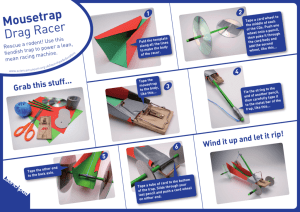
The Relationship between Processing and Microstructure of Tape Cast Green Sheets
advertisement

The Relationship between Processing and Microstructure of Tape Cast Green Sheets by Ming Liu B. A., Physics Hamilton College. 1992 Submitted to the Department of Materials Science and Engineering in Partial Fulfillment of the Requirements for the Degree of MASTER OF SCIENCE in Materials Science and Engineering at the Massachusetts Institute of Technology May 1994 1994 Massachusetts Institute of Technology All rights reserved Signature of Author ... ........ 41. A Departmen ......... \ ............... aterials Science and Engineering May 6 1994 Certifiedby ....... Accepted by ................ V ......... .............. Michael J. Cima, Associate Professor of Ceramics Department of Materials Science and Engineering Thesis Advisor I "W ........... Carl V. Thompson H Professor of Electronic Materials Chair, Departmental Committee on Graduate Students science AUG 1 8 1994 2 The Relationship between Processing and Microstructure of Tape Cast Green Sheets by Ming Liu Submitted to the Department of Materials Science and Engineering on May 6 1994 in Partial Fufillment of the Requirements for the Degree of MASTER OF SCIENCE in Materials Science and Engineering Abstract Tape casting is used to produce thin layers of ceramic-loaded polymers for electronic packaging. his project focused on two aspects of tape casting: (1) the measurement of plasticizer concentration across tape thickness, and (2) the examination of drying behavior and green tape densities for three different polymer to plasticizer ratios. A high resolution method was developed to measure the ratio of plasticizer (Benzyl Butyl Phthalate) to binder (Poly(vinyl butyral)) as a function of position within ceramic green tapes. Diamond microtome sections, 07 trri thick, were taken parallel to the surface of the tape. Each section was analyzed using Fourier transform infrared microspetroscopy (TIR-M measure the absorption intensity of unique absorption to bands for the plasticizer and binder. 'Me plasticizer concentration as a function of depth from the tape surface was determined by measuring the absorption intensity of each band. The measurements are the first high resolution composition measurements performed on tape cast sheets. We have discovered by using this tool, that common polymer-plasticizer combinations forrn twophase mixtures. We have also demonstrated that solubility limits correlated with the solubility parameter for the plasticizer. The purpose of the second study was to find an optimal way to process the green tape, so that dewetting and pores could be controlled. he study emphasized the relationship between the ratio of the binder to plasticizer and the densities of cast tapes. We have made the first in situ density measurements of a drying tape. There was less porosity in the green tape with the higher polymer to plasticizer ratio. Dewetting and porosity could be eliminated tinder slower drying rate. Thesis Supervisor: Dr. Michael J Cima Title: Associate Professor of Ceramics 3 4 ACKNOWLEDGMENTS First, I would like to express my gratitude to Professor Michael Cma. I have benefited both professionally and personally from his unwavering enthusiasm, high intellectual caliber, as well as his keen guidance and moral support throughout the entire course of this work. I also would like to express my appreciation to Dr. Wendell Rhine, who provided me with numerous helpful suggestions, critiques and comments. My thanks are also due to Lenny Rigione for setting up the drying bag, drying box and the tape caster for me; John Centorino for providing me with various technical assistance. I cannot thank Dr. Lunhan Peng enough for teaching me FrIR; and Dr., Jiang Yue enough for teaching me microtoming. thanks go ot My special to Dr. Yuying Tang for teaching me tape casting and encouraging e throughout this project; to Dr. Yaping Liu, Dr. Tae Sung, Man Fai Ng and Bill Rowe for doing TEM for me. 'Me successful completion of the work would not have been possible without some of my wonderful lab mates: Resourceful Man Fai Ng, smiling Keigo Hirakata, fun loving Satbir KhanuJa, considerate Dr. Tae Sung, courteous Jae Yoo, cheerful Karina Rigby, cooperative Bill Rowe, athletic Kevin Ressler, thoughtful Bertha Chang, humorous Dr. Neville Sonnenberg, helpful Barbara Layne and many others. They have not only created a positive working environment, but also provided me with tremendous amount of assistance and support that I can never forget. Finally, I would like to express my gratitude to all my friends and family for moral support in all forrns. In particular I would like to thank my parents for being there with me during all these years of my education; and my boyfriend Yethun Goh for his loving and caring. 5 6 TABLE OF CONTENTS TITLE PAGE 1 ABSTRACT 3 ACKNOWLEDGMENTS 5 LIST OF EXHIBITS 9 CHAPTER 1. INTRODUCTION 14 CHAPTER 2 BACKGROUND 18 2.1 2.2 Tape Casting 18 2.1.1 GreenTapeComponentsandTheirFunctionl8 2.1.2 Tape Casting Procedures 19 Drying and Density Measurement 20 2.2.1 Drying 20 2.2.2 Density Measurement 21 2.3 Microtoming 22 2.4 Phase Separation 23 2.4.1 Microscopic Techniques 23 2.4.2 Staining 24 2.4.3 DSC Technique 26 CHAPTER 3 PLASTICIZER DISTRIBUTION WITHIN CERAMIC GREEN TAPES 37 3.1 Introduction 37 3.2 Experimental Procedures 38 3.3 Observations 40 3.4 Discussion 42 3.5 Conclusion 45 7 CHAPTER 4 DRYING AND DENSITY MEASUREMENTS 66 4.1 Introduction 66 4.2 Experimental Procedures 67 4.3 Observations 68 4.4 Discussion 69 4.5 Conclusion 7 CHAPTER 5. CONCLUSION AND FUTURE WORK 85 5.1 Major conclusions 85 5.2 Industrial Applications 86 5.3 Suggestions for Future Work 87 Bibliography 88 8 LIST OF FIGURES Page Figure 21 Schematic of the tape casting process (Ref. 5). 30 Figure 22 Two stage drying process (Ref. 5). 31 Figure 23 Schematic of the microtorning process (Ref 6. 32 Figure 24 Microtomed section adhering together in ribbon fon-nation while floating on a liquid surface (Ref 6. 32 Figure 25 Osmimium tetroxide reacts with the carbon-carbon double bonds (Ref 9. Figure 26 33 Hydrazine or hydroxylamine reacts with polymer containing acid and ester groups (Ref. 12). Figure 2.7a Phase contrast of a stained cryosection through optical microscope showing dispersed phase particles in a matrix (Ref. 14). Figure 2.7b 34 Phase contrast through TEM of a stained cryosection showing dispersed phase particles in a matrix (Ref. 14). Figure 28 33 34 DSC traces of polyisoprene/polybutadiene and polybutadiene/polybutadiene immiscible blends exhibiting two Tg's (Ref. 14). Figure 31 35 Binder burnout TGA curves for thin sections cut from the top and button of the tape (ref. 5). 48 Figure 32 The chemical structure of PVB. 49 Figure 33 'Me chemical structure of BBP. 49 Figure 34 Sample selection for nkrotoming. 50 Figure 35 IR spectra representative of PVB-BBP tape. 51 Figure 36 (calibration curve relating plasticizer concentration and band ratio. 52 9 Page Figure 37 IR spectra representative of Al,)03-PVB-BBP 53 tape. Figure 3.8a Plasticizer composition profile for = feet/min, with 54 Al')03- Figure 3.8b Plasticizer composition profile for = feet/min, with 54 Al')03- Figure 39 Plasticizer composition profile for = 200 feet/ min, with 55 A'203Figure 3 10 Plasticizer composition profile for green tape with binder B55 79. Figure 311 Plasticizer composition profile for 40% (Vol.) BBP, without 56 A'203Figure 312 Plasticizer composition profile for 35% (Vol.) BBP, without 56 All03Figure 313 Plasticizer composition profile for 30% (Vol.) BBP, without 57 A'203Figure 314 TEM print Of S04 stained PVB-BBP cross section.56 Figure 3.15a Plasticizer composition profile for Dioctyl Phthalate. 58 Figure 3.15b Plasticizer composition profile for BBP. 59 Figure 3.15c Plasticizer composition profile for Dibutyl Phthalate. 59 Figure 3.15d Plasticizer composition profile for Dimethyl Phthalate. 60 Figure 3.16 Phase separation as a function of 8. 61 Figure 3.17 Phase separation during dying. 62 10 I 'age Figure 3.18a Phase separation during dying, ft/min. 63 = 200 ft/min. 614 = Figure 3.18b Phase separation during drying, Figure 41 The "glass rod table" for tape casting (ref 3. Figure 42 A schematic of in situ density measurement (ref 4. Figure 43 F4 A representative plot of green tape weight loss during its drying for sample B. Figure 44 77 In situ density measurement for samples B and C from the "glass rod table". Figure 47 78 Pore volume fraction as a function of solvent remaining for sample C. Figure 48 r6 A plot of thickness reduction vs. weight loss for samples B and C. Figure 46 F5 A schematic of the wet tape on the microscopic glass substrate 10 minutes after casting. Figure 45 F3 79 In situ density measurements for samples A, B and C from the tape caster. 30 Figure 49 In situ density measurements for sample C. Figure 4 10 The variation of the tensile strength of an Alumina 31 green sheet as a function of plasticizer concentration. (ref. 8) 32 I 1 LIST OF TABLES Page# Table 21 Listing of microscopy techniques (Ref 9. 36 Table 31 The composition of green tape for plasticizer gradient measurement. Table 32 65 Solubility parameters for plasticizers used in this experiment. Table 41 65 The composition of green tape for insitu density measurements. 83 Table 42 PVB-BBP tape densities. 83 Table 43 Final densities and drying times for samples A, B and C Table 44 84. A summary of density and dewetting for sample C under different drying rates. 12 84 13 CHAPTERI INTRODUCTION Tape casting has been used to produce thin layers of ceramic-loaded polymers for multilayer ceramic (MLQ packaging 1. structures, such as electronic Organic binder systems used in the electronic packaging are critical to the final products, and they have to be completely removed prior to the sintering. The binder system of the ceramic tape has to provide the tape with high tensile and yield strength, sufficient flexibility for handling and machining, dimensional stability over time, and a uniform and reproducible shrinkage during binder removal and sintering 2 A binder system which satisfies these requirements should include a long chain polymer to provide strength to the film, a plasticizer to increase the flexibility, and a deflocculant to ensure a uniform dispersion of the ceramic powder. Proper selection of the binder and plasticizer is very important for successful multilayer fabrication. Many different types of binders have been used, but the most common ones for electronic applications are acrylic and polyvinyl butryal resins. Most tape-casting processes start with a milling procedure to thoroughly mix the plasticizer, binders and ceramic powder. The mixed slurry is ready to be conditioned for the actual tape-casting process. casting processes Most tape are based upon continuous casting machines where the doctor blade is stationary and the casting surface is moving. Dried tapes are then cut to size, punched with via holes, screened with metallic paste and laminated together to form multilayer greenware.[21 A major problem in the electronic packaging is the difficulty for precise dimensional control, which is critical for 14 MLC manufacturing. Most projections suggest that dimensional requirements complexity and the size of the package increase. will increase Shrinkage variations within 0.2% linear and camber within 0. 0 I "fin. are often specified 3 is complicated as the by the use of many processing steps. This variation Other problems in electronic packaging include retention of carbon in fired products, delamination and defects such as voids and cracks. Structural inhornogenity can also exist in tape cast ceramic sheets, such as a density gradient across the tape thickness 4 The inhomogeneity in the green sheets tends to be amplified during the binder removal and the firing processes 5. he resulting Inhornogeneous shrinkage creates stresses which result in defect formation. Ile origin of the above problems must be related to the microstructure of the green tape. Few experiments have been reported to our knowledge that describe the microstructure of the tape and its relationship to the processing conditions. Therefore, in this project, we will study the relationship between processing and microstructure of tape cast green sheets. We will focus on two problems: density of the green tape, and the composition gradient in the green tape. The first problem rises because most ceramic packaging is produced so that the final ceramic structure is near full density . Thus, variation in shrinkage is largely caused by variation in the component green density. Similarly, camber can be caused by variation in packing density through the thickness of the green sheet. This study will enhance control of dimension and camber. The eason for the second study is that structural homogeneity requires that a the phases be uniformly distributed throughout the tape. We discovered that blends of binder and plasticizer may be immiscible and exhibit a two phase morphology. As a result, plasticizer distributes unevenly across the 15 green tape thickness. Such a phase separation will result different microstructure across the green tape thickness, and thus, complicate the MLC manufacturing. The study of plasticizer gradient across the green tape win help us to understand the cause of camber and delamination. This will help in the development of new ceramic fori-ning operations and new binder systems. This thesis starts with a literature review (Chapter 2 which includes tape casting, drying, microton-ling and phase separation. Plasticizer distribution across ceramic green sheets are described in Chapter 3 In this study, we win introduce a method for measuring composition gradients on a scale that has never be done before for green tapes. We will show the discovery that common polymer - plasticizer combinations fon-n two-phase mixtures. We will also demonstrate the solubility limits correlate with the solubility parameter for the plasticizer. Chapter 4 is devoted to the relationship between the binder to plasticizer ratio and the density of cast tapes. This is also the first time insitu density measurements of a drying tape is made. The major conclusions of this project are given in Chapter 5, followed suggestions for future work. 16 by industrial application and References 1. R. Mistler, "Tape Casting: The Basic Process for Meeting the Needs of the Electronic Industry", Ceramic Bulletin, Vol. 69, NO. 6 1990. 2. Y. Y. Tang, Ph.D. Thesis, Department of Materials Science and Engineering, Massachusetts Institute of Technology, 1994. 3. M. J. Cirna, Y. Y. Tang and M. Liu, "Inhomogeneity and anisotropy of Tape Cast Ceramic Films For Multilayer Structures", Ceramics Processing Laboratory, Massachusetts Institute of Technology. 4. Roosen, "Basic Requirement for Tape Casting of Ceramic Powders", PP. 675-92 in Ceramic Transactions, Vol. 1, Ceramic Powder Science I 1, B/ Edited by G. L. Messing, E. R. Fuller and H. Hausner. American Ceramic Society, Westerville, OH, 1988. 5. T. Ueyarna and N. Kaneko, "Effect of Agglomerated Particles on Properties of Ceramic Green Sheets", pp. 1451-58 in High Tech Ceramics. Edited by P. Vincenzini. Elsevier, Amsterdam, Netherlands, 1987. 17 CHAPTER 2 BACKGROUND In this chapter, we will review other studies on tape casting and drying. Some common techniques used for analyzing the microstructures of polymer blends will also be described. 2.1 Tape Casting Tape casting has been used to produce thin layers of ceramic-loaded polymers which can be stacked and aminated into multilayered structures. Today, cast tapes find wide use in making multilayer chip capacitors and in other electronic components, such as then-nistors, ferrites, inductors, and piezoelectrics 1.1. 2.1.1 Green Tape Components and Their Functions The organic additives in the tape-casting dispersing agent, binder and plasticizer. systems are: solvent, Their functions are summarized as follows: Solvent: the solvent dissolves the other organic materials and distributes them unifon-nly throughout the slurry. It carries the ceramic particles in an ordered dispersion. As solvent evaporates, the green sheet made up of organic-ceramic composite on the substrate become dense. 9 Dispersing agent: The dispersant coats the ceramic particles and keeps them in a stable suspension in the slurry due to steric hindrance and electric repulsion. With aluminum oxide and most other oxides it has been found that Menhaden fish oil and glyceral trioleate are effective dispersants. Hyatt 2 concluded that non-nally, as much as 3 n-d of dispersant may be used for each 100 g of inorganics. 18 * Binder: the binder dissolves in the solvent and enhances its viscosity to fon-n a slurry. As the solvent evaporates, the binder-coated ceramic particles bond together to fon-n a strong dense green tape. The temporary binder is used to fon-n an adhering film around the inorganic particles. Yan 3 suggested that Polyvinyl butyral (PVB) and various acrylic polymers are used commonly for this purpose. *Plasticizer: the plasticizer structurally expands the binder and improves the distribution of the binder in the slurry. Properties of the binders may be changed by selected use of a plasticizer. It also makes the green tape flexible. The common plasticizers include butyl benzyl phthalate (BBP), and polyethylene glycol (PEG) Roosen et al 3]. suggested that the non-optimized use of these organic 4 additives can have disadvantages, e.g. density gradients, low green strength, poor shrinkage reproducibility, crack fon-nation during sintering, and physical and chemical inhornogenities. 2.1.2 Tape Casting Procedures Most tape-casting processes start with a milling procedure. The first stage of milling produces a low viscosity slurry by breaking down powder agglomerates and uniformly distributing a dispersing agent in the solvent. In the second stage, the plasticizer and binder are dissolved into the solvent/ceramic slurry. After the slurry has been thoroughly n-dxed, it is ready to be conditioned for the actual tape-casting process. The conditioning procedure consists of de-airing by a vacuum. Chong et al [51 concluded that standard vacuums which are used are in the range of 635 to 710 mmHg. The viscosity of the slurry usually range from 1000 to 5000 mPa for standard tape formulation 6]. 19 Most tape casting processes are based upon continuous casting machines where, the doctor blade is stationary and the casting surface is moving. For laboratory operations, batch casting with a mobile doctor blade and stationary casting surface can be used. Thickness control is a function of several parameters which can be adjusted, including slurry viscosity, casting carrier speed, doctor-blade gap setting, and reservoir depth behind the doctor blade. Chou et al 7 suggested that the ratio of blade gap setting to final dried green tape is approximately 2 . 'Me tape can be either rolled onto a spool after drying for use in a rollto-roll process or stripped and cut into integral lengths for use in a subsequent procedure. Figure 21 is a schematic of the tape casting process 81. 2.2 Drying and Density Measurement 2.2.1 Drying The layer of slip dries slowly while it is carried through the machine on the substrate. Filtered air is blown through the machine in a direction opposite to that of the moving slip. This arrangement allows dry air to come in contact with dried tape at the exit end. Moreover, air passes through the length of cast tape and gets saturated with solvent vapor before coming in contact with freshly cast tape at the other end. The difference in solvent content between the slip and the air controls the rate of solvent evaporation, allowing the remaining solvent in the slip layer to redistribute itself with only a small vertical gradient of concentration. This small gradient of concentration tends to minimize the cracking of the slip as it dries. A two-stage drying process of cast tapes is observed by Mistler et al [8]. The first stage of drying proceeds at a constant rate, and the second stage at a gradually decreasing rate as shown in Figure 22. In either stage of drying, the solvent must move through three consecutive steps of transport: (1) solvent 20 flows vertically through the slip to the surface, 2) solvent then evaporates at the surface, and 3) solvent vapor is swept away at the surface. In Step I the slip is still fluid and solvent is easily transported through it by liquid diffusion or capillary action. The drying rate in Step 2 is limited by the inflow of heat required to supply the latent heat of vaporization. Step 3 can also be slow due to the accumulation of nearly saturated vapor at the surface. A constant rate is usually observed in Step . 2.2.2 Density Measurement The density of unfired tape (often called the green" density) is an important parameter for characterizing a tape-casting process. The green tape density measurement can be useful in detecting poor packing of the powder or in detecting excessive polymeric binder content. Mistler et al (81 have developed a method to determine the bulk green density. The volume measurement could be made with a flat-faced micrometer caliper, provided about 15 thickness measurements were taken and averaged. The same volume could be obtained by using mercury porosimeter. Typicalbulk densities for the Alun-finatapes produced were 25 to 26 g/cc. The use of porosimeter reported some degree of permanent penetration of the tape by the mercury, proving that there was some open porosity. However, the weight gain due to Hg absorption could not be used as a quantitative separation of porosity from compressibility because some mercury flows back out of elastic porous materials when the applied pressure was removed. The weight gain is only a qualitative test, but nevertheless it is a definite indication of unoccupied space in the original tape. Karas et al 9 determined green density of cast BaT403tape from geometrical measurements using a thickness gage accurate to 00025 mm on a 21 carefully cut 1-cm2 section. They also concluded that there was little effect of binder to plasticizer ratio on the powder packing density. 2.3 Microtome Urtramicrotomy is a technique of producing sections of materials thin enough for examination under the electron microscope. It has been used successfully to study all types of plant and animal tissue and other materials such as metals, plastics, synthetic fibers and even samples from the moon. Ile production of sections by ultran-licrotomy involves a type of cutting action similar to that used in the machining of metals. A prepared specimen is moved past a cutting edge so that a thin layer of the material is sliced off. Considerable friction is encountered as the section moves down the sectioning facet of the blade, but this becomes almost negligible if water is present at the knife edge. The slice then floats easily onto the liquid surface, and will straighten out if it is allowed to float freely on the surface. In the ideal situation, the section would be exactly the same size and shape as the face of the specimen block from which it is cut. However, in practice the sliced piece is not an entire cross section of the specimen block. schematic of the microtoming process. Figure 23 As shown in Figure 24 101 is a 101, as the slices are produced they will adhere to one another along one edge forming "ribbons". Goodhew 111 suggested that in ultramicrotomy, as in both machining and normal microtomy, variations of the knife angle and clearance angle win affect the amount of stress introduced into the material and thus vary the quality of the results. Other factors influencing the quality of the sections are the speed of the cut, the temperature, and the sharpness of the cutting edge. The production of good sections is dependent conditions for the material under observation. 22 on finding the optimum 2.4 Phase Separation Blends of two or more polymers are often used to combine the desired physical properties of each polymer to obtain an improved product. polymers, when blended, may be thermodynamically The miscible, exhibiting a single phase morphology, or the polymers may be immiscible and exhibit a two phase morphology. The most common instruments used to study the multiphase polymer structures are: microscopic instruments and differential scanning calorimeter (DSQ 121. McBrierty 131 has conducted studies by using nuclear magnetic resonance (NMR) to test the multiphase behavior. In the following sections, we will introduce these two most conu-non techniques. 2.4.1 Microscopic Techniques There are ranges of general techniques information relating to polymer microstructures. commonly employed optical and electron Table 21 141 with used to provide useful A listing of the more icroscopy techniques is shown in type of features that are commonly imaged, the size range of the structures and typical magnifications. Optical microscopy and transmission electron microscopy (TEM) will be introduced in the following sections. OpticalMicroscopy Optical microscopy techniques are useful in providing a rapid overview of structures in relation to the whole specimen. Phase contrast and Nomarski techniques provide differentiation in multiphase polymers where small differences in refractive index can provide information regarding the dispersed phase size and distribution. Sawyer et al 141 summarized the advantages of this technique: Optical microscopy is shown to provide a rapid and informative overview. Sample preparation is minimum. Samples for optical study are not placed in a vacuum, as with electron microscopy, and thus volatiles are not removed. 23 Beam damage, which is common in electron in an optical microscope. icroscopy of polymers, does not occur It also provides less chance of artifacts in the preparation of a thin section for optical study than in the preparation of an ultrathin section for TEM. The limitations of optical magnification range and microscopy a decreasing depth are the limited resolution, of field with increasing magnification. Tadokoro [151 suggested that the resolution limit is on the order of 02 gm; and the magnification limit is 2000x. TEM Techniques Fundamental polymer characterizations generally involve the application of TEM techniques. They offer the best image resolution of a of the microscopy techniques and structural infon-nation relating to crystallinity and crystallite sizes. Dispersed phase structures can often be observed and quantified over size range from less than 10 nm up to I tm. The disadvantages associated with the use of TEM are: high capital expenditure, time consuming in specimen preparation, and two dimensional image interpretation. There are two reasons for the time consuming nature of the specimen preparation for TEM: the specimens must be extremely thin (50 nm thick), and extra steps are often required to increase the contrast in polymer specimens (section 24.2). Most of the methods for producing ultrathin sections are tedious such as ultramicrotomes with diamond knives for sectioning (section 23). Image interpretation is difficult due to variation of the specimen in the vacuum and under the electron beam, and artifacts caused by sample preparations. 2.4.2 Staining Image contrast in TEM is the result of variations in electron density among the structures present. Since most polymers are composed of low 24 atomic number elements, they exhibit little variation in electron density. As a result, TEM micrographs of multiphase polymers often do not provide enough contrast to image the phase clearly. One of the most common methods which has proven useful in contrast enhancement is staining, by the addition of heavy atoms to specific structures. Staining involves the incorporation of electron dense atoms into the polymer, in order to increase the density and thus enhance contrast. Staining of polymers can. be conducted either before or after microton-ling. The sample is immersed in the stain solution or exposed to the vapor. The most common staining method staining, introduced by Andrews and Stubbs synthetic rubbers. is osr-nium tetroxide 161, (S04) who stained unsaturated Such technique reveals the nature of the dispersed phase domains of multiphase polymers. Osmium tetroxide reacts with the carboncarbon double bonds (Figure 25) in unsaturated rubber phases enhancing the contrast in TEM by the increased electron scattering of the heavy metal in the rubber compared to the unstained matrix. The reaction is slow, often taking days to weeks. The high vapor pressure Of S04 is beneficial, making vapor staining of sections viable; however, this vapor pressure, combines with the toxicity of the stain, makes it very dangerous to use. Several two step reactions have extended the range of OsO4 staining to materials that cannot be stained directly. For example, alkaline saponification at boiling temperature followed by reaction of the hych-oxylgroups with was used to study poly(vinyl chloride)/ethylene-vinyl acetate systems. [171 has S04 Kanig developed a staining method for butyl acrylate rubber by treatment with hydrazine or hydrozylanime and post staining with SO4, which works for polymers containing acid and ester groups. The Os is deposited after the ester is reduced by the hydrazine (Figure 26). 25 Exhibit 28 shows an example of microscopy techniques and methods for polymers. Phase contrast optical and TEM of a stained cryosection (Figure 27) sO4 stain icroscopy (Figure 2.7a) 141 141 all show dispersed phase particles in a matrix. 2.4.3 DSC Technique: The glass transaction temperature is the main characteristic temperature of the amorphous solid and liquid states. A liquid becomes a solid on cooling through the glass transition temperature. The microscopic process involved is the freezing of large-scale molecular motion without change in structure. The glass-liquid transition occurs at a recognizable "transition temperature" because of a rather large temperature dependence of the relaxation time for large-scale molecular motion. The reported Tg is the temperature measured at the onset of the glass transition. A blend with a single Tg is defined as miscible and one with two Tg's was defined as being immiscible. DSC is the most common instrument used to measure Tg of polymers. Two types of DSC instrument have been widely used: the heat-flux DSC (e.g. DuPont 910 DSQ and the power-compensational DSC (Perkin-Elmer and Setarurn 11) temperature [181. loop. The theory of DSC can be explained with the differential The signals representing temperatures are fed to the differential-temperature the sample and reference amplifier via a comparator circuit, which determines whether the reference or the sample temperature is greater. he differential-temperature amplifier output then supplies power to the reference or sample heater as necessary to correct any temperature difference between them. A signal proportional to this differential power is also transmitted to the pen of the recorder, giving a curve of differential power versus time or temperature. The area under a peak is then directly proportional to the thermal energy absorbed or liberated in the transition. 26 As an example of the above application, Massie et al 191conducted DSC studies as shown in Figure 28. and polybutadiene/Polybutadiene Traces of polyisoprene/polybutadiene immiscible blends exhibiting two Tg's. 27 References R. Mistler, "Tape Casting: The Basic Process for Meeting the Needs of the Electronic Industry", Am. Ceramic Soc. Bulletin, Vol. 69, NO. 6, 1990. 2. E. Hyatt, "Making Thin, Flat Cerarnics-A Review", Am. Ceramic Bulletin, Vol. 65, NO. 41986. 3. M. F. Yan, "Microstructural Control in the Processing of Electronic Ceramics," Mater. Sci. Eng., 48, 53-72 1981). 4. A. Roosen, F. Hessel, H. Fischer, F. Aldinger, "Interaction of Polyvinylbutyral with Alumina", Ceramic Powder Science, 1990. 5. J. S. Chong, E. B. Christiansen, and A. D. Baer, "Rheology of Concentrated Suspensions," Journal of Concentrated Suspensions, 15,2007-2021, 6. 1971). E. S. Tormey, R. L. Pober, H. K. Bowen, and P. D. Calvert, "Tape Casting--Future Development," pp 140-49 in advances in Ceramics, Vol. 9 Forming of Ceramics, Edited by J. A. Mangels, and D. L. Messing, American Ceramic Society, Columbus, Ohio, 1984. 7. Y. T. Chou, Y.T. Ko, and M. F. Yan, "Fluid Flow Model for Ceramic Tape Casting", J. Am. Ceram. Soc., 70 [10], C-280-C-282, 1987. 8. R. Mistler, D. Shanefield, and R. Runk, "Tape Casting of Ceramics," pp 411-418, in Ceramic Processing Before Firing. Edited by G. Y. Onoda and L. L. Hench. Wiley, New York, 1978. 9. A. Karas, T. Kumagai, and R. Cannon, "Casting Behavior and Tensile Strength of Cast BaTi4O3Tape", Advanced Ceramic materials, 34] 374-77 1988). 10. Reid, "Ultramicrotomy", pp 217-223, 1980. 28 11. Goodhew, P. F. 1972) Specimen preparation in materials science, in: Practical methods in electron microscopy, A. M. Glauert, ed. NorthHolland, Amsterdam). 12. T. K. Sherwood, "The Drying of Solids", Ind. Eng. Chem., 21 (1), ppl2-16, 1929. 13. V. J. McBrierty, "Heterogeneity in Polymers as Studied by Nuclear Magnetic Resonance", Faraday Discussions of the Chemical Society, NO. 68, 1979. 14. L. Sawyer, D. T. Grubb, "Polymer Microscopy", Chapman and Hall, London, New York. 15. H. Tadokoro, "Structure of Crystalline Polymers", Wiley-Interscience, New York, 1979. 16. E. H. Andrews and J. M. Stubbs, J. R. Microsc. Soc. 82 pp 221, 1964. 17. G. Kanig, Proc. Colloid Polym. Sci. 57 pp 176, 1975. 18. Thermal Characterization of Polymeric Materials, edited by E. Turi, Academic Press Inc. 19. J. M. Massie, A. F. Halasa, R. N. Thudium, and C. W. Burkhart, "Miscibility and Phase Behavior of Polyisoprene/polybutadiene and Polybutadiene/Polybutadiene Blends", ANTEC'92. 29 SLIP CARRIER I FILM IA Figure 21 Schematic of the tape casting process (Ref. 5). 'A0 0 RUN 2 L PM AIR FLOW 0 zW -.1 0(A U. 0 0 z0 P U 4 (z U. 0z (, Z- 4 2W Et 0 5 10 Is DRYING TIME Figure 22 20 25 30 MINUTES Two stage drying process (Ref. 5). 31 '.14ier,81detacnea 4* block I II Act.al . " -, r , secto. size , , Theoretcai section e LIQUID Figure 23 Schematic of the microton-iing process (Ref 6. spe Wm of ections Figure 24 Microtomed section adhering together in ribbon formation while floating on a liquid surface (Ref. 6). 32 H H I I -C C-C =-I H + 0 S 04 C- I0 0\ I H 0S 0/ _CC_ I I I H I H H I H I I I U\ /U Os 0/ \0 1 I H Figure 25 1 -C -C I H Osmimium tetroxide reacts with the carbon-carbon double bonds (Re 9. __T_ + H2N-NH2 Pc\ 0 0 DEC,\N H1-11N H2 O-CH3 C30H Figure 26 Hydrazine or hydroxylamine reacts with polymer containing acid and ester groups (Ref. 12). 33 Figure 2.7a Phase contrast of a stained cryosection through optical microscope showing dispersed phase particles in a matrix (Ref 14). Figure 2.7b Phase contrast through TEM of a stained cryosection. showing dispersed phase particles in a matrix (Ref. 14). 34 I 01. :i Figure 28 DSC traces of polyisoprene/polybutadiene and polybutadiene/polybutadiene two Tg's (Ref. 14). 35 immiscible blends exhibiting Type Features Size range Mastnification Macro-microstructures, color, homogeneity Spherulitic textures Phase variants, refractive index differences I cm - 02 gm IX-100OX I cm - 0.2gm I cm 0.2 gm 5OX-1200x 30%-1200x I cm -5 nm 100 g. 0 nm 0.1 mm 03 nm lOx-50000x lox-1000OX 3000x-5000000x 10 gm -Inm 300x-300000x Optical Bright field Polarized light phase contrast Conventional electron Scanning (SET) Backscatter Transmission STEM Tablell Surface topography Atomic number contrast Internal morphology, lamellar I Structures and crystallinity Internal morphology and I crystallinity Usting of microscopy techniques (ref 9) 36 CHAPTER 3 PLASTICIZER DISTRIBUTION WITHIN CERAMIC GREEN TAPES 3.1. Introduction Tape-casting is a well-known ceramic forming technology and has been widely applied to the production of thin ceramic sheets. The primary components ofthe ceramic sheets are polymer binder, organic plasticizer and ceramic powder. Blends of binder and plasticizer are often used to obtain desired physical properties. Low molecular weight diluents are often blended with polymers to increase flexibility by shifting the glass transition to a lower temperature. 'Me mixture, when blended, may be thermodynamically miscible, exhibiting a single phase morphology, or the mixture may be immiscible and exhibit a two phase morphology. Differential Scanning Calorimeter Ill and Nuclear Magnetic Resonance relaxation measurements [21 have been used to study the phase diagram of several blends which exhibited phase separation behavior. One related study 3 in this field concerned inhomogeneity and anisotropy of tape cast ceramic films for multilayer structures. A significant result of this study was that shrinkage and camber during annealing was higher in the tape casting direction than in the transverse direction. Another related study 4 was on binder thermolysis on the diffusivities of dialkyl phthalates (DAP) in plasticized binder. The results of this study demonstrated that the distribution of binder and the kinetics of the removal process were intimately coupled. The development of a penetrating porosity during the initial stages of binder removal as a result of capillary redistribution could reduce the path 37 length over which species must diffuse to a length scale on the order of the particle size. Mistler et al. [51 showed binder burnout TGA curves for thin sections cut from the top and bottom of the tape (Figure 3 ). They proposed that the difference between the curves was due to segregation during iring. They suggested that if segregation of the solids and organics took place as a result of settling, there would be large difference in binder content from top to bottom. Thus, they believed that the uniformity observed was a good measure of the degree of deflocculation, even during the drying state. No detailed study has been done on the inhomogeneity of composition across the green tape thickness. herefore, we have developed a method to measure the ratio of plasticizer to binder as a function of position in green tape. Diamond microtome sections, 07 [im thick, were taken parallel to the surface of the tape. Each section was analyzed using Fourier transform infrared microspetroscopy (FI'IR-M) to measure the absorption intensity of unique absorption bands for the plasticizer and binder. The plasticizer concentration as a function of depth from the tape surface was determined by measuring the absorption intensity of each band. The measurements are the first high resolution composition measurements performed on tape cast sheets. Composition inhomogeneity across the tape thickness is evidence of polymer plasticizer immiscibility for PVB-BBP systems. 3.2 Experimental Procedure The binder (PVB) used in this study has a molecular weight range of 90,000 to 120,000g/mol (B-76 6 The chemical structure of PVB is shown in Figure 32: The plasticizer (BBP) used in the study has the chemical structure shown in Figure 33. We chose 03 as the ceramic powder, because A'203 has no absorption peak in the wavenumber region under study. 38 A doctor blade was used to cast A203-PVB-BBP then dried. flow rate = tape, which was Two drying rates used in this experiment were feet/min), and = (linear air = 200 (linear air flow rate = 200 feet/min). The dried tape thickness varied from 130 trn to 150 tm. The composition of the green tape can be summarized in Table 3 . A piece of green sample was first epoxied with Epofix "' for eight hours. It was then polished to 02 mm in diameter for icrotoming A diamond knife was used to microtome the sample parallel to the surface of the tape from the top to the bottom (sample A). A second sample was taken from the tape immediately next to sample A and was n-ticrotomed bottom to top (sample B). The third sample was obtained 10 cm from the previous two and was also microtomed (sample thick. (Figure 34). Each thin slice was 07 tm hese thin slices were collected on round Pyrex silver mirrors and carefully recorded so that the measurements of each slide could be indexed with position in the tape. The sliced thin fUmswere examined at room temperature by MR 181 The silver mirror underneath the sample enhanced the signal to noise ratio of the reflected IR beam. he IR spectra of the films were collected in the reflectance mode for 30 scans at a resolution of 4 cm- The aperture was set to a circular area of 10 [tm in diameter. These conditions remained the same for the rest of the experiments. Plots of peak intensity vs. wavenumber were obtained. he relative band ratio of C=O (at wavenumber 1720 cm- I) to C-H band (at wavenumber 2870- 1) was measured. Plasticizer concentration as a function of position in green tape was plotted. Binder PVB-B-76 was replaced by B-79 which has a lower molecular weight (50,000 g/mol to 80,000 g.mol) 6]. According to the composition ratio as shown in Table 3 1, Green tape with B-79, BBP and 39 All-03 was cast. Several PVB-BBP films (without A1203) were cast at different polymer to plasticizer ratios on silver mirrors for calibration purposes. These thin films were first spin dried and then placed in vacuum at the room temperature to remove any remaining solvent. The final thickness of the PVB- BBP films was approximately gm. These films were also examined at room temperature by FTIR, and relative peak ratios of C=O/C-H were obtained. A calibration curve relating the peak ratio and the plasticizer concentration was plotted. Three PVB-BBP films (with 40 vol. % BBP, 35% vol. BBP and 30% vol. BBP) were also cast to thickness 80 m by using a doctor blade. These films were also microtomed to 07 gm thin slices and were examined by FITR40 vol. % PVB-BBP film was cross section microtomed to 07 thick. m he thin slices were collected on the copper grids and the samples were stained in s0,j vapor for half hour. plasticizer reacted with the S04 The ester functional group in the as shown in Figure 26. As a result, plasticizer density was increased and its contrast relative to the binder was enhanced. TEM micrographs were obtained in order to study the top to bottom microstructure variation of the sample. Dioctyl Phthalate, Dibutyl Phthalate and Dimethyl Phthalate with different solubility parameters (defined in section 33) were used to replace BBP in the green tapes. These green tapes were also microtomed and examined under TIR. Plots of C=O/C-H ratio as functions of tape depth were obtained. 3.3 Observations Figure 35 is an IR spectra representative of the PVB-BBP tape. The pertinent IR frequency range for those films is between 3000 to 1700 cm The following absorbence bands are observed in this range: CH3 at 2960 cm- 40 CH2 at 2870 cm- I ,and C=O at 1720 cm -I . The band at 1720 cm - I is present only in films which contain BBP since the ester content of PVB is quite small. The intensity of the 1720 m- I band is measured in the absorbency units and is linear with respect to the concentration of the plasticizer. The band at 2870 cm-1 has contributions from both plasticizer and binder since both contain C-H bonds. The relative band ratios of C=O (at wavenumber 1720 cm- I) to C-H band (at wavenumber 2870 cm- I) represents the ratio of plasticizer to binder. A calibration curve relating the plasticizer concentration and band ratio is shown in Figure 36. An IR spectra representative of the thin sliced A203-PVB-BBP shown in Figure 37. The relative band ratios of CO tape is (at wavenumber 1720 cm- 1) to C-H band (at wavenumber 2870 cm- I) were measured again. The band ratios were converted to the plasticizer concentration according to the calibration curve. Figure 38 a b ( = ) show a substantial plasticizer gradient giving evidence that the top surface of the tape was plasticizer rich. Figure 39 (S =200) displays a random plasticizer distribution pattern through the depth of the green tape. The data are reproducible from both "top to bottom rnicrotorning" and "bottom to top microtoming" from samples A and B. A larger degree of plasticizer distribution deviation was observed between samples A and C. Plasticizer-rich regions (55 vol. %) and plasticizer - poor regions 32 vol. %) were observed in all cases. Figure 3 10 displays the plasticizer composition profile for binder PVB- B-79 under the slower drying rate ( = ). Plasticizer gradient with the same upper and lower boundaries was observed in the sample. Figures 311 tapes without A203 concentration. 312 show plasticizer composition profile of PVB-BBP powder at 40% and 35% by volume plasticizer Plasticizer gradient with the upper (55 vol.%) and lower 41 32 vol.%) boundaries was also observed in these samples. In the 35% sample, the plasticizer richregion was smaller than in the 40% sample. Figure 313 shows the plasticizer composition profile of PVB-BBP tape with 30% by volume plasticizer concentration. There is no plasticizer composition gradient across the tape. The correct plasticizer composition level was also verified. Figure 314 is a TEM micrograph of the cross section of PVB-BBP film with 40% by volume plasticizer concentration. It shows larger plasticizer domains at the top of the tape; and fine grained plasticizer at the bottom of the tape. The dimensions of the domains varied from 0.8 m to 0 I gm. Table 32 lists 8's for four plasticizers used in this experiment. Figures 3.15a, b, c and d are the plots of C=O/C-H ratio as functions of tape depth for Dioctyl Phthalate, BBP, Dibutyl Phthalate and Dimethyl Phthalate green tapes respectively. Plasticizer gradient is also observed in these samples. As shown in Figure 316, a larger plasticizer solubility parameter leads to a smaller difference in C=O/C-H ratios. 3.4 Discussion One explanation for the plasticizer gradient is based on two component diffusion and convection during drying 3 by evaporation. The solvent leaves the top surface The plasticizer flux through the top surface is negligible since its vapor pressure is so low. The molar flux of plasticizer at any point, z, in the tape can be written i = XP (Js + J) -cDP ayaz) where Equation 31 p and J, are the molar flux of the plasticizer solvent, respectively, P and DP are the mole fraction and diffusivity of the plasticizer, and c is the molar concentration of solvent and plasticizer. 'Me second term on the right represents simple diffusion of the plasticizer toward regions of lower concentration. The first term on the right, however, represents the convective 42 flux toward the surface of the tape which always has positive contribution of solvent flux toward the surface. It is clear from Equation 32 that the solvent flux convectively carries the plasticizer to the surface where diffusion must carry the plasticizer back in to the interior of the tape. The diffusion is unable to overcome the relatively rapid convective flux of solvent because Lewis et al. 141 have measured plasticizer diffusivities in polymers and shown them to be rather small. Thus, the concentration of plasticizer must be highest at the drying surface. Due to the convective flow of solvent model, the concentration profile should arise such as a higher drying rate leads to higher plasticizer concentration at the drying surface. However, Figure 39 (S =200) displays a random plasticizer distribution pattern through the depth of the green tape. This observation does not fit the convective flow of solvent model. The concentration profiles shown in Figures 38-3.13 can thus only be explained by te existence of an immiscibility gap in the BBP-PVB phase diagram. The 55 vol. % and 32 vol. % regions are the upper and lower phase boundaries, respectively. Other plasticizer-polymer systems are known to have a maximum solubility of plasticizer. Studies of Poly (vinyl chloride) polymer /di-isodecylphthalate (plasticizer) blends with nuclear magnetic resonance (NMR) techniques show that plasticizer molecules can be m icroscopically distributed into regions of either high plasticizer concentration or high polymer concentration units [21 . This technique was based on two characteristics of NMR: the short-range nature of the dipole-dipole interaction and the transport of spin energy. The manufacturer [61 of PVB suggests that the maximum recommended BBP content is 40% by volume. The BBP-PVB-solvent solution is of course, initially single phase. Immiscibility must occur at some point during drying. The situation is depicted 43 in Figure 317 where the immiscibility gap extends to an unknown concentration of solvent. Coarsening could be used to explain the variation in second phase size across the tape thickness observed in Figure 314. Its driving force is the lower surface energy of the large plasticizer domains compared with the high surface energy of smaller plasticizer domains. 91The particle size grows at the expense of smaller second phase regions. The top surface of the tape dries earlier than the bottom surface. Therefore, phase separation occurs first at the top and the plasticizers there has more time to coarsen. As a result, fine-grained plasticizers increase in average size earlier at the top of the tape and the plasticizer domain there becomes larger. The second explanation for Figure 3.14 is that larger plasticizer particles with lower density raise to the top under buoyancy force (Figure 3.18a). Under faster drying condition (S=200), the time difference in phase separation between the top and bottom of the tape is minimized. Therefore, the morphology of the second phase under the fast drying rate is depicted in Figure 3.18b. The explanations above fit our observation such as under slower drying rate (S=O), the top surface of the green tape was plasticizer rich (Figures 38); and under faster drying rate, plasticizer-rich regions distributed more or less uniformly throughout the tape (Figure 39). The solubility parameter = AE/V is defined as: Equation 32 12 where AE is the energy of vaporization to a gas at zero pressure, and V is the molar volume. The dimensions of are (cal/cM3)1/2. The relationship between 8 and phase separation (Figure 316) can be explained by using the theories of thermodynamics of polymer solutions. 'Me n-fixingof plasticizer and binder can be understood as the interaction between the solvent molecules and 44 polymer monomers. The process of dissolving a polymer in a solvent is governed by the free energy equation: AF =A H -T AS Equation 33 where AF = the change in Gibb's free energy, AH = the heat of mixing, T = the absolute temperature and AS = the entropy of ixing. A negative AF predicts that a process wl occur spontaneously. Since the dissolution of a polymer is always connected with a larger increase in entropy, the magnitude of the heat term AH is the deciding factor in determining the sign of the free energy change. Hilebrand and Scott 101proposed that AH = where V M151-5212 VIV, Equation 34 = total volume of the mixture; 1 and 8 are the solubility parameter of the solvent and polymer; V, and V2 are the volume fraction of solvent and polymer in the mixture. It may thus be seen that the unit heat of mixing of two substances is dependent on [51-8,)]2. If the heat of mixing is not so large as to prevent mixing, then [81-82]2 has to be relatively small. In fact, f 5 1-52]2 = , solution is assured by the entropy factor. This is mathematically equivalent to saying that if the values of two substances are nearly equal, the substances will be miscible. We were not able to calculate the solubility parameter for PVB monomer. However, the observation in Figure 316 shows that (3PVB i larger than of the plasticizer. As a result, least phase separation was observed in the Dimethyl Phthalate (with the largest 5) green tape. The correlation again supports the idea that phase separation is occurring in the green tape. 3.5 Conclusion: Inhomogeneous composition across the tape thickness was studied in this chapter. For the first time, green tapes are found to be plasticizer-rich near the top surface under slow drying conditions. 45 Plasticizer-rich regions are distributed more uniformly across the tape thickness at faster drying rates. Molecular weight of the polymer did not affect the iscibility of binder and plasticizer. This type of phase separation was also observed in other Phthalate plasticizers. We have discovered that solubility limits correlated solubility parameter for the plasticizer. A larger plasticizer degree of phase separation. 46 with the leads to a smaller References: 1. J. Massie, A. Halasa, R. Thudium, and C Burkhart, "Miscibility and Phase Behavior of Polyisoprene/Polybutadiene and Polybutadiene/Polybutadiene Blends", Antec 92. 2. V. J. McBrierty, "Heterogeneity in Polymers as Studied by Nuclear Magnetic Resonance", Faraday Discussions of the Chemical Society, No. 68, 1979. 3. M.J. Cima, Y. Tang and M Liu, "Inhomogeneity and Anisotropy of Tape Cast Ceramic films for Multilayer structures", Massachusetts Institute of Technology. 4 J.A. Lewis and M.J. Cima, "Diffusivities of Dialkyl Phthalates in Plasticized Poly(vinyl butyral) Impact on Binder Thermolysis" J. Am. Ceram. Soc.,73 9] 2702-2707 1990). 5 R. E. Mistler, D. J. Shanefield, and R. B. Runk, "Tape Casting of Ceramics"; pp.411-48 in Ceramic Processing Before Firing. Edited by G. Y. Onoda and L. L. Hench. Wiley, New York, 1978. 6 Monsanto Plastics, St. Louis, MO. 7 Struers Company, Pennsylvania. 8 IBM System 9000. 9 Kingery, Bowen and Uhlmann, "Introduction to Ceramics", Wiley Interscience, P 448-P 515 10 J. Hildebrand, R. Scott, "The solubility of Non-electrolytes", Reinhold Publishing Crop., N. Y., 1949. 47 3rd ed., 100 - %%1307 TOM '<Il x 90 I (D w 3: so I--- 0 100 ---- I - W-- 200 TEMPERATURE, Figure 3.1 I I 1 300 400 50C C Binder burnout TGA curves for thin sections cut from the top and button of the tape (ref. 5). 48 H -Cll ( H -C C 0 0 C H Figure 32 CA The chemical structure of PVB. -O(CH)3CI-I3 -- OCHi-- 0 Figure 33 The chemical structure of BBP. 49 tb .E C p U .E 49 0 u 1) V cn .2Cl. r:s cn Cri 2 -p 8 0 50 -:t C2 C) a 11 z x x i 5 Tr, N cic w CD m i C2 zw > It W m < am at I I I x I 11 I 9C2 f C3 ts C; 1: CNI C; 0 a 33NMIOSSY 51 a 0 C3, I -1 1- Cl) "I X:" m "I 0 U. 0. 0 11-10. 0 m 6. 4- a) Q 0. 0 U (I.) .N 0. 5 C4 Ct n- 0. 0 0.2 0.4 0.6 0.8 1 1.2 1.4 1.6 1.8 The Ratio of C=O to C-H peaks Figure 36 Calibration curve relating plasticizer concentration and band ratio. 2 di V 0. m Q C3 C3 4 k 0ll ,L)041 VI I .Z 5 4) ll E ea 1= ud.) Q CD FM 5 -* TS I P 4 bi t- rn a 1. I E u CY w R A I ga ai 1: 9 K :D zw iSt V,.IC, - - - - WI9 mZ; at: IL -C 1% r_ 4) C bf j 1- a ICI A0 CD C) C2 FC.) X: -i F- (1) X: (14 1-1 (1) ile 3ONYSaOSSV X " U X: W C) Cl w 11 U.(/-I I- - -70 - 10 C .0.5 C 0.4- 6- 6a IL -... v2 a a -I K NO a a I-W x PIN > I.-I 3 - Al It.,R.. E a I -WW a := 0. 'IN U U - 0 (j $-. U N 0.2 'U CA 0. .2 1 C-, I . 0- I ' ' . 20 0 t I i . I A-i . . . i , I I-T - I $ i . I I I I 40 60 80 100 IZO 140 160 Tape Depth (Micron) _ U-07I I.-I I 0 C-' --0; 0. 5 5 0.4- or n U Q 0.3- 0 C.) U. 0.2 (U I .N Top to Bottom Cutting, Point A. a Cn*0.1- I- Bottom to Top Cutting, Point C. E: 0- iI 0 . I I 20 i . 40 I I I 60 I I I I I I I I - 80 100 120 140 160 Tape Depth (Micron) Figure 3.8a Plasticizer composition profile for = feet/min, with A1203- Figure 3.8b Plasticizer composition profile for A120354 = feet/min, with 0. U 7 -7750, 0.5- :M ::X PI I a 0.4- a a7- 5 U U Z ._%WMQL-7-: ZKKAOU Wn- t) Q 0.3- a WE 0.2, a RON - Top to Bottom Cuttin2, Point A. :: Bottom to Top Cutting, Point CI 2a. 0.1 Cn I - 7 .111,7 1- 7 - 1 0 0 20 40 60 80 i''.1, i I 100 120 140 160 Tam Devth (Micron) 0 0 m0 2- L IF so as or 0 CZ Ce.i.5 - 0 0 a m 0 1- a 11 U 0.50- I I I 0 20 Figure 39 I I 0 ME a a m 80% lipa a 0 NJ a -I i A-, -, v ft I I on OmIn no a I I I I I 40 60 100 120 140 160 Tape Depth (Micron) Plasticizer composition profile for = 200 feet/ min, with A1203- Figure 3 0 Plasticizer composition profile for green tape with binder 79. 55 Ml I- C Ca a) Q a U 6(U ZN C4 .E2 a-, 20 0 40 60 80 100 i2O Tape Depth (Micron) 01 -a C 0 U- C 0U 0 U U. 0 .5N -O.W M C- 0 Figure 311 10 20 30 40 50 60 70 80 90 100 Tape Depth (Micron) Plasticizer composition profile for 40% (Vol.) BBP, without A1203- Figure 312 Plasticizer composition profile for 35% (Vol.) BBP, without Al')0356 0.5" 0. - ON "0 0.45- I-I - I- 3 0.4Z 55 U - 0. 3 5 0 - - L-M e no a.) N - .5 Cn CZ - 0.25- n- x 0 no 5 0 0 N M 0 0 0 N 0 a a - - 0.2 I . 0 Figure 313 I 10 I I I I 20 T Ii , 30 40 I I I I I I I I 50 60 I I I 70 .1111.1.1 8 90 I 100 Plasticizer composition profile for 30% (Vol.) BBP, without Al')03- 57 0 I 0 t% P. 2 E .1 's 0 '*O I eni L 91 P. 9 d Ak A A t; 9 11 114 58 -7 w &O v 1. 0 -I h am 0 0 1.6 - 0 0 Ip so Now O a N on a on 1.4no & a CZ CIO-, 1.2- a so I a 0 O 0.811 U 0.6. lisp no N 0.4- a WAS 0.27 0 I 0-. 0 I ' -- 20 i -1 I -' -'-F-7- i d I M i , I - a - P I I I 120 140 160 40 60 Tape Depth (Micron) 100 2.5 2- 0 I-on OR mm oil L a a 0 0 v a a 0 0 4k % N N W% v E ae. 1.5- a a II N a 0 0 0 1 0 0 on 11 N wI 160 U an 0 mmAN . fto 0.5. . . I. .- 0 20 I I 40 60 I I I I I I. .. .I I . 80 10 120 Tape Depth (Micron) . . I . 140 I 160 Figure 3.15a Plasticizer composition profile for Dioctyl Phthalate. Figure 3.15b Plasticizer composition profile for BBP. 9 II 0.9 E 0 0.87 0E 0 0 0.7- - 50 ON so 0 P CF" 06 a onU - 2 a U son a C 0.57 0 0 a a N C: 04 E 0a 11 II noun 41 IN U 0.37 of &Mal 0 v OMEN 0.20.1 I I D I I 20 I I I I I I 40 I I 60 80 I I I 100 120 Tape Depth (Micron) 0 CZ 01-1 C 11 U 0 10 20 30 40 50 60 70 80 90 IC9 Tape Depth (Micron) Figure 3.15c Plasticizer composition profile for Dibutyl Phthalate. Figure 3.15d Plasticizer composition profile for Dimethyl Phthalate. 60 1.1 Q Q I., 12 L . IU a0 M 0. C-, *1 0. U Ii U 0. 0. 0.02 0025 003 0035 004 0.0,15 005 Plasticizer Solubility Parameter Figure 316 Phase separation as a function of . 61 Cj z Cfu . F Ei-. rl e 11-1 4 p a E O 4- 0 C W u aE (1) 0 C.( q d 914 CLO 0 8 pq pq 10 . V) 4-4 0 .40 0rN tR e Q 0144, AO tR a0 1-0 C: 0 I= a 0 A4 94 P 7; Q P ,4-.4zft e%) tT n 62 ,I= u "-o T* la 0 "-4 "a ...I. /5 j .1 0 n I .9 ba vW2 0 V4 *.b CA M U co F--o 04 cn 0 m t"4) ;g q4 ,= n 4-6 Om$ W W-4 I 9 I a at co 1-4 0114 9LO (Vi L X 63 i A ITZ i i 4u . 6 (1) v M _-4-.4 iI ;1.0 \\", ,4 9L4 914 10 (1) ... P--o "-O el 5 C14 A 93 A 1= .9 W 0 Q A *.b 116.4 = Cn V.-.4 CZ >. a) =4 .,.4 . .o n ,0-4 -4 0= CL-4 4 9 la 0 am 4) U *n .1 op" rA I 2 co el-; L X 64 Binder Plasticizer Powder Dispersant, Solvent PVB BBP . A120 Fish Oil Toluene Poly vinyl butyral) Benzyl Butyl Phthalate Particle size: 02-0.5 Ethyl Alcohol Table 31 7.110 g 4.309mi 43.12g 0.04g 5 ml I 11MI The composition of green tape for plasticizer gradient measurement. Plasticizer Solubility Parameter (cal/cc)112 DiocIvI Phthalate 0.021 Butyl Benzyl Phthalate Dibutyl Phthalate Dimethvi Phthalate 0.027 0.032 0.049 Table 32 Solubility parameters for plasticizers used in this experiment 65 CHAPTER 4 DRYING AND DENSITY MEASUREMENTS 4.1 Introduction Ceramic green tape is generally considered a composite of ceramic powder, polymer and plasticizer. In reality, however, gas filled pores exist in the green tape. The production of multi-layer ceramic devices requires specific structural properties of the green tapes, such as no density gradient. To achieve such an uniformity, the optimization of slurry composition is crucial. Fiori and DePortu III suggested some rules for the preparation of a tape-casting slurry. These rules are: (1) the plasticizer to binder ratio must be less than 2 2) the amount of dispersant must be chosen from within the range where the adsorption is constant, 3 the ratio between organic components and ceramic powder must be as low as possible, and 4) the amount of solvent must be fixed at the minimum to ensure a good dissolution of organic components and a good homogenization for the slurry. An optimized slurry leads to tapes which satisfy the following criteria: no cracking during drying, a good cohesion to allow the manipulation of the dried sheets, and good microstructure. The drying behaviors of aluminum nitride tape-cast sheets was recently studied. 2 A high apparent density, a low volume of pores, and a high resistance to cracking require a low ratio of binder to plasticizer. The purpose of the current study is to find an optimal way to process the green tape, so that dewetting and pores could be controlled. The study emphasized on the relationship between the ratio of binder to plasticizer and the green tape density. Air flow rates, drying time and tape dewetting were also examined. 66 4.2 Experimental Procedures 'Me composition summarized in Table 4 . of the slurry used in this experiment A203-PMMA-BBP drying. The "glass rod table" (Figure 4 ) can be slurry was cast followed by E31 was piece of microscope glass served as the substrate. used for tape casting. A The thickness of the tape could be adjusted by the height between the top and bottom plates with the height adjustment screws. For more accuracy, a tape caster was then used for tape casting. A six by eight inch glass plate was the substrate. Samples A, , and C were produced by using these two techniques. Immediately after casting, the wet tape was placed under a microscope, so that its thickness was measured through the drying. We chose the uniform central square region for this thickness measurement, due to the small deviation in thickness (5 trn difference across the square region). At the same time, a balance was used to weigh the tape during its drying (Figure 42 "" As a result, the intermediate densities of the tape, its thickness reduction and its pore volume fraction were calculated from its final density, thickness and weight during drying. This whole setting was enclosed in a Polyethylene bag, where N, flew through. We have developed three methods to measure the final green tape densities. (1 a piece the center region. measured. Of _CM2 square shaped green tape sample was cut from Its thickness (with a micrometer) and its weight were 2 to obtain a more accurate area measurement, this piece of green tape sample was photographed and then its print was enlarged. We calculated the area of the green tape sample using the enlargement ratio and the area of the larger print. 3 Mercury porosimeter tape densities. 67 [51 was also used to verify the green The theoretical density of the green tape was calculated assuming that there was no porosity remaining: Densitymeoy= WtPwdr + WtBinder + WtP1atiied/(VPowder + Vp1asticized + VBinder Equation 41 Three different drying rates 6 S =0 feet/niin; S =100 feet/min; and =200 feet/min) were used for sample C. Its final densities under these rates were also measured. 4.3 Observations Figure 43 is a representative plot of green tape weight loss during its drying for sample B. 80% of the solvent weight loss occurred during the first quarter of the drying time. The last 7% of the solvent was removed during the second half of the drying time. Figure 44 is a schematic of the wet tape on the substrate 10 icroscopic glass inutes after casting. Dewetting occurred when 50% of the solvent was removed. Figure 45 is a plot of thickness reduction vs. weight loss for samples B and C. 'Me initial sample thickness was 500 tm. Thickness reduction was larger for sample B than that of sample C for the same amount of solvent weight loss. The data were reliable due to the high degree of reproducibility. Table 42 includes the density measurements of PVB-BBP tape with 35% (vol.) and 30% (vol.) plasticizer concentration. There is 06% difference in their experimental densities. Table 43 summarizes the final densities and drying times for these three samples. was the shortest. Sample C contained the most plasticizer, and its drying time The density trend was consistent with Figure 45 such that the higher the polymer concentration of the sample, the less porosity it had. The density difference between sample C and sample A is 66%. 68 Table 44 summarizes the density and dewetting results for sample C. The three density measurement techniques provided a consistent trend such that a faster drying rate resulted in less density in the final green tape. Under = 200 feet/min, final green tape density was 8% less than that under stagnant air. On the other hand, the faster the drying rate, the more dewetting occurred in the green tape. Figure 46 is a plot of tape densities during its drying. The densities of sample matched well with the theory. The densities of sample C started to deviate from the theory when 30% (weight) of the solvent remained. The results were reproducible, for three repeated experiments. As 30% of the solvent remained in the tape, the volume fraction of the organic component was 72.4%; the composition of the organic phase (by weight) was: PMMA 23.5%, BBP =21.5% and solvent = 55%. Figure 47 shows the pore volume fraction as a function of the solvent remaining for sample C. Again, when 30% of the solvent remained, porosity increased to 22% by volume and stayed at this level through drying. Dewetting still occurred at the edge of the tape as we switched to use the tape caster. However, the area of the glass plate was big enough so that the central square region was assumed unaffected. Figure 48 summarizes the in situ density measurements for samples A, and C. This again demonstrated that sample A correlated the most with the theory; sample C deviated most from the theory. Figure 49 summarizes three repeated in situ density measurements for sample C. The reproducibility also verified the above result. 4.4 Discussion Dewetting occurred 10 minutes after casting. This is an indication of the slurry particles' movement during the early stage of the drying. After 50% of the solvent is removed, slurry particles are stationed, due to their high 69 viscosity. Because of the small area of the microscope glass, the central square region could be affected by such a particle movement. As a result, the sample thickness and weight data are only considered after the removal of 50% of the solvent. High surface tension is evidence of dewetting. The surface energy of the substrate must be greater than the sum of the surface tension of the slurry and the substrate for a slurry to spread 9 If the surface tension of the slurry was too high, the slurry would not spread evenly. A high drying rate caused an inhornogeneity in solvent distribution, which resulted surface tension gradient across the tape length. The retraction of the tape (or dewetting) occurred, when it experienced such a surface tension gradient in different directions. The observations in Figures 45-4.9 indicate that a low polymer to plasticizer ratio resulted in low green tape density. Such a reduction in green tape density 66%) is not caused by the addition of plasticizer (Density reduced 06% for 5% vol. plasticizer concentration increase.). The effect of plasticizer on binder was the cause for the decrease in density. molecular-weight The lower- organic specie plasticizer was an additive that intimately mixed with the binder as a single material. The plasticizer disrupted the close aligning and bonding of the binder molecules, thereby increasing the flexibility of the material. The plasticizer tended to reduce the strength of binder while softening it 7]. Figure 410 shows the variation of the tensile strength of an Alumina green sheet as a function of plasticizer concentration, which proves that the plasticizer additions significantly decreased the tape strength. The vaporization of the solvent left vacancies inside the slurry. Ceramic particles which embedded in the binder matrix, rearranged themselves to fffl up these vacancies. A binder matrix with excessive plasticizer (sample Q could not provide the particles enough strength. As a result, the particle 70 rearrangement was slower than the solvent vaporization and porosity were created. Under a faster drying rate, the particle rearrangement was slow enough, so that porosity could be created as well. 4.5. Conclusion We have made the first in situ density measurements of a drying tape. The drying behavior and densities of tapes with three different polymer to plasticizer ratios were studied. Green tapes with different drying rates were also examined. 80% of the solvent weight loss occurred during the first quarter of the drying time. The "glass rod table" casting method was not an effective way for tape casting, because the microscope glass area was small enough so that dewetting at the tape edge affected the central region. Figures 45 to 49 indicated that the higher polymer to plasticizer ratio resulted in high green tape density. 0.02) Green tape A PMMA:BBP = 18:1) had a final density = 2.57 ± g/CM3, which was almost consistent with the theory 2.56 g/CM3). Its densities during drying also demonstrated the most correlation with the theory. Green tape C (PMMA :BBP = 11) deviated most from the theory, with 22% by volume porosity remaining as it dried. Its final density was 2.40 ± 002) g/CM3. For the same initial thickness, the drying time for sample C was 16% less than that of sample A. Dewetting and low final density occurred when a tape was dried under the faster drying rate (S = 200 feet/min). 71 References 1. C. Fiori and G. DePortu, Br. Ceram. Proc. 38 1986) 213. 2. E. Streicher, T. Chartier, "Study of cracking and microstructural. evolution during drying of tape-cast aluminum nitride sheets", Chapman and Hall Ltd. 1991. 3. Manufactured and drawn by Bill Rowe. 4. Drawn by Sam Gido. 5. Micromeritics Autopore 9220. 6. Tape caster at BP Research. 7. G. Y. Onoda, Jr., "The Rheology of Organic Binder Solutions" in Ceramic Processing Before Firing, Wiley, New York. 8. R. Moreno, "The Role of Slip Additives in Tape Casting Technology, Part 2-Binders and Plasticizers", Ceramic Bulletin, Vol. 71, No. I , Nov. 1992. 9. "Avoid Coating and Drying Defects", Chemical Engineering Progress, Jan. 1993. 72 ht atment ";,V's T-T-1. Adjustment Screws Figure 41 'Me "glass rod table" for tape casting (ref 3. 73 Camera Optical 1croscope Balance Figure 42 A schematic of in situ density measurement (ref 4. 74 0.) C. E 'rCl. LI-C 0 tb -E 22 l lc in t1i 0Slo r- c .2 W. IRT c c .r_ c-4 E W c E v u zEa ICZ c00 c?, c\C .p 01.) t-b z C-1 u ZZ: C ,,I-t, cu V. IU Cl. u c SSO-1 IPZA 75 te '5 9 17 -S r tr r.4 U .5:- 5 4 2 C u E t d tri I d d V. < "IT u EL 76 u 1= I'll, ,C d" 0 4U Irl 0 P4 U) EA 4) AC-) 10 20 30 40 50 Weight "ss Figure 45 60 70 (%) A plot of thickness reduction vs. weight loss for samples and C. 77 80 90 100 -3 T530jurT-3)8pmPMNMIJBP--l.5:1 i f 2.8 - T =529pa Tf--3W pm P MMA.BB P--1.51 T;=53'7pmT 2.6-1 209pmPMMA:BBP--131 T530 pm Tf=;248pm PMMA:BBP=1I 2.4- T.=533pmTf7-239pm P"M:BBP-11 It I--, M E _6 2.2- *0 0 T,=535pmTf=;241pm PMNIA.BBP=lI b i-- .;; z0 -'nwcre&:a1 Densiy U 2- - v0. 1.8M 5, U C) "M 0 0 C3qm a i:D - 0 1.6Z 1")C A, *A L F.] C-Z., 1.41.2I 1 0 Figure 4.6 I . I I 0.1 . . . . I , . . I I I I . I . I 0.2 0.3 0.4 Wt Fraction of Solvent Remaining In situ density measurement for samples B and C from the "glass rod table". 78 I 0.5 I 0.3N T(Mpm"r=346pm I f * T530 pm T =244pm I It 0.25- 0 0 0 ILA IA IA A, A = ky ...- A A ) A A 0 4Lft won I =420 pm T =205 pm I f 0 0 0 A I f 0 * An *A 0 of MA A A, 0 U CIj 4 U g 0.15-6 A a M a 0 U 0 C" A, A t I 0 0.05- I .1 7 0 Figure 47 . I . I I . 0.1 I I --A . I I - - - - I , 0.2 0.3 0.4 Wt Fraction of Solvent Remaining - - - I 0. 5 Pore volume fraction as a function of solvent remaining for sample C. IPMMA:BBP=I:l 9 0 PMMA:BBP =1.5:1 4 2.5 - A PMMA:BBP =.8:1 A $b A ----Theoretical Density i A V M a rn A a a A A 0 -u :h 1. a we - A A 0 A a - A --,- A.. e M K It A, n p 0 u r') I -. A,... 8 II It - 0. - 0I- 0 RQUre 48 I 0.1 I 0.2 I , -1 - - - I- 1 , , 1 7 ; ; , I I 0.5 0.6 07 0.8 0.3 0.4 Wt Fraction of Solvent Remaining In situ density measurements for samples A, 80 ; 1 0.9 I I and C from the tape caster. I N PMMA:BBP=I:ITrv I * PMMA:BBP=I:ITry2 2.5 j A * .- Theoretical Density --- .4 " n% 'nI- PMMA:BBP=I:ITry3 n-- A A IA r 0 -b - It A, WE Hn 1. - 11 V) N 0 A V I 0 A I 0 0 0 Figure 49 V I 0.1 0.2 I I I I I : - -T- ----- i I ; I 0.3 0.4 0.5 0.6 0.7 0.8 Wt Fraction of Solvent Remaining In situ density measurements for sample C. 81 A 1 0.9 3 15 T i 3 a. M G cm a 10 2 5D 'A I? 6 7 .2 G 'D 5 1 1 I 1.0 Figure 410 I 1.4 1.6 Dibutyl phthalate YA/6) The variation of the tensile strength of an Alumina green sheet as a function of plasticizer concentration. (ref. 8) 82 Binder: PNINIA Polv(methvi methacrviate) Plasticizer: BBP BenzvIButvlPhthalate Powder: A1101 (Particle size: 02-0.5 um) Dispersant: Fish Oil Solvent: Toluene Solvent:powder:oryanic = 6.8: 1 I ol.) - PNINIA:BBP = 1.8:1 (ol.) Sample A PNINIA:BBP = 1.5:1 (ol.) Sample I PNIMA:BBP = 1 I (Vol.) Table 41 Composition BBP Concentration 30% ,Sample C of green tape for insitu density measurements. (Vol.%) Theoretical Density (g,,/cm3) 1.168 Experimental Density igcm 3) 1.150 ± 0029 1.163 1.143 ± 0032 35% Table 42 - PVB-BBP tape densities. 83 - - -- Binder Plasticizer Vol.) 1.8: 1.5:1 1:1 Table 43 2.54 2.40 = = 100 = 0 DrvinL Time iNfin.) 200 0.03 1.02 180 169 Final densities and dyingy times for samples A. Drying Rate (Linear Air Velocitv: ft/min) Table 44 Green TaDe Densitv ivcm3) 2.57 0.02 and C. Gren Tape Densitv Pcm3) Square 2-55 009 2.41 ± 0.08 2.39 ± 003 Dewetting 2.47 2.45 2.44 Hg 0.03 0.02 0.04 Photo 2.53 ± 003 2.42 ± 002 2.33 ± 003 No Yes (slightly) Yes (severeiv) A summary of density and dewetting for sample C under different drying rates. 84 CHAPTER CONCLUSION AND FUTURE WORK 5.1 Major Conclusions This project investigated the relationship between processing and microstructure of tape cast green sheets. Plasticizer distribution across the tape thickness was measured. The drying behavior and densities of tapes with three different polymer to plasticizer ratios were studied. Green tapes with different drying rates were also examined. The major conclusions and accomplishments of this research are summarized below. 1. We have made the first in situ density measurements of the drying tape. During drying, a microscope was used to measure the tape thickness; and a balance was used to record its weight. As a result, the inten-nediate densities of the tape, its thickness reduction and its pore volume fraction were calculated from its final density, thickness and weight during drying. We found that the higher the polymer to plasticizer ratio, the less porosity existed in the dried tape. Green tape A PMMA:BBP = 18:1) had a final density = 257 ± 0.02) g/CM3, which was almost consistent with the theory 256 g/CM3) ts densities during drying also demonstrated the most correlation with the theory. Green tape C (PMMA :BBP = 11) deviated most from the theory, with 22% by volume porosity remaining as it dried. Its final density was 240 ± 002) g/CM3. Dewetting and low final density occurred when a tape was dried under a faster drying rate (S = 200 feet/min). 2. We developed method for measuring composition gradients on the 0.7 gm scale, this has never be done before for green tapes. Each microtomed thin section was analyzed using FrIR-M to measure the absorption intensity of unique absorption bands for the plasticizer and binder. 85 The plasticizer concentration as a function of depth from the tape surface was determined by measuring the absorption intensity of each band. 3. We found evidence for the first time for phase separation in PVB- BBP system. Green tapes were found to be plasticizer-rich near the top surface under slow drying conditions. Plasticizer-rich regions were distributed more uniformly across the tape thickness at faster drying rates. The upper boundary was 55% by volume plasticizer concentration, and the lower boundary was 32% by volume plasticizer concentration. 4. Phase separation occurs when other plasticizers are used such as Dioctyl Phthalate, Dibutyl Phthalate and Dirnethyl Phthalate. We also demonstrated for the first time that the solubility lmits between the binder and plasticizer correlated with the solubility parameter for the plasticizer. Plasticizer with larger solubility parameter resulted smaller degree of phase separation in the PVB green tape. 5.2 Industrial.Applications The plasticizer and polymer phase separation phenomena has not been reported to our knowledge in the context of tape-casting formulations. The implications for devising tape casting formulations are that truly homogeneous tapes can not be formed if the binder phase actually is composed of two phases. This situation is particularly serious if the phases completely separate. In this case, the top of the tape differs significantly in composition and physical properties from the bottom. manufacture he resulting anisotropy considerably complicates of multilayer devices. To inimize the above problem, we suggest a plasticizer that is more soluble with the binder. In other words, the plasticizer should have a solubility parameter closest to that of the binder. For 86 example, in the PVB binder system, Dimethyl Phthalate is more recommended than BBP. To reduce the porosity in the green tape, moderate high polymer to plasticizer ratio and slow drying rate are recommend. For example, in the PMMA-BBP system, the binder to plasticizer ratio should not be lower than 1.5 :1 by volume. 5.3 Suggestions for future work 1. More details of the ternary phase diagram of the solvent-plasticizerbinder system should be investigated. 2. The solubility parameter of PVB monomer should be estimated. 3. Other techniques such as NMR should be used to verify the plasticizer-binder phase separation. 87 Bibliography 1. R. Mistler, "Tape Casting: The Basic Process for Meeting the Needs of the Electronic Industry", Ceramic Bulletin, Vol. 69, NO. 6 1990. 2. Y. Y. Tang, Ph.D. Thesis, Department of Materials Science and Engineering, Massachusetts Institute of Technology, 1994. 3. M. J. Cima, Y. Y. Tang and M. Liu, "Inhomogeneity and anisotropy of 4. Tape Cast Ceramic Films For Multilayer Structures", Ceramics Processing Laboratory, Massachusetts Institute of Technology, 1993. Roosen, "Basic Requirement for Tape Casting of Ceramic Powders", PP. 675-92 in Ceramic Transactions, Vol. 1, Ceramic Powder Science I I, B/ Edited by G. L. Messing, E. R. Fuller and H. Hausner. American Ceramic Society, Westerville, OH, 1988. 5. T. Ueyama and N. Kaneko, "Effect of Agglomerated Particles on Properties of Ceramic Green Sheets", pp. 1451-58 in High Tech Ceramics. Edited by P. Vincenzini. Elsevier, Amsterdam, Netherlands, 6. 1987. E. Hyatt, "Making Thin, Flat Ceramics-A Review", Ceramic Bulletin, 7. Vol. 65 NO. 41986. M. F. Yan, "Microstructural Control in the Processing of Electronic Ceramics," Mater. Sci. Eng., 48, 53-72 1981). 5. A. Roosen, F. Hessel, H. Fischer, F. Aldinger, "Interaction of 6. Polyvinylbutyral with Alumina", Ceramic Powder Science, 1990. J. S. Chong, E. B. Christiansen, and A. D. Baer, "Rheology of Concentrated Suspensions," Journal of Concentrated Suspensions, 7. 8. 9. 15,2007-2021, 1971). E. S. Tormey, R. L. Pober, H. K. Bowen, and P. D. Calvert, "Tape Casting--Future Development," pp 140-49 in Advances in Ceramics, Vol. 9 Forming of Ceramics, Edited by J. A. Mangels, and D. L. Messing, American Ceramic Society, Columbus, Ohio, 1984. Y. T. Cou, Y.T. Ko, and M. F. Yan, "Fluid Flow Model for Ceramic Tape Casting", J. Am. Ceram. Soc., 70 101,C-280-C-282,1987. R. Mistler, D. Shanefield, and R. Runk, "Tape Casting of Ceramics," pp 411418, in Ceramic Processing Before Firing. Edited by G. Y. Onoda and L. L. Hench. Wiley, New York, 1978. 10. 11. 12. 13. A. Karas, T. Kumagai, and R. Cannon, "Casting Behavior and Tensile Strength of Cast BaTi4O3 Tape", Advanced Ceramic materials, 34] 374-77 1988). Reid, Utramicrotomy", pp 217-223,Wiley, New York, 1980. Goodhew, P. F. 1972) Specimen preparation in materials science in: Practical methods in electron microscopy, A. M. Glauert, ed. NorthHolland, Amsterdam). T. K. Sherwood, "The Drying of Solids", Ind. Eng. Chem., 21 (1), ppl2-16, 1929. 88 1.4. 15. 16. V. J. McBrierty, "Heterogeneity in Polymers as Studied by Nuclear Magnetic Resonance", Faraday Discussions of the Chemical Society, NO. 68,1979. L. Sawyer, D. T. Grubb, "Polymer Microscopy", pp 213-18 Chapman and Hall, London, New York, 1965. H. Tadokoro, "Structure of Crystalline Polymers",pp 543-567, WileyInterscience, New York, 1979. 17. 18. E. H. Andrews and J. M. Stubbs, J. R. Microsc. Soc. 82 pp 221, 1964. G. Kani g, Proc. Colloid Polyrn. Sci. 57 pp 176, 197 5. 19. Thermal Characterization of Polymeric Materials, edited by E. Turi, 20. Academic Press Inc. J. M. Massie, A. F. Halasa, R. N. Thudium, and C. W. Burkhart, "Miscibility and Phase Behavior of Polyisoprene/polybutadiene and Polybutadiene/Polybutadiene Blends", ANTEC'92. 21 J.A. Lewis and M.J. Cirna, "Diffusivities of Dialkyl Phthalates in 22 23 Plasticized Poly(vinyl butyral) Impact on BinderThermolysis" J. Am. Ceram. Soc.,73 91 2702-2707 1990). Kingery, Bowen and Uhlmann, "Introduction to Ceramics", Wiley Interscience, P 448-P 515,1985. J. Hildebrand, R. Scott, "The solubility of Non-electrolytes", 3rd ed., 24 Reinhold Publishing Crop., N. Y., 1949. C. Fiori and G. DePortu, Br. Ceram. Proc. 38 1986) 213. 25 26. 27. E. Streicher, T. Chartier, "Study of cracking and microstructural evolution during drying of tape-cast aluminum nitride sheets", Chapman and Hall Ltd. 1991. G. Y. Onoda, Jr., "The Rheology of Organic Binder Solutions" in Ceramic Processing Before Firing, Wiley, New York. R. Moreno, "The Role of Slip Additives in Tape Casting Technology, Part 2-Binders and Plasticizers", Ceramic Bulletin, Vol. 7 , No. 11, Nov. 1992. 28. "Avoid Coating and Drying Defects", Chemical Engineering Progress, J an. 1993. 89