Thermodynamic processes…
advertisement

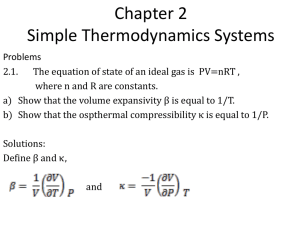
Thermodynamic processes… We are interested in changes in internal energy, the heat transferred to or from the system and the work done by the system - ideal the gas under piston In principle, all we need to know are the ideal gas law and the 1st law of thermodynamics: BUT… There are many, processes, conditions (idealistic or realistic) and applications… PV = nRT "U = Q ! W Thermodynamic processes in ideal gas… Usually presented as P-V diagrams… A diagram suggests that both pressure and volume are well defined in every point. ⇒ The gas is in a thermodynamic equilibrium in every point, every moment of time… We call that a quasi-static process. Equilibrium with what?.. At least with itself! That is, different parts of the gas are in equilibrium with each other. Can be implemented if we change things slowly…. Quasi-static processes…. Heating and boiling water on a stove. Quasi-static? Why? The least we can say is that the water is in contact with burning gas from below and cool air from above. So, its temperature is not likely to be the same at the top and bottom, which precludes thermodynamic equilibrium and quasistatic process. Quasi-static processes…. Is this one any better? Practically, how slowly you should go to be quasi-static? Quasi-static processes are in principle reversible … You can go back and forth along the same line of well defined equilibrium states. Can there be multiple paths to get from 1 to 2? Work done and heat transferred – our major concerns! Work done by the gas W = F!x = PA!x = P!V P – pressure of the gas; Δx – displacement of the piston, A – area of the piston The work is positive when the gas expands! Differential form: dW = Fdx = PAdx = PdV Integral form – also good for varying pressure: W = ! dW = ! PdV Work done and heat transferred – Work done by the gas our major concerns! W = ! dW = ! PdV P – (varying) pressure of the gas; dV – differential volume change P !W = P !V V Work, W, - area under curve on a P-V diagram Multiple ways to get (quasi-statically !) from an initial to a final state… Is work going to be the same for different processes? NO! Is heat going to be the same? NO! Is the change in internal energy going to be the same? YES! Internal energy is a function of state and will be the same as well as it variation between the states. Isothermal process – T = const Isotherm nRT const P= = V V V2 V2 nRT W = ' PdV = ' dV = V V1 V1 V2 & V2 # dV = nRT ' = nRT ln$$ !! V % V1 " V1 "U = Q ! W "U = 0 ! Q =W Constant volume (isochoric) process – V = const. "U = Q ! W "W = P"V ! 0 !U = Q = nCv !T n – number of moles of the gas; Cv – molar specific heat at constant volume – heat capacity of one mole of the gas in an constant volume process. (Compare with Q = mc!T ) Why bother introducing a new parameter? 1 !U Cv = n !T Measuring Cv we learn about internal energy of the gas as a function of temperature! Constant pressure (isobaric) W = P !V P What about internal energy and heat? "U = Q ! W V From the 1st law and equation for Cv: PV = nRT Q = !U + W = nCv !T + P!V Ideal gas at constant pressure: P!V = nR!T Q = !U + W = nCv !T + nR!T = n(Cv + R ) !T Molar specific heat at constant pressure (definition): nC p !T = n(Cv + R ) !T C p = Q /( n!T ) C p = Cv + R