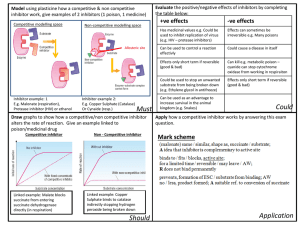
JUN 3 0 LIBRARIES ARCHNES
advertisement

Increasing IFN-y Productivity in CHO Cells through CDK Inhibition MASSACHUSETTS INSTITUTE OF TECHNOLOGY David Alan McClain JUN 3 0 2010 B.S. Chemical Engineering Cornell University, Ithaca, NY, 2004 LIBRARIES ARCHNES SUBMITTED TO THE DEPARTMENT OF CHEMICAL ENGINEERING IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY IN CHEMICAL ENGINEERING at the MASSACHUSETTS INSTITUTE OF TECHNOLOGY MAY 2010 0 2010 Massachusetts Institute of Technology. All Rights Reserved. Author: Department of Chemical Engineering May 2010 Certified by: Daniel I.C. Wang Institute Professor Thesis Supervisor Accepted by: William M. Deen Professor Chemical Engineering Chairman, Committee for Graduate Students 2 Increasing IFN-y Productivity in CHO Cells through CDK Inhibition by David Alan McClain Submitted to the Department of Chemical Engineering on May 2010 In Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy in Chemical Engineering ABSTRACT Approximately 60-70% of all recombinant human glycoproteins are produced in Chinese Hamster Ovary (CHO) cells. Production in CHO cells, however, is often plagued by low productivity when compared with other host cell lines, including bacteria and yeast. For this reason, investigating ways of improving the productivity of CHO cells producing recombinant proteins has been an active area of research for many decades. The induction of growth arrest is one such area that shows particular promise. Through the use of siRNA and chemical cyclin dependent kinase (CDK) inhibitors, we have developed new methods to improve and better understand recombinant protein production during growth arrest. In this study, we have shown that the specific inhibition of the CDK2-CcnE complex through chemical inhibition leads to growth arrest and a subsequent increase in specific productivity. In addition, we have shown that the knockdown of CcnEl alone leads to increases in specific productivity. With the advent of improved shRNA expression systems, we believe that the targeted knockdown of CcnE1 has the potential to induce growth arrest and improve total recombinant protein production The relationship between growth-arrested cell cycle phase and productivity is very poorly understood. In this work, we have used various CDK inhibitors to better understand the relationship between growth-arrested cell cycle phase, specific growth rate, and productivity. We have shown that increases in specific productivity are cell-cycle independent following growth arrest induced by CDK inhibition. Instead, specific productivity increases correlate strongly with a decreasing specific growth rate. Lastly, in this work, we have identified an interesting CDK2 inhibitor that inhibits mitosis and induces a subsequent growth arrest. Following its addition, we observe a decrease in specific growth rate, an increase in DNA content, and a drastic increase in the specific productivity of a recombinant protein (IFN-y). We used this inhibitor to increase total IFN-y productivity by 73% in a modified batch culture. With the development of an optimized feed medium, we believe that this CDK2 inhibitor could also be used to increase recombinant protein production in fed-batch cultures. Thesis Supervisor: Title: Daniel I.C. Wang Institute Professor 4 DEDICATION This work is dedicated to my parents, Richard and Sara McClain, and my sister, Erin McClain. You instilled in me the importance of life-long learning from a young age. Without your constant support and encouragement, none of this work would have been possible. ACKNOWLEDGEMENTS As I sit here, I'm overwhelmed by the thought of trying to thank everyone who has helped me up until to this point in my life, thesis, and career. I'm particularly glad that I'm writing this section last, as the crippling writer's block that has set in would surely have discouraged me from writing the rest of this thesis. I'd like to take this time to thank all of those people who have been particularly influential and supportive throughout my thesis. I'd first like to thank my thesis committee members: Professor Charlie Cooney, Professor Harvey Lodish, Professor Kris Prather, and Professor Greg Stephanopoulos. Your advice and support, especially during the times when my experiments just weren't working, is greatly appreciated. Having five committee members made it particularly challenging to get everyone in the same room at one time, and I appreciate your patience in helping to ensure that we could all get together at least a few times. I also would like to thank my thesis advisor, Professor Daniel Wang. I truly appreciate the intellectual freedom you gave me, both inside and outside of lab. You provided me with many opportunities that I would not have otherwise had and have been very encouraging of my career goals. For that I thank you. I'd also like to thank a number of members of the Stephanopoulos and Colton groups that helped me throughout the years. Thanks to both Jeff Millman and Jit Hit Tan for putting up with my use of the Guava. Thanks to Dr. Keith Tyo for initial help with my qRT-PCR work, and Paulo Gameiro for putting up with my use of the iCycler and help with the YSI. I'd also like to thank both past and present members (and visitors) of our own lab: Frederyk Ngantung, Lam Raga Markeley, Andres Abin, and Jin Kiat Ng (SMA). Our conversations have had an immeasurable impact on this thesis. Notably absence from this previous list is Sohan Patel, who deserves particular mention. It wasn't until you left for Singapore that I realized how important it was to have someone with whom to share the highs and the lows of lab work. Your calming presence was certainly something I took for granted, and was missed over these past months. A number of people in the Chemical Engineering Department deserve special recognition. Thank you to the ChemE Student Office, especially Suzanne Easterly, Mary Wesolowski, and Katie Lewis for you help throughout the years. Also, a special thank you to Susan Lanza for everything she has done for the Wang Lab, even while working in BCS. At MIT, we are lucky to have a number of world-class facilities at our fingertips. All of which made my work possible. Thank you to a number of the core facilities at the CCR: the FACS Facility, specifically Glen Paradis, the Biopolymers Facility, and the Microscopy and Imaging Facility. Special thanks also to the Biotechnology Process Engineering Center (BPEC), especially the members of the Griffith group (past and present), Michelle Berry, Dan Darling, Cathy Greene, and Aran Parillo. You've done more than I can thank you for to make my life at MIT easier. I'd also like to say a special thank you to the VWR Stockroom, especially Steve and Monica. I don't think a week (or sometimes day) went by without me frantically searching the shelves for that reagent I forgot to order the week before. Lastly, thanks to Rich Lay and Sara Darcy in the ASO. You were always polite with your reminders that I hadn't submitted my packing slips in months. "I'm not sure what is more amazing, that the people I've met at MIT are as weird as I expected or that I was able to find people who I had so much in common with." That quote is attributed to Brower, who couldn't have put it any better. Thanks to all of the great friends I've made at MIT, specifically the trivia team (Kevin and Jenny, Nate, Dave, and Matt), the lunch group (Melanie, John, Kurt, and Sohan), and Kevin and Rachel Krogman for the Derby and Holiday parties that brought us all back to our "Louisville" roots. I would also like to thank Christos Tsokos for thoughtful discussion. It was your suggestion nearly 3 years ago that led me to work with CDK inhibitors, which eventually formed the backbone of my thesis. Although that conversation has had an immeasurable impact on my work, in comparison with others we have had, it was one of the least important. Christos, thank you for being a true friend. Lastly, I want to thank Leslie Mebane. It is rare to have the opportunity to thank one's significant other for contributions made to a thesis. Thank you for lending your time and microscopy expertise. Without you, the microscope images in Chapter 6 would not have been possible. More importantly, your love and support over this past year have been amazing. I couldn't have done this without you. TABLE OF CONTENTS ABSTRACT .................................................................. 3 ...-...---...... ....... DEDICATION .............................................................. ACKNOW LEDGEMENTS ....................................................................... ........ 5 ..... 7 TABLE OF CONTENTS................................................................. ... 9 LIST OF FIGURES........................................................................ ... LIST OF TABLES .................................................................. ...13 ........... 15 1 INTRODUCTION ...................................................................... 1.1 1.2 1.3 1.4 2 3 BACKGROUND .............................................-----..----------------------------................................. 17 17 18 --.................................................... -...... MOTIVATION .........................................-18 .......................... .. THESIS OBJECTIVES ........................................... 19 THESIS ORGANIZATION .......................................................................... 21 LITERATURE REVIEW ............................................................................... .................... 21 2.1 DEFINING PRODUCTIVITY .................................................. 22 2.2 INCREASING INTEGRATED VIABLE CELL DENSITY (IVCD) ........................................... 22 2.2.1 Medium Design andNutritional Control.............................................................. 2.2.2 Anti-apoptosis Engineering....................................................................................23 23 2.2.2.1 Gene overexpression...................................................................................... 25 2.2.2.2 RNA interference (RNAi) .................................................................................. 2.3 INCREASING SPECIFIC PRODUCTIVITY THROUGH CONTROLLED PROLIFERATION............26 28 2.3.1 Cell Cycle Overview ............................................................................................. 33 2.3.2 Hypothermic Cell Culture ...................................................................................... 34 2.3.3 Culture A dditives ................................................................................................... 36 2.3.4 CellularEngineering............................................................................................. 38 2.3.5 Cell Cycle P hase.................................................................................................... MATERIALS AND METHODS.....................................43 43 43 .......................... 3 .1.1 CellL in es ....................................................................................... 3.1.2 Culture Medium and Maintenance.........................................................................43 43 3.1.2.1 Adherent Cell Cultures .................................................................................... 44 3.1.2.2 Suspension Cell Cultures............................................................................... 44 ................................................................................. 3.1.2.3 Cell Bank Maintenance 45 3.1.3 Cell Enum eration.................................................................................................... 3.1.3.1 Trypan Blue Staining......................................................................................45 45 3.1.3.2 Guava ViaCount ............................................................................................ 46 ............................................. Plates Culture 3.1.4 Pseudo-perfusionCulture in Tissue 46 3.1.5 Fed-batch Culture in Shake Flasks ........................................................................ 47 Concentration................................. Lactate 3.1.6 Measuring Glucose, Glutamine and 47 3.2 CYCLIN-DEPENDENT KINASE ATP-COMPETITIVE INHIBITORS ....................................... 49 ...... 3.3 MRNA ANALYSIS........................................................................... 3.1 CELL CU LTURE .......................................................----..- .. -------------------------------------------- 3.3.1 3.3.2 3.3.3 3 .3 .4 3.3.5 3.3.6 3.4 RNA Pur f cation ....................................................................................................... RNA Quantificationand Quality Assessment ........................................................ First-StrandcDNA Synthesis..................................................................................50 R T-P CR ...................................................................................................................... Real-time PCR Quantification of Ccnel and /-Actin cDNA Levels ..................... CalculatingRelative mRNA Expression............................................................... 49 49 50 51 58 CYCLIN El sIRNA METHODOLOGY .............................................................................. 58 3.4.1 Cyclin Sequencing ................................................................................................. 3.4.2 Cyclin El siRNA Design........................................................................................ 3.4.3 PreparationofsiRNA Duplexes ............................................................................ 3.4.4 siRNA Transfection and RNA Collection................................................................61 3.5 CREATION OF AN INDUCIBLE SHRNA IFN-y CELL LINE .................................................... 3.5.1 PlasmidDescription and Design........................................................................... 3.5.2 PlasmidProduction and Purification.................................................................... 3.5.3 Transfection and Selection of Stable Cell Lines .................................................... 3.5.4 Stable Cell Line Screening: tTS Expression...........................................................68 3.5.5 Stable Cell Line Screening: Inducible shRNA Expression .................................... 3.6 IFN -y EL ISA ..................................................................................................................... 3.7 CALCULATING IFN-y SPECIFIC PRODUCTIVITY ............................................................. 3.7.1 Specific Productivity, qp .......................................................................................... 59 61 61 3.7.2 DifferentialSpecific Productivity, qp sif5.................................................................... 3.8 CELL CYCLE ANALYSIS.................................................................................................73 3.9 IMMUNOSTAINING AND CELL IMAGING ......................................................................... 3.10 GENOMIC DNA PURIFICATION AND QUANTIFICATION ................................................ 3.11 MEASUREMENT OF INTRACELLULAR PROTEIN CONTENT.............................................76 3.12 NOTE ON STATISTICAL ANALYSIS/SIGNIFICANCE ........................................................ 4 CDK2-CCNE1 TARGET VALIDATION .................................................... 4 .1 4.2 4.3 4 .4 IN TR OD U CTION .................................................................................................................. CDK2 INHIBITION WITH A SELECTIVE ATP-COMPETITIVE INHIBITOR ............................ CcNEI SIRNA KNOCKDOWN........................................................................................ C ON CLU SION S ................................................................................................................... 62 62 68 68 70 72 72 73 73 74 75 76 77 77 78 82 92 5 STABLE SHRNA EXPRESSING CELL LINE: A PATH FORWARD ....... 93 5.1 IN TROD U CTION .................................................................................................................. 93 5.2 5.3 5.4 PSINGLE-TTS-ANTI CCNE1 SHRNA ............................................................................... 94 5.5 TETRACYCLINE-CONTROLLED TRANSCRIPTIONAL SUPPRESSOR (TTS) CELL LINE ............ 96 PSILENCER-TETO 7 -CMV-ANTI CCNE1 SHRNA EXPRESSING CELL LINE ........................ 102 C ON CLU SION S ................................................................................................................. 114 6 SHORT TERM CDK INHIBITION: GROWTH, CELL CYCLE, AND PRODUCTIVITY ............................................................................................ 6.1 6.2 6.3 6.4 6.5 IN TRODU CTION ................................................................................................................ 115 115 116 RELATIONSHIP BETWEEN SPECIFIC PRODUCTIVITY AND CELL CYCLE DISTRIBUTION ..... 130 RELATIONSHIP BETWEEN SPECIFIC PRODUCTIVITY AND SPECIFIC GROWTH RATE .......... 133 FURTHER CHARACTERIZATION OF INHIBITOR CDK2IV ..................................................... 137 CDK INHIBITOR CHARACTERIZATION ............................................................................. 6.6 C ON CLU SION S .................................................-----..-. -------------------------------------------.------ 146 INCREASING TOTAL PRODUCTIVITY THROUGH CDK...........149 ..... 149 INHIBITION.......................................................................--149 7.1 IN TRODU CTION ..................................................---....----.-.------.---.------------.------.-----.-.------.--149 7.2 ADDITION OF CDK INHIBITORS IN BATCH CULTURES ..................................................... 7 7.3 7.4 7.5 8 ADDITION OF INHIBITOR CDK2IV IN A MODIFIED BATCH CULTURE .................................. ADDITION OF INHIBITOR CDK2IV IN A MODIFIED FED-BATCH CULTURE ......................... CONCLUSIONS ....................................................----..................................................... CONCLUSIONS AND RECOMMENDATIONS .................... 8.1 THESIS CONCLUSIONS ...................................................... 8.2 RECOMMENDATIONS FOR FUTURE WORK....................................................... 153 158 164 167 167 169 169 8.2.1 Inducible shRNA Expression Systems...................................................................... 1 70 .... .. ................................... 8.2.2 Overexpression of m iRNA ..................................... 1 71 8.2.3 Screeningfor Less Toxic Inhibitors......................................................................... 171 ................................... Culture Cell Arrested Growth 8.2.4 Optimized Feed Medium for 172 8.2.5 InhibitorAddition in Perfusion Culture .................................................................. 1 72 8 .2 .6 Con clusion ............................................................................................................... NOMENCLATURE..............................................................................................175 REFERENCES........................................................................................179 APPENDIX ................................................................................ 11 -........ 191 12 LIST OF FIGURES FIGURE 2-1. REGULATION OF THE G1 TO S-PHASE CHECKPOINT IN THE MAMMALIAN CELL CYCLE.31 FIGURE 2-2. REGULATION OF THE G2 TO M-PHASE CHECKPOINT IN THE MAMMALIAN CELL CYCLE. .3 2 . ----------------------------------------- .---------............................ ......................................................... FIGURE 3-1. REAL-TIME PCR FLUORESCENCE VERSUS PCR CYCLE NUMBER. ........................... 54 FIGURE 3-2. THRESHOLD CYCLE (CT) AS A FUNCTION OF THE LOG OF DNA COPY NUMBER...........55 FIGURE 3-3. MELTING CURVE ANALYSIS...............................................56 64 FIGURE 3-4. PLASMID MAP OF PSINGLE-TTS-SHRNA VECTOR. ............................................ FIGURE 3-5. PLASMID MAPS OF PTTS-NEO AND A MODIFIED PSILENCER 4.1 VECTOR.................65 66 .... .......................... FIGURE 3-6. THE TTS SYSTEM............................................ FIGURE 3-7. PLASMID MAP OF THE MODIFIED PSINGLE VECTOR, PSINGLE-NO TTS-ANTI-LUC 71 SHRN A ..................................................................-......................................................... FIGURE 4-1. GROWTH CURVES OF ADHERENT CULTURES WITH OR WITHOUT THE ADDITION OF 2 [iM 80 - - - - - - --..................................................... INHIBITOR CDK2III. ............................................--..-.. FIGURE 4-2. IFN-y PRODUCTION VERSUS INTEGRATED VIABLE CELL DENSITY (IVCD) WITH AND 81 WITHOUT THE ADDITION OF 2 IM INHIBITOR CDK2III. ................................................. FIGURE 4-3. RELATIVE CCNE] MRNA EXPRESSION IN CHO CELLS TRANSFECTED WITH 13 UNIQUE 86 SIR N AS . ................................................................................................................................. FIGURE 4-4. GRAPHICAL REPRESENTATION OF FIGURE 4-3..........................................87 FIGURE 4-5. CONSISTENCY OF RELATIVE CcNE1 MRNA EXPRESSION FOLLOWING TRANSFECTION 88 W ITH MOST EFFECTIVE SIRN A S .......................................................................................... 97 5-1. RT-PCR OF TTS EXPRESSING SINGLE CELL COLONIES. ............................................ 100 5-2. LUCIFERASE KNOCKDOWN IN TTS EXPRESSING CELL LINES. ................................... FIGURE 5-3. INDUCTION OF LUCIFERASE KNOCKDOWN IN TTS-2..................................................101 FIGURE 5-4. FIRST ROUND SCREEN OF PSILENCER-TETO 7-ANTI CCNEl SHRNA EXPRESSING SINGLE -----......................................................-- 104 CELL COLONIES...................................................... FIGURE 5-5. SECOND ROUND SCREEN OF BEST PERFORMING SINGLE CELL COLONIES FROM THE FIRST FIGURE FIGURE 105 . - - - - - . - - --...................................................... ROUND SCREEN. .................................................DOXYCYCLINE. OF LEVELS INCREASING TO FIGURE 5-6. DOSE-RESPONSE OF SINGLE CELL COLONIES 10 7 ............................................................................................................................................. 100 OF ADDITION THE WITHOUT OR WITH CLONES CELL SINGLE FIGURE 5-7. GROWTH CURVES OF 12 .... NG MUL DOXYCYCLINE......................................................---............ CELL GROWTH CURVES IN THE PRESENCE OF INHIBITORS CDK2III AND CDKlIV......... 118 CELL GROWTH CURVES IN THE PRESENCE OF INHIBITORS CDK4 AND CDK2IV............ 119 CELL CYCLE DISTRIBUTION IN EXPONENTIALLY GROWING CELLS. ........................... 123 CUMULATIVE IFN-y PRODUCTION PLOTTED AGAINST INTEGRATED VIABLE CELL 129 DENSITY (IVCD) FOR EACH INHIBITOR ............................................................. CYCLE CELL AND FIGURE 6-8. CORRELATIONS BETWEEN NORMALIZED SPECIFIC PRODUCTIVITY 132 PHASE FOLLOWING INHIBITOR ADDITION. .................................................................. SPECIFIC FIGURE 6-9. COR RELATION BETWEEN NORMALIZED SPECIFIC PRODUCTIVITY AND FIGURE 6-1. FIGURE 6-2. FIGURE 6-3. FIGURE 6-7. GROWTH RATE FOLLOWING INHIBITOR ADDITION. .............................................................. 135 FIGURE 6-10. FIGURE 6-11. FIGURE 6-14. THE EFFECT OF NORMALIZED SPECIFIC PRODUCTIVITY AND SPECIFIC GROWTH RATE ON TOTAL IFN -y PRODUCTION . ............................................................................................. 136 CELL CYCLE DISTRIBUTIONS FOLLOWING THE ADDITION OF THE INHIBITOR CDK2IV. ............................................................................................................................................. 13 9 FIGURE 6-12. 60X IMAGES OF STAINED 7-CHO CELLS 24 HOURS POST-INHIBITOR TREATMENT... 140 FIGURE 6-13. CELL GROWTH CURVES FOLLOWING THE ADDITION AND SUBSEQUENT REMOVAL OF IN H IBITO R CD K 2IV . ............................................................................................................... 143 CUMULATIVE IFN-y PRODUCTION PLOTTED AGAINST INTEGRATED VIABLE CELL DENSITY (IVCD) FOLLOWING THE ADDITION OF INHIBITOR CDK2IV.....................................144 FIGURE 6-15. RELATIVE GENOMIC DNA CONTENT FOLLOWING INHIBITOR CDK2IV ADDITION. ... 145 7-1. SUSPENSION y-CHO GROWTH AND VIABILITY CURVES IN THE PRESENCE OF IN H IB IT O R S. .......................................................................................................................... FIGURE FIGURE 7-2. CDK 15 1 CDK INHIBITORS............152 FIGURE 7-3. CUMULATIVE IFN-7 TITER PLOTTED AGAINST INTEGRATED VIABLE CELL DENSITY FOR EA CH IN H IBITOR ............................................................................................................. 152 FIGURE 7-4. SUSPENSION CHO-IFNy GROWTH AND VIABILITY CURVES IN THE PRESENCE OF CDK INHIBITORS IN A MODIFIED BATCH CULTURE ......................................................................... 154 CUMULATIVE IFN-y TITER FOLLOWING THE ADDITION OF FIGURE 7-5. CUMULATIVE IFN-y TITER FOLLOWING THE ADDITION AND REMOVAL OF A CDK INHIBITOR IN A MODIFIED BATCH CULTURE...........................................................................156 FIGURE 7-6. CUMULATIVE IFN-y TITER PLOTTED AGAINST INTEGRATED VIABLE CELL DENSITY FOLLOWING THE ADDITION AND REMOVAL OF A CDK INHIBITOR IN A MODIFIED BATCH CU L TUR E ............................................................................................................................... 15 6 FIGURE 7-7. TOTAL PROTEIN CONTENT FOLLOWING THE ADDITION OF INHIBITOR CDK2IV. .......... 157 CHO-IFNy GROWTH AND VIABILITY CURVES IN THE PRESENCE OF INHIBITOR CDK2IV IN A MODIFIED FED-BATCH CULTURE.......................................................159 FIGURE 7-9. OVERLAY OF MODIFIED BATCH AND FED-BATCH CHO-IFNr' SUSPENSION GROWTH FIGURE 7-8. SUSPENSION CURVES FOLLOWING THE ADDITION OF INHIBITOR CDK2IV. .................................................. 160 FIGURE 7-10. CUMULATIVE IFN-y TITER FOLLOWING THE ADDITION AND REMOVAL OF INHIBITOR CK2IV IN A MODIFIED FED-BATCH CULTURE..........................................................................162 FIGURE 7-11. CUMULATIVE IFN-y TITER PLOTTED AGAINST INTEGRATED VIABLE CELL DENSITY FOLLOWING THE ADDITION AND REMOVAL OF INHIBITOR CDK2IV IN A MODIFIED FED-BATCH CU L TU RE ............................................................................................................................... FIGURE 7-12. OVERLAY OF CUMULATIVE IFN-7 TITER PLOTTED AGAINST IVCD FOR MODIFIED BATCH AND FED-BATCH CULTURES FOLLOWING THE ADDITION OF INHIBITOR CDK2IV. ........ 16 2 163 LIST OF TABLES TABLE 2-1. SUMMARY OF METHODS TO IMPROVE VOLUMETRIC PRODUCTIVITY BY INCREASING THE IVCD .....................................................................----------------------------------------......................... TABLE 2-2. SUMMARY OF METHODS TO IMPROVE VOLUMETRIC PRODUCTIVITY BY INCREASING 41 SPECIFIC PRODUCTIVITY, Qp...................................................................--42 3-1. 1C5 0 AND K, VALUES OF ATP-COMPETITIVE CDK INHIBITORS ..................................... 48 52 TABLE 3-2. REAL-TIM E PCR PRIMERS ....................................................................................... TABLE 3-3. QRT-PCR EFFICIENCY FOR CDNA STANDARD CURVES............................................57 TABLE 3-4. PRIMER SEQUENCES FOR THE AMPLIFICATION OF CYCLIN CDNA TEMPLATES.............60 TABLE 3-5. SHRNA HAIRPIN SEQUENCES FOR INSERTION INTO INDUCIBLE EXPRESSION VECTORS.67 TABLE 4-1. A SUMMARY OF THE EFFECT OF INHIBITOR CDK2III ON SPECIFIC..................................81 TABLE 4-2. CCNE1 SIRNA SEQUENCES DESIGNED USING MODIFIED TUSCHL RULES DEVELOPED BY 85 REYNOLDS ET AL. (2004) AND UI-TEI ET AL. (2004) .......................................................... TABLE 4-3. FOLD-INCREASE IN Qp, DIFF RELATIVE TO A NON-SILENCING CONTROL FOLLOWING ...... 91 SIRNA KNOCKDOWN OF CcNEJ EXPRESSION (N=2) .............................................. TABLE TABLE 5-1. SCREENING SUMMARY OF INDUCED CcNE1 KNOCKDOWN FOLLOWING 100 NG ML' 109 ........................... DOXYCYCLINE ADDITION .................................................. 1 CELL SINGLE TO ADDITION TABLE 5-2. SUMMARY OF THE EFFECTS OF 100 NG ML DOXYCYCLINE CLONES.......................................................-----.................................................................... TABLE 6-1. TIME-INTEGRATED (WEIGHTED) CELL CYCLE DISTRIBUTION ..................................... TABLE Al. SEQUENCING SUMMARY OF KNOWN CHO CYCLINS ............................................. 13 127 191 16 1 INTRODUCTION 1.1 Background There are currently over 165 biopharmaceuticals approved for use in humans with a projected market size of $70 billion by the end of 2010 (Durocher and Butler, 2009; Walsh, 2006). Approximately 60-70% of these are recombinant proteins produced in mammalian cells, and the most common production host is the immortalized Chinese hamster ovary (CHO) cell (Birch and Racher et al., 2006; Durocher and Butler, 2009; Hacker et al., 2009; Wurm, 2004). In 2006, CHO cells were used to produce 62 of the approximately 107 protein therapeutics (58%) manufactured in mammalian cells (Walsh, 2006). Mammalian cell culture is the preferred method of producing recombinant proteins because of its inherent ability to provide posttranslational modification, specifically glycosylation, to secreted proteins. CHO cells, in particular, produce glycan structures that are very similar to those naturally isolated from humans (Walsh and Jefferis, 2006). In addition to their ability to perform post-translational modifications, CHO cells are used in industrial scale production of therapeutic proteins for a number of other reasons. CHO cells allow for the stable introduction of chromosomally integrated heterologous transgenes (Schimke, 1984), they have the ability to adapt to protein-free suspension culture (Meents et al., 2002) and there are readily available dihydrofolate reductase mutants (DHFR~) that enable the amplification of recombinant product-encoding regions of chromosomes (Kaufman et al., 1983; Urlaub and Chasin, 1980) which results in high expression levels (Fox, 2005a). In this work, we use a CHO cell line expressing interferon-gamma (IFN-y) created from a DHFR- CHO cell line (Scahill et al., 1983). One disadvantage of using CHO cells, when compared with bacteria and yeast cells, is that they produce comparatively smaller amounts of recombinant proteins. Over the past two decades, order-of-magnitude improvements have been made to the productivity of CHO cells (Wurm, 2004). However, work is still ongoing to improve the production of glycoproteins from mammalian cells by improving glycosylation quality and consistency, and increasing overall productivity. 1.2 Motivation It is estimated that 15 different protein therapeutics with total sales of over $20 billion will lose patent protection over the next 5 years (Lanthier et al., 2008). Also, according to the PhRMA, there are currently more than 600 biopharmaceuticals currently in the clinic. Any improvements that can be made in the production of therapeutic proteins will result in substantial cost savings for the biotechnology industry and patients taking these medications. Our work specifically focuses on improving total volumetric recombinant protein production in CHO cells. 1.3 Thesis Objectives The central goal of this thesis is to develop new ways of increasing total volumetric productivity through induced growth arrest. Within this main goal, the thesis has three major objectives. First, to determine if the inhibition or silencing of the CcnE 1-CDK2 protein complex is sufficient to induce growth arrest and subsequently increase specific productivity. Second, to better understand the relationship between the specific growth rate, cell cycle phase, and the specific productivity of IFN-y. Third, to develop a method to improve total productivity through growth arrest by inhibiting the CcnEl-CDK2 protein complex. 1.4 Thesis Organization This thesis is divided into eight chapters. Chapter 2 provides a literature review of the previous methods used to improve productivity in CHO cell cultures. In Chapter 3, the materials and methods used in this thesis are explained in detail. Chapter 4 demonstrates that the silencing or inhibition of the CcnEl-CDK2 complex results in growth arrest and increases specific IFN-y productivity. Chapter 5 details the work performed towards the creation of a stable cell line expressing shRNA against CcnE1. In Chapter 6, the relationship between specific growth rate, cell cycle distribution, and specific productivity is analyzed. In addition, the characterization of an interesting CDK2 inhibitor, cdk2iv, is described. In Chapter 7, the inhibitor cdk2iv is used to improve total volumetric productivity of IFN-y in suspension culture. Chapter 8 presents the concluding remarks and recommendations for future research. A nomenclature section and a list of cited references follow Chapter 8. The mRNA sequences of CHO cyclins are presented in the Appendix. 20 2 LITERATURE REVIEW Our main goal in this thesis is to develop methods for improving total recombinant protein production. Towards this end, it is useful to better understand the work that has already been done in this area. To begin, we would like to briefly describe how productivity is defined and what key factors contribute to it. 2.1 Defining Productivity The specific productivity, qp (g protein cell-' day-'), is a measure of the amount of protein produced per cell per unit of time. At any give time, it is defined as the slope of the production, P (g protein mL'), vs. time curve, divided by the total number of viable cells, dP = dt qpN,,ajlf Nviable (viable cells (2-1) Because we assume that all of the recombinant protein that is produced is secreted from the cell, and not released by cell death or following cell lysis, we can also assume that the qp remains constant over the course of a cell culture (Renard et al., 1988). This allows us to define the total volumetric production of recombinant protein, P, as: P = qpJ Ndt = qp -IVCD (2-2) The total amount of protein produced is therefore directly related to the specific productivity, qp, and the Integrated Viable Cell Density, IVCD (cells day mL'), which can be visualized as the area under the viable cell density curve (Renard et al., 1988). Therefore, in order to increase total volumetric production one must increase the specific productivity, the IVCD, or both. We will focus this review on past approaches that have led to increases in total volumetric productivity. A summary of all of the methods used to increase total volumetric productivity is shown at the end of this chapter in Tables 2-1 and 2-2. 2.2 Increasing Integrated Viable Cell Density (IVCD) The approaches for improving productivity by increasing the IVCD in a cell culture generally fall into one of two categories: (1) medium design and nutritional control and (2) cellular engineering. 2.2.1 Medium Design and Nutritional Control Nutritional control and medium design are ways that cell cultures can be improved without changing or modifying the CHO cells that are used in a production process. Using cell culture medium to increase the IVCD was one of the major motivations behind fed-batch cell culture development. It is well understood that ammonia and lactate, waste metabolites of glycolysis and glutamine catabolism, inhibit cell growth which results in a decrease in the maximum achievable cell density and limits recombinant protein production (Hassell et al., 1991; Lao and Toth, 1997). The presence of high concentrations of glucose in culture medium results in the majority being converted to lactate by lactate dehydrogenase (LDH) (Kim and Lee, 2007; Neermann and Wagner, 1996). Ammonia is the direct byproduct of glutamine degradation to glutamate by glutaminase and the end product of glutamine catabolism (Glacken et al., 1986; Nelson and Cox, 2000). By feeding glucose, glutamine, and other nutrients into cell cultures at controlled rates, waste metabolite formation can be minimized. Stoichiometric fed-batch cultures have been used in a number of different cell culture systems to drastically extend culture time, to increase the maximum achievable cell density, and to improve the overall production of recombinant proteins (Xie et al., 1997; Xie and Wang, 1994). Xie et al. (1997) performed nutrient feeding every 12-24 hours, which resulted in a 10-fold increase in IVCD and a resulting 4.5-fold increase in the final IFN-y titer. Although an improvement from batch culture, the addition of nutrients every 12-24 hours still results in the addition of excess glucose and glutamine to a cell culture. Improved dynamic online feeding strategies have been developed to further tighten control of nutrient feeding, resulting in more efficient metabolism (Glacken et al., 1986; Wong et al., 2004; Zhou et al., 1995). As an example, in a 12-hour feeding strategy, the glutamine setpoint is typically 1 mM (Lim et al., 2006). However, by dynamically feeding to a glutamine set point of 0.3 mM, Wong et al. (2004) increased total IFN-y productivity by 10-fold. 2.2.2 Anti-apoptosis Engineering The prolonged culturing of cells is almost always limited by cell death caused by a multitude of factors including nutrient deprivation, oxygen limitation, and shear stress. The largest source of cell death in bioreactors has been attributed to apoptosis or programmed cell death (Goswami et al., 1999; Vives et al., 2003). Apoptosis occurs in fed-batch cultures even when there are no oxygen or nutrient limitations, and is the result of a complex network of signaling pathways that ultimately activate cyteine aspartate proteases (caspases) that perform the final stages of cell death (Wong et al., 2006a; Majors et al., 2007). Inhibiting or decreasing apoptotic signaling can extend cell cultures, leading to increases in IVCD. 2.2.2.1 Gene overexpression Possibly the most common genetic approach to minimizing apoptotic signaling is through the overexpression of Bcl-2 (Goswami et al., 1999; Meents et al., 2002; Tey et al., 2000) and Bcl-xL. (Chiang and Sisk, 2005; Meents et al., 2002). Both Bcl-2 and Bcl-xL, are characterized as antiapoptotic proteins and act to maintain the mitochondria membrane potential and prevent the release of cytochrome c during apoptotic insults (Majors et al., 2007). Although the overexpression of both Bcl-2 and Bcl-xL has been shown to increase cell viability and extend culture time, only the overexpression of Bcl-xL consistently results in an increase in overall productivity (Meents et al., 2002). Chiang and Sisk (2005) increased both specific and total productivity of a monoclonal antibody by 2-fold through the overexpression of Bcl-xL. In addition, the overexpression of Bcl-xL has been shown to increase specific productivity, contributing to increases in product titer (Fussenegger et al., 1998; Meents et al., 2002). The use of selection markers, such as neomycin, has been shown to enhance naturally occurring levels of Bcl-2 and makes it more difficult to determine the effect of Bcl-2 overexpression on productivity (Tey et al., 2000). Another approach towards increasing the IVCD is the overexpression of naturally occurring caspase inhibitors like the X-linked inhibitor of apoptosis protein (XIAP) (Sauerwald et al., 2002). While the overexpression of XIAP has been shown to inhibit apoptosis and protect against various insults including serum deprivation and viral infection, the impact on recombinant protein production is not known. With the advent of new genomic tools, production cell lines can be examined in entirely new ways (Kantardjieff et al., 2009). Most recently, the transcriptome analysis of apoptosis signaling pathways in fed-batch cultures, showed downregulation of Faim, a pro-survival gene, and upregulation of FADD, which is involved in death receptor apoptosis signaling, upon entry into stationary phase (Wong et al., 2006a). By overexpressing Faim and a dominant negative of FADD (FADD DN), apoptosis was inhibited, resulting in increases in. IVCD and 1.5-fold increases in recombinant IFN-y product titer (Wong et al., 2006b). Dominant negative overexpression is one of the easiest ways to down regulate the activity of a particular protein. However, it is often the case that the functional domains of particular proteins are not well defined, making it difficult to design a dominant negative approach to down-regulation (Wong et al., 2006b). In these cases, RNA interference represents a potential solution. 2.2.2.2 RNA interference (RNAi) RNA interference (RNAi) refers to the specific post-transcriptional down-regulation of gene expression by small-double stranded RNA (Dykxhoorn et al., 2003; McManus and Sharp, 2002). Originally discovered in C. elegans (Fire et al., 1998), RNAi has been shown to occur in a number of other cell types including CHO cells. The mechanism of RNAi is comprised of two main steps: (1) the processing of long double-stranded RNA by the RNase III-like enzyme Dicer to 21-23 bp small interfering RNA (siRNA) and (2) the cleavage of the homologous mRNA through incorporation of siRNA into RNA-induced silencing complex (RISC). (Dykxhoorn et al., 2003). The use of RNAi has been used extensively in the recent past to improve the productivity of CHO cells (Wu, 2009). One striking advantage of RNAi when compared with gene overexpression is that it does not overcharge the translational machinery of the host cell (Muller et al., 2008). A number of groups have recently targeted the pro-apoptotic genes Bax and Bak (Lim et al., 2006), Alg-2 and Requiem (Wong et al., 2006b), and Caspase-3 and Caspase-7 (Sung et al., 2007) with RNAi. The simultaneous knockdown of Bax and Bak, increased resistance to a variety of apoptotic stimuli like nutrient depletion and high-osmolality medium, extended the culture lifespan, and increased total IFN-y product titer by 35% (Lim et al., 2006). The separate knockdown of Alg-2 and Requiem suppressed caspase activation, drastically increased the maximum achievable cell density in a fed-batch bioreactor, and increased overall IFN-y product titer by 2.5-fold (Wong et al., 2006b). Lastly, the simultaneous knockdown of caspase-3 and caspase-7 increased the achievable maximum cell density, extended the culture lifespan, and increased the final product titer of thrombopoietin by 1.5-fold in the presence of 1 mM sodium butyrate (Sung et al. 2007). Often, anti-apoptotic engineering increases IVCD mainly through the extension of culture time and not by increasing the maximum attainable cell density. In these cases, the benefits of increased product titer must be balanced with the cost of bioreactor maintenance. 2.3 Increasing Specific Productivity through Controlled Proliferation The alternative to increasing the IVCD, either by increasing the maximum achievable cell density or culture lifetime, is to increase the amount of recombinant protein produced per cell per time. This can be done in a number of ways, including by improving expression technology (Ng et al., 2007; Prentice et al., 2007b), by secretion engineering (Tigges and Fussenegger, 2006), by host cell development (Prentice et al., 2007a), and by targeted vector insertion (Barron et al., 2007). All of these techniques are considered outside the scope of this thesis. Another common method used to increase specific productivity is through controlled proliferation. Controlled proliferation has a number of advantages: (i) it extends culture times, sometimes resulting in increases in IVCD, (ii) it lowers the release of intracellular proteases and glycosidases resulting in improved product quality, (iii) it decreases medium and nutrient consumption, (iv) it lowers genetic drift of the cell population because of reduced cell growth, and most importantly (v) it increases specific productivity as intracellular resources can be redirected towards product production in the absence of growth (Fussenegger and Bailey, 1999). We will be focusing on this last point for the remainder of this thesis. The potential of controlled proliferation was demonstrated in the pioneering modeling and experimental work of Suzuki and Ollis (1989; 1999), who showed an inverse relationship between growth and productivity (Fussenegger and Bailey, 1999). This initial work in controlled proliferation involved the addition of toxic chemicals to the culture medium of hybridoma cells, including the DNA-synthesis inhibitors hydroxyurea and thymidine (Al-Rubeai at. al., 1992; Suzuki and Ollis, 1990), the protein synthesis inhibitors cycloheximide and potassium acetate, and the rRNA synthesis inhibitor Actinomycin D (Suzuki and Ollis, 1990). Hydroxyurea, thymidine, and potassium acetate addition all led to a slowed growth and a subsequent increase in specific productivity. However, the toxicity of these compounds prevented extended cultivation in their presence. Interestingly, the addition of chemicals inhibiting protein elongation and rRNA synthesis produced non-growth or positive-growth associated responses (Suzuki and Ollis, 1990). It is important to note that the addition of some chemicals may slow growth through metabolic or unknown mechanisms that are detrimental to recombinant protein production, and should not be used in controlled proliferation studies. While most of this pioneering work was performed in hybridoma cells, an inverse relationship between growth and productivity in CHO cells has also been shown by a number of researchers. The first such demonstration was performed by Jenkins and Hovey (1993) where the use of temperature to control growth resulted in increases in the productivity of recombinant tissue inhibitor of metalloproteinases (TIMP). 2.3.1 Cell Cycle Overview We will begin with a brief overview of cell cycle progression because the mechanisms involved are critically important in controlled proliferation strategies. The cell cycle can be described as an organized series of events that leads to cell division and the creation of two daughter cells each with a pair of chromosomes identical to the parent cell (Lodish et al., 2004). The cell cycle itself was originally thought to compose two distinct phases: interphase and mitosis. Interphase is further comprised of three phases, S-phase where the genetic material of the cell is replicated and two gap phases, G1 and G2, where the cell prepares for entry into either S-phase or mitosis (M-phase) respectively (Sunley and Butler, 2010; Vermeulen et al., 2003). Progression through the cell cycle is tightly controlled by a network of signaling pathways referred to as cell cycle checkpoints that are shown in Figures 2-1 and 2-2 (Lukas et al., 2004). The key regulatory proteins in this process are the cyclin-dependent kinases (CDK), which are activated following the association with their positive regulatory subunit, the cyclin (Ekholm and Reed, 2000; van den Heuvel and Harlow, 1993). CDK activity can be repressed by cell cycle inhibitory proteins (CKI), which either bind to the CDK alone or the CDK-cyclin complex (Vermeulen et al., 2003). There are two distinct families of CKIs, the INK4 family (p1 5 INK4b INK4a INK4c 4 INK d), which specifically inactive the G1 CDKs (CDK4 and CDK6) prior to cyclin binding, and the Cip/Kip family (p21 wafi/cipI and p27 Cip2), which specifically inactivate G1 CDK-cyclin complexes after cyclin binding (Harper et al., 1993; Toyoshima and Hunter, 1994; Vermeulen et al., 2003). To prevent entry into S-phase with damaged DNA, checkpoint kinases phosphorylate Cdc25A phosphatase and the p53 transcription factor (Kastan et al., 1991; Lukas et al., 2004). The phosphorylation of Cdc25a prevents the activating dephosphorylation of CDK2 (Falck et al., 2001; Mailand et al., 2000). Phosphorylation of p53 contributes to its stabilization and increased activity. The key effector of p53-dependent transcription is the p21 wafi/cip CKI (El-Deiry et al., 1994; Lukas et al., 2004). The accumulation of p21wafl/ciPl to levels capable of blocking the Gi/S-promoting complex CDK2-cyclin E (CKD2-CcnE) can require several hours, which complements the more transient and acute inhibition of CDK2 through Cdc25A. Once cells pass the restriction point (R) in G, and then enter S-phase they are committed to progress through the entire cell cycle. Additional controls and checkpoints also mediated by Cdc25A and p53 exist to ensure an orderly sequence of events (Vermeulen et al., 2003). The downstream target of G-phase CDK regulation is the retinoblastoma protein (Rb), which upon phosphorylation leads to the disruption of its complex with histone deacetylase protein (HDAC) and the release of transcription factors E2F-1 and DP-1. E2F-1 and DP-1 positively regulate the transcription of genes whose products are necessary for S phase progression (Magae et al., 1996; Vermeulen et al., 2003; Weinberg, 1995). Cancer can be described as a breakdown in the cell cycle regulatory process caused by DNA damage (Kastan and Bartek 2004). This results in fundamental alterations in the genetic control of cell cycle progression, which often results in unrestrained cell division. Some examples of these alternations include, inactivation of tumor suppressor genes like pRb and p53, and mutations of proteins important at different levels of the cell cycle like CDKs, cyclins, CKIs, and checkpoint proteins (Vermeulen et al., 2003). The evidence that CDKs, their regulators, and substrates are often targets of genetic alterations in different types of human cancers has encouraged the search for CDK inhibitors. All of the CDK inhibitors that have been identified act by competitively inhibiting the binding of ATP to the CDK (Vermeulen et al., 2003). A larger number of these inhibitors are commercially available, many of which we use in our work and will be described in more detail later. Growth Factor Receptor Activation 1-Ubiquination Genes: E2F/DP Target Tb A FOFasL --- > Apopioss TRAIL - EF2c2. -RanGAP, c-Myc. p107, TK, DHFR, PCNA. K2A, etc. -I 'popiois OFF ON Figure 2-1. Regulation of the G, to S-phase checkpoint in the mammalian cell cycle. Diagram reproduced from Cell Signaling Technology, Inc. (www.cellsignal.com). PLNuctir Exusio n cdc,25 sCF Figure 2-2. Regulation of the G2 to M-phase checkpoint in the mammalian cell cycle. Diagram reproduced from Cell Signaling Technology, Inc. (www.cellsignal.com). 2.3.2 Hypothermic Cell Culture Typically, mammalian cells are cultured at 37 *C. However, a number of researchers have shown that culturing CHO cells at temperatures below 37 "C results in a decrease in specific growth rate and a subsequent increase in specific productivity (Furukawa and Ohsuye, 1998; Hendrick et al., 2001; Kaufmann et al., 1999; Yoon et al., 2003). This increase in specific productivity has been shown to be due, at least in part, to an increase in mRNA stability (Fox et al., 2005b; Furukawa and Ohsuye, 1998; Sonna et al., 2002). In addition to increases in productivity, decreasing culture temperature causes a specific Go/Gi-phase growth arrest, suppression of apoptosis, and an overall reduction in metabolism (Moore et al., 1997). The Go/Gi-phase arrest has been shown to occur through p21 Wavf/cIP by a p53-dependent mechanism (Ohnishi et al., 1998). A decrease in specific growth rate is related to a decrease in IVCD and therefore the increase in specific productivity caused by low temperature cultivation does not necessarily result in an increase in volumetric productivity. In a number of circumstances, cultivation at 30 "C did not result in an increase in total recombinant protein productivity (Furukawa and Ohsuye, 1998). In order to increase total productivity, one must balance decreases in IVCD with increases in qp, which can be done in one of two ways: (1) a biphasic culture, where cells are grown to an elevated cell density at 37 C followed by a temperature shift to 30-32 "C, or (2) a slightly hypothermic culture (i.e. 33-35 0C). A number of researchers have utilized biphasic growth to improve the production of human granulocyte macrophage colony stimulating factor (Bollati-Fogolin et al., 2005), t-PA (Hendrick et al., 2001), secreted alkaline phosphatase (Kaufmann et al., 1999), and an X-amidating enzyme (Furukawa and Ohsuye, 1999). The time at which the temperature shift occurs can be optimized to yield the largest possible increase in recombinant protein productivity. In the case of IFN-y production, Fox et al. (2004) showed that a temperature shift to 32 "C after 3 days of growth resulted in a 90% increase in total production when compared to a 37 "C control. Rather than utilizing a temperature shift culture, researchers culturing CHO cells at 35 C were able to increase the total production of an a-amidating enzyme by 1.8-fold (Furukawa and Ohsuye, 1998) and researchers culturing CHO cells at 33 "C and lower were able to increase the total production of erythropoietin in both batch (Yoon et al., 2003) and perfusion cultures (Ahn et al., 2008) by 2.5- and 6.5-fold, respectively. Lastly, a particularly novel approach, developed by members in our own lab, involves mutagenizing and selecting for cells that grow under hypothermic conditions. By growing cells under hypothermic conditions, specific productivity can be increased without a subsequent decrease in IVCD resulting in a 7.7-fold increase in total productivity when compared to a 37 "C control (Fox, 2005a; Fox et al., 2005c). This work also suggests that improvements in specific productivity in low temperature culture may mainly be caused by increases in mRNA stability rather than growth arrest. 2.3.3 Culture Additives The addition of chemicals to cell cultures with the purpose of improving specific productivity began with toxic chemicals like thymidine and hydroxyurea (Suzuki and Ollis, 1990). The disadvantages of using these chemicals have been described previously. The most common culture additive used industrially is sodium butyrate (NaBu), a sodium salt of butyric acid. It has been used extensively in CHO cells to increase the productivity of t-PA (Hendrick et al., 2001; Palermo et al., 1991), EPO (Chang et al., 1999), IFN- (Rodriguez et al., 2005), and other recombinant proteins (Dorner et al., 1989). It has long been known that butyrate is a reversible non-competitive inhibitor of histone deacetylase and causes the accumulation of hyperacetylated histones (Cousens et al., 1979; Riggs et al., 1977). Histones are the DNA-binding proteins that comprise nucleosomes, which bind DNA and repress transcription in vivo and in vitro. In unacetylated histones, the positively charged n-terminal lysines interact strongly with negatively charged DNA phosphates. The addition of butyrate acts to make DNA more accessible, resulting in an increase in transcription and translation (Berger, 2002; Grunstein, 1997; Jiang and Sharfstein, 2008). In addition, butyrate is also known to cause a Go/Gi-phase arrest (D'Anna et al., 1980; Hendrick et al., 2001). It does this in two ways, by activating the p2 1 WAF/Cip1 promoter in a p53-independent manner (Lagger et al., 2002; Nakano et al., 1997) and by inhibiting phosphorylation and inducing dephosphorylation of Rb (Buquet-Fagot et al., 1996; Schwartz et al., 1998). Unfortunately, the addition of butyrate is also known to induce apoptosis, preventing its use in extended fermentations (Kim and Lee, 2001). In order to increase total recombinant protein production, anti-apoptosis engineering methods have been combined with butyrate addition. By adding butyrate to CHO cells overexpressing Bcl-2 (Kim and Lee, 2001) or silencing Caspase-3 and Caspase-7 (Sung et al., 2007), recombinant protein production was increased by 2- and 1.5fold respectively. A number of other small molecule additives have been screened for their effect on total volumetric recombinant protein productivity. Following an extensive screening of common nucleotides, nucleosides and bases, Carvalhal et al. (2003b) found that the addition of adenosine monophosphate (AMP) to CHO cells producing secreted alkaline phosphatase (SEAP) resulted in a 1.6-fold increase in total productivity. However, the culture time was also extended by more than 2-fold. Interestingly, it was observed that AMP caused an accumulation of cells in S-phase, though the exact reason for this accumulation is unknown. 2.3.4 Cellular Engineering So far we have discussed a number of externally applied methods that induce growth arrest and subsequently increase the specific productivity of recombinant protein producing cell lines. These methods, including the lowering of culture temperature and the addition of chemicals to the culture media, impact the cell cycle signaling network by up and down regulating the expression of a number of different genes. Genetic engineering, on the other hand, allows for the specific modification in the expression of a small number of genes. One of the first attempts to control cell growth genetically was by overexpressing interferon regulatory factor 1 (IRF-1) (Koster et al., 1995). IRF-1 acts as a tumor suppressor and its overexpression caused an inhibition in cell growth. In addition, reporter gene expression driven by IRF-1 responsive promoters increased up to 20-fold following overexpression. However, the effect on total recombinant protein productivity was not shown, and cell viability was not enhanced nor was the cell culture extended. Both sodium butyrate treatment and hypothermic cell culture up-regulate the expression of a number of CKIs, including: p21ciP1, p 2 7KPJ, and p53. The first successful attempts to induce growth arrest and increase productivity through genetic means began with the overexpression of these particular proteins (Fussenegger et al., 1997; Fussenegger et al., 1998; Mazur et al., 1998; Sunley and Butler, 2010). Initially, the transient transfection of plasmids coexpressing SEAP and either p21, p27 or p53175P (a p53 mutant showing specific loss of apoptotic function) inhibited cell proliferation and increased the specific SEAP productivity 4.6-fold, 3.9-fold, and 3.9-fold respectively (Fussenegger et al., 1997). However, when stably expressed, only p27 resulted in an increase in specific SEAP productivity (Mazur et al., 1998). It wasn't until p21 was stably overexpressed along with the CCAAT/enhancer-binding protein a (C/EBPa) that growth arrest was induced and multi-fold increases in specific SEAP productivity were observed (Fussenegger et al., 1998). C/EBPa stabilizes p21 at the protein level, increasing its half-life, which suggests that it is difficult to stably overexpress p21 to effective levels. Bi et al. (2004), were able to stably overexpress p21 using a LacSwitch expression system driven by a RSV promoter, as opposed to the Tet-off/minCMV system used by Mazur et al. (1998), and observed 4-fold increases in specific IgG4 productivity. In all of the above systems, the Gi-phase of the cell cycle was specifically targeted for arrest. There are two major disadvantages to the overexpression of CKIs. Although the induced growth arrest results in an increase in specific productivity, the resulting decrease in cell density (and IVCD) does not result in a volumetric productivity increase. Secondly, the cells overexpressing CKIs eventually escape growth arrest, either because of the strong selective pressure to grow or because of a damaged or genetically unstable expression system. Certainly more work could be performed in this area to determine if it is possible to increase total volumetric productivity by inhibiting cell cycle progression with a genetic engineering approach. Lastly, non-cell-cycle related proteins have also been overexpressed with some success. Cells trigger an active response to hypothermic culture, resulting in the up and down-regulation of particular proteins, including the cold-inducible RNA-binding protein (CIRP) (Sonna et al., 2002). Tan et al. (2008) recently overexpressed CIRP in actively growing cultures at 37 "C, a temperature at which it is not normally expressed. The overexpression led to a 1.4-fold increase in both specific and volumetric productivity with no impact on cell growth. 2.3.5 Cell Cycle Phase In the vast majority of controlled proliferation studies, cells are arrested in the Gi-phase of the cell cycle because it is believed to be the ideal phase for recombinant protein production. In some cases, like in hypothermic culture (Furukawa and Ohsuye, 1998; Hendrick et al., 2001; Kaufmann et al., 1999; Moore et al., 1997; Yoon et al., 2003) and following butyrate addition (Chang et al., 1999; Hendrick et al., 2001; Palermo et al., 1991; Rodriguez et al., 2005), arrest in the Gi-phase is a secondary effect of the changes made to the culture. And in other cases, like CKI overexpreesion (Carvalhal et al., 2003a; Fussenegger et al., 1997; Fussenegger et al., 1998; Mazur et al,. 1998), arrest is specifically targeted to occur in Gi-phase. The mechanisms responsible for increases in specific productivity during G1 have not been described (Sunley and Butler, 2010). In addition, both S-phase (Carvalhal et al., 2003b; Lloyd et al., 2000; Fox et al., 2005) and G2/M-phases (Tey and Al-Rubeai, 2005) have been shown to correlate with an increase in recombinant protein productivity. Clearly, the relationship between cell cycle and specific productivity during growth arrest is not very well understood. No systematic studies have been performed using the same expression and culture system to try and elucidate such a relationship, and work could still be done in this area. 40 Table 2-1. Summary of methods to improve volumetric productivity by incr Increasing IVCD Fed-batch Dynamic Feeding 12 hour sampling 12 hour sampling On-line Feeding Wong et al., 2004 Xie et al., 1997 Xie and Wang, 1994 Zhou et al., 1995 Anti-apoptosis Engineering Gene Overexpression Bc-XL Bc/2 XIAP Faim/Fadd Chiang and Sisk, 2005 Fussenegger et al., 1998 Meents et al., 2002 Goswami et al., 1999 Meents et al., 2002 Tey et al., 2000 Sauerwald et al., 2002 Wong et al., 2006b CHO CHO Hybridoma Hybridoma IFN-y IFN-y IgG 1.2-fold 1.1-fold 2.0-fold 10-fold 4.5-fold 11-fold IgG - - CHO CHO CHO CHO CHO CHO CHO / 293 HEK CHO mAb SEAP slCAM IFN-y sICAM cB72.3 N/A IFN-y 2-fold 30-fold* 1.3-fold 2-fold decreased no increase - CHO CHO CHO IFN-y IFN-y hTPO no increase RNA Interference Bax/Bak Alg2/Requiem Caspase-3/7 Lim et al., 2006 Wong et al., 2006b Sung et al., 2007 Note: '-': no data available; *: overexpression of Bcl-xL and p27 - - no increase - 1.5-fold 1.4-fold 2.5-fold 1.5-fold G2 /M Table 2-2. Summary of methods to imnrove volumetric nrndietivitv hv inertnc Increasing qp Hypothermic Cell Culture 35 C 33 OC Furukawa and Ohsuye, 1998 Yoon et al., 2003 CHO CHO 799Bg/llx-AE EPO Ahn et al., 2008 Bollati-Fogolin et al., 2005 Fox et al., 2004 Furukawa and Ohsuye, 1999 Hendrick et al., 2001 Kaufmann et al., 1999 Rodriguez et al., 2005 CHO CHO CHO CHO CHO CHO CHO EPO hGM-CSF IFN-y 799Bg/lla-AE t-PA SEAP IFN- Fox et al., 2005c CHO Chang et al., 1999 Hendrick et al., 2001 Rodriguez et al., 2005 Kim and Lee, 2001 Sung et al,. 2007 Carvalhal et al,. 2003b - 4-fold 1.8-fold 2.5-fold G1 Biphasic Temperature Shift 37 0C to 33 0C 37 0C to 32 'C 37 *C to 30 0C 4-fold - 6.5-fold 6-fold 1.9-fold 1.6-fold 1.7-fold 3.4-fold 4-fold IFN-y - 7.7-fold CHO CHO CHO CHO CHO CHO EPO t-PA IFN-P SH-0.32 hTPO SEAP - 3-fold 3-fold 2.7-fold no increase 3.1-fold 2-fold 2.1-fold no increase 2-fold 1.5-fold 1.6-fold Tan et al., 2008 CHO IFN-y 1.4-fold 1.4-fold Bi et al., 2004 Fussenegger et al., 1998 CHO CHO IgG Carvalhal et al., 2003a Fussenegger et al., 1998 SEAP 4-fold 15-fold - CHO CHO SEAP SEAP 2-fold 30-fold - - 2-fold 2-fold 1.7-fold G1 G1 G1 Active Growth S Culture Additives Butyrate alone Butyrate w/ Bc/2 Butyrate w/o caspases 3 & 7 AMP G1 S Gene Overexpression (stable) CIRP p21 CipI p21 C' w/ c/ebpa p27K'l p27K'P'w/ BcI-xL Note: '-': no data available - G1 G1 G1 3 MATERIALS AND METHODS 3.1 Cell Culture 3.1.1 Cell Lines The main cell line used in this thesis was a Chinese Hamster Ovary (CHO) cell line expressing IFN-y driven by the SV40 promoter. The CHO IFN-y (y-CHO) cell line was obtained many years ago from Dr. Walter Fiers (Scahill et al., 1983) and has been used extensively in our laboratory (Ngantung, 2005; Fox, 2005a; Yuk, 2001; Nyberg, 1998; Gu, 1997). The cell line was created from a DHFR CHO cell line by cotransfecting the cells with genes for both DHFR and IFN-y. CHO IFN-y is grown in the presence of methotrexate, which is a competitive inhibitor of DHFR, and increases the copy number of genes cotransfected with DHFR. This cell line has also been adapted to suspension culture (Nyberg, 1998), and both formats were used throughout this thesis. The cell lines have been passaged several hundred times prior to this project. 3.1.2 Culture Medium and Maintenance 3.1.2.1 Adherent Cell Cultures The basal medium used for all adherent cultures was Dulbecco's Modified Eagle Medium (DMEM) (Invitrogen, Grand Island, NY). All supplements were obtained from Invitrogen and all chemicals were obtained from Sigma Aldrich (St. Louis, MO) unless otherwise noted. Prior to 0.22 gm filtration DMEM was supplemented with 0.25 jiM methotrexate, 20 U/mL penicillin - 20 gg/mL streptomycin mix, and 10% heat inactivated fetal bovine serum (IFS). Adherent cell cultures were incubated at 37 'C with a 5-10% CO 2 overlay in a humidified incubator. Cells were grown in surface-treated 6-well plates and T-75 flasks. The cells were passaged every 3-4 days at 1x10 5 cells/mL. 3.1.2.2 Suspension Cell Cultures The basal medium used for all suspension cultures discussed in this thesis was protein-free HyQ PF-CHO (HyClone, Logan, UT). Prior to 0.22 pm filtration, HyQ-PF CHO was supplemented with 4mM L-glutamine, 0.25 gM methotrexate, 20 U/mL penicillin - 20 Rg/mL streptomycin mix, and 0.1% Pluronic-F68. Suspension cell cultures were incubated at 37 *C with a 5% CO 2 overlay on shaker platforms set at 115 RPM in a humidified incubator. The cells were grown in 125-mL Erlenmeyer flasks and passaged every 3-4 days at 2.5x10 5 cells/mL. 3.1.2.3 Cell Bank Maintenance Frozen stocks were prepared from cells in their growth phase with viability greater than 95%. Cells were centrifuged at 1000 RPM for 5 minutes and resuspended to 107 cells/mL in fresh growth medium containing 14% (v/v) dimethylsulfoxide (DMSO). Freezing medium for adherent cells also contained 10% IFS. Cryogenic vials containing 1 mL of the cell suspension were placed in a NalgeneTM Cryo 1 0 C min-' freezing container and placed in a -80 C freezer overnight. The following day, the vials were transferred to a liquid nitrogen cell bank for longterm storage. New cultures were started from the stock vials by quickly thawing the cells in a 37 0 C water bath for 5-10 minutes. The cryovials were gently flicked and sprayed with 70% ethanol before being opened in a sterile biosafety cabinet. These cells were immediately diluted into 25 mL of prewarmed growth medium and transferred to the appropriate growth vessel (either T-75 or 125 mL Erlenmeyer flask). Growth medium was changed within 24 hours of plating. 3.1.3 Cell Enumeration 3.1.3.1 Trypan Blue Staining Cell enumeration was performed with a hemacytometer. Briefly, anchorage dependent CHO cells were collected in suspension after washing with Dulbecco's PBS (DPBS) (Invitrogen, 0 Grand Island, NY) and treating with 0.05% Trypsin/EDTA (Invitroen) for 2 minutes at 37 C The cells were stained with trypan blue and diluted appropriately. Viable cells exclude the trypan blue dye while non-viable cells, which lack membrane integrity, are stained blue. The cell number was then determined by counting the stained and unstained cells under a microscope and multiplying by the known dilution number. 3.1.3.2 Guava ViaCount Cells were collected in suspension following the same protocol as in Trypan Blue Staining 5 (Section 3.1.3.1) and diluted to a concentration no higher than 5x10 cells/mL using the Guava ViaCount Reagent (Guava Technologies, Millipore). Similarly to trypan blue, the ViaCount Reagent differentially stains viable and non-viable cells based on their permeability to the DNAbinding dyes in the reagent. Samples were then analyzed using a flow cytometer (Guava Personal Cell Analysis (PCA) System, Guava Technologies, Hayward, CA). Typically, PM1 voltage was set at 430V and PM2 voltage at 450V. PM2 threshold was usually set to approximately 100. Forward scatter (FSC) gain was set at 2x, and the FSC gate was set to about 100 and debris was excluded. Data analysis was performed using the Guava Express Software and 1000 events were acquired per sample. 3.1.4 Pseudo-perfusion Culture in Tissue Culture Plates Following 2-4 passages of stock cells, between 1 to 2x1 05 mid exponential cells with viability greater then 95% were seeded in fresh growth medium in 6-well plates. Cells were incubated at 37 C in humidified incubators with 5% CO 2 overlay. Cultures were run in batch mode for the first 2 to 4 days, at which point 2-mL medium changes occurred every 2 days. At each time point, 3 wells per condition were sacrificed for cell counting. The supernatant from the sacrificed wells was centrifuged at 1000 RPM for 5 minutes and stored at -80 C for later analysis and the cells were saved for RNA analysis and/or fixed for cell cycle analysis, using the procedure outlined below. 3.1.5 Fed-batch Culture in Shake Flasks Feb-batch cultures were performed in 125-mL Erlenmeyer flasks. Cultures were seeded 2x1 05 cells mL 1 in 25-mL volumes and incubated at 37 0 C under 5% CO 2 and agitated at 115 RPM. The basal medium was the same for both batch and fed-batch cultures and was HyQ PF-CHO (Hyclone) supplemented with 4 mM L-glutamine, 0.1% Pluronic F-68, 0.25 gM methotrexate, and 20 U/mL penicillin - 20 gg/mL streptomycin. Feed forward control was employed in the fed-batch setup. Control of glutamine and glucose concentrations was achieved by feeding the projected amount of glutamine and glucose that will be consumed by the cells over the forecast interval. Glucose and glutamine set points were 0.5 and 0.15 g L-, respectively. The glutamine feed was prepared from a loX nutrient concentrate of salt free DMEM/F12 and IX DMEM/F12 salts (Hyclone), 100 mM L-glutamine, 0.1% Pluronic F-68 (Invitrogen), 0.25 gM methotrexate (Sigma), and 20 U/mL penicillin - 20 [tg/mL streptomycin (Invitrogen). The glucose feed was a 2M glucose solution (Sigma). Samples were taken every 8 to 12 hours for offline analysis and feeding. 3.1.6 Measuring Glucose, Glutamine and Lactate Concentration Glucose, glutamine and lactate concentrations in the medium were measured using the YSI Model 2700 SELECT Biochemistry Analyzers (YSI Incorporated, Yellow Springs, OH). Glutamine was also often measured using a Glutamine and Glutamate Determination Kit (Sigma). 3.2 Cyclin-dependent Kinase ATP-competitive Inhibitors A number of different ATP-competitive inhibitors of CDKs are used in our work, including: CDK2 Inhibitor III (cdk2iii)( (Cat. No. 238803; Brooks et al., 1997; Bhattacharjee et al., 2001), CDK2 Inhibitor IV (cdk2iv) (Cat. No. 238804; Pennati et al., 2005), CDK1 Inhibitor IV (cdkliv) Cat. No. 217699; Vassilev et al., 2006), and CDK4 Inhibitor (cdk4) (Cat. No. 219476; RetzerLidl et al., 2007). All inhibitors were purchased from Calbiochem (EMD Biosciences). Stock solutions were made by dissolving inhibitors in dimethyl sulfoxide (DMSO) to a final concentration of 2 mM and stored at -20 "C. These inhibitors are used in our experiments at a number of different concentrations based mostly on their IC50 values, which are summarized in Table 3-1, below. It should be noted that the inhibitors generally have selectivity towards a particular CDK, but also bind to other CDKs at elevated concentrations. Table 3-1. 1C 5 o and Ki values of ATP-compeetitive CDK inhibitors Inhibitor Structure ICSo/K ID Concentration CcnA/E-CDK2 (0.5 gM)> CcnB-CDK1 (4.2 gM) > CcnD-CDK4 (215 pM) cdk2iii cdk2iv Typical N0 CcnA-CDK2 (0.41 jM) > CcnD-CDK2 (5.5 pM) > CcnB-CDK1 (6.6 pM) 0 N N H4 cdkliv N 1-15 gM 1-10 piM H 0 CcnB-CDK1 (Ki = 35 nM) > N 5-20 gM CcnA-CDK1 (Ki= 110 nM)> VN S CDK2 (Ki =340 nM) H O 7 N O cdk4 ______ CcnD-CDK4 (76 nM) > CcnE-CDK2 (520 nM) > CcnB-CDK1 (2.1 gM) H H_ 1-5 M _ _ _ _ 3.3 mRNA Analysis 3.3.1 RNA Purification RNA isolation was performed on at least 1x106 cells with the RNeasy Mini Kit (Qiagen) following the manufacturer's instructions. Cell lysis was performed with a QIAShredder column (Qiagen) as suggested. On-column digestion with RNAse free DNAse (Qiagen) was performed during the RNA purification to eliminate genomic DNA contamination. During the development and screening of a cell line that inducibly expresses anti-CcnEl shRNA, we performed RNA isolation with the TurboCapture 96 mRNA Kit (Qiagen) following the manufacturer's protocol. This kit allows for higher throughput RNA isolation and subsequent quantitative reverse transcription PCR (qRT-PCR) in a single plate 3.3.2 RNA Quantification and Quality Assessment Total purified RNA was diluted 25-fold in RNase free water and the concentration and quality of RNA was determined from the absorbance at 260nm and 280nm. Absorbance measurements were performed on either an Eppendorf Biophotometer (cuvettes) or a Molecular Devices SpectraMax M2 Microplate Reader (microplates). The RNA concentration is calculated from the absorbance at 260nm (assuming 40 gg mL' for 1.0 OD of single-stranded RNA) and the RNA quality can be estimated from the ratio of the absorbance at 260nm to the absorbance at 280nm. A ratio greater than 1.8 is considered an indication of high RNA quality and was achieved regularly. 3.3.3 First-Strand cDNA Synthesis First-strand cDNA was synthesized from 1 pg of total RNA using Superscript III Reverse Transcriptase (Invitrogen) as follows. In a nuclease-free tube, 1 gg of RNA was combined with 1 gL of oligo-dT mix, 1 gL of 10 mM dNTP mix, and DEPC-treated water was added to a volume of 10 gL. The mixture was heated at 65 "C for 5 minutes and placed on ice briefly. Then, 10 pL of cDNA Synthesis Mix (2X RT Buffer, 4 gL 25mM MgCl 2, 2 gL O.1M DTT, 1 mL 40 U gL 1 RNaseOUT, and 1 pL 200U gL- 1) was added and the final solution was incubated for 50 minutes at 50 "C and for 5 minutes at 85 "C. Finally, 1 gL of RNase H was added and the solution incubated for 20 minutes at 37 C. 3.3.4 RT-PCR Reverse transcription PCR (RT-PCR) was frequently used to check primer specificity, amplify cDNA standards, and prepare samples for cDNA sequencing. RT-PCR was typically performed with Platinum PCR Supermix (Invitrogen) following the manufacturer's instructions. Briefly, the first strand reaction was chilled on ice before the addition of 45 gL Platinum PCR Supermix and 0.5 gL of each primer stock (20 gM) to 1 pL of cDNA. Cycling conditions were 94 "C for 2 minutes followed by 20-30 cycles of 94 "C for 30 seconds, 55 *C for 30 seconds, and 72 C for 2 minutes. The product was stored at 4 "C following PCR, and analyzed via agarose gel electrophoresis. 3.3.5 Real-time PCR Quantification of Ccnel and /-Actin cDNA Levels Real-time PCR assays were developed for a number of CHO cDNAs, including: CcnA2, CcnB1, CcnD], CcnE1, CcnE2, and Rnl8s. We also performed real-time PCR on #-A ctin, which acted as an internal control with the assumption that its expression was constant over most experimental conditions. We also refer to real-time PCR as quantitative reverse transcription PCR (qRT-PCR). This assay was previously developed and performed by a number of other researchers in our group (Ngantung, 2005; Fox, 2005a). Primers sequences were selected based on trial and error RT-PCR experiments with many different sets of primers. The specificity and efficacy of each primer was assessed qualitatively by observing the gel-electrophoresis of the RT-PCR products. The primers for these assays can be found in Table 3-1. Annealing temperatures were selected based on gradient PCR optimization by testing annealing temperatures between 50 and 60 0C. An annealing temperature of 55 *C was chosen for all genes 0 with the exception of CcnB1, which used an annealing temperature of 52 C. A qRT-PCR assay was performed in a total reaction volume of 50 pL. This consists of 25 gL iQ SYBR Green Supermix (Bio-Rad, Hercules, CA), 0.2 gM of each primer, 2 pL of cDNA, and RNase free water. Real-time PCR was conducted in 96-well plates using the iCycler RT-PCR 'machine (Bio-Rad). Cycling conditions were 3 minutes at 95 "C followed by 50 cycles of 30 seconds at 95 "C, 1 minute at the Tanneai, and 1 minute at 72 "C, during which time the fluorescence was measured. Melting curve analysis was performed by adding a cycle consisting of 1 minute at 95 *C followed by a temperature decrease in 1 "C increments to reach 4 C. A 10 second hold was introduced between every step decrease. Table 3-2. Real-time PCR primers cDNA Real-time PCR Primers Product Size (bp) /#-Actin 5'5'- AGCTGAGAGGGAAATTGTGCG -3' GCAACGGAACCGCTCATT -3' 163 CcnA2 5'- GGAAAGCAACCAGTAAACAGCC -3' 136 5'- CCAGGTAAAGAGACAGCAGCATT 5'- ACTGTGTACCCAAGAAGATGCTGC -3' 5'- CGAAGTCACCTATTTCTGGAGGG CcnD] 5'5'- AGAGGCGGATGAGAACAAGC -3' GGGTGGGTTGGAAATGAACTTC -3' 94 CcnE1 5'5'- ATAGCAGTCAGCCTTGGGATG -3' CATGATCCTCCAAACCTCTTCTC -3' 194 CcnE2 5'5'- 128 Rn]8s 5'- ATCTGTGTATCCTCGGCATTGACT -3' AGGCACCATCCAGTCTACACATTC -3' TTGACGGAAGGGCACCACCA -3' 5'- GCACCACCACCCACGGAATCG CcnB1 -3' -3' 99 -3' 109 SYBR Green dye binds double stranded DNA. The resulting DNA-dye complex absorbs blue light (Xmax = 488 nm) and emits green light (kmax = 522 nm), and therefore the fluorescence measured at the end of each amplification cycle is proportional to the amount of DNA in the reaction vessel. The cycle at which a given sample crosses a threshold cycle (CT) during the exponential part of the amplification is used to calculate the initial template concentration. A standard curve is generated by simultaneously running real-time PCR on 10-fold serial dilutions of #-Actin, Cyclin, or Rn18s cDNA standards of known concentration. analysis was performed on CcnE1 and #-Actin, The most common and examples of fluorescence versus cycle number for these two genes are shown in Figure 3-1. A plot of CT versus the log of concentration is linear and can be extrapolated to find the concentration of unknown samples. Example standard curves are shown in Figure 3-2. Both the samples and standards were at least run in duplicate and final CT values represent the average of these samples. Melting curve analysis of CcnE] and #-Actin RT-PCR products from control cells is shown in Figure 3-3. A single peak ensures that only one PCR product was amplified and ensures the specificity of the RT-PCR assay. In order to use the AACT Method, Section 3.3.6, the efficiencies (E)of the target (CcnE1) and endogenous control (3-Actin) must be approximately the same. The efficiency values (E) were measured using the CT slope method and are shown in Table 3-2. Using the slopes from Figure 3-2, we calculate efficiency using the following equation: E= slope -1 (3-1) A: -Actin 4500 4500 4000 3500 -3500 u.3000 U_ u-. 300 U U 2500 a 2000-' C ~- 1500 00 000 445000 0 _30 -500 -- -1-I- -a ~ 2-500 0 2 4 6 8 10121416182022242628303234363840424446485052 Cycle BOCcnE1 5000TTT -5000 4500--t * U 0V UU'U -o W, U, .44 4 4 4 4 4500 .44 4000- -4000 3500 -3500 3000 - 1 4 4 *3000 ~44 2500 2500 4 2000? 44 In 1000 4 4 44.4.44.4.4444444.. t 1500 ~ 44 2000 .1. *1500 A T T 4 L~ ~ 4 ~-4 4 44 1000 44 r r .4 500. 500 ~~1__ 0 0 0 2 4 6 8 10 12 14 16 18 20 22 24 26 28 30 32 34 36 38 40 42 44 46 48 50 52 Cycle Figure 3-1. Real-time PCR fluorescence versus PCR cycle number. An example of normalized fluorescence versus PCR cycle number for triplicates of (A) /-Actin standards (108-103 copies mL-1) and (B) CcnE1 standards (106-101 copies mL-). Threshold fluorescence was calculated using iCycler Software Version 3.1 and is shown by the horizontal red line. A: -Actin y = -3.3229x + 36.815 R2 = 0.9994 8 4 6 log (copies mL-1) 2 B: CcnE1 y = -3.3914x + 34.12 R2 = 0.9999 0 2 4 log (copies mL1) 6 8 Figure 3-2. Threshold cycle (Ct) as a function of the log of DNA copy number. The date from Figure 3-1 is plotted here. The concentration of known standards is plotted against the threshold cycle determined by the iCycler Software Version 3.1. (A) /#-Actin and (B) CcnEl. A: $-Actin 800700600- i~ ~.,-----~ i-i 4 ~~t 4 500- ~ 400300200 100- 20 25 30 35 40 ~ 44 144 .... -100 45 55 50 60 65 70 75 80 85 90 95 100 Temperature, Celsius B: CcnE1 900 800 700 600.. u 400 m 300 200 20 100 20 25 30 35 40 45 50 55 60 65 70 Temperature, Celsius 75 80 85 90 95 100 Figure 3-3. Melting curve analysis. The presence of a single sharp peak in the melting curve analysis indicates that our method is specific for our cDNA of interest. The absence of smaller broad peaks indicates that our primers are not amplifying off-target cDNAs. (A) #-Actin and (B) CcnE1. Table 3-3. gRT-PCR efficiency for cDNA standard curves fiiny(PC ~ ~ cDNA #-Actin 1.00 CcnA2 1.01 CcnBi 0.929 CcnDl 1.00 CcnEJ 0.972 CcnE2 1.00 Rn18s 0.935 3.3.6 Calculating Relative mRNA Expression Relative mRNA expression was calculated using the 2001). 2 -AACt method (Livak and Schmittgen, For the AACT method to be valid, the amplification efficiencies of the target and reference must be approximately equal, Table 3-2. In addition, the internal control (f-Actin) must be expressed at a constant level over all of the experimental conditions. All relative expression calculations are calculated with respect to a non-silencing, 'mock', siRNA control (Dharmacon) and normalized against P-Actin expression. 3.4 Cyclin El siRNA Methodology The cyclin El (CcnEl) CHO mRNA represented a challenging target for silencing RNA (siRNA). The CHO genome is not available publicly, so we first had to sequence any mRNA that we wished to target with siRNA. Secondly, we are not aware of any validated primary antibodies against CHO CcnE], nor were we able to validate any ourselves (data not shown). Therefore we were not able to confirm knockdown of CcnElat the protein level. Nevertheless, by following well-annotated methods for confirming the specificity of RNAi experiments (Cullen, 2006), we are confident that we were able to determine the phenotype of CcnE] knockdown. Namely, we observed a similar phenotype following transfection with multiple unrelated and effective siRNAs targeting different regions of the CcnE] mRNA. When quantifying siRNA knockdown we always compare to a non-silencing 'mock' siRNA (Dharmacon). 3.4.1 Cyclin Sequencing Sequencing primers were designed based on the open reading frames of cyclins from the mus musculus transcriptome and ordered from Integrated DNA Technologies (Coralville, IA). The cDNA sequencing templates for each cyclin were amplified with primer sets in Table 3-4. These templates were also used as standards in our real-time PCR assays, Section 3.3.5. Effective sequencing primers were validated through touchdown PCR (Tanneal = 65 to 55 0 C) with the specific cyclin template, and submitted along with the template for sequencing. Sequencing was performed by the Biopolymers Laboratory at the Center for Cancer Research at MIT using the Applied Biosystems Model 3730 capillary DNA sequencer which employs the Sanger method (Sanger et al., 1977). I able .- 4. Primer se uences for the amplification of cyclin cDNA temp late s mRNA Targret CcnA2 Primers 5' -ACTCGACGGGTTGCTCCTCTTA-3' 5'-CTGGTGGGTTGAGAAGAGAAAC-3' CcnB] 5' -CTCAGGGTCACTAGGAACACGA-3' 5'-GACTACATTCTTAGCCAGGTGCTG-3' CcnDl 5' -TGTGCTGCGAAGTGGAGA-3' 5'-GGAGGGTGGGTTGGAAATGA-3' CcnE1 5' -CGCTCCAGAAAAAGGAAGGC-3' 5'-CGAACGGAACCATCCATTTG-3' CcnE2 5' -CACCCCATAAAGAAATAGGAACAAG-3' 5' -GCTTCACTGGACTCACACTTTTTAC-3' 3.4.2 Cyclin El siRNA Design An updated modification to the Tuschl rules (Elbashir et al., 2002), developed by Dharmacon, (Reynolds et al., 2004) was used to design highly effective siRNAs. Briefly, the eight criteria used to design siRNA were: G/C content between 36% and 52%, three or more A/U base pairs at positions 15-19 of the sense strand (low internal stability of the 3' end of the sense strand), a lack of internal repeats, A at positions 3 and 19 of the sense strand, U at position 10 at the sense strand, no G/C at position 19 of the sense strand, and no G at position 13 of the sense strand. All siRNA sequences were generated with siDirect (http://des]itn.RNAi.jp/) (Naito et al., 2004) and ordered from Dharmacon Research (Lafayette, CO). At least 12 sequences from different locations along the CcnE1 cDNA sequence were selected. A BLAST search against the mus musculus transcriptome was performed to minimize potential off-target effects. Both unlabelled (ON-TARGET plus Non-Targeting siRNA, Dharmacon) and fluorescein (FAM)-labeled (Invitrogen, cat. 2013) non-silencing siRNAs were used as transfection and experimental controls. 3.4.3 Preparation of siRNA Duplexes The siRNA was shipped from Dharmacon as a dried pellet in the 2'-deprotected and duplexed form. The pellet was centrifuged briefly and resuspended to 20 RM in siRNA universal buffer. The concentration of each siRNA oligo was verified using an Eppendorf Biophotometer. 3.4.4 siRNA Transfection and RNA Collection CHO cells were typically plated at a concentration of 2x1 05 cells/well in a 6-well plate 18 hours prior to transfection. On the day of transfection, 100 pmol of siRNA duplex was added to 250 RL of OptiMEM (Invitrogen). Separately 5 gL of Lipofectamine 2000 (Invitrogen) was added to 250 pL of OptiMEM. After a five minute incubation, the two solutions were mixed and incubated for twenty minutes at room temperature. During the incubation period, the CHO cell cultures were aspirated and the cells washed with DPBS and 1.5 mL of warmed DMEM (no additives) was added to each well. Next, 500 gL of siRNA-Lipo2000 solution was added to each well and allowed to incubate at 37 0 C for 24 hours. After 24 hours, each well was washed once with DPBS and fresh DMEM (supplemented with 10% IFS) was added. The transfection controls (FAM-labeled siRNA) are analyzed via FACS to ensure >95% transfection efficiency. At 48 hours post-transfection, the cells were harvested, washed with DPBS and pelleted prior to RNA purification. 3.5 Creation of an Inducible shRNA IFN-y Cell Line 3.5.1 Plasmid Description and Design A number of different plasmid vectors were used in this study. Figure 3-4 shows the vector map of pSingle-tTS-shRNA which is a commercially available vector (Clontech, Mountain View, CA) for the single step selection of inducible mRNA knockdown. Figure 3-5, shows the vector maps of the two commercially available plasmids (Clontech) necessary for a two-step selection strategy. The inducible shRNA expression vector, shown in Figure 3-5B, is a modification of the commercially available pSilencer 4.1 -CMV-hygro vector. Seven repeats of the tetracycline operon (tetO 7) were inserted upstream of the CMV promoter. The cDNA encoding these seven repeats of the tetracycline operon (tetO7 ) were amplified from the pSingle-tTS-shRNA, Figure 3-4, (Clontech) vector using the 5' primer CGCCCGGTACCTCTTCACTTGAGTTTACTCCC with an underline showing an added KpnJ site and 3' primer GGCCAGATCTTACACGCCTACCTCGACATA with an underline showing an added BgII site. The effectiveness of this modification has previously been shown to control the knockdown of GFP expression in CHO cells (Malphettes and Fussenegger, 2004). The insertion was verified by DNA sequencing. A visual description of the tetracycline transcriptional silencer (tTS) controlled shRNA expression is shown in Figure 3-5. In the absence of doxycycline the tTS binds to the tet operon (tetO7 ) and represses shRNA expression. Upon addition, doxycycline preferentially binds to the tTS, which allows for expression of shRNA driven by either a U6 or CMV promoter. The tTS is a fusion of the Tet repressor protein (TetR) and the KRAB-AB silencing domain of the Kid-1 protein (SDKid%1), which is a powerful transcriptional suppressor (Freundlieb et al., 1999; Witzgall et al., 1994). shRNA expression vectors were created to express the shRNA hairpins: El-1, El-2, El-5, El-6, El-7 and El-8. Hairpins were annealed and inserted into the appropriate vectors by digestion with either HindIl and XhoI (pSingle-tTS-shRNA) or BamHI and HindIII (pSilencer-tetO7CMV-shRNA). All hairpins were order from Integrated DNA Technologies (Coralville, IA) and the appropriate sequences are shown in Table 3-5. sequencing. All insertions were verified by DNA SV40 Promoter Ampicillin Neomycin TK pA CoIE1 origin pSingle-tTS-shRNA 7.0 kb Promoter ICMV -EcoRI(2199) shRNA Xho HindIll(4717) tTS Tight(tetO7)U6 Promoter b-globin pA Figure 3-4. Plasmid map of pSingle-tTS-shRNA vector. This is a vector map of the commercially available plasmid pSingle-tTS-shRNA (Clontech, Mountain View, CA). An shRNA of interest can be directionally inserted downstream of the inducible U6 promoter using the HindIIl and XhoI restriction sites. This plasmid allows for transfection and a single-step selection with neomycin for the inducible knockdown of a target mRNA. A SV40 Promoter Ampicillin TK pA CoIE1 Origin CMV Promoter b-globin pA SV40 pA B HindIII(463) BamHI(517) -- SV40 Promoter shRNA KpnI(1 118) tet 7 Hygromycin pSilencer 4.1 - tetO7 CMV hygro 5.5 kb - 8g111(1390) CoIE1 origin SV40 pA Ampicillin Figure 3-5. Plasmid maps of ptTS-Neo and a modified pSilencer 4.1 vector. Both vectors are commercially available pre-modification (Clontech, Mountain View, CA). These plasmids allow for a two-step transfection and selection process for the inducible knockdown of a target mRNA. A ptTS-Neo (A) transfection and neomycin selection is followed by a pSilencer 4.1 -tetO 7-CMV-hygro (B) selection and hygromycin selection. The tTS System tTS ( 1) Remove Dox bindsTRE, and suppresses transcription in the absence of Dox tTS / 1TS Transcription Knockdown TetR SDKid-l TRE U6 TRE. U6 Anti-X shRNA Add Dox TREmod U6 Figure 3-6. The tTS System. This diagram shows how U6-driven shRNA expression responds to the addition or removal of doxycycline. Also applies to CMV-driven expression in the case of the two-step selection CMVdriven shRNA expression system. (KnockoutTM Single Vector Inducible RNAi System User Manual, 2007). Table 3-5. ShK.NA hair in seguences tor inserton into inuuile expresswin vectors pSingle-tTS-shRNA El-i 3' 5' TCGAGGGAGAAGATTTACCTAAGATTCAAGAGATCTTAGGTAAATCTTCTCCTTTTTTACGCGTA CCCTCTTCTAAATGGATTCTAAGTTCTCTAGAATCCATTTAGAAGAGGAAAAAATGCGCATTCGA 5' 3' El-2 3' 5' TCGAGGTTACATGGCATCACAACAATTCAAGAGATTGTTGTGATGCCATGTAACTTTTTTACGCGTA CCAATGTACCGTAGTGTTGTTAAGTTCTCTAACAACACTACGGTACATTGAAAAAATGCGCATTCGA 5' 3' El-5 3' 5' TCGAGGTTTGGAGGATCATGTTAAATTCAAGAGATTTAACATGATCCTCCAAACTTTTTTACGCGTA CCAAACCTCCTAGTACAATTTAAGTTCTCTAAATTGTACTAGGAGGTTTGAAAAAATGCGCATTCGA 5' 3' El-6 3' 5' TCGAGGACAGCTCATTGGGATTTCATTCAAGAGATGAAATCCCAATGAGCTGTCTTTTTTACGCGTA CCTGTCGAGTAACCCTAAAGTAAGTTCTCTACTTTAGGGTTACTCGACAGAAAAAATGCGCATTCGA 5' 3' El-7 3' 5' TCGAGGCCAGTTTGCTTACGTTACATTCAAGAGATGTAACGTAAGCAAACTGGCTTTTTTACGCGTA 5' CCGGTCAAACGAATGCAATGTAAGTTCTCTACATTGCATTCGTTTGACCGAAAAAATGCGCATTCGA 3' EI-8 3' 5' TCGAGGCCCTTAAGTGGCGTTTAATTCAAGAGATTAAACGCCACTTAAGGGCTTTTTTACGCGTA CCGGGAATTCACCGCAAATTAAGTTCTCTAATTTGCGGTGAATTCCCGAAAAAATGCGCATTCGA 5' 3' pSilencer-tetO 7-CMV-shRNA El- 3' 5' GATCCGGAGAAGATTTACCTAAGATTCAAGAGATCTTAGGTAAATCTTCTCCTTA GCCTCTTCTAAATGGATTCTAAGTTCTCTAGAATCCATTTAGAAGAGGAATTCGA 5' 3' El-2 3' 5' GATCCTTACATGGCATCACAACAATTCAAGAGATTGTTGTGATGCCATGTAACGA GAATGTACCGTAGTGTTGTTAAGTTCTCTAACAACACTACGGTACATTGCTTCGA 5' 3 El-5 3' 5' GATCCTTTGGAGGATCATGTTAAATTCAAGAGATTTAACATGATCCTCCAAACCA 3' GAAACCTCCTAGTACAATTTAAGTTCTCTAAATTGTACTAGGAGGTTTGGTTCGA 5' El-6 3' 5' GATCCACAGCTCATTGGGATTTCATTCAAGAGATGAAATCCCAATGAGCTGTAAA ' GTGTCGAGTAACCCTAAAGTAAGTTCTCTACTTTAGGGTTACTCGACATTTTCGA 5' El3- 3' 5' GATCCCCAGTTTGCTTACGTTACATTCAAGAGATGTAACGTAAGCAAACTGGTGA GGGTCAAACGAATGCAATGTAAGTTCTCTACATTGCATTCGTTTGACCACTTCGA 5' El-8 3' 5' GATCCGCCCTTAAGTGGCGTTTAATTCAAGAGATTAAACGCCACTTAAGGGCCTA 3' GCGGGAATTCACCGCAAATTAAGTTCTCTAATTTGCGGTGAATTCCCGGATTCGA 5' 3.5.2 Plasmid Production and Purification Plasmids were produced by transformation in DH5a competent cells (Invitrogen) grown in LB medium and selected using 100 jig mUL ampicillin. Plasmids were purified with a QIAfilter Plasmid Maxi Kit (Qiagen). 3.5.3 Transfection and Selection of Stable Cell Lines Cells were plated at 1x10 5 cells mUL in 6-well plates one day prior to transfection. All transfections were performed with CLONfectin (Clontech) according to the manufacturer's instructions. Briefly, on the day of transfection 2 pg of plasmid was combined with 4 pg of diluted CLONfectin in DMEM and allowed to incubate for 20 minutes before being added to the cells. The CLONfectin/DNA solution was removed after 4 hours, cells were washed once with DPBS, and growth medium was added to the cells. Selection with either Geneticin/G418 (600 jg mL-1) or hygromycin (600 jg mL-') was initiated 48 hours post-transfection. Once colonies began to appear in our 6-well plates, single cells were separated by serial dilution into 96-well plates. Cells were plated at 1x10 4 cells ml' in the upper-left most well and diluted down and across a 96-well plate. Conditioned medium was added to each well and selection at 600 jg ml' was continued. Wells with single cells were expanded with either neomycin or hygromycin at a concentration of 100 pg mUL and screened. 3.5.4 Stable Cell Line Screening: tTS Expression Following neomycin selection single cell colonies expressing tTS were screened by RT-PCR for tTS mRNA expression and iWith a functi<onal luciferase expression assay to test the induction of shRNA expression. RT-PCR was performed with forward primer 5'- GAGTTGGCAGCAGTTTCTCC-3' and reverse primer 5'-AAACCCTGCATCGCATAGAC-3' on all single cell colonies to find the colonies with the highest levels of tTS mRNA transcript. Cells that were shown to have high levels of the tTS mRNA transcript were then screened with a luciferase knockdown assay. In the luciferase knockdown assay, the luciferase expression vector pGL2 (Clontech) was cotransfected with a modified pSingle vector, Figure 3-7, expressing anti-luciferase shRNA (pSingle-no tTS-anti-luc shRNA). The pSingle vector has been modified through the removal of its tTS expression cassette by digestion with EcoRI followed by religation. This ensures that the only tTS involved in silencing the expression of the anti-luciferase shRNA is the tTS that is stably expressed from within the cell. Elimination of the tTS cassette was verified through both a restriction digest and DNA sequencing. Luciferase expression was quantified using the Luciferase Assay System (Promega, Madison, WI). 5 Briefly, on the day before transfection, cells were plated at a density of 1x10 cells well-' in 6- well plates. On the day of transfection, 1 Rg of pGL2, 1 gg of pSingle-no tTS-anti-luc shRNA, and 4 pg of CLONfectin (Clontech) (2:4 DNA:CLONfectin) were combined in DMEM and incubated for 20 minutes before applying the solution to the cells. Four hours following the transfection, growth medium was added to the cultures along with varying amounts of doxycycline (from 0-10,000 ng mL-1). Two days later, Reporter Lysis Buffer (Promega) was added to each sample followed by subjecting the samples to a single freeze-thaw cycle. Luciferase expression was quantified using a SpectraMax Luminometer Micorplate Reader (Molecular Devices, Sunnyvale, CA), by adding 100 gL of Luciferase Assay Reagent (Promega) to 20 gL of cell lysate in white opaque 96-well plates, and measuring the luminescence for a period of 10 seconds. 3.5.5 Stable Cell Line Screening: Inducible shRNA Expression Cell lines that were selected for the insertion of vectors inducibly expressing shRNA were first screened in 24-well plates by measuring CcnE1 knockdown 48 hours after the addition of 1000 ng mL- 1 (pSingle-tTS-shRNA) or 100 ng mL-1 (pSilencer-tetO 7-CMV-shRNA) doxycyline. RNA purification was performed using the RNeasy Mini Kit for pSingle screening and the TurboCapture Kit for pSilencer screening. In the first screen of the pSilencer colonies, knockdown was not normalized to P-Actin. Cell colonies that showed an induced knockdown in CcnE1 expression were expanded and rescreened in triplicate in 6-well plates. SV40 Promoter Ampicillin Neomycin TK pA pSingle - no tTS - anti-luc shRNA 5.5 kb CoIE1 Origin shRNA EcoRI(2199) Xhol(3 Hindill(3217) Tight(tetO7)/U6 Promoter Figure 3-7. Plasmid map of the modified pSingle vector, pSingle-no tTS-anti-luc shRNA. This is a vector map of the commercially available pSingle vector (Clontech) with the tTS expression cassette removed. 3.6 IFN-y ELISA IFN-y concentration was measured using an ELISA kit (Invitrogen) by following the manufacturer's protocol. Cell culture supernatants were centrifuged for 5 minutes at 1600 RPM and stored at -80 "C for future analysis. On the day of analysis, supernatants were thawed and serially diluted using phosphate buffer saline (PBS) and diluent buffer (provided in kit) to obtain samples within the range of the standard curve (15.6 - 1000 pg mL'). Each sample was measured in duplicate. 3.7 Calculating IFN-y Specific Productivity The specific productivity, or the amount of IFN-y produced on a per cell basis in a given amount of time, qp, is generally defined by the following equation, where P is the total cumulative IFNy production and Nviable is the total number of viable cells: dP = dt PN,,ab,, (2-1) By rearranging Equation 2-1 we are able to calculate qp in two different ways depending on the data that is available. The first is through the integral method which is described in Section 3.7.1 and is used when more than three data points are taken over the course of an experiment. We will refer to this specific productivity as qp. The second method is a differential method, which is described in Section 3.7.2 and is used when two data points are known. We will refer to this specific productivity as qp, diff- 3.7.1 Specific Productivity, qp The integral method of calculating specific productivity is performed by plotting the cumulative production of IFN-y against the integrated viable cell density (IVCD) at each time point. The slope of a linear regressive fit of the data is the specific productivity, as is seen in the following equation: JdP= qp Ndt= qp -IVCD (3-2) A typical plot and regressive fit is shown in Figure 4-2. 3.7.2 DifferentialSpecific Productivity, qp. diff The differential specific productivity, qp. dif; simply involves calculating the amount of IFN-y produced in a given time and dividing by the average number of viable cells, Nv,avg, in that time, as seen below: AP qPp, dAt diff N" (3-2) The differential specific productivity is used to compare productivity over very short time frames. Notably, differential specific productivity is used to compare the effects of CcnE] knockdown on IFN-y productivity. 3.8 Cell Cycle Analysis At least 1 x 106 cells were collected by centrifugation (5 minutes at 1600 RPM) and washed once with 1 mL of PBS. After a second centrifugation step, 500 tL of cells were added drop-wise to 1 mL of ice cold 100% ethanol stored at -20 "C. Ethanol fixed cells cin be stored up to one month at -20 C prior to analysis. On the day of analysis, fixed cells were underlayed with 300500 jiL of IFS, centrifuged (5 minutes at 400 G) and the ethanol/IFS was aspirated. The cells were washed once with PBS and centrifuged again (5 minutes at 400 G). Finally the cells were resuspended in 1 mL of the propidium iodide (PI)/Triton X-100 straining solution [0.1% (v/v) Triton X-100; 10 gg mL PI; and 0.2 mg mL-1 RNase A in PBS] and incubated at room temperature for at least 1 hour prior to flow cytometry analysis. The PI/Triton X-100 straining solution is prepared as a IOX stock and is stored at 4 C for up to one month. Flow cytometry analysis was performed using the FACScan flow cytometer (Becton Dickinson, NJ). The laser was tuned to 488 nm, and a band-pass of 585/42 filter (FL2) was used for red fluorescence detection. The intensity of fluorescence was characterized by its area (FL2-A). Single cells were selected for analysis by using the distribution of PI area (FL2-A) against PI width (FL2-W) to discriminate doublets and debris. The number of events collected per sample was set at 25,000 and the fluorescence parameter (FL2-A) was always collected in linear mode. Histogram fitting was performed with ModFit LT Software. 3.9 Immunostaining and Cell Imaging Round glass coverslips, #1 thickness, were treated with 1 mL of 5 jig mL-1 fibronectin (Sigma) for 45 minutes at 37 0 C before being plated with 10,000 cells mL-1 in 12-well plates. On the day following plating, the cells were treated with our CDK ATP-competitive inhibitors for 24 hours before being fixed prior to immunostaining. On the day of fixation cells were washed once with PBS and then 1 mL of pre-warmed fixation buffer (0.2% Triton-X 100, 20 mM PIPES pH 6.8, 1 mM MgCl- 10 mM EGTA, 4% p-formaldehyde) was added to each well and allowed to sit for at least 20 minutes. The slides were then washed three times with PBS and stored at 4 0 C for up to two weeks. The coverslips were blocked in a 3% bovine serum albumin (BSA)/PBS solution at room temperature for 30 minutes and washed once in PBS. The coverslips were then incubated with 0 the y-tubulin primary antibody, Gtu.88 y-tubulin (Sigma), in 3% BSA/PBS at 37 C for 1 hour. The anti-mouse Gtu.88 y-tubulin antibody is used as a centrosome marker and was used at a dilution of 1:5000. The coverslips were washed three times before being incubated with the secondary antibody and phalloidin. We incubated the coverslipes in Alexa Fluor 488 donkeyanti-mouse secondary antibody (Invitrogen) at a 1:250 dilution (8 jig mL) and Alexa Fluor 594 0 phalloidin (Invitrogen) at a 1:100 dilution (2 U mL-) in 3% BSA/PBS at 37 C for 1 hour. Alex Fluor 594 phalloidin is a high affinity probe for F-actin that is conjugated with a photostable Alexa Fluor 594. The coverslips were again washed three times before a final 5 minute treatment with 10 gg mL Hoechst 33258 (Invitrogen), a DNA binding dye. Stained cells were imaged with a DeltaVision Core deconvolution microscope (MIT CCR) with a 60X objective. Gtu.88 y-tubulin was detected using a green, fluorescein, filter, Alexa Fluor 594 phalloidin was detected using a red, Texas Red, filter, and Hoechst 33528 was detected using a blue, DAPI, filter. Image processing was performed with Deltavision Softworx. 3.10 Genomic DNA Purification and Quantification Genomic DNA was typically purified from 5 x 105 cells after washing 1X with PBS using the Wizard SV Genomic DNA Purification System (Promega, Madison, WI). DNA was quantified using the Quant-iT PicoGreen dsDNA Reagant (Invitrogen) using spectrophotometer cuvettes. Briefly, a standard curve was created from 2000 to 0 ng mL' using Salmon Sperm. DNA samples and the standard curve were diluted in 1X TE (10 mM Tris-HCl, 1 mM EDTA, pH 8.0). A working solution of the Quant-iT PicoGreen Reagent was made by diluting the concentrated buffer 20-fold with DNase-free water. 1 mL of the diluted Quant-iT PicoGreen Reagent was added to 1 mL of diluted standards and samples. The sample fluorescence was measured using a SpectraMax M2 Spectrophotometer and standard fluorescein wavelengths (Xex = 480 nm; Xem= 520 nm). Genomic DNA content was normalized to growth control cultures at all time points. 3.11 Measurement of Intracellular Protein Content Typically, 2.50 x 105 suspension CHO cells were centrifuged for 5 minutes at 1600 RPM. Cell pellets were lysed with 125 gL CelLytic TM Cell Lysis Reagant (Sigma) and frozen at -80 0 C until further analysis. On the day of analysis, cell lysates were thawed and protein content was measured using the Pierce BCA Assay Kit (Pierce, Rockford, IL). Bovine serum albumin (BSA) was used to make a standard curve. 3.12 Note on Statistical Analysis/Significance In general, data is analyzed following the statistical rules outlined by Cumming et al. (2007). Unless otherwise noted, all error bars are standard error bars. The number of experiments for each graph and/or table will be noted. Statistical significance is assumed to occur when p < 0.05 and will also be noted. 4 CDK2-CcnEl TARGET VALIDATION 4.1 Introduction Most controlled proliferation strategies are known to slow cell growth by inhibiting the cell cycle machinery. Some of these strategies, like lowering culture temperature (Yoon et al., 2003) and cell engineering based approaches, like overexpressing cyclin-dependent kinase inhibitors (CKIs) (Mazur et al., 1998), arrest growing cell populations in the Gi-phase of the cell cycle and lead to an increase in specific recombinant protein productivity. Decreasing culture temperature stabilizes p53 (Ohnishi et al., 1998), a transcription factor that under normal conditions is extremely unstable, and subsequently increases p21cipi protein levels (Ohnishi et al., 1998). It is well understood that p53 transcriptionally activates p21cipI expression by directly interacting with its regulatory elements (El-Diery et al., 1994). p2lc'P itself is known as a potent, tightbinding inhibitor of cyclin A-CDK2, cyclin E-CDK2, cyclin D1-CDK4, and cyclin D2-CDK4 complexes, all of which act at the Gi-S phase transition (Harper et al., 1993). Cell engineering based approaches are inspired by the effects of hypothermic cell culture. In these approaches, the CKIs p2lciP, p2 7 KP, and a non-apoptotic p53 mutant, p53175P, are inducibly overexpressed to induce growth arrest in the Giphase of the cell cycle (Mazur et al., 1998). Unlike p2 1 ci1, p 2 7 KiP1 is not induced by p53 but rather is expressed in response to the anti-mitogenic cytokine transforming growth factor b (TGF-$) (Reynisdottir et al., 1995). Like p21cipI, p2 7 KiP1 exerts G1 cell-cycle arrest by binding to cyclin E-CDK2, cyclin A-CDK2, and cyclin D-CDK4 (Toyoshima and Hunter, 1994). These approaches are shown to dramatically increase the specific productivity of secreted alkaline phosphatase (SEAP), a model protein. For these reasons, inhibition of the cyclin-cyclin dependent kinase (Ccn-CDK) complex represents a good starting point for developing new growth arrest strategies. We wanted to first determine if specifically inhibiting only the cyclin El-CDK2 (CcnEl-CDK2) complex was sufficient to induce growth arrest and increase recombinant protein specific productivity. We did this in two distinct ways: by adding a selective ATP-competitive CDK2 inhibitor to CHO cell cultures, and by using RNA interference (RNAi) to knockdown CcnE1 expression. We targeted CcnE1 for RNAi knockdown, as opposed to directly knocking down CDK2, for a number of reasons. In a wide variety of cell lines, CDKl has been shown to compensate for loss in CDK2 activity (Berthet and Kaldis, 2007) and cell cycle progression can occur even in the absence of CDK2. In addition, CcnE] expression fluctuates throughout the cell cycle (Lodish et al., 2004) making it a more natural target for knockdown. Also, its interaction with CDK2 is known to promote progression from the G1 to S-phase of the cell cycle (Malumbres, 2008). Lastly, the targeted knockdown in CcnE] by siRNA in various hepatocellular carcinoma (HCC) cell lines reduced cell growth following transfection (Li et al., 2003). 4.2 CDK2 Inhibition with a Selective ATP-competitive Inhibitor In order to determine if inhibiting only CDK2 was sufficient to induce growth arrest we added the CDK2 inhibitor, cdk2iii, to adherent y-CHO cell cultures 2 days after plating at 1 x 105 cells mL' in 6-well plates. Inhibitor cdk2iii was added to a concentration of 2 gM based on its known IC50 of 0.5 pM in vitro (Brooks et al., 1997). Growth curves with and without inhibitor addition are shown in Figure 4-1. Although viability data is not shown, viability is greater than 90% across all data points. The slight decrease in viable cell count on day 6, four, ays following inhibitor addition, can be explained by a small amount of cells detaching from the plates. Slowed growth was observed by day 4, two days following inhibitor addition. The effect of growth arrest on specific IFN-y productivity is shown in Figure 4-2, a representative plot of the cumulative IFN-y production versus the integrated viable cell density for this 6-day period. On day 6, the cumulative IFN-y production following the addition of the inhibitor cdk2iii is equal to that of the growth control even though the final viable cell density is approximately 50% lower. This suggests that by timing the induction of growth arrest, we may be able to strike a balance between cell density and productivity, potentially improving total IFN-y titer. Table 4-1 summarizes the results of three independent inhibitor cdk2iii experiments. By adding the cd2iii inhibitor to y-CHO cell cultures we were able to increase specific productivity by 66%, or 1.66-fold, over the 6 day time period. 7 -9- cdk2iii 65 .a; 0 S4 - 2 1 - 0 0 2 4 6 8 Time (days) Figure 4-1. Growth curves of adherent cultures with or without the addition of 2 gM inhibitor cdk2iii. Cells were grown with (o) and without (0) the addition of 2 gM inhibitor cdk2iii for 6 days. Starred points represent a statistically significant difference in cell density (*: p < 0.05, **: p < 0.01) (Error bars: S.E., n = 3) 80 * Growth Control o cdk2iii 5- + 0 4V 0 a- z 3- LL 2E 015 Integrated Viable Cell Density (106 cells d) 5 0 10 z Figure 4-2. IFN-y production versus Integrated Viable Cell Density (IVCD) with and without the addition of 2 gM inhibitor cdk2iii. The slope of each line is the calculated specific productivity with (o) and without (C) the addition of 2 gM of inhibitor cdk2iii. The regression lines are linear least-squares fits of each data set. (Error bars: S.E., n = 1) Table 4-1. A summary of the effect of inhibitor cdk2iii on specific Specific Productivity ( xg 10~6 cell d') Growth cdk2iii Avg 0.28 0.46 0.39 0.38 0.04 0.05 0.06 0.43 0.75 0.71 0.63 0.08 0.01 0.06 Foldincrease (X) 1.53 1.63 1.82 11.66 ± 0.09 4.3 CcnEJ siRNA Knockdown Now that we have shown that specifically inhibiting CDK2 is sufficient to induce growth arrest and increase productivity, we next sought to determine if inhibiting the CDK2-CcnEl complex alone using RNAi produced similar results. To do this we specifically target CcnE1 for knockdown. This is necessary because CDK2 is known to interact with CcnEl, CcnE2, and CcnA2 during cell cycle progression from G1 to S phase. The first step that we took in determining the effect of CcnE1 knockdown by siRNA was validating that we could reproducibly knockdown CcnE1 mRNA. Because we were unable to validate knockdown on the protein level, due to a lack of primary antibodies against CHO cyclins, we needed to ensure that we observed the same phenotype following knockdown with multiple unique siRNAs targeting different parts of the CcnE1 mRNA (Cullen, 2006). Prior to designing our siRNAs, we first needed to sequence the CHO cyclin mRNA because it is not available in public databases. The reader can refer to the Appendix for a summary of the cyclin mRNA sequences and a summary of our sequencing coverage. We then designed siRNAs targeting the entirety of the CcnE] mRNA, outlined in Table 4-2, using rules developed by Reynolds et al. (2004) and Ui-Tei et al. (2004). A screen of all siRNAs was performed following the protocol outlined in Section 3.4 and the results are shown in Figure 4-3, which shows relative CcnE] expression 48 hours posttransfection. Measurement of mRNA expression at 48 hours post-transfection was shown to be optimal by previous members of our lab group (Ngantung, 2005). As described in Section 3.3.6, all expression calculations were performed relative to a non-silencing siRNA control, and utilized an internal control (either p-Actin or Rnl8s) to adjust for differences in initial RNA levels. As one can see, a number of siRNA sequences (El-7, -1, -8, -5, -2, and -6) reduced CcnEl expression to less than 10% of that in the non-silencing controls. Because off-target effects are often a problem when using RNAi, we calculated relative expression compared with two different non-silencing controls to verify knockdown consistency. In addition, in order to ensure that we are using an appropriate internal control, we calculated relative expression using both /-Actin and Rn18s (ribosomal RNA) and compared our results. As seen in Figure 4-3, we observe consistency across all non-silencing and internal controls, especially when the largest levels of knockdown were observed. Figure 4-4 is a reinterpretation of the data from Figure 4-3. Moving forward, we wanted to choose one internal control and one non-silencing control for all of our future experiments. Based on the data in Figure 4-4, we chose to use #-Actin and non-silencing control #1 for a couple of reasons. For the AACT method to be valid, the amplification efficiencies of control and target mRNA primers must be approximately equal. The amplification efficiency of our #-Actin primers is closest to the efficiency of our CcnE1 primers (Table 3-3). combination of #-Actin In addition, the and non-silencing control #1 provides more conservative expression levels when expression was knocked down by at least 60%, or in other words the relative expression was less than 0.4. This is reflected in the inset graph, where the slopes of the best-fit lines are both less than unity, and the CcnE1 mRNA levels are all slightly higher with NS #1 than with NS #2. Ultimately, this choice of controls should matter very little because we observe very good agreemeint amongst all of them The siRNA transfections were than repeated 2 more times with the most highly effective siRNA sequences (El-1, -2, -5, -6, -7, and -8), and the data is shown in Figure 4-5. Each of these siRNAs consistently reduces CcnEl expression to < 10% of the non-silencing control. In addition, the transfection itself does not significantly alter the levels of CcnE] expression at 48 hours when we compare the non-silencing control with the growth control. Table 4-2. CcnE1 siRNA sequences designed using modified Tuschl rules developed by Reynolds et al. (2004) and Ui-Tei et al. (2004) sIRNA ID El-I Target sequence starting position Design Rules (R/U) GGAGAAGAUUUACCTAAGAUU 3' 5' 36l 361 R siRNA Sequence 5' 3' UUCCUCUUCUAAAUGGAUUCU E1-2 5' UUACAUGGCAUCACAACAAUU 3' 5' 517 R E1-3 UCAGCCUUGGGAUGAUAAUUU 3' 5' 51 3' UUAGUCGGAACCCUACUAUUA 166 R E1-4 5' GCCUUGGGAUGAUAAUUCAUU 3' 5# 169 E1-5 UUUGGAGGAUCAUGUUAAAUU 5' 3E' UUAAACCUCCUAGUACAAUUU 3' 5 ' 339 R E1-6 5' ACAGCUCAUUGGGAUUUCAUU 3' 5' 556 R E1-7 CCAGUUUGCUUACGUUACAUU 3' 5' 51 3' UUGGUCAAACGAAUGCAAUGU 625 R U E1-8 5' GCCCUUAAGUGGCGUUUAAUU 3' 5' 698 R U E1-9 5' E1-10 5' 3' UUGGUUCACCGAAUACAGUUA 754 R U E1-11 5' 3' AGAAAGCCAUAUUGUCAGAUU UUUCUUUCGGUAUAACAGUCU E1-12 5' E1-13 CUAAACUUGAGGAAAUCUAUU 3' 5' 51 3' UUGAUUUGAACUCCUUUAGAU 3' UUAAUGUACCGUAGUGUUGUU 3' UUCGGAACCCUACUAUUAAGU 3' 3' 3' UUUGUCGAGUAACCCUAAAGU UUCGGGAAUUCACCGCAAAUU UAAGCCCAAUGACCAUUGUUU UUAUUCGGGUUACUGGUAACA U R 3' 51 CCAAGUGGCUUAUGUCAAUUU 3' 51 3' 5 ' 1119 R UGAGCAAACCUGCCAAAGAUU 3' 5' 1223 R 591 RU 3' UUACUCGUUUGGACGGUUUCU (Note: R/U refers to Reynolds et al. (2004) and Ui-Tei et al. (2004), respectively) DActin NS #1 Rn18s NS #1 SActin NS #2 Rn 18s NS #2 rr-6..rfL E1-7 E1-1 rFLM .Rita. ffl Lmm,. E1-8 E1-5 E1-2 EI-6 E1-4 EI-10 sIRNA Sequence ~rii E1-13 E1-3 E1-12 El-11 E1-9 Figure 4-3. Relative Ccnel mRNA expression in CHO cells transfected with 13 unique siRNAs. mRNA expression at 48 hours post-transfection is calculated relative to two different nonsilencing controls (NS #1 and NS #2). Two different housekeeping genes (#-Actin and Rn18s) were used as internal controls. 0.4 1.4 slope 0.3 0-91 0.19 0.2 V1.2 0 01 COe slope 0.84 0.17 0.1 1.0 0 0l 0-- 0.2 0-1 0.0 08 0-3 0.4 E -0.6 QNon-silencing control #2 0.0 0.0 0.1 0.2 0.7 0.6 0.5 0.4 0.3 (fiActin Control) Relative CCnEI mRNA Expression 0.8 0.9 1.0 Figure 4-4. Graphical representation of Figure 4-3. The inset graph shows those data points with a relative expression level <40% of the internal controls. mRNA expression is calculated relative to non-silencing control #1 (dashed) and nonsilencing control #2 (solid). Best fit lines are linear regressions of either data set (slope ±S.E.). E 0.4 ,0.8 0.2 > NS Control Growth Control E1-1 E1-2 E1I-5 siRNA Sequence E1-6 E1-7 E1-8 Figure 4-5. Consistency of relative CcnE1 mRNA expression following transfection with most effective siRNAs. Relative expression is calculated 48 hours post-transfections and is relative to non-silencing control #1 with 8-Actin used as an internal control. The 'Growth Control' is an untransfected cell population. (Error bars: S.E., n = 3) Now that we have identified a number of unique and highly effective siRNAs that target different parts of the CcnEl mRNA we wanted to determine the effect of CcnE1 knockdown on IFN-y productivity. We sought determine if targeting CcnE1 alone was sufficient to improve recombinant protein production. Differential specific productivities were calculated and compared between day 2 and 4 following siRNA transfection. Our experiments were not continued beyond day 4 as mRNA knockdown generally recovers within 96 hours post-siRNA transfection (Ngantung, 2005). The fold-increases in differential specific productivity, qp, diff, following CcnE1 siRNA transfection relative to a non-silencing control are shown in Table 4-3. When comparing the growth, or untransfected, control with the non-silencing control it is clear that the transfection with siRNA alone negatively impacts IFN-y productivity. However, in order to understand the effect of CcnE 1 knockdown, we compare changes in productivity with the nonsilencing control. In all but one case, transfection with El-5, we are able to significantly increase IFN-y productivity (p < 0.01) by knocking down CcnE1 expression. On average, we are able to increase productivity by 137 ± 35% when compared with the non-silencing control, and by 159 ± 13% when El-5 is not included. When compared with the growth control, the specific productivity is halved following transfection with the non-silencing control. However, this reduction either does not occur or is not as drastic following transfection with all of the CcnE1 siRNA. In other words, knockdown in CcnE1 expression appears to cause the transfected cells to recover from the negative effects of the transfection. In addition to determining the effect CcnE] knockdown has on productivity, we also hoped to determine the effect of knockdown on cell growth. Referring back to Figure 4-1, it is clear that large differences in cell density are not generally observed on day 4. In our experiments, three of the siRNA transfections (El-5, -6, and -8) showed a reduction in growth (data not shown, p < 0.05) by day 4 when compared with the non-silencing control. One of the drawbacks of transient transfection is that we cannot draw conclusions about the effect of knockdown beyond day 4. We are encouraged that some of the knockdowns showed a reduction in growth by day 4 and believe that extended shRNA expression may lead to a reduction in growth beyond day 4. Table 4-3. Fold-increase in qp, diff relative to a non-silencing control following siRNA knockdown of CcnEl expression (n=2) Fold-increase (X) in p < 0.01? qp,diff (D2-D4) SE 0.00 0.95 0.53 0.55 0.36 0.21 0.93 0.22 NS Control #1 1.00 Growth Control 2.07 E1-1 2.35 E1-2 3.76 E1-5 1.27 E1-6 2.44 E1-7 2.64 E1-8 1.76 Avg 2.37 ± 0.35 Avg (w/o E1-5)1 2.59 ± 0.13 4.4 Conclusions This chapter has shown that inhibiting the CcnEl-CDK2 complex, both through chemical inhibitors and siRNA targeting of CcnE1, results in a significant increase in IFN-y specific productivity. In addition, prolonged chemical inhibition (more than 48 hours) of the CcnEl- CDK2 complex also results in growth arrest. Since the time that we began our initial study into CcnE1 and CDK2 inhibition, March and Bentley (2007) have performed similar work in Drosphila S2. In their work, the transient transfection of antisense RNA against CcnE] increased the specific productivity of green fluorescent protein (GFP). Interestingly, they found that complete silencing of CcnE1 had detrimental effects on GFP expression and that there was an optimal level of silencing that resulted in the greatest increase in per cell GFP expression. GFP, however, is not the best model protein to use in these types of studies because it is not a secreted recombinant protein. In addition, the effect of long term CcnE] silencing on overall productivity was not analyzed. Our work, on the other hand, aims to apply CcnE1 knockdown to a production relevant cell line, secreting a recombinant protein. In addition, by designing an inducible shRNA expression system we will be able to tune the level of CcnE] knockdown to its optimum level. Because of limitations associated with transient transfection methods, namely the relatively quick recovery of mRNA knockdown, we are unable to determine the long-term effects of CcnE1 siRNA knockdown on growth and productivity in these experiments. We address this limitation in the next chapter, where we discuss the work we performed in developing a cell line that inducibly expresses shRNA targeting CcnE] over extended periods of time. 5 STABLE shRNA EXPRESSING CELL LINE: A PATH FORWARD 5.1 Introduction RNA interference (RNAi) technology, or the stable expression of shRNAs, has been used to improve recombinant protein production in CHO cells in a number of different ways. These approaches include the silencing of apoptotic genes (Lim et al., 2006; Sung et al., 2007; Wong et al., 2006b), silencing genes involved in glycosylation (Ngantung et al., 2006; Mori et al., 2004), and silencing lactate dehydrogenase (Kim and Lee, 2007). The typical shRNA expression systems employed in all of these studies are either the pSUPER system (H1 promoter, OligoEngine, Seattle, WA) or the pSilencer system (U6 or CMV promoter, Ambion, Austin, TX). In all of these cases, the shRNA is continuously expressed from a U6, HI, or CMV promoter. We planned to use a similar expression system for our work. However, because our target is CcnE], which acts to control progression through the cell cycle, we needed to modify these systems to control CcnEl shRNA expression. Controllable systems for the expression of proteins (Tet-On/Tet-Off, Clontech, Mountain View, CA) and shRNA (Tet-Inducible shRNA System, Clontech, Mountain View, CA) are commercially available and consist of a modified tetracycline repressor (tetR) protein, which binds to the tetracycline operon (tetO) placed upstream of a promoter of interest. The CKIs p21 and p27 along with the CCAAT/enhancerbinding protein a (C/EBPa) and the survival factor bcl-xL have been controllably over expressed with a Tet-On system in CHO cells (Fussenegger et al., 1998; Mazur et al., 1998). In the Tet-On system, the tetR protein is fused with the activating domain of virion protein 16 (VP16) of the herpes simplex virus (Gossen and Bujard, 1992), which in the presence of tetracycline or doxycycline, binds to tetO sequences and activates expression from a minimal CMV promoter. As far as we are aware, there have been no reports in the literature involving the controlled expression of shRNA targeting a native constitutively expressed gene in CHO cells. However, such a systems has been shown to be able to control the knockdown of green fluorescent protein (GFP) expression in CHO cells (Malphettes and Fussenegger, 2004). In this work, anti-GFP shRNA expression was suppressed in the absence of tetracycline or doxycycline by a tetracycline-controlled transcriptional suppressor (tTS) protein, which is a fusion of the tetR protein and the KRAB domain of human Kox1 (Deuschle et al., 1995). Following the addition of doxycycline, the shRNA is expressed from a CMV promoter and GFP expression is knocked down. Our work represents, to the best of our knowledge, the first attempt to inducibly knockdown a native CHO gene with RNAi. We tried two different systems for the controllable expression of shRNA: (1) pSingle-tTS-shRNA, a commercially available expression vector that allows for the one-step selection of shRNA inducible expression, and (2) a modified version of pSilencer 4.1CMV, which requires first stably selecting for a cell population expressing the tTS protein and then selecting for inducible shRNA expression. 5.2 pSingle-tTS-anti CcnEl shRNA y- CHO cell lines were created that stably express the pSingle-tTS-anti CcnE 1 shRNA expressing each of our highly effective shRNA sequences. Following transfection, the cells were selected with 600 pg mL-1 neomycin for over 2 weeks followed by the isolation of single cell colonies by serial dilution in 96-well plates. In total, 58 single cell colonies were isolated and screened for CcnE1 mRNA knockdown, compared with the manufacturer's recommendation of 30 (Knockout TM Single Vector Inducible RNAi System User Manual, 2007). CcnE] knockdown was quantified 72 hours following the addition of 1000 ng mL- to the medium. None of the isolated colonies were able to induce a knockdown that was greater than 30% (data not shown). Our hypothesis is that the U6 pol II promoter is not strong enough to express the necessary amount of shRNA needed to silence CcnE1. A number of previous researchers have reported some difficulty using U6 driven shRNA expression in y-CHO cells (Ngantung et al., 2006; Lim et al., 2006). Ngantung et al. (2006) screened over 500 single cell colonies expressing anti-sialidase shRNA driven by a U6 promoter and was unable to identify a single clone with over 50% reduction in sialidase activity. Lim et al. (2006) screened approximately 350 single cell colonies expressing anti-Bax or anti-Bak shRNA and found only 3 colonies that significantly reduced Bax or Bak expression. Lastly, Mori et al. (2004) were unable to detect any significant reduction in al,6 fucosyltransferase (FUT8) expression in puromycin-resistant colonies expressing siRNA driven by a U6 promoter. One alternative to the U6 pol II promoter is a CMV pol III promoter (Malphettes and Fussenegger, 2004). CMV driven expression has been shown to be a more effective in reducing mRNA expression in our y-CHO cell line (Ngantung et al., 2006). In addition, during discussions with colleagues familiar with the induced expression of proteins, it was mentioned that it is easier to develop inducible systems via a two-step process. In this two-step process, we would first create a cell line stably expressing the tetracycline-controlled transcriptional silencer (tTS) followed by the introduction of the shRNA expression plasmid. For these reasons, we chose to stop our work with the pSingle vector and instead focus on developing the modified pSilencer system discussed in Section 5.3. 5.3 Tetracycline-controlled Transcriptional Suppressor (tTS) Cell Line The first step in creating our CMV driven shRNA expressing cell line was to first create a stable cell line expressing the tetracycline-controlled transcriptional suppressor (tTS). As was mentioned previously, the tTS protein is a fusion of the tetracycline repressor (tetR) protein and the KRAB silencing domain of human Koxl (SDKid-) (Deuschle et al., 1995). When 7 repeats of the tetracycline operator (tetO7 ) are placed upstream of a CMV promoter, the tTS protein binds to tetO 7 and represses expression from the upstream promoter. The tTS protein preferentially binds to doxycycline or tetracycline, and CMV driven expression is induced following doxycycline addition, reaching a peak expression at 48 hours post-addition. Stable expression of the tTS occurs following stable transfection with the commercially available plasmid ptTS-neo (Clontech, Mountain View, CA) and selection with 600 pg mL- neomycin. Once single cell colonies were isolated and expanded, we performed RT-PCR to determine which colonies had the highest expression levels of tTS. Figure 5-1 shows the results from the four best single cell colonies. All four single cell colonies show reasonable expression of the tTS mRNA. The negative control is the original IFN-y CHO cell line, which was transfected with ptTS-neo vector and, as expected, showed no tTS expression. The expression of /3-Actin was used as a positive control. tTS Actin 2 15 18 2D . + Figure 5-1. RT-PCR of tTS expressing single cell colonies. Gel electrophoresis of the four highest tTS expressing single cell colonies. The tTS product is shown on the top of the gel. The original IFN-y CHO cell line acted as the negative control (-), while the positive control (+) was the mRNA from a commercially available HeLa tTS cell line (Clontech, Mountain View, CA). Once we had a handful of single cell colonies that showed high levels of tTS expression, we screened them for their ability to control shRNA expression. This was done with an anti- luciferase shRNA induction assay. In this assay, we introduce two vectors via transfection, pGL2 (a luciferase expression plasmid) and a modified version of the pSingle-anti-luciferase shRNA plasmid (Figure 3-7) in which the tTS expression cassette has been removed. Varying amounts of doxycycline were added to the medium 4 hours post-transfection and luciferase expression was quantified 48 hours post-addition, the results are shown in Figure 5-2. All data is normalized to luciferase expression with no doxycycline addition. As one can see, the addition of doxycycline has a small impact on luciferase expression in the growth control. However, we observe a marked decline in luciferase expression when compared to the growth control in the cells that are transfected with the pSingle-anti-luciferase shRNA plasmid. This suggests that we are able to control expression of shRNA in our tTS cell lines. Of the four single cell colonies that were tested, tTS-2 (Figure 5-2A) performed the best in this assay. Ideally we would like to observe the induction of knockdown at the lowest possible concentration of doxycycline (dox). Doxycycline can be cytotoxic at concentrations as low as 200 ng mL-1 and can have begin to have detrimental effects in tet-on systems at concentrations nearing 2000 ng mL-1 (Ermak et al., 2003). We hypothesize that this cytotoxicity is the reason for the reduction in luciferase expression in the growth control as the doxycycline concentration increases. We would like to minimize these cytotoxic effects by choosing the cell colony that responds to the lowest concentrations of doxycycline. Both tTS-2 and tTS-6 showed significant reduction in luciferase activity at 10 ng mL-I. Comparing these two single cell colonies over the range of doxycycline concentrations used, the tTS-2 cell line performs slightly better over all concentrations. Luciferase expression continues to predictably decrease as more dox is added the medium. In addition, the tTS-2 cell line reduces luciferase expression 2-fold upon the addition of 10000 ng mL-1 doxycycline. We then repeated the same luciferase assay with only the tTS-2 cell line, and the results are shown in Figure 5-3. The data is shown in two different ways, luciferase expression is shown relative to no doxycycline addition in A and is normalized to the growth control in B. As one can see, we are able to reproducibly induce the expression of anti-luciferase shRNA and knockdown luciferase expression with the addition of as little as 1 ng mL' of doxycycline. In addition, we are able to knockdown luciferase expression up to 3-fold, which compares favorably to other protein level knockdowns by shRNA in our IFN-y CHO cell line (Ngantung et al., 2006). 1.2 1.2 00 Z 1.01.0 a00 X 0.8 - X 0.8- 0.6 0.6 ig 0.4- - 0.4 >A - 0.2 - -- B tTS-2 0.2 -- Growth -- 0.0 Growth 0.0 0 10 100 1000 Doxycycline (ng mL-) 10000 0 10 100 1000 Doxycycline (ng mL) 10000 1.2 -1.2 0 - 0 - 1.0 1.0 x 0.8 wU 1 X 0.8 w * a) 0.6 -0.6 ~ - 0.4 - - 0.4 C 0.2 -9- tTS-18 'U -+- 0.0-11 0 D) 0.2- ~0.2 Growth 10 100 1000 Doxycycline (ng mL') -o- tTS-20 --+- 0.0 11 0 10000 Growth 10 100 1000 Doxycycline (ng mL~) 10000 Figure 5-2. Luciferase knockdown in tTS expressing cell lines. Relative luciferase expression is shown for 4 tTS expressing cell lines: tTS-2 (A), tTS-6 (B), tTS-18 (C), tTS-20 (D). Luciferase expression is measured 48 hours post doxycycline addition and is shown relative to no doxycycline addition. The growth control is the original y-CHO cell line. 100 1.4 1.2 1 0.8 0.6 0.4 0.2 0.1 10 1 100 1000 10000 1000 10000 Doxycycline (ng mL1 ) 1.2 1 0.8 0.6 0.4 0.2 0 0.1 10 1 100 Doxycycline (ng mL') Figure 5-3. Induction of luciferase knockdown in tTS-2. Repeat of the luciferase knockdown experiment with only the tTS-2 colony. Data is shown in two ways: (A) Expression relative to no doxycycline addition and (B) expression relative to no doxycyline addition and normalized against the growth control at each doxycycline concentration. (Error bars: S.E., n=3) 101 5.4 pSilencer-tetO 7-CMV-anti CcnE1 shRNA Expressing Cell Line We stably transfected our tTS IFN-y CHO, from Section 5.3, cell line with our modified pSilencer plasmids expressing four different shRNAs: El-1, El-2, E1-6, and E1-7. We decided to focus our efforts on the best performing shRNAs from our initial siRNA screening (Chapter 4). Referring back to Table 4-3, El-1, E1-2, E1-6, and E1-7 all yielded the largest increases in specific productivity. The appropriate shRNAs were then designed from these siRNA sequences, as can be seen in Table 3-5. Following transfection, selection, and single cell colony isolation, we performed a series of screens to determine which single cell colonies had the ability to consistently induce CcnE] knockdown following the addition of doxycycline to the medium. All of the initial screening experiments used 100 ng mL of doxycycline for induction. Although Figure 5-3 suggests that as little as 1 ng mL' doxycycline is enough to induce shRNA expression, knocking down constitutively expressed native genes likely requires higher levels of intracellular shRNA. Also, because doxycycline can potentially be cytotoxic at 200 ng mL' (Ermak et al., 2003) we used a concentration slightly lower to ensure the doxycycline itself did not cause any aberrant observations The first round screen was performed in duplicate in 24-well plates, and a sampling of the knockdown results 48 hours after doxycycline addition are shown in Figure 5-4. Overall we screened 139 single cell clones across all 4 shRNA sequences (El-1: 38, El-2: 33, El-6: 32, El7: 36) and found 22 clones (20%) that reduced CcnE1 mRNA expression by 30% or greater. This further strengthened our initial hypothesis that the polymerase III promoter in the pSingle expression system, from Section 5.1, did not yield high levels of intracellular shRNA. Only five colonies reduced CcnE] expression greater than 50% (El-1BD, El-2BF, El-2BC, El-2E and 102 El-7Q). The El-2 shRNA seems to be the most effective shRNA sequence in this first screen, with 3 of the best performing clones expressing this shRNA. A second follow-up screen was performed on 24 single cells colonies (the 22 colonies that showed greater than 30% knockdown, plus 2 colonies with 29% knockdown). This second screen was performed in triplicate and in 6-well plates and the results are shown in Figure 5-5. Of the 24 total screens that were performed, 20 are shown in the figure. The single cell colonies that were omitted showed an increase in CcnE1 expression 48 hours following doxycycline addition. Overall, 7 single cell colonies showed an induced CcnE] knockdown of greater than 30% (El-H, El-2J, El-2BD, El-61, El-7K, El-7AI, El-7AF). This second screen had a slightly higher success rate than the first (29% compared with 20%); however, we would have expected it to be much larger. In addition, none of the five colonies that reduced CcnE1 expression below 50% in the first screen, showed any effectiveness in this second screen. We are inclined to believe that the results of the second follow-up screen are more accurate because it was performed in triplicate and the data was normalized to should be noted, that #-Actin #-Actin mRNA levels. However, it normalization does not drastically affect the final results (data not shown). 103 0.9 0.8 0 0.7 - C. W 0.6 z E 0.5 w r~ 0.4 - - 0.3 0.2 0.1 0 1 . . . . . . . . 1 .. . C LUL LJLU LI. LU LU U LU C ~ ~ ~ C- WL L LUI LU LU . .. D LUJ U - LU -LU LLU LU W U W LU - LU LU LU WL Colony ID Figure 5-4. First round screen of pSilencer-tetO 7 -anti CcnE1 shRNA expressing single cell colonies. Relative knockdown is measured 48 hours after the addition of 100 ng mL-1 of doxycycline, and all CcnE1 mRNA expression levels are shown relative to no doxycycline addition. CcnE1 mRNA levels are not normalized to #-A ctin in this first round screen. 104 1.4 E1-1 I I EI-2 EI-6 I E1-7 1.2- 0 1 0 C. z 0.8- E 0.6- 0.4- 004 0.2- - 0ZM CC - ' M CM M M LU uCj UJ . - - - -1 - Colony ID Figure 5-5. Second round screen of best performing single cell colonies from the first round screen. Relative knockdown is measured 48 hours after the addition of 100 ng mL- of doxycycline and all CcnE1 mRNA expression is shown relative to no doxycycline addition. -Actin is used as an internal control in this screen. 105 According to well-annotated methods for confirming the specificity and consistency of shRNA knockdown (Cullen, 2006), it is important to test for a dose-response when inducing shRNA expression. Therefore, we further analyzed our 7 best single cell colonies by adding increasing amounts of doxycycline to them (0, 10, 100, and 1000 ng mL-) and measured the effect that this had on CcnE] mRNA expression. The results are shown in Figure 5-6. As expected CcnE1 mRNA levels do not change drastically in the tTS control colony until the addition of 1000 ng mL' doxycycline when CcnE1 expression is reduced by 36%. This further validates our initial assumption that all screening should be done with doxycycline concentrations of 100 ng mUL1 . The five colonies that exhibited a dose-response behavior are also shown. Colonies El-2BD and E -7K have lost the ability to induce CcnE] knockdown and are not shown. Of the remaining 5 colonies, both El-1H and El-2J showed an induction of CcnE] expression following the addition of 10 ng mL- of doxycycline, but did show an induced knockdown at the elevated doxycycline concentration of 100 ng mUL. Single cell colonies El-61, El-7AI, and El-7AF showed reasonable reductions in CcnE1 expression following the addition of 10 ng mL doxycycline. 106 2 1.8 1.6 1.4 0 ng/mL 10 ng/mL 100 ng/mL 1000 ng/mL - 1.2 1 0.8 0.6 0.4 0.2 tTS E1-1H E1-2J E1-61 E1-7Al E1-7AF Colony ID Figure 5-6. Dose-response of single cell colonies to increasing levels of doxycycline. Relative knockdown is measured 48 hours after the addition of doxycycline and all CcnE1 mRNA expression is shown relative to no doxycycline addition. #-Actin is used as an internal control (Error bars: S.E., n=1). 107 The results of all the screens have been summarized in Table 5-1. None of the 5 best performing single cell colonies from the first screen showed any knockdown in the subsequent screen and were not brought forward for further analysis. Of the 22 single cell colonies, 7 exhibited a knockdown in CcnE1 expression of greater than 30% in the second screen and are also shown in the table. - The extremely low relative CcnE1 expression for clone El -7AF in the second round screen is likely a result of the loss of some sample during the screening process and was not included in the final average for that clone. During the third 'dose-response' screen, 2 of the single cell colonies (E1-7K and El-2BD) lost the ability to induce CcnE1 knockdown. A total of 5 single cell colonies, however, showed the ability to consistently knockdown CcnE1 levels 48 hours following the addition of 100 ng mL I doxycycline in all of the screens. These results are combined in the table and show that clone El-61 was able to induce the largest decrease in CcnE1 expression, while clone El-7AI was the most consistent. 108 Table 5-1. Screening summary of induced CcnEJ knockdown following 100 ng mLdoxycycline addition Most Successful 1" Round screens El-1BD 0.46 0.93 E1-2BF 0.17 1.13 E1-2BC 0.26 0.79 E1-2E 0.44 2.31 E1-7Q 0.44 4.12 Lost Ability to Induce Knockdown El-7K 0.54 0.23 2.01 El-2BD 0.56 0.60 1.85 Most Consistent Colonies Overall El-1H 0.71 0.22 0.80 0.58 0.18 E1-2J 0.70 0.43 0.83 0.65 0.12 El-61 0.71 0.37 0.61 0.56 E1-7AI 0.61 0.67 0.63 0.63 0.02 E1-7AF 0.68 0.04 0.81 0.75 0.07 109 t 0.10 We then further analyzed our 5 best performing clones to determine what affect the induction of CcnE1 knockdown had on cell growth and specific productivity. There experiments were again performed in 6-well plates in triplicate and lasted 6 days. We measured relative CcnE1 mRNA levels, cell density, and IFN-y titer in the supernatant. The growth curves from these experiments are shown in Figure 5-7. Viability remained above 95% for all clones throughout the experiment (data not shown). We observe a decrease in cell growth following the addition of doxycycline to clones El-2J and El-7AF. Importantly, the growth of the base tTS cell line was unaffected by doxycycline addition. None of the other clones slowed in growth following doxycycline addition. Table 5-2 further summarizes the results from our 5 best performing clones on the induced knockdown of CcnE1 and the impact of knockdown on specific productivity. In addition, clone E 1-61 is the only single cell clone that maintains CcnE1 knockdown until day 3. We hypothesize that this is because our expression system is not expressing or maintaining high levels of intracellular shRNA. For this reason, the actual phenotypes that we observe are called into question. Nevertheless, only two colonies, El-2J and El-7AF, show a reduction in growth. Although CcnE1 levels are reduced for the longest time in the clone El-61, it does not slow in growth. Clone El-2J is the only clone that shows an increase in specific productivity and a decrease in growth following doxycycline addition In general, there is very little consistency over time amongst our results. Knockdown does not necessarily result in the slowing of growth (El-7AI, for example), and the slowing of growth does not necessarily result in an increase in specific productivity (El-7AF, for example). We 110 hypothesize that this is likely because our expression system is not expressing shRNA to levels high enough to induce a consistent phenotype. In addition, it is conceivable that expression from the shRNA cassette is reduced or eliminated through common cellular events like DNA methylation or the inherent genetic instability of CHO cells. 111 tTS -- -dox -- 0 +dox 2 ---.. -9- 0 2 0 2 4 6 8 4 Time (d) 6 8 Time (d) Time (d) 6 8 E1-61 ox +dox 2 0 4 2 0 4 Time (d) 2 4 Time (d) 6 4 6 Time (d) 8 Figure 5-7. Growth curves of single cell clones with or without the addition of 100 ng mL 1 doxycycline. Total viable cells per 6-well plate is shown as a function of time. Single cell clones were grown in the (o) presence or (+) absence of 100 ng mL' doxycycline. Doxycycline addition occurred on day 2. (Asterisk represents a statistically significant difference in cell density, Ip< 0.05) 112 Table 5-2. Summary of the effects of 100 ng mL 1 doxycycline addition to single cell clones tTS 1.15 ± 0.00 0.87 ± 0.09 El-1H 0.89 0.08 1.18 0.25 N N 0.56 El-2J 0.80 0.06 1.17+0.37 Y Y 1.55 El-61 0.72 0.04 0.66 0.15 N N 0.90 El-7AF 0.92 0.09 1.18 0.37 Y N 0.62 El-7AI 0.40 ±0.15 1.24 0.49 N N 1.15 + 0.80 113 5.5 Conclusions We created a functional tetracycline-controlled transcriptional silencer (tTS) expressing IFN-y CHO cell line expressing high levels of the tTS protein with the ability to control shRNA expression. However, we were unable to express shRNA to high enough levels, regardless of promoter type, to consistently knockdown CcnE] mRNA levels by more than 30%. We hypothesize that this level of knockdown is not large enough to induce a consistent functional change in phenotype. Although tTS controlled shRNA expression systems have been used to control to the expression of GFP (Malphettes and Fussenegger, 2004), in these systems, GFP mRNA is likely not expressed to the same high levels as an endogenously expressed gene like CcnE1. In addition, over time the single cell colonies slowly lost their modest ability to knockdown CcnE] mRNA. The reason for this is currently unknown, however we hypothesize that common cellular events, like DNA methylation and genetic instability, could lead to loss of shRNA expression. Because of these issues with our expression system, we are unable to answer the over-arching question of whether prolonged CcnE1 knockdown can result in overall increases in total IFN-y productivity. We think that this type of system, however, has the potential to work in the future. With improvements in shRNA expression systems, that can express very high levels of shRNA with low levels of inducing chemicals (doxycycline), this approach could be quite successful. Our proof-of-concept experiments provide compelling evidence to suggest that inhibition or knockdown of CcnE] can, at the very least, result in improvements in specific productivity. 114 6 SHORT TERM CDK INHIBITION: GROWTH, CELL CYCLE, AND PRODUCTIVITY 6.1 Introduction It is important to understand the relationships between cell cycle, growth and productivity when developing a recombinant protein production process in order to best optimize the medium, culture conditions, and bioreactor operations. A number of studies have been performed attempting to systematically determine the relationship between cell cycle and the specific productivity of both secreted (i.e. tPA, IFN-y and SEAP) and intracellular proteins (i.e. PGalactosidase and DHFR). The vast majority of these studies were performed in normal exponentially growing CHO cell cultures and these studies have elucidated relationships between productivity and Gi-phase (de Boer et al., 2004; Kubbies and Stockinger, 1990), S-phase (Gu et al., 1993; Kubbies and Stockinger, 1990; Lloyd et al., 1999, Mariani et al., 1981), G2/M-phase (Lloyd et al., 2000; Yokota and Tanji, 2008), aphasic (Feder et al., 1989) and inversely related to Gi-phase (Yamaji et al., 2004). These apparently contradictory results generally can be explained by differences in the expression system, protein of interest, promoter construct, culture conditions, and experimental design. Very few systematic studies have been performed that elucidate the relationship between cell cycle and productivity in growth arrested cells. Increases in the specific and total productivity of recombinant proteins have been shown to occur during Gi-phase growth arrest in low temperature culture (Hendrick et al., 2001; Kaufmann et al., 1999; Yoon et al., 2003), following the addition of butyrate to cell cultures (D'Anna et al., 1980, Hendrick et al., 2001), and following. the overexpression of CKIs (Bi et al., 2004; Carvalhal et al., 2003a; Fussenegger et al., 1997). In most of the above work, the G, phase of the cell cycle was specifically targeted for 115 growth arrest. In addition, increases in productivity have also been shown to occur during Sphase growth arrest following the addition of various nucleotides and nucleosides, including AMP, to culture medium (Carvalhal et al., 2003b). It is unclear whether these improvements in productivity are directly related to the specific cell cycle phase in which arrest occurs, or if growth arrest alone is sufficient to improve productivity. To our knowledge, there have been no reports of studies that determine the effect of specific differences in cell cycle distribution have on either arrested or slowly growing cells. We proposed to induce specific changes in cell cycle distribution through the addition of exogenous chemical CDK inhibitors, Table 3-1. We have shown previously (Chapter 3) that the addition of a CDK2 inhibitor results in extended growth arrest and increases in specific productivity. By adding inhibitors of other CDKs, we were able alter cell cycle distribution in slowly growing or arrested cells and quantitatively assess the impact on specific productivity. Most importantly, because we are targeting highly specific cell cycle regulating proteins, we believe we are able to determine the relationship between cell cycle, productivity, and growth without metabolic disruption. 6.2 CDK Inhibitor Characterization All of the inhibitors that were used in this thesis have been previously characterized in the literature (Brooks et al., 1997; Bhattacharjee et al., 2001; Pennati et al., 2005; Vassilev et al., 2006; Retzer-Lidl et al., 2007). Starting from the known IC50 concentrations (Table 3-1), we added varying amounts of the inhibitors to growing y-CHO cells. Cells were plated at 1x10 5 cells/well and inhibitors were typically added during exponential growth 2 days later when the 116 viable cell density was approximately 1x10 6 cells well-1. In addition, because our inhibitors are dissolved in DMSO, we added equal volumes of DMSO to our control cultures to ensure that our observations are not caused by DMSO-induced growth arrest (Fiore and Degrassi, 1999). Finally, we added inhibitors at a cell density of 1x106 cells well' to prevent cell density related growth arrest within our experimental time frame of 36 hours. Figures 6-1 and 6-2 show representative growth curves following the addition of inhibitors cdk2iii, cdkliv, cdk4, and cdk2iv at varying concentrations. Viability remained above 90% across all inhibitors and concentrations (data not shown). We are able to drastically slow cell growth following the addition of the inhibitors cdk2iii and cdkliv as can be seen in Figure 6-1. Shedding occurs following the addition of 10 and 20 gM cdk2iii, and is the reason for the decline in viable cell density. The actual growth rate in these cultures is actually zero. Shown in Figure 6-2, 2.5 gM was the only cdk4 inhibitor concentration that slowed growth without killing the cells. All concentrations higher than 2.5 gM that were tried resulted in massive cell death prior to 36 hours. This cell death even occurred following the addition of only 3.5 gM cdk4 inhibitor. The addition of inhibitor cdk2iv drastically slowed cell growth, Figure 6-2. We will go into more detail regarding inhibitor cdk2iv in Section 6.3. The specific growth rate was estimated for each inhibitor concentration assuming an exponential model of cell growth and assuming no cell death was occurring. assumptions given the short time frame of our experiments. 117 These are both reasonable 3- Growth Control A cdk2iii - 10 uM x cdk2iii - 20 uM = 2.5 0 2cdk2iii - 5 pM 1.5 - cdk2iii -10 pM 0.5cdk2iii - 20 pLM 0 20 40 Time (hours) 60 80 3.5 Growth Control o Growth A cdkliv- 10 uM x cdkliv - 20 uM B cdkliv - 10 piM 2.5 - )2 .01.5 204 0 08 0.5 0 20 40 60 80 Time (hours) Figure 6-1. Cell growth curves in the presence of inhibitors cdk2iii and cdkliv. The figures show representative growth curves of y-CHO cells in the presence of inhibitors (A) cdk2iii and (B) cdkliv. (Error bars: S.E., n=1) 118 6 * Growth Growth Control o cdk2iv - 5 uM A cdk4 - 2.5 uM 5 O cdk4 - 2.5 jM 3 - a] 0 20 40 Time (hours) c dk2iv - 5 60 MN 80 Figure 6-2. Cell growth curves in the presence of inhibitors cdk4 and cdk2iv. The figure shows representative growth curves of y-CHO cells in the presence of inhibitors (A) cdk4 and (o) cdk2iv, and in control cultures (Error bars: S.E., n=1) 119 We also determined the effect of each inhibitor on cell cycle distribution and compared our results with those previously reported in the literature. Cells that were growing in 6-well plates, in the presence and absence of inhibitors, were sacrificed at predetermined intervals, fixed in ethanol, propidium iodide (PI) stained, and analyzed by FACS. Figure 6-3A, shows the cell cycle distribution over a 36 hour time frame in exponentially growing y-CHO cells. Between 8 and 12 hours we observe cells transitioning from the Go/Gj to the S-phase of the cell cycle. Between 12 and 16 hours we observe cells transitioning from the S to the G2/M-phase of the cell cycle. Also, it is clear that accumulation begins to occur in the Go/Gi-phase of the cell cycle after 36 hours, Figure 6-3B. The accumulation of cells in G, during confluence is observed in nontransformed and immortalized cell lines (Fiore and Degrassi, 1999) and is the reason we stop our experiments at 36 hours. In this way, we can eliminate the effects that cell density can have on cell cycle distribution. Figures 6-4, 6-5, and 6-6, show the effect of each of the inhibitors on cell cycle distribution. The inhibitor cdkliv, Figure 6-4, noticeably increases the number of cells in G2 /M, in a concentration dependent manner. The G2 /M peak is much larger in Figure 6-4B when compared to 6-4A. In addition, the G2 /M peak is delayed at the higher inhibitor concentration. This is partially because inhibitor was added to cells with different cell cycle distributions at time 0. Part of this delay can be explained by the fact that these two experiments were performed at different times, and the inhibitors were added at different cell densities. The delay is also due, in part, to the inhibition of CDK2, which is also inhibited by cdkliv at elevated concentrations. 120 The inhibitor cdk2iii acts by delaying progression from the Gi to the S-phase of the cell cycle, Figure 6-5, and like the inhibitor cdkliv this delay is concentration dependent. Although we did not take a 12-hour time point when we added 5 tM cdk2iii, we can still compare the data in Figure 6-5 to the growth control, Figure 6-3. Comparing Figure 6-5B to Figure 6-3A, the peak in S-phase cells is delayed by 4 hours and slightly decreased following the addition of 10 pM inhibitor cdk2iii. Comparing Figure 6-5A and Figure 6-3B, when a smaller amount of inhibitor is added, we do not observe a large change in the S-phase peak, however, progression through Sphase is delayed. In addition, inhibitor cdk2iii also causes an increase in the number of cells in G2/M, which is caused the inhibition of CDK1, a secondary target of cdk2iii. Lastly, the inhibitor cdk4 acts by drastically increasing the number of cells in the Gi-phase of the cell cycle, and inhibits normal progression through the cell cycle, as can be seen in Figure 6-6. This change in cell cycle progression occurs even though cell growth is only slowed slightly. As we mentioned previously, increased concentrations of cdk4 rapidly kill cells in culture. It is not clear if the inhibitor is the cause of death or if cells cannot remain in G1 for extended periods of time. It should be noted that because CDK4 is inhibited these cells are likely accumulating in early-G 1 , although there is no way to distinguish between early and late-G 1 with PI staining. In order to quantify these changes in cell-cycle distribution, we calculated a time-integrated cell cycle distribution for the entire 36-hour experiment. This time-integrated cell cycle distribution is the area under the cell cycle distribution curves from Figures 6-3, 6-4, 6-5, and 6-6 divided by the total experiment time of 36 hours. This takes the cell cycle distribution and time-weights the average distribution, capturing the general changes that occur over the entire 36-hour period. 121 This value is also sometimes referred to as the average value of the cell cycle distribution. We hypothesized that a relationship may exist between this quantitative metric and overall changes in specific productivity. Table 6-1, shows our calculated values for the time integrated cell cycle distribution for the growth control and all inhibitors. These values verify our qualitative observations: cdkliv increases cells in G2 /M, cdk4 drastically increases cells in G1 , and cdk2iii slightly increases cells in G2 /M. Perhaps most interesting, and slightly unexpected, is the effect that the inhibitor cdk2iii has on cells in G1 . Because the inhibitor cdk2ii acts to slow progression through the cell cycle, we observe an overall decrease in the proportion of cells in G1 when compared to the growth control. 122 A 7060. [8 UG2AM G1G -+- -M--S -G2IM 8 0 16 12 Time (hours) 24 8 0 36 G2/M 16 12 Time (hours) 24 36 B 80 80 70 70 GOG1 + GO/G1 -BS *GZ'Mv 60 60 3 50 a 50 40 40 $ 30 30 G21M 0 8 24 16 Time (hours) 36 60 0 8 24 16 Time (hours) 36 60 Figure 6-3. Cell cycle distribution in exponentially growing cells. The cell cycle distribution is shown as a function of time over two slightly different time periods: (A) 0-36 hours and (B) 0-60 hours. The same data is plotted in two different ways. 123 A 10 tM 70 70 1GO/GI1 60 50 -p-GOiG 1 -ES S GOYG1 +G21M A G2M ~40 )40 30 0 30 20 20 10 G2M 0 0 8 12 16 Time (hours) 24 16 12 Time (hours) 24 36 0 8 12 16 Time (hours) B 20 M 0 8 36 0 8 12 16 24 36 Time (hours) Figure 6-4. Cell cycle distribution following the addition of inhibitor cdkliv. Representative cell cycle distributions are shown as a function of time and the same data is plotted in two different ways. Inhibitors were added at (A) 10 pM and (B) 20 pM. 124 A 5gM 80 . 70 - -*- GO/G1 -- S -k-- G2/M 60 - 2 50 40 - $ 30 20 10 - 0 0 8 12 16 Time (hours) 24 36 0 8 12 16 Time (hours) 24 36 B 10 tM 70 70 60 60 50 50 40 0%40 30 30 20 20 10 10 0 0 0 8 12 16 Time (hours) 24 0 36 0 8 12 16 Time (hours) 24 36 Figure 6-5. Cell cycle distribution following the addition of inhibitor cdk2iii. Representative cell cycle distributions are shown as a function of time and the same data is plotted in two different ways. Inhibitors were added at (A) 5 pM and (B) 10 pM. Data was not available at 12 hours in the top graph (A). 125 80 70 60 50 40 30 20 10 0 0 8 12 16 24 36 - - --..... Time (hours) I- 80 70 - 60 - 50 - 40 - - - - - - --- GOIG1 -I SO/G1 &S A- G2/M 30- S 0-- 0 8 12 16 24 36 Time (hours) Figure 6-6. Cell cycle distribution following the addition of inhibitor cdk4. A representative cell cycle distribution is shown as a function of time and the same data is plotted in two different ways. The cdk4 inhibitor was added to 2.5 M. 126 Table 6-1. Time-integrated (w ted) cell cycle distribution ± 1.1 ± 2.2 37.6 ± RM 34.7 2.2 38.5 ± 1.9 26.8 ± 3.5 20 jtM 28.9 1.7 28.8 ± 1.0 42.3 ± 2.8 5 ptM 43.8 0.1 33.8 ± 0.0 22.4 ± 0.1 10 M 36.7 3.4 39.1 ± 2.0 24.2 ± 2.5 2.5 gM 67.1 3.6 24.5 ± 5.5 8.4 ± 1.9 10 3.5 10.9 51.3 Growth cdkliv cdk2iii cdk4 (Error bars: ± S.E.) 127 We also determined the effect that inhibitor addition had on specific productivity, qp. Specific productivity is a measure of the amount of IFN-y produced per cell in a given amount of time, and is calculated as shown in Section 3.7.1. To reiterate, it is the slope of a linear regression of cumulative IFN-y production and the integrated viable cell density (IVCD), as shown in Figure 6-7. We show a representative sampling of this data for all of our inhibitors. In most cases, the addition of CDK inhibitors acts to increase specific productivity, with the lone exception being 10 gM of inhibitor cdkliv, Figure 6-7A, which has little impact on qp. In addition, as more inhibitor is added to the cell cultures we generally observe an increase in specific productivity. Now that we have fully characterized our CDK inhibitors in terms of how they effect changes in growth, cell cycle distribution, and specific productivity, we used this information to determine the relationships between qp, growth, and cell cycle distribution. 128 A 1.8 1.6 Growth Control (0.026) 1.4 cdkliv 20 pM (0033) . 1.2 cdkliv 10 IM (0,025) 1 0.8 0.6 *Growth Control 0.4 Ucdktiv - 10 pM 0.2 Ocdkliv - 20 pM 0 0 50 40 30 20 10 70 60 80 Integrated Viable Cell Density (10' cells hr) 2 B 1.8 1.6 cdk2li 10 (0.046) pM 4 14 Growth Control (0.026) cdk2i 20 pM (0053) 1.2 1 0.8 +Gfowth Contro 0.6 cdk2i - 5 pi 0.4 Ocdk,2i- 0.2 10pM Ocdk2ini- 20 OM 0 20 10 0 50 40 30 60 70 80 Integrated Viable Cell Density (10' cells hr) C Growth Control (0.042) 4 z 3 IOcdk4 OGrowth Control -2.5 pM 0 0 20 40 60 80 100 120 140 Integrated Viable Cell Density (100cells hr) Figure 6-7. Cumulative IFN-y production plotted against Integrated Viable Cell Density (IVCD) for each inhibitor. Data is plotted for inhibitors (A) cdkliv (B) cdk2iii and (C) cdk4. The slope of a best-fit line through the plotted data is defined as the specific productivity, qp. Specific productivity for each inhibitor concentration is shown in parentheses in units of pg cell' h- (Error bars: S.E., n=1) 129 6.3 Relationship between Specific Productivity and Cell Cycle Distribution If a relationship exists between a specific cell cycle phase and specific productivity we should be able to observe a correlation between the two. While this approach does not reveal any causality between cell cycle phase and productivity, it can reveal the best cell cycle phase to target in a growth arrest strategy. A typical way of quantifying cell cycle distributions involves calculating the time spent in each cell cycle phase during one doubling (Hendrick et al. 2001; Slater et al. 1977). We cannot employ this method using our experimental data, because our doubling times approach infinity as cell growth decreases. Instead, we were able to capture the drastic changes we made in cell cycle distribution quantitatively by defining a time-integrated cell cycle distribution. We then sought to determine if there was a correlation between this quantitative metric and specific productivity. The time-integrated cell distributions are plotted against normalized specific productivity below in Figure 6-8. Each of the inhibitor experiments was performed two or more times and the specific productivities were normalized to the growth control in each experiment. As one can see from the linear regression correlation coefficients (Pearson's correlation coefficients), there is a very weak inverse relationship between G1 and specific productivity and a weak positive relationship between G2/M and specific productivity. In general, we do not observe any strong correlations between specific productivity and cell cycle distribution. Interestingly, regardless of the type of inhibitor added we almost always observe an increase in specific productivity, regardless of the changes made to cell cycle progression. It is possible that a linear regression is not the best metric to determine whether a relationship exists between cell cycle distribution and specific productivity. 130 Another commonly used correlation coefficient is the Spearman's rank correlation coefficient, which is a non-parametric measure of dependence between two variables. It determines how well the relationship between two variables, in our case cell cycle distribution and specific productivity, can be described by a monotonic function. The Spearman's rank correlation coefficients agree extremely well with the Pearson's correlation coefficients and are as follows: Go/G 1 - -0.43, S - 0.09, and G2/M - 0.43. Lastly, rather than using a time-integrated cell cycle distribution to capture the changes in cell cycle distribution, we could have used the normalized time distributions defined by Slater et al. (1977). This analysis yields the fraction of time that is spent in each cell cycle phase during a single doubling. This approach does not substantially change any of the correlation coefficients (data not shown). Had we taken a more qualitative approach to this analysis, we could have easily determined that the G2/M-phase of the cell cycle has a much stronger impact on specific productivity that it actually does. The inhibitors cdk2iii and cdkliv increase specific productivity the most and also increase the proportion of cells in the G2/M-phase of the cell cycle. Rather, our analysis with CDK inhibitors shows that specific productivity does not depend strongly on cell cycle distribution in slowed growth and growth-arrested cell cultures. This emphasizes the importance of taking a more quantitative approach to determining the relationship between cell cycle and productivity. 131 n Growth o cdk2iii 2.0 - * cdkl iv x cdk4 2.5 R = -0.43 R = 0.13 * Growth 10 pM 2.0 o cdk2iii - * cdkliv 10 PM x cdk4 20 pM 5PM 25pM 10 PM Fi 0.5 - - 1.5 - 20pM 1.0 - 2 5M 0.5 A 5pM B - nn 0 20 40 % GoIG 1 0 10 20 30 40 50 2.5 - o Growth o cdk2iii 2.0 - +4cdk1iv cdk4 1.5 - R = 0.43 0 PM 5 PM 2.5 pM 1.0 20 pM - 0.5 - 00 0 C . 10 20 30 40 50 % G2IM Figure 6-8. Correlations between normalized specific productivity and cell cycle phase following inhibitor addition. qp is shown normalized to the growth control and plotted with respect to the time-weighted average cell cycle distribution over the entire 36 hour experiment (A) Go/G 1 (B) S and (C) G2 /M. R = Pearson's correlation coefficients. (Error bars: S.E; n = 6: Growth; n = 3: 10 p.M cdkliv, 1ORM cdk2iii; n = 2: 5 p.M cdk2iii, 20 gM cdkliv, and 2.5 pM cdk4) 132 6.4 Relationship between Specific Productivity and Specific Growth Rate We also sought to determine the relationship between specific productivity and specific growth rate following the addition of our CDK inhibitors. Figure 6-9 below shows the same normalized productivity shown in Figure 6-8 plotted against specific growth rate for all of our inhibitor experiments. As one can see from the linear correlation coefficients (Pearson's correlation coefficients) there is a very strong relationship between specific growth rate and specific productivity. Most importantly, regardless of the type of inhibitor that was added to the culture, specific productivity always increased as growth decreased. The only exception is that 10 gM of inhibitor cdkliv had no impact on productivity. While increasing specific productivity is a notable achievement in itself, it is only the first step in increasing overall productivity of a cell culture system. As we know, there is often a trade-off between specific productivity and the IVCD, and balancing them is necessary to improve total recombinant protein productivity, (Equation 3-2). The total IFN-y production at a specific time can be expressed as a function of specific productivity and specific growth rate, p, as shown in Equation 6-2: J dP= qp Ndt (3-2) eudt (6-1) t N P=q, 0 = ] PNvO[ kt 133 (6-2) In Figure 6-10, we have added a dashed line to the data from Figure 6-9 that is defined by Equation 6-3, where P is a constant, and represents total IFN-y production in the growth control at 36 hours, and No,, refers to the number of viable cells during inoculation. qp = N,, 0 [e#' -1] (6-3) From Figure 6-10, it is clear that there are a number of inhibitor concentrations, 10 gM cdk2iii and 20 gM cdkl iv, that can cause an increase in total IFN-y production over the course of our 36 hour batch experiments. This theoretical calculation also agrees with our experimental results, both cdk2iii and cdkliv have been shown to slightly increase IFN-y production over 36 hours. Although these experiments do not represent a production relevant cell culture system, this observation does provide us with some optimism that these inhibitors may be able to improve total productivity in a batch or fed-batch production scale cell culture. 134 2.5 2.0 - o Growth o cdk2iii +cdkliv x cdk4 -10 MN C 1.5 E;VV N E 0 1.0Z - 2. M - 10 pUM =-0.93 0.5R I 0.0 - -0.01 0.00 0.02 0.01 0.03 0.04 t (hr-) Figure 6-9. Correlation between normalized specific productivity and specific growth rate following inhibitor addition. qp is shown relative to the growth control and plotted with respect to the specific growth rate, p. R = Pearson's correlation coefficients. (Error bars: S.E; n = 6: Growth; n = 3: 10 gM cdkliv, 10gM cdk2iii; n = 2: 5 gM cdk2iii, 20 gM cdkliv, and 2.5 pM cdk4) 135 2.5 2.0 -.. 10 M + cdkl1iv x cdk4 L1.5 -- 1.5 20 M M. M 0 1.0 2.5gM Z . 10M .... 0.5 - 0.0- -0.01 0.00 0.01 0.02 0.03 0.04 p (hr') Figure 6-10. The effect of normalized specific productivity and specific growth rate on total IFN-y production. qp is shown relative to the growth control and plotted with respect to the specific growth rate, p. The dashed line represents a line of constant total production at 36 hours and is defined by Equation 6-3. (Error bars: S.E; n = 6: Growth; n = 3: 10 gM cdkliv, 10 gM cdk2iii; n = 2: 5 [tM cdk2iii, 20 gM cdkliv, and 2.5 gM cdk4) 136 6.5 Further Characterization of Inhibitor cdk2iv While we have observed that the addition of inhibitor cdk2iv results in an apparent growth arrest as shown in Figure 6-2, we have not included it in our previous analysis. The reason for this is that inhibitor cdk2iv behaves differently from the other CDK inhibitors. Rather than induce an accumulation of cells in G2 /M, as suggested by the manufacturer (Pennati et al., 2005), we observe that cdk2iv actually induces tetraploidy, or an increase in chromosomes or chromosomal DNA, in our y-CHO cells. We first observed this behavior during the cell cycle analysis shown in Figure 6-11, which shows the cell cycle distribution 0, 8, and 24 hours after the addition of 5 gM inhibitor cdk2iv to growing cells. Within 24 hours, we observe that the entire cell population is in a tetraploid state. To further verify that the inhibitor cdk2iv was inducing tetraploidy we visualized the cells postinhibitor treatment using immunofluorescence. Cells were plated on fibronectin-treated coverslips and fixed 24 hours after the addition of each inhibitor (cdk2iii, cdkliv, cdk4, and cdk2iv). The cells were stained with three different dyes/antibodies. DNA was stained with Hoechst 33258 and detected with a DAPI (blue) filter. y-tubulin, which is found mainly in centrosomes, was detected with a primary antibody (Gtu.88 y-tubulin antibody) followed by an AlexaFluor 488 secondary antibody and detected with a fluorescein (green) filter. F-Actin was stained with AlexaFluor 594 phalloidin and detected with a Texas Red (red) filter. The cells were then imaged at 60X with a DeltaVision Core deconvolution microscope. The merged images are shown in Figure 6-12. The right hand pictures in each group are cropped versions of the original 60X image on the left. This was done to enhance visualization of the centrosome. 137 Normal cells have one centrosome comprised of two centrioles (Lodish et al., 2004). The centrosome is the primary microtubule-organizing center in G2-phase cells. During mitosis, the two centrioles separate and migrate to opposite sides of the cell, separate the duplicated chromosomes, and establish the bipolarity of a dividing cell (Lodish et al., 2004). The addition of inhibitors cdk2iii, cdkliv, and cdk4 does not affect the ploidy of our y-CHO cell line and, therefore, these cells all have two centrioles as can be seen in Figure 6-12A, B, C, and D. However, following cdk2iv treatment, Figure 6-12 E, we observe a significant increase in the number of centrioles present in our cells, which is a characteristic of polyploidy. In addition, we see a noticeable increase in both the cell size as well as the DNA content (stained blue) in our cells. Taken together, these observations verify that we are inhibiting mitosis and inducing tetraploidy in our cell line through the addition of inhibitor cdk2iv. 138 A 8- 50 0 0 50 0n 15 Channals(FL2-A) 100 290 25 150 Channels (FL2-A) C 0 50 150 100 Channels(FL2-A) 200 250 Figure 6-11. Cell cycle distributions following the addition of the inhibitor cdk2iv. Cell cycle distributions are shown (A) Oh, (13) 8h, and (C) 24h after the addition of the cdk2iv inhibitor. The graphs are histograms of DNA content (FL2-A). 2N cells in Go/Gi have an FL2A of 50, 4N cells in G2/M have an FL2-A of 100, and 8N cells have an FL2-A of 200. 139 Figure 6-12. 60X images of stained y-CHO cells 24 hours post-inhibitor treatment. CHO cells were treated for 24 hours with (A) DMSO control, (B) 5 iM cdk2iii, (C) 10 gM cdkliv, (D) 5 [LM cdk4, and (E) 5 pM cdk2iv. Cells were fixed prior to immunostaining. FActin was stained with AlexaFluor 594 phalloidin and detected with a Texas Red (red) filter. DNA was stained with Hoechst 33258 and detected with a DAPI (blue) filter. y-tubulin was detected with a primary antibody followed by an AlexaFluor 488 secondary antibody and detected with a fluorescein (green) filter. All images were taken with a DeltaVision Core deconvolution microscope. 140 We wanted to further determine the effect that inhibitor cdk2iv has on growth and specific productivity. Figure 6-13 shows growth curves following the addition of 2.5 gM of inhibitor cdk2iv, and following the addition and subsequent removal of the same inhibitor 24 hours later. Time points were taken every 12 hours for 48 hours total. The viability remained above 90% throughout the experiment. As can be seen in the graph, the cells undergo a strong growth arrest following addition of the inhibitor cdk2iv, which we also observed in Figure 6-2. By adding the inhibitor cdk2iv and subsequently removing it through a medium replacement 24 hours later, the cells are able to start growing again. Figure 6-14 plots the total cumulative IFN-y production against the IVCD. As can be seen, the addition of the inhibitor cdk2iv drastically increases specific productivity. This observation is somewhat expected, partly because we observe large increases in cell size following inhibitor addition. Intuitively, larger cells are likely to produce more IFN-y on a per cell basis. It is also interesting to note, that after removing the inhibitor cdk2iv at 24 hours, the specific productivity still remains quite high. We hypothesized that the increase in specific productivity may also be caused by an increase in intracellular genomic DNA content. At each 12-hour time point we purified the genomic DNA from 500,000 cells and measured the DNA content with Picogreen dye. In Figure 6-15 we show that exposure to the cdk2iv inhibitor doubles the genomic DNA content in a cell within 12 hours of addition when compared with a regularly growing cell. Following the removal of the inhibitor after 24 hours (light gray bars), the amount of genomic DNA in the cells levels out at approximately double the genomic DNA content of a regularly growing cell. Interestingly, the 141 increase in specific productivity, with and without the removal of the inhibitor, trends with DNA content. The specific productivity increases 2.39 (± 0.5)-fold (± S.E., n=2) without the removal of the inhibitor and 2.17 (± 0.2)-fold with the removal of the inhibitor while the DNA content increases 2.8-fold (n=1) without the removal of the inhibitor and 2.3-fold with the removal of the inhibitor. The genomic and phenotypic instability of CHO cells have been shown to be proportional to their degree of aneuploidy (Duesberg et al., 1998). For this reason, the addition and subsequent removal of the inhibitor cdk2iv may represent an interesting strategy to balance the advantages of tetraploid cells, increased specific productivity, with the disadvantages, genetic instability and eventual cell death. Such a strategy may increase total IFN-y production in an extended cell culture. 142 *'Growth Control Acdk2jv Ocdk2iv w/ Arnedia 5 4w 0 40 20 60 80 Time (hours) 4C' Figure 6-13. Cell growth curves following the addition and subsequent removal of inhibitor cdk2iv. Total viable cell density is shown over time following the addition of the inhibitor cdk2iv to growing cells. The cdk2iv inhibitor was either (0) not added, (n) added at 5 gM, or (x) added at 5 piM and removed 24 hours later. (Error bars: S.E., n=1) 143 cdk2iv w/ Lrmedia (0.08) 6 4 (0.08) Growth Control (0.04) 2 dGrowth Control 1Ocdk2iv 6cdk2iv w/ Amedia 0 20 40 60 80 100 120 140 IVCD (106 cells h) Figure 6-14. Cumulative IFN-y production plotted against Integrated Viable Cell Density (IVCD) following the addition of inhibitor cdk2iv. The slope of a best-fit line through the plotted data is defined as the specific productivity, qp. The cdk2iv inhibitor was either (0) not added, (o) added at 5 RM, or (A) added at 5 [LM and removed 24 hours later. Specific productivity for each data set is shown in parentheses in units of pg cell-1 h-1. (Error bars: S.E., n=1) 144 4 - Ocdk2iv 0 cdk2iv w!Amedia 3.5 3 2.5 2- 1.5- 0.5 - 0 - 24 Time (hours) 36 Figure 6-15. Relative genomic DNA content following inhibitor cdk2iv addition. Genomic DNA content is measured with PicoGreen dye and shown relative to the growth control, either without (black) or with (gray) the removal of the inhibitor at 23 hours. 145 6.6 Conclusions We were successfully able to use a variety of CDK inhibitors to explore the relationship between cell cycle, growth, and productivity. Although qualitatively it appears that the G2/M phase of the cell cycle positively impacts specific productivity, we have shown that cell cycle only has a very modest effect. To our knowledge we are the first to report even a mild relationship between the G2/M-phase of the cell cycle and specific productivity. It is possible, however, that this relationship is merely coincidental. Both of the CDK inhibitors that have the largest impact on cell growth, cdkliv and cdk2iii, at least partially target CDK1 and increase the number of cells in G2/M-phase. This is analogous to inferring a qualitative relationship between the Gi-phase of the cell cycle and specific productivity in past studies involving low temperature (Hendrick et al., 2001; Kaufmann et al., 1999; Yoon et al., 2003), butyrate (Hendrick et al., 2001), and CKI overexpression (Bi et al., 2004; Carvalhal et al., 2003a; Fussenegger et al., 1997). Instead it is likely that growth arrest alone contributed to those specific productivity increases. We are not the first group to suggest that cell cycle may not play an important role in increasing specific productivity in both growing and arrested cultures. A number of previous researchers have suggested that other factors like size (Lloyd et al., 2000), medium composition (Lloyd et al., 1999), and mRNA transcript level (Fox et al., 2005b; Yoon et al., 2003) can have a much more important effect on productivity than cell cycle phase. In our y-CHO cell line, IFN-y expression is driven by the SV40 promoter. Although the SV40 promoter is known to be S-phase specific (Banik et al., 1996), we did not observe any relationship between the proportion of cells in S-phase and productivity as has often been 146 predicted. This may be due, in part, to the fact that our cells continued to cycle in the presence of the CDK inhibitors. We were able to show that the specific growth rate inversely correlates with specific productivity, or in other words, as cell growth decreases specific productivity increases. This inverse relationship has been observed previously in hybridoma cultures (Suzuki and Ollis, 1990). Perhaps our most interesting observation is that irrespective of which inhibitor we added to the culture, whenever growth slowed we observed an increase in productivity. This contradicts a previous study where various nucleosides and nucleotides were added to cell cultures (Carvalhal et al., 2003b). In this study, growth arrest led to an increase in specific productivity with the exception of the addition of ATP, NAD, NADH, NADP and NADPH. Because the precise mechanisms by which these molecules induce growth arrest is unknown, it is possible that they are drastically changing intracellular metabolism resulting in decreased productivity. During our inhibitor characterization, we found an interesting CDK2 inhibitor that induces tetraploidy in our CHO cells. This inhibitor cdk2iv drastically increases the size of our y-CHO cells, intracellular genomic DNA content, and specific productivity. We hypothesize that by adding the inhibitor cdk2iv and subsequently removing it after 24 hours we may be able to drastically increase total production of IFN-y in an extended culture. 147 148 7 INCREASING TOTAL PRODUCTIVITY THROUGH CDK INHIBITION 7.1 Introduction In Chapter 6, we hypothesized that we may be able to use our chemical CDK inhibitors to increase total IFN-y production in an extended culture. In this chapter, we aim to increase total recombinant protein production by adding these CDK inhibitors to suspension y-CHO cells in batch, modified batch, and fed-batch cultures. Adding small molecules to cultures in order to improve volumetric productivity is not uncommon. The addition of butyrate (Kim and Lee, 2001; Sung et al., 2007), AMP (Carvalhal et al., 2003b), and hexanohydroxamic acid (HHA) (Allen et al., 2008) has been shown to increase total productivity in various culture systems. The effects of AMP and HHA on recombinant protein production were determined following the screening of dozens of compounds in the case of AMP, and hundreds of compounds in the case of HHA. To our knowledge, our study represents the first rational addition of small molecules to cell cultures, since the addition of butyrate, with the goal of inducing growth arrest and improving total recombinant protein production. 7.2 Addition of CDK Inhibitors in Batch Cultures We used the effective inhibitor concentrations from our short-term studies (Chapter 6) as a starting point for longer-term shake flask experiments. Shake flasks were inoculated at 2x10 5 cells mL- and allowed to grow for two days before inhibitors were added. After 48 hours and once the viable cell density exceeded 1x10 6 cells mL, the CDK inhibitors were added to the cultures at the following concentrations: 2.5 gM cdk2iii, 1 gM cdk2iv, 10 pM cdkliv, and 2.5 pM cdk4. The inhibitors cdk2iii and cdk4 drastically decrease the viability of the suspension 149 CHO cells (data not shown). This effect is not entirely surprising, as these two inhibitors were the most toxic in the short-term studies. Elevated concentrations of cdk4 resulted in rapid decreases in viability in out 36-hour experiments and extended exposure to cdk2iii resulted in the shedding of the viable cells from 6 well plates. For these reasons we immediately stopped using these inhibitors in extended cultures. The effect of inhibitors cdkliv and cdk2iv on growth is shown in Figure 7-1. Both inhibitors slow cell growth following their addition at 48 hours without substantially affecting viability, and the results reinforce our observations from the short-term experiments. The addition of inhibitor cdkliv results in a slight decrease in growth rate while the addition of cdk2iv results in a strong growth arrest following inhibitor addition. The addition of the inhibitors cdkliv and cdk2iv does not result in a statistically significant increase in total volumetric IFN-y production, as can be seen in Figure 7-2. Rather, there is no change in total IFN-y production following inhibitor addition, even though the IVCD decreased in both cases. In other words, the addition of inhibitors cdkliv and cdk2iv increased the specific IFN-y productivity from 0.7 pg IFN-y cell-' day~', by 1.5-fold (1.09 pg IFN-y cell-' day-) and 1.8-fold (1.24 pg IFN-y cell-' day-) respectively (Figure 7-3). As we know, increasing total production in growth-arrested cells involves a trade-off between increasing specific productivity and decreasing IVCD. Although we have not fully optimized the concentration of inhibitors or the timing of addition, we do not expect to achieve drastic increases in total productivity in this batch system. One reason, in particular, is that the nutrient depletion that occurs by the time inhibitors are added prevents an extended culture, which is necessary to increase total production in a growth-arrested culture. Secondly, inhibitor toxicity can limit culture time. 150 A -6 Growth -9- cdk2iv 3 -- -cdkliv 2 E2.5 105 1 00 0.5 0 24 48 72 96 120 144 168 192 Time (hours) I0 90 - 6- Growth 80 D-cdk2iv B 0-cdkliv 70 Q 60 50 40 30 20 10 0 0 24 48 72 96 120 144 168 192 Time (hours) Suspension y-CHO growth and viability curves in the presence of CDK Figure 7-1. inhibitors. Both (A) viable cell density and (B) viability curves are shown following the addition of (A) DMSO, (0) 1 jiM cdk2iv, and (0) 10 gM cdkliv at 48 hours. (Error bars: SsE., n=2) 151 IU -A-Growth -B- cdk2iv -0 cdkliv 8 7 6 5 4 3 2 0 24 48 72 96 120 144 168 192 216 Time (hours) Figure 7-2. Cumulative IFN-y titer following the addition of CDK inhibitors. Data is plotted following the addition of (A) DMSO, (0) 1 gM cdk2iv, and (0) 10 jiM cdklv. (Error bars: S.E., n=2) 7 6 S4 z 2 A Growth Ncdk2iv 0 cdkliv 0 ----- 0 50 150 100 200 250 IVCD (101 cells mL hr) Figure 7-3. Cumulative IFN-y titer plotted against Integrated Viable Cell Density for each inhibitor. Data is plotted following the addition of (A) DMSO, (E) 1 piM cdk2iv, and (0) 10 RM cdklv. The slope of a best-fit line through the plotted data is defined as the specific productivity, qp. (n=2) 152 7.3 Addition of Inhibitor cdk2iv in a Modified Batch Culture Looking back to Section 6.5, the addition and subsequent removal of cdk2iv resulted in an increase in JVCD with only a marginal change specific productivity, Figures 6-13 and 6-14. We hypothesized that we may be able to increase total volumetric IFN-y productivity by applying a similar methodology in shake flask cultures. After inoculating 125-mL shake flask at 2 x 105 cells mL- 1 in 25 mL, we allowed the cultures to grow for 48 hours, reaching a cell density of at least 1 x 106 cells mL'. We then added the inhibitor cdk2iv to a concentration of 1 gM, and subsequently removed the inhibitor 24 hours later by performing a full medium change. The same process was performed in a control culture where DMSO was added in place of the inhibitor. Figure 7-4 shows the affect of inhibitor cdk2iv addition and its subsequent removal on growth in this modified batch culture. Comparing the growth curve to that in Figure 7-1, the maximum viable cell density in the control culture was significantly higher. This is the result of the complete medium change that occurred at 72 hours. As in the batch culture, the addition of cdk2iv does not have any significantly detrimental effects on viability. While viability does begin to drop slightly faster at 144 hours, ultimately the culture is extended by approximately 1 day. In addition, the culture length is the same in both batch and modified batch cultures. Interestingly, unlike in the 6-well plate cultures, the removal of the inhibitor cdk2iv through medium replacement did not induce growth in the shake flask cultures. The 6-well plate experiments were performed in the presence of serum, which is the likely cause of growth following medium replacement. Although the culture time is not extended when comparing batch and modified batch cultures, the medium change did act to maintain a higher viable cell density in the cdk2iv cultures through 144 hours. 153 A -- Growth Control -8- cdk2iv 5 4 3 2 0 24 48 72 96 120 144 168 192 216 Time (hours) 100 90 -*-Growth Control & cdk2Iv B1 80 70 50 40 30 20 10 24 48 72 120 96 Time (hours) 144 168 192 216 Figure 7-4. Suspension CHO-IFNy growth and viability curves in the presence of CDK inhibitors in a modified batch culture. Both (A) viable cell density and (B) viability curves are shown following the addition of (A) DMSO and (E) 1 uM cdk2iv at 48 hours. Full medium change was performed at 72 hours (Error bars: S.E., n=4) 154 The effect of inhibitor treatment and its subsequent removal on the total volumetric productivity of IFN-y is significant, as shown in Figure 7-5. The addition of the inhibitor cdk2iv results in 1.73-fold or 73% increase in total recombinant protein titer. This translates into an over 4-fold increase in specific productivity, from 0.8 to 3.3 pg IFN-y cell-1 day' as shown in Figure 7-6. Moreover, the final IFN-y titer in the modified batch culture is even higher than that achieved in a normal batch culture (Figure 7-2). We also sought to ensure that the cells were metabolically active following the addition of the inhibitor cdk2iv. We did this by measuring the total intracellular protein content following the' addition of the cdk2iv inhibitor. Every 12 hours following inhibitor addition, 2.5 x 10 5 cells were lysed and the total protein was measured with a BCA assay for both the growth control culture and the inhibitor culture, and the results are shown in Figure 7-7. Following the inhibitor addition at 48 hours, and subsequent medium change at 72 hours, intracellular protein content continues to increase in the inhibitor culture while remaining constant in the growth control as expected. In the growth control, cell division maintains an approximately constant intracellular protein content of 3.2 ng cell-'. In the cdk2iv inhibitor culture, the inhibition of cell division causes a continued increase in intracellular protein content, reaching a maximum of 11.9 ng cell' at 108 hours. The cell cultures are still metabolically active following inhibitor addition, allowing for the continued replication of DNA (Figure 6-15) and creation of intracellular protein. 155 14 -6- Grx wth Control -B- ed k21v 12 10 8 LA. 4 2 0 0 24 72 48 96 120 Time (hours) 144 168 192 Figure 7-5. Cumulative IFN-y titer following the addition and removal of a CDK inhibitor in a modified batch culture. Data is plotted following the addition of (A) DMSO and (E) 1 gM cdk2iv at 48 hours and the full medium change that was performed at 72 hours (Error bars: S.E., n=4) (*: p < 0.05) AGrowth Control 12 Ocdk2ov 0 cdk2lv 10 (0.14) / Growth Control (0.03) 2 0~ 0 50 100 150 200 250 300 Integrated Viable Cell Density (105 cells mL-1 hr) Figure 7-6. Cumulative IFN-y titer plotted against Integrated Viable Cell Density following the addition and removal of a CDK inhibitor in a modified batch culture. Data is plotted following the addition of (A) DMSO and (E) 1 pM cdk2iv at 48 hours and the full medium change that was performed at 72 hours. The slope of a best-fit line through the plotted data is defined ao the specific productivity, qp, and is in parentheses (pg cell-1 hr1) (n=4) 156 14 1 3Growth Control 4*cdk2iv S12 12 o 10 C C IWO 8 A media 0 4 0 2 48 60 72 84 96 108 120 Time (hours) Figure 7-7. Total protein content following the addition of inhibitor cdk2iv. Total protein content per cell is shown following the addition of either DMSO (white) or 1 pLM cdk2iv (black) at 48 hours. Full medium change was performed at 72 hours (modified batch). (n=1) 157 7.4 Addition of Inhibitor cdk2iv in a Modified Fed-Batch Culture We next sought to determine if the addition of inhibitor cdk2iv could improve total productivity in a fed-batch culture. Fed-batch cultures were started in the same way as the batch cultures. Shake flasks were inoculated 2 x 105 cells mL- 1 and allowed to grow for 48 hours, reaching a cell density of at least 1 x 106 cells mL~1. We then added the inhibitor cdk2iv to a concentration of 1 pM, and subsequently removed the inhibitor 24 hours later through a full medium change. We then followed the fed-batch strategy described by Lim et al. (2006) and outlined in Section 3.1.5, by feeding glucose and glutamine to set points of 0.5 and 0.15 g L-1 every 8 to 12 hours Growth and viability curves for the fed-batch cultures are shown in Figure 7-8. These growth curves compare well with those achieved by Lim et al. (2006). Interestingly, the addition of the inhibitor cdk2iv had a detrimental affect on viability starting at 168 hours in the fed-batch culture when compared with the growth control. This observation was not made in the batch culture. Figure 7-9 shows an overlay of the growth curves for both the batch and fed-batch cultures. The fed-batch culture growth control reaches a slightly higher maximum cell density, 5.87x1 06 cells ml compared with 4.94x106 cells mL-1. Because we perform a medium change in the batch culture at 72 hours, we already achieve a higher than normal maximum cell density in those experiments, and this is the reason that we do not observe a larger increase in maximum cell density in the fed-batch cultures. The increase in maximum cell density, along with the extension of the culture by a day, increases the IVCD by 39% in the growth control before viability declines. We do not expect there to be as large of an increase in IVCD in the inhibitor cultures, because the maximum cell density does not increase. The extension of the culture by a day increases in IVCD by 11% following inhibitor addition in the fed-batch culture. 158 -a- Growth A -02 cdk2iv 6 6 E 5 0 0 24 48 72 96 120 144 168 192 216 240 Time (hours) 100 90 Growth 80 -9-cdk2"B 70 i60 K 150 > 40 30 20 10 0 0 24 48 72 96 120 144 168 192 216 240 Time (hours) Figure 7-8. Suspension CHO-IFNy growth and viability curves in the presence of inhibitor cdk2iv in a modified fed-batch culture. Both (A) viable cell density and (B) viability curves are shown following the addition of (A) DMSO and (LI) 1 pM cdk2iv at 48 hours. Full medium change was performed at 72 hours followed by fed-batch feeding at 8-hour intervals. 159 - 6 ' Growth - Batch &-cdk2iv - Batch - Fed-batch +Growtlh -U- cdk2iv - Fed-batch 0 24 48 72 96 120 144 168 192 216 240 Time (hours) Figure 7-9. Overlay of modified batch and fed-batch CHO-IFNy suspension growth curves following the addition of inhibitor cdk2iv. Data from batch (open symbols) and fed-batch (closed symbols) cultures are shown for easy comparison. Inhibitor cdk2iv or DMSO was added at 48 hours followed by a full medium change at 72 hours. Fed-batch feedings occurred at 8-hour intervals. (Error bars: S.E., n=4) 160 The addition of the inhibitor cdk2iv does not increase the total production of IFN-y in this fedbatch culture, as shown in Figure 7-10. In fact, total IFN-y production decreases by 43% (or 0.57-fold lower than the growth control). Part of this decrease can be explained by the comparatively larger increase in IVCD in the growth control. The extension of the cultures by a day in the fed-batch cultures has a much larger impact on the growth control because the maximum cell density is much higher than in the inhibitor cultures. The decrease in total productivity is also partly explained by a decrease in specific productivity in the fed-batch inhibitor cultures. While the specific productivity increases in the fed-batch culture following inhibitor cdk2iv addition, Figure 7-11, it actually decreases when compared with the batch culture, Figure 7-12. Typically, specific productivity should not change from a batch to a fedbatch culture (Xie et al., 1997), as can be seen in Figure 7-12. This 2-fold decrease in specific productivity following inhibitor addition severely limits the amount of total IFN-y that can be produced in the culture. We hypothesize that this decrease is the result of the feed medium having been optimized for growing CHO cells, and not for maintaining cells in a growth arrested state. By using an optimized medium, that at least maintains specific productivity following inhibitor addition, inhibitor cdk2iv has the potential to increase total productivity in a fed-batch culture 161 -- Growth 8- cdk2iv 14 12 10 _j S6; 4' 0 0 24 48 96 72 120 144 168 192 216 240 Time (hours) Figure 7-10. Cumulative IFN-y titer following the addition and removal of inhibitor ck2iv in a modified fed-batch culture. Data is plotted following the addition of (A) DMSO and (0) 1 gM cdk2iv at 48 hours and the full medium change that was performed at 72 hours followed by fed-batch feedings at 8-hour intervals. A Growth *cdk2iv 14 12 10 8 6 4 2 0 100 200 300 400 500 600 VCD (106 cells mL I hr) Figure 7-11. Cumulative IFN-y titer plotted against Integrated Viable Cell Density following the addition and removal of inhibitor cdk2iv in a modified fed-batch culture. Data is plotted following the addition of (A) DMSO and (U) 1 pM cdk2iv at 48 hours and the full medium change that was performed at 72 hours. The slope of a best-fit line through the plotted data is defined as the specific productivity, qp. 162 AGrowth - Batch 14 Ocdk2iv - Batch AGrowth - Fed-batch 12 Ucdk2iv - Fed-batch 10 100 200 300 400 500 600 IVCD (106 cells mL-1 hr) Figure 7-12. Overlay of cumulative IFN-y titer plotted against IVCD for modified batch and fed-batch cultures following the addition of inhibitor cdk2iv. Data from batch (open symbols) and fed-batch (closed symbols) cultures are shown for easy comparison. Inhibitor cdk2iv or DMSO was added at 48 hours followed by a full medium change at 72 hours. Fed-batch feedings occurred at 8-hour intervals. The slope of a best-fit line through the plotted data is defined as the specific productivity, qp. 163 7.5 Conclusions In this chapter, we have shown the potential of using a CDK2 inhibitor to improve total volumetric IFN-y productivity. In addition to improving the specific productivity of IFN-Y by over 4-fold, we were also able to increase total productivity in a modified batch culture by 73%. The addition of nocodazole, an anti-mitotic microtubule disrupting agent, causes growth arrest in the G2/M-phase of the cell cycle and is often characterized by an increase in both cell size and DNA content. Tait et al. (2004) used nocodazole to enhance the transient production of both SEAP and an IgG 4 monoclonal antibody. Nocodazole addition was shown to increase both the transfection efficiency and the specific production rate of transfected cells. However, when nocodazole was added to GS-CHO cells stably transfected with IgG 4 , no significant increase in total productivity was observed, although specific productivity was increased. We attribute this apparent discrepancy with our results to a number of causes. Most importantly, the effect of our modified batch culture on productivity cannot be overstated. When we added our cdk2iv inhibitor to cultures in a batch format, we also observed no increase in total productivity. Only by performing a single medium replacement at 72 hours were we able to drive total productivity higher in our inhibitor cultures. We hypothesize that the nutrient depletion that occurs in batch cultures over the first 48 hours prevents the drastic increase in productivity following inhibitor addition from being observed. In addition, while growth arrest following the addition of inhibitor cdk2iv exhibits a number of the same traits as a nocodazole induced arrest, notably an increase in cell size and DNA content, the mechanism of arrest is different. Nocodazole induces arrest by disrupting microtubules, while cdk2iv specifically inhibits CDK2 (Pennati et al., 2005). 164 The addition of inhibitor cdk2iv in a modified fed-batch culture does not lead to an increase in total IFN-y production. This observed decrease in total production following inhibitor addition was caused by both a decrease in IVCD and specific productivity. While a decrease in IVCD in the fed-batch inhibitor culture was expected, we did not expect to observe a decrease in specific productivity. We hypothesize that our feed medium is not properly optimized for use in a growth arrested culture. By improving the feed medium and maintaining specific productivity, we believe that we can increase total IFN-y productivity though the addition of inhibitor cdk2iv in fed-batch cultures. While we are not the first group to utilize a G2 /M-like arrest to increase total productivity (Tait et al., 2004) in a cell culture system, we are the first to increase recombinant protein titer from a stable recombinant protein expressing cell line. The ability to increase total recombinant protein production through the addition of a small molecule to the culture medium has many advantages. Such an addition could potentially be used in current batch and fed-batch processes without many modifications. In addition, like other general methods to improve protein production such as hypothermic culture and the addition of butyrate, cell engineering and cell line development is not required, decreasing the time to implementation. Lastly, the addition of a small molecule enhancer could be used in many types of cell culture systems, making it generally applicable. 165 166 8 CONCLUSIONS AND RECOMMENDATIONS 8.1 Thesis Conclusions Past researchers have shown that growth arrested CHO cell cultures can lead to increases in both the specific and total productivity of recombinant proteins. The majority of these studies utilize general methodologies (i.e. hypothermic culture and butyrate addition) that induce growth arrest through multiple cellular mechanisms. The overexpression of CKIs represents the first successful attempt to induce growth arrest and increase specific productivity through a single cellular mechanism. However, total productivity was not improved. In this thesis, we have sought to determine if growth arrest can be induced through the specific inhibition of the cell cycle and what effect this inhibition has on specific and total productivity. In addition, the relationship between growth-arrested cell cycle phase and productivity is very poorly understood and no studies have sought to elucidate such a relationship. In this work, we have established such a relationship between cell cycle phase, growth, and productivity. We have shown that the specific inhibition of the CDK2-CcnE complex through chemical inhibition leads to growth arrest and a subsequent increase in specific productivity. In addition, through the use of chemical inhibitors we have been able to increase specific productivity through the inhibition of CDK1 and CDK4. By inhibiting different CDKs, we were able to slow cell cycle progression and alter cell cycle distribution. Through this work, we have shown that increases in specific productivity are independent of cell cycle phase, and instead are strongly correlated with a decreasing specific growth rate. This implies that the targeting of specific cell cycle phases for growth arrest is largely unnecessary. 167 Instead, focus should be placed on maintaining cells at an elevated cell density and in a growth arrested state for the longest period of time, independent of cell cycle phase. We have also shown that the knockdown of CcnE1 leads to increases in specific productivity. However, we were unable to determine if these increases in specific productivity can be translated into increases in total productivity because of limitations with our expression system. It would be interesting to revisit this work when controllable shRNA expression systems have improved, allowing the complete repression and the strong induction of shRNA expression. Lastly, we have shown that the addition of CDK inhibitors directly to suspension cultures may be a promising strategy to increase recombinant protein productivity. Unfortunately, commercially available CDK inhibitors are quite toxic and prevent extended culturing in their presence. The inhibitor cdk2iv has shown particular promise towards increasing total productivity. When added to suspension cultures, inhibitor cdk2iv induces growth arrest, increases both cell size and DNA content, and drastically increases specific productivity. Through its addition and subsequent removal, we are able to increase total IFN-y production by 73% when compared with a control culture. We believe that with the development of an optimized supplemental feed medium for growth arrested cells, this methodology could also be applied to fed-batch cultures. In summary, we have shown that chemical CDK inhibitors not only represent an interesting tool to explore the effects of cell cycle phase and growth rate on productivity, but can also be used to improve total productivity in commercially relevant cell cultures. 168 8.2 Recommendations for Future Work 8.2.1 Inducible shRNA Expression Systems One of the major limitations in our anti-CcnEl shRNA expression work was the expression system itself. The potential for the success of an inducible shRNA expression system can be determined based on two general criteria: (1) the ability of the system to drive high levels of shRNA expression, and (2) the ability to finely control shRNA expression. A number of researchers have used shRNA to silence gene expression in CHO cell lines (Kim and Lee, 2007; Lim et al., 2006; Mori et al., 2004; Ngantung et al., 2006; Sung et al., 2007; Wong et al., 2006b). In all of these studies, shRNA expression was constitutive and the researchers only had to screen for the knockdown of their target. Even so, hundreds and sometimes thousands of clones had to be screened in order to find a handful that had high levels of shRNA expression. Alternatively, high levels of shRNA expression can be driven by either the directed insertion of the expression system into highly active regions of the CHO chromosome, or the engineering of stronger promoters. Any improvements in either insertion location or expression level should make the screening for high expressing clones faster and more likely to yield a desired phenotype. We are not aware of any researchers who have successfully expressed a stable and inducible shRNA expression system targeting an endogenous gene in CHO cells. We believe this is because commercially available systems to do not adequately control shRNA expression, neither fully repressing nor fully inducing expression when necessary. The most common system to control shRNA expression is the tetracycline responsive system used in this study, and we have 169 shown that this system does not fully repress shRNA expression nor induce shRNA expression to adequate levels. Improvements in controllable systems still need to be made before a study like ours should be carried out again. 8.2.2 Overexpression of miRNA MicroRNAs (miRNAs) are a class of non-coding and naturally expressed RNAs that are structurally and mechanistically similar to siRNA (Bartel, 2004). Most importantly, a single miRNA can post-transcriptionally regulate the gene expression of up to 100 targets (Lim et al., 2005). miRNAs are known to be critical in the regulation of cell proliferation and death (Brennecke et al., 2003; Liu et al., 2008), and apoptosis (Xu et al., 2003), all of which are, for reasons covered extensively in this thesis, important to the production of recombinant proteins in CHO cells. It has been suggested that the overexpression of miRNAs may serve as a useful tool for improving the production of recombinant proteins (Muller et al., 2008). Like hypothermic culture and butyrate addition, miRNA expression has the potential to regulate the expression of multiple genes at one time, potentially increasing the chance of success when compared to an single shRNA expression strategy. In addition, another advantage of miRNA expression is that it does not overcharge the transcriptional machinery of the host cell. As of the writing of this thesis, only one CHO-derived miRNA, cgr-miR-21 (http://microrna.sanger.ac.uk/), can be found in the miRNA registry. Both miR-21 and miR-24 have been shown to modulate growth, and are up regulated in stationary phase cultures induced by either temperature shift or during normal batch culture (Gammell et al., 2007). The overexpression of either of these miRNAs during exponential growth phase may lead to an 170 induced growth arrest and a subsequent improvement in productivity. In addition miR-16 and let-7b have been shown to down regulate CcnE] and CDK6 expression respectively in the production cell line, HEK-293 (Koh et al., 2009), and the expression of miR-16 alone is known to induce cell cycle arrest (Liu et al., 2008). The overexpression of either of these miRNAs may have the potential to induce growth arrest and increase recombinant protein productivity in other cell lines. 8.2.3 Screening for Less Toxic Inhibitors The chemical inhibitors used in this study were originally created as potential cancer treatments. For this reason, they have toxicity profiles that may make them well suited as chemotherapy, but not well suited for use in extended cultures. All of the inhibitors are known to induce apoptosis in cells following exposure at elevated concentrations. Although it is not known whether the induction of apoptosis is the result of inhibitor toxicity or the result of CDK inhibition, it would be useful to screen for compounds that selectively inhibit CDKs without inducing apoptosis. The screening of small molecules for enhancements in recombinant protein production is not uncommon (Allen et al., 2008; Carvalhal et al., 2003b), and may represent an easy way to find less toxic inhibitors. 8.2.4 Optimized Feed Medium for Growth Arrested Cell Culture In order to achieve improvements in recombinant protein production through the addition of inhibitor cdk2iv in fed-batch cultures, a supplemental feed medium optimized for a growth arrested cell culture needs to be designed. During growth arrest, many changes occur to the cells that ultimately yield drastic increases in the specific productivity of recombinant proteins. 171 Although not measured in this thesis, there are likely lots of metabolic changes following growth arrest that likely result in different nutritional demands when compared with growing cells. These changing nutritional demands are likely not met by the feed and supplemental media used in this thesis because they have been designed for growing cells. Newly optimized feed and supplemental media for growth-arrested cell cultures would go a long way towards improving the potential of growth-arrested cultures. 8.2.5 Inhibitor Addition in Perfusion Culture While we have shown that our inhibitors are potentially effective at increasing productivity in both batch and fed-batch cultures, it would be useful to extend the application of these inhibitors to other culture systems. Long-term perfusion cultures may present a particularly challenging application because of the toxicity issues mentioned in Section 8.2.3. However, if less toxic CDK inhibitors could be identified, it would be interesting to determine their effect in long-term culture. Perfusion cultures offer a unique opportunity to employ an inhibitor removal strategy similar to our modified batch culture experiments. G2/M arrest and the induction of tetraploidy could be achieved by spiking inhibitor cdk2iv into the perfusion medium over a short period of time. The following flow of fresh medium would remove the inhibitor from the cells, potentially allowing for its application in extended perfusion cultures. 8.2.6 Conclusion Growth arrest methodologies have the potential to drastically improve the production of recombinant therapeutic proteins. By expanding on the genetic approaches introduced in this thesis, developing new methods to induce growth arrest, and designing media and cell culture 172 processes that are optimized for growth arrested cultures, we can capitalize on the advantages that growth arrested cultures offer. 173 174 NOMENCLATURE IVCD Integrated viable cell density (cell hr) Nviable Viable cell density (viable cell mL-1) Nv, avg Average viable cell density (viable cell mL~') N, o Initial viable cell density (viable cell mL') P Volumetric IFN-y production (gg mL') qp Specific IFN-y production rate (pg cell-' hr~') qP,diff Differential specific IFN-y production rate (jg cell~ hr- ) t Time (hr) Tanneal Annealing temperature ("C) Greek Letters E qPCR efficiency Xem Emission wavelength (nm) Xex Excitation wavelength (nm) Specific growth rate (hr-d) Abbreviations AMP Adenosine 5'-monophosphate ATP Adenosine triphosphate cDNA complementary DNA C/EPBax CCAAT/enhancer-binding protein x 175 CcnX Cyclin X [where X is A1, A2, B1, B2, D1, D2, D3, E1, E2] CDK Cyclin dependent kinase CHO Chinese hamster ovary CIRP Cold-inducible RNA-binding protein CKI Cyclin dependent kinase inhibitor CMV Cytomegalovirus Ct quantitative PCR threshold cycle DHFR Dihydrofolate reductase DMEM Dulbecco's modified Eagle's medium DMSO Dimethyl sulfoxide Dox Doxycycline DPBS Dulbecco's phosphate buffered saline DTT Dithiothreitol EDTA Ethylene diamine tetraacetic acid ELISA Enzyme-linked Immunosorbent Assay EPO Erythropoietin FACS Fluorescence activated cell sorting FAM Fluorescein FBS Fetal Bovine Serum FUT8 al,6 fucosyltransferase y-CHO Chinese hamster ovary cells expressing recombinant human interferon-gamma Go Quiescent cell state indistinguishable from G1 G1 First gap phase of the cell cycle 176 G2 Second gap phase of the cell cycle G2 /M Combined G2 and M phases of the cell cycle GFP Green fluorescent protein HI Polymerase III promoter that drives small RNA expression (rRNA, tRNA, etc.) IC5 0 Half maximal inhibitory concentration IFN-y Interferon-gamma IFS Heat-inactivated fetal bovine serum IRF- 1 Interferon regulatory factor 1 kDa Kilo-dalton Ki Dissociation constant for inhibitor binding LDH Lactate dehydrogenase M Mitosis phase of the cell cycle mRNA Messenger RNA NaBu Sodium butyrate Oligo(dT) Oligodeoxythymidine PBS Phosphate buffered saline PCR Polymerase chain reaction PhRMA Pharmaceutical Researchers and Manufacturers of America PI Propidium iodide pRb Retinoblastoma protein qRT-PCR Quantitative reverse-transcription PCR (a.k.a. Real time PCR) RNAi RNA interference rRNA Ribosomal RNA 177 RT-PCR Reverse-transcription PCR S DNA synthesis phase of the cell cycle SDKid-I Silencing KRAB domain of human Kox-1 SEAP Secreted alkaline phosphatase shRNA Short hairpin RNA siRNA Silencing RNA SV40 Simian virus 40 TE Tris-EDTA Tet Tetracycline tetO 7 7 repeats of the tetracycline operon tetR Tetracycline repressor TIMP Tissue inhibitor of metalloproteinases t-PA Tissue-type plasminogen activator Tris Tris(hydroxymethyl)aminomethane tRAN Transfer RNA tTS Tetracycline-controlled transcriptional suppressor U6 Polymerase III promoter that drives small RNA expression (rRNA, tRNA, etc.) VP16 Virion protein 16 XIAP X-linked inhibitor of apoptosis 178 REFERENCES Ahn WS, Jeon J, Jeong Y, Lee SJ and Yook SK. 2008. Effect of culture temperature on erythropoietin production and glycosylation in a perfusion culture of recombinant CHO cells. Biotech Bioeng. 101:1234-1244. Allen MJ, Boyce JP, Trentalange MT, Treiber DL, Rasmussen B, Tillotson B, Davis R and Reddy P. 2008. Identification of novel small molecule enhancers of protein production by cultured mammalian cells. Biotech Bioeng. 100:1193-1204. Al-Rubeai M, Emery AN, Chalder S and Jan DC. 1992. Specific monoclonal antibody productivity and the comparisons of batch, continuous and perfusion cultures. Cytotechnology. 9:85-97. Anonymous. 2007. KnockoutTm Single Vector Inducible RNAi System User Manual. Clontech, Mountain View, CA. 24 p. Banik GG, Todd PW and Kompala DS. 1996. Foreign protein expression from S phase specific promoters in continuous cultures of recombinant CHO cells. Cytotechnology. 22:179-184. Barron N, Piskareva 0 and Muniyappa M. 2007. Targeted genetic modification of cell lines for recombinant protein production. Cytotechnology. 53:65-73. Bartel DP. 2004. MircoRNAs: Genomics, Biogenesis, Mechanism, and Function. Cell. 116:281297. Berger SL. 2002. Histone modifications in transcriptional regulation. Curr Opin Gen Dev. 12:142-148. Berthet C and Kaldis P. 2007. Cell-specific responses to loss of cyclin-dependent kinases. Oncogene. 26:4469-4477. Bhattacharjee RN, Banks GC, Trotter KW, Lee H and Archer TK. 2001. Histone HI phosphorylation by CDK2 selectively modulates mouse mammary tumor virus transcription through chromative remodeling. Mol Cell Bio. 21:5417-5425. Bi J, Shuttleworth J and Al-Rubeai M. 2004. Uncoupling of cell growth and proliferation results in enhancement of productivity in p21ciP-arrested CHO cells. Biotech Bioeng. 85: 741-749. Birch JR and Racher AJ. 2006. Antibody production. Adv Drug Del Rev. 58:671-685. de Boer L, Gray PP and Sunstrom NA. 2004. Enhanced productivity of GI phase Chinese hamster ovary cells using the GADD 153 promoter. Biotech Letters. 26:61-65. 179 Bollati-Fogolin M, Forno G, Nimtz M, Conradt HS, Etcheverrigaray M and Kratje R. 2005. Temperature reduction in cultures in hGM-CSF-expressing CHO cells: effect on productivity and product quality. Biotech Prog.21:17-21. Brennecke J, Hipfner DR, Stark A, Russell RB and Cohen SM. 2003. Bantam encodes a developmentally regulated microRNA that controls cell proliferation and regulates the proapoptotic gene hid in drosophila. Cell. 113:25-36. Brooks EE, Gray NS, Joly A, Kerwar SS, Lum R, Mackman RL, Norman TC, Rosete J, Rowe M, Schow SR, Schultz PG, Wang X, Wick MM, Shiffman D. 1997. CVT-313, a specific and potent inhibitor of CDK2 that prevents neointimal proliferation. JBio Chem. 272:29207-29211. Buequet-Fagot C, Lallemand F, Charollais R and Mester J. 1996. Sodium butyrate inhibits the phosphorylation of the retinoblastoma gene product in mouse fibroblasts by a transcriptiondependent mechanism. J Cell Phys. 166:631-636. Carvalhal AV, Marcelino I and Carrondo MJT. 2003a. Metabolic changes during cell growth inhibition by p27 overexpression. Appl Microbiol Biotech. 63:164-173. Carvalhal AV, Santos SS, Calado J, Haury M and Carrondo MJT. 2003b. Cell growth arrest by nuclueotides, nucleosides and basses as a tool for improved production of recombinant proteins. Biotech Prog. 19:69-83. Chang KH, Kim KS and Kim JH. 1999. N-acetylcysteine increases the biosynthesis of recombinant EPO in apoptotic Chinese hamster ovary cells. Free Rad Res. 30:85-91. Chiang G and Sisk WP. 2005. Bcl-xL mediates increased production of humanized monoclonal antibodies in Chinese hamster ovary cells. Biotech Bioeng. 91:779-792. Cousens LS, Gallwitz D and Alberts BM. 1979. Different accessibilities in chromatin to histone acetylase. JBio Chem. 254:1716-1723. Cullen BR. 2006. Enhancing and confirming the specificity of RNAi experiments. Nat Genetics. 3:677-681. Cumming G, Fidler F and Vaux DL. 2007. Error bars in experimental biology. J Cell Bio. 177:711. D'Anna JA, Tobey RA and Gurley LR. 1980. Concentration -dependent effects of sodium butyrate in Chinese hamster cells: cell-cycle progression, inner-histone acetylation, histone hi dephosphorylation, and induction of hl-like protein. Biochemistry. 19:2656-2671. Deuschle U, Meyer WKH and Thiesen HJ. 1995. Tetracycline-reversible silencing of eukaryotic promoters. Mol Cell Bio. 15:1907-1914. -4 180 Dorner AJ, Wasley LC and Kaufman RJ. 1989. Increased synthesis of secreted proteins induces expression of glucose-related proteins in butyrate-treated Chinese hamster ovary cells. J Bio Chem. 34:20602-20607. Duesberg P, Rausch C, Rasnick D and Hehlmann R. 1998. Genetic instability of cancer cells is proportional to their degree of aneuploidy. PNAS. 95:13692-13697. Durocher Y and Butler M. 2009. Expression systems for therapeutic glycoprotein production. CurrOpin Biotech. 20:700-707. Dykxhoorn DM, Novina CD and Sharp PA. 2003. Killing the messenger: short RNAs that silence gene expression. Nat Rev Mol Cell Bio. 4:457-467. Ekholm SV and Reed SI. 2000. Regulation of G1 cyclin-dependent kinases in the mammalian cell cycle. Curr Opin Cell Bio. 12:676-684. El-Deiry WS, Harper JW, O'Connor PM, Velculescu VE, Canman CE, Jackman J, Pietenpol JA, Burrell M, Hill DE, Wang Y, Wiman KG, Mercer WE, Kastan MB, Kohn KW, Elledge SJ, Kinzler KW, Vogelstein B. 1994. WAFI/CIPI is induced in p53-mediated G1 Arrest and Apoptosis. CancerRes. 54:1169-1174. Elbashir SM, Harboth J, Weber K and Tuschl T. 2002. Analysis of gene function in somatic mammalian cells using small interfering RNAs. Methods. 26:199-213. Ermak G, Cancasca VJ and Davies KJA. 2003. Cytotoxic effect of doxycycline and its implications for tet-on gene expression systems. Anal Biochem. 318:152-154. Falck J, Mailand N, Syljuasen RG, Bartek J and Lukas J. 2001. The ATM-Chk2-Cdc25A checkpoint pathway guards against radioresistant DNA synthesis. Nature. 410:842-847. Feder JN, Assaraf YG, Seamer LC and Schimke RT. 1989. The pattern of dihydrofolate reductase expression through the cell cycle in rodent and human cultured cells. J Bio Chem. 264:20583-20590. Fiore M and Degrassi F. 1999. Dimethyl sulfoxide restores contact inhibition-induced growth arrest and inhibits cell density-dependent apoptosis in hamster cells. Exp Cell Res. 251:102-110. Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. 1998. Potent and specific genetic interference by double-stranded RNA in Caenorhabditiselegans. Nature. 391:806-11. Fox SR, Patel UA, Yap MGS and Wang DIC. 2004. Maximizing interferon-g production by Chinese hamster ovary cells through temperature shift optimization: experimental and modeling. Biotech Bioeng. 85:177-184. Fox SR. 2005a. Active hypothermic growth: a novel means for increasing total recombinant protein production in CHO cells. Ph. D. thesis. Massachusetts Institute of Technology. 181 Fox SR, Tan HK, Tan MC, Wong SC, Yap MG, Wang DIC. 2005b. A detailed understanding of the enhanced hypothermic productivity of interferon-gamma by Chinese hamster ovary cells. Biotech Appl Biochem. 41:255-264. Fox SR, Yap MX, Yap MG, Wang DIC. 2005c. Active hypothermic growth: a novel means for increasing total interferon-gamma production by Chinese hamster ovary cells. Biotechnol Appl Biochem. 41:265-272. Freundlieb S, Schirra-Muller C and Bujard H. 1999. A tetracycline controlled activation/repression system with increased potential for gene transfer into mammalian cells. J Gene Med.1:4-12. Furukawa K and Ohsuye K. 1998. Effect of culture temperature on a recombinant CHO cell line producing a C-terminal a-amidating enzyme. Cytotechnology. 26:153-164. Furukawa K and Ohsuye K. 1999. Enhancement of productivity of recombinant a-amidating enzyme by low temperature culture. Cytotechnology. 31:85-94. Fussenegger M, Mazur X and Bailey JE. 1997. A novel cytostatic process enhances the productivity of Chinese hamster ovary cells. Biotech Bioeng. 55:927-939. Fussenegger M, Schlatter S, Datwyler D, Mazur X and Bailey JE. 1998. Controlled proliferation by multigene metabolic engineering enhances the productivity of Chinese hamster ovary cells. Nat Biotech. 16:468-472. Fussenegger M and Bailey JE. 1999. Control of mammalian cell proliferation as an important strategy in cell culture technology, cancer therapy and tissue engineering. In: Cell Engineering; Al-Rubeai, Hauser, Jenkins, Betenbaugh, McDonald (eds.), Kluwer Academic Publishers, Norwell, MA, p. 186-219. Gammell P, Barron N, Kumar N and Clynes M. 2007. Initial identification of low temperature and culture stage induction of miRNA expression in suspension CHO-Ki cells. J Biotech. 130:213-218. Glacken MW, Fleischaker RJ and Sinskey AJ. 1986. Reduction of waste product excretion via nutrient control: possible strategies for maximizing product and cell yields on serum in cultures of mammalian cells. Biotech Bioeng. 28:1376-1389. Gossen M and Bujard H. 1992. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. PNAS. 89:5547-5551. Goswami J, Sinksey AJ, Steller H, Stephanopoulos GN and Wang DIC. 1999. Apoptosis in batch cultures of Chinese hamster ovary cells. Biotech Bioeng. 62:632-640. Grunstein M. 1997. Histone acetylation in chromatin structure and transcription. Nature. 389:349-352. 182 Gu MB, Todd P and Kompala DS. 1993. Foreign gene expression (b-galactosidase) during the cell cycle phases in recombinant CHO cells. Biotech Bioeng. 42:1113-1123. Gu X. 1997. Characterization and improvement of Interferon-g glycosylation in Chinese hamster ovary cell culture. Ph. D. thesis. Massachusetts Institute of Technology. Hacker DL, Jesus MD and Wurm FM. 2009. 25 years of recombinant proteins from reactorgrown cells - where do we go from here? Biotech Adv. 27:1023-1027. Harper JW, Adami GR, Wei N, Keyomaris K and Elledge SJ. 1993. The p21 cdk-interacting protein CipI is a potent inhibitor of GI cyclin-dependent kinases. Cell. 75:805-816. Hassell T, Gleave S and Butler M. 1991. Growth inhibition in animal cell culture. Appl Biochem Biotech. 30:29-41. Hendrick V, Winnepenninckw P, Abdelkafi C, Vandeputte 0, Cherlet M, Marique T, Renemann G, Loa A, Kretzmer G and Werenne J. 2001. Increased productivity of recombinant tissular plasminogen activator (t-PA) by butyrate and shift of temperature: a cell cycle phases analysis. Cytotechnology. 36:71-83. van den Heuvel S and Harlow E. 1993. Distinct roles for cyclin-dependent kinases in cell cycle control. Science. 262:2050-2054. Jenkins N and Hovey A. 1993. Temperature control of growth and productivity in mutant Chinese hamster ovary cells synthesizing a recombinant protein. Biotech Bioeng. 42:1029-1036. Jiang Z and Sharfstein S. 2008. Sodium butyrate stimulates monoclonal antibody overexpression in CHO cells by improving gene accessibility. Biotech Bioeng. 100:189-94. Kantardjieff A, Nissom PM, Chuah SH, Yusufi F, Jacob NM, Mulukutla BC, Yap M and Hu WS. 2009. Developing genomic platforms for Chinese hamster ovary cells. Biotech Adv. 27:1028-1035. Kastan MB and Bartek J. 2004. Cell-cycle checkpoints and cancer. Nature. 32:316-323. Kastan MB, Onyekwere 0, Sidransky D, Vogelstein B and Craig RW. 1991. Participation of p53 protein in the cellular response to DNA damage. CancerRes. 51:6304-6311. Kaufman RJ, Sharp PA and Latt SA. 1983. Evolution of chromosomal regions containing transfected and amplified dihydrofolate reductase sequences. Mol Cell Bio. 3:699-711. Kaufmann H, Mazur X, Fussenegger M, Bailey JE. 1999. Influence of low temperature on productivity, proteome and protein phosphorylation of CHO cells. Biotech Bioeng. 63:573-582. 183 Kim SH and Lee GM. 2001. Overexpressiong of bcl-2 inhibits sodium butyrate-induced apoptosis in Chinese hamster ovary cells resulting in enhanced humanized antibody production. Biotech Bioeng. 71:184-193. Kim SH and Lee GM. 2007. Down-regulation of lactate dehydrogenase-A by siRNAs for reduced lactic acid formation of Chinese hamster ovary cells producing thrombopoietin. Appi Micro Biotech. 74:152-159. Koh T, Lee Y, Chang S and Nissom PM. 2009. Identification and expression analysis of miRNAs during batch culture of HEK-293 cells. JBiotech. 140:149-155. Koster M, Kirchhoff S, Schaper F and Hauser H. 1995. Proliferation control of mammalian cells by tumor suppressor IRF- 1. Cytotechnology. 18:67-75. Kubbies M and Stockinger H. 1990. Cell cycle-dependent DHFR and tPA production in cotransfected, MTX-amplified CHO cells revealed by dual-laser flow cytometry. Exp Cell Res. 188:267-271. Lagger G, O'Carroll D, Rembold M, Khier H, Tischler J, Weitzer G, Schuettengruber B, Hauser C, Brunmeir R, Jenuwein T and Seiser C. 2002. Essential function of histone deacetylase 1 in proliferation control and CDK inhibitor repression. EMBO. 21:2672-2681. Lanthier M, Behrman R and Nardinelli C. 2008. Economic issues with follow-on protein products. Nat Rev Drug Dis. 7:733-737. Lao M and Toth D. 1997. Effects of ammonium and lactate on growth and metabolism of a recombinant Chinese hamster ovary cell culture. Biotech Prog. 13:688-691. Li K, Lin S, Brunicardi FC and Seu P. 2003. Use of RNA interference to target cyclin Eoverexpressing hepatocellular carcinoma. Oncogene. 63:3593-3597. Lim LP, Lau NC, Garrett-Engele P, Grimson A, Schelter JM, Castle J, Bartel DP, Linsley PS and Johnson JM. 2005. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature. 433:769-773. Lim SF, Chuan KH, Liu S, Loh SO, Chung BY, Ong CC, Song Z. 2006. RNAi suppression of Bax and Bak enhances viability in fed-batch cultures in CHO cells. Met Eng. 8:509-522. Liu Q, Fu H, Sun F, Zhang H, Tie Y, Zhe J, Xing R, Sun Z and Zheng X. 2008. miR-16 family induces cell cycle arrest by regulating multiple cell cycle genes. Nucl Acids Res. 36:5391-5404. Livak KJ and Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and 2 -DDCt method. Methods. 25:402-408. Lloyd DR, Leelavatcharamas V, Emery AN and Al-Rubeai M. 1999. The role of the cell cycle in determining gene expression and productivity in CHO cells. Cytotechnology. 30:49-57. 184 Lloyd DR, Holmes P, Jackson LP, Emery AN and Al-Rubeal M. 2000. Relationship between cell size, cell cycle and specific recombinant protein productivity. Cytotechnology. 34:59-70. Lodish H, Berk A, Matsudaira P, Kaiser CA, Krieger M, Scott MP, Zipursky SL, Darnell JE. 2004. Molecular Cell Biology, 5 th Edition, W.H. Freeman and Company, New York, NY. Lukas J, Lukas C and Bartek J. 2004. Mammalian cell cycle checkpoints: signaling pathways and their organization in spacing and time. DNA Repair. 3:997-1007. Magae J, Wu C, Illenye S, Harlow S and Heintz NH. 1996. Nuclear localization of DP and E2F transcription factors by heterodimeric partners and retinoblastoma protein family members. J Cell Sci. 109:1717-1726. Mailand N, Falck J, Lukas C, Syljuasen RG, Welcker M, Bartek J and Lukas J. 2000. Rapid destruction of human Cdc25A in response to DNA damage. Science. 288:1425-1429. Majors BS, Betenbaugh MJ and Chiang GG. 2007. Links between metabolism and apoptosis in mammalian cells: applications for anti-apoptosis engineering. Met Eng. 9:317-326. Malphettes L and Fussenegger M. 2004. Macrolide- and Tetracycline- Adjustable siRNAMediated Gene Silencing in Mammalian Cells Using Polymerase II-Dependent Promoter Derivatives. Biotech Bioeng. 88:417-425. Malumbres M. 2008. Cyclin-dependent kinases and their regulators as potential targets for anticancer therapeutics. In: Principles of Molecular Oncology; Bronchud, Foote, Giaccone, Olopade, Workman (eds.), Humana Press, Totowa, NJ, p.207-237. March JC and Bethley WE. 2007. RNAi-based tuning of cell cycling in Drosphila S2 cellseffects on recombinant protein yield. Appl Microbiol Biotechnol. 73:1128-1135. Mariani BD, Slate DL and Schimke RT. 1981. S phase-specific synthesis of dihydrofolate reductase in Chinese hamster ovary cells. ProcNatl Acad Sci. 78:4985-4989. Mazur X, Fussenegger M, Renner WA and Bailey JE. 1998. Higher Productivity of GrowthArrested Chinese Hamster Ovary Cells Expressing the Cyclin-Dependent Kinase Inhibitor p27. Biotech Prog. 14:705-713. McManus MT and Sharp PA. 2002. Gene silencing in mammals by small interfering RNAs. Nat Rev Gen. 3:737-747. Meents H, Enenkel B, Eppenberger HM, Werner RG and Fussenegger M. 2002. Impact of coexpression and coamplification of sICAM and antiapoptosis determinants bcl2/bcl-xL on productivity, cell survival, and mitochondria number in CHO-DG44 grown in suspension and serum-free media. Biotech Bioeng. 80:706-716. 185 Moore A, Mercer J, Dutina G, Donahue CJ, Bauer KD, Mather JP, Etcheverry T and Ryll T. 1997. Effects of temperature shift on cell cycle, apoptosis and nucleotide pools in CHO cell bath cultures. Cytotechnology. 23:47-54. Mori K, Kuni-Kamochi R, Yamane-Ohnuki N, Wakitani M, Yamano K, Imai H, Kanda Y, Niwa R, Iida S, Uchida K, Shitara K and Satoh M. 2004. Engineering Chinese hamster ovary cells to maximize effector function of produced antibodies using FUT8 siRNA. Biotech Bioeng. 88:901908. Muller D, Katinger H and Grillari J. 2008. MicroRNAs as targets for engineering of CHO cell factories. Trends Biotech. 26:359-365. Naito Y, Yamada T, Ui-Tei K, Morishita S and Saigo K. 2004. siDirect: highly effective, targetspecific siRNA design software for mammalian RNA interference. Nuc. Acid Res. 32:Web Server Issue. Nakano K, Mizuno T, Sowa Y, Orita T, Yoshino T, Okuyama Y, Fujita T, Ohtani-Fujita N, Matsukawa Y, Tokina T, Yamagishi H, Oka T, Nomura H and Sakai T. 1997. Butyrate activates the WAF1/Ci11 gene promoter through SpI sites in a p53-negative human colon cancer cell line. JBiol Chem. 272:22199-22206. Neermann J and Wagner R. 1996. Comparative analysis of glucose and glutamine metabolism in transformed mammalian cell lines, insect and primary liver cells. J Cell Physio. 166:152-169. Nelson DL and Cox MM. 2000. Lehninger Principles of Biochemsitry. 3rd ed. Worth Publishers, New York, NY. Ng SK, Wang DIC, Yap MGS. 2007. Application of destabilizing sequences on selection marker for improved recombinant protein productivity in CHO-DG44. Met Eng. 9:304-316. Ngantung F. 2005. Engineering mammalian cell line to improve sialylation. Ph. D. thesis. Massachusetts Institute of Technology. Ngantung FA, Miller PG, Brushett FR, Tang GL and Wang DIC. 2006. RNA interference of sialidase improves glycoprotein sialic acid content consistency. Biotech Bioeng. 95:106:119. Nyberg GB. 1998. Glycosylation site occupancy heterogeneity of Chinese hamster ovary cell culture. Ph. D. thesis. Massachusetts Institute of Technology. Ohnishi T, Wang X, Ohnishi K and Takahasi A. 1998. p53-dependent induction of WAFl by cold shock in human glioblastoma cells. Oncogene. 16:1507-1511. Palermo DP, DeGraaf ME, Marotti KR, Rehberg E and Post LE. 1991. Production of analytical quantities of recombinant proteins in Chinese hamster ovary cells using sodium butyrate to elevate gene expression. JBiotech. 19:35-48. 186 Pennati M, Campbell AJ, Curto M, Binda M, Cheng Y, Wang L, Curtin N, Golding BT, Griffin RJ, Hardcastle IR, Henderson A, Zaffaroni N and Newell DR. 2005. Potentiation of paclitaxelinduced apoptosis by the novel cyclin-dependent kinase inhibitor NU6140: a possible role for surviving down-regulation. Mol Cancer Ther. 4:1328-1337. Prentice HL, Ehrenfels BN and Sisk WP. 2007a. Improving performance of mammalian cells in fed-batch processes through "bioreactor evolution". Biotech Prog.23:458-464. Prentice HL, Tonkin CJD, Caamano L and Sisk WP. 2007b. High level expression of proteins using sequences from the ferritin heavy chain gene locus. JBiotech. 128:50-60. Renard JM, Spagnoli R, Mazier C, Salles MF and Mandin E. 1988. Evidence that monoclonal antibody production kinetics is related to the integral of the viable cells curve in batch systems. Biotech Let. 10:91-96. Retzer-Lidl M, Schmid RM and Schneider G. 2007. Inhibition of CDK4 impairs proliferation of pancreatic cancer cells and sensitizes towards TRAIL-induced apoptosis via downregulation of survivin. Int J Cancer. 121:66-75. Reynisdottir I, Polyak K, Iavarone A and Masague J. 1995. Kip/Cip and Ink4 inhibitors cooperate to induce cell-cycly arrest in response to TGF-b. Genes Dev. 9:1831-1845. Reynolds A, Leake D, Boese Q, Scaringe S, Marshall WS and Khvorova A. 2004. Rational siRNA design for RNA interference. Nat. Biotech. 22:326-330. Riggs MG, Whittaker RG, Neumann JR and Ingram VM. 1977. n-Butyrate causes histone modification in HeLa and Friend erythroleukaemia cells. Nature. 268:462-464. Rodriguez J, Spearman M, Huzel N and Butler M. 2005. Enhanced production of monomeric interferon-b by CHO cells through the control of culture conditions. Biotech Prog.21:22-30. Sanger F, Nicklen S and Coulson AR. 1977. inhibitors. Proc.Natl. Acad. Sci. 74:5463-5467. DNA sequencing with chain-terminating Sauerwald TM, Betenbaugh MJ and Oyler GA. 2002. Inhibiting apoptosis in mammalian cell culture using the caspase inhibitor XIAP and deletion mutants. Biotech Bioeng. 77:704-716. Scahill SJ, Devos R, Van der Heyden J and Fiers W. 1983. Expression and characterization of the product of a human immune interferon cDNA gene in Chinese hamster ovary cells. Proc. Natl. Acad Sci. 80:4654-4658. Schwartz B, Avivi-Green C and Polak-Charcon S. 1998. Sodium butyrate induces retinoblastoma protein dephosphorylation, p16 expression and growth arrest of colon cancer cells. Mol Cell Bio. 188:21-30. Schimke RT. 1984. Gene amplification in cultured animal cells. Cell. 37:705-713. 187 Slater ML, Sharrow SO and Gart JJ. 1977. Cell cycle of Saccharomyces cerevisiae in populations growing at different rates. PNAS. 74:3850-3854. Sonna LA, Fujita J, Gaffin SL and Lilly CM. 2002. Effects of heat and cold stress on mammalian gene expression. JAppl Physiol. 92:1725-1742. Sung YH, Lee JS, Park SH, Koo J and Lee GM. 2007. Influence of co-down-regulation of caspase-3 and caspase-7 by siRNAs on sodium butyrate-induced apoptotic cell death of Chinese hamster ovary cells producing thrombopoietin. Met Eng. 9:452-464. Sunley K and Butler M. 2010. Strategies for the enhancement of recombinant protein production from mammalian cells by growth arrest. Biotech Adv. 28:385-394. Suzuki E and Ollis DF. 1989. Bioeng. 34:1398-1402. Cell cycle model for antibody production kinetics. Biotech Suzuki E and Ollis DF. 1990. Enhanced antibody production at slowed growth rates: experimental demonstration and a simple structured model. Biotech Prog. 6:231-236. Tait AS, Brown CJ, Galbraith DJ, Hines MJ, Hoare M, Birch JR and James DC. 2004. Transient production of recombinant proteins by Chinese hamster ovary cells using polyethyleneimine/ DNA complexes in combination with microtubule disrupting anti-mitotic agents. Biotech Bioeng. 88:707-721. Tan HK, Lee MM, Yap MGS and Wang DIC. 2008. Overexpression of cold-inducible RNAbinding protein increases interferon-g production in Chinese hamster ovary cells. Biotechnol Appl Biochem. 49:247-257. Tey BT and Al-Rubeai M. 2005. Effect on bcl-2 overexpression on cell culture and antibody productivity in chemostat cultures of myeloma NSO cells. JBiosci Bioeng. 100:303-3 10. Tey BT, Singh RP, Piredda L, Piacentini M and Al-Rubeai M. 2000. Influence of bcl-2 on cell death during the cultivation of a Chinese hamster ovary cell line expressing a chimeric antibody. Biotech Bioeng. 68:31-43. Tigges M and Fussenegger M. 2006. Xbp 1-based engineering of secretory capacity enhances the productivity of Chinese hamster ovary cells. Met Eng. 8:264-272. Toyoshima H and Hunter T. 1994. p27, a novel inhibitor of G1 cyclin-CDK protein kinase activity, is related to p21. Cell. 78:67-74. Ui-Tei K, Naito Y, Takahashi F, Haraguchi T, Ohki-Hamazaki H, Juni A, Ueda R and Saigo K. 2004. Guidelines for the selection of highly effective siRNA sequences for mammalian and chick RNA interference. Nuc Acids Res. 32:936-948. 188 Urlaub G and Chasin LA. 1980. Isolation of Chinese hamster cell mutants deficient in dihydrofolate reductase activity. PNAS. 77:4216-4220. Vassilev LT, Tovar C, Chen S, Knezevic D, Zhao X, Sun H, Heimbrook DC and Chen L. 2006. Selective small-molecule inhibitor reveals critical mitotic functions of human CDK1. PNAS. 103:10660-10665. Vermeulen K, van Bockstaele DR and Berneman ZN. 2003. The cell cycle: a review in regulation, deregulation and therapeutic targets in cancer. Cell Prolif 36:131-149. Vives J, Juanola S, Cairo JJ and Godia F. 2003. Metabolic engineering of apoptosis in cultured animal cells: implications for the biotechnology industry. Met Eng. 5:124-132. Walsh G. 2006. Biopharmaceutical benchmarks 2006. Nat Biotech. 24:769-776. Walsh G and Jefferis R. 2006. Post-translational modifications in the context of therapeutic proteins. Nat Biotech. 24:1241-1252. Weinberg RA. 1995. The retinoblastoma protein and cell cycle control. Cell. 81:323-330. Witzgall R, O'Leary E, Leaf A, Onaldi D and Bonventre J. 1994. The Kruppel-associated box-A (KRAB-A) domain of zinc finger proteins mediates transcriptional repression. PNAS. 91:45144518. Wong CFD, Wong TKK, Goh TL, Heng CK and Yap MGS. 2004. Impact of dynamic online fed-batch strategies on metabolism, productivity and N-glycosylation quality in CHO cell cultures. Biotech Bioeng. 89:164-177. Wong CFD, Wong TKK, Lee YY, Morin PN, Heng CK and Yap MGS. 2006a. Transcriptional profiling of apoptotic pathways in batch and fed-batch CHO cell cultures. Biotech Bioeng. 94:373-382. Wong CFD, Wong TKK, Nissom PM, Heng CK and Yap MGS. 2006b. Targeting early apoptotic genes in batch and fed-batch CHO cell cultures. Biotech Bioeng. 95:350-361. Wu S. 2009. RNA interference technology to improve recombinant protein production in Chinese hamster ovary cells. Biotech Adv. 27:417-422. Wurm FM. 2004. Production of recombinant protein therapeutics in cultivated mammalian cells. Nat Biotech. 22:1393-1398. Xie L and Wang DIC. 1994. Fed-batch cultivation of animal cells using different medium design concepts and feeding strategies. Biotech Bioeng. 43:1175-1189. 189 Xie L, Nyberg G, Gu X, Li H, Mollbom F and Wang DIC. 1997. Gamma-interferon production and quality in stoiciometric fed-batch cultures of Chinese hamster ovary (CHO) cells under serum-free conditions. Biotech Bioeng. 56:577-582. Xu P, Vernooy SY, Goo M and Hay BA. 2003. The DrosphilamicroRNA mir-14 suppresses cell death and is reguired for normal fat metabolism. CurrBio. 13:790-795. Yamaji H, Sakai K, Joho T, Izumoto E and Fukuda H. 2004. Cell cycle analysis of Chinese hamster ovary cells stimulated by phosphatidic acid in serum-free culture. J Biosci Bioeng. 6:487-498. Yokota M and Tanji Y. 2008. Analysis of cell-cycle-dependent production of tissue plasminogen activator analogue (pamiteplase) by CHO cells. Biochem Eng J 39:297-304. Yoon SK, Song JY and Lee GM. 2003. Effect of low culture temperature on specific productivity, transcription level, and heterogeneity of Erythropoietin in Chinese hamster ovary cells. Biotech Bioeng. 82:289-298. Yuk IHY. 2001. Protein expression and glycosylation in Chinese Hamster ovary cells. Ph. D. thesis. Massachusetts Institute of Technology. Zhou W, Rehm J and Hu WS. 1995. High viable cell concentration fed-batch cultures of hybridoma cells through on-line nutrient feeding. Biotech Bioeng. 46:579-587. 190 APPENDIX Cyclin Sequences This section contains the cDNA sequences of six of the eight known cyclins that are expressed in CHO cells. Sequencing was performed following the methodology outlined in Section 3.2.1. Capital letters represent CHO base pairs, while lower case letters are taken from mus musculus cDNA. Table 9-1 outlines our sequencing coverage. Table Al. Sequencing summary of known CHO cyclins Gene Mouse ORF (bp) CHO ORF (bp) % complete NOT TRANSCRIBED IN MATURE CHO CELLS CcnA] (ONLY SEX CELLS) CcnA2 1266 1080 85.3 CcnB1 1290 1287 100 CcnB2 PRESENT - NOT SEQUENCED YET CcnD1 885 591 66.8 CcnD2 867 867 100 CcnD3 PRESENT - NOT SEQUENCED YET CcnE] 1473 1227 83.3 CcnE2 1215 1119 92.1 191 Cyclin A2 (CcnA2) 1 61 121 181 241 301 361 421 481 541 601 661 721 781 841 901 961 1021 1081 1141 1201 1261 atgccgggca caggaagacc caggcggtgc CGACGGGTTG TCATGGAAAG ACTCAGAAAA GCTGCTGTCT GGTAGCTTTG GTGAATGTTA GAGGTTAAAT ATGCGGGCCA GAGACTCTGC AGAGGAAAGC ATATACCCCC CAGGTTCTGA ACAGTAAATC GAAAGCTTAG TATTTGCCAT CAAAGCTGGC TGTCTCATGG AGGGAAAAGT ctaagtgtgt cctcgaggca aagagaatgt tgaaggccgg CTCCTCTTAA CAACCAGTAA TGCCAGCTGA CTTTACCTGG AATCACCACA ATGAAGTACC GTAAACCTAA TCCTTGTGGA ATTTGGCTGT TTCAACTTGT CAGAAGTAGC GAATGGAGCA AGTTTCTTAA CAATGTTTTT CACTCATTGC CTGAGTCATT ACCTTCACCA ACAAACATTC ga ttcgggtcgc caaccccgaa gaacgtgcgt AGACCTTTCT ACAGCCGGCC ACATAAAGAA AGCAAGAAAA TGCTATGGAC CGACTATCAT AGTGGGTTAT CTGGCTAGTT GAACTACATT GGGCACTGCT AGAGTTTGTG CCTAGTATTG CCAGTACTTT GGGAGAATTA TGGAGCTGCC GGTACAAAAG GACCTACCTC AAAATACCAT cagccctgct cagcccagca cgcagcagaa AACATGTCGC ATGTGGATGA AAGATGCCCT CTCTTGATTA TCTTAGAAGA ACACATACCT AGCCAGACAT AAGAATATAA TCTCTTCCAT TAGCTTCAAA ACGATACCTA CTTTTGACTT AGCCTGCAAA ATGCTGACCC CACTCTATAC CCCTGGAAAG AGCATGCTCA TTCTCAACCC ctcgctgca.t gccgcgggcg gctcaaTACT CGTTGGTCCT AGAAGAGGAG GGCTTTTAAT TCCAATGGAT CGAAAAGCCA TAGGGAAATG CACTAACAGT ATTACAGAAT GTCTGTGTTA ATTTGAAGAA TTCCAAGAAG AGCTGCGCCA CTGCAAAGTT ATACCTAAAG AGTCACAGGA TCTTAAACCT ACAGTCAATA ACCAGAgaca CTTAACACAG AAAATAAGGC ATTGCTGCAG CCTCCAAGCC AAAGTCAGCG AACAGGCACA ACTGGAAAAG TTTCAGCTAA GAGCCAGAGG TGGAACTTGC CTTTCTCCTG AACCTATCTT TGTGCCCCTG CAGAAGAATA GATGTGGATG CAGATGATGG TATGCTTATC TCAGACAGCT CGTGAAGTCA CTGGAAACAT AAATTTAGGC TGCTGCAAGA CAGGATAACT GTGTACCCAA GCAAGCAAAT ATGAAGAAAT AATACTTACA CTAAGCACCA TTCAGTTTGG GTCGCCCTCT GTTGACGTTG AACAGCATAC GACATGGTGC ACTTTGCTCC ATTCTTGACA ACGGTGAATG TCCCTTCTGC CTGTCATGCA ACAAAGCACA TGACTATCAA CTGGCACAGC TGAATTGTAC CTCAAGTGGA CCACTACACT TAGTATACAG TGTTTACTTG ATTTGAATGT TGGTTGTTCT ATTTTTGAAA CTGCTTTTAG TATGTTTATG TACTTCCTTC AGCCCTCTGC CTAGCTCTTG CAAGGTCAGC CGGGCTGAGA GGCAAGAGTG AATCCCACCA TGAGCCTGAA GGTTGATAAT CCTGTGTCAG CGCTGATCCA GGAGGAAGAA GAGAGCCATC GACTATGTAC GAAGATGCTG GTACCCTCCA AATCAGACAG GCCTCTGCAC TTTGGCCAAA TTCTCAGATT GACACCGACC GCACCTGGCT GAACAAGTAT ACTAGTTCAG ACATCGGCAA CTCTTCAATA GACTCTAGGA TTTAACAGAG TT TAATGTGT TTTAAACTGT gatgcgggct aaactggcgc ggacccgcgc ATAAACGATG TTCACTATTC ACACAGTGTG CCACTGGTAC ATGTCTATTG GAAGATATTC ATGAAGAAGC GAAGTGGGAG GATCGGTTCC GCTATGCTGC TATATTACAG AAAGTCCTTG CTGCACCAGC AGTTTGATAG TTTCATTTAG ACTGGATATA AGAGCAGCAC GGTGTTTCTC Cyclin B1 (CcnBi) 1 61 121 181 241 301 361 421 481 541 601 661 721 781 841 901 961 1021 1081 1141 1201 1261 1321 1381 1441 1501 1561 ATGGCTCTCA ATGACAGGCG CCGAGAACCG CCTCTGAAAA CCAAAACCTC CCTGAACCTG CCCTCTCCAA GCTTTCTCTG AACCTCTGTA CAATCAGTTA CTAATTGACT ATGACTGTTT CAGCTAGTTG GAAATAGGTG ATGGAAATGA TTCCTCCGTA TACCTCATGG GCAGCTGGAG CTGCAGCACT AAGAATGTAG GCAACATCCA AATTTGTCCA ATGCAATTGG AAGGCTGTTT TAACAAGGGT AATCCAAGTA CCTGGTCATA GGGTCACTAG GAACACGAAA CCAAGCGTGT GCCTGTGGCG CTCTAGGGGA TATTGGTAAC AGGAACTAAA AACCTCAGTT AGGAGAAGGT TCCTGTGAGT AACCTGTTAT GGAGGAAAAG GCCCAATGGA AACATCTGGA ATGTAATTCT TGCCGTGAGC GTGAATATGT GAAAGACATA GACCAAGATA TCTACTGGGC GGCTGATACA GGTTCAAATG CCATTATTGA CCGGTTCATG GTGTGACTGC CATGTTTATT ACTTCGCTTT TGTGACTAAT AGATTCTAAG AGTTCTAAAC GAGCCTCTAA GATTGGAGAG AGCTCACTAT GTTGGACTAT CTTTTTGCTT AGCACTGAAA ACCTGTCCTA TACTGAAGAA TCATGGTGAA CCGTGGCCTT AGCATGCTAA GATCAGCACT AGGCTGTGTC AAAGGCATAA CACCATGTGC CACCTGTACA TACTTTTCAT AGCTTACCTC TGTCTAAAAC ATTTAGGATT ATGTACGTAA TTGTAGCCTA ,TCTTTTAAGT CATCCTGCAA 192 1621 1681 1741 1801 1861 1921 CAATTTACCA CTGTGTCTTG TTGTTAATTG TTAATCTCAA ATTTTTTTCA ACTGTATGTA GCTTGTCCTT AGCTATGGAC GCTTGGGAAA TGTCCTATAT ACAATATTCC ATGTATTCAG AGTTCCCTTT TTTCAGATCT TAGCATGTTT AAATGTAATC TTTTAATGCC TGATCCTTTA TCTATTTCTT GAACCCCAGT AAAATTAAAG ATGCATATGT TATATTGCAT CAATACATTC CAGGTGGTTG TTTCTTGTGT GTATAAAAAG TGTCAAGGAG TTCCTAAGTG AAATTGCATT CTGTCCTTCA AATTATTTAT AATTTGCCCC ATATGGACTC TACATTTCAT CTCTC agcTCCTGTG ACGACCGAGT ACTTCAAGTG TGCTGGAGGT ACCTGGACCG CCACCTGCAT TGTGCATCTA TGGTGAACAA TCCTCTCCAA CCTTTGTGGC ctgctgggag tctcctgcta tccgtgcctg agaacgtcga cctgcacgcc CTGCGAAGTG GCTGCGAGCC CGTGCAGAGG CTGCGAGGAG TTTCCTGTCT GTTCGTGGCC CACTGACAAC ACTTAAGTGG AATGCCAGAG CCTCTGTGCC cgtggtggct ccgcacaacg ccaggaacag ccccaaggcc caccgacgtg GAGACCATCC ATGCTCAAGA GAGATTGTGC CAGAAGTGCG CTGGAGCCCC TCCAAGATGA TCTATCCGGC AACCTGGCCG GCGGATGAGA ACAGACGTGA gcgatgcaag cactttcttt attgaagccc actgaggagg cgagatgtgg GCCGCGCGTA CCGAGGAGAC CGTCCATGCG AAGAGGAGGT TAAAAAAGAG AGGAGACCAT CCGAGGAGCT CCATGACTCC ACAAGCAGAT AGTTCATTTC gcctgaacct ccagagtcat ttctggagtc agggggaagt acatctga CCCTGACACC CTGCGCGCCC GAAAATCGTG CTTCCCTCTG CCGCCTGCAG TCCTTTGACC GCTGCAAATG CCACGATTTC CATCCGCAAG CAACCCACCC gggcagcccc caagtgtgac aagcctgcgc ggaggaagag TGTGCTGCGA GCGTCCTGCA AGTGCGTGCA AGGTCTGCGA ACCGTTTTTT GCATGTTCCT TTTACACCGA GAAAACTGAA GCAAGCTGCC TCGCTCTGTG GAAGTGTGGG GTGATGCACT CCTGCCAGGA AGCATAATGG GGGATGTTGA GGTGGACCCG GAACCTGTTG GAAGGACATT GGAACAGAAG GGCTGGAGTC AGCCTCTAAG CAACTCCGTG GTGGAACCTG CCAGCAGAAG TGCTACAGAC AGCAGCCATC GACTGAGCTG GCAGATTGAA ATCCAAGTCT CCTGTGA GTCCGCAGGG ACCATCGAGG CAGCCATACA TGTGAAGAGG CCGACTCCTA CTCAAAGAGA AAGCCCCAGG GCGGCAGTCA GAGAAGCTAT TTCAAGTTTG TGTGGGCTTC CTGGCCAAGA GCTGTGCTGC GTGGAAGATC CTGTGCCGGA AGCGCTACCT TGCGCAGGAT AAGTCTTTCC AGACCCACCT CCATCCCCCT AGCTGCTGGA CCCCTCACGA CCCTGATTCG CCATGTACCC AGCAGGATGA TCACCCACAC TCAACAGCCT TGGACCAAGC CCGCAACCTG CCCGCAGTGC GGTGGCCACC TTTGGCCATG TCAGCTCCTG GACGGCTGAG GTGGGAGCTG CTTCATTGAG CAAGCATGCG ACCATCGATG AGAAGAGAAC TGATGTGGAT GCAGCAGTTC CACCACCCCT agagagactc gctccagaaa CCAGAGTTGA CCTGTGTGGA AGTACCCCAA CAGTCTTGAA gacggaccac aaggaaggca CAAGACTGTG CCCATGCGCT GACTGCGTTT CTGGGGCAAT agcaacatga aaGGCGGCTG AAAAGCCAGG TTCATCCCCA CAGCCTCGGA AGAGAAGAGG aagaagaagg TTTTTCAACA ATAGCAGTCA CCCCTAATAA AAATCAGGCC TTTGGAGGAT tggctccgac TGCTCCAGAA GCCTTGGGAT AGAAGAGGAC ACCCAGGGCC CATGTTAAAC Cyclin Dl (CcnDi) 1 61 121 181 241 301 361 421 481 541 601 661 721 781 841 atggaacacc AACCTCCTGA TCCGTGTCTT GCCACCTGGA GCCATGAACT CTGCTAGGGG GCCGAGAAGT GAACTCCTTC ATCGAACACT CATGCGCAGA TCCAtggtag aacaacttcc ccggactgcc caggcccagc gctggtctgg Cyclin D2 (CcnD2) 1 61 121 181 241 301 361 421 481 541 601 661 721 781 841 ATGGAGCTGC CTGGAAGACC TCCTATTTCA TGGATGCTAG AATTATCTGG GGCGCAGTGT AAGCTGTGTA GTGGTCCTGG CATATCCTGC CAGACCTTCA ATCGCAACCG ACACTCACGT TGTCTCAAAG CGTCAAGAGC ACAGATGTGC Cyclin E1 (CcnEl) 1 61 121 181 241 301 atgccaaggg ctttcagtcc GAAGTAATCG GATAATTCAG GATGAGCTTG TCCCCACTTC 193 361 421 481 541 601 661 721 781 841 901 961 1021 1081 1141 1201 1261 1321 1381 1441 1501 AAGGAGAAGA AGGATGCGAG AGGGAGACAT ATACTGAAAA GAGGAAATCT GGAGATGAAA CCAATGACCA AGCGAGGTGC GATCTGTGTG GCCTTGTATC GACATAGAGA AGCTCCAAGC CACGCCAACA CAGAACAGGA CAGAACAGTG CTGCCTGGAG GGCGTATCTC TGGAATGCTA GAGTGCTCCA CAAAAGCTTG TTTACCTAAG CAGTTCTTCT TCTATCTGGC CACTTTTACA ACCCTCCAAA TTCTCCAGAT TTGTGTCCTG TACTGCCTCA TCCTGGATGT ATTTCTCCTC AATGTGTCAA TCAAGCACTT GCTTGGATTT TCTCTCCTCC AGGAGGAGAC CTCCTTTGCT TTCCAGAGCA GTTGTTTTAA GAAGCTGCTA AAATTAAGGA AGATGAGCAC GGATTGGCTA GCAAGACTTC GCTCATTGGG GTTGCACCAG GGAATTAATG GCTGAACGTG GTACCCACAG CGGCTGCTTA CATGGAACTG GTGGATGGTT CCGGGGAGTT GCTGGACAAA TCCCAGTGGG AGAATGAGCA GCCTAGGAGG GCGGGCGTCT CAATAGGGTT AGGAGGGTGC GGCCACGGTT TTTCTGCAAC ATGGAGGTGT TTTGATCGTT ATTTCAGCTT TTTGCTTACG ATGATGAAGG TATGTCCAAG CAGGTCTTTG GAATTTCCTT ATGCAGAAGG CCGTTCGCCA CCCATGGAAA GCCCAGGCAA GTCCTGACAC AACCTGCCAA TTGGGAGCCC TCAAAATGGC GAGGACACCA TACTTGACCC GCTGCTGGCC GCCATCCTCT GCGAAGTCTA ACATGGCATC TATTTATTGC TTACAGATGG CCCTTAAGTG TGGCTTATGT TGCAGATTGC ATGGTGTCCT TTTCAGGGTA TGGCCTTCCG ACGCCCACAA AGAAAGCCAT CCCCACCCAG AGACAGTGTT CTGCTGATGC CTCGGACAGA GCCACCTCCA ACTGGACTCT TCTGCCCGGG CCTGCAGGCA TAAGCTTCAC ACAACAAAAT TTCTAAACTT CGCTTGTTCC GCGTTTAAGC CAATGACACA TGAGCTTTTA TGCTGCTTCT TCAGTGGTGC GGAGGTGGGA CATCCAGACC ATTGTCAGAA CAGTAAGAAG GTGGAAGCAG TCTGCGGAAA AGTGCTTGTA GAACGCCGCT TCACACATGA TGTTGTA Cyclin E2 (CcnE2) 1 61 121 181 241 301 361 421 481 541 601 661 721 781 841 901 961 1021 1081 1141 1201 atgtcaagac gcagtagccg tttacaagct aagcaacatg cccagcccaa ccagccagac tctcCGCAAG AAAAGAAAAG GTACTGTCTG ACAAGTGACT CCTCTGCCAG GAGAGCAGAT ATGAGGTCAA GAGACATTTT AATAAAAATA GAAATCTACG GTAGATATCT GTAACAGTCA AAGGTTCTTC CTGTGTATCC TTGTGCCATT ATTTCAGAAT GTGAAGCTGA ACGAATTATT CAGTTGTCAC ccaggaaaac AAGTCCAGAT AGGAGATCAC GAGGAATCAG TCTCTAGATT ATTTAAGCTG ACGTGCATGA TACTTTTAGA ACCTTGCCCA TGCTTCAACT CTCCCAAACT TAAAGATGGA TTTCCTGGTT TACCTCAATA TCGCCATTGA TTACCTCCAT GTGTAGACTG AGACTTTTAA TGGCTTTGCT CAGTGTGTAA actga AATTCAGGCC CAAGAAGCAT TCCTTGCATT TACAAATTAC GGGGTGTTCA CAAACATTTT CTGGCTTTTA AGACTTTTTT CATTGGGATT CCAAGAGTTT ACTCTCTATA GAATCTTTTT TTCTCAGGAG CTCATTAGAA TGAAGTAGTT GATGGTGCCT GAAGATACCC GAATGAAGTA TGGAGGCATT 194 AAGAAGAGAA CAGTATGAGA ATCATTGAAA AGATTTAAAA CAGGAGGTTT GAAGTTCTGC GAGGTTTGTG GACAGATTTA ACCTCATTGT GCTTATGTCA TTAAAGGCTT CTCCAAGTTG ACATTCATCC TTCCAATACA AAGAAAGCCT TTTGTCAGTG ATGGAAGACA AACTACGTGA ATGACACCAC AAACAGCACA TCAGGAATTG CGCCCCATAA ATCTTTTTAT GGCTAAACAT ATTCTGACCT AAGTATACAC TGTTGACACA TCATTGCTTC CTGATGGTGC TAAAATGGGA ATGCTGTTAA AGATAGCTCA GAATTCTGGC CAGGTTTGGA TAGTAAAAAG GACATAATAT ACACCTTCAG CAAagagtac GGATGTCAAA TTGGCCACCT AGAAATAGAA TAATCCTTCA GCTACAAAAG GGAACCACAG ACTTCATAGG GAAAGATGTA TAAACTTGAG TTGCAGTGAA ACTTTGTCCT AGATGTTCCT GCTTTTAGAT AGCTGCTGCT ATGGGATGAC TGTAAGTCCA CCAGACACAC AAAAGGAGGG tgaaaaacca