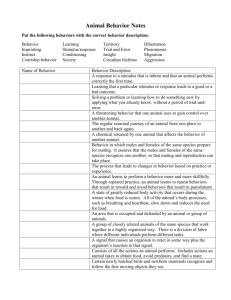
AN ABSTRACT OF THE DISSERTATION OF
advertisement

AN ABSTRACT OF THE DISSERTATION OF Leslie A. Dyal for the degree of Doctor of Philosophy in Zoology presented on April 8, 2005. Title: Variation in Female Reproductive Success in Amphibians Redacted for Privacy Abstract approved: My dissertation focuses on the factors that influence variation in female reproductive success in plethodontid salamanders and in toads. Variation in reproductive success fuels evolutionary change. Although, females often have been overlooked in studies of reproductive success due to perceived lower levels of variation when compared to variation in male reproductive success, understanding factors influencing variation in female reproductive success is critical for several reasons. First, female reproduction is usually the limiting factor on population growth. Second, the factors affecting female reproduction provide the impetus for current and evolving patterns of sexual dimorphism. Lastly, male reproductive success inevitably is determined by the reproductive success of the females with which they mate. Recent theoretical developments of sexual conflict have contributed significantly to a renewed emphasis on studies of female reproductive success. Sexual conflict theory elucidates important factors, from the perspective of females, affecting female behavior and reproductive success. Sexual conflict assumes that a female will benefit, in terms of reproductive success and offspring viability, when she is able to freely choose among males, unconstrained by social and environmental factors. Female choice would be constrained if a non-preferred male (i.e., one that would be rejected by a freely choosing female) coerced a female to mate in order to increase his own mating success. The dynamics between discriminating females and non-preferred males will lead to a coevolutionary "arms race", referred to as sexual conflict. From this perspective, secondary male traits may not reflect their intrinsic quality, but rather their ability to manipulate or coerce female mating decisions. The current debate lies in the importance and pervasiveness of sexual conflict. To address the current view of sexual conflict, I investigated whether sexual conflict plays a role in the mating systems of amphibians. In particular, my results support the main assumption that females benefit from freely expressed female mate choice in toads. In addition, I explored the potential influence of sexual conflict in plethodontid salamanders. During mating trials, I documented novel female courtship behaviors. I also examined the effects of male courtship pheromones on female reproductive success to assess the potential role of male pheromones in sexual conflict. My results suggest that sexual conflict also may be an important factor in plethodontid mating systems. My investigations of sexual conflict theory have provided new insights and novel predictions for understanding sexual selection and sexual dimorphism. ©Copyright by Leslie A. Dyal April 8, 2005 All Rights Reserved Variation in Female Reproductive Success in Amphibians Leslie A. Dyal A DISSERTATION submitted to Oregon State University in partial fulfillment of the requirements for the degree of Doctor of Philosophy Presented April 8, 2005 Commencement June 2005 Doctor of Philosophy dissertation of Leslie A. Dyal presented on April 8, 2005 APPROVED: Redacted for Privacy Major Zoology Redacted for Privacy Chair of Zoology Redacted for Privacy Dean of the Gdhat'e School I understand that my dissertation will become part of the permanent collection of Oregon State University libraries. My signature below authorizes release of my dissertation to any reader upon request. Redacted for Privacy Leslie AAYval. Author ACKOWLEDGEMENTS Above all, I want thank my advisor, Lynne I-buck. Lynne was always helpful and willing to share her time, knowledge, and money. Lynne was there when I needed her and provided much more than financial and intellectual support. When we were not discussing science, we could spend hours talking about our pets and their "issues". I owe a debt of gratitude for all that Lynne has done for me over the years. My appreciation goes out to Steve Arnold who support was critical. Steve was always willing to listen to and appreciate my "tangential" theories. His encouragement and knowledge were critical at the times I needed it most. I'll always be grateful for Steve's involvement in my graduate career. I also want to thank Joe Beatty for too many things to list. It suffices to say that I do not think I would have made it through this endeavor without his encouragement and support. Joe is the best! I would also like to thank my committee members, Deanna Olson, Paul Murtaugh, Jeff Arthur, and Bruce Coblentz. Paul and Jeff, I thank for critical statistical counsel. Thanks to Deanna for providing the inspiration for my research with toads. I especially enjoyed the rare occasion when we could actually do field work together. I would like to thank Bruce for his support and perspective. I especially appreciate the support and guidance of Bob Mason and Barb Taylor. I want to thank the Zoology Department for the financial support and the office staff, Sarah Cain, Traci Durrell-Khalife, Mary Crafts, and Tara Bevandich for their critical assistance. I would like to thank my friends and lab mates, Heather Waye, Catherine Palmer, Mollie Manier, Jerod Sapp, Doug Degross, Erika Adams, Mark Christie, Chris Stallings, Amy Picard, Mike Westphal, and Karen Kiemnic. I'm thankful for the theoretical debates with Adam Jones and Mike Phrender. The Ethology Writing Workshop was helpful in developing a portion on this dissertation. I would also like to thank Richard and Pamela Feidhoff for the pheromone contributions. I thank all the undergraduates that helped with my research, Keeta Owens, Renee Fox, and Randa Mills. I also owe thanks to Rebecca Hundt who drew the fabulous illustration of Plethodon dorsalis in Chapter 2. I would also like to thank Micheal Blouin and his lab for the chance to broaden my horizons into population genetics. I appreciated all the chats with Becky Cooper, Jacob Tennessan, Charles Criscione, and Roman Vilas. In addition, I would like to thank Richard Watts for his sharing his implacable knowledge of molecular biology. On a more personal note, I would like to thank my parents, Christa Wiggins and Conrad Dyal, for everything including the "field work grants". I would like to thank my grandmother, Margaret Summerlin for her love and support. I am indebted to Kevin Hood for his love and friendship. Without him, I would never have been able to leave the house and do field work since he was the only affordable and competent house and pet sitter. I also want to thank Kelly Gibbs and all her horses for my weekly horse riding therapy. Last, but not least, I want to thank my pets, Cheese, Bodhi, Moksha, Alex, G, Fly, Nima, Leto, Old Man, Veda, Sophie, Spea, Clyde, Ged, Nansen, Slick and Turtie for keeping me going. Squirt, CONTRJBUTION OF AUTHORS My advisor, Lynne D. Houck, provided design and financial assistance for all of the projects included in this dissertation. Dr. Stevan J. Arnold also helped with project design and provided ideas for side projects that ended up being critical components of my dissertation. TABLE OF CONTENTS CHAPTER 1. INTRODUCTION CHAPTER 2. NOVEL COURTSHIP BEHAVIOR IN THREE SMALL EASTERN PLETHODON SPEICES ABSTRACT INTRODUCTION METHODS RESULTS DISCUSSION CHAPTER 3. MALE COURTSHIP PHEROMONES AND FEMALE REPRODUCTIVE SUCCESS ABSTRACT INTRODUCTION METHODS RESULTS DISCUSSION CHAPTER 4. FEMALES BENEFIT FROM FREELY EXPRESSED MATE CHOICE IN THE WESTERN TOAD, BUFO BOREAS ABSTRACT INTRODUCTION METHODS DISCUSSION 18 18 19 23 25 38 48 48 49 54 58 63 66 66 66 74 78 84 CHAPTER 5. CONCLUSIONS AND FUTURE DIRECTIONS 90 BIBLIOGRAPHY 93 LIST OF FIGURES Figure 1.1 A model of social behavior in sexual species from Gowaty 1997 3 1 .2 Factors that primarily limit the reproductive success of females and males 4 1.3 Interactions between the sexes, comprising the dialectics of sex 5 1.4 Effects of male efforts to control females on female fitness 7 1.5 Female resistance, variation in mechanisms of male-male competition and mating systems 9 2.1 Courtship sequence for Plethodon angusticlavius 42 2.2 Character states of behavioral features mapped onto a partial phylogeny of Plethodon and the related genus Aneides 46 Relationship between female SVL and clutch size for Plethodon shermani in 2002 60 Relationship between female SVL and clutch size for Plethodon shermani in 2003 62 The total number of eggs retained by female Plethodon shermani after oviposition in each treatment group in 2003 62 The relative size of male and female Western toads represented as the relation between snout-urostyle length and mass 72 4.2 Schematic of female choice enclosures 76 4.3 The average proportion of hatching tadpoles for preferred and nonpreferred matings for Western toads at Todd Lake and Little Three Creeks Lake 81 3.1 3.2 3.3 4.1 LIST OF FIGURES (Continued) Figure 4.4 4.5 Tadpole length at hatching averaged for preferred and non-preferred matings for Western toads at Todd Lake and Little Three Creeks 84 Differences in Western toad male SUL at Little Three Creeks Lake and Todd Lake 89 LIST OF TABLES Table 2.1 A comparison of female and male courtship behavior for each species 31 2.2 A summary of the duration of the phases of courtship for Plethodon cinereus 34 A summary of the duration of the phases of courtship for Plethodon angusticlavius 36 A summary of the duration of the phases of courtship for Plethodon richmondi 39 3.1 A summary of results for each year of the study for Plethodon shermani 58 3.2 Linear regression estimates for Plethodon sherman! clutch size 59 4.1 Clutch data for Western toads from Little Three Creeks Lake 79 4.2 Clutch data for Western toads from Todd Lake 80 4.3 Mean tadpole length per clutch for preferred and non-preferred matings for Western toads at Little Three Creeks Lake 82 Mean tadpole length per clutch for preferred and non-preferred matings for Western toads at Todd Lake 83 2.3 2.4 4.4 DEDICATION This dissertation is dedicated In memory of Keeta Owens, an adventurer, biologist, and friend VARIATION IN FEMALE REPRODUCTIVE SUCCESS IN AMPHIBIANS CHAPTER 1 INTRODUCTION Variation in reproductive success is the basis of evolutionary change. Until recently, the study of mating behavior and its effects on variation in reproductive success has focused primarily on males. There are three main reasons for this focus. First, male mating success and reproductive success usually are directly related. The more often a male mates, the higher his reproductive success. In contrast, female mating success is rarely a determinate of female reproductive success (unless males are limited in numbers or a single male does not produce enough sperm to fertilize all of a female's eggs). Even under scenarios where multiply-mating females incur some fitness benefits, the consequent increase in reproductive success is considerably less than that in males. Second, male mating success and reproductive success typically are highly variable among males, depending on mating system dynamics. As there is less variation in female mating success and thus a weaker correlation with reproductive success, variation in reproductive success of females has not been a main focus of studies of sexual selection. Third, Darwin (1871) defined sexual selection in terms of intra-sexual selection (e.g., male-male competition) and inter-sexual selection (e.g., female choice). As both of these mechanisms of sexual selection influence male variation, variation and selection in females received less attention. The focus of sexual selection research, however, has recently broadened its scope to include potential conflicting selective pressures acting on males and females 2 (Parker 1979, Rice 1996, Drickamer et al. 2000, Moore et al., 2000, Arnqvist and Rowe 2002, Chapman et al. 2003, Bluhm and Gowaty 2004a). While much research of traditional sexual selection has been conducted, these findings do not exclude or clarify the role of sexual conflict (Figure 1.1) (see reviews in Andersson 1994, Pizarri and Snook 2003, Eberhard and Cordero 2003, Cordoba-Aguilar and Contreras-Garduno 2003). I discuss the general concept of sexual conflict first, and then describe particular examples of well-studied systems. Sexual conflicts occur when the reproductive interests of males and females are different (Figure 1.2). In general, sexual conflicts can occur at many stages of reproduction, including the decision to mate, the sex ratio of offspring, and allocation of parental care. The theoretical basis for my research is focused on sexual conflicts over mating (also termed "sexual dialectics"; Gowaty 1997). For simplicity, I will use the term sexual conflict. The main assumption of sexual conflict is that "freely expressed" female mate choice is adaptive. In other words, female reproductive success is higher when under female control (i.e., when a female can select the mate that she prefers) than when the female is manipulated into suboptimal mating by non-preferred males. Sexual conflict describes the behavioral and/or physiological interactions when there is a conflict over mating between discriminating females and non-preferred males (Figure 1.3). The conflict between females and non-preferred males leads to selection favoring males that are able to manipulate or coerce female mating decisions. If female reproductive success is higher under her own control, selection will favor resistance by females. 3 Mechanisms of Sexual Selection Legend same-sex behavioral competition choice 4 ' counterchoice behavior e I 4 'U Figure 1.1. A model of social behavior in sexual species from Gowaty 1997. The thin lines represent traditional sexual selection view of male-male competition and female choice (sexual selection on males). The thick lines represent what is usually left out of the traditional discussion, female-female interactions and male mate choice (sexual selection on females). The medium line with double headed arrows from male to female represents sexual dialectics (sexual conflict over mating), mediating sexual selection on either males or females. Factors Primarily Limiting RS Females Males Females symmetry Nutrients, nest sites, refugia "typical" asymmetry mammals, birds, insects Males Nutrients, nest sites, refugia "role-reversed" asymmetry symmetry or asymmetry Figure 1.2. Factors that primarily limit the reproductive success (RS) of individuals may include access to the opposite sex or to other resources for reproduction, such as nutrients, nest sites, and refugia. In typical species, reproductive success of females is thought to be primarily limited by access to nutrients and nest sites; males are most limited by access to females. 5 Sexual Dialectics Males attempt to control females directly (e.g., forced copulation; affiliative conditioning) Females resist "nice" "nasty" Males attempt to control females by brokering females' access to resources Females resist Females freely choose so that males compete through honest signals of quality Figure 1.3. Interactions between the sexes, comprising the dialectics of sex (i.e., sexual conflict over mating). The dialectics of sex are engaged whenever females fail to freely choose a particular male as a gametic partner. Selection acts on non-preferred males to manipulate female mating decisions using "nice", "null", and "nasty" mechanisms. When these attempts have deleterious effects on female fitness, females are under selection to resist. When females are successful at resisting direct mechanisms, males are under selection to manipulate females by brokering females' access to resources necessary for reproduction. If male resource brokering is deleterious to female fitness, females will be under selection to resist. If females are successful at resistance, females will be free to choose among males based on honest signals of intrinsic male quality. Under the scenario proposed by sexual conflict, non-preferred males initially attempt to control females by using direct mechanisms. One such mechanism is aggressive conditioning or forced copulation. Another mechanism is affiliative conditioning (Gowaty 1997). Affiliative conditioning refers to situations in which males manipulate female mating decisions without costs or sometimes with benefits to females' survival (Figure 1.4). Affiliative conditioning may seem like cooperation between the sexes when it is actually "helpful coercion" (Gowaty 1997). This kind of conditioning occurs on a continuum, based on the effects on the survival components of female fitness. An example of a situation in which a female's survival is lowered by male behavior is mate guarding in which guarding makes the female more vulnerable to predators (as in water striders Aquarius sp., Gerris sp., Rowe 1994). On the other end of the continuum, male behavior may enhance female survival (e.g., nuptial feeding). Females should still resist these behaviors, even though they may seem helpful, because of the costs of suboptimal mating on the reproductive components of female fitness. Females will inherently vary in their abilities to resist male coercion. Female resistance may take the form of social patterns, such as kin coalitions facilitated by female philopatry in primates (Smuts 1992, 1995). Females may resist through ecological advantage such as niche separation and availability of refugia (Rowe et al. 1994). Also, female resistance can take the form of morphological adaptation, including female size relative to male size (RaIls 1976) and female genital spines that prevent forced ii copulation in water striders (Fairbairn et al. 2003). In addition, physiological adaptations may include facultative control of ovulation, implantation, and sperm storage. Effects of Males' Efforts to Control Females on Females' Fitness Effects on Females' Survival + 0 Effects on Females' RS - - 0 - + 0 0-000+ + +o'++ Figure 1.4. Matrix of the effects of males' efforts to manipulate or coerce females. Effects may be on either the survival component or reproductive success (RS) component of a female's fitness. Because manipulation of females' reproductive capabilities theoretically is almost always costly to the reproductive success component of a female's fitness, the continuum of manipulation efforts is defined in terms of their effects on the survival component of a female's fitness only, and ranges from "nasty" to "null" to "nice." The full matrix is present to show the entire range of possible interactions, even though this discussion is based on the first row of the matrix. 8 If females are able to resist direct male attempts to control mating, selection should favor males that indirectly attempt to control females by brokering females' access to critical resources. Such males will benefit through increased mating success (Figure 1.2). Female success at resisting indirect control will depend on the environmental potential for females to resist resource brokering. This environment potential for resistance includes factors such as the spatial distribution of resources, male-female niche separation and arrival times at critical resources. If the environmental potential favors female resistance, females will freely choose so that males compete through honest signals of quality (Figure 1.5). Sexual conflict and the consequent male-female coevolution often result in male traits that stem from females discriminating against non-preferred males. This discrimination can lead to male-male competition as a mechanism to manipulate female mating decisions (Gowaty 1997). When considering the potential conflict between males and females, we cannot assume that females prefer male traits that function in male-male competition. For example, males may compete for territories that have nesting sites needed by females. Thus, a male can monopolize a resource needed by females. In this scenario, a female's inherent preferences that confer a selective advantage, the "freely expressed choice", are constrained by her need for a resource monopolized by males. The resulting "choice" is a compromise between mate compatibility and access to a needed resource. In fact, there is evidence of the conflicting nature of male-male competition and free female choice in both invertebrate Female Resistance, Variation In Mechanisms of Male-Male Competition and Mating Systems gh Environmental potential for female resistance to resource brokering Success of female resistance to direct control by males II Honest signals of male intrinsic quality I 1'ree Female Choice I Territoriality Female-male contests for resources Malemale contests for resources Exploitation of sensory bias Affiliative conditioning Helpful coercion Mate herdlng."guarding" Aggressive coercion Forced copulation Male ResourceBrokering Mating Systems Direct Control of Females Mating Systems Figure 1.5. The success of females at resisting direct mechanisms of manipulation by males, coupled with the environmental potential for females to resist resource brokering by males, determines the type of mating system. Note that the success or failure of females' resistance determines the type of male-male competitive interactions and thus affects variance in male reproductive success. From a female's perspective, there are three types of mating systems: free female choice mating systems, male resource brokering mating systems, and direct control of females mating systems. 10 and vertebrate systems (discussed below). Male traits are potentially influenced by sexual conflict if these traits directly or indirectly manipulate or coerce females into mating. The consequent patterns of intra- and inter-specific mating system variation are generated through variation in females and their interaction with the environment (Figure 1.5). The sources of mating system variation are (1) the female's ability to physically resist attempts to mate with a manipulative, non-preferred male, and (2) the environmental and social factors that indirectly affect females' ability to resist matings. These factors include the availability of food, niche separation between males and females, refugia, the operational sex ratio experienced by females, and the density of individuals (Rowe et al. 1994, Lauer et al., 1996, Gowaty 1997). The accumulating evidence suggests that sexual conflict is more pervasive than previously thought and that determining the presence and role of conflict will expand our understanding of male-female coevolution and the resulting sexual dimorphism. Much debate centers on what role is played by sexual conflict, particularly in terms of the evolution of mating systems and sexual dimorphism (Gowaty 1997, Chapman et al. 2003, Cordoba-Aguilar and Contreras-Garduno 2003, Eberhard and Cordero 2003, Kokko et al. 2003, Pizarri and Snook 2003). Most researchers agree that sexual conflict and traditional sexual selection are not mutually exclusive. The behavioral manifestations of intra- and inter-sexual selection are often behaviorally and theoretically interconnected, and thus are difficult to distinguish (Gowaty 1997, Chapman et al. 2002). The method of untangling the most important mechanisms of selection requires predictions appropriate to the mating system of interest, while 11 addressing all potential behavioral pathways of intra- and inter-sexual selection. Recent studies revealed that conflicts over whether or not to mate often play a pivotal role. In certain well-studied systems, sexual conflicts are the causative pathway of sexual selection acting on male traits (Rowe et al. 1994, Crean and Gilburn 1998, Shukar and Day 2002, Bonduriansky and Rowe 2003). These systems are discussed below. Sexual conflict has been most thoroughly investigated in several invertebrate systems. The phylogenetic footprint of sexual conflict and the consequent coevolution of male and female traits have been confirmed in water striders (Gerris sp., Rowe et al. 1994), dragonflies and damselflies (Odonata: Anisoptera and Zygoptera, Finke 1997), diving beetles (Coleoptera: Dystiscidae, Soulier-Perkins 2001) and planthoppers (Hemiptera: Lophopidae, Miller 2003). Additionally, the interplay between traditional forms of sexual selection (e.g., female choice, male-male competition) and sexual conflict has been clarified in several insect systems (Bonduriansky and Rowe 2003, Rice 1996), and also in meadow voles (Spritzer et al. 2005)). For example, in the cockroach (Nauphoeta cinerea), males produce a social pheromone that has multiple components. One component of this pheromone indicates social dominance amongst males, but another component (usually absent in dominant males) is most attractive to females (Moore and Moore 1999). In cockroaches, therefore, the outcome of malemale competition is in conflict with female mating preferences (Moore et al. 2000). Also, water striders exhibit a premating struggle, a behavior that has led to this system being thoroughly studied in terms of a conflict over mating (Arnqvist et al. 1997, Arnqvist and Rowe 1995, Rowe et al. 1994, Fairburn et al. 2003). Variables thought to 12 influence the outcome of the pre-mating struggles included: morphology, refugia, sex ratio, density, diet and mating status. Results from experimental manipulation of each of these variables supported the predictions of sexual conflict as determined by female resistance behavior (Rowe et al. 1994, Watson et al. 1998, Ortigosa and Rowe 2003, Arnqvist and Rowe 2002). The possibility that sexual conflict drives traditional sexual selection also has been supported by research in the seaweed fly (Coleopafrigida). Selection favoring large male size occurs as a result of sexual conflict over mating (Crean and Gilburn 1998, Shukar and Day 2002). The seaweed fly mating system is characterized by scramble competition without obvious active mate choice. Although females do not actively choose males, female rejection behaviors select for large males that are best able to overcome female resistance and force copulations (Shukar and Day 2002). In addition to identifying examples of sexual conflicts, the costs and benefits of many aspects of sexual conflict have been investigated in the previously mentioned invertebrate systems (also Drosophila, see Rice 1996). There also is accumulating evidence of sexual conflict in a few vertebrate systems, including mice (Mus musculus, see Drickamer et al. 2000), mallards (Anas platyrhinos, see Bluhm and Gowaty 2004a,b), and fish (Gadus morhua, Rudolfsen et al. 2005; Poecilia reticulata, Rosenqvist et at., 2001; Symphodus ocellatus, Alonzo and Warner 2000). In mice and guppies, females benefited from "freely expressed choice" in many reproductive components of fitness, including offspring survival, viability, and performance (Drickamer et al. 2000, Rosenqvist et al. 2001). In another example, female mallards 13 incurred reproductive costs associated with non-preferred matings occurring as a result of social constraints on female preferences (Bluhm and Gowaty 2004a). Also, with increasing female age and experience, female mallards were able to compensate for costs associated with non-preferred matings by increasing investment in offspring (Bluhm and Gowaty 2004b; also found in guppies, Reynolds and Gross 1992, Nicoletto 1995, Nicoletto et al. 2001). In another vertebrate study, compatible male-female crosses in Atlantic cod resulted in higher offspring survival, and this higher suvivorship was not correlated with male traits such as sexual ornaments or sperm quality (Rudolfsen et al. 2005). Lastly, sexual conflict has been shown to influence costs of courtship in female garter snakes (Shine et al. 2003, 2004). In garter snakes, female resistance behavior may explain size-assortative mating, temporal patterns in dispersal from overwintering dens, and female mimicry (Shine et al. 2000). Since most studies of sexual conflict focused on invertebrates as study organisms, it will be necessary to include more vertebrate systems in studies of sexual selection and sexual dimorphism to understand the pervasiveness of sexual conflict. The work reported below addresses this need. My research has primarily focused on variation in female mating behavior and corresponding effects on female reproductive success. Amphibians provide an opportunity to examine the nature of variation in reproduction in a range of mating systems, from systems having prolonged courtships and breeding seasons (plethodontid salamanders) to systems characterized by explosive breeding with very brief breeding seasons (Western toads). I focused on amphibians as a model for addressing three 14 questions not usually considered within the context of reproductive success. The questions are described in Chapters 2, 3, and 4. In Chapter 2, I ask whether females can play an active role in courtship interactions in plethodontid salamanders. Courtship in this family is highly stereotyped and courtship behaviors have been well documented for many species. The general observation is that the male plays the more active role in courtship (Noble and Brady 1930; Organ 1960; Stebbins 1949; Arnold 1972, 1976, 1977). In addition, Plethodon males deliver courtship pheromones by two methods, vaccination and olfaction (Arnold 1977, Houck and Sever 1994). These courtship pheromones alter female reluctance to mate (Houck 1986, Houck and Reagan 1990, Houck et al. 1998, Rollmann et al. 1999), and thus may reflect sexual conflict. If courtship pheromones were involved with sexual conflict, I would expect to observe variation in female courtship behavior within the genus Plethodon, since variation in female behavior is a critical tenet of sexual conflict theory. I investigate courtship behavior in three salamander species to address the potential for variation in the female's role in courtship. Previous studies of social interactions in one of the study species (Plethodon cinereus) found that females defended territories, were socially monogamous, and exhibited differential aggressive behavior between familiar and unfamiliar individuals (Gabor and Jaeger 1995, Jaeger and Peterson 2002, Lang and Jaeger 2000, Gillette et al. 2000). Thus, the potential for active female participation in courtship seemed possible for P. cinereus since these females were, in general, active participants in social situations. I chose P. richmondi as a study species due to a lack of behavioral information and their close relationship to 15 P. cinereus. Plethodon angusticlavius was chosen because they are phylogenetically intermediate between species using olfactory and vaccination courtship pheromone delivery. I made observations of courtship behavior for each of these three salamander species. I documented novel female courtship behaviors in all three species. The active role of these females may reflect a more complex social structure within these species. The use of courtship pheromones that alter female resistance in plethodontid salamanders may reflect sexual conflict if it is used to manipulate female mating decisions. If sexual conflict were occurring, I would expect to see variation in female behaviors reflecting attempts by females to resist manipulation by males. I found that females actively attempted to persuade males to mate, and both males and females played a leading role in courtship interactions. My observations of courtship broaden the range of documented female courtship behavior and provide insight into the potential for sexual conflict in plethodontid salamanders. In Chapter 3, I address more directly the potential role of courtship pheromones in sexual conflict. In particular, I test the hypothesis that female reproductive success is affected by male pheromone delivery in plethodontid salamanders. I focused on a single well-studied species, Plethodon shermani. For P. shermani, the male courtship pheromone has already been shown to alter female reluctance to mate, as indicated by a decreased duration of courtship (Rollmann et al. 1999, Rollmann et al. 2003). Precedence for courtship chemicals to have multiple effects on females has been documented in several insects (Drosophila, Rice 1996, cockroaches, Moore et al. 2003). Consequently, I investigated other potential consequences of male courtship pheromone 16 delivery on female reproductive success in P. shermani. I examined male courtship pheromone delivery from the perspectives of sexual conflict. If female choice is adaptive and is operating in this system, then the pheromone may function to overcome female resistance to mating with a non-preferred male. In this case, the pheromone should negatively affect female reproduction, even if the pheromone is beneficial in terms of survival (e.g., decreased exposure to predators due to shorter courtships). If, on the other hand, the male pheromone is an honest signal of quality or only decreases courtship time in response to predator exposure, then the effects on female reproduction will be neutral or positive. I discovered that distinguishing between these two alternatives is difficult, but my results provide incentive for more research. In Chapter 4, I test the main assumption of sexual conflict theory that freely expressed female choice is selectively advantageous. In particular, is female choice important in a species of toad (Bufo boreas) in which a female's mating decisions typically are constrained by male behavior? I conducted an experiment to determine if females benefit from freely expressed mate choice, even when the mating systems seems to prevent the expression of the preferences of females. In addition, I conducted a post hoc analysis of male traits that females may be using to differentiate among males. My results support the assumption that females benefit from mate choice. The benefits were independent of particular male traits and occurred in a mating system that constrains free female mate choice. My post-hoc analysis of male traits indicates that preferred males are often smaller than are non-preferred males or the original male with 17 which a female was amplexed. My results suggest that the observed mating system patterns of B. boreas are influenced by female choice and sexual conflict. In conclusion, the theoretical developments based on sexual conflict theory now allow us to evaluate reproductive success from the perspective of females. These developments help to identify potential sources of variation in female reproductive success. I have addressed three different amphibian examples in which female variation in reproductive success may be affected by sexual conflict. In addition, a new perspective on the role of females clarifies what factors may influence male and female traits involved with male-female coevolution, sexual dimorphism, and speciation. 18 CHAPTER 2 NOVEL COURTSHIP BEHAVIOR IN THREE SMALL EASTERN PLETHODON SPECIES Leslie A. Dyal ABSTRACT Plethodontid salamanders provide an opportunity to investigate mating behaviors and pheromonal communication within the context of well-established phylogenetic relationships. Courtship in many members of the genus Plethodon has been well studied, but critical gaps exist in observations of pheromone delivery methods (vaccination or olfactory) in transitional species, those species phylogenetically intermediate between those having the two pheromone delivery methods. I observed courtship interactions in three small eastern plethdontids, P. cinereus, P. angusticlavius, and P. richmondi. I documented several novel female behaviors, as well as interspecific variation in courtship patterns that otherwise have been highly conserved for over 70 million years. I confirmed that olfactory pheromone delivery occurs in P. angusticlavius, a species that is phylogenetically in the transition from vaccination to olfactory pheromone delivery within the genus Plethodon. The deviation from typical courtship patterns observed for the three study species revealed that females play a more active role in courtship than has previously been documented for Plethodon species. INTRODUCTION Studies of the evolutionary dynamics of courtship behavior have been facilitated by molecular data and other independent information providing the basis for wellestablished phylogenies (Hoikkala and Kaneshiro 1984, Rowe 1994, Greenspan and Ferveur 2000). Mapping behaviors and associated morphologies onto phylogenies often can provide insight into the evolution of mating patterns by elucidating how differences among species could have resulted from sexual selection and/or sexual conflict (Rowe 1994, Fincke 1997, Weller et al. 1999, Soulier-Perkins 2001, Miller 2003). Invertebrates, in particular, have contributed significantly to our understanding of these processes of selection. In water striders (Aquarius sp., Gerris sp.) and diving beetles (Dytiscidae), for example, independently established phylogenetic patterns reveal the order and alternation of traits that are related to a sexual conflict over mating (Rowe 1994, Miller 2003). In tiger moths (Arctiidae), a phylogenetic study of mating signals and displays showed that these signals originated as visual and chemical defenses against predators (Weller et al. 1999). Studies based on robust phylogenies also reveal behavioral trends in vertebrates, particularly in terms of female mate choice. The determination that pre-existing sensory biases affect female mating behaviors has been supported by phylogenetic information in swordtail fish (Xiphophorus) (Basolo 1990). Less attention has been devoted to the evolution of courtship behavior and chemical reproductive signals in other vertebrate taxa. For salamanders, however, a 20 recent summary (Houck and Arnold 2003) reviewed both stability and dynamic changes in basic courtship patterns across all nine families. More basal families of salamanders (Sirenidae, Cryptobranchidae and Hynobiidae) have external fertilization, while the remaining, more recently derived families have internal fertilization that presumably enhances fertilization success (Houck and Arnold 2003). Another trend is the long-term stability of particular aspects of courtship behavior. For example, male egg-guarding in Cryptobranchidae and Hynobiidae (Kerbert 1904, Smith 1907, Sato 1992, Usuda 1993, Hasumi 1994, Kawamichi and Ueda 1998, Park and Park 2000) and ventral amplexus with "pinwheel" sperm transfer in Salamandridae (Arnold 1977, Rehberg 1986, Fleck 1992) are behaviors conserved for tens of millions of years. Similarly, a strikingly conserved behavior is seen in the plethodontid salamanders (Houck and Arnold 2003). All plethodontid species for which courtship has been observed are characterized by a linear "tail-straddling walk" (Noble and Brady 1930, Stebbins 1949, Arnold 1977, described below). Phylogenetic evidence supports the estimate that the linear tail- straddling walk has been retained for over 70 million years (Highton et al. 2000). The stability of the linear tail-straddling walk behavior is most likely attributable to the increased success of sperm transfer since courtship in the Plethodontidae involves the deposition of only one or very few spermatophores. In other families, a male may deposit several or scores of spermatophores per courtship (Houck and Arnold 2003). Courtship in plethodontid salamanders, and particularly in the genus Plethodon, is typically initiated when the male orients to and approaches the female (Organ 1961, Arnold 1977). Initially, the male will make head contact with the female, and the pair 21 later engages in tail-straddling walk. During tail-straddling walk, the female follows the male with her chin on the base of his tail while having her forelimbs on either side of the male's tail (Noble and Brady 1930, Stebbins 1949, Arnold 1977; see Figure 2.2). During the courtship season, the male develops a mental gland that secretes pheromone proteins that can alter female behavior (Houck and Reagan 1990, Rollmann et al. 1999, Rollmann et al. 2003). Courtship pheromones are delivered to the female before or during the tail-straddling walk. Most plethodontids maintain the ancestral mode of pheromone delivery by "vaccination." Vaccination occurs when the male uses his premaxillary teeth to scratch and abrade the skin on the female's dorsum, and rubs his mental gland over the same area (Organ 1961, Arnold 1972, Houck and Arnold 2003). Also, males of several species use a rapidly executed snapping or puUing behavior (described below) to deliver pheromone to the female (Arnold 1972, Marvin and 1-lutchison 1996). These vaccination behaviors presumably allow the pheromone produced by the mental gland to enter the female's superficial circulatory system (Organ 1961, Arnold 1972, Houck and Arnold 2003). In contrast, large eastern plethodontids have a derived mode of pheromone delivery in which the male "slaps" his mental gland directly on the female's nares (Arnold 1976, 1977; and Figure 1 in Rollmann et al. 2000). Pheromone stimulation by direct olfactory delivery is processed by the female's accessory olfactory system (Wirsig-Wiechmann et al. 2002). Regardless of the delivery mode, the majority of pheromone delivery occurs during tailstraddling walk (1-buck and Arnold 2003). 22 During courtship, the male is traditionally considered the more active participant with female behavior being more subtle. For example, the female initially demonstrates receptivity by no longer fleeing when the male approaches her and contacts her with his head. When the male initiates tail-straddling walk, the female steps over his tail and places her chin on the base of his tail. Spermatophore deposition timing presumably is coordinated by the ability of the male to assess the female's forward movement during linear tail-straddling walk (Arnold 1976). If a female moves in a coordinated fashion with the male (i.e., does not lag behind), the male is more likely to deposit a spermatophore without further courtship interactions. After spermatophore deposition, the female obtains the sperm mass by moving forward and lowering her cloaca onto the spermatophore, thus lodging the sperm mass in her cloaca. Courtship in the Plethodontidae is highly stereotyped in that the same behaviors are observed in many species (Arnold 1977, Marvin and 1-lutchison 1996, Houck and Arnold 2003). However, more recent observations of previously unstudied species have revealed novel variations in courtship behavior between species (Sapp 2002). In particular, these recent studies have revealed unsuspected variation in the tail-straddling walk. Species of Aneides, for example, engage in a tail-straddling walk that proceeds in a circle, instead of in the traditional straight line (Sapp 2002). Aneides females also exhibit behaviors previously considered to be stereotypical male behaviors. For example, a female will rub the male's back with her chin, a behavior that in other species is typically performed only by males. Adding to the examples of variation in 23 tail-straddling walk and more active female behavior in Aneides, I describe below the courtship behavior of P. cinereus, P. angusticlavius, and P. richmondi. MATERIALS AND METHODS Animals were collected from two locations in Virginia, USA and one location in Arkansas, USA in August 2000 and February 2001. Twenty-eight P. richmondi and eight P. cinereus were collected from Grayson County, VA (36 37.73 N, 81 35.23). Another twelve P. cinereus were collected from a second site in Grayson County, VA, on Mount Rogers (36 47' N, 81 32.96' W). Five P. angusticlavius were collected from Madison County Wildlife Management Area in Arkansas (36 14' 42"N, 93 40'45W). Animals were sexed using the "candling" method (Gillette and Peterson 2001). There were 14 P. richmondi of each sex and 14 females and 6 males of P. cinereus. For P. angusticlavius, there were 4 males and 1 female. Although the sample size for P. angusticlavius observations are small, the highly stereotyped nature of behavior in these salamanders increases the likelihood that behaviors observed are robust. In courtships of P. angusticlavius and P. richmondi, males and females used in trials were from the same population. Since there were two populations of P. cinereus, males and females may or may not have been from the same population due to the shortage of males. Salamanders were transported to Oregon State University, Corvallis, Oregon, USA and housed in a climate-controlled room (13 - 16 °C) with a natural photoperiod. Animals were housed individually in plastic shoeboxes (12 x 17 x 6 cm). Boxes were lined with moist paper towels and several crumpled paper towels were added to provide 24 cover. The animals were fed white worms (Galleria ne/Ia) and/or small crickets (Acheta domestica) weekly. Separate from observed courtship stagings, males and females were paired randomly within each species and allowed to interact overnight to maintain levels of reproductive and social behavior in the laboratory. These unobserved courtships were conducted on nights when staged courtships were not recorded on video. Infrequently, and in all species, spermatophores were found the morning following unrecorded social interactions, indicating the maintenance of reproductive condition. Staged video taped courtship trials began in October 2000 and continued until behavioral interactions diminished (mid-December 2000 for P. cinereus, P. richmondi, through March 2001 for P. angusticlavius). The trials were staged at night, beginning at 1500-1900 hr , in the same room in which the animals were housed. Each courtship arena consisted of two glass plates separated by a plexiglass frame (17 x 17 x 5 cm). The lower glass sui-face was covered with a moist paper towel. Trials lasted until the following morning (0700-0900 hr). Animals were given at least 3 days of rest between staged interactions or after successful unrecorded courtships occurring between video taped encounters. Courtships were recorded using a low light camera (Panasonic VW BD400) and a closed circuit time-lapse video tape recorder (Panasonic AG 6540). Up to four different pairs of animals were recorded each night, each at four images per second. Thirty-seven courtship interactions were recorded, including 13 spermatophore depositions. Behavioral observations are described using summary statistics (mean, standard deviation, and range). 25 RESULTS Courtship behaviors are cataloged below in ethogram format for P. cinereus, P. richmondi, and P. angusticlavius. The catalog describes in detail all behaviors observed in any of the three species and generally follows nomenclature in previous ethograms from Arnold (1972, 1976). Following the catalog, I clarified which behaviors were performed by a particular species and by sex (Table 2. 1). I also described the temporal relations of behaviors observed during courtship interactions for each species, and compare the duration of each stage of courtship among species (Tables 2.2-2.4). Behaviors performed by males or females Approach Retreat Nudging One individual moves toward the other (within 2 cm). One individual moves away after an approach by the other. One individual moves its head along the sides of the other's body. Rubbing and sliding One individual moves its head in a side-to-side (rubbing) or forward-to-backward (sliding) motion while in contact with body/dorsum. When rubbing is performed by males, the pre-maxillary teeth may abrade the female's skin, accomplishing a type of vaccination pheromone delivery. Foot shuffling While stationary, the animal lifts one foot as the other descends. This behavior is confined to rear legs. It is similar to foot dancing (Arnold 1976), which includes all four legs. 26 Cloacal nudging The snout of one animal contacts the cloacal region of the other salamander. Head contact Contact to the head is initiated by one individual (cheek to cheek, snout to cheek, snout to snout). Head rubbing I-lead to head contact is performed by one individual by making a side- to-side or forward-to-backward motion while its head is on top of the other's head. Turning back During female-first tail-straddling walk (ffTSW) or during linear tail- straddling walk, the female or male turns back towards the other's dorsal and/or sacral region. When performed by a female, her body remains in contact with (or close to) the substrate. In contrast, a male turning back raises his body off the ground to better reach the female's nares for olfactory pheromone delivery. Tail wagging One individual will undulate the distal portion of its tail (one-half to one-third of the tail). Tail wagging usually occurs throughout the courtship, often (but not necessarily) in contact with the other salamander Isimilar to tail undulation (see below) but faster and with only the tail tip moving I. Behaviors performed by females only Cloacal rubbing The female crawls over the male and rubs her cloaca with quick forward-backward movements while in contact with the male dorsuni (usually toward the rear of the male). High amplitude tail undulations When the male is in contact with female's tail, usually with his chin touching her tail, she will undulate her tail in a slow, high 27 amplitude wave-like motion while her tail is either in full contact with the substrate or with the tail base slightly arched. High amplitude head swinging The female swings her head side to side at approximately 45-degree arcs. She may be a short distance from the male (<5cm) and in visual contact, or the male may be rubbing or nudging the female's back or tail. Pulling The female turns back towards the male, places her head on the posterior portion of the male's body, and pulls her head quickly over the male's back. This behavior is similar to snapping (see male behaviors), except the female is not displaced from the male. Pulling usually occurs during female-first tail-straddling walk. Stop and lower vent The female halts her forward movement and lowers her vent close to (or over) the spermatophore. She may or may not retrieve the sperm mass. Pass over spermatophore After the male has deposited a spermatophore, the female walks forward while elevating her body over the spermatophore. At this point, the male has moved his tail to one side, but the female continues forward with her chin on his tail base. Transfer sperm mass After the female lowers her vent on to the spermatophore, she will undulate her tail and sway forward and backward. Lift off The female lifts her cloaca upward after retrieving sperm and moves away. Resume tail straddling walk The female does not move away after spermatophore deposition, but re-engages in tail-straddling walk. This re-engagement occurs when the female is not inseminated and usually leads to additional spermatophore depositions (up to 4). 28 Behaviors performed by males only Snapping The male's head moves backward over the female's dorsum in a very rapid pulling motion, either at the base of the female's tail (from ffl'SW position) or perpendicular to the female's body. This usually places male 1-4cm from the female (Arnold and Houck 1982). Snapping is one type of "vaccination" pheromone delivery in which the male's pre-maxillary teeth abrade the skin of the female. Slapping While in tail-straddling walk, the male curls back toward the female and 'slaps' his mental gland on the female's nares (olfactory pheromone delivery). Crossing under The male walks under the female's chin until her chin is at the base of his tail. When the male positions himself so that his tail base is under her chin, he may straighten his hind limbs to lift up the female's chin. If the female is receptive, she will enter tail-straddling walk from this position. Spermatophore deposition and sperm transfer The male stops, lowers his vent to the substrate, and undulates his tail. When deposition is complete several mm later, the male flexes his tail to one side, raises his body off the spermatophore and resumes forward motion with the female's chin on his tail base. When the female stops to obtain the sperm mass, the male pushes backward under the female's chin by entending his hind limbs. Tail undulation The male arches and undulates his tail, which involves the full length of the tail. This is usually performed when the base of the male's tail is in contact with any part of the female's body. Head swinging The male moves head laterally in a side-to-side motion slightly above the substrate, alternating sides at a 15-degree arc. Behaviors performed jointly by males and females Linear Tail straddling walk (TSW) The female follows the male with her head on his tail base, straddling his tail. Female-first tail straddling walk (ffTSW) The male follows the female, initially with his head on the distal portion of her tail but moving towards the base of her tail, the male straddles her tail as he moves forward. An unreceptive female will pull her tail away from the male or walk fast to prevent ffTSW. Joint head swinging The male and female sway heads laterally, usually alternating sides relative to each other. The female's head moves in +I 45 degree arcs while the male's head swings at +1- 15 degree arcs. The pair either faces one another or the male is on top of the female and facing the same direction. Circling From female-first tail-straddling walk, the female turns back towards the male and the pair forms a circle. The female may or may not straddle the male's tail. If the female does straddle his tail, a circular form of tail-straddling walk ensues later leading to the traditional linear tail-straddling walk. Temporal Relations Plethodon cinereus I observed 11 complete courtship sequences (i.e., each sequence included spermatophore deposition and sperm transfer). Also, 13 observations were made of 30 initial courtship interactions not leading to spermatophore deposition (Table 2.2). In total, these observations involved 6 males and 13 females and included over 90 hours of courtship. Below, I categorize the courtship interactions using the three stages of courtship previously described for members of the plethodontid family (Organ 1960, Arnold 1972): (1) orientation (approach); (2) persuasion (head contact and pheromone delivery); and (3) sperm transfer (tail-straddling walk, pheromone delivery and spermatophore deposition). When first introduced into the courtship enclosure, both the male and the female explored the area. During the orientation stage, contact was initiated by males in 43% and by females in 56% of the staged encounters (N=24). During orientation, the male and female usually made contact by nudging, head contact, and, in one case, cloacal rubbing. This stage of the courtship interactions lasted, on average, 22 mm (SD=62.1; N24). The persuasion stage of courtship began after initial contact and both the male and female maintained interest in the courtship by slowing their movements around the arena, allowing for more direct contact. The main behavioral components of this stage were rubbing, tail wagging, and female-first tail-straddling walk. The male generally contacted the female's body, moving his head from posterior to anterior in a variety of ways, including rubbing, nudging, cloacal nudging, and snapping. Tail wagging by males usually occurred simultaneously with these behaviors, either while in contact with, or while following, the female. These male behaviors were often performed while in female-first tail-straddling walk. Snapping occurred sporadically, either while in the 31 Table 2. 1. A comparison of female (F) and male (M) courtship behaviors for each species. See text for description of the behaviors. + = behavior present, = behavior not present 0 = behavior not observed, and ? = uncertainty due to limited observations. Behaviors Female or Male Approach Retreat Nudging Rubbing/Sliding Cloacal nudging Head contact Head rubbing Foot shuffling Tail wagging Turning back Female Only Cloacal rubbing High amplitude tail undulations Tail wagging High amplitude head swinging Pulling Stopandlover cloaca Pass over spermatophore Retreive sperm Resume tailstraddling walk P. angusliclavius F M P. richmondi F M + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + - - + + + 0 ? + ? + + + + + - + + + 0 + + 0 + + + 0 0 Male Only Snapping Pulling Slapping Head swinging Spermatophore deposition Eat spermatophore Tail undulation Tail wagging Crossing under Male and Female Linear tailstraddling walk Female first tailstraddling walk Circling Joint head swinging P. cinereus F M + 0 + + + 0 + - + + + 0 0 0 + 0 + + + + + + + + + + + + 0 0 + + + + + + + + + + + + + + 32 female-first tail-straddling walk position or from a position perpendicular to the female. While in female-first tail-straddling walk, a female may arch and undulate her tail under the male's chin. Females also may perform high amplitude tail undulations, usually when in contact with the male. A female occasionally turned back towards the male while in female-first tail-straddling walk. In one courtship interaction, the female repeatedly turned back towards the male and the pair walked briefly in a circle. During the persuasion stage, females initiated rubbing, nudging, head contact and head rubbing. In courtship interactions that did not result in spermatophore deposition, the persuasion stage accounted for most of the time spent in courtship. In the 11 complete courtship sequences, the persuasion stage (as well as the entire courtship) was relatively short and many of the behaviors described above were not performed. The sperm transfer stage began when the male crossed under the female's chin, moved forward, and the female entered tail-straddling walk (TSW). While in TSW, the male undulated his tail under the female's chin and body as the pair walked forward in tandem. The amount of time in TSW ranged from 2 to 240 mm. The mean amount of time in TSW was 35 mm (SD=62.6; N=14) when courtships with more than two spermatophore depositions were included. Since the length of TSW increased with each successive spermatophore deposition, a few observations of multiple spermatophore deposition increased the mean substantially. If courtships with more than two spermatophore depositions are excluded, the mean amount of time in TSW was 5.7 mm (SD=4.82; N=7). After the TSW, the pair ceased moving forward as the male lowered his vent. While the male's vent was lowered, tail undulations increased in 33 speed, particularly as spermatophore deposition was nearing completion, which was within 6 mm. The male then raised his vent and shifted his tail to one side at a 90degree angle to his body (cf Arnold 1976). The pair moved forward until the female was positioned over the spermatophore. The female lowered her cloaca and swayed/rocked on the spermatophore to lodge the sperm mass in her cloaca. While the female retrieved sperm, she foot shuffled. In one instance, the female then crawled over the male and rubbed her cloaca on the base of the male's tail, dislodging the sperm mass from her cloaca. A male typically deposited one spermatophore, but occasionally two to four spermatophores were deposited in the course of a single courtship interaction. Plethodon angusticlavius I observed four courtship sequences with spermatophore deposition occurring in two of these courtships. Two of the four courtship sequences involved the same male and I had only one female in the study. Observations include over 4.5 hours of courtship (Table 2.3). When introduced into the courtship arena, a pair initially remained stationary and in close proximity, usually with one animal on top of the other. Once the pair began exploring the area, both the male and female would approach one another repeatedly. The male would attempt to make head contact with the female while tail wagging, in a manner similar to that described above for P. cinereus. The approach 34 Table 2.2. A summary of the duration (mm) of the phases of courtship for Plethodon cinereus. The phases are separated into seven categories: approach, HC/TW= head contact and tail wagging, fITS W=female-first tail-straddling walk, ITSW = linear tailstraddling walk, SD=spermatophore deposition, ST=sperm transfer. See text for a more detailed description of these phases. The total time of each courtship sequence is presented (far right column), along with means, standard deviations, and percent of total for each courtship component (zeros were not included in calculations). The number in parentheses next to the sequence number denotes the number of multiple courtship sequences by the same pair that occurred sequentially on the same night. A blank in the ST column reflects a courtship without SD. Plethodon cinereus Phases of Courtship Approach HCTTW ffTSW ITSW SD ST 1(1) 14 103 0 2 5 2(1) 3(1) 4(1) 5(1) 6(2) 7(2) 8(3) 9(4) 0 0 0 5 0 5 5 0 0 0 9 0 0 0 0 25 22 35 240 6 6 0 6 0 0 0 0 0 0 No No No No Sequence 10(5) 11(6) 12(7) 13(8) 14(8) 15(8) 16(9) 17(9) 18(9) 19(9) 20(9) 21(10) 22(11) 23(12) 24(13) 240 2 2 1 1 I 1 1 60 0 0 450 150 287 520 62 243 30 0 420 10 8 0 0 0 0 1 6 0 0 690 291 245 195 238 Mean 337 22.5 3952 219.6 SD 62.1 % 6.2 199.5 73.1 Total of total 1 1 1 54 39 I 1 1 0 0 0 0 0 0 0 0 6 83 249 1 460 46 76.6 8.5 3 0 0 0 0 0 0 2 4 II 5 0 3 0 6 14 5 26 58 64 0 0 0 0 5 485 34.6 62.6 9.0 6 0 0 0 0 0 59 5.4 0.7 1.1 Yes Total (mm) 124 15 28 41 480 22 477 208 332 750 No Yes No No Yes No 75 333 96 18 420 27 25 33 64 754 346 298 195 246 5407 225.3 224.5 35 phase of courtship accounted for 29% of courtship duration (mean=27 mm; SD 36 mm, N=4). When the female became more receptive, the male began head contact by rubbing and sliding his head on the female, beginning at the tip of her tail and working his way to her head. While the male rubbed the female's head, he seemed to focus on and around the nares of the female, perhaps accomplishing olfactory pheromone delivery. The female would slow her walk, allowing for female-first tail-straddling walk. The mean time spent in female-first tail-straddling walk was 10.25 mm (SD=7.6 mm; N=4). While the male straddled the female's tail, she undulated her tail in response to the male's contact. From female-first tail-straddling walk, either the male would break contact and move axially along the female and attempt to cross under her chin, or else the female curled back towards the male's tail base (forming a circle) and then the pair entered linear tail-straddling walk. If the male broke contact, he made several attempts to initiate tail-straddling walk by crossing under the female's chin while walking forward to give the female an opportunity to step over his tail and enter tail-straddling walk. Tail-straddling walk lasted an average of 6.3 mm (SD=2.9 mm) for the two complete sequences with spermatophore deposition. During tail-straddling walk, the male would undulate his tail under the female's body. In one sequence, the male turned back towards the female's head and seemed to tap her nares in a manner similar to that observed for pheromone delivery in Plethodon shermani (see Figure 1, Rollmann et al. 2000). However, due to the angle of the view, I was unable to confirm if the male made 36 contact with the female's nares. I also did not observe snapping or pulling in P. angusticlavius. After a short period of linear tail-straddling walk, the male stopped forward movement and lowered his cloaca onto the substrate and deposited a spermatophore. Spermatophore deposition consistently took 6 mm. Once the spermatophore was deposited, the pair moved forward. The female in these two courtships did not attempt to become inseminated (i.e., in both cases she did not move her cloaca down onto the spermatophore). Table 2.3. A summary of the duration (mm) of each phase of courtship for Plethodon angusticlavius. The phases are separated into seven categories: approach, HC!TW= head contact and tail wagging, fffSW=female-first tail-straddling walk, ITSW = linear tail-straddling walk, SD=spermatophore deposition, ST=sperm transfer. See text for a more detailed description of the courtship phases. The total time of each courtship sequence is presented (far right column) along with means, standard deviations, and percent of total of each courtship component. The number in parentheses next to the sequence number denotes the number of multiple courtship sequences by the same pair that occurred sequentially on the same night. A blank in the ST column reflects a courtship without SD. Plethodon angusticlavius Phases of Courtship Approach HC/TW 1(1) 2(1) 3(2) 68 64 4 9 3 3 59 4(2) 0 8 21 8 Total 81 Mean SD 27 35.6 29.0 134 33.5 32.5 41 10.3 48.0 14.7 Sequence % of total ffTSW 9 7.6 ITSW SD 6 8 0 3 8 0 6 0 19 12 6.3 2.9 6.8 6 0 4.3 ST No No Total (mm) 155 10 90 24 279 69 66.7 37 Plethodon richinondi I observed five initial courtship interactions. Spermatophore deposition only occurred during courtship encounters that were not recorded on video-tape. These observations involved two females and two males and included over 27 hours of courtship (Table 2.4). I describe below the male-female interactions during the orientation and persuasion courtship stages since sperm transfer behaviors were not observed. A male and female were equally likely to initiate contact by approaching the other. During this stage, the male and female made contact by nudging or head contact. This phase of courtship was relatively brief lasting on average 5.6 mm (SD=7.4 mm; N=5). Most of the courtship interactions occurred during the persuasion stage and included head contact and tail wagging. On average, this stage lasted 257 mm (SD= 172 mm; N=5) and included behaviors such as male rubbing and sliding, head rubbing, male and female tail undulations, high amplitude head swinging, joint head swinging, cloacal nudging, female-first tail-straddling walk, and snapping. A male initiated the head contact stage by following the female axially along her dorsum. A male began rubbing and sliding on all parts of the female's body, approaching the female's head last. Once the male reached the female's head, the female often performed high amplitude head swinging and, in one courtship, the pair performed joint head swinging. The male focused his rubbing around the cloacal region of the female. During this stage the male and female undulated their tails when contact occurred. Female-first tail-straddling walk also occurred during the persuasion phase of courtship. A receptive female allowed the male to rub and slide while straddling her tail. An unreceptive female kept her tail curled away from the male in response to his rubbing her tail, preventing ffTSW. The male moved forward over her tail until he reached the tail base. Snapping typically occurred while in this position, although males infrequently will snap while perpendicular to the female. During female-first tail-straddling walk, the female sometimes turned back towards the male's tail base and nudged the male. The male arched and undulated his tail, presumably in an attempt to persuade the female to enter linear tail-straddling walk. When not in female-first tailstraddling walk, the male occasionally lifted his tail base under the female's chin and arched and undulated his tail. The female usually broke contact with the male and he then ceased tail undulations. On average, female-first tail-straddling walk lasted 21 mm (SD=36 mm; N=5). DISCUSSION Courtship behaviors observed in three Plethodon species represent a significant deviation from the typical courtship pattern previously described for this salamander genus. The typical pattern involves a linear, tail-straddling walk led by the male, a pattern found in all major clades of the family (Houck and Arnold 2003). This type of tail-straddling walk is consistently linear except when the male turns back for pheromone delivery (e.g., Fig. 1 Rollmann et al. 2000) or turns for obstacle avoidance. In contrast, the courtship behaviors of Plethodon cinereus, P. angusticlavius, and P. 39 Table 2.4. A summary of the duration (mm) of the phases of courtship for Plethodon richrnondi. The phases are separated into seven categories: approach, HC/TW= head contact and tail wagging, fffSW=female-first tail-straddling walk. See text for a more detailed description of the courtship phases. The total time of each courtship sequence are presented (far right column) along with means, standard deviations, and percent of total for each courtship component. Tail-straddling walk, spermatophore deposition, and sperm transfer were not observed in this species. Plethodon richmondi Sequence 1 2 3 Phases of Courtship Approach 1 I 1 4 18 5 7 Total Mean HC/TW 447 299 377 33 132 ffVSW 84 1 17 2 1 Total (mm) 519 330 577 61 139 28 1288 105 1626 257.6 21 SD 5.6 7.4 171.8 % of total 1.7 79.2 35.9 6.5 325.2 226.6 richmondi all included a novel, female-led tail-straddling walk. Moreover, females in these species more actively participated in courtship than did females of other Plethodon species. The females I observed initiated courtship as often as did males, performed tail undulations or tail wagging, and also approached, nudged, and rubbed males. Additionally, these females occasionally turned back towards the male while in female-first tail-straddling walk. Other notable female behaviors seen in at least one of these species included head swinging (P. cinereus and P. richmondi), cloacal rubbing (P. cinereus) and pulling (P. richmondi). While small sample sizes in two species, P. angusticlavius and P. richmondi, provided limited observations, plethodontid salamanders exhibit highly stereotyped behavior within a species. Consequently, my results provide strong predictions of female behavior under average conditions. The female-first tail-straddling walk seen in these three species may represent a less frequent segue into the male-led linear tail-straddling walk (Figure 2.1), which typically leads to spermatophore deposition. Such a sequence of behavior happened when, during female-first tail-straddling walk, the female turned back towards the male (forming a circle) and placed her chin on the base of his tail. The male then began the linear male-led tail-straddling walk. I did not observe this complete sequence in each of the three species, but P. angusticlavius performed this sequence of behaviors and the courtship led to insemination. Although P. richmondi performed female-first tailstraddling walk, I did not observe the male-led tail-straddling walk or insemination. In P. cinereus, pairs that performed female-first tail-straddling walk either did not engage in male-led tail-straddling walk or terminated the female-first tail-straddling walk and only later entered the male-led tail-straddling walk. However, females in all three species turned back towards the male during female-first tail-straddling walk. Recent observations of plethodontids in the genus Aneides also revealed a circular tail- straddling walk that led to the traditional male-led linear form (Sapp 2002). Based on the phylogeny of these salanianders (Larson et al. 2003), it seems likely that the circular form of tail-straddling walk evolved twice within this group (Figure 2.2). The delivery of pheromones by male Plethodon salamanders typically occurs during the linear tail-straddling walk (Arnold and Houck 1982, Houck 1986). In these three species, however, most pheromone delivery occurred during female-first tail- straddling walk. The male scratched the female's tail from the tip until he arrived at the base of her tail. In this position, the male snapped (a quick pulling back of his teeth across her dorsum), which is another type of vaccination pheromone delivery. As a courtship progressed, the female walked more slowly, allowing for the maintenance of contact during the female-first tail-straddling walk. In P. angusticlavius, one courtship interaction included a "slap" from the male as he led tail-straddling walk. Also, P. richmondi and P. angusticlavius exhibited persistent head-head rubbing, which may result in olfactory pheromone delivery to the female's nares. The earlier-timed pheromone delivery in these species may indicate the pheromone's function to persuade reluctant females to mate suboptimally, perhaps indicative of a sexual conflict over mating. In an earlier description of courtship in P. cinereus, Gergits and Jaeger (1990) observed 10 courtships and four inseminations in the field. They concluded that the courtship sequence of this species generally followed the pattern of other plethodontid salamanders. In contrast, my observations show that female behavior in this species is much more variable than previously thought. The difference between field observations and my laboratory observations of courtship behaviors may be due to laboratory artifact. However, studies of agonistic behavior in P. cinereus conducted in the field and laboratory indicate that laboratory-based inferences are a reasonable representation of field behavior, at least in P. cinereus (Gergits and Jaeger 1990). Another explanation for the differences between my observations and those of Gergits and Jaeger (1990) may be the unusual weather conditions that led to their observation of 10 pI Figure 2.1. Courtship sequence for P. angusticlavius. This sequence began with female-first tail-straddling walk (top). The female (lighter animal) turned around towards the male, formed a circle (bottom right). From this position, the female entered tail-straddling walk (bottom left). This drawing has been created from videotaped footage of P. angusticlavius. The depicted courtship led to spermatophore deposition. 43 coiirtships in one night. Prior to their field observations, multiple weeks of dry conditions led to limited courtship opportunities. After one rainy day, the density and activity of salamanders on the surface dramatically increased and the females apparently were highly receptive. Courtships in which the female is highly receptive often do not include pheromone delivery (Arnold 1977, Halliday 1990, Houck and Reagan 1990). Thus, it is not surprising that the courtship observations of Gergits and Jaeger (1990) did not include pheromone delivery and the associated behaviors of snapping and female-first tail-straddling walk. Previous attempts to observe the complete courtship of these species have had limited success (Arnold 1972). In general, my observations of male behavior in P. cinereus are in agreement with earlier observations of Arnold (1972). However, the female behaviors I observed in this species have not been reported previously. Similarly in P. richmondi, many of my observations are novel, but in general my observations of male behavior agree with those of Arnold (1972). Interestingly, the courtship observations of P. vandykei, a western species, reveal some similarities to those of P. richmondi (Lynch and Wallace 1987). In particular, these are the only two Plethodon species that exhibit joint head swinging. Similar to my observations and those of Aneides, female P. vandykei, also have a more active role in courtship than most plethodontids (Sapp 2002, Lynch and Wallace 1987). Prior observations of P. angusticlavius were not available, but following my observations, a colleague observed courtship in a northern population of a closely related species P. dorsalis, both species are within the P. dorsalis complex (Picard 2005). These observations are in general agreement with those presented here. In particular, slapping was observed as the mode of pheromone delivery, but occurred prior to, instead of during, the male-led tail-straddling walk. In addition, female-first tail-straddling walk was observed, but females did not turn back towards the males. These populations are now considered to be different species within the P. dorsalis complex (Highton 1997), perhaps accounting for differences between courtship behaviors. The courtship of P. welleri, closely related to P. angusticlavius, has been observed, but the mode of pheromone delivery is still unclear (Organ 1960b, Arnold 1972). Based on observations of pheromone delivery in P. angusticlavius, I would expect to see olfactory delivery in P. welleri as well. Documentation of courtship pheromone delivery suggests that most small eastern Plethodon species have the ancestral delivery mode of "vaccination" while large eastern Plethodon have a derived mode of olfactory pheromone delivery. My additional observations of pheromone delivery correspond to the phylogeny established from molecular data for the eastern Plethodon species (Figure 2.2) (Highton and Larson 1979, Larson et al. 1981, Highton 1991, Mahoney 2001). In particular, P. cinereus and P. richrnondi are among the vaccinating Plethodon species, and my observations of scratching, pulling and snapping in these species confirm their more basal placement in the group. Plethodon angusticlavius lies in an intermediate position on the phylogeny in that this species is more closely related to large eastern Plethodon species than to other small eastern Plethodon species. Until now, the method of pheromone delivery in this intermediate group had been undetermined. My observations suggest that the P. 45 angusticlavius method of pheromone delivery is intermediate between those having vaccination and olfactory delivery since males perform behaviors associated with both delivery modes (i.e., rubbing and slapping, respectively). In addition, mental gland morphology, pre-maxillary teeth, and behavioral observations of slapping prior to linear tail-straddling walk supports the placement of this species as intermediate between vaccination and olfactory pheromone delivery (Highton 1962, Coss 1974, Picard 2005). As compared to other well-studied Plethodon, females of these three species participate more actively in courtship during the course of this study. The more assertive courtship behaviors of these females could have social and phylogenetic implications. In particular, courtship behaviors performed by females of these species may represent a derived condition, one reflected in salamanders that have a more complex social structure relative to other conspecifics. Many laboratory and field studies have revealed such a complex social structure for P. cinereus. Females and males each maintain territories that often overlap, adults are socially monogamous, both males and females co-defend territories during the breeding season and females are less aggressive towards familiar females than towards unfamiliar females (Gabor and Jaeger 1995, Gillette et al. 2000, Lang and Jaeger 2000, Jaegar et al. 2001, Jaeger and Peterson 2002). Male P. (inereus are more aggressive towards polyandrous females than towards monogamous females during the courtship and non-courtship season (Jaegar et al. 2002). In addition, individuals in pairs co-defending territories are not aggressive towards opposite-sex intruders, suggesting a potential conflict between male and female remating strategies (Jaeger et al. 2002). If more active female participation in courtship is a derived jHS SN P IH f1-TSW SL Aneide aeneu D D Aneidex/erreus. E flaipunciatus. lugubris 0 0 0 Plethodon OlE 1E1 lEE 1 (largeeastern) Plethodon angusticlavius Plethodon dorsalic I 0 I Plethodon welleri Plethodoncinereus Plethodon richmondi I 0 0 IU I0 I0 IU IU 0 0 0 0 U U 0 0 U 0 0 0 IU i I i I U U U U U U Plethodon vandykei fl Figure 2.2. Character states of behavioral features mapped onto a partial phylogeny of Plethodon and the related genus Aneides: P (pulling), I-TSW (linear tail-straddling walk), jHS (joint head swinging), SN (snapping), SL (slapping), ff-TSW (female-first tail-straddling walk), fH (female head rubbing), and fHT (female head rubbing and/or tail wagging). Behaviors are described in the text. Minus sign denotes evolutionary loss. This tree is based on information in Mahoney (2001) and Larson et al. (2003). Dark squares denote observed behaviors, gray squares denote hypothesized behavior presence, and open squares indicate a lack of observations or absence of behavior. Gray rectangles represent proposed phylogenetic transitions. condition corresponding to a more complex social structure, I predict that western species of the genus Aneides would have independently evolved more active female behaviors (e.g., Sapp 2002) and a complex social structure similar to that found in P. 47 cinereus. Future studies of social and courtship behavior in Aneides could test this prediction. My observations of courtship in P. cinereus, P. angusticlavius, and P. richmondi revealed novel behaviors that have implications for the phylogeny and sociality of plethodontid salamanders. In particular, the method of pheromone delivery in P. angusticlavius is behaviorally intermediate between courtship behaviors for Plethdon species having vaccination or olfactory delivery methods. In addition, more active female participation in courtship is present in at least one species (P. cinereus) that has well-documented, complex social structure. Documenting previously unknown courtship behaviors contributes to a broader foundation of courtship observations in these salamanders and facilitates the generation of novel, testable predictions. For example, Plethodon species that are closely related to P. angusticlavius (P. wehrlei, P. welleri, and P. punctatus) should also perform slapping as a method of pheromone delivery. Consequently, courtship descriptions enhance our understanding of the relationships between closely related species and provide insight into the direction and impetus for behavioral evolution. CHAPTER 3 MALE COURTSHIP PHEROMONES AND FEMALE RERPRODUCTIVE SUCCESS Leslie A. Dyal, Lynne D. Houck, and Amy Picard ABSTRACT Male plethodontid salamanders produce a pheromone that is delivered to females during courtship. Courtship pheromone communication between males and females provides an opportunity to investigate the costs and benefits of a signaling system from the perspective of sexual conflict. The courtship pheromone alters a female's reluctance to mate, leading to courtships that are shorter in duration. If the pheromone functions to manipulate female mating decisions, it may negatively affect some aspect of female reproductive success. We examined the consequences of pheromone delivery on clutch size, egg size, and early mortality in Plethodon shermani. Our results do not provide conclusive evidence that male courtship pheromones negatively affect female reproductive success. However, the pheromone appears to alter clutch size in some females under particular circumstances. In addition, the pheromone treatment is correlated with higher egg retention in females. Our results provide incentive for more research on whether the male courtship pheromones mediate sexual conflicts over mating. INTRODUCTION The evolution of signaling systems has long been a popular area of research and a subject of considerable controversy (Darwin 187!, Huxley 1914, Tinbergen 1959, Johnstone 1996). The debate centers on the degree of honesty and cheating between signal senders and receivers (Grafen 1990, Zuk et al. 1990, Guilford and Dawkins 1991, Dawkins and Guilford 1991, Michod and Hasson 1990, Krebs and Dawkins 1984, Burk 1988). If signals are predominately honest, both the sender and receiver will benefit from communication. However, if signals are dishonest and manipulative, then the benefits of communication may predominantly lie with the sender. The concept of honest or manipulative signals can be examined in communication systems involved with female choice of mates with exaggerated secondary sexual traits. Understanding the evolution of signaling between males and females for the purposes of reproduction requires addressing the theoretical perspectives of both sexual selection and signal systems (honesty vs. manipulation). Proponents of the view that signaling systems are predominately honest suggest that, if the signal is costly, then cheating will be rare. In this scenario, the signal is an honest indicator of quality due to the costs of producing the signal (Williams 1966, Trivers 1972, Emlen 1973, Zahavi 1975, Grafen 1990, Kodric-Brown and Brown 1984, Rich and Hurst 1998). Costs associated with signal production and maintenance ensure that only individuals of high quality can handle the costs (Zahavi 1975, Clutton-Brock and Albon 1979, Hamilton and Zuk 1982, Zuk et al. 1990). The honest signaling perspective focuses mainly on the fitness consequences of the signal on its sender (e.g., 50 males) (Fisher 1958, Michod and Hasson 1990, Arnold 1983, Kirkpatrick 1982, Lande 1981, Grafen 1990, Kodric-Brown and Brown 1984). The frequency of cheating in such systems is rare due to the phenotypic constraints associated with signal production (e.g., Hill 1992, Hill and Montgomerie 1994). While the idea of condition-dependent costs of signal production seem compelling, the experimental evidence linking condition-dependent male traits to female mate choice based on mate quality is lacking in most animal systems (Johnstone 1996, Navara et al. 2001, Mays and Hill 2004, Hill et al. 2004). Alternatively, the manipulative or dishonest signaling perspective addresses the fitness consequences on signal receivers (typically, females) and argues that costs to receivers are essential to understanding the evolution of communication systems (Guilford and Dawkins 991). At the very least, receivers incur costs that include the time spent determining the honesty of a signal, as well as predation and disease risks arising from proximity of the sender (Krebs and Dawkins 1991). In other words, if the signal is costly to produce and send, it may also be costly to receive. When receiver costs are included, the honesty of communication systems becomes questionable and is likely to be less frequent than expected (Dawkins and Krebs 1978, Krebs and Dawkins 1984). Thus, females may settle for less informative signals that are less costly to receive, even when reception costs are less than signaling costs. These receiver dynamics are predicted to lead to a less honest, but more conventional, signaling system (Guilford and Dawkins 1991). Conventional signals occur when signals do not reflect an underlying quality in the signaler (even if the signal is somewhat costly to produce) because of selection pressures to reduce costs on both signaler and receiver (Dawkins and Guilford 1991). One common example is prey with warning coloration associated with distastefulness. The predator learns that prey with warning colors are distasteful (i.e., category recognition). Asssessing the honesty of this signal is costly to the receiver such that cheats in the form of Batesian mimics exist in low frequencies (Guilford and Dawkins 1991). Conventional signals may or may not be limited by phenotype, depending on the costs of cheating, in addition to signal production costs. If cheaters can avoid punishment, their frequency may increase (Barnard and Burk 1979). In fact, the high diversity of signals can be attributed to variation in the receivers of conventional signals (Dawkins and Guilford 1991). Additionally, if receivers benefit from of an accurate assessment of male quality, selection on receivers would need to be incorporated to determine the cooperative or conflicting nature of the communication system (Trivers 1972, Clutton-Brock and Vincent 1991, Clutton-Brock and Parker 1992, Johnstone et al. 1996, Johnstone 1996). Proponents of the conventional signaling perspective suggest that an honest indicator of quality in the form of one clear signal would be beneficial to both senders and receivers. However, most signals have multiple components that provide visual, tactile, acoustic, and/or olfactory information. Conventional signaling theory predicts that multi-component signals contain no strategic message about sender quality (Guilford and Dawkins 1991). Instead, multi-component signals are constrained by efficiency and are designed to enhance the effectiveness in eliciting the appropriate receiver response (Johnstone 1996). Communication systems with conventional, multicomponent signals are more likely to be invaded by cheaters. 52 Predictions about dishonest or manipulative signals are similar to those proposed by sexual dialectics theory, which addresses conflicts over mating decisions (Gowaty 1997). Sexual dialectics suggests that a conflict between discriminating females and non-preferred males will result in sexually dimorphic traits in males that function to manipulate or coerce female mating decisions. The main assumption of the theory is that freely expressed female mating preferences are adaptive, such that female reproductive success is higher when mating is under female control than when coerced or manipulated by males. Sexual dialectics theory predicts that a signal that alters a female's mating decisions could negatively affect female reproductive success and/or offspring fitness. This is a key point. Thus, the most direct method of distinguishing between the alternative theories of the evolution of animal communication systems lies in the fitness consequences to signal receivers (Guilford and Dawkins 1991, Dawkins and Guilford 1991, Krebs and Dawkins 1984, Gowaty 1997). Plethodontid salamanders have a sexual communication system that permits examination of the fitness consequences of signal reception. During the breeding season, a male produces a multi-component pheromone cocktail from a seasonally enlarged gland under its chin (I-buck and Sever 1994, Feldhoff et al. 1999, Rollmann et al. 2000). The courtship chemical signal is delivered only after courtship has begun (Arnold 1977, Houck 1986). This direct male-to-female delivery within the context of courtship means that there are no unintended receivers of the pheromone signal. Males within a population vary significantly in the relative composition of pheromone components (Rollmann et al. 2000). This variation is a means by which a female might prefer one male over others. Experimental delivery of pheromone to females decreases 53 the length of courtship, interpreted as increasing female receptivity (Houck and Reagan 1990, Rollmann et al. 1999). Alternatively, sexual dialectics and conventional signaling theories would suggest that the pheromone could manipulate female mating decisions. Based on the theories discussed above, the chemical communication system of plethodontids may be one that is vulnerable to dishonest or manipulative signaling for several reasons. First, signal production is limited to the breeding season so that any costs of signal production are limited in time, which would make cheating easier. Second, females selecting mates would only discriminate against a subset of males, so that the number of males under selection to cheat would be smaller than the total number of males in the population. In addition, if females vary in their preferences, then the pool of non-preferred males would not be fixed. As a consequence, honest signaling males would predominate over cheaters, preventing the collapse of the communication system. The frequency of courtships that lack pheromone delivery, but contain visual and tactile signals (Arnold 1976), supports the idea that some males are successful without attempts to alter female receptivity to mating. The lack of pheromone delivery may occur because these particular females are initially receptive to mating with preferred males. In addition, the pheromone signal itself has multiple components that are highly variable and often combined with tactile cues. Thus, this pheromone signal is likely designed for effectiveness in eliciting the preferred responses from receivers (e.g., receptivity to mating) (Krebs and Dawkins 1984, Guilford and Dawkins 1991). Finally, the cost of producing the pheromone is likely to be small due to the seasonal constraints on production relative to benefits in terms of increased mating success experienced by males that use the pheromone. 54 The male courtship pheromone of plethodontid salamanders may manipulate female mating decisions. Accordingly, we experimentally addressed the fitness consequences of signal reception in terms of female reproductive success in Plethodon sherinani. The main goal was to distinguish between the alternative functions of the pheromone signaling system. If the courtship pheromone functions as a dishonest signal that manipulates female mating decisions due to a sexual conflict over mating, then female reproduction should be negatively affected. On the other hand, if the pheromone is an honest signal of male quality, then it should be beneficial to both males and females or at least neutral in its effects on female reproductive success. We measured clutch size, egg size, egg retention, and early mortality to assess whether pheromone delivery affected female reproductive success. METHODS Adult male and female P. shermani were collected from a single site in Macon County, North Carolina, USA, during August and early September in 2001, 2003. 2002, and The year of collection was used to refer to results for each year, although treatment results were measured in the following January or February. Females were determined to be gravid by the presence of enlarged ova, visible through the body wall. Each male had an enlarged mental gland, known to produce courtship pheromones. Following collection, all salamanders were transported to Oregon State University (Corvallis, OR) and housed in a climate-controlled room at 13 16° C photoperiod. Animals were housed individually in plastic shoeboxes with a natural (9x13x17 cm). Boxes were lined with moist paper towels and several moist, crumpled paper towels 55 were added to provide cover. Each salamander was fed two waxworms (Galleria mellonella) once per week. Total sample sizes for control and experimental treatments were: 29, 72, and 76 females in 2001, 2002, and 2003, respectively. Beginning in August and ending in mid September, each female was repeatedly paired with a randomly selected male until insemination was confirmed by observation of spermatophore deposition and sperm transfer (from one to five pairings) beginning in August and ending in mid-September. In 2002, each female that mated more than once was inseminated by the same male during subsequent matings, thus decreasing the potential for multiple paternity within a clutch. The size measurements of all males in 2002 were collected as a covariate in the analysis of factors influencing fecundity. Additionally, the females from 2003 were not randomly chosen. Females were selected in 2003 such that there were relatively similar numbers of females distributed across the size range. This selection procedure was inspired by results from 2002, in which a relatively truncated distribution of females may have affected the assessment of treatment effects. We prepared a pooled whole pheromone extract by anesthetizing males in 4% ether and excising their mental glands. Glands were placed in a vial containing a solution of 0.8 mM acetylcholine chloride in amphibian Ringer's solution for 30 mm. Glands were kept on ice for 30 mm, after which the gland was removed and the solution containing pheromone components was centrifuged (14,000 g) for 10 mm at 4 °C. The pooled extract was frozen at 80°C. The pheromone solution was later processed in the laboratory of Richard Feldhoff (Univ. of Louisville School of Medicine, Louisville, KY), as described in Rollmann etal. (1999). This processing involved removal of the 56 acetylcholine chloride, and standardizing the protein content of multiple aliquots that were subsequently thawed and used for treating females. Pheromone treatment began towards the end of the breeding season (November 5 in 2001 and in 2002, November 13 in 2003). Females were randomly assigned to a treatment type (pheromone or saline control), with the condition that both treatment types were represented in approximately equal numbers. A treatment was applied to each female in the box in which it was housed. Males were not present during the experiment to ensure that male behavior would not influence the outcome. Pheromone or saline was applied to a female's nares using a pipette. A treatment solution was delivered every other day for one hour. During this hour, a female received the appropriate solution at 10-minute intervals. In 2001 and 2002, females received treatments during the first two weeks of November (5 drops of 3tL for each of 5 days for a total of 751iL per female). Due to limited availability of pheromone in 2003, pheromone delivery was altered to the following scheme (each delivery occurring during one hour): Day 1 = 3 drops of 3tL, Day 2 = 3 drops of 3tL, Day 3 = 4 drops of 3tL, Day 4 = drops of 3tL, Day 5 = 5 drops of 3tL (total volume of pheromone or saline was 57jiL per female). Female P. shermani typically do not oviposit spontaneously in the laboratory, so each female was injected with gonadotropin (human pregnancy urine) every third day, beginning in mid-December, until oviposition occurred. If a female failed to oviposit by the 6Ih injection, she was excluded from subsequent analyses. Females that failed to oviposit included 9, 12, and 10 females in 2001, 2002, and 2003, respectively. Sample sizes reported in Table 3. 1 exclude females that did not oviposit. Injections occurred 57 between December 11 and 20 of each year. Gonadotropin (1mg) was diluted with 2mL of Ringers solution (500m1 DI water, 2.98g NaCL, 0.19g KCI, 0.12g CaCI). Eggs were collected from each female immediately following oviposition. Eggs were counted and yolk diameters were measured at the widest point using digital calipers. The relationship between female size and clutch size for treatment groups in 2002 suggested that treatment differences might affect the number of ova that were oviposited, with some females retaining more ova than did others. Accordingly, in the protocol for 2003, retained ova (visible through the body wall) were counted in addition to the number of eggs actually oviposited. In 2001 and 2002, each clutch was maintained in a separate jar under a water drip system (constant water movement minimized fungal growth). Clutches were recounted once per week for the first three weeks to determine if the treatment affected mortality rates during this time. Clutch size and mean egg size for each clutch were analyzed using linear regression (JMP by SAS), beginning with a model that included all factors believed to potentially influence these measures. Factors were dropped from the regression model if the p-value was greater than 0.05. The explanatory variables initially included female snout-vent length (SVL), male SVL, treatment (pheromone or saline), and the pairwise interactions between these variables. Early estimates for mortality (collected in 2002) and the proportion of eggs retained after oviposition (collected in 2003) were compared between treatments with logistic regression (alpha = 0.05). 58 RESULTS Measures of female body size (SVL), clutch size, and egg size are summarized in Table 3.1 for females in each treatment group. For all years combined, the mean female size was 62.49mm in SVL (range = 52 74mm), the mean clutch size was 18 eggs (range = 10- 38 eggs), and the mean egg size was 4.3mm in diameter (range = 3.5 5.1mm). Table 3.1. A summary of results for each year of the study for Plethodon shermani. Means were calculated over both treatment groups (0) and separately for each treatment group (P=pheromone; S=saline). All SVL data is in mm. Egg size was measured at the widest diameter in mm. Year 2001 n=20 n=59 2002 2003 n=75 0 P S 0 P S o P s 63.2 63.1 63.3 61.8 62.6 61.0 62.5 62.0 63 Largest Female SVL 70.3 69.0 70.3 71.8 67.8 71.8 74.0 71.0 74.0 Smallest Female SVL 56.9 59.6 56.9 52.6 58.9 52.6 52.0 52.0 54.0 Mean Clutch Size 15 15 15 19 19 19 21 21 20. 4.00 4.01 3.99 4.62 4.64 4.59 na na na Mean Female SVL Mean Egg 59 Clutch Size In 2001, there was no correlation between female SVL and clutch size, and no treatment effects on clutch size. In 2002, the sample size was increased (from 20 females in 2001) to 59 females. There was a significant positive relationship between Table 3.2. Linear regression anlysis of Plethodon shermani clutch size. The explanatory variables are female snout-vent length (SVL), treatment (saline or pheromone), and the interaction between female snout-vent length and treatment. YEAR 2001 (n=20) 2002 (n=59) 2003 (n=75) Variable Coefficient SE t-stat p-value SVL 0.39 0.41 -0.94 0.362 Treatment -0.33 1.41 -0.24 0.81 SVLxTreatment -0.19 0.41 -0.46 0.65 SVL 1.073 0.223 4.81 <0.0001 * Treatment 55.584 24.623 2.26 0.028 * SVLxTreatment -0.909 0.396 -2.29 0.0257 * SVL 0.4997 0.158 3.17 0.0022* Treatment 0.2703 13.59 0.02 0.984 SVLxTreatment 0.0167 0.2168 0.08 0.939 I In particular, the female SVL coefficient was 1.07 and 0.97 with and without two females, respectively, and the p-value for this variable went from 0.003 to 0.019. Similarly, the p-values for treatment and the interaction between treatment and female SVL were 0.028 and 0.026 with all females, and 0.095 and 0.093 when these females were omitted. In addition, the overall variance explained by the regression model decreased from 26% to 10% when the smallest and largest females were excluded (p- value increased from 0.0002 to 0.04). In 2002, sire SVL was not correlated with clutch size. The 2003 data were analyzed separately due to differences in female selection criteria and quantity of pheromone available for treatment. In 2003, there was a significant positive relationship between female SVL and clutch size (Figure 3.2). However, there was no significant treatment effect on clutch size. Overall, females retained from 1-9 eggs (mean 2.7 eggs). Pheromone-treated females retained proportionally more ova than did saline-treated females (t-ratio=-2.39; p-value=0.02; Figure 3.3). The number and size of pheromone-treated and saline-treated females that retained eggs was not significantly different. Of females that retained ova, pheromonetreated females averaged 60.9mm in SVL (n = 20) and oviposited 19.5 eggs and saline- treated females averaged 62.1mm (n = 13) and laid 18.6 eggs. Females (n = 15 pheromone-treated, n = 13 saline-treated) that oviposited an entire clutch averaged 64mm in SVL and oviposited an average of 22 eggs. 62 40 35 A S 30 w 25 - N .5 20 15 10 5 0 50 55 60 65 70 75 Female SVL Figure 3.2. Relationship between female SVL (mm) and clutch size (number of eggs) for Plethodon shermani in 2003. The dark circles represent the pheromone-treated females and light triangles represent saline-treated females. The solid line represents the group of females receiving pheromone treatment. The dotted line represents the females receiving saline. 800 F, 700 600 500 400 300 200 100 0 Treat Figure 3.3. The total number of eggs retained by female Plethodon shermani after oviposition in each treatment group in 2003. Pheromone-treated females retained a significantly larger proportion of eggs than did saline-treated females (t-ratio=-2.39, p = 63 0.02, df=42, N=46). Lighter bars represent the total number of eggs oviposited and the dark bars represent retained ova. Egg Size In 2001, there was no significant effect of any explanatory variable on egg size, presumably due to high variation and low sample sizes. In 2002, there was no significant treatment effect. However, female size was positively correlated with mean egg size (effect estimate = 0.029, p 0.0009). In addition, clutch size was negatively correlated with mean egg size (effect estimate 0.014, p = 0.0083). In 2002, male SVL was not correlated with egg size. In 2003, we did not measure egg sizes due to lack of treatment effects in a previous experiment. Early Mortality We monitored early mortality in 2001 and 2002. Early mortality was not related to any explanatory variable. DISCUSSION Tests for the influence of male courtship pheromones on measures of female reproductive success revealed different effects in different years. In 2002, smaller females treated with pheromone had an increased clutch size, while larger females had a smaller clutch size. This effect is puzzling in that there is no obvious physiological mechanism that would account for differential effects due to female body size. The pheromone is detected by the vomeronasal system (Wirsig-Wiechmann et al., 2002) and there is no reason to think that smaller females have a different response to vomeronasal stimulation than would larger females It is possible that the observed pheromone effect is spurious because, a few "outlier" females representing the tails of the female size distribution may have substantially affected the results. In contrast, the 2003 data documented a lack of pheromone effect on clutch size. The amount of pheromone delivered to each female was lower than that in the 2002 experiment. However, the 57 tL delivered to each female was likely a substantial increase over amounts normally delivered by males during the mating season. While it is possible that multiple paternity in 2003 altered within-clutch variation, there is no evidence in plethdontid salamanders that males influence clutch size (no significant effect of male body size on any results in 2002). While there was no treatment effect on clutch size in 2003, pheromone-treated females retained more eggs than did saline- treated females. It is possible that the decrease in clutch size observed for larger, pheromone-treated females in 2002 resulted from egg retention, similar to that found in 2003. The lack of correlation in 2001 between female SVL and clutch size was unusual because a positive correlation typically is seen in plethodontid salamanders (Houck 1977a, b). Clutch size is highly variable in plethodontid salamanders, however, such that there can be a wide range of clutch size for females of a given SVL (Houck 1977a, b). Thus, small sample size (N=20 females) and relatively narrow range of SVL probably precluded seeing any positive correlation between female SVL and clutch size, as well as any effect of pheromone treatment. However, after combining 2001 and 2002 data for analysis, we found the same significant interaction between female size and pheromone treatment, similar to 2002 alone, but slightly higher p-values (combined analysis not included). A significant interaction between year of the study and female size in addition to a year effect on clutch size suggests that these years are different and should be analyzed separately. Larger sample sizes in 2002 and 2003 facilitated the demonstration of the expected female SVL and clutch size relationship. The positive correlation between female size and clutch size was consistent with that found in other plethodontid studies that had a sufficient sample size and range of female SVL (Houck 1977a, b). Drawing conclusions based on sexual conflict theory is difficult due to the inconsistency of results. If the male courtship pheromone functions to manipulate or coerce female mating decisions, then we would expect it to negatively affect female reproductive success. In fact, the pheromone did alter clutch size and egg retention for particular females in particular years. It may be that a female can alter the number of eggs oviposited in response to receiving pheromone due to perceived suboptimal mating with non-preferred males. Evidence that females can alter clutch characteristics relative to her or her mate's condition and quality has been found in many taxa (Blaxter 1986, Rothchild 1986, Bailey and Houde 1989, Chambers et al. 1989, Kaplan 1992, Leggett and Deblois 1994, Clark and Galef 1995, Parichy and Kaplan 1995, Gil et al. 1999, Olsson et al. 2002, Rutkowska and Cichon 2002, Pilz et al. 2003). Based on the results of this study, the possibility that male courtship pheromones function in the context of sexual conflict warrants further investigation. 66 CHAPTER 4 FEMALES BENEFIT FROM FREELY EXPRESSED MATE CHOICE IN THE WESTERN TOAD (BUFO BOREAS) Leslie A. Dyal ABSTRACT The fitness benefits of freely expressed female mate choice are assumed to drive sexual conflict over mating, also termed sexual dialectics (Gowaty 1997). I tested this assumption in two populations of the Western Toad, Bufo boreas because male-male scramble competition and forceful, indiscriminate amplexus by males seem to preclude the opportunity for a female to exercise her mating preferences. I asked whether female toads that mate with preferred males benefit in terms of offspring survivorship and size at hatching. Females mated to preferred males had a higher proportion of offspring surviving to hatching at one population. In addition, females mated to preferred males had longer tadpoles at hatching than did females that mated to non-preferred males at both populations. My results provide the first evidence in amphibians, anurans in particular, that freely expressed female mate choice is selectively advantageous. I discuss these results in terms of female resistance behavior and consequent mating system patterns. INTRODUCTION The theories on the evolution of female mate choice have undergone considerable transformation since they were recognized as one mechanism of sexual selection explaining the evolution of elaborate male traits (Darwin 1871, review in Andersson 1994). Darwin also proposed male-male competition as another force of sexual selection on males. The initial focus of both mechanisms was on how sexual selection affected male traits. The evolution of female preferences, however, was not considered in depth until Fisher's 1958 publication. Fisher argued that female preferences could be genetically correlated to the male traits that were preferred. Thus, the evolution of female preference could be driven by selection on males (Lande 1980, Kirkpatrick 1982). Over time, other work suggested that selection on female preferences might indirectly benefit females through genetic benefits to their offspring from mating with particular males (Fisher 1915; Williams 1966; Trivers 1972; Emlen 1973; Zahavi 1975; Partridge 1980, 1983; Hamilton and Zuk 1982; Kodric-Brown and Brown 1984; Andersson 1986; Pomiankowski 1988; Grafen 1990a,b; Iwasa etal. 1991; review in Andersson 1994). Studies also have suggested that female mate choice may be beneficial through non-genetic effects on female survival and fecundity (review in Andersson 1994). Most recently, the development of sexual dialectics theory suggests that female choice is adaptive through direct effects on female reproductive success and indirectly by accruing fitness benefits for offspring (Gowaty 1997). The main assumption of sexual dialectics theory is that a female mated with a male of her choice will benefit more than if she mated with a non-preferred male (Gowaty 1997). If females benefit from freely expressed mate choice, then male-female interactions that result in females mating with a non-preferred male will reflect sexual conflict. The behavioral manifestations of this conflict between potential mates, also termed sexual dialectics, results from selection acting on non-preferred males to 68 manipulate or coerce females' mating decisions. In turn, selection will favor females that are able to resist manipulation, if these females are better off, in terms of reproductive success and/or offspring viability, when mating decisions are under their own control. As a consequence, mating system variation, both within and among species, should be a function of females' abilities to resist direct and indirect attempts of manipulation or coercion (see Gowaty 1997). Recent research has revealed the importance of sexual conflicts over mating decisions. In certain species, conflicts over mating are critical in determining many significant factors: individual fitness, selection on males and females, physiological, behavioral, and morphological evolution, mating system variation and speciation (e.g., Rowe et al. 1994, Parker and Partridge 1998, Arnqvist et al. 2000, Drickamer et al. 2000, Moore et al. 2001, Arnqvist and Rowe 2002,). Studies of invertebrates, in particular, have contributed significantly to our understanding of sexual conflicts (Rice 1996, Finke 1997, Holland and Rice 1999, Arnqvist et al. 2000, Shukar and Day 2001, Soulier-Perkins 2001, Blankenhorn et al. 2002, Miller 2003). In water striders (Aquarius sp., Gerris sp.), planthoppers (Lophopidae), and diving beetles (Dystiscidae), for example, independently established phylogenetic patterns reveal the order and alternation of traits that are related to sexual conflict over mating (Rowe et al. 1994, Soulier-Perkins, 2001, Miller 2003). Water striders, in particular, are one of the most studied systems in terms of sexual conflict over mating. The focus on these insects can be attributed to the visually obvious conflict over mating as seen in pre- and post- mating struggles between males and females (Rowe et al. 1994, Watson et al. 1998, Ortigosa and Rowe 2003). Research on mating conflicts in water striders has focused on the costs and benefits to each sex, environmental and social factors influencing conflict outcomes, behavioral and morphological evolution, and phylogenetics (Fairbairn 1990, Rowe 1992, Arnqvist and Rowe 1995, Watson et al. 1998, Arnqvist and Danielsson 1999, Fairbairn et al. 2003). In the water strider Gerris buenoi, mating is costly to a female in terms of slower skating speed due to carrying a male (Rowe 1992). The slower speed leads to decreased foraging efficiency and increased risk of predation. Consequently, females frequently are reluctant to mate and this reluctance leads to pre-mating struggles (Rowe et al. 1994). However, when the sex ratio is highly skewed towards males, a female is more likely to accept a mate to offset the costs of repeated harassment by males while being single. The results of manipulating social and environmental variables (density, sex ratio) follow the prediction of increased female reluctance as a function of declining male harassment rate (Rowe 1992). In another water strider, G. odontogaster, males have enlarged clasping appendages that function to overcome female resistance during pre-mating struggles (Arnqvist 1989, Andersen 1991). Female resistance biases mating frequency according to male clasper size (Arnqvist 1992). Traditionally, clasper size would be viewed as a trait under sexual selection by female mate choice, even though clasper size is driven by sexual conflict and female resistance behavior. In addition, other components of male size appear to be related to the ability to subdue females, resulting in nonrandom mating patterns by body size and leg size in several other water strider species (Rubenstein 1984, J-Jayashi 1985, Fairbairn 1988, 1990, Kaitala and Dingle 1993, Preziosi and Fairbairn 2000). 70 Studies of sexual conflict are increasing, but the importance and taxonomic distribution of sexual conflicts is unclear (Gowaty 1997, Chapman et al. 2003, CordobaAguiler and Contreras-Garduno 2003, Eberbard and Cordero 2003). We need concise and well-controlled experiments that allow us to differentiate between traditional forms of sexual selection (e.g., unhindered mate choice) and sexual conflict in a broader variety of taxa (Chapman et al. 2003, Cordoba-Aguiler and Contreras-Garduno 2003, Eberbard and Cordero 2003). The distinction between these selective forces hinges on determining the fitness consequences of mating for females and their offspring (Watson et al. 1998, Chapman et al. 2003, Cordoba-Aguilar and Contreras-Garduno 2003, Eberhard and Cordero 2003, Pizarri and Snook 2003). In particular, the confounding effects of potential benefits associated with male traits (versus the benefits from free choice) should be controlled without a priori assumptions about traits in males that females may use to differentiate mates (Gowaty 1997, Drickamer et al. 2000). Other important factors such as male-male competition, male mate choice, male-female competition, and male coercion of females need to be experimentally controlled or eliminated if these elements constrain freely expressed female choice (Zuk et al. 1990, Gowaty 1997, Drickamer et al. 2000). Although research on sexual conflict has increased in the past several years, amphibians are a group that has been overlooked (but see Roesli and Reyer 2000). Anurans, in particular, provide an opportunity to assess fitness consequences of freely expressed female choice while limiting other selective events that may potentially influence reproductive success and offspring viability. Anurans typically have external fertilization, so factors that may alter reproductive success such as sperm competition 71 and cryptic female choice are absent or limited. Additionally, in most North American anurans, parental care is not provided by either sex. Aspects of the mating system of the Western toad (Bufo boreas) make this species a promising candidate for studies of sexual conflict. While many anuran males display and chorus, the Western toad does not. In non-chorusing, non-displaying anurans, there are no obvious traits that females may use to differentiate males, except for male body size and clasping vigor. Western toads are explosive breeders with a very limited breeding season (Olson 1988). Consequently, the likelihood of sexual conflicts over mating seem high due to male-male scramble competition and male clasping behaviors (discussed below) that preclude females' abilities to exercise their preferences. In this sense, the Western toad mating system is similar to other systems in which sexual conflicts over mating have been documented (e.g., water striders-Rowe 1994, seaweed flies-Shukar and Day 2001, Crean and Gilburn 1998, and mosquito fishWatson et al. 1998). One aspect of the mating system that likely contributes to sexual conflict is that male B. boreas amplex other individuals vigorously and indiscriminately, without prior courtship behavior. Males appear to exhibit preference for larger individuals, whether male, female, dead, alive, or inanimate floating objects (Marco et al. 1998, pers. ohs.). This preference presumably increases the likelihood of amplexing a female, as most adult females are larger than males (Marco et al. 1998, see Figure 4.1). Amplexus is prolonged (longer than required for oviposition) and resembles pre-copulatory mate guarding in arthropods (e.g., water striders Rowe et al. 1994, seaweed flies and Day 2001, amphipods Shukar Cothran 2004). No direct investigations of female mating 72 preferences have been made because male-male competition and a highly male biased sex ratio seem to preclude the opportunity for female mate choice. 160 140 . . 120 100 40 (0 80 60 . 55 S 40 S $S S S 20 0 5 5 5 6 6.5 7 7.5 8 8.5 9 9.5 SUL Figure 4.1. The relative size of male and female Western toads represented as the relation between snout-urostyle length and mass. Females (large diamonds) generally are larger than males (small circles), although there is overlap between large males and small females in this population at Little Three Creeks Lake. Previous research with B. boreas confirmed that females can influence male clasping success, and that females are not passive during the clasping process, appearing to avoid encounters (Olson 1988). In order to evade an approaching male, a female B. boreas can exhibit a suite of behaviors, including kicking, diving, swimming away, and retracting (slight backward movement on the water surface) when approached by a male (Olson 1988). In experimental closures at the natural breeding site, male clasping success varied depending on which behavior the female performed 73 (Olson 1988). For example, when a female dived away from a male, amplexus was less frequent (28.9%). However, retracting from the male often led to amplexus (64.3%). In experimental enclosures, the consequences of female behavior during an encounter were dependent on male size (Olson 1988). Successful clasps by small males were frequently associated with female retractions, whereas large males were more successful when females responded to the encounter by diving, swimming, or kicking. Also, most females initially attempted to dislodge males when amplexed (pers. obs.). These behaviors may be attempts by females to express mate choice. In this paper, I test the main assumption of sexual dialectics theory that freely expressed female choice is selectively advantageous to female Western toads. Specifically, females that mate with preferred males should benefit in terms of higher reproductive success as compared to females that mate with non-preferred males. In measuring this selective advantage, care must be taken so the investigator does not bias a female's options (Gowaty 1997, Drickamer 2000). A bias could be introduced, for example, by only offering females a choice between toads that differed greatly in body mass. The assumption here is that a female selects a male based on phenotypic attributes, which may include traits (e.g., odor) not easily measured by the investigator. To test the sexual dialectics assumption, therefore, I first evaluated female preferences using randomly selected males. I then staged matings between females and preferred or non-preferred males. For all mating pairs, I recorded offspring survivorship to hatching (a measure of reproductive success) and average size at hatching (a measure of offspring viability). 74 METHODS Study Species I studied two populations of B. boreas: Little Three Creeks Lake (Deschutes County, elevation 2000m) and Todd Lake (Deschutes County, 1 860m) in the Cascade Range of Oregon during 2003. The length of the breeding season ranged from 5 - 15 consecutive days and occurred between May and July. Breeding population size at Little Three Creeks Lake ranged from 260-438 individuals and at Todd Lake from 131 to 497 individuals. Over the breeding season, the sex ratio of males to females ranged from 2.5 to 3.4 at Little Three Creeks Lake and 0.72 to 2.25 at Todd Lake (Olson 1988). However, on any day during breeding, there can be up to 9.5 males per female at Little Three Creeks Lake and 3.3 males per female at Todd Lake (personal obs., Olson 1988). Both populations had significant large male mating advantages (larger males experience increased mating success, Olson 1988). However, the pattern was not consistently significant across days or years. Futhermore, the apparent size assortative mating pattern was not related to fertilization success, as is the case in other anurans (Olson et al. 1986, Olson 1988). Oviposition is communal, usually occurring in an area approximately Sm x Sm. Female Choice Trials At each lake, animals were collected from the periphery of the oviposition site to minimize disturbance of the breeding aggregation. I captured gravid females in 75 amplexus, removed the male, and immediately placed the female in a choice enclosure (described below) to acclimate. The original male was measured and released. Two single males were randomly collected from the aggregation and assigned as mate choice candidates. Trials were conducted one at a time to decrease handling stress. By selecting males at random, I did not bias the male phenotypic traits that characterized the mate choice candidates. As the focus was on the benefits of choice, not on a particular male trait (such as body size), it was necessary to eliminate potentially confounding variables by randomization. The choice enclosure consisted of a PVC frame (150 x 50 x 100cm) covered with nylon mesh (see Figure 1). The enclosure had three compartments created by mesh dividers. One male was placed in each of the two end compartments and the female was allowed to move freely throughout the center compartment. The female could smell, hear, and make some tactile contact with each male. The divisions within each enclosure excluded other forms of reproductive competition, such as male-male competition, male-female competition, male mate choice, and male coercion of females (cfZuk et al. 1990). Each trial consisted of a 10-minute focal period. Females used in the experiment met the following criteria for choice: (a) the female spent at least 300 seconds inspecting males (i.e., the female was < 15 cm from a male) and (b) 60% of the inspection time was spent in close proximity (<15 cm) to one male, defined as the preferred male. Twenty-two trials were conducted at Little Three Creeks Lake and 18 trials at Todd Lake. All 40 females met both criteria. In all trials, I recorded standard measurements for each individual, snout-urostyle length and body mass. Trials were conducted at peak breeding activity time, between 1000 and 1800 hr. 76 Figure 4.2. Schematic of female choice enclosures (150 x 50 x 100 cm). The dash-dot lines represent the mesh divisions within the enclosure. The dotted lines represent the proximity threshold (15cm) for demarking female preference. The female's head must cross this line to be considered in close proximity to a male. Mating Pair Manipulations Once a female met the choice criteria described above, she was paired with a male and allowed to oviposit. The type of male was assigned such that each female was paired with either the preferred or non-preferred male from her trial. On a given day, a coin flip determined whether the first female to be paired was assigned the preferred or non-preferred treatment. The remaining treatment assignments on that day alternated to assure equal sample sizes in each treatment group. Each pair was placed in an oviposition enclosure 5-lOm from the oviposition site. These oviposition enclosures were mesh baskets suspended from a Styrofoam flotation frame (individual baskets: 20 x 30 x 30cm). All paired females oviposited within 48 hours, except for one female in a non-preferred mating. This pair was released into the lake and no data from this pair was included in the analysis of treatment effects. Toads were released from the 77 enclosures immediately following the completion of oviposition, as indicated by the pair no longer being in amplexus. Survivorship and Hatching Size Following oviposition, I randomly collected a subset of eggs from each clutch due to logistical problems counting entire clutches that usually contain 12,000 eggs. Depending on location, the subset consisted of 200-300 eggs per mating pair. Eggs from each pair were placed in Styrofoam-framed, floating mesh baskets (similar to oviposition enclosures) at the main oviposition site. Embryos were reared in these enclosures. During the embryonic period, I closely monitored the enclosures until hatching. Eggs from all clutches were reared at the same depth of water, equidistant from the shore, and the mesh enclosure allowed water flow between clutches. I recorded survivorship to hatching and hatching size, measured as total length. I released all tadpoles at the oviposition sites after hatching to minimize disturbance to the breeding population. As toad clutches contain about 12,000 eggs and mortality is usually high, survivorship to hatching may be a better estimate of female reproductive success than is the number of eggs laid for this species. Statistical Analyses From each study population, I obtained two sets of dependent variables for preferred and non-preferred matings, survivorship to hatching and size at hatching. I averaged tadpole size for each mated pair (N21 pairs at Little Three Creeks Lake and N=l 8 pairs at Todd Lake). I analyzed the body size data (tadpole body length) with linear regression, including treatment (preferred or non-preferred) and parental size as covariates. I used logistic regression to analyze treatment effects on the proportion of tadpoles that survived until hatching. RESULTS Reproductive Success: survivorship to hatching There was no significant difference in the percentage of eggs surviving to hatching between preferred (3 9%) and non-preferred (42%) matings at Little Three Creeks Lake (t-ratio-O.21, pO.84, df 19, N21; Table 4.1; Figure 4.3). However, the percentage of eggs surviving to hatching at Todd Lake was significantly higher in preferred matings (61%) than in non-preferred (33%) matings (t-ratio-3.Ol, p = 0.009, df 16, N18; Table 4.2; Figure 4.3). Parental size did not explain a significant amount of variation in survivorship at either population. Overall, mortality was higher at Little Three Creeks Lake. The source of mortality is unknown, although spotted sandpipers (Actitus macularia) were regularly seen perched on my floating enclosures. 78 79 Table 4.1. Clutch data for Western toads from Little Three Creeks Lake. The total number of eggs sampled and number of tadpoles present at hatching are given for preferred and non-preferred matings. Initial Egg No. Clutch No. Hatched 1 315 315 328 308 309 342 358 339 350 309 348 330 338 358 334 434 313 356 339 351 315 81 2 3 4 5 6 7 9 10 11 12 13 14 15 16 17 18 19 20 21 22 PAvg NP Avg 3787 3302 96 328 60 179 216 210 137 136 193 81 86 215 224 115 99 299 6 2 7 84 1548 1304 Proportion Hatched Treatment 0.26 p 0.30 np 1.00 p 0.19 np 0.58 p 0.63 np 0.59 p 0.40 p 0.39 np 0.62 p 0.23 np 0.26 p 0.64 np 0.63 p 0.34 np 0.22 p 0.96 np 0.02 p 0.01 np 0.02 p 0.27 np 0.42 0.39 p np 80 Table 4.2. Clutch data for Western toads from Todd Lake. The total number ofeggs sampled and number of tadpoles present at hatching are given for preferred and nonpreferred matings. Initial Egg No. Clutch No. 1 227 232 249 258 245 238 255 257 239 234 219 231 244 233 211 226 237 214 Hatched 114 112 201 77 108 97 172 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 PAvg NP Avg 2126 2123 14 131 102 206 45 28 141 199 82 116 23 1023 827 Proportion Hatched Treatment 0.50 p 0.48 np 0.81 p 0.30 np 0.44 p 0.41 np 0.67 p 0.05 np 0.55 p 0.44 np 0.94 p 0.19 np 0.11 p 0.61 rip 0.94 p 0.36 np 0.49 p 0.11 np 0.61 0.33 p np 81 0 0.6 0.5 VI C 0.3 ox 0 0.2 o I- 0.1 0. Little Three Creeks Lake Todd Lake Location Figure 4.3. The average proportion of hatching tadpoles for preferred (dark bars on the right) and non-preferred matings (light bars on the left) for Western toads at Todd Lake and Little Three Creeks Lake. ** denotes p-value below 0.05. Offspring Viability: Hatching Size At Little Three Creeks Lake, the average length of newly hatched tadpoles was significantly greater from preferred matings than from non-preferred matings (t-ratio = - 2.35, p-value = 0.03 18, df19, N21; Table 4.3; Figure 4.4). Tadpoles from preferred matings averaged 8.58mm (SD = 0.23mm) in length and tadpoles from non-preferred matings averaged 7.66mm (SD 0.23mm) in length. Similarly, at Todd Lake, tadpole length at hatching was significantly greater in preferred matings than in non-preferred matings (t-ratio = -4.82, p-value = 0.0002, df16, N18; Table 4.4; Figure 4.4). On average at Todd Lake, tadpoles from preferred matings were 7.72mm (SD = 0.17mm) in length and tadpoles from non-preferred matings were 6.8 8mm (SD 0.17mm). Female and male size (snout-urostyle length) did not explain a significant amount of variation in tadpole size at hatching. 82 Table 4.3. Mean tadpole length per clutch for preferred and non-preferred matings for Western toads at Little Three Creeks Lake. Clutch 1 2 3 4 5 6 7 9 10 11 12 13 14 15 16 17 18 19 20 21 22 PAvg NP Avg Mean Tadpole Length 9.15 7.71 8.58 7.54 8.38 7.72 8.32 7.75 8.59 8.33 8.01 7.44 7.72 7.92 6.91 7.42 7.6 7.7 7.1 8.61 7.65 8.58 7.66 Treatment p np p np p np p p np p np p np p np p np p np p np p np Table 4.4. Mean tadpole length per each clutch from preferred and non-preferred matings for Western toads at Todd Lake. Clutch 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 PAvg NP Avg Mean Tadpole Length 7.8 6.29 6.92 7.2 8.11 6.93 7.76 6.24 7.95 7.29 7.3 7.12 7.58 7.02 8.2 6.96 7.88 6.89 7.72 6.88 Treatment p np p np p np p np p np p np p np p np p np p np 83 84 Figure 4.4. Tadpole length at hatching averaged for preferred (dark bar on right) and non-preferred (light bar on left) matings for Western toads at Todd Lake and Little Three Creeks Lake. ** denotes p-values below 0.05 DISCUSSION Two main differences were found when female Western toads were allowed to select their mates. Female toads that mated with preferred males (a) oviposited eggs that were more likely to survive until hatching and (b) produced tadpoles that had larger average size at hatching. It is possible that fertilization success may have contributed to differences in hatching success. However, a previous study of B. boreas found that fertilization success was nearly 100% for all pairs. Thus, the successful development of zygotes to hatching is inferred as the mechanism by which females mating with preferred males produced proportionally more hatchlings. These differences in hatchling survivorship and body size presumably translate into fitness benefits for females that freely express mate choice. Thus, the benefits of free female choice can be 85 found even in a mating system that normally constrains a female's ability to discriminate between potential mates. Additionally, these results provide the first evidence in amphibians, and anurans in particular, that females benefit from mate choice that is independent of any specific male trait (e.g., body size) that females may use to differentiate among males. Together, these results are consistent with the main assumption of sexual dialectics theory that females benefit from freely expressed mate choice. The assumption that survivorship and hatching size are beneficial is supported by evidence from other anuran studies. These studies have shown that larger tadpoles can have higher competitive ability, better predator avoidance, increased survival, and shorter larval periods (Wilbur and Collins, 1973, Smith-Gill and Gill 1978, Steinwascher 1979, Travis et al. 1987, Woodward 1987). In addition, reduced larval periods in anurans are linked to other fitness measures, such as age at first reproduction, overwintering survival, and survival to adulthood (CaIdwell et al. 1980, Morin 1983, Berven and Gill 1983, Smith 1987, Travis et al. 1987, Woodward et al. 1988, Mitchell 1990, Berven 1990, Blouin 1992). These offspring fitness measures may be especially important in toad populations since tadpoles experience selection to metamorphose quickly due to drought and lake drying at breeding sites (Blaustein and Olson 1991). My experimental design was conservative with respect to observing potential differences between preferred and non-preferred males (cf Drickamer 2000). First, only two males were used in each choice trial, which limits the potential differences that were the basis for choice by a given female. Another conservative feature of the experimental design was that eggs were kept in mesh enclosures, which limited egg 86 predation from aquatic predators and potentially decreased pathogen exposure. These design features increased the likelihood that observed differences in survivorship and hatching size were related primarily to preferred versus non-preferred matings. The focus of this study was to determine whether preferred matings provided benefits. No attempt was made, a priori, to determine the mechanism by which females might benefit from mate choice. However, several possible mechanisms that might be responsible for the observed results. Other studies have identified two agents as responsible for high embryo mortality in the Western toad: exposure to ultraviolet-B light and S. ferax (Kiesecker and Blaustein 1995). A female might choose a male such that offspring are more resistant to evolving pathogens or better suited to changing environmental conditions. Hamilton and Zuk (1982) and Gowaty (1997) propose that mate choice is based on evolving resistance to disease or adaptation to changing environmental conditions, as opposed to a static trait (e.g., ozone depletion causing higher levels of ultraviolet B or drought leading to premature lake drying). In addition, if mate choice is based on genetic compatability between males and females, then nonpreferred matings could result in incompatable genetic recombinations in some proportion of offspring. In contrast, food availability and competition for food are not potential explanatory factors as the experiment was terminated at hatching (before tadpoles begin to feed) and adult toads do not forage while at the breeding aggregation. Another possible mechanism that may explain the advantages of preferred mating is that mating with non-preferred males could be stressful and result in increased cortisol levels. Numerous studies of other vertebrate taxa have revealed negative effects of stress on offspring survivorship or size (Weiner et al. 1986, Tam et al. 1990, 87 Foo and Lam 1993, Campbell et al. 1994, Pankhurst et al. 1995, Kerrigan 1996). McCormick (1998), for example, determined that cortisol from stress influenced yolk size of larval progeny and larval morphology in a tropical damselfish. Small effects on larval stages are of critical importance because: (a) these effects can alter developmental trajectories, and (b) small changes in mortality can have large effects on recruitment into the breeding population (Houde 1987, Underwood and Fairweather 1989, De Jesus et al. 1990, Galton 1990, Leggett and Deblois 1994, Bernardo 1996, McNamara and Houston 1996, McCormick 1998). In addition, females of many species can alter nutrient and hormone allocations to their clutches, and even to different sexes within the clutch, based on her or her mate's quality (Pilz et al. 2003, Rutkowska and Cichon 2002, Olsson et al. 2002, Gil et al. 1999, Clark and Galef 1995, Leggett and Deblois 1994, Blaxter 1986, Bailey and Houde 1989, Rothschild 1986, Chambers et al. 1989). Studies of maternal investment and offspring development in anurans suggest that differential investment may have consequences for offspring fitness (Parichy and Kaplan 1995, Kaplan 1992). These non-genetic maternal effects also may be responsible for the observed differences in toad progeny from preferred versus non-preferred matings. The benefits of free female mate choice found in Western toads have implications in terms of how a female may attempt to control mating with a non- preferred male. A female already in amplexus is not easily able to resist mating with a non-preferred male due to the strength of male clasping. However, females control the movements of the pair prior to and during oviposition. Consequently, some females oviposit in close proximity to other ovipositing pairs, while others oviposit at the 88 periphery of the breeding sites where other pairs are not abundant. Since most anurans have external fertilization, it may be possible that a female amplexed by a non-preferred male can position herself at the oviposition site to increase the chances of multiple paternity. In addition, females are rarely found single at the breeding site where males amplex forcefully and indiscriminately and the operational sex ratio is highly male biased. It is possible that females could choose males at a distance from the actual breeding site to increase their ability to exercise their preferences. Similary, females that arrive at the lake early experience a less skewed sex ratio and lower density of individuals than do late arriving females because late in the season most males remain at the lake and most females have oviposited and left the lake. In addition to predictions of female resistance behavior, my results have implications for observed mating system patterns. In particular, Western toads have prolonged amplexus (similar to pre-copulatory mate guarding in invertebrates) where the female carries the male on her back. Prolonged amplexus is likely to incur energetic costs, decrease mobility, and increase her risk of predation (as evidence by higher mortality of females from raven predation, Olson 1988). As mentioned earlier, Western toad populations exhibit a significant, but inconsistent size-based mating pattern, this pattern does not necessarily reflect female choice (see Figure 4.5). Larger males may be better able to overcome female resistance behaviors, as shown by Olson (1988). Similar size-based mating patterns reflecting sexual conflicts over mating have been found in water striders and seaweed flies (Ortigosa and Rowe 2003, Fairburn et al. 2003, Shukar and Day 2001, Rowe et al. 1994, Crean and Gilburn 1998, Watson et al. 1998). 89 Todd Lake Little Three Creeks Lake D-- 7.6 ab 7.4 7.2 a 7 68 '1±. 62 6 5.8 Preferred Non-preferred Original Preferred Non-preferred Original Male Category Figure 4.5. Differences in Western toad male SUL at Little Three Creeks Lake and Todd Lake. Preferred males were smaller on average than non-preferred males and the original males with which females were amplexed. Significant differences are denoted with different letters. The previously discussed predictions of female resistance behavior and consequent mating patterns in B. boreas are novel and testable. Female mate choice in explosive breeding anurans has often been overlooked due to social constraints (e.g., male clasping behavior and male-biased sex ratios) and seasonal constraints (short breeding season) within the system. My findings suggest that free mate choice by females can play a role in the dynamics of social behavior and mating patterns and, consequently, can be an important source of evolutionary change. To my knowledge, this is the first study in anurans to address the critical assumption of sexual conflict that freely expressed female mate choice is selectively advantageous. Consequently, this work contributes to our understanding of the pervasiveness of sexual conflicts over mating across taxa. CHAPTER 5 GENERAL CONCLUSIONS AND FUTURE DIRECTIONS The research presented here addresses the causes and consequences of variation in female mating behavior and female reproductive success in plethodontid salamanders and toads. Sexual conflict theory provided the theoretical background from which to address variation in female reproductive success from the perspective of females. In Chapter 2, I assessed the potential for sexual conflict to play a role in plethodontid courtship behavior and male courtship pheromone delivery. The results of this study provided evidence that, even in plethodontid salamanders with highly stereotyped courtship behavior, there is significant variation among species in the roles females play in courtship interactions. Female Plethdon cinereus, P. angusticlavius, and P. richmondi exhibited behaviors previously considered as male behaviors. In addition, females of these species performed behaviors that had never been reported prior to this study. The timing of male courtship pheromone delivery occurred earlier in the courtship interactions (prior to tail-straddling walk) than had been observed for other well-studied plethodontids (pheromone delivery during tail-straddling walk). I confirmed that P. angusticlavius males deliver courtship pheromones by "slapping," and that "vaccination" pheromone delivery occurs in P. cinereus and P. richmondi. A more active female role in courtship interactions and pheromone delivery occurring during early stages of courtship are consistent with, but do not confirm, the predictions that sexual conflict may be operating in these salamanders. My observations provide incentive for more research from the perspective of females and sexual conflict. 91 In Chapter 3, I address more directly the effects of male courtship pheromone delivery on selected measures of female reproductive success. My results suggest that male courtship pheromones may have other effects on females, in addition to decreasing female reluctance to mate. The overall results vary between years, and thus are not conclusive. In one year of the study, the pheromone altered the positive relationship between female size and clutch size. In particular, the normal slope of the relationship between female size and clutch size is depressed in the group of females that received pheromone. However, the second year of the study failed to support this result, and there was no treatment effect on clutch size/female size relationships. At least in certain circumstances and in particular females, however, the male courtship pheromone apparently can alter female clutch size. My results suggest that the male courtship pheromone may function to overcome female resistance in interactions between a female and a non-preferred male. The potential for male courtship pheromones to function in sexual conflict provides incentive for more research to clarify the role courtship pheromones play in plethodontid salamanders. Chapter 4 addressed the main assumption of sexual conflict theory that a female accrues benefits for herself and her offspring through freely expressed mate choice. My results provided the first evidence in amphibians, and anurans in particular, that female reproductive success (offspring survivorship to hatching) and offspring viability (size at hatching) is higher in females that mated with preferred males. In a post-hoc analysis of male traits, I found that, contrary to observed mating patterns favoring large males (large male mating advantage), preferred males were often the smaller male used in the choice trial. Thus, the large male mating advantage did not occur as a result of female 92 mate choice. Instead, larger, non-preferred males were better able to maintain amplexus with reluctant females. In addition, my results provide the basis for novel predictions of female resistance behaviors that enable a female to more easily exercise her mating preferences. In general, my research broadens the scope of sexual conflict by investigating its role in the mating systems of amphibians. Behavioral information critical to reproductive success may contribute to future management strategies for threatened or endangered species. Also, my research provides incentive for a new perspective on quantitative genetic models of sexual selection. Models of sexual selection previously assumed that female mate choice evolved as a by-product of selection on male traits or at a cost to females that spent time discriminating among males (Lande 1981; Arnold 1982; Iwasa and Pomiankowski 1995; Pomiankowski and Iwasa 1998; Mead and Arnold 2004). My research supports the incorporation of substantial fitness benefits for females into models of sexual selection. The addition of variation in sexual selection attributable to female mate choice yields models that more accurately represent the natural variation in animal mating systems. Lastly, by broadening the taxa studied from the perspective of sexual conflict, my research further supports ideas that sexual conflict plays a critical role in sexual dimorphism, reproductive isolation, and speciation (Chapman and Partridge 1996; Parker and Partridge 1998; Arnqvist et al., 2000; Gavrilets 2000a, b; Blankenhorn et al. 2002). Freely expressed mate choice and subsequent sexual conflict are important in many vertebrate and invertebrate mating systems. BIBLIOGRAPHY Alonzo, S. H. and R. R. Warner (2000). "Female choice, conflict between the sexes, and the evolution of male alternative reproductive behaviors." Evolutionary Ecological Research 2: 149-170. Andersson, M. (1986). "Evolution of condition-dependent sex ornaments and mating preferences: sexual selection based on viability differences." Evolution 40: 804-8 16. Andersson, M. (1994). Sexual Selection. Princeton, NJ, Princeton University Press. Andersson, 5. (1991). "Bowers on the savanna: Display courts and mate choice in a lekking widowbird." Behavioral Ecology 2: 210-218. Arnold, S. J. (1972). The evolution of courtship behavior in salamander. Ann Arbor, University of Michigan. Arnold, S. J. (1976). "Sexual behavior, sexual interference, and sexual defense in the salamanders Ambystoma maculatum, Ambystoma tigrinum, and Plethodonjordoni." Zeitschrift fur Tierpsychologie 42: 247-3 00. Arnold, S. J. (1977). The evolution of courtship behavior in new world salamanders with some comments on old world salamanders. The Reproductive Biology of Amphibians. D. H. Taylor and S. I. Guttman. New York, Plenum Press: 141 - 183. Arnold, S. J. and L. D. Houck. (1982). Courtship pheromones: evolution by natural and sexual selection. Biochemical Aspects of Evolutionary Biology. M. Nitecke. Chicago, University of Chicago Press: 173-211. Arnold, S. J. (1983). Sexual selection: the interface of theory and empiricism. Mate Choice. Bateson. Cambridge, Cambridge University Press: 67-107. Arnqvist, G. (1989). "Sexual selection in a water strider: the function, mechanism of selection and heritability of a male grasping apparatus." Oikos 56: 344-350. Arnqvist, G. (1992). "The effects of operational sex ratio on the relative mating success of extreme male phenotypes in the water strider Gerris odontogaster." Animal Behavior 43: 68 1-683. Arnqvist, G. and L. Rowe (1995). "Sexual conflict and arms races between the sexes: a morphological adaptation for control of mating in a female insect."Proceedings of the Royal Society of London Series B 261: 123-127. Arnqvist, G., R. Thornhill and L. Rowe (1997). "Evolution of animal genitalia: Morphological correlates of fitness components in a water strider. "Journal of Evolutionary Biology 10(4): 613-640. Arnqvist, G. and I. Danielsson (1999). "Copulatory behavior, gential morphology, and male fertilization success in water striders." Evolution 53: 147-156. Arnqvist, G., M. Edvardsson, U. Friberg, and T. Nilsson (2000). "Sexual conflict promotes speciation in insects." Proceedings of the National Academy of Science 97: 10460-10464. Arnqvist, G. and L. Rowe. (2002). "Antagonistic coevolution between the sexes in a group of insects." Nature 415: 787-789. Bailey, K. M., and E. D. Houde (1989). "Predation on eggs and larvae of marine fishes and the recruitment problem." Advances in Marine Biology 26: 1-83. Barnard, C. J. and T. Burk (1979). "Dominance hierarchies and the evolution of "individual recognition"." Journal of Theoretical Biology 81: 65-73. Basolo, A. L. (1990). "Female preference predates the evolution of the sword in swordtailfish." Science 250: 808-8 10. Bernardo, J. (1996). "Maternal effects in animal ecology." American Zoologist 36: 83105. Bervin, K. A. (1990). "Factors affecting population fluctuations in larval and adult stages of the wood frog (Rana sylvatica)." Ecology 71: 1599-1608. Berven, K. A. and D. E. Gill (1983). "Interpreting geographic variation in life history traits." American Zoologist 23: 85-97. Blanckenhorn, W. U., D. J. Hosken, 0. Y. Martin, C. Reim,Y. Teuschl, and P. I. Ward (2002). "The costs of copulating in the dung fly Sepsis cynipsea." Behavioral Ecology 13: 353-358. Blaustein, A.R. and D.H. Olson. 1991. "Amphibian population declines." Science 253: 1467. Blaxter, J. H. 5. (1986). "Development of sense organs and behaviour in teleost larvae with special reference to feeding and predator avoidance." Transactions of the American Fisheries Society 115: 98-114. Blouin, M. S. (1992). "Genetic correlations among morphometric traits and rates of growth and differentiation in the green tree frog, Hyla cinerea." Evolution 46: 735-744. Bluhm, C. K. and P. A. Gowaty (2004). "Reproductive compensation for offspring 95 viability deficits by female mallards, Anasplatyrhynchos." Animal Behavior 68: 985992. Bluhm, C. K. and P. A. Gowaty (2004). "Social constraints on female mate preferences in mallards, Anas platyrhynchos, decrease offspring viability and mother productivity." Animal Behavior 68: 977-983. Bonduriansky, R. and L. Rowe (2003). "Interactions among mechanisms of sexual selection on male body size and head shape in a sexually dimorphic fly." Evolution 57: 2046-2053. Burk, T. (1988). "Acoustic signals, arms races and the costs of honest signalling." Florida Entomologist 71: 400-409. Caidwell, J. P., J. H. Thorp, and T. 0. Jervey (1980). "Predator-prey relationships among larval dragonflies, salamanders, and frogs." Oecologia 46: 285-289. Campbell, P. M., R. G. Pottinger, and J. P. Sumpter (1994). "Preliminary evidence that chronic confinement stress reduces quality of gametes produced by brown and rainbow trout." Aguaculture 120: 151-169. Chambers, R. C., W. C. Leggett, and J. A. Brown (1989). "Egg size, female effects, and the correlation between early life history traits of capelin, Mallotus villosus: an appraisal at the individual level." Fisheries Bulletin 87: 5 15-523. Chapman, T. G. and L. Partridge (1996). "Sexual conflict as fuel for evolution." Nature 381: 189-190. Chapman, T., G. Arnqvist, J. Bangham, and L. Rowe (2003). "Sexual conflict." Trends in Ecology and Evolution 18(1): 41-47. Clark, M. M. and B. G. Galef (1995). "Prenatal influences on reproductive life history strategies." Trends in Ecology and Evolution 10: 15 1-153. Clutten-Brock, T. H. and S. D. Albon (1979). "The roaring red deer and the evolution of honest advertisement." Behaviour 69: 145-170. Clutten-Brock, T. H. and G. A. Parker (1992). "Potential reproductive rates and the operation of sexual selection." Quarterly Review in Biology 67: 437-455. Clutton-Brock, T. H. and A. C. J. Vincent (1991). "Sexual selection and the potential reproductive rates in males and females." Nature 352: 5 8-60. Cordero, A. and J. A. Andres (2002). "Male coercion and convenience polyandry in a calopterygid damselfly." Journal of Insect Science 2: 1-7. Cordoba-Aguilar, A. and J. Contreras-Garduno (2003). "Sexual conflict." Trends in Ecology and Evolution 18: 48. Coss, H. T. (1974). Maxillary and premaxillary dentition of salamanders of the tribe Plethodontini. Clemson, Clemson University. Cothran, R. D. (2004). "Precopulatory mate guarding affects predation risk in two freshwater amphipod species." Animal Behavior 68: 1133-1138. Crean, C. S. and A. S. Gilburn (1998). "Sexual selection as a side-effect of sexual conflict in the seaweed fly." Animal Behavior 56(6): 1405-1410. Darwin, C. (1871). The Descent of Man and Selection in Relation to Sex. London, John Murray. Dawkins, R. and J. R. Krebs (1978). Animal signals: information or manipulation? Behavioural Ecology: an Evolutionary Approach. J. R. Krebs and N. B. Davies. Oxford, Blackwell Scientific Publications: 282-309. Dawkins, M. S. and T. Guilford (1991). "The corruption of honest signalling." Animal Behavior 41: 865-873. De Jesus, E. G., Y. Inui, and T. Hirano (1990). "Cortisol enhances the stimulating action of thyroid hormones on dorsal fin-ray resorption of flounder larvae in vitro." General and Comparative Endocrinology 79: 167-173. Drickamer, L. C., P. A. Gowaty, and C. M. Holmes (2000). "Free female mate choice in house mice affects reproductive success and offspring viability and performance." Animal Behavior 59: 37 1-378. Eberhard, W. G. and C. Cordero (2003). "Sexual conflict and female choice." Trends in Ecology and Evolution: 18: 122. Emlen, J. M. (1973). Ecology: An Evolutionary Approach. Reading, Mass, AddisonWesley. Fairbairn, D. J. (1988). "Sexual selection for homogamy in the gerridae: an extensionof Ridley's comparative approach." Evolution 42; 1212-1222. Fairbairn, D. J. (1990). "Factors influencing sexual dimorphism in temperate water striders." American Naturalist 136: 6 1-86. Fairbairn, D. J., R. Vermette, N. Kapoor and N. Zahiri (2003). "Functional morphology of sexually selected genetalia in the water strider Aquarius remigis.' Canadian Journal 97 of Zoology 81(3): 400-4 13. Feldhoff, R. C., S. M. Rollmann, and L. D. Houck (1999). Chemical analysis of courtship pheromones in a plethodontid salamander. Advances in Chemical signals in vertebrates. R. E. Johnson, D. Muller-Schwarze, and P. W. Sorenson. New York, Kluwer Academic / Plenum Press. Fincke, 0. M. (1997). "Conflict resolution in the Odonata: Implications for understanding female mating patterns and female choice." Biological Journal of the Linnean Society 60: 20 1-220. Fisher, R. A. (1915). "The evolution of sexual preference." Eugenics Review 7: 184192. Fisher, R. A. (1958). The genetical theory of natural selection. New York, Dover Publications, Inc. Fleck, J. (1992). "Haithung und Nachzucht von Tylototriton kweichowensis." Salamandra 28: 97-105. Foo, J. T. W. and T. J. Lam (1993). "Retardation of ovarian growth and depression of serum steroid levels in the tilapia, Oreochromis mossambicus, by cortisol implantation." Aquaculture 115: 133-143. Gabor, C. R. and R. G. Jaeger (1995). "Resource quality affects the agonistic behavior of territorial salamanders." Animal Behavior 49: 71-79. Galton, V. A. (1990). "Mechanism underlying the acceleration of thyroid hormoneinduced tadpole metamorphosis by corticosterone." Endocrinology 127: 2997-3 002. Gavrilets, S. (2000). "Rapid evolution of reproductive barriers driven by sexual conflict." Nature 403: 886-889. Gavrilets, S. (2000). "Sexual conflict and speciation." Nature 407: 150. Gergits, W. F. and R. G. Jaeger (1990). "Field observations of the behavior of the Redbacked Salamander (Plethodon cinerus): courtship and agonistic interactions." Journal of Herpetology 24: 93-95. Gil, D., J. Graves, N. Hazon, and A. Wells (1999). "Male attractiveness and differential testosterone investment in zebra finches." Science 286: 126-128. Gillette, J. R., R. G. Jaeger, and M. C. Peterson (2000). "Social monogamy in a territorial salamander." Animal Behavior 59: 124 1-1250. Gillette, J. R. and M. C. Peterson. (2001). "The benefits of transparancy: candling as a simple method for determining sex in red-backed salamanders (Plethodon cinereus)." Herpetological Review 32: 233-235. Gowaty, P. A. (1997). Sexual dialectics, sexual selection, and variation in reproductive behavior. Feminism and Evolutionary Biology: Boundaries, Intersections, and Frontiers. P. A. Gowaty. New York, Chapman and Hall: 35 1-384. Grafen, A. (1990a). "Biological signals as handicaps." Journal of Theoretical Biology 144: 5 17-546. Grafen, A. (1990b). "Sexual selection unhandicapped by the Fisher process." Journal of Theoretical Biology 144: 473-5 16. Greenspan, R. J. and J. F. Fervour. (2000). "Courtship in Drosophila." Annual Review in Genetics 34: 205-32. Guilford, T. and M. S. Dawkins (1991). "Receiver psychology and the evolution of animal signals." Animal Behavior 42: 1-14. Halliday, T. R. (1990). "The evolution of courtship behavior in newts and salamanders." Advanced Studies of Animal Behavior 19: 137-169. Hamilton, W. D. and M. Zuk. (1982). "Heritable true fitness and bright birds: A role for parasites?" Science 218: 384-387. Hasumi, M. (1994). "Reproductive behavior of the salamander Hynobius nigrescens: monopoly of egg sacs during scramble competition." Journal of Herpetology 28: 264267. Highton, R. (1962). "Revision of North American salamanders of the genus Plethodon." Bulletin of the Florida State Museum 6: 235-367. Highton, R. and A. Larson (1979). "The genetic relationship of the salamanders of the genus Plethodon." Annual Review of Ecological Systematics 26: 5 79-599. Highton, R. (1991). "Molecular phylogeny of plethodontine salamanders and hylid frogs: statistical analysis of protein comparisons." Molecular Biology & Evolution 8: 796-8 18. Highton, R. (1997). "Geographic protein variation and speciation in the Plethodon dorsalis complex." Herpetologica 53: 345-356. Highton, R. and R. B. Peabody (2000). Geographic protein variation and speciation in salamanders of the Plethodonjordoni and Plethodon glutinosus complexes in the 99 southern Appalachian Mountains with descriptions of four new species. The Biology of Plethodontid Salamanders. R. G. Jaeger, R. C. Bruce, and L. D. Houck. New York, Plenum Press: 3 1-94. Hill, G. E. (1992). "Proximate basis of variation in carotenoid pigmentation in male house finches." Auk 109: 1-12. Hill, 0. E. and R. Montgomerie (1994). "Plumage colour signals nutritional condition in the house finch." Proceedings of the Royal Society of London Series B 258: 47-52. Hill, G. E. and R. Montgomerie. (2002). "Dietary carotenoids predict plumage coloration in wild house finches." Proceedings of the Royal Society of London Series B 269: 1119-1124. Hill, G. E., K. L. Farmer, and M. L. Beck (2004). "The effect of mycoplasmosis on carotenoid plumage coloration in male house finches." Journal of Experimental Biology 207: 2095-2099. Hiyashi, K. (1985). "Alternative mating strategies in the water strider Gerris thoracicus as an adaptation to food and habitat variation." Behavioral Ecology and Sociobiology 16: 301-306. Hoikkala, A. and K. Y. Kaneshiro (1994). "Courtship songs of the picture-winged Drosophila plantibia subgroup species." Animal Behavior 47: 1363-1374. Holland, B. and W. R. Rice (1999). "Experimental removal of sexual selection reverses intersexual antagonistic coevolution and removes a reproductive load." Proceedings of the National Academy of Science 96: 5083-5088. Houck, L. D. (1 977a). Reproductive patterns in neotropical salamanders. Department of Zoology. Berkley, University of California. Houck, L. D. (1977b). Life history patterns and reproductive biology of neotropical salamanders. Reproductive Biology of Amphibians. D. H. Taylor and S. I. Guttman. New York, Plenum Press: 43-72. Houck, L. D. (1986). The evolution of salamander courtship pheromones. Chemical Signals in Vertebrates: IV : Ecology, Evolution, and Comparitive Biology. D. Duvall, D. Muller-Schwarze, and R. M. Siverstein. New York, Plenum Press: 173-190. Houck, L. D. and N. L. Reagan (1990). "Male courtship pheromones increase female receptivity in a plethodontid salamander." Animal Behavior 39: 729-734 Houck, L. D. and D. M. Sever. (1994). The role of the skin in reproduction and behavior. Amphibian Biology, Vol. 1, The Integument. H. Heatwole and G. 100 Barthalamus. Chipping Norton, Australia, Surrey Beatty and Sons: 351-381. Houck, L. D., A. M. Bell, N. L. Reagan-Wallin, and R. C. Feidhoff (1998). "Effects of experimental delivery a male courtship pheromones on the timing of courtship in a terrestrial salamander, Plethodonjordani." Copeia 1998(1): 214-219. Houck, L. D. and S. J. Arnold. (2003). Courtship and mating. Phylogeny and Reproductive Biology of Urodela. D. M. Sever. Enfield, New Hampshire, Science Publishers: 3 83-424. Houde, F. D. (1987). "Fish early life dynamics and recruitment variability." American Fisheries Society Symposium 2: 17-29. Huxley, J. S. (1914). "The courtship-habits of the great crested grebe (Podiceps cristatus); with an addition to the theory of sexual selection." Proceedings of the Zoological Society of London 35: 491-562. Iwasa, Y., A. Pomiankowski, and S. Nee (1991). "The evolution of costly mate preferences. II. The "handicap" principle." Evolution 45: 143 1-1442. Iwasa, Y. and A. Pomiankowski. (1995). "Continual change in mate preferences." Nature 377: 420-423. Jaeger, R. G., M. G. Peterson, G. Gollmann, B. Gollmann, and V. R. Townsend Jr. (2001). "Salamander social strategies: living together in male-female pairs." Journal of Herpetology 35: 335-338. Jaeger, R. G. and M. G. Peterson. (2002). "Familiarity affects agonistic interactions between female red-backed salamanders." Copeia 2002: 865-869. Jaeger, R. G., J. R. Gillette, and R. C. Cooper (2002). "Sexual coercion in a territorial salamander: males punish socially polyandrous female partners." Animal Behavior 63: 87 1-877. Johnstone, R. A. (1996). "Multiple displays in animal communication: 'backup signals' and 'multiple messages'." Philosophical Transactions of the Royal Society of London Series B 351: 328-338. Johnstone, R. A., J. D. Reynolds, and J. C. Deutsch (1996). "Mutual mate choice and sex differences in choosiness." Evolution 50: 1382-1391. Kaitala, A. and H. Dingle (1993). "Wing dimorphism, territoriality, and mating frequency of the waterstrider Aquarius remigis." Ann. Zool. Fenn. 30: 163-168. Kaplan, R. H. (1992). "Greater maternal investment can decrease offspring survival in 101 the frog Bomb/na oriental/s." Ecology 73: 280-288. Kawamichi, T. and H. Ueda (1998). "Spawning at nests of extra-large males in the Giant Salamander A ndrias japonicus." Journal of Herpetology 32: 133-136. Kerbert, G. (1904). "Zur Fortpflanzung von Megalobatrachus maximus Schiegel." Zoologischer Anzeiger 27: 305-320. Kerrigan, B. A. (1996). "Variability in larval development of a tropical reef fish (Pomacentridae: Pomacentrus amboinensis): the parental legacy." Marine Biology 127: 395-402. Kiesecker, J. M. and A. R. Blaustein (1995). "Synergism between UV-B radiation and a pathogen magnifies amphibian embryo mortality in nature." Proceedings of the National Academy of Science 92: 11049-11052. Kirkpatrick, M. (1982). "Sexual selection and the evolution of female mate choice." Evolution 36: 1-12. Kodric-Brown, A. and J. H. Brown (1984). "Truth in advertising: the kinds of traits favored by sexual selection." The American Naturalist 124: 309-323. Kokko, H., R. Brooks, M. D. Jennions, and J. Morley (2003). "The evolution of mate choice and mating biases." Proceedings of the Royal Society of London Series B 270: 653-664. Krebs, J. R. and R. Dawkins (1984). Animal signals: Mind-reading and manipulation. Behavioral Ecology - An evolutionary approach. J. R. Krebs and N. B. Davies, Blackwell Scientific. Krebs, J. R. and A. Kacelnik (1991). Decision-making. Behavioural Ecology: An Evolutionary Approach. J. R. Krebs and N. B. Davies. Oxford, Blackwell Scientific Publications: 105-136. Lande, R. (1980). "Sexual dimorphism, sexual selection, and adaptation in polygenic characters." Evolution 34: 292-305. Lande, R. (1981). "Models of speciation by sexual selection on polygenic traits." Proceedings of the National Academy of Science 78: 3721-3725. Lang, C. and R. G. Jaeger. (2000). "Defense of territories by male-female pairs in the Red-backed Salamander." Copeia 2000: 169-177. Larson, A., D. B. Wake, L. R. Maxson, and R. Highton (1981). "A molecular phylogenetic perspective on the origins of morphological novelties in the salamanders 102 of the tribe Plethodontini." Evolution 35: 405-422. Larson, A., D. W. Weisrock, and K. H. Kozak (2003). Phylogenetic systematics of salamanders, a review. Phylogeny and Reproductive Biology of Urodela. D. M. Sever. Enfield, New Hampshire, Science Publishers: 3 83-424. Lauer, M. J., A. Sih, and J. J. Krupa (1996). 'Male density, female density, and intersexual conflict ma stream-dwelling insect." Animal Behavior 52: 929-939. Leggett, W. C. and E. Deblois (1994). "Recruitment in marine fishes - is it regulated by starvation and predation in the egg and larva! stages." Netherlands Journal of Sea Research 32: 119-134. Mahoney, M. (2001). "Molecular systematics of Plethodon and Aneides: phylogenetic analysis of an old and rapid radiation." Molecular Phylogenetics and Evolution 18: 174188. Marco, A., J. M. Kiesecker, D. P. Chivers, and A. R. Blaustein (1998). "Sex recognition and mate choice by male western toads, Bufo boreas." Animal Behavior 55: 163 1-1635. Marvin, G. A. and V. H. Hutchison. (1996), "Courtship behavior of the Cumberland Plateau Woodland Salamander, Plethodon kentucky, with a review of courtship in the genus Plethodon." Ethology 102: 285-303. Mays, H. L. J. and G. E. Hill (2004). "Choosing mates: good genes versus genes that are a good fit." Trends in Ecology and Evolution 19: 554-559. McCormick, M. 1. (1998). "Behaviorally induced maternal stress in a fish influences progeny quality by a hormonal mechanism." Ecology 79: 1873-1883. McNamara, J. M. and A. I. Houston (1996). "State-dependent life histories." Nature 380: 215-221. Michod, R. E. and 0. Hasson. (1990). "On the evolution of reliable indicators of fitness." The American Naturalist 135: 788-808. Miller, K. A. (2003). "The phylogeny of diving beetles and the evolution of sexual conflict." Biological Journal of the Linnean Society 79(3): 359-388. Mitchell, S. L. (1990). "The mating system genetically affects offspring performance in Woodhouse's toad (Bufo woodhousei)." Evolution 44: 502-5 19. Moore, A. J. and P. J. Moore. (1999). "Balancing sexual selection through opposing mate choice and male competition." Proceedings of the Royal Society of London B 266: 711-716. 103 Moore, A. J., P. A. Gowaty, W. G. Wallin, and P. J. Moore (2000). "Sexual conflict and the evolution of female mate choice and male social dominance." Proceedings of the Royal Society of London Series B 268: 517-523. Moore, A. J., P. A. Gowaty and P. J. Moore (2003). "Females avoid manipulative males and live longer." Journal of Evolutionary Biology 16: 523-530. Morin, P. J. (1983). "Predation, competition, and the composition of larval anuran guilds." Ecological Monographs 53: 119-138. Navara, K. J., G. E. Hill, and M. T. Mendonca (2001). "Are there immunological costs to carotenoid display in songbirds?" American Zoologist 41: 1535-1536. Nicoletto, P. F. (1995). "Offspring quality and female choice in the guppy, Poecilia reticulata." Animal Behavior 49: 377-387. Nicoletto, P. F. and A. Kodric-Brown (2001). "Age and experience affect female choice in the guppy (Poecilia reticulata)." The American Naturalist 157: 3 16-323. Noble, G. K. and M. K. Brady. (1930). "The courtship of plethodontid salamanders." Copeia 1930: 100-117. Olson, D. H. (1988). The ecological and behavioral dynamics of breeding in three sympatric anuran amphibians. Zoology. Corvallis, Oregon State University. Olsson, M., E. Wapstra, and C. Olofsson (2002). "Offspring size-number strategies: experimental manipulation of offspring size in a viviporous lizard." Functional Ecology 15: 135-140. Organ, J. A. (1960a). The courtship and spermatophore of the salamander Plethodon glutinosus." Copeia 1960: 34-40. Organ, J. A. (1960b). "Studies on the life history of the salamander, Plethodon welleri." Copeia 1960: 287-297. Ortigosa, A. and L. Rowe. (2003). "The role of mating history and male size in determining mating behaviors and sexual conflict in a water strider." Animal Behavior 65: 851-858. Pankhurst, N. W., G. Van Der Kraaak, and R. E. Peter (1995). "Evidence that the inhibitory effects of stress on reproduction in teleost fish are not mediated by the action or cortisol on ovarian steroidogenesis." General and Comparative Endocrinology 99: 249-257. 104 Parichy, D. M. and R. H. Kaplan. (1995). "Maternal investment and developmental plasticity: functional consequences for locomotor performance of hatchling frog larvae." Functional Ecolov 9: 606-6 17. Park, D. and S. R. Park. (2000). "Multiple insemination and reproductive biology of Hynobius leechii." Journal of Herpetology 34: 594-598. Parker, G. A. (1979). "Seuxal selection and sexual conflict." Sexual Selection and Reproductive Competition in Insects. M. S. Blum and N. A. Blum. New York, Academic Press. Parker, G. A. and L. Partridge. (1998). "Sexual conflict and speciation." Philosophical Transactions of the Royal Society of London Series B 353: 26 1-274. Partridge, L. (1980). "Mate choice increases a component of offspring fitness in fruitflies." Nature 283: 290-291. Partridge, L. (1983). "Non-random mating and offspring fitness." Mate Choice. P. Bateson. Cambridge University Press, Cambridge, UK Picard, A. (2005). "Courtship in the zig-zag salamander (Plethodon dorsalis): insights into a transition in pheromone delivery behavior." Ethology. In press. Pilastro, A., B. Stefano and A. Bisazza (2003). "Female aggregation and male competition reduce the costs of sexual harassment in the mosquitofish Gambusia hoibrooki." Animal Behavior 65: 1161-1167. Pilz, K. M., H. G. Smith, M. I. Sandell, and H. Schwabl (2003). "Interfemale variation in egg yolk androgen allocation in the European starling: do high-quality females invest more." Animal Behavior 65: 84 1-850. Pizzari, T. and R. R. Snook. (2003). "Perspective: sexual conflict and sexual selection: chasing away paradigm shifts." Evolution 57: 1223-1236. Pomiankowski, A. (1988). "The evolution of female mate preferences for male genetic quality." Oxford Surv. Evol. Biol. 5: 136-184. Pomiankowski, A. and Y. Iwasa (1998). "Runaway ornament diversity caused by Fisherian selection." Proceedings of the National Academy of Science 95: 5206-5 111. Preziosi, R. F. and D. J. Fairbairn (2000). "Lifetime selection on adult body size and components of body size in a waterstrider: Opposing selection and maintenance of sexual size dimorphism." Evolution 54: 558-566. Rails, K. (1976). "Mammals in which females are larger than males." Quarterly Review 105 in Biology 51: 245-276. Rehberg, F. (1986). "Haltung und Zucht der Krokodilmolches Tylototriton verrucosus Anderson 1871." Herpetofauna8: 11-17. Reynolds, J. D. and M. R. Gross (1992). "Female mate preference enhances offspring growth and reproduction in a fish, Poecilia reticulata." Proceedings of the Royal Society of London Series B 250: 57-62. Rice, W. R. (1996). "Sexually antagonistic male adaptation triggered by experimental arrest of female evolution." Nature 361: 232-2344. Rich, T. J. and J. L. Hurst (1998). "Scent marks as reliable signals of the competitive ability of mates." Animal Behavior 56: 727-735. Roesli, M. and H. Reyer. (2000). "Male vocalizations and female choice in the hybridogenetic Rana lessonae/Rana esculenta complex." Animal Behavior 60: 745-755. Rollmann, S. M., L. D. Houck, and R. C. Feldhoff (1999). "Proteinaceous pheromon affecting female receptivity in a terrestrial salamander." Science 285: 1907-1909. Rollmann, S. M., L. D. Houck, and R. C. Feldhoff (2000). "Population Variation in Salamander Courtship Pheromones." Journal of Chemical Ecology 26: 2713-2724. Rosenqvist, G., A. N. Bordal, M. E. Aune, and P. A. Gowaty (2001). "Implications of freely expressed mate choice in the guppy." Advances in Ethology 36: 50. Rothchild, B. J. (1986). Dynamics of Marine Fish Populations. Harvard University Press, Cambridge, Massachusetts, USA. Rowe, L. (1992). "Covenience polyandry in a water strider: foraging conflicts and female control of copulation frequency and guarding duration." Animal Behavior 44: 189-202. Rowe, L. (1994). "The costs of mating and mate choice in water striders." Animal Behavior 48: 1049-1056. Rowe, L., G. Arnqvist, A. Sib, and J. J. Krupa (1994). "Sexual conflict and the evolutionary ecology of mating patterns: water striders as a model system." Trends in Ecology and Evolution 9: 289-293. Rubenstein, D. J. (1984). "Resource acquisition and alternative mating strategies in water striders." American Zoologist 24: 345-3 53. Rudolfsen, G., L. Figenschou, I. Folstad, J. T. Nordeide, and E. Soreng (2005). 106 "Potential fitness benefits from mate selection in the Atlantic cod (Gadus morhua)." Journal of Evolutionary Biology 18: 172-179. Rutkowska, J. and M. Cichon (2002). "Maternal investment during egg laying and offspring sex: an experimental study of zebra finches." Animal Behavior 64: 817-822. Sapp, J. (2002). Courtship behavior in the salamander genus Aneides. Zoology. Corvallis, Oregon, Oregon State University. "Reproductive behavior in the Japanese salamander Hynobius retardatus." Japanese Journal of Herpetology 14: 184-190. Sato, T. (1992). Shine, R., O'Connor, D. and R.T. Mason (2000). "Sexual conflict in the snake den." Behavioral Ecology & Sociobiology 48: 392-401. Shine, R., Langkilde, T. and R.T. Mason (2003). "Cryptic forcible insemination: male snakes exploit female physiology, anatomy, and behavior to obtain coercive matings." American Naturalist 162: 653-667. Shine, R., Phillips, B., Langkilde, T., Lutterschmidt, D.I., Waye, H. and R.T. Mason (2004). "Mechanisms and consequences of sexual conflict in garter snakes (Thamnophis sirtalis, Colubridae)." Behavioral Ecology 15: 654-660. Shuker, D. M. and T. H. Day (2001). "The repeatability of a sexual conflict over mating." Animal Behavior 61: 755-762. Shuker, D. M. and T. H. Day (2002). "Mate sampling and the sexual conflict over mating in seaweed flies." Behavioral Ecology 13: 83-86. Smith, B. G. (1907). "The life history and habits of the mole salamander, Ambystoma talpoideum, in southeastern Louisiana." Tulane Studies of Herpeto1oy 8: 65-82. Smith, D. C. (1987). "Adult recruitment in chorus frogs: effects of size and date at metamorphosis." Ecology 68: 344-350. Smuts, B. B. (1992). "Male aggression against females in primates: Data and implications for sexual selection theory." Human Nature 3: 1-44. Smuts, B. B. (1995). "The origins of patriarchy: an evolutionary perspective." Human Nature 6: 1-32. Smith-Gill, S. J. and D. E. Gill (1978). "Curvilinearities in the competition equations: an experiment with ranid tadpoles." American Naturalist 112: 557-570. 107 Smuts, B. B. (1992). 'Male aggression against females in primates: data and implications for sexual selection theory." Human Nature 3: 1-44. Soulier-Perkins, A. (2001). "The phylogeny of the Lophopidae and the impact of sexual selection and coevolutionary sexual conflict." Cladistics 17: 56-78. Spritzer, M. D., D. B. Meikle, and N. G. Solomon (2005). "Female choice based on male spatial ability and aggressiveness among meadow voles." Animal Behavior. In press. Stebbins, R. C. (1949). "Courtship of the plethodontid salamander, Ensatini eschsholtzii." Copeia 1949: 274-28 1. Steinwascher, K. and J. Travis (1983). "Influence of food quality and quantity on early larval growth of two anurans." Copeia 1983: 238-242. Tam, W. H., J. N. Fryer, B. Valentine, and R. J. J. Roy (1990). "Reduction in oocyte production and gonadotrope activity, and plasma levels of estrogens and vitellogenin, in brook trout exposed to low environmental pH." Canadian Journal of Zoology 68: 24682479. Tinbergen, N. (1939). "The behaviour of the snow bunting during spring." Transactions of the Linnean Society 5: 1-94. Travis, J., S. B. Emerson, and M. Blouin (1987). "A quatitative genetic analysis of fitness-related traits in Hyla cruc/er. I. Larval development patterns." Evolution 41: 145-156. Trivers, R. L. (1972). Parental investment and sexual selection. Sexual Selection and the Descent of Man. B. Campbell. Chicago, Aldine: 136-179. Underwood, A. J. and P. G. Fairweather (1989). "Supply-side ecology and benthic marine assemblages." Trends in Ecology and Evolution 4: 16-20. Usuda, H. (1993). "Reproductive behavior of Hynobius nigrescens, with special reference to male midwife behavior." Japanese Journal of Herpetology 15: 64-70. Watson, P. J., G. Arnqvist, and R. R. Stallmann (1998). "Sexual conflict and the energetic costs of mating and mate choice in water striders." The American Naturalist 151: 46-58. Weiner, C. S., C. B. Schreck, and H. W. Li (1986). "Effect of low pH on reproduction in rainbow trout." Transactions of the American Fisheries Society 115: 75-82. Wilbur, H. M. and J. P. Collins (1973). "Ecological aspects of amphibian 108 metamorphosis." Science 182: 1305-13 14. Williams, G. C. (1966). Adaptation and Natural Selection: A Critique of Some Current Evolutionary Thought. Princeton, NJ, Princeton University Press. Wirsig-Wiechmann, C. R., L. D. Houck, P. W. Feldhoff, and F. C. Feldhoff (2002). "Pheromonal activation of vomeronasal neurons in plethodontid salamanders." Brain Research Interactive 952: 335-344. Woodward, B. D. (1987). "Paternal effects on offspring traits in Scaphi opus couchi (Anura: Pelobatidae." Oecologia 73: 626-629. Woodward, B. D., J. Travis, and S. Mitchell (1988). "The effects of mating system on progeny performance in Hyla crucfer." Evolution 42: 784-794. Woodward, B. D., J. Travis, and S. L. Mitchell (1988). "Nonrandom genetic consequences of the spring peeper (Hyla crucfer) mating system." Evolution 42: 784794. Zahavi, A. (1975). "Mate selection - A selection for a handicap." Journal of Theoretical Biology 53: 205-2 14. Zuk, M., R. Thornhill, J. D. Ligon, K. Johnson, S. Austad, S. H. Ligon, N. W. Thornhill, and C. Costin (1990). "The role of male ornaments and courtship behavior in female mate choice of red jungle fowl." The American Naturalist 136: 459-473.