Document 11164687
advertisement
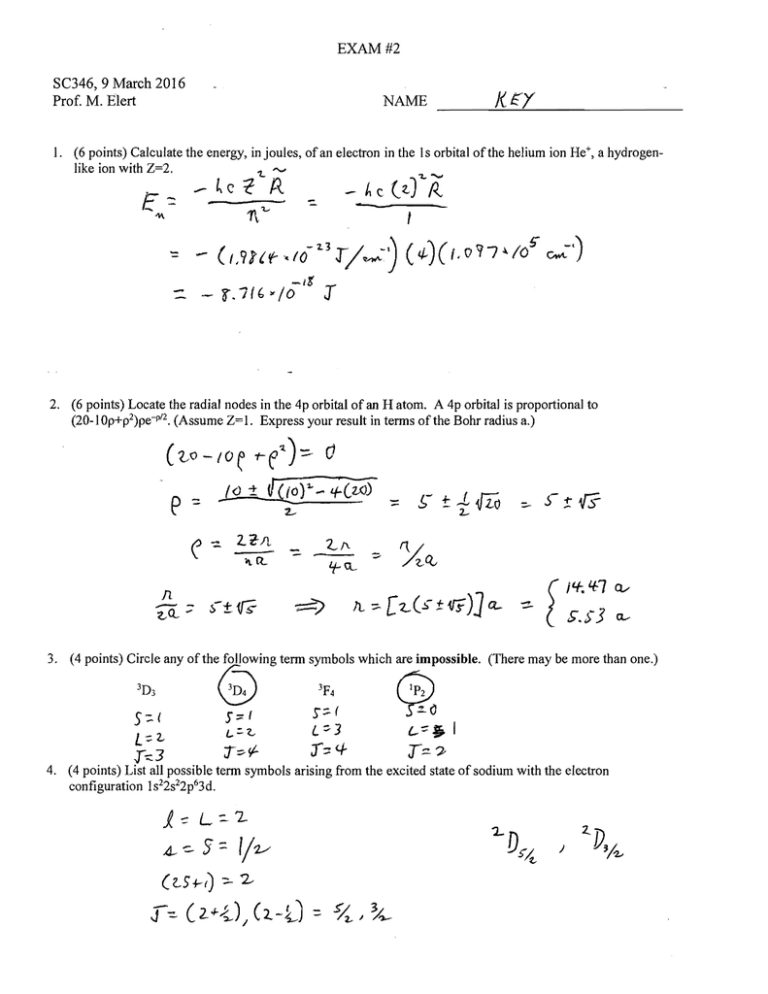
EXAM #2 SC346, 9 March 2016
Prof. M. Elert NAME
1. (6 points) Calculate the energy, in joules, of an electron in the Is orbital of the helium ion He+, a hydrogen­
like ion with
t
-
(L9t(lf~/O-2.3T/~-) (q.,)(f,O'Y7~/OG" c..:')
-lg
J
- o.l{b"lo
2. (6 points) Locate the radial nodes in the 4p orbital of an H atom. A 4p orbital is proportional to
(20-1 Op+p2)pe-p/ 2. (Assume Z= 1. Express your result in tenns of the Bohr radius a.)
( 00 - I 0 ~ r
p::.
f ~ 0
1. )
tQ ± {((o)l- -
-~
+cWf
'::=
3. (4 points) Circle any of the following tenn symbols which are impossible. (There may be more than one.)
G
3D3 5::( L:::.
r::.(
S;;(
,::.z.,
2.,.....
,["-::3
3F,
j::;.t/-
L:::'r]
~
~o l--:::a.
I
.,. 1'-;:.4-
T:::-":P 4. (4 points) List all possible tenn symbols arising from the excited state of sodium with the electron
configuration 1s22s22p63d.
J-:::L-=-2
4.-
-=- g:::
(~S .pt)
'::-
1/
}
V
2­
J -=- (l ~ -l) (~- LJ
I
::
~) ~')-
5. (6 points) Identify the shortest and longest wavelength lines in the Paschen series (nl == 3) for the hydrogen
atom.
~ - 17.,1'10
~
.A
I
/1J
-:=.-
L~ tJ r IiJT' :
-5'
-= [,1..-<)«- -J./6
1'\. 'L ~
Y~
A :::;.
~~ 'iJ. tf.
-::.
OM
-I
~
1VIVI­
Li-
It (f.-. -+~) " (. ~t G) 'it '" (.
0
(
0---
-4
I.f1S">-/()
?
(/1M-
C'I
u) (fo 970 OJ ~
1,,{7.j- ~
-;:.
6. (6 points) The orbital energies ofXe5p and 02p electrons are -12.1 eV and -13.6 eV, respectively.
- Calculate the energies of the bonding and antibonding orbitals ofXeO in the zero overlap
l
approximation. Use p = -1.2 eV. ex ~"C.
~8
-
t 1:
fLI
~ ~(
-;. ... (? ,
f3;:
J.
-I,
<­
=-
±(-
+
O(A
~iS) l' {l4<.n-o(:s) [{ + I ~)Z.} ~
ltt:1J O(B
{t. ( -
'3. ,) t i (-{(..I +- (S, b) ~ I
l
- - It,ifr + (o.IS)
.-, - n ,8' s ±
f.
[I . . z..;-d
-I-
/2l ~ r. ~)
l;-n./ +-/3.(,
)2-] '").
'i..
</-1.,.,­
(-II.tfJ ~V
_
Z- If. 'L1 V
2
7. (6 points) A simplified form of the bonding and antibonding molecular orbitals for H2 is
1fI±
= (l/.J2)(A ± B)
where + gives the bonding orbital and
gives the antibonding orbital. Here A
andBarethe Iswavefunctions: A=lsA=(7rao3rll2e-rA'oo andB
lSB
(1Cao3rIl2e-rslau. [SeeBrief
Illustration lOB. 1 p. 408 for a picture.] Suppose that the bond length between atoms A and B in H2
is R 2ao. Calculate the numerical values of IfI+ and of 1fI- at the midpoint between the two atoms.
In<P
-l-
,(14~ f'1'iJPbIN~
~ ~
'V
<.
-
{
(t(({
7
J) ~
(j
ilr'l -;: 118 :::
[ fo
o
-)ta
-JLII!Ci.-
~
Q
Q
+- ~
1rt..<4J
l
=:
(
r{{(7TQ3)~
0
(7 -')
Jf2(£
=-;c rTcct )~ -:::. --;-[-7T-C-">-.-L-""i-~-. . {,-()--'-{~-)""'j~
~ 1,(2. v./o(<./.. ~3/'V
<....Q
z
8. (6 points) What is the value of the overlap integral S for the situation described in problem 7 above,
i.e. two Is wave functions separated by a distance R 2ao?
t
(AB.
a.
~-... 'I
""
f
S~ l
~
(r. f( ) ~(.>
f(
I y~. """j L~
[ I + 2-
-- R.let c
J0
~ ~ (<-y- 5;-
'L
::: .SF-6
"~. 9~' (i) p'o'lnts)"A' Htickel calculation' for naphthalene, CloHg, gives the molecular orbital energies
E = a + m~, where the ten values ofm are ±2.303, .618, ±1.303, ±l.OOO, and ±O.618. Calculate
the x-electron binding energy & and the delocalization energy of naphthalene.
f 11 -;:.
-
L (I
L
.J-
2. ~ -;.... <-- r'3..J-
~ E",
'j-
L
~'-
[Co<-.. U()3 f3) + Cox+ (. C' 'i p,) + C"'-~ (,,03 fl) -I- ( "'-+fi)'" (0<. P8'JI8
-- /0 ()(.
l'
+-
13. {,8' fi