advertisement

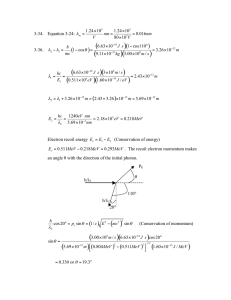
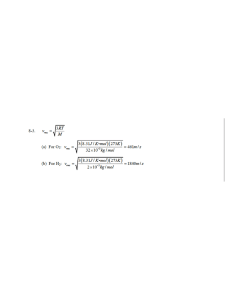
3-20. (a) mT 2.898 103 m K m (Equation 3-5) 2.898 103 m K 1.45 107 m 145nm 4 2 10 K (b) m is in the ultraviolet region of the electromagnetic spectrum. 3-21. Equation 3-4: R T 4 Pabs 1.36 103W / m 2 RE2 m 2 where RE = radius of Earth Pemit RW / m 2 4 RE2 1.36 103W / m 2 RE2 m 2 RE2 R 1.36 103W / m 2 2 4 RE T4 1.36 103 W T 4 2 4 m 1.36 103W / m 2 T 278.3K 5.3C 4 5.67 108W / m 2 K 4 3-22. (a) mT 2.898 103 m K m f m c / m 2.898 103 m K 8.78 107 m 878nm 3300 K 3.00 108 m / s 3.42 1014 Hz 8.78 107 m (b) Each photon has average energy E hf and NE 40 J / s. N 40 J / s 40 J / s 1.77 1020 photons / s 34 14 hf m 6.63 10 J s 3.42 10 Hz (c) At 5m from the lamp N photons are distributed uniformly over an area A 4 r 2 100 m 2 . The density of photons on that sphere is N / A / s m 2 . The area of the pupil of the eye is 2.5 103 m , so the number of photons 2 entering the eye per second is: n N / A 2.5 103 m 2 1.77 10 20 / s 2.5 103 m 2 100 m 2 1.77 1020 / s 2.5 103 m 1.10 1013 photons / s 2 3-50. (a) mT 2.898 103 mK (b) Equation 3-18: T 2.898 103 mK 3.50 104 K 9 82.8 10 m 70nm / ehc / 70nmkT 1 u 82.8nm 82.8nm 5 / e hc / 82.8 nm kT 1 5 u 70nm 6.63 1034 J s 3.00 108 m / s hc where 5.88 and 70nm kT 70 109 m 1.38 1023 J / K 3.5 104 K 5 100nm / e4.12 1 0.924 u 82.8nm 82.8nm 5 / e 4.97 1 5 u 100nm 3-53. (a) Equation 3-18: u gives u (b) 70nm / e5.88 1 0.929 u 82.8nm 82.8nm 5 / e 4.97 1 u 70nm hc 4.97 82.8nm kT Similarly, 8 hc 5 Letting C 8 hc and a hc / kT e hc / kT 1 C 5 ea / 1 5 1 e a / a 2 du d C 5 5 6 a/ C 2 a/ d d ea / 1 e 1 1 e 6 6 a / C a a/ C e a e 5 ea / 1 5 1 ea / 0 2 2 ea / 1 ea / 1 The maximum corresponds to the vanishing of the quantity in brackets. Thus, 5 1 e a / a (c) This equation is most efficiently solved by trial and error; i.e., guess at a value for a / in the expression 5 1 e a / a , solve for a better value of a / ; substitute the new value to get an even better value, and so on. Repeat the process until the calculated value no longer changes. One succession of values is 5, 4.966310, 4.965156, 4.965116, 4.965114, 4.965114. Further iterations repeat the same value (to seven digits), so we have a m 4.965114 6.63 1034 J s 3.00 108 m / s hc (d) mT 4.965114 k 4.965114 1.38 1023 J / K Therefore, mT 2.898 103 mK hc m kT Equation 3-5 1.24 103 1.24 103 3-34. Equation 3-24: m 0.016nm nm 80 103V V 3-36. 6.63 1034 J s 1 cos110 h 3.26 1012 m 2 1 1 cos 8 31 mc 9.1110 kg 3.00 10 m / s 6.63 1034 J s 3 108 m / s hc 2.43 1012 m 1 6 19 E1 0.511 10 eV 1.60 10 J / eV 2 1 3.26 1012 m 2.43 3.26 1012 m 5.69 1012 m E2 hc 2 1240eV nm 2.18 105 eV 0.218MeV 3 5.69 10 nm Electron recoil energy Ee E1 E2 (Conservation of energy) Ee 0.511MeV 0.218MeV 0.293MeV . The recoil electron momentum makes an angle θ with the direction of the initial photon. PE θ h/λ1 110° h/λ2 20° h 2 cos 20 pe sin 1/ c E 2 mc 2 sin 2 3.00 10 m / s 6.63 10 8 sin 5.69 10 12 (Conservation of momentum) 34 J s cos 20 2 2 m 0.804MeV 0.511MeV 1/ 2 1.60 10 13 J / MeV 0.330 or 19.3 3-37. 2 1 1 100 h 1 cos 100 0.00243nm 1 cos 90 0.243nm mc hc 3-38. (a) E1 1 (b) 2 1 (c) E2 h 1 cos 0.011 Equation 3-25 mc hc 2 1240eV nm 1.747 104 eV 0.0711nm h 1 cos 0.0711 0.00243nm 1 cos180 0.0760nm mc 1240eV nm 1.634 104 eV 0.0760nm (d) Ee E1 E2 1.128 103 eV 3-41. (a) Compton wavelength = h mc h 6.63 1034 J s electron: 2.43 1012 m 0.00243nm 31 8 mc 9.1110 kg 3.00 10 m / s proton: (b) E hc h 6.63 1034 J s 1.32 1015 m 1.32 fm mc 1.67 1027 kg 3.00 108 m / s (i) electron: E (ii) proton: E 4-1. 1 mn 1240eV nm 5.10 105 eV 0.510 MeV 0.00243nm 1240eV nm 9.39 108 eV 939 MeV 6 1.32 10 nm 1 1 R 2 2 where R 1.097 107 m 1 (Equation 4-2) n m The Lyman series ends on m =1, the Balmer series on m =2, and the Paschen series on 1 m =3. The series limits all have n , so 0 . n 1 1 R 2 1.097 107 m 1 L 1 L limit 1.097 107 m 1 91.16 109 m 91.16nm 1 R 2 1.097 107 m 1 / 4 B 2 B limit 4 / 1.097 107 m 1 3.646 107 m 364.6nm 1 1 R 2 1.097 107 m 1 / 9 P 3 1 P limit 9 / 1.097 107 m 1 8.204 107 m 820.4nm 4-2. 1 mn 1 1 R 2 2 where m 2 for Balmer series (Equation 4-2) n m 1 1.097 107 m 1 1 1 2 9 2 379.1nm 10 nm / m 2 n 1 1 109 nm / m 0.2405 4 n 2 379.1nm 1.097 107 m 1 1 0.2500 0.2405 0.0095 n2 n2 1 0.0095 n 1 / 0.0095 1/ 2 10.3 n 10 n 10 n 2 1 4-3. mn 1 1 R 2 2 where m 1 for Lyman series (Equation 4-2) n m 1 1.097 107 m 1 1 1 2 9 164.1nm 10 nm / m n 1 109 nm / m 1 1 0.5555 0.4445 n2 164.1nm 1.097 107 m 1 n 1 / 0.4445 1/ 2 1. 5 No, this is not a hydrogen Lyman series transition because n is not an integer. 4-4. 1 mn 1 1 R 2 2 n m (Equation 4-2) For the Brackett series m = 4 and the first four (i.e., longest wavelength lines have n = 5, 6, 7, and 8. 1 45 1 1 1.097 107 m 1 2 2 2.468 105 m 1 5 4 45 1 4.052 106 m 4052nm. Similarly, 2.68 105 m 1 46 1 2.625 106 m 2625nm 1 5 3.809 10 m 47 1 2.166 106 m 2166nm 1 5 4.617 10 m 48 1 1.945 106 m 1945nm 5.142 105 m 1 These lines are all in the infrared.