild-type versus the heparin-binding domain mutant and evaluation of tumor formation would be an interesting study. 6.3 Future directions: HB-EGF localization change in vivo. As discussed in section 5.4, proHB-EGF may undergo a change in localization as seen with the addition of heparin in vivo. This could be stimulated by heparin administered intravenously as an anti-coagulant, heparin release by degranulating mast cells, loss of heparan sulfate from cell surface proteoglycans by heparanase or Hsulfl action, or by heparan sulfate chains generated by degradation of HSPGs with these enzymes. Therefore it would be interesting to determine if any of these mechanisms do indeed change the localization HB-EGF in vivo, and if the localization changed led to any differences in tissue phenotype. 103 6.4 Future directions: Structure of the HSPG-proHBEGF-CD9 complex ProHB-EGF interacts with both HSPGs and CD9. A peptide corresponding to the second extracellular loop of CD9 binds to a peptide consisting of the heparin-binding domain of HB-EGF with a Kd of 37 pM (Sakuma et al., 1997). However, the Kd for binding of heparin to the heparin-binding domain of HB-EGF is much lower (~28 nM) (Sakuma et al., 1997). Interestingly, ideas in the literature have been presented the large second extracellular loop of CD9 might have a structure, including disulfide bridges, and that this region may control tetraspanin-tetraspanin homo and heterodimerization (Zoller, 2009). Therefore, despite the high Kd of a CD9 peptide binding to the heparin-binding domain, the Kd of the fully formed, folded protein may be lower. The EGF-like domain has also been demonstrated to interact with CD9 and play a crucial role in upregulation of juxtacrine activity (Nakamura et al., 2000) and the membrane-anchoring domain of proHB-EGF is also required for juxtacrine signaling (Dong et al., 2005). Therefore, the proHB-EGF-CD9 interaction may span many domains of proHB-EGF. As HB-EGF, CD9, and HSPGs all cluster at cell-cell contact sites (Nakamura et al., 2001), we suggest that multi-protein complex may be required to regulate juxtacrine activity. Ternary complexes with HSPGs have been discovered before, as fibroblast growth factor (FGF) requires HSPG interaction along with the FGF receptor to form an active signaling complex (reviewed in (Harmer, 2006)). 6.5 Future Directions: Computational analysis To better understand the role of HSPGs in juxtacrine and autocrine/paracrine signaling via the EGFR and ErbB4, computational modeling could provide useful insight in this network. We hypothesize here that proHB-EGF is primarily localized to cell-cell contact sites between an HB-EGF expressing cell and a non-producing cell because competition for binding between two cells expressing proHB-EGF reduces the amount accumulated at cell-cell contact sites. Computational modeling could be employed to determine if this is possible under physiological conditions of HB-EGF and HSPG expression. Additionally, as the interaction between proHB-EGF and HSPGs inhibits 104 cleavage of the ligand, computational modeling of the balance between juxtacrine and autocrine/paracrine signaling of HB-EGF could employed to better understand the balance between the two. Additionally, this model could shed insight onto the role of HSPG altering enzymes, such as HSulfI and heparanase, on the balance between HBEGF juxtacrine vs. autocrine/paracrine signaling in cancer. 105 References Abraham, J.A., D. Damm, A. Bajardi, J. Miller, M. Klagsbrun, and R.A. Ezekowitz. 1993. Heparin-binding EGF-like growth factor: characterization of rat and mouse cDNA clones, protein domain conservation across species, and transcript expression in tissues. Biochem Biophys Res Commun. 190:125-33. Ancha, H.R., R.R. Kurella, C.A. Stewart, G. Damera, B.P. Ceresa, and R.F. Harty. 2007. Histamine stimulation of MMP- 1(collagenase-1) secretion and gene expression in gastric epithelial cells: role of EGFR transactivation and the MAP kinase pathway. Int JBiochem Cell Biol. 39:2143-52. Anderson, H.D., F. Wang, and D.G. Gardner. 2004. Role of the epidermal growth factor receptor in signaling strain-dependent activation of the brain natriuretic peptide gene. JBiol Chem. 279:9287-97. Anklesaria, P., J. Teixido, M. Laiho, J.H. Pierce, J.S. Greenberger, and J. Massague. 1990. Cell-cell adhesion mediated by binding of membrane-anchored transforming growth factor alpha to epidermal growth factor receptors promotes cell proliferation. Proc Natl Acad Sci USA. 87:3289-93. Armant, D.R., B.A. Kilburn, A. Petkova, S.S. Edwin, Z.M. Duniec-Dmuchowski, H.J. Edwards, R. Romero, and R.E. Leach. 2006. Human trophoblast survival at low oxygen concentrations requires metalloproteinase-mediated shedding of heparinbinding EGF-like growth factor. Development. 133:751-9. Artuc, M., U.M. Steckelings, and B.M. Henz. 2002. Mast cell-fibroblast interactions: human mast cells as source and inducers of fibroblast and epithelial growth factors. JInvest Dermatol. 118:391-5. Asakura, M., M. Kitakaze, S. Takashima, Y. Liao, F. Ishikura, T. Yoshinaka, H. Ohmoto, K. Node, K. Yoshino, H. Ishiguro, H. Asanuma, S. Sanada, Y. Matsumura, H. Takeda, S. Beppu, M. Tada, M. Hori, and S. Higashiyama. 2002. Cardiac hypertrophy is inhibited by antagonism of ADAM12 processing of HB-EGF: metalloproteinase inhibitors as a new therapy. Nat Med. 8:35-40. Beckett, D., E. Kovaleva, and P.J. Schatz. 1999. A minimal peptide substrate in biotin holoenzyme synthetase-catalyzed biotinylation. Protein Sci. 8:921-9. Block, E.R., A.R. Matela, N. SundarRaj, E.R. Iszkula, and J.K. Klarlund. 2004. Wounding induces motility in sheets of corneal epithelial cells through loss of spatial constraints: role of heparin-binding epidermal growth factor-like growth factor signaling. JBiol Chem. 279:24307-12. Capila, I., and R.J. Linhardt. 2002. Heparin-protein interactions. Angew Chem Int Ed Engl. 41:391-412. Carson, D.D., J.P. Tang, and J. Julian. 1993. Heparan sulfate proteoglycan (perlecan) expression by mouse embryos during acquisition of attachment competence. Dev Biol. 155:97-106. Cha, J.H., J.S. Brooke, K.N. Ivey, and L. Eidels. 2000. Cell surface monkey CD9 antigen is a coreceptor that increases diphtheria toxin sensitivity and diphtheria toxin receptor affinity. JBiol Chem. 275:6901-7. Chansel, D., M. Ciroldi, S. Vandermeersch, L.F. Jackson, A.M. Gomez, D. Henrion, D.C. Lee, T.M. Coffman, S. Richard, J.C. Dussaule, and P.L. Tharaux. 2006. Heparin 106 binding EGF is necessary for vasospastic response to endothelin. FASEB J. 20:1936-8. Chen, I., M. Howarth, W. Lin, and A.Y. Ting. 2005. Site-specific labeling of cell surface proteins with biophysical probes using biotin ligase. Nat Methods. 2:99-104. Chien, K.R. 1999. Molecular Basis of Cardiovascular Disease. W.B. Saunders Company, Philadelpha. Chu, E.K., J.S. Foley, J. Cheng, A.S. Patel, J.M. Drazen, and D.J. Tschumperlin. 2005. Bronchial epithelial compression regulates epidermal growth factor receptor family ligand expression in an autocrine manner. Am J Respir Cell Mol Biol. 32:373-80. Chuang, Y.J., R. Swanson, S.M. Raja, and S.T. Olson. 2001. Heparin enhances the specificity of antithrombin for thrombin and factor Xa independent of the reactive center loop sequence. Evidence for an exosite determinant of factor Xa specificity in heparin-activated antithrombin. JBiol Chem. 276:14961-71. Citri, A., and Y. Yarden. 2006. EGF-ERBB signalling: towards the systems level. Nat Rev Mol Cell Biol. 7:505-16. Conrad, H.E. 1998. Heparin-Binding Proteins. Academic Press. 527 pp. Cook, P.W., P.A. Mattox, W.W. Keeble, M.R. Pittelkow, G.D. Plowman, M. Shoyab, J.P. Adelman, and G.D. Shipley. 1991. A heparin sulfate-regulated human keratinocyte autocrine factor is similar or identical to amphiregulin. Mol Cell Biol. 11:2547-57. Cribbs, R.K., M.H. Luquette, and G.E. Besner. 1998. Acceleration of partial-thickness bum wound healing with topical application of heparin-binding EGF-like growth factor (HB-EGF). JBurn Care Rehabil. 19:95-101. Das, S.K., X.N. Wang, B.C. Paria, D. Damm, J.A. Abraham, M. Klagsbrun, G.K. Andrews, and S.K. Dey. 1994. Heparin-binding EGF-like growth factor gene is induced in the mouse uterus temporally by the blastocyst solely at the site of its apposition: a possible ligand for interaction with blastocyst EGF-receptor in implantation. Development. 120:1071-83. Davies, B., P.D. Brown, N. East, M.J. Crimmin, and F.R. Balkwill. 1993. A synthetic matrix metalloproteinase inhibitor decreases tumor burden and prolongs survival of mice bearing human ovarian carcinoma xenografts. Cancer Res. 53:2087-91. DeWitt, A.E., J.Y. Dong, H.S. Wiley, and D.A. Lauffenburger. 2001. Quantitative analysis of the EGF receptor autocrine system reveals cryptic regulation of cell response by ligand capture. J Cell Sci. 114:2301-13. Dong, J., L.K. Opresko, W. Chrisler, G. Orr, R.D. Quesenberry, D.A. Lauffenburger, and H.S. Wiley. 2005. The membrane-anchoring domain of epidermal growth factor receptor ligands dictates their ability to operate in juxtacrine mode. Mol Biol Cell. 16:2984-98. Dong, J., L.K. Opresko, P.J. Dempsey, D.A. Lauffenburger, R.J. Coffey, and H.S. Wiley. 1999. Metalloprotease-mediated ligand release regulates autocrine signaling through the epidermal growth factor receptor. Proc Natl Acad Sci US A. 96:6235- 40. Du, J., and P.D. Wilson. 1995. Abnormal polarization of EGF receptors and autocrine stimulation of cyst epithelial growth in human ADPKD. Am JPhysiol.269:C48795. 107 Elenbaas, B., L. Spirio, F. Koerner, M.D. Fleming, D.B. Zimonjic, J.L. Donaher, N.C. Popescu, W.C. Hahn, and R.A. Weinberg. 2001. Human breast cancer cells generated by oncogenic transformation of primary mammary epithelial cells. Genes Dev. 15:50-65. Elenius, K., S. Paul, G. Allison, J. Sun, and M. Klagsbrun. 1997. Activation of HER4 by heparin-binding EGF-like growth factor stimulates chemotaxis but not proliferation. EMBO J. 16:1268-78. Farach, M.C., J.P. Tang, G.L. Decker, and D.D. Carson. 1987. Heparin/heparan sulfate is involved in attachment and spreading of mouse embryos in vitro. Dev Biol. 123:401-10. Farach, M.C., J.P. Tang, G.L. Decker, and D.D. Carson. 1988. Differential effects of pnitrophenyl-D-xylosides on mouse blastocysts and uterine epithelial cells. Biol Reprod. 39:443-55. Ferrara, N., and W.J. Henzel. 1989. Pituitary follicular cells secrete a novel heparinbinding growth factor specific for vascular endothelial cells. Biochem Biophys Res Commun. 161:851-8. Galinsky, D.S., and H. Nechushtan. 2008. Mast cells and cancer-No longer just basic science. Crit Rev Oncol Hematol. 68:115-30. Geiser, M., R. Cebe, D. Drewello, and R. Schmitz. 2001. Integration of PCR fragments at any specific site within cloning vectors without the use of restriction enzymes and DNA ligase. Biotechniques. 31:88-90, 92. Gill, G.N., T. Kawamoto, C. Cochet, A. Le, J.D. Sato, H. Masui, C. McLeod, and J. Mendelsohn. 1984. Monoclonal anti-epidermal growth factor receptor antibodies which are inhibitors of epidermal growth factor binding and antagonists of epidermal growth factor binding and antagonists of epidermal growth factorstimulated tyrosine protein kinase activity. JBiol Chem. 259:7755-60. Goishi, K., S. Higashiyama, M. Klagsbrun, N. Nakano, T. Umata, M. Ishikawa, E. Mekada, and N. Taniguchi. 1995. Phorbol ester induces the rapid processing of cell surface heparin-binding EGF-like growth factor: conversion from juxtacrine to paracrine growth factor activity. Mol Biol Cell. 6:967-80. Gospodarowicz, D., J. Cheng, G.M. Lui, A. Baird, and P. Bohlent. 1984. Isolation of brain fibroblast growth factor by heparin-Sepharose affinity chromatography: identity with pituitary fibroblast growth factor. Proc Natl Acad Sci U S A. 81:6963-7. Hamatani, T., T. Daikoku, H. Wang, H. Matsumoto, M.G. Carter, M.S. Ko, and S.K. Dey. 2004. Global gene expression analysis identifies molecular pathways distinguishing blastocyst dormancy and activation. Proc Natl Acad Sci U S A. 101:10326-31. Harmer, N.J. 2006. Insights into the role of heparan sulphate in fibroblast growth factor signalling. Biochem Soc Trans. 34:442-5. Higashiyama, S., J.A. Abraham, and M. Klagsbrun. 1993. Heparin-binding EGF-like growth factor stimulation of smooth muscle cell migration: dependence on interactions with cell surface heparan sulfate. J Cell Biol. 122:933-40. Higashiyama, S., J.A. Abraham, J. Miller, J.C. Fiddes, and M. Klagsbrun. 1991. A heparin-binding growth factor secreted by macrophage-like cells that is related to EGF. Science. 251:936-9. 108 Higashiyama, S., R. Iwamoto, K. Goishi, G. Raab, N. Taniguchi, M. Klagsbrun, and E. Mekada. 1995. The membrane protein CD9/DRAP 27 potentiates the juxtacrine growth factor activity of the membrane-anchored heparin-binding EGF-like growth factor. J Cell Biol. 128:929-38. Higashiyama, S., and D. Nanba. 2005. ADAM-mediated ectodomain shedding of HBEGF in receptor cross-talk. Biochim Biophys Acta. 1751:110-7. Hinkle, C.L., S.W. Sunnarborg, D. Loiselle, C.E. Parker, M. Stevenson, W.E. Russell, and D.C. Lee. 2004. Selective roles for tumor necrosis factor alpha-converting enzyme/ADAM 17 in the shedding of the epidermal growth factor receptor ligand family: the juxtamembrane stalk determines cleavage efficiency. J Biol Chem. 279:24179-88. Holmes, W.E., M.X. Sliwkowski, R.W. Akita, W.J. Henzel, J. Lee, J.W. Park, D. Yansura, N. Abadi, H. Raab, G.D. Lewis, and et al. 1992. Identification of heregulin, a specific activator of p185erbB2. Science. 256:1205-10. Howarth, M., and A.Y. Ting. 2008. Imaging proteins in live mammalian cells with biotin ligase and monovalent streptavidin. Nat Protoc. 3:534-45. Inui, S., S. Higashiyama, K. Hashimoto, M. Higashiyama, K. Yoshikawa, and N. Taniguchi. 1997. Possible role of coexpression of CD9 with membrane-anchored heparin-binding EGF-like growth factor and amphiregulin in cultured human keratinocyte growth. J Cell Physiol. 171:291-8. Itoh, Y., T. Joh, S. Tanida, M. Sasaki, H. Kataoka, K. Itoh, T. Oshima, N. Ogasawara, S. Togawa, T. Wada, H. Kubota, Y. Mori, H. Ohara, T. Nomura, S. Higashiyama, and M. Itoh. 2005. IL-8 promotes cell proliferation and migration through metalloproteinase-cleavage proHB-EGF in human colon carcinoma cells. Cytokine. 29:275-82. Iwamoto, R., K. Handa, and E. Mekada. 1999. Contact-dependent growth inhibition and apoptosis of epidermal growth factor (EGF) receptor-expressing cells by the membrane-anchored form of heparin-binding EGF-like growth factor. J Biol Chem. 274:25906-12. Iwamoto, R., H. Senoh, Y. Okada, T. Uchida, and E. Mekada. 1991. An antibody that inhibits the binding of diphtheria toxin to cells revealed the association of a 27kDa membrane protein with the diphtheria toxin receptor. J Biol Chem. 266:20463-9. Iwamoto, R., S. Yamazaki, M. Asakura, S. Takashima, H. Hasuwa, K. Miyado, S. Adachi, M. Kitakaze, K. Hashimoto, G. Raab, D. Nanba, S. Higashiyama, M. Hori, M. Klagsbrun, and E. Mekada. 2003. Heparin-binding EGF-like growth factor and ErbB signaling is essential for heart function. Proc Natl Acad Sci U S A. 100:3221-6. Jackson, L.F., T.H. Qiu, S.W. Sunnarborg, A. Chang, C. Zhang, C. Patterson, and D.C. Lee. 2003. Defective valvulogenesis in HB-EGF and TACE-null mice is associated with aberrant BMP signaling. EMBO J. 22:2704-16. Jakobsson, L., J. Kreuger, K. Holmborn, L. Lundin, I. Eriksson, L. Kjellen, and L. Claesson-Welsh. 2006. Heparan sulfate in trans potentiates VEGFR-mediated angiogenesis. Dev Cell. 10:625-34. 109 Jennings, L.K., J.T. Crossno, Jr., C.F. Fox, M.M. White, and C.A. Green. 1994. Platelet p24/CD9, a member of the tetraspanin family of proteins. Ann N Y Acad Sci. 714:175-84. Johnson, G.R., and L. Wong. 1994. Heparan sulfate is essential to amphiregulin-induced mitogenic signaling by the epidermal growth factor receptor. J Biol Chem. 269:27149-54. Kain, K.H., and R.L. Klemke. 2001. Inhibition of cell migration by Abl family tyrosine kinases through uncoupling of Crk-CAS complexes. JBiol Chem. 276:16185-92. Kalesnikoff, J., and S.J. Galli. 2008. New developments in mast cell biology. Nat Immunol. 9:1215-23. Kinugasa, Y., M. Hieda, M. Hori, and S. Higashiyama. 2007. The carboxyl-terminal fragment of pro-HB-EGF reverses Bcl6-mediated gene repression. J Biol Chem. 282:14797-806. Kramer, K.L., and H.J. Yost. 2002. Ectodermal syndecan-2 mediates left-right axis formation in migrating mesoderm as a cell-nonautonomous Vgl cofactor. Dev Cell. 2:115-24. Kreitman, R.J. 2009. Recombinant immunotoxins containing truncated bacterial toxins for the treatment of hematologic malignancies. BioDrugs. 23:1-13. Lin, J., L. Hutchinson, S.M. Gaston, G. Raab, and M.R. Freeman. 2001. BAG-1 is a novel cytoplasmic binding partner of the membrane form of heparin-binding EGF-like growth factor: a unique role for proHB-EGF in cell survival regulation. JBiol Chem. 276:30127-32. Lodish, H. 2003. Molecular Cell Biology. W.H. Freeman. Loeb, J.A., and G.D. Fischbach. 1995. ARIA can be released from extracellular matrix through cleavage of a heparin-binding domain. J Cell Biol. 130:127-35. Low, J.A., M.D. Johnson, E.A. Bone, and R.B. Dickson. 1996. The matrix metalloproteinase inhibitor batimastat (BB-94) retards human breast cancer solid tumor growth but not ascites formation in nude mice. Clin Cancer Res. 2:1207-14. Maatta, J.A., K. Olli, T. Henttinen, M.T. Tuittila, K. Elenius, and M. Salmivirta. 2009. Removal of cell surface heparan sulfate increases TACE activity and cleavage of ErbB4 receptor. BMC Cell Biol. 10:5. Maciag, T., T. Mehlman, R. Friesel, and A.B. Schreiber. 1984. Heparin binds endothelial cell growth factor, the principal endothelial cell mitogen in bovine brain. Science. 225:932-5. MacRae Dell, K., R. Nemo, W.E. Sweeney, Jr., and E.D. Avner. 2004. EGF-related growth factors in the pathogenesis of murine ARPKD. Kidney Int. 65:2018-29. Maheshwari, G., H.S. Wiley, and D.A. Lauffenburger. 2001. Autocrine epidermal growth factor signaling stimulates directionally persistent mammary epithelial cell migration. J Cell Biol. 155:1123-8. Maly, I.V., H.S. Wiley, and D.A. Lauffenburger. 2004. Self-organization of polarized cell signaling via autocrine circuits: computational model analysis. Biophys J. 86:1022. Marikovsky, M., K. Breuing, P.Y. Liu, E. Eriksson, S. Higashiyama, P. Farber, J. Abraham, and M. Klagsbrun. 1993. Appearance of heparin-binding EGF-like growth factor in wound fluid as a response to injury. Proc Natl Acad Sci U S A. 90:3889-93. 110 Massague, J., and A. Pandiella. 1993. Membrane-anchored growth factors. Annu Rev Biochem. 62:515-41. Mathay, C., S. Giltaire, F. Minner, E. Bera, M. Herin, and Y. Poumay. 2008. Heparinbinding EGF-like growth factor is induced by disruption of lipid rafts and oxidative stress in keratinocytes and participates in the epidermal response to cutaneous wounds. J Invest Dermatol. 128:717-27. McCarthy, D.W., M.T. Downing, D.R. Brigstock, M.H. Luquette, K.D. Brown, M.S. Abad, and G.E. Besner. 1996. Production of heparin-binding epidermal growth factor-like growth factor (HB-EGF) at sites of thermal injury in pediatric patients. JInvest Dermatol. 106:49-56. Mifune, M., H. Ohtsu, H. Suzuki, H. Nakashima, E. Brailoiu, N.J. Dun, G.D. Frank, T. Inagami, S. Higashiyama, W.G. Thomas, A.D. Eckhart, P.J. Dempsey, and S. Eguchi. 2005. G protein coupling and second messenger generation are indispensable for metalloprotease-dependent, heparin-binding epidermal growth factor shedding through angiotensin II type-i receptor. JBiol Chem. 280:26592-9. Mine, N., R. Iwamoto, and E. Mekada. 2005. HB-EGF promotes epithelial cell migration in eyelid development. Development. 132:4317-26. Mitamura, T., S. Higashiyama, N. Taniguchi, M. Klagsbrun, and E. Mekada. 1995. Diphtheria toxin binds to the epidermal growth factor (EGF)-like domain of human heparin-binding EGF-like growth factor/diphtheria toxin receptor and inhibits specifically its mitogenic activity. JBiol Chem. 270:1015-9. Miyagawa, J., S. Higashiyama, S. Kawata, Y. Inui, S. Tamura, K. Yamamoto, M. Nishida, T. Nakamura, S. Yamashita, Y. Matsuzawa, and et al. 1995. Localization of heparin-binding EGF-like growth factor in the smooth muscle cells and macrophages of human atherosclerotic plaques. J Clin Invest. 95:404-11. Miyamoto, S., M. Hirata, A. Yamazaki, T. Kageyama, H. Hasuwa, H. Mizushima, Y. Tanaka, H. Yagi, K. Sonoda, M. Kai, H. Kanoh, H. Nakano, and E. Mekada. 2004. Heparin-binding EGF-like growth factor is a promising target for ovarian cancer therapy. Cancer Res. 64:5720-7. Miyamoto, S., H. Yagi, F. Yotsumoto, T. Kawarabayashi, and E. Mekada. 2006. Heparinbinding epidermal growth factor-like growth factor as a novel targeting molecule for cancer therapy. Cancer Sci. 97:341-7. Miyamoto, S., H. Yagi, F. Yotsumoto, T. Kawarabayashi, and E. Mekada. 2008. Heparinbinding epidermal growth factor-like growth factor as a new target molecule for cancer therapy. Adv Exp Med Biol. 622:281-95. Morimoto-Tomita, M., K. Uchimura, Z. Werb, S. Hemmerich, and S.D. Rosen. 2002. Cloning and characterization of two extracellular heparin-degrading endosulfatases in mice and humans. JBiol Chem. 277:49175-85. Naglich, J.G., J.E. Metherall, D.W. Russell, and L. Eidels. 1992. Expression cloning of a diphtheria toxin receptor: identity with a heparin-binding EGF-like growth factor precursor. Cell. 69:1051-61. Nakagawa, T., Y. Hayase, M. Sasahara, M. Haneda, R. Kikkawa, S. Higashiyama, N. Taniguchi, and F. Hazama. 1997. Distribution of heparin-binding EGF-like growth factor protein and mRNA in the normal rat kidneys. Kidney Int. 51:1774-9. Nakamura, K., R. Iwamoto, and E. Mekada. 1995. Membrane-anchored heparin-binding EGF-like growth factor (HB-EGF) and diphtheria toxin receptor-associated 111 protein (DRAP27)/CD9 form a complex with integrin alpha 3 beta 1 at cell-cell contact sites. J Cell Biol. 129:1691-705. Nakamura, K., T. Mitamura, T. Takahashi, T. Kobayashi, and E. Mekada. 2000. Importance of the major extracellular domain of CD9 and the epidermal growth factor (EGF)-like domain of heparin-binding EGF-like growth factor for upregulation of binding and activity. JBiol Chem. 275:18284-90. Nakamura, T., K. Nawa, and A. Ichihara. 1984. Partial purification and characterization of hepatocyte growth factor from serum of hepatectomized rats. Biochem Biophys Res Commun. 122:1450-9. Nakamura, Y., K. Handa, R. Iwamoto, T. Tsukamoto, M. Takahasi, and E. Mekada. 2001. Immunohistochemical distribution of CD9, heparin binding epidermal growth factor-like growth factor, and integrin alpha3betal in normal human tissues. J Histochem Cytochem. 49:439-44. Nakata, A., J. Miyagawa, S. Yamashita, M. Nishida, R. Tamura, K. Yamamori, T. Nakamura, S. Nozaki, K. Kameda-Takemura, S. Kawata, N. Taniguchi, S. Higashiyama, and Y. Matsuzawa. 1996. Localization of heparin-binding epidermal growth factor-like growth factor in human coronary arteries. Possible roles of HB-EGF in the formation of coronary atherosclerosis. Circulation. 94:2778-86. Nanba, D., Y. Kinugasa, C. Morimoto, M. Koizumi, H. Yamamura, K. Takahashi, N. Takakura, E. Mekada, K. Hashimoto, and S. Higashiyama. 2006. Loss of HBEGF in smooth muscle or endothelial cell lineages causes heart malformation. Biochem Biophys Res Commun. 350:315-21. Nanba, D., A. Mammoto, K. Hashimoto, and S. Higashiyama. 2003. Proteolytic release of the carboxy-terminal fragment of proHB-EGF causes nuclear export of PLZF. J Cell Biol. 163:489-502. Neville, D.M., Jr., and T.H. Hudson. 1986. Transmembrane transport of diphtheria toxin, related toxins, and colicins. Annu Rev Biochem. 55:195-224. Nguyen, H.T., S.H. Bride, A.B. Badawy, R.M. Adam, J. Lin, A. Orsola, P.D. Guthrie, M.R. Freeman, and C.A. Peters. 2000. Heparin-binding EGF-like growth factor is up-regulated in the obstructed kidney in a cell- and region-specific manner and acts to inhibit apoptosis. Am JPathol.156:889-98. Noli, C., and A. Miolo. 2001. The mast cell in wound healing. Vet Dermatol. 12:303-13. Ongusaha, P.P., J.C. Kwak, A.J. Zwible, S. Macip, S. Higashiyama, N. Taniguchi, L. Fang, and S.W. Lee. 2004. HB-EGF is a potent inducer of tumor growth and angiogenesis. CancerRes. 64:5283-90. Paizis, K., G. Kirkland, T. Khong, M. Katerelos, S. Fraser, J. Kanellis, and D.A. Power. 1999. Heparin-binding epidermal growth factor-like growth factor is expressed in the adhesive lesions of experimental focal glomerular sclerosis. Kidney Int. 55:2310-21. Pan, B., K. Sengoku, K. Goishi, N. Takuma, T. Yamashita, K. Wada, and M. Ishikawa. 2002. The soluble and membrane-anchored forms of heparin-binding epidermal growth factor-like growth factor appear to play opposing roles in the survival and apoptosis of human luteinized granulosa cells. Mol Hum Reprod. 8:734-41. Paria, B.C., K. Elenius, M. Klagsbrun, and S.K. Dey. 1999. Heparin-binding EGF-like growth factor interacts with mouse blastocysts independently of ErbB 1: a possible 112 role for heparan sulfate proteoglycans and ErbB4 in blastocyst implantation. Development. 126:1997-2005. Piepkorn, M., C. Lo, and G. Plowman. 1994. Amphiregulin-dependent proliferation of cultured human keratinocytes: autocrine growth, the effects of exogenous recombinant cytokine, and apparent requirement for heparin-like glycosaminoglycans. J Cell Physiol. 159:114-20. Piepkom, M., M.R. Pittelkow, and P.W. Cook. 1998. Autocrine regulation of keratinocytes: the emerging role of heparin-binding, epidermal growth factorrelated growth factors. J Invest Dermatol. 111:715-21. Prenzel, N., E. Zwick, H. Daub, M. Leserer, R. Abraham, C. Wallasch, and A. Ullrich. 1999. EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature. 402:884-8. Raab, G., and M. Klagsbrun. 1997. Heparin-binding EGF-like growth factor. Biochim Biophys Acta. 1333:F179-99. Raab, G., K. Kover, B.C. Paria, S.K. Dey, R.M. Ezzell, and M. Klagsbrun. 1996. Mouse preimplantation blastocysts adhere to cells expressing the transmembrane form of heparin-binding EGF-like growth factor. Development. 122:637-45. Robinson, C.R., and R.T. Sauer. 1998. Optimizing the stability of single-chain proteins by linker length and composition mutagenesis. Proc Natl Acad Sci U S A. 95:5929-34. Rodland, K.D., N. Bollinger, D. Ippolito, L.K. Opresko, R.J. Coffey, R. Zangar, and H.S. Wiley. 2008. Multiple mechanisms are responsible for transactivation of the epidermal growth factor receptor in mammary epithelial cells. J Biol Chem. 283:31477-87. Sadoshima, J., and S. Izumo. 1997. The cellular and molecular response of cardiac myocytes to mechanical stress. Annu Rev Physiol. 59:551-71. Sahin, U., G. Weskamp, K. Kelly, H.M. Zhou, S. Higashiyama, J. Peschon, D. Hartmann, P. Saftig, and C.P. Blobel. 2004. Distinct roles for ADAM10 and ADAM17 in ectodomain shedding of six EGFR ligands. J Cell Biol. 164:769-79. Saito, M., T. Iwawaki, C. Taya, H. Yonekawa, M. Noda, Y. Inui, E. Mekada, Y. Kimata, A. Tsuru, and K. Kohno. 2001. Diphtheria toxin receptor-mediated conditional and targeted cell ablation in transgenic mice. Nat Biotechnol. 19:746-50. Sakuma, T., S. Higashiyama, S. Hosoe, S. Hayashi, and N. Taniguchi. 1997. CD9 antigen interacts with heparin-binding EGF-like growth factor through its heparin-binding domain. JBiochem. 122:474-80. Schilling, D., I.J. Reid, A. Hujer, D. Morgan, E. Demoll, P. Bummer, R.A. Fenstermaker, and D.M. Kaetzel. 1998. Loop III region of platelet-derived growth factor (PDGF) B-chain mediates binding to PDGF receptors and heparin. Biochem J. 333 ( Pt 3):637-44. Schlessinger, J., I. Lax, and M. Lemmon. 1995. Regulation of growth factor activation by proteoglycans: what is the role of the low affinity receptors? Cell. 83:357-60. Sheikh-Hamad, D., K. Youker, L.D. Truong, S. Nielsen, and M.L. Entman. 2000. Osmotically relevant membrane signaling complex: association between HB-EGF, beta(l)-integrin, and CD9 in mTAL. Am JPhysiol Cell Physiol. 279:C136-46. 113 Shing, Y., G. Christofori, D. Hanahan, Y. Ono, R. Sasada, K. Igarashi, and J. Folkman. 1993. Betacellulin: a mitogen from pancreatic beta cell tumors. Science. 259:1604-7. Shing, Y., J. Folkman, R. Sullivan, C. Butterfield, J. Murray, and M. Klagsbrun. 1984. Heparin affinity: purification of a tumor-derived capillary endothelial cell growth factor. Science. 223:1296-9. Shirakata, Y., R. Kimura, D. Nanba, R. Iwamoto, S. Tokumaru, C. Morimoto, K. Yokota, M. Nakamura, K. Sayama, E. Mekada, S. Higashiyama, and K. Hashimoto. 2005. Heparin-binding EGF-like growth factor accelerates keratinocyte migration and skin wound healing. J Cell Sci. 118:2363-70. Shishido, Y., K.D. Sharma, S. Higashiyama, M. Klagsbrun, and E. Mekada. 1995. Heparin-like molecules on the cell surface potentiate binding of diphtheria toxin to the diphtheria toxin receptor/membrane-anchored heparin-binding epidermal growth factor-like growth factor. JBiol Chem. 270:29578-85. Singethan, K., N. Muller, S. Schubert, D. Luttge, D.N. Krementsov, S.R. Khurana, G. Krohne, S. Schneider-Schaulies, M. Thali, and J. Schneider-Schaulies. 2008. CD9 clustering and formation of microvilli zippers between contacting cells regulates virus-induced cell fusion. Traffic. 9:924-35. Singh, A.B., K. Sugimoto, P. Dhawan, and R.C. Harris. 2007a. Juxtacrine activation of EGFR regulates claudin expression and increases transepithelial resistance. Am J Physiol Cell Physiol. 293:C 1660-8. Singh, A.B., K. Sugimoto, and R.C. Harris. 2007b. Juxtacrine activation of epidermal growth factor (EGF) receptor by membrane-anchored heparin-binding EGF-like growth factor protects epithelial cells from anoikis while maintaining an epithelial phenotype. JBiol Chem. 282:32890-901. Sorensen, H.P., R.R. Vives, C. Manetopoulos, R. Albrechtsen, M.C. Lydolph, J. Jacobsen, J.R. Couchman, and U.M. Wewer. 2008. Heparan sulfate regulates ADAM12 through a molecular switch mechanism. JBiol Chem. 283:31920-32. Soulet, F., S.L. Schmid, and H. Damke. 2006. Domain requirements for an endocytosisindependent, isoform-specific function of dynamin-2. Exp Cell Res. 312:3539-45. Sporn, M.B., and G.J. Todaro. 1980. Autocrine secretion and malignant transformation of cells. NEnglJMed. 303:878-80. Stein, B.S., K.G. Bensch, and H.H. Sussman. 1984. Complete inhibition of transferrin recycling by monensin in K562 cells. JBiol Chem. 259:14762-72. Tada, H., R. Sasada, Y. Kawaguchi, I. Kojima, W.J. Gullick, D.S. Salomon, K. Igarashi, M. Seno, and H. Yamada. 1999. Processing and juxtacrine activity of membraneanchored betacellulin. J Cell Biochem. 72:423-34. Takazaki, R., Y. Shishido, R. Iwamoto, and E. Mekada. 2004. Suppression of the biological activities of the epidermal growth factor (EGF)-like domain by the heparin-binding domain of heparin-binding EGF-like Growth Factor. JBiol Chem. 279:47335-43. Takemura, T., S. Hino, H. Kuwajima, H. Yanagida, M. Okada, M. Nagata, S. Sasaki, J. Barasch, R.C. Harris, and K. Yoshioka. 2001. Induction of collecting duct morphogenesis in vitro by heparin-binding epidermal growth factor-like growth factor. JAm Soc Nephrol. 12:964-72. 114 Takemura, T., S. Hino, Y. Murata, H. Yanagida, M. Okada, K. Yoshioka, and R.C. Harris. 1999a. Coexpression of CD9 augments the ability of membrane-bound heparinbinding epidermal growth factor-like growth factor (proHB-EGF) to preserve renal epithelial cell viability. Kidney Int. 55:71-81. Takemura, T., S. Hino, M. Okada, Y. Murata, H. Yanagida, M. Ikeda, K. Yoshioka, and R.C. Harris. 2002. Role of membrane-bound heparin-binding epidermal growth factor-like growth factor (HB-EGF) in renal epithelial cell branching. Kidney Int. 61:1968-79. Takemura, T., S. Kondo, T. Homma, M. Sakai, and R.C. Harris. 1997. The membranebound form of heparin-binding epidermal growth factor-like growth factor promotes survival of cultured renal epithelial cells. JBiol Chem. 272:31036-42. Takemura, T., Y. Murata, S. Hino, M. Okada, H. Yanagida, M. Ikeda, and K. Yoshioka. 1999b. Heparin-binding EGF-like growth factor is expressed by mesangial cells and is involved in mesangial proliferation in glomerulonephritis. J Pathol. 189:431-8. Tanaka, Y., S. Miyamoto, S.O. Suzuki, E. Oki, H. Yagi, K. Sonoda, A. Yamazaki, H. Mizushima, Y. Maehara, E. Mekada, and H. Nakano. 2005. Clinical significance of heparin-binding epidermal growth factor-like growth factor and a disintegrin and metalloprotease 17 expression in human ovarian cancer. Clin Cancer Res. 11:4783-92. Thompson, S.A., S. Higashiyama, K. Wood, N.S. Pollitt, D. Damm, G. McEnroe, B. Garrick, N. Ashton, K. Lau, N. Hancock, and et al. 1994. Characterization of sequences within heparin-binding EGF-like growth factor that mediate interaction with heparin. JBiol Chem. 269:2541-9. Tokumaru, S., S. Higashiyama, T. Endo, T. Nakagawa, J.I. Miyagawa, K. Yamamori, Y. Hanakawa, H. Ohmoto, K. Yoshino, Y. Shirakata, Y. Matsuzawa, K. Hashimoto, and N. Taniguchi. 2000. Ectodomain shedding of epidermal growth factor receptor ligands is required for keratinocyte migration in cutaneous wound healing. J Cell Biol. 151:209-20. Vlodavsky, I., N. Ilan, Y. Nadir, B. Brenner, B.Z. Katz, A. Naggi, G. Torri, B. Casu, and R. Sasisekharan. 2007. Heparanase, heparin and the coagulation system in cancer progression. Thromb Res. 120 Suppl 2:S112-20. Wang, F., R. Liu, S.W. Lee, C.M. Sloss, J. Couget, and J.C. Cusack. 2007. Heparinbinding EGF-like growth factor is an early response gene to chemotherapy and contributes to chemotherapy resistance. Oncogene. 26:2006-16. Wang, H., and S.K. Dey. 2006. Roadmap to embryo implantation: clues from mouse models. Nat Rev Genet. 7:185-99. Xie, H., H. Wang, S. Tranguch, R. Iwamoto, E. Mekada, F.J. Demayo, J.P. Lydon, S.K. Das, and S.K. Dey. 2007. Maternal heparin-binding-EGF deficiency limits pregnancy success in mice. Proc Natl Acad Sci US A. 104:18315-20. Xu, K.P., Y. Ding, J. Ling, Z. Dong, and F.S. Yu. 2004. Wound-induced HB-EGF ectodomain shedding and EGFR activation in corneal epithelial cells. Invest Ophthalmol Vis Sci. 45:813-20. Xu, K.P., J. Yin, and F.S. Yu. 2007. Lysophosphatidic acid promoting corneal epithelial wound healing by transactivation of epidermal growth factor receptor. Invest Ophthalmol Vis Sci. 48:636-43. 115 Yahata, Y., Y. Shirakata, S. Tokumaru, L. Yang, X. Dai, M. Tohyama, T. Tsuda, K. Sayama, M. Iwai, M. Horiuchi, and K. Hashimoto. 2006. A novel function of angiotensin II in skin wound healing. Induction of fibroblast and keratinocyte migration by angiotensin II via heparin-binding epidermal growth factor (EGF)like growth factor-mediated EGF receptor transactivation. J Biol Chem. 281:13209-16. Yamazaki, S., R. Iwamoto, K. Saeki, M. Asakura, S. Takashima, A. Yamazaki, R. Kimura, H. Mizushima, H. Moribe, S. Higashiyama, M. Endoh, Y. Kaneda, S. Takagi, S. Itami, N. Takeda, G. Yamada, and E. Mekada. 2003. Mice with defects in HB-EGF ectodomain shedding show severe developmental abnormalities. J Cell Biol. 163:469-75. Yang, X., M.J. Zhu, N. Sreejayan, J. Ren, and M. Du. 2005. Angiotensin II promotes smooth muscle cell proliferation and migration through release of heparin-binding epidermal growth factor and activation of EGF-receptor pathway. Mol Cells. 20:263-70. Yano, S., R.J. Macleod, N. Chattopadhyay, J. Tfelt-Hansen, 0. Kifor, R.R. Butters, and E.M. Brown. 2004. Calcium-sensing receptor activation stimulates parathyroid hormone-related protein secretion in prostate cancer cells: role of epidermal growth factor receptor transactivation. Bone. 35:664-72. Yin, J., and F.S. Yu. 2009. ERK1/2 mediate wounding- and G-protein-coupled receptor ligands-induced EGFR activation via regulating ADAM 17 and HB-EGF shedding. Invest Ophthalmol Vis Sci. 50:132-9. Yoshioka, J., R.N. Prince, H. Huang, S.B. Perkins, F.U. Cruz, C. MacGillivray, D.A. Lauffenburger, and R.T. Lee. 2005. Cardiomyocyte hypertrophy and degradation of connexin43 through spatially restricted autocrine/paracrine heparin-binding EGF. Proc Natl Acad Sci U S A. 102:10622-7. Young, E., V. Douros, T.J. Podor, S.G. Shaughnessy, and J.I. Weitz. 2004. Localization of heparin and low-molecular-weight heparin in the rat kidney. Thromb Haemost. 91:927-34. Zhan, L., A. Rosenberg, K.C. Bergami, M. Yu, Z. Xuan, A.B. Jaffe, C. Allred, and S.K. Muthuswamy. 2008. Deregulation of scribble promotes mammary tumorigenesis and reveals a role for cell polarity in carcinoma. Cell. 135:865-78. Zhang, H., S.W. Sunnarborg, K.K. McNaughton, T.G. Johns, D.C. Lee, and J.E. Faber. 2008. Heparin-binding epidermal growth factor-like growth factor signaling in flow-induced arterial remodeling. Circ Res. 102:1275-85. Zhao, H., W. Tian, H. Xu, and D.M. Cohen. 2003. Urea signalling to immediate-early gene transcription in renal medullary cells requires transactivation of the epidermal growth factor receptor. Biochem J. 370:479-87. Zhou, H., J.R. Casas-Finet, R. Heath Coats, J.D. Kaufman, S.J. Stahl, P.T. Wingfield, J.S. Rubin, D.P. Bottaro, and R.A. Byrd. 1999. Identification and dynamics of a heparin-binding site in hepatocyte growth factor. Biochemistry. 38:14793-802. Zoller, M. 2009. Tetraspanins: push and pull in suppressing and promoting metastasis. Nat Rev Cancer. 9:40-55. 116