Document 10825872
advertisement

NAME:
MON“KEY”
CHEMISTRY 419, SPRING, 2010 (2103)
Midterm Examination 2, April 22, 2010
Answer each question in the space provided; use back of page if extra space is needed. Answer questions so the grader can READILY
understand your work; only work on the exam sheet will be considered. Write answers, where appropriate, with reasonable numbers of
significant figures. You may use only the "Student Handbook," a calculator, and a straight edge.
1. (10 points) In the figure is shown the potential-energy surface for the reaction
AB C BC
DO NOT WRITE
IN THIS SPACE
A.
# 1________/10
Indicate the following things by putting points or lines with labels:
(a)
the transition state (or saddle point)
(b)
the entrance to the reactant channel for the reaction as written
(c)
the exit from the product channel for the reaction as written
(d)
a reactive event
(e)
an unreactive event.
# 2________/15
(Be sure to label any points or lines you may draw on the graph carefully.)
# 6________/10
# 3________/20
# 4________/15
# 5________/15
# 7________/15
=============
TOTAL PTS
/100
The entrance and exit channels are determined by which diatomic molecule starts (and it will have the smallest
separation); in this case RAB must be small. The transition state is the saddle point in the surface. A reactive event starts
in the reactant channel and ends in the product channel. An unreactive event starts in the in reactant channel and
ultimately moves back out through the reactant channel.
NAME:
CHEM 419, Midterm Exam 2, Spring, 2010, page 2
2. (15 points) Lindemann developed a simple mechanism to explain certain unimolecular reactions. The mechanism
involves the interaction of the reactant molecule (A) with a “mediator” (M) to produce an excited-state molecule (A*), which
ends up reacting to give product (P). The overall reaction is simple:
A P
Here is the mechanism:
A M
A*
M
A*
k1
A*
M
k2
A M
k
P
v1
k1 [ A][ M ]
v2
k 2 [ A* ][ M ]
v3
k 3 [ A* ]
(a)
To the right of each step (as indicated), write an equation for the velocity of the step in terms of the concentrations
and rate constant.
(b)
Assume that the excited-state molecule is a reactive intermediate. Give an expression for the steady-state
concentration of the reactive intermediate in terms of other concentrations and rate constants.
d [ A* ]
0
dt
d [ A* ]
v1
dt
v2
v3
k1 [ A][ M ] k 2 [ A* ][ M ] k 3 [ A* ] 0
Solving this equation gives the steady-state concentration:
[ A* ]
(c)
k1 [ A][ M ]
k3 k 2 [M ]
Give the rate law for the Lindemann mechanism as a function of concentrations and rate constants.
The rate of reaction is given by the rate of creation of product, which happens only in step 3.
v k 3 [ A* ]
Substitution of the steady-state concentration of the excited A molecules gives the final rate law:
v
k 3 k1 [ A][ M ]
k3 k 2 [M ]
Score for Page
NAME:
CHEM 419, Midterm Exam 2, Spring, 2010, page 3
3. (20 points) Insert the proper word or phrase (from the list below) into each of the following sentences to complete it
appropriately.
a.
A reaction in which the reactant disappears exponentially is said to be of
b.
A reaction in which the rate constant for disappearance of a reactant is equal to the sum of the rate constants for
the appearance of two products is said to be a
c.
FIRST
PARALLEL REACTION
order.
.
One means of measuring reactions that occur on short time scales is the
PERTURBATION-RELAXATION
method.
d.
The activity of an enzyme is modeled by
MICHAELIS-MENTEN KINETICS.
e.
In competitive inhibition, the
of the Lineweaver-Burk plot depends on the concentration of inhibitor.
f.
The unit of light intensity is WATT PER METER2.
g.
The most common means of describing the temperature dependence of a rate constant is by reporting the
ARRHENIUS
h.
SLOPE
parameters.
The so-called inversion of the rate constant for electron transfer has been successfully predicted by MARCUS
theory.
i.
In determining rate laws, the concentrations of reactive intermediates are often determined by invoking the
STEADY-STATE
j.
approximation.
Fluorescence resonance energy transfer involves the formation of a
Arrhenius
Eyring
Joule
Michaelis-Menten kinetics
Quenching
Steady-state
Diffusion control
First
Lifetime
Parallel reaction
Second
Stern-Volmer plot
DONOR-ACCEPTOR COMPLEX.
Donor-acceptor complex
Intercept
Lineweaver-Burk plot
Perturbation-relaxation
Sequential reaction
Third
Elementary step
Intersystem crossing
Marcus
Pre-equilibrium
Slope
Watt per meter2
Score for Page
NAME:
CHEM 419, Midterm Exam 2, Spring, 2010, page 4
4. (15 points) The decarboxylation of a -keto acid is catalyzed by a decarboxylase to produce CO2. The rate of reaction
can be determined by measuring the rate of appearance of CO2. The table lists the initial reaction velocity (as the rate of
formation of CO2) for various concentrations of the acid. (In these experiments, the enzyme concentration was fixed.) From
these data, determine the maximum reaction velocity and the Michaelis-Menten constant for this reaction.
[-keto acid]
(mole dm-3)
Initial velocity
(micromoles dm-3 s-1)
1/[-keto acid]
(dm3 mole-1)
2.500
1.000
0.714
0.526
0.250
0.588
0.500
0.417
0.370
0.256
0.400
1.000
1.401
1.901
4.000
The appropriate plot is a Lineweaver-Burk plot:
1
v0
v max [ S ]0
}
[ S ]0 K m
{HINT: The reaction rate law for Michaelis-Menten kinetics is: v 0
1/(Initial velocity)
(s dm3 micromole1
)
1.701
2.000
2.398
2.703
3.906
1
v max
Km
v max
1
[ S ]0
. In the third and fourth rows of the table
are the inverses of the concentration and initial velocity. A plot is shown below. The slope of the line, as determined in
EXCEL is 0.617 s mole micromole-1 = 0.617 106 s. Similarly, the intercept is 1.467 s dm3 micromole-1 = 1.467 106 s dm3
mole-1.
From the inverse of the intercept,
v max
6.817 10 7 mole dm 3 s 1 .
From this value and the slope, the Michaelis-Menten constant is
Km
0.421 mole dm 3
1/(initial velocity (s dm 3 micromole -1)
4.500
4.000
3.500
3.000
2.500
2.000
1.500
1.000
0.500
0.000
0.000
0.500
1.000
1.500
2.000
2.500
3
3.000
3.500
4.000
4.500
-1
1/[ -keto acid] (dm mole )
Score for Page
NAME:
CHEM 419, Midterm Exam 2, Spring, 2010, page 5
5. (15 points) Water has several vibrational modes. Water has been of concern because the 13 million tons of water in the
atmosphere may participate in so-called global warming. In the infrared spectrum, a vibrational transition of water (the
symmetric stretch mode) occurs at an energy of 3657.05 cm-1.
(a) What is the frequency of a photon that corresponds to this energy?
c
2.99792458 10 8 m s 1 3657.05 10 2 m 1
1.09636 1014 Hz
(b) Express that energy in joules.
E h
hc
6.6260693 10 34 J s 1.09636 1014 Hz
7.264557 10 20 J
(c) The solar flux at Mars is approximately 610 W m-2. Assume (a wild assumption) that all the energy absorbed by the
Martian surface ultimately ends up as vibrational excitation of water (if it is present on the surface of Mars). To maintain a
constant temperature of Mars, that energy must ultimately be reemitted into space. There are three vibrations of the water
molecule, so assume that 1/3 of the absorbed energy is radiated through the symmetric vibration at 3657.05 cm-1. Assume,
at equilibrium, only one radiative event per molecule per second. How many water molecules would be in one square meter
of Martian surface to keep the temperature constant by this radiative-emission process?
Only 1/3 of the flux ends up in this vibrational mode, so one must divide the total flux by 3 to find out how much energy ends
up in that mode:
Emode
610W m 2
3
203.3W m 2
All of this energy is reradiated through the mode, one photon per molecule. Thus, one can divide this energy by the energy
of the photon, to find the number of photons that must be emitted per second.
N photons
203.3W m 2
7.264557 10 20 J photon 1
2.79898 10 21 photon m 2 s 1
Since each of these photons corresponds to one molecule (In actuality, molecule probably re-emit much more often, but we
are just doing a simple calculation here.), this number of photons is the number of water molecules:
N molecule
2.79898 10 21 molecule m 2
About 27 millimoles per square meter.
Score for Page
NAME:
CHEM 419, Midterm Exam 2, Spring, 2010, page 6
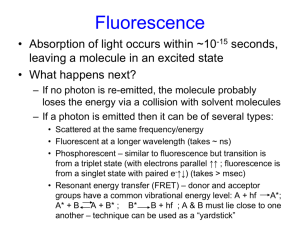
6. (10 points) A Jablonski diagram from your textbook is shown in the figure. (a) On it are indicated several processes. In
the table, circle the phrase to right that most correctly describes each lettered process. There is only one correct answer for
each process. (b) The letter S on the diagram stands for what? SINGLET
Process Label
A
Absorption from the ground electronic
state
Emission from the ground
electronic state
Vibrational relaxation
B
Internal conversion
Intersystem crossing
Vibrational relaxation
C
Fluorescence
Internal conversion
Intersystem crossing
D
Emission from the ground electronic
state
Internal conversion
Vibrational relaxation
E
Fluorescence
Internal conversion
Phosphorescence
F
Internal conversion
Intersystem crossing
Vibrational relaxation
G
Fluorescence
Internal conversion
Intersystem crossing
H
Emission from the ground electronic
state
Fluorescence
Intersystem crossing
I
Fluorescence
Internal conversion
Vibrational relaxation
Score for Page
NAME:
CHEM 419, Midterm Exam 2, Spring, 2010, page 7
7. (15 points) Barker et al. (Anal. Chem, 71, 1767 – 1772 (1999).) reported a device consisting of fluorescent-dye-labeled
cytochrome c’ supported on colloidal gold particles. The device is intended to be a detector of nitric oxide in biological
systems. The basis of the detection is the quenching of the dye’s fluorescence by nitric oxide that interacts with the
cytochrome, thus shortening the lifetime of the dye fluorescence. The figure below, modified from one of their figures, shows
the fluorescence decay of the dye-labeled cytochrome in the absence of nitric oxide following excitation with a laser pulse.
Determine the fluorescence lifetime of the dye in the absence of nitric oxide. [Show all work clearly.]
The fluorescence decay, after the initial excitation, is predicted by theory to be exponential. That is, the fluorescence is
predicted to follow the equation
I f (t ) I f , 0 e
t / f
,
where f is the fluorescence lifetime. One may observe that a plot of the natural logarithm of the fluorescence versus time
If
ln
I
f ,0
should be linear and obey the following equation:
t
f
The plot happens to show the logarithm to the base 10 of the fluorescence versus time. However, these two logarithms are
related by the following formula:
log10 y
1
ln y . So, the log10 of the fluorescence would also be
2.30259
expected to be linear and of the form:
If
log10
I
f ,0
If
1
ln
2.30259 I f , 0
t
2.30259 f
The red line shown in the figure is an estimated straight line through the data (excluding the part during the excitation with a
laser pulse at short times). Its slope is estimated, as shown by the blue lines, to be
slope
2.35 2.85
8 2ns
0.0833 ns 1
From this slope, one easily finds the fluorescence lifetime. Using these numbers gives a value:
f
5.2 ns .
Depending on how one estimated the slope, the value could be between 4.5 ns and 6.0 ns.
Score for Page